- 1College of Marine Sciences, Shanghai Ocean University, Shanghai, China
- 2The Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources, Ministry of Education, Shanghai, China
- 3National Engineering Research Centre for Oceanic Fisheries, Shanghai Ocean University, Shanghai, China
- 4The Key Laboratory of Oceanic Fisheries Exploration, Ministry of Agriculture and Rural Affairs, Shanghai, China
Stable isotope analysis (SIA) has proven to be a powerful tool in reconstructing diets and characterizing trophic relationships for pelagic predators. Ethanol has been a common preservative solution for biopsy samples from remote areas and archived collections. It is still under debate whether the effects of ethanol (ET) would bias the trophic interpretation of the stable isotope values. Further, lipid extraction (LE) is becoming more popular as a general treatment for standardization prior to SIA, particularly for investigating intra and interspecific variation of sympatric species, because lipids have lower δ13C values. In this study, the long-term (up to 448 days) effects of treatment ET and combined treatments ET and LE (ET+LE) on stable carbon and nitrogen isotope values (δ13C and δ15N, respectively) of twelve pelagic predators from the open ocean were evaluated. Results showed that compared with control values, δ15N values displayed a positive change (δ15Nmean offset was 0.71 ± 0.56‰) but δ13C values had variable results (δ13Cmean offset was 0.42 ± 0.64‰) among all species following treatment with ET during the first 28 days and then remained stable throughout the experiment. Compared with treatment LE results, no difference was observed in δ13C, δ15N values, and C/N ratios through time following treatment ET+LE. These results indicated that treatment ET may have species-specific effects on stable isotope values, and the shifts from treatment LE could counter the changes caused by treatment ET. In addition, after 28 days of preservation, the values following treatment ET were similar to those following treatment LE in low C/N species (C/N<3.5), which suggested ethanol may also affect some of lipid contents from muscle tissues. Nevertheless, further research is needed to focus on the mechanisms that control changes in stable isotope composition in tissues stored in ethanol. Given the effects on pelagic predators, muscle tissue samples stored in ethanol from the open ocean or a museum after LE treatment could be used to develop SIA.
Highlight
1. Ethanol may have species-specific effects on stable isotope values within a short period.
2. Lipid extraction could counter the changes caused by ethanol.
3. Ethanol may affect some of lipid contents from muscle tissues.
4. Muscle tissue samples stored in ethanol from the open ocean or museum following LE could be used for stable isotope analysis.
Introduction
Understanding the trophic ecology of pelagic predators is essential as they can profoundly regulate the structure of marine communities (Baum et al., 2003). Studies using stable isotopes (SIA) to access foraging behaviors and migration patterns of pelagic predators are increasing (Li et al., 2016; Gallagher et al., 2017; Bird et al., 2018; Wyatt et al., 2019; Prieto-Amador et al., 2022). These applications are based on the premise that the stable carbon and nitrogen isotope ratios (δ13C and δ15N, respectively), fractionate systematically throughout the food web as predators consume preys (Peterson and Fry, 1987; Boecklen et al., 2011). Specifically, δ13C values are generally used to determine the consumer’s original dietary carbon source, whereas δ15N values can provide knowledge of trophic relationships since 15N relatively enriched in consumer tissues (Peterson and Fry, 1987; Post, 2002).
Muscle is the common biopsy tissue, mostly used in genetic studies since it can be easily assessed and is increasingly used for other new biochemical prospective studies such as trophic ecology studies based on SIA (Boecklen et al., 2011; Kiszka, 2014). Compared with most neritic species, trophic studies of pelagic predators often involve a delay between sample collection and laboratory processing. Although freezing is the preferred method of samples preservation due to the limited effects on organisms’ δ13C and δ15N values (Kaehler and Pakhomov, 2001), this method is inconvenient for samples collected from remote areas or archived samples (Hobson et al., 1997; Carabel et al., 2009). Ethanol (ET) is the most commonly used preservative to store fish tissues. Since the effects of treatment ET on muscle δ13C and δ15N values of marine organisms were reported to be variable and inconsistent among species, conducting SIA directly using treatment ET samples was not recommended (Barrow et al., 2008; Boecklen et al., 2011; Kim and Koch, 2012). In addition, lipid extraction (LE) was recommended as a standardization step prior to SIA since lipids were 13C-depleted relative to proteins and carbohydrates and could potentially cause the δ13C values of organisms to be negatively biased relative to their diet with increasing lipid content (Wessels and Hahn, 2010; Elliott and Elliott, 2016; Li et al., 2016; Bennett-Williams et al., 2022). However, there was less evidence to indicate whether treatment LE affected the stable isotope values of treatment ET samples. Thus, biases introduced by combining treatments ET and LE (ET+LE) must be weighed against the magnitude of effects expected to be documented.
In this context, prior to SIA, treatments ET and ET+LE were performed on muscle tissue of twelve pelagic predators from tuna longline fisheries to 1) investigate the temporal and species-specific effects of treatments ET and ET+LE on δ13C, δ15N values, and C/N ratios; and 2) determine the relationship between treatments ET and LE in stable isotopic effects and whether treatment ET samples could be used in further SIA.
Materials and methods
Sampling methods
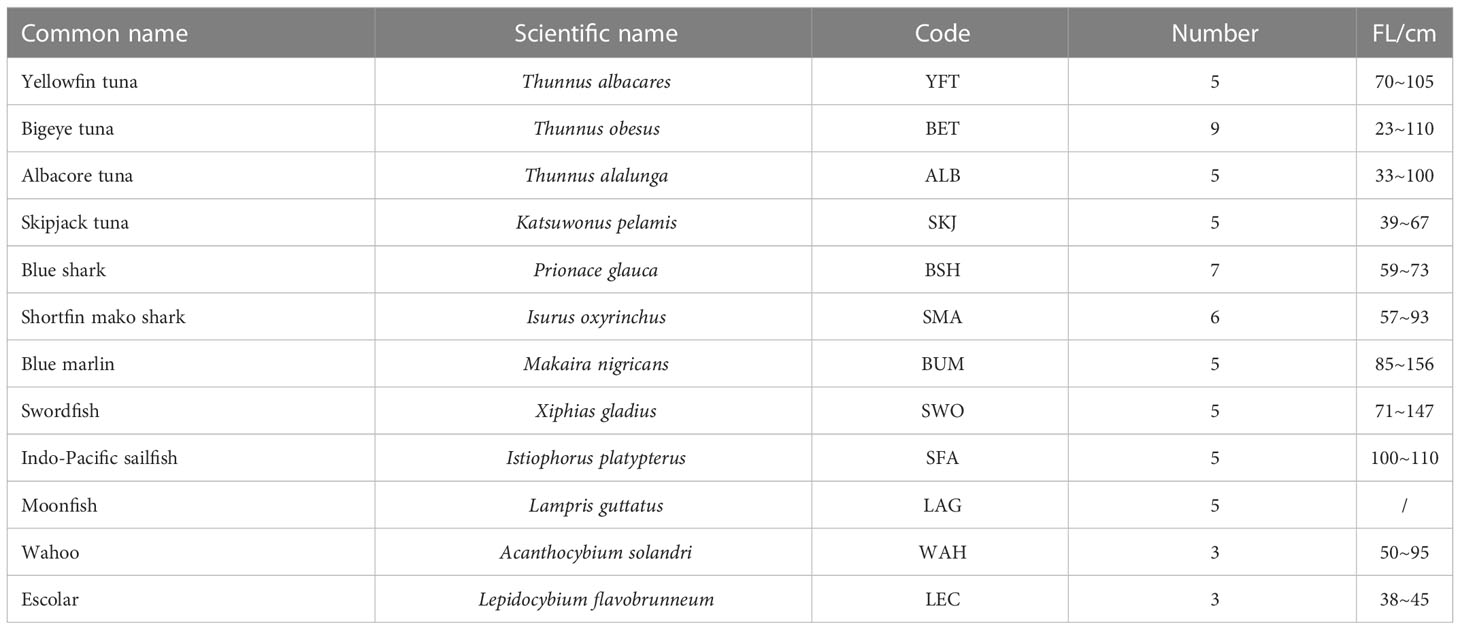
The muscle tissues of 63 individuals from twelve fish species were sampled from pelagic longline fisheries operating in the Atlantic (32°9′S~18°32′S, 5°19′W~7°13′E) in October 2015. For each individual, fork length (FL) was measured to the nearest cm, and a white muscle sample was excised from the dorsal area and immediately frozen at -50°C before being transferred back to the lab (Table 1).
Sample preparation
Each muscle sample was equally divided into seven subsamples. The first one was handled immediately (referred to as “Control”). The others were preserved in 95% ethanol (referred to as “ET”), and were assigned to different periods (7, 28, 56, 112, 238, 448 days). At the assigned date of analysis, each subsample was freeze-dried at -55°C for 48 hours using a Christ Alpha 1-4 LD plus Freeze Dryer (Martin Christ; Osterode am Harz, GER) and homogenized using a Retsch Mixer Mill MM 400 (RETSCH; Haan, GER). Then, in the 2:1 chloroform/methanol mixture, half of each subsample was lipid extracted (referred to as “ET+LE”, ET+LE for 0 days could be regarded as “LE”). The mixture was vortexed for 1 min and left undisturbed overnight at room temperature, then centrifuged for 10 min and decanted. This process was repeated three times, and the samples were re-dried overnight at 80°C to eliminate excess solvent. And then a 1.0~2.0 mg powdered muscle tissue from each subsample was prepared for SIA (Li et al., 2016).
Stable isotope analysis
Approximately 1.0-1.5 mg of prepared sample were weighed into 0.3 mg tin capsules and analyzed using an IsoPrime 100 isotope ratio mass spectrometer (IsoPrime Corporation; Cheadle, UK) and a vario ISOTOPE cube elemental analyzer (Elementar Analysensysteme GmbH; Hanau, Germany) with analytical error at Shanghai Ocean University Stable Isotope Laboratory.
The isotope compositions of the samples were expressed as δ13C and δ15N notation using the following equations:
where 13C/12C and 15N/14N are the atomic ratios of 13C and 15N in the sample or standard, respectively, and δ is the measure of the heavy-to-light isotope in the sample, expressed in parts per thousand (‰). The standard reference materials for C and N were Pee Dee Belemnite carbonate (VPDB) and air (Air-N2), respectively. Reference standards USGS 24 (-16.049‰) and USGS 26 (53.7‰) were used to quantify 13C and 15N stable isotope values, respectively. Every tenth sample was run in triplicate of Organic Analytical Standard (Protein (-26.98‰ and 5.96‰)) to correct for linearity and instrument drift, and a blank sample was run every ten samples to clear off residual gases. The analytical errors were approximately 0.20‰ for both δ13C and δ15N values. All C/N ratios were calculated based on atomic mass.
Statistical analysis
Paired Student’s t-test was performed on each treatment (ET and ET+LE) on δ13C, δ15N values, and on C/N ratios to assess the effect of each period of preservation on those parameters. Then we used analysis of variance (ANOVA) to assess the effect of time of preservation on stable isotope values, and used exponential relationships to determine when preservation alters stable isotope values. The difference in δ13C and δ15N values between the day 0 and each time (δ0 day- δx days) was calculated for each sample (hereafter referred to as the δ13C and δ15N offsets), and Analysis of Variance (ANOVA) was then performed to compare the differences. Finally, a paired Student’s t-test was performed to compare the differences between treatments ET and LE samples. All statistical analyses were performed in SPSS 22.0 and/or R 4.1.0.
Results
The means of the δ13C, δ15N, and the C/N ratios for all treatments of the different periods are shown in Table S1. Our results showed that treatment ET produced species-specific effects on the δ13C, δ15N values, and C/N ratios during 28 days of the experiment and then these values remained stable throughout the experiment. Meanwhile, stable isotope values and C/N ratios of all the species did not change following treatment ET+LE (Figure 1). Paired t-tests revealed no significant difference in δ13C, δ15N values, and C/N ratios between treatments LE and ET+LE for all twelve species at each period (Figure 1).
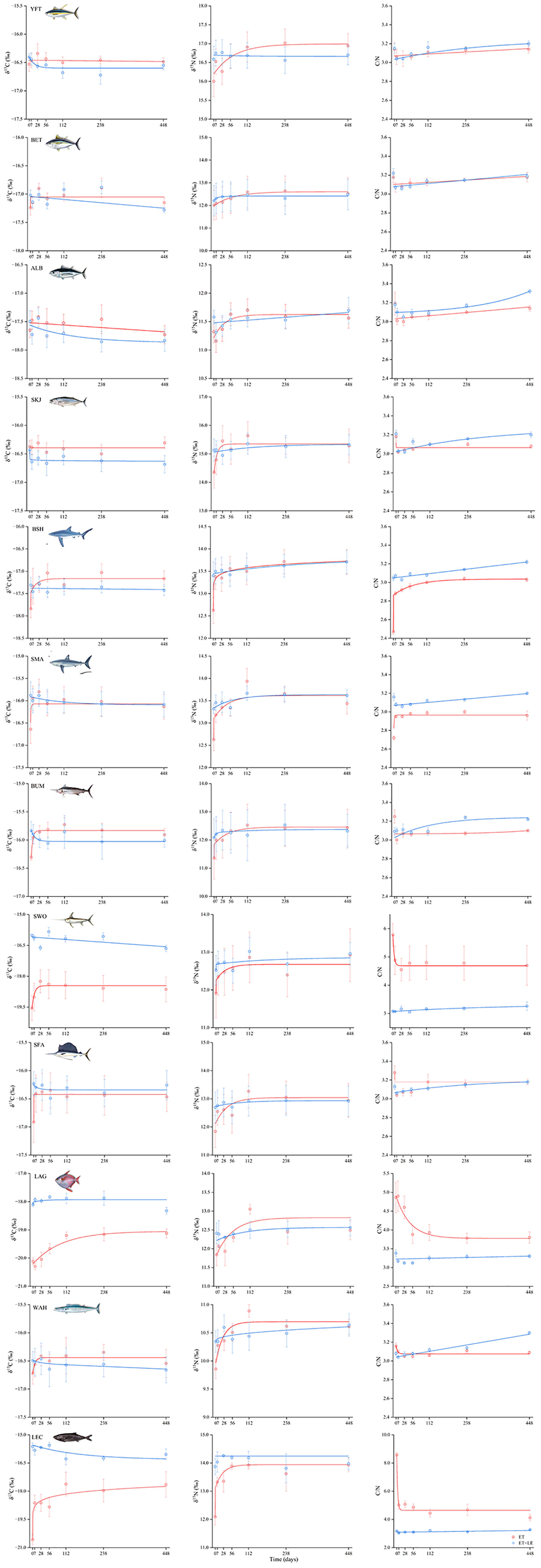
Figure 1 Time series data of mean±SE of δ13C and δ15N value and C/N ratios in twelve pelagic predators’ muscle stored ethanol (red) and combined ethanol storage and lipid extraction (blue).
Specifically, the effects of treatment ET on δ13C values were variable through time until reaching an asymptote, where the direction and magnitude of change varied among taxa. Compared with control values, positive shifts were observed in P. glauca, I. oxyrinchus, M. nigricans, X. gladius, I. platypterus, L. guttatus, A.solandri and L. flavobrunneum (Table S1), while substantial variation was detected in δ13C offsets among these species (Figure 2). There were no changes in δ13C values of tuna species following treatment ET (Table S1), but a mean negative change occurred in K. pelamis (P<0.05). The largest offsets were observed in L. flavobrunneum (2.36 ± 0.64‰). Treatment LE caused positive shifts in δ13C values across all species except for tuna species (Figure 1; Table S1) and the δ13C offsets of L. flavobrunneum were utmost (4.86 ± 0.16‰), which were similar to the treatment ET. Paired t-test results between treatments LE and ET+LE showed limited shifts in δ13C values among all twelve species at each period (Figure 1; Table S1), and the δ13C offsets displayed little individual variation (Figure 2).
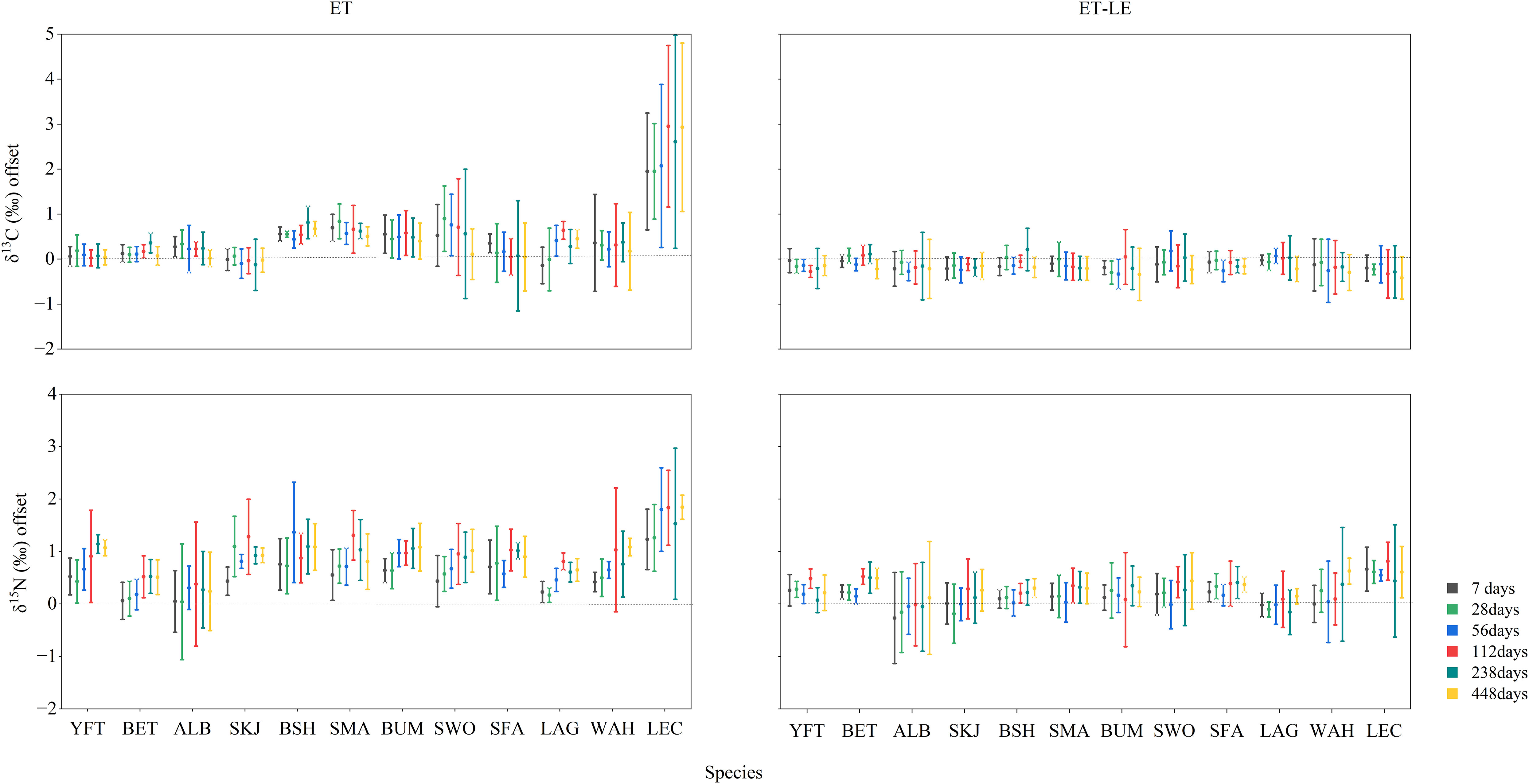
Figure 2 The δ13C and δ15N offsets for all treatments (ET, ET+LE) in muscle tissue across each species.
For δ15N values, both treatments ET and LE caused a positive shift for all the species during a short period (Figure 1; Table S1), with L. flavobrunneum showing the largest δ15N offsets (ET: 2.15 ± 0.56‰, LE: 1.45± 0.17‰, Figure 2). The δ15N values showed no changes among all the species at each period between treatments LE and ET+LE, which was consistent with the effects of δ13C values (Figure 1). The C/N ratios of P. glauca and I. oxyrinchus in either treatment ET (ranged from 2.63 to 3.11) or LE (ranged from 2.97 to 3.25) were generally higher than control (ranged from 2.37 to 2.80, Table S1). On the contrary, the other teleost species displayed negative shifts in C/N ratios following both treatments, LE and ET (Table S1). Furthermore, no difference in C/N ratios was found between treatments LE and ET+LE (Figure 1). In addition, stable isotope values and C/N ratios of muscles following treatment ET were different from the treatment LE samples on the 7th dayand/or the 28th day of preservation (P<0.05), and then simulation results were observed throughout the experiment, except for the δ13C values of X.gladius, L.guttatus, and L.flavobrunneum.
Discussion
SIA has the potential to significantly improve our understanding of predator’s trophic ecology, but it is dependent on the removal of known biases caused by the treatment method. Understanding the effects of treatments ET and ET+LE on predator muscle tissue stable isotope values is valuable for accurately interpreting data in food-web studies. Removing these biases is essential for acquiring standardized stable isotope values among different species, and a reasonable adjustment could be helpful for the use of the valuable biopsy samples obtained from remote areas and archived collections (Kim and Koch, 2012; Li et al., 2016; Bennett-Williams et al., 2022). In this study, the results for twelve pelagic predators suggested that both treatments ET and ET+LE caused inconsistent effects in δ13C, δ15N values, and C/N ratios during the 28 days of preservation and then could remain stable throughout the experiment, yielding new insights into the effects of these treatments.
Post et al. (2007) considered the increases in tissue δ13C values to be positively correlated to the amount of lipids. In this study, twelve species were affected inconsistently with different δ13C offsets following treatments either ET or LE suggesting potentially different lipid content and species-specific metabolic pathways (Javornik et al., 2019). Von Endt (1994) found that a variety of lipids in the ethanol storage media, especially some nonpolar lipids such as triglycerides, could be extracted by ethanol. Consequently, ethanol and the 2:1 chloroform/methanol mixture extraction of 13C-depleted and carbon-rich lipids from tissues may lead to changes in δ13C values. Meanwhile, our results showed the δ13C values could remain stable after the initial change, which was similar to previous researches (Le Bourg et al., 2020; Sarakinos et al., 2002). A possible mechanism is that protein lysis in muscle and integration of C from the preservative liquid into the samples may occur in a short period (Sarakinos et al., 2002). However, δ13C values of treatment ET+LE in muscle were similar to the LE, suggesting that 13C-depleted compounds such as lipids have been removed, potentially counteracting the changes in δ13C values caused by the treatment ET.
This study discovered unexpected effects of treatment ET on δ15N in all species. Such δ15N enrichment following treatment ET was reported in other fishes and molluscs (Kelly et al., 2006; Sweeting et al., 2006; Syväranta et al., 2011; Liu et al., 2013). Ethanol causing tissue hydrolysis, leaching, and extracting certain constituents containing nitrogen from the muscle tissue in addition to lipids could explain these shifts (Horii et al., 2015; Sarakinos et al., 2002). Furthermore, we discovered that treatment LE resulted in a significant increase in δ15N values in muscle tissues. It conflicts with previous research showing no effects or a small positive shift (Javornik et al., 2019; Le Bourg et al., 2020), but agrees with Li et al. (2016). Polar solvents, such as chloroform-methanol and the 2:1 chloroform/methanol mixture extraction, are commonly used for lipid extraction since they can extract both nonpolar lipids (e.g., triglycerides) and polar lipid compounds (e.g., phospholipids and free fatty acids) (Schlechtriem et al., 2003; Doucette et al., 2010). Giménez et al. (2017) reported that polar solvents could increase δ15N values in tissues as a substantial amount of non-lipids, including some hydrophobic essential amino acids, were extracted (Elliott and Elliott, 2016). In addition, the C/N ratios were between 3 and 3.5 in both treatments ET+LE and LE treatments, suggesting lipid extraction was sufficient. No major effects on δ15N between the two treatments instructed that lipid extraction was sufficient. Thus, sufficient lipid extraction may reduce the variability of treatment ET effects on stable isotope signatures in marine organisms (Lesage et al., 2010; Ruiz-Cooley et al., 2011). Meanwhile, our study showed no time-dependent variation of δ15N signatures with chemical preservatives. Sweeting et al. (2004) also reported that the most significant effects of ethanol preservation on stable isotope signatures generally occurred within a short period. We observed a decrease in C/N ratios of all teleost species following two treatments because of removing of carbonaceous waste from lipids. (Kaehler and Pakhomov, 2001; Javornik et al., 2019). However, shark species showed the opposite effect, suggesting that the 2:1 chloroform/methanol mixture could also remove urea leading to depletes in 15N (Hussey et al., 2012; Li et al., 2016).
Previous research showed that it is not necessary to account for lipids in aquatic animal samples when lipid content is consistently lower than 5% (C/N< 3.5) (Post et al., 2007). However, in our study, ethanol and the 2:1 chloroform/methanol mixture could significantly affect the stable isotope values of eight species with low lipid content (C/N<3.5), suggesting removing lipids are also necessary for these species. In addition, the stable isotope values following treatment ET were similar to those following treatment LE in nine species after the 28 days of preservation, indicating ethanol could also affect some of lipid contents from muscle tissues, though this effect is not significant in species with high C/N ratios (X.gladius, L.guttatus, and L.flavobrunneum). However, further research is required to understand the mechanisms behind ethanol-induced changes in stable isotope values.
In summary, when using archived samples to SIA, the effects of preservation method should be considered. Our results indicated that ethanol could produce species-specific effects on stable isotope values and C/N ratios in pelagic predators within a short period. For LE samples, we can ignore the effects of ethanol since the shifts from LE could counter the changes caused by ET. In addition, researchers could use low C/N samples (C/N<3.5) following treatment ET after 28 days for stable isotope analysis instead of treatment LE samples, since the ethanol and the 2:1 chloroform/methanol mixture may have similar effects in these species. Treatment ET+LE is thus recommended as the optimal method for obtaining stable isotope values from archived samples.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Shanghai Ocean University.
Author contributions
YS and YL conceived and designed the experiments. FW provided the tissue samples. YS performed the experiments and analyzed the data with the help of MD, YG and YL. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (42276092, 31872573) and program on the Survey, Monitoring and Assessment of Global Fishery Resources sponsored by the Ministry of Agriculture and Rural Affairs.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1118013/full#supplementary-material
References
Barrow L. M., Bjorndal K. A., Reich K. J. (2008). Effects of preservation method on stable carbon and nitrogen isotope values. Physiol. Biochem. Zool. 81, 688–693. doi: 10.1086/588172
Baum J. K., Myers R. A., Kehler D. G., Worm B., Harley S. J., Doherty P. A. (2003). Collapse and conservation of shark populations in the Northwest Atlantic. Science 299, 389–392. doi: 10.1126/science.1079777
Bennett-Williams J., Skinner C., Wyatt A. S. J., McGill R. A. R., Willis T. J. (2022). A multi-tissue, multi-species assessment of lipid and urea stable isotope biases in mesopredator elasmobranchs. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.821478
Bird C. S., Veríssimo A., Magozzi S., Abrantes K. G., Aguilar A., Al-Reasi H., et al. (2018). A global perspective on the trophic geography of sharks. Nat. Ecol. Evol. 2 (2), 299–305. doi: 10.1038/s41559-017-0432-z
Boecklen W. J., Yarnes C. T., Cook B. A., James A. C. (2011). On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 42, 411–440. doi: 10.1146/annurev-ecolsys-102209-144726
Carabel S., Verísimo P., Freire J. (2009). Effects of preservatives on stable isotope analyses of four marine species. Estuar. Coast. Shelf Sci. 82 (2), 348–350. doi: 10.1016/j.ecss.2009.01.01
Doucette J. L., Wissel B., Somers C. M. (2010). Effects of lipid extraction and lipid normalization on stable carbon and nitrogen isotope ratios in double-crested cormorants: implications for food web studies. Waterbirds 33, 273–284. doi: 10.1675/063.033.0302
Elliott K. H., Elliott J. E. (2016). Lipid extraction techniques for stable isotope analysis of bird eggs: Chloroform-methanol leads to more enriched 13C values than extraction via petroleum ether. J. Exp. Mar. Biol. Ecol. 474, 54–57. doi: 10.1016/j.jembe.2015.09.017
Gallagher A. J., Shiffman D. S., Byrnes E. E., Hammerschlag-Peyer C. M., Hammerschlag N. (2017). Patterns of resource use and isotopic niche overlap among three species of sharks occurring within a protected subtropical estuary. Aquat. Ecol. 51 (3), 435–448. doi: 10.1007/s10452-017-9627-2
Giménez J., Ramírez F., Forero M. G., Almunia J., Stephanis R., Navarro J. (2017). Lipid effects on isotopic values in bottlenose dolphins (Tursiops truncatus) and their prey with implications for diet assessment. Mar. Biol. 164, 1–9. doi: 10.1007/s00227-017-3154-5
Hobson K. A., Gibbs H. L., Gloutney M. L. (1997). Preservation of blood and tissue samples for stable-carbon and stable-nitrogen isotope analysis. Can. J. Zool. 75, 1720–1723. doi: 10.1139/z97-799
Horii S., Takahashi K., Furuya K. (2015). Effects of ethanol-preservation on stable carbon and nitrogen isotopic signatures in marine predators. Plankton. Benthos. Res. 10 (2), 91–97. doi: 10.3800/pbr.10.91
Hussey N. E., MacNeil M., Olin J., McMeans B. C., Kinney M. J., Chapman D. D., et al. (2012). Stable isotopes and elasmobranchs: tissue types, methods, applications and assumptions. J. Fish Biol. 80 (5), 1449–1484. doi: 10.1111/j.1095-8649.2012.03251.x
Javornik J., Hopkins III J B, Zavadlav S., Levanič T., Lojen S., Polak T., et al. (2019). Effects of ethanol storage and lipids on stable isotope values in a large mammalian omnivore. J. Mammal. 100 (1), 150–157. doi: 10.1093/jmammal/gyy187
Kaehler S., Pakhomov E. A. (2001). Effects of storage and preservation on the δ13C and δ15N signatures of selected marine organisms. Mar. Ecol. Prog. Ser. 219, 299–304. doi: 10.3354/meps219299
Kelly B., Dempson J. B., Power M. (2006). The effects of preservation on fish tissue stable isotope signature. J. Fish Biol. 69, 1595–1611. doi: 10.1111/j.1095-8649.2006.01226.x
Kim S. L., Koch P. L. (2012). Methods to collect, preserve, and prepare elasmobranch tissues for stable isotope analysis. Environ. Biol. Fish. 95 (1), 53–63. doi: 10.1007/s10641-011-9860-9
Kiszka J. (2014). Effect of ethanol preservation on stable carbon and nitrogen isotope values in cetacean epidermis: Implication for using archived biopsy samples. Mar. Mammal Sci. 30 (2), 788–795. doi: 10.1111/mms.12058
Le Bourg B., Lepoint G., Michel L. N. (2020). Effects of preservation methodology on stable isotope compositions of sea stars. Rapid Commun. Mass Spectrom. 34, e8589. doi: 10.1002/rcm.8589
Lesage V., Morin Y., Rioux È, Pomerleau C., Ferguson S. H., Pelletier É. (2010). Stable isotopes and trace elements as indicators of diet and habitat use in cetaceans: Predicting errors related to preservation, lipid extraction, and lipid normalization. Mar. Ecol. Prog. Ser. 419, 249–265. doi: 10.3354/meps08825
Li Y., Zhang Y., Hussey N. E., Dai X. (2016). Urea and lipid extraction treatment effects on δ15N and δ13C values in pelagic sharks. Rapid Commun. Mass Spectrom. 30 (1), 1–8. doi: 10.1002/rcm.7396
Liu B., Liu Y., Li Y., Wang H., Xu J. (2013). An assessment of sample preservation methods for the determination of stable carbon and nitrogen isotope ratios in mollusks. Anal. Lett. 46, 2620–2634. doi: 10.1080/00032719.2013.805415
Peterson B. J., Fry B. (1987). Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 18, 293–320. doi: 10.1146/annurev.es.18.110187.001453
Post D. M. (2002). Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecol. 83, 703–718. doi: 10.2307/3071875
Post D. M., Layman C. A., Arrington D. A., Takimoto G., Quattrochi J., Montana C. G. (2007). Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecol. 152, 179–189. doi: 10.1007/s00442-006-0630-x
Prieto-Amador M., Medina A., Varela J. L. (2022). Trophic niche segregation between the sympatric tunas Thunnus alalunga and Katsuwonus pelamis in the gulf of cadiz (East Atlantic). Mar. Biodivers. 52, 18. doi: 10.1007/s12526-021-01256-y
Ruiz-Cooley R. I., Garcia K. Y., Hetherington E. D. (2011). Effects of lipid removal and preservatives on carbon and nitrogen stable isotope ratios of squid tissues: Implications for ecological studies. J. Exp. Mar. Biol. Ecol. 407, 101–107. doi: 10.1016/j.jembe.2011.07.002
Sarakinos H. C., Johnson M. L., Vander Zanden M. J. (2002). A synthesis of tissue preservation effects on carbon and nitrogen stable isotope signatures. Can. J. Zool. 80 (2), 381–387. doi: 10.1139/z02-007
Schlechtriem C. H., Focken U., Becker K. (2003). Effect of different lipid extraction methods on δ13C of lipid and lipid-free fractions of fish and different fish feeds. Isotopes Environ. Health Stud. 39, 135–140. doi: 10.1080/1025601031000113565
Sweeting C. J., Polunin N. V. C., Jennings S. (2004). Tissue and fixative dependent shifts of δ13C and δ15N in preserved ecological material. Rapid Commun. Mass Spectrom. 18, 2587–2592. doi: 10.1002/rcm.1661
Sweeting C. J., Polunin N. V. C., Jennings S. (2006). Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid Comm. Mass Spectrom. 20, 595–601. doi: 10.1002/rcm.2347
Syväranta J., Martino A., Kopp D., Céréghino R., Santoul F. (2011). Freezing and chemical preservatives alter the stable isotope values of carbon and nitrogen of the Asiatic clam (Corbicula fluminea). Hydrobiologia 658, 383–388. doi: 10.1007/s10750-010-0512-4
Von Endt D. W. (1994). Spirit collections: a preliminary analysis of some organic materials found in the storage fluids of mammals. Collection Forum. 10, 10–19. doi: 10.14351/0831-4985-34.1.53
Wessels F. J., Hahn D. A. (2010). Carbon 13 discrimination during lipid biosynthesis varies with dietary concentration of stable isotopes: implications for stable isotope analyses. Funct. Ecol. 24, 1017–1022. doi: 10.1111/j.1365-2435.2010.01716.x
Keywords: ethanol storage, lipid extraction, pelagic predator, muscle, stable isotopes
Citation: Shen Y, David M, Gong Y, Wu F and Li Y (2023) Effects of ethanol storage and lipid extraction on stable isotope compositions of twelve pelagic predators. Front. Mar. Sci. 10:1118013. doi: 10.3389/fmars.2023.1118013
Received: 08 December 2022; Accepted: 30 January 2023;
Published: 08 February 2023.
Edited by:
Siu Gin Cheung, City University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Ichiro Tayasu, Research Institute for Humanity and Nature, JapanKyung-Hoon Shin, Hanyang University, Republic of Korea
Copyright © 2023 Shen, David, Gong, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunkai Li, eWtsaUBzaG91LmVkdS5jbg==; Feng Wu, Znd1QHNob3UuZWR1LmNu
 Yongfu Shen
Yongfu Shen Mboglen David
Mboglen David Yi Gong
Yi Gong Feng Wu
Feng Wu Yunkai Li
Yunkai Li