- 1Key Laboratory of Marine Ecosystem Dynamics, Second Institute of Oceanography, Ministry of Natural Resources (MNR), Hangzhou, China
- 2Southern Marine Science and Engineering Guangdong Laboratory, Zhuhai, China
- 3Department of Biology, Faculty of Sciences, University of Porto, Porto, Portugal
- 4MARE, Marine and Environmental Sciences Centre, ISPA- University Institute, Lisbon, Portugal
Knowledge about marine tardigrades from the South China Sea is very scarce, with only four species from shallow waters recorded to date. The present study investigated the structure and diversity of tardigrade communities from the deep sea (1517-1725 m) at 8 stations in a polymetallic nodule area of the northern South China Sea. A total of 151 arthrotardigrades were collected belonging to 11 genera (Angursa, Batillipes, Coronarctus, Euclavarctus, Exoclavarctus, Halechiniscus, Moebjergarctus, Raiarctus, Rhomboarctus, Tanarctus and Tholoarctus), representing 17 species. Two Angursa species (Angursa sp. 4 and Angursa sp. 3) were the most abundant (25.2% and 14.6%, respectively), followed by Moebjergarctus sp. (13.9%). Specimens were mostly (90.7%) distributed in the upper layer of the sandy-mud sediment (0-1 cm). The SIMPROF test showed that the composition of tardigrade communities at all stations was not significantly different. At different stations, the number of species, Shannon-Wiener diversity index and Pielou’s evenness index ranged from 4 to 10, 1.94 to 2.87, and 0.75 to 1.00, respectively. The average taxonomic distinctness (Δ+) ranged from 72.50 to 90.00, and the variation in taxonomic distinctness (Λ+) ranged from 316.67 to 1181.25. This study provides some basic information about the biodiversity of the marine tardigrade community in the South China Sea.
1 Introduction
Meiofaunal organisms are very important in energy conversion processes and as indicators of environmental health, as they provide links in the marine benthic food web (Higgins and Thiel, 1988; Montagna, 1995; Zeppilli et al., 2015). However, studies carried out to date all over the world have mainly focused on nematodes, as they are the most abundant meiofaunal group (Grove et al., 2006; Giere, 2009). Tardigrades are usually less numerous, although occasionally they can reach more than 2500 specimens per 50 cm3 (Martinez, 1975), and are poorly understood. Hansen and Kristensen (2020) reported 223 known marine species, including all arthrotardigrades and all species of the three genera of the order Echiniscoidea, as well as six eutardigrade species of the order Parachela. Marine tardigrades can be found from intertidal to abyssal depths, inhabiting a great variety of sediments and living associated with algae, barnacles, holothurians and bryozoans (Nelson et al., 2018). Marine tardigrades have been recorded from all seas; they may live interstitially in the intertidal zone and subtidal coarse coralline sand, tend to be epibenthic in finer sands and mud because of decreased oxygen availability (Giere, 2009), may drift or swim weakly above the substrate thus being considered semibenthic (Kristensen and Renaud-Mornant, 1983), and may live in crevices on polymetallic nodules, abyssal mud and deep-sea ooze (Bussau, 1992; Giere, 2009; Nelson et al., 2018; Bai et al., 2020). Deep-sea tardigrades were reported for the first time by Thiel (1966) from 2600 to 4690 m depth in the Indian Ocean. The populations of marine tardigrades tend to be small and patchy (Nelson, 2002). Basic knowledge of trophic interactions in marine tardigrades is very scarce. Most marine tardigrades are likely to feed on algal cells, including macroalgae and diatoms, while others may be detritivores, bacteriovores, or ectoparasites (Kristensen and Sørensen, 2004).
Studies on marine tardigrades are mainly focused on the discovery of new species. The few tardigrade ecological approaches are focused on intertidal or shallow subtidal tardigrades and are related to the environmental factors influencing their distribution, in particular the type of substrate and characteristics of the sediment and seasonal distribution (Renaud-Debyser, 1956; Renaud-Debyser, 1959a; Renaud-Debyser, 1959b; Renaud-Debyser, 1963; Renaud-Debyser and Salvat, 1963; de Zio and Grimaldi, 1966; Pollock, 1975; Renaud-Mornant, 1979; D’Addabbo Gallo et al., 1987; Gallo D’Addabbo et al., 1999). More recently, Accogli et al. (2011) studied the structure and diversity of tardigrade communities along a Mediterranean coastline, trying to understand the effect of particular environmental factors such as sulfur springs, mussel farms and seasonal tourism; Rubal et al. (2016a) studied the diversity of tardigrades along the northern Portuguese coast, and Tilbert et al. (2019) studied the spatial and seasonal variation in tardigrade densities in a Brazilian estuary. On the other hand, Bartels et al. (2020) found some signals that, at least in the Northern Hemisphere, tardigrade body size was related to latitudinal gradients, which have an effect on minimum body size, with smaller species disappearing at higher latitudes, but not with net primary productivity. The only known biodiversity study focused on deep-sea tardigrades was carried out in the Gulf of Mexico (NW Atlantic) by Romano III et al. (2011). In that study, 54 tardigrades from 43 stations at depths of 625-3159 m were collected in total, and the authors found a slight positive correlation between the number of species and depth and a slight negative correlation between the number of species and longitude. Higgins (1972) mentioned that in aquatic environments, tardigrades were most commonly found in the interstitial habitats (coarse to medium-fine sand) of beaches and submerged sediments. Mu et al. (2020) reported that marine tardigrade abundance had a significant positive correlation with interstitial seawater temperature and median grain size in tidal zones.
The South China Sea is a marginal sea of the western Pacific Ocean. It extends across the tropical and subtropical zones. The Kuroshio Current intrudes from the Philippine Sea through the Luzon Strait to the northern South China Sea year-round (Nan et al., 2015). The fauna of the southern South China Sea possesses typical tropical elements paralleled with the typical tropical faunal center of the Philippine-New Guinea-Indonesia Coral triangle. The shelf of the northern South China Sea holds abundant distributions of decapod crustaceans and bivalve mollusks (Zhong, 1986; Song et al., 2003; Liu, 2013). Generally, most studies have focused on micro- and megaplankton, as well as macrofauna and nekton. For example, Miao and Thunell (1993) investigated benthic foraminiferal distributions in the South China and the Sulu Sea. Li et al. (2022) studied the geographical meroplankton distribution pattern among the South China Sea and Philippine Sea. Meiofauna studies carried out in the South China Sea have mainly focused on nematodes with studies on spatial distribution (Du et al., 2010), seasonal distribution (Tang et al., 2012a; Tang et al., 2012b; Jia et al., 2020), effects of environmental factors (Wang et al., 2009; Cai et al., 2012) and abundance and biomass (Liu et al., 2014; Liu et al., 2015; Qiao et al., 2021). The structure of communities of free-living nematodes was reported in the northeastern part of the South China Sea by Jia et al. (2020) and in both deep-sea (313-1600 m) and shallow waters (87 m) by Liu et al. (2014); Liu et al. (2015). However, the marine tardigrades in the South China Sea are deeply understudied, with only four shallow-water (from 0–4 m) species reported until now: Batillipes mirus Richters, 1909 (Renaud-Mornant and Serène, 1967); Batillipes philippinensis Chang and Rho, 1997 (Chang and Rho, 1997); Florarctus kwoni Chang and Rho, 1997 and Parastygarctus higginsi Renaud-Debyser, 1965 (Renaud-Mornant, 1967; Renaud-Mornant and Serène, 1967). Such a scarcity of records justifies the great need for ecological information in this previously ignored area.
In this paper, we investigated for the first time the diversity of deep-sea tardigrade communities (1517-1725 m) from the South China Sea. This study provides some basic information about the biodiversity of the marine tardigrade community in the South China Sea.
2 Materials and methods
2.1 Sampling stations, procedures and sampling treatment
The South China Sea is a semienclosed body of water with complex bottom topography that has wide continental shelves in the north and south and steep slopes in the east and west (Liu, 2013). Tardigrades were obtained from eight sampling stations (20.97°N–21.19°N, 117.98°E –118.10°E): M1, M2, M8, J1, J4, S1, S2, and S3 at depths of 1517-1725 m from the northern South China Sea. Sampling stations were divided into three areas: area I (Station J4), area II (Stations S2, S3 and M2) and area III (Stations S1, M1, M8 and J1). The distances from J4 (area I) to S2 (area II) and M8 (area III) were 12 km and 25 km, respectively. It was 15 km from S2 (area II) to M8 (area III). Rod and oval nodules were collected at Stations J1 and M8. Samples from Station M8 were collected in May 2019; samples from Stations M1 and M2, J1 and J4, and S1, S2 and S3 were collected in May, July, and August of 2021, respectively (Figure 1). The sampling sediments were collected by an MCS-1-type multiple corer with an inner diameter of 9.5 cm and extruded into the following layers: 0-1, 1-2, 2-4, and 4-6 cm. Then, the sampled sediments were preserved in 7% neutralized formalin. In the laboratory, to release the meiofauna from the sediment, a number of subsamples at each station were washed through a set of sieves of 250-µm, 125-µm, 63-µm, and 32-µm mesh size and then were extracted from the sediment using the Ludox centrifugation technique (de Jonge and Bouwman, 1977; Wang et al., 2010). Prior to sorting, the meiofauna were stained with “Rose Bengal” for two hours, which binds to their cuticle, making specimens easier to find. The trapped tardigrades were sorted under a stereomicroscope (Leica M205 A).
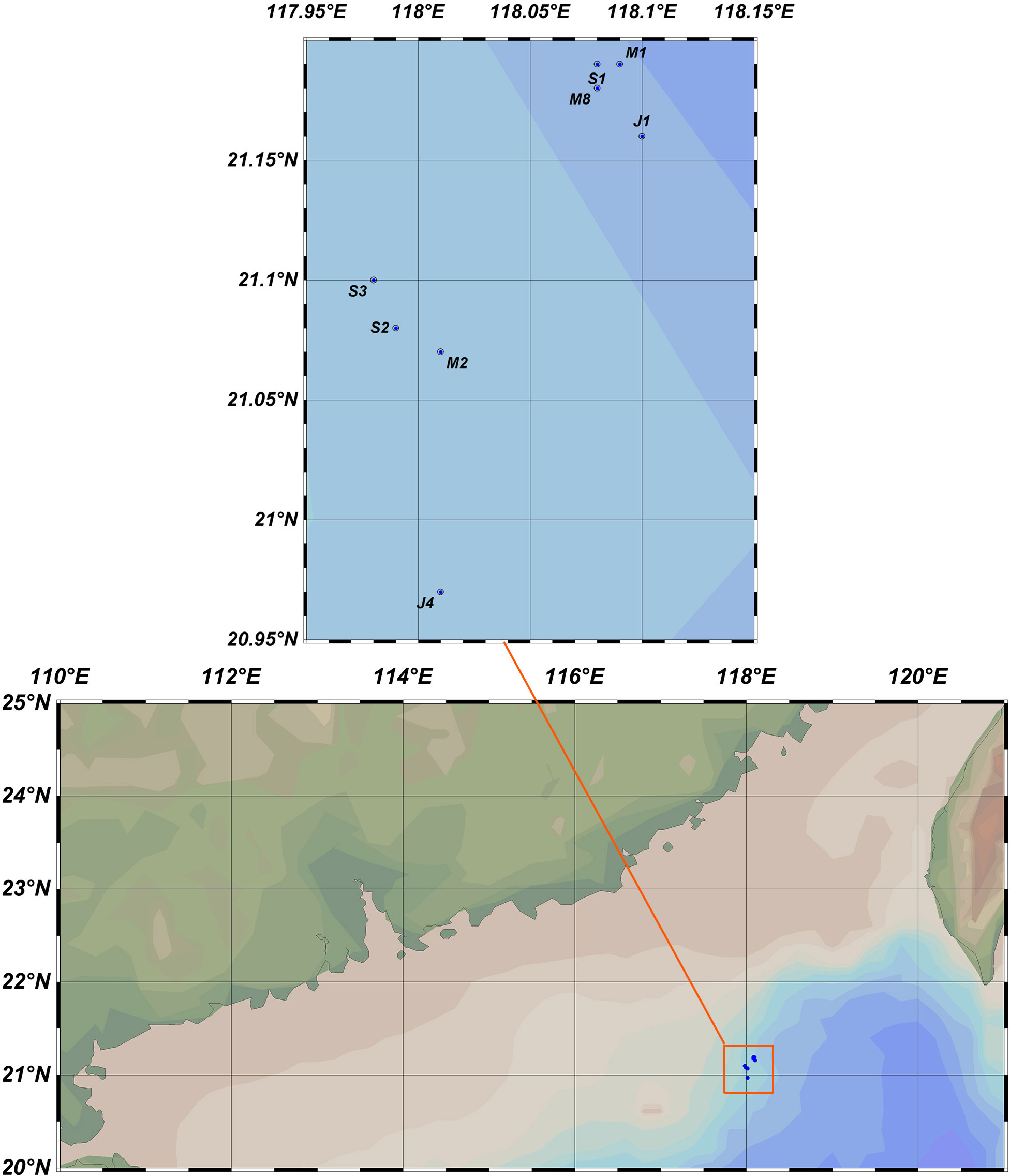
Figure 1 Sampling stations in the South China Sea (map made by Ocean Data View, Schlitzer, Reiner, Ocean Data View, odv.awi.de, 2021).
Species were identified based on information from Fontoura et al. (2017); Hansen and Kristensen (2020) and original descriptions and redescriptions. For that purpose, specimens were prepared for light microscopy (LM) and scanning electron microscopy (SEM). Specimens used for light microscopy were mounted on microscope slides in glycerin with a cover slip and sealed with transparent nail polish. Images were taken and measured by a Leica DM6B microscope and a Zeiss Axio imager A2 microscope under 20×, 40× and 100× oil immersion using the bright field mode (BF) and differential interference contrast (DIC). Specimens prepared for SEM were dehydrated in a graded series of ethanol prior to critical point drying or hexamethyldisilazane drying (Shively and Miller, 2009; Zhou et al., 2017). The dehydrated specimens were then mounted on stubs, coated with platinum and observed in a HITACHI TM-1000 scanning electron microscope.
2.2 Structural parameters of the tardigrade community
The biological information about tardigrade communities was synthesized by means of the number of species and individuals. To analyze tardigrade diversity, the Shannon−Wiener diversity index (H´), Pielou’s evenness index (J´), average taxonomic distinctness (Δ+) and variation in taxonomic distinctness (Λ+) were calculated (Clarke and Gorley, 2006).
The comparison among stations was performed by means of group average CLUSTER analysis of the SIMPROF test, SIMPER analysis, calculations of diversity indices and TAXDTEST using the PRIMER version 6 software package. The SIMPROF test was carried out using the Bray−Curtis similarity measure with a significance level of 5%. The data were transformed by square root before CLUSTER and SIMPER analysis (Clarke and Gorley, 2006).
3 Results
3.1 Composition, diversity and abundance of fauna
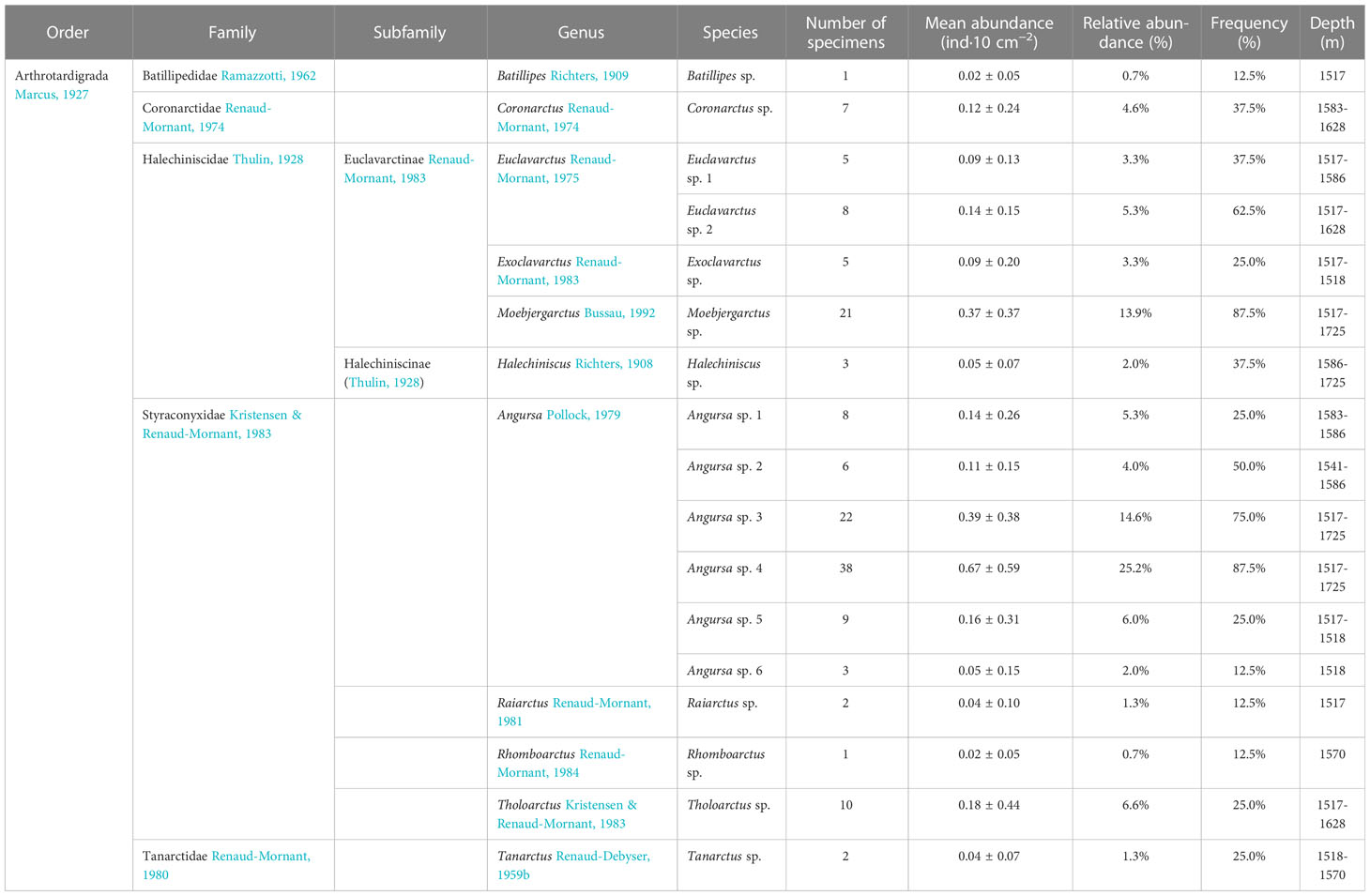
A total of 151 specimens of Tardigrada representing 17 arthrotardigrade species from 5 families and 11 genera were collected (Table 1). The most relatively abundant families were Styraconyxidae (65.6%) and Halechiniscidae (27.8%). Angursa was the most relatively abundant genus, accounting for 57.0% of the total number of tardigrades, followed by Moebjergarctus (13.9%), Euclavarctus (8.6%), Tholoarctus (6.6%), Coronarctus (4.6%), Exoclavarctus (3.3%) and Halechiniscus (2.0%). Other genera were rare. Tanarctus and Raiarctus accounted for 1.3% each, and Batillipes and Rhomboarctus each accounted for 0.7% (Table 1).
Three species were clearly more abundant than all the others: Angursa sp. 4 (25.2%), with an average abundance of 0.67 ± 0.59 ind·10 cm−2, followed by Angursa sp. 3 (14.6%) and Moebjergarctus sp. (13.9%), with average abundances of 0.39 ± 0.38 and 0.37 ± 0.37 ind·10 cm−2, respectively. The relative abundances of each of the other species did not exceed 7% (Table 1).
The abundance of the marine tardigrade fauna at all stations ranged from 0.56 to 4.09, with an average abundance of 2.66 ± 1.13 ind·10 cm−2 (Table 2). The number of species at different stations ranged from 4 at Station J4 to 10 at Station J1. There were 7 species at Stations M1, S1 and S2. The Shannon−Wiener diversity index (H′) and Pielou’s evenness index (J′) ranged from 1.94 to 2.87 and 0.75 to 1, respectively. The highest value of H’ (2.87) was recorded at Station J1 with 23 specimens from 10 species, while the lowest value of the Shannon−Wiener diversity index (H′=1.94) was recorded at Station S3 with 17 specimens from 6 species (Table 2). The highest value of Pielou’s evenness index (J′=1) was recorded at Station J4, with only 4 specimens from 4 species: Angursa sp. 3, Angursa sp. 4, Moebjergarctus sp. and Halechiniscus sp.
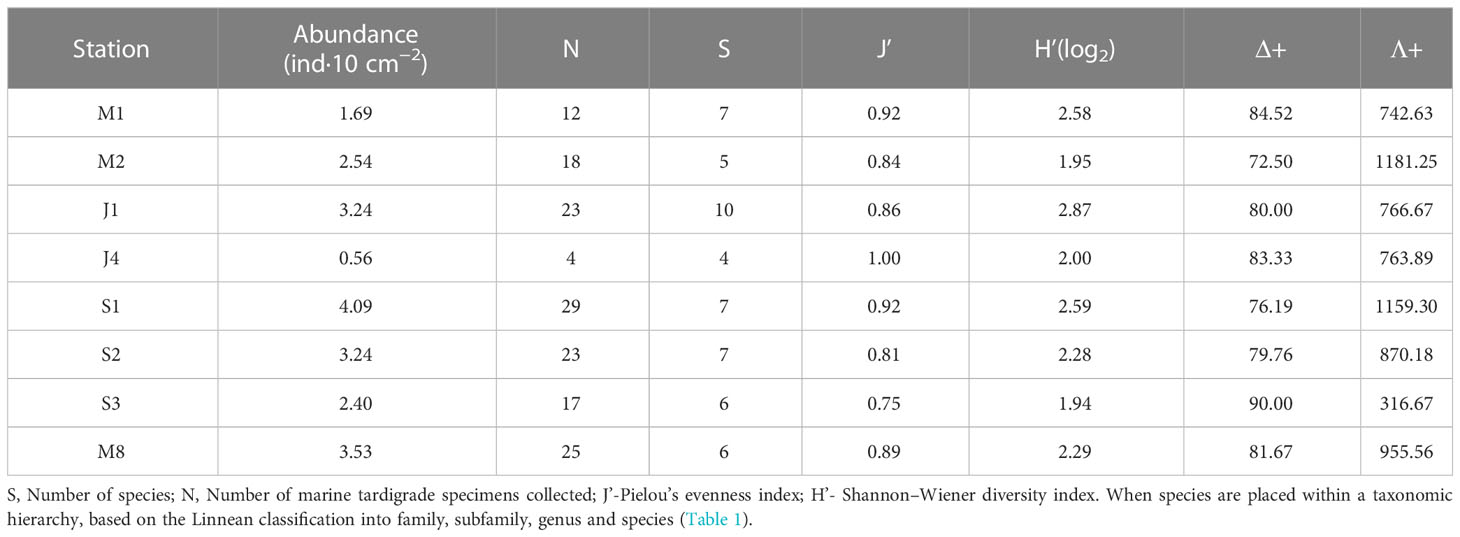
Table 2 Biodiversity and abundance of marine tardigrade fauna from different stations in the South China Sea.
The highest value of Δ+ was recorded at Station S3 (90.00), while the lowest value was recorded at Station M2 (72.50). The Δ+ values of Stations J4, M8, M1 and S3 were above the simulated mean Δ+, while the Δ+ values of Stations M2, S1, S2 and J1 were lower than the simulated mean Δ+. The variation amplitude of Λ+ ranged from 316.67 at Station S3 to 1181.25 at Station M2, representing a large change in unevenness in the tardigrade community in terms of taxonomy. The values of Λ+ at all stations except Station S3 were higher than the simulated mean Λ+ (Table 2). Both the values of Δ+ and Λ+ at all stations fell inside the 95% probability limit.
3.2 Distribution in sediment layers
Tardigrades were mainly distributed in the upper 0–1cm sediment layer (Table 3) at all sampling stations (mean > 90%). The proportion of specimens recorded in deeper layers was residual (mean values in the 2–4 cm and 4–6 cm layers were 1.0% and 1.5%, respectively).
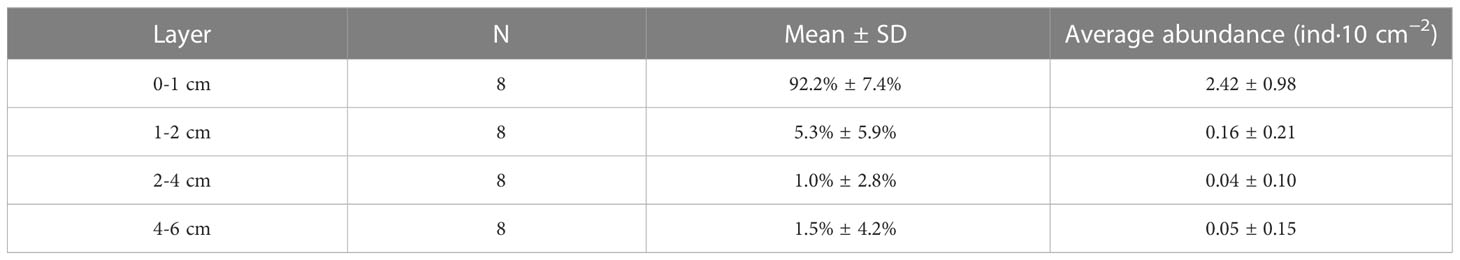
Table 3 Percentage and mean abundance (mean values and standard deviation - SD) of tardigrade specimens collected in each sediment layer at the eight stations in the South China Sea.
All tardigrade specimens at Stations M2, J1 and J4 were collected from the upper 0–1 cm layers of sediments. Tardigrades were collected from both the 0–1 cm and 1–2 cm layers at Stations M1, S1, S2 and S3. However, at Station M8, specimens were collected from the upper 0–1 cm layer and then from the deeper layers (2-4 cm and 4-6 cm) (Figure 2).
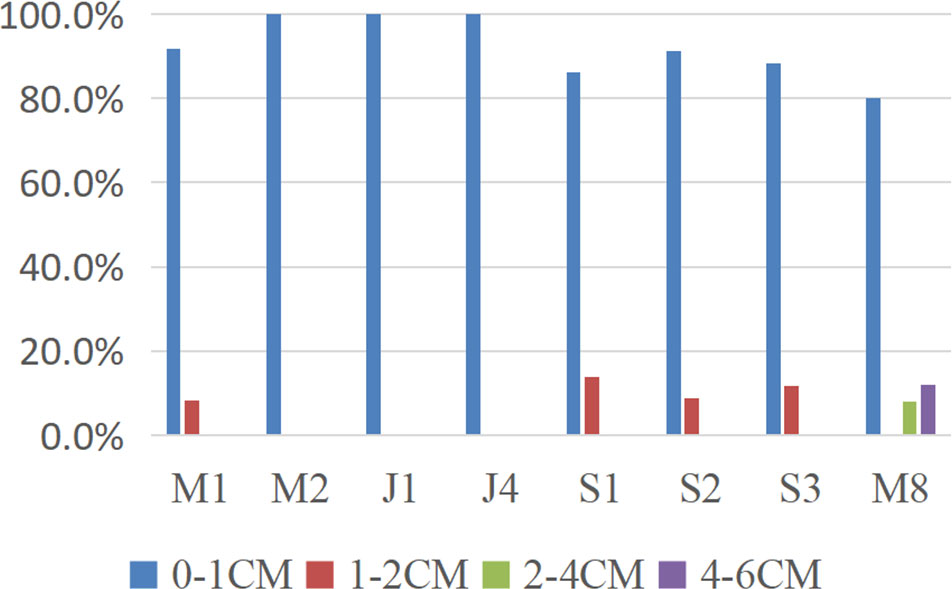
Figure 2 Vertical distribution of the marine tardigrade fauna. Percentage of specimens found in each sediment layer at different stations.
3.3 Similarity analysis
According to CLUSTER analysis of the Bray−Curtis similarity matrix for the tardigrade species composition at different stations, all eight stations belonged to one group. This finding meant that the composition of tardigrade communities among all three areas and between depths of 1500-1600 m and above 1600 m was not significantly different (SIMPROF test, p >0.05) (Figures 3A, B).
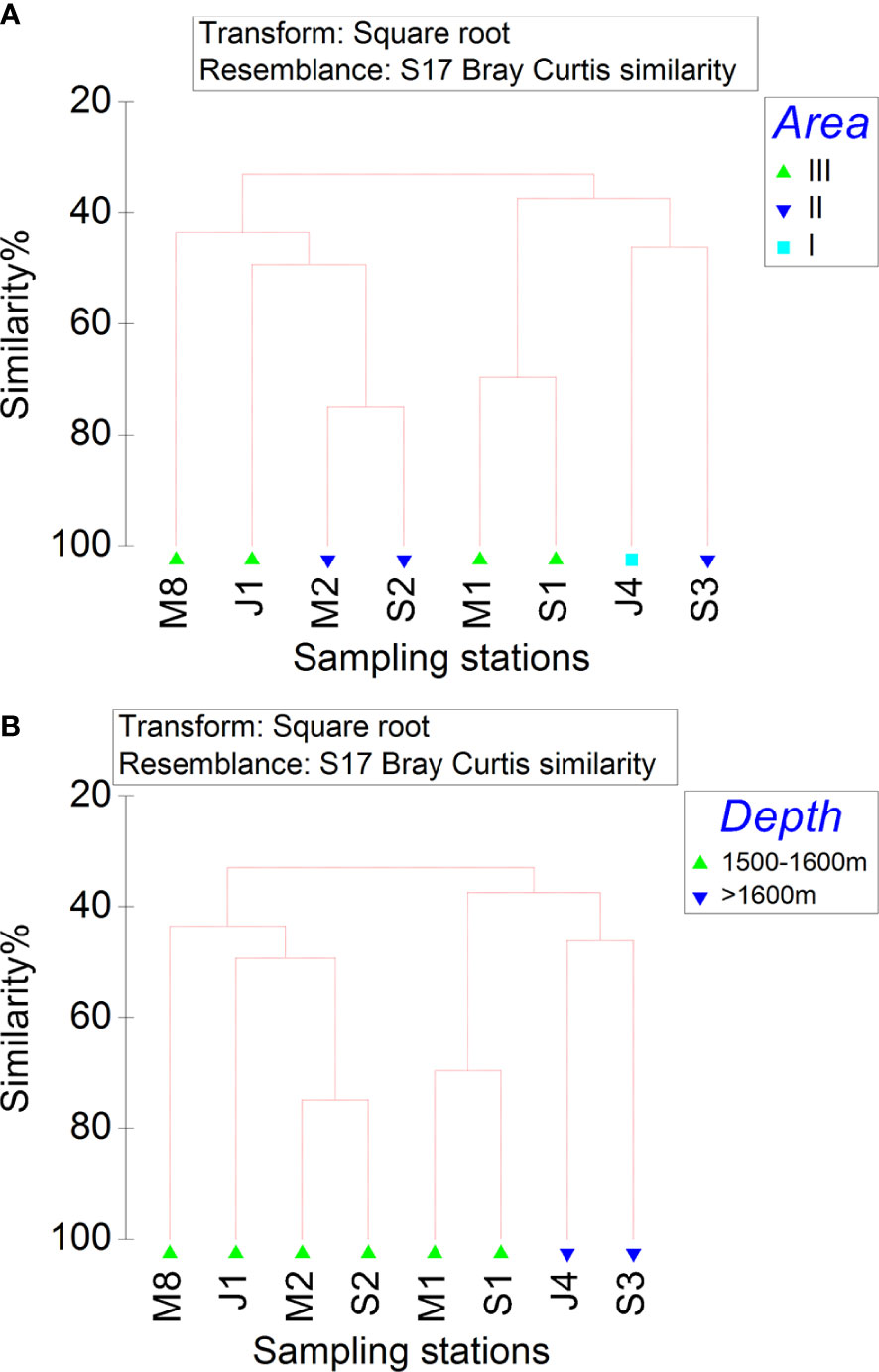
Figure 3 Species composition of marine tardigrade fauna at all stations in the deep South China Sea based on CLUSTER analysis. (A) Area factor, (B) Depth factor.
SIMPER analysis showed that the average similarity among the eight stations was 39.20%. The three most dominant species that contributed strongly to the average similarities were Angursa sp. 4, Moebjergarctus sp., and Angursa sp. 3, which contributed 28.99%, 23.77% and 19.16%, respectively, to the average similarity. Angursa sp. 4, Moebjergarctus sp., and Angursa sp. 3 were present in all three areas (Tables 4, 5).
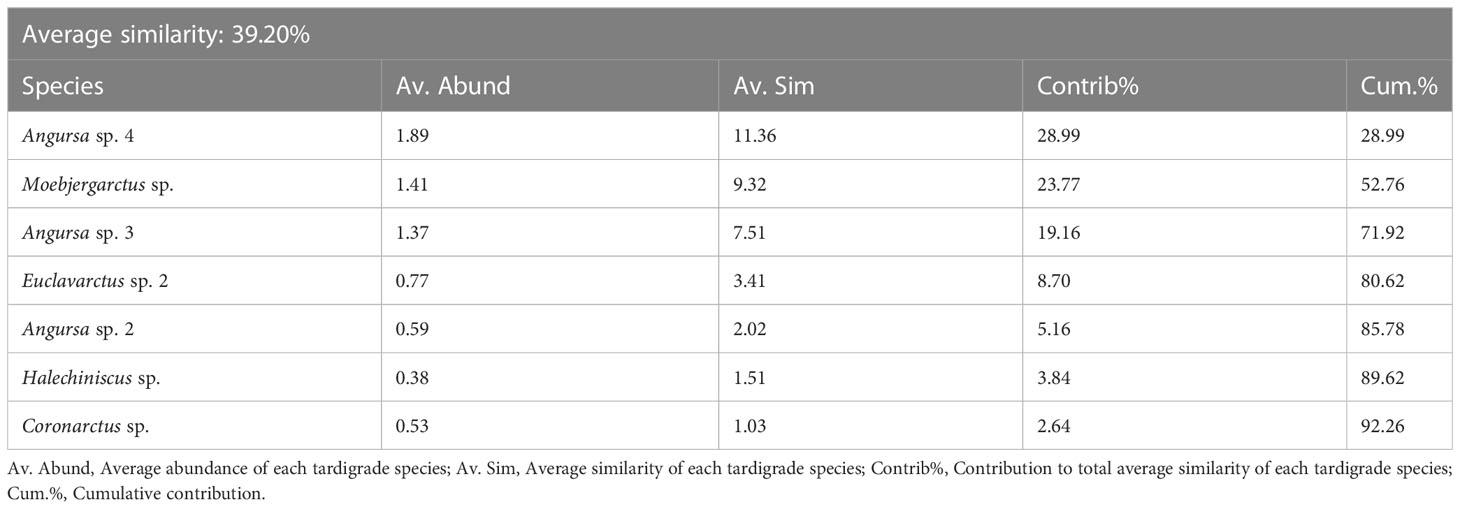
Table 4 SIMPER analysis for the contributions of species to the average similarity among all stations in the deep South China Sea.
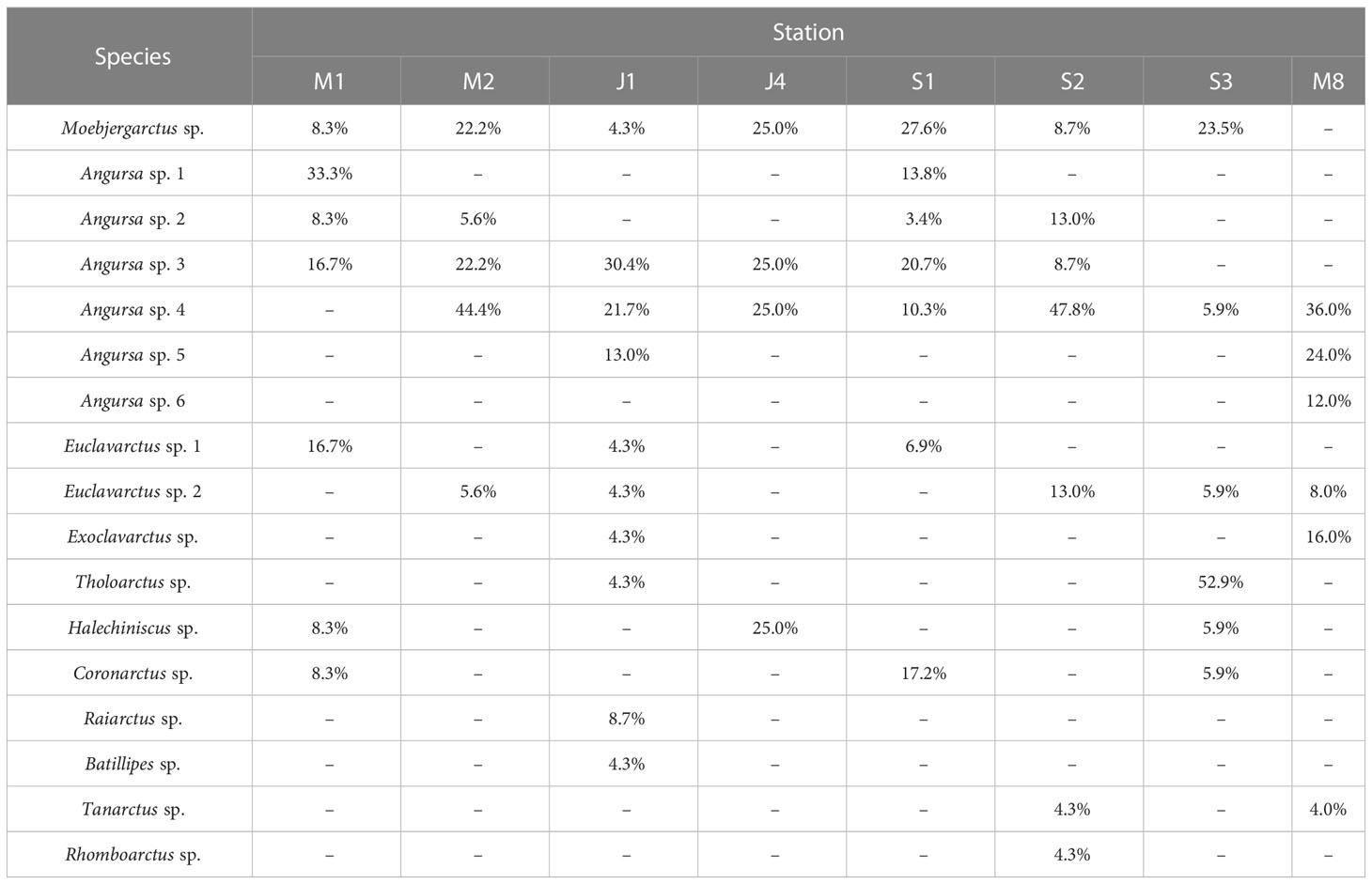
Table 5 Species composition of marine tardigrade fauna at different stations in the deep South China Sea.
Some species were distributed widely. The most frequent species were Angursa sp. 4 and Moebjergarctus sp., which was only absent at one station with a frequency of 87.5%. However, four species were only present at a particular station with a frequency of 12.5%: Angursa sp. 6 only existed at Station M8, Batillipes sp. and Raiarctus sp. at Station J1, and Rhomboarctus sp. at Station S2 (Tables 1, 4).
At each station, the dominant species belonged to only three genera: Angursa sp. 4 was the most relatively abundant species at three stations, namely, S2 (47.8%), M2 (44.4%) and M8 (36.0%); Tholoarctus sp. (52.9%) was the most relatively abundant species at Station S3; Angursa sp. 1 (33.3%) was the most relatively abundant species at Station M1; Angursa sp. 3 was the most relatively abundant species at Station J1 (30.4%); and Moebjergarctus sp. was the most relatively abundant species at Station S1 (27.6%) (Table 5).
4 Discussion
4.1 Comparison of the abundance of marine tardigrade fauna in the South China Sea to that in other deep-sea areas
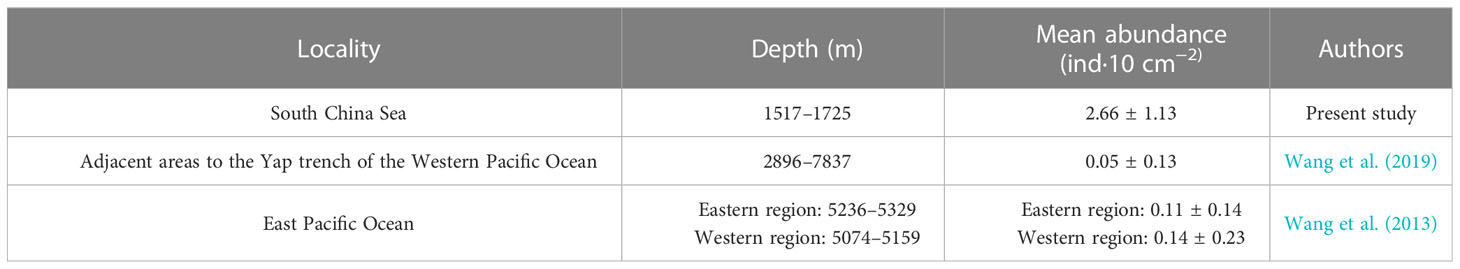
This study showed that the marine tardigrade abundance in the deep South China Sea was much higher than that in the abyssal East Pacific Ocean and the adjacent areas to the Yap Trench of the Western Pacific Ocean (Table 6) (Wang et al., 2013; Wang et al., 2019). Many studies have indicated that food availability is an important factor that affects meiofauna abundance (Danovaro et al., 2002; Gambi and Danovaro, 2016; Leduc et al., 2016). The water depth of this study ranged from 1517 to 1725 m. Although the analysis of the organic matter content of sediments in the stations of this study was not carried out, since the South China Sea is a marginal sea of the western Pacific Ocean, ocean margins are the repository for approximately 90% of the organic carbon buried in marine sediments. The Pearl River, which is the second largest river in China, discharges onto the northern shelf of the South China Sea (Hedges and Keil, 1995; Hu et al., 2006). Studies have shown that the organic matter content of sediments usually decreases with increasing water depth (Billett et al., 1993). Therefore, the organic matter in the northern South China Sea is generally higher than that in both the abyssal East Pacific Ocean and the adjacent areas to the Yap Trench. The data from Liu et al. (2015) showed that the organic matter of the northern South China Sea ranged from 1.31% to 1.55% at depths of 313-1600 m. It was much higher than that adjacent areas of the Yap trench (2896-7837 m), with just 0.02%-0.37% at 22 of 23 sites (except 0.76% at one site) (Wang et al., 2019). This likely caused the abundance of marine tardigrade fauna in the South China Sea to be much higher than that in the abyssal East Pacific Ocean and the adjacent areas to the Yap Trench of the Western Pacific Ocean.
4.2 Community of the tardigrade fauna in the South China Sea compared to other areas
In this study, deep-sea tardigrades were surveyed in the South China Sea for the first time. A total of 151 specimens from 5 families, 11 genera and 17 species were recorded. The South China Sea connects with the Pacific Ocean through the Bashi straits and connects with the Indian Ocean through the Malacca straits. The marine tardigrade fauna from the South China Sea shows the connection between the Pacific Ocean and the Indian Ocean. All the genera of the South China Sea were recorded either in the Pacific Ocean or the Indian Ocean except the genus Exoclavarctus, which has been recorded only in the Atlantic Ocean until now (Table 7). The genera Exoclavarctus and Moebjergarctus are likely to be present in the Indian Ocean and have not yet been collected.
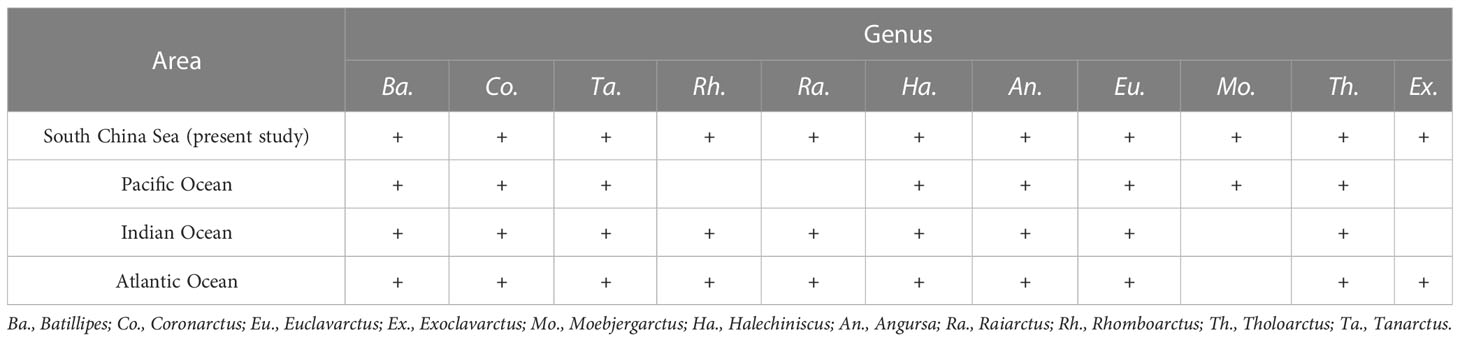
Table 7 Comparison of the genus composition of the marine tardigrade fauna of the South China Sea to others.
Kaczmarek et al. (2015) divided the meiofauna habitat into four zones: littoral (intertidal), shallow sublittoral (below low tide to 200 m), deep sublittoral (200-2000 m) and abyssal (>2000 m). All tardigrade specimens collected in this study belong to the exclusively marine order Arthrotardigrada, and the family Echiniscoididae (order Echiniscoidea) is not found in the deep sublittoral South China Sea. Marine heterotardigrades belonging to Echiniscoididae represent the dominant tardigrade group in intertidal zones worldwide, and the evolution of this family is interesting because it holds key evidence as to how heterotardigrades colonize terrestrial and freshwater environments (Møbjerg et al., 2016). The absence of this family infers that it is probably not adapted to deep-sea environments.
Thirteen recorded species belong to seven genera (Angursa, Coronarctus, Moebjergarctus, Euclavarctus, Exoclavarctus, Tanarctus, and Tholoarctus) frequently found in the deep sublittoral and abyssal zones (Kaczmarek et al., 2015). Two other species, namely, Halechiniscus sp. and Rhomboarctus sp., belong to genera already found in the deep sublittoral zone but not in the abyssal zone (Kaczmarek et al., 2015). Batillipes sp. and Raiarctus sp. belong to genera found in both littoral and shallow sublittoral zones (Kaczmarek et al., 2015). All the described species of the genus Batillipes have been recorded in that zone (Kaczmarek et al., 2015; Rubal et al., 2016b; Menechella et al., 2017; Santos et al., 2017; Santos et al., 2018; Bartels et al., 2021). However, taking into account the record from the South China Sea and the records of two new undescribed Batillipes species from the deep sublittoral zone (260 m) in the Faroe Bank, Atlantic Ocean (Hansen et al., 2001; Hansen, 2005), this assumption needs to be reevaluated.
Species of three genera, namely, Exoclavarctus, Raiarctus and Rhomboarctus, were recorded for the first time in the Pacific Ocean. Exoclavarctus is a monotypic deep-sea genus only known from the Atlantic Ocean at 1369 m (Renaud-Mornant, 1983). The genus Rhomboarctus includes 3 species: Rhomboarctus aslaki Hansen et al., 2003 known from the Faroe Islands in the North Atlantic Sea, between 138.5 and 260 m; Rh. duplicicaudatus Hansen et al., 2003, a subtidal species recorded in the Tyrrhenian Sea (Italy, Europe); and Rh. thomassini Renaud-Mornant, 1984, known only from the Mozambique Channel (770 m), Indian Ocean (Renaud-Mornant, 1984; Hansen et al., 2003).
A species of the genus Moebjergarctus was dominant in samples from the South China Sea. Three species in this genus have been described: Moebjergarctus manganis Bussau, 1992 and Mo. clarionclippertonensis Bai et al., 2020, both recorded in polymetallic nodule areas in the Eastern South Pacific (Peru Basin) and in the Northeastern Pacific (Clarion-Clipperton Fracture Zone) (Bussau, 1992; Bai et al., 2020), and Mo. okhotensis in the North-Western Pacific (Saulenko et al., 2022). In the sampling area of this study in the South China Sea, polymetallic nodules were also collected, suggesting that species of the genus Moebjergarctus can be closely associated with this kind of substrate.
Saulenko et al. (2022) reported that representatives of nine marine tardigrade genera were found only deeper than 200 m, with the exception of Coronarctus neptunus found at depths of 150-1300 m. Four of them, that is, Coronarctus, Moebjergarctus, Euclavarctus, and Exoclavarctus, were found in this study, while Bathyechiniscus, Clavarctus, Ligiarctus, Parmursa and Proclavarctus have not yet been found in the South China Sea. It is also interesting to note that the Halechiniscus species found in the South China Sea is new to science and has already been described (Halechiniscus janus Bai et al., 2022).
4.3 Biodiversity of the marine tardigrade communities
In the absence of other studies that could be used for comparison, the obtained biodiversity values should be used for future reference. The results of the present study show that 13 genera and at least 20 species, including the four previously recorded shallow-water species, are present in the South China Sea at present. This study increases the diversity estimation of both the South China Sea and the Pacific Ocean. To date, only 51 tardigrade species from 23 genera have been recorded from the deep sea (> 200 m depth), including the deep sublittoral and abyssal zones (Kaczmarek et al., 2015; Gomes-Júnior et al., 2020; Hansen and Kristensen, 2020; Degma et al., 2009-2023; Saulenko et al., 2022). Eleven genera and 17 species from the South China Sea accounted for 48% of the recorded deep-sea genera and 33% of the recorded deep-sea species. Considering the inadequate deep-sea sampling in the South China Sea (Liu, 2013) and the generally poor knowledge about marine tardigrades, it can be expected that the diversity of deep-sea tardigrades in the South China Sea, where only a minute area was surveyed coupled with the fact that a very low sampling effort was made in the present study, may be much higher. Bartels et al. (2016) suggested that with the continuing exploration of marine habitats, marine tardigrade species diversity will be similar to that of limnoterrestrial tardigrades.
The results of the SIMPROF test showed that the composition of tardigrade communities between depths of 1500-1600 m and above 1600 m was not significantly different at the stations of this study in the South China Sea. Hansen et al. (2001) reported that samples of the Faroe Bank with similar sediment at depths of 104–260 m had similar species distributions, indicating that the type of sediment was the key factor related to the species distribution and that depth was less important. The results of the present study also agreed with that conclusion. The locations of all stations in this study are quite near, with the same type of sandy mud sediment, which is probably the factor that caused the species composition of marine tardigrade communities at all stations to be very similar.
Although marine tardigrade biodiversity was investigated in this study, unnamed species are urgently in need of identification to further understand the marine tardigrade biodiversity in the South China Sea. Large-scale and large-depth studies are also urgently required to investigate the environmental factors responsible for the distribution of marine tardigrades in the South China Sea.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Author contributions
XW and CW conceived the study, and XW and LB drafted the manuscript. XW, BL, YL, QL and XH sampled the sediments. LB conducted experiments in the laboratory with instructions of PF. PF, BL and CW revised and improved the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key R&D Program of China (No. 2022YFC2803902). PF (Portugal) was partially funded by Fundação para a Ciência e a Tecnologia (FCT) through the strategic project UID/MAR/04292/2019 granted to MARE.
Acknowledgments
All scientists and crew are gratefully acknowledged for sampling the sediments with Tardigrada. Fang Chen is acknowledged for helping with PRIMER v6 software. Warm appreciation goes to the team of the Key Laboratory of Marine Ecosystem Dynamics (China) who helped in the preparation of the material for SEM by the critical point drying technique and in providing suggestions for the manuscript. We sincerely thank the reviewers for the suggestions to improve the quality of this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Accogli G., Gallo M., D’Addabbo R., Hansen J. G. (2011). Diversity and ecology of the marine tardigrades along the Apulian Coast. J. Zool. Syst. Evol. Res. 49, 53–57. doi: 10.1111/j.1439-0469.2010.00598.x
Bai L., Wang X., Gao X., Li Y., Fontoura P. (2022). First record of a deep-sea tardigrade from the South China Sea, Halechiniscus janus sp. nov. (Arthrotardigrada: Halechiniscidae). Zootaxa 5159 (3), 425–439. doi: 10.11646/zootaxa.5159.3.7
Bai L., Wang X., Zhou Y., Lin S., Meng F., Fontoura P. (2020). Moebjergarctus clarionclippertonensis a new abyssal tardigrade (Arthrotardigrada, Halechiniscidae, Euclavarctinae) from a Clarion-Clipperton Fracture Zone, North-East Pacific. Zootaxa 4755 (3), 561–575. doi: 10.11646/zootaxa.4755.3.8
Bartels P. J., Apodaca J. J., Mora C., Nelson D. R. (2016). A global biodiversity estimate of a poorly known taxon: Phylum Tardigrada. Zool. J. Linn. Soc 178 (4), 730–736. doi: 10.1111/zoj.12441
Bartels P. J., Fontaneto D., Roszkowska M., Nelson D. R., Kaczmarek Ł. (2020). Latitudinal gradients in body size in marine tardigrades. Zool. J. Linn Soc 188 (3), 820–838. doi: 10.1093/zoolinnean/zlz080
Bartels P. J., Fontoura P., Nelson D. R., Orozco-Cubero S., Mioduchowska M., Gawlak M., et al. (2021). A trans-isthmus survey of marine tardigrades from Costa Rica (Central America) with descriptions of seven new species. Mar. Biol.Res. 17 (2), 167–171. doi: 10.1080/17451000.2021.1901936
Billett D. S. M., Lampitt R. S., Rice A. L., Mantoura R. F. C. (1993). Seasonal sedimentation of phytoplankton to the deep-sea benthos. Nature 302, 520–522. doi: 10.1038/302520a0
Bussau C. (1992). New deep-sea Tardigrada (Arthrotardigrada, Halechiniscidae) from manganese nodule area of the eastern South Pacific. Zoo. Scr. 21 (1), 79–91. doi: 10.1111/j.1463-6409.1992.tb00311.x
Cai L., Fu S., Yang J., Zhou X. (2012). Distribution of meiofaunal abundance in relation to environmental factors in Beibu Gulf, South China Sea. Acta Oceanol. Sin. 31 (6), 92–103. doi: 10.1007/s13131-012-0256-2
Chang C. Y., Rho H. S. (1997). Two new marine tardigrades from Palawan Island, the Philippines. Korean J. Biol. Sci. 1, 419–423.
D’Addabbo Gallo M., Morone De Lucia M. R., Grimaldi de Zio S. (1987). “Heterotardigrada of the Amendolara Shoal, High Ionian Sea,” in The Biology of Tardigrades. Ed. Bertolani R.. Selected Symposia and Monographs U.Z.I. 1. Mucchi, Modena, Italy, pp. 93–101.
Danovaro R., Gambi C., Croce N. D. (2002). Meiofauna hotspot in the Atacama Trench, eastern South Pacific Ocean. Deep Sea Res. Part Oceanogr. Res. Pap. 49, 843–857. doi: 10.1016/S0967-0637(01)00084-x
Degma P., Bertolani R., Guidetti R. (2022–2023). Actual Checklist of Tardigrada Species, (42th Edition: 01-09-2023). doi: 10.25431/11380_1178608. (accessed on 20 March 2023)
de Jonge V. N., Bouwman L. A. (1977). A simple density separation technique for quantitative isolation of meiobenthos using the colloidal silica ludox-TM. Mar. Biol. 42, 143–148. doi: 10.1007/BF00391564
de Zio S., Grimaldi P. (1966). Ecological aspects of Tardigrada distribution in South Adriatic beaches. Veröff. Inst. Meeresf. Bremerh. 2, 87–94.
Du Y., Xu K., Meng Z., Wang J. (2010). Spatial distribution of meiofauna in relation to environmental factors in the South China Sea. Oceanol. Limnol. Sin. 41 (2), 199–207. doi: 10.1007/s13131-012-0256-2
Fontoura P., Bartels P. J., Jørgensen A., Kristensen R. M., Hansen J. G. (2017). A dichotomous key to the genera of the Marine Heterotardigrades (Tardigrada). Zootaxa 4294 (1), 1–45. doi: 10.11646/zootaxa.4294.1.1
Gallo D’Addabbo M., de Zio Grimaldi S., Morone De Lucia M. R., Pietanza R., D’Addabbo R., Todaro M. A. (1999). Diversity and dynamics of an interstitial Tardigrada population in the Meloria Shoals, Ligurian Sea, with a redescription of Batillipes similis (Heterotardigrada, Batillipedidae). Ital. J. Zoo. 66, 51–61. doi: 10.1080/11250009909356237
Gambi C., Danovaro R. (2016). Biodiversity and life strategies of deep-sea meiofauna and nematode assemblages in the Whittard Canyon (Celtic margin. NE Atlantic Ocean). Deep Sea Res. Part Oceanogr. Res. Pap. 108, 13–22. doi: 10.1016/j.dsr.2015.12.001
Giere O. (2009). Meiobenthology: the microscopic motile fauna of aquatic sediments. 2nd edn (London: Springer).
Gomes-Júnior E., Santos É., da Rocha C. M. C., Santos P. J. P., Fontoura P. (2020). The deep-Sea genus Coronarctus (Tardigrada, Arthrotardigrada) in Brazil, South-Western Atlantic Ocean, with the description of three new species. Diversity 12, 63. doi: 10.3390/d12020063
Grove S. L., Probert P. K., Berkenbusch K., Nodder S. D. (2006). Distribution of bathyal meiofauna in the region of the subtropical front, Chatham Rise, South-West Pacific. J. Exp. Mar. Biol. Ecol. 330 (1), 342–355. doi: 10.1016/j.jembe.2005.12.038
Hansen J. G. (2005). The ongoing investigation of the Faroe Bank tardigrade fauna. Frodskaparrit supplementum: Proceedings from the BIOFAR Symposium, Tórshavn, Faroe Islands, 24.- 26. April 2003, North-East Atlantic marine benthic organisms in the Faroes - taxonomy, distribution and ecology, 220–223.
Hansen J. G., D'Addabbo Gallo M., Grimaldi De Zio S. (2003). A comparison of morphological characters within the genus Rhomboarctus (Tardigrada: Heterotardigrada) with the description of two new species. Zool. Anz. 242) 1, 83–96. doi: 10.1078/0044-5231-00089
Hansen J. G., Jørgensen A., Kristensen R. M. (2001). Preliminary studies of the tardigrade fauna of the Faroe Bank. Zool. Anz. 240, 385–393. doi: 10.1078/0044-5231-00046
Hansen J. G., Kristensen R. M. (2020). “Tardigrada,” in Guide to the identification of marine meiofauna. Ed. Schmidt-Rhaesa A. (Munich: Friedrich Pfeil), 428–444. Verlag Dr.
Hedges J. I., Keil R. G. (1995). Sedimentary organic matter preservation: an assessment and speculative synthesis. Mar. Chem. 49, 81–115. doi: 10.1016/0304-4203(95)00008-F
Higgins R. P. (1972). Tardigrada of the Chesapeake Bay. Chesapeake Sci. 13 (1), S103–S104. doi: 10.2307/1350659
Higgins R. P., Thiel H. (1988). Introduction to the study of meiofauna (Washington, DC: Smithsonian Institution Press), 1–488.
Hu J., Peng P., Jia G., Mai B., Zhang G. (2006). Distribution and sources of organic carbon, nitrogen and their isotopes in sediments of the subtropical Pearl River estuary and adjacent shelf, Southern China. Mar. Chem. 98, 274–285. doi: 10.1016/j.marchem.2005.03.008
Jia S., Qiao C., Huang Y. (2020). Study on summer meiofauna in the northeastern South China Sea. Oceanol. Limnol. Sin. 51 (3), 564–571. doi: 10.11693/hyhz20191100216
Kaczmarek Ł., Bartels P. J., Roszkowska M., Nelson D. R. (2015). The zoogeography of marine Tardigrada. Zootaxa 4037 (1), 1–189. doi: 10.11646/zootaxa.4037.1.1
Kristensen R. M., Renaud-Mornant J. (1983). Existence d’arthrotardigrades semi-benthiques de genres nouveaux de la sous-famille des Styraconyxinae subfam. nov. Cah. Biol. Mar. 24, 337–353.
Kristensen R. M., Sørensen M. V. (2004). “Phylum: Tardigrada (Water bears),” in Grzimek’s animal life encyclopedia, vol 2. protostomes. Thompson Gale, Detroit, pp 115–123.
Leduc D., Rowden A. A., Glud R. N., Wenzhöfer F., Kitazato H., Clark M. R. (2016). Comparison between infaunal communities of the deep floor and edge of the Tonga Trench: possible effects of differences in organic matter supply. Deep Sea Res. PartI Oceanogr. Res. Pap. 116, 264–275. doi: 10.1016/j.dsr.2015.11.003
Li Q., Chai Y., Shao Q., Wang Z., Xie W., Zhou Y., et al. (2022). Metabarcoding survey of meroplankton communities in the South China Sea and Philippine Sea: shedding light on inter-basin biogeography in the West Pacific. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.968666
Liu J. Y. (2013). Status of marine biodiversity of the China seas. PloS One 8 (1), e50719. doi: 10.1371/journal.pone.0050719
Liu X., Xu M., Zhang J., Liu D., Li X. (2015). Community structure and biodiversity of free-living marine nematodes in the northern South China Sea. Acta Oceanol. Sin. 34 (6), 77–85. doi: 10.1007/s13131-014-0549-8
Liu X., Xu M., Zhang J., Mu. G., Liu D., Li X. (2014). Abundance and biomass of deep-sea meiofauna in the northern South China Sea. J. Trop. Oceanogr. 33, 52–59. doi: 10.3969/j.issn.1009-5470.2014.02.007
Møbjerg N., Kristensen R. M., Jørgensen A. (2016). Data from new taxa infer Isoechiniscoides gen. nov. and increase the phylogenetic and evolutionary understanding of echiniscoidid tardigrades (echiniscoidea: tardigrada). Zool. J. Linn. Soc 178 (4), 804–818. doi: 10.1111/zoj.12500
Martinez E. A. (1975). Marine meiofauna of a New York City Beach, with particular reference to Tardigrada. Estuar. Coast. Mar. Sci. 3, 337–348. doi: 10.1016/0302-3524(75)90033-x
Menechella A. G., Bulnes V. N., Cazzaniga N. J. (2017). Two new species of Batillipes (Tardigrada, Arthrotardigrada, Batillipedidae) from the Argentinean Atlantic coast, and a key to all known species. Ma. Biodiversity 48, 239–247. doi: 10.1007/s12526-017-0640-4
Miao Q., Thunell R. C. (1993). Recent deep-sea benthic foraminiferal distributions in the South China and Sulu seas. Mar. Micropaleontol. 22 (1–2), 1–32. doi: 10.1016/0377-8398(93)90002-F
Mu F., Zhang T., Li J., Hua E. (2020). Spatiotemporal distribution of meiofauna and its influencing factors at the Dadeji Beach, Xiamen. Periodical Ocean Univ. China 50 (9), 34–45. doi: 10.16441/j.cnki.hdxb.20200122
Nan F., Xue H., Yu F. (2015). Kuroshio intrusion into the South China Sea:A review. Prog. Oceanography 137, 314–333. doi: 10.1016/j.pOcean.2014.05.012
Nelson D. R. (2002). Current status of the Tardigrada: evolution and ecology. Integr. Comp. Biol. 42 (3), 652–659. doi: 10.1093/icb/42.3.652
Nelson D. R., Bartels P. J., Guil N. (2018). “Tardigrade ecology,” in Water bears: the biology of tardigrades. Ed. Schill R. O., (Cham, Switzerland: Springer), Switzerland, Zoological Monographs 2, chapter 7 163–210. doi: 10.1007/978-3-319-95702-9_7
Pollock L. W. (1975). The role of three environmental factors in the distribution of the interstitial tardigrade Batillipes mirus Richters. Memorie Ist. Ital. Idrobiol 32 Suppl, 305–322.
Pollock L. W. (1979). Angursa bicuspis n. sp., a marine arthrotardigrade from the western north Atlantic. Trans. Amer. Microsc. Soc 98, 558–565. doi: 10.2307/3225907
Qiao C., Hao Y., Lu Y., Huang Y. (2021). Preliminary study on biomass and biodiversity of free-living nematodes in intertidal zone of northern South China Sea. J. Liaocheng Univ. (Nat. Sci.) 34 (2), 65–72. doi: 10.19728/j.issn1672-6634.2021.02.009
Renaud-Debyser J. (1956). Répartition de deux tardigrades, Batillipes mirus Richters et Stygarctus bradypus Schulz dans un segment de plage du bassin d’Arcachon. C. R. Acad. Sc. (Paris) 213, 1365–1369.
Renaud-Debyser J. (1959b). Étude sur la faune interstitielle des iles Bahamas. III tardigrades. Vie Millieu 10, 296–302.
Renaud-Debyser J. (1963). Recherches écologiques sur la faune interstitielle des sables (Bassin d’Arcachon, Île de Bimini, Bahamas). Vie Millieu 15, 1–157.
Renaud-Debyser J. (1965). Études sur un Stygarctidae (Tardigrada) nouveau de Madagascar. Bull. Soc Zool. France 90, 31–38.
Renaud-Debyser J., Salvat B. (1963). Élements de prospérité des biotopes des sediments meubles intertidaux et écologie de leurs populations en microfaune et macrofaune. Vie Millieu 14, 463–550.
Renaud-Mornant J. (1967). Parastygarctus higginsi Renaud-Debyser, 1965, Sur la côte orientale de Malaisie. Description de la femelle (Tardigrada). Bull. Mus. Natl. Hist. Nat. Série 2e. 39, 205–208.
Renaud-Mornant J. (1974). Une nouvelle famille de tardigrades marins abyssaux: les Coronarctidae fam. nov. (Heterotardigrada). C. R. Acad. Sc. (Paris) 278, 3087–3090.
Renaud-Mornant J. (1975). Deep Sea Tardigrada from «Meteor» Indian Ocean expedition. «Meteor» Forsch.-Ergeb Ser. D 21, 54–61.
Renaud-Mornant J. (1979). Tardigrades marins de Madagascar. II. Stygarctidae et Oreellidae. III. Considérations écologiques générales. Bull. Mus. Hist. Nat. 2, 339–351. (Paris) Série 4e, 1 (Séction A).
Renaud-Mornant J. (1980). Description de trois espèces nouvelles du genre Tanarctus Renaud-Debyser 1959, et création de la sous-famille des Tanarctinae, subfam. nov. (Tardigrada, Heterotardigrada). Bull. Mus. Natl. Hist. Nat. (Paris) Série 4e 2 (1), 129–141.
Renaud-Mornant J. (1981). Raiarctus colurus n. g., n. sp., et R. aureolatus n. sp., Tardigrades (Arthrotardigrada) marins nouveaux de sédiments calcaires. Bull. du Muséum Natl. d’Histoire Naturelle, (Paris), Série 4e (Section A), 3 (2), 515–522.
Renaud-Mornant J. (1983). Tardigrades abyssaux nouveaux de la sous-famille des Euclavarctinae n. subfam. (Arthrotardigrada, Halechiniscidae). Bull. Mus. Natl. Hist. Nat. (Paris) 5 (1), 201–219. Séries 4e, 5e (Section A, n° 1).
Renaud-Mornant J. (1984). Halechiniscidae (Heterotardigrada) de la campagne Benthedi, canal du Mozambique. Bull. Mus. Natl. Hist. Nat. (Paris), Série 4e, 6e (Section A), 6 (1), 67–88.
Renaud-Mornant J., Serène P. (1967). Note sur la microfaune de la côte orientale de la Malaisie. Cah. Pac. 11, 51–73.
Romano III F., Gallo M., D’Addabbo R., Accogli G., Baguley J., Montagna P. (2011). Deep-sea tardigrades in the northern gulf of Mexico with a description of a new species of Coronarctidae (Tardigrada: Arthrotardigrada), Coronarctus mexicus. J. Zool. Sys. Evol. Res. 49, 48–52. doi: 10.1111/j.1439-0469.2010.00597.x
Rubal M., Veiga P., Fontoura P., Santos E., Sousa-Pinto I. (2016a). Biodiversity of marine tardigrades from the northern coast of Portugal (Iberian Peninsula). Zool. J. Linn. Soc 178, 747–754. doi: 10.1111/zoj.12462
Rubal M., Veiga P., Fontoura P., Sousa-Pinto I. (2016b). A new Batillipes (Tardigrada, Heterotardigrada, Batillipedidae) from north Portugal (Atlantic Ocean). Mar. Biodiv. 47, 921–928. doi: 10.1007/s12526-016-0526-x
Santos E., Da Rocha C. M. C., Gomes J. E., Fontoura P. (2017). Three new Batillipes species (Arthrotardigrada: Batillipedidae) from the Brazilian coast. Zootaxa 4243 (3), 483–502. doi: 10.11646/zootaxa.4243.3.4
Santos É., Rubal M., Veiga P., da Rocha C. M. C., Fontoura P. (2018). Batillipes (Tardigrada, Arthrotardigrada) from the Portuguese coast with the description of two new species and a new dichotomous key for all species. Eur. J.of Taxon. 425, 1–32. doi: 10.5852/ejt.2018.425
Saulenko A. A., Maiorova A. S., Martínez Arbizu P., Mordukhovich V. V. (2022). Deep-Sea tardigrades from the North-Western Pacific, with descriptions of two new species. Diversity 14, 1086. doi: 10.3390/d14121086
Shively S., Miller W. R. (2009). The use of HMDS (hexamethyldisilazane) to replace critical point drying (CPD) in the preparation of tardigrades for SEM (scanning electron microscope) imaging. Trans. Kans. Acad. Sci. 112 (3/4), 198–200. doi: 10.1660/062.112.0407
Song H., Yao G., Yu C., Lv H. (2003). Compositing and of shrimp species in the East China Sea. Acta Oceanologica 1, 8–12.
Tang L., Li H., Yan Y. (2012a). Preliminary study on meiofauna in Daya Bay of the South China Sea in spring. Mar. Environ. Sci. 31 (3), 405–409.
Tang L., Zhang H., Li H., Yan Y. (2012b). Meiofauna in autumn in Daya Bay of South China Sea. J. Trop. Oceanogr. 31 (4), 104–111. doi: 10.3969/j.issn.1009-5470.2012.04.014
Thiel H. (1966). Quantitative Untersuchungen über die Meiofauna des Tiefseebodens. (Vorlaufiges Erge buis des “Meteor”- Expedition in den Indischen Ozean). Veroff. Inst. Meeresforsch. Bremerh. 2, 131–148.
Thulin G. (1928). Über die Phylogenie und das System der Tardigraden. Hereditas 11, 207–266. doi: 10.1111/j.1601-5223.1928.tb02488.x
Tilbert S., de Castro F. J. V., Tavares G., Nogueira Júnior M. (2019). Spatial variation of meiofaunal tardigrades in a small tropical estuary (~ 6°S; Brazil). Mar. Freshw. Res. 70 (8), 1094–1104. doi: 10.1071/MF18222
Wang J., Lei Y., Xu K., Meng Z. (2009). Spatial distribution of meiofauna in autumn and their relation to environmental factors in China seas. Mar. Sci. 33 (9), 62–70.
Wang X., Liu X., Xu J. (2019). Distribution patterns of meiofauna assemblages and their relationship with environmental factors of deep sea adjacent to the Yap trench, Western Pacific Ocean. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00735
Wang X., Wang C., Zhang D., Hong L. (2010). An improved method for separating meiofauna from deep sea sediments using colloidal silica Ludox@HS-40. J. Mar. Sci. 28 (3), 79–84. doi: 10.3969/j.issn.1001-909X.2010.03.011
Wang X., Zhou Y., Zhang D., Hong L., Wang C. (2013). A study of meiofauna in the OMRA’s contracted area during the summer of 2005. Acta Ecol. Sin. 33, 492–500. doi: 10.5846/stxb201111251801
Zeppilli D., Sarrazin J., Leduc D., Martinéz Arbizu P., Fontaneto D., Fontanier C., et al. (2015). Is the meiofauna a good indicator for climate change and anthropogenic impacts? Mar. Biodiv. 45 (3), 505–535. doi: 10.1007/s12526-015-0359-z
Zhong Z. (1986). Characteristics of shrimp resources of South China Sea and distributiong of economic important species. Mar. fieheries 3, 99–103.
Keywords: marine tardigrades, heterotardigrada, meiofauna, diversity indexes, deep sea, South China Sea
Citation: Wang X, Bai L, Wang C, Lu B, Li Y, Lin Q, Huang X and Fontoura P (2023) Preliminary studies of the tardigrada communities from a polymetallic nodule area of the deep South China Sea. Front. Mar. Sci. 10:1110841. doi: 10.3389/fmars.2023.1110841
Received: 29 November 2022; Accepted: 14 March 2023;
Published: 21 April 2023.
Edited by:
Stefanie Kaiser, Senckenberg Research Institute and Natural History Museum Frankfurt, GermanyReviewed by:
Krzysztof Pabis, University of Łódź, PolandDaniel Pech, El Colegio de la Frontera Sur, Mexico
Copyright © 2023 Wang, Bai, Wang, Lu, Li, Lin, Huang and Fontoura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunsheng Wang, d2FuZ3Npb0BzaW8ub3JnLmNu
 Xiaogu Wang1
Xiaogu Wang1 Lifen Bai
Lifen Bai Chunsheng Wang
Chunsheng Wang Qinyi Lin
Qinyi Lin Paulo Fontoura
Paulo Fontoura