
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 14 April 2023
Sec. Coral Reef Research
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1077805
 Tullia I. Terraneo1*
Tullia I. Terraneo1* Mustapha Ouhssain1
Mustapha Ouhssain1 Carolina Bocanegra Castano1
Carolina Bocanegra Castano1 Manuel Aranda1,2
Manuel Aranda1,2 Benjamin C. C. Hume3
Benjamin C. C. Hume3 Fabio Marchese1
Fabio Marchese1 Silvia Vimercati1,2
Silvia Vimercati1,2 Giovanni Chimienti4,5
Giovanni Chimienti4,5 Ameer A. Eweida6,7
Ameer A. Eweida6,7 Christian R. Voolstra3
Christian R. Voolstra3 Burton H. Jones1,2†
Burton H. Jones1,2† Sam J. Purkis7,8
Sam J. Purkis7,8 Mattie Rodrigue9
Mattie Rodrigue9 Francesca Benzoni1,2
Francesca Benzoni1,2Introduction: The northern Red Sea has been coined a refuge for reef corals due to the exceptional thermal tolerance of these organisms. With ocean warming threatening coral reefs worldwide, a panoptic characterization of corals living in extreme conditions may provide insights into future responses of corals to environmental change. Among other factors, the genotype of the endosymbiotic algae in the family Symbiodiniaceae has been shown to have major implications on the distribution and resilience of their coral hosts. In this study, we aim at genotyping the Symbiodiniaceae communities associated with three depth generalist and one depth specialist coral species, characterized by the ability to withstand environmental conditions that are apparently limiting for other corals and occurring in a unique geographical region.
Methods: We sampled 50 corals from the northern Saudi Arabian Red Sea and the Gulf of Aqaba, covering a 97 m bathymetric gradient. We used high-throughput ITS2 gene sequencing and recovered different patterns of host–algal associations.
Results and discussion: The majority of the recovered algal genotypes appeared host- and environment-specific, while others were more widely distributed. At large, coral specimens were overwhelmingly associated with symbionts from the genus Cladocopium and specifically with many previously undescribed genotypes. This suggests the selection of specific genotypes, which might confer resistance and/or resilience to their host counterparts. Interestingly, we found a limited association with Durusdinium spp. and other known tolerant taxa in mesophotic corals in the northern Red Sea, but not in the Gulf of Aqaba. The broad absence of Durusdinium spp., typically ascribed to be stress tolerant, warrants further investigation into Symbiodiniaceae species that convey environmental resilience. Our data will serve as a baseline to explore the occurrence of specific symbionts that might be contributing to coral acclimation and adaptation and to assay how biodiversity might be impacted if subjected to increasing stressors.
The Red Sea is a semi-enclosed basin with unique abiotic regimes (temperature, light, oxygen concentration, etc.) due to latitudinal and bathymetric environmental gradients (Purkis et al., 2012; Rowlands et al., 2014; Berumen et al., 2019). Temperature, salinity, and nutrient concentration vary from the north to the south (Raitsos et al., 2013; Wafar et al., 2016; Chaidez et al., 2017), and these conditions shape the diversity, distribution, and evolution of Red Sea marine organisms (Berumen et al., 2019). Being one of the warmest and saltiest extant seas on Earth, the Red Sea is often referred to as a “natural laboratory,” and the organisms adapted to live in it are studied to provide insights into the genetic adaptations of marine organisms to potential environmental scenarios driven by environmental change (Berumen et al., 2019). In particular, corals living in the basin regularly experience thermal conditions predicted for reefs elsewhere in the upcoming 50 years (Grimsditch and Salm, 2006). Within the Red Sea, its northernmost area (i.e., the north Red Sea and Gulf of Aqaba) has been attracting increasing attention, as its fauna seems adapted to withstand exceptional thermal conditions in comparison with the prevailing temperature regimes (Fine et al., 2013; Osman et al., 2018). Indeed, the northern Red Sea hosts coral species living below their bleaching thresholds, despite the thermal extremes to which they are exposed, rendering this unique region a potential thermal refuge in a rapid climate change context. In corals, thermal thresholds are in part determined by the symbiont type, and associating with more resilient symbionts is one of the mechanisms allowing corals to thrive under thermal extremes (Ziegler et al., 2015). Yet, the composition of the algal community of these corals and the molecular mechanisms which might help them thrive under such extremes remain largely unknown. As ocean warming is threatening the survival of coral reef ecosystems worldwide (Hughes et al., 2017), genotyping algal communities associated with corals living in extreme environments may provide insights into specific algal genotypes able to drive future responses of corals to climate change (Riegl et al., 2012a; Hume et al., 2013; D’Angelo et al., 2015; Davies et al., 2018; Howells et al., 2020).
The Red Sea organisms have also adapted to warm and salty conditions in deeper waters (>30 m), as well as to an oligotrophic and oxygen-limited environment (Roder et al., 2013; Qurban et al., 2014). Elsewhere in the world, mesophotic and deep-water corals occur at temperatures between 12°C and 4°C (Roberts et al., 2006). The Red Sea water temperature, however, stabilizes at 21°C–22°C even below 2,000 m depth (Sofianos and Johns, 2007; Roder et al., 2013); notwithstanding, it is inhabited by peculiar deep-sea coral species (e.g., Qurban et al., 2014; Chimienti et al., 2022). In the Red Sea, high water transparency due to its oligotrophic conditions increases the lower depth limit of the photic zone allowing the survival of photoautotrophic organisms deeper in the water column when compared with other localities (Kahng et al., 2014; Kheireddine et al., 2018). Several zooxanthellate coral species are unable to survive at depths deeper than 60 m where photosynthetic active radiation is limited (Loya et al., 2016). Yet, mesophotic coral ecosystems (MCEs), i.e., light-dependent communities between approximately 30-40 and 150 m depth, include symbiotic coral species that persist along the tropical and subtropical belts (Lesser et al., 2009; Hinderstein et al., 2010; Baker and Harris, 2016; Pyle and Copus, 2019). Adaptations of corals to depth regimes remain largely unknown (Schlesinger et al., 2018), but evidence indicates that among other factors, the bathymetric niche partitioning of photoautotrophic symbiont communities might play a fundamental role (Sampayo et al., 2009; Cooper et al., 2011; Bongaerts et al., 2013; Einbinder et al., 2016; Eckert et al., 2020). Overall, the symbionts’ genotype contributes to the distribution of Scleractinia across environmental regimes, with some coral species occurring both at shallow and mesophotic depths (i.e., depth generalist species) and others specialized to particular depths (i.e., depth specialist species).
Corals are most often associated with a single dominant alga at any given time, the identity of which is often primarily driven by a combination of host identity and the environment. Association with multiple algae from distinct genera is also not uncommon, especially with the increasing prevalence of stress events (e.g., increasing global bleaching events) with an iconic example being the (sometimes transient) association with Durusdinium trenchii LaJeunesse, 2018 in stress-characterized environments (LaJeunesse et al., 2009). There is a large genetic and phenotypic diversity within Symbiodiniaceae, with some algal symbionts displaying a high host fidelity, while others may associate with a broader range of host genotypes. The coral host may make use of the large diversity by associating with algae that are best suited to prevailing conditions. These algal symbionts are often resolved using the ITS2 rDNA gene, where the algae are characterized according to one or more abundant ITS2 sequences found in their genomes (e.g., the nomenclature of C11–C11N4 would represent an alga characterized by a dominance of the C11 and C11N4 ITS2 sequences; this will be used throughout this manuscript). For example, it has been shown that the symbiont C11–C11N4 lineage appears deeper than 20-30 m depth and might enable adaptation to low light regimes in Agaricia lamarcki Milne Edwards and Haime, 1851 and Helioseris cucullata (Ellis and Solander, 1786) in Curaçao (Bongaerts et al., 2013; Bongaerts et al., 2015a; Bongaerts et al., 2015b) and Puerto Rico (Lucas et al., 2016). Similarly, in the central Red Sea, C39 and C63, respectively, only occur in the upper mesophotic in Leptoseris Milne Edwards and Haime, 1849 corals and C39 appear between 20 and 30 m depth in Podabacia Milne Edwards and Haime, 1849 (Ziegler et al., 2015). However, not all symbionts are partitioned along the host’s depth gradient. For example, depth generalist lineages such as C3/t and C3nt consistently span the depth distribution of Seriatopora hystrix Dana, 1846 on the Great Barrier Reef (Bongaerts et al., 2011) and the Gulf of Eilat, respectively (Nir et al., 2011), as well as C15 in Porites Link, 1807 from the central Red Sea (Ziegler et al., 2015).
Genotyping efforts of Symbiodiniaceae communities along the depth gradient have been, so far, restricted to a few localities comprising the Caribbean (Frade et al., 2008a; Bongaerts et al., 2013; Bongaerts et al., 2015a; Bongaerts et al., 2015b), the Great Barrier Reef (Bongaerts et al., 2011; Bongaerts et al., 2013), and Hawaii (Wagner et al., 2011; Pochon et al., 2015; Padilla-Gamiño et al., 2019). In the Red Sea, the vast majority of the publications addressing Symbiodiniaceae characterization in MCEs are restricted to the Eilat coast (Gulf of Aqaba) and few model coral species (Winters et al., 2009; Nir et al., 2011; Byler et al., 2013; Nir et al., 2014; Einbinder et al., 2016; Turner et al., 2017; Ben-Zvi et al., 2020), with only one study from the central Saudi Arabian coast (Ziegler et al., 2015). To date, the symbiont communities along depth gradients in this region have been characterized in nine coral species distributed from the shallow to the upper mesophotic [see Goulet et al. (2019) for a review]. Therefore, the composition and zonation patterns of Symbiodiniaceae communities associated with scleractinian corals in Red Sea MCEs are unknown in the majority of the hosts.
In this study, we aim at filling this knowledge gap by genotyping, for the first time, the Symbiodiniaceae community associated with three depth generalist corals, namely, Coscinaraea monile (Forskål, 1775), Blastomussa cf. merleti (Wells, 1961), and Psammocora profundacella Gardiner, 1898, and the depth specialist Craterastrea levis Head, 1983. The four species have been reported to broadcast spawn their gametes in the Red Sea (Bouwmeester et al., 2015; Bouwmeester et al., 2016; Baird et al., 2021), which theoretically should reduce algal specificity as opposed to specific vertically transmitted symbionts in brooders (Baird et al., 2009). The investigated coral species occur within and outside the Red Sea with different distributions. In particular, P. profundacella is reportedly from all over the Indo-Pacific, B. cf. merleti and C. monile occur from the Indian Ocean to the western Pacific Ocean, while C. levis is only known in the Red Sea and the western Indian Ocean (Veron, 2000). Both P. profundacella and C. monile are common components of the coral communities all around the Arabian Peninsula, and their distribution extends into the Arabian Gulf (Sheppard and Sheppard, 1991; Claereboudt, 2006; Pichon et al., 2010; Riegl and Purkis, 2012c; Riegl et al., 2012b). There, they can withstand what are arguably among the harshest environmental conditions for scleractinian corals in terms of temperature and salinity extremes as well as environmental fluctuations and anthropogenic impacts (Sheppard et al., 2010; Riegl et al., 2012b). Blastomussa cf. merleti is not recorded from the Arabian Gulf but is common in the Gulf of Oman and the Arabian Sea, where it survives the pseudo-high latitude effect of the seasonal upwelling limiting other coral species and the formation of coral reefs (Sheppard et al., 1992; Benzoni et al., 2003; Claereboudt, 2006). Craterastrea levis is known to be restricted to low-light conditions and can occur at depths above 30 m exclusively in turbid and low-light environments (Benzoni et al., 2012). The remaining corals, although considered shallow water species, were previously recorded below 30 m in the Red Sea (Fricke and Schuhmacher, 1983; Scheer and Pillai, 1983; Eyal et al., 2019). As none of the corals examined in this study is considered a model species for research on coral biology and ecology, their symbionts remain largely uncharacterized thus far, despite their widespread distribution and ability to withstand environmental conditions that are apparently limiting for other corals. Therefore, we decided to focus on these to contribute to the unraveling of the role of different symbiont genotypes in their ecology. Our study covers a 97-m bathymetric range, comprising shallow and mesophotic communities in the northern Saudi Arabian Red Sea (NEOM region) and encompassing latitudinal and environmental depth gradients. We used next-generation sequencing of the ITS2 amplicon to generate ITS2 profiles representative of putative symbiont genotypes associated with each host species to investigate the distribution of algal assemblages along depth and latitudinal gradients.
The four scleractinian coral species targeted in this study (Figure 1) belong to the Robust clade (Kitahara et al., 2016), and their systematic position has been revised through an integrated morphomolecular approach (Benzoni et al., 2010; Benzoni et al., 2012; Benzoni et al., 2014).
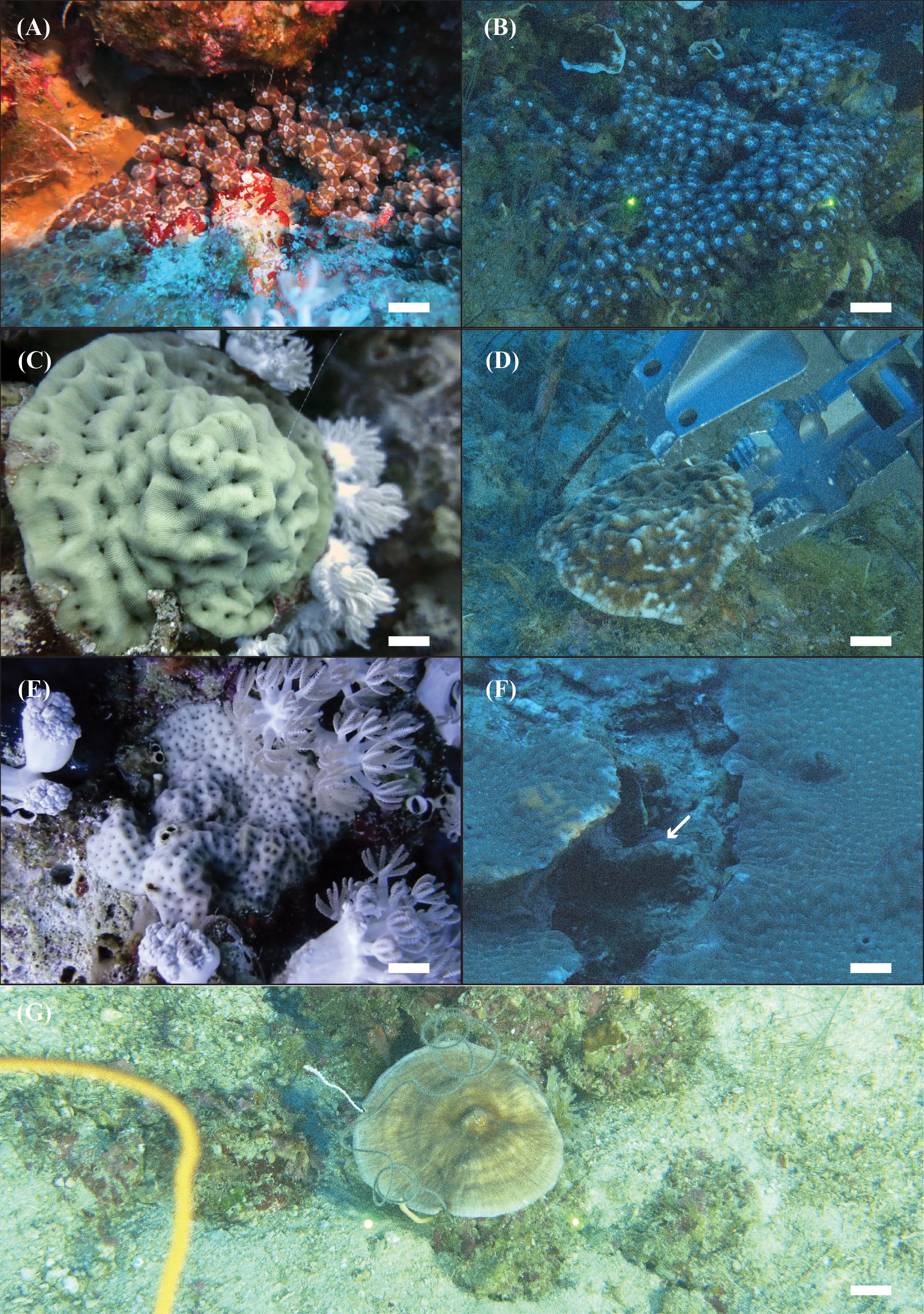
Figure 1 In situ images of the four Scleractinia host species analyzed in the present study. Blastomussa cf. merleti: (A) SA4621—shallow and (B) NTN0060-19—mesophotic. Coscinaraea monile: (C) SA4639—shallow and (D) NTN0060-22—mesophotic. Psammocora profundacella: (E) SA4775—shallow and (F) NTN0048-14B—mesophotic. Craterastrea levis: (G) NTN0043-20—mesophotic.
In October and November 2020, 50 coral colonies were collected during the Red Sea Deep Blue Expedition on board the M/V OceanXplorer. A total of nine coral colonies belonging to the species B. cf. merleti (1), C. monile (3), P. profundacella (1), and C. levis (4) were collected using a Triton 3300/3 submersible (Sub) with a Schilling T4 hydraulic manipulator between 57 and 97 m depth in the Saudi Arabian Gulf of Aqaba (three sites) and in the northern Red Sea (three sites). One colony of B. cf. merleti was sampled using an Argus Mariner XL Remotely Operated Vehicle (ROV) and ROV Suction Sampler (Cellula Robotics Ltd., Burnaby, Canada) at 95 m in the northern Red Sea (one site). The Sub and ROV dives were video-recorded, and frame grabs of the colonies were extracted from the videos using the open-source software MPC-HC (Media Player Classic – Home Cinema) available at https://github.com/mpc-hc/mpc-hc. A total of 14 colonies of B. cf. merleti, 16 colonies of C. monile, and 10 colonies of P. profundacella were collected between 0 and 30 m depth while scuba diving, using a hammer and chisel (Figure 1). Prior to sampling, the colonies were photographed in situ using a Nikon Coolpix W300 underwater digital camera. Scuba sampling occurred at eight sites in the Gulf of Aqaba and 14 sites in the northern Red Sea (Figure 2, Table 1).
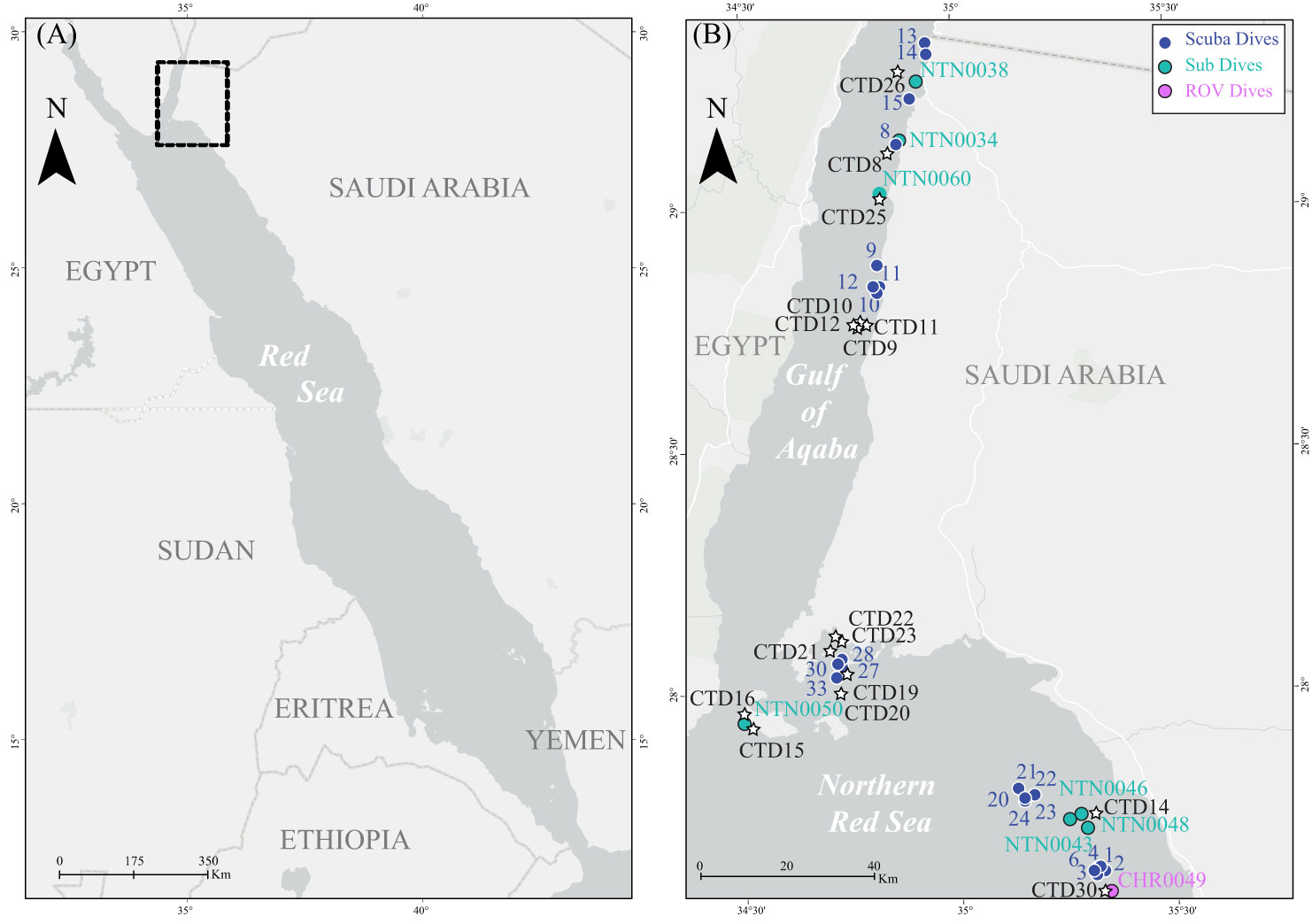
Figure 2 (A) Map of the Saudi Arabian Red Sea. (B) Close-up map showing sampling sites for the current study. Blue sequential numbers indicate scuba diving sites, light blue NTN codes indicate submarine sampling sites, and pink CHR codes indicate ROV sampling sites. Black CTD codes indicate CTD cast sites.
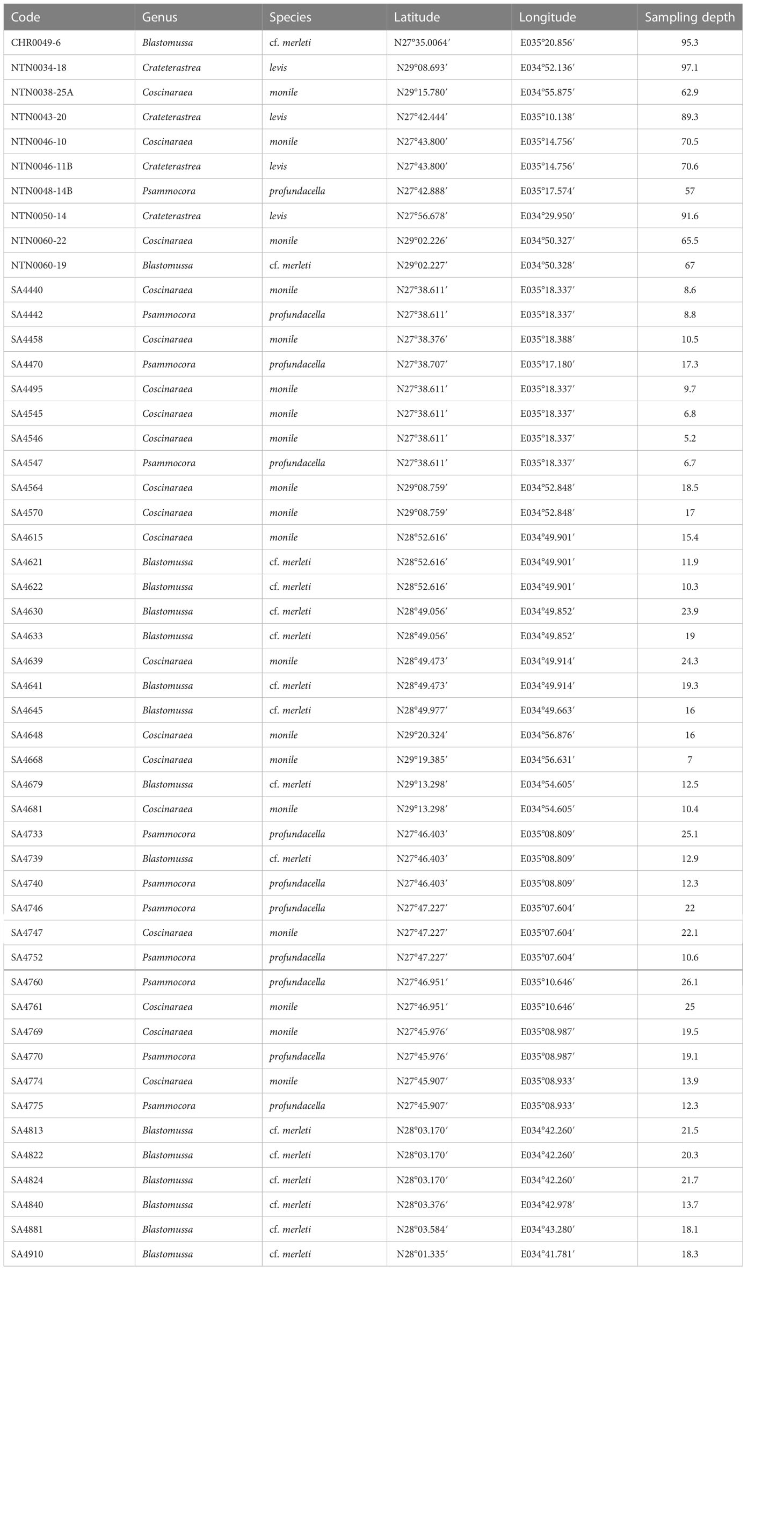
Table 1 List of coral specimens collected in the Saudi Arabian Red Sea for the current study, including voucher code, genus and species identification, and collection metadata.
After collection, approximately 1 cm2 of coral tissue from each colony was preserved in 99% EtOH for molecular analyses. The rest of the corallum was bleached in sodium hypochlorite to remove fresh tissue and then air-dried. The coral skeletons and tissue samples are stored at the King Abdullah University of Science and Technology (KAUST, Saudi Arabia).
Environmental data (conductivity, temperature, depth) profilings were recorded using a Sea-Bird SBE 19+ CTD mounted on the submersible for sub dives (RBR data corresponding to submersible dive sites) or a Sea-Bird 911 CTD mounted on a rosette from the mother ship (CTD data). Both CTDs were calibrated prior to the cruise. Data from both sensors were extracted and processed using SBE’s Seasoft software.
Symbiodiniaceae genomic DNA was extracted from coral tissues using the DNeasy® Blood and Tissue Kit (Qiagen Inc., Hilden, Germany). Symbiodiniaceae library preparation and sequencing the ITS2 rDNA gene (ITS2) on the Illumina MiSeq platform. The primers ITSintfor2 and ITS2-reverse (LaJeunesse, 2002) with Illumina adapters were used. For each sample, PCRs were run with 11 µl of Qiagen Multiplex PCR Kit (Qiagen, Hilden, Germany), 2 µl of 10 µM primers, and 5 µl of DNA, in a total volume of 25 µl. The following PCR conditions were used: 15 min at 94°C, followed by 35 cycles of 94°C for 30 s, 51°C for 30 s, 72°C for 30 s, and a final extension step of 10 min at 72°C. PCR products were using the QIAxcel capillary electrophoresis machine, with the DNA Screening Kit (2400), a 3-kb marker, and the AM320 method (Qiagen, Hilden, Germany). The PCR products were pooled and cleaned with Agencourt AMPure XP magnetic bead system (Beckman Coulter, Brea, CA, USA). Nextera XT indexing and sequencing adapters were added via PCR (8 cycles) following the manufacturer’s instructions. Samples were normalized and pooled using SequalPrep Normalization Plate Kit (Thermo Fisher Scientific, Waltham, MA, USA). The samples were then quantified on Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA) and qPCR (Thermo Fisher Scientific, Waltham, MA, USA) to check library size and concentration, respectively. Libraries were sequenced using the Illumina MiSeq platform (Illumina, San Diego, CA, USA) and kit reagents v3 (2 × 300 bp pair-ended reads) at KAUST Bioscience Core Lab (Thuwal, Saudi Arabia), following the manufacturer’s protocol.
Demultiplexed paired fastq.gz files were submitted online to SymPortal (https://symportal.org, Hume et al., 2019). The SymPortal pipeline consists of running sequence quality control (QC) for each sample using mothur 1.39.5 (Schloss et al., 2009), BLAST+ executables against the symClade.fa-based BLAST database (Camacho et al., 2009), and finally, minimum entropy decomposition (MED; Eren et al., 2015). Then, ITS2 profiles are discovered by searching for re-occurring sets of ITS2 sequences and assigned to samples. The post-MED ITS2 sequence relative abundance count table (Appendix S2) and the ITS2 profile relative abundance count table (Appendix S3), together with the respective absolute counts, between sample distances and between profile distances, were downloaded from the online framework. Here, we use the ITS2 profile predictions as proxies for Symbiodiniaceae genotypes. We produced stacked bar plots to associate the Symbiodiniaceae genotypes with different categorical levels (host species, body of water, sampling depth) with the R package ggplot2 (Wickham et al., 2016). Patterns of specificity and generalism were visualized using Venn diagrams.
The environmental parameters measured during the expeditions are summarized in Appendices S1, S2 (CTD data and RBR data). The abiotic profiles recovered evidenced the presence of two distinct masses at the time of sampling for this study: the northern Red Sea and the Gulf of Aqaba (Figure 3). The upper water column in the northern Red Sea is warmer and less saline (28°C ± 0.7°C, 40.8 ± 0.1) than the Gulf of Aqaba, which is cooler and more saline (26.7°C ± 0.2°C, 44 ± 0.1). The northern Red Sea is marked by higher variability due to the physical dynamics in this region (i.e., the presence of a cyclonic circulation, the eastern boundary current, and the influence of the Gulf of Aden inflow water).
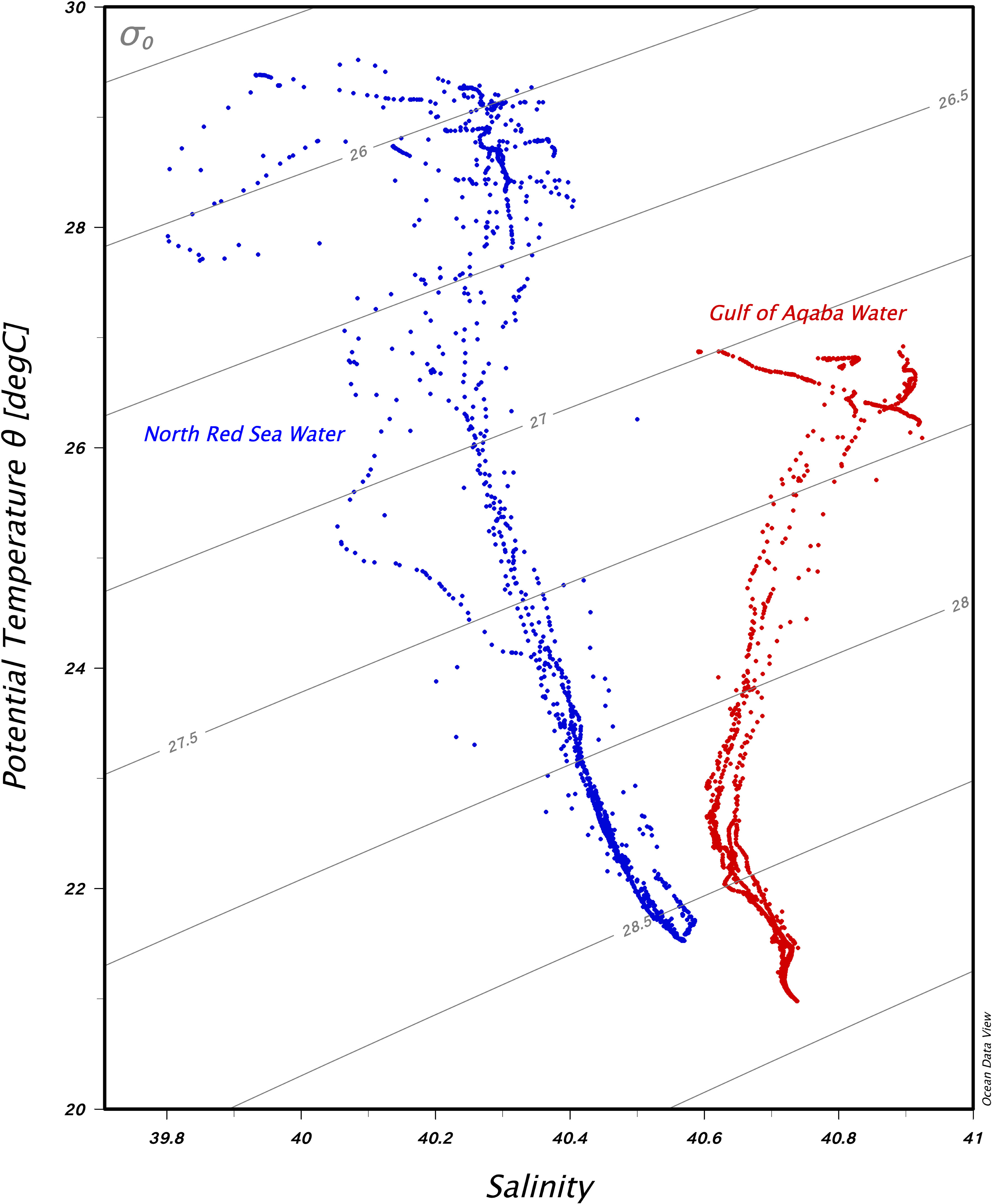
Figure 3 Temperature–salinity diagram with isopycnal contours in the water column of the Gulf of Aqaba stations (red dots) and the northern Red Sea stations (blue dots).
Using Illumina MiSeq, 4,363,239 ITS2 sequences were generated, with a mean of 87,264 sequences per sample (n = 50) (Appendix S2). After submission to the SymPortal, the post-MED output consisted of 268 different ITS2 sequences, of which 237 belonged to the Symbiodiniaceae genus Cladocopium LaJeunesse and Jeong, 2018, 30 sequences belonged to Durusdinium LaJeunesse, 2018, and one sequence belonged to Symbiodinium Freudenthal, 1962 (Appendix S4). Durusdinium-derived sequences were only found in the north Red Sea at depths of >30 m, but not in the Gulf of Aqaba, while Symbiodinium was found in one sample from the Gulf of Aqaba. A total of 19 ITS2 profiles were identified (Appendix S3; Figures 4, 5). Overall, 96% of ITS2 profiles belonged to the C1 radiation (Appendix S3; Figures 4, 5). The remaining profiles (hereby represented by their most abundant sequences) were C39, C116, C115, C3, and D1. The ITS2 profiles were not evenly distributed among the samples, with four all belonging to the C1 radiation, which accounted for 68% of the total diversity (Appendix S3; Figures 4, 5).
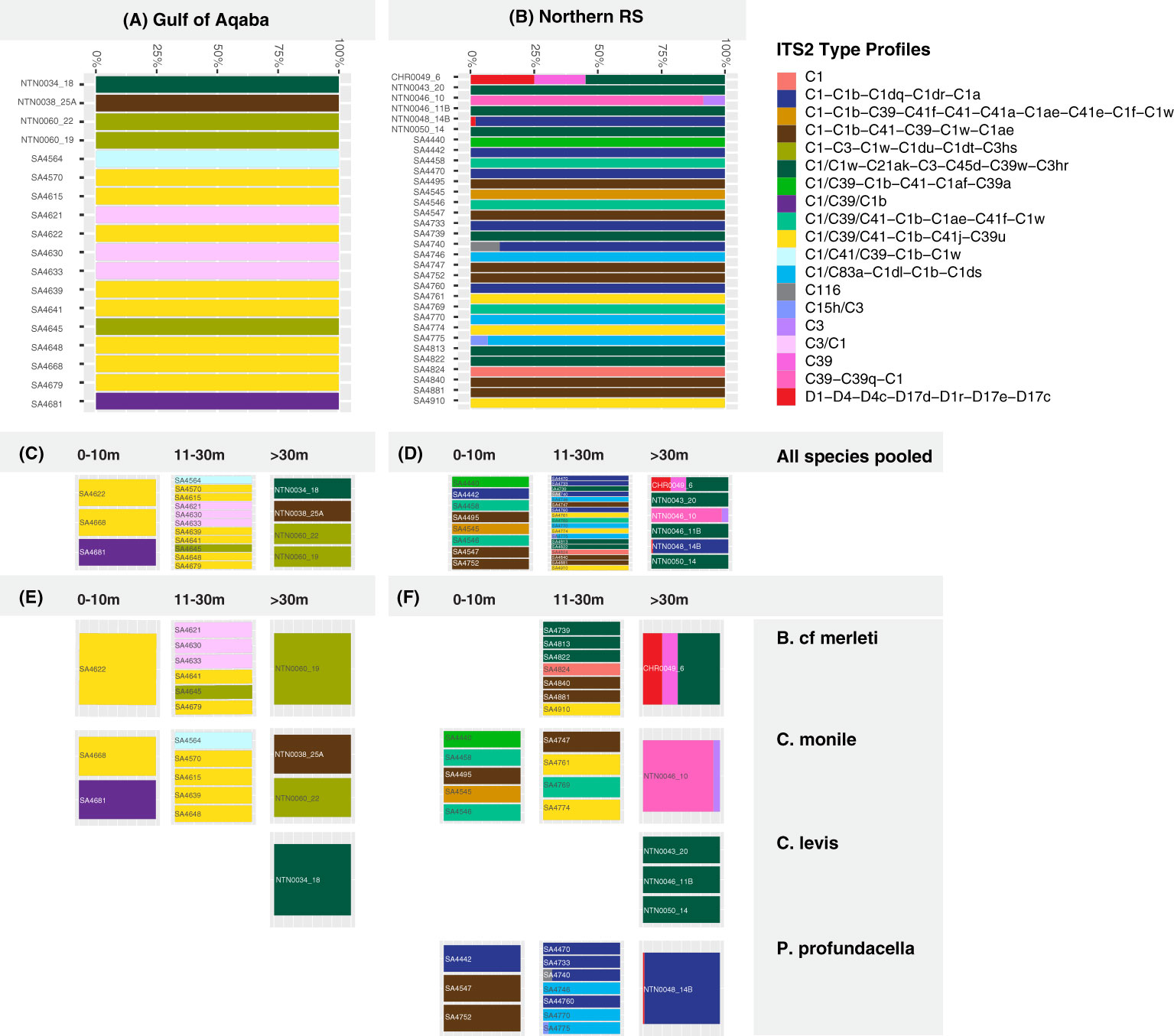
Figure 4 Predicted Symbiodiniaceae ITS2 profiles for the analyzed coral hosts in the Gulf of Aqaba (A) and in the northern Red Sea (B) at (C, D) three depth ranges (0-10, 11-30, >30 m) and for (E, F) four Scleractinia host species (Blastomussa cf. merleti, Coscinaraea monile, Craterastrea levis, Psammocora profundacella) at three depth ranges (0-10, 11-30, >30 m).
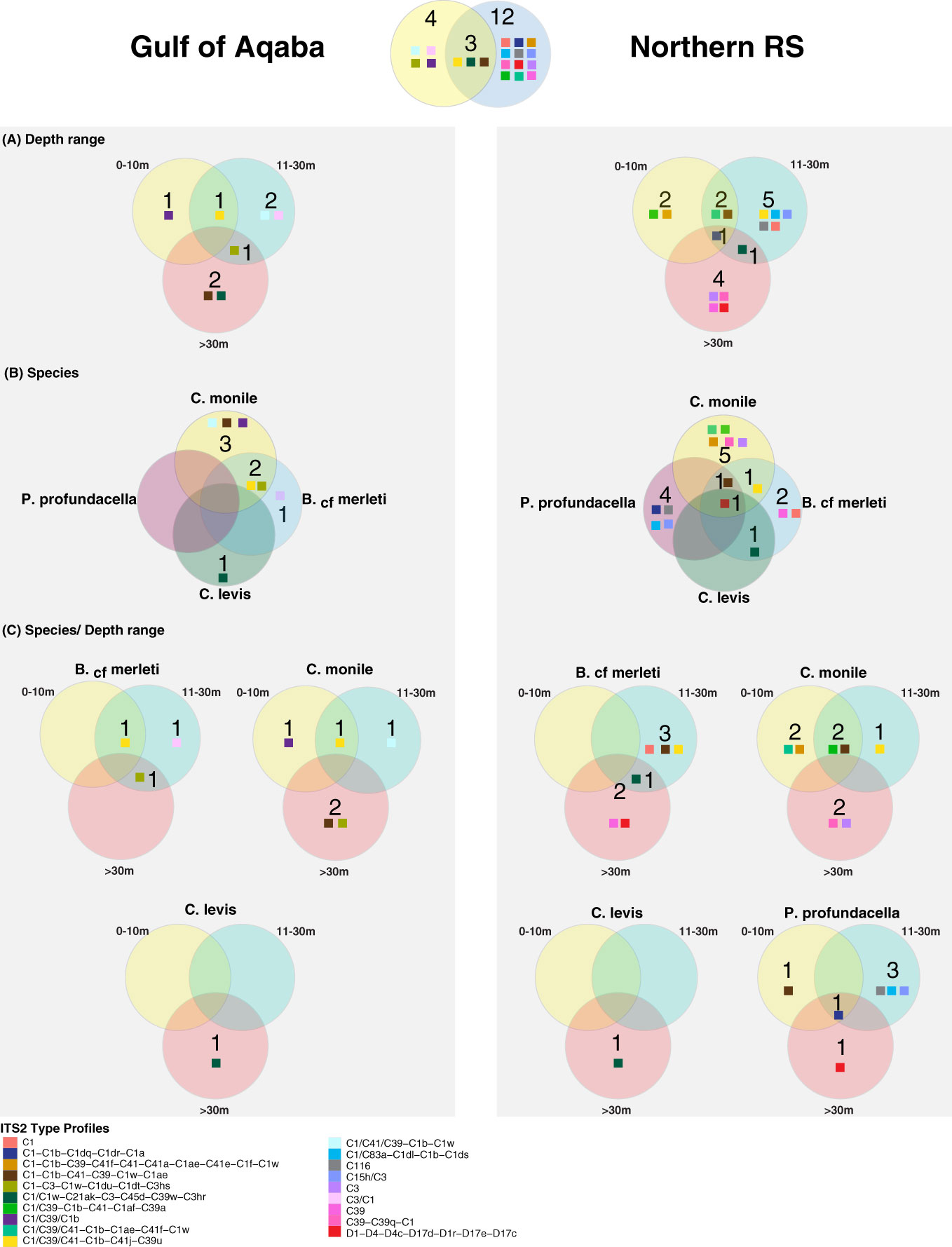
Figure 5 Venn diagrams representing the unique and shared Symbiodiniaceae ITS2 profiles in the Gulf of Aqaba and the northern Red Sea over (A) depth range, (B) species, and (C) species/depth range. Colored squares refer to Symbiodiniaceae ITS2 profiles as outlined in the legend.
Overall, the majority of the ITS2 profiles (14/19) were recovered from specific coral host species, sites, or depth ranges, indicating high levels of specificity (specialist ITS2 profiles). Only a few (5/19) were recovered from multiple hosts, sites, and/or depths (generalist ITS2 profiles). All five generalist profiles belonged to the C1 radiation. Of these, three occurred in multiple hosts and sites but appeared specific to a certain depth range, one was host and depth generalist but site-specific, while one was exclusively a depth generalist taxon but at the same time species- and site-specific (Figures 4, 5).
Among the examined host taxa, C. monile was the species with the most diverse Symbiodiniaceae ITS2, found in association with 10 ITS2 profiles, 80% of which belonged to the C1 radiation (Figure 5). Blastomussa cf. merleti was associated with eight ITS2 profiles (five of which were represented by C1, and one belonged to Durusdinium), while P. profundacella was associated with five ITS2 profiles (3/5—C1). Craterastrea levis was exclusively associated with one profile also belonging to the C1 radiation. In total, 15 ITS2 profiles were found to be host specialist in C. monile, B. cf. merleti, and P. profundacella, while no specific profile was exclusively associated with C. Levis. Focusing on the site, the Gulf of Aqaba harbored the least diverse Symbiodiniaceae community in association with the examined coral species, represented by seven ITS2 profiles, while 15 occurred in the northern Red Sea. The two basins shared three type profiles, all belonging to the C1 radiation. Site specialist profiles occurring in the Gulf of Aqaba also belonged to the C1 radiation and represented newly discovered genotypes, while in the north Red Sea, we also retrieved C15h/C3, C116, C39-C39q-C1, D1-D4-D4c-D17d-D1r-D17e-D17c, C39, and C3 (Figures 4, 5).
The host-specific profiles were distinct in the water masses, suggesting a latitudinal signature (Figures 4, 5). In the Gulf of Aqaba, we recovered three specific ITS2 profiles: two in C. monile belonging to the C1 radiation and one in B. cf. merleti (C3/C1). In the northern Red Sea, we recovered more ITS2 profiles in terms of number and diversity in association with the four coral species, including not only representatives of the C1 radiation, which were confirmed to be the most abundant but also specific profiles like C39-C39q-C1 and C3 exclusively associated with C. monile, D1-D4-D4c-D17d-D1r-D17e-D17c the only profile representative of Durusdinium, C39 associated with B. cf. merleti, and C15h/C3 and C116 associated with P. profundacella. In total, five ITS2 profiles belonging to the C1 radiation were host generalist and shared among species independently of the body of water. Craterastrea levis hosted a profile shared with other species, yet all the samples were associated exclusively with it, suggesting a specific community composition (Figures 4, 5).
Focusing on the depth, the range which presented the highest Symbiodiniaceae profile diversity was 11-30 m depth, with the majority of the ITS2 profile diversity from the northern Red Sea. Instead, between 0 and 10 and >30 m, we found seven profiles. Overall, we recovered two depth generalist and 17 depth specialist ITS2 profiles. The only profile occurring in all depth ranges was C1/C39/C1b associated with P. profundacella in the northern Red Sea from 0 to >30 m. Exclusive profiles occurred at different depth ranges. In particular, between 0 and 10 m depth, we found four specific profiles belonging to the C1 radiation; between 11 and 30 m, C15h/C3 and C116 also appeared; and finally, below 30 m, C39, C39-C39q-C1, and D1-D4-D4c-D17d-D1r-D17e-D17c were specialized (Figures 4, 5).
With this work, we aimed at characterizing the identity of algal genotypes which might contribute to coral acclimation and adaptation in a unique region of the world. We first provided an environmental characterization of the Gulf of Aqaba and northern Red Sea waters, areas that harbor corals tolerant to thermal stress that can cause bleaching and mortality elsewhere. We then genotyped for the first time the Symbiodiniaceae diversity associated with four coral species known to withstand environmental conditions that are normally limiting for other corals. We used the NGS of the ITS2 marker and the SymPortal online framework to identify dominant algal genotypes and compare their diversity across latitudinal and bathymetric gradients.
Interest is growing in identifying regions where corals present high resistance and resilience to stressors, as well as the ecological and physiological mechanisms able to confer such abilities. In this work, we investigated the presence of unique tolerant algal genotypes along a latitudinal gradient, comprising the Saudi Arabian Gulf of Aqaba and the northern Red Sea. The coral holobiont plays a crucial role in thermal tolerance and adaptation (Bang et al., 2018). In particular, evolutionary distinct Symbiodiniaceae genotypes have undergone radiations in relation to environmental conditions, on both a global and local scale (LaJeunesse, 2005). For example, even if genotypes of the C3 radiation have been long considered generalist and sensitive to thermal stress, C. thermophilum has been identified in corals of the gulf and shown to confer thermal tolerance to the hosts living under these extreme environmental conditions (D’Angelo et al., 2015; Hume et al., 2015). The genotypes recovered in our work mainly represented the genus Cladocopium at all sites, while only one belonged to the generally known thermal tolerant genus Durusdinium, and it exclusively appeared in one mesophotic colony, together with Cladocopium. The dominance of Cladocopium sequences in the Gulf of Aqaba corals was previously recorded in the coral genera Porites (Terraneo et al., 2019; Osman et al., 2020), Pocillopora Lamarck, 1816 (Sawall et al., 2014), Dipsastraea Blainville, 1830 (Osman et al., 2020), and Stylophora Schweigger, 1820 (Winters et al., 2009; Arrigoni et al., 2016; Ezzat et al., 2017), as well as in the zoanthid Palythoa tuberculosa (Esper, 1805) (Reimer et al., 2017). When considering symbiont patterns at the genus level, we did not encounter latitudinal differences in association with the four hosts, as they all presented Cladocopium-dominated communities both in the Gulf of Aqaba and in the northern Red Sea. Contrary to our findings, previous studies evidenced a community break in the coral genus Pocillopora in the Saudi Arabian Red Sea south of the Gulf of Aqaba, with a switch from Cladocopium-dominated to Symbiodinium-dominated communities (e.g., Sawall et al., 2014). This was attributed to a correlation of the Symbiodiniaceae diversity with Red Sea environmental latitudinal gradients. Yet, other studies showed a mix of Cladocopium and Durusdinium genotypes in shallow water Porites south of the Gulf of Aqaba, and Osman et al. (2020) evidenced a continuity in the communities associated with Porites and Dipsastrea outside of the Gulf of Aqaba along the Egyptian coast.
A community break could have been expected in our data as the Gulf of Aqaba and the northern Red Sea have been recently identified as two distinct bioregions (Kheireddine et al., 2021). This has been attributed to the phytoplankton phenology, which is linked to the physical conditions occurring in their respective areas. The Gulf of Aqaba is characterized by a winter phytoplankton bloom that is related to deep vertical mixing. This supplies cold, nutrient-rich waters at the surface due to thermohaline circulation as well as wind forces (Kheireddine et al., 2021 and references therein). The northern Red Sea is influenced by the general cyclonic circulation observed in this area, as well as the permanent presence of mesoscale features (i.e., cyclonic and anticyclonic eddies) (Kheireddine et al., 2021; Papagiannopoulos et al., 2021). For example, in coastal areas where coral reefs are present, summer phytoplankton bloom can be observed due to local circulation features and the presence of corals that lead to an injection of nutrients within the surface layer (Papagiannopoulos et al., 2021). This region is also characterized by a shallower mixing and a shorter duration of the phytoplankton bloom than in the Gulf of Aqaba (Kheireddine et al., 2021). Such conditions could lead to a unique area with a specific biodiversity regarding coastal habitats. Yet, this was not recovered in our data when considering genus-level Symbiodiniaceae–host associations. Nevertheless, the Symbiodiniaceae within genus diversity is proving increasingly diverse and ecologically significant as opposed to genus level associations (Sampayo et al., 2008; Sampayo et al., 2009; Hume et al., 2019; Hume et al., 2020). As our ability to resolve within the Symbiodiniaceae has increased, it has become clear that there is diversity within each of the genera, with Cladocopium likely having the highest diversity of all the genera. With this genetic diversity comes phenotypic diversity exemplified by the wide range of ecosystems and environments in which Cladocopium associations can be found. While some genus-level generalizations may be true for the majority of the members (i.e., thermal tolerance of Durusdinium spp.), phenotypes should likely be associated with finer-scale resolutions. Indeed, in our data, we identified a lower number of unique genotypes in the Gulf of Aqaba compared to the more diverse northern Red Sea. Two of these genotypes were new to science, belonging to the C1 radiation, the remaining being C3/C1 and C39/C1 represented. No genotypes commonly associated with thermal tolerance such as Durusdinium radiations were recovered in the Gulf of Aqaba, while we did encounter a small proportion of D1 radiation in two samples from the northern Red Sea, together with Cladocopium-dominated genotypes, indicating that we still lack knowledge of specific symbionts, which can convey acclimation and adaptation of corals under stress conditions.
From a bathymetric perspective, in our study, both shallow and mesophotic communities were dominated by Cladocopium. Ezzat et al. (2017) compared dominant symbiotic communities associated with Sylophora pistillata from shallow to mesophotic reefs in the Gulf of Aqaba and recovered Cladocopium in mesophotic colonies, as opposed to Symbiodinium associations in shallow water samples. This was interpreted as a possible advantage of Cladocopium to fix carbon in low-light environments. Interestingly, in our data, Durusdinium was only encountered in mesophotic hosts, possibly conferring higher resilience to its hosts. Specifically, in our data, we found unique profiles between 0 and 10 m in C. monile and P. profundacella and between 11 and 30 m in C. monile, B. cf. merleti, and P. profundacella. In the mesophotic, unique profiles appeared, possibly associated with the changing environment. This is well exemplified by the recovery of the only Durusdinium-related genotype in B. cf. merleti and P. profundacella, C3 and C39 radiation in C. monile, and C39 radiation in B. cf. merleti. Communities dominated by C3 sequences were already shown in association with Pachyseris speciosa (Dana, 1846) colonies at 40-50 m depth in the central Red Sea, while C39 ITS2 sequences were previously found dominant between 20 and 40 m depth in Podabacia sp., Leptoseris sp., and P. speciosa in the central Red Sea (Ziegler et al., 2015). As hosts persist under different environmental regimes, it is because they harbor specific symbionts fulfilling their physiological requirements. Frade et al. (2008b) highlighted variation in photosynthetic symbionts of coral species in the genus Madracis over a depth gradient, showing host specificity and depth-driven symbiont community composition. Bongaerts et al. (2015a) and Lucas et al. (2016) evidenced depth zonation of Symbiodiniaceae in Agaricia lamarcki in the Caribbean. Nevertheless, not all corals exhibit the same adaptation to depths by showing symbiont zonation. Indeed, we also found an overlap in symbiont genotypes at different depths, as in the case of C1/C39/C1b, which occurred from shallow to mesophotic colonies in P. profundacella. The genotype of the photosynthetic symbionts over depth has long been considered one of the main responsible factors for Scleractinia persistence over wide depth ranges (Rowan and Knowlton, 1995), and the physiological differences among Symbiodiniaceae have been related to coral distribution patterns (González-Pech et al., 2019). At the same time, Ziegler et al. (2015) evidenced an influence from the host on the composition of the photosynthetic pigments of the Symbiodiniaceae. It, thus, remains difficult to draw conclusions on the actual contribution from the host’s genotype and the Symbiodiniaceae counterpart, as they both likely play key roles in shaping distribution and resilience patterns.
When considering the algal genotype predictions of our study, we recovered different combinations of host, site, and depth patterns of specificity and generalism. The generalist genotypes were less in number (5/19 host generalist, 3/19 site generalist, 2/19 depth generalist) but were encountered in the majority of explored diversity. Four were host and site generalist but depth-specific or host and depth generalist but site-specific, while one was exclusively a depth generalist taxon. The specialist genotypes were higher in number (14/19 host specialist, 16/19 site specialist, 17/19 depth specialist) but represented a smaller proportion of the community. The host has previously been shown to be a major driver in shaping the distribution of Symbiodiniaceae (Tonk et al., 2013). In our study, C. levis, the only depth specialist coral taxon included, exhibited a single genotype belonging to the C1 radiation, and this was independent of the sampled basin, highlighting high fidelity. Nevertheless, this genotype was not unique to C. levis and was found also in B. cf. merleti from 11 m to the mesophotic, indicating that such a profile is not exclusive to C. levis nor is a depth-specific taxon.
Most of the algal genotypes associated with the four selected hosts belonged to the C1 radiation. The majority of these were recovered in the northern Red Sea and between 11 and 30 m depth. On the contrary, a lower number of genotypes was present in the Gulf of Aqaba, as well as at shallower and deeper ranges. In the mesophotic, we encountered specific tolerance-related algal genotypes, enforcing the idea that these depths might also represent resilience areas for corals. Host, site, and depth-specific genotypes were higher in number, while a smaller number were host, site, and depth generalist. Craterastrea levis was always associated with the same single genotype, while C. monile presented the most diversity. To which extent the host or the symbionts play a role in driving the host distribution remains unclear, and due to the limited sample size for some of the species investigated in our study, we should not draw strict conclusions, as further sampling might have revealed more genotypes. However, this work provides the basis for a better understanding of the ecology and distribution of corals in a unique region of the world over a 97 m bathymetric range. Moreover, the discovery of new genotypes informs that selection to withstand particular environmental conditions might be happening and that further investigation encompassing other coral species and including more localities and samples might help us understand the processes driving such diversity. Finally, the presence of unique genotypes calls for the environmental protection of such unique fauna.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: GenBank of NCBI at https://www.ncbi.nlm.nih.gov/sra. The associated **BioProject** and **Bio-Samples** numbers are PRJNA925301, and SAMN32786842 to SAMN32786891 respectively.
TT and FB conceived and designed the project. TT, FM, SV, GC, SP, and FB were involved in coral sampling and processing. TT performed the NGS library preparation, and wrote the manuscript. MO collected and extracted the environmental parameters and wrote the environmental section of the methods and results. CC performed the NGS library preparation with TT. AE and MR were the scientific coordinators for the expedition. MO, MA, BH, CV, BJ, SP, MR, and FB revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
This project was supported by funding from NEOM and KAUST (award FCC/1/1973-50-01 and baseline research funds to FB).
This research was undertaken in accordance with the policies and procedures of the King Abdullah University of Science and Technology (KAUST). Permissions relevant for KAUST to undertake the research were obtained from the applicable governmental agencies in the Kingdom of Saudi Arabia. We thank NEOM for the facilitation and coordination of the Red Sea Deep Blue expedition and, specifically, A. A. E., Thamer Habis, Justin Mynar, Paul Marshall, Giacomo Palavicini, Peter Mackelworth, and Abdulaziz Alghamdi. We would like to thank OceanX and the crew of OceanXplorer for their operational and logistical support for the duration of this expedition. In particular, we would like to acknowledge the ROV and submersible teams for sample collection and OceanX for the support of scientific operations on board the OceanXplorer. We would also like to thank OceanX Media for documenting and communicating this work to the public.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1077805/full#supplementary-material
Appendix S1 | Physical parameters measured with the CTD at different locations in the Gulf of Aqaba and the northern Red Sea. Location of each station is shown in Figure 1.
Appendix S2 | Physical parameters measured with the RBR at different locations in the Gulf of Aqaba and the northern Red Sea during submarine dives. Location of each station is shown in .
Appendix S3 | SymPortal output, including relative abundance of post MED ITS2 sequences.
Appendix S4 | SymPortal output, including relative abundance of predicted ITS2 Profiles.
Appendix S5 | Composition of algal communities associated with four coral species in the Saudi Arabian Red Sea. Each stacked bar refers to a Scleractinia host sample. The top panel shows the ITS2 Symbiodiniaceae Post-MED genus annotated sequences relative abundances. The lower panel shows the predicted ITS2 Profiles representative of putative Symbiodiniaceae genotypes for each coral sample.
Arrigoni R., Benzoni F., Terraneo T. I., Caragnano A., Berumen M. L. (2016). Recent origin and semi-permeable species boundaries in the scleractinian coral genus Stylophora from the red Sea. Sci. Rep. 6 (1), 1–13. doi: 10.1038/srep34612
Baird A. H., Guest J. R., Edwards A. J., Bauman A. G., Bouwmeester J., Mera H., et al. (2021). An indo-pacific coral spawning database. Sci. Data 8 (1), 35. doi: 10.1038/s41597-020-00793-8
Baird A. H., Guest J. R., Willis B. L. (2009). Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecology Evolution Systematics 40, 551–571. doi: 10.1146/annurev.ecolsys.110308.120220
Baker E. K., Harris P. T. (Eds.) (2016). Mesophotic coral ecosystems: A lifeboat for coral reefs? (Arendal: United Nations Environment Programme and GRID). Retrieved from https://policycommons.net/artifacts/2390329/mesophotic-coral-ecosystems/3411646/ on 30 Mar 2023. CID: 20.500.12592/mq8sjt.
Bang C., Dagan T., Deines P., Dubilier N., Duschl W. J., Fraune S., et al. (2018). Metaorganisms in extreme environments: do microbes play a role in organismal adaptation? Zoology 127, 1–19. doi: 10.1016/j.zool.2018.02.004
Benzoni F., Arrigoni R., Stefani F., Stolarski J. (2012). Systematics of the coral genus Craterastrea (Cnidaria, anthozoa, scleractinia) and description of a new family through combined morphological and molecular analyses. Systematics Biodiversity 10 (4), 417–433. doi: 10.1080/14772000.2012.744369
Benzoni F., Arrigoni R., Waheed Z., Stefani F., Hoeksema B. W. (2014). Phylogenetic relationships and revision of the genus Blastomussa (Cnidaria: Anthozoa: Scleractinia) with description of a new species. Raffles Bull. Zool. 62, 358–378.
Benzoni F., Bianchi C. N., Morri C. (2003). Coral communities of the northwestern gulf of Aden (Yemen): Variation in framework building related to environmental factors and biotic conditions. Coral Reefs 22 (4), 475–484. doi: 10.1007/s00338-003-0342-1
Benzoni F., Stefani F., Pichon M., Galli P. (2010). The name game: morpho-molecular species boundaries in the genus Psammocora (Cnidaria, scleractinia). Zoological J. Linn. Soc. 160 (3), 421–456. doi: 10.1111/j.1096-3642.2010.00622.x
Ben-Zvi O., Tamir R., Keren N., Tchernov D., Berman-Frank I., Kolodny Y., et al. (2020). Photophysiology of a mesophotic coral 3 years after transplantation to a shallow environment. Coral Reefs 39 (4), 903–913. doi: 10.1007/s00338-020-01910-0
Berumen M. L., Voolstra C. R., Daffonchio D., Agusti S., Aranda M., Irigoien X., et al. (2019). “The red Sea: Environmental gradients shape a natural laboratory in a nascent ocean,” in Coral reefs of the red Sea (Cham: Springer), 1–10.
Bongaerts P., Carmichael M., Hay K. B., Tonk L., Frade P. R., Hoegh-Guldberg O. (2015a). Prevalent endosymbiont zonation shapes the depth distributions of scleractinian coral species. R. Soc. Open Sci. 2 (2), 140297. doi: 10.1098/rsos.140297
Bongaerts P., Frade P. R., Hay K. B., Englebert N., Latijnhouwers K. R., Bak R. P., et al. (2015b). Deep down on a Caribbean reef: lower mesophotic depths harbor a specialized coral-endosymbiont community. Sci. Rep. 5 (1), 1–9. doi: 10.1038/srep07652
Bongaerts P., Frade P. R., Ogier J. J., Hay K. B., Van Bleijswijk J., Englebert N., et al. (2013). Sharing the slope: Depth partitioning of agariciid corals and associated Symbiodinium across shallow and mesophotic habitats (2-60 m) on a Caribbean reef. BMC Evolutionary Biol. 13 (1), 1–15. doi: 10.1186/1471-2148-13-205
Bongaerts P., Sampayo E. M., Bridge T. C., Ridgway T., Vermeulen F., Englebert N., et al. (2011). Symbiodinium diversity in mesophotic coral communities on the great barrier reef: A first assessment. Mar. Ecol. Prog. Ser. 439, 117–126. doi: 10.3354/meps09315
Bouwmeester J., Baird A. H., Chen C. J., Guest J. R., Vicentuan K. C., Berumen M. L. (2015). Multi-species spawning synchrony within scleractinian coral assemblages in the red Sea. Coral Reefs 34, 65–77. doi: 10.1007/s00338-014-1214-6
Bouwmeester J., Gatins R., Giles E. C., Sinclair-Taylor T. H., Berumen M. L. (2016). Spawning of coral reef invertebrates and a second spawning season for scleractinian corals in the central red Sea. Invertebrate Biol. 135 (3), 273–284. doi: 10.1111/ivb.12129
Byler K. A., Carmi-Veal M., Fine M., Goulet T. L. (2013). Multiple symbiont acquisition strategies as an adaptive mechanism in the coral Stylophora pistillata. PloS One 8 (3), e59596. doi: 10.1371/journal.pone.0059596
Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., et al. (2009). BLAST+: Architecture and applications. BMC Bioinf. 10 (1), 1–9. doi: 10.1186/1471-2105-10-421
Chaidez V., Dreano D., Agusti S., Duarte C. M., Hoteit I. (2017). Decadal trends in red Sea maximum surface temperature. Sci. Rep. 7 (1), 1–8. doi: 10.1038/s41598-017-08146-z
Chimienti G., Terraneo T. I., Vicario S., Marchese F., Purkis S. J., Abdulla Eweida A., et al. (2022). a new species of Bathypathes (Cnidaria, anthozoa, antipatharia, schizopathidae) from the red Sea and its phylogenetic position. ZooKeys 1116, 1–22. doi: 10.3897/zookeys.1116.79846
Claereboudt M. R. (2006). Reef corals and coral reefs of the gulf of Oman (Historical Association of Oman, Al-Roya Publishing).
Cooper T. F., Ulstrup K. E., Dandan S. S., Heyward A. J., Kühl M., Muirhead A., et al. (2011). Niche specialization of reef-building corals in the mesophotic zone: metabolic trade-offs between divergent Symbiodinium types. Proc. R. Soc. B: Biol. Sci. 278 (1713), 1840–1850. doi: 10.1098/rspb.2010.2321
D’Angelo C., Hume B. C., Burt J., Smith E. G., Achterberg E. P., Wiedenmann J. (2015). Local adaptation constrains the distribution potential of heat-tolerant Symbiodinium from the Persian/Arabian gulf. ISME J. 9 (12), 2551–2560. doi: 10.1038/ismej.2015.80
Davies S. W., Ries J. B., Marchetti A., Castillo K. D. (2018). Symbiodinium functional diversity in the coral Siderastrerrucosrea is influenced by thermal stress and reef environment, but not ocean acidification. Front. Mar. Sci. 5, 150. doi: 10.3389/fmars.2018.00150
Eckert R. J., Reaume A. M., Sturm A. B., Studivan M. S., Voss J. D. (2020). Depth influences symbiodiniaceae associations among Montastraea cavernosa corals on the Belize barrier reef. Front. Microbiol. 11, 518. doi: 10.3389/fmicb.2020.00518
Einbinder S., Gruber D. F., Salomon E., Liran O., Keren N., Tchernov D. (2016). Novel adaptive photosynthetic characteristics of mesophotic symbiotic microalgae within the reef-building coral, Stylophora pistillata. Front. Mar. Sci. 3, 195. doi: 10.3389/fmars.2016.00195
Ellis J., Solander D. C. (1786). The natural history of many curious and uncommon zoophytes: collected from various parts of the globe.
Eren A. M., Morrison H. G., Lescault P. J., Reveillaud J., Vineis J. H., Sogin M. L. (2015). Minimum entropy decomposition: Unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J. 9 (4), 968–979. doi: 10.1038/ismej.2014.195
Esper E. J. C. (1805). Die pflanzenthiere in abbildungen nach der natur mit farben erleuchtet nebst beschreibungen Vol. 3 (Nuremberg: Raspischen Buchhandlung).
Eyal G., Tamir R., Kramer N., Eyal-Shaham L., Loya Y. (2019). “The red Sea: Israel,” in Mesophotic coral ecosystems. coral reefs of the world, vol. vol 12 . Eds. Loya Y., Puglise K., Bridge T. (Cham: Springer). doi: 10.1007/978-3-319-92735-0_11
Ezzat L., Fine M., Maguer J. F., Grover R., Ferrier-Pages C. (2017). Carbon and nitrogen acquisition in shallow and deep holobionts of the scleractinian coral S. pistillata. Front. Mar. Sci. 4, 102. doi: 10.3389/fmars.2017.00102
Fine M., Gildor H., Genin A. (2013). A coral reef refuge in the red Sea. Global Change Biol. 19, 3640–3647. doi: 10.1111/gcb.12356
Forskål P. (1775). Post Mortem Auctoris editit Carsten Niebuhr. Adjuncta est materia Med. Kahirina. Mölleri Hafniae 19 xxxiv, 164.
Frade P. R., De Jongh F., Vermeulen F., Van Bleijswijk J., Bak R. P. M. (2008a). Variation in symbiont distribution between closely related coral species over large depth ranges. Mol. Ecol. 17 (2), 691–703. doi: 10.1111/j.1365-294X.2007.03612.x
Frade P. R., Englebert N., Faria J., Visser P. M., Bak R. P. M. (2008b). Distribution and photobiology of Symbiodinium types in different light environments for three colour morphs of the coral Madracis pharensis: is there more to it than total irradiance? Coral Reefs 27 (4), 913–925. doi: 10.1007/s00338-008-0406-3
Fricke H. W., Schuhmacher H. (1983). The depth limits of red Sea stony corals: an ecophysiological problem (a deep diving survey by submersible). Mar. Ecol. 4 (2), 163–194. doi: 10.1111/j.1439-0485.1983.tb00294.x
González-Pech R. A., Bhattacharya D., Ragan M. A., Chan C. X. (2019). Genome evolution of coral reef symbionts as intracellular residents. Trends Ecol. Evol. 34 (9), 799–806. doi: 10.1016/j.tree.2019.04.010
Goulet T. L., Lucas M. Q., Schizas N. V. (2019). “Symbiodiniaceae genetic diversity and symbioses with hosts from shallow to mesophotic coral ecosystems,” in Mesophotic coral ecosystems (Cham: Springer), 537–551.
Grimsditch G. D., Salm R. V. (2006). Coral reef resilience and resistance to bleaching (Gland, Switzerland), 52 p.
Hinderstein L. M., Marr J. C. A., Martinez F. A., Dowgiallo M. J., Puglise K. A., Pyle R. L., et al. (2010). Theme section on “Mesophotic coral ecosystems: Characterization, ecology, and management”. Coral Reefs 29 (2), 247–251. doi: 10.1007/s00338-010-0614-5
Howells E. J., Bauman A. G., Vaughan G. O., Hume B. C., Voolstra C. R., Burt J. A. (2020). Corals in the hottest reefs in the world exhibit symbiont fidelity not flexibility. Mol. Ecol. 29 (5), 899–911. doi: 10.1111/mec.15372
Hughes T. P., Kerry J. T., Álvarez-Noriega M., Álvarez-Romero J. G., Anderson K. D., Baird A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543 (7645), 373–377. doi: 10.1038/nature21707
Hume B. C., D'Angelo C., Smith E. G., Stevens J. R., Burt J., Wiedenmann J. (2015). Symbiodinium thermophilum sp. nov., a thermotolerant symbiotic alga prevalent in corals of the wo’ld's hottest sea, the Persian/Arabian gulf. Sci. Rep. 5 (1), 1–8. doi: 10.1038/srep08562
Hume B. C., D’Angelo C., Burt J., Baker A. C., Riegl B., Wiedenmann J. (2013). Corals from the Persian/Arabian gulf as models for thermotolerant reef-builders: prevalence of clade C3 Symbiodinium, host fluorescence and ex situ temperature tolerance. Mar. Pollut. Bull. 72 (2), 313–322. doi: 10.1016/j.marpolbul.2012.11.032
Hume B. C., Mejia-Restrepo A., Voolstra C. R., Berumen M. L. (2020). Fine-scale delineation of symbiodiniaceae genotypes on a previously bleached central red Sea reef system demonstrates a prevalence of coral host-specific associations. Coral Reefs 39 (3), 583–601. doi: 10.1007/s00338-020-01917-7
Hume B. C., Smith E. G., Ziegler M., Warrington H. J., Burt J. A., LaJeunesse T. C., et al. (2019). SymPortal: A novel analytical framework and platform for coral algal symbiont next-generation sequencing ITS2 profiling. Mol. Ecol. Resour. 19 (4), 1063–1080. doi: 10.1111/1755-0998.13004
Kahng S. E., Copus J. M., Wagner D. (2014). Recent advances in the ecology of mesophotic coral ecosystems (MCEs). Curr. Opin. Environ. sustainability 7, 72–81. doi: 10.1016/j.cosust.2013.11.019
Kheireddine M., Mayot N., Ouhssain M., Jones B. H. (2021). Regionalization of the red Sea based on phytoplankton phenology: A satellite analysis. J. Geophysical Research: Oceans 126 (10), e2021JC017486. doi: 10.1029/2021JC017486
Kheireddine M., Ouhssain M., Organelli E., Bricaud A., Jones B. H. (2018). Light absorption by suspended particles in the red Sea: Effect of phytoplankton community size structure and pigment composition. J. Geophysical Research: Oceans 123 (2), 902–921. doi: 10.1002/2017JC013279
Kitahara M. V., Fukami H., Benzoni F., Huang D. (2016). “The new systematics of scleractinia: Integrating molecular and morphological evidence,” in The cnidaria, past, present and future. Eds. Goffredo S., Dubinsky Z. (Netherlands: Springer), 41–59. doi: 10.1007/978-3-319-31305-4_4
LaJeunesse T. C. (2002). Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar. Biol. 141 (2), 387–400. doi: 10.1007/s00227-002-0829-2
LaJeunesse T. C. (2005). “Species” radiations of symbiotic dinoflagellates in the Atlantic and indo-pacific since the Miocene-pliocene transition. Mol. Biol. Evolution. 22 (3), 570–581. doi: 10.1093/molbev/msi042
LaJeunesse T. C., Smith R. T., Finney J., Oxenford H. (2009). Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral ‘bleaching’event. Proc. R. Soc. B: Biol. Sci. 276 (1676), 4139–4148. doi: 10.1098/rspb.2009.1405
LaJeunesse T. C., Parkinson J. E., Gabrielson P. W., Jeong H. J., Reimer J. D., Voolstra C. R., et al. (2018). Systematic revision of symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 28 (16), 2570–2580.
Lesser M. P., Slattery M., Leichter J. J. (2009). Ecology of mesophotic coral reefs. J. Exp. Mar. Biol. Ecol. 375 (1-2), 1–8. doi: 10.1016/j.jembe.2009.05.009
Loya Y., Eyal G., Treibitz T., Lesser M. P., Appeldoorn R. (2016). Theme section on mesophotic coral ecosystems: Advances in knowledge and future perspectives. Coral Reefs 35 (1), 1–9. doi: 10.1007/s00338-016-1410-7
Lucas M. Q., Stat M., Smith M. C., Weil E., Schizas N. V. (2016). Symbiodinium (internal transcribed spacer 2) diversity in the coral host Agaricia lamarcki (Cnidaria: Scleractinia) between shallow and mesophotic reefs in the northern Caribbean (20–70 m). Mar. Ecol. 37 (5), 1079–1087. doi: 10.1111/maec.12367
Nir O., Gruber D. F., Einbinder S., Kark S., Tchernov D. (2011). Changes in scleractinian coral Seriatopora hystrix morphology and its endocellular Symbiodinium characteristics along a bathymetric gradient from shallow to mesophotic reef. Coral Reefs 30 (4), 1089–1100. doi: 10.1007/s00338-011-0801-z
Nir O., Gruber D. F., Shemesh E., Glasser E., Tchernov D. (2014). Seasonal mesophotic coral bleaching of Stylophora pistillata in the northern red Sea. PloS One 9 (1), e84968. doi: 10.1371/journal.pone.0084968
Osman E. O., Smith D. J., Ziegler M., Kürten B., Conrad C., El‐Haddad K. M., et al. (2018). Thermal refugia against coral bleaching throughout the northern Red Sea. Glob. Change Biol. 24 (2), e474–e484. doi: 10.1111/gcb.13895
Osman E. O., Suggett D. J., Voolstra C. R., Pettay D. T., Clark D. R., Pogoreutz C., et al. (2020). Coral microbiome composition along the northern red Sea suggests high plasticity of bacterial and specificity of endosymbiotic dinoflagellate communities. Microbiome 8 (1), 1–16. doi: 10.1186/s40168-019-0776-5
Padilla-Gamiño J. L., Roth M. S., Rodrigues L. J., Bradley C. J., Bidigare R. R., Gates R. D., et al. (2019). Ecophysiology of mesophotic reef-building corals in HawaI ‘i is influenced by symbiont–host associations, photoacclimatization, trophic plasticity, and adaptation. Limnology Oceanography 64 (5), 1980–1995. doi: 10.1002/lno.11164
Papagiannopoulos N., Raitsos D. E., Krokos G., Gittings J. A., Brewin R. J., Papadopoulos V. I., et al. (2021). Phytoplankton biomass and the hydrodynamic regime in NEOM, red Sea. Remote Sens. 13 (11), 2082. doi: 10.3390/rs13112082
Pichon M., Benzoni F., Chaineau C. H., Dutrieux E. (2010). Field guide to the hard corals of the southern coast of Yemen (Paris: BIOTOPE Parthenope), 256.
Pochon X., Forsman Z. H., Spalding H. L., Padilla-Gamiño J. L., Smith C. M., Gates R. D. (2015). Depth specialization in mesophotic corals (Leptoseris spp.) and associated algal symbionts i’ hawai'i. R. Soc. Open Sci. 2 (2), 140351. doi: 10.1098/rsos.140351
Purkis S. J., Harris P. M., Ellis J. (2012). Patterns of sedimentation in the contemporary red Sea as an analog for ancient carbonates in rift settings. J. Sedimentary Res. 82 (11), 859–870. doi: 10.2110/jsr.2012.77
Pyle R. L., Copus J. M. (2019). “Mesophotic coral ecosystems: introduction and overview,” in Mesophotic coral ecosystems (Cham: Springer), 3–27.
Qurban M. A., Krishnakumar P. K., Joydas T. V., Manikandan K. P., Ashraf T. T. M., Quadri I. I., et al. (2014). In-situ observation of deep water corals in the northern red Sea waters of Saudi Arabia. Deep Sea Res. Part I: Oceanographic Res. Papers 89, 35–43. doi: 10.1016/j.dsr.2014.04.002
Raitsos D. E., Pradhan Y., Brewin R. J., Stenchikov G., Hoteit I. (2013). Remote sensing the phytoplankton seasonal succession of the red Sea. PloS One 8 (6), e64909. doi: 10.1371/journal.pone.0064909
Reimer J. D., Herrera M., Gatins R., Roberts M. B., Parkinson J. E., Berumen M. L. (2017). Latitudinal variation in the symbiotic dinoflagellate Symbiodinium of the common reef zoantharian Palythoa tuberculosa on the Saudi Arabian coast of the red Sea. J. Biogeography 44 (3), 661–673. doi: 10.1111/jbi.12795
Riegl B. M., Benzoni F., Samimi-Namin K., Sheppard C. (2012b). “The hermatypic scleractinian (hard) coral fauna of the gulf,” in Coral reefs of the gulf (Dordrecht: Springer), 187–224.
Riegl B. M., Bruckner A. W., Rowlands G. P., Purkis S. J., Renaud P. (2012a). Red Sea coral reef trajectories over 2 decades suggest increasing community homogenization and decline in coral size. PloS One 7 (5), e38396. doi: 10.1371/journal.pone.0038396
Riegl B. M., Purkis S. J. (2012c). “Coral reefs of the gulf: adaptation to climatic extremes in the world’s hottest sea,” in Coral reefs of the gulf (Dordrecht: Springer), 1–4.
Roberts J. M., Wheeler A. J., Freiwald A. (2006). Reefs of the deep: The biology and geology of cold-water coral ecosystems. Science 312 (5773), 543–547. doi: 10.1126/science.1119861
Roder C., Berumen M. L., Bouwmeester J., Papathanassiou E., Al-Suwailem A., Voolstra C. R. (2013). First biological measurements of deep-sea corals from the red Sea. Sci. Rep. 3 (1), 1–10. doi: 10.1038/srep02802
Rowan R., Knowlton N. (1995). Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc. Natl. Acad. Sci. 92 (7), 2850–2853. doi: 10.1073/pnas.92.7.2850
Rowlands G., Purkis S., Bruckner A. (2014). Diversity in the geomorphology of shallow-water carbonate depositional systems in the Saudi Arabian red Sea. Geomorphology 222, 3–13. doi: 10.1016/j.geomorph.2014.03.014
Sampayo E. M., Dove S., LaJeunesse T. C. (2009). Cohesive molecular genetic data delineate species diversity in the dinoflagellate genus Symbiodinium. Mol. Ecol. 18 (3), 500–519. doi: 10.1111/j.1365-294X.2008.04037.x
Sampayo E. M., Ridgway T., Bongaerts P., Hoegh-Guldberg O. (2008). Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc. Natl. Acad. Sci. 105 (30), 10444–10449. doi: 10.1073/pnas.0708049105
Sawall Y., Al-Sofyani A., Banguera-Hinestroza E., Voolstra C. R. (2014). Spatio-temporal analyses of symbiodinium physiology of the coral Poerrucose verrucosa along large-scale nutrient and temperature gradients in the red Sea. PloS One 9 (8), e103179. doi: 10.1371/journal.pone.0103179
Schlesinger T., Grinblat M., Rapuano H., Amit T., Loya Y. (2018). Can mesophotic reefs replenish shallow reefs? Reduced coral reproductive performance casts a doubt. Ecology 99 (2), 421–437. doi: 10.1002/ecy.2098
Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75 (23), 7537–7541. doi: 10.1128/AEM.01541-09
Sheppard C., Al-Husiani M., Al-Jamali F., Al-Yamani F., Baldwin R., Ishop J., et al. (2010). The gulf: A young sea in decline. Mar. Pollut. Bull. 60 (1), 13–38. doi: 10.1016/j.marpolbul.2009.10.017
Sheppard C., Price A., Roberts C. (1992). Marine ecology of the arabian region: patterns and processes in extreme tropical environments (London, UK: Academic Press), 1992ISBN: ISBN 978-012-639-490-0.
Sheppard C. R., Sheppard A. L. S. (1991). Corals and coral communities of Arabia. (Saudi Arabia: Fauna) 12, 3–170.
Sofianos S. S., Johns W. E. (2007). Observations of the summer red Sea circulation. J. Geophysical Research-Oceans 112 (C6), ArtnC06025. doi: 10.1029/2006jc003886
Terraneo T. I., Fusi M., Hume B. C., Arrigoni R., Voolstra C. R., Benzoni F., et al. (2019). Environmental latitudinal gradients and host-specificity shape symbiodiniaceae distribution in red Sea Porites corals. J. Biogeography 46 (10), 2323–2335. doi: 10.1111/jbi.13672
Tonk L., Sampayo E. M., Weeks S., Magno-Canto M., Hoegh-Guldberg O. (2013). Host-specific interactions with environmental factors shape the distribution of Symbiodinium across the great barrier reef. PloS One 8 (7), e68533. doi: 10.1371/journal.pone.0068533
Turner J. A., Babcock R. C., Hovey R., Kendrick G. A. (2017). Deep thinking: A systematic review of mesophotic coral ecosystems. ICES J. Mar. Sci. 74 (9), 2309–2320. doi: 10.1093/icesjms/fsx085
Wafar M., Ashraf M., Manikandan K. P., Qurban M. A., Kattan Y. (2016). Propagation of gulf of Aden intermediate water (GAIW) in the red Sea during autumn and its importance to biological production. J. Mar. Syst. 154, 243–251. doi: 10.1016/j.jmarsys.2015.10.016
Wagner D., Pochon X., Irwin L., Toonen R. J., Gates R. D. (2011). Azooxanthellate? most Hawaiian black corals contain Symbiodinium. Proc. R. Soc. B: Biol. Sci. 278 (1710), 1323–1328. doi: 10.1098/rspb.2010.1681
Wells J. W. (1961). ). notes on indo-pacific scleractinian corals. part 3, a new reef coral from new Caledonia. Pacific Sci. 15, 189–191.
Wickham H., Chang W., Wickham M. H. (2016). Package ‘ggplot2’. create elegant data visualisations using the grammar of graphics. version, Vol. 2. New York: Springer-Verlag. 1–189.
Winters G., Beer S., Zvi B. B., Brickner I., Loya Y. (2009). Spatial and temporal photoacclimation of Stylophora pistillata: Zooxanthella size, pigmentation, location and clade. Mar. Ecol. Prog. Ser. 384, 107–119. doi: 10.3354/meps08036
Keywords: symbiotic algae, MCES, next-generation sequencing - NGS, ITS2, SymPortal
Citation: Terraneo TI, Ouhssain M, Castano CB, Aranda M, Hume BCC, Marchese F, Vimercati S, Chimienti G, Eweida AA, Voolstra CR, Jones BH, Purkis SJ, Rodrigue M and Benzoni F (2023) From the shallow to the mesophotic: a characterization of Symbiodiniaceae diversity in the Red Sea NEOM region. Front. Mar. Sci. 10:1077805. doi: 10.3389/fmars.2023.1077805
Received: 23 October 2022; Accepted: 20 March 2023;
Published: 14 April 2023.
Edited by:
Aldo Cróquer, The Nature Conservancy, Dominican RepublicReviewed by:
Nikolaos V. Schizas, University of Puerto Rico at Mayagüez, Puerto RicoCopyright © 2023 Terraneo, Ouhssain, Castano, Aranda, Hume, Marchese, Vimercati, Chimienti, Eweida, Voolstra, Jones, Purkis, Rodrigue and Benzoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tullia I. Terraneo, dHVsbGlhaXNvdHRhLnRlcnJhbmVvQGthdXN0LmVkdS5zYQ==
†ORCID: Burton H. Jones, orcid.org/0000-0002-9599-1593
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.