
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 29 May 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1063210
This article is part of the Research TopicHilsa Shad: Progress in Science and ManagementView all 4 articles
 Simanku Borah1
Simanku Borah1 Amiya Kumar Sahoo2
Amiya Kumar Sahoo2 Vaisakh Gopinathapillai3
Vaisakh Gopinathapillai3 Dharmendra Kumar Meena2
Dharmendra Kumar Meena2 Ashok Kumar Jaiswar4
Ashok Kumar Jaiswar4 Geetanjali Deshmukhe4
Geetanjali Deshmukhe4 Anil Kumar Yadav1
Anil Kumar Yadav1 Pranab Gogoi5
Pranab Gogoi5 Debasmita Mohanty2
Debasmita Mohanty2 Karankumar Ramteke4
Karankumar Ramteke4 Birendra Kumar Bhattacharjya1
Birendra Kumar Bhattacharjya1 Basanta Kumar Das2*
Basanta Kumar Das2*The Hilsa, Tenualosa ilisha, commands a very high value as food fish. The present study was carried out to understand the breeding phenology of T. ilisha in relation to climatic variables. Monthly fish samples were collected from two landing centres, namely, Uzanbazar (Guwahati) and Shri Ramghat, Dhubri, of River Brahmaputra during May 2018 to April 2019. The assessment of gonadosomatic index (GSI) of T. ilisha revealed higher GSI values during October to February, and showed temporal variations with respect to sex. In males, highest GSI value was observed in the month January followed by February, whereas in females, GSI value was found to be highest in November followed by October. GSI (pooled) value was negatively correlated with air temperature, indicating vulnerability of the species to climate change. The highest percentage of mature males was observed during October–February, and mature females during October–December. The length at first maturity was recorded to be 290 mm for female(s) and 259 mm for male(s). The absolute fecundity ranged from 103,164 to 583,456 ova for fishes in the size range of 229–403 mm, with an average of 250,532 ova per female. Relative fecundity was found to range from 306 to 1096 ova per gram body weight, with an average of 791 ova per gram body weight. The diameter of ova of the studied fishes ranged from 414.6 to 738.2 µm, with a mean value of 546.73 ± 7.18 µm. The percentage frequency distribution of mature ova shows a distinct single peak or mode. Sex ratio (male: female) was found to be 1:0.87, indicating dominance of males over females. The chi-square test on observed sex ratio against the hypothetical ratio of 1:1 did not reveal a significant difference (p > 0.05). The findings of the present study can provide impetus toward successful management of this highly prized, transboundary, and migratory resource of River Brahmaputra, in the context of changing climate.
Brahmaputra and Barak, with 53 tributaries, are two major river systems of Assam, north-east India. Originating at 5,300 msl, in the glacial mass of Kailash Range in the mighty Himalayas, Brahmaputra flows by the name of Yarlung Tsangpo for a distance of 1,625 km in Tibet and enters India at Gelling near Tuting in Upper Siang district of Arunachal Pradesh. Brahmaputra flows for a distance of 278 km through the state Arunachal Pradesh, with name Siang or Dihang. The river then enters Assam where it unites with Dibang and Lohit, two large Himalayan rivers, and further flows by the name of Brahmaputra through a distance of 640 km from Sadiya to Dhubri. After travelling for a distance of 918 km in India, Brahmaputra flows 337 km in Bangladesh, where it is popular as Jamuna (Bhattacharjya et al., 2017). The diversity of fish fauna in the river stretch along Assam comprises a blend of torrential, warm-water, and cold-water species (Sinha, 1994; Sen, 2000; Vishwanath, 2002). Bhattacharjya et al. (2003) reported 217 species, under 36 families, from the state of Assam. Motwani et al. (1962) reported 126 fish species belonging to 26 families, from Brahmaputra in Assam, which was revised to 141 species belonging to 84 genera and 29 families by Bhattacharjya et al. (2017). Major carps, minor carps, catfishes, featherbacks, and Hilsa contribute to commercial fishery in the Indian stretch of River Brahmaputra (Bhattacharjya et al., 2017).
T. ilisha (Hamilton, 1822) commonly known as Hilsa migrates from sea to rivers for breeding and has a wide range of distribution in foreshore zones, estuarine zones, brackish water systems, and freshwater lotic ecosystems of the western part of the Indo-Pacific region. In marine waters, the distribution of this species ranges from Persian Gulf to the west and east coasts of India in the Arabian Sea and Bay of Bengal, respectively. The species has also been reported by researchers from the coastal belt of Sri Lanka and from Laos (Pillay and Rosa, 1963). Hilsa fishery both in inland and offshore regions was confined to the artisanal sector, particularly traditional non-mechanized and small mechanized boats (Raja, 1985), and is at present mainly exploited by gill nets in inland waters. The species spends a major part of its life in inshore areas and estuaries of rivers and migrates upstream into freshwater bodies, particularly rivers for breeding. Post breeding, the spent fish and their young ones return to the inshore areas (Reuben et al., 1992). The highest catch of Hilsa from across the globe is reported from the Ganga–Brahmaputra–Meghna deltaic region of India and Bangladesh and commands an extremely higher value as compared with other fish species reported from the region. Global catch of Hilsa stands at around 0.72 million tonnes year-1, with Bangladesh contributing more than half (50%–60%), followed by Myanmar (20%–25%) and India (15%–20%). The remaining 5%–10% comes from nations like Iraq, Kuwait, Pakistan, Malaysia, and Thailand (Milton, 2010; Hossain et al., 2019). The species has a great cultural and economic importance in India and Bangladesh. It has a very high consumer preference and is highly prized with an average price of around US$ 12 per kg in the region (Sahoo et al., 2016). The importance of this species can be derived from the fact that the economic value of Hilsa fishery is worth over US$ 2 billion and generates employment opportunities and acts as a livelihood source for millions of people in India, Bangladesh, and Myanmar (BOBLME, 2012). It is designated as national fish of Bangladesh, and Hilsa of Bangladesh is also given the tag of a geographical indicator.
In India, Hilsa is reported from varied riverine and estuarine ecosystems ranging from the snow-fed Himalayan rivers like Ganga, Bhagirathi, Hooghly, Rupnarayan, and Brahmaputra and their estuaries to rain-fed peninsular rivers like Godavari and their estuaries, all of which drains into Bay of Bengal. The species is also reported from rain-fed peninsular rivers like Narmada and Tapti and their estuaries (Bhaumik and Sharma, 2012) draining into the Arabian Sea. The rivers Hooghly, Brahmaputra, and Ganga and their tributaries contribute around 70% of total Hilsa catch in India (Raja, 1985; Borah et al., 2019a; Borah et al., 2019b). Although widely distributed along the Indian coast, it forms a commercial fishery in the north-east coast of India comprising the states of West Bengal and Orissa. The species is known for its delicious taste and hence fetches a high price in markets across India (Reuben et al., 1992). In addition to its cultural and economic importance, the species also holds an important position in terms of nutritional value (Mohanty et al., 2012) and is popular for its unique taste (Nath and Banerjee, 2012).
ICAR-CIFRI, Barrackpore, has been collecting data on fish catch and catch composition across different landing centres of River Brahmaputra in Assam, especially from Uzanbazar landing centre in Guwahati since 1973. Analysis of data till 2006–2007 indicated that miscellaneous species have started contributing a major chunk of the total fish catch (40%–50%) with dominance of Aspidoparia morar, a minor carp, across all major landing centres of River Brahmaputra in Assam (Borah et al., 2014). At the same time, catch of highly prized species like major carps and Hilsa has declined considerably, with a marked decline (81%) in Hilsa catch (Vaas and Moza, 2011), which formed a major commercial fishery along the lower stretch of the river from Dhubri to Guwahati. Yadav et al. (2022a) documented the highly variable catch trend of prized Hilsa from Uzanbazar (Guwahati) landing centre of River Brahmaputra in Assam. Average landing of the species during the period 1987–1999 was 7,022.1 kg year-1, which increased by 40% during 2000–2009 (9,825.7 kg year-1). The last decade from 2010 to 2019 showed a drastic decline in average catch of the species to 3,424.9 kg year-1, a cause of serious concern and which needs immediate attention. In this context, studies on biology of the species can help researchers and policymakers in formulating measures for sustainable species management.
Detailed research has been carried out on the biology and fishery of this species from Bangladesh waters (Miah, 2015; Pramanik et al., 2017; Hossain et al., 2019; Das et al., 2022). These studies along with other past reports have formed the base for formulating management regulations toward reviving Hilsa fishery in the country. A few selected studies on food and feeding habits (Borah et al., 2022a), population characteristics (Borah et al., 2022b), catch trend (Yadav et al., 2022a), and stock structure (Vaisakh et al., 2020) of this species have been carried out from Brahmaputra system, India. However, detailed studies on the biology of this species from the Indian part of River Brahmaputra in Assam, which is one of the major migratory route and breeding ground, are lacking. The present study has the potential to act as a knowledge base toward formulating necessary conservatory measures to revive this declining fishery from River Brahmaputra in India and also serve as additional information for policymakers of neighbouring countries to reframe legislations. Hilsa of River Brahmaputra has its origin in Bay of Bengal (Miah, 2015), and being a migratory species, any management measure implemented in the Indian part of River Brahmaputra will have its implications in Hilsa fishery of Bangladesh. Hence, the present attempt to document the reproductive biology of the species from Brahmaputra River in India holds significance from a wider policy perspective in the Bay of Bengal region.
During the present study, a total of 275 individuals of T. ilisha (male = 147 no., length: 146–352 mm and weight: 26.75–406 g; and female = 128 no., length: 162–403 mm and weight: 37.93–762 g) were collected from Uzanbazar, Guwahati (26°11′44.33″ N and 91°45′23.94″ E), and Shri Ramghat, Dhubri (26°0′36.50″ N and 89°58′41.61″ E), landing centres of Brahmaputra River (Figure 1). Sampling was carried out at monthly intervals for 1 year from May 2018 to April 2019.
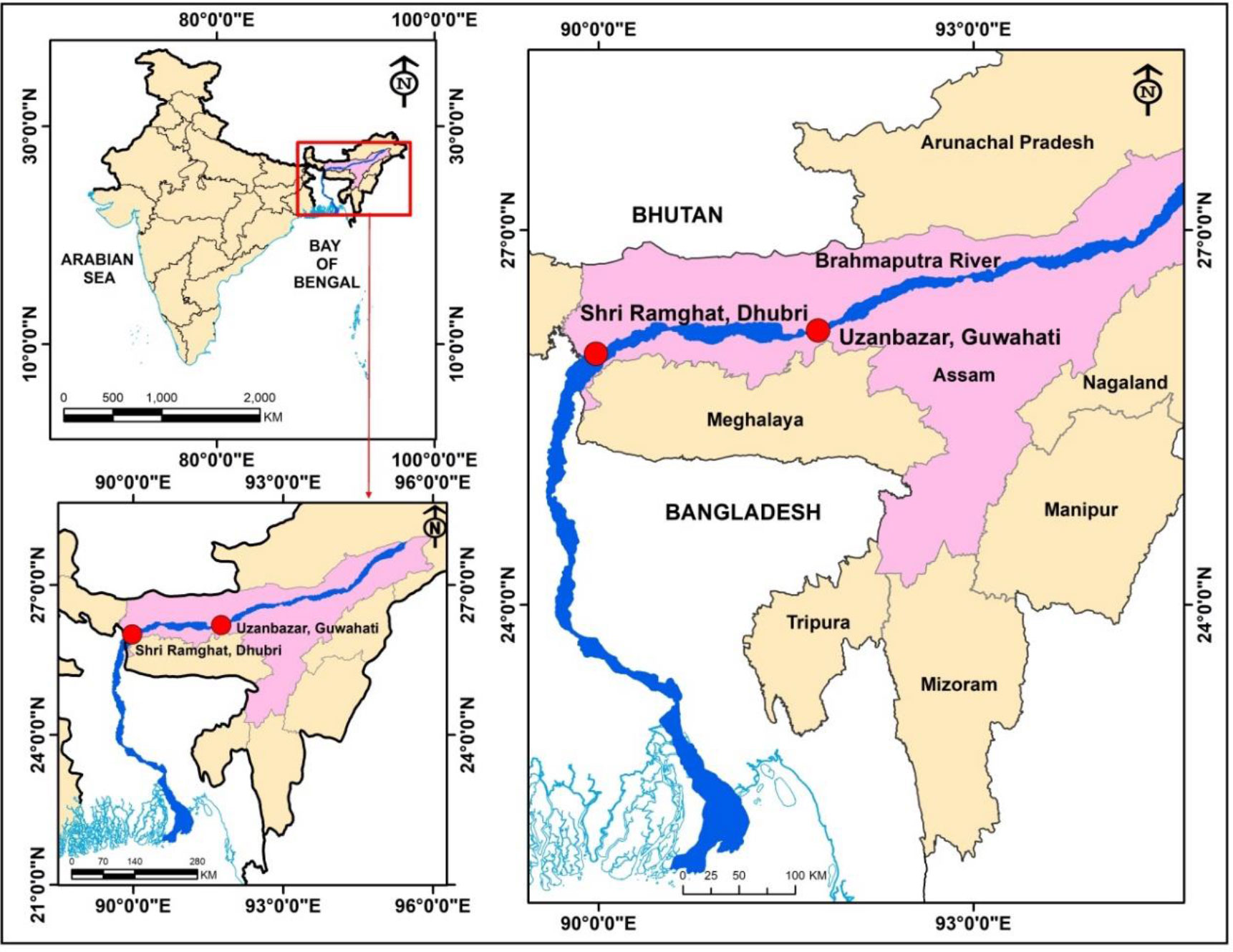
Figure 1 Sampling stations in River Brahmaputra, Assam (ArcGIS Desktop: Release 9.3. (ESRI, 2008).
The total length and weight of the collected specimens were measured by using a measuring scale and an electronic precision balance (0.01 g accuracy), respectively. The total weight of gonad of individual fishes was also measured after dissecting out the gonad, and the gonadosomatic index (GSI) was calculated by applying the following formula, described by Vladykov (1956) and Hopkins (1979). GSI was calculated for males (n = 147) and females (n = 128) separately and in combination.
Simple linear regression was carried out to examine the relationship between GSI values of both the sexes separately and in combination, in relation to climate variable, mean monthly air temperature (°C), using SPSS version 16.0 (SPSS Inc., 2008). Simple correlation analysis was carried out to examine the degree of relationship between GSI values of both the sexes separately and in combination with climatic variable. The mean monthly air temperature (°C) data were obtained from Regional Meteorological Centre, Indian Meteorological Department, Guwahati.
The maturity scale for Hilsa in the present study was adopted from De (2014). The number of individuals belonging to stage V (mature) and stage VI (ripe) was used in calculating the length group-wise and month-wise percentages of mature individuals. Length at first maturity (Lm) was determined by plotting the cumulative percentage of mature individuals (stages V and VI) against length groups (Rajesh et al., 2015). Histological study was carried out through double staining method using the stains haematoxylin and eosin. Gonads were fixed in 10% buffered formalin for a period of around 24 h. The specimens were dehydrated in a series of graded ethanol (30%, 50%, 70%, 90%, 95% and absolute alcohol). The alcohol present in the tissues was then removed with the help of an organic solvent, xylene. The tissues were then embedded in paraffin blocks with the help of suitable containers. Paraffin used for embedding the tissues was kept in molten condition overnight. Five-micron sections were cut using a microtome (Leica, Tokyo, Japan) and deparaffinised with xylol. The sections were rehydrated in 90%, 70%, and 50% ethanol followed by a 10-min wash in water and stained with haematoxylin and eosin (Karmakar et al., 2016). Ova at different stages of maturation were identified following Walsh et al. (1990) and Carnevali et al. (2019).
After dissection, the ovaries were removed out of the body cavity, cleaned, dried in blotting paper, and weighed, and then random subsamples of matured ova from the anterior, mid, and posterior portions of ovaries were taken. Prior to counting the number of eggs present in each subsample, the subsamples were weighed with the help of an electronic precision balance (0.01 g accuracy). A total of 30 ovaries were studied for this purpose. Absolute fecundity was estimated following gravimetric method of Biswas (1992).
where n = number of matured ova in the subsample, OW = weight of the ovary (g), and w = weight of the sub-sample (g).
In the present study, relative fecundity in relation to body weight (Toots, 1951) was estimated using the following equation:
Linear regression was performed to study the relationships between absolute fecundity and total length (mm), absolute fecundity and body weight (g), and absolute fecundity and gonad weight (g). All sets of data were log transformed prior to analysis. The relationship between absolute fecundity and each of the independent variables was represented using the following equation:
where F is the absolute fecundity, and x is the total length (mm) or body weight (g) or gonad weight (g), depending on the relationship.
For spawning periodicity, ova diameter studies were carried out from preserved ovaries (preserved in 5% formalin). A method of subsampling by taking mature ova from the anterior, posterior, and mid regions of the ovary in a random manner was followed, and the ova were measured (Biswas, 1992). The ova were observed under a stereo zoom microscope (Nikon; Model: SMZ 745T) (2× objective), and the image was captured using 5.0 MP Digital Camera (model: UCB 500). The diameter of individual ova was measured to the nearest 1 µm using VImage 2016 image processing software. The ova diameter (n = 200) recorded from the digitised samples was grouped into class intervals ranging from 401 to 750 µm with an interval of 50 µm. Spawning periodicity was determined by plotting the number of ova expressed in percentage against the class intervals (Prabhu, 1956). Sexes were identified by dissecting individual specimens. A total of 275 specimens were dissected to identify the sexes during the study period. The sex ratio (male: female) was estimated length group-wise and month-wise. The observed sex ratio was tested using chi-square (χ²) test to determine the deviation from normal sex ratio of 1:1, if any. All statistical analysis was carried out using SPSS 16.0 (SPSS Inc., 2008).
Assessment of GSI of T. ilisha samples shows that GSI values were higher during the months from October to February. High GSI values during these months from October to February indicate that the species breed during this part of the year in Brahmaputra River, Assam. From the month of March onward, a decline in GSI values of males and females as well as in pooled data was noticed during the present study and GSI values were found to be on the lower side during the months from March to September. The peak breeding period was found to vary in the case of both males and females. In males, the highest GSI value which can be correlated with the peak breeding period was in January followed by February, whereas in females, the GSI value was found to be highest in November followed by October. In the case of pooled data, GSI value was recorded maximum during November (Figure 2). GSI (male) and GSI (pooled) were significantly negatively correlated with mean air temperature (Table 1). The relationships between GSI (male) versus mean air temperature (F statistic = 14.54; p = 0.003) and between GSI (pooled) and mean air temperature (F statistic = 12.11; p = 0.006) were significant at 1% level of significance. However, the relationship between GSI (female) and mean air temperature (F statistic = 3.49; p = 0.091) was found to be statistically insignificant (p > 0.05). Statistical significance of the relationship between climate variable and GSI of T. ilisha can be ascertained by t-statistic and p-value (Table 2). It can be concluded that mean air temperature has a significant effect on GSI of T. ilisha.
In case of pooled data, highest percentage of mature individuals (stages V and VI) was observed during October–February. A high percentage of mature males was observed during October–February with 100% mature males observed during January–February. The highest percentage of mature females was observed during October–December with 100% mature females during November–December (Figure 3).
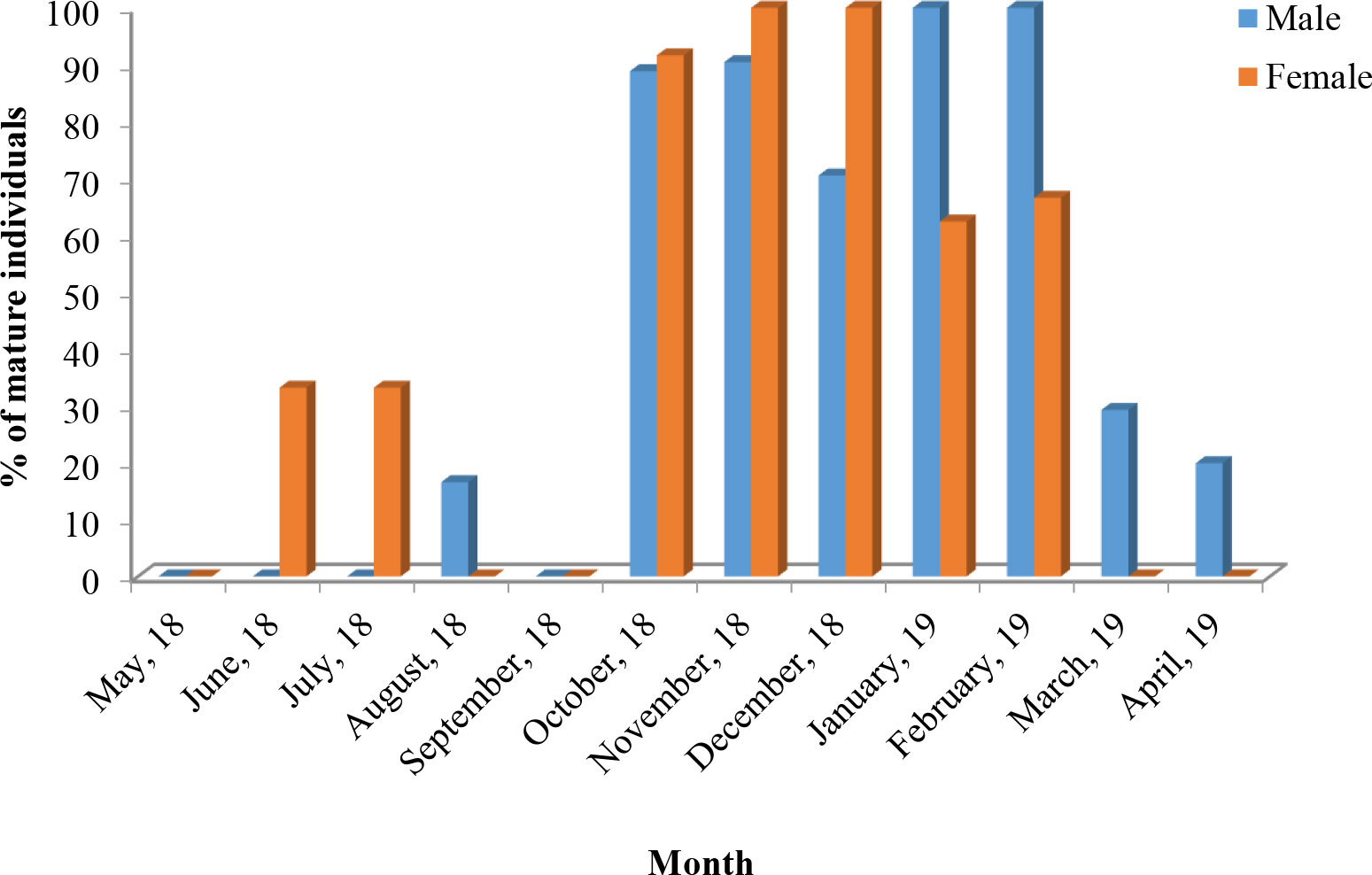
Figure 3 Monthly occurrence of mature individuals (male and female) of T. ilisha (expressed in percentage) from River Brahmaputra, Assam.
Length at first maturity, i.e., the length at which 50% of the total population attains maturity, was found to be 290 mm for female(s) (Figure 4) and 259 mm for male(s) (Figure 5). It indicates that males mature at a smaller size as compared to females. The absolute fecundity was found to range from 103,164 to 583,456 ova for fishes in the size range of 229–403 mm with an average of 250,532 ova per female. Relative fecundity was found to range from 306 to 1096 ova per gram body weight with an average of 791 ova per gram body weight. Analysis showed that a significant linear relationship exists between absolute fecundity and gonad weight (p < 0.01) (Figure 6), between absolute fecundity and fish weight (p < 0.01) (Figure 7), and between absolute fecundity and fish length (p < 0.01) (Figure 8). Based on the value of coefficient of determination (R2), the relationship between absolute fecundity (F) and gonad weight of fish (Gwt) showed a greater degree of fitting (R2 = 0.95), followed by the relationship between absolute fecundity and weight of the fish (W) (R2 = 0.82), followed by the relationship between absolute fecundity and total length of fish (L) (R2 = 0.74). The regression coefficient (slope of the regression equation) was found to be statistically significant as ascertained from the ‘t’ statistic and p value (p < 0.01). Histological sections of mature female gonad (stage VI) is shown in Figure 9.
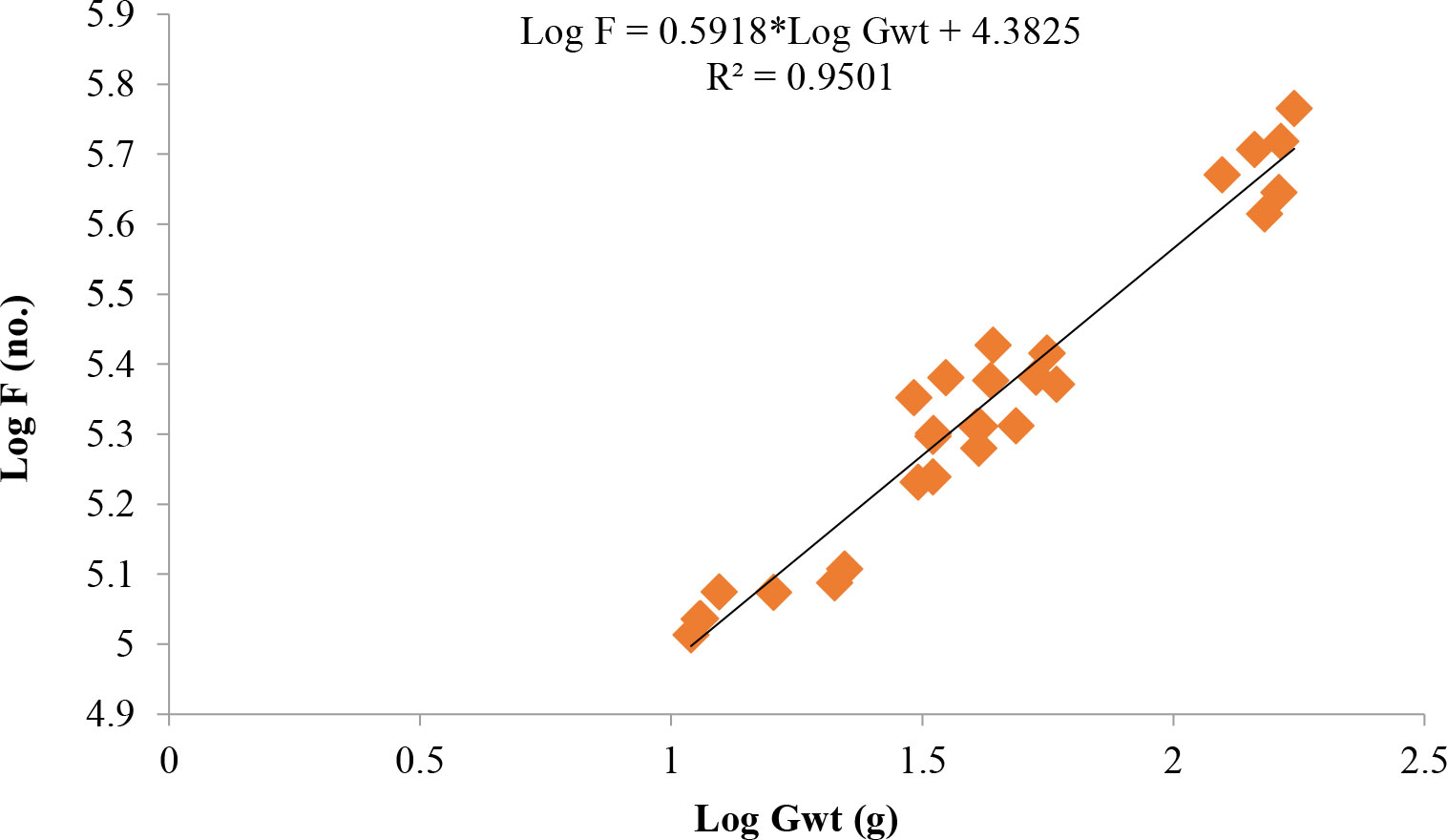
Figure 6 Relationship between absolute fecundity (F) and gonad weight (Gwt) in T. ilisha from River Brahmaputra, Assam.
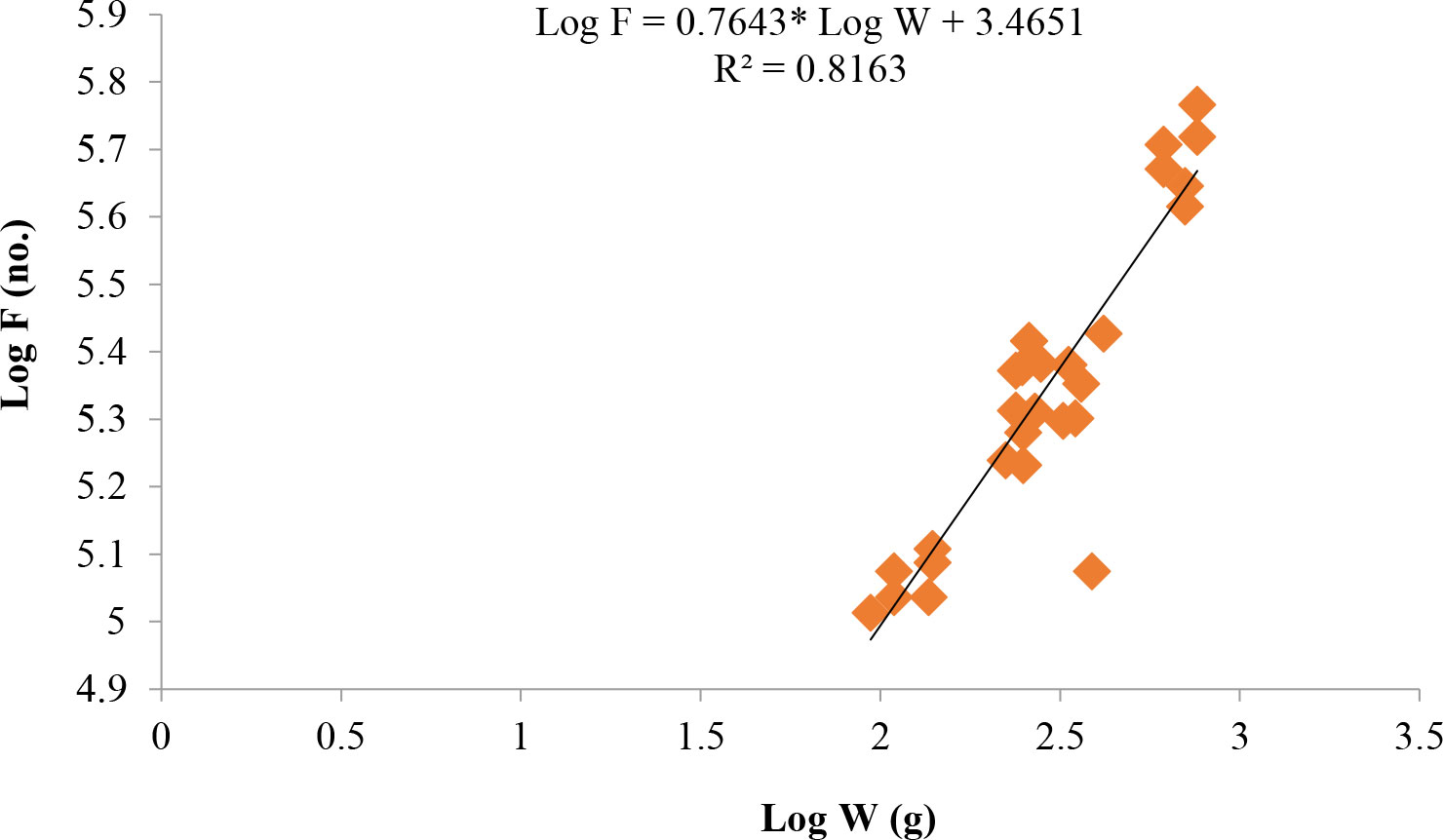
Figure 7 Relationship between absolute fecundity (F) and fish weight (W) in T. ilisha from River Brahmaputra, Assam.
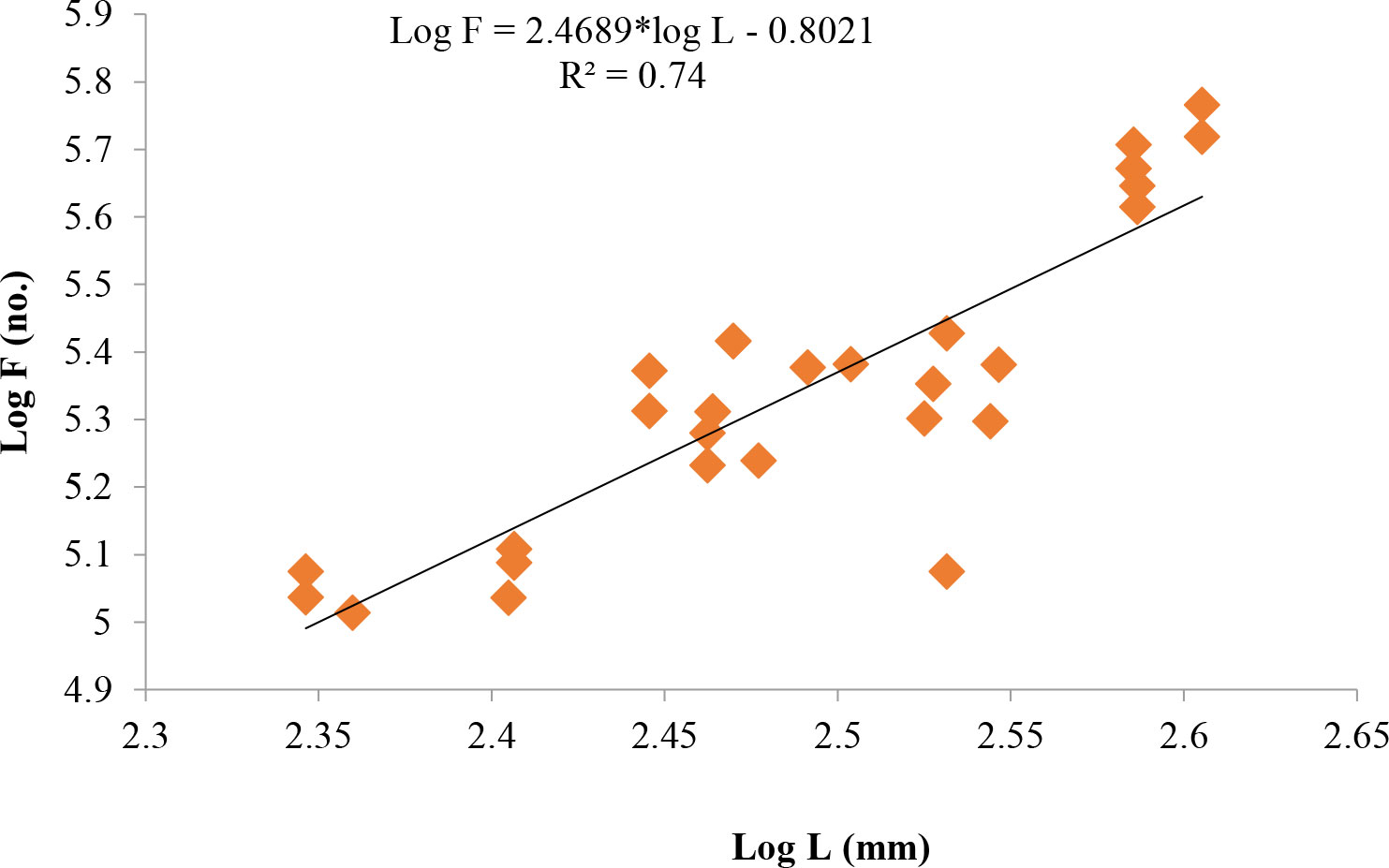
Figure 8 Relationship between absolute fecundity (F) and total length of fish (L) in T. ilisha from River Brahmaputra, Assam.
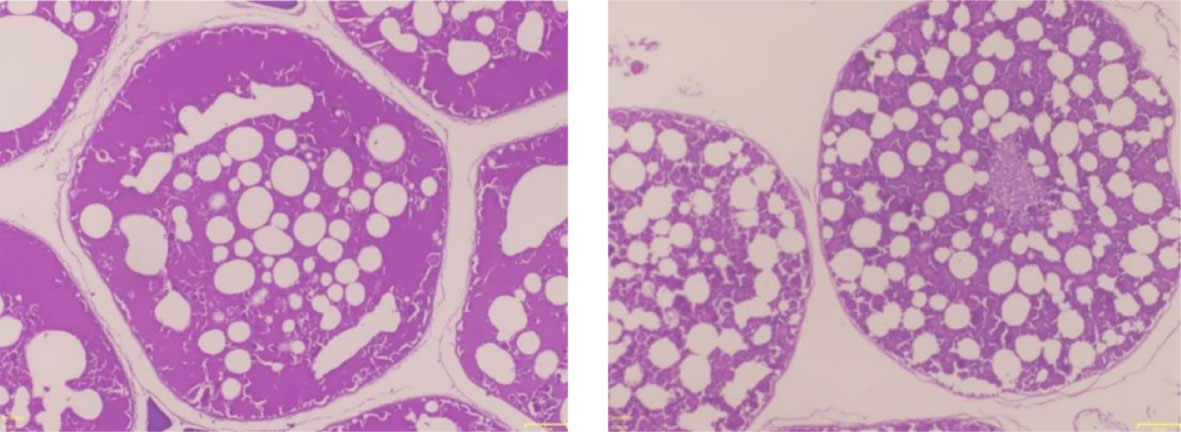
Figure 9 Haematoxylin–eosin-stained histological sections of mature female gonad (stage VI) of T. ilisha from River Brahmaputra, Assam.
Ova obtained from mature females in stages V and VI of maturity were observed under a stereo zoom microscope (Nikon; Model: SMZ 745T) (2× objective), and the image was captured. The objective of the microscope was adjusted such that it focused along the centre of the ovum. The ova diameter ranged from 414.6 to 738.2 µm. The mean ova diameter was found to be 546.73 ± 7.18 µm.
The percentage frequency distribution of mature ova shows that there is a distinct single peak or mode (Figure 10). This clearly indicates that a single batch of ova undergoes maturation at a time. The occurrence of a single mode as evident from the percentage frequency distribution of ova diameter establishes the fact that T. ilisha spawns once in a year in Brahmaputra River system.
The sex ratio (male: female) of T. ilisha collected from River Brahmaputra in Assam was found to be 1:0.87. This showed that males were dominant over females. This observed sex ratio was tested against the hypothetical ratio of 1:1 using chi-square test, and it was found that there was no significant difference (p > 0.05) between the two. However, the chi-square test showed that there was a significant difference (p < 0.05) in the observed sex ratio from the hypothetical sex ratio of 1:1 during the months of January and March (Table 3). Length group-wise analysis showed that males were dominant in smaller length groups (100–250 mm), whereas in the larger length groups (>250 mm) females were dominant.
The gonadosomatic index (GSI) is an indicator of reproductive status of fish (Saud et al., 2015; Jan and Jan, 2017) and spawning season (Arruda et al., 1993). Many researchers have used the GSI value in determining the spawning season in different fish species (Islam et al., 2008; Ghaffari et al., 2011; Kingdom and Allison, 2011). During the present study, GSI values of Hilsa were recorded to be higher during the months from October to February for females and October to April for males. It was also seen that the highest percentage of mature individuals (stages V and VI) was observed during October–February with 100% mature males observed during January–February and 100% mature females during November–December. High GSI values during these months from October to February (females) and from October to April (males) along with the occurrence of high percentage of mature individuals indicate that the species breed during this part of the year in Brahmaputra River, Assam. This is also supported by the low gastro-somatic index (GaSI) values observed in the species during this point of time (Borah et al., 2022a). GaSI value in fishes is found to be low during spawning season due to cessation of feeding and high during post-spawning period (Sattar and Adam, 2005). Das et al. (2022) studied the reproductive behaviour of T. ilisha from Meghna and Tentulia rivers of Bangladesh and found that the species has a prolonged breeding season with peak in October–November, which support our findings. During our study, we observed a temporal variation in peak GSI values across sexes. Highest GSI values in females were observed in November followed by October, whereas in males it was highest in January followed by February. Iossa (2019) stated that male fertility is comparatively less resilient to temperature-related stress than females and susceptibility to rising temperature may induce swift evolution and rapid local adaptation in biological organisms (Grossen et al., 2011). This is corroborated by our finding on the male GSI of T. ilisha being significantly negatively correlated with air temperature, whereas that of females had a non-significant relationship. Thus, rising temperature is one of the factors that might have played a role in the temporal variation in GSI across sexes in the species. Potential consequences of such temporal variation in GSI across sexes include limited spawning success, limited recruitment, and biased sex ratios, which will affect the fishery of the species.
De (1980) and De (1986) observed that T. ilisha has an extended spawning season from September to March, which is in line with our present study. However, Rao and Pathak (1972) observed that Hilsa spawns once in a year in River Brahmaputra and the spawning season of the species lies between May to July, whereas in our present study the spawning season was found to occur between October to February (females) and from October to April (males). The shift in spawning season of Hilsa in Brahmaputra River may be due to impact of climate change. Studies conducted on the reproductive biology of the species from Bangladesh waters revealed that Hilsa has two spawning peaks, with major peaks during monsoon and minor spawning peaks in all other months (Ahmed, 1954; Raja, 1985). In our present study, we have noticed that the species has a single spawning peak during post-monsoon and winter (October–February). The shift in phenology of fish species as a response or adaptive mechanism to climate change is well-documented. Studies conducted in walleye pollock (Gadus chalcogrammus) in Gulf of Alaska showed that there is a shift in spawning season of the species by over 3 weeks throughout the last 3 decades (Rogers and Dougherty, 2019).
Sharma et al. (2014) studied the relationship between GSI and temperature in Indian major carps (IMCs) and found that temperature has an impact on GSI of IMCs. They further reported that water temperature has a significant impact on GSI and subsequent spawning in T. ilisha and water temperature in the range of 29°C–32°C is conducive for spawning. In our study, we analysed the impact of air temperature (climate parameter) on GSI of T. ilisha. It was observed that mean air temperature and GSI of T. ilisha (both male and female) were negatively correlated. Temperature of air and water have a significant correlation as reported by Zhu et al. (2018) in Missouri River. Thus, air temperature can be used as a proxy for water temperature in situations where information on the latter is limited. Sharma et al. (2014) stated that a rise in temperature above the desired range will definitely affect spawning of T. ilisha, which reiterates our findings. Research carried out in American shad, Alosa sapidissima, showed that temperature is one of the most important factors affecting spawning migration of the species (Leach, 1925; Talbot, 1953). The breeding season of Chelon parsia was reported from November to March, and it was found that water temperature has a negative correlation with the GSI of C. parsia (Sharma et al., 2014), which is in line with our present findings. The negative correlation between GSI and temperature as observed in our study indicates that T. ilisha is vulnerable to climate change. Shift in spawning season, shift in spawning grounds from freshwater toward marine, change in migration pattern, reduced spawning success, limited recruitment, lower fecundity, and reduced survival rate in juveniles are some of the potential impacts of climate change in T. ilisha. Climate change coupled with anthropogenic activities led to shifting of T. ilisha fishery from inland waters toward marine, resulting in a decline of inland catch by 20% and an increase in marine catch by three times (Miah, 2015).
Length at first maturity, i.e., the length at which 50% of the total population attains maturity, was found to be 290 mm for female(s) and 259 mm for male(s). It was found that males mature at a smaller size as compared with females. A series of studies have been made on the length at first maturity in Hilsa from different water bodies across India, viz., 350 mm for males and 356 mm for females from Godavari river (Chacko and Ganapati, 1949); 280 mm for males from Godavari river (Chacko and Krishnamurthy, 1950); 420–430 mm in Godavari river (Rajyalakshmi, 1973); 216–254 mm in males and 267–305 mm in females from Hooghly river, Chilka Lake, and Mahanadi River (Jones and Menon, 1951); 266 mm in females from Narmada River (Karamchandani, 1961); and 200 mm in males and 350 mm in females from Ganga (Mathur, 1964). The length at first maturity of the species from Bangladesh waters was found to be 210 mm in males and 320 mm for females (Shafi et al., 1978b) and 400 mm for both males and females (Dunn, 1982). The minimum size at maturity was found to be 160–170 mm in males and 190–200 mm in females from Hooghly river (Pillay, 1958). Amin et al. (2005) found that the size at first maturity for Hilsa from Bangladesh waters was 240–250 mm in males and 280–300 mm in females, whereas Mutlak (2012) found that it was 242 mm in males and 256 mm in females from Al-Hammar. All these studies report that males mature at a smaller size as compared with females, which is in line with our present findings. As females contribute the major genetic material along with energy reserve (yolk) for offspring, they divert more energy for reproduction as compared with males and hence mature at a later period and at a larger size as compared with males (Trindade-Santos and Freire, 2015).
De (1980) and De (1986) reported that fecundity of T. ilisha was found to range from 373,120 to 1,475,676 ova for fishes in the size range of 343–522 mm with an average of 740,971 ova per female. The author reported that the relative fecundity of this species ranges from 502 to 1,350 ova per gram body weight with an average of 1,190 ova per gram body weight. De (2014) reviewed the work done on fecundity of the species and reported that fecundity of the species ranges from 250,000 to 2,917,000 ova as reported by various authors for specimens in the size range of 253–566 mm. Fecundity of the species from Bangladesh waters was found to range from 90,000 to 2,000,000 (Table 4). During our study, fecundity was found to range from 103,164 to 583,456 ova with an average of 250,532 ova per female. Relative fecundity was found to range from 306 to 1096 ova per gram body weight with an average of 791 ova per gram body weight. It is seen that estimated fecundity in our present study was found to be slighter lower than most of earlier records from Bangladesh waters. It might be due to the availability of higher percentage of samples in lower size range of 229–403 mm during our present study. De (2014) stated that a significant linear relationship exists between absolute fecundity and gonad weight (R2 = 0.7741), absolute fecundity and fish weight (R2 = 0.7215), and between absolute fecundity and fish length (R2 = 0.6741), which is in line with our present findings, where we have found that a significant linear relationship exists between fecundity and body parameters and the best-fit relation based on the R2 value was between fecundity and gonad weight, followed by fish weight and then fish length. Fecundity in fishes has a direct correlation with size, length, and age of the fish, population density, mate choice, and environmental variability (Bradshaw and McMahon, 2008). Sadovy (1996) stated that fecundity in fishes decreases interspecifically with size.
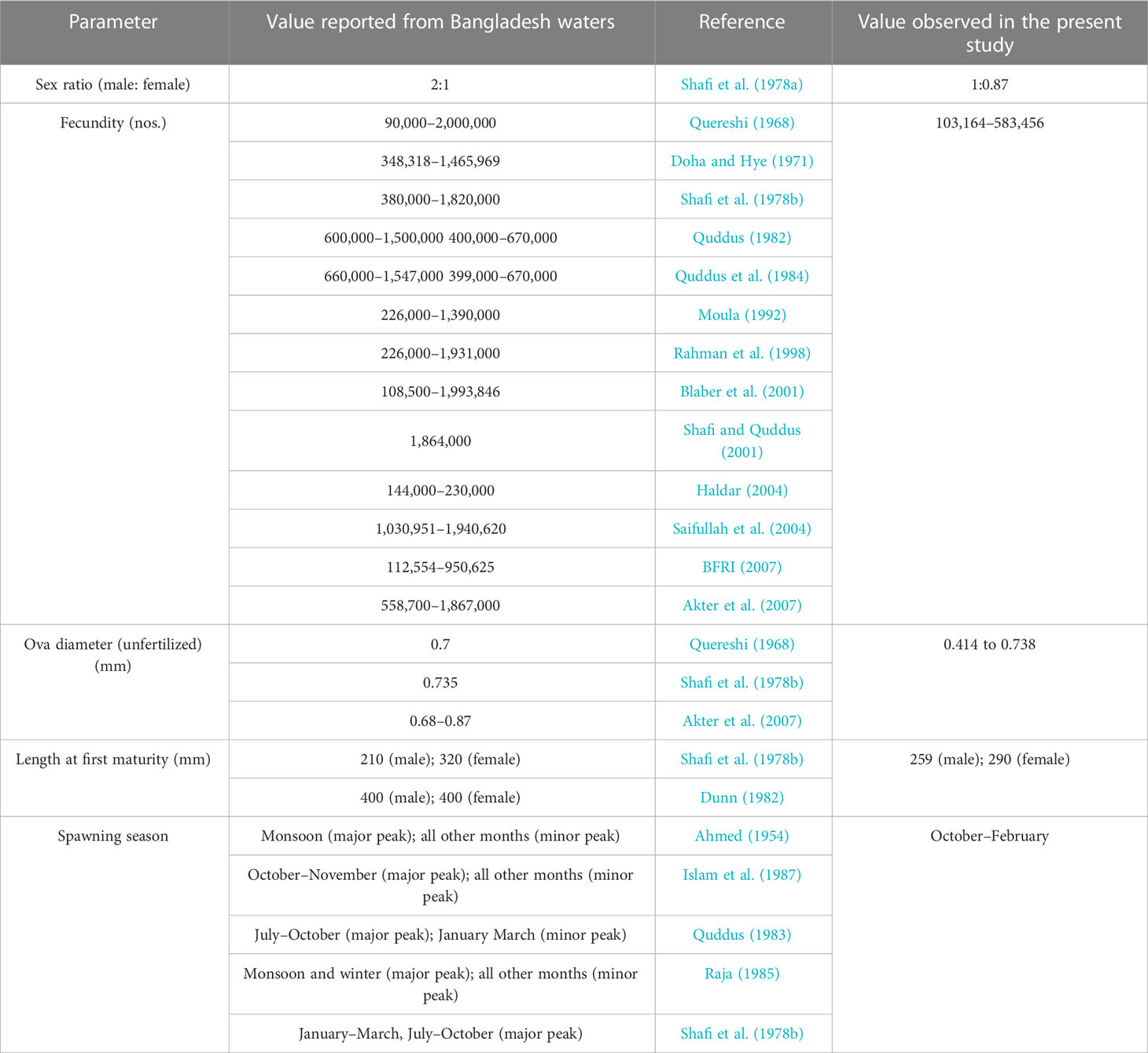
Table 4 Comparative analysis of selected biological parameters of T. ilisha from Brahmaputra River, India, and Bangladesh waters.
Reports of various workers revealed that Hilsa spawns only once in a year in Brahmaputra (Rao and Pathak, 1972), Hooghly (De, 1980; De, 1986), Godavari (Pillay and Rao, 1963), Chilka Lake (Ramakrishnaiah, 1972), and Narmada (Karamchandani, 1961), which is line with our present findings, where we have found that the species has an extended spawning season with a single peak in Brahmaputra River, Assam. On the contrary studies from Bangladesh waters have revealed two spawning peaks (major and minor) exist in the species, with major peak observed either during monsoon, post-monsoon, or winter (Quddus, 1983; Raja, 1985; Islam et al., 1987). Sharma et al. (2014) stated that temperatures of 29°C–32°C are congenial for spawning of the species. Warmer waters of Bangladesh might provide a comparatively better environment for breeding of the species as compared with the Indian part of Brahmaputra, where the average temperature is around 24.9°C during 2018–2019 (Source: RMC, IMD, Guwahati). Furthermore, researchers have reported that access to suitable spawning ground is also one of the most important factors in inducing spawning in fishes (Ganapati et al., 1951). Suitable spawning grounds may be easily accessible to the species and that too within a short period of time in rivers of Bangladesh as compared with Indian part of Brahmaputra, where the species must travel large distances for spawning, which consumes a lot of time and energy. Doha and Hye (1971) reported that the ova diameter of the species ranges from 430 to 729.2 µm, which is similar to our present findings of 414.6 to 738.2 µm. Reports available from Bangladesh waters revealed that ova diameter of the species ranges from 680 to 870 µm, which is slightly higher than our present findings. The difference in ova diameter may be attributed to fish body dimensions as egg size has a direct correlation with the size, length, and age of fishes (El-Sayed, 2020).
De (1986) reported that the sex ratio of T. ilisha from Hooghly river was 1:0.93 (male: female) with a dominance of males over females. In our present study also, we have observed dominance of males over females (1: 0.87; male: female). However, chi-square test did not reveal any significant deviation of observed sex ratio against hypothetical sex ratio of 1:1. This observation agrees with the findings of Jones and Menon (1951) and Pillay (1958) from Hooghly estuary; Mathur (1964) from Ganga River; and Quereshi (1968) from Padma and Meghna rivers of Bangladesh. Length group-wise analysis showed that males are dominant in smaller length groups (100–250 mm), which reiterates the findings of De (2014) that males are dominant over females in juveniles of T. ilisha. Dominance of males over females as observed in our present study has also been reported by Shafi et al. (1978a) from Meghna river, Bangladesh. Dominance of males over females in nature, specifically in fishes with an external mode of fertilization, is a well-established and universal fact. Higher number of males provides a better chance of fertilizing a higher percentage of eggs, thereby ensuring higher genetic diversity and a better chance of species survival. Further exposure to fluctuations in temperature is known to have impact on sex ratios of many fish species. It has been documented that warm- and cold-water temperatures masculinize fish population with mid-range conditions producing males and females in the ratio of 1:1 approximately (Honeycutt et al., 2019). Dominance of males over females as observed in the present study may be attributed to the relatively warm waters of the Bay of Bengal region.
Hilsa has a dominant presence in the entire Bay of Bengal region and its adjoining freshwater zones. Being a transboundary and highly migratory resource, specific management measures need to be catered. Sociocultural significance in the Indian subcontinent makes this anadromous fish highly sought after. The present study on the reproductive biology of this species from Brahmaputra River has its significance in north-east India and more importantly in Bangladesh, downstream. Policy measures like fish sanctuaries and minimum legal size (MLS) for capture based on length at first maturity are few examples of management implications that can revive Hilsa fishery in Brahmaputra. Size at which females become sexually mature (29 cm as observed in the present study) can be set as MLS for Hilsa in Assam. It is to be noted that MLS for catching Hilsa in Bangladesh is 25 cm (Pramanik et al., 2017) and the allowable mesh size for Hilsa gill nets is 6.5 cm (Anon, 2020). The selection range of fish caught in 6.5-cm-mesh-sized gill nets ranged from 31.4 to 34.2 cm (Pramanik et al., 2017), which is almost similar to the MLS suggested in the present study. These regulations along with fish sanctuaries have resulted in revival of Hilsa fisheries in Bangladesh (Rahman et al., 2020; Dutta et al., 2021). In the case of Assam, MLS of 23 cm is already in place for carps (Phukan, 2006), whereas for Hilsa there is no such recommendation. Implementing evidence-based management measures in the Indian part of Brahmaputra will definitely boost Hilsa fishery, which is otherwise declining (Yadav et al., 2022b). This will have an anticipated positive impact on fishers’ livelihood and income in the region. Inferences derived from the present study can have burgeoning effects at the international level. Again, we have also observed in our study that the species is vulnerable to rising temperature. There lies a scope to carry out further studies to understand the impact of other factors like rainfall, salinity, water flow, siltation, turbidity, and lunar periodicity on the breeding phenology of Hilsa in Brahmaputra River, India.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by ICAR-Central Inland Fisheries Research Institute.
SB: conceptualization, major contribution in writing, data collection, and analysis. AS, VG, DM and DM: manuscript writing and correction. AY, PG and KR: data analysis and interpretation of data. AJ, GD and BB: revision of the draft for final submission. BD: overall guidance and manuscript correction. All authors contributed to the article and approved the submitted version.
The authors are thankful to the Indian Council of Agricultural Research, New Delhi, for financial support to carry out the research work under the NEH Component of ICAR-CIFRI, Barrackpore.
The authors are grateful to the Director, ICAR-CIFE, Mumbai, for providing necessary facilities to carry out the research work. The first author is thankful to the Director, ICAR-CIFRI, Barrackpore, Kolkata, for providing necessary laboratory facilities and valuable guidance for completing the research work. The authors are also thankful to the fisher community of River Brahmaputra, Assam, India.
The authors declare that the research was conducted in the absence of any commercial and financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akter A. M., Hossain M. D., Hossian K. M., Afza R., Bhuyian A. S. (2007). The fecundity of Hilsa ilisha from the river Padma near Godagari of Rajshahi district. Univ. J. Zool. Rajshahi Univ. 20, 97–103. doi: 10.3329/ujzru.v26i0.696
Amin S. M. N., Arshad A., Halder G. C., Shohaimi S., Ara R. (2005). Estimation of size frequency distribution, sex ratio and length weight relationship of Hilsa (Tenualosa ilisha) in Bangladesh waters. Res. J. Agric. Biol. Sci. 1, 61–66.
Anon (2020). Gazette notification, Department of Fisheries, Government of Bangladesh (Dhaka, Bangladesh: Government of Bangladesh).
Arruda L. M., Azevedo I. N., Neto A. I. (1993). Abundance, age structure and growth and reproduction of gobies in the Riade Averio lagoon (Portugal). Estuar. Coast. Shelf S. 37, 509–523. doi: 10.1006/ecss.1993.1070
BFRI (2007). Hilsa fishery management research: annual progress report 2007 (Bangladesh: Bangladesh Fisheries Research Institute), 10.
Bhattacharjya B. K., Bhaumik U., Sharma A. P. (2017). Fish habitat and fisheries of Brahmaputra river in Assam, India. Aquat. Ecosyst. Health Manage. 20 (1-2), 102–115. doi: 10.1080/14634988.2017.1297171
Bhattacharjya B. K., Choudhury M., Sugunan V. V. (2003). “Icthyofaunistic resources of Assam with a note on their sustainable utilization,” in The participatory approach for fish biodiversity conservation in northeast India (Lucknow, India: NBFGR), 87–105.
Bhaumik U., Sharma A. P. (2012). Present status of Hilsa in Hooghly-Bhagirathi river (India: ICAR-CIFRI, Barrackpore, Bulletin no. 179).
Biswas S. P. (1992). Manual of methods in fish biology (India: South Asian Publishers Pvt. Ltd., New Delhi), 131.
Blaber S. J. M., Milton D. A., Brewer D. T., Salini J. P. (2001). “The shads (genus Tenualosa) of tropical Asia: an overview of their biology, status and fisheries,” in The proceedings of the international terubok conference. Eds. Blader S., Bewer D., Milton D., Baiano C. (Malaysia: Sarawark Development Institute (SDI), Kuching, Sarawark), 9–17.
BOBLME (2012). Report of the Hilsa fisheries assessment working group II (Thailand: BOBLME, Phuket), 29.
Borah S., Jaiswar A. K., Bhattacharjya B. K., Deshmukhe G., Sahoo A. K., Gogoi P., et al. (2022a). Food spectrum dynamics of anadromous hilsa, Tenualosa ilisha (Hamilton 1822) inhabiting river Brahmaputra, India curtailing apprehension of food selectivity: an insight into its domestication. Indian J. Mar. Sci. 51 (01), 67–77. doi: 10.56042/ijms.v51i01.41641
Borah S., Landge A. T., Bhattacharjya B. K., Chakraborty S. K., Ramteke K. K., Barman J., et al. (2014). Variation in morphometric and meristic traits of Aspidoparia morar from Brahmaputra and Barak rivers of Assam, India. J. Appl. Nat. Sci. 6 (1), 262–266. doi: 10.31018/jans.v6i1.412
Borah S., Vaisakh G., Jaiswar A. K., Bhattacharjya B. K., Sahoo A. K., Deshmukhe G., et al. (2022b). On the population characteristics of anadromous Tenualosa ilisha (Hamilton 1822) occurring from river Brahmaputra, India. Aquat. Ecosyst. Health Manage. 25 (2), 44–52. doi: 10.14321/aehm.025.02.44
Borah S., Vaisakh G., Jaiswar A. K., Bhattacharjya B. K., Sahoo A. K., Deshmukhe G., et al. (2019b). Association pattern between dimensions of fish and otolith to expedite morphometric variations of three geographically isolated stocks of Tenualosa ilisha (Hamilton 1822) from diverse ecosystems. Indian J. Fish. 66 (3), 48–52. doi: 10.21077/ijf.2019.66.3.91245-06
Borah S., Vaisakh G., Jaiswar A. K., Bhattacharjya B. K., Sahoo A. K., Deshmukhe G., et al. (2019a). Length-weight relationship and condition factor of three geographically isolated populations of Hilsa shad, Tenualosa ilisha (Hamilton 1822). J. Inland Fish. Soc India 51 (1), 49–54.
Bradshaw C. J. A., McMahon C. R. (2008). “Fecundity,” in The encyclopaedia of ecology. Eds. Jorgensen S. E., Fath B. D. (Amsterdam, Netherlands: Elsevier Inc), 1535–1543.
Carnevali O., Candelma M., Sagrati A., Pignalosa P., Giorgini E., Gioacchini G. (2019). Macromolecular characterization of swordfish oocytes by FTIR imaging spectroscopy. Sci. Rep. 9, 8850. doi: 10.1038/s41598-019-45065-7
Chacko P. I., Ganapati S. V. (1949). On the bionomics of Hilsa ilisha (Hamilton) in the Godavari river. Madras Univ. J. 18, 16–22.
Chacko P. I., Krishnamurthy B. (1950). A biometrical study of Hilsa ilisha (Ham.) in the Godavari river. J. Bombay Nat. Hist. Soc 49, 315–316.
Das B. C., Swati S. N., Khatun T., Moniruzzaman M., Rashid H. (2022). Study of the spawning season of hilsa, Tenualosa ilisha in Bangladesh. Bangladesh J. Fisheries 34, 87–94. doi: 10.52168/bjf.2022.34.9
De D. K. (1980). Maturity, fecundity and spawning of post-monsoon run of hilsa, Hilsa ilisha (Hamilton) in the upper stretches of the Hooghly estuarine system. J. Inland Fish. Soc India 12, 54–63.
De D. K. (1986). Studies on the food and feeding habit of Hilsa ilisha (Hamilton) of the Hooghly estuarine system and some aspects of its biology. PhD Thesis (Calcutta, India: Calcutta University), 285.
De D. K. (2014). The shad hilsa, Tenualosa ilisha (Hamilton): its fishery and biology (India: Narendra Publishing House, Delhi), 227.
Doha S., Hye M. A. (1971). Fecundity of Padma river hilsa, Hilsa ilisha (Hamilton). Pak. J. Sci. 22, 176–184.
Dunn I. C. (1982). The Hilsa fishery of Bangladesh 1982: an investigation of the present status with an evaluation of the current data (Rome: FAO, Field document 2), 71.
Dutta S., Al-Abri I., Paul S. (2021). Bio-economic trends of Hilsa (Tenualosa ilisha) fishery: perspectives of transboundary management between India and Bangladesh. Mar. Policy 128, 104483. doi: 10.1016/j.marpol.2021.104483
ESRI (2008). ArcGIS desktop: release 9.3 (Redlands, California: Environmental Systems Research Institute).
Ganapati S. V., Alikunhi K. H., Thivy F. (1951). On an interesting case of carp spawning in the river Cauvery at Bhavani during June 1947. J. Bombay Nat. Hist. Soc 50, 140–146.
Ghaffari H., Ardalan A. A., Sahafi H. H., Babaei M. M. (2011). Annual changes in gonadosomatic index (GSI), hepatosomatic index (HSI) and condition factor (K) of largescale tonguesole Cynoglossus arel (Bloch & Schneider 1801) in the coastal waters of Bandar abbas, Persian gulf. Aust. J. Basic Appl. Sci. 5, 1640–1646.
Grossen C., Neuenschwander S., Perrin N. (2011). Temperature-dependent turnovers in sex-determination mechanisms: a quantitative model. Evolution 65, 64–78. doi: 10.1111/j.1558-5646.2010.01098.x
Hamilton F. (1822). An account of the fishes found in the river Ganga and its branches (UK: Archibald Constable and Company, Edinburgh, London).
Honeycutt J. L., Deck C. A., Miller S. C., Severance M. E., Atkins E. B., Luckenbach J. A., et al. (2019). Warmer waters masculinize wild populations of a fish with temperature-dependent sex determination. Sci. Rep. 9, 1–13. doi: 10.1038/s41598-019-42944-x
Haldar G. C. (2004). Present status of the hilsa fishery in Bangladesh. Report of studies conducted under ARDMCS GEF component FFP Rep. No. 38.8 (Dhaka, Bangladesh: Department of Fisheries), 70.
Hopkins C. L. (1979). Reproduction in Galaxias fasciatus Gray (Salmoniformes: Galaxiidae). New Zeal. J. Mar. Fresh. 13, 225–230. doi: 10.1080/00288330.1979.9515797
Hossain M. A., Das I., Genevier L., Hazra S., Rahman M., Barange M., et al. (2019). Biology and fisheries of Hilsa shad in Bay of Bengal. Sci. Total Environ. 651, 1720–1734. doi: 10.1016/j.scitotenv.2018.10.034
Iossa G. (2019). Sex-specific differences in thermal fertility limits. Trends Ecol. Evol. 34, 490–492. doi: 10.1016/j.tree.2019.02.016
Islam M. A., Begum M., Pal H. K., Alam M. J. (2008). Studies on the gonadosomatic index and fecundity of Mystus gulio (Ham.). Progress. Agric. 19, 161–166. doi: 10.3329/pa.v19i2.16957
Islam M. S., Huq Q. M., Hossian M. M., Azad S. A., Das N. N. (1987). “Maturity and spawning of Hilsa shad, Hilsa ilisha of Bangladesh,” in Hilsa investigation in Bangladesh. Marine Fishery resources management (Colombo: Bay of Bengal Programme), 82–95.
Jan M., Jan N. (2017). Studies on the fecundity (F), gonadosomatic index (GSI) and hepatosomatic index (HSI) of Salmo trutta fario (Brown trout) at Kokernag trout fish farm, Anantnag, Jammu and Kashmir. Int. J. Fish. Aquat. Stud. 5, 170–173.
Jones S., Menon P. M. G. (1951). Observations on the life-history of the Indian shad, HiIsa ilisha (Hamilton). Proc. Indian Acad. Sci. 31, 101–125. doi: 10.1007/BF03049975
Karmakar S., Patra K., Jana S., Mandal D. P., Bhattacharjee S. (2016). Exposure to environmentally relevant concentrations of malathion induces significant cellular, biochemical and histological alterations in Labeo rohita. Pestic. Biochem. Physiol. 126, 49–57. doi: 10.1016/j.pestbp.2015.07.006
Karamchandani S. J. (1961). On the location of spawning grounds of Indian shad, Hilsa ilisha (Hamilton) in freshwater regions of the Narmada river. Curr. Sci. 30, 373–375.
Kingdom T., Allison M. E. (2011). The fecundity, gonadosomatic and hepatosomatic indices of Pellonula leonensis in the lower Nun river, Niger delta, Nigeria. Curr. Res. J. Biol. Sci. 3, 175–179.
Leach G. C. (1925). Artificial propagation of shad (Washington D. C.: Department of US commercial fisheries), 459–486.
Mathur P. K. (1964). Studies on the maturity and fecundity of the hilsa, Hilsa ilisha (Hamilton) in the upper stretches of the Ganga. Indian J. Fish. 11, 423–448.
Miah M. S. (2015). Climatic and anthropogenic factors changing spawning pattern and production zone of Hilsa fishery in the Bay of Bengal. Weather and Climate Extremes 7, 109–115. doi: 10.1016/j.wace.2015.01.001
Milton D. A. (2010). Status of Hilsa (Tenualosa ilisha) management in the Bay of Bengal: an assessment of population risk and data gaps for more effective regional management (Phuket, Thailand: BOBLME).
Mohanty B. P., Paria P., Mahanty A., Behera B. K., Mathew S., Shankar T. V., et al. (2012). Fatty acid profile of Indian shad Tenualosa ilisha. Natl. Acad. Sci. Lett. 35, 263–269. doi: 10.1007/s40009-012-0042-x
Motwani M. P., Jayram K. C., Sehgal K. L. (1962). Fish and fisheries of the Brahmaputra river system, Assam. Fish fauna with observations on their zoo-geographical distribution. Trop. Ecol. 3, 17–43.
Moula G. (1992). Studies on some biological aspects of hilsa (Chandpur: National Workshop on Hilsa Fishery Development and Management, Fisheries Research Institute), 12.
Mutlak M. F. (2012). Stock assessment of some fish species from East Al-hammar marsh, Southern Iraq (Iraq: University of Basrah), 195. PhD Thesis.
Nath A. K., Banerjee B. (2012). Comparative evaluation of body composition of hilsa, Tenualosa ilisha in different size groups with special reference to fatty acids, in Hooghly estuarine system, West Bengal. Indian J. Fish. 59, 141–146.
Pillay T. V. R. (1958). Biology of the hilsa, Hilsa ilisha (Ham.) of the river Hooghly. Indian J. Fish. 5, 201–257.
Pillay S. R., Rao K. V. (1963). “Observations on the biology and fishery of the hilsa, Hilsa ilisha (Hamilton) of river Godavari,” in Proceedings of the 10th Indo-Pacific fisheries council meeting (Thailand, Bangkok: Indo-Pacific Fisheries Council), 37–61.
Pillay S. R., Rosa H. Jr. (1963). Synopsis of biological data on hilsa, Hilsa ilisha (Hamilton) Vol. 38 (Rome: FAO Fisheries Biology Synopsis, FAO).
Prabhu M. S. (1956). Maturation of intra-ovarian eggs and spawning periodicities in some fishes. Indian J. Fish. 3, 59–90.
Pramanik M. M. H., Rahman M. A., Ahmed T., Flura H. M., Hasan M. M., Khan M. M., et al. (2017). Gill net selectivity of Hilsa (Tenualosa ilisha) in the Meghna river estuary of Bangladesh. J. Aquac. Res. Dev. 8, 1000483. doi: 10.4172/2155-9546.1000483
Quddus M. M. A. (1982). Two types of Hilsa ilisha and their population biology from Bangladesh waters (Tokyo, Japan: University of Tokyo), 180.
Quddus M. M. A., Shimizu M., Nose Y. (1984). Meristic and morphometric differences in two types of Hilsa ilisha in Bangladesh waters. Bull. Japan Soc Sci. Fish. 50, 43–49. doi: 10.2331/suisan.50.43
Rahman M. J., Mustafa M. G., Rahman M. A. (1998). “Population dynamics and recruitment pattern of hilsa, Tenualosa ilisha,” in Proceedings of the BFRI/ACIAR/CSIRO workshop on Hilsa fisheries research in Bangladesh (Mymensingh, Bangladesh: BFRI), 28–36.
Rahman M. J., Wahab M. A., Nahiduzzaman M., Haque A. B. M. M., Cohen P. (2020). Hilsa fishery management in Bangladesh. IOP Conf. Series: Earth Environ. Sci. 414, 012018.
Raja B. T. A. (1985). A review of the biology and fisheries of Hilsa ilisha in the upper Bay of Bengal. (FAO, Rome: Bay of Bengal Programme), 53.
Rajesh K. M., Rohit P., Vase V. K., Sampathkumar G., Sahib K. (2015). Fishery, reproductive biology and stock status of the large head hairtail Trichiurus lepturus Linnaeus 1758 off Karnataka, South-west coast of India. Indian J. Fish. 62, 28–34.
Rajyalakshmi T. (1973). The population characteristics of the Godavari Hilsa over the years 1963-1967. Indian J. Fish. 20, 78–94.
Ramakrishnaiah M. (1972). Biology of Hilsa ilisha (Hamilton) from the Chilka lake with an account on its racial status. Indian J. Fish. 19, 35–53.
Rao K. V., Pathak S. C. (1972). A note on the occurrence of spawning of Hilsa ilisha (Hamilton) in the river Brahmaputra (Assam). Proc. Natl. Acad. Sci. India Sec. B. 42, 231–233.
Reuben S., Dan S. S., Somaraju M. V., Philipose V., Sathianandan T. V. (1992). The resources of Hilsa shad, Hilsa ilisha (Hamilton), along the northeast coast of India. Indian J. Fish. 39, 169–181.
Rogers L. A., Dougherty A. B. (2019). Effects of climate and demography on reproductive phenology of a harvested marine fish population. Global Change Biol. 25, 708–720. doi: 10.1111/gcb.14483
Sadovy Y. J. (1996). “Reproduction of reef fishery species,” in Reef fisheries. Eds. Polunin N. V. C., Roberts C. M. (Dordrecht, NL: Springer), 15–59.
Sahoo A. K., Wahab M., Phillips M., Rahman A., Padiyar A., Puvanendran V., et al. (2016). Breeding and culture status of Hilsa (Tenualosa ilisha, Ham. 1822) in South Asia: a review. Rev. Aquacult. 10, 96–110. doi: 10.1111/raq.12149
Saifullah A. S. M., Rahman M. S., Khan S. A. Y. (2004). Fecundity of Hilsa ilisha (Hamilton 1822) from the Bay of Bengal. Pakistan J. Biol. Sci. 7, 1394–1398. doi: 10.3923/pjbs.2004.1394.1398
Sattar S. A., Adam M. S. (2005). Review of grouper fishery of the Maldives with additional notes on the faafu atoll fishery Vol. 54 (Male (Maldives: Marine Fishery Research Centre).
Saud B. J., Landge A. T., Borah S. (2015). Gonado-somatic index and fecundity of Heteropneustes fossilis (Bloch) from the lower reaches of Brahmaputra river, Assam, India. J. Exp. Zool. India 18, 657–660.
Sen N. (2000). “Occurrence, distribution and status of diversified fish fauna of northeast India,” in Fish biodiversity of northeast India. Eds. Ponniah A. G., Sarkar U. K. (Lucknow, India: NBFGR), 31–48.
Shafi M., Quddus M. M. A., Hossain M. (1978a). Studies on length-girth relationship, sex ratio, size composition, gears and abundance of Hilsa ilisha (Hamilton-Buchanan) in the river Padma. Dacca Univ. Stud. B. 26, 123–127.
Shafi M., Quddus M. M. A., Islam N. (1978b). Maturation and spawning of Hilsa ilisha (Hamilton-Buchanan) of the river Meghna. Dacca Univ. Stud. B. 26, 63–71.
Sharma A. P., Naskar M., Joshi K. D., Bhattacharjya B. K., Sahu S. K., Das S., et al. (2014). Impact of climate variation on breeding of major fish species in inland waters (India: Central Inland Fisheries Research Institute, Kolkata, Bulletin No. 185).
Sinha M. (1994). Fish genetic resources of the north-eastern region of India. J. Inland Fish. Soc India 26, 1–19.
SPSS Inc. (2008). Statistical package for social sciences (SPSS) version 16.0 (Chicago III: SPSS Inc.).
Talbot G. B. (1953). The passage of shad at the Bonneville fishways. Special scientific report: fisheries no. 94 (Washington D.C.: US Department of the Interior Fish and Wildlife Service), 1–30.
Toots H. (1951). Number of eggs in different populations of whitefish (Coregonus). Rep. lnst. Freshwat. Res. Drottningholm 32, 133–138.
Trindade-Santos I., Freire K. D. M. F. (2015). Analysis of reproductive patterns of fishes from three large marine ecosystems. Front. Mar. Sci. 2, 38. doi: 10.3389/fmars.2015.00038
Vaas K. K., Moza U. (2011). “Riverine fisheries,” in Handbook of fisheries and aquaculture (New Delhi, India: Indian Council of Agricultural Research), 169–207.
Vaisakh G., Borah S., Deshmukhe G., Jaiswar A. K., Sahoo A. K., Srihari M., et al. (2020). On the morphological variations of geographically isolated migratory and non-migratory populations of tropical shad, Tenualosa ilisha (Hamilton 1822) from three distinct tropical ecosystems. Indian J. Mar. Sci. 49 (07), 1189–1196.
Vishwanath W. (2002). Fishes of northeast India: a field guide to species identification (India: Manipur University, Imphal).
Vladykov V. (1956). Fecundity of wild speckled trout (Salvelinus fontinalis) in Quebec lakes. J. Fish. Res. Board Can. 13, 799–841. doi: 10.1139/f56-046
Walsh M., Hopkins P., Witthames P., Greer Walker M., Watson J. (1990). Estimation of total potential fecundity and atresia in the western mackerel stock 1989 (Copenhagen, Denmark: ICES).
Yadav A. K., Borah S., Bhattacharjya B. K., Das K. K., Das B. K. (2022b). Decadal shift in fish landings and catch composition in Brahmaputra river, Assam, India. Res. Bio. 4, 132–138. doi: 10.54083/ResBio/4.3.2022/132-138
Yadav A. K., Borah S., Das K. K., Raman R. K., Das P., Das B. K. (2022a). Modeling of Hilsa (Tenualosa ilisha) landings in the lower stretch of river Brahmaputra, Assam, India under time-series framework. ScienceAsia 48, 367–372. doi: 10.2306/scienceasia1513-1874.2022.042
Keywords: Indian shad, reproductive biology, climate parameters, Brahmaputra, sustainable management
Citation: Borah S, Sahoo AK, Gopinathapillai V, Meena DK, Jaiswar AK, Deshmukhe G, Yadav AK, Gogoi P, Mohanty D, Ramteke K, Bhattacharjya BK and Das BK (2023) Understanding the breeding phenology of anadromous fish Tenualosa ilisha (Hamilton, 1822) in relation to climatic variables in Brahmaputra River, India. Front. Mar. Sci. 10:1063210. doi: 10.3389/fmars.2023.1063210
Received: 06 October 2022; Accepted: 02 May 2023;
Published: 29 May 2023.
Edited by:
Michael Phillips, WorldFish, MalaysiaReviewed by:
Md. Nahiduzzaman, WorldFish, MalaysiaCopyright © 2023 Borah, Sahoo, Gopinathapillai, Meena, Jaiswar, Deshmukhe, Yadav, Gogoi, Mohanty, Ramteke, Bhattacharjya and Das. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Basanta Kumar Das, YmFzYW50YWt1bWFyZEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.