
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 27 February 2023
Sec. Marine Conservation and Sustainability
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1040123
This article is part of the Research Topic Identifying and Comparing Important Areas for Marine Sustainable Use and Conservation View all 19 articles
We delineated and scored Biologically Important Areas (BIAs) in the Arctic region. The Arctic region extends from the Bering Strait to the Chukchi Sea, Beaufort Sea, Amundsen Gulf, and Viscount Melville Sound. This NOAA-led effort uses structured elicitation principles to build upon the first version of NOAA BIAs (BIA I) for cetaceans. In addition to narratives, maps, and metadata tables, BIA II products incorporated a scoring and labeling system to improve their utility and interpretability. BIAs are compilations of the best available science and have no inherent regulatory authority. They have been used by NOAA, other federal agencies, and the public to support marine spatial planning and marine mammal impact assessments, and to inform the development of conservation measures for cetaceans. Supporting evidence for Arctic BIA II came from data derived from aerial-, land-, and vessel-based surveys; satellite telemetry; passive acoustic monitoring; Indigenous knowledge; photo-identification; aboriginal subsistence harvests, including catch and sighting locations and stomach contents; and prey studies. BIAs were identified for bowhead (Balaena mysticetus), gray (Eschrichtius robustus), humpback (Megaptera novaeangliae), fin (Balaenoptera physalus), and beluga (Delphinapterus leucas) whales. In total, 44 BIAs were delineated and scored for the Arctic, including 12 reproduction, 24 feeding, and 8 migration BIAs. BIAs were identified in all months except January-March. Fifteen candidate areas did not have sufficient information to delineate as BIAs and were added to a watch list for future consideration in the BIA process. Some BIAs were transboundary between the Arctic region and the Aleutian Islands-Bering Sea region. Several BIAs were transnational, extending into territorial waters of Russia (in the Chukchi Sea) and Canada (in the Beaufort Sea), and a few BIAs were delineated in international waters.
Cetacean seasonal distributions in the Pacific Arctic overlap with numerous anthropogenic activities, including offshore energy exploration, development, and extraction; shipping; recreational vessels; military operations; and aboriginal subsistence hunting. The Arctic ecosystem is also changing rapidly due to a warming climate driven by increases in atmospheric greenhouse gas concentrations (Overland et al., 2019). These changes include decreases in seasonal sea ice (Stroeve et al., 2012), large warm winter air temperature anomalies, record low winter sea ice extent and expanded terrestrial ice melt seasons (Overland et al., 2019), and increased net primary production (Frey et al., 2015; Hill et al., 2018). These ecosystem perturbations are expected to increase in magnitude, space, and time. To inform location-based marine conservation and management efforts in the region, we delineated and scored Biologically Important Areas (BIAs) for cetaceans in the Arctic as part of a nationwide process led by the U.S. National Oceanic and Atmospheric Administration (NOAA).
BIAs represent places and periods (months or seasons) that are important to cetacean species, stocks, or populations for feeding, migration, or activities related to reproduction (Ferguson et al., 2015; Harrison et al., in review). BIAs may also be defined to encompass the range or core areas of small resident populations occupying a limited geographic extent. This BIA II effort builds on NOAA’s inaugural BIA process (BIA I; Van Parijs, 2015) by revising existing Arctic BIAs (Clarke et al., 2015b) and creating new Arctic BIAs based on new information. In addition, each BIA II delineation was scored based on Intensity of use, Data Support, Spatiotemporal Variability, and Boundary Certainty (see section 2.1; Harrison et al., in review).
The Arctic region as defined in this paper encompasses the U.S. Exclusive Economic Zone (EEZ) and areas beyond the U.S. EEZ in the Beaufort and Chukchi seas where cetacean seasonal distribution extends into transnational or international waters (Harrison et al., in review). This area is covered by seasonal sea ice in the winter and is largely free of sea ice in summer. The Chukchi Sea is characterized by a continental shelf that extends north ~800 km from the Bering Strait. Depths are generally <50-60 m, except for shoals near Hanna and Herald shoals and Wrangel Island, where depths are shallower (Weingartner et al., 2021) (Figure 1). The shallower depths near the Chukotkan and Alaskan coastlines, Wrangel Island, and shoals define channels for three branches of Pacific-derived waters that bring heat, fresh water, and nutrients northward from the Bering Sea (Weingartner et al., 2021). Transport loads are not equal among currents, with variation due to temperature, salinity, and sea ice extent, among other factors.
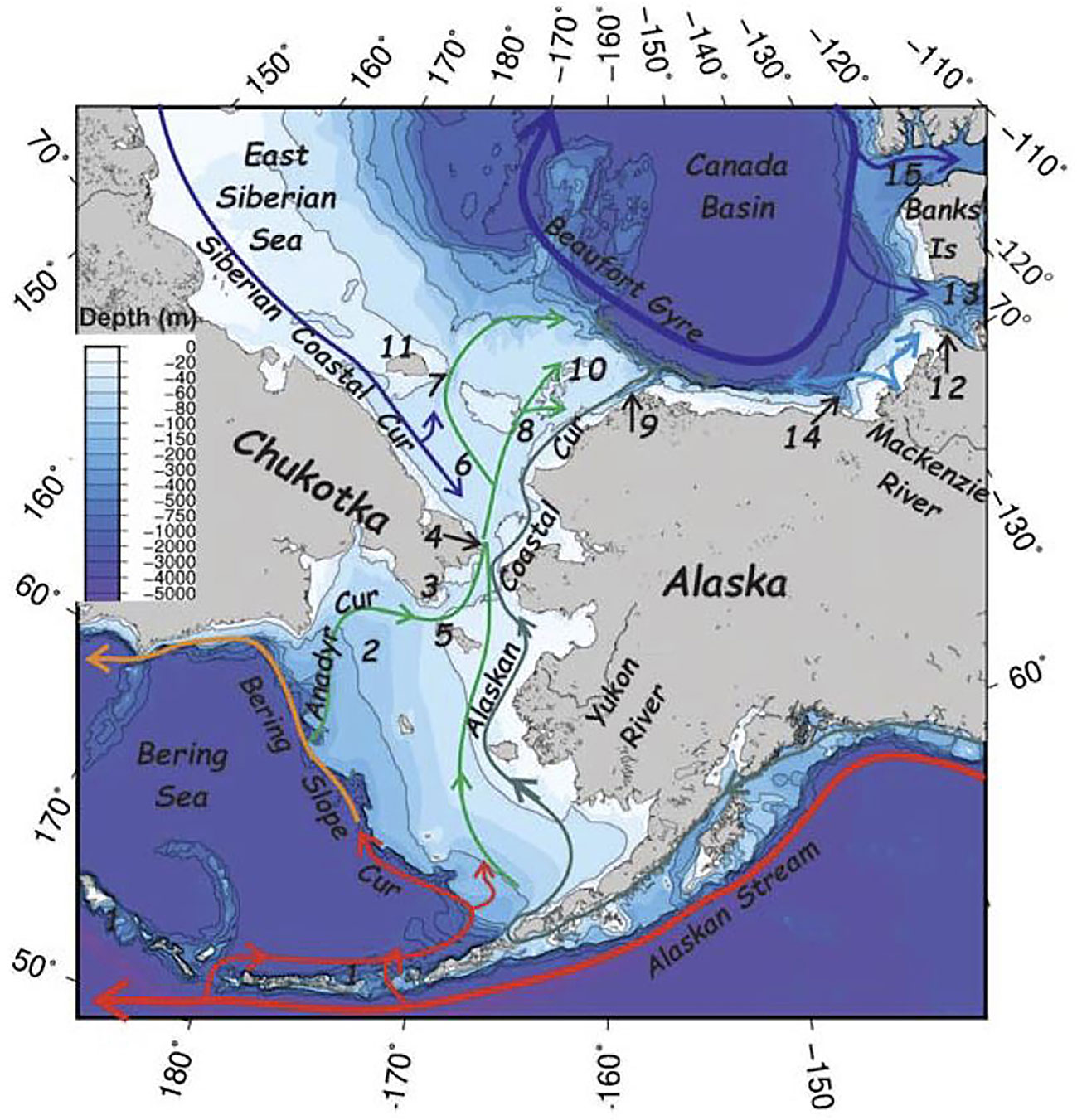
Figure 1 Bathymetric map of the Bering-Chukchi-Beaufort seas overlain with major currents depicted from warmest (red) to coldest (blue). Major geomorphic features are (1) Aleutian Island Archipelago, (2) Gulf of Anadyr, (3) Anadyr Strait, (4) Bering Strait, (5) St. Lawrence Island, (6) Hope Valley, (7) Herald Valley, (8) Central Channel, (9) Barrow Canyon, (10) Hanna Shoal, (11) Wrangel Island, (12) Cape Bathurst, (13) Amundsen Gulf, (14) Mackenzie Canyon, and (15) McClure Strait. Reprinted from The Bowhead Whale, Weingartner, T.J., Okkonen, S.R., Danielson, S.L., Physical Oceanography, pages 385-402, Copyright 2021, with permission from Elsevier.
The Beaufort Sea shelf is much narrower than the Chukchi Sea shelf, only ~80 km wide north of Alaska (Weingartner et al., 2021) and ~120 km wide near the Mackenzie delta north of Canada. Hydrography on the Mackenzie shelf is heavily influenced by year-round runoff from the Mackenzie River. There are several small, shallow rivers that empty onto the Alaskan Beaufort shelf as well, but their impact on hydrography is less than on the Mackenzie shelf. Strong northeasterly winds promote upwelling of nutrient rich water at the Beaufort Sea shelf break.
In both the Chukchi and Beaufort seas, primary production is driven by ice algae and phytoplankton, particularly in marginal ice zones, polynyas, and leads (linear openings in sea ice) (Ashjian et al., 2021). Secondary production, including the pelagic zooplankton prey of most Arctic mysticete whales, depends on primary production together with physical processes like advection, vertical mixing, and upwelling. Benthic organisms, including the preferred prey of gray whales, benefit from primary production not consumed by pelagic zooplankton which falls to the sea floor. Secondary producers also provide food for pinnipeds, seabirds, and fish.
Within the Arctic region identified here, five species of baleen whale (bowhead whale [Balaena mysticetus], gray whale [Eschrichtius robustus], humpback whale [Megaptera novaeangliae], fin whale [Balaenoptera physalus], and minke whale [Balaenoptera acutorostrata]) and three species of toothed whale (beluga [Delphinapterus leucas]), harbor porpoise [Phocoena phocoena], and killer whale [Orcinus orca]) occur seasonally. Bowhead whales and belugas, species that are endemic to the Arctic, migrate northward through the Bering Strait in spring from wintering grounds in the northern Bering Sea, remain in high northern latitudes throughout summer, and undertake a return migration to the Bering Sea in autumn. Gray, humpback, fin, and minke whales occur in the Arctic during summer and early autumn, migrating south to the North Pacific Ocean for winter. Harbor porpoises are present in the Arctic during summer and may occur year-round in some coastal areas (Castellote et al., 2017). Killer whales are also present in the Arctic region in summer and autumn but are less frequently observed than other subarctic cetacean species (see Stafford et al., 2022a).
The goals of this paper are to provide: (1) insight into the process used to delineate and score BIAs in the Arctic; (2) a summary of results; and (3) information on where to find additional materials, including supplementary supporting information, online access to all BIA II graphic shapefiles, and metadata. We present detailed information on the data sources and decision-making process used to delineate and score five example Arctic BIAs. The example BIAs were selected to span a range of BIA types, intensities, available information, and spatiotemporal variability characteristics. We then summarize information for all Arctic BIAs, present watch list areas (areas that may be important, but which currently lack sufficient information to be delineated as BIAs), and provide recommendations to facilitate future conservation and management efforts.
BIAs for all seven regions around the U.S. were delineated, scored, and labeled using consistent application of the methodology described in the Introductory chapter included in this special edition (Harrison et al., in review). Additionally, Harrison et al. (in review) highlight the changes in BIA delineation since Van Parijs (2015) and describe the intended use of the BIAs, specifically addressing common mischaracterizations of the BIA I products to try to eliminate inappropriate use of BIAs in the future. Fundamentally, BIAs are compilations of the best available science and have no inherent or direct regulatory power. We provide a brief overview of the methods outlined in Harrison et al. (in review) below.
A regional lead with cetacean expertise oversaw the identification, delineation, and scoring of Arctic BIAs, engaging with additional subject matter experts (SMEs) as needed to ensure all available information and necessary expertise were included for all cetacean taxa. Four types of BIAs were defined (Supplementary Table 1): feeding areas (F-BIAs), reproductive areas (R-BIAs), migratory routes (M-BIAs), and small and resident populations (S-BIAs). Each BIA was delineated only for the times and areas for which direct information exists on a particular cetacean species, population, or stock. Any reliable published or unpublished information from scientific research, Indigenous or local knowledge, or community science, including both data and personal observations, were considered valid. Spatial optimization modeling, incorporating spatial information about whale relative density under variable thresholds for minimum cluster size (Ferguson et al., in review), was used for delineating some BIAs. Intentional “buffers” or other “precautionary” additions of area or time were not allowed. Similarly, predictions of potential habitat alone were insufficient to support a BIA delineation.
All candidate BIAs were scored and labeled using five metrics: Intensity, Data Support, Importance, Boundary Certainty, and Spatiotemporal Variability (Supplementary Table 2). All scoring metrics except Spatiotemporal Variability were assigned an integer value ranging from 1 (“low”) to 3 (“high”). For each candidate BIA, Intensity and Data Support were independently scored using scoring rules specific to each BIA type. Then, Intensity and Data Support scores were combined to determine an overall Importance score using the Importance Score matrix (Figure 2), which was the same for all BIA types. Candidate BIAs with an Importance score of 0 were added to a “watch list” of areas for future consideration and were not included as BIAs. Boundary Certainty and Spatiotemporal Variability (dynamic, ephemeral, or static) were assigned for each BIA, using the same rules across BIA types, and were independent of the Intensity and Data Support scores.
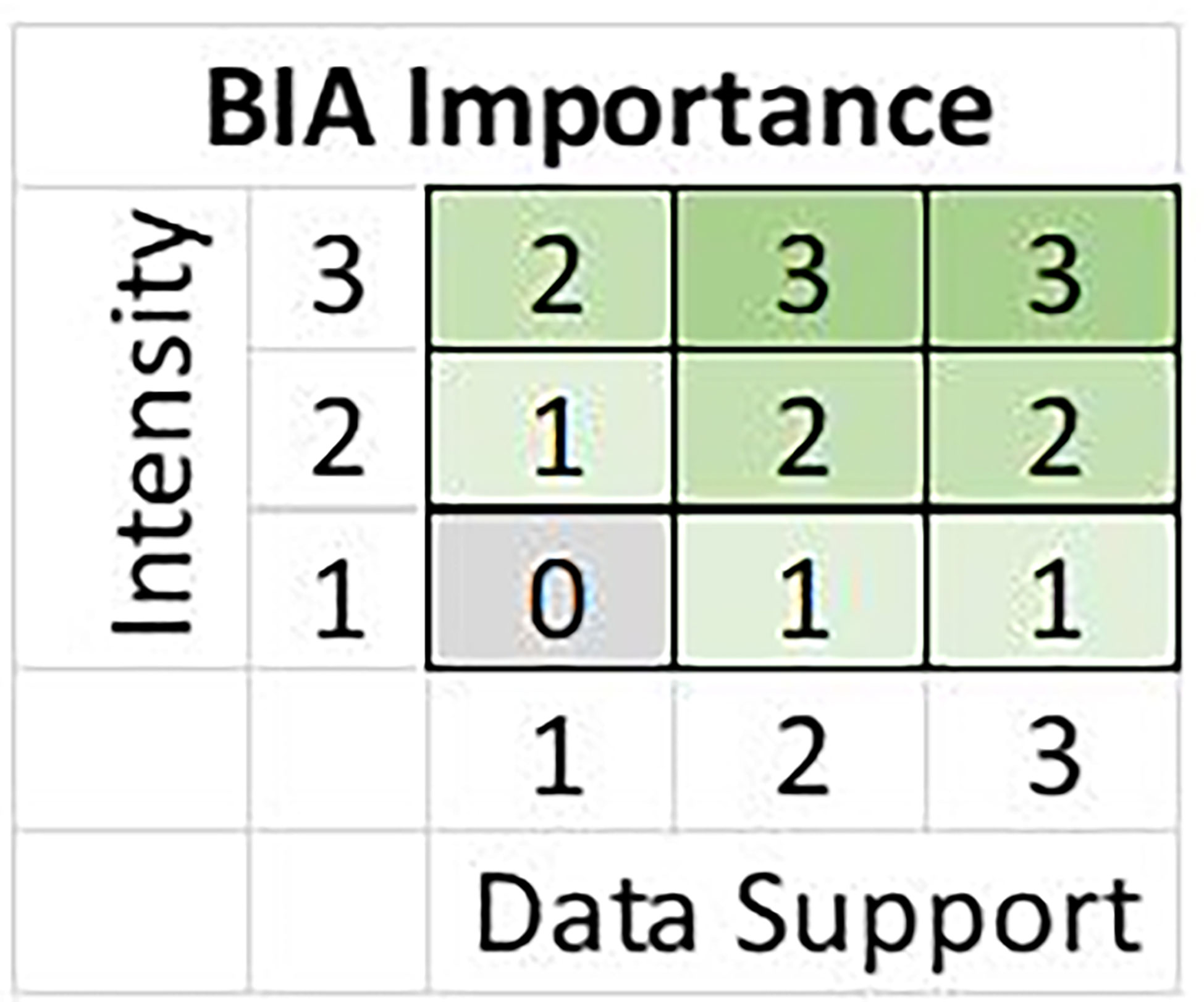
Figure 2 Importance matrix illustrating the relationship between Intensity and Data Support in determining the Importance score for all BIA types.
The definition of a BIA unit was expanded for this BIA II process. In the simplest case, a BIA unit corresponds to a single polygon and one continuous period within which a species engages in a particular biologically important activity, or it corresponds to the range of a small resident population. However, multiple non-contiguous polygons of the same type of BIA for a species could exist in a single region and period. In this case, a “cluster” of BIA polygons could be delineated, scored, and labeled as a single unit, regardless of whether they share common boundaries, as long as the scores for all metrics were identical across all polygons in the cluster. Another new feature of this BIA II process was the option to identify “hierarchical” BIAs for cases in which high-resolution information is available and it is appropriate and helpful to reflect a gradation in animal use (Intensity), available information (Data Support), Boundary Certainty, or ecological characteristics (Spatiotemporal Variability) across a broader area. For example, in some cases data may support a single core area (a “child” BIA) identified within the larger “parent” BIA. In other cases, one or more clusters of variably scored polygons may appropriately be identified as child BIAs within a larger parent BIA. For R-, F-, and M-BIAs, the Intensity score for the parent BIA must be less than the highest Intensity score among the child BIAs. For S-BIAs, when hierarchical scoring is used to identify core habitat within the population’s range, the Intensity score may be the same for the core habitat (the child BIA) and the overall range (the parent BIA), as S-BIAs have quantitative scoring protocols and the parent BIA could score a 3.
Each BIA unit (individual, parent, child, cluster, and watch list) has a label, which identifies the BIA type, Importance score, Spatiotemporal Variability, Boundary Certainty score, region, identification number, and suffix that indicates hierarchical or non-hierarchical structure, in that order (e.g., Supplementary Table 3). The Intensity and Data Support scores underlying the Importance score are not included in the label but are indicated in the metadata for each BIA. For example, a BIA with label “R-BIA3-d-b2” refers to a reproductive (R-) BIA with the highest (3) of three possible Importance scores, generally dynamic (d) in nature, with medium confidence (b2) in the accuracy of the boundary delineation itself. The BIA II effort did not artificially limit coverage to areas only within the U.S. EEZ. Where reliable information was available for areas beyond the U.S. EEZ, BIAs in the Arctic region extended into transnational waters in the east (Canada) and west (Russia) and international waters north of the U.S. EEZ. Furthermore, areas completely outside of the U.S. EEZ were also considered if reliable information was available and the areas in question were of potential importance for species occurring seasonally in U.S. Arctic waters.
The BIA II effort applied principles of expert elicitation to create a more structured and consistent manner for the identification, delineation, and scoring of BIAs across regions, as well as to ensure that information that was not incorporated during BIA I (e.g., Indigenous knowledge) was included. Expert elicitation is a formal, structured process for obtaining experts’ opinions and knowledge to help inform decision-making, particularly in an information-limited situation. The framework for expert elicitation included wider-ranging information solicitation: extensive communication of purpose, intention, and protocols; clearer documentation of methods; and extensive consistency review. Additional details on expert elicitation are included in Harrison et al. (in review).
Information used to identify and score Arctic region BIAs included peer-reviewed publications, gray literature, raw data, spatial optimization modeling (Ferguson et al., in review), expert elicitations, Indigenous knowledge, and subsistence harvest records. Arctic region BIA delineation benefited from numerous recent (since 2000) multiyear studies that focused specifically on marine mammal occurrence in areas of the Chukchi and Beaufort seas where offshore oil and gas exploration was occurring or planned (e.g., U.S. Department of the Interior, 2008; U.S. Department of the Interior, 2018). These studies, including aerial line transect surveys, satellite telemetry, and systematic vessel surveys, provided extraordinary databases to support BIA II delineation and resulted in more than 100 individual BIAs, including hierarchical (parent and child BIAs), cluster, transboundary (with the Aleutian Islands-Bering Sea region), and transnational (extending beyond U.S. EEZ waters) and international (completely outside of any national EEZ) BIAs, as well as watch list areas. Nine experts familiar with Arctic cetacean species through field work and data analysis provided data and personal observations and helped interpret information for the BIA II assessment.
One primary data source for Arctic BIAs was aerial line transect surveys conducted from summer (July-August) through autumn (September-October) in the northeastern Chukchi and western Beaufort seas that collected visual sighting data under the auspices of the Aerial Surveys of Arctic Marine Mammals (ASAMM) project (e.g., Clarke et al., 2020). Although spatiotemporal variation in effort occurred over the course of the ASAMM study, no other single research project in the Alaskan Arctic compares with the spatiotemporal scope of ASAMM. The ASAMM project was conducted from 1982-2019; however, data used for BIA II focused on surveys conducted from 2000-2019 which corresponds to the “new Arctic” regime, when sea ice loss driven by atmospheric processes has accelerated (Wood et al., 2013; Frey et al., 2015). Another primary source of information was bowhead whale satellite telemetry data collected from 2006-2018 (Citta et al., 2021), which provided year-round data on the movements of over 70 whales. A third primary source of data was systematic vessel surveys conducted from 2009-2019 as part of the Distributed Biological Observatory (DBO) project (Grebmeier et al., 2019), which contributed valuable cetacean sighting data during the open water season in areas that were difficult to cover with aerial surveys. Additionally, Arctic BIA II delineation and scoring efforts benefitted from smaller-scale aerial survey projects (e.g., Mocklin et al., 2012; Harwood et al., 2014; Hornby et al., 2016), ice- and shore-based observations (e.g., George et al., 2004; Noongwook et al., 2007; Huntington and Quakenbush, 2009a; Huntington and Quakenbush, 2009b; Melnikov, 2019), Indigenous knowledge (e.g., Galginaitis, 2014; Collings et al., 2018; Noongwook et al., 2007; Huntington and Quakenbush, 2009a; Huntington and Quakenbush, 2009b), aboriginal subsistence harvest records (e.g., Suydam et al., 2011; Suydam et al., 2012; Suydam et al., 2013; Ilyashenko and Zharikov, 2014; Suydam et al., 2014; Ilyashenko and Zharikov, 2015; Suydam et al., 2015; Ilyashenko and Zharikov, 2016; Suydam et al., 2016; Ilyashenko and Zharikov, 2017; Suydam et al., 2017; Suydam et al., 2018; Suydam et al., 2019; Suydam et al., 2020), passive acoustic monitoring (e.g., Chou et al., 2019; Halliday et al., 2019; Scharffenberg et al., 2019a; Scharffenberg et al., 2019b; Escajeda et al., 2020; Alaska Fisheries Science Center, 2021), and gray whale and beluga satellite telemetry data (e.g., Richard et al., 2001; Suydam et al., 2001; Hauser et al., 2014; Hauser et al., 2015; Hauser et al., 2016; Kennedy et al., 2017; Storrie et al., 2022).
In the following narrative, detailed examples for five Arctic BIAs are presented to illustrate how available information was used to delineate and score them (Supplementary Table 3). These BIAs were selected because they present a variety of BIA types, including hierarchical, cluster, transboundary, transnational, and international BIAs. Each example BIA includes species’ life history and background information, the available information sources that were used to assess BIA status, the process used to delineate the BIA in space and time, and details of how each score was determined.
Information presented here is assumed to represent the Bering-Chukchi-Beaufort (BCB) or Western Arctic stock (Muto et al., 2020) only. BCB bowhead whales are migratory, ranging from subarctic to Arctic waters (Citta et al., 2021). The BCB stock winters primarily in the northwestern Bering Sea. Most of the stock migrates annually in spring (April-May) past the western side of St. Lawrence Island, through the Bering Strait and northeast through the eastern Chukchi Sea, traveling through nearshore leads that develop each year. In the northeastern Chukchi Sea, the lead system is relatively well defined due to warm water transported from the Pacific Ocean, the high percentage of first-year ice compared to multiyear ice, and variable surface winds that move ice towards and away from the coastline (Mahoney, 2012). The migration turns east near Point Barrow, where it crosses the Beaufort Sea in continental slope and Canadian basin waters. Leads in the Beaufort Sea are fewer and more isolated, due to the movement of sea ice parallel to the coastline and the higher percentage of multiyear ice (Mahoney, 2012).
Information used in delineating the April bowhead whale M-BIA (Figure 3) in the western Bering Sea included satellite telemetry (Figure 4.3 in Citta et al., 2021, 50% kernel density), Indigenous knowledge from St. Lawrence Island (Noongwook et al., 2007), aboriginal subsistence harvest data from the villages of Gambell and Savoonga on St. Lawrence Island (Suydam et al., 2011; Suydam et al., 2012; Suydam et al., 2013; Suydam et al., 2014; Suydam et al., 2015; Suydam et al., 2016; Suydam et al., 2017; Suydam et al., 2018; Suydam et al., 2019; Suydam et al., 2020), and shore observations from the village of Sireniki on the Chukotka peninsula (Melnikov et al., 2004). The April bowhead whale M-BIA in the eastern Chukchi and western Alaskan Beaufort seas was delineated using data from satellite telemetry (Figure 3A in Olnes et al., 2020, 50% kernel density; Figure 4.3 in Citta et al., 2021, 50% kernel density), passive acoustic monitoring (Figure 3 in Clark et al., 2015, 9 moorings; Alaska Fisheries Science Center, 2021, moorings BF2, WT1, IC1 CL1, PH1, KZ1), aerial photographic surveys (Mocklin et al., 2012), and Indigenous knowledge from the northeastern Chukchi Sea villages of Wainwright (Huntington and Quakenbush, 2009a) and Utqiaġvik (Huntington and Quakenbush, 2009b), Alaska. Notably, data from ASAMM aerial surveys conducted from 1979-1984 that were used in BIA I were not included here because those data are several decades old, the Arctic ecosystem has undergone considerable charges in recent decades, and newer data were available. This M-BIA extends across the U.S. EEZ into Russian waters and is also transboundary, extending to the Aleutian Islands-Bering Sea region; it was included in the Arctic Region because most of the information available was from the Arctic region.
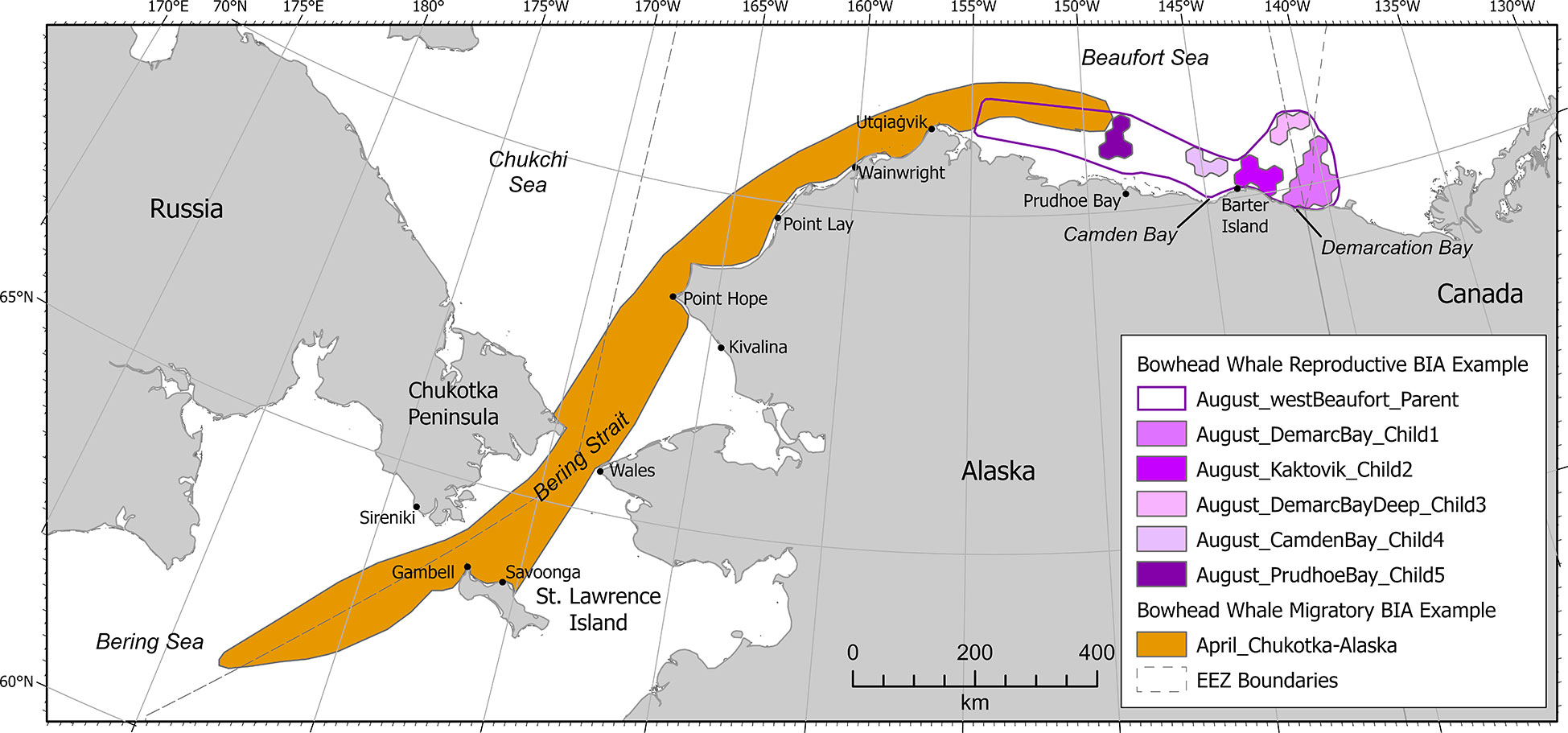
Figure 3 Example Arctic region BIAs showing bowhead whale transboundary-international (Bowhead Whale Migratory) and hierarchical (Bowhead Whale Reproductive) BIAs.
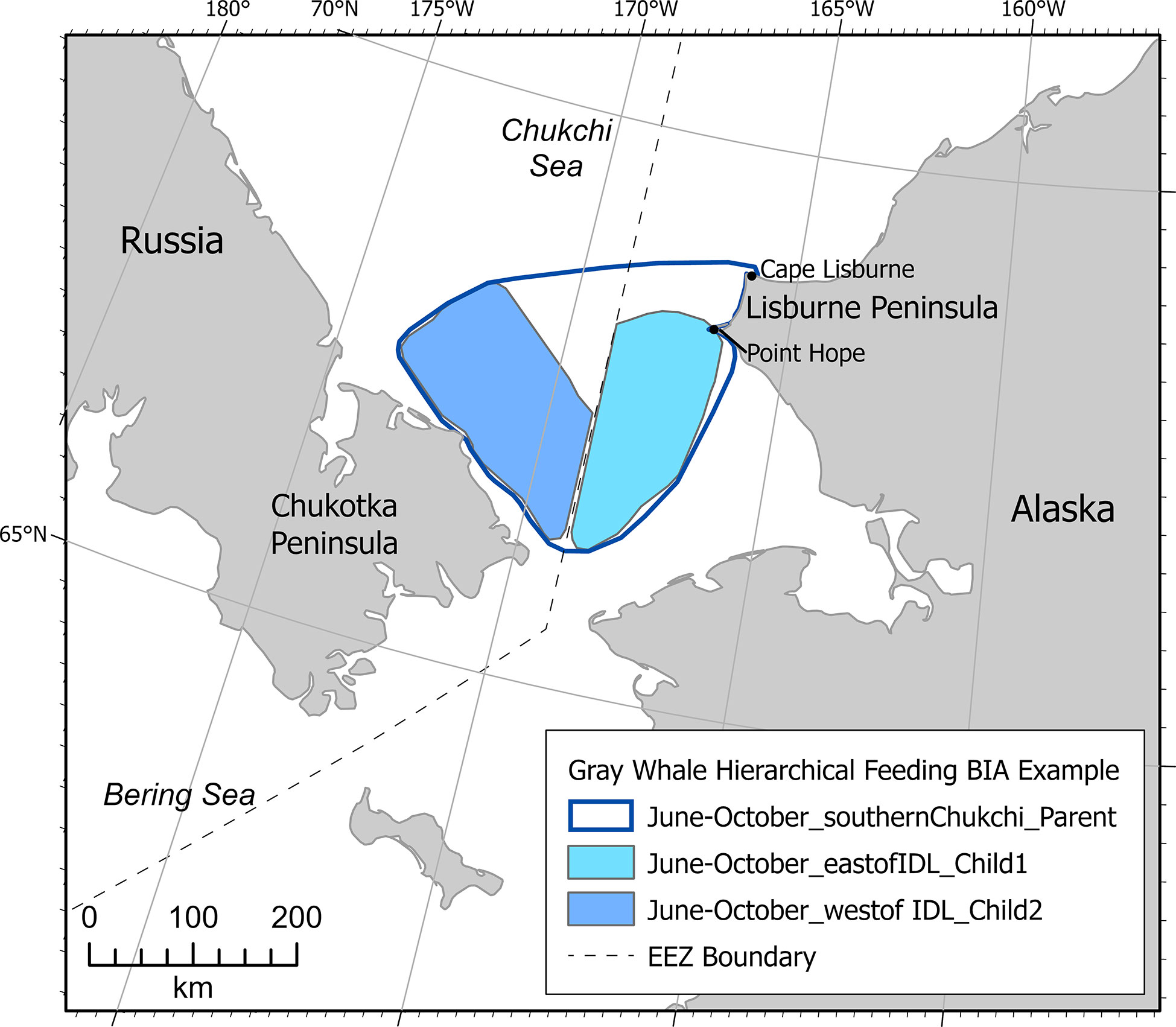
Figure 4 Example Arctic region BIA showing gray whale hierarchical-international (Gray Whale Hierarchical Feeding) BIA.
Scoring the April bowhead whale M-BIA required a stepwise assimilation of information (Supplementary Table 3). First, Intensity was scored as moderate (2) because, although the Bering Strait is the only migration corridor for BCB bowhead whales, the proportion of BCB bowhead whales that migrate through this area in April is unknown relative to other spring/summer months (e.g., March, May, June). Data Support was scored high (3) due to the number and diversity of information sources available for April. Using the Importance matrix (Figure 2) the resulting Importance score was 2. Spatiotemporal variability was characterized as dynamic because, while the migration occurs every year, the timing and location of the migration varies annually due to differences in the formation of spring ice leads. The Boundary Certainty of this BIA was scored high (3) based primarily on the 50% kernel density distribution from satellite telemetry data (Olnes et al., 2020; Citta et al., 2021) augmented by the additional data sources (e.g., Melnikov et al., 2004; Noongwook et al., 2007; Huntington and Quakenbush 2009a; Huntington and Quakenbush 2009b). Given these scores, the label for the bowhead whale April M-BIA is M-BIA2-d-b3-ARC049-0 (Supplementary Table 3).
Bowhead whales calve primarily between the beginning of April and the end of May (Tarpley et al., 2021), although calving may occur as late as August (Koski et al., 1993). Important areas for newborn calves have been described near Wainwright (Huntington and Quakenbush, 2009a) and Utqiaġvik (Huntington and Quakenbush, 2009b) from Indigenous knowledge. The most extensive dataset of BCB bowhead whale calf observations was from ASAMM for the periods July-August 2012-2019 and September-October 2000-2019 (Clarke et al., 2022). During ASAMM surveys, calves of the year were identified based on a combination of characteristics, including noticeably smaller size, lighter gray color, smaller head compared to overall body size, and close association with an adult. The ASAMM dataset was the primary data source for delineating and scoring bowhead whale reproductive BIAs in the western Beaufort Sea and provided high-resolution data that were amenable to hierarchical scoring (Harrison et al., in review).
A hierarchical R-BIA was delineated for bowhead whales in the western Beaufort Sea in August, extending from just east of the U.S.-Canada border to just east of Point Barrow (Figure 3). ASAMM calf sightings and survey effort were analyzed using a spatially explicit optimization model (Ferguson et al., in review) to assist with BIA delineation and scoring. To identify the optimal BIA configuration that resulted in the maximum number of bowhead whale calves within the smallest total area, the model selected hexagonal cells arranged on a lattice (25 km between cell midpoints) that covered the western Beaufort Sea study area. The model input three parameters: relative density of bowhead whale calves per cell during August 2012-2019; contiguity threshold, defined as the minimum size of a single BIA polygon or cluster of cells, ranging from one to five contiguous cells; and total area threshold, defined as the proportion of cells with calves in the study area that were enclosed by BIA boundaries, ranging from 0.1 to 1.2, in 0.1 increments (see Ferguson et al., in review). The optimal solutions to each combination of contiguity threshold and total area threshold were mapped and visually inspected by experts with 14-28 years of experience conducting ASAMM surveys in the western Beaufort Sea. The experts selected a single scenario (i.e., contiguity threshold and total area threshold) comprising clusters of contiguous cells, and each of these clusters was defined as a child BIA (Harrison et al., in review). Each child BIA was scored independently for Intensity, Data Support, Importance, Spatiotemporal Variability, and Boundary Certainty. Ferguson et al. (in review) examined the interannual variability in the relative density of calves in each BIA cluster using a generalized linear mixed effects model. Intensity was based on the parameter estimates from Ferguson et al.'s (in review) generalized linear mixed effects models: the fixed intercept was a function of the overall mean relative density of calves, and the standard deviation (SD) in the random effect for year reflected the deviation from the mean relative density of calves that can be attributed to interannual variability. Intensity was scored high (3) for child BIAs with high fixed intercept and low SD; moderate (2) for child BIAs that had moderate fixed intercept and SD, and for clusters that did not fit cleanly into high or low Intensity; and low (1) for child BIAs with low fixed intercept and high SD (Supplementary Table 3). Data Support was scored high (3) for all child BIAs due to the comprehensive survey coverage from ASAMM in August 2012-2019. The resulting Importance scores ranged from high (3) for three of the child BIAs (near Barter Island, north of Camden Bay, and north of Prudhoe Bay); moderate (2) for one child BIA (offshore north of Barter Island); and low (1) for one child BIA (north of Demarcation Bay). Spatiotemporal variability was labeled dynamic for all child BIAs because, while calves use the western Beaufort Sea in August every year, calf sighting location varies annually. Boundary Certainty for all child BIAs was scored high (3) due to the extensive information in the ASAMM database and the quantitative delineation methods described above. The delineation of the parent R-BIA for August included >90% of all bowhead whale calves sighted during ASAMM in August 2012-2019, regardless of whether the sightings were part of a child BIA group, and incorporated all five child BIAs identified in the process described above. As outlined in Harrison et al. (in review), the Intensity score of the parent R-BIA must be less than the highest Intensity for any child BIA.
The hierarchical delineation and scoring method described above for bowhead whale R-BIAs in the western Beaufort Sea in August was also used for bowhead whale R-BIAs in the western Beaufort Sea in July, September, and October, and for bowhead whale F-BIAs (feeding BIAs) in the western Beaufort Sea in July through October.
The information presented here is assumed to represent the Eastern North Pacific (ENP) stock of gray whales (Muto et al., 2020) only. There is no evidence that gray whales from the Western North Pacific stock summer in the U.S. Arctic. Gray whales of the ENP stock migrate each spring from Baja California, Mexico, along the west coast of the U.S. and Canada, across the Gulf of Alaska and into the Bering, Chukchi, and extreme western Beaufort seas (west of 155°W). Gray whales are occasionally seen farther east in the Beaufort Sea, but their occurrence there is probably extralimital. Gray whales remain in the U.S. Arctic throughout summer and early autumn before making a return migration south. The predominant behavior of gray whales in Arctic waters is feeding (Clarke et al., 2016; Brower et al., 2017; Moore et al., 2022). Benthic feeding is easily identified via the presence of mud plumes visible at the surface that are produced when whales surface after feeding on benthic or epibenthic species (Nerini, 1984). However, gray whales are generalist feeders and not limited to benthic or epibenthic prey (e.g., Bluhm et al., 2007). Therefore, mud plumes may not always accompany gray whale feeding events, leading to an overall underestimation of feeding behavior in the ASAMM database. The ASAMM project documented gray whale feeding in the eastern Chukchi Sea from summer through autumn with moderate variability in feeding location within these seasons (Clarke et al., 2020). The two main areas for gray whale feeding were in the northeastern Chukchi Sea within about 120 km of shore from Icy Cape to Point Barrow, Alaska, (Moore et al., 2022) and in the southern Chukchi Sea southwest of Point Hope, Alaska. These areas were delineated as BIAs previously (Clarke et al., 2015b), although the southern area was truncated west of the U.S. EEZ.
In BIA II, a hierarchical feeding BIA was delineated for gray whales in the southern Chukchi Sea for June through October that extends from the Lisburne peninsula in Alaska across the U.S. EEZ to the Chukotka peninsula (Figure 4). The optimization model described for defining bowhead whale R-BIAs was not available for gray whales. Rather, data used in delineating the June-October gray whale F-BIA child east of the U.S. EEZ included gray whale sightings during eleven years of ASAMM surveys in 2009-2019 (Clarke et al., 2020), eleven years of sightings during standardized marine mammal watches during DBO cruises (Grebmeier et al., 2019; IUCN, 2021), and passive acoustic data during seven years of year-round monitoring (Alaska Fisheries Science Center, 2021). Data used for the June-October gray whale F-BIA child west of the U.S. EEZ included two years of DBO sightings in 2009-2010 (Grebmeier et al., 2019; IUCN, 2021).
Intensity was scored as moderate (2) for both child BIAs because, while these areas are important for gray whale feeding, there are known gray whale feeding areas elsewhere in the northeastern Chukchi Sea and in the Bering Sea (Brower et al., 2022) that overlap temporally. Data Support and Boundary Certainty were very different for the two child BIAs, however (Supplementary Table 3). Both values were scored high for the child BIA within the U.S. EEZ due to the preponderance of standardized data (e.g., ASAMM, passive acoustic) outlined above, and low for the child BIA west of the U.S. EEZ due to the comparative lack of standardized data. Note that there are data available for gray whale feeding nearshore along the Chukotka peninsula coast (e.g., Heide-Jørgensen et al., 2012; Blohkin et al., 2013; Blokhin et al., 2017; Zdor, 2021) but those data differ spatiotemporally from this child BIA and were used to support a completely unique F-BIA (https://oceannoise.noaa.gov/biologically-important-areas). Spatiotemporal variability was designated ephemeral because, while both areas are used for feeding every year, feeding locations within these areas may change annually due to changes in prey abundance (Moore et al., 2022). The delineation of the June-October gray whale parent F-BIA included the two child BIAs identified in the process described above and >90% of all ASAMM gray whale sightings south of Cape Lisburne, Alaska, from June-October. As outlined in Harrison et al. (in review), the Intensity score of the parent R-BIA must be less than the highest Intensity for any child BIA. Note that data from ASAMM aerial surveys conducted from 1979-1984 that were used in BIA I were not included here because those data are several decades old, evidence suggests that the ecosystem has changed in recent decades (e.g., Grebmeier, 2012), and newer data were available.
Two stocks of belugas are found in the Pacific Arctic: the Beaufort Sea (BS) or Mackenzie stock, and the Eastern Chukchi Sea (ECS) stock (Muto et al., 2020). Both stocks winter in the Bering and southern Chukchi seas. Migration north through the Chukchi Sea and east through the Beaufort Sea is stock-specific, occurring in spring (BS) and summer (ECS). BS belugas calve and molt in June and July in the Mackenzie River estuary, Yukon and Northwest Territories, Canada (Department of Fisheries and Oceans, 2000; Richard et al., 2001). Satellite telemetry data indicated that BS belugas tagged in the Mackenzie River delta stayed in the Canadian Beaufort Sea for the entire month of July and most of August, in an area extending from the Mackenzie delta east into Amundsen Gulf and north to Viscount Melville Sound (Richard et al., 2001). BS belugas migrate into the western Beaufort Sea and northern Chukchi Sea in September and are found primarily in the Chukchi Sea in October (Hauser et al., 2016). There is some overlap in ECS and BS beluga ranges in September and October, but core areas do not overlap (Hauser et al., 2014; Hauser et al., 2016).
A cluster R-BIA was delineated for BS belugas from mid-June to July in the Mackenzie River estuary (Figure 5). Even though this R-BIA is entirely outside of the U.S. EEZ, it was included in the BIA II effort because the Mackenzie River estuary is the only known reproductive region for this stock that migrates through U.S. EEZ waters in spring and autumn. Six aggregation areas (defined by 50% kernel density) were defined based on data collected during strip transect aerial surveys from late June-July 1977-1985, and 1992 (Harwood et al., 2014). The same aerial survey data were used in analyses of seabed habitat (Whalen et al., 2019) showing use of sandy shoal habitat (perhaps for molting) and avoidance of deep channel habitat. Additional aerial survey data collected in 2012 and 2013 indicated that use of the estuary remained spatiotemporally similar to estuary use in 1977-1992 and expanded on associations between belugas’ use of the estuary and the breakup of land-fast ice (Hornby et al., 2014), and associations with turbidity (Hornby et al., 2016). Visual sightings and passive acoustic detections from June to August 2017 indicated that belugas did not use the aggregation areas identified in Harwood et al. (2014) during periods of strong winds and also indicated a preference for warmer, fresher water rather than colder, saltier water (Scharffenberg et al., 2019a).
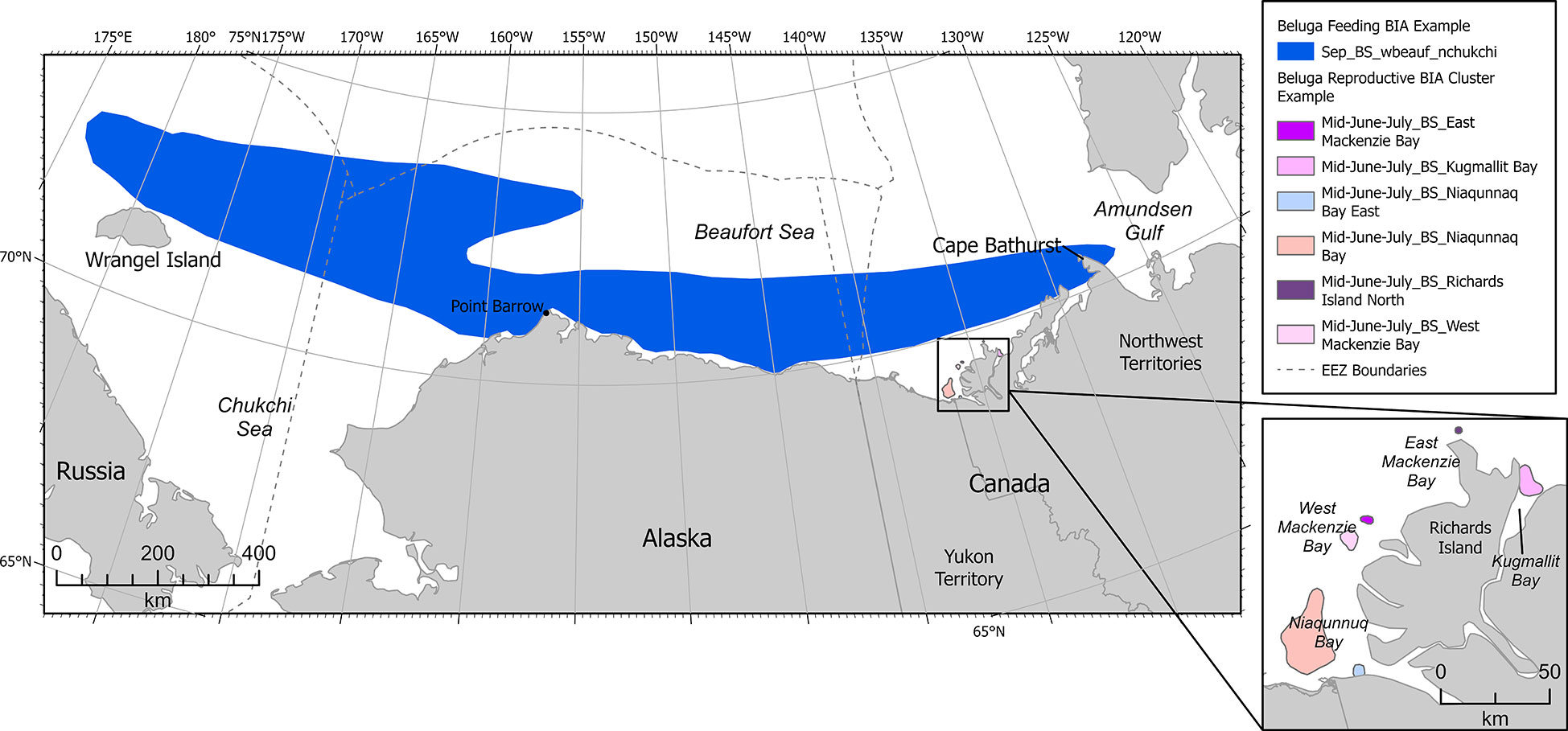
Figure 5 Example Arctic region BIAs showing beluga international (Beluga – Beaufort Sea Stock - Feeding) and cluster (Beluga – Beaufort Sea Stock - Reproductive BIA, inset map) BIAs.
Because 50% kernel densities from analyses of aerial survey data were identified for each of the six individual clusters within a continuous 1.5-month period, all Intensity, Data Support, and Boundary Certainty scores were identical, and a hierarchical R-BIA was not necessary. Each cluster, including Niaqunnuq Bay, Niqunnuq Bay East, West Mackenzie Bay, East Mackenzie Bay, Richards Island North, and Kugmallit Bay, was scored high (3) Intensity and high (3) Data Support, resulting in a high (3) Importance score (Supplementary Table 3). Boundary Certainty was also high (3) and spatiotemporal variability was designated dynamic due to annual variation in spring land-fast ice breakup and access to the estuary.
Occasionally, BS belugas, including newborn calves, have been observed upriver in the Mackenzie Delta (Scharffenberg et al., 2020). These occurrences have all been observed under high-water level conditions and are comparatively rare. This may be an important R-BIA for BS belugas, but presently both Intensity and Data Support were scored low (1), making this a watch list area. Additional information on watch list areas is included in Section 3.3.
Feeding behavior in belugas can be inferred from stomach content analysis (Loseto et al., 2018; Quakenbush et al., 2015), dive patterns from satellite telemetry, direct observations (although rare), and studies of the distribution and abundance of known beluga prey species. Both ECS and BS belugas are known to feed on a wide variety of prey (Quakenbush et al., 2015). Satellite telemetry indicated that, while BS belugas in shallow shelf regions dive to the seafloor to forage on invertebrates (e.g., shrimp and echiurid worms), pelagic dives were frequently to ~300 m, suggesting that belugas dive to depths to maximize prey encounters (Hauser et al., 2015). Choy et al. (2020) found that BS belugas in offshore areas primarily consume Arctic cod (Boreogadus saida). Therefore, although direct observations of feeding by belugas in offshore areas are extremely rare, dive data exist to support the designation of most beluga BIAs in the offshore areas of the Beaufort and Chukchi seas as F-BIAs.
The F-BIA designated for BS belugas in September extended from Cape Bathurst, Northwest Territories, Canada, to north of Wrangel Island, Russia (Figure 5). This extraordinarily large F-BIA was based primarily on the 50% utilization distribution (core area) (Figure 2 in Halliday et al., 2021) that used satellite telemetry data combined from two periods, 1993-1997 (Richard et al., 2001) and 2004-2006 (Hauser et al., 2014). Dive data within the core area indicated that foraging was the principal behavior. Satellite telemetry data were augmented by aerial line transect survey data collected during ASAMM in September 2009-2019 in the western Beaufort and eastern Chukchi seas (Clarke et al., 2018; Clarke et al., 2020; Givens et al., 2020).
Intensity for this transnational and international F-BIA (encompassing waters in the Canadian, U.S., and Russian EEZs, and beyond the U.S. and Russian EEZs; Figure 5) was scored moderate (2) (Supplementary Table 3) because the home range of BS belugas (shown as the 90-95% utilization distribution) far exceeds the core area boundary, sometimes extending several hundred kilometers farther offshore (Hauser et al., 2014; Hauser et al., 2016), and feeding occurs throughout the home range. Data Support was scored high (3) because of the combination of satellite telemetry data from 1993-2006 (and subsequent analyses) and eleven years of cetacean-focused aerial surveys. The overall Importance score was moderate (2). Boundary Certainty was also scored moderate (2) and Spatiotemporal Variability was designated ephemeral because feeding opportunities are contingent on oceanographic phenomena that may differ between years, although Majewski et al. (2017) indicated that depth, a static variable, was the strongest parameter in fish community structure in the Canadian Beaufort Sea.
In the Arctic region, 44 BIAs were designated for five species, including two stocks of belugas (Supplementary Table 4). Bowhead whales accounted for the highest percentage (48%, n=21) of BIAs, followed by BS belugas (18%, n=8) and ECS belugas (16%, n=7). Of the 44 total BIAs, 13 BIAs were hierarchical, resulting in an additional 44 child BIAs (Supplementary Table 4). This is the highest BIA total for any of the seven BIA II regions; the next closest total BIA count was for the Hawaii region with 39 BIAs (including 16 child BIAs). The total number of Arctic BIAs is nearly three times as many BIAs as delineated during BIA I (Clarke et al., 2015b). The designation of BIAs in the Arctic benefitted from the numerous comprehensive, multiyear, cetacean-focused studies undertaken since the early 2000s which allowed for the specificity needed to differentiate BIAs between months. The Arctic region also borders Canadian and Russian EEZs on the east and west frontiers, respectfully, and 15 migratory, feeding, and reproductive BIAs were transboundary. Some BIAs (n=10) were completely outside of the U.S. EEZ but were included because the research that supported those BIAs also yielded BIAs completely or partially in U.S. EEZ waters and it would have been non-comprehensive to exclude them. Three BIAs extended into international waters north of the U.S. and Russian EEZs.
The most common type of BIA in the Arctic region was feeding area, representing 52% (n=46) of all BIAs, followed by reproductive area (39%, n=34) (Supplementary Table 5). Relatively few migratory route BIAs (9%, n=8) were identified. Migratory route and reproductive BIAs were delineated for belugas and bowhead and gray whales only. Spatiotemporal variability for feeding BIAs was overwhelmingly categorized as ephemeral, underscoring the patchiness of feeding opportunities based on physical and biological factors for most species.
Due to the number of BIAs delineated for the Arctic (Supplementary Table 6), this summarization of BIAs for each species or stock is necessarily succinct. Details for each Arctic BIA are included in Supplementary Information Tables and graphic representations for each BIA and are available on the BIA website (https://oceannoise.noaa.gov/biologically-important-areas).
Bowhead whale F-BIAs in the Arctic (n=9, not including child BIAs) were primarily located in the Beaufort Sea and Amundsen Gulf, with one BIA situated along the northern Chukotka peninsula coast (Figure 6). Temporally, bowhead whale F-BIAs in the Arctic region encompassed May-December. Most of the data supporting bowhead whale F-BIA scoring originated from ASAMM aerial line transect surveys in the western Beaufort Sea and an optimization model that incorporated ASAMM survey effort and feeding and milling whales (Ferguson et al., in review), while boundary and temporal parameters were supported by both aerial survey and satellite telemetry data (Citta et al., 2015; Citta et al., 2021; Halliday et al., 2021). Bowhead whale F-BIAs were designated for the Aleutian Islands-Bering Sea region for December through April (Brower et al., 2022).
Bowhead whale M-BIAs in the Arctic (n=5) extended from Amundsen Gulf in the east to the Chukotka peninsula in the west and south through the Bering Strait (Figure 7). Some M-BIAs overlapped spatiotemporally with F-BIAs, particularly in August-September in the western Beaufort Sea. Feeding behavior in the western Beaufort Sea is ephemeral and can differ dramatically between years (Ferguson et al., 2021), accounting for as little as 10% to over 50% of whales seen annually from 2012-2019 (Clarke et al., 2013a, Clarke et al., 2014; Clarke et al., 2015a; Clarke et al., 2017a; Clarke et al., 2017b; Clarke et al., 2018; Clarke et al., 2019, Clarke et al., 2020). Most bowhead whales observed in any given year in the western Beaufort Sea were not feeding, however, supporting delineation of M-BIAs.
Bowhead whale R-BIAs in the Arctic (n=7, not including child BIAs) were also located primarily in the Beaufort Sea (Figure 8). Most bowhead whale R-BIAs relied on an optimization model that used ASAMM data (Ferguson et al., in review), described previously in Section 2.4.2. R-BIAs designated for spring and early summer (April to mid-June) near Wainwright and Utqiaġvik relied on Indigenous knowledge (Huntington and Quakenbush, 2009a; Huntington and Quakenbush, 2009b) and a photographic aerial survey (Mocklin et al., 2012).
Gray whale F-BIAs in the Arctic (n=3, not including child BIAs) were located entirely in the Chukchi Sea, with one F-BIA situated along the northwestern Alaska coast and two F-BIAs in the southern Chukchi Sea (Figure 9). Temporally, gray whale F-BIAs in the Arctic encompassed May-November. The gray whale hierarchical F-BIA in the southern Chukchi was described in Section 2.4.3. The gray whale hierarchical F-BIA for the northeastern Chukchi Sea was based primarily on data from ASAMM aerial line transect surveys conducted from 2009-2019 (Clarke et al., 2020), with additional data support from vessel sightings (Grebmeier et al., 2019; IUCN, 2021; Alaska Fisheries Science Center, 2021), passive acoustic monitoring (Alaska Fisheries Science Center, 2021), and limited satellite telemetry (one whale in 2012, four whales in 2013; Kennedy et al., 2017). The F-BIA along the Chukotka peninsula coast was based on aboriginal subsistence harvest data from several Chukotka villages (Blohkin et al., 2013; Ilyashenko, 2013; Ilyashenko and Zharikov, 2014; Blokhin and Litovka, 2015; Ilyashenko and Zharikov, 2015; Ilyashenko and Zharikov, 2016; Blokhin et al., 2017; Ilyashenko and Zharikov, 2017; Ilyashenko, 2018; Zagrebelnyy, 2018; Zharikov, 2018; Zharikov et al., 2019; Zharikov et al., 2020) and a summary of present-day Chukotka whaling (Zdor, 2021). Most of the gray whales taken during Chukotka subsistence hunts for which stomach content data were available had full or half-full stomachs, indicating that gray whales were actively feeding in the area (Blohkin et al., 2013; Blokhin and Litovka, 2015; Blokhin et al., 2017).
Gray whale R-BIAs in the Arctic (n=2) were located entirely in the eastern Chukchi Sea (Figure 10). Gray whales from the ENP stock calve mainly in the protected lagoons of Baja California from early January through mid-February (Rice et al., 1984). Gray whale calves grow quickly in the first year, increasing in length from ~4.5 m at birth to ~7 m at weaning, which occurs at 7-9 months (Sumich, 1986). Because growth slows considerably after weaning, two-year old gray whales may be only 8 m in length, which makes differentiating them from calves-of-the-year difficult. Gray whale R-BIAs in the Arctic were based entirely on visual data from ASAMM aerial line transect surveys from 2009-2019 (Clarke et al., 2020); there were no other known sources of information specific to gray whale calf occurrence. Gray whale calves were identified in the ASAMM database as whales that were appreciably smaller in size and in close association with an adult. R-BIAs were designated separately for July-August and September-October based on differences in Intensity and spatial extent.
Additional gray whale BIAs were designated for the Aleutian Islands-Bering Sea (Brower et al., 2022) and Gulf of Alaska (Wild et al., in review) regions.
Humpback whale F-BIAs in the Arctic (n=2, not including child BIAs) were located entirely in the southcentral Chukchi Sea (Figure 11). One BIA was transnational and one BIA was located entirely in Russian waters. Temporally, humpback whale F-BIAs in the Arctic encompassed July-October. The humpback whale hierarchical F-BIA was based primarily on data from ASAMM aerial line transect surveys conducted from 2009-2019 (Clarke et al., 2013b; Brower et al., 2018; Clarke et al., 2020), with additional data support from vessel sightings from 2009-2019 (Grebmeier et al., 2019; IUCN, 2021), and passive acoustic monitoring from 2012-2018 (Alaska Fisheries Science Center, 2021) for the child BIA within the U.S. EEZ. Data collected from DBO vessel surveys in 2009-2011 (Grebmeier et al., 2019; IUCN, 2021) were the only data available for the child BIA west of the U.S. EEZ. The small F-BIA delineated for the Chukotka peninsula just north of Bering Strait was supported by data from shore-based observations during October in some years between 1993-2012 (Mel'nikov, 2000; Melnikov, 2019).
Additional humpback whale F-BIAs were designated for the Aleutian Islands-Bering Sea (Brower et al., 2022) and Gulf of Alaska (Wild et al., in review) regions.
The fin whale F-BIA in the Arctic (n=1, not including child BIAs) was located entirely in the southcentral Chukchi Sea (Figure 11) and extended into the Russian EEZ. This hierarchical BIA extended spatially from west of Wainwright to the Bering Strait and temporally from July to October. ASAMM aerial line transect surveys conducted from 2009-2019 (Brower et al., 2018; Clarke et al., 2013b; Clarke et al., 2020) were the principal data set for the two northernmost child BIAs, with additional data support from passive acoustic monitoring from 2007-2018 (Delarue et al., 2013; Escajeda et al., 2020; Furumaki et al., 2021; Tsujii et al., 2016; Alaska Fisheries Science Center, 2021) and vessel sightings (Grebmeier et al., 2019; IUCN, 2021) for the southernmost child BIA. There are no recent data for fin whales west of the U.S. EEZ; information on fin whale distribution and occurrence from Soviet-era whaling in the 1960s and 1970s (e.g., Ivashin and Votrogov, 1982; Ivashchenko et al., 2013; Votrogov and Ivashin 1980) were considered outdated for F-BIA purposes. A single acoustic detection of a fin whale near Point Barrow in 2012 (Crance et al., 2015) did not meet BIA criteria.
Additional fin whale F-BIAs were designated for the Aleutian Islands-Bering Sea (Brower et al., 2022) and Gulf of Alaska (Wild et al., in review).
Beluga F-BIAs for BS and ECS stocks in the Arctic (n=9) were delineated from eastern Amundsen Gulf and Viscount Melville Sound to the western Chukchi Sea, and were the most spatially extensive of all Arctic BIAs (Figure 12). Temporally, F-BIAs in the Arctic for both stocks encompassed July-October. Data supporting beluga F-BIA scoring, boundary, and temporal parameters originated primarily from satellite telemetry studies (Richard et al., 2001; Suydam et al., 2001; Hauser et al., 2014; Hauser et al., 2016; Halliday et al., 2021; Storrie et al., 2022), augmented by passive acoustic monitoring (Halliday et al., 2018; Stafford et al., 2018) and aerial surveys (Harwood and Kingsley, 2013; Hornby et al., 2017; Clarke et al., 2018; Clarke et al., 2020). At first glance, it appears that there is substantial overlap between the two stocks, but a close examination of Figure 12 reveals the spatiotemporal distinctions between the BS and ECS stocks (Hauser et al., 2014; Hauser et al., 2016).
Beluga M-BIAs in the Arctic (n=3) delineated important areas for the BS stock in spring and late autumn, and the ECS stock in autumn only (Figure 13). As discussed above in the example BS F-BIA, belugas are assumed to be feeding most of the time, so relatively few M-BIAs were defined; it is highly likely that belugas feed during the times and areas identified here as M-BIAs. Autumn M-BIAs were delineated based primarily on data from satellite telemetry (Hauser et al., 2014; Hauser et al., 2016) boundaries used the 50% utilization density.
Beluga R-BIAs in the Arctic (n=3) were located near Kasegaluk Lagoon in the northeastern Chukchi Sea for ECS belugas, and in the Mackenzie estuary in the eastern Beaufort Sea for BS belugas (Figure 14). The latter area was discussed in detail in Section 2.4.4. There is surprisingly little direct information specific to calving for the ECS beluga stock. This stock returns annually to the area from Omalik Lagoon to Kasegaluk Lagoon and is observed molting and feeding at inlets between barrier islands; the assumption is that calving occurs primarily near Omalik Lagoon prior to the migration into Kasegaluk (Huntington and the communities of Buckland, Elim, Koyuk, Point Lay and Shaktoolik, 1999). O’Corry-Crowe et al. (2020) indicated that most social aggregations observed in and near Kasegaluk Lagoon were mixed age herds, not adult-calf dyads or adult calf groups which were observed at other known beluga calving areas (e.g., Svalbard, Norton Sound, Mackenzie delta, Cunningham Inlet), but Kasegaluk Lagoon is assumed to provide a more hospitable environment to newborn calves due to predator avoidance and warmer water. The ECS beluga R-BIA was based on ASAMM aerial line transect surveys (Clarke et al., 2020), Indigenous knowledge from the village of Point Lay, Alaska (Huntington and the communities of Buckland, Elim, Koyuk, Point Lay and Shaktoolik, 1999), and aerial surveys conducted in the early 1990s (e.g., Frost and Lowry, 1990; DeMaster et al., 2001). The aerial survey data from the early 1990s were included because, although those data are dated, there is no evidence indicating that ECS beluga calving areas have changed over time.
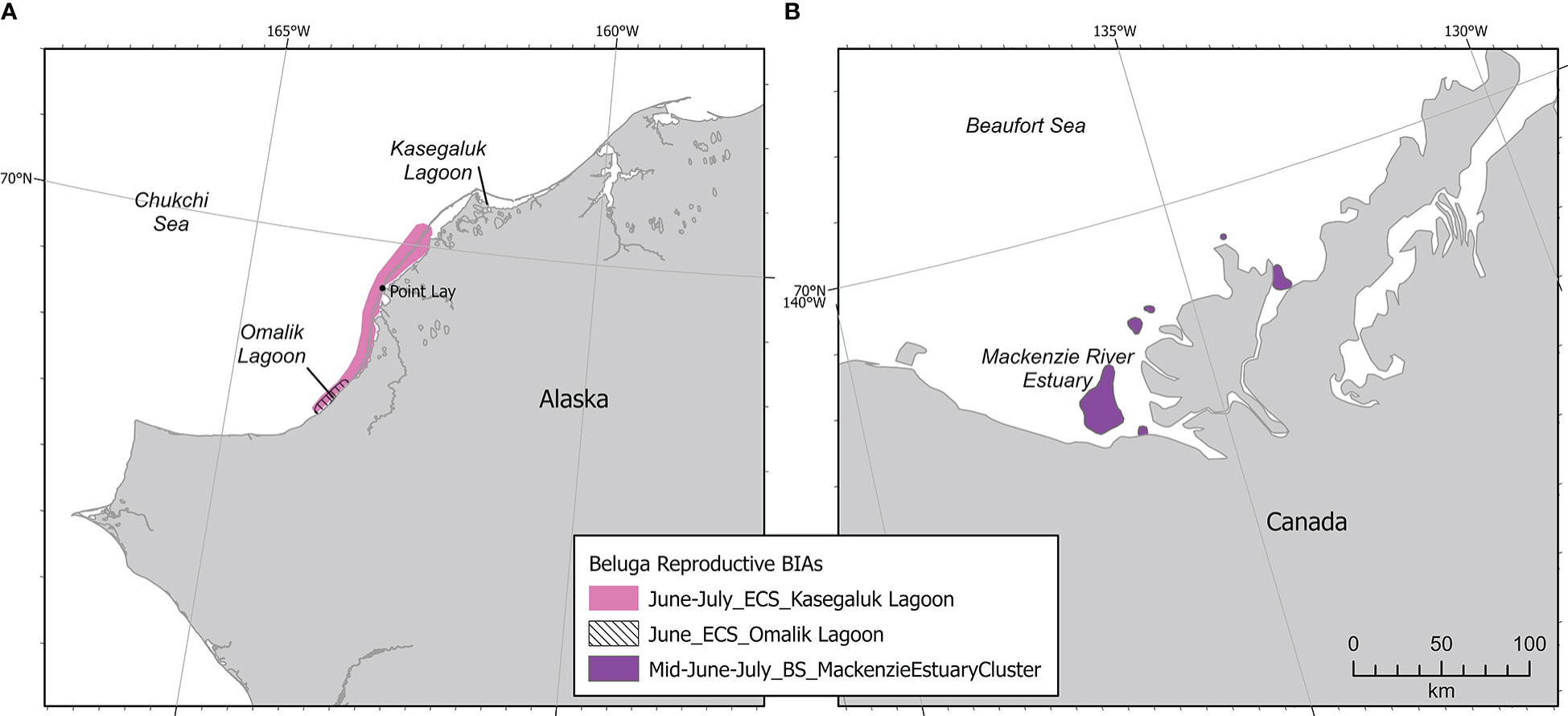
Figure 14 Arctic region beluga reproductive BIAs. (A) Eastern Chukchi Sea Stock; (B) Beaufort Sea Stock.
Fifteen watch list areas (Figure 15) were identified in the Arctic indicating that, while they may be important, the areas currently lack sufficient information to reliably be scored and delineated spatiotemporally (Supplementary Table 7). Arctic watch list areas expand spatial or temporal extents for four species; one area was delineated because it may represent use by an as-yet undefined beluga stock. The two watch list areas in Kotzebue Sound were the only Arctic watch list areas within the U.S. EEZ (Figure 15), representing areas that may be important for belugas in Kotzebue Sound in June and July and harbor porpoises year-round (Castellote et al., 2017; O’Corry-Crowe et al., 2021). Watch list areas beyond the boundaries of the U.S. EEZ included two in the Russian EEZ, nine in the Canadian EEZ, and two in international waters north of the U.S. and Russian EEZs, all of which represent areas of potential importance for species that occur seasonally in U.S. waters. Eight of the watch list areas were for bowhead whales, with half in the northern Chukchi Sea and half in Amundsen Gulf, Canada. Many of the bowhead whale watch list areas were identified based on passive acoustic monitoring via a single hydrophone (e.g., Halliday et al., 2018; Halliday et al., 2019; Halliday et al., 2020; Insley et al., 2021; Moore et al., 2012a; Stafford et al., 2022b) or use of an area by 1-2 satellite tagged whales (Citta et al., 2015; Halliday et al., 2021), so Intensity, Data Support, and spatiotemporal parameters were difficult to determine. Two of the bowhead whale watch list areas in the northern Chukchi Sea were in international waters north of U.S. and Russian EEZs. One watch list feeding area was designated for gray whales in the eastern Beaufort Sea, based on a relatively large number of gray whales (n=15, including one calf) observed there on one day in August 2019 (Clarke et al., 2020). Four watch list areas were designated for BS belugas in Amundsen Gulf or far upriver in the Mackenzie estuary. Most (80%) watch list areas were designated for feeding although, in many cases, the actual behavior was assumed.
Identified BIAs in the Arctic region included feeding areas, reproductive areas, and migratory corridors for bowhead whales and two stocks of belugas; reproductive and feeding areas for gray whales; and feeding areas for humpback and fin whales. Some of the information gaps identified during BIA I for the Arctic region, including bowhead whale use of the western Beaufort Sea in summer, existence of a bowhead whale autumn migratory corridor in the Chukchi Sea, and the extent and nature of beluga use of outer continental shelf and slope habitat in the Beaufort Sea (Clarke et al., 2015b), were resolved during the BIA II delineation process. Other previously identified gaps, including the existence or location of gray whale migratory corridors in spring and autumn and the degree to which gray whales move between known feeding hotspots, remain unanswered. BIAs designated for humpback and fin whales during BIA II were made possible due to broad-scale, multiyear studies (e.g., ASAMM, DBO), but were limited to F-BIAs even though calves have also been observed (Brower et al., 2018; Clarke et al., 2020). Watch list areas were designated for bowhead whales, gray whales, and belugas in the Canadian Beaufort Sea, bowhead whales in the northern Chukchi Sea, and belugas and harbor porpoises in Kotzebue Sound.
Minke whales, killer whales, and harbor porpoises were increasingly observed in the Chukchi Sea starting in 2008 (Brower et al., 2018; Clarke et al., 2013b, Clarke et al., 2020; Stafford et al., 2022a; Willoughby et al., 2020), but visual observations and passive acoustic detections were not as frequent or regular as other cetaceans for which BIAs were designated. A transboundary (Aleutian Islands-Bering Sea and Arctic regions) watch list area was delineated for minke whales (Brower et al., 2022). There is insufficient information to delineate any Arctic BIAs or watch list areas for killer whales. As noted above, a watch list area was delineated for harbor porpoise in a relatively small geographic area in the Arctic. Some of the broad-scale, multiyear studies (e.g., ASAMM) that provided visual observations are no longer occurring, and opportunities for continued data collection on all cetaceans have lessened as interests in offshore oil and gas development in U.S. and Canadian waters have recently waned.
Watch list areas for bowhead whales and BS belugas in Amundsen Gulf (n=8) and bowhead whales in the northern Chukchi Sea (n=4) suggest more extensive use of those areas, spatiotemporally, than previously documented. Visual observations in these areas have been generally limited to the open water season (i.e., July-October) when vessel access is possible and daylight is suitable for visual observations, but passive acoustic monitoring and satellite telemetry have revealed near year-round occurrence in at least some years. As environmental conditions in the Arctic continue to dramatically alter (e.g., diminished sea ice in all seasons), the continuation of passive acoustic monitoring (e.g., Stafford et al., 2022a) and satellite telemetry studies (Citta et al., 2021) that allow for year-round data collection will be paramount. Despite the discontinuation of ASAMM visual surveys, there are several ongoing research efforts, most of which are multidisciplinary, but which include a marine mammal component. The Chukchi Ecosystem Observatory (https://aoos.org/project-page/ecosystems/chukchi-ecosystem-observatory/) includes passive acoustic sampling for marine mammal sounds from year-round moorings. Satellite tags were deployed on several BCB bowhead whales in autumn 2022 to continue to contribute to our understanding of how bowhead whale behaviors and distribution are changing with decreasing sea ice, different wind patterns, warmer water, and increasing human activity in the Arctic (http://www.adfg.alaska.gov/index.cfm?adfg=marinemammalprogram). Visual monitoring of marine mammals is incorporated in the ongoing DBO collaboration (https://dbo.cbl.umces.edu/about.html). Autonomous underwater vehicles (AUV), or underwater gliders, have been deployed in the Pacific Arctic since 2013, providing the means to monitor marine mammal sounds near real-time (Baumgartner et al., 2014). To augment these efforts, unmanned aircraft (i.e., drones) could be used to visually survey areas that are otherwise too remote (e.g., northern Chukchi Sea) to be surveyed via manned aircraft. Unmanned aircraft could also be deployed from research vessels for targeted surveillance of localized areas of interest, for example, dense aggregations of feeding whales or to collect photographs for potential stock identification. Unmanned aircraft have previously been used for cetacean studies in Alaska (Ferguson et al., 2018), Norway (Aniceto et al., 2018), Australia (Christiansen et al., 2020), and elsewhere. Unmanned aircraft and passive acoustic monitoring studies could assess potential watch list areas for multiple species; satellite telemetry is species-specific.
The southern Chukchi Sea, including Kotzebue Sound and areas west of the U.S. EEZ, and the greater Bering Strait area would benefit from an increase in cetacean research. This area may be undergoing an ecosystem regime shift to one characterized by subarctic conditions, species, and interactions more like that observed in the east-central Bering Sea shelf (Huntington et al., 2020). Studies have been conducted in regional subareas, revealing increased presence of humpback and fin whales in the southcentral Chukchi Sea (Brower et al., 2018; Clarke et al., 2013b, Clarke et al., 2020), linkages between humpback whales near the Chukotka Peninsula and two lower latitude breeding grounds (Titova et al., 2017; Titova et al., 2020), the possible presence of harbor porpoise year round in Kotzebue Sound (Castellote et al., 2017), and the continued existence of the Kotzebue Sound beluga stock (O’Corry-Crowe et al., 2021), but broader scale effort is lacking in the region.
To maintain utility and relevance in the rapidly changing Arctic, BIAs should continue to be reevaluated and revised as new information becomes available. Furthermore, non-cetacean Arctic marine mammals, including walruses, ice seals, and polar bears, should be included in future BIA analyses. The frequency at which BIAs should be updated is difficult to define. The Arctic is one of seven regions evaluated for BIAs in this issue, each with inherent challenges that may be shared or unique. Funding for marine mammal research fluctuates depending on national and international interests, anthropogenic impacts, and other factors. The magnitude, quality, and availability of new information will continue to vary between regions which will make it difficult to set specific timelines for BIA updates, but reevaluation and revisions should likely occur no less frequently than every five years.
The Arctic remains a strategically important region. Maritime traffic in the Arctic is governed by global, regional, and bilateral agreements and national policies, some of which contradict one another (Boylan, 2016). International shipping and national security interests, both military and commercial, coupled with decreased sea ice, are expected to allow increased vessel traffic across the Northern Sea Route (NSR) north of Russia, the Northwest Passage (NWP) north of the U.S., Canada, and Greenland, and across the North Pole in the decades to come (Stephenson et al., 2011; Smith and Stephenson, 2013). The negative implications for cetaceans, including behavioral disturbance, masking of important sounds, physiological stress or physical injury, hearing loss, and impacts to prey species, have been well documented (Moore et al., 2012b; Southall et al., 2019). The only pathway out of the Pacific Arctic, for marine mammals and vessels traversing the NSR, NWP or polar route alike, is through the 85-km wide but shallow Bering Strait, underscoring the need for continued identification of and revisions to cetacean BIAs.
Publicly available datasets were analyzed in this study. This data can be found here: Alaska Fisheries Science Center long-term passive monitoring, https://www.fisheries.noaa.gov/inport/item/17343; Aerial Surveys of Arctic Marine Mammals, https://www.fisheries.noaa.gov/alaska/marine-mammalprotection/aerial-surveys-arctic-marine-mammals; Distributed Biological Observatory (DBO) vessel sightings, 2009-2019, arcticdata.io (doi:10.18739/A26T0GX06).
JC, MF and AB identified and compiled available information sources. MF, as part of the BIA planning team, provided guidance on BIA delineation and scoring methods. JC delineated and scored BIAs, with guidance and input from MF and AB. JC wrote the manuscript with review by MF and AB. EF created the BIA Tool website and summary tables. SD created BIA maps. All authors contributed to the article and approved the submitted version.
This process would not have been possible without inputs from several Arctic cetacean experts. We are grateful to the following subject matter experts for their valuable insights into Pacific Arctic cetacean occurrence, including directing us to several data sources that had originally eluded us: Jessica Crance, John “Craig” George, William Halliday, Donna Hauser, Lisa Loseto, Sue Moore, Kate Stafford, Alex Whiting, and Alex Zerbini. Special thanks to William Halliday and Sue Moore for their review of draft BIAs – their comments and suggestions were particularly helpful, and we greatly appreciate their time and attention to detail in assisting with BIA delineation. The draft manuscript was reviewed by Jessica Crance, Bonnie Easley-Appleyard, Lisa Loseto, and Sue Moore. The BIA II team, including Jesse Cleary, Corrie Curtice, Pat Halpin, Jolie Harrison, Leslie New, Reny Tyson Moore, and Sofie Van Parijs, provided valuable guidance during all stages of BIA II planning and development. This work was completed through a collaboration between NOAA and the U.S. Navy to better describe areas and time periods in which cetacean populations are known to concentrate for breeding, feeding, and migration, and areas within which small and resident populations occupy a limited geographic extent.
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1040123/full#supplementary-material
Alaska Fisheries Science Center (2021) AFSC/NMML: Acoustics long-term passive monitoring using moored autonomous recorders in the Bering, Chukchi, and Western Beaufort seas 2007-2012. Available at: https://www.fisheries.noaa.gov/inport/item/17343.
Aniceto A. S., Biuw M., Lindstrøm U., Solbø S. A., Broms F., Carroll J. L. (2018). Monitoring marine mammals using unmanned aerial vehicles; quantifying detection certainty. Ecosphere 9 (3). doi: 10.1002/ecs2.2122
Ashjian C. J., Campbell R. G., Okkonen S. R. (2021). “Biological environment,” in The bowhead whale Balaena mysticetus: Biology and human interactions. Eds. George J. C., Thewissan J. G. H. (London: Academic Press), 403–416.
Baumgartner M. F., Stafford K. M., Winsor P., Statscewich H., Fratantoni D. M. (2014). Glider-based passive acoustic monitoring in the Arctic. Mar. Tech. Soc J. 48 (5), 40–51. doi: 10.4031/MTSJ.48.5.2
Blokhin S. A., Litovka D. I. (2015). Brief results of gray whale Eschrichtius robustus research off Chukotka, Russian Federation 2012-2014, IWC paper SC/66a/BRG14. Available at: https://www.iwc.int/en.
Blohkin S. A., Litovka D. I., Vinnikov A. V. (2013). Brief results of gray whale Eschrichtius robustus research off Chukotka, Russian Federation 2012, IWC paper SC/65a/BRG12. Available at: https://www.iwc.int/en.
Blokhin S. A., Litovka D. I., Shkarupa M. A. (2017). Results of gray whale Eschrichtius robustus monitoring research off Chukotka, Russian Federation 2012-2016, IWC paper SC/67A/AWMP/11. Available at: https://www.iwc.int/en.
Bluhm B., Coyle K. O., Konar B., Highsmith R. (2007). High gray whale relative abundances associated with an oceanographic front in the south-central Chukchi Sea. Deep Sea Res. Part II: Topical Stud. Oceano. 54 (23-26), 2919–2933. doi: 10.1016/j.dsr2.2007.08.015
Boylan B. M. (2016). Increased maritime traffic in the Arctic: Implications for governance of Arctic sea routes. Mar. Pol. 131, 104566. doi: 10.1016/j.marpol.2021.104566
Brower A. A., Clarke J. T., Ferguson M. C. (2018). Increased sightings of subarctic cetaceans in the eastern Chukchi Sea 2008-2016: population recovery, response to climate change, or increased effort? Pol. Bio 41, 1033-1039. doi: 10.1007/s00300-018-2257-x
Brower A. A., Ferguson M. C., Clarke J. T., Fujioka E., Deland S. (2022). Biologically important areas II for cetaceans within U.S. and adjacent waters - Aleutian Islands and Bering Sea region. Front. Mar. Sci 9, 1055398. doi: 10.3389/fmars.2022.1055398
Brower A. A., Ferguson M. C., Schonberg S. V., Jewett S. C., Clarke J. T. (2017). Gray whale distribution relative to benthic invertebrate biomass and abundance: northeastern Chukchi Sea 2009-2012. Deep-Sea Res. II 144, 156–174. doi: 10.1016/j.dsr2.2016.12.007
Castellote M., Stafford K., Small R. J. (2017). Acoustic monitoring of cetaceans in Kotzebue Sound: 2014-2016. Final report submitted to the Alaska Beluga Whale Committee. Available at: north-slope.org/departments/wildlife-management/co-management-organizations/Alaska-beluga-whale-committee.
Chou E., Antunes R., Sardelis S., Stafford K. M., West L., Spagnoli D., et al. (2019). Seasonal variation in Arctic marine mammal acoustic detection in the northern Bering Sea. Mar. Mam. Sci 36 (2), 522-547. doi: 10.1111/mms.12658
Choy E. S., Giraldo C., Rosenberg B., Roth J. D., Ehrman A. D., Majewski A., et al. (2020). Variation in the diet of beluga whales in response to changes in prey availability: insights on changes in the Beaufort Sea ecosystem. Mar. Ecol. Prog. Ser. 647, 195–210. doi: 10.3354/meps13413
Christiansen F., Nielsen M. L., Charlton C., Bejder L., Madsen P. T. (2020). Southern right whales show no behavioral response to low noise levels from a nearby unmanned aerial vehicle. Mar. Mam. Sci. 36 (3), 953–693. doi: 10.1111/mms.12699
Citta J. J., Quakenbush L., George J. C. (2021). “Distribution and behavior of Bering-Chukchi-Beaufort bowhead whales as inferred by telemetry,” in The bowhead whale Balaena mysticetus: Biology and human interactions, vol. 31-56 . Eds. George J. C., Thewissan J. G. H. (London: Academic Press).
Citta J. J., Quakenbush L. T., Okkonen S. R., Druckenmiller M. L., Maslowski W., Clement-Kinney J., et al. (2015). Ecological characteristics of core-use areas used by Bering-Chukchi-Beaufort (BCB) bowhead whales 2006-2012. Prog. Oceano. 136, 201–222. doi: 10.1016/j.pocean.2014.08.012
Clark C. W., Berchok C. L., Blackwell S. B., Hannay D. E., Jones J., Ponirakis D., et al. (2015). A year in the acoustic world of bowhead whales in the Bering, Chukchi and Beaufort seas. Prog. Ocean. 136, 223–240. doi: 10.1016/j.pocean.2015.05.007
Clarke J. T., Brower A. A., Christman C. L., Ferguson M. C. (2014). Distribution and relative abundance of marine mammals in the northeastern Chukchi and western Beaufort seas 2013 annual report, OCS study BOEM 2014-018 (7600 Sand Point Way NE, F/AKC3, Seattle, WA: National Marine Mammal Laboratory, Alaska Fisheries Science Center, NMFS, NOAA), 98115–96349.
Clarke J. T., Brower A. A., Ferguson M. C., Kennedy A. S., Willoughby A. L. (2015a). Distribution and relative abundance of marine mammals in the eastern Chukchi and western Beaufort seas 2014. annual report, OCS study BOEM 2015-040 (7600 Sand Point Way NE, F/AKC3, Seattle, WA: National Marine Mammal Laboratory, Alaska Fisheries Science Center, NMFS, NOAA), 98115–96349. Available at: https://www.fisheries.noaa.gov/alaska/marine-mammal-protection/aerial-surveys-arctic-marine-mammals.
Clarke J. T., Brower A. A., Ferguson M. C., Willoughby A. L (2017a). Distribution and relative abundance of marine mammals in the eastern Chukchi and western Beaufort seas 2015. annual report, OCS study BOEM 2017-019 (7600 Sand Point Way NE, F/AKC, Seattle, WA: National Marine Mammal Laboratory, Alaska Fisheries Science Center, NMFS, NOAA), 98115–96349. Available at: https://www.fisheries.noaa.gov/alaska/marine-mammal-protection/aerial-surveys-arctic-marine-mammals.
Clarke J. T., Brower A. A., Ferguson M. C., Willoughby A. L (2017b). Distribution and relative abundance of marine mammals in the eastern Chukchi and western Beaufort seas 2016. annual report, OCS study BOEM 2017-078 (7600 Sand Point Way NE, F/AKC3, Seattle, WA: Marine Mammal Laboratory, Alaska Fisheries Science Center, NMFS, NOAA), 98115–96349. Available at: https://www.fisheries.noaa.gov/alaska/marine-mammal-protection/aerial-surveys-arctic-marine-mammals.
Clarke J. T., Brower A. A., Ferguson M. C., Willoughby A. L. (2018). Distribution and relative abundance of marine mammals in the eastern Chukchi and western Beaufort seas 2017. annual report, OCS study BOEM 2018-023 (7600 Sand Point Way NE, F/AKC3, Seattle, WA: Marine Mammal Laboratory, Alaska Fisheries Science Center, NMFS, NOAA), 98115–96349. Available at: https://www.fisheries.noaa.gov/alaska/marine-mammal-protection/aerial-surveys-arctic-marine-mammals.
Clarke J. T., Brower A. A., Ferguson M. C., Willoughby A. L. (2019). Distribution and relative abundance of marine mammals in the eastern Chukchi and western Beaufort seas 2018. annual report, OCS study BOEM 2019-021 (7600 Sand Point Way NE, F/AKC3, Seattle, WA: Marine Mammal Laboratory, Alaska Fisheries Science Center, NMFS, NOAA), 98115–96349. Available at: https://www.fisheries.noaa.gov/alaska/marine-mammal-protection/aerial-surveys-arctic-marine-mammals.
Clarke J. T., Brower A. A., Ferguson M. C., Willoughby A. L., Rotrock A. D. (2020). Distribution and relative abundance of marine mammals in the eastern Chukchi Sea, eastern and western Beaufort Sea, and Amundsen Gulf 2019. annual report, OCS study BOEM 2020-027 (7600 Sand Point Way NE, F/AKC3, Seattle, WA 98115-6349: Marine Mammal Laboratory, Alaska Fisheries Science Center, NMFS, NOAA). Available at: https://www.fisheries.noaa.gov/alaska/marine-mammal-protection/aerial-surveys-arctic-marine-mammals.
Clarke J. T., Christman C. L., Brower A. A., Ferguson M. C. (2013a). Distribution and relative abundance of marine mammals in the northeastern Chukchi and western Beaufort seas 2012. annual report, OCS study BOEM 2013-00117 (National Marine Mammal Laboratory, Alaska Fisheries Science Center, NMFS, NOAA). Available at: https://www.fisheries.noaa.gov/alaska/marine-mammal-protection/aerial-surveys-arctic-marine-mammals.
Clarke J. T., Ferguson M. C., Curtice C., Harrison J. (2015b). 8. Biologically important areas for cetaceans within U.S. waters - Arctic region. Aq. Mam. 41 (1), 94–103. doi: 10.1578/AM.41.1.2015.94
Clarke J. T., Ferguson M. C., Okkonen S. R., Brower A. A., Willoughby A. L. (2022). Bowhead whale calf occurrence in the Beaufort Sea. Arc. Sci 8 (2). doi: 10.1139/as-2021-0020
Clarke J. T., Ferguson M. C., Willoughby A. L., Brower A. A. (2018). Bowhead and beluga whale distributions, sighting rates, and habitat associations in the western Beaufort Sea in summer and fall 2009-16, with comparison to 1982-91. Arctic 71 (2), 115–138. doi: 10.14430/arctic4713
Clarke J. T., Kennedy A. S., Ferguson M. C. (2016). Bowhead and gray whale distributions, sighting rates, and habitat associations in the eastern Chukchi Sea, summer and fall 2009 – 15, with a retrospective comparison to 1982 – 91. Arctic 69 (4), 359–377. doi: 10.14430/arctic4597
Clarke J., Stafford K., Moore S., Rone B., Aerts L., Crance J. (2013b). Subarctic cetaceans in the southern Chukchi Sea: Evidence of recovery or response to a changing ecosystem. Oceano. 24 (4), 136–149. doi: 10.5670/oceanog.2013.81
Collings P., Pearce T., Kann J. (2018). "We don't know anything about whales": ecological knowledge and ways of knowing in Ulukhaktok, Northwest Territories, Canada. Arc. Sci. 4, 233–241. doi: 10.1139/as-2017-0030
Crance J. L., Berchok C. L., Bonnel J., Thode A. M. (2015). Northeasternmost record of a north Pacific fin whale (Balaenoptera physalus) in the alaskan Chukchi Sea. Pol. Bio. 38 (10), 1767–1773. doi: 10.1007/s00300-015-1719-7
Delarue J., Martin B., Hannay D., Berchok C. L. (2013). Acoustic occurrence and affiliation of fin whales detected in the northeastern Chukchi Sea, July to October 2007-10. Arctic 66 (2), 159–172. doi: 10.14430/arctic4287
DeMaster D. P., Lowry L. F., Frost K. J., Bengtson R. A. (2001). The effect of sea state on estimates of abundance for beluga whales (Delphinapterus leucas) in Norton sound, Alaska. Fish. Bull. 99 (1), 197–201. Available at: https://spo.nmfs.noaa.gov.
Department of Fisheries and Oceans (2000). Eastern Beaufort Sea Beluga. DFO science stock status report. E5–38. Available at: https://www.north-slope.org/departments/wildlife-management/co-management-organizations/alaska-beluga-whale-committee/abwc-research-projects/beaufort-sea-stock-projects-dfo-funded/.
Escajeda E., Stafford K. M., Woodgate R. A., Laidre K. L. (2020). Variability in fin whale (Balaenoptera physalus) occurrence in the Bering strait and southern Chukchi Sea in relation to environmental factors. Deep-Sea Res. II 177, 104782. doi: 10.1016/j.dsr2.2020.104782
Ferguson M., Angliss R. P., Kennedy A., Lynch B., Willoughby A., Helker V., et al. (2018). Performance of manned and unmanned aerial surveys to collect visual data and imagery for estimating arctic cetacean density and associated uncertainty. J. Unman. Veh. Sys. 6 (3), 128–154. doi: 10.1139/juvs-2018-0002
Ferguson M. C., Clarke J. T., Brower A. A., Willoughby A. L., Okkonen S. R. (2021). “Ecological variation in the western Beaufort Sea,” in The bowhead whale Balaena mysticetus: Biology and human interactions. Eds. George J. C., Thewissan J. G. H. (London: Academic Press), 365–379.
Ferguson M. C., Harrison J., Van Parijs S. M. (2015). 1. Biologically important areas for cetaceans within U.S. waters – overview and rationale. Aq. Mam. 41 (1), 2–16. doi: 10.1578/AM.41.1.2015.2
Ferguson M. C., Toth S. F., Clarke J. T., Willoughby A. L., Brower A. A., White T. P. Biologically important areas for bowhead whales (Balaena mysticetus): optimal site selection with linear integer programming. Front. Mar. Sci. (in review).
Frey K. E., Moore G. W. K., Cooper L. W., Grebmeier J. M. (2015). Divergent patterns of recent sea ice cover across the Bering, Chukchi, and Beaufort seas of the Pacific Arctic region. Prog. Ocean 136, 32–49. doi: 10.1016/j.pocean.2015.05.009
Frost K. J., Lowry L. F. (1990). Distribution, abundance, and movements of beluga whales, Delphinapterus leucas, in coastal waters of western Alaska. Can. Bull. Fish. Aq. Sci. 224, 39-57.
Furumaki S., Trujii K., Mitani Y. (2021). Fin whale (Balaenoptera physalus) song pattern in the southern Chukchi Sea. Pol. Bio 44, 1021-1027. doi: 10.1007/s00300-021-02855-y
Galginaitis M. (2014). Monitoring cross island whaling activities, Beaufort Sea, Alaska: 2008-2012 final report, incorporating ANIMIDA and cANIMIDA, (2001-2007) (Alaska Region, Anchorage, AK: U.S. Dept. of the Interior, Bureau of Ocean Energy Management), 208. OCS Study BOEM 2013-212.
George J. C., Zeh J., Suydam R., Clark C. (2004). Abundance and population trend (1978-2001) of western Arctic bowhead whales surveyed near Barrow, Alaska. Mar. Mam. Sci. 20 (4), 755–773. doi: 10.1111/j.1748-7692.2004.tb01191.x
Givens G. H., Ferguson M. C., Clarke J. T., Willoughby A., Brower A., Suydam R. (2020). Abundance of the eastern Chukchi Sea stock of beluga whales 2012-17. Arctic 73, 485–498. doi: 10.14430/arctic71592
Grebmeier J. M (2012). Shifting patterns of life in the Pacific Arctic and Sub-Arctic seas. Ann.Rev. Mar. Sci 4, 63–78. doi: 10.1146/annurev-marine-120710-100926
Grebmeier J. M., Moore S. E., Cooper L. W., Frey K. E. (2019). The distributed biological observatory: a change detection array in the Pacific Arctic – an introduction. Deep-Sea Res. II 162, 1–7. Vessel sighting data, 2009-2019, available through NSF Arctic Data Center doi: 10.1016/j.dsr2.2019.05.005
Halliday W. D., Insley S. J., de Jong T., Mouy X. (2018). Seasonal patterns in acoustic detections of marine mammals near Sachs Harbour, Northwest Territories. Arc. Sci. 4, 259–278. doi: 10.1139/as-2017-0021
Halliday W. D., Pine M. K., Citta J. J., Harwood L., Hauser D. D. W., Casey Hilliard R., et al. (2021). Potential exposure of beluga and bowhead whales to underwater noise from ship traffic in the Beaufort and Chukchi Sea. Ocean Coast. Man. 204, 105473. doi: 10.1016/j.ocecoaman.2020.105473
Halliday W. D., Pine M. K., Insley S. J., Soares R. N., Kortsalo P., Mouy X. (2019). Acoustic detections of Arctic marine mammals near Ulukhaktok, Northwest Territories, Canada. Can. J. Zool 97, 72–80. doi: 10.1139/cjz-2018-0077
Halliday W. D., Pine M. K., Mouy X., Kortsalo P., Hilliard R. C., Insley S. J. (2020). The coastal Arctic marine soundscape near Ulukhaktok, Northwest Territories, Canada. Pol. Bio. 43, 623–636. doi: 10.1007/s00300-020-02665-8
Harrison J., Ferguson M. C., New L., DeLand S., Fujioka E., Curtice C., et al. Biologically important areas for cetaceans: updates and the application of a new scoring system. Front. Mar. Sci. (in review).
Harwood L. A., Kingsley M. C. S. (2013). Trends in the offshore distribution and relative abundance of Beaufort Sea belugas 1982-85 vs 2007-09. Arctic 66 (3), 247–256. doi: 10.14430/arctic4304
Harwood L., Iacozza J., Auld J., Norton P., Loseto L. L. (2014). Belugas in the Mackenzie River estuary, NT, Canada : Habitat use and hotspots in the Tarium Niryutait Marine Protected Area. Ocean. Coast. Man. Sci. 100, 128–138. doi: 10.1016/j.ocecoaman.2014.08.004
Hauser D. D. W., Laidre K. L., Parker-Stetter S. L., Horne J. K., Suydam R. S., Richard P. R. (2015). Regional diving behavior of Pacific Arctic beluga whales Delphinapterus leucas and possible associations with prey. Mar. Ecol. Prog. Ser. 541, 245–264. doi: 10.3354/meps11530
Hauser D. D. W., Laidre K. L., Stafford K. M., Stern H. L., Suydam R. S., Richard P. R. (2016). Decadal shifts in autumn migration timing by Pacific Arctic beluga whales are related to delayed annual sea ice formation. Glob. Change Biol 23 (6), 2206-2217. doi: 10.1111/gcb.13564
Hauser D. D. W., Laidre K. L., Suydam R. S., Richard P. R. (2014). Population-specific home ranges and migration timing of Pacific Arctic beluga whales (Delphinapterus leucas). Pol. Biol. 37, 1171–1183. doi: 10.1007/s00300-014-1510-1
Heide-Jørgensen M. P., Laidre K. L., Litovka D., Villum Jensen M., Grebmeier J. M., Sirenko B. I. (2012). Identifying gray whale (Eschrichtius robustus) foraging grounds along the Chukotka Peninsula, Russia, using satellite telemetry. Pol. Bio. 35, 1035–1045. doi: 10.1007/s00300-011-1151-6
Hill V., Ardyna M., Lee S. H., Varela D. E. (2018). Decadal trends in phytoplankton production in the Pacific Arctic region from 1950 to 2012. Deep-Sea Res. II 152, 82–94. doi: 10.1016/j.dsr2.2016.12.015
Hornby C., Hoover C., Joynt A., Torontow V., Hynes K., Loseto L. (2014). Arrival of beluga (Delphinapterus leucas) to the Mackenzie estuary in relation to sea ice: report on spring 2011-2013 aerial surveys. Can. Data Rep. Fish. Aq. Sci. 1251, vii+25. Available at: https://nwtdiscoveryportal.enr.gov.nt.ca/geoportal/catalog/search/resource/details.page?uuid=%7BA5BDD733-A7C7-49FF-8F65-452647488585%7D.
Hornby C., Iacozza J., Hoover C., Barber D., Loseto L. L. (2016). Spring habitat use of beluga whales (Delphinapterus leucas) during arrival to the Mackenzie river estuary. Pol. Bio 39, 2319-2334. doi: 10.1007/s00300-16-1899-9
Hornby C., Iacozza J., Hoover C., Barber D., Loseto L. L. (2017). Beluga whale Delphinapterus leucas late summer habitat use and support for foraging areas in the Canadian Beaufort Sea. Mar. Ecol. Prog. Ser. 574, 243–257. doi: 10.3354/meps12178
Huntington H. P., Danielson S. L., Wiese F. K., Baker M., Boveng P., Citta J. J., et al. (2020). Evidence suggests potential transformation of the Pacific Arctic ecosystem is underway. Nat. Clim Chang 10, 342–348. doi: 10.1038/s41558-020-0695-2
Huntington H. P., Quakenbush L. T. (2009a). “Traditional knowledge of bowhead whale migratory patterns near Kaktovik and Barrow, Alaska. Report to the Alaska Eskimo Whaling Commission and the Barrow and Kaktovik Whaling Captains. Appendix K,” in Satellite tracking of western Arctic bowhead whales. study prepared by the Alaska Department of Fish and Game for the Bureau of Ocean Energy Management, Regulation and Enforcement. Eds. Quakenbush L. T., Small R. J., Citta J. J. (OCS Study BOERME), 2010–2033. 2010. Available at: https://www.boem.gov/newsroom/library/alaska-scientific-and-technical-publications.
Huntington H. P., Quakenbush L. T. (2009b). “Traditional knowledge of bowhead whale migratory patterns near Wainwright, Alaska. Report to the Alaska Eskimo Whaling Commission and the Wainwright Whaling Captains. Appendix l,” in Satellite tracking of western Arctic bowhead whales. study prepared by the Alaska Department of Fish and Game for the Bureau of Ocean Energy Management, Regulation and Enforcement. Eds. Quakenbush L. T., Small R. J., Citta J. J. (OCS Study BOERME), 2010–2033. 2010. Available at: https://www.boem.gov/newsroom/library/alaska-scientific-and-technical-publications.
Huntington H. P.,, the communities of Buckland, Elim, Koyuk, Point Lay and Shaktoolik. (1999). Traditional knowledge on the ecology of beluga whales (Delphinapterus leucas) in the eastern Chukchi and northern Bering seas, Alaska. Arctic 52 (1), 49–61. doi: 10.14430/arctic909
Ilyashenko V. Y. (2013). Aboriginal harvest of gray and bowhead whales in the Russian Federation in 2008-2012, IWC paper SC/65a/BRG24. Available at: https://www.iwc.int/en.
Ilyashenko V. Y. (2018). Needs of Chukotka indigenous people in gray whale harvest products and rationale for updates to the paragraph 13(b)(2) of the schedule (the gray whale catch limit), IWC paper SC/67b/AWMP17. Available at: https://www.iwc.int/en.
Ilyashenko V., Zharikov K. (2014). Aboriginal harvest of gray and bowhead whales in the Russian Federation in 2013, IWC paper SC/65b/BRG03 plus supplemental table with catch statistics. Available at: https://www.iwc.int/en.
Ilyashenko V., Zharikov K. (2015). Aboriginal subsistence whaling in the Russian Federation in 2014, IWC paper SC/66a/BRG7. Available at: https://www.iwc.int/en.
Ilyashenko V., Zharikov K. (2016). Aboriginal subsistence whaling in the Russian Federation in 2015, IWC paper SC/66b/BRG22 plus supplemental table with catch statistics. Available at: https://www.iwc.int/en.
Ilyashenko V., Zharikov K. (2017). Aboriginal subsistence whaling in the Russian Federation in 2016, IWC paper SC/67A/AWMP/03 plus supplemental table with catch statistics. Available at: https://www.iwc.int/en.
Insley S. J., Halliday W. D., Mouy X., Diogou N. (2021). Bowhead whales overwinter in the Amundsen Gulf and eastern Beaufort Sea. R. Soc Open Sci. 8, 202268. doi: 10.1098/rsos.202268
IUCN (2021). Baleen whales in the cross hairs: potential for increased ship strike risk in and near Bering Strait. SSC Cetacean Specialist Group/Arctic. Available at: https://iucn-csg.org/baleen-whales-in-the-cross-hairs-potential-for-increased-ship-strike-risk-in-and-near-bering-strait/.
Ivashchenko Y. V., Clapham P. J., Brownell J. R. L. (2013). Soviet catches of whales in the north Pacific: revised totals. J. Cet. Res. Manage. 13 (1), 59–71. doi: 10.3354/esr00443
Ivashin M. V., Votrogov L. M. (1982). Occurrence of baleen and killer whales off Chukotka. Rep. Int. Whal. Commun. 32, 499–501. Available at: https://www.iwc.int/en.
Kennedy A. S., Rone B. K., Zerbini A. N., Berchok C., Clapham P. J. (2017). Visual and tagging results for multidisciplinary marine mammal research cruises in the northern Bering, Chukchi, and Beaufort seas between 2010 and 2016 (Anchorage, AK: Poster presented at the Alaska Marine Sciences Symposium).
Koski W. R., Davis R. A., Miller G. W., Withrow D. E. (1993). “Reproduction,” in The bowhead whale, vol. 2 . Eds. Burns J. J., Montague J. J., Cowles C. J. (Lawrence, KS: The Society for Marine Mammalogy), 409–489.
Loseto L. L., Brewster J. D., Ostertag S. K., Snow K., MacPhee S. A., McNicholl D. G., et al. (2018). Diet and feeding observations from an unusual beluga harvest in 2014 near Ulukhaktok, Northwest Territories, Canada. Arc. Sci. 4, 421–431. doi: 10.1139/as-2017-0046
Mahoney A. R. (2012). Sea Ice conditions in the Chukchi and Beaufort seas (Washington, DC: Pew Charitable Trusts, U.S. Arctic Program).
Majewski A. R., Atchison S., McPhee S., Eert J., Niemi A., Michel C., et al. (2017). Marine fish community structure and habitat associations on the Canadian Beaufort shelf and slope. Deep-Sea Res. I 121, 169–182. doi: 10.1016/j.dsr.2017.01.009
Mel'nikov V. V. (2000). Humpback whales Megaptera novaeangliae off Chukchi Peninsula. Oceanology 40 (6), 844–849.
Melnikov V. V. (2019). Observation of humpback whales (Megaptera novaeangliae) in the waters adjacent to the Chukotka Peninsula with comparisons to historical sighting data. Open Access Library J. 6, e5407. doi: 10.4236/oalib.1.1105407
Melnikov V. V., Livotka D. I., Zagrebin L. A., Zelensky G. M., Ainana L. I. (2004). Shore-based counts of bowhead whales along the Chukotka Peninsula in May and Jun 1999-2001. Arctic 57 (3), 290–298. Available at: https://journalhosting.ucalgary.ca/index.php/arctic/issue/view/4878.
Mocklin J., George J. C., Ferguson M., Vate Brattström L., Beaver V., Rone B., et al. (2012). Aerial photography of bowhead whales near Barrow, Alaska, during the 2011 spring migration, IWC paper SC/64/BRG3. Available at: https://www.iwc.int/en.
Moore S. E., Clarke J. T., Okkonen S. R., Grebmeier J. M., Berchok C. L., Stafford K. M. (2022). Changes in gray whale phenology and distribution related to prey variability and ocean biophysics in the northern Bering and eastern Chukchi seas. PloS One 17 (4), e0265934. doi: 10.1371/journal.pone.0265934
Moore S. E., Reeves R. R. (1993). “Distribution and movement,” in The bowhead whale, vol. 2 . Eds. Burns J. J., Montague J. J., Cowles C. J. (Lawrence, KS: The Society for Marine Mammalogy), 409–489.
Moore S. E., Reeves R. R., Southall B. L., Ragen T. J., Suydam R. S., Clark C. W. (2012a). A new framework for assessing the effects of anthropogenic sound on marine mammals in a rapidly changing arctic. Bioscience 62, 289–295. doi: 10.1525/bio.2012.62.3.10
Moore S. E., Stafford K. M., Melling H., Berchok C., Wiig O., Kovacs K. M., et al. (2012b). Comparing marine mammal acoustic habitats in Atlantic and Pacific sectors of the high Arctic: year-long records from Eschrichtius and the Chukchi Plateau. Pol. Bio. 35, 475–480. doi: 10.1007/s00300-011-1086-y
Muto M. M., Helker V. T., Delean B. J., Angliss R. P., Boveng P. L., Breiwick, et al. (2020). Alaska Marine mammal stock assessments 2019 (U.S. Dep. Commer.: NOAA Tech. Memo. NMFS-AFSC-404), 395.
Nerini M. (1984). “A review of gray whale feeding ecology,” in The gray whale, Eschrichtius robustus. Eds. Jones M. L., Swartz S. L., Leatherwood S. (New York: Academic Press), 423–450. doi: 10.1016/B978-0-08-092372-7.50024-8
Noongwook G.,, the Native Village of Savoonga, the Native Village of Gambell, Huntington H. P., George J. C. (2007). Traditional knowledge of the bowhead whale (Balaena mysticetus) around St. Lawrence Island, Alaska. Arctic 60 (1), 47–54. Available at: https://journalhosting.ucalgary.ca/index.php/arctic/issue/view/4864.
O’Corry-Crowe G., Ferrer T., Citta J. J., Suydam R., Quakenbush L., Burns J. J., et al. (2021). Genetic history and stock identity of beluga whales in Kotzebue Sound. Pol. Res. 40, 7623. doi: 10.33265/polar.v40.7623
O’Corry-Crowe G., Suydam R., Quakenbush L., Smith T. G., Lydersen C., Kovacs K. M., et al. (2020). Group structure and kinship in beluga whale societies. Scient. Rep. 10, 11462. doi: 10.1038/s41598-020-67314-w
Olnes J., Citta J., Quakenbush L., George C., Harwood L., Lea E., et al. (2020). Use of the Alaskan Beaufort Sea by bowhead whales (Balaena mysticetus) tagged with satellite transmitters 2006 – 2018. Arctic 73 (3), 278–291. doi: 10.14430/arctic70865
Overland J., Dunlea E., Box J. E., Corell R., Forsius M., Kattsov V., et al. (2019). The urgency of Arctic change. Pol. Sci. 21, 6–13. doi: 10.1016/j.polar.2018.11.008
Quakenbush L. T., Suydam R. S., Bryan A. L., Lowry L. F., Frost K. J., Mahoney B. A. (2015). Diet of beluga whales, Delphinapterus leucas, in Alaska from stomach contents, March-November. Mar. Fish. Rev. 77 (1), 70–84. doi: 10.7755/MFR.77.1.7
Rice D. W., Wolman A. A., Braham H. W. (1984). The gray whale, Eschrichtius robustus.Mar. Fish. Rev. 46 (4), 7–14. Available at: https://spo.nmfs.noaa.gov.
Richard P. R., Martin A. R., Orr J. R. (2001). Summer and autumn movements of belugas of the eastern Beaufort Sea stock. Arctic 54 (3), 223–236. doi: 10.14430/arctic783
Scharffenberg K., MacPhee S., Loseto L. (2020). Upriver sightings of beluga whales (Delphinapterus leucas) follow storm surges and high-water in the Mackenzie delta, Northwest Territories, Canada. Arc. Sci. 7 (3). doi: 10.1139/as-2020-0010
Scharffenberg K., Whalen D., MacPhee S., Marcoux M., Iacozza J., Davoren G., et al. (2019a). Oceanographic, ecological, and socio-economic impacts of an unusual summer storm in the Mackenzie estuary. Arc. Sci. 6, 62–76. doi: 10.1139/as-2018-0029
Scharffenberg K., Whalen D., Marcoux M., Iacozza J., Davoren G., Loseto L. (2019b). Environmental drivers of beluga whale (Delphinapterus leucas) habitat use in the Mackenzie estuary. Mar. Ecol. Prog. Ser. 626, 209–226. doi: 10.3354/meps13011
Smith L. C., Stephenson S. R. (2013). New trans-Arctic shipping routes navigable by midcentury. Proc. Natl. Acad. Sci. Unit. States Am. 110, E1191–E1195. doi: 10.1073/pnas.1214212110
Southall B. L., Finneran J. J., Reichmuth C., Nachtigall P. E., Ketten D. R., Bowles A. E., et al. (2019). Marine mammal noise exposure criteria: updated scientific recommendations for residual hearing effects. Aq. Mam. 45, 125–232. doi: 10.1578/AM.45.2.2019.125
Stafford K. M., Farley E. V., Ferguson M., Kuletz K. J., Levine R. (2022a). Northward range expansion of subarctic upper trophic level animals into the Pacific Arctic region. Oceanography 35 (3-4), 158-166. doi: 10.5670/oceanog.2022.101
Stafford K. M., Ferguson M. C., Hauser D. D. W., Okkonen S. R., Berchok C. L., Citta J. J., et al. (2018). Beluga whales in the western Beaufort Sea: current state of knowledge on timing, distribution, habitat use and environmental drivers. Deep-Sea Res. II 152, 182–194. doi: 10.1016/j.dsr2.2016.11.017
Stafford K. M., Melling H., Moore S. E., Berchok C. L., Braen E. K., Brewer A., et al. (2022b). Marine mammal detections on the Chukchi Plateau 2009-2020. J. Acoust. Soc Am. 151 (4), 2521–2529. doi: 10.1121/10.0010208
Stephenson S. R., Smith S. C., Agnew J. A. (2011). Divergent long-term trajectories of human access to the Arctic. Nat. Clim. Change 1, 156–160. doi: 10.1038/nclimate1120
Storrie L., Hussey N. E., MacPhee S. A., O'Corry-Crowe G., Iacozza J., Barber D. G., et al. (2022). Year-round dive characteristics of male beluga whales from the eastern Beaufort Sea population indicate seasonal shifts in foraging strategies. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.715412
Stroeve J. C., Kattsov V., Barrett A., Serreze M., Pavlova T., Holland M., et al. (2012). Trends in Arctic sea ice extent from CMIP5, CMIP3 and observations. Geophys. Res. Lett. 39, L16502. doi: 10.1029/2012GL052676
Sumich J. L. (1986). Growth in young gray whales (Eschrichtius robustus). Mar. Mam. Sci. 2 (2), 145–152. doi: 10.1111/j.1748-7692.1986.tb00035
Suydam R., George J. C., Person B. T., Hanns C., Sheffield G. (2011). Subsistence harvest of bowhead whales (Balaena mysticetus) by Alaskan Eskimos during 2010, Paper SC/63/BRG2 presented to the International Whaling Commission. Available at: https://www.iwc.int/en.
Suydam R., George J. C., Person B. T., Hanns C., Stimmelmayr R., Pierce L., et al. (2012). Subsistence harvest of bowhead whales (Balaena mysticetus) by Alaskan Eskimos during 2011, Paper SC/64/BRG2 presented to the International Whaling Commission. Available at: https://www.iwc.int/en.
Suydam R., George J. C., Person B. T., Hanns C., Stimmelmayr R., Pierce L., et al. (2013). Subsistence harvest of bowhead whales (Balaena mysticetus) by Alaskan Eskimos during 2012, Paper SC/65a/BRG19 presented to the International Whaling Commission. Available at: https://www.iwc.int/en.
Suydam R., George J. C., Person B. T., Hanns C., Stimmelmayr R., Pierce L., et al. (2014). Subsistence harvest of bowhead whales (Balaena mysticetus) by Alaskan Eskimos during 2013, Paper SC/65b/BRG/08 presented to the International Whaling Commission. Available at: https://www.iwc.int/en.
Suydam R., George J. C., Person B. T., Ramey D., Hanns C., Stimmelmayr R., et al. (2015). Subsistence harvest of bowhead whales (Balaena mysticetus) by Alaskan Eskimos during 2014, Paper SC/66a/BRG/6 presented to the International Whaling Commission. Available at: https://www.iwc.int/en.
Suydam R., George J. C., Person B. T., Ramey D., Stimmelmayr R., Sformo T. L., et al. (2016). Subsistence harvest of bowhead whales (Balaena mysticetus) by Alaskan Eskimos during 2015 and other aspects of bowhead biology and science, Paper SC/66B/BRG/03 presented to the International Whaling Commission. Available at: https://www.iwc.int/en.
Suydam R., George J. C., Person B. T., Ramey D., Stimmelmayr R., Sformo T. L., et al. (2017). Subsistence harvest of bowhead whales (Balaena mysticetus) by Alaskan Natives during 2016, Paper SC/67A/AWMP/02 Rev1 presented to the International Whaling Commission. Available at: https://www.iwc.int/en.
Suydam R., George J. C., Person B. T., Stimmelmayr R., Sformo T. L., Pierce L., et al. (2018). Subsistence harvest of bowhead whales (Balaena mysticetus) by Alaskan Natives during 2017, Paper SC/67b/AWMP presented to the International Whaling Commission. Available at: https://www.iwc.int/en.
Suydam R., George J. C., Person B. T., Stimmelmayr R., Sformo T. L., Pierce L., et al. (2019). Subsistence harvest of bowhead whales (Balaena mysticetus) by Alaskan Natives during 2018, Paper SC/68a/ASW presented to the International Whaling Commission. Available at: https://www.iwc.int/en.
Suydam R., George J. C., Person B. T., Stimmelmayr R., Sformo T. L., Pierce L., et al. (2020). Subsistence harvest of bowhead whales (Balaena mysticetus) by Alaskan Natives during 2019, Paper SC/68b/ASW presented to the International Whaling Commission. Available at: https://www.iwc.int/en.
Suydam R. S., Lowry L. F., Frost K. J., O'Corry-Crowe G. M., Pikok D. Jr. (2001). Satellite tracking of eastern Chukchi Sea beluga whales in the Arctic ocean. Arctic 54 (3), 237–243. doi: 10.14430/arctic784
Tarpley R. J., Hillmann D. J., George J. C., Thewissen J. G. M. (2021). “Female and male reproduction,” in The bowhead whale Balaena mysticetus: Biology and human interactions. Eds. George J. C., Thewissan J. G. H. (London: Academic Press), 185–211.
Titova O. V., Fedutin I. D., Filatova O. A., Antipin M. A., Burdin A. M., Hoyt E. (2020). The characteristics of the feeding aggregation formed by humpback whales, Megaptera novaeangliae (Borowski 1781) in Senyavin Strait, off the eastern Chukotka coast, according to photo-identification data. Russ. J. Mar. Bio. 46 (5), 330–337. doi: 10.1134/S1063074020050119
Titova O. V., Filatova O. A., Fedutin I. D., Ovsyanikova E. N., Okabe H., Kabayashi N., et al. (2017). Photo-identification matches of humpback whales (Megaptera novaeangliae) from feeding areas in Russian Far East seas and breeding grounds in the north Pacific. Mar. Mam. Sci 34 (1), 100-112. doi: 10.1111/mms.12444
Tsujii K., Otsuki M., Akamatsu T., Matsuo I., Amakasu K., Kitamura M., et al. (2016). The migration of fin whales into the southern Chukchi Sea as monitored with passive acoustics. ICES J. Mar. Sci 73 (8), 2085-2092. doi: 10.1093/icesjms/fsv271
U.S. Department of the Interior (2008). Alaska annual studies plan final FY 2009 (Anchorage, Alaska: Minerals Management Service, Alaska Outer Continental Shelf Region). Available at: https://www.boem.gov/environment/environmental-studies/previous-years-studies-plans-alaska-region.
U.S. Department of the Interior (2018). Alaska annual studies plan FY 2018-2019 (Anchorage, Alaska: Bureau of Ocean Energy Management, Alaska Outer Continental Shelf Region). Available at: https://www.boem.gov/environment/environmental-studies/previous-years-studies-plans-alaska-region.
Van Parijs S. M. (2015). Letter of introduction to the Biologically important areas issue. Aq. Mam. 41 (1), 1. doi: 10.1578/AM.41.1.2015.1
Votrogov L. M., Ivashin M. V. (1980). Sightings of fin and humpback whales in the Bering and Chukchi seas. Rep. Int. Whal. Commn 30, 247–248. Available at: https://www.iwc.int/en.
Weingartner T. J., Okkonen S. R., Danielson S. L. (2021). “Physical oceanography,” in The bowhead whale Balaena mysticetus: Biology and human interactions. Eds. George J. C., Thewissan J. G. H. (London: Academic Press), 383–402.
Whalen D., Loseto L. L., Hornby C., Harwood L., Hansen-Craik K. (2019). Mapping and understanding the role of seabed morphology with respect to beluga whale hotspots and habitat use in the Mackenzie estuary, NT. Estuaries Coasts 43, 161–173. doi: 10.1007/s12237-019-00653-8
Wild L. A., Riley H. E., Pearson H., Gabriele C. M., Deland S. Biologically important areas II for cetaceans in U.S. waters – gulf of Alaska region. Front. Mar. Sci. (in review).
Willoughby A. L., Ferguson M. C., Stimmelmayr R., Clarke J. T., Brower A. A. (2020). Bowhead whale (Balaena mysticetus) and killer whale (Orcinus orca) co-occurrence in the U.S. Pacific Arctic 2009-2018: evidence from bowhead whale carcasses. Pol. Bio. 43, 1669–1679. doi: 10.1007/s00300-020-02734-y
Wood K. R., Overland J. E., Salo S. A., Bond N. A., Williams W. J., Dong X. (2013). Is there a “new normal” climate in the Beaufort Sea? Pol. Res. 32, 19552. doi: 10.3402/polar.v32i0.19552
Zagrebelnyy S. V. (2018). Whaling in Chukotka from 2013 till 2017, IWC paper SC/67B/AWMP/20 Rev1. Available at: https://www.iwc.int/en.
Zdor E. (2021). Subsistence whaling of the Chukotka indigenous peoples. Senri Ethnological Stud. 104, 75–92. doi: 10.15021/00009656
Zharikov K. (2018). Aboriginal subsistence whaling the Russian Federation in 2017, IWC paper SC/67B/AWMP/WP/02/. Available at: https://www.iwc.int/en.
Zharikov K. A., Litovka D. I., Vereshagin E. V. (2019). Aboriginal subsistence whaling in the Russian Federation during 2018, IWC paper SC/68a/ASW03. Available at: https://www.iwc.int/en.
Zharikov K. A., Litovka D. I., Vereshagin E. V. (2020). Aboriginal subsistence whaling in the Russian Federation during 2019, IWC paper SC/68b/ASW05. Available at: https://www.iwc.int/en.
Keywords: Arctic, cetacean, feeding, migratory, reproductive, species distribution
Citation: Clarke JT, Ferguson MC, Brower AA, Fujioka E and Deland S (2023) Biologically important areas II for cetaceans in U.S. and adjacent waters - Arctic region. Front. Mar. Sci. 10:1040123. doi: 10.3389/fmars.2023.1040123
Received: 08 September 2022; Accepted: 19 January 2023;
Published: 27 February 2023.
Edited by:
Piers Dunstan, Oceans and Atmosphere (CSIRO), AustraliaReviewed by:
Michael Jasny, Natural Resources Defense Council, United StatesCopyright © 2023 Clarke, Ferguson, Brower, Fujioka and Deland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janet T. Clarke, amNsYXJrZUB3aWxleWxhYnMubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.