- 1Archipelagos - environment and development, Lourdata, Greece
- 2Hellenic Center of Marine Research, Institute of Marine Biological Resources and Inland Waters, Heraklion, Greece
- 3Mediterranean Center for Environmental Monitoring (MedCEM), Sutomore, Montenegro
- 4Regional Administrate of Protected Areas (RAPA), Vlora, Albania
- 5Department of Philosophy and Cultural Heritage, Ca’ Foscari University of Venice, Venice, Italy
- 6Archipelagos - ambiente e sviluppo, Italia, Venice, Italy
Occasional but recurrent sightings indicate that the endangered Mediterranean monk seal is still present in most of its historical range within the Adriatic-Ionian region in the central Mediterranean Sea. However, in most of the adjacent countries, the species’ abundance and distribution are practically unknown. An actively reproducing sub-population with a minimum of 25 adult/sub-adult seals lives in the Greek central Ionian Sea. The latter can form a nucleus from which the entire Adriatic-Ionian Basin could be re-colonized if adequate conservation measures are implemented throughout the area and in a coordinated manner. We examine the historical presence in the region as a baseline for providing a benchmark for conservation. We further look into the species’ habitat availability, possibilities for a rapid population assessment and various parameters that are considered crucial for its conservation, such as the existence of Marine Protected Areas (MPAs), corridors for connectivity purposes as well as needs for raising public awareness. We recommend a series of interlinked actions within the framework of a conservation strategy the implementation of which will ensure the conditions for maintaining ecologically, demographically, and genetically viable sub-populations of this species emblematic for the entire Mediterranean Sea. To achieve this goal, a coalition of partners from this area is required in order to adopt the strategy and jointly implement the measures required.
1 Introduction
The first description of the Mediterranean monk seal, Monachus monachus (hereafter MMS) was made upon examination of an individual from Croatia in the Adriatic Sea by Hermann (1779). Yet, its distribution and abundance in the area is poorly known as is also for much of the rest of the Mediterranean. In ancient times, the species was described as abundant in its entire range: the Mediterranean and Black Seas, the NE Atlantic coasts of Africa and the Canaries, Azores and Madeira islands. The species’ massive depletion started in Roman times followed by the exploitation of large numbers in the Atlantic by Spaniards/Portuguese in the Middle Ages (Johnson, 2004).
Nowadays, the Mediterranean population of the endangered MMS is estimated to consist of about half of the world population (~700 individuals), primarily concentrated in Greek-Turkish waters (Karamanlidis et al., 2016). Although photo-identification projects are being carried out in the Aegean and Levantine Seas for more than a decade (Dendrinos et al., 2008; Karamanlidis et al., 2010; Kurt and Gücü, 2021), overall actual seal numbers remain largely unknown.
The MMS is listed in all relevant international Conventions and in Annex II (priority species) and Annex IV of the EU Habitats Directive. It has officially been declared as regionally extinct, vanished or vanishing by the IUCN (International Union for Conservation of Nature), UNEP-MAP (United Nation Environment Programme-Mediterranean Action Plan), SPA-RAC (Specially Protected Areas-Regional Activity Centre) and the GFCM (General Fisheries Commission for the Mediterranean) in many Mediterranean countries. A recent search, however, confirmed occasional sightings in most of these countries (Bundone et al., 2019). Nevertheless, its endangered status along with our limited knowledge on basic biological parameters dictate the immediate implementation of protection measures.
The Adriatic-Ionian Basin (hereafter Basin), in the crossroad between the eastern and western Mediterranean, is crucial for the overall recovery of the MMS. We discuss here a strategy framework for its conservation in this region focusing on the best studied areas: the eastern coasts of the Basin and the area of Salento/Italy. The supporting bibliographic research includes a total of 65 citations: 49 published papers (75%, peer-reviewed in magazines or in congresses) and 21 publications in the grey literature (25%, technical reports and PhD theses). It was mainly based on our own published research (31% of total data used), supplemented by bibliography gathered through the authors´ involvement in various relevant matters over the years, e.g. oceanography, fisheries and pollution (66% of total data used). The remaining 3% were peer-reviewed publications on issues we are not involved in, i.e. environmental noise and alien species.
2 Distribution and abundance - Past and present
2.1 General features of the area
The Basin (Figure 1) consists of the semi-enclosed Adriatic Sea and the Ionian Sea, open to the south. The Adriatic Sea is a generally shallow basin with sandy beaches along most of the Italian coast and mostly rocky eastern coasts. Water exchange with the Mediterranean takes place through the narrow Otranto Straits. Two currents dominate the circulation: the West Adriatic Current flows toward the southeast along the western coast while the East Adriatic Current flows northwest along the eastern coast. The Ionian Sea, with mostly rocky coasts along the western parts of its islands, is the deepest Mediterranean sub-basin exceeding 4500 m in depth. The general circulation typically consists of a northward flow along the Hellenic Trench and a southward flow along the Italian coastline (Artegiani et al., 1997a; Artegiani et al., 1997b).
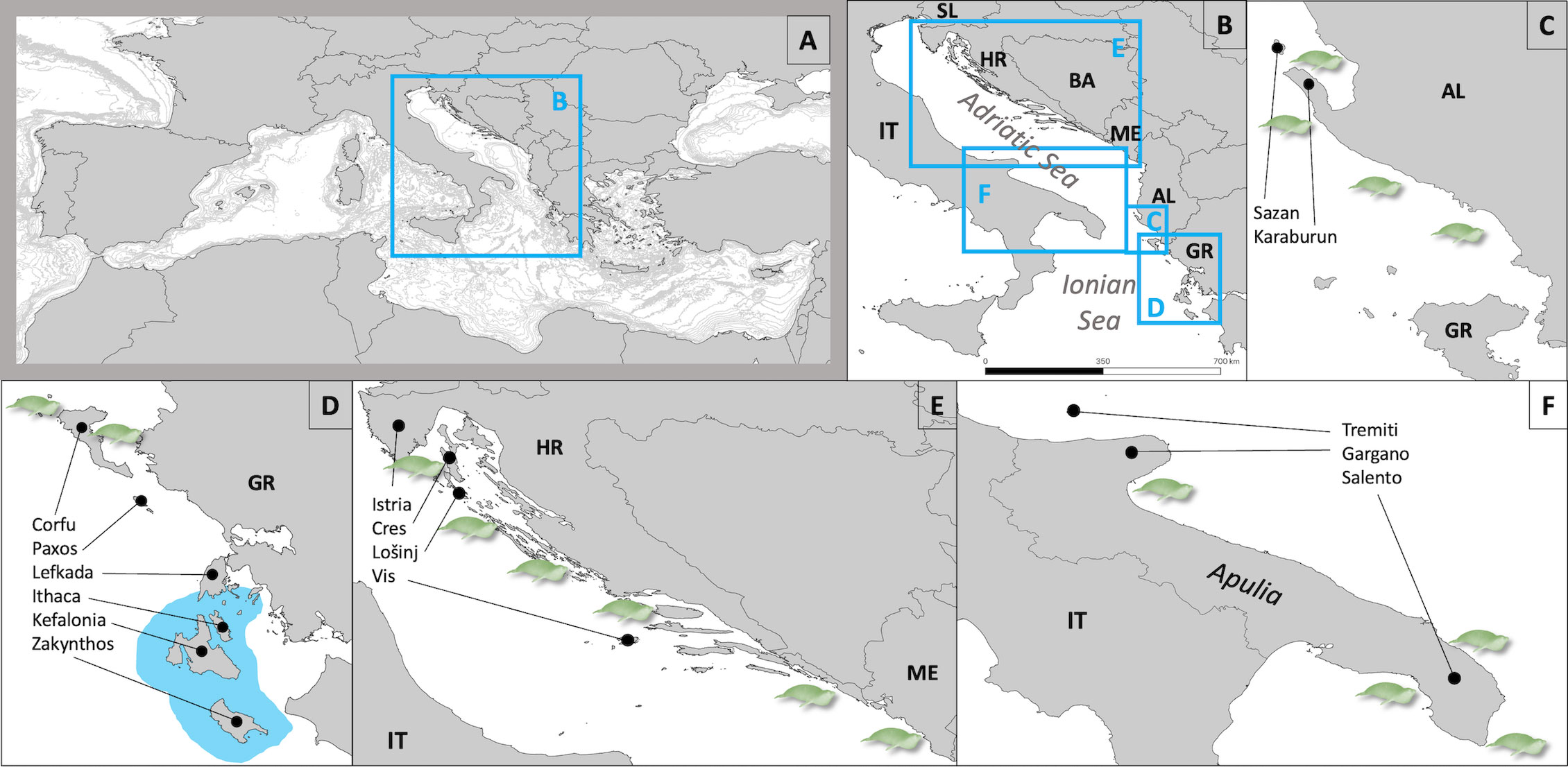
Figure 1 Map of the study area. (A) Mediterranean Sea and location of the study area. (B) Adriatic-Ionian Basin (country indication according to the IO 3166 international standard alpha-2 code). Main areas of study are displayed in detail in (C–F) In D, the distribution of the permanent reproductive population in the Ionian Sea is indicated by the blue color around the islands. Seal icons display the main localities of seal sightings as outlined in the text, they do not represent numbers of sightings.
The entire Basin is currently under strong overfishing pressure with around 20700 medium and small-scale vessels (FAO, 2020). Recently, ten out of thirteen demersal/small pelagic stocks were found exploited beyond safe biological limits (GFCM, 2022a; GFCM, 2022b).
The Adriatic Sea is a highly polluted area with densely populated coasts and intense commercial, touristic and industrial activities. Contamination/pollution includes hydrocarbons, heavy metals, pesticides, endocrine disrupters, faecal parasites, plastics/microplastics, all potentially affecting the MMS (Perić et al., 2016; Zeri et al., 2018; Klinčić et al., 2020; Bruschi et al., 2021; Spagnoli et al., 2021). The less populated Ionian Sea (with fewer studies though), seems to be less affected by pollutants such as oil spills, heavy metals and plastics/microplastics (Vlachogianni et al., 2017; Carpenter and Kostianoy, 2018; Molina Jack et al., 2020; Hatzonikolakis et al., 2022).
In the entire Basin, various activities often compete for the same space and resources such as fisheries, aquaculture farms and maritime traffic. Several MPAs and Fishing Restricted Areas with various levels of protection exist in the study area alongside maritime infrastructure such as cables, pipelines, oil, gas and wind installations.
2.2 Monk seal presence
The species’ status in the Basin is summarized below by country except for Slovenia and Bosnia & Herzegovina the short and heavily used coastlines of which are not particularly suitable for supporting a permanent MMS population (Figure 1; Table 1). Here, we focus on the species´ most critical habitat: marine caves with a beach inside, essential for resting and reproduction.
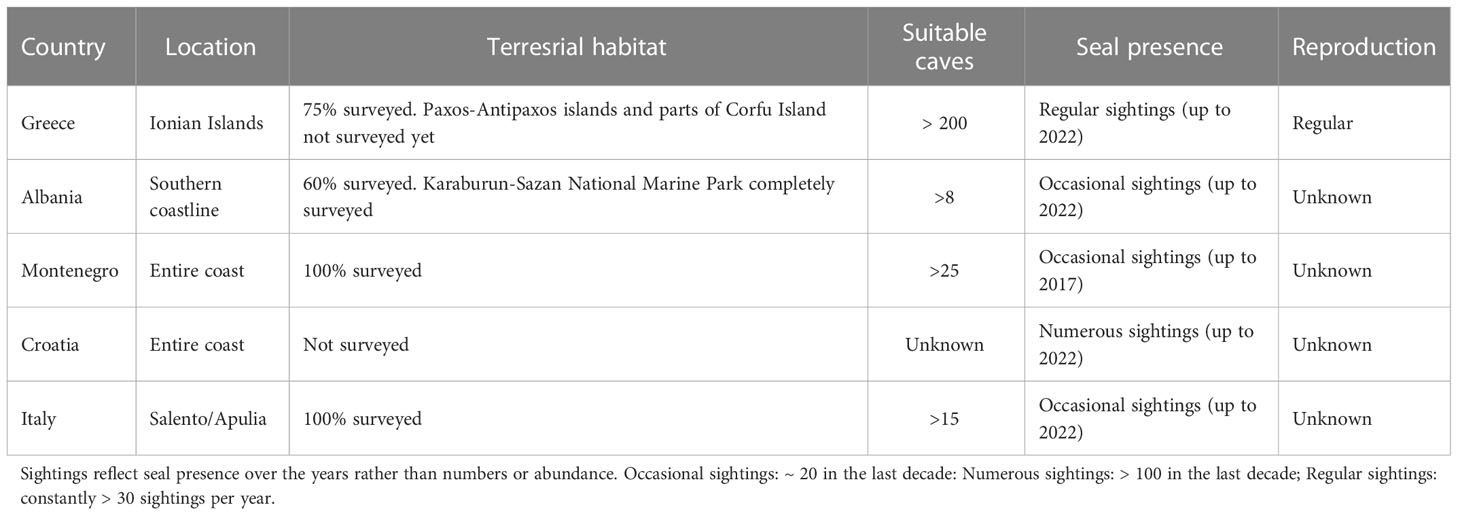
Table 1 Surveys of terrestrial habitats, suitable marine caves and monk seal presence in the Adriatic-Ionian Basin.
2.2.1 Ionian Sea, western Greece
The MMS has been quite abundant in the Ionian islands at least until the 19th century with “animals on Kefalonia/Zayknthos being more abundant than in the Adriatic” (Mohr, 1852) and regular seal hunting was reported from the area (Grasset de Saint Sauveur, 1800; Archduke Salvator, 1889). In the 1950’s, seals were still abundant in Kefalonia (Marinatos, 1962) but, in the 1970’s, numbers started declining (Marchessaux and Duguy, 1977).
In 1985-2002, systematic surveys of marine caves supported by observers’ and our own sightings, were conducted in the central Ionian Sea: Kefalonia, Ithaca and the islets around (Jacobs and Panou, 1988; Archipelagos, 1996). Cautious estimates revealed a minimum of 15-20 individuals of all age classes and up to 4 pups/year (Panou, 2009). In total, 38 caves/overhangs were used by seals, 5 of which also for pupping (Panou et al., 1993; Panou et al., 2002a). Parallel projects on Zakynthos in 1989-1999 revealed 25 caves along the W and NE coastline used by seals, 6 of which also for pupping with up to 4 pups/year (Panou et al., 2002b).
In the central Ionian Sea, a systematic photo-identification project was launched in 2018. Presently, 25 adult/sub-adult individuals are fully identified (animals not fully identified yet, juveniles and pups not included). Increased birth numbers (11 pups/year in 2020-2021) in 8 pupping caves indicate an increase in seal numbers (Panou et al., 2022). In 2021, a parallel photo-ID project was launched in Corfu (occasional sightings). Information on numbers from Zakynthos and Paxos is currently lacking.
2.2.2 Albania
Most publications related to the MMS presence in Albania provide no details on numbers, habitat use, reproduction, captures or killings. The most solid data were reported from the 1940’s onwards, most referring to single individuals. A total of 24 sightings from 2000-2020 were reviewed or directly documented, mostly of single animals (Bundone et al., 2021). Most sightings were recorded along the southern coast where the majority of suitable caves are located. During a photo-identification project, 8 marine caves were mapped in the Karaburun-Sazan National Marine Park as suitable habitat, the use of two caves was documented for the first time (Bundone et al., 2022) and, in 2022, two seals were found together in one cave (RAPA Vlore, unpublished data).
2.2.3 Montenegro
The scant information about the species’ historical presence in Montenegro is summarized by Panou et al. (2017). Data refer mainly to 3 animals captured before 1970, one of which was killed in 1969 as the “last” monk seal. Since this event, no further presence had been officially recorded and the species was officially considered as regionally extinct. A thorough survey revealed more than 25 caves potentially suitable for the MMS (Mačić et al., 2019) although no evidence of its presence was found. Parallel investigations however, revealed a total of 14 sightings in 1985-2010 throughout the country’s coastline (Panou et al., 2017). These findings confirm the species’ presence after 1969 and at least indicate movements of animals from neighboring countries.
2.2.4 Croatia
The MMS presence along the Croatian coast was known since ancient times. Mohr (1852) mentions “big enough colonies in Lošinj and Vis islands”. Until the 19th century, and despite the population being considered already heavily depleted, reports of sightings were numerous. Sightings and captures of animals continued throughout the coast until the 1980’s though with a decreasing trend, and reproduction was reported from some locations (Bruno, 1976; Gamulin-Brida et al., 1979). During the 1990’s, reports became rather rare referring to fewer animals per sighting (Cebrian, 1998). More than 300 sightings from the entire coast in 2000-2014 were ascribed only to a limited number of animals (Bundone et al., 2019). Habitat surveys were conducted only in the Vis Archipelago, in Istria and in the island of Cres (Antolovic et al., 2001; Bundone et al., 2013).
2.2.5 Eastern Italy, Southern Apulia
The generally sandy eastern Italian coast north of the Gargano peninsula is not particularly suitable as MMS habitat. The species was known to frequent the Tremiti Islands and the Gargano peninsula (Di Turo, 1984). A recent study focusing on Salento/Apulia summarized the historical information on the species’ presence, documented since the Paleolithic Period. However, already in the 17th century, the species was considered rare. Historical data, mostly of single seals, are documented up until 1988. Ten sightings of single seals and one of two seals were recorded in 2009-2014. A total of 15 caves suitable for the MMS were mapped in the area and 5 exhibited the best morphological characteristics but no evidence of use has been verified in the last decades (Bundone, 2016).
3 Discussion
Altogether, we deal here with a thriving and actively reproducing open sub-population of the MMS in the Greek Ionian Sea (possibly genetically slightly different from the Aegean population, Karamanlidis et al., 2016), and scattered seal sightings in the other countries of the Basin. Sightings continue being recorded in the last 3-5 years with an increasing rate including Sicily, Calabria and central-northern Apulia. However, basic data on abundance, distribution, use of caves and reproduction are lacking.
Given the ability of the MMS to travel up to 300 km within a few months (Adamantopoulou et al., 2011) and up to 70 km over the open sea (Ryan et al., 2014), we cannot exclude the possibility that seals from Greece may travel into the Adriatic Sea and, eventually, re-colonize the entire Basin while the prevailing currents as also the available habitats along the eastern coast are rather favorable. Thus, the existing network of protected terrestrial/marine habitats is extremely important for the species´ recovery and would facilitate the safe passage of seals through the Basin ensuring gene flow within the area but possibly also from more distant regions during potential long migratory movements (Hilty et al., 2020; Salmona et al., 2022).
An essential first step for MMS conservation is, thus, to determine habitat availability/suitability in areas not surveyed yet (Calabria, Sicily, parts of Apulia, Croatia and of the Greek Ionian Sea). The e-DNA analysis of samples from areas throughout the Basin might yield useful results (Valsecchi et al., 2022) as a non-invasive, low-cost method on where to concentrate conservation activities, compared to time-consuming, expensive questionnaire/field surveys with often questionable results. Questionnaire surveys focusing on fishers in areas already pre-selected via e-DNA analysis, along with broad public awareness actions encouraging the reporting of sightings, may further help to prioritize core areas with recurring sightings. Data on historical presence may be collected in parallel, thus, setting a baseline for the carrying capacity of each area, useful for formulating achievable conservation goals.
As a second step, a systematic long-term monitoring system in all promising areas is needed (White, 2019) for identifying (a) the most important caves and (b) actual seal numbers and other important parameters such as reproduction rates, pup survival and the population structure in general, necessary for the design of effective conservation measures. IR camera traps installed in caves for photo-identification purposes is an effective, inexpensive method (Kurt and Gücü, 2021; Bundone and Panou, 2022) since field surveys scattered in time and space may not yield satisfactory results (Jacobs and Panou, 1988). Implementing the same methodology throughout the Basin after a comprehensive training of the participants would be particularly useful. A common monk seal data base as a matching tool via machine learning and as a management tool for facilitating transboundary seal identification is of utmost importance here.
As home ranges, feeding grounds, mating areas and migratory patterns of the MMS are practically unknown, the existing MPAs may not cover sufficient core areas for resting/breeding and feeding. The use of telemetry may significantly fill the existing knowledge gaps. Accordingly, the extension of existing and/or the designation of additional MPAs may prove necessary in order to (a) function as connectivity corridors and (b) counterbalance one main threat to the MMS: the increasing loss of habitat the species has suffered in the last decades due to coastal development, mainly tourism.
The MPA status should ideally protect the MMS from human disturbance but mitigation measures should be implemented also in key areas outside MPAs since a decreasing tendency in cave use has been registered in areas with recent coastal development eventually resulting in a permanent loss of habitat (Ok et al., 2019).
Among potentially useful measures, habitat restoration in specific areas may be taken into consideration. However, this tool should be considered of minor priority as long as natural habitats are available, their natural regeneration remains possible and seal numbers are still low.
Site-based measures are probably unlikely to adequately protect this mobile species from Basin-wide diffuse pollution and other threats associated with fisheries and tourism (Fortuna et al., 2018). The implementation of the EU Marine Strategy Framework Directive may significantly contribute in reducing those threats. Tools such as Marine Spatial Planning (e.g. Krassanakis and Vassilopoulou, 2018) and Systematic Conservation Planning (e.g. Giakoumi et al., 2012) may greatly facilitate the selection of future MPAs on an ecosystem-based approach.
All measures for preventing and/or reducing pollution levels should be taken, particularly within MPA borders, especially regarding highly toxic heavy metals, organochlorine compounds and oil spills that may pollute critical caves for long periods of time. Marine litter is generally a major threat to marine mammals. Additionally, microplastics may pose a new threat to the MMS (Hernandez-Milian et al., 2020) as their impact on marine fauna is as yet largely unknown and methods for their reduction still under development. So far, effects of noise pollution on the MMS are unknown and research into the matter is required for the design of adequate mitigation measures, especially for establishing baseline data as already launched in Croatia (Rako et al., 2013).
Fisheries are an important issue as they affect the basis of the MMS’ food source but also are a potential direct threat to the species: in Greece, accidental entanglement in fishing gear is a main cause of human-induced mortality, mainly of sub-adults (Panou et al., 1993; Karamanlidis et al., 2008). The adoption of technical measures to reduce depredation on fishing gear is recommended along with possible fishing restrictions close to breeding caves.
Deliberate killing of seals has been and still is among the main causes of mortality: fishers traditionally have been killing seals throughout the Basin because of the considerable damage the MMS may cause to catch and gear (Archipelagos, 1998). Long-term public awareness actions may mitigate the levels of friction but the problem will not be solved unless beneficial measures for fishers are implemented, especially with increasing seal numbers due to effective protection measures and while marine resources remain at low levels.
Although there is no clear evidence that food shortage due to overfishing may significantly affect the MMS, an adequate number of MPAs strategically covering the entire Basin may partly counterbalance the reduction in marine resources and enhance their regeneration through the spill-over of stocks, especially if no-take zones are established within their borders and/or elsewhere. Other measures re-directing the fisheries branch towards sustainability should be considered together with the responsible fisheries authorities (e.g. GFCM, 2021a; GFCM, 2021b).
Climate change may also pose a threat to the survival of the MMS as the sea level rise may leave critical caves with no suitable beaches in some areas, and biodiversity along with ecosystem functioning may generally be disrupted (Coll et al., 2010) Additionally, the ever-increasing number of marine alien species may alter the ecosystem functioning (Steger et al., 2022) and thereby possibly affect the living conditions of the MMS.
Further research into various aspects such as the species’ genetics, movements, dietary preferences, etc., is required for improving our knowledge and, thus, support effective management options. Novel, non-invasive techniques are available such as e-DNA analysis (Valsecchi et al., 2022) or microplastics analysis (Hernandez-Milian et al., 2021), and may greatly facilitate the filling of gaps in the species’ biology and ecology.
The Annex IV, EU Habitats Directive requires the protection of every single individual. Trained and fully operational teams including veterinarians should be established in each country for the rescue and rehabilitation of ill, wounded or orphaned MMSs in appropriate rescue centers. Here, avoiding the habituation of seals to humans and proper plans for release are of utmost importance. Of course, the in situ conservation is the absolute priority with respect to the overall conservation goals.
All above interconnected measures may fail if not accompanied by a wide array of public awareness actions providing timely and accurate information in regular intervals and aiming at directly involving the relevant local stakeholders in conservation activities.
Main gaps in the species biology/ecology to be considered are habitat availability, population numbers and demographic parameters, movements, feeding grounds and, possibly, the genetic isolation. One main problem to deal with is the vast area to be covered, preferably simultaneously and using the same methodologies. Thus, the pre-selection of adequate and promising sites is necessary. Here, one main problem is the potential bias in the evaluation/verification of sightings, e.g. via questionnaires. Sightings reflect seal presence rather than numbers or abundance since they depend on the observers´ distribution in time and space as also on their ability to accurately describe their observations. Furthermore, the identification process should be carried out following a common and accurate methodology for obtaining comparable data, avoiding an overestimation of seal numbers.
4 Conclusion
The actively reproducing population in in the Greek Ionian Sea is crucial for the conservation and recovery of the species in the entire Basin. We believe that the MMS could regularly (re)use the Adriatic-Ionian coastlines if adequate conservation measures are implemented ensuring safe terrestrial habitats and sufficient food availability. We propose the establishment of an informal consortium of experts from each country adjacent to the Basin for adopting a common strategy and jointly implementing the measures required, in particular those with a transboundary component as also suggested by Mackelworth et al. (2019).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AP conceptualized and led the project, wrote and edited the manuscript including revisions. LB contributed substantially to the conceptualization, writing, editing and revisions. MG and LB elaborated the Figure. All authors contributed to the article and approved the submitted version.
Acknowledgments
We wish to thank the Monk Seal Alliance, Monaco, and the Department of Philosophy and Cultural Heritage of the Ca’ Foscari University of Venice, Italy, for funding the fees for this publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adamantopoulou S., Androukaki E., Dendrinos P., Kotomatas S., Paravas V., Psaradellis M., et al. (2011). Movements of Mediterranean monk seal (Monachus monachus) in the Eastern Mediterranean Sea. Aquat. Mamm. 37, 256–261. doi: 10.1578/AM.37.3.2011.256
Antolovic J., Vaso A., Kashta L., Shutina V., Anagnosti S., Bogdanovic S., et al. (2001). Protection of the Mediterranean monk seal (Monachus monachus) and its habitat. Rapp. Commun. Int. Mer. Médit. 36, 230.
Archduke Salvator L. (1889). Paxos und antipaxos im ionischen meere (Würzburg und Wien, Austria: L. Woerl).
Archipelagos (1996). Monk seal conservation in Greece. part 3, central Ionian Sea, Final Report to the European Commission, contract B4-3040/95/009/AO/D2. Compiled by A. Panou. Published by Archipelagos - environment and development, Lourdata, Kefalonia, Greece.
Archipelagos (1998). Technical-economical investigation of the effects of the Mediterranean monk seal on coastal fisheries on the western coasts of Zakynthos Island, Final report, sub-project WWF Greece, EU programme LIFE96NAT/GR/3225, contract B4-3200/96/500 «The Mediterranean monk seal in Greece: Conservation in action», co- ordinated by the Ηellenic Society for the Study and Protection of the Monk Seal HSSPMS- MOm). Compiled by A. Panou. Published by Archipelagos - environment and development, Lourdata, Kefalonia, Greece.
Artegiani A., Paschini E., Russo A., Bregant D., Raicich F., Pinardi N. (1997a). The Adriatic Sea general circulation. part I: air–sea interactions and water mass structure. J. Phys. Oceanogr. 27, 1492–1514. doi: 10.1175/1520-0485(1997)027<1492:TASGCP>2.0.CO;2
Artegiani A., Paschini E., Russo A., Bregant D., Raicich F., Pinardi N. (1997b). : The Adriatic Sea general circulation. part II: baroclinic circulation structure. J. Phys. Oceanogr. 27, 1515–1532. doi: 10.1175/1520-0485(1997)027<1515:TASGCP>2.0.CO;2
Bruno S. (1976). “Considerazioni sulla foca monaca mediterranea. storia, distribuzione e stato di monachus monachus (Hermann 1779) nel mare adriatico (mammalia, pinnipedia, phocidae),” in Scritti in memoria di augusto toschi, vol. 8. (supplemento alle Ricerche di Biologia della Selvaggina). Savignano, Italy. 91–110.
Bruschi A., Lisi I., De Angelis R., Querin S., Cossarini G., Di Biagio V., et al. (2021). Indexes for the assessment of bacterial pollution in bathing waters from point sources: The northern Adriatic Sea CADEAU service. J. Environ. Manage. 293, 112878. doi: 10.1016/j.jenvman.2021.112878
Bundone L. (2016). Piano di valutazione e conservazione della foca monaca monachus monachus in aree a bassa densità del mediterraneo (Venice, Italy: Università Ca’ Foscari di Venezia).
Bundone L., Antolovic J., Coppola E., Zalac S., Hervat M., Antolovic N., et al. (2013). Habitat use, movement and sightings of monk seals in Croatia between 2010 and 2012-2013. Rapp. Commun. Int. Mer. Médit. 40, 608.
Bundone L., Hernandez-Milian G., Hysolakoj N., Bakiu R., Mehillaj T., Lazaj L. (2021). Mediterranean Monk seal in Albania: Historical presence, sightings and habitat availability. J. Nat. Tech. Sci. 53, 89–100.
Bundone L., Hernandez-Milian G., Hysolakoj N., Bakiu R., Mehillaj T., Lazaj L., et al. (2022). First documented uses of caves along the coast of Albania by Mediterranean monk seals (Monachus monachus, Hermann 1779): Ecological and conservation inferences. Animals 12, 2620. doi: 10.3390/ani12192620
Bundone L., Panou A. (2022). Improvement of knowledge on the Mediterranean monk seal sub-population in the central Ionian Sea, Greece, using photo-identification. In 33rd Conference of the European Cetacean Society, 5–7 April 2022, Ashdod, Israel, 106. Book of Abstracts.
Bundone L., Panou A., Molinaroli E. (2019). On sightings of (vagrant?) monk seal, Monachus monachus, in the Mediterranean basin and their importance for the conservation of the species. Aquat. Conserv.: Mar. Freshw. Ecosyst. 29, 554–563. doi: 10.1002/aqc.3005
Carpenter A., Kostianoy A. G. (2018). “Oil pollution in the Mediterranean Sea: Part i. the international context,” in The handbook of environmental chemistry 84 (Springer Cham: Springer Nature Switzerland AG). doi: 10.1007/698_2018_368
Cebrian D. (1998). La foca monje (Monachus monachus Hermann 1779) en el Mediterraneo oriental (Grecia y Croacia) (Madrid, Spain: Universidad Complutense De Madrid).
Coll M., Piroddi C., Steenbeek J., Kaschner K., Ben Rais Lasram F., Aguzzi J., et al. (2010). The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS One 5, e11842. doi: 10.1371/journal.pone.0011842
Dendrinos P., Karamalidis A. A., Paravas V., Adamantopoulou S. (2008). Report of a new Mediterranean monk seal (Monachus monachus) breeding colony in the Aegean Sea, Greece. Aquat. Mamm. 34, 355–361. doi: 10.1578/AM.34.3.2008.355
Di Turo P. (1984). Presenza della foca monaca (Monachus monachus) nell’area mediterranea con particolare riferimento alla puglia. Thalassia Salentina 14, 66–84.
FAO (2020). The state of Mediterranean and black Sea fisheries -At a glance (Rome: General Fisheries Commission for the Mediterranean).
Fortuna C. M., Canadas A., Holcer D., Brecciaroli B., Donovan G. P., Lazar B., et al. (2018). The coherence of the European union marine natura 2000 network for wide-ranging charismatic species: A Mediterranean case study. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.356
Gamulin-Brida H., Mikulic Z., Simunovic A. (1979). Le statut recent du phoque moine - Monachus monachus (Hermann 1779) en Adriatique. Rapp. Commun. Int. Mer Médit. 25/26, 141–142.
GFCM (2021a). “Recommendation GFCM/43/2019/5 on a multiannual management plan for fisheries exploiting small pelagic stocks in geographical subarea 17 (northern Adriatic Sea) and on transitional conservation measures for fisheries exploiting small pelagic stocks in geographical subarea 18 (southern Adriatic Sea)”. In Compendium of GFCM decisions. Revised version 5.0. Food and Agriculture Organization of the United Nations (FAO) and General Fisheries Commission for the Mediterranean (GFCM). Rome, Italy, 192–200.
GFCM. (2021b). “Recommendation GFCM/37/2013/1 on a multiannual management plan for sustainable demersal fisheries in the Adriatic Sea (geographical subareas 17 and 18)”. In Compendium of GFCM decisions. Revised version 5.0. Food and Agriculture Organization of the United Nations (FAO) and General Fisheries Commission for the Mediterranean (GFCM). Rome, Italy, 47–59.
GFCM. (2022a). Report of the Scientific Advisory Committee on Fisheries (SAC), Working Group on Stock Assessment of Demersal Species (WGSAD). Food and Agriculture Organization of the United Nations (FAO) and General Fisheries Commission for the Mediterranean (GFCM). Rome, Italy.
GFCM. (2022b). Report of the Scientific Advisory Committee on Fisheries (SAC), Working Group on Stock Assessment of Small Pelagic Species (WGSASP). Food and Agriculture Organization of the United Nations (FAO) and General Fisheries Commission for the Mediterranean (GFCM). Rome, Italy.
Giakoumi S., Katsanevakis S., Vassilopoulou V., Panayotidis P., Kavadas S., Issaris Y., et al. (2012). Could European marine conservation policy benefit from systematic conservation planning? Aquat. Conserv.: Mar. Freshw. Ecosyst. 22, 762–775. doi: 10.1002/aqc.2273
Grasset de Saint Sauveur A. (1800). Voyage historique, littéraire et pittoresque dans les isles et possesions ci-devant vénitiennes du levant (Paris: Tome 3. Tavernier).
Hatzonikolakis Y., Giakoumi S., Raitsos D. E., Tsiaras K., Kalaroni S., Triantafyllidis G., et al. (2022). Quantifying transboundary plastic pollution in marine protected areas across the Mediterranean Sea. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.762235
Hermann J. (1779). Beschreibung der münchsrobbe. Beschäftigungen der Berlinischen Gesellschaft Naturfoschender Freunde 4, 456–509.
Hernandez-Milian G., Tsangaris C., Anestis A., Βundone L., Panou A. (2021). “Using monk seal faeces as a non-invasive technique to monitor the incidence of microdebris,” in 17th International Conference on Environmental Science and Technology (CEST2021)(Athens, Greece: CEST).
Hernandez-Milian G., Tsangaris C., Anestis A., Panou A. (2020). “More threats for the Mediterranean monk seal: Presence of microplastics in their diet. 532,” in Fate and impact of microplastics: Knowledge and responsibilities (Lanzarote, Spain), 23–27. Marine Sciences For Society (MSFS) & World Network of Island and Coastal Biosphere Research (WNICBR) Eds, Proceedings of “MICRO 2020”
Hilty J., Worboys G. L., Keeley A., Woodley S., Lausche B., Locke H., et al. (2020). Guidelines for conserving connectivity through ecological networks and corridors. best practice protected area guidelines series n°30. IUCN Published by IUCN, Gland, Switzerland. doi: 10.2305/IUCN.CH.2020.PAG.30.en
Jacobs J., Panou A. (1988). Conservation of the Mediterranean monk seal, monachus monachus, in kefalonia, Ithaca and lefkada isl., Ionian Sea, greece. institute of zoology, university of Munich, germany. final report, contract 6611/28 «Descriptive analysis and pilot project for the establishment of a conservation strategy for the monk seal and its habitat (Brussels, Belgium: Institut Royal des Sciences Naturelles de Belgique).
Johnson W. (2004). Monk seals in post-classical history. the role of the Mediterranean monk seal (Monachus monachus) in European history and culture, from the fall of Rome to the 20th century. mededelingen 39 (Netherlands: Netherlands Commission for International Nature Protection, Leiden).
Karamanlidis A. A., Androukaki E., Adamantopoulou S., Chatzispyrou A., Johnson W. M., Kotomatas S., et al. (2008). Assessing accidental entanglement as a threat to the Mediterranean monk seal Monachus monachus. Endang. Species Res. 5, 205–213. doi: 10.3354/esr00092
Karamanlidis A. A., Dendrinos P., Fernández de Larrinoa P., Gücü A. C., Johnson W. M., Kiraç W. M., et al. (2016). The Mediterranean monk seal Monachus monachus: Status, biology, threats, and conservation priorities. Mamm. Rev. 45, 92–105. doi: 10.1111/mam.12053
Karamanlidis A. A., Paravas V., Trillmich F., Dendrinos P. (2010). First observation of parturition and postpartum behaviour in the Mediterranean monk seal (Monachus monachus) in the Eastern Mediterranean. Aquat. Mamm. 36, 27–32. doi: 10.1578/AM.36.1.2010.27
Klinčić D., Romanić S. H., Katalinić M., Zandona A., Čadež T., Sarić M. M., et al. (2020). Persistent organic pollutants in tissues of farmed tuna from the Adriatic Sea. Mar. pollut. Bull. 158, 111413. doi: 10.1016/j.marpolbul.2020.111413
Krassanakis V., Vassilopoulou V. (2018). Introducing a data-driven approach towards the identification of grid cell size threshold (CST) for spatial data visualization: An application on marine spatial planning (MSP). J. Urban Environ. Eng. 12, 3–14. doi: 10.4090/juee.2018.v12n1.3-14
Kurt M., Gücü A. C. (2021). Demography and population structure of northeastern Mediterranean monk seal population. Mediterr. Mar. Sci. 22, 78–87. doi: 10.12681/mms.2913
Mačić V., Panou A., Bundone L., Varda D., Pavićević M. (2019). First inventory of the semi-submerged marine caves in south dinarides karst (Adriatic coast) and preliminary list of species. Turkish J. Fish. Aquat Sci. 19, 765–774. doi: 10.4194/1303-2712-v19_9_05
Mackelworth P. C., Seker Y. T., Vega-Fernández T., Marques M., Lopes-Alves F., D’Anna G., et al. (2019). Geopolitics and marine conservation: Synergies and conflicts. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00759
Marchessaux D., Duguy R. (1977). Le phoque moine (Monachus monachus) en grece. Mammalia 41, 419–440. doi: 10.1515/mamm.1977.41.4.419
Marinatos S. N. (1962). Cephalonia (a recent historical and archaeological sketch) (Argostoli, Kefalonia, Greece: Publication of the Local Tourism Committee of Cephalonia), 51–82.
Mohr E. (1852). Die Robben der Europäischen Gewässer. Monographien der Wildsäugetiere, Vol. 12. P. Schöps, Frankfurt am Main, Germany. 221–229.
Molina Jack M. E., Bakiu R., Castelli A., Čermelj B., Fafandel M., Georgopoulou C., et al. (2020). Heavy metals in the Adriatic Ionian seas: A case study to illustrate the challenges in data management when dealing with regional datasets. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.571365
Ok M., Sakinan S., Gücü A. C. (2019). “A story of how a critical habitat of an endangered species is lost: The Mediterranean monk seal in the northeastern (NE) Mediterranean,” in World Marine Mammal Conference, Barcelona, Spain. 521, 7–12. Book of Abstracts (Barcelona, Spain: Society for Marine Mammalogy and European Cetacean Society)
Panou A. (2009). “Monk seal sightings in the central Ionian Sea - a network of fishermen for the protection of the marine resources. presented in the workshop «Who are our seals? moving towards a standardised population estimate approach for Monachus monachus,” in 23rd Annual Conference of the European Cetacean Society, Istanbul, Turkey. 2–4 (Istanbul, Turkey). Monachus Guardian, on-line database: https://www.monachus-guardian.org/catalog.htm.
Panou A., Anestis A., Ioannou G., Karavellas D. P., Liontou E., Potamitis A., et al. (2002b). “Zakynthos island, Ionian Sea, Greece: An important habitat for the Mediterranean monk seal, Monachus monachus,” in 9th International Congress on the Zoogeography and Ecology of Greece and Adjacent Regions. 119, 22–25(Thessaloniki, Greece: ICZEGAR).
Panou A., Aravantinos P., Kokkolis T., Kourkoulakos S., Lekatsa T., Minetou L., et al. (2002a). “The Mediterranean monk seal, Monachus monachus,” in 9th International Congress on the Zoogeography and Ecology of Greece and Adjacent Regions, Thessaloniki, Greece. 22–25 May 2002, Thessaloniki, Greece. 118. Book of Proceedings (Thessaloniki, Greece: ICZEGAR)
Panou A., Bundone L., Aravantinos P., Kokkolis T., Chaldas X. (2022). “Mediterranean Monk seal, a sign of hope: increased birth numbers and enlarged terrestrial habitat,” in 33rd Conference of the European Cetacean Society, Ashdod, Israel. 5–7. (Ashdod, Israel: European Cetacean Society)
Panou A., Jacobs J., Panos D. (1993). The endangered Mediterranean monk seal, Monachus monachus, in the Ionian Sea, Greece. Biol. Conserv. 64, 129–140. doi: 10.1016/0006-3207(93)90649-L
Panou A., Varda D., Bundone L. (2017). “The Mediterranean monk seal, Monachus monachus, in Montenegro,” in 7th International Symposium of Ecologist, Sutomore, Montenegro. 94–101. (Sutomore, Montenegro: ISEM7)
Perić T., Komadina P., Račić N. (2016). Wastewater pollution from cruise ships in the Adriatic Sea. Promet -Traffic &Transportation 28, 425–433. doi: 10.7307/ptt.v28i4.2087
Rako N., Picciulin M., Vilibić I., Fortuna C. (2013). Spatial and temporal variability of sea ambient noise as an anthropogenic pressure index: The case of the cres-lošinj archipelago, Croatia. J. Mar. Biolog. Assoc. U.K. 93, 27–36. doi: 10.1017/S0025315412001233
Ryan C., Cucknell A. C., Romagosa M., Boisseau O., Moscrop A., Frantzis A., et al. (2014). A visual and acoustic survey for marine mammals in the Eastern Mediterranean Sea during summer 2013 (Kelvedon, UK: International Fund for Animal Welfare, Marine Conservation Research International).
Salmona J., Dayon J., Lecompte E., Karamanlidis A. A., Aguilar A., Fernandez de Larrinoa P., et al. (2022). The antique genetic plight of the Mediterranean monk seal (Monachus monachus). Proc. R. Soc B 289, 20220846. doi: 10.1098/rspb.2022.0864
Spagnoli F., De Marco R., Dinelli E., Frapiccini E., Frontalini F., Giordano P. (2021). Sources and metal pollution of sediments from a coastal area of the central western Adriatic Sea (Southern marche region, Italy). Appl. Sci. 11, 1118. doi: 10.3390/app11031118
Steger J., Bošnjak M., Belmaker J., Galil B. S., Zuschin M., Albano P. G. (2022). Non-indigenous molluscs in the eastern Mediterranean have distinct traits and cannot replace historic ecosystem functioning. Glob. Ecol. Biogeogr. 31, 89–102. doi: 10.1111/geb.13415
Valsecchi E., Coppola E., Pires R., Parmegiani A., Casiraghi M., Galli P., et al. (2022). A species-specific qPCR assay provides new insight into range expansion of the Mediterranean monk seal (Monachus monachus) by means of eDNA analysis. Biodivers. Conserv. 31, 1175–1196. doi: 10.1007/s10531-022-02382-0
Vlachogianni Th., Anastasopoulou A., Fortibuoni T., Ronchi F., Zeri Ch. (2017). Marine litter assessment in the Adriatic and Ionian seas, IPA-Adriatic DeFishGear Project. Mediterranean Information Office for Environment, Culture and Sustainable Development (MIO-ECSDE) Athens-Greece, Hellenic Centre for Marine Research (HCMR) Athens-Greece, and Italian National Institute for Environmental Protection and Research (ISPRA) Rome-Italy.
White E. R. (2019). Minimum time required to detect population trends: The need for long-term monitoring programs. BioScience 69, 40–46. doi: 10.1093/biosci/biy144
Keywords: Mediterranean monk seal, Adriatic-Ionian Basin, distribution, abundance, conservation, re-colonization
Citation: Panou A, Giannoulaki M, Varda D, Lazaj L, Pojana G and Bundone L (2023) Towards a strategy for the recovering of the Mediterranean monk seal in the Adriatic-Ionian Basin. Front. Mar. Sci. 10:1034124. doi: 10.3389/fmars.2023.1034124
Received: 01 September 2022; Accepted: 10 January 2023;
Published: 06 February 2023.
Edited by:
Milica Mandic, University of Montenegro, MontenegroReviewed by:
Maria Sini, University of the Aegean, GreeceCopyright © 2023 Panou, Giannoulaki, Varda, Lazaj, Pojana and Bundone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aliki Panou, YWxpa2kucGFub3VAeWFob28uZ3I=
 Aliki Panou
Aliki Panou Marianna Giannoulaki
Marianna Giannoulaki Dušan Varda
Dušan Varda Lorela Lazaj
Lorela Lazaj Giulio Pojana
Giulio Pojana Luigi Bundone
Luigi Bundone