
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 16 March 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1011630
This article is part of the Research TopicInnovations in Fishing Technology Aimed at Achieving Sustainable FishingView all 23 articles
 Giacomo Sardo1*†
Giacomo Sardo1*† Luca Vecchioni2†
Luca Vecchioni2† Giacomo Milisenda3,4
Giacomo Milisenda3,4 Fabio Falsone1
Fabio Falsone1 Michele Luca Geraci1,5
Michele Luca Geraci1,5 Daniela Massi1
Daniela Massi1 Pietro Rizzo1
Pietro Rizzo1 Danilo Scannella1
Danilo Scannella1 Sergio Vitale1,4
Sergio Vitale1,4Discards remain among the main negative impacts of fishing activities, and their reductions are strengthened by the European Common Fisheries Policy (European Regulation 1380/2013). Trammel net fisheries appear more sustainable compared with other fishing techniques, especially from an ecological viewpoint. Despite this, reports show that trammel net fisheries deliver discard quantities between 10% and 43% of the total catch biomass. To supplement existing information, this current work attempts to address the discard reduction using guarding net in the small-scale fisheries of Egadi Islands MPA (Western Sicily, Central Mediterranean Sea). To assess the reduction of unwanted catches, 48 experimental fishing trials were conducted within a 6-month period. The experimental fishing trial employed a trammel net made up of 20 panels alternated with two different net configurations. The control panels (CN) held a large outer (180 mm) and small inner (31.25 mm) meshes. The test panels (GN) with guarding net constituted a three-mesh-high (50-mm mesh size) net placed between trammel net panels and a lead line. A total of 3,310 individuals belonging to 106 taxa and nine phyla were caught. Crustaceans were the most abundant unwanted catches in the control panels, whereas bioconstructions occurred in the guarding net panels. The discard ratios of CN and GN panels were statistically different (t-value = –2.55; p< 0.05). The analysis of catch per unit effort showed higher catches of CN panels for both commercial and discard fractions (p< 0.05). Moreover, the guarding net panels caught the main discarded species at 20% lower compared with the control. The overall value of the catch at the CN panels (€ 3,366.90) was higher than the total income (€ 2,043.70) generated using the GN panels, which suggests a significant commercial loss of 40% (p< 0.05).
Small-scale fishery (SSF) remains a tradition that has long been performed across countries bordering the Mediterranean Sea (Lucchetti et al., 2020) and is an important socio-economic sector at the local level for tourism, cultural implications, and fisher employment (Colloca et al., 2004; Vitale et al., 2011; Cohen and Foale, 2013; Falsone et al., 2020). The main fishing gears employed in SSF include set nets (trammel nets and gillnets) or longlines, which catch a variety of demersal resources (Tzanatos et al., 2005; Stergiou et al., 2006). Specifically, trammel nets with different characteristics are crucial in southern European countries such as Spain, Italy, and Greece (Salas and Gaertner, 2004; Tzanatos et al., 2005; Erzini et al., 2006; Grati et al., 2022). The trammel nets are more selective compared with the towed gears (Vitale et al., 2011; Petetta et al., 2021) and, in the Mediterranean Sea, are characterized by moderate discarding rates (Veiga et al., 2016). Moreover, small-scale fleets account for 83% (71.400 vessels) of the EU Mediterranean fleet and contribute to 15% of the EU catches (Maynou et al., 2013; FAO, 2020). The estimate of SSF discards in European waters depends on such factors as the fishing practices and market influence as well as métier and varies between 10% and 43% of the total catch biomass (European Commission (EC), 2002; Vassilopoulou, 2011; Tsagarakis et al., 2014). Typically, SSFs have moderate levels of discards per vessel, but the large small-scale fleets (e.g., Falsone et al., 2020) in the Mediterranean can produce a considerable amount of discards (Bellido et al., 2011). Discards are an integral part of most fishing operations since all gears catch species that are thrown back into the sea (Bellido et al., 2011; Roda et al., 2019). However, the discards involving marine organisms are not negligible and depict a waste of natural resources that impact negatively the marine ecosystem and become a worldwide problem for the sustainable management of marine fisheries (Kelleher 2005; Vitale et al., 2018; Sardo et al., 2020; Geraci et al., 2021a). Over the past few decades, several efforts by the European Commission (EC) have aimed to tackle fishery discard-related challenges/issues—for instance, the European Commission introduced the landing obligation in Art. 15 of the EU Common Fisheries Policy (EC Regulation 1380/2013) (European parliament and Council (EU), 2013) and adopted measures such as spatio-temporal fishery closures (i.e., fisheries restricted areas), landing quotas (e.g., tuna and swordfish) as well as minimum mesh sizes (EU STECF, 2008; Damalas and Vassilopoulou, 2013; De Vos et al., 2016; European parliament and Council (EU), 2019; Di Maio et al., 2022). Importantly, the EU encourages the adoption of more selective fishing gears and better control about the record of catches (Catanese et al., 2018). As trammel nets are one of the most commonly used gears in coastal waters worldwide (Gonçalves et al., 2008; Gökçe et al., 2016), there have been some studies on discards (Purbayanto et al., 2001; Coelho et al., 2005; Martínez-Baños and Maynou, 2018), but as with discard studies for most gears, the emphasis was on vertebrates and commercially valuable or protected species (Catanese et al., 2018; Brownell et al., 2019; Geraci et al., 2019; Swimmer et al., 2020; Buscaino et al., 2021). Nevertheless, ecologically important species such as habitat-builder species and invertebrates, species at risk, or small-sized individuals are also affected by discards, and such concerns regarding the ecological impact of trammel net fisheries on benthic communities within the coastal zone (Gonçalves et al., 2008; Metin et al., 2009; Aydin et al., 2013; Gökçe et al., 2016) and the unwanted by-catch (according to the ICES, 2020 classification) of benthic species with no commercial interest—for instance, crustaceans (crabs and hermit crabs), echinoderms (starfish, sea urchins and sea cucumbers), and gastropods (Gökçe et al., 2016). Previous works have focused on trammel net modifications (hanging ratio, different materials used to construct trammel nets, and mesh size) and devices such as acoustic deterrents and artificial lights to improve the selectivity of the gear and to reduce the interaction with unwanted by-catch species (Aydin et al., 2011; Maccarrone et al., 2014; Martínez-Baños and Maynou, 2018; Bruno et al., 2021). Other investigations have highlighted that adding a mono-cloth net with a larger mesh from 10 to 30 cm in height, called “guarding net”, at the bottom of the trammel net could represent an efficient solution to reduce the amount of unwanted species in trammel net fisheries (Sartor et al., 2007; Metin et al., 2009; Aydin et al., 2013; Gökçe et al., 2016). To supplement the existing information, this current work attempts to address the discard reduction using a guarding net in the small-scale fisheries of Egadi Island MPA (Western Sicily, Central Mediterranean Sea). The current study was designed within the framework of Project “GRECA”, a pilot project based on a measure of 3.5 of the EFF (European Fishery Fund) 2007/2013 which targets the importance of adopting low-impact fishing gears in the marine protected areas (MPAs).
The study took place at the Egadi Islands Marine Protected Area (Central Mediterranean, Italy; hereinafter referred to as “E-MPA”), located at about 7–9 km from the western coast of Sicily, across the Strait of Sicily and the Tyrrhenian Sea (Figure 1). This archipelago comprises three main islands (Favignana, Marettimo, and Levanzo) plus a few small rocky outcrops (Galeotta, Galera, Preveto, Formica, and Maraone) (Mannino et al., 2017). The 53.992-ha E-MPA, one of the largest in the Mediterranean Sea, was created in 1991 and subdivided into four management zones (Guidetti et al., 2008; D’Anna et al., 2016): (A) fully protected zone, where only scientific research is allowed, (B) buffer zone, where small-scale fishing gears are allowed, (C) peripheral zone, in which stipulated selective fishing gears and recreational activities are permitted, and (D) regulated trawling zone, where all legal fishery activities are permitted, including trawl fishing. Specifically, both SSF and trawl fishery activities require a prior authorization of the E-MPA managing body. In particular, local management rules allow the SSF activities within zones B and C using a minimum mesh size (knot to knot) and a total length of trammel nets of 50 mm and 2.000 m, respectively (DM 715/2010). In the E-MPA, SSF is practiced throughout the year in the shallow waters below 100 m in depth surrounding the Egadi Islands and characterized by soft muds with fluid surface film (Garofalo et al., 2004), playing an important role in terms of employment and harvest of fishery products (Maccarrone et al., 2014). Trammel nets are normally set on the seafloor in inshore areas during the afternoon and retrieved in the morning of the next day. In particular, Sepia officinalis (Linnaeus, 1758), Palinurus elephas (Fabricius, 1787) as well as Mullus surmuletus Linnaeus, 1758 are generally targeted.
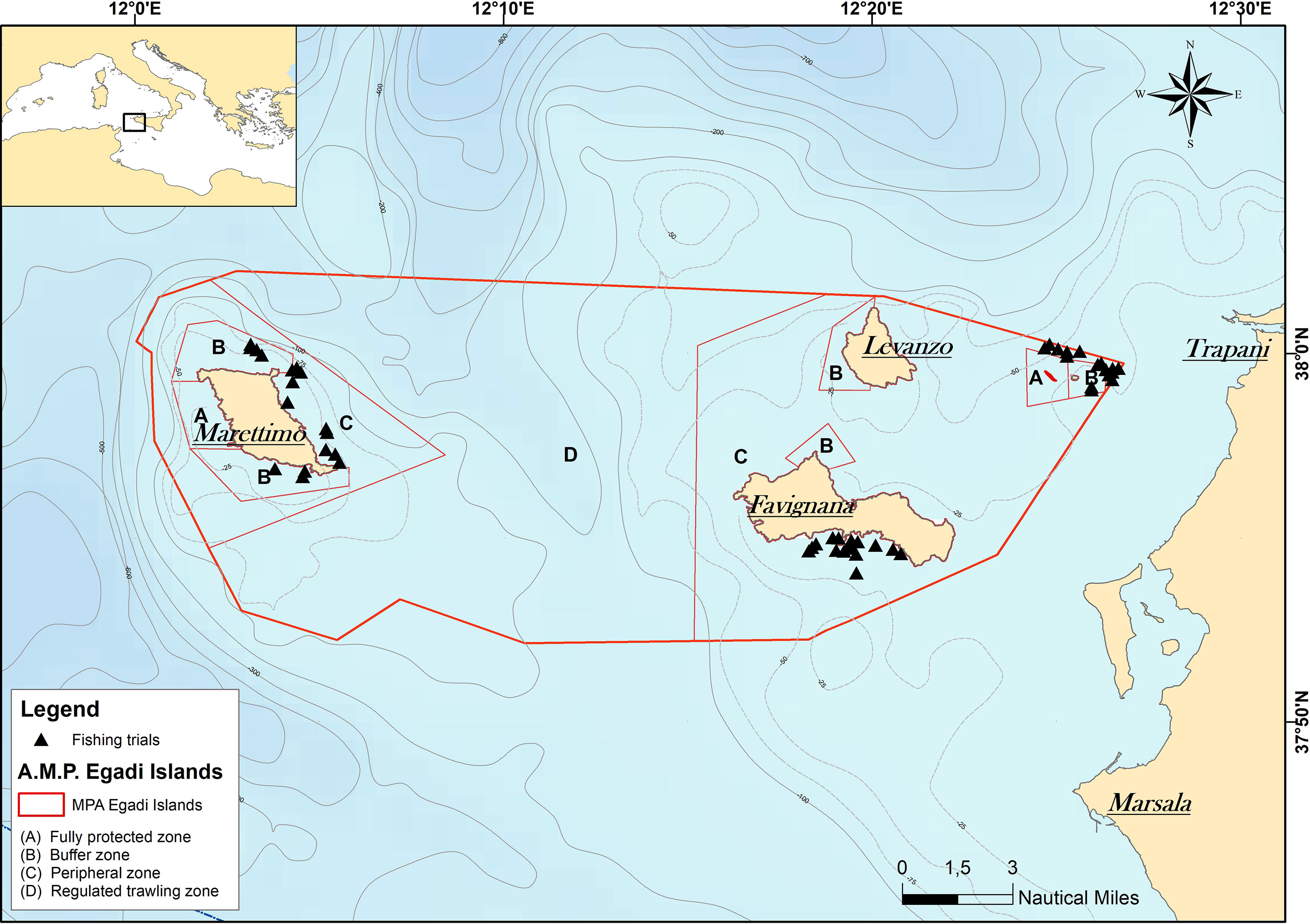
Figure 1 Map showing the location of the study. Red line, boundaries of the Egadi Islands MPA; Black triangles: sites of fishing trials; letters, existing sectoral initiatives in the marine protected area.
The experimental trammel net was designed so as to alternate standard commercial panels, designated as control (CN), and modified panels with guarding net (GN), designated as test. Thus, 10 control panels were constructed with two large mesh outer panels (225 meshes in depth, 180-mm mesh size, twine thickness 210/d12 PA, four meshes in height, and hanging ratio 0.6) and one smaller mesh inner panel (1,770 meshes in depth, 31-mm mesh size, twine thickness 210/d3 PA, 40 meshes in height, and hanging ratio 0.4). Moreover, 10 modified panels that differed with a monofilament guarding net made of three-mesh-high polyamide mesh (50 mm mesh size, 1,000 meshes in depth, and twine thickness 210/d6 PA) between the trammel net and the lead line and a different height of the mesh of the outer (3.5 meshes in height) and inner (35 meshes in height) panels were constructed (Figures 2A, B). In particular, the mesh size was measured from knot to knot. The floatline and the lead line of each panel were 50- and 52-m long, with 4- and 3-mm-diameter polyester, respectively. The donut-shaped polyester floats were 50 mm in diameter. The experimental trammel net that comprised 20 panels, each of 50 m of two different combinations (CN and GN) reached 1,000 m in length and 1.8 m in height (Figure 2C).
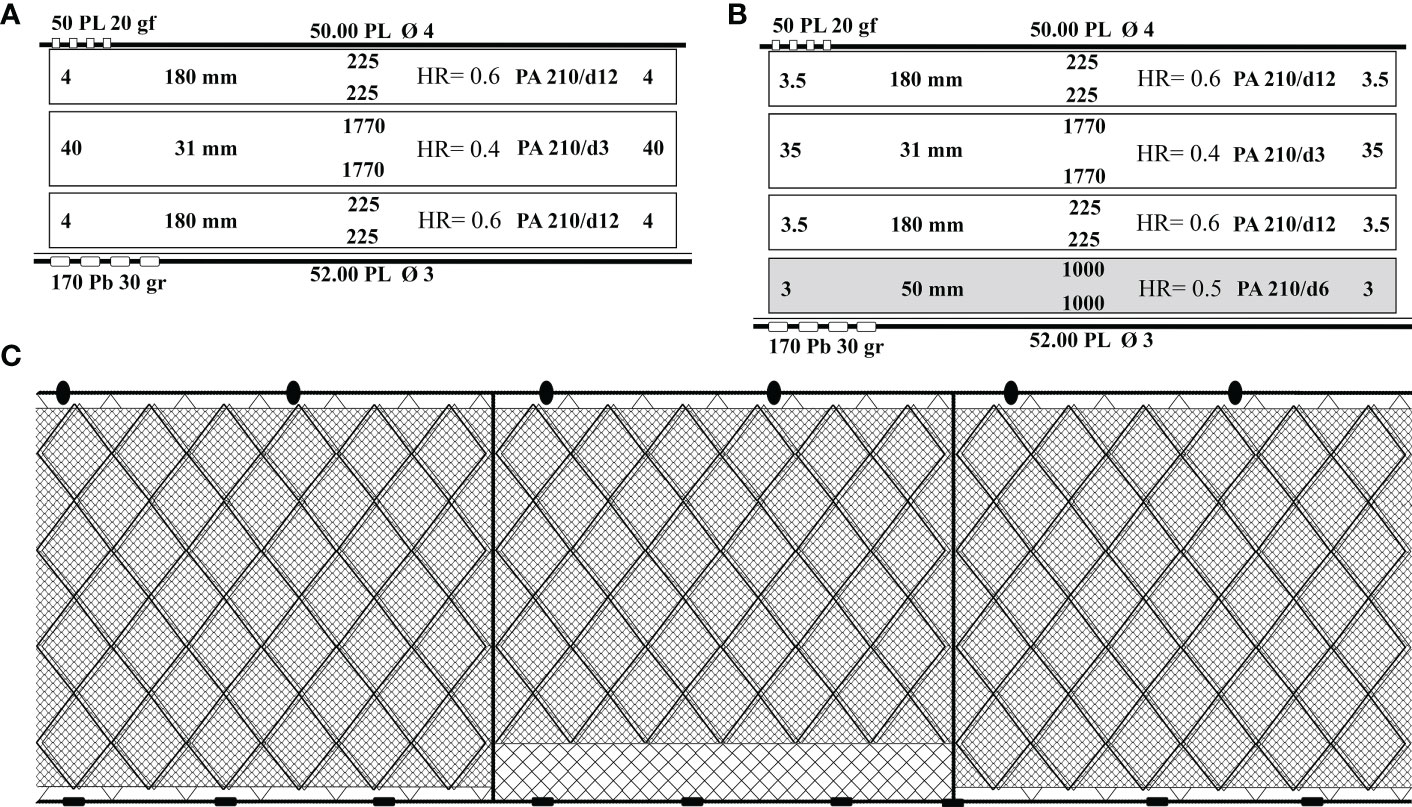
Figure 2 (A–C) Technical plans and illustrations of panels used in the trials. (A) Commercial panel (CN), (B) modified panel with guarding net (GN), and (C) illustration of the experimental trammel net. HR, hanging ratio; PL, polyester; PA, polyamide; Pb, lead; gf, floatability; gr, grams.
A schematic outlay of the experimental design is shown in Figure 3. Overall, there were six fishing vessels, with a length overall (LOA) comprised between 5.5 and 6.5 m, harbored in Favignana, Marettimo, and Trapani. Three different areas—namely, Favignana, Marettimo, and Formica—were randomly chosen within the “B” and “C” zones of the E-MPA (Figure 3). In particular, the vessels deployed in each area were the same during the entire period of experimental fishing trials and adhered to normal fishing practices. Eight fishing trials, with the setting of the gear in the afternoon (17:00 p.m.) and the hauling after sunrise (6:00 a.m.) of the next day (soak time of about 12 hours), were performed per vessel at a depth between 15 and 30 m. A total of 48 experimental fishing trials were carried out from January to June 2015, during the fishing season of Sepia officinalis. In total, 10 replicates were carried out per panel during each fishing trial, which amounted to a total of 960 replicates. Catches of each replicate were classified by the fishers as commercial or discard and labeled according to panel type and their order in the trammel net. Thereafter, the samples were stored in ice and transported to the laboratory of CNR–IRBIM of Mazara del Vallo where the identification of species at the lowest taxonomic level possible and biometric data were implemented. All organisms were numbered and weighed (0.1-g accuracy) both individually and as total by species.
The emergent data were standardized as the number of individuals per square meter (N/m2) and grams per square meter (g/m2) and divided into two groups, namely: (i) commercial catch and (ii) discards. Furthermore, the discard fraction comprised non-marketable undersized specimens, species with low commercial value, non-commercial invertebrate discard such as crustaceans, molluscs, echinoderms, bioconstructions, as well as the seagrass Posidonia oceanica (Linnaeus) Delile, 1813 and algae. Specifically, bioconstructions are characterized by non-identified species that belonged to Alcyonacea and Bryozoa taxa. Hence, three matrices (total catch, commercial catch, and discards) of species density (N/m2) per CN and GN panel combination and area were constructed to analyze the composition of catch and discard. Data were transformed using the square root transformation to minimize the dominant effect of abundant species (Field et al., 1982). Variables were checked for collinearity using a Pearson’s correlation matrix and a scatterplot plot of each pair of variables. Furthermore, the homoscedasticity assumption was assessed with a scatter plot of the residuals (Zuur et al., 2009). Matrices were computed into triangular matrices of similarities using the Bray–Curtis similarity index (Bray and Curtis, 1957). Subsequently, a four-way permutational multiple analysis of variance (PERMANOVA, Anderson, 2001) was performed, which considered four factors, namely: net type, fixed with two levels (CN and GN); area, fixed and orthogonal with three levels (Favignana, Marettimo, and Formica); month, random and orthogonal, with five levels (number of the months); and vessel, fixed and nested in area, with two levels (number of vessels per area). Furthermore, the similarity of percentages (SIMPER) analysis (Clarke, 1983) was performed in order to identify species that mainly contribute to the similarity either within CN and GN panels and across the groups of total catch, commercial catch, and discards. In addition, non-metric multidimensional scaling (NMDS) of the three matrices was performed to graphically highlight differences in assemblage of catches across control and experimental panels. In particular, due to the high number of replicates, only the centroids from the interaction between factors month, area, and net were considered. Stress coefficient values<0.2 (Field et al., 1982) were considered as a good representation (Clarke, 1993). The probability level of p<0.05 was considered as statistically significant. Furthermore, catch per unit effort (CPUE; expressed as g/km of commercial and discard fractions) as well as the most abundant commercial species were subjected to Shapiro–Wilk normality, which resulted in a non-parametric (p< 0.05) outcome. Thus, a Kruskal–Wallis H-test was performed to determine the overall differences in the CPUEs of commercial and discard fractions between CN and GN panels. In addition, a Dunn post-hoc test was performed to establish differences in the CPUEs of taxa between commercial and discard fractions. Probability level p< 0.05 was considered statistically significant. Furthermore, fishers involved in the fishing trials set the price per species according to the local fish markets (Favignana, Marettimo, and Trapani). Thus, total income per vessel and panel, respectively, were recorded, and one-way analysis of variance (ANOVA) was performed to compare the economic performance of the two types of panels, which were subsequently converted to economic mean value (€).
Discard ratio was defined as the discard fraction of the total catch for each type of panel (CN and GN) and calculated as:
with the biomass expressed in weight and the Dratio ranging between 0 and 1.
The GAMLSS model was fitted by specifying a beta error family distribution, which allowed for responded variables of a value between 0 and 1 (Stasinopoulos and Rigby, 2007). Through the model, it was possible to test whether the discard rate (discard ratio) was significantly different between the two levels of the explanatory variable “trammel type” (CN vs. GN). The model validation was performed through checking the absence of a residual pattern and their normality. The statistical analysis employed Primer 6 and Permanova+ (Clarke & Gorley, 2006; Anderson et al., 2008) software for Windows (Plymouth Routines in Multivariate Ecology Research, Auckland, New Zealand) and R Statistical Environment (R Core Team, 2022) using the gamlss library (Wood, 2006).
A total of 106 taxa, including 98 species and five genera, were caught by the experimental trammel nets (Supplementary Table S1). Specifically, the fishing trials resulted in a catch of 3,310 individuals belonging to seven zoobenthic phyla: Cnidaria (Anthozoa), Mollusca (Gastropoda, Bivalvia, and Cephalopoda), Arthropoda (Malacostraca), Echinodermata (Asteroidea, Echinoidea, and Holothuroidea), Chordata (Chondrichthyes and Osteichthyes), and two phytobenthic phyla: Tracheophyta and Rhodophyta. The biomass of taxa using control and experimental panels per area are shown in Table 1. The total catch weight was 498 kg, and 30.5% of it was represented by discards.
The NMDS ordination for the matrices of total catch, commercial catch, and discards showed patterns of catches in relation to CN and GN panels (Figures 4A–C). Furthermore, the PERMANOVA results showed a significant value for area/net type as well as the interaction between month and vessel for both total catch and non-commercial invertebrate discard (Supplementary Tables S2, S4), whereas net type/vessel and the interaction between month and vessel were significant for the commercial catch (Supplementary Table S3). The absence of a significant interaction between area and net type for both commercial catch and discards suggests that species assemblages for CN and GN panels in different areas of the E-MPA did not differ. The results of the SIMPER analysis identified species that contributed to the separation between CN and GN panels among commercial and discard catches. In particular, Scorpaena porcus Linnaeus, 1758 and Sepia officinalis Linnaeus, 1758 were the most abundant species, with S. porcus having a contribution to the similarity for the total catch and commercial catch matrices of 18.92% in CN and of 24.16% in GN, while S. officinalis contributed 27.26% in CN and 28.33% in GN (Tables 2, 3). Specific to the discards, the SIMPER analysis showed the most representative groups using CN and GN panels to be Crustacea and bioconstructions, with 28.21% and 15.13% similarity, respectively (Table 4). Regarding CN panels, Crustacea was followed by bioconstructions, Osteichthyes, Gastropoda, and Asteroidea, whereas Crustacea, Osteichthyes, Gastropoda, and Chondrichthyes followed bioconstructions for GN panels (Table 4).
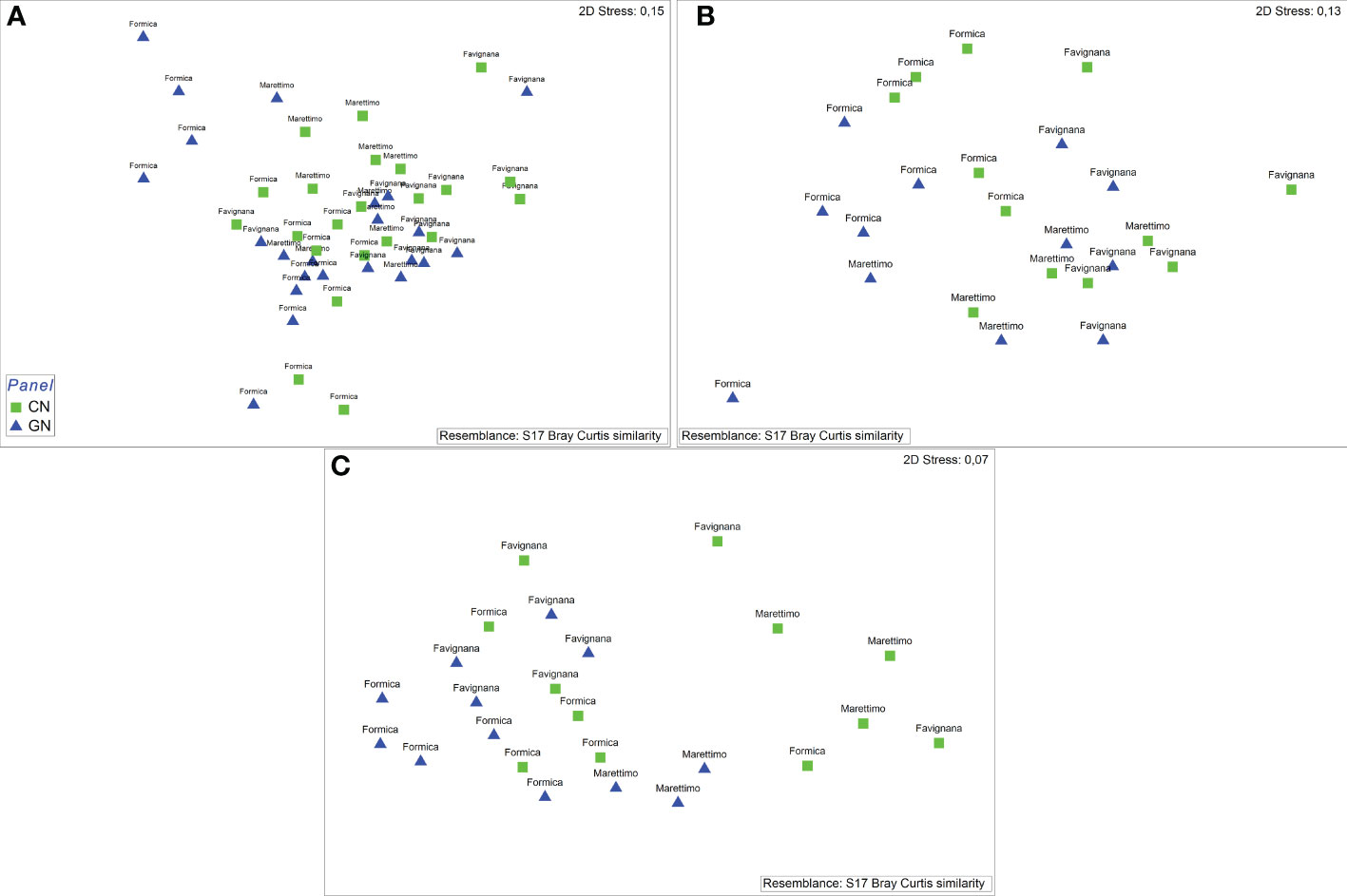
Figure 4 (A–C) Non-metric multidimensional scaling plot of total catch abundance (A), commercial catch (B), and non-commercial invertebrate discard (C) caught in 48 experimental fishing trials with standard (CN) and modified (GN) panels by area.
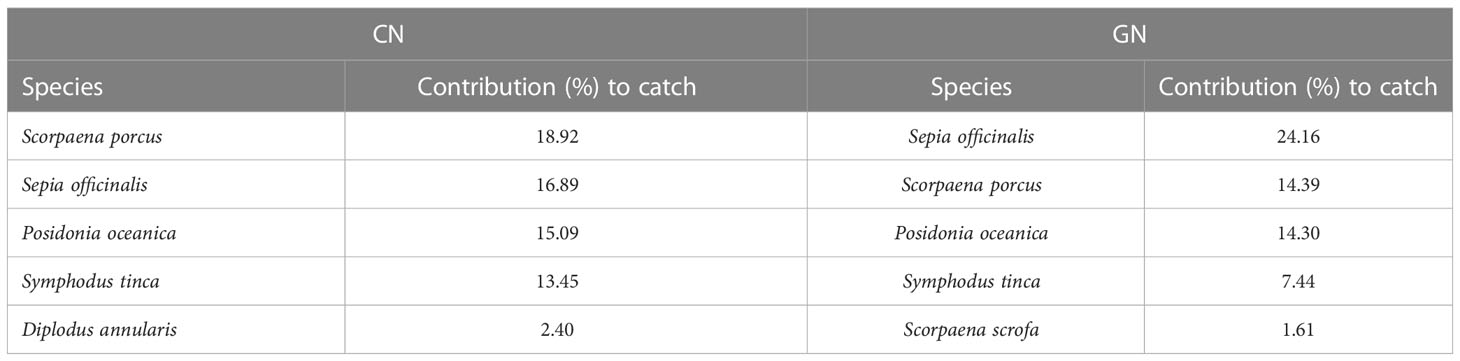
Table 2 Percentage contribution of the first five typifying species (over 1.5%) to within-group similarity for the standard (CN) and modified (GN) panels of total catch as assessed by similarity percentage analysis.
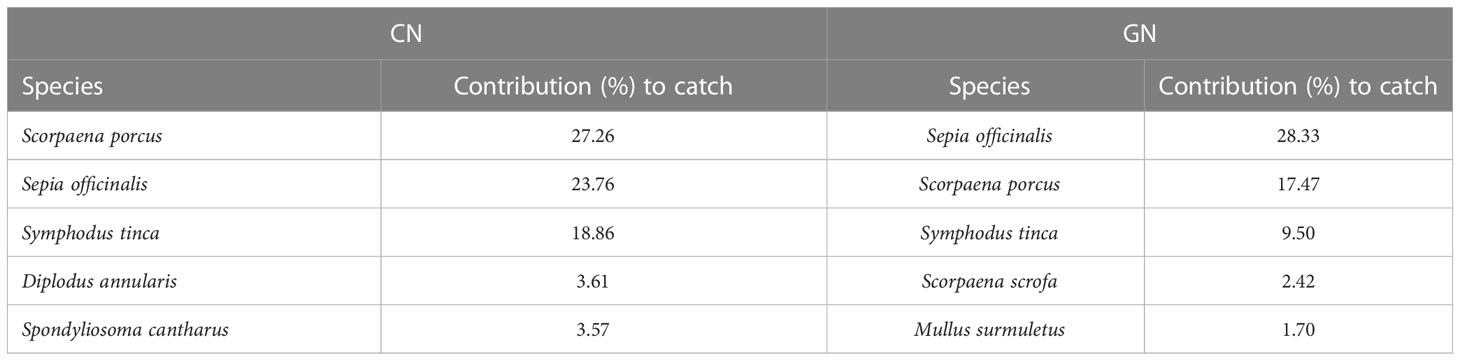
Table 3 Percentage contribution of the first five typifying species (over 1.5%) to within-group similarity for the standard (CN) and modified (GN) panels of commercial catch as assessed by similarity percentage analysis.
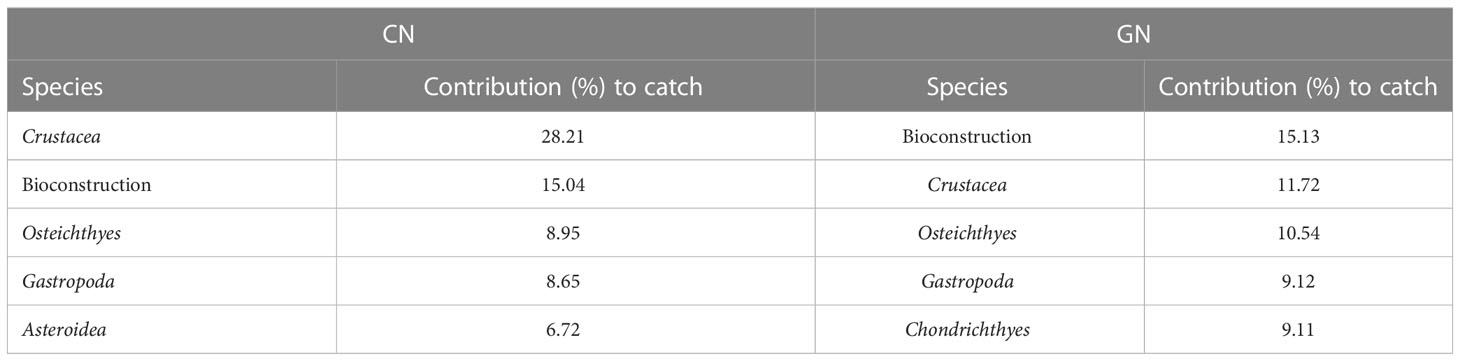
Table 4 Percentage contribution of the invertebrate discard (over 1.5%) to within-group similarity for the standard (CN) and modified (GN) panels as assessed by similarity percentage analysis.
The analysis of CPUEs showed higher catches of CN panels for both commercial (CN, 6,442 ± 1,887; GN, 3,377 ± 1,294) and discard (CN, 1,437 ± 685; GN, 659 ± 521) fractions (p< 0.05) (Figure 5). Moreover, Osteichthyes (CN, 5,393 ± 1,367; GN, 2,510 ± 681), Cephalopoda (CN, 38,333 ± 6,009; GN, 26,000 ± 4,932), and Crustacea (CN, 3,328 ± 1,032; GN, 1,395 ± 1,050) were the most caught taxa of CN panels (p< 0.05). As for discard, Mollusca (CN, 33,333 ± 4,409; GN, 18,918 ± 4,441), Echinodermata (CN, 4,100 ± 1,209; GN, 972 ± 366), bioconstructions (CN, 11,000 ± 2,081; GN, ±) (Figure 6), and P. oceanica (CN, 1,198 ± 178; GN, 721 ± 86) (Figure 7) were the most caught taxa/groups (p< 0.05) of CN panels, while Chondrichthyes (CN, 16,678 ± 4,602; GN, 29,238 ± 5,524) was the most caught taxon of GN panels (p< 0.05) (Figure 6).
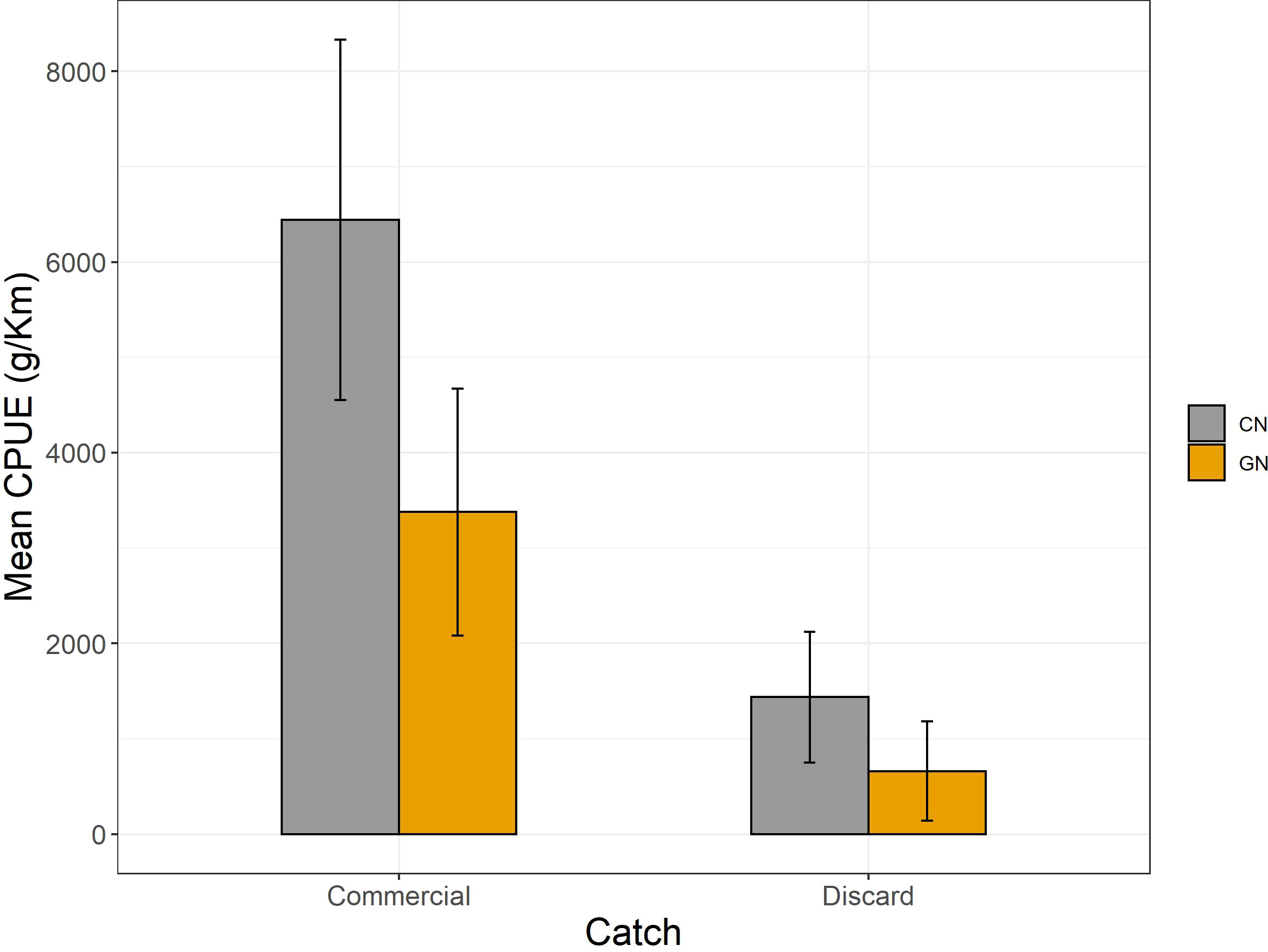
Figure 5 Mean catch per unit effort expressed as g/km for commercial and discarded catches per commercial (CN) and modified (GN) panel, respectively.
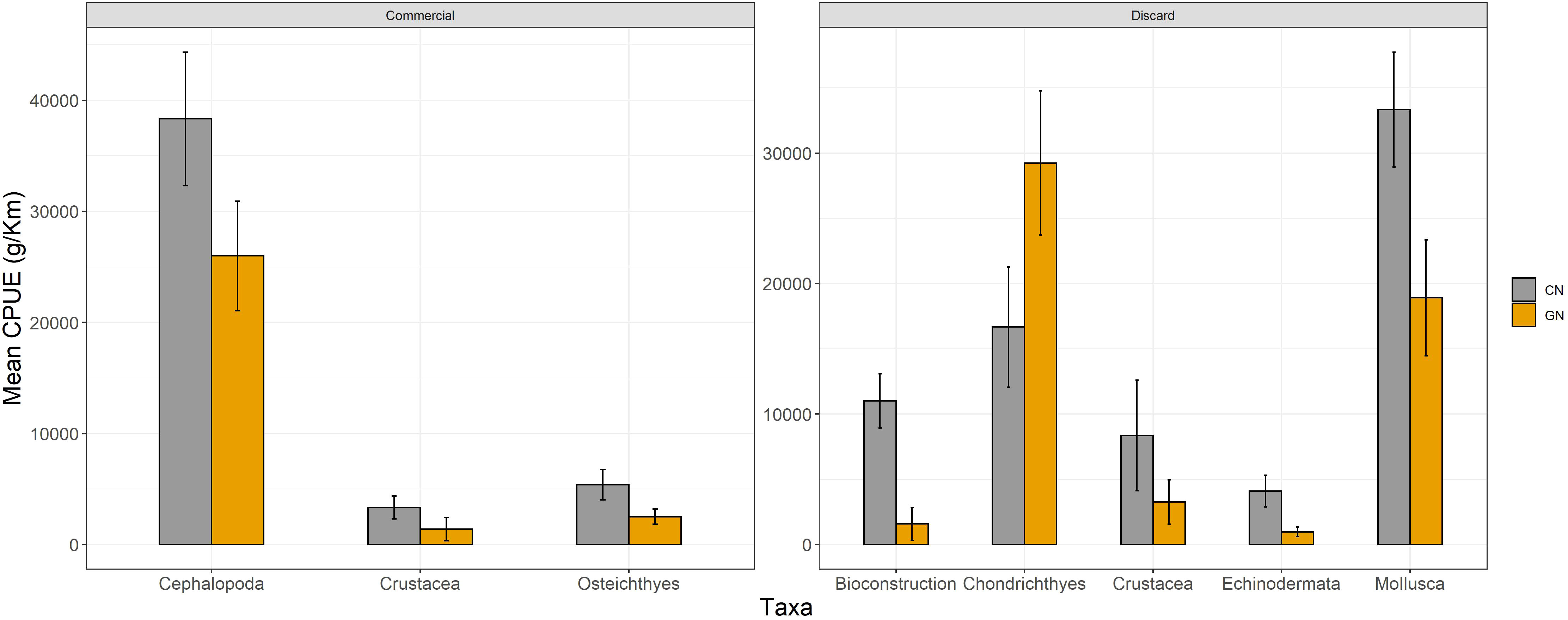
Figure 6 Mean catch per unit effort expressed as g/km of most abundant taxa for commercial and discarded fractions per commercial (CN) and modified (GN) panel, respectively.
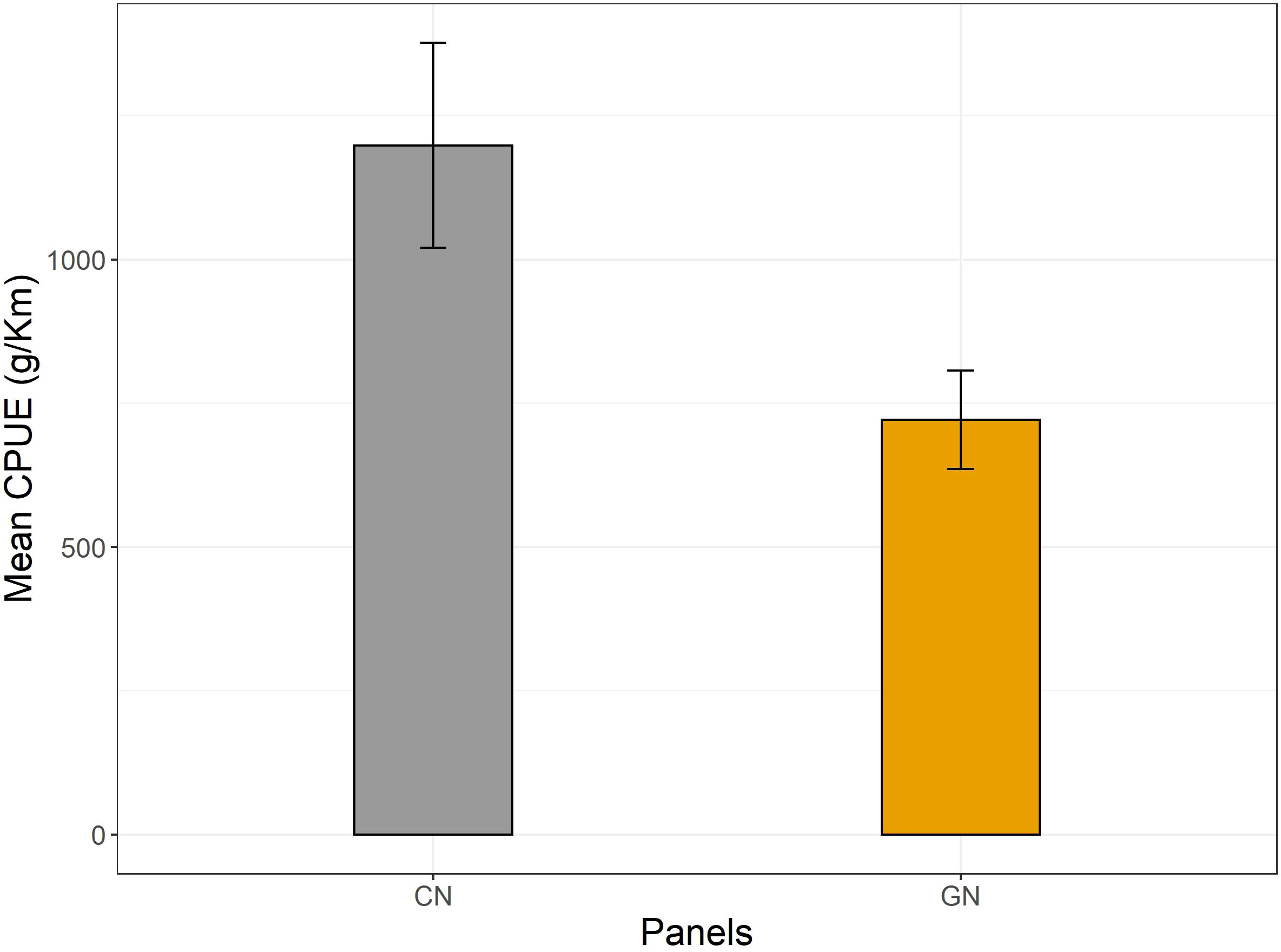
Figure 7 Mean catch per unit effort expressed as g/km of Posidonia oceanica per commercial (CN) and modified (GN) panel, respectively.
The discard composition was constituted by 50 species, mainly crustaceans—such as Hexaplex trunculus (Linnaeus, 1758), Liocarcinus corrugatus (Pennant, 1777), and Dardanus calidus (Risso, 1827)—and bioconstructions (Alcyonacea, Actiniaria, and Bryozoa) for control and experimental panels, respectively (Supplementary Table S1). The discard composition was significantly different upon comparing area and vessel (p< 0.05). The discarded species with the highest weight at CN panels included P. oceanica (9.7 kg), Torpedo marmorata Risso, 1810 (8.4 kg), and Aplysia spp. (4.6 kg), while those at GN panels included T. marmorata (21.6 kg) and P. oceanica (6.1 kg). Overall, there was a significant reduction (20%) in the total weight of discards of GN (35 kg) compared with CN (43 kg). The discard ratios of CN and GN panels were statistically different (t value = –2.55; p< 0.05). The GN panels showed a lower discard ratio (0.18) than the CN panels (0.23).
The income and loss of commercial catch for both CN and GN panels are shown in Table 5. The overall value of the catch at CN panels (€ 3,366.90) was higher than the total income (€ 2,043.70) using the GN panels, which suggests a significant commercial loss of 40% (p< 0.05). Moreover, a higher loss of income was recorded in Marettimo (41.17%). The mean CPUEs of commercial species contributing to the difference in revenues between the two types of panels are shown in Figure 8. Notably, catches of Sepia officinalis (CN, 9,754 ± 736; GN, 9,102 ± 641) seem unaffected by guarding net panels (p > 0.05), whereas non-targeted commercial species such as Symphodus tinca (Linnaeus, 1758) (CN, 4,092 ± 292; GN, 2,525 ± 118), Mullus surmuletus (CN, 5,470 ± 808; GN, 2,641 ± 227), Scorpaena scrofa Linnaeus, 1758 (CN, 6,888 ± 847; GN, 4,999 ± 542), Scorpaena porcus Linnaeus, 1758 (CN, 3,392 ± 204; GN, 2,379 ± 145), Diplodus annularis (Linnaeus, 1758) (CN, 3,052 ± 606; GN, 1,916 ± 196), and Spondyliosoma cantharus (Linnaeus, 1758) (CN, 1,888 ± 198; GN, 1,113 ± 169) appeared with somewhat noticeable reduced catches (p< 0.05).
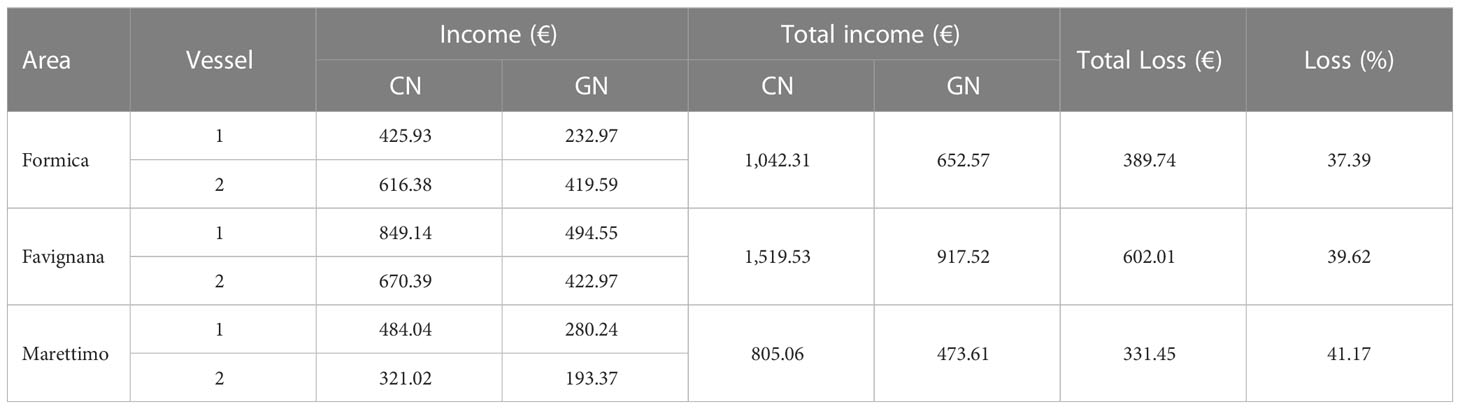
Table 5 Comparison of income and loss per area and vessel with standard (CN) and modified (GN) panels.
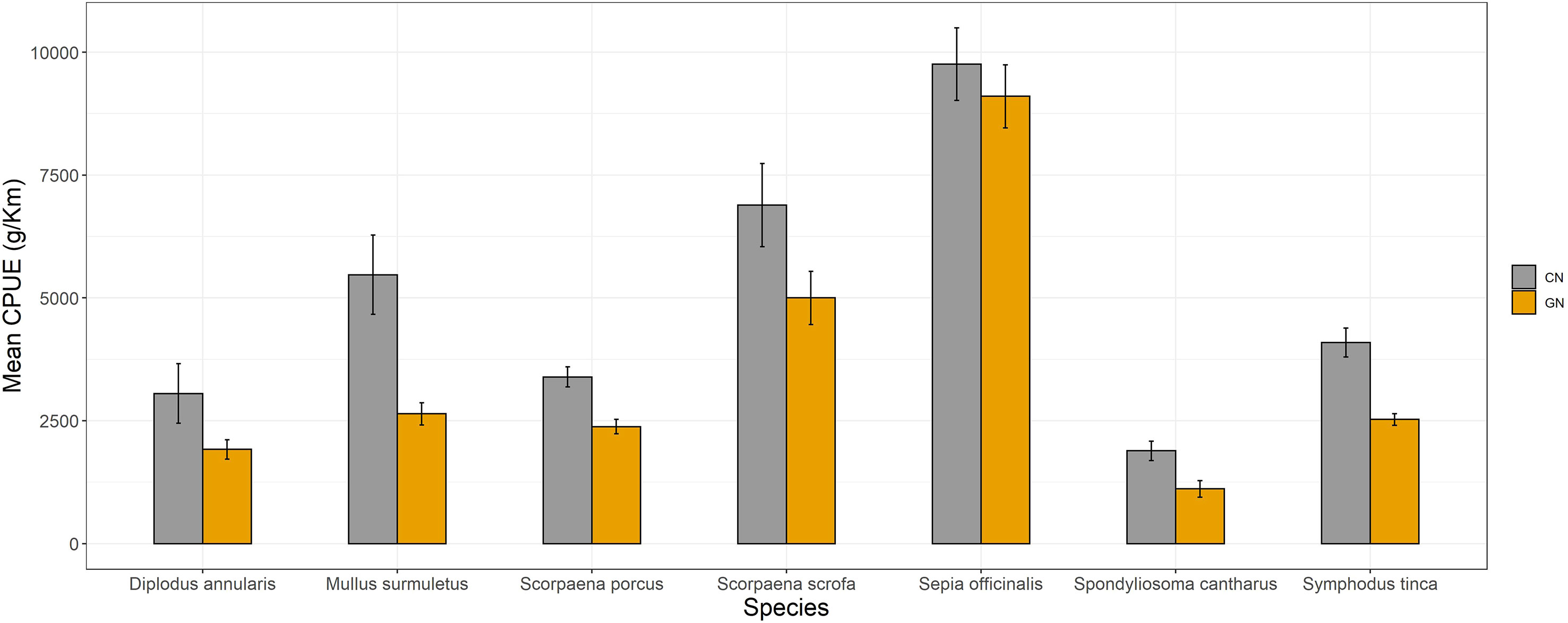
Figure 8 Mean catch per unit effort expressed as g/km of commercial species per commercial (CN) and modified (GN) panel, respectively.
This current study has demonstrated that the adoption of a guarding net fitted to the lead line of net reduced the catch of benthic invertebrates, and our findings appear to be in agreement with previous studies in the Mediterranean Sea (Sartor et al., 2007; Metin et al., 2009; Aydin et al., 2013; Gökçe et al., 2016; Martínez-Baños and Maynou, 2018; Szynaka et al., 2018). The composition of bycatch taxa in the control panels suggests that the discards, both in terms of abundance and biomass, comprised mainly of invertebrates such as crustaceans and gastropods. High fishing mortality of crustaceans would produce cascading effects on the various species that depend on this trophic resource, which suggests that their removal could reduce the diversity and complexity of the benthic community. Disentanglement of discarded invertebrates might increase the labor time on board and result to some damage to the net with the addition of weight and increasing contact with the seabed (Catanese et al., 2018; Szynaka et al., 2018). Among the benefits associated with the guarding net could be the catch reduction of predatory epifaunal invertebrates, which would be potentially feasible when a physical barrier is effected to the climbing scavengers that might damage the capture (Metin et al., 2009; Szynaka et al., 2018). Furthermore, the fragments of sessile organisms such as rhizomes and leaves of P. oceanica represented a non-negligible fraction of the discard and provided an insight about the interaction between the trammel net and the seagrass bed. In this study, the control panels caught more P. oceanica than the test panels. There is evidence that seagrass meadows serve as source of food and shelter as well as nursery for numerous marine species (Vlachopoulou et al., 2013)—for instance, crustaceans and gastropods were among the best-represented groups in the vagile fauna of P. oceanica beds (Russo and Terlizzi, 1997). Given its important role in coastal ecosystems and the status of endangered species, P. oceanica is protected through the EC Habitats Directive (92/43/EEC, 1992), and this encourages the establishment of MPAs for priority habitats. In addition, reductions in the bycatch of P. oceanica especially in sensitive ecosystems should be considered an important issue for sustainable fisheries. In particular, the E-MPA includes the best-preserved and the largest P. oceanica meadow of the Mediterranean Sea (about 7,700 ha) (Agius and Chaperon, 2021). Therefore, the adoption of the guarding net is advisable in order to reduce the impact of the trammel net on P. oceanica. Nevertheless, further investigations to identify technical devices aimed to mitigate interactions with P. oceanica should be considered.
In this study, some specimens of elasmobranchs such as T. marmorata showed great importance for the total discard biomass both in control and experimental panels despite being captured in low numbers. Plausibly, the higher catches of T. marmorata in the guarding net panels might be related to the size of the specimens. Small individuals could most likely pass through the guarding net as larger individuals are retained. The bycatch and discards of elasmobranchs would, therefore, be considered high (33.2%) for trawl fisheries (Coelho and Erzini, 2008; FAO, 2020; Geraci et al., 2021b; Falsone et al., 2022). Nevertheless, artisanal fishery such as trammel net (37.8%) and longline (7.7%) also provide a significant bycatch of elasmobranch species (Erzini et al., 2002; Baeta et al., 2010; Catanese et al., 2018; FAO, 2020). Notably, the yields of SSF depend on environmental and seasonal conditions as well as the quantity of target species caught during the fishing trip (Battaglia et al., 2010). It is important to mention that fishers in the Egadi Islands SSF seem to retain bycatch species with potential commercial value, such as Raja spp., especially when catches are low, probably to sustain their economic activity. Either the retention or discarding of elasmobranchs could differ based on the local fisher’s behavior—for instance, T. marmorata is retained for fishers’ own consumption, and large-sized specimens are marketed along the southern coasts of Sicily (Tiralongo et al., 2018), Algarve (Gonçalves et al., 2007), and Portugal (Baeta et al., 2010). On the contrary, T. marmorata is among the discards in the Ionian coast of Sicily (Tiralongo et al., 2018), Gulf of Cadiz (Gonçalves et al., 2007), and E-MPA. According to Gil et al. (2018), T. marmorata showed a low survival rate, using trammel nets, when immediately released to the sea.
Concerning the catch composition of commercial species, the present study corroborate with the seasonal dynamics of Sicilian SSF (Grati et al., 2018; Falautano et al., 2018; Falsone et al., 2020). Trammel net fishery in southwestern Sicily might provide high species variability of landings during the year according to the seasonal patterns of target species. Specifically, S. officinalis is exploited mostly in winter–spring, when mature specimens aggregate inshore for spawning from late fall to spring (Gharbi and Ktari, 1981; Colloca et al., 2004). In this study, guarding net panels caught more S. officinalis than S. porcus when compared with the control panels. Thus, guarding net panels might improve the catchability of trammel nets’ target cuttlefish within the E-MPA. The potential loss of revenue of about 40% seems likely in the commercial value of landings when a commercial trammel net is replaced with an alternative net aiming to reduce by-catch and discards. Such loss might be due to the lower catches of non-target marketable species, such as Spondyliosoma cantharus, Scorpaena scrofa, and Mullus surmuletus, that have been highly priced. Such occurrence might plausibly corroborate the mesh size of the guarding net, which could decrease the chance for the abovementioned fish species to be trapped. To the best of our knowledge, the contrasting results about the effects of guardian nets in SSF appears to be the situation that we found, which is consistent with those reported in scientific literature (Sartor et al., 2007; Metin et al., 2009; Martínez-Baños and Maynou, 2018). In particular, Sartor et al. (2007) showed that discards in trammel nets in the Tyrrhenian Sea were significantly reduced along with abundance of the target species caramote prawn Penaeus kerathurus (Forskål, 1775). On the other hand, Metin et al. (2009) in the Aegean Sea showed that the height of the guarding net is an important factor for discard reduction, whereas the decrease of commercial catch was considered not significant for the fishermen at Izmir Bay. Martínez-Baños and Maynou (2018) showed that guarding net reduced unwanted by-catch and increased the catches of commercial species in the Murcia Region, which would target cuttlefish and some fishes, by 30%. Sartor et al. (2018) considered that the economic loss that could happen due to the reduced catch of commercial species is offset by decreased sorting time and labor costs in the immediate short term. Other research, nonetheless, believe that a difference of incomes between the standard and test panels might create resistance toward the adoption of the guarding net by fishers (Szynaka et al., 2018). However, overcoming the economic loss might require the adoption of an eco-label marker for fishery products harvested with more sustainable fishing gears mounting the guarding net, as supported by the E-MPA managing body. The use of guarding net on the lead line of the trammel nets at Egadi Islands significantly reduced the catch rate of discards, which benefits the benthic invertebrate species. Nevertheless, further investigation on catch composition, specific to commercial and discarded fraction within the Sicilian SSF, should consider the effectiveness and suitability of guarding net by métier (Szynaka et al., 2018), season and depth (Stergiou et al., 2006), soak time, fishing grounds as well as the use of different combinations of mesh sizes both for inner and outer panels (Gonçalves et al., 2008).
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
LV, GM, and SV: conceptualization. LV and GM: formal analysis. GS, MG, FF, DS, and DM: data curation. MG, FF, DS, PR, and DM: data collection and figures. SV, DS, FF, and GS: validation. GS, LV, and GM: writing—original draft. MG, DS, FF, DS, DM, and SV: writing—review and editing. All authors contributed to the article and approved the submitted version.
The funding support for this study was from the project GRECA, FEP 2007/2013.
The authors express their appreciation to the fishers who provided access to their fishing vessels during the experimental sampling as well as to the technicians of the CNR-IRBIM laboratory for their assistance. We are grateful to Dr. Fabio Fiorentino and Dr. Charles O.R. Okpala for their useful comments that improved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1011630/full#supplementary-material
Anderson M. J. (2001). Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 58, 626–639. doi: 10.1139/f01-004
Anderson M., Gorley R. N., Clarke K. R. (2008). Permanova+ for primer: Guide to software and statisticl methods (Primer-E Limited).
Aydin I., Gokce G., Metin C. (2013). Using guarding net to reduce regularly discarded invertebrates in trammel net fisheries operating on seagrass meadows (Posidonia oceanica) in İzmir bay (Eastern Aegean Sea). Mediterr. Mar. Sci. 14 (2), 282–291. doi: 10.12681/mms.425
Baeta F., Batista M., Maia A., Costa M. J., Cabral H. (2010). Elasmobranch bycatch in a trammel net fishery in the Portuguese west coast. Fish. Res. 102, 123–129. doi: 10.1016/j.fishres.2009.10.016
Battaglia P., Romeo T., Consoli P., Scotti G., Andaloro F. (2010). Characterization of the artisanal fishery and its socio-economic aspects in the central Mediterranean Sea (Aeolian islands, Italy). Fish. Res. 102, 87–97. doi: 10.1016/j.fishres.2009.10.013
Bellido J. M., Santos M. B., Pennino M. G., Valeiras X., Pierce G. J. (2011). Fishery discards and bycatch: solutions for an ecosystem approach to fisheries management? Hydrobiologia 670, 317. doi: 10.1007/s10750-011-0721-5
Bray J. R., Curtis J. T. (1957). An ordination of the upland forest communities of southern wisconsin. Ecol. Monogr. 27 (4), 326–349. doi: 10.2307/1942268
Brownell R. L. Jr., Reeves R. R., Read A. J., Smith B. D., Thomas P. O., Ralls K., et al. (2019). Bycatch in gillnet fisheries threatens critically endangered small cetaceans and other aquatic megafauna. endanger. Species Res. 40, 285–296. doi: 10.3354/esr00994
Bruno C. A., Caserta V., Salzeri P., Bonanno Ferraro G., Pecoraro F., Lucchetti A., et al. (2021). Acoustic deterrent devices as mitigation tool to prevent dolphin-fishery interactions in the aeolian archipelago (Southern tyrrhenian Sea, Italy). Med. Mar. Sci. 22 (2), 408–421. doi: 10.12681/mms.23129
Buscaino G., Ceraulo M., Alonge G., Pace D. S., Grammauta R., Maccarrone V., et al. (2021). Artisanal fishing, dolphins, and interactive pinger: A study from a passive acoustic perspective. Aquat. Conserv.: Mar. Freshw. Ecosyst. 31 (8), 2241–2256. doi: 10.1002/aqc.3588
Catanese G., Hinz H., del Mar Gil M., Palmer M., Breen M., Mira A., et al. (2018). Comparing the catch composition, profitability and discard survival from different trammel net designs targeting common spiny lobster (Palinurus elephas) in a Mediterranean fishery. PeerJ 6, e4707. doi: 10.7717/peerj.4707
Clarke K. R. (1993). Non-parametric multivariate analisys of changes in community structure. Aust. J. Ecol. 18 (1), 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Coelho R., Erzini K. (2008). Effects of fishing methods on deep water shark species caught as by-catch off southern Portugal. Hydrobiologia 606, 187–193. doi: 10.1007/s10750-008-9335-y
Coelho R., Erzini K., Bentes L., Correia C., Lino P. G., Monteiro P., et al. (2005). Semipelagic longline and trammel net elasmobranch catches in southern Portugal: catch composition, catch rates and discards. J. Northw. Atl. Fish. Sci. 35, 531–537. doi: 10.2960/J.v35.m482
Cohen P. J., Foale S. J. (2013). Sustaining small-scale fisheries with periodically harvested marine reserves. Mar. Policy 37, 278–287. doi: 10.1016/j.marpol.2012.05.010
Colloca F., Crespi V., Cerasi S., Coppola S. R. (2004). Structure and evolution of the artisanal fishery in a southern Italian coastal area. Fish. Res. 69 (3), 359–369. doi: 10.1016/j.fishres.2004.06.014
Di Maio F., Geraci M. L., Scannella D., Russo T., Fiorentino F. (2022). Evaluation of the economic performance of coastal trawling off the southern coast of Sicily (Central Mediterranean Sea). Sustainability 14 (8), 4743. doi: 10.3390/su14084743
Damalas D., Vassilopoulou V. (2013). Slack regulation compliance in the Mediterranean fisheries: A paradigm from the Greek Aegean Sea demersal trawl fishery, modelling discard ogives. Fish. Manage. Ecol. 20 (1), 21–33. doi: 10.1111/j.1365-2400.2012.00860.x
Dimitriadis C., Sini M., Trygonis V., Gerovasileiou V., Sourbès L., Koutsoubas D. (2018). Assessment of fish communities in a Mediterranean MPA: Can a seasonal no-take zone provide effective protection? Estuar. Coast. Shelf Sci. 207, 223–231. doi: 10.1016/j.ecss.2018.04.012
D’Anna G., Fernández T. V., Pipitone C., Garofalo G., Badalamenti F. (2016). Governance analysis in the egadi islands marine protected area: a Mediterranean case study. Mar. Policy 71, 301–309. doi: 10.1016/j.marpol.2015.12.009
Decreto Ministeriale (DM) (2010). Available at: https://www.mite.gov.it/normative/decreto-ministeriale-1-giugno-2010-n-715-regolamento-di-esecuzione-ed-organizzazione.
De Vos B. I., Döring R., Aranda M., Buisman F. C., Frangoudes K., Goti L., et al. (2016). New modes of fisheries governance: Implementation of the landing obligation in four European countries. Mar. Policy 64, 1–8. doi: 10.1016/j.marpol.2015.11.005
Erzini K., Costa M. E., Bentes L., Borges T. C. (2002). A comparative study of the species composition of discards from five fisheries from the Algarve (Southern Portugal). Fish. Manage. Ecol. 9, 31–40. doi: 10.1046/j.1365-2400.2002.00284.x
Erzini K., Gonçalves J. M., Bentes L., Moutopoulos D. K., Casal J. A. H., Soriguer M. C., et al. (2006). Size selectivity of trammel nets in southern European small-scale fisheries. Fish. Res. 79 (1-2), 183–201. doi: 10.1016/j.fishres.2006.03.004
European Commission (EC) (2002) Communication from the commission to the council and the European parliament on a community action plan to reduce discards of fish (COM, (2022) 656 final). Available at: http://ec.europa.eu/transparency/regdoc/rep/1/2002/EN/1-2002-656-EN-F1-1.Pdf.
European Commission (EC) (2015) EU Fleet register database: Number of active vessels as of December, European commission fisheries & maritime affair. Available at: http://ec.europa.eu/fisheries/fleet/index.cfm?method¼Search.SearchAdvanced%3E (Accessed 13.06.22).
European parliament and Council (EU) (2013). Regulation (EU) no 1380/2013 of the European parliament and of the council of 11 December 2013 on the common fisheries policy, amending council regulations (EC) no 1954/2003 and (EC) no 1224/2009 and repealing council regulations (EC) no 2371/2002 and (EC) no 639/2004 and council decision 2004/585/EC. Off. J. Eur. Union L 354/22 L354, 22–61.
European parliament and Council (EU) (2019). Regulation (EU) 2019/1241 of the European parliament and of the council of 20 June 2019 on the conservation of fisheries resources and the protection of marine ecosystems through technical measures, amending council regulations (EC) no 1967/2006, (EC) no 1224/2009 and regulations (EU) no 1380/2013, (EU) 2016/1139, (EU) 2018/973, (EU) 2019/472 and (EU) 2019/1022 of the European parliament and of the council, and repealing council regulations. (EC) no 894/97, (EC) no 850/98, (EC) no 2549/2000, (EC) no 254/2002, (EC) no 812/2004 and (EC) no 2187/2005. Off. J. Eur. Union L 198/105.
EU STECF (2008). Scientific, technical and economic committee for fisheries (STECF) evaluation of the STECF/SGMOS 07-04 working group on discards (Hamburg:Luxembourg), 156.
Falautano M., Castriota L., Cillari T., Vivona P., Finoia M. G., Andaloro F. (2018). Characterization of artisanal fishery in a coastal area of the strait of Sicily (Mediterranean sea): Evaluation of legal and IUU fishing. Ocean Coast. Manage. 151, 77–91. doi: 10.1016/j.ocecoaman.2017.10.022
Falsone F., Gancitano V., Geraci M. L., Sardo G., Scannella D., Serena F., et al. (2022). Assessing the stock dynamics of elasmobranchii off the southern coast of Sicily by using trawl survey data. Fishes 7, 136. doi: 10.3390/fishes7030136
Falsone F., Scannella D., Geraci M. L., Vitale S., Colloca F., Di Maio F., et al. (2020). Identification and characterization of trammel net métiers: A case study from the southwestern Sicily (Central Mediterranean). Reg. Stud. Mar. 39, 101419. doi: 10.1016/j.rsma.2020.101419
FAO (2020). The state of Mediterranean and black Sea fisheries 2020 (Rome: General Fisheries Commission for the Mediterranean). doi: 10.4060/cb2429en
Field J. G., Clarke K. R., Warwick R. M. (1982). A practical strategy for analysing multispecies distribution patterns. Mar. Ecol. Prog. Ser. 8, 37–52.
Forskal (1775). And bycatch species in trammel nets from izmir bay (Aegean sea). Turkey. J. Anim. Vet. Adv. 10 (24), 3316–3320.
Garofalo G., Gristina M., Toccaceli M., Giusto G. B., Rizzo P., Sinacori G. (2004). “Geostatistical modelling of biocenosis distribution in the strait of sicily. GIS/Spatial analyses in fishery and acquatic science. (2),” in Proceeding of the second international symposium on GIS/Spatial analyses in fishery and aquatic sciences. Eds. Nishida T., Kailola P. J., Hollingworth C. E. (Brighton: University of Sussex), 241–250. (Vol. 2).
Geraci M. L., Colloca F., Di Maio F., Falsone F., Fiorentino F., Sardo G., et al. (2021a). How is artificial lighting affecting the catches in deep water rose shrimp trawl fishery of the central Mediterranean Sea? Ocean Coast. Manage. 217, 105970. doi: 10.1016/j.ocecoaman.2021.105970
Geraci M. L., Falsone F., Scannella D., Sardo G., Vitale S. (2019). Dolphin-fisheries interactions: An increasing problem for Mediterranean small-scale fisheries. Examines Mar. Biol. Oceanogr. 3 (1), EIMBO.000552.2019. doi: 10.31031/EIMBO.2019.03.000552
Geraci M. L., Ragonese S., Scannella D., Falsone F., Gancitano V., Mifsud J., et al. (2021b). Batoid abundances, spatial distribution, and life history traits in the strait of Sicily (Central Mediterranean sea): Bridging a knowledge gap through three decades of survey. Animals 11, 2189. doi: 10.3390/ani11082189
Gharbi H., Ktari M. H. (1981). Croissance des rougets en tunisie. Bull. Inst. Oceanogr. Peche Salammbo 8, 5–40.
Gil M. M., Catanese G., Palmer M., Hinz H., Pastor E., Mira A., et al. (2018). Commercial catches and discards of a Mediterranean small-scale cuttlefish fishery: Implications of the new EU discard policy. Sci. Mar. 82, 155–164. doi: 10.3989/scimar.04735.03B
Gökçe G., Bozaoğlu A. S., Eryaşar A. R., Özbilgin H. (2016). Discard reduction of trammel nets in the northeastern Mediterranean prawn fishery. J. Appl. Ichthyol. 32 (3), 427–431. doi: 10.1111/jai.13015
Gonçalves J. M. S., Stergiou K. I., Hernando J. A., Puente E., Moutopoulos D. K., Arregi L., et al. (2007). Discards from experimental trammel nets in southern european small-scale fisheries. Fish. Res. 88 (1-3), 5–14. doi: 10.1016/j.fishres.2007.06.017
Gonçalves J. M. S., Bentes L., Coelho R., Monteiro P., Ribeiro J., Correia C., et al. (2008). Non-commercial invertebrate discards in an experimental trammel net fishery. Fish. Manage. Ecol. 15 (3), 199–210. doi: 10.1111/j.1365-2400.2008.00607.x
Grati F., Aladzuz A., Azzurro E., Bolognini L., Carbonara P., Çobani M., et al. (2018). Seasonal dynamics of small-scale fisheries in the adriatic sea. Med. Mar. Sci. 19 (1), 21–35. doi: 10.12681/mms.2153
Grati F., Azzurro E., Scanu M., Tassetti A. N., Bolognini L., Guicciardi S., et al. (2022). Mapping small-scale fisheries through a coordinated participatory strategy. Fish Fish. 00, 1–13. doi: 10.1111/faf.12644
Guidetti P., Milazzo M., Bussotti S., Molinari A., Murenu M., Pais A., et al. (2008). Italian Marine reserve effectiveness: Does enforcement matter? Biol. Conserv. 141 (3), 699–709. doi: 10.1016/j.biocon.2007.12.013
ICES (2020). ICES workshop on innovative fishing gear (WKING). ICES Sci. Rep. 2 (96), 130. doi: 10.17895/ices.pub.7528
Kelleher K. (2005). “Discards in the world’s marine fisheries. an update,” in FAO fisheries technical paper 470 (Rome, Italy: FAO), 1–134.
Lucchetti A., Virgili M., Petetta A., Sartor P. (2020). An overview of gill net and trammel net size selectivity in the Mediterranean Sea. Fish. Res. 230, 105677. doi: 10.1016/j.fishres.2020.105677
Maccarrone V., Buffa G., Stefano V. D., Filiciotto F., Mazzola S., Buscaino G. (2014). Economic assessment of dolphin depredation damages and pinger use in artisanal fisheries in the archipelago of egadi islands (Sicily). Turkish J. Fish. Aquat. Sci. 14 (1), 173–181. doi: 10.4194/1303-2712-v14_1_19
Mannino A. M., Parasporo M., Crocetta F., Balistrieri P. (2017). An updated overview of the marine alien and cryptogenic species from the egadi islands marine protected area (Italy). Mar. Biodiv. 47, 469–480. doi: 10.1007/s12526-016-0496-z
Martínez-Baños P., Maynou F. (2018). Reducing discards in trammel net fisheries with simple modifications based on a guarding net and artificial light: contributing to marine biodiversity conservation. Sci. Mar. 82 (S1), 9–18. doi: 10.3989/scimar.04710.03A
Maynou F., Morales-Nin B., Cabanellas-Reboredo M., Palmer M., García E., Grau ,. A. M. (2013). Small-scale fishery in the balearic islands (W mediterranean): A socio-economic approach. Fish. Res. 139, 11–17. doi: 10.1016/j.fishres.2012.11.006
Metin C., Gökçe G., Aydın İ., Bayramiç İ. (2009). Bycatch reduction in trammel net fishery for prawn (Melicertus kerathurus) by using guarding net in izmir bay on Aegean coast of Turkey. Turkish J. Fish. Aquat. Sci. 9 (2), 133–136. doi: 10.4194/trjfas.2009.0202
Petetta A., Virgili M., Guicciardi S., Lucchetti A. (2021). Pots as alternative and sustainable fishing gears in the Mediterranean Sea: an overview. Rev. Fish. Biol. Fisheries 31, 773–795. doi: 10.1007/s11160-021-09676-6
Purbayanto A., Tsunoda A., Akiyama S., Arimoto T., Tokai T. (2001). Survival of Japanese whiting sillago japonica and by-catch species captured by a sweeping trammel net. Fish. Sci. 67 (1), 21–29. doi: 10.1046/j.1444-2906.2001.00194.x
R Core Team (2022). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Russo G. F., Terlizzi A. (1997). Structural patterns in the mollusc assemblages of posidonia oceanica beds: methodological, edaphic or biogeographical product? Bollettino Malacologico 33, 89–94.
Salas S., Gaertner D. (2004). The behavioural dynamics of fishers: management implications. Fish Fish 5 (2), 153–167. doi: 10.1111/j.1467-2979.2004.00146.x
Sardo G., Okpala C. O. R., Geraci M. L., Fiorentino F., Vitale S. (2020). The effects of different artificial light wavelengths on some behavioural features of juvenile pelagic Atlantic horse mackerel, Trachurus trachurus (Actinopterygii: Perciformes: Carangidae). Acta Ichthyol. Piscat. 50 (1), 85–92. doi: 10.3750/AIEP/02709
Sartor P., Silvestri R., Sbrana M., Voliani A., Rossetti I., Bulgheri G. (2007). Experimentation of technical devices for the discard reduction in the set net fishery along the livorno coast. Biol. Mar. Mediterr. 14 (2), 360–361.
Sartor P., Veli D. L., De Carlo F., Ligas A., Massaro A., Musumeci C., et al. (2018). Reducing unwanted catches of trammel nets: experimental results of the “guarding net” in the caramote prawn, Penaeus kerathurus, small-scale fishery of the ligurian Sea (western Mediterranean). Sci. Mar. 82 (S1), 31–140. doi: 10.3989/scimar.04765.15B
Stasinopoulos D. M., Rigby R. A. (2007). Generalized additive models for location scale and shape (GAMLSS) in r. J. Stat. Software 23 (7), 1–46. doi: 10.18637/jss.v023.i07
Stergiou K. I., Moutopoulos D. K., Soriguer M. C., Puente E., Lino P. G., Zabala C., et al. (2006). Trammel net catch species composition, catch rates and métiers in southern European waters: A multivariate approach. Fish. Res. 79 (1-2), 170–182. doi: 10.1016/j.fishres.2006.03.003
Swimmer Y., Zollett E. A., Gutierrez A. (2020). Bycatch mitigation of protected and threatened species in tuna purse seine and longline fisheries. Endang. Species. Res. 43, 517–542. doi: 10.3354/esr01069
Szynaka M. J., Bentes L., Monteiro P., Rangel M., Erzini K. (2018). Reduction of by-catch and discards in the Algarve small-scale coastal fishery using a monofilament trammel net rigged with a guarding net. Sci. Mar. 82 (S1), 121–129. doi: 10.3989/scimar.04734.16B
Tiralongo F., Messina G., Lombardo B. M. (2018). Discards of elasmobranchs in a trammel net fishery targeting cuttlefish, Sepia officinalis linnaeus 1758, Along the coast of Sicily (central Mediterranean Sea). Reg. Stud. Mar. Sci. 20, 60–63. doi: 10.1016/j.rsma.2018.04.002
Tsagarakis K., Palialexis A., Vassilopoulou V. (2014). Mediterranean Fishery discards: review of the existing knowledge. ICES J. Mar. Sci. 71 (5), 1219–1234. doi: 10.1093/icesjms/fst074
Tzanatos E., Dimitriou E., Katselis G., Georgiadis M., Koutsikopoulos C. (2005). Composition, temporal dynamics and regional characteristics of small-scale fisheries in Greece. Fish. Res. 73 (1-2), 147–158. doi: 10.1016/j.fishres.2004.12.006
Vassilopoulou V. (2011). “Review of existing knowledge on fisheries by-catch in the GFCM area,” in Report of the 2nd transversal working group on by-catch (Antalya, Turkey: General Fisheries Commission for the Mediterranean, Scientific Advisory Committee), 39.
Veiga P., Pita C., Rangel M., Gonçalves J. M., Campos A., Fernandes P. G., et al. (2016). The EU landing obligation and European small-scale fisheries: What are the odds for success? Mar. Policy 64, 64–71. doi: 10.1016/j.marpol.2015.11.008
Vitale S., Cannizzaro L., De Stefano G., Milazzo A., Salvo G. (2011). Sicilian Coastal biodiversity through small-scale fishery: an innovative approach. J. Coast. Res. 64, 1931–1935.
Vitale S., Enea M., Milisenda G., Gancitano V., Geraci M. L., Falsone F., et al. (2018). Modelling the effects of more selective trawl nets on the productivity of European hake (Merluccius merluccius) and deep-water rose shrimp (Parapenaeus longirostris) stocks in the strait of Sicily. Sci. Mar. 82 (S1), 199–208. doi: 10.3989/scimar.04752.03A
Vlachopoulou E. I., Wilson A. M., Miliou A. (2013). Disconnects in EU and Greek fishery policies and practices in the eastern Aegean Sea and impacts on Posidonia oceanica meadows. Ocean Coast. Manage. 76, 105–113. doi: 10.1016/j.ocecoaman.2013.02.008
Wood S. N. (2006). Generalized additive models: an introduction with r (New York: Taylor & Francis Group). doi: 10.1201/9781420010404
Zuur A. F., Ieno E. N., Walker N., Saveliev A. A., Smith G. M. (2009). “Mixed effects models and extensions in ecology with r,” in Statistics for biology and health, vol. vol. XXII. (New York, NY, USA: Springer-Verlag). Available at: http://link.springer.com/10.1007/978-0-387-87458-6.
Keywords: multivariate analysis, benthic assemblages, sustainability, unwanted catches, discard, by-catch reduction device, conservation
Citation: Sardo G, Vecchioni L, Milisenda G, Falsone F, Geraci ML, Massi D, Rizzo P, Scannella D and Vitale S (2023) Guarding net effects on landings and discards in Mediterranean trammel net fishery: Case analysis of Egadi Islands Marine Protected Area (Central Mediterranean Sea, Italy). Front. Mar. Sci. 10:1011630. doi: 10.3389/fmars.2023.1011630
Received: 04 August 2022; Accepted: 27 February 2023;
Published: 16 March 2023.
Edited by:
Jure Brčić, University of Split, CroatiaReviewed by:
Gaetano Catanese, Government of the Balearic Islands, SpainCopyright © 2023 Sardo, Vecchioni, Milisenda, Falsone, Geraci, Massi, Rizzo, Scannella and Vitale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giacomo Sardo, Z2lhY29tby5zYXJkb0BpcmJpbS5jbnIuaXQ=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.