
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 31 January 2023
Sec. Marine Pollution
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.991650
This article is part of the Research Topic Advances in Quantification, Degradation and Ecotoxicology of Microplastics in Marine Resources View all 10 articles
Increasing shares of microfibers are being detected in environmental samples and a closer look to identify the risk associated with them using ecologically relevant endpoints, especially at sensitive early life stages, is needed. To assess exposure hazards, we used rope samples representative of fiber types ubiquitous in coastal systems, where microfibers are often the most common debris type found in the water column. To compare responses to natural vs. synthetic microfibers, we used rinsed “natural” cotton, polyester, and polypropylene microfibers (80-150 µm length, 8-20 µm width) created from the rope. Larval and juvenile estuarine indicator species Inland Silverside (Menidia beryllina) and mysid shrimp (Americamysis bahia), respectively, were exposed to these three microfiber types at three concentrations (3, 10, 30 particles/ml) along a 5-25 PSU salinity gradient to mimic estuarine conditions. Behavioral responses, growth, and ingestion were measured. The cotton microfibers were not detected in the digestive tracts of Silversides, however, both the polyester and polypropylene microfibers were detected in the Silversides’ stomach and gut lining. None of the fiber types were detected in mysid shrimps. Mysids exposed to cotton microfibers had fewer behavioral effects compared to Silversides, who responded more to cotton. Cotton exerted no effect on growth in Silversides but did cause reduced growth in the mysids at the two lower salinities. In contrast, polyester and polypropylene were identified to have a significant dose dependent effect on mysid and Silverside behavior as well as growth was affected in at least one of the three salinities at concentrations as low as 3 particles/ml. Cotton impacted both the organism’s behavior more at higher salinities, whereas polyester and polypropylene had more impacts at lower salinities. This raises concerns for microfiber impacts on estuarine ecosystems and the need for policies to limit microfiber production and outfall into the aquatic environment.
There are a wide range of contaminants entering the environment from anthropogenic sources, with the largest component being from marine litter (Bergmann et al., 2015; Auta et al., 2017). Recently, micro-sized particles (<5 mm), particularly microfibers, have gained great attention due to increased identification in samples and identification of adverse impacts on organisms and ecosystems (Mishra et al., 2020; Granek et al., 2022; Kwak et al., 2022). In coastal systems, most microfiber are produced by the ropes and fishing gear that contribute 1277 ± 431 microplastic pieces m−1, with an estimated 44% from fishing rope and 49% from nets (Wright et al., 2021). Additional sources of microfibers are natural and synthetic textiles (Granek et al., 2022). Synthetic microfiber consist of persistent polymers including nylon, polyester, polyethylene terephthalate (PET), polypropylene, acrylic or spandex (Athey and Erdle, 2021; Granek et al., 2022), while natural fibers can be cotton, wool, linen, etc. The ubiquity of microfibers in the environment ranges from the atmosphere to the deepest parts of the ocean (Krüger et al., 2020; Mishra et al., 2020; Reineccius et al., 2020; Suaria et al., 2020; Acharya et al., 2021; Brahney et al., 2021, Caldwell et al., 2022).
From 1975 to 2020, total global fiber production increased from 32 to120 million metric tons, and is expected to increase to 146 million metric tons by 2030 (Truscott, 2020). Microfibers are released into the environment due to their propensity for shedding and are sourced from household washing machines, cloth dryers and also from vacuum cleaning (Hartline et al., 2016; Sillanpää and Sainio, 2017; Yang et al., 2019), and most recently from protective personal equipment during the pandemic (e.g., masks) (Akhbarizadeh et al., 2021; Morgana et al., 2021). Of the total fabric produced in 2015, approximately 75% either went to a landfill or was incinerated (Ellen MacArthur Foundation, 2017). Accumulation of microfibers is of great concern due to their potential environmental impacts as well as their carbon footprint (Zhu, 2021; O’Brien et al., 2022).
Natural microfibers (e.g., cellulose based), may be less persistent and can break apart during metabolic processes as well as through aerobic biodegradation processes (Zambrano et al., 2019; Zambrano et al., 2020). However, their fate and role in the aquatic ecosystems due to different chemical dyes, functional finishes (e.g., fluorinated compounds) coupled with a higher propensity for shedding compared to synthetics is unknown, and they may provide more surface area to adsorb contaminants (Salvador Cesa et al., 2017; Henry et al., 2019; Mishra et al., 2019; Athey et al., 2020). For synthetic microfibers, their nonbiodegradable nature and high tensile strength likely leads to increased persistence in the environment (Li et al., 2010), entrapment in the gills or digestive tract, or once ingested a false sense of satiation and subsequent loss of nutrition (Watts et al., 2015; Watts et al., 2016; Stienbarger et al., 2021). Given organisms are exposed to both natural and synthetic fibers (e.g. Caldwell et al., 2022, Granek et al., 2022), it is essential to study both.
Estuaries have high variability in physio-chemical conditions and provide a wide range of favorable habitat for a diverse suite of taxa (Costanza et al., 1997). Being first to receive direct inflow from rivers transporting contaminants into surface waters, they can concentrate microplastics (Browne et al., 2010; Wright et al., 2013). Regardless of this, data on estuarine organisms for microplastic ingestion and effects are fairly limited (Possatto et al., 2011; Vendel et al., 2017; Bessa et al., 2018; Athey et al., 2020) and this data gap has been noted (Granek et al., 2020; Kutralam-Muniasamy et al., 2020; Kwak et al., 2022).
Additionally, behavioral endpoints are very poorly studied in relation to emerging contaminants, including microplastics and especially for microfibers, even though behavioral alterations are demonstrated to impact fitness and survival in the wild (McCormick et al., 2020; Brodin et al., 2014; Scott and Sloman, 2004; Weis et al., 2001). To address these knowledge gaps, this study describes behavioral and growth impacts of natural cotton and synthetic (polyester, polypropylene) microfiber (80-120 µm) in two estuarine indicator species (mysid shrimp and Silversides) across a salinity gradient. We hypothesized that sublethal effects would be caused following exposure to both synthetic and non-synthetic fibers. To the best of our knowledge, this is the first paper to measure and compare natural verses synthetic microfiber responses in the early life stages of both fish and invertebrates.
Suwanee River Natural organic matter (NOM) - 2R101N used to create suspensions of microparticles in exposure wells was purchased from the International Humic Substance Society, St. Paul, MN. Tissue-Clearing Reagent CUBIC-R+ [for Animals] (T3741) and Tissue-Clearing Reagent CUBIC-L [for Animals] (T3740) for visualization of particles within organisms following exposures were purchased from Tokyo Chemical Industry Co., Ltd. Ropes were purchased from SGT knots and the “natural” cotton rope was purchased from the local craft store.
Detailed microfiber preparation protocol has been provided in Figure S1. Various studies reported microfiber production in the lab (Saborowski et al., 2019; Ward et al., 2019; Gambino et al., 2020; Knauss et al., 2021; Ma et al., 2021); however, very few demonstrated efficient microfiber generation with limited equipment. In this study, we adapted microfiber production methods described by Cole (2016). Briefly, microfibers were untwisted and cut into short sections. After adding a freezing solution (Surgipath® FSC 22® Frozen Section Embedding Medium, Leica), each cut section was frozen in a -80°C freezer for 5 minutes. Next, each frozen block was shredded through a coffee grinder (>1 mm) with some filtered DI water. The shortest microfibers from the wall of the coffee grinder cup were collected and mixed with warm DI water (40 °C) before filtering through 120 micron filter paper (polycarbonate). Filtered microfiber of <120 µm in length was collected and washed with ethanol. The resultant stock solution was then used to prepare the required amount for the exposures. The micron (80-120 µm) sample particle count is determined by triplicate sampling of the suspension and the particle count analysis confirmed using a light microscope (Leica EZ4) and Sedgwick rafter slide without a coverslip to avoid miscounting (Stienbarger et al., 2021). The microfiber size range found in the natural environment is from 50 to 100 µm (about 53% of total sampled microfiber) (Pirc et al., 2016; Conley et al., 2019). The concentration of microfibers used in this study was 3-30 particles/ml. Since these microfibers are generated from ropes, which are used in the coastal environment during commercial and recreational fishing activities, their bioavailability increases with more use. They are potentially a higher cause of concern, as they do not go through wastewater treatment process, which tends to reduce microfibers significantly in treated effluent (Granek et al., 2022). In part the aim of these concentrations was also to make microfibers available for the organism to ingest at levels comparable to what is found in larval fish in the wild (e.g. Lasdin et al. in revision), reported later in this paper. Using this method, cotton microfiber generation was higher compared to polyester and polypropylene fiber. This is a general trend reported by others, that generation of microfiber per gram of textile laundered is significantly higher in cellulose-based fabrics compared to polyester (Sillanpää and Sainio, 2017; Cesa et al., 2020; Zambrano et al., 2021).
Americamysis bahia larvae were purchased from Aquatic Biosystems in Fort Collins, Colorado, and reared in three tanks to adulthood at 15, 20, and 25 PSU salinities with filtered artificial seawater prepared (AFSW). Following EPA protocol 833-C-09-001 (USEPA, 2009; Siddiqui et al., 2022). when adult A. bahia reproduced, larvae were moved to additional tanks of the same salinity and reared for seven days. Microfiber exposures with mysids were initiated at seven days post fertilization (dpf) (n=9) under static renewal conditions for seven days. Menidia beryllina embryos were harvested from broodstock held at the OSU Hatfield Marine Science Center and placed into three acclimation aquaria of 5, 15, and 25 PSU salinities with filtered AFSW following modified methods from Middaugh et al. (1987), as done in previous studies in the Brander lab (DeCourten et al., 2020; Hutton et al., 2021; Siddiqui et al., 2022). Larvae were placed into exposure vessels at 6 ± 1 days post fertilization (dpf) (n=6) and maintained under static renewal conditions for 96 h.
Each model species was exposed to a total of 26 treatments: each in covered beakers containing water control, NOM control with each of the three-microfiber types with concentration treatments (3,10 and 30 particles/ml) across three salinities per species as described above (Figure S2). Nominal water concentrations with detailed QA/QC are provided in SI table 1. Water quality parameters were measured daily just prior to and following 80% water renewal. Cumulative hatching and mortality were recorded daily. A. bahia were fed concentrated brine shrimp (Artemia franciscana) ad libitum, and M. beryllina were fed Gemma Microdiet 0.2 mg/beaker/day (Skretting, Westbrook, Maine). Both organisms were fed daily and allowed to feed for at least two hours before water was changed. Tables SI 2, 3 provides water quality parameters maintained throughout the experiment. A control filter was set up to measure background contamination in the air.
Following microfiber exposures of 7d (A. bahia) and 96 h (M. beryllina), behavioral assays were performed post-exposure for each treatment using a DanioVision Observation Chamber (Noldus, Wageningen, the Netherlands) for the Dark: Light cycle as described previously (Mundy et al., 2021; Segarra et al., 2021; Siddiqui et al., 2022). Briefly, A. bahia and M. beryllina larvae were randomized and placed in individual 10 ml glass beaker in a 12 well plate frame in the Ethovision Observation Chamber (EOB) to observe natural photo motor response. Larvae were allowed to be acclimatized for at least 1 hour before placing into the EOB. After acclimatization outside, another 5-minute acclimatization time was provided inside the dark chamber, followed by three 2-minute intervals of dark stimuli and three 2-minute intervals of light stimuli. Behavior and activity were recorded and tracked by a Basler Gen 1 Camera using Ethovision XT15 software. Velocity thresholds were determined for swimming parameters between 0.5 cm/s (freezing) – 2.0 cm/s (moving) (Segarra et al., 2021; Siddiqui et al., 2022). A virtual center zone (1.6 cm diameter) was established to measure the time that larvae spent in the center of the 2.2 cm diameter in the beaker. All behavioral tests were conducted between 09:00 and 18:00 h. The resolution was set at 1280 x 960, light cycles were programmed at 10,000 lux, and the frame rate was set at 25/s. A total of eight variables were analyzed in this study, the p values and other statistics for which are included in Table 1. Following behavioral analysis, organisms were euthanized humanely, Silversides per IACUC protocol #0035, and fixed in paraformaldehyde (PFA) to preserve tissues for examination of microfibers internalization.
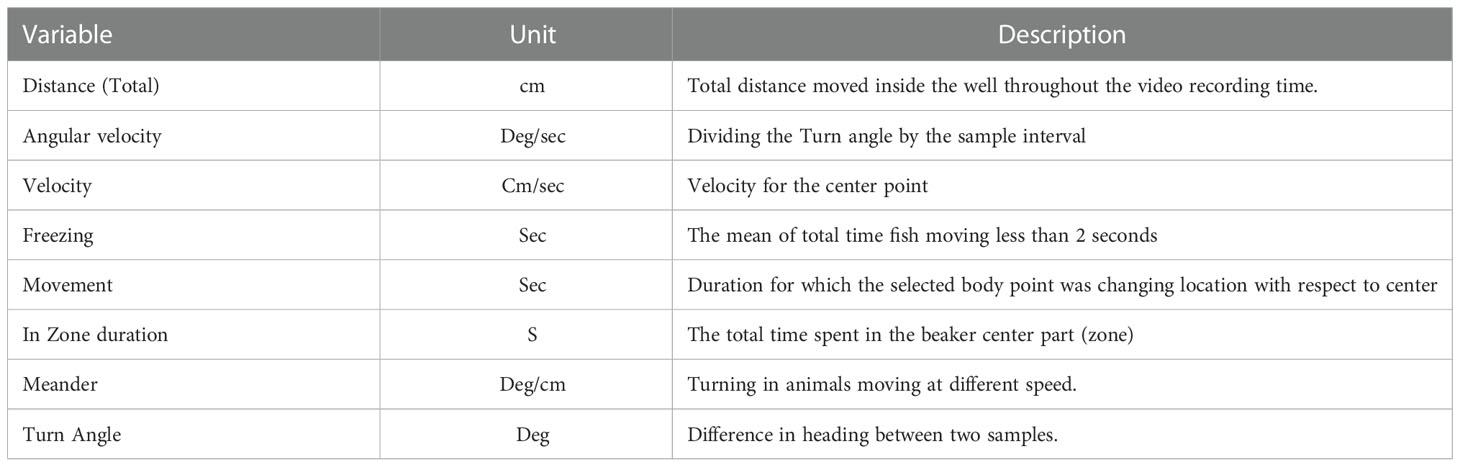
Table 1 Behavioral variables from Noldus ethovision software used in this study to analyze Mysid shrimp (A. bahia) larvae and Silverside (M. beryllina) larvae behavioral response.
Per Siddiqui et al. (2022), at least three individuals from each species per replicates were collected for growth. Length and width measurements were collected via dissecting scope equipped with Moticam visual software, and particle uptake was visualized on a Zeiss Axio Observer inverted microscope (Carl Zeiss, White Plains, NY). Growth data were assessed by creating a growth index with the following formula:
Where W is the Width of the organism, L is the length, and d is the number of days the organism is exposed to the microfiber. This relationship provides the index used to plot the final growth curve. Organisms were then cleared using a protocol adapted for larval organisms with CUBIC™ clearing reagents (Susaki et al., 2015; Ohnuma et al., 2017). Briefly, to remove pigmentation and allow visualization of internalized microplastics (1-20 um), individual organisms fixed in 3% PFA were washed in 5 ml phosphate-buffered saline (PBS) for 30 minutes and incubated in 5 ml CUBIC-L at 37° C for seven days to encourage lipid removal. Following this step, organisms were washed again in 5 ml PBS for an additional two hours and then transferred to CUBIC-R + for an additional seven days to clear the remaining tissue.
Statistical analysis was performed using RStudio Version 1.0.153. Dose-response curves were generated to evaluate larval swimming behavior and growth effects across concentration treatments. The growth data and concentration dependent dose response curves (C-DRC) for behavioral data were prepared by drm function in r using the DRC package by Ritz et al. (2010) (Ritz, 2010) and lines were fit via ggplot (Wickham, 2005). The Shapiro–Wilk test was used to test normality, and Levene’s test was used for homogeneity testing. After confirming normality and homogeneity of data, a 3-4 parameter model using a nonlinear regression approach was used to prepare the model at each salinity and combined using ggplot2 function to create graphs in R. Analysis of Variance (ANOVA) was used to evaluate differences among treatment groups. A Tukey HSD post-hoc test was used to compare particle concentrations between treatments, (α =< 0.05). Responses between the NOM control and water only control where combined into only one control treatment per salinity for statistical analysis. Radar (or spider web) plots were used to visualize behavioral effects of microfibers across multiple salinities. To effectively convey the large amount of data produced by behavioral assays, radar plots are commonly employed (Mundy et al., 2020; Segarra et al., 2021). Radar plots are particularly useful when trying to compare multiple variables to each other with highly dynamic data, such as those presented from behavioral assays. For radar plots, all data were normalized to a 0-1 scale per behavioral analyses published in Segarra et al. (2021).
Average A. bahia larvae survival for control and exposure treatments was 99 ± 1% and 95 ± 2%, respectively, with no significant difference across the treatments (ANOVA (Normal distribution, Tukey HSD post-hoc, p > 0.05). Average M. beryllina larvae survival for control and exposure treatments was 98 ± 2% and 93 ± 1%, respectively, with no significant difference across the treatments (ANOVA (Normal distribution, Tukey HSD post-hoc, p< 0.05). As such, these were truly sublethal exposures.
When Silversides were observed under a Zeiss model microscope (Carl Zeiss, White Plains, NY) following tissue clearing for microfiber presence, cotton microfibers were not detected in the Silverside stomach or gut (Figure S3A). However, short pieces (15-60 µm length, shorter than length of added fibers ranging 80-100 µm) of synthetic microfiber (polyester and polypropylene) were observed in both the stomach and gut in the samples examined (Figures S3B, C). Similar partitioning results were reported by other studies in different aquatic organisms (Kolandhasamy et al., 2018; Woods et al., 2018). Other model species (invertebrates and fish) when exposed to synthetic microfibers, showed particle ingestion (Bour et al., 2020; Knauss et al., 2021; Ma et al., 2021). When looking at body tissue partitioning, blue mussel (Mytilus edulis), accumulated 81.3% of ingested microfibers in the digestive gland, followed by 14.4% in gills and 4.3% in soft tissues (Woods et al., 2018). No fibers of either type, synthetic or natural, could be detected in the cleared mysids. This finding is in line with other studies showing that crustaceans are more effective than fishes in breaking down microplastics (Cau et al., 2020; Matteos-Cárdenos et al., 2020).
Aquatic organisms seem to be more interested in capturing and consuming plastic microfibers, which may relate to visual or tactile misidentification or be a result of flavoring by organic compounds on their surfaces (Brillant and Macdonald, 2002; Moore, 2008). Other studies suggest plastics may act as phagostimulants that cause organisms to ingest them (Allen et al., 2017). Cotton is primarily made up of enriched cellulose which can be easily digested and broken down by living organisms (Stickney and Shumway, 1974), whereas polyester is a hydrophobic and non-sugar based compound that is not as easily degraded (Li et al., 2010). Additionally, the breaking load of cotton yarn at the dry and wet stage is much higher when compared to polyester (Zambrano et al., 2019), which can increase digestibility in organisms when internalized.
Another aspect of internalization can lead to bioaccumulation, uptake, and particle movement to higher trophic levels. Low feeding rates were reported in freshwater diving beetle Cybister japonicus (a top predator in freshwater ecosystems) after consumption of zebrafish (Danio rerio) exposed to polyethylene microspherest (diameter- 247.5 ± 14.4 μm; concentration 92± 7 particles/ml) with a trophic transfer rate of 13-18% (Kim et al., 2018). The observation of internalization of synthetic fibers in the larval fish studied herein, but not in juvenile shrimp, suggests a longer residence time in comparison to the mysid shrimp. This should be further investigated over longer exposure times to determine if sublethal effects caused by internalization are more severe over longer timescales. The lack of detection for cotton in either taxa is perhaps not surprising given the comparably easier digestibility of natural materials.
Although it was not detected in their digestive tract, mysids exposed to cotton had significantly lower growth relative to the control in the lowest and middle salinities (Figure 1A). However, silversides displayed no decrease in growth following cotton exposure at any salinity (Figure 2A). While the mysids did have decreased growth compared to the controls it was not decreased in a concentration dependent manner, nor was there a concentration dependent response in Silversides (Figures 1B, 2B). Polyester decreased growth in all three salinities in mysids but only the lowest and highest salinities in Silversides (Figures 1A, B). Polyester also caused a significant concentration dependent decrease in mysid growth from the medium and high salinity exposures and in Silversides in the highest salinity (Figure 1). When both organisms were exposed to polypropylene both mysids and silversides had decreased growth relative to the controls from all three salinities. Polypropylene exposed mysids had a concentration dependent decrease in growth in the highest salinity as did Silversides in the lowest salinity (Figures 1B, 2B). The decrease in mysids but not Silversides may be a result of species differences in feeding, where in mysid shrimp feed prolifically from hatching while Silversides can feed following hatching, it is not necessary until 6-7 dph (personal observation). Different feeding rates may explain the differences in growth if microfiber ingestion results in satiation without caloric value. Salinity may also play a role in altering the agglomeration, density, and therefore settling of microfibers, contributing to varied growth responses. Microplastic agglomeration differs across salinities (Shupe et al., 2021), and this may also hold true for microfibers, however few data currently exist on the sinking rates of fibers across salinities, although a recent paper suggests there may be a strong influence of salinity on the transport and fate of microfibers in the global ocean (Lima et al., 2021).
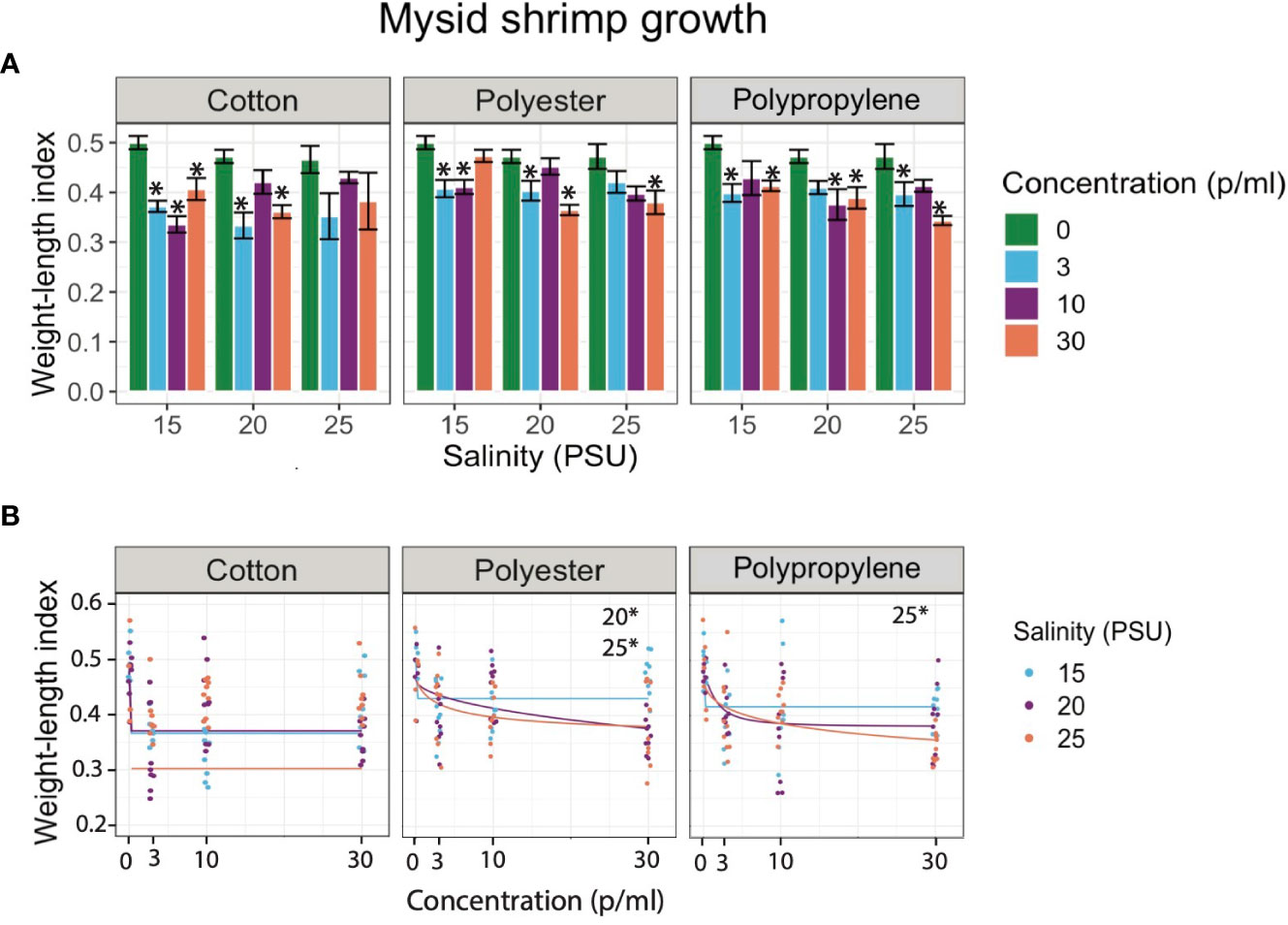
Figure 1 (A) Bar graphs of juvenile mysid shrimp (A. bahia) larvae growth exposed to cotton, polyester, and polypropylene microfibers across 0 - 30 p/mL concentration and 15-25 PSU salinity gradient respectively. Asterisks represent statistically significant differences relative to the controls. Only effects relative to the control are shown (α < 0.5; TukeyHSD). (B) Dose response curves (DRCs) of juvenile mysid shrimp (A. bahia) larvae growth exposed to cotton, polyester, and polypropylene microfibers across 0 - 30 p/mL concentration and 15-25 PSU salinity gradient respectively. Each circle in the DRCs represents the rescaled growth index mean of one larva (n=9) and regression lines are plotted from the DRC model from the R drc package. Data are presented on a log scale.
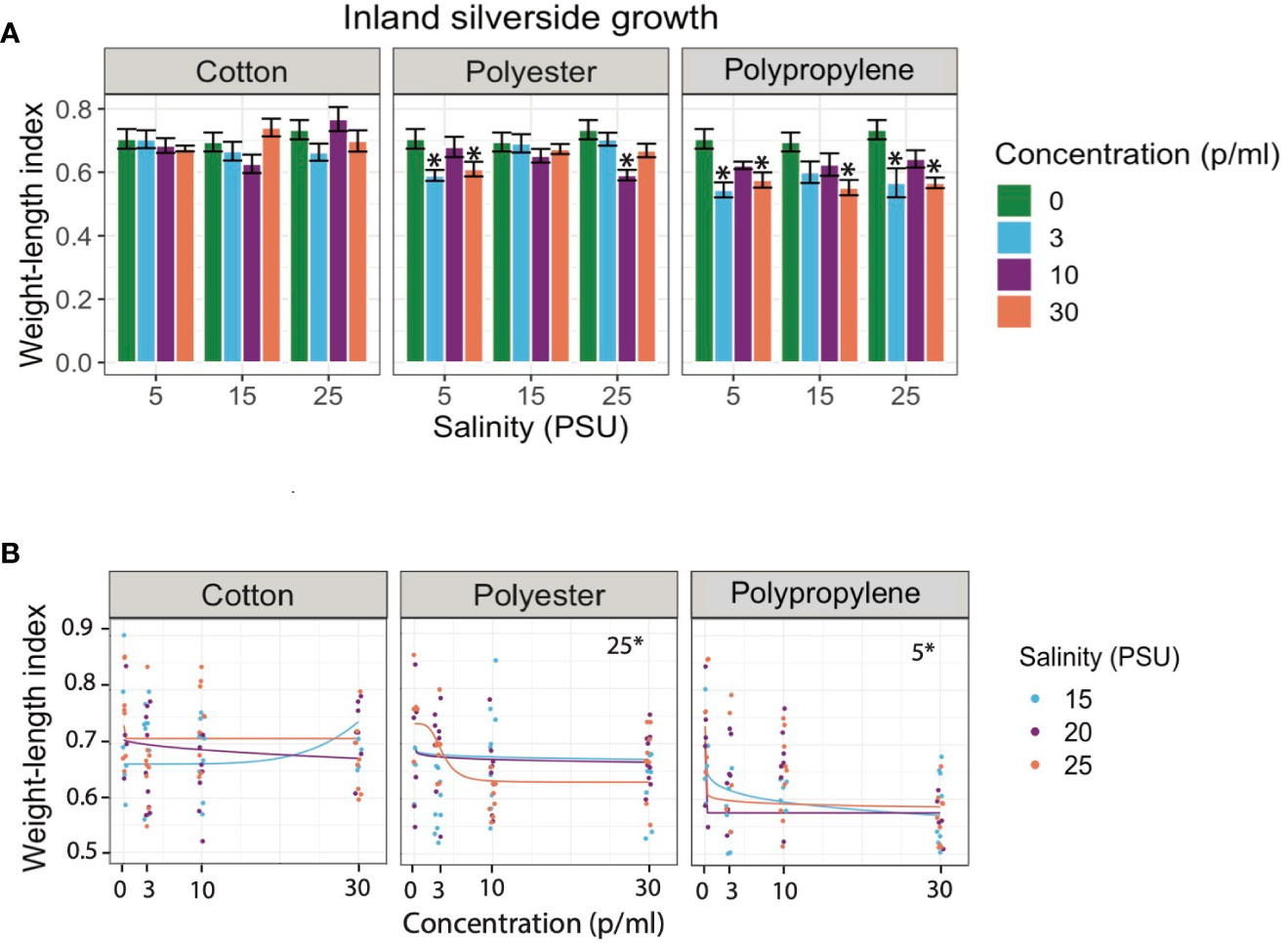
Figure 2 (A) Bar graphs of juvenile Silverside (M. beryllina) larvae growth exposed to cotton, polyester, and polypropylene microfibers across 0 - 30 p/mL concentration and 15-25 PSU salinity gradient respectively. Asterisks represent statistically significant differences relative to the controls. Only effects relative to the control are shown (α < 0.5; TukeyHSD). (B) Dose response curves (DRCs) of juvenile Silverside (M. beryllina) larvae growth exposed to cotton, polyester, and polypropylene microfibers across 0 - 30 p/mL concentration and 15-25 PSU salinity gradient respectively. Each circle in the DRCs represents the rescaled growth index mean of one larva (n=9) and regression lines are plotted from the DRC model from the R drc package. Data are presented on a log scale.
Contaminants, microplastics in particular, can impair feeding behavior by demotivating feeding preferences due to false satiation, resulting in reduced search effectiveness and prey capture ability. Various studies have linked foraging behavior alterations to reduced feeding resulting in lower growth (Little et al., 1990; Chapron et al., 2018; Lo and Chan, 2018; Cole et al., 2019; Coppock et al., 2019; Naidoo and Glassom, 2019). In general, when organisms are exposed to microplastics, reduced growth and energy reserves are reported (Yin et al., 2018; Jacob et al., 2020). Studies specifically examining microfibers reported similar results to ours in other organisms (crab, Carcinus maenas) where they observed reduced energy available for growth (scope for growth) when exposed to polypropylene rope (1–5 mm in length) (Watts et al., 2015). Other studies also show the selection preference depends upon the type of microplastic fibers in addition to the presence and absence of food. For example, sea anemone (Exaiptasia pallida) ingested a higher percentage of nylon microfiber compared to the other polymers in the absence of brine shrimp, whereas; anemones ingest all types of synthetic microfibers when offered in the presence of brine shrimp (Romanó de Orte et al., 2019). In another study, Norway lobster (Nephrops norvegicus) demonstrated reduced body mass in addition to blood and stored lipid when exposed to polypropylene rope microfibers (Welden and Cowie, 2016). Similarly, growth impacts on fishes, including Silversides, were observed following exposure to other microplastic types (Critchell and Hoogenboom, 2018; Athey et al., 2020; Siddiqui et al., 2022). When planktivorous reef fish (Acanthochromis polyacanthus) were exposed to polyethylene terephthalate (300-125 µm diam), they showed decreased growth under limited food availability conditions (Critchell and Hoogenboom, 2018). Collectively these results show that there may be more the negative health impacts of synthetic microfibers compared to cotton, however additional studies comparing synthetic fibers to cellulose-based fibers across many types and origins (e.g. clothing, rope, carpet, etc.) are needed, especially given the variability in composition, additives used, tensile strength, and other industrial treatments fabrics undergo prior to shedding into the environment (Athey and Erdle, 2021; Granek et al., 2022)
In the case of the cotton treatment, out of eight mysid behavioral activities (Table 1), only 20% of behavioral variables were affected in a concentration dependent manner in at least one salinity (Figures 3, S4A). In contrast, 90% of behavioral variables measured in mysids exposed to polyester were affected in a concentration dependent manner at least at one salinity (Figure S4B). For polypropylene, 50% of behavioral variable measured in mysids were impacted in a concentration dependent manner in at least at one salinity (Figures 3, S4C).
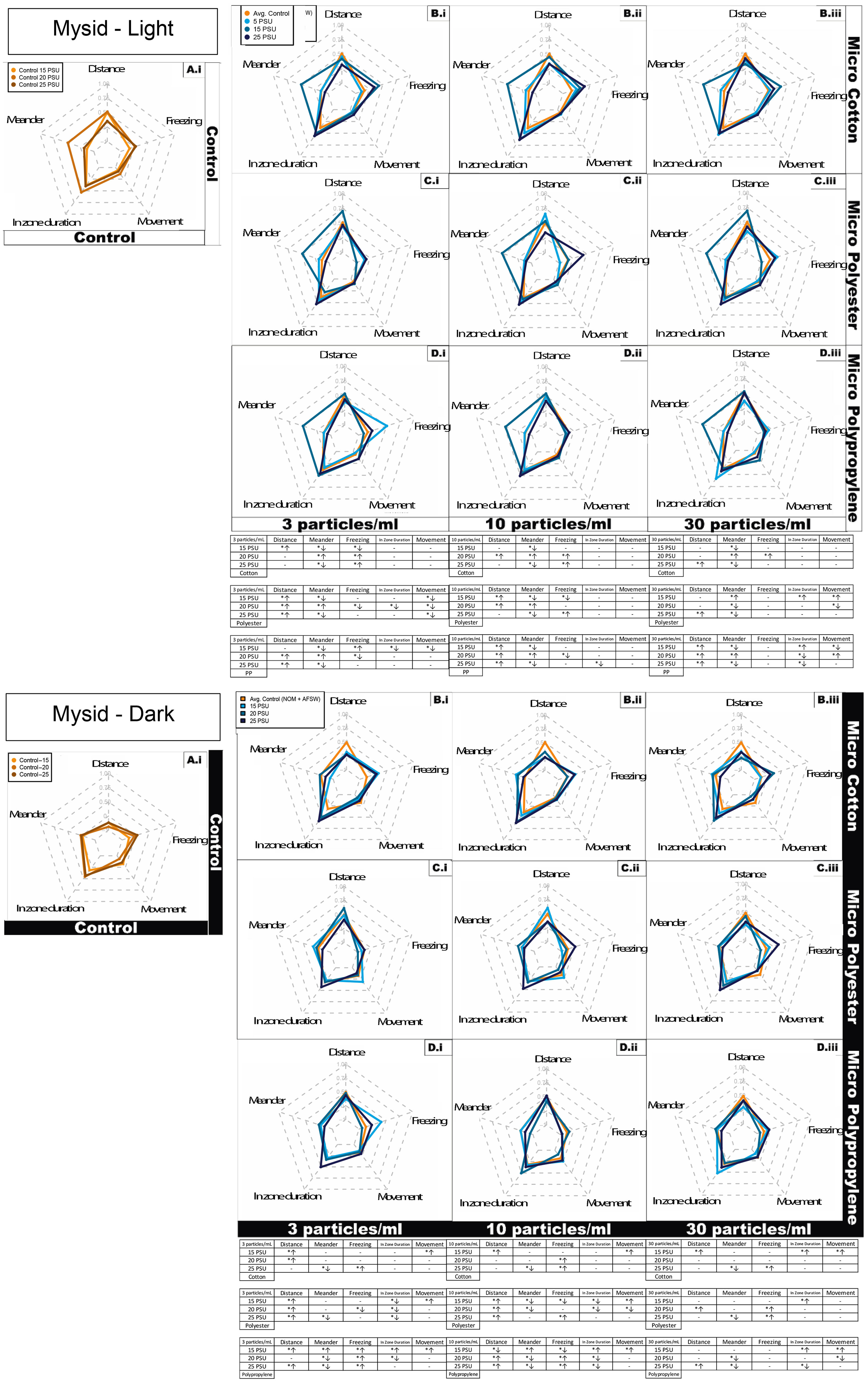
Figure 3 Juvenile Mysid shrimp (A. bahia) behavioral response represented as radar plot after 7 days exposure to cotton, polyester, and polypropylene microfibers in dark and light cycles across a salinity gradient 15PSU – 25PSU. (A) Control in water and NOM, combined for each salinity; (B) Cotton microfiber with average control; (C) Polyester microfiber with average control; (D) Polypropylene microfiber with average control. Data are representative of the calculated Z-scores normalized to controls (per Segarra et al., 2021), which are presented on the 0 axis in the middle of each figure in the panel. In the tables, asterisks represent statistically significant differences relative to the controls. The arrows indicate an increase or decrease in response for that particular behavioral endpoint. Only effects relative to the control within each salinity are shown (α < 0.5; TukeyHSD).
To summarize the significantly- impacted behavioral endpoints collectively described above, cotton microfiber exposed mysids exhibited a significant concentration dependent increase in freezing activity from medium to highest concentration within the highest salinity under dark cycle (Figures 3, S4A). There was a significant concentration dependent increase in time spent in the central portion of the beaker (change in zone) at medium salinity (dark cycle) and highest salinity (light cycle). The lowest salinity demonstrated a significantly higher turn angle activity under dark conditions, but with no concentration dependence. Overall, cotton exposed mysids exhibited more significant behavioral variations at highest salinity (50% of total activity; Figures 3, S4), compared to lowest salinity (40% of total activity; Figures 3, S4).
A concentration dependent decline in angular velocity during light cycle conditions was observed in polyester microfiber-exposed mysids, with a significant increase at the medium salinity from lowest to highest concentration (Figures 3, S4B). Mysids exposed to polyester also demonstrated a significant increase in total distance moved at the medium salinity from the lowest to the highest concentration. There was a significant concentration dependent increase in freezing within the lowest and highest salinity in both dark and light cycles, with a significant decline in medium salinity in the light cycle. Mysids in the medium salinity exhibited a significantly higher incidence of meandering activity under the light conditions with no concentration dependence. In the case of turn angle, mysids held at the lowest salinity (15 ppt) demonstrated significantly higher activity with no concentration dependent activity, whereas the highest salinity (25 ppt) demonstrated a concentration dependent increase from the medium to highest concentration under dark conditions. In contrast, mysids exposed at a medium salinity demonstrated significantly decreased concentration dependent activity under light conditions. Mysids in the lowest salinity had significantly higher activity under the dark condition with no concentration dependent activity (Table SI4). Overall, polyester-exposed mysids had more significant behavioral variations at highest salinity (94% of total activity; Figures 3, S4), compared to lowest salinity (56% of total activity; Figures 3, S4).
Polypropylene microfiber exposed mysids demonstrated a significant concentration dependent decrease in freezing within the lowest salinity in both dark and light cycles (Figures 3, S4C). There was a concentration dependent increase at the lowest salinity observed in time duration in the center of the beaker (zone) at both dark and light cycle conditions. Mysids in medium salinity demonstrated significantly higher meandering activity under light conditions with no concentration dependent activity. In contrast, mysids in the lowest salinity demonstrated significantly decreasing concentration-dependent velocity under the dark condition with no concentration dependent activity in the light cycle. Overall, polypropylene exposed mysids displayed similar behavioral variations at both highest and lowest salinity (56% of total activity; Figure S4).
Micro cotton, polyester and polypropylene all demonstrated a significant decrease in meander at all particle concentrations, except in polyester at 15 ppt in highest concentration, where it increased over time (Figure 3A). There was no impact observed on freezing behavior in all microfiber types at two highest concentrations. In medium particle concentration, there was no effect on movement observed across all microfiber types. In both synthetic microfiber treatments, there was increased distance moved by mysids in at least at one salinity. Meander was affected at all salinities in both the synthetic microfiber treatments.
Silversides exposed to the cotton microfiber treatment had 30% of measured behaviors affected in a concentration dependent manner in at least one salinity (Figure 4, S5A). In the case of polyester microfibers, 90% of behavioral endpoints exhibited a concentration dependent effect in at least one salinity (Figure S4B). For polypropylene microfiber exposed Silversides, 100% of behavioral activities demonstrated a concentration dependent effect in at least one salinity (Figure S5C). The behavioral variables are discussed below with each treatment. To summarize specific impacts on fish behavior, Silversides exposed to cotton microfiber had overall significantly higher activity at the medium salinity in light conditions (Figure 4, and Figure S5A). In the medium salinity total distance moved had significantly increasing concentration dependent activity under the dark condition in contrast to significantly lower activity in the light cycle. Silversides in the lowest salinity demonstrated significantly increased concentration dependent freezing under both dark and light conditions. Silversides also spent increasing time in the zone (center of beaker, as described above for mysids) in a significant concentration dependent manner in the lowest salinity under both light and dark conditions. Overall, at the lowest salinity Silversides demonstrated significantly lower meandering activity under both, light and dark conditions compared to other salinities. Silversides at the lowest salinity also demonstrated a significant concentration dependent decreasing movement under both light and dark conditions, whereas the medium salinity only demonstrated decreasing concentration dependent activity in the light cycle. At the medium salinity Silversides exhibited significant concentration dependent increasing velocity under dark conditions. Overall, cotton exposed silversides shown significant behavioral variations at highest salinity (94% of total activity; Figures 4, S5), compared to lowest salinity (56% of total activity; Figures 4, S5).
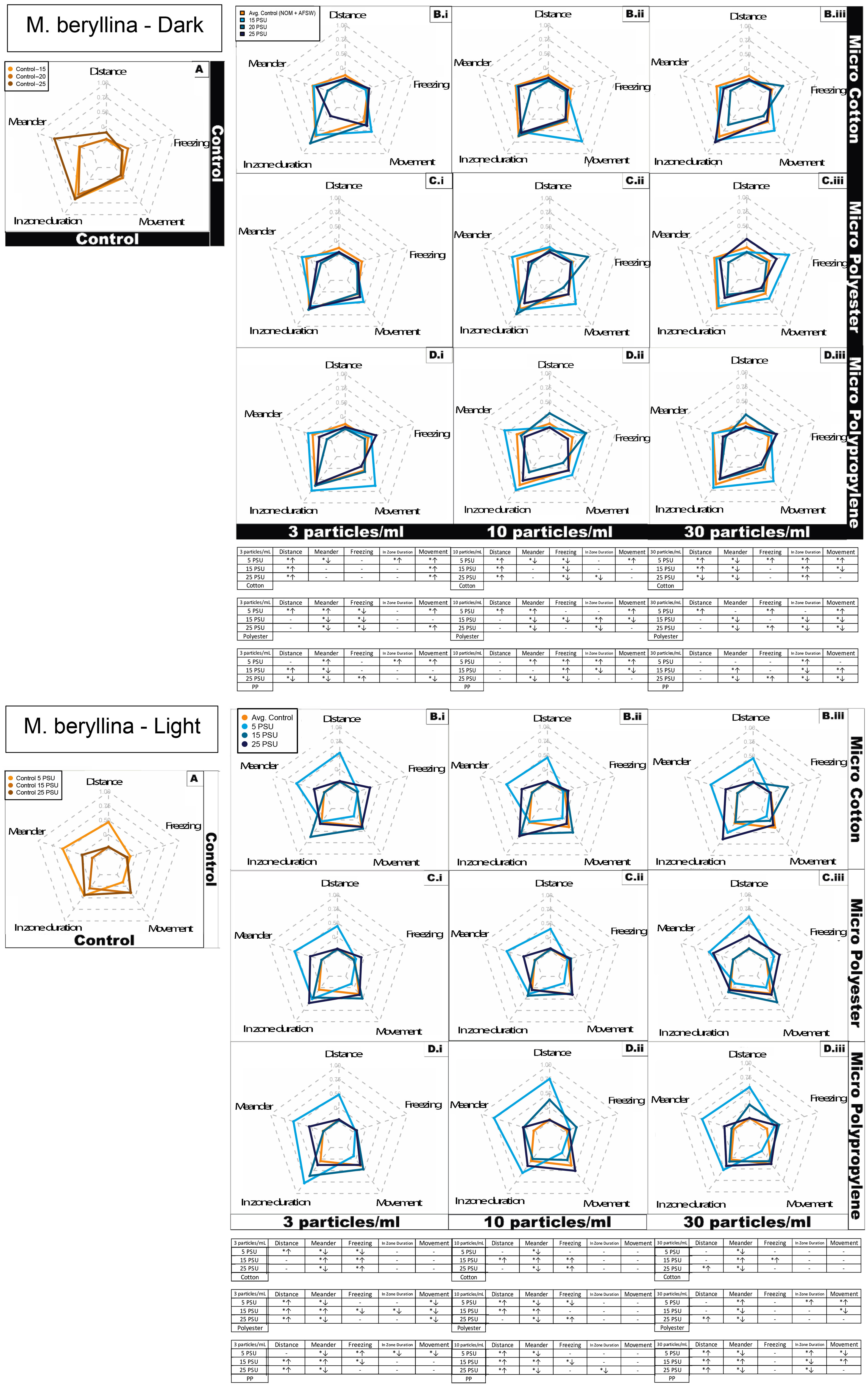
Figure 4 Silverside (M. beryllina) larvae behavioral response represented as radar plot after 4 days exposure to cotton, polyester and polypropylene microfibers in dark and light cycles at both the cycles across a salinity gradient 5 PSU – 25 PSU. S = salinity, arrows indicate significant increases or decreases for that particular behavioral endpoint. (A) Control in water and NOM, combined for each salinity; (B) Cotton microfiber with average water and NOM control; (C) Polyester microfiber with average water and NOM control; (D) Polypropylene microfiber with average water and NOM control. Data are representative of the calculated Z-scores normalized to controls (per Segarra et al., 2021), which are presented on the 0 axis in the middle of each figure in the panel. In the tables, asterisks represent statistically significant differences relative to the controls. The arrows indicate an increase or decrease in response for that particular behavioral endpoint. Only effects relative to the control within each salinity are shown (α < 0.5; TukeyHSD).
Polyester microfiber exposed Silversides in medium and high salinity demonstrated significantly increasing concentration dependent angular velocity under both dark and light conditions with increasing concentration dependent activity only within highest salinity in dark cycle (Figures 4, S5B). Total distance moved was significantly increased in a concentration dependent manner in both the dark and light cycles. Both the medium and high salinities Silversides demonstrated significantly increasing concentration dependent freezing under both, light and dark condition. Silversides exposed at all three salinities showed decreasing activity in the zone in a significant concentration dependent manner under both light and dark conditions. In the highest salinity, Silversides showed significant concentration dependent increasing meandering activity in both light and dark conditions. The high salinity also showed significantly decreasing concentration dependent movement in the dark cycles. In contrast, Silversides in low and medium salinity exhibited significantly increasing concentration dependent movement under light conditions. Silversides in highest salinity also showed significant concentration dependent increasing turn angle under both, light and dark conditions. Velocity was only altered in the medium salinity under light conditions, where Silversides demonstrated significant concentration dependent increasing velocity. Overall, polyester exposed silversides shown similar behavioral variations at both, highest and lowest salinities (62.5% of total activity; Figures 4, S5).
Polypropylene microfiber exposed Silversides showed significantly increasing concentration dependent angular velocity in the lowest salinity under dark conditions whereas in the light cycle, highest and medium salinities demonstrated concentration dependent increasing angular velocity (Figures 4, S5C). Silversides in the lowest salinity showed significantly higher total distance moved in the light conditions followed by the highest and medium salinity. In the case of freezing activity, fish exposed at the lowest salinity demonstrated significantly increasing concentration dependent activity under both, light and dark conditions whereas, the highest salinity demonstrated significantly increasing concentration dependent activity in the light condition only. In zone duration was decreased in all three salinities in a significant concentration dependent manner under dark conditions. Meandering activity was significantly increased in the medium salinity dark cycle, in contrast to the lowest activity in the light cycle. Silversides at lowest salinity showed a significant concentration dependent increase in turn angle under both, light and dark conditions. At the lowest salinity Silversides’ velocity was significantly decreased in a concentration dependent manner under light conditions. Overall, polypropylene exposed silversides exhibited significant behavioral variations at lowest salinity (87% of total activity; Figures 4, S5), compared to lowest salinity (50% of total activity; Figures 4, S5).
In case of overall behavioral variation, there was significant increased distance observed in all three microfiber types in at least one salinity (Figure 3b). Meandering activity decreased in all concentrations at the highest salinity in all three microfiber types. Increased freezing behavior was observed in polypropylene at all the salinity whereas cotton caused increased freezing at highest salinity in both the lowest and medium concentration. There was no increase in zone (center of beaker) duration observed in cotton at lowest concentration compared to both the synthetic microfiber types. At lowest salinity all microfiber types caused increased movement over time at all concentration ranges. During medium concentration range cotton microfiber caused least behavioral variations compared to both the synthetic microfibers. At highest concentration overall behavioral variation was less in all microfiber types.
It is important to discuss the implications of these mixed behavioral responses in the context of the current body of literature, which is still somewhat limited in terms of describing and understanding behavioral responses to microplastics. Rather than being caused by a specific molecular interaction, such as in the case of pesticides which are designed to act as neurotoxicants, microplastic and microfiber effects from particles that are too large to translocate may be caused by a combination of particle ingestion causing an altered physiological state, coupled with encountering foreign objects in the water column (Jacob et al., 2020). Plastics of other types besides microfibers, such as polystyrene microbeads (from 0.001 to 10 mg L−1) reduced mobility and altered swimming speed in sea urchin, Paracentrotus lividus, and also reduced mobility (with increased mortality) in the rotifer Brachionus plicatilis (Gambardella et al., 2018). Similarly, for blue mussel (Mytilus edulis) reduced filtering activity was reported following exposure to 100-nm polystyrene beads (Wegner et al., 2012). Another study reported that swimming area and a total distance of Chinese rice fish (Oryzias sinensis) and Korean dark chub (Zacco temminckii) were affected by nano-sized polystyrene exposure (Chae et al., 2018). Juvenile jacopever (Sebastes schlegelii) had reduced swimming speed and range of movement following exposure to polyesterene microplastics (Yin et al., 2018).
When organisms are exposed to synthetic microfibers, in particular, several behavioral effects have been observed. For example, blue mussels (Mytilus edulis) fed concentrations up to 30 MPF mL−1 had reduced filtration rates with a concentration dependent increase in microfiber tissue accumulation (Woods et al., 2018). Similarly, Kolandhasamy et al. (2018) reported microfiber retention by mussel foot and mantle that affected their adherence properties. In another study, copepods (Calanus finmarchicus) had altered prey selectivity when exposed to 50 particles/ml nylon microfibers for 6 days (Cole et al., 2019). Long term exposure to polypropylene microfibers are reported to be toxic at high concentrations (3 particles/L, ~1000 µm length) to crab (Emerita analoga), in addition to reducing egg clutches retention and affecting embryonic development (Horn et al., 2020).
Typically, behavior in fish is triggered by an external stimulus acting via neural networks (Weber and Speiler, 1994). If these neural networks disrupt by some external stimuli in the form of contaminants, that can result in altered behavior. Silverside behavior, for example, is also impacted by tire particle exposure with salinity-dependent differences (Siddiqui et al., 2022). Another study on planktivorous reef fish (Acanthochromis polyacanthus) exposed to PET (300-125 µm diam), under limited food availability conditions showed altered behavior (Critchell and Hoogenboom, 2018). Similarly, juvenile European seabass, Dicentrarchus labrax exhibited reduced swimming velocity and resistance time when exposed to fluorescent red polymer microspheres (1–5 μm diameter) (Barboza et al., 2018). Another study on Crucian Carp (Carassius carassius) reported groups exposed to live Daphnia enriched with nanoparticles moved much more slowly, exhibiting stronger shoaling behavior, occupied less space in the aquarium and did not hunt as actively as control fish (Mattsson et al., 2015). Due to limited studies reporting behavioral variability in fish following microfiber digestion, these results can be considered as the first to report behavioral alterations in fish subsequent to microfiber exposure, particularly considering the inclusion of cellulose-based fibers which is novel. Traditionally, growth, development and reproduction are considered important ecotoxicological endpoints that can help identify the severity of risk associated with contaminants. However, microplastics and microfibers are often present below the quantities that can cause significant toxicity, such as mortality or deformities. Under such low doses, endpoints such as behavior serve as a link between physiological and ecological processes to study environmental contaminants, and changes in behavior can affect an organism’s chances of surviving, as well as its fitness (Weis et al., 2001). The need to include behavioral indicators for ecologically relevant monitoring has been proposed from the late 80s (Atchison et al., 1987) and has continued to date (Ford et al., 2021).
In summary, natural microfibers were identified as least toxic when compared to the other two synthetic microfiber types in this study, but they still caused adverse responses in terms of growth in mysids and altered behavior in both taxa. Similar results were reported in brine shrimp Artemia franciscana when exposed to two commonly synthetic microfibers (polypropylene and polyethylene terephthalate) and one natural fiber (lyocell) (Kim et al., 2021). Based on behavioral variability mysids shrimps are more affected by polyester microfibers whereas Silverside larvae were more impacted by polypropylene microfibers. Both organisms demonstrated the lowest impact from the cotton microfibers. When considering salinity, cotton impacted both fish and invertebrate behavior more at higher salinities, whereas polyester and polypropylene had higher impacts at lower salinities. Also, overall, in terms of internalization and effects on growth, cotton has a lesser impact compared to synthetic fibers. This study contributes to our understanding of impacts to growth and the behavioral ecotoxicology of microfiber exposure and identifies potential risks associated with each. Our results support proposed efforts to reduce the loading of microfibers into the environment, such as potentially requiring filtration devices on washing machines and clothes dryers (Erdle et al., 2021). The impact of microfibers on growth and behavior in both organisms tested here is concerning, and further investigations are needed to better understand the behavior of microfibers across salinities, as well as the potential relationship between changes in apical endpoints, such as growth, and altered behavior.
The original contributions presented in the study are included in the article/Supplementary Materials. Data and code used in this analysis can be found at https://github.com/Brander-Harper/microfiber_acute_ss_mysid. For further inquiries please contact the corresponding authors.
The animal study was reviewed and approved by Oregon State University IACUC.
Conceptualization, SB, SH. Methodology, investigation, formal analysis, data curation, writing SS, JD, SB. Review, editing and investigation SJH, EP, SB. Visualization SJH, SS. Supervision, project administration, funding acquisition, SB. All authors have read and agreed to the published version of the manuscript.
This research was funded by the National Science Foundation Growing Convergence Research Big Idea (to SH, SB), 1935028 and supported by ideas and behavioral assay protocols developed under California Delta Science agreement # 18206 (to SB). The ideas presented in this publication are those solely of the authors and do not necessarily reflect the opinions of the granting agency.
Authors would like to thank Christopher Markgraf for their assistance in experimental preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.991650/full#supplementary-material
Acharya S., Rumi S. S., Hu Y., Abidi N. (2021). Microfibers from synthetic textiles as a major source of microplastics in the environment: A review. Textile Res. J. 91, 2136–2156. doi: 10.1177/0040517521991244
Akhbarizadeh R., Dobaradaran S., Nabipour I., Tangestani M., Abedi D., Javanfekr F., et al. (2021). Abandoned covid-19 personal protective equipment along the bushehr shores, the Persian gulf: An emerging source of secondary microplastics in coastlines. Mar. pollut. Bull. 168, 112386. doi: 10.1016/j.marpolbul.2021.112386
Allen A. S., Seymour A. C., Rittschof D. (2017). Chemoreception drives plastic consumption in a hard coral. Mar. pollut. Bull. 124, 198–205. doi: 10.1016/j.marpolbul.2017.07.030
Atchison G. J., Henry M. G., Sandheinrich M. B. (1987). Effects of metals on fish behavior: A review. Environ. Biol. Fishes 18, 11–25. doi: 10.1007/BF00002324
Athey S. N., Adams J. K., Erdle L. M., Jantunen L. M., Helm P. A., Finkelstein S. A., et al. (2020). The widespread environmental footprint of indigo denim microfibers from blue jeans. Environ. Sci. Technol. Lett. 7, 840–847. doi: 10.1021/acs.estlett.0c00498
Athey S. N., Erdle L. M. (2021). Are we underestimating anthropogenic microfiber pollution? a critical review of occurrence, methods, and reporting. Environ. Toxicol. Chem., 5173. doi: 10.1002/etc.5173
Auta H. S., Emenike C. U., Fauziah S. H. (2017). Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 102, 165–176. doi: 10.1016/j.envint.2017.02.013
Barboza L. G. A., Vieira L. R., Guilhermino L. (2018). Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchus labrax): Changes in behavioural responses and reduction of swimming velocity and resistance time. Environ. pollut. 236, 1014–1019. doi: 10.1016/j.envpol.2017.12.082
Bergmann M., Gutow L., Klages M. (2015). Marine anthropogenic litter (Cham: Springer International Publishing). doi: 10.1007/978-3-319-16510-3
Bessa F., Barría P., Neto J. M., Frias J. P. G. L., Otero V., Sobral P., et al. (2018). Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. pollut. Bull. 128, 575–584. doi: 10.1016/j.marpolbul.2018.01.044
Bour A., Hossain S., Taylor M., Sumner M., Carney Almroth B. (2020). Synthetic microfiber and microbead exposure and retention time in model aquatic species under different exposure scenarios. Front. Environ. Sci. 8. doi: 10.3389/fenvs.2020.00083
Brahney J., Mahowald N., Prank M., Cornwell G., Klimont Z., Matsui H., et al. (2021). Constraining the atmospheric limb of the plastic cycle. Proc. Natl. Acad. Sci. U.S.A. 118, e2020719118. doi: 10.1073/pnas.2020719118
Brillant M., Macdonald R. (2002). Postingestive selection in the sea scallop (Placopecten magellanicus) on the basis of chemical properties of particles. Mar. Biol. 141, 457–465. doi: 10.1007/s00227-002-0845-2
Brodin T., Piovano S., Fick J., Klaminder J., Heynen M., Jonsson M. (2014). Ecological effects of pharmaceuticals in aquatic systems–impacts through behavioural alterations. Philos. Trans. R. Soc. B: Biol. Sci. 369, 20130580.
Browne M. A., Galloway T. S., Thompson R. C. (2010). Spatial patterns of plastic debris along estuarine shorelines. Environ. Sci. Technol. 44, 3404–3409. doi: 10.1021/es903784e
Cau A., Avio C. G., Dessì C., Moccia D., Pusceddu A., Regoli F., et al. (2020). Benthic crustacean digestion can modulate the environmental fate of microplastics in the deep Sea. Environ. Sci. Technol. 54, 4886–4892. doi: 10.1021/acs.est.9b07705
Caldwell A., Brander S., Wiedenmann J., Clucas G., Craig E. (2022). Incidence of microplastic fiber ingestion by common terns (Sterna hirundo) and roseate terns (S. dougallii) breeding in the Northwestern Atlantic. Mar. Pollut. Bull. 177, 113560. doi: 10.1016/j.marpolbul.2022.113560
Cesa F. S., Turra A., Checon H. H., Leonardi B., Baruque-Ramos J. (2020). Laundering and textile parameters influence fibers release in household washings. Environ. pollut. 257, 113553. doi: 10.1016/j.envpol.2019.113553
Chae Y., Kim D., Kim S. W., An Y.-J. (2018). Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci. Rep. 8, 284. doi: 10.1038/s41598-017-18849-y
Chapron L., Peru E., Engler A., Ghiglione J. F., Meistertzheim A. L., Pruski A. M., et al. (2018). Macro- and microplastics affect cold-water corals growth, feeding and behaviour. Sci. Rep. 8, 15299. doi: 10.1038/s41598-018-33683-6
Cole M. (2016). A novel method for preparing microplastic fibers. Sci. Rep. 6, 34519. doi: 10.1038/srep34519
Cole M., Coppock R., Lindeque P. K., Altin D., Reed S., Pond D. W., et al. (2019). Effects of nylon microplastic on feeding, lipid accumulation, and moulting in a coldwater copepod. Environ. Sci. Technol. 53, 7075–7082. doi: 10.1021/acs.est.9b01853
Conley K., Clum A., Deepe J., Lane H., Beckingham B. (2019). Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Water Res. X 3, 100030. doi: 10.1016/j.wroa.2019.100030
Coppock R. L., Galloway T. S., Cole M., Fileman E. S., Queirós A. M., Lindeque P. K. (2019). Microplastics alter feeding selectivity and faecal density in the copepod, calanus helgolandicus. Sci. Total Environ. 687, 780–789. doi: 10.1016/j.scitotenv.2019.06.009
Costanza R., d’Arge R., de Groot R., Farber S., Grasso M., Hannon B., et al. (1997). The value of the world’s ecosystem services and natural capital. Nature 387, 253–260. doi: 10.1038/387253a0
Critchell K., Hoogenboom M. O. (2018). Effects of microplastic exposure on the body condition and behaviour of planktivorous reef fish (Acanthochromis polyacanthus). PloS One 13, e0193308. doi: 10.1371/journal.pone.0193308
DeCourten B. M., Forbes J. P., Roark H. K., Burns N. P., Major K. M., White J. W., et al. (2020). Multigenerational and transgenerational effects of environmentally relevant concentrations of endocrine disruptors in an estuarine fish model. Environ. Sci. Technol. 54, 13849–13860. doi: 10.1021/acs.est.0c02892
Erdle L. M., Nouri Parto D., Sweetnam D., Rochman C. M. (2021). Washing machine filters reduce microfiber emissions: Evidence from a community-scale pilot in parry sound, Ontario. Front. Mar. Sci. 1703.
Ford A. T., Ågerstrand M., Brooks B. W., Allen J., Bertram M. G., Brodin T., et al. (2021). The role of behavioral ecotoxicology in environmental protection. Environ. Sci. Technol. 55, 5620–5628. doi: 10.1021/acs.est.0c06493
Gambardella C., Morgana S., Bramini M., Rotini A., Manfra L., Migliore L., et al. (2018). Ecotoxicological effects of polystyrene microbeads in a battery of marine organisms belonging to different trophic levels. Mar. Environ. Res. 141, 313–321. doi: 10.1016/j.marenvres.2018.09.023
Gambino G., Falleni A., Nigro M., Salvetti A., Cecchettini A., Ippolito C., et al. (2020). Dynamics of interaction and effects of microplastics on planarian tissue regeneration and cellular homeostasis. Aquat. Toxicol. 218, 105354. doi: 10.1016/j.aquatox.2019.105354
Granek E. F., Brander S. M., Holland E. B. (2020). Microplastics in aquatic organisms: Improving understanding and identifying research directions for the next decade. Limnol. Oceanogr. Lett. 5, 1–4. doi: 10.1002/lol2.10145
Granek E., Traylor S., Tisot A., Hurst P., Woods R., Brander S. M. (2022). “Clothes encounters of the microfibre kind: The effects of synthetic and natural textiles on organisms,” in Microplastics: Environmental problems and textile solutions. Ed. Weis J. S.. (Abingdon UK: Taylor and Francis).
Hartline N. L., Bruce N. J., Karba S. N., Ruff E. O., Sonar S. U., Holden P. A. (2016). Microfiber masses recovered from conventional machine washing of new or aged garments. Environ. Sci. Technol. 50, 11532–11538. doi: 10.1021/acs.est.6b03045
Henry B., Laitala K., Klepp I. G. (2019). Microfibres from apparel and home textiles: Prospects for including microplastics in environmental sustainability assessment. Sci. Total Environ. 652, 483–494. doi: 10.1016/j.scitotenv.2018.10.166
Horn D. A., Granek E. F., Steele C. L. (2020). Effects of environmentally relevant concentrations of microplastic fibers on pacific mole crab (Emerita analoga) mortality and reproduction. Limnol. Oceanogr. 5, 74–83. doi: 10.1002/lol2.10137
Hutton S. J., St. Romain S. J., Pedersen E. I., Siddiqui S., Chappell P. E., White J. W., et al. (2021). Salinity alters toxicity of commonly used pesticides in a model euryhaline fish species (Menidia beryllina). Toxics 9, 114. doi: 10.3390/toxics9050114
Jacob H., Besson M., Swarzenski P. W., Lecchini D., Metian M. (2020). Effects of virgin micro- and nanoplastics on fish: Trends, meta-analysis, and perspectives. Environ. Sci. Technol. 54, 4733–4745. doi: 10.1021/acs.est.9b05995
Kim S. W., Kim D., Chae Y., An Y.-J. (2018). Dietary uptake, biodistribution, and depuration of microplastics in the freshwater diving beetle cybister japonicus: Effects on predacious behavior. Environ. pollut. 242, 839–844. doi: 10.1016/j.envpol.2018.07.071
Kim L., Kim S. A., Kim T. H., Kim J., An Y.-J. (2021). Synthetic and natural microfibers induce gut damage in the brine shrimp artemia franciscana. Aquat. Toxicol. 232, 105748. doi: 10.1016/j.aquatox.2021.105748
Knauss C. M., Dungan C. F., Lehmann S. A. (2021). A paraffin microtomy method for improved and efficient production of standardized plastic microfibers. Environ. Toxicol. Chem., 5216. doi: 10.1002/etc.5216
Kolandhasamy P., Su L., Li J., Qu X., Jabeen K., Shi H. (2018). Adherence of microplastics to soft tissue of mussels: A novel way to uptake microplastics beyond ingestion. Sci. Total Environ. 610–611, 635–640. doi: 10.1016/j.scitotenv.2017.08.053
Krüger L., Casado-Coy N., Valle C., Ramos M., Sánchez-Jerez P., Gago J., et al. (2020). Plastic debris accumulation in the seabed derived from coastal fish farming. Environ. pollut. 257, 113336. doi: 10.1016/j.envpol.2019.113336
Kutralam-Muniasamy G., Pérez-Guevara F., Elizalde-Martínez I., Shruti V. C. (2020). An overview of recent advances in micro/nano beads and microfibers research: Critical assessment and promoting the less known. Sci. Total Environ. 740, 139991. doi: 10.1016/j.scitotenv.2020.139991
Kwak J. I., Liu H., Wang D., Lee Y. H., Lee J.-S., An Y.-J. (2022). Critical review of environmental impacts of microfibers in different environmental matrices. Comp. Biochem. Physiol. Part C.: Toxicol. Pharmacol. 251, 109196. doi: 10.1016/j.cbpc.2021.109196
Li L., Frey M., Browning K. J. (2010). Biodegradability study on cotton and polyester fabrics. J. Engineered Fibers Fabrics 5. doi: 10.1177/155892501000500406
Lima A. R. A., Ferreira G. V. B., Barrows A. P. W., Christiansen K. S., Treinish G., Toshack M. C. (2021). Global patterns for the spatial distribution of floating microfibers: Arctic ocean as a potential accumulation zone. J. Hazardous Mater. 403, 123796. doi: 10.1016/j.jhazmat.2020.123796
Little E. E., Archeski R. D., Flerov B. A., Kozlovskaya V. I. (1990). Behavioral indicators of sublethal toxicity in rainbow trout. Arch. Environ. Contam. Toxicol. 19, 380–385. doi: 10.1007/BF01054982
Lo H. K. A., Chan K. Y. K. (2018). Negative effects of microplastic exposure on growth and development of crepidula onyx. Environ. pollut. 233, 588–595. doi: 10.1016/j.envpol.2017.10.095
Ma C., Li L., Chen Q., Lee J.-S., Gong J., Shi H. (2021). Application of internal persistent fluorescent fibers in tracking microplastics in vivo processes in aquatic organisms. J. Hazardous Mater. 401, 123336. doi: 10.1016/j.jhazmat.2020.123336
Mattsson K., Ekvall M. T., Hansson L.-A., Linse S., Malmendal A., Cedervall T. (2015). Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ. Sci. Technol. 49, 553–561. doi: 10.1021/es5053655
McCormick M. I., Chivers D. P., Ferrari M. C. O., Blandford M. I., Nanninga G. B., Richardson C., et al. (2020). Microplastic exposure interacts with habitat degradation to affect behaviour and survival of juvenile fish in the field. Proc. R. Soc B. 287, 20201947. doi: 10.1098/rspb.2020.1947
Middaugh D. P., Hemmer M. J., Goodman L. R. (1987). Methods for spawning, culturing and conducting toxicity-tests with early life stages of four atherinid fishes: the inland silverside, menidia beryllina, Atlantic silverside, m. menidia, tidewater silverside, m. peninsulae and California grunion, leuresthes tenuis (Washington DC: Office of Research and Development, U.S. Environmental Protection Agency), 20460.
Mishra S., Rath C., Das A. P. (2019). Marine microfiber pollution: A review on present status and future challenges. Mar. pollut. Bull. 140, 188–197. doi: 10.1016/j.marpolbul.2019.01.039
Mishra S., Singh R. P., Rath C. C., Das A. P. (2020). Synthetic microfibers: Source, transport and their remediation. J. Water Process Eng. 38, 101612. doi: 10.1016/j.jwpe.2020.101612
Moore C. J. (2008). Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 108, 131–139. doi: 10.1016/j.envres.2008.07.025
Morgana S., Casentini B., Amalfitano S. (2021). Uncovering the release of micro/nanoplastics from disposable face masks at times of COVID-19. J. Hazardous Mater. 419, 126507. doi: 10.1016/j.jhazmat.2021.126507
Mundy P., Huff Hartz K., Fulton C., Lydy M., Brander S., Hung T., et al. (2021). Exposure to permethrin or chlorpyrifos causes differential dose- and time-dependent behavioral effects at early larval stages of an endangered teleost species. Endang Species Res. 44, 89–103. doi: 10.3354/esr01091
Mundy P. C., Carte M. F., Brander S. M., Hung T.-C., Fangue N., Connon R. E. (2020). Bifenthrin exposure causes hyperactivity in early larval stages of an endangered fish species at concentrations that occur during their hatching season. Aquat. Toxicol. 228, 105611. doi: 10.1016/j.aquatox.2020.105611
Naidoo T., Glassom D. (2019). Decreased growth and survival in small juvenile fish, after chronic exposure to environmentally relevant concentrations of microplastic. Mar. pollut. Bull. 145, 254–259. doi: 10.1016/j.marpolbul.2019.02.037
O’Brien A. M., Lins T. F., Yang Y., Frederickson M. E., Sinton D., Rochman C. M. (2022). Microplastics shift impacts of climate change on a plant-microbe mutualism: Temperature, CO2, and tire wear particles. Environ. Res. 203, 111727. doi: 10.1016/j.envres.2021.111727
Ohnuma S., Katagiri T., Maita M., Endo M., Futami K. (2017). Application of tissue clearing techniques to 3D study of infectious disease pathology in fish. Fish. Pathol. 52, 96–99. doi: 10.3147/jsfp.52.96
Pirc U., Vidmar M., Mozer A., Kržan A. (2016). Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ. Sci. pollut. Res. 23, 22206–22211. doi: 10.1007/s11356-016-7703-0
Possatto F. E., Barletta M., Costa M. F., Ivar do Sul J. A., Dantas D. V. (2011). Plastic debris ingestion by marine catfish: An unexpected fisheries impact. Mar. pollut. Bull. 62, 1098–1102. doi: 10.1016/j.marpolbul.2011.01.036
Reineccius J., Appelt J.-S., Hinrichs T., Kaiser D., Stern J., Prien R. D., et al. (2020). Abundance and characteristics of microfibers detected in sediment trap material from the deep subtropical north Atlantic ocean. Sci. Total Environ. 738, 140354. doi: 10.1016/j.scitotenv.2020.140354
Ritz C. (2010). Toward a unified approach to dose-response modeling in ecotoxicology. Environ. Toxicol. Chem. 29, 220–229. doi: 10.1002/etc.7
Romanó de Orte M., Clowez S., Caldeira K. (2019). Response of bleached and symbiotic sea anemones to plastic microfiber exposure. Environ. pollut. 249, 512–517. doi: 10.1016/j.envpol.2019.02.100
Saborowski R., Paulischkis E., Gutow L. (2019). How to get rid of ingested microplastic fibers? a straightforward approach of the Atlantic ditch shrimp palaemon varians. Environ. pollut. 254, 113068. doi: 10.1016/j.envpol.2019.113068
Salvador Cesa F., Turra A., Baruque-Ramos J. (2017). Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 598, 1116–1129. doi: 10.1016/j.scitotenv.2017.04.172
Segarra A., Mauduit F., Amer N. R., Biefel F., Hladik M. L., Connon R. E., et al. (2021). Salinity changes the dynamics of pyrethroid toxicity in terms of behavioral effects on newly hatched delta smelt larvae. Toxics 9, 40. doi: 10.3390/toxics9020040
Shupe H. J., Boenisch K. M., Harper B. J., Brander S. M., Harper S. L. (2021). Effect of nanoplastic type and surface chemistry on particle agglomeration over a salinity gradient. Environ. Toxicol. Chem. 40, 1820–1826. doi: 10.1002/etc.5030
Siddiqui S., Dickens J. M., Cunningham B. E., Hutton S. J., Pedersen E. I., Harper B., et al. (2022). Internalization, reduced growth, and behavioral effects following exposure to micro and nano tire particles in two estuarine indicator species. Chemosphere, 133934. doi: 10.1016/j.chemosphere.2022.133934
Sillanpää M., Sainio P. (2017). Release of polyester and cotton fibers from textiles in machine washings. Environ. Sci. pollut. Res. 24, 19313–19321. doi: 10.1007/s11356-017-9621-1
Stickney R. R., Shumway S. E. (1974). Occurrence of cellulase activity in the stomachs of fishes. J. Fish. Biol. 6, 779–790. doi: 10.1111/j.1095-8649.1974.tb05120.x
Stienbarger C. D., Joseph J., Athey S. N., Monteleone B., Andrady A. L., Watanabe W. O., et al. (2021). Direct ingestion, trophic transfer, and physiological effects of microplastics in the early life stages of centropristis striata, a commercially and recreationally valuable fishery species. Environ. pollut. 285, 117653. doi: 10.1016/j.envpol.2021.117653
Suaria G., Achtypi A., Perold V., Lee J. R., Pierucci A., Bornman T. G., et al. (2020). Microfibers in oceanic surface waters: A global characterization. Sci. Adv. 6, eaay8493. doi: 10.1126/sciadv.aay8493
Susaki E. A., Tainaka K., Perrin D., Yukinaga H., Kuno A., Ueda H. R. (2015). Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat. Protoc. 10, 1709–1727. doi: 10.1038/nprot.2015.085
Vendel A. L., Bessa F., Alves V. E. N., Amorim A. L. A., Patrício J., Palma A. R. T. (2017). Widespread microplastic ingestion by fish assemblages in tropical estuaries subjected to anthropogenic pressures. Mar. pollut. Bull. 117, 448–455. doi: 10.1016/j.marpolbul.2017.01.081
Ward J. E., Zhao S., Holohan B. A., Mladinich K. M., Griffin T. W., Wozniak J., et al. (2019). Selective ingestion and egestion of plastic particles by the blue mussel (Mytilus edulis) and Eastern oyster (Crassostrea virginica): Implications for using bivalves as bioindicators of microplastic pollution. Environ. Sci. Technol. 53, 8776–8784. doi: 10.1021/acs.est.9b02073
Watts A. J. R., Urbina M. A., Corr S., Lewis C., Galloway T. S. (2015). Ingestion of plastic microfibers by the crab Carcinus maenas and its effect on food consumption and energy balance. Environ. Sci. Technol. 49, 14597–14604. doi: 10.1021/acs.est.5b04026
Watts A. J. R., Urbina M. A., Goodhead R., Moger J., Lewis C., Galloway T. S. (2016). Effect of microplastic on the gills of the shore crab Carcinus maenas. environ. Sci. Technol. 50, 5364–5369. doi: 10.1021/acs.est.6b01187
Weber D. N., Speiler R. E. (1994). “Behavioral mechanisms of metal toxicity in fishes,” in Aquatic toxicology: Molecular, biochemical and cellular perspectives. Eds. Malins D. C., Ostrander G. K. (London, UK.: CRC Press), 421–467.
Wegner A., Besseling E., Foekema E. M., Kamermans P., Koelmans A. A. (2012). Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis l.). Environ. Toxicol. Chem. 31, 2490–2497. doi: 10.1002/etc.1984
Weis J. S., Smith G., Zhou T., Santiago-Bass C., Weis P. (2001). Effects of contaminants on behavior: Biochemical mechanisms and ecological consequences. BioScience 51, 209. doi: 10.1641/0006-3568(2001)051[0209:EOCOBB]2.0.CO;2
Welden N. A. C., Cowie P. R. (2016). Long-term microplastic retention causes reduced body condition in the langoustine, nephrops norvegicus. Environ. pollut. 218, 895–900. doi: 10.1016/j.envpol.2016.08.020
Woods M. N., Stack M. E., Fields D. M., Shaw S. D., Matrai P. A. (2018). Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis). Mar. pollut. Bull. 137, 638–645. doi: 10.1016/j.marpolbul.2018.10.061
Wright L. S., Napper I. E., Thompson R. C. (2021). Potential microplastic release from beached fishing gear in great britain’s region of highest fishing litter density. Mar. pollut. Bull. 173, 113115. doi: 10.1016/j.marpolbul.2021.113115
Wright S. L., Thompson R. C., Galloway T. S. (2013). The physical impacts of microplastics on marine organisms: A review. Environ. pollut. 178, 483–492. doi: 10.1016/j.envpol.2013.02.031
Yang L., Qiao F., Lei K., Li H., Kang Y., Cui S., et al. (2019). Microfiber release from different fabrics during washing. Environ. pollut. 249, 136–143. doi: 10.1016/j.envpol.2019.03.011
Yin L., Chen B., Xia B., Shi X., Qu K. (2018). Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J. Hazardous Mater. 360, 97–105. doi: 10.1016/j.jhazmat.2018.07.110
Zambrano M. C., Pawlak J. J., Daystar J., Ankeny M., Cheng J. J., Venditti R. A. (2019). Microfibers generated from the laundering of cotton, rayon and polyester based fabrics and their aquatic biodegradation. Mar. pollut. Bull. 142, 394–407. doi: 10.1016/j.marpolbul.2019.02.062
Zambrano M. C., Pawlak J. J., Daystar J., Ankeny M., Goller C. C., Venditti R. A. (2020). Aerobic biodegradation in freshwater and marine environments of textile microfibers generated in clothes laundering: Effects of cellulose and polyester-based microfibers on the microbiome. Mar. pollut. Bull. 151, 110826. doi: 10.1016/j.marpolbul.2019.110826
Zambrano M. C., Pawlak J. J., Daystar J., Ankeny M., Venditti R. A. (2021). Impact of dyes and finishes on the microfibers released on the laundering of cotton knitted fabrics. Environ. pollut. 272, 115998. doi: 10.1016/j.envpol.2020.115998
Keywords: rope, cotton, polyester, polypropylene, inland silversides, mysid shrimp, sublethal, marine
Citation: Siddiqui S, Hutton SJ, Dickens JM, Pedersen EI, Harper SL and Brander SM (2023) Natural and synthetic microfibers alter growth and behavior in early life stages of estuarine organisms. Front. Mar. Sci. 9:991650. doi: 10.3389/fmars.2022.991650
Received: 11 July 2022; Accepted: 06 December 2022;
Published: 31 January 2023.
Edited by:
Hans Uwe Dahms, Kaohsiung Medical University, TaiwanReviewed by:
Mengyang Liu, City University of Hong Kong SAR, ChinaCopyright © 2023 Siddiqui, Hutton, Dickens, Pedersen, Harper and Brander. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. Siddiqui, c2FtcmVlbl9zaWRkaXF1aUBvdXRsb29rLmNvbQ==; S. M. Brander, c3VzYW5uZS5icmFuZGVyQG9yZWdvbnN0YXRlLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.