- 1Key Laboratory of Sichuan Province for Fishes Conservation and Utilization in the Upper Reaches of the Yangtze River, Neijiang Normal University, Neijiang, China
- 2Guangdong Provincial Key Laboratory of Marine Biology, Shantou University, Shantou, China
This study aimed to evaluate the impacts of dietary pyrroloquinoline quinone (PQQ) supplement on growth performance, serum biochemical parameters, antioxidant status, and growth-related genes expressions in juvenile yellow catfish, Pelteobagrus fulvidraco. Triplicate groups of fish (n = 40) with an average weight of 5 g were fed with five gradient levels PQQ-incorporated diets (0 (basal), 1.5 mg/kg; 3.0 mg/kg; 4.5 mg/kg, 6.0 mg/kg) for 56 days. Our findings revealed that fish fed with the diets containing PQQ at the level of 3.0-6.0 mg/kg showed significantly higher final body weight, weight gain rate, and specific growth rate than those of that in the control group (P < 0.05). The activities of protease were observed significantly increased in fish fed with diets containing 4.5 mg/kg and 6 mg/kg PQQ (P < 0.05). Meanwhile, fish in 4.5 mg/kg PQQ group showed significantly lower levels of serum total cholesterol, triglycerides, and low-density lipoprotein cholesterol, and significantly higher level of the high-density lipoprotein cholesterol (P < 0.05). The antioxidant-related parameters of superoxide dismutase and total antioxidant capacity were markedly elevated (P < 0.05), while malondialdehyde content was significantly reduced in 3.0-6.0 mg/kg PQQ group (P < 0.05). Meanwhile, the mRNA expression levels of growth-related genes (growth hormone, insulin-like growth factor 1, and insulin-like growth factor 2) were dramatically up-regulated in the liver of fish fed with the diets containing 3-6 mg/kg PQQ in comparison with the control group (P < 0.05). In conclusion, dietary PQQ could improve the growth performance, serum biochemical parameters, antioxidant status, and growth-related genes expressions in juvenile yellow catfish, and the optimal dietary PQQ level was evaluated to be 4.92 mg/kg of dry diet for juvenile yellow catfish.
Introduction
Yellow catfish, Pelteobagrus fulvidraco is an important aquaculture species in China and its production has greatly improved over the past 10 years due to its suitability for aquaculture, marketability, good taste, and high nutritional value (Shi et al., 2021). Unsurprisingly, this kind of fish has been largely cultured to meet the increasing market demands. As a result, lots of outbreaks of infectious diseases that caused by microorganisms (such as viruses, bacteria fungi, or parasites) are commonly seen and which usually leads to large economic losses (Zhang et al., 2014; Jiang et al., 2018). Antibiotics have been commonly used as a traditional strategy to control the outbreak of various infectious disease (Ramesh and Souissi, 2018). However, the over and continuous application of antibiotics may cause the emergence of antimicrobial resistance, environmental hazards, and food safety problems (Hollis and Ahmed, 2014). Meanwhile, the interest in the safety and quality of aquatic products of customers is obviously increasing with the growing problems of contaminants, antibiotics, and carcinogens in aquatic industry (Rama and Manjabhat, 2014). Therefore, antibiotics have been banned or restricted for utilization in aquaculture and which has encouraged researchers to develop alternative strategies. Thus far, many research teams in this field have devoted themselves to evaluating the positive effects of eco-friendly bio-active components as functional feed supplements on the growth, feed utilization, and enzymatic profiles in different fish including yellow catfish (Gabriel et al., 2017; Safari et al., 2020; Park et al., 2021; Fu et al., 2022).
Pyrroloquinoline quinone (PQQ), a water-soluble thermo-stable triglyceride-quinone (Zhang et al., 2006), is initially identified in methylotrophic bacteria and characterized as a redox cofactor of bacterial dehydrogenases, such as alcohol and glucose dehydrogenases (Killgore et al., 1989). PQQ is an essential nutrient for animals, and intake of PQQ-deficient diet usually leads to multifarious illnesses (Akagawa et al., 2016). PQQ has caused considerable attention, as it is exactly important for mammalian growth, development, reproduction, and immune function (Steinberg et al., 2003; Ikemoto et al., 2017). PQQ is also an effective antioxidant that can protect mitochondria from oxidative stress-induced lipid peroxidation, protein carbonyl formation, and mitochondrial respiratory chain inactivation (Hwang and Willoughby, 2018). On a molar basis, PQQ exhibited 15-fold effects than ascorbic acid in reducing chemiluminescence from xanthine-xanthine oxidase reaction and 7-fold effects than alpha-tocopherol in preventing lipid peroxidation in rat brain preparations (Hamagishi et al., 1990). In addition, PQQ inhibits the apoptosis of cardiomyocytes under conditions of oxygen/glucose deprivation (Xu et al., 2014). Because of its versatile functions, PQQ-containing products have been certified by authorities in Canada as a Natural Health Product (Health Canada, 2012) and have also been authorized as a new type of food for use in food supplement by the European Commission in 2018. Until now, no published studies concerning the physiological responses to PQQ-supplemented diets has been reported in yellow catfish. Meanwhile, it is also unclear whether PQQ can be used as a supplement in aquaculture. According to studies in broilers (Samuel et al., 2015; Liu et al., 2020; Zheng et al., 2020) and pigs (Zhang et al., 2019; Yin et al., 2019), we hypothesized that PQQ may benefit aquaculture by affecting growth, plasma parameters, and antioxidant status of fish. To verify this hypothesis, the present study evaluate the effects of PQQ supplementary diets on the growth performance, serum biochemical parameters, antioxidant status, and growth-related gene expression such as growth hormone (GH), insulin-like growth factor 1 (IGF-1), and insulin-like growth factor 2 (IGF-2) in juvenile yellow catfish.
Materials and methods
Experimental diets and feeding trials
PQQ (purity, ≥98 mg/kg; Shanxi Boke Biological Technology Co., Ltd., Xian, China) was diluted with wheat flour to a concentration of 1 g/kg mixture before being mixed into the diet. Five experiment diets containing 0 (control), 1.5, 3.0, 4.5, and 6 mg per kg of PQQ in this experiment were produced at Neijiang Normal University, Neijiang, China, as described by Shi et al. (2021). The amount of cellulose was reduced in compensation. The dried experimental diets were stored at −20 °C for further use. The composition of the experimental diets was shown in Table 1.
The current study was performed in polyvinyl chloride round aquarium (Diameter × High: 100 × 120 cm) located at Neijiang Normal University (Sichuan, China). Fish provided by a private fishery farm (Meishan, China) were transported to the rearing facilities with air pumps, acclimated for two weeks and fed with the basal diet during this period. After two weeks of acclimatization to aquarium conditions, a total of 600 fish (average body weight of 5 g) were randomly divided into five treatment groups with three replicates (40 fish per replicate). During the feeding period, fish were fed with the designed diet twice daily (7:30 and 18:30) for 56 days. Daily feeding rates were 4-6% of the total body weight for each aquarium. The detailed food intake was recorded. Uneaten pellets were collected at 30 min after feeding, gathered, dried, and weighted in turn, and the data were used to calculate the actual food intake. This trial was carried out under natural photoperiod. About 25-30% of the water in the aquarium was replaced per day and the water temperature, dissolved oxygen, and pH were maintained at 25.0 ± 2.5°C, 7.3 ± 0.2 mg/L, and 7.6 ± 0.2, respectively. Furthermore, the level of ammonia was kept below 0.3 mg/L.
Samples collection
At the end of the feeding trial, fish were fasted for 24 h, weighed and counted to calculate their growth performance. Blood samples (9 fish per replicate) were drawn from the caudal vein, separated by centrifugation after clotting (10 min at 4000 rpm). The supernatant was stored at −80°C for plasma biochemical and antioxidant enzyme activity analysis. After that, the liver and intestine (6 fish per replicate) were removed immediately using sterile forceps, frozen rapidly by dipping in liquid nitrogen, and then stored at −80 °C until for analysis.
Digestive enzyme activity
The intestine samples were removed onto the ice, homogenized in a 1:9 (m/v) ratio of physiological saline solution, and then centrifuged with 3000 rpm at 4 °C for 10 min. After that, the supernatant comprising enzymes was stored at −80 °C until further utilization. The amylase and lipase activities were determined in triplicate using commercial assay kits supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The protease activity of each sample were analyzed in triplicate was detected using Folin method with reference to the professional standards of People’ s Republic of China to determine protease activity SB/T 10317-1999 (SB/T 10317-1999 (1999)). The total amount of protein in the intestine was determined by using the Bradford method (Bradford, 1976). All enzyme activities were measured as the change in absorbance using a Microplate Reader (UV-2802S; Unico, Shanghai, China).
Serum biochemical parameters
The serum biochemical parameters, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and blood urea nitrogen (BUN) were measured with commercial kits producted by Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer’s instructions.
Antioxidant parameters
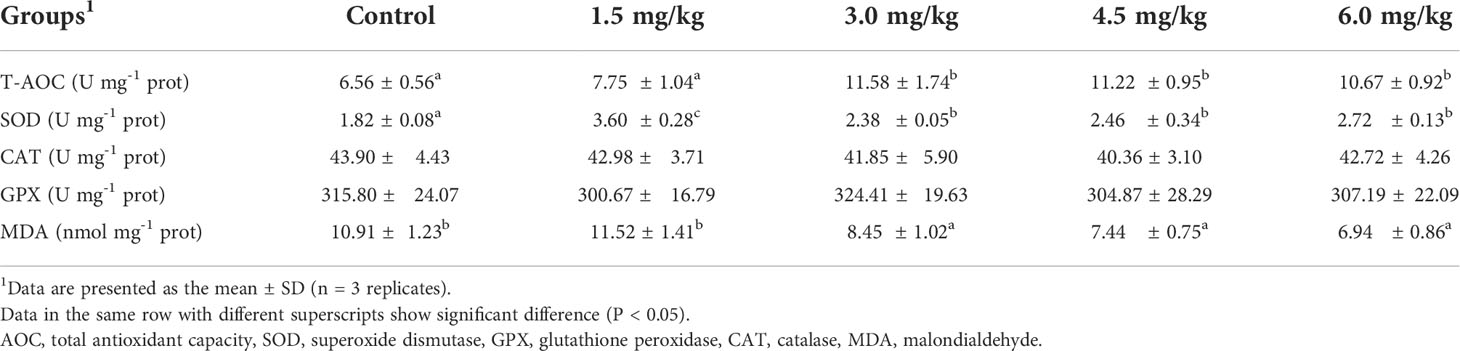
The antioxidant parameters including superoxide dismutase (SOD), catalase (CAT), total antioxidant capacity (T-AOC), glutathione peroxidase (GPX), and malondialdehyde (MDA) were all determined using kits from the same manufacturer as described by an our previous study (Shi et al., 2019).
RNA isolation and gene expression
The RNA isolation and detection process were performed according to the method described by Shi et al. (2019). In brief, the total RNA was extracted from liver sample (approximately for 50 mg) using 1 mL Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions. Thereafter, RNA quality and purity were verified by agarose gel (1 mg/kg) electrophoresis and UV-spectroscopic analysis. A fixed concentration of RNA (2 μg) was used for cDNA synthesis using Prime Script II 1st Strand cDNA Synthesis Kit (Tiangen, Beijing, China) based on the manufacturer’s protocol. For qRT-PCR, specific primers for GH, IGF-1, and IGF-2 genes were designed with online Primer 5 software (PREMIER Biosoft International, San Francisco, CA, USA), based on an our transcriptome data of yellow catfish (Table 2). The SYBR Green qPCR Master Mix Kit (Glpbio, USA) was used for qRT-PCR analysis on a Bio-Rad CFX Connect System (Bio-Rad, Hercules, CA, USA). The qRT-PCR program was designed as follows: 95°C for 5 min, followed by 95°C for 15 s, annealing at specific temperatures (Table 1) for each gene for 30 s, a total of 40 cycles, and 72°C for 30 s. The reaction volume was 20 μL. Each transcript was analyzed in triplicate (3 fish for each replicate). The 2−ΔΔCT method was used to calculate the relative gene expression levels of selected genes.
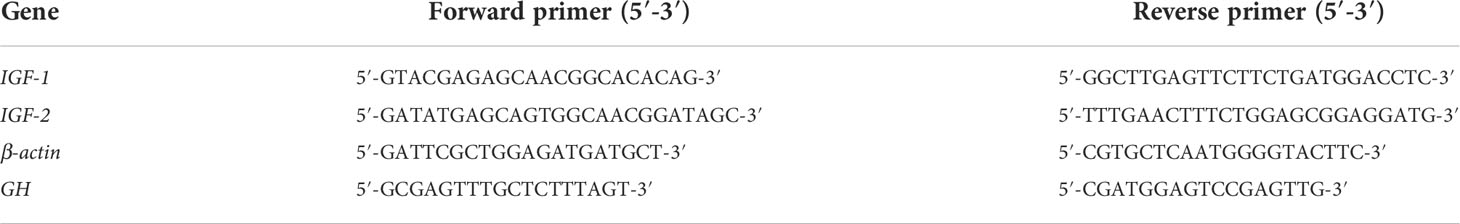
Table 2 The oligonucleotide sequences of primers for quantitative real-time PCR analysis in this study.
Statistical analysis
The weight gain rate (WGR), the specific growth rate (SGR), the feed conversion ratio (FCR), the survival rate (SR), hepatosomatic index (HSI), and condition factor (CF) were calculated as follows according to the report by Park et al. (2021):
All data were presented as the mean ± standard error, analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Data in different groups was considered to be significant if P < 0.05.
Results
Growth performance
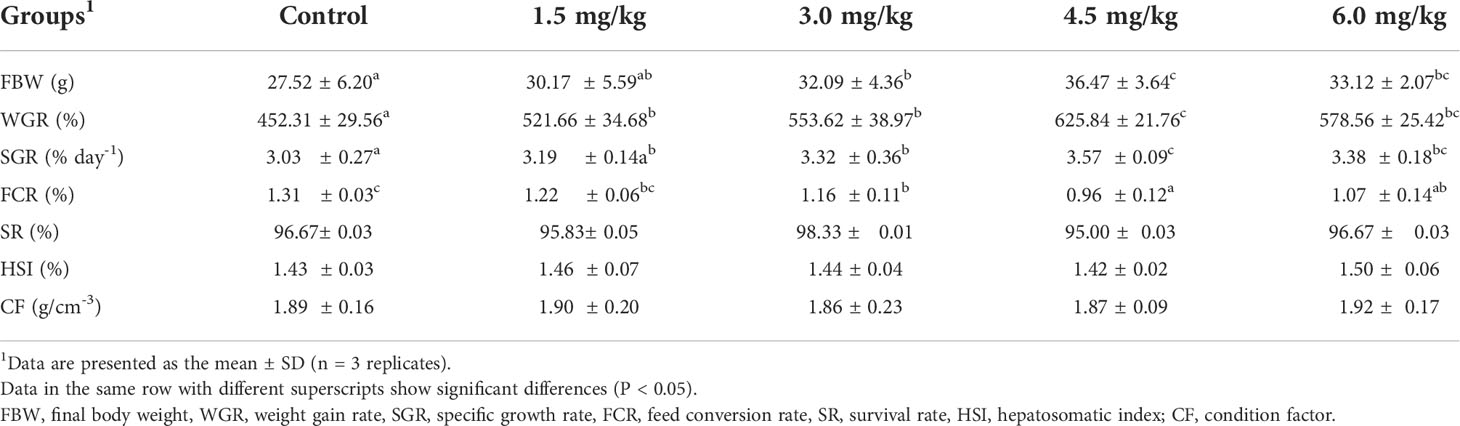
The effect of PQQ-supplemented diets on growth parameters of juvenile yellow catfish is displayed in Table 3. Compared with the control group, dietary PQQ supplementation at 3-6 mg/kg significantly increased the growth parameters such as FBW, WGR, and SGR of the juvenile yellow catfish (P < 0.05), and the maximum values of these parameters were appeared at 4.5 mg/kg group. On the contrary, FCR was decreased significantly with the administration of 3-6 mg/kg PQQ to the diet and the minimum value was observed in the 4.5 mg/kg group (P < 0.05). No significant change was detected in SR, HSI, and CF in all groups (P > 0.05). The relationship between FBW and dietary PQQ levels for yellow catfish juveniles can be well expressed by the following secondary curve equation: y = − 0.30286 x2 + 2.98381 x + 27.01114 (R2 = 0.83237) (Figure 1).
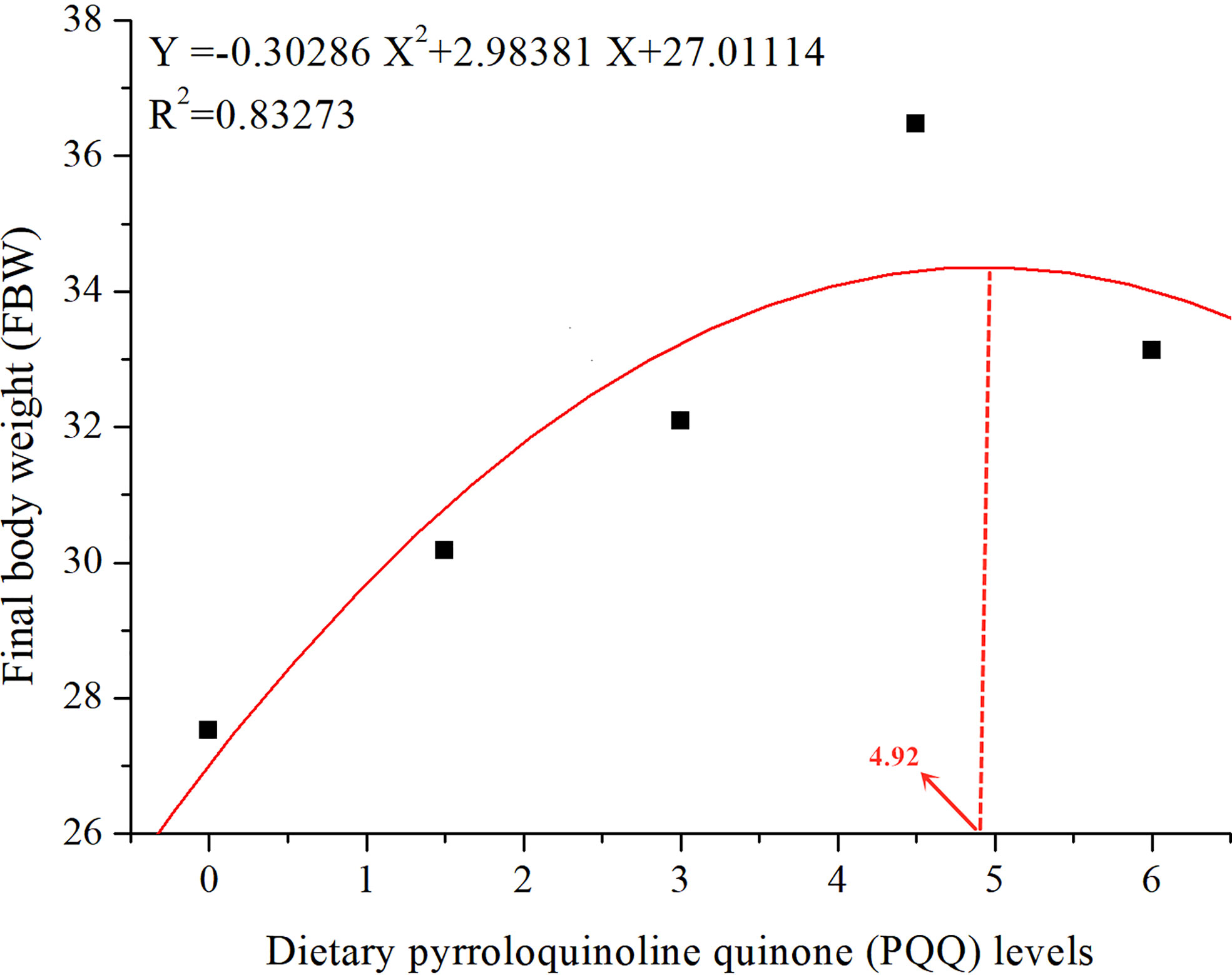
Figure 1 The relationship between the final body weight (FBW) of juvenile yellow catfish and different levels of PQQ supplemented diet after feeding for 8 weeks.
Digestive enzyme activity
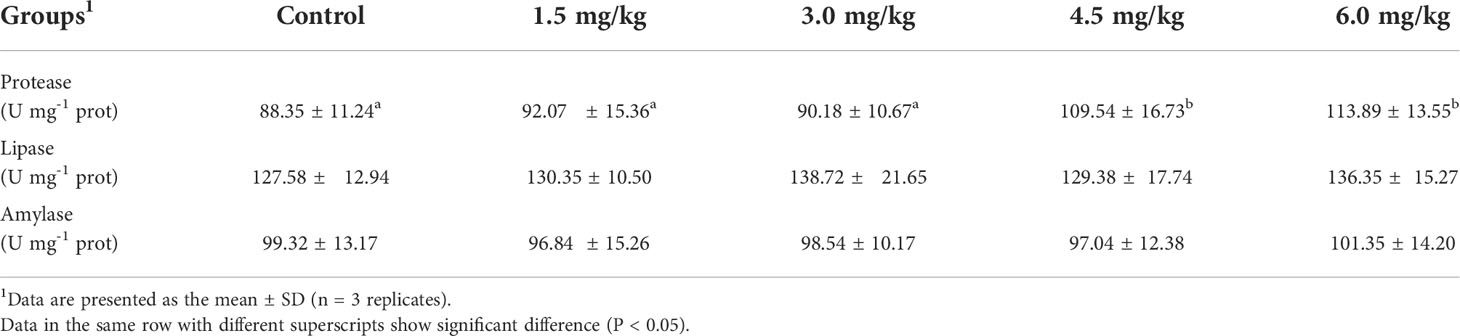
As shown in Table 4. Dietary PQQ supplementation had no significant effect on the amylase and lipase activities in the intestine of yellow catfish (P > 0.05). However, significantly higher protease activities were observed in fish fed with 4.5 mg/kg and 6 mg/kg PQQ groups compared with those of that in the control group (P < 0.05).
Serum biochemical parameters
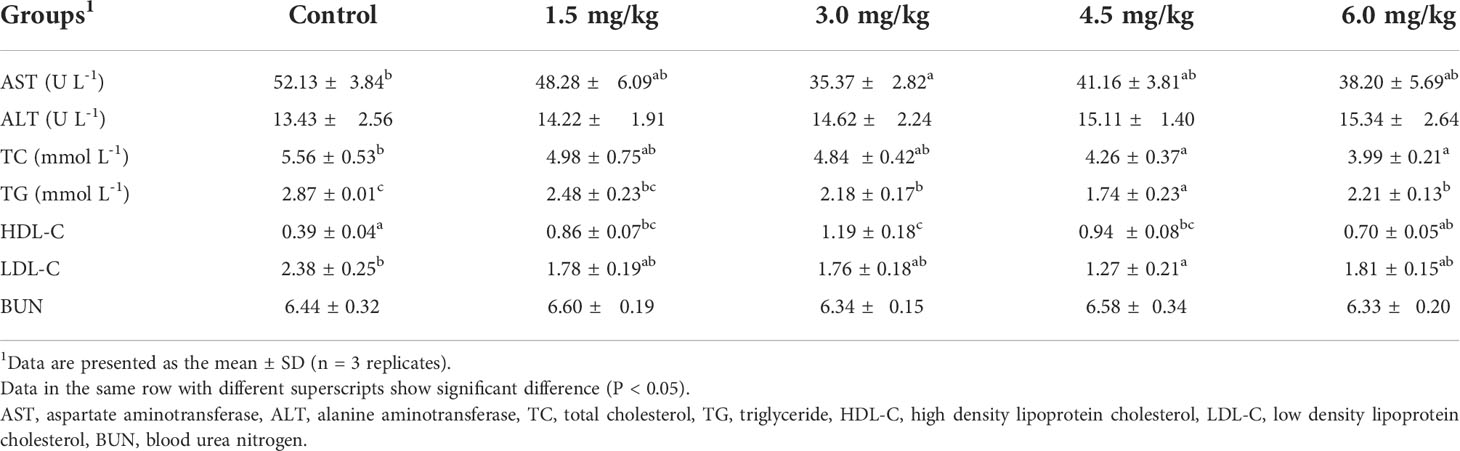
Table 5 presents the effect of dietary PQQ supplementation on serum biochemical parameters in juvenile yellow catfish. The activity of AST in the 3 mg/kg PQQ group and the level of LDL-C in the 4.5 mg/kg PQQ group were all significantly lower than those of that in the control group (P < 0.05). Except for 6 mg/kg group, HDL-C level was significantly higher than the control group (P < 0.05), and the highest value was recorded in the 3 mg/kg PQQ group. Besides, the levels of TG and TC in the 4.5 mg/kg PQQ and 6 mg/kg PQQ groups were significantly lower in comparison with the control group (P < 0.05). However, dietary PQQ supplementation did not cause significant changes in the serum ALT and BUN contents (P > 0.05).
Serum antioxidant status
As displayed in Table 6, the activities of SOD in the serum significantly increased in fish fed with PQQ diets than that of those in the control group (P < 0.05), of which in 1.5 mg/kg PQQ group was highest among various groups (P < 0.05). The levels of T-AOC in fish fed with PQQ diets (except 1.5 mg/kg) were significantly increased (P < 0.05), but no significant difference was observed among the various PQQ diets (P > 0.05). Unlike SOD and T-AOC, remarkable decrease of MDA contents was found in yellow catfish fed with 3 mg/kg, 4.5 mg/kg, and 6 mg/kg of PQQ compared to the fish from the control group. However, no significant difference was observed regarding the serum GPX and CAT activities in the PQQ supplementation groups and the control group (P > 0.05).
Gene expression
Relative gene expression levels of growth-related genes GH, IGF-1, and IGF-2 were shown in Figure 2. The mRNA expression levels of GH in the liver of fish fed with higher concentration of PQQ in diets (4.5 mg/kg and 6 mg/kg) were significantly increased than that of those fed with the lower concentration (1.5 mg/kg and 3 mg/kg) and control diet (P < 0.05). Except for 1.5 mg/kg group, relative mRNA levels of live IGF-1 in various PQQ groups were significantly higher than that of those in the control group (P < 0.05). Dietary PQQ supplementation significantly increased the mRNA expressions of the IGF-2 in the liver of yellow catfish (P < 0.05), especially in the 4.5 mg/kg PQQ group, with representing 12.06-fold higher in comparison with the control group.
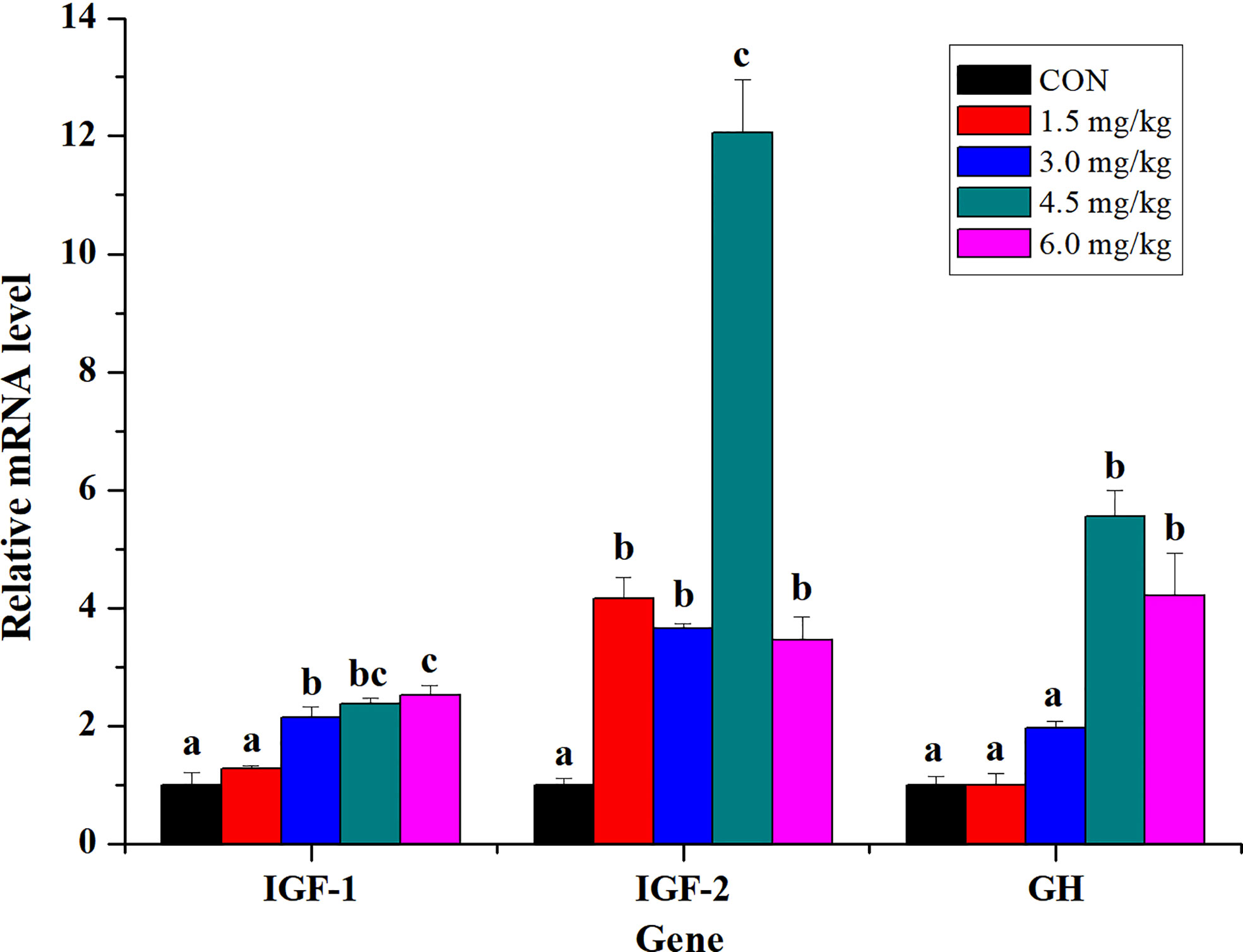
Figure 2 Effects of PQQ on the gene expression of growth-related genes GH, IGF-1, and IGF-2 in the liver of yellow catfish. Bars with different letters in the same gene indicate significant difference between the corresponding treatment (P < 0.05) (n = 3).
Discussion
In the present study, dietary with PQQ (3-6 mg/kg) supplementation resulted in significantly higher FBW, WGR, and SGR, as well as significantly lower FCR. Thus, dietary SHE was advantageous for the growth of yellow catfish. Similarly, growth was enhanced by adding PQQ to the basal diet in mice (Steinberg et al., 2003). Meanwhile, PQQ was a feed additive that can effectively promote the utilization of nutrients and stimulate the development of breast muscle in broiler chicks (Samuel et al., 2015; Liu et al., 2020), and improved the growth of weaned pigs, feed efficiency, and reduces the incidence of diarrhea in weaned pigs (Yin et al., 2019). The promotion of growth by dietary PQQ might be attributed to the modulation of mitochondrial function (Bauerly et al., 2006). PQQ was found to stimulate mitochondrial biogenesis through promoting the phosphorylation of cAMP response element-binding protein and increasing the expression of peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1α) (Chowanadisai et al., 2010). PQQ could also improve intestinal health to promote the growth of yellow catfish. Yin et al. (2019) revealed that PQQ can enhance intestinal morphology, promote intestinal barrier integrity, and improve the antioxidant status of the intestine. Wang et al. (2020) indicated that PQQ can alter the composition or metabolism of intestine microbiota, especially to increase the abundance of Firmicutes and decrease the levels of Actinobacillus and Escherichia, resulting in a more balanced bacterial structure. Moreover, the promotion of growth by PQQ supplementation could be also due to the increase of digestive enzyme activity. The digestive enzymes, such as protease, lipase, and amylase, etc., play a major role in food digestion and assimilation (Duan et al., 2017). An increase in the production of these enzymes is usually associated with an improvement in overall body metabolism (Midhun et al., 2019). In the present study, fish fed with 4.5 mg/kg and 6 mg/kg PQQ increased significantly the activities of protease. This observation indicated that PQQ in the diet might benefit for protein digestion and absorption in yellow catfish intestine, and thus improve growth performance in yellow catfish. The potential mechanism is that PQQ may have synergistic action along the intestinal tract and promote the development of intestinal tissue (Zheng et al., 2020), modulate intestinal microbial status (Wang et al., 2020), and ultimately stimulate enzyme expression.
Blood analysis plays a key role in the nutrition and physiology of fish, which can indirectly reflect the health status of fish (Hossain et al., 2016). AST and ALT are two of the most important aminotransferases in fish and are usually considered sensitive tools for indications of liver tissue damage. The decline of serum enzymes mentioned above in response to the nutritional agent is usually thought to improve liver function (Wang et al., 2016). In this study, the AST decreased significantly in fish fed with 3 mg/kg PQQ, suggesting that PQQ had a beneficial effect on liver health. Consistently, a previous study reported that PQQ showed a better hepatoprotective effect against oxidative stress by reducing the elevated AST activity in the serum caused by oxidized sunflower oil (Zhao et al., 2014). Serum TG and TC, are two important indicators of lipid levels in fish, which reflect the metabolism, and increase energy storage of lipids in fish, and high contents of TG and TC in the serum are believed to be involved in cardiovascular diseases (Castro et al., 2015). The present study revealed that PQQ could reduce the levels of serum TC and TG, suggesting that PQQ possessed a hypolipidemic effect in yellow catfish. Similar to our results, Zhao et al. (2014) demonstrated that PQQ can significantly inhibit the elevation of triglyceride and total cholesterol in the liver of laying hens induced by high-energy and low-protein diets. One possible mechanism that might explain these observations could be due to PQQ can protect the integrity of mitochondria in hepatocytes, promote β-oxidation of fatty acids, regulate the level of lipid metabolism in the body, increase the uptake and reduce the accumulation of TG in the liver tissues, and thus decrease serum and/or liver cholesterol levels (Chowanadisai et al., 2010; Bauerly et al., 2011). However, the exact mechanisms are still needed to be further investigated. Our results also revealed that PQQ significantly reduced serum LDL-C and increased serum HDL-C levels in yellow catfish. Similarly, Zhang et al. (2015) showed that PQQ can significantly increase HDL-C levels in the serum of broilers after 21 days of feeding. The decline of LDL-C is especially linked with HDL-C. Several researchers have reported that HDL plays antioxidant roles due to its antioxidant proteins and enzymes (Mackness and Mackness, 2012; Soran et al., 2015; Islam et al., 2018). Apolipoprotein-AI, the major structural protein of HDL, is considered the main antioxidant factor in HDL, and which is capable of removing LDL lipid hydroperoxides (Islam et al., 2018). The increased concentration of serum HDL in our study was accompanied by decreased levels of LDL and MDA.
The anti-oxidative enzymes CAT, SOD, and GPX are essential for the protection of important organelles and macromolecules in cells from oxidation-related damage by scavenging or neutralizing the pro-oxidants produced by normal animal metabolism (Rashidian et al., 2021). T-AOC directly reflected the antioxidant capacity of fish, which prevents reactive oxygen species’ negative effects (Tan et al., 2017). MDA, an end-product of lipid peroxidation, indirectly reflected the extent of lipid peroxidation in tissue cells from free radicals attack (Cai et al., 2016). Usually, higher levels of SOD, CAT, and GPX activities revealed an increased antioxidant defense in fish. In this study, although PQQ supplementation had no impact on CAT and GPX activity, the activity of SOD and level of T-AOC in the serum were significantly increased by 3-6 mg/kg PQQ supplementation, while the MDA levels were markedly decreased. These data revealed that PQQ may improve antioxidant capacity and reduce lipid oxidation damage in yellow catfish. Similar results were also observed in the previous studies in laying hens (Wang et al., 2016), weaned pigs (Ming et al., 2021), and broilers (Samuel et al., 2015). PQQ was reported to be a potent non-enzymatic antioxidant, and its reduced form (pyrroloquinoline quinol, PQQH2) can directly eliminate reactive oxygen species (superoxide anion, hydrogen peroxide, and lipid radicals), with PQQH2 having a scavenging capacity 7.4 times higher than that of vitamin C, which is the most active water-soluble antioxidant (Ouchi et al., 2009). On the other hand, PQQ seems to enhance the antioxidant defense system by inducing antioxidant enzymes (Misra et al., 2004), consistent with our previous finding. A recent study had shown that PQQ could increase antioxidant enzyme activity by stimulating the PGC-1α and Nrf2-ARE signaling pathways of the peroxisome proliferator-activated receptor (Chowanadisai et al., 2010).
It is well known that the GH/IGF axis plays an important role in the regulation of fish growth (Picha et al., 2008). GH can bind to the growth hormone receptor (GHR) in targeted tissues, then promote IGFs production and release in the liver and in most peripheral tissues, which mediates many of the growth-promoting effects of GH (Tan et al., 2017). There are two principle IGFs referred to as IGF-1 and IGF-2 (Gabillard et al., 2006). In particular, IGF-1 promotes growth in large part depending on nutrient availability (Fox et al., 2010). IGF-2 is indicated to show a high structural homology with IGF-1 and extensively expressed in juvenile and adult fish (Terova et al., 2007). IGFs stimulate strongly growth, inducing an anabolic effect on protein and carbohydrate metabolism (Perez-Sanchez and Le Bail, 1999; Amin et al., 2019). Previous studies had showed that the increase in the number of mRNA copies of GH and IGFs expression levels likely reflects the improved growth performance of teleosts under the same nutrition conditions (Picha et al., 2008; Asaduzzaman et al., 2017; El-Kassas et al., 2020). In the current study, fish fed with 4.5 mg/kg and 6 mg/kg PQQ increased significantly the expressions of GH, IGF-1, and IGF-2 in the liver of yellow catfish. This was in accordance with the result obtained in growth performance. We infer that PQQ could stimulate the growth of yellow catfish via its action on the GH/IGF axis. Currently, studies on the effects of dietary PQQ supplemented on the GH/IGF axis in fish are rarely reported. Hence, future studies are needed to better understand the underlying mechanisms of PQQ affecting GH/IGF axis. Moreover, the expressions of IGF-1 and IGF-2 in the liver were in parallel with that of GH in this study. This finding suggest that GH may directly promote the mitosis and differentiation of cells to indirectly trigger the production and release of IGF (Delgadin et al., 2015).
In conclusion, our study revealed that dietary PQQ supplementation had beneficial effects on growth performance, serum biochemical parameters, and antioxidant status of juvenile yellow catfish, and the optimum supplemental level of PQQ is 4.92 mg/kg.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by The Institutional Animal Care and Use Committee of Neijiang Normal University, the Institutional Ethics Committee of the Chinese Institute of Chemical Biology guidelines. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
QS: Project administration, writing – original draft, Writing – review & editing. ZW: Review & editing. JW: Software, Formal analysis. PH and YZ: Investigation, Methodology. SW: Supervision. CQ: Review & editing, Funding acquisition. All authors read and approved the final manuscript.
Funding
This work was supported by the foundation of Guangdong Provincial Key Laboratory of Marine Biotechnology (No. GPKLMB202004), and the Sichuan Science and Technology Program (No. 2021YFYZ0015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akagawa M., Nakano M., Ikemoto K. (2016). Recent progress in studies on the health benefits of pyrroloquinoline quinone. Biosci. Biotech. Bioch. 80, 13–22. doi: 10.1080/09168451.2015.1062715
Amin A., El Asely A., El-Naby A. S. A., Samir F., El-Ashram A., Sudhakaran R., et al. (2019). Growth performance, intestinal histomorphology and growth-related gene expression in response to dietary Ziziphus mauritiana in Nile tilapia (Oreochromis niloticus). Aquaculture 512, 734301. doi: 10.1016/j.aquaculture.2019.734301
Asaduzzaman M., Ikeda D., Abol-Munafi A. B., Bulbul M., Ali M. E., Kinoshita S., et al. (2017). Dietary supplementation of inosine monophosphate promotes cellular growth of muscle and upregulates growth-related gene expression in Nile tilapia. Oreochromis niloticus. Aquacult. 468, 297–306. doi: 10.1016/j.aquaculture.2016.10.033
Bauerly K., Harris C., Chowanadisai W., Graham J., Havel P. J., Tchaparian E., et al. (2011). Altering pyrroloquinoline quinone nutritional status modulates mitochondrial, lipid, and energy metabolism in rats. PLoS One 6, 21779. doi: 10.1371/journal.pone.0021779
Bauerly K. A., Storms D. H., Harris C. B., Hajizadeh S., Sun M. Y., Cheung C. P., et al. (2006). Pyrroloquinoline quinone nutritional status alters lysine metabolism and modulates mitochondrial DNA content in the mouse and rat. BBA-Gen Subjects. 1760, 1741–1748. doi: 10.1016/j.bbagen.2006.07.009
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Cai Z., Feng S., Xiang X., Mai K., Ai Q. (2016). Effects of dietary phospholipid on lipase activity, antioxidant capacity and lipid metabolism-related gene expression in large yellow croaker larvae (Larimichthys crocea). Comp. Biochem. Phys. B. 201, 46–52. doi: 10.1016/j.cbpb.2016.06.007
Castro C., Corraze G., Panserat S., Oliva-Teles A. (2015). Effects of fish oil replacement by a vegetable oil blend on digestibility, postprandial serum metabolite profile, lipid and glucose metabolism of European sea bass (Dicentrarchus labrax) juveniles. Aquacult. Nutr. 21, 592–603. doi: 10.1111/anu.12184
Chowanadisai W., Bauerly K. A., Tchaparian E., Wong A., Cortopassi G. A., Rucker R. B. (2010). Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1α expression. J. Biol. Chem. 285, 142–152. doi: 10.1074/jbc.M109.030130
Delgadin T. H., Pérez Sirkin D. I., Di Yorio M. P., Arranz S. E., Vissio P. G. G. H. (2015). IGF-I and GH receptors mRNA expression in response to growth impairment following a food deprivation period in individually housed cichlid fish. Cichlasoma dimerus. Fish. Physiol. Biochem. 41, 51–60. doi: 10.1007/s10695-014-0005-x
Duan Y., Zhang Y., Dong H., Zheng X., Wang Y., Li H., et al. (2017). Effect of dietary poly-β-hydroxybutyrate (PHB) on growth performance, intestinal health status and body composition of pacific white shrimp Litopenaeus vannamei (Boone, 1931). Fish. Shellfish Immunol. 60, 520–528. doi: 10.1016/j.fsi.2016.11.020
El-Kassas S., Abdo S. E., Abosheashaa W., Mohamed R., EI-Naggar K. (2020). Growth performance, serum lipid profile, intestinal morphometry, and growth and lipid indicator gene expression analysis of mono-sex Nile tilapia fed Moringa oleifera leaf powder. Aquacult. Rep. 18, 100422. doi: 10.1016/j.aqrep.2020.100422
Fox B. K., Breves J. P., Davis L. K., Pierce A. L., Hirano T., Grau E. G. (2010). Tissue-specific regulation of the growth hormone/insulin-like growth factor axis during fasting and re-feeding: Importance of muscle expression of IGF-I and IGF-II mRNA in the tilapia. Gen. Comp. Endocr. 166, 573–580. doi: 10.1016/j.ygcen.2009.11.012
Fu W., Amenyogbe E., Yang E., Luo J., Huang J. S., Xie R. T., et al. (2022). Effects of dietary supplementation of ferulic acid on growth performance, antioxidant ability, non-specific immunity, hepatic morphology and genes expression related to growth and immunity in juvenile hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus polyphekadion♂). Aquaculture. 552, 737988. doi: 10.1016/j.aquaculture.2022.737988
Gabillard J. C., Kamangar B. B., Nuria M. (2006). Coordinated regulation of the GH/IGF system genes during refeeding in rainbow trout (Oncorhynchus mykiss). J. Endocr. 191, 15–24. doi: 10.1677/joe.1.06869
Gabriel N. N., Qiang J., Ma X. Y., Xu P., Nakwaya D. N. (2017). Effects of dietary Aloe vera crude extracts on digestive enzyme activities and muscle proximate composition of GIFT tilapia juveniles. S Afr. J. Anim. Sci. 47, 904–913. doi: 10.4314/sajas.v47i6.18
Hamagishi Y., Murata S., Kamei H., Oki T., Adachi O., Ameyama M. (1990). New biological properties of pyrroloquinoline quinone and its related compounds: Inhibition of chemiluminescence, lipid peroxidation and rat paw edema. J. Pharmacol. Exp. Ther. 255, 980–985.
Health Canada (2012). Licensed natural health products database (Ottawa, ON: Natural Health Products Directorate (NHPD). Available at: http://www.hc-sc.gc.ca/ahc-asc/branch-dirgen/hpfb-dgpsa/nhpd-dpsn/index-eng.php. NPN 80030871 [PQQ disodium salt]Health Canada.
Hollis A., Ahmed Z. (2014). The path of least resistance: Paying for antibiotics in non-human uses. Health Policy. 118, 264–270. doi: 10.1016/j.healthpol.2014.08.013
Hossain M. S., Koshio S., Ishikawa M., Yokoyama S., Sony N. M., Ono S., et al. (2016). Comparison of the effects of inosine and inosine monophosphate on growth, immune response, stress resistance and gut morphology of juvenile red sea bream. Pagrus. major. Aquacult. 458, 64–74. doi: 10.1016/j.aquaculture.2016.02.032
Hwang P., Willoughby D. S. (2018). Mechanisms behind pyrroloquinoline quinone supplementation on skeletal muscle mitochondrial biogenesis: Possible synergistic effects with exercise. J. Am. Coll. Nutr. 37, 738–748. doi: 10.1080/07315724.2018.1461146
Ikemoto K., Mori S., Mukai K. (2017). Synthesis and crystal structure of pyrroloquinoline quinol (PQQH(2)) and pyrroloquinoline quinone (PQQ). Acta Crystallogr. B. 73, 489–497. doi: 10.1107/S2052520617002281
Islam R. M., Pourmousa M., Sviridov D., Gordon S. M., Neufeld E. B., Freeman L. A., et al. (2018). Structural properties of apolipoprotein a-I mimetic peptides that promote ABCA1-dependent cholesterol efflux. Sci. Rep. 8, 2956. doi: 10.1038/s41598-018-20965-2
Jiang R., Zhang G. R., Zhu D. M., Shi Z. C., Liao C. L., Fan Q. X., et al. (2018). Molecular characterization and expression analysis of IL-22 and its two receptors genes in yellow catfish (Pelteobagrus filvidraco) in response to Edwardsiella ictaluri challenge. Fish. Shellfish Immunol. 80, 250–263. doi: 10.1016/j.fsi.2018.06.012
Killgore J., Smidt C., Duich L., Romero-Chapman N., Tinker D., Reiser K., et al. (1989). Nutritional importance of pyrroloquinoline quinone. Science. 245, 850–852. doi: 10.1126/science.2549636
Liu G. Q., Sun G. M., Liao X. D., Huang J. Z., Guo M. J., Zhang L. Y., et al. (2020). Effect of dietary supplementation of pyrroloquinoline quinone disodium on growth performance, meat quality and antioxidative ability of broilers. J. Integr. Agr. 19, 1850–1856. doi: 10.1016/S2095-3119(19)62851-0
Mackness B., Mackness M. (2012). The antioxidant properties of high-density lipoproteins in atherosclerosis. Panminerva Med. 54, 83–90.
Midhun S. J., Neethu S., Arun D., Vysakh A., Divya L., Radhakrishnan E. K., et al. (2019). Dietary supplementation of Bacillus licheniformis HGA8B improves growth parameters, enzymatic profile and gene expression of Oreochromis niloticus. Aquaculture. 505, 289–296. doi: 10.1016/j.aquaculture.2019.02.064
Ming D., Huang C., Wang W., Wang Z., Shi C., Yin X., et al. (2021). Effects of diet supplemented with excess pyrroloquinoline quinone disodium on growth performance, blood parameters and redox status in weaned pigs. Animals. 11 (2), 359. doi: 10.3390/ani11020359
Misra H. S., Khairnar N. P., Barik A., Indira P. K., Mohan H., Apte S. K. (2004). Pyrroloquinoline-quinone: A reactive oxygen species scavenger in bacteria. FEBS Lett. 578, 26–30. doi: 10.1016/j.febslet.2004.10.061
Ouchi A., Nakano M., Nagaoka S., Mukai K. (2009). Kinetic study of the antioxidant activity of pyrroloquinolinequinol (PQQH(2), a reduced form of pyrroloquinolinequinone) in micellar solution. J. Agr. Food Chem. 57, 450–456. doi: 10.1021/jf802197d
Park Y., Park M., Hamidoghli A., Kim C. H., Bai S. C. (2021). Optimum dietary processed sulfur (Immuno-f) level has antibiotic effects on the growth, hematology and disease resistance of juvenile olive flounder. Paralichthys olivaceus. Anim. Feed Sci. Tech. 279, 115035. doi: 10.1016/j.anifeedsci.2021.115035
Perez-Sanchez J., Le Bail P. Y. (1999). Growth hormone axis as marker of nutritional status and growth performance in fish. Aquaculture. 177, 117–128. doi: 10.1016/S0044-8486(99)00073-3
Picha M. E., Turano M. J., Beckman B. R., Borski R. J. (2008). Endocrine biomarkers of growth and applications to aquaculture: A minireview of growth hormone, insulin-like growth factor (IGF)-I, and IGF-binding proteins as potential growth indicators in fish. N Am. J. Aquacult. 70, 196–211. doi: 10.1577/A07-038.1
Rama S., Manjabhat S. N. (2014). Protective effect of shrimp carotenoids against ammonia stress in common carp. Cyprinus carpio. Ecotox Environ. Safe. 107, 207–213. doi: 10.1016/j.ecoenv.2014.06.016
Ramesh D., Souissi S. (2018). Antibiotic resistance and virulence traits of bacterial pathogens from infected freshwater fish. Labeo rohita. Microb. Pathog. 116, 113–119. doi: 10.1016/j.micpath.2018.01.019
Rashidian G., Abedian K. A., Nikkhah M. (2021). Evaluation of antioxidative and antibacterial activities of fractionated hydrolysate from shrimp Litopenaeus vannamei head wastes against aquatic pathogenic bacteria. Aquacult. Res. 52, 3696–3704. doi: 10.1111/are.15214
Safari R., Hoseinifar S. H., Imanpour M. R., Mazandarani M., Sanchouli H., Paolucci M. (2020). Effects of dietary polyphenols on mucosal and humoral immune responses, antioxidant defense and growth gene expression in beluga sturgeon (Huso huso). Aquaculture. 528, 735494. doi: 10.1016/j.aquaculture.2020.735494
Samuel K. G., Zhang H. J., Wang J., Wu S. G., Yue H. Y., Sun L. L., et al. (2015). Effects of dietary pyrroloquinoline quinone disodium on growth performance, carcass yield and antioxidant status of broiler chicks. Animal. 9, 409–416. doi: 10.1017/S1751731114002328
SB/T 10317-1999(1999). People’s Republic of China Professional Standard.Measurement of Proteinase Activity (SB/T 10317-1999). (Beijing: Ministry of Industry and Information Technology of the People’s Republic of China). doi: 10.3389/fmicb.2022.926515
Shi Q., Rong H., Hao M., Zhu D., Aweya J. J., Li S., et al. (2019). Effects of dietary Sargassum horneri on growth performance, serum biochemical parameters, hepatic antioxidant status, and immune responses of juvenile black sea bream Acanthopagrus schlegelii. J. Appl. Phycol. 31, 2103–2113. doi: 10.1007/s10811-018-1719-4
Shi Q., Wang J., Qin C., Yu C., Wang S., Jia J. (2021). Growth performance, serum biochemical parameters, immune parameters and hepatic antioxidant status of yellow catfish Pelteobagrus fulvidraco supplemented with Sargassum horneri hot-water extract. Aquacult. Rep. 21, 100839. doi: 10.1016/j.aqrep.2021.100839
Soran H., Schofield J. D., Durrington P. N. (2015). Antioxidant properties of HDL. Front. Pharmacol. 6, 222. doi: 10.3389/fphar.2015.00222
Steinberg F., Stites T. E., Anderson P., Storms D., Chan I., Eghbali S., et al. (2003). Pyrroloquinoline quinone improves growth and reproductive performance in mice fed chemically defined diets. Exp. Biol. Med. 228, 160–166. doi: 10.1177/153537020322800205
Tan X., Sun Z., Chen S., Chen S., Huang Z., Zhou C., et al. (2017). Effects of dietary dandelion extracts on growth performance, body composition, plasma biochemical parameters, immune responses and disease resistance of juvenile golden pompano. Trachinotus ovatus. Fish. Shellfish Immunol. 66, 198–206. doi: 10.1016/j.fsi.2017.05.028
Tan X., Sun Z., Huang Z., Zhou C., Lin H., Tan L., et al. (2017). Effects of dietary hawthorn extract on growth performance, immune responses, growth- and immune-related genes expression of juvenile golden pompano (Trachinotus ovatus) and its susceptibility to Vibrio harveyi infection. Fish. Shellfish Immunol. 70, 656–664. doi: 10.1016/j.fsi.2017.09.041
Terova G., Rimoldi S., Chini V., Gornati R., Bernardini G., Saroglia M. (2007). Cloning and expression analysis of insulin-like growth factor I and II in liver and muscle of sea bass (Dicentrarchus labrax, l.) during long-term fasting and refeeding. J. Fish. Biol. 70, 219–233. doi: 10.1111/j.1095-8649.2007.01402.x
Wang J., Zhang H. J., Xu L., Long C., Samuel K. G., Yue H. Y., et al. (2016). Dietary supplementation of pyrroloquinoline quinone disodium protects against oxidative stress and liver damage in laying hens fed an oxidized sunflower oil-added diet. Animal. 10, 1129–1136. doi: 10.1017/S175173111600001X
Wang C., Zhang B., Zhang H. (2020). Effect of dietary pyrroloquinoline quinone disodium in sows on intestinal health of the offspring. Food Funct. 11, 7804–7816. doi: 10.1039/D0FO01403F
Xu F., Yu H., Liu J., Cheng L. (2014). Pyrroloquinoline quinone inhibits oxygen/glucose deprivation-induced apoptosis by activating the PI3K/AKT pathway in cardiomyocytes. Mol. Cell Biochem. 386, 107–115. doi: 10.1007/s11010-013-1849-6
Yin X., Ming D., Bai L., Wu F., Liu H., Chen Y., et al. (2019). Effects of pyrroloquinoline quinone supplementation on growth performance and small intestine characteristics in weaned pigs. J. Anim. Sci. 97, 246–256. doi: 10.1093/jas/sky387
Zhang Y., Feustel P. J., Kimelberg H. K. (2006). Neuroprotection by pyrroloquinoline quinone (PQQ) in reversible middle cerebral artery occlusion in the adult rat. Brain Res. 1094, 200–206. doi: 10.1016/j.brainres.2006.03.111
Zhang X., Li Y. W., Mo Z. Q., Luo X. C., Sun H. Y., Liu P., et al. (2014). Outbreak of a novel disease associated with Vibrio mimicus infection in fresh water cultured yellow catfish, Pelteobagrus fulvidraco. Aquaculture 432, 119–124. doi: 10.1016/j.aquaculture.2014.04.039
Zhang Y., Qi B., Wu S. G., Yue H. Y., Wang J., Zhang H., et al. (2015). Effects of pyrroloquinoline quinine disodium on growth performance, plasma lipid metabolism and antioxidant ability in broilers fed oxidized duck oil. Chin. J. Anim. Nutr. 27, 2884–2893. doi: 10.3969/j.issn.1006-267x.2015.09.028
Zhang B., Wang C., Yang W., Zhang H., Meng Q., Shi B., et al. (2019). Transcriptome analysis of the effect of pyrroloquinoline quinone disodium (PQQ·Na(2)) on reproductive performance in sows during gestation and lactation. J. Anim. Sci. Biotechnol. 10, 62. doi: 10.1186/s40104-019-0369-y
Zhao Q., Zhang H. J., Wu S. G., Yue H. Y., Wang J., Qi G. H., et al. (2014). Protective mechanisms of dietary pyrroloquinoline quinine on fatty liver laying hens. Chin. J. Anim. Nutr. 26, 651–658. doi: 10.3969/j.issn.1006-267x.2014.03.014
Zheng Y. W., Zhang J. Y., Zhou H. B., Guo Y. P., Ma Q. G., Ji C., et al. (2020). Effects of dietary pyrroloquinoline quinone disodium supplementation on inflammatory responses, oxidative stress, and intestinal morphology in broiler chickens challenged with lipopolysaccharide. Poultry Sci. 99, 5389–5398. doi: 10.1016/j.psj.2020.08.007
Keywords: pyrroloquinoline quinone, growth, antioxidant status, gene expression, Pelteobagrus fulvidraco
Citation: Shi Q, Wen Z, Wang J, Hu P, Zou Y, Wang S and Qin C (2022) Effects of dietary pyrroloquinoline quinone on growth performance, serum biochemical parameters, antioxidant status, and growth-related genes expressions in juvenile yellow catfish, Pelteobagrus fulvidraco. Front. Mar. Sci. 9:989948. doi: 10.3389/fmars.2022.989948
Received: 09 July 2022; Accepted: 06 September 2022;
Published: 16 September 2022.
Edited by:
Zhendong Qin, Zhongkai University of Agriculture and Engineering, ChinaReviewed by:
Bimal Prasanna Mohanty, Indian Council of Agricultural Research (ICAR), IndiaHector Abelardo Gonzalez-Ocampo, Instituto Politécnico Nacional (IPN), Mexico
Copyright © 2022 Shi, Wen, Wang, Hu, Zou, Wang and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanjie Qin, cWluY2h1YW5qaWVAMTI2LmNvbQ==
 Qingchao Shi
Qingchao Shi Zhengyong Wen
Zhengyong Wen Jun Wang
Jun Wang Peng Hu1
Peng Hu1 Shuqi Wang
Shuqi Wang