- 1Key Laboratory of Healthy Mariculture for the East China Sea, Ministry of Agriculture and Rural Affairs, Fisheries College, Jimei University, Xiamen, China
- 2Key Laboratory of Cultivation and High-value Utilization of Marine Organisms in Fujian Province, Institute of Fisheries of Fujian, Xiamen, China
- 3Putian Institute of Aquaculture Science of Fujian Province, Putian, China
- 4MOE Key Laboratory of Marine Genetics and Breeding, Ocean University of China, Qingdao, China
Improving the progress of breeding is the main purpose of breeders, and shorting the reproductive cycle is one of the ways to improve the breeding progress. Although the reproductive cycle of Crassostrea angulata can be shortened from one year to half a year by selecting precocious individuals, little is known about whether this selection will accelerate their gonadal development or disturb their selection for productive traits. Here, the heritability of gonadal development traits (gametogenic stage and egg diameter) and their genetic correlations with both growth traits (shell height at 4 and 12 months and total body weight at 12 months) and heat tolerance trait were estimated. The heritability for gametogenic stage was low (0.18 ± 0.09), while the egg diameter showed a high heritability (0.78 ± 0.30). These results suggest that selection for precocious individuals has little effect on the rate of gonadal development in the C. angulata, despite the effect may be greater in females than in males. The genetic correlations between gonadal development traits and both growth (-0.02–0.30) and heat tolerance traits (-0.08–0.34) were low and non-significant. These results suggest that selection for precocious individuals has negligible effects on their productive traits. Therefore, it is feasible to double the breeding efficiency of C. angulata by halving their reproductive cycle.
Introduction
Improving the progress of breeding is the main purpose of breeders, and shorting the reproductive cycle is one of the ways to improve the breeding progress. Oysters are the top productive molluscs cultured worldwide, and usually reproduce only one generation per year for most species (see review by Gjedrem and Rye, 2018). However, the reproductive cycles of oysters are strongly related to energy storage-utilization cycles and environmental factors such as temperature and photoperiod (Li et al., 2006). For example, the reproductive cycle of the Pacific oyster can be shortened from one year to half a year under experimental conditions (Fabioux et al., 2005; Normand et al., 2009). Additionally, some oyster species distributed in subtropical and tropical coasts naturally have multiple reproductive cycles per year, with fast gametogenesis, prolonged spawning, and rapid recovery of gonads after spawning (Aldana Aranda et al., 2014; Castilho-Westphal et al., 2015; Shi et al., 2019). The first functional maturation of gametes of some oyster species can be achieved as young as about 2 or 3 months after fertilization, and these first mature gametes can develop normally and produce viable progeny (Thompson et al., 1996; Zhang et al., 2019). These reproductive characteristics of these oysters offer the opportunity to shorten their reproductive cycle, thereby increasing their breeding efficiency. It is worth noting, however, that this practice may lead to the selection of individuals with rapid gonadal development when the individuals used as parents are in their first reproductive cycle. It is then necessary to determine whether this selective process leads to the improvement of gonadal developmental rate in oysters, and whether the improved rate of gonadal development disturb their selection for production traits.
To answer the above two questions, the heritability of gonadal development and its genetic correlations with production traits should be elucidated. Unfortunately, limited studies estimated the genetic parameters of reproductive effort in mollusks and only focus on fecundity, the number of oocytes produced per individual or the ratio of the gamete weight to total body weight, rather than gonadal developmental rate. For example, low heritability for fecundity were reported in the Caribbean scallop (Barros et al., 2018) and the Pacific oyster (Ernande et al., 2004), and the genetic correlations between fecundity and both survival and growth of the Pacific oyster were not consistent across environments (Ernande et al., 2004). These genetic parameters of fecundity, however, cannot be used to infer the genetic basis of gonadal developmental rate, because the relationship between these two traits remains unclear. For oysters, a phenotypic correlation between fecundity and gametogenic stage was found in wild population (Royer et al., 2008). By contrast, a similar distribution of gametogenesis stage was observed between two selected lines with significant different fecundity (Huvet et al., 2010). Therefore, genetic parameters specific to the rate of gonadal developmental are necessary to assess its effect on productive traits.
Fujian oyster (Crassostrea angulata) also known as Portuguese oyster, is one of the most commonly farmed molluscan species around the world, especially in China, Japan, Vietnam, Portugal and France (Wang et al., 2010; Sekino et al., 2016; In et al., 2017). It was the main bivalve species cultured in in Europe and annual production reached approximately 100,000 tons until the 1970’s when massive mortalities caused it to now present in only a few regions of Europe (Batista et al., 2015). Nowadays, the main producer of C. angulata is Asia. For example, the annual production of C. angulata in China is more than 2 million tons, accounting for about 50% of their total oyster production (Qin et al., 2012), while the production of C. angulata in Vietnam was about 50,000 tons in 2018 (O’Connor et al., 2019). It usually reproduces only one generation per year in current breeding programs (Wu et al., 2019; Vu et al., 2020a; Vu et al., 2020b), but it has multiple reproductive cycles per year (Shi et al., 2019). In southern China, most of larvae of C. angulata hatched in May were found to be reproductively mature in September, with a shell height of 2-5 cm and a fecundity of 1-8 million eggs per individual. This fecundity of precocious C. angulata can produce enough progeny for selective breeding, considering that the progeny from precocious broodstock are similar to that of adult broodstock in terms of fertilization, hatching, survival, growth and metamorphosis (Zhang et al., 2019). If these matured individuals are then used as parents to produce zygotes in September, these zygotes will be reproductively mature in May of next year. In this way, the breeding cycle of C. angulata has the opportunity to be shortened by half and its breeding progress can be doubled.
In order to assess the feasibility to improve the breeding progress of C. angulata by shortening their breeding cycle, the heritability of gonadal development traits (gametogenic stage and egg diameter) and their genetic correlations with both growth traits (shell height at 4 and 12 months and total body weight at 12 months) and stress tolerance trait (heat tolerance) were estimated by 21 full-sib families of C. angulata.
Materials and method
Family construction and breeding
In May 2021, 1-year-old gonadal mature individuals of wild C. angulata collected from the Zhaoan bay in Fujian province, China were transferred to Zhaoan breeding base. Thirty dams and ten sires were randomly selected as parents to produce thirty full-sib families following a nested mating design (each male was mated with three different females). For all families, artificial fertilization and larval rearing management were conducted following the standard procedure described by Li et al. (2011). Briefly, gametes of each parent were collected into separate buckets by stripping its gonad. Eggs from each dam were fertilized by matching sperm at a sperm: egg ratio of 30. Twenty-four hours after fertilization at 25 °C, D-larvae of each family were collected into 280 L plastic bucket for larval rearing. Algae diets of Isochrysis galbana were fed three times a day until larvae reached 120 μm and then Nitzschia closterium supplemented gradually. Seawater filtered through sand filters and non-woven polypropylene fabric was changed once a day. When the larvae with eyespots and feet approached 50%, string of oyster shells were placed into the bucket to provide a substrate for eyed larvae to set. After all eyed larvae metamorphosed to spat, all families were marked and transferred to an outdoor nursery pond for two-week temporary rearing. In July, all families were placed in 10-layer lantern nets and hung 1-3 meters under the water surface of Futou Bay (23.92° N, 117.71° E) in Fujian province. The annual average water temperature of Futou Bay is 21°C. Nine families lost during grow-out stage, and 21 families used in next experiments.
Histology
To determine the sample time for histology, pre-examination of gonadal development was performed monthly between July and September. Individuals with mature gonad were firstly observed in September, with less than 10% of individuals with a small amount of gonadal development in the visceral mass were observed in August, whereas no gonadal development was observed in other individuals sampled in August and all individual sampled in July. Therefore, in September 2021, six to twenty oysters were randomly selected from each family, and a total of 320 oysters were used for histological examination. A transverse cut was made across the middle part of the soft body of each oyster and a 5-mm-thick section was fixed in 4% formaldehyde solution. Sections were then dehydrated in ascending ethanol solutions, embedded in paraffin wax, slice cut (5 μm) and stained with Mayer’s hematoxylin and eosin. Each slide was examined microscopically to assess the sex and stage of gametogenesis. The gametogenic stages were classified into resting stage, early developmental stage, late developmental stage, mature stage and spawning and reabsorb stage and scored on a 0 to 4 scale according to a scale of maturity (Li et al., 2006; Huvet et al., 2010). Moreover, the diameter of 30 oocytes of each female was microscopically measured.
Growth traits
Thirty oysters were randomly selected from each family at 4 (September 2021) and 12 months of age (May 2022). The shell height of oysters at 4 and 12 months were measured by digital calipers (0.01 mm), and body weights of oysters at 12 months were determined by a digital electronic balance (0.01 g).
Heat tolerance
The acute heat tolerance trait can reflect the stress tolerance of oysters, and the selection of this trait can effectively improve the survival rate of oysters in summer (Ding et al., 2020). Therefore, the acute heat tolerance traits of all families were examined in this study. After the shell heights of 4-month-old oysters were measured, 19 individuals from each family were randomly selected and conditioned at 27 °C and 30 ppt for 10 days prior to later heat challenge. No dead oyster was observed in all families during this period. After these 10 days, all oysters were transferred to pre-heated seawater at 44 °C and maintained at this temperature for 1 hour, then returned to 27 °C for a further 10 days. For C. angulata, 44 °C is the semi-lethal temperature for 1 h based on the results of pre-experiment. Dead oysters from each family were recorded and removed every 12 hours after heat challenge. Mortality were record as binary data, with 1 for survivors and 0 for dead oysters. At the end of experiment, mortality data at the 10th day after heat challenge were used to estimate the heritability of heat tolerance trait. During the experiment, all oysters were fed with concentrated Chlorella pyrenoidosa twice per day, and seawater was kept aerated and changed daily. Before each water change, the seawater will be filtered, UV sterilized, and adjusted to the set temperature by immersed heaters or water chiller in advance. To ensure all families were under the same conditions during experiment, all individuals were in a 300 L tank with a circulating water temperature control system, and different families were separated by grids.
Statistical analysis
Heritability of each trait was estimated by the Restricted Maximum Likelihood method using ASReml-R 3.0 package (Gilmour et al., 2009). The variance for each trait was calculated using the animal model as follows:
Where yijk was the observation on the individual k from sire i and dam j, μ was the overall mean, aijk was the additive genetic effects for the ijkth animal, fij was the random effect common to each full-sib family, and eijk was the residual error. Heritability was calculated with the following equation:
Where was the additive genetic variance, was the random effect variance, and was the residual variance. The heat tolerance recorded as binary data, so the heritability of heat tolerance was calculated using logit model, and residual variance was π2/3 ≈ 3.28987. Then, the significance level of heritability was tested by the t test, with t value calculated by (Liu et al., 2005).
SPSS 22.0 was used to calculate correlations. Phenotype and genetic correlations between gonadal development traits, growth traits and heat tolerance trait were expressed as Pearson correlation between phenotype values and breeding values of each family for these traits, respectively. The breeding value of each family was the average of the breeding values of its sire and dam predicted by the above model.
Results
Gonadal development
The gametogenic stages of 320 oysters were identified by paraffin section, including 67 individuals at resting stage, 27 individuals at early development stage, 79 individuals at later development stage, 147 individuals at mature stage, and no individual at spawning and reabsorb stage was found in this study (Figures 1 and 2A). Besides individuals at resting stage, 85 females, 167 males and 1 hermaphrodite were identified. Not only were males twice as many as females, but the proportion of mature individuals in males (69.46%) was also nearly double that of females (35.29%) (Figure 2A).
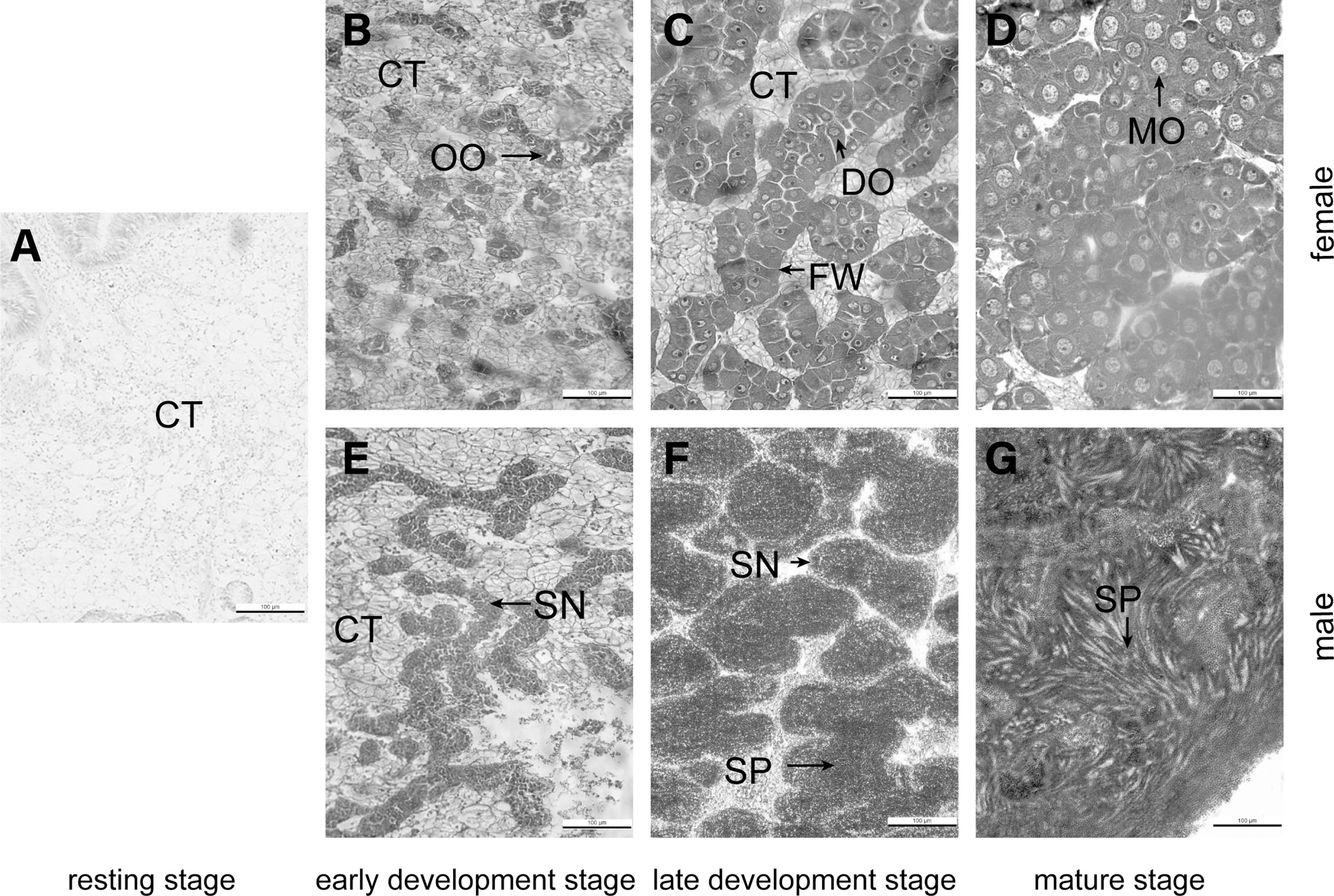
Figure 1 Histological observation on the gonad of Crassostrea angulata at 4 months of age. (A) resting stage, (B, E) early developmental stage, (C, E) late developmental stage, (D, G) mature stage. (B–D) female, (E–G) male. CT, connective tissue; FW, follicle wall; OO, oogonia; DO, developing oocyte; MO, mature oocyte; SN, spermatogonia, SP, sperm; bar, 100 μm.
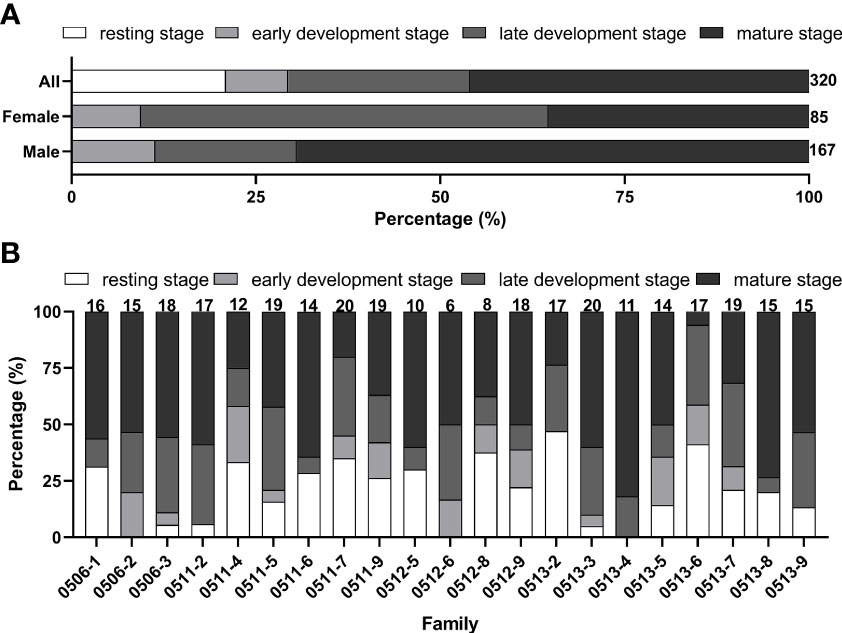
Figure 2 Distribution of gametogenic stages in different sexes (A) and families (B) of Crassostrea angulata at 4 months of age. Numbers above each column indicate sample sizes.
Three or four different stages were observed in all but one family, and the percentage of gametogenic stages were different among families (Figure 2B). For the percentage of oysters at resting stage, the highest one was found in family 0513-2 (47.06%), and the lowest one was found in family 0513-3 (5.00%). For the percentage of oysters at mature stage, the highest one was found in family 0513-4 (81.82%), and the lowest one was found in family 0513-6 (5.88%).
The mean egg diameters of all families ranged from 26.75 to 40.34 μm, and the largest one was found in family 0506-3 (40.34 ± 4.88 μm), and the smallest one was found in family 0513-6 (26.75 ± 1.63 μm) (Table 1).
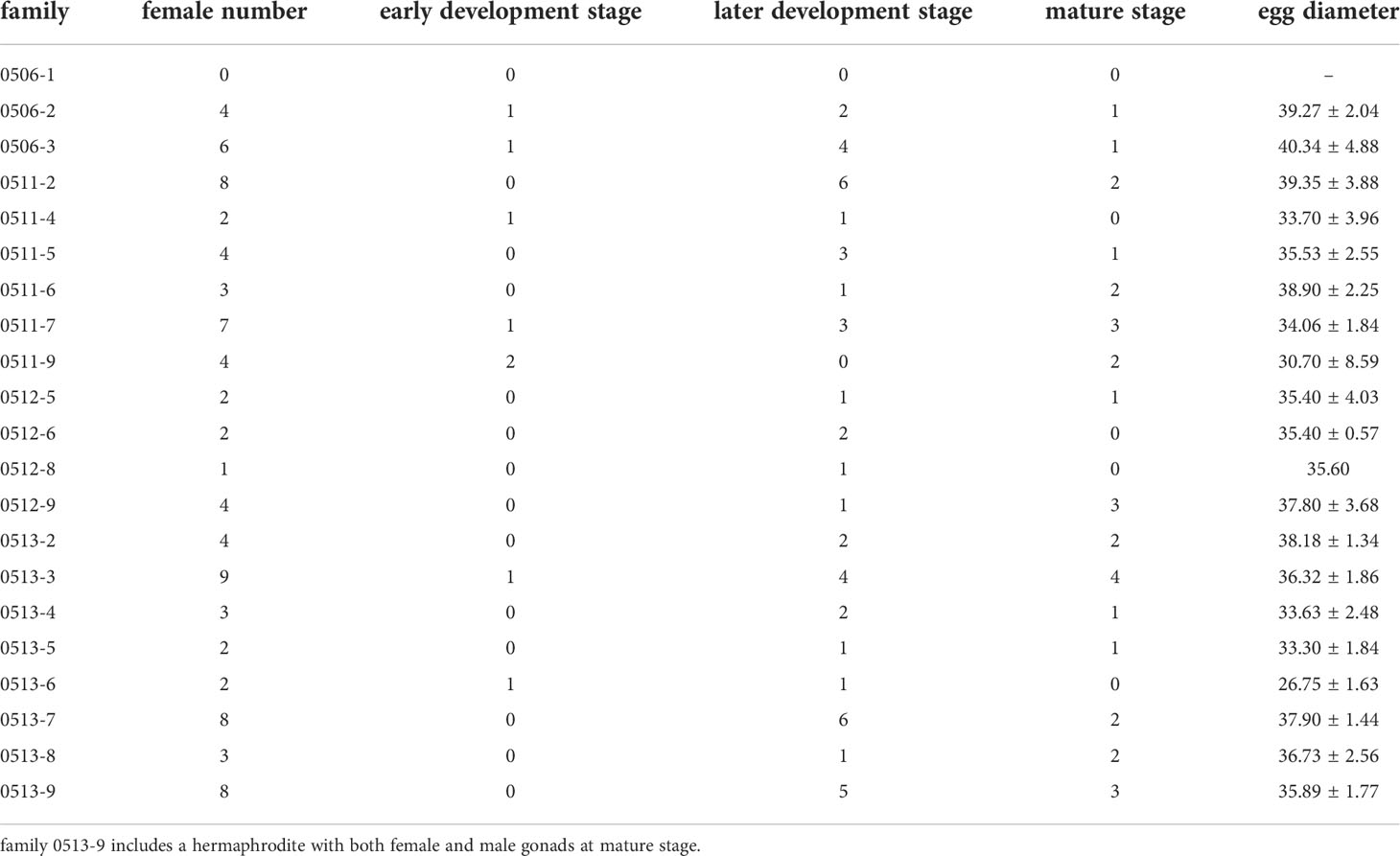
Table 1 Distribution of gametogenic stages and egg diameter of females in each family of Crassostrea angulata.
Growth traits
The shell height at 4 and 12 months and total weight at 12 months were shown in Figure 3. The mean shell heights of all families ranged from 28.34 to 42.25 mm at 4 months, and the largest one was found in family 0513-8 (42.25 ± 6.89 mm), and the smallest one was found in family 0512-5 (28.34 ± 4.79 mm). At 12 months, both the largest shell height and total weigh were found in family 0513-3 (shell height: 59.87 ± 8.66 mm; total weight: 31.13 ± 12.43 g), and both the smallest shell height and total weight were found in family 0512-6 (43.36 ± 8.62 mm; 14.05 ± 6.24 g).
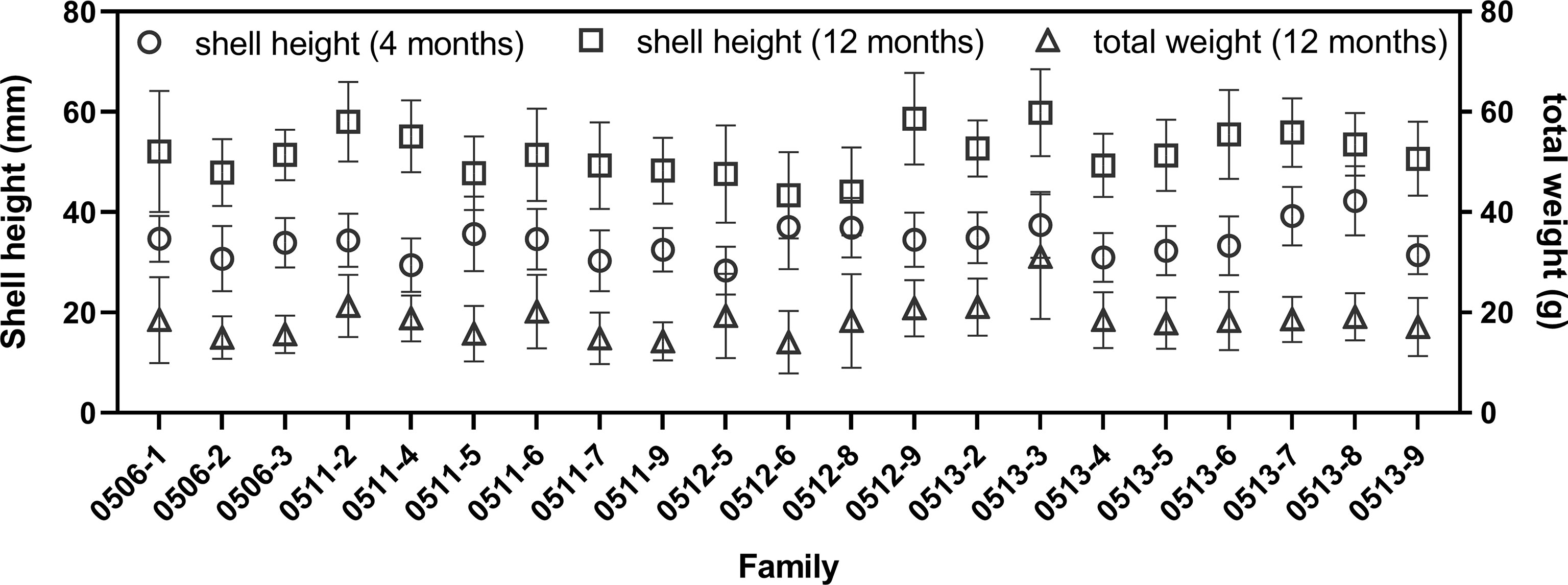
Figure 3 Shell height at 4- and 12-month-old and total weight at 12-month-old of different families of Crassostrea angulata.
Heat tolerance
For all families, oysters began to die at 24 hours after heat challenge, and reached peak of deaths at 36 hours, then basically stable after 156 hours (Figure 4A). the overall survival rate was 52.38%, but there were differences among families (Figure 4B). For example, family 0511-7 had an 82.61% survival rate at 240 hours after heat challenge, while the oysters of family 0512-8 were all died at 72 hours.
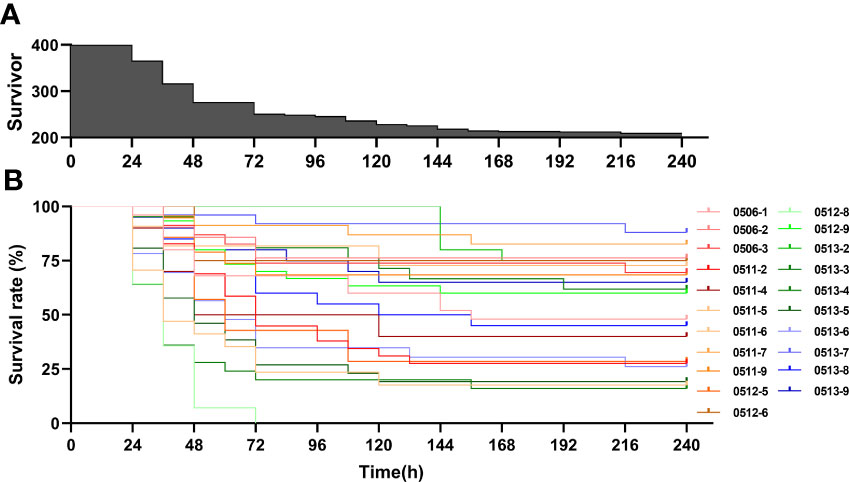
Figure 4 Number of survivors overall (A) and survival curve of different families (B) of Crassostrea angulata after heat challenge (44 °C, 1 h).
Heritability and correlations
Estimated heritability, phenotype and genetic correlations of gonad developmental traits (gametogenic stage and egg diameter), growth traits (SH-juvenile, SH-adult and TW-adult) and heat tolerance trait were present in Table 2. A low heritability was estimated for gametogenic stage (0.18 ± 0.09), while the egg diameter showed a high heritability (0.78 ± 0.30). Medium heritability estimates were found for three growth traits (0.38–0.47) and heat tolerance trait (0.25 ± 0.07).
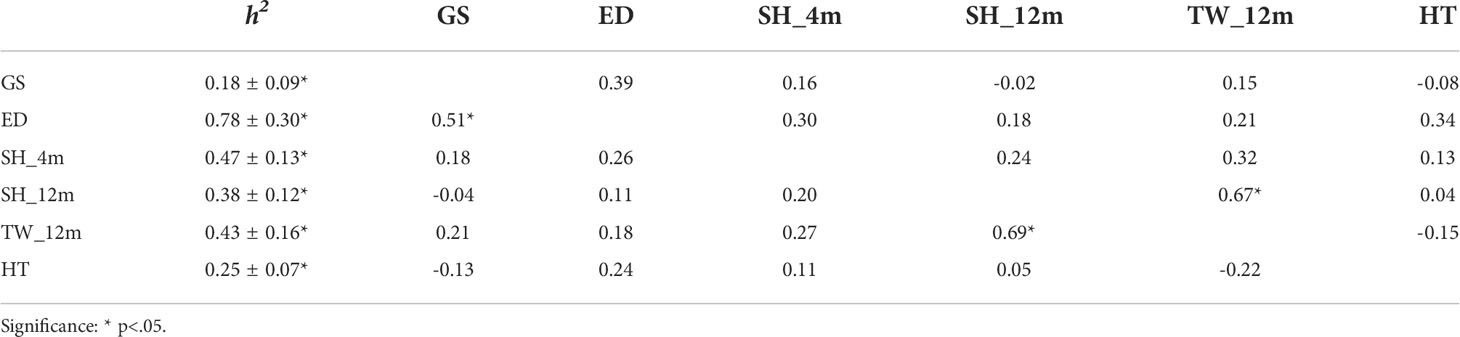
Table 2 Heritability estimates of gametogenic stage (GS), egg diameter (ED), shell height at 4 (SH_4m) and 12 months (SH_12m), total weight at 12 months (TW_12m), heat tolerance traits (HT), and genetic (above diagonal) and phenotypic (below diagonal) correlations between these traits.
Gametogenic stage and heat tolerance showed low and non-significant negative phenotype (-0.13) and genetic correlations (-0.08), while low and non-significant positive phenotype (0.24) and genetic correlations (0.34) were found between egg diameter and heat tolerance. The phenotype (-0.04–0.26) and genetic correlations (-0.02–0.30) between gonad developmental traits and growth traits were low and non-significant. Further, the phenotype and genetic correlations between gametogenic stage and shell height decreased form 0.18 and 0.16 to -0.04 and -0.02, respectively, with growth. Similar downward trends were also observed in the phenotype and genetic correlations between egg diameter and shell height.
As expected, the phenotype correlations between gametogenic stage and egg diameter (0.51, p<.05), and between shell height and total weight at 12 months (0.69, p<.05) were high and significant positive, and a high and significant positive genetic correlation was also observed between shell height and total weight at 12 months (0.67, p<.05). In addition, low and non-significant positive phenotype (0.05–0.11) and genetic correlations (0.04–0.13) between heat tolerance and shell height were found at 4 and 12 months, while heat tolerance and total weight at 12 months showed low and non-significant negative phenotype (-0.22) and genetic correlations (-0.15).
Discussion
Although the reproductive cycle of some oyster species can be shortened by selecting precocious individuals, little is known about whether this selection will accelerate their gonadal development or disturb the selection of productive traits. The present work estimated the heritability of gonadal developmental traits and their correlations with growth and heat tolerance traits in C. angulata. These results will provide a rationale for the feasibility of improving the breeding progress of oysters by shortening their reproductive cycle.
Heritability of gonadal development traits
Phenotypic variation among full-sib families implies the effect of genetic factors on these traits. The heritability of a specific trait estimates the proportion of the phenotypic variation attributable to the additive effect of genes, and it can be used to infer the potential selection response for the trait in a population (Falconer and Mackay, 1996). Different from previous studies about reproductive traits, the present work estimated the heritability of gonadal developmental rate rather than fecundity related traits. In order to quantify the gonadal developmental rate, the gametogenic stage of C. angulata at 4 months were determined by histology method, and no individuals at spawning and re-absorbing stage were observed. This result, combined with the finding that oysters starting to develop gonads at 3 months, suggests that individuals used in present study were all in their first reproductive cycle. In this way, the interference of multiple reproductive cycles of C. angulata on the quantification of gonadal developmental rate can be avoided. Therefore, the gonadal developmental rates of oysters can be reflected by their gametogenic stages.
The heritability for gametogenic stage was low, in consistent with the heritability estimates for fecundity in other bivalves (Ernande et al., 2004; Barros et al., 2018). The low heritability for gametogenic stage suggests that selection for precocious individuals has little effect on the rate of gonadal development of the population. Moreover, egg diameter can be used as an indicator of the rate of gonadal development of females. This is the first time that the heritability for egg diameter was estimated in bivalves, and it was higher than the heritability for average egg weight or egg diameter in fishes (0.04-0.05) (Trong et al., 2013; Srimai et al., 2019). This difference maybe because the eggs used in this study were at different gametogenic stages, while those in fishes all at mature stage. Then, the high heritability for egg diameter suggests that selection for precocious individuals may have a greater effect on females than males in the population, although the effect is small for the population as a whole. It is worth noting that the high heritability of egg diameter obtained in present study maybe biased by the number of females.
Correlations between gonadal development and productive traits
Genetic correlation between two traits can be used to infer the effect of one selected trait on the other (Falconer and Mackay, 1996). Low and non-significant phenotype (-0.13–0.26) and genetic correlations (-0.08–0.34) between gonadal development traits (gametogenic stage and egg diameter) and both growth and heat tolerance traits were observed in C. angulata. Furthermore, downward trends for the phenotype and genetic correlations between gonadal development speed and shell height were observed with growth. These results suggest that selection for precocious individuals has negligible effects on productive traits of C. angulata. Therefore, it is feasible to double the breeding efficiency of C. angulata by halving their reproductive cycle. Then, a scenario can be put forward for C. angulata that two generations are bred a year in a selective breeding stage, and the successfully selected spat are still cultivated for one year in farms to reach commercial size.
Although rare study estimated the correlation between gonadal developmental rate and productive traits, correlations between fecundity and productive traits had been reported in some aquatic species. It is generally believed that sexual maturation results in decreased body growth by shunting the energy for growth (Piferrer et al., 2009), and this phenomenon had been demonstrated in some studies comparing the growth of triploid with diploid oysters (Nell, 2002; Qin et al., 2020). However, the correlation between fecundity and growth is inconclusive in studies that estimate genetic parameters based on diploid full-sib families. For example, low and non-significant correlations were observed in tilapias (Charo-Karisa et al., 2007; Thoa et al., 2017), while moderate positive correlations were observed in rainbow trout (Su et al., 2002) and other population of tilapias (Trong et al., 2013). The differences in magnitude in consistent with the finding reported in the Pacific oyster that the genetic correlations between fecundity and both growth and survival traits related to food abundance and were negative in poor treatments, but positive in rich ones (Ernande et al., 2004). Similarly, the food availability can also affect the growth advantage of triploids (Racotta et al., 2008). Given the importance of environmental factors, the above scenario of doubling the breeding efficiency of C. angulata through two generations one year may require a rich environment.
Overall, the fecundity and gonadal developmental rate are conceptually different. The genetic correlation between gonadal developmental rate and fecundity and whether the genetic correlations of gonadal developmental rate with productive traits are also related to the environment require further research.
Genetic parameters of growth and heat tolerance traits
The heritability for growth and survival traits have been broadly reported in oysters (Chi et al., 2022; De Melo et al., 2018; Dégremont et al., 2007; Vu et al., 2020a; Vu et al., 2020b). Similarly, medium heritability estimates were found for three growth traits (0.38–0.47) and heat tolerance trait (0.25 ± 0.07), suggesting favorable selection response for these traits in C. angulata in China. In addition, growth traits and heat tolerance trait showed low and non-significant phenotype and genetic correlations in present study, in consistent with the results reported in the Pacific oyster (Chi et al., 2020; Dégremont et al., 2007) and the Caribbean scallop (Barros et al., 2018). These similar results found in present study with previous reports, indicate that the genetic parameters estimated in this study is reasonable, despite the relatively small number of families.
Conclusion
The heritability for gametogenic stage was low, and the genetic correlations between gonadal development traits and both growth and heat tolerance traits were low and non-significant. These results suggest that the selection for precocious individuals has little effect on the rate of gonadal development in the population, and has negligible effects on their productive traits. Therefore, it is feasible to double the breeding efficiency of C. angulata by halving their reproductive cycle.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZH conceived the study, conducted the data analysis, and drafted the manuscript. XG, ZL, YS, RC, XH, SY, KT and LL carried out the field and laboratory work. QH revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Open Program of Key Laboratory of Cultivation and High-value Utilization of Marine Organisms in Fujian Province (2020fjscq06), Special Projects Supporting the Construction of Academies of Agricultural Sciences in Districts and Cities of Fujian Province (2021N0041), Education and Research Project for Young and Middle-aged Teachers in Fujian Province (JAT200245), Natural Science Foundation of Fujian Province (2021J05159), Start-up Funds for Scientific Research of Jimei University (ZQ2020023). Key Laboratory of Healthy Mariculture for the East China Sea, Ministry of Agriculture and Rural Affairs, P.R.China (2022ESHML19).
Acknowledgments
We thank Fujian Baozhi Aquatic Technology Co., Ltd. for providing the experimental sites. We are also grateful to the reviewers for reviewing our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aldana Aranda D., Enriquez Diaz M., Lango Reynoso F., Brule T., Montero J., Baqueiro Cardenas E. (2014). Reproductive strategies of the Eastern oyster Crassostrea virginica (Gmelin 1791) in tropical lagoons of the Mexican gulf of Mexico. J. Shellfish Res. 33, 145–152. doi: 10.2983/035.033.0114
Barros J., Velasco L. A., Winkler F. M. (2018). Heritability, genetic correlations and genotype by environment interactions in productive traits of the Caribbean scallop, Argopecten nucleus (Mollusca: Bivalvia). Aquaculture 488, 39–48. doi: 10.1016/j.aquaculture.2018.01.011
Batista F. M., López-Sanmartín M., Grade A., Morgado I., Valente M., Navas J. I., et al. (2015). Sequence variation in ostreid herpesvirus 1 microvar isolates detected in dying and asymptomatic Crassostrea angulata adults in the Iberian peninsula: insights into viral origin and spread. Aquaculture 435, 43–51. doi: 10.1016/j.aquaculture.2014.09.016
Castilho-Westphal G. G., Magnani F. P., Ostrensky A. (2015). Gonad morphology and reproductive cycle of the mangrove oyster Crassostrea brasiliana (Lamarck 1819) in the baía de guaratuba, paraná, Brazil. Acta Zool. 96, 99–107. doi: 10.1111/azo.12055
Charo-Karisa H., Bovenhuis H., Rezk M. A., Ponzoni R. W., van Arendonk J. A. M., Komen H. (2007). Phenotypic and genetic parameters for body measurements, reproductive traits and gut length of Nile tilapia (Oreochromis niloticus) selected for growth in low-input earthen ponds. Aquaculture 273, 15–23. doi: 10.1016/j.aquaculture.2007.09.011
Chi Y., Jiang G. W., Liang Y. X., Xu C. X., Li Q. (2022). Selective breeding for summer survival in pacific oyster (Crassostrea gigas): Genetic parameters and response to selection. Aquaculture 556, 738271. doi: 10.1016/j.aquaculture.2022.738271
Chi Y., Li Q., Liu S. K., Kong L. F. (2020). Genetic parameters of growth and survival in the pacific oyster Crassostrea gigas. Aquac. Res. 52, 282–290. doi: 10.1111/are.14891
Dégremont L., Ernande B., Bedier E., Boudry P. (2007). Summer mortality of hatchery-produced pacific oyster spat (Crassostrea gigas). i. estimation of genetic parameters for survival and growth. Aquaculture 262, 41–53. doi: 10.1016/j.aquaculture.2006.10.025
De Melo C. M. R., Morvezen R., Durland E., Langdon C. (2018). Genetic by environment interactions for harvest traits of the pacific oyster Crassostrea gigas (Thunberg) across different environments on the west coast, USA. J. Shellfish Res. 37, 49–61. doi: 10.2983/035.037.0104
Ding F. F., Li A., Cong R. H., Wang X. X., Wang W., Que H. Y., et al. (2020). The phenotypic and the genetic response to the extreme high temperature provides new insight into thermal tolerance for the pacific oyster Crassostrea gigas. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00399
Ernande B., Boudry P., Clobert J., Haure J. (2004). Plasticity in resource allocation based life history traits in the pacific oyster, Crassostrea gigas. i. spatial variation in food abundance. J. Evol. Biol. 17, 342–356. doi: 10.1046/j.1420-9101.2003.00674.x
Fabioux C., Huvet A., Le Souchu P., Le Pennec M., Pouvreau S. (2005). Temperature and photoperiod drive Crassostrea gigas reproductive internal clock. Aquaculture 250, 458–470. doi: 10.1016/j.aquaculture.2005.02.038
Falconer D. S., Mackay T. F. C. (1996). Introduction to quantitative genetic, fourth ed (Essex: Pearson Education Ltd. Press).
Gilmour A., Gogel B., Cullisand B., Thompson R. (2009). ASReml user guide release 3.0 (Hemel Hempstead: VSN International Ltd. Press).
Gjedrem T., Rye M. (2018). Selection response in fish and shellfish: A review. Rev. Aquac. 10, 168–179. doi: 10.1111/raq.12154
Huvet A., Normand J., Fleury E., Quillien V., Fabioux C., Boudry P. (2010). Reproductive effort of pacific oysters: A trait associated with susceptibility to summer mortality. Aquaculture 304, 95–99. doi: 10.1016/j.aquaculture.2010.03.022
In V. V., O’Connor W., Sang V. V., Van P. T., Knibb W. (2017). Resolution of the controversial relationship between pacific and Portuguese oysters internationally and in Vietnam. Aquaculture 473, 389–399. doi: 10.1016/j.aquaculture.2017.03.004
Li Q., Liu W., Shirasu K., Chen W., Jiang S. (2006). Reproductive cycle and biochemical composition of the zhe oyster Crassostrea plicatula gmelin in an eastern coastal bay of China. Aquaculture 261, 752–759. doi: 10.1016/j.aquaculture.2006.08.023
Liu X. L., Chang Y. Q., Xiang T. H., Cao X. B. (2005). Estimates of genetic parameters for growth traits of the sea urchin, Strongylocentrotus intermedius. Aquaculture 243, 27–32. doi: 10.1016/j.aquaculture.2004.10.014
Li Q., Wang Q., Liu S., Kong L. (2011). Selection response and realized heritability for growth in three stocks of the pacific oyster Crassostrea gigas. Fish. Sci. 77, 643–648. doi: 10.1007/s12562-011-0369-0
Nell J. A. (2002). Farming triploid oysters. Aquaculture 210, 69–88. doi: 10.1016/S0044-8486(01)00861-4
Normand J., Ernande B., Haure J., McCombie H., Boudry P. (2009). Reproductive effort and growth in Crassostrea gigas: Comparison of young diploid and triploid oysters issued from natural crosses or chemical induction. Aquat. Biol. 7, 229–241. doi: 10.3354/ab00190
O’Connor W., Dove M., O’Connor S., In V. V., Lien V. T. N., Van P. T. (2019). Enhancing bivalve production in northern Vietnam and Australia (Canberra, Australia: ACIAR).
Piferrer F., Beaumont A., Falguière J., Flajšhans M., Haffray P., Colombo L. (2009). Polyploid fish and shellfish: Production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293, 125–156. doi: 10.1016/j.aquaculture.2009.04.036
Qin J., Huang Z. X., Chen J., Zou Q., You W. W., Ke C. H. (2012). Sequencing and de novo analysis of Crassostrea angulata (Fujian oyster) from 8 different developing phases using 454 GSFlx. PloS One 7, e43653. doi: 10.1371/journal.pone.0043653
Qin Y. P., Li X. Y., Noor Z., Li J., Zhou Z. H., Ma H. T., et al. (2020). A comparative analysis of the growth, survival and reproduction of Crassostrea hongkongensis, Crassostrea ariakensis, and their diploid and triploid hybrids. Aquaculture 520, 734946. doi: 10.1016/j.aquaculture.2020.734946
Racotta I. S., Palacios E., Ibarra A. M., Ramírez J. L., Arcos F., Arjona O., et al. (2008). Comparative biochemical composition of ploidy groups of the lion-paw scallop (Nodipecten subnodosus Sowerby) supports the physiological hypothesis for the lack of advantage in triploid mollusc's growth in food-rich environments. Mar. Biol. 153, 1245–1256. doi: 10.1007/s00227-007-0897-4
Royer J., Seguineau C., Park K. I., Pouvreau S., Choi K. S., Costil K. (2008). Gametogenetic cycle and reproductive effort assessed by two methods in 3 age classes of pacific oysters, Crassostrea gigas, reared in normandy. Aquaculture 277, 313–320. doi: 10.1016/j.aquaculture.2008.02.033
Sekino M., Ishikawa H., Fujiwara A., Yamashita H. (2016). Occurrence of the Portuguese oyster Crassostrea angulata (Lamarck 1819) along the coast of Shikoku island, Japan. Plankton Benthos Res. 11, 71–74. doi: 10.3800/pbr.11.71
Shi B., Wu Y. D., Zhou L., You W. W., Ke C. H. (2019). Influence of gonadal development on metal accumulation in the Portuguese oyster, Crassostrea angulata, in subtropical areas. Aquac. Res. 50, 1142–1152. doi: 10.1111/are.13988
Srimai W., Koonawootrittriron S., Manee-aphai W., Chaivichoo P., Phu-onnim A., Koolboon U., et al. (2019). Genetic parameters of reproductive traits in male and female north African catfish, Clarias gariepinus (Burchell 1822). Aquaculture 513, 734431. doi: 10.1016/j.aquaculture.2019.734431
Su G. S., Liljedahl L. E., Gall G. A. E. (2002). Genetic correlations between body weight at different ages and with reproductive traits in rainbow trout. Aquaculture 213, 85–94. doi: 10.1016/s0044-8486(01)00809-2
Thoa N. P., Hamzah A., Nguyen N. H. (2017). Genetic variation and correlated changes in reproductive performance of a red tilapia line selected for improved growth over three generations. Anim. Reprod. Sci. 184, 94–101. doi: 10.1016/j.anireprosci.2017.07.003
Thompson R. J., Newell R. I. E., Kennedy V. S., Mann R. (1996). “Reproductive process and early development,” in The Eastern oyster crassostrea virginica. Eds. Kennedy V. S., Newell R. I. E. (College Park, MD: Maryland Sea Grant Book), 335–370.
Trong T. Q., van Arendonk J. A. M., Komen H. (2013). Genetic parameters for reproductive traits in female Nile tilapia (Oreochromis niloticus): II. fecundity and fertility. Aquaculture 416, 72–77. doi: 10.1016/j.aquaculture.2013.08.031
Vu S. V., Knibb W., Nguyen N. T., Vu I. V., O’Connor W., Dove M., et al. (2020b). First breeding program of the Portuguese oyster Crassostrea angulata demonstrated significant selection response in traits of economic importance. Aquaculture 518, 734664. doi: 10.1016/j.aquaculture.2019.734664
Vu S. V., Knibb W., O’Connor W., Nguyen N. T. H., In V. V., Dove M., et al. (2020a). Genetic parameters for traits affecting consumer preferences for the Portuguese oyster, Crassostrea angulata. Aquaculture 526, 735391. doi: 10.1016/j.aquaculture.2020.735391
Wang H. Y., Qian L. M., Liu X. A., Zhang G. F., Guo X. M. (2010). Classification of a common cupped oyster from southern china. J. Shellfish Res. 29, 857–866. doi: 10.2983/035.029.0420
Wu Y. D., Shi B., Zhou L., Dong C. Y., You W. W., Ke C. H. (2019). Heritability estimates for copper/zinc accumulation capabilities and correlation with growth/quality traits in the fujian oyster, Crassostrea angulata. Aquaculture 499, 212–219. doi: 10.1016/j.aquaculture.2018.09.021
Keywords: heritability, genetic correlation, gonadal development, productive traits, Crassostrea angulata
Citation: Han Z, Guo X, Lu Z, Song Y, Chen R, Han X, Yu S, Tu K, Liu L and Que H (2022) Heritability estimates for gonadal development traits and their genetic correlations with growth and heat tolerance traits in the Fujian Oyster Crassostrea angulata. Front. Mar. Sci. 9:986441. doi: 10.3389/fmars.2022.986441
Received: 05 July 2022; Accepted: 05 September 2022;
Published: 16 September 2022.
Edited by:
Bin Xia, Qingdao Agricultural University, ChinaReviewed by:
Weijun Wang, Ludong University, ChinaSang Vu, Research Institute for Aquaculture number 1, Vietnam
Yanping Qin, Chinese Academy of Sciences (CAS), China
Copyright © 2022 Han, Guo, Lu, Song, Chen, Han, Yu, Tu, Liu and Que. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziqiang Han, aGFuenFAam11LmVkdS5jbg==; Huayong Que, aHF1ZUBqbXUuZWR1LmNu
 Ziqiang Han
Ziqiang Han Xiang Guo2
Xiang Guo2 Zuoliang Lu
Zuoliang Lu Huayong Que
Huayong Que