- 1Guangdong Research Center on Reproductive Control and Breeding Technology of Indigenous Valuable Fish Species, Guangdong Provincial Key Laboratory of Aquatic Animal Disease Control and Healthy Culture, Fisheries College, Guangdong Ocean University, Zhanjiang, China
- 2Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang), Zhanjiang, China
In the aquaculture industry, fish oil is widely used as a nutritional supplement to promote the gonadal maturation of broodstocks, while the mechanism of fish oil on ovary development remain unclear. Herein, female adult spotted scat (Scatophagus argus) with most ovaries at phase II were fed with diets containing 8% soybean oil (SO) or 8% fish oil (FO) for 60 days. The final average fish body weight was similar between FO and SO groups. The average gonadosomatic index (GSI) of FO group was higher (non-significant) than that of SO group. Finally, the phase IV ovary of the FO and SO groups were 7 and 5 out of 10, respectively. The serum estradiol (E2) level of the FO group was significantly higher than that of the SO group. The proportions of n-3 long-chain polyunsaturated fatty acids (LC-PUFA, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)) in the ovaries were much higher in the FO group than in the SO group. These results suggest that the maturity level of the FO group is relatively higher than that of the SO group. Transcriptome sequencing was used to detect the ovary gene expression. Comparatively, the ovary transcriptome analysis showed 68 up-regulated and 193 down-regulated genes in the FO group. The expression levels of Jund and Jun necessary for the ovary maturation were up-regulated in the FO group, while Pparγ and Cxcl12, which could inhibit the ovary development, were down-regulated in the FO group. Cyp3a27 coding the enzyme for degrading the estrogen was significantly down-regulated in the FO group and coincided with the increase of its serum E2. Kif5b which could regulate the glucose metabolism was up-regulated in the FO group. Serum insulin level was also increased in the FO group. Additionally, Aldh3a2 and Plin2 related to lipid metabolism were significantly down-regulated in the FO group. Briefly, dietary fish oil can influence the expression of genes related to steroid hormone, glucose and lipid metabolism. This study will clarify the mechanism of dietary fish oil in promoting ovary development in teleost fish.
Introduction
The ovarian follicle releases oestradiol in response to pituitary gonadotropins and acts on the liver to create the yolk protein vitellogenin. This is then sequestered into the developing oocyte by pinocytosis as ovarian recrudescence begins in teleost fish (Kime, 1993). Following the completion of the vitellogenic phase, the oocyte in fish undergoes meiotic resumption. The quick and tightly synchronized events, such as granulosa cell proliferation, cumulus cell-matrix formation, and chromosome segregation, are energy-intensive processes that necessitate enough ATP generation from cellular energy stores (Song et al., 2018). Many fish species tend to reduce their food intake during sexual maturation; thus, the final stages of gonadal growth are dependent on the mobilization and re-allocation of endogenous reserves (Perez et al., 2007). The presence of lipids in in vitro maturation systems is reported to affect oocyte lipid content and developmental competence (Dunning et al., 2014). As a result, lipid metabolism is one of the most researched topics in reproductive and developmental biology today (Pawlak et al., 2020). The suitable source of dietary oil for broodstock is widely analyzed in different aquaculture species (Araújo et al., 2016; Hilbig et al., 2019; Leng et al., 2019).
Fish oil is a unique and abundant source of omega-3 long-chain polyunsaturated fatty acids (n−3 LC-PUFA), particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Tocher, 2015). Some fish species absorb dietary unsaturated fatty acids into eggs readily during the spawning season. LC-PUFA, mainly DHA and EPA, affect fish steroidogenesis and maturation directly or via their metabolites (Izquierdo et al., 2001). By boosting E2 secretion and Vtg production, high dietary n-3 LC-PUFAs stimulate ovarian development in silver pomfret (Pampus argenteus) (Peng et al., 2015). Similarly, a high DHA diet in Siberian sturgeon (Acipenser baeri) broodstock improves serum E2, fertility, egg hatchability and overall larval quality (Luo et al., 2015). Increasing the dietary intake of n-3 LC-PUFA appeared to increase oocyte growth, resulting in a faster progression of ovarian development in European eels (Anguilla anguilla) after hormonal therapy (Da Silva et al., 2016). These findings imply that an adequate supplemental level of n-3 LC-PUFA can aid in the development of fish ovaries. We recommend other research to be conducted to unearth the exact mechanisms causing such changes of the dietary fish oil.
In nutrigenomics, nutrients are well-thought-out to be signals through which cells deduce information about the environment (diet) and respond, according to necessity, by adjusting the metabolic pathways via the regulation of protein and gene expression towards homeostasis (De Santis et al., 2015). Nutrigenomics contributes to our awareness of the powerful effects of various fatty acids on several metabolic pathways, and homeostatic control in the cell, and thus on overall health. Transcriptomics, which permits assessing genome-wide changes in gene expression of thousands of genes simultaneously in a single sample, has been the most widely used genomics technique thus far (Afman and Müller, 2012). Ovary transcriptomics analyses were used to evaluate dietary lipid levels that benefit the steroid hormone synthesis and improve gonad development in female Chinese sturgeon (Acipenser sinensis) (Leng et al., 2019). The application of transcriptomics analyses of ovaries affected by fish oil in teleost is still rarely reported.
Spotted scat (Scatophagus argus) is a well-known aquarium fish due to its colorful appearance, hardiness, moderate growth, and quiet temperament. It is also used as food fish, particularly in South and South-East Asian countries, because of its high protein content and flavor (Gupta, 2016). In addition, female gonad maturation in spotted scat is delayed compared to male gonad maturation (Gandhi et al., 2014). At the moment, most commercially available broodstock diets for cultured fish species are just larger sized “on-growing” diets (Izquierdo et al., 2001). In our practice, spotted scat hatcheries improve their broodstock nutrition by feeding only fresh oysters and lobworm or by combining them with commercial diets. The usage of these unprocessed marine animals frequently raises the risk of disease transmission from the parents to offspring. However, research on broodstock nutrition is restricted. Fish oil supplementation in the diet up-regulated 497 and down-regulate 267 genes in the liver of spotted scat, including reproduction-related genes (Wang et al., 2021). While the direct influence of dietary fish oil on the ovarian genes expression is still unknown at the transcriptome level.
In this study, we investigated how fish oil influences ovarian gene expression and blood hormone content in spotted scat, and the regulatory mechanism of fish oil on lipid metabolism and reproductive endocrine during ovarian development in female adult spotted scat. This study gives an essential theoretical foundation for investigating of nutrients that promote teleost fish reproductive performance.
Materials and methods
Ethical considerations
With approval from Guangdong Ocean University’s Committee on the Ethics of Animal Experiments, this experiment was carried out in compliance with the guidance, care, and use of laboratory animals in China.
Experimental fish rearing
The Zhuhai Hengda Cultivation Base (Guangdong, China) provided two-year-old female spotted scats. The female to male ratio of spotted scats is similar to 1:1 as shown in Mustapha et al. (2021). The sexual anatomical difference in the head, anal fin, body color, body weight, and genital pore empirically was used to judge the female spotted scat from the mixed sex fish (Barry and Fast, 1992). The fish were brought to our laboratory and bred at the Donghai island experimental base in Guangdong Province, China. The fish were grown in a concrete pond (12 m × 5 m × 2 m) for two weeks to adjust to the rearing conditions. The tanks were fully aerated, and the average water temperature was 31.1 ± 0.8°C. The salinity was 8‰, and the pH and dissolved oxygen levels were 7.5 to 8.5 and 6.0 to 7.0 mg L-1, respectively. Commercial fish pellets containing 43% crude protein and 12% crude fat were supplied to the fish at 2.5% of their body weight. Throughout the adaption phase, fish were fed twice a day at 06:00 and 18:00 h. The pond’s water was not constantly circulated. Every three days, about twenty percent of the water in the pond was drained and refilled with fresh water.
Experimental design
Following the acclimation period, 100 females (average initial weights of 242.83 ± 50.90 g and lengths of 19.48 ± 1.13 cm, 90% ovaries at phase II) were randomly assigned to fish oil (FO) and soybean oil (SO) treatment groups (each with 50 fish; ingredients are listed in Supplementary Table S1). The FO group was assigned to the diet containing 8% fish oil, while the control group diet containing 8% soybean oil was marked SO. Fatty acid composition of the experimental diets is shown in Supplementary Table S2. Each treatment group was repeated, and each net cage had 25 fish (5 m × 3.5 m × 1.8 m). Two separate concrete ponds (12 m × 5 m × 2 m) were used, each with two net cages for both FO and SO treatments. Before sampling, the fish were fed the two diets for 60 days. The culturing environment was appropriate for the adaption stage.
Growth measurements and sampling
The spotted scat were sampled at the end of the experiment (after 24 hours of starvation). For sampling, five spotted scats per cage were sacrificed. Individual measurements of the body weight, body length, viscera weight, liver weight, and gonad weight were taken. The following formulas were used to compute the survival rate (SR), condition factor (CF), viscerosomatic index (VSI), hepatosomatic index (HSI), and gonadosomatic index (GSI):
SR (%) = final number of fish/initial number of fish × 100;
CF = final body weight (g)/final body length (cm) 3 × 100;
VSI (%) = final viscera weight (g)/final body weight (g) × 100;
HIS (%) = final liver weight (g)/final body weight (g) × 100;
GSI (%) = final gonad weight (g)/final body weight (g) × 100
Ovary samples were excised from each fish and snap-frozen in liquid nitrogen immediately. Blood samples were taken from the caudal vein and stored at 4°C overnight and centrifuged at 3000 rpm for 10 minutes. Following that, all samples were frozen at -80°C for further analysis.
Fatty acid composition of the fish ovary
After acid hydrolysis, the total lipid from the ovary (n = 6 per group) were extracted with diethyl ether according to the protocol of National Standards of the People’s Republic of China (5009.168—2016). Using NaOH-methanol, the extracted lipid was saponified. Their constitutive fatty acids were methylated using boron trifluoride-methanol, separated by gas chromatography (Agilent 7890B) using a capillary column (SPTM-2560; 100 m * 0.25 mm * 0.25 μm) and quantified using glyceryl triundecanoate as an internal standard.
RNA extraction, library construction and sequencing
Transcriptome analysis was used to assess the impact of fish oil on the genes expressed in the ovary. Six samples in each group were obtained from randomly mixed samples of 10 fish according to the ovary development stages. Stage II or III ovarian samples were randomly merged into three different premature ovary samples, and stage IV ovary samples were also randomly merged into three mature ovary samples. Reasons for merging samples include the fact that (1) the representativeness of transcriptome samples has a significant impact on the results; (2) individual genetic factors affect ovarian development; (3) the gene expression of samples in the prophase and anaphase ovary development was different; (4) Stage II and Stage III ovaries are grouped together for their GSI is very close. Considering the above factors, we decided to avoid the effect of ovarian staging and to study the differences between fish oil and soybean oil on the ovarian development. Total RNA was isolated from fish ovaries using RNeasy® Mini Kit reagents according to the manufacturer’s instructions (AMBION, Inc). An Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) was used to assess and quantify RNA quality, which was then confirmed using RNase-free agarose gel electrophoresis. Oligo (dT) beads were used to enhance the mRNA, and the mRNA was interrupted. The first strand of cDNA was synthesized using the M-MuLV reverse transcriptase system, while the second strand was made with the DNA polymerase I system using dNTPs. The segments of cDNA were then ligated to Illumina sequencing adapters. Ligation products were size-selected using agarose gel electrophoresis, PCR amplified them, then sequenced them using novaseq 6000 by Guangzhou Genedenovo Biotechnology Co (Guangzhou, China). The NCBI Sequence Read Archive (SRA) database received all raw sequencing data (Accession No: PRJNA833283).
Data filtering, reads mapping, and differential gene expression analysis
The original data was filtered before being analyzed to ensure high-quality data. Fastp (version 0.18.0) was used to filter the raw reads. Over 50% of low-quality nucleotides (Q-value 20) were eliminated from the adapters, which read with more than 10% unknown nucleotides. The alignment tool Bowtie2 (version 2.2.8) was used to remove the ribosomal RNA (rRNA) readings. Then, using HISAT2.2.4, these clean reads were mapped to the spotted scat reference genome (Huang et al., 2021). Novel genes were defined as those with unknown transcripts or those found in intergenic regions. Gene expression levels were estimated using the fragments per kilobase of transcript per million mapped reads (FPKM) technique. The EdgeR software package was used to identify the differentially expressed genes (DEGs) in two groups (each with six biological replicates). Genes with |log2 (fold change) | > 1 and P-value < 0.05 were determined as significant DEGs. Enrichment analysis of Gene Ontology (GO) functions and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were used to annotate DEGs. With a P-value of 0.05, the KEGG pathway was considered a significant enrichment route.
Validation by real-time PCR
Ten differentially expressed genes associated to lipid and glucose metabolism and ovarian development were chosen for Real-time PCR analysis to confirm the gene expression profiles from the RNA-seq data. The PerfectStart™ Green qPCR SuperMix was used for real-time PCR (TRAN, China). In a total volume of 20 μl, the PCR reaction was carried out with 10 μl of 2 × PerfectStart™ Green qPCR SuperMix, 0.4 μl of each primer, and 2 μl cDNA. An initial denaturation at 94°C for 30 s was followed by 40 cycles of 94 °C for 5 s, 60°C for 15 s, and 72°C for 10 s in the thermal cycling program. The internal reference genes for ovary were identified in spotted scat (Liu et al., unpublished data). Accordingly, the reference gene B2M was employed in the present study. The relative expression of the target genes was calculated using the 2−ΔΔCt method. Each group consists of ten separate samples, each of which was detected in triplicate. Supplementary Table S3 lists the primer sequences used in Real-time PCR.
E2 and insulin levels of serum
According to the manufacturer’s instructions, the serum E2 and insulin content were determined using the Fish Estradiol ELISA Kit and the Fish Insulin ELISA Kit (CUSABIO, Inc). A total of ten fish from each group had their serum samples analyzed.
Statistical analysis
Data presented are given as means ± standard errors, and an independent-samples T-test with a significance level of P < 0.05 was utilized. SPSS 17.0 was used to conduct the statistical analysis of the data (SPSS Inc., Chicago, IL, USA).
Results
Survival rate, growth performance and body indices
At the end of the experiment, the survival rate was 100% in both FO and SO groups. There was no significant difference between the final body weight and length, GSI, HSI and VSI in the SO and FO groups (P > 0.05, Table 1). GSI and VSI in the FO group were higher than that in the SO group, although their differences were insignificant. The CF was significantly higher in the FO group than that in the SO group (P < 0.05).

Table 1 Growth performance of the female adult spotted scat fed with different types of oil sources.
Effect of fish oil on ovarian development
The GSI values positively correlate with the ovary development phases in both SO and FO groups. The phase IV ovary of the SO group (n=5) is less than that of FO group (n=7) indicating that the maturity level of the FO group is higher than that of SO group (Figure 1; Supplementary Table S4).

Figure 1 Paraffin section of the adult spotted scat ovary. (A–J), SO group. (K–T), FO group. The ovaries were at phase II, III or IV. Scale = 100 µm.
Note: n=10. II, III and IV represent the developmental stages of the ovary. Sample numbers corresponding to the transcriptome: SO1 (A), SO2 (B, C), SO3 (D, E), SO4 (F), SO5 (G, H), SO6 (I, J); FO1 (K), FO2 (L), FO3 (M), FO4 (N, O), FO5 (P, Q, R), FO6 (S, T).
Fatty acid composition of the adult spotted scat ovary
The main fatty acids in the ovaries of adult spotted scat are palmitic acid (C16:0) and oleic acid (C18:1n-9). The absolute content of myristic acid (C14:0), pentadecanoic acid (C15:0), heptadecanoic acid (C17:0), palmitoleic acid (C16:1n-7), erucic acid (C22:1n-9), arachidonic acid (C20:4n-6), EPA, DHA and n-3 PUFA/n-6 PUFA ratio in the FO group were significantly higher than those in the SO group (Table 2).
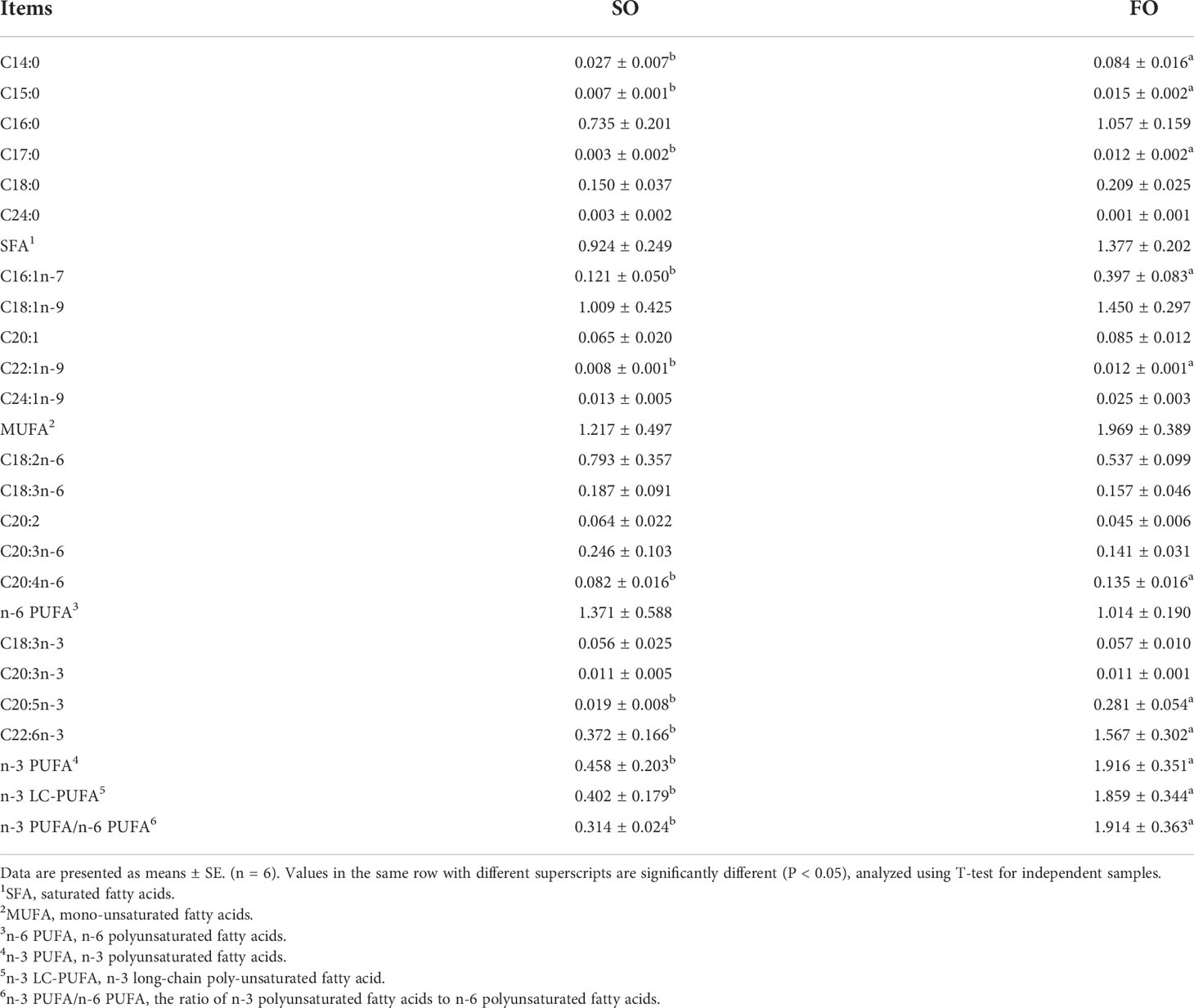
Table 2 Fatty acid composition in the ovary of the adult spotted scat fed with different types of oil sources (g/100g).
Raw sequencing reads and quality statistics
We constructed twelve cDNA libraries using ovary from the SO and FO groups in this study. A total of 266, 067, 512 and 252, 834, 414 clean reads in the SO and FO groups were obtained from the transcriptome sequencing, respectively. The sequence quality was high in all samples, with a percentage of Q30 bases of more than 93.43% (Table 3). The transcriptome sequence data was aligned with the spotted scat’s previous genome (Huang et al., 2021). The results showed total mapped reads were 482, 981, 867. The percentage of mapped reads for each library ranged from 90.93% to 94.56%. 22, 609 genes were predicted, including 21, 948 known genes and 661 novel genes. This accounts for 90.48% of the reference genomes. Supplementary Table S5 shows the number of genes detected in each sample.
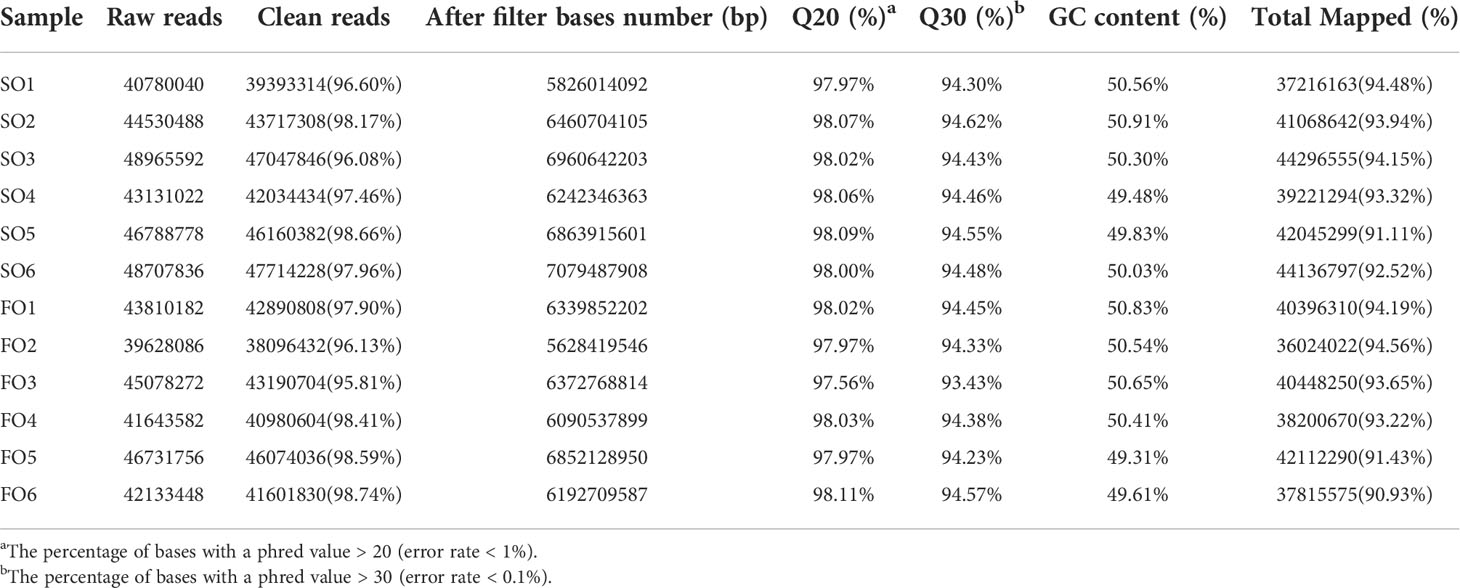
Table 3 Summary of the sequencing data in the ovary of the adult spotted scat fed with different types of oil sources.
GO and KEGG enrichment analysis of the DEGs
This study annotated a total of 261 genes to be DEGs (P-value < 0.05, |log2FC| > 1). Comparatively, in the SO and FO groups, 68 and 193 genes were up-regulated and down-regulated, respectively (Figure 2).
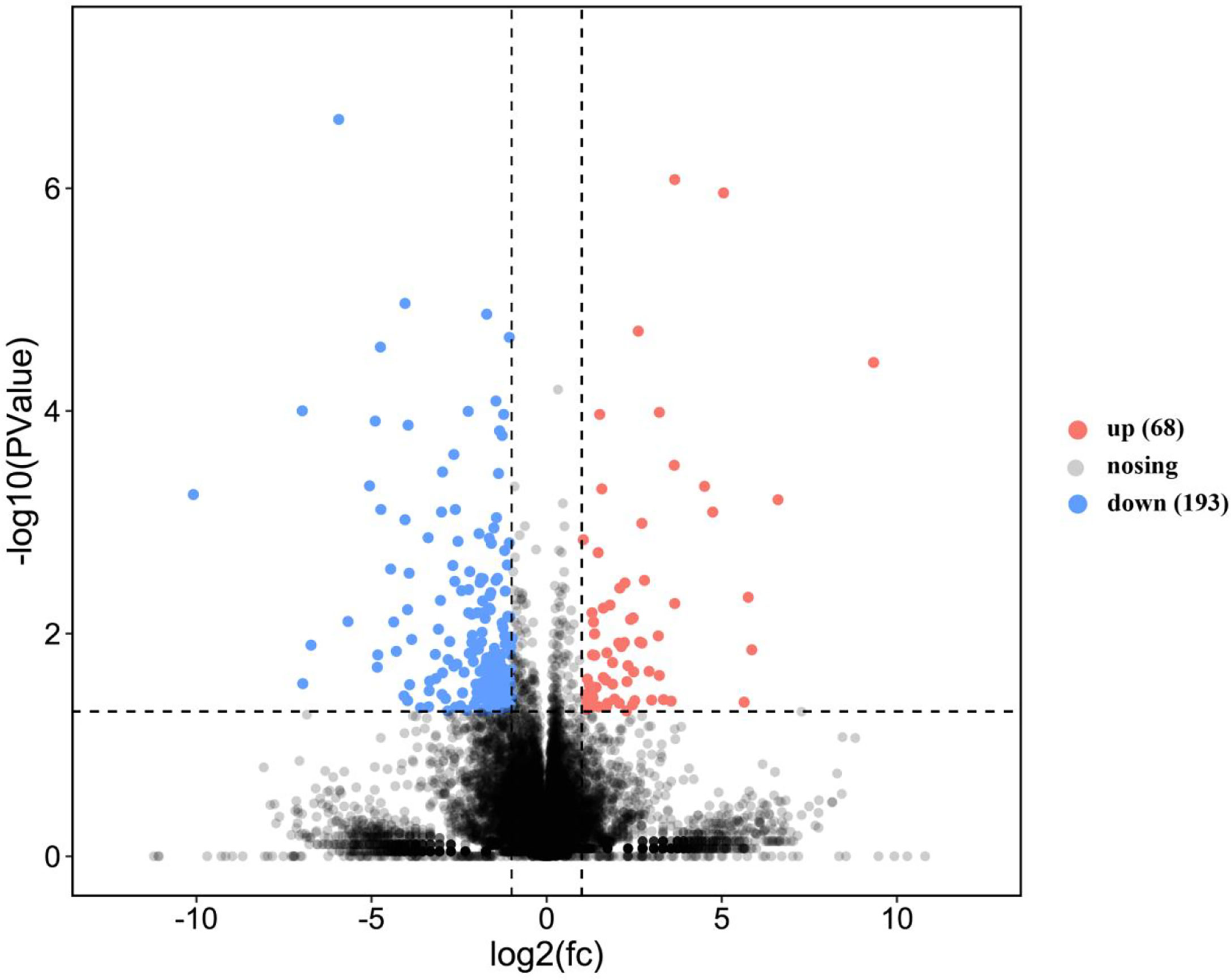
Figure 2 Volcano plot of the differentially expressed genes in the ovary of adult spotted scat fed with different oil sources.
GO, KEGG enrichment analyses were explored to ascertain the biological functions of the DEGs. The DEGs were annotated into three GO term categories: cellular component, biological process, and molecular function (Figure 3). In the cellular component categories, membrane part (29), membrane (31), macromolecular complex (7), organelle (15), cell (19), cell part (19) were the most enriched GO terms. In the biological process categories, single-organism process (55), biological regulation (34), signaling (19), biological regulation (34), growth (1), cellular process (48) and metabolic process (35) were the most enriched GO terms. In the molecular function categories, signal transducer activity (14), molecular transducer activity (14), nucleic acid binding transcription factor activity (5), binding (37) and catalytic activity (25) were the most enriched GO terms.
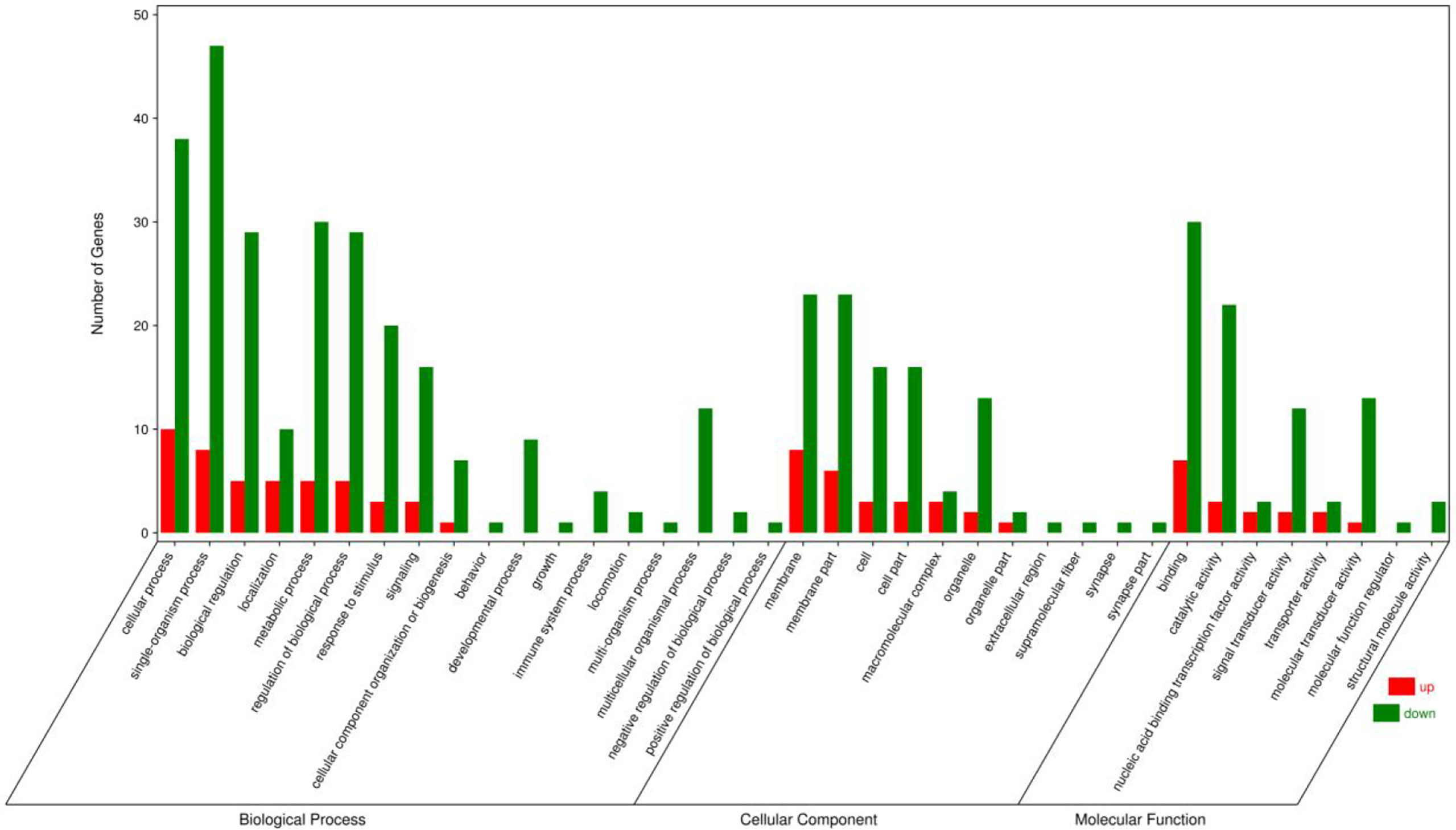
Figure 3 Gene ontology annotations of the differentially expressed unigenes in the ovary of adult spotted scat fed with different oil sources.
Figure 4 shows the top 20 biological pathways revealed by KEGG analysis are shown. The DEGs are primarily classified into cellular processes, environmental information processing, metabolism, organismal systems and human diseases. Key genes for Phagosome (Tubb4b), Linoleic acid metabolism (Pla2g4c, Cyp3a27), Cytokine-cytokine receptor interaction (Cxcl12, Ccl21, Il12b), PPAR signaling pathway (Pparγ, Plin2, Acsbg2), Fatty acid degradation (Acsbg2, Aldh3a2), as well as additional crucial genes that play roles in GnRH signaling pathway (Pla2g4c, Jun, Hbegf) were identified. In addition, DEGs fell into alpha-Linolenic acid metabolism (Pla2g4c) and Endocytosis (Kif5b, Mr1).
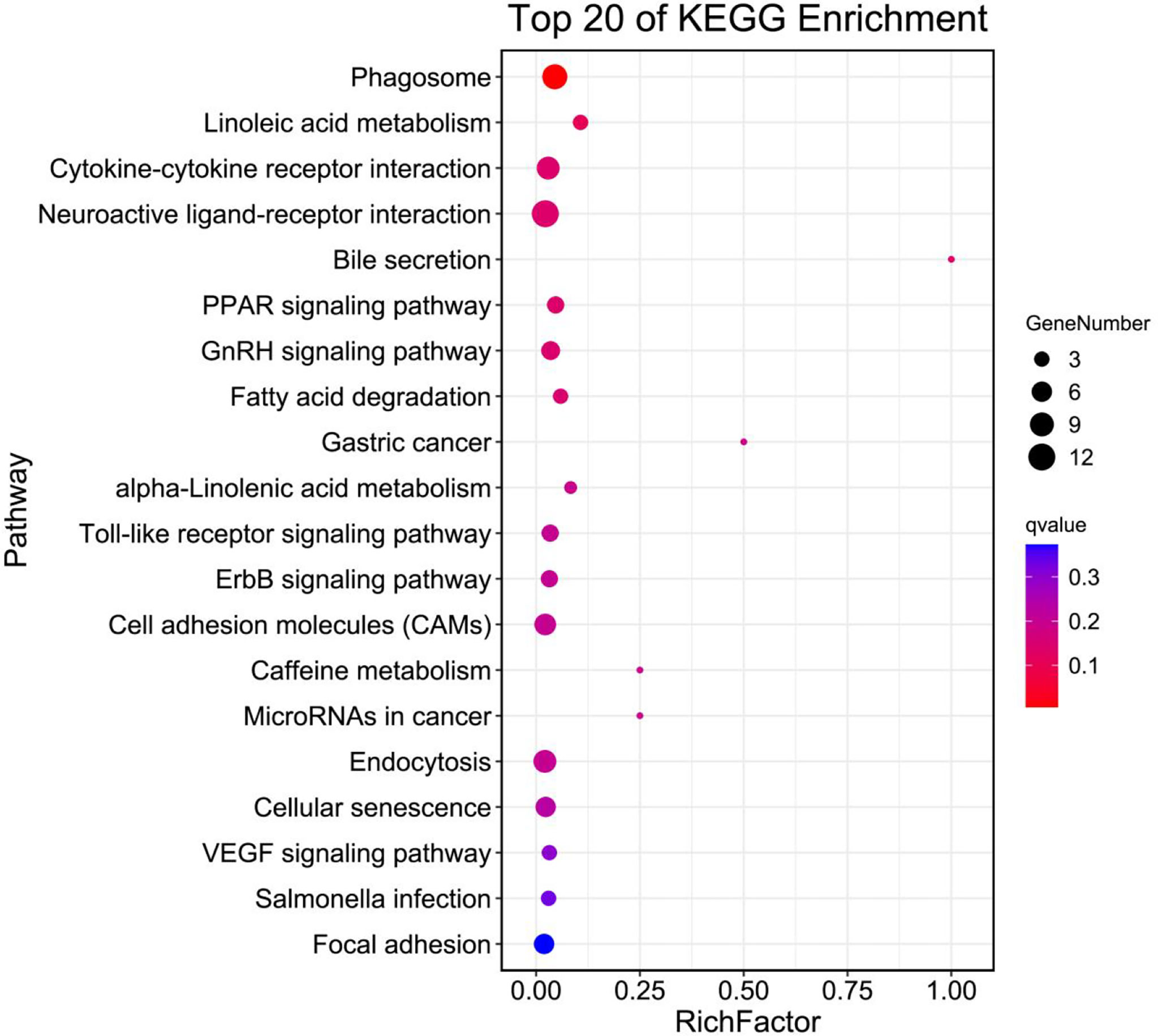
Figure 4 Top 20 pathway enrichments analysis of differentially expressed unigenes in the ovary of adult spotted scat fed with different oil sources.
DEG verification using real-time PCR
Ten DEGs related to lipid and glucose metabolism and ovarian development from RNA-Seq were verified by real-time PCR (Figure 5). The results showed consistent expression levels of the selected genes between RNA-Seq and real-time PCR, indicating the reliability of the RNA-Seq results.
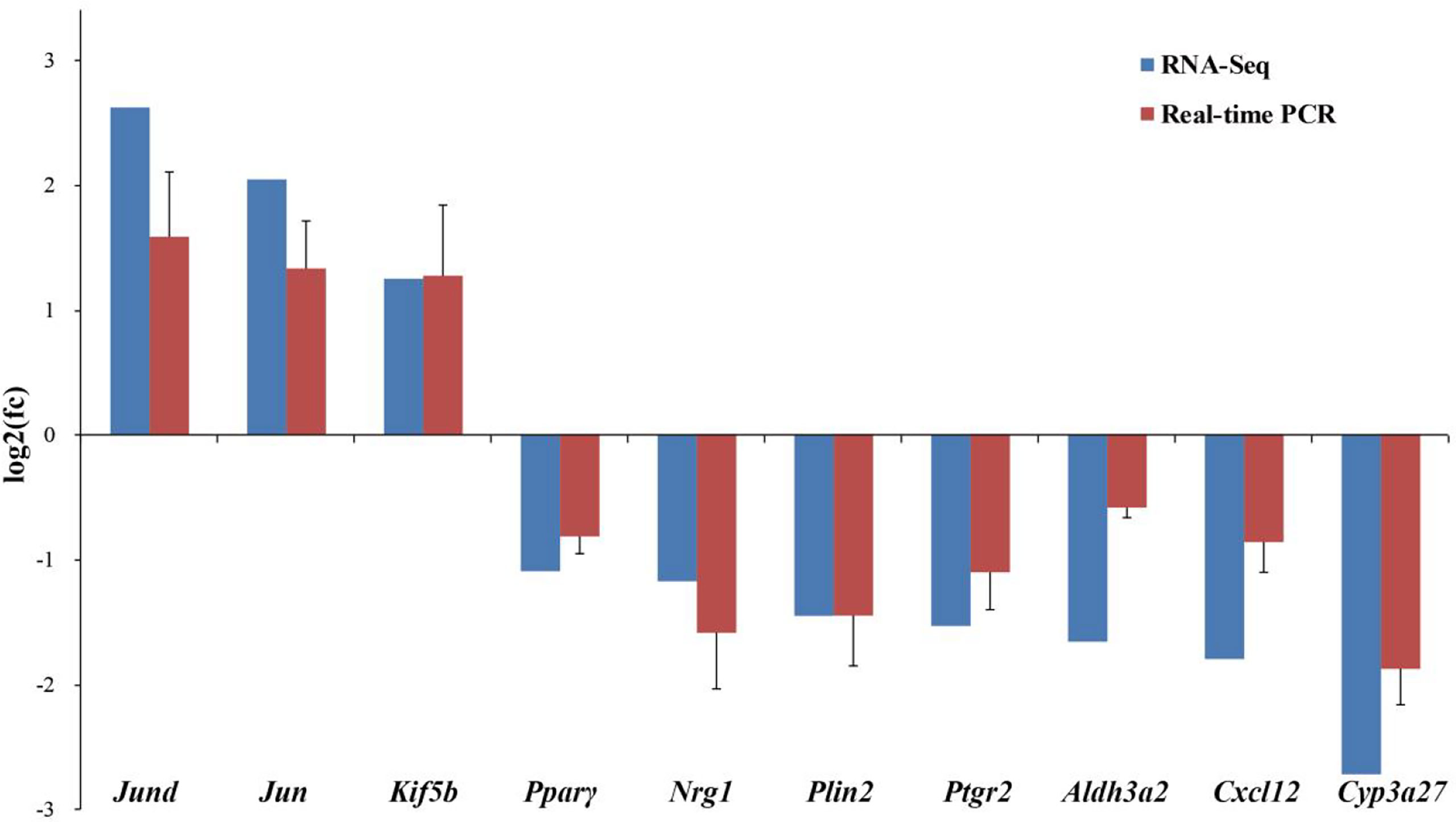
Figure 5 Comparison of gene expression data between RNA-Seq and Real-time PCR in the ovary of adult spotted scat fed with different oil sources. Data are presented as means ± SE. (n = 10), analyzed using T-test for independent samples.
Change in serum E2 and insulin levels
The E2 and insulin levels in the serum were detected. The results indicated that the serum E2 and insulin levels in the FO group fish were significantly higher than in the SO group (P < 0.05, Figure 6).
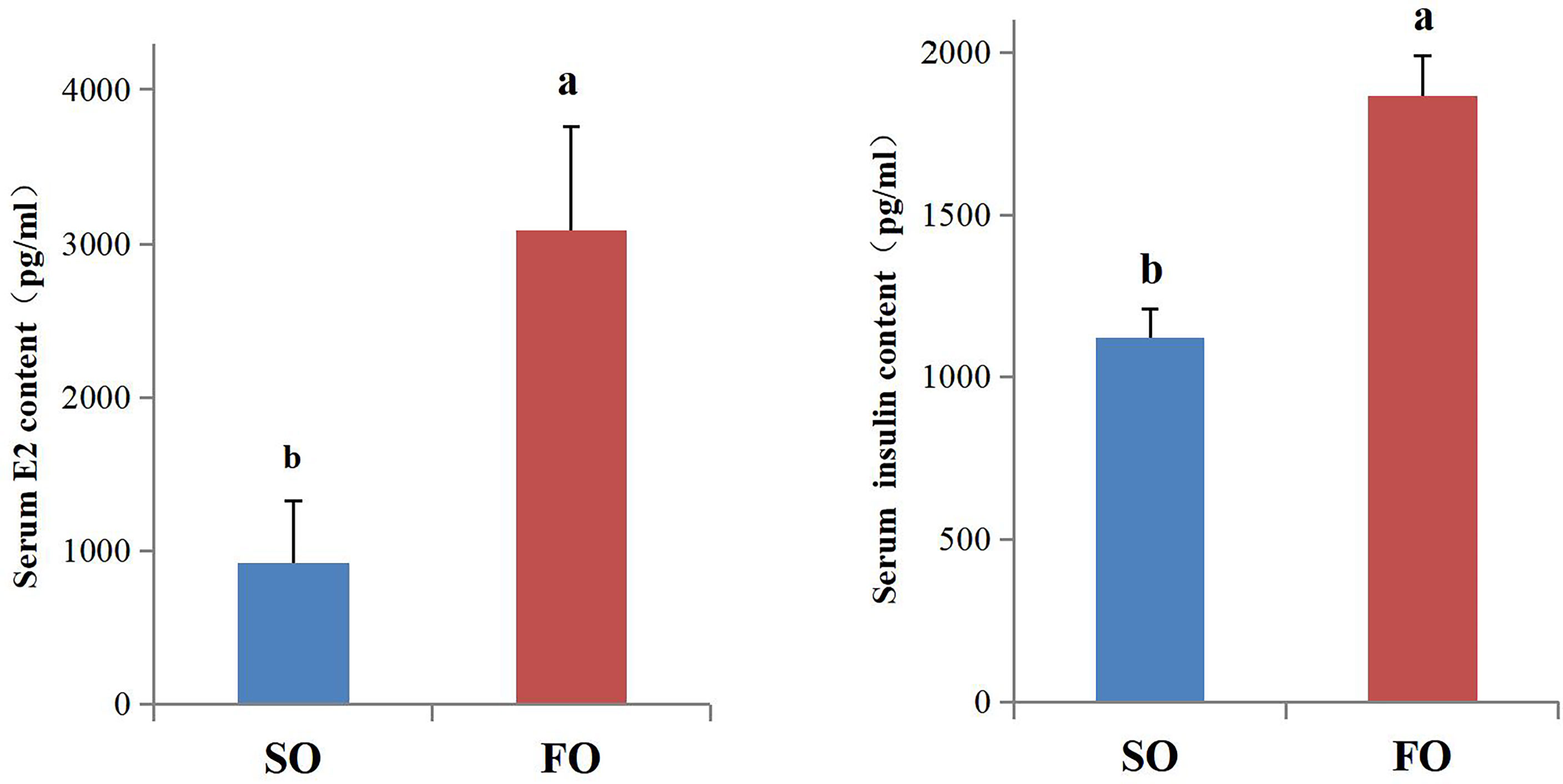
Figure 6 Serum E2 and insulin content of the female adult spotted scat fed with different oil sources. Data are presented as means ± SE. (n = 10). Different letters denote statistically significant differences (P < 0.05), analyzed using T-test for independent samples.
Discussion
Growth performance and ovarian development in spotted scat
Herein, the fish growth performances of the FO and SO groups after 60 days of culturing trial were similar. This study has revealed that supplementation of soybean oil, which is rich in oleic and linoleic acid, as the sole dietary lipid source resulted in comparable fish growth performance to the fish oil-based diet. Spotted scat is omnivorous and prefers algae as food (Sivan and Radhakrishnan, 2011). Its dietary requirement for fish oil level might be relatively low for normal growth. In addition, the residual fish oil from the fish meal in the SO group might be enough and meet the minimum dietary requirements for n-3 LC-PUFA.
The spotted scat GSI values correlate with the gonadal maturation (Gandhi et al., 2014). Gonadal maturation is associated with lipid accumulation and changes in the ratios of lipid classes during gonadal development (Perez et al., 2007). In wild Japanese catfish (Silurus asotus), the phosphatidylethanolamine and phosphatidylcholine from ovary in the spawning season contained more EPA and DHA (Shiraia et al., 2001). The high level of the ovarian n-3 LC-PUFA indicated that it is critical for the ovary development in fish. Dietary lipids can alter the fatty acid composition of follicular fluid, cumulus cells and oocytes (Dunning et al., 2014). Ewe diets containing n-3 PUFA fish oil or n-6 PUFA vegetable oil increased the contents of n-3 PUFA or n-6 PUFA composition of both granulosa cells and oocytes, respectively (Wonnacott et al., 2010). Consistently, n-3 LC-PUFA in the ovary of the FO group were significantly higher than those in the SO group, indicating that fish oil was absorbed into the ovaries. The increasing GSI and ovarian n-3 LC-PUFA level in the FO group indicated that dietary fish oil is beneficial for ovary maturation in spotted scat.
Dietary fish oil affected steroid hormone metabolism and reproduction -related genes expressions in the ovary
Different from the previous study, the fish oil supplementation might increase the serum E2 level via up-regulating the ovarian Cyp19a1a gene expression (Peng et al., 2015). In the present study, the Cyp19a1a expression levels were not different between the FO group and SO group, indicating that the up-regulation of the serum E2 in the FO group is due to other reasons. In human, estrogens are removed from the body by metabolic conversion to estrogenically inactive metabolites excreted in the urine and/or feces (Tsuchiya et al., 2005). Cyp3a activities provide biochemical protection against bioaccumulation of endogenous and exogenous lipophilic compounds to toxic levels (McArthur et al., 2003). CYP3A4 metabolizes many steroids including E2 and estrone (Pikuleva, 2006). In the endometrium, estrogen down-regulates CYP3A4 and CYP3A43 in humans. In addition, the mRNA expression of CYP3A subfamily increased in the liver with age (Williams et al., 2004). Studies have shown a similar structural functional relationship between human CYP3A4 and rainbow trout (Oncorhynchus mykiss) Cyp3a27 (Lee and Buhler, 2002). The highest expression sites of the Cyp3a27 mRNA in 2 year-old female was the upper small intestine, ovary, liver and stomach, respectively. Female rainbow trout expressed considerably more Cyp3a27 mRNA than males (Lee et al., 1998). Female rainbow trout liver was analyzed, and Cyp3a27 mRNA and protein levels decreased in the E2-treated group (Buhler et al., 2000). In this experiment, the expression of Cyp3a27 in the ovary of the FO group was significantly decreased, while the content of serum E2 was significantly increased in FO group. The up-regulation of the serum E2 level in the FO group might be attributed to the decreasing of Cyp3a27 expression which leads to the degradation of E2 restricted. 1.5-year-old chickens were fed the 15% flaxseed-supplemented (rich in linolenic acid) diet, and Cyp3a4 significantly decreased in the ovaries (Dikshit et al., 2015). In an in vitro study using rat intestinal microsomes, the intestinal Cyp3a activity was inhibited by DHA (Hirunpanich et al., 2008). Cyp3a27 mRNA level might be also decreased by the dietary DHA in spotted scat, while the detailed mechanisms remain to be elucidated.
Peroxisome proliferator-activated receptors (Pparα, Pparβ and Pparγ) are a family of nuclear receptors activated by natural binding of ligands, such as PUFA or synthetic ligands. Ppars are expressed in different sections of the reproductive system (pituitary, hypothalamus, testis, ovary and uterus). A strong expression of Pparγ has been described in ovarian granulosa cells (Froment et al., 2006). In human granulosa cells, Bisphenol A induces PPARγ to mediate the down-regulation of FSH-stimulated E2 (Kwintkiewicz et al., 2010). During all tested stages of the estrous cycle and pregnancy, Pparγ activator reduces E2 secretion by the porcine corpora lutea explants (Kurzynska et al., 2014). In cattle, Pparγ activation blocked follicle development (Ferst et al., 2020). In mammals, Pparγ participate in follicular atresia by influencing β-oxidation of fatty acids, synthesis of cholesterol and sex steroid hormones (Cheng et al., 2021). In this experiment, transcriptome and real-time PCR results showed that Pparγ in FO group decreased significantly, and negatively correlated with the serum E2 levels. Thereafter the Pparγ might be under the regulation of LC-HUFA in the fish oil and might also affect the E2 level in spotted scat.
Studies have shown that pancreatic β-cell-specific Pparγ overexpression in diet-induced obese mice increased obesity-induced glucose intolerance with increased islet cell apoptosis, decreased β-cell mass and serum insulin (Hogh et al., 2014). The insulin signaling pathway has been demonstrated to positively control vitellogenesis and oocyte growth in insects (Badisco et al., 2013). Reduction in the Caenorhabditis elegans insulin signaling activates Daf-16/Foxo, which reduce the transcription of intestinal and germline genes required to transport PUFAs to oocytes in lipoprotein complexes (Edmonds et al., 2010). In addition, in this experiment serum insulin levels were elevated in the FO group. Thus, the Pparγ expression in the pancreatic tissues and the possible role of insulin in the ovary development should also be studied further in spotted scat in the future.
The Activator Protein 1 (AP-1) is a dimeric protein complex of leucine zipper transcription factors. Three Jun proteins (Junb, c-Jun, Jund) and four Fos proteins (Fosb, c-Fos, Fra-1, Fra-2) form AP-1 dimer (Hasenfuss et al., 2014). In the human ovary, FOS expression and its heterodimeric binding partners JUN, JUNB, and JUND increase in periovulatory follicles (Choi et al., 2021). FOS, JUNB and JUND expression drastically increased in ovulatory follicles after HCG administration in humans (Choi et al., 2018). The up-regulation of Jun and Jund in the FO group indicates that the maturity level of the ovary is relatively higher and consistent with the GSI and serum E2 level in the FO group.
Stromal-derived factor-1 (Sdf1, aka Cxcl12) is a chemokine expressed in ovaries and serves as the ligand to the Cxcr4 receptor (Rojo et al., 2018). The autocrine expression of the chemokine Sdf1-α and its receptor Cxcr4 in the female gamete during the early developmental and perinatal period of the female mouse germ cell development indicates that the signaling axis may play a vital role in impeding follicle activation (Holt et al., 2006). The ovary expression of Sdf-1/Cxcr4 was increased in premature ovarian failure mice (Luo et al., 2017). In this experiment, the expression level of Cxcl12 in the SO group increased significantly, indicating that the ovaries of the SO group may have a higher possibility of premature ovarian failure.
Dietary fish oil affected the expression of lipid and glucose metabolism related genes in the ovary
Physiological levels of reactive oxygen species (ROS) in females play a significant role in regulating various signaling transduction pathways in oocyte maturation, folliculogenesis, luteolysis, endometrial cycle, embryogenesis, implantation and pregnancy (Agarwal et al., 2008). Aldehyde dehydrogenase 3 member A2 (Aldh3a2), is a ubiquitous enzyme involved in the detoxification of lipids. One of the cellular responses to lipid peroxidation is to increase the expression of Aldh3a2 enzyme in response to ROS. In women with no ovarian pathology, ALDH3A2 expression in granulosa-lutein cells positively correlates with age and negatively with the number of total and mature oocytes (Gonzalez-Fernandez et al., 2016). In mice liver Aldh3a2 was up-regulated in the high-fat-corn oil diet compared to high fat diet-EPA/DHA and control at 8 weeks (Soni et al., 2015). Consistently, the expression level of Aldh3a2 in the ovary was significantly reduced in FO group. The lower expression of Aldh3a2 in FO group might indicate that the oxidative stress in the ovary of SO group was more substantial than that of the FO group.
Perilipin2 (Plin2) belongs to the PAT family and participates in lipid droplet formation in the liver and other tissues (Takahashi et al., 2016). The decrease in the expression of Plin2 enhanced triglyceride hydrolysis through an increase in the access of lipase to the surface of lipid droplets, resulting in the release of more fatty acids for β-oxidation to support porcine oocyte maturation. Plin2 protein is a potential marker for cellular lipid metabolism during oocytes maturation in porcine (Zhang et al., 2014). In mice, there were significant increases in cytosolic Plin2 in the fat cake fraction in omental/mesenteric fat from ovariectomy, demonstrating that the signaling mechanisms that regulate basal and stimulate lipolytic function were impaired in ovariectomy (Wohlers et al., 2011). Supplementing overweight and obese women with n–3 LC-PUFA during pregnancy, the mRNA expression of placental PPARγ and its target genes PLIN2 significantly decreased, and the ability of the placenta to esterify and store lipids were inhibited (Calabuig-Navarro et al., 2016). Herein, Pparγ and Plin2 were both decreased in the FO group indicating that both of them might be regulated by the dietary n-3 LC-PUFA in spotted scat. The Pparγ and Plin2 might influence the lipid metabolism in the ovary of spotted scat. On the other hand, cellular glucose uptake mediated by insulin negatively correlated with Plin2 expression in mouse myoblasts in vitro (Cho and Kang, 2015). As Plin2 decreased in the ovary and serum insulin increased, the insulin-mediated cellular glucose uptake in the ovary might be enhanced, thereafter promoting the ovary development in the FO group in the present study.
During the meiotic process in pig oocytes, Kif5b protein transports molecules and organelles moving along the microtubules (Brevini et al., 2007). Adipose-specific Kif5b knockout mice had hyperlipidemia and significant glucose intolerance and insulin resistance aggravated by the harmful impact of a high-fat diet on body weight gain. These changes influenced insulin signaling, and increased serum Leptin level (Cui et al., 2017). The expression level of Kif5b in the FO group was significantly increased. Since there are few studies on Kif5b in fish, the authors speculated that its up-regulation might be helpful for the glucose and lipid metabolism in the FO group.
Conclusion
Feeding fish oil-rich diet can promote n-3 LC-PUFA precipitation in the ovary of adult spotted scat, improve GSI, and promote ovary maturation. The serum E2 and insulin levels of the FO group were significantly higher than that of the SO group. The down-regulation of Cyp3a27 in the FO group might be the reason for the E2 up-regulation. Several genes involved in lipids and glucose metabolism were also differentially expressed between the FO and SO groups. The present study help understand the mechanism of fish oil promoting the maturation of oocytes in spotted scat.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA83328.
Ethics statement
The animal study was reviewed and approved by Guangdong Ocean University’s Committee on the Ethics of Animal Experiments.
Author contributions
D-NJ and TW designed and took part in the whole process of the experiment, and wrote the draft of this manuscript. Z-LL, UFM and CBN revised the draft. Z-LL, H-JS, C-HZ, H-PC, and YH participated in the experiments. D-NJ and G-LL supervised the experiments. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (32172971); Guangdong Basic and Applied Basic Research Foundation (2021A1515010430); the Key Project of “Blue Granary Science and Technology Innovation” of the Ministry of Science and Technology (2018YFD0901203).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.935968/full#supplementary-material
References
Afman L. A., Müller M. (2012). Human nutrigenomics of gene regulation by dietary fatty acids. Prog. Lipid. Res. 51, 63–70. doi: 10.1016/j.plipres.2011.11.005
Agarwal A., Gupta S., Sekhon L., Shah R. (2008). Redox considerations in female reproductive function and assisted reproduction: From molecular mechanisms to health implications. Antioxid. Redox Sign. 10 (8), 1375–1404. doi: 10.1089/ars.2007.1964
Araújo F. G., Costa D. V., Machado M. R. F., Paulino R. R., Okamura D., Rosa P. V. (2016). Dietary oils influence ovary and carcass composition and embryonic development of zebrafish. Aquacult. Nutr. 23, 651–661. doi: 10.1111/anu.12432
Badisco L., Van Wielendaele P., Vanden Broeck J. (2013). Eat to reproduce: A key role for the insulin signaling pathway in adult insects. Front. Physiol. 4. doi: 10.3389/fphys.2013.00202
Barry T. P., Fast A. W. (1992). Biology of the spotted scat (Scatophagus argus) in the Philippines. Asian. Fish. Sci. 5, 163–179.
Brevini T. A. L., Cillo F., Antonini S., Gandolfi F. (2007). Cytoplasmic remodelling and the acquisition of developmental competence in pig oocytes. Anim. Reprod. Sci. 98 (1-2), 23–38. doi: 10.1016/j.anireprosci.2006.10.018
Buhler D. R., Miranda C. L., Henderson M. C., Yang Y. H., Lee S. J., Wang-Buhler J. L. (2000). Effects of 17β-estradiol and testosterone on hepatic mRNA/protein levels and catalytic activities of CYP2M1, CYP2K1, and CYP3A27 in rainbow trout (Oncorhynchus mykiss). Toxicol. Appl. Pharmacol. 168 (2), 91–101. doi: 10.1006/taap.1999.9016
Calabuig-Navarro V., Puchowicz M., Glazebrook P., Haghiac M., Minium J., Catalano P., et al. (2016). Effect of ω-3 supplementation on placental lipid metabolism in overweight and obese women. Am. J. Clin. Nutr. 103 (4), 1064–1072. doi: 10.3945/ajcn.115.124651
Cheng J., Pan Y., Yang S., Wei Y., Lv Q., Xing Q., et al. (2021). Integration of transcriptomics and non-targeted metabolomics reveals the underlying mechanism of follicular atresia in Chinese buffalo. J. Steroid. Biochem. Mol. Biol. 212, 105944. doi: 10.1016/j.jsbmb.2021.105944
Choi Y., Jeon H., Akin J. W., Curry T. E., Jo M. (2021). The FOS/AP-1 regulates metabolic changes and cholesterol synthesis in human periovulatory granulosa cells. Endocrinology 162 (9), 1–18. doi: 10.1210/endocr/bqab127
Choi Y., Rosewell K. L., Brannstrom M., Akin J. W., Curry T. E. Jr., Jo M. (2018). FOS, a critical downstream mediator of PGR and EGF signaling necessary for ovulatory prostaglandins in the human ovary. J. Clin. Endocr. Metab. 103 (11), 4241–4252. doi: 10.1210/jc.2017-02532
Cho K. A., Kang P. B. (2015). PLIN2 inhibits insulin-induced glucose uptake in myoblasts through the activation of the NLRP3 inflammasome. Int. J. Mol. Med. 36 (3), 839–844. doi: 10.3892/ijmm.2015.2276
Cui J., Pang J., Lin Y. J., Gong H., Wang Z. H., Li Y. X., et al. (2017). Adipose-specific deletion of Kif5b exacerbates obesity and insulin resistance in a mouse model of diet-induced obesity. Faseb. J. 31 (6), 2533–2547. doi: 10.1096/fj.201601103R
Da Silva F. F. G., Støttrup J. G., Kjørsvik E., Tveiten H., Tomkiewicz J. (2016). Interactive effects of dietary composition and hormonal treatment on reproductive development of cultured female European eel, Anguilla anguilla. Anim. Reprod. Sci. 171, 17–26. doi: 10.1016/j.anireprosci.2016.05.007
De Santis C., Bartie K. L., Olsen R. E., Taggart J. B., Tocher D. R. (2015). Nutrigenomic profiling of transcriptional processes affected in liver and distal intestine in response to a soybean meal-induced nutritional stress in Atlantic salmon (Salmo salar). Comp. Biochem. Phys. D. 15, 1–11. doi: 10.1016/j.cbd.2015.04.001
Dikshit A., Gomes Filho M. A., Eilati E., McGee S., Small C., Gao C., et al. (2015). Flaxseed reduces the pro-carcinogenic micro-environment in the ovaries of normal hens by altering the PG and oestrogen pathways in a dose-dependent manner. Br. J. Nutr. 113 (9), 1384–1395. doi: 10.1017/S000711451500029X
Dunning K. R., Russell D. L., Robker R. L. (2014). Lipids and oocyte developmental competence: The role of fatty acids and β-oxidation. Reproduction 148 (1), R15–R27. doi: 10.1530/REP-13-0251
Edmonds J. W., Prasain J. K., Dorand D., Yang Y., Hoang H. D., Vibbert J., et al. (2010). Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev. Cell. 19 (6), 858–871. doi: 10.1016/j.devcel.2010.11.005
Ferst J. G., Rovani M. T., Dau A. M. P., Gasperin B. G., Antoniazzi A. Q., Bordignon V., et al. (2020). Activation of PPARG inhibits dominant follicle development in cattle. Theriogenology 142, 276–283. doi: 10.1016/j.theriogenology.2019.10.032
Froment P., Gizard F., Defever D., Staels B., Dupont J., Monget P. (2006). Peroxisome proliferator-activated receptors in reproductive tissues: from gametogenesis to parturition. J. Endocrinol. 189 (2), 199–209. doi: 10.1677/joe.1.06667
Gandhi V., Venkatesan V., Ramamoorthy N. (2014). Reproductive biology of the spotted scat scatophagus argus (Linnaeu) from mandapam waters, south-east coast of India. Indian J. Fish. 61 (4), 55–59.
Gonzalez-Fernandez R., Hernandez J., Martin-Vasallo P., Puopolo M., Palumbo A., Avila J. (2016). Expression levels of the oxidative stress response gene ALDH3A2 in granulosa-lutein cells are related to female age and infertility diagnosis. Reprod. Sci. 23 (5), 604–609. doi: 10.1177/1933719115607996
Gupta S. (2016). An overview on morphology, biology, and culture of spotted scat scatophagus argus (Linnaeus 1766). Rev. Fish. Sci. Aquac. 24 (2), 203–212. doi: 10.1080/23308249.2015.1119800
Hasenfuss S. C., Bakiri L., Thomsen M. K., Williams E. G., Auwerx J., Wagner E. F. (2014). Regulation of steatohepatitis and PPARgamma signaling by distinct AP-1 dimers. Cell. Metab. 19 (1), 84–95. doi: 10.1016/j.cmet.2013.11.018
Hilbig C. C., Nascimento N., Heinen A. L., Neto A. T., Nakaghi L. (2019). Effects of dietary fatty acids on the reproduction of South American female catfish Rhamdia quelen (quoy & gaimard 1824). Lat. Am. J. Aquat. Res. 47 (3), 456–466. doi: 10.3856/vol47-issue3-fulltext-8
Hirunpanich V., Murakoso K., Sato H. (2008). Inhibitory effect of docosahexaenoic acid (DHA) on the intestinal metabolism of midazolam: In vitro and in vivo studies in rats. Int. J. Pharm. 351 (1-2), 133–143. doi: 10.1016/j.ijpharm.2007.09.037
Hogh K. L., Craig M. N., Uy C. E., Nygren H., Asadi A., Speck M., et al. (2014). Overexpression of PPARγ specifically in pancreatic beta-cells exacerbates obesity-induced glucose intolerance, reduces beta-cell mass, and alters islet lipid metabolism in male mice. Endocrinology 155 (10), 3843–3852. doi: 10.1210/en.2014-1076
Holt J. E., Jackson A., Roman S. D., Aitken R. J., Koopman P., McLaughlin E. A. (2006). CXCR4/SDF1 interaction inhibits the primordial to primary follicle transition in the neonatal mouse ovary. Dev. Biol. 293 (2), 449–460. doi: 10.1016/j.ydbio.2006.02.012
Huang Y., Mustapha U. F., Huang Y., Tian C., Yang W., Chen H., et al. (2021). A chromosome-level genome assembly of the spotted scat (Scatophagus argus). Genome. Biol. Evol. 13 (6), evab092. doi: 10.1093/gbe/evab092
Izquierdo M. S., Fernandez-Palacios H., Tacon A. G. J. (2001). Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 197 (1-4), 25–42. doi: 10.1016/B978-0-444-50913-0.50006-0
Kime D. E. (1993). "Classical" and "non-classical" reproductive steroids in fish. Rev. Fish. Biol. Fisher. 3 (2), 160–180. doi: 10.1007/BF00045230
Kurzynska A., Bogacki M., Chojnowska K., Bogacka I. (2014). Peroxisome proliferator activated receptor ligands affect progesterone and 17β-estradiol secretion by porcine corpus luteum during early pregnancy. J. Physiol. Pharmacol. 65 (5), 709–717.
Kwintkiewicz J., Nishi Y., Yanase T., Giudice L. C. (2010). Peroxisome proliferator-activated receptor-γ mediates bisphenol a inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ. Health Perspect. 118 (3), 400–406. doi: 10.1289/ehp.0901161
Lee S. J., Buhler D. R. (2002). Functional properties of a rainbow trout cyp3A27 expressed by recombinant baculovirus in insect cells. Drug Metab. Dispos. 30 (12), 1406–1412. doi: 10.1124/dmd.30.12.1406
Lee S. J., Wang-Buhler J.-L., Cok I., Yu T.-S., Yang Y.-H., Miranda C. L., et al. (1998). Cloning, sequencing, and tissue expression of CYP3A27, a new member of the CYP3A subfamily from embryonic and adult rainbow trout livers. Arch. Biochem. Biophys. 360 (1), 56–61. doi: 10.1006/abbi.1998.0943
Leng X. Q., Zhou H., Tan Q. S., Du H., Wu J. P., Liang X. F., et al. (2019). Integrated metabolomic and transcriptomic analyses suggest that high dietary lipid levels facilitate ovary development through the enhanced arachidonic acid metabolism, cholesterol biosynthesis and steroid hormone synthesis in Chinese sturgeon (Acipenser sinensis). Brit. J. Nutr. 122, 1230–1241. doi: 10.1017/S0007114519002010
Luo L., Ai L., Li T., Xue M., Wang J., Li W., et al. (2015). The impact of dietary DHA/EPA ratio on spawning performance, egg and offspring quality in Siberian sturgeon (Acipenser baeri). Aquaculture, 437, 140–145. doi: 10.1016/j.aquaculture.2014.11.036
Luo Q., Yin N., Zhang L., Yuan W., Zhao W., Luan X., et al. (2017). Role of SDF-1/CXCR4 and cytokines in the development of ovary injury in chemotherapy drug induced premature ovarian failure mice. Life Sci. 179, 103–109. doi: 10.1016/j.lfs.2017.05.001
McArthur A. G., Hegelund T., Cox R. L., Stegeman J. J., Liljenberg M., Olsson U., et al. (2003). Phylogenetic analysis of the cytochrome P450 3 (CYP3) gene family. J. Mol. Evol. 57 (2), 200–211. doi: 10.1007/s00239-003-2466-x
Mustapha U. F., Huang Y., Huang Y.-Q., Assan D., Shi H.-J., Jiang M.-Y., et al. (2021). Gonadal development and molecular analysis revealed the critical window for sex differentiation, and E2 reversibility of XY-male spotted scat, Scatophagus argus. Aquaculture 544, 737147. doi: 10.1016/j.aquaculture.2021.737147
Pawlak P., Malyszka N., Szczerbal I., Kolodziejski P. (2020). Fatty acid induced lipolysis influences embryo development, gene expression and lipid droplet formation in the porcine cumulus cellsdagger. Biol. Reprod. 103 (1), 36–48. doi: 10.1093/biolre/ioaa045
Peng S., Gao Q., Shi Z., Zhang C., Wang J., Yin F., et al. (2015). Effect of dietary n–3 LC-PUFAs on plasma vitellogenin, sex steroids, and ovarian steroidogenesis during vitellogenesis in female silver pomfret (Pampus argenteus) broodstock. Aquaculture 444, 93–98. doi: 10.1016/j.aquaculture.2015.03.031
Perez M. J., Rodriguez C., Cejas J. R., Martin M. V., Jerez S., Lorenzo A. (2007). Lipid and fatty acid content in wild white seabream (Diplodus sargus) broodstock at different stages of the reproductive cycle. Comp. Biochem. Phys. B. 146 (2), 187–196. doi: 10.1016/j.cbpb.2006.10.097
Pikuleva I. A. (2006). Cytochrome P450s and cholesterol homeostasis. Pharmacol. Therapeut. 112 (3), 761–773. doi: 10.1016/j.pharmthera.2006.05.014
Rojo J. L., Linari M., Young K. A., Peluffo M. C. (2018). Stromal-derived factor 1 directly promotes genes expressed within the ovulatory cascade in feline cumulus oocyte complexes. J. Assist. Reprod. Genet. 35 (5), 785–792. doi: 10.1007/s10815-018-1150-4
Shiraia N., Suzuki H., Toukairinc S., Wadaa S. (2001). Spawning and season affect lipid content and fatty acid composition of ovary and liver in Japanese catfish (Silurus asotus). Comp. Biochem. Phys. B. 129, 185–195. doi: 10.1016/S1096-4959(01)00378-5
Sivan G., Radhakrishnan C. K. (2011). Food, feeding habits and biochemical composition of Scatophagus argus. Turk. J. Fish. Aquat. Sc. 11, 603–608. doi: 10.4194/1303-2712-v11_4_14
Song Y. F., Tan X. Y., Pan Y. X., Zhang L. H., Chen Q. L. (2018). Fatty acidβ-oxidation is essential in leptin-mediated oocytes maturation of yellow catfish pelteobagrus fulvidraco. Int. J. Mol. Sci. 19 (5), 1457. doi: 10.3390/ijms19051457
Soni N. K., Nookaew I., Sandberg A.-S., Gabrielsson B. G. (2015). Eicosapentaenoic and docosahexaenoic acid-enriched high fat diet delays the development of fatty liver in mice. Lipids Health Dis. 14, 74. doi: 10.1186/s12944-015-0072-8
Takahashi Y., Shinoda A., Kamada H., Shimizu M., Inoue J., Sato R. (2016). Perilipin2 plays a positive role in adipocytes during lipolysis by escaping proteasomal degradation. Sci. Rep. 6, 20975. doi: 10.1038/srep20975
Tocher D. R. (2015). Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449, 94–107. doi: 10.1016/j.aquaculture.2015.01.010
Tsuchiya Y., Nakajima M., Yokoi T. (2005). Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 227 (2), 115–124. doi: 10.1016/j.canlet.2004.10.007
Wang T., Jiang D. N., Shi H. J., Mustapha U. F., Deng S. P., Liu Z. L., et al. (2021). Liver transcriptomic analysis of the effects of dietary fish oil revealed a regulated expression pattern of genes in adult female spotted scat (Scatophagus argus). Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.784845
Williams E. T., Leyk M., Wrighton S. A., Davies P. J., Loose D. S., Shipley G. L., et al. (2004). Estrogen regulation of the cytochrome P450 3A subfamily in humans. J. Pharmacol. Exp. Ther. 311 (2), 728–735. doi: 10.1124/jpet.104.068908
Wohlers L. M., Jackson K. C., Spangenburg E. E. (2011). Lipolytic signaling in response to acute exercise is altered in female mice following ovariectomy. J. Cell. Biochem. 112 (12), 3675–3684. doi: 10.1002/jcb.23302
Wonnacott K. E., Kwong W. Y., Hughes J., Salter A. M., Lea R. G., Garnsworthy P. C., et al. (2010). Dietary omega-3 and -6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Reproduction 139 (1), 57–69. doi: 10.1530/REP-09-0219
Keywords: spotted scat, adult female, fish oil, ovary, transcriptomic, estrogen
Citation: Wang T, Liu Z-L, Li G-L, Mustapha UF, Ndandala CB, Shi H-J, Zhu C-H, Chen H-P, Huang Y and Jiang D-N (2022) Ovary transcriptomic analysis reveals regulation effects of dietary fish oil on hormone, lipid, and glucose metabolism in female adult spotted scat (Scatophagus argus). Front. Mar. Sci. 9:935968. doi: 10.3389/fmars.2022.935968
Received: 04 May 2022; Accepted: 20 July 2022;
Published: 08 August 2022.
Edited by:
Eduardo Almansa, Spanish Institute of Oceanography (IEO), SpainReviewed by:
Yao Zheng, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, ChinaTao Han, Zhejiang Ocean University, China
Copyright © 2022 Wang, Liu, Li, Mustapha, Ndandala, Shi, Zhu, Chen, Huang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Neng Jiang, ZG5qaWFuZ0BnZG91LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Tuo Wang1†
Tuo Wang1† Guang-Li Li
Guang-Li Li Umar Farouk Mustapha
Umar Farouk Mustapha Hong-Juan Shi
Hong-Juan Shi Chun-Hua Zhu
Chun-Hua Zhu Dong-Neng Jiang
Dong-Neng Jiang