
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 06 January 2023
Sec. Marine Ecosystem Ecology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.935324
 Igal Berenshtein1,2,3,4*
Igal Berenshtein1,2,3,4* Skyler R. Sagarese3*
Skyler R. Sagarese3* Matthew V. Lauretta3
Matthew V. Lauretta3 Amy M. Schueller5
Amy M. Schueller5 David D. Chagaris6*
David D. Chagaris6*Gulf menhaden (Brevoortia patronus) support the largest fishery by yield in the Gulf of Mexico (GoM) and are a key forage species for many marine predators. While menhaden stock assessments indicated that overfishing was not likely to have occurred in the past, concerns have been raised regarding the possible effects of menhaden fishing on their predators. In this study, we used a US Gulfwide Ecopath with Ecosim (EwE) model to explore the predicted effects of increased menhaden harvest on the GoM ecosystem and focused our analyses on Gulf menhaden predators. Key menhaden predators identified included king mackerel (Scomberomorus cavalla), Spanish mackerel (Scomberomorus maculatus), sea trout (Cynoscion spp.), red drum (Sciaenops ocellatus), and pelagic coastal piscivores [e.g., bluefish (Pomatomus saltatrix)]. As expected, these predators exhibited reduced biomass in response to increased Gulf menhaden harvest, with a predicted 11% decrease in predator biomass at simulated fishing levels near historical highs. Our results indicate strong relationships between the effects of menhaden fishing and the predator fishing mortality for king mackerel and intermediate relationships for Spanish mackerel, blacktip shark (Carcharhinus limbatus), red drum, large coastal sharks, and pelagic coastal piscivores. Biomass of predator groups such as demersal coastal invertebrate feeders [e.g., drums and croakers (Sciaenidae)] are more affected by menhaden harvest (through trophodynamics interactions and bycatch removal) compared to the isolated effect of their fishing mortality. For almost all the groups examined in the trade-off analysis, with the exception of sea trout, current biomass (2016) was higher than their target biomass representing 75% of their biomass at maximum sustainable yield. In comparison to the time series of fishing mortality rates estimated by the most recent Gulf menhaden stock assessment, the mean ecological reference point (ERP) of 0.862 was exceeded in all but 1 year from 1977 to 2007; however, neither the target nor threshold upper ERP value has been exceeded since 2008. The observed Gulf menhaden landings from 2003 to the present were generally within the range of the projected equilibrium landings (i.e., within confidence intervals) at both the ERP target and threshold values except for three recent years.
Forage species provide key food-web linkages in marine ecosystems, directly and indirectly sustaining predators and fisheries (Pikitch et al., 2014; Robinson et al., 2014; Siple et al., 2019). Forage species are commonly defined by their ecological importance to upper-trophic-level predators (Pikitch et al., 2012), although the reliance on forage species as prey can vary considerably from predator to predator (e.g., due to prey switching). An analysis of more than 70 ecosystem models worldwide found that forage species make up 50% and 75% of diets for at least one predator in 75% and 29% of ecosystems examined, respectively (Pikitch et al., 2012). At the same time, forage fish represent nearly 30% of total global landings (Tacon and Metian, 2009). The important role of forage fish as prey as well as fishery harvest warrants a detailed examination of the effect of their harvest on ecosystem dynamics and the sustainability of other fishery stocks.
Considerable debate remains surrounding the effects of forage fish harvest on predator populations (Hilborn et al., 2017; Pikitch et al., 2018). While examples of strong linkages between forage fish and the abundance of predators have been identified and reported for numerous ecosystems throughout the world (reviewed in Pikitch et al., 2012; Pikitch et al., 2018), other studies have concluded that the abundance of forage fish has little impact on predators (Hilborn et al., 2017; Free et al., 2021). Hilborn et al. (2017) cautioned the use of ecosystem models for evaluating the relationships between predators and forage fish (as done in Pikitch et al., 2012) and instead focused efforts on evaluating empirical relationships between assessment model-derived abundance of forage fish and predators for United States (US) fisheries (Hilborn et al., 2017). They found that connections between forage fish abundance and the rate of change in the abundance of their predators broke down after accounting for the high variability in forage fish dynamics, the weak relationship between forage fish recruitment and spawning stock biomass (along with the role of environmental productivity regimes), prey size selectivity of predators compared to fisheries selectivity, and spatial distributions of forage fish. While their conclusions continue to be debated (Pikitch et al., 2018), there is consensus that a case-specific approach is necessary to evaluate the impact of forage fish removal on marine ecosystems (Hilborn et al., 2018; Pikitch et al., 2018).
Menhaden (Brevortia spp.) of the family Clupeidae are important fishes in both the Atlantic Ocean and Gulf of Mexico (GoM) because of their economic and ecological value (SEDAR (Southeast Data Assessment and Review), 2018; SEDAR (Southeast Data Assessment and Review), 2020). Within the GoM, Gulf menhaden (B. patronus) support the second largest commercial fishery in the US by weight (NMFS, 2021). A commercial “reduction” fishery uses large purse seines to harvest an average of 600,000 metric tons of Gulf menhaden each year (SEDAR (Southeast Data Assessment and Review), 2018), with record landings in the 1980s reaching 982,800 metric tons and the lowest landings of 387,200 in 2010 due to fishing closures following BP’s Deep Water Horizon Oil Spill (SEDAR (Southeast Data Assessment and Review), 2018). Menhaden are processed into fish meal, oil, and fish soluble that are used in domestic animal feeds, as lubrication components, and for human consumption (Smith, 1991). Menhaden form massive schools, making them relatively easy to target and, due to their high oil content, produce up to 97% of US fish oil production and a variety of fish meal products (Hale et al., 1991; Alder et al., 2008).
As a forage species, menhaden provide sustenance for higher trophic level predators such as mackerels, sharks, and coastal fishes (Geers et al., 2016; Sagarese et al., 2016; Leaf and Oshima, 2019), and thus provide indirect ecosystem services supporting multiple commercial and recreational fisheries. The Gulf menhaden Regional Management Plan recognized their importance to the ecosystem, but highlighted the lack of understanding of their trophic role (GSMFC (Gulf States Marine Fisheries Commission), 2015). The effect of forage species harvest on predators is likely dependent on the biomass of predator populations, such that when predators are highly abundant, there is more demand for food, and they are more sensitive to forage removals. Maintaining predators near their biomass target may therefore require more available forage than if they were maintained in a depleted state, and this must be considered when developing ecological reference points (ERPs).
Ecosystem models represent key tools to elucidate trade-offs between species, thereby enabling the management of fisheries in a more holistic manner as emphasized in the federal Reauthorized Magnuson-Stevens Fishery Conservation and Management Act (MSFCMA (Magnuson–Stevens Fishery Conservation and Management Act), 2007). These models integrate multiple data sources to evaluate ecosystem-based fisheries management options (EBFM; Pikitch et al., 2004), guide focused research on dominant species interactions, and provide insight into possible unforeseen and adverse outcomes. The recent development of ERPs for Atlantic menhaden (B. tyrannus) using an ecosystem model of intermediate complexity provides a success story on incorporating quantitative ecosystem considerations into fisheries management (Chagaris et al., 2020; Anstead et al., 2021; Drew et al., 2021; Howell et al., 2021). These ERPs were based on the trade-off relationship between menhaden harvest and their predator biomass, when the predator was fished at their own target fishing mortality rate (Chagaris et al., 2020). That is, the ERP fishing mortality rates of the forage species should not impede the ability to achieve single-species targets of the predator.
Management objectives for Gulf menhaden are explicit about the need to account for their role in the ecosystem, and managers have called for development of maximum sustainable yield (MSY)-based reference points that consider predator demands and an improved understanding of the cumulative impacts of freshwater flow, habitat loss, and hypoxia on menhaden population dynamics (Chagaris et al., 2019). The overall goal of this study was to evaluate the effects of menhaden harvest on its predators and develop new management reference points that account for their role as a forage species. The first objective was to develop and calibrate an ecosystem model of the GoM, with a central focus on Gulf menhaden and their predators. Upon satisfactory calibration of the model, the ecosystem effects of alternative fishing policies on menhaden were assessed, with a particular focus on how menhaden predator species would respond to changes in fishing pressure, i.e., fishing mortality (F) of Gulf menhaden (Brevoortia sp.) or purse seine fishing effort. While the purse seine fishery generally targets Gulf menhaden, bycatch species can contribute about 2% to 3% by weight of total landings (e.g., Guillory and Hutton, 1982). Trade-offs between menhaden harvest and predator biomass are identified in order to develop management reference points. Ecological indicators are also presented to enable comparison of ecosystem structure and function across alternative levels of fishing.
The Ecopath with Ecosim (EwE) ecosystem modeling package has been widely used to address a variety of marine resource and ecosystem management challenges (Colléter et al., 2015), including fisheries (Chagaris et al., 2015; Chagaris et al., 2020), invasive species (Chagaris et al., 2017), marine pollution (Walters and Christensen, 2018), habitat alteration (de Mutsert et al., 2021), river discharge and drought conditions (Sinnickson et al., 2021), and climate change (Coll et al., 2020). The modeling framework has been described extensively elsewhere (Christensen and Walters, 2004; Christensen et al., 2005), including best practices (Link, 2010; Heymans et al., 2016), and the software is freely available at www.ecopath.org. Briefly, EwE is an end-to-end trophic–dynamic model that consists of a static food web representation (Ecopath), which contains the initial conditions for dynamic simulations in the Ecosim module. While the complexity of EwE models (i.e., number of modeled species/age classes) is specific to each application and modeled region, all EwE models represent dynamics across several trophic levels, from detritus and primary producers to top predators. Required inputs to Ecopath are biomass, consumption rates, mortality rates, diet compositions, and fisheries removals. Ecosim consists of a set of coupled differential equations that simulate biomass dynamics forward from Ecopath starting conditions, at a monthly time step, based on changes in fishing mortality, predator–prey abundances, and environmental drivers, such as nutrient loading and primary production.
In Ecosim, consumption by predators is modeled based on the foraging arena theory (Ahrens et al., 2012), which states that predation rates are limited by the flux of prey biomass from invulnerable states (resting, hiding) into vulnerable “foraging arenas”. The exchange rate between these states is determined by the Ecosim vulnerability parameter (v), and there is one for each predator–prey interaction. The vulnerability parameters function as upper limits on predation mortality rates, such that high values (>10) assume strong top-down control and allow predators to increase consumption of prey, leading to greater biomass increases of the predator and a reciprocal decline in their prey. Low vulnerability settings assume bottom-up control, which limits consumption (and biomass gains) of predators and keeps predation mortality rates of the prey near the Ecopath baseline value. The vulnerability parameters are highly influential in Ecosim models and can be estimated by fitting models to time series of observed abundance and landings. Ecosim also accounts for bottom-up drivers on primary production using a Michaelis–Menten nutrient uptake model, multipliers on primary production rates, and estimated primary production anomaly multipliers (Christensen et al., 2005).
Ecosim models are especially useful for examining ecosystem impacts of forage species harvest because they simultaneously account for both the bottom-up (food availability) and top-down (predation and fishing) effects on prey and predators. For example, a suite of modeling approaches was developed for Atlantic menhaden (Drew et al., 2021), and EwE was selected for management use because it was the only model capable of evaluating the bottom-up impacts of menhaden harvest on their predators, which is also the key management question for Gulf menhaden. Other approaches such as multispecies surplus production (Uphoff and Sharov, 2018) and statistical catch-at-age models (Curti et al., 2013) are currently only capable of addressing the top-down effect that predators have on forage species (i.e., estimation of predation mortality rates). Additionally, Gulf menhaden filter feed on phytoplankton and particulate organic matter and their dynamics are tightly coupled to the Mississippi River (Grimes, 2001; Leaf, 2017; Adams et al., 2018), which provides nutrients that stimulate phytoplankton growth throughout the area where menhaden are concentrated. Thus, the need to evaluate the effects of menhaden harvest on predators, while also considering changes in primary production and predator harvest rates, makes EwE an appropriate model for this analysis.
The US Gulfwide EwE model focuses on federally (i.e., National Marine Fisheries Service) and internationally [International Commission for the Conservation of Atlantic Tunas (ICCAT)] managed species as well as protected species such as dolphins and seabirds (Supplementary Table 1). The region considered in the model includes the US territorial waters from Brownsville, Texas, to the Florida Keys out to 400 m depth, covering an area of approximately 310,000 km2. While the US Gulfwide EwE model has many structural similarities to the 2005–2009 Ecopath model of Sagarese et al. (2017), the Ecopath model was reparametrized to reflect 1980 conditions and serve as a starting point for Ecosim. In addition, refinements to functional groups (marine mammals and age-structure for key fisheries species) were made following feedback from stakeholders during a 2017 Scoping Workshop (Chagaris et al., 2019) and subsequent discussions. For example, age classes of Gulf menhaden were modeled individually from 0 incrementally to 4+ years to align with the Gulf menhaden stock assessment and better represent recruitment, fishery, and predation processes.
A total of 78 functional groups are represented in the US Gulfwide EwE model, including three marine mammal groups, an aggregate seabird group, an aggregate sea turtle group, eight elasmobranch groups, 52 fish groups (18 of which are subdivided into multiple life stages), nine invertebrate groups, three primary producers, and one detritus group (Supplementary Table 2; detailed in Supplementary Table 1). The diet matrix input into the US Gulfwide Ecopath model was based on a meta-analysis conducted of diet studies obtained from nearly 600 references (Sagarese et al., 2016). Both landings and discards (i.e., bycatch) are modeled where possible for 12 commercial fleets and 4 recreational fleets. The Ecopath model representing the base year of 1980 was mass-balanced and diagnostics were evaluated following approaches in Link (2010) and Darwall et al. (2010). Ecopath diagnostics indicated that model inputs and estimates are biologically realistic. Additional details on model structure, data inputs, parameterization, and diagnostics can be found in Berenshtein et al. (2021).
A considerable portion of the impact of the menhaden purse-seine fishery on predatory species occurs through bycatch. In our Gulfwide Ecopath model for the snapshot year of 1980, we implemented the menhaden purse-seine bycatch on other species through both retained (i.e., landed) and discarded (i.e., released) bycatch. According to previous studies, relative bycatch quantities of the menhaden purse-seine fleet generally account for <2.5% of total catch (Berenshtein et al., 2021). Estimates of purse seine bycatch in units of weight are less common than estimates in units of number, but have ranged from 0.66% by weight (de Silva et al., 1996) to 3.1% by weight (Guillory and Hutton, 1982). Working inside three reduction plants in Louisiana in 1980–1981, Guillory and Hutton (1982) reported that bycatch varied spatially from 0 to 10.5% of total catch weight, with a mean across plants and years of 2.35%. However, they considered these estimates low because large specimens such as sharks and jacks were typically removed from the catch during the harvesting or unloading process and discarded. Although classified as a “small amount of incidental catch” by Guillory and Hutton (1982), a few percent of 700,000 metric tons can still be a substantial amount and negatively affect species since the effect of bycatch on species can differ based on their species-specific abundance and life history.
Retained bycatch of the menhaden purse seine reduction fishery was calculated based on assumptions regarding species composition and magnitude of retained bycatch derived from Guillory and Hutton (1982) and de Silva and Condrey (1997). Using the species composition of retained bycatch by weight from Guillory and Hutton (1982), and assuming 2.5% of Gulf menhaden landings in 1980 by weight were attributed to bycatch, bycatch estimates were allocated by species and summed by functional groups. Since Guillory and Hutton (1982) did not distinguish shark species within the bycatch, species-specific resolution for sharks within the bycatch was based on de Silva et al. (2001), which sampled dead bycatch aboard commercial menhaden fishing vessels. Using the species composition of retained bycatch by weight from de Silva and Condrey (1997), and assuming about 2% of Gulf menhaden landings in 1994–1995 by weight were attributed to bycatch, we allocated bycatch by species and summed them by functional groups. We then scaled these estimates derived from de Silva and Condrey (1997) back to 1980 using the ratio of Gulf menhaden landings between these two time periods, assuming the proportions and species composition would be similar. Lastly, estimates of retained bycatch for each functional group were averaged between both approaches to determine the final estimates [see Berenshtein et al. (2021) for additional details].
Released bycatch computation was based on de Silva and Condrey (1997). First, an average number of released bycatch was determined using the 1994 and 1995 estimates weighted by their respective sample sizes (N1994 = 235 sets; N1995 = 257 sets). Second, the numbers of released bycatch were converted to weights using the average weight of species that were retained. Since these estimates were for 1994–1995, we scaled these estimates back to 1980 using the ratio of Gulf menhaden landings between time periods, under the assumption of static proportions and species compositions [see Berenshtein et al. (2021) for additional details]. Released bycatch was treated as dead discards in Ecopath.
The time-dynamic Ecosim component of EwE was constructed based on reference time series spanning 1980 to 2016. Reference time series (N = 109) included group biomass (relative) and landings and were treated as observed values for model fitting. The majority of the biomass reference time series were obtained directly from stock assessments or monitoring surveys in the absence of stock assessments (Supplementary Table S3). Time series of landings included both commercial and recreational landings (in tons) and estimated retained bycatch from the commercial menhaden purse seine fishery (annual estimates obtained as described as above and rescaled each year). Time series weights were applied to reference time series for biomass and landings (Heymans et al., 2016), such that the weight of each reference time series was calculated as the inverse of the mean coefficient of variation (CV) across all years with data. Importantly, CV per group was computed as the mean CV across years. The standard deviation divided by the mean for each year determines the year-specific CV, and that represents the uncertainty of the year-specific mean estimate. Hence, higher weights were assigned to more precise (i.e., low CV) time series and provided more influence on model fit for these time series. Forcing time series (N = 51) were used to drive group or fleet dynamics and included annual group-specific fishing mortality estimates from stock assessments (Supplementary Table 3), annual fleet-specific fishing effort derived from the Vessel Operating Units database (used when stock assessments are unavailable), and an environmental forcing function (Mississippi River nutrient input), which was used to drive primary production.
To fit Ecosim predicted time series to the reference time series, first, five recursive vulnerability searches were carried out, and next, three consecutive primary production anomaly searches were applied based on the approach of Chagaris et al. (2020). The primary production (PP) anomaly and the vulnerability search algorithms are both computed as part of the automatic Ecosim fitting routine and are based on minimizing the errors’ sum of squares (SSE, i.e., the sum of squared residuals) using an iterative search algorithm (Christensen et al., 2005). For the vulnerability search, the maximum number of predator–prey vulnerability parameters (vij) estimated during any iteration was 108, which is one fewer than the number of reference time series (N = 109) (Heymans et al., 2016). No further reduction (>1) in the SSE was obtained after five iterations. The PP anomaly captures the temporal variation in the PP of the ecosystem that helps explain the difference between observed and predicted biomass and landings. It represents process error or an unknown environmental effect (e.g., physical transport of nutrients or ecophysiological responses of phytoplankton) that can interact with nutrient loading to affect primary production and have a bottom-up effect on the ecosystem. The PP anomaly time series estimated by the EwE fitting routine is significantly and positively correlated to the sea surface temperature (SST) anomaly time series for the northern GoM (Supplementary section S1). This correlation suggests that an increase in SST has a positive effect on fisheries productivity, as was also demonstrated elsewhere (Heenan et al., 2020). The PP anomaly was not estimated in the last year (2016) because there was no subsequent year information to constrain the estimate.
A limitation of the automatic fitting routine in Ecosim is that the SSE minimization is unconstrained, meaning that there are no penalized bounds in the optimization function and parameters are not informed by priors or specification of the variance. Poor contrast in the data can lead to vulnerability parameters estimated at upper and lower bounds (1.0 and 1e10), which can cause unstable dynamics in simulations, and especially in future long-term projections. Ecosim models are most sensitive to changes in the vulnerability parameters at the lower end than with larger values, such that going from 1 to 10 has a larger effect than going from 100 to 1000. Vulnerability values that are too high may result in unrealistic predation estimates compared to their natural mortality estimates. To address this, vulnerability caps (Vcap) were calculated as the ratio of theoretical maximum predation mortality (M2) and baseline Ecopath M2 rates, where Vcap = (M2cap *M)/M2base, such that M2cap is a multiplier on the Ecopath prey M, representing how high predation mortality can go relative to the prey’s total natural mortality. For example, an M2cap of 0.5 would prevent predation mortality by a single predator on a single prey item from exceeding half of the total natural mortality of that prey. In our model, we restricted M2 capto 1, such that M2 cannot exceed M. Adjustments were made for several groups to improve biological realism (e.g., exhibit unrealistic increase or depletion of a given group under status quo conditions). The vulnerability caps were applied after the repeated search was complete (Chagaris et al., 2020). As an additional diagnostic, the model was projected 20 years into the future to evaluate the groups’ response to no-fishing and extremely high fishing mortalities to make sure that the modeled ecosystem responds as expected, i.e., there are no exponential growth or depletion trends, which could indicate issues with parameter configuration. Additional details on diagnostics can be found in Berenshtein et al. (2021).
Ecosim accounts for bottom-up drivers on primary production using a Michaelis–Menten nutrient uptake model. Specifically, the EwE modeling framework assumes that the total nutrient concentration can be divided between that which is available (free) and unavailable for uptake (e.g., bound in biomass). The total nutrient content is computed as the ratio between the total nutrient inflow rate from all nutrient loading sources, and the total loss rate considering all loss pathways (e.g., advection, sedimentation, and harvest). The Ecopath base estimate of total nutrient concentration is calculated by the EwE software based on the biomass pool value, its fixed nutrient content per unit of biomass pool, and the free nutrient concentration (Walters et al., 2008).
The base free nutrient proportion, which was set in our simulations to the default value of 0.5, provides the basis for the time-dependent nutrient forcing, which is simulated using monthly multipliers of the Ecopath-based nutrient content estimate that represent the relative nutrient loading. Here, nutrient loading is represented by total monthly nitrogen and phosphorus input from the Mississippi River, scaled by the mean of the entire time period. In each simulation, the free and bound nutrient fractions must sum to the total nutrient content, which results in a depletion of free nutrients under conditions of increased growth, limiting PP. The PP and the free nutrient concentrations are linked through a Michaelis–Menten uptake relationship, in which nutrient uptake increases asymptotically up to a maximum in response to increasing free nutrient concentrations (Walters et al., 2008).
Equilibrium analysis employing the fishing mortality rate associated with achieving MSY (FMSY; Walters et al., 2005) was used as an additional diagnostic for the model (Heymans et al., 2016). For this analysis, long-term (40 years) simulations were run in Ecosim over a range of constant fishing mortality rate (F) values for each species one at a time and the resultant response in group landings and biomass was recorded (Christensen and Walters, 2004; Christensen et al., 2005). The stationary FMSY analysis, which fixes the parameters of all other groups at their Ecopath inputs so that they cannot respond to changes in the target group, can be compared to single-species MSY estimates. In contrast, the non-stationary (compensatory) option allows other groups (both predators and prey) to respond to changes in biomass of the target group. We compared the values derived from the stationary equilibrium MSY analysis with those estimated from stock assessments where possible, or assuming an FMSY proxy equal to M as a reference for unassessed species. For several groups, prey vulnerabilities were manually adjusted (typically very small increases to vij estimated near 1) to increase the correspondence between the EwE-derived and the assessment FMSY. These final adjustments had little effect on overall model fit, but greatly improved the model’s ability to generate more realistic responses to fishing pressure. The compensatory FMSY analysis was used to develop ERPs for Gulf menhaden. A schematic representation of FMSY and the respective ERPs is given in Figure 1.
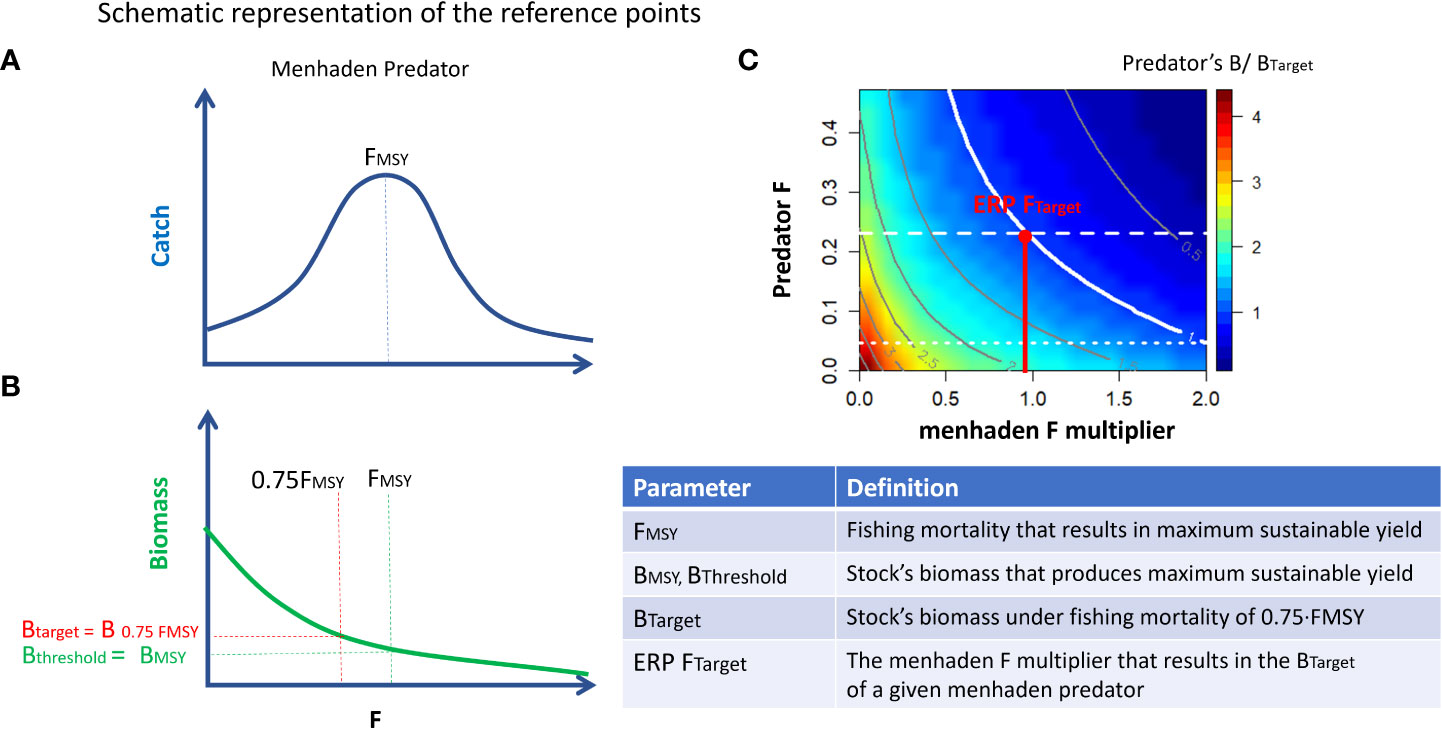
Figure 1 Schematic representation of reference points. (A) FMSY is defined as the fishing mortality that results in maximum sustainable yield. (B) BThreshold and BTarget are defined as the biomass that corresponds to FMSY and 0.75·FMSY, respectively. (C) Ratios (color map) of scenario’s biomass relative to the target biomass (Btarget) for menhaden predators as a function of variation in fishing mortalities for Gulf menhaden and their predators. A contour value of 1 indicates a state in which the predators’ biomass equals Btarget. The x-value of the intersect between the “1” contour and the horizontal dashed line represents the ERP Ftarget. ERP FTarget is the multiplier of menhaden F that results in the BTarget of a given menhaden predator and is represented as a vertical red line.
Using the calibrated Ecosim model, an ecological sensitivity analysis was conducted to identify the species that are most responsive to changes in Gulf menhaden harvest. This analysis was akin to a hierarchical risk assessment that first identifies units (e.g., species, habitats, and fleets) subject to decline under some hazard or management option and then uses integrative modeling to quantify those effects and assess risk (Hobday et al., 2011). Projections were run from 2017 through 2036, over a range of menhaden F and/or purse seine effort multipliers, ranging from 0 to 40 times the geometric mean of the last 3 years of the historic period (2014–2016) rates by increments of 0.1. Most groups require a high multiplication factor to reach their stabilized response biomass. Importantly, in this section, we are interested in the biomass change after 20 years given menhaden fishing pressure (menhaden F and menhaden purse seine fleet effort). Using the multisim routine, we ran multiple simulations examining the biomass change after 20 years such that menhaden fishing pressure is constant within each simulation but differs between the simulations. Based on this, to evaluate the effects caused by menhaden harvest removal versus purse seine bycatch, a set of sensitivity analyses was conducted. Alternative simulations included (1) varying both menhaden F and purse seine effort simultaneously using the multiplier range, (2) varying menhaden F with constant purse seine effort (i.e., 2014–2016 geometric mean), and (3) constant menhaden F and varying purse seine effort. Fishing mortality and fishing effort were held constant at 2016 levels for all other species and fleets in the projections. A total of 1,200 alternative scenarios were run (400 F or fishing effort scenarios × 3 treatments). For each scenario, changes in relative biomass were expressed as the ratio of terminal year (2036) biomass in the projection scenario to the terminal year (2036) biomass under status quo F and purse seine effort, i.e., multiplying the geometric mean of the last three data years (2014–2016) by 1. The 12 functional groups that exhibited the largest decline in biomass under the combined F and purse seine effort scenario were considered in the trade-off analysis discussed below. In order to enable the development of ERPs, declining species were selected because they would likely limit fishing mortality of menhaden. For this analysis and for the trade-off analysis below, we used the multisim plugin in Ecosim, which allows runs of multiple simulations at a time based on predefined sets of input parameters.
To evaluate the trade-offs associated with menhaden harvest, a series of 40-year projection scenarios were run iterating across a range of menhaden F and purse seine effort multipliers in combination with predator F rates. For menhaden, 20 different multipliers were applied to both menhaden F and purse seine effort, ranging from 0 to 2× the 2016 values. Here, we use a lower multiplication factor compared to the former analysis, since we modify the fishing mortality of both menhaden and its predators. F×2 is sufficient for the second analysis because (1) it represents a realistic range of values and (2) it is more reasonable in terms of the number of simulations considering the multitude of combinations and the required computational power. By manipulating both menhaden F and purse seine effort simultaneously, these runs incorporated trophic effects associated with both menhaden harvest and purse seine bycatch. For predators, the range of multipliers used (N = 20) extended from 0 to 2× the compensatory FMSY from the equilibrium analysis. Compensatory FMSY is the FMSY resulting from the full compensation of the EwE FMSY routine, which allows all groups’ biomass to change in response to the biomass changes of the focal FMSY groups (in contrast to stationary, which keeps the rest of the groups fixed at their last year’s biomass). The compensation in this case represents the trophic dynamics that result from the depletion of a certain group in the system, i.e., decreased prey availability and/or decreased predation. For each predator, a target fishing mortality rate (Ftarget) was defined as 75% of the FMSY estimated from the Ecosim compensatory equilibrium FMSY analysis and a biomass target Btarget was defined as the equilibrium biomass achieved at Ftarget. Fishing mortality and effort of all other model groups and fleets were held constant at their 2016 level. For multi-stanza groups including king and Spanish mackerels, F estimates of both stanzas (0–1 year and 1+ year) were iterated simultaneously. A total of 4,000 40-year Ecosim projections (2017–2056) were run to generate the trade-off frontiers (20 menhaden F × 20 predator F × 10 predators). For each projection, the ratio of predator biomass in the terminal year to the Btarget was calculated and used to describe the trade-off relationship between menhaden harvest and predator biomass. A schematic representation of the trade-off analysis is given in Figure 1C.
Ecological indicators computed for this study included the biomass of menhaden predators (mt/km2), mean trophic level of the catch (TLC), total consumed menhaden biomass (mt/km2), and total menhaden consumed: landed biomass ratio. Traditionally, high TLC indicates that there is more fishing pressure on the ecosystem predators, while a declining trend of TLC can indicate “fishing down marine food webs” (Pauly et al., 1998) or “fishing through marine food webs” (Essington et al., 2006). Consumed menhaden biomass simply represents the total menhaden biomass consumed by all predators, which indicates the trophic importance of menhaden given the variation of menhaden fishing pressure. The ratio between consumed and landed menhaden biomass represents the proportion of menhaden production allocated to predation and fishing, such that higher ratios represent a larger emphasis on the ecosystem services provided by menhaden to support predator populations. Indicator values were extracted from each simulation as well as on four menhaden fishing mortality (F) and menhaden purse seine fleet effort (E) values including no fishing (E_F0), fishing at the historical minimum (E_Fmin) and maximum (E_Fmax), and fishing at the current 2016 levels (E_FCurrent). For each scenario, changes in relative biomass were expressed as the ratio of terminal year (2036) biomass in the projection scenario to the terminal year biomass under status quo F and purse seine effort (i.e., 2014–2016 geometric mean assumed throughout the projection).
ERPs were developed based on the menhaden–predator trade-off relationships. For each predator, we defined a target menhaden fishing mortality reference point (ERP Ftarget) and a threshold menhaden fishing mortality reference point (ERP Fthreshold). These benchmarks were previously developed and adopted for management of Atlantic menhaden (Chagaris et al., 2020). The ERP Ftarget is defined as the menhaden fishing mortality that would sustain the predator at its biomass target (B at 75% FMSY), when the predator is fished at its Ftarget (75% FMSY). This is represented in the trade-off plots as the point on the x-axis where the horizontal dashed Ftarget line intersects the 1.0 contour. The ERP Fthreshold is the menhaden fishing mortality that would sustain the predator at its biomass threshold (assumed here to be equal to BMSY), when the predator is fished at its Ftarget. Because the trade-off analysis manipulated all menhaden F stanzas and purse seine effort using a common multiplier to capture both prey and bycatch effects, the ERPs are expressed relative to the current (2016) menhaden fishing mortality and effort values. A schematic representation of the ERP Ftarget computation is given in Figure 1C.
Projections of the above-defined ERP target, threshold, and upper and lower confidence intervals (CIs) of each were completed to determine the range in equilibrium landings that would result, which could be converted into a Total Allowable Catch. The code used was the same as the code developed and reviewed during the SEDAR 63 (2018) benchmark stock assessment for Gulf menhaden. Projections used a constant fishing mortality rate and were run for 5 years (2018–2022, as the terminal year of the single-species assessment was 2017). Uncertainty in runs was promulgated from the assessment to the projections using the Monte Carlo bootstrap (MCB) runs, which included uncertainty in the input data, specifically the landings, index values, composition data, natural mortality, and selectivity for the commercial reduction fishery for ages 3 and 4+. Numbers at age in the population were projected based on the results from the base run and each MCB run, while recruitment was projected using the underlying stock–recruitment curve from the base run and each MCB run. The projections provide a distribution of landings, fecundity, and recruitment that result from the constant, input fishing mortality rate.
Based on the mass balanced Ecopath model, Gulf menhaden represent a forage group for 32 predators spanning marine mammals, sea birds, sea turtles, sharks, and finfish (Figure 2). Eight predator groups feed upon age 0 menhaden in the model, primarily coastal species such as red drum (Sciaenops ocellatus), sea trout (Cynoscion spp.), sea birds [e.g., brown pelican (Pelecanus occidentalis)], and coastal piscivores [e.g., ladyfish (Elops saurus), tarpon (Megalops atlanticus), and snook (Centropomus undecimalis); Figure 2]. Sea trout and red drum also consume age 1+ menhaden, along with other important predators including king and Spanish mackerel (Figure 2). The largest diversity of predators occurred for ages 2+ menhaden due to predation by numerous shark species, coastal dolphins [e.g., bottlenose dolphin (Tursiops truncatus)], mackerels, sea trout, and red drum (Figure 2). Predation mortality (M2) was estimated to be less than 0.001 year−1 for 19 out of the 32 predators of age 2 and age 3 menhaden, and 25 out of 32 predators of age 4+ menhaden. Overall, M2 for menhaden in the Ecopath base year of 1980 were 0.06 year−1 for age 0, 0.06 year−1 for age 1, 0.11 year−1 for age 2, 0.25 year−1 for age 3, and 0.42 year−1 for age 4+ (Supplementary Table 2). Fishing mortality of Gulf menhaden in the Ecopath model showed a slight dome-shaped relationship with age, where F at age 0 was near zero, increased to a maximum of 0.63 year−1 at age 2, and declined to 0.22 year−1 by age 4+ (Supplementary Table 2).
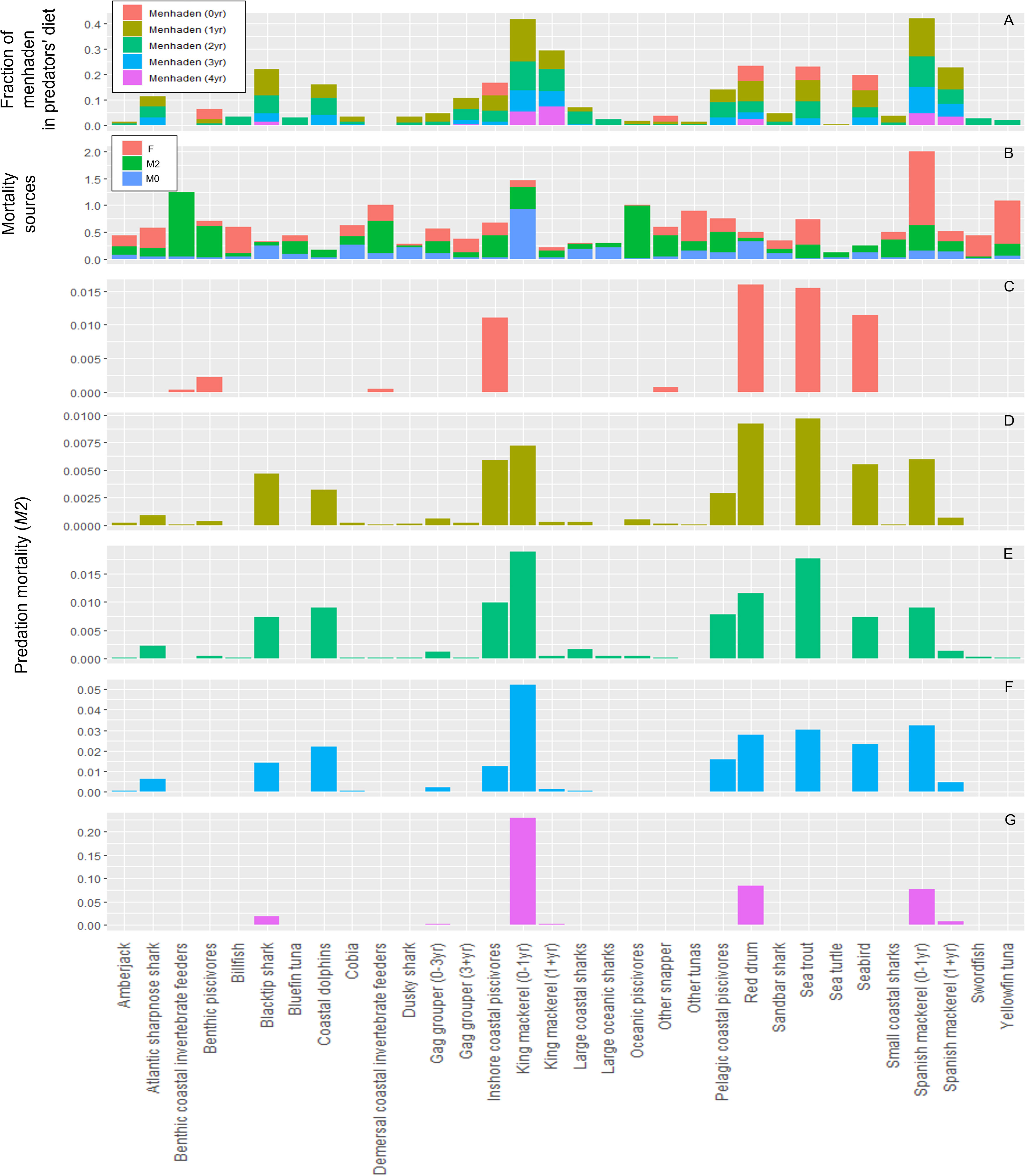
Figure 2 (A) The contribution of Gulf menhaden to the diets of its predators in the US Gulfwide Ecopath model. (B) Different sources of predators’ mortality (F: fishing mortality, M2: predation mortality; and M0: other mortality; units: year−1). (C–G) The contribution of predators to predation mortality (M2) of Gulf menhaden.
Predation mortality rates were much higher for other teleost forage groups including anchovy (Engraulidae)–silverside (Atherinidae)–killifish (e.g., Fundulidae) (M2 = 1.34 year−1), surface pelagics [e.g., flyingfish (Exocoetidae); M2 = 1.32 year−1], butterfish (Stromateidae; M2 = 1.31 year−1), and sardine–herring (Clupeidae)–scad (Decapterus spp.) (M2 = 0.95 year−1; Supplementary Table 2). These forage groups collectively supported a large spectrum of predators, including predators not strongly associated with menhaden due to different habitats [e.g., offshore groupers (Serranidae) and snappers (Lutjanidae)].
Of the menhaden predators, species with the largest Ecopath (1980) F estimates included juvenile Spanish mackerel (F = 1.37 year−1), tunas (Thunnus spp.; 0.11–0.80 year−1), billfish (Istiophoridae; 0.40–0.51 year−1), and sea trout (0.47 year−1; Supplementary Table 2; Figure 2). For these groups, the primary fisheries were the commercial shrimp trawl (indirectly) for juvenile Spanish mackerel (92% of F), the commercial pelagic longline for yellowfin tuna (Thunnus albacares; 87% of F) and bluefin tuna (Thunnus thynnus; 42% of F), the commercial purse seine (targeting species other than menhaden) for other tunas (Thunnus spp.; 86% of F), the recreational private fishery for billfish (40% of F), the commercial pelagic longline for swordfish (Xiphias gladius; 51% of F), and both the recreational private fishery and commercial purse seine fishery (as bycatch) for sea trout (each accounting for 29% of F). In addition to capturing menhaden, the purse seine fishery indirectly captures other forage species including anchovies–silversides–killifish, zooplankton [e.g., jellyfish (Cyaneidae)], butterfish, cephalopods (e.g., squid), sardine–herring–scad, surface pelagics, mullet (Mugilidae), and invertebrates (Supplementary Table 2). Of menhaden predators, unexplained mortality (M0), which is not attributed to fishing or predation, was largest for blacktip shark (78% of total mortality), dusky shark (74% of total mortality), and red drum (65% of total mortality; Figure 2).
Ecosim predictions matched historical biomass trends for many species (Figure 3), with reasonable agreement exhibited with observed biomass trends for Spanish mackerel, king mackerel, cobia (Rachycentron canadum), and greater amberjack (Seriola dumerili), as well as aggregate groups such as pelagic coastal piscivores and snappers (Figure 3). Predicted biomass of menhaden showed cyclical oscillations driven by nutrient loading from the Mississippi River. Fits were notably good for younger menhaden, including age 0 (Figure 3). This was in contrast to most other age 0 fish stages, which Ecosim fit poorly in general. Ecosim predictions diverged from observed biomass series for sharks and highly migratory species (tunas and billfish) as a result of high uncertainty and low assigned weights to these time series (Supplementary Table 3). For some of these species, the trend was generally correct, but the model missed the high-frequency fluctuations (e.g., biomass of billfish, bluefin tuna, and swordfish). When compared to observed landings, Ecosim predictions replicated historical trends for many species such as greater amberjack, Spanish mackerel, adult gag grouper, and snappers (Figure 4). As observed for biomass, fits for sharks and highly migratory species were poor due to lower weights attributed to these inputs (Supplementary Table 3).
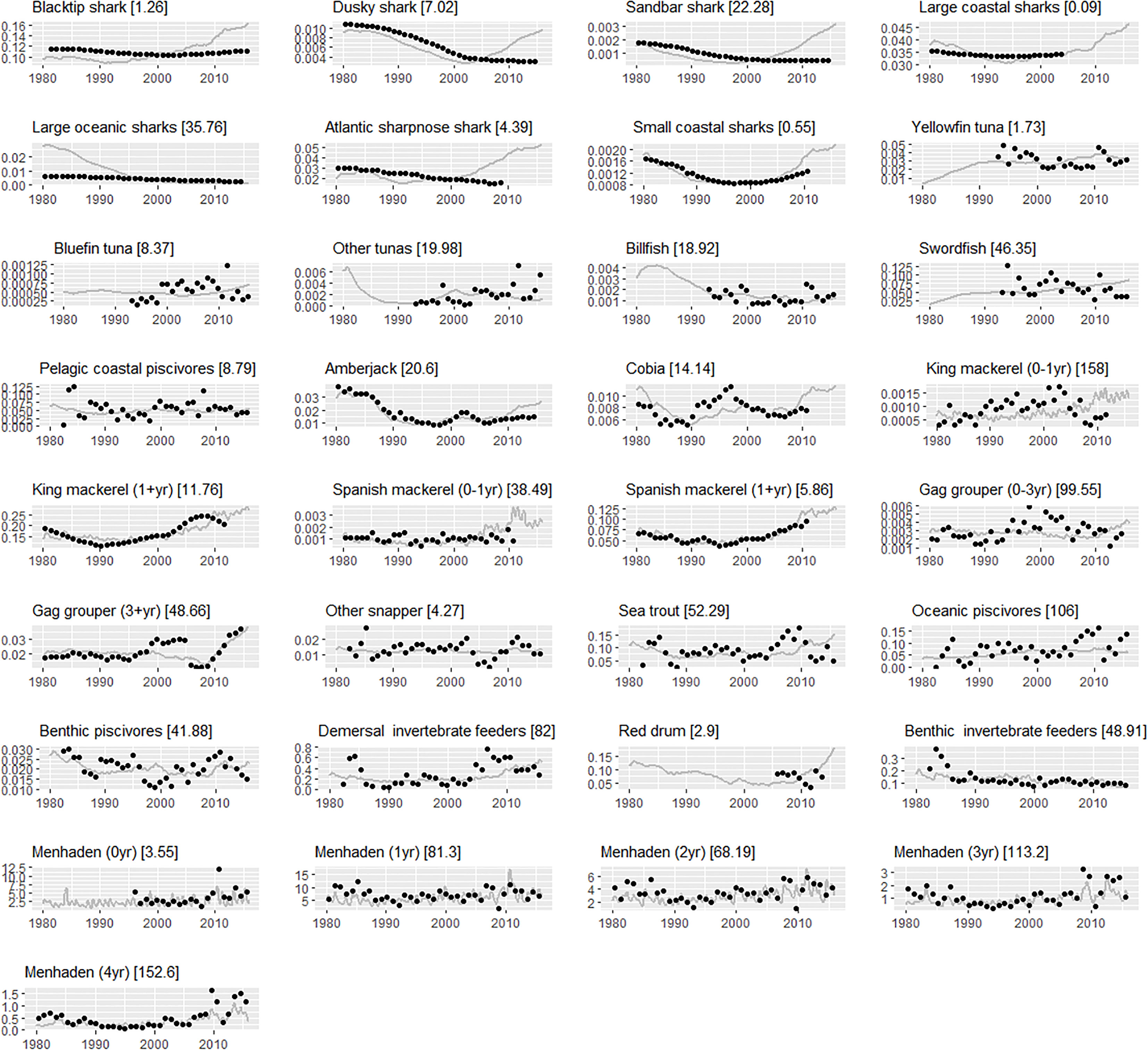
Figure 3 Ecosim fits to biomass (units: t km2 year−1) reference time series of select Gulf menhaden predators and Gulf menhaden age classes. The gray solid line represents the biomass predicted by Ecosim and the black circles represent the biomass reference time series. The sum-of-squared errors (SSE) of model fits are given in squared brackets. Note that SSE values provided here for each time series are dependent on the series specific weight (see Supplementary Table 3).
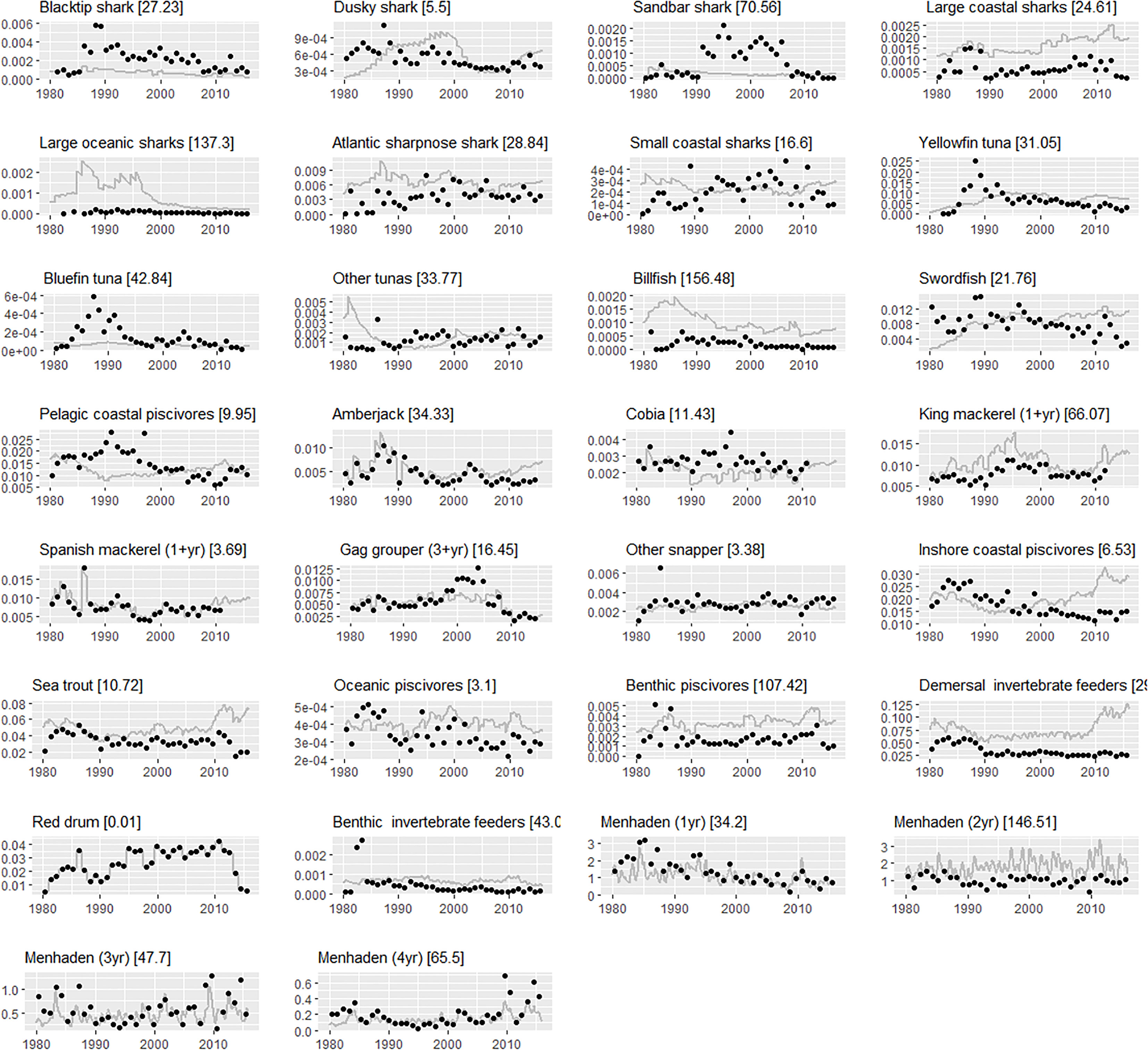
Figure 4 Ecosim fits to landings (units: t km2 year−1) reference time series of select Gulf menhaden predators and Gulf menhaden age classes. The gray solid line represents the landings predicted by Ecosim and the black circles represent the landings reference time series. The sum-of-squared errors (SSE) of model fits are given in squared brackets. Note that SSE values provided here for each time series are dependent on the series specific weight (see Supplementary Table 3).
For the key menhaden predators of interest, the evaluation of equilibrium FMSY estimates from Ecosim showed fairly good agreement with those derived from single-species stock assessments (Figure 5), with the exception of large coastal sharks and adult king mackerel, for which FMSY_EwE estimates were substantially higher than that of the assessment. For groups that used natural mortality (M) as a proxy for FMSY, FMSY_EwE estimates were substantially lower than FMSY = M for demersal coastal invertebrate feeders [e.g., drums and croakers (Sciaenidae)] and blacktip shark, and substantially higher than FMSY = M for red drum. Sea trout and pelagic coastal piscivores showed good correspondence between FMSY_EwE and FMSY = M. For all groups except sea trout, FMSY_EwE estimates are higher than the 2016 FCurrent estimate, indicating that most menhaden predators are not experiencing overfishing according to dynamics of this Ecosim model. In addition, all the groups displayed a non-symmetrical “long tail” curve such that the catch rate decreases only slightly under high levels of F because of strong compensatory responses implied by the vulnerability parameters that allow stocks to withstand higher levels of fishing (Christensen et al., 2005).
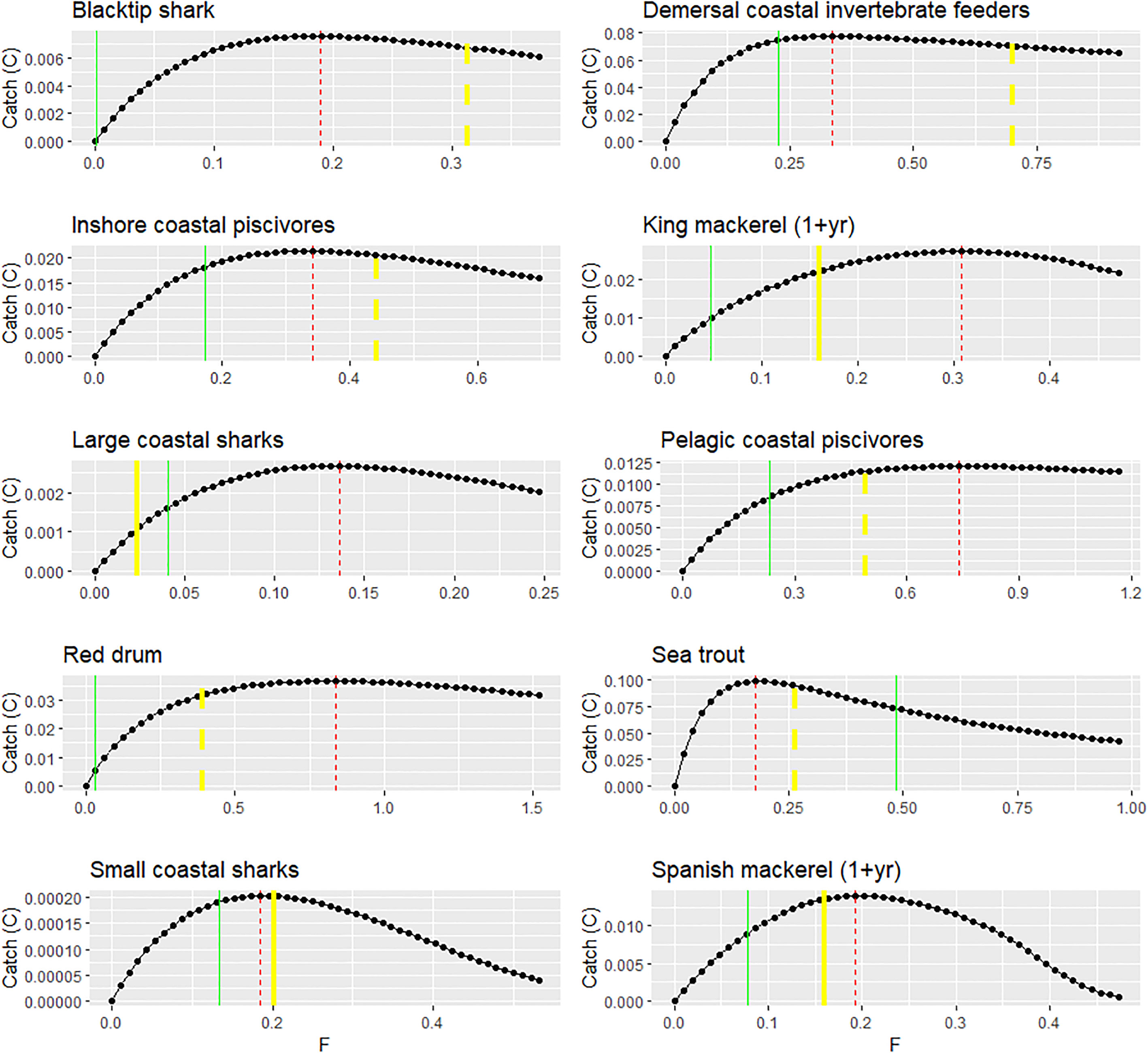
Figure 5 FMSY estimated from the Gulfwide EwE model compared to single-species stock assessment estimates or proxies for key Gulf menhaden predators. Red lines represent the EwE FMSY estimates (F units: year−1; catch units: t km2 year−1). Green lines represent FCurrent (for 2016). Yellow lines represent the FMSY estimated by a single-species stock assessment (solid line) or using the proxy FMSY = natural mortality (M) (dashed line).
Gulf menhaden predatory groups that are the most sensitive to changes in menhaden F and purse seine effort include inshore coastal piscivores, sea trout, demersal coastal invertebrate feeders, king mackerel (both age stanzas), large coastal sharks, Spanish mackerel (0–1 year), small coastal sharks [e.g., finetooth shark (Carcharhinus isodon)], pelagic coastal piscivores, Spanish mackerel (1+ year), blacktip shark, and red drum (Figure 6A). Predatory groups that responded with an increase in biomass given increased menhaden F and purse seine effort include yellowfin tuna, benthic coastal invertebrate feeders, greater amberjack, large oceanic sharks (e.g., Lamnidae), other snappers, and bluefin tuna. This counterintuitive increase with the removal of Gulf menhaden is likely due to reduced competition and larger biomass of other forage that is more important in their diets (Supplementary Figure 1), as well as reduced abundance of predators and/or competitors (Figure 6A). For example, the biomass of cephalopods (a key prey item for yellowfin tuna) is expected to increase under high fishing pressure of Gulf menhaden (Supplementary Figure 1A), potentially due to reduced competition for resources. This effect occurred both through direct harvest of menhaden (Supplementary Figure 1B) and bycatch removal (Supplementary Figure 1C).
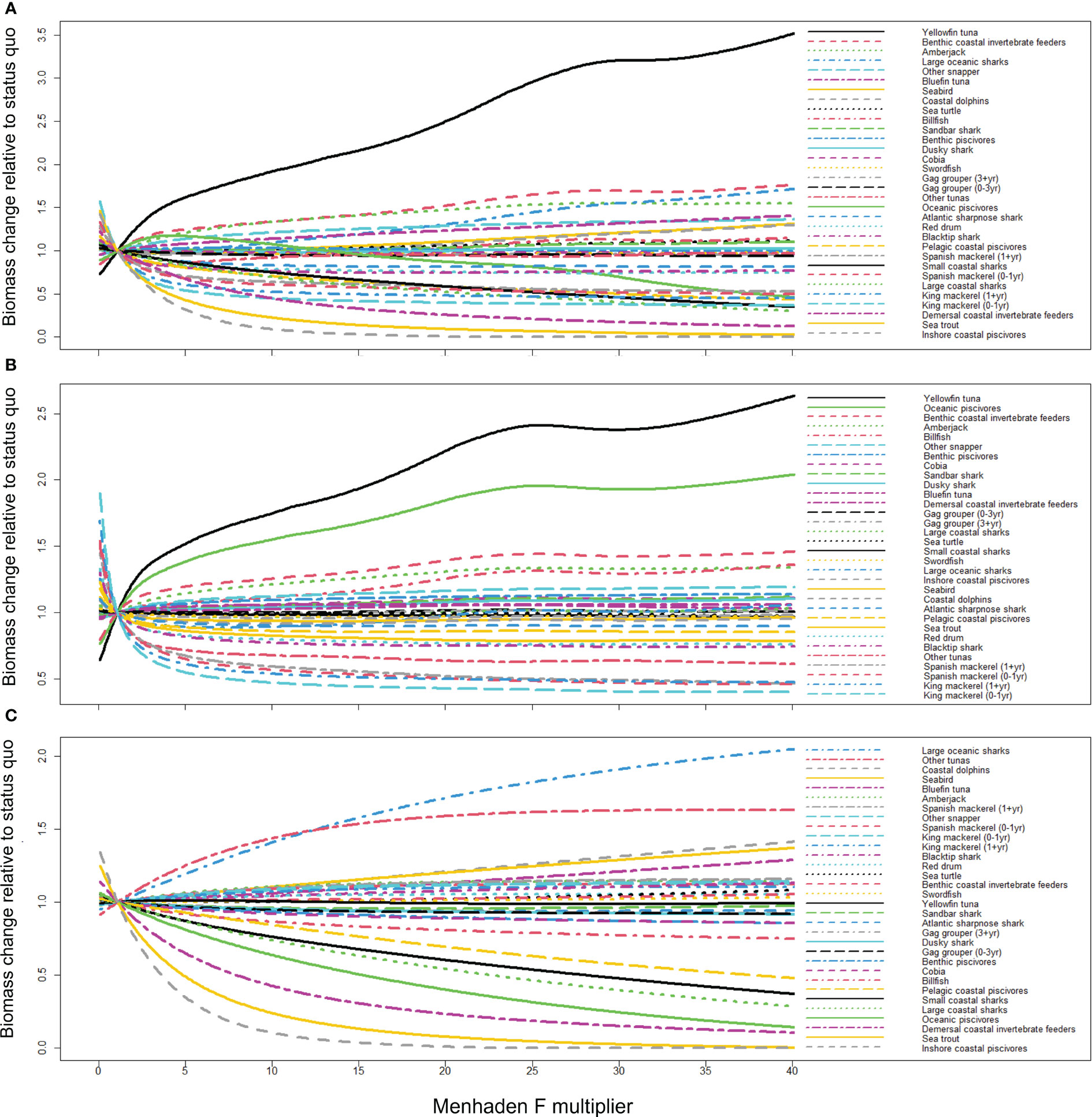
Figure 6 The change in biomass (scenario biomass/2014–2016 geometric mean biomass) of Gulf menhaden predators in response to increasing (A) menhaden F and purse seine effort (prey and bycatch effects), (B) menhaden F only (prey), and (C) purse seine effort only (bycatch). Multipliers for each driver ranged from 0 to 40× for reasons discussed in the text. The response of groups that are not considered Gulf menhaden predators is given in Supplementary Figure 1 in the supplementary material. Note that the x-axis represents the multiplier value of the 2014–2016 geometric mean biomass on menhaden F and purse seine effort (A), menhaden F (B), and purse seine effort (C).
When considering only menhaden F, hence excluding the effect of bycatch by the menhaden purse seine fleet and isolating the trophic impact of menhaden removals, the most affected species included king and Spanish mackerels in addition to other tuna, blacktip shark, red drum, and sea trout (Figure 6B). Groups for which biomass increased included yellowfin tuna, oceanic piscivores [e.g., Atlantic cutlassfish (Trichiurus lepturus)], benthic coastal invertebrate feeders, greater amberjack, billfish, and other snapper. When considering only purse seine effort, hence considering the effect of bycatch only, the most depleted groups included inshore coastal piscivores, sea trout, demersal coastal invertebrate feeders, oceanic piscivores, and large and small coastal sharks. For this scenario, the groups for which biomass increased include large oceanic sharks, other tunas, coastal dolphins, seabirds, bluefin tuna, and greater amberjack (Figure 6C). Note that coastal dolphins and seabirds are not included in the menhaden purse seine fleet bycatch due to the lack of data quantifying mortality.
The results from Figure 6A can be used to quantify differences in predicted biomass under each of the menhaden F or purse seine effort scenarios tested. For example, the biomass of coastal dolphins and seabirds was predicted to increase 31% under conditions of increased menhaden F and purse seine effort (F and E multiplier ×40). This increase is attributed to indirect influence on these groups’ predators and prey through fishing mortality (Figure 6B) and bycatch removal (Figure 6C). For example, the increase in the biomass of the coastal dolphins in response to increased menhaden fishing pressure may be explained by the biomass decrease in the dolphins’ predators—blacktip sharks and large coastal sharks—as well as by the increase in key prey groups such as cephalopods and sardine–herring–scad. For these groups, the positive indirect effects on predation (i.e., reduction of predators) and consumption (i.e., increase in prey) outweigh the negative effects of fishing effort and the reduction of Gulf menhaden as prey. For the recreationally important red drum, the effect of increased menhaden F and purse seine effort results in a reduction of 25.6% red drum biomass at the maximal modeled scenario (E and F multiplier ×40; Figure 6A). This reduction is mainly attributed to the effect of fishing mortality for menhaden (Figure 6B) rather than bycatch removal (Figure 6C).
The equilibrium dynamics in Figure 6 indicate reciprocal trends, passing through the point of F and E multiplier value = 1 (x-axis) and relative biomass = 1 (y-axis). Groups that exhibit declines under high Gulf menhaden fishing pressure, such as inshore coastal piscivores, exhibit high biomass values under Gulf menhaden fishing pressure conditions closer to zero. Similarly, groups that exhibit an increase under high Gulf menhaden fishing pressure, such as yellowfin tuna, exhibit low biomass values when F and E multiplier values are close to zero. This pattern characterizes all three combinations of fishing pressure (Figures 6A–C).
The equilibrium dynamics differ among the predatory groups such that some groups are characterized by an initial steep slope (decreasing or increasing) and subsequent rapid stabilization [e.g., king mackerel (0–1 year) and Spanish mackerel (0–1 year)], compared to other groups in which the dynamics are more gradual, with a relatively constant slope (e.g., small coastal sharks and cobia; Figure 6A). Oceanic piscivores exhibit an initial increase under the initial F multiplication values up to about 5×, with a subsequent depletion thereafter (Figure 6A).
The equilibrium trade-off analysis incorporated the combined bottom-up effect of menhaden removals, bycatch by the purse seine fleet, and direct exploitation of predatory groups. The results were summarized as trade-off plots, which indicate the relative effect of Gulf menhaden and predator fishing rates in achieving Btarget (Figure 7). Some groups exhibited a hyperbolic relationship between the effects of menhaden fishing and the predator F, leading to a gradient in the B/Btarget ratios from the bottom left corner (i.e., high values, no fishing on menhaden and the predator) to the upper right corner (low biomass ratios, maximal fishing on menhaden, and the predator). Such a relationship is expressed for king mackerel, Spanish mackerel, and inshore coastal piscivores. For other groups, such as demersal coastal invertebrate feeders, and small coastal sharks, the effect of menhaden fishing substantially outweighs the effect of the predatory group’s fishing, resulting in a stronger gradient associated with menhaden F (Figure 7). For most groups, Bcurrent (representing 2016, the last year of the model time series) is higher than Btarget (B at 75% FMSY), indicating a sustainable state of the fishery that can tolerate higher F rates (Table 1). However, for sea trout, Bcurrent is lower than the Btarget, indicating an unsustainable state for this group and that target biomass can be achieved by adjusting seatrout and menhaden F. Under current seatrout F, menhaden fishing would need to be reduced to near 0 to achieve Btarget, whereas if sea trout were fished at their own Ftarget (0.134), then menhaden F would need to be reduced to 18.9% of current rates (Figure 7). Spanish mackerel are currently above their Btarget, and Ftarget is higher than current F. Under current Spanish mackerel and menhaden F rates, Spanish mackerel will reach equilibrium at about 80% of their biomass target. To achieve Btarget at current Spanish mackerel F, menhaden fishing can be reduced to 64% of current levels. Alternatively, if Spanish mackerel exploitation is increased to the target rate, then menhaden fishing would need to be reduced further to 50% of current rates.
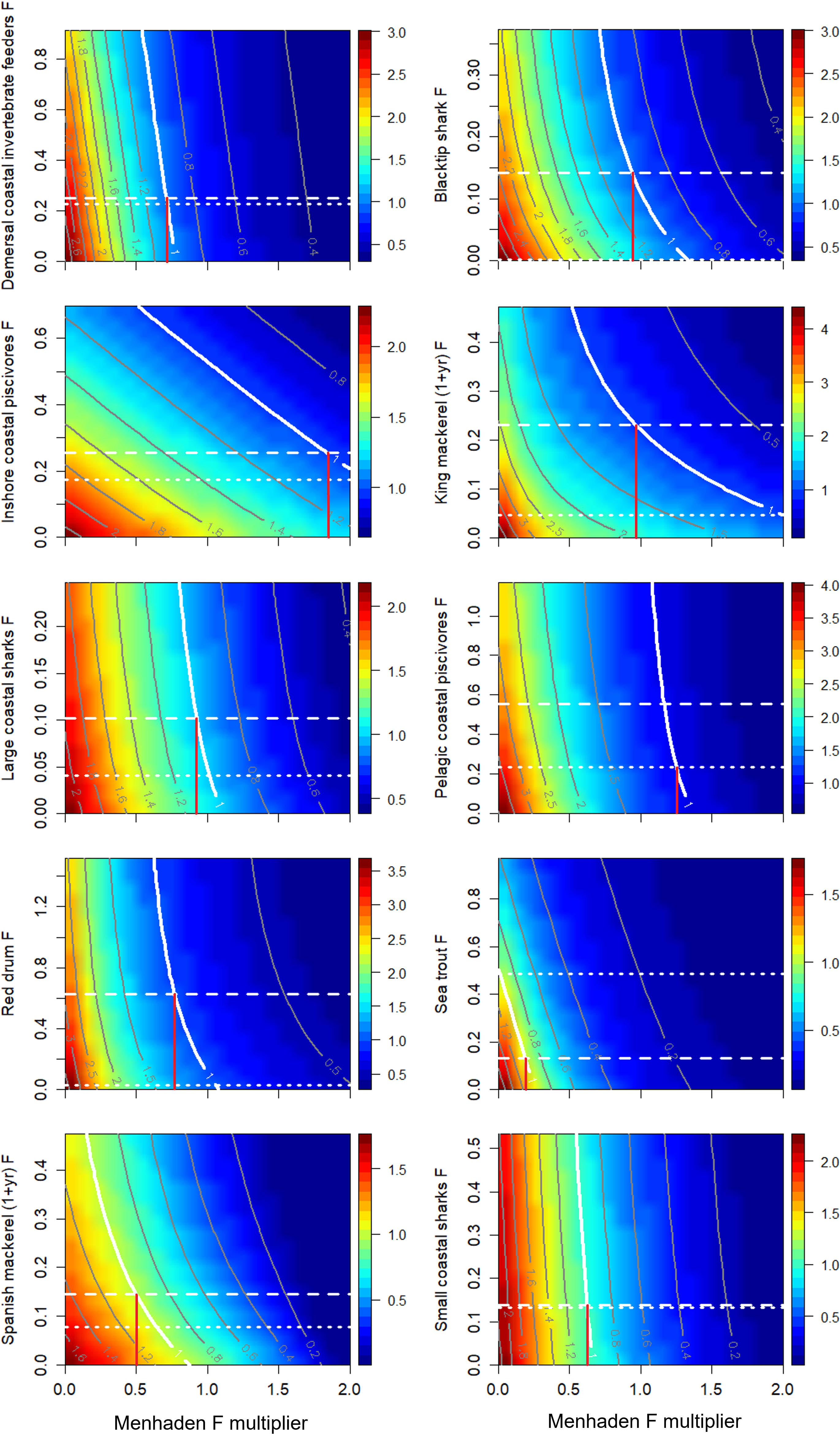
Figure 7 Ratios (color map) of scenario’s terminal year (2036) biomass relative to the target biomass (Btarget) for menhaden predators as a function of variation in fishing mortalities for Gulf menhaden and their top predators. Note that the x-axis represents the menhaden F multiplier (and not the actual menhaden F per se). Btarget was computed as the equilibrium biomass achieved when fishing at Ftarget, defined as 75% of FMSY from the equilibrium FMSY analysis. Dotted and dashed white lines represent 2016 fishing mortality estimates (FCurrent) and 0.75 FMSY, respectively. A contour value of 1 indicates a state in which the predators’ terminal year (2036) biomass equals Btarget. The x-value of the intersect between the “1” contour and the predator’s Btarget (horizontal dashed line) represents the ERP Ftarget (vertical red line). ERP Ftarget values are given in Table 1.
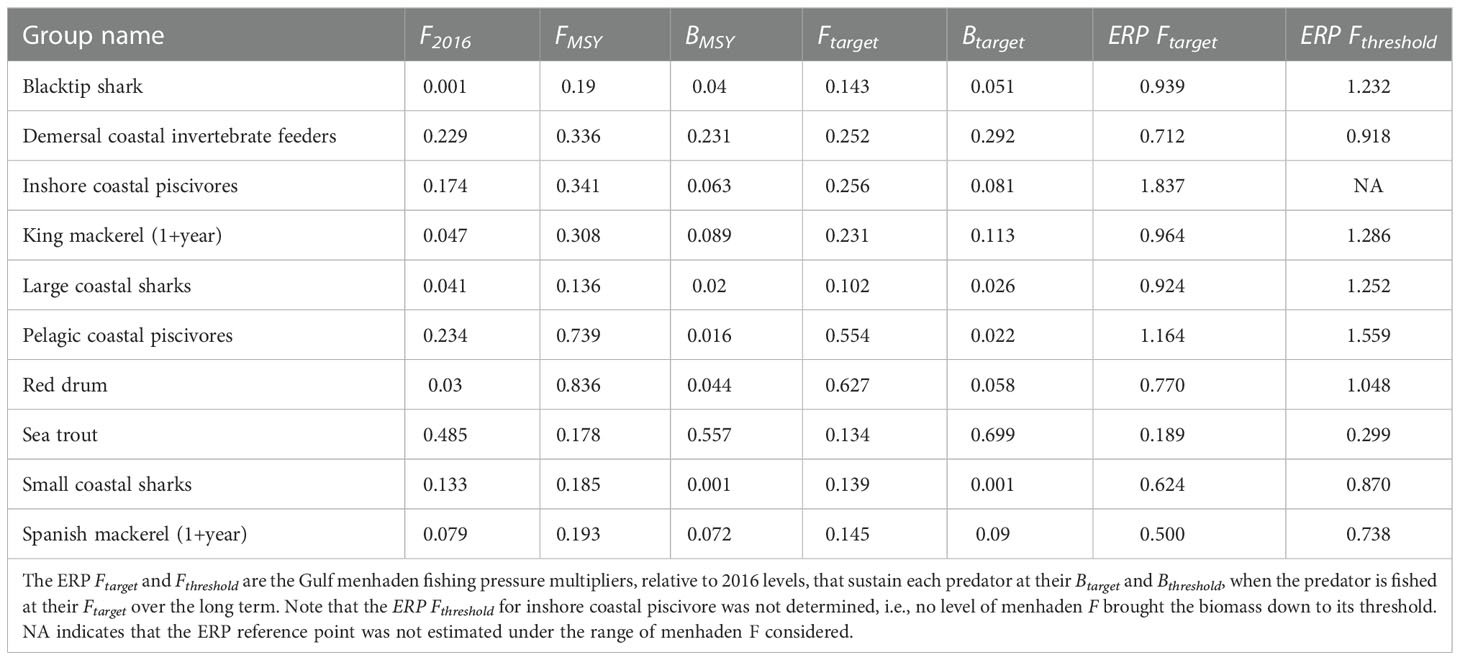
Table 1 Reference points for Gulf menhaden predators, including current F (2016) and equilibrium FMSY and BMSY from the compensatory Ecosim equilibrium MSY analysis; target biomass (Btarget) is computed as the equilibrium biomass achieved when fishing at Ftarget, which was assumed to be 75% of FMSY.
Variation in menhaden F and purse seine effort affects the GoM ecosystem on a broader scale, which is demonstrated by examining ecological indicators. All metrics exhibit nonlinear declining trends in response to increasing menhaden F and purse seine effort (Figure 8). The indicator values of the 2016 fishing pressure (F_Ecurrent) is higher than the indicator values under maximal historical fishing mortality (F_Emax). Specifically, the biomass of menhaden predators increased by 11% from 1.47 to 1.63 t/km2/year, the ratio of menhaden biomass consumed to menhaden biomass landed increased by 46% from 0.34 to 0.73, the total menhaden consumed increased by 36% from 1.1 to 1.5, and the TLC increased slightly from 2.35 to 2.38. Indicator values under no menhaden F or purse seine effort (F_E0) are substantially higher than those under historical F values. Specifically, indicator values under this scenario increased by 21.2% from 1.65 to 1.95 t/km2/year in menhaden predator biomass, by 66.6% from 1.51 to 2.51 in total menhaden consumed, and by 14.3% from 2.35 to 2.72 in the TLC, compared to the 2016 levels.
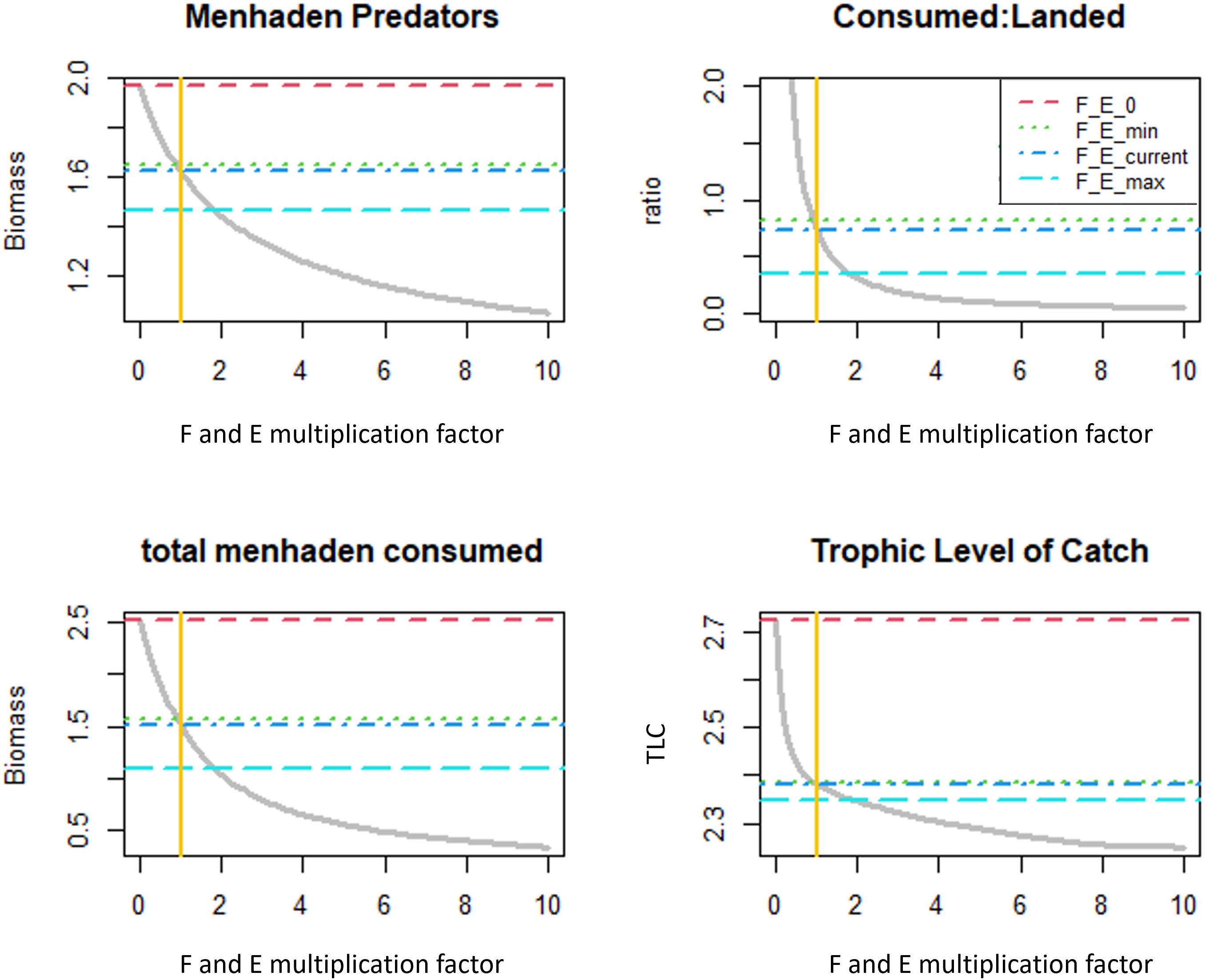
Figure 8 Ecological indicators under increasing menhaden F and purse seine effort. Horizontal lines represent the indicator values of four menhaden F and effort scenarios: F and purse seine effort = 0 (F_E_0), minimal historic F and purse seine effort (F_E_min), current (2016) F and purse seine effort (F_E_current), and maximal historic F and purse seine effort (F_E_max). The current Gulf menhaden F and purse seine effort (F,E multiplier = 1 in the x-axis) are marked by the yellow vertical line.
The ERPs were developed specific to each predator and represent the amount of menhaden fishing pressure, relative to 2016, that would sustain predators at their biomass target (B at 75% FMSY) and threshold (BMSY) when they are fished at their target rates (Ftarget = 75% FMSY) over the long term. Across all species, the ERP Ftarget ranged from 0.19 to 1.84, with a mean of 0.86 (SD = 0.44) and only two instances (inshore coastal piscivores and pelagic coastal piscivores) occurred where the target was greater than current menhaden F (ERP Ftarget > 1) (Table 1). Of predators with high commercial and recreational value, sea trout had the lowest ERP Ftarget at 0.19, followed by Spanish mackerel (0.50), red drum (0.77), and king mackerel (0.96). The ERP Fthreshold values were roughly 40% higher than their targets, ranging from 0.30 to 1.56, with a mean of 1.02 (SD = 0.37) and five instances where ERPthreshold > 1.
The mean ERP Ftarget and Fthreshold (expressed as a ratio to the 2016 F in Ecosim) were converted to a fully selected F (Ffull) for comparison with historical F estimates by multiplying the ratio by the 2016 Ffull from the stock assessment. This equates to mean ERP Ftarget and Fthreshold values in Ffull units of 0.69 and 0.82, respectively. In comparison to the time series of fishing mortality rates estimated by the most recent Gulf menhaden stock assessment, the mean ERP Ftarget of 0.86 (equivalent to an Ffull of 0.69) was exceeded in all but 1 year from 1977 to 2007 (Figure 9). From the mid-1980s to early 2000s, fishing mortality exceeded the upper value (mean + 1 SD) of both the ERP Ftarget and Fthreshold, indicating a period of possible ecosystem overfishing. In contrast, since 2008, the mean ERP Ftarget was exceeded in only 5 years and the mean ERP Fthreshold (equivalent to Ffull of 0.82) was exceeded in only 3 years. Since 2008, neither the ERP target nor threshold upper value (mean + 1 SD) was exceeded in a single year.
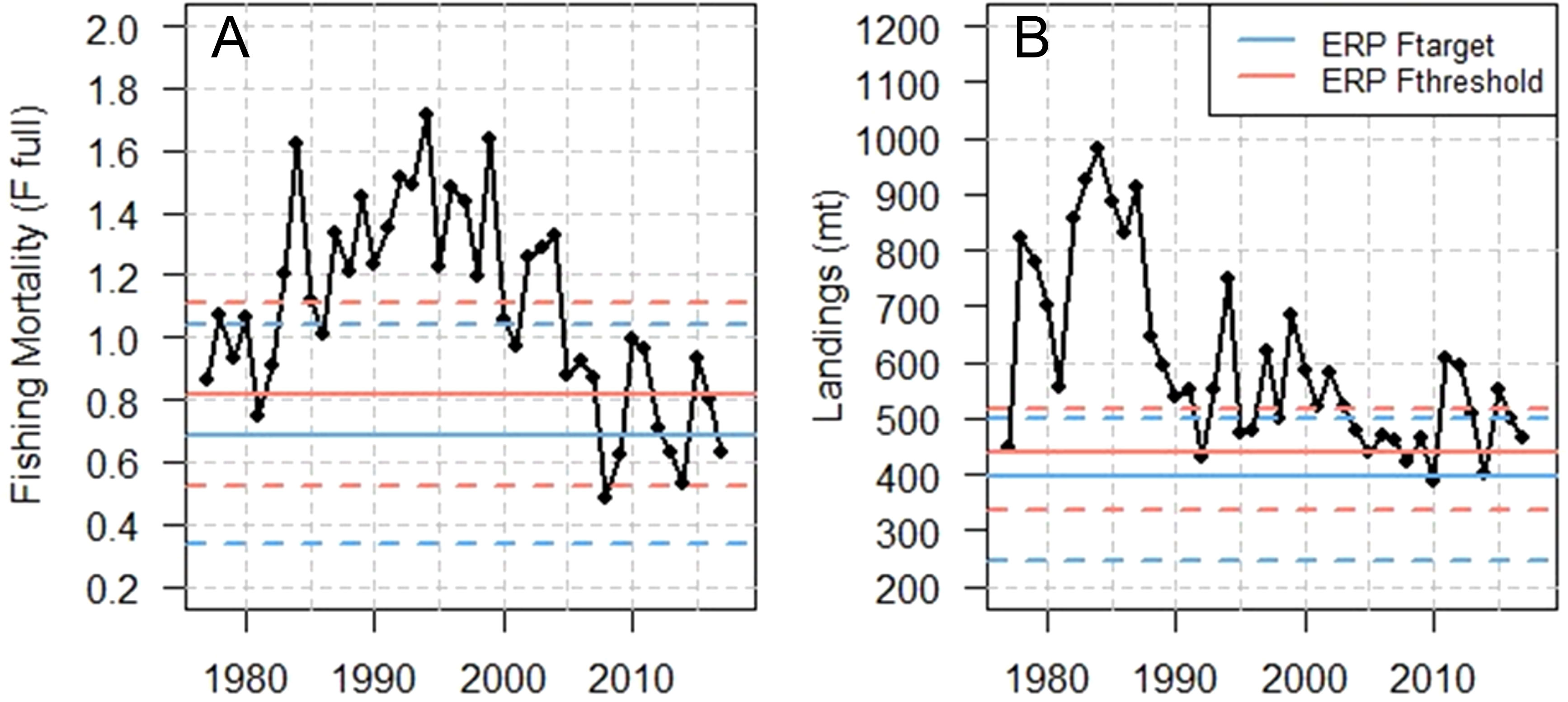
Figure 9 Full fishing mortality rate for Gulf menhaden from 1977 to 2017 from the 2019 Beaufort Assessment Model (BAM) and total menhaden removals, along with horizontal lines referencing the mean ERP Ftarget and Fthreshold (solid lines), averaged over all 10 predator species, ± 1 standard deviations (dashed lines) and the projected equilibrium landings from the BAM model associated with each ERP. Note that the mean/SD is referring to the ERP F rates (A), whereas the median/CIs refer to the projected landings from BAM (B), that are associated with the mean/SD of the ERPs. The BAM projections were run in Monte Carlo bootstrap mode, and the medians were used to get the landings associated with the ERPs. The CIs associated with the landings are reported in the text but are not shown in the figure.
Due to the short life span of Gulf menhaden, landings projections reached a rapid equilibrium (~4 years). Thus, the landings for 2022 were assumed to represent long-term equilibrium values. Median projected landings at the ERP target for Gulf menhaden were 397,774 mt (367,840 to 446,364 mt; 95th percentiles), with median landings being 245,484 mt (223,892 to 270,424 mt; 95th percentiles) for the lower confidence interval of the ERP target and 498,529 mt (458,597 to 572,304 mt; 95th percentiles) for the upper confidence interval of the ERP target. Median projected landings at the ERP threshold for Gulf menhaden were 438,979 mt (404,983 to 496,370 mt; 95th percentiles), with median landings being 334,022 mt (307,533 to 371,262 mt; 95th percentiles) for the lower confidence interval of the ERP threshold and 515,710 mt (473,485 to 593,982 mt; 95th percentiles) for the upper confidence interval of the ERP threshold. The observed landings from 2003 to the present were generally within the range of the projected equilibrium landings except for three recent years (Figure 9).
Overall, we developed and calibrated the Gulfwide EwE model and provided key mortality estimates for Gulf menhaden and associated predators. We found that Gulf menhaden supports a wide range of predatory species, and menhaden harvest substantially affects their abundance via direct predator–prey interactions, indirect bycatch removal, or the combination of the two. Furthermore, trade-off analysis demonstrated that for most predators, the harvest of menhaden had a stronger effect on predators’ biomass compared to the effect of the harvest of the predators. Ecological indicators demonstrated an improvement of the current state (2016) compared to the state of historical maximum fishing pressure of menhaden, showing an increase in the predators’ biomass. Ecological indicators of the current state were similar to those of the historical minimum fishing pressure. The ratio of current (2016) menhaden F to the mean ERP Ftarget and Fthreshold was 1.16 and 0.98, respectively, indicating that a small reduction in menhaden fishing pressure is needed to reach the target and move away from the threshold. The mean ERP Ftarget converted to Ffull units used in the stock assessment was 0.69, which is about 30% lower than the single-species target fishing mortality rate of 0.99. Thus, these ERPs provide a buffer from the single-species reference points that is intended to provide food for predators and reduce bycatch.
We exposed several key predator–prey trade-offs associated with Gulf menhaden harvest and developed trophic dynamics-integrated reference points that appropriately sustain predator populations accounting for optimal prey harvest levels. This analysis demonstrated changes in perceptions of stock status given the interplay between the fishing mortalities of Gulf menhaden and its predators. Specifically, the analysis demonstrated how Btarget could be achieved for a given predatory group, by modifying menhaden and/or the group’s fishing pressure, and based on this relationship, ERPs were established. Based on these ERPs, menhaden overfishing likely occurred from the early 1980s to early 2000s. This period extended beyond the time period of overfishing estimated for Gulf menhaden based on the single-species reference points from stock assessment (GSMFC (Gulf States Marine Fisheries Commission), 2021). It was also evident that recent fishing mortality on Gulf menhaden was notably lower than pre-2005 levels, even when accounting for predator forage requirements. Overall, the average estimated fishing mortality rate from 2008 to 2017 fell between the mean ERP Ftarget and Fthreshold values, indicating that current fishing pressure is within reasonable limits to support the consumptive demands of predators at their current (2016) F levels. The results caution against expansion of the fishery to levels observed in decades prior to 2005, in order to avoid long-term decline in fish biomass across multiple trophic levels.
In the case of Atlantic menhaden, an ERP was selected based on the relationship with a single dominant predator, striped bass Morone saxatilis, which is of high cultural and recreational value (Chagaris et al., 2020). In the GoM, no single menhaden-dependent predator was identified. As such, we evaluated ERPs for a suite of predators and proposed the average value across predators as a threshold of menhaden mortality and target for management. Furthermore, we computed broader, ecosystem-level indicators, which showed that recent (2016) conditions represented an improvement in status compared to the levels of fishing pressure experienced in the past. While the reference points presented here represent multiple approaches to establishing ERPs for Gulf menhaden, a final ERP used for management could be selected through an inclusive and transparent process (Anstead et al., 2021) that applies a suite of modeling approaches (Drew et al., 2021) and accounts for how managers and stakeholders value different components of the ecosystem (Anstead et al., 2021).
The average ERP Ftarget across all 10 predator species and functional groups evaluated here was 0.86, which translated to a 14% reduction in fishing pressure relative to recent (2016) levels and a median projected landings estimate of 397,774 mt (~20% lower compared to the average landings from 2018 to 2020). This menhaden purse seine fishery extracts more biomass than any GoM fishery (Karnauskas et al., 2017). While bycatch has historically been considered negligible compared to menhaden landings (de Silva and Condrey, 1998), estimated at approximately 2.35% of total fleet landings by weight (Guillory and Hutton, 1982), this percentage can equate to a substantial amount of bycatch, as first recognized by de Silva and Condrey (1998). Our trade-off analysis revealed depletion of some groups attributed to increased mortality through bycatch of the purse seine menhaden fleet, particularly for large and small coastal sharks, oceanic piscivores (e.g., cutlassfish), demersal coastal invertebrate feeders (e.g., croaker), sea trout (Cynoscion spp.), and coastal piscivores (e.g., ladyfish). For species such as sea trout, estimates of fishing mortality derived from the menhaden purse seine fleet rivaled recreational private estimates in 1980, largely due to peak menhaden landings during this time period. Other species that exhibited relatively high fishing mortality (≥ 50% of F) due to purse seine bycatch included oceanic piscivores, coastal piscivores, and skates–rays.
The development and calibration of the US Gulfwide EwE model represents a substantial advancement to support EBFM in the GoM by providing a valuable tool to build upon our current understanding of the trophic role of Gulf menhaden in the ecosystem. Several trophic groups, including menhaden, passed a defined set of diagnostics aimed at gauging ecosystem model performance, which included appropriate fits to relative biomass and absolute landings time series, as well as agreement between predicted fishing mortality reference points (e.g., FMSY) and stock assessment estimates of FMSY or related proxies. Our findings provide a foundational building block towards implementing EBFM, by identifying key predators of juvenile and adult Gulf menhaden, and quantifying trade-offs across fisheries. One overall pattern that emerged is that menhaden harvest was predicted to result in a reduction in predator biomass, whether by varying fishing mortality on menhaden, by varying the menhaden purse seine effort (representing the effect of bycatch), or by varying both mechanisms. Notably, the decoupling between menhaden fishing mortality and menhaden purse seine effort was applied in EwE to tease apart the effects of fishing mortality and bycatch; however, in reality, such decoupling is not possible. The biomass depletion of groups such as king and Spanish mackerels was largely attributed to the decreased biomass of menhaden as prey. Other groups such as coastal dolphins and seabirds did not conform to expectations of reduced abundance as menhaden harvest increased. This result emphasized the interconnectedness of the ecosystem and diversity in the availability of prey in the GoM. In particular, several forage species (e.g., cephalopods) increased with higher menhaden harvest, potentially due to reduced competition for food. However, it is important to note that estimates of coastal dolphin biomass caught in the menhaden purse seine were not incorporated into this study due to lack of information on biomass scale and historic trend.
Gulf menhaden were characterized by relatively low ecotrophic efficiency (EE) estimates (range 0.03–0.46; Supplementary Table 2), which suggested that a majority of the species production was not accounted for in the model. Low EE translates to higher other mortality (M0), and in such cases, larger portions of “dead menhaden” are directed towards detritus. Previous EwE models of the GoM (Sagarese et al., 2016) produced similar EE estimates. One possible reason may be that the consumption of menhaden was limited by relatively low abundance of predators compared to the menhaden biomass estimates from the stock assessment (SEDAR (Southeast Data Assessment and Review), 2018). Another explanation is that unknown sources of mortality are missing in the model, including environmental stressors such as oxygen-limited dead zones or diseases. A third possibility is underrepresentation of menhaden consumption in the diet matrix, which may stem from inadequate sampling of key predators, robustness of the diet data, rapid degradation of menhaden in predator stomachs, or the coarse taxonomic resolution of prey items in diet studies (Sagarese et al., 2016). If menhaden consumption is significantly greater than we estimated, the results presented here may represent an underestimate of the impact that menhaden harvest can have on their predators.
Increased consideration of forage fish life history and trophic dynamics is warranted (Hilborn et al., 2017); however, our study highlights the challenges of collecting the necessary data to parameterize such classifications, particularly when it comes to age-specific predation and consumption. Additional diet studies aimed at quantifying ontogenetic diet compositions are necessary to deepen our understanding of the trophic role of menhaden in the GoM ecosystem. Confirmation of menhaden predator plausibility in the GoM was partially validated based on spatial overlap in both purse seine bycatch and Louisiana state sampling surveys. Nonetheless, additional research is needed to further validate prey selectivity for Gulf menhaden. Clear gaps exist in available diet data, particularly for juvenile fishes in the GoM, and higher trophic level predators for which comprehensive regional data are lacking. Future data collection efforts focused on Gulf menhaden as prey could include comprehensive stomach sampling in a single year throughout all seasons (e.g., a “Year of the stomachs” such as conducted in the North Sea; Plagányi, 2007) or more comprehensive sampling programs across the range of the menhaden stock. In addition to traditional diet studies, genetic and microchemistry approaches such as DNA barcoding or stable isotope analyses may help gain a better understanding of predation on age 0, juvenile, and adult Gulf menhaden.
While the current Gulfwide EwE model reflects the best available information, our results highlight areas for improvement, and therefore, caution should be exercised in interpreting results for groups exhibiting poor diagnostics. For example, poor fits were noted for many of the highly migratory species, potentially due to population distributions outside the GoM (we assumed trends from basin-wide assessments would hold true in the GoM) or migration effects on biomass or energetics. Additional datasets (e.g., relative abundance from the NMFS Bottom Longline Survey) could be more appropriate for tracking population trends of sharks within the region. Similarly poor fits were noted for many of the age 0 fish groups because EwE does not directly estimate annual recruitment deviations as is common practice within stock assessments. Increased understanding of the drivers behind recruitment for assessed species could enable such mechanisms to be incorporated into the ecosystem model.
Ecosim models are sensitive to the predator–prey vulnerabilities, which ultimately influence the productivity estimates of the trophic groups, how sensitive the fish are to harvest, and the relative influence of top-down (predation, fishing) versus bottom-up (prey, primary production) effects. For example, some of the trade-off plots indicated a weak gradient in biomass ratios associated with predator F rates; i.e., the change in the predator’s fishing pressure has a smaller effect on the predator’s biomass compared to menhaden fishing pressure. This can be explained by the limited response to fishing in the equilibrium analysis, which is often observed when Ecosim estimates very low vulnerabilities, which implies strong compensatory responses in food consumption when biomass decreases due to harvest. We estimated these key parameters by fitting the model to long-term biomass and landings time series, and applying biologically plausible upper and lower bounds. However, future work should evaluate the uncertainty associated with the vulnerability parameters and sensitivity of target and threshold reference points to vulnerability assumptions.
We directly modeled nutrient input from the Mississippi River as a driver of PP. This is a simplistic representation of PP that does not consider the effects of upwelling, water column–nutrient availability, light limitation, and interplay between the different primary producers. In addition, further work remains to integrate environmental factors into the analysis. Given the potential importance of bottom-up environmental drivers on Gulf menhaden abundance and recruitment, additional variables could be incorporated, such as water temperature, ocean circulation, and additional nutrient sources (e.g., upwelling). Examples of important ecosystem components for future consideration include the effects of harmful algal blooms, hypoxia, climate change, and sea level rise. The implementation of Ecospace, the spatial component of EwE, will be beneficial to address such questions. In addition, expanding the model to include a spatial component will likely prove critical for further refining predator–prey interactions concerning Gulf menhaden.
Bycatch by the Gulf menhaden purse seine fishery received considerable attention recently, with debates about the potential impacts on the ecosystem. To our knowledge, this is the first attempt to standardize and incorporate bycatch into an ecosystem model for the northern GoM. Our analysis incorporated the limited information available on bycatch in terms of weight within the purse seine fishery in the 1980s (Guillory and Hutton, 1982) and the mid-1990s (de Silva and Condrey, 1997; de Silva et al., 2001). From a US Gulfwide perspective (the scale on which management is based for many of these species), the results suggested that biomass removals of non-target species could potentially be large for several species. Overall, purse seine bycatch accounted for at least 10% of fishery removals in 6 of the 10 predators considered in the ERPs, with as much as 73% of F attributed to bycatch of inshore coastal piscivores. We note that overestimates of bycatch for some predators would result in lower menhaden ERPs, and vice versa. In the present study, we implemented a static bycatch estimation based on numerous assumptions regarding species composition and magnitude of bycatch by weight based on available studies. While our estimates are realistic as they were all within the bounds of bycatch percentages from the literature, this estimate is likely dynamic. Additional efforts should explore alternative hypotheses regarding purse menhaden bycatch, as incorporation of other scenarios and uncertainty in these estimates will help capture the effects on bycatch species. These limitations call for additional studies to obtain an updated characterization and temporally explicit quantification of the bycatch by weight of the menhaden purse seine fishery to reduce uncertainty and facilitate decision-making, such as whether bycatch removals for some species are substantial enough for consideration during stock assessments. Further development of the US Gulfwide EwE model to include a spatial component (e.g., into regions and depth strata) may also improve the model information content related to Gulf Menhaden and their essential habitat in the northern GoM. The current model structure does not fully capture ecosystem spatial dynamics, including fleet bycatch, previously observed to be spatially restricted (de Silva and Condrey, 1998).
Achieving and operationalizing an ecosystem-based approach to fisheries management is a monumental task that requires substantial resources in the form of data collection and technical expertise by ecosystem modelers. Given the large public interest in forage fish and fishery management in the GoM, successful EBFM is likely going to hinge on collaborative efforts between state, federal, academic, and non-government organizations. Menhaden support large-scale fisheries and predator populations in both the GoM and the Atlantic; however, EBFM for Gulf menhaden in the GoM is challenging for several reasons. First and foremost, the GoM lacks the comprehensive diet information like that provided through regional surveys in the Atlantic, such as the Northeast Fisheries Science Center bottom trawl survey (Link and Almeida, 2000; Smith and Link, 2010) and the Northeast Area Monitoring and Assessment Program survey (Bonzek et al., 2017). Second, Gulf menhaden do not have a history of multispecies modeling efforts as in the Atlantic, where multispecies virtual population analyses were developed between 2005 and 2015 (Garrison et al., 2010; Anstead et al., 2021), which provided a foundation for the ecosystem models that were later used to develop the ERPs (Buchheister et al., 2017; Chagaris et al., 2020). Lastly, acceptance of ERPs for Atlantic menhaden was largely due to broad public support for amendment 3 to the fishery management plan, which called for the ERPs, and the management authority provided to the Atlantic States Marine Fisheries Commission for both Atlantic menhaden and their predators (Anstead et al., 2021). This type of management structure does not yet exist for Gulf menhaden. Therefore, management actions should focus on (1) obtaining additional data to reduce uncertainties in the model, specifically trophic interactions data; (2) co-production of alternative modeling approaches; and (3) implementing changes to the management structure of this fishery.
Our collaborative study provides a comprehensive tool to begin exploring ecosystem dynamics and developing ecologically sensible reference points for managing Gulf menhaden. However, we recommend a multimodel approach to better address key uncertainties in model dynamics and provide a more robust analysis of ecosystem dynamics. For example, models of intermediate complexity and smaller spatial domain (Plagányi et al., 2014) could focus exclusively on menhaden and their key predators, as highlighted by the Atlantic menhaden application (Chagaris et al., 2020; Anstead et al., 2021; Drew et al., 2021; Howell et al., 2021). Focusing on a subset of key species could help reduce the number of parameters and their uncertainty and allow for in-depth evaluation of the sensitivity of the model results to key trophic dynamics (e.g., prey switching; Chagaris et al., 2020). Although this analysis did not elucidate a strong dependence of any single predator on menhaden, our results corroborated other researcher findings (e.g., Leaf and Oshima, 2019) and highlighted multiple coastal predator groups that could be incorporated into ERPs. Moreover, this work is the first to implement a quantitative consideration of the impact of the menhaden purse seine fishery on bycatch species in relation to these species’ population dynamics. This may have regulatory and/or policy implications as assessments for these species may need to explicitly account for bycatch from the menhaden purse seine fishery. In addition, other modeling platforms such as OSMOSE (Shin and Cury, 2001) could also be explored, which would eliminate the need for a diet matrix by requiring different inputs such as gape size and spatial distribution for functional groups (e.g., Grüss et al., 2015).
In order for ecosystem models to be useful for either strategic or tactical purposes, these models will likely need to undergo a technical peer review process (Kaplan and Marshall, 2016; Reum et al., 2021). Simultaneous review of a stock assessment and the ecosystem model(s) would help align ecosystem model products with the data requirements or other needs (e.g., understanding the mechanism) of the stock assessment, as was done for Atlantic menhaden (SEDAR (Southeast Data Assessment and Review), 2020). Such a review process could help build trust in the process and fine-tune data inputs via a collaborative process that could include output from stakeholders and other scientists. Such interdisciplinary collaborations will be essential as fisheries management moves towards ecosystem-based approaches.
Our findings indicated that, in general, a moderate reduction of menhaden fishing pressure would result in a favorable trend of predators’ biomass towards their target value. The fact that this result was consistent for most predatory groups supports the robustness of this finding. This information should be weighted along with the broad set of considerations related to the degree of harvest limitation, taking into account the limitations and uncertainties of our methodology as mentioned above.
The original contributions presented in the study are included in the article/Supplementary Material. The raw data supporting the conclusions of this article will be made available by the authors, upon reasonable request. Further inquiries can be directed to the corresponding authors.
DC, IB, SS, and ML developed the Gulfwide EwE model. AS provided data inputs and conducted the single-species projections. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the National Oceanic and Atmospheric Administration, Resources and Ecosystems Sustainability, Tourist Opportunities and Revived Economies of the Gulf Coast States Act (NOAA RESTORE) Science Program, USA Award Number NA17NOS4510098.
We thank our RESTORE research team, notably K. de Mutsert, R. Ahrens, M. Allen, and N. Farmer, for insightful discussions throughout the development of this model. We are also indebted to all the federal, state, and academic researchers, scientists, staff, and volunteers who have helped acquire the vast amount of fishery-dependent and fishery-independent data and diet composition estimates used within model parameterization. This research was carried out (in part) under the auspices of the Cooperative Institute for Marine and Atmospheric Studies (CIMAS), a Cooperative Institute of the University of Miami and the National Oceanic and Atmospheric Administration, cooperative agreement #NA10OAR4320143. The scientific results and conclusions, as well as any views and opinions expressed herein, are those of the authors and do not necessarily reflect those of any government agency.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.935324/full#supplementary-material
Adams G. D., Leaf R. T., Wu W., Hernandez F. J. (2018). Environmentally driven fluctuations in condition factor of adult gulf menhaden (Brevoortia patronus) in the northern gulf of Mexico. ICES. J. Mar. Sci. 75 (4), 1269–1279. doi: 10.1093/icesjms/fsy002
Ahrens R. N. M., Walters C. J., Christensen V. (2012). Foraging arena theory. Fish. Fish. 13 (1), 41–59. doi: 10.1111/j.1467-2979.2011.00432.x
Alder J., Campbell B., Karpouzi V., Kaschner K., Pauly D. (2008). Forage fish: From ecosystems to markets. Annu. Rev. Environ. Resour. 33, 153–166. doi: 10.1146/annurev.environ.33.020807.143204
Anstead K. A., Drew K., Chagaris D., Schueller A. M., McNamee J. E., Buchheister A., et al. (2021). The path to an ecosystem approach for forage fish management: A case study of Atlantic menhaden. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.607657
Berenshtein I., Sagarese S. R., Lauretta M. V., Chagaris D. D. (2021). Technical documentation of a U.S. gulf of Mexico-wide ecosystem model. NOAA. Tech. Memo. NMFS-SEFSC-751., 229. doi: 10.25923/zj8t-e656
Bonzek C. F., Gartland J., Gauthier D. J., Latour R. J. (2017). Northeast area monitoring and assessment program (NEAMAP) 2016 data collection and analysis in support of single and multispecies stock assessments in the mid-Atlantic: Northeast area monitoring and assessment program near shore trawl survey. Virginia. Inst. Mar. Sci. William. Mary. doi: 10.25773/7206-KM61
Buchheister A., Miller T. J., Houde E. D. (2017). Evaluating ecosystem-based reference points for Atlantic menhaden. Mar. Coast. Fish. 9 (1), 457–478. doi: 10.1080/19425120.2017.1360420
Chagaris D., Binion-Rock S., Bogdanoff A., Dahl K., Granneman J., Harris H., et al. (2017). An ecosystem-based approach to evaluating impacts and management of invasive lionfish. Fisheries 42, 421–431. doi: 10.1080/03632415.2017.1340273
Chagaris D., Drew K., Schueller A., Cieri M., Brito J., Buchheister A. (2020). Ecological reference points for Atlantic menhaden established using an ecosystem model of intermediate complexity. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.606417
Chagaris D. D., Mahmoudi B., Walters C. J., Allen M. S. (2015). Simulating the trophic impacts of fishery policy options on the West Florida shelf using ecopath with ecosim. Mar. Coast. Fish. 7 (1), 44–58. doi: 10.1080/19425120.2014.966216
Chagaris D., Sagarese S., Farmer N., Mahmoudi B., de Mutsert K., Vanderkooy S., et al. (2019). Management challenges are opportunities for fisheries ecosystem models in the gulf of Mexico. Mar. Policy 101, 1–7. doi: 10.1016/j.marpol.2018.11.033
Christensen V., Walters C. J. (2004). Ecopath with ecosim: Methods, capabilities and limitations. Ecol. Model. 172, 109–139. doi: 10.1016/j.ecolmodel.2003.09.003
Christensen V., Walters C. J., Pauly D. (2005). “Ecopath with ecosim: A user’s guide,” in Fish (Vancouver: Centre, Univ. Br. Columbia), 154.
Colléter M., Valls A., Guitton J., Gascuel D., Pauly D., Christensen V. (2015). Global overview of the applications of the ecopath with ecosim modeling approach using the EcoBase models repository. Ecol. Model. 302, 42–53. doi: 10.1016/j.ecolmodel.2015.01.025
Coll M., Steenbeek J., Pennino M. G., Buszowski J., Kaschner K., Lotze H. K., et al. (2020). Advancing global ecological modeling capabilities to simulate future trajectories of change in marine ecosystems. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.567877
Curti K. L., Collie J. S., Legault C. M., Link J. S. (2013). Evaluating the performance of a multispecies statistical catch-at-age model. Can. J. Fish. Aquat. Sci. 70, 470–484. doi: 10.1139/cjfas-2012-0229
Darwall W. R., Allison E. H., Turner G. F., Irvine K. (2010). Lake of flies, or lake of fish? a trophic model of lake Malawi. Ecol. Model. 221, 713–727. doi: 10.1016/j.ecolmodel.2009.11.001
de Mutsert K., Lewis K. A., White E. D., Buszowski J. (2021). End-to-End modeling reveals species-specific effects of Large-scale coastal restoration on living resources facing climate change. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.624532
de Silva J., Condrey R. (1997). “Bycatch in the U.S. gulf of Mexico menhaden fishery,” in Results of onboard sampling conducted in the 1994 and 1995 fishing season (Baton Rouge, Louisiana: Louisiana State University), 125.
de Silva J. A., Condrey R. E. (1998). Discerning patterns in patchy data: a categorical approach using gulf menhaden, Brevoortia patronus, bycatch. Fish. Bull. 96, 193–209.
de Silva J., Condrey R., Anglin K., Rester J. (1996). “Bycatch in the united states gulf of Mexico menhaden fishery,” in Fisheries bycatch consequences & management., Dearborn, Michigan, August 27 & 28, 1996. (Fairbanks, AK: University of Alaska Sea Grant College Program, University of Alaska Fairbanks) 4, Alaska Sea Grant Report 97-02.
de Silva J. A., Condrey R. E., Thompson B. A. (2001). Profile of shark bycatch in the U.S. gulf of Mexico menhaden fishery. N. Am. J. Fish. Manage. 21, 111–124. doi: 10.1577/1548-8675(2001)021<0111:POSBIT>2.0.CO;2
Drew K., Cieri M., Schueller A. M., Buchheister A., Chagaris D., Nesslage G., et al. (2021). Balancing model complexity, data requirements, and management objectives in developing ecological reference points for Atlantic menhaden. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.608059
Essington T. E., Beaudreau A. H., Wiedenmann J. (2006). Fishing through marine food webs. Proc. Natl. Acad. Sci. U.S.A. 103 (9), 3171–3175. doi: 10.1073/pnas.0510964103
Free C. M., Jensen O. P., Hilborn R. (2021). Evaluating impacts of forage fish abundance on marine predators. Conserv. Biol. 35 (5), 1540–1551. doi: 10.1111/cobi.13709
Garrison L. P., Link J. S., Kilduff D. P., Cieri M. D., Muffley B., Vaughan D. S., et al. (2010). An expansion of the MSVPA approach for quantifying predator–prey interactions in exploited fish communities. ICES. J. Mar. Sci. 67 (5), 856–870. doi: 10.1093/icesjms/fsq005
Geers T. M., Pikitch E. K., Frisk M. G. (2016). An original model of the northern gulf of Mexico using ecopath with ecosim and its implications for the effects of fishing on ecosystem structure and maturity. Deep. Sea. Res. Part II. Top. Stud. Oceanogr. 129, 319–331. doi: 10.1016/j.dsr2.2014.01.009
Grimes C. B. (2001). Fishery production and the Mississippi river discharge. Fisheries 26 (8), 17–26. doi: 10.1577/1548-8446(2001)026<0017:FPATMR>2.0.CO;2
Grüss A., Schirripa M. J., Chagaris D., Drexler M., Simons J., Verley P., et al. (2015). Evaluation of the trophic structure of the West Florida shelf in the 2000s using the ecosystem model OSMOSE. J. Mar. Syst. 144, 30–47. doi: 10.1016/j.jmarsys.2014.11.004
GSMFC (Gulf States Marine Fisheries Commission) (2015). “The gulf menhaden fishery of the gulf of Mexico a regional management plan,” in Gulf states marine fisheries commission, vol. 240. (Mississippi: Ocean Springs), 220. Available at: https://www.gsmfc.org/publications/GSMFC%20Number%20240.pdf.
GSMFC (Gulf States Marine Fisheries Commission) (2021). “GDAR 03 gulf menhaden stock assessment 2021 update,” in Gulf states marine fisheries commission (Mississippi: Ocean Springs), 73. Available at: https://www.gsmfc.org/publications/GSMFC%20Number%20308.pdf.
Guillory V., Hutton G. (1982). A survey of bycatch in the Louisiana gulf menhaden fishery. Proc. Annu. Conf. Southeastern. Assoc. Fish. Wildlife. Agencies. 36, 213–233.
Hale M. B., Bauersfeld P. E., Galloway S. B., Joseph J. D. (1991). New products and markets for menhaden, Brevoortia spp. Mar. Fish. Rev. 53, 42–48.
Heenan A., Williams G. J., Williams I. D. (2020). Natural variation in coral reef trophic structure across environmental gradients. Front Ecol Environ 18 (2), 69–75. doi: 10.1002/fee.2144
Heymans J. J., Coll M., Link J. S., Mackinson S., Steenbeek J., Walters C., et al. (2016). Best practice in ecopath with ecosim food-web models for ecosystem-based management. Ecol. Model. 331, 173–184. doi: 10.1016/j.ecolmodel.2015.12.007
Hilborn R., Amoroso R. O., Bogazzi E., Jensen O. P., Parma A. M., Szuwalski C., et al. (2017). When does fishing forage species affect their predators? Fish. Res. 191, 211–221. doi: 10.1016/j.fishres.2017.01.008
Hilborn R., Amoroso R. O., Bogazzi E., Jensen O. P., Parma A. M., Szuwalski C., et al. (2018). Response to pikitch et al. Fish. Res. 198, 224. doi: 10.1016/j.fishres.2017.07.025
Hobday A. J., Smith A. D. M., Stobutzki I. C., Bulman C., Daley R., Dambacher J. M., et al. (2011). Ecological risk assessment for the effects of fishing. Fish. Res. 108, 372–384. doi: 10.1016/j.fishres.2011.01.013
Howell D., Schueller A. M., Bentley J. W., Buchheister A., Chagaris D., Cieri M., et al. (2021). Combining ecosystem and single-species modeling to provide ecosystem-based fisheries management advice within current management systems. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.607831
Kaplan I. C., Marshall K. N. (2016). A guinea pig's tale: Learning to review end-to-end marine ecosystem models for management applications. ICES. J. Mar. Sci. 73 (7), 1715–1724. doi: 10.1093/icesjms/fsw047
Karnauskas M., Kelble C. R., Regan S., Quenée C., Allee R., Jepson M., et al. (2017). 2017 ecosystem status report update for the gulf of Mexico. NOAA. Tech. Memo. NMFS-SEFSC-706., 51 pp.
Leaf R. T. (2017). Environmental determinants of gulf menhaden (Brevoortia patronus) oil content in the northern gulf of Mexico. Ecol. Indic. 82, 551–557. doi: 10.1016/j.ecolind.2017.07.031
Leaf R. T., Oshima M. C. (2019). Construction and evaluation of a robust trophic network model for the northern gulf of Mexico ecosystem. Ecol. Inform. 50, 13–23. doi: 10.1016/j.ecoinf.2018.12.005
Link J. S. (2010). Adding rigor to ecological network models by evaluating a set of pre-balance diagnostics: A plea for PREBAL. Ecol. Model. 221, 1580–1591. doi: 10.1016/j.ecolmodel.2010.03.012
Link J. S., Almeida F. P. (2000). An overview and history of the food web dynamics program of the northeast fisheries science center, woods hole, Massachusetts. NOAA. Tech. Memo. NMFS-NE-159., 64.
MSFCMA (Magnuson–Stevens Fishery Conservation and Management Act) (2007). Magnuson-Stevens fishery conservation and management act. U.S. department of commerce, national oceanic and atmospheric administration. Natl. Mar. Fish. Service., 170.
NMFS (National Marine Fisheries Service) (2021) Fisheries of the united states 2019. U.S. department of commerce, NOAA. current fishery statistics no. 2019. Available at: https://www.fisheries.noaa.gov/national/sustainable-fisheries/fisheries-united-states.
Pauly D., Christensen V., Dalsgaard J., Froese R., Torres F. (1998). Fishing down marine food webs. Science 279 (5352), 860–863. doi: 10.1126/science.279.5352.860
Pikitch E. K., Boersma P. D., Boyd I. L., Conover D. O., Cury P., Essington T. E., et al. (2018). The strong connection between forage fish and their predators: A response to hilborn et al., (2017). Fish. Res. 198, 220–223. doi: 10.1016/j.fishres.2017.07.022
Pikitch E., Boersma P. D., Boyd I. L., Conover D. O., Cury P., Essington T., et al. (2012). “Little fish, big impact: Managing a crucial link in ocean food webs,” in Lenfest ocean program (Washington, DC: Lenfest Ocean Program), 108.
Pikitch E. K., Rountos K. J., Essington T. E., Santora C., Pauly D., Watson R., et al. (2014). The global contribution of forage fish to marine fisheries and ecosystems. Fish. Fish. 15 (1), 43–64. doi: 10.1111/faf.12004
Pikitch E. K., Santora C., Babcock E. A., Bakun A., Bonfil R., Conover D. O., et al. (2004). Ecosystem-based fishery management. Science 305 (5682), 346–347. doi: 10.1126/science.1098222
Plagányi É. E. (2007). Models for an ecosystem approach to fisheries Vol. 477 (Rome, FAO: FAO Fisheries Technical Paper) 1, 108. doi: 10.25607/OBP-392
Plagányi É. E., Punt A. E., Hillary R., Morello E. B., Thébaud O., Hutton T., et al. (2014). Models of intermediate complexity for ecosystem assessment to support tactical management decisions in fisheries and conservation. Fish. Fish. 1, 1–22. doi: 10.1111/j.1467-2979.2012.00488.x
Reum J. C., Townsend H., Gaichas S., Sagarese S., Kaplan I. C., and Grüss A. (2021). It’s not the destination, it’s the journey: Multispecies model ensembles for ecosystem approaches to fisheries management. Front. Mar. Sci. 8, 631839. doi: 10.3389/fmars.2021.631839
Robinson K. L., Ruzicka J. J., Decker M. B., Brodeur R. D., Hernandez F. J., Quiñones J., et al. (2014). Jellyfish, forage fish, and the world’s major fisheries. Oceanogr 27 (4), 104–115. doi: 10.5670/oceanog.2014.90
Sagarese S. R., Lauretta M. V., Walter J. F. III (2017). Progress towards a next-generation fisheries ecosystem model for the northern gulf of Mexico. Ecol. Model. 345, 75–98. doi: 10.1016/j.ecolmodel.2016.11.001
Sagarese S. R., Nuttall M. A., Geers T. M., Lauretta M. V., Walter J. F., Serafy J. E. (2016). Quantifying the trophic importance of gulf menhaden Brevoortia patronus within the northern gulf of Mexico ecosystem. Mar. Coast. Fish. 8, 23–45. doi: 10.1080/19425120.2015.1091412
SEDAR (Southeast Data Assessment and Review) (2018). “SEDAR 63: Gulf of Mexico menhaden,” (North Charleston, SC: SEDAR) 352. Available at: http://sedarweb.org/docs/sar/S63_GulfMenSAR_12.17.2018_FINAL.pdf.
SEDAR (Southeast Data Assessment and Review) (2020). “SEDAR 69: Atlantic menhaden benchmark stock assessment report,” (North Charleston, SC: SEDAR, north Charleston), 691. Available at: http://sedarweb.org/sedar-69.
Shin Y. J., Cury P. (2001). Exploring fish community dynamics through size-dependent trophic interactions using a spatialized individual-based model. Aquat. Living. Resour. 14 (2), 65–80. doi: 10.1016/S0990-7440(01)01106-8
Sinnickson D., Chagaris D., Allen. M. (2021). Exploring impacts of river discharge on forage fish and predators using ecopath with ecosim. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.689950
Siple M. C., Essington T. E., Plagányi É. (2019). Forage fish fisheries management requires a tailored approach to balance trade-offs. Fish. Fish. 20 (1), 110–124. doi: 10.1111/faf.12326
Smith J. (1991). The Atlantic and gulf menhaden purse seine fisheries: Origins, harvesting technologies, biostatistical monitoring, recent trends in fisheries statistics, and forecasting. Mar. Fish. Rev. 53 (4), 28–41.
Smith B., Link J. (2010). The trophic dynamics of 50 finfish and 2 squid species on the northeast US continental shelf. U.S. dep. commer. NOAA. Tech. Memo. NMFS-NE-21., 29.
Tacon A. G., Metian M. (2009). Fishing for aquaculture: non-food use of small pelagic forage fish—a global perspective. Rev. Fish. Sci 17 (3), 305–317. doi: 10.1080/10641260802677074
Uphoff J. H. Jr, Sharov A. (2018). Striped bass and Atlantic menhaden predator–prey dynamics: Model choice makes the difference. Mar. Coast. Fish. 10, 370–385. doi: 10.1002/mcf2.10030
Walters W. J., Christensen V. (2018). Ecotracer: analyzing concentration of contaminants and radioisotopes in an aquatic spatial-dynamic food web model. J. Environ. Radioact. 181, 118–127. doi: 10.1016/j.jenvrad.2017.11.008
Walters C. J., Christensen V., Martell S. J., Kitchell J. F. (2005). Possible ecosystem impacts of applying MSY policies from single-species assessment. ICES. J. Mar. Sci. 62 (3), 558–568. doi: 10.1016/j.icesjms.2004.12.005
Keywords: ecosystem-based fisheries management, forage fish, Gulf menhaden, trophic interactions, fishing mortality, bycatch
Citation: Berenshtein I, Sagarese SR, Lauretta MV, Schueller AM and Chagaris DD (2023) Identifying trade-offs and reference points in support of ecosystem approaches to managing Gulf of Mexico menhaden. Front. Mar. Sci. 9:935324. doi: 10.3389/fmars.2022.935324
Received: 03 May 2022; Accepted: 02 December 2022;
Published: 06 January 2023.
Edited by:
Catherine Sarah Longo, Marine Stewardship Council (MSC), United KingdomReviewed by:
Cameron Ainsworth, University of South Florida, United StatesCopyright © 2023 Berenshtein, Sagarese, Lauretta, Schueller and Chagaris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Igal Berenshtein, iberensht@univ.haifa.ac.il; Skyler R. Sagarese, skyler.sagarese@noaa.gov; David D. Chagaris, dchagaris@ufl.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.