
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 22 August 2022
Sec. Marine Megafauna
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.935235
This article is part of the Research Topic Risks, Threats, and Conservation Status of Cetaceans in the Mediterranean and Black Seas View all 15 articles
 Daniela Silvia Pace1,2*
Daniela Silvia Pace1,2* Sara Ferri1
Sara Ferri1 Giancarlo Giacomini1
Giancarlo Giacomini1 Chiara Di Marco1
Chiara Di Marco1 Elena Papale2,3
Elena Papale2,3 Margherita Silvestri4
Margherita Silvestri4 Giulia Pedrazzi1
Giulia Pedrazzi1 Daniele Ventura1
Daniele Ventura1 Edoardo Casoli1
Edoardo Casoli1 Giandomenico Ardizzone1
Giandomenico Ardizzone1Sociality and ecological drivers that can influence individual association patterns are infrequently considered in wildlife management, although they are essential aspects affecting animals’ responses to both human-related pressures and conservation strategies. In common bottlenose dolphins (Tursiops truncatus), sex-specific social dynamics and interactions with anthropogenic activities may affect grouping and induce changes in relationships between individuals. Out of a total of 347 individuals, we assessed the level of association among 68 bottlenose dolphins that have been sighted more than five times near the Roman coast (central Mediterranean Sea, Italy). The half-weight index (HWI) of dyadic associations, their network relations, and stability over time were investigated by using the SOCPROG software. Outcomes showed that females were more strongly associated than other individuals, with both preferred constant short-term associations and random long-term associations, possibly resulting in greater success in rearing young. Individuals interacting with the bottom trawl fishery showed weaker and short-term associations. Temporary disruption of individual associations during interaction with fishery and the relatively low number of females with calves participating in depredation seem to denote both the opportunistic nature of interactions with fishing vessels and the offspring-related protection strategy. The results show that the dolphins in this region maintain a complex but flexible social structure that varies with local biological requirements and is resilient to anthropogenic pressures.
Differences in gregariousness and social relationships are features of many group-living mammalian species, influencing numerous traits of an individual’s life, from feeding success, mate selection, and grouping in different life stages, to habitat preference (Pace et al., 2014a; Majolo and Huang, 2018; Pace et al., 2018) and defense against predators (Heithaus and Dill, 2006; Wirsing et al., 2008; Miller et al., 2022). Group formation is a highly dynamic process subjected to many variations in size and member composition over different temporal scales. Groups can separate (fission) or merge (fusion) (Couzin, 2006; Couzin and Laidre, 2009), and this fluid joining and splitting possibly evolved as an adaptive strategy to minimize competition in relation to the fickleness of resources, the mating access, and the habitat type (Majolo and Huang, 2018). The flexible scheme, within fission–fusion societies, is assumed to enable mammals to react to highly mobile resources and/or rapid-changing pressures. It allows to adjust the number of associates and to shift the identity of the individuals with which they relate (Archie and Chiyo, 2012). However, ‘strategic’ non-random association patterns and long-term social preferences may emerge in specific conditions or contexts. They can guide, for example, the development of sympatric communities, as in some common bottlenose dolphin (Tursiops truncatus, hereinafter ‘bottlenose dolphins’) populations with specializations in hunting/foraging techniques (Chilvers and Corkeron, 2001; Wiszniewski et al., 2009; Daura–Jorge et al., 2012; Pace et al., 2012). In the fluid fission–fusion societies of bottlenose dolphins, the composition of group members changes over hours, days, or seasons (Gowans et al., 2007). Differences in interaction patterns and social affinity between and within genders are also recognized (Moreno and Acevedo-Gutiérrez, 2016). For example, sex-specific social dynamics, such as sex-age segregation (in Florida: Wells et al., 1987), hierarchical male alliances (in Australia: Randić et al., 2012; Connor and Krützen, 2015), strong or preferred associations between/within sexes (in New Zealand and Australia: Lusseau et al., 2003; Gero et al., 2005, respectively), and female–male affiliations with the absence of male alliances (in Ireland: Baker et al., 2020), may have effects on social structure.
Bottlenose dolphin social structure shows a high degree of flexibility and adaptations, with changes in the association patterns relative to different pressures acting on local populations (Papale et al., 2017; Louis et al., 2018). The environmental conditions, habitat features (Lusseau et al., 2003; Wiszniewski et al., 2009), behavioral states (Moreno and Acevedo-Gutiérrez, 2016), and prey availability and predictability (Gowans et al., 2007; Wiszniewski et al., 2009), are considered drivers for variations in bottlenose dolphin social structure (Díaz López and Shirai, 2008; Blumstein, 2010; Pace et al., 2012; Blasi and Boitani, 2014; Genov et al., 2019; Bonizzoni et al., 2021; Frau et al., 2021). Furthermore, anthropogenic factors and other mediating forces that alter resource accessibility and distribution can influence the association pattern among individuals. For example, individuals opportunistically exploiting both aquaculture cages and trawling fishery may form long-term preferred companionship (Pace et al., 2012). However, during opportunistic feeding behavior at marine fish farms, the number of dolphin associations is described to decrease, as it is easier to capture prey, and cooperation is not as necessary (Díaz López and Shirai, 2008). A trawler efficiently herds species that are part of the bottlenose dolphin diet, letting dolphins decrease the effort spent feeding, in both energy and time, thus enhancing foraging effectiveness (Fertl and Leatherwood, 1997; Pace et al., 2012). The energetic benefit of depredation (Tixier et al., 2015) comes with an increased risk of injury for individuals, incidental capture (bycatch), and/or mortality during the interaction with the fishing gear, leading to a risk–reward trade-off that can modify individual behavior and social dynamics (Santana–Garcon et al., 2018; Buscaino et al., 2021). Several common bottlenose dolphin populations are known to interact with different fishing gears (e.g., Blasi and Boitani, 2014; Pennino et al., 2015; Buscaino et al., 2021), and a number of reports are related to trawling boats (e.g., Pace et al., 2012; Genov et al., 2019; Bonizzoni et al., 2021; Bonizzoni et al., 2022), with individuals intentionally entering the nets and actively take advantage of fisheries through depredation (i.e., injuries or removal of captured fish from a fishing gear; Chilvers and Corkeron, 2001; Hamer et al., 2012).
The interaction with trawling vessels highlights conservation issues and management implications as well (e.g., Chilvers and Corkeron, 2001; Pace et al., 2012; Bonizzoni et al., 2021; Vella et al., 2021; Bonizzoni et al., 2022). It clearly influences social dynamics and changes the pattern of interactions at the group level, possibly affecting demographic parameters such as survival and reproduction (Maldonado-Chaparro and Chaverri, 2021). The disruption of groups and the loss of individuals that may play central roles within a social network, possibly holding key information (e.g., the location of food resources or the fulfillment of specific feeding strategies), could induce the loss of behavioral diversity (Kühl et al., 2019) and may result in a reduction of adaptability to changing conditions (He et al., 2019). Sociality and ecological drivers that affect population dynamics and select for individual association patterns are infrequently considered in wildlife management (Bolaños-Jiménez et al., 2021), although they affect animals’ responses to both human-related pressures and conservation strategies (Díaz López, 2019). Considering that different geographic units may have highly variable sizes, distribution patterns, degrees of exposure to potential anthropogenic threats, and flexible social structures, understanding drivers affecting association patterns could be crucial for dolphin conservation (Avila et al., 2018; Díaz López, 2019). Information on the social structure of bottlenose dolphin units in the Mediterranean Sea is scattered and not fully reported yet (Blasi and Boitani, 2014). Some of the available data seem to suggest that sex composition (Blasi and Boitani, 2014) and operational trawlers (Pace et al., 2012; Genov et al., 2019; Bonizzoni et al., 2021; Bonizzoni et al., 2022) may play a pivotal role in shaping social structure and patterns of individual arrangements. Here, the social structure of the bottlenose dolphins living nearby the Tiber River estuary (central Mediterranean Sea, Italy) is reported, with the aim of providing an initial assessment of the relationships between individuals in this geographical unit under local conditions. Furthermore, the following goals are achieved: a) evaluating the level of association between individuals within groups, b) examining if any evidence occurred for sex-specific patterns, c) assessing eventual changes in groups opportunistically interacting with trawlers, and d) discussing ecological implications and associated conservation issues.
The study area (Figure 1) is located in the central Tyrrhenian Sea (Mediterranean Sea, Italy). The area is characterized by a variety of habitats (seagrass meadows; hard and soft bottom communities within coastal banks and cliffs; Ventura et al., 2015; Ardizzone et al., 2018; Ventura et al., 2018; Bonifazi et al., 2019; Casoli et al., 2019) and includes the Tiber River estuary. The southern portion of the seabed of the Tiber River’s mouths presents several habitats of biological importance (i.e., coralligenous outcrops and Posidonia oceanica meadows, extending on both sandy and rocky substrata), which are included in the EU Natura 2000 network Sites of Community Importance and the Marine Protected Area of Secche di Tor Paterno (MPA IT6000010, 1,387 ha).
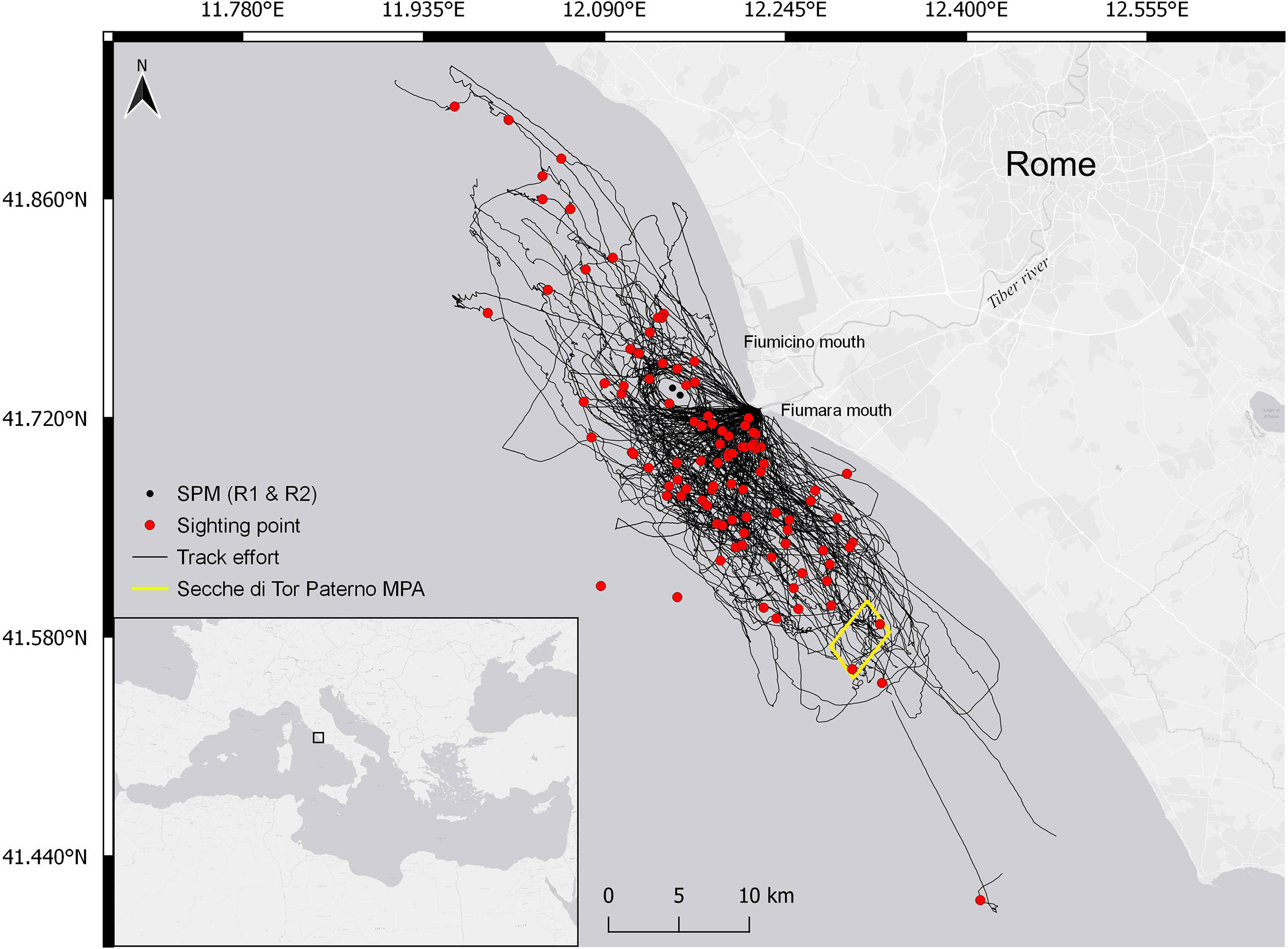
Figure 1 Study area in Central Tyrrhenian Sea (Mediterranean Sea, Italy). Black lines represent the 2017–2020 survey tracks, red dots correspond to the location of the bottlenose dolphin encounters, black dots identify the two single-point moorings (SPMs), and the yellow square delimits the Secche di Tor Paterno marine protected area (MPA).
At about 3 nautical miles off the two Tiber River mouths is situated a terminal including two single-point moorings (SPMs) handling crude and petroleum products. Navigation, anchoring, diving, and fishing are banned within a radius of 750 m from each SPM. These structures are reported to attract some dolphin species (Triossi et al., 2013; Todd et al., 2020), as confirmed by the regular presence of groups of bottlenose dolphins around them (Pace et al., 2019; Pace et al., 2021). Bottlenose dolphins are also commonly reported at the mouths of the Tiber River and the Secche di Tor Paterno MPA (Pace et al., 2019; Pace et al., 2021; Martino et al., 2021). Three hundred forty-seven unique individuals have been photo identified, with females showing a high degree of site fidelity, low levels of dispersion, and localized movements (Pace et al., 2021). Mother–calf dyads have been observed throughout all survey seasons (May–November). Bottlenose dolphin occurrence is possibly favored by the ecological conditions and heterogeneity of morphological features of the region (Ardizzone et al., 2018). Water mass circulation generates upwellings that support high productivity, making the area a suitable site for both feeding and nursing bottlenose dolphins (Pace et al., 2019; Pedrazzi et al., 2022). The study area is considered a valuable ground for the commercial fishery as well (Ardizzone et al., 2018) and is characterized by a high proportion of small-scale artisanal fishery and larger trawling vessels (Ardizzone et al., 2018). Bottom trawlers belonging to the Fiumicino fleet, consisting of 25–30 vessels typically 18–25 m long, operate on both the continental shelf and the slope, running 1-day fishing trips from Monday to Friday year-round. Interactions with fishery were commonly observed in the bottlenose dolphin population frequenting the area (Pace et al., 2019; Pace et al., 2021; Pace et al., 2022). Dolphins have been observed foraging almost evenly across all the study sites, where target prey species (Blanco et al., 2001; Bearzi et al., 2009) are distributed. These include demersal species (European hake, Merluccius merluccius; red mullets, Mullus barbatus and Mullus surmuletus), small pelagic fishes (sardine, Sardina pilchardus; anchovy, Engraulis encrasicolus), cephalopods (common octopus, Octopus vulgaris; horned octopus Eledone cirrhosa), and crustaceans (shrimps, Parapenaeus longirostris; Norway lobster, Nephrops norvegicus), which are also main fishery targets (Ardizzone et al., 2018). Prey abundance appears to peak in August, with anchovies representing more than 20% of the fishery catches (EMODnet, 2022; EUMOFA, 2022).
Focal follows and photo-identification protocols over four survey seasons, from August 2017 to November 2020, were conducted. Data were collected onboard a sailing vessel Beneteau, model Oceanis, length 41.1 ft, powered by a 55-hp Volvo diesel engine. Daily surveys were carried out principally during summer, in favorable weather conditions (i.e., sea state ≤ 3 Douglas, wind force ≤ 3 Beaufort, no rain, and no fog). Surveys were conducted by three to six observers alternating between 7 × 50 and 7 × 80 binoculars and the naked eye, at a steady speed of 4–6 knots. Survey track lines did not follow a standardized scheme but an adaptive procedure (Dawson et al., 2008; La Manna et al., 2016; La Manna et al., 2020; Martino et al., 2021). Acoustic data were also collected (Papale et al., 2021; Pace et al., 2022).
A group of dolphins were defined as a number of individuals with relatively close spatial cohesion (i.e., each member within 10 m of any other member) engaged in similar predominant behavioral activities (Parra et al., 2006). When a dolphin group was sighted, location, time, direction, behavior, size, age classes (see below), and the presence of concomitant anthropogenic activities (e.g., fishing vessels, fishing gears, and pleasure boats) were recorded. More specifically, the occurrence of trawlers and the presence of dolphins interacting with the fishing vessels were evaluated. Dolphins were considered to interact with trawlers when following the operating vessel at a variable distance (from less than 100 to 400 m; similarly to Pace et al., 2012; Genov et al., 2019), alternating sequences of short surfacing with dives of different duration (from 2 to 6 min), and often showing surface behaviors (e.g., rushes and leaps).
Two observers collected photographs of the dorsal fins using Canon digital 5D and 6D cameras and Canon 70–300 and 100–400 mm f/4.5–5.6L lenses. Once observers were confident that the best possible photographs had been acquired, or the animals were lost, dolphin sighting ended. Total group size and age class composition were estimated in the field and then corrected (if needed) via photo-identification analysis. Age class was defined following Pace et al. (2021): adult = an individual generally of a length of about 2.8–3.0 m; juvenile = a poorly scarred and rarely nicked individual of about 2/3 the length of an adult; calf = an individual of about 1/2 the length of an adult, with often visible fetal folds, always in echelon swimming position close to an adult mid-lateral flank; and newborn = an individual of about 1/3 the length of an adult, with visible fetal folds, swimming uncoordinatedly always in echelon position, very close to an adult. Sex was determined whenever possible using the following procedures (Pace et al., 2021): the collection of photographs of the genital area of individuals or the observation of constant adult–offspring associations during one or more encounters (the adult was assumed to be a female). Each sighting and related photo-identification analysis were considered an independent sample.
Photographs were classified considering their quality (see Würsig and Jefferson, 1990), and a quality grade (G) of between 1 and 5 was assigned to each image. Only high-quality photos with G ≥ 4 were used. The occurrence and position of permanent natural markings on the dorsal fins (such as nicks and notches) and on the body were used to univocally recognize dolphins (Pulcini et al., 2014; Urian et al., 2015; Mariani et al., 2016; Mussi et al., 2021). The individual distinctiveness was scored as well-marked (individuals with highly distinctive dorsal fins and scars on the body), fairly marked (individuals with moderately distinctive dorsal fins), and unmarked (individuals with no distinctive features on dorsal fins) (see Pace et al., 2021 for further details).
The ‘gambit of the group’ (GoG) assumption, i.e., each animal in a group or a cluster is associating and interacting with every other animal in that group, was adopted to examine dolphins’ association patterns (Whitehead and Dufault, 1999; Whitehead, 2008a; Franks et al., 2010). To reduce biases (Chilvers and Corkeron, 2001; Bouveroux et al., 2019), and to include bottlenose dolphins regularly frequenting the study area, only distinctive individuals encountered on ≥5 occasions in two to four different years were used for the association analysis (all individuals, AI dataset; n = 68). The number of 68 individuals resulted in a powerful sample size considering an estimated abundance of 80 resident bottlenose dolphins in the study area (see Pace et al., 2021, for details on population abundance). The sample size was calculated using G*Power 3 software (Faul and Erdfelder, 1992; Faul et al., 2007). Then, association patterns were specifically examined in a) the subgroup of individuals classified as ‘females’ (females only, FO dataset; n = 23), b) the subgroup of individuals interacting with trawling vessels at least 70% of their total encounter occasions (individuals in the presence of trawls, PT dataset; n = 23), and c) the subgroup of individuals not interacting with trawling vessels at least 70% of their total encounter occasions (individuals in the absence of trawls, AT dataset; n = 27). We used the 70% threshold since about 30% of the sightings occurred when trawling vessels were not operating (every Saturday and Sunday, and during the fishing break period for biological recovery each year). Daily sampling periods were used to remove demographic effects occurring during the study period, such as birth, death, immigration, and emigration (Whitehead, 2008b; Bouveroux et al., 2019).
Three basic approaches were considered using the software SOCPROG version 2.9 (Whitehead, 2009a): 1) the dyadic association levels, 2) the network metrics, and 3) the type and temporal stability of the associations. Dyadic associations were evaluated with the half-weight index (HWI) (Cairns and Schwager, 1987). HWI measures the proportion of times a pair of individuals was associated, ranging from 0 to 1 (with 0 indicating a pair never observed together and 1 a pair always observed together). Following Quintana–Rizzo and Wells (2001), HWIs were classified into categories based on strength of associations. Mean and maximum levels of association (HWIave and HWImax, respectively) were examined for each individual. The social differentiation (S), i.e., the coefficient of variation (CV) of the true association indices, represents how varied the social system is. This was estimated by maximizing the likelihood of observed dyadic associations using the algorithm available in SOCPROG. S values close to 0 reveal a very homogeneous society, S close to 0.5 indicates quite well-differentiated societies, and S > 2 indicates extremely differentiated societies (Whitehead, 2008b).
To characterize social bonds between individuals, a mean linkage hierarchical cluster analysis was performed. Results were represented as dendrogram only if the cophenetic correlation coeficient (CCC, i.e., the correlation between real HWIs and the levels of clustering between individuals)—which ranges from 0 to 1—was greater than about 0.8 (which indicates a reliable separation among clusters; Whitehead, 2008b). The modularity clustering technique (Newman, 2006) was then applied to understand whether the population is divided into clusters of individuals based on social affiliations. The modularity coefficient (Q), i.e., the difference between the observed and the expected proportion of the total of the HWIs within clusters (Newman, 2006; Dungan et al., 2016), was calculated in SOCPROG. Q values ≥ 0.3 reveal strong divisions in the population (Newman, 2006).
The presence of preferred (non-random) associations among dolphins was tested through a modified permutation test against the null hypothesis that the dolphins were randomly associated. The Manly and Bëjder permutation technique (Manly, 1995; Bëjder et al., 1998) in SOCPROG—with extensions advanced by Whitehead (1999); Whitehead et al. (2005) and corrections introduced by Krause et al. (2009)—was used. The association matrices were randomly permutated 10,000 times with 1,000 flips per permutation, with HWIs being calculated after each permutation, at which point the p-values stabilized (Whitehead, 2009a and b). Since the p-value cannot be considered as a statistical threshold to identify significant associations (Whitehead, 2008a), an arbitrary threshold was fixed to identify the significant associations at twice the mean association index of the population, including zero values (Gero et al., 2005; Frau et al., 2021). The hypothesis of non-random associations (i.e., preferred companionships in the population) in the observed matrix was accepted if the value of the standard deviation (SD) and the coefficient of variation (CV) were significantly higher than those computed from the randomly permuted data (Whitehead and Dufault, 1999; Whitehead, 2008b).
Then, the social structure was examined through specific network metrics (Wey et al., 2008; Croft et al., 2011). To measure how individuals were connected and/or central in the groups (Whitehead, 2008b), the following parameters were estimated in SOCPROG. a) The strength: it indicates the gregariousness of each individual, so larger values suggest a broad preference for larger groups. b) The affinity: it measures if individuals are strongly connected to other individuals that have also strong connections, so an individual with high affinity has relatively high associations with individuals that have high strength. c) The eigenvector centrality: it determines an individual’s relevance (connectedness) in the network, so higher values indicate that individuals generally have high gregariousness and/or are connected to individuals with high gregariousness. d) The reach: it evaluates the indirect connectedness of an individual, so a high value indicates that individuals are indirectly linked to many others in the population. e) The clustering coefficient: it measures how well the associates of an individual are themselves associated, so a value of 0 indicates none of an individual’s associates are associated with each other, and a value of 1 indicates that are all associates of each other with equal weight (Whitehead, 2008b; Titcomb et al., 2015; Dungan et al., 2016). In a well-connected network, all these measurements are likely significantly higher than expected at random. To graphically display network relationships and illustrate the structure of each network, sociograms were obtained with NetDraw 2.123 (Borgatti et al., 2002) using double HWIave values and their multiples (Diaz-Aguirre et al., 2020).
Finally, to determine the stability over time of associations, the standardized lagged association rate (SLAR; Whitehead, 1995) was calculated for the AI, FO, PT, and AT datasets. The SLAR estimates the probability of resighting two individuals in association at t(x), after having found them associated at t(0). The following four exponential models in SOCPROG were fitted to SLAR to describe the temporal patterning of bottlenose dolphin associations at the Tiber River estuary. 1) Preferred companions: some pairs of individuals have a preference for associating, which is constant over time, suggesting permanent associations. 2) Casual acquaintances: some pairs of individuals associate for some time, disassociate, and may reassociate. 3) Constant companions and casual acquaintances: association followed by disassociation at some time lag to a lower level of associations where associations stabilize. 4) Two levels of casual acquaintances: association and disassociation occurring on two different time scales. The best-fitting model was chosen according to the lowest quasi-Akaike information criterion (QAIC) (Burnham and Anderson, 2002).
A total of 137 surveys were conducted between 2017 and 2020, covering a total of 4,967 km on-effort within the study area (Figure 1). As reported in Pace et al. (2021), 105 bottlenose dolphin groups were encountered during surveys; their distribution within the study area is shown in Figure 1. Group size ranged from one to 65 animals, with an average value of 15. The typical group composition consisted of several adults, mostly accompanied by calves (70% of sightings). Three hundred forty-seven (347) unique individuals were identified from 104,781 high-quality images (40% of the total photographs collected). The maximum number of re-sighting was 30 for a single animal, while 65% of individuals (n = 226) were recorded only once or twice (Figure 2A). The discovery curve for the overall number of identified individuals regularly increased throughout the study, while the curve of the 68 identified at least five times (AI dataset) showed a stabilization over time (Figure 2B).
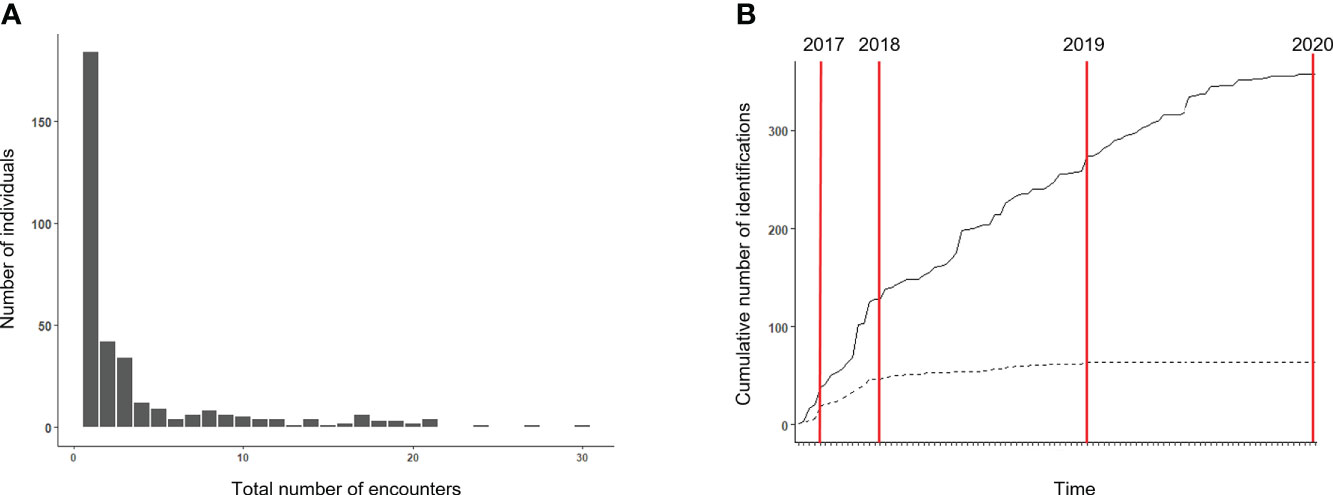
Figure 2 (A) Frequency distribution of the number of encounters of individual bottlenose dolphins. (B) Cumulative discovery curves for bottlenose dolphin individuals over the study period. The solid line shows the discovery curve for the overall 347 individuals identified; the broken line represents the discovery curve for the 68 individuals seen at least five times.
The estimate of social differentiation (S) indicates a good representation of the social pattern, with a quite well-differentiated population (S = 0.54, SE = 0.06, n = 100 bootstrap replicates). The overall HWIave obtained for the individuals in the AI dataset (n = 68) was 0.18 ± 0.06, suggesting very low levels of associations. However, the overall HWImax was considerably higher (0.58 ± 0.13), with 14 individuals (21% of the total) showing HWImax ≥ 0.70. Five of these individuals with higher HWImax were females. Indeed, females (FO dataset, n = 23) showed a higher HWIave (0.25 ± 0.08) than the overall individuals, with 13 individuals (57%) strongly associated (HWIave > 0.5), including a pair (seen between 18 and 27 times) having HWIave > 0.7 (Figure 3, top panel, CCC ≥ 0.80). The modularity coeficient (Q = 0.11) also indicates divisions in the female network (the best division into clusters was at an association index of 0.3), resulting in two main clusters of six and 10 individuals.

Figure 3 Hierarchical cluster analysis of female bottlenose dolphins (FO dataset, n = 23; top panel) and individuals mostly seen without interactions with trawling vessels (AT dataset, n = 27; bottom panel) using the half-weight index.
Monte Carlo permutation tests for equal variances (n = 10,000) showed a significant lower HWIave (F = 6.565; p = 0.0001) and HWImax (F = 2.418; p = 0.0087) for individuals mostly seen in association with trawling vessels (PT dataset; HWIave = 0.21 ± 0.04; HWImax = 0.57 ± 0.12) compared to dolphins preferentially observed without interactions (AT dataset; HWIave = 0.25 ± 0.11; HWImax = 0.65 ± 0.18). The modularity coeficient without trawling vessels (AT dataset; Q = 0.15) indicates divisions in the network (the best division into clusters was at an association index of 0.3), resulting in four main clusters of two, three, four, and 15 individuals; Figure 3, bottom panel; CCC ≥ 0.80).
The results of the permutation tests on possible preferred or avoided associations in the population are reported in Table 1. In the overall population (AI dataset), in female individuals (FO dataset), and dolphins preferentially observed without interactions with trawling vessels (AT dataset), permutation tests hinted at preferred companionships, as revealed by a significantly higher SD and CV of the observed data compared with random data. The significantly smaller proportion of non-zero HWIs in the observed data (AI, FO, and AT datasets) seem to indicate that avoidance between individuals may also occur within the investigated population. Short-term preferred associations emerged in individuals mostly seen in association with trawling vessels (PT dataset), whereby random HWIave was lower than the observed data.
The values of the social metric parameters in the investigated datasets are shown in Table 2. The characterization of the social network of overall individuals (AI dataset) emphasized a well-connected network. All metrics (strength, affinity, eigen-centrality, reach, and clustering coeficient) were significantly different from random, indicating a general preference for larger groups, with individuals strongly connected to each other and indirectly linked to many others in the population. The same pattern was observed for female individuals (FO dataset) and dolphins preferentially observed without interactions with trawling vessels (AT dataset) as well, while all network metrics were not significantly different from random for individuals mostly observed in association with trawling vessels (PT dataset).
The sociograms generated with levels of double HWIave showed the well-connected bottlenose dolphin network at the Tiber River estuary and confirmed several strong associations among females (Figure 4, upper panels). The networks in the absence or presence of fishing vessels (Figure 4, lower panels, respectively) were different, with the former characterized by multiple connections between female individuals and the latter by a lower number of links between individuals of unknown sex. Both networks showed a triad separated from the principal ones.
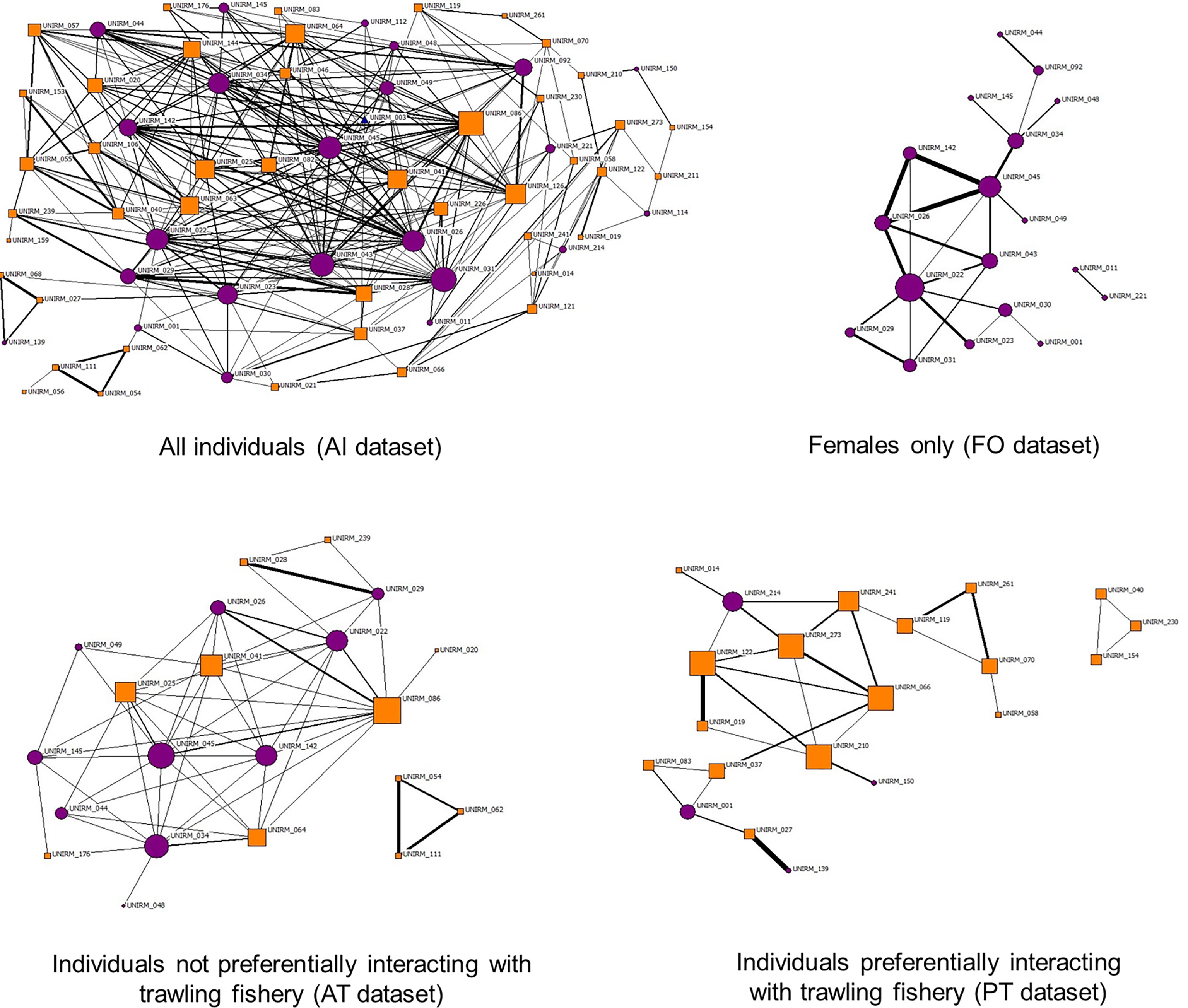
Figure 4 Sociograms of individuals in the different datasets. Sociograms were set with HWI values at least twice the HWIave for all datasets. Top left: sociogram of overall individuals (AI dataset, HWI ≥ 0.36). Top right: sociogram of female individuals (FO dataset, HWI ≥ 0.50). Bottom left: sociogram of individuals not preferentially interacting with trawlers (AT dataset, HWI ≥ 0.50). Bottom right: individuals preferentially interacting with trawlers (PT dataset, HWI ≥ 0.42). Purple circles indicate females, and orange squares mark individuals of unknown gender. HWI, half-weight index.
The best-fitting model (Table 3) obtained for overall (AI dataset) and female individuals (FO dataset) was ‘two levels of casual acquaintance’ (a short, casual level of association and a longer‐term one). However, in the female dataset, a model containing ‘constant companions and casual acquaintances’ strongly supported the SLAR as well, suggesting that sex-specific patterns of association may persist over time between females at two levels of association, one of ‘constant companions’(preferred and constant short-term associations) and one of ‘casual acquaintances’ (random long-term associations). Thus, although the social structure appeared to be driven by short‐term relationships, female individuals also had longer‐term and constant companions over the 4-year study period. A similar result was highlighted for individuals mainly observed in the presence of trawling vessels (PT dataset). The model containing ‘casual acquaintances’(random long-term associations) was the best-fitting one in the case of individuals principally sighted without trawls (AT dataset), although a model containing ‘constant companions and casual acquaintances’ strongly supported the SLAR as well.
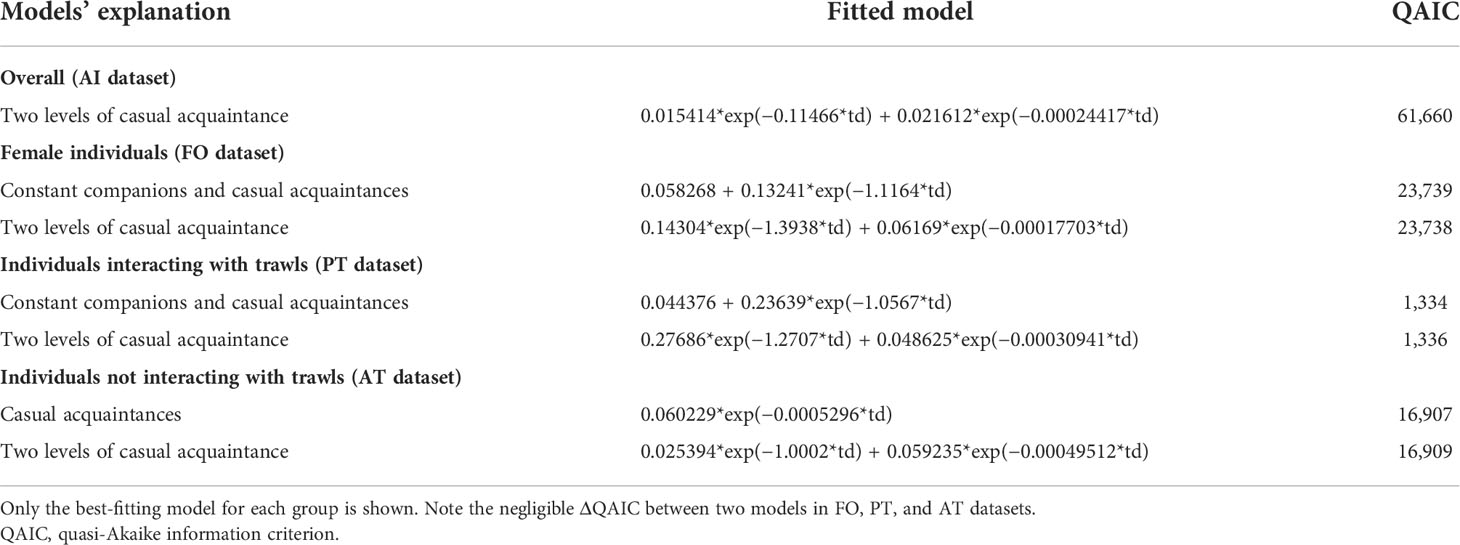
Table 3 Exponential decay models fitted to the standardized lagged association rate (SLAR) among bottlenose dolphin individuals at the Tiber River estuary.
This study investigated for the first time the social structure of T. truncatus in the Tiber River estuary (Italy) over 4 years. The discovery curve for these individuals (AI dataset) showed a stabilization after 15 encounters, highlighting that the portion of individuals within the population showing a considerable degree of site fidelity was captured, similarly to bottlenose dolphins at the Shannon Estuary (Baker et al., 2018) and in a lagoon in the Gulf of Mexico (Morteo et al., 2017). Results also showed that common bottlenose dolphins in the Tiber River estuary were organized into a quite well-differentiated fission–fusion society encompassing both extremely fluid and stable associations between individuals, which seem to be a common pattern among bottlenose dolphin populations (e.g., Moreno and Acevedo-Gutiérrez, 2016; Morales-Rincon et al., 2019). HWIave was significantly higher in both females and individuals not interacting with trawling vessels if compared to the overall individuals. Association levels in female bottlenose dolphins are generally related to reproductive state (Connor et al., 2000), calf protection and food access (Mann et al., 2000; Möller et al., 2006), defense against predators and male coercion (Connor et al., 2000; Galezo et al., 2018), or lifetime fitness (Möller et al., 2006). Here, the stronger associations between females seem analogous to other populations (e.g., Papale et al., 2017), where females tend to associate with other females sharing similar energy requirements to obtain greater success in rearing the young and maximize the chances of offspring survival (Wells, 2003; Möller and Harcourt, 2008; Rendell et al., 2019; Diaz-Aguirre et al., 2020).
From a behavioral ecology perspective, the higher levels of associations here observed between individuals in the absence of trawling vessels could be related to possible benefits from 1) increased cooperation and reduced intragroup competition to advance information sharing when the patchy and uneven distribution of prey occur, since cooperative foraging strategies may increase the foraging efficiency (Methion and Díaz López, 2020); 2) safer contexts to improve calf care, social behaviors, or resting in females; and 3) foraging specializations preferences (not related to bottom trawling fishery) possibly transferred from mother to offspring through the social learning process (vertical transmission; Rendell and Whitehead, 2001; Weiss, 2006). Although our study did not find separate bottlenose dolphin ‘trawling’ and ‘non-trawling’ communities (Chilvers and Corkeron, 2001; Genov et al., 2019), despite overlapping spatial ranges, significantly lower association levels were obtained when individuals interact with trawls. The opportunistic interaction behind the trawling vessel makes it possible to feed on organisms captured by the trawl, picking out fish entangled in the nets or possibly feeding on fish passing through the net meshes (Fertl and Leatherwood, 1997), even behaviorally impaired (Ryer et al., 2004). Concentrated food sources and increased prey availability are key attracting factors for bottlenose dolphins (Fertl and Leatherwood, 1997), which may act individually with lower association levels. However, despite these positive aspects, animals are exposed to the risk of bycatch (dolphins that spend more time in the vicinity of fishing nets are more likely to get caught than dolphins that avoid the interaction; Fortuna et al., 2010), although there is no reported evidence of entanglement or bycatch in the study area (Carpentieri et al., 2021).
Similarly, to the estuarine population of bottlenose dolphins in the Indian River (Titcomb et al., 2015), social network structures governed by preferred and avoided companionships were not homogeneous. The networks varied from a few vertices with multiple links to only one or two links. The overall dataset (AI) network appeared cohesive and well-connected, showing multiple links between individuals. Females seemed to have a central role in this network, being strongly associated with each other. This pattern of association between females appeared clearer in the female-only network (FO) and in the network of individuals not preferentially interacting with trawls (AT), where central positions were occupied almost by the same female individuals. However, individuals not identified as females composed the network of animals mostly seen in association with trawling vessels. Non-random associations are common in many terrestrial and marine mammals that exhibit fission–fusion grouping patterns [e.g., African elephants, Loxodonta africana (Wittemyer et al., 2005); Indian ocean humpback dolphins, Sousa plumbea (Bouveroux et al., 2019); killer whale, Orcinus orca (Ford et al., 2000), particularly in female clusters [e.g., bottlenose dolphins (Connor et al., 2000); grey kangaroos, Macropus giganteus (Best et al., 2014); zebras, Equus grevyi (Sundaresan et al., 2007); and giraffes, Giraffa camelopardalis (Carter et al., 2013)]. Here, non-random associations within different groups may indicate that not all individuals have the same role in this society or play a similar part in the network’s cohesion (Lusseau et al., 2003). Individual female centrality and the strength of associations with other females, for example, are likely to change over time because of variable interbirth intervals (Mann et al., 2000; Barrett and Henzi, 2002), thus altering the networks’ configuration. It is known that kinship may also play an important role in shaping female associations (Diaz-Aguirre et al., 2020), but it is not known the degree of genetic relatedness between females in the study area. This aspect needs further investigations in the future.
The type and temporal stability of the associations of the bottlenose dolphin groups in the Tiber River estuary were best described by models containing a) associations or dissociations at two different time scales (‘two levels of casual acquaintances’), where the associations eventually decay completely, and b) short-term preferred mates and occasional long-term acquaintances with individuals that associate over a period of time, disassociate, and re-associate later (‘constant companions and casual acquaintances’). In females, the tendency to form strong temporary associations with other females appears to be a defense technique to reduce harassment by groups of males (Moreno and Acevedo-Gutiérrez, 2016; Galezo et al., 2018), but prey type may play an important role in the decision-making regarding leaving and/or bonding specific individuals in a group as well (Lusseau et al., 2004). Indeed, the wide-open habitat at the Tiber River mouths allows bottlenose dolphins both to pursue and circle schooling fish, with a few individuals at a time preying (Connor et al., 2000) and to individually target isolated prey items throughout the water column. These strategies may favor associations or disassociations at different temporal scales depending on changing foraging opportunities (Gregorietti et al., 2021).
In the Tiber River estuary, bottlenose dolphins appear to be organized in a fission–fusion society characterized by both free and fluctuating, but also strong and preferred associations. Strong social bonds can be attributed to differences in habitat use and residency patterns of dolphin groups inhabiting the study region (Pace et al., 2021) and to the regular presence of females with their recent offspring (Pedrazzi et al., 2022). The River mouths are likely to represent a key nursery ground and a valuable habitat with an important availability of suitable food sources for bottlenose dolphins since nutrient transport influences primary production and the whole trophic web. These favorable local conditions also support the exploitation by trawling fishery, which in turn affects the social dynamics of the population. Furthermore, the presence of trawling vessels can influence relationships and bonds between individuals because the behavioral complexity required to advantageously complete this opportunistic feeding activity possibly implies a specific type of cooperation (Pace et al., 2012). Nevertheless, in this study, common bottlenose dolphins established weaker associations in the presence of trawling, letting us presume that they might prefer limiting the risk of bycatch by avoiding associating during this activity. This hypothesis seems to be also supported by the lower presence of identified females (prevalently recognized by the occurrence of associated calves) following fishing trawlers, suggesting that females with calves are possibly using other foraging strategies (not related to operating bottom trawlers) to secure food more easily when resources are generally abundant (as in the study area), in order to meet their daily energetic requirement and prevent unnecessary risk (Kovacs et al., 2017). From a conservation perspective, both aspects (weaker associations between individuals and mother–calf pairs avoidance) may be pivotal since they represent a temporary disruption of adult social bonds due to fishing activity but may also denote that dolphin’s social structure may be a complex adaptive system resilient to anthropogenic disturbance (Ansmann et al., 2012; Díaz López, 2019; Genov et al., 2019; Frau et al., 2021), as bonds are restored when fishing trawlers are absent. Different anthropogenic activities have been demonstrated to possibly alter population structure in terms of age and sex composition, by influencing the survival rate (Senigaglia et al., 2019; Tenan et al., 2020) or affecting the relationships among individuals (Marley et al., 2017), with the potential of eventually influencing population dynamics (Tenan et al., 2020). Thus, assessing how social structure changes and adapts in response to human activities is essential to investigate the possible consequences of anthropogenic disturbance on a population level. This study reports information on a bottlenose dolphin population over a 4-year period, which is a short time frame that does not allow for analyzing interannual or even generational changes. Further, long-term data collection is therefore needed to investigate population dynamics over a wider time frame (Pace et al., 2014b).
The present work provides new evidence on the common bottlenose dolphin that could be useful for future management plans and practical conservation efforts for the species. To date, current management approaches focus on the conservation of numbers of animals, yet this study emphasizes the importance of individual variations and the necessity to preserve behaviors that allow adaptation to the local environment. The bottlenose dolphin is included in Annex II of the EU Habitats Directive (92/43/CEE) as priority species and listed as Least Concern in the last International Union for Conservation of Nature (IUCN) Red List of Threatened species regional assessment (Natoli et al., 2021). This new assessment strongly indicated to monitor the effects of human-related stressors, to guarantee the preservation of intra-species diversity and the survival across its range (Natoli et al., 2021). In addition, the Tiber River estuary area was identified as an ‘Area of Interest’ for bottlenose dolphin during the first Important Marine Mammal Areas (IMMA) Mediterranean workshop organized by the IUCN Marine Mammal Protected Areas Task Force (IUCN, 2017). This was the first significant step toward the recognition of this discrete area as important for feeding and reproduction of the common bottlenose dolphin, thus having the potential to be managed for conservation.
The data supporting the conclusions of this article will be made available by the authors upon request.
Ethical review and approval was not required for the animal study because this is a non-invasive, observational study.
DSP, GG, and GA designed the study and managed the funding acquisition. DSP, GG, SF, CD, MS, GP, EC, and DV performed the field work. SF, CD, and DSP completed the photo-identification analysis. SF and DSP analyzed the data. DSP and EP wrote the first version of the manuscript. All authors discussed the results and implications, commented on the manuscript at all stages, and contributed extensively to the work here presented.
In 2017–2018, this work was supported by OceanCare (Switzerland) and in 2019–2020 by Cooperativa Pelagos (Italy). In 2020, part of the data were collected within the framework of the ‘DelPHEos’ project funded by the Lazio Region (Italy) within the PO FEAMP 2014/2020-Misura 1.40 framework.
We would like to thank the Secche di Tor Paterno (MPA) and Roma Natura for the logistic support; ‘I Barcaroli del Dollaro’ and the local professional/recreational fishing community for their help; Alessandro Frachea and Sara Marini for their assistance in 2019 field season; Sara Verni and Carla Tumino their assistance in the 2020 field season; and Maria Cristina Gambi, Raffaella Tizzi, Caterina Lanfredi, Junio Fabrizio Borsani, Sabina Airoldi, Giovanna Jona‐Lasinio, Gianni Pavan, Antonella Arcangeli, and Francesca Triossi for the constant encouragements.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ansmann I. C., Parra G. J., Chilvers B. L., Lanyon J. M. (2012). Dolphins restructure social system after reduction of commercial fisheries. Anim. Behav. 84, 575–581. doi: 10.1016/j.anbehav.2012.06.009
Archie E. A., Chiyo P. I. (2012). Elephant behaviour and conservation: social relationships, the effects of poaching, and genetic tools for management. Mol. Ecol. 21, 765–778. doi: 10.1111/j.1365–294X.2011.05237
Ardizzone G. D., Belluscio A., Criscoli A. (2018). Atlante degli habitat dei fondali marini del Lazio (Rome, Italy: Sapienza Università Editrice).
Avila I. C., Kaschner C., Dormann C. F. (2018). Current global risks to marine mammals: Taking stock of the threats. Biol. Conservat. 221, 44–58. doi: 10.1016/j.biocon.2018.02.021
Baker I., O'Brien J., McHugh K., Berrow S. (2020). Fine-scale sociality reveals female–male affiliations and absence of male alliances in bottlenose dolphins (Tursiops truncatus) in the Shannon estuary, Ireland. Mar. Mam. Sci. 36, 66–88. doi: 10.1111/mms.12631
Baker I., O’Brien J., McHugh K., Ingram S. N., Berrow S. (2018). Bottlenose dolphin (Tursiops truncatus) social structure in the Shannon estuary, Ireland, is distinguished by age– and area–related associations. Mar. Mam. Sci. 34, 458–487. doi: 10.1111/mms.12462
Barrett L., Henzi S. P. (2002). Constraints of relationship formation among female primates. Behaviour 139, 263–289. doi: 10.1163/156853902760102672
Bearzi G., Fortuna C. M., Reeves R. R. (2009). Ecology and conservation of common bottlenose dolphins Tursiops truncatus in the Mediterranean Sea. Mammal. Rev. 39, 92–123. doi: 10.1111/j.1365–2907.2008.00133.x
Bëjder L., Fletcher D., Bräger S. (1998). A method for testing association patterns of social animals. Anim. Behav. 56, 719–725. doi: 10.1006/anbe.1998.0802
Best E. C., Dwyer R. G., Seddon J. M., Goldize A. W. (2014). Associations are more strongly correlated with space use than kinship in female eastern grey kangaroos. Anim. Behav. 89, 1–10. doi: 10.1016/j.anbehav.2013.12.011
Blanco C., Salomón O., Raga J. A. (2001). Diet of the bottlenose dolphin (Tursiops truncatus) in the western Mediterranean Sea. J. Mar. Biol. Assoc. UK 81, 1053–1058. doi: 10.1017/S0025315401005057
Blasi M., Boitani L. (2014). Complex social structure of an endangered population of bottlenose dolphins (Tursiops truncatus) in the aeolian archipelago (Italy). PloS One 9 (12), e114849. doi: 10.1371/journal.pone.0114849
Blumstein D. T. (2010). “Social behaviour in conservation” in Social behaviour: genes, ecology and evolution. Eds. Szèkely T., Moore A. J., Komdeur J. (Cambridge: Cambridge University Press), 654–672.
Bolaños-Jiménez J., Morteo E., Delfín-Alfonso C. A., Fruet P. F., Secchi E. R., Bello-Pineda J. (2021). Population dynamics reveal a core community of the common bottlenose dolphin (Tursiops truncatus) in open waters of the South-Western Gulf of Mexico. Frontiers in Marine Science, 1610. doi: 10.3389/fmars.2021.753484
Bonifazi A., Lezzi M., Ventura D., Lisco S., Cardone F., Gravina M. F. (2019). Macrofaunal biodiversity associated with different developmental phases of a threatened Mediterranean Sabellaria alveolata (Linnaeus 1767) reef. Mar. Environ. Res. 145, 97–111. doi: 10.1016/j.marenvres.2019.02.009
Bonizzoni S., Furey N. B., Bearzi G. (2021). Bottlenose dolphins (Tursiops truncatus) in the north-western Adriatic Sea: Spatial distribution and effects of trawling. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 635–650. doi: 10.1002/aqc.3433
Bonizzoni S., Hamilton S., Reeves R. R., Genov T., Bearzi G. (2022). Odontocete cetaceans foraging behind trawlers, worldwide. Rev. Fish Biol. Fish. 32, 827–877. doi: 10.1007/s11160–022–09712–z
Borgatti S. P., Everett M. G., Freeman L. C. (2002). Ucinet 6 for Windows: Software for Social Network Analysis. Harvard, MA: Analytic Technologies.
Bouveroux T., Kirkman S. P., Conry D., Vargas–Fonseca O. A., Pistorius P. A. (2019). The first assessment of social organisation of the Indian ocean humpback dolphin (Sousa plumbea) along the south coast of south Africa. Can. J. Zool. 97, 855–865. doi: 10.1139/cjz–2018–0244
Burnham K. P., Anderson D. R. (2002). Model selection and multimodel inference: a practical information–theoretic approach (New York: Springer).
Buscaino G., Ceraulo M., Alonge G., Pace D. S., Grammauta R., Maccarrone V., et al. (2021). Artisanal fishing, dolphins, and interactive pinger: A study from a passive acoustic perspective. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 2241–2256. doi: 10.1002/aqc.3588
Cairns S. J., Schwager S. J. (1987). A comparison of association indices. Anim. Behav. 35, 1454–1469. doi: 10.1016/S0003–3472(87)80018–0
Carpentieri P., Nastasi A., Sessa M., Srour A. (Eds.) (2021). “Incidental catch of vulnerable species in Mediterranean and black Sea fisheries – a review,” in Studies and reviews no. 101 (Rome: General Fisheries Commission for the Mediterranean, FAO). doi: 10.4060/cb5405en
Carter K. D., Seddon J. M., Frere C. H., Carter J. K., Goldizen A. W. (2013). Fission–fusion dynamics in wild giraffes may be driven by kinship, spatial overlap and individual social preferences. Anim. Behav. 85, 385–394. doi: 10.1016/j.anbehav.2012.11.011
Casoli E., Bonifazi A., Ardizzone G., Gravina M. F., Russo G. F., Sandulli R., et al. (2019). Comparative analysis of mollusc assemblages from different hard bottom habitats in the central tyrrhenian Sea. Diversity 11, 74. doi: 10.3390/d11050074
Chilvers B. L., Corkeron P. J. (2001). Trawling and bottlenose dolphins’ social structure. Proc. R. Soc B 268, 1901–1905. doi: 10.1098/rspb.2001.1732
Connor R. C., Krützen M. (2015). Male Dolphin alliances in shark bay: changing perspectives in a 30–year study. Anim. Behav. 103, 223–235. doi: 10.1016/j.anbehav.2015.02.019
Connor R. C., Wells R. S., Mann J., Read A. J. (2000). “The bottlenose dolphin: social relationships in a fission–fusion society”, in Cetacean Societies: Field Studies of Dolphins and Whales. Eds. Mann J., Connor R. C., Tyack P. L., Whitehead H. (Chicago: The University of Chicago Press), 91–126.
Couzin I. D. (2006). Behavioral ecology: social organization in fission–fusion societies. Curr. Biol. 16, R169–R171. doi: 10.1016/j.cub.2006.02.042
Couzin I. D., Laidre M. E. (2009). Fission–fusion populations. Curr. Biol. 19, R633–R635. doi: 10.1016/j.cub.2009.05.034
Croft D. P., Madden J. R., Franks D. W., James R. (2011). Hypothesis testing in animal social networks. Trends Ecol. Evol. 26, 502–507. doi: 10.1016/j.tree.2011.05.012
Daura–Jorge F. G., Cantor M., Ingram S. N., Lusseau D., Simões–Lopes P. C. (2012). The structure of a bottlenose dolphin society is coupled to a unique foraging cooperation with artisanal fishermen. Biol. Lett. 8, 702–705. doi: 10.1098/rsbl.2012.0174
Dawson S., Wade P., Slooten E., Barlow J. (2008). Design and field methods for sightings surveys of cetaceans in coastal and riverine habitats. Mammal Rev. 38, 19–49. doi: 10.1111/j.1365-2907.2008.00119.x
Diaz-Aguirre F., Parra G. J., Passadore C. (2020). Kinship and reproductive condition correlate with affiliation patterns in female southern Australian bottlenose dolphins. Sci. Rep. 10, 189. doi: 10.1038/s41598–020–58800–2
Díaz López B. (2019). “Hot deals at sea”: responses of a top predator (Bottlenose dolphin, Tursiops truncatus) to human–induced changes in the coastal ecosystem. Behav. Ecol. 30 (2), 291–300. doi: 10.1007/s00265–007–0512–1
Díaz López B., Shirai J. A. B. (2008). Marine aquaculture and bottlenose dolphins’ (Tursiops truncatus) social structure. Behav. Ecol. Sociobiol. 62, 887–894. doi: 10.1093/beheco/ary162
Dungan S. Z., Wang J. Y., Araujo C. C., Yang S. C., White B. N. (2016). Social structure in a critically endangered indo–pacific humpback dolphin (Sousa chinensis) population. Aquat. Conserv. Mar. Freshw. Ecosyst. 26, 517–529. doi: 10.1002/aqc.2562
European Marine Observation and Data Network (2022) Human activities. Available at: https://www.emodnet-humanactivities.eu (Accessed June 6, 2022).
European Market Observatory for Fisheries and Aquaculture products (2022) Data. Available at: https://eumofa.eu/web/eumofa/data (Accessed June 6, 2022]).
Faul F., Erdfelder E. (1992). GPOWER a priori-, post hoc-, and compromise power analyses for MS-DOS (Bonn, Germany: Bonn University). Available at: https://www.psychologie.hhu.de/fileadmin/redaktion/Fakultaeten/Mathematisch-Naturwissenschaftliche_Fakultaet/Psychologie/AAP/gpower/GPowerManual.pdf.
Faul F., Erdfelder E., Lang A. G., Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. doi: 10.3758/BF03193146
Fertl D., Leatherwood S. (1997). Cetacean interactions with trawls: A preliminary review. J. Northw. Atl. Fish. Sci. 22, 219–248. doi: 10.2960/J.v22.a17
Ford J. K. B., Ellis G. M., Balcomb K. C. (2000). Killer whales: The natural history and genealogy of orcinus orca in British Columbia and Washington (Vancouver, Canada: UBC Press).
Fortuna C. M., Vallini C., Filidei E., Ruffino M., Consalvo I., Di Muccio S., et al. (2010). By–catch of cetaceans and other species of conservation concern during pair trawl fishing operations in the Adriatic Sea (Italy). Chem. Ecol. 26, 65–76. doi: 10.1080/02757541003627662
Franks D. W., Ruxton G. D., James R. (2010). Sampling animal association networks with the gambit of the group. Behav. Ecol. Sociobiol. 64, 493–503. doi: 10.1007/s00265–009–0865–8
Frau S., Ronchetti F., Perretti F., Addis A., Ceccherelli G., La Manna G. (2021). The influence of fish farm activity on the social structure of the common bottlenose dolphin in Sardinia (Italy). Peer J 9, e10960. doi: 10.7717/peerj.10960
Galezo A. A., Krzyszczyk E., Mann J. (2018). Sexual segregation in indo–pacific bottlenose dolphins is driven by female avoidance of males. Behav. Ecol. 29, 377–386. doi: 10.1093/beheco/arx177
Genov T., Centrih T., Kotnjek P., Hace A. (2019). Behavioural and temporal partitioning of dolphin social groups in the northern Adriatic Sea. Mar. Biol. 166, 11. doi: 10.1007/s00227–018–3450–8
Gero S., Bejder L., Whitehead H., Mann J., Connor R. C. (2005). Behaviourally specific preferred associations in bottlenose dolphins, tursiops spp. Can. J. Zool. 83, 1566–1573. doi: 10.1139/z05–155
Gowans S., Würsig B., Karczmarski L. (2007). The social structure and strategies of delphinids: predictions based on an ecological framework. Adv. Mar. Biol. 53, 195–294. doi: 10.1016/S0065–2881(07)53003–8
Gregorietti M., Papale E., Ceraulo M., de Vita C., Pace D. S., Tranchida G., et al. (2021). Acoustic presence of dolphins through whistles detection in Mediterranean shallow waters. J. Mar. Sci. Eng. 9, 78. doi: 10.3390/jmse9010078
Hamer D. J., Childerhouse S. J., Gales N. J. (2012). Odontocete bycatch and depredation in longline fisheries: A review of available literature and of potential solutions. Mar. Mamm. Sci. 28, E345–E374. doi: 10.1111/j.1748–7692.2011.00544.x
Heithaus M. R., Dill L. M. (2006). Does tiger shark predation risk influence foraging habitat use by bottlenose dolphins at multiple spatial scales? Oikos 114 (2), 257–264. doi: 10.1111/j.2006.0030-1299.14443.x
He P., Maldonado–Chaparro A. A., Farine D. R. (2019). The role of habitat configuration in shaping social structure: a gap in studies of animal social complexity. Behav. Ecol. Sociobiol. 73, 9. doi: 10.1007/s00265–018–2602–7
IUCN Marine Mammal Protected Areas Task Force (2017) Final report of the workshop: First IMMA regional workshop for the Mediterranean, chania, Greece, 24–28 October 2016. Available at: https://www.marinemammalhabitat.org/download/final–report–regional–workshop–mediterranean–sea–important–marine–mammal–areas/?ind=1501742106612&filename=First%20IMMA%20Regional%20Workshop%20Mediterranean%20Region%20Final%20Report_01.08.17_rev.2.pdf&wpdmdl=1648&refresh=62661bf8240ba1650859000.
Kovacs C. J., Perrtree R. M., Cox T. M. (2017). Social differentiation in common bottlenose dolphins (Tursiops truncatus) that engage in human-related foraging behaviors. PloS One 12 (2), e0170151. doi: 10.1371/journal.pone.0170151
Krause S., Mattner L., James R., Guttridge T., Corcoran M. J., Gruber S. H., et al. (2009). Social network analysis and valid Markov chain Monte Carlo tests of null models. Behav. Ecol. Sociobiol. 63, 1089–1096. doi: 10.1007/s00265-009-0746-1
Kühl H. S., Boesch C., Kulik L., Haas F., Arandjelovic M., Dieguez P., et al. (2019). Human impact erodes chimpanzee behavioral diversity. Science 363, 1453–1455. doi: 10.1126/science.aau4532
La Manna G., Ronchetti F., Sarà G. (2016). Predicting common bottlenose dolphin habitat preference to dynamically adapt management measures from a marine spatial planning perspective. Ocean Coast. Manage. 130, 317–327. doi: 10.1016/j.ocecoaman.2016.07.004
La Manna G., Ronchetti F., Sarà G., Ruiu A., Ceccherelli G. (2020). Common bottlenose dolphin protection and sustainable boating: Species distribution modeling for effective coastal planning. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.542648
Louis M., Simon–Bouhet B., Viricel A., Lucas T., Gally F., Cherel Y., et al. (2018). Evaluating the influence of ecology, sex and kinship on the social structure of resident coastal bottlenose dolphins. Mar. Biol. 165 (5), 80. doi: 10.1007/s00227–018–3341–z
Lusseau D., Schneider K., Boisseau O. J., Haase P., Slooten E., Dawson S. M. (2003). The bottlenose dolphin community of doubtful sound features a large proportion of long–lasting associations. can geographic isolation explain this unique trait? Behav. Ecol. Sociobiol. 54, 396–405. doi: 10.1007/s00265–003–0651–y
Lusseau D., Williams R. J., Wilson B., Grellier K., Barton T. R., Hammond P. S., et al. (2004). Parallel influence of climate on the behaviour of pacific killer whales and Atlantic bottlenose dolphins. Ecol. Lett. 7, 1068–1076. doi: 10.1111/j.1461–0248.2004.00669.x
Majolo B., Huang P. (2018). “Group living” in Encyclopedia of animal cognition and behaviour. Eds. Vonks J., Shackelford T. (New York: Springer International Publishing).
Maldonado-Chaparro A. A., Chaverri G. (2021). Why do animal groups matter for conservation and management? Conserv. Sci. Pract. 3 (12), e550. doi: 10.1111/csp2.550
Manly B. F. J. (1995). A note on the analysis of species co–occurrences. Ecology 76, 1109–1115. doi: 10.2307/1940919
Mann J., Connor R. C., Barre L. M., Heithaus M. R. (2000). Female reproductive success in bottlenose dolphins (Tursiops sp.): life history, habitat, provisioning, and group–size effects. Behav. Ecol. 11, 210–219. doi: 10.1093/beheco/11.2.210
Mariani M., Miragliuolo A., Mussi B., Russo G. F., Ardizzone G., Pace D. S. (2016). Analysis of the natural markings of risso's dolphins (Grampus griseus) in the central Mediterranean Sea. J. Mammal. 97, 1512–1524. doi: 10.1093/jmammal/gyw109
Marley S. A., Salgado Kent C. P., Erbe C., Parnum I. M. (2017). Effects of vessel traffic and underwater noise on the movement, behaviour and vocalisations of bottlenose dolphins in an urbanised estuary. Sci. Rep. 7, 13437. doi: 10.1038/s41598-017-13252-z
Martino S., Pace D. S., Moro S., Casoli E., Ventura D., Frachea A., et al. (2021). Integration of presence–only data from several sources: a case study on dolphins' spatial distribution. Ecography 44, 1533–1543. doi: 10.1111/ecog.05843
Methion S., Díaz López B. (2020). Individual foraging variation drives social organization in bottlenose dolphins. Behav. Ecol. 31, 97–106. doi: 10.1093/beheco/arz160
Miller P. J., Isojunno S., Siegal E., Lam F. P. A., Kvadsheim P. H., Curé C. (2022). Behavioral responses to predatory sounds predict sensitivity of cetaceans to anthropogenic noise within a soundscape of fear. PNAS 119 (13), e2114932119. doi: 10.1073/pnas.2114932119
Möller L. M., Beheregaray L. B., Allen S. J., Harcourt R. G. (2006). Association patterns and kinship in female indo–pacific bottlenose dolphins (Tursiops aduncus) of south–eastern Australia. Behav. Ecol. Sociobiol. 61, 109–177. doi: 10.1007/s00265–006–0241–x
Möller L. M., Harcourt R. G. (2008). Shared reproductive state enhances female associations in dolphin. Res. Lett. Ecol. 5, 498390. doi: 10.1155/2008/498390
Morales-Rincon N., Morteo E., Delfín–Alfonso C. A. (2019). Influence of artisanal fisheries on the behaviour and social structure of Tursiops truncatus in the south–western gulf of Mexico. J. Mar. Biolog. Assoc. UK 9, 1841–1849. doi: 10.1017/S002531541900078X
Moreno K., Acevedo-Gutiérrez A. (2016). The social structure of golfo dulce bottlenose dolphins (Tursiops truncatus) and the influence of behavioural state. R. Soc Open Sci. 3, 160010. doi: 10.1098/rsos.160010
Morteo E., Rocha–Olivares A., Abarca–Arenares L. G. (2017). Abundance, residency, and potential hazards for coastal bottlenose dolphins (Tursiops truncatus) off a productive lagoon in the gulf of Mexico. Aquat. Mamm. 43, 308–319. doi: 10.1578/AM.43.3.2017.308
Mussi B., Vivaldi C., Zucchini A., Miragliuolo A., Pace D. S. (2021). The decline of short-beaked common dolphin (Delphinus delphis) in the waters off the island of Ischia (Gulf of Naples, Italy). Aquat. Conserv. Mar. Freshw Ecosyst. 31, 87–100. doi: 10.1002/aqc.3061
Natoli A., Genov T., Kerem D., Gonzalvo J., Holcer D., Labach H., et al. (2021). Tursiops truncatus (Mediterranean subpopulation). The IUCN Red List of Threatened Species 2021, e.T16369383A50285287. https://dx.doi.org/10.2305/IUCN.UK.2021–3.RLTS.T16369383A50285287.en. Accessed on 1 May 2022.
Newman M. E. J. (2006). Modularity and community structure in networks. Proc. Natl. Acad. Sci. U.S.A. 103, 8577–8582. doi: 10.1073/pnas.0601602103
Pace D. S., Arcangeli A., Mussi B., Vivaldi C., Ledon C., Lagorio S., et al. (2018). Habitat suitability modelling in sperm whale (Physeter macrocephalus) social groups. Jour. Wild. Manage. 82, 1062–1073. doi: 10.1002/jwmg.21453
Pace D. S., Di Marco C., Giacomini G., Ferri S., Silvestri M., Papale E., et al. (2021). Capitoline dolphins: residency patterns and abundance estimate of Tursiops truncatus at the Tiber river estuary (Mediterranean Sea). Biology 10, 275. doi: 10.3390/biology10040275
Pace D. S., Giacomini G., Campana I., Paraboschi M., Pellegrino G., Silvestri M., et al. (2019). An integrated approach for cetacean knowledge and conservation in the central Mediterranean Sea using research and social media data sources. Aquat. Conserv. Mar. Freshw. Ecosyst. 29, 1302–1323. doi: 10.1002/aqc.3117
Pace D. S., Miragliuolo A., Mariani M., Vivaldi C., Mussi B. (2014a). Sociality of sperm whale off ischia island (Tyrrhenian Sea, Italy). Aquat. Conserv. Mar. Freshw. Ecosyst. 24, 71–82. doi: 10.1002/aqc.2459
Pace D. S., Mussi B., Wurtz M., Gordon J. D. C. (2014b). Foreword. Aquat. Conserv. Mar. Freshw. Ecosyst. 24, 1–3. doi: 10.1002/aqc.2457
Pace D. S., Pulcini M., Triossi F. (2012). Anthropogenic food patches and association patterns of bottlenose dolphin (Tursiops truncatus) at lampedusa island, Italy. Behav. Ecol. 23, 254–264. doi: 10.1093/beheco/arr180
Pace D. S., Tumino C., Giacomini G., Silvestri M., Pedrazzi G., Ceraulo M., et al. (2022). Bray–call sequences in the Mediterranean common bottlenose dolphin (Tursiops truncatus) acoustic repertoire. Biology 11 (3), 367. doi: 10.3390/biology11030367
Papale E., Ceraulo M., Giardino G., Buffa G., Filiciotto F., Grammauta R., et al. (2017). Association patterns and population dynamics of bottlenose dolphins in the strait of Sicily (Central Mediterranean sea): implication for management. Popul. Ecol. 59, 55–64. doi: 10.1007/s10144–016–0566–x
Papale E., Alonge G., Caruso F., Grammauta R., Mazzola S., Mussi B., et al (2021). The higher, the closer, the better? influence of sampling frequency and distance on the acoustic properties of short-beaked common dolphins burst pulses in the Mediterranean Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 51–60. doi: 10.1002/aqc.3158
Parra G. J., Corkeron P. J., Marsh H. (2006). Population sizes, site fidelity and residence patterns of Australian snubfin and indo–pacific humpback dolphins: implications for conservation. Biol. Conserv. 129, 167–180. doi: 10.1016/j.biocon.2005.10.031
Pedrazzi G., Giacomini G., Pace D. S. (2022). First report of epimeletic and acoustic behaviour in Mediterranean common bottlenose dolphins (Tursiops truncatus) carrying dead calves. Biology 11 (2), 337. doi: 10.3390/biology11020337
Pennino M. G., Rotta A., Pierce G. J., Bellido J. M. (2015) Interaction between Bottlenose Dolphin (Tursiops truncatus) and Trammel Nets in the Archipelago de La Maddalena, Italy. Hydrobiologia 747, 69–82 doi: 10.1007/s10750-014-2127-7
Pulcini M., Pace D. S., Triossi F., La Manna G., Galante I., Fortuna M. C. (2014). Distribution and abundance estimates of bottlenose dolphins (Tursiops truncatus) around lampedusa island (Sicily channel, Italy) – implications for their management. J. Mar. Biol. Assoc. UK 94, 1175–1184. doi: 10.1017/S0025315413000842
Quintana–Rizzo E., Wells R. S. (2001). Resighting and association patterns of bottlenose dolphins (Tursiops truncatus) in the cedar keys, Florida: insights into social organization. Can. J. Zool. 79, 447–456. doi: 10.1139/cjz–79–3–447
Randić S., Conno R. C., Sherwin W. B., Krützen M. (2012). A novel mammalian social structure in indo–pacific bottlenose dolphins (Tursiops sp.): complex male alliances in an open social network. Proc. Math. Phys. Eng. Sci. 279, 3083–3090. doi: 10.1098/rspb.2012.0264
Rendell L., Cantor M., Gero S., Whitehead H., Mann J. (2019). Causes and consequences of female centrality in cetacean societies. Phil. Trans. R. Soc B. 374, 20180066. doi: 10.1098/rstb.2018.0066
Rendell L., Whitehead H. (2001). Culture in whales and dolphins. Behav. Brain Sci. 24 (2), 309–324. doi: 10.1017/S0140525X0100396X
Ryer C. H., Ottmar M. L., Sturm E. A. (2004). Behavioral impairment after escape from trawl cod ends may not be limited to fragile fish species. Fish. Res. 66, 261–269. doi: 10.1016/S0165-7836(03)00197-8
Santana–Garcon J., Wakefield C. B., Dorman S. R., Denham A., Blight S., Molony B. W., et al. (2018). Risk versus reward: interactions, depredation rates, and bycatch mitigation of dolphins in demersal fish trawls. Can. J. Fish. Aquat. Sci. 75, 2233–2240. doi: 10.1139/cjfas–2017–0203
Senigaglia V., Christiansen F., Sprogis K. R., Symons J., Bejder L. (2019). Food-provisioning negatively affects calf survival and female reproductive success in bottlenose dolphins. Sci. Rep. 9 (1), 8981. doi: 10.1038/s41598-019-45395-6
Sundaresan S. R., Fischhoff I. R., Dushoff J., Rubenstein D. I. (2007). Network metrics reveal differences in social organization between two fission–fusion species, grevy’s zebra and onager. Oecologia 151, 140–149. doi: 10.1007/s00442–006–0553–6
Tenan S., Hernández N., Fearnbach H., de Stephanis R., Verborgh P., Oro D. (2020). Impact of maritime traffic and whale-watching on apparent survival of bottlenose dolphins in the strait of Gibraltar. Aquat. Conserv.: Mar. Freshw. Ecosyst. 30 (5), 949–958. doi: 10.1002/aqc.3292
Titcomb E. M., O’Corry–Crowe G., Hartel E. F., Mazzoil M. S. (2015). Social communities and spatiotemporal dynamics of association patterns in estuarine bottlenose dolphins. Mar. Mamm. Sci. 31, 1314–1337. doi: 10.1111/mms.12222
Tixier P., Authier M., Gasco N., Guinet C. (2015). Influence of artificial food provisioning from fisheries on killer whale reproductive output. Anim. Conserv. 18, 207–218. doi: 10.1111/acv.12161
Todd V. L. G., Lazar L., Williamson L. D., Peters I. T., Hoover A. L., Cox S. E., et al. (2020). Underwater visual records of marine megafauna around offshore anthropogenic structures. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00230
Triossi F., Willis T., Pace D. S. (2013). Occurrence of bottlenose dolphins Tursiops truncatus in natural gas fields of the northwestern Adriatic Sea. Mar. Ecol. 34, 373–379. doi: 10.1111/maec.12020
Urian K., Gorgone A., Read A., Balmer B., Wells R. S., Berggren P. (2015). Recommendations for photo–identification methods used in capture–recapture models with cetaceans. Mar. Mamm. Sci. 31, 298–321. doi: 10.1111/mms.12141
Vella A., Murphy S., Giménez J., De Stephanis R., Mussi B., Vella J. G., et al. (2021). The conservation of the endangered Mediterranean common dolphin (Delphinus delphis). Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 110–136. doi: 10.1002/aqc.3538
Ventura D., Bonifazi A., Gravina M. F., Belluscio A., Ardizzone G. (2018). Mapping and classification of ecologically sensitive marine habitats using unmanned aerial vehicle (UAV) imagery and object–based image analysis (OBIA). Remote Sens. 10, 1331. doi: 10.3390/rs10091331
Ventura D., Jona Lasinio G., Ardizzone G. (2015). Temporal partitioning of microhabitat use among four juvenile fish species of the genus Diplodus (Pisces: Perciformes, sparidae). Mar. Ecol. 36, 1013–1032. doi: 10.1111/maec.12198
Weiss J. (2006). Foraging habitats and associated preferential foraging specializations of bottlenose dolphin (Tursiops truncatus) mother-calf pairs. Aquat. Mamm. 32 (1), 10–19. doi: 10.1578/AM.32.1.2006.10
Wells R. S. (2003). “Dolphin social complexity: Lessons from long–term study and life history” in Animal social complexity: Intelligence, culture, and individualized societies. Eds. de Waal F. B. M., Tyack P. L. (Harvard: Harvard University Press), 32–56. doi: 10.4159/harvard.9780674419131.c4
Wells R. S., Scott M. D., Irvine A. B. (1987). The social structure of free–ranging bottlenose dolphins. Curr. Mammal. 1, 247–305. doi: 10.1007/978–1–4757–9909–5_7
Wey T., Blumstein D. T., Shen W., Jordán F. (2008). Social network analysis of animal behaviour:a promising tool for the study of sociality. Anim. Behav. 75, 333–344. doi: 10.1016/j.anbehav.2007.06.020
Whitehead H. (1995). Investigating structure and temporal scale in social organizations using identified individuals. Behaviour 6, 199–208. doi: 10.1093/beheco/6.2.199
Whitehead H. (2008a). Analyzing animal societies: quantitative methods for vertebrate social analysis (Chicago, IL: University of Chicago Press).
Whitehead H. (2008b). Precision and power in the analysis of social structure using associations. Anim. Behav. 75, 1093–1099. doi: 10.1016/j.anbehav.2007.08.022
Whitehead H. (2009a). SOCPROG: programs for analyzing social structure (Halifax: Nova Scotia, Dalhousie University). Available at: http://whitelab.biology.dal.ca/SOCPROG/Manual.pdf.
Whitehead H. (2009b). SOCPROG programs: analysing animal social structures. Behav 63, 765–778. doi: 10.1007/s00265–008–0697–y
Whitehead H., Bejder L., Ottensmeyer A. C. (2005). Testing association patterns: issues arising and extensions. Anim. Behav. 69, e1–e6. doi: 10.1016/j.anbehav.2004.11.004
Whitehead H. A. L. (1999). Testing association patterns of social animals. Anim. Behav 57(6), F26–F29.
Whitehead H., Dufault S. (1999). Techniques for analyzing vertebrate social structure using identified individuals. Adv. Study Behav. 28, 33–74. doi: 10.1016/S0065–3454(08)60215–6
Wirsing A. J., Heithaus M. R., Frid A., Dill L. M. (2008). Seascapes of fear: evaluating sublethal predator effects experienced and generated by marine mammals. Mar. Mamm. Sci. 24 (1), 1–15. doi: 10.1111/j.1748-7692.2007.00167.x
Wiszniewski J., Allen S. J., Möller L. M. (2009). Social cohesion in a hierarchically structured embayment population of indo–pacific bottlenose dolphins. Anim. Behav. 77, 1449–1457. doi: 10.1016/j.anbehav.2009.02.025
Wittemyer G., Douglas–Hamilton I., Getz M. (2005). The socioecology of elephants: analysis of the processes creating multitiered social structures. Anim. Behav. 69, 1357–1371. doi: 10.1016/j.anbehav.2004.08.018
Keywords: social structure, Tursiops truncatus, trawling fishery, conservation, ecology, Tyrrhenian Sea
Citation: Pace DS, Ferri S, Giacomini G, Di Marco C, Papale E, Silvestri M, Pedrazzi G, Ventura D, Casoli E and Ardizzone G (2022) Resources and population traits modulate the association patterns in the common bottlenose dolphin living nearby the Tiber River estuary (Mediterranean Sea). Front. Mar. Sci. 9:935235. doi: 10.3389/fmars.2022.935235
Received: 03 May 2022; Accepted: 25 July 2022;
Published: 22 August 2022.
Edited by:
Sandro Mazzariol, University of Padua, ItalyCopyright © 2022 Pace, Ferri, Giacomini, Di Marco, Papale, Silvestri, Pedrazzi, Ventura, Casoli and Ardizzone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniela Silvia Pace, ZGFuaWVsYXNpbHZpYS5wYWNlQHVuaXJvbWExLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.