- 1School of Life Sciences, Ludong University, Yantai, China
- 2Shandong Breeding Environmental Control Engineering Laboratory, Ludong University, Yantai, China
- 3Shandong Provincial Key Laboratory of Quality Safty Monitoring and Risk Assessment for Animal Products, Shandong Center for Quality Control of Feed and Veterinary Drug, Ji’nan, China
- 4Internal Collection Department, Linyi Central Blood Station, Linyi, China
- 5Quality Control Department, Shandong New Times Pharmaceutical Co., LTD, Linyi, China
- 6Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing, China
Cancer is the leading lethal disease worldwide. Natural products have contributed significantly to the development of approved therapeutic agents. Therefore, research into new bioactive naturally sourced metabolites with lead potential is urgently needed. It is well-known that marine microorganisms are by far one of the most notable and prolific sources of bioactive natural products. Among them, deep-sea-derived fungi are extraordinarily adapted and metabolically active under extreme environmental conditions, which enable them to produce a large number of novel secondary metabolites. Chemical examination of deep-sea-derived fungi has yielded enormous amounts of cytotoxic natural products and potential drug leads. This review summarizes a total of 229 cytotoxic compounds isolated from deep-sea-derived fungi from 2010 to 2021. The emphasis is on the unique chemical diversity of these metabolic products, together with their relevant cytotoxic properties. Among the isolated metabolites, 82 compounds have been found to possess moderate to potent cytotoxic activities. Meanwhile, we also highlight some compounds with potent cytotoxicities (namely “star molecules”) considering their high drug lead potential. This review reveals deep-sea-derived fungi as considerable resources for the development of new drugs and the potential of the newly discovered secondary metabolites as valuable antitumor lead compounds.
1 Introduction
Covering approximately 72% of the Earth’s surface, the oceans are considered to make significant contributions to the development of novel pharmaceutical resources. Of the total sea areas, 60% are deep seas that are covered by seawater at a depth of more than 2,000 m. The deep sea is quite a complicated and extreme ecosystem characterized by elevated hydrostatic pressure, low or high temperature (such as hydrothermal vents), absence of light, fickle salinity, oligotrophy, and low oxygen concentration (Zeng et al., 2010). It is the largest remaining unexplored aqueous habitat on Earth, and organisms in this realm are confronted by various fundamental challenges (Wu J. et al., 2013). To overcome these multiple extreme stresses, organisms that reside in deep sea ecosystems have evolved specific genetic capabilities to produce a large number of metabolic products, including small molecules such as secondary metabolites, proteins and enzymes, saccharides, and so on. These deep-sea-derived metabolic products have played important roles in adaptation to species communications and biotechnological and pharmaceutical applications.
Fungi are regarded as the richest and most varied eukaryotes on Earth, and their existence in every possible extreme environment makes them a valuable source for new drug discovery (Zain Ul Arifeen et al., 2019). Marine-derived fungi have proven to be untapped sources of novel marine natural products for exploitation in medicine. In addition to fungi living in terrestrial environments, marine-derived fungi suffer from the abovementioned extreme environmental stresses, and therefore, they have enjoyed specific metabolic pathways to synthesize structurally creative metabolites with remarkable biological activities (Zhang et al., 2020). However, although massive metabolites have been reported from marine-derived fungi thus far (Zhang et al., 2020; Carroll et al., 2021), it is a matter of fact that the search for new marine natural products is gradually approaching saturation. As a result, the discovery of new marine natural products from unexplored environments has become an alternative pathway. Extremophiles isolated from the deep sea, hydrothermal vents, cold water, and polar regions, have attracted much attention (Soldatou and Baker, 2017). They are extraordinarily adapted and metabolically active under extreme environmental conditions, which affords a large number of marine natural products.
Cancer is the leading lethal disease worldwide. Although localized surgery and radiation approaches play an important role in the treatment of cancer, it is impossible to prevent the dissemination of tumor cells. Chemotherapy has become the most preferred treatment of choice for patients, which has aroused an urgent necessity and priority to discover new molecules (Yuan et al., 2020). Natural products have benefited greatly from the growth of the pharmaceutical industry, especially pharmacologically attractive leads and potential clinical therapeutic drugs. It is estimated that among all 75 small-molecule approved antitumor drugs from 1946 to 1980, 53.3% are derived from unaltered natural products or their derivatives (Newman and Cragg, 2020). Among them, marine natural product-originated drugs have attracted more and more attention. As for antitumor drugs, Figure 1 listed representative marine natural products originated antitumor drugs, which have been approved and in phase III, II, and I clinical trials. For example, cytarabine obtained from a marine sponge is mainly used to treat acute and chronic lymphocyte in clinic (Deshmukh et al., 2018) (Figure 1). Eribulin (E7389), which was isolated from a marine sponge, was approved by FDA for metastatic breast cancer. In addition, plinabulin, which was previously isolated from a marine-derived fungus, is in a phase II clinical trial for the treatment of non-small-cell lung cancer (Zhou et al., 2016).

Figure 1 Marine natural products originated antitumor drugs (approved and in phase III, II, and I clinical trial).
As previously mentioned, deep-sea-derived fungi (depth > 1000 m) have been recognized as valuable treasure houses for structurally novel and biologically active secondary metabolites. Many interesting reviews of deep-sea-derived secondary metabolites have been published in recent years. For example, Sun et al. summarized 442 new molecules obtained from deep-sea-derived fungi, actinomycetes, bacteria, and archaea, with emphasis on structural characteristics, biological activities, and biogenetic origins (Sun et al., 2020). Wang et al. described 98 secondary metabolites with various bioactivities such as antitumor, antibacterial, antiviral, and anti-inflammatory isolated from deep-sea fungi and bacteria during 2018–2020 (Wang et al., 2020a). Wang et al. reported 180 metabolites with anticancer, antimicrobial, antifungal, antiprotozoal, and antiviral activities from deep-sea fungi (Wang et al., 2015). However, to the best of our knowledge, there are no reviews particularly focused on cytotoxic secondary metabolites isolated from deep-sea fungi. Considering their interesting chemical structures and potent lead potential, in this review, we summarize a total of 229 cytotoxic compounds isolated from deep-sea fungi from 2010 to 2021. The emphasis is on their unique chemical diversity, their relevant cytotoxic properties, and their potential as drug leads. This review will reveal deep-sea-derived fungi as considerable resources for the development of new drugs and the potential of newly discovered secondary metabolites as valuable antitumor lead compounds.
2 Cytotoxic Secondary Metabolites From Deep-Sea Fungi
2.1 Alkaloids and N-Containing Compounds
2.1.1 Diketopiperazines
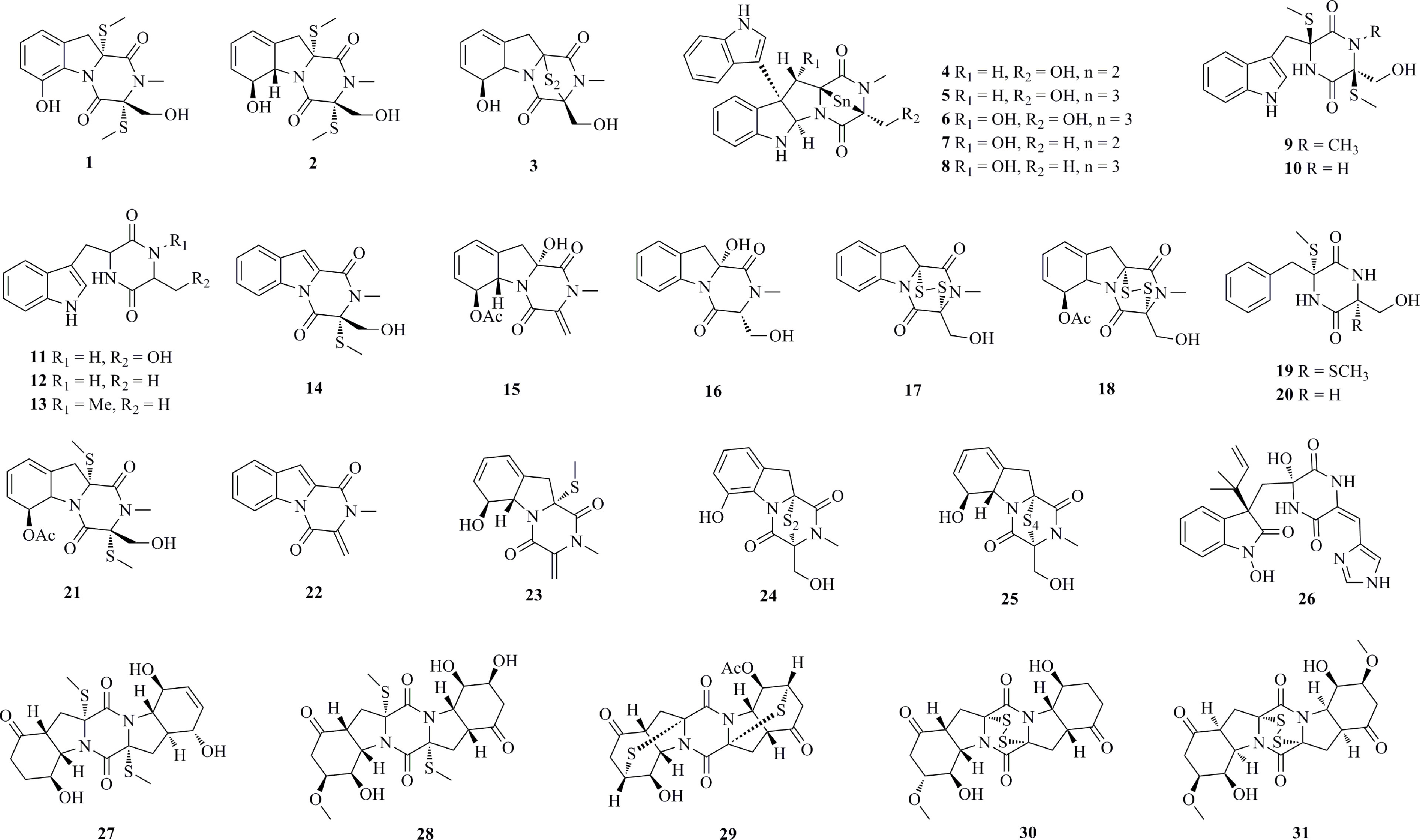
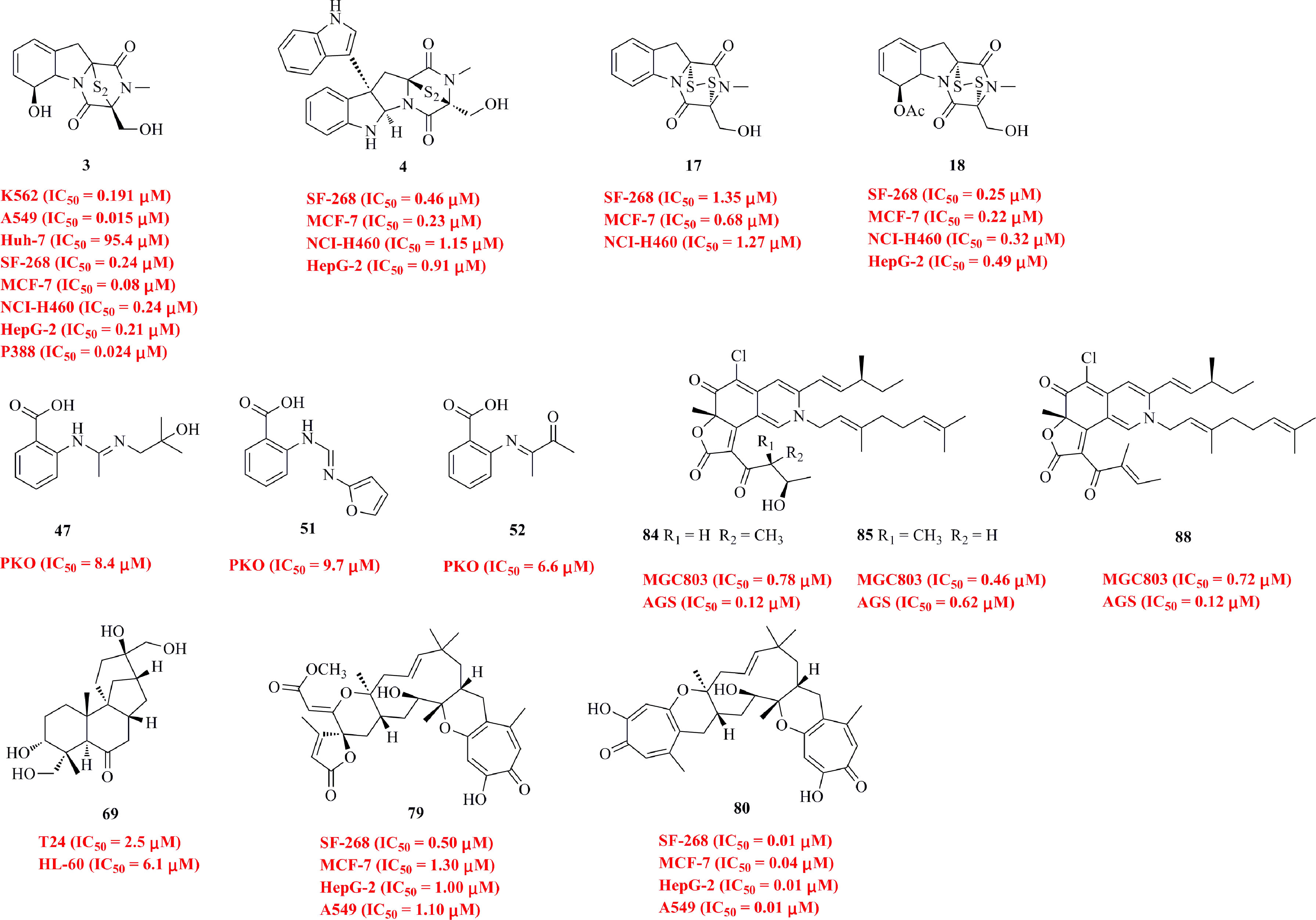
Thirty-one diverse diketopiperazines (1−31, Figure 2) with considerable cytotoxic activity were isolated from deep-sea fungi. Three diketopiperazines with a sulfur bridge, bisdethiobis(methylthio)-dehydrogliotoxin (1), bisdethiobis-(methylthio)gliotoxin (2), and gliotoxin (3), were isolated from Aspergillus sp. SCSIO Ind09F01, which was obtained from deep-sea sediment collected from the Indian Ocean (Lat: 82.04513333’ N, Long: 0.497883333’ E) at a depth of 4530 m (Luo et al., 2017). Compound 3 displayed significant cytotoxicity against the K562, A549, and Huh-7 cell lines, with IC50 values of 0.191, 0.015 and 95.4 μM, respectively. Two new bisindole diketopiperazines, luteoalbusins A−B (4−5), along with eight known compounds (6−13), were isolated from the fungus Acrostalagmus luteoalbus SCSIO F457 originating from deep-sea sediment (South China Sea, N 21°28.567’, E 118°57.297’; 2801 m depth) (Wang et al., 2012). The bisindole diketopiperazines 4−8 showed more potent cytotoxicities against SF-268, MCF-7, NCI-H460, and HepG-2 cell lines than monoindole compounds (9−13), especially for the new compounds 4−5, which were stronger than the positive control cisplatin (Supplementary Table 1). The polysulfide bridge of 4−8 can contribute more to their cytotoxicity. Three new diketopiperazines, dichotocejpins A−C (14−16), together with eight known analogs (2−3, 17−22), were isolated from Dichotomomyces cejpii FS110 (isolated from a deep-sea sediment sample from the South China Sea, N 19°0.368’, E 117°58.233’; 3941 m depth) (Fan et al., 2016). Compounds 17, 3, and 18, which contain a disulfide bond, exhibit the most potent inhibitory activities against SF-268, MCF-7, NCI-H460, and HepG-2 cell lines, with IC50 values in the range of 0.08−1.52 μM. Chemical studies of Penicillium sp. JMF034, which was isolated from deep-sea sediments collected from Fujikawa, Suruga-Bay, Japan, at a depth of 1151 m, yielded seven gliotoxin-related metabolites, 1−3, 17, and 23−25 (Sun et al., 2012). All of them show significant activity against P388 murine leukemia cells. Compounds 3, 17, 24, and 25 exhibit the most potent activity, with IC50 values of 0.024, 0.058, 0.056, and 0.020 μM, respectively. A new indolyl diketopiperazine derivative, penilline C (26), was isolated from P. chrysogenum SCSIO 07007, separated from a deep-sea hydrothermal vent environment sample (Western Atlantic, 126.8983°E, 27.7875°N, 1028 m depth) (Han et al., 2020). Four new thiodiketopiperazines, 5’-hydroxy-6’-ene-epicoccin G (27), 7-methoxy-7’-hydroxyepicoccin G (28), 8’-acetoxyepicoccin D (29), and 7’-demethoxyrostratin C (30), as well as a known analog 31 were isolated from Epicoccum nigrum SD-388, a fungus obtained from deep-sea sediments (West Pacific, 4500 m depth) (Chi et al., 2020). Compounds 30 and 31 display strong activity against Huh7.5 liver tumor cells with IC50 values of 9.52 and 4.88 μM, respectively, which were comparable to that of the positive control, sorafenib (IC50 = 8.2 μM).
2.1.2 N-Containing Compounds
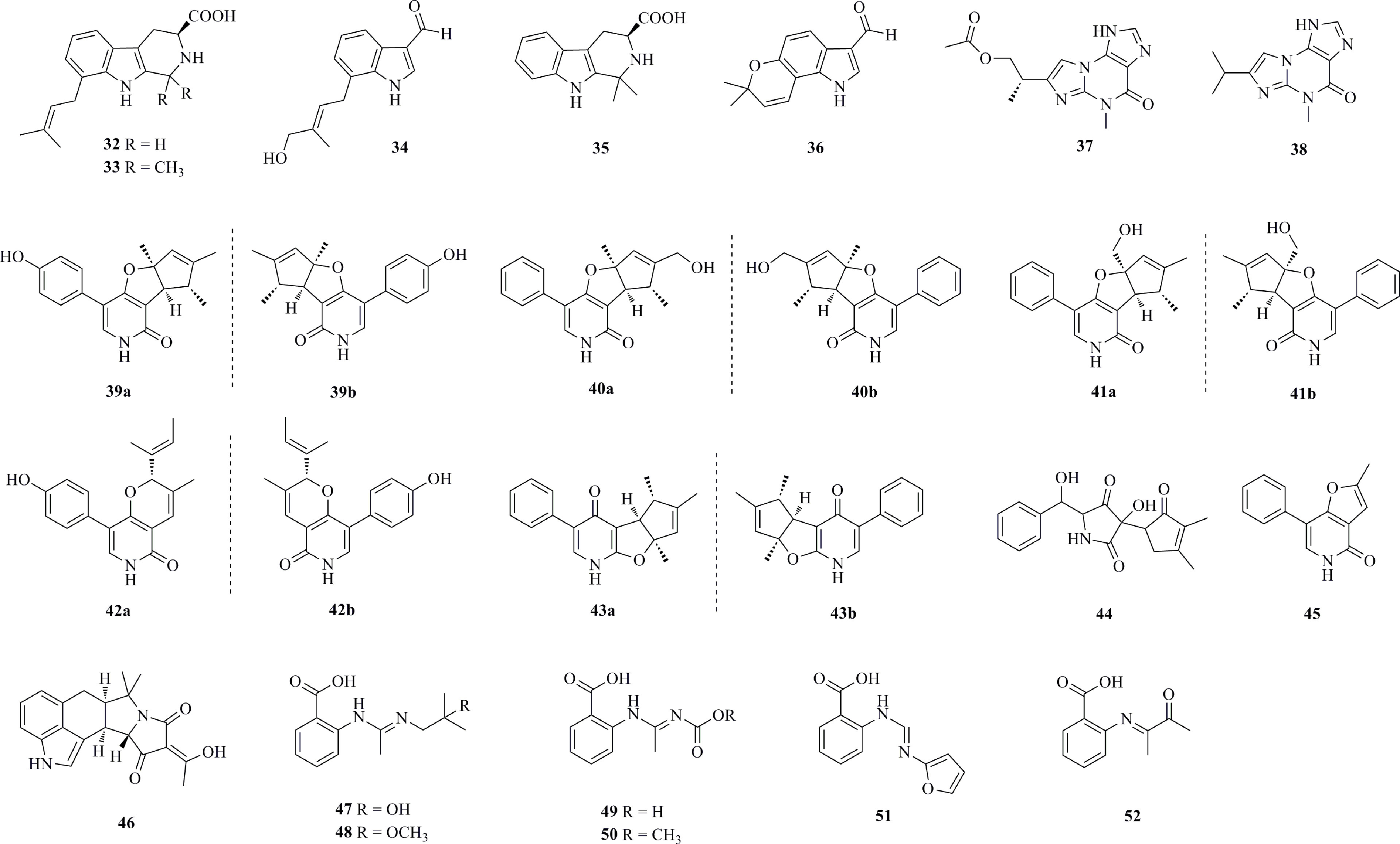
Cytotoxic N-containing compounds (32−52) isolated from deep-sea fungi are shown in Figure 3. Three new prenylated indole alkaloids, penipalines A−C (32−34), as well as two known analogs 35−36, were isolated from the deep-sea-sediment-derived fungus P. paneum SD-44 (Li et al., 2014). The new compounds 32 and 33 are β-carbolines, while 34 is an indole carbaldehyde derivative. Compounds 33 and 34 are active against the A-549 and HCT-116 cell lines, with IC50 values of 20.44 and 21.54 μM for A-549 cells and 14.88 and 18.54 μM for HCT-116 cells, respectively. A new acremolin-type alkaloid acremolin D (37) and a known compound 38, both containing an unprecedented 1H-azirine unit, were isolated from A. sydowii MCCC 3A00324, which was obtained from the deep sea sediment (2246 m) of the South Atlantic Ocean (W13.6639°, S14.2592°) (Niu et al., 2021). Compound 37 shows certain effects against HeLa-S3 and K562 cells, with inhibition rates of 30.6% and 25.1%, respectively, at a concentration of 20 μM, whereas 38 is active against A549, HepG2, and K562 cells, with inhibition rates of 20.9−35.5%. Seven new pyridone alkaloids (39−45) were isolated from the deep-sea fungus Phomopsis tersa FS441 obtained from a sediment sample (Indian Ocean, 88°58’640’’ E, 0°00’307’’ S, 3000 m depth) (Chen et al., 2019). Structurally, 39−41 and 43 represent phenylfuropyridone racemates with a rare 6-6/5/5 ring system, and 42 was reported as a phenylpyridone racemate with a 6-6/6 core. In addition, 45 is the first 5-phenylpyridone derivative with an unprecedented furo [3,2-c]pyridin-4(5H)-one skeleton. Compound 43b possesses mild cytotoxic activities against SF-268, MCF-7, HepG-2 and A549 cell lines with IC50 values of 32.0, 29.5, 39.5 and 33.2 μM, respectively. 46 was isolated from the fungus A. flavus SCSIO F025 derived from deep-sea sediments in the South China Sea (117.062°E, 20.077°N, at a depth of 1781 m) (Xiang et al., 2021). Compound 46 shows broad-spectrum cytotoxicity against SF-268, HepG-2, MCF-7, and A549 cell lines with IC50 values ranging from 24.38 to 48.28 μM. Six anthranilic acid derivatives 47−52 were isolated from the marine sediment-derived fungus P. paneum SD-44 (Li et al., 2013). Compounds 47 and 51 exhibit higher inhibitory activity against the RKO cell line with IC50 values of 8.4 and 9.7 μM, respectively, while 52 exhibits higher inhibitory activity against the HeLa cell line with an IC50 value of 6.6 μM than against the positive control fluorouracil (with IC50 values of 25.0 and 14.5 μM).
2.1.3 Peptides
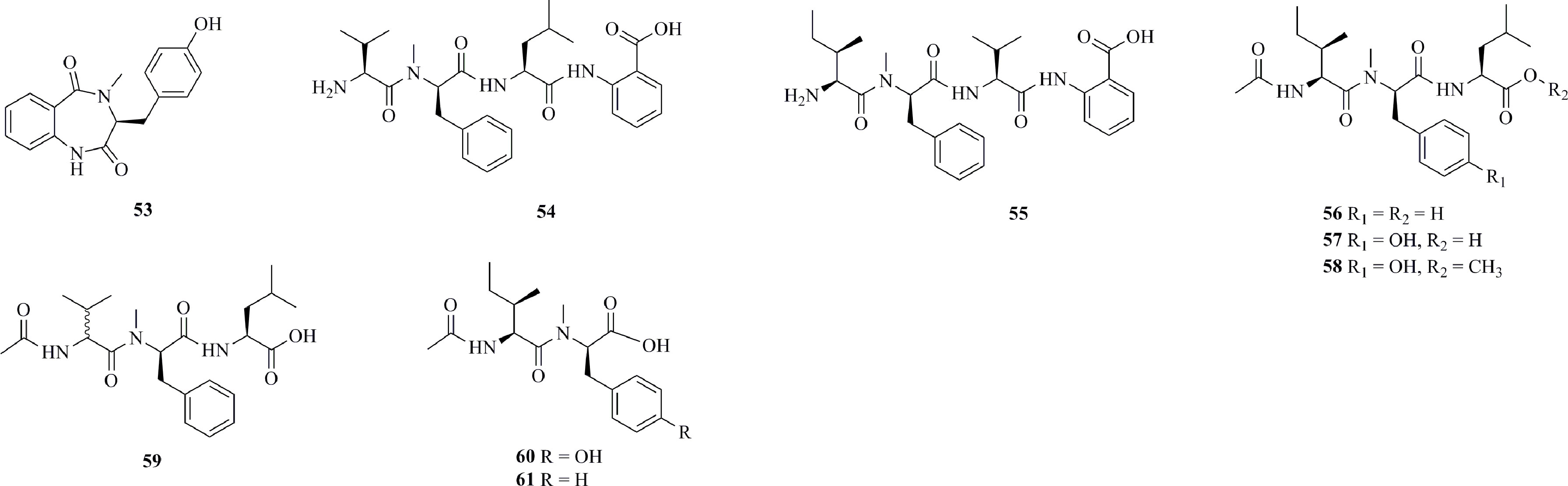
Figure 4 lists nine cytotoxic peptides isolated from deep-sea fungi. A bioassay-guided chemical investigation of Aspergillus sp. SCSIOW2 [separated from a deep marine sediment sample in the South China Sea (112°30.203E, 18°1.654N) at a depth of 2439 m] yielded a novel cyclic dipeptide, 14-hydroxy-cyclopeptine (53) (Zhou et al., 2016). This cyclodipeptide possesses NO production inhibitory activity and no cytotoxicity at the tested dose range (30−100 μg/mL). The fungus Simplicillium obclavatum EIODSF 020 was isolated from deep sea sediment collected from the East Indian Ocean (10°00’ N, 84°33’ E; 4571 m depth) (Liang et al., 2016). Eight new peptides, simplicilliumtides A−H (54−61), were isolated from this fungal strain. 54 and 55 are linear tetrapeptides bearing a 2-aminobenzoic acid residue, while 56−61 are acetylated linear tri- or dipeptides. Only weak cytotoxicity was observed for 54 and 60 toward the human leukemia HL-60 cell line and for 58 and 61 toward the K562 cell line.
2.2 Terpenoids and Steroids
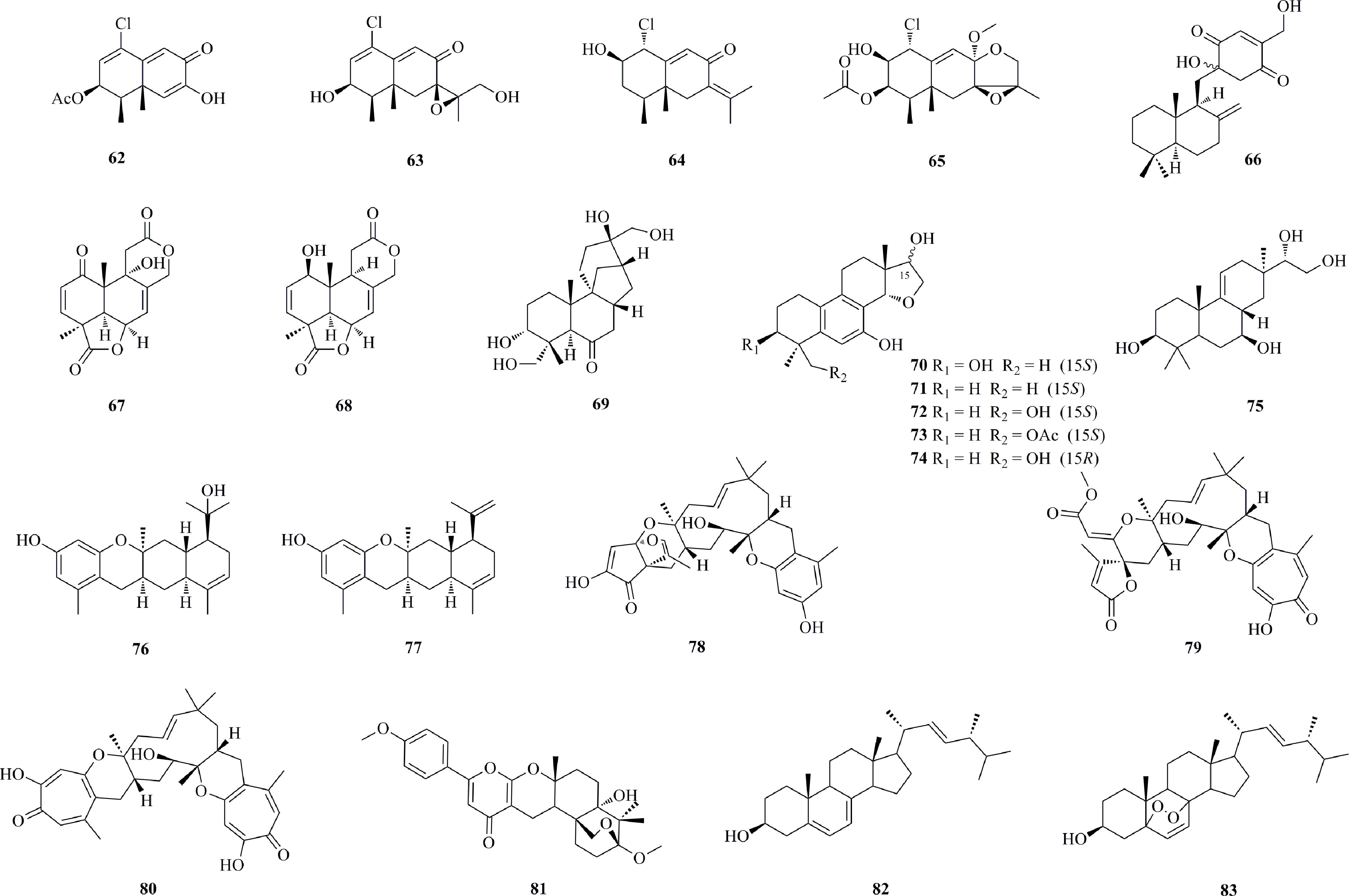
As shown in Figure 5, four new chlorinated eremophilane-type sesquiterpenes 62−65 were isolated from an Antarctic deep-sea fungus, Penicillium sp. PR19N-1, which was obtained from marine sludge in Prydz Bay (1000 m depth), Antarctica (Wu G. et al., 2013). 62 demonstrated moderate activity against HL-60 and A549 cell lines, with IC50 values of 11.8 and 12.2 μM, respectively. The fungal strain Penicillium sp. F00120 isolated from a deep sea sediment sample collected at a depth of 1300 m produced a new sesquiterpene quinone, named penicilliumin A (66) (Lin et al., 2012). Compound 66 is active against the A375, B16 and HeLa cell lines with GI50 values of 22.88, 27.37, and 44.05 μg/mL, respectively. Two new tetranorlabdane diterpenoids, asperolides D (67) and E (68), were isolated from A. wentii SD-310, a fungus obtained from a deep sea sediment sample in the South China Sea at a depth of 2038 m (Li et al., 2016a). Compound 68 shows certain activities against HeLa, MCF-7, and NCI-H446 cell lines, with IC50 values of 10.0, 11.0, and 16.0 μM, respectively. A systematic isolation of Botryotinia fuckeliana MCCC 3A00494, a fungus isolated from the western Pacific Ocean (5572 m depth), provided 71 new and eight known aphidicolin derivatives (structures in Figure S1 and Figure S2 in Supplementary Material) (Niu et al., 2019a). Among them, aphidicolin A8 (69) is found to observably induce apoptosis in T24 (IC50, 2.5 μM) and HL-60 (IC50, 6.1 μM) cells by causing DNA damage, suggesting that it is a promising lead compound. Asperethers A−E (70−74), five new 20-nor-isopimarane diterpenoids, were isolated from the abovementioned A. wentii SD-310 (Li et al., 2016b). 70−74 possess a cycloether unit with a unique 6/6/6/5 tetracyclic skeleton, which has not been reported up to date. Compounds 70−74 exhibit cytotoxic activities against the A549, HEK293, MCF-7, SMMC-7721, and T-47D cell lines, with IC50 values of 10−48 μM. A new pimarane-type diterpenoid, botryopimarene A (75), was discovered from the deep-sea fungus B. fuckeliana MCCC 3A00494 (Niu et al., 2019b). Photeroids A (76) and B (77), two unique phenol-sesquiterpene meroterpenoids, were isolated from Phomopsis tersa FS441, a fungus separated from a sediment sample that was collected at a depth of 3000 m in the Indian Ocean (88°58.640′ E, 0°00.307′ S) (Chen et al., 2020a). 76 and 77 represent the first examples of phenolic sesquiterpene meroterpenoids featuring a highly fused 6/6/6/6 tetracyclic ring skeleton. Compounds 76 and 77 exert mild cytotoxicities against SF-268, MCF-7, HepG-2, and A549 cell lines, with IC50 values ranging from 20.0 to 26.2 μM. Three novel meroterpenoids 78−80 were isolated from the same P. tersa FS441 fungus (Chen et al., 2020b). 78 represents the first tropolonic sesquiterpene having a highly fused 6/6/11/6/5/5 ring system, while 79 is the first meroterpenoid featuring a rare 7/6/11/6-5 spiral core skeleton. 79 and 80 exhibit potent antiproliferative effects against SF-268, MCF-7, HepG-2, and A549 cell lines, with IC50 values of 0.01−1.30 μM, which were even higher than the positive control adriamycin. A new merosesquiterpenoid, yaminterritrem C (81), was isolated from the deep-sea-derived strain P. chrysogenum SCSIO 41001, which was obtained from the deep sea sediment of the Indian Ocean (Lat: 10.00371667°N, Long: 88.72803333°E) at a depth of 3386 m (Chen et al., 2017). 81 possesses a rare naphtho[2,1-b]pyrano-[3,2-e]pyran moiety. P. chrysogenum strain S003, a fungus isolated from Red Sea deep sediment, yielded two cytotoxic steroids 82 and 83 (Alshehri et al., 2020). 82 and 83 show cytotoxic effects against A-549, DU-145, MCF-7, and HepG2 cell lines, with IC50 values ranging from 1.5 to 21.26 μM.
2.3 Polyketides
2.3.1 Azaphilones
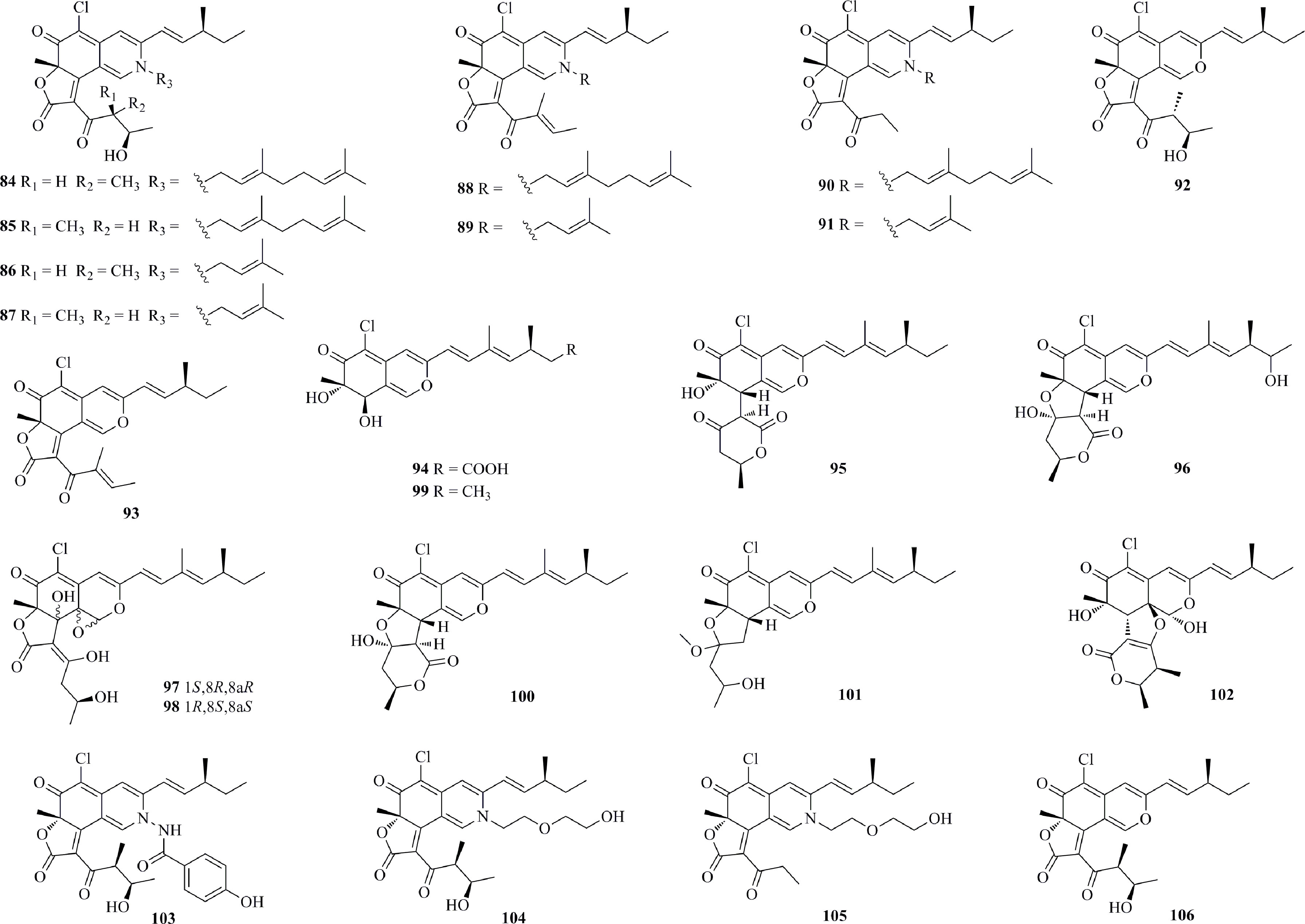
A total of 23 azaphilones with an oxabicyclic core were isolated from deep-sea fungi (Figure 6). Eight new nitrogenated azaphilones (84−91) and two known compounds (92 and 93) were isolated from Chaetomium globosum MP4-S01-7, a fungus obtained from a water sample collected at a depth of 4300 m (19°57′32.9205″ N, 161°51′47.3181″ E) in the West Pacific Ocean (Wang et al., 2020b). 84, 85, and 88 exhibit potent cytotoxicities against MGC803 and AGS cell lines, with IC50 values less than 1 μM. Moreover, 85 arrests the cell cycle in the G1 phase. 84 and 85 induce apoptosis in a concentration-dependent manner. Eight chlorinated azaphilone derivatives (94−101), including five new derivatives (94−98), were isolated from the deep-sea fungus Phomopsis tersa FS441 (Chen et al., 2021). Structurally, 95 features a unique 6/6-6 carbon skeleton, rather than a tetrahydrofuranyl ring such as that in 96. 97 and 98, a pair of diastereomers with a characteristic and rare epoxide ring, exhibit potent cytotoxicity against MCF-7, SF-268, and A549 cell lines with IC50 values ranging from 5.4 to 8.3 μM. Another five chlorinated azaphilone pigments (102−106) were produced by a Chaetomium sp. strain NA-S01-R1, which was isolated from seawater at a depth of 4050 m (20°25′11.0321′′ N, 155°51′22.1549′′ E) in the West Pacific Ocean (Wang et al., 2018). 103 shows the strongest activity toward HepG2 cells, with an IC50 value of 3.9 μM, while 102 and 104 were found to be active against HeLa cells, with IC50 values of 5−8 μM.
2.3.2 Tetramic Acid and Sorbicillinoid Derivatives
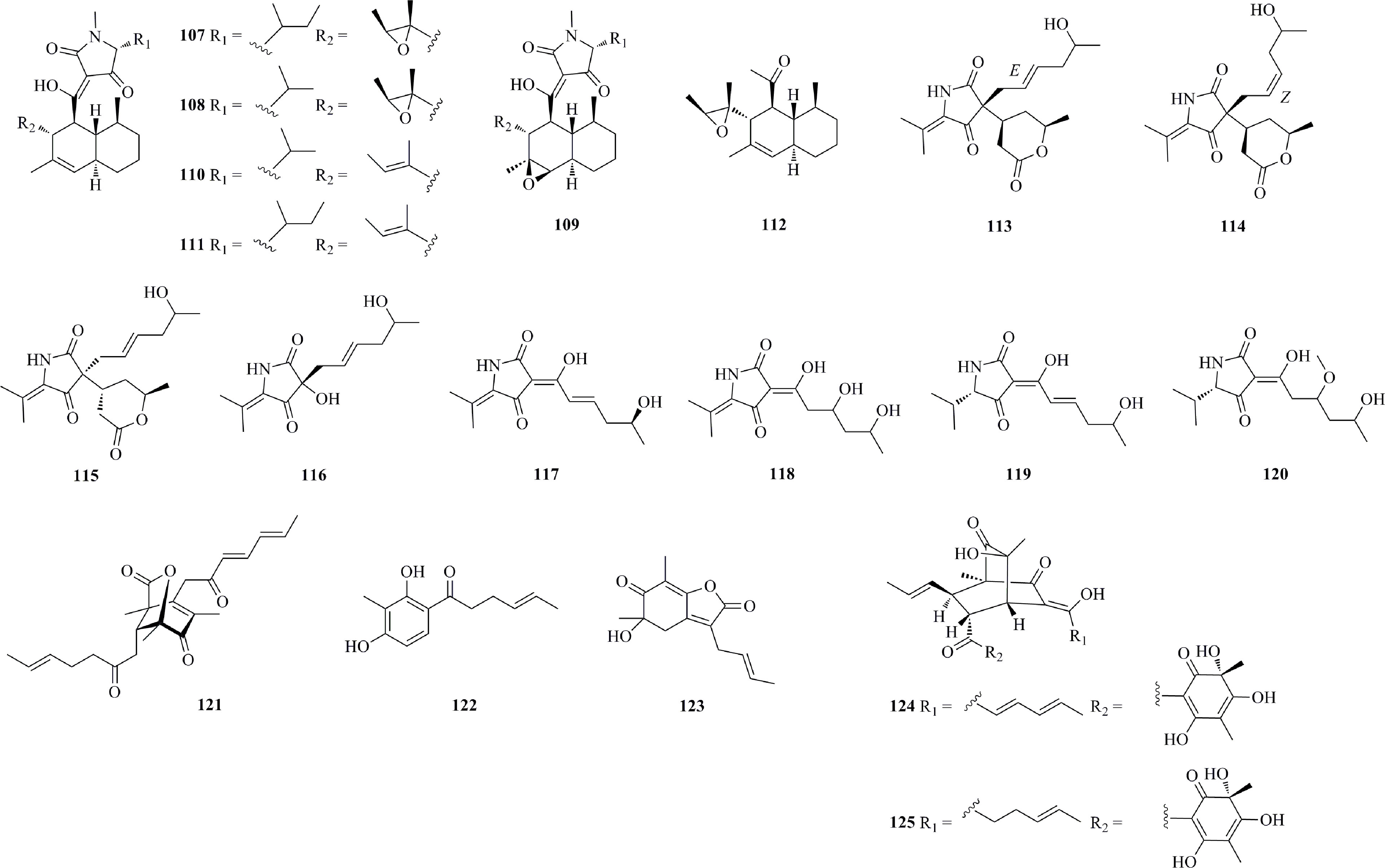
Tetramic acid derivatives (107−120, Figure 7) are characterized as simple heterocycles with pyrrolidine-2,4-dione. Six new tetramic acid derivatives with a decalin ring (107−112) were characterized from the fungus Trichobotrys effuse DFFSCS021 derived from the deep sea sediment of the South China Sea (Sun et al., 2015). 107, 108, and 112 potently inhibit the KG-1a cell line with IC50 values of 5.44, 8.97, and 6.16 μM. Cladosporium sp. SCSIO z0025 derived from deep-sea sediment (at a depth of 1330 m from the Okinawa Trough, 27°48.12′N and 126°58.89′E) produced eight new tetramic acid derivatives, cladosporiumins A−H (113−120) (Huang et al., 2018). 113−115 were characterized as 3-acyltetramic acids incorporating a hexyl enic alcohol side chain and a six-membered lactone ring. Three new sorbicillinoids 121−123 were isolated from a deep-sea sediment-derived fungus, Phialocephala sp. FL30r (obtained from an underwater sample from the east Pacific site W2003-03, W154°04′57′′, N8°30′20′′, 5059 m depth) (Li et al., 2011). 121 possesses the rare bicyclo[3.2.1] lactone skeleton and displays moderate cytotoxic activity against P388 (IC50, 11.5 μM) and K562 (IC50, 22.9 μM) cell lines. 122 and 123 were found to display submicromolar activities against P388, with IC50 values of 0.1 and 0.2 μM, respectively. Two new bisorbicillinoids, 124 and 125, were isolated from Phialocephala sp. FL30r derived from deep-sea sediment from ES304 (W145°23′03′′, N8°19′50′′, depth 5059 m) (Li et al., 2007). Both 124 and 125 only show weak cytotoxic activities against the P388, HL60, BEL7402, and K562 cell lines.
2.3.3 Chromones
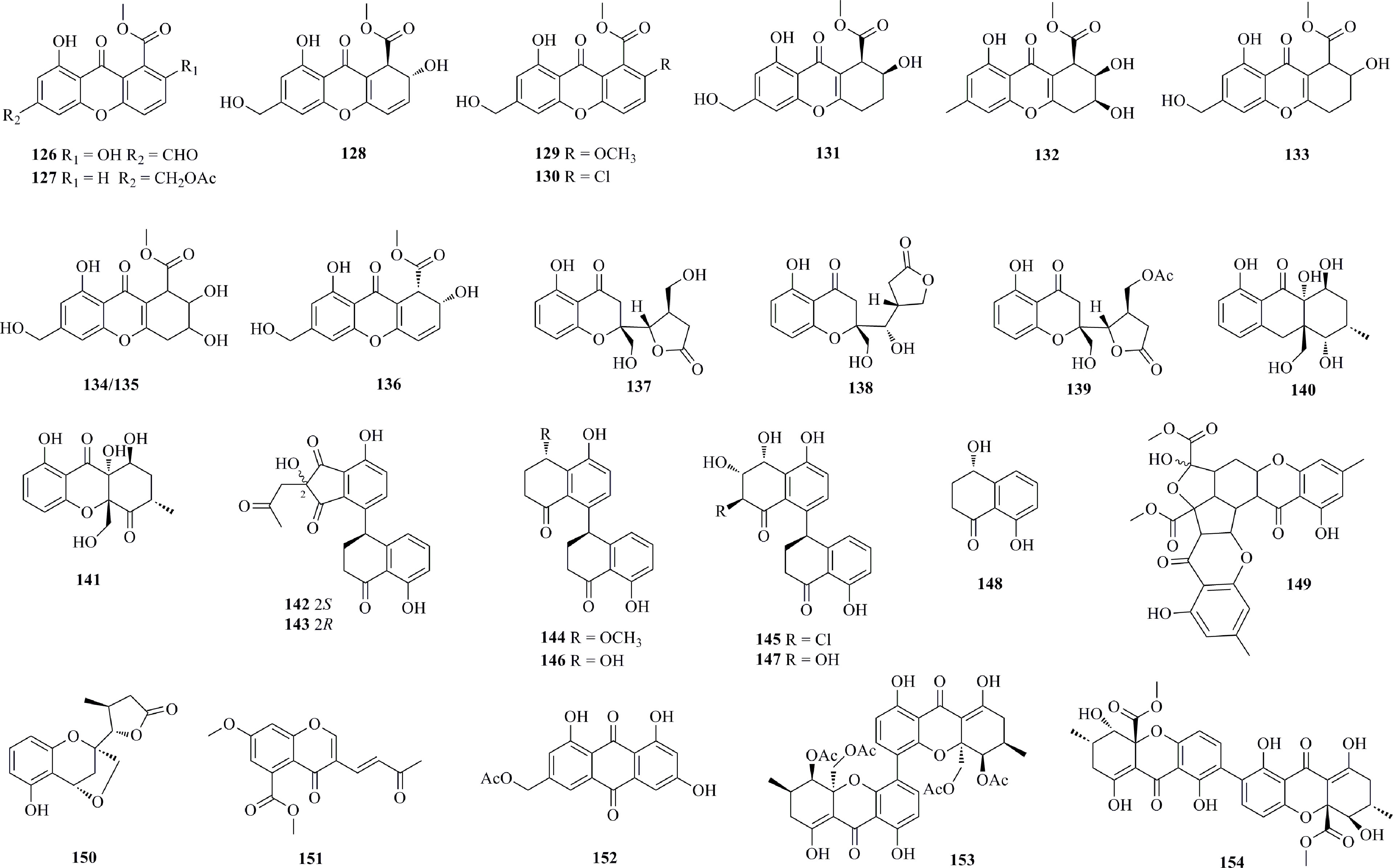
Cytotoxic chromone polyketides (126−154) isolated from deep-sea fungi are listed in Figure 8. Two new xanthones 126−127 and a known compound 128 were isolated from an A. sydowii C1-S01-A7, separated from a seawater sample obtained at a depth of 4950 m (20°07′02.7264′′ N, 158°46′52.3352′′ E) from the West Pacific Ocean (Wang et al., 2019a). Compound 128 displays selective cytotoxicity against the A549 cell line with an IC50 value of 8.1 μM. The fungal strain Engyodontium album DFFSCS021 isolated from a marine sediment sample in the South China Sea (19°00′368″N, 117°58′223″E, 3739 m depth) was found to produce eight new chromones, 129−136 (Yan et al., 2014). Compound 136 shows strong cytotoxicity against the U937 cell line with an IC50 value of 4.9 μM. Five new chromone polyketides 137−141 were isolated from the deep-sea sediment-derived fungus Diaporthe phaseolorum FS431 collected from the Indian Ocean (depth 3605 m, 7°57.75944′ N, 89°19.43851′ E) (Guo et al., 2019). 138 was first reported as an unprecedented chromone with a recombined five-member lactone ring. Four new tetralone derivatives 142−145 and three known polyketides 146−148 were isolated from the deep-sea derived fungus Cladosporium cladosporioides HDN14-342 (collected from the Indian Ocean, depth 3471 m) (Zhang et al., 2016a). The 1-tetralone dimers linked by a C–C bond are widespread fungal polyketides. 142 and 143 are new dimeric forms of indanone and 1-tetralone adducts, and compound 145 is the first halogenated cladosporols. 145 shows relatively higher cytotoxic activity against HeLa cells with an IC50 value of 3.9 μM. The deep-sea sediment-derived fungus Penicillium sp. SCSIO Ind16F01 afforded a cytotoxic chromone dimer 149 against K562, MCF-7, and SGC7901 cells, with IC50 values of 16.6, 16.3, and 15.8 μM, respectively (Liu et al., 2017). Two new chromones 150 and 151, one new anthraquinone 152, and one known chromone dimer 153 were isolated from the fungus D. phaseolorum FS431 (Niu et al., 2019c). 153 shows strong activity against MCF-7, HepG-2, and A549 cells, with IC50 values of 2.60, 2.55, and 4.64 μM, respectively. Secalonic acid F (154) was isolated from the fungus Penicillium sp. F11 derived from deep-sea sediment samples at a depth of 1744 m in the Southwest Pacific (Li et al., 2012). 154 shows significant cytotoxicity and induced apoptosis in HL60 cells with an IC50 value of 4.1 μg/mL.
2.3.4 Benzophenones
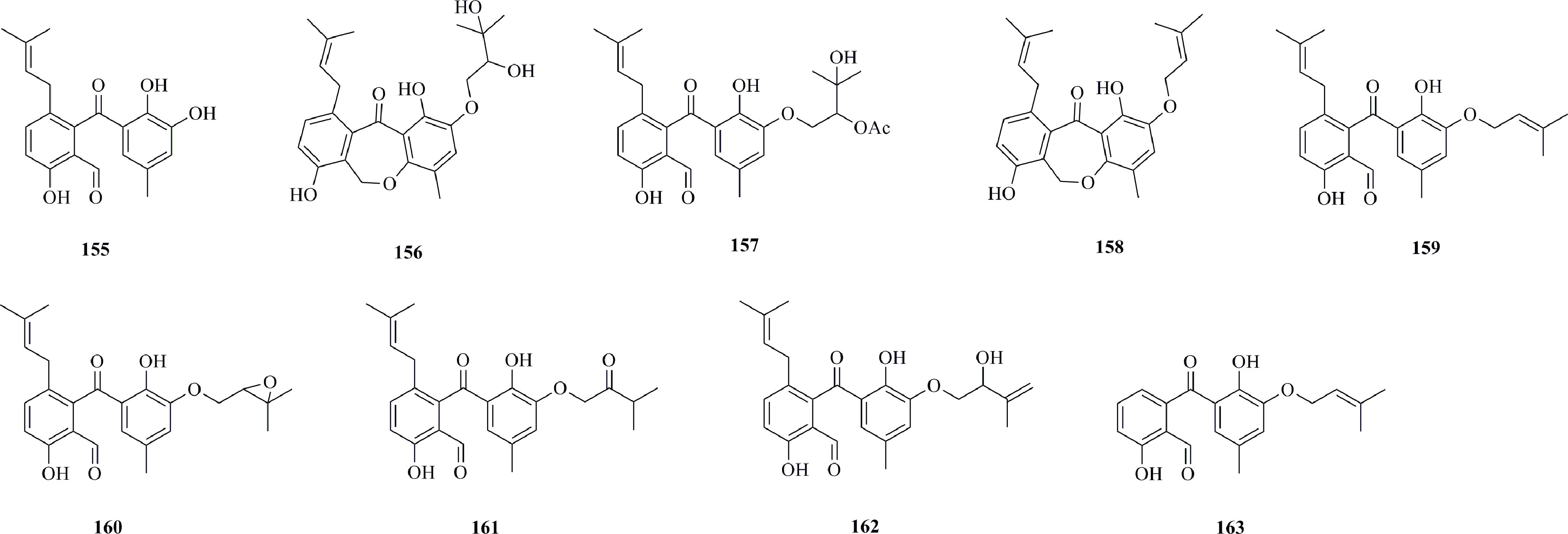
Figure 9 shows benzophenones (155−163) from deep-sea fungi. Four new benzophenones named tenellones J−M (155−158) were produced by culturing of Phomopsis lithocarpus FS508 (isolated from a deep-sea sediment sample collected from the Indian Ocean, 111°53.335′ E, 16°50.508′ N, depth 3606 m) (Liu et al., 2021a). 156 moderately inhibits the SF-268 cell line with an IC50 value of 11.36 μM. Five new highly substituted benzophenone derivatives, tenellones D−H (159−163), were isolated from the deep-sea sediment-derived fungus Phomopsis lithocarpus FS508, which was collected at a depth of 3606 m from the Indian Ocean (111°53.335′ E, 16°50.508′ N) (Xu et al., 2018). Compounds 159−163 possess naturally occurring aldehyde functionalities, which are rare in natural products. 163, in particular, displays modest cytotoxic activity against HepG-2 and A549 cell lines, with IC50 values of 16.0 and 17.6 μM, respectively.
2.3.5 Other Polyketides
Other types of polyketides (164−212) isolated from the deep-sea are shown in Figures 10 and 11. Five new 2,3-dihydro-1H-indene derivatives (164−168) were isolated from the previously mentioned deep-sea sediment-derived fungus Phomopsis lithocarpus FS508 (Liu et al., 2021a). They possess a weak ability against SF-268, MCF-7, HepG-2, and A-549 cells (IC50 > 50 μM). Furthermore, chemical investigations of this fungal strain also led to the isolation of lithocarols A−F (169−175) possessing a novel highly oxygenated isobenzofuran (Xu et al., 2019). 169−173 were characterized as the first examples of polyketal derivatives in the tenellone family, while 174 is a rarely observed tenellone lactone. These compounds exerted moderate cytotoxicities against HepG-2, MCF-7, SF-268, and A549 cell lines (IC50, 10.5−38.7 μM). A new isopentylated dibenzodioxocinone 176 and a new isopentylated pyran-3,5-dione derivative 177 were isolated from P. canescens SCSIO z053, a fungus collected from deep-sea sediment from the Okinawa Trough (27°33.07′ N, 126°58.36′ E, 1387 m depth) (Dasanayaka et al., 2020). An Alternaria sp. fungus MCCC 3A00467 was isolated from a sediment of the Pacific Ocean at a depth of 5295 m. This fungus was found to produce three new phomalone derivatives 178−180 and seven known analogs 181−187 (Zhong et al., 2022). 179 shows cytotoxic activity against the U266 cell line with an IC50 value of 24.99 μg/mL, while 187, the most active compound, possesses cytotoxicity against U266, HepG2, and A549 cells with IC50 values of 13.26, 14.69 and 24.39 μg/mL, respectively. A new dihydrobenzofuran-phenyl acrylate hybrid 188 was isolated from the culture of A. terreus CC-S06-18 obtained from a seawater sample at a depth of 5250 m from the North Pacific Ocean (Wang et al., 2020c). 188 shows selective cytotoxicity against HGC27, MGC803, BGC823, and AGS cells, with IC50 values of 3.4, 7.0, 6.2, and 8.2 μM, respectively. Further pharmacological studies indicate that 188 inhibits cell cycle progression and induced apoptosis. Two new citrinin dimers, 189 and 190 were isolated from the fungus P. citrinum NLG-S01-P1 obtained from a seawater sample at a depth of 4650 m (Wang et al., 2019b). 189 is active against the HeLa cell line with an IC50 value of 4.1 μM. A dimeric isocoumarin, bipenicilisorin (191), a citrinin dimer, penicitrinone F (192), and a δ-valerolacton 193 were isolated from the deep-sea fungus P. chrysogenum SCSIO 41001 (Chen et al., 2017). Cytotoxic evaluation indicated that 193 significantly inhibits K562, A549, and Huh-7 cell lines with IC50 values of 6.78, 6.94, and 2.59 μM, respectively, whereas 192 specifically shows inhibitory activity against EV71 with an IC50 value of 14.50 μM.
The fungus P. chrysogenum MCCC 3A00292 derived from the South Atlantic Ocean at a depth of 2076 m yielded five versiol-type derivatives 194−198 and two novel γ-lactones 199−200 (Niu et al., 2019d). 194−198 represent a rare class of fungal polyketides with an alkylated decalin nucleus, while 199−200 are the first report of γ-lactones bearing a 1,3-dihydroxy-5-methylbenzene unit. 194 shows potent inhibitory activity against the BIU-87 cell line with an IC50 value of 10.21 μM. 197 and 198 are active against the ECA109 cell line with IC50 values of 12.41 and 15.60 μM, respectively. Two new cytotoxic heteroatom-containing compounds 201 and 202 were isolated from the deep-sea sediment-derived fungus P. citreonigrum XT20-134 (Tang et al., 2019). Their IC50 values toward the Bel7402 cell line were 7.63 and 13.14 μM and toward the HT1080 cell line were 10.22 and 16.53 μM, respectively. Ten new C9 polyketides belonging to the aspyrone derivatives 203−212 were isolated from deep-sea-derived A. ochraceus (Zou et al., 2020). Compounds 207−210 exert cytotoxic effects on the BV-2 cell line with inhibition rates ranging from 72.81% to 50.29%.
2.4 Phenolic Derivatives
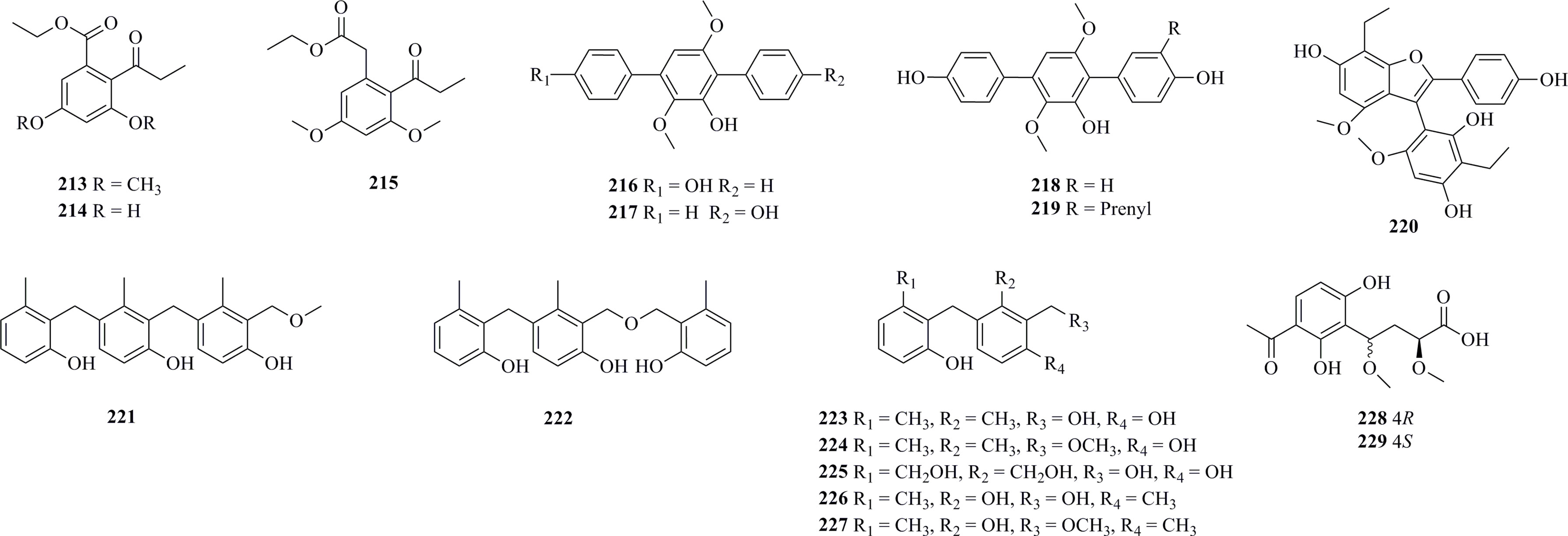
As shown in Figure 12, seventeen phenolic derivatives (213−229) have been isolated. Engyodontium album, isolated from marine sediments collected at a depth of 2530 m in the Pacific Ocean (176.45° W, 21.449° S), produced two new benzoate derivatives 213 and 214 and a new phenylacetate derivative 215 (Wang et al., 2017). However, all of them only show weak cytotoxicity against HeLa cells (IC50 > 50 μM). One new (216) and three known (217−219) p-terphenyl derivates were isolated from the fungus A. candidus collected from the Atlantic Ocean at a depth of 3542 m (Lin et al., 2021). 213 and 214 display strong antiproliferative effects against HeLa, Eca-109, Bel-7402, and PANC-1 cells, with IC50 values ranging from 5.5 μM to 9.4 μM. A highly substituted phenol derivative 220 was isolated from the deep-sea-derived fungus Trichobotrys effuse FS524 (Liu et al., 2020). 220 with an interesting 6-5/6/6 tetracyclic ring system exhibits moderate activities against SF-268, MCF-7, HepG-2, and A549 cell lines with IC50 values ranging from 30.1 to 43.3 μM (compared with the positive control cisplatin, 2.5−3.2 μM). P. fellutanum HDN14-323, isolated from a sediment sample collected at a depth of 5725 m from the Indian Ocean, produced seven new 6-methylsaligenin derivatives, including two trimeric derivatives, 221 and 222, and five dimeric derivatives, 223−227 (Zhang et al., 2016b). 224 was found to possess the best activity against the HeLa cell line with an IC50 value of 9.3 μM. Two new globoscin derivatives, 228 and 229, were isolated from the deep-sea-derived fungus A. fischeri FS452 (Liu et al., 2021b). 229 demonstrates potential activities against SF-268, MCF-7, HepG-2, and A549 cell lines with IC50 values of 7.56–9.98 μM.
3 Discussion
3.1 Structural Diversity of the Described Compounds Isolated From Deep-Sea Fungi
It is estimated that over 500 secondary metabolites have been isolated from deep-sea-derived fungi (> 1000 m). However, these microorganisms remain a relatively untapped source of bioactive molecules both structurally and biologically compared to the 24000 reported marine natural products (Carroll et al., 2021). This review first summarizes a total of 229 cytotoxic compounds isolated from deep-sea fungi from 2010 to 2021. They are further classified into diketopiperazines (1−31), alkaloids (32−52), peptides (53−61), terpenoids and steroids (62−83), azaphilones (84−106), tetramic acid and sorbicillinoid derivatives (107−125), chromones (126−154), benzophenones (155−163), other polyketides (164−212), and phenolic derivatives (213−229), according to their putative biogenetic sources. As shown in Figure 13A, among the 229 active compounds, approximately 56.33% are polyketides, which include 10.04% azaphilones, 8.30% tetramic acid and sorbicillinoid derivatives, 12.66% chromones, 3.93% benzophenones, and 21.40% other polyketides. These findings indicated that molecules grouped as polyketides are one of the most promising compounds as novel antitumor drug leads. Alkaloids are also the main structure type for these compounds. Taking into account diketopiperazines (13.54%) and peptides (3.93%), alkaloids account for 26.64% of the isolated compounds. In addition, compounds isolated from deep-sea fungi often contain heteroatoms, such as sulfur and chlorine. For example, compounds 1−9, 17−20, and 27−31 are rare sulfur-containing diketopiperazines, which were isolated exclusively from extreme marine environments. As mentioned earlier, the extreme marine environment can produce more natural products with novel structures, which is a potential resource for new antitumor drugs.
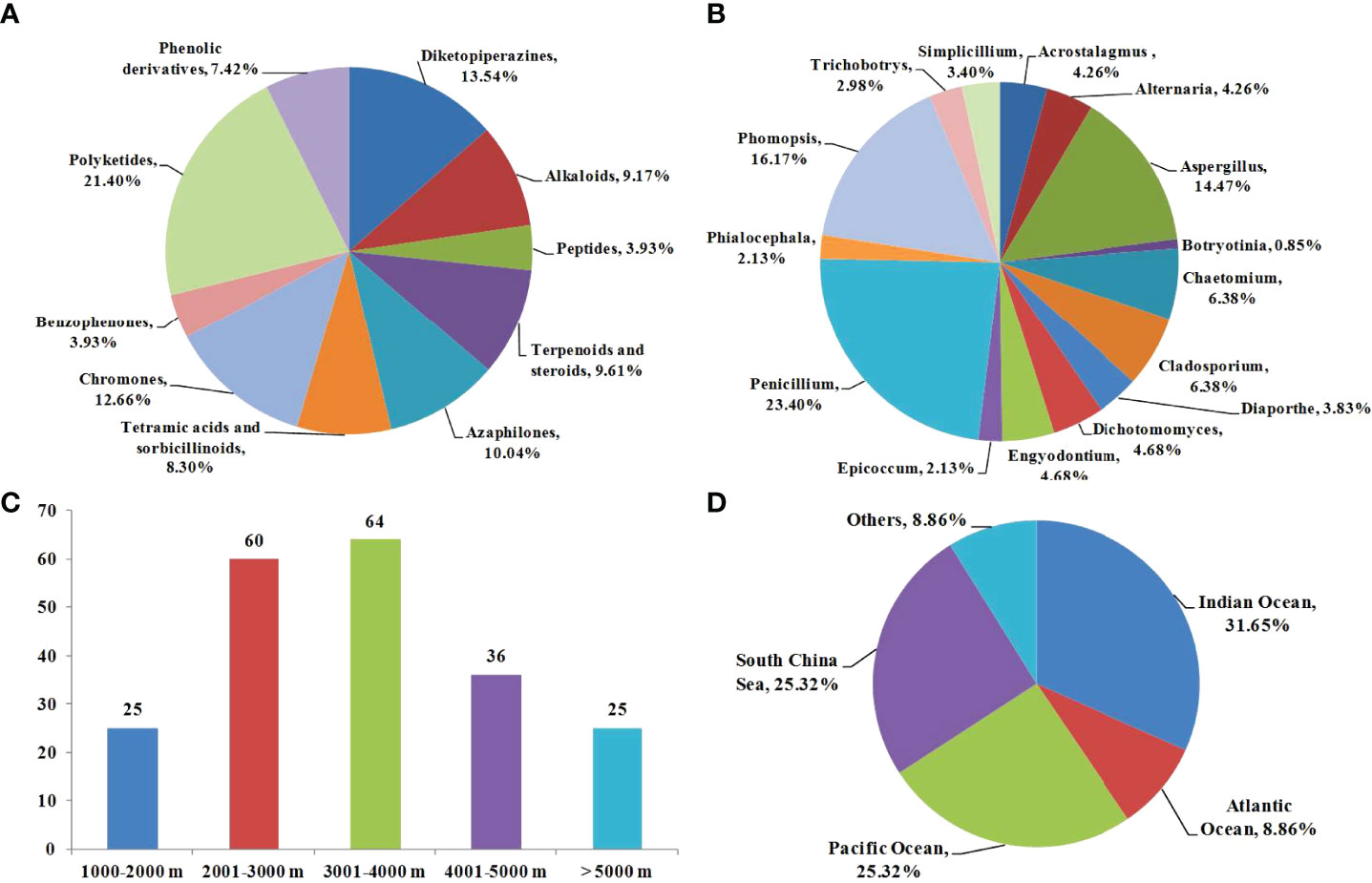
Figure 13 (A) Chemical structures categories of the described compounds isolated from deep-sea fungi; (B) Producing strains categories of the isolated compounds; (C) Distribution categories of the producing strains; (D) Geographic origins of the isolated compounds.
3.2 Diverse Fungal Species as Producers of Isolated Compounds
As shown in Figure 13B, a total of 15 fungal species in this review, including Acrostalagmus, Alternaria, Aspergillus, Botryotinia, Chaetomium, Cladosporium, Diaporthe, Dichotomomyces, Engyodontium, Epicoccum, Penicillium, Phialocephala, Phomopsis, Trichobotrys, and Simplicillium, have been reported as producing strains for these cytotoxic compounds. Among them, Penicillium, Phomopsis and Aspergillus are the most prolific fungal strains, with 55 (accounting for 23.40%), 38 (accounting for 16.17%), and 34 (accounting for 14.47%) compounds produced, respectively. The genera Aspergillus and Penicillium are regarded as the most widely studied fungal groups in nature. Interestingly, the deep-sea environment contains rare fungal species, such as Diaporthe, Dichotomomyces, and Engyodontium, which are rarely observed in terrestrial environments. Moreover, the distributions of the producing strains are shown in Figure 13C. The deep-sea is defined as that more than 1000 m below the water surface. Among these compounds, a total of 25, 60, 64, 36, and 25 compounds were isolated from deep-sea samples at depths of 1000−2000 m, 2001−3000 m, 3001−4000 m, 4001−5000 m, and > 5000 m, respectively. It is clear that more compounds can be obtained at depths of 2000−4000 m. Organisms living in this area are considered to be more adapted to the environment. Therefore, fungi have evolved diverse metabolic pathways to produce more novel bioactive metabolites. Conversely, deeper depths mean much more demanding environments, which is disadvantageous for marine-derived fungi, although they still can produce metabolites when subjected to extreme environmental stresses. Regarding the geographic origins of deep-sea natural products in Figure 13D, 31.65% of them were isolated from the Indian Ocean, followed by the South China Sea (25.32%) and Pacific Ocean (25.32%).
3.3 Some “Star Molecules” With High Drug Lead Potential
Among the 229 isolated metabolites, a total of 82 compounds were found to possess moderate to potent cytotoxic activities. Among them, as shown in Figure 14 and Supplementary Table 1, we highlight some compounds with potent cytotoxicities and name them “star molecules” considering their high drug lead potential. These molecules include diketopiperazines with sulfur bridges (such as compounds 3, 4, 17, and 18), anthranilic acid derivatives (such as compounds 47, 51, and 52), nitrogenated azaphilones (such as compounds 84, 85, and 88), aphidicolin (compound 69), and meroterpenoids (compounds 79 and 80). Their structures are classified into polyketides, alkaloids, and terpenoids according to their putative biogenetic sources. A variety of chemical structures helps synthetists design better antitumor molecules. All of these compounds not only possess diverse chemical structures but also show significant activities, even higher than those of the positive controls (usually clinical drugs such as adriamycin, cisplatin, taxol, and doxorubicin). For example, compound 3 shows remarkable cytotoxicity at the nanomolar or low micromolar level (IC50 values of 0.191, 0.015, and 0.008 μM against K562, A549, and MCF-7 cells), which are 10−100 times higher than that of the positive control. Compound 80, in particular, shows potent activities against SF-268, MCF-7, HepG-2, and A549 cell lines, with IC50 values of 0.01−0.04 μM, approximately 100 times stronger than the positive control adriamycin. Table 1 lists the common target cell types and cytotoxic activity of the isolated compounds. The A549, HepG2, and MCF-7 cell lines are the main tested cell lines. Meanwhile, the structure-activity relationship and mechanism of action has been studied for some of the isolated compounds. For the diketopiperazines, the polysulfide bridge contributes significantly to their cytotoxicities (Wang et al., 2012). Aphidicolin A8 (69) is found to observably induce apoptosis in T24 and HL-60 cells by causing DNA damage (Niu et al., 2019a). Nitrogenated azaphilone 85 arrests the cell cycle in the G1 phase, while 84 and 85 induced apoptosis in a concentration-dependent manner (Wang et al., 2020b). These “star molecules”, with potent activities and clear mechanisms of action, are considered to be potential alternatives to antitumor drugs.
4 Conclusions
In summary, deep-sea fungi are an untapped source of valuable marine natural products. Although a large number of metabolites have been isolated from deep-sea fungi, the further excavation of novel metabolites is expected. This review first summarizes 229 cytotoxic compounds isolated from deep-sea fungi. Among them, 82 members have been found to possess moderate to potent cytotoxic activities. Most importantly, some of these compounds, namely, “star molecules” herein, show potent cytotoxic activities (higher than that of the positive controls). It is believed that in the near future, studies of cytotoxic compounds isolated from deep-sea fungi will become more prolific, which will certainly be beneficial for the discovery of new antitumor drugs.
Author Contributions
GZ, WT, and JZ wrote this manuscript; PS, YL, JW, QS, HS, LJ, XY, HZ, and GC collected and reorganized the literature data; JZ, XZ, and HJ supervised the research work and revised the manuscript; all authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2021QC167, ZR2020KC028, ZR2020QC227), Major Science and Technology Innovation Project of Shandong Province (2017GGH5129), Innovation Team Project for Modern Agricultural Industrious Technology System of Shandong Province (SDAIT-11-10), Cooperation Project of University and Local Enterprise in Yantai of Shandong Province (2020XDRHXMPT34, 2021XDRHXMXK23), Key Research and Development Plan of Yantai (2020XDRH101, 2021XDHZ076, 2021YT06000060), and Yantai Research Institute for Replacing Old Growth Drivers with New Ones (2020XJDN003).
Conflict of Interest
Authors QS and HS are employed by Shandong New Times Pharmaceutical Co., LTD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.929561/full#supplementary-material
References
Alshehri S. O., Malatani R. T., Bogari H. A., Noor A. O., Ibrahim A. K., Elhady S. S., et al. (2020). LAMA-1: A Cerebroside Isolated From the Deep-Sea-Derived Fungus Penicillium Chrysogenum. Metabolites 10, 75. doi: 10.3390/metabo10020075
Carroll A. R., Copp B. R., Davis R. A., Keyzers R. A., Prinsep M. R. (2021). Marine Natural Products. Nat. Prod. Rep. 38, 362–413. doi: 10.1039/D0NP00089B
Chen S., Liu Z., Chen Y., Tan H., Liu H., Zhang W. (2021). Tersaphilones A–E, Cytotoxic Chlorinated Azaphilones From the Deep-Sea-Derived Fungus Phomopsis Tersa FS441. Tetrahedron 78, 131806. doi: 10.1016/j.tet.2020.131806
Chen S. C., Liu Z. M., Tan H. B., Chen Y. C., Li S. N., Li H. H., et al. (2019). Tersone A–G, New Pyridone Alkaloids From the Deep-Sea Fungus Phomopsis Tersa. Mar. Drugs 17, 394. doi: 10.3390/md17070394
Chen S., Liu Z., Tan H., Chen Y., Li S., Li H., et al. (2020b). Phomeroids A and B: Two Novel Cytotoxic Meroterpenoids From the Deep-Sea-Derived Fungus Phomopsis Tersa FS441. Org. Chem. Front. 7, 557–562. doi: 10.1039/C9QO01365B
Chen S., Liu Z., Tan H., Chen Y., Zhu S., Liu H., et al. (2020a). Photeroids A and B, Unique Phenol-Sesquiterpene Meroterpenoids From the Deep-Sea-Derived Fungus Phomopsis Tersa. Org. Biomol. Chem. 18, 642–645. doi: 10.1039/C9OB02625H
Chen S., Wang J., Wang Z., Lin X., Zhao B., Kaliaperumal K., et al. (2017). Structurally Diverse Secondary Metabolites From a Deep-Sea-Derived Fungus Penicillium Chrysogenum SCSIO 41001 and Their Biological Evaluation. Fitoterapia 117, 71–78. doi: 10.1016/j.fitote.2017.01.005
Chi L. P., Li X. M., Li L., Li X., Wang B. G. (2020). Cytotoxic Thiodiketopiperazine Derivatives From the Deep Sea-Derived Fungus Epicoccum Nigrum SD-388. Mar. Drugs 18, 160. doi: 10.3390/md18030160
Dasanayaka S. A. H. K., Nong X. H., Liang X., Liang J. Q., Amin M., Qi S. H. (2020). New Dibenzodioxocinone and Pyran-3,5-Dione Derivatives From the Deep-Sea-Derived Fungus Penicillium Canescens SCSIO Z053. J. Asian Nat. Prod. Res. 22, 338–345. doi: 10.1080/10286020.2019.1575819
Deshmukh S. K., Prakash V., Ranjan N. (2018). Marine Fungi: A Source of Potential Anticancer Compounds. Front. Microbiol. 8, 2536. doi: 10.3389/fmicb.2017.02536
Fan Z., Sun Z. H., Liu Z., Chen Y. C., Liu H. X., Li H. H., et al. (2016). Dichotocejpins A–C: New Diketopiperazines From a Deep-Sea-Derived Fungus Dichotomomyces Cejpii FS110. Mar. Drugs 14, 164. doi: 10.3390/md14090164
Guo H., Liu Z. M., Chen Y. C., Tan H. B., Li S. N., Li H. H., et al. (2019). Chromone-Derived Polyketides From the Deep-Sea Fungus Diaporthe Phaseolorum FS431. Mar. Drugs 17, 182. doi: 10.3390/md17030182
Han W., Cai J., Zhong W., Xu G., Wang F., Tian X., et al. (2020). Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitorsfrom the Deep-Sea Fungus Penicillium Chrysogenum SCSIO 07007. Bioorg. Chem. 96, 103646. doi: 10.1016/j.bioorg.2020.103646
Huang Z. H., Nong X. H., Liang X., Qi S. H. (2018). New Tetramic Acid Derivatives From the Deep-Sea-Derived Fungus Cladosporium Sp. SCSIO Z0025. Tetrahedron 74, 2620–2626. doi: 10.1016/j.tet.2018.04.010
Liang X., Zhang X. Y., Nong X. H., Wang J., Huang Z. H., Qi S. H. (2016). Eight Linear Peptides From the Deep-Sea-Derived Fungus Simplicillium Obclavatum EIODSF 020. Tetrahedron 72, 3092–3097. doi: 10.1016/j.tet.2016.04.032
Li D. H., Cai S. X., Zhu T. J., Wang F. P., Xiao X., Gu Q. Q. (2011). New Cytotoxic Metabolites From a Deep-Sea-Derived Fungus, Phialocephala Sp., Strain FL30r. Chem. Biodivers. 8, 895–901. doi: 10.1002/cbdv.201000134
Li C. S., Li X. M., An C. Y., Wang B. G. (2014). Prenylated Indole Alkaloid Derivatives From Marine Sediment-Derived Fungus Penicillium Paneum SD-44. Helv. Chim. Acta 97, 1440–1444. doi: 10.1002/hlca.201400035
Li C. S., Li X. M., Gao S. S., Lu Y. H., Wang B. G. (2013). Cytotoxic Anthranilic Acid Derivatives From Deep Sea Sediment-Derived Fungus Penicillium Paneum SD-44. Mar. Drugs 11, 3068–3076. doi: 10.3390/md11083068
Li X., Li X. M., Li X. D., Xu G. M., Liu Y., Wang B. G. (2016b). 20-Nor-Isopimarane Cycloethers From the Deep-Sea Sediment-Derived Fungus Aspergillus Wentii SD-310. RSC. Adv. 6, 75981. doi: 10.1039/C6RA17638K
Li X. D., Li X., Li X. M., Xu G. M., Zhang P., Meng L. H., et al. (2016a). Tetranorlabdane Diterpenoids From the Deep Sea Sediment-Derived Fungus Aspergillus Wentii SD-310. Planta. Med. 82, 877–881. doi: 10.1055/s-0042-102965
Lin Y. K., Xie C. L., Xing C. P., Wang B. Q., Tian X. X., Xia J. M., et al. (2021). Cytotoxic P-Terphenyls From the Deep-Sea-Derived Aspergillus Candidus. Nat. Prod. Res. 35, 1627–1631. doi: 10.1080/14786419.2019.1633651
Lin X., Zhou X., Wang F., Liu K., Yang B., Yang X., et al. (2012). A New Cytotoxic Sesquiterpene Quinone Produced by Penicillium Sp. F00120 Isolated From a Deep Sea Sediment Sample. Mar. Drugs 10, 106–115. doi: 10.3390/md10010106
Liu H., Chen S., Zhang X., Dong C., Chen Y., Liu Z., et al. (2020). Structural Elucidation, Total Synthesis, and Cytotoxic Activity of Effphenol A. Org. Biomol. Chem. 18, 9035–9038. doi: 10.1039/D0OB01985B
Liu Z., Li S., Chen Y., Li M., Liu H., Zhang W. (2021b). Cytotoxic Polyketides From the Deep-Sea-Derived Fungus Aspergillus Fischeri FS452. Nat. Prod. Res. doi: 10.1080/14786419.2021.2015595
Liu F. A., Lin X., Zhou X., Chen M., Huang X., Yang B., et al. (2017). Xanthones and Quinolones Derivatives Produced by the Deep-Sea-Derived Fungus Penicillium Sp. SCSIO Ind16f01. Molecules 22, 1999. doi: 10.3390/molecules22121999
Liu H. B., Liu Z. M., Chen Y. C., Tan H. B., Li S. N., Li D. L., et al. (2021a). Cytotoxic Diaporindene and Tenellone Derivatives From the Fungus Phomopsis Lithocarpus. Chin. J. Nat. Med. 19, 874–880. doi: 10.1016/S1875-5364(21)60095-X
Li D., Wang F., Cai S., Zeng X., Xiao X., Gu Q., et al. (2007). Two New Bisorbicillinoids Isolated From a Deep-Sea Fungus, Phialocephala Sp. FL30r. J. Antibiot. 60, 317–320. doi: 10.1038/ja.2007.40
Li N., Yi Z., Wang Y., Zhang Q., Zhong T., Qiu Y., et al. (2012). Differential Proteomic Analysis of HL60 Cells Treated With Secalonic Acid F Reveals Caspase 3-Induced Cleavage of Rho GDP Dissociation Inhibitor 2. Oncol. Rep. 28, 2016–2022. doi: 10.3892/or.2012.2062
Luo X., Zhou X., Lin X., Qin X., Zhang T., Wang J., et al. (2017). Antituberculosis Compounds From a Deep-Sea-Derived Fungus Aspergillus Sp. SCSIO Ind09f01. Nat. Prod. Res. 31, 1958–1962. doi: 10.1080/14786419.2016.1266353
Newman D. J., Cragg G. M. (2020). Natural Products as Sources of New Drugs Over the Nearly Four Decades From 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803. doi: 10.1021/acs.jnatprod.9b01285
Niu Z., Chen Y., Guo H., Li S. N., Li H. H., Liu H. X., et al. (2019c). Cytotoxic Polyketides From a Deep-Sea Sediment Derived Fungus Diaporthe Phaseolorum FS431. Molecules 24, 3062. doi: 10.3390/molecules24173062
Niu S., Chen Z., Pei S., Shao Z., Zhang G., Hong B. (2021). Acremolin D, A New Acremolin Alkaloid From the Deep-Sea Sediment Derived Aspergillus Sydowii Fungus. Nat. Prod. Res. doi: 10.1080/14786419.2021.1913587
Niu S., Peng G., Xia J. M., Xie C. L., Li Z., Yang X. W. (2019b). A New Pimarane Diterpenoid From the Botryotinia Fuckeliana Fungus Isolated From Deep-Sea Water. Chem. Biodivers. 16, e1900519. doi: 10.1002/cbdv.201900519
Niu S., Xia M., Chen M., Liu X., Li Z., Xie Y., et al. (2019d). Cytotoxic Polyketides Isolated From the Deep-Sea-Derived Fungus Penicillium Chrysogenum MCCC 3a00292. Mar. Drugs 17, 686. doi: 10.3390/md17120686
Niu S., Xia J. M., Li Z., Yang L. H., Yi Z. W., Xie C. L., et al. (2019a). Aphidicolin Chemistry of the Deep-Sea-Derived Fungus Botryotinia Fuckeliana MCCC 3a00494. J. Nat. Prod. 82, 2307–2331. doi: 10.1021/acs.jnatprod.9b00705
Soldatou S., Baker B. J. (2017). Cold-Water Marine Natural Products 2006 to 2016. Nat. Prod. Rep. 34, 585–626. doi: 10.1039/C6NP00127K
Sun C., Mudassir S., Zhang Z., Feng Y., Chang Y., Che Q., et al. (2020). Secondary Metabolites From Deep-Sea Derived Microorganisms. Curr. Med. Chem. 27, 6244–6273. doi: 10.2174/0929867326666190618153950
Sun Y., Takada K., Takemoto Y., Yoshida M., Nogi Y., Okada S., et al. (2012). Gliotoxin Analogues From a Marine-Derived Fungus, Penicillium Sp., and Their Cytotoxic and Histone Methyltransferase Inhibitory Activities. J. Nat. Prod. 75, 111–114. doi: 10.1021/np200740e
Sun Y. L., Wang J., Wang Y. F., Zhang X. Y., Nong X. H., Chen M. Y., et al. (2015). Cytotoxic and Antiviral Tetramic Acid Derivatives From the Deep-Sea-Derived Fungus Trichobotrys Effuse DFFSCS021. Tetrahedron 71, 9328–9332. doi: 10.1016/j.tet.2015.10.010
Tang X. X., Liu S. Z., Yan X., Tang B. W., Fang M. J., Wang X. M., et al. (2019). Two New Cytotoxic Compounds From a Deep-Sea Penicillum Citreonigrum XT20-134. Mar. Drugs 17, 509. doi: 10.3390/md17090509
Wang W., Chen R., Luo Z., Wang W., Chen J. (2017). Two New Benzoate Derivatives and One New Phenylacetate Derivative From a Marine-Derived Fungus Engyodontium Album. Nat. Prod. Res. 31, 758–764. doi: 10.1080/14786419.2016.1242002
Wang W., Gao M., Luo Z., Liao Y., Zhang B., Ke W., et al. (2019a). Secondary Metabolites Isolated From the Deep Sea-Derived Fungus Aspergillus Sydowii C1-S01-A7. Nat. Prod. Res. 33, 3077–3082. doi: 10.1080/14786419.2018.1519561
Wang F. Z., Huang Z., Shi X. F., Chen Y. C., Zhang W. M., Tian X. P., et al. (2012). Cytotoxic Indole Diketopiperazines From the Deep Sea-Derived Fungus Acrostalagmus Luteoalbus SCSIO F457. Bioorg. Med. Chem. Lett. 22, 7265–7267. doi: 10.1016/j.bmcl.2012.08.115
Wang W., Liao Y., Chen R., Hou Y., Ke W., Zhang B., et al. (2018). Chlorinated Azaphilone Pigments With Antimicrobial and Cytotoxic Activities Isolated From the Deep Sea Derived Fungus Chaetomium Sp. NA-S01-R1. Mar. Drugs 16, 61. doi: 10.3390/md16020061
Wang W., Liao Y., Zhang B., Gao M., Ke W., Li F., et al. (2019b). Citrinin Monomer and Dimer Derivatives With Antibacterial and Cytotoxic Activities Isolated From the Deep Sea-Derived Fungus Penicillium Citrinum NLG-S01-P1. Mar. Drugs 17, 46. doi: 10.3390/md17010046
Wang Y. N., Meng L. H., Wang B. G. (2020a). Progress in Research on Bioactive Secondary Metabolites From Deep-Sea Derived Microorganisms. Mar. Drugs 18, 614. doi: 10.3390/md18120614
Wang Y. T., Xue Y. R., Liu C. H. (2015). A Brief Review of Bioactive Metabolites Derived From Deep-Sea Fungi. Mar. Drugs 13, 4594–4616. doi: 10.3390/md13084594
Wang W., Yang J., Liao Y. Y., Cheng G., Chen J., Cheng X. D., et al. (2020b). Cytotoxic Nitrogenated Azaphilones From the Deep-Sea-Derived Fungus Chaetomium Globosum MP4-S01-7. J. Nat. Prod. 83, 1157–1166. doi: 10.1021/acs.jnatprod.9b01165
Wang W., Yang J., Liao Y. Y., Cheng G., Chen J., Mo S., et al. (2020c). Aspeterreurone A, a Cytotoxic Dihydrobenzofuran-Phenyl Acrylate Hybrid From the Deep-Sea-Derived Fungus Aspergillus Terreus CC-S06-18. J. Nat. Prod. 83, 1998–2003. doi: 10.1021/acs.jnatprod.0c00189
Wu J., Gao W., Johnson R. H., Zhang W., Meldrum D. R. (2013). Integrated Metagenomic and Metatranscriptomic Analyses of Microbial Communities in the Meso- and Bathypelagic Realm of North Pacific Ocean. Mar. Drugs 11, 3777–3801. doi: 10.3390/md11103777
Wu G., Lin A., Gu Q., Zhu T., Li D. (2013). Four New Chloro-Eremophilane Sesquiterpenes From an Antarctic Deep-Sea Derived Fungus, Penicillium Sp. PR19N-1. Mar. Drugs 11, 1399–1408. doi: 10.3390/md11041399
Xiang Y., Zeng Q., Mai Z. M., Chen Y. C., Shi X. F., Chen X. Y., et al. (2021). Asperorydines N–P, Three New Cyclopiazonic Acid Alkaloids From the Marine-Derived Fungus Aspergillus Flavus SCSIO F025. Fitoterapia 150, 104839. doi: 10.1016/j.fitote.2021.104839
Xu J. L., Liu H. X., Chen Y. C., Tan H. B., Guo H., Xu L. Q., et al. (2018). Highly Substituted Benzophenone Aldehydes and Eremophilane Derivatives From the Deep-Sea Derived Fungus Phomopsis Lithocarpus FS508. Mar. Drugs 16, 329. doi: 10.3390/md16090329
Xu J., Liu Z., Chen Y., Tan H., Li H., Li S., et al. (2019). Lithocarols A–F, Six Tenellone Derivatives From the Deep-Sea Derived Fungus Phomopsis Lithocarpus FS508. Bioorg. Chem. 87, 728–735. doi: 10.1016/j.bioorg.2019.03.078
Yao Q., Wang J., Zhang X., Nong X., Xu X., Qi S. (2014). Cytotoxic Polyketides From the Deep-Sea-Derived Fungus Engyodontium Album DFFSCS021. Mar. Drugs 12, 5902–5915. doi: 10.3390/md12125902
Yuan X. L., Li X. Q., Xu K., Hou X. D., Zhang Z. F., Xue L., et al. (2020). Transcriptome Profiling and Cytological Assessments for Identifying Regulatory Pathways Associated With Diorcinol N-Induced Autophagy in A3 Cells. Front. Pharmacol. 11, 570450. doi: 10.3389/fphar.2020.570450
Zain Ul Arifeen M., Ma Y. N., Xue Y. R., Liu C. H. (2019). Deep-Sea Fungi Could be the New Arsenal for Bioactive Molecules. Mar. Drugs 18, 9. doi: 10.3390/md18010009
Zeng X., Xiao X., Li D., Gu Q., Wang F. (2010). Isolation, Identification and Screening of Microorganisms for Cytotoxic Activities From Deep Sea Sediments at Different Pacific Stations. World J. Microbiol. Biotechnol. 26, 2141–2150. doi: 10.1007/s11274-010-0396-5
Zhang Z., Guo W., He X., Che Q., Zhu T., Gu Q., et al. (2016b). Peniphenylanes a–G From the Deep-Sea-Derived Fungus Penicillium Fellutanum HDN14-323. Planta. Med. 82, 872–876. doi: 10.1055/s-0042-102885
Zhang Z., He X., Liu C., Che Q., Zhu T., Gu Q., et al. (2016a). Clindanones A and B and Cladosporols F and G, Polyketides From the Deep-Sea Derived Fungus Cladosporium Cladosporioides HDN14-342. RSC. Adv. 6, 76498. doi: 10.1039/C6RA14640F
Zhang P., Wei Q., Yuan X., Xu K. (2020). Newly Reported Alkaloids Produced by Marine-Derived Penicillium Species (Covering 2014–2018). Bioorg. Chem. 99, 103840. doi: 10.1016/j.bioorg.2020.103840
Zhong T. H., Zeng X. M., Feng S. B., Zhang H. T., Zhang Y. H., Luo Z. H., et al. (2022). Three New Phomalone Derivatives From a Deep-Sea-Derived Fungus Alternaria Sp. MCCC 3a00467. Nat. Prod. Res. 36, 414–418. doi: 10.1080/14786419.2020.1771706
Zhou X., Fang P., Tang J., Wu Z., Li X., Li S., et al. (2016). A Novel Cyclic Dipeptide From Deep Marine-Derived Fungus Aspergillus Sp. SCSIOW2. Nat. Prod. Res. 30, 52–57. doi: 10.1080/14786419.2015.1033623
Keywords: deep-sea, fungi, secondary metabolites, cytotoxic activity, lead compounds
Citation: Zhao G, Tang W, Zhang J, Shi P, Li Y, Wang J, Shen Q, Si H, Jiang L, Yu X, Zhu H, Chen G, Zhang X and Jia H (2022) Deep-Sea-Derived Fungi as Valuable Producers of Cytotoxic Secondary Metabolites and Their Leads Potential. Front. Mar. Sci. 9:929561. doi: 10.3389/fmars.2022.929561
Received: 27 April 2022; Accepted: 16 May 2022;
Published: 27 June 2022.
Edited by:
Ahmed M. Sayed, AlMaaqal University, IraqCopyright © 2022 Zhao, Tang, Zhang, Shi, Li, Wang, Shen, Si, Jiang, Yu, Zhu, Chen, Zhang and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianlong Zhang, emhhbmdqaWFubG9uZ0BsZHUuZWR1LmNu; Xingxiao Zhang, emhhbmd4aW5neGlhb0BsZHUuZWR1LmNu; Hong Jia, aG9uZ2ppYTIwMjExMkAxNjMuY29t
†These authors have contributed equally to this work
 Guangrong Zhao1,2†
Guangrong Zhao1,2† Jianlong Zhang
Jianlong Zhang Linlin Jiang
Linlin Jiang Xingxiao Zhang
Xingxiao Zhang Hong Jia
Hong Jia