
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 27 June 2022
Sec. Marine Pollution
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.925567
This article is part of the Research Topic Coastal Environmental and Ecological Data Analysis View all 30 articles
 Fengxia Zhou1,2,3
Fengxia Zhou1,2,3 Mengqi Xiong1,2,3
Mengqi Xiong1,2,3 Shuangling Wang1,2,3
Shuangling Wang1,2,3 Sheng Tian1,2,3
Sheng Tian1,2,3 Guangzhe Jin1,2,3*
Guangzhe Jin1,2,3* Fajin Chen1,2,3
Fajin Chen1,2,3 Chunqing Chen1,2,3
Chunqing Chen1,2,3 Xuan Lu1,2,3
Xuan Lu1,2,3 Qingmei Zhu1,2,3
Qingmei Zhu1,2,3 Yafei Meng1,2,3
Yafei Meng1,2,3This study investigated the total concentrations and geochemical compositions of metals (Cd, Cr, Cu, Ni, Pb, Zn, Fe and Mn) in surface sediments of Zhanjiang Bay (ZJB) in spring and summer, to assess the contamination status, mobility and influencing factors of spatial-seasonal changes of these metals. The average total concentration for each studied metal in the surface sediments of ZJB was 0.173 μg/g for Cd, 58.25 μg/g for Cr, 17.11 μg/g for Cu, 16.89 μg/g for Ni, 28.70 μg/g for Pb, 67.91 μg/g for Zn, 30.18 mg/g for Fe, and 275.5 μg/g for Mn during the investigation period. Generally higher total concentrations of metals were found in the channel and coastal sediments of ZJB compared with those in the central ZJB, which may be probably resulted by the input of Suixi river, domestic sewage and industrial wastewater. The grain size compositions and TOC contents also had influences on the distributions of metals in ZJB. In the channel, total metals and reducible and bioavailable fractions of metals generally showed decreased concentrations in summer compared with those in spring, suggesting the release of metals from sediments. Organic matter degradation and Fe and Mn (hydr)oxides reduction processes may contribute much to this phenomenon. Relatively high proportions of Cd and Zn (average of 21.7% and 14.6%, respectively) were associated with the acid soluble fraction, indicating their high risk to the environment. The combined assessment results of enrichment factor, contaminated factor and the percentages of acid soluble fraction indicated that Cd and Zn in the surface sediments of ZJB were generally contaminated and they had medium to high risk to the environment. The average values of pollution load index in the channel, coastal and central ZJB were 1.28, 0.93 and 0.81, respectively, indicating the deterioration of surface sediments in the channel of ZJB. More attention should be paid on the metals in surface sediments of the channel of ZJB.
Metals are one of the major anthropogenic contaminants in coastal environments (Yu et al., 2008; Hossain et al., 2020). Some metals (e.g. Fe, Mn, Zn etc.) are essential for living organisms when they are in very low concentrations (Hossain et al., 2020). While some metals (e.g. Cd, Cu, Zn etc.) are very toxic when they are supplied above a certain concentration (Pais and Jones, 1997; USEPA, 2000). For example, Cu is an essential element which serves as a cofactor in a number of enzyme systems. However, high intake of Cu can cause adverse health effect problems for most living organisms (Mohanraj et al., 2021). Therefore, assessment of metals in marine environments is useful to determine contamination levels and provide information for determining health risks.
Metals in the coastal environment have natural and anthropogenic sources. With the rapid industrialization and economic development in coastal regions, metals are continuing to be introduced to coastal environment through river runoff and land-based point source discharge (Yu et al., 2008; Anandkumar et al., 2018; Hossain et al., 2020). Anthropogenic sources such as industrial sewage and domestic sewage have led to an increase of metal concentrations in the coastal environments (Gao et al., 2014; Freitas et al., 2019). A major fraction of metals entering into the aquatic systems are rapidly transported into sediments (Wang and Chen, 2000; Gu et al., 2016). Sediments are recognized as an important sink of heavy metals in coastal ecosystems (Pekey, 2006; Hossain et al., 2020).
In sediments, metals can exist in many chemical species and exhibit different behaviors (Akcay et al., 2003; Gao et al., 2010). Investigations on the geochemical forms of metals by sequential extraction give further information about the fundamental reactions that govern the behavior of metals in sediments (Tessier et al., 1979; Gao and Chen, 2012; Prabakaran et al., 2020; Shibini Mol and Sujatha, 2020; Chakraborty et al., 2021; Zhao et al., 2021). The study of metal speciation in sediments is essential for estimating the mobility and bioavailability of metals (Chakraborty et al., 2015; Chakraborty et al., 2017). The chemical forms that are weakly bounded to the sediment (such as acid soluble, reducible and oxidizable forms) are considered as the bioavailable forms (Peña-lcart et al., 2014; Freitas et al., 2019). Under certain conditions, the bioavailable forms of metals in sediments can be released from sediment. For, example, changes in the redox conditions of sediment can lead to the reductive dissolution of Fe and Mn (hydr)oxides in sediments and result in the release of associated metals from sediment to water (Charriau et al., 2011; Dang et al., 2015). The study of Duan et al. (2019) found that obvious seasonal variations of metals occurred at the surface sediments of Changjiang Estuary, which was related to the seasonal variation of temperature, dissolved oxygen (DO) and organic matter in the overlying waters. Many previous studies also indicated that, benthic diffusive fluxes of metals from sediments to water column are equivalent to or even exceed riverine influxes in many coastal areas (Rivera-Duarte and Flegal, 1997; Santos-Echeandia et al., 2009; Duan et al., 2019). Therefore, the release of metals from sediment can cause water quality and ecosystem degradation (Lee et al., 2017; Nagarajan et al., 2019; Li et al., 2020).
Coastal areas, especially the semi-enclosed bays, are particularly at risk from metal contamination since the strong influence of anthropogenic activities in these areas (Gao et al., 2014; Hossain et al., 2020). The Zhanjiang bay (ZJB), located at the northwestern South China Sea, is a typical semi-enclosed bay with a very narrow entrance (< 2 km). It is also an important aquaculture area in China. However, it has been increasingly contaminated by the industrial, agricultural and domestic wastes. Industries discharges hazardous contaminants like metals into ZJB, which may be consumed by planktons, shellfishes or fishes, and finally magnified and transferred to humans. Metal contamination is one of the key environmental problems for ZJB. Zhang et al. (2018) reported the total metal concentrations in the surface sediments of ZJB. A combined study that addresses the seasonal variations and mobility of metals in the sediments of ZJB is limited. In addition, the influences of anthropogenic activities and environmental changes on the spatial-seasonal variations of metals in sediments of ZJB are not completely understood.
The main objectives of this study were to assess the spatial and seasonal variations of metals (Cd, Cr, Cu, Ni, Pb, Zn, Fe and Mn) in surface sediments of ZJB, to assess the potential mobility/bioavailability of metals in different subregions of ZJB, to identify possible sources of metals in ZJB, and to evaluate the contamination status of metals in sediments of ZJB based on different evaluation methods. The results of this study will provide scientific basis for improving the environment of ZJB and protection of aquatic flora and fauna.
Zhanjiang Bay is influenced by seasonally reversing monsoon winds and has a dynamic environment. It has higher precipitation in summer than other seasons. The water temperature, DO concentration and chlorophyll a (Chl a) concentration of water in ZJB also vary seasonally (Zhou et al., 2020). The water of ZJB is mainly influenced by the freshwater from Suixi river and seawater from South China Sea (Figure 1). These environmental characteristics of ZJB may probably have significant influences on the spatial-seasonal variations of metals in the surface sediments of ZJB.
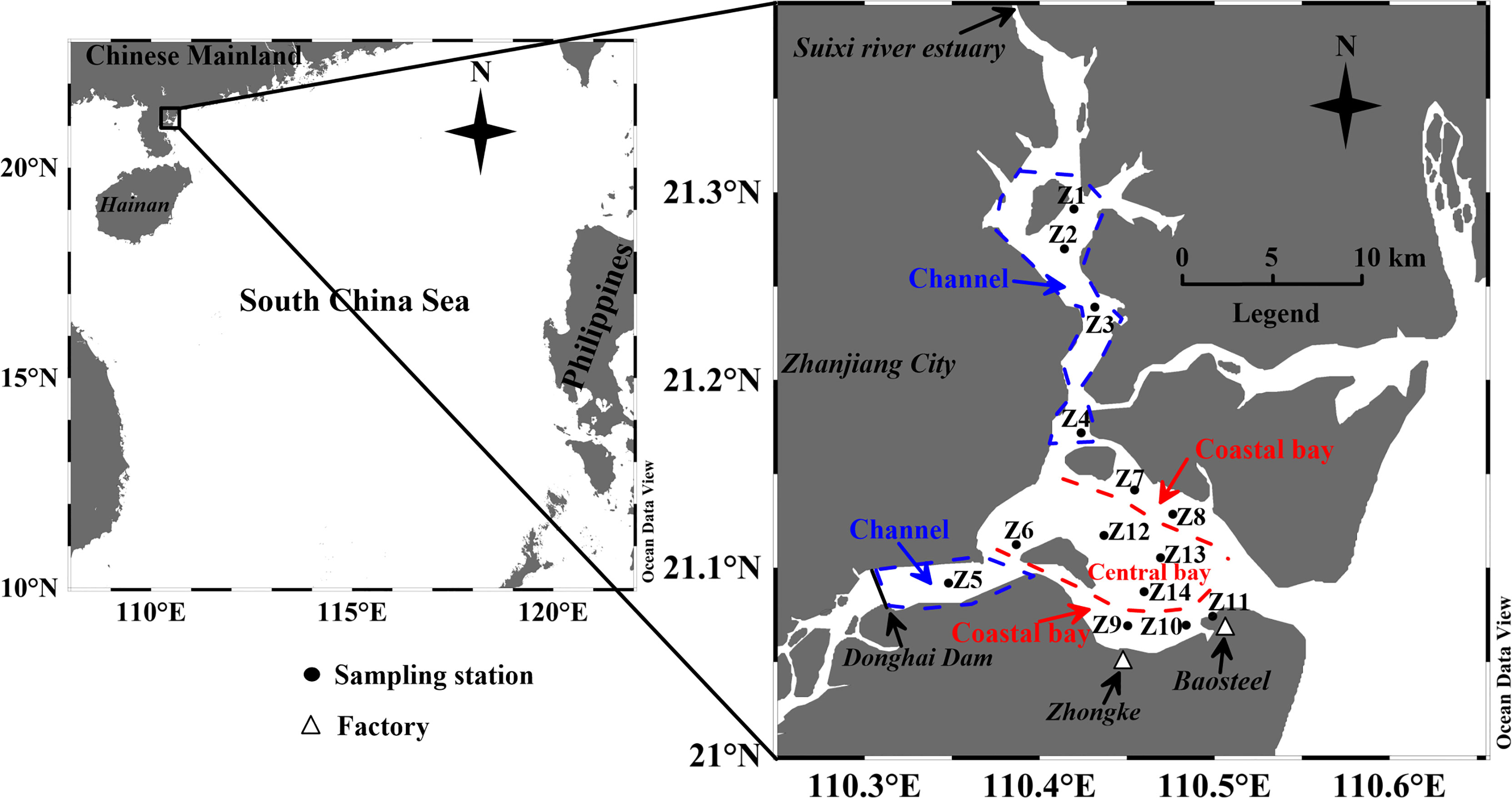
Figure 1 Sampling stations in the Zhanjiang Bay. The Zhanjiang bay is divided into three subregions: channel, coastal bay and central bay. Zhongke: Zhongke (Guangdong) Refining and Chemical Co., Ltd. Baosteel: Baosteel Zhanjiang Iron Steel Co., Ltd.
According to the environmental characteristics of ZJB, the study area can be divided into three subregions: channel, coastal bay and central bay (Figure 1). Suixi river has a strong influence on the channel. Besides, large amounts of domestic sewage from Zhanjiang city have been discharged to this area. There are many factories along the coast of ZJB, especially the south coast of ZJB (Figure 1). Baosteel Zhanjiang Iron Steel Co., Ltd. (Baosteel, an iron and steel company) and Zhongke (Guangdong) Refining and Chemical Co., Ltd (Zhongke, a refining and chemical company) are two of the large enterprises locating at the south coast of ZJB (Figure 1). Large amounts of industrial sewage have been discharged into the coastal area of ZJB. The central bay is relatively less influenced by river runoff and sewage input.
Bottom water and surface sediment samples were collected from ZJB during two cruises in April (spring) and August (summer) 2017. A total of fourteen stations were selected for investigation according to the environment of ZJB. The sampling stations Z1, Z2, Z3, Z4 and Z5 were located in the channel, stations Z7, Z8, Z9, Z10 and Z11 in the coastal bay, and stations Z6, Z12, Z13 and Z14 in the central bay. The temperature of bottom water was measured by a conductivity-temperature-depth meter in situ. Bottom water was collected by a plexiglass water sampler for the measurements of DO and Chl a. The depths of the collected bottom water samples ranged from 3 to 18 m. The bottom water samples for DO analysis were collected first. DO was determined by the Winkler method. Water samples for Chl a analysis were filtered by glass-fiber filters immediately after collection. The filter was extracted with 90% acetone in laboratory. The extract solution was measured for Chl a concentration using a Turner fluorometer. A stainless steel grab sampler was used to collect sediment. The surface sediment (0-2 cm) was collected using a plastic spatula at each station, and packed in polyethylene bags. Then the samples were kept on ice in a cooler and immediately transported to the laboratory. In laboratory, the samples were kept frozen at -20°C until further analysis.
A portion of each sediment sample was first pretreated with 30% H2O2 to remove organic matter and then with 1 M HCl to remove carbonates. The pretreated samples were washed to neutral with deionized water. Then, the solids were dispersed with 0.05 M (NaPO3)6 and analyzed for grain size using a Malvern Masterizer 2000 laser diffractometer (Malvern Instruments, UK). The percentages of the clay (< 4 μm), silt (4 – 63 μm) and sand (> 63 μm) fractions were determined. For the analysis of TOC and metal concentrations, sediment samples were freeze-dried and grounded, then passed through a mesh sieve (150 μm in pore size). They were stored in cleaned polyethylene bags until further analysis. For the analysis of TOC, the freeze-dried and grounded sediment samples were treated with 1M HCl to remove carbonates. Then they were washed to neutral with deionized water and dried at 60°C. Concentrations of TOC were determined using an elemental analyzer (Flash EA 1112 HT, Thermo Fisher Scientific, USA). Replicate analysis of one sample (n = 5) gave a relative standard deviation less than 0.8% for TOC.
For the analysis of total metal concentrations, the sediment samples were freeze-dried and grounded. Then, they were digested with HNO3-HCl-HF (3:1:2) using microwave high-pressure digestion (Multiwave PRO 41HVT56, Austria). The digestion was diluted and determined by inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500cx, USA) for metals (Cd, Cr, Cu, Ni, Pb, Zn, Fe and Mn). The fractionation of metals in the sediment was determined according to the method reported by Rauret et al. (1999), which has been successfully applied in many studies (Zhuang and Gao, 2014; Akhbarizadeh et al., 2017; Anandkumar et al., 2022). Cd, Cr, Cu, Ni, Pb and Zn associated with four operationally defined geochemical fractions (acid soluble, reducible, oxidizable and residual) were identified by this method. For the acid soluble fraction, 0.5 g sediment was extracted with 0.11 M acetic acid (Step 1). The residual of Step 1 was shaken with 0.5 M hydroxylamine hydrochloride for 16 h to extract the reducible fraction (Step 2). The residual of Step 2 was digested with 8.8 M hydrogen peroxide and then shaken with 1 M ammonium acetate for 16 h to extract the oxidizable fraction. The detailed sequential extraction protocol has been described by Gao et al. (2010). The metal concentration of the extraction solution for each step was determined by ICP-MS (Agilent 7500cx, USA). The residual fraction was calculated by the difference between the total concentrations of metals and the sums of the acid soluble, reducible and oxidizable fractions.
For the measurement of metals, each sample was analyzed at least in duplicate. The precision of the analysis was <10%. Procedural blanks were analyzed with each batch of samples. Certified reference material for coastal sediment (GBW07314, the Chinese national reference material) was analyzed to control metal analytical quality. The determined values for all metals were consistent with the reference values (Table S1). All plastic and glassware were soaked for at least 2 days in 10% HNO3, followed by soaking and rinsing with de-ionized water.
Several methods have been used to evaluate the contamination status, potential risk, enrichment and sources of metals in surface sediments of ZJB, including comparison with related sediment quality guidelines, enrichment factor analysis, contamination factor analysis, pollution load index, and potential risk assessment based on geochemical compositions of metals.
The National Standard of China for Marine Sediment Quality (MSQ) (SEPA, 2002) can be used to assess the potential risk of metals in sediments (Gao et al., 2014). This standard classifies marine sediments into three classes based on the function and protection targets of the area (SEPA, 2002). Enrichment factor (EF) is an important tool to assess metal enrichment. It can be used to determine the anthropogenic influence and the contamination status of sediments. The following equation was used to estimate the EF of metals in sediments using Fe as a normalizer (Ergin et al., 1991; Mucha et al., 2003; Keskin, 2012; Shibini Mol and Sujatha, 2020).
EF = (Ms/Ns)/(Mb/Nb)
where Ms and Ms represent metal concentration of sample and background concentration respectively, and Ns and Nb are the metal concentration used for normalization in the sample and the background respectively. The metal concentration of China Shelf Sea sediment (Zhao et al., 1995) was used as the background concentration in this study. The EF is classified into many groups to denote the degree of enrichment factor (Chen et al., 2007). EF ≤ 1 denotes no enrichment; 1< EF ≤ 3 denotes minor enrichment; 3< EF ≤ 5 denotes moderate enrichment; 5< EF ≤ 10 denotes moderately severe enrichment; 10 < EF ≤ 25 denotes severe enrichment; 25 < EF ≤ 50 denotes very severe enrichment; and EF > 50 denotes extremely severe enrichment.
Contamination factor (CF) can be used to assess contamination level (Pekey et al., 2004). The following equation was used to estimate the CF of metals (Shibini Mol and Sujatha, 2020).
CF = Ms/Mb
where Ms and Mb represent metal concentration of sample and background concentration. The metal concentration of Chin Shelf Sea sediment (Zhao et al., 1995) was used as the background concentration in this study. CF ≤ 1 denotes low contamination; 1< CF ≤3 denotes moderate contamination; 3 < CF ≤ 6 denotes considerable contamination; and CF > 6 denotes high contamination. Pollution load index (PLI) can be used to assess the contamination extent of metals in surface sediments of ZJB. It can provide an overall indication of metal pollution contamination (Tomlinson et al., 1980). The following equation was used to calculate PLI.
where n is the number of studied metals and the CF is the contamination factor of the metal calculated as above. PLI provides a comparative mean for assessing a site quality. PLI < 1 denotes uncontaminated area; PLI = 1 denotes baseline level of pollutants; and PLI > 1 denotes deterioration of site quality (Tomlinson et al., 1980).
The former assessment methods of sediment metal contamination are based on the total concentrations of metals. In addition to the total concentrations, the geochemical composition of metals is equally important to determine their potential toxicity and threat to ecosystems (Sahuquillo et al., 2003; Gao et al., 2016). According to the study of Perin et al. (1985), the acid soluble fraction (F1) of metal with no more than 1% of its total concentration is considered to have no risk to the environment; the percentage of metal in this fraction falling in the range of 1-10% indicates a low risk to the environment; its falling in the range of 10-30% indicates a medium risk to the environment; its falling in the range of 30-50% indicates a high risk to the environment; and the percentage higher than 50% indicates a very high risk to the environment.
The surface sediments of ZJB were mainly composed of silt fraction (average of 60.0% and 63.3% in spring and summer, respectively); the clay and sand fractions were generally lesser than 40% (Table 1). Based on the environmental characteristics, the ZJB was divided into three subregions – channel, coastal bay and central bay (Section 2.1; Figure 1). In spring, the channel had relatively fine sediments (average of fine (clay + silt) fraction: 46.1%) compared with the coastal bay (33.8%) and central bay (38.3%). Similar distribution pattern was also found in summer. The reason is that the flow of Suixi river is small. The fine particles from Suixi river are mainly settled at the channel of ZJB (Lu et al., 2020). Besides, the channel has large-scale, cage-based mariculture, which can weaken the hydrodynamic conditions and contribute to the settlement of fine particles (Cai et al., 2006; Ke et al., 2014; Pondell and Canuel, 2017; Pan et al., 2019). EBCBS (1999) also indicated that the channel of ZJB had finer sediments compared with the other regions of ZJB. The TOC content in surface sediments of ZJB ranged from 0.10% to 1.46% (average: 0.74%) in spring and from 0.15% to 1.04% (average: 0.60%) in summer (Table 1). The decreased average TOC content in summer indicates organic matter degradation in this season. Higher TOC content was observed at the channel compared with those of the other two subregions, indicating the strong influences of river input and/or anthropogenic activities (Lu et al., 2020).
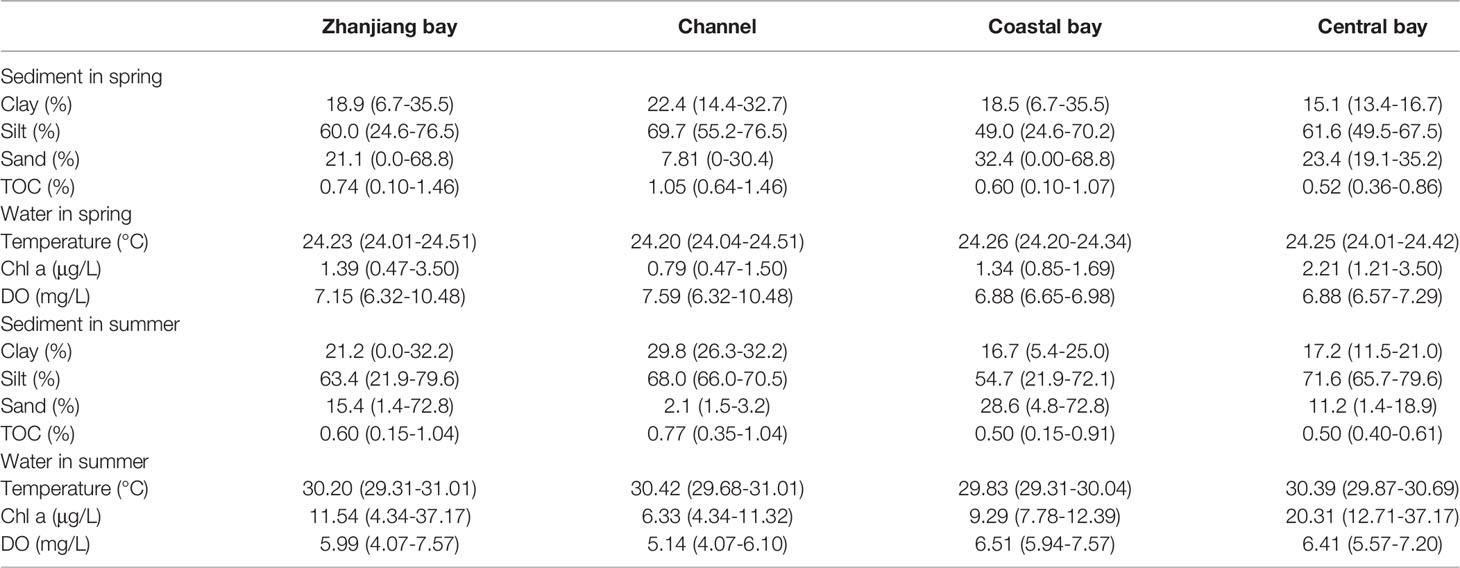
Table 1 Average (minimum-maximum) of surface sediment and bottom water parameters in Zhanjiang bay in spring and summer.
The temperature of bottom water in ZJB ranged from 24.01 to 24.51°C (average: 24.23 °C) in spring and from 29.31 to 31.01 °C (average: 30.20 °C) in summer (Table 1). Significant increase of temperature was found in summer compared with that in spring. The Chl a concentration of bottom water in ZJB ranged from 0.47 to 3.50 μg/L (average: 1.39 μg/L) in spring and from 4.34 to 37.17 μg/L (average: 11.54 μg/L) in summer (Table 1). Significant increase of Chl a was found in summer compared with that in spring. This indicates that the supply of organic matter in summer increased compared with that in spring. The DO of bottom water in ZJB ranged from 6.32 to 10.48 mg/L (average: 7.15 mg/L) in spring and from 4.07 to 7.57 mg/L (average: 5.99 mg/L) in summer (Table 1). This indicates an aerobic environment of the bottom water in ZJB in both spring and summer. In channel, the average DO of bottom water decreased obviously in summer compared with that in spring (Table 1). Increased water temperature and organic matter decomposition may contribute to the decrease of DO in bottom water of this area. The seasonal variations of bottom water environment may have significant influences on the concentrations of metals in surface sediments of ZJB.
Total metal concentrations in sediments collected from ZJB in spring and summer are summarized in Table 2 and Figures 2, S1. The concentration range for each studied metal in the surface sediments of ZJB was 0.023 to 0.464 μg/g for Cd, 1.67 to 100.71 μg/g for Cr, 1.86 to 37.36 μg/g for Cu, 3.88 to 30.49 μg/g for Ni, 4.72 to 70.10 μg/g for Pb, 8.38 to 161.18 μg/g for Zn, 7.99 to 45.73 mg/g for Fe, and 125.1 to 584.5 μg/g for Mn during the investigation period. In comparison with the previous work of ZJB, the concentrations of the studied metals in this study were generally comparable with those reported by Zhang et al. (2018) (Table 2). Table 2 also shows the metal values of other coastal areas in China. The average concentrations of the studied metals in this study were comparable to the values reported for the surface sediments of the Taiwan Strait (Gao et al., 2016), Xiamen bay (Lin et al., 2014), and Laizhou Bay (Zhuang and Gao, 2014) (Table 2). The metal concentrations in this study were apparently lower than the values reported for the Jinzhou Bay (Li et al., 2012), which is one of the most heavily polluted coastal region in China (Gao et al., 2014; Gao et al., 2016).
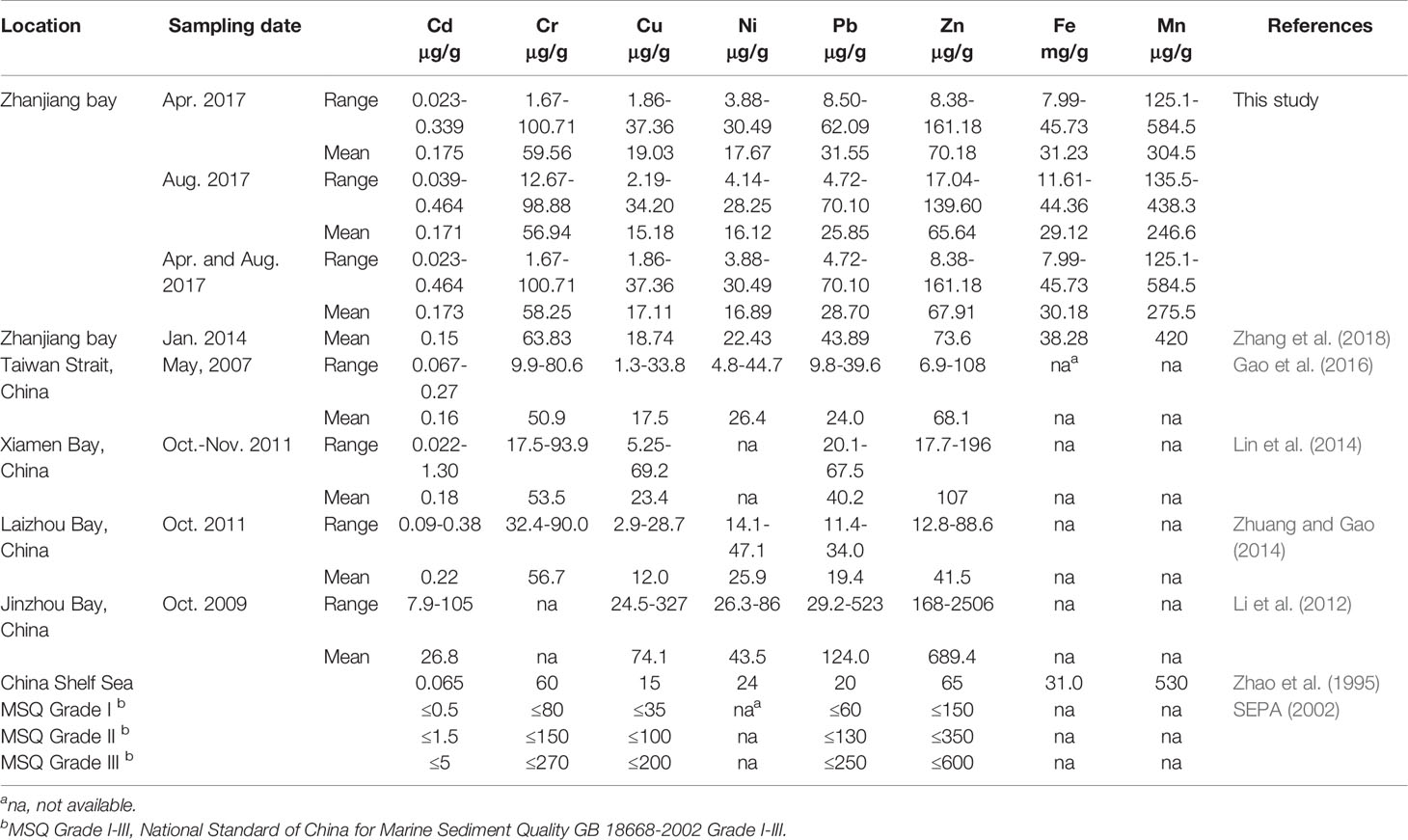
Table 2 Metal concentrations in the surface sediments of ZJB and other coastal areas of China. Related sediment quality guidelines are also shown for comparison purpose.
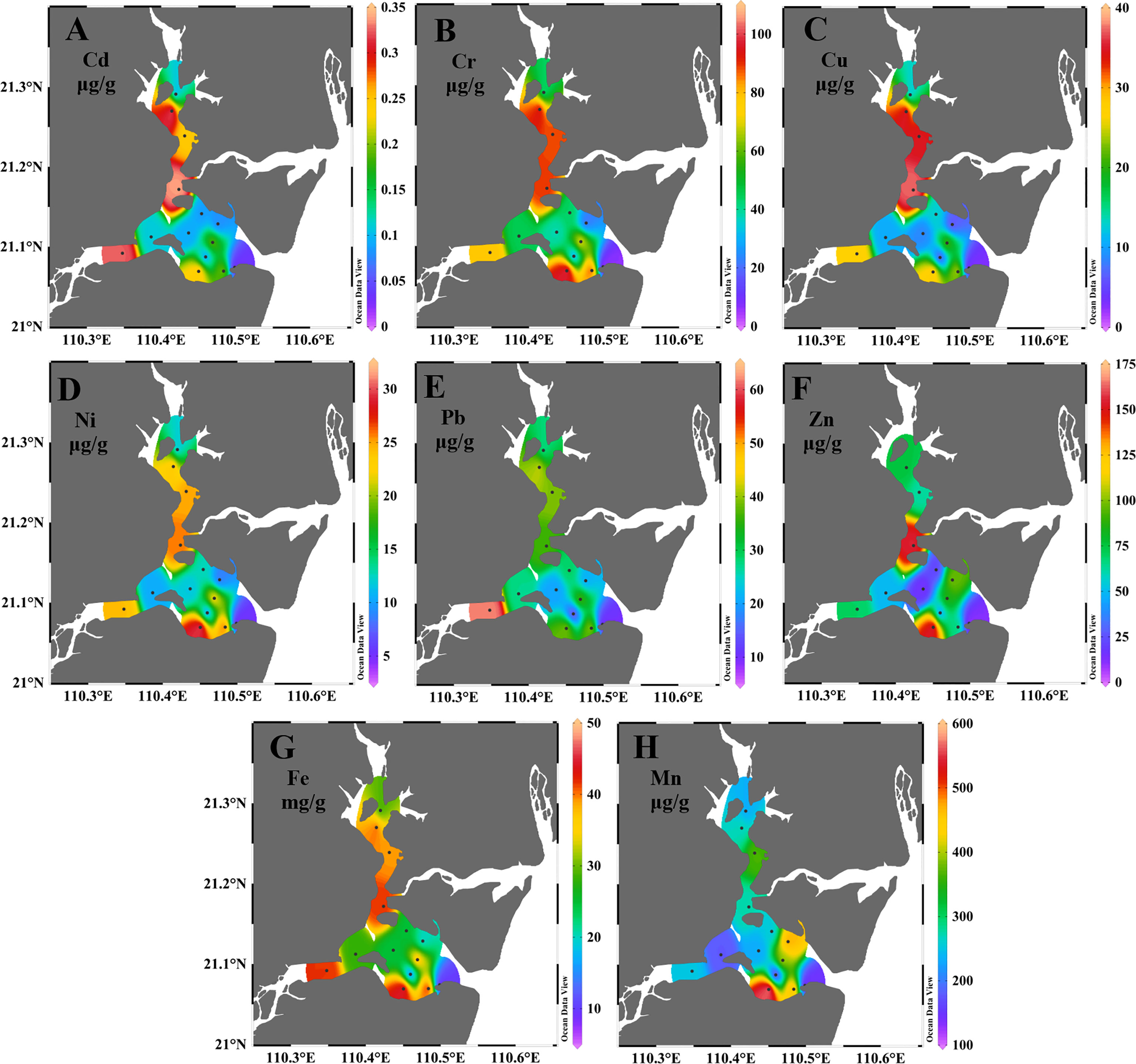
Figure 2 The distribution of total metal concentrations for Cd (A), Cr (B), Cu (C), Ni (D), Pb (E), Zn (F), Fe (G) and Mn (H) in surface sediments of Zhanjiang bay in spring.
The distribution patterns of studied metals were generally similar with relatively high concentrations at the channel and coastal bay, and relatively low concentrations in the central bay (Figures 2, S1). Spatial distribution is a useful tool for determining hotspot area with high metal concentrations (Hossain et al., 2020). The high concentrations of metals at the north channel may probably be resulted by the input of Suixi river and/or the discharge of domestic sewage from coastal area. The high concentrations of metals at the south channel (station Z5) may probably be related to the construction of the Donghai Dam. Previous studies indicated that the construction of the Donghai Dam could lead to long residence time of water near the Donghai Dam (Li, 2008; Zhou et al., 2020), which is conducive to the settlement of fine particles (Lu et al., 2020; Anandkumar et al., 2022). Fine grained sediments are conducive to adsorb more metals (Salomons and Förstner, 1984). Therefore, high metal concentrations can be found in the south channel of ZJB (Figures 2, 3; Lu et al., 2020). Besides, point sources of metals may also contribute to the high concentrations of metals in this area. More research should be carried out to find the possible sources of metals in the south channel of ZJB. Chemical manufacturing units can act as point sources of metal contamination. Many factories are located on the south coast of ZJB (Figure 1). The high concentrations of almost all studied metals at the south coast of ZJB (station Z9) may probably indicate the influence of industrial wastewater from coastal factories.
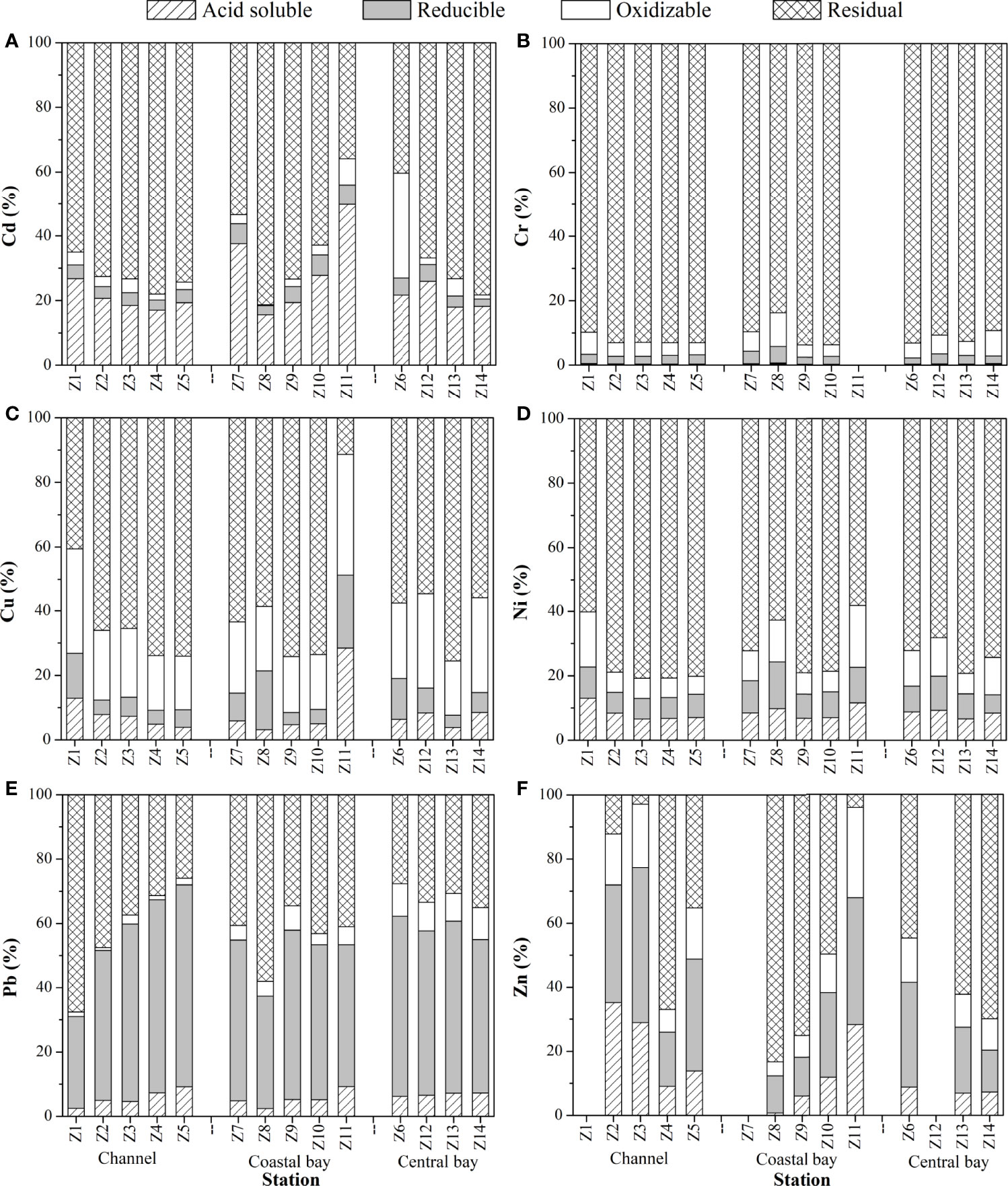
Figure 3 Distributions of Cd (A), Cr (B), Cu (C), Ni (D), Pb (E) and Zn (F) in different geochemical fractions in the surface sediments of Zhanjiang bay in spring.
Table 3 showed the average total concentrations of metals in different subregions. In spring, the average total concentrations of Cd, Cr, Cu, Ni, Pb, Zn and Fe in different subregions followed the order of channel > coastal bay > central bay, while for Mn, the order is coastal bay > channel > central bay. This indicates that in spring the main source of Cd, Cr, Cu, Ni, Pb, Zn and Fe in surface sediments of ZJB was probably the input of Suixi river, while for Mn the main source was probably the industrial wastewater from south coast. In summer, the average total concentrations of Cd, Cr, and Cu in different subregions followed the order of channel > coastal bay > central bay; for Ni, Pb and Fe, the order is channel > central bay > coastal bay; for Zn, the order is coastal bay > channel > central bay; and for Mn, the order is coastal bay > central bay > channel (Table 3). The distribution pattern of Cd, Cr and Cu in summer was similar with that in spring, while the distribution pattern of Ni, Pb, Fe, Zn and Mn showed some differences compared with that in spring. For Ni, Pb and Fe, the average total concentrations in central bay were higher than those in coastal bay and lower than those in channel. This distribution pattern suggests that the central bay may probably be influenced by river runoff in summer. High rainfall in summer could cause high river runoff, resulting in terrestrial materials transporting to a far distance from Suixi river estuary. This phenomenon has also been found in the study of Zhou et al. (2020). For Zn, the average total concentration in coastal area was higher than that in channel and central bay. This indicates that the main source of Zn in summer was industrial waste from coastal factories, which was different from that in spring. For Mn, the average total concentration in central area was higher than that in channel and lower than that in coastal bay. This indicates that industrial waste water can also be transported to the central bay from the coastal bay in summer. The dynamic environment of ZJB between spring and summer (e.g. increased river runoff in summer) contributed to the spatial variations of Ni, Pb, Zn, Fe and Mn in these two seasons. In spring, due to the low rainfall in this season, the Suixi river had a small runoff and the surface runoff was also weak. The influence from the Suixi river was mainly trapped in the channel, resulting in relatively high metal concentrations in this area, and the pollutants from coastal area cannot be transported far away from coast. While in summer, increased rainfall resulted in increased terrestrial input (Zhou et al., 2020), which may bring pollutants to the central ZJB through river input and/or coastal transportation.
In addition to the variations of spatial distribution pattern of some metals, the average total concentrations of the studied metals in different subregions also showed variations (Table 3). For the channel, all the studied metals showed decreasement in summer compared with those in spring, with the maximum decrease of 32.4% for Zn. Similar seasonal variations of metals were also found in the surface sediment of Changjiang Estuary (Duan et al., 2019). Though increased rainfall in summer brought terrigenous pollutants to the surface sediment of channel bay, seasonal variations of environmental characteristics promoted metal release from sediment (Table 1, Section 3.1; Duan et al., 2019). The reduced Fe, Mn and TOC concentration in surface sediment, increased Chl a concentration in bottom water and the aerobic environment of bottom water in summer in the channel may probably indicate that the increase in organic matter degradation resulted in some Fe and Mn (hydr)oxides being reduced and released into the water (Table 1, Section 3.1; Duan et al., 2019). The decrease of other studied metals in channel may probably be related to organic matter degradation and Fe and Mn (hydr)oxides reduction processes. High temperature in summer enhanced bacterial activities, which may also contribute to the release of metals in summer (Table 1; Duan et al., 2019).
However, in the central bay, Cr, Ni and Zn showed some increases in summer compared with those in spring. These increases of metals in the central bay may be mainly due to the increased discharge of river input (Zhou et al., 2020) and/or industrial sewage from coastal areas in summer. In the coastal bay, the total concentrations of Cu, Ni, Pb, Fe and Mn decreased and Cd, Cr and Zn increased in summer compared with those corresponding concentrations in spring. These metals’ seasonal variations in the coastal area may be the comprehensive impact of environmental changes (the change of temperature, DO and/or organic matter degradation) and increased terrigenous input, with the former five metals mainly influenced by environmental changes and the latter three metals mainly influenced by increased terrigenous input.
Correlation analysis was made based on the spring and summer data. Most of the studied metals showed significant positive correlations with each other (Table 4), indicating their similar sources and/or behaviors. Besides, almost all the studied metals showed significant positive correlations with TOC content, clay fraction and silt fraction, and showed significant negative correlations with sand fraction. This indicates that sediment grain size and TOC content both had significant influences on the distribution of metals in the surface sediments of ZJB, which has also been found in other areas such as the coastal Bohai Bay (Gao and Chen, 2012) and the mouth of São Francisco Channel (Freitas et al., 2019). Fine-grained sediments (clay + silt) have a larger specific surface area than coarser fraction of sediments (sand), providing them with more binding sites for the adsorption of organic matter and metals (Salomons and Förstner, 1984; Keil et al., 1994; Mayer, 1994). Organic matter is also an important mechanism of metals complexation in sediment (Freitas et al., 2019). The high concentrations of studied metals in the channel and coastal sediments of Zhanjiang bay were also related to the grain size compositions and TOC contents of sediments in these areas. Under oxidizing conditions, Fe and Mn tend to precipitate in the forms of oxides/hydroxides (Salomons and Förstner, 1984; Freitas et al., 2019). In this study, significant positive correlations between Fe, Mn and other studied trace metals were found (Table 4). This suggests that metals in surface sediment of ZJB were related to Fe and Mn oxides and hydroxides (Freitas et al., 2019).
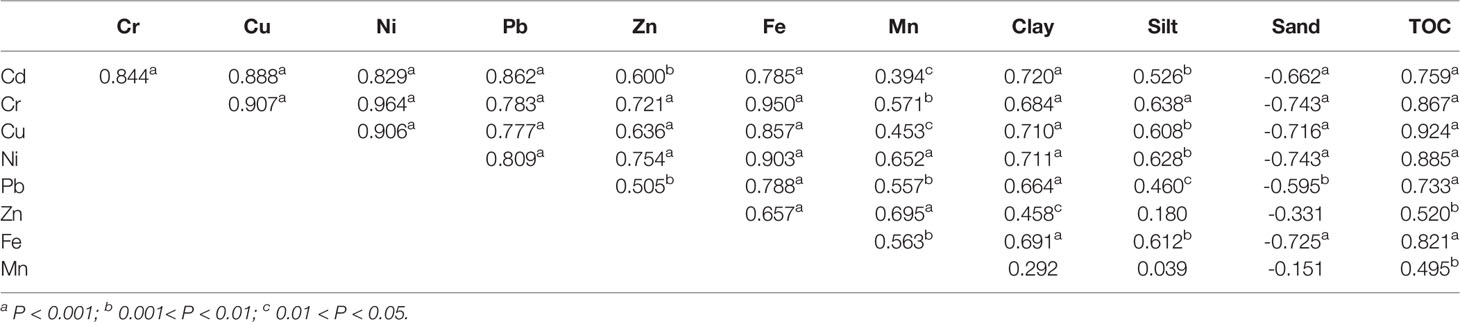
Table 4 Pearson correlation analysis for total concentrations of metals and related parameters (n = 28).
Principal component analysis (PCA) was used to identify principal components from the studied metals and related environmental parameters in surface sediments of ZJB (Table 5). Based on the results of PCA, two principal components (PC1 and PC2) were identified which accounted for 84.6% of the total data variance. PC1, accounting for 71.6% of the total data variance, had high positive loadings for TOC, Clay, silt and all studied metals. Considering the spatial distributions of these parameters (Table 1 and Figures 2, S1) and the aforementioned discussion, we think that PC1 mainly represented the sources of river input and/or domestic sewage from Zhanjiang city. PC2, accounting for 12.9% of the total data variance, had high positive loadings for Mn, Zn and sand. Considering their high concentrations in the coastal bay (Tables 1, 3), we think that PC1 represented the sources from coastal region.
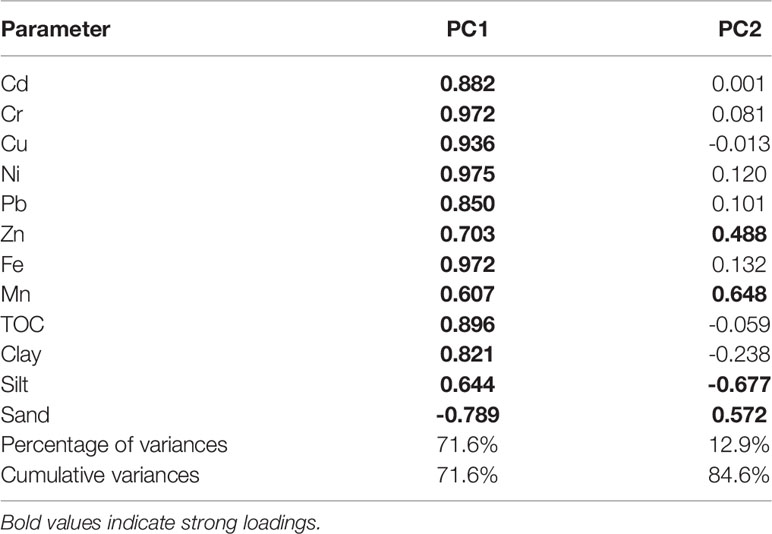
Table 5 Loadings of experimental variables on significant principal components for the data from Zhanjiang bay.
The geochemical compositions of Fe and Mn were not determined in sediments of ZJB. The average values of metal fractions in surface sediments of ZJB are summarized in Table S2. Among the studied metals, Pb and Zn were mainly associated with non-residual fractions (acid soluble fraction, reducible fraction and oxidizable fraction), and Cd, Cr, Cu and Ni were mainly associated with the residual fraction (Table S2).
The acid soluble fraction (F1) of metals in sediments (exchangeable and bound to carbonates components) is in equilibrium with metals dissolved in water. It is labile, highly toxic and is the most bioavailable fraction. In the surface sediments of ZJB, the mean proportion of metals in F1 was Cd 24.0%, Cr 0.4%, Cu 7.9%, Ni 8.5%, Pb 5.9%, Zn 14.3% in spring, and Cd 19.4%, Cr 0.6%, Cu 5.8%, Ni 6.8%, Pb 6.5%, Zn 14.8% in summer. Relatively high proportions of Cd and Zn were associated with F1, indicating their high risk to the environment of ZJB. Low proportions of Cr, Cu, Ni and Pb were associated with F1. This indicates that these metals had low risk to the environment of ZJB.
The distribution of metal fractions is presented in Figures 3, S2. In spring, the average percentage of Zn in F1 was 21.8% at the channel, which was higher than that in the coastal bay (average: 11.7%) and the central bay (average: 7.6%). The acid soluble fraction has the greatest tendency to move from sediment to overlying water (Wang et al., 2011). This indicates that the channel was subject to more anthropogenic inputs of Zn compared with the coastal and central bay. Similar phenomenon was found in summer. In spring, a considerable fraction of Cd in F1 was observed at the coast of ZJB (average: 30.0%), which was higher than that in the channel (average: 20.4%) and the central bay (average: 20.9%). This suggests that the coastal bay was subject to more anthropogenic inputs of Cd compared with the channel and central bay. While in summer, relatively high percentage of Cd in F1 was observed at the channel. This indicates the main sources of acid soluble fraction of Cd varied with seasons.
Reducible fraction (F2) of metal is bound to amorphous Fe and Mn oxides and hydroxides (Rauret et al., 1999; Nemati et al., 2011). This fraction can become dissolved under reducing environment (Morillo et al., 2004). Among the studied metals, Pb had the highest percentage in this fraction (average of 47.4% in spring and summer) in the surface sediments of ZJB, indicating its potential risk to the environment under reducing conditions. Other studied metals had low proportions in F2. The average proportion in this fraction for both seasons can be summarized as: Pb (49.4%) > Zn (26.6%) > Cu (8.7%) > Ni (8.5%) > Cd (4.4%) > Cr (2.8%) in spring and Pb (45.2%) > Zn (16.4%) > Ni (3.9%) > Cd (3.8%) > Cu (3.4%) > Cr (2.5%) in summer.
The oxidizable fraction (F3) of metal is bound to organic matter and sulfides components (Rauret et al., 1999; Nemati et al., 2011). Cu in this fraction was generally higher in surface sediments of ZJB, with an average proportion of 23.0% in spring and 32.8% in summer. The presence of other metals in this fraction was generally low in both seasons. The average proportion of metals in this fraction can be summarized as follows: Cu (23.0%) > Zn (13.1%) > Ni (9.8%) > Cr (5.4%) > Cd (5.3%) > Pb (5.1%) (spring) and Cu (32.8%) > Zn (15.2%) > Pb (9.9%) > Ni (8.4%) > Cr (4.5%) (summer). Due to contamination in the operation process, Cd in oxidizable fraction in summer was not available. Related parameters (Cd in residual and bioavailable fraction in summer) were also not available.
Metals in the residual fraction (F4) are associated with silicate mineral lattices (Rauret et al., 1999; Nemati et al., 2011). A major proportion of Cr (average of 91.4% and 92.4% in spring and summer, respectively) and Ni (average of 73.2% and 81.0% in spring and summer, respectively) were associated with this fraction, indicating their less mobility and bioavailability in the surface sediments of ZJB. The average proportion of studied metals in residual fraction was summarized as follows: Cr (91.4%) > Ni (73.2%) > Cd (66.3%) > Cu (60.4%) > Zn (46.0%) > Pb (39.6%) (spring) and Cr (92.4%) > Ni (81.0%) > Cu (58.0%) > Zn (53.5%) > Pb (38.4%) (summer).
The bioavailable fraction (BF) of metals is the summing results of the acid soluble, reducible and oxidizable fraction. The residual fraction is the non-bioavailable fraction. Metals associated with BF are bounded through weak bonds on sediments and are readily available to aquatic biota (Forstner, 1989; Pempkowiase et al., 1999). The concentration of metals in the BF is a serious environmental concern (Sundaray et al., 2011).
The average concentrations of bioavailable metals in different subregions are shown in Figures 4A, B. All the studied metals in BF had the highest concentrations in the channel sediments, suggesting that terrestrial input and/or domestic sewage may be their main sources. The percentage of the metals in BF decreased in the order of Pb (60.4%) > Zn (54.0%) > Cu (39.6%) > Cd (33.7%) > Ni (26.8%) > Cr (8.6%) in spring and Pb (61.6%) > Zn (46.5%) > Cu (42.0%) > Ni (19.0%) > Cr (7.6%) in summer (Table S2). This indicates that Cr and Ni were the least bioavailable studied metals in the sediments of ZJB. High percentages of Pb and Zn recorded in the BF indicate their greater mobility in the sediments of ZJB, which may be mainly from shipping activities (ship repair or painting), industrial effluent or domestic sewage.
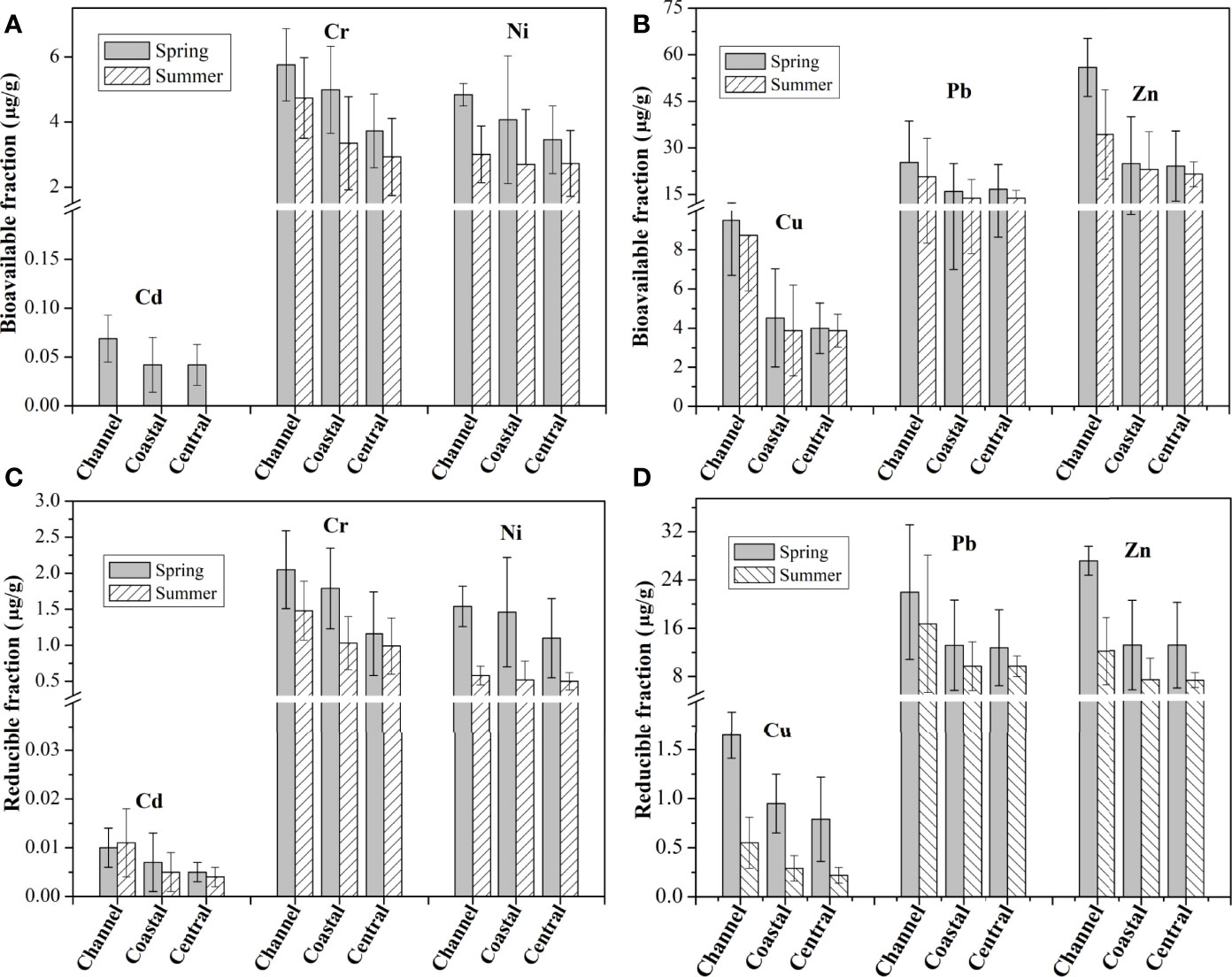
Figure 4 Average concentrations of bioavailable (A, B) and reducible (C, D) fraction of metals in different subregions of Zhanjiang bay.
Seasonal changes on bioavailability of metals were observed in the surface sediments of ZJB. The average concentrations of bioavailable fractions for all studied metals (except for Cd) decreased in summer compared with those in spring, with the maximum decrease of 52% for bioavailable Zn (Figures 4A, B). These changes indicate the release of bioavailable metals in summer, which were able to increase ecological risks. The concentrations of reducible fraction (bound to Fe/Mn oxyhydroxides components) for all studied metals (except for Cd) decreased in summer compared with those in spring (Figures 4C, D). For example, the average concentration of reducible Zn in channel was 27.19 μg/g in spring and 12.21 μg/g in summer. The seasonal variations of metals in this fraction may indicate the influence of relatively low DO in summer (Table 1). Decreased DO concentration in summer can promote the reduction of Fe and Mn oxides and hydroxides and the release of related metals (Morillo et al., 2004; Duan et al., 2019).
The average total concentrations of Cd, Cr, Cu, Pb and Zn in the surface sediments of ZJB in both seasons were all within the range of MSQ Grade I (Table 2), suggesting that the surface sediments of ZJB were generally not contaminated by these metals.
According to the calculated EF values (Figure 5), Cr, Ni and Mn in the sediment of ZJB (average of 0.93, 0.70 and 0.58, respectively) generally showed no enrichment in both spring and summer; Cu showed minor enrichment in spring (average: 1.12); and Cd, Pb and Zn showed minor enrichment in both spring and summer. Overall, the calculated average EF values were in the descending order of Cd (2.47) > Pb (1.56) > Cu (1.12) > Zn (1.04) > Cr (0.90) > Ni (0.71) > Mn (0.62) in spring and Cd (2.61) > Pb (1.33) > Zn (1.05) > Cu (0.97) > Cr (0.96) > Ni (0.70) > Mn (0.54) in summer. Cr, Ni and Mn in the sediment of ZJB originated mainly from the natural input. Cd, Cu, Pb and Zn were influenced by anthropogenic activities to some extent.
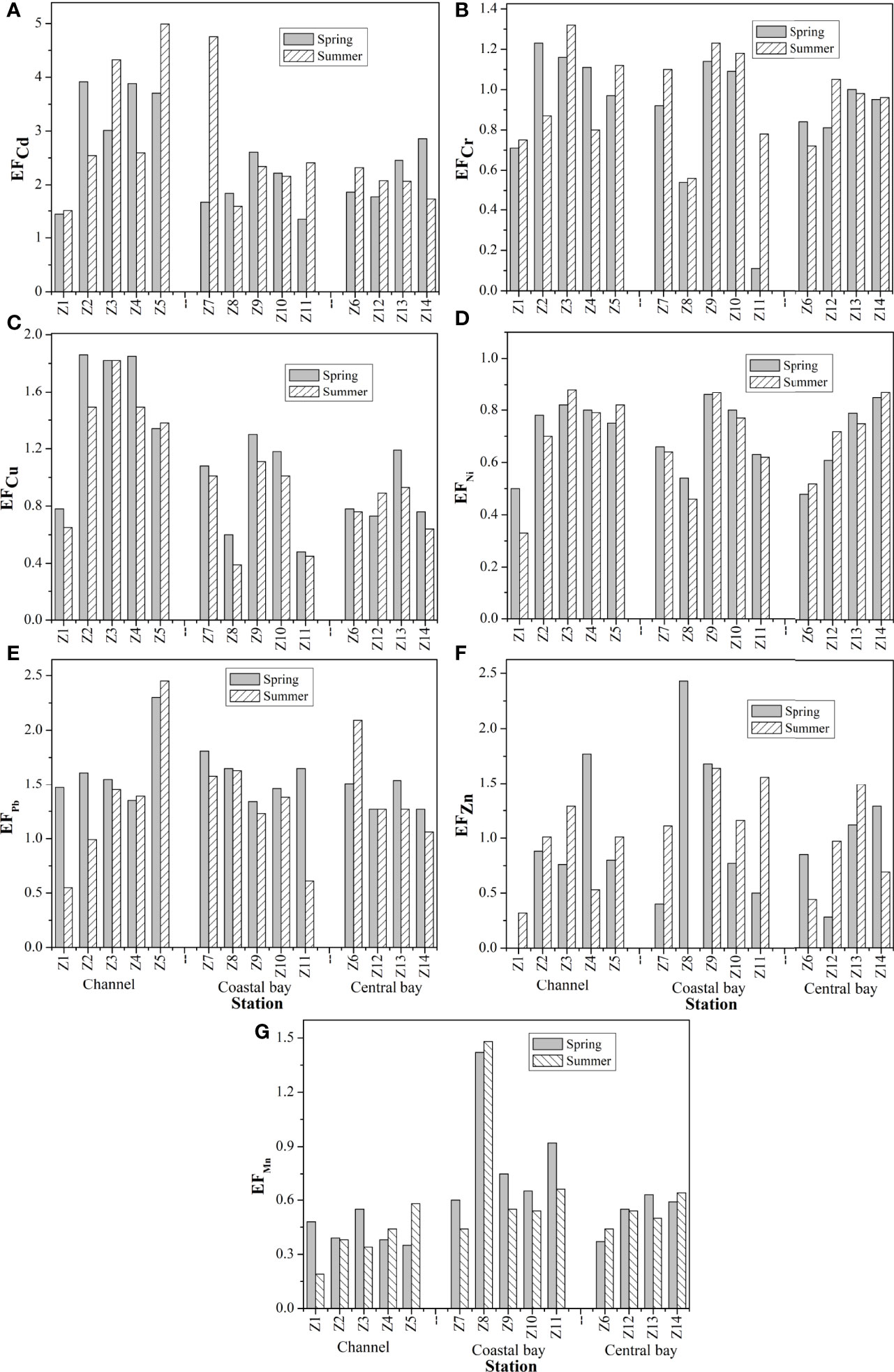
Figure 5 Distributions of enrichment factor (EF) for Cd (A), Cr (B), Cu (C), Ni (D), Pb (E), Zn (F) and Mn (G) in surface sediments of Zhanjiang bay.
The average CF values of the studied metals decreased in the order of Cd (2.69) > Pb (1.58) > Cu (1.27) > Zn (1.08) > Fe (1.01) > Cr (0.99) > Ni (0.74) > Mn (0.57) in spring and Cd (2.63) > Pb (1.29) > Zn (1.01) = Cu (1.01) > Cr (0.95) > Fe (0.94) > Ni (0.67) > Mn (0.47) in summer (Figure 6). The channel sediments of ZJB were considerably contaminated by Cd during both seasons (average CF of 4.10 in spring and 3.76 in summer) (Figure 6). The coastal and central bay sediments are moderately contaminated by Cd. The three subregions of ZJB were moderately contaminated by Pb. For Cu, only the channel sediments showed moderately contaminated by this metal. For Zn, the station (Z9) near the Zhongke (Guangdong) Refining and Chemical Co., Ltd recorded the highest CF values in both spring and summer (2.48 in spring and 2.15 in summer) (Figures 1, 6). This indicates that station Z9 was moderately contaminated by Zn, which may be probably sourced from the sewage of Zhongke (Guangdong) Refining and Chemical Co., Ltd. Other studied metals generally had low contamination to the surface sediments of ZJB (Figure 6).
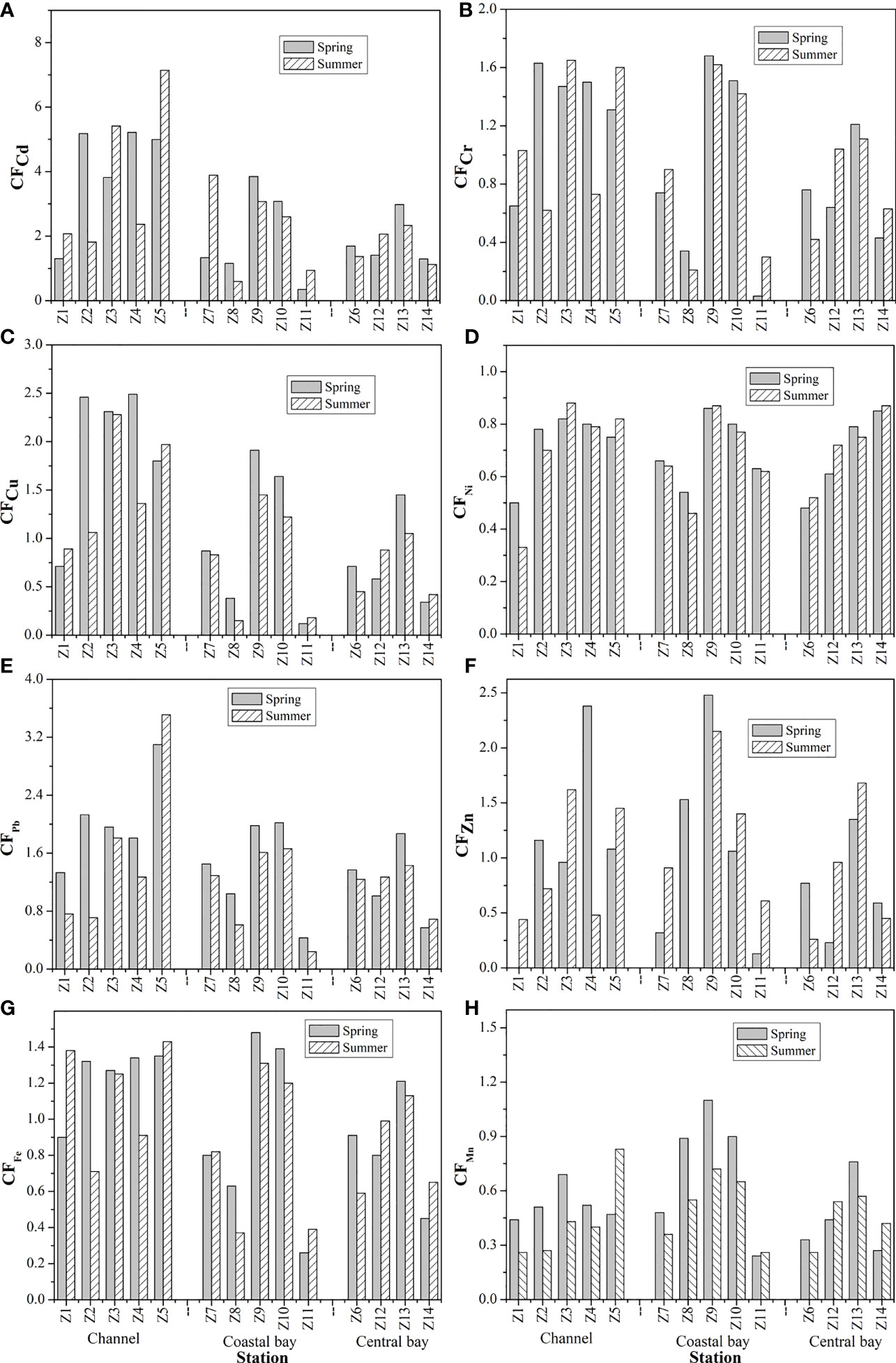
Figure 6 Distributions of contamination factor (CF) for Cd (A), Cr (B), Cu (C), Ni (D), Pb (E), Zn (F), Fe (G) and Mn (H) in surface sediments of Zhanjiang bay.
The result of the hierarchical cluster analysis of the sampling stations based on the calculated CF in the surface sediment of ZJB in spring and summer is shown in Figure 7. Three main different clusters could be observed. Cluster 1 involved several stations (Z1, Z6, Z7, Z8, Z11, Z12, Z14) which were less contaminated. Cluster 2 involved several stations (Z2, Z3, Z4, Z9, Z10, Z13) which were generally contaminated. Cluster 3 involved one station (Z5) near the Donghai Dam which was moderately contaminated according to the calculated CF.
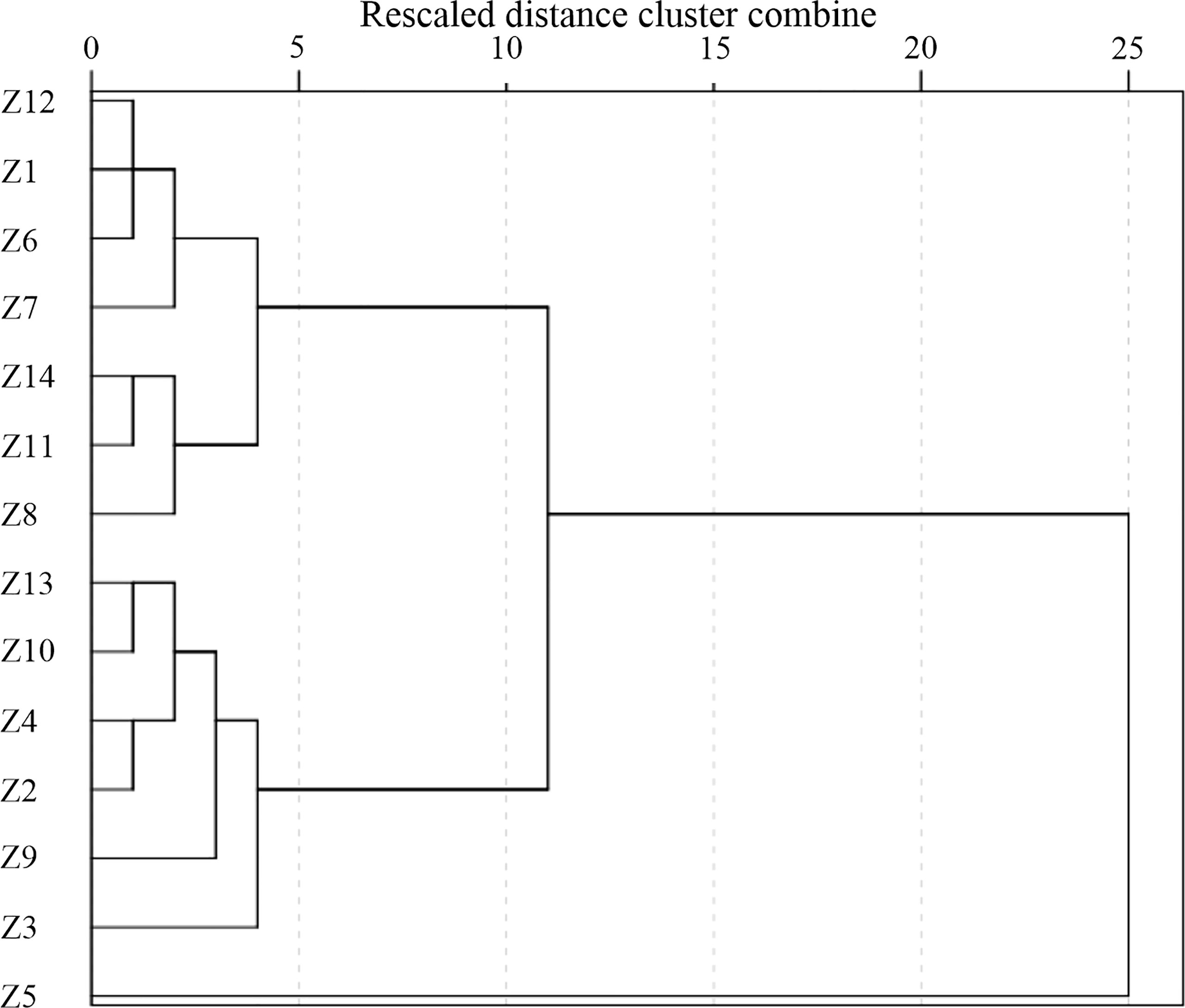
Figure 7 Hierarchical dendrogram in terms of the sampling stations based on the data of contaminant factor in spring and summer.
The calculated PLI values for metals in the surface sediment of ZJB are summarized in Figure 8. PLI ranged from 0.17 to 1.83 in spring and from 0.33 to 1.88 in summer. The average PLI values in the channel, coastal and central ZJB were 1.28, 0.93 and 0.81, respectively (spring and summer). This indicates that the channel of ZJB was contaminated by the studied metals. Besides, the two stations locating at the south coast of ZJB also had PLI values larger than 1, indicating the deterioration of sediment quality in this area.
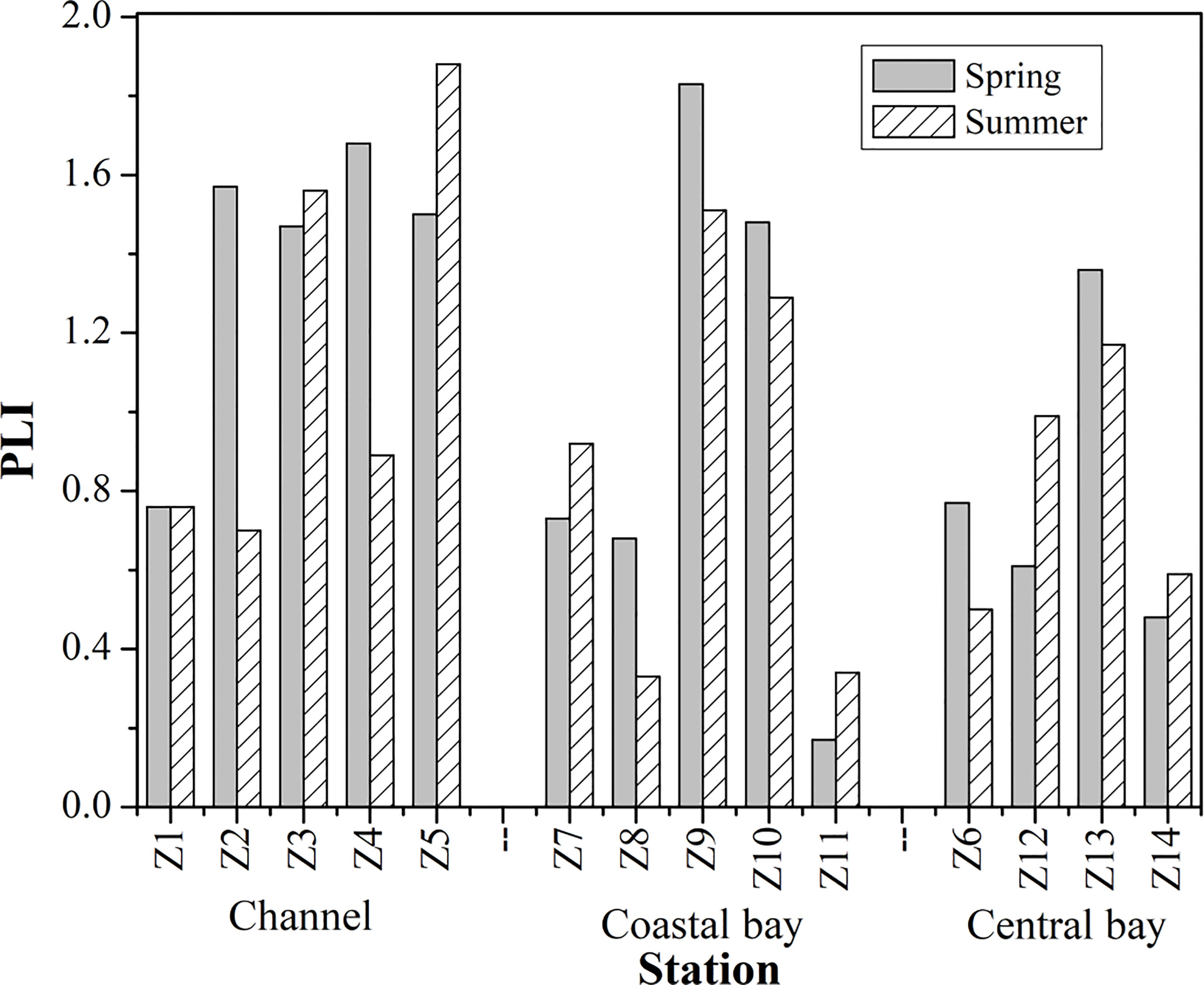
Figure 8 Distributions of pollution load index (PLI) for metals in surface sediments of Zhanjiang bay.
The risk of metals in surface sediments of ZJB was evaluated based on the percentage of acid soluble fraction (F1) of metals (Section 2.3). The percentages of Cd in F1 in the channel and coastal bay and the percentages of Zn in F1 in the channel were generally in the range of 30-50% (Figures 3, S2), indicating the high risk of Cd and Zn to the environment in these subregions of ZJB. The percentages of Cd in F1 in the central bay and the percentages of Zn in F1 in the coastal and central bay were generally in the range of 10-30% (Figures 3, S2), indicating their medium risk to the environment in these subregions. Cu, Ni, Pb and Cr in the surface sediments of Zhanjiang bay generally had low risk or no risk to the environment based on their percentages in F1 (Figures 3, S2).
The results of EF and CF, which were calculated based on total metal concentrations, also indicated the contamination of Cd and Zn in surface sediments of ZJB (Section 3.5.1 and 3.5.2). Combined the assessment results of EF, CF and the percentages of acid soluble fraction, we concluded that Cd and Zn in the surface sediments of Zhanjiang bay were generally contaminated and they had medium to high risk to the environment.
Total concentrations and geochemical compositions of metals in surface sediments of Zhanjiang bay were studied. The total concentrations of metals were generally higher in the channel and coastal sediments of Zhanjiang bay. River discharge, domestic sewage, industrial wastewater, grain size compositions and TOC contents of sediments contributed much to the high concentrations of metals in these subregions of Zhanjiang bay. The spatial distribution pattern of some studied metals (Ni, Pb, Fe, Zn and Mn) showed seasonal variations. The dynamic environment of Zhanjiang bay between spring and summer contributed to the spatial variations of these metals. In the channel, all the studied metals showed decreased concentrations in summer compared with those in spring, indicating the release of metals from sediments. Organic matter degradation and Fe and Mn (hydr)oxides reduction processes contributed to that phenomenon.
Relatively high proportions of Cd and Zn were associated with the acid soluble fraction (average of 21.7% and 14.6%, respectively), indicating their high risk to the environment of Zhanjiang bay. The concentrations of reducible and bioavailable fractions of metals generally decreased in summer compared with those in spring. These changes may probably suggest the release of bioavailable metals in summer, which could be able to increase ecological risks. More research should be conducted to confirm this conclusion. Based on the assessment results of enrichment factor, contamination factor and the percentages of acid soluble fraction, Cd and Zn in the surface sediments of Zhanjiang bay were generally contaminated and they had medium to high risk to the environment. The pollution load index indicated the deterioration of sediment quality in the channel and the south coast of Zhanjiang bay. More attention should be paid on these contaminated areas in Zhanjiang bay. Some bioremediation techniques like using aquatic plants to remove the contaminated metals can be used to reduce the contamination of Zhanjiang bay.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conceptualization: FZ. Data curation: FZ, MX, SW, and ST. Funding acquisition: FZ, SW, and FC. Investigation: FZ, GJ, FC, XL, QZ, and YM. Methodology: FZ, MX, ST, and CC. Writing – original draft: FZ. Writing – review and editing: FZ, GJ, and FC. All authors contributed to the article and approved the submitted version.
This work was supported by the Foundation for Young Talents in General Colleges and Universities of Guangdong Province (2019KQNCX044), the Guangdong Povincial College Innovation Team Project (2019KCXTF021), the Guangdong Basic and Applied Basic Research Foundation (2021A1515110172), the National Natural Science Foundation of China (U1901213), the Guangdong Natural Science Foundation of China (2019B1515120066, 2016A030312004), and the First-class Discipline Plan of Guangdong Province (080503032101, 231420003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.925567/full#supplementary-material
Akcay H., Oguz A., Karapire C. (2003). Study of Heavy Metal Pollution and Speciation in Buyak Menderes and Gediz River Sediments. Water Res. 37, 813–822. doi: 10.1016/S0043-1354(02)00392-5
Akhbarizadeh R., Moore F., Keshavarzi B., Moeinpour A. (2017). Microplastics and Potentially Toxic Elements in Coastal Sediments of Iran’s Main Oil Terminal (Khark Island). Environ. pollut. 220, 720–731. doi: 10.1016/j
Anandkumar A., Nagarajan R., Gounder E. S., Prabakaran K. (2022). Seasonal Variation and Mobility of Trace Metals in the Beach Sediments of NW Borneo. Chemosphere 287, 132069. doi: 10.1016/j.chemosphere.2021.132069
Anandkumar A., Nagarajan R., Prabakaran K., Bing C. H., Rajaram R. (2018). Human Health Risk Assessment and Bioaccumulation of Trace Metals in Fish Species Collected From the Miri Coast, Sarawak, Borneo. Mar. pollut. Bull. 133, 655–663. doi: 10.1016/j.marpolbul.2018.06.033
Cai J. G., Xu J. L., Yang S. Y., Bao Y. J., Lu L. F. (2006). The Fractionation of an Argillaceous Sediment and Difference in Organic Matter Enrichment in Different Fractions. Geological. J. China Universities. 12, 234–241. doi: 10.1111/j.1745-4557.2006.00081.x
Chakraborty S., Chakraborty P., Hathorne E., Sarkar A., Linsy P., Frank M., et al. (2021). Evidence for Increasing Anthropogenic Pb Concentrations in Indian Shelf Sediments During the Last Century. Sci. Total. Environ. 760, 143833. doi: 10.1016/j.scitotenv.2020.143833
Chakraborty S., Chakraborty P., Sarkar A., Nagender Nath B. (2017). Kinetic and Equilibrium Based Fractionation Study of Pb in Continental Shelf Sediment of India. Mar. pollut. Bull. 123 (1-2), 188–196. doi: 10.1016/j.marpolbul.2017.08.063
Chakraborty P., Sarkar A., Vudamala K., Naik R., Nagender Nath B. (2015). Organic Matter — A Key Factor in Controlling Mercury Distribution in Estuarine Sediment. Mar. Chem. 173 (20), 302–309. doi: 10.1016/j.marchem.2014.10.005
Charriau A., Lesven L., Gao Y., Leermakers M., Baeyens W., Ouddane B., et al. (2011). Trace Metal Behavior in Riverine Sediments: Role of Organic Matter and Sulfides. Appl. Geochem. 26, 80–90. doi: 10.1016/j.apgeochem.2010.11.005
Chen C. W., Kao C. M., Chen C. F., Dong C. D. (2007). Distribution and Accumulation of Heavy Metals in the Sediments of Kaohsiung Harbor, Taiwan. Chemosphere 66, 1431–1440. doi: 10.1016/j.chemosphere.2006.09.030
Dang D. H., Lenoble V., Durrieu G., Omanović D., Mullot J. U., Mounier S., et al. (2015). Seasonal Variations of Coastal Sedimentary Trace Metals Cycling: Insight on the Effect of Manganese and Iron (Oxy) Hydroxides, Sulphide and Organic Matter. Mar. pollut. Bull. 92, 113–124. doi: 10.1016/j.marpolbul.2014.12.048
Duan L. Q., Song J. M., Liang X. M., Yin M. L., Yuan H. M., Li X. G., et al. (2019). Dynamics and Diagenesis of Trace Metals in Sediments of the Changjiang Estuary. Sci. Total. Environ. 675, 247–259. doi: 10.1016/j.scitotenv.2019.04.190
EBCBS (Editorial Board of China Bay Survey) (1999). Survey of China Bays Vol. Vol. 10: Western Gulf of Guangdong Province) (Beijing: China Ocean Press).
Ergin M., Saydam C., Ba¸stürk O., Erdem E., Yörük R. (1991). Heavy Metal Concentrations in Surface Sediments From the Two Coastal Inlets (Golden Horn Estuary and Izmit Bay) of the Northeastern Sea of Marmara. Chem. Geology. 91 (3), 269–285. doi: 10.1016/0009-2541(91)90004-B
Freitas A. R. D., Rodrigues A. P. D. C., Monte C. D. N., Freire A. S., Santelli R. E., Machado W., et al. (2019). Increase in the Bioavailability of Trace Metals After Sediment Resuspension. SN. Appl. Sci. 1, 1288. doi: 10.1007/s42452-019-1276-8
Gao X. L., Chen C. T. A. (2012). Heavy Metal Pollution Status in Surface Sediments of the Coastal Bohai Bay. Water Res. 46, 1901–1911. doi: 10.1016/j.watres.2012.01.007
Gao X. L., Chen C. T. A., Wang G., Xue Q. Z., Tang C., Chen S. Y. (2010). Environmental Status of Daya Bay Surface Sediment Inferred From a Sequential Extraction Technique. Estuarine. Coast. Shelf. Sci. 86 (3), 369–378. doi: 10.1016/j.ecss.2009.10.012
Gao X. L., Zhou F. X., Chen C. T. A. (2014). Pollution Status of the Bohai Sea: An Overview of the Environmental Quality Assessment Related Trace Metals. Environ. Int. 62, 12–30. doi: 10.1016/j.envint.2013.09.019
Gao X. L., Zhou F. X., Lui H. K., Lou J. Y., Chen C. T. A., Zhuang W. (2016). Trace Metals in Surface Sediments of the Taiwan Strait: Geochemical Characteristics and Environmental Indication. Environ. Sci. Pollut. Res. 23, 10494–10503. doi: 10.1007/s11356-015-5669-y
Gu Y. G., Wang X. N., Lin Q., Du F. Y., Ning J. J., Li Y. F. (2016). Fuzzy Comprehensive Assessment of Heavy Metals and Pb Isotopic Signature in Surface Sediments From a Bay Under Serious Anthropogenic Influences: Daya Bay, China. Ecotoxicol. Environ. Saf. 126, 38–44. doi: 10.1016/j.ecoenv.2015.12.011
Hossain M. S., Ahmed M. K., Sarker S., Rahman M. S. (2020). Seasonal Variations of Trace Metals From Water and Sediment Samples in the Northern Bay of Bengal. Ecotoxicol. Environ. Saf. 193, 110347. doi: 10.1016/j.ecoenv.2020.110347
Ke S., Zhao L. R., Sun S. L. (2014). Distribution characteristics and sources of PAHs in sea water of the land-based outlet of Zhanjiang Bay. Marine Environ. Sci. 33, 71–77. doi: CNKI:SUN:HYHJ.0.2014-01-013
Keil R. G., Montluçon D. B., Prahl F. G., Hedges J. I. (1994). Sorptive Preservation of Labile Organic Matter in Marine Sediments. Nature 370, 549–552. doi: 10.1038/370549a0
Keskin S. (2012). Distribution and Accumulation of Heavy Metals in the Sediments of Akkaya Dam, Nigde, Turkey. Environ. Monit. Assess. 184, 449–460. doi: 10.1007/s10661-011-1979-9
Lee P. K., Kang M. J., Yu S., Ko K. S., Ha K., Shin S. C., et al. (2017). Enrichment and Geochemical Mobility of Heavy Metals in Bottom Sediment of the Hoedong Reservoir, Korea and Their Source Apportionment. Chemosphere 184, 74–85. doi: 10.1016/j.chemosphere.2017.05.124
Li X. B. (2008). Research on the Effects of Donghai Dam on the Hydrodynamic Environment of Zhanjiang Bay. Master’s Thesis (China: Ocean University of China, Qingdao).
Li Y. Y., Gao B., Xu D. Y., Peng W. Q., Liu X. B., Qu X. D., et al. (2020). Hydrodynamic Impact on Trace Metals in Sediments in the Cascade Reservoirs, North China. Sci. total. Environ. 716, 136914. doi: 10.1016/j.scitotenv.2020.136914
Li X. Y., Liu L. J., Wang Y. G., Luo G. P., Chen X., Yang X. L., et al. (2012). Integrated Assessment of Heavy Metal Contamination in Sediments From a Coastal Industrial Basin, NE China. PloS One 7, e39690. doi: 10.1371/journal.pone.0039690
Lin C., Liu Y., Li W. Q., Sun X. W., Ji W. D. (2014). Speciation, Distribution, and Potential Ecological Risk Assessment of Heavy Metals in Xiamen Bay Surface Sediment. Acta Oceanol. Sin. 33 (4), 13–21. doi: 10.1007/s13131-014-0453-2
Lu X., Zhou F. X., Chen F. J., Lao Q. B., Zhu Q. M., Meng Y. F., et al. (2020). Spatial and Seasonal Variations of Sedimentary Organic Matter in a Subtropical Bay: Implication for Human Interventions. Int. J. Environ. Res. Public Health 17, 1362. doi: 10.3390/ijerph17041362
Mayer L. M. (1994). Surface Area Control of Organic Carbon Accumulation in Continental Shelf Sediments. Geochimica. Cosmochimica. Acta 58, 1271–1284. doi: 10.1016/0016-7037(94)90381-6
Mohanraj T., Sheeba M., Cross S. R. T. S., Rajathy T. J. (2021). Seasonal Variation of Heavy Metals in the Intertidal Gastropod Trochus Radiatus of Gulf of Mannar. Open Jornal. Mar. Sci. 11, 92–102. doi: 10.4236/ojms.2021.112007
Morillo J., Usero J., Gracia I. (2004). Heavy Metal Distribution in Marine Sediments From the Southwest Coast of Spain. Chemosphere 55 (3), 431–442. doi: 10.1016/j.chemosphere.2003.10.047
Mucha A. P., Vasconcelos M. T. S. D., Bordalo A. A. (2003). Macrobenthic Community in the Douro Estuary: Relations With Trace Metals and Natural Sediment Characteristics. Environ. pollut. 121 (2), 169–180. doi: 10.1016/S0269-7491(02)00229-4
Nagarajan R., Kumar A., Hussain S. M., Jonathan M. P., Ramkumar M., Gounder E. S., et al. (2019). “Geochemical Characterization of Beach Sediments of Miri, NW Borneo, SE Asia: Implications on Provenance, Weathering Intensity, and Assessment of Coastal Environmental Status,” in Coastal Zone Management (Amsterdam, The Netherlands: Elsevier), 279–330.
Nemati K., Bakar N. K. A., Abas M. R., Sobhanzadeh E. (2011). Speciation of Heavy Metals by Modified BCR Sequential Extraction Procedure in Different Depths of Sediments From Sungai Buloh, Selangor, Malaysia. J. Hazardous. Materials. 192 (1), 402–410. doi: 10.1016/j.jhazmat.2011.05.039
Pan Z., Gao Q. F., Dong S. L., Wang F., Li D. H., Zhao K., et al. (2019). Effects of Abalone (Haliotis Discus Hannai Ino) and Kelp (Saccharina Japonica) Mariculture on Sources, Distribution, and Preservation of Sedimentary Organic Carbon in Ailian Bay, China: Identified by Coupling Stable Isotopes ( δ13c and δ15n) With C/N Ratio Analyses. Mar. pollut. Bull. 141, 387–397. doi: 10.1016/j.marpolbul.2019.02.053
Pekey H. (2006). The Distribution and Sources of Heavy Metals in Izmit Bay Surface Sediments Affected by a Polluted Stream. Mar. pollut. Bull. 52 (10), 1197–1208. doi: 10.1016/j.marpolbul.2006.02.012
Pekey H., Karakaş D., Ayberk S., Tolun L., Bakoǧlu M. (2004). Ecological risk assessment using trace elements from surface sediments of Izmit Bay (Northeastern Marmara Sea) Turkey. Mar. Pollut. Bull. 48 (9–10), 946–953. doi: 10.1016/j.marpolbul.2003.11.023
Pempkowiase J., Sikora A., Biernacka E. (1999). Speciation of Heavy Metals in Marine Sediments vs Their Bioaccumulation by Mussels. Chemosphere 39 (2), 313–321. doi: 10.1016/S0045-6535(99)00112-5
Peña-lcart M., Mendiguchía C., Villanueva-tagle M. E., Pomares-Alfonso M. S., Moreno C. (2014). Revisiting Methods for the Determination of Bioavailable Metals in Coastal Sediments. Mar. Pollut. Bull. 89 (1-2), 67–74. doi: 10.1016/j.marpolbul.2014.10.034
Perin G., Craboledda L., Lucchese L., Cirillo R., Dotta L., Zanette M. L., et al. (1985). “Heavy metal speciation in the sediments of Northern Adriatic Sea: a new approach for environmental toxicity determination,” in Heavy metal in the environment, vol. vol 2 . Ed. Lekkas T. D. (Edinburgh: CEP Consultant), pp 454–pp 456.
Pondell C. R., Canuel E. A. (2017). The Role of Hydrodynamic Sorting on the Accumulation and Distribution of Organic Carbon in an Impoundment: Englebright Lake, California, USA. Biogeochemistry 133, 129–145. doi: 10.1007/s10533-017-0319-8
Prabakaran K., Eswaramoorthi S., Nagarajan R., Anandkumar A., Franco F. M. (2020). Geochemical Behaviour and Risk Assessment of Trace Elements in a Tropical River, Northwest Borneo. Chemosphere 252, 126430. doi: 10.1016/j.chemosphere.2020.126430
Rauret G., Lopez-Sanchez J. F., Sahuquillo A., Rubio R., Davidson C., Ure A., et al. (1999). Improvement of the BCR Three Step Sequential Extraction Procedure Prior to the Certification of New Sediment and Soil Reference Materials. J. Environ. Monit. 1 (1), 57–61. doi: 10.1039/a807854h
Rivera-Duarte I., Flegal A. R. (1997). Porewater Gradients and Diffusive Benthic Fluxes of Co, Ni, Cu, Zn, and Cd in San Francisco Bay. Croatica. Chemica. Acta 70, 389–417.
Sahuquillo A., Rigol A., Rauret G. (2003). Overview of the Use of Leaching/Extraction Tests for Risk Assessment of Trace Metals in Contaminated Soils and Sediments. TrAC. Trends Analytical. Chem. 22 (3), 152–159. doi: 10.1016/S0165-9936(03)00303-0
Santos-Echeandia J., Prego R., Cobelo-García A., Millward G. E. (2009). Porewater Geochemistry in a Galician Ria (NW Iberian Peninsula): Implications for Benthic Fluxes of Dissolved Trace Elements (Co, Cu, Ni, Pb, V, Zn). Mar. Chem. 117, 77–87. doi: 10.1016/j.marchem.2009.05.001
SEPA (State Environmental Protection Administration of China) (2002). Marine Sediment Quality (GB 18668-2002) (Beijing: Standards Press of China).
Shibini Mol P. A., Sujatha C. H. (2020). Distribution and Geochemical Speciation of Sediment Bound Heavy Metals in the Specific Zones of Central Kerala, India. Environ. Nanotechn. Monit. Manage. 14, 100358. doi: 10.1016/j.enmm.2020.100358
Sundaray S. K., Nayak B. B., Lin S., Bhatta D. (2011). Geochemical Speciation and Risk Assessment of Heavy Metals in the River Estuarine Sediments-a Case Study: Mahanadi Basin. India. J. Hazardous. Materials. 186, 1837–1846. doi: 10.1016/j.jhazmat.2010.12.081
Tessier A., Campbell P. G. C., Blsson M. (1979). Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Analytical. Chem. 51 (7), 844–851. doi: 10.1021/ac50043a017
Tomlinson D. L., Wilson J. G., Harris C. R., Jeffrey D. W. (1980). Problems in the Assessment of Heavy Metal Levels in Estuaries and the Formation of a Pollution Index. Helgoland. Mar. Res. 33, 566–575. doi: 10.1007/BF02414780
USEPA, United States Environmental Protection Agency (2000). “Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories,” in Fish Sampling and Analysis, 3rd edition, vol. Volume I. (Washington, D.C: Office of Science and Technology).
Wang F., Chen J. (2000). Relation of Sediment Characteristics to Trace Metal Concentrations: A Statistical Study. Water Res. 34 (2), 694–698. doi: 10.1016/S0043-1354(99)00184-0
Wang Y., Yang Z., Shen Z., Tang Z., Niu J., Gao F. (2011). Assessment of Heavy Metals in Sediments From a Typical Catchment of the Yangtze River, China. Environ. Monit. Assess. 172, 407–417. doi: 10.1007/s10661-010-1343-5
Yu R., Yuan X., Zhao Y., Hu G., Tu X. (2008). Heavy Metal Pollution in Intertidal Sediments From Quanzhou Bay, China. J. Environ. Sci. 20 (6), 664–669. doi: 10.1016/S1001-0742(08)62110-5
Zhang J. B., Zhou F. X., Chen C. L., Sun X. L., Shi Y. Z., Zhao H., et al. (2018). Spatial Distribution and Correlation Characteristics of Heavy Metals in the Seawater, Suspended Particulate Matter and Sediments in Zhanjiang Bay, China. PloS One 13 (8), e0201414. doi: 10.1371/journal.pone.0201414
Zhao M., Shenghui Z., Haitao H., Pan D. (2021). Heavy Metals in Sediments of Yellow Sea and East China Sea: Chemical Speciation, Distribution, Influence Factor, and Contamination. J. Oceanol. Limnol. 39, 1277–1292. doi: 10.1007/s00343-020-0237-9
Zhao Y. Y., Yan M. C., Jiang R. H. (1995). Abundance of Chemical Elements in Continental Shelf Sediment of China. Geo-Marine. Lett. 15, 71–76. doi: 10.1007/BF01275409
Zhou F. X., Lu X., Chen F. J., Zhu Q. M., Meng Y. F., Chen C. Q., et al. (2020). Spatial-Monthly Variations and Influencing Factors of Dissolved Oxygen in Surface Water of Zhanjiang Bay, China. J. Mar. Sci. Eng. 8, 403. doi: 10.3390/jmse8060403
Keywords: metals, distribution, geochemical forms, environmental assessment, seasonal variations, influencing factors
Citation: Zhou F, Xiong M, Wang S, Tian S, Jin G, Chen F, Chen C, Lu X, Zhu Q and Meng Y (2022) Impacts of Human Activities and Environmental Changes on Spatial-Seasonal Variations of Metals in Surface Sediments of Zhanjiang Bay, China. Front. Mar. Sci. 9:925567. doi: 10.3389/fmars.2022.925567
Received: 21 April 2022; Accepted: 26 May 2022;
Published: 27 June 2022.
Edited by:
Meilin Wu, South China Sea Institute of Oceanology (CAS), ChinaReviewed by:
Arindam Sarkar, Bidhan Chandra Krishi Viswavidyalaya, IndiaCopyright © 2022 Zhou, Xiong, Wang, Tian, Jin, Chen, Chen, Lu, Zhu and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangzhe Jin, amluZ3Vhbmd6aGVAbGl2ZS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.