
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
DATA REPORT article
Front. Mar. Sci., 13 July 2022
Sec. Marine Pollution
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.914240
This article is part of the Research TopicCoastal and Marine Environmental Quality AssessmentsView all 28 articles
 Nithyanandam Marimuthu1*
Nithyanandam Marimuthu1* James Jerald Wilson2
James Jerald Wilson2 Abdulmohsin Abdullah Al-Sofyani3
Abdulmohsin Abdullah Al-Sofyani3 Arumugam Kuppuswamy Kumaraguru4
Arumugam Kuppuswamy Kumaraguru4Island ecosystems possess pristine environmental characteristics; human influence poses a serious threat to the fragile and susceptible biological processes on the islands (Sahu et al., 2013; Jha et al., 2015). Isolated oceanic islands support a highly sensitive and fragile coral reef ecosystem that offers unique possibilities to study the ecological changes and consequences that come with human settlement (Jha et al., 2011; Connor et al., 2012; Jha et al., 2013). Coral reefs are vital and core economic assets for any country that lies in the tropical and sub-tropical marine environment. Globally, the estimated economic support from this habitat has been calculated to be $375 billion per year (Cesar and Beukering, 2004; Brander et al., 2007). The important ecological services provided by these coral reef habitats have been identified as fish production, control of soil erosion on land, carbon sequestration, breeding grounds, etc. The coral reefs of Lakshadweep Islands are predominantly occupied by Scleractinian corals at various levels of the benthic substrate such as reef flat lagoon, reef crest, and reef slope. They are under great threat due to natural disturbances (Kumaraguru et al., 2005; Wilson et al., 2005) as well as anthropogenic disturbances (Wilson, 2010). The assessment of the biological indicators of benthic reef habitat is a key factor that helps in understanding the health status of any coral reef ecosystem (Al-Sofyani et al., 2014). The Crown-of-thorns Starfish (Acanthaster planciLinnaeus, 1758) is a major coral predator reported from various coral reef ecosystems. Their devastating population outbreaks have posed a great threat to coral reefs of the Indo-Pacific coastal region in the last five decades (Birkeland and Lukas, 1990; Fabricius et al., 2010). Besides the Crown-of-thorns Starfish, zooxanthellae-consuming gastropods are also reported as indicators for assessing the health status of corals in the Red Sea reef ecosystem (Mohamed et al., 2012; Al-Sofyani et al., 2014). Various studies on Drupella cornusRöding 1798 from its original combination (Röding 1798) to the corallivorous capability on Scleractinian corals of various coastal environments were presented through a timeline series (Supplementary Figure 1). The present study aims to interpret the impact of this indicator species and the relationship of physicochemical variables on the coral species cover along the Lakshadweep Archipelago by using the multivariate analytic tool.
From Minicoy Island in the south to Chetlet Island in the north, 42 locations (Figure 1) were chosen to assess the diversity of coral communities and the impact of Drupella cornus on these reef habitats in the Lakshadweep group of Islands. Sessile benthic communities were studied using the line intercept transect method (English et al., 1997) during the post-monsoon Season (December-February) between 2015 and 2020. Five replicates of a 50-m long flexible underwater tape were laid on the reefs at a depth of (1) 10m on the reef slope and, (2) 3m on the reef flat, nearly parallel to the shore. Video transects were also taken for further analysis and the benthos present under the transition points were recorded using international codes (English et al., 1997; Al-Sofyani et al., 2014; Ravindran et al., 2014). In the distinct community structure of the reef habitat of Lakshadweep Islands, live coral species cover was recorded in all the selected study sites. The scleractinian fauna was identified up to the generic level from the video transects and verified (Veron, 2000). The abundance of Drupella cornus assembled with the coral colonies below the transect line was estimated manually and documented during the monitoring period. Voucher specimens collected from Minicoy Island (latitude 8.317794°N and longitude 73.031218°E), Lakshadweep Island, were deposited (M-29888/7) in the National Zoological Collection of the Zoological Survey of India (ZSI), Kolkata, India. To check the influence of physicochemical variables on the coral species cover, the data on Sea surface temperature (SST), salinity, chlorophyll-a, and total suspended matter (TSM) were collected from the Indian National Centre for Ocean Information Services (INCOIS) live access portal. The pH variable was assessed in-situ by using Oakton PCS Testr 35 waterproof tester.
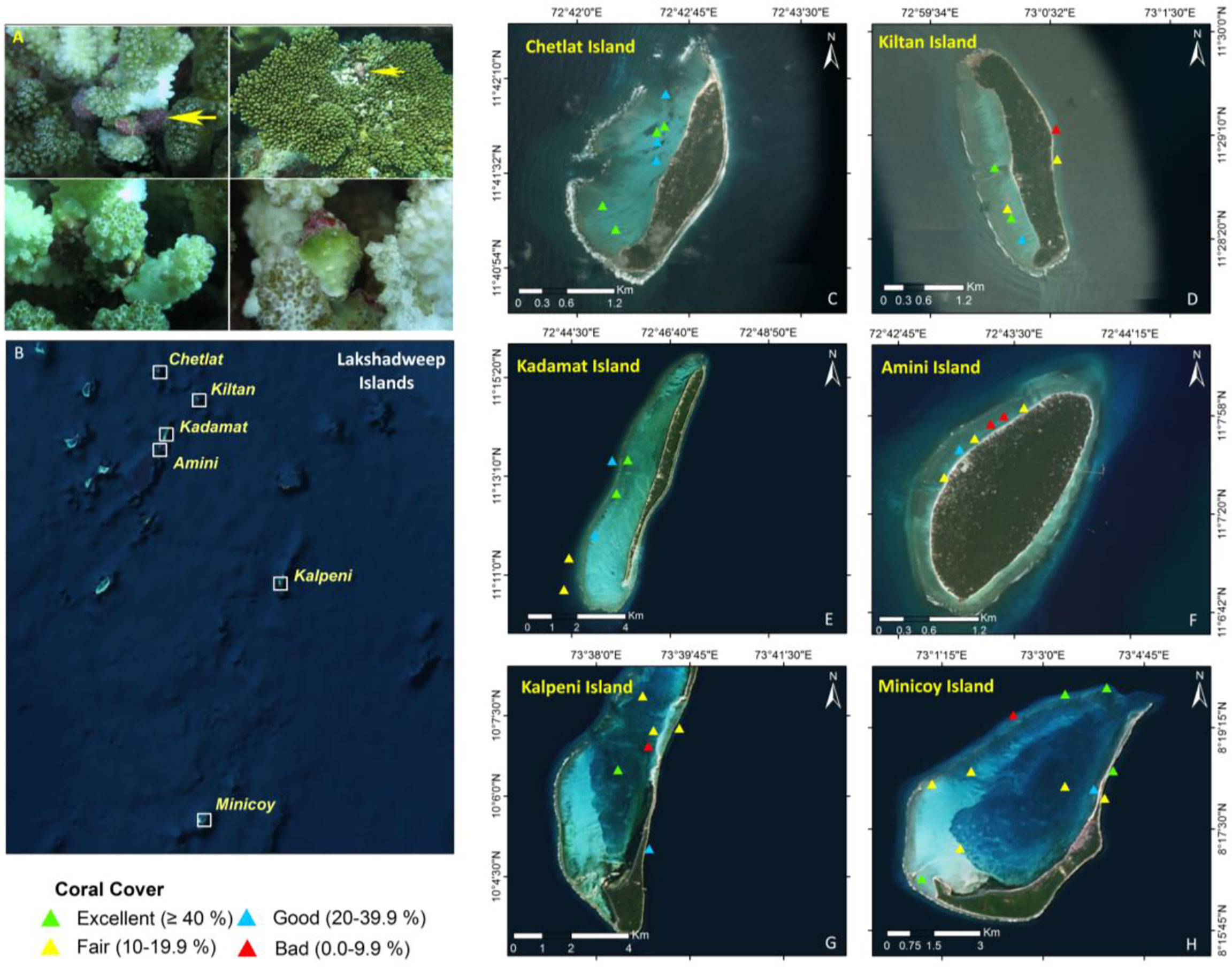
Figure 1 Map showing the study sites. (A)Drupella cornus on the coral; (B) Lakshadweep Archipelago; (C–H) Islands of Lakshadweep Archipelago.
Benthic raw data was sorted and assessed using the Reef Monitoring Data Entry System of AIMS (ARMDES V1.6 Data Entry Program – Long-term reef monitoring project, Australian Institute of Marine Sciences) (ARMDES, 1996). The percentage cover of live coral and Seaweed cover were taken for this study to assess the live coral index, corresponding to the study sites (Murdoch and Murdoch, 2016) by categorizing them into four different classes viz., (1) Excellent (≥40% of live corals), (2) Good (20-39.9%), (3) Fair (10-19.9%) and (4) Poor or Bad (0-9.9%). Principal component analysis (PCA) and Shade plot with Bray-curtis cluster analysis were carried out using PRIMER software (Version 7.0.5) (Clarke and Gorley, 2015) for identifying preferred coral species by Drupella cornus and its similarity matrix in different islands. Redundancy analysis (RDA) (Leps and Smilauer, 2003) was also carried out for identifying the correlation between environmental [SST, salinity, chlorophyll-a, and TSM] and biological variables using CANOCO 4.5.
The live coral index is presented in Figure 1. The coral index of the lagoon portion of Chetlet, Kadamat, and Minicoy Islands was “Excellent”. The coral index was between “Fair” and “Poor” in the reef slope region of all the selected Islands. However, in the case of Minicoy Island, three out of five selected study sites of the reef slope region showed an “excellent” coral index. During this extensive reef monitoring of the different Islands of Lakshadweep Archipelago in connection with the health of the reef ecosystem, localized bleaching on the branching form of corals mediated by the corallivorous snail Drupella cornus was observed (Figure 1A). This impact was significant on the species, Pocillopora verrucosa. Supplementary Figure 2 shows the impact of the assemblage of corallivorous Drupella on Pocillopora verrucosa as compared to Acropora sp. Based on the coefficient of determination value (R2 = 0.844), it was found that 84% of the variance in the dependant variable (i.e. Abundance of Drupella cornus) is explained by the derived regression equation (y = 1.6927x + 1.576). A positive correlation between Drupella cornus and Pocillopora verrucosa abundance was observed from the collected data (Supplementary Figure 2C). Moreover, the assemblage of this species was also high in the offshore environment rather than in the lagoon region. Drupella cornus abundance contributed more to the coral Pocillopora verrucosa than to other corals, according to the PCA. In this assessment, a strong variability (PC1: 62.9% variance) was observed between a group of Islands based on the assemblage of Drupella cornus (Figure 2A). The most influencing variable as a negative biological indicator was the impact of Drupella cornus on Pocillopora verrucosa and Acropora sp. in the reef slope region. Chetlet Island is separated from all the other Islands due to the presence of only lagoon sites which resulted in the relatively low occurrence of Drupella cornus on Pocillopora verrucosa. It was also observed that there was a moderate variance (PC2: 29.6% variance) between the group of Islands, and the most influencing variable was the impact of Drupella cornus on Pocillopora verrucosa followed by Acropora sp.
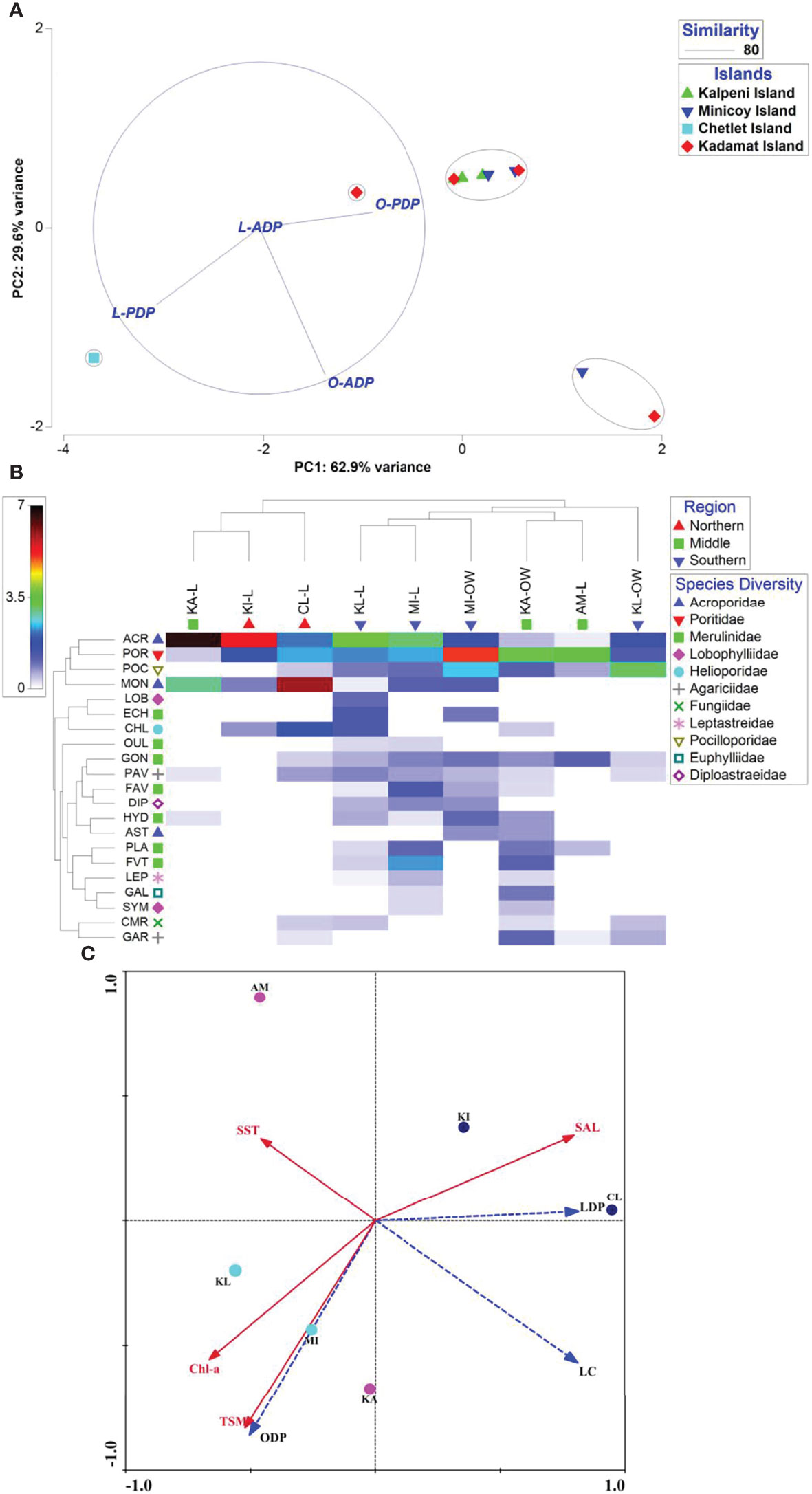
Figure 2 Multivariate analyses. (A) PCA and Bray-curtis cluster analysis based on the abundance of Drupella cornus on coral colonies. L Lagoon region; O Openwater Reef slope region; PDP Drupella cornus on Pocillopora verrucosa; ADP Drupella cornus on Acropora sp.;(B) Shade plot combined with Bray-Curtis cluster analysis showing coral species occurrence with respect to the Islands. MI, Minicoy Is.; CL, Chetlet Is.; KA, Kadamat Is.; AM, Amini Is.; KL, Kalpeni Is.; OW, Openwater Reef slope region; L Lagoon region; ACRAcropora sp.; POR,Porites sp.; GON,Goniastrea sp.; PLA,Platygyra sp.; LOB,Lobophyllia sp.; OUL,Oulophyllia sp.; HYD,Hydnophora sp.; CHL, Coral Heliopora; PAV,Pavona sp.; ECH,Echinopora sp.; FAV,Favia (Dipsastraea) sp.; FVT,Favites sp.; CMR, Coral Mushroom; LEP,Leptastrea sp.; MON,Montipora sp.; DIP,Diploastrea sp.; POC,Pocillopora sp.; GAR,Gardineroseris sp.; GAL,Galaxea sp.; SYM,Symphyllia (Lobophyllia) sp.; AST,Astreopora sp; (C) Redundancy Analysis Triplot visualizing the distribution of live coral cover and Drupella cornus abundance in relation to various environmental variables (SST, salinity, chlorophyll-a and TSM). Red arrows indicate the environmental variables and blue coloured arrows represent the biological variables [live coral cover (LC), Offshore Drupella cornus abundance (ODP) and Lagoon Drupella cornus abundance (LDP)]. The circles indicates respective Islands (MI & KL, Minicoy and Kalpeni Islands of southern part of Lakshadweep; KA & AM, Kadamat and Amini Islands of middle part; CL & KI, Chetlet and Kilthan Islands of Lakshadweep Archipelago).
Bray-curtis cluster analysis under paired linkage (Figure 2A) was also done based on the assemblage of Drupella cornus on Pocillopora verrucosa and Acropora sp. Two major mixed clusters were observed with 80% similarity. Among these clusters, a cluster was formed between the sites, Minicoy and Kadamat Islands due to the occurrence of Drupella cornus on Acropora sp. even though the impact of this snail on Pocillopora verrucosa was very high in this site. Hence, the remaining cluster was formed due to the higher contribution and occurrence of the snail on Pocillopora verrucosa in the reef slope region. On the other hand, due to the lower effect observed in the lagoon region, Chetlet Island was categorized as an outlier. The intensity of the impact of Drupella cornus on Pocillopora verrucosa was observed to be higher than that on Acropora sp. and it was assessed by merging the cluster on the PCA plot (Figure 2A).The PCA (Supplementary Figure 3) of coral species abundance from the reef flat to reef slope regions indicated that there was significant variability (PC1: 32.1% variance) between the lagoon and reef slope regions concerning coral species abundance. The most influencing variable on the reef slope was Pocillopora verrucosa whereas Acropora spp. and Montipora sp. contributed more in the lagoon part. This was interpreted as the reason behind the highest intensity of Drupella cornus observed on the reef slope area. Moreover, there was little variation (PC2: 19.7% variance) between the lagoon part of a cluster of Islands (Chetlet Island, Kadamat Island, and Kilthan Island) and the rest of the study sites of Lakshadweep concerning the intensity of Montipora sp. followed by Heliopora sp. It also indicated their monospecific species occurrence in the lagoon part of Chetlet Island and that there was no impact of Drupella cornus in this site. Shade plot merged with Bray-curtis cluster analysis (Figure 2B) showed the contribution of coral species richness for Islands and benthic substrate (Reef slope and Lagoon). It perfectly supports PCA that the impacted colony, Pocillopora verrucosa on a greater intensity in the reef slope where as Acropora spp. and Montipora sp. in the lagoonal environment. More over, there were three complete clusters formed based on the coral species diversity. One formed in the Southern region irrespective of benthic substrates with 54.56% similarity; the second formed in the middle region with 56.09% similarity; the third one formed completely on the lagoon portion of the northern and middle region with 47.37% similarity (Figure 2B).In addition to the impact of indicator species, the relationship between the environmental (SST, salinity, chlorophyll-a, and TSM) and biological variables were also studied and presented as RDA triplots (Figure 2C). Arrows indicating in the same direction represent a high positive correlation between those variables, whereas variables with negative correlation are represented by arrows oriented in opposite direction. SST plays a significant negative role in live coral cover. TSM and Chlorophyll-a also slightly influenced the coral cover of the Lakshadweep Archipelago. The impact and assemblage of Drupella cornus [Offshore abundance] were positively correlated towards the reef slope region of the southern part of the Lakshadweep Archipelago followed by the middle part (Kadamat Island). Based on the coefficient of determination value, a negative correlation (r = -0.11) of the mean percentage of live coral cover with the abundance of Drupella cornus is observed from the collected data. There was no significant variation in pH observed between Southern (7.9 to 8.35), Northern regions (7.9 to 8.4) and central region (7.99 to 8.32) of the Lakshadweep Archipelago.
In recent years, Drupella has been considered a marine organism that promotes coral reef degradation. This is the first study to report the localized bleaching impact caused by Drupella cornus in the Lakshadweep Archipelago. Until 2009, the population of Drupella (Drupella cornus, Drupella fragum, and Drupella rugosa) were reported only in three major coastal regions viz., Japan, northern Red Sea, and Ningaloo Reef of Western Australia (Ayling, 2000; Cumming, 2009). In addition, D. eburnea and D. minuta have also been recorded on the Japanese coast (Tsuchiya, 1992). Cumming (2009) also reported such assemblages of Drupella spp. as a normal population (density of 0-2 per m2), but recently Koido et al. (2017) recorded its assemblage as an outbreak, by collecting 13.8 kg of Drupella spp. off the coast of Cape Toi, Miyazaki, Japan. Al-Sofyani et al. (2014) also reported its impact on the coral reef ecosystem of the Red Sea during their spatial assessment of different growth forms of Scleractinian and non-Scleractinian corals. Recently, the population has considerably declined after a bleaching event (Saponari et al., 2021) that happened near the reef habitat of Maldives. This impact was significant on the species, Pocillopora verrucosa. Globally, Drupella spp. has expanded its geographical range and posed a serious threat to the coral reef ecosystem worldwide.
During the present large-scale benthic reef monitoring near different Islands of the Lakshadweep Archipelago (Minicoy, Kalpeni, Kilthan, Amini, Kadamat and Chetlet Islands), while studying the health of the reef ecosystem, localized bleaching was observed in some coral colonies that were affected by the corallivorous gastropod, Drupella cornus. It was observed only in the coral species Pocillopora verrucosa. It is inferred that the bleaching effect (Baird, 1999) in the coral colonies is due to the loss of symbiotic algae, i.e., zooxanthellae, as Drupella cornus consume them as food. Information is lacking on the species description and the interaction of Drupella cornus on coral species from the Indian coastal region. Marimuthu and Tripathy (2018) reported this species for the first time as a biological indicator of the coral reef habitat of Lakshadweep Archipelago. Antonius and Riegl (1997) reported the outbreak of coral disease caused by this species. In the present study, this outbreak was observed in the reef slope area of the Lakshadweep Archipelago.
Ayling (2000) also correlated the outbreak of this indicator organism on the reef habitat with the result of the impact of various natural and anthropogenic factors. Rosenau et al. (2021) pointed out that commercial vessels are considered a potential vector for coral habitat degradation through coral tissue loss. Drupella assemblage may impact reef resilience in association with SST-induced mortality, coral diseases, pollution, and other corallivores (Saponari et al., 2021). Similarly, a redundancy analysis of the temporal data of physical parameters showed SST mediated less coral cover along the coast of Lakshadweep Islands.
Al-Horani et al. (2011) found that the branching forms such as Acropora sp. and Stylophora sp. were occupied by Drupella cornus during their studies on prey selection and feeding rates on corals in the Gulf of Aquaba, Red Sea. On the other hand, Pocillopora sp. was reported in other parts of the Red Sea coast (Al-Sofyani et al., 2014). Similarly, it was observed that Pocillopora verrucosa was dominantly chosen as prey by Drupella cornus in the Lakshadweep group of Islands. Regression analysis also confirmed that a significant positive correlation exists between the abundance of Drupella cornus and Pocillopora verrucosa. In the case of the Gulf of Mannar, the corals of Acroporidae, Acropora muricata, A. cytherea, and Montipora sp. were infested with Drupella cornus at Shingle Island and Vaan Island (Raj et al., 2016). Hence, it can be inferred that there is a close relationship of the Drupella populations between the Lakshadweep Archipelago and the Red Sea (Al-Sofyani et al., 2014) because of similar prey preferences (Pocillopora sp.).
The spatial variability of the abundance of negative biological indicator, Drupella cornus was assessed to find out their assemblage and preferred coral species in the benthic environment of Lakshadweep Archipelago. Pocillopora verrucosa was identified as an impacted/preferred coral species during the study period. The dataset on the coral species diversity, interpretation of the impact of Drupella cornus, and physical variables on the coral diversity through multivariate analyses would be helpful for marine managers to study the present status of reef habitats and indicator species of the tropical environments.
Conceived the study, survey, and multivariate analyses: NM; Ecological interpretation and review: NM, JW, and AAA; Wrote the manuscript: NM and AK. All authors contributed to the article and approved the submitted version.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
There are no known sources of funding to pay for article processing fees. The payment toward article processing fees is to be borne by the author(s) only.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are thankful to the Directors, Zoological Survey of India; National Centre for Sustainable Coastal Management (all under the Ministry of Environment, Forest and Climate Change, Government of India) for the materials and facilities provided to conduct this study. The authors are also thankful to the ESRI for providing the Digital Globe imagery for map preparation. NM would like to thank the Lakshadweep Administration and Department of Environment and Forests, Union Territory of Lakshadweep for the entry permit and logistic support to carry out the study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.914240/full#supplementary-material
Al-Horani F. A., Hamdi M., Al-Rousan S. A.(2011)Prey Selection andFeeding Rates of Drupella Cornus (Gastropoda: Muricidae) on Corals From the Jordanian Coast of the Gulf of Aqaba, Red Sea. Jordan. J. Biol. Sci. 4 (4), 191–198. https://jjbs.hu.edu.jo/FILES/v4n4/Paper%20Number%201.pdf
Al-Sofyani A. A., Marimuthu N., Wilson J. J. (2014). A Rapid Assessment of Scleractinian and Non-Scleractinian Coral Growth Forms Along the Saudi Arabian Coast, Red Sea. J. Ocean. Univ. China. 13 (2), 243–248. doi: 10.1007/s11802-014-2308-z
Antonius A., Riegl B. (1997). A Possible Link Between Coral Diseases and a Corallivorous Snail (Drupella Cornus) Outbreak in the Red Sea. Atoll. Res. Bull. 443-449, 1–9. doi: 10.5479/si.00775630.447.1
ARMDES (1996). AIMS Reef Monitoring Data Entry System; Version 1.6 Long Term Reef Monitoring Project (Townsville: Australian Institute of Marine Science).
Ayling A. M. (2000). “The Effects of Drupella Spp,” in Grazing on Coral Reefs in Australia. Available at: http://www.SeareSearch.com.au/reports/drupella/drupella.html.
Baird A. (1999). A Large Aggregation of Drupella Rugosa Following the Mass Bleaching of Corals on the Great Barrier Reef. Reef. Res. 9 (2), 6–7.
Birkeland C., Lucas J. S. (1990). Acanthaster Planci: Major Management Problem of Coral Reefs (Boca Raton, FL: CRC press),1990 272 p.
Brander L. M., Beukering P. J. H. V., Cesar H. S. J. (2007). The Recreational Value of Coral Reefs: A Meta-Analysis. Ecol. Econ. 63 (1), 209–218. doi: 10.1016/j.ecolecon.2006.11.002
Cesar H. S. J., Beukering P. J. H. V (2004). Economic Valuation of the Coral Reefs of Hawaii. Pacific. Sci. 58 (2), 231–242. doi: 10.1353/psc.2004.0014
Clarke K. R., Gorley R. N. (2015). PRIMER V7. PRIMER-E. Ltd., Devon PL21 9RH, UK. http://updates.primer-e.com/primer7/manuals/User_manual_v7a.pdf
Connor S. E., van Leeuwen J. F., Rittenour T. M., van der Knaap W. O., Ammann B., Björck S. (2012). The Ecological Impact of Oceanic Island Colonization—a Palaeoecological Perspective From the Azores. J. Biogeogr. 39, 1007–1023. doi: 10.1111/j.1365-2699.2011.02671.x
Cumming R. L. (2009). Population Outbreaks and Large Aggregations of Drupella on the Great Barrier Reef (Townsville QLD: ReSearch publication No. 96. Great Barrier Reef Marine Park Authority), 26p. Available at: http://elibrary.gbrmpa.gov.au/jspui/bitstream/11017/437/1/Population-outbreaks-large-aggregations-drupella-GBR.pdf.
English S., Wilkinson C., Baker V. (1997). Survey Manual for Tropical Marine Resource (Townsville: Australian Institute of Marine Science), 390 p. Available at: https://www.aims.gov.au/sites/default/files/Survey%20Manual-sm01.pdf.
ESSO-INCOIS (2022) Live Access Server. Available at: http://las.incois.gov.in/las/UI.vm# (Accessed 02.05.2022).
Fabricius K. E., Okaji K., De’ath G. (2010). Three Lines of Evidence to Link Outbreaks of the Crown-of-Thorns Seastar, Acanthaster Planci to the Release of Larval Food Limitation. Coral. Reefs. 29, 593–605. doi: 10.1007/s00338-010-0628-z
Jha D. K., Vinithkumar N. V., Marimuthu N., Baskar B., Sahu B. K., Das A. K., et al. (2013). Field and GIS Based Post-Tsunami Assessment of Scleractinian Coral Cover in the Aerial Bay Group of Islands, North Andaman, India. J. Coast. Conserv. 17, 671–677. doi: 10.1007/s11852-013-0266-z
Jha D. K., Vinithkumar N. V., Sahu B. K., Dheenan P. S., Das A. K., Mehmuna B., et al. (2015). Multivariate and Geo-Spatial Approach for Seawater Quality of Chidiyatappu Bay, South Andaman Islands, India. Mar. pollut. Bull. 96 (1–2), 463–470. doi: 10.1016/j.marpolbul.2015.05.004
Jha D. K., Vinithkumar N. V., Santhanakumar J., Nazar A., Kirubagaran R. (2011). Post Tsunami Coral Reef Resource Assessment in Pongi Balu Coast, South Andaman Island. Curr. Sci. 100 (4), 530–534. https://www.currentscience.ac.in/Volumes/100/04/0530.pdf
Koido T., Oku Y., Fucuda M., Nakano A., Fukami H. (2017). Drupella Outbreak in a Large Coral Community Off the Coast of Cape Toi, Miyazaki, Japan. Galaxea. J. Coral. Reef. Stud. 19, 31–32. doi: 10.3755/galaxea.19.1_31
Kumaraguru A. K., Jeyakumar K., Wilson J. J., Ramakritinan C. M. (2005). Impact of the Tsunami of 26 December 2004 on the Coral Reef Environment of Gulf of Mannar and Palk Bay in the Southeast Coast of India. Curr. Sci. 89 (10), 1729–1741. https://www.currentscience.ac.in/Volumes/89/10/1729.pdf
Leps J., Smilauer P. (2003). Multivariate Analysis of Ecological Data Using C.A.N.O.C.O. Cambridge University Press, Cambridge, CB2 8EA.
Linnaeus C. (1758). Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata [10th revised edition], vol. 1: 824 pp.
Marimuthu N., Tripathy B. (2018). First Report of Drupella Cornus Röding, 1978 (Gastropoda: Muricidae), a Biological Indicator of Coral Reef Habitat of Lakshadweep Archipelago, India. Rec. zool. Surv. India. 118 (1), 97–99. doi: 10.26515/rzsi/v118/i1/2018/122305
Mohamed A. R., Aham A., Abdel-Salam H. A. (2012). “Status of Coral Reef Health in the Northern Red Sea, Egypt,” in Proc 12th International Coral Reef Symposium, Cairns, Australia. Available at: (eds.) Yellowlees D., Hughes T. P. ., ARC Centre of Excellence for Coral Reef Studies James Cook University Townsville, Queensland, Australia.,
Murdoch T. J. T., Murdoch J. M. H. (2016). “Baseline Condition of the Coral Reefs and Fishes Across Three Depth Zones of the Fore Reef of Bermuda,” in BREAM: Bermuda Reef Ecosystem Analysis and Monitoring Programme Report (Flatts Bermuda: Bermuda Zoological Society), BBP–2016-237.
Nelson N. (2009). Acanthaster planci population dynamics on Nyange Reef, Zanzibar, Tanzania: Implications for resource management and economic sustainability. Independent Study Project, Knox College Department of Biology; University of Dar es Salaam Institute of Marine Science, Mji Mkongwe, Unguja, Tanzania.
Raj K. D., Aeby G. S., Mathews G., Bharath M. S., Rajesh S., Laju R. L., Aeby G. S, et al. (2016). “Patterns in the abundance of Fish and Snail Corallivores to an Outbreak of Acute Tissue Loss Disease on the Reefs of Vaan Island in the Gulf of Mannar, India,” in Proc 13th International Coral Reef symposium, (eds.) . Hawaii Institute of Marine Biology, University of Hawaii HI 96744 USA.,
Ravindran J., Manikandan B., Venkatesh M., Murali R. M., Marimuthu N., Wafar M. V. M. (2014). Repercussions of Embarkation Wharves in Lakshadweep Islands on Coral Communities and Their Ecology. Ind. J. Geo-Mar. Sci. 43 (7), 1391–1400. http://nopr.niscair.res.in/bitstream/123456789/34459/1/IJMS%2043%287%29%201391-1400.pdf
Röding P. F. (1798). Museum Boltenianum sive Catalogus cimeliorum e tribus regnis naturæ quæ olim collegerat Joa. Fried Bolten, M. D. p. d. per XL. annos proto physicus Hamburgensis. Pars secunda continens Conchylia sive Testacea univalvia, bivalvia & multivalvia. Trapp, Hamburg. viii, 199 pp.
Rosenau N. A., Gignoux-Wolfsohn S., Everett R. A., Miller A. W., Minton M. S., Ruiz G. M. (2021). Considering Commercial Vessels as Potential Vectors of Stony Coral Tissue Loss DiSease. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.709764
Sahu B. K., Begum M., Khadanga M. K., Jha D. K., Vinithkumar N. V., Kirubagaran R. (2013). Evaluation of Significant Sources Influencing the Variation of Physico-Chemical Parameters in Port Blair Bay, South Andaman, India by Using Multivariate Statistics. Mar. pollut. Bull. 66, 246–251. doi: 10.1016/j.marpolbul.2012.09.021
Saponari L., Dehnert I., Galli P., Montano S. (2021). Assessing Population Collapse of Drupella Spp. (Mollusca: Gastropoda) 2 Years After a Coral Bleaching Event in the Republic of Maldives. Hydrobiologia. 848, 2653–2666. doi: 10.1007/s10750-021-04546-5
Wilson J. J., Marimuthu N., Kumaraguru A. K. (2005) Sedimentation of silt in the coral reef environment of southeast coast of India. J Mar Biol Ass India. 47 (1), 83-87.
Keywords: corals, coral reef, corallivorous snail, Drupella cornus, biological indicator, multivariate analyses
Citation: Marimuthu N, Wilson JJ, Al-Sofyani AA and Kumaraguru AK (2022) Coral Reef Health Status versus Muricid Bioindicator in the Lakshadweep Archipelago – A Multivariate Approach. Front. Mar. Sci. 9:914240. doi: 10.3389/fmars.2022.914240
Received: 06 April 2022; Accepted: 06 June 2022;
Published: 13 July 2022.
Edited by:
Dilip Kumar Jha, National Institute of Ocean Technology, IndiaReviewed by:
Pankaj Verma, National Institute of Ocean Technology, IndiaCopyright © 2022 Marimuthu, Wilson, Al-Sofyani and Kumaraguru. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nithyanandam Marimuthu, bWFyaW11dGh1QHpzaS5nb3YuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.