- 1CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 2Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
- 3Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao, China
- 4College of Marine Science, University of Chinese Academy of Sciences, Qingdao, China
Understanding the life history strategy of organisms is key to predicting their population dynamics. The population of scyphozoan jellyfish has displayed an increasing trend in recent decades, yet its life history strategy is not fully understood. To interpret the reproduction strategy of scyphozoan jellyfish from an evolutionary ecology perspective, we dissected 10 asexual generations of Aurelia coerulea polyps to investigate the relationships between transgenerational effects on their budding reproduction and strobilation. Our results reveal that a polyp’s average budding reproduction rate declined 32.82% through asexual generations within the experimental time. Furthermore, a longer culture duration counteracted the transgenerational effects on budding rates and strobilation afterward. Thus, this effort provides insight into the necessity of sexual reproduction in organisms involving a metagenic life cycle, i.e., to renew the asexual reproduction ability of a population. Besides this, we suggest taking note that it is necessary to know the “asexual age” of polyps when performing experimental studies and mathematical modeling to explore their population dynamics. Our results also present a valuable data set to interpret the evolution of the scyphozoan jellyfish’s life history strategy under multifactorial environments.
Introduction
Life history strategies of organisms have important implications from both evolutionary and demographic points of view; therefore, they have attracted many biologists’ attention (e.g., Partridge and Harvey, 1988; Llodra, 2002; Biro and Stamps, 2008). Reproduction is a key life history trait that determines the survival of a species that allows parental generation to produce offspring sexually or/and asexually (Combosch and Vollmer, 2013; Subramoniam, 2018). It plays a critical role in regulating an organism’s population dynamic and genetic inheritance.
Many aquatic invertebrates are capable of both sexual and asexual reproduction in their life history (Subramoniam, 2018). The moon jellyfish Aurelia spp. are cosmopolitan and common bloom-forming species, yet its reproductive strategy is not fully understood. In recent decades, jellyfish have drawn increasing attention in the light of global warming because of their occasional aggregations that have caused significant ecological and socioeconomic impacts (Condon et al., 2013; Tiller et al., 2017; Fuentes et al., 2018). The moon jellyfish exhibits a metagenic life cycle (Arai, 1997), involving a benthic polyp stage and a pelagic medusa stage (Figure 1A). The metagenic life cycle makes Aurelia spp. model organisms gain insights into the evolutionary development of its life history strategy. Yet the prediction of the rise and fall of jellyfish outbreaks remains challenging, regarding which attributes pertain to their complex life history. Though many efforts have been spent on explaining their population fluctuation, most studies examine polyps in response to environmental cues in a single or unknown asexual generation (e.g., Schiariti et al., 2015; Hubot et al., 2017; Treible and Condon, 2019). In addition, the modeling prediction often assumes polyps reproduce asexually at a constant rate along with time and through different asexual generations (e.g., Hočevar et al., 2018). To date, transgenerational effects are barely explored in scyphozoan polyps. But, in a recent study, transgenerational acclimation was found that may benefit invasive jellyfish species by facilitating budding reproduction under experimental conditions (Lu et al., 2020). Nevertheless, the long-term transgenerational effects on the asexual reproduction of polyps have not been explored.
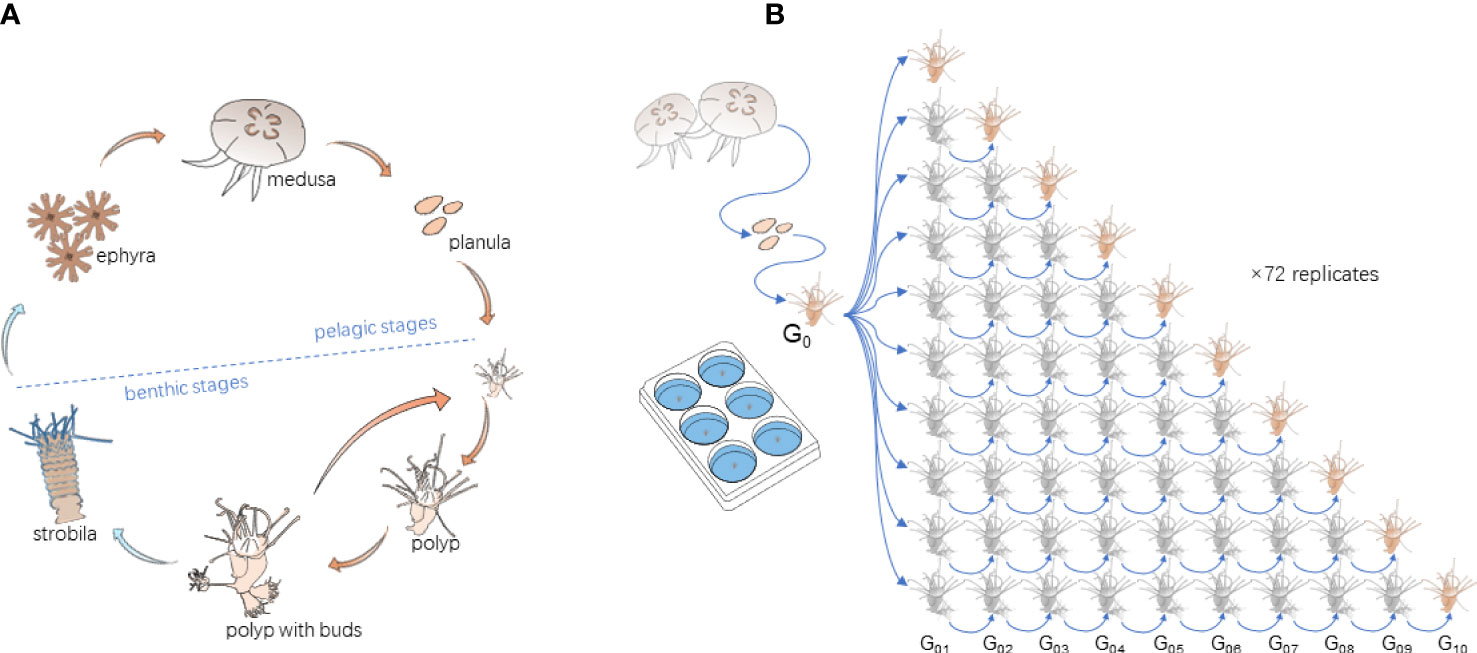
Figure 1 (A) Life cycle of Aurelia spp. and (B) the experimental design, consisting of 10 asexual generations and 72 replicates. Each experimental unit contained one polyp.
Besides the transgenerational effects on polyp population dynamics, getting insight into the energy allocation and shifts between different reproduction modes is a comprehensive understanding of their life history (Lucas, 2001). Strobilation that leads to ephyra production is a benthic-pelagic coupling process in the moon jellyfish’s life cycle. It is the key process of phase transition from the benthic polyp to the pelagic medusa stage and shifts to sexual reproduction afterward. The success of strobilation directly determines the recruitment of the medusae population (Lucas et al., 2012). From an evolution and ecology perspective, the shifting from budding reproduction to strobilation in the moon jellyfish is allowing polyps to escape the unfavorable environmental conditions to expand their habitat and improve genetic diversity through sexual reproduction (Sun, 2012; Gerber et al., 2018). However, less attention has been paid to understanding the transgenerational effects on energy allocation and mode shifting during this process. It is noteworthy that, even in the same treatment of an experimental study, usually not all of the polyps can strobilate (e.g., Treible and Condon, 2019; Loveridge et al., 2021). Similarly, in a field polyp population, strobilation observed about a 63% shift to the pelagic stage via strobilation (Purcell et al., 2009). As the polyps were cultured or lived under the same environmental conditions, the potential transgenerational difference draws our attention and might determine which polyp could strobilate and enter its pelagic stage.
In this study, we aim to interpret the life history strategy of scyphozoan jellyfish from an evolutionary ecology perspective. To dissect the relationships between transgenerational effects and reproduction traits, we capitalize on polyps of the moon jellyfish Aurelia coerulea in 10 asexual generations under the microcosm of experimental conditions. The experiment lasted for 56 weeks, and it was conducted under three mimicked temperatures and excess food conditions. This effort provides unique insight into the understanding of asexual reproduction as well as the trade-offs in life history strategy in scyphozoan jellyfish.
Materials and methods
Study organism
Polyps used in this experiment originated from matured medusae that were collected in August 2019 from the pier of Yangma Island, China (37°26’43” N, 121°34’27” E). Planula larvae collected from the oral arms of several ripe female medusae were mixed. Every 20 planula individuals were randomly allocated to each well of 12 pieces of six-well polycarbonate culture plates, thus making up 72 replicates. All the research units were filled with filtered (20 μm) seawater. All plates were maintained at 20°C ± 1°C under darkness and ensured by a temperature-controlled incubator for a week. During this period, no water exchange and no feeding were performed. When planula larvae metamorphosed into polyps, only the single most healthy polyp was left in each research unit, and others were removed using forceps. The remaining polyps in the experimental units were treated as the mother polyps (G0). They were maintained in the initial conditions at 20°C and fed newly hatched Artemia sp. nauplii in excess three times a week. The seawater was renewed before each feeding.
Experimental setup
We defined the “asexual generation” of polyps for the experiment in the case of budding reproduction. When a new bud was generated by vegetative budding, stolon formation, or pedal laceration, etc., it was treated as the offspring of the mother polyp. We cultured 10 asexual generations of each polyp to test their reproduction traits. To keep their same genetic background, 10 asexual generations of polyps in each replicate originated from the same mother polyp. To achieve the target, the mother polyps were cultured for two weeks till more than 10 buds were generated. The 10 most healthy buds confirmed to be the first asexual generation (G01) of the mother polyp were selected and randomly transferred to each of 10 prepared experimental units. One of them was labeled as G01, and its reproduction performance was quantified and recorded from the time when it was transferred. The other units were labeled as G02 to G10. To achieve the other target asexual generations, when a new bud was generated, the old one was removed. The process was repeated till the newly generated polyp reached the aimed generation (Figure 1B). For example, for the G02 group, when the polyp generated a new bud, the old one (G01) was removed, and the asexually reproduced new bud made the target G02 polyp and so forth for the other generations. Their reproduction data were collected when each targeted generation was achieved. The procedure was replicated 72 times, thus resulting in 720 research units in total (Figure 1B).
The experiment consisted of three temperature levels, 20°C, 13°C, and 5°C, representing the common summer, late autumn/early spring, and winter temperatures in the study area, respectively. The chosen temperatures fit the polyp’s budding, strobilation, and overwintering tolerance range (Chi et al., 2019). The target temperatures were achieved by increasing or decreasing 1°C d-1 from the previous temperature, following the temperature variation protocol as follows (Figure 2A). After most polyps (more than 70%) in the G10 group achieved the target asexual generation, polyps were cultured at 20°C for eight more weeks for collecting their budding reproduction data. Then, the temperature was decreased and increased to 13°C to mimic the temperature variation before and after overwintering (5°C). The final temperature was raised to 20°C to represent the average temperature condition in the next summer. During the experiment, polyps were fed with newly hatched Artemia nauplii in excess of three times a week. The research units were cleaned, and the water was renewed with filtered (20 μm) seawater maintained in the same incubator before each feeding. The experiment lasted for 56 weeks (w01~w56).
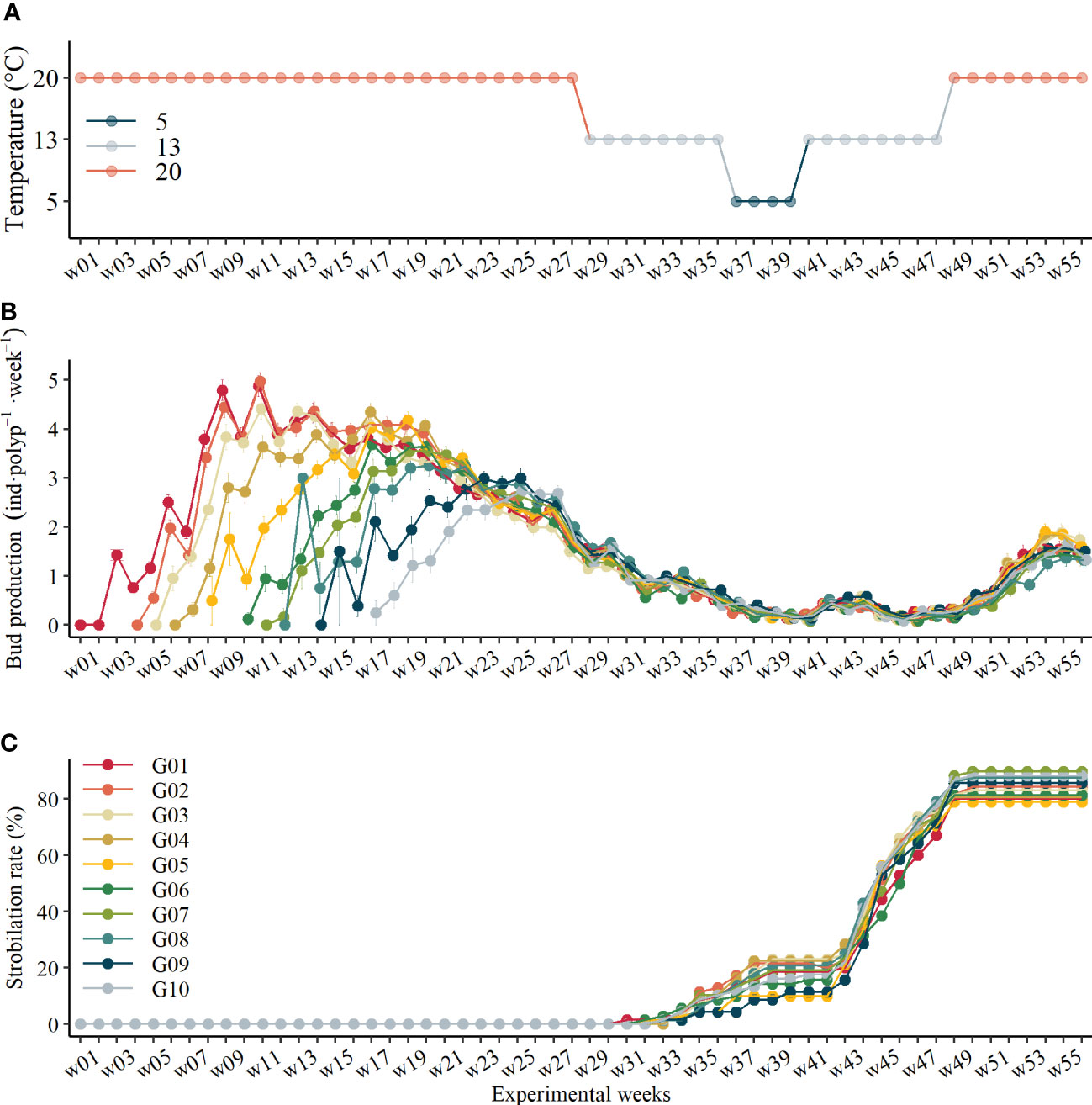
Figure 2 (A) Time series of temperature change protocol, (B) bud production rate variations of polyps in each asexual generation over time (mean ± se), and (C) accumulative strobilation rate during the experiment.
Data collection
Each polyp’s status was checked before each water exchange and feeding performance; polyps that had decomposed were recorded as dead, and their data were not included in the analyses. Reproduction data were collected once a week. When signs of strobilation occurred, they were recognized as strobilae. Strobilation rates were calculated based on the number of vital surviving polyps. Ephyra production of each strobila was also recorded and quantified.
Statistical analysis
Data were checked for outliers, homogeneity, and collinearity in both response and explanatory variables before applying statistical models (Zuur et al., 2010). Following our research aims and hypotheses, generalized linear models (GLMs) were used to analyze the response variables, namely, survival, budding reproduction, strobilation rate, and ephyra production, respectively. The explanatory variables, transgeneration, temperature, and experimental time were included in the GLMs. Optimum models were selected by applying a stepwise backward method via an Akaike information criterion index (Zuur et al., 2009). The count data, including bud and ephyra production, were fitted in a Poisson family distribution, and survival rate and strobilation rate were fitted in a binomial family distribution. All data visualizations and statistical analyses in this study were conducted using R software (R Core Team, 2021).
Results
Time series of budding reproduction and strobilation overview
At the end of this experiment, 29 out of 720 (c.a. 4%) polyps died, and the final survival rate did not differ in transgenerations (GLM, χ2 = 15.42, df = 9, p = .08).
In general, budding reproduction was affected significantly by polyps’ asexual transgeneration, temperature, and experimental time (Table 1). Interestingly, the asexual transgeneration effect on budding reproduction at 20°C has not been detected since experimental time w23 (GLM, χ2 = 6.95, df = 9, p = .64, Figure 2B) nor when the temperature changed afterward. At 20°C, there was a steady increase in budding reproduction rates (BRR) of all polyps before they reach the maximum values; after that, their reproduction rates dropped along with time (Figure 2B). The BRR declined to similar values (1.75 ± 1.18 inds·polyp-1·week-1, mean ± sd and thereafter) at the end of the first 20°C period (w28, Figure 2B). After that, the BRR of polyps did not differ between asexual generations but varied with temperature changes (Figure 2B). Following the temperature decrease, BRR continuously declined during the mimicked late autumn (the first 13°C) and overwintering (5°C) period. It is noteworthy that the BRR followed a bell-shaped line during the mimicked spring (the second 13°C) period. The BRR reached the minimum values at the beginning and the end of the second 13°C period at experimental time w40 and w46 with the minimum values of 0.074 ± 0.032 inds·polyp-1·week-1. When the temperature was raised to 20°C again, the BRR recovered to similar values at the end of the first 20°C periods, but they cannot reach the previous maximum values during another 8 weeks of culture (Figure 2B).
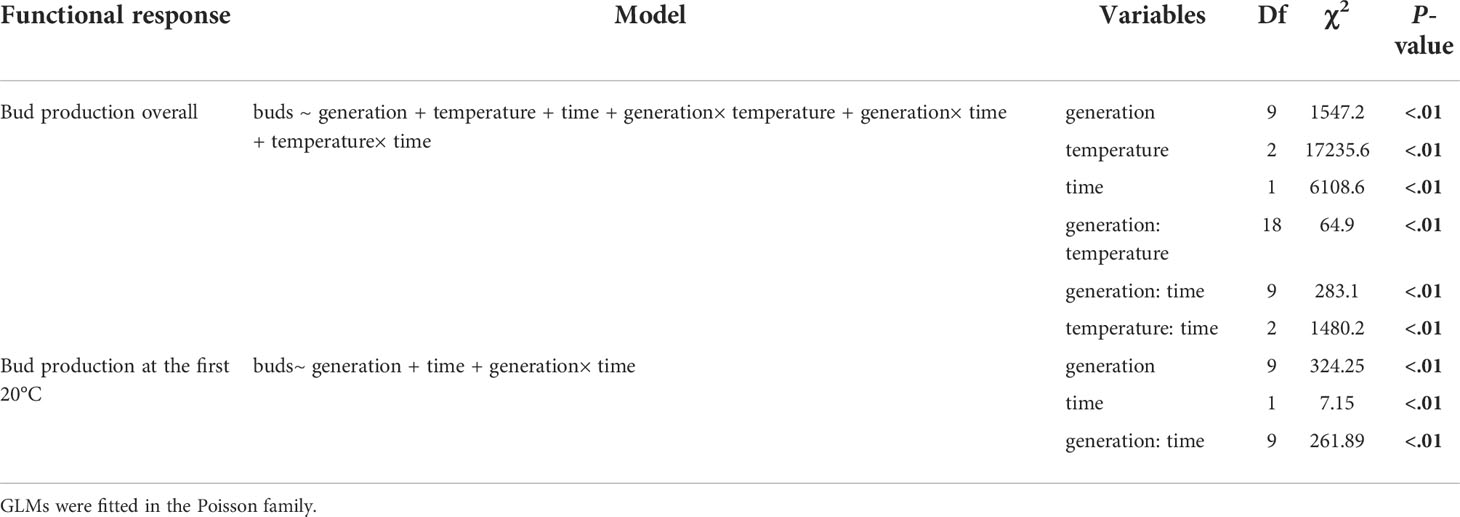
Table 1 Statistical outputs from GLMs selected by a stepwise backward method on budding reproduction overall and during the first 20°C period.
Strobilation of polyps occurred 2~3 weeks after the temperature shifted to 13°C before and after overwintering (5°C). About 14.6% of polyps strobilated during the first 13°C period before overwintering, and 83.2% of polyps strobilated in total at the end of the experiment, among which 8.7% of polyps strobilated twice. Asexual transgeneration was not found to significantly affect strobilation rate (GLM, χ2 = 6.67, df = 9, p = .67).
Effects of transgeneration on budding reproduction
To compare bud production rates between asexual generations, we standardized the starting time of each generation from the time when the target asexual generations were achieved. As Figure 3A shows, there is a significant difference in BRR of polyps through asexual generation and over time (Table 1). The BRR of all polyps in each asexual generation followed a bell-shaped curve, which increased once they were generated and decreased after they reached maximum values. As shown in Figure 3B, the maximum BRR decreased through asexual generations from G01 to G10 (GLM, χ2 = 86.3, df = 9, p <.01). Besides this, when comparing the average BRR during the first 20°C periods (Figure 3C), it generally declined with generations (GLM, χ2 = 27.13, df = 9, p <.01) except for the first generation. The average BRR declined from 3.23 ± 0.60 inds·polyp-1·week-1 in G02 to 2.17 ± 0.80 inds·polyp-1·week-1 in G10, which dropped 32.82% during the 20°C period.
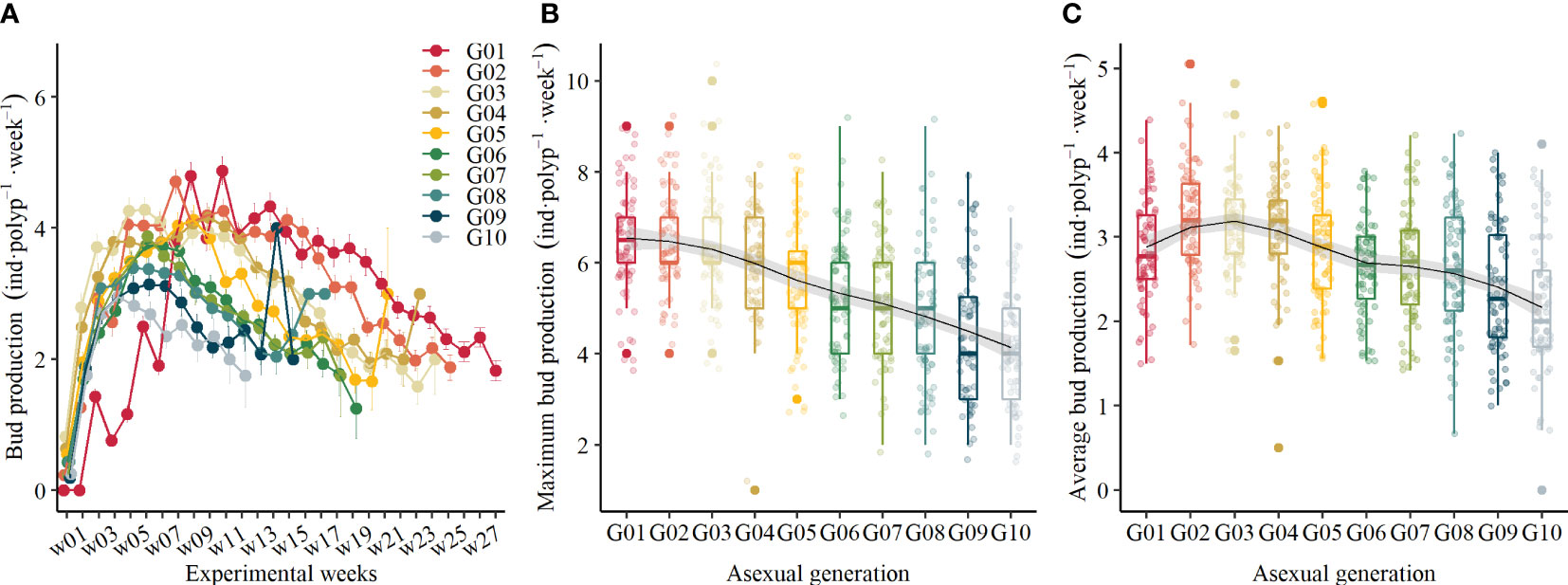
Figure 3 (A) Standardized bud production of polyps in 10 different asexual generations over time (mean ± se), (B) the maximum bud production in different asexual generations, and (C) the average bud production in different asexual generations.
The BRR finally declined to 0.55 ± 0.66 inds·polyp-1·week-1 when the temperature decreased to 13°C (Figure 4A), and it was not significantly different through transgenerations (GLM, χ2 = 3.04, df = 9, p = .96). During the overwintering period, the average BRR ranged from 0.213 ± 0.029 inds·polyp-1·week-1 to 0.30 ± 0.028 inds·polyp-1·week-1 (Figure 4B, GLM, χ2 = 1.59, df = 9, p = .996). When the temperature increased to 13°C again, the average BRR ranged from 0.246 ± 0.025 inds·polyp-1·week-1 to 0.341 ± 0.024 inds·polyp-1·week-1 (Figure 4C, GLM, χ2 = 2.11, df = 9, p = .99). When the temperature increased to 20°C, which mimicked the next summer, the BRR did not recover the maximum values during the first 20°C periods. It varied between 0.84 ± 0.087 inds·polyp-1·week-1 and 1.14 ± 0.088 inds·polyp-1·week-1 (Figure 4D), which was not significantly different between asexual generations (GLM, χ2 = 7.71, df = 9, p = .56).
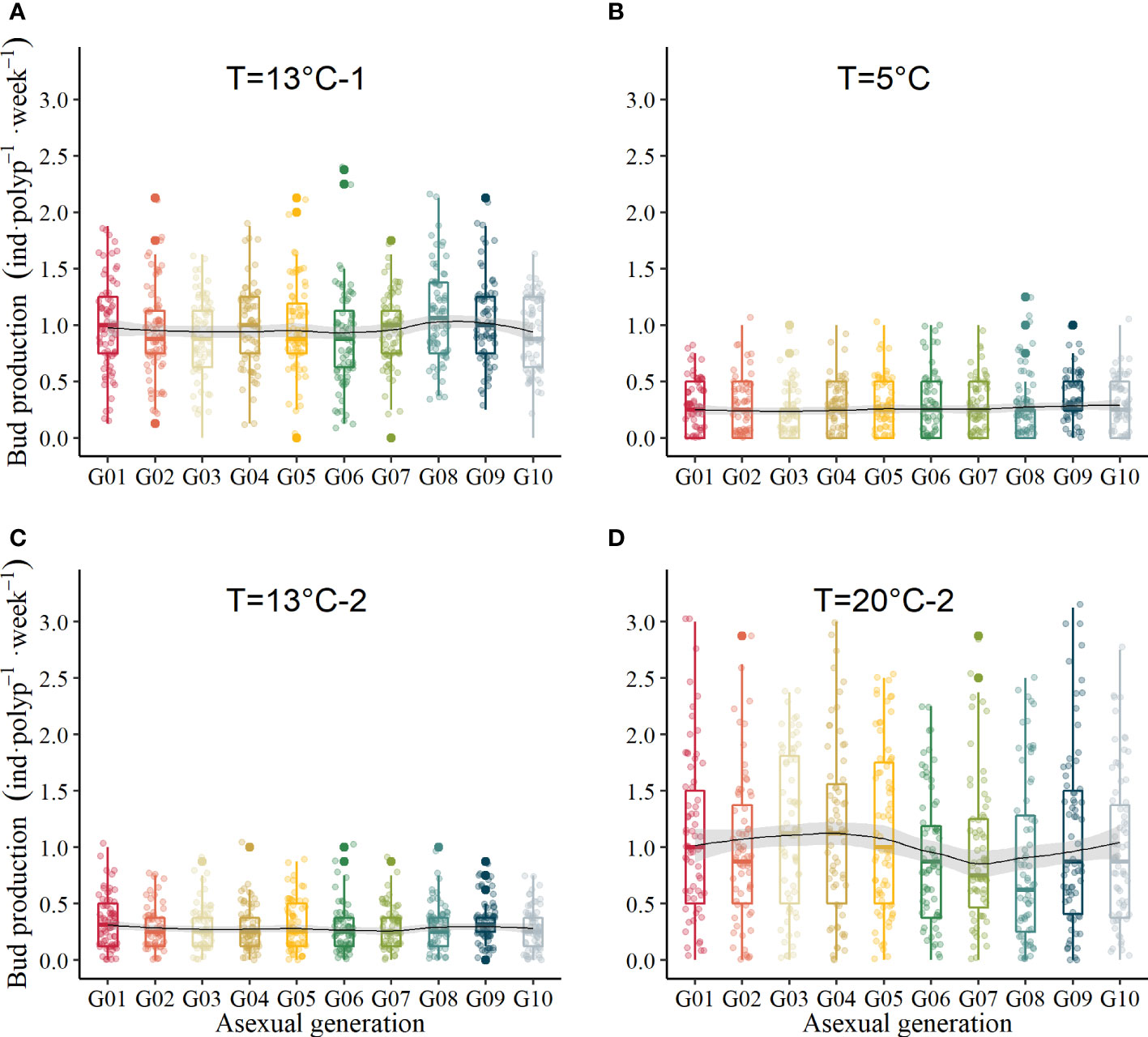
Figure 4 Bud production with temperature variation. (A) temperature at the first 13°C in mimicked autumn, (B) temperature during overwintering at 5°C, (C) temperature at the second 13°C in mimicked spring, (D) temperature at the second 20°C in mimicked the next summer.
At the end of this experiment, the G01 polyps produced the most buds with 99.93 ± 21.9 inds·polyp-1 on average, and the G10 polyps produced the least buds with 37.94 ± 12.53 inds·polyp-1 on average.
Effects of transgeneration on strobilation
In this study, strobilation occurred twice, i.e., before and after overwintering (Figure 2C). Polyps started strobilation 3 weeks after the temperature decreased to 13°C before overwintering and 2 weeks after the temperature increased to 13°C when going through overwintering.
As presented above, the strobilation rate was higher in the second strobilation than that in the first strobilation. Strobilation was paused during overwintering (5°C) and stopped 2 weeks after the temperature increased to 20°C. At the end of the experiment, ephyra production of each strobila was 15.86 ± 6.49 inds·polyp-1 on average. However, the fate of strobila was different in the two strobilation periods. From our observation, there was a strong trend of deformities when strobila experience an extremely low temperature of 5°C, whereas most strobilae can finish strobilation and release ephyrae successfully when the temperature increased from 13°C to 20°C. Interestingly, we also observed that several polyps that just showed signs of strobilation at the final 13°C period shifted to polyp mode when the temperature increased to 20°C, rather than metamorphosing into strobilae.
Discussion
The benthic polyp is recognized to be the key life stage regulating the population dynamics of scyphozoan jellyfish in the field (Lucas et al., 2012; Fuchs et al., 2014). Our results highlight the transgenerational effects on the asexual reproduction strategy of polyps. It reveals that budding reproduction rates of polyps declined through transgeneration and along with time. However, polyps from different asexual generations ended with similar reproduction rates after a long-run experimental culture. Although transgenerational effects were not detected significantly affecting strobilation in the current study, we suggest further in situ studies be performed to verify polyps’ reproduction strategy under fluctuating and multifactorial environmental conditions.
Transgenerational effects on asexual reproduction of scyphozoan jellyfish
Budding is one of the asexual reproduction modes of many invertebrates (Molnar and Gair, 2015). Temperature and food conditions were recognized as the main environmental factors regulating bud production of scyphozoan jellyfish (Amorim et al., 2018; Treible and Condon, 2019; Loveridge et al., 2021). Besides environmental factors, our results highlight the importance of transgenerational effects on budding reproduction and the variable budding reproduction rates along with time. Given the moderate temperature and excess food supply conditions, the budding reproduction of jellyfish polyps varies in two dimensions. First, the mother polyps and their daughter polyps are not equal; their budding reproduction rates declined through asexual transgeneration. Moreover, in all asexual generations, their budding reproduction rates varied with time and finally declined to similarly low levels. These results suggest that the physiological conditions of polyps are affected not only by environmental conditions but also by their “asexual age.” Under this circumstance, we suggest considering the “asexual age” of scyphozoan polyps in two aspects: (i) the age of the polyp sexually developing from a planula and (ii) the age of the polyp asexually budding from a mother polyp.
More interestingly, we found the transgeneration effect on budding reproduction rates disappeared after long-term experimental culture under the same environmental conditions as well as when temperature varied afterward. The budding reproduction rates did not recover when the temperature increased to the initial values. It implies that only the “new” polyps recruited from summer via sexual reproduction can reach the maximum budding reproduction rate. For a polyp population from permanent laboratory culture, the budding reproduction rates in mixed asexual generations cannot reach the maximum values of the new recruited population. For example, for those experiments applying polyps with mixed asexual generations under the same temperature (20°C) and food supply condition (excess Artemia nauplii), the budding reproduction rate exhibited 0.27 inds·polyp-1·day-1 in the North Sea population (Chi et al., 2019) or 0.25 inds·polyp-1·day-1 in the Adriatic Sea population (Hubot et al., 2017). These results are equivalent to the low values after long-run experimental culture in the current study. Therefore, to predict polyp population dynamics, the transgenerational effects on budding reproduction rates together with multifactorial environmental factors should be taken into account. For future experimental studies to explore polyps’ budding reproduction, it is necessary to know their “asexual age”.
Strobilation is suggested to be a strategy in response to physiological stress when polyps encounter unfavorable conditions, e.g., low temperature and food scarcity (Lucas et al., 2012; Treible and Condon, 2019; Loveridge et al., 2021). The optimum temperature for strobilation of Aurelia sp. in temperate regions ranges from 13°C~15°C (Lucas et al., 2012). Thus, there are two opportunities for polyps to fit their optimum temperature ranges of strobilation annually in the field, i.e., when temperatures decrease from summer to late autumn and when temperatures increase from winter to early spring. This is confirmed in our results as well as in a field observation (Marques et al., 2019). However, the strobilation rate of these two strobilation processes is significantly different. Our result implies that the second strobilation event after overwintering is the main process to recruit pelagic medusae in temperate regions. It is in line with one of our field observations in Kiel Fjord, Germany (Chi et al., unpublished). However, because of the widely distributed Aurelia spp. populations, the strobilation of polyps might show site-specific strobilation patterns (Lucas et al., 2012). For example, in the Mediterranean Sea, it was observed that there was a significantly higher strobilation rate that occurred in November than that from February to April (Marques et al., 2019). In addition, we found the state of ephyrae and strobilae varied during the temperature transformation period. There were more deformities when the temperature shifted from 13°C to 5°C than that from 13°C to 20°C. Thus, the fate of ephyrae produced before overwintering should be further investigated as they will encounter severe low temperature and food scarcity conditions during overwintering.
In addition, there is no evidence of transgenerational effects on strobilation in the current study. A possible explanation is that those polyps have adapted to moderate temperature and sufficient food conditions after a long-run culture, thus maintaining similar physiological situations. However, it should be taken into account that the duration for polyp acclimatization in the field is not too long because the new polyp population is established. They might be in different physiological conditions when temperature shifts to the first strobilation tolerance range. Thus, we suggest the transgenerational effect on polyps’ strobilation and whether it contributes to its mode shifting should be further studied under in situ conditions.
Energy allocation between different reproduction modes
Trade-offs play a central role in the evolution of life history strategy (Zera and Harshman, 2001; Smallegange, 2016). In our study, trade-offs between somatic growth and reproduction as well as between different reproduction modes were observed. Besides supporting the somatic growth of each newly generated bud, the excess food supply enables sufficient energy allocated to budding reproduction. When shifting to the strobilation temperature range, budding reproduction was limited; energy tended to be allocated to strobilation and ephyrae. Whereas during overwintering, the extremely low temperature inhibited both budding reproduction and strobilation, polyps grow to larger sizes and tend to stay in low metabolism activities (Han and Uye, 2010; Chi et al., 2019).
It is worth noting that a bell-shaped curve of budding reproduction rate was observed when the temperature rose to 13°C after overwintering. It seems that, to trigger strobilation of polyps, not only is “nutritive preparation” required (Thiel, 1962), but also the “time preparation” at the appropriate temperature range is needed. When the temperature shifted from 5°C to 13°C, the energy was first allocated to budding reproduction, which displayed a temporary increase in bud production rates. After two weeks under 13°C, strobilation was triggered, meanwhile, their budding reproduction rates declined. Afterward, the situation of energy allocation between budding reproduction and strobilation was altered again when the temperature was raised to 20°C. From this point of view, the sensitive threshold of the reproduction strategy of polyps to seasonal temperature variation is about 2~3 weeks. Therefore, following temperature and food supply variations in the temperate field conditions (Sommer et al., 2012), the annual life cycle of jellyfish involves sexual reproduction, major and minor budding reproduction, and strobilation. The total energy budget is allocated as a trade-off between survival and different reproduction modes (Figure 5). Thus, in light of future global warming, a long period of warm winter potentially contributes to a large polyp population and recruits more pelagic medusae once strobilation is triggered.
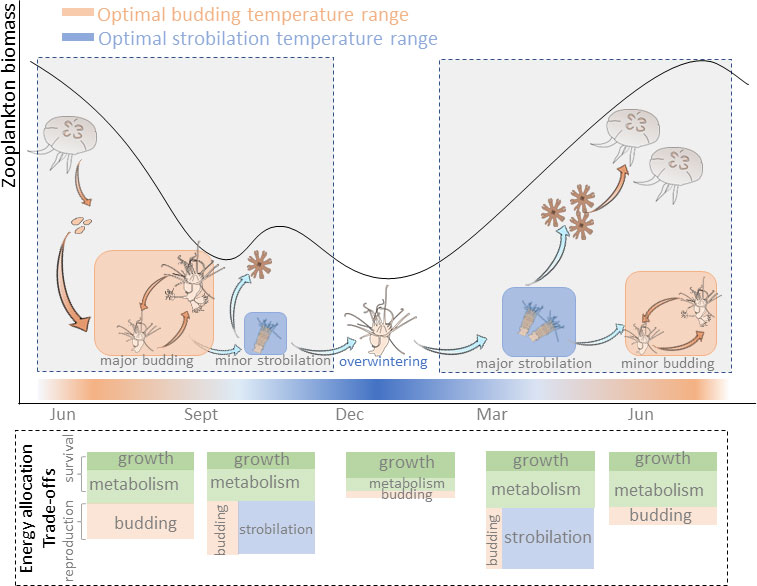
Figure 5 A speculative scheme for trade-offs of energy allocation in Aurelia sp. life history with seasonal temperature and food variations.
Ecological implications
Jellyfish polyps are capable of tolerance to a wide range of environmental conditions, the plasticity of their life history strategy enables them to respond to seasonal environmental variations via shifting between different reproduction modes (Chi et al., 2019). From evolutionary and ecological implications, the moon jellyfish develops a metagenic life cycle that possesses a mixed strategy of asexual propagation and sexual reproduction. Polyps developing from planulae rapidly expand population size via asexually budding reproduction when settling in a new habitat. From a general perspective, the energy investment in asexual reproduction is typically lower than that required for sexual reproduction (Sebens, 1979), and it avoids the “twofold cost of male” (Smith and Maynard-Smith, 1978; Shuo et al., 2020). Thus, asexually budding seems to be the more efficient and advantageous reproduction strategy of jellyfish in terms of converting resources into offspring compared with sexual reproduction. However, our results highlight that the reproduction rate of asexual budding is not always constant even under the same environmental conditions. The reproduction rates decrease with time once they reach the maximum values and decline through asexual generations, which seems to be “asexually aging” in polyps. Therefore, asexual reproduction is not always an efficient reproduction strategy for scyphozoan jellyfish.
When budding reproduction declines, strobilation is the process for jellyfish to transition from benthic to pelagic life stages, which is a mechanism for persisting under harsh and unpredictable environments (Schiariti et al., 2014). The following sex reproduction enables more evolutionary advantages in genetic recombination and maintains genetic diversity (Nei, 1967; Barton and Charlesworth, 1998; Hartfield and Keightley, 2012). Besides contributing to genetic recombination, from our results, another advantage of sexual reproduction is to recover polyps’ asexual budding ability. Thus, in the case of Aurelia spp., the actual costs of sex are reduced by intermittent sexuality, i.e., a series of asexual generations followed by an occasional sexual generation. During this process, there are two opportunities for polyps to adapt to the new environment, i.e., polyps produced by budding reproduction (Lu et al., 2020) and polyps produced by sexual reproduction. Thus, from an evolutionary perspective, the long survival of the jellyfish population is attributed to the complexity and plasticity of their life history under fluctuating and multifactorial environmental conditions, which also is the attribution to the challenge to predict their occasional population outbreaks.
We suggest that special attention should be given to the experimental conditions. To be comparable with the most published results, we also supplied polyps with Artemia sp. as food. However, the lack of essential nutrients, such as highly unsaturated fatty acids in this very common experimental food, may cause bias in polyps’ budding reproduction (Chi et al., 2019), not to mention the interaction with other environmental cues such as light, salinity, etc. Thus, further studies should be performed to investigate the transgenerational effects on polyp’s asexual reproduction under in situ conditions. Anyhow, the asexual age of polyps plays an essential ecological significance in scyphozoan’s metagenetic life history strategy.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://figshare.com/s/bb1d0d06c813d06b1ff8.
Author contributions
XC conceptualized the study. XC and FZ designed the experiment, XC performed the experiment and collected the data, conducted the analyses, and wrote the first draft of the manuscript. FZ and SS contributed substantially to data analyses and manuscript revisions. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the National Natural Science Foundation of China (No.42130411, No.42076166), Key deployment projects of the Center for Ocean Mega-Science,Chinese Academy of Sciences (COMS2019Z01) and Mount Tai Scholar Climbing Plan to SS.
Acknowledgments
We would like to thank Jinxian Liu for his suggestion during the experiment design. We thank Zhijun Dong, Lei Wang, and Dongjie Guo for their support to collect matured medusae for this experiment, and Dongchen Li for his help when we performed the experiment. We also thank three reviews whose suggestions improved this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amorim K., Mattmüller R. M., Algueró-Muñiz M., Meunier C. L., Alvarez-Fernandez S., Boersma M., et al. (2018). Winter river discharge may affect summer estuarine jellyfish blooms. Mar. Ecol. Prog. Ser. 591, 253–265. doi: 10.3354/meps12356
Barton N. H., Charlesworth B. (1998). Why sex and recombination? Science 281, 1986–1990. doi: 10.1126/science.281.5385.1986
Biro P. A., Stamps J. A. (2008). Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. doi: 10.1016/j.tree.2008.04.003
Chi X., Mueller-Navarra D. C., Hylander S., Sommer U., Javidpour J. (2019). Food quality matters: Interplay among food quality, food quantity and temperature affecting life history traits of aurelia aurita (Cnidaria: Scyphozoa) polyps. Sci. Total Environ. 656, 1280–1288. doi: 10.1016/j.scitotenv.2018.11.469
Combosch D. J., Vollmer S. V. (2013). Mixed asexual and sexual reproduction in the I ndo-p acific reef coral p ocillopora damicornis. Ecol. Evol. 3, 3379–3387. doi: 10.1002/ece3.721
Condon R. H., Duarte C. M., Pitt K. A., Robinson K. L., Lucas C. H., Sutherland K. R., et al. (2013). Recurrent jellyfish blooms are a consequence of global oscillations. Proc. Natl. Acad. Sci. 110, 1000–1005. doi: 10.1073/pnas.1210920110
Fuchs B., Wang W., Graspeuntner S., Li Y., Insua S., Herbst E.-M., et al. (2014). Regulation of polyp-to-jellyfish transition in aurelia aurita. Curr. Biol. 24, 263–273. doi: 10.1016/j.cub.2013.12.003
Fuentes V. L., Purcell J. E., Condon R. H., Lombard F., Lucas C. H. (2018). Jellyfish blooms: advances and challenges. Mar. Ecol. Prog. Ser. 591, 3–5. doi: 10.3354/meps12536
Gerber N., Kokko H., Ebert D., Booksmythe I. (2018). Daphnia invest in sexual reproduction when its relative costs are reduced. Proc. R. Soc. B: Biol. Sci. 285, 20172176. doi: 10.1098/rspb.2017.2176
Han C.-H., Uye S.-i. (2010). Combined effects of food supply and temperature on asexual reproduction and somatic growth of polyps of the common jellyfish aurelia aurita sl. Plankton Benthos Res. 5, 98–105. doi: 10.3800/pbr.5.98
Hartfield M., Keightley P. D. (2012). Current hypotheses for the evolution of sex and recombination. Integr. zool 7, 192–209. doi: 10.1111/j.1749-4877.2012.00284.x
Hočevar S., Malej A., Boldin B., Purcell J. E. (2018). Seasonal fluctuations in population dynamics of aurelia aurita polyps in situ with a modelling perspective. Mar. Ecol. Prog. Ser. 591, 155–166. doi: 10.3354/meps12387
Hubot N., Lucas C. H., Piraino S. (2017). Environmental control of asexual reproduction and somatic growth of aurelia spp.(Cnidaria, scyphozoa) polyps from the Adriatic Sea. PLoS One 12, 16. doi: 10.1371/journal.pone.0178482
Llodra E. R. (2002). Fecundity and life-history strategies in marine invertebrates. Adv. Mar. Biol. 43, 87–170. doi: 10.1016/S0065-2881(02)43004-0
Loveridge A., Lucas C. H., Pitt K. A. (2021). Shorter, warmer winters may inhibit production of ephyrae in a population of the moon jellyfish aurelia aurita. Hydrobiologia 848, 739–749. doi: 10.1007/s10750-020-04483-9
Lucas C. H. (2001). Reproduction and life history strategies of the common jellyfish, aurelia aurita, in relation to its ambient environment. Hydrobiologia 451, 229–246. doi: 10.1023/A:1011836326717
Lucas C. H., Graham W. M., Widmer C. (2012). Jellyfish life histories: Role of polyps in forming and maintaining scyphomedusa populations. Adv. Mar. Biol. 63, 133–196. doi: 10.1016/B978-0-12-394282-1.00003-X
Lu Y., Lucas C., Loveridge A. (2020). Transgenerational acclimation influences asexual reproduction in aurelia aurita jellyfish polyps in response to temperature. Mar. Ecol. Prog. Ser. 656, 35–50. doi: 10.3354/meps13517
Marques R., Darnaude A. M., Schiariti A., Tremblay Y., Molinero J. -C., Soriano S., et al. (2019). Dynamics and asexual reproduction of the jellyfish aurelia coerulea benthic life stage in the thau lagoon (northwestern Mediterranean). Mar. Biol. 166, 74. doi: 10.1007/s00227-019-3522-4
Nei M. (1967). Modification of linkage intensity by natural selection. Genetics 57, 625. doi: 10.1093/genetics/57.3.625
Partridge L., Harvey P. H. (1988). The ecological context of life history evolution. Science 241, 1449–1455. doi: 10.1126/science.241.4872.1449
Purcell J. E., Hoover R. A., Schwarck N. T. (2009). Interannual variation of strobilation by the scyphozoan aurelia labiata in relation to polyp density, temperature, salinity, and light conditions in situ. Mar. Ecol. Prog. Ser. 375, 139–149. doi: 10.3354/meps07785
R Core Team (2021) R: A language and environment for statistical computing. Available at: https://www.R-project.org/.
Schiariti A., Melica V., Kogovšek T., Malej A. (2015). Density-dependent effects control the reproductive strategy and population growth of aurelia aurita sl scyphistomae. Mar. Biol. 162, 1665–1672. doi: 10.1007/s00227-015-2704-y
Schiariti A., Morandini A. C., Jarms G., von Glehn Paes R., Franke S., Mianzan H. (2014). Asexual reproduction strategies and blooming potential in scyphozoa. Mar. Ecol. Prog. Ser. 510, 241–253. doi: 10.3354/meps10798
Sebens K. P. (1979). The energetics of asexual reproduction and colony formation in benthic marine invertebrates. Am. Zool. 19, 683–699. doi: 10.1093/icb/19.3.683
Shuo Y., Wang W.-x., Jie S. (2020). Reproductive polyphenism and its advantages in aphids: Switching between sexual and asexual reproduction. J. Integr. Agric. 19, 1447–1457. doi: 10.1016/S2095-3119(19)62767-X
Smallegange I. M. (2016). “Life history trade-offs,” in Encyclopedia of evolutionary biology. Ed. Kliman R. M. (Oxford: Academic Press Oxford), 390–393.
Smith J. M., Maynard-Smith J. (1978). The evolution of sex (Cambridge, New York: Cambridge University Press Cambridge).
Sommer U., Adrian R., De Senerpont Domis L., Elser J. J., Gaedke U., Ibelings B., et al. (2012). Beyond the plankton ecology group (PEG) model: Mechanisms driving plankton succession. Annu. Rev. Ecol Evolution Systematics 43, 429–448. doi: 10.1146/annurev-ecolsys-110411-160251
Subramoniam T. (2018). Mode of reproduction: Invertebrate animals. Encyclopedia Reprod. 6, 32–40. doi: 10.1016/B978-0-12-809633-8.20533-5
Sun S. (2012). New perception of jellyfish bloom in the East China Sea and yellow Sea (in Chinese). Oceanologia Et Limnologia Sin. 43, 406–410. doi: 10.11693/hyhz201203002002
Thiel H. (1962). Untersuchungen über die strobilisation von aurelia aurita lam. an einer population der kieler förde. Kieler meeresforschungen 18, 198–230.
Tiller R. G., Borgersen Å.L., Knutsen Ø., Bailey J., Bjelland H. V., Mork J., et al. (2017). Coming soon to a fjord near you: Future jellyfish scenarios in a changing climate. Coast. Manage. 45, 1–23. doi: 10.1080/08920753.2017.1237239
Treible L. M., Condon R. H. (2019). Temperature-driven asexual reproduction and strobilation in three scyphozoan jellyfish polyps. J. Exp. Mar. Biol. Ecol. 520, 151204. doi: 10.1016/j.jembe.2019.151204
Zera A. J., Harshman L. G. (2001). The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126. doi: 10.1146/annurev.ecolsys.32.081501.114006
Zuur A. F., Ieno E. N., Elphick C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1, 3–14. doi: 10.1111/j.2041-210X.2009.00001.x
Keywords: asexual reproduction, life history, transgenerational effect, trade-off, jellyfish bloom, Aurelia
Citation: Chi X, Zhang F and Sun S (2022) Transgenerational effects and temperature variation alter life history traits of the moon jellyfish. Front. Mar. Sci. 9:913654. doi: 10.3389/fmars.2022.913654
Received: 06 April 2022; Accepted: 18 July 2022;
Published: 11 August 2022.
Edited by:
Cathy Lucas, University of Southampton, United KingdomReviewed by:
Maria Pia Miglietta, Texas A&M University at Galveston, United StatesRoberto Simonini, University of Modena and Reggio Emilia, Italy
Otto Oliveira, Federal University of ABC, Brazil
Copyright © 2022 Chi, Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Zhang, emhhbmdmYW5nQHFkaW8uYWMuY24=; Song Sun, c3Vuc29uZ0BxZGlvLmFjLmNu
 Xupeng Chi
Xupeng Chi Fang Zhang
Fang Zhang Song Sun1,2,3,4*
Song Sun1,2,3,4*