
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 22 June 2022
Sec. Aquatic Microbiology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.910327
This article is part of the Research Topic The Impacts of Anthropogenic Activity and Climate Change on the Formation of Harmful Algal Blooms (HABs) and Its Ecological Consequence View all 16 articles
Surface sediments were collected from three sea areas of the Qingdao coast, the Yellow Sea, China, namely, the inner Jiaozhou Bay, the Laoshan coast, and the Amphioxus Reserve area in November to December 2017. Dinoflagellate cysts were observed in the sediments, focusing on the distribution of toxic and harmful species. Contents of biogenic elements were analyzed to reveal their relationships to cysts. A total of 32 cyst taxa were identified, including 23 autotrophic and 9 heterotrophic taxa. Cyst concentrations ranged from 83.3 to 346.5 cysts/g D Wt with an average of 210.7 cysts/g D Wt. Generally, cysts of autotrophic dinoflagellates dominated in sediments from the Qingdao coast with proportions of 41.05%–90.25%. There were no dominant group in cyst assemblages; cysts of Protoperidiniaceae, Suessiales, and Calciodinelloideae showed similar contributions. Cyst assemblages were quite different in the inner Jiaozhou Bay reflected by the lower species richness, diversity, and cyst concentration. Results from the redundancy analysis (RDA) demonstrated the influence of biogenic elements on cyst assemblages, which explained well why the three sea areas with different degrees of human activities showed different dinocyst storages. Notably, 17 harmful algal bloom (HAB) dinoflagellate cysts were identified in this study, including cysts of those producing toxins that may damage human health and marine animals. Some of these cysts occurred widely and dominantly in this study, such as cysts of Gonyaulax spinifera, Azadinium trinitatum, Scrippsiella acuminata, and Biecheleria halophila, suggesting the potential risk of HABs in the Qingdao coastal area.
Dinoflagellates are the second largest group of marine phytoplankton and also an important group of toxic and harmful algal bloom (HAB) species (Gomez, 2012). HABs affect marine organisms negatively, degrade the environment, cause severe economic losses, and, in some cases, threaten human health (Hallegraeff et al., 2003). HABs only concern about 5% of marine phytoplanktonic species (Zingone and Enevoldsen, 2000), of which dinoflagellates represent 75% (Smayda, 1997). Many dinoflagellate species form resting cysts during their life cycles, which thicken cell walls and sink to the sea floor as part of marine sediments (Bravo and Figueroa, 2014). Resting cysts can survive in benthic sediments for decades or even centuries due to their thick, tolerant walls (Ellegaard and Ribeiro, 2018). Dinoflagellate cysts (hereafter referred to as dinocysts) help the population survive from the harsh environment, and the germination of cysts provides large amounts of vegetative cells to the water column. Therefore, dinocysts are considered as the “seed bank” for the occurrence of HAB (Anderson et al., 2014; Castaneda-Quezada et al., 2021). Although only 10%–20% of dinoflagellates can form resting cysts, many toxic and HAB species are cyst-forming, especially those causing recurrent blooms (Genovesi-Giunti et al., 2006; Bravo and Figueroa, 2014). The distribution of dinocysts in sediments provides essential information in giving early warnings of the presence of toxic species and possible continuing recurrence of HABs in a given area (Anderson et al., 2014; Joyce et al., 2015; Sidabutar and Srimariana, 2021).
The coastal zone is the most active area of human activity, provides abundant resources, and has high ecological value and environmental functions. However, many coastal environments have been suffering from environmental degradation, decline in biodiversity, and bio-invasion such as the rapid economic development, population expansion, and overexploitation of marine resources (Yu et al., 2019). Qingdao is located in northeastern China, southeast of Shandong Peninsula, and east of the Yellow Sea. It is the economic center of Shandong Province and functions as an important international port, a modern marine industry zone, an international shipping hub in Northeast Asia, and a maritime sports base. Qingdao City covers an area of 758.16 km2 with a population of 9.50 million in 2019. Due to the rapid increase of economic development and population size as well as the increase of aquaculture, the pollution level in Qingdao coastal waters has been increasing recently (Yuan et al., 2018). The degradation of water quality results in the frequent occurrence of algal blooms. A total of 36 algal blooms occurred in the coastal areas of Qingdao between 1990 and 2017, of which more than 80% occurred in Jiaozhou Bay (Zhou et al., 2020). Though intensive surveys have been carried out to investigate phytoplankton community in Jiaozhou Bay during the past 50 years (reviewed by Liu and Chen, 2021), dinocysts have been barely reported (Li et al., 2017).
In order to understand the effects of human activities on the distribution of dinocysts and the potential of HABs in the Qingdao coast, surface sediments were sampled from three different function sea areas of the Qingdao coast in this study, namely, a eutrophic enclosed bay (the inner Jiaozhou Bay), a conservation area (the Qingdao Amphioxus Reserve area), and a scenic and aquacultural area (the Laoshan coast). Biogenic elements including total organic carbon (TOC), organic matter (OM), total nitrogen (TN), total phosphorus (TP), and biogenic silicon (BSi) were analyzed, and the relationships between dinocysts and biogenic elements were discussed. The purposes of this study were to compare dinocyst composition among the three sea areas with different degrees of human activities, and to discuss the distribution of cysts of HAB dinoflagellates in sediments from the Qingdao coast.
Qingdao is located in the southeast of the Shandong Peninsula, which borders the Yellow Sea in the east and the south. Surface sediments were collected in three sea areas along the Qingdao coast, i.e., the inner Jiaozhou Bay (JZ, J1–J4), the Laoshan coast (LS, L1–L9), and the Qingdao Amphioxus Reserve area (AR, A1–A4) (Figure 1). Jiaozhou Bay, in the northwest of the center Qingdao City, is a large semi-closed bay connected to the Yellow Sea by a narrow bay mouth. Jiaozhou Bay has multiple functions (port, transportation, and aquaculture). The Laoshan coast is located in the eastern part of Qingdao City and surrounds the famous tourist attraction, the Laoshan Scenic Area. There are a lot of aquaculture farms in the Laoshan coast. The Amphioxus Reserve area is located in the southern part of Qingdao City, just outside the mouth of Jiaozhou Bay and close to the Qingdao Port. The Amphioxus Reserve area was established in August 2004 to protect wild amphioxus population and its habitat.
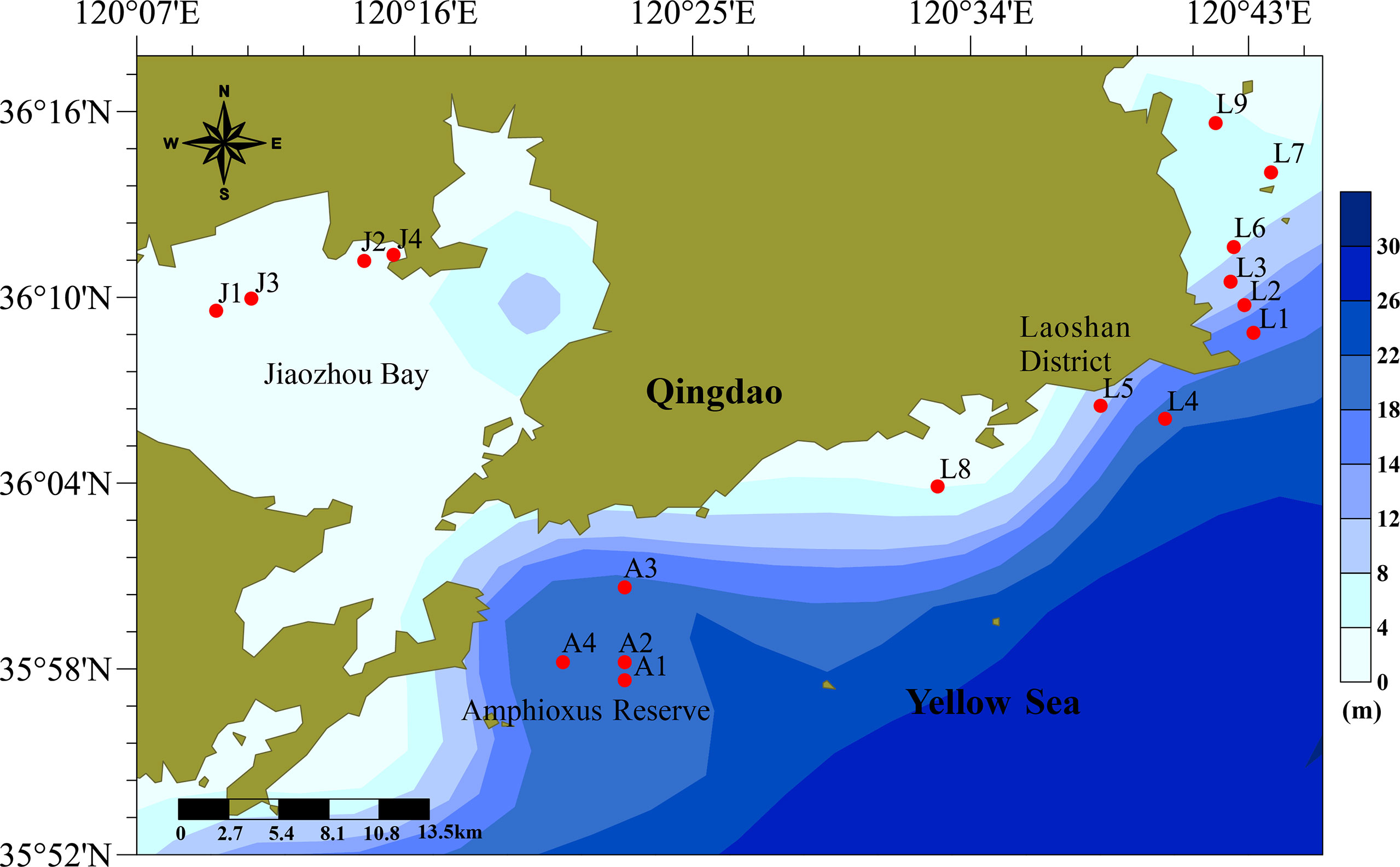
Figure 1 Sampling stations in the Qingdao coastal sea area. Stations J1–J4 are in the inner Jiaozhou Bay, stations A1–A4 are in the Amphioxus Reserve area, and stations L1–L9 are in the Laoshan coast.
Surface sediments were collected from seventeen stations in the three sea areas using a Peterson grab between November and December 2017. The top 2 cm of sediments was sampled with a polyethylene spatula and sealed in a polyethylene bag, and then stored at −20°C for further treatment. All sediment samples were processed after transporting to the lab within 24 h.
Sediments for biogenic elements analysis were dried in an oven at 40°C until a constant weight was reached, and the water content of the sediments was calculated. The dried sediments were ground gently with an agate mortar and pestle, sieved through a 100-μm mesh for homogenization, and stored in sealed glass vials. In order to reduce the influence of external contamination on biogenic elements, sediment processing was carried out in a clean cupboard. All vessels were washed and soaked in acid for more than 24 h, and then fully rinsed with distilled water and dried before re-used. Total organic carbon (TOC) and total nitrogen (TN) were measured by a Perkin-Elmer 2400 Series II CHNS/O Analyzer (Perkin Elmer Inc., USA). Total phosphorus (TP) was measured using the potassium persulfate digestion method (Thien and Myers, 1992). Organic matter (OM) was determined by ignition loss in a muffle furnace (SG-XL1200, Honglang, Shanghai, China). Biogenic silica (BSi) was measured by the molybdate blue spectrophotometric method after removing the carbonates and organics by 1 mol/L HCl and 10% H2O2 and digested using 0.5 mol/L Na2CO3 solution (Mortlock and Froelich, 1989). The quality assurance/quality control (QA/QC) was assessed by the analyses of blank reagents and five replicates of the certified reference material (Offshore Marine Sediment, GBW 07314). The analytical precision was controlled to within 5% for biogenic elements.
Approximately 5 g of wet sediments was weighted for dinocyst observation, placed in a beaker mixed with 50 ml of filtered seawater, and then sonicated for 60 s in a water bath. The sonicated materials were successively sieved through 125- and 10-μm sieves, and the slurry remaining on the 10-μm sieve with grain sizes between 10 and 125 μm was collected and filled to a final volume of 10 ml with filtered seawater, and then fixed with 3% formalin. An aliquot of 0.5–1 ml of treated sample was placed on a 1-ml counting chamber and diluted with appropriate distilled water. Dinocysts were identified and counted with an inverted light microscope (Nikon ECLIPSE) at 400× magnification according to Matsuoka and Fukuyo (2000) and Zonneveld and Pospelova (2015). At least 100 cysts were counted in each sample. Cyst concentration was expressed by the numbers of cyst per gram of dry sediments (cysts/g D Wt).
The sampling map was drawn by the software Sufer13.0. The Shannon–Wiener diversity index (H’), Pielou’s Evenness index (J), and the correlation analysis (CA) between the cysts and biogenic elements were calculated by the SPSS 20.0 software. The bar charts and line charts were drawn by Excel 2016. Venn diagram was drawn using the Venn Diagram function package of the software R4.1.0. The bubble chart of cysts arranged by their average abundance was drawn using the ggplot2 function package of R4.1.0. The vegan function package of R4.1.0 was used to standardize the cyst data, and the vegdist function was used to calculate the Bray–Curtis distance, and then a cluster diagram was drawn by the gclus and plot function packages of R4.1.0 based on the Bray–Curtis distance. A detrended correspondence analysis (DCA) was first achieved to test the character of variability in the dinocyst assemblages. The length of the first DCA gradient was 0.78 standard deviations for our dataset, which justified the further use of the redundancy analysis (RDA). DCA and RDA were performed using Canoco 5 software.
A total of 32 dinocyst taxa were identified in the surface sediments from the Qingdao coast, namely, 9 taxa in Gonyaulacales, 4 taxa in Calciodinelloideae, 1 taxon in Suessiales, 7 taxa in Gymnodiniales, 8 taxa in Protoperidiniaceae, and 3 taxa in genus Azadinium (Dinophyceae incertae sedis) (Table 1). The species richness identified at each station ranged between 6 and 23 taxa, and only 4 core species were shared among all stations (Figure 2A). The species richness in the inner Jiaozhou Bay (JZ) was 13 taxa, and 25 taxa were identified in the other two sea areas, the Laoshan coast (LS) and the Amphioxus Reserve area (AR), respectively (Table 1). Only 11 species were shared among the three sea areas (Figure 2B). No unique species occurred in JZ, while 6 unique species were recorded in the other two sea areas (Figure 2B). The Shannon–Wiener diversity index (H’) ranged from 1.37 to 2.63, and values of Pielou’s Evenness index (J) were between 0.69 and 0.89 (Figure 3A). JZ had the lowest cyst diversity with an average H’ value of 1.57, while the average H’ values in LS and AR were 2.07 and 2.28, respectively. The mean values of the evenness index (J) were similar in the three sea areas, ranging from 0.79 to 0.82 (Figure 3B).
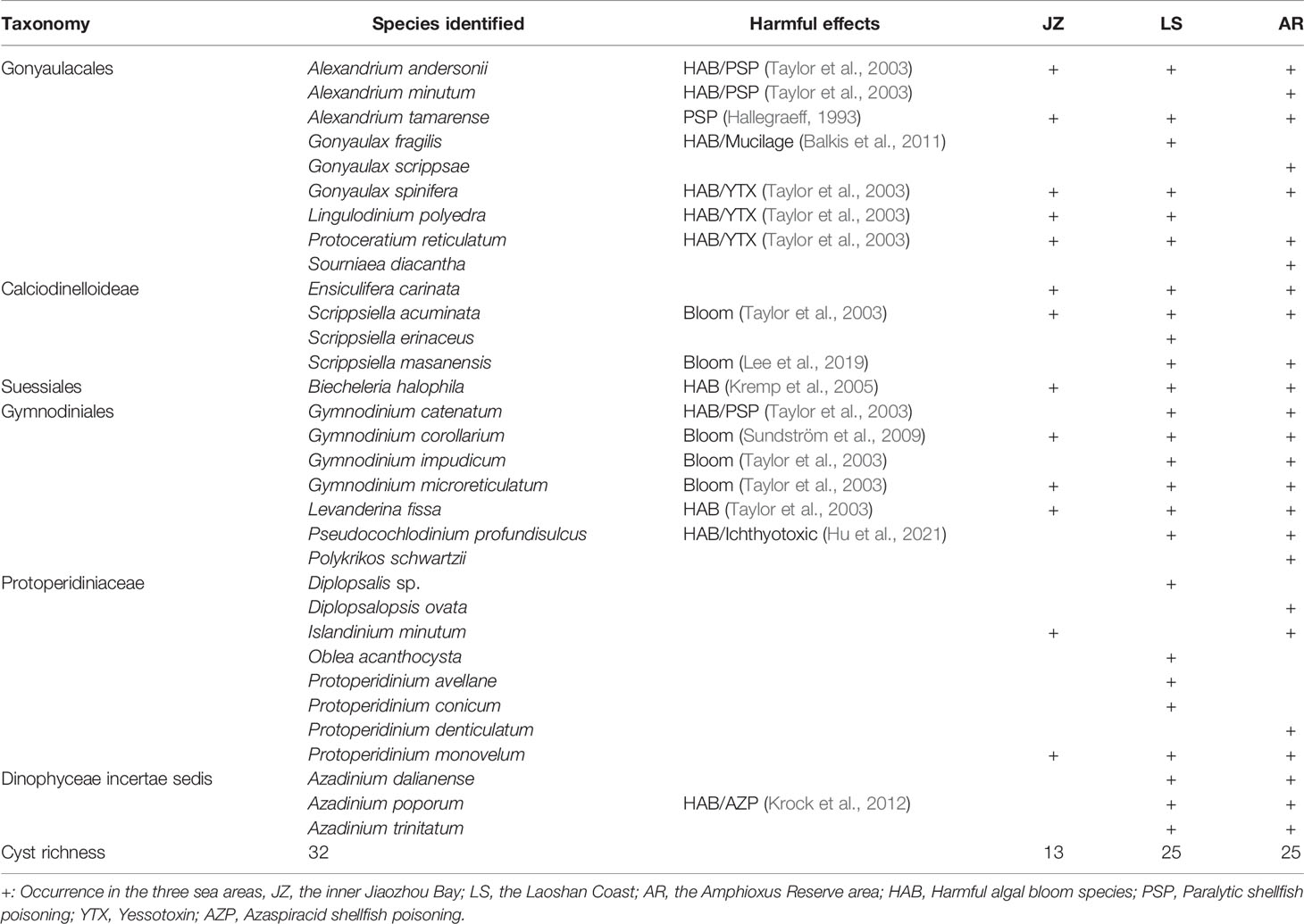
Table 1 Information and distribution of dinoflagellate cysts in surface sediments from the Qingdao coast.
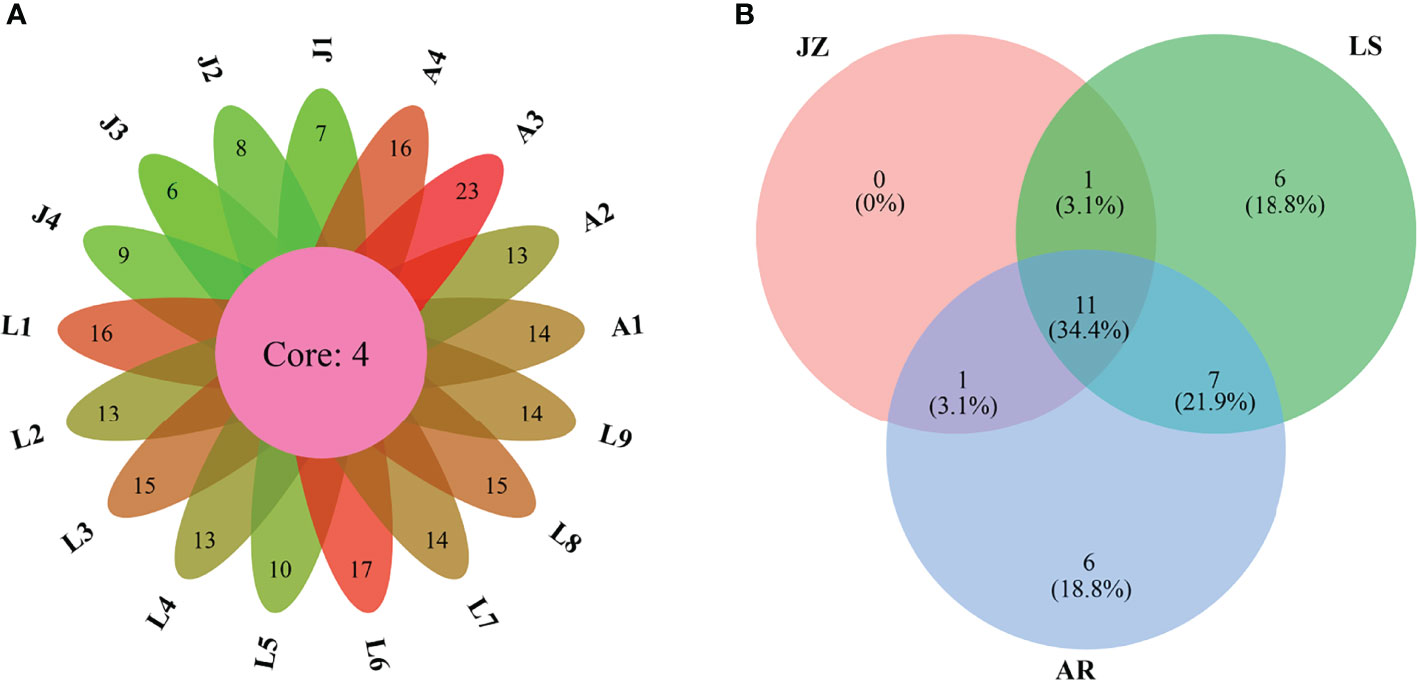
Figure 2 Venn diagrams highlighting the degree of overlap of dinocyst taxa among the seventeen samples (A) and among the three sea areas (B). JZ, the inner Jiaozhou Bay; LS, the Laoshan coast; AR, the Amphioxus Reserve area.
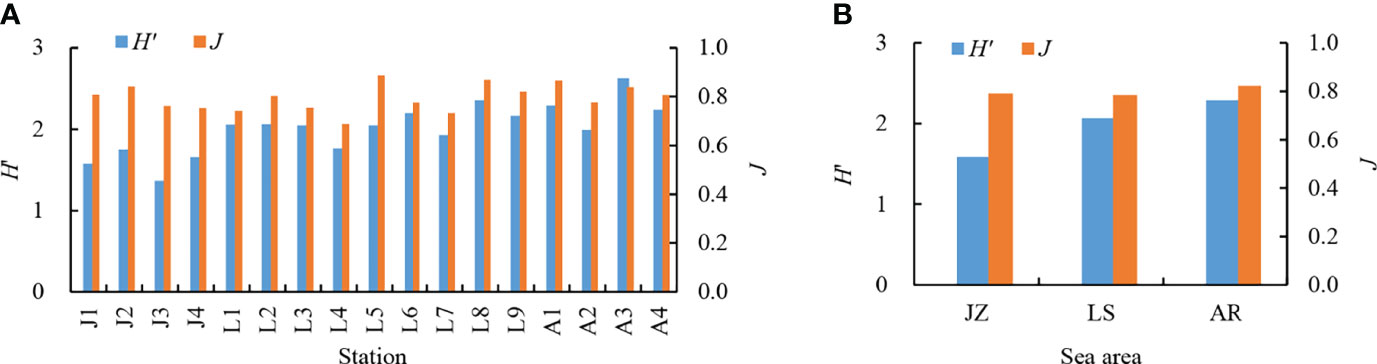
Figure 3 Shannon–Wiener diversity index (H’) and Pielou’s evenness index (J) of dinocysts in the seventeen samples (A) and the three sea areas (B). JZ, the inner Jiaozhou Bay; LS, the Laoshan coast; AR, the Amphioxus Reserve area.
Notably, 17 cysts of the potentially toxic/harmful and/or bloom dinoflagellates were detected in this study (Table 1), including the paralytic shellfish poisoning (PSP) producers Alexandrium andersonii, A. minutum, A. tamarense, and Gymnodinium catenatum; the yessotoxin (YTX) producers Gonyaulax spinifera, Lingulodinium polyedra and Protoceratium reticulatum; the Azaspiracid shellfish poisoning (AZP) producer Azadinium poporum; and cysts of other bloom species (Biecheleria halophila, Gonyaulax fragilis, Gymnodinium corollarium, Gy. impudicum, Gy. microreticulatum, Levanderina fissa, Pseudocochlodinium profundisulcus, Scrippsiella acuminata, and S. masanensis). There were 10, 16, and 15 bloom species recorded in JZ, LS, and AR, respectively, nine of which occurred in all of the three sea areas.
Dinoflagellates have autotrophic and heterotrophic lifestyles, in which Gonyaulacales, Calciodinelloideae, Suessiales, and Azadinium (Dinophyceae incertae sedis) are autotrophic dinoflagellates, Gymnodiniales have both autotrophic and heterotrophic ones, and all of Protoperidiniaceae are heterotrophic. A total of 23 taxa of cysts of autotrophic dinoflagellates and 9 taxa of heterotrophic dinoflagellates were identified. The heterotrophic dinoflagellates included 7 taxa in Protoperidiniaceae and cysts of Polykrikos Schwartzii in Gymnodiniales. Generally, cysts of autotrophic dinoflagellates dominated in sediments from the Qingdao coast (Figure 4A), and the percentage proportions of autotrophic dinoflagellate cysts ranged from 41.05% to 90.25%, with an average of 76.48%. The percentages of autotrophic cysts in the three sea areas showed a gradually increasing trend from JZ to AR, from 60.44% in JZ to 79.36% in LS, and 86.04% in AR, while those of heterotrophic cysts showed an opposite trend (Figure 4B).
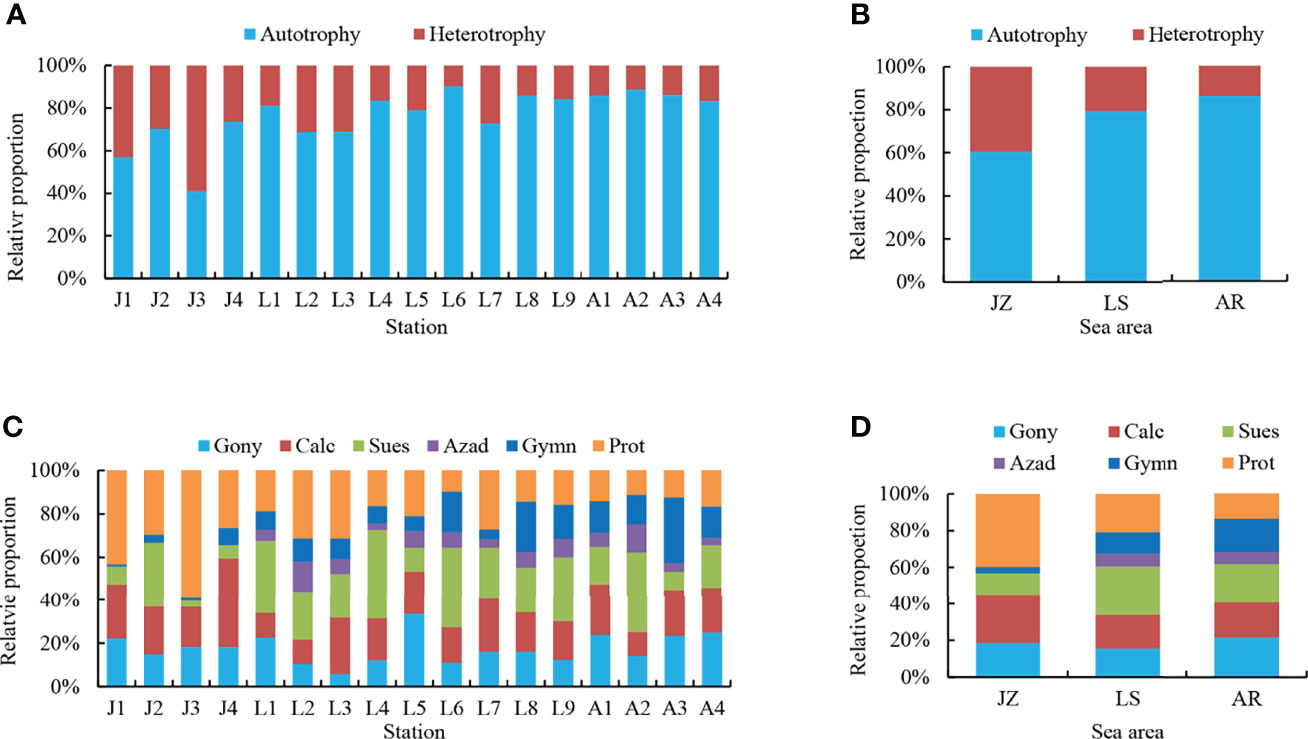
Figure 4 Cyst profile in the seventeen samples (A, C) and the three sea areas (B, D). (A, B) Percentage proportions of cysts of autotrophic and heterotrophic dinoflagellates. (C, D) Percentage proportions of cysts of each dinoflagellate group. Gony, Gonyaulacales; Calc, Calciodinelloideae; Sues, Suessiales; Azad, Azadinium (Dinophyceae incertae sedis); Gymn, Gymnodiniales; Prot, Protoperidiniaceae.
Relative abundances of dinocyst groups in each station andsea area are illustrated in Figures 4C, D, respectively. There were no dominant groups in cyst assemblages, and Protoperidiniaceae, Suessiales, and Calciodinelloideae showed similar contributions with the average relative abundances of 23.45%, 21.75%, and 20.52%, respectively, followed by cysts in Gonyaulacales (17.66%) and Gymnodiniales (11.24%) (Figure 4C). Cyst composition varied in different sea areas (Figure 4D). Cyst assemblages in JZ were dominated by Protoperidiniaceae (averagely 39.56%), followed by the Calciodinelloideae (26.71%). Cysts in Suessiales slightlydominated in LS with an average proportion of 26.69%, and cysts in Protoperidiniaceae, Calciodinelloideae, and Gonyaulacales made similar contributions to the overall cyst assemblages with proportions of 15.54%–20.64%. Cysts in Gonyaulacales, Calciodinelloideae, Suessiales, and Gymnodiniales equally contributed to cyst assemblages in AR with a relative abundance of ca. 20%, and cysts in Protoperidiniaceae and Azadinium accounted for 13.66% and 6.81%, respectively.
Cyst concentrations ranged from 83.3 to 346.5 cysts/g D Wt with an average of 210.7 cysts/g D Wt, with the highest at station L6 and the lowest at station J3 (Figure 5). Lower cyst concentrations were recorded in JZ, ranging between 83.3 and 165.8 cysts/g D Wt, with an average of 127.2 cysts/g D Wt. Cyst concentrations in LS varied from 109.4 to 346.5 cysts/g D Wt, with an average of 230.6 cysts/g D Wt. There were few differences in cyst concentrations between samples from AR, ranging from 221.2 to 281.4 cysts/g D Wt, with an average of 249.5 cysts/g D Wt.
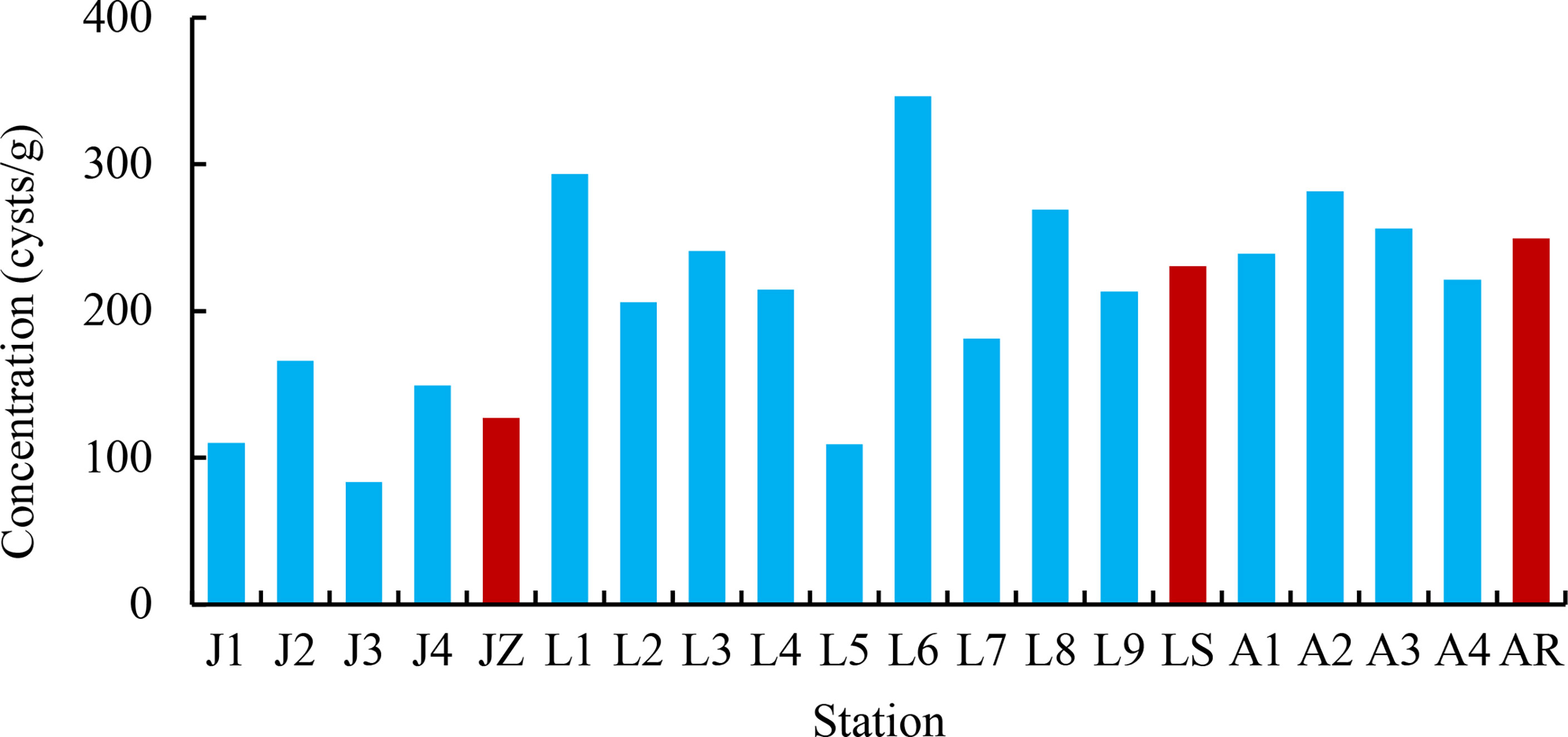
Figure 5 Cyst concentration in the seventeen samples and the three sea areas. The blue bar shows cyst concentration in each sample, and the red bars present averages in the three sea areas.
Distribution of the 32 dinocyst taxa by dominance is shown in Figure 6. Only four cyst types were distributed at all stations, and they were also the top 4 most abundant cyst taxa in this study, including the bloom species Biecheleria halophila and Scrippsiella acuminata, and the YTX producer Gonyaulax spinifera, which ranked first, third, and fourth in cyst abundance, respectively. Protoperidinium monovelum was also found at all stations and ranked second in dominance. The average concentrations of the top 4 cyst types ranged between 23.8 and 51.0 cysts/g D Wt. Cysts of the bloom species Gymnodinium corollarium were distributed at 14 stations except stations J1–J3 in Jiaozhou Bay with an average of 12.2 cysts/g D Wt. Cysts of Azadinium trinitatum, Alexandrium andersonii, Gymnodinium impudicum, Gymnodinium microreticulatum, and Ensiculifera carinata occurred in most stations, with average concentrations of 4.5–11.3 cysts/g D Wt, while other cyst types rarely occurred.
Cluster analysis of 17 sediment samples showed that four samples in the inner Jiaozhou Bay (JZ1-JZ4) were clustered together with station L5, while other LS and AR samples were clustered together into a large group (Figure 7). The results indicated that dinocyst assemblages in the inner Jiaozhou Bay and station L5 were quite different from other samples.
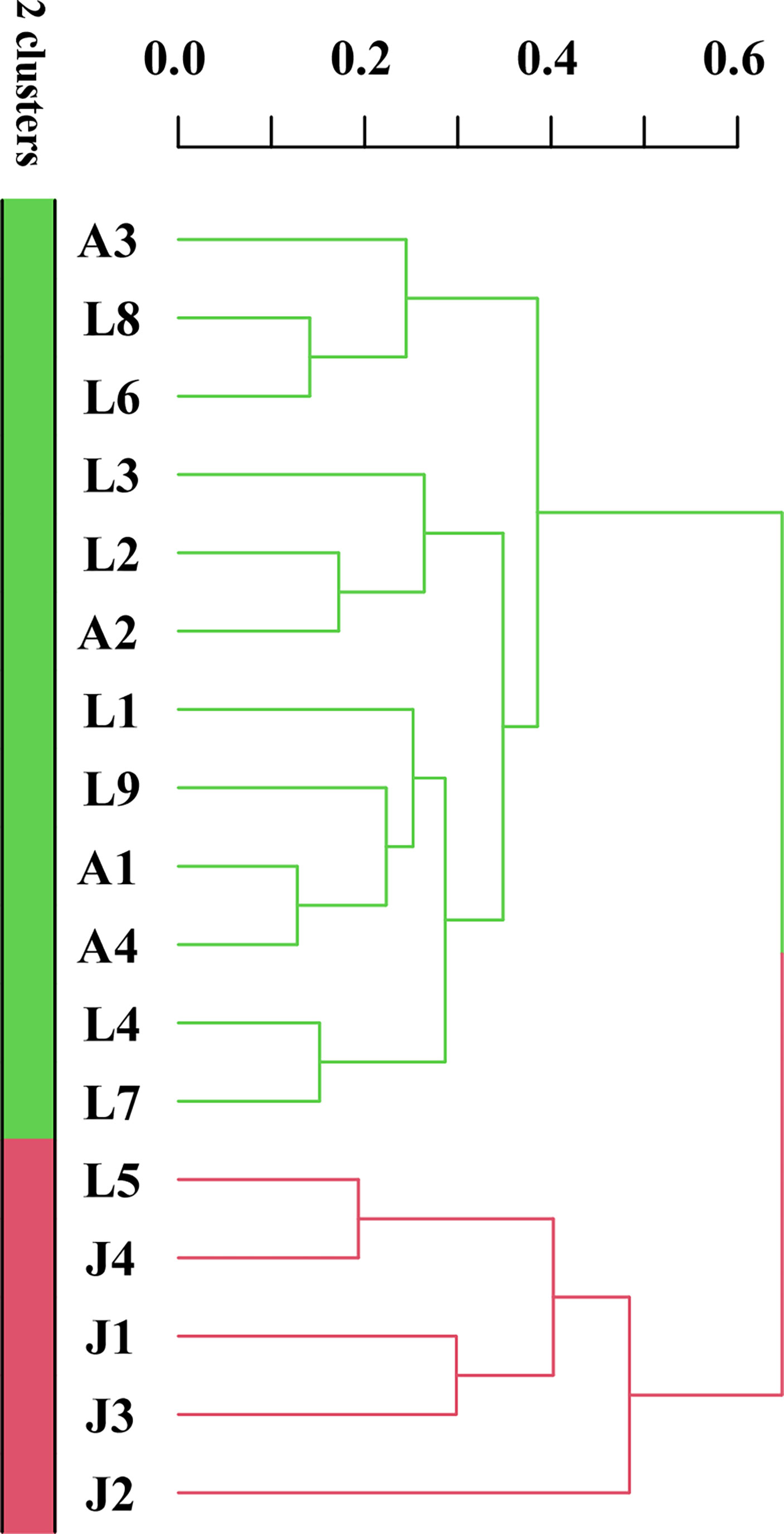
Figure 7 Cluster analysis of seventeen sediment samples from the three sea areas of the Qingdao coast based on Bray–Curtis distance of dinocysts.
RDA analysis was conducted based on biogenic elements and dinocysts (Figure 8A). RDA1 and RDA2 explained 55.21% and 35.18% of the environmental and biological variables, respectively. The biogenic elements and dinocysts scattered in all of the four quadrants. Cysts in Calciodinelloideae showed a narrow intersection angle with BSi, indicating the significant influence of BSi on the distribution of the Calciodinelloideae cysts. Cysts in other classes scattered in the coordinates far away from the biogenic elements, indicating few effects of biogenic elements on their distribution. The ordination sampling station showed that samples in the three sea areas were separately grouped (Figure 8B). Samples in the inner Jiaozhou Bay were clustered along the negative axis of RDA2, which indicated the low BSi content and the high organic matter (TN, OM, and TOC) in this area. Samples in the Amphioxus Reserve area were distributed along the positive axis of RDA2, indicating the low organic matter (TOC, OM, and TN) and high BSi in this area. Meanwhile, the arrows of the biogenic elements were distributed within the group of samples of the Laoshan area, indicating the high content of biogenic elements in this area.
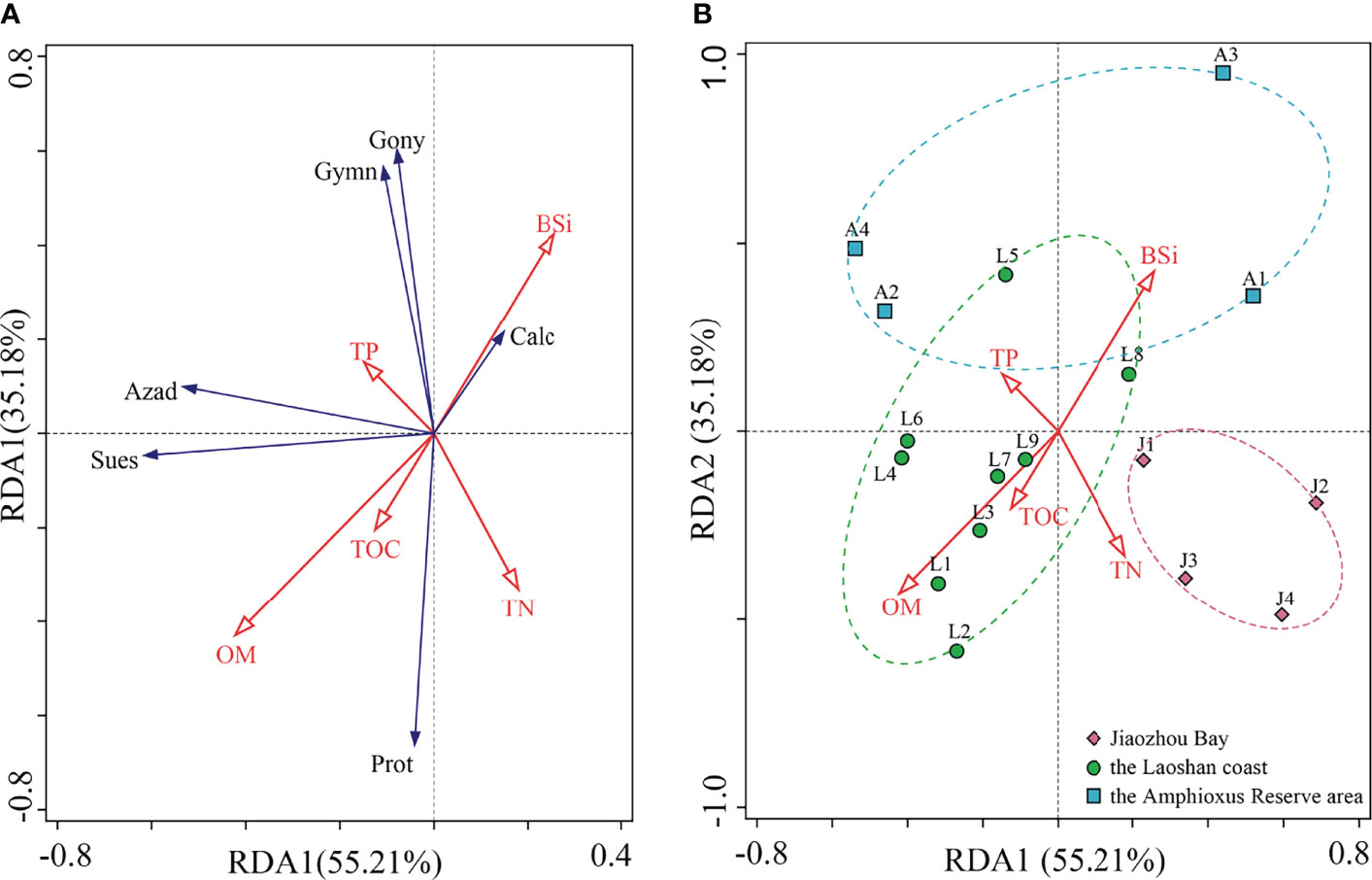
Figure 8 Redundancy analysis (RDA) of biogenic elements and dinocysts in surface sediments from the Qingdao coast, showing cyst distribution and the directions of biogenic elements to the first two RDA axes, RDA1 (55.21%) and RDA2 (35.18%). The length of arrows indicates the importance of biogenic elements in explaining the distribution of cysts. The direction of the arrows shows approximate correlation to the ordination axes. (A) Ordination dinocyst groups and (B) ordination sampling stations, showing the distribution of the three areas.
Generally, there were no significant correlations between dinocysts and biogenic elements except for a significant positive correlation between cysts in Calciodinelloideae and BSi (Table 2). Species richness showed significant correlations with concentrations of overall cysts and cysts in Gonyaulacales, Calciodinelloideae, and Gymnodiniales (p < 0.05 or p < 0.01). The concentration of overall cysts was positively correlated with the concentration of most cyst groups except for cysts of Protoperidiniaceae. Cysts of autotrophic dinoflagellates were positively correlated with each other, while cysts of heterotrophic Protoperidiniaceae were negatively correlated with other cyst groups.
Resting cyst is a specific dormant stage in the life cycle of many dinoflagellates.
Nearly 10%–20% of modern dinoflagellates form resting cysts (Head, 1996), which constitute the coupling between benthic and pelagic stages, and support bloom development and recurrence (Genovesi-Giunti et al., 2006; Anderson et al., 2014). However, the morphological characteristics of many resting cysts are not clear right now, and the corresponding relationships between some cysts and vegetative cells are still unknown (Gu et al., 2022). Therefore, the diversity and abundance of dinocysts are often underestimated based on the morphological characteristics under microscopic observations. Nevertheless, cyst identification by traditional morphological classification is the basis for the study of cysts in sediments. In this study, a total of 32 cyst taxa were recorded in 17 surface sediments from the Qingdao coast. The cyst species richness was comparable to those reported in sediments from the other coastal sea areas around the world, which were generally 20–40 taxa recorded (Pospelova et al., 2005; Limoges et al., 2010; Liu et al., 2012; Aydin et al., 2015; Lu et al., 2017).
Although the three sea areas in this study are not far from each other, cyst assemblages differed among sea areas. Only 4 species were shared among all of the 17 stations, and only 34.4% of the species were shared among the three sea areas (Figure 2). The inner Jiaozhou Bay had lower cyst diversity and concentration, but a higher proportion of cysts of heterotrophic dinoflagellates. The cluster and RDA analyses showed that samples from Jiaozhou Bay were separately clustered from the other sea areas. Jiaozhou Bay is a semi-enclosed sea area with only a narrow mouth open to the Yellow Sea, which makes it a specific ecosystem and microalgal community, while the Laoshan coast and the Amphioxus Reserve area are connected to the Yellow Sea, which share similar cyst compositions. Eutrophication in Jiaozhou Bay has greatly increased recently, which resulted in frequent algal blooms and decreased phytoplankton biodiversity (Shi et al., 2020). In addition, Jiaozhou Bay is an important sea area for shellfish culturing, and feeding of shellfish also reduces the diversity of phytoplankton to a certain extent (Yu et al., 2019) and thus results in low cyst diversity and abundance. On the other hand, high sedimentation rate (0.19–3.96 cm/a, Li et al., 2011) and low water depth might result in the low abundance of cysts in Jiaozhou Bay. Station L5 showed similar cyst composition to the inner Jiaozhou Bay (Figure 7), as it is also a shallow nearshore site close to Qingdao City (Figure 1).
Generally, the productivity of heterotrophic dinoflagellates is lower than that of autotrophic ones because heterotrophs need to prey on diatoms or other small planktons. Therefore, the yield of cysts of autotrophic dinoflagellates should be higher than those of heterotrophs. The increased proportion of heterotrophic cysts indicates sufficient food resources for them, which has been regarded as an indicator of eutrophication (Matsuoka, 1999; Kang et al., 2021). Cyst assemblages in the Qingdao coast were dominated by cysts of autotrophic dinoflagellates with an average proportion of 76.48%, suggesting that the water quality in the Qingdao coast has not reached eutrophication level yet. Though nutrient levels had greatly increased in Jiaozhou Bay in recent decades (Shen et al., 2016), nutrient concentrations decreased gradually during 2010–2016, and the water quality had a slightly enriched level based on the national standard of the Marine Water Quality of China (GB3097-1997) (Yuan et al., 2018). Results from pollution assessment of biogenic elements indicated that TOC in the surface sediments from the Qingdao coast belonged to the uncontaminated level, while TN and TP reached moderate pollution levels (Lei et al., 2021). However, cysts of heterotrophic Protoperidiniaceae significantly contributed to cyst assemblages, especially in Jiaozhou Bay with an average proportion of 39.56% (Figure 4D). Diatoms generally dominated in phytoplankton community in Jiaozhou Bay, and related blooms have occurred frequently (Yao et al., 2010; Shen et al., 2016). Sufficient food supply (diatoms) promotes the growth of heterotrophic dinoflagellates and thus leads to the increased production of cysts of heterotrophic dinoflagellates.
Seventeen cyst taxa of HAB dinoflagellates were identified in this study, namely, 8 toxin-producing species and 9 bloom species (Table 1). Cysts of the potential YTX producer, Gonyaulax spinifera, Lingulodinium polyedra, and Protoceratium reticulatum (Chikwililwa et al., 2019), were detected in this study. Cysts of G. spinifera were recorded at all stations and ranked 4th in abundance (Figure 6). Coincidentally, large numbers of G. spinifera sequences were analyzed in our metabarcoding study using the same sediments (Wang et al., 2022). Blooms of G. spinifera occurred in Jiaozhou Bay in 2003 and 2007, respectively (Zhou et al., 2020). As a cyst-forming species, G. spinifera can form various types of cysts, mostly belonging to the cyst genus Spiniferites with wide morphological variations (Rochon et al., 2009). The wide and abundant occurrence of G. spinifera cysts in the Qingdao coast indicated high numbers of its vegetative cells in the water column and the high risk of its blooms.
Species in Alexandrium are the major producers of PSP (Lundholm et al., 2009 onwards). Alexandrium blooms have occurred frequently in the Chinese coastal waters (Yu et al., 2020), and cysts of Alexandrium were widely distributed in sediments of the China coasts (Tang et al., 2021). Meanwhile, PSP toxins have also been detected in phytoplankton and shellfish samples (Zou et al., 2014; Liu et al., 2017). Four cyst types of the PSP producers were detected in this study, i.e., Alexandrium andersonii, A. minutum, A. tamarense, and Gymnodinium catenatum, in which A. Andersonii was widely distributed with high concentrations, indicating a potential risk of Alexandrium blooms and PSP events in the Qingdao coast.
The potential AZP producer, Azadinium poporum, occurred in all stations except for those in the inner Jiaozhou Bay, and ranked the sixth most abundant cyst type (Figure 6). A. poporum was reported to distribute widely in surface sediments from the Chinese coasts (Gu et al., 2013; Liu et al., 2020; Tang et al., 2021), and 13 out of 16 strains of A. poporum from different geographic locations along the Chinese coastline contained AZPs (Krock et al., 2014). However, species in Azadinium has been seldom reported in the phytoplankton survey, which might be ignored due to their small sizes.
Biecheleria halophila was the most abundant cyst type in sediments from the Qingdao coast in our study. However, this species was rarely observed in the previous routine phytoplankton surveys due to the small size and frangible thin wall. Biecheleria has become more frequently detected in the phytoplankton communities as the development of molecular biological techniques (Sundstrom et al., 2010). Taxa in Biecheleria generally form resting cysts (Moestrup et al., 2009), which made them common dominant eukaryotes in sediments based on metabarcoding analysis (Dzhembekova et al., 2018; Liu et al., 2020; Rhodes et al., 2020). B. halophila was reported to form a co-occurring bloom with Scrippsiella hangoei in the Baltic Sea (Kremp et al., 2005). The wide and abundant occurrence of cysts of B. halophila in the Qingdao coast suggests that it is a common dominant dinoflagellate species in this sea area, but might be ignored during the microscopic observation because of their fragility and small size.
Cysts of Scrippsiella acuminata occurred in all stations and ranked second in abundance (Figure 6). S. acuminata is a common bloom species in coastal waters (Wang et al., 2007; Zinssmeister et al., 2011), which is easy to form cysts, making its cysts dominant in sediments from worldwide coasts (Tang et al., 2021). In China, the recurrent blooms of S. acuminata have occurred in Daya Bay, South China Sea since the end of the 1990s (Wang et al., 2007). Pseudocochlodinium profundisulcus and Levanderina fissa are common bloom species in the Pearl River Estuary of the South China Sea, and their blooms have frequently occurred in the recent two decades (Wang et al., 2001; Shen et al., 2012; Dong et al., 2020). Their cysts were detected in most stations in this study, and ranked the 13th and 14th most abundant cyst taxa (Figure 6). Shang et al. (2022) detected cysts of P. profundisulcus in the ballast tank of an international ship arriving at the Jiangyin Port (China), and successfully germinated cysts into the vegetative cells, and thus suggested the feasibility of the bio-invasion risk via the transport of live resting cysts by ship’s ballast tanks. Although these algal blooms have not occurred in the coastal waters of Qingdao, cysts of the toxic and harmful dinoflagellates were distributed widely and abundantly in surface sediments, indicating the potential risk of these algal blooms in the Qingdao coast to some extent.
This study provides an overview of dinocyst assemblages (including those of HAB species) from three different sea areas in the Qingdao coast, the Yellow Sea, China. Our results suggested the quite different cyst assemblages in the inner Jiaozhou Bay, which is a shallow enclosed embayment with intensive human activities, reflected by lower cyst diversity and concentration, and higher proportion of cysts of heterotrophic dinoflagellates. Notably, 17 HAB dinocysts were identified in this study, including cysts of the PSP producers Alexandrium andersonii, A. minutum, A. tamarense, and Gymnodinium catenatum; the YTX producers Gonyaulax spinifera, Lingulodinium polyedra, and Protoceratium reticulatum; the AZP producer Azadinium poporum; and the ichthyotoxic species Pseudocochlodinium profundisulcus. Cysts of the toxic and harmful dinoflagellates were distributed widely and abundantly in surface sediments, indicating the potential risk of HABs in the Qingdao coast to some extent.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
ZW, YT, and RH designed the experiment and prepared the manuscript. YZ, ML, and SJ completed the experiment. JC and HZ conducted statistical analyses. All authors contributed to the article and approved the submitted version.
This work was supported by the Science and Technology Basic Resources Investigation Program of China (No. 2018FY100200) and the National Natural Science Foundation of China (No. 42076141), and Key Program of Marine Economy Development (Six Marine Industries) Special Foundation of Department of Natural Resources of Guangdong Province (No. GDNRC[2021]37).
The handling Editor AL declared a past co-authorship with the author ZW. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Prof. Tao Jiang for collecting sediment samples. We also thank the funding program from the National Natural Science Foundation of China and Science and Technology Basic Resources Investigation Program of China.
Anderson D. M., Keafer B. A., Kleindinst J. L., McGillicuddy D. J. Jr., Martin J. L., Norton K., et al. (2014). Alexandrium Fundyense Cysts in the Gulf of Maine: Long-Term Time Series of Abundance and Distribution, and Linkages to Past and Future Blooms. Deep-Sea Res. PT II. 103, 6–26. doi: 10.1016/j.dsr2.2013.10.002
Aydin H., Yurur E. E., Uzar S., Kucuksezgin F. (2015). Impact of Industrial Pollution on Recent Dinoflagellate Cysts in Izmir Bay (Eastern Aegean). Mar. pollut. Bull. 94, 144–152. doi: 10.1016/j.marpolbul.2015.02.038
Balkis N., Atabay H., Türetgen I., Albayrak S., Balkis H., Tüfekçi V. (2011). Role of Single-Celled Organisms in Mucilage Formation on the Shores of Büyükada Island (the Marmara Sea). J. Mar. Biol. Assoc. UK 91, 771–781. doi: 10.1017/S0025315410000081
Bravo I., Figueroa R. I. (2014). Towards an Ecological Understanding of Dinoflagellate Cyst Functions. Microorganisms 2, 11–32. doi: 10.3390/microorganisms2010011
Castaneda-Quezada R., García-Mendoza E., Ramírez-Mendoza R., Helenes J., Rivas D., Romo-Curiel A., et al. (2021). Distribution of Gymnodinium Catenatum Graham Cysts and its Relation to Harmful Algae Blooms in the Northern Gulf of California. J. Mar. Biol. Assoc. UK 101, 895–909. doi: 10.1017/S0025315421000795
Chikwililwa C., McCarron P., Waniek J. J., Schulz-Bull D. E. (2019). Phylogenetic Analysis and Yessotoxin Profiles of Gonyaulax Spinifera Cultures From the Benguela Current Upwelling System. Harmful Algae 85, 101626. doi: 10.1016/j.hal.2019.101626
Dong Y., Cui L., Cao R., Cen J., Zou J., Zhou X., et al. (2020). Ecological Characteristics and Teratogenic Retinal Determination of Cochlodinium Geminatum Blooms in Pearl River Estuary, South China. Ecotoxicol. Environ. Safe. 191, 110226. doi: 10.1016/j.ecoenv.2020.110226
Dzhembekova N., Moncheva S., Ivanova P., Slabakova N., Nagai S. (2018). Biodiversity of Phytoplankton Cyst Assemblages in Surface Sediments of the Black Sea Based on Metabarcoding. Biotechnol. Biotechnol. Equip. 32, 1507–1513. doi: 10.1080/13102818.2018.1532816
Ellegaard M., Ribeiro S. (2018). The Long-Term Persistence of Phytoplankton Resting Stages in Aquatic 'Seed Banks'. Biol. Rev. Camb. Philos. Soc 93, 166–183. doi: 10.1111/brv.12338
Genovesi-Giunti B., Laabir M., Vaquer A. (2006). The Benthic Resting Cyst: A Key Actor in Harmful Dinoflagellate Blooms-Areview. Vie Milieu / Life Environ. 56, 327–337. doi: 10.1016/j.tree.2006.09.005
Gomez F. (2012). A Quantitative Review of the Lifestyle, Habitat and Trophic Diversity of Dinoflagellates (Dinoflagellata, Alveolata). System. Biodivers 10, 267–275. doi: 10.1080/14772000.2012.721021
Gu H., Luo Z., Krock B., Witt M., Tillmann U. (2013). Morphology, Phylogeny and Azaspiracid Profile of Azadinium Poporum (Dinophyceae) From the China Sea. Harmful Algae 21-22, 64–75. doi: 10.1016/j.hal.2012.11.009
Gu H., Mertens K. D., Amelie D., Gwenael B., Zhen L., Philipp H., et al. (2022). Unravelling the Gonyaulax Baltica Species Complex: Cyst-Theca Relationship of Impagidinium Variaseptum, Spiniferites Pseudodelicatus Sp. Nov. And S. Ristingensis (Gonyaulacaceae, Dinophyceae), With Descriptions of Gonyaulax Bohaiensis Sp. Nov, G. Amoyensis Sp. Nov. And G. Portimonensis Sp. Nov. J. Phycol. doi: 10.1111/jpy.13245
Hallegraeff G. M. (1993). A Review of Harmful Algal Blooms and Their Apparent Global Increase. Phycologia 32, 79–99. doi: 10.2216/i0031-8884-32-2-79.1
Hallegraeff G. M., Anderson D. M., Cembella A. D. (2003). Manual on Harmful Marine Microalgae (Paris: UNESCO Publishing).
Head M. J. (1996). ““Modern Dinoflagellate Cysts and Their Biological Affinities”,” in The Palynology: Principles and Applications. Eds. Jansonius J., McGregor D. C. (U.S.A: AASP), 1197–1248.
Hu Z., Xu N., Gu H., Chai Z., Takahashi K., Li Z., et al. (2021). Morphomolecular Description of a New HAB Species, Pseudocochlodinium Profundisulcus Gen. Et Sp. Nov., and its LSU rRNA Gene Based Genetic Diversity and Geographical Distribution. Harmful algae 108, 102098. doi: 10.1016/j.hal.2021.102098
Joyce L. B., Pitcher G. C., Randt A. D., Monteiro P. M. S. (2015). Dinoflagellate Cysts From Surface Sediments of Saldanha Bay, South Africa: An Indication of the Potential Risk of Harmful Algal Blooms. Harmful Algae 5, 309–318. doi: 10.1016/j.hal.2004.08.001
Kang Y., Kim H. J., Moon C. H. (2021). Eutrophication Driven by Aquaculture Fish Farms Controls Phytoplankton and Dinoflagellate Cyst Abundance in the Southern Coastal Waters of Korea. J. Mar. Sci. Eng. 9, 362. doi: 10.3390/jmse9040362
Kremp A., Elbrachter M., Schweikert M., Wolny J. L., Gottschling M. (2005). Woloszynskia Halophila (Biecheler) Comb. Nov.: A Bloom-Forming Cold-Water Dinoflagellate Co-Occurring With Scrippsiella Hangoei (Dinophyceae) in the Baltic Sea. J. Phycol 41, 629–642. doi: 10.1111/j.1529-8817.2005.00070.x
Krock B., Tillmann U., Voss D., Koch B. P., Salas R., Witt M., et al. (2012). New Azaspiracids in Amphidomataceae (Dinophyceae). Toxicon 60, 830–839. doi: 10.1016/j.toxicon.2012.05.007
Krock B., Tillmann U., Witt M., Gu H. (2014). Azaspiracid Variability of Azadinium Poporum (Dinophyceae) From the China Sea. Harmful Algae 36, 22–28. doi: 10.1016/j.hal.2014.04.012
Lee S. Y., Jeong H. J., Kim S. J., Lee K. H., Jang S. H. (2019). Scrippsiella Masanensis Sp. Nov. (Thoracosphaerales, Dinophyceae), a Phototrophic Dinoflagellate From the Coastal Waters of Southern Korea. Phycologia 58, 287–299. doi: 10.1080/00318884.2019.1568794
Lei M., Wang Z., Jiang T. (2021). Distribution and Pollution Assessment of Biogenic Elements in Surface Sediments From Qingdao Coastal Area. Mar. Environ. Sci. 40, 93–100. doi: 10.13634/j.cnki.mes.2021.01.013
Li F., Li X., Qi J., Song J. (2011). Accumulation of Heavy Metals in the Core Sediments From the Jiaozhouwan Bay During Last Hundred Years and its Environmental Significance. J. Mar. Sci. 29, 35–45. doi: 10.3969/j.issn.1001-909X.2011.02.004
Limoges A., Kielt J. F., Radi T., Ruiz-Fernandez A. C., Vernal A. D. (2010). Dinoflagellate Cyst Distribution in Surface Sediments Along the South-Western Mexican Coast (14.76 Degrees N to 24.75 Degrees N). Mar. Micropaleontol 76, 104–123. doi: 10.1016/j.marmicro.2010.06.003
Li Y., Tang Y., Shen P., Gu H., Tan Y. (2017). Distribution of Dinoflagellate Resting Cysts in Surface Sediment of Jiaozhou Bay, China. Oceanol. Limnol. Sin. 48, 760–766. doi: 10.11693/hyhz20161200283
Liu S., Chen N. (2021). Advances in Biodiversity Analysis of Phytoplankton and Harmful Algal Bloom Species in the Jiaozhou Bay. Mar. Sci. 45, 170–188. doi: 10.11759/hykx20201021003
Liu D., Shi Y., Di B., Sun Q., Wang Y., Dong Z., et al. (2012). The Impact of Different Pollution Sources on Modern Dinoflagellate Cysts in Sishili Bay, Yellow Sea, China. Mar. Micropaleontol. 84-85, 1–13. doi: 10.1016/j.marmicro.2011.11.001
Liu L., Wang Z., Lu S. (2020). Diversity and Geographical Distribution of Resting Stages of Eukaryotic Algae in the Surface Sediments From the Southern Chinese Coastline Based on Metabarcoding Partial 18S rDNA Sequences. Mar. Ecol. 41, e12585. doi: 10.1111/maec.12585
Liu Y., Yu R., Kong F., Chen Z., Dai L., Gao Y., et al. (2017). Paralytic Shellfish Toxins in Phytoplankton and Shellfish Samples Collected From the Bohai Sea, China. Mar. pollut. Bull. 115, 324–331. doi: 10.1016/j.marpolbul.2016.12.023
Lundholm N., Churro C., Hoppenrath M., Iwataki M., Larsen J., Mertens K., Moestrup Ø., Zingone A. (2009 onwards) IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. Available at: https://www.marinespecies.org/habon2022-03-11.
Lu X., Wang Z., Gu X., Gu Y., Liang W., Liu L. (2017). Impacts of Metal Contamination and Eutrophication on Dinoflagellate Cyst Assemblages Along the Guangdong Coast of Southern China. Mar. pollut. Bull. 120, 239–249. doi: 10.1016/j.marpolbul.2017.05.032
Matsuoka K. (1999). Eutrophication Process Recorded in Dinoflagellate Cyst Assemblages-a Case of Yokohama Port, Tokyo Bay, Japan. Sci. Total. Environ. 231, 17–35. doi: 10.1016/S0048-9697(99)00087-X
Matsuoka K., Fukuyo Y. (2000). Technical Guide for Modern Dinoflagellate Cyst Study (Tokyo: Japan Society for the Promotion of Science). WESTPAC-HAB.
Moestrup O., Lindberg K., Daugbjerg N. (2009). Studies on Woloszynskioid Dinoflagellates IV: The Genus Biecheleria Gen. Nov. Phycol. Res. 57, 203–220. doi: 10.1111/j.1440-1835.2009.00540.x
Mortlock R. A., Froelich P. N. (1989). A Simple Method for the Rapid Determination of Biogenic Opal in Pelagic Marine Sediments. Deep-Sea Res. Part I. 36, 1415–1426. doi: 10.1016/0198-0149(89)90092-7DEEP-SEARESPTI
Pospelova V., Chmura G. L., Boothman W., Latimer J. S. (2005). Spatial Distribution of Modern Dinoflagellate Cysts in Polluted Estuarine Sediments From Buzzards Bay (Massachusetts, USA) Embayments. Mar. Ecol. Prog. Ser. 292, 23–40. doi: 10.3354/meps292023
Rhodes L. L., Smith K. F., MacKenzie L., Moisan C. (2020). Checklist of the Planktonic Marine Dinoflagellates of New Zealand. New Zeal. J. Mar. Fresh 54, 86–101. doi: 10.1080/00288330.2019.1626746
Rochon A., Lewis J., Ellegaard M., Harding L. C. (2009). The Gonyaulax Spinifera (Dinophyceae) "Complex": Perpetuating the Paradox? Rev. Palaeobot. Palynol 155, 52–60. doi: 10.1016/j.revpalbo.2008.12.017
Shang L., Zhai X., Tian W., Liu Y., Han Y., Deng Y., et al. (2022). Pseudocochlodinium Profundisulcus Resting Cysts Detected in the Ballast Tank Sediment of Ships Arriving in the Ports of China and North America and the Implications in the Species' Geographic Distribution and Possible Invasion. Int. J. Environ. Res. Public Health 19, 299. doi: 10.3390/ijerph19010299
Shen P., Li Y., Qi Y., Zhang L., Tan Y., Huang L. (2012). Morphology and Bloom Dynamics of Cochlodinium Geminatum (Schütt) Schütt in the Pearl River Estuary, South China Sea. Harmful Algae 13, 10–19. doi: 10.1016/j.hal.2011.09.009
Shen Z., Yao Y., Wu Y. (2016). Silica Supply and Diatom Blooms in the Jiaozhou Bay, China. Acta Oceanol. Sin. 35, 20–27. doi: 10.1007/s13131-016-0917-7
Shi J., Leng Q., Zhu J., Gao H., Guo X., Mao X. (2020). Influences of Nutrient Sources on the Alternation of Nutrient Limitations and Phytoplankton Community in Jiaozhou Bay, Southern Yellow Sea of China. Sustainability 12, 2224. doi: 10.3390/su12062224
Sidabutar T., Srimariana E. S. (2021). Dinoflagellate Cyst Assemblages in Surface Sediment From Jakarta Bay. IOP Conf. Ser.: Earth Environ. Sci. 718, 12091. doi: 10.1088/1755-1315/718/1/012091
Smayda T. (1997). Harmful Algal Blooms: Their Ecophysiology and General Relevance to Phytoplankton Blooms in the Sea. Limnol. Oceanogr 42, 1137–1153. doi: 10.4319/lo.1997.42.5_part_2.1137
Sundström A. M., Kremp A., Daugbjerg N., Moestrup Ø., Ellegaard M., Hansen R., et al. (2009). Gymnodinium Corollarium Sp. Nov. (Dinophyceae)- a New Cold-Water Dinoflagellate Responsible for Cyst Sedimentation Events in the Baltic Sea. J. Phycol. 45, 938–952. doi: 10.1111/j.1529-8817.2009.00712.x
Sundstrom A. M., Kremp A., Tammilehto A., Tuimala J., Larsson U. (2010). Detection of the Bloom-Forming Cold-Water Dinoflagellate Biecheleria Baltica in the Baltic Sea Using LSU rRNA Probes. Aquat. Microb. Ecol. 61, 129–140. doi: 10.3354/ame01442
Tang Y., Gu H., Wang Z., Liu D., Wang Y., Lu D., et al. (2021). Exploration of Resting Cysts (Stages) and Their Relevance for Possibly HABs-Causing Species in China. Harmful Algae 107, 102050. doi: 10.1016/j.hal.2021.102050
Taylor F. J. R., Fukuyo Y., Hallegraeff G. M. (2003). “Taxonomy of Harmful Dinoflagellates,” in Manual on Harmful Marine Microalgae. Eds. Hallegraeff G. M., Anderson D. M., Cembella A. D. (Paris; UNESCO), 389–432.
Thien S. J., Myers R. (1992). Determination of Bioavailable Phosphorus in Soil. Soil Sci. Soc Am. J. 56, 814–818. doi: 10.2136/sssaj1992.03615995005600030023x
Wang Z., Lei M., Ji S., Xie C., Chen J., Li W., et al. (2022). Comparison in Diversity of Eukaryotic Algae in Surface Sediments From Different Functional Sea Areas of Qingdao Coast, the Yellow Sea, China: A Metabarcoding Approach. J. Oceanol. Limnol. doi: 10.1007/s00343-021-1200-0
Wang Z., Qi Y., Yang Y. (2007). Cyst Formation: An Important Mechanism for the Termination of Scrippsiella Trochoidea (Dinophyceae) Bloom. J. Plankton Res. 29, 209–218. doi: 10.1093/plankt/fbm008
Wang Z., Qi Y., Yin Y., Jiang T., Xie L. (2001). Studies on the Cause and the Occurrence Reasons of a Gyrodinium Instriatum Red Tides in Shenzhen Bay in Spring of 1998. Mar. Sci. 25, 47–49. doi: 10.3969/j.issn.1000-3096.2001.05.015
Yao P., Yu Z., Deng C., Liu S., Zhen Y. (2010). Spatial-Temporal Distribution of Phytoplankton Pigments in Relation to Nutrient Status in Jiaozhou Bay, China. Estuar. Coast. Shelf. Sci. 89, 234–244. doi: 10.1016/j.ecss.2010.07.003
Yuan H., Song J., Xing J., Li X., Li N., Duan L. (2018). Spatial and Seasonal Variations, Partitioning and Fluxes of Dissolved and Particulate Nutrients in Jiaozhou Bay. Cont. Shelf Res. 171, 140–149. doi: 10.1016/j.csr.2018.11.004
Yu R., Lu S., Qi Y. (2020). Progress and Perspectives of Harmful Algal Bloom Studies in China. Oceanol. Limnol. Sin. 51, 768–788. doi: 10.11693/hyhz20200400127
Yu L., Wu X., Zheng X., Zheng T., Xin J., Walther M. (2019). An Index System Constructed for Ecological Stress Assessment of the Coastal Zone: A Case Study of Shandong, China. J. Environ. Manage. 232, 499–504. doi: 10.1016/j.jenvman.2018.11.084
Zhou J., Wang W., Wu Z., Wang Q., Wang Y., Gao X. (2020). The Basic Characteristics and Prevention Countermeasures of Red Tide in Shandong Coast Waters. Chin. J. Mar. Environ. Sci. 39, 537–543. doi: 10.13634/j.cnki.mes.2020.04.006
Zingone A., Enevoldsen H. O. (2000). The Diversity of Harmful Algal Blooms: A Challenge for Science and Management. Ocean Coast. Manag 43, 725–748. doi: 10.1016/S0964-5691(00)00056-9
Zinssmeister C., Soehner S., Facher E., Kirsch M., Meier K. S. J., Gottschling M. (2011). Catch Me If You can: The Taxonomic Identity of Scrippsiella Trochoidea (F. Stein) AR Loebl. (Thoracosphaeraceae, Dinophyceae). Syst. Biodivers. 9, 145–157. doi: 10.1080/14772000.2011.586071
Zonneveld K. A. F., Pospelova V. (2015). A Determination Key for Modern Dinoflagellate Cysts. Palynology 39, 387–409. doi: 10.1080/01916122.2014.990115
Keywords: dinoflagellate cysts, sediment, harmful algal bloom, Qingdao Coast, the Yellow Sea, biogenic elements
Citation: Wang Z, Zhang Y, Lei M, Ji S, Chen J, Zheng H, Tang Y and Hu R (2022) Distribution of Dinoflagellate Cysts in Surface Sediments From the Qingdao Coast, the Yellow Sea, China: The Potential Risk of Harmful Algal Blooms. Front. Mar. Sci. 9:910327. doi: 10.3389/fmars.2022.910327
Received: 01 April 2022; Accepted: 23 May 2022;
Published: 22 June 2022.
Edited by:
Aifeng Li, Ocean University of China, ChinaReviewed by:
Pengbin Wang, Ministry of Natural Resources, ChinaCopyright © 2022 Wang, Zhang, Lei, Ji, Chen, Zheng, Tang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaohui Wang, dHd6aEBqbnUuZWR1LmNu; Yali Tang, bGl0YW5neWFsaUAxNjMuY29t; Ren Hu, dGh1cmVuQGpudS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.