- 1Bazaruto Center for Scientific Studies (BCSS), Benguerra Island, Inhambane, Mozambique
- 2Marine Biodiversity, Exploitation and Conservation (MARBEC), IRD/CNRS/IFREMER/University of Montpellier, Montpellier, France
- 3Department of Statistics, J. Londer Laboratories, Detroit, MI, United States
- 4Marine Ecology, Universidad de Las Palmas de Gran Canaria, Las Palmas, Spain
- 5National Oceanography Centre, University of Southampton Waterfront Campus, Southampton, United Kingdom
Post-collapse of seasonal blooms of gelatinous zooplankton (Cnidaria, Ctenophora, and Thaliacea) sinking carcasses transports labile carbon (jelly-C) to benthic continental margins and the deep sea. In recent decades, bloom frequency and intensity have increased globally; however, how sinking jelly-C affects benthic communities is poorly known. Further, as climate change and other anthropogenic impacts may increase gelatinous blooms in the future, understanding the contributions and impacts of jelly-C upon benthic communities is of pivotal importance. Thus, in this study, we assessed jelly-C deposits post-intense blooming of a pelagic species of marine colonial gelatinous tunicate in the Thaliacean class, Pyrosoma atlanticum. We studied the seabed using a remotely operated vehicle (ROV) from 26 to 1,276 m, documenting jelly-C deposits and species of the megafaunal benthic community. Environmental variables from water column profiles at transects near our own off the Ivory Coast of West Africa were used in assessments. Jelly-C biomass peaked at 400 m (1,500 grC 100 m−2) and remained at the average (300 grC 100 m−2) through 1,276 m. Typically, in depth strata between 300 and 800 m, peaks in jelly-C biomass (500 to 1,500 grC 100 m−2) corresponded to areas with significantly reduced benthic megafaunal species abundances as well as diversity. From 800 to 1,276 m, we noted patchier jelly-C biomass deposits where individual megafauna species abundances and diversity correspondingly increased, yet total organism counts remained low. We observed 11 species from 5 phyla directly feeding on jelly-C and 10 single-species aggregations triggering megafaunal dominance events at various localized depth strata. Although such dynamics have been hypothesized and examined for some time with respect to phytodetritus biomass, they have been rarely described for jelly-C. Thus, our novel findings for jelly-C dynamics in the offshore regions can help to provide a better understanding of the ecological role that this component plays in marine benthic ecosystems and continental margins.
Introduction
Marine zooplankton play prominent roles as ecosystem engineers in coastal and open ocean areas, as they link primary production, higher trophic levels, and deep-sea communities via carbon export (Purcell, 2005; Billett et al., 2006; Henschke et al., 2019; Lebrato et al., 2019). In particular, gelatinous zooplankton (Cnidaria, Ctenophora, and Thaliacea), herein referred to as jelly-carbon (jelly-C) (Lebrato et al., 2012), rapidly reproduce and, under favorable conditions, form large seasonal blooms. Globally, jelly-C accounts for 38.3 Tg C (1 Tg = 1012 g) of carbon as living biomass in the upper 200 m of the ocean (Lucas et al., 2014), with 0.038 Pg C year−1 being exported to the ocean interior (Lebrato et al., 2019). Despite being an important carbon export mechanism (Buesseler et al., 2007; Lebrato et al., 2012; Lebrato et al., 2013; Lebrato et al., 2019), its contribution is smaller than global primary production (50 Pg C year−1) (Field et al., 1998), which translates into export estimates close to 6 Pg C year−1 below 100 m (Moore et al., 2004; Siegel et al., 2014). Sinking and laterally transported jelly-C can fuel benthic ecosystem production in continental margins and the deep sea (Lebrato and Jones, 2009; Robinson et al., 2010; Lebrato et al., 2013; Smith et al., 2014), even supporting some commercial fisheries (Dunlop et al., 2017).
Sinking carbon reaching the seafloor shapes spatiotemporal ecological patterns in benthic communities, driving biological responses along bathymetric gradients (Pearson and Rosenberg, 1978; Gage and Tyler, 1991; Glover et al., 2010). Benthic organisms appear in successions that lag food supply, a dynamic that often depends upon a balance between the depth (Rex et al., 2006), organic matter input (Ruhl, 2007), and migratory capabilities within respective physiological and ecological limits (Drazen et al., 2012). The spatial structure of benthic communities often depends upon spatiotemporally variable and relatively localized food supplies; however, it is also driven by habitat heterogeneity, i.e., substrate (Gooday et al., 2010). Because of the inherent complexity and difficulty of studying biological, bathymetric, and chemical effects of organic matter, and other aspects related to jelly-C dynamics, their impacts upon benthic communities in vast oceanic areas including continental margins and deep-sea regions have been challenging to assess and characterize.
Recent assessments highlighted large amounts of jelly-C depositions in coastal areas, continental shelves, and slopes (Billett et al., 2006; Lebrato et al., 2012; Smith et al., 2014; Sweetman et al., 2014; Archer et al., 2018). Jelly-C depositions upon the seabed occur after seasonal accumulations of gelatinous zooplankton and subsequent die-offs and sinking through the water column. The bloom-bust dynamics of many gelatinous zooplankton populations exacerbates jelly-C export to the benthic environment, and biological processes at the seabed inundated with these materials can become limited. For example, such resulting seabed jelly-C can impact biogeochemical and ecological processes including reductions in available dissolved oxygen (DO); affecting dissolved inorganic carbon (DIC) levels; inducing the leaching of nitrogen and phosphorus (non-Redfield stoichiometry), a pH decline, and undesirable bacterial growth; and creating opportunities for meio-, macro-, and megafauna scavengers (West et al., 2009; Frost et al., 2012; Sweetman et al., 2016). Recent examinations of jelly-C biogeochemistry revealed decay-based pathways similar to other types of biomasses. In these pathways, jelly-C enters into food webs through benthic scavengers or microbial loops that promote decomposition in both the water column and at the seabed (Tinta et al., 2016). Microbial decay ensues rapidly in the initial days post-settlement and as materials become increasingly labile (Sempere et al., 2000). The rate of decay then declines, being partially controlled by temperature (Lebrato et al., 2011), until all material is microbially respired.
Despite the emergence of gelatinous creatures in imbalance with ecosystems in recent decades due to climate change and other anthropogenic factors, it still has taken a relatively long time to assemble field-based evidence describing some of the roles that jelly-C plays as an important source of labile materials to marine benthic communities. Congregations of brittle stars on jelly-C were first documented by Jumars (1976) at 1,000 m in the Pacific Ocean. Since then, a number of studies on jelly-C have been published, but its importance as a food source has not been acknowledged until recently (reviewed in Ates, 2017). Multiple studies have examined jelly-C using photographs and video-based evidence of organisms scavenging at different depths (Billett et al., 2006; Lebrato and Jones, 2009; Lebrato et al., 2012; Sweetman et al., 2014; Dunlop et al., 2017; Archer et al., 2018). In the Atlantic, Pacific, and Indian oceans, the taxonomic list of organisms scavenging on benthic jelly-C mostly includes fish, echinoderms, cnidaria (actinaria), crustaceans, and arthropods. In the Mediterranean Sea, jelly-C from the salp Soestia zonaria, the pyrosome Pyrosoma atlanticum, and the scyphozoan Periphylla periphylla has often been found in the guts of crustaceans (Fanelli and Cartes, 2008) and fishes (Carrasson and Cartes, 2002). Pelagic organisms also consume jelly-C, accounting for 20% to 40% of the diet in some species and up to 80% in others, including tuna, billfish, mackerel and penguin species, sun fish, turtles, fish larvae, and juveniles (Sampey et al., 2007; Llopiz et al., 2010; Cardona et al., 2012; Thiebot et al., 2017; Hays et al., 2018). Although jelly-C is low in energy content at only 0.5–6 gross energy dry mass kJ g−1, as compared to other food sources like fish (5–22 gross energy dry mass kJ g−1) or algae (>10 gross energy dry mass kJ g−1) (Doyle et al., 2007), reduced energy spent searching for carcasses and the lability of jelly-C compared to other sources of detritus may cancel such bioenergetic costs to scavengers (Sweetman et al., 2014).
When jelly-C reaches the seafloor, it is often scavenged by megafaunal organisms (Sweetman et al., 2014); sometimes, it is partly consumed (Lebrato and Jones, 2009); and occasionally, it remains intact, being respired by microbial communities (Billett et al., 2006). In all cases, however, jelly-C depositions impact the dynamics of seawater carbon and nutrient cycling, as well as overall water and localized benthic chemistry and microbial respiration (West et al., 2009; Sweetman et al., 2016). To date, assessments of the responses of macro- and megafaunal communities along bathymetric gradients in continental margins that are naturally enriched with jelly-C are unknown, except from experiments that used manually placed bait (Sweetman et al., 2014; Dunlop et al., 2017; Dunlop et al., 2018) and examinations of fixed stations only at single depths (Smith et al., 2014). Thus, in this study, we sought to combine observations from a benthic remotely operated vehicle (ROV) video survey and from water column profiles off the Ivory Coast of West Africa. Further, we sought to assess how freshly deposited jelly-C can shape benthic ecological processes and megafaunal diversity, as well as dominance, aggregation, and opportunistic events spanning sample locations across a vast bathymetric gradient. We expected that data from field observations could help provide strong supporting evidence for the importance of jelly-C and its roles in the dynamics of species in marine benthic ecosystems and continental margins.
Methods
Study Area
The Ivory Coast of West Africa faces the central Atlantic Ocean and has warm waters dominated by strong surface as well as deep currents, which can trigger seasonal upwelling (Bakun, 1978; Colin, 1988) (Figure 1A). The study area experiences zones of seasonal minima of DO; however, based upon assessments spanning from 1973 to 2010, these are not as strong as in other tropical areas such as the Arabian Sea (Gooday et al., 2010) (Figure 2B). However, biological production in our study area still remains relatively high with strong phytoplankton production consequently regularly triggering pelagic gelatinous zooplankton blooms exceeding hundreds of km2 (Goy, 1977; Roger, 1982; Le-Borgne, 1983). Few publications have assessed benthic ecology in the area (Buchanan, 1958; Koranteng, 2001). For our study, the benthic data were opportunistic and derived from oil and gas industry prospecting, off the Ghanaian Ivory Coast, West Africa (SPTEC Advisory; SERPENT Project; Lebrato and Jones, 2009) (Figure 1).
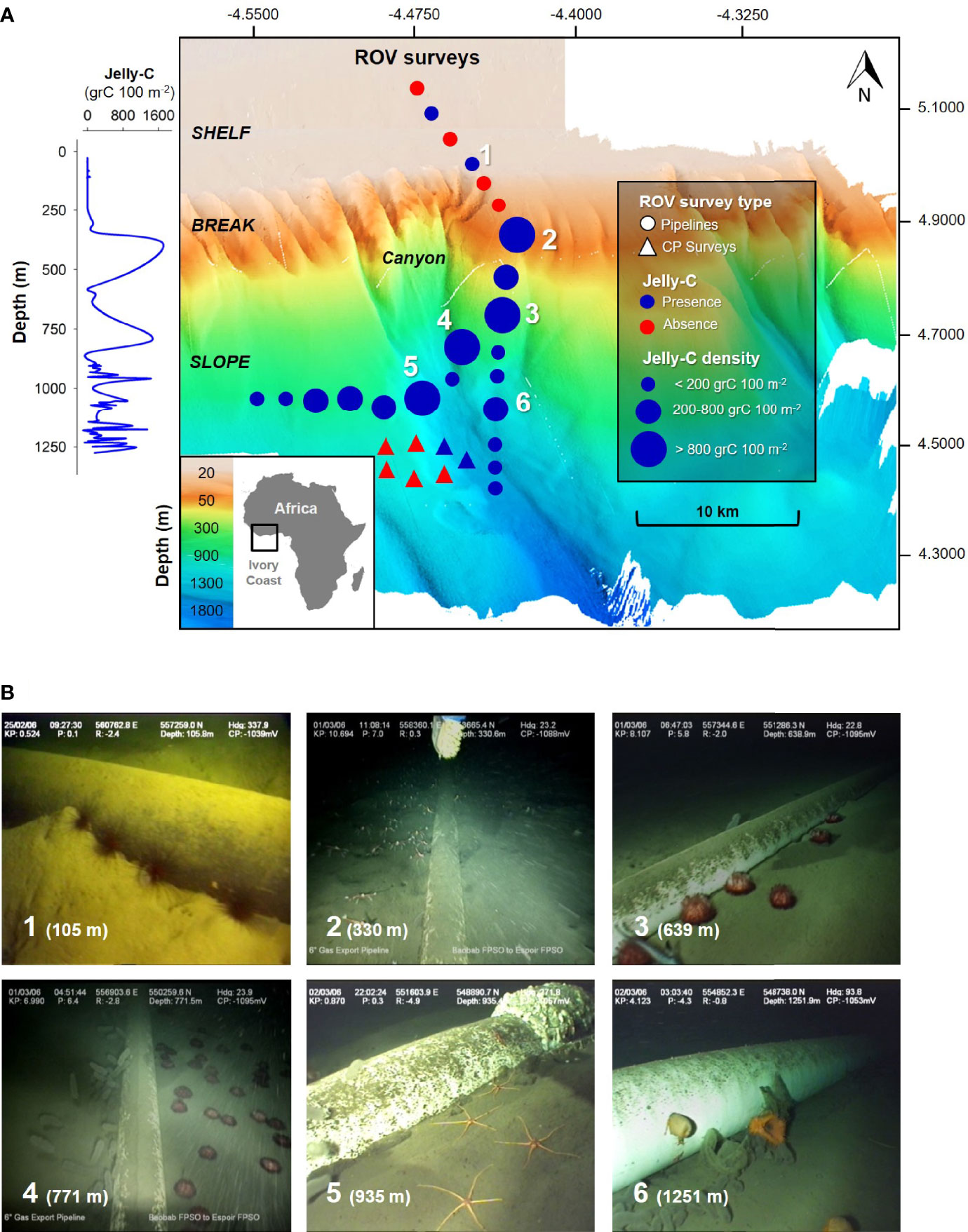
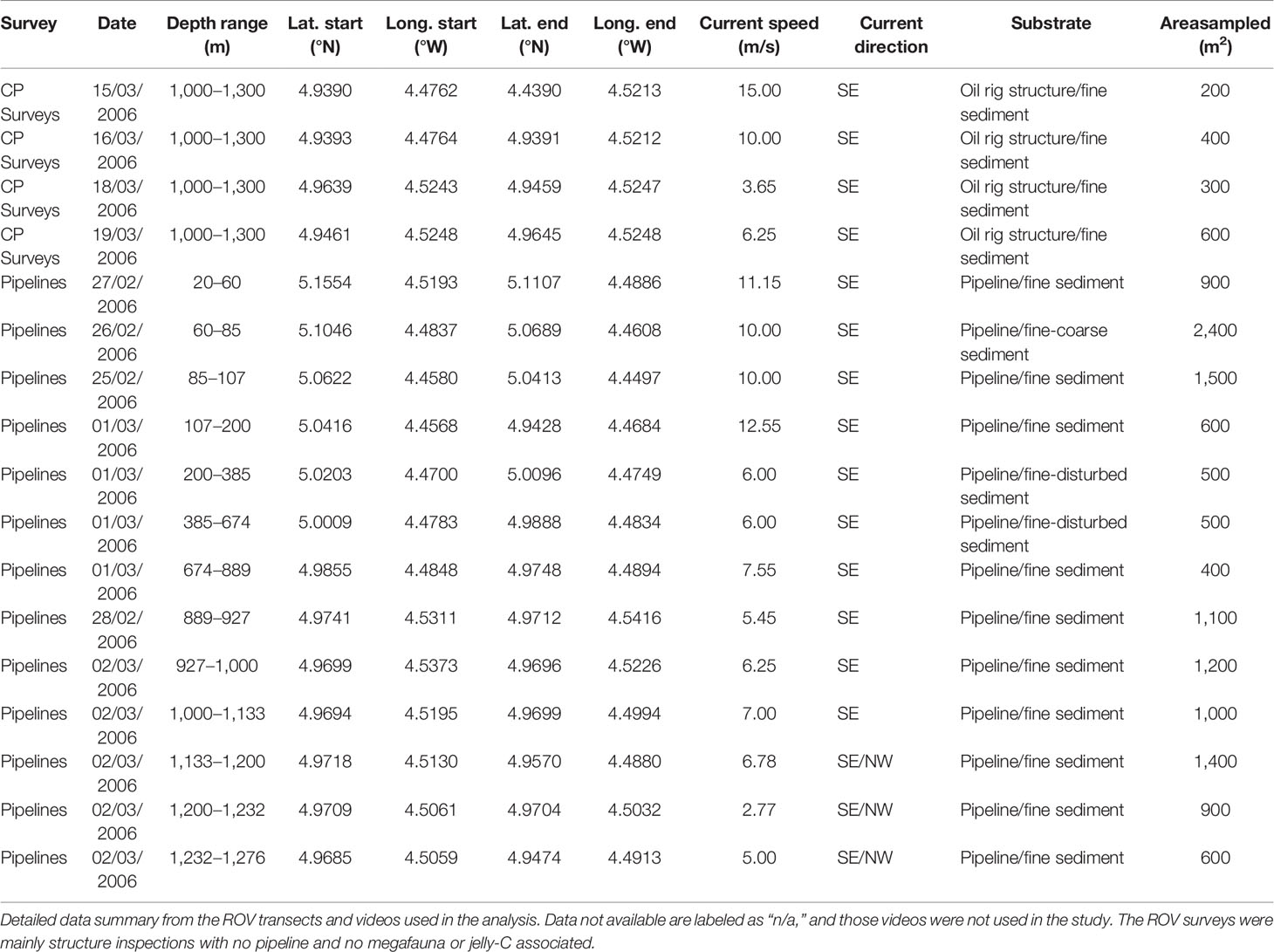
Figure 1 Map of the remotely operated vehicle (ROV) benthic surveys off the Ghanaian Ivory Coast (Africa) continental margin. (A) Pyrosome jelly-C benthic observations, biomass, and carbon estimations at the seafloor from 20 to 1,276 m near an oil pipeline in 2006 using an ROV. Survey types include “Pipelines” and “CP Surveys” alongside a canyon. (B) Examples of megafaunal organisms’ aggregations triggering dominance events by single species: 1, Diadema spp.; 2, Galathea sp.; 3–4, Phormosoma sp.; 5, Ophiolepadidae; 6, Actinostola sp. The numbers in panel A correspond to the areas of the observations/organisms in panel (B) A full taxonomic list of organisms from this study is available in Table 2. The 3D seabed map was rendered by Sonsub technical staff and was produced from the DP Reel Vessel using multibeam technology.
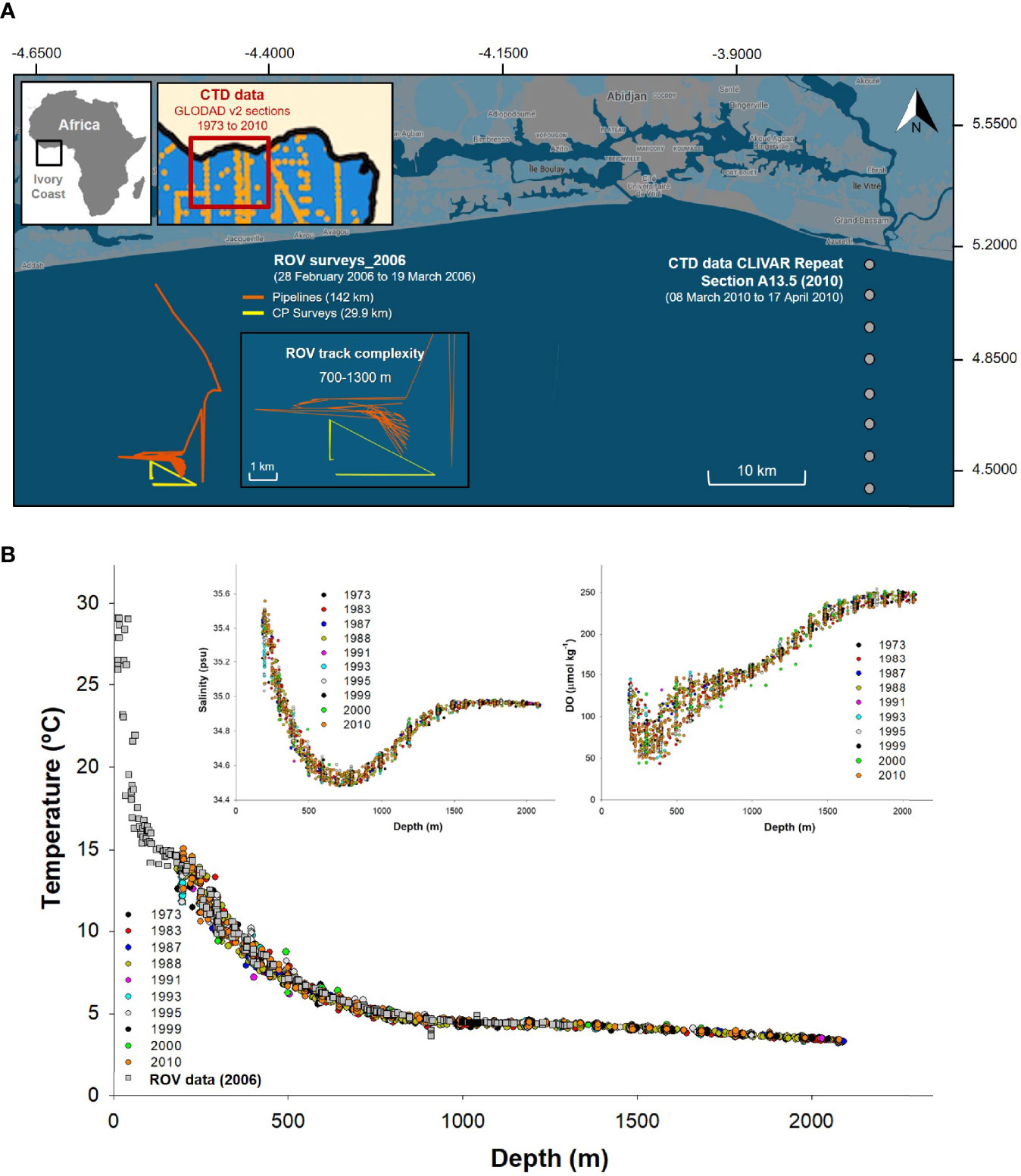
Figure 2 Detailed map of the remotely operated vehicle (ROV)-based surveys. Also included is the wider area from which water column profile data from GLODAP v2 were derived. Profiles of benthic environmental data vs. depth over time to justify the GLODAP v2 data use. (A) Detailed ROV-based survey map with tracks to help understand the complexity and positioning of the CLIVAR Repeat Section A13.5 2010 (closest year to 2006 with reliable data available) with respect to the ROV survey. Also included maps of the GLODAP v2 (https://www.nodc.noaa.gov/ocads/oceans/GLODAPv2/) transects from 1973 to 2010 used to understand water column profiles environmental data variability in the area. (B) Near-bottom water profiles and ROV-derived temperature, salinity, and dissolved oxygen (DO) profiles, from 1973 to 2010, were compared to justify the use of 2010 data to analyze the 2006 events. All 11 profiles were remarkably similar near the seabed, despite covering an area wider than 500 km across the Ghanaian Ivory Coast continental margin. The base map was retrieved from https://www.google.com/maps/d/u/0/edit?hl=en&mid=118kWIJnxdfJ_aa79Qq6JFqf-aedacoi7&ll=5.049633727701252%2C-4.504481&z=12, and then survey data were plotted on it (Google, n.d.).
The sample region off the Ghanaian Ivory Coast spanning the continental margin is very narrow. This facilitates relatively rapid carbon transfer to the deep sea upon the sinking of jelly-C biomass post-blooms. With steep slopes beginning at approximately 200 m, the seabed topography has many canyons and complex structures, making an assessment of relatively localized jelly-C deposits difficult but important. Thus, in our study, we sought to use ROV data in the form of video over a continuous depth range starting at 20 m through 1,276 m (also see Delteil et al., 1974; Basile et al., 1993). Our sampling region covered a broad range of a suite of environmental characteristics and parameters that we felt could help to objectively capture some of the dynamics underlying jelly-C in offshore benthic systems.
Remotely Operated Vehicle Benthic Transects
During an ongoing collaboration between the oil industry and marine sciences (SERPENT project; Gates et al., 2016), we gained access to ROV videos (Sonsub vehicle type, operated from the DP Reel Vessel) in the Baobab oil field (discovered in 2001) off Ghanaian Ivory Coast. The ROV video footage we selected was originally meant to study the mechanical state of the Baobab Oil Pipelines and their structures using umbilical-based inspections and cone penetrometer (CP) surveys. The ROV footage spanned dives completed upon days from February 28, 2006, to March 19, 2006, and coverage ranged from only 26 to a depth of 1,276 m (Figure 1A; Tables 1, 2). Most oil field structures coincided with large areas of seabed, and the ROV thoroughly inspected the entirety of the pipeline route from 26- to 1,276-m depth, mimicking a planned scientific survey (Figure 1A). The total pipeline-seabed distance covered and analyzed was 142 km of Pipelines and 29.9 km of CP Surveys (Figure 2A; Table 1). Surveys allowed for the identification of large depositions of jelly-C composed of P. atlanticum and megafaunal assemblages that covered over 10,000 m2 of the seafloor (Figure 1). A large number of ROV video transects were conducted for areas of relative plain seabed, or for very low-profiled pipelines (sometimes buried) showing megafaunal organisms aggregating and scavenging on jelly-C.
Remotely Operated Vehicle Videos and Ecological Data Acquisition
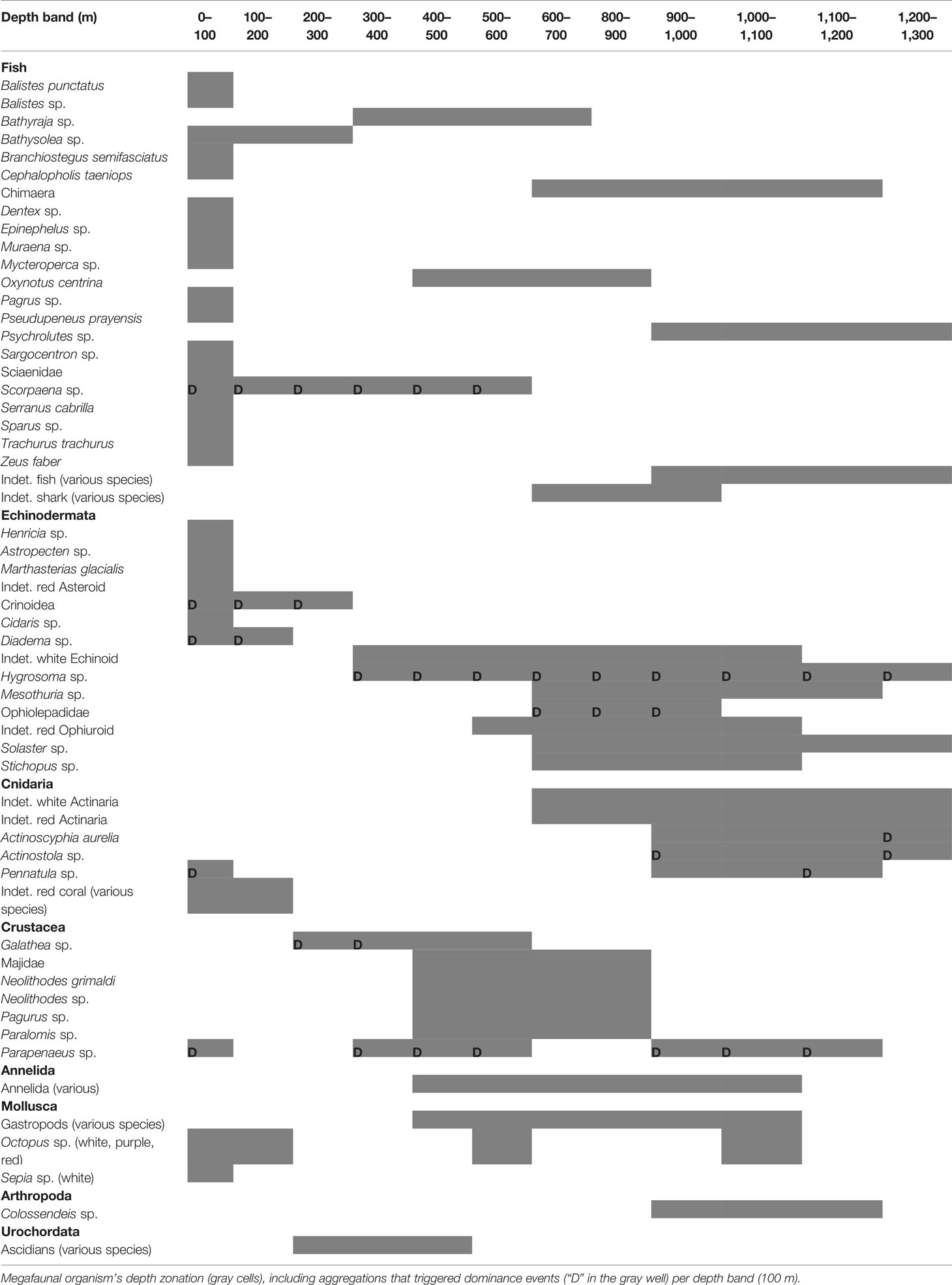
The ROV benthic transects were first analyzed in 2007–2008 using 309 videos with a focus on P. atlanticum biomass and jelly-C export (Lebrato and Jones, 2009), with a qualitative basic description of the ecology. Thus, herein we sought to re-analyze the benthic video surveys (“Pipelines” in Table 1), including additional videos (“CP Surveys” in Table 1), to assess quantitatively the importance of jelly-C depositions on major benthic ecological processes. All videos were assessed using VisualReview 6.5 (VisualSoft Limited). From a total of 705 initial videos including pipelines, seabed, and underwater machinery (summing over 150 h), 148 (summing over 33 h) showed concomitant jelly-C and megafaunal communities and thus were selected for further analyses. Following the methods outlined in Lebrato and Jones (2009), a sampling unit of video representing 100 m2 of the seabed was used. Using data quantified based on ROV position and scaled sizes of the nearby pipeline, we were able to assess P. atlanticum colony densities, sizes, and conditions (fresh, degraded, and decomposing, visually categorized), to provide a general overview (Figure 3). We also analyzed current speed and direction by using particle tracking in video images and estimated substrate type (pipeline or land-based materials, pipeline and fine sediments, pipeline and coarse sediment, and pipeline and disturbed sediment). Further, both the pipeline diameter (15, 25, and 30 cm) (Figure 3; Table 1) and megafauna size were estimated (for taxa > 50 mm in size) (see Jones et al., 2007) from the sub-selections of video. Megafaunal assessments for fine-scale patterns analyses were based upon 50-m2 sampling units in video transects. This was novel, in that this was at a higher spatial resolution for the visual assessments. Subsequently, our approach resulted in high megafaunal counts at similar individual depths where the seabed profile sloped gently (resulting in often extensive replication of measurements within a similar depth), but sometimes there were abrupt depth changes with fewer counts. We resolved this issue by estimating depth band averages for organisms every 50 and 100 m for statistical analyses to avoid the weighting of certain individual depths overriding others. Further, the averaging was performed so as to integrate zero measurements at certain depths. All megafaunal organisms were characterized by operational taxonomic units (OTUs) for further analyses. These OTUs were identified to at least the genus level and in many cases to the species level. In cases lacking positive identification, we allocated the respective organisms to family or class with respect to the most distinctive morphologies or colors (see Table 2 for a complete taxonomic list). As Supplementary Materials, the 705 transect videos were used to create two videos summarizing the findings: 1) a summary 12-min presentation (Video S1, https://www.youtube.com/watch?v=Ai0QODpqh1A) and 2) a detailed 130-min presentation (Video S2, https://www.youtube.com/watch?v=KejvhIczDXU). Videos include commentary from the first author herein. From visualizations, we provided an annotated document including any relevant feature, behavior, or event as well as working comments on observations (Text S1).
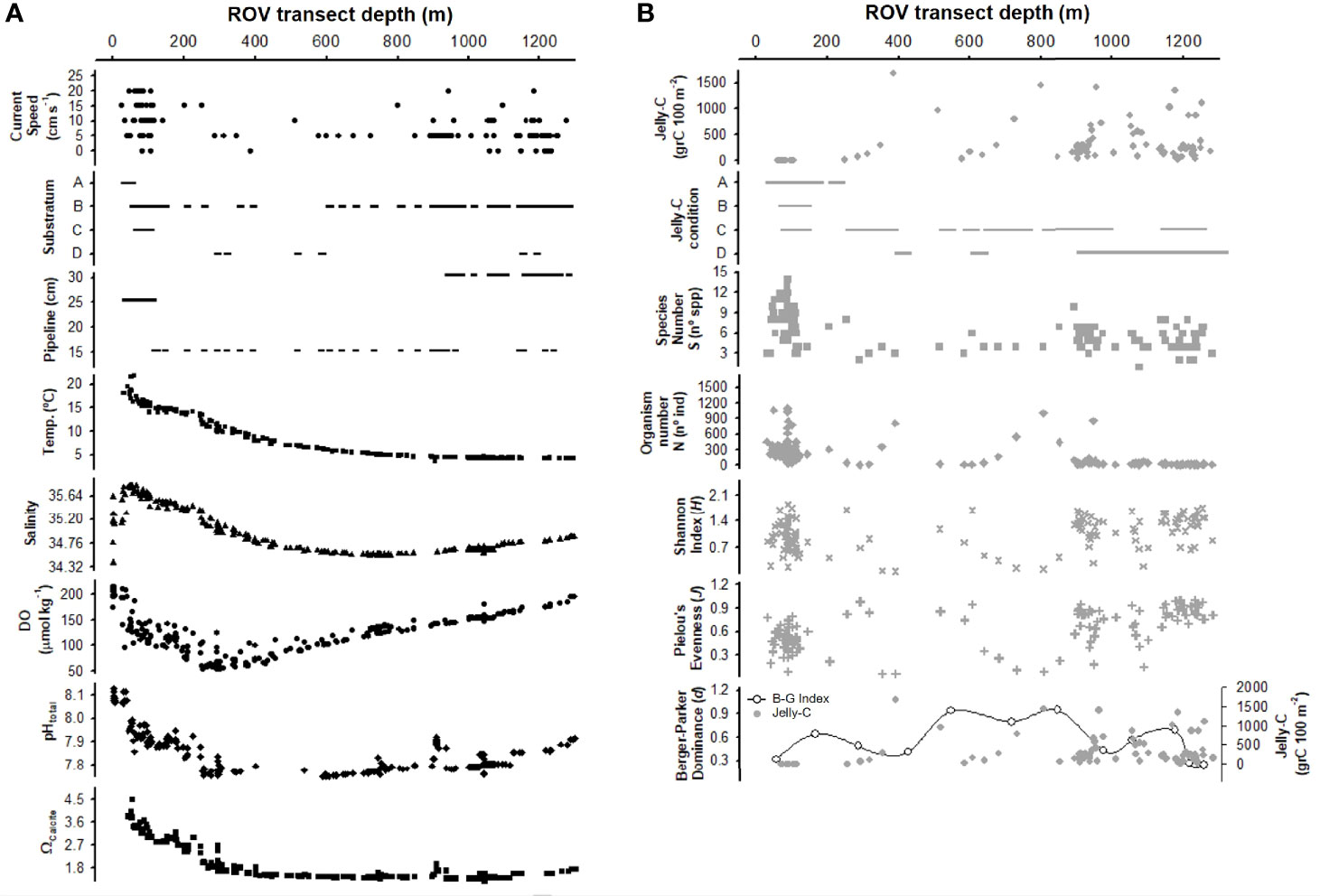
Figure 3 Broad-scale vertical comparison of abiotic and biotic variables along the bathymetric gradient. Abiotic (A) and biotic (B) variables used with 100-m2 transect data. Data were dependent upon the information available from remotely operated vehicle (ROV) videos: current speed, substratum (A, pipeline/land material; B, pipeline/fine sediment; C, pipeline/coarse sediment; D, pipeline/disturbed sediment), pipeline size, jelly-C per unit area, jelly-C condition (A, no jelly-C; B, fresh jelly-C; C, degraded jelly-C; D, decomposing jelly-C), species number (S), organism number (N), Shannon index (H), Pielou’s evenness (J), and Berger–Parker dominance (d), and the environmental data obtained from the water column surveys (temperature, salinity, dissolved oxygen (DO), pHtotal, and ΩCalcite). All data were plotted in raw format (no transformation) using 100-m2-sized sampling transects to provide an overall view of the system.
Jelly-C Analytics
Pyrosome carcasses were counted and measured for total length (nearest cm) individually from the video. Counts and metrics were extracted from still photos and imported into ImageJ software (Version 1.31; Schreider et al., 2012) to provide an average for each analyzed depth band using 50- and 100-m2 transects. Published equations were used to convert length to dry weight using P. atlanticum data (Mayzaud, 2007):
Pyrosome dry weight was converted to carbon units per 50- and 100-m2 transects using a value = 34.93% (organic carbon) measured in Lebrato and Jones (2009) and used by Lucas et al. (2011). Biomass and jelly-C data were used as the “organic” input to facilitate the assessment of the influence of the averaged depth bands (50 and 100 m), jelly-C food sources, and environmental variables upon the observances of benthic megafaunal assemblages (Figures 2B, 3).
Environmental Data and Limitations
Aspects of our study were limited by the absence of seawater environmental data obtained concurrently with the ROV surveys. However, measures for seawater temperature were obtained and taken from an ROV-based probe ( ± 0.43°C). Our limitations resulted from opportunistic uses of industry ROVs not equipped with complex oceanographic sensors but rather meant for visual-based monitoring. Of course, seawater chemistry influences benthic megafaunal assemblages beyond depth and food supplies (jelly-C). To account for the lack of ROV environmental parameters (excepting temperature), we used the GLODAPv2 database (https://www.nodc.noaa.gov/ocads/oceans/GLODAPv2/) to download data for sample sites in the Gulf of Guinea as relatively close as possible to our ROV survey sites (Key et al., 2015; Figure 2). We compiled water column profile data from 1973 to 2010 for individual sections A13, A13.5, and A14 to compare and understand the historical benthic environmental variability in the area near our bathymetric transects. Cubic line-adjusted regression equations were fitted to salinity, DO, total pH (pHtotal), and seawater saturation state of calcite (ΩCalcite) data using log10 data transformations (for normality, Anderson–Darling p < 0.005), adjusting to 95% CIs of individual profiles. The data that achieved the best fit were identified within the CLIVAR Repeat Section A13.5 2010 (https://www.nodc.noaa.gov/ocads/oceans/RepeatSections/clivar_a13.html). The best-fit data were used to represent average values for environmental conditions and parameters, along the bathymetric gradient (Figure 2). The CLIVAR Repeat Section A13.5 2010, for which we used parameters estimates other than the temperature for environmental conditions, lies only 60–70 km to the east of our ROV benthic survey (Figure 2). Further, assessments of the CLIVAR Repeat Section A13.5 2010 had overlapping dates with our own ROV-based surveys dates (ROV surveys from February 28, 2006, through March 19, 2006; CLIVAR A13.5 section assessed March 8, 2010, through April 17, 2010). We used water column profiles selecting only casts where the sample was taken <5 m from the seabed such as to best mimic near-seabed conditions. Furthermore, when ROV-derived temperature data were plotted concomitantly with the 1973–2010 CLIVAR Repeat Section A13.5 2010 temperature data, all data fit within the 95% CIs. In general, in deep-sea habitats, benthic seawater conditions deeper than 100–200 m tend to be stable, with lower variability with depth, likely alleviating some of the concerns regarding the use of nearby data instead of strictly ROV-derived data.
Ecological Indices
The total number of megafaunal individuals (“organism number N”) and the total number of species accounted for by those individuals (“species number S”) were calculated from the video-based megafaunal observations (Figures 3, 4). Ecological indices for diversity (Shannon; Shannon and Weaver, 1949), evenness (Pielou’s; Pielou, 1969), and dominance (Berger–Parker; Berger and Parker, 1970) were also calculated.
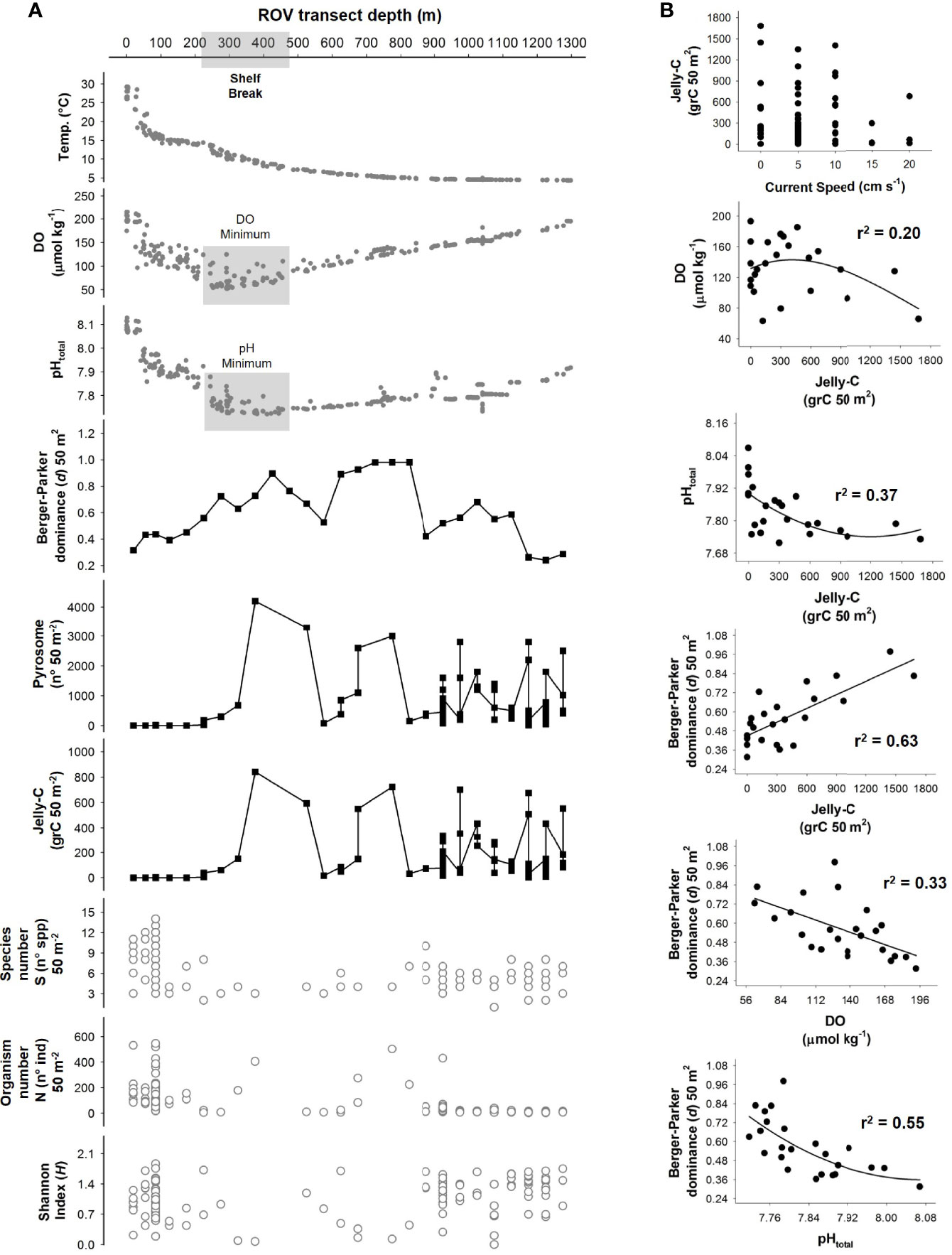
Figure 4 Fine-scale vertical comparison of abiotic and biotic variables along the bathymetric gradient as well as correlations of main explanatory variables. (A) Main environmental parameters compared with biological data on a detailed 50-m2 re-analysis of the remotely operated vehicle (ROV) transect-based data. This visualization helps to link the megafaunal aggregations, triggering dominance events to environmental context with respect to depth. (B) Individual correlations between the main variables driving the system were assessed according to multiple regression and principal component analysis (PCA). All data are plotted in raw format (no transformation) using 50-m2-sized sampling transects.
Data Analyses
The benthic megafaunal data (N, S, Shannon, Pielou, and Berger–Parker), other data extracted from the images (substratum, pipeline presence, jelly-C biomass, and jelly-C condition), and the environmental parameters (depth, temperature, pHtotal, and Ωcalcite) were analyzed using principal component analysis (PCA). PCA was used to facilitate the assessment of the main effects of biomass, environmental parameters, and ecological responses. In order to further analyze Berger–Parker dominance as a function of environmental parameters (depth, jelly-C, DO, pHtotal, and temperature), we used a multiple regression model following a normal distribution (K-S > 0.05). All statistics were calculated in Minitab 17 (Minitab LLC., 2016), plots were built in SigmaPlot 12 (SigmaPlot, 2013), and GLODAPv2 were data visualized with Ocean Data View (ODV 5.1.7; Schlitzer, 2015).
Results
Benthic surveys included areas of featureless seabed, shelf, slope, and canyon mouths (Figure 1A). We excluded deep canyons because many surveys were parallel to the coastline. Numerous visual observations of aggregation, triggering dominance events (wherein one or a few species dominated the seabed for a long period of time and or a wide area), occurred across the shelf and slope off the Ghanaian Ivory Coast (Figure 1B). At the depths noted, there were notable aggregations of Diadema sp. (105 m), Galathea sp. (330 m), Hygrosoma sp. (639 and 771 m), Ophiolepadidae (935 m), and Actinoscyphia aurelia and Actinostola sp. (1,251 m) (Figure 1; full taxonomic list in Table 2). Pyrosome jelly-C and megafauna were abundant from the entirety of our sampling depth range, essentially from the shelf to the deep slope; however, abundances and distributions were mosaic in pattern (Videos S1, S2; Table 2).
Shelf and slope environmental conditions varied considerably with depth. Benthic currents were strong on the shelf (up to 20 cm s−1), sharply decreased at the shelf break and in the shallow slope (5 cm s−1), and then increased again from 800 to 1,200 m (range 5 to 15 cm s−1) (Figure 2A). Current speed affected jelly-C transport and accumulation (see Videos S1, S2), with a critical threshold of 8–10 cm s−1 resuspending and moving carcasses downslope (Figure 2A). The substratum was mainly composed of soft and fine sediments with some presence of land-based debris and coarse sediment, which included rocks and pebbles (Figure 2A). Many small channels and depressions were observed. The seafloor was dominated by the pipeline, which itself had different textures, from the smooth metal surface of the pipeline to being covered in soft sediment and calcifying/crustose organisms (Figure 5). In many cases, the pipeline was completely buried in the seabed, producing deep channels. The pipeline varied in size (15, 25, and 30 cm wide) and occasionally trapped jelly-C at current speeds under 8 cm s−1 (Figures 2A, 3A). The pipeline did not exert any trapping effect with current speeds >8–10 cm s−1 (Figure 2A); however, the pipeline margins served as a shelter for organisms and as a substrate for attachment (Videos S1, S2). Bottom temperature profiles indicated a sharp decrease moving towards the shelf break and further decreases thereafter (Figure 2A). Bottom salinity varied in the first 50 m, remained constant through about 600 m when it decreased, and then increased again at 1,276 m. Bottom levels of DO sharply declined from transect initiation at 50 m to the shelf break at 200–250 m where minima occurred. Then, DO continually increased through 1,276 m. The bottom pHtotal had a similar profile to DO with a notably sharp decrease through the shelf break (Figure 2A). Bottom levels of Ωcalcite also declined through the shelf break where after levels remained relatively constant through 1,276 m (Figure 2A).
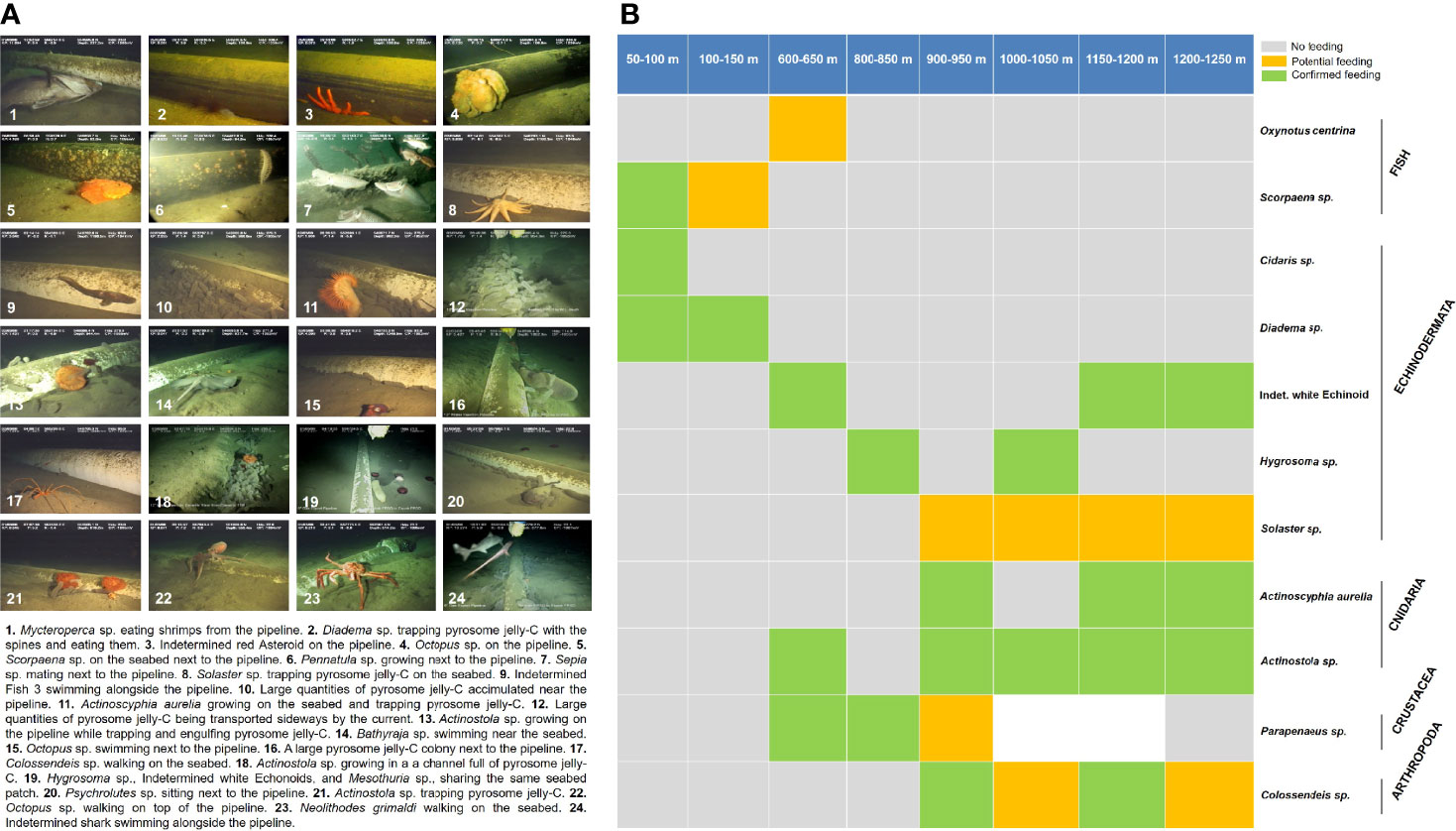
Figure 5 Remotely operated vehicle (ROV) survey photo summary and feeding events. (A) Photo summary of the benthic surveys including descriptions of interesting ecological events. (B) Details of the feeding events observed in the surveys along the bathymetric gradient. “No feeding” means the organism was not seen interacting with the jelly-C, “potential feeding” means the organism was seen manipulating the jelly-C but not feeding, and “confirmed feeding” means the organism was seen actively feeding, biting, or nibbling the jelly-C. A summary and the complete surveys are provided in Videos S1 and S2. A full taxonomic list of organisms from this study is available in Table 2.
Jelly-C standing stock biomass was relatively low on the shelf (under 10 grC 100 m−2) starting approximately 20 m and down through 200 m (Figure 2B). On the slope (deeper than 200 m), the jelly-C standing stock was much higher but variable with water depth (from 10 to 1,600 grC 100 m−2 near the 400-m depth zone). Relatively dense but patchy jelly-C distributions covered hundreds of meters of the seafloor (Videos S1, S2) and were mainly trapped by the pipeline on the shelf. In the slope, we made frequent observations of jelly-C being transported at 50 cm to 1 m above the seabed/pipeline downslope; however, jelly-C accumulation decreased linearly with increasing current speed, especially above the 8–10 cm s−1 threshold (Figure 2A). The jelly-C condition was fresh only within the shelf, and on the slope, P. atlanticum was degraded and decomposing (Figure 2B). In many cases, relatively fluffy in appearance jelly-C material was resuspended, which indicated advanced states of decomposition (see Videos S1, S2).
Many organisms were observed feeding or attempting to feed on the jelly-C because they were manipulating (e.g., sharks, fish, crustaceans, and pycnogonids), biting, nibbling, trapping, or engulfing (e.g., cnidaria-actiniaria, echinoids, and asteroids) the materials (Table 2; Figure 5B; Videos S1, S2). Very similar observations to our study were also described in Archer et al. (2018). Yet an important distinction in this study was that most feeding events we observed occurred in the slope region, below 600 m, concomitant with localized areas heavily dominated by a few species, which in many cases were attributed as single-species dominance events (Table 2). Aggregations triggering dominance were patchily distributed along with the whole depth range, within the species’ natural zonation (Table 2; Figure S1). On a few occasions, there were overlapping species (motile and sessile feeding-mode) during the dominance event at different depths, but in most cases, the dominance was caused by single species (motile feeding-mode) at a time. Feeding strategies were observed to have included active jelly-C scavenging (mostly fish, including wait and feed as jelly-C, moved to them), ambushing associated organisms (fish, sharks, and rays), jelly-C trapping/engulfing (cnidarians-actiniarians and asteroids), jelly-C retaining/trapping with spines and nibbling (echinoids), and jelly-C picking/tearing/nibbling (crustaceans). In other cases, jelly-C was actively being carried along the seabed by crustaceans and arthropods, suggesting feeding elsewhere (Table 2; Figure 5; see Videos S1, S2 for examples).
The number of species varied between 3 and 15 across 100 m−2 transects on the shelf, with a total of 15 different species identified. At the shelf break, the species richness was the lowest (3–4 spp.), with a notable absence of fish species (Figure 3B). The number of species on the upper slope (300 to 800 m), which was an area with high jelly-C, was also low (3–6 spp.) but then increased in deeper water. The density of organisms followed a similar trend to the species richness along the depth zones and the transects within them. Shannon (H) and Pielou’s (J) indices reflected these changes in species and counts of individuals but also varied (Figure 3).
The Berger–Parker dominance index (d) measures were calculated, and results were compared with the measures of biomass of jelly-C. This index indicated aggregation events triggering dominance, potentially caused by high abundances of a few species that appeared in visual observations along with high levels of jelly-C (Figures 3, 4). At the shelf break (depths below 200 m), DO minima (~50 µmol kg−1), pHtotal minima (~7.70), and jelly-C maxima (1,600 grC 100 m−2) all concomitantly occurred (Figures 2, 3). The Berger–Parker index and the jelly-C were significantly correlated (Spearman’s rank correlation: r2 = 0.37, p < 0.050), but it was difficult to separate and elucidate the effect of depth (Figures 3B, 4). There were sharp increases in the Berger–Parker dominance index associated with low DO, pHtotal, and high levels of jelly-C (Spearman’s rank correlation: r2 = 0.33 DO, 0.55 pHtotal, 0.63 jelly-C, p < 0.050, Figures 3B, 4). As the ROV-based surveys moved deeper, increased DO, pHtotal, and jelly-C occurred concomitantly. Aggregation events, triggering dominance, were more common, especially from 300- to 800-m depths than in the shelf (<200 m), with further notable peaks at 1,000 m. Aggregation events and dominance, then, slowly decreased with depths beyond 1,000 m, with increased megafaunal diversity through 1,276 m and correspondingly variable levels of jelly-C (Table 2). We further analyzed the Berger–Parker dominance index as a function of depth, current speed, jelly-C, DO, pHtotal, and temperature using a multiple regression model. The model explained 88% of the dominance variability (p < 0.05), confirming not only that jelly-C was responsible for shaping dominance and ecological trends but also that parameters used to assess environmental variability along the bathymetric gradient also played key roles (Figure 4).
The results from PCA showed that the first two principal component axes (PCs) captured 67% of the total environmental variance (Figure 6). PC axis 1 (PC1; 49.60%) showed a decreasing trend along with depth that was mainly driven by temperature followed in importance by depth, pHtotal and Ωcalcite changes, jelly-C biomass, and jelly-C condition. PC2 (17.06%) showed a decline in the layer of 300- to 600-m depth that rose afterward with respect to jelly-C biomass. This pattern was mainly driven by DO and pHtotal, as well as associated with the dynamics of the pipeline (Figure 6). The projection of species number (S), abundance (N), and ecological indices (Shannon and Pielou’s) showed that both S and N displayed a positive relationship with current speed (via jelly-C distribution, Figure 3), while the ecological indices (Shannon and Pielou’s) were positively associated with temperature, depth, jelly-C, jelly-C condition, and Ωcalcite.
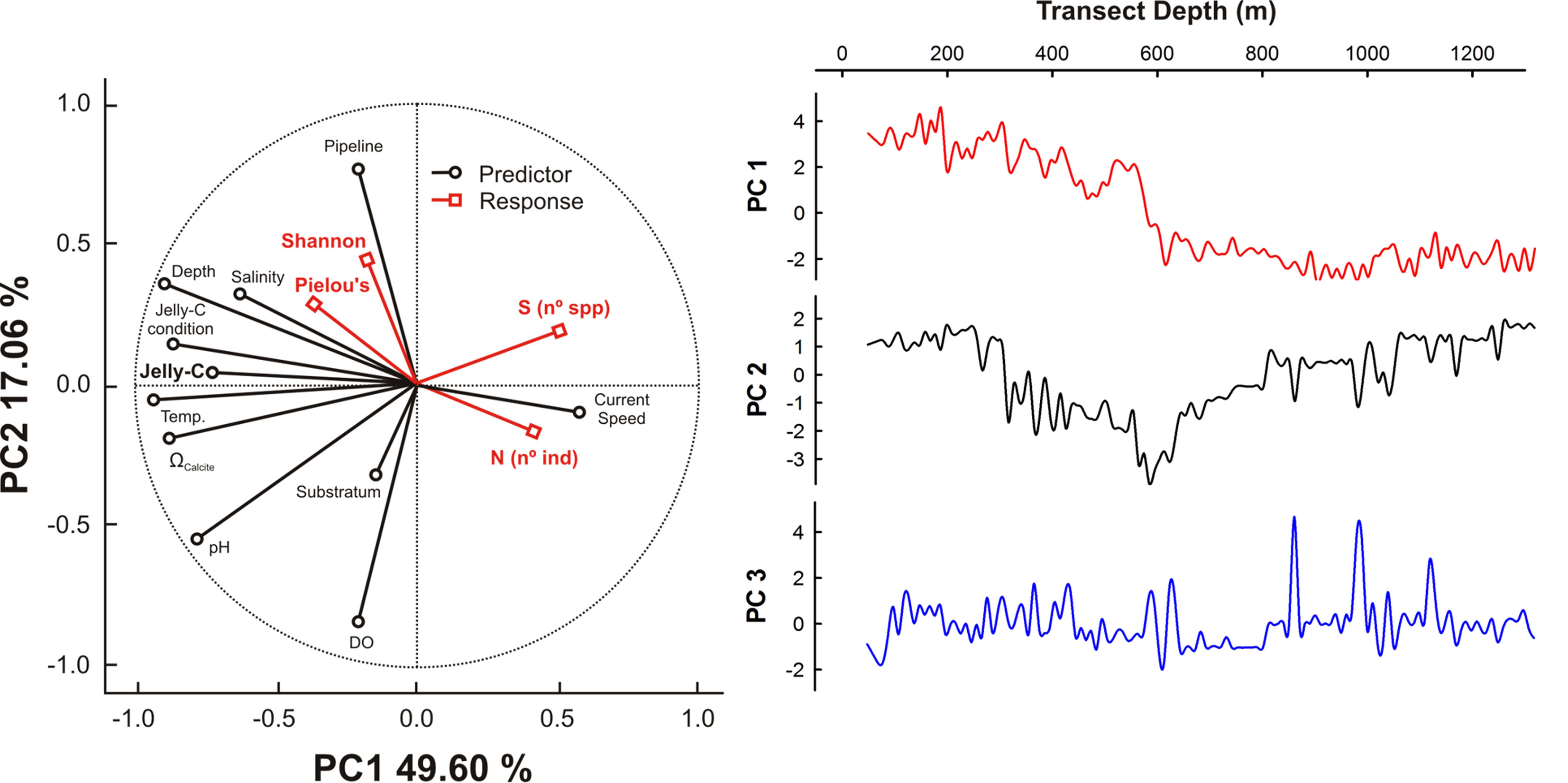
Figure 6 Principal component analysis (PCA) describing ecological and environmental patterns. PC axis 1 and PC axis 2 percentages are presented in the circular plot. PC axis 1 and PC axis 3 results also were plotted against transect depth to facilitate analysis of bathymetric-based distributions.
Discussion
We examined a uniquely derived dataset consisting of oceanic ROV field-based observations of massive-scaled jelly-C depositions and their influence on the shelf and slope benthic ecosystem. Our assessments spanned a wide and deep bathymetric gradient off the Ghanaian Ivory Coast in West Africa. To facilitate the assessment of the influences of environmental parameters upon jelly-C biomass depositions and counts of megafauna across the bathymetric gradients we sampled, we supplemented field-based ROV observations with water column ambient data sampled nearby to our own sites and during overlapping time frames. The area covered, as well as the large dataset used, allowed us to comprehensively explore the influence of jelly-C depositions upon benthic megafauna and to shed light on their importance for benthic ecosystems and inhabitant megafauna in continental margins. We acknowledge that interpretations were conditioned by the presence of artificial structures and pipelines that can trap jelly-C as well as attract megafauna. Yet the results shed light on the jelly-C impact upon benthic megafaunal assemblages along large bathymetric gradients (Figures 1, 2; Table 2).
Jelly-C Deposition Events and Deep-Sea Benthic Macrofauna
The large quantities of jelly-C found off the Ghanaian Ivory Coast promoted some megafaunal aggregations, triggering opportunistic dominance driven by scavengers along the entire bathymetric gradient (Table 2; Figures 3, 4; Video S2). Yet it remains unclear if similar assemblages would congregate on the site during non-bloom episodes. Most likely, megafauna are scattered on the seabed, and they congregate on opportunistic feeding opportunities such as the one described herein. Similar events have been observed before, but not discussed in great detail. For example, jelly-C and megafauna in the Arabian Sea (Billett et al., 2006) were assessed, and likewise, in our findings, crab species fed only through depths up to 350 m, whereas holothuroids (which we noted at levels of dominance at our relatively deepest sites) fed at high abundances in areas as deep as 3,000 m. Surprisingly, for areas actually directly above most of the very dense and large-sized jelly-C mats in the Arabian Sea, no megafauna were observed to have been ingesting or undertaking other similar behaviors with the materials as we observed off the Ghanaian Ivory Coast. This result could imply that in a productive region like the Arabian Sea, jelly-C and especially the decaying proportions of the biomass are not attractive as a food source to a high number or diversity of species and that only some megafaunal organisms prefer to feed on this resource. Another hypothesis could be that microbially driven and chemically mediated competition exists whereby microbes make the food resource unpalatable for megafauna, which could vary in one region or ecosystem compared to another due to the secretion of specific compounds (Burkepile et al., 2006). Off the Ghanaian Ivory Coast where we obtained our samples, dense jelly-C patches were observed to have been likely exploited by a few types of species of fish, echinoderms, cnidarians, crustaceans, and arthropods (Table 2; Figure 5B). Such observations occurred for single species on many occasions, and across many different depth intervals (with overlapping), especially for transects spanning the slope (Figure 5B; Video S2). It is possible that increasing microbial density (Sweetman et al., 2016) diminished the attractiveness of otherwise relatively large jelly-C falls either by way of inducing reductions in DO/sulfidic conditions or by making jelly-C less attractive via compounds segregated by microbes. For example, microbial respiration can lead to reduced DO, and sulfide/ammonium builds up within jelly-C (Chelsky et al., 2016). Yet in our study, DO levels were high enough for microbial degradation to occur and for megafauna to respire owing to anoxy likely not reaching a point of increasing sulfide and ammonium. In our study, jelly-C was categorized as being fresh, degraded, or decomposing, with over 90% of the observations having been recorded as degraded or decomposing. We did not assess the biochemical and physical constituents, which could have contributed to reducing attractiveness. However, on many occasions, different levels of decomposition of overlapping constituents of jelly-C biomass were noted across the bathymetric gradients sampled (Figure 2B). In this situation, the degraded and decomposing jelly-C dominating the seabed suggests that microbes could have displaced megafauna with respect to its use as a food source in some areas (e.g., very advanced state of decomposition) but also made it attractive in other cases (nitrogen source from bacteria). This would extend the ecosystem roles of such recyclers to include them also as competitors (Burkepile et al., 2006). A similar situation to the Arabian Sea and off the Ivory Coast for dense jelly-C patches was described at the Station M abyssal seafloor Californian Coast sampling site (United States), where 6 months of salp jelly-C export to the benthos triggered a subsequent significant holothuroid response (Ruhl et al., 2008; Smith et al., 2014). No other megafaunal organisms were described in that study, but it is difficult to say if this was a dominance event or an artifact of low densities of other megafauna because of the extreme depth at 4,000 m (Ruhl et al., 2008). However, though relatively limited, all other studies with individual or a low number of carcasses constituting jelly-C have shown a large number of species feeding upon the jelly-C and a higher scavenger diversity (Jumars, 1976; Miyake et al., 2005; Yamamoto et al., 2008; Sweetman and Chapman, 2011; Sweetman et al., 2014). In our study, we find both scenarios of high and low diversity coexisting with various levels of the decomposing substrate (jelly-C), and we attribute it to different decomposition stages. Further, many of the previous studies were conducted at single depths; thus, it is also difficult to separate the influence of bathymetric gradient-based responses and zonation of jelly-C impacts from seabed heterogeneity and niche exploitation (Figure S1). Recently, it was discovered in a shallow coastal environment that manual additions of jelly-C detritus to the seabed decreased macrofaunal C-uptake and increased bacterial C-uptake and that the jelly-C decay was capable of producing sulfides, which could further change benthic organisms’ responses and abundances (Chelsky et al., 2016). While similar dynamics could have been at play in some localized areas during our sampling, we suggest that the overall good water circulation and oxygen availability did not impact megafaunal assemblages at large.
Varying Community Responses Over a Bathymetric Gradient
Our findings indicate that a wide range of community-based variations and associated responses to jelly-C exist along an extensive and deep transect, which has not typically been captured in other studies of jelly-C (Smith et al., 2014; Sweetman et al., 2014). Yet variability can also be introduced via a substantial environmental change from ongoing water column processes. The effect of jelly-C needs to be understood as an episodic one, adding variability to a continuously changing system. The dense aggregations were followed by dominance events that we observed in which one species (different ones along the transect) was hyper-abundant in conjunction with a relatively large jelly-C mass but often covered as much as 50 to 100 m2 per event. This did not correspond with the scale of deposition of jelly-C, which extended over much greater spatial scales (hundreds of square meters). Thus, peaks in jelly-C biomass did not uniformly elicit the same aggregation response of the same species, throughout the extent of the deposit. We believe that this is attributed to megafaunal communities’ own zonation; thus, the jelly-C arriving in such depths triggers an aggregation response within their preferred depth ranges. Published megafaunal distribution assessments for other regions far from our own demonstrated zonation and turnover in species compositions with increasing depth (Haedrich et al., 1980; Rowe et al., 1982). The natural decrease in megafaunal biomass with depth is a common phenomenon in the deep sea below 200 m and is linked to the exponential decrease in food supply distances from coastal waters and deeper waters (Rex and Etter, 2010). Megafaunal biomass is the most affected by energy reduction with depth, owing to its large size (Smith et al., 2008). Thus, jelly-C inputs, and especially those that are palatable in deeper waters, are particularly important in driving patterns in megafaunal assemblages. The longer life spans, greater energy reserves, and mobility of larger-sized fauna also mean they are more able to benefit from spatially and temporally variable food inputs like jelly-C.
Jelly-C and Dissolved Oxygen
We found DO minima in our study area coinciding with jelly-C maxima. However, DO minima never fell below oxygen minimum zone (OMZ) concentrations (below 0.5 mg L−1). As a result, the role of DO in controlling the megafaunal response to jelly-C is, and was for us, difficult to assess (Levin and Gage, 1998). Although higher megafaunal densities in low DO areas tend to occur in upwelling oxygenated water (Smallwood et al., 1999; Levin, 2003), the Ivory Coast area we sampled is not an OMZ such as the Arabian Sea (DO 200–400 m = 0.1/0.2 mg L−1). In the coastal areas of the Pakistani margin, megafaunal organisms were nearly or entirely almost absent from all areas with low DO despite shallow depths and abundant food (Murty et al., 2009), which suggested that DO had the potential to limit megafaunal activity regardless of other environmental conditions or food resources. Megabenthic organisms tolerate DO as low as 0.15–0.3 mg L−1 depending upon the species and physiological dynamics (Murty et al., 2009). Such levels are relatively well below the Ivory Coast minima that we observed at 1.6–1.76 mg L−1. Thus, the findings reinforce the idea that jelly-C exerted a strong control upon megafauna beyond the influence of other environmental variables, in particular for DO. Notably, many environmental conditions at the relatively deep depths we sampled and seabed substrates were almost identical everywhere. As calcifiers are less tolerant to hypoxia than other organisms (Levin, 2003), we expected that they could be less prevalent in relatively low oxygen conditions at our sites. However, we found such taxa in all surveys (Table 2; Figure 5B; Videos S1, S2), and they were especially prevalent in areas of high jelly-C and relatively low DO. Galatheids, ophiuroids, actinians, gastropods, arthropods (sea spiders), and pennatulaceans are organisms that characteristically can be found in OMZs and deep-sea zones (Bett, 1995), and we also found abundances of these taxa at our sample sites off the Ghanaian Ivory Coast using ROV visual-based surveys. These taxa were the most important ones in the aggregations that triggered dominance events (Table 2; Figures 1B, 3, 4).
Jelly-C as Food Resource in Continental Margins
Megafauna were observed mostly congregating and feeding upon freshly and degraded jelly-C (based on visual inspections), rather than on decomposing patches. Beyond the proposed microbially mediated competition, this could be due to jelly-C decay reducing seawater DO at the sediment interface, increasing sediment-related DO demand, decreasing the pH, and increasing the DIC (Smith et al., 2014). It has also been reported that anoxic layers can trigger megafauna dominance events (Pearson and Rosenberg, 1978). Large megafaunal aggregation and dominance events occurred 7 times along the bathymetric gradient occupying hundreds of meters, corresponding, in particular to 5 species, namely, the echinoid Diadema sp., the crustacean Galathea sp., the echinoid Phormosoma sp., ophiuroids (brittle star) identified as Ophiolepadidae, and the holothuroid Mesothuria sp. (Table 2). There were also aggregation events triggering dominance in certain patches (smaller than 100 m) along the bathymetric transect, for other species such as the fish Scorpaena sp., the pycnogonid (arthropod) Colossendeis sp., the cnidaria Actinostola sp., and A. aurelia, and for the asteroid Solaster sp., but at a smaller scale (Table 2).
Substrate appeared not to have an important role, and many areas of seabed, sediment, and pipeline remained empty of megafauna, especially far away from the jelly-C food source (Figures 2A, 6). In this study, some of the large stretches of jelly-C covering the seabed also had no feeding events that aligned with findings from the Arabian Sea (Billett et al., 2006). Thus, we suggest that areas experiencing regular jelly-C depositions experience a transition from grazing and scavenging to a detritus-based food chain for detritus-feeders, followed by a transition to a bacteria-mediated community (Sweetman et al., 2016). Field surveys, such as this one, provide a more representative indication of scavenging activity than the results from isolated baits in lander experiments (Sweetman et al., 2014), although both approaches are required to fully understand jelly-C dynamics and carbon transfer.
We also examined the influence of benthic currents, as they resuspend and transport organic matter and jelly-C downslope and sideways. We found that a threshold of 8–10 cm s−1 was required to lift carcasses, transport, and re-distribute them elsewhere. It was likely that such transport indirectly shaped megafaunal distribution. Our results show the effects of current speeds above 10 cm s−1, which reduced jelly-C accumulation to low levels (Figures 2A, 3). This indirectly impacted ecological patterns following and with respect to jelly-C depositions. In other deep-sea areas outside the Ghanaian Ivory Coast, bottom currents have been found to interact with the terrain and control phytodetrital distribution at spatial scales as small as tens of meters (Morris et al., 2016). When comparing where jelly-C was accumulating with the current speed, our results seemed to indicate that most jelly-C depositions occurred at the lowest current speeds from 300 to 900 m, which in turn coincided with most megafaunal aggregation events, triggering dominance (Figure 2). Benthic current is a key feature to accumulate organic material of any kind around seabed features (deep reefs, rocks, channels, depressions, etc.). We suggest that the pyrosome jelly-C originally sunk elsewhere following major bloom collapse, and then once it reached the seabed, the predominant current transported the material, trapping it against the pipeline. This resembles what would happen around other naturally occurring seabed features that could have also trapped the jelly-C.
Overall, the spatial distributions of megafaunal communities observed off the Ghanaian Ivory Coast were controlled by combinations of environmental factors (bathymetry and seawater chemistry) and biological factors (organismal zonation and aggregation, followed by dominance) but heavily driven by an episodic and opportunistic manner by jelly-C mass deposits. The jelly-C impact is transient in nature and subjected to the bloom dynamics in the water column, but it exerts an impact on short time-scales of several weeks. In addition to the role of jelly-C in providing a pivotal carbon export mechanism and food resource in continental margins and the deep sea, our novel findings indicate that jelly-C has the capacity to impact the short-term dynamics of benthic megafaunal assemblages at broad scales including deeper habitats than have been previously accounted for.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval were not required for the animal study because. We only did observational work using cameras. We did not touch or interact with animals.
Author Contributions
In this study, ML and DJ conducted the fieldwork and surveys, ML revised the videos and obtained the data, ML and JM did the data analyses, and EG worked on the bulk videos revision. ML, J-CM, JM-L, EG, and DJ wrote the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was co-financed by a grant from the Federal Ministry of Education and Research (BMBF) to ML and J-CM under contract No. 03F0722A. DJ was supported by a funding from the European Union’s Horizon 2020 research and innovation program under the MERCES (Marine Ecosystem Restoration in Changing European Seas) project, grant agreement No. 689518, and the Norwegian Research Council project Jelly Farm (Grant No. 244572). ML was further supported by the funding provided to the Ocean Observatory at the Bazaruto Center for Scientific Studies (BCSS) - www.bcssmz.org/.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.902674/full#supplementary-material
References
Archer S. K., Kahn A. S., Leys S. P., Norgard T., Girard F., Du Preez C., et al. (2018). Pyrosome Consumption by Benthic Organisms During Blooms in the Northeast Pacific and Gulf of Mexico. Ecology 99, 981–984. doi: 10.1002/ecy.2097
Ates R. M. L. (2017). Benthic Scavengers and Predators of Jellyfish, Material for a Review. Plankt. Benth. Res. 12, 71–77. doi: 10.3800/pbr.12.71
Basile C., Mascle J., Popoff M., Bouillin J. P., Mascle G. (1993). The Côte D’ivoire-Ghana Transform Margin: A Marginal Ridge Structure Deduced From Seismic Data. Tectonophysics 222, 1–19. doi: 10.1016/0040-1951(93)90186-N
Berger W. H., Parker F. L. (1970). Diversity of Planktonic Foraminifera in Deep-Sea Sediments. Science 168, 1345–1134. doi: 10.1126/science.168.3937.1345
Billett D. S. M., Bett B. J., Jacobs C. L., Rouse I. P., Wigham B. D. (2006). Mass Deposition of Jellyfish in the Deep Arabian Sea. Limnol. Oceanogr. 51, 2077–2083. doi: 10.4319/lo.2006.51.5.2077
Buchanan J. B. (1958). The Bottom Fauna Communities Across the Continental Shelf Off Accra, Ghana (Gold Coast). Proc. Zoo. Soc Lond. 130, 1–56. doi: 10.1111/j.1096-3642.1958.tb00562.x
Buesseler K. O., Lamborg C. H., Boyd P. W., Lam P. J., Trull T. W., Bidigare R. R., et al. (2007). Revisiting Carbon Flux Through the Ocean’s Twilight Zone. Science 316, 567–570. doi: 10.1126/science.1137959
Burkepile D. E., Parker J. D., Woodson C. B., Mills H. J., Kubanek J., Sobecky P. A., et al. (2006). Chemically-Mediated Competition Between Microbes and Animals: Microbes as Consumers in Food Webs. Ecology 87, 2821–2831. doi: 10.1890/0012-9658(2006)87[2821:CMCBMA]2.0.CO;2
Cardona L., de Quevedo I. A., Borrel A., Aguilar A. (2012). Massive Consumption of Gelatinous Plankton by Mediterranean Apex Predators. PLoS One 7, e31329. doi: 10.1371/journal.pone.0031329
Carrasson M., Cartes J. E. (2002). Trophic Relationships in a Mediterranean Deep-Sea Fish Community Partition of Food Resources, Dietary Overlap and Connections Within the Benthic Boundary Layer. Mar. Ecol. Prog. Ser. 241, 41–55. doi: 10.3354/meps241041
Chelsky A., Pitt K. A., Ferguson A. J., Bernnett W. W., Teasdale P. R., Welsh D. T. (2016). Decomposition of Jellyfish Carrion In Situ: Short-Term Impacts on Infauna, Benthic Nutrient Fluxes and Sediment Redox Conditions. Sci. Total. Environ. 566-567, 929–937. doi: 10.1016/j.scitotenv.2016.05.011
Colin C. (1988). Coastal Upwelling Events in Front of the Ivory Coast During the FOCAL Program. Oceanol. Acta. 11, 2.
Delteil J. R., Valery P., Montadert C., Fondeur C., Patriat P., Mascle J. (1974). “Continental Margin in the Northern Part of the Gulf of Guinea,” in Geology of Continental Margins. Eds. Burk C. A., Drake C. L. (New York: Springer), 297–311.
Doyle K. T., Houghton J. D.R., Buckley S. M., Hays G. C., Davenport J. (2007). The Broad-Scale Distribution of Five Jellyfish Species Across a Temperate Coastal Environment. Hydrobiologia 579, 29–39. doi: 10.1007/s10750-006-0362-2
Drazen J. C., Bailey D. M., Ruhl H. A., Smith K. L. Jr. (2012). The Role of Carrion Supply in the Abundance of Deep-Water Fish Off California. PLoS One 7, e49332. doi: 10.1371/journal.pone.0049332
Dunlop K. M., Jones D. O. B., Sweetman A. K. (2017). Direct Evidence of an Efficient Energy Transfer Pathway From Jellyfish Carcasses to a Commercially Important Deep-Water Species. Sci. Rep. 7, 17455. doi: 10.1038/s41598-017-17557-x
Dunlop K. M., Jones D. O. B., Sweetman A. K. (2018). Scavenging Processes on Jellyfish Carcasses Across a Fjord Depth Gradient. Limnol. Oceanogr. 63, 1146–1155. doi: 10.1002/lno.10760
Fanelli E., Cartes J. E. (2008). Spatio-Temporal Variability in the Diet of Two Pandalid Shrimps in the Western Mediterranean Evidenced From Gut Contents and Stable Isotope Analysis: Influence on the Reproductive Cycle. Mar. Ecol. Prog. Ser. 355, 219–233. doi: 10.3354/meps07260
Field C., Behrenfeld M., Randerson J., Falkowski P. (1998). Primary Production in the Biosphere: Integrating Terrestrial and Oceanic Components. Science 281, 237–241. doi: 10.1126/science.281.5374.237
Frost J. R., Jacoby C. A., Frazer T. K., Zimmerman A. R. (2012). Pulse Perturbations From Bacterial Decomposition of Chrysaora Quinquecirrha (Scyphozoa: Pelagiidae). Hydrobiologia 690, 247–256. doi: 10.1007/s10750-012-1042-z
Gage J. D., Tyler P. A. (1991). Deep-Sea Biology: A Natural History of Organisms at the Deep-Sea Floor (London: Cambridge University Press).
Gates A. R., Benfield M. C., Booth D. J., Fowler A. M., Skropeta D., Jones D. O. B. (2016). Deep-Sea Observations at Hydrocarbon Drilling Locations: Contributions From the SERPENT Project After 120 Field Visits. Deep-Sea Res. 137, 463–479.
Glover A. G., Gooday A. J., Bailey D. M., Billett D. S. M., Chevaldonné P., Colaço A., et al. (2010). Temporal Change in Deep-Sea Benthic Ecosystems: A Review of the Evidence From Recent Time-Series Studies. Adv. Mar. Biol. 58, 1–95. doi: 10.1016/B978-0-12-381015-1.00001-0
Gooday A. J., Bett B. J., Escobar E., Ingole B., Levin L. A., Neira C., et al. (2010). Habitat Heterogeneity and Its Influence on Benthic Biodiversity in Oxygen Minimum Zones. Mar. Ecol. 31, 125–147. doi: 10.1111/j.1439-0485.2009.00348.x
Haedrich R. L., Rowe G. T., Polloni P. T. (1980). The Megabenthic Fauna of the Deep-Sea South of New England, USA. Mar. Biol. 57, 165–179. doi: 10.1007/BF00390735
Hays G. C., Doyle T. K., Houghton D. R. (2018). A Paradigm Shift in the Trophic Importance of Jellyfish. T. Ecol. Evol. 33, 11. doi: 10.1016/j.tree.2018.09.001
Henschke N., Pakhomov E. A., Kwong L. E., Everett J. D., Laiolo L., Coghlan A. R., et al. (2019). Large Vertical Migrations of Pyrosoma Atlanticum Play an Important Role in Active Carbon Transport. JGR. Biogeosci. 124, 1056–1070. doi: 10.1029/2018JG004918
Jones D. O. B., Wigham B. D., Hudson I. R., Bett B. J. (2007). Anthropogenic Disturbance of Deep-Sea Megabenthic Communities Investigated Using Remotely Operated Vehicles (Faroe-Shetland Channel, NE Atlantic). Mar. Biol. 151, 1731–1741. doi: 10.1007/s00227-007-0606-3
Jumars P. A. (1976). Deep-Sea Species Diversity. Does it Have a Characteristic Scale? J. Mar. Res. 34, 217–246.
Key R. M., Olsen A., van Heuven S., Lauvset S. K., Velo A., Xiaohua L., et al. (2015). Global Ocean Data Analysis Project, Version 2. ORNL/CDIAC-162 NDP-093 (Oak Ridge, Tennessee: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy). doi: 10.3334/CDIAC/OTG.NDP093_GLODAPv2.
Koranteng K. A. (2001). Structure and Dynamics of Demersal Assemblages on the Continental Shelf and Upper Slope Off Ghana, West Africa. Mar. Ecol. Prog. Ser. 20, 1–12. doi: 10.3354/meps220001
Lebrato M., de Jesus Mendes P., Steinberg D. K., Cartes J. E., Jones B. M., Birsa L. M., et al. (2013). Jelly Biomass Sinking Speed Reveals a Fast Carbon Export Mechanism. Limnol. Oceanogr. 58, 1113–1122. doi: 10.4319/lo.2013.58.3.1113
Lebrato M., Jones D. O. B. (2009). Mass Deposition Event of Pyrosoma Atlanticum Carcasses Off Ivory Coast (West Africa). Limnol. Oceanogr. 54, 1197–1209. doi: 10.4319/lo.2009.54.4.1197
Lebrato M., Pahlow M., Frost J. R., Kuter M., de Jesus Mendes P., Molinero J.-C., et al. (2019). Sinking of Gelatinous Zooplankton Biomass Increases Deep Carbon Transfer Efficiency Globally. G. Biogeochem. Cy. 33, 2019GB006265. doi: 10.1029/2019GB006265
Lebrato M., Pahlow M., Oschlies A., Pitt K. A., Jones D. O. B., Molinero J.-C., et al. (2011). Depth Attenuation of Organic Matter Export Associated With Jelly Falls. Limnol. Oceanogr. 56, 1917–1928. doi: 10.4319/lo.2011.56.5.1917
Lebrato M., Pitt K. A., Sweetman A. K., Jones D. O. B., Cartes J. E., Oschlies A., et al. (2012). Jelly-Falls Historic and Recent Observations: A Synthesis to Drive Future Research Directions. Hydrobiologia 690, 227–245. doi: 10.1007/s10750-012-1046-8
Levin L. A. (2003). Oxygen Minimum Zone Benthos: Adaptation and Community Response to Hypoxia. Oceanogr. Mar. Biol. Annu. Rev. 41, 1–45.
Levin L. A., Gage J. D. (1998). Relationships Between Oxygen, Organic Matter and the Diversity of Bathyal Macrofauna. Deep-Sea Res. 45, 129–163. doi: 10.1016/S0967-0645(97)00085-4
Llopiz J. K., Richardson D. E., Shiroza A., Smith S. L., Cowen R. K. (2010). Distinctions in the Diets and Distributions of Larval Tunas and the Important Role of Appendicularians. Limnol. Oceanogr. 55, 983–996. doi: 10.4319/lo.2010.55.3.0983
Lucas C. H., Jones D. O. B., Hollyhead C. J., Condon R. H., Duarte C. M., Graham W. M, et al. (2014). Gelatinous Zooplankton Biomass in the Global Oceans: Geographic Variation and Environmental Drivers. G. Ecol. Biogeogr 23, 701–714. doi: 10.1111/geb.12169
Lucas C. H., Pitt K. A., Purcell J. E., Lebrato M., Condon R. H. (2011). What’s in a Jellyfish? Proximate and Elemental Composition and Biometric Relationships for Use in Biogeochemical Studies. Ecology 92, 1704.
Mayzaud P. (2007). Polar and Neutral Lipid Composition in the Pelagic Tunicate Pyrosoma Atlanticum. Lipids 42, 647–657. doi: 10.1007/s11745-007-3066-0
Minitab, LLC. (2016). Minitab. Available at: https://www.minitab.com.
Miyake H., Lindsay D. L., Kitamura M., Nishida S. (2005). Occurrence of the Scyphomedusa Parumbrosa Olylobate Kishinouye 1910 in Suruga Bay, Japan. J. Plankt. Biol. Ecol. 52, 58–66.
Moore J. K., Doney S. C., Lindsay K. (2004). Upper Ocean Ecosystem Dynamics and Iron Cycling in a Global Three-Dimensional Model. G. Biogeochem. Cy. 18, GB4028. doi: 10.1029/2004GB002220
Morris K., Bett B., Durden J., Benoist N., Huvenne V., Jones D., et al. (2016). Landscape-Scale Spatial Heterogeneity in Phytodetrital Cover and Megafauna Biomass in the Abyss Links to Modest Topographic Variation. Sci. Rep. 6, 34080. doi: 10.1038/srep34080
Murty S. J., Bett B. J., Gooday A. J. (2009). Megafaunal Responses to Strong Oxygen Gradients on the Pakistan Margin of the Arabian Sea. Deep-sea. Res. 56, 472–487. doi: 10.1016/j.dsr2.2008.05.029
Pearson T. H., Rosenberg R. (1978). Macrobenthic Succession in Relation to Organic Enrichment and Pollution of the Marine Environment. Oceanogr. Mar. Biol. Annu. Rev. 16, 229–311.
Purcell J. E. (2005). Climate Effects on Formation of Jellyfish and Ctenophore Blooms: A Review. J. Mar. Biol. Assoc. 85, 461–476. doi: 10.1017/S0025315405011409
Rex M. A., Etter R. J. (2010). Deep-Sea Biodiversity: Pattern and Scale (Cambridge, MA: Harvard University Press).
Rex M. A., Etter R. J., Morris J. S., Crouse J., McClain C. R., Johnson N. A., et al. (2006). Global Bathymetric Patterns of Standing Stock and Body Size in the Deep-Sea Benthos. Mar. Ecol. Prog. Ser. 317, 1–8. doi: 10.3354/meps317001
Robinson C., Steinberg D. K., Anderson T. R., Aristegui J., Carlson C. A., Frost J. R., et al. (2010). Mesopelagic Zone Ecology and Biogeochemistry – A Synthesis. Deep-sea. Res. 57, 1504–1518. doi: 10.1016/j.dsr2.2010.02.018
Roger C. (1982). Macroplankton and Micronecton of the Tropical Atlantic. I. Biomass and Taxonomic Composition. Oceanogr. Trop. 17, 85–96.
Rowe G. T., Polloni P. T., Haedrich R. L. (1982). The Deep-Sea Macrobenthos on the Continental Margin of the Northwest Atlantic Ocean. Deep-Sea. Res. 29, 257–278. doi: 10.1016/0198-0149(82)90113-3
Ruhl H. A. (2007). Abundance and Size Distribution Dynamics of Abyssal Epibenthic Megafauna in the Northeast Pacific. Ecology 88, 1250–1262. doi: 10.1890/06-0890
Ruhl H. A., Ellena J. A., Smith J. K.L. (2008). Connections Between Climate, Food Limitation, and Carbon Cycling in Abyssal Sediment Communities. Proc. Natl. Acad. Sci. U. S. A. 105, 17006–17011. doi: 10.1073/pnas.0803898105
Sampey A., McKinnon A. D., Meekan M. G., McCormick M. I. (2007). Glimpse Into Guts: Overview of the Feeding of Larvae of Tropical Shorefishes. Mar. Ecol. Prog. Ser. 339, 243–257. doi: 10.3354/meps339243
Schlitzer R. (2015). Data Analysis and Visualization With Ocean Data View. CMOS. Bull. SCMO. 9–13, 43.
Schreider C. A., Rasband W. S., Eliceiri K. W. (2012). NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Sempere R., Yoro S. C., van Wambeke F., Charriere B. (2000). Microbial Decomposition of Large Organic Particles in the Northwestern Mediterranean Sea: An Experimental Approach. Mar. Ecol. Prog. Ser. 198, 61–72. doi: 10.3354/meps198061
Shannon C. E., Weaver W. (1949). The Mathematical Theory of Communication (Urbana: University of Illinois Press).
Siegel D. A., Buesseler K. O., Doney S. C., Sailley S. F., Behrenfeld M. J., Boyd P. W. (2014). Global Assessment of Ocean Carbon Export by Combining Satellite Observations and Food-Web Models. G. Biogechem. Cy 28, 181–196. doi: 10.1002/2013GB004743
Smallwood B. J., Wolff G. A., Bett B. J., Smith C. R., Hoover D., Gage J. D., et al. (1999). Megafauna can Control the Quality of Organic Matter in Marine Sediments. Naturwissenschaften 86, 320–324. doi: 10.1007/s001140050624
Smith C. R., De Leo F. C., Bernardino A. F., Sweetman A. K., Arbizu P. M. (2008). Abyssal Food Limitation, Ecosystem Structure and Climate Change. Trends Ecol. Evol. 23, 518–528. doi: 10.1016/j.tree.2008.05.002
Smith K. L. Jr., Sherman A. D., Huffard C. L., McGill R. Henthorn P. R., Von Thun S., Ruhl H. A., et al. (2014). Large Salp Bloom Export From the Upper Ocean and Benthic Community Response in the Abyssal Northeast Pacific: Day to Week Resolution. Limnol. Oceanogr. 59, 745–757. doi: 10.4319/lo.2014.59.3.0745
Sweetman A. K., Chapman A. C. (2011). First Observations of Jelly-Falls at the Seafloor in a Deep-Sea Fjord. Deep-Sea. Res. I. 58, 1206–1211. doi: 10.1016/j.dsr.2011.08.006
Sweetman A. K., Chelsky A., Pitt K. A., Andrade H., van Oevelen D., Renaud P. E. (2016). Jellyfish Decomposition at the Seafloor Rapidly Alters Biogeochemical Cycling and Carbon Flow Through Benthic Food-Webs. Limnol. Oceanogr. 61, 1449–1461. doi: 10.1002/lno.10310
Sweetman A. K., Smith C. R., Dale T., Jones D. O. B. (2014). Rapid Scavenging of Jellyfish Carcasses Reveals the Importance of Gelatinous Material to Deep-Sea Food Webs. Proc. R. Soc B. 281, 20142210. doi: 10.1098/rspb.2014.2210
Thiebot J.-B., Arnould J. P. Y., Gomez-Laich A., Ito K., Kato A., Mattern T., et al. (2017). Jellyfish and Other Gelata as Food for Four Penguin Species – Insights From Predator-Borne Videos. Front. Ecol. Environ. 15, 437–441. doi: 10.1002/fee.1529
Tinta T., Kogovsek T., Turk V., Shiganova T. A., Mikaelyan A. S., Malej A. (2016). Microbial Transformation of Jellyfish Organic Matter Affects the Nitrogen Cycle in the Marine Water Column – A Black Sea Case Study. J. Exp. Mar. Biol. Ecol. 475, 19–30. doi: 10.1016/j.jembe.2015.10.018
West E. J., Welsh D. T., Pitt K. A. (2009). Influence of Decomposing Jellyfish on Sediment Oxygen Demand and Nutrient Dynamics. Hydrobiologia 616, 151–160. doi: 10.1007/s10750-008-9586-7
Keywords: jellyfish, carbon, gelatinous, zooplankton, benthic, ecology, deep sea
Citation: Lebrato M, Molinero J-C, Mychek-Londer JG, Gonzalez EM and Jones DOB (2022) Gelatinous Carbon Impacts Benthic Megafaunal Communities in a Continental Margin. Front. Mar. Sci. 9:902674. doi: 10.3389/fmars.2022.902674
Received: 23 March 2022; Accepted: 19 April 2022;
Published: 24 May 2022.
Edited by:
Stefano Aliani, National Research Council (CNR), ItalyReviewed by:
Ulisses Miranda Azeiteiro, University of Aveiro, PortugalFrederico Pereira Brandini, University of São Paulo, Brazil
Copyright © 2022 Lebrato, Molinero, Mychek-Londer, Gonzalez and Jones. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario Lebrato, bWFyaW8ubGVicmF0b0BiY3NzbXoub3Jn
 Mario Lebrato
Mario Lebrato Juan-Carlos Molinero
Juan-Carlos Molinero Justin G. Mychek-Londer
Justin G. Mychek-Londer Elena Mateo Gonzalez4
Elena Mateo Gonzalez4