- 1Faculty of Agro-Industry, Chiang Mai University, Chiang Mai, Thailand
- 2National Center for Genetic Engineering and Biotechnology, Thailand Science Park, Pathum Thani, Thailand
- 3Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
- 4Faculty of Agriculture and Natural Resources, University of Phayao, Phayao, Thailand
- 5Department of Food Science, College of Agriculture and Life Science, Cornell University, Ithaca, NY, United States
- 6College of Maritime Studies and Management, Chiang Mai University, Samut Sakhon, Thailand
Sea cucumber is rich in protein that can be used to prepare a potential derived bioactive peptide for antioxidant and protective effect against UV-B induced skin cell damage. This study aimed to optimize preparation of sea cucumber hydrolysate with both UV-B protective and antioxidant activities using three commercial enzymes using response surface methodology (RSM) with a face-centered central composite design (face- centered CCD). Hydrolysis time and concentration of enzyme effects on the degree of hydrolysis (DH), yield, antioxidant and UV-B protective activities of sea cucumber hydrolysates were determined. The optimum conditions for sea cucumber hydrolysis using papain (SCP), alcalase (SCA), or flavourzyme (SCF) were 3.6, 5.0, and 4.1% (w/w protein), respectively, and a hydrolysis time of 360 min. The resulting hydrolysates had a DH of 81-91%, yield of 13-14%, IC50 for DPPH radical scavenging activity of 0.3-4.1 mg/mL, FRAP of 0.5-0.6 mmol FeSO4/mL, and IC50 for ABTS radical scavenging activity of 1.3-1.6 mg/mL. The UV-B protective activity was reported as the HaCaT cell viability percentage after UV-B treatment. The SCP, SCA, and SCF hydrolysates showed 72.4, 74.5, and 71.3% cell viability, respectively. The concentration of hydrolysates with 80% survival of HaCaT cells was 0.21, 0.15 and 0.20 mg/mL for SCP, SCA and SCF, respectively. Thus, the SCP was selected for bioactive peptide isolation and characterization. The SCP contained hydrophilic and hydrophobic amino acids of 42.4 and 57.6%, respectively. The ultrafiltration and Sephadex G-25 gel filtration chromatography were done for peptide isolation from the SCP. Six potential peptides were identified using LC-MS/MS as Leu-Val-Asn-Glu-Leu-Thr-Glu-Phe-Ala-Gln (1163 Da), Leu-Val-Asn-Glu-Val-Thr-Glu-Phe-Ala-Gln (1149 Da), Phe-Val-Asp-Ser-Ser-Ala-Thr-Thr (826 Da), Phe-Asn-Asp-Leu-Gly-Ala-Trp (821 Da), Phe-Pro-Asp-Thr-Thr-Thr-Leu (793 Da), and Lys-Phe-Gly-Glu-Gly-Lys (664).
Introduction
Marine invertebrates have been received particular attention on discovering bioactive and derivative natural compounds for health benefit proposes (Ghosh et al., 2022). Sea cucumber are one of the highest value edible marine invertebrates. They have been consumed in Asian countries as an ideal tonic and culinary delicacy (Dewi et al., 2020; Lin et al., 2020; Li et al., 2021). A total of 52 species are commercially used for food processing and consumption, mainly from the families of Stichopodidae and Holothuriidae, including the four genera of Stichopus, Holothuria, Bohadschia, and Actinopyga (Choo, 2008). Sea cucumbers are nutritious since they are low in fat and high in protein content, and are rich in essential amino acids such as lysine, arginine and tryptophan as well as polyunsaturated fatty acids (Li et al., 2021). Sea cucumbers can also produce valuable secondary metabolites such as saponins, peptides, minerals, vitamins, fatty acids, carotenoids, collagen, chondroitin sulfates, and amino acids that have biological activities, including anti-atherosclerotic, anti-blood clotting, antimicrobial, antioxidation, antitumor, anti-inflammatory, and angiotensin I-converting enzyme (ACE) inhibitory effects (Han et al., 2012; Kim et al., 2012; Liu et al., 2012; Bahrami et al., 2014; Xue et al., 2015; Dewi et al., 2020).
In several studies, enzymatic hydrolysis has been widely applied to improve the biological or functional properties of native protein in food resources (Mongkonkamthorn et al., 2021). Enzymatic protein hydrolysis is one of the processes used for preparation of functional peptides. Many protein sources have been studied, especially marine organisms. Research has shown various bioactive properties of marine peptides, especially sea cucumber, such as an ACE inhibitory activity (Dewi et al., 2020), and antioxidant and anti-aging activities (Lin et al., 2020). Ye et al. (2019) reported that 10 peptides from oysters (Ostrea rivularis) showed a protective activity against UV-induced photodamage in keratinocyte (HaCaT) cells. Chen et al. (2007) found that a polypeptide derived from scallops (Chlamys farreri) could inhibit UV-B-induced apoptosis in murine thymocytes. Two peptides isolated from cod skin gelatin hydrolysates showed a protective role against UV-B induced damage in mouse skin fibroblasts (Lu et al., 2017). Fish bone peptides showed bioactivity and photoprotective properties against UV-B irradiation in both fibroblasts and HaCaT cell (Iosageanu et al., 2021).
Currently, the chemical structure, physical and biological properties as well as application of bioactive compound from sea cucumber have attracted more attention (Siahaan et al., 2017). However, there are a few of information on optimal process for preparation of enzymatic hydrolysate from sea cucumber with both antioxidant and UV-B protective activities as well as amino acid sequencing of bioactive peptide. Thus, the present study was undertaken to optimize the production of sea cucumber protein hydrolysates with maximizing the antioxidant and UV-B protective activities. Three commercial proteases (i.e., papain, alcalase and flavourzyme) were used. The optimum hydrolysis conditions (i.e., hydrolysis time and enzyme concentration) were investigated using the RSM with a face-centered CCD. The characterization and peptide isolation and identification of the hydrolysates prepared using the optimum hydrolysis conditions were also studied.
Materials and Methods
Sea Cucumber and Preparation
Plain dried, gutted sea cucumber (Holothuria scabra) (Figure 1A) were obtained from local fisheries on Pu Island (Krabi Province, Thailand). Upon arrival (about 3 h in transit, 25-27°C) at the laboratory (College of Maritime Studies and Management, Chiang Mai University, Samut Sakhon, Thailand), the samples were separately packed with a 200 g/bag before storage in a freezer (-18 to -20°C) until further preparation (<3 months). To prepare rehydrated samples, the dried sea cucumbers were cut into small pieces with a knife (5-6 mm squares), soaked in distilled water at the ratio of 1:2 (w:v) for 1 h at room temperature (25 ± 1°C). The rehydrated sea cucumbers were then drained, left to drain in a plastic basket for 5 min, and packed in polyethylene bag in order to store in freezer until further use (not over than 3 months).
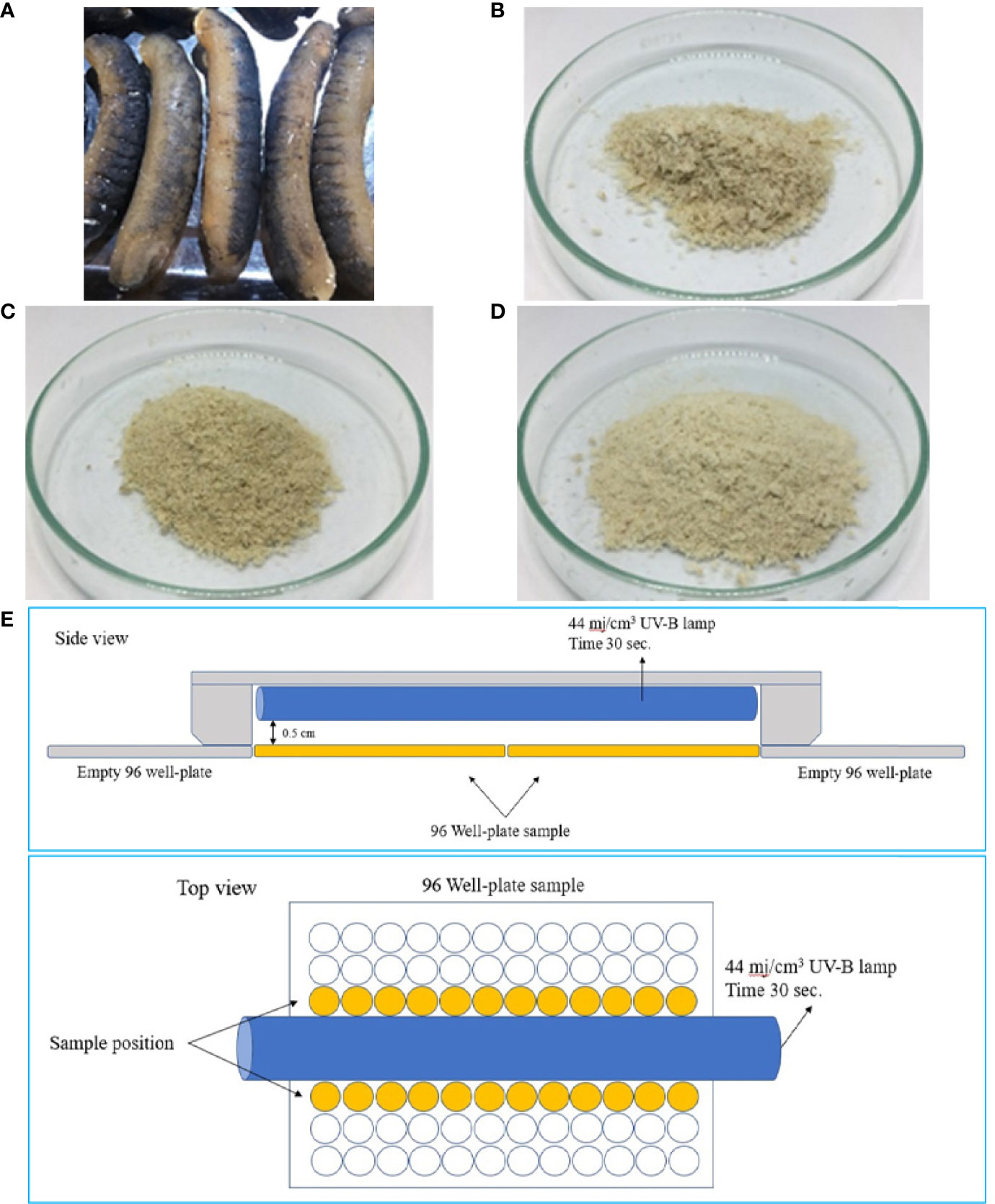
Figure 1 Dried sea cucumber (A) and lyophilized protein hydrolysates obtained using papain (B), alcalase (C), and flavourzyme (D). Diagram illustrates the experimental setup for UV radiation using a UV-B lamp (E).
Enzymes, Cell Line and Chemicals
The papain from Carica papaya (activity ≥30,000 USP-U/mg) was purchased from Merck KGaA (Darmstadt, Germany). Alcalase from Bacillus licheniformis (activity ≥2.4 U/g), and flavourzyme from Aspergiluss oryzae (activity ≥500 U/g), 2,2-diphenyi-l-picrylhydrazyl radical (DPPH), 2,4,6-tripyridyl-s-triazine (TPTZ), 2,4,6-trinitrobenzenesulfonic acid (TNBS) solution, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), and were purchased from Sigma-Aldrich Co. (MO, USA). Human keratinocyte cell lines (HaCaT, 300493, CLS Cell Lines Service) and all cell culture mediums were purchased from XL Biotec Co., Ltd. (Bangkok, Thailand). All other reagents and chemicals used in this study were at least analytical grade (AR).
Sea Cucumber Protein Hydrolysates Preparation
Fifty g of initial rehydrated sea cucumbers were mixed with distilled water at ratio 1:5 (w:v), homogenized at 10,000 rpm using Cole-Parmer T-25 digital ULTRA-TURRAX (Wertheim, Germany) for 10 min, and boiled at 95°C for 15 min for inactivation of endogenous enzyme. The homogenate was then enzymatically hydrolyzed using the papain, alcalase, or flavourzyme singularly. Enzyme concentration varied from 1.0 to 5.0% (w:w crude protein in homogenized sea cucumber) with hydrolysis times of 60 to 360 min in a shaking water bath (Memmert WNB45, Schwabach, Germany) at 150 rpm at temperature 50°C for papain and flavozyme and 60°C for alcalase. Immediately following the hydrolysis, the sample were incubated at 95°C for 15 min to stop the enzymatic reaction and cooled in running tap water. The mixtures were then centrifuged at 2100×g (5,500 rpm) for 10 min (1736R, LaboGene, Lynge, Denmark). The supernatants were then collected and freeze-dried (GFD-3H, Grisrianthong, Samut Sakorn, Thailand) to obtain sea cucumber protein hydrolysate powders. Dried sea cucumbers and the lyophilized hydrolysates as SCP, SCA, and SCF are shown in Figures 1A–D, respectively.
Proximate Composition
Proximate compositions of dried and rehydrated sea cucumber were obtained using the official methods (AOAC, 2000: 954.01 for crude protein, 934.01 for moisture, 991.36 for lipid, and 942.05 for ash. A 6.25 conversion factor was used to convert nitrogen content to crude protein.
Determination of Yield
Yield of hydrolysate powder was calculated following the method of Mongkonkamthorn et al. (2020) and calculated using the following equation: yield (%) = [dried sea cucumber protein hydrolysate weight (g)/rehydrated sea cucumber weight (g)] × 100.
Degree of Hydrolysis (DH) Assay
After hydrolysis, the DH of sea cucumber hydrolysate solution was determined following Mongkonkamthorn et al. (2020) method. Briefly, 125 µL of appropriately diluted sea cucumber hydrolysate solutions were mixed thoroughly with 2 mL 0.2 mol/L sodium phosphate buffer (Na3PO4/Na2HPO4) (pH 8.2) and 1.0 mL 0.01% TNBS solution and kept at 50°C in the dark for 30 min. Na2SO3 (2 mL 0.1 mol/L) was added to inactivate the reaction. The mixture was cooled at room temperature for 15 min and obtained the absorbance at 420 nm was done using a Thermo Scientific, Varioskan Lux microplate reader (Thermo Fisher Scientific Inc., Singapore). The α-amino acid content was expressed in terms of the L-leucine (Sigma-Aldrich) used for the calibration curve and DH was calculated using the following equation (1):
Where At = amount of Leu equivalence obtained from sea cucumber protein hydrolysates. A0 = amount of Leu equivalence in the beginning rehydrated sea cucumber. Amax = total amount of Leu equivalence in rehydrated sea cucumber, which was obtained after hydrolysis by 6 mol/L of HCl (100°C, 24 h), which probably underestimates the total α-amino groups as all the tryptophan and small amounts of other amino acids are destroyed while some dipeptides remain.
Determination of DPPH Radical Scavenging Activity
DPPH radical scavenging activity was investigated according to the method of Ahn et al. (2014). The 100 µL of sample solution was mixed with 100 µL of 0.05 mM DPPH solution (in 95% ethanol) and kept in the dark for 30 min. The absorbance (Abs) at 515 nm was obtained using the microplate reader. The sample was replaced by an equal volume of distilled water as the control. The percentage of DPPH radical scavenging activity was expressed as the difference in the Abs between the control and the sample relative to the Abs of the control. The scavenging activity plot was done using the sea cucumber hydrolysate concentration versus scavenging activity. The IC50 or the concentration obtaining 50% DPPH radical scavenging activity was estimated from the plot using an equation of linear regression analysis. The DPPH radical scavenging activity was expressed from the following equation (2).
Where DPPH0 is the Abs of the DPPH solution. DPPH1 is the Abs of the sample mixed with DPPH.
ABTS Radical Scavenging Activity
The ABTS radical scavenging activity was based on Ahn et al. (2014) method. A stock solution was generated (mixing of 7 mmol/L ABTS and 2.45 mmol/L K2S2O8) and keeping it in the dark at 4°C for 16 to 18 h. The working solution was generated by diluting the stock solution with 95% ethanol to obtain 0.70 ± 0.02 of Abs at 734 nm. The 20 µL of sea cucumber hydrolysate solution was mixed with the 200 µL of working solution and incubated in the dark at room temperature for 8 min. The Abs at 734 nm was obtained. The ABTS scavenging activity and the IC50 values were obtained with a previous explain as used for the DPPH.
Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP assay was done according to the method described by Wangtueai et al. (2016). The working FRAP solution (containing 10 volumes of 300 mmol/L acetate buffer (pH 3.6), 1 volume of 10 mmol/L FeCl3.6H2O, and 1 volume of 10 mmol/L TPTZ in 40 mmol/L HCl) was kept at 37°C for 30 min. In reaction, a 10 mg/mL of sample (150 µL) was mixed with working FRAP solution (2.85 mL) and incubated at room temperature in the dark for 30 min. The Abs was measured at 593 nm. The solutions of 0-5 mmol FeSO4.7H2O were used for a standard. FRAP value was performed in mmol FeSO4/mL for optimization and ultrafiltration membrane, while FeSO4/g protein for gel filtration chromatography.
UV-B Protective Activity
The UV-B protective activity was determined based upon the cell viability according to a slightly modification of Torita et al. (2004) method.
HaCaT Cell Culture
HaCaT cells were maintained in the DMEM supplemented with 10% fetal bovine serum (FBS), 2 mmol/L Glu, and 1% penicillin using a gas mixture of 95% air and 5% CO2. Cells were cultured until they reached 80% confluence and sub-cultured following trypsinization (Das et al., 2014). The cell from the 33rd to 44th passages were used for the experiments.
Determination of Cell Viability
The cell viability was determined based on a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) tetrazolium reduction assay, a colorimetric assay for assessing cells metabolic activity (the MTT assay). After HaCaT cell were seeded at a density of 1×104 cell/well, counted with a hemacytometer (Hausser scientific, Horsham, USA), (100 µL) in a 96-well plate for 24 h, the cells were treated along with an untreated (control) with various concentrations of sea cucumber hydrolysates for 24 h. Subsequently, 15 µL MTT (5 mg/mL in phosphate-buffered saline (PBS) was added into each well, followed by incubation at 37°C for 4 h. The medium was then aspirated and 100 µL DMSO added to solubilize the colored product. After 10 min, optical density (OD) was read using a scanning multi-well microplate reader (SpectraMax i3x, Molecular Devices, San Jose, CA, USA) at 540 and 630 nm. The %cell viability was calculated using the following equation (3).
Where ODtreated = (OD of treated at 540 nm - OD treated at 630); ODcontrol = (OD of control at 540 nm - OD of control at 630 nm).
UV-B Irradiation
HaCaT cells (1x104 cells/well) in culture medium were mixed with 10 µg/mL of sea cucumber hydrolysates solution for 24 h, and the culture medium mixed with sea cucumber hydrolysate was replaced with 100 µL of PBS. The cells were subjected to an irradiation with a single dose of UV-B radiation (G5, Lamptan, Bangkok, Thailand) at 44 mj/cm3 (according to the manufacturer) for 30 s in a laminar hood (Figure 1E). Afterwards, the culture medium was replaced again with DEME adding a 2 mM glutamine, 10% FBS, and 1% penicillin. After 24 h of incubation at 37°C and 5% CO2, the viable cells were determined using the MTT assay as above mentioned.
Amino Acid Determination
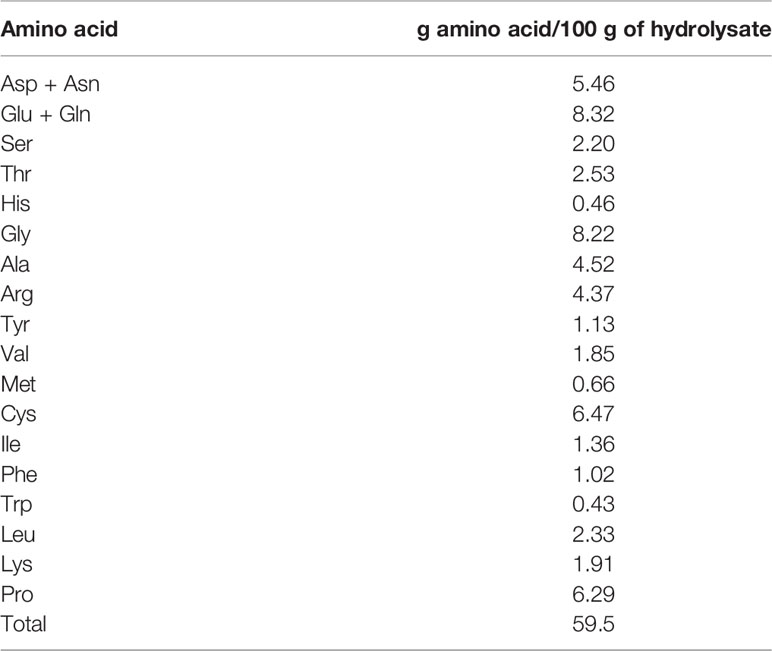
Amino acids of the sea cucumber hydrolysate powder were determined according to the method of Herbert et al. (2000) using HPLC with fluorescence detector (HP 1260, Agilent Technologies, Waldbronn, Germany). A standard amino acid mixture from Sigma-Aldrich was used to construct calibration curves where peak areas of each amino acids were plotted against their known concentrations. The content of amino acids was calculated and reported in g/100 g of hydrolysates.
HaCaT Cell Cytotoxicity Assay
The HaCaT cell line was cultured in DMEM medium added with 10% FBS, 2 mmol/L glutamine, and 1% penicillin. The cells were seeded at 1x104 cells/100 µL in 96-well plates and allowed to adhere for 24 h. The culture medium was then replaced with a new medium containing the sea cucumber hydrolysates at various concentrations. The maximum concentration of sea cucumber hydrolysates used in this experiment was 1000 µg/mL and diluted serially (0, 63, 125, 250, 500 and 1000 µg/mL). The incubation was subsequently continued for 4 h at 37°C and 5% carbon dioxide. The viability of cell was evaluated using the MTT assay.
Ultrafiltration of Hydrolysate
The fractionation of sea cucumber hydrolysates was done using a stirred cell (Amicon®, Merck KGaA) with ultrafiltration membranes (UF) of regenerated cellulose (Merck KGaA). Two hundred mL of 10% SCP solution was placed in the Amicon® stirred cell and fractionated using a series of nominal MW cut-off membranes of 10, 3, and 1 kDa. The 4 fractions of >10, 10-3, 3-1, and <1 kDa was obtained as SCP I, SCP II, SCP III, and SCP IV, respectively. Those each fraction was dried using freeze dryer. Freeze-dried yield, antioxidant and UV-B protective activities of the four fractions were determined.
Gel Filtration Chromatography
Gel filtration chromatography of high antioxidant and UV-B protective activities fraction from UF was fractioned using a Sephadex G-25 2.6×70 cm column packed (GE Healthcare Bio-Science AB, Uppsala, Sweden). The system of BioLogic low-pressure chromatography (Bio-Rad, Hercules, CA, USA) connecting with a Biofrac fraction collector (Bio-Rad) was used to collect each 3 mL of the eluted. In brief, 50 mg/mL of sample solution in distilled water (DW) was filtered with 0.45 µm before loaded onto a column. The DW was used for elution at 0.5 mL/min of flow rate. All fraction was subjected to absorbances measurement at 220 and 280 nm together with determination of antioxidant and UV-B protective activity. Peptides MW were estimated from MW protein standards.
Peptide Identification by De-Novo Sequencing
The peptide sequence and accurate molecular mass of potential antioxidant and UV-B protective peptide fraction was conducted following a previous method of Krobthong and Yingchutrakul (2020) with minor modifications. Briefly, the 3 mg of peptide fraction was onloaded to equilibrated Sep-Pak C18 column (Waters, MA, USA) and eluted with 40-35% of acetonitrile per water. The collected supernatant was evaporated using rotatory evaporator. The peptide fraction was reconstituted in 0.1% formic acid and subjected to Orbitrap HF LC-MS/MS (ThermoScientific, MA, USA). The peptides were separated using LC-MS/MS with a 120 min gradient of 0.1% formic acid/water (mobile phase A) and 0.1% formic acid/80% acetonitrile (mobile phase B) at 250 nL/min flow rate. Mass spectra (m/z range from 600 to 2200) were fully acquired in positive mode using a data dependent acquisition mode (TopN5) and collected at +2, +3, +4, and +5 charge state. The peptides were fragmented by a higher-energy collisional dissociation (HCD) at collision energy = 34. an AGC target set at 3 × 106 ions and a resolution of 120k. MS/MS scan was initiated when the ACG target reached 105 ions and a resolution of 30k. Raw mass spectra was analyzed using a PeakX studio 10.0 program (Bioinformatics Solution Inc., Ontario, Canada). A de novo peptide sequencing of the highest peptide ion intensity was shown with default parameters. The 20 ppm of mass error and 0.05 Da of MS/MS was used. The HCD-Fragmentation series were used to constructed peptide sequence (Ma et al., 2003). Acceptability of de novo peptide sequences were obtained with a filtering of ≥85% of average local confidence (ALC).
Response Surface Methodology (RSM) and Statistical Analysis
RSM with a 2-factor, 3-level, face-centered CCD was done to identify the optimize sea cucumber hydrolysis conditions. Two independent variables (hydrolysis time; X1 and enzyme concentration; X2) were selected for the optimization. Six of dependent variables (responses) include Y1=IC50 value of ABTS radical scavenging activity (mg/mL), Y2=IC50 value of DPPH radical scavenging activity (mg/mL), Y3=FRAP value (mmol FeSO4/mL), Y4=DH (%), Y5=yield (%), and Y6=UV-B protective activity as cell viability (%). The experiment units (11 treatments for each enzyme with including 8 fraction-factorials and 3 center points) are shown in Table 1.

Table 1 The experiment units and responses for sea cucumber hydrolysate using papain, alcalase or flavourzyme hydrolysis.
The software of Design Expert version 11 (Stat-Ease, Inc., Minneapolis, MN, USA) was used to design of the experiments, analysis of data, and the 3D and contour plots. A mathematic model of full quadratic for dependent variables was established. This model can be shown with real variables using the following equation (4).
Where Y represents the predicted response or dependent variable, while B0, Bi, Bii, and Bij are the constant and the coefficients for the linear, quadratic, and interactions, respectively (i = 1 and j = 2).
One-way analysis of variance was done to determine the significant differences (P ≤ 0.05) and Duncan’s new multiple range tests were subsequently used to analyze the tests for the differences between means (P ≤ 0.05) using the Statistical Package for the Social Sciences, SPSS Statistical Package 17.0 software (SPSS Inc., Chicago, IL, USA).
Results and Discussion
Proximate Compositions of Sea Cucumber
The proximate composition of plain dried sea cucumber (gutted) are shown in Table 2. The results were consistent with the previous studies of Wen et al. (2010), in which the content of crude protein, fat, and ash ranged from 40.7 to 63.3%, 0.3 to 10.1%, and 15.4 to 39.6%, respectively, and of Li et al. (2021), in which 52.4 to 54.2% of protein, 0.21 to 0.41% of fat, and 14.0 to 30.7% of ash content was addressed. Previous studies consistently showed that commercial dried sea cucumbers contained low fat and high ash with crude protein >40% (Li et al., 2021). Since fat content in the rehydrated sea cucumber (1.9%), the pre-treatment step of defatting was not necessary.
Optimization of Sea Cucumber Hydrolysis Conditions
Response Surface Models and Plots
The effects of independent variable X1 (hydrolysis time) and X2 (enzyme concentration) of sea cucumber hydrolysates are shown in Table 1. To observe the influence of hydrolysis condition on responses, the response surface was created, and the regression analysis was done using a full-quadratic response surface as shown in Table 3. The models had significant (P ≤ 0.05) terms and high coefficients of determination (R2) (0.83-0.97), except for the IC50 of DPPH radical scavenging models of alcalase and papain hydrolysis and the yield models of papain and flavourzyme hydrolysis that were not significant (P>0.05). The models were appropriated to indicate the correlations among the interested parameters with a high confidence that they were able to explain the effects of the variables in the observed data (Wangtueai et al., 2020).
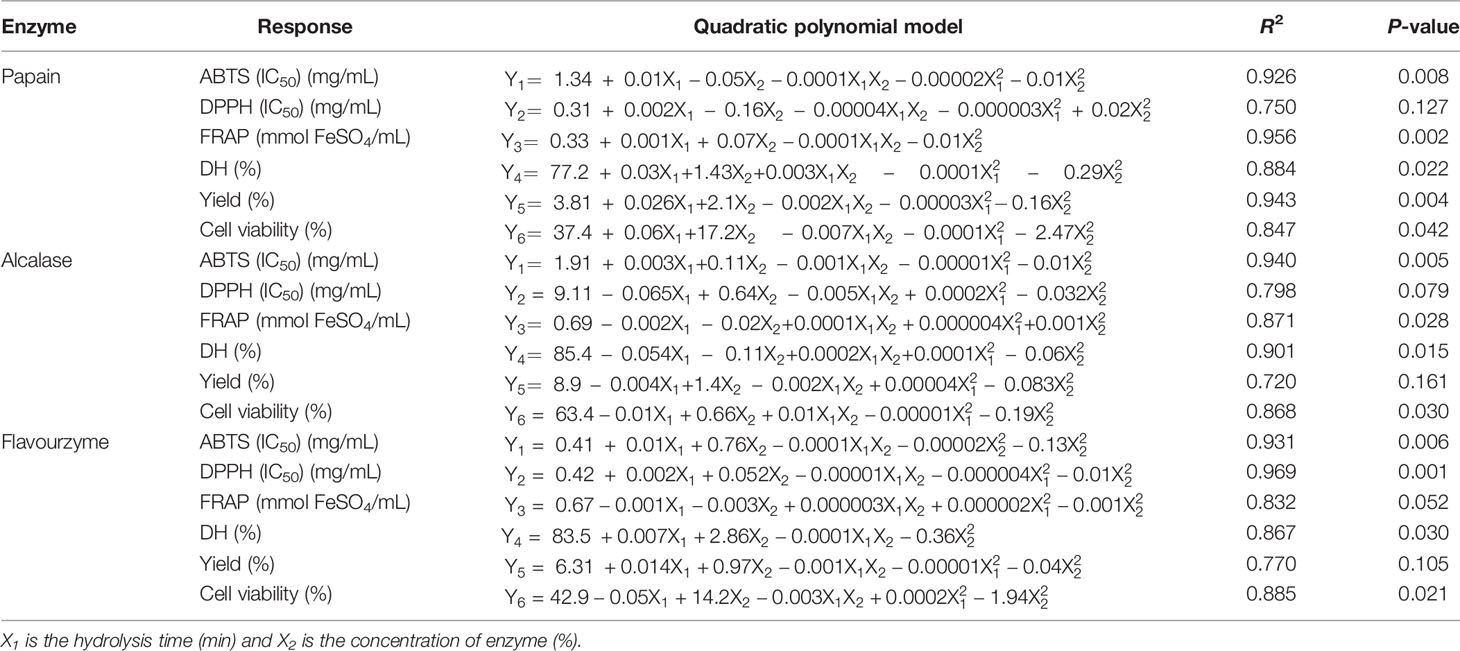
Table 3 Response surface models for sea cucumber hydrolysates using papain (SCP), alcalase (SCA) and flavourzyme (SCF).
The response surface plots predicting response functions and effects of independent variables (X1 and X2) on the dependent variables or responses as present in the 3D and contour response surface plots are shown in Figure 2. Figures 2A1–A3 show the effects of the independent variables on DH. The hydrolysis time of papain was the main significant effects on DH, while the concentrations of alcalase and flavourzyme was the main effects that was significant for DH. The interaction between the two factors was absent with among all three enzymes (Table 4). Increases in hydrolysis time and enzyme concentration might enhance the breakdown of polypeptide chains; hence, the DH was increased (Saidi et al., 2014; Mongkonkamthorn et al., 2020). Hamzeh et al. (2017) reported that increasing hydrolysis time to 180 min contributed to a high DH for protein hydrolysates obtained from squid (Sepia pharaonis). Both X1 and X2 were the main effects on DH for preparation of tuna blood hydrolysate (Mongkonkamthorn et al., 2020) and tuna dark meat hydrolysates (Mongkonkamthorn et al., 2021). Dewi et al. (2020) showed the range of 66-90% for DH and 0.40-0.72 mg glutathione/mg of hydrolysates for the peptide content in sea cucumber hydrolysates using enzyme hydrolysis.
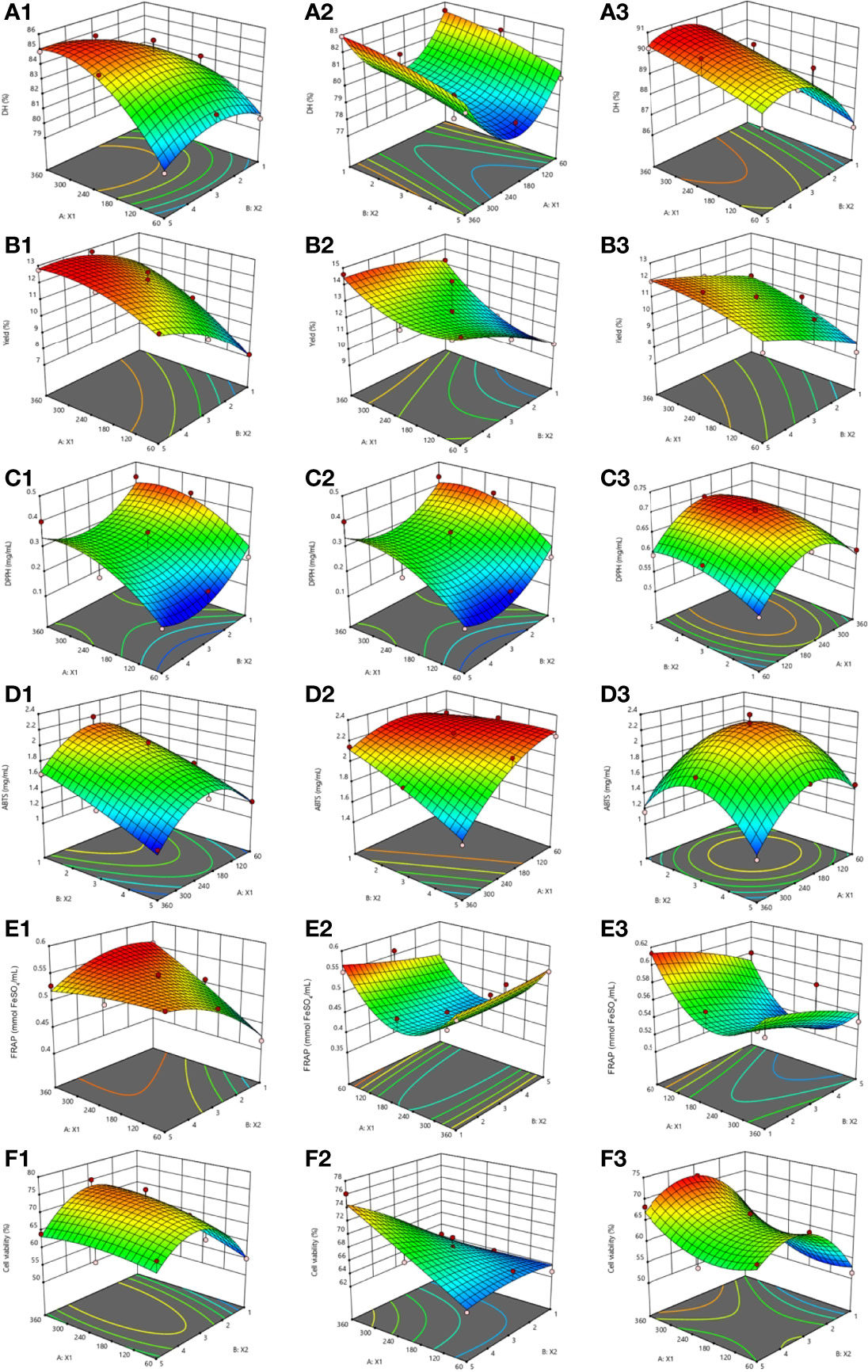
Figure 2 The 3D and contour plots of hydrolysis time (X1: min) against the concentration of papain (A1-F1), alcalase (A2-F2), and flavourzyme (A3-F3) (X2; %) for the degree of hydrolysis (%DH), yield (%), IC50 of DPPH radical scavenging activity (mg/mL), IC50 of ABTS radical scavenging activity (mg/mL), FRAP value (mmol FeSO4/mL), and UV-B protective activity express as the cell viability (%).
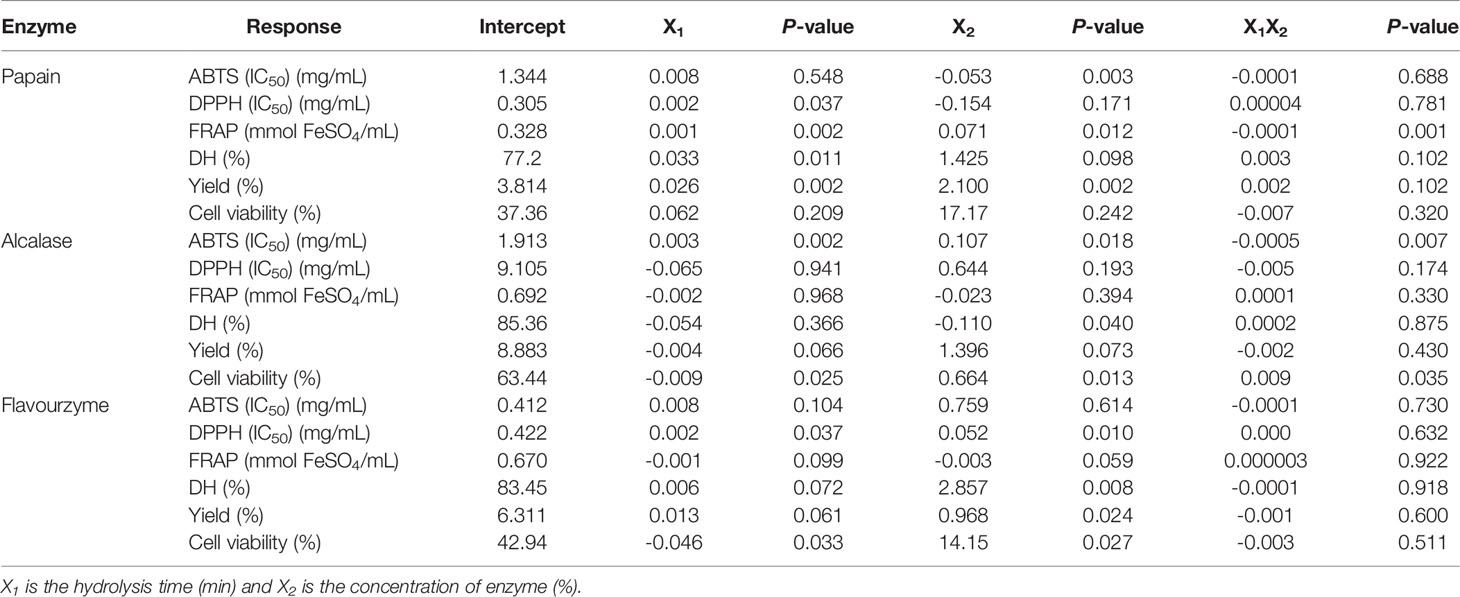
Table 4 The constants, coefficients and P-values of independent variables and interactions for all responses of sea cucumber hydrolysis with different three enzyme.
The influence of the X1 and X2 on hydrolysate yields are shown in Figures 2B1–B3. The main significant effects on yield were both independent variables for papain and enzyme concentration for flavourzyme hydrolysis. Both X1 and X2 showed insignificant effects for alcalase hydrolysis. No interaction on yields were observed (Table 4). In agreement with DH, the yield of hydrolysate was increased with increasing hydrolysis time and concentration of enzyme (Figure 3). The findings were similar to the results reported for hydrolysates prepared from Indonesian sea cucumber (Dewi et al., 2020), tuna blood (Mongkonkamthorn et al., 2020), and tuna dark meat (Mongkonkamthorn et al., 2021).
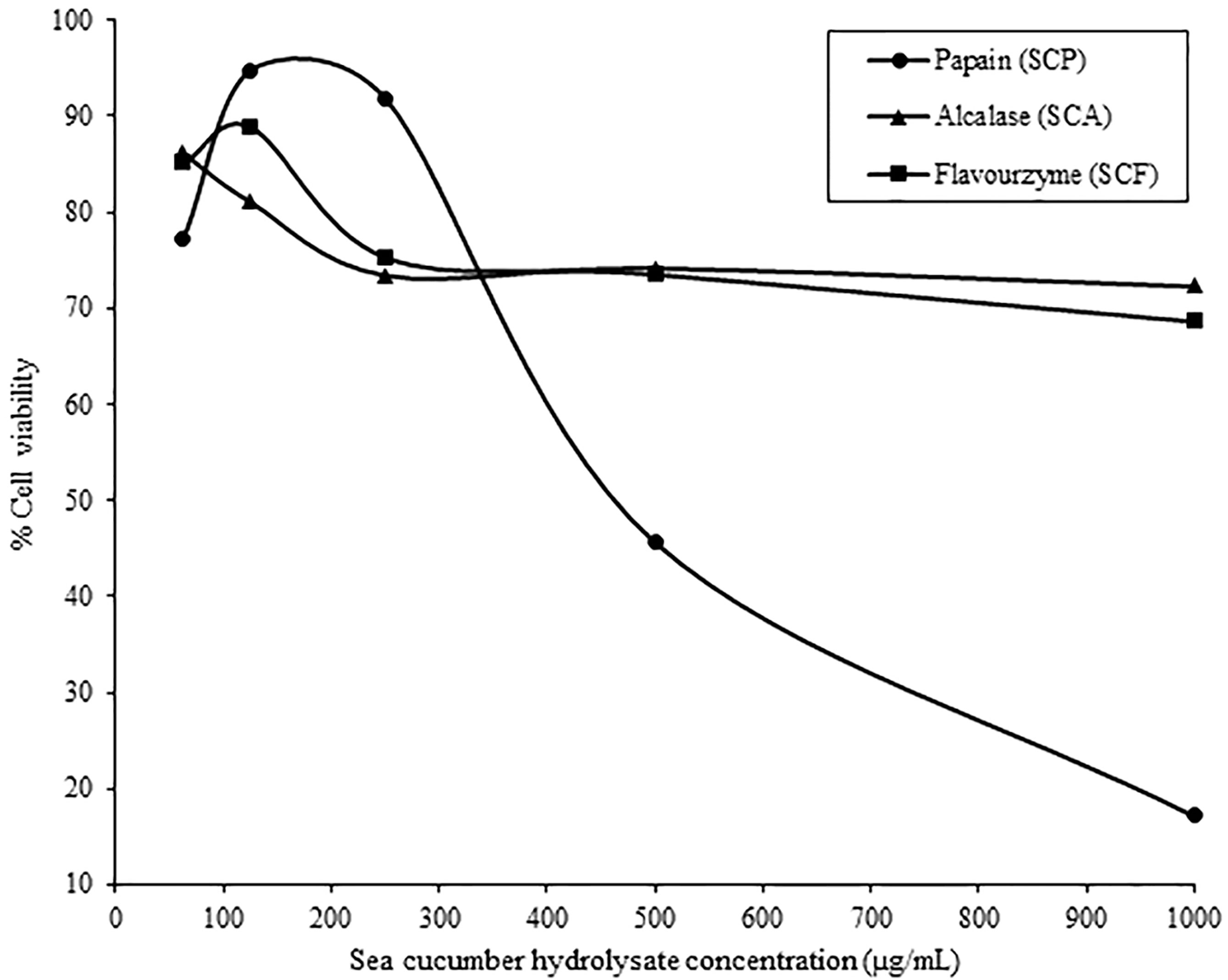
Figure 3 Cell cytotoxicity of sea cucumber hydrolysates prepared with papain (SCP), alcalase (SCA) and flavourzyme (SCF) on human keratinocyte cells lines (HaCaT cells).
The results for the IC50 of DPPH radical scavenging activity are shown in Figures 2C1–C3. The main significant effect for papain was the hydrolysis time whereas both independent variables were significant for flavourzyme (Table 4). No interactions between the two factors on the IC50 of DPPH radical scavenging activity were observed for any enzymes (Table 4). The effects of the X1 and X2 on the IC50 of ABTS radical scavenging activity are shown in Figures 2D1–D3. Enzyme concentration was identified for papain while both independent variables were significant for alcalase hydrolysis. There were interaction of the two factors on the IC50 of ABTS radical scavenging activity for alcalase (Table 4). The results for the FRAP assay are shown in Figures 2E1–E3. Both factors were significant for papain but not for alcalase and flavourzyme. Interaction effects for these two factors on FRAP was only found with papain (Table 4). The results indicated that as hydrolysis time and enzyme concentration increased, the values of IC50 for DPPH radical scavenging activity and ABTS radical scavenging activity were decreased while FRAP values were increased (Figure 2C1-3, D1-3, E1-3). These results were consistent with the previous studies showing that peptides with smaller MW showed greater antioxidant properties than the ones with larger MW (Khantaphant et al., 2011; Zhong et al., 2011). The enhanced cleavage of the polypeptides by increasing enzyme concentration and hydrolysis time would release more bioactive peptides, hence increasing antioxidant activities.
The effects of X1 and X2 on the UV-B protective activity showed in Figures 2F1–F3. Both independent variables showed significant effect for alcalase and flavourzyme hydrolysis but not for papain hydrolysis. The interaction effects of the two factors on the cell viability were found only when alcalase was used (Table 4). The greater degree of UV-B protective activity, when hydrolysis time and enzyme concentration were increased, was more likely related to the release of more bioactive peptides. In addition, molecular size, MW and specific sequence of the resulting peptide chains influenced their bioactive activities and indicated that these peptides might prevent skin cell death due to UV-B radiation. The UV radiation stimulates the production of reactive oxygen species (ROS), including the superoxide anion radical (·O−2), hydrogen peroxide (H2O2), hydroxyl radical (·OH) and singlet oxygen (1O2), which causes the death of skin cell (Jessica et al., 2000; Zhuang et al., 2009). Consistent with these results, the low MW peptides from a fish gelatin hydrolysate exhibited a protective effect against the UV irradiation because it had antioxidant activity that promoted a prevention of skin cell destruction in rats (Sun et al., 2013). Chen et al. (2007) reported that a polypeptide from Chlamys farreri with MW of 879 Da showed a protective effect against UV-B irradiation of murine thymocytes. Peptides prepared from Ostrea rivularis and fish bone showed cytoprotective properties against UV-B irradiation, reduced ROS levels and apoptosis of HaCaT keratinocytes (Ye et al., 2019; Iosageanu et al., 2021).
Multiple Responses Optimization and Model Validation
The optimal enzyme concentration to obtain the highest yield, antioxidant activity (DPPH, ABTS, FRAP) and UV-B protective activity were 3.58%, 5.00% and 4.08% for papain, alcalase and flavouzyme, respectively, with a hydrolysis time of 360 min. Desirability value of individual and composite for various responses, predicted values, and validated experimental values are shown in Table 5. A predicted value was not significantly different from the experimental values (P>0.05) for all responses, indicating that the models were good predictors. Based on its higher yield and better bioactivity to the other hydrolysates, SCP was selected for amino acid profiling as well as further purification.
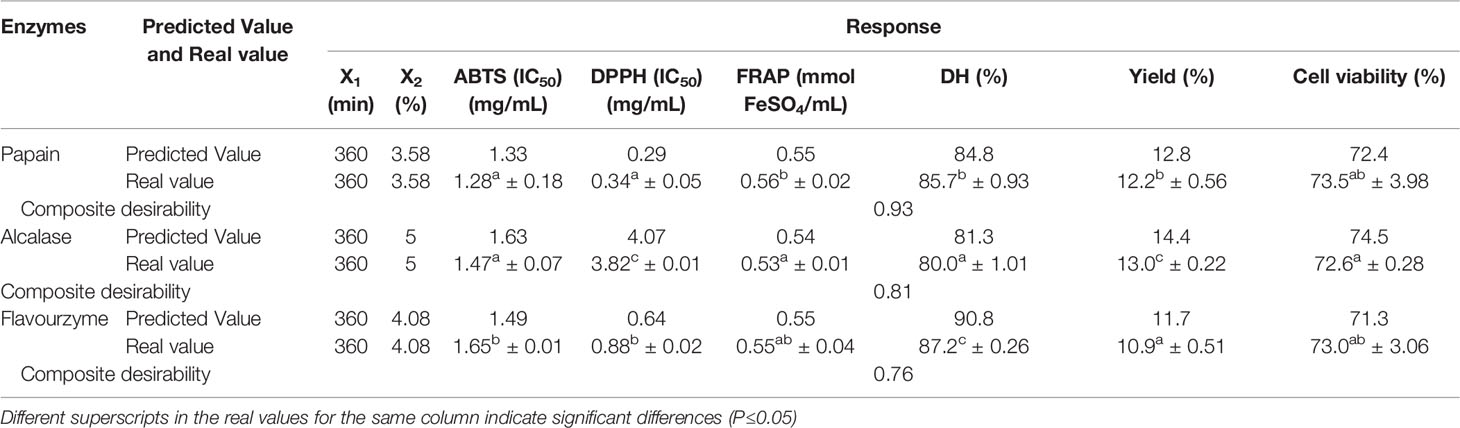
Table 5 The optimize condition, predicted and experiment values from the model verification for all responses of sea cucumber hydrolysates using papain, alcalase, and flavourzyme.
Amino Acids of Sea Cucumber Hydrolysate
The choice of SCP for amino acid composition and fractionation was based on both high antioxidant and UV-B protective activities. The amino acid composition of SCP is shown in Table 6. The SCP was rich in Asx, Glx, Gly, Ala, Arg, Cys and Pro. About 42.4% of the SCP total amino acids were hydrophilic, comprising Asx, Glx, Ser, Thr, Arg, Lys, His whereas the other 57.6% of SCP were hydrophobic amino acids. The high antioxidant and UV-B protective activities of the SCP powder might be dependent on amino acids and their sequences. The antioxidant efficiency of amino acids might depend on their polarity and non-polarity with the greater hydrophobicity, the higher antioxidant activity (Mendis et al., 2005). The hydrophobic amino acids can be proton or electron donors which stabilize free radicals. Mendis et al. (2005) reported that peptides from the skins of jumbo squid showed a strong antioxidant activity and inhibited peroxide formation. Based on peptide sequencing, they found that the resulting peptides consisted of Phe-Asp-Ser-Gly-Pro-Ala-Gly-Val-Leu (880 Da) and Asn-Gly-Pro-Leu-Gln-Ala-Gly-Gln-Pro-Gly-Glu-Arg (1242 Da), of which most of the peptide molecular chain consisted of hydrophobic amino acids. Furthermore, an increase in the antioxidant and photoprotective activities were found with the presence of Gly-Pro and Gly-Ala peptide sequences and hydrophobic amino acids at the C-terminus and within the polypeptide chain (Chen et al., 2016). The current results were consistent with the report of Zhuang et al. (2009) in which peptide from a jellyfish (Rhopilema esculentum) showed anti UV-A, which adversely affects human skin, with most peptides contained Gly, Pro, Asp, Ala and Glx. Chen et al. (2016) studied 23 peptides of cod skin for anti UV radiation and found Arg, Gly-Pro, Gly-Leu at the C-terminus along with the ring-shaped amino acids within the peptide chains. Most contained the 5 amino acids (Arg, Glu, Val, Ile, and Leu) with specific ratio in peptides can show anti-aging properties of skin cells (Ye et al., 2017).
Cell Cytotoxicity of Sea Cucumber Hydrolysate
The three hydrolysates were tested for cytotoxicity effects on HaCaT cells reported as the concentration such that cells had 80% survival. The increasing of cell survival was observed at the concentration range from 63 to 125 µg/mL for SCP and SCF, respectively, and the cell survival was decreased afterwards. On the other hand, cell survival decreased as SCA concentration increased (Figure 3). Parichat et al. (2011) studied the effects of heated whey protein concentrate on survival of human epithelial colorectal carcinoma (Caco-2). Their results showed a cytotoxicity of whey protein concentrate on Caco-2 cells when concentration was added up to 2.0 mg/mL and observing almost 60% cell survival at 2.5 mg protein/mL. Klarić et al. (2008) also reported that the cell death rates of porcine kidney PK15 depended on the duration of the contact time and the extract concentration. The concentration of SCP, SCA, and SCF with 80% survival of HaCaT cell was 0.21, 0.15 and 0.20 mg/mL, which might be used as maximize concentration limited for further applications.
Isolation and Characterization Peptide From the SCP Sea Cucumber Hydrolysate
The antioxidant, UV-B protective activities, and fraction yields of the SCP are shown in Table 7. The SCP I showed the highest FRAP assays, yield and UV-B protective activity, while the SCP II showed the highest antioxidant activity based on ABTS and DPPH as well as good UV-B protective activity. The results were in accordance with the previous studies in which protein hydrolysates derived from tilapia and cod that showed antioxidant activity had a MW range of 3.5 to 10 kDa or <10 kDa (Raghavan and Kristinsson, 2008; Je et al., 2008). The structural characteristics of peptides such as MW, hydrophilic and hydrophobic amino acids, inner ring structures, N-terminal or C-terminal positions affected the antioxidant and photoprotective activities (Zou et al., 2016). Peng et al. (2020) also reported that the effect of oyster hydrolysates on inhibiting of UV-B induced photodamage in HaCaT cells was possibly related with antioxidant activity of hydrolysates. Although the peptide of SCP I showed high FRAP value, yield and UV-B protective activity when considering in both activities of UV-B protective and antioxidant, especially stronger in DPPH and ABTS radical scavenging activities of SCP II that might important for skin cell protection from UV-induced free radicals mainly by oxidative stress (Peng et al., 2020). Therefore, the SCP II was selected for further isolation and identification process.
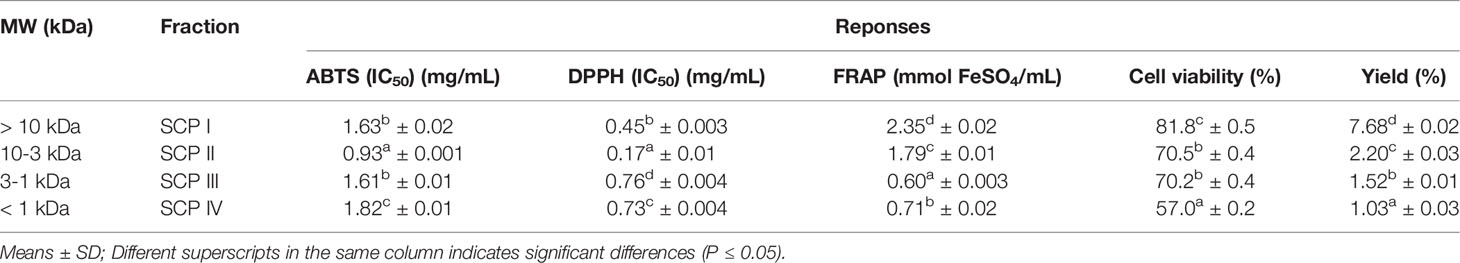
Table 7 Antioxidant and UV-B protective activities, and fraction yield from the fractionation of sea cucumber hydrolysates prepared with papain.
The peptides of SCP II sea cucumber hydrolysate were fractioned according to size of MW by Sephadex G-25 gel filtration chromatography. The elution profile of A220 and A280 were plotted to monitor the content of peptide bonds and peptide and protein/amino acid with aromatic groups, respectively (Figure 4A). The A220 was higher than A280 as showing a similar trend for both absorbances profile. A high peptide bonds and amino acid content was exhibited in the fractions 52-78 with an estimated MW ranging from 2503 to 12885 Da. The antioxidant activities of fractioned peptides are shown in Figure 4B. The highest ABTS and DPPH radical scavenging activities were observed in fraction 105, MW of 456 Da, while the highest of FRAP value was showed at fraction 103, MW of 518 Da. This result corresponded with the peptide fraction of seabass skin gelatin hydrolysate with MWs ranging from 256 to 1610 Da had the best ABTS radical activity (Sae-Leaw et al., 2016) and the fractioned peptide of oyster hydrolysate with a MW range of 842-1787 Da had higher DPPH, ABTS, and ·OH radical scavenging activities (Zhang et al., 2022). Based on a high content of protein and peptide from A220 and A280 profile and antioxidant activities, several sharp peak of peptide fractions was chosen to determine the UV-B protective activity as shown in Figure 4C. A higher 90% HaCaT cell viability was found in the fractions 95 and 103 with estimated MW of 857 and 518 Da, respectively. Ye et al. (2019) reported that shellfish (Ostrea rivularis) peptide with a MW range of 634-1123 Da had good inhibition activity of the UV-induced photodamage, having a HaCaT cell viability of 80-90% at treated concentration of 500-1500 µg/mL. The photoprotective activity of peptide might be related with the inhibition of excessed reactive oxygen species (ROS) from skin cell after UV-irradiation (Zhang et al., 2022). Therefore, those two fractions were collected to amino acid sequencing.
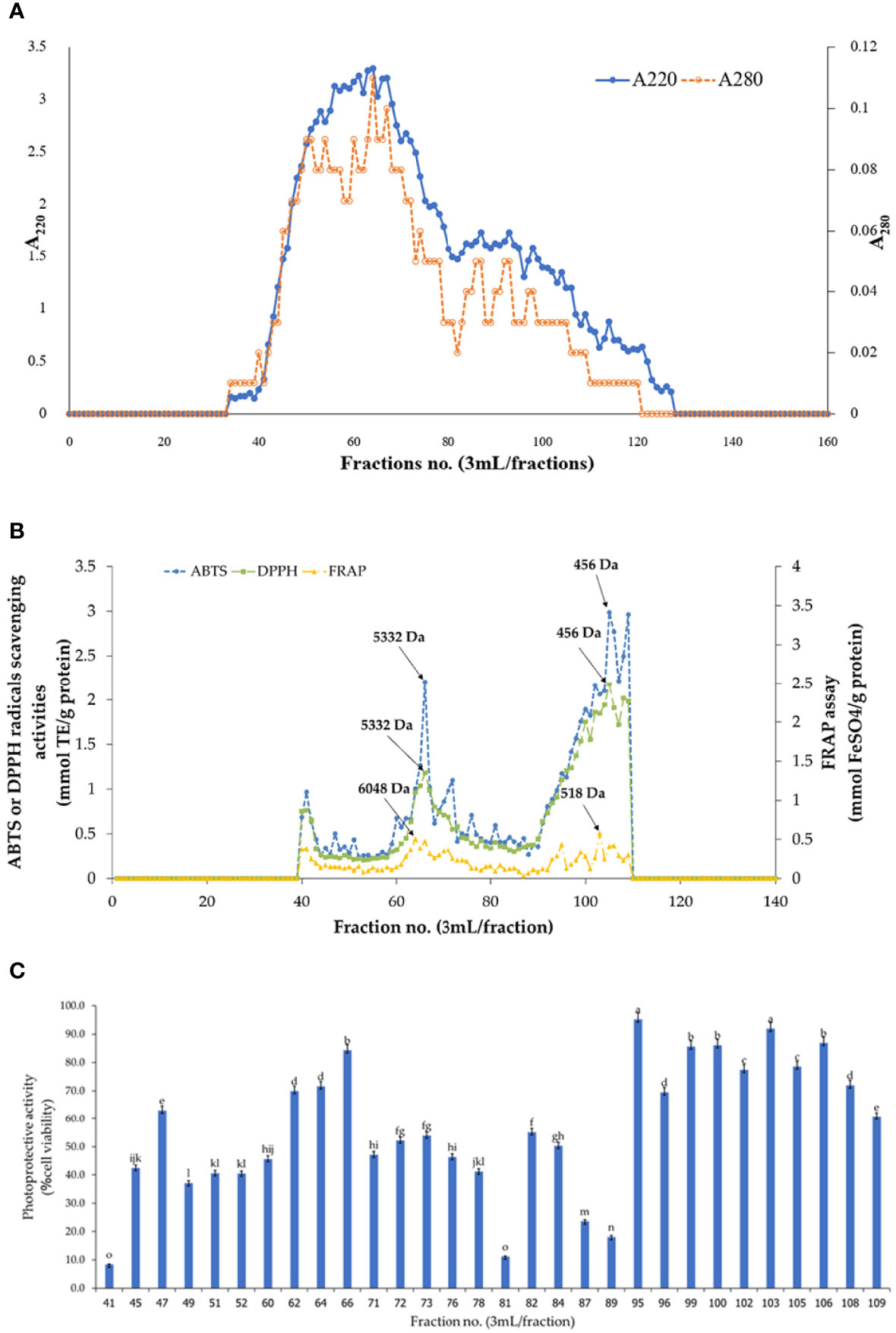
Figure 4 Elution plot (A) absorbance at 220 and 280, (B) antioxidant activities, and (C) UV-B protective activity as %cell viability. Different small letters in bar graph (C) indicate significant differences (P≤0.05).
The selected peptide fractions from gel filtration chromatography (95 and 103) were identified using LC-MS/MS. The amino acid sequences of UV-B protective and antioxidant peptide from sea cucumber hydrolysate are shown in Table 8. The LC-MS/MS showed that a selected fraction consisted of peptides with a MW range of 664-1443 Da and 6 to 14 amino acids in the length. Considering the identified peptide sequence with a ≥90% of de novo peptide sequencing score and ALC, the six peptide sequences with a potent antioxidant and UV-B protective effect were Leu-Val-Asn-Glu-Val-Thr-Glu-Phe-Ala-Gln (1149 Da), Phe-Asn-Asp-Leu-Gly-Ala-Trp (821 Da), Phe-Val-Asp-Ser-Ser-Ala-Thr-Thr (826 Da), Leu-Val-Asn-Glu-Leu-Thr-Glu-Phe-Ala-Gln (1163 Da), Phe-Pro-Asp-Thr-Thr-Thr-Leu (793 Da), and Lys-Phe-Gly-Glu-Gly-Lys (664). Generally, aromatic and hydrophobic amino acids containing in peptides are believed to be a high radical scavenging activity (Zhou et al., 2012; Sae-Leaw et al., 2016). Previous studies also reported that strong radical scavenging peptides could be due to the presence of non-aromatic amino acids such as Pro, Ala, Val, and Leu in the sequences (Mendis et al., 2005; Ngo et al., 2010). In this study, most identified peptides were rich in Thr, Phe, Leu, Val, Glu, and Ala, that might contribute to high antioxidant and UV-B protective activities. The peptides containing photoprotective and antioxidant activities have been identified such as Phe-Leu-Asn-Glu-Phe-Leu-His-Val (1019 Da) from salmon by-product hydrolysate (Ahn et al., 2014), Ala-Thr-Pro-Gly-Asp-Glu-Gly (752 Da) from boiled abalone (Chen et al., 2019), Ala-Ile-Val-Ala-Glu-Val-Asn-Glu-Ala-Ala-Lys (1114 Da) from oysters (Crassostrea hongkongensis) (Zhang et al., 2022), Leu-Ser-Gly-Tyr-Gly-Pro (592 Da) from tilapia gelatin peptides (Sun et al., 2013), and Ser-Ser-Asp-Asn-Asn-Asp-Glu-Ala-Lys (1036 Da) from Pacific oyster (Crassostrea gigas) (Han et al., 2019).
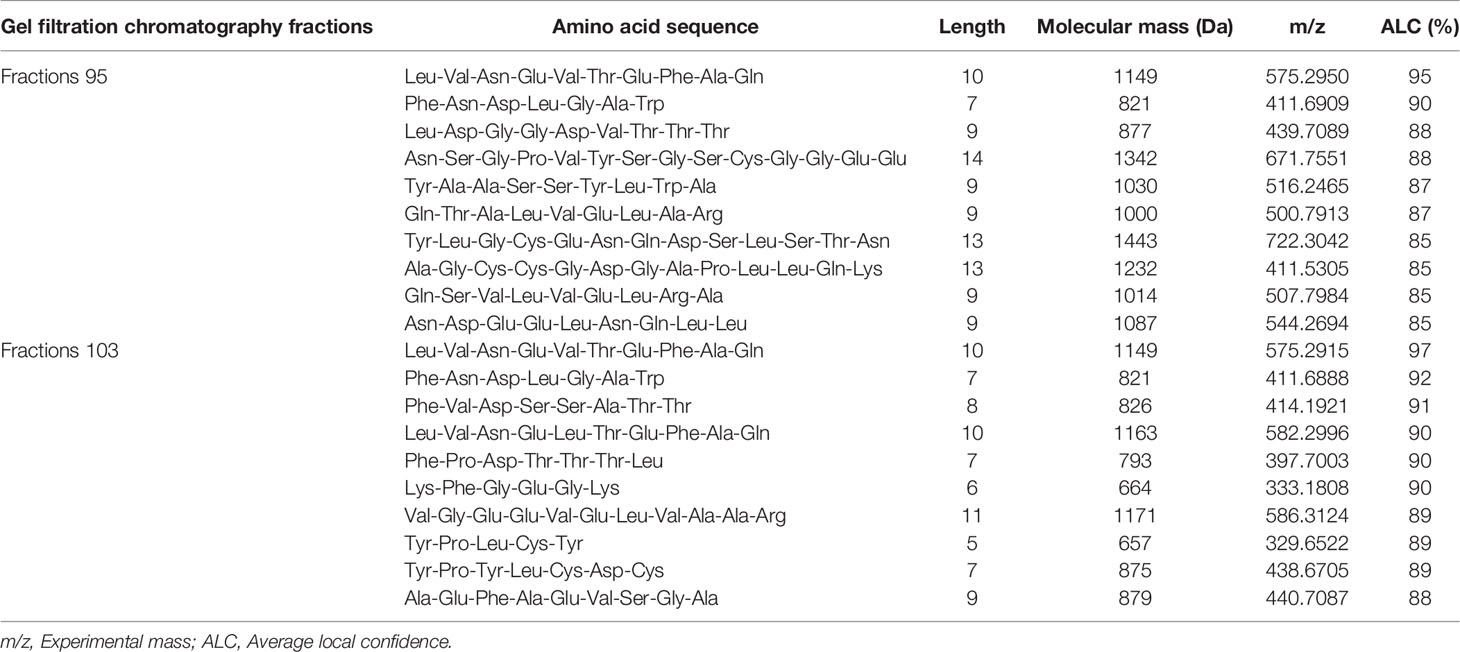
Table 8 Amino acid sequence of peptide in the selected fractions from gel filtration chromatography of sea cucumber (Holothuria scabra) hydrolysate prepared with papain.
Conclusion
To prepare sea cucumber hydrolysates, the optimum concentrations of papain, alcalase, and flavourzyme were 3.58, 5.00, and 4.08% (w/w protein), respectively, with an optimum hydrolysis time of 360 min. The models fit the experimental values. The fractionated peptides of SCP II with MW of 10 to 3 kDa was selected for characterizing the peptide sequences based on its high antioxidant and UV-B protective activities. Six potential peptides were identified as Leu-Val-Asn-Glu-Leu-Thr-Glu-Phe-Ala-Gln (1163 Da), Leu-Val-Asn-Glu-Val-Thr-Glu-Phe-Ala-Gln (1149 Da), Phe-Val-Asp-Ser-Ser-Ala-Thr-Thr (826 Da), Phe-Asn-Asp-Leu-Gly-Ala-Trp (821 Da), Phe-Pro-Asp-Thr-Thr-Thr-Leu (793 Da), and Lys-Phe-Gly-Glu-Gly-Lys (664). This study provided useful information for further development of an inexpensive enzymatic hydrolysis for producing high-value sea cucumber peptides. The peptides showed a potential to be applied as bio-functional ingredients for the food, pharmaceutical, or cosmetic industries. However, it is recommended that those six peptides to be further in-depth studied for its application efficacy, reaction mechanism, and bioavailability in animal or human trial.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Author Contributions
CD: investigation, data curation, writing-original draft preparation. TS and YM: supervision, validation, writing-review and editing. NA, SY, SM, and KJ: validation and supervision. JMR: writing-review and editing, validation, and supervision. SW: conceptualization, methodology, validation, investigation, writing-original draft preparation, writing-review and editing, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This research work was financial support from the National Research Council of Thailand (NRCT), Chiang Mai University (Thailand), College of Maritime Studies and Management, Chiang Mai University, and the program of the Thailand Graduate Institute of Science and Technology (TGIST, TG-22-10-61-049M) of the National Science and Technology Development Agency (NSTDA, Thailand).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Research Council of Thailand (NRCT) provided by Chiang Mai University (Thailand) and the program of the Thailand Graduate Institute of Science and Technology (TGIST, TG-22-10-61-049M) of the National Science and Technology Development Agency (NSTDA, Thailand).
References
Ahn C. B., Kim J. G., Je J. Y. (2014). Purification and Antioxidant Properties of Octapeptide From Salmon Byproduct Protein Hydrolysate by Gastrointestinal Digestion. Food Chem. 147, 78–83. doi: 10.1016/j.foodchem.2013.09.136
Association of Official Analytical (AOAC) (2000). “Official Methods of Analysis,” in The Association of Official Analytical Chemists, 17th Edn (Gaithersburg: MD: AOAC).
Bahrami Y., Zhang W., Franco C. (2014). Discovery of Novel Saponins From the Viscera of the Sea Cucumber Holothuria Lessoni. Mar. Drugs 12, 2633–2667. doi: 10.3390/md12052633
Chen H. Y., Chu X., Yan C. L., Chen X. H., Sun M., Wang Y. J., et al. (2007). Polypeptide From Chlamys Farreri Attenuates Murine Thymocytes Damage Induced by Ultraviolet B. Acta Pharmacol. Sin. 28, 1665–1670. doi: 10.1111/j.1745-7254.2007.00621.x
Chen T., Hou H., Fan Y., Wang S., Chen O., Si L., et al. (2016). Protective Effect of Gelatin Peptides From Pacific Cod Skin Against Photoaging by Inhibiting the Expression of MMPs via MAPK Signaling Pathway. J. Photochem. Photobiol. B. Biol. 15, 711–718. doi: 10.1016/j.jphotobiol.2016.10.015
Chen J., Liang P., Xiao Z., Chen M. F., Gong F., Li C., et al. (2019). Antiphotoaging Effect of Boiled Abalone Residual Peptide ATPGDEG on UVB-induced Keratinocyte HaCaT Cells. Food Nutri. Res. 63, 3508. doi: 10.29219/fnr.v63.3508
Choo P. S. (2008). ““Population Status, Fisheries and Trade of Sea Cucumbers in Asia”, In Sea Cucumbers,” in A Global Review of Fisheries and Trade, FAO Technical Paper No. 516. Eds. Toral-Granda V., Lovatelli A., Vasconcellos M. (Rome, Italy: FAO), 81–118.
Das M. K., Sahu P. K., Rao G. S., Mukkanti K., Silpavathi L. (2014). Application of Response Surface Method to Evaluate the Cytotoxic Potency of Ulva Fasciata Delile, a Marine Macro Alga. Saudi. J. Biol. Sci. 21 (6), 539–546. doi: 10.1016/j.sjbs.2014.02.003
Dewi A. S., Patantis G., Fawzya Y. N., Irianto H. E., Sádiah S. (2020). Angiotensin−converting Enzyme (ACE) Inhibitory Activities of Protein Hydrolysates From Indonesian Sea Cucumbers. Int. J. Pept. Res. Ther. 26, 2485–2493. doi: 10.1007/s10989-020-10035-5
Ghosh S., Sarkar T., Pati S., Kari Z. A., Edinur H. A., Chakraborty R. (2022). Novel Bioactive Compounds From Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.832957
Hamzeh A., Rezaei M., Khodabandeh S., Motamedzadegan A., Noruzinia M. (2017). Antiproliferative and Antioxidative Activities of Cuttlefish (Sepia Pharaonis) Protein Hydrolysates as Affected by Degree of Hydrolysis. J. Food Meas. Charact. 12, 721–727. doi: 10.1007/s11694-017-9685-0
Han J. H., Bang J. S., Choi Y. J., Choung S. Y. (2019). Anti-Melanogenic Effects of Oyster Hydrolysate in UVB Irradiated C57BL/6J Mice and B16F10 Melanoma Cells via Downregulation of cAMP Signaling Pathway. J. Ethnopharmacol. 229, 137–144. doi: 10.1016/j.jep.2018.09.036
Han H., Li L., Yi Y. H., Wang X. H., Pan M. X. (2012). Triterpene Glycosides From Sea Cucumber Holothuria Scabra With Cytotoxic Activity. Chin. Herb. Med. 4, 183–188. doi: 10.3969/j.issn.1674-6384.2012.03.002
Herbert P., Barros P., Ratola N., Alves A. (2000). HPLC Determination of Amino Acids in Musts and Port Wine Using OPA/FMOC Derivatives. J. Food Sci. 65 (7), 1130–1133. doi: 10.1111/j.1365-2621.2000.tb10251.x
Iosageanu A., Ilie D., Craciunescu O., Seciu-Grama A. M., Oancea A., Zarnescu O., et al. (2021). Effect of Fish Bone Bioactive Peptides on Oxidative, Inflammatory and Pigmentation Processes Triggered by UVB Irradiation in Skin Cells. Molecules 26, 2691. doi: 10.3390/molecules26092691
Je J. Y., Qian Z. J., Lee S. H., Byun H. G., Kim S. K. (2008). Purification and Antioxidant Properties of Bigeye Tuna (Thunnus Obesus) Dark Muscle Peptide on Free Radical-Mediated Oxidative Systems. J. Med. Food. 11, 629–637. doi: 10.1089/jmf.2007.0114
Jessica H. R., Adam J. M., Patrick J. S., Warwick L. M., Daniel N. S., Baltimore M. (2000). Photoaging: Mechanisms and Repair. J. Am. Acad. Dermatol. 55, 1–19. doi: 10.1016/j.jaad.2005.05.010
Khantaphant S., Benjakul S., Ghomi M. R. (2011). The Effects of Pretreatments on Antioxidative Activities of Protein Hydrolysate From the Muscle of Brownstripe Red Snapper (Lutjanus Vitta). LWT-Food. Sci. Technol. 44 (4), 1139–1148. doi: 10.1016/j.lwt.2010.10.009
Kim S. K., Himaya S. W., Kang K. H., Kim S. K. (2012). “Sea Cucumber Saponins: Realization of Their Anticancer Effects”, in In Marine Pharmacognosy: Trends and Applications (New York, NY: CRC Press), 119–128.
Klarić M. S., Rumora L., Ljubanovic D., Pepeljnjak S. (2008). Cytotoxicity and Apoptosis Induced by Fumonisin B1, Beauvericin and Ochratoxin A in Porcine Kidney PK15 Cells: Effects of Individual and Combined Treatment. Arch. Toxicol. 82, 247–255. doi: 10.1007/s00204-007-0245-y
Krobthong S., Yingchutrakul Y. (2020). Identification and Enhancement of Antioxidant P1-Peptide Isolated From Ganoderma Lucidum Hydrolysate. Food Biotechnol. 34 (4), 338–351. doi: 10.1080/08905436.2020.1844228
Li M., Gao Y., Qi Y. X., Song Z. Y., Li Z. B., Lin Y. T., et al. (2021). Assessment of the Nutritional Value of Cultured Sea Cucumber Apostichopus Japonicus. J. Aquat. Food Prod. Technol 30 (7), 868–879. doi: 10.1080/10498850.2021.1949769
Lin L., Zhu Q., Zheng L., Zhao M., Fan J., Liu S. (2020). Preparation of Sea Cucumber (Stichopus Variegates) Peptide Fraction With Desired Organoleptic Property and its Anti-Aging Activity in Fruit Flies and D-Galactose-Induced Aging Mice. J. Funct. Food. 69, 103–954. doi: 10.1016/j.jff.2020.103954
Liu X., Sun Z., Xhang M., Meng X., Xia X., Yuan W., et al. (2012). Antioxidant and Antihyperlipidemic Activities of Polysaccharides From Sea Cucumber Apostichopus Japonicus. Carbohydr. Polym. 90, 1664–1670. doi: 10.1016/j.carbpol.2012.07.047
Lu J., Hou H., Fan Y., Yang T., Li B. (2017). Identification of MMP-1 Inhibitory Peptides From Cod Skin Gelatin Hydrolysates and the Inhibition Mechanism by MAPK Signaling Pathway. J. Funct. Foods. 33, 251–260. doi: 10.1016/j.jff.2017.03.049
Ma B., Zhang K., Hendrie C., Liang C., Li M., Doherty-Kirby A., et al. (2003). PEAKS: Powerful Software for Peptide De Novo Sequencing by Tandem Mass Spectrometry. Rapid Commun. Mass. Spectrom. 17 (20), 2337–2342. doi: 10.1002/rcm.1196
Mendis E., Rajapakse N., Kim S. K. (2005). Antioxidant Properties of a Radicals Scavenging Peptide Purified From Enzymatically Prepared Fish Skin Gelatin Hydrolysate. J. Agric. Food Chem. 53, 581–587. doi: 10.1021/jf048877v
Mongkonkamthorn N., Malila Y., Regenstein J. M., Wangtueai S. (2021). Enzymatic Hydrolysis Optimization for Preparation of Tuna Dark Meat Hydrolysate With Antioxidant and Angiotensin I-Converting Enzyme (ACE) Inhibitory Activities. J. Aquat. Food Prod. Technol. 30 (9), 1090–1108. doi: 10.1080/10498850.2021.1974138
Mongkonkamthorn N., Malila Y., Yarnpakdee S., Makkhun S., Regenstein J. M., Wangtueai S. (2020). Production of Protein Hydrolysate Containing Antioxidant and Angiotensin -I-Converting Enzyme (ACE) Inhibitory Activities From Tuna (Katsuwonus Pelamis) Blood. Processes 8 (11), 15–18. doi: 10.3390/pr8111518
Ngo D. H., Qian Z. J., Ryu B. M., Park J. W., Kim S. K. (2010). In Vitro Antioxidant Activity of a Peptide Isolated From Nile Tilapia (Oreochromis Niloticus) Scale Gelatin in Free Radical-Mediated Oxidative Systems. J. Funct. Food. 2, 107–117. doi: 10.1016/j.jff.2010.02.001
Parichat H., Piyathida K., Premwadee S. (2011). Lowering the Maillard Reaction Products (MRPs) in Heated Whey Protein Products and Their Cytotoxicity in Human Cell Models by Whey Protein Hydrolysate. Int. Food Res. J. 44 (3), 748–754. doi: 10.1016/j.foodres.2011.01.010
Peng Z., Chen B., Zheng Q., Zhu G., Cao W., Qin X., et al. (2020). Ameliorative Effects of Peptides From the Oyster (Crassostrea Hongkongensis) Protein Hydrolysates Against UVB-Induced Skin Photodamage in Mice. Mar. Drugs 18, 288. doi: 10.3390/md18060288
Raghavan S., Kristinsson H. G. (2008). Antioxidative Efficacy of Alkali-Treated Tilapia Protein Hydrolysates: A Comparative Study of Five Enzymes. J. Agric. Food Chem. 56 (4), 1434–1441. doi: 10.1021/jf0733160
Sae-Leaw T., Karnjanapratum S., O’Callaghan Y. C., O’Keeffe M. B., FitzGerald R. J., O’Brien N. M., et al. (2016). Purification and Identification of Antioxidant Peptides From Gelatin Hydrolysate of Seabass Skin. Food Biochem. 41 (3), e12350. doi: 10.1111/jfbc.12350
Saidi S., Deratani A., Belleville M. P., Amar R. B. (2014). Antioxidant Properties of Peptide Fractions From Tuna Dark Muscle Protein by-Product Hydrolysate Produced by Membrane Fractionation Process. Int. Food Res. J. 65, 329–336. doi: 10.1016/j.foodres.2014.09.023
Siahaan E. A., Pangestuti R., Munandar H., Kim S. K. (2017). Cosmeceuticals Properties of Sea Cucumbers: Prospects and Trends. Cosmetics 4 (3), 26. doi: 10.3390/cosmetics4030026
Sun L., Zhang Y., Zhuang Y. (2013). Antiphotoaging Effect and Purification of an Antioxidant Peptide From Tilapia (Oreochromis Niloticus) Gelatin Peptides. J. Funct. Foods. 5, 154–162. doi: 10.1016/j.jff.2012.09.006
Torita A., Liu Y. C., Hasega Y. (2004). Photoprotective Activity of Scallop Shell Water-Extract in Keratinocyte Cells. Fish. Sci. 70 (5), 910–915. doi: 10.1111/j.1444-2906.2004.00886.x
Wangtueai S., Phimolsiripol Y., Vichasilp C., Regenstein J. M., Schoenlechner R. (2020). Optimization of Gluten-Free Functional Noodles Formulation Enriched With Fish Gelatin Hydrolysates. LWT-Food. Sci. Technol. 133, 10997. doi: 10.1016/j.lwt.2020.109977
Wangtueai S., Siebenhandl-Ehn S., Haltrich D. (2016). Optimization of the Preparation of Gelatin Hydrolysates With Antioxidative Activity From Lizardfish (Saurida Spp.) Scales Gelatin. Chiang. Mai. J. Sci. 43 (1), 68–79.
Wen J., Hu C., Fan S. (2010). Chemical Composition and Nutritional Quality of Sea Cucumbers. J. Sci. Food Agric. 90 (14), 2469–2474. doi: 10.1002/jsfa.4108
Xue Z., Li H., Wang X., Li X., Liu Y., Sun J. (2015). A Review of the Immune Molecules in the Sea Cucumber. Fish. Shellfish. Immunol. 44 (1), 1–11. doi: 10.1016/j.fsi.2015.01.026
Ye Y., Sun-Waterhouse D., You L., Abbasi A. M. (2017). Harnessing Food-Based Bioactive Compounds to Reduce the Effects of Ultraviolet Radiation: A Review Exploring the Link Between Food and Human Health. Int. J. Food Sci. 52, 595–607. doi: 10.1111/ijfs.13344
Ye Y., You L., Deng Q., Li X., Zhao M. (2019). Preparation, Structure Identification and the Antiphotoaging Activity of Peptide Fraction OP-Ia From Ostrea Rivularis. RSC. Adv. 9, 44–51. doi: 10.1039/C8RA08137A
Zhang C., Lv J., Qin X., Peng Z., Lin H. (2022). Novel Antioxidant Peptides From Crassostrea Hongkongensis Improve Photo-Oxidation in UV-Induced HaCaT Cells. Mar. Drugs 20, 100. doi: 10.3390/md20020100
Zhong S., Ma C., Lin Y. C., Luo Y. (2011). Antioxidant Properties of Peptide Fractions From Silver Carp (Hypophthalmichthys Molitrix) Processing by-Product Protein Hydrolysates Evaluated by Electron Spin Resonance Spectrometry. Food Chem. 126, 1636–1642. doi: 10.1016/j.foodchem.2010.12.046
Zhou X., Wang C., Jiang A. (2012). Antioxidant Peptides Isolated From Sea Cucumber Stichopus Japonicus. Eur. Food Res. Technol. 234, 441–447. doi: 10.1007/s00217-001-1610-x
Zhuang Y., Hou H., Zhao X., Zhang Z., Li B. (2009). Effects of Collagen and Collagen Hydrolysate From Jellyfish (Rhopilema Esculentum) on Mice Skin Photoaging Induced by UV Irradiation. J. Food Sci. 74 (6), 183–188. doi: 10.1111/j.1750-3841.2009.01236.x
Keywords: sea cucumber, Holothuria scabra, antioxidant, UV-B protective activity, protein hydrolysate, peptide
Citation: Doungapai C, Siriwoharn T, Malila Y, Autsavapromporn N, Makkhun S, Yarnpakdee S, Jantanasakulwong K, Regenstein JM and Wangtueai S (2022) UV-B Protective and Antioxidant Activities of Protein Hydrolysate From Sea Cucumber (Holothuria scabra) Using Enzymatic Hydrolysis. Front. Mar. Sci. 9:892255. doi: 10.3389/fmars.2022.892255
Received: 08 March 2022; Accepted: 19 May 2022;
Published: 13 June 2022.
Edited by:
Wolfram Brück, University of Applied Sciences and Arts of Western Switzerland, SwitzerlandReviewed by:
Meivelu Moovendhan, Sathyabama Institute of Science and Technology, IndiaAphichart Karnchanatat, Chulalongkorn University, Thailand
Baoguo Sun, Beijing Technology and Business University, China
Copyright © 2022 Doungapai, Siriwoharn, Malila, Autsavapromporn, Makkhun, Yarnpakdee, Jantanasakulwong, Regenstein and Wangtueai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sutee Wangtueai, c3V0ZWUud0BjbXUuYWMudGg=
 Chitpon Doungapai1
Chitpon Doungapai1 Narongchai Autsavapromporn
Narongchai Autsavapromporn Joe M. Regenstein
Joe M. Regenstein Sutee Wangtueai
Sutee Wangtueai