- 1Laboratory of Ichthyology and Coastal Fishery, Institute of Oceanography and Fisheries, Split, Croatia
- 2Faculty of Science, University of Split, Split, Croatia
- 3Ocean Graduate School and the UWA Oceans Institute, The University of Western Australia, Crawley, WA, Australia
- 4Division for Marine and Environmental Research, Ruđer Bošković Institute, Zagreb, Croatia
- 5Ministry of Agriculture, Directorate of Fisheries, Zagreb, Croatia
The study describes recent decadal changes (2008–2017) in the landing biomass, fishing effort and CPUE (kg/day) data of European lobster Homarus gammarus in the eastern Adriatic Sea region, and relates these changes to increases of sea bottom temperatures detected at long-term in situ stations and modelled by an ocean numerical model (ROMS, Regional Ocean Modelling System). Modelling results were further used to quantify spatial and temporal differences of bottom temperature changes over different fishing zones. Trends of sea bottom temperature were positive and statistically significant between stations. Temporal trends of landing, effort and CPUE were also positive and significant for the northern Adriatic. Correlation analysis was used to test the relationship between winter and spring sea bottom temperatures and CPUE data of H. gammarus, separately for the northern and central Adriatic Sea, resulting in statistically significant correlations for both areas. Whether the increased CPUE in the northern Adriatic is due to increased abundance or catchability is discussed. The observed temperature changes likely reflect climate system changes recognised at the regional level and as such, lobster management measures will need to be revised and updated in the future.
Introduction
Climate change is reshaping ecosystems in ways that affect resources and ecosystem services (Nelson et al., 2013). Generally, fisheries depends first and foremost on the biomass of fishing resources, and fishing has often been the dominant driver of the status of resources. A failure to detect changes in the environment, or to act appropriately when changes are detected, can jeopardize fisheries (Holling, 2001). In term of climate change, fisheries are most often affected by rising sea temperatures and changes in ocean current systems. Cold water species are generally negatively affected by water warming, while thermophilus species benefit from it (Chaikin et al., 2021).
Climate change is already provoking changes in the spatial distribution of lobster species and therefore has the potential to alter territorial behaviour of fishermen and their landings as a consequence (Briones-Fourzán and Lozano-Álvarez, 2015). Such changes have already been reported in lobster populations worldwide, and are mainly related to sea warming (Cockcroft et al., 2008; Pecl et al., 2009; Caputi et al., 2010; Steneck and Wahle, 2013; Wahle et al., 2015; Rheuban et al., 2017; Le Bris et al., 2018). Boavida-Portugal et al. (2018) projected that clawed lobsters will contract their climatic envelope between 40 and 100% by the end of the century. Clawed lobsters of the genera Homarus and Nephrops are projected to shift their envelope to northern latitudes, likely affecting the North European, North American and Canadian fisheries, with potential detrimental effects on coastal communities (Greenan et al., 2019). Increasing temperatures and overfishing in coastal areas may result in sudden changes of environmental conditions and loss of benthic habitat (Caputi et al., 2013).
Ocean temperatures above an optimal thermal range can reduce lobster survival, growth, and reproduction as a result of stress, decreased recruitment and increased susceptibility to disease (Aiken and Waddy, 1986; ASMFC, 2015). The scale and characteristics of lobster responses to warming vary across the range of warming (Boudreau et al., 2015; Le Bris et al., 2018). For example, an overall increase of abundance was reported for of American lobster, but different trajectories were observed within the range of the species in the Gulf of Maine (Le Bris et al., 2018), with the fishery increasing dramatically in the central and northern part while it effectively collapsed at the warmer southern limit (ASMFC, 2015). Similarly, a major shift in resource availability of rock lobster Jasus lalandii to higher latitudes on the western coast of Africa was reported, with declined landings in lower latitudes at the end of 20th century (Cockcroft et al., 2008). A difference in the dependence on environmental variability in relation to geographic position was also determined for Scottish H. gammarus fisheries (Lizárraga-Cubedo et al., 2015). However, there are no published data regarding recent changes in landings and CPUE in relation to sea warming for the European lobster in southern European regions.
European lobster (Homarus gammarus) is a species of boreal origin inhabiting coastal shelf seas of northern Europe, with the Mediterranean Sea representing the southern limit of its distribution range (Holthius, 1991; Mercer et al., 2001). Previous studies have suggested that warming beyond the temperature optimum will lead to lower juvenile survival, lower recruitment, suboptimal growth conditions, and reduced fishery productivity in the future (Pere et al., 2019). Temperature changes might play a major role for the future southern distribution of H. gammarus populations, with excessively high temperatures leading to reduced population abundance at the southern boundaries (Triantafyllidis et al., 2005). In line with this, it is presumed that the coldest parts of the Mediterranean Sea (Gulf of Lyon and the northern Adriatic) could initially serve as a sanctuary for cold-temperate species (Ben Rais Lasram et al., 2010).
The European lobster fishery is one of the most valuable fisheries in northern Europe, mainly in the United Kingdom, Ireland, and northern France (Bennett and Lovewell, 1977; Bennet et al., 1993; Browne et al., 2001) with total annual landings around 5000 t in last 10 years (FAO, 2021). Beside European lobster, economically important lobster species within European water include Palinurus elephas and Scyllarides latus (Kampouris et al., 2020). The fishery is based on traps of various designs, shapes, and sizes (Cobb and Castro, 2006). Along the Mediterranean coast, H. gammarus is not a target species and is more often a by-catch occurring in trammel nets targeting the common spiny lobster Palinurus elephas (Quetglas et al., 2004) or in gillnets targeting fish (Kampouris et al., 2020) during the fishing season (Goñi and Latrouite, 2005; Pere et al., 2019). Mediterranean landings of H. gammarus were around 140 t for the period 2006–2015 (FAO, 2021) though this may be a general underestimation in the Mediterranean (Le Manach et al., 2011; Pere et al., 2019). Data for the Greek fleet, which reported more than 50% of Mediterranean landings in previous years, are missing for the years 2016 and later (FAO, 2021) and some data and clarifications are available (Kampouris et al., 2020). For lobster catches in Spain, there are indications of significant declines (Lloret and Riera, 2008). The lack of historical landing datasets prevents us from concluding on the reliable abundance of H. gammarus. However, increasing signs of possible failure in egg production and recruitment overfishing have already been reported in Irish fisheries (Tully et al., 2001) and can be worrying in the context of a lack of knowledge of the current population (stock) status of H. gammarus in the Mediterranean.
With regard to the current higher landings reported in some Mediterranean sub-areas, failure to recognise the impacts of sea warming on the European lobster may contribute to potential overfishing in the coming years. This fishery depends on sound management, though the size of the stock certainly depends on future temperature conditions. This study analyses recent positive changes in the reported landing biomass and catch per unit effort (CPUE) of H. gammarus in the official fishing zones in the Adriatic Sea and links them with positive sea bottom temperature trends at the sub-regional level. We hypothesised that a substantial increase in the winter and spring sea bottom temperatures in the period 2008–2017 is reflected in the positive trends in landings and catch per unit effort (CPUE) in the northern Adriatic. Ocean numerical model results were examined to quantify the observed spatial and temporal temperature trends. The correlations between increased abundance and the observed increased landings are discussed in detail.
Material and Methods
Study Area
Located in the northernmost part of the central Mediterranean, the semi-enclosed Adriatic Sea is divided into the northern, central and southern Adriatic. The Jabuka Pit depression (280 m depth) separates the northern shallow shelf (depths up to 80 m) from the deeper central Adriatic, while the northwest perimeter of the South Adriatic Pit (1200 m depth) separates the central and the southern Adriatic (Figure 1).
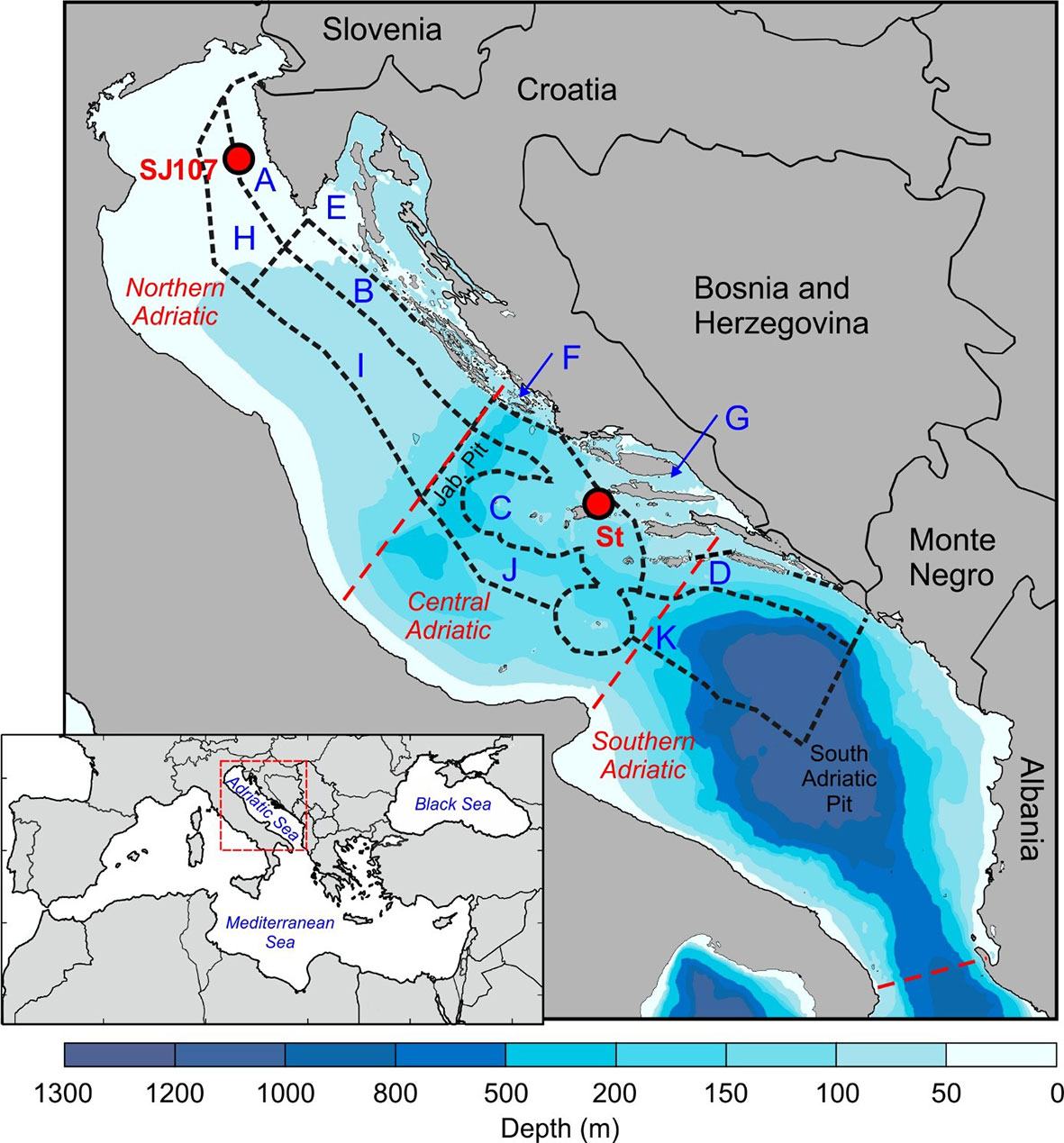
Figure 1 Bathymetric map of the study area, sampling stations SJ107 and Stončica (St) with 11 fishery zones corresponding to northern, central and southern Adriatic indicated.
The most important physical parameter affecting lobster abundance, and thus landings and CPUE, is sea bottom temperature (Green et al., 2014; Zhao et al., 2019). Throughout the Adriatic, this is dominantly affected by bathymetry and seasonal changes of heat flux (Artegiani et al., 1997). Over the shallow northern Adriatic, temperatures vary from 6°C during severe winter cooling events to 20°C during periods of vertical mixing in the autumn. Deeper regions of the central Adriatic exhibit less seasonal changes with temperatures ranging from 13 to 17°C (Buljan and Zore-Armanda, 1976; Lipizer et al., 2014), and within deepest regions of southern Adriatic temperatures vary even less, from 12.4 to 13.7°C (Lipizer et al., 2014; Cardin et al., 2020).
Environmental Variables and Modelling System
Temperature data were retrieved from measurements and the numerical model Regional Ocean Modelling System (ROMS) (Shchepetkin and McWilliams, 2005; Shchepetkin and McWilliams, 2009). Temperature measurements were collected at the stations Stončica (depth: 95 m) in the central Adriatic (43°0’0”N, 16°20’0”E) and SJ107 (depth: 35 m) in the northern Adriatic (45°2′52″N, 13°19′0″E) in the period 2008–2017 (Figure 1). These stations are parts of two oceanographic transects monitored for at least a half of century: Stončica by the Institute of Oceanography and Fisheries (Split) and SJ107 by Institute Ruđer Bošković (Rovinj) (Marić et al., 2012; Vilibić et al., 2012). The stations are highly representative in term of depth for the northern and central Adriatic. Also, the depths at which the measurements were performed correspond to the typical inhabitation and catch depths of the analysed lobster species in the northern (20–35 m) and central (50–100 m) Adriatic. Temperature measurements were mostly carried out once a month or once every two months. At Stončica measurements were performed in 64%, and at SJ107 in 75% of months within the study period. Measurements were taken at Stončica using CTD probes (IDRONAUT 316, SeaBird-25 and 911+) with an accuracy of ±0.003°C, and at SJ107 using protected reversing thermometers (Richter and Wiese, Berlin; precision ±0.01°C) and reversing digital thermometers (SiS RTM 4002; precision ±0.003°C) attached to Niskin bottles. For this research, we used the deepest sampling, which was usually taken 2 m above the seabed.
Daily values of the ROMS were used to reproduce temperature changes in the Adriatic Sea between 2008 and 2017. The ROMS model is a 3D hydrostatic, nonlinear, free surface, sigma coordinate, time splitting finite difference primitive equation model (Shchepetkin and McWilliams, 2005; Shchepetkin and McWilliams, 2009). The lateral boundary conditions were taken from the AREG model of the Adriatic Forecasting System (AFS) (Oddo et al., 2006), while atmospheric forcing was prescribed via bulk formulation (Fairall et al., 1996), using all the required variables from the operational local area model ALADIN/HR (Tudor et al., 2013). Horizontal resolution was 2 km, with 20 sigma vertical layers in the model. River discharges were introduced to the ocean model following climatology by Vilibić et al. (2016) for all rivers except the River Po, for which real daily discharges were used. All other details about the model setup are provided by Janeković et al. (2014) and Vilibić et al. (2016). Temperatures modelled at the lowest sigma coordinate, roughly corresponding to sea bottom temperatures, were analysed in the paper.
Additionally, bottom temperature averages were calculated for each fishing zone and year/season by considering each model point within the fishing zone polygons. Trend significance of sea bottom temperature was tested by the Mann-Kendall nonparametric test.
Fisheries Data
For management purposes and data collection, the Croatian marine fishing area has been administratively divided into smaller units (11 fishing zones; OG, 2011) (Figure 1). All available data on H. gammarus landings in the Adriatic Sea for fishing zones A, B, C, D, E, F, G (coastal zones) and H, I, J, K (offshore zones) for the period 2008–2017 were obtained from the Fisheries Directorate (Croatian Ministry of Agriculture) based on fisher’s logbooks (fishery dependant data). These data correspond with open season for lobsters (May–August) and the MED EU minimum legal size of 105 mm carapace length (CL) (EU Regulation 1967/2006). During the study period, insignificant landings (less than 50 kg per year) were reported in fishing zones H, I, J and K and therefore these zones were excluded from further analysis. For analysis purposes, data were standardised as the catch per unit effort (CPUE), expressed as the biomass of H. gammarus caught per fishing trip of a single fisher. For this study, the available data at the scale of fishing zones were aggregated to three general regions: northern, central and southern following the natural geographical division of the Adriatic Sea. Each fishing zone has boundaries expressed by geographical position. Thus, fishing zones A, B and E corresponds to the northern, zones C, F and G to the central, and zone D to the southern Adriatic. As with temperature, the trend significance in H. gammarus landings, fishing effort and CPUE was tested with the Mann-Kendall nonparametric test. Pearson correlation analysis was used to test the linear relationship between sea bottom temperature and CPUE (kg/day) data of H. gammarus.
Results
Time Series of Landings and CPUE
A total of 22.83 t of H. gammarus was landed on the eastern Adriatic coast during the study period (data of the Fisheries Directorate for 2008–2017), distributed by region as follows: northern 13.93 t, central 8.65 t and southern Adriatic 0.25 t. Particularly, H. gammarus was mostly captured by pots and gillnets in the fishing zones A and E in the northern Adriatic (Table 1). In that area, as shown in Figure 2A, landings averaged over three fishing zones (A, B, E) fluctuated from 0.21 t (2008; landings = 631.5 kg; CPUE = 2.5 kg/day) to 0.75 t (2016; landings = 2244 kg; CPUE=3.41 kg/day). Landings averaged over the three fishing zones corresponding to the central Adriatic (C, G, F) ranged from 0.23 t (2010; landings = 685.7 kg; CPUE = 1.77 kg/day) to 0.37 t (2014; landings = 1112 kg; CPUE = 2.18 kg/day). In the same period, landings in the southern Adriatic (zone D) were less than 50 kg per year, namely from 14 kg (2008; 10 fishing days corresponding to CPUE of 1.4 kg/day) to 41.1 (2010; 25 fishing days and CPUE of 1.6 kg/day) and therefore the data from that zone, i.e. the southern Adriatic, were not included in the further analysis. Temporal trends (2008–2017) of H. gammarus landings in the Adriatic Sea indicate a statistically insignificant and weak increase in the central Adriatic (4.5 kg/year; p > 0.05; R2 = 0.008) and a statistically significant increase in the northern Adriatic (160.9 kg/year; p < 0.05; R2 = 0.755).

Table 1 Reported landings of European lobster, Homarus gammarus among seven fishing zones corresponding to the northern, central and southern Adriatic Sea across the study period (data of the Fisheries Directorate for 2008–2017).
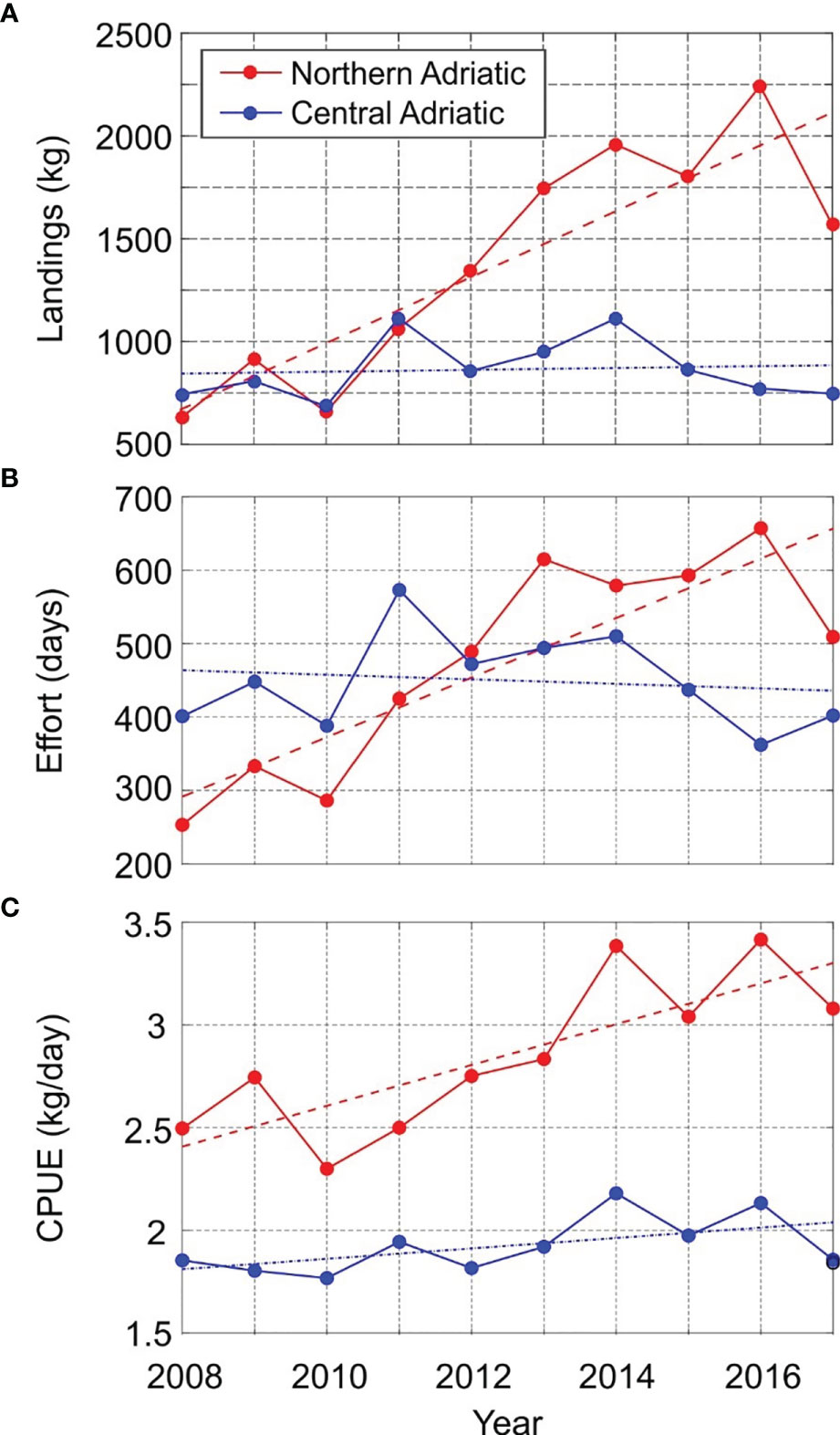
Figure 2 Time series of: (A) landings; (B) effort; (C) CPUE. Significant (p < 0.05) and insignificant (p > 0.05) trends are plotted with dashed and dot-dash line, respectively.
The temporal trend across the study period (2008–2017) of fishing effort (Figure 2B) was weakly negative and statistically insignificant (-3.1 days/year, p > 0.05; R2 = 0.021) in the central Adriatic, while fishing effort was positive and statistically significant (40.6 days/year, p < 0.05; R = 0.728) in the northern Adriatic. As a consequence, the decennial temporal trend of H. gammarus of catch per unit effort followed a similar pattern as landings: the CPUE trend over time in the central Adriatic was weakly positive but statistically insignificant (0.03 kg/effort, p > 0.05, R2 = 0.307) while CPUE was positive and statistically significant (0.09 kg/effort, p < 0.05, R2 = 0.643) in the northern Adriatic (Figure 2C). The negative trend of fishing effort in the central Adriatic affects the CPUE calculation in the same area and should be carefully considered.
Time Series of Bottom Temperature
Time series of the yearly averages of measured bottom temperatures at the SJ107 and Stončica stations and modelled bottom temperatures in the selected fishing zones are shown in Figure 3. The yearly averages of measured temperatures at SJ107 for 2012 and 2017 were not calculated, since data were lacking at SJ107 for February to June 2012, i.e. during the part of year characterised by the lowest bottom temperatures, while in 2017, measurements are lacking for September to December, i.e., the part of the year characterised by the highest bottom temperatures. Including these values would thus result in a significant overestimation (or underestimation) of the measured yearly averages for these two years.
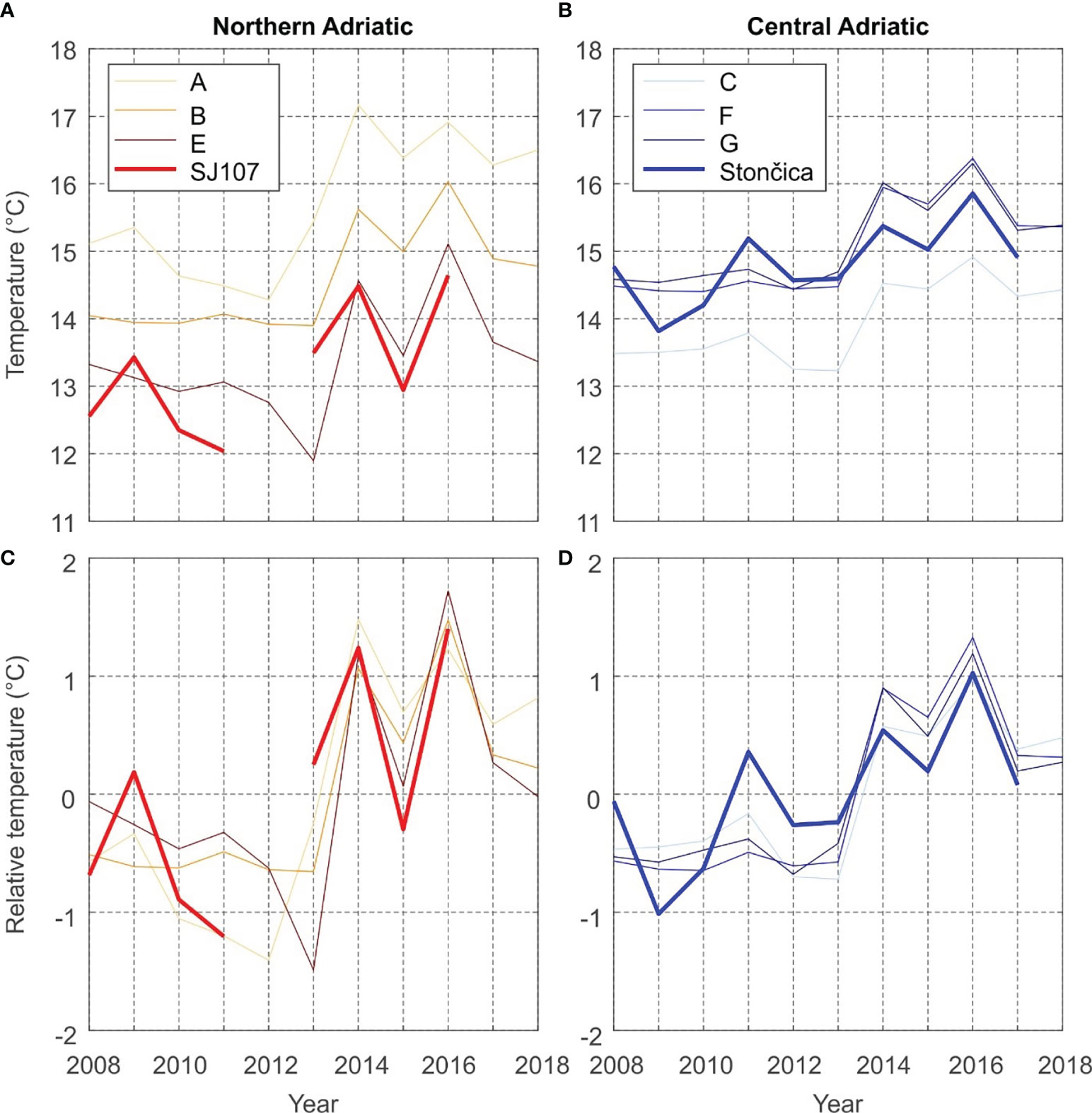
Figure 3 Model to measurement comparison of annual mean value of sea bottom temperatures at: (A) measurement station SJ107 and the northern Adriatic fishing zones; (B) measurement station Stončica and the central Adriatic fishing zones. (C, D) same as (A, B) but with average values removed.
The bottom temperature trends at both stations (0.15°C/yr, p < 0.05 at SJ107; 0.14°C/yr, p < 0.05 at Stončica) and over fishing zones were positive and statistically significant. The temperature trend is apparently governed by a pronounced jump of bottom temperatures values, starting in 2013 (Figure 3). This jump is evident in both the measurements and the model, in both the central and northern Adriatic, though it is more pronounced in the northern Adriatic (~1.5-2.5°C) than in the central Adriatic (~1°C). Figure 3 clearly shows that the model is biased when it comes to reproducing absolute values of bottom sea temperature, in particularly in the shallow northern Adriatic (Figure 3A). Nonetheless, the reproduction of the variability of yearly (Figures 3C, D) and seasonal (Figure 4) temperature was satisfactory for both areas, and thus we chose to use the model for more detailed analysis.
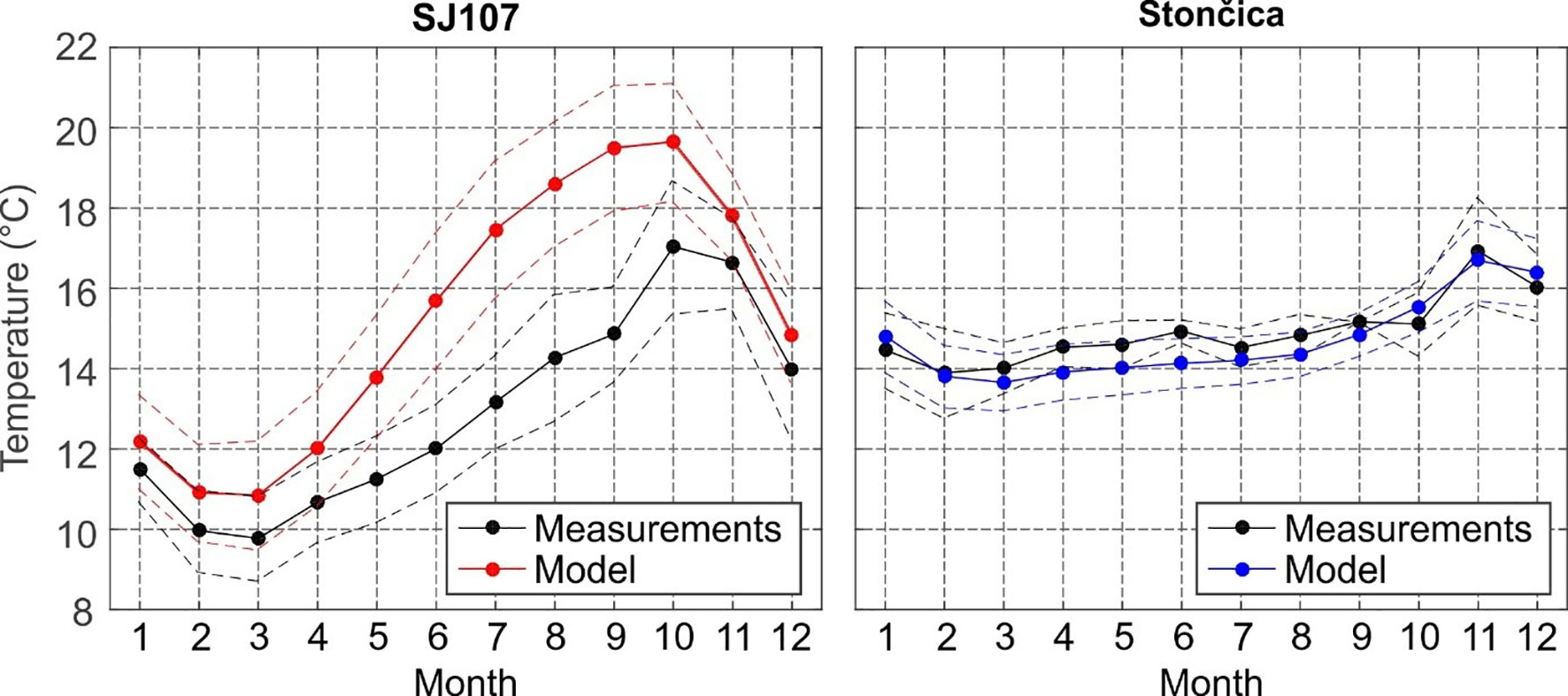
Figure 4 Monthly averages (2008–2017) of measured (black) and modelled (red) near bottom temperature (full line) and corresponding standard deviation (dashed line) at (left) SJ107; (right) Stončica. For the model, temperature series were taken from the grid cell nearest to SJ107 and Stončica.
Seasonal Changes of Bottom Temperature
The seasonal changes of near-bottom temperature at SJ107 (34 m) and Stončica (95 m) are shown in Figure 4. These changes were more pronounced in the northern Adriatic, where their range reaches ~7°C, than in the deeper central Adriatic (~3°C in range). The northern Adriatic is much colder during most of the year, particularly during the winter period (February-March). October is the only month in which the sea bottom temperature is higher in the deep northern Adriatic than in the deep central Adriatic.
The modelled values were overestimated at SJ107 by 0.6-0.9°C during the winter months and even more during the period of developed thermocline (May through October; up to 4.0°C). In contrast, the model slightly underestimated the bottom temperature at the Stončica station (model-to-measurements bias is up to -0.8°C in June). The offset of model values might be due to systematic offsets of values of atmosphere-ocean heat fluxes, or of lateral boundary conditions propagating from Otranto towards the central and northern Adriatic. Also, it might be due to an inadequate reproduction of vertical mixing processes. Nonetheless, it should be noted that the phase of the seasonal signal and its variability and range are well reproduced. Standard deviations of bottom temperatures are consistent between model and measurements, and are higher in the northern Adriatic (~1°C) than in the central Adriatic (~0.5°C). The model best reproduced the JFM (January-February-March) and AMJ (April-May-June) seasonal bottom temperatures. This is particularly important for this study, as JFM and AMJ were found to be the two most relevant periods in which changes can affect lobster landings and CPUE. JFM and AMJ also correspond to periods preceding and during the open fishing season.
Correlation Analysis of Modelled JFM and AMJ Temperature and European Lobster CPUE
Correlations between bottom sea temperature and CPUE (kg/day) for the fishing areas (Northern Adriatic - zones A, B and E; central Adriatic - zones C, G and F) for the period from 2008 to 2017 were found to be statistically significant both for winter (JFM) and spring (AMJ) (Table 2). The simultaneous time series of JFM and AMJ temperatures and CPUE (kg/day) of European lobster are shown in Figure 5 for the northern and in Figure 6 for the central Adriatic. A high correspondence between the two variables is evident: in both areas, the CPUE values significantly increases in 2013/2014, at the same time as the bottom temperatures. Most individual peaks in CPUE also correspond with individual peaks of the temperature time series (2014 and 2016 for the northern Adriatic; 2011, 2014 and 2016 for the central Adriatic).
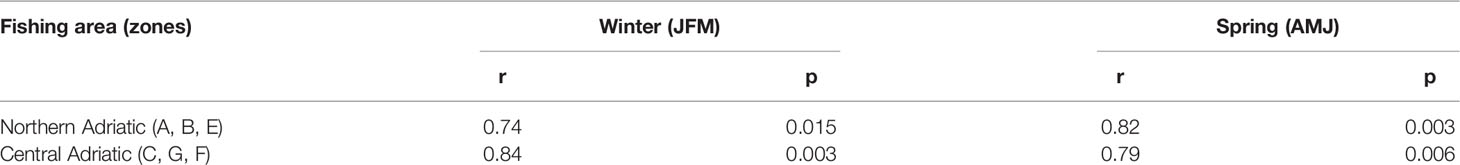
Table 2 Correlation indices for time series of bottom sea temperature and catch per unit effort CPUE (kg/day) of European lobster Homarus gammarus for the grouped fishing zones of the northern and central Adriatic in winter (JFM) and spring (AMJ) period (r-correlation coefficient; p – significance value).
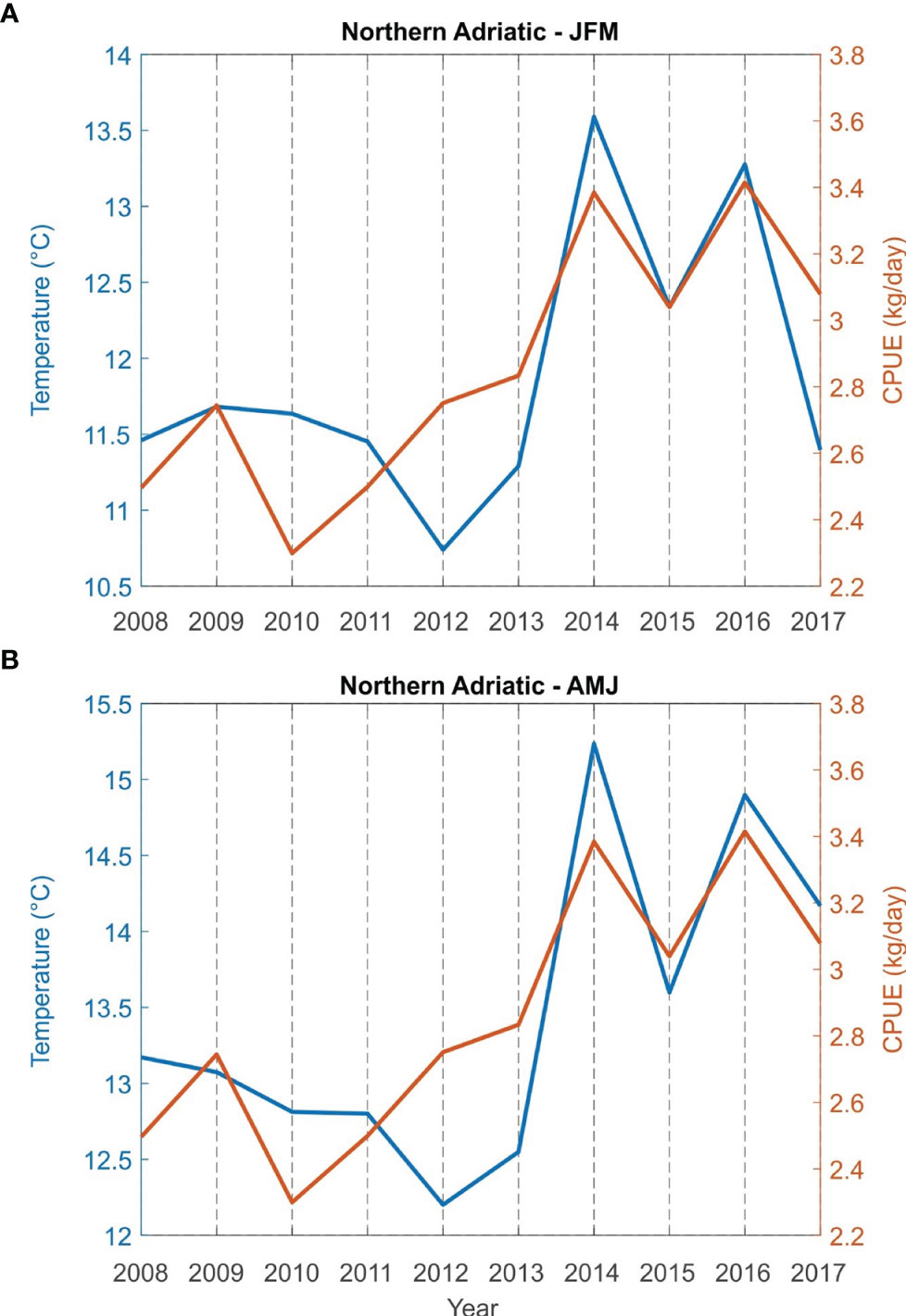
Figure 5 Time series of sea bottom temperature and catch per unit effort CPUE (kg/day) of European lobster Homarus gammarus in winter (JFM, A) and spring (AMJ, B) for grouped fishing zones of the northern Adriatic (zones A, B and E).
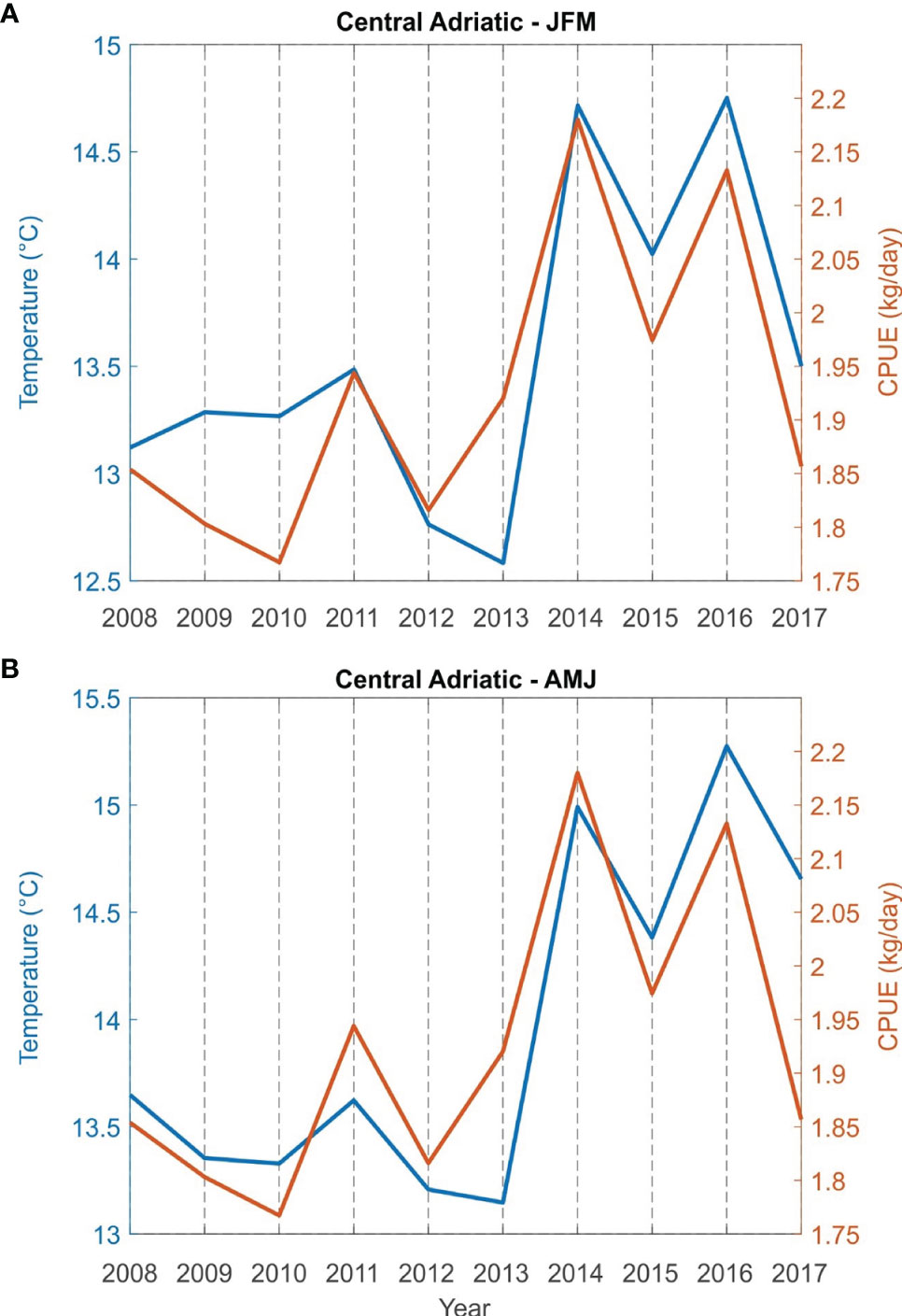
Figure 6 Time series of sea bottom temperature and catch per unit effort CPUE (kg/day) of European lobster Homarus gammarus in winter (JFM, A) and spring (AMJ, B) for grouped fishing zones of the central Adriatic (zones C, G and F).
Discussion
Changes of Sea Bottom Temperatures in the Period 2008–2017
The observed temperature changes might be the result of ongoing climate changes or just a local or regional phenomenon. Analyses of satellite-derived sea surface temperature documented the largest trend in June, with a rate of 4.3°C over 100 years over the whole Mediterranean and some 30% higher rates over the northern Adriatic (Pastor et al., 2018). This has been attributed quasi-equally to multi-decadal oscillations (like Atlantic Multidecadal Oscillation, Knight et al., 2006) and to real warming trends (Iona et al., 2018). Vilibić et al. (2019) found a substantial increase in temperature in the northern Adriatic between 1979 and 2017, particularly high on the surface and during the summer season. Modelled bottom temperatures in the present study show a substantial increase in winter (JFM) and spring (AMJ) values over the whole Adriatic between the first (2008–2012) and the last (2013–2017) five years of the simulation. These changes are clearly visible on the corresponding difference plots for JFM and AMJ (Figure 7). The largest increase in both seasons was found in the shallow northern Adriatic, particularly in zone A (also the zone where most lobster is caught), thereby confirming the findings of Pastor et al. (2018). The increase is less pronounced in the deeper central and southern parts of the Adriatic, as the seasonal thermocline is far shallower than the ocean depth there (Buljan and Zore-Armanda, 1976; Lipizer et al., 2014). The JFM temperature increase in zone A was between 1 and 2°C, and between 0 and 1°C over most of the central Adriatic. The AMJ temperature increases were even higher in both areas: up to 2.5°C in zone A of the northern Adriatic, and up to 1.5°C over the central Adriatic zones. Despite the observed changes, JFM temperatures remained lower in the northern than in the central Adriatic. On the other hand, the northern AMJ temperatures remained higher than central ones, with the temperature difference between the two areas increasing over time, due to the more efficient heat transfer towards the bottom in shallow waters (Artegiani et al., 1997).
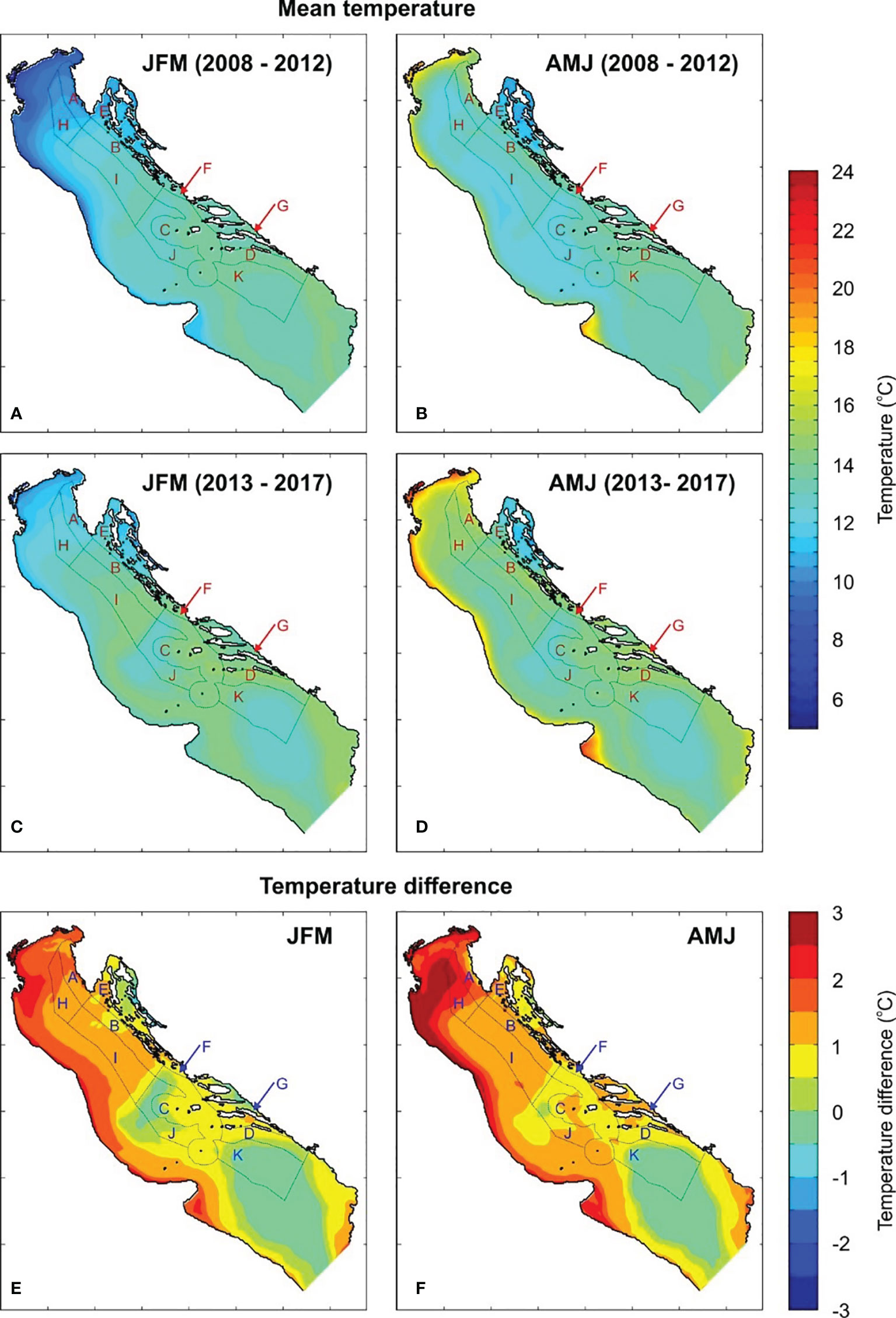
Figure 7 Modelled averaged JFM (A, C) and AMJ (B, D) sea bottom temperatures for 2008–2012 and 2013–2017; temperature difference between two periods (E, F). Fishery zones are also indicated (JFM, January, February, March; AMJ, April – May – June).
Increase in Lobster Landings: Higher Abundance or Increased Catchability?
The total annual European landings of H. gammarus over the last 10 years has been approximately 5000 tonnes (FAO, 2021). However, northern European countries have reported considerably higher landings than those in the Mediterranean basin, and a pattern of low H. gammarus abundance is evident throughout the Mediterranean when compared with Atlantic stocks. However, statistics of H. gammarus landings obtained by small-scale fisheries in general should be considered with caution across the Mediterranean, since they are difficult to evaluate and are often underestimated (Lloret et al., 2018; Pere et al., 2019). The Fisheries Department of the Croatian Ministry of Agriculture have been collecting data in a uniform manner since 2008 due to the pre-requisites of entering the EU. Thus, the ten years of data selected for the present study can be considered to have a higher degree of reliability. However, the reliability of catch statistics may be insufficient, since fishery-independent data were not available for validation of the landings and CPUE data with reference to population abundance, as suggested by Salas et al. (2007). As an example of how drastically different official statistics can be from actual ones, shows the study of Kleiven et al. (2012) where total estimated catch of European lobster was 14 times higher than officially reported. Also, CPUE data could be biased due to improvement of fishing technology, a phenomenon known as technological creep (Kleiven et al., 2022). For sure, on board surveys representing fishery-independent data together with scientific surveys will increase the reliability of catch data issue. Fluctuations in lobster abundance may occur as a consequence of the combination of environmental and fishery-related processes (Lizárraga-Cubedo et al., 2015). Since an increase in landings in the northern Adriatic was also associated with an increase in CPUE, this suggests higher abundance and/or increased catchability (Bueno-Pardo et al., 2020).
In this study, a positive and strong correlation was observed between winter (JFM) and spring (AMJ) sea bottom temperatures and CPUE. Similarly, McCleese and Wildner (1958) detected a strong correlation between long-term catch rates of the American lobster and sea surface temperature (SST) at the largest spatial scales, with lags of 0–3 years. More recently, Zhao et al. (2019) also reported that a temperature rise in Gulf of Maine led to increased catchability of American lobster over many years, with an expanded juvenile habitat in the north (Steneck and Wahle, 2013; Tanaka and Chen, 2016). These environmental changes have also been accompanied by the decline of large predators (Le Bris et al., 2018). On the contrary, warming waters have been associated with declined landings related to decreased juvenile habitat availability (Tanaka and Chen, 2015; Wahle et al., 2015) and increased prevalence of epizootic shell disease in the southern Gulf of Maine (Glenn and Pugh, 2006). Although there is no reference about the temperature range limits for European lobster in the published literature, Caputi et al. (2013) and Green et al. (2014) warn that H. gammarus may be sensitive to climate change, as rising temperature is the most important factor driving shifts in its distribution range, with increased abundance found at higher latitudes and decreased abundance at lower latitudes (Le Bris et al., 2018 and references therein). Sea temperature is one of the most important environmental factors affecting the fluctuations of lobster abundance, but how it contributes to total variation in the catch rate, and whether are there any temporal and spatial differences, remains unclear.
In homarid lobsters, sea temperature influences behaviour, which in turn affects their availability to fisheries (Lizárraga-Cubedo et al., 2015). Catchability is related to lobster movement and affected by numerous factors, including feeding behaviour and moulting status (Wahle et al., 2013), both of which are closely related to temperature (Green et al., 2014). Higher water temperatures affect lobster movements and catchability by encouraging increased movement, thereby increasing catchability (Smith et al., 1999; Moland et al., 2011). Rising seawater temperatures at the beginning of fishing season causes higher mobility and lobster feeding activity (Bennett and Lovewell, 1977; Lizárraga-Cubedo et al., 2015). This is followed by reduced mobility attributable to moulting activity (Miller, 1990; Sheehy et al., 1999), and then increased mobility due to higher activity observed during the post-moult period in summer. Moland et al. (2011) indicated limited movement of adult European lobsters, while American lobster exhibits higher movement and migration patterns (Estrella and Morrissey, 1997). Also, Moland et al. (2011) reported that seasonal variation in H. gammarus activity was correlated to water temperature, where lobster activity declined during the winter, with a minimum during February and March, and resumed again in April. This is in line with the winter (JFM) and spring (AMJ) temperatures chosen in this study, preceding and during the fishing season. Temperature is also positively correlated with growth rate among crustaceans, due to the within species thermal tolerance (Hartnoll, 2001). Higher temperature can positively stimulate growth rate by decreasing the time of the intermoult period or by increasing the moult increment (Green et al., 2014). This of course reduces the period in which lobsters are most susceptible to predatory mortality, while also increasing their fitness and the potential to affect growth rate and size at maturity.
Most crustaceans have synchronised spawning and time their reproduction based primarily on temperature (Lawrence and Soame, 2004). Harmonising hatching with food abundance increases the larval survival rate (Cushing, 1972). In this study, the reported positive trends in seawater temperature could possibly be responsible for more successful spawning and increased recruitment in the northern Adriatic, particularly due to the fact that positive trends were observed even before 2008 (Vilibić et al., 2019). The egg-bearing females of H. gammarus spawn in late summer (Tully et al., 2001), and egg hatching occurs in late spring to early summer (Phillips, 2013). The duration of egg development is largely influenced by temperature, with increasing temperature shortening the egg incubation process (Green et al., 2014). In the context of climate change in the North Sea, regimes with elevated temperatures (mild winters) resulted in a strong seasonal forward shift of larval hatching, while experiments showed that larval duration decreased and survival increased significantly at higher temperatures (Schmalenbach and Franke, 2010). The first few weeks post-hatching are characterised by a pelagic phase and the duration of this phase is temperature-dependent and reported to last for 14–35 days (Jørstad et al., 2001; Browne et al., 2009). Although specific observations of benthic post-hatch larvae of European lobsters in the wild are still lacking (Linnane et al., 2001), it is assumed they settle and remain cryptic in shelter-providing rocky substrata and emerge from their shelters only once they reach capapace lengths (CL) between 25 and 40 mm (Linnane et al., 2000a; Linnane et al., 2000b; Ball et al., 2001). Therefore, high temperatures in shallow coastal waters during summer in the Mediterranean could play a major role in juvenile survival and the recruitment success (Pere et al., 2019). Moreover, a recent study in the northern Adriatic reported a high number of pre-adult lobster (Pavičić et al., 2021).
Consequences for Fisheries Management in the Near Future
Regulatory measures of lobster management in Croatia include a minimum landing size, closed season and prohibition of catching berried females (OG, 2016). Currently, the fishing season is open from 5 May until 1 September. Given the possible earlier increased movement due to elevated temperature, a trend of illegal lobster catching was observed in Croatia before the open season, mainly in March and April (pers. comm). Modifications of the existing time frame of the fishing ban should be considered in the future. Also in recent years, V-notching schemes for berried females (Tully, 2001) and protected areas were implemented (Moland et al., 2021) in Europe, and the implementation of these measures should also be considered in Croatia. Recently established MPA network for European lobster in Norway provides good example of management measures (Knutsen et al 2022). After establishment of MPAs protection effects started to manifest, including effects on density, growth, demography, behaviour, and phenotypic diversity. Climate change will surely provoke changes in all fishery sectors, professional and recreational. Artisanal and industrial professional fishers may adapt to these changes mainly through the expansion of fishing grounds following the distribution of target species, which will consequently increase operation costs. The effect on the fishing community is highest when the socio-ecological system is already under pressure, such as with overfishing in the Mediterranean (Miller et al., 2010; Colloca et al., 2011; Pranovi et al., 2013) related to decreased demographic structure, limitations of geographic distribution and diversity loss (Rijnsdorp et al., 2009; Perry et al., 2010; Planque et al., 2010). It is well known that differences in lobster size at maturity, fecundity and population size structure between different areas may occur as a response to the local environmental conditions and fishing strategies (Lizárraga-Cubedo et al., 2015). Since lobster fisheries are most often regulated only through the minimum landing size (MLS) (Pere et al., 2019), fishery managers need to be sure that MLS regulation currently in force corresponds to real size at maturity, throughout the distribution range. Thus, the EU Directive recognises and prescribes different minimum landing size for H. gammarus for northern European countries and the Mediterranean (European Union, 2006). It is possible that this should also be considered at the subregional level, since different genetic populations have been documented in the distribution range (Triantafyllidis et al., 2005; Ellis et al., 2017), while Adriatic populations are panmictic (Pavičić et al., 2020b). It can be expected that if these warming trends continue, the differences in the main biological points between Atlantic and Mediterranean stocks will become even more pronounced. In this study, we confirm that the northern Adriatic as a particularly vulnerable area to climate change. However, it is configured as a cul-de-sac (Ben Rais Lasram et al., 2010; Pranovi et al., 2016), preventing further northward migration of temperate and boreal affinity species like H. gammarus. Considering its shallowness, it is questionable how these species will behave in the future. Further on, recently, the American lobster (Homarus americanus) was reported in Adriatic Sea (Pavičić et al., 2020a) and Aegean Sea (Kampouris et al., 2021). If American lobster establish population in the Mediterranean Sea it can negatively affect European lobster populations, since these two species compete for the same habitat and hybridization is possible (Jørstad et al., 2007). For certain, the observed changes require a deeper and more complex analysis of the lobster stocks and updating of current management measures in the Mediterranean region.
Conclusions
In summary, the present study revealed significant increase in landings and CPUE in the northern Adriatic, particularly after 2013, coinciding with significant rises in sea bottom temperature in both the northern and central Adriatic. We hypothesise that rising sea temperatures have resulted in greater lobster mobility and thus its availability to fishing, which is reflected consequently in higher landings and CPUE. It is still unknown how the increase in temperature will affect early developing stages in the coming years. With this in mind, fishery managers need to be very careful in considering the frequent requests of local fishers to open the fishing season earlier, which would impart an even higher fishing effort and pressure on the reproductive part of the lobster population.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Authors Contributions
MP, SM-S, DV, and AV were involved in data collection. JŠ, IV, and IJ were involved in ROMS model setup and analysis. MP, DV, NS, JŠ, and TŠ-B analyzed the data. MP led the writing of the manuscript with contribution of JŠ and SM-S. All authors have reviewed and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
To all scientists, crew and people engaged in data collection. Also, we are grateful to both Institute of Oceanography and Fisheries and Institute Ruđer Bošković for many years of collecting and maintaining oceanographic data series at stations SJ 107 and Stončica through various projects. This study was supported by the Croatian Science Foundation (project NurseFish, HrZZ Grant IP-2016-06-9884, project ADIOS, HrZZ Grant IP-06-2016-1955, project StVar-Adri, HRZZ Grant IP-2019-04-5875, and project MAUD, HRZZ Grant IP-2018-01-9849).
References
Aiken D. E., Waddy S. L. (1986). Environmental Influence on Recruitment of the American Lobster, Homarus Americanus: A Perspective. Can. J. Fish. Aquat. Sci. 43, 2258–2270. doi: 10.1139/f86-277
Artegiani A., Bregant D., Paschini E., Pinardi N., Raicich F., Russo A. (1997). The Adriatic Sea General Circulation, Part I: Air– Sea Interactions and Water Mass Structure. J. Phys. Oceanogr. 27, 1492–1514. doi: 10.1175/1520-0485(1997)027<1492:TASGCP>2.0.CO;2
Atlantic States Marine Fisheries Commission (2015). American Lobster Benchmark Stock Assessment and Peer Review Reports (Arlington, VA: Atl States Mar Fish Comm).
Ball B., Linnane A., Munday B., Browne R., Mercer J. P. (2001). The Effect of Cover on in Situ Predation in Early Benthic Phase European Lobster Homarus Gammarus. J. Mar. Biol. Assoc. U. K. 81, 639–642. doi: 10.1017/S0025315401004301
Bennet D., Casey J., Dare P., Dawson W., Flatman S., Hulme T., et al. (1993). Identification Biogéographique Des Principaux Stocks Exploités En Manche, Relations Avec Ceux Des Régions Voisines. RI. DRV., 93–028. Available at: https://archimer.ifremer.fr/doc/1993/rapport-719.pdf.
Bennett D. B., Lovewell S. R. J. (1977). The Effects of Pot Immersion Time on Catches of Lobsters Homarus Gammarus (L) in the Welsh Coast Fishery (Lowestoft: Great Britain Ministry of Agriculture, Fisheries and Food).
Ben Rais Lasram F., Guilhaumon F., Albouy C., Somot S., Thuiller W., Mouillot D. (2010). The Mediterranean Sea as a ‘Cul De-Sac’ for Endemic Fishes Facing Climate Change. Glob. Change Biol. 16, 3233–3245. doi: 10.1111/j.1365-2486.2010.02224.x
Boavida-Portugal J., Rosa R., Calado R., Pinto M., Boavida-Portugal I., Araújo M. B., et al. (2018). Climate Change Impacts on the Distribution of Coastal Lobsters. Mar. Biol. 165, 186. doi: 10.1007/s00227-018-3441-9
Boudreau S. A., Anderson S. C., Worm B. (2015). Top-Down and Bottom-Up Forces Interact at Thermal Range Extremes on American Lobster. J. Anim. Ecol. 84, 840–850. doi: 10.1111/1365-2656.12322
Briones-Fourzán P., Lozano-Álvarez E. (2015). Lobsters: Ocean Icons in Changing Times. ICES. J. Mar. Sci. 72, i1–i6. doi: 10.1093/icesjms/fsv111
Browne R., Benavente G. P., Uglem I., Marino Balsa J. C. (2009). An Illustrated Hatchery Guide for the Production of Clawed Lobsters Using a Green Water Technique. Aquacult. Explained. 23, 1–36.
Browne R. M., Mercer J. P., Duncan M. J. (2001). An Historical Overview of the Republic of Ireland's Lobster (Homarus Gammarus Linnaeus) Fishery, With Reference to European and North American (Homarus Americanus Milne Edwards) Lobster Landings. Hydrobiologia 465, 49–62. doi: 10.1023/A:1014517614770
Bueno-Pardo J., Pierce G. J., Cabecinha E., Grilo C., Assis J., Valavanis V., et al. (2020). Trends and Drivers of Marine Fish Landings in Portugal Since its Entrance in the European Union. ICES. J. Mar. Sci. 77, 3, 988–1001. doi: 10.1093/icesjms/fsaa010
Buljan M., Zore-Armanda M. (1976). Oceanographic Properties of the Adriatic Sea. Oceanogr. Mar. Biol. 14, 11–98.
Caputi N., Lestang S., Frusher S., Wahle R. A. (2013). “The Impact of Climate Change on Exploited Lobster Stocks,” in Lobsters: Biology, Management, Aquaculture, and Fisheries. Ed. Phillips B. F (Oxford, UK: Blackwell Publishing Ltd.), pp 84–pp112.
Caputi N., Melville-Smith R., de Lestang S., Pearce A., Feng M. (2010). The Effect of Climate Change on the Western Rock Lobster (Panulirus Cygnus) Fishery of Western Australia. Can. J. Fish. Aquat. Sci. 67, 85–96. doi: 10.1139/F09-167
Cardin V., Wirth A., Khosravi M., Gačić M. (2020). South Adriatic Recipes: Estimating the Vertical Mixing in the Deep Pit. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.565982
Chaikin S., Dubiner S., Belmaker J. (2021). Cold-Water Species Deepen to Escape Warm Water Temperatures. Glob. Ecol. Biogeogr. 31, 75–88. doi: 10.1111/geb.13414
Cobb J. S., Castro K. M. (2006). “Homarus Species,” in Lobsters: Biology, Management, Aquaculture, and Fisheries. Ed. Phillips B. F (Oxford, UK: Blackwell Publishing Ltd.), 310–339.
Cockcroft A. C., van Zyl D., Hutchings L. (2008). Large-Scale Changes in the Spatial Distribution of South African West Coast Rock Lobsters: An Overview. Afr. J. Mar. Sci. 30, 149–159. doi: 10.2989/AJMS.2008.30.1.15.465
Colloca F., Cardinale M., Giannoulaki M., Scarcella G., Jenko K., Fiorentino F., et al. (2011). Rebuilding Mediterranean fisheries: Toward a New Paradigm for Ecological Sustainability in Single Species Population Models. Fish. Fish. 14, 89–109. doi: 10.1111/j.1467-2979.2011.00453.x
Cushing D. H. (1972). The Production Cycle and the Numbers of Marine Fish. Symp. Zool. Soc Lond. 29, 213–232.
Ellis C. D., Hodgson D. J., Daniels C. L., Collins M., Griffiths A. G. F. (2017). Population Genetic Structure in European Lobsters: Implications for Connectivity, Diversity and Hatchery Stocking. Mar. Ecol. Prog. Ser. 563, 123–137. doi: 10.3354/meps11957
Estrella B. T., Morrissey T. D. (1997). Seasonal Movement of Off-Shore American Lobster, Homarus Americanus, Tagged Along the Eastern Shore of Cape Cod, Massachusetts. Fish. Bull. 95, 466–476.
European Union (2006). Council Regulation (EC) No 1967/2006 of 21 December 2006 Concerning Management Measures for the Sustainable Exploitation of Fishery Resources in the Mediterranean Sea, Amending Regulation (EEC) No 2847/93 and Repealing Regulation (EC) No 1626/94 (Brussels, Belgium: European Union).
Fairall C. W., Bradley E. F., Rogers D. P., Edson J. B., Young G. (1996). Bulk Parameterization of Air-Sea Fluxes for Tropical Ocean-Global Atmosphere Coupled-Ocean Atmosphere Response Experiment. J. Geophys. Res. 101 (C2), 3747–3764. doi: 10.1029/95JC03205
FAO (2021)Fishery and Aquaculture Statistics. Global Capture Production 1950-2019 (FishstatJ). In: FAO Fisheries Division (Rome). Available at: www.fao.org/fishery/statistics/software/fishstatj/en (Accessed December 15, 2021).
Glenn R. P., Pugh T. L. (2006). Epizootic Shell Disease in American Lobster (Homarus Americanus) in Massachusetts Coastal Waters: Interactions of Temperature, Maturity, and Intermolt Duration. J. Crustac. Biol. 26, 639–645. doi: 10.1651/S-2754.1
Goñi R., Latrouite D. (2005). Review of the Biology, Ecology and Fisheries of Palinurus Spp. Species of European Waters: Palinurus Elephas (Fabricius 1787) and Palinurus Mauritanicus (Gruvel 1911). Cah. Biol. Mar. 46, 127–142. Available at: https://archimer.ifremer.fr/doc/00000/3625/.
Greenan B. J. W., Shackell N. L., Ferguson K., Greyson P., Cogswell A., Brickman D., et al. (2019). Climate Change Vulnerability of American Lobster Fishing Communities in Atlantic Canada. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00579
Green B. S., Gardner C., Hochmuth J. D., Linnane A. (2014). Environmental Effects on Fished Lobsters and Crabs. Rev. Fish. Biol. Fish. 24, 613–638. doi: 10.1007/s11160-014-9350-1
Hartnoll R. G. (2001). Growth in Crustacea—twenty Years on. Hydrobiologia 49, 111–122. doi: 10.1023/A:1017597104367
Holling C. (2001). Understanding the Complexity of Economic, Ecological, and Social Systems. Ecosystems 4, 390–405. doi: 10.1007/s10021-001-0101-5
Holthius L. B. (1991). FAO Species Catalogue. Marine Lobsters of the World. An Annotated and Illustrated Catalogue of Species of Interest to fisheries Known to Date. FAO Fish. Synop. 125, 13.
Iona A., Theodorou A., Sofianos S., Watelet S., Troupin C., Beckers J. M. (2018). Mediterranean Sea Climatic Indices: Monitoring Long-Term Variability and Climate Changes. Earth. Syst. Sci. Data. 10, 1829–1842. doi: 10.5194/essd-10-1829-2018
Jørstad K. E., Agnalt A. L., Kristiansen T. S., Nøstvold E. (2001). High Survival and Growth of European Lobster Juveniles (Homarus Gammarus) Reared Communally on a Natural-Bottom Substrate. Mar. Freshw. Res. 52, 1431–1438. doi: 10.1071/MF01065
Jørstad K. E., Prodohl P. A., Agnalt A.-L., Hughes M., Farestveit E., Ferguson A. F. (2007). Comparison of Genetic and Morphological Methods to Detect the Presence of American Lobsters, Homarus Americanus H. Milne Edwards 1837 (Astacidea: Nephropidae) in Norwegian Waters. Hydrobiologia 590, 103–114. doi: 10.1007/s10750-007-0762-y
Janeković I., Mihanović H., Vilibić I., Tudor M. (2014). Extreme Cooling and Dense Water Formation Estimates in Open and Coastal Regions of the Adriatic Sea During the Winter of 2012. J. Geophys. Res. 119, 3200–3218. doi: 10.1002/2014JC009865
Kampouris T. E., Gkafas G. A., Sarantopoulou J., Exadactylos A., Batjakas I. E. (2021). An American in the Aegean: First Record of the American Lobster Homarus Americanus H. Milne Edwards 1837from the eastern Mediterranean Sea. BioInvasions. Rec. 10(1), 170–180. doi: 10.3391/bir.2021.10.1.18
Kampouris T. E., Koutsoubas D., Milenkova D., Economidis G., Tamvakidis S., Batjakas I. E. (2020). New Data on the Biology and Fisheries of the Threatened Palinurus Elephas (Fabricius 1787) (Decapoda, Achelata, Palinuridae) From the North-West Aegean Sea, Greece. Water 12, 2390. doi: 10.3390/w12092390
Kleiven A. R., Espeland S. H., Stiansen S., Ono K., Zimmermann F., Moland Olsen E. (2022). Technological Creep Masks Continued Decline in a Lobster (Homarus Gammarus) Fishery Over a Century. Sci. Rep. 12, 3318. doi: 10.1038/s41598-022-07293-2
Kleiven A. R., Olsen E. M., Vølstad J. H. (2012). Total Catch of a Red-Listed Marine Species Is an Order of Magnitude Higher Than Official Data. PloS One 7(2), e31216. doi: 10.1371/journal.pone.0031216
Knight J. R., Folland C. K., Scaife A. A. (2006). Climate Impacts of the Atlantic Multidecadal Oscillation. Geophys. Res. Lett. 33, L17706. doi: 10.1029/2006GL026242
Knutsen J. A., Kleiven A. R., Olsen E. M., Knutsen H., Espeland S. H., Sørdalen T. K., et al. (2022). Lobster Reserves as a Management Tool in Coastal Waters: Two Decades of Experience in Norway. Mar. Policy. 136, 104908. doi: 10.1016/J.MARPOL.2021.104908
Lawrence A. J., Soame J. M. (2004). The Effects of Climate Change on the Reproduction of Coastal Invertebrates. Ibis 146, 29–39. doi: 10.1111/j.1474-919X.2004.00325.x
Le Bris A., Mills K. E., Wahle R. A., Chen Y., Alexander M. A., Allyn A. J., et al. (2018). Climate Vulnerability and Resilience in Fisheries. PNAS. 115(8), 1831–1836. doi: 10.1073/pnas.1711122115
Le Manach F., Dura D., Pere A., Riutort J. J., Lejeune P., Santoni M. C., et al. (2011). “Preliminary Estimate of Total Marine Fisheries Catches in Corsica, France, (1950-2008)”, in Fisheries Catch Reconstructions: Islands, Part Ii. Eds. Harper S., Zeller D.(Univ. British Columbia: Fisheries Centre).
Linnane A., Ball B., Mercer J. P., Browne R., van der Meeren G. I., Ringvold H., et al. (2001). Searching for the Early Benthic Phase (EBP) of the European Lobster: A Trans-European Study of Cobble Fauna. Hydrobiologia 465, 63–72. doi: 10.1023/A:1014547618888
Linnane A., Ball B., Munday B., Mercer J. P. (2000a). On the Occurrence of Juvenile Lobster Homarus Gammarus in Intertidal Habitat. J. Mar. Biol. Assoc. U. K. 80, 375–376. doi: 10.1017/S0025315499002039
Linnane A., Mazzoni D., Mercer J. P. (2000b). A Long-Term Mesocosm Study on the Settlement and Survival of Juvenile European Lobster Homarus Gammarus L. @ in Four Natural Substrata. J. Exp. Mar. Biol. Ecol. 249, 51–64. doi: 10.1016/S0022-0981(00)00190-8
Lipizer M., Partescano E., Rabitti A., Giorgetti A., Crise A. (2014). Qualified Temperature, Salinity and Dissolved Oxygen Climatologies in a Changing Adriatic Sea. Ocean. Sci. 10, 771–797. doi: 10.5194/os-10-771-2014
Lizárraga-Cubedo H. A., Tuck I., Bailey N., Pierce G. J., Zuur A. F., Bova D. (2015). Scottish Lobster fisheries and Environmental Variability. ICES. J. Mar. Sci. 72, i211–i224. doi: 10.1093/icesjms/fsu248
Lloret J., Cowx I. G., Cabral H., Castro M., Font T., Gonçalves J. M. S., et al. (2018). Coastal Fisheries in European Seas are Not What They Were: Ecological, Social and Economic Changes in Small Scale Fisheries. Mar. Policy 98, 176–186. doi: 10.1016/j.marpol.2016.11.007
Lloret J., Riera V. (2008). Evolution of a Mediterranean Coastal Zone: Human Impacts on the Marine Environment of Cape Creus. Environ. Manage. 42, 977–988. doi: 10.1007/s00267-008-9196-1
Marić D., Kraus R., Godrijan J., Supić N., Djakovac T., Precali R. (2012). Phytoplankton Response to Climatic and Anthropogenic Influences in the North-Eastern Adriatic During the Last Four Decades. Estuar. Coast. Shelf. Sci. 115, 98–112. doi: 10.1016/j.ecss.2012.02.003
McCleese D. W., Wildner D. G. (1958). Overview of the Inshore Lobster Resources in the Scotia-Fundy Region (Dartmouth, Nova Scotia: Canadian Atlantic Fisheries Scientific Advisory Committee (CAFSAC) Research Document 85), 20 pp.
Mercer J. P., Bannister R. C. A., van der Meeren G. I., Debuse V., Mazzoni M., Lovewell S., et al. (2001). An Overview of the LEAR (Lobster Ecology and Recruitment) Project: Results of field and Experimental Studies on the Juvenile Ecology of Homarus Gammarus in Cobble. Mar. Freshw. Res. 52, 1291–1301. doi: 10.1071/MF01216
Miller R. J. (1990). Effectiveness of Crab and Lobster Traps. Can. J. Fish. Aquat. Sci. 47, 1228–1251. doi: 10.1139/f90-143
Miller K., Charles A., Barange M., Brander K., Gallucci V. F., Gasalla M. A., et al. (2010). Climate Change, Uncertainty, and Resilient fisheries: Institutional Responses Through Integrative Science. Prog. Oceanogr. 87, 338–346. doi: 10.1016/j.pocean.2010.09.014
Moland E., Fernández-Chacón A., Sørdalen T. K., Villegas-Ríos D., Thorbjørnsen S. H., Halvorsen K. T., et al. (2021). Restoration of Abundance and Dynamics of Coastal Fish and Lobster Within Northern Marine Protected Areas Across Two Decades. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.674756
Moland E., Olsen E. M., Knutsen H., Knutsen J. A., Enersen S. E., Andre C., et al. (2011). Activity Patterns of Wild European Lobster Homarus Gammarus in Coastal Marine Reserves: Implications for Future Reserve Design. Mar. Ecol. Prog. Ser. 429, 197–207. doi: 10.3354/meps09102
Nelson E. J., Kareiva P., Ruckelshaus M., Arkema K., Geller G., Girvetz E., et al. (2013). Climate Change's Impact on Key Ecosystem Services and the Human Well-Being They Support in the US. Front. Ecol. Environ. 11, 483–893. doi: 10.1890/120312
Oddo P., Pinardi N., Zavatarelli M., Coluccelli A. (2006). The Adriatic Basin Forecasting System. Acta Adriat. 47, 169–184.
OG (Official Gazette of the Republic of Croatia) (2011) Pravilnik O Granicama U Ribolovnom Moru Republike Hrvatske. No. 86/5/2011. Available at: https://narodne-novine.nn.hr/eli/sluzbeni/2011/5/86.
OG (Official Gazette of the Republic of Croatia) (2016) Pravilnik O Zaštiti Riba I Drugih Morskih Organizama. No. 1096/42/2016. Available at: https://narodne-novine.nn.hr/eli/sluzbeni/2016/42/1096.
Pastor F., Valiente J. A., Palau J. L. (2018). Sea Surface Temperature in the Mediterranean: Trends and Spatial Patterns, (1982-2016). Pure. Appl. Geophys. 175, 4017–4029. doi: 10.1007/s00024-017-1739-z
Pavičić M., Dragičević B., Žužul I., Vrdoljak D., Matić-Skoko S., Šegvić-Bubić T. (2020a). First Record of American Lobster, Homarus Americanus (H. Milne Edwards 1837), in the Mediterranean Sea. Bioinvasions. Rec. 9, 83–88. doi: 10.3391/bir.2020.9.1.11
Pavičić M., Matić-Skoko S., Vrdoljak D., Vujević A. (2021). Population Characteristics of the European Lobster, Homarus Gammarus in the Adriatic Sea: Implications for Sustainable Fisheries Management. Water 13, 1072. doi: 10.3390/w13081072
Pavičić M., Žužul I., Matić-Skoko S., Triantafyllidis A., Grati F., Durieux E.D. H., et al. (2020b). Population Genetic Structure and Connectivity of the European Lobster Homarus Gammarus in the Adriatic and Mediterranean Seas. Front. Genet. 11. doi: 10.3389/fgene.2020.576023
Pecl G., Frusher S., Gardner C., Haward M., Hobday A., Jennings S., et al. (2009). “The East Coast Tasmanian Rock Lobster Fishery—Vulnerability to Climate Change Impacts and Adaptation Response Options,” in Report to the Department of Climate Change (Australia: Commonwealth of Australia).
Pere A., Marengo M., Lejeune P., Durieux E. D. H. (2019). Evaluation of Homarus Gammarus (Crustacea: Decapoda: Nephropidae) Catches and Potential in a Mediterranean Small-Scale Fishery. Sci. Mar. 83 (1), 69–77. doi: 10.3989/scimar.04862.22B
Perry R. I., Cury P., Brander K., Jenning S., Möllmann C., Planque B. (2010). Sensitivity of Marine Systems to Climate and fishing: Concepts, Issues and Management Responses. J. Mar. Syst. 79, 427–435. doi: 10.1016/j.jmarsys.2008.12.017
Phillips B. F. (2013). Lobsters: Biology, Management, Aquaculture and Fisheries. Ed. Phillips B. F. (Oxford, UK: John Wiley & Sons, Ltd). doi: 10.1002/9781118517444
Planque B., Fromentin J. M., Cury P., Drinkwater J. F., Jennings S., Perry R. I., et al. (2010). How Does fishing Alter Marine Populations and Ecosystems Sensitivity to Climate? J. Mar. Syst. 79, 403–417. doi: 10.1016/j.jmarsys.2008.12.018
Pranovi F., Caccin A., Franzoi P., Malavasi S., Zucchetta M., Torricelli P. (2013). Vulnerability of Artisanal fisheries to Climate Change in the Venice Lagoon. J. Fish. Biol. 83 (4), 847–864. doi: 10.1111/jfb.12124
Pranovi F., Colla S., Valeri P., Anelli M. (2016). Present and Future Status of Artisanal Fisheries in the Adriatic Sea (Western Mediterranean Sea). Ocean. Coast. Manage. 122, 49–56. doi: 10.1016/j.ocecoaman.2016.01.004
Quetglas A., Gaamour A., Reñones O., Missaoui H., Zarrouk T., El Abed S., et al. (2004). Common Spiny Lobster (Palinurus Elephas Fabricius 1787) Fisheries in the Western Mediterranean: A Comparison of Spanish and Tunisian Fisheries. Bolletí. la. Societat. d'Història. Natural les. Balears. 47, 63–80.
Rheuban J. E., Kavanaugh M. T., Doney S. C. (2017). Implications of Future Northwest Atlantic Bottom Temperatures on the American Lobster (Homarus Americanus) Fishery. J. Geophys. Res.: Oceans. 122, 9387–9398. doi: 10.1002/2017JC012949
Rijnsdorp A. D., Peck M. A., Engelhard G. H., Möllmann C., Pinnegar J. K. (2009). Resolving the Effect of Climate Change on fish Populations. ICES. J. Mar. Sci. 66, 1570–1583. doi: 10.1093/icesjms/fsp056
Salas S., Chuenpagdee R., Seijo J. C., Charles A. (2007). Challenges in the Assessment and Management of Small-Scale Fisheries in Latin America and the Caribbean. Fish. Res. 87, 5e16. doi: 10.1016/j.fishres.2007.06.015
Schmalenbach I., Franke H. D. (2010). Potential Impact of Climate Warming on the Recruitment of an Economically and Ecologically Important Species, the European Lobster (Homarus Gammarus) at Helgoland, North Sea. Mar. Biol. 157, 1127–1135. doi: 10.1007/s00227-010-1394-8
Shchepetkin A. F., McWilliams J. C. (2005). The Regional Oceanic Modelling System: A Split-Explicit, Free-Surface, Topography-Following Coordinate Ocean Model. Ocean. Model. 9, 347–404. doi: 10.1016/j.ocemod.2004.08.002
Shchepetkin A. F., McWilliams J. C. (2009). Correction and Commentary for ‘‘Ocean Forecasting in Terrain-Following Coordinates: Formulation and Skill Assessment of the Regional Ocean Modeling System’’ by Haidvogel Et Al., J. Comput. Phys., 227, 3595–3624. J. Comput. Phys. 228, 8985–9000. doi: 10.1016/j.jcp.2009.09.002
Sheehy M. R. J., Bannister R. C. A., Wickins J. F., Shelton P. M. J. (1999). New Perspectives on the Growth and Longevity of the European Lobster (Homarus Gammarus). Can. J. Fish. Aquat. Sci. 56, 1904–1915. doi: 10.1139/f99-116
Smith I. P., Collins K. J., Jensen A. C. (1999). Seasonal Changes in the Level and Diel Pattern of Activity in the European Lobster Homarus Gammarus. Mar. Ecol. Prog. Ser. 186, 255–264. doi: 10.3354/meps186255
Steneck R. S., Wahle R. A. (2013). American Lobster Dynamics in a Brave New Ocean. Can. J. Fish. Aquat. Sci. 70, 1612–1624. doi: 10.1139/cjfas-2013-0094
Tanaka K., Chen Y. (2015). Spatiotemporal Variability of Suitable Habitat for American Lobster (Homarus Americanus) in Long Island Sound. J. Shellfish. Res. 34, 531–543. doi: 10.2983/035.034.0238
Tanaka K., Chen Y. (2016). Modeling Spatiotemporal Variability of the Bioclimate Envelope of Homarus Americanus in the Coastal Waters of Maine and New Hampshire. Fish. Res. 177, 137–152. doi: 10.1016/j.fishres.2016.01.010
Triantafyllidis A., Apostolidis A., Katsares V., Kelly E., Hughes M., Jorstad K., et al. (2005). Mitochondrial DNA Variation in the European Lobster Throughout the Range. Mar. Biol. 146, 223–235. doi: 10.1007/s00227-004-1435-2
Tudor M., Ivatek-Šahdan S., Stanešić A., Horvath K., Bajić A. (2013). “Forecasting Weather in Croatia Using ALADIN Numerical Weather Prediction Model”, in Climate Change and Regional/Local Responses. Eds. Zhang Y., Ray P. (Rijeka, Croatia: InTech), 59–88.
Tully O. (2001). Impact of the V-Notch Technical Conservation Measure on Reproductive Potential in a Lobster (Homarus Gammarus L.) Fishery in Ireland. Mar. Freshw. Res. 52, 1551–1557. doi: 10.1071/MF01046
Tully O., Roantree V., Robinson M. (2001). Maturity, Fecundity and Reproductive Potential of the European Lobster (Homarus Gammarus) in Ireland. J. Mar. Biol. Assoc. U. K. 81, 61–68. doi: 10.1017/S002531540100340X
Vilibić I., Matijević S., Šepić J., Kušpilić G. (2012). Changes in the Adriatic Oceanographic Properties Induced by the Eastern Mediterranean Transient. Biogeosciences 9, 2085–2097. doi: 10.5194/bg-9-2085-2012
Vilibić I., Mihanović H., Janeković I., Šepić J. (2016). Modelling the Formation of Dense Water in the Northern Adriatic: Sensitivity Studies. Ocean. Model. 101, 17–29. doi: 10.1016/j.ocemod.2016.03.001
Vilibić I., Zemunik P., Šepić J., Dunić N., Marzouk O., Mihanović H., et al. (2019). Present-Climate Trends and Variability in Thermohaline Properties of the Northern Adriatic Shelf. Ocean. Sci. 15, 1351–1362. doi: 10.5194/os-15-1351-2019
Wahle R. A., Castro K. M., Tully O., Cobb J. S. (2013). ““Homarus”,” in Lobsters: Biology, Management, Aquaculture and Fisheries, 2nd Ed. Ed. Phillips B. F. (New York: Wiley), 221–258.
Wahle R. A., Dellinger L., Olszewski S., Jekielek P. (2015). American Lobster Nurseries of Southern New England Receding in the Face of Climate Change. ICES. J. Mar. Sci. 72, 69–78. doi: 10.1093/icesjms/fsv093
Keywords: higher CPUE, landings, climate change, Homarus gammarus, Northern Adriatic Sea
Citation: Matić-Skoko S, Pavičić M, Šepić J, Janeković I, Vrdoljak D, Vilibić I, Stagličić N, Šegvić-Bubić T and Vujević A (2022) Impacts of Sea Bottom Temperature on CPUE of European Lobster Homarus gammarus (Linnaeus, 1758; Decapoda, Nephropidae) in the Eastern Adriatic Sea. Front. Mar. Sci. 9:891197. doi: 10.3389/fmars.2022.891197
Received: 07 March 2022; Accepted: 29 March 2022;
Published: 25 April 2022.
Edited by:
Milica Mandic, University of Montenegro, MontenegroReviewed by:
Olivera Marković, University of Montenegro, MontenegroThodoros E. Kampouris, University of the Aegean, Greece
Copyright © 2022 Matić-Skoko, Pavičić, Šepić, Janeković, Vrdoljak, Vilibić, Stagličić, Šegvić-Bubić and Vujević. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mišo Pavičić, cGF2aWNpY0Bpem9yLmhy
 Sanja Matić-Skoko
Sanja Matić-Skoko Mišo Pavičić
Mišo Pavičić Jadranka Šepić2
Jadranka Šepić2 Dario Vrdoljak
Dario Vrdoljak Ivica Vilibić
Ivica Vilibić Tanja Šegvić-Bubić
Tanja Šegvić-Bubić Ante Vujević
Ante Vujević