
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 03 June 2022
Sec. Marine Ecosystem Ecology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.890219
This article is part of the Research TopicMultiple Stressors and Ecological Response in Marine Fishery EcosystemsView all 18 articles
 Ming-Zhi Liu1,2,3,4
Ming-Zhi Liu1,2,3,4 Ri-jin Jiang2,3,4*
Ri-jin Jiang2,3,4* Hui Zhang5,6
Hui Zhang5,6 Fan Yang1,2,3,4
Fan Yang1,2,3,4 Xia-Fang Li1,2,3,4
Xia-Fang Li1,2,3,4 Guang-Peng Feng6
Guang-Peng Feng6 Rui Yin2,3,4
Rui Yin2,3,4 Feng Chen2,3,4
Feng Chen2,3,4Mass stock enhancement and release are excellent ways to recover Coilia nasus resources. However, it is challenging to evaluate stock enhancement effectively, and it is important to establish a method suitable for estimating C. nasus populations. We explored the effectiveness of marking otoliths in these fish with strontium by immersing C. nasus in hexahydrate strontium chloride solutions. We used laser ablation inductively coupled plasma mass spectrometry to measure the strontium content of otoliths and fish bodies. The larvae (40 d post hatch) were reared in five different concentrations of strontium (0, 12, 24, 48, and 60 mg/L) for 7 d, followed by treatment in non-additive water for 3 wk. The results showed that the cumulative mortality rate was not significantly different between treatment and control groups (P>0.05), except in the group treated with 24 mg/L strontium. The swimming and feeding behaviors did not change significantly, indicating that strontium did not negatively affect survival in this species. The strontium/calcium ratios of otoliths in the control group were stable (1.78–2.32 mmol/mol), whereas those of the experimental (marked) groups ranged widely (4.47–61.02 mmol/mol). The strontium/calcium ratios increased with increasing strontium concentration, but gradually returned to baseline values, resulting in a 100% success rate of marking with strontium. Following immersion in 12 mg/L strontium, strontium levels in the body returned to normal after 24 d. In summary, a treatment of 12 mg/L strontium for 4 d was identified as viable for marking. We confirmed the feasibility of strontium marking for the mass marking and release of C. nasus. This marking method does not affect the physiology of the fish and may provide a new approach for reasonable and scientific stock assessment of C. nasus post hatch.
Coilia nasus is an estuarine migratory fish belonging to the family Engraulidae of the order Clupeiformes. Previously, it was one of the most economically important fish species in China and was widely distributed in the East China Sea. However, the species is now threatened by overexploitation and loss of habitat that has devastated its population. Accordingly, catch sizes have decreased substantially, and it is difficult to establish a fishing season (Figure 1). Moreover, the majority of fish are caught at a younger age and reach sexual maturity earlier than they did before the 1970s (Zhang et al., 2005).
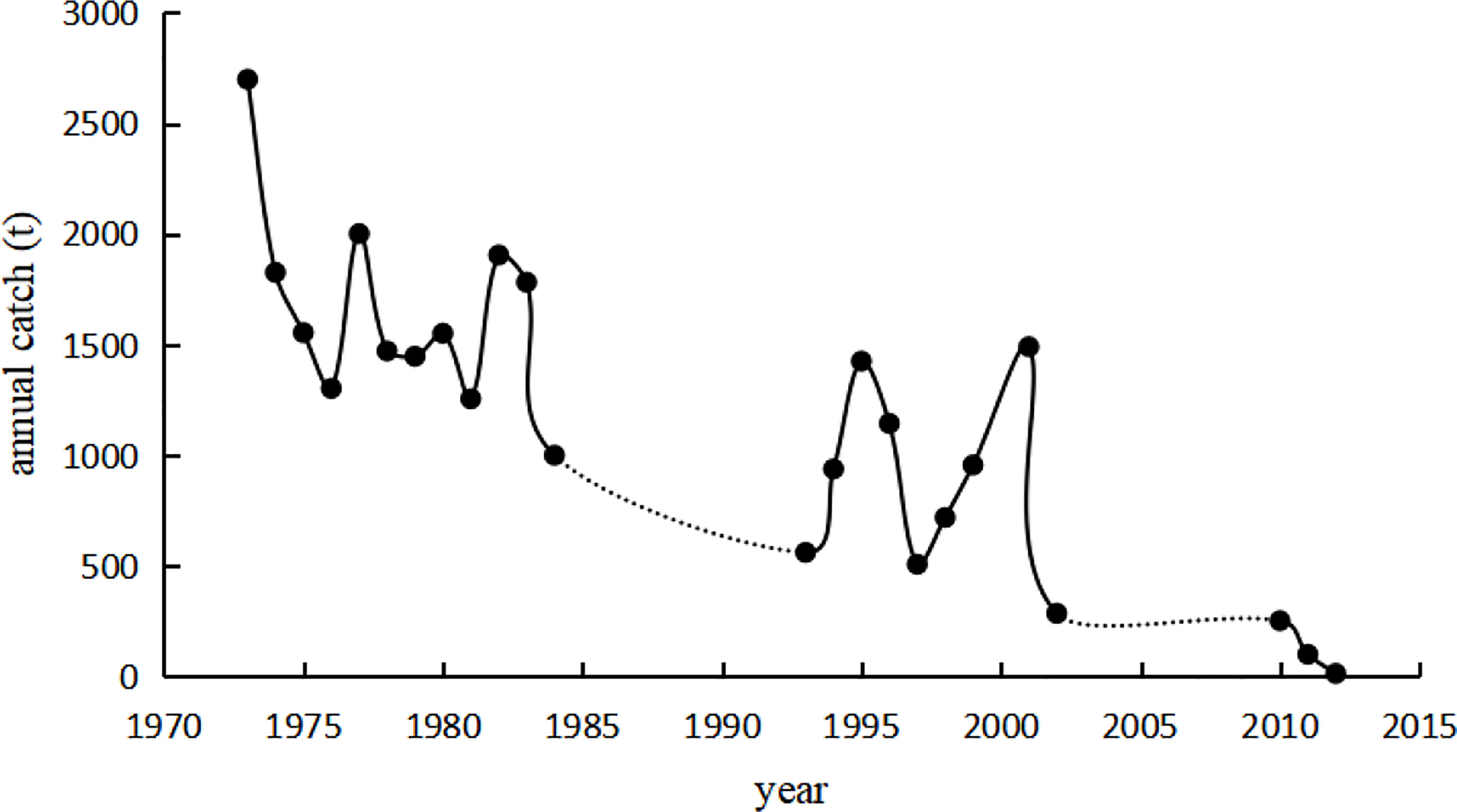
Figure 1 Annual catch of Coilia nasus in the lower reaches of the Yangtze River. The data for 1973–1984, 1993–2002, and 2010–2012 are from Yuan (1988); Zhang et al. (2005), and Liu et al. (2012), respectively. The dashed lines indicate where data was not available.
Enhancement and release initiatives are an effective way of restoring fishery resources (He et al., 2011; Yan et al., 2015), but evaluating the results is essential for protecting and using fishery resources. At present, there are several practical methods for assessing stock conditions, including external (fin-clipping and cold-branding) and internal (fluorescence marking) marking techniques. However, these may cause issues such as high mortality and difficult recognition (Nielsen, 1992; Yang et al., 2013). Therefore, there is an urgent need to explore a method of stock assessment that is suitable for releasing C. nasus.
Some studies have reported that the strontium content of otoliths is associated with seawater strontium content, and strontium marking technology has been applied in several released species (Pollard et al., 1999; Schroder et al., 2001). For example, strontium has been used successfully to mark Aristichthys nobilis, Cyprinus carpio, and Sparus macrocephalus (Li et al., 2017; Zhang et al., 2018; Qiu et al., 2019b). Researchers have reported the steady deposition of a given concentration of strontium in otoliths through a complex metabolic process with demonstrable reliability. Moreover, deposition is not assimilated by the body. However, to our knowledge, no studies have reported the application of this marking technique in Clupeiformes. Therefore, we investigated the optimal strontium concentration and treatment duration for marking in C. nasus. In this study, we evaluated a marking technique and providing a theoretical basis for its application in enhancement and release practices.
Larvae were cultivated by the Fisheries Research Institute, Shanghai, China. We directly collected the samples at 40 d post hatch. Hexahydrate strontium chloride was obtained from China, Sinopharm Chemical Reagent Co., Ltd (batch number is 20210114). The reagent was dissolved in fresh water. Four strontium concentrations (12, 24, 48, and 60 mg/L) were tested according to the strontium content of seawater. A total of 15 containers (300 L) were installed with 150 samples per container. There were three containers for each experimental group, and the remaining containers were regarded as the control group. Fresh water for cultures was obtained from the institute. All containers were stored at 27.5–28°C and the water salinity was maintained at 1.3‰–1.4‰. Blackout fabric was installed to prevent exposure to sun and light, as these impact water temperature and larval survival. The tanks were cleaned, and half of the water was changed daily.
The experiment was divided into three phases. In the first phase, all samples were kept for 1 d in containers equipped with an oxygenation device composed of an aerator and an air-transporting soft pipe. None of the groups were fed during this period. In the second phase, cultivation with added strontium lasted for 7 d. The fish larvae were fed Cladoceran prey twice per day. In the third phase, the immersed groups were transferred to water without added strontium for 3 wk and cultured as above. The activity and number of larvae were monitored daily.
To evaluate the effect of strontium deposition in fish, 30 samples were collected from each concentration group every 3 d during the second phase, and 30 samples were collected from the experimental (12 mg/L) group every 7 d during the third phase. After the marking process, specimens from experimental and control groups were collected for the analysis of body length (accurate to 0.01 mm) and weight (accurate to 0.001 g) (Table 1). Body length was defined as the distance from the rostral side to the end of the tail fin. An electronic balance was used for weighing (Mettler Toledo PL403). Before weighing, blotting paper was used to remove water from the fish bodies to minimize error.
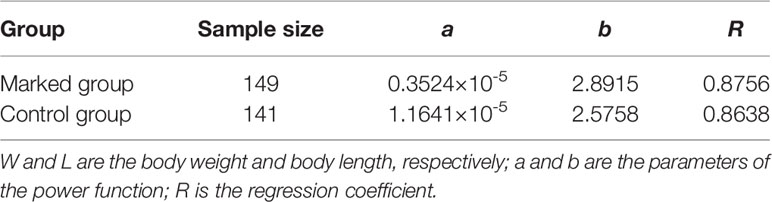
Table 1 Parameter values of the equation (W = aLb) fitted to body length and body weight in Coilia nasus larvae in the marked and control groups.
We attempted to collect three pairs of otoliths (sagittaes, asteriscus, and lapillus) for the analyses; however, only sagittaes could be used as experimental materials because of their size. Laser ablation- inductively coupled plasma-mass spectrometry (LA-ICP-MS) (Thermo X Series II, Thermo Fisher Scientific, Bremen, Germany) was used to measure the strontium and calcium concentrations in the otoliths and fish bodies (Zhang et al., 2013; Zhang et al., 2019). The LA system was equipped with an excimer laser operating at 213 nm. The otoliths were analyzed using a spot size of 32 μm and a laser repetition rate of 2 μm/s. The measurements were conducted under conditions of pure helium (He) to minimize the re-deposition of ablated material, and the samples were entrained into the argon (Ar) carrier gas flow to the ICP-MS. We used Plasmalab (Thermo Fisher Scientific, Bremen, Germany) to complete sensor calibration to achieve the best performance indicators. Based on Warren-Myers et al. (2014), making success was determined with 100% accuracy when the mean in the marking area was 3.3 times that of the control group.
The power function was tested to examine the growth pattern of C. nasus during its early life stages as follows (Zhan, 1995):
where W (g) and L (mm) are the body weight and body length, respectively; and a and b are the parameters of the power function. Statistical analysis was performed using Excel 2019 (Microsoft Corp., Redmond, WA, USA) and IBM SPSS Statistics v.24.0 (IBM Corp., Armonk, NY, USA).
Mortality occurred in each group throughout the experiment but was relatively low overall (Figure 2). During the second phase, mortality was the highest at a strontium concentration of 24 mg/L. A paired sample t-test showed a significant difference between the control and 24 mg/L groups (P < 0.05); however, no significant differences were observed between the other experimental and control groups groups (P > 0.05). Mortality was not significantly different between groups in the third phase (P > 0.05). Additionally, swimming and feeding behaviors were not observed to be significantly different between groups, indicating that the treatment was safe. The mortality rate was the highest when the concentration was 24 mg/L; however, this may have been due to improper suction of sewage. The cumulative mortality increased with time, probably due to increasing ammonia and nitrogen and human operational mistakes.
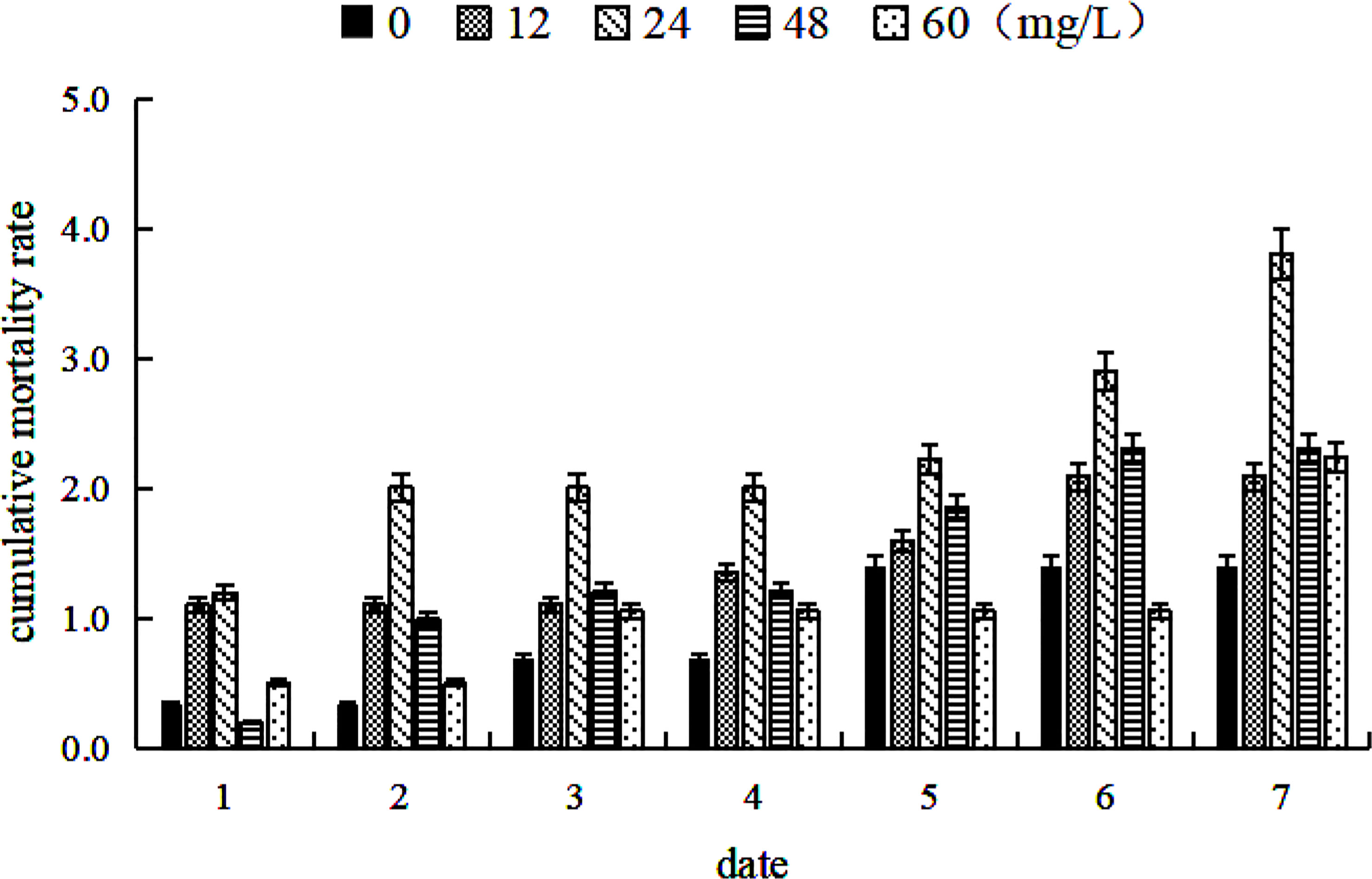
Figure 2 Cumulative mortality of Coilia nasus larvae in the second phase (cultivation with added strontium for 7 d) with different strontium concentrations. Cumulative mortality refers to the sample death as a percentage of total sample size.
To analyze the growth patterns of fish cultivated in different strontium concentrations, we used the body length and weight of 7 d-immersed larvae (Figure 3). The body length and weight were 43.19 ± 6.89 mm and 0.02 ± 0.08 g in the marked groups and 39.46 ± 9.39 mm and 0.17 ± 0.09 g in the control groups, respectively.
The parameters of power equation were estimated using the linear least square method (Table 1). The b parameters of the fitted function in the marked and control groups were below 3. This indicates that populations showed allometric growth. One-way analysis of variance (ANOVA) and Duncan’s test indicated no differences in the growth parameters among groups (P > 0.05), indicating that strontium had little effect on the growth of fish. These findings suggested that the results of strontium marking in C. nasus were reliable.
Strontium-to-calcium (Sr/Ca) ratios were used for quantitative analysis of the marking process. The ratio in otoliths in the control group was stable (1.78–2.32 mmol/mol), and the range did not fluctuate significantly during the experiment. However, the Sr/Ca ratios varied widely in the experimental groups (4.47–61.02 mmol/mol; Table 2) and were 3.3 times the value in the control group.The Sr/Ca ratios from the core to the edge of the otoliths (0.37–0.57 mm) increased after 1 d after marking and stabilized after 3 d. The distances varied between samples because of the difference in the sizes of otoliths (Figure 4). In areas with significant differences in Sr/Ca ratios, the ratios increased with increased strontium concentration. There were significant differences in Sr/Ca ratios between experimental and control groups, as indicated by paired sample t-tests (P < 0.01).
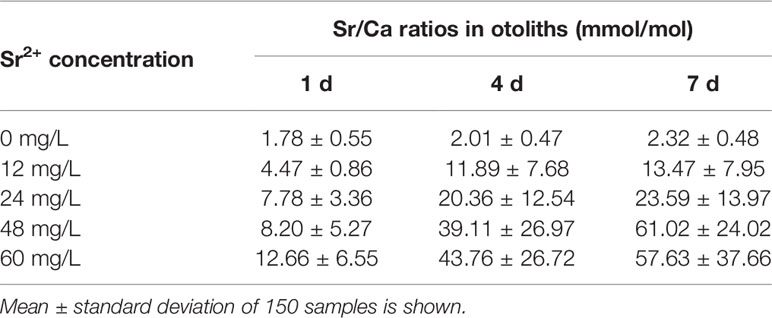
Table 2 Sr/Ca ratios in the strontium-marked otoliths of Coilia nasus larvae in different treatment groups.
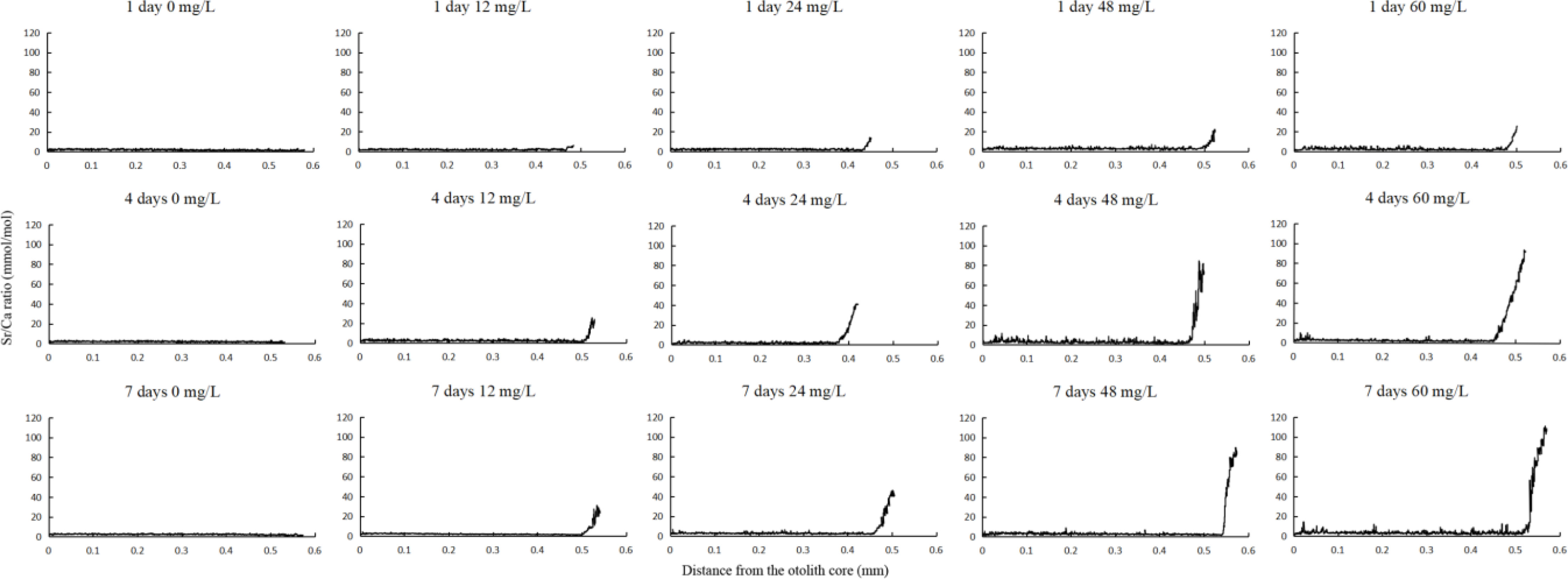
Figure 4 Fluctuations in strontium-to-calcium (Sr/Ca) ratios along a line transect from the core (0 mm) to the edge of otoliths in Coilia nasus larvae cultivated at different strontium concentrations (0, 12, 24, 48, and 60 mg/L) for 1, 4, and 7 d in the second phase (cultivation with added strontium for 7 d).
During the third phase, the Sr/Ca ratio in the experimental groups reflected crests with a width of approximately 0.11 mm (0.42–0.54 mm from the edge of otoliths). For the groups cultivated in 12 mg/L strontium for 14, 21, and 28 d, the Sr/Ca ratios were 10.87 ± 5.65, 11.86 ± 6.15, and 12.32 ± 5.44 (mmol/mol), respectively. Subsequently, the ratios returned to baseline levels. The marking rate was significantly higher than the of non-marking rate (Figure 5), indicating a 100% success rate of marking by exogenous strontium treatment.
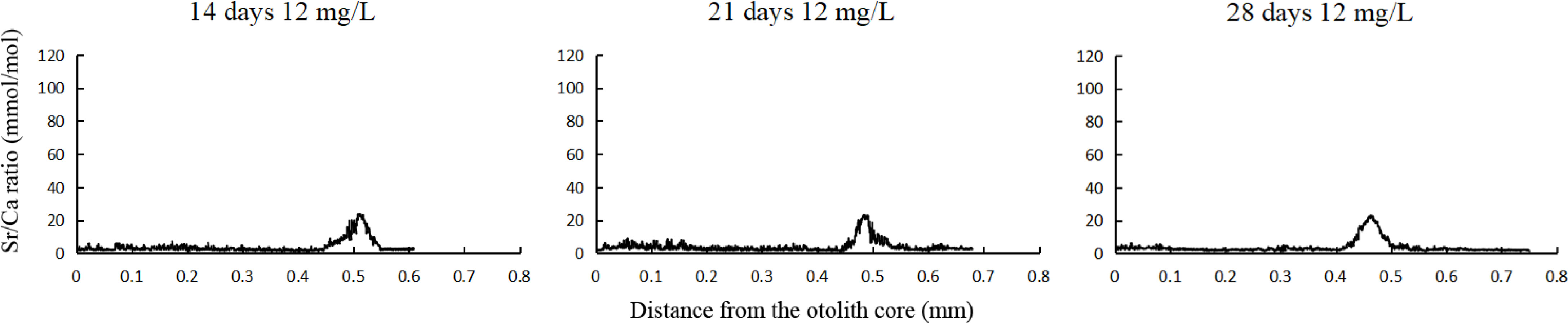
Figure 5 Fluctuations in Sr/Ca ratios along a line transect from the core (0 mm) to the edge of otoliths in Coilia nasus larvae cultivated under different immersion durations (14, 21, and 28 d) at a 12 mg/L concentration of strontium in the third phase (groups were transferred to water without added strontium for 3 wk).
The otolith samples were below the minimum weight required for detection with LA-ICP-MS; therefore, we used the whole body as a testing material. Paired sample t-tests revealed no significant differences in strontium content in phase one (P > 0.05). However, strontium levels increased significantly in the second phase (P < 0.05), and then decreased gradually until the end of the treatment period (Figure 6). The strontium content in the marking group was approximately twice that of the control group. From 7–28 d, the variation in strontium content fit the following formula:
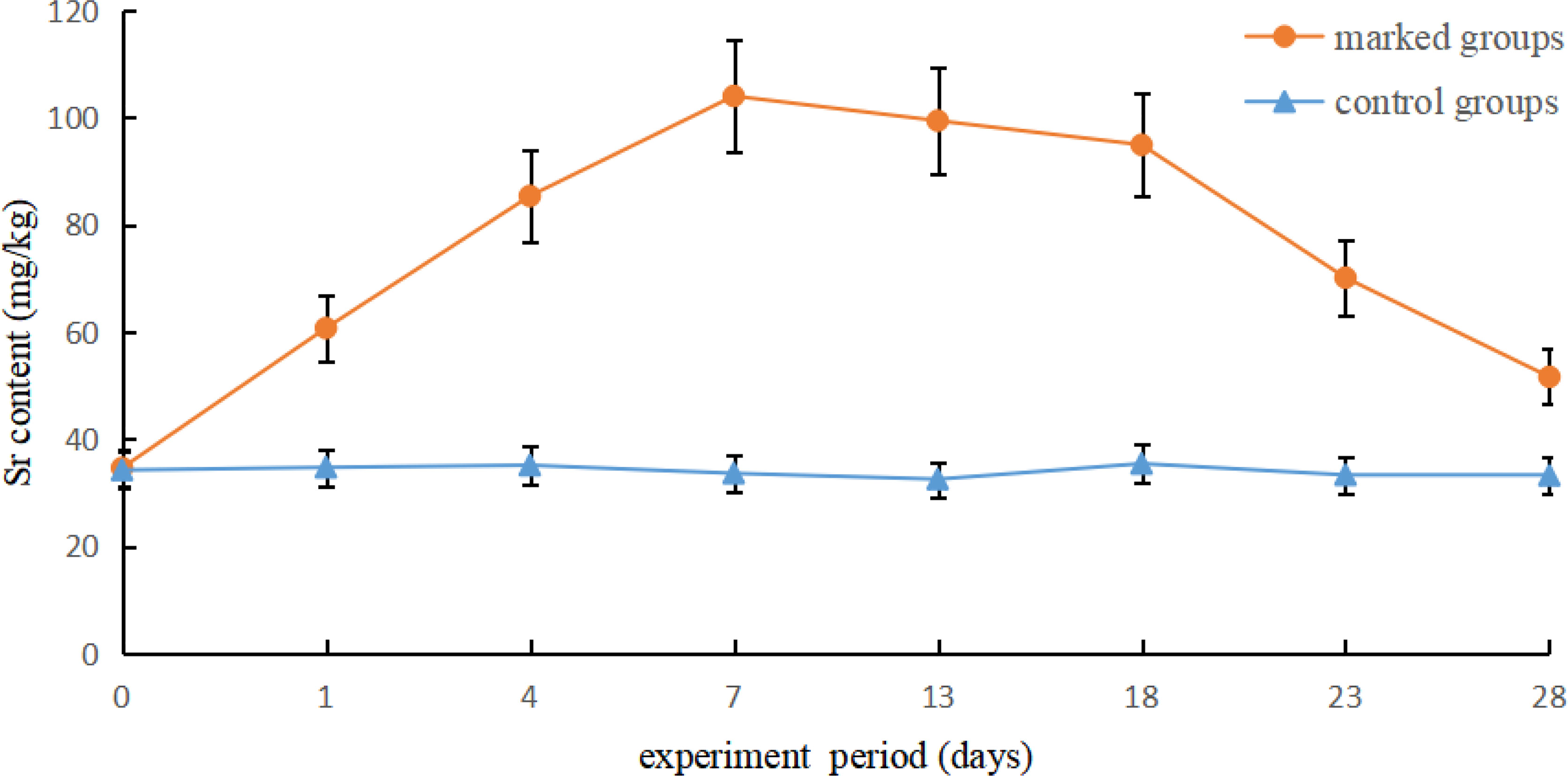
Figure 6 Variation in Sr concentrations between the marked and control groups of Coilia nasus larvae.
where x represents the experimental period and y axis represents the Strontium levels in the body returned to baseline levels similar to that of the control group at approximately 31 d.
Strontium commonly exists in natural water. A literature survey reported that average Sr/Ca ratios (molar ratio) are 2.7 ± 1.5 in freshwater, 5.6 ± 1.1 in estuarine water, and 8.3 ± 4.5 in seawater (Yang et al., 2011). The Environmental Protection Agency (USA) stipulates that the level of strontium ions in standard public drinking water should have an upper limit of 4 mg/L (ATSDR, 2004); in China, the limit is 0.2–5 mg/L. As a necessary trace element, strontium plays an important role in the growth and development of organisms (Buehler et al., 2001; Dahl et al., 2001) by promoting the growth and formation of bone cells and enhancing bone strength (Caverzasio, 2008). Strontium strongly inhibits calcium absorption in the early life stages of fish (Chowdhury and Blust, 2002), and high levels of strontium can cause larval and juvenile mortality in rainbow trout (Birge et al., 1981). Pyle et al. (2002) found that Sr2+ ions in the fresh water did not cause substantial mortality in larvae but caused a decline in egg hatching rates. A study on chum salmon showed that Ca2+-ATPase activity and growth indices were the highest at a strontium mass concentration of 10 mg/L, and that mortality increased with an increase in Sr2+ concentration (Song, 2013). At a strontium mass concentration of 50 mg/L, sedimentation values of an experimental group were 50 times that of the control group (accompanied by a strong marking effect), although the growth and feeding statuses remained unchanged (Wang et al., 2015). These findings indicate that an appropriate concentration of Sr2+ can promote the development of fish, whereas outlier values are not conducive to growth. Thus, when exogenous strontium exceeds optimal levels, it affects the enrichment of otolith elements. Different populations exhibit differing ranges of adaptation to strontium. Strontium marks in otoliths were confirmed to exist for at least 20 mo in C. carpio (Qiu et al., 2021). In the current study, we evaluated the effects of immersion in concentrations of 0, 12, 24, 48, and 60 mg/L of strontium, nearly 2–10 times the levels found in natural seawater. These concentrations were used because it is usually difficult to distinguish between conditions at release and in the wild. However, the concentrations had no negative effect on the development of fish, indicating that they would not cause a toxic response and were safe and reliable.
To recover from a decline in fishery resources, it is important to develop strategies for stock enhancement and release. Accurately distinguishing between release and catches and evaluating the associated responses have become urgent scientific problems. To this end, several methods have been employed, including marking techniques such as fin-clipping and scutcheon-tagging (Pan et al., 2010). However, these traditional methods often involve a heavy workload, and the markers may fall off or make identification difficult; therefore, these methods are unsuitable for large-scale use.
Coilia nasus exhibit high sensitivity and die immediately in the absence of water (Wang, 2015), because of which some common marking methods are inapplicable in this species. Otoliths are used as a natural indicator of the ontogeny of fish, and strontium is a biomarker used to track and reconstruct habitat events (Melancon et al., 2009). Combining these factors provides a new approach for mark–release technologies. Owing to anthropogenic factors that increase the strontium content in water bodies, strontium is expected to be deposited in otoliths. In this study, most of the Sr/Ca ratios measured in the experimental group were 3.3 times the values in the control group. The results of otolith marking with strontium showed that specific peaks were formed under different strontium concentration and immersion duration conditions, with a 100% success rate. A slight curve was detected in Sr/Ca ratios when the concentration was 24 mg/L or after immersion for 1 d. The Sr/Ca ratios were higher on day four than on day one and higher on day seven than on day four. This is because the marking process operated at near capacity for the first 4 d. The fitted function indicates that 4 d is an appropriate duration for marking. Of the various concentrations at which the Sr/Ca ratio could be measured, 12 mg/L strontium was chosen based on economic feasibility. Strontium is a long-lasting trace element that is not lost over time (even during the third phase of this experiment). Accordingly, its persistence is positively correlated with immersion time and concentration. Remarkably, there was no time lag for marking in C. nasus, contrary to the results of Qiu et al. (2019a), which can be explained by the different detection principles of the two instruments. However, further studies are needed to test whether its deposition has any lingering effects.
Experimental samples are occasionally caught by fishermen after the large-scale release of marked individuals. Although strontium has low toxicity (Qin and Pan, 2001), it may still cause food safety issues. If the strontium content in the fish body is not fully absorbed and metabolized over time, excessive consumption of strontium may have adverse effects on the physical health of consumers, including nausea, digestive upsets, and other phenomena (Wu, 2012). Therefore, we analyzed the metabolic rate of strontium to avert potential side-effects of marking practices. The levels of Sr2+ increased considerably after immersion in 12 mg/L strontium for 7 d, and decreased gradually thereafter (Figure 5). Statistical analysis revealed that the strontium levels of marked groups decreased to baseline levels after 24 d. Therefore, we recommend that after large-scale marking, fish should be cultured in natural water to ensure the removal of excess strontium and avoid its side effects.
The paper reported that strontium has little impact on Coilia nasus larvae in different marked groups, and strontium can deposit on otoliths stably. Moreover, the level of strontium also toward lower with body’s metabolism. Strontium can be used as a natural biomarker to evaluate the effect of stock enhancement. According to the strontium marking of otoliths in the early stages, the released populations can be identified through mark-recapture, and the length of released fish can be determined according to the growth equation. The ratio of strontium and calcium can be used to distinguish wild fish from the released population. The effect of the release can be analyzed according to the recapture rate; for rapidly declining fish species, this method can be used to artificially increase stock.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Zhejiang Marine Fisheries Research Institute.
M-ZL wrote the paper; HZ performed the experiment; FY helped with the analysis and discussions; X-FL assisted in the experiment; G-PF coordinated the sampling; RY adjusted and tested equipment; FC offered technical support; R-jJ contributed to the conception of the study. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the Chinese Ministry of Science and Technology through the National Key Research and Development Program of China (2020YFD0900805), and the Public Welfare Technology Application Research Project of Zhejiang Province (LGN20C190012).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank HZ and the technical staff of the East China Sea Fisheries Research Institute of the Chinese Academy of Fishery Sciences, who assisted with LA-ICP-MS analyses.
Agency for Toxic Substances and Disease Registry (ATSDR) (2004) Toxicological Profile for Strontium (Atlanta: US Department of Health and Human Services, Public Health Service). Available at: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=656&tid=120 (Accessed May 4, 2022).
Birge W. J., Black J. A., Ramey B. A. (1981). “The Reproductive Toxicology of Aquatic Contaminants,” in Hazard Assessment of Chemicals: Current Developments. Eds. Saxena J., Fisher F. (New York: Academic Press), 59–115.
Buehler J., Chappuis P., Saffar J. L., Tsouderos Y., Vignery A. (2001). Strontium Ranelate Inhibits Bone Resorption While Maintaining Bone Formation in Alveolar Bone in Monkeys (Macaca Fascicularis). Bone. 29, 176–179. doi: 10.1016/s8756-3282(01)00484–7
Caverzasio J. (2008). Strontium Ranelate Promotes Osteoblastic Cell Replication Through at Least Two Different Mechanisms. Bone. 42, 1131–1136. doi: 10.1016/j.bone.2008.02.010
Chowdhury M. J., Blust R. (2002). Bioavailability of Waterborne Strontium to the Common Carp, Cyprinus Carpio, in Complexing Environments. Aquat. Toxicol. 58, 215–227. doi: 10.1016/S0166–445X(01)00230–2
Dahl S. G., Allain P., Marie P. J., Mauras Y., Boivin G., Ammann P. (2001). Incorporation and Distribution of Strontium in Bone. Bone. 28, 446–453. doi: 10.1016/s8756–3282(01)00419–7
He J., Gu X. H., Zhang X. Z. (2011). Study on Structural Characteristics and Driving Mechanism of Natural Fishery in Taihu Lake. J. Jiangsu. Agric. Sci. 39, 287–291. doi: 10.3969/j.issn.1002-1302.2011.03.116
Li X. Q., Cong X. R., Shi J. H. (2017). Feasibility Analysis of Releasing Individuals of Aristichthys Nobilis Identification Based on Otolith Sr Markers. J. Lake. Sci. 29, 914–922. doi: 10.18307/2017.0415
Liu K., Duan J. R., Zhang M. Y., Fang D. A., Shi D. A., Shi W. G. (2012). Present Situation of Coilia Nasus Population Features and Yield in Yangtze River Estuary Waters in Fishing Season. Chin. J. Ecol. 12, 3138–3143. doi: 10.13292/j.1000-4890.2012.0407
Melancon S., Fryer B. J., Markham J. L. (2009). Chemical Analysis of Endolymph and the Growing Otolith: Fractionation of Metals in Freshwater Fish Species. Environ. Toxicol. Chem. 28, 1279–1287. doi: 10.1897/08–358.1
Nielsen L. A. (1992). Methods of Marking Fish and Shellfish (New York: American Fisheries Society Special Publication).
Pan X. W., Yang L. L., Ji L. L., Liu Z. L. (2010). Research Progress on Technique of Fish Stock Enhancement. Jiangsu. Agric. Sci. 4, 263–240. doi: 10.15889/j.issn.1002-1302.2010.04.151
Pollard M. J., Kingsford M. J., Battaglene S. C. (1999). Chemical Marking of Juvenile Snapper, Pagrus Auratus (Sparidae), by Incorporation of Strontium Into Dorsal Spines. Fish. Bull. 97, 118–131.
Pyle G. G., Swanson S. M., Lehmkuhl D. M. (2002). Toxicity of Uranium Mine Receiving Waters to Early Life Stage Fathead Minnows (Pimephales Promelas) in the Laboratory. Environ. Pollut. 116, 243–255. doi: 10.1016/s0269–7491(01)00130–0
Qin J. F., Pan W. Q. (2001). Limited Amount Index of Sr in Drinking Nature Mineral Water. Guangdong Trace Elem. Science 1, 16–23. doi: 10.16755/j.cnki.issn.1006-446x.2001.01.003
Qiu C., Jiang T., Chen X. B., Liu H. B., Yang J. (2019a). Characteristics of Otolith Strontium Marking and its Time Lags of Larval Cyprinus Carpio. Oceanologia. Limnol. Sin. 50, 903–912.
Qiu C., Jiang T., Chen X. B., Liu H. B., Yang J. (2019b). Effectiveness of Otolith Strontium Marking for Juvenile Cyprinus Carpio. Chin. J. Appl. Ecol. 30, 2093–2100. doi: 10.13287/j.1001–9332.201906.027
Qiu C., Jiang T., Chen X. B., Liu H. B., Yang J. (2021). Persistence Evaluation of Strontium Marking on Otoliths in Larvae Common Carp (Cyprinus Carpio). Hubei. Agric. Sci. 60, 114–117. doi: 10.14088/j.cnki.issn0439-8114.2021.08.023
Schroder S. L., Volk E. C., Hagen P. (2001). Marking Salmonids With Strontium Chloride at Various Life History Stages. NPAFC. Tech. Rep. 3, 9–10.
Song H. J. (2013). Allometric Growth of Chum Salmon Larvae and Effects of Strontium on Physiological Indices of Their Juveniles (Harbin, IL: Northeast Agricultural University).
Wang Y. (2015). Effects of Acute Handling Stress on Coilia Nasus. [Master’s Thesis] Hainan (IL: University of Hainan). doi: 10.13287/j.1001-9332.20150921.033
Wang C., Liu W., Zhan P. R., Wang J. L., Li P. L. (2015). Exogenous Sr2+ Sedimentation on Otolith of Chum Salmon Embryos. Chin. J. Appl. Ecol. 26, 3189–3194.
Warren–Myers F., Dempster T., Fjelldal P. G., Hansen T., Jensen A. J., Swearer S. E. (2014). Stable Isotope Marking of Otoliths During Vaccination: A Novel Method for Mass-Marking Fish. Aquacult. Environ. Interact. 5, 143–154. doi: 10.3354/aei00103
Wu M. J. (2012). The Relationship Between Strontium and Human Health. Stud. Trace Elem. Health 29, 66–67. doi: 10.3969/j.issn.1005-5320.2007.01.030
Yang J., Jiang T., Liu H. (2011). Are There Habitat Salinity Markers of the Sr: Ca Ratio in the Otolith of Wild Diadromous Fishes? A Literature Survey. Ichthyol. Res. 58, 291–294. doi: 10.1007/s10228–011–0220–8
Yang X. J., Pan X. F., Chen X. Y., Wang X. A., Zhao Y. P., Li J. Y. (2013). Overview of the Artificial Enhancement and Release of Endemic Freshwater Fish in China. Zool. Res. 34, 267–280. doi: 10.11813/j.issn.0254–5853.2013.4.0267
Yan C. M., Zheng W., Zhang Y. B. (2015). Study on Germplasm Identification and Resource Restoration of Cyprinus Carpio Linnaeus. Fish. Sci. Technol. Inf. 42, 30–35. doi: 10.16446/j.cnki.1001-1994.2015.01.007
Yuan C. M. (1988). Changes of Resources and Population Composition of Coilia Nasus in the Middle and Lower Reaches of the Yangtze River and its Causes. J. Zool. 03, 12–15. doi: 10.13859/j.cjz.1988.03.005
Zhang Y., Jiang Y. Z., Xu K. D., Jiang H. L., Jiao H. F., Zhang H., et al. (2018). Evaluation on Effectiveness of Marking Juvenile Sparus Macrocephalus Otoliths With Strontium. Mar. Fish. 40, 171–178. doi: 10.3969/j.issn.1004-2490.2018.02.006
Zhang H., Jiang Y. Z., Yuan X. W., Zhang Y., Jiang H. L., Jiao H. F., et al. (2019). Effects of Strontium-Enriched Water on Strontium Content in the Otolith and Body of Hatchery–Reared Larimichthys Crocea. Mar. Fish. 41, 338–345. doi: 10.13233/j.cnki.mar.fish.2019.03.009
Zhang Y., Li Y. X., Xu X. M., Huang J. H., Gao Y. S., Xu H., et al. (2013). Effects of Environmental Factors on Otolith Sr Incorporation in Juvenile Larimichthys Crocea. Mar. Fish. 35, 278–288. doi: 10.3969/j.issn.1004-2490.2013.03.004
Keywords: Coilia nasus, otolith, strontium marking, enhancement and release, stock assessment
Citation: Liu M-Z, Jiang R-j, Zhang H, Yang F, Li X-F, Feng G-P, Yin R and Chen F (2022) Otolith Marking With Strontium for Stock Assessment in Coilia nasus. Front. Mar. Sci. 9:890219. doi: 10.3389/fmars.2022.890219
Received: 05 March 2022; Accepted: 06 May 2022;
Published: 03 June 2022.
Edited by:
Jun Xu, Institute of Hydrobiology (CAS), ChinaReviewed by:
Xiujuan Shan, Chinese Academy of Fishery Sciences (CAFS), ChinaCopyright © 2022 Liu, Jiang, Zhang, Yang, Li, Feng, Yin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ri-jin Jiang, amlhbmdyaWRnZUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.