- School of Marine and Atmospheric Sciences, Stony Brook University, Stony Brook, NY, United States
The adhesion between food particles and mucus is a fundamental process in particle sorting in suspension-feeding bivalves that requires specific recognition. Interactions between carbohydrate-binding proteins (lectins) expressed on the feeding organs and carbohydrates present on microbial cell surface can provide this specificity. Microalga cell surface carbohydrates (MCSC) represent unique patterns that can be considered as species-specific fingerprints. In this study, sorting efficiencies in blue mussels Mytilus edulis fed with microalgae having modified MCSC and engineered microspheres coated with target carbohydrates was measured. The nature and quantities of surface carbohydrates required to trigger sorting in mussels was evaluated and the relationship between ligand quantities and sorting efficiency (SE) was determined. Mussels fed with Chlamydomonas which MCSC were blocked with ConA or PEA lectins (affinity to mannose and glucose) led to a significant decrease of the sorting efficiencies, not observed when the lectin UEA (affinity to fucose) was used. The ability of commercial lectins to inhibit sorting was not linear and a threshold was noted between 30 and 45 ug lectins per million algae cells. Further, mussels were fed with microspheres coated with neoglycoproteins. Results showed that glucose-BSA, but not fucose-BSA, has an effect on particle sorting in mussels, and 1.08 x 109 molecules of glucose per microspheres, corresponding to a density of 6.99 x 106 molecules of glucose per µm2, triggers particle selection. These findings support that selection of food particles by mussels rely on the strength of the bond between suspended particle and the mucosal layer that mediate sorting, and that these bonds depend on the quantity of compatible ligands on each particle.
Introduction
The elementary functions of carbohydrates are to provide energy to the body and to offer structural support to cell walls. Beyond these basic functions, carbohydrates are also recognized as key elements in various molecular recognition and interaction processes (Varki, 1993; Sears and Wong, 1996; Wang and Boons, 2011; Varki, 2017). In fact, almost all cell surface molecules and secreted proteins undergo glycosylation resulting in their modification by covalently-linked carbohydrate moieties. A comprehensive overview of about fifty biological functions of glycans, including intercellular signaling, intercellular adhesion, cell–matrix interactions, recognition of pathogen-associated molecular patterns or immune modulation of host by symbionts (including parasites), is provided by Varki (2017). In marine organisms, carbohydrates are also involved in many recognition processes including reproduction, chemical communication and host-symbiont interactions (Caldwell and Pagett, 2010). For example, the endosymbiotic dinoflagellate (Symbiodinium C1f) cell surface displays glycan ligands, including mannose/glucose and galactose, that are involved in recognition during initial contact at the onset of symbiosis with their coral host larvae, Fungia scutaria (Wood-Charlson et al., 2006). Similarly, a 40-kDa glycoprotein (gp40) presents on the cell surface of the heliozoon Actinophrys sol and displaying mannose- and/or glucose-related residues was found to be involved in the immobilization and ingestion of prey flagellates (Sakaguchi et al., 2001). Finally, several heterotrophic dinoflagellates are known to use a similar mechanism involving the same carbohydrate residues (i.e., mannose and/or glucose) to catch their prey (Ucko et al., 1999; Wootton et al., 2007; Martel, 2009).
The role of carbohydrate residues in food capture is not unique to unicellular organisms and has also been demonstrated in suspension feeding bivalves, including mussels (Pales Espinosa et al., 2008; Pales Espinosa and Allam, 2013). The strikingly-efficient ability of suspension feeding bivalves to select their food particles have been recognized for decades (Newell and Jordan, 1983; Pastoureaud et al., 1996). To optimize energy uptake from their surroundings, these organisms preferentially ingest nutrient-rich particles while rejecting poor quality ones in pseudofeces (Loosanoff and Engle, 1947; Morton, 1960). This ability was shown to be mediated by interactions between carbohydrates associated with the cell surface of microalgae and C-type lectins present in mucus covering the feeding organs (Pales Espinosa et al., 2010; Pales Espinosa and Allam, 2018). Microalga cell surface carbohydrates (MCSC) represent unique patterns that can be considered as species-specific fingerprints (Cho, 2003; Pales Espinosa et al., 2016; Jones et al., 2020). This characteristic allows, for example, the discrimination of toxic and non-toxic microalgae (e.g., dinoflagellates) presenting similar morphology (Hou et al., 2008). Experimental modification of MCSC significantly reduced the recognition of endosymbiotic microalgae by corals (Wood-Charlson et al., 2006) and microalgae sorting by suspension feeding bivalves (Pales Espinosa et al., 2009) highlighting the fundamental role of these epitopes in diverse mechanisms. Further, the MCSC profile represents an excellent predictor of the fate (ingestion vs. rejection) of different microalgae in the feeding process of Crassostrea virginica (eastern oyster), Mytilus edulis (blue mussel), Mercenaria mercenaria (hard clam) and Argopecten irradians irradians (Northern bay scallop) (Pales Espinosa et al., 2016; Jones et al., 2020). In particular, statistical models (e.g., decision trees) showed that the relative abundance of mannose and glucose residues on microalgae cell surface represents a major driver of particle selection in these species (Pales Espinosa et al., 2016; Jones et al., 2020). Specifically, results showed that of two microalgae used in experimental diets, the one richer in mannose/glucose residues on the cell surface would be more likely selected.
The objective of this study was to determine the nature and quantity of carbohydrates mediating particle selection in the blue mussel M. edulis. Specifically, we wanted to quantitatively assess the sorting behavior to determine whether a minimal quantity of carbohydrates associated with cell surface is needed to trigger selection. To do so, mussels were given a choice between synthetic microspheres coated or uncoated with different quantities of various neoglycoproteins (e.g., glucose-BSA), or with microalgae having “modified” MCSC (i.e., having target MCSC blocked using different quantities of commercial lectins). Results underline to role of mannose/glucose residues in sorting and provide the quantities of carbohydrates required on microalgae cell surface to trigger selection.
Material and Methods
Bivalves
Blue mussels (35 ± 2.15 mm in length, mean ± SD), Mytilus edulis, were collected from Long Island Sound (Port Jefferson, NY, USA). Their external shell surface was scrubbed to remove mud and fouling organisms. Mussels were then acclimated in the laboratory for at least 1 week (salinity 28, 15°C) and fed daily (15% dry mass) using fresh cultures of Pavlova lutheri, Isochrysis sp. and DT’s Live Marine Phytoplankton (Sustainable Aquatics, Jefferson City, TN, USA; Pales Espinosa and Allam, 2006). Animals were unfed for 1 day prior to being used in particle-sorting assays.
Microalgal Cultures
Two microalgae species, Chlamydomonas sp. (11/35, Milford Microalgal Culture Collection) and Tetraselmis chuii (PLY429, same source), were used in lectin-binding assays and feeding experiments because their cell size (ca. 10 µm) and their unique cell surface carbohydrate signature are suitable for particle selection experiments using bivalves (Pales Espinosa et al., 2016; Jones et al., 2020). In lectin-binding assays, strains were grown in triplicate in 250 ml Erlenmeyer flasks using F/2-enriched media (Guillard, 1982) at 15°C under a 12 h light/12 h dark cycle. Cultures were harvested in the exponential phase of growth and used in further assays as described below. For feeding experiments, microalgae were produced in 3 L cultures under the conditions described above.
Binding of FITC-labeled Lectins to Chlamydomonas sp.
The cultures of Chlamydomonas sp. were centrifuged at 400 g for 10 min, washed once with filtered (0.22 µm) artificial seawater (FSW), and resuspended in FSW. FITC-conjugated lectins (Supplementary Table 1, Con A, Canavalia ensiformis lectin; PEA, Pisum sativum agglutinin; UEA, Ulex europaeus agglutinin; EY laboratories, Inc., Mateo, CA, USA), were diluted in FSW to 1 mg/ml. The lectins ConA and PEA have affinity for mannose and glucose residues and were used to block these carbohydrates on microalgae cell surface. The lectin UEA has affinity for fucose and was used to evaluate the role of this carbohydrate in sorting as prior studies suggested that it does not mediate particle sorting in mussel (Pales Espinosa et al., 2016). Lectins (final concentration ranging from 0 to 100 µg/ml) were separately added to microcentrifuge tubes containing 1 ml of washed microalgae (106 cells). Microalgae were then incubated in the dark at room temperature for 1 h, washed 3 times (400 g, 10 min) and resuspended in FSW for flow cytometry analysis. Each assay was performed in triplicate.
Microsphere Coupling
Two types of carboxylated polystyrene microspheres (7µm diameter, Magsphere Inc., Pasadena, CA, USA) were used: red fluorescent (CAFR007UM) and white plain (CA007UM). These were covalently coupled with one of the following compounds: bovine serum albumin (BSA; control) or either one of the neoglycoproteins glucose-BSA or fucose-BSA (GLYcoDiag Applied Genomics, France). These neoglycoproteins were selected because previous results showed that glucose is one of the main drivers of particle selection in suspension feeding bivalves whereas fucose appears to play a minor role (Pales Espinosa et al., 2016; Jones et al., 2020). The coupling procedure was performed using the PolyLink Protein Coupling kit (Polysciences Inc., Warrington, PA, USA) according to the manufacturer’s instructions. Briefly, aqueous suspensions of microspheres (equivalent to 12.5 mg or 108 beads per reaction) were transferred into 1.5 ml microcentrifuge tubes, washed 3 times (1500g, 5 min) and resuspended in 170 μl of PolyLink Coupling buffer. The EDAC solution (200 mg/ml) was prepared just before use and added (20 µl) to each tube. Beads were then shaken for 20 min, washed once, and transferred into new tubes previously coated with 1% BSA to limit beads adherence to the tube wall. A solution of BSA (control) or neoglycoproteins (final quantity ranging from 0.03 to 0.71 mg) was finally added to each tube and the beads were incubated for 4 hours at room temperature with gentle agitation. After coupling, the beads were washed twice (1500g, 5 min) with nuclease free water. The supernatants were kept and used to calculate the coupling efficiency. The protein concentrations in the initial neoglycoprotein solutions and in the supernatant were measured with a Pierce BCA protein assay reagent kit (Pierce, Rockford, IL), and the difference between both values represented proteins/neoglycoproteins that adhered to the microspheres. The coupled microbeads were then stored at 4°C and used in feeding experiments within a week.
Feeding Experiments
To evaluate the involvement of MCSC in particle selection by mussels and determine the threshold quantity of carbohydrates above which selection is possible, cells of Chlamydomonas sp. (2000 ml) were treated with commercially available FITC-lectins (i.e., Con A, PEA and UEA; 15, 30 and 45 µg lectin per 106 cells per ml) or seawater (i.e., control) following the protocol described in the section “Binding of FITC-labeled lectins to Chlamydomonas sp.”. Each labeled Chlamydomonas sp. suspension was then mixed with Tetraselmis chuii (unlabeled) to formulate three experimental diets. In addition, a control experimental diet was also prepared by mixing unlabeled Chlamydomonas sp. and unlabeled T. chuii. Equal concentrations of each particle type (i.e., Chlamydomonas sp. and T. chuii) were suspended in filtered seawater (105 cells/ml final concentration) and delivered to 12 mussels maintained in individual 500-ml tanks. Microalgae were kept in suspension by means of gentle manual mixing with a pipette every 15 min, and water samples were taken periodically to determine potential differential sedimentation. Pseudofeces were collected from each tank as soon as they are produced by mussels, typically within 30 min. They were then vortexed to disrupt particle aggregates, passed through a 35-µm nylon-mesh sieve and their composition was determined using flow cytometry.
In parallel, carboxylated polystyrene microspheres (red and white) were coated with gradually increasing amounts of neoglycoproteins and BSA (i.e., 0.03 to 0.71 mg) in order to determine the threshold of carbohydrates that triggers selection. Six aliquots of beads were coated with glucose-BSA (0.04, 0.33 and 0.53 mg) and BSA (0.03, 0.48 and 0.56 mg) and used in 3 feeding experiments (i.e., Exp. 1, Exp. 2 and Exp. 3). The final quantity of glucose-BSA coated per bead was measured to be 0.26, 3.54 and 5.66 of pg of protein, corresponding to a density of 0.17 x 10-2, 2.30 x 10-2 and 3.68 x 10-2 pg/µm2 (Table 1). Further, the number of molecules of glucose coated per bead was calculated to be 0.05 x 109, 0.67 x 109 and 1.08 x 109 respectively, corresponding to a density of 0.32 x 106, 4.37 x 106 and 6.99 x 106 molecules of glucose per µm2. Similarly, the final quantity of BSA in control beads was calculated to be 0.27, 3.80 and 5.89 of pg which correspond to a density of 0.17 x 10-2, 2.47 x 10-2 and 3.82 x 10-2 pg/µm2. Two additional feeding experiments (Exp. 4 and Exp. 5) were performed using beads coated with fucose-BSA (0.39 and 0.60 mg) and BSA (0.62 and 0.71 mg). The final quantity of fucose-BSA coated per bead was measured to be 2.86 and 5.94 of pg, corresponding to a density of 1.86 x 10-2 and 3.86 x 10-2 pg/µm2 or 3.87 x 106 and 8.05 x 106 molecules of fucose per µm2 (Table 1). In parallel, the final quantity of BSA per beads was measured to be 2.84 and 6.02 of pg, corresponding to a density of 1.84 10-2 and 3.91 10-2 pg/µm2. The coupling efficiencies of the different assays varied between 42.2 to 97.4% (Table 1). Microspheres (e.g., red beads coated with 0.03 mg of BSA and white beads coated with 0.04 mg of glucose-BSA) were then mixed and suspended in filtered seawater (105 cells/ml final concentration) and delivered to 12 mussels maintained in individual 300-ml tank. Sorting experiments were then run as described for microalgae. Preliminary trials showed that microsphere color does not have any effect on particle sorting by mussels or BSA/neoglycoprotein binding efficiency.
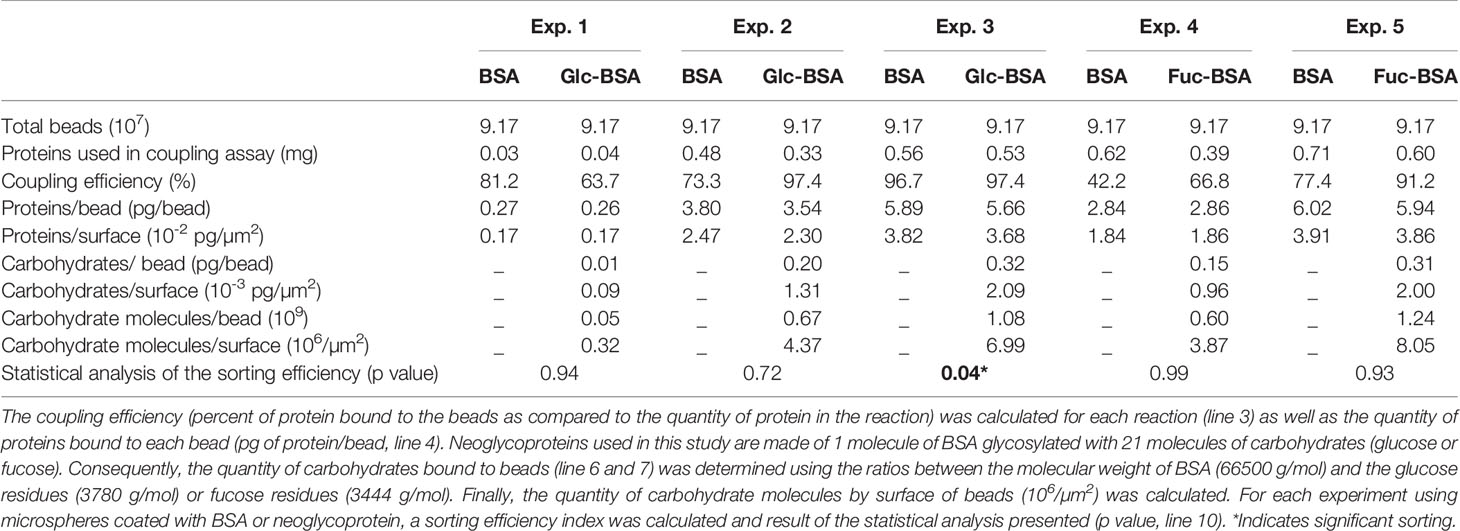
Table 1 Parameters and outcomes of each of the 5 experiments. Microspheres (107 beads) were coated with different quantities of BSA (Bovin Serum Albumin, used as control) or one of neoglycoproteins, glucose-BSA (Glc-BSA) or fucose-BSA (Fuc-BSA).
Flow Cytometry Analysis
Microalgae and microspheres were analyzed using a FACSCalibur flow cytometer (Becton Dickinson Biosciences, CA, USA). A minimum of 104 events were analyzed. The 488-nm argon laser was used for excitation, and test particles (microspheres, labeled and unlabeled microalgae) were identified and characterized on the basis of one or more of the following parameters: forward (FSC) and side (SSC) light scatters, FITC fluorescence (FL1, 535 nm), and photosynthetic pigments auto-fluorescence (FL3, 675 nm and FL4, 695 nm). Microalgal size (FSC) and intracellular complexity (SSC) were evaluated after each treatment (lectins) to check for cell alterations.
Data Treatment and Statistical Analysis
All data are presented as mean ± standard deviation. For the feeding experiments, data were analyzed using goodness-of-fit tests (G test). Two series of tests were performed comparing the proportion of each particle type in samples of the diet and pseudofeces collected from the mussels. The first series of tests ensured that within each treatment, replicate samples of the diet and pseudofeces were homogeneous. The second series tested the null hypothesis that, within each treatment, the proportion of each particle type (i.e., cells treated or untreated, or microspheres) in diets and pseudofeces were not different. In addition to the comparison of raw counts, a sorting efficiency (SE) index was calculated to examine particle selection (Iglesias et al., 1992). This index was defined as:
where PP and DP represent the proportion of the particle of interest in pseudofeces and diet, respectively. A positive SE for a given particle type indicates that it is preferentially ingested (particle type is depleted in pseudofeces, compared to diet), a negative SE indicates rejection (particle type is enriched in pseudofeces compared to diet), and zero indicates the absence of active selection. After confirming their normal distributions, calculated SE values obtained for each of the two particles in each treatment were compared to zero using a one-sample Student’s t-test (two-tailed). The null hypothesis was that the selection efficiencies were equal to zero (i.e., no selection). Two sample t-tests were then used to examine differences in SE between the two particle types in each treatment.
To facilitate the schematic representation of the results, an SE percent change was calculated as: SE % Change = 100 x (SE control - SE test)/SE control,
Where SE control represent efficiencies measured in untreated controls (e.g., untreated microalgae, or uncoated spheres) and SE test representing efficiencies measured in each respective treatment.
Results
The fluorescence of Chlamydomonas sp. exposed to several concentrations of FITC-lectins (ConA, PEA and UEA, up to 100 µg/ml) was first evaluated. Results showed a linear increase in cell fluorescence (FL1) as lectin concentrations increased from 0 to 35 µg/ml and then remained unchanged up to 50 µg/ml (Figure 1). Lectin concentrations > 50 µg/ml induced another increase of the green fluorescence (FL1) which microscopic observations showed it to be due to the penetration of lectins inside the cell instead of just staining the carbohydrates present on cell surface. In parallel, preliminary assays showed that cell integrity was not affected when 50 µg of lectin/ml was used while a slow decrease in cell integrity was noticed when lectin concentrations approached 100 µg of lectin/ml. Consequently, three concentrations of lectins lower than 50 µg/ml (i.e., 15, 30 and 45 µg/ml) were chosen for the feeding assays.
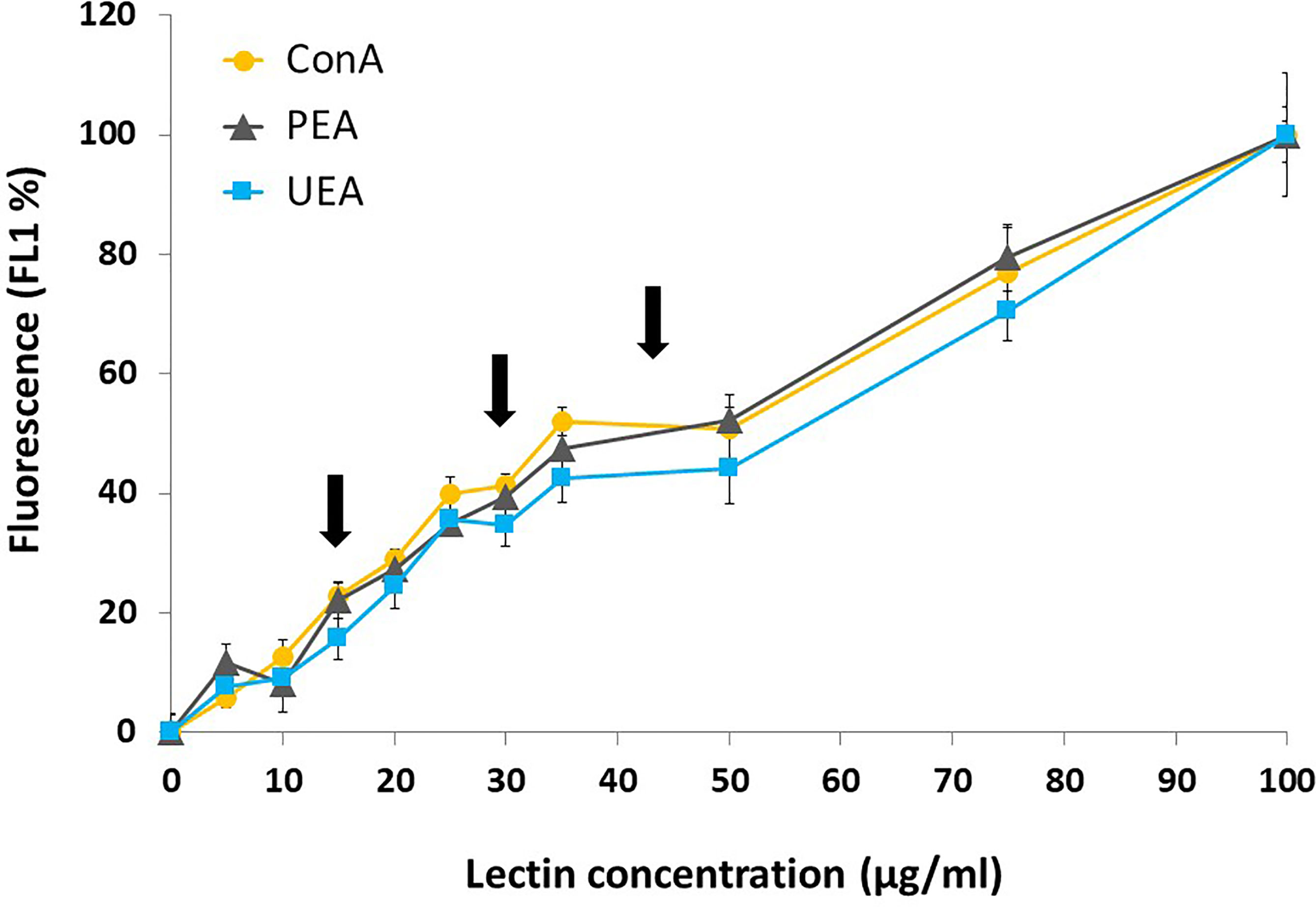
Figure 1 Fluorescence intensity (mean ± standard deviation, 3 biological replicates per data point) of Chlamydomonas sp. labeled with several concentrations of FITC-lectins (ConA, PEA and UEA, up to 100 µg/ml). Black arrows indicate concentrations (15, 30 and 45 µg/ml) used in feeding experiments.
In the control treatment (i.e., involving the pairing of unlabeled Chlamydomonas sp. and unlabeled Tetraselmis chuii), mussels significantly ingested Chlamydomonas sp. while they rejected T. chuii (Figure 2). The sorting efficiency indexes (SE) for Chlamydomonas sp. varied between 0.41 and 0.47.
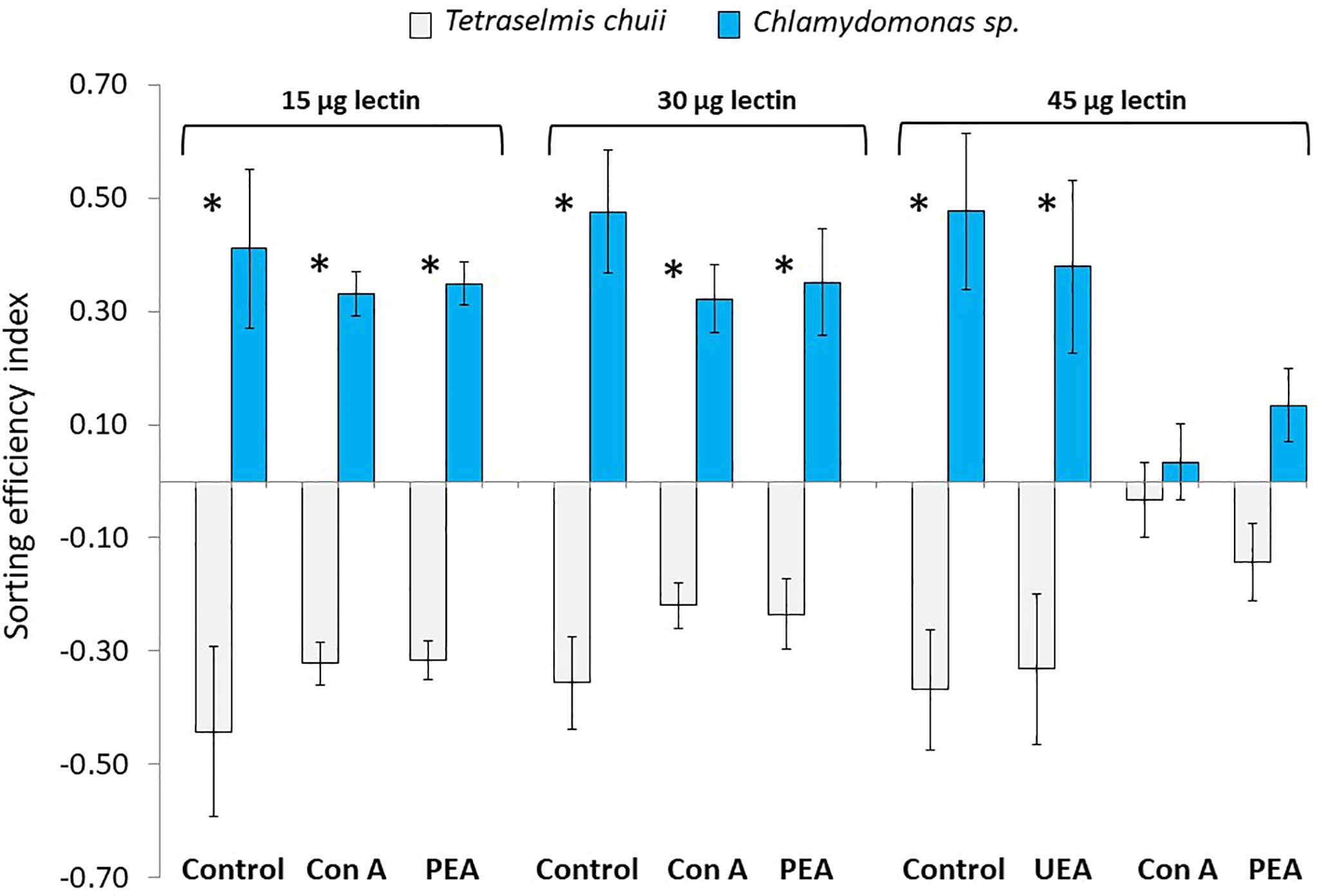
Figure 2 Sorting efficiencies (mean ± standard deviation, n = 12 per data point) of M. edulis fed a mixture of Tetraselmis chuii and Chlamydomonas sp. treated with FITC-lectins (Con A, PEA and UEA; 15, 30 and 45 µg lectin per 106 cells per ml) or seawater (control). *Indicates significant sorting.
When Chlamydomonas sp. was treated with 15 and 30 µg/ml of ConA or PEA, mussels continue to preferentially ingest Chlamydomonas sp. as compared to T. chuii. The SE slightly decreased to 0.32 and 0.35 when the algae were labelled with 30 µg/ml ConA and PEA, respectively. When Chlamydomonas sp. was labelled with 45 µg/ml of ConA or PEA, mussels stopped selecting microalgae and the SE dropped to 0.03 and 0.13, respectively. Interestingly, when Chlamydomonas sp. was labelled with 45 µg/ml of UEA, a lectin specific to fucose residues, the sorting activity was not affected and mussels continued to preferentially ingest Chlamydomonas sp. as compared to T. chuii with an SE equal to 0.38.
The percent change in the sorting efficiency confirmed trends detected with raw SE values. In addition, the best fit for the data was not given by a linear regression but by a polynomial regression model yielding R2 values of 0.96 and 0.95 for ConA and PEA, respectively (Figure 3).
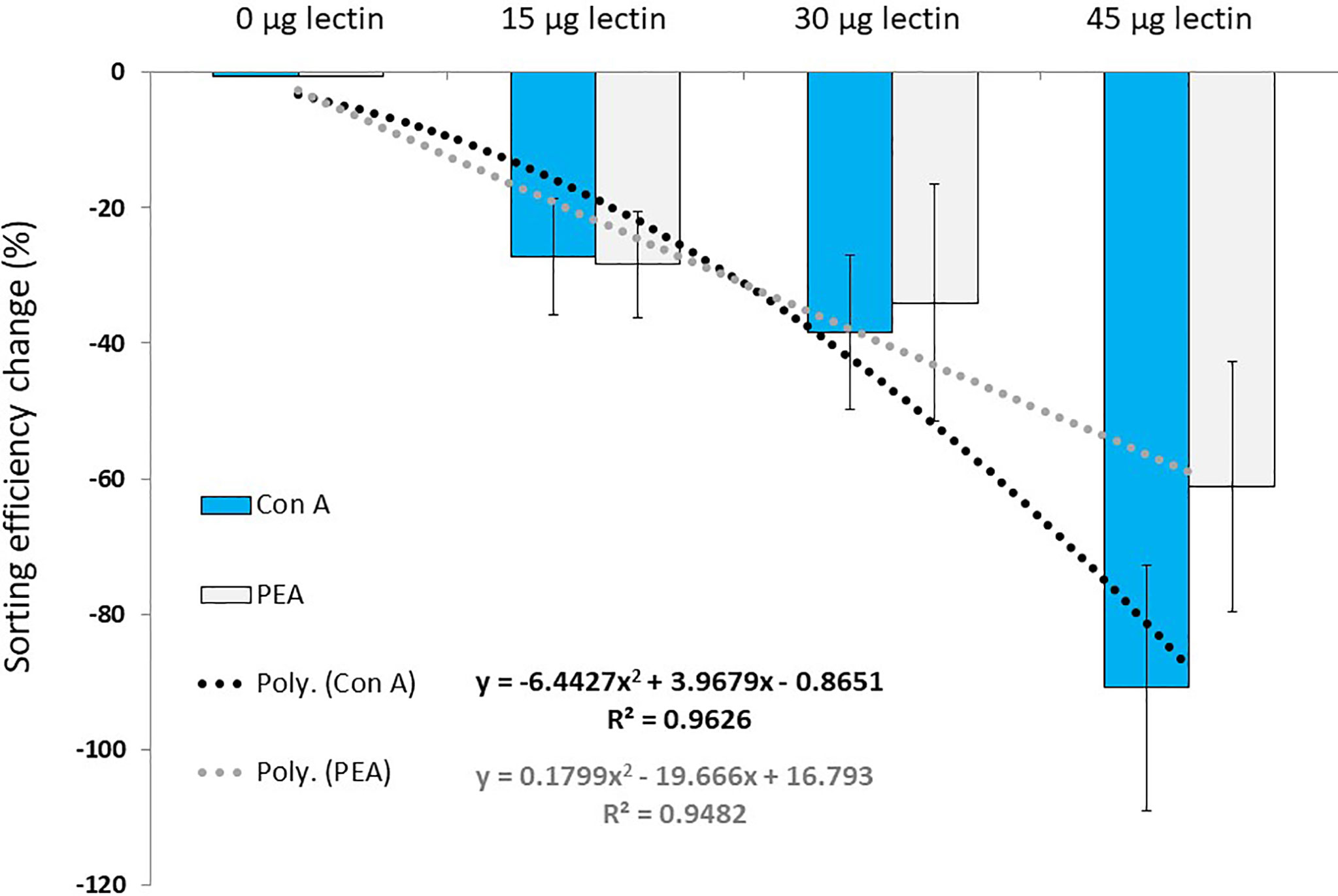
Figure 3 Percent change in sorting efficiency (mean ± standard deviation, n = 12 per data point) for microalgae treated with different quantities of lectins as compared to controls (0 µg lectin). Dashed lines and equations represent the best fitting models for each lectin.
Experimental diets were then made by mixing 2 types of beads (e.g., red beads coated with BSA and white beads coated with glucose-BSA) suspended in filtered seawater (105 beads/ml final concentration) and delivered to 12 mussels maintained in individual 300-ml tank. The number of each bead type in diet and pseudofeces was determined using flow cytometry and used to calculate sorting efficiencies. In the feeding Experiments 1 and 2 (i.e., 0.05 x 109 and 0.67 x 109 molecules of glucose coated per bead respectively, Table 1 and Figure 4), the sorting efficiency indexes (SE) were not significantly different (p = 0.94 and 0.72 respectively) and no preferential ingestion for either type of microspheres (coated with BSA or glucose-BSA) was recorded. In experiment 3 (i.e., 1.08 x 109 molecules of glucose coated per bead), mussels preferentially ingested microspheres coated with glucose-BSA and the SE was significantly different between coated microspheres and uncoated controls (p = 0.04). The percent change in the sorting efficiency index showed that the increasing quantity of glucose-BSA coated on microspheres (0.5 x 109 pg/bead to 1.08 x 109 pg bead) induces a gradual increase in sorting (Figure 5). In the fucose-BSA experiments (i.e., Exp. 4 and Exp. 5 with 0.60 x 109 and 1.24 x 109 of molecules of fucose coated per bead respectively, Table 1 and Figure 4), the sorting efficiency indices were not significantly different between experimental (fucose-BSA) and control (BSA alone) microspheres (p = 0.99 and 0.92 respectively).
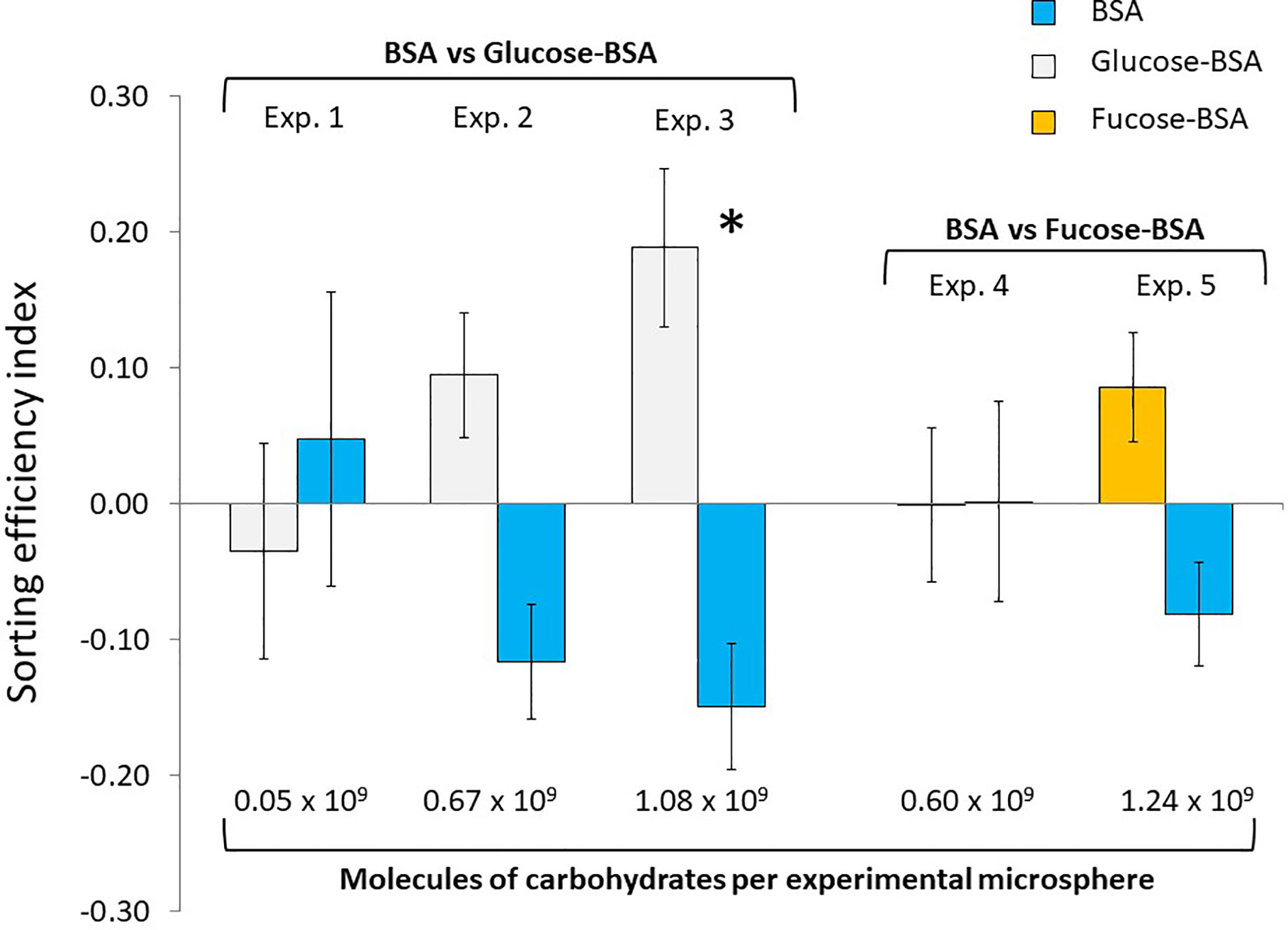
Figure 4 Sorting efficiencies (mean ± standard deviation, n = 12 per data point) of M. edulis fed a mixture of BSA- and neoglycoprotein-coated microspheres (glucose-BSA or fucose-BSA; from 0.05 to 1.24 109 molecules of carbohydrates per microsphere). *Indicates significant sorting.
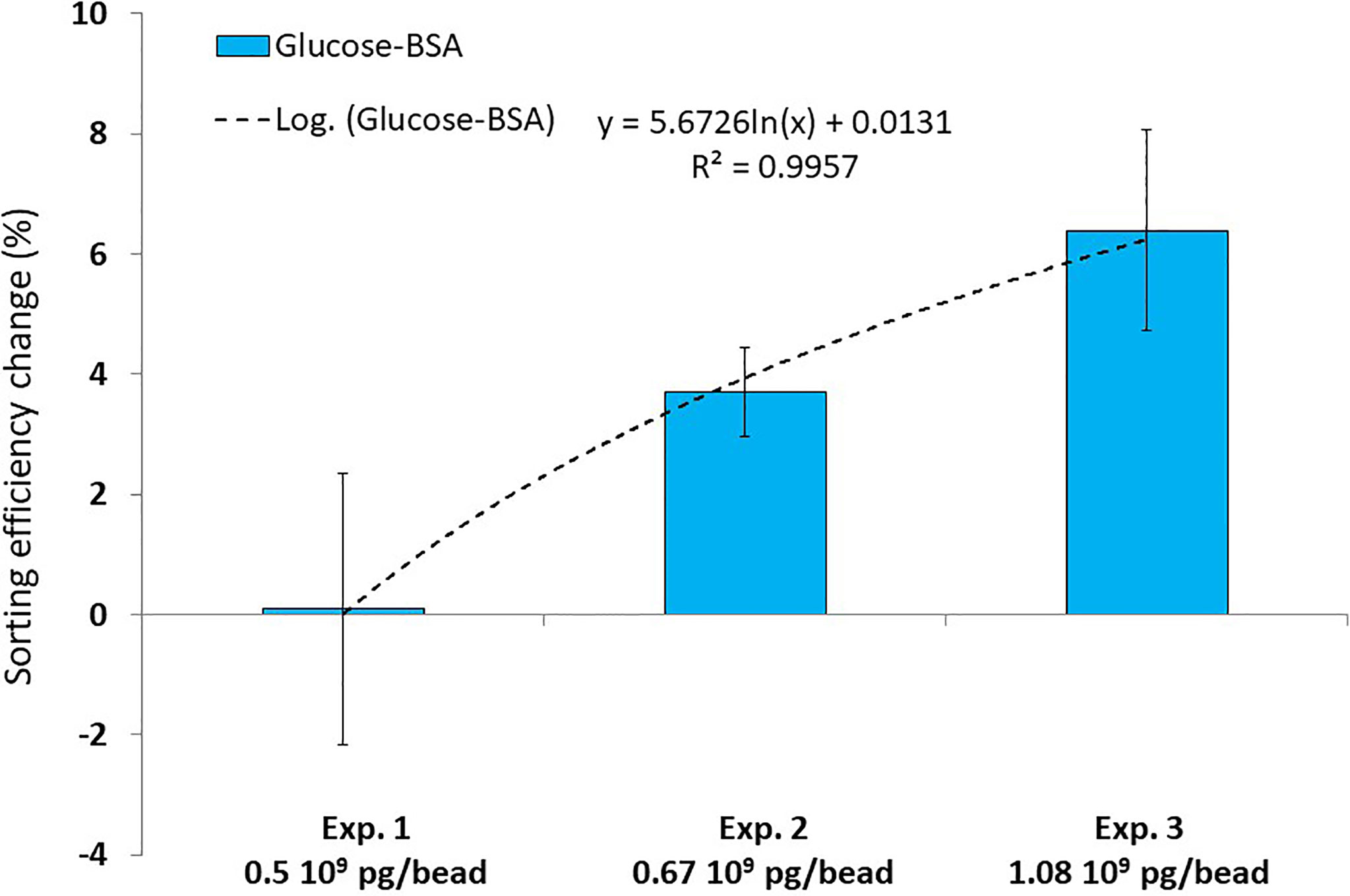
Figure 5 Percent change in sorting efficiency (mean ± standard deviation, n = 12 per data point) for microspheres coated with different quantities of glucose-BSA. The dashed line and equation represent the best fitting model.
Discussion
This study aimed to evaluate the nature and the quantity of surface carbohydrates required to trigger sorting of food particles in the blue mussel and to determine the relationship between the quantity of MCSC and sorting efficiencies. The results presented here prove that carbohydrates and more specifically mannose and glucose residues present on particle surface (as opposed to fucose residues), drive particle selection in the blue mussel. For instance, lectins specific for mannose and glucose (i.e., ConA and PEA) were the most effective at inhibiting particle selection likely via the blocking of cognate carbohydrates present on algae cell surface. Furthermore, results with engineered microspheres showed that particles coated with glucose-BSA, but not fucose-BSA or BSA (at least at similar quantities as glucose-BSA), induce particle selection in mussels. Cell surface carbohydrates play key roles in a broad range of functions involving cellular recognition such as host-pathogen interactions (Allen and Dawidowicz, 1990; Stahl and Ezekowitz, 1998), reproduction (Kim and Fritz, 1993; Benoff, 1997) and symbiosis establishment and maintenance (Wood-Charlson et al., 2006; Vidal-Dupiol et al., 2009). These carbohydrates are ligands for carbohydrate-binding proteins (i.e., lectins) that function as recognition molecules in a wide range of biological systems (Sharon and Lis, 2004). Previous work focusing on predator-prey interactions showed that mannose and glucose residues present on microalgae cell surfaces are used by some members of the heterotrophic dinophyceae (Ucko et al., 1999; Wootton et al., 2007; Martel, 2009) and tintinid ciliates (Cobb, 2017) to select and capture their food particles. Similarly, our previous findings demonstrated that mannose and glucose residues associated with microalgae cell surface represents the main cue for selection in a broad range of suspension-feeding bivalves (Jing et al., 2011; Pales Espinosa et al., 2016; Pales Espinosa and Allam, 2018; Jones et al., 2020). In these organisms, MCSC, particularly mannose and glucose, interact with lectins present in mucosal secretions covering feeding organs, triggering selection (Jing et al., 2011; Pales Espinosa et al., 2016; Pales Espinosa and Allam, 2018). It is important to note that preferential ingestion or rejection is relative and depends on the particles present in the diet. For instance, of two microalgae, the one richer in mannose/glucose residues on the cell surface would be more likely selected by suspension feeding bivalves (Pales Espinosa et al., 2016; Jones et al., 2020). In the present study, the microalgae Chlamydomonas sp. (which displays more mannose/glucose residues among its MCSC than T. chuii; Pales Espinosa et al., 2016) was preferentially ingested by mussels as compared to T. chuii, confirming results obtained by Jones et al. (2020) in clams and scallops. The current study builds on this previous information and provides a quantitative estimation of MCSC required to mediate selection.
The number of glucose molecules (in the form of glucose-BSA) needed to trigger particle selection in mussels was estimated to be 1.08 x109 molecules per 7 µm-bead which was equivalent to 6.99 106 molecules per µm2. Very little information is available on the quantity of carbohydrate present at the surface of cells. For example, it has been reported that HeLa, K562 and BGC cells (cancerous human cell lines) have about 4 x 1010, 4.7 x 109 and 5.3 x 107 molecules of mannose/glucose at their cell surface, respectively (Cheng et al., 2008; Zhang et al., 2010; Ding et al., 2011). These human cell lines, which sizes are approximatively 20 µm, are known to harbor more carbohydrates (~ 2 fold) than normal cells (Chen et al., 2016). Given this information, the estimated quantity of glucose (i.e., 1.08 109 molecules) attached to the beads and able to mimic live microalgae and initiate a biologic response seems plausible.
In another type of cell recognition, carbohydrate-carbohydrate interactions provide dissociated cells from marine sponges the ability to re-aggregate through a 200-kD glycan associated with their cell surface (Fernandez-Busquets and Burger, 2003; Bucior et al., 2004). The 200-kD glycan density per cell able to initiate an aggregation response was calculated to be 828 molecules/µm2, representing a molecular weight of 160,000 kD/µm2 (Bucior et al., 2004). In our study, it was estimated that the weight of 6.99 106 molecules of glucose/µm2, which are able to enable sorting in our experiment, corresponds to 1,260,000 kD/µm2. This is nearly 8 times higher than the weight of the 200-kD glycan able to trigger cellular recognition in sponge. This difference can be caused by multiple reasons. First and foremost, the strength of the bonds required to trigger selection in mussel is likely higher than that of bonds needed to initiate an aggregation response in sponge. In the former, selected particles have to resist the flow of water that mussels pump through the gill to capture food particles, as weakly bound particles are transferred to the food rejection tract. In contrast, cellular aggregation in sponge does not face the same physical stress and may simply require the initiation of recognition signaling. Further, the difference might be due to the fact that the interactions do not involve the same type of ligand-receptor interactions (one being carbohydrate-lectin and the other one being carbohydrate-carbohydrate) and/or to the size or the glycan. In our case, the carbohydrate is delivered as a neoglycoprotein meaning a molecule of BSA linked to 21 glucose residues, whereas the BSA (not used for the molecular mass comparisons discussed here) is simply used as a backbone to attach glucose molecules and does not interact with mucosal lectins to initiate sorting. In contrast, the 200-KD glycan, although larger that our neoglycoproteins on a molecule-to-molecule comparison, is solely made of carbohydrates (Bucior et al., 2004) and may include more active binding sites per molecule as compared to BSA-glucose.
Overall, results showed that the relationship between the biological response (sorting) and the density of available ligands (i.e., carbohydrates coated to microspheres able to trigger particle selection or quantity of commercial lectins added to microalgae cell surface to inhibit sorting) seems to follow a non-linear model. This “all-or-none” principle is common in biology, with a good example being the action potential observed in neurons or muscle cells where activation occurs only when a voltage threshold is reached. This nonlinear dynamic is also a hallmark of cell adhesion mechanisms, and has been described to enhance the stability and the strength of the receptor-ligand bonds (Kong et al., 2008; Lagunas et al., 2012). For example, Lagunas et al. (2012) demonstrated that binding of mouse embryonic fibroblast cells to substrate coated with RGD (Arg-Gly-Asp) is non-linear to the density of RGD, where a threshold of 4.0 pmol RGD/cm2 is required for successful cell attachment. In the particle selection mechanism, bonds between mucosal lectins and carbohydrates present on algae cell surface may follow the same principle and a minimal number of bonds is required to initiate selection. In other words, the strength of the bonds between food particles and the mucus covering the feeding organs will determine whether a particle is captured and transferred to the food selection tract or directed to the “default” tract for particle rejection.
Conclusion
This study represents an important step for understanding particle selection in suspension feeders, and evaluates the nature and quantities of surface carbohydrates required to trigger sorting in the blue mussel. Further, the relationship between carbohydrate quantities and sorting efficiencies was determined. These results confirm the major role of mannose/glucose residues in this process and provide a quantitative assessment of the sorting behavior. We estimate the quantity of glucose ligand needed to trigger selection to be about 1.08 x 109 molecules on a 7 µm in diameter polystyrene microsphere. These findings represent a first step for evaluating whether these ligand density thresholds are conserved among other suspension-feeding invertebrates.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
EPE and BA led the conceptualization and secured the funding. EPE and ME contributed to investigation and methodology. EPE and BA contributed to the formal analysis, writing the original draft. All authors participated in manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Science Foundation for funding this work (IOS-1656753).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Natarajan Aaradhana and Nora Straquadine for help during this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.882356/full#supplementary-material
References
Allen P. G., Dawidowicz E. A. (1990). Phagocytosis in Acanthamoeba.1. A Mannose Receptor Is Responsible for the Binding and Phagocytosis of Yeast. J. Cell. Physiol. 145 (3), 508–513. doi: 10.1002/jcp.1041450317
Benoff S. (1997). Carbohydrates and Fertilization: An Overview. Mol. Hum. Reprod. 3 (7), 599–637. doi: 10.1093/molehr/3.7.599
Bucior I., Scheuring S., Engel A., Burger M. M. (2004). Carbohydrate–carbohydrate Interaction Provides Adhesion Force and Specificity for Cellular Recognition. J. Cell Biol. 165 (4), 529–537. doi: 10.1083/jcb.200309005
Caldwell G. S., Pagett H. E. (2010). Marine Glycobiology: Current Status and Future Perspectives. Mar. Biotechnol. 12 (3), 241–252. doi: 10.1007/s10126-010-9263-5
Cheng W., Ding L., Lei J., Ding S., Ju H. (2008). Effective Cell Capture With Tetrapeptide-Functionalized Carbon Nanotubes and Dual Signal Amplification for Cytosensing and Evaluation of Cell Surface Carbohydrate. Analytic. Chem. 80 (10), 3867–3872. doi: 10.1021/ac800199t
Chen J., Liu T., Gao J., Gao L., Zhou L., Cai M., et al. (2016). Variation in Carbohydrates Between Cancer and Normal Cell Membranes Revealed by Super-Resolution Fluorescence Imaging. Adv. Sci. 3 (12), 1600270. doi: 10.1002/advs.201600270
Cho E. S. (2003). Cluster Analysis on the Lectin Binding Patterns of Marine Microalgae. J. Plankt. Res. 25 (3), 309–315. doi: 10.1093/plankt/25.3.309
Cobb S. (2017). Investigation of Contact-Based Cues Mediating Food Uptake in the Marine Tintinnid Ciliate Favella Sp. Pg 440. Available at: https://scholarworks.calstate.edu/concern/theses/kp78gg976.
Ding C., Qian S., Wang Z., Qu B. (2011). Electrochemical Cytosensor Based on Gold Nanoparticles for the Determination of Carbohydrate on Cell Surface. Analytic. Biochem. 414 (1), 84–87. doi: 10.1016/j.ab.2011.03.007
Fernandez-Busquets X., Burger M. (2003). Circular Proteoglycans From Sponges: First Members of the Spongican Family. Cell. Mol. Life Sci. CMLS. 60 (1), 88–112. doi: 10.1007/s000180300006
Guillard R. R. L. (1982). “Culture of Phytoplankton for Feeding Marine Invertebrates”. in Culture of Marine Invertebrate Animals. Smith W.L., Chanley M.H. (Eds.) (New York: Plenum Press), pp. 108–132.
Hou J., Huang B., Hu J., Lin L., Hong H. (2008). Fourteen FITC-Conjugated Lectins as a Tool for the Recognition and Differentiation of Some Harmful Algae in Chinese Coastal Waters. J. Appl. Phycol. 20 (1), 35–46. doi: 10.1007/s10811-007-9178-3
Iglesias J. I. P., Navarro E., Jorna P. A., Armentia I. (1992). Feeding, Particle Selection and Absorption in Cockles Cerastoderma Edule (L) Exposed to Variable Conditions of Food Concentration and Quality. J. Exp. Mar. Biol. Ecol. 162 (2), 177–198. doi: 10.1016/0022-0981(92)90200-T
Jing X., Pales Espinosa E., Perrigault M., Allam B. (2011). Identification, Molecular Characterization and Expression Analysis of a Mucosal C-Type Lectin in the Eastern Oyster, Crassostrea Virginica. Fish. Shellfish. Immunol. 30 (3), 851–858. doi: 10.1016/j.fsi.2011.01.007
Jones J., Allam B., Pales Espinosa E. (2020). Particle Selection in Suspension-Feeding Bivalves: Does One Model Fit All? Biol. Bull. 238 (1), 41–53. doi: 10.1086/707718
Kim G., Fritz L. (1993). Gamete Recognition During Fertilization in a Red Alga, Antithamnion Nipponicum. Protoplasma 174 (1), 69–73. doi: 10.1007/BF01404044
Kong D., Ji B., Dai L. (2008). Nonlinear Mechanical Modeling of Cell Adhesion. J. Theor. Biol. 250 (1), 75–84. doi: 10.1016/j.jtbi.2007.09.028
Lagunas A., Comelles J., Martínez E., Prats-Alfonso E., Acosta G. A., Albericio F., et al. (2012). Cell Adhesion and Focal Contact Formation on Linear RGD Molecular Gradients: Study of non-Linear Concentration Dependence Effects. Nanomed.: Nanotech. Biol. Med. 8 (4), 432–439. doi: 10.1016/j.nano.2011.08.001
Loosanoff V. L., Engle J. B. (1947). Feeding of Oysters in Relation to Density of Microorganisms. Science 105 (2723), 260–261. doi: 10.1126/science.105.2723.260
Martel C. M. (2009). Conceptual Bases for Prey Biorecognition and Feeding Selectivity in the Microplanktonic Marine Phagotroph Oxyrrhis Marina. Microbial. Ecol. 57 (4), 589–597. doi: 10.1007/s00248-008-9421-8
Morton J. E. (1960). The Function of the Gut in the Ciliary Feeders. Biol. Rev. Cambr. Philos. Soc. 35 (1), 92–139. doi: 10.1111/j.1469-185X.1960.tb01463.x
Newell R. I. E., Jordan S. J. (1983). Preferential Ingestion of Organic Material by the American Oyster Crassostrea Virginica. Mar. Ecol. Prog. Ser. 13 (1), 47–53. doi: 10.3354/meps013047
Pales Espinosa E., Allam B. (2006). Comparative Growth and Survival of Juvenile Hard Clams, Mercenaria Mercenaria, Fed Commercially Available Diets. Zoo Biol. 25 (6), 513–525. doi: 10.1002/zoo.20113
Pales Espinosa E., Allam B. (2013). Food Quality and Season Affect Gene Expression of the Mucosal Lectin MeML and Particle Sorting in the Blue Mussel Mytilus Edulis. Mar. Biol. 160 (6), 1441–1450. doi: 10.1007/s00227-013-2196-6
Pales Espinosa E., Allam B. (2018). Reverse Genetics Demonstrate the Role of Mucosal C-Type Lectins in Food Particle Selection in the Oyster Crassostrea Virginica. J. Exp. Biol. 221 (6), jeb174094. doi: 10.1242/jeb.174094
Pales Espinosa E., Allam B., Ford S. E. (2008). Particle Selection in the Ribbed Mussel Geukensia Demissa and the Eastern Oyster Crassostrea Virginica: Effect of Microalgae Growth Stage. Estua. Coast. Mar. Sci. 79 (1), 1–6. doi: 10.1016/j.ecss.2008.02.022
Pales Espinosa E., Cerrato R. M., Wikfors G., Allam B. (2016). Modeling Food Choice in Suspension-Feeding Bivalves. Mar. Biol. 163, 2–13. doi: 10.1007/s00227-016-2815-0
Pales Espinosa E., Perrigault M., Allam B. (2010). Identification and Molecular Characterization of a Mucosal Lectin (MeML) From the Blue Mussel Mytilus Edulis and its Potential Role in Particle Capture. Comp. Biochem. Physiol. A-Molecul. Integr. Physiol. 156, 495–501. doi: 10.1016/j.cbpa.2010.04.004
Pales Espinosa E., Perrigault M., Ward J. E., Shumway S. E., Allam B. (2009). Microalgal Cell Surface Carbohydrates as Recognition Sites for Particle Sorting in Suspension Feeding Bivalves. J. Shellfish. Res. 28 (3), 695–695. doi: 10.1086/BBLv217n2p130
Pastoureaud A., Heral M., Prou J., Razet D., Russu P. (1996). Particle Selection in the Oyster Crassostrea Gigas (Thunberg) Studied by Pigment HPLC Analysis Under Natural Food Conditions. Oceanolog. Acta 19 (1), 79–88.
Sakaguchi M., Murakami H., Suzaki T. (2001). Involvement of a 40-kDa Glycoprotein in Food Recognition, Prey Capture, and Induction of Phagocytosis in the Protozoon Actinophrys Sol. Protist 152 (1), 33–41. doi: 10.1078/1434-4610-00041
Sears P., Wong C.-H. (1996). Intervention of Carbohydrate Recognition by Proteins and Nucleic Acids. Proc. Natl. Acad. Sci. 93 (22), 12086–12093. doi: 10.1073/pnas.93.22.12086
Sharon N., Lis H. (2004). History of Lectins: From Hemagglutinins to Biological Recognition Molecules. Glycobiology 14 (11), 53R–62R. doi: 10.1093/glycob/cwh122
Stahl P. D., Ezekowitz R. A. B. (1998). The Mannose Receptor is a Pattern Recognition Receptor Involved in Host Defense. Curr. Opin. Immunol. 10 (1), 50–55. doi: 10.1016/S0952-7915(98)80031-9
Ucko M., Shrestha R. P., Mesika P., Bar-Zvi D., Arad S. M. (1999). Glycoprotein Moiety in the Cell Wall of the Red Microalga Porphyridium Sp. (Rhodophyta) as the Biorecognition Site for the Crypthecodinium Cohnii-Like Dinoflagellate. J. Phycol. 35 (6), 1276–1281. doi: 10.1046/j.1529-8817.1999.3561276.x
Varki A. (1993). Biological Roles of Oligosaccharides: All of the Theories are Correct. Glycobiology 3 (2), 97–130. doi: 10.1093/glycob/3.2.97
Vidal-Dupiol J., Adjeroud M., Roger E., Foure L., Duval D., Mone Y., et al. (2009). Coral Bleaching Under Thermal Stress: Putative Involvement of Host/Symbiont Recognition Mechanisms. BMC Physiol. 9 (1), 1–16. doi: 10.1186/1472-6793-9-14
Wang B., Boons G.-J. (2011). Carbohydrate Recognition: Biological Problems, Methods, and Applications (Hoboken, New Jersey: John Wiley & Sons).
Wood-Charlson E. M., Hollingsworth L. L., Krupp D. A., Weis V. M. (2006). Lectin/glycan Interactions Play a Role in Recognition in a Coral/Dinoflagellate Symbiosis. Cell. Microbiol. 8 (12), 1985–1993. doi: 10.1111/j.1462-5822.2006.00765.x
Wootton E. C., Zubkov M. V., Jones D. H., Jones R. H., Martel C. M., Thornton C. A., et al. (2007). Biochemical Prey Recognition by Planktonic Protozoa. Environ. Microbiol. 9 (1), 216–222. doi: 10.1111/j.1462-2920.2006.01130.x
Keywords: carbohydrate, ligand, lectin, feeding, selection, suspension-feeding
Citation: Pales Espinosa E, Eckstein M and Allam B (2022) Density of Compatible Ligands on the Surface of Food Particles Modulates Sorting Efficiency in the Blue Mussel Mytilus edulis. Front. Mar. Sci. 9:882356. doi: 10.3389/fmars.2022.882356
Received: 28 February 2022; Accepted: 06 April 2022;
Published: 11 May 2022.
Edited by:
Inna Sokolova, University of Rostock, GermanyReviewed by:
Bent Vismann, University of Copenhagen, DenmarkBojan Hamer, Rudjer Boskovic Institute, Croatia
Copyright © 2022 Pales Espinosa, Eckstein and Allam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emmanuelle Pales Espinosa, RW1tYW51ZWxsZS5QYWxlc2VzcGlub3NhQHN0b255YnJvb2suZWR1; orcid.org/0000-0003-1779-6757
 Emmanuelle Pales Espinosa
Emmanuelle Pales Espinosa Margot Eckstein
Margot Eckstein Bassem Allam
Bassem Allam