
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 20 May 2022
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.880210
The Hebei Spirit oil spill (HSOS) accident in December 2007 on the west coast of Korea devastated the intertidal oyster farms along the Taean coast, resulting in the shut-down of the farming for three years. In 2010, two years after the accident, the level of residual oil in the water, sediment, and oyster tissue in the spilled area became similar to the level before the accident, although the fitness of the oysters in the spilled area remained unknown. In an attempt to resume the oyster culture in the spilled area, we monitored the growth and reproduction of the Pacific oyster Crassostrea gigas in an area the most heavily impacted. The oyster spats used in this study were harvested from the oil-damaged area in September 2011 and hardened for 9 months before being transplanted into the grow-out facilities in May 2012. The transplanted oysters demonstrated a fast shell growth for the first 5 months, reaching 53.9 mm in shell length (SL) in October 2011. At the end of the survey in November 2013, the oyster became 65 mm, suggesting an additional year of cultivation to reach the market size. Histology indicated that the annual gametogenesis was synchronous, as the males and females initiated the gametogenesis in January when the water temperature was 5.2°C. The female oysters spawned from July to September, as the water temperature ranged from 21.2 to 24.8°C. An indirect enzyme-linked immunosorbent assay (ELISA) applied in the reproductive effort measurement indicated that the fully ripe female oysters produced 20-23% of their body weight as eggs prior to spawning, which was comparable to the reproductive effort of oysters in oil-spill-free areas. The data suggested that off-bottom rack cultured Pacific oysters at the Euhangri beach successfully recovered from HSOS stresses 4 years after the accident.
On December 7, 2007, the oil tanker Hebei Spirit collided with a crane barge 10 km off the west coast of Korea, leaking 10,900 tons of crude oil (Yim et al., 2017). This accident is recorded as the largest marine oil spill in Korean history. Within a few days after the accident, the crude oil released covered and damaged more than 150 km of shoreline due to the semi-diurnal tidal currents on the west coast of Korea and prevailing northwesterly wind in the winter season. The spilled crude oil covered the entire coast of Taean and damaged most of the intertidal shellfish aquaculture facilities. Consequently, the shellfish aquaculture was suspended officially until the petroleum hydrocarbon in shellfish tissues and the environment returned to the level before the accident [Ministry of Land, Transport and Maritime Affairs, Republic of Korea (MLTM), 2009].
Although most of the spilled oil in the coastal Taean was removed one to two months after the accident due to intensive clean-up activities (Kim et al., 2010; Kim et al., 2013), the survived Pacific oysters exposed to the crude oil demonstrated a slow somatic tissue growth and retarded gametogenesis (Lee, 2010; Mondol et al., 2015). Due to the detrimental effects of the petroleum hydrocarbons, a reduction in immune competence of the Pacific oyster was also reported a year after the accident (Donaghy et al., 2010). Two years after HSOS, polycyclic aromatic hydrocarbons (PAHs) in the water column and oyster tissues declined to the level before the accident [Ministry of Land, Transport and Maritime Affairs, Republic of Korea (MLTM), 2009; Ministry of Land, Transport and Maritime Affairs, Republic of Korea (MLTM), 2010; Kim et al., 2013]. According to Mondol et al. (2015), wild Pacific oysters in the HSOS area showed substantially higher somatic tissue growth and reproductive performance than the control oysters in the none-oil-spill area two years after the oil spill accident, suggesting that the oysters successfully recovered from the adverse effects of the oil contamination.
In Korea, oysters are produced chiefly from small bays on the south coast, where the oyster industry uses submerged long lines to suspend and raise oysters in an estuarine environment (Choi, 2008). The Pacific oysters are also cultivated in tidal flats on the west coast using an off-bottom rack culture system (Choi, 2008; Jeong et al., 2016). The tidal flats in the Taean coast are the primary oyster farming site on the west coast, where the oyster strings are suspended on a wooden rack installed near the low-tide line. Shortly after the HSOS, oyster farming in this area became suspended until the petroleum hydrocarbon concentrations in oyster tissues returned to the acceptable level. In an attempt to resume the oyster farming in the HSOP areas, Lim et al. (2012) transplanted seed oysters (2–3 mm in SL) from a bay on the south coast to the tidal flats in HSOS areas and cultivated over 12 months in 2010. It was noticeable that the density of oyster larvae in the water column and the spat attached to spat collectors increased dramatically from late summer to early fall in 2010, suggesting that the transplanted oyster spawned and subsequently enhanced the oyster aquaculture in the HSOS areas. Based on the transplantation experiment results, oyster aquaculture was fully resumed in tidal flats in Taean in 2011, and the petroleum carbon levels in the environment and shellfish tissues dropped and returned to the level before the accident (Lim et al., 2012; Yim et al., 2017).
Despite residual oil concentrations in the environment and oyster tissues declining to background within two years after HSOS (Yim et al., 2017), information about the effects of spilled oil on the growth and reproduction of Pacific oysters is still insufficient. In an attempt to understand the recovery of oysters from the HSOS accident, we surveyed the seasonal variation in the shell, somatic tissue growth, the annual gametogenesis, and the reproductive effort of the Pacific oysters cultivated at Euhangri beach, one of the most heavily damaged oyster farming areas by HSOS, from July 2012 to December 2013. Here, we report the growth and reproductive performance of the Pacific oysters raised on off-bottom racks at the HSOS site four years after the accident.
The study site, Euhangri beach (36°50’N, 126° 9’E), is one of the areas heavily damaged by HSOS (Figure 1). According to the report by the Oil and Persistent Organic Pollutants (POPs) research group of Korea Institute of Ocean Science and Technology (KIOST), 16 PAHs in the water column immediately after the incident were as high as 5,710 ng/L. However, 16 PAH levels in the water column dropped dramatically to 100 ng/L only 1 month after the accident (Ministry of Land, Transport and Maritime Affairs, Republic of Korea (MLTM), 2010; Yim et al., 2017). The 16 and alkylated PAH levels in oyster tissue at Euhangri beach in January 2008 reached 412 and 36,970 ng/g respectively (Ministry of Land, Transport and Maritime Affairs, Republic of Korea (MLTM), 2010). However, the residual oil concentrations in the oyster tissue declined dramatically to 51 and 672 ng/g, respectively, 10 months after the incident [Ministry of Land, Transport and Maritime Affairs, Republic of Korea (MLTM), 2010], which is similar to the pre-spilled level.
During the monitoring, sea surface temperature and salinity (SST/SSS) were recorded monthly using a YSI 85 multi-parameter (YSI Inc., USA). In addition, chlorophyll-a level in the water column was referred from the Giovanni database (http://giovanni.gsfc.nasa.gov). The sea surface temperature (SST) ranged from 4.6 (January 2013) to 24.8°C (September 2012), and the salinity varied from 27.2 (March 2013) to 32.3 (August 2013) g/L (Figure 2). The chlorophyll-a level in the water column displayed a clear seasonality with two annual peaks; one in spring (March to April) and the other one in late summer to early autumn (August to September), ranging from 2.25 (August 2012) to 4.49 µg/L (April 2013).
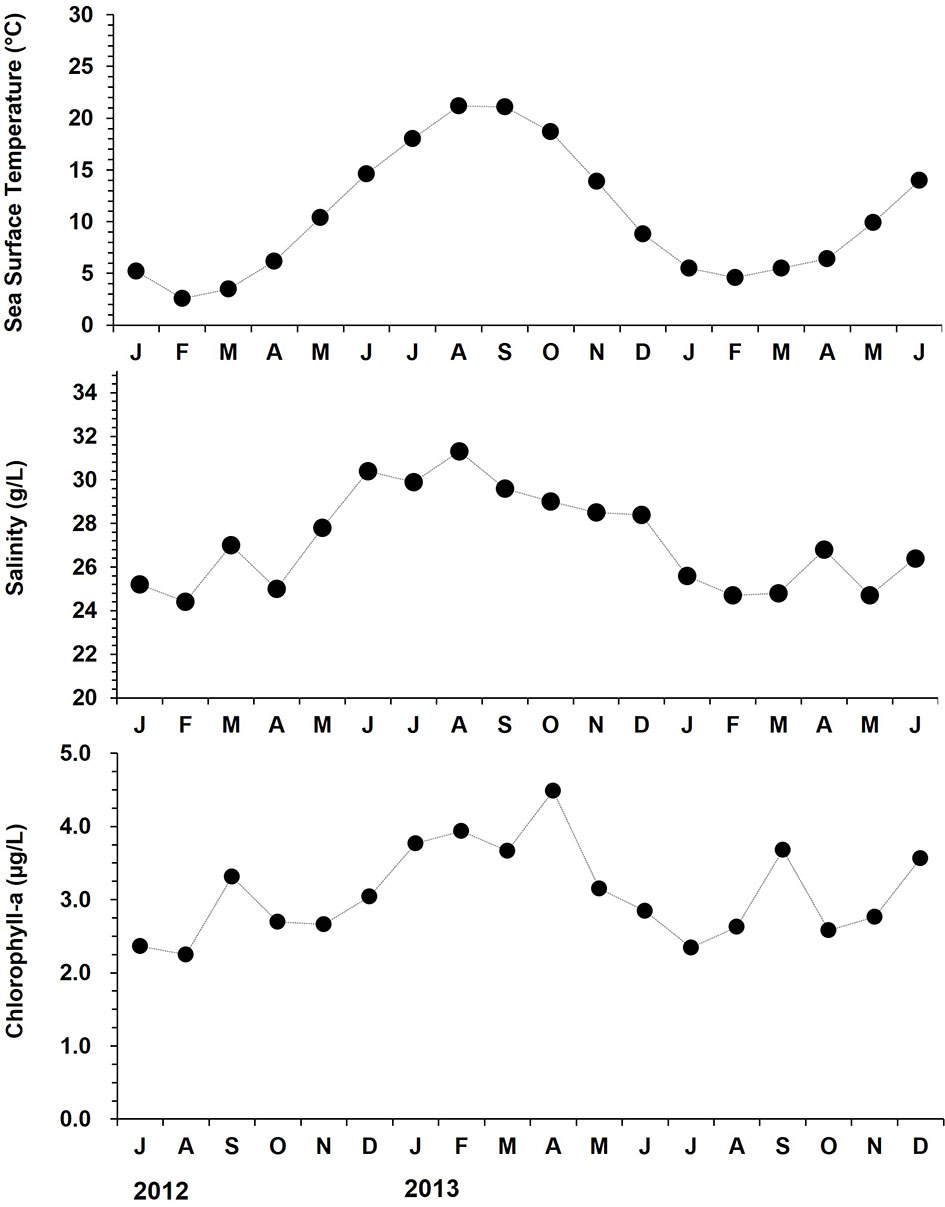
Figure 2 Monthly changes in surface seawater temperature, salinity, and chlorophyll-a level at the study site from July 2012 to December 2013.
The Euhangri oyster farming industry supplies the seeds from adjacent subtidal areas during the post-spawning period in late summer (Lim et al., 2012), using 1.5 m-long plastic wires tied with numerous empty mussel shells as spat collectors. The oyster strings with the oyster larvae are hardened for several months by suspending the strings on wooden racks placed in the upper intertidal area. Then, in May 2012, the hardened seed oysters were transplanted to the racks installed near the low tide line for grow-out. The density of transplanted oysters averaged 119.7 individuals per 1.5 m-long string tied with 10 mussel shells. For the study, we collected 3 oyster strings monthly from July 2012 to December 2013 (Table 1).
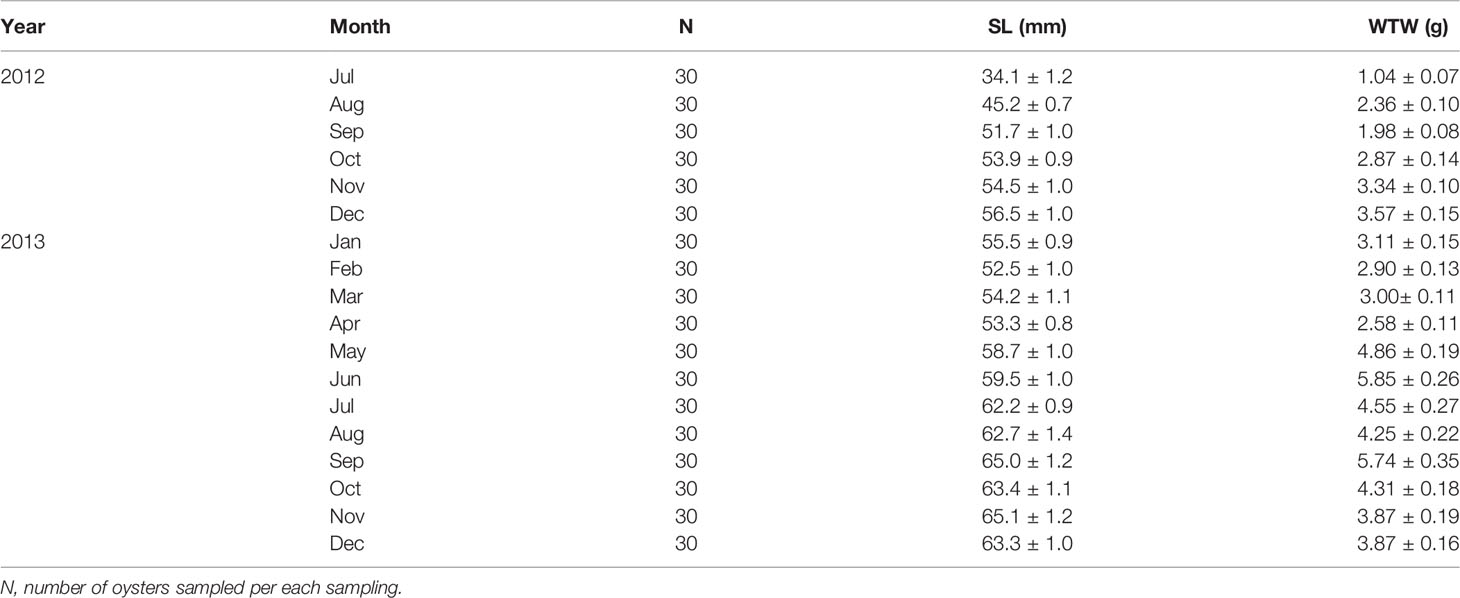
Table 1 Shell length (SL) and wet tissue weight (WTW) of the off-bottom rack cultured oysters at the Euhangri beach from July 2012 to December 2013.
We first counted the total number of oysters attached to the entire string, then identified the fresh dead oysters exhibiting opened shells. Finally, the mortality was calculated as the ratio of the dead oysters to the total oysters. The oysters were separated from the mussel substrate, and barnacles and other epifauna encrusted on the shell surface were removed. Thirty oysters were collected randomly from the 3 oyster strings monthly for further analyses. Shell length (SL), the longest axis of the shell, was assessed using a Vernier caliper. After measuring the total weight using an electronic balance, somatic tissue was separated from the shell and weighed. The oyster shells were dried and weighed to mg using an electronic balance.
For histology, a 3 mm thick dorso-ventral section was cut in the middle of the body and preserved in Davidson’s fixative. The remaining tissues were lyophilized and weighed to determine the dry tissue weight. The lyophilized tissues were homogenized using pestle and mortar then stored at -70°C to measure the egg mass and glycogen content. After dehydrating water in the tissues using a series of ascending ethanol, the tissue sections were embedded in paraffin, sliced to 5 µm, stained with hematoxylin, and counter-stained with eosin Y. The stained tissue sections were examined under a light microscope to identify the sex and evaluate the level of gonad maturation. Based on the microscopic appearance of the gonad, the reproductive stage of individual oysters was categorized into 1) early developing, 2) late developing, 3) ripe, 4) spawning, 5) spent, and 6) resting/resorbing (Kang et al., 2010).
The reproductive effort of female oysters was estimated using the rabbit anti-oyster egg protein antibody developed by Kang et al. (2003) as the primary antibody in an indirect enzyme-linked immunosorbent assay (ELISA). We followed the indirect ELISA assay protocol reported by Kang et al. (2003); Ngo et al. (2006), and Mondol et al. (2016). For the assay, 20–25 mg aliquot of the lyophilized tissue containing the eggs was homogenized using an ultrasonifier and diluted in phosphate-buffered saline (PBS, 0.15 M NaCl, pH 7.6) up to 5,000 times. The egg protein level in the tissue homogenate was estimated from a standard curve that plotted the optical density and known quantity of the Pacific oyster egg protein included in each ELISA plate. Finally, the quantity of the egg mass in each oyster was expressed as the gonad somatic index (GSI), which is a ratio of the dry egg weight to the whole oyster dry tissue weight. The potential fecundity of ripe female oysters prior to spawning was estimated from the ratio of total egg weight measured by ELISA to the weight of an individual egg as 13 ng (Kang et al., 2003).
Glycogen in the homogenized tissue was precipitated with absolute ethanol after extracting in 15% trichloroacetic acid. The glycogen contents were then determined using the phenol-sulfuric acid method described by Dubois et al. (1956). Dextrose anhydrous was used as the standard material in the assay. Finally, glycogen level was expressed as mg of glycogen per g of dry tissue weight (DTW).
We determined the condition index (CI) of oysters as a ratio of the total dry tissue weight (DTW) to the internal shell cavity volume (SCV), CI=(DTW/SCV)x100 (Kang et al., 2010). The whole oyster volume was determined first by submerging a whole oyster in a cylinder filled with a known volume of water. Secondly, we removed the soft body from the oyster then submerged the shells in a cylinder containing a known volume of water. Accordingly, the internal shell cavity volume was determined as the difference between the whole oyster and the empty shell.
Figure 3 shows monthly oyster mortality determined from November 2012 to December 2013. The oyster mortality ranged from 11.3 (July 2013) to 33.3% (November 2013). It was noticeable that the mortality increased from August to November 2013 at a fast rate, which coincided with the high sea surface temperature, spawning, and subsequent post-spawning condition (Figure 3).
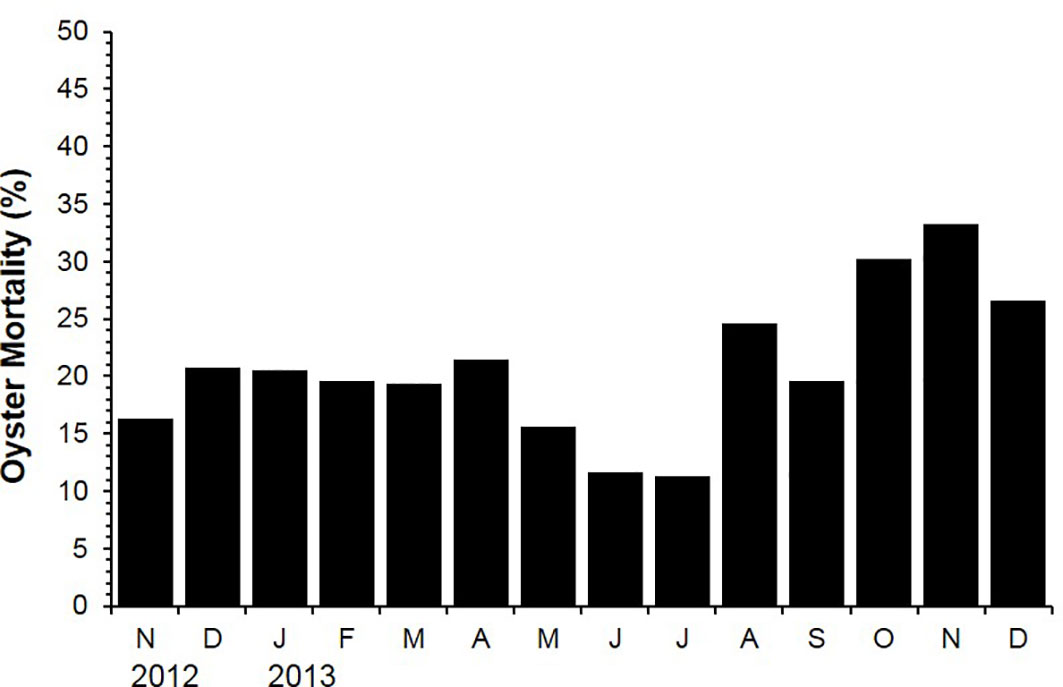
Figure 3 Mortality of the off-bottom rack cultured oysters at the Euhangri beach from July 2012 to December 2013.
Over 18 months of grow-out in the low tide zone, the SL increased from 34.1 (July 2012) to 65.1 mm (November 2013, Figure 4). The off-bottom rack cultured oysters showed a fast shell growth from July to December (56.5 mm), and then the growth remained stable until April 2013. The shell growth also exhibited a linear increase from April (53.3 mm) to September (65.0).
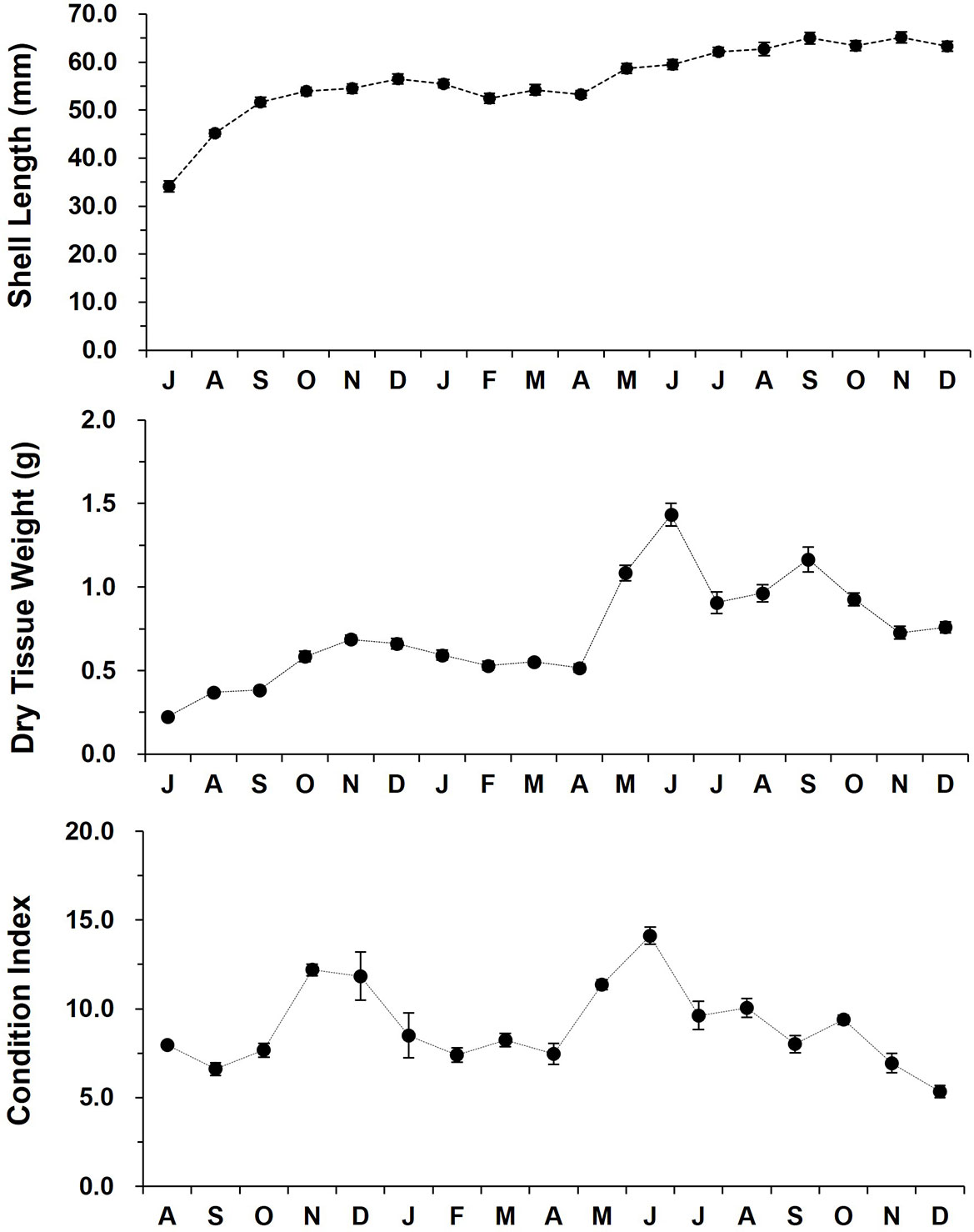
Figure 4 The monthly variation in the shell length, dry tissue weight, and condition index of the off-bottom rack cultured oysters at the Euhangri beach from July 2012 to December 2013. Condition index was calculated as (dry tissue weight/dry shell weight) x 100.
CI showed a distinct inter-annual and seasonal variability. In 2012 fall, oysters showed a dramatic increase in CI from September (6.61) to November (12.19), then CI declined gradually from December to April 2013 (7.45). CI elevated markedly from April to July 2013, reaching an annual highest of 14.12. Lowest CI was recorded in December 2013 as 5.34 (Figure 4).
Histology revealed that the females collected in July 2012 were in the ripe stage and ready to spawn (Figure 5). The females were actively engaged in spawning during August and September as the water temperature reached 24°C. Shortly after the resting stage in December, the females initiated the gonial in January 2013 (4.6°C), and the early developing stage peaked in March and April. In 2013, most of the females spawned during July and August, indicating that the spawning was a month earlier compared to 2012. The annual gametogenesis of the males followed the same pattern of the females.
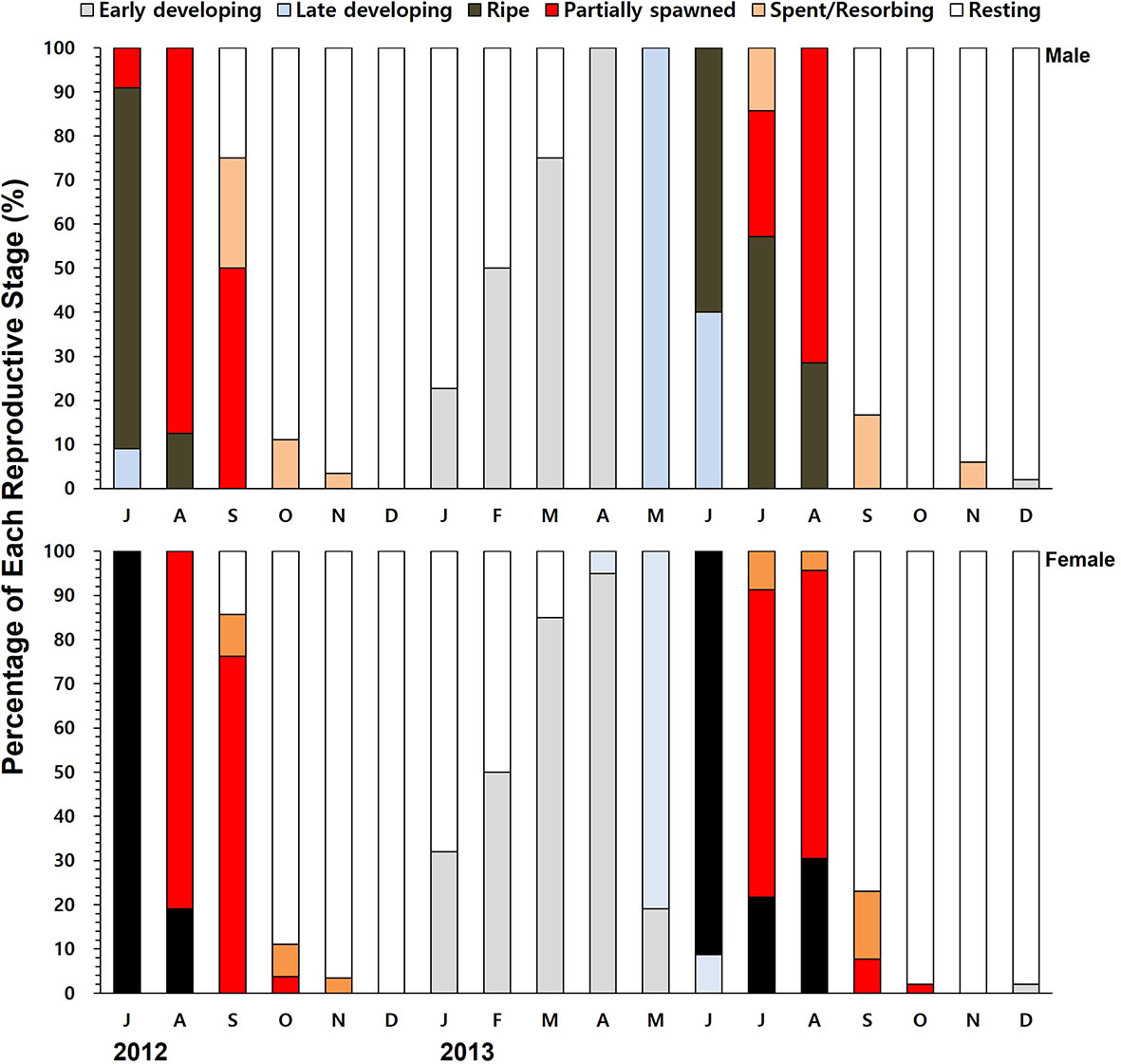
Figure 5 The monthly percentage composition of different gametogenic stages of the males and females.
Indirect ELISA detected the egg proteins in the oysters collected during spawning and post-spawning from July to November in 2012 (Figure 6). In May 2013, the egg proteins could be detected first in May when the females were in the developing stage. GSI of the females collected in July 2012 was 24.2, suggesting that approximately one-year-old oysters produced as much as 24.2% of the body weight as eggs. The GSI dropped dramatically from July (24.2%) to August (11.4%), suggesting that the females spawned during this period. In June 2013, GSI of the females was recorded as 22.0%, which was somewhat compared to the value recorded in July 2012. In 2013, the GSI declined markedly from June (22.0%) to July (10.0%), indicating a massive spawning of the females during this period (Figure 6).
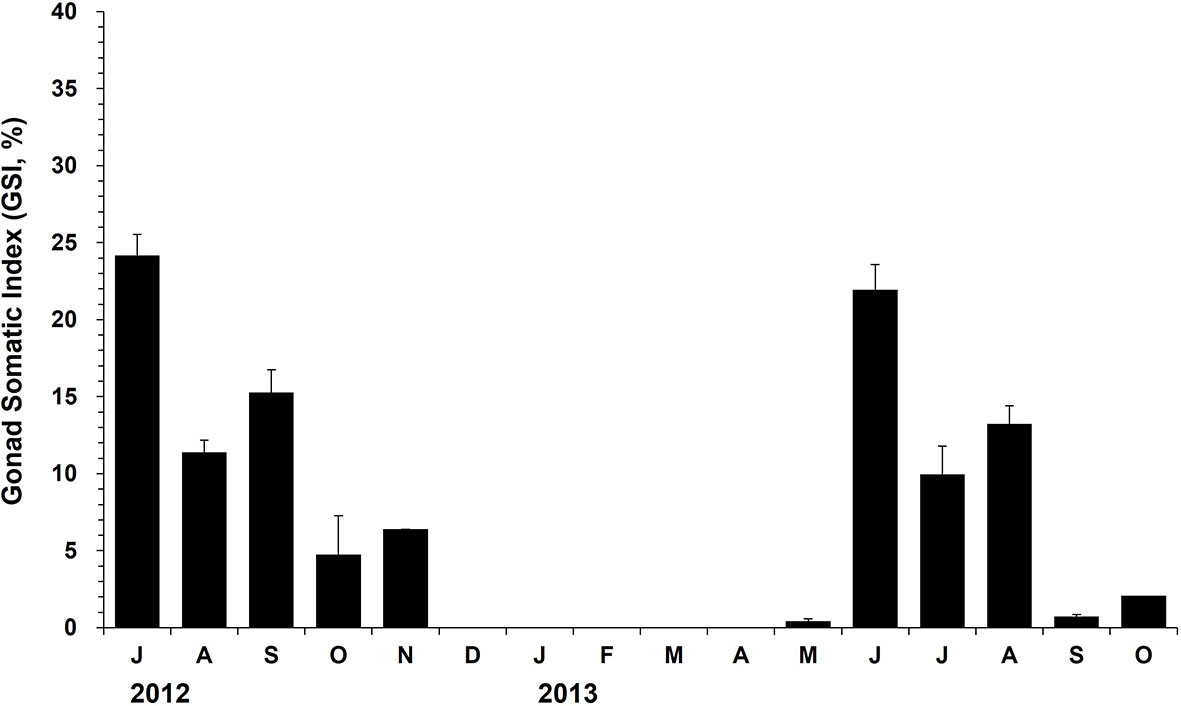
Figure 6 The monthly mean gonad somatic index (GSI) of the female oysters determined using ELISA. The vertical bars on each monthly mean represent the standard error.
The potential fecundity of ripe female oysters varied depending on oyster age. The fecundity was estimated to be 4.1 to 4.5 million eggs for one-year-old oysters, whereas the fecundity ranged from 11.0 to 17.6 million eggs for two-year-old oysters, indicating that the two-year-old produce approximately two to three times more eggs than a year old oysters during the spawning period.
The glycogen level in oyster tissue declined dramatically from August (94.9 mg/g DTW) to September (33.3 mg/g DTW), suggesting high energy catabolism during this period, possibly due to the spawning (Figure 7). The glycogen content elevated slowly during the autumn-winter period (September to December). The glycogen level increased markedly from April (36.5 mg/g DTW) to May (116.6 mg/g DTW) in 2013 when the chlorophyll-a level in the water column increased. From May to July, the level declined noticeably from 116.6 mg/g DTW to 39.7 mg/g DTW, which was coincided with the advance of gonad maturation and subsequent spawning.
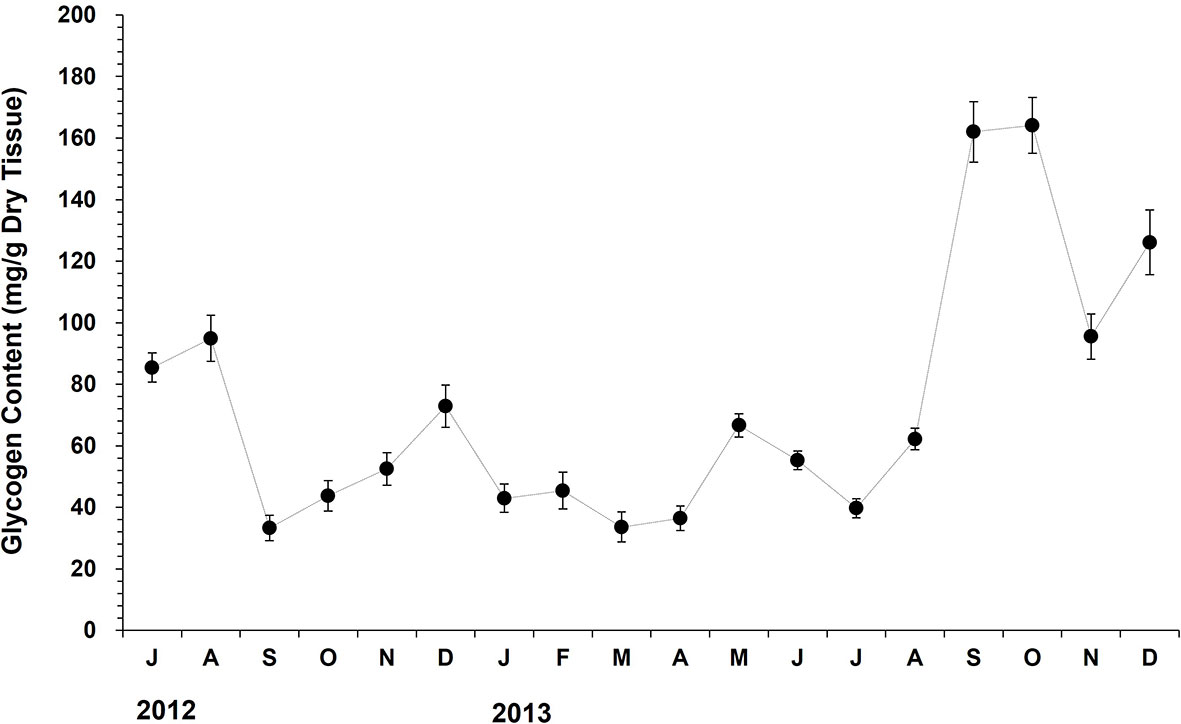
Figure 7 The monthly variation in the tissue glycogen content of the off-bottom rack cultured oysters at the Euhangri beach from July 2012 to December 2013. The vertical bars on the monthly mean represent the standard error.
One year after the Hebei Spirit oil spill, the level of residual oil in the water, sediment, and oyster tissues in the oil spill area became similar to the level before the accident (Yim et al., 2017). Although the low PAH levels in water and sediment were too low to be lethal to oysters, long-term sub-lethal effects on growth and reproduction were unknown. Mondol et al. (2015) demonstrated the recovery of the wild Pacific oyster, C. gigas encrusting on the rocky intertidal shore in the oil-contaminated area in terms of growth. They also examined the reproductive performance of the wild oysters by monitoring the annual gametogenesis, reproductive effort, and energy storage approximately two years after the HSOS accident. The data revealed that in 2010, the shell and somatic tissue growth, annual gametogenic pattern, reproductive effort, and glycogen level of the oysters in the oil impacted area were similar or higher than those in the control site.
Growth and mortality are two main factors that directly govern the success of the oyster culture. In Korean and Japanese oyster industries, the oyster spats are hardened in the intertidal area for up to six months before they are transplanted onto the long-line culture system to minimize the mortality during growth-out (Ventilla, 1984; Ngo et al., 2006; Kang et al., 2010; Mondol et al., 2016). Ventilla (1984) reported that the mortality of hardened oysters in Hiroshima water during the grow-out was only 10–30%, whereas mortality of non-hardened ordinary oysters reached 80%. Similarly, Park et al. (1988) reported mortality of the hardened oysters during the growth-out in subtidal suspended culture system as 18.2%, which is much lower than mortality of non-hardened oysters as 42.6% (Park et al., 1988). In this study, the mortality rate of the hardened oysters over a two-year culture period ranged from 11.3 to 33.3%, which is comparable to the rate observed by Park et al. (1988). In 2013, the mortality elevated from August onwards and peaked in November at 33.3%. High mortality of oysters observed in the 2013 fall coincided with the post-spawning condition, which could be a consequence of high water temperature and spawning stresses in late summer (Mondol et al., 2016). Several studies have reported that the high summer mortality rate of oysters is often coupled with high temperature and high level of physiological stresses (Berthelin et al., 2000; Cho and Jeoung, 2005; Delaporte et al., 2006; Li et al., 2007; Huvet et al., 2010).
The hardened Pacific oyster spats reached 6.5 cm in SL and 1.43 g in TDW after 12 mo of growth-out in the intertidal suspended long line system, which is quite similar to the shell growth reported by Lim et al. (2012). According to Lim et al. (2012), the transplanted Pacific oysters (mean SL 2.8 cm) raised on off-bottom racks over 12 months at the Euhangri oil spill site in 2010 reached a mean shell length of 6.1 cm, suggesting that there is no significant difference in the shell growth between 2010 and 2012 at the study site. However, the oyster shell growth observed in this study is lower than that of oysters recorded in small bays on the south coast. According to Oh et al. (2002), the mean SL of C. gigas in Goseong Bay off the south coast of Korea reached 8.5 cm after ten months of grow-out in subtidal long-lines. Mondol et al. (2016) also reported the shell growth of the Pacific oyster raised using submerged long-lines on the south coast as 8.1 cm after seven months of grow-out. The slow shell growth rate observed in this study can be, in part, explained by a relatively shorter period of feeding due to recurrent exposure to the atmosphere during low tide. In this study, the oyster strings are suspended on steel racks installed near the low tide line, and we estimate that the intertidal oysters are 20-30% less submerged than those in the subtidal long-lines leading to the observed difference in the shell growth. Bishop and Peterson (2006) also reported a slow shell growth in the triploid Suminoe oysters raised in a tidal creek in North Carolina compared to the oysters cultured in the subtidal rack system. Although the shell growth of the oyster used in this study was relatively slow, the DTW of oysters recorded at the end of the study (1.2g) is considered to be a marketable size (Lee et al., 2018).
Marine bivalves exposed to hydrocarbons often show extended time in gonad maturation and decreased reproductive effort, as the hydrocarbons interrupt the physiological processes (Bayne et al., 1982; McDowell et al., 1999; Chu et al., 2003; Ortiz-Zarragoitia et al., 2011). In 2008 summer, a few months after the HSOS accident, Lee (2010) also observed declined reproductive effort and delayed spawning of the Pacific oysters exposed to the crude oils at the Shinduri coast, a few kilometers apart from the study site. However, according to Lee (2016), such sub-lethal impacts on oyster reproduction were no longer observed two to three years after the accident, coinciding with the lowered level of hydrocarbons in the oyster tissues. Mondol et al. (2015) also examined the annual gametogenesis of the wild Pacific oysters occurring on a rocky shore near the present study site. Two years after HSOS, the wild oysters at the damaged site demonstrated superior growth and reproductive performance than the control oysters, suggesting that oysters achieved a certain level of recovery in their ecophysiology.
Histology revealed that the off-bottom rack cultured oysters at the Euhangri beach spawned in summer, from July to September, when SST ranged from 22 to 24°C. Mondol et al. (2015) also reported the spawning period of the wild Pacific oysters collected from a rocky shore near the present study site in 2010, two years after HSOS, as August and September when SST was in a range of 22 to 25°C. Mondol et al. (2016) also reported that the Pacific oysters suspended on submerged long-lines on the south coast spawn from June to September with a peak in August as SST reached 24°C. Accordingly, we believe that the off-bottom rack cultured oysters at the Euhangri beach recovered from HSOS stresses four years after the accident, as the annual gametogenic pattern followed that of unimpacted oysters on the west and south coast.
We determined the reproductive effort of oysters using indirect ELISA, which provides the quantity of the eggs in an individual oyster and expressed as GSI, a ratio of the egg mass to the dry tissue weight (Kang et al., 2003; Ngo et al., 2006; Mondol et al., 2015; Mondol et al., 2016). In an annual reproductive cycle, oysters show their highest GSI when they are fully ripe and ready to spawn, although the values vary spatio-temporally (Ngo et al., 2006; Mondol et al., 2015; Mondol et al., 2016). The monthly highest GSIs recorded in this study, 24.2% (July 2012) and 22.0% (June 2013), are somewhat comparable to the GSIs reported from the west coast by Jeong et al. (2016) as 22.2%, although it is much lower than those of the wild oysters from HSOS site reported by Mondol et al. (2015) as 43.7 and 48.1%, respectively (Table 2). Royer et al. (2008) also the reproductive effort of the Pacific oysters reared in intertidal off-bottom bags in the Bay of Veys (Normandy, France). They reported GSIs of the one-year-old female as 36% and two-year-old as 61.0%, which are markedly higher than the GSIs assessed in this study (Table 2). These differences in the reproductive effort could, in part, be explained by the different food levels in the environment. According to Hofmann et al. (1992); Hofmann et al. (1994) and Enríquez-Díaz et al. (2009), food availability in the water column is one of the main factors determining oysters’ reproductive effort. Compared to the annual range and the highest chlorophyll-a level observed in the Bay of Veys (Enríquez-Díaz et al., 2009) is noticeably higher than those observed in this study.
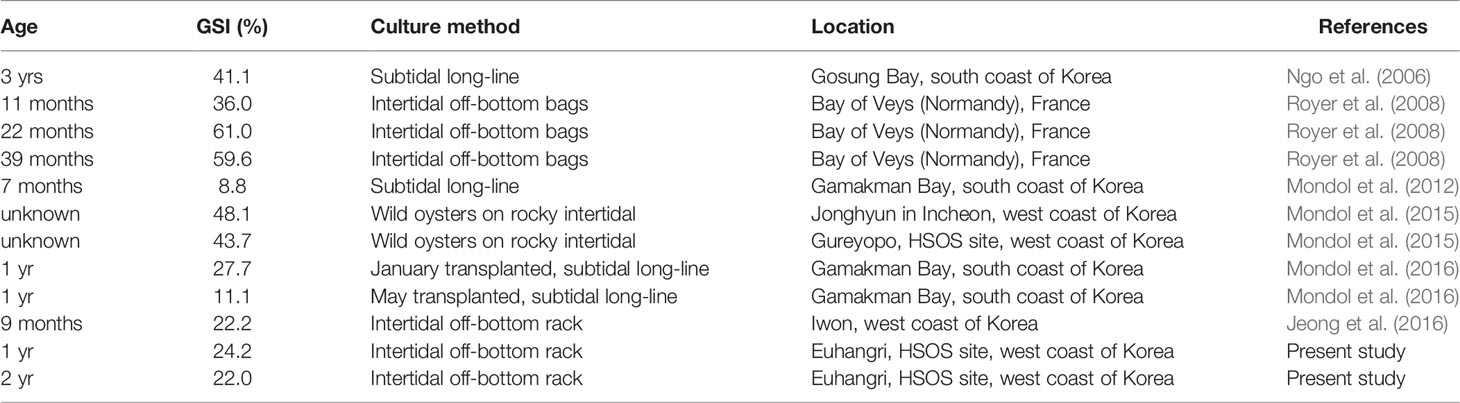
Table 2 Summary of the gonad somatic index (GSI) and fecundity of female Crassostrea gigas previously reported from elsewhere and the present study.
We also calculated CI of C. gigas as a ratio of the tissue weight to the shell weight since numerous studies have evaluated CI of the Pacific oyster using this ratio. As the Table 3 shows, the annual CI range of C. gigas reared in this study, from 3.9 to 8.7 is similar to the CIs of oysters raised in a tidal flat on the west coast as 1.7 to 8.6 (Lim et al., 2014), or somewhat higher than the values reported from Marennes–Oléron Bay, France (Patrick et al., 2006). However, CI of oysters observed in this study is lower than the oysters cultured in New Zealand using subtidal long-line, from 5.3 to 12.1. Such variance in CI of oysters could be, again, attributable to the environmental factors including the water temperature and food availability in the environment (Hofmann et al., 1992; Hofmann et al., 1994, Hyun et al., 2001).
The reproductive effort of oysters is also age- and size-dependent, as larger and older oysters produce more eggs (Hofmann et al., 1994; Royer et al., 2008), although the age-dependent GSI was not recognized in this study. In the Bay of Veys in France, the GSIs of two to three-year-old oysters C. gigas (59.6–61.0%) are markedly higher than one-year-old oyster (36.0%). Such a positive correlation between the age and GSI in oysters has also been reported from the south coast, where the Pacific oysters are cultured using submerged suspended long-lines. The GSIs estimated by indirect ELISA increased with age, as Mondol et al. (2012) reported GSI of 7-month-old oysters as 8.8%, 27.7% in 1-year-old oysters (Mondol et al., 2016), and 41.1% in 3-year-old oysters (Ngo et al., 2006). According to Hoffman et al. (1994), the young oysters may preferably allocate more net energy to shell and somatic tissue growth, whereas more aged oysters devote comparatively more net energy to reproduction.
CI is widely used as an aquaculture performance indicator and an overall health index of marine bivalves (Lawrence and Scott, 1982; Lucas and Beninger, 1985; Brown and Hartwick, 1988; Filgueira et al., 2013). CI of marine bivalves is often defined as a ratio of the somatic tissue weight to the shell weight or length (Lucas and Beninger, 1985). We determined CI of oysters as a ratio of the somatic tissue weight to the internal shell cavity volume since the oyster-shell shape is irregular and the shell weight varies widely (Lawrence and Scott, 1982; Abbe and Albright, 2003; Kang et al., 2010). In Table 3 and Figure 8, we summarize CIs of the Pacific oysters reported from elsewhere to evaluate the growth performance of oysters in Euhangri beach four years after HSOS. The annual CI ranges recorded in this study, 5.3–12.2, is comparable to the CI of cultured (4.0 to 12.2, Jeong et al., 2016) and wild oysters (5.2 to11.5, Mondol et al., 2015) reported from the west coast of Korea, suggesting that the overall health or growth performace of oyster observed in this study is normal.
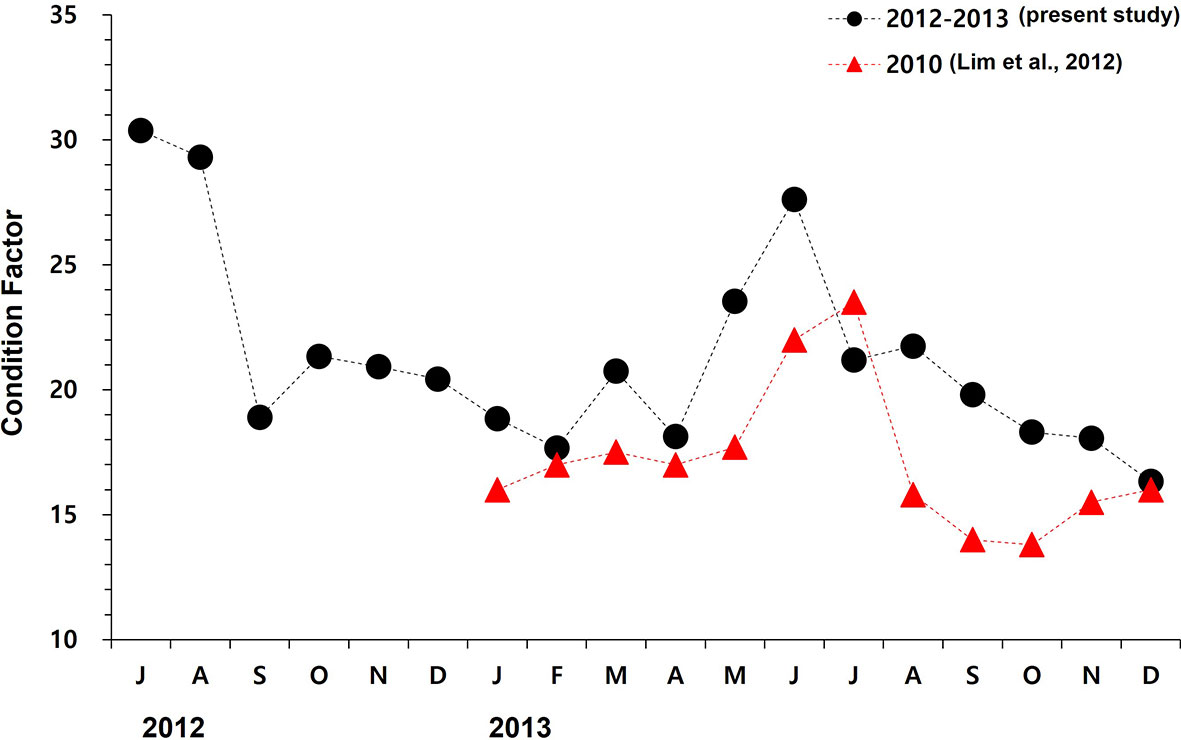
Figure 8 The monthly mean condition factor (i.e., ((wet tissue weight/total weight) x 100) of oysters cultured in this study (July 2012 to December 2013) and the data reported by Lim et al. (2012) measured from the study site Euhangri beach.
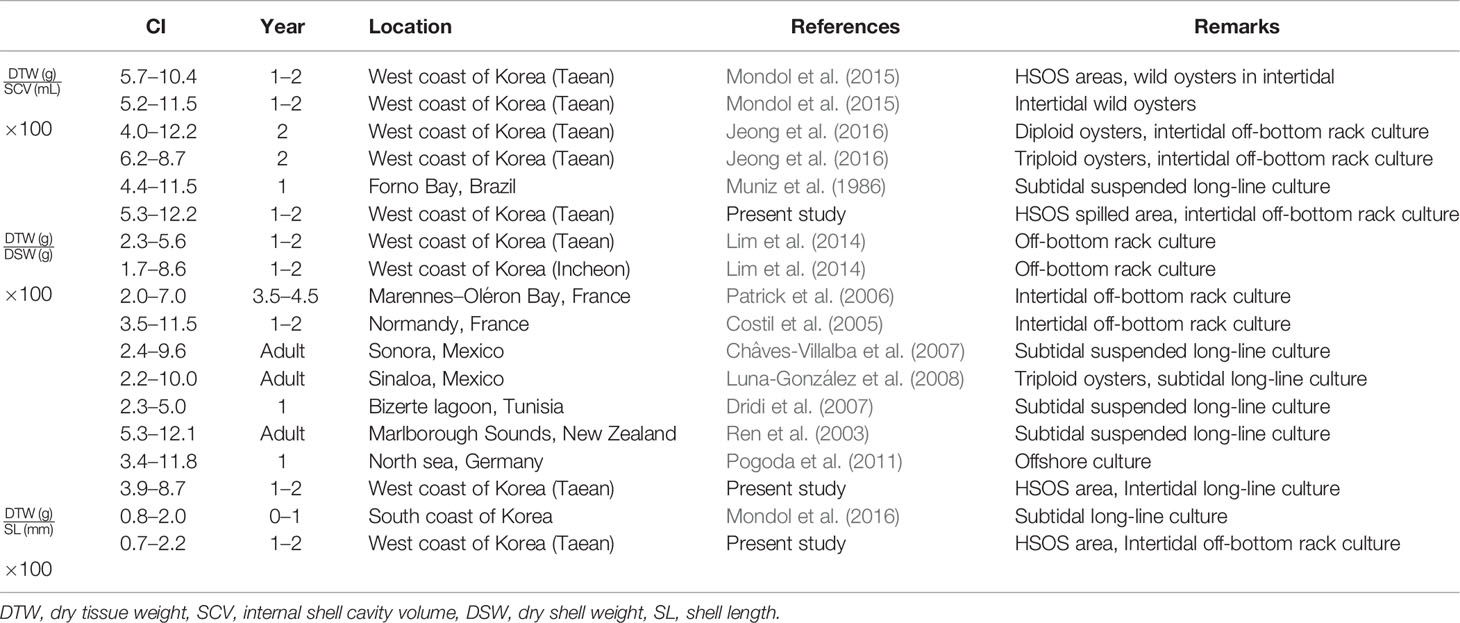
Table 3 Summary of the condition index (CI) of Crassostrea gigas previously reported from elsewhere and the present study.
The present study revealed that the Pacific oysters raised in intertidal off-bottom rack culture system in Euhangri in Taean off the west coast of Korea, one of the most heavily damaged beached by the HSOS, successfully recovered from the adverse effects of petroleum contamination with respect to growth and reproduction. CI, the oyster health or culture performance index recorded from the Euhangri tidal flat 4 years after HSOS were also comparable to those of oysters observed from other tidal flats on the west and south coasts of Korea, supporting the idea that oysters at HSOS site recovered from physiological stresses caused by the petrolium carbon contamination.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
H-KH, H-SP, and K-SC designed this study. H-KH processed and analyzed the datasets and worked on interpretations of the results with K-SC. H-KH wrote the manuscript. K-SC reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Korea Ministry of Education (2019R1A6A1A03033553). This study was also, in part, supported by the funding from the Ministry of Oceans and Fisheries of Korea, through a project, "Responses of Species-Populations to Climate Change Scenario (20220559).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the staff of the Shellfish Aquaculture and Research Laboratory at Jeju National University for their assistance in sampling and laboratory work.
Abbe G. R., Albright B. W. (2003). An Improvement to the Determination of Meat Condition Index for the Eastern Oyster Crassostrea Virginica (Gmelin 1791). J. Shellfish. Res. 22 (3), 747–752.
Bayne B. L., Widdows J., Moore M. N., Salkeld P., Worral C. M., Donkin P. (1982). Some Ecological Consequences of the Physiological and Biochemical Effects of Petroleum Compounds on Marine Molluscs. Philos. Trans. R. Soc Lond. B.: Biol. Sci. 297, 219–238. doi: 10.1098/rstb.1982.0039
Berthelin C., Kellner K., Mathieu M. (2000). Storage Metabolism in the Pacific Oyster (Crassostrea Gigas) in Relation to Summer Mortalities and Reproductive Cycle (West Coast of France). Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 125, 359–369. doi: 10.1016/S0305-0491(99)00187-X
Bishop M. J., Peterson C. H. (2006). Direct Effects of Physical Stress can be Counteracted by Indirect Benefits: Oyster Growth on a Tidal Elevation Gradient. Oecologia 147 (3), 426–433. doi: 10.1007/s00442-005-0273-3
Brown J. R., Hartwick E. B. (1988). Influence of Temperature, Salinity and Available Food Upon Suspended Culture of the Pacific Oyster, Crassostrea Gigas: II. Condition Index and Survival. Aquaculture 70 (3), 253–267. doi: 10.1016/0044-8486(88)90100-7
Chávez-Villalba J., Villelas-Ávila R., Cáceres-Martínez C. (2007). Reproduction, Condition and Mortality of the Pacific Oyster Crassostrea gigas (Thunberg) in Sonora, México. Aquac. Res. 38 (3), 268–278. doi: 10.1111/j.1365-2109.2007.01662.x
Choi K. S. (2008). "Oyster Capture-Based Aquaculture in the Republic of Korea" in Capture-Based Aquaculture: Global Overview. Eds. Lovatelli A., Holthus P. E. (Rome: Food and Agriculture Organization of the United Nations), 271–286.
Cho S., Jeoung W. G. (2005). Spawning Impact on Lysosomal Stability of the Pacific Oyster, Crassostrea Gigas. Aquaculture 244, 383–387. doi: 10.1016/j.aquaculture.2004.12.013
Chu F. E., Soudant P., Hale R. C. (2003). Relationship Between PCB Accumulation and Reproductive Output in Conditioned Oysters Crassostrea Virginica Fed a Contaminated Algal Diet. Aquat. Toxicol. 65, 293–307. doi: 10.1016/S0166-445X(03)00152-8
Costil K., Royer J., Ropert M., Soletchnik P., Mathieu M. (2005) Spatio-Temporal Variations in Biological Performances and Summer Mortality of the Pacific Oyster Crassostrea gigas in Normandy (France). Helgol. Mar. Res. 59, 286–300. doi: 10.1007/s10152-005-0004-5
Delaporte M., Soudant P., Lambert C., Moal J., Pouvreau S., Samain J. F. (2006). Impact of Food Availability on Energy Storage and Defense Related Hemocyte Parameters of the Pacific Oyster Crassostrea Gigas During an Experimental Reproductive Cycle. Aquaculture 254, 571–582. doi: 10.1016/j.aquaculture.2005.10.006
Donaghy D., Hong H. K., Lee H. J., Jun J. C., Park Y. J., Choi K. S. (2010). Hemocyte Parameters of the Pacific Oyster, Crassostrea Gigas, a Year After the Hebei Spirit Oil Spill Off the West Coast of Korea. Helgol. Mar. Res. 64, 349–355. doi: 10.1007/s10152-010-0190-7
Dubois M., Gilles K. A., Hamilton J. K., Rebers P. A., Smith F. (1956). Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 28 (3), 350–356. doi: 10.1021/ac60111a017
Dridi S., Romdhane M. S., Elcafsi M. (2007). Seasonal Variation in Weight and Biochemical Composition of the Pacific Oyster, Crassostrea gigas in Relation to the Gametogenic Cycle and Environmental Conditions of the Bizert lagoon, Tunisia. Aquaculture 263, 238–248. doi: 10.1016/j.aquaculture.2006.10.028
Enríquez-Díaz M., Pouvreau S., Chávez-Villalba J., Le Pennec M. (2009). Gametogenesis, Reproductive Investment, and Spawning Behavior of the Pacific Giant Oyster Crassostrea Gigas: Evidence of an Environment-Dependent Strategy. Aquac. Int. 17, 491–506. doi: 10.1007/s10499-008-9219-1
Filgueira R., Comeau L. A., Landry T., Grant J., Guyondet T., Mallet A. (2013). Bivalve Condition Index as an Indicator of Aquaculture Intensity: A Meta-Analysis. Ecol. Indic. 25, 215–229. doi: 10.1016/j.ecolind.2012.10.001
Hofmann E. E., Klinck J. M., Powell E. N., Boyles S., Ellis M. (1994). Modeling Oyster Populations II. Adult Size and Reproductive Effort. J. Shellfish. Res. 13, 165–182.
Hofmann E. E., Powel E. N., Klinck J. M., Wilson E. A. (1992). Modeling Oyster Populations III. Critical Feeding Peridos, Growth and Reproduction. J. Shellfish. Res. 11, 399–416.
Huvet A., Normand J., Fleury E., Quillien V., Fabious C., Boudry P. (2010). Reproductive Effort of Pacific Oyster: A Trait Associated With Susceptibility to Summer Mortality. Aquaculture 304, 95–99. doi: 10.1016/j.aquaculture.2010.03.022
Hyun K.-H., Pang I.-C., Klinck J. M., Choi K. S., Lee J.-B., Powell E. N., et al. (2001). The Effet of Food Composition on Pacific Oyster Crassostrea Gigas (Thunberg) Growth in Korea: A Modelling Study. Aquaculture 199, 41–62. doi: 10.1016/S0044-8486(01)00509-9
Jeong H. D., Keshavmurthy S., Lim H. J., Kim S. K., Choi K. S. (2016). Quantification of Reproductive Effort of the Triploid Pacific Oyster, Crassostrea Gigas Raised in Intertidal Rack and Bag Oyster Culture System Off the West Coast of Korea During Spawning Season. Aquaculture 464, 374–380. doi: 10.1016/j.aquaculture.2016.07.010
Kang S. G., Choi K. S., Bulgakov A. A., Kim Y., Kim S. Y. (2003). Enzyme-Linked Immunosorbent Assay (ELISA) Used in Quantification of Reproductive Output in the Pacific Oyster, Crassostrea Gigas, in Korea. J. Exp. Mar. Biol. Ecol. 282, 1–21. doi: 10.1016/S0022-0981(02)00444-6
Kang D. H., Chu F. L. E., Yang H. S., Lee C. H., Koh H. B., Choi K. S. (2010). Growth, Reproductive Condition, and Digestive Tubule Atrophy of Pacific Oyster Crassostrea Gigas in Gamakman Bay Off the Southern Coast of Korea. J. Shellfish. Res. 29 (4), 1–7. doi: 10.2983/035.029.0418
Kim M., Hong S. H., Won J., Yim U. H., Jung J. H., Ha S. Y., et al. (2013). Petroleum Hydrocarbon Contaminations in the Intertidal Seawater After the Hebei Spirit Oil Spill – Effect of Tidal Cycle on the TPH Concentrations and the Chromatographic Characterization of Seawater Extracts. Water Res. 47, 758–768. doi: 10.1016/j.watres.2012.10.050
Kim M., Yim U. H., Hong S. H., Jung J. H., Choi H. W., An J., et al. (2010). Hebei Spirit Oil Spill Monitored on Site by Fluorometric Detection of Residual Oil in Coastal Waters Off Taean, Koera. Mar. Pollut. Bull. 60, 383–389. doi: 10.1016/j.marpolbul.2009.10.015
Lawrence D. R., Scott G. I. (1982). The Determination and Use of Condition Index of Oysters. Estuaries 5, 23–27. doi: 10.2307/1352213
Lee H. J. (2010). Impacts of 2007 Hebei Spirit Oil Spill on Reproductive Physiology of Pacific Oyster, (Crassostrea Gigas Thunberg 1973) in Taean, Off the West Coast of Korea (Jeju: Jeju National University).
Lee H. J. (2016). Recovery of Nutrient Storage and Reproductive Physiology of Cultured Pacific Oyster Crassostrea Gigas (Tunberg 1793) 2 Years After the Hebei Spirit Oil Spill (Jeju: Jeju National University).
Lee Y. J., Han E., Wilberg M. J., Lee W. C., Choi K. S., Kang C. K. (2018). Physiological Processes and Gross Energy Budget of the Submerged Longline-Cultured Pacific Oyster Crassostrea Gigas in a Temperate Bay of Korea. PloS One 13 (7), e0199752. doi: 10.1371/journal.pone.0199752
Li Y., Qin J. G., Abbott C. A., Li X., Benkendorff K. (2007). Synergistic Impacts of Heat Shock and Spawning on the Physiology and Immune Health of Crassostrea Gigas: An Explanation for Summer Mortality in Pacific Oysters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R2253–R2262. doi: 10.1152/ajpregu.00463.2007
Lim H. J., Back S. H., Lim M. S., Choi E. H., Kim S. K. (2012). Regional Variations in Pacific Oyster, Crassostrea Gigas Growth and the Number of Larvae Occurrence and Spat Settlement Along the West Coast, Korea. Kor. J. Malacol. 28 (3), 259–267. doi: 10.9710/kjm.2012.28.3.259
Lim H. J., Lim M. S., Lee W. Y., Choi E. H., Yoon J. H., Park S. Y., Lee S. M., et al. (2014). Condition Index and Hemocyte Apoptosis as a Heath Indicator for the Pacific Oysters, Crassostrea gigas Cultured in the Western Coastal Waters of Korea. Kor. J. Malacol. 30 (3), 189–196. doi: 10.9710/kjm.2014.30.3.189
Lucas A., Beninger P. G. (1985). The Use of Physiological Condition Indices in Marine Bivalve Aquaculture. Aquaculture 44 (3), 187–200. doi: 10.1016/0044-8486(85)90243-1
Luna-González A., Romero-Geraldo M. D.J., Campa-Córdova Á., Orduña-Rojas J., Valles-Jiménez R., Ruíz-Verdugo C. A., et al. (2008). Seasonal Variations in the Immunological and Physiological Parameters of the Pacific Oyster Crassostrea gigas Cultured in Bahía de Macapule (Sinaloa, Mexico). Aqua. Res. 39 (14), 1488–1497. doi: 10.1111/j.1365-2109.2008.02017.x
McDowell J. E., Lancaster B. A., Leavitt D. F., Rantamaki P., Ripley B. (1999). The Effects of Lipophilic Organic Contaminants on Reproductive Physiology and Disease Processes in Marine Bivalve Molluscs. Limnol. Oceanogr. 44, 903–909. doi: 10.4319/lo.1999.44.3_part_2.0903
Ministry of Land, Transport and Maritime Affairs, Republic of Korea (MLTM). (2009). “Environmental Impact Assessment of the Hebei Spirit Oil Spill”, in MLTM Reports Number 11-1611000-000392-01 (in Korean With English Summary). (Seoul: MLTM).
Ministry of Land, Transport and Maritime Affairs, Republic of Korea (MLTM). (2010). “Environmental Impact Assessment of the Hebei Spirit Oil Spill”, in MLTM Reports Number 11-1611000-001143-01 (in Korean With English Summary). (Seoul: MLTM).
Mondol M. R., Keshavmurthy S., Lee H. J., Hong H. K., Park H. S., Park S. R., et al. (2015). Recovery of Wild Pacific Oyster, Crassostrea Gigas in Terms of Reproduction and Gametogenesis Two-Years After the Hebei Spirit Oil Spill Accident Off the West Coast of Korea. Cont. Shelf. Res. 111, 333–341. doi: 10.1016/j.csr.2015.08.019
Mondol M. R., Kim C. W., Kang C. K., Park S. R., Noseworthy R. G., Choi K. S. (2016). Growth and Reproduction of Early Growth-Out Hardened Juvenile Pacific Oysters, Crassostrea Gigas in Gamakman Bay, Off the South Coast of Korea. Aquaculture 463, 224–233. doi: 10.1016/j.aquaculture.2016.05.047
Mondol M. R., Kim C. W., Kim B. K., Kang C. K., Choi K. S. (2012). Early Growth and Reproduction of Hatchery-Produced Pacific Oyster Crassostrea Gigas in Gamakman Bay Off the Southern Coast of Korea. Fish. Sci. 78, 1285–1292. doi: 10.1007/s12562-012-0553-x
Muniz E. C., Jacob S. A., Helm M. M. (1986). Condition Index, Meat Yield and Biochemical Composition of Crassostrea Brasiliana and Crassostrea Gigas Grown in Cabo Frio, Brazil. Aquaculture 59, 235–250. doi: 10.1016/0044-8486(86)90006-2
Ngo T. T. T., Kang S. G., Kang D. H., Sorgeloos P., Choi K. S. (2006). Effect of Culture Depth on the Proximate Composition and Reproduction of the Pacific Oyster, Crassostrea Gigas, From Gosung Bay, Korea. Aquaculture 253, 712–720. doi: 10.1016/j.aquaculture.2005.09.009
Oh K. H., Pang I. C., Hogmann E. E., Kim Y., Kim S. Y., Park Y. J., et al. (2002). Modeling Oyster Population Dynamics I. Effects of Available Food on Growthz of the Pacific Oyster Crassostrea Gigas in Goseong Bay, Korea. J. Kor. Fish. Soc 35, 327–335. doi: 10.5657/kfas.2002.35.4.327
Ortiz-Zarragoitia M., Garmendia L., Barbero M. C., Serrano T., Marigomez I., Cajaraville M. P. (2011). Effects of the Fuel Oil Spilled by the Prestige Tanker on Reproduction Parameters of Wild Mussel Populations. J. Environ. Monit. 13, 84–94. doi: 10.1039/C0EM00102C
Park B. H., Park M. S., Kim B. Y., Hur S. B., Kim S. H. (1988). Culture of the Pacific Oyster (Crassostrea Gigas) in the Republic of Korea. Available at: https://www.fao.org/3/ab706e/AB706E00.htm#TOC
Patrick S., Faury N., Goulletquer P. (2006). Seasonal Changes in Carbohydrate Metabolism and Its Relationship with Summer Mortality of Pacifc Oysters Crassostrea gigas (Thunberg) in Marennes-Oléron Bay (France). Aquaculture 252, 328–338. doi: 10.1016/j.aquaculture.2005.07.008
Pogoda B., Buck B. H., Hagen W. (2011). Growth Performance and Condition of Oysters (Crassostrea gigas and Ostrea edulis) Farmed in an Offshore Environment (North Sea, Germany). Aquaculture 319, 484–492. doi: 10.1016/j.aquaculture.2011.07.017
Ren J. S., Marsden I. D., Ross A. H., Schiel D. R. (2003). Seasonal Variation in the Reproductive Activity and Biochemical Composition of the Pacific Oyster (Crassostrea gigas) from the Marlborough Sounds, New Zealand. N. Z. J. Mar. Freshwater Res. 37, 171–182. doi: 10.1080/00288330.2003.9517155
Royer J., Seguineau C., Park K. I., Pouvreau S., Choi K. S., Costil K. (2008). Gametogenetic Cycle and Reproductive Effort Assessed by Two Methods in 3 Age Classes of Pacific Oysters, Crassostrea Gigas, Reared in Normandy. Aquaculture 277, 313–320. doi: 10.1016/j.aquaculture.2008.02.033
Ventilla R. F. (1984). Recent Developments in the Japanese Oyster Culture Industry. Adv. Mar. Biol. 21, 1–57. doi: 10.1016/S0065-2881(08)60098-X
Keywords: Hebei Spirit oil spill, intertidal off-bottom rack culture, growth, reproductive cycle, reproductive effort, Crassostrea gigas
Citation: Hong H-K, Park H-S and Choi K-S (2022) Growth and Reproduction of the Pacific Oyster Crassostrea gigas Cultured on Tidal Flat in Hebei Spirit Oil Spill Area on the West Coast of Korea Four Years After the Accident. Front. Mar. Sci. 9:880210. doi: 10.3389/fmars.2022.880210
Received: 21 February 2022; Accepted: 03 May 2022;
Published: 20 May 2022.
Edited by:
Wei Huang, Ministry of Natural Resources, ChinaReviewed by:
Mohamed Mohsen, Institute of Oceanology, ChinaCopyright © 2022 Hong, Park and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kwang-Sik Choi, c2tjaG9pQGplanVudS5hYy5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.