
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 22 July 2022
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.865390
This article is part of the Research TopicThe Ecological Function of MaricultureView all 14 articles
Shellfish and salmonid aquaculture operations in Eastern Canada attract several mobile epibenthic species as a result of added structural complexity and increased food availability (bivalve fall-off and waste salmonid feed). It is not clear whether the aggregation of predators and scavengers below coastal farms contributes positively or negatively to their population dynamics, due to concerns about the quality of food items found under farms. We conducted an 18-month laboratory study to investigate the effect of diets composed of 1) mixed items, 2) mussels (Mytilus edulis), and 3) salmonid feed on the performance and condition of the rock crab, Cancer irroratus. Diet had no impact on crab survival but several negative consequences were observed in crabs fed the salmonid feed diet when compared to the mixed diet: reduced 1) moulting rates during the second growing season, 2) inter-molt growth, 3) gonad and hepatopancreas indices, 4) hemolymph dissolved compounds, 5) hepatopancreatic glycogen, and 6) shell hardness. Crabs fed the mussel diet had similar performance and condition when compared to the mixed diet. Fatty acid composition of muscle, gonad, and hepatopancreas tissues revealed that a salmonid feed diet decreased n3/n6 ratio when compared to a mixed or a mussel diet; those differences were mostly due to increases in the proportions of terrestrial (18:1n9 and 18:2n6) and decreases in proportions of marine essential (20:5n3 and 22:6n3) fatty acids. Together, these results point to a minimal impact of a mussel-only diet on crabs, whereas the salmonid feed diet resulted in negative impacts on condition. Our experimental results explored the consequences of a ‘worst-case scenario’ in which crabs were forced to feed on a single item for a long period of time; the realized impact in field settings will depend on other factors such as consumption of alternate food items underneath a farm, proportion of time spent in farms, and level of overlap between crab habitat and aquaculture facilities.
Coastal aquaculture has been developing rapidly over the past few decades with well-documented negative impacts on the biodiversity of infaunal organisms located directly below and in the immediate vicinity of farms, largely due to organic enrichment (Weise et al., 2009; Holmer, 2010; Weitzman et al., 2019). However, farms also have positive impacts by adding structural complexity to otherwise featureless bottoms and water columns, offering substrate for sessile organisms and shelter for mobile organisms (McKindsey, 2011; Theuerkauf et al., 2021). In addition, large quantities of food can fall to the bottom under and in the proximity of farms. For instance, in mussel farms, many individuals fall to the bottom through the process of self-thinning as mussels grow in size and compete for space on suspended lines (McKindsey et al., 2011; Fréchette, 2012). In finfish net farms, a non-negligible proportion of food pellets are not consumed by the farmed fish and sink to the bottom (Holmer et al., 2005; Wang et al., 2012). In both cases, several species of mobile predators and scavengers tend to be more abundant under farms compared to surrounding areas (Drouin et al., 2015; Callier et al., 2018; Barrett et al., 2019; Theuerkauf et al., 2021). Several taxa have been determined to assimilate energy coming from farms, either directly or indirectly; these include sponges (Laroche et al., 2021), polychaetes (Salvo et al., 2015; White et al., 2017a; Woodcock et al., 2019), echinoderms (White et al., 2017b; Woodcock et al., 2018), bivalves (Handå et al., 2012; Irisarri et al., 2015), and decapods (Olsen et al., 2012; Izquierdo-Gomez et al., 2015; Woodcock et al., 2018; Sardenne et al., 2019; Baltadakis et al., 2020; Sardenne et al., 2020). Therefore, these anthropogenic inputs of resources might impact natural population dynamics, including the productivity of several commercially-important species.
It remains unclear if farm-related food subsidies have overall positive or negative effects on associated wild populations. If individuals frequenting farms are able to use the energy added to the ecosystem to improve their condition and reproductive outputs, then farms could contribute positively to population dynamics. This has been suggested for the sea star Coscinasterias muricata in a New Zealand mussel farm, where sea star aggregations might improve their fertilization rates during broadcast spawning (Inglis and Gust, 2003). Fish associated with salmonid farms often have elevated condition indices when compared to fish collected away from farms (Fernandez-Jover et al., 2007; Dempster et al., 2011; Fernandez-Jover et al., 2011; Barrett et al., 2018) with limited documented consequences on reproductive output (Barrett et al., 2018). Alternately, if individuals aggregated in farms consume large amounts of salmonid feed or mussels, then nutritional requirements might not be met and farms may have overall negative impacts on population dynamics and stock productivity. For example, American lobsters (Homarus americanus) feeding exclusively on mussels in a laboratory experiment had lowered survival and condition when compared to those fed a mixed diet of fish, crustaceans and mollusks (Wang and McGaw, 2016), suggesting that a mussel diet might not meet all dietary requirements of decapod crustaceans. Similarly, salmonid feed may lack some essential dietary components for marine organisms; over the last few decades, fish oil supplies for the production of fish feed could not keep up with growing demand and therefore, sources of proteins and fatty acids in fish feed have shifted progressively from marine to terrestrial sources (Ytrestøyl et al., 2015). Nowadays, most commercial diets include substantial proportions of soy, wheat, rapeseed, linseed oil, and other terrestrial ingredients (Turchini et al., 2009; Ytrestøyl et al., 2015). Modern fish feeds are rich in terrestrial short-chained monounsaturated and omega-6 polyunsaturated fatty acids (e. g., 18:1n9 and 18:2n6) and poor in marine long-chain omega-3 polyunsaturated fatty acids (e. g., 20:5n3 and 22:6n3; Sardenne et al., 2020). This lipidic imbalance may have negative effects for marine species for which long-chained omega-3 are often considered essential fatty acids. For example, White et al. (2016; 2018) found that a diet with high concentrations of omega-6 polyunsaturated fatty acids had negative impacts on reproduction and larval development for two sea urchin species. Similar effects on other marine invertebrates are likely, with possible deleterious consequences on individuals’ condition and population dynamics.
In Eastern Canada, the crabs Cancer irroratus, C. borealis and Hyas spp. are fished commercially with landings of 3.5-6 kt annually (2015-2019), for a market value of CAD$ 5-8 million (Fisheries and Oceans Canada, 2021). The rock crab, C. irroratus, inhabits coastal rocky and soft-bottom habitats where they feed on polychaetes, mussels, sea urchins, and various other animals and debris (Scarratt and Lowe, 1972). They form reproductive couples, by which a male will grasp a pre-moult female, mate shortly after her moulting, and then release her after her carapace hardens; fertilized eggs are extruded the following year and the female will carry the embryos on her abdomen until hatching (Bigford, 1979). Field studies showed that C. irroratus is found at higher density below both mussel and salmonid farms compared to surrounding areas (Clynick et al., 2008; D’Amours et al., 2008; Drouin et al., 2015; McKindsey, unpublished). Acoustic telemetry revealed that they remain relatively sedentary under mussel farms for periods of weeks; the same pattern is observed under salmon farms, although more movement is observed (McKindsey, unpublished data). Under mussel farms, crabs actively feed on mussel fall-off since analyses of gut content showed a greater proportion of mussels compared to those of crabs in surrounding areas (McKindsey, unpublished data). Crabs also consume salmon waste or waste feed as analyses of muscle, hepatopancreas and gonads showed terrestrial fatty acid and stable isotope fingerprints of salmonid feed in rock crabs collected under salmon farms (Sardenne et al., 2020). In addition, diet breadth in crabs collected under farms was reduced when compared to crabs collected further from farms (Sardenne et al., 2020). The relatively long residence time of rock crabs under farms and apparent dietary preference for mussels and salmonid feed make them likely to be impacted by their diet in the long term, either negatively or positively. This may be particularly relevant since rock crabs are a preferred and essential prey for American lobsters (Gendron et al., 2001), the most lucrative Canadian fishery with an annual value of CAD$ 1.5 billion (Fisheries and Oceans Canada, 2021). Lobsters also frequent mussel and salmonid farms, where they consume a mix of crab, mussels and waste salmonid feed (Sardenne et al., 2019; Sardenne et al., 2020). Therefore, crab condition, whether enhanced or decreased, may cascade down to lobster stock productivity.
In this study, we evaluated the long-term effects of feeding rock crabs with a 1) mixed diet, 2) exclusively salmonid feed, and 3) exclusively mussels to investigate potential impacts of aquaculture operations on rock crab population dynamics. We maintained individuals for 18 months in the laboratory and monitored survival, moulting, and growth. At the end of the experiment, individuals were dissected and several physical and physiological variables measured as proxies for condition and detailed lipid profiles obtained from various tissues. The results directly address concerns raised by fisherman about impacts of aquaculture on their activities, and contribute to our knowledge of the impact of aquaculture-related anthropogenic food subsidies to the benthic ecosystem.
The study was conducted in the wetlabs at the Maurice-Lamontagne Institute (Fisheries and Oceans Canada, Mont-Joli, QC, Canada) under natural photoperiod regimes. Rock crabs were collected between April 27 and May 23 2018 using baited-traps deployed at the institute’s wharf. Crabs were transported to the laboratory within one hour of being collected from traps and maintained in 1-m3 tanks with flow-through seawater pumped from a depth of ~15 m in the Saint Lawrence Estuary, approximately 2 km from shore, through a sand-filter. Crabs were fed a mixture of blue mussels (Mytilus edulis), fish (frozen Atlantic mackerel Scomber scombrus) and green sea urchins (Strongylocentrotus droebachiensis) until they were transferred to the experimental tanks on June 14th 2018. Experimental tanks consisted of 76 L plastic containers (80 × 45 × 30 cm) with the bottom covered in sand; rocks and small plastic buckets cut in half longitudinally provided shelter. Each experimental tank was supplied with flow-through seawater at ambient temperature and salinity (measured twice weekly using a Hanna multiparameter water quality meter model HI98194; range of temperature: 2-12°C and salinity: 22-30 PPT; Figure S1) and the flow was adjusted at 2.5 L min-1 using a valve and flow meter. We randomly assigned 3 females and 2 males (individually tagged using numbered plastic circles glued to the carapace; carapace width mean ± SD = 50.91 ± 4.76 mm, n = 84 for females and 68.64 ± 4.09 mm, n = 56 for males) to each of 28 experimental tanks. Those crab sizes were chosen because they represent the onset of sexual maturity in females and females tend to mate with males that are slightly larger than themselves (Bigford, 1979).
Tanks were randomly assigned one of three diets: 1) mixed (n=10), 2) blue mussels only (n=9), and 3) salmonid feed only (n=9). The mixed diet was designed to recreate a natural diet and consisted, in random alternation, of whole blue mussels M. edulis (20-30 mm in shell length), thawed fish pieces (10-20 cm3; Atlantic mackerel S. scombrus, rainbow smelt Osmerus mordax or Atlantic herring Clupea harengus, depending on availability), green sea urchin S. droebachiensis with the test broken open, and whole common periwinkles Littorina littorea. The mussel diet consisted of whole M. edulis (20-30 mm shell length) whereas the salmonid feed diet consisted of commercially available formulated pellets (5.5 mm in diameter). Tanks were maintained twice weekly during the growing season (May to November) and weekly during the rest of the year. During maintenance, food remains (e.g. uneaten flesh, shells, and tests) were removed using a fish net and weighed. Then rocks and caches were carefully moved and the substrate cleaned using an aquarium siphon and gravel cleaner; care was taken to minimize stress. New food items were weighed and distributed to each tank according to its assigned diet. Food consumption varied considerably over the course of the experiment because of natural changes in water temperature and increases in crab size; we thus developed a protocol to ensure that crabs fed to satiation while minimizing wastes. If the entire amount of food allotted to a tank of a particular diet was eaten since the last maintenance, the quantity of food given was increased by 20% for all replicates of that diet; the same logic was used to reduce the rations. Whole wet weight per tank varied as follows: blue mussels (~15 to 70 g), salmonid feed (~0.5 to 8 g), common periwinkles (~4 to 40 g), green sea urchins (~15 to 100 g) and fish (~2 to 15 g) for maintenance period.
Based on the amounts of food consumed, the caloric content of the different food items and the weight of crabs present in each tank, we estimated the overall energy consumption (Kcal/100 g of crab) for each inter-maintenance period. We estimated food consumption for each tank based on the amount given during a maintenance and the amount remaining at the following maintenance. For fish, periwinkles, and sea urchins (test broken and body fluids drained), wet weight of each ration given was measured and the uneaten prey remains (flesh, intact shells and shell fragments) carefully collected and weighed; the difference between the weights given and recovered provides a good estimation of the amount of soft tissue consumed. For mussels, the number of individuals given and total whole wet weight was recorded. Because mussels can trap water within their valves and were weighed whole, a correction factor was applied to remove contribution of water to the measurements. This was done by relating wet soft tissue weight to whole mussel weight following dissection of a total of 224 individuals (18-32 mm in shell length) on 6 different dates during summer-fall 2018. We then used the linear relationship between whole wet weight and tissue weight (tissue = 0.21 wet weight -0.01, R = 0.67), the number of individuals consumed, and the average weight of individuals given to calculate flesh consumption. For salmonid feed, we weighted the whole ration and counted the number of pellets given and the number of pellets that were still intact after an inter-maintenance period to estimate weight of pellets consumed.
Samples were prepared to measure the caloric content of each food item. Periwinkles (17-30 mm in shell length), sea urchins (45-67 mm in test diameter) and mussels (20-30 mm shell length) were dissected and soft tissues isolated; individuals were dissected until we obtained a pooled sample of ~100 g wet weight for each species (> 100 periwinkles, 6-9 sea urchins, and > 200 mussels). This was repeated 4 times for periwinkles and sea urchins and 6 times for mussels between May and November 2018. In addition, single samples (~ 100 g) of the three species of fish (mackerel, smelt, and herring) and salmonid feed pellets were prepared. All samples were homogenized and preserved frozen until further processing in a commercial laboratory, where the energy content (kcal/g) was measured.
For each inter-maintenance period, the energy consumed by crabs in a given tank was estimated by multiplying the weight of food consumed by its caloric content (average from multiple samples for periwinkles, sea urchins, and mussels). This value was then divided by the weight of crab present in a tank (taking into account the mortalities and the weight increases due to moulting) to calculate energy consumption in Kcal/100 g of crab. We then calculated averages from all inter-maintenance periods for each tank from mid-June 2018 to early-July 2019. Tanks were monitored regularly and dead individuals were noted and removed. In 2018, a tank was closed if there was not at least one male and one female still alive in the tank since we originally planned to focus on reproduction; remaining individuals were removed and the tank was no longer followed. In 2019, this practice was stopped and all individuals were kept as we approached the end of the experiment and dissection of individuals. The date at which individuals moulted was noted and newly-moulted crabs were placed on floating cages to protect them from cannibalism. After the carapace had hardened, carapace width was measured with a digital vernier caliper, individuals were re-tagged, and returned to their respective tanks. The date and duration of couple formation were also noted.
At the end of the experiment (October 27th 2019), all remaining crabs (n = 23 [8 males and 15 females], 11 [6 males and 5 females], and 13 [7 males and 6 females] for the mixed, salmonid feed and mussel diets, respectively) were dissected in the laboratory to measure several indices of condition. Wet crab body weight was measured and a final measure of carapace width was taken. We removed gonads and hepatopancreas tissues and measured their wet weight.
We estimated concentration of hemolymph dissolved compounds using the Brix index, a measure based on refraction of hemolymph which relates well to general physiological condition of crustaceans (Wang and McGaw, 2014). We collected a 1 ml sample of hemolymph from the articulations of walking legs using a 5 ml syringe and a 21 gauge needle and placed the sample on the cell of an Atago Digital Pocket Refractometer (model 3810 PAL-1) to measure the refraction index.
We measured shell hardness using a PTC Shell Hardness Durometer Model 307HF, which provides a dimensionless comparison of breaking point of materials (Hicks and Johnson, 1999). Readings were taken at three locations on the dorsal side of the carapace: at the posterior end (on the middle line) and at the anterior end (both right and left sides).
Glycogen content analyses were used to evaluate levels of stored energy. During the dissection, 60 mg hepatopancreas samples were collected and preserved at -80°C. Glycogen assays were performed using a commercial Glycogen Assay Kit (Cayman Chemical) at the Centre de Recherche sur les Biotechnologies Marines (CRBM, Rimouski, QC, Canada). Hepatopancreases were homogenized using a Precellys 24 tissue homogenizer, at a concentration of 200 mg/mL with the buffer solution of the kit supplemented with protease inhibitors (Protease Inhibitor Cocktail powder, Sigma-Aldrich) in tubes containing 1.4 mm ceramic balls. The assay was performed with excitation wavelengths between 530 and 540 nm and emission wavelengths from 585 to 595 nm. The glycogen content was reported as mg/g of wet weight.
During the dissection, two 200 mg samples of hepatopancreas, gonads, and muscle (collected from the crusher claw) were collected from the 47 crabs. One sample was oven-dried at 60°C for 120 h to estimate water content; this value was subsequently used to calculate ratio of fatty acid weight to tissue dry weight. The other sample was frozen at -80°C until further processed. Fatty acids (FA) were extracted and methylated from frozen samples following a one-step extraction/methylation procedure (direct methylation; modified from Lepage and Roy, 1984). Samples were homogenized in a conical tissue grinder with 4 ml 2:1 dichloromethane:methanol solution. FA were then transesterified using 3 ml of 3:2 MeOH : Toluene and 2 ml of Hilditch reagent (2:98 H2SO4:MeOH). Samples were heated (100°C; 10 min), cooled (10 min) and centrifuged at 1000 rpm for 1 minute; the supernatant was collected and transferred into 2 ml amber vials prior to nitrogen blowdown. 300 µl of Hexane were added to each vial as a final step before purification.
A purification was done to eliminate the sterols that can obstruct the mass spectrometer. To form a column, a small pipette was filled with fiberglass wool and 0.5 cm of previously burned silica. Columns were conditioned with 1.5 ml of 1:1 hexane:ethyl acetate solution. Samples (300 µl) were injected into the column. When the sample was well distributed into the silica, 1.5 ml of 1:1 solution was added to rinse and collect all the lipids. Samples were blown down under nitrogen and stored at -80°C for FA analyses.
Fatty acid methyl esters (FAME) were analyzed by a gas chromatograph (Trace GC Ultra) equipped with a Omegawax 250 column (30 m x 0.25 mm) and coupled to an ion trap mass spectrometer (Trace ITQ900) (GC-MS, ThermoScientific) at the Institut des Sciences de la Mer de Rimouski (ISMER, Rimouski, QC, Canada). FA were identified and quantified by comparing retention times and mass spectra with known standards calibration curves (37 Component FAME Mix, Supelco) with Xcalibur v.2.1 software.
Variation in estimated energy consumption was analyzed with a one-way ANOVA with the fixed factor Diet as the independent variable and the energy consumption per 100 g of live crab (average for all inter-maintenance periods) as the dependent variable. Data were log-transformed to meet parametric assumptions and multiple comparisons among diets were done using Tukey HSD post-hoc test.
We used a Kaplan-Meier log-rank test (survival analysis; Fox, 2001) to compare survival over the whole experiment among diets; individuals that were present in tanks that were closed were considered censored data, i.e. their status was considered as unknown after the closure date. The same test was used to analyze moulting for the 2018 and 2019 growing seasons separately with the proportion of individuals that had not moulted at a particular date as the dependent variable. The moulting analysis for 2018 only included individuals that were still alive in the fall (i.e., 150 days after the start of the experiment) and those that died during moulting; for 2019, we included individuals that were alive in June 2019 and survived until the end of the experiment in fall 2019 or that died during moulting. Multiple comparisons among diets were done using the Holm-Sidak method following a significant overall test.
Growth was assessed using inter-molt proportional increase in carapace width ([Widthpostmoult – Widthpremoult]/Widthpremoult). Data was analyzed using a one-way ANOVA with the fixed factor Diet; the dependent variable was the mean growth of all moults recorded within a replicate experimental tank in 2018 and 2019 combined. Assumptions of normality and homoscedasticity were assessed by inspection of residual plots and no transformation was deemed required. Multiple comparisons among diets were conducted using Tukey HSD post-hoc tests.
Condition indices were analyzed with one-way ANOVAs with the fixed factor Diet; the variables analyzed were: 1) Gonad index (wet gonad weight/wet total weight; females that were ovigerous at the time of dissection were removed from the analysis since their gonads were very small), 2) Hepatopancreas index (wet hepatopancreas weight/wet total weight), 3) Brix index, 4) Hepatopancreatic glycogen concentration and 5) Carapace hardness, with a separate analysis for the 3 carapace positions. In all cases, the dependent variable was the average for a replicate experimental tank. Assumptions of normality of residuals and homoscedasticity were assessed by inspection of residual plots and appropriate transformations were used when necessary (see results). When a significant Diet effect was detected, multiple comparisons were conducted using Tukey HSD post-hoc tests.
Lipid profiles were analyzed using the software Primer v7 with PERMANOVA. Bray-Curtis similarity matrices were built using the mean proportion of each fatty acid in a given tank as dependent variables. Only fatty acids with proportion greater than 0.5% (average from all samples) were retained in the analysis. A two-way factorial PERMANOVA was conducted using Diet and Tissue as fixed factors. This analysis showed a significant interaction between the two factors; we thus conducted a one-way analysis for each tissue separately. Following a significant Diet effect, we conducted pair-wise tests to determine which pairs of treatment differed significantly and SIMPER analyses to determine which fatty acids contributed the most to significant differences among pairs of diets. Finally, we used principal component analyses (PCA) and plotted replicate values on the plane composed of the two dominant PCs and the ordination of the fatty acids that contributed the most to differences.
Proportion of each fatty acid, total proportion of saturated, monounsaturated, n-6 and n-3 polyunsaturated, total polyunsaturated fatty acids, n-3/n6 ratios and total fatty acid content were analyzed with one-way ANOVAs with the fixed factor Diet for each tissue separately. Various transformations (log or arcsin-square root) were used to meet assumptions of normality of residuals and homoscedasticity; when no transformation succeeded in meeting assumptions, we used Kruskall-Wallis tests. Detection of significant Diet effect was followed by Tukey HSD post-hoc comparisons (ANOVA) or multiple comparison of ranks (Kruskall-Wallis tests).
Estimates of caloric intake per 100 g of live crab varied significantly among diets (ANOVA: MS = 0.43, F2,25 = 50.88, p < 0.001). Estimates were lowest for the mussel diet, intermediate for the mixed diet, and highest for the salmonid feed diet (Figure 1); all pairwise comparisons were significant.
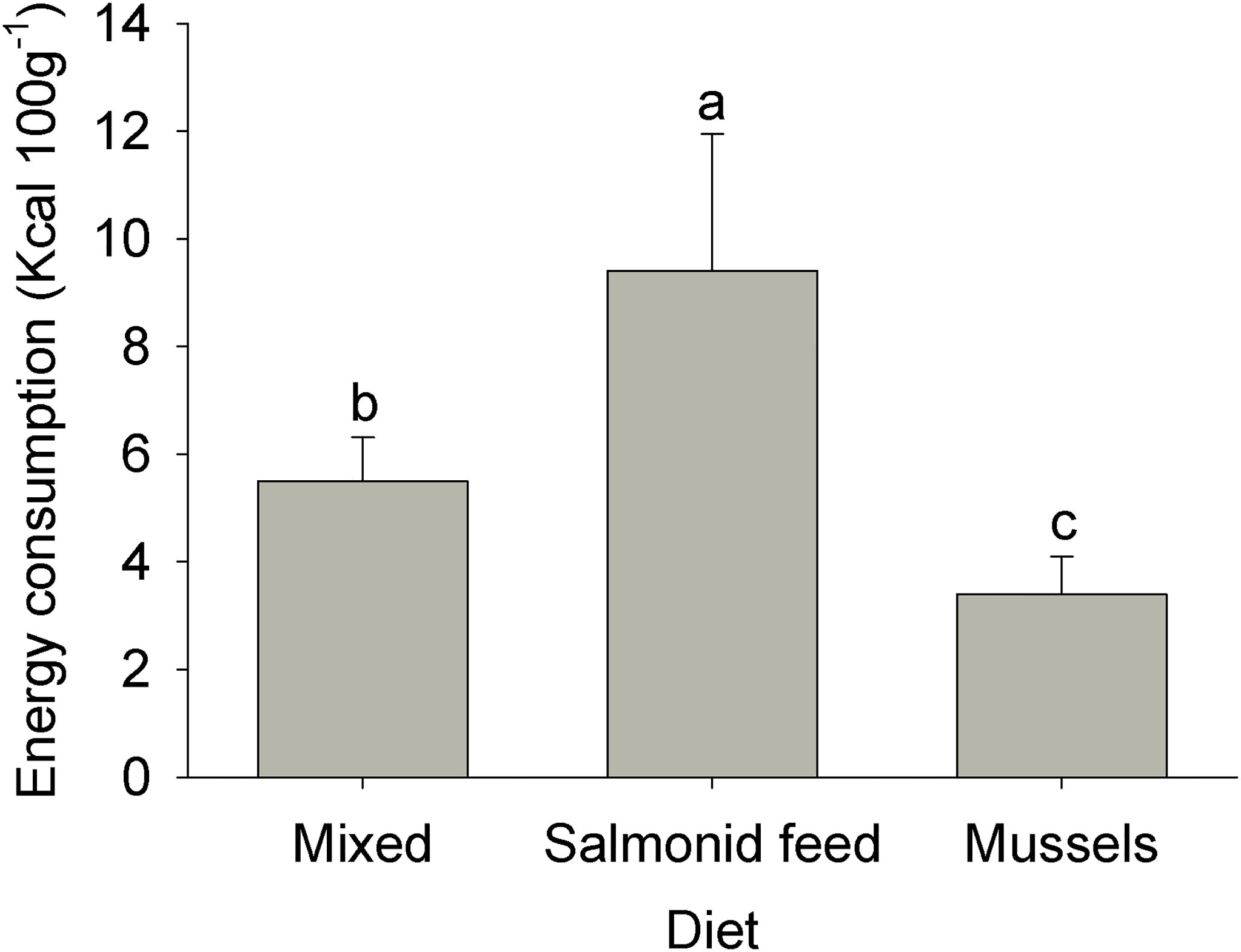
Figure 1 Estimated energy consumption for rock crabs Cancer irroratus fed the different experimental diets. Replicates are the mean values for each experimental tank. Log-transformed data was analyzed, but raw data is presented. Bars show mean +SE and columns not sharing a common letter are significantly different (Tukey HSD post-hoc tests, n = 9-10).
We observed considerable mortality over the course of the experiment (34% of individuals survived to the end of the experiment) but survival was not related to Diet (Figure 2; Kaplan-Meier log-rank test statistic = 1.06, df = 2, p = 0.59). Most mortality occurred early in the experiment as a result of 1) an unidentified disease expressed as the presence of black spots on the legs and carapace (~70% of recorded mortalities) and 2) individuals being killed by their conspecifics following moulting (~20% of recorded mortalities). Occurrence of the disease greatly diminished in the fall and important outbreaks did not happen afterwards; this resulted in low mortality during the winter. Removing the period of high disease-related mortality from the statistical analysis (i.e. starting the analysis in October 2018) did not change the results (Kaplan-Meier log-rank test statistic = 0.51, df = 2, p = 0.78). A few individuals died during the 2019 growing season, mostly as a result of individuals being killed by conspecifics during moulting (Figure 2). At the end of the experiment, the number of tanks that still had live individuals had decreased to 9 for the mixed diet and 5 for each of the salmonid feed and mussel diets.
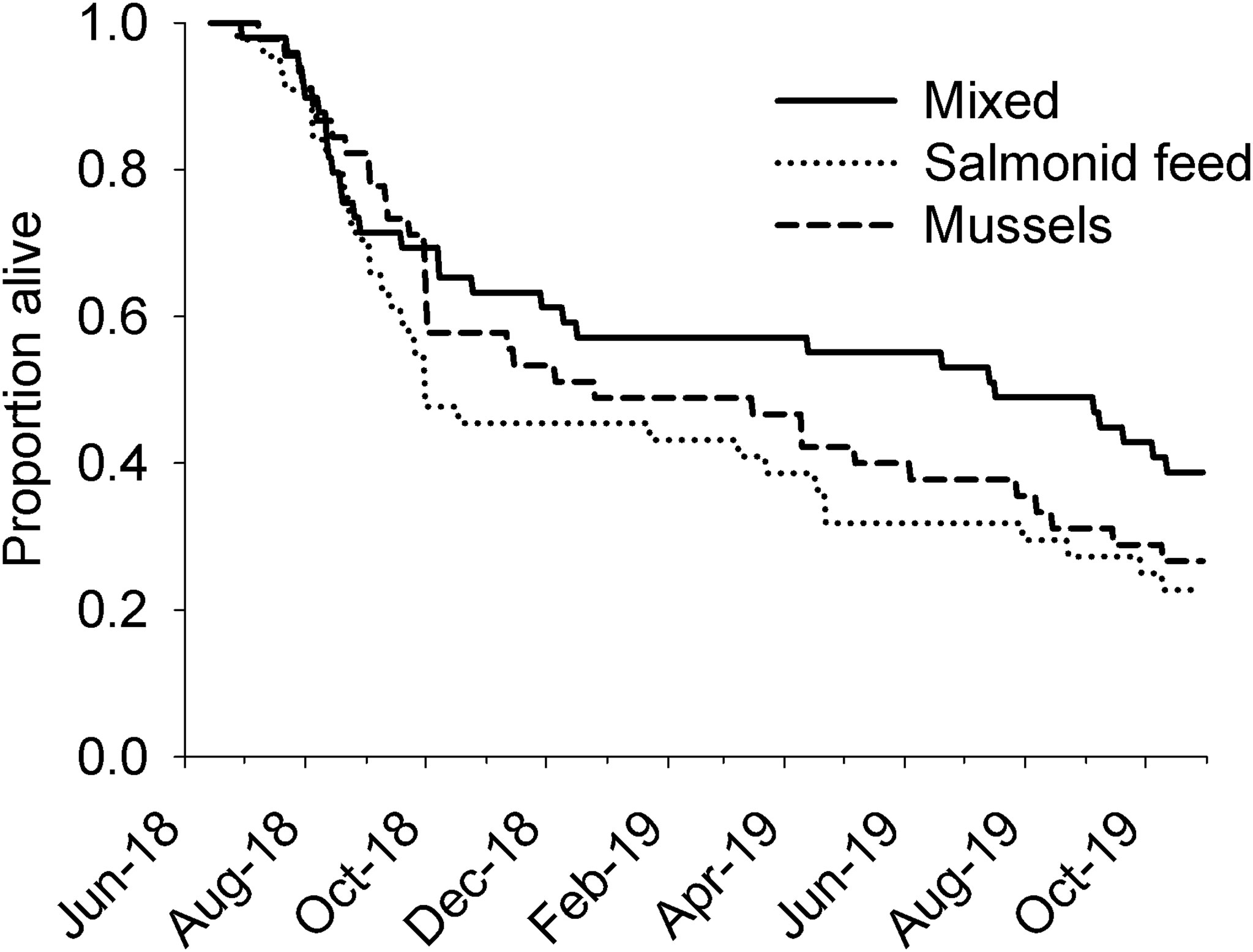
Figure 2 Survival of rock crabs Cancer irroratus fed different aquaculture-related diets in a laboratory experiment. Survival was not significantly influenced by diet (Kaplan-Maier log-rank survival analysis, n = 45-50 for each treatment).
In 2018, moulting was not affected by Diet (Figure 3A; Kaplan-Meier log-rank test statistic = 1.78, df = 2, p = 0.41). Among individuals included in the analysis, ~75% were observed moulting. Moulting activity was maximal between the end of August and the end of September and the last moult observed was on October 9th (Figure 3A); no moult was observed until the following summer. In 2019, the moulting pattern was significantly affected by Diet (Figure 3B; Kaplan-Meier log-rank test statistic = 6.32, df = 2, p = 0.04). Of the individuals included in the analysis, the proportion that moulted was highest for the mixed diet, intermediate for the mussel diet, and lowest for the salmonid feed diet; the only statistically significant multiple comparison was between the mixed and salmonid feed diets (Figure 3B).
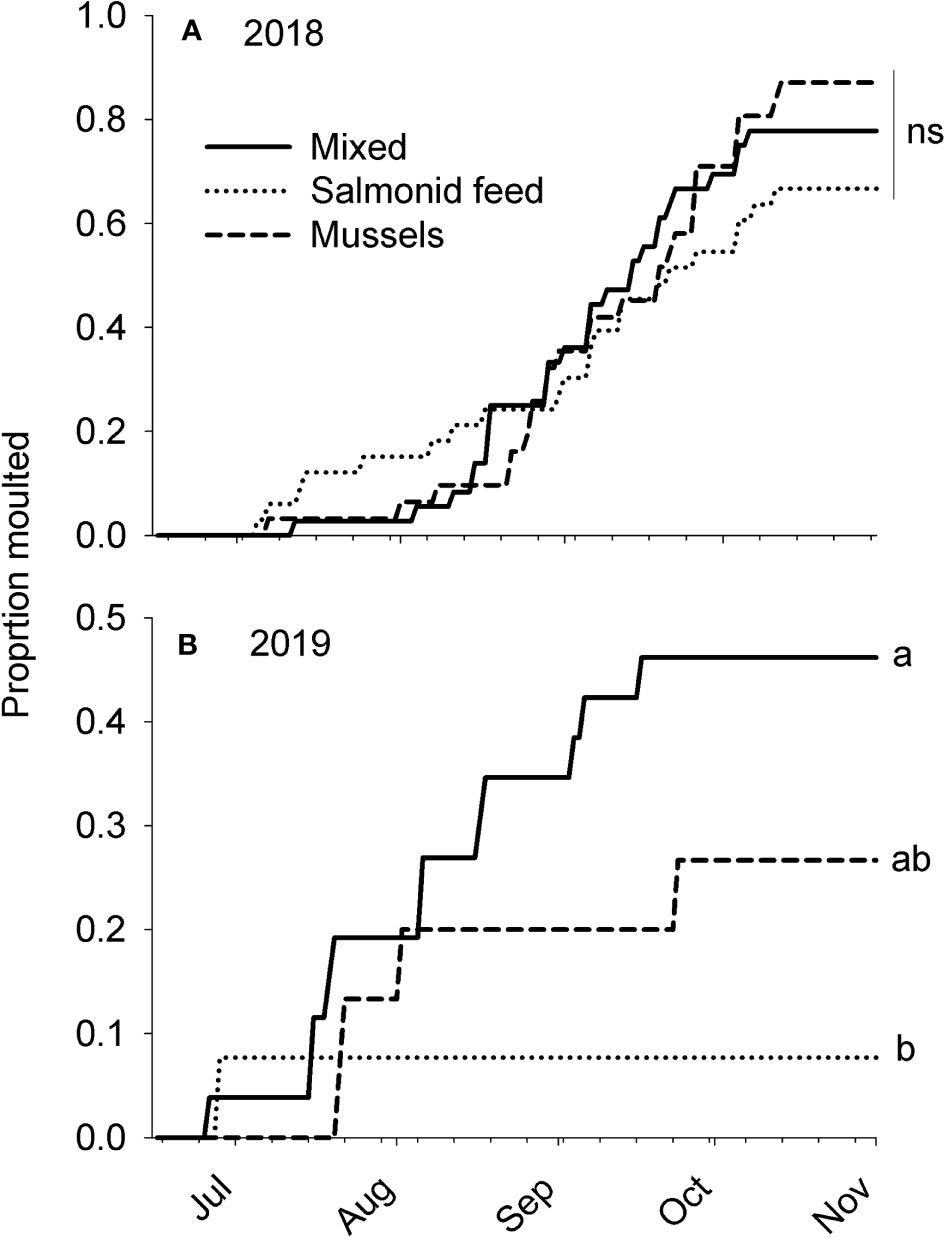
Figure 3 Moulting of rock crabs Cancer irroratus fed different aquaculture-related diets in a laboratory experiment in (A) 2018 and (B) 2019. Proportion not moulted was analyzed through survival analyses, but proportion moulted is presented. Lines not sharing a common letter are significantly different (Holm-Sidak post-hoc test), ns = not significant. The number of individuals included in the analysis in 2018 was 36, 33, and 31 for the mixed, salmonid feed and mussel diets, respectively and, in the same order, 26, 13, and 15 in 2019.
Diet impacted growth over the course of the experiment (ANOVA: MS = 7.11 E-3, F2,24 = 6.02, p = 0.008). Carapace width following a moult increased by ~ 25% in experimental tanks fed a mixed or a mussel diet but significantly less (~ 20%) in those fed the salmonid feed diet (Figure 4).
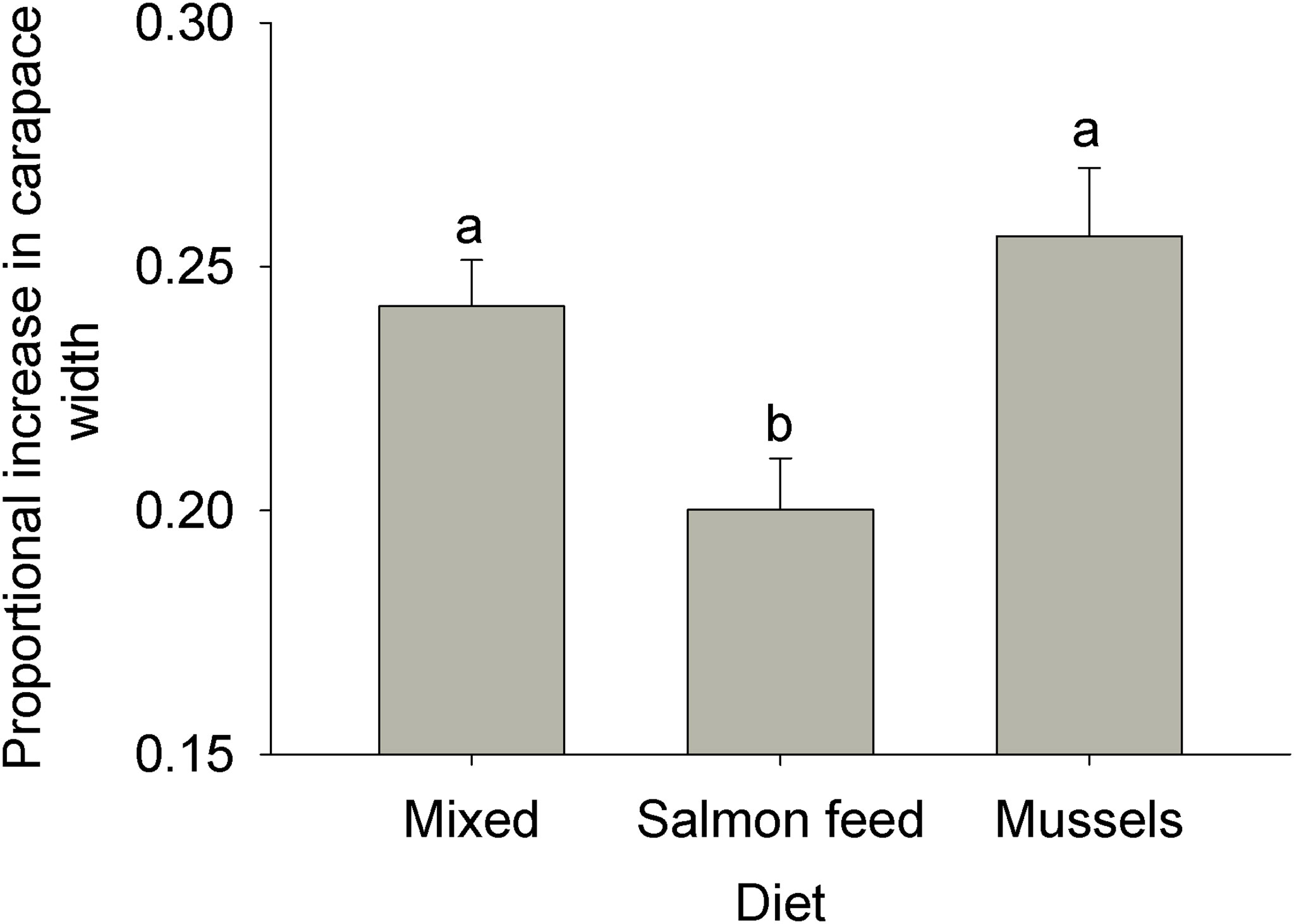
Figure 4 Impact of experimental diets on the growth of rock crabs Cancer irroratus. Replicate values are the average proportional increases in carapace width for each experimental tank. Bars show mean + SE and columns not sharing a common letter are significantly different (Tukey HSD post-hoc tests, n = 5-9). The total number of moults for which growth was recorded was 37, 17, and 29 for the mixed, salmonid feed and mussel diets, respectively.
Both gonad and hepatopancreas indices were significantly affected by Diet (ANOVAs: log-transformed gonad index, MS = 0.28, F2,18 = 5.05, p = 0.02; hepatopancreas index, MS = 1.80E-3, F2,18 = 11.18, p < 0.001). Gonad index was greatest for individuals fed the mixed diet, intermediate for the mussel diet, and lowest for the salmonid feed diet; only the mixed and salmonid feed diets differed significantly (Figure 5). Hepatopancreas index was significantly lower for individuals from the salmonid feed diet compared to other treatments (Figure 5). Concentration of hemolymph dissolved compounds (Brix index) and hepatopancreatic glycogen were both affected by diet (ANOVAs: Brix index, MS = 18.50, F2,18 = 16.14, p < 0.001, log-transformed glycogen, MS = 1.29, F2,18 = 11.41, p < 0.001). Brix index was significantly lower in the salmonid feed diet when compared to other treatments (Figure 6). Hepatopancreatic glycogen concentration was highest in the mussel diet, intermediate in the mixed diet, and lowest in the salmonid feed diet; all treatments differed significantly from each other (Figure 6). Finally, carapace hardness was significantly affected by diet for the 3 positions evaluated (ANOVAs, MS = 184.60, 520.39, and 327.36, F2,18 = 3.64, 4.08, and 5.18, p = 0.05, 0.04, and 0.02 for the posterior, anterior left and anterior right positions, respectively). Post-hoc comparisons found no differences for the posterior position. Anterior carapace hardness was highest for the mixed diet, intermediate for the mussel diet and lowest for the salmonid feed diet; only the mixed and salmonid feed diets differed significantly (Figure 7).
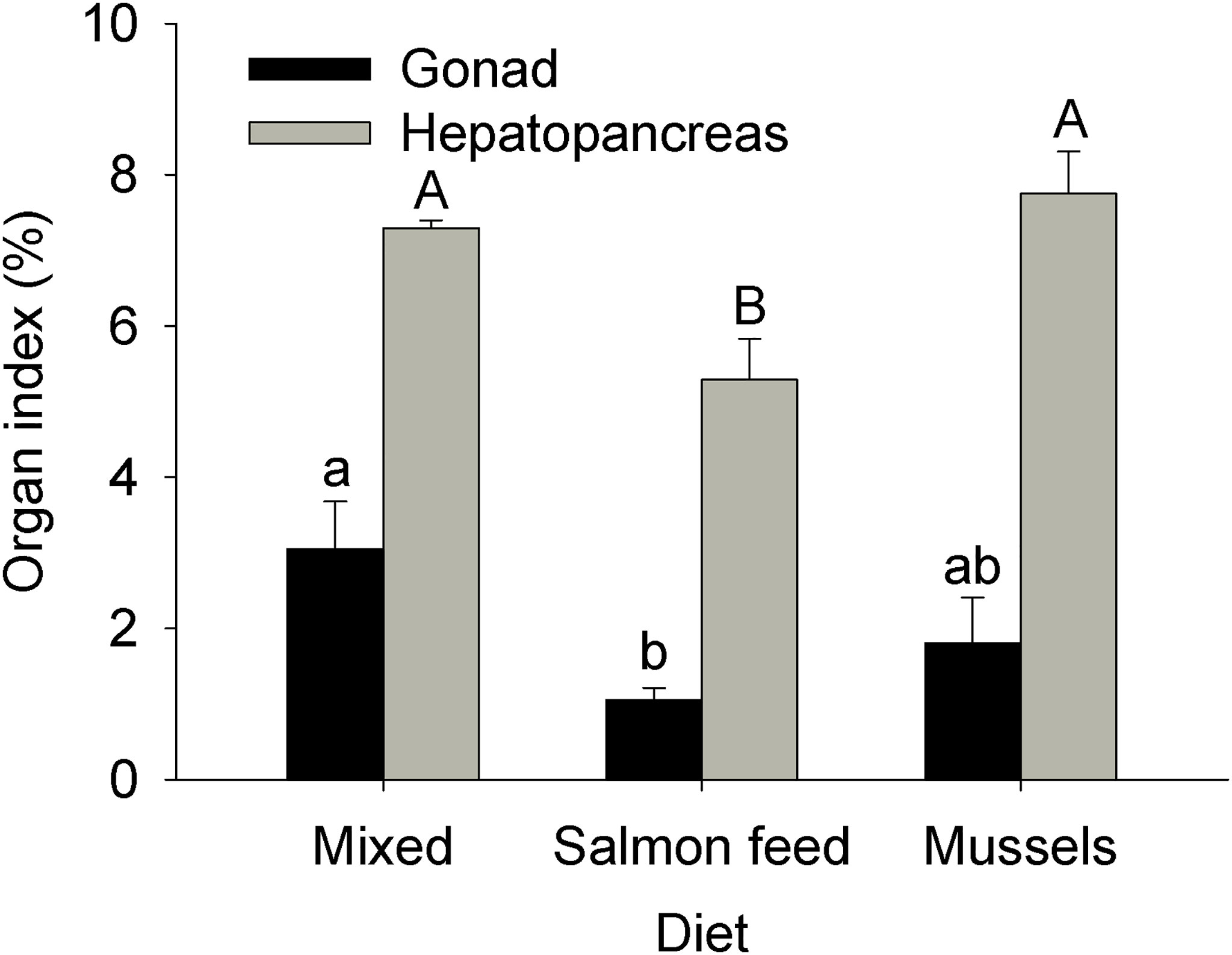
Figure 5 Impact of experimental diets on gonadal and hepatopancreatic indices in the rock crab Cancer irroratus. Replicates are the mean values for each experimental tank. Log-transformed gonad index was analyzed, but raw data is presented. Bars show mean +SE and columns not sharing a common letter are significantly different (Tukey HSD post-hoc tests, n = 5-9). The total number of individuals included in the analyses was 23, 11, and 13 for the mixed, salmonid feed and mussel diets, respectively.
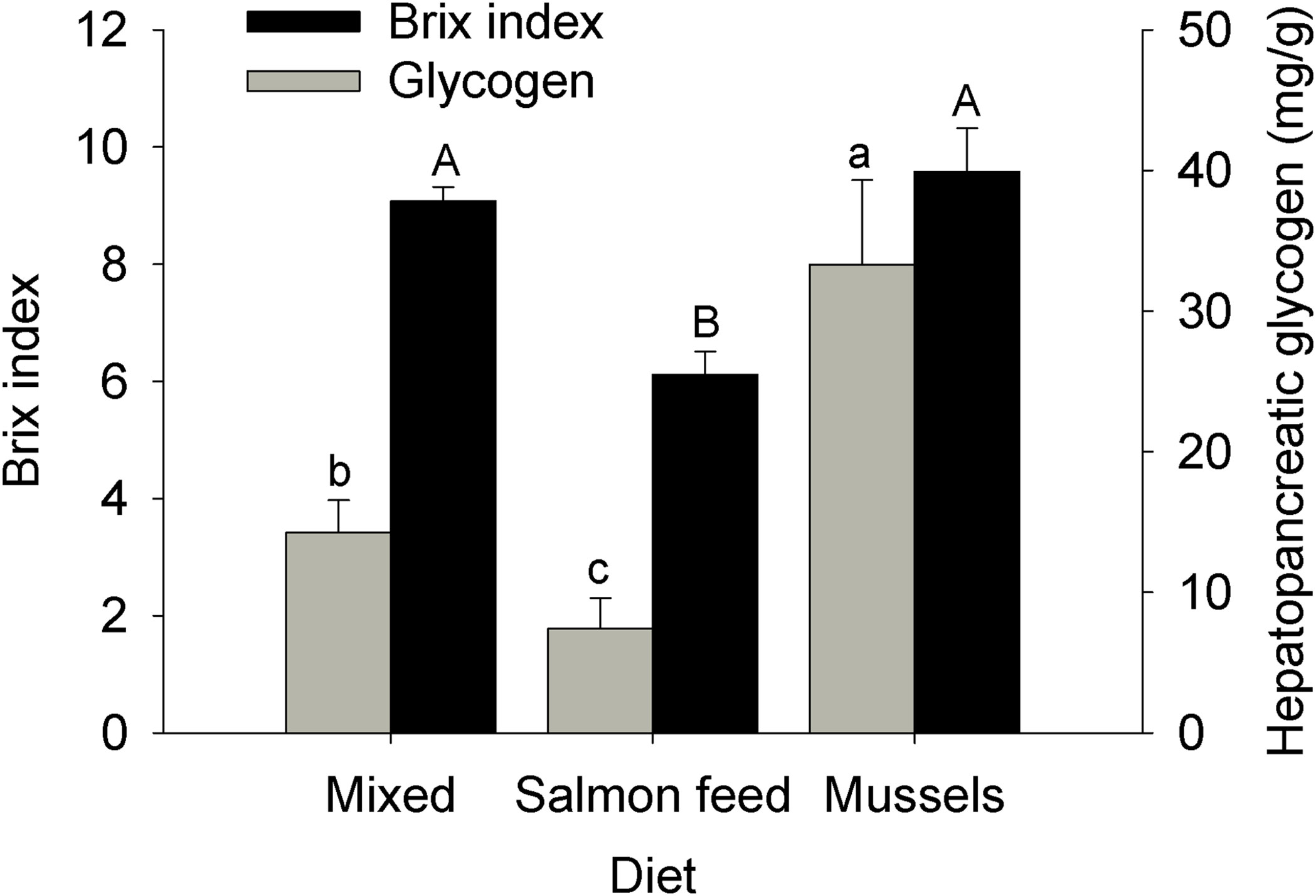
Figure 6 Impact of experimental diets on concentration of hemolymph dissolved compounds (Brix index) and hepatopancreatic glycogen in the rock crab Cancer irroratus. Replicates are the mean values for each experimental tank. Log-transformed glycogen content was analyzed, but raw data is presented. Bars show mean +SE and columns not sharing a common letter are significantly different (Tukey HSD post-hoc tests, n = 5-9). The total number of individuals included in the analyses was 23, 11, and 13 for the mixed, salmonid feed and mussel diets, respectively.
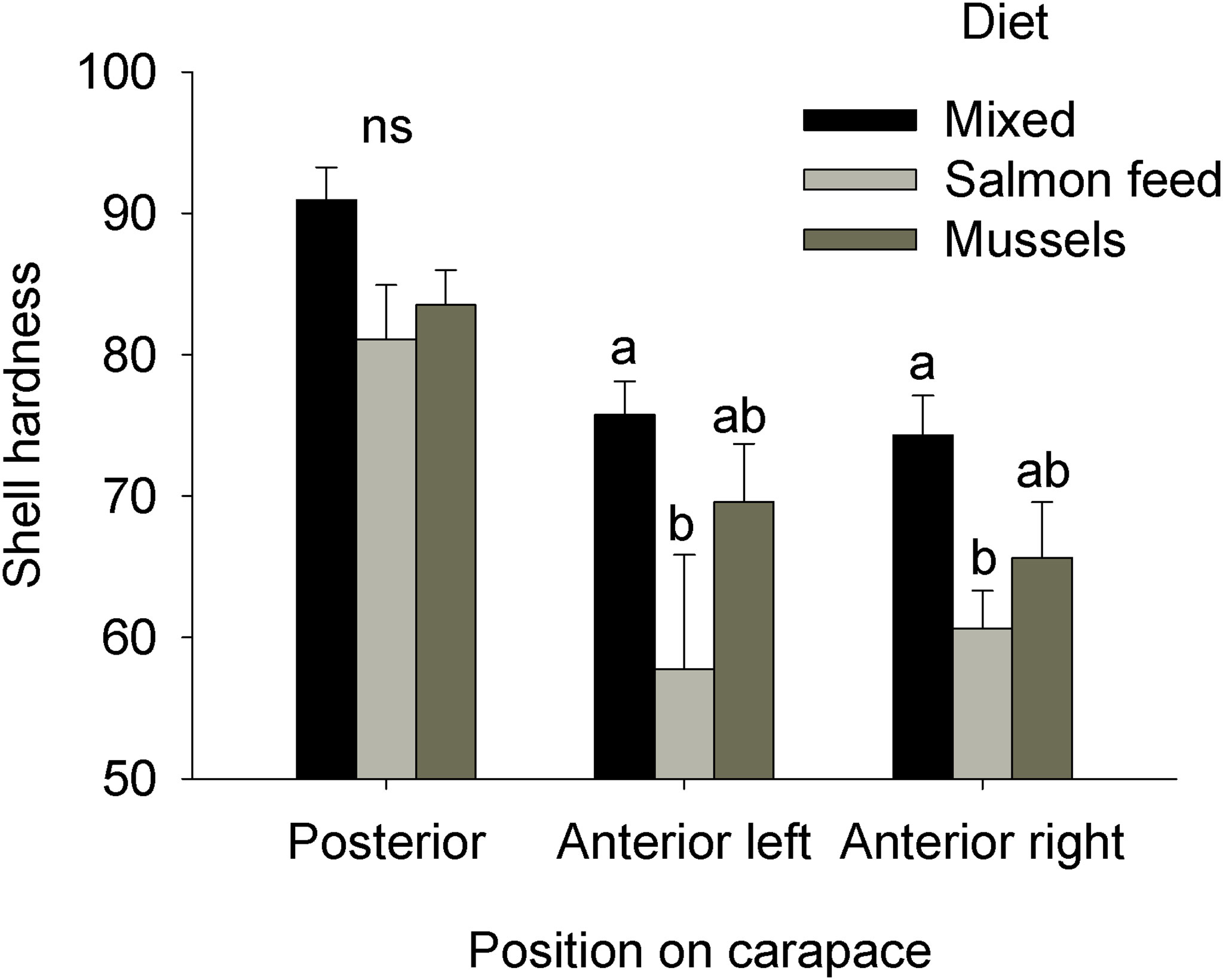
Figure 7 Impact of experimental diets on carapace hardness measured at three positions on rock crabs Cancer irroratus. Replicates are the mean values for each experimental tank. Bars show mean +SE and columns not sharing a common letter are significantly different, ns = not significant (Tukey HSD post-hoc tests, n = 5-9). The total number of individuals included in the analyses was 23, 11, and 13 for the mixed, salmonid feed and mussel diets, respectively.
Fatty acid profiles were significantly affected by diet in all three tissues (PERMANOVA, MS = 923.54, 1774.30, and 3014.60, pseudo-F2,16 = 42.44, 5.41, and 42.12 for muscles, gonads, and hepatopancreas, respectively; p < 0.003 for all tissues). Pairwise tests were all significant with the exception of the Mixed and Mussel comparison for gonads (Figures 8A–C). Individual fatty acids that contributed most consistently to differences among diets were terrestrial markers (18:1n9 and 18:2n6) that were found in higher proportions, and marine essential fatty acids (20:5n3 and 22:6n3) in lower proportion in the salmonid feed diet than the mixed and mussel diets for all organs (Figures 8A–C; Table 1). Bacterial markers (16:0 and 16:1; Kelly and Scheibling, 2012) were generally found in higher proportion in the mussel diet than the other two diets (Figures 8A–C; Table 1). There was a greater proportion of zooplankton markers (20:1n9 and 22:1n9; George and Parrish, 2015) in the muscle and hepatopancreas of crabs fed the mixed diet when compared to those fed the other two diets (Figure 8C; Table 1).
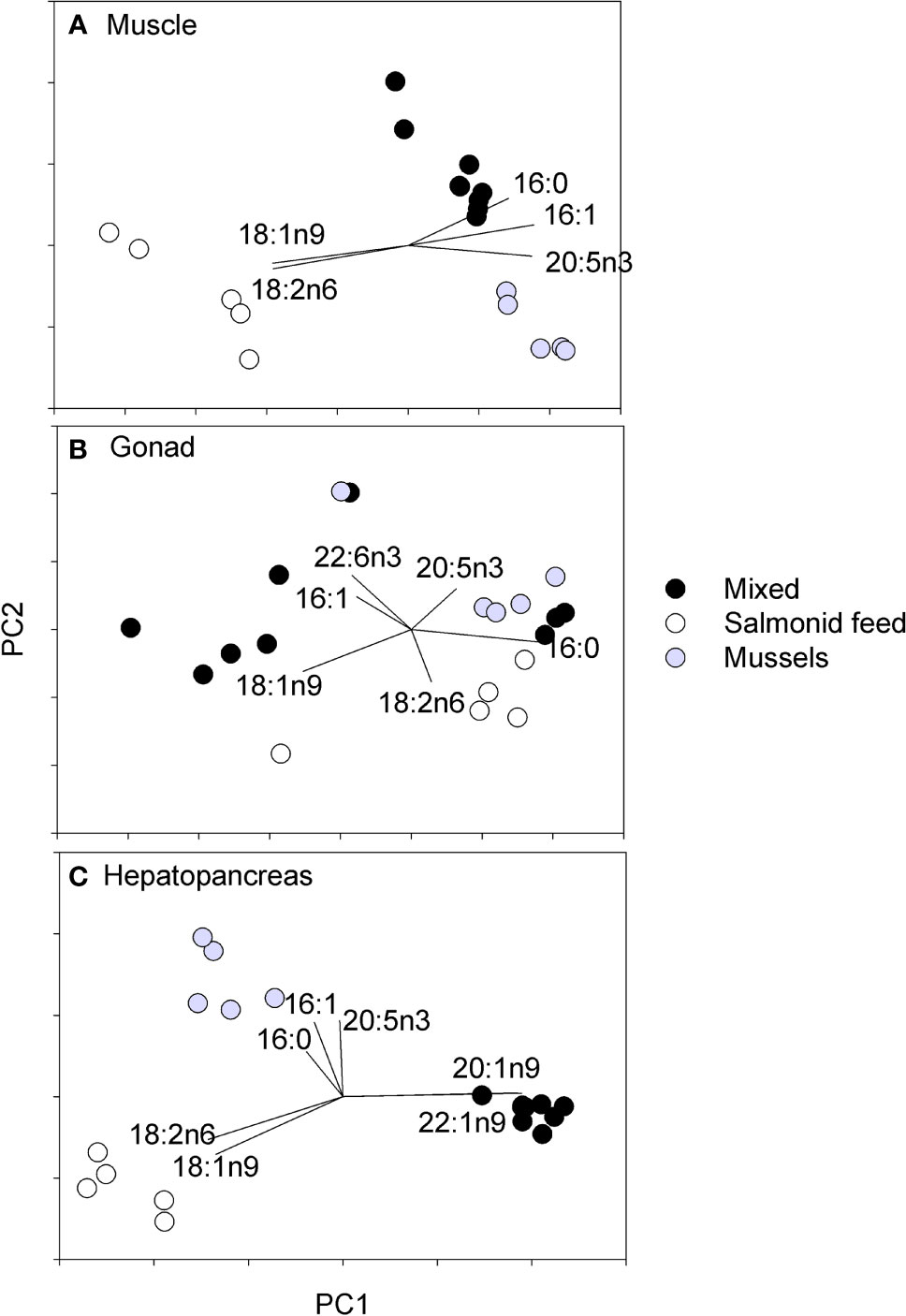
Figure 8 Principle component (PCA) analysis of the lipidic profiles in (A) muscle, (B) gonad, and (C) hepatopancreas of rock crabs, Cancer irroratus fed different experimental diets. Dots show means for each experimental tank (n = 5-9 for the different diets) and lines show loading of the fatty acids that contributed the most to diet differences (SIMPER analysis) within each tissue. The total number of individuals included in the analyses was 23, 11, and 13 for the mixed, salmonid feed and mussel diets, respectively.
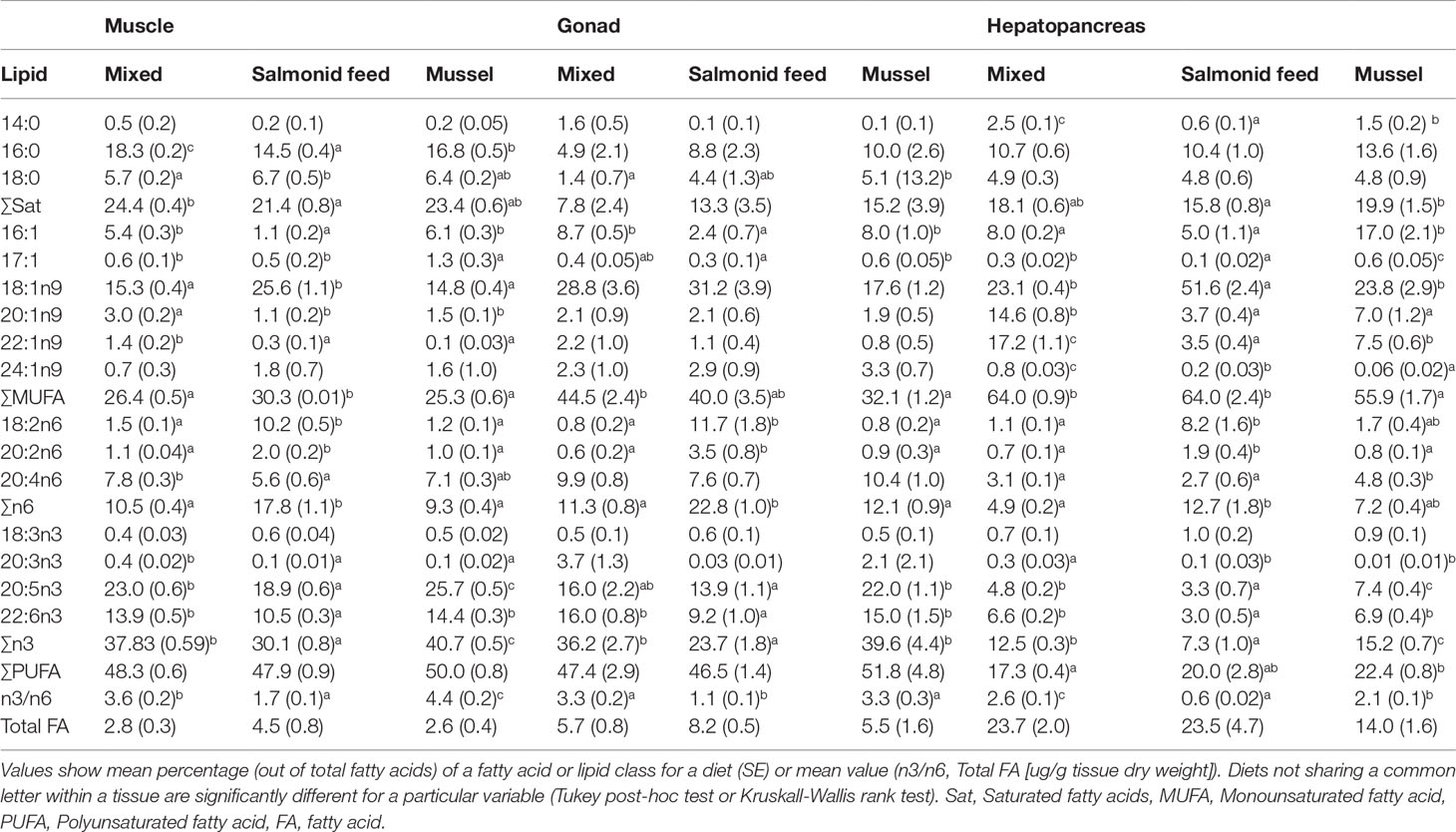
Table 1 Fatty acid profiles of muscle, gonad and hepatopancreas of rock crabs, Cancer irroratus, fed different experimental diets.
Total proportions of saturated and monounsaturated fatty acids often varied significantly as a function of diet, but the diet effect was inconsistent among the three tissues. For all three tissues, proportions of n-3 and n-6 and their ratio varied with diet with more n-6, less n-3 and a smaller n-3 to n-6 ratio for the salmonid feed diet when compared to the mixed and mussel diets (Table 1). Total fatty acid content was marginally affected by Diet (p = 0.041, 0.085, and 0.049 for muscle, gonad, and hepatopancreas respectively) but no multiple comparison was significant; overall, fatty acid tended to be more abundant in the salmonid feed diet (Table 1)
Recent reviews have highlighted that many wild species are more abundant within coastal aquaculture facilities when compared to the surrounding areas and that farms act as anthropogenic food subsidies to natural populations (Callier et al., 2018; Barrett et al., 2019; Theuerkauf et al., 2021). However, it is not clear if farms have a positive (acting as source), neutral, or negative (acting as a sink) effect on individuals, populations, and fisheries, and the factors determining whether farms act as population sources or sinks. The present study is part of a larger research program designed to address concerns raised by fisherman on long-term impacts of aquaculture on decapod fisheries in Eastern Canada. One concern was that farms may function as ecological sinks (Fernandez-Jover et al., 2011), attracting individuals from the surrounding area, where these could consume highly available mussels or salmonid feed, resulting in detrimental effects, because of poor nutritional quality of these readily available food items. Our study was designed to experimentally investigate the ‘worst-case scenarios’ in terms of individual performance when they consumed exclusively mussels or salmonid feed.
An important aspect of our work was to make sure that crabs had a comparable energy intake among diets to avoid potential effects of partial starvation. This was done by adjusting the amounts of each food item based on previous consumption (i.e. increase the amount when all the food was consumed in a previous period) to ensure crabs were fed to satiation. A good indication that starvation did not occur is the amount of food left; on average for all feedings, the amounts of edible food left (total weight of fish left, soft tissue left for shelled organisms, and pellets left untouched for salmonid feed) corresponded to 24.7, 22.1, and 16.2% of the amount given for the mixed, salmonid feed and mussel diets, respectively. In addition, our estimates of energy consumption did not relate to overall condition; the two diets for which estimated energy intake was lowest (mixed and mussel diets) were the diets for which indices of condition were better. In contrast, condition was poorest in the salmonid feed diet for which estimated energy consumption was highest. It is clear that these estimates are biased because it was not possible to recover all the small fragments of shells and small pieces of soft tissue (for fish and shelled organisms). The largest bias, probably leading to an over-estimation of energy intake, was for the salmonid feed diet; when crabs handled pellets, they tended to disintegrate into fine particles that were too small to be handled by the mouth pieces of crabs. However, based on visual observations, we are confident that the proportion of the pellets that disintegrated corresponded to less than 50% of the pellets that were consumed (estimates of energy consumed in the salmonid feed treatment was ~ twice that of the other diets). Although it cannot be formally excluded, it is unlikely that the differences observed in this study resulted from differential energy consumption as crabs in all treatments probably fed to satiation.
Overall, rock crabs fed exclusively mussels had performance and condition that were comparable to those fed a mixed diet designed to mimic a natural one that could be expected outside of farm areas. Differences were found in the lipidic profiles between the two diets for somatic tissues; the main fatty acids contributing to the differences (increased proportions of 16:0, 16:1, and 20:5n3 in the mussel diet) are found in high concentration in mytilid mussels (e.g. Alkani et al., 2007; Dernekbasi et al., 2015). A mussel diet thus appears to provide crabs all their required essential fatty acids, which is not surprising given that phytoplankton (the main producer of marine n-3 polyunsaturated fatty acids; Jónasdóttir, 2019) is an important part of mussel diets (e.g. Rouillon et al., 2005).
In contrast to the mussel diet, crabs feeding solely on salmonid feed had hindered performance and condition; with the exception of survival, all variables measured were negatively affected when compared to the mixed diet. These effects on condition and performance were accompanied by important switches in fatty acid profiles of somatic tissues (muscle and hepatopancreas) typical of wild organisms frequenting finfish farms, i.e., high lipid content, and low n3-n6 ratios driven by increases in proportions of 18:1n9 and 18:2n6 and decreases in 20:5n3 and 22:6n3 (reviewed in White et al., 2019). Specific dietary fatty acid requirements are not available for adult C. irroratus, but several studies have investigated essential fatty acids in larval and post-larval decapod crustaceans. Substituting fish oils for terrestrial plant oils had detrimental impacts (Noordin et al., 2015; Zhu et al., 2019) and polyunsaturated fatty acids, including 20:5n3, 20:4n6, and 22:6n3, are essential for proper development and various physiological processes (Harrison, 1991; Xu et al., 1994; Sheen and Wu, 2002; Thériault and Pernet, 2007; van der Meeren et al., 2009). It is possible that the observed deficiencies in essential fatty acids, especially 20:5n3 and 22:6n3, related to consumption of salmonid feed could be responsible for the whole suit of negative effects observed in adult C. irroratus. Other unmeasured dietary components may also have contributed to these effects. Essential minerals, such as calcium, may be assimilated when crabs ingest fragments of shells when they feed on natural prey items; low levels of minerals in salmonid feed may lead to deficiencies. Our results contrast with those of previous studies based in the field, where somatic condition of fish and invertebrates frequenting finfish farms was improved when compared to reference areas (Fernandez-Jover et al., 2007; Fernandez-Jover et al., 2011; Dempster et al., 2011; Izquierdo-Gomez et al., 2015), and invertebrate condition and/or growth was unaffected or positively affected by salmon farm wastes in trials pertaining to integrated multi-trophic aquaculture (Sarà et al., 2009; Lander et al., 2012; Baltadakis et al., 2020); only two studies found negative impacts on some variables measured in mussels (Handå et al., 2012; Irisarri et al., 2015).
One of our main objective was to evaluate the effect of diets on the reproductive output of crabs, i.e., clutch size, embryo development, larval development, and survival. Unfortunately, we were unable to get enough embryos to conduct this work. We were, however, able to gather information on other aspects of reproduction; we found no difference in the proportion of females that were observed forming a couple among diets (data not shown), suggesting the absence of diet-related behavioral changes. However, rock crabs fed the salmonid feed diet accumulated terrestrial fatty acids in gonads and had smaller gonads compared to crabs fed the mixed diet. Relatively few studies have investigated the impacts of a finfish feed diet on reproductive processes. Barrett et al. (2018) found no accumulation of terrestrial fatty acids in gonads of Atlantic cod (Gadus morhua) and minimal difference in the condition of eggs, embryos, and larvae in wild Atlantic cod caught in heavily-farmed areas compared to reference ones. In contrast, Gonzalez-Silvera et al. (2020) found accumulation of terrestrial fatty acids in four species of fish and accelerated ovarian development for individuals collected close to farms when compared to more distant areas. In a laboratory experiment, White et al. (2016) found no differences in lipid profiles in gonads or eggs in the sea urchin Heliocidaris erythrogramma fed a natural (mixed), current feed (n3-n6 ratios currently used by the industry), and future feed diets (very low n3-n6 ratios that could be used if the proportion of terrestrial fatty acids keep increasing in feed used by the industry); current feed did not influence reproductive processes, but future feed had serious consequences on egg diameter, fertilization, and larval development and survival. Finally, in the sea urchin Echinus acutus, a laboratory diet of fish feed increased gonad size, gonad, and egg terrestrial fatty acid content when compared to a natural diet (White et al., 2017b), resulting in reduced egg and larval quality (White et al., 2018).
While we observed negative impacts of feeding rock crabs solely with salmonid feed, it is important to remember that the experiment was designed to investigate the ‘worst-case scenarios’ where individuals are obliged to feed on a single item. The realized net impact in a field situation will depend on the contribution of salmonid feed in the overall diet. This in turn will depend on food selection when crabs are inside a farm and amount of time spent in that farm. Future studies should investigate dietary choices made by individuals in presence of several food items. It would also be useful to design laboratory experiments in which individuals receive various proportions of their total energy from salmonid feed and evaluate how this relates to lipidic composition and performance on a continuous scale. From there it would be possible to sample crabs from farm areas and estimate how much salmonid feed they consume in the wild. Preliminary comparisons are possible using data from Sardenne et al. (2020) in which detailed profiles were obtained from crabs collected in farms and surrounding areas. They found n3-n6 ratios slightly higher than our mixed diet in crabs collected outside farms (average from muscle, gonads, and hepatopancreas: Sardenne et al. 3.93 compared to 3.17 in this study) and also slightly higher under farms compared with that of crabs fed the salmonid feed diet (Sardenne et al. 1.60 compared to 1.13 in this study). While preliminary, this comparison suggests that crabs consume a good proportion of salmonid feed in farms as they have ratios similar to laboratory individuals feeding solely on fish food for 1.5 years. Even if crabs eat a great proportion of salmonid feed while below farms, the effect at the individual level will depend on the proportion of time spent there. Crabs could consume only salmonid feed when under a farm, but the negative effects might not be manifest if they only spend a few days or weeks under farms and return to unaffected areas where they resume a natural diet. Hydroacoustic telemetry data is currently being analyzed and this will inform on farm frequentation habits (McKindsey, unpublished data). These two factors (i.e. food selection and time spent under farms) may explain why field studies typically find positive or neutral effects of farms on somatic condition and reproductive processes. While fish feed can be a dominant component of stomach content of fish caught near fish farms, other natural prey items are also found (Fernandez-Jover et al., 2007; Fernandez-Jover et al., 2011); thus fish either still prey on other items or feed outside of farms. Similarly, suspension-feeding invertebrates ingest farm wastes (Sarà et al., 2009; Handå et al., 2012; Lander et al., 2012; Irisarri et al., 2015) but still have access to natural suspended matter and plankton communities. In contrast, White et al. (2016; 2017b; 2018 ) found negative impacts in sea urchins fed solely of specific pelletized food in the laboratory, a design similar to the present study. There likely exists a balance between essential fatty acid and/or minerals requirements and overall energy consumption. If an individual can fulfill its essential dietary requirements by feeding on a minimum amount of natural items, then the energy subsidy coming from consumption of salmonid feed could improve condition and reproduction. In contrast, if a specific combination of natural items and salmonid feed consumed does not meet essential requirements, then deleterious impacts might occur. Future studies should verify this idea and try to identify potential thresholds which are likely to vary according to specific composition of finfish feed used and taxon-specific dietary requirements.
Even if individuals spend significant time under farms and consume predominantly finfish feed, the effects at the individual level will translate into changes at the population level based on the proportion of a population that frequents farms (White et al., 2018). In turn, this value will depend on the overall proportion of the habitat where farms are present and farm attraction. Given the current extent of farming in Eastern Canada, the overall effect at the population level for C. irroratus is likely small, but could increase if aquaculture operations end up covering an important proportion of rock crab habitat.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DD, CR, RE, SR, BG and CM contributed to the design and concept of the study. CR was in charge of the experimental setup, with active contributions from RE and SR. DD and BG performed the statistical analyses. DD and CM obtained funding. DD was in charge of writing the original draft of the manuscript, together with CR, RE and SR. All authors reviewed and edited the manuscript contributing to the final version. All authors contributed to the article and approved the submitted version.
This research was funded by Fisheries and Oceans Canada through the Program for Aquaculture Regulatory Research to DD and CM (PARR-2017-Q-04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to R. Léger-Daigle, O. Larouche, L. Landry, E. Vadboncoeur and M. Chevrinais for help with the experiment, to R. Gagnon, Y. Gagnon, J. Gagnon, and F. Tremblay for their help with the laboratory setup, and M. Babin and R. Tremblay for help with the lipid analyses.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.865390/full#supplementary-material
Alkani T., Parrish C. C., Thompson R. J., McKenzie C. H. (2007). Role of Fatty Acids in Cultured Mussels, Mytilus edulis, Grown in Notre Dame Bay, Newfoundland. J. Exp. Mar. Biol. Ecol. 348, 33–45. doi: 10.1016/j.jembe.2007.02.017
Baltadakis A., Casserly J., Falconer L., Sprague M., Telfer T. C. (2020). European Lobsters Utilise Atlantic Salmon Wastes in Coastal Integrated Multi-Trophic Aquaculture Systems. Aquac. Environ. Interact. 12, 485–494. doi: 10.3354/aei00378
Barrett L. T., Swearer S. E., Dempster T. (2019). Impacts of Marine and Freshwater Aquaculture on Wildlife: A Global Meta-Analysis. Rev. Aquac. 11, 1022–1044. doi: 10.1111/raq.12277
Barrett L. T., Swearer S. E., Harboe T., Karlsen Ø, Meier S., Dempster T. (2018). Limited Evidence for Differential Reproductive Fitness of Wild Atlantic Cod in Areas of High and Low Salmon Farming Density. Aquac. Environ. Interact. 10, 369–383. doi: 10.3354/aei00275
Bigford T. E. (1979). “Synopsis of Biological Data on the Rock Crab, Cancer Irroratus Say,” in NOAA Technical Report NMFS Circular, 426. ( National Marine Fisheries Service, Woods Hole, MA, 1979)
Callier M. D., Byron C. J., Bengtson D. A., Cranford P. J., Cross S. F., Focken U., et al. (2018). Attraction and Repulsion of Mobile Wild Organisms to Finfish and Shellfish Aquaculture: A Review. Rev. Aquac. 10, 924–949. doi: 10.1111/raq.12208
Clynick B. G., McKindsey C. W., Archambault P. (2008). Distribution and Productivity of Fish and Macroinvertebrates in Mussel Aquaculture Sites in the Magdalen Islands (Québec, Canada). Aquaculture 283, 203–210. doi: 10.1016/j.aquaculture.2008.06.009
D’Amours O., Archambault P., McKindsey C. W., Johnson L. E. (2008). Local Enhancement of Epibentic Macrofauna by Aquaculture Activities. Mar. Ecol. Prog. Ser. 371, 73–84. doi: 10.3354/meps07672
Dempster T., Sanchez-Jerez P., Fernandez-Jover D., Bayle-Sempere J., Nilsen R., Bjørn P.-A., et al. (2011). Proxy Measures of Fitness Suggest Coastal Fish Farms can Act as Population Sources and Not Ecological Traps for Wild Gadoid Fish. PLoS One 6, e15646. doi: 10.1371/journal.pone.0015646
Dernekbasi S., Oksuz A., Celik M. Y., Karay I., Karay S. (2015). The Fatty Acid Composition of Cultured Mussels (Mytilus Galloprovincialis Lamarck 1819) in Offshore Longline System in the Black Sea. J. Aquac. Mar. Biol. 2, 254–258. doi: 10.15406/jamb.2015.02.00049
Drouin A., Archambault P., Clynick B., Richer K., McKindsey C. W. (2015). Influence of Mussel Aquaculture on the Distribution of Vagile Benthic Macrofauna in Îles De La Madeleine, Eastern Canada. Aquac. Environ. Interact. 6, 175–183. doi: 10.3354/aei00123
Fernandez-Jover D., Lopez Jimenez J. A., Sanchez-Jerez P., Bayle-Sempere J., Gimenez Casalduero P., Martinez Lopez F. J., et al. (2007). Changes in Body Condition and Fatty Acid Composition of Wild Mediterranean Horse Mackerel (Trachurus Mediterraneus, Steindachner 1868) Associated to Sea Cage Fish Farms. Mar. Environ. Res. 63, 1–18. doi: 10.1016/j.marenvres.2006.05.002
Fernandez-Jover D., Martinez-Rubio L., Sanchez-Jerez P., Bayle-Sempere J. T., Lopez Jimenez J. A., Martínez Lopez F. J., et al. (2011). Waste Feed From Coastal Fish Farms: A Trophic Subsidy With Compositional Side-Effects for Wild Gadoids. Estuar. Coast. Shelf. Sci. 91, 559–568. doi: 10.1016/j.ecss.2010.12.009
Fisheries and Oceans Canada (2021) Seafisheries Landings. Available at: https://www.dfo-mpo.gc.ca/stats/commercial/sea-maritimes-eng.htm (Accessed November 25, 2021).
Fox G. A. (2001). “Failure-Time Analysis”, in Design and Analysis of Ecological Experiments. Eds. Scheiner S. M., Gurevitch J. (New York, NY: Oxford University Press), 235–266.
Fréchette M. (2012). Self-Thinning, Biodeposit Production, and Organic Matter Input to the Bottom in Mussel Suspension Culture. J. Sea. Res. 67, 10–20. doi: 10.1016/j.seares.2011.08.006
Gendron L., Fradette P., Godbout G. (2001). The Importance of Rock Crab (Cancer irroratus) for Growth, Condition and Ovary Development of Adult American Lobster (Homarus americanus). J. Exp. Mar. Biol. Ecol. 262, 221–241. doi: 10.1016/s0022-0981(01)00297-0
George E. M., Parrish C. P. (2015). Invertebrate Uptake of Lipids in the Vicinity of Atlantic Salmon (Salmo Salar) Aquaculture Sites in British Columbia. Aquac. Res. 46, 1044–1065. doi: 10.1111/are.12259
Gonzalez-Silvera D., Izquierdo-Gomez D., Sanchez-Jerez P., Elbal M. T., López-Jiménez J. A., Martínez-López F. J. (2020). Influence of Aquaculture Waste on Fatty Acid Profiles and Gonad Maturation of Wild Fish Aggregations at Fish Farms. Mar. Environ. Res. 156, 104902. doi: 10.1016/j.marenvres.2020.104902
Handå A., Min H., Wang X., Broch O. J., Reitan K. I., Reinertsen H., et al. (2012). Incorporation of Fish Feed and Growth of Blue Mussels (Mytilus edulis) in Close Proximity to Salmon (Salmo Salar) Aquaculture: Implications for Integrated Multi-Trophic Aquaculture in Norwegian Coastal Waters. Aquaculture, 356−357. doi: 10.1016/j.aquaculture.2012.04.048
Harrison K. E. (1991). Essential Fatty Acid Metabolism and Requirements of the Lobster, Homarus Americanus (Halifax: Dalhousie University).
Hicks D., Johnson B. A. (1999). A Device to Measure Shell Hardness of Dungeness Crabs and Trial Application in the Kodiak Island, Alaska, Commercial Fishery. N. Am. J. Fish. Manage. 19, 581–590. doi: 10.1577/1548-8675(1999)019<0581:ADTMSH>2.0.CO;2
Holmer M. (2010). Environmental Issues of Fish Farming in Offshore Waters: Perspectives, Concerns and Research Needs. Aquac. Environ. Interact. 1, 57–70. doi: 10.3354/aei00007
Holmer M., Wildish D. J., Hargrave B. (2005). “Organic Enrichment From Marine Finfish Aquaculture and Effects on Sediment Biogeochemical Processes”, in The Handbook of Environmental Chemistry, Vol 5m. Water Pollution. Ed. Hargrave B. T. (Berlin: Springer-Verlag), 181–206.
Inglis G. J., Gust N. (2003). Potential Indirect Effects of Shellfish Culture on the Reproductive Success of Benthic Predators. J. Appl. Ecol. 40, 1077–1089. doi: 10.1111/j.1365-2664.2003.00860.x
Irisarri J., Fernández-Reiriz M. J., Labarta U., Cranford P. J., Robinson S. M. C. (2015). Availability and Utilization of Waste Fish Feed by Mussels Mytilus Edulis in a Commercial Integrated Multi-Trophic Aquaculture (IMTA) System: A Multi-Indicator Assessment Approach. Ecol. Indic. 48, 673–686. doi: 10.1016/j.ecolind.2014.09.030
Izquierdo-Gomez D., Sanchez-Jerez P., Bayle-Sempere J. T., Loader N. J., Garcia de Leaniz C. (2015). Effects of Coastal Fish Farms on Body Size and Isotope Composition of Wild Penaeid Prawn. Fish. Res. 172, 50–56. doi: 10.1016/j.fishres.2015.06.017
Jónasdóttir S. H. (2019). Fatty Acid Profiles and Production in Marine Phytoplankton. Mar. Drugs 17, 151. doi: 10.3390/md1730151
Kelly J. R., Scheibling R. E. (2012). Fatty Acids as Dietary Tracers in Benthic Food Webs. Mar. Ecol. Prog. Ser. 446, 1–22. doi: 10.3354/meps09559
Lander T. R., Robinson S. M. C., MacDonald B. A., Martin J. D. (2012). Enhanced Growth Rates and Condition Index of Blue Mussels (Mytilus Edulis) Held at Integrated Multitrophic Aquaculture Sites in the Bay of Fundy. J. Shellfish. Res. 31, 997–1007. doi: 10.2983/035.031.0412
Laroche O., Meier S., Mjøs S. A., Keeley N. (2021). Effects of Farm Activities on the Sponge, Weberella Bursa, and Its Associated Microbiota. Ecol. Indic. 129, 107879. doi: 10.1016/j.ecolind.2021.107879
Lepage G., Roy C. C. (1984). Improved Recovery of Fatty Acids Through Direct Transesterification Without Prior Extraction or Purification. J. Lipid Res. 25, 1391–1396. doi: 10.1016/S0022-2275(20)34457-6
McKindsey C. W. (2011). Aquaculture-Related Physical Alterations of Habitat Structure as Ecosystem Stressors (Ottawa, Canada:DFO Can. Sci. Advis. Sec. Res. Doc), viii + 154.
McKindsey C. W., Archambault P., Callier M. D., Olivier F. (2011). Influence of Suspended and Off-Bottom Mussel Culture on the Sea Bottom and Benthic Habitats: A Review. Can. J. Zool. 89, 622–646. doi: 10.1139/z11-037
Noordin N. M., Zeng C., Southgate P. C., Romano N. (2015). Effects of Dietary Fish Oil to Soybean Ratio on Survival, Development, and Growth of Early Juveniles of the Blue Swimmer Crab Portunus Pelagicus. J. Shellfish. Res. 34, 1065–1072. doi: 10.2983/035.034.0333
Olsen S. A., Ervik A., Grahl-Nielsen O. (2012). Tracing Fish Farm Waste in the Northern Shrimp Pandalus Borealis (Krøyer 1838) Using Lipid Biomarkers. Aquac. Environ. Interact. 2, 133–144. doi: 10.3354/aei00036
Rouillon G., Guerra Rivas J., Ochoa N., Navarro E. (2005). Phytoplankton Composition of the Stomach Contents of the Mussel Mytilus Edulis L. From Two Populations: Comparison With its Food Supply. J. Shellfish. Res. 24, 5–14. doi: 10.2983/0730-8000(2005)24[5:PCOTSC]2.0.CO;2
Salvo F., Dufour S. C., Hamoutene D., Parrish C. C. (2015). Lipid Classes and Fatty Acids in Ophryotrocha Cyclops, a Dorvilleid From Newfoundland Aquaculture Sites. PLoS One 10, e0136772. doi: 10.1371/journal.pone.0136772
Sarà G., Zenone A., Tomasello A. (2009). Growth of Mytilus Galloprovincialis (Mollusca, Bivavlia) Close to Fish Farms: A Case of Integrated Multi-Trophic Aquaculture Within the Tyrrhenian Sea. Hydrobiologia 636, 129–136. doi: 10.1007/s10750-009-9942-2
Sardenne F., Forget N., McKindsey C. W. (2019). Contribution of Mussel Fall-Off From Aquaculture to Wild Lobster Homarus Americanus Diet. Mar. Environ. Res. 149, 126–136. doi: 10.1016/j.marenvres.2019.06.003
Sardenne F., Simard M., Robinson S. M. C., McKindsey C. W. (2020). Consumption of Organic Wastes From Coastal Salmon Aquaculture by Wild Decapods. Sci. Total Environ. 711, 134863. doi: 10.1016/j.scitotenv.2019.134863
Scarratt D. J., Lowe R. (1972). Biology of Rock Crab (Cancer Irroratus) in Northumberland Strait. J. Fish. Res. Bd. Canada 29, 161–166. doi: 10.1139/f72-026
Sheen S. S., Wu S. W. (2002). Essential Fatty Acid Requirements of Juvenile Mud Crab, Scylla Serrata (Forskål 1775) (Decapoda: Scyllaridae). Crustaceana 75, 1387–1401. doi: 10.1163/156854002321629817
Thériault I., Pernet F. (2007). Lipid Nutrition and Settlement Behaviour in American Lobster Homarus americanus. Aquat. Biol. 1, 121–133. doi: 10.3354/ab00015
Theuerkauf S. J., Barrett L. T., Alleway H. K., Costa-Pierve B. A., St. Gelais A., Jones R. C. (2021). Habitat Value of Bivalve Shellfish and Seaweed Aquaculture for Fish and Invertebrates: Pathways, Synthesis and Next Steps. Rev. Aquac. 14, 54–72. doi: 10.1111/raq.12584
Turchini G. M., Tortensen B. E., Ng W.-K. (2009). Fish Oil Replacement in Finfish Nutrition. Rev. Aquac. 1, 10–57. doi: 10.1111/j.1753-5131.2008.01001.x
van der Meeren G., Tlusty M. F., Metzler A., van der Meeren T. (2009). Effects of Dietary DHA and EPA on Neurogenesis, Growth and Survival of Juvenile American Lobster, Homarus americanus. N. Z. J. Mar. Freshw. Res. 43, 225–232. doi: 10.1080/00288330909509996
Wang G., McGaw I. J. (2014). Use of Serum Protein Concentration as an Indicator of Quality and Physiological Condition in the Lobster Homarus americanus (Milne-Edwards 1837). J. Shellfish. Res. 33, 805–813. doi: 10.2983/035.033.0315
Wang G., McGaw I. J. (2016). Potential Use of Mussel Farms as Multitrophic on-Growth Sites for American Lobster, Homarus Americanus (Milne Edwards). Fish. Aquacult. J. 7, 161. doi: 10.4172/2150-3508.1000161
Wang X., Olsen L. M., Reitan K. I., Olsen Y. (2012). Discharge of Nutrient Wastes From Salmon Farms: Environmental Effects, and Potential for Integrated Multi-Trophic Aquaculture. Aquac. Environ. Interact. 2, 267–283. doi: 10.3354/aei00044
Weise A. M., Cromey C. J., Callier M. D., Archambault P., Chamberlain J., McKindsey C. W. (2009). Shellfish-DEPOMOD: Modelling the Biodeposition From Suspended Shellfish Aquaculture and Assessing Benthic Effects. Aquaculture 288, 239–253. doi: 10.1016/j.aquaculture.2008.12.001
Weitzman J., Steeves L., Bradford J., Filgueira R. (2019). “Far-Field and Near-Field Effects of Marine Aquaculture,” in World Seas: An Environmental Evaluation, 2nd Edition, Volume III: Ecological Issues and Environmental Impacts. Ed. Sheppard C. (London, England: Academic Press, London Annual Review of Ecology, Evolution, and Systematics), 197–220.
White C. A., Bannister R. J., Dworjanyn S. A., Husa V., Nichols P. D., Dempster T. (2018). Aquaculture-Derived Trophic Subsidy Boosts Populations of an Ecosystem Engineer. Aquac. Environ. Int. 10, 279–289. doi: 10.3354/aei00270
White C. A., Bannister R. J., Dworjanyn S. A., Husa V., Nichols P. D., Kutti T., et al. (2017b). Consumption of Aquaculture Waste Affects the Fatty Acid Metabolism of a Benthic Invertebrate. Sci. Total Environ. 586, 1170–1181. doi: 10.1016/j.scitotenv.2017.02.109
White C. A., Dworjanyn S. A., Nichols P. D., Mos B., Dempster T. (2016). Future Aquafeeds may Compromise Reproductive Fitness in a Marine Invertebrate. Mar. Environ. Res. 122, 67–75. doi: 10.1016/j.marenvres.2016.09.008
White C. A., Nichols P. D., Ross D. J., Dempster T. (2017a). Dispersal and Assimilation of an Aquaculture Waste Subsidy in a Low Productivity Coastal Environment. Mar. pollut. Bull. 120, 309–321. doi: 10.1016/j.marpolbul.2017.05.042
White C. A., Woodcock S. H., Bannister R. J., Nichols P. D. (2019). Terrestrial Fatty Acids as Tracers of Finfish Aquaculture Waste in the Marine Environment. Rev. Aquac. 11, 133–148. doi: 10.1111/raq.12230
Woodcock S. H., Meier S., Keeley N. B., Bannister R. J. (2019). Fate and Longevity of Terrestrial Fatty Acids From Caged Fin-Fish Aquaculture in Dynamic Coastal Marine Systems. Ecol. Indic. 103, 43–54. doi: 10.1016/j.ecolind.2019.03.057
Woodcock S. H., Strohmeier T., Strand Ø., Olsen S. A., Bannister R. J. (2018). Mobile Epibenthic Fauna Consume Organic Waste From Coastal Fin-Fish Aquaculture. Mar. Environ. Res. 137, 16–23. doi: 10.1016/j.marenvres.2018.02.017
Xu X. L., Ji W. J., Castell J. D., O’Dor R. K. (1994). Essential Fatty Acid Requirements of the Chinese Prawn, Penaeus Chinensis. Aquaculture 127, 29–40. doi: 10.1016/0044-8486(94)90189-9
Ytrestøyl T., Aas T. S., Åsgård T. (2015). Utilisation of Feed Resources in Production of Atlantic Salmon (Salmo salar) in Norway. Aquaculture 448, 365–374. doi: 10.1016/j.aquaculture.2015.06.023
Keywords: waste salmonid feed, mussel fall-offs, anthropogenic food subsidies, fatty acid composition, shellfish aquaculture, salmonid aquaculture
Citation: Drolet D, Riley C, Robert S, Estrada R, Gianasi BL and McKindsey CW (2022) Effect of Aquaculture-Related Diets on the Long-Term Performance and Condition of the Rock Crab, Cancer irroratus. Front. Mar. Sci. 9:865390. doi: 10.3389/fmars.2022.865390
Received: 29 January 2022; Accepted: 22 June 2022;
Published: 22 July 2022.
Edited by:
Øivind Strand, Norwegian Institute of Marine Research (IMR), NorwayCopyright © 2022 Drolet, Riley, Robert, Estrada, Gianasi and McKindsey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Drolet, ZGF2aWQuZHJvbGV0QGRmby1tcG8uZ2MuY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.