- 1Shenzhen Key Laboratory of Marine Microbiome Engineering, Institute for Advanced Study, Shenzhen University, Shenzhen, China
- 2Key Laboratory of Optoelectronic Devices and Systems, College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, China
- 3Shenzhen International Graduate School, Tsinghua University, Shenzhen, China
- 4College of Life Science, Hainan Normal University, Haikou, China
- 5South Laboratory of Ocean Science and Engineering (Guangdong, Zhuhai), Zhuhai, China
Few studies have systematically assessed the ecological status of mangrove wetlands following the stress of anthropogenic activities in China. This study investigated the spatial and seasonal distribution of benthic macroinvertebrate communities and assessed the ecological quality of mangrove habitats on an island scale in Hainan, China (containing the third largest mangrove area of China and the highest mangrove species richness). For the benthic macrofauna community structure, a total of 102 macrobenthic taxa belonging to 50 families were identified, with Crustaceans, Molluscs, Polychaetes, and Oligochaeta having relative abundances of 52.3%, 36.1%, 10.8%, and 0.8%, respectively. Decapoda and Gastropoda dominated the benthic community abundance. Non-metric multidimensional scaling and an analysis of similarities revealed significantly different macroinvertebrate assemblages among the regions during the two seasons. The South mangroves had the lowest macrofauna species numbers, biodiversity, richness, and abundance. The macrofaunal species richness, Shannon index, Margalef index, abundance, and biomass markedly affected by region and season. As indicated by the biotic indices AMBI (AZTI’s Marine Biotic Index) and M-AMBI, more than half of the mangrove habitats on Hainan Island were slightly to heavily disturbed and had poor to moderate ecological quality. Our results recommend long-term monitoring for evaluating the quality status of mangrove wetlands and avoiding extensive land-use conversion of mangroves. Holistic approaches considering ecological characteristics and combining information on both floral and faunal functionality would contribute to the effective management and conservation of mangroves in disturbed areas.
Introduction
Mangrove forests, which are distributed on tropical and subtropical coastlines, provide excellent habitats for marine organisms. Due to the rapid development of coastal regions, mangroves are under increasing threats from human activities (e.g., tourism, industry, agriculture, and aquaculture). To conserve and restore mangrove ecosystems, it is of particular significance to monitor their quality and health under different anthropogenic impacts.
Benthic macrofauna are poorly mobile and sensitive to environmental changes, yet they play an important role in linking the primary producers and higher trophic levels in marine ecosystems (Bouillon et al., 2002; Lee, 2008). Disturbances such as human activities and natural factors can lead to changes in their habitats, which in turn leads to changes in the species composition of macrobenthic communities (Lee et al., 2006). Marine macrobenthos are thus widely used as ecological indicators to assess the health of marine ecosystems (Ni et al., 2019; Dimitriou et al., 2020; Dong et al., 2021).
Hainan Island contains the third largest mangrove area of China and the highest mangrove species richness (Li and Lee, 1997; Chen et al., 2009). In recent decades, due to the rapid development of the economy, mangrove wetlands on Hainan Island have suffered from fragmentation and habitat degradation (Liao et al., 2019; Herbeck et al., 2020). Although previous studies have reported on the diversity of the mangrove benthos (e.g., crabs, mollusks, foraminifera, etc.) in some areas (Gu, 2017; Ma et al., 2018; Li et al., 2021), the benthic macrofauna diversity and ecological status of mangrove wetlands on Hainan Island as a whole are still unclear.
In the present study, we chose Hainan Island as a case study with the aim of (1) investigating the macrofaunal distribution and community composition of the mangrove wetlands on the whole island and (2) evaluating the ecological quality of the mangrove wetlands by examining the changes in species composition of macrobenthic communities over time and space. The biological and nonbiological factors (mangrove vegetation, land usage, and other environmental factors) shaping the composition of the macrofaunal community were also discussed to link mangrove wetland quality with different anthropogenic impact pressures. Given that mangrove system is such an important and distinctive coastal habitat and are currently threatened by natural and anthropogenic pressures, such studies will help to better understand their ecological responses to environmental pressures and guide mangrove wetland management.
Materials and Methods
Study Area
Hainan Island (18°10’–20°09’ N, 108°37’–111°01’ E) is located in the South China Sea, with a coastline of 1944.35 km. In this study, seven mangrove wetlands covering 97.5% of the mangrove areas on Hainan Island were selected (Figure 1), and divided into four regions (North, East, West, and South) based on their geographic locations. These were the Dongzhai Harbor Mangrove Reserve with 1508.14 ha of mangroves (North); the Bamen Bay Mangrove Reserve with 1036.03 ha (East); the Danzhou, Lingao, and Chengmai mangroves with 915.35 ha (West); and the Lingshui and Sanya mangroves with 143.5 ha (South) (Wang et al., 2019). The Bamen Bay (35 species), followed by the Dongzhai Harbor mangrove wetlands (23 species) was reported to contain the most number of mangrove species in China (Chen et al., 2009).
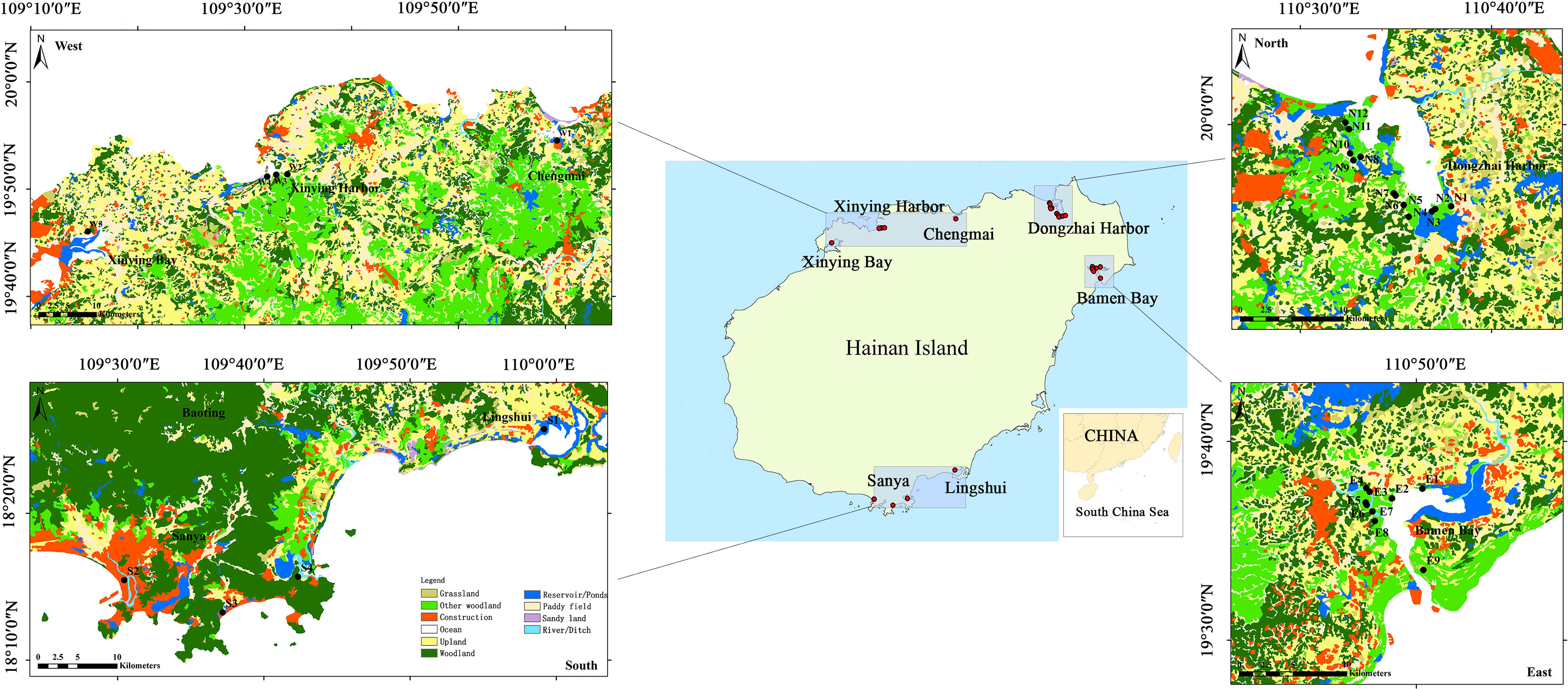
Figure 1 Land use map for mangrove wetlands with the sediment sample locations on Hainan Island in China. Note: mangrove areas are included in the other woodland.
Sampling and Analysis
Field campaigns were carried out in these seven mangrove wetlands during December 2018 (dry season) and August 2019 (wet season). As shown in Figure 1 and Supplementary Table 1, a total of 30 sampling sites (transects) were selected as the representative areas based on “Technical specification for eco-monitoring of mangrove ecosystem” (HY/T 081-2005, China). Surface sediments (0 –5 cm) were collected for determining the heavy metal content (Cr, Zn, Pb, Cu, As, and Cd) and performing other physicochemical analyses such as water content, nitrogen content, carbon content, etc. (Li et al., 2022). Sediment samples were transported in a cooler and stored at -20°C until analysis. The carbon and nitrogen contents of sediment samples were measured based on the combustion method (Schumacher, 2002) by a MARCO Cube Elemental Analyzer (Elementar, Germany). The temperature, pH, and salinity of water and sediments were measured in situ using a Thermo Scientific A321 pH Portable Meter (Thermo Fisher, USA), YSI Pro30 Salinity Instrument (YSI Inc, USA), and HM-TY soil salinity instrument (HM Inc, CHN), respectively. Heavy metals in the sediments were extracted according to the US EPA 3052, and determined by ICP-MS (Agilent 7700X, Agilent Technologies, USA). The mangrove species diversity, density, and structural characteristics were also investigated (Bai et al., 2021). The diameters of trees at breast height (DBH) in each plot were measured (Kauffman and Donato, 2012). The species, basal diameter, height and live/dead status of trees were recorded at the same time. More details of methodology were presented in Supplementary Material.
Benthic macrofauna samples were collected from the 30 sampling sites mentioned above. Three field plots (10 × 10 m) were designed at each site. Only one field plot could be designed for S2, S4 and W3 sites due to narrow mangrove areas. Three to five sediment samples were collected randomly from each plot using a 25 × 25 cm quadrat at a depth of 25 cm below the sediment surface. All the sediment from a quadrat was passed through a 0.5 mm mesh sieve to retrieve the macrofauna. The macrofauna collected were stored in 70% ethanol and then transported to the laboratory for further analysis. The processing, identification, counting, and weighing of the collected samples were carried out according to the Guidelines for Marine Biological Surveys (GB/T 12763.6-2007). Species richness, abundance, biomass, and diversity were determined to characterize the macrofaunal communities within the different habitats.
Biodiversity Analysis
The dominant macrofaunal species were determined by the Index of Relative Importance (IRI) (Pinkas, 1971) as follows:where N and W represents the proportions of abundance and biomass of each species, respectively, and F represents the percentage of each species at all sampling sites.
The diversity of the macrofaunal community was quantified using the Shannon diversity index (H′), Margalef richness index (D), and Pielou evenness index (J). The three indices are most well known and frequently used for biodiversity assessment of benthic macrofauna (Ni et al., 2019; Delfan et al., 2021; Yang et al., 2021).
Shannon index considers the proportional abundances of species and is more sensitive to changes in the rare species (Peet, 1974). It is calculated as follows (Shannon, 1948):Margalef richness index considers both abundances and species numbers, and is calculated as follows (Margalef, 1958):Pielou evenness index considers abundance and species occurrence, displaying the relations between the class frequencies (Palaghianu, 2014). It is calculated as follows (Pielou, 1969):where ni represents the number of individuals in the ith group, N represents the total number of individuals at each site, and S is the number of species at each site.
Statistical Analysis
Two-way ANOVA was used to assess effects of season and region on macrofaunal species richness, abundance, biomass, and diversity indices. Normality and homogeneity of variances were examined by the Shapiro–Wilk test and Levene’s test, respectively. One-way ANOVA with Tukey’s HSD test and independent-Samples T Test were applied to evaluate differences in macrofaunal abundance and diversity indices among regions and between seasons. All these analyses were conducted using SPSS 19.0 software. To visualize the spatial differences of the macrofaunal community structures, non-metric multidimensional scaling (NMDS) analyses were performed using Bray-Curtis dissimilarity between samples. For ordination analyses, only sites in which there were more than one species were included. The data was standardized in terms of relative abundance of the identified species in each site prior to analysis. An analysis of similarity (ANOSIM) test on Bray-Curtis distances was conducted to assess significant differences in the community structures between samples. NMDS and ANOSIM analyses were conducted using the vegan package in R 4.1.2 (Oksanen et al., 2019).
To assess the benthic ecological quality of the study area, AZTI’s Marine Biotic Index (AMBI) and multivariate AMBI (M-AMBI) were calculated using AMBI version 6.0 (http://ambi.azti.es). Considering that the mangrove areas in Hainan were affected by different levels of human activities, the M-AMBI reference conditions were obtained following the method based on previous studies (Borja and Tunberg, 2011; Cai et al., 2013; Li et al., 2017; Zhou et al., 2018; Yan et al., 2020): the highest values of the Shannon index H′ and species richness (S) in the present study were both increased by 15%, and the lowest AMBI value were selected as the M-AMBI reference condition under high quality status, e.g.,AMBI=0, H′=4.42, S = 20; and under bad quality status, AMBI = 6, H′=0, S = 0.
Results
Community Structure and Distribution of Benthic Macrofauna
As shown in Supplementary Table 2, a total of 102 species were observed in the four mangrove regions over the two seasons. These consisted of Crustacea (43.1% Decapoda, 9.8% Amphipoda, 3.9% Isopoda, and 1% each Tanaidacea, Stomatopoda, and Sessilia), Mollusca (20.6% Gastropoda and 10.8% Bivalvia), and Annelida (7.8% Polychaeta and 1.0% Oligochaeta). A total of 67 species were found in the dry season and 59 species in the wet season. Some species were only observed during one season, e.g., Corophium sp. and Audouinia comosa were only found in the dry season. The number of species found in the Hainan mangrove wetlands followed the order of North (72 species) >East and West (33 species each) > South (25 species). The North mangroves had the highest number of macrofaunal species in both seasons, while the mangroves in the South had the lowest number. Thus, the species richness varied not only by season, but also by region.
In the dry season, Gastropoda (25.7% of total individuals) were the most abundant, followed by Decapoda (23.3%) and Tanaidacea (21.2%) (Figure 2A). In the East and North, Tanaidacea predominated, with abundances of 43.2% and 31.9%, respectively. In contrast, Gastropoda (48.1%) dominated in the West and Decapoda (37.7%) in the South. The most abundant species were Geloina expansa in the East and South (IRI values of 5366.1 and 1543.6, respectively), Paradoxapseudes mortoni in the North (IRI 3036.8), and Assiminea sp. in the West (IRI 3256.4; Supplementary Table 2).
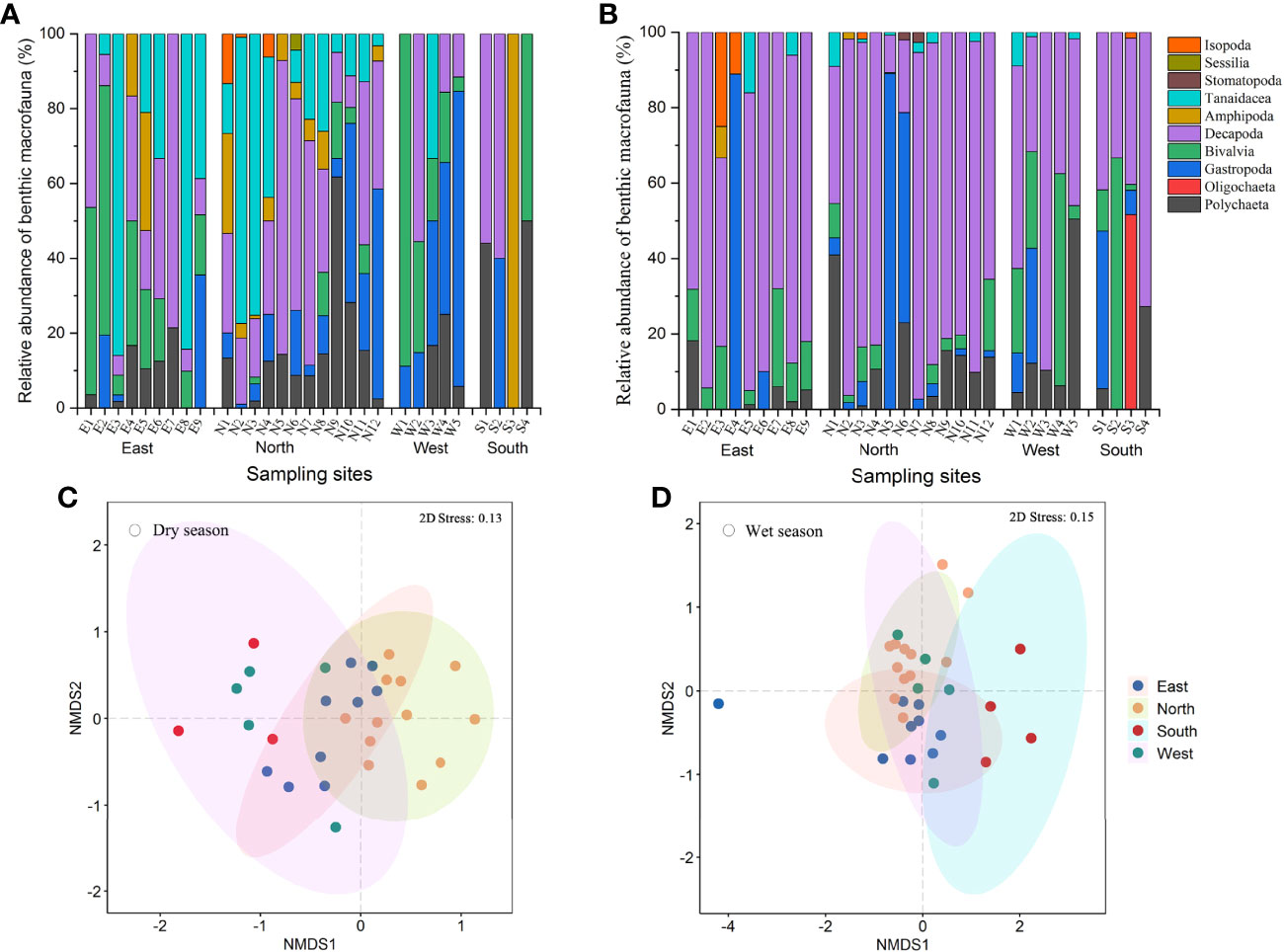
Figure 2 Benthic macrofauna in the mangrove wetlands on Hainan Island in different seasons. Community structure during the (A) dry and (B) wet seasons and non-metric multidimensional scaling (NMDS) maps during the (C) dry and (D) wet seasons.
During the wet season, Decapoda (53.6%) and Gastropoda (25.6%) predominated in the Hainan mangrove wetlands (Figure 2B). Decapoda was dominant in all regions, accounting for 77.3%, 49.0%, 52.1%, and 45.8% in the East, North, West, and South, respectively. The most predominant species in the East and North was Perisesarma bidens, with IRI values of 8327.0 and 4779.9, respectively, while Geloina expansa prevailed in the West (IRI 4322.3) and South (IRI 4202.8).
The NMDS ordinations showed that the four mangrove regions had distinct macrofaunal communities during the two seasons (Figures 2C, D). The two-dimensional ordinations had acceptably low stress values: 0.13 for the dry season dataset and 0.15 for the wet season dataset, respectively. The macrofaunal community at Site S3 in the dry season was not subjected to the NMDS analysis, as only one species of macrofauna was found. The statistically significant clustering of the macrofaunal communities was confirmed by an ANOSIM test based on the sample locations in the dry (R = 0.441, p = 0.001) and wet (R = 0.385, p = 0.001) seasons.
Abundance, Biomass, and Diversity of Benthic Macrofauna
Macrofaunal species richness, abundance, Shannon index, Margalef index, and Pielou index in the mangrove wetlands on Hainan Island did not differ significantly between seasons (Independent-Samples T Test, p > 0.05) (Figures 3A, B, D–F). The average biomass of the macrofauna in the Hainan mangroves was 92.3 ± 104.8 g·m−2 in the dry season, which was significantly lower than that in the wet season (190.0 ± 134.4 g·m−2) (Independent-Samples T Test, F = 0.723, p = 0.003) (Figure 3C). In the dry season, macrofaunal abundance in the West (One-way ANOVA, F = 4.022, p = 0.018), species richness (F = 19.519, p < 0.0001), Shannon index (F = 3.739, p = 0.023), and Margalef index (F = 23.099, p < 0.0001) in the North was significantly higher than that in other regions. During the wet season, macrofaunal species richness (F = 5.310, p = 0.005), Shannon index (F = 3.464, p = 0.031), and Margalef index (F = 4.042, p = 0.017) in the West was significantly higher than that in other regions. There was no significant variance observed in the macrofaunal biomass and Pielou index among the different regions in either season (One-way ANOVA, p > 0.05).
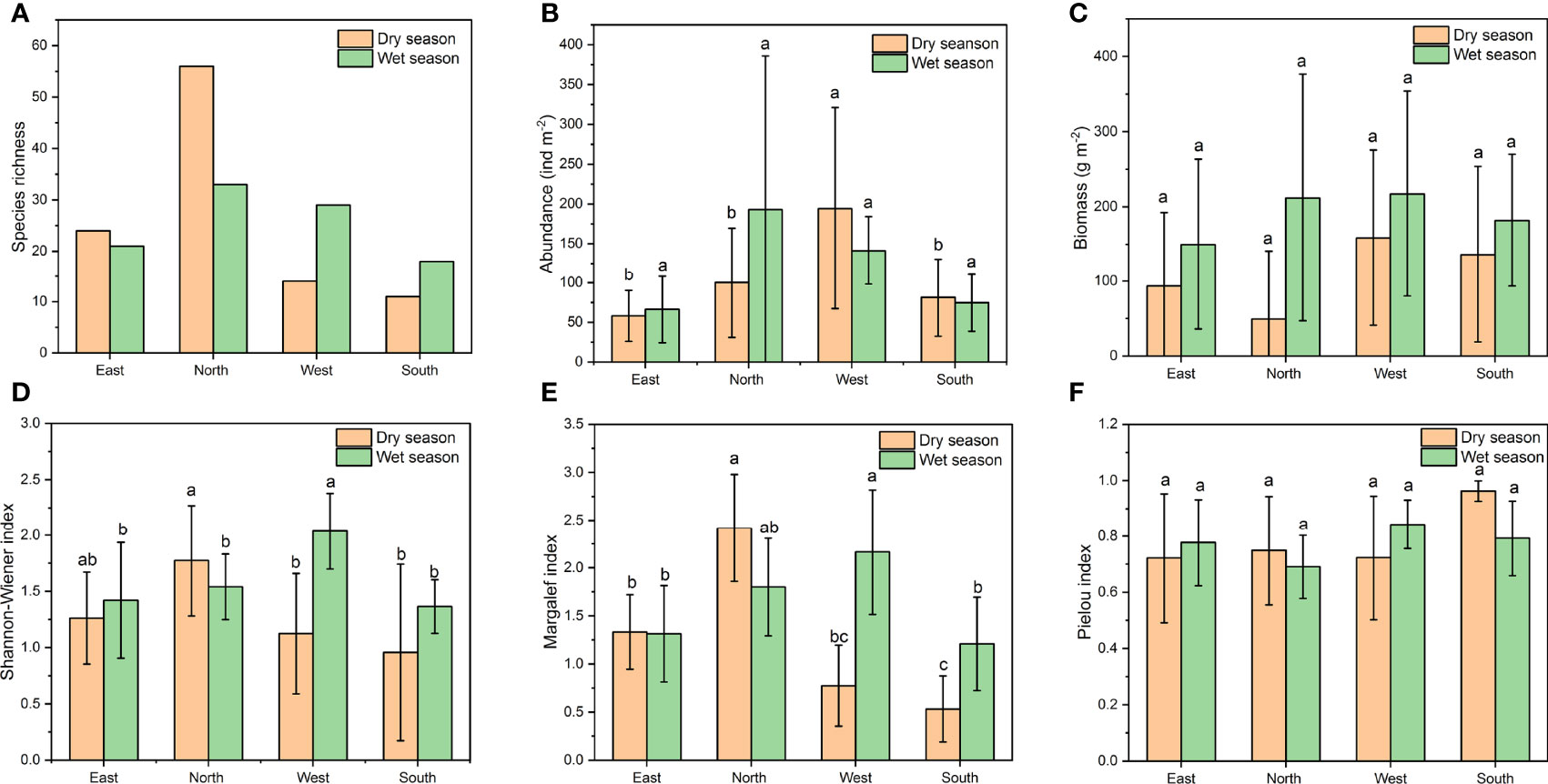
Figure 3 Species richness (A), abundance (B), biomass (C), and diversity indices [(D) Shannon-Wiener index; (E) Margalef index; (F) Pielou index] for the mangrove benthic macrofaunal communities on Hainan Island in different seasons.
Two-way ANOVA test for effects of region and season revealed that both factors significantly influenced the species richness (region: F = 14.719, p < 0.0001; season: F = 9.352, p = 0.004), Shannon index (region: F = 3.272, p = 0.028; season: F = 5.852, p = 0.019), and Margalef index (region: F = 15.460, p < 0.0001; season: F = 6.126, p = 0.017) for the benthic macrofauna (Supplementary Table 3). Moreover, there was significant interaction effect between region and season for the species richness (F = 5.679, p = 0.002), Shannon index (F = 4.016, p = 0.012), and Margalef index (F = 10.253, p < 0.0001). Macrofaunal abundance and biomass was significantly affected by region (F = 3.450, p = 0.023) and season (F = 5.485, p = 0.023), respectively. Neither region nor season had a significant effect on the Pielou index (region: F = 1.641, p = 0.191; season: F = 0.088, p = 0.768). There was no significant interaction between region and season for the abundance, biomass and Pielou index.
Ecological Quality Status of the Mangrove Habitats
The AMBI and M-AMBI indices were used to evaluate the ecological quality of the mangrove habitats on Hainan Island (Figure 4). The AMBI is based on the abundance of ecological macrofaunal groups, from sensitive species to opportunistic species (Supplementary Figure 1). Generally, an AMBI <1.2 suggests an undisturbed area, whereas 1.2–3.3, 3.3–4.3, 4.3–5.5, and >5.5 indicate slightly, moderately, heavily, and extremely disturbed areas, respectively (Muxika et al., 2005). The average AMBI value for the mangrove wetlands did not differ significantly between seasons (Independent-Samples T Test, p = 0.064), although the average values during the wet season was slightly higher than that in the dry season. In the dry season, 56.7% of the mangrove habitats on Hainan Island were slightly or moderately disturbed (1.2 < AMBI < 4.3). The AMBI values followed the order of South (2.8) > West (1.7) > East (1.5) > North (1.3), indicating the mangrove ecosystem in the South was more strongly disturbed than the other regions. Among the sites, E7, N9, and S1 were moderately disturbed during this season. In the wet season, nearly 86.7% of the mangroves on Hainan Island suffered from slight to heavy disturbances. The East and West had the highest AMBI values (2.5), followed by the North (2.0) and South (1.4), indicating the East and West were more disturbed than other regions. Sites N4 and N9 were moderately disturbed, and W5 was heavily disturbed in this season.

Figure 4 Habitat ecological quality of the mangrove wetlands on Hainan Island based on AZTI’s Marine Biotic Index (AMBI) during the (A) dry and (B) wet seasons and the multivariate AMBI in the (C) dry and (D) wet seasons.
The threshold values for M-AMBI classifications are as follows. An M-AMBI <0.2 refers to bad ecological quality, while 0.20–0.38, 0.38–0.53, 0.53–0.77, and >0.77 indicate poor, moderate, good, and high ecological habitat quality, respectively (Borja et al., 2007; Borja et al., 2009). The average M-AMBI values in the Hainan mangroves were 0.54 ± 0.14 in the dry season and 0.52 ± 0.12 in the wet season, indicating the overall ecological quality was good and moderate, respectively. Over 50.0% of the mangroves on Hainan Island had a poor to moderate ecological quality in both seasons. In the dry season, the M-AMBI values followed the order of North (0.66) > East (0.52) > West (0.43) > South (0.37), which suggests the ecological quality of the mangroves in the North was better than other regions and that the South region was the worst. It should be noted that, the ecological quality of the mangroves at Sites W1 in the West, S3 and S4 in the South was poor (M-AMBI <0.38). In contrast, during the wet season, the M-AMBI values in the West were the highest (0.61), indicating that the West mangrove wetlands had the better ecological quality. The West was followed by the North (0.55), South (0.50), and East (0.45), indicating the East had the worst ecological quality, with 77.8% of the mangrove wetlands rated as poor to moderate. In particular, the ecological quality of the mangroves at Sites E4, E6 and N9 was at the poor level (M-AMBI <0.38).
Discussion
The macrofauna communities in the mangrove wetlands of Hainan Island in this study were similar to the one observed in June 2009 (Zhang et al., 2016), which was dominated by polychaetes, crustaceans, and mollusks. Variations in environmental parameters such as rainfall, water temperature, pH, and salinity might contribute to shaping macrofaunal communities in different seasons and regions. Our previous results found that the physicochemical characters of the sediments were significantly distinct between these four regions, e.g., the East sediments were found to contain significantly higher concentrations of total organic nitrogen, total nitrogen, total organic carbon, and total carbon. The mean water salinity and pH in the Hainan mangroves were higher in the dry season than in the wet season, and the water content of the sediments was lower in the dry season (Bai et al., 2021; Wang et al., 2021). Salinity has been reported as the main environmental factor affecting benthic macroinvertebrate community composition and structure in estuarine ecosystems (Conde et al., 2013; Verdelhos et al., 2015; Little et al., 2017). For instance, Mariano and Barros (2014) found that several abundant macrofaunal species showed specific preferences for different salinities along a salinity gradient; among these, the polychaete families Capitellidae, Nereididae, and Spionidae appeared mainly at low salinity.
In addition to variation in environmental parameters, anthropogenic contamination can also affect benthic communities (Johnston and Roberts, 2009). In this study, the sediments in the North contained the highest heavy metal (Cr, Zn, Pb, Cu, As, and Cd) contents (Li et al., 2022), but they also had the higher macrofaunal species richness and diversity. Metal contamination increasing macrofaunal richness may be due to species replacement with those having a higher tolerance to pollutants than others. Infaunal communities in metal-contaminated sediments are generally governed by metal-resistant opportunistic deposit-feeding polychaetes (Belan, 2004; Lancellotti and Stotz, 2004). The high heavy-metal enriched North also showed the clear abundance of Capitella sp., which is a typical indicator species for pollution (Grassle and Grassle, 1976; Pearson and Rosenberg, 1977). Metal contamination in the Hainan mangroves is mainly from aquacultural sewage and agricultural runoff (Li et al., 2022), which may lead to the increased availability of nutrients with a concomitant increase in species richness (Grall and Chauvaud, 2002). The presence of these nutrients at low concentrations does not affect the macrofauna, which may even benefit from the additional supply (Ribeiro et al., 2016); however, an oversupply of nutrients will cause an opposite effect (Giles, 2008), especially in species with low tolerance to stressful conditions (Weisberg et al., 1997; King et al., 2005).
The number of macrofaunal species observed in this study during the wet season was comparable to the same period in 2009 (56 species). The macrofaunal biomass was higher than in 2009, but the average abundance and diversity (Shannon index) were much lower (Zhang et al., 2016). Reduction of macrofaunal diversity might be caused by habitat loss or habitat degradation (Airoldi et al., 2008; Carugati et al., 2018). The mangrove areas in Hainan Island was reported to decrease by 9.3% between 1987 and 2017, which was likely driven by human activities, such as land conversion for aquaculture, tourism development, and wastewater discharge (Liao et al., 2019). Region and season were revealed to significantly influence the species richness, Shannon index, Margalef index, abundance, and biomass for the benthic macrofauna in this study. The macrofaunal biomass during the wet season was higher than those in the dry season, which is consistent with previous findings. Zou et al. (1999) found that macrofaunal biomass in the mud flat of the Dongzhai Harbor mangroves on Hainan Island was higher in summer (June) than in winter (December).
Comparing the benthic macrofauna regionally, the higher species numbers, Shannon index and Margalef index were found in the North, while the lowest were in the South. Such spatial differences may be related to the land-use patterns of the mangrove wetlands in different regions. The North region has a higher proportion of mangrove area (accounting for 30.8% of the total area) than other regions, while the South has the lowest (0.5%) (Li et al., 2022). In general, structurally complex habitats support a higher density of benthic organisms than non-vegetated habitats due to their lower predation pressure, greater number of settlement areas, and enhanced nutrient availability (Alfaro, 2006). The complex structures of mangroves can provide shelter for various benthic fauna from predation, while the mangrove litter provides a direct or indirect food source for some macrobenthos such as gastropods and crabs (Nagelkerken et al., 2008). Thus, the loss of mangroves in the South might have resulted in a reduction in biodiversity and macrofaunal abundance, which is consistent with previous studies’ findings (Ribeiro et al., 2016; Carugati et al., 2018).
According to the results of AMBI and M-AMBI analysis, the degree of disturbance to the Hainan mangroves and the ecological quality of the mangrove habitats might be related to the mangrove area and land-use patterns. For instance, Sites S3 and S4, determined as habitats with poor ecological quality, had the smallest mangrove areas (with proportions of the total area of only 0.001% and 2.03%, respectively). Site W5, determined as heavily disturbed area, had a mangrove area ratio of less than 0.001%. Mangrove wetland at W5 was strongly affected by agriculture, aquaculture, and construction activities, which accounted for more than 50% of the total area (Li et al., 2022). It indicates the importance of the mangrove habitat to benthic community structure and that a loss of mangrove habitat can lead to a decline in ecological quality. Importantly, the impact of human activities on the ecological quality of mangroves cannot be ignored.
Conclusion
With the current intensive anthropogenic pressures on coastal marine ecosystems, it is crucial to conduct investigations on the quality and health status of these important habitats. Taking Hainan Island as an example, this study provides a comprehensive investigation of the community structure and spatial distribution of benthic macrofauna within different mangrove wetlands and evaluates the ecological status of the mangrove habitats. The results showed that Crustaceans were the most abundant group, followed by Molluscs, Polychaetes, and Oligochaeta. Among them, Decapoda and Gastropoda dominated the benthic community abundance. Except for biomass, macrofaunal species richness, abundance, Shannon index, Margalef index, and Pielou index did not differ significantly between seasons. Macrofaunal species richness, Shannon index, and Margalef index were significantly varied in different regions in both seasons. Seasonal fluctuations of environmental factors and different land usage patterns may explain the macrofaunal community variations by season and region, respectively. Based on the macrofaunal community, the AMBI and M-AMBI indices revealed that more than half of the mangrove habitats on Hainan Island were slightly to heavily disturbed and had poor to moderate ecological quality in both seasons. The study implies that the impact of human activities on the ecological quality of mangroves cannot be ignored. We recommend long-term monitoring of the composition and traits of resident fauna to evaluate the quality status of mangrove ecosystem. Moreover, extensive land-use conversion of mangrove wetlands into aquaculture ponds or construction land etc. should be avoided. Finally, holistic approaches considering ecological characteristics and combining information on both floral and faunal functionality would be benefit for effective management, conservation, and restoration for these threatened mangrove ecosystems.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
PL: Conceptualization, Data Curation, Writing-original draft. JL: Visualization, biological data analysis. JB, YT, and YM: Investigation, Formal analysis. XD: Methodology. KP: Validation, Resources. XZ: Project administration, Supervision, Writing- review and editing. GL: Supervision, Resources. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Ministry of Science and Technology of China (2017FY100703), Shenzhen Fundamental Research project (WDZC202008191733 45002) and Shenzhen Key Laboratory of Marine IntelliSense and Computation (ZDSYS20200811142605016). It was also supported in part by the fellowship of China Postdoctoral Science Foundation (2020M672780) and Guangdong Basic and Applied Basic Research Foundation (2019A1515110930).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all scientists who participated in sampling throughout the years. We wish to thank the editors and the reviewers for their valuable comments and suggestions on this paper.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.861718/full#supplementary-material
References
Airoldi L., Balata D., Beck M. W. (2008). The Gray Zone: Relationships Between Habitat Loss and Marine Diversity and Their Applications in Conservation. J. Exp. Mar. Biol. Ecol. 366, 8–15. doi: 10.1016/j.jembe.2008.07.034
Alfaro A. C. (2006). Benthic Macro-Invertebrate Community Composition Within a Mangrove/Seagrass Estuary in Northern New Zealand. Estuar. Coast. Shelf. Sci. 66, 97–110. doi: 10.1016/j.ecss.2005.07.024
Bai J., Meng Y., Gou R., Lyu J., Dai Z., Diao X., et al. (2021). Mangrove Diversity Enhances Plant Biomass Production and Carbon Storage in Hainan Island, China. Funct. Ecol. 35, 774–786. doi: 10.1111/1365-2435.13753
Belan T. A. (2004). Marine Environmental Quality Assessment Using Polychaete Taxocene Characteristics in Vancouver Harbour. Mar. Environ. Res. 57, 89–101. doi: 10.1016/S0141-1136(03)00062-X
Borja A., Josefson A. B., Miles A., Muxika I., Olsgard F., Phillips G., et al. (2007). An Approach to the Intercalibration of Benthic Ecological Status Assessment in the North Atlantic Ecoregion, According to the European Water Framework Directive. Mar. Pollut. Bull. 55, 42–52. doi: 10.1016/j.marpolbul.2006.08.018
Borja A., Miles A., Occhipinti-Ambrogi A., Berg T. (2009). Current Status of Macroinvertebrate Methods Used for Assessing the Quality of European Marine Waters: Implementing the Water Framework Directive. Hydrobiologia 633, 181–196. doi: 10.1007/s10750-009-9881-y
Borja A., Tunberg B. G. (2011). Assessing Benthic Health in Stressed Subtropical Estuaries, Eastern Florida, USA Using AMBI and M-AMBI. Ecol. Indic. 11, 295–303. doi: 10.1016/j.ecolind.2010.05.007
Bouillon S., Koedam N., Raman A., Dehairs F. (2002). Primary Producers Sustaining Macro-Invertebrate Communities in Intertidal Mangrove Forests. Oecologia 130, 441–448. doi: 10.1007/s004420100814
Cai W., Meng W., Zhu Y., Zhou J., Liu L. (2013). Assessing Benthic Ecological Status in Stressed Liaodong Bay (China) With AMBI and M-AMBI. Chin. J. Oceanol. Limnol. 31, 482–492. doi: 10.1007/s00343-013-2177-0
Carugati L., Gatto B., Rastelli E., Lo Martire M., Coral C., Greco S., et al. (2018). Impact of Mangrove Forests Degradation on Biodiversity and Ecosystem Functioning. Sci. Rep. 8, 13298. doi: 10.1038/s41598-018-31683-0
Chen L., Wang W., Zhang Y., Lin G. (2009). Recent Progresses in Mangrove Conservation, Restoration and Research in China. J. Plant Ecol. 2, 45–54. doi: 10.1093/jpe/rtp009
Conde A., Novais J. M., Dominguez J. (2013). Intertidal Macrofauna and Environmental Stress at a Riverine-Marine Boundary. Mar. Environ. Res. 92, 1–9. doi: 10.1016/j.marenvres.2013.07.002
Delfan N., Shojaei M. G., Naderloo R. (2021). Patterns of Structural and Functional Diversity of Macrofaunal Communities in a Subtropical Mangrove Ecosystem. Estuar. Coast. Shelf. Sci. 252, 107288. doi: 10.1016/j.ecss.2021.107288
Dimitriou P. D., Chatzinikolaou E., Arvanitidis C. (2020). Ecological Status Assessment Based on Benthic Macrofauna of Three Mediterranean Ports: Comparisons Across Seasons, Activities and Regions. Mar. Pollut. Bull. 153, 110997. doi: 10.1016/j.marpolbul.2020.110997
Dong J.-Y., Sun X., Zhang Y., Zhan Q., Zhang X. (2021). Assessing Benthic Habitat Ecological Quality Using Four Benthic Indices in the Coastal Waters of Sanshandao, Laizhou Bay, China. Ecol. Indic. 129, 107980. doi: 10.1016/j.ecolind.2021.107980
Giles H. (2008). Using Bayesian Networks to Examine Consistent Trends in Fish Farm Benthic Impact Studies. Aquaculture 274, 181–195. doi: 10.1016/j.aquaculture.2007.11.020
Grall J., Chauvaud L. (2002). Marine Eutrophication and Benthos: The Need for New Approaches and Concepts. Global Change Biol. 8, 813–830. doi: 10.1046/j.1365-2486.2002.00519.x
Grassle J. P., Grassle J. F. (1976). Sibling Species in the Marine Pollution Indicator Capitella (Polychaeta). Science 192, 567–569. doi: 10.1126/science.1257794
Gu K. (2017). Distribution Characteristics and Influence Factors of Crabs Among Mangrove Communities (Dongzhaigang Bay, Hainan: Xiamen University).
Herbeck L. S., Krumme U., Andersen T. J., Jennerjahn T. C. (2020). Decadal Trends in Mangrove and Pond Aquaculture Cover on Hainan (China) Since 1966: Mangrove Loss, Fragmentation and Associated Biogeochemical Changes. Estuar. Coast. Shelf. Sci. 233, 106531. doi: 10.1016/j.ecss.2019.106531
Johnston E. L., Roberts D. A. (2009). Contaminants Reduce the Richness and Evenness of Marine Communities: A Review and Meta-Analysis. Environ. Pollut. 157, 1745–1752. doi: 10.1016/j.envpol.2009.02.017
King R. S., Hines A. H., Craige F. D., Grap S. (2005). Regional, Watershed and Local Correlates of Blue Crab and Bivalve Abundances in Subestuaries of Chesapeake Bay, USA. J. Exp. Mar. Biol. Ecol. 319, 101–116. doi: 10.1016/j.jembe.2004.05.022
Kauffman J. B., Donato D. C. (2012). Protocols for the Measurement, Monitoring and Reporting of Structure, Biomass and Carbon Stocks in Mangrove Forests. Working Paper 86. Bogor, Indonesia: Center for International Forestry Research (CIFOR). Available at: https://www.researchgate.net/publication/264892201_W_o_r_k_i_n_g_P_a_P_e_r_Protocols_for_the_measurement_monitoring_and_reporting_of_structure_biomass_and_carbon_stocks_in_mangrove_forests.
Lancellotti D., Stotz W. (2004). Effects of Shoreline Discharge of Iron Mine Tailings on a Marine Soft-Bottom Community in Northern Chile. Mar. Pollut. Bull. 48, 303–312. doi: 10.1016/j.marpolbul.2003.08.005
Lee S. Y. (2008). Mangrove Macrobenthos: Assemblages, Services, and Linkages. J. Sea. Res. 59, 16–29. doi: 10.1016/j.seares.2007.05.002
Lee S. Y., Dunn R. J. K., Young R. A., Connolly R. M., Dale P. E. R., Dehayr R., et al. (2006). Impact of Urbanization on Coastal Wetland Structure and Function. Austral Ecol. 31, 149–163. doi: 10.1111/j.1442-9993.2006.01581.x
Liao J., Zhen J, Zhang L., Metternicht G. (2019). Understanding Dynamics of Mangrove Forest on Protected Areas of Hainan Island, China: 30 Years of Evidence From Remote Sensing. Sustainability 11, 5356. doi: 10.3390/su11195356
Li T., Cai G., Zhang M., Li S., Nie X. (2021). The Response of Benthic Foraminifera to Heavy Metals and Grain Sizes: A Case Study From Hainan Island, China. Mar. Pollut. Bull. 167, 112328. doi: 10.1016/j.marpolbul.2021.112328
Li M. S., Lee S. Y. (1997). Mangroves of China: A Brief Review. For. Ecol. Manage. 96, 241–259. doi: 10.1016/S0378-1127(97)00054-6
Li P., Li X., Bai J., Meng Y., Diao X., Pan K., et al. (2022). Effects of Land Use on the Heavy Metal Pollution in Mangrove Sediments: Study on a Whole Island Scale in Hainan, China. Sci. Tot. Environ. 824, 153856. doi: 10.1016/j.scitotenv.2022.153856
Li B., Li X., Bouma T. J., Soissons L. M., Cozzoli F., Wang Q., et al. (2017). Analysis of Macrobenthic Assemblages and Ecological Health of Yellow River Delta, China, Using AMBI & M-AMBI Assessment Method. Mar. Pollut. Bull. 119, 23–32. doi: 10.1016/j.marpolbul.2017.03.044
Little S., Wood P. J., Elliott M. (2017). Quantifying Salinity-Induced Changes on Estuarine Benthic Fauna: The Potential Implications of Climate Change. Estuar. Coast. Shelf. Sci. 198, 610–625. doi: 10.1016/j.ecss.2016.07.020
Mariano D. L. S., Barros F. (2014). Intertidal Benthic Macrofaunal Assemblages: Changes in Structure Along Entire Tropical Estuarine Salinity Gradients. J. Mar. Biol. Assoc. Unit Kingdom. 95, 5–15. doi: 10.1017/S002531541400126X
Ma W., Wang M., Wang W., Liu Y., Luo L., Tang C. (2018). Biodiversity of Mangrove Mollusks in the West Coast of Hainan Island, China. Biodiver. Sci. 26, 707–716. doi: 10.17520/biods.2018104
Muxika I., Borja A., Bonne W. (2005). The Suitability of the Marine Biotic Index (AMBI) to New Impact Sources Along European Coasts. Ecol. Indic. 5, 19–31. doi: 10.1016/j.ecolind.2004.08.004
Nagelkerken I., Blaber S. J. M., Bouillon S., Green P., Haywood M., Kirton L. G., et al. (2008). The Habitat Function of Mangroves for Terrestrial and Marine Fauna: A Review. Aquat. Bot. 89, 155–185. doi: 10.1016/j.aquabot.2007.12.007
Ni D., Zhang Z., Liu X. (2019). Benthic Ecological Quality Assessment of the Bohai Sea, China Using Marine Biotic Indices. Mar. Pollut. Bull. 142, 457–464. doi: 10.1016/j.marpolbul.2019.03.055
Oksanen J., Blanchet F., Friendly M., Kindt R., Legendre P., McGlinn D., et al. (2019). Vegan: Community Ecology Package. R Package Version 2.5-5.
Palaghianu C. (2014). A Tool for Computing Diversity and Consideration on Differences Between Diversity Indices. J. Landscape Manage. 5, 78–82. doi: 10.48550/arXiv.1602.04005
Pearson T. H., Rosenberg R. (1977). Macrobenthic Succession in Relation to Organic Enrichment and Pollution of the Marine Environment. Oceanog. Mar. Biol.: An. Annu. Rev. 16, 229–311.
Peet R. K. (1974). The Measurement of Species Diversity. Annu. Rev. Ecol. Systemat. 5, 285–307. doi: 10.1146/annurev.es.05.110174.001441
Pinkas L. (1971). Food Habits of Albacore, Bluefin Tuna and Bonito in California Waters. Calif. Dept. Fish. Game. Fish. Bull. 152, 1–105.
Ribeiro L. F., Eca G. F., Barros F., Hatje V. (2016). Impacts of Shrimp Farming Cultivation Cycles on Macrobenthic Assemblages and Chemistry of Sediments. Environ. Pollut. 211, 307–315. doi: 10.1016/j.envpol.2015.12.031
Schumacher B. A. (2002). Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments. Ecological Risk Assessment Support Center, Office of Research and Development, US Environmental Protection Agency, NCEA-C-1282, 2002: 1-23. Available at: http://bcodata.whoi.edu/LaurentianGreatLakes_Chemistry/bs116.pdf. (Accessed January 12, 2019).
Shannon C. E. (1948). A Mathematical Theory of Communication. Bell. Sys. Tech. J. 27, 379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
Verdelhos T., Marques J. C., Anastácio P. (2015). The Impact of Estuarine Salinity Changes on the Bivalves Scrobicularia Plana and Cerastoderma Edule, Illustrated by Behavioral and Mortality Responses on a Laboratory Assay. Ecol. Indic. 52, 96–104. doi: 10.1016/j.ecolind.2014.11.022
Wang H., Tian T., Gong Y., Ma S., Altaf M. M., Wu H., et al. (2021). Both Environmental and Spatial Variables Affect Bacterial Functional Diversity in Mangrove Sediments at an Island Scale. Sci. Tot. Environ. 753, 142054. doi: 10.1016/j.scitotenv.2020.142054
Wang D., Wan B., Qiu P., Zuo Z., Wang R., Wu X. (2019). Mapping Height and Aboveground Biomass of Mangrove Forests on Hainan Island Using UAV-LiDAR Sampling. Remote Sens. 11, 2156. doi: 10.3390/rs11182156
Weisberg S. B., Ranasinghe J. A., Dauer D. M., Schaffner L. C., Diaz R. J., Frithsen J. B. (1997). An Estuarine Benthic Index of Biotic Integrity (B-IBI) for Chesapeake Bay. Estuaries 20, 149–158. doi: 10.2307/1352728
Yang X., Ma S., Li T., Gan M., Chen M. (2021). Community Characteristics and Distribution Patterns of Soil Fauna After Vegetation Restoration in the Northern Loess Plateau. Ecol. Indic. 122, 107236. doi: 10.1016/j.ecolind.2020.107236
Yan J., Sui J., Xu Y., Li X., Wang H., Zhang B. (2020). Assessment of the Benthic Ecological Status in Adjacent Areas of the Yangtze River Estuary, China, Using AMBI, M-AMBI and BOPA Biotic Indices. Mar. Pollut. Bull. 153, 111020. doi: 10.1016/j.marpolbul.2020.111020
Zhang G., Lan J., Wu R., Wang D. (2016). Preliminary Study on Macrozoobenthos Diversity in Mangrove Area of Hainan Province. Chin. J. Trop. Agric. 36, 37–42. doi: 10.12008/j.issn.1009-2196.2016.11.008
Zhou Z., Li X., Chen L., Li B., Liu T., Ai B., et al. (2018). Macrobenthic Assemblage Characteristics Under Stressed Waters and Ecological Health Assessment Using AMBI and M-AMBI: A Case Study at the Xin’an River Estuary, Yantai, China. Acta Oceanol. Sin. 37, 77–86. doi: 10.1007/s13131-018-1180-x
Keywords: mangrove ecosystems, benthic macrofauna, community structure, ecological status, Hainan Island
Citation: Li P, Liu J, Bai J, Tong Y, Meng Y, Diao X, Pan K, Zhu X and Lin G (2022) Community Structure of Benthic Macrofauna and the Ecological Quality of Mangrove Wetlands in Hainan, China. Front. Mar. Sci. 9:861718. doi: 10.3389/fmars.2022.861718
Received: 25 January 2022; Accepted: 25 April 2022;
Published: 24 May 2022.
Edited by:
Ana Carolina Ruiz-Fernández, National Autonomous University of Mexico, MexicoReviewed by:
Michal Kowalewski, University of Florida, United StatesIñigo Muxika, Technological Center Expert in Marine and Food Innovation (AZTI), Spain
Copyright © 2022 Li, Liu, Bai, Tong, Meng, Diao, Pan, Zhu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoshan Zhu, emh1LnhpYW9zaGFuQHN6LnRzaW5naHVhLmVkdS5jbg==; orcid.org/0000-0003-1223-1415
 Ping Li
Ping Li Jingli Liu
Jingli Liu Jiankun Bai
Jiankun Bai Yifan Tong
Yifan Tong Yuchen Meng3
Yuchen Meng3 Xiaoping Diao
Xiaoping Diao Ke Pan
Ke Pan Xiaoshan Zhu
Xiaoshan Zhu