- 1Institute of Oceanography and Fisheries, Split, Croatia
- 2Natural History Museum Rijeka, Rijeka, Croatia
This study tested generality in the settlement and recruitment patterns of juvenile fish in the coastal Mediterranean as driven by interannual environmental differences. A multivariate analysis of juvenile fish community data, sampled over three consecutive years, was conducted to elucidate the interannual changes of new settlers’ occurrence and abundance in different nurseries along the eastern Adriatic coast. Sites were assigned to four groups of nurseries based on water type (marine or transitional) and geographical position (north or south). Statistically significant interannual differences were found in temperature but not in salinity. In general, species occurrence significantly fluctuated between years and seasons. The highest total abundance of juveniles was observed in the significantly warmer year 2018 within all study groups. Defined groups expressed significant annual differences in species richness and abundance related to variations in water temperature and salinity as environmental factors for the same consecutive years. Nurseries within transitional waters in the north are more prone to interannual water temperature changes. The associated community composition differed most from those recorded in southern marine waters, where groups were mostly defined by salinity influence and were least sensitive to interannual temperature fluctuations. The cold and rainy spring in 2019 caused late settlement and longer retention of specific economically and ecologically important fish species in the nurseries. The results suggested that settlers’ delay or retention due to negative temperature deviation in the spawning period were linked to the nurseries located in the northern transitional waters that are under a stronger coastal influence. These delays can have ecological consequences on population dynamics and on inter- and intraspecific relationships within specific nursery communities.
Introduction
Coastal areas are commonly acknowledged as highly productive and valuable ecosystems that provide many favourable habitats for fish, while also supporting fundamental ecological links with other environments (Duarte, 2000; Beck et al., 2001; Hinz et al., 2019). Their role in early life stages, and also as foraging and spawning grounds for non-coastal fish species (Harmelin-Vivien, 1984; Francour et al., 1999) is recognised throughout the Mediterranean and worldwide (Francour, 1997; Deudero et al., 2008). There is ambiguous evidence for the importance of habitat characteristics in driving patterns of population dynamics (Vasconcelos et al., 2013). Highly valuable habitats generally provide shelter and an abundance of food to juveniles that may facilitate their survival and growth processes, thus contributing to overall production and population stability (Scharf et al., 2006; Dahlgren and Eggleston, 2014; Cheminée et al., 2016). Knowing the role of habitat use is particularly valuable from a conservation and management perspective, since the vulnerability of coastal habitats to anthropogenic stressors is increasing (Sala, 2004; Halpern et al., 2008; Sala et al., 2012). Marine ecosystems have been degraded in many areas, particularly in the Mediterranean, and many critical coastal habitats are no longer available or adequate to provide nursery, feeding, or reproductive functions, with negative consequences on production and renewal of populations (Guidetti et al., 2002; Barbier et al., 2011; Matić-Skoko et al., 2020).
Population dynamics, as a complex process including reproduction, growth, survival, and demographic rates, are under environmental influences (Vasconcelos et al., 2013) and therefore they are reflected in the fisheries dynamics of exploited species (Hixon and Johnson, 2009). However, the variability of spawning success and the relationship between spawning and recruitment are among the least understood aspects of fish population dynamics. The process starts with the transition between pelagic and benthic stages (Brothers et al., 1983). Settler supply and nursery habitat availability are key factors affecting settlement and recruitment processes, determining the renewal of populations and shaping the structure of adult assemblages (Cheminee et al., 2011). In the Mediterranean, a variety of fish species inhabit coastal nurseries, some of them for life while others just for a part of life (Dulčić et al., 2007; La Mesa et al., 2011; Félix-Hackradt et al., 2014; Cheminée et al., 2017).
Settlement of juvenile fishes occurs year-round, though most species have a settlement peak between early spring and late summer (García-Rubies and Macpherson, 1995; Dulčić et al., 1997, 2007; Biagi et al., 1998). Specific settlers often have strict microhabitat requirements for potential nurseries. After settling until recruitment, their survival within a habitat largely depends on the environmental conditions encountered at the site of settlement (Beck et al., 2001). This makes temperature, salinity and bottom depth preferences even more important when choosing a nursery area. Global warming or climate change has driven significant changes in marine ecosystems in recent decades, particularly in coastal areas facing compositional and structural changes. Specifically, alterations in species distributions and ranges, composition of species assemblages, and biodiversity have all been convincingly linked to rising temperatures (Bellard et al., 2012; Doney et al., 2012) in regions where climate change has substantially altered the physical environment (see Barceló et al., 2016 and references therein). These are the main biological responses to temperature and salinity as factors of environmental modifications. Generally, changes in growth rates, shifts in the spawning season, and shifts in the spawning area (latitude) are expected after a rise in sea temperature due to global warming. Recently, shifts in the physical and biological regimes of the Adriatic Sea were connected with the climate change in the northern hemisphere (Grbec et al., 2015). Matić-Skoko et al. (2020) highlighted the importance of investigating temporal changes in littoral juvenile fish assemblages through responses to environmental factors as important causal factors of habitat modifications due to climate change. These responses represent the environmental tolerances of individual species (Martins et al., 2019). Further, the absence or occurrence of certain species may be a signal of a poleward shift in the spawning area and a temporal shift in spawning timing to avoid higher temperatures (Shoji et al., 2011).
Previous studies have generally focused on the intrinsic ecological functions of microhabitats at a local scale, e.g., for sparids along the rocky shore of Marseilles (NW Mediterranean) (Cheminee et al., 2011). The whole eastern Adriatic coast represents a mosaic of different coastal nurseries, from coastal lagoons to shallow, semi-closed coves with varying annual influence of freshwater springs, which is highest in the winter-spring period after snow melt and intensive rain (Novosel et al., 2005). These coves were previously identified as having higher fish species richness (Dulčić et al., 1997) than coastal lagoons, due to the wide connection with the sea that favours the inflow of fish eggs, larvae and young of-the-year by the tides and currents from spawning areas (Blaber et al., 1995; Potter et al., 2010; Araújo et al., 2016). However, the extent of interannual differences in settler supply along the coast and across different habitats is unknown, and little is also known about which habitats and consequently which fish species are most sensitive to such changes. Several hypotheses have thus been defined for the main aims of this study: (1) there are significant interannual variations in species presence and species abundance of settled juvenile fish along the eastern Adriatic coast, (2) different habitat types (related to water type and geographical position) respond differently to interannual changes of environmental factors, and (3) estuarine nursery areas are more susceptible to such changes than marine ones. The present study aims to further understand the relative importance of interannual changes of environmental parameters and nursery attributes at different spatial scales in settlement and post-settlement processes and how they influence recruitment level.
Materials and Methods
Sampling Procedure
Sampling on a seasonal basis (March, June, September, and December) was conducted in three consecutive years (2017, 2018, and 2019) at 20 sites (Table 1 and Figure 1) across marine and transitional waters along the eastern Adriatic coast. The specific ecological characteristics of each site are presented in Table 1. All sites were selected based on knowledge where juvenile fish occurrence had been previously described (Dulčić et al., 1997, 2005, 2007; Matić-Skoko et al., 2007) over a similar biotope characterised mainly by sandy (S1, S3, S5, S6, S9, S10, S12, S15, S18, and S20), rocky (S11 and S16), or mixed sandy-rocky-pebbles (S2, S4, S7, S8, S13, S14, S17, and S19) substrata covered by photophilic algae alternating with patches of Cymodocea nodosa seagrass beds. At each site, three replicates separated by tens of meters were performed with a specially constructed small shore seine (L = 25 m; minimum mesh size 4 mm). To ensure comparability, the same net was used during the entire study period at all sites. Hauls were also performed in the same bathymetric range, from 0 to 2.2 m depth. A total of 36 samples per site were taken over a 3-year period. Sites were divided into four groups of five locations based on water type (marine or transitional) and geographical position (north or south): transitional north (T_N), transitional south (T_S), marine north (M_N), and marine south (M_S). The border between the northern and southern sites is Cape Ploča (43°29.648′N; 15°58.184′E) as the most prominent point of land along the eastern Adriatic coast. This cape represents the geographic and climatological boundary between the northern and the southern Adriatic, and is often characterised by strong sea currents and swells as weather systems from the north and the south come in contact (Gloginja and Mitrović, 2021). Surface and bottom temperature (°C) and salinity (‰) measurements were performed using a multi-parameter probe model YSI 85. All fish individuals were identified to the species level according to Jardas (1996) and Dulčić and Kovačić (2020), measured (total length to the nearest 0.1 cm) and weighed (total body weight to the nearest 0.1 g). Sampling covered all stages of fish, but only juveniles were selected for further analysis. Further on, the species Atherina sp. were excluded, since they were the most dominant and most common species in all samples, and the majority of specimens were adults, while Sardina pilchardus and Engraulis encrasicolus were caught only sporadically in high abundance, thus skewing the results. The following species were excluded from further analysis as only adults, and no juvenile specimens, were caught: Lichia amia, Pseudocaranx dentex, Sciena umbra, Uranoscopus scaber, and Zeus faber.
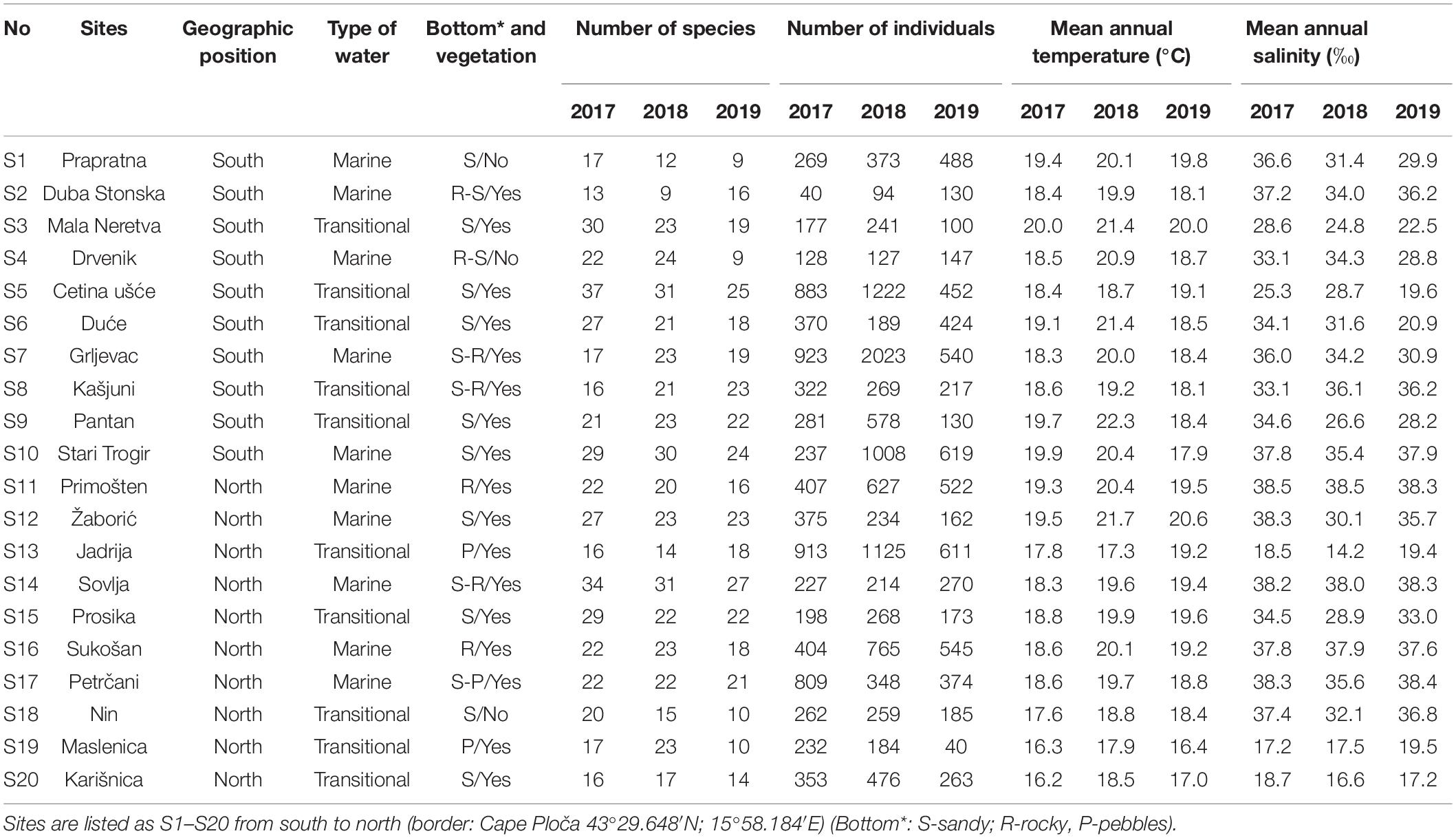
Table 1. Summary of sampling data for juvenile fish communities along the eastern Adriatic coast within selected sites in the period 2017–2019.
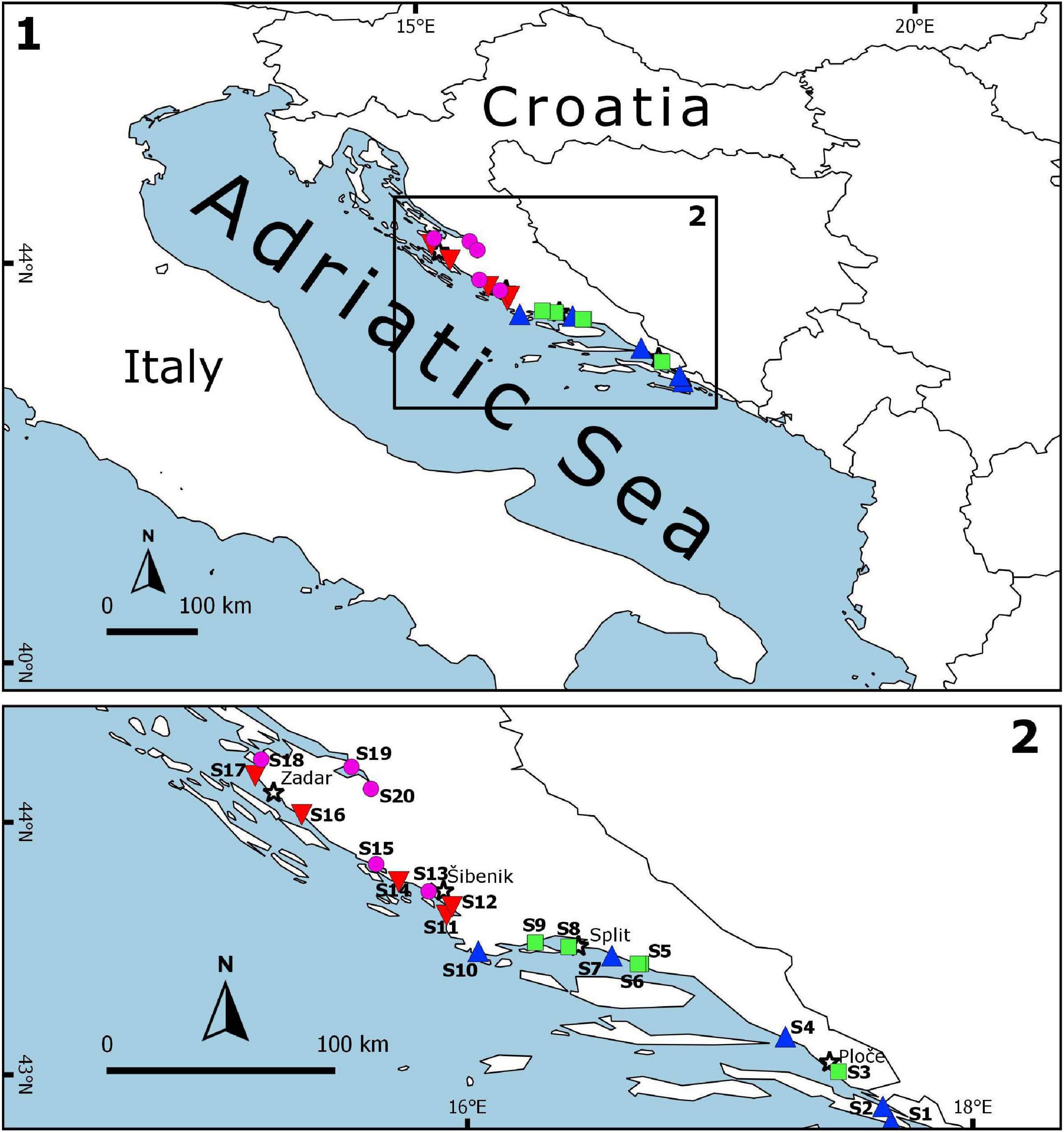
Figure 1. Sampling area representing nursery areas along the eastern Adriatic coast with 20 sites across the four groups: marine_south (M_S;  ), marine_north (M_N;
), marine_north (M_N;  ), transitional_south (T_S;
), transitional_south (T_S;  ) and transitional_north (T_N;
) and transitional_north (T_N;  ). The map was created using QGIS Desktop 2.18.27 (version Las Palmas).
). The map was created using QGIS Desktop 2.18.27 (version Las Palmas).
Data Analysis
Total abundance (A), as the number of individuals (N) per species, and species richness (R), as the number of species (S), were counted, while the mean number of individuals and species per haul were recorded for each site and each period.
All analyses were performed using PRIMER-E software (Clarke and Gorley, 2015) with the add-on package PERMANOVA+ (Anderson, 2001; Anderson et al., 2008). Graphs were prepared using SigmaPlot (v. 13.0; Systat Software Inc., San Jose, CA, United States).
Multivariate statistical testing of abundance data for juvenile fish assemblages used a four-factor design [Year (Ye)/Season (Se)/Geographic position (Ge)/Type of water (Ty)], within one factor Period (1–12): 2017 (1–4), 2018 (5–8), and 2019 (9–12) constructed for cyclicity purposes. Factors Ye and Se were considered fixed while factors Ge and Ty were randomly nested in Se. The additional factor Period_Type of water_Geographic position was combined. Data sets for each site were imported in the PRIMER workspace and combined into a single matrix. Variability in the numbers of individual species in the samples was used to carry out dispersion weighting for each species. The dispersion-weighted data was transformed by a milder square-root transformation, as demonstrated by Clarke et al. (2014) for fish communities, particularly those in estuaries and nearshore coastal waters, where the prevalence of juvenile and small schooling species is high. Bray–Curtis similarity was then calculated on the dispersion weighted, transformed data.
Metric Multidimensional Scaling (mMDS) ordination used data at the same level of sample averaging for each Period, Geographical position, and Type of water combination to visualise the extents to which ichthyofaunal composition differed across Period_Type of water_Geographic position. All plots were constructed by calculating the distances between each pair of group centroids, i.e., the relevant average in the “Bray–Curtis space” of all samples (Anderson et al., 2008).
The RELATE statistic (P) was employed to test whether the pattern of temporal catch composition change conformed to cyclicity. If there is no tendency to cyclicity, then P will be close to zero (Clarke and Warwick, 2001). Separate two-way crossed analysis of similarities ANOSIM was used to interpret the relative size of the overall spatio-temporal effects on fish compositions, using the same resemblances as for the PERMANOVA tests. The species that mainly contribute to the separation between the factor Period_Type of water_Geographic position were determined by the SIMPER procedure, which shows the average contribution of each species to the dissimilarity between the groups. The results were presented for the specific season along all three consecutive years, characterised by most significant changes in communities.
To explore the data further, we used canonical variate plot (CAP) analysis. CAP is a routine for performing canonical analysis by calculating principal coordinates among groups of samples to predict group membership, positions of samples with another single continuous variable, or finding axes having maximum correlations with another set of variables (Anderson and Willis, 2003). This was initially run separately for each of the two factors: “Geographic position” and “Type of water” and then merged into a scatter plot. Particularly, CAP was used to estimate the accuracy of fish composition and environmental variables (temperature and salinity).
Results
Interannual Variations of Abiotic Factors
The mean monthly variation in water temperature and salinity averaged over four groups (M_S, M_N, T_S, and T_N) are shown in Figure 2. The observed sea surface temperatures followed a seasonal cycle, with the highest temperatures in June-September (particularly in 2018) and the lowest in December in all three years (Figure 2A). Observing the differences between marine and transitional waters, less variation was observed among waters in the south, regardless of the type of water (Supplementary Table 1). The mean seasonal temperature in T_N was always lower than in the remaining groups, though this is especially evident in December when higher and more similar results were recorded in the other three groups compared to T_N. Namely, northern transitional waters had the lowest mean values recorded in December 2017 (10.12 ± 1.81°C) and December 2018 (10.44 ± 1.39°C). It is evident that the mean temperature in December 2019 was higher for the T_N group than in the two previous years, and this was much more uniform among the groups (Figure 2A). Further, the mean temperature in June 2019, regardless of water type, was 2.78°C lower than in 2017 and 4.78°C lower compared to 2018. Analysis of variance revealed significant differences in temperature between years (PERMANOVA: Pseudo-F = 10.371; P = 0.0004), seasons (Pseudo-F = 50.181; P = 0.0013), interactions of years and seasons (Pseudo-F = 10.624; P = 0.0002), and among groups (M_S, M_N, T_S, and T_N) in relation to seasonal changes in temperature (Pseudo-F = 4.6813; P = 0.0008) (Table 2).
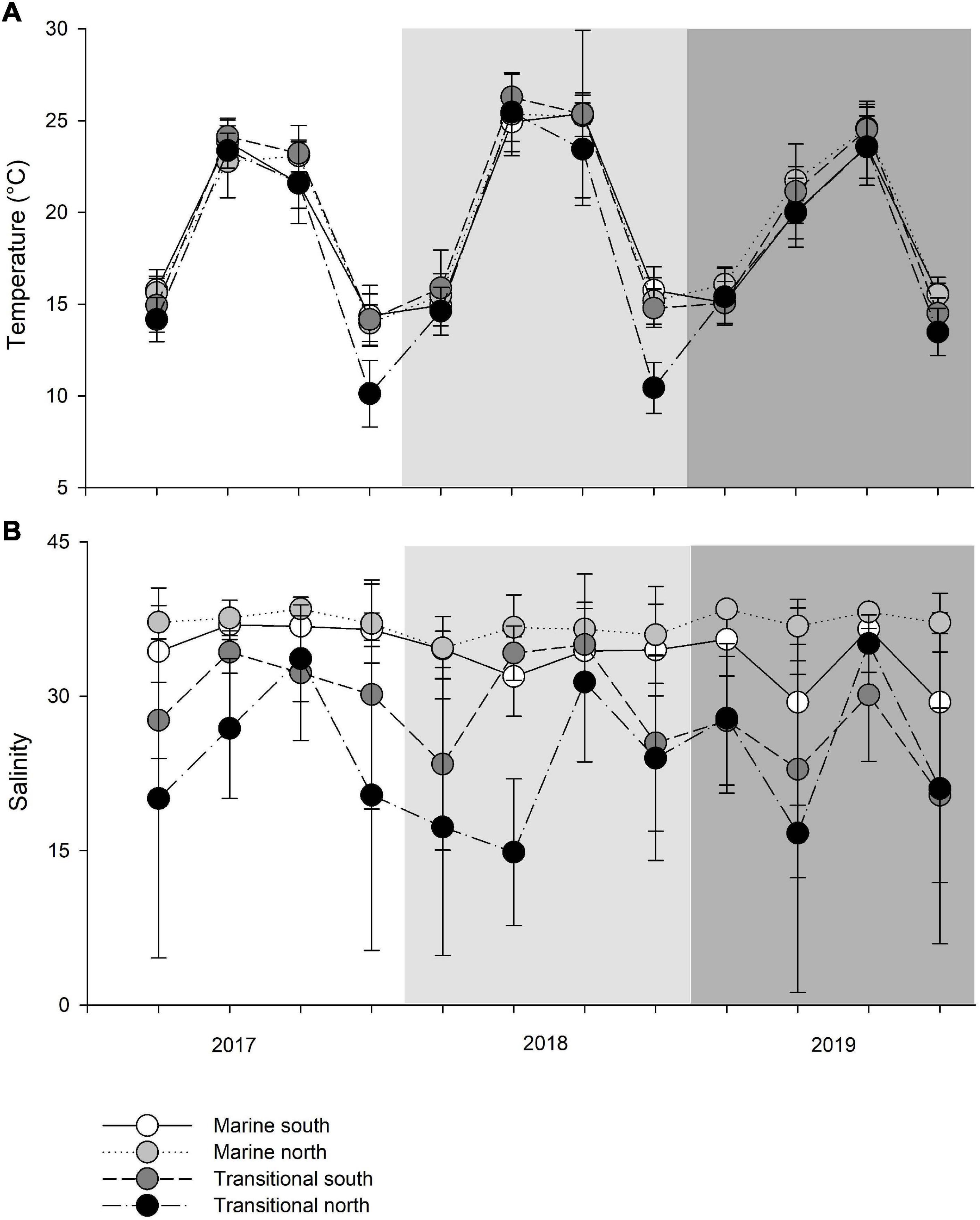
Figure 2. Mean seasonal values of (A) temperature (°C) and (B) salinity (‰) among transitional and marine waters in the north and south (M_S, M_N, T_S, and T_N).
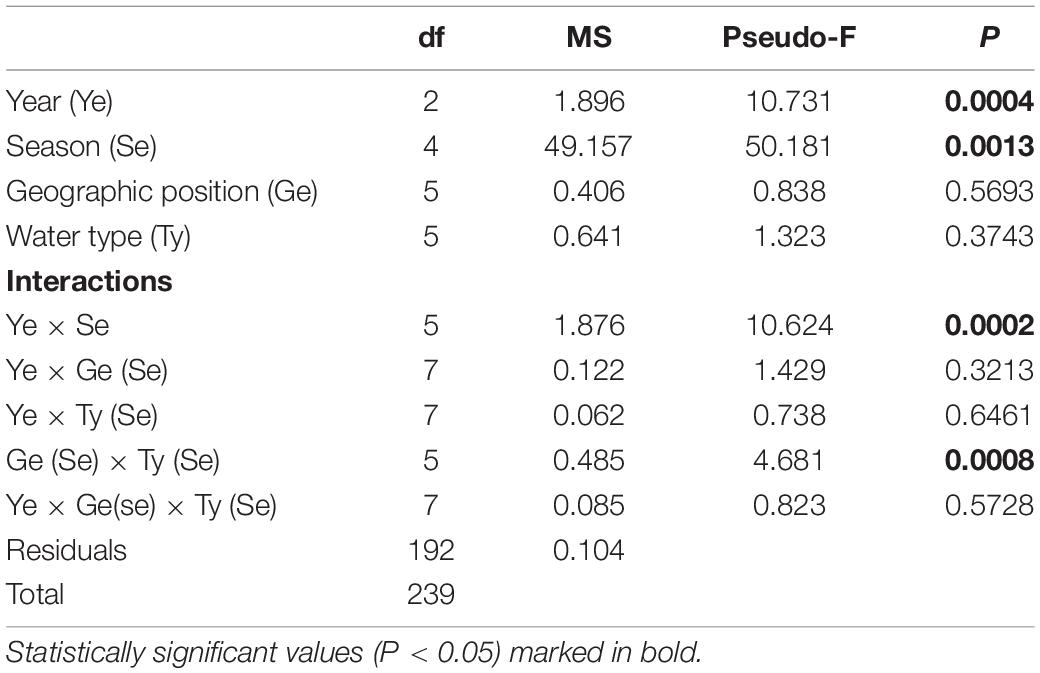
Table 2. Summary of PERMANOVA results for the multivariate analysis of the overall matrix constructed from seasonal temperature data measured in three consecutive years (2017, 2018, 2019) along the eastern Adriatic coast for 20 sites with assigned affiliation according to water type (marine or transitional) and geographic position (south or north).
As expected, the measured salinity values showed greater variation between water types such that marine areas showed less seasonal fluctuations and continuous higher values (Figure 2B and Supplementary Table 1). Lower salinity values were observed for northern transitional waters (mean salinity value across the sampling period was 23.53 ± 6.23‰) than southern transitional waters. Two negative peaks of mean salinity values were observed in June and December 2019, regardless of water type. The mean salinity was always higher in 2017 and 2018 in the south, regardless of water type, while an inversion occurred in 2019 and mean salinity values were higher in the north in both types of waters. Also, every September in all three years, the mean salinity values were highest and most similar among the groups following the summer drought (Figure 2B). However, analysis of variance did not reveal significant differences in salinity between years, seasons, type of waters, and geographic position and their interactions, except for strong statistically significant annual variations among water type/geographic position groups (M_S, M_N, T_S, and T_N) in relation to seasonal changes in salinity, i.e., as nested in season (Pseudo-F = 1.9354; P = 0.0001) (Table 3).
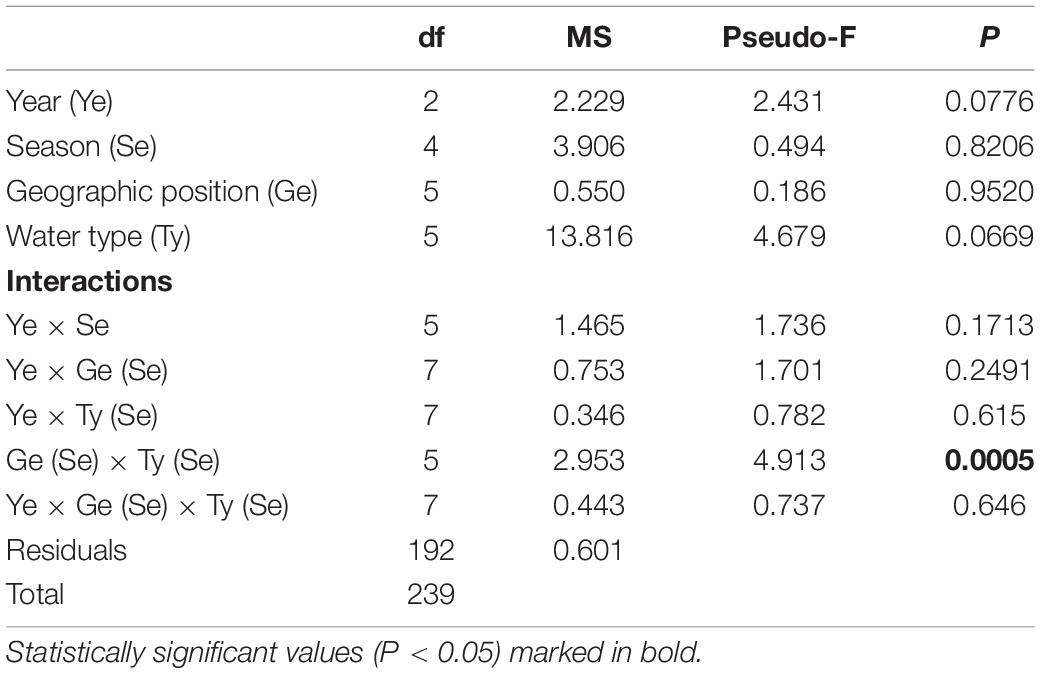
Table 3. Summary of PERMANOVA results for the multivariate analysis of overall matrix constructed from seasonal salinity data measured in three consecutive years (2017, 2018, 2019) along the eastern Adriatic coast for 20 sites with assigned affiliation according to water type (marine or transitional) and geographic position (south or north).
Interannual Variations of Juvenile Fish Composition
At all sites, a total of 720 small beach seine samples were taken across three consecutive years (Tables 1, 4). A total of 24,807 fish juveniles belonging to 23 families and 92 species were included in this survey. The most abundant species were Sarpa salpa (2,805 individuals in total), Pomatoschistus marmoratus (2,028), Pagellus acarne (1,806), Chelon ramada (1712), Diplodus annularis (1,683), Chelon auratus (1,550), and Diplodus vulgaris (1,074), which together comprised 51.03% of the total sample. A high number of species were sporadically present throughout the 3-year study period (though with <30 individuals). Some species, such as Symphodus roissali and Chelidonichthys lucerna, were completely absent in 2019. Although the collection effort (as the total number of samples per year, month and site) was the same, the total number of species and individuals clearly fluctuated across sites (Table 4, Figure 3, and Supplementary Table 2) and across groups. The number of species varied among sites from 1 (at S18 and S19 in June and September 2019, respectively) to 24 species (at S16 in June 2018) and among the groups from 1 (T_N in December 2019) to 25 species (M_N in June 2018). The number of species varied less over the 3-year period at marine stations than at transitional ones, with the largest interannual changes observed in the T_N group. Moreover, PERMANOVA analysis of the number of juvenile fish species found significant differences along three consecutive years (Pseudo-F = 1.7459; P = 0.0362), across seasons among years (Pseudo-F = 1.4222; P = 0.0406) and among groups [M_S, M_N, T_S, and T_N, as Ge(Se) × Wa (Se) interactions] in relation to seasonal changes in the number of species (Pseudo-F = 2.0571; P = 0.0002) (Table 5).
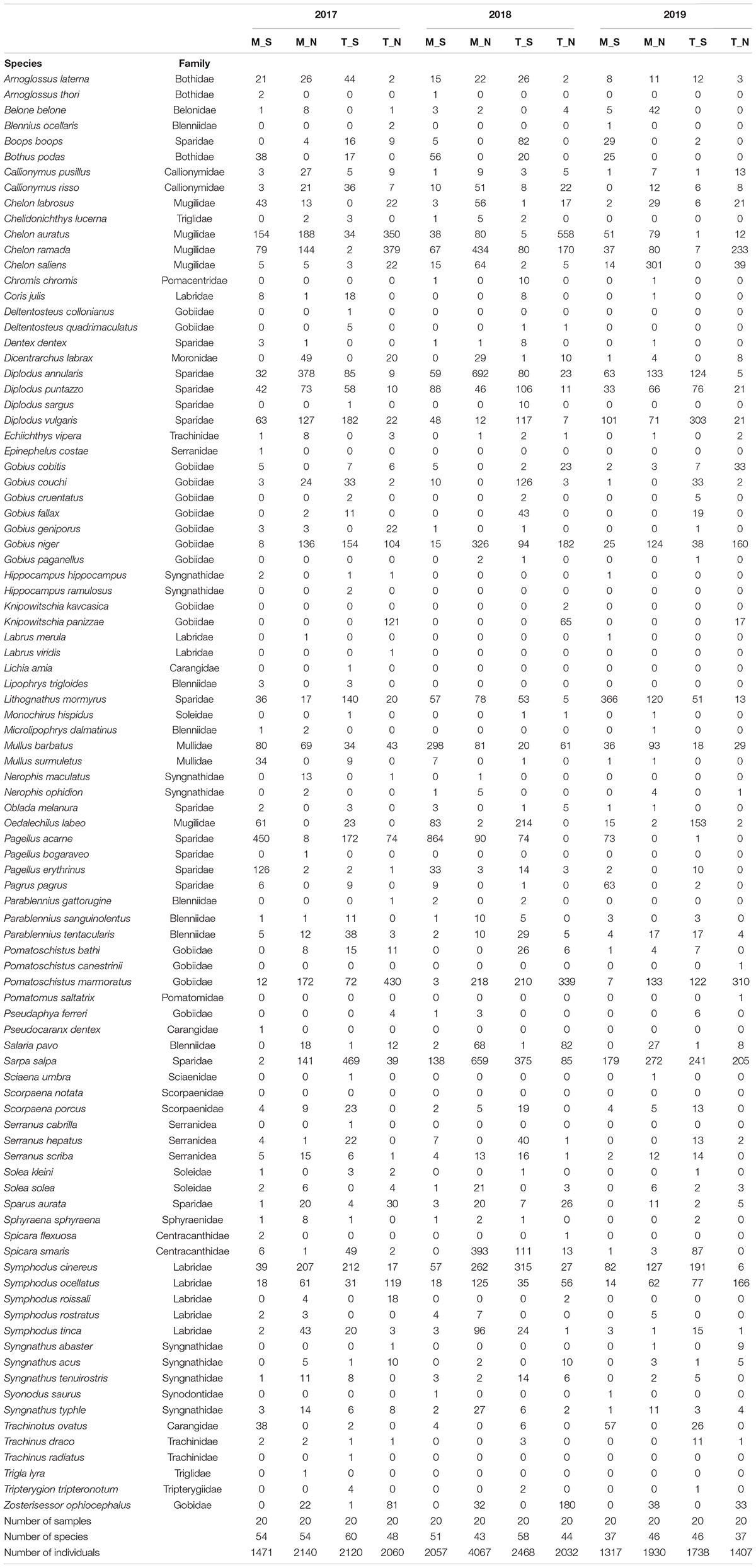
Table 4. Summary of abundance data (number of individuals) for 92 juvenile fish species sampled in three consecutive years (2017–2019) along the eastern Adriatic coast with assigned affiliation to four groups according to water type (marine or transitional) and geographic position (south or north): M_S, M_N, T_S, and T_N.
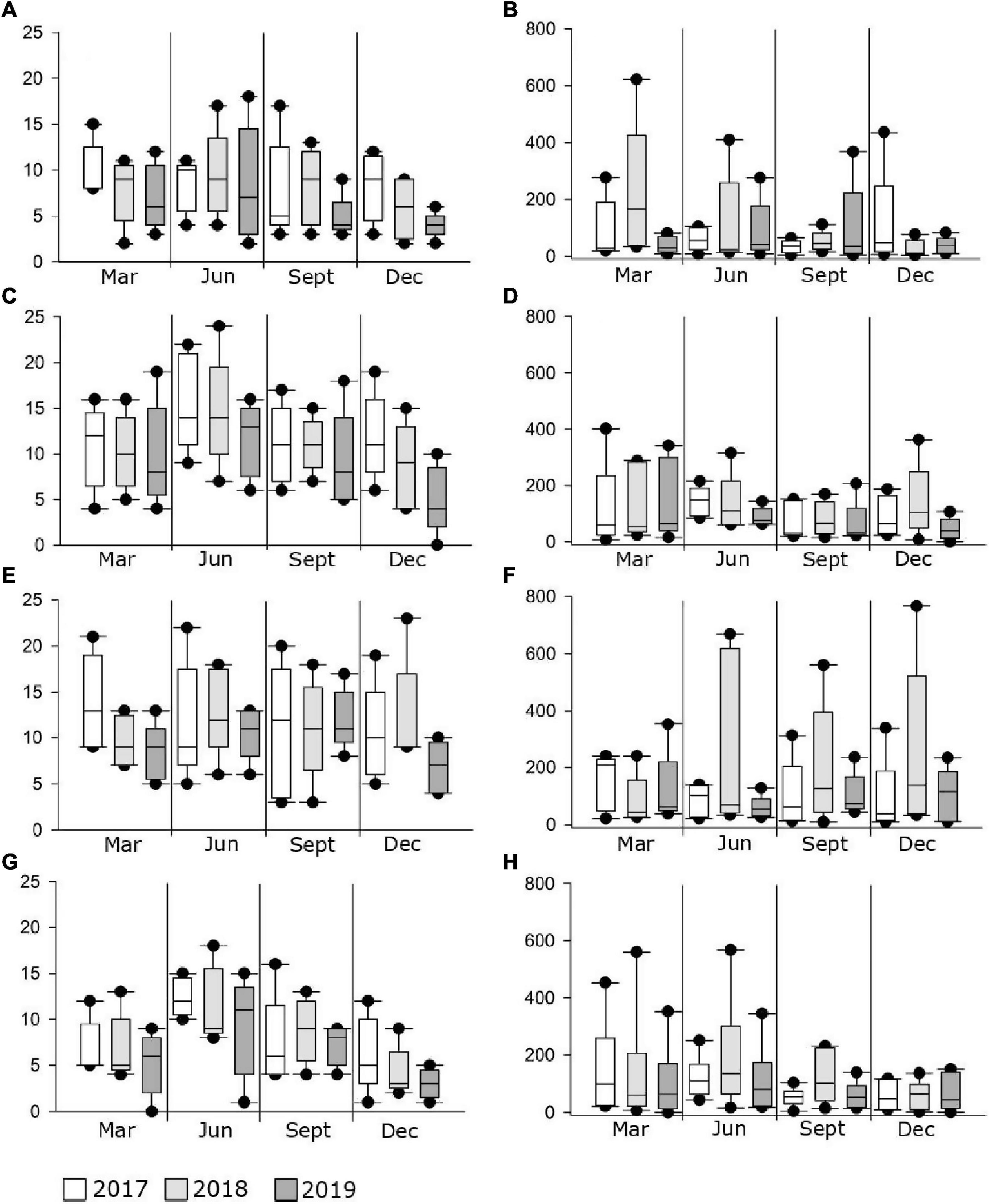
Figure 3. Box plots of median values (± standard deviation) for the number of juvenile fish species and abundance across marine _south (A,B), marine_north (C,D), transitional_south (E,F), transitional_north (G,H) groups during the sampling period (2017–2019) along the eastern Adriatic Sea.
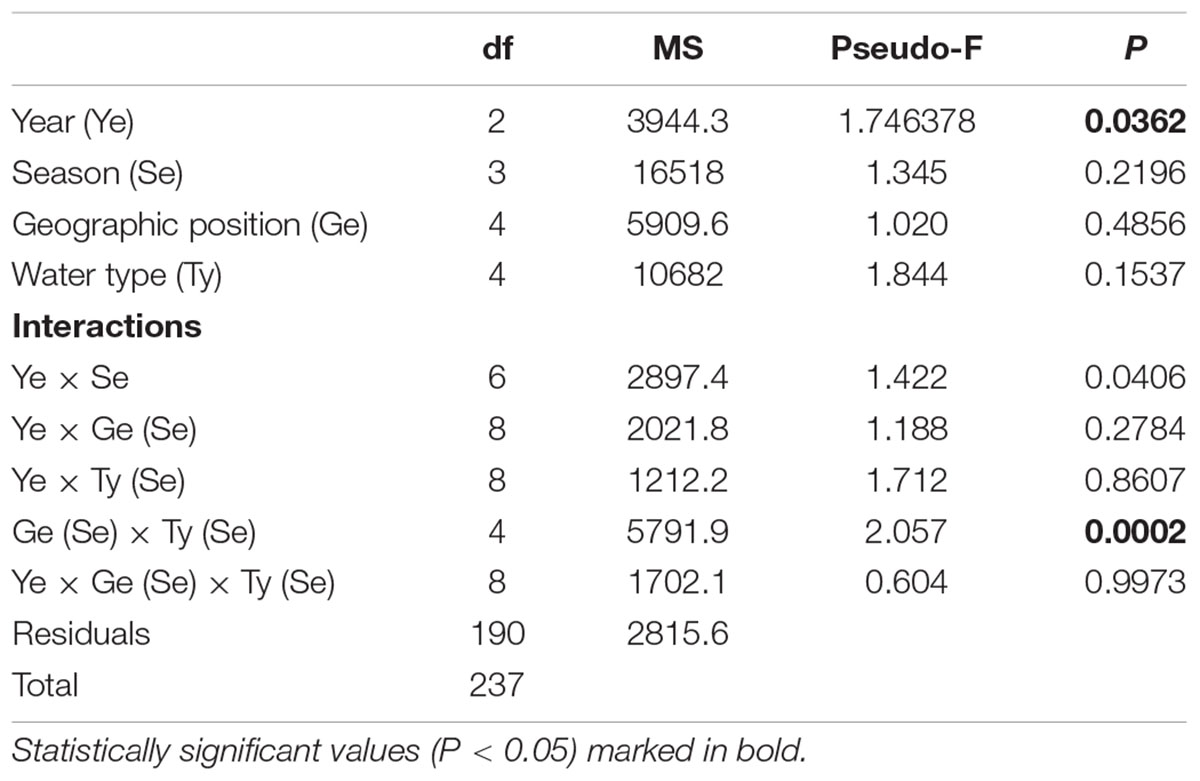
Table 5. Summary of PERMANOVA results for the multivariate analysis of overall matrix constructed from juvenile fish species data sampled in three consecutive years (2017, 2018, 2019) along the eastern Adriatic coast for 20 sites with assigned affiliation according to water type (marine or transitional) and geographic position (south or north).
Similar to the number of species, abundance fluctuated considerably less in marine waters and more in transitional ones, especially in the north. The abundance of individuals was the highest in 2018, and the highest in M_N group (4067 individuals). On the contrary, abundance was lowest in 2019, especially in the M_S (1,317) and T_N groups (1,407). Although some fluctuations in abundance were evident, especially the 52.5% reduction in the M_N group in 2019 (1,930), PERMANOVA analysis of juvenile fish abundance for all sites found no significant differences for the three consecutive years, seasons, type of water and geographical position together with their interactions, except among groups in relation to seasonal changes in the number of individuals (Pseudo-F = 1.9354; P = 0.0001) (Table 6). Regarding species, the abundance of Boops boops, Pagellus acarne, Pagellus erythrinus, Symphodus tinca, and S. roissali was drastically decreased in 2019 compared to previous years (Table 4). Additionally, P. acarne was only present in southern marine waters in 2017 and 2018.
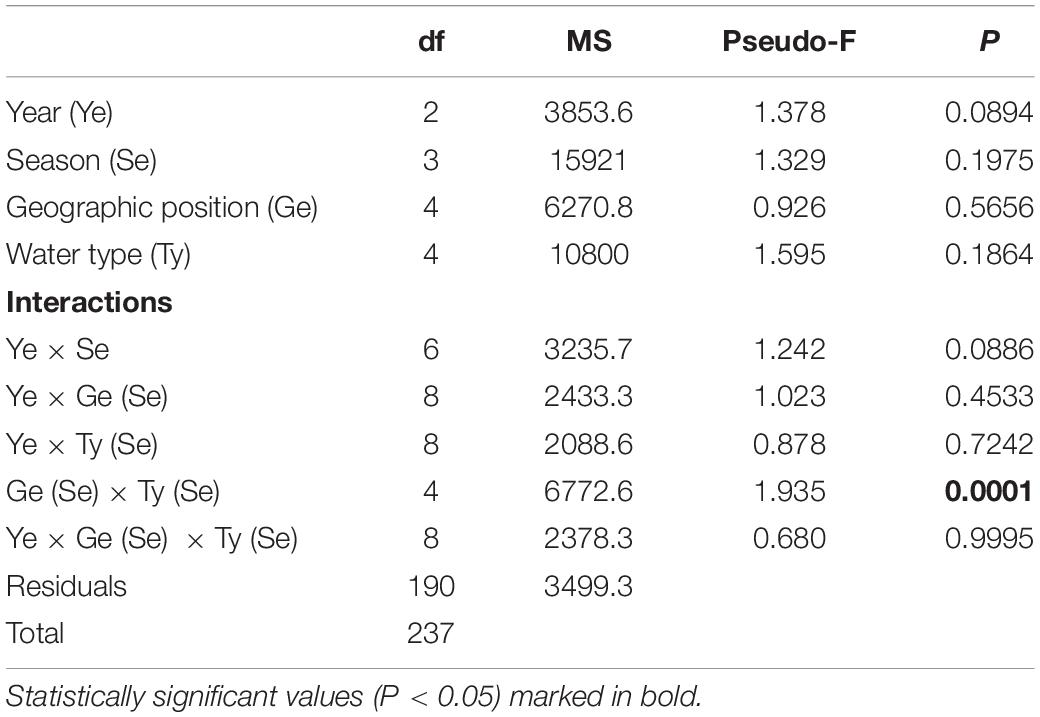
Table 6. Summary of PERMANOVA results for the multivariate analysis of overall matrix constructed from juvenile fish abundances sampled in three consecutive years (2017, 2018, 2019) along the eastern Adriatic coast for 20 sites with assigned affiliation according to water type (marine or transitional) and geographic position (south or north).
On the metric MDS ordination plot, derived from the distance among centroid matrices, four groups were defined, clearly separating both geographical position and type of waters (Table 3 and Figure 3) ordinating groups from transitional to marine and then from north to south (T_N, T_S, M_N, and M_S), indicating that the greatest differences were found between the groups T_N and M_S (Figure 4). The cyclicity of abundance data, obtained from the RELATE test, following a seasonal pattern and was less visible for T_N. It seems that there were similar patterns for marine groups, namely between M_S in 2017 (Rho = 0.621, P = 0.839) and 2018 (Rho = 0.414, P = 0.665) and M_N in 2017 (Rho = 0.828, P = 0.335) and 2018 (Rho = 0.828, P = 0.333) while in the final year of the study period, similarity dropped drastically. Interestingly, there was no similar pattern between the three consecutive years at T_S (Rho = 0.014, P = 0.365). Species composition in T_N was governed by a similar pattern in 2018 (Rho = 0.414, P = 0.679) and 2019 (Rho = 0.414, P = 0.035). Two-way crossed ANOSIM tests showed and confirmed that the fish fauna composition was significantly related to both factors, types of water (R = 0.106, P = 0.001) and geographic position (R = 0.188, P = 0.001), with the latter factor more prominent. Moreover, one-way ANOSIM for the interaction type of water_geographic position confirmed that the magnitude of difference was lower between groups in 2017 (R = 0.419; P = 0.001) and 2018 (R = 0.458; P = 0.001), and highest in 2019 (R = 0.899; P = 0.001).
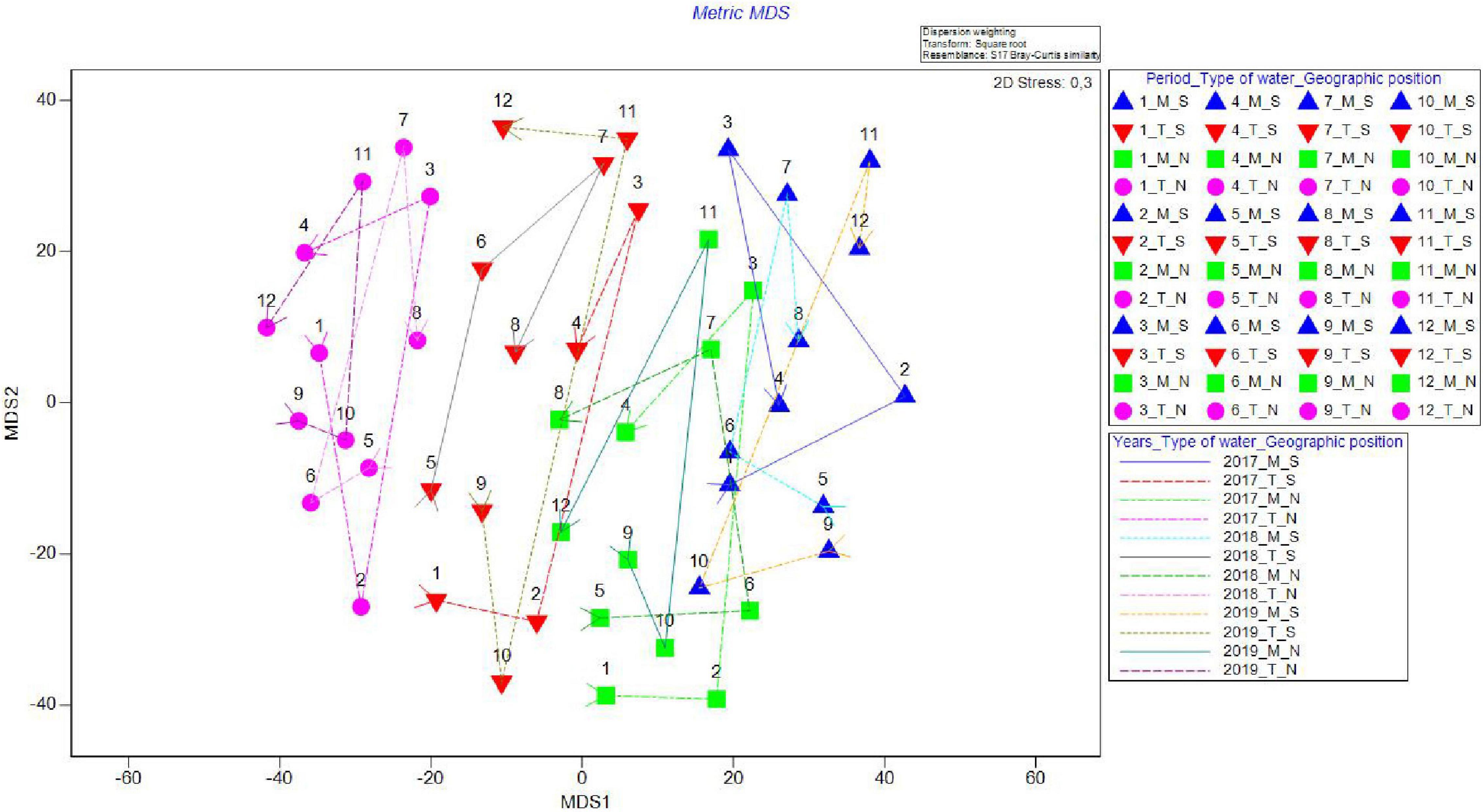
Figure 4. Metric MDS for juvenile fish abundance for Period_Water type_Geographic position (periods 1–4, 5–8, and 9–12 corresponding to 2017, 2018, and 2019, water types marine and transitional and geographic positions south and north) in the four groups in the Adriatic Sea with marked cyclicity for all four groups (M_S, M_N, T_S, and T_N).
We additionally ran a CAP analysis and related the environmental data to the community composition information. Temperature and salinity values for the four water types/geographic position groups of factors were plotted as distances among centroids based on community data (Figure 5), revealing that temperature had a prominent influence on community composition in T_N, less on T_S, while salinity had a positive effect on M_S and M_N. A separate CAP analysis was run for each of the two factors (“type of waters” and “geographical position”), which also gave successful discrimination. In particular, 84.03 and 78.99% of samples from marine and transitional waters, respectively, were correctly allocated based on the community composition information. Further, 72.88 and 71.67% of samples were correctly allocated to north or south, respectively. These results suggested that transitional waters are more sensitive to interannual differences, especially in the north (Figure 6).
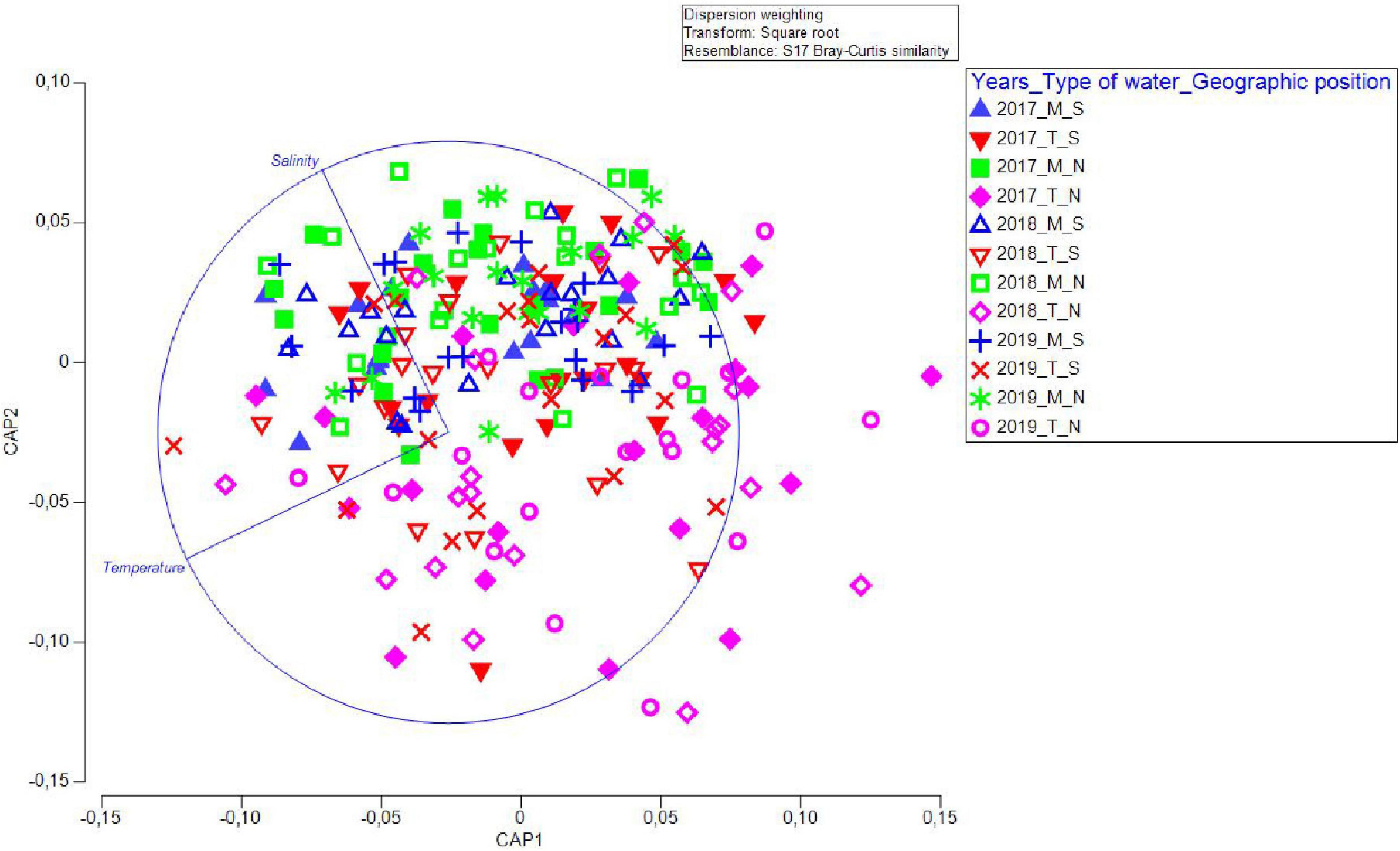
Figure 5. Canonical variate plot (CAP) of juvenile fish abundance data against environmental (continuous quantitative) values of temperature and salinity showing dependence on geographical position and water type. The distance matrix based on community abundance data was related to the distance matrix based on environmental data.
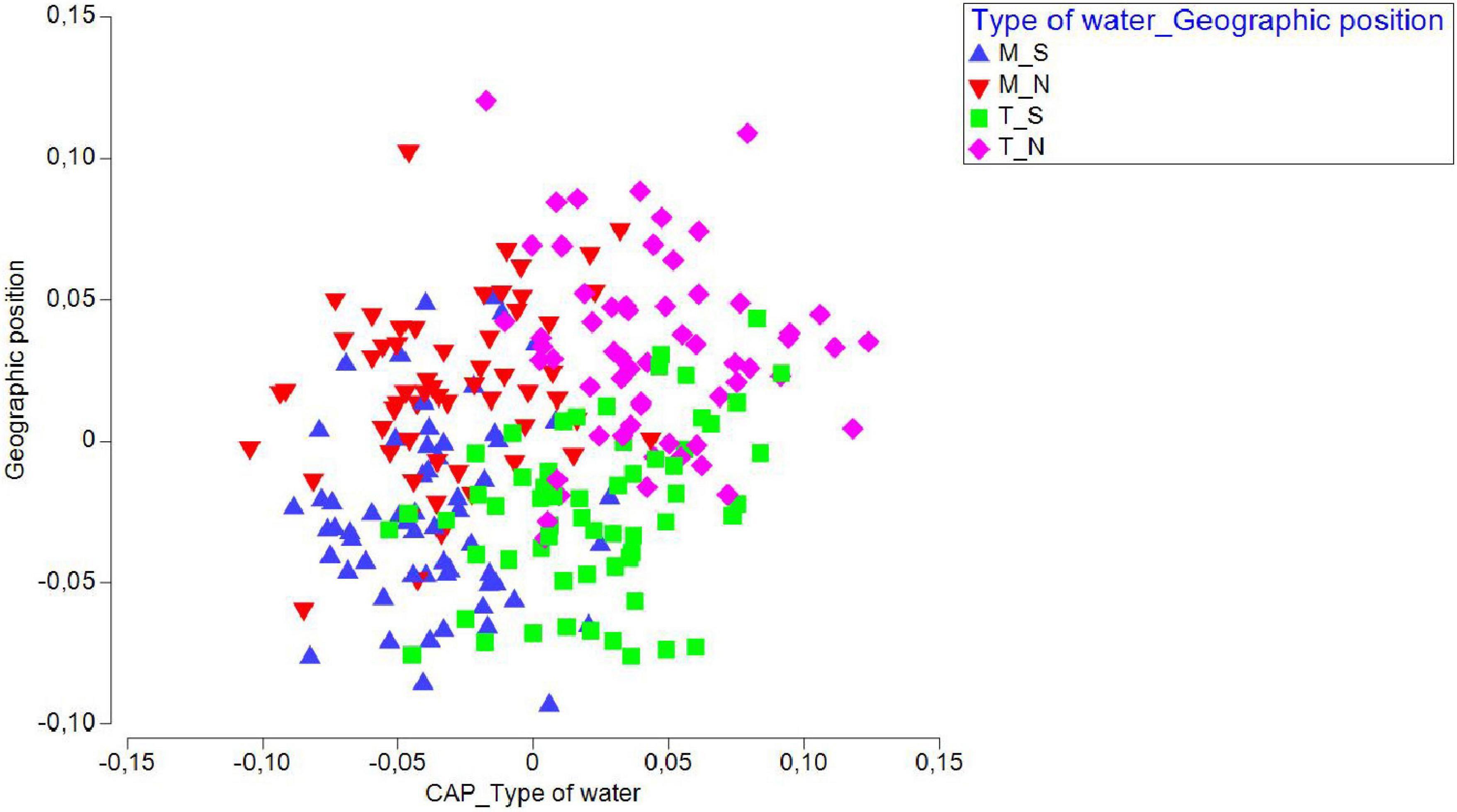
Figure 6. Canonical variate plot (CAP) for juvenile fish abundances sampled in three consecutive years (2017, 2018, 2019) at 20 sites in the Adriatic Sea, grouped by “Water type” and “Geographic position.”
The SIMPER routine revealed that 14 species (Table 7) in varying order of percentage contribution were responsible for the most (>50%) dissimilarities in the interactions between the composition of juvenile fish sampled in June among the three consecutive years. It was evident that five species that gave a significant contribution to T_N in 2017 (e.g., Sparus aurata, Diplodus vulgaris, and Symphodus roissali) were not present in 2018, while other species appeared (Sarpa salpa and Chelon ramada). SIMPER revealed that the average dissimilarity was highest between M_S and T_N (90.51%) while T_S and M_N were more similar (79.41%).
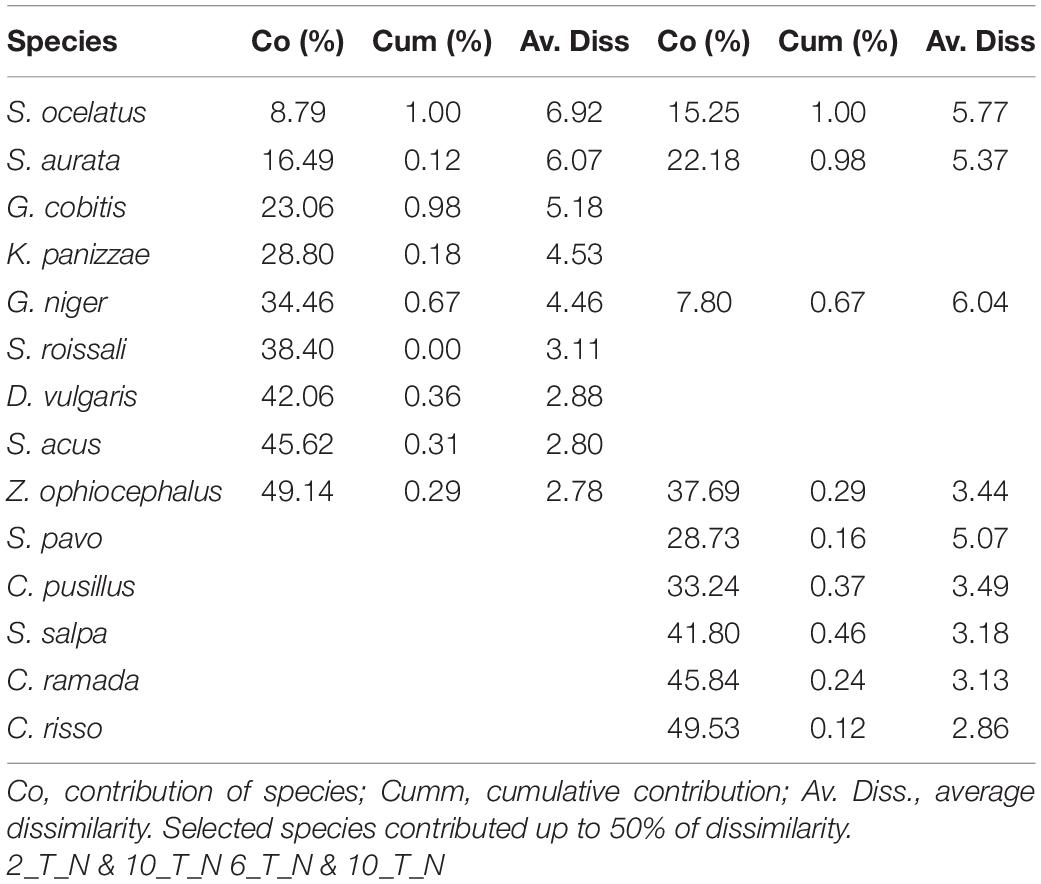
Table 7. SIMPER: species contributing most to the dissimilarity, in terms of abundance of juvenile fish abundances among transitional waters in the north (T_N) in June 2017 (Period 2), 2018 (Period 6) and 2019 (Period 10) in the Adriatic Sea.
Discussion
The present study focussed on the patterns of use of coastal nurseries by juvenile fish species along the eastern Adriatic coast. In particular, the study sought to answer whether species occurrence and density differed between habitats and sites over three consecutive years and whether certain sites or habitats could be highlighted as important nursery areas for juveniles, and whether they are highly sensitive to environmental and other changes. Moreover, by modelling the response of juvenile occurrence and density to the environmental variables, this study identified which variables best explained the intra- and inter-coastal variability in juvenile fish distribution and abundance. Also, the strength of these parameter influences can characterise the features that define important sites for juveniles and how they varied among systems. Last but not least, more sensitive species in this context were defined.
Interannual Variations of Abiotic Factors
Generally, the determined fluctuations in sea temperature and salinity during the study period followed the well-known seasonal cycle specific to the Adriatic Sea (Grbec and Morović, 1997). More pronounced interannual fluctuations in water temperature were found for transitional waters, especially those situated in the north. According to the Croatian Meteorological and Hydrological Service, mean monthly air temperatures for most of 2018 were above the average of the reference period 1961-1990, while in May 2019, temperatures were below the reference average along the entire coast. Specially in the area gravitating toward T_N, temperatures in May 2019 were nearly 5°C below average (DHMZ, 2019). Sites within this group are related to the transitional waters of the Zrmanja and Krka Rivers, whose water systems are more exposed to the mainland due to relief characteristics (heavy rains, snow melt from nearby mountains and cold north-easterly winds in spring) and the maritime influence is less pronounced. Usually, salinity fluctuations are associated with freshwater river flows, though the significantly lower temperatures of these waters, especially in the spring months are due to their origin and flow through extremely mountainous terrain. On the contrary, the transitional waters of the Neretva and Cetina Rivers have wider mouths toward the open sea, especially the Neretva Delta, and thus are more influenced by the gentle maritime environment (Krvavica and Ružić, 2020). Unlike temperature, there were no prominent interannual fluctuations in salinity. However, reduced salinity was evident in the northern transitional waters for the same reasons as mentioned above. In late spring 2019, reduced salinity was recorded along the entire coast as a consequence of an extremely rainy period. For example, the amount of precipitation in May (120 mm) and November (210 mm) 2019 in the middle of study area (S8 and S9) was twice the average cumulative amount of precipitation in the reference period 1961–1990 for those months (DHMZ, 2019). In 2017 and 2018, the average salinity in the south, regardless of water type, was always higher than in the north, confirming the unusually high rainfall in the north (DHMZ, 2019). As expected, statistically significant interannual differences in mean seasonal water temperatures also led to a change in the species occurrence among the three consecutive years. Thus, June 2019 was characterised by markedly low salinity and low temperature after a cold and rainy winter and spring, which likely caused the absence of the following species: B. boops, B. podas, P. acarne, P. erythrinus, S. tinca, S. roisalli, and C. auratus. All these species, except C. auratus, spawn in spring (Bartulović et al., 2011; Dulčić and Kovačić, 2020) and their settlers likely delayed settlement due to the prevailing conditions. Interestingly, a significantly reduced abundance of B. boops and B. podas were observed in southern transitional waters (T_S) in the same period. These species also spawn in early spring (Dulčić et al., 2004; Matić-Skoko et al., 2007; Dobroslavić et al., 2017; Zorica et al., 2020) and apparently their juveniles did not yet inhabit the nursery areas by June. Late spawning and a longer retention in the nursery area could be the reason for increased abundance of L. mormyrus in September 2019. Increased abundance is most often a positive response to average warmer habitat conditions (Jones et al., 2009). It seems that the longer the spawning season for a population, the higher its probability of finding suitable conditions for larval survival, resulting in a higher recruitment level (Sale, 2006). This may imply that if environmental conditions change significantly, species with a shorter spawning period may experience a more pronounced shift in spawning time and consequently in settlement onset. Interannual variability in temperature has been linked with changes in fish year-class strength for a number of species (Planque and Fox, 1998; Laurel et al., 2017; Barbeaux and Hollowed, 2018). Temperatures affect the onset of spawning and spawning period, but also the ecology of early developmental stages of fish, thus defining the moment of their migration or entry into benthic nursery areas (García-Rubies and Macpherson, 1995; Dulčić et al., 1997, 2007; Biagi et al., 1998). Also, positive relationships between temperature and impacts on settlement and recruitment are generally observed in cold-temperate regions, whilst negative relationships are observed in warm-temperate regions (Planque and Fox, 1998; Barbeaux and Hollowed, 2018; Downie et al., 2021). Environmental changes at higher latitudes that are under greater coastal influence appear to be more drastic or more rapidly reflected in changes at the community, species or individual level.
Interannual Variations of Juvenile Fish Composition
The influence of environmental factors on species composition or occurrence is specifically evident at the fish community level for both species richness and abundance. Moreover, water temperature appears to have a greater impact on transitional waters that are often retracted deep into the mainland. This is even more relevant since more than 64% of fish juveniles representing 68 species and 28 families were recorded in estuarine nurseries, and most are species of commercial interest (Dulčić et al., 2007). On the other hand, marine waters appear to be a less volatile environment, and the establishment of the juvenile fish community was defined primarily by salinity. This is supported by the fact that the same species were recorded in marine waters in all three years. Dulčić et al. (1997) investigated several shallow marine coves on distant islands and found a similar temporal pattern and the prevalence of five dominant species, suggesting that only a low amount of variation in the juvenile fish abundance in the real marine environment can be explained by temperature and salinity. The absence of certain species (e.g., S. roissali and C. lucerna) in 2019 in both marine and transitional waters could be regulated by a factor that is more regional in nature. Similarly, Stagličić et al. (2011) reported that species abundance within littoral fish assemblages could be a consequence of factors occurring on a wider spatial scale (i.e., fisheries mismanagement, pollution, climate change). Contrary, C. saliens and S. salpa showed a higher abundance in northern marine waters (M_N) in 2019 and 2018, respectively, while S. cinereus was most abundant in T_S in 2018 in all four groups. However, there is the possibility that the sites where certain species were recorded are not essential or preferable nurseries for those species (i.e., poor site selection, low sample size, sampling gear selectivity). For example, P. erythrinus or T. ovatus were both lacking in southern marine areas in both 2018 and 2019. These species use other nursery areas, like marine coves on distant islands or at greater depths that are characterised by high and constant salinity (Dulčić et al., 1997; Biagi et al., 1998; La Mesa et al., 2011). In this study, P. erythrinus was found in 2017 exclusively at one station (S12) that is wide open toward the sea. Another typical marine species, P. pagrus, was also caught only at this site. The dispersal strategies of certain small migratory, shoaling species, such as Spicara sp. or B. belone, could also be the reason for their spatio-temporal absence (Hoare et al., 2000).
More Sensitive Species
If transitional waters geographically located at higher latitudes are more susceptible to interannual change, then the assumption is that more vulnerable species will select those waters for their nurseries, especially the more abundant species in the northern-central Adriatic (Dulčić et al., 2004; Matić-Skoko et al., 2007; Dulčić and Kovačić, 2020). Firstly, species of economic interest such as D. labrax, S. salpa and S. aurata should be highlighted here. It is also important to mention the small, resident species that also have ecological significance in the benthic communities of transitional waters, e.g., Salaria pavo, S. ocellatus, and Z. ophiocephalus, as these herbivorous and omnivorous fishes seem to control both algae and invertebrates in rocky sub-littoral zones (Ruitton et al., 2000). S. ocellatus showed a significant association with an algal habitat, and its abundance can certainly be related to the strong seasonal differences in algal cover (Hinz et al., 2019). If species preferring transitional waters at higher latitudes also spawn in winter and/or early spring, such as D. vulgaris, D. sargus, S. aurata and D. labrax, this makes them particularly sensitive to interannual differences in environmental conditions. As mentioned earlier, they can potentially be affected by late spawning, causing delays in settlement, leaving nurseries, or even causing individuals to remain in nurseries year round. Particularly, this delay can lead to the fact that at the moment of entry there is no prey of suitable size or in sufficient quantity for all incoming settlers (Machado et al., 2017). Decreased growth can make individuals too weak to join adult populations in the open seas that season (Lanier and Scharf, 2007). Since many marine species undergo long-distance dispersal during the pelagic larval phase before settling in benthic habitats where they remain (Di Franco et al., 2012), and many species also undergo further ontogenetic changes in restricted nursery grounds, pre- and post-settlement emigration by juveniles can then have significant population-level consequences (Vasconcelos et al., 2013). Sensitivity to physical-chemical changes of the aquatic environment, lack of suitable prey, and predation are the main causes of juvenile fish mortality (Guidetti, 2001; Cuadros et al., 2018).
Conclusion
Durrieu de Madron et al. (2011) highlighted the need to improve our understanding of species’ distributions and ecological preferences to determine marine ecosystem responses to different anthropogenic impacts in the Mediterranean that affect juvenile survival in nursery areas. Matić-Skoko et al. (2020) suggested that significantly modified juvenile fish communities in recent decades may be the result of constant human embankment and marine infrastructure construction along the coast. Such an undesirable continuous practice on a wider spatial scale could potentially lead to biological homogenisation (Cacabelos et al., 2016; Pastro et al., 2016) due to changes in sediment or bottom type. In that sense, although there is a critical need to define the value of coastal habitats as nurseries for population abundance and growth of economically important or exploited species, quantifying the value of coastal habitats at the population level is a complex task (Beck et al., 2001; Duarte et al., 2008; Fodrie et al., 2009; Vasconcelos et al., 2011). The variability or similarity of structural features among different habitats illustrates species tolerance to available nursery conditions and should provide valuable information toward future spatially explicit predictions of species’ distribution responses and resilience to potential changes in coastal conditions. Furthermore, variability of spatial and environmental use patterns in coastal areas should provide knowledge on the relevance of local regulation in defining nursery function. Ultimately, determining important sites for juveniles is a fundamental step toward the accurate identification of coastal nursery areas (Beck et al., 2001) that are essential for these species’ metapopulations.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Ethics Committee of the Institute of Oceanography and Fisheries in Split.
Author Contributions
SM-S, DV, HU and MP analysed the data end existing literature in collaboration with PT and DB. SM-S and DV wrote the draft of the manuscript. All authors conceived the research and participated in the improvement and revision of the document.
Funding
This study was fully supported by the Croatian Science Foundation (HRZZ) under project IP-2016-06-9884 (NurseFish).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Marti J. Anderson (Director of PRIMER-e) for the knowledge shared with us during the PERMANOVA workshop at the University of Trieste, Italy (September 2019).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.849092/full#supplementary-material
References
Anderson, M. J. (2001). Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 58, 626–639. doi: 10.1139/f01-004
Anderson, M. J., Gorley, R. N., and Clarke, K. R. (2008). PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth: PRIMER-E, 214.
Anderson, M. J., and Willis, T. J. (2003). Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84, 511–525. doi: 10.1890/0012-9658(2003)084[0511:caopca]2.0.co;2
Araújo, F. G., Azevedo, M. C. C., and Guedes, A. P. P. (2016). Interdecadal changes in fish communities of a tropical bay in southeastern Brazil. Reg. Stud. Mar. Sci. 3, 107–118. doi: 10.1016/j.rsma.2015.06.001
Barbeaux, S. J., and Hollowed, A. B. (2018). Ontogeny matters: climate variability and effects on fish distribution in the eastern Bering Sea. Fish. Ocean. 27, 1–15. doi: 10.1111/fog.12229
Barbier, E. B., Hacker, S. D., Kennedy, C., Koch, E. W., Stier, A. C., and Silliman, B. R. (2011). The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193. doi: 10.1890/10-1510.1
Barceló, C., Ciannelli, L., Olsen, E. M., Johannessen, T., and Knutsen, H. (2016). Eight decades of sampling reveal a contemporary novel fish assemblage in coastal nursery habitats. Glob. Chang. Biol. 22, 1155–1167. doi: 10.1111/gcb.13047
Bartulović, V., Dulčić, J., Matić-Skoko, S., and Glamuzina, B. (2011). Reproductive cycles of Mugil cephalus, ramada and Liza aurata (Teleostei: Mugilidae). J. Fish. Biol. 78, 2067–2073. doi: 10.1111/j.1095-8649.2011.02953.x
Beck, M. W., Heck, K. L., Able, K. W., Childers, D. L., Eggleston, D. B., Gillanders, B. M., et al. (2001). The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51, 633–641. doi: 10.1641/0006-3568(2001)051[0633:ticamo]2.0.co;2
Bellard, C., Bertelsmeier, C., Leadley, P., Thuiller, W., and Courchamp, F. (2012). Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377. doi: 10.1111/j.1461-0248.2011.01736.x
Biagi, F., Gambaccini, S., and Zazzetta, M. (1998). Settlement and recruitment in fishes: the role of coastal areas. Ital. J. Zool. 65, 269–274. doi: 10.1080/11250009809386831
Blaber, S. J. M., Brewer, D. T., and Salini, J. P. (1995). Fish communities and the nursery role of the shallow inshore waters of a tropical bay in the gulf of Carpentaria, Australia. Est. Coast. Shelf Sci. 40, 177–193. doi: 10.1016/S0272-7714(05)80004-6
Brothers, E. B., Williams, D. McB, and Sales, P. F. (1983). Length of larval life in twelve families of fishes at “one tree lagoon,” Great Barrier Reef, Australia. Mar. Biol. 76, 319–324. doi: 10.1007/bf00393035
Cacabelos, E., Martins, G. M., Thompson, R., Prestes, A. C. L., Azevedo, J. M. N., and Neto, A. I. (2016). Material type and roughness influence structure of inter−tidal communities on coastal defences. Mar. Ecol. 37, 801–812. doi: 10.1111/maec.12354
Cheminee, A., Francour, P., and Harmelin-Vivien, M. (2011). Assessment of Diplodus spp. (Sparidae) nursery grounds along the rocky shore of Marseilles (France, NW Mediterranean). Sci. Mar. 75, 181–188. doi: 10.3989/scimar.2011.75n1181
Cheminée, A., Merigot, B., Vanderklift, M. A., and Francour, P. (2016). Does habitat complexity influence fish recruitment? Mediterr. Mar. Sci. 17, 39–46. doi: 10.12681/mms.1231
Cheminée, A., Pastor, J., Bianchimani, O., Thiriet, P., Sala, E., Cottalorda, J. M., et al. (2017). Juvenile fish assemblages in temperate rocky reefs are shaped by the presence of macro-Algae canopy and its three-dimensional structure. Sci. Rep. 7, 1–11. doi: 10.1038/s41598-017-15291-y
Clarke, K. R., Tweedley, J. R., and Valesini, F. J. (2014). Simple shade plots aid better long-term choices of data pre-treatment in multivariate assemblage studies. J. Mar. Biol. Ass. U.K. 94, 1–16. doi: 10.1017/S0025315413001227
Clarke, K. R., and Warwick, R. M. (2001). Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd Edn. Plymouth, UK: Primer-E Ltd.
Cuadros, A., Basterretxea, G., Cardona, L., Cheminée, A., Hidalgo, M., and Moranta, J. (2018). Settlement and post-settlement survival rates of the white seabream (Diplodus sargus) in the western Mediterranean Sea. PLoS One 13:e0190278. doi: 10.1371/journal.pone.0190278
Dahlgren, C. P., and Eggleston, D. B. (2014). Ecological Processes Underlying Ontogenetic Habitat Shifts in a Coral Reef Fish. Ecology 81, 2227–2240. doi: 10.2307/177110
Deudero, S., Morey, G., Frau, A., Moranta, J., and Moreno, I. (2008). Temporal trends of littoral fishes at deep Posidonia oceanica seagrass meadows in a temperate coastal zone. J. Mar. Syst. 70, 182–195. doi: 10.1016/J.JMARSYS.2007.05.001
DHMZ (2019). Meteorological and Hydrological Service. Available online at: www.meteo.hr, (accessed on Dec 17, 2021)
Di Franco, A., Coppini, G., Pujolar, J. M., De Leo, G. A., Gatto, M., Lyubartsev, V., et al. (2012). Assessing Dispersal Patterns of Fish Propagules from an Effective Mediterranean Marine Protected Area. PLoS One 7:e52108. doi: 10.1371/journal.pone.0052108
Dobroslavić, T., Mozara, R., Glamuzina, B., and Bartulović, V. (2017). Reproductive patterns of bogue, Boops boops (Sparidae), in the southeastern Adriatic Sea. Acta Adria. 58, 117–125. doi: 10.32582/aa.58.1.9
Doney, S. C., Ruckelshaus, M., Duffy, J. E., Barry, J. P., Chan, F., English, C. A., et al. (2012). Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 4, 11–37. doi: 10.1146/annurev-marine-041911-111611
Downie, A. T., Leis, J. M., Cowman, P. F., McCormick, M. I., and Rummer, J. L. (2021). The influence of habitat association on swimming performance in marine teleost fish larvae. Fish Fish. 22, 1187–1212. doi: 10.1111/faf.12580
Duarte, C. M. (2000). Marine biodiversity and ecosystem services: an elusive link. J. Exp. Mar. Biol. Ecol. 250, 117–131. doi: 10.1016/S0022-0981(00)00194-5
Duarte, C. M., Dennison, W. C., Orth, R. J. W., and Carruthers, T. J. B. (2008). The Charisma of Coastal Ecosystems: addressing the Imbalance. Estuar. Coast 31, 233–238. doi: 10.1007/s12237-008-9038-7
Dulčić, J., Fencil, M., Matić-Skoko, S., Kraljević, M., and Glamuzina, B. (2004). Diel catch variations in a shallow-water fish assemblage at Duće-Glava, eastern Adriatic (Croatian coast). J. Mar. Biol. Ass. U.K. 84, 659–664. doi: 10.1017/S0025315404009701h
Dulčić, J., and Kovačić, M. (2020). Ihtiofauna Jadranskog mora. Zagreb: Golden marketing – Tehnička knjiga.
Dulčić, J., Kraljević, M., Grbec, B., and Pallaoro, A. (1997). Composition and temporal fluctuations of inshore juvenile fish populations in the Kornati Archipelago, eastern middle Adriatic. Mar. Biol. 129, 267–277. doi: 10.1007/s002270050167
Dulčić, J., Matić-Skoko, S., Kraljević, M., Fencil, M., and Glamuzina, B. (2005). Seasonality of a fish assemblage in shallow waters of Duće-Glava, eastern middle Adriatic. Cybium 29, 57–63.
Dulčić, J., Tutman, P., Matić-Skoko, S., Kraljević, M., Skaramuca, B., Glavić, N., et al. (2007). Y-O-Y fish species richness in the littoral shallows of the Neretva and Mala Neretva river estuaries (Eastern Adriatic. Croatian coast). Acta Adriat. 48, 89–94.
Durrieu de Madron, X., Guieu, C., Sempéré, R., Conan, P., Cossa, D., D’Ortenzio, F., et al. (2011). Marine ecosystems’ responses to climatic and anthropogenic forcings in the Mediterranean. Prog. Oceanogr. 91, 97–166. doi: 10.1016/j.pocean.2011.02.003
Félix-Hackradt, F. C., Hackradt, C. W., Treviño-Otón, J., Pérez-Ruzafa, A., and García-Charton, J. A. (2014). Habitat use and ontogenetic shifts of fish life stages at rocky reefs in South-western Mediterranean Sea. J. Sea Res. 88, 67–77. doi: 10.1016/j.seares.2013.12.018
Fodrie, F. J., Levin, L. A., and Lucas, A. J. (2009). Use of population fitness to evaluate the nursery function of juvenile habitats. Mar. Ecol. Prog. Ser. 385, 39–49. doi: 10.3354/meps08069
Francour, P. (1997). Fish assemblages of Posidonia oceanica beds at Port-Cros (France. Mar. Ecol. 18, 157–173. doi: 10.1111/j.1439-0485.1997.tb00434.x
Francour, P., Ganteaume, A., and Poulain, M. (1999). Effects of boat anchoring in Posidonia oceanica seagrass beds in the Port-Cros national park (northwestern Mediterranean Sea). Aquat. Conserv 9, 391–400. doi: 10.1002/(SICI)1099-0755(199907/08)9:43.0.CO;2-8
García-Rubies, A., and Macpherson, E. (1995). Substrate use and temporal pattern of recruitment in juvenile fishes of the Mediterranean littoral. Mar. Biol. 124, 35–42. doi: 10.1007/BF00349144
Gloginja, B., and Mitrović, L. (2021). “Hydrographic and Oceanographic Characteristics of the Southern Part of the Adriatic Sea,” in The Montenegrin Adriatic Coast, eds A. Joksimović, M. Ðurović, I. S. Zonn, A. G. Kostianoy, and A. V. Semenov (Springer: Springer Link).
Grbec, B., and Morović, M. (1997). Seasonal thermohaline fluctuations in the middle Adriatic Sea. Il Nuovo Cimento 20, 561–576.
Grbec, B., Morović, M., Matić, F., Ninčević, Ž, Marasović, I., Vidjak, O., et al. (2015). Climate regime shifts and multi-decadal variability of the Adriatic Sea pelagic ecosystem. Acta Adriat. 56, 47–66.
Guidetti, P. (2001). Population dynamics and post-settlement mortality of the ornate wrasse, Thalassoma pavo, in the Tyrrhenian sea (western Mediterranean). Ital. J. Zool. 68, 75–78. doi: 10.1080/11250000109356386
Guidetti, P., Fanelli, G., Fraschetti, S., Terlizzi, A., and Boero, F. (2002). Coastal fish indicate human-induced changes in the Mediterranean littoral. Mar. Environ. Res. 53, 77–94. doi: 10.1016/s0141-1136(01)00111-8
Halpern, B. S., Walbridge, S., Selkoe, K. A., Kappel, C. V., Micheli, F., D’Agrosa, C., et al. (2008). A global map of human impact on marine ecosystems. Science 319, 948–952. doi: 10.1126/science.1149345
Harmelin-Vivien, M. (1984). “Ichtyofaune des herbiers de posidonies du parc naturel régional de Corse,” in Internation, Workshop Posidonia oceanica Beds, Boudouresque, eds C. F. Jeudy, A. de Grissac, and J. Olivier (Marseille: GIS Posidonie Publ.), 291–301.
Hinz, H., Reñones, O., Gouraguine, A., Johnson, A. F., and Moranta, J. (2019). Fish nursery value of algae habitats in temperate coastal reefs. PeerJ 7:e6797. doi: 10.7717/peerj.6797
Hixon, M. A., and Johnson, D. W. (2009). Density dependence and independence. eLS (Encyclopedia of life sciences). New Jersey: John Wiley & Sons, Ltd.
Hoare, D. J., Krause, J., Peuhkuri, N., and Godin, J.-G. J. (2000). Body size and shoaling in fish. J. Fish Biol. 57, 1351–1366. doi: 10.1006/jfbi.2000.1446
Jones, G. P., Almany, G. R., Russ, G. R., Sale, P. F., Steneck, R. S., van Oppen, M. J. H., et al. (2009). Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28, 307–325. doi: 10.1007/s00338-009-0469-9
Krvavica, N., and Ružić, I. (2020). Assessment of sea-level rise impacts on salt-wedge intrusion in idealized and Neretva River Estuary. Estua. Coast. Shelf Sci. 234:106638. doi: 10.1016/j.ecss.2020.106638
La Mesa, G., Molinari, A., Gambaccini, S., and Tunesi, L. (2011). Spatial pattern of coastal fish assemblages in different habitats in North-western Mediterranean. Mar. Ecol. 32, 104–114. doi: 10.1111/j.1439-0485.2010.00404.x
Lanier, J. M., and Scharf, F. S. (2007). Experimental investigation of spatial and temporal variation in estuarine growth of age-0 juvenile red drum (Sciaenops ocellatus). J. Exper. Mar. Biol. Ecol. 349, 131–141. doi: 10.1016/j.jembe.2007.05.004
Laurel, B. J., Cote, D., Gregory, R. S., Rogers, L. A., Knutsen, H., and Olsen, E. M. (2017). Recruitment signals in juvenile cod surveys depend on thermal growth conditions. Can. J. Fish. Aqua. Sci. 74, 511–523. doi: 10.1139/cjfas-2016-0035
Machado, I., Calliari, D., Denicola, A., and Rodríguez-Graña, L. (2017). Coupling suitable prey field to in situ fish larval condition and abundance in a subtropical estuary. Estuar. Coast. Shelf Sci. 187, 31–42. doi: 10.1016/j.ecss.2016.12.021
Martins, G. M., Harley, C. D. G., Faria, J., Vale, M., Hawkins, S. J., Neto, A. I., et al. (2019). Direct and indirect effects of climate change squeeze the local distribution of a habitat-forming seaweed. Mar. Ecol. Prog. Ser. 626, 43–52. doi: 10.3354/meps13080
Matić-Skoko, S., Peharda, M., Pallaoro, A., Cukrov, M., and Baždarić, B. (2007). Infralittoral fish assemblages in the Zrmanja estuary, Adriatic Sea. Acta Adria. 48, 45–55.
Matić-Skoko, S., Vrdoljak, D., Uvanović, H., Pavičić, M., Tutman, P., and Bojanić Varezić, D. (2020). Early evidence of a shift in juvenile fish communities in response to conditions in nursery areas. Sci. Rep. 10:21078. doi: 10.1038/s41598-020-78181-wda0b5c49-0119-4a19-9b2c-81d12c0d245c
Novosel, M., Olujić, G., Cocito, S., and Požar-Domac, A. (2005). “Submarine freshwater springs in the Adriatic Sea: a unique habitat for the bryozoan Pentapora fascialis,” in Proceedings of the 13th International Conference of the International Bryozoology Association, ed. G. Moyano, I. Hugo, J. M. Cancino, and P. N. Wyse Jackson (London: Balkema Publishers (London)), 215–221.
Pastro, G., Dias, G. M., Pereira, G. H., and Gibran, F. Z. (2016). The consequences of small-scale variations in habitat conditions driven by a floating marina on reef fish assemblages of SE Brazil. Ocean Coast. Manag. 141, 98–106. doi: 10.1016/j.ocecoaman.2017.03.004
Planque, B., and Fox, C. J. (1998). Interannual variability in temperature and the recruitment of Irish Sea cod. Mar. Ecol. Prog. Ser. 172, 101–105. doi: 10.3354/meps172101
Potter, I. C., Chuwen, B. M., Hoeksema, S. D., and Elliott, M. (2010). The concept of an estuary: a definition that incorporates systems which can become closed to the ocean and hypersaline. Estuar. Coast. Shelf Sci. 87, 497–500. doi: 10.1016/j.ecss.2010.01.021
Ruitton, S., Francour, P., and Boudouresque, C. (2000). Relationships between Algae. Estuar. Coast. Shelf Sci. 50, 217–230. doi: 10.1006/ecss.1999.0546
Sala, E. (2004). The past and present topology and structure of the Mediterranean subtidal rocky-shore food webs. Ecosystem 7, 333–340. doi: 10.1007/s10021-003-0241-x
Sala, E., Ballesteros, E., Dendrinos, P., Di Franco, A., Ferretti, F., Foley, D., et al. (2012). The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 7:e32742. doi: 10.1371/journal.pone.0032742
Sale, P. F. (2006). Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem. Amsterdam: Academic Press.
Scharf, F. S., Manderson, J. P., and Fabrizio, M. C. (2006). The effects of seafloor habitat complexity on survival of juvenile fishes: species-specific interactions with structural refuge. J. Exp. Mar. Biol. Ecol. 335, 167–176. doi: 10.1016/j.jembe.2006.03.018
Shoji, J., Toshito, S., Mizuno, K., Kamimura, Y., Hori, M., and Hirakawa, K. (2011). Possible effects of global warming on fish recruitment: shifts in spawning season and latitudinal distribution can alter growth of fish early life stages through changes in daylength. ICES J. Mar. Sci. 68, 1165–1169.
Stagličić, N., Matić-Skoko, S., Pallaoro, A., Grgičević, R., Kraljević, M., Tutman, P., et al. (2011). Long term trends in the structure of eastern Adriatic littoral fish assemblages: consequences for fisheries management. Estuar. Coast. Shelf Sci. 94, 263–271. doi: 10.1016/j.ecss.2011.07.005
Vasconcelos, R. P., Eggleston, D. B., Le Pape, O., and Tulp, I. (2013). Patterns and processes of habitat-specific demographic variability in exploited marine species. ICES J. Mar. Sci. 71, 638–647. doi: 10.1093/icesjms/fst136
Vasconcelos, R. P., Reis-Santos, P., Costa, M. J., and Cabral, H. N. (2011). Connectivity between estuaries and marine environment: integrating metrics to assess estuarine nursery function. Ecol. Ind. 11, 1123–1133. doi: 10.1016/j.ecolind.2010.12.012
Keywords: juvenile fish, community, settlement, nurseries, interannual changes
Citation: Matić-Skoko S, Vrdoljak D, Uvanović H, Pavičić M, Tutman P, Bojanić Varezić D and Kovačić M (2022) Effects of Interannual Environmental Changes on Juvenile Fish Settlement in Coastal Nurseries: The Case of the Adriatic Sea. Front. Mar. Sci. 9:849092. doi: 10.3389/fmars.2022.849092
Received: 05 January 2022; Accepted: 27 January 2022;
Published: 25 February 2022.
Edited by:
Stylianos Somarakis, Institute of Marine Biological Resources and Inland Waters, Hellenic Center for Marine Research, GreeceReviewed by:
Francesco Tiralongo, University of Catania, ItalyStefanos Kalogirou, Swedish Agency for Marine and Water Management, Sweden
Copyright © 2022 Matić-Skoko, Vrdoljak, Uvanović, Pavičić, Tutman, Bojanić Varezić and Kovačić. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanja Matić-Skoko, c2FuamFAaXpvci5ocg==
 Sanja Matić-Skoko
Sanja Matić-Skoko Dario Vrdoljak
Dario Vrdoljak Hana Uvanović
Hana Uvanović Mišo Pavičić
Mišo Pavičić Pero Tutman
Pero Tutman Dubravka Bojanić Varezić1
Dubravka Bojanić Varezić1 Marcelo Kovačić
Marcelo Kovačić