
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci., 12 April 2022
Sec. Marine Megafauna
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.824071
This article is part of the Research TopicRisks, Threats, and Conservation Status of Cetaceans in the Mediterranean and Black SeasView all 15 articles
 Danny Morick1,2,3*
Danny Morick1,2,3* Nadav Davidovich1,2,4
Nadav Davidovich1,2,4 Ziv Zemah-Shamir1,2
Ziv Zemah-Shamir1,2 Eyal Bigal1,2
Eyal Bigal1,2 Assaf Rokney5
Assaf Rokney5 Merav Ron5
Merav Ron5 Shlomo E. Blum6
Shlomo E. Blum6 Marcelo Fleker6
Marcelo Fleker6 Esteban Soto7
Esteban Soto7 Taylor I. Heckman7
Taylor I. Heckman7 Stanley C. K. Lau8,3
Stanley C. K. Lau8,3 Natascha Wosnick9
Natascha Wosnick9 Dan Tchernov1,2,3
Dan Tchernov1,2,3 Aviad P. Scheinin1,2
Aviad P. Scheinin1,2Streptococcosis is an infectious bacterial disease of both homeotherms and poikilotherms. Among the Streptococcus species that infect marine animals, Streptococcus agalactiae has the broadest host spectrum, including different aquatic organisms in freshwater and marine environments. The common dolphin (Delphinus delphis) is categorized as Endangered in the Mediterranean Sea. There are few reports of a streptococcal infection of D. delphis, caused by Streptococcus phocae and Streptococcus iniae. Here we report the isolation and identification of S. agalactiae in a stranded, wild male common dolphin that was found dead in September 2020 on the seashore next to the city of Bat-Yam, Israel. The carcass was fresh with a moderate nutritional status and with no apparent fishing gear or other anthropogenic-related signs. A post-mortem examination did not reveal an apparent cause of death, but further laboratory analysis demonstrated a S. agalactiae bacterial presence in urine, lungs and pericardial fluid that was characterized as type Ia-ST7 by whole genome sequencing. Interestingly, this isolate was found to be almost identical to another isolate recently recovered from a wild sandbar shark (Carcharhinus plumbeus) in the same area in Israel, the eastern Mediterranean Sea.
Bacterial infections are considered to be a significant threat to marine mammals, causing significant morbidity and mortality. Members of the genus Streptococcus are among the most commonly reported pathogens in marine mammals, including cetaceans (Dunn et al., 2001; Numberger et al., 2021). Although these bacteria have been isolated from apparently healthy individuals, they have also been associated with significant pathological changes and zoonotic potential (Díaz-Delgado et al., 2017). Streptococcosis is a septicemic disease that affects freshwater, brackish and marine animals in wild and farmed populations. Ten streptococcal species have been isolated from marine mammals, including Streptococcus agalactiae (Group B Streptococcus, GBS) and other closely related streptococci like S. phocae and S. iniae (Numberger et al., 2021). In the area of the eastern Mediterranean Sea, there are very limited data regarding the presence and importance of streptococcal infection in the marine environment and Streptococcus spp. are reported in wild marine fish (Zlotkin et al., 1998; Corloni et al., 2002; Berzak et al., 2019). The common dolphin (Delphinus delphis) is a small cetacean species with a wide distribution. Delphinus delphis was once abundant in the Mediterranean Sea, but from the 1960s the species declined dramatically in the region and the Mediterranean subpopulation contains fewer than 2,500 mature individuals (Notarbartolo di Sciara and Tonay, 2021). It is estimated to continue and decline of at least 20% in two generations, and a reduction of 66% is suspected in the past three generations (Bearzi et al., 2003; Del Mar Otero and Conigliaro, 2012; Notarbartolo di Sciara and Tonay, 2021). At a global level, the species is classified as “Least Concern” (Braulik et al., 2021). However, due to its dramatic decrease, the Mediterranean subpopulation was listed as “Endangered” in the International Union for Conservation of Nature (IUCN) Red List (Bearzi, 2012). In the Mediterranean, D. delphis are found in both pelagic and neritic environments, forming mixed groups along with Striped (Stenella coeruleoalba) and Risso’s Dolphins (Grampus griseus) (Frantzis and Herzing, 2002). Isolated groups are also recorded, with about 50–70 individuals (Bearzi et al., 2003). Threats to the species in the region include competition with commercial fisheries (Bearzi et al., 2003), PCB pollution (Borrell et al., 2001), bycatch and climate change (Bearzi, 2012). As with other Mediterranean marine mammals, data on health status is limited, and few publications described Erysipelothrix rhusiopathiae, Toxoplasma gondii and dolphin morbillivirus as the primary infectious agents, affecting the common dolphin in the Mediterranean Sea area (Vella et al., 2021). Here we report the first isolation and identification of S. agalactiae type Ia-ST7 in a common dolphin from the Mediterranean subpopulation, and the first report of this infection for the species worldwide.
In September 2020, a male common dolphin was found stranded nearby Bat-Yam, Israel. The carcass was collected for necropsy at the Morris Kahn Marine Research Station in Ashdod, Israel. A post-mortem examination was conducted, based on the procedure described by Kuiken and García Hartmann (1993) and Ijsseldijk et al. (2019), and tissue samples were aseptically collected to avoid cross contamination. An exhaustive pathological examination was not performed, as other research groups were involved in the necropsy and removed the brain and gastrointestinal tract for functional MRI and microplastic analysis, respectively. Due to several ongoing studies described above, the sampling protocol was also performed with limitations. Samples of the penis, spleen, testicle, liver and several sections of the lung and kidney were fixed in 10%-buffered formalin for 48 h. Subsequently, the fixed samples were reduced in size, trimmed, dehydrated, embedded in Paraplast®, and stained with Hematoxyline & Eosin and Gram stain for routine histological evaluation. Liver, spleen, kidney and lungs were sampled by sterile swabs, and pericardial fluid and urine (from the urinary bladder) were collected aseptically via sterile needle and syringe during the necropsy. Before swabbing, the surface of the organ was seared with a hot blade, then incised with a sterile scalpel and, finally, a sterile swab was inserted into the incision. Two aliquots, one for PCR (stored at -20°C) and one for microbiological investigations (stored at 4°C), were collected. These samples were sent to the department of bacteriology at the Kimron Veterinary Institute were the pericardial fluid and urine were aseptically swabbed and further used for the following tests. All the mentioned above swabs were screened by molecular tools for canine distemper virus (CDV) following Elia et al. (2006). For bacteriology, all swabs were inoculated onto tryptic soy agar (TSA), blood agar (5% sheep blood enriched TSA), and MacConkey agar, and incubated for 24-48 h at 37°C. The samples were inoculated onto Brucella agar as previously described (Markey et al., 2013) for 10 days and onto Mycoplasma broth followed by Mycoplasma agar incubated at 37°C for up to 10 days in CO2 enriched atmosphere as described before (Blum et al., 2010). Confirmation of bacteria species was initially done by Matrix Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS), according to the manufacturing protocol (Autoflex, Bruker). Antimicrobial sensitivity test was performed to this strain by in vitro susceptibility testing by standard disk diffusion method and inhibition zones were measured as previously described (Elad et al., 2018). DNA was extracted from bacterial colonies isolated from the urine using a Wizard SV Genomic System (Promega, WI, USA) by genomic DNA purification protocol following the manufacturer’s instructions for tissue lysates. The quantity and purity of the DNA were estimated using NanoDrop One (Thermo Scientific, Rockford, CA, USA). The genomic DNA obtained was stored at −20°C until use. The quantity and purity of the DNA were estimated using NanoDrop One (Thermo Scientific, Rockford, CA, USA). The isolates were further serotyped at the Public Health National Reference Laboratory (Ministry of Health, Israel) using a molecular serotyping method by multiplex-PCR for species confirmation and direct identification of capsular type (Poyart et al., 2007). Additionally, whole-genome sequencing (WGS) was performed. The DNA for WGS was extracted using the QIAsymphony® SP system and the QIAsymphony® DNA mini kit (Qiagen) according to the manufacturers’ recommendations. A DNA library was prepared using the Nextera XT library preparation kit (Illumina, CA, USA), followed by WGS using the Illumina MiSeq system with the read length of 250 bp paired-end. Reads were assembled using SPAdes by the BioNumerics 8.0 platform.
The dolphin weighed 85.5 kg, had a girth measuring 107 cm, a total length of 211 cm and was classified as mature according to Murphy and Rogan (2006); we assumed that the animal was not of old age base on mild erosion of the teeth. At necropsy, no external signs of interaction with fishing gear were observed. The decomposition state of the carcass was classed as ‘fresh’ (condition code 2; DCC2), as defined by Ijsseldijk et al. (2019), with a moderate nutritional status. Little gross changes were evident, including an assumed papilloma at the end of the penis. Emphysema was found in the cranial parts of both lungs along with a white foam material in the bronchioles. Five ml of mucopurulent discharge was observed in the urinary bladder (Figure 1). Histological examination of lungs revealed numerous bacterial colonies in subpleural alveoli and numerous bacterial colonies in pulmonary blood vessels (Figure 2A). The splenic architecture appeared within normal ranges with no evidence of lymphoid depletion. The liver was partially autolyzed. However, extracellular bacteria were present inside hepatic blood vessels but no lesions were observed in the parenchyma (Figure 2B). Bacterial colonies were also observed in the capillaries of the kidneys (Figure 2C). Aerobic culture yielded pure bacterial colonies of spherical or ovoid cocci, 1-2 μm in diameter, grayish-white, smooth, glossy and translucent, with a narrow zone of β hemolysis. Colonies, consistent with the genus Streptococcus, appeared on the blood agar plates 48 h post-inoculation from the mucopurulent discharge from the urine, lungs and pericardial fluid. Gram staining confirmed the presence of Gram-positive bacteria (Figures 2D, E). The bacteria were confirmed as S. agalactiae by MALDI-TOF MS, and further by the mentioned above molecular methods. No other bacteria were isolated from the tested samples. The isolate was resistant to Gentamicin with intermediate susceptible to Amoxicillin/clavulanic acid, Ampicillin, Fluoroquinolones and Tetracyclines and susceptible to Erythromycin, Sulfamethoxazole/Trimethoprim, Florfenicol, Penicillin, Penicillinase resistant penicillins, First gen. cephalosporins and Clindamycin. The assembled genome was submitted to the PubMLST S. agalactiae database as ICLGBS002 (ST35685). The strain was identified as ST-7 strain by wgMLST comparison analysis of ST-7 strains. Using the Genome Comparator (GC) tool, allelic profiles of the 2,207 loci were retrieved and imported to BioNumerics 8.0 (Applied Maths) in order to generate a phylogenetic tree (Figure 3). The present isolate is almost identical with a two-SNP difference in comparison of 1,969,033 bases (96.7% genome size), to an isolate described by Morick et al. (2020), collected from a moribund sandbar shark (Carcharhinus plumbeus) found on Netanya’s shoreline (Israel – east Mediterranean coast) in 2018. Other tests that were performed and described in the methods section produced negative results.
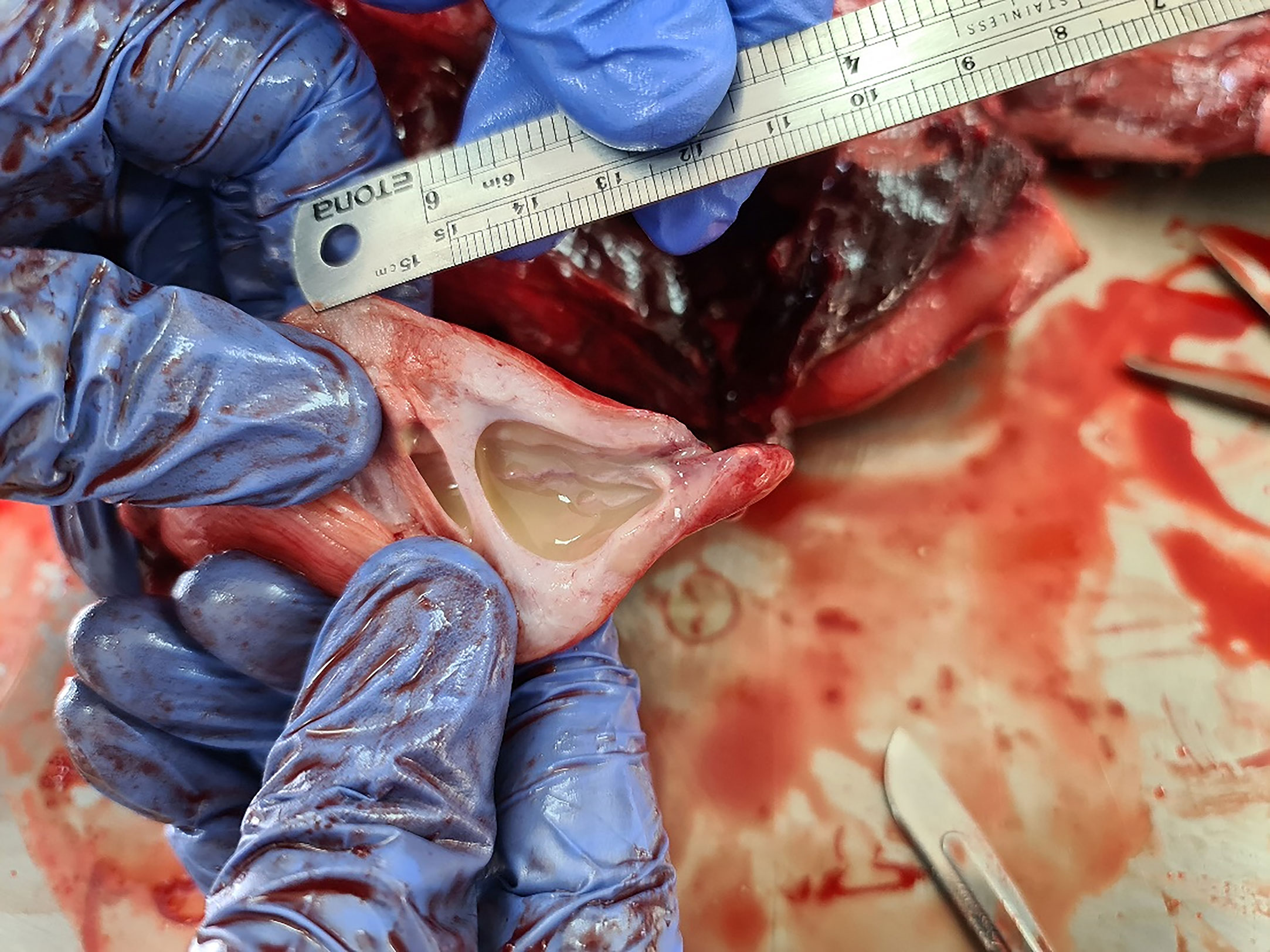
Figure 1 Gross pathology of the urinary bladder of a common dolphin (Delphinus delphis). About five ml of mucopurulent discharge was observed in the urinary bladder.

Figure 2 Streptococcus agalactiae in lungs, liver, and kidney of a common dolphin. (A) Colonies of spherical or ovoid cocci, 1-2 μm in diameter, detected in subpleural alveoli and lung blood vessels (H&E stain). (B) Colonies identified inside hepatic blood vessels. No lesions or pathology was observed in the parenchyma (H&E stain). (C) Colonies and detached bacteria detected in the capillaries of the kidneys (H&E stain). Higher magnification of the kidney (D) and Gram-positive cocci confirmation in kidney samples (E); (Gram stain).

Figure 3 Whole genome MLST phylogenetic analysis of 55 publicly available global ST-7 sequences. Minimum spanning tree was based on wgMLST scheme (2,207 genes). The numbers on branches indicate allelic differences between strains. Group B Streptococcus (GBS) serotype is shown on top of the nodes. Color-coding is by country of origin ND = no data available regarding the serotype of the isolate.
Although rapid population declines have been reported for the species in the Mediterranean, the causes are still a matter of speculation. Studies indicate that synergistic environmental and anthropogenic causes contributed to the reduction reported in the region (Vella et al., 2021). Overexploitation of their prey (Bearzi et al., 2008) and niche competition with striped dolphins (Giménez et al., 2018) are also potential causes. Nevertheless, quantitative data on abundance and decline rates are not available for this subpopulation, so a reduction in population size was inferred at more than 50% over a three-generation period (30 to 45 years) (Bearzi, 2012). This subpopulation was listed as “Endangered” based not only on population declines but also on its extent of occurrence, as well as a deterioration of habitat quality (Bearzi et al., 2003). One of the most commonly used proxies to assess a population (and its habitat) status is the systemic health of individuals. Environmental alterations can affect the immune response of cetaceans, making them more susceptible to infectious diseases and associated mortality (Romano et al., 2002). Concerning diseases affecting this subpopulation (both infectious and non-infectious), neoplasms with metastatic potential (Di Guardo et al., 2005; Días-Delgado et al., 2012), Erysipelothrix rhusiopathiae infection (Fernández-Maldonado, 2016), toxoplasmosis (Sobrino et al., 2007) and intestinal parasites (Quiñones et al., 2013) have already been described for D. delphis Mediterranean subpopulation. There is also evidence that shared habitat and foraging resources between common and stripped dolphins may lead to disease outbreaks, as morbillivirus epizootics are reported for both species since the 1990s (Birkun et al., 1999; Raga et al., 2008). The immunosuppressive effect of morbillivirus in wild populations can make common dolphins predisposed to other infections that can seriously compromise population health and recovery from declines caused by other stressors.
Streptococcus agalactiae is a Gram-positive coccus, and S. agalactiae ST-261 was first reported in 1988 from farmed Nile tilapia (Oreochromis niloticus) leading to outbreaks in Israel (Eldar et al., 1994). Streptococcus outbreaks were also reported in farmed cows in the region (and worldwide), but due to a successful eradication program, it is no longer a sanitary issue in Israel (Lavon et al., 2019). In the late 1980s, it had also been described in association with marine mammal infections and cause of death, when it was isolated from wounds and tissue lesions of grey seals in Scotland (Halichoerus grypus) and Antarctic fur seals (Arctocephalus gazella) on Bird Island (Baker, 1988; Baker and McCann, 1989). The first cetacean isolations were in the early 2000s, as a fatal fasciitis and myositis in a captive common bottlenose dolphin (Tursiops truncatus), then a wild animal with no associated pathology (Zappulli et al., 2005; Evans et al., 2006). Streptococcus agalactiae has also been described as part of the nasal flora of healthy captive Hawaiian monk seals (Monachus schauinslandi) in Hawaii (Kissel et al., 2011). Consumption of diseased fish was a means of bacterial transmission to marine mammals, as S. agalactiae is a significant piscine pathogen (Numberger et al., 2021). The results of the antibacterial drug sensitivity test showed susceptibility of this isolate to the drugs that are most frequently used in human and veterinary medicine in this region. The resistance to gentamicin is reasonable and was described before for S. agalactiae isolated from a captive common bottlenose dolphin (Tursiops truncatus) (Zappulli et al., 2005). Additional studies should be conducted to increase the knowledge of GBS sensitivity profile to antimicrobials in marine animals. Streptococcus agalactiae was isolated from mucopurulent discharge from the urinary bladder, lungs and pericardial fluid, suggesting possible antemortem peracute septicemia. There was no significant gross nor microscopical findings available that could be associated with streptococcal infection. Unfortunately, the cause of death or primary causes for disease in this common dolphin remains undefined, but the dissemination of S. agalactiae may have contributed to stranding and death. The current isolate was assigned to ST7, which is a human pathogenic lineage, associated with septicemia and meningitis of immunocompromised people (Harris et al., 2011). Streptococcus agalactiae, previously reported in association with marine mammals (Zappulli et al., 2005), but also associated with outbreaks of fish disease, suggesting links between human, fish, and cetacean cases (Evans et al., 2006; Evans et al., 2008; Delannoy et al., 2013).
This is the first published report of a Streptococcus agalactiae infection in a common dolphin, with the potential to dispersion throughout the already endangered Mediterranean subpopulation. To date, conservation measures for the species are recommendations from the Convention on Biological Diversity, the Barcelona Convention (trough the Protection of the Marine Environment and Coastal Region of the Mediterranean), the Bern Convention (trough the Convention on the Conservation of European Wildlife and Natural Habitat), the Bonn Convention (trough the Convention on Migratory Species), legal protection trough the Legal Notice 203 (2003, Malta), the Convention on International Trade in Endangered Species of Wild of Flora and Fauna (CITES, Appendix II), and the Habitats Directive (92/43/EEC) (Vella et al., 2021). Marine Protected Areas are cited as a promising strategy to protect common dolphin populations, especially from human-induced disturbances (Giménez et al., 2021). Although such measure is extremely relevant for the species, infectious agents with zoonotic potential adhere to statutory delineations, thus confounding the management of diseases that might compromise species conservation. Long-term monitoring should be done, and funds should be constantly allocated to better understand the demographic and evolutionary effects of infectious diseases (Vella et al., 2021), as well as the genetic basis of susceptibility. This pathogen has been diagnosed in other marine fish (Morick et al., 2020) and now in a marine mammal in the eastern Mediterranean Sea. Possible transmission routes of this pathogen into marine mammals can include infected prey, sea bird feces and human and terrestrial animals wastes via sewage and rivers (Numberger et al., 2021). Further inquiry into the importance of S. agalactiae in wild marine animals in this area and its zoonotic potential is needed.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://pubmlst.org/bigsdb?db=pubmlst_sagalactiae_isolates&page=profiles, ICLGBS002 (ST35685).
This animal study was authorized with permits issued by Israel Nature and Parks Authority (n. 42548).
DM, ND, EB, and AS contributed to field collections, necropsy procedure, and sample processing. ZZ-S, TH, SL, DT, NW, and ES contributed to data processing, pathological interpretation, and writing of the manuscript. MR, AR, SB, and MF perform bacterial isolation and molecular characterization. All authors participated in drafting the manuscript, contributed to writing the article, and approved the submitted version.
This study was financially supported by the Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou, China (SMSEGL20SC02) and by the Kahn Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Dr. Ana Maria Botero- Anug DVM, DVSc, Diplomate ACVP, for her assistance with histology.
Baker J. R. (1988). Further Studies on Grey Seal (Halichoerus Grypus) Pup Mortality on North Rona. Br. Vet. J. 144 (5), 497–506. doi: 10.1016/0007-1935(88)90090-5
Baker J. R., McCann T. S. (1989). Pathology and Bacteriology of Adult Male Antarctic Fur Seals, Arctocephalus Gazella, Dying at Bird Island, South Georgia. Br. Vet. J. 145 (3), 263–275. doi: 10.1016/0007-1935(89)90079-1
Bearzi G. (2012). “Delphinus Delphis,” in The IUCN Red List of Threatened Species 2012. Downloaded on 28 November 2021. doi: 10.2305/IUCN.UK.2012-1.RLTS.T134817215A195829089
Bearzi G., Agazzi S., Gonzalvo J., Costa M., Bonizzoni S., Politi E., et al. (2008). Overfishing and the Disappearance of Short-Beaked Common Dolphins From Western Greece. Endanger. Species Res. 5, 1–12. doi: 10.3354/esr00103
Bearzi G., Reeves R. R., Notarbartolo Di Sciara G., Politi E., Canadas A., Mussi B. (2003). Ecology, Status and Conservation of Short-Beaked Common Dolphins Delphinus Delphis in the Mediterranean Sea. Mamm. Rev. 33, 224–252. doi: 10.1046/j.1365-2907.2003.00032.x
Berzak R., Scheinin A., Davidovich N., Regev Y., Diga R., Tchernov D., et al. (2019). Prevalence of Nervous Necrosis Virus (NNV) and Streptococcus Species in Wild Marine Fish and Crustaceans From the Levantine Basin, Mediterranean Sea. Dis. Aquat. Org. 133 (1), 7–17. doi: 10.3354/dao03339
Birkun A. Jr., Kuiken T., Krivokhizhin S., Haines D. M., Osterhaus A. D. M. E., Van de Bildt M. W. G., et al. (1999). Epizootic of Morbilliviral Disease in Common Dolphins (Delphinus Delphis Ponticus) From the Black Sea. Vet. Rec. 144, 85–92. doi: 10.1136/vr.144.4.85
Blum S., Elad D., Zukin N., Lysnyansky I., Weisblith L., Perl S., et al. (2010). Outbreak of Streptococcus Equi Subsp. Zooepidemicus Infections in Cats. Vet. Microbiol. 144 (1-2), 236–239. doi: 10.1016/j.vetmic.2009.12.040
Borrell A., Cantos G., Pastor T., Aguilar A. (2001). Organochlorine Compounds in Common Dolphins (Delphinus Delphis) From the Atlantic and Mediterranean Waters of Spain. Environ. Pollut. 114 (2), 265–274. doi: 10.1016/s0269-7491(00)00213-x
Braulik G., Jefferson T. A., Bearzi G. (2021). “Delphinus Delphis (Amended Version of 2021 Assessment),” in The IUCN Red List of Threatened Species 2021. Downloaded on 28 November 2021. doi: 10.2305/IUCN.UK.2021-2.RLTS.T134817215A199893039
Corlorni A., Diamant A., Eldar A., Kvitt H., Zlotkin A. (2002). Streptococcus iniae Infections in Red Sea Cage-Cultured and Wild Fishes. Dis. Aquat. Org. 49 (3), 165–170. doi: 10.3354/dao049165
Delannoy C. M., Crumlish M., Fontaine M. C., Pollock J., Foster G., Dagleish M. P., et al. (2013). Human Streptococcus Agalactiae Strains in Aquatic Mammals and Fish. BMC Microbiol. 13 (1), 1–9. doi: 10.1186/1471-2180-13-41
Del Mar Otero M., Conigliaro M. (2012). Marine Mammals and Sea Turtles of the Mediterranean and Black Seas Vol. 32 (Gland: IUCN), ISBN: ISBN: 978-2-8317-1478-3.
Díaz-Delgado J., de los Monteros A. E., Fernández-Maldonado C., Arbelo M., Quesada-Canales O., Andrada M., et al. (2012). Mixed Testicular Neoplasia in a Short Beaked Common Dolphin Delphinus Delphis. Dis. Aquat. Org. 101, 257–260. doi: 10.3354/dao02525
Díaz-Delgado J., Sierra E., Vela A. I., Arbelo M., Zucca D., Groch K. R., et al. (2017). Coinfection by Streptococcus Phocae and Cetacean Morbillivirus in a Short-Beaked Common Dolphin Delphinus Delphis. Dis. Aquat. Org. 124 (3), 247–252. doi: 10.3354/dao03124
Di Guardo G., Marruchella G., Affronte M., Zappulli V., Benazzi C. (2005). Heterotopic Kidney Tissue in the Lung of a Free-Living Common Dolphin (Delphinus Delphis). Vet. Pathol. 42, 213–214. doi: 10.1354/vp.42-2-213
Dunn L. J., Buck J. D., Robeck T. R. (2001). “Bacterial Diseases of Cetaceans and Pinnipeds,” in Handbook of Marine Mammal Medicine: Health, Disease, and Rehabilitation. Eds. Dierauf L. A., Gulland F. M. D. Boca Raton, Florida: (CRC Press), 309–336.
Elad D., Blum S. E., Perreten V., Fleker M., Avni Z., Weisbelith L. (2018). Emergence and Prevalence Decline of a Phenotypically Multidrug-Resistant Staphylococcus Pseudintermedius in Israel. Israel J. Vet. Med. 73 (3), 14–18. doi: 10.7892/boris.121224
Eldar A., Bejerano Y., Bercovier H. (1994). Streptococcus Shiloi and Streptococcus Difficile: Two New Streptococcal Species Causing a Meningoencephalitis in Fish. Curr. Microbiol. 28 (3), 139–143. doi: 10.1007/BF01571054
Elia G., Decaro N., Martella V., Cirone F., Lucente M. S., Lorusso E., et al. (2006). Detection of Canine Distemper Virus in Dogs by Real-Time RT-PCR. J. Virol. Methods 136 (1-2), 171–176. doi: 10.1016/j.jviromet.2006.05.004
Evans J. J., Bohnsack J. F., Klesius P. H., Whiting A. A., Garcia J. C., Shoemaker C. A., et al. (2008). Phylogenetic Relationships Among Streptococcus Agalactiae Isolated From Piscine, Dolphin, Bovine and Human Sources: A Dolphin and Piscine Lineage Associated With a Fish Epidemic in Kuwait is Also Associated With Human Neonatal Infections in Japan. J. Med. Microbiol. 57 (11), 1369–1376. doi: 10.1099/jmm.0.47815-0
Evans J. J., Pasnik D. J., Klesius P. H., Al-Ablani S. (2006). First Report of Streptococcus Agalactiae and Lactococcus Garvieae From a Wild Bottlenose Dolphin (Tursiops Truncatus). J. Wildl. Dis. 42 (3), 561–569. doi: 10.7589/0090-3558-42.3.561
Fernández-Maldonado C. (2016). Patologías Y Causas De La Muerte De Los Cetáceos Varados En Andalucí–2014) (PhD Thesis) (Universidad de las Palmas de Gran Canaria, Las Palmas, Spain).
Frantzis A., Herzing D. L. (2002). Mixed Species Associations of Striped Dolphin (Stenella Coeruleoalba), Short-Beaked Common Dolphin (Delphinus Delphis) and Risso’s Dolphin (Grampus Griseus), in the Gulf of Corinth (Greece, Mediterranean Sea). Aquat. Mamm. 28 (2), 188–197.
Giménez J., Cañadas A., de Stephanis R., Ramírez F. (2021). Expanding Protected Areas to Encompass the Conservation of the Endangered Common Dolphin (Delphinus Delphis) in the Alboran Sea. Mar. Environ. Res. 168, 105305. doi: 10.1016/j.marenvres.2021.105305
Giménez J., Cañadas A., Ramírez F., Afán I., García-Tiscar S., Fernández-Maldonado C., et al. (2018). Living Apart Together: Niche Partitioning Among Alboran Sea Cetaceans. Ecol. Indic. 95, 32–40. doi: 10.1016/j.ecolind.2018.07.020
Harris P., Siew D. A., Proud M., Buettner P., Norton R. (2011). Bacteraemia Caused by Beta-Haemolytic Streptococci in North Queensland: Changing Trends Over a 14-Year Period. Clin. Microbiol. Infect. 17 (8), 1216–1222. doi: 10.1111/j.1469-0691.2010.03427.x
Ijsseldijk L. L., Brownlow A. C., Mazzariol S. (2019). “Best Practice on Cetacean Post-Mortem Investigation and Tissue Sampling,” in Joint ACCOBAMS and ASCOBANS Document.
Kissel L. N., Bankowski M. J., Koyamatsu T. L., Nagai R. Y., Seifried S. E., Crow G. L. (2011). Aerobic Microorganisms Identified Over a Fourteen-Month Period From the Upper Respiratory Tract of Captive Hawaiian Monk Seals (Monachus Schauinslandi). Aquat. Mamm. 37 (3), 377–385. doi: 10.1578/AM.37.3.2011.377
Kuiken T., García Hartmann M. (1993). Cetacean Pathology: Dissection Techniques and Tissue Sampling. ECS Newslett. 17, Special Issue, 1–43.
Lavon Y., Leitner G., Kressel Y., Ezra E., Wolfenson D. (2019). Comparing Effects of Bovine Streptococcus and Escherichia Coli Mastitis on Impaired Reproductive Performance. J. Dairy Sci. 102 (11), 10587–10598. doi: 10.3168/jds.2019-16673
Markey B., Leonard F., Archambault M., Cullinane A., Maguire D. (2013). Clinical Veterinary Microbiology E-Book (Amsterdam: Elsevier Health Sciences), 857.
Morick D., Davidovich N., Bigal E., Rosenbluth E., Bouznach A., Rokney A., et al. (2020). Fatal Infection in a Wild Sandbar Shark (Carcharhinus Plumbeus), Caused by Streptococcus Agalactiae, Type Ia-St7. Animals 10 (2), 284. doi: 10.3390/ani10020284
Murphy S., Rogan E. (2006). External Morphology of the Short-Beaked Common Dolphin, Delphinus Delphis: Growth, Allometric Relationships and Sexual Dimorphism. Acta Zool. 87 (4), 315–329. doi: 10.1111/j.1463-6395.2006.00245.x
Notarbartolo di Sciara G., Tonay A. M. (2021). Conserving Whales, Dolphins and Porpoises in the Mediterranean Sea, Black Sea and Adjacent Areas: An ACCOBAMS Status Report, (2021) (Monaco: ACCOBAMS), 160.
Numberger D., Siebert U., Fulde M., Valentin-Weigand P. (2021). Streptococcal Infections in Marine Mammals. Microorganisms 9 (2), 350. doi: 10.3390/microorganisms9020350
Poyart C., Tazi A., Réglier-Poupet H., Billoët A., Tavares N., Raymond J., et al. (2007). Multiplex PCR Assay for Rapid and Accurate Capsular Typing of Group B Streptococci. J. Clin. Microbiol. 45 (6), 1985–1988. doi: 10.1128/JCM.00159-07
Quiñones R., Giovannini A., Raga J. A., Fernández M. (2013). Intestinal Helminth Fauna of Bottlenose Dolphin, Tursiops Truncatus, and Common Dolphin, Delphinus Delphis, From the Western Mediterranean. J. Parasitol. 99, 576–579. doi: 10.1645/GE-3165.1
Raga J. A., Banyard A., Domingo M., Corteyn M., Van Bressem M.-F., Fernández M., et al. (2008). Dolphin Morbillivirus Epizootic Resurgence, Mediterranean Sea. Emerg. Infect. Dis. 14, 471–473. doi: 10.3201/eid1403.071230
Romano T. A., Olschowka J. A., Felten S. Y., Quaranta V., Ridgway S. H., Felten D. L. (2002). “Immune Response, Stress, and Environment: Implications for Cetaceans,” in Cell and Molecular Biology of Marine Mammals. Ed. Pfeiffer C. J. (Malabar, Florida: Krieger Publishing Co., Inc).
Sobrino R., Cabezón O., Millán J., Pabón M., Arnal M. C., Luco D. F., et al. (2007). Seroprevalence of Toxoplasma Gondii Antibodies in Wild Carnivores From Spain. Vet. Parasitol. 148, 187–192. doi: 10.1016/j.vetpar.2007.06.038
Vella A., Murphy S., Giménez J., de Stephanis R., Mussi B., Vella J. G., et al. (2021). The Conservation of the Endangered Mediterranean Common Dolphin (Delphinus Delphis): Current Knowledge and Research Priorities. Aquat. Conserv.: Mar. Freshw. Ecosyst. 31, 110–136. doi: 10.1002/aqc.3538
Zappulli V., Mazzariol S., Cavicchioli L., Petterino C., Bargelloni L., Castagnaro M. (2005). Fatal Necrotizing Fasciitis and Myositis in a Captive Common Bottlenose Dolphin (Tursiops Truncatus) Associated With Streptococcus Agalactiae. J. Vet. Diagn. Invest. 17 (6), 617–622. doi: 10.1177/104063870501700620
Keywords: Delphinus delphis, common dolphin, Streptococcus agalactiae, streptococcosis, type Ia-ST7, whole-genome sequencing (WGS), phylogeny
Citation: Morick D, Davidovich N, Zemah-Shamir Z, Bigal E, Rokney A, Ron M, Blum SE, Fleker M, Soto E, Heckman TI, Lau SCK, Wosnick N, Tchernov D and Scheinin AP (2022) First Isolation and Characterization of Streptococcus agalactiae From a Stranded Wild Common Dolphin (Delphinus delphis). Front. Mar. Sci. 9:824071. doi: 10.3389/fmars.2022.824071
Received: 28 November 2021; Accepted: 21 March 2022;
Published: 12 April 2022.
Edited by:
Sandro Mazzariol, University of Padua, ItalyReviewed by:
Carla Grattarola, Experimental Zooprophylactic Institute for Piedmont, Liguria and Valle d’Aosta (IZSTO), ItalyCopyright © 2022 Morick, Davidovich, Zemah-Shamir, Bigal, Rokney, Ron, Blum, Fleker, Soto, Heckman, Lau, Wosnick, Tchernov and Scheinin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danny Morick, ZG1vcmlja0B1bml2LmhhaWZhLmFjLmls
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.