
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 09 March 2022
Sec. Marine Biology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.803354
This article is part of the Research TopicProtists as Model Ecological and Evolutionary Study Systems: Emerging Methodologies of the 21st centuryView all 8 articles
Photosymbiosis is one of the key features characterizing planktonic foraminifera; the number of symbiont cells within a single host has been reported to be well over thousands, meaning that photosynthesis by photosymbiosis may be a “hot spot” for primary production, especially in oligotrophic oceans. As microenvironmental conditions around foraminifera are greatly affected by rapid biological activities—such as photosynthesis and respiration—information on the photosynthetic activities of symbionts is essential to interpret the geochemical proxies recorded in foraminiferal tests (e.g., δ13C and δ18O). Recently, active chlorophyll fluorometry has been increasingly employed as a useful tool for immediate estimation of photosynthesis. However, carbon assimilation rates are the only direct indicator of the photosynthetic carbon flux. Therefore, before utilizing active fluorescence methods to understand carbon dynamics in foraminiferal symbiosis, it is necessary to confirm the relationship between the fluorescence-based photosynthetic rate [electron transport rate (ETR)] and carbon assimilation rate (P). Here, these two rates were compared for two species, Trilobatus sacculifer and Globigerinella siphonifera Type II, using 14C-tracer experiments and active fluorometric measurements by fast repetition rate fluorometry. The results showed a significant positive correlation between the P and ETR of the two species, indicating that carbon assimilation can be estimated by the fluorometric method. However, the regression slopes, which represent the apparent electron requirement for carbon assimilation (e–/C), were significantly different in the two species, and were estimated at 26.2 for T. sacculifer and 96.5 for G. siphonifera. These are strikingly high, considering the theoretically and empirically realistic e–/C values. We hypothesized that the high e–/C observed may be due in part to the use of unlabeled respiratory carbon (underestimation of P). A simple mass balance calculation suggests that a significant amount of carbon should derive from the host’s respired CO2, whose contribution is higher in G. siphonifera than in T. sacculifer. Within the context of using test geochemical parameters, such as δ13C, as paleoceanographic proxies, it is important to note that the potential magnitude of the photosynthetic effect varies among species. This attempt to couple ETR and P could comprehensively reveal an interesting perspective on the intimate interactions existing within photosymbiotic systems.
Photosymbiosis is one of the styles of acquired phototrophy observed in marine protistan organisms, such as acantharians, radiolarians, phaeodarians, and foraminifera (test-forming rhizarians) (Caron, 2000; Stoecker et al., 2009; Decelle et al., 2015). Although their biomass in the ocean is not so large compared to that of phytoplankton, the total primary production of photosymbiotic rhizarians is estimated to be as high as 5% of the annual primary production of the oceans (Caron et al., 1995). In particular, in oligotrophic oceans, photosynthesis mediated by symbiotic systems can be considered as a “hot spot” of primary production (Rink et al., 1998; Köhler-Rink and Kühl, 2005). Photosynthetic rates of more than four orders of magnitude are estimated within these symbiotic consortia compared to primary production in an equivalent volume of surrounding seawater (Spero and Parker, 1985; Caron et al., 1995). Among these photosymbiotic rhizarians, planktonic foraminifera that precipitate calcite tests contribute to carbonate production in oligotrophic open oceans. They play an important role in the global carbon cycle by sinking massive amounts of carbonate to the seafloor (Schiebel, 2002). Therefore, photosymbiotic planktonic foraminifera contribute to both inorganic and organic carbon production through calcification and photosynthesis of their symbionts, respectively.
Information on the photosynthetic activity of symbiotic algae in intact association with the host (in hospite) is also valuable when evaluating the reliability of geochemical proxies recorded in foraminiferal calcite tests (Bemis et al., 1998; Hönisch et al., 2003; Zeebe et al., 2008). Passively floating tiny organisms like planktonic foraminifera (<ca. 1 mm) are living in a diffusion-limited environment that is smaller than the smallest scale of a turbulent eddy (Lazier and Mann, 1989). Therefore, the microenvironmental conditions in the vicinity of these organisms are greatly affected by rapid biological activities such as photosynthesis and respiration (Jørgensen et al., 1985; Rink et al., 1998). Altered geochemical compositions in the surrounding seawater are eventually recorded in the tests through specific physicochemical mechanisms (Spero et al., 1991; Wolf-Gladrow et al., 1999; Hönisch et al., 2003). In this context, the examination of a biochemical activity like photosynthesis in this proxy-bearer is fundamentally important to better understand geochemical proxies such as stable carbon isotope (δ13C) of foraminiferal tests, reflecting δ13C of dissolved inorganic carbon in the seawater. In addition, elucidating the dynamics between photosynthesis and test geochemistry can also contribute to the potential development of a new proxy for paleo-photosymbiotic activities. In order to clarify the dynamics of inorganic carbon in the vicinity of foraminifera, it is necessary to quantify photosynthetic carbon incorporation. However, the existing knowledge on the subject in relation to photosymbiotic planktonic foraminifera is still limited. Previous studies measured the photosynthetic rates of the consortia of these organisms (holobionts) by either 14C-tracer technique (Erez, 1983; Spero and Parker, 1985; Caron et al., 1995) or oxygen micro-sensor technique (Jørgensen et al., 1985; Rink et al., 1998; Köhler-Rink and Kühl, 2005; Lombard et al., 2009). The former approach estimates carbon assimilation rates directly, while the latter estimates oxygen production rates, and each measure is related to a different photosynthetic process. These two measures have not been directly compared in the photosymbiotic system of planktonic foraminifera, thus a potential uncertainty exists when attempts are made to derive carbon assimilation rates from oxygen production rates. In addition, except for the study by Spero and Parker (1985) conducting both carbon assimilation measurements and symbiont counting, most of the previous studies estimated the photosynthetic rate on a per foraminifera basis, and did not provide the amount of symbionts (Erez, 1983; Jørgensen et al., 1985; Caron et al., 1995; Rink et al., 1998; Köhler-Rink and Kühl, 2005; Lombard et al., 2009). Therefore, the inter-comparison of photosynthetic rates published to date is not straightforward, due not only to the different methods used, but also to uncertainties in terms of phototroph masses. The photosynthetic rates reported ranged from 0.5 to 18 nmol C (foraminifera) –1 h–1 even for the same foraminiferal species (Trilobatus sacculifer) when a photosynthetic quotient of 1 was applied (Erez, 1983; Jørgensen et al., 1985). As a result, because the photosynthetic rate per foraminifera is undoubtedly affected by the quantity of symbionts, an accurate comparison is not possible without the volumetric information regarding the symbionts or the chlorophyll content.
Recently, active chlorophyll fluorometry has increasingly been employed as one of the most useful and convenient tools to estimate primary production (Kolber and Falkowski, 1993; Suggett et al., 2011). The estimation is based on the photochemical transport of electrons through photosystem II, which is closely related to the rate of oxygen production (Falkowski and Raven, 2007). However, the amount of transported electrons is not directly related to the subsequent production of energy used to assimilate carbon (Edwards and Baker, 1993; Suggett et al., 2009; Lawrenz et al., 2013). As the carbon assimilation rate is the only direct expression of the photosynthetic rate associated with carbon flow, it must be correlated with the convenient measure –electron transport rate (ETR)–using chlorophyll fluorescence. Moreover, as chlorophyll fluorometry can provide a large amount of photophysiological information and can simultaneously quantify the content of chlorophyll a (Chl a), it has great potential to provide both quantitative estimations of the photosynthetic rate, and information on the qualitative photophysiological characteristics of the symbionts in hospite.
Here, we performed paired measurements of the photosynthetic rates of holobionts through fast repetition rate fluorometry and 14C-tracer experiments. The purpose of this study was to elucidate the relationship between the ETRs and carbon assimilation rates of planktonic foraminiferal holobionts. The results will contribute to future investigations of the flow of dissolved inorganic carbon from seawater into symbionts via photosynthesis, using a rapid and non-destructive method based on chlorophyll fluorescence. This study represents an indispensable step toward reaching an integrated view of the physiological factors that can affect foraminiferal test geochemistry.
Planktonic foraminifera were sampled in Sagami Bay (35°10.5′N, 139°12.5′E, 922-m depth) on October 28th, 2014. Sagami Bay is located under the influence of the Kuroshio Warm Current that usually yields subtropical to temperate species of planktonic foraminifera. Samples were collected either by surface net tows drifted for 5 min, or by vertical tows from 500–0 m, and 50–0 m (100-μm mesh, 45-cm aperture opening). Foraminifera were selected under a stereoscopic microscope soon after sampling. The isolated specimens were rinsed with filtered seawater (0.22 μm-filtrated) several times, then they were put into culture wells (Nunclon MultiDish 12-well, Thermo Fisher Scientific) filled with filtered seawater, with one individual per well. Trilobatus sacculifer (dinoflagellate-bearing) and Globigerinella siphonifera Type II (pelagophyte-bearing) were selected for the experiment. The latter was differentiated based on the following phenotypic characters observed under a stereomicroscope: darker color, longer spines compared to G. siphonifera Type I, and presence of small, coccoid symbionts densely aligned along the spines (Faber et al., 1988, 1989; Huber et al., 1997; Bijma et al., 1998; Supplementary Figure 1), (the term G. siphonifera is used hereafter to indicate G. siphonifera Type II). The largest test dimension (test size) was measured for each specimen with a stereoscopic microscope using a micrometer with a calibrated eyepiece. To obtain data reflecting natural physiological states when they lived in the ocean, the experiments were conducted 1 or 2 days after the sampling (October 29th or 30th 2014, see Supplementary Table 1 for experimental date and time for each specimen). No food was provided since feeding would alter their nutritional and health status. Specimens that recovered spines were considered to be in good health and were used in the experiments. Three irradiance groups (70, 150, and 220 μmol quanta m–2 s–1) and a dark-control group per species were set for the experiment. The highest irradiance was chosen based on previous studies demonstrating that these two species grew well under light condition 200 ± 30 μmol quanta m–2 s–1 (Takagi et al., 2016, 2018), indicating that the irradiance 220 μmol quanta m–2 s–1 would not induce severe damage even for relatively low-light adapted species G. siphonifera. The lower two intensities were chosen to be approximately 1/3 and 2/3 of the highest intensity. In total, 24 specimens were used for T. sacculifer (n = 7 for low-, middle-, and high-light, and 3 for the dark-control group), and 15 for G. siphonifera (n = 4 for low- and middle-light, 5 for high-light, and 2 for the dark-control group).
Fast repetition rate (FRR) fluorometry was used to assess the electron transport rate (ETR) of the photosystem II (PSII) of the symbiotic algae in hospite. A FRR fluorometer (Diving Flash, Kimoto Electric Co., Ltd., see Fujiki et al., 2008 for details) was used to obtain the fluorescence induction curve of PSII for specimens in a dark-adapted state and under actinic light conditions. FRR fluorometric measurements were operated following the protocol described in Fujiki et al. (2014), providing a saturating flash sequence (wave length of 470 nm, 25 nm bandwidth) consisting of 50 subsaturation flashlets (2 μs duration separated by a 4 μs interval). One measurement consisted of a series of 50 flash sequences. The PSII parameters were derived from the fluorescence induction curve using the numerical fitting procedure described in Kolber et al. (1998). The dark parameters of PSII, minimum fluorescence (F0), maximum fluorescence (Fm), and functional absorption cross-section of PSII (σPSII)—and the corresponding light parameters, F′, Fm′, and σPSII′—were provided. The parameters and the other terms derived from them are listed in Table 1. All parameters obtained under actinic light conditions are denoted by a prime (′). Of these, Fq′/Fm′ estimates the quantum efficiency of photosystem II photochemistry. It is actually the product of two other parameters, Fv′/Fm′ and qP (=Fq′/Fv′): the former represents the maximum quantum efficiency of PSII photochemistry, while the latter is a factor that relates the PSII maximum efficiency (Fv′/Fm′) to the PSII operating efficiency (Fq′/Fm′), determining the level of photochemical quenching of chlorophyll fluorescence (Baker and Oxborough, 2005).

Table 1. List of fast repetition rate fluorometry parameters and the derived terms used in this study.
Firstly, the fluorescence induction curve for the dark-adapted state was measured to obtain F0, Fm, and σPSII. Dark-adaptation was set for at least 10 min. A foraminiferal holobiont was put into a customized quartz cuvette with filtered seawater, then set to the fluorometer. One measurement for each specimen consisted of four sequential measurements obtained by rotating the cuvette clockwise by 90° (Fujiki et al., 2014). Subsequently, the measurements under light conditions were conducted. The actinic light originating from white light-emitting diodes (LEDs) with a bandpass filter of 450 nm (BPB45, Fuji Film) was set at the cuvette holder. Photosynthetically active radiation (PAR) from the LEDs was set to 70, 150, and 220 μmol quanta m–2 s–1 for low-, middle-, and high-light groups, respectively. The irradiance was measured by a PAR sensor (LI-1400 data-logger, LI-COR). Each foraminiferal specimen was exposed to the assigned irradiance level for 5 min in total, through a 1.25 min-exposure on each side of the cuvette. The measurement procedure was the same as the dark-adapted one, except for the actinic light exposure during the process. The background fluorescence level was measured with the same cuvette filled with filtered seawater. The measurements were conducted during the light hours; 8:30 to 14:40 local time (JST) for T. sacculifer and 10:30 to 14:30 for G. siphonifera (Supplementary Table 1) on the same day of the 14C-tracer experiments. Note that the measurement time was taken into consideration to avoid any bias among the experimental groups (Supplementary Figure 2).
Based on Suggett et al. (2009), the chlorophyll-specific electron transport rate, ETRChl mol e– (mol Chl)–1 s–1, was calculated using the following equation:
whose parameters are defined in Table 1. The concentration of the reaction center (RCII) per chlorophyll (nPSII = RCII/Chl) was derived from the equation of Babin et al. (1996; Table 1). The constant terms are the numerical factors used for the unit-conversion of E from μmol quanta m–2 s–1 to mol quanta m–2 s–1, and σPSII’ from 10–20 m2 quanta–1 to m2 (mol RCII) –1.
Chl a content was calculated from Fm values based on a previously established linear relationship between Fm of individual foraminifera and their Chl a content extracted with N,N-dimethylformamide (Fujiki et al., 2014; Takagi et al., 2016, see Supplementary Figures 3–5 for details). The relationship used here was established using both dinoflagellate-bearing species and pelagophyte-bearing species. We have also examined the relationship for dinoflagellate-bearing species and pelagophyte-bearing species separately, and eventually confirmed that the Fm-Chl a relationship was almost the same regardless of the symbiont type (Supplementary Figure 5).
Using the estimated Chl a values, the specific ETR for a single foraminifera-symbiont system, ETRForam nmol e– (foraminifera)–1 h–1, can be written as follows:
where the constant terms are the unit-conversion factors of Chl a content from ng foraminifera–1 to nmol foraminifera–1 (molecular weight of Chl a = 893.49), and of unit time from s–1 to h–1.
14C-tracer experiments using a photosynthetron with white LEDs as light source (Fujiki et al., 2007) were conducted to investigate the carbon assimilation rates of holobionts. The same light conditions used in the previous FRR fluorometric measurement were set for each specimen. Blue bandpass filters were set above the white LEDs at the bottom of the photosynthetron, whose irradiance levels were controlled to either 70, 150, or 220 μmol quanta m–2 s–1.
The experiments were performed in glass scintillation vials (Pico Vial, 6 mL, PerkinElmer Life Sciences). After the FRR fluorometric measurement, individual holobionts were pipetted into scintillation vials filled with 1,950 μL of filtered seawater (0.22 μm-filtrated). The assay was started by adding 50 μL of 1,480 kBq mL–1 NaH14CO3 solution (filtered seawater solvent) to each vial containing one individual holobiont. The initial activity was 74 kBq mL–1. Three vials were incubated under dark condition as dark-controls to calibrate the non-photosynthetic carbon uptake and possible experimental errors. For blank measurements, 55 μL of 1,480 kBq mL–1 NaH14CO3 solution was added to three vials containing 2,145 μL of filtered seawater (74 kBq mL–1). For the initial total activity measurement, a 200 μL-aliquot was removed from the blank vials and was 10 times diluted by adding 2,000 μL of filtered seawater (7.4 kBq mL–1). The assay was conducted for 1 h from 15:00 to 16:00 local time at 25°C (room temperature). The carbon assimilation rate is known to vary with time of day, and this time slot corresponds to the maximum photosynthetic rates across the day period (Spero and Parker, 1985).
At the end of the assay, 200 μL of 1N-HCl was added to each sample vial, dark-control, and blank in order to stop the photosynthetic activity. Then the vials were well shaken so that all the residual inorganic carbon was reacted, and subsequently removed. The foraminiferal tests were dissolved as well. The vials were settled in a draft chamber for more than 24 h to complete the reaction, and then 3,800 μL of scintillation cocktail (Insta-Gel Plus, PerkinElmer Life Sciences) was added to them. All the sample vials were ultra-sonicated three times for a few seconds to allow the assimilated radiocarbon to disperse homogeneously in the gel.
Radioactivity was measured with a liquid scintillation counter (Tri-Carb 2900TR, PerkinElmer Life Sciences) at the Japan Agency for Marine-Earth Science and Technology. The scintillation counting of the total activity and of the samples was performed for 20 min for each vial. The dark-controls and the blank samples were counted for 60 min to obtain an accurate measurement of the small count rate. The counting procedure was repeated three times, and the results for each sample were averaged. The equation presented in Barber et al. (1996) was used to calculate the carbon assimilation rate for each vial (for each foraminiferal specimen). The assimilation rate, PForam nmol C (foraminifera) –1 h–1, can be written as follows:
where Asample, Ablank and Atotal are the radioactivity values counted for the sample, blank, and total activity vials (cpm), respectively. Atotal is multiplied by 10 to convert it to the initial total seawater activity of the samples (total activity vials were diluted 10 times before the counting). t is the duration of incubation (=1 h), and the seawater volume that contained one foraminifera was 2 × 10–3 L. 25,000 μg C L–1 was used for the concentration of dissolved inorganic carbon (DIC) (Knap et al., 1996). This is a general value for seawater, and any effects which may alter the concentration is not considered at this moment (see section “Discussion” for the detail). The constant value of 1.05 is the discrimination factor of 12C to 14C (Peterson, 1980).
The chlorophyll-specific carbon assimilation rate, PChl mmol C (mol Chl)–1 s–1, for each foraminiferal specimen was calculated using the following equation:
The mean Fv/Fm values (±1 standard deviation) of T. sacculifer and G. siphonifera were 0.48 ± 0.05 (n = 24) and 0.50 ± 0.03 (n = 15), respectively, and they were not statistically different between the two species (Welch’s t-test, p = 0.2, Figure 1A). The σPSII of T. sacculifer was 565 ± 44 × 10–20 m2 quanta–1, and that of G. siphonifera was 858 ± 74 × 10–20 m2 quanta–1. The mean σPSII of G. siphonifera was statistically higher than that of T. sacculifer (Welch’s t-test, p < 0.001, Figure 1B).

Figure 1. Box plots of the dark-adapted photophysiological parameters. (A) Fv/Fm and (B) σPSII. Boxes and horizontal lines represent the first and the third quartiles and medians. Values spanning more than 1.5 times the length of the box, from either end, are considered as outliers. The statistically significant difference is denoted with *.
The photochemical efficiency under actinic light conditions (Fq′/Fm′) decreased as a response to increasing irradiance in T. sacculifer (Figure 2A). In G. siphonifera, the Fq′/Fm′ was highest in the low-light group as well, and lowest in the middle-light group. A similar relationship to the irradiance levels was observed in qP for both species (Figure 2B), however, the Fv′/Fm′ did not largely differ among the three irradiance groups (Figure 2C). The difference of the size of the light-harvesting antenna, σPSII′, did not largely differ among the irradiance groups, but was significantly higher in the middle-light group for G. siphonifera (Figure 2D). The results of statistical tests are shown in Supplementary Table 3.
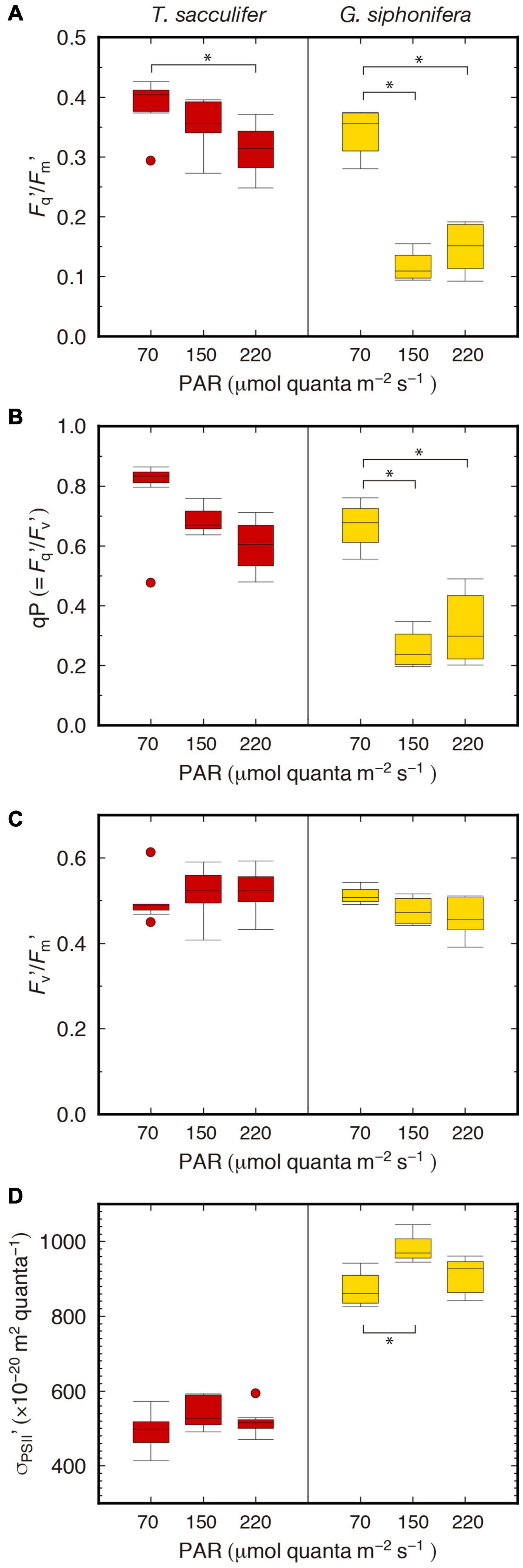
Figure 2. Box plots of photophysiological parameters under actinic light. (A) Fq′/Fm′, (B) qP, (C) Fv′/Fm′, (D) σPSII′. Boxes and horizontal lines represent the first and the third quartiles and medians. Values spanning more than 1.5 times the length of the box, from either end, are considered as outliers. Statistically significant differences are denoted with *.
The Chl a content was ranged from 7 to 84 ng foraminifera–1 in T. sacculifer and 19 to 226 ng foraminifera–1 in G. siphonifera (Supplementary Table 1). Although there was considerable variation in each species, the differences between the three irradiance groups were not significant (Kruskal–Wallis test for multiple comparisons, p = 0.22 and 0.89 for T. sacculifer and G. siphonifera, respectively). The wide range of Chl a contents was originally intended to cover wide range of ETRForam and PForam for correlation.
Firstly, Chl a-specific electron transport rates (ETRChl) were calculated. The higher the irradiance was, the higher the rates of electron transport observed in T. sacculifer (Figure 3A). The median value of each irradiance group was 0.15, 0.38, and 0.48 mol e– (mol Chl)–1 s–1 for the irradiance levels of 70, 150, and 220 μmol quanta m–2 s–1, respectively. However, such an apparent ordered ranking between groups with different irradiance levels was not clearly seen in G. siphonifera. For this species, the ETRChl of the middle-light group was the lowest. The median values were 0.29, 0.23, and 0.46 mol e– (mol Chl)–1 s–1 for 70, 150, and 220 μmol quanta m–2 s–1, respectively. Then, by multiplying the Chl a content per foraminifera estimated from Fm, the electron transport rates for bulk host-symbiont systems were calculated (ETRForam) (Supplementary Table 1).
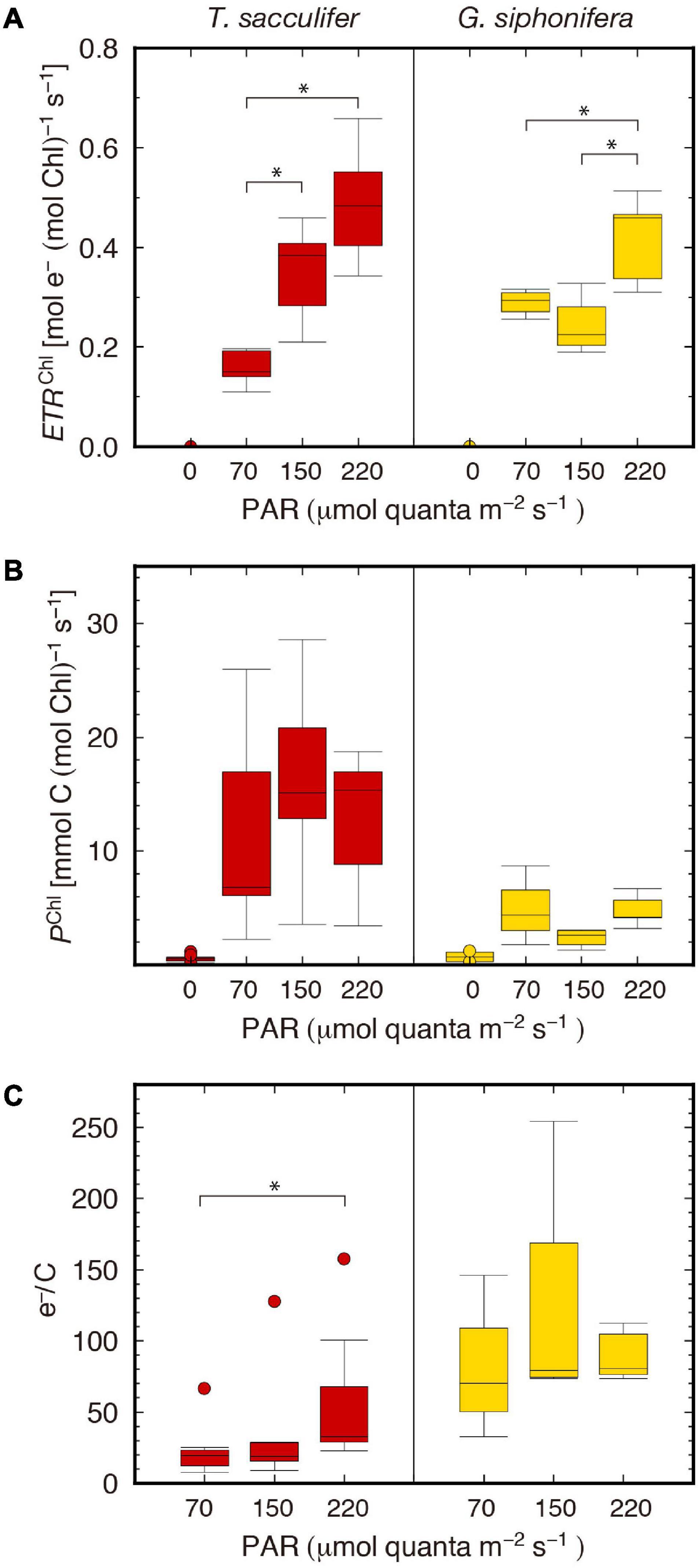
Figure 3. Box plots of the two different photosynthetic rates and their quotient. (A) Chl a-specific electron transport rate (ETRChl), (B) carbon assimilation rate (PChl), (C) e–/C. Boxes and horizontal lines represent the first and the third quartiles and medians. Values spanning more than 1.5 times the length of the box, from either end, are considered as outliers. Statistically significant differences are denoted with *.
Individual-based carbon assimilation rates (PForam) were calculated directly from the scintillation counting results. Then, the Chl a-specific carbon assimilation rates (PChl) were calculated by dividing the PForam by the Chl a content of each individual. The rates were much smaller in G. siphonifera than T. sacculifer (Figure 3B). For the latter, the median values of the PChl of each irradiance group at levels of 70, 150, and 220 μmol quanta m–2 s–1 were 6.8, 15.1, and 15.4 mmol C (mol Chl) –1 s–1, respectively. In G. siphonifera, the median values for each irradiance group were 4.4, 2.6, and 4.2 mmol C (mol Chl) –1 s–1, also, respectively. The middle-light group (150 μmol quanta m–2 s–1) showed the lowest rate in G. siphonifera as seen in the ETRChl of this species (Figure 3A).
Apart from our previous studies of foraminiferal photophysiology (Fujiki et al., 2014; Takagi et al., 2016, 2018, 2019), FRR parameters for T. sacculifer were most recently reported by another group (Hönisch et al., 2021). Their results (Fv/Fm = 0.39 ± 0.05, σPSII = 546 ± 59 × 10–20 m2 quanta–1) were similar to ours (Fv/Fm = 0.48 ± 0.03, σPSII = 565 ± 44 × 10–20 m2 quanta–1), though the Fv/Fm was higher in ours indicating that the photophysiological condition of our specimens were even better. The higher σPSII observed in G. siphonifera (pelagophyte-bearer) than in T. sacculifer (dinoflagellate-bearer) was consistent with the results of previous studies based on specimens collected from different regions (e.g., Northwestern Pacific Ocean, Fujiki et al., 2014; Takagi et al., 2019; East China Sea, Takagi et al., 2016). The higher σPSII represents a more efficient absorption of light by the symbionts in G. siphonifera, which implies a low-light adaptation for their deeper habitat depths in the photic zone (Bijma et al., 1998; Takagi et al., 2016). The actual difference in terms of light conditions between these two species is recognized in the Fq′/Fm′ parameter, which represents the photochemical efficiency of PSII (sometimes denoted as ΔF/Fm′, Kromkamp and Forster, 2003), and usually decreases due to light exposure (Lawson et al., 2002; Suggett et al., 2003). In our results, the decline in the higher irradiance groups was greater in G. siphonifera than in T. sacculifer (Figure 2A). The decline in Fq′/Fm′ (=qP × Fv′/Fm′) observed here was mainly derived from the decline in qP rather than in Fv′/Fm′, which indicates a limitation of the electron transport capacity downstream of PSII, for example, in the cytochrome b6/f complex and/or photosystem I (Suggett et al., 2003). The greater decline of the qP parameter in G. siphonifera indicates the lower potential efficiency of this species in processing high-light energy, suggesting its low-light adapted nature.
In addition, the observed low relevance of the Fv′/Fm′ parameter to the irradiance (Figure 3C) indicates that energy dissipation via non-photochemical quenching was low (Baker and Oxborough, 2005). This interpretation is supported by the observed σPSII′, which was almost unchanged throughout the exposure to sequential light levels in both species (Figure 2D). This parameter reflects the size of the light-harvesting antenna and is generally reduced when non-photochemical quenching in the antenna bed increases (Suggett et al., 2006). Although σPSII′ was higher in the middle light group in G. siphonifera, it was consistent with the results of σPSII measured before actinic light exposure (dark-adapted state). So we assume that the difference in σPSII′ (under actinic light) was not due to the difference in light exposure, but to the intrinsic properties of the specimens used in this study (see Supplementary Table 1 for σPSII data for each specimen).
The Chl a contents estimated in this study were within the range of reported values measured during the course of ontogenetic development of these species (Takagi et al., 2016). Overall, it was higher in G. siphonifera than in T. sacculifer, which was also in agreement with the previous study (Takagi et al., 2016). In general, Chl a per unit volume is higher in smaller algae (Agusti, 1991), so it is likely that the smaller pelagophyte symbionts (ca. 1.5–3.5 μm, Gastrich, 1987; Faber et al., 1988) would have denser Chl a than the larger dinoflagellate symbionts (ca. 5–9 μm, Spero, 1987). So if the two symbionts occupy comparable host volumes, pelagophyte-bearing G. siphonifera would have a higher Chl a content per foraminifera. The higher Chl a in G. siphonifera is also consistent with our photophysiological results indicating that the pelagophyte symbionts are more low-light adapted than dinoflagellate symbionts—higher content of Chl a is likely to be achieved for species living in lower-light habitat.
To compare the symbiont number per foraminifera with those reported in literatures, we attempted to estimate the number of symbionts using the PForam values. According to the light response curve of Spero and Parker (1985) for Orbulina universa—the same dinoflagellate symbiont bearer as T. sacculifer, the maximum carbon assimilation rate at low-light (70 μmol quanta m–2 s–1), middle-light (150 μmol quanta m–2 s–1), and high-light (220 μmol quanta m–2 s–1) were 0.31, 0.67, and 0.98 × 10–12 mol C symbiont–1 h–1, respectively. Applying these values to our PForam results of T. sacculifer, the number of symbionts can be estimated from 111 to 5,420 cells foraminifera–1 (Supplementary Table 2). The numbers are not largely different from the ones of T. sacculifer reported in Hönisch et al. (2021) (400–1,600 cells foraminifera–1, n = 3) and the other dinoflagellate-bearing species (132–3,300 cells foraminifera–1 for O. universa, Spero and Parker, 1985; 250–1,900 and 300–3,000 cells foraminifera–1 for O. universa and Globigerinoides ruber, respectively, Hönisch et al., 2021).
For T. sacculifer, a significant increase in ETRChl was observed as the irradiance level increased (Figure 3A). However, the difference in PChl was not significant (Figure 3B), indicating that under higher irradiance the rate of carbon assimilation became saturated, whereas the electron transport still increased.
There are a number of processes to consume electrons other than carbon fixation through a series of photosynthetic reactions. For example, the Mehler reaction (Mehler, 1957; Asada, 1999), chlororespiration via a plastid terminal oxidase (Bennoun, 1982), photorespiration via oxygenase activity of the enzyme RuBisCO (Badger et al., 2000), and nutrient assimilation (Holmes et al., 1989) are well known biochemical processes that can serve as alternative electron sinks. The excess electrons in the high-light group were likely processed by these mechanisms.
Unlike the profile in T. sacculifer, the smallest PChl was recorded in the middle-light group in G. siphonifera, and it was consistent with the ETRChl (Figures 3A,B). A notable point is that the qP was the lowest in the middle-light group as well (Figure 2B). The values were less than 50%, relative to the qP of the low-light group. The low qP values reflect the low electron transport capacity downstream of PSII, toward PSI. This lowered electron transport should result in the decrease of ETRChl and PChl. One might also think the time of day for photosynthetic measurements mattered since it is known that photosynthetic rates varies through the day (Spero and Parker, 1985). However, since the experimental time for each irradiance group were set not to be biased (Supplementary Table 1) and the ETRChl did not show any tendency due to the experimental time (Supplementary Figure 2), we can rule out this possibility. In summary, the reason for the resultant smallest photosynthetic rate in the middle-light group for G. siphonifera cannot be fully explained. Unfortunately, equivalent conditions for all the specimens cannot be guaranteed in this kind of experiments that are based on field-collected samples. It is possible that the specimens in the middle-light group were photophysiologically less healthy, relative to the specimens in the other irradiance groups. To understand the light response of these G. siphonifera pelagophyte symbionts, repeated experiments on the species and/or analysis of isolated symbiont cultures, when established, are required as well.
Previous studies on the carbon assimilation and oxygen production rates of symbiotic foraminifera are listed in Table 2, together with the highest PForam observed in this study for each experimental group. A direct comparison with the previous studies is not possible, because experimental conditions differed among them, and the Chl a content of the tested individuals, which should have considerably varied, was not provided in most of the studies. The photosynthetic rate per foraminifera should be obviously related to the Chl a content within the hosts, as shown in Figure 4. Nevertheless, through a rough comparison, a tendency in the magnitude of photosynthetic rates can be recognized depending on the method used.
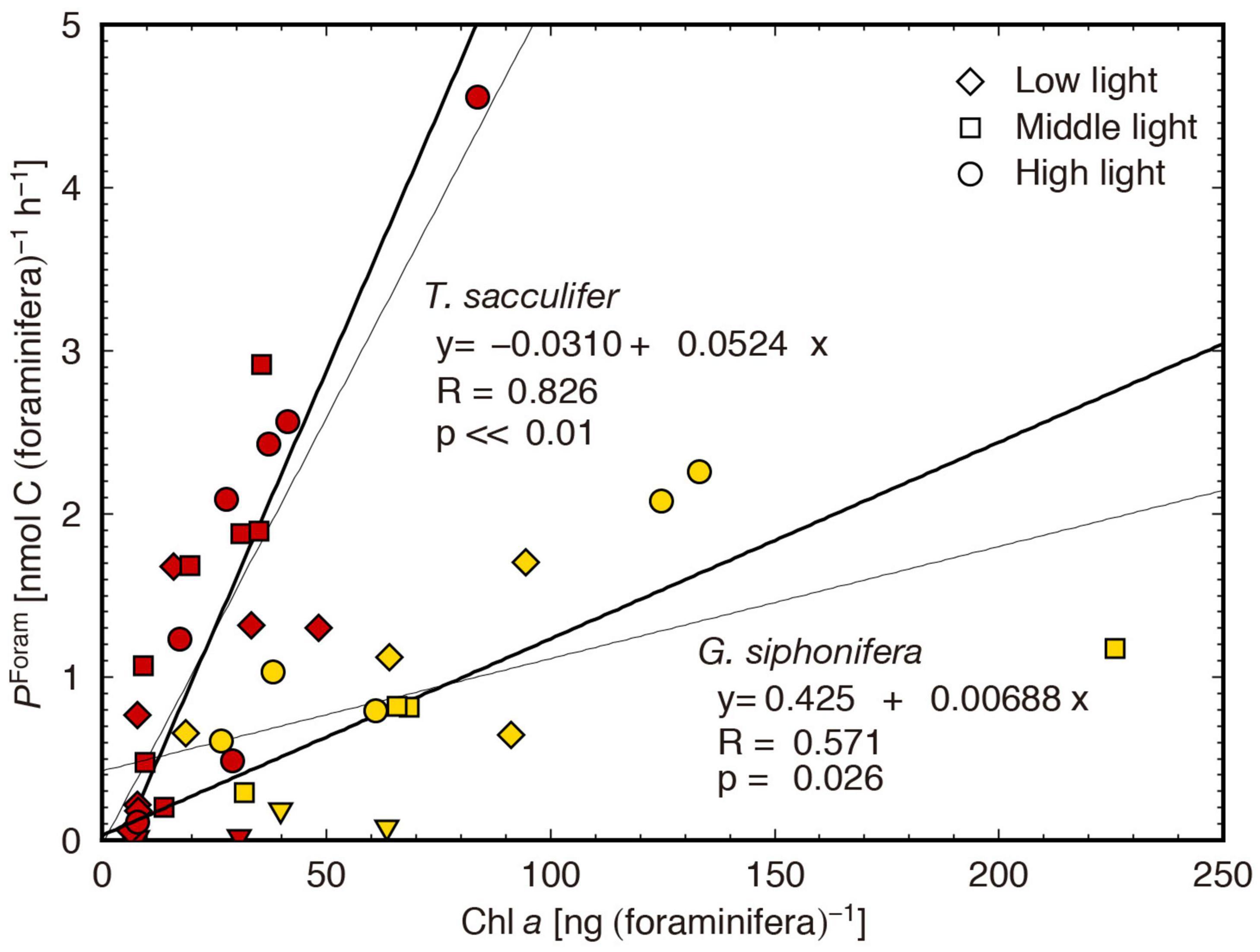
Figure 4. Relation between Chl a content and individual-based carbon assimilation rate (PForam). The bold and thin lines are obtained from reduced major axis regression and least square regression, respectively.
The carbon assimilation rates estimated by the 14C-tracer method were generally lower than the oxygen production rates estimated by micro-sensor measurements (Table 2). A similar observation was previously reported by Caron et al. (1995), arguing that “the symbiont production rates measured by the uptake of NaH14CO3 may be significantly underestimated because of the possibility that the symbionts utilized the inorganic carbon produced by the host’s respiration” (Caron et al., 1995). This means that there might be an additional source of unlabeled inorganic carbon other than the labeled DIC; therefore, the actual rate of carbon assimilation was underestimated by the 14C experiment. The paired estimation of ETRForam and PForam performed on the same individuals in this study can provide an insight into this supposition.
The ETRForam and PForam results were significantly positively correlated in both species (p < 0.01, Figure 5). The proportional relationship means that the carbon assimilation rate can be estimated from the ETR. Here, the regression slope represents the mean value of the apparent electron requirement for the carbon assimilation, e–/C.
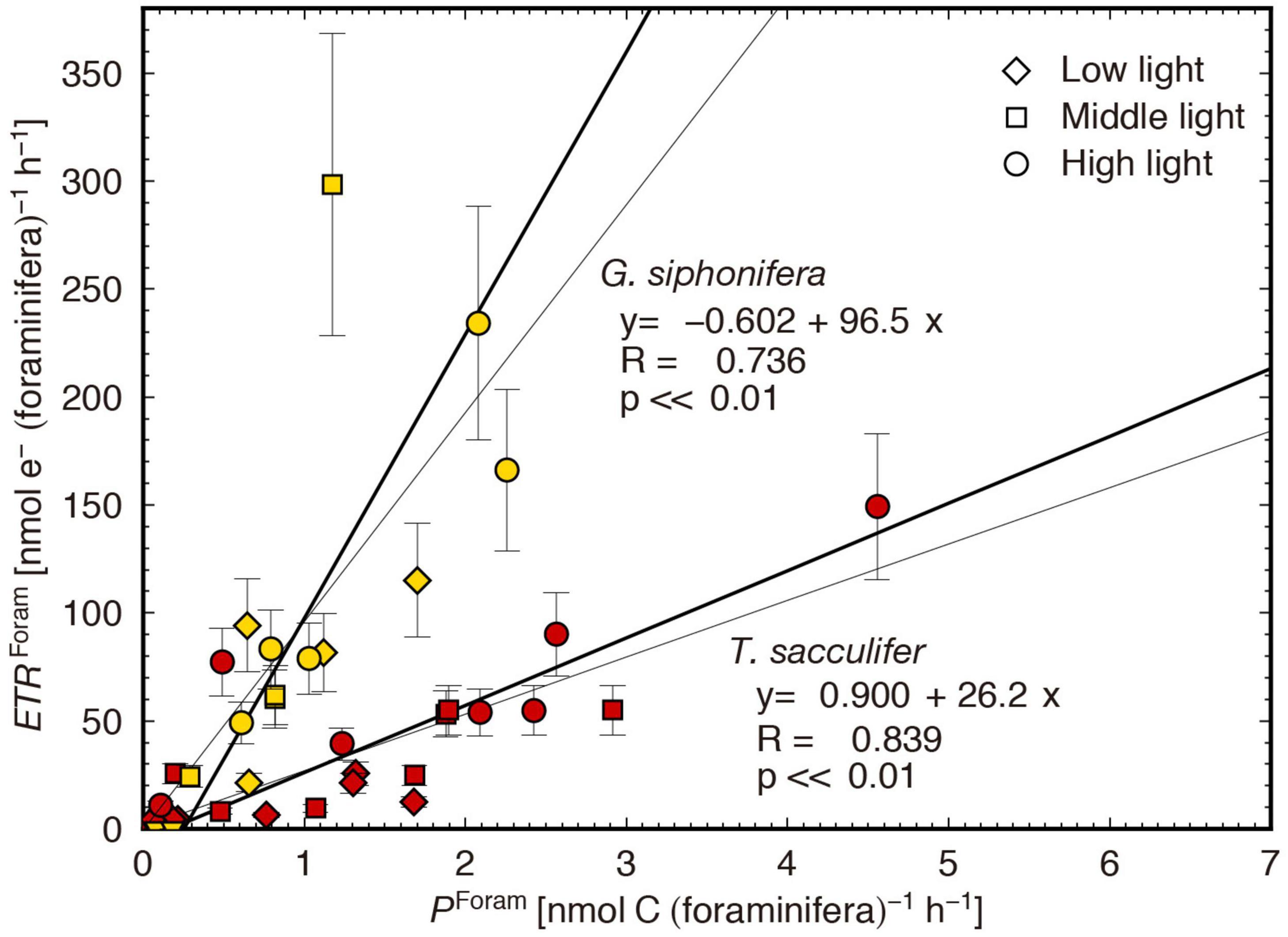
Figure 5. Comparison of individual-based electron transport rates (ETRForam) and carbon assimilation rates (PForam). The bold and thin lines are obtained from reduced major axis regression and least square regression, respectively. The ETRForam error is based on the Chl a error estimation with 95% confidence interval.
The regression slopes of the two species were largely different (Figure 5); the e–/C was estimated at 26.2 mol e– (mol C) –1 for T. sacculifer, and 96.5 mol e– (mol C)–1 for G. siphonifera. These values are, in fact, strikingly high. Theoretically, under optimal growth conditions for phototrophs, the e–/C should be 4 mol e– (mol C)–1 (Genty et al., 1989; Suggett et al., 2009). The ratio of 4 is based on the minimum number of electrons derived from two water molecules to generate one oxygen molecule [4 mol e– (mol O2)–1]. Using the proportion of oxygen produced to 1 mol O2 (mol C)–1 of carbon assimilated (termed as photosynthetic quotient) (Laws, 1991), the minimum e–/C should be 4, in theory. In reality, however, the ratio is usually higher than 4, because—apart from carbon fixation—other possible electron sinks or cycles may be present during electron transport from PSII to PSI, such as photorespiration, chlororespiration, and cyclic electron flow around PSII and PSI (Bennoun, 1982; Prášil et al., 1996; Badger et al., 2000). Based on various studies that provided the e–/C of phytoplankton cultures, the value usually does not exceed ca. 12 mol e– (mol C)–1 (Suggett et al., 2009; Table 3). However, considerably higher e–/C values were obtained in the present study. In general, the estimation of ETR presents potential inaccuracies in the algorithms used for calculation. It is often pointed out that the accuracy in ETR calculations depends on the assumption of constant nPSII, which is 0.002 mol RCII (mol Chl a)–1 for eukaryotic microalgae (Suggett et al., 2011; Lawrenz et al., 2013). The nPSII used in this study was not constant, but it was calculated using a fluorescence-based algorithm considering the functional RCII (Falkowski and Kolber, 1995). Although potential error in nPSII estimation is not ruled out—as the resultant nPSII value was low compared to the other reported values (Supplementary Table 1)—the high e–/C obtained in this study would not be attributed to such estimation.
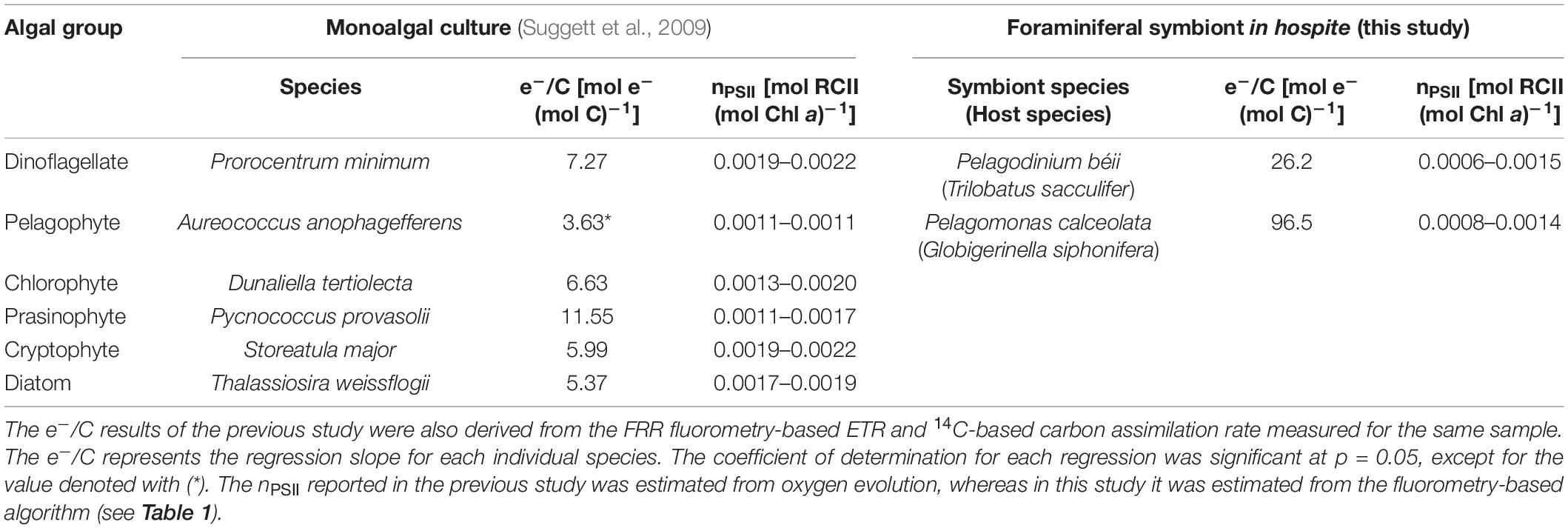
Table 3. Electron requirement for carbon assimilation (e–/C) and estimated nPSII of monoalgal cultures and foraminiferal symbionts.
There are two factors attributable to the high e–/C; (1) alternative sinking of electrons, and (2) the underestimation of the carbon assimilation rates of foraminiferal holobionts, due to the contribution of unlabeled carbon sources. In fact, these possibilities are not mutually exclusive, and the results in this study cannot precisely determine the contribution of the two factors quantitatively. Nevertheless, their careful consideration will contribute to the understanding of the complex physiology of symbiotic systems, and to the identification of issues that need to be addressed in the future. We here examine the two factors separately, from a comparative point of view, in the two species, T. sacculifer and G. siphonifera.
The first factor—alternative sinking of electrons which were not eventually used to assimilate carbon—is difficult to evaluate without information on isolated cultures of the same symbiont species under the same environmental conditions. The mechanism itself is important because excess electrons generate harmful reactive oxygen species. As shown by the higher e–/C observed in G. siphonifera than in T. sacculifer, the symbionts in the former species may have a higher potential to treat excess electrons. However, in general, such treatment process is developed in phototrophs exposed to higher stressful conditions such as high light, high salinity, and low temperature, etc., as an adaptive strategy (Mackey et al., 2008). Because the symbionts in G. siphonifera are regarded as low-light adapted in nature (as discussed above), the hypothesis of a more effective treatment of excess electron in G. siphonifera than in T. sacculifer is not associated with their light preferences. Moreover, the higher decline of qP in the higher light group of G. siphonifera also supports the theory that their photosynthetic apparatus is vulnerable to higher irradiance levels, which is opposite to the interpretation of the higher e–/C as a greater potential to treat excess electrons. Therefore, the second factor proposed—the underestimation of carbon assimilation rates—would largely account for the high e–/C, at least in G. siphonifera. Nevertheless, estimation of the true exchange ratio of electrons to carbon in these algal species is certainly required.
In the endosymbiotic system assessed in this study, a certain proportion of the carbon assimilated by symbionts possibly derived from other sources—i.e., their host’s metabolite (unlabeled respired CO2)—and not only from the 14C-labeled DIC present in the seawater. In this context, we simply calculated the relative amount of carbon from the labeled seawater (carbon from seawater) for both species, using the apparent e–/C observed here and assuming the naturally expected (true) e–/C as an independent variable (Figure 6). For T. sacculifer, if the true e–/C corresponded to the minimum theoretical ratio of 4, the observed e–/C of 26.2 could be achieved with only 16% of carbon contribution from seawater. This means that the remaining 84% of carbon derived from the host’s metabolic carbon. Similarly, for the observed e–/C of 96.5 in G. siphonifera, the symbionts needed almost all of the assimilated carbon, 96%, from the host. When assuming the empirical e–/C of 12 mol e– (mol C)–1 for monoalgal cultures (Suggett et al., 2009; Table 3), the proportion of the host-derived carbon should be ca. 54% in T. sacculifer, while it should still remain very high in G. siphonifera, reaching more than 88% (Figure 6). Although the extent can vary depending on the true e–/C for the symbionts in hospite, the above calculations, assuming the empirical range of the e–/C ratio, imply that a significant amount of carbon had to be derived from the host, and the quantity was by far larger in G. siphonifera.
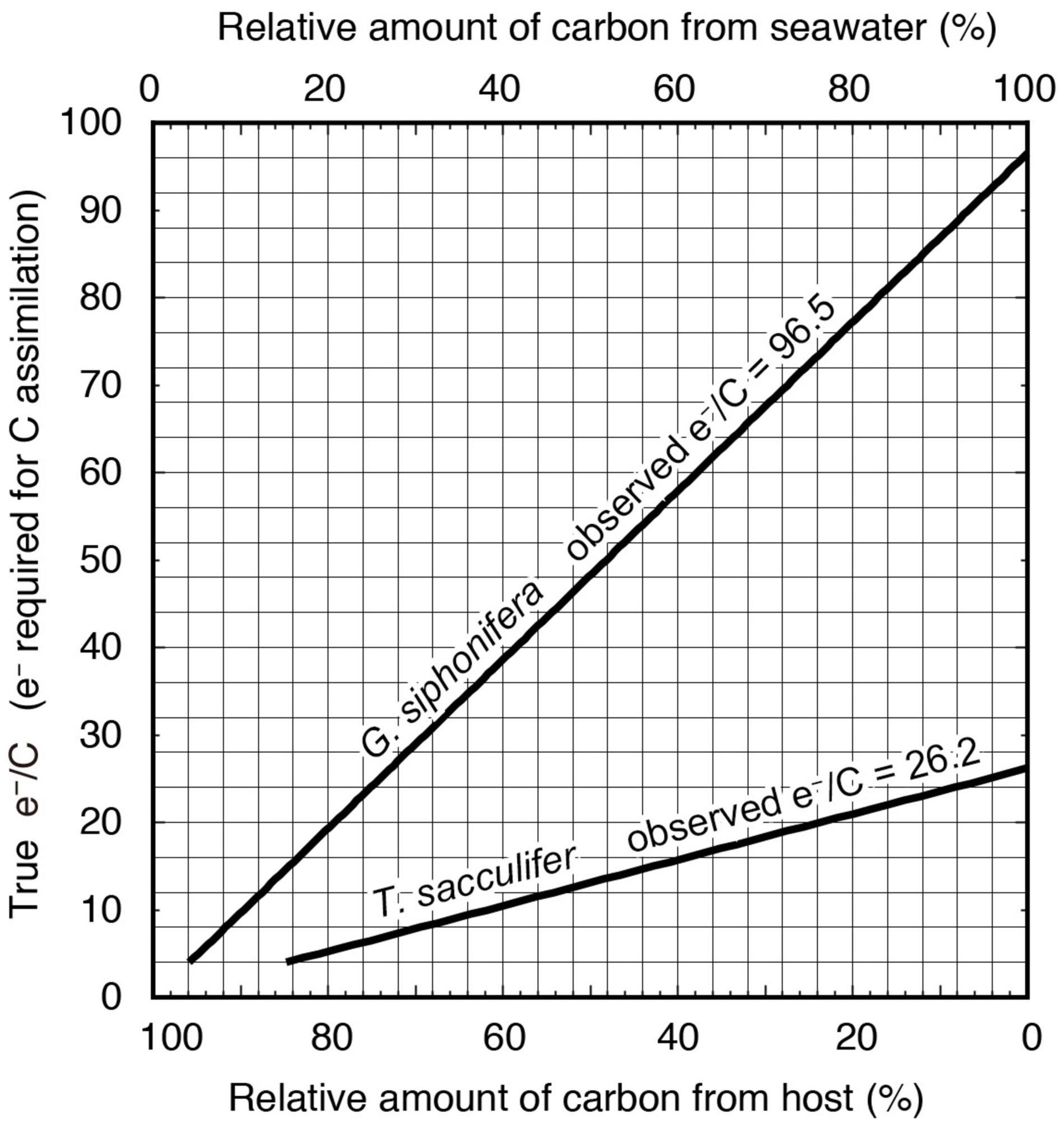
Figure 6. Diagram estimating the proportion of inorganic carbon and host-derived carbon for photosynthesis based on the observed e–/C in this study (bold lines in the diagram), and the actual e–/C (vertical axis). When the true e–/C is the theoretical value of 4 mol e– (mol C)–1, the contribution of the host-derived carbon should be more than 84%. Assuming the true e–/C as a reported empirical value of 12 mol e– (mol C)–1 (Suggett et al., 2009, 2011), more than 54% of carbon is estimated to be derived from the host.
This difference between species is possibly due to the concentration of symbionts within the host and their spatial distribution. When symbionts are densely packed within the test of the host, only those located near the test surface can access the labeled inorganic carbon. When comparing the e–/C to Chl a content, only G. siphonifera showed a significant positive correlation (p < 0.01, Figure 7). This indicates a less effective utilization of labeled carbon in high-density symbiont population, which supports the above interpretation. Moreover, when considering the general distribution of symbionts, T. sacculifer usually distributes the symbiotic algal cells almost homogeneously within the spherical area outside of its test (Supplementary Figure 1A), a phenomenon called symbiont halo (cf. Wolf-Gladrow et al., 1999). In contrast, G. siphonifera usually holds a certain amount of symbionts inside the test (Supplementary Figure 1D), and often forms a thick algal mat on the test surface (Supplementary Figure 1E). Even when it distributes its symbionts along the spines, the symbionts cling densely (Bijma et al., 1998; Supplementary Figure 1F), sometimes forming a chunk of algal cells in the middle of the spines (Supplementary Figure 1G). These distributional differences appear to be associated with the utilization of carbon from seawater as well. As a result, 14C in seawater was incorporated less effectively into the pelagophyte symbionts in G. siphonifera compared to the dinoflagellate symbionts in T. sacculifer.
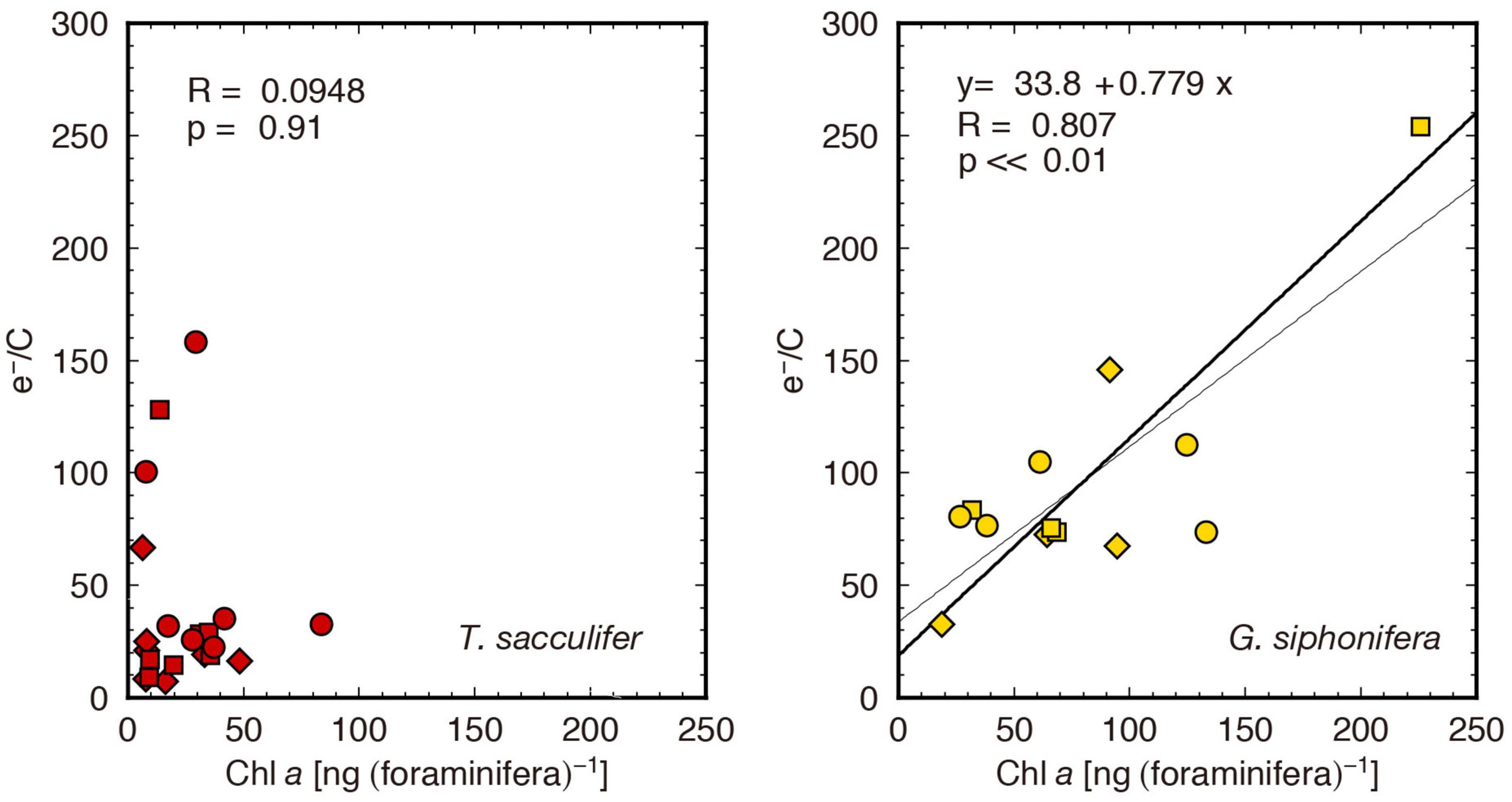
Figure 7. Cross plots of Chl a content and e–/C. The bold and thin lines are obtained from reduced major axis regression and least square regression, respectively. A statistically significant correlation is shown only in G. siphonifera.
A particular specimen which had the highest concentration of Chl a in the samples (specimen ID: siph4, Supplementary Table 1), yielded the highest e–/C of 254 mol e– (mol C)–1 (the highest point in Figure 7). This is, in fact, unrealistically high. It may partly be due to the underestimation of the carbon assimilation rate, as discussed above, and in addition, to the overestimation of the ETR on a per foraminifera basis. In fact, the ETRForam × 0.25 (assuming minimum 4 e– required for 1 O2 evolution) is still high when compared to reported O2 evolution results (Table 3), especially for G. siphonifera. The ETRForam value was calculated assuming that all the symbionts were under the same irradiance level, which, in reality, may not be true. When symbionts are densely packed within the host, the innermost ones would be shaded. Therefore, it is necessary to be aware that specimens with higher Chl a contents produce underestimated PForam and overestimated ETRForam values.
Generally speaking, as seawater is limited in terms of CO2 content, algae need enzymes (e.g., carbonic anhydrase) to convert HCO3– to CO2, which implies a higher cost compared to the direct acquirement of CO2 from their host. In this context, it is reasonable to use the easily accessible, low-cost respired CO2 from the host for photosynthesis. Although it has long been considered as advantageous in photosymbiosis (Bé et al., 1977; Caron, 2000), the contribution of the host’s metabolic carbon as a resource for symbiont photosynthesis has not been quantitatively evaluated yet. In fact, a series of pulse-chase experiments by LeKieffre et al. (2018, 2020) investigating the source of carbon and nitrogen assimilation for O. universa–dinoflagellate photosymbiotic system using NanoSIMS demonstrated that the HCO3– in the environmental seawater were incorporated by the symbionts, but the carbon derived from food did not significantly appear in the symbiont. Their former finding agrees with our results that 14C-labeled HCO3– were assimilated. Their latter result seems to contradict to our argument, however, it does not rule out the possibility that the respired CO2 is incorporated to the symbionts. Their observation was conducted for specimens fed after 8 h (n = 2), and there still existed the undigested food vacuoles with labeled C, indicating that the heterotrophic carbon needs more time to be digested and respired. The other culturing study also showed that respired CO2 derived from digestion of natural prey affected foraminifera at least until a first new chamber was formed after collection (Spero and Lea, 1996).
Our comparison of observed e–/C and empirically realistic e–/C revealed that respired CO2 does play an important role for photosynthesis, and that the proportion may differ depending on species. As the species analyzed in this study harbor different types of symbionts, whether this difference is derived from the host or the symbionts is unclear. Further investigations of photosymbiotic consortia presenting the same symbionts—e.g., Globigerinoides ruber and Orbulina universa for dinoflagellates, and Neogloboquadrina dutertrei for pelagophytes—together with the isolation cultures of the algae alone, will contribute to a more comprehensive understanding of photosymbiotic relationships.
Previous oxygen micro-sensor studies have revealed that a considerable amount of oxygen is generated during symbiont photosynthesis, while the respiration rate of host foraminifera is approximately only one-tenth of the gross photosynthetic rate (Jørgensen et al., 1985; Lombard et al., 2009). Therefore, it was concluded that holobionts are highly autotrophic. Based on the experimental results of the above-mentioned studies, the concept that photosynthetically assimilated inorganic carbon is largely derived from the surrounding seawater is generally accepted (Wolf-Gladrow et al., 1999; Zeebe et al., 1999). In the microenvironmental model of photosymbiotic foraminifera proposed by Wolf-Gladrow et al. (1999) and Zeebe et al. (1999), it was assumed that the flux of the host’s respired CO2 diffuses freely from the spherical surface of the test. The model predicted significant alteration of the δ13C of DIC in the vicinity of a foraminiferal test surface, caused by both the symbiont photosynthesis and holobiont respiration. The model’s prediction of the chemical distribution in the microenvironment was generally in line with experimental results (Köhler-Rink and Kühl, 2005). The present study demonstrated that the efficiency in the utilization of respired CO2 differed between species. Specifically, the pelagophyte-bearing G. siphonifera relied mostly on the host-derived respired CO2 for photosynthesis, while the dinoflagellate-bearing T. sacculifer relied on it as well, but to a much smaller extent. Considering the mechanisms that determine the geochemical composition of foraminiferal tests proposed by the existing model, such difference in the efficiency of internal CO2 utilization may in turn cause the difference in the magnitude of the offset of δ13C between foraminiferal tests and DIC. This also suggests that the existing model needs to be partly revised to accommodate the case of highly effective utilization of respired CO2 for photosynthesis, as observed in G. siphonifera.
A previous study based on culture experiments and subsequent isotopic analyses of G. siphonifera showed that a higher feeding frequency—thus a higher respiration rate (Bijma et al., 1998)—did not affect the stable carbon isotope ratio of G. siphonifera Type II (Bijma et al., 1998). It was argued that the symbionts in this species assimilate most of the respired CO2 before it reaches the calcification site. As a result, the δ13C of foraminiferal carbonate tests is less affected by the respired CO2. In contrast, another experiment conducted on T. sacculifer, involving the modification of the δ13C of its food (different strains of Artemia), revealed that the respired CO2 accounted for ca. 17% of the resultant δ13C change in the test calcite (Bijma et al., 1999), which was higher than the results obtained in G. siphonifera Type II (Bijma et al., 1998). The reported higher influence of respired CO2 on the test δ13C in T. sacculifer may be associated with the less effective utilization of CO2 by its symbionts. However, another experiment conducted for non-symbiotic species Globigerina bulloides showed that the contribution of respired CO2 was only ∼10% (Spero and Lea, 1996), though it would be expected to be higher due to the lack of photosynthetic CO2 uptake. Evidence from these previous experimental studies is invaluable and very insightful, but still data is limited and not yet necessarily conclusive. Apart from the scenario of effective elimination of respired CO2 in symbiont-bearing foraminifera, another mechanism that reduces the impact of respired CO2 on test calcite needs to be further studied.
Even though the effective elimination of respired CO2 by symbionts may not be the perfect scenario, the above considerations for G. siphonifera and T. sacculifer δ13C changes in the test calcite (Bijma et al., 1998, 1999) are in line with the experimental results obtained in this study; the most effective consumption of respired CO2 derived from the host, occurred in G. siphonifera. Possibly, this may explain why pelagophyte-bearing species do not show a clear isotopic signal that can be regarded as a symbiotic-specific feature; for example, an ontogenetic increase of the test δ13C, which is widely accepted as a signal of photosymbiosis, is absent in them (Bijma et al., 1998; Bornemann and Norris, 2007; Ezard et al., 2015). Even if the symbiont photosynthetic activity increases with ontogeny in G. siphonifera, it may not contribute to altering the microenvironmental geochemical composition at the calcification site in order to finally affect the δ13C of their tests.
There are many existing foraminiferal species that harbor pelagophyte symbionts (Gastrich, 1987; Hemleben et al., 1989), and their tests have been used in paleoceanographic studies (Kroon and Darling, 1995; Spero et al., 2003). Yet, experimental information on these species is scarce. The considerations on G. siphonifera provided in this study will contribute to the understanding of test geochemistry in pelagophyte-bearing species. Nevertheless, in order to provide a general theory on the effect of photosynthesis on test geochemistry in other host species, it is necessary to further consider morphological characters, such as presence or absence of spines, their length, and distribution of symbionts. It still remains challenging to elucidate the geochemical signature related to the photosynthetic activities of the symbionts.
The FRR fluorometry-based electron transport rates and the 14C-tracer based carbon assimilation rates were compared for the two species of foraminiferal-algal consortia—the T. sacculifer-dinoflagellate and G. siphonifera-pelagophyte symbioses—and the photosynthetic rates for each individual holobiont were determined.
The highlight of this study was the strikingly high electron requirement for carbon assimilation (e–/C) observed in these holobionts compared to that previously reported in monoalgal cultures and in situ oceanographic observations. The high e–/C detected in the foraminiferal holobionts is partly attributable to the incorporation of the unlabeled respiratory carbon used for photosynthesis that causes the underestimation of carbon assimilation rates. Through a model that assumed theoretical and empirically realistic e–/C values, and included the apparent e–/C observed, we estimated the carbon source proportion used for photosynthesis. The results showed that a significant amount of photosynthetic carbon should be derived from the host’s respired CO2. A higher contribution of respired CO2 exists in G. siphonifera than in T. sacculifer.
Therefore, from the viewpoint of making use of test geochemical signatures—such as δ13C—as paleoceanographic proxies, it is necessary to consider that the potential magnitude of the effect of photosynthetic activities can differ between foraminiferal species and/or algal species. In the cases of T. sacculifer and G. siphonifera, the latter showed a smaller magnitude in terms of photosynthetic inorganic carbon incorporation from seawater, which indicates that this species would be less susceptible to the alteration of geochemical composition by photosynthesis. At the same time, the more efficient utilization of the host-derived carbon by symbionts in G. siphonifera can also reduce the possible effect of respiration on their tests. This attempt to couple the ETR and carbon assimilation rate could comprehensively reveal an interesting perspective on the intimate interactions existing within photosymbiotic consortia. Further examinations using isolation cultures are required to verify more detailed nature of the symbionts, in order to obtain new information on the physiological interactions between foraminifera and symbionts.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
HT, KK, and TF conceived the study. HT and KK collected the samples. HT conducted the experiments and measurements with help from KK and TF, analyzed the data, and took the lead on the writing of the manuscript. All authors commented on the drafts and approved the submission.
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (grant numbers 13J05477 and 21K14896).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors HT.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge Tomohiko Kikuchi and Shinji Shimode (Yokohama National University) for their assistance during sampling. We thank Yoshihisa Mino (Nagoya University) for the use of the photosynthetron, Yoshikazu Mochizuki (JAMSTEC) for the assistance of 14C experiments, and Kazuyoshi Moriya (Waseda University) for fruitful discussion on theoretical consideration of carbon incorporation sources. We also thank two reviewers for their valuable discussion and the manuscript was greatly improved by their suggestions.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.803354/full#supplementary-material
Agusti, S. (1991). Allometric scaling of light-absorption and scattering by phytoplankton cells. Can. J. Fish. Aquat. Sci. 48, 763–767.
Asada, K. (1999). THE WATER-WATER CYCLE IN CHLOROPLASTS: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639. doi: 10.1146/annurev.arplant.50.1.601
Babin, M., Morel, A., Claustre, H., Bricaud, C., Kolber, Z., and Falkowski, P. G. (1996). Nitrogen- and irradiance-dependent variations of the maximum quantum yield of carbon fixation in eutrophic, mesotrophic and oligotrophic marine systems. Deep Sea Res. 43, 1241–1272.
Badger, M. R., von Caemmerer, S., Ruuska, S., and Nakano, H. (2000). Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1433–1446. doi: 10.1098/rstb.2000.0704
Baker, N. R., and Oxborough, K. (2005). “Chlorophyll fluorescence as a probe of photosynthetic productivity,” in Chlorophyll a Fluorescence: A Signature of Photosynthesis, eds G. C. Papageorgiou Govindjee (Dordrecht: Springer), 65–82. doi: 10.1007/978-1-4020-3218-9_3
Barber, R. T., Sanderson, M. P., Lindley, S. T., Chai, F., Newton, J., Trees, C. C., et al. (1996). Primary productivity and its regulation in the equatorial Pacific during and following the 1991–1992 El Ninõ. Deep Sea Res. II 43, 933–969. doi: 10.1016/0967-0645(96)00035-5
Bé, A. W. H., Hemleben, C., Anderson, O. R., Spindler, M., Hacunda, J., and Tuntivate-Choy, S. (1977). Laboratory and field observations of living planktonic foraminifera. Micropaleontology 23, 155–179.
Bemis, B. E., Spero, H. J., Bijma, J., and Lea, D. W. (1998). Reevaluation of the oxygen isotopic composition of planktonic foraminifera: experimental results and revised paleotemperature equations. Paleoceanography 13, 150–160. doi: 10.1029/98pa00070
Bennoun, P. (1982). Evidence for a respiratory chain in the chloroplast. Proc. Natl. Acad. Sci. U.S.A. 79, 4352–4356. doi: 10.1073/pnas.79.14.4352
Bijma, J., Hemleben, C., Huber, B. T., Erlenkeuser, H., and Kroon, D. (1998). Experimental determination of the ontogenetic stable isotope variability in two morphotypes of Globigerinella siphonifera (d’Orbigny). Mar. Micropaleontol. 35, 141–160.
Bijma, J., Spero, H. J., and Lea, D. W. (1999). “Reassessing foraminiferal stable isotope geochemistry: impact of the oceanic carbonate system (experimental results),” in Use of Proxies in Paleoceanography: Examples From the South Atlantic, eds G. Fischer and G. Wefer (Berlin: Springer-Verlag), 489–512. doi: 10.1007/978-3-642-58646-0_20
Bornemann, A., and Norris, R. D. (2007). Size-related stable isotope changes in Late Cretaceous planktic foraminifera: implications for paleoecology and photosymbiosis. Mar. Micropaleontol. 65, 32–42.
Caron, D. A. (2000). “Symbiosis and mixotrophy among pelagic microorganisms,” in Microbial Ecology of the Oceans, ed. D. L. Kirchman (Hoboken, NJ: Wiley), 495–523.
Caron, D. A., Michaels, A. F., Swanberg, N. R., and Howse, F. A. (1995). Primary productivity by symbiont-bearing planktonic sarcodines (Acantharia, Radiolaria, Foraminifera) in surface waters near Bermuda. J. Plankton Res. 17, 103–129.
Collos, Y., Descolas-Gros, C., Fontugne, M., Mortain-Bertrand, A., Chrétiennot-Dinet, M. J., and Frikha, M. G. (1993). Chemical, isotopic and enzymatic monitoring of free and enclosed seawater implications for primary production estimates in incubation bottles. Mar. Ecol. Progr. Ser. 93, 49–54.
Decelle, J., Colin, S., and Foster, R. A. (2015). “Photosymbiosis in marine planktonic protists,” in Marine Protists, eds S. Ohtsuka, T. Suzaki, T. Horiguchi, N. Suzuki, and F. Not (Tokyo: Springer), 465–500.
Edwards, G. E., and Baker, N. R. (1993). Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynth. Res. 37, 89–102.
Erez, J. (1983). “Calcification rates, photosynthesis and light in planktonic foraminifera,” in Biomineralization and Biological Metal Accumulation, eds P. Westbroek and E. W. de Jong (Dordrecht: D. Reidel Publishing Company), 307–312.
Ezard, T. H. G., Edgar, K. M., and Hull, P. M. (2015). Environmental and biological controls on size-specific δ13C and δ18O in recent planktonic foraminifera. Paleoceanography 30, 151–173.
Faber, W. W. Jr., Anderson, O. R., and Caron, D. A. (1989). Algal–foraminiferal symbiosis in the planktonic foraminifer Globigerinella aequilateralis. II. Effects of two symbiont species on foraminiferal growth and longevity. J. Foraminifer. Res. 19, 185–193.
Faber, W. W. Jr., Anderson, O. R., Lindsey, J. L., and Caron, D. A. (1988). Algal–foraminiferal symbiosis in the planktonic foraminifer Globigerinella aequilateralis. I. Occurrence and stability of two mutually exclusive chrysophyte endosymbionts and their ultrastructure. J. Foraminifer. Res. 18, 334–343.
Falkowski, P. G., and Kolber, Z. S. (1995). Variations in chlorophyll fluorescence yields in phytoplankton in the worlds oceans. Aust. J. Plant Physiol. 22, 341–355. doi: 10.3389/fmicb.2018.01589
Falkowski, P. G., and Raven, J. A. (2007). Aquatic Photosynthesis, 2nd Edn. Princeton, NJ: Princeton Press.
Fujiki, T., Hosaka, T., Kimoto, H., Ishimaru, T., and Saino, T. (2008). In situ observation of phytoplankton productivity by an underwater profiling buoy system: use of fast repetition rate fluorometry. Mar. Ecol. Progr. Ser. 353, 81–88.
Fujiki, T., Suzue, T., Kimoto, H., and Saino, T. (2007). Photosynthetic electron transport in Dunaliella tertiolecta (Chlorophyceae) measured by fast repetition rate fluorometry: relation to carbon assimilation. J. Plankton Res. 29, 199–208.
Fujiki, T., Takagi, H., Kimoto, K., Kurasawa, A., Yuasa, T., and Mino, Y. (2014). Assessment of algal photosynthesis in planktic foraminifers by fast repetition rate fluorometry. J. Plankton Res. 36, 1403–1407.
Gastrich, M. D. (1987). Ultrastructure of a new intracellular symbiotic alga found within planktonic foraminifera. J. Phycol. 23, 623–632.
Gastrich, M. D., and Bartha, R. (1988). Primary productivity in the planktonic foraminifer Globigerinoides ruber (d’Orbigny). J. Foraminifer. Res. 18, 137–142.
Genty, B., Briantais, J.-M., and Baker, N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990, 87–92.
Hemleben, C., Spindler, M., and Anderson, O. R. (1989). Modern Planktonic Foraminifera. Berlin: Springer-Verlag.
Holmes, J. J., Weger, H. G., and Turpin, D. H. (1989). Chlorophyll a fluorescence predicts total photosynthetic electron flow to CO2 or NO3–/NO2– under transient conditions. Plant Physiol. 91, 331–337.
Hönisch, B., Bijma, J., Russell, A. D., Spero, H. J., Palmer, M. R., Zeebe, R. E., et al. (2003). The influence of symbiont photosynthesis on the boron isotopic composition of foraminifera shells. Mar. Micropaleontol. 49, 87–96.
Hönisch, B., Fish, C. R., Phelps, S. R., Haynes, L. L., Dyez, K., Holland, K., et al. (2021). Symbiont photosynthesis and its effect on boron proxies in planktic foraminifera. Paleoceanogr. Paleoclimatol. 36:e2020A004022.
Huber, B. T., Bijma, J., and Darling, K. (1997). Cryptic speciation in the living planktonic foraminifer Globigerinella siphonifera (d’Orbigny). Paleobiology 23, 33–62. doi: 10.1017/s0094837300016638
Jørgensen, B. B., Erez, J., Revsbech, N. P., and Cohen, Y. (1985). Symbiotic photosynthesis in a planktonic foraminiferan Globigerinoides sacculifer (Brady), studied with microelectrodes. Limnol. Oceanogr. 30, 1253–1267. doi: 10.4319/lo.1985.30.6.1253
Knap, A., Michaels, A., Close, A., Ducklow, H., and Dickson, A. (1996). Protocols for the Joint Global Ocean Flux Study (JGOFS) Core Measurements. JGOFS Report Nr. 19. Reprint of the IOC Manuals and Guides No. 29, UNESCO 1994. Paris: UNESCO-IOC.
Köhler-Rink, S., and Kühl, M. (2005). The chemical microenvironment of the symbiotic planktonic foraminifer Orbulina universa. Mar. Biol. Res. 1, 68–78. doi: 10.1080/17451000510019015
Kolber, Z. S., and Falkowski, P. G. (1993). Use of active fluorescence to estimate phytoplankton photosynthesis in situ. Limnol. Oceanogr. 38, 1646–1665. doi: 10.4319/lo.1993.38.8.1646
Kolber, Z. S., Prášil, O., and Falkowski, P. G. (1998). Measurements of variable chlorophyll fluorescence using fast repetition rate techniques: defining methodology and experimental protocols. Biochim. Biophys. Acta 1367, 88–106. doi: 10.1016/s0005-2728(98)00135-2
Kromkamp, J. C., and Forster, R. M. (2003). The use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. Eur. J. Phycol. 38, 103–112. doi: 10.1080/0967026031000094094
Kroon, D., and Darling, K. (1995). Size and upwelling control of the stable isotope composition of Neogloboquadrina dutertrei (d’Orbigny), Globigerinoides ruber (d’Orbigny) and Globigerina bulloides d’Orbigny: examples from the Panama Basin and Arabian Sea. J. Foraminifer. Res. 25, 39–52. doi: 10.2113/gsjfr.25.1.39
Lawrenz, E., Silsbe, G., Capuzzo, E., Ylöstalo, P., Forster, R. M., Simis, S. G. H., et al. (2013). Predicting the electron requirement for carbon fixation in seas and oceans. PLoS One 8:e58137. doi: 10.1371/journal.pone.0058137
Laws, E. A. (1991). Photosynthetic quotients, new production and net community production in the open ocean. Deep Sea Res. 38, 143–167. doi: 10.1016/0198-0149(91)90059-o
Lawson, T., Oxborough, K., Morison, J. I. L., and Baker, N. R. (2002). Responses of photosynthetic electron transport in stomatal guard cells and mesophyll cells in intact leaves to light, CO2 and humidity. Plant Physiol. 128, 52–62. doi: 10.1104/pp.128.1.52
Lazier, J. R. N., and Mann, K. H. (1989). Turbulence and the diffusive layers around small organisms. Deep-sea Research 36, 1721–1733.
LeKieffre, C., Spero, H. J., Fehrenbacher, J. S., Russell, A. D., Ren, H., Geslin, E., et al. (2020). Ammonium is the preferred source of nitrogen for planktonic foraminifer and their dinoflagellate symbionts. Proc. R. Soc. B 287:20200620. doi: 10.1098/rspb.2020.0620
LeKieffre, C., Spero, H. J., Russell, A. D., Fehrenbacher, J. S., Geslin, E., and Meibom, A. (2018). Assimilation, translocation, and utilization of carbon between photosynthetic symbiotic dinoflagellates and their planktic foraminifera host. Mar. Biol. 165:104.
Lombard, F., Erez, J., Michel, E., and Labeyrie, L. (2009). Temperature effect on respiration and photosynthesis of the symbiont-bearing planktonic foraminifera Globigerinoides ruber, Orbulina universa, and Globigerinella siphonifera. Limnol. Oceanogr. 54, 210–218. doi: 10.4319/lo.2009.54.1.0210
Mackey, K., Paytan, A., Grossman, A., and Bailey, S. (2008). A photosynthetic strategy for coping in a high-light, low nutrient environment. Limnol. Oceanogr. 53, 900–913.
Mehler, A. H. (1957). Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch. Biochem. Biophys. 33, 65–77. doi: 10.1016/0003-9861(51)90082-3
Oxborough, K., and Baker, N. R. (1997). Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components— calculation of qP and Fv’/Fm’ without measuring F0’. Photosynth. Res. 54, 135–142.
Peterson, B. J. (1980). Aquatic primary productivity and the 14C-CO2 method: a history of the productivity problem. Annu. Rev. Ecol. Syst. 11, 359–385.
Prášil, O., Kolber, Z., Berry, J. A., and Falkowski, P. G. (1996). Cyclic electron flow around photosystem II in vivo. Photosynth. Res. 48, 395–410.
Rink, S., Kühl, M., Bijma, J., and Spero, H. J. (1998). Microsensor studies of photosynthesis and respiration in the symbiotic foraminifer Orbulina universa. Mar. Biol. 131, 583–595.
Schiebel, R. (2002). Planktic foraminiferal sedimentation and the marine calcite budget. Glob. Biogeochem. Cycles 16, 3–1–3–21. doi: 10.1029/2001GB001459
Spero, H. J. (1987). Symbiosis in the planktonic foraminifer, Orbulina universa, and the isolation of its symbiotic dinoflagellate, Gymnodinium béii snov. J. Phycol. 23, 307–317.
Spero, H. J., and Lea, D. W. (1996). Experimental determination of stable isotope variability in Globigerina bulloides: implications for paleoceanographic reconstruction. Mar. Micropaleontol. 28, 231–246.
Spero, H. J., and Parker, S. L. (1985). Photosynthesis in the symbiotic planktonic foraminifer Orbulina universa, and its potential contribution to oceanic primary productivity. J. Foraminifer. Res. 15, 273–281. doi: 10.2113/gsjfr.15.4.273
Spero, H. J., Lerche, I., and Williams, D. F. (1991). Opening the carbon isotope “vital effect” black box, 2, quantitative model for interpreting foraminiferal carbon isotope data. Paleoceanography 6, 639–655.
Spero, H. J., Mielke, K. M., Kalve, E. M., Lea, D. W., and Pak, D. K. (2003). Multispecies approach to reconstructing eastern equatorial Pacific thermocline hydrography during the past 360 kyr. Paleoceanography 18:A1022.
Stoecker, D. K., Johnson, M. D., de Vargas, C., and Not, F. (2009). Acquired phototrophy in aquatic protists. Aquat. Microb. Ecol. 57, 279–310. doi: 10.3354/ame01340
Suggett, D. J., Maberly, S. C., and Geider, R. J. (2006). Gross photosynthesis and lake community metabolism during the spring phytoplankton bloom. Limnol. Oceanogr. 51, 2064–2076.
Suggett, D. J., MacIntyre, H. L., Kana, T. M., and Geider, R. J. (2009). Comparing electron transport with gas exchange: parameterising exchange rates between alternative photosynthetic currencies for eukaryotic phytoplankton. Aquat. Microb. Ecol. 56, 147–162.
Suggett, D. J., Moore, C. M., and Geider, R. J. (2011). “Estimating aquatic productivity from active fluorescence measurements,” in Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications, eds D. J. Suggett, O. Prášil, and M. A. Borowitzka (Cham: Springer), 103–127. doi: 10.1007/978-90-481-9268-7_6
Suggett, D. J., Oxborough, K., Baker, N. R., MacIntyre, H. L., Kana, T. M., and Geider, R. J. (2003). Fast repetition rate and pulse amplitude modulation chlorophyll a fluorescence measurements for assessment of photosynthetic electron transport in marine phytoplankton. Eur. J. Phycol. 38, 371–384. doi: 10.1080/09670260310001612655
Takagi, H., Kimoto, K., Fujiki, T., and Moriya, K. (2018). Effect of nutritional condition on photosymbiotic consortium of cultured Globigerinoides sacculifer (Rhizaria, Foraminifera). Symbiosis 76, 25–39. doi: 10.1007/s13199-017-0530-3
Takagi, H., Kimoto, K., Fujiki, T., Kurasawa, A., Moriya, K., and Hirano, H. (2016). Ontogenetic dynamics of photosymbiosis in cultured planktic foraminifers revealed by fast repetition rate fluorometry. Mar. Micropaleontol. 122, 44–52. doi: 10.1016/j.marmicro.2015.10.003
Takagi, H., Kimoto, K., Fujiki, T., Saito, H., Schmidt, C., Kucera, M., et al. (2019). Characterizing photosymbiosis in modern planktonic foraminifera. Biogeosciences 16, 3377–3396. doi: 10.5194/bg-16-3377-2019
Wolf-Gladrow, D. A., Bijma, J., and Zeebe, R. E. (1999). Model simulation of the carbonate system in the microenvironment of symbiont bearing foraminifera. Mar. Chem. 64, 181–198. doi: 10.1016/s0304-4203(98)00074-7
Zeebe, R. E., Bijma, J., and Wolf-Gladrow, D. A. (1999). A diffusion-reaction model of carbon isotope fractionation in foraminifera. Mar. Chem. 64, 199–228. doi: 10.1016/s0304-4203(98)00075-9
Keywords: planktonic foraminifera, photosymbiosis, photosynthesis, fast repetition rate fluorometry, carbon assimilation
Citation: Takagi H, Kimoto K and Fujiki T (2022) Photosynthetic Carbon Assimilation and Electron Transport Rates in Two Symbiont-Bearing Planktonic Foraminifera. Front. Mar. Sci. 9:803354. doi: 10.3389/fmars.2022.803354
Received: 27 October 2021; Accepted: 28 January 2022;
Published: 09 March 2022.
Edited by:
Raphael Morard, University of Bremen, GermanyReviewed by:
Howard Spero, University of California, Davis, United StatesCopyright © 2022 Takagi, Kimoto and Fujiki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haruka Takagi, aHRha2FnaUBjaGliYS11Lmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.