
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 06 January 2023
Sec. Marine Pollution
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1098232
This article is part of the Research Topic Nuclear Power Cooling-Water System Disaster-Causing Organisms: Outbreak and Aggregation Mechanisms, Early-warning Monitoring, Prevention and Control View all 15 articles
The intake safety of nuclear power cooling-water systems (NPCSs) is an important aspect of operational safety of nuclear power plants (NPPs). The blockages caused by aberrant outbreaks of various aquatic organisms have seriously affected operational safety. Large jellyfish constitute the main groups of marine organisms responsible for these blockages. The processes of aggregation and the relationships of two major disaster-causing scyphozoan jellyfish species, Nemopilema nomurai and Aurelia coerulea, with four environmental factors at the intake area of an NPCS in Eastern Liaodong Bay, China, were investigated in 2019 and 2020. The findings revealed that A. coerulea ephyrae were present in the surrounding ports in mid-May; however, N. nomurai ephyrae were absent during the survey period in this study, and the medusae of N. nomurai started appearing from late May. The individual growth and relative biomass (RB) of the jellyfish increased rapidly from late June to July and decreased rapidly thereafter, in September. The RB of N. nomurai was highly correlated to the sea surface temperature (SST) and levels of dissolved oxygen (DO) in the region. The RB increased with increasing SST and decreased at increasing DO levels. The RB of A. coerulea was significantly negatively correlated with that of N. nomurai, and the peak biomass of the two species alternated over time, which could be attributed to the fact that the jellyfish species share similar ecological niches. The bell diameters were significantly positively correlated with the individual wet weights, and the value of one could be inferred from the value of the other. Although the processes of jellyfish aggregation are attributed to several factors, including interactions with environmental factors and human activities, such as fishing, the results obtained in this study would serve as an important reference and provide a basis for the prevention of jellyfish blooms in waters adjacent to NPPs. The prevention and control of jellyfish disasters at the intake area of NPCSs are not only local concerns. Therefore, remediation from the source combined with the maximum utilization of social resources for monitoring and early warning would immensely improve the efficacy of such preventive strategies.
Jellyfish constitute one of the most important groups of gelatinous zooplankton and play critical roles in marine ecosystems (Mills, 1995; Hamilton, 2016). Compared with most pelagic metazoans, jellyfishes have a high water content (95% or above) but a low carbon content (Lucas et al., 2011). This explains why they are larger than non-gelatinous animals with comparable carbon contents (Pitt et al., 2013). Jellyfish can grow faster and demonstrate competitive advantages in various marine ecosystems owing to their higher metabolic rate, good adaptability, and lack of natural enemies, among other characteristics (Schneider, 1992; Dawson and Hamner, 2009; Berwald, 2017).
Blooms caused by jellyfish, especially those of the class Scyphozoa, which have metagenic life cycles, have become a common phenomenon in recent years owing to various environmental pressures, including global climate change and anthropogenic activities (Purcell et al., 2007; Richardson et al., 2009; Purcell, 2012; Dawson et al., 2015; Quinones et al., 2018; Goldstein and Steiner, 2020; Rekstad et al., 2021; Riyas et al., 2021). The sudden or aberrant increase in jellyfish biomass has caused jellyfish disasters in several parts of the globe’s oceans, affecting fisheries, and damaging the safety of nuclear power cooling-water systems (NPCSs), especially in Europe, Asia, and North America (Lucas and Dawson, 2014; Schiariti et al., 2015). Jellyfish blooms were responsible for the blockage of the cooling water intakes of the Madras Atomic Power Station, in the south-western Bay of Bengal at Kalpakkam, which led to the shutdown of the power station in 1995–1996 (Masilamoni et al., 2000). An unusually large flow of jellyfish caused the shutdown of the filtering equipment in reactors 1, 2, and 3 of the Kashiwazaki Kariwa Nuclear Power Station in Japan, which forced it to reduce power output on 7 July 1999 (Takizawa, 2005). Jellyfish blooms have been appearing frequently in Korean waters since 2003 and have clogged coastal power plant cooling-water intakes (Yoon et al., 2014). In 2011, while jellyfish outbreaks in the United States, Japan, Israel, and Scotland led to the shutdown of nuclear power plants (NPPs) in these countries (Schrope, 2012).
Nemopilema nomurai is a common species of jellyfish belonging to class Scyphozoa. It has a widespread distribution and is responsible for frequent blooms in East Asian marginal seas. This jellyfish is primarily observed in the waters of China, Korea, and Japan from late spring to autumn (Dong et al., 2018). The northern parts of the East China Sea (ECS), Yellow Sea (YS), and Bohai Sea (BS) in China are considered to be the main habitats of N. nomurai (Kawahara et al., 2006; Dong et al., 2010). Although the origin of this large jellyfish remains controversial, field surveys and physical modeling studies have demonstrated that the Yangtze River Estuary and adjacent sea areas are possible sources of N. nomurai (Yoon et al., 2008; Moon et al., 2010; Sun et al., 2015). Previous studies reported that the benthic polyps of N. nomurai develop into medusae and are released between April and June in the Yangtze River Estuary and the adjacent sea areas (Moon et al., 2010; Dong et al., 2018). The medusae subsequently migrate to the northern side of the YS, eastern side of the ECS, and the East Sea (ES) (Moon et al., 2010; Dong et al., 2018). It has been reported that their biomass increases at rising temperatures and peaks by August (Zhang et al., 2012; Sun et al., 2015). Various environmental factors shape the distribution characteristics of jellyfish to a certain extent. Previous studies have demonstrated that the temperature and salinity of water significantly affect the distribution and abundance of N. nomurai, and that there is a positive relationship between the abundance of N. nomura and the low salinity of Changjiang Diluted Water (Yoon et al., 2008; Kitajima et al., 2020). Juvenile jellyfishes have been found in Liaodong Bay (LDB), where the waters have low salinity and high temperatures (Dong et al., 2018). During development, the juveniles and small medusae drift to the central and southern regions of the LDB, where the waters have lower temperatures and higher salinity (Dong et al., 2018).
Aurelia coerulea is one of the most common species of jellyfish living in offshore regions, and is widely distributed in tropical, subtropical, and temperate marine areas. A. coerulea outbreaks have been reported in China, Japan, and Korea (Mills, 2001). The outbreaks of A. coerulea are different from those of N. nomurai, and A. coerulea blooms primarily occur in coastal and estuarine areas, where anthropogenic activities are higher (Sun et al., 2012). Outbreaks and aggregation of A. coerulea are primarily observed in the bays and temperate areas of YS and BS in China (Dong et al., 2010; Wang et al., 2012). The biomass of A. coerulea is particularly high in July and August in the northern coastal sea of China, and this species causes frequent disaster events in Qinhuangdao in the Hebei province, Dalian in the Liaoning province, and in Yantai, Weihai, and Qingdao in the Shandong province (Dong et al., 2010). A. coerulea is highly adaptable and can adapt to a wide range of temperature and salinity conditions. For instance, populations of A. coerulea can migrate through winter ice caps and survive at an upper temperature range of 31–32°C (Hamner et al., 1982; Hernroth and Gröndahl, 1985). A. coerulea can be found in waters with salinity levels of less than 10‰ up to levels of 38‰ (Russell, 1970; Papathanassiou et al., 1987; Olesen and Riisgard, 1994). The previous study had demonstrated that jellyfish outbreaks are primarily mediated by a temporal shift from polyp-dominated to medusa-dominated populations (Goldstein and Steiner 2020). The temperature and variations in temperature are key factors that control the initiation and cessation of strobilation, and an optimal increase in temperature facilitates the release of larvae and the reproduction of polyps (Kroiher et al., 2000; Ishii and Takagi, 2003; Han and Uye, 2010; Prieto et al., 2010; Wang et al., 2014). Low levels of salinity can delay or inhibit the reproduction of polyps (Purcell et al., 2009). Nutrient availability can be another important ecological driver of jellyfish blooms because it facilitates a shift in the population structure from a polyp-dominated to a medusa-dominated population (Goldstein and Steiner 2020).
The Hongyanhe Nuclear Power Plant (HYHNPP) is located in the eastern part of LDB and is currently the only operational NPP in the northern seas of China. Six units of HYHNPP, with a total installed capacity of 6.7 million kilowatts, have been fully completed and put into operation in 2022, making HYHNPP the largest operational NPP in China and the third largest operational NPP in the world. However, the intake area of the NPCS of HYHNPP has been troubled by jellyfish blooms, primarily caused by N. nomurai and A. coerulea since 2014, and this has affected the normal operation of the HYHNPP. In July 2014, a large jellyfish population entered the inlet region of the recirculating water filtration system, resulting in the shutdown of units 1 and 2 of the HYHNPP. In July 2015, a large jellyfish population flooded the inlet as a result of the rupture of the first and third barrier nets. Although massive human and material resources have been used for coping with jellyfish disasters to date, the approaches have met with limited success. The distribution characteristics of dominant disaster-causing jellyfish at the intake area of NPCSs have been reported in few studies. In addition, the regions from which these jellyfish species originate have always been a concern for managers and researchers. The sudden gathering of jellyfish can block the intake area of NPCSs, which poses as a huge safety risk and causes economic losses to NPPs. Further studies are therefore necessary for obtaining better insights into the patterns of jellyfish blooms for planning targeted measures against jellyfish disasters.
In this study, we investigated the process of aggregation and relationships of the two major disaster-causing scyphozoan jellyfish species, N. nomurai and A. coerulea, with four environmental factors at the intake area of the NPCS in Eastern LDB in 2019 and 2020. The present study aimed to elucidate the mechanism of distribution of the two jellyfish species, and the findings provide an important reference and supporting data for preventing jellyfish blooms in waters adjacent to NPPs. The study also discussed various measures for predicting and controlling jellyfish outbreaks near the HYHNPP.
A total of 61 surveys were conducted at the intake area of HYHNPP in the eastern part of LDB from May to September of 2019 and 2020. Five sampling stations were set up at the intake area (Figure 1), of which the central station was set up at the inlet area of the NPCS (HYH03), two stations (HYH01 and HYH02) were located at the north-eastern side of the central station, and two stations (HYH04 and HYH05) were set up at the south-western side of the central station. The distance between two stations was approximately 2 km. Large jellyfish species were sampled using a plane anchor drift net (mesh size: 10 mm, length: 110 m, width: 15 m; Figure 2). The direction of net casting was perpendicular to the direction of the flow, and the nets were hauled with the current. The soak time usually lasted from 30 minutes to 1 hour and was adjusted according to the size of the catch. As individual N. nomurai and A. coerulea jellyfish are highly fragmented and difficult to count accurately at high density, the relative biomass (RB) was used for expressing the abundance of jellyfish in this study. The crane of the fishing vessel was used for lifting large numbers of jellyfish, which were weighed using a large hanging hook scale. Small numbers of jellyfish and individuals were placed into sample bags and weighed using a small hanging hook scale. The precision of wet weight weighed by the large and small scale was 1 kg and 0.1 g, respectively. The RB of the jellyfish was expressed in kg net–1 h–1. The numbers, bell diameters (BDs), and wet weights (WWs) of individual jellyfish were noted; the BD was measured with a straight edge. The surface environmental parameters, including the sea surface temperature (SST), dissolved oxygen (DO), surface salinity (SS), and pH, were measured in situ using a YSI ProQuatro water quality meter. Ephyrae and juveniles were collected using a shallow-water type II plankton net (mesh size: 160 μm, diameter: 31.6 cm) and the ephyrae collected from Jiangjunshi port, located 22 km from HYHNPP, were investigated (Figure 1).
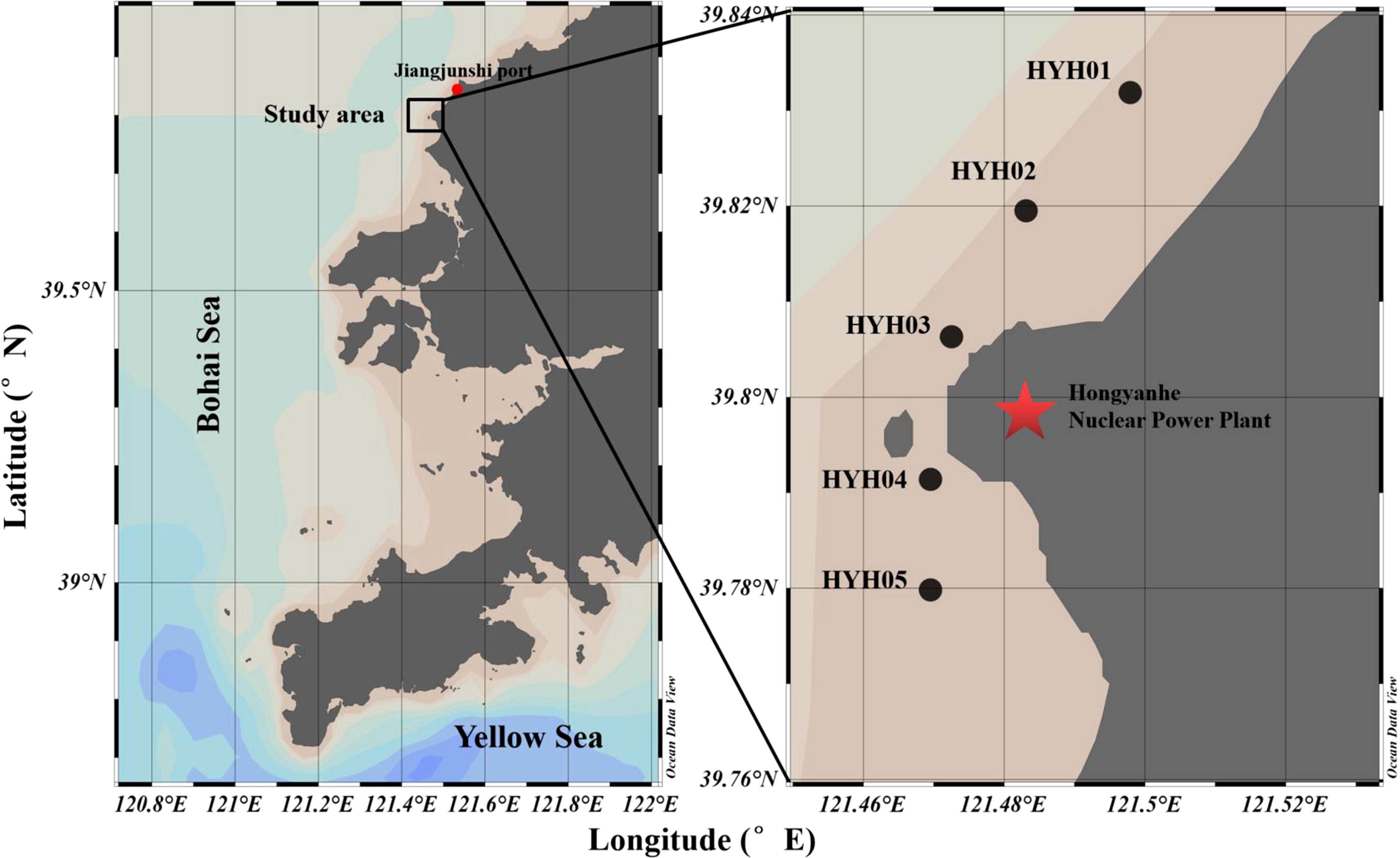
Figure 1 Location of the study area. The area from where the ephyrae were collected and the HYHNPP are delimited by the red dot and the red pentacle, respectively.
The normality and homogeneity of the variance were confirmed using the one-sample Kolmogorov–Smirnov test and Levene’s test, respectively. The Kruskal–Wallis H-test was performed when the data did not approach normality or homogeneity of variance. Multiple parametric comparisons were performed as the data were abnormal or exhibited non-homogeneous variance. Simple correlation analyses were performed for evaluating the statistical correlation between the RB and environmental factors (SST, SS, DO, and pH) using Spearman’s correlation with SPSS version 16.0. The graphs were prepared with Microsoft 2016 and R version 4.2.2.
In this study, the first ephyrae of A. coerulea appeared at Jiangjunshi port on 15 May 2019 and were collected for further analyses. However, N. nomurai ephyrae were absent during the entire period of the survey. The first juvenile medusae of N. nomurai appeared on 31 May 2019, with a BD of 4–10 cm. The medusae grew rapidly thereafter and reached a maximum BD of 76 cm at the beginning of September. Medusae of A. coerulea were first collected on 8 July, and these had BDs of 12–21 cm and had reached the size of adult organisms. The size of the medusae did not alter much thereafter (Figure 3). The time of appearance of A. coerulea ephyrae in 2020 was the same as that in 2019, and no ephyrae of N. nomurai were detected in 2020. The first juvenile medusae of N. nomurai appeared on 11 June with a BD of 4–6 cm, which was comparable to the BD observed in 2019; however, the medusae appeared at a later period in 2020. There was a difficulty in measuring the BD of the subsequent samples, as the majority of samples were fragments. The results of available survey data revealed that the BD had increased rapidly since the first appearance of juvenile medusa. In this study, the BDs reached a maximum of 130 cm in mid-August 2020, which was significantly greater than the observations in 2019. Compared with 2019, the medusae of A. coerulea appeared earlier the following year, on 11 June 2020. The BDs of A. coerulea medusae collected in 2020 were 3–10 cm. The BDs increased rapidly and were similar to the BDs observed in the same period in 2019 (Figure 3).
The RB of the two large jellyfish species, N. nomurai and A. coerulea, are depicted in Figure 4. N. nomurai and A. coerulea populations alternated at the intake area of the NPCS, and the RB was not high during the period when they appeared simultaneously. In 2019, large numbers of N. nomurai began appearing in late June, and the average relative biomass (ARB) peaked in early July. The RB was highest on 2 July, when it reached a value of 2,806.00 kg net–1 h–1, and decreased rapidly thereafter. The ARB of A. coerulea increased subsequently and A. coerulea gradually became the dominant species from mid-July to mid-August. In 2020, large numbers of large jellyfish started appearing in July, and A. coerulea remained the dominant jellyfish species during the whole of July. The abundance of A. coerulea was highest on 7 July, when the ARB reached 2,234.40 kg net–1 h–1. A. coerulea decreased rapidly thereafter and N. nomurai gradually dominated from August, during which the RB remained continually low. The abundance of N. nomurai was highest on 22 August, when the RB reached 412.50 kg net–1 h–1. There were no significant patterns in the abundance of the large jellyfish species across the five stations. Overall, the RB of both species remained consistently lower at HYH04 than at the other stations (Figure 5).
The relationship between the BD of the individual jellyfish species (BDa for A. coerulea, BDn for N. nomurai) and the WW (Wa for A. coerulea, Wn for N. nomurai) could be described using the following equations (Figure 6): and .
The fluctuations in the SST, DO level, SS, and pH at the intake area of the NPCS between 2019 and 2020 are depicted in Figure 7. The SST increased from May to early August, gradually stabilized from August, and decreased from September. The annual SST in 2020 was generally lower than that during the same period in 2019. In contrast, the DO levels exhibited a decreasing trend from May to early August, gradually stabilized from August, and decreased from September. Overall, the annual DO level in 2020 was higher than that during the same period in 2019. In 2019, the SS was relatively stable until August, decreased significantly from the beginning of August, and was subsequently stabilized. In 2020, the SS decreased slightly from late August and increased gradually thereafter. The pH fluctuated and remained relatively stable in 2019 and 2020, with a range of 8.34–8.45. The fluctuations in the DO level, SS, and pH exhibited a similar trend across the five stations; however, the SST fluctuated significantly more at HYH04 than at the other stations and was generally higher than at the other stations (Figure 8).
The survival, SST, DO, SS, and pH range of the two large jellyfish species were similar during the survey period. A. coerulea was distributed in waters with SSTs of 16.7–32.2°C, DO levels of 4.37–7.71 mg/l, SS of 31.37–32.82‰, and pH of 8.12–8.50, while N. nomurai was distributed in waters with SSTs of 15.6–32.2°C, DO levels of 4.48–7.71 mg/l, SS of 31.27–32.82‰, and pH of 8.12–8.50 (Figure 9). The RB of these two jellyfish species was similar, and greater than 60 kg net–1 h–1. The RB of A. coerulea was relatively high in waters with SSTs of 20.9–30.9°C, DO levels of 4.37–6.69 mg/l, SS of 31.38–32.61‰, and pH of 8.26–8.50. The RB of N. nomurai was relatively high in waters with SSTs of 20.6–32.2°C, DO levels of 4.48–6.69 mg/l, SS of 31.27–32.79‰, and pH of 8.26–8.50 (Figure 9).
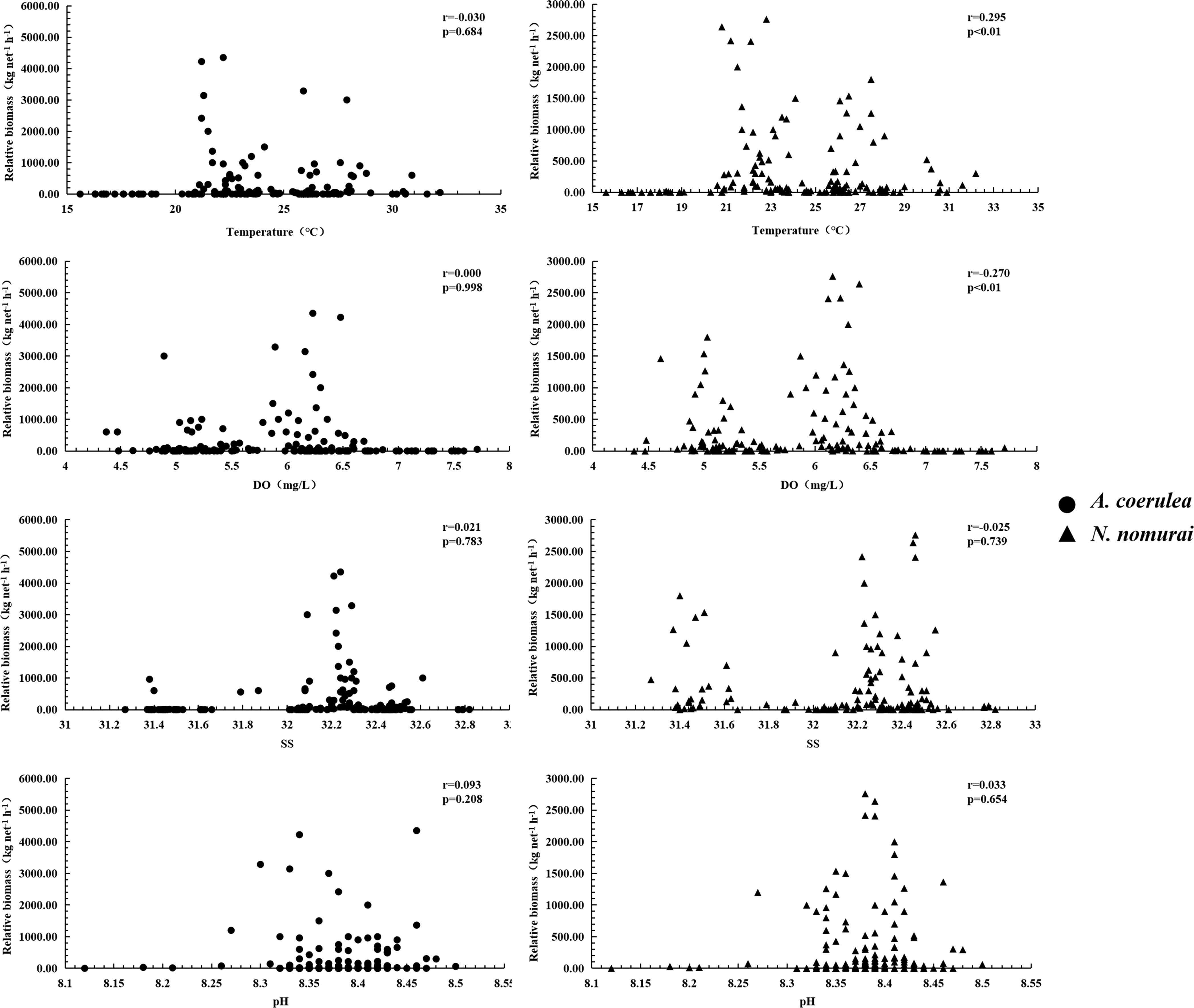
Figure 9 Relationship between the RBs of N. nomurai and A. coerulea and the environmental parameters.
The results of simple correlation analyses demonstrated a highly significant negative correlation between the RB of A. coerulea and the RB of N. nomurai (Figure 9) (r = –0.609, p< 0.01). There was no significant correlation between the RB of A. coerulea and the environmental parameters (SST, DO, SS, and pH); however, the RB of N. nomurai had a highly significant positive correlation with the SST and a highly significant negative correlation with the DO level (p< 0.01).
Determining the sources of jellyfish is essential for deciding appropriate measures for the prevention and control of jellyfish disasters at an early stage; it is currently one of most effective approaches for addressing this concern. A pure waterjet and a scraper have been applied to remove polyps after determining the sources in Korean waters, which proved to be effective (Yoon et al., 2018); however, the sources of large jellyfish at the intake area of the HYHNPP remain to be clearly determined to date.
It has been demonstrated that artificial structures such as ports provide additional habitats for the asexual stage of jellyfish (Feng et al., 2017). Ephyrae of A. coerulea were observed at Jiangjunshi port for two consecutive years in this study but were not detected at the intake area. The discovery of ephyrae at Jiangjunshi port indicated that the ephyrae had been released at the region, from where they might have gradually migrated outward to regions around the intake area from mid-May. This finding provides evidence regarding one of the habitats of A. coerulea. Moreover, the phenomenon observed at the intake area also coincides with the findings of other studies on the time of appearance of different life history stages of A. coerulea (Dong et al., 2008; Dong et al., 2014; Wang and Sun, 2015; Feng et al., 2018). A previous study indicated that the duration between the release of ephyrae and the development of small medusae of A. aurita could be less than 1 month at 18°C (Båmstedt et al., 2001). The newly liberated ephyrae of A. aurita developed into young medusae in only 20–28 days in the innermost part of Tokyo Bay (Ishii et al., 2004). In this study, juvenile medusae of A. coerulea were collected from the intake area approximately 1.5 and 1 months after the appearance of ephyrae in 2019 and 2020, respectively. Therefore, the findings possibly indicate a potential source of A. coerulea around the intake area. However, it is necessary to assess the association between the released ephyrae and the medusae collected at the intake area by continuous monitoring, and to determine whether there are more sources of A. coerulea.
Notably, N. nomurai ephyrae did not appear at the nearby ports or intake area during the two years of the survey, and the presence of N. nomurai ephyrae in this region has not been reported in similar studies (Wang et al., 2013; Dong et al., 2018). These findings indicate that N. nomurai did not originate in this region. It has been demonstrated that the northern estuarine area of LDB is the main habitat of N. nomurai in the study area. The ephyrae were possibly produced in mid-spring, and the rapid growth period lasted from late spring to early summer. The juveniles migrate toward the central and southern waters during development, reach maturity, and prepare to spawn in mid-autumn, and the populations decrease rapidly in late autumn (Dong et al., 2018). The intake area was located in the central region of LDB, and the overall timing of each stage was delayed by half a month in the central region, compared with that in the northern area of LDB. It was speculated that, following their release, the ephyrae and some juveniles migrated from the northern region of the LDB and continued to grow during their southward migration, and reached the intake area in early summer, which corresponded to the period of delay discovering juveniles at the study area. The RB increased rapidly in this area during the period of continuous migration, and the individuals grew rapidly, with the BDs reaching more than 50 cm in early July. This situation possibly continued until late autumn, following which the RB declined rapidly owing to the lack of source replenishment and the commencement of fishing activities in early September. The results obtained herein support the findings of previous studies which suggested that the estuarine area is possibly one of the sources of N. nomurai in the intake area; however, direct evidence is necessary for confirming the conjecture.
Jellyfish feed heavily on plankton, fish eggs, and juveniles, and populations require large quantities of food for supporting the rapid growth period (Greve, 1994; Hansson et al., 2005). Food availability can become a pivotal variable in limiting the viability and fecundity of jellyfish populations when the quantity of food decreases following a rapid increase in the density of large jellyfish species (Goldstein and Steiner 2020; Kitajima et al., 2020). A partial similarity in food composition induced competition between N. nomurai and A. coerulea (Wang et al., 2021). The study revealed that the RB of large jellyfish increased exponentially at the intake area from late June or early July and reached a plateau thereafter, and finally declined from September. The alternating occurrence and population distribution of these two species with similar niches could indicate passive adaptation and balance under the extremely high biomass and density in a restricted space with limited food availability.
Food composition not only controlled the population size, but also affected the spatial distribution of the two species. The main food for large jellyfish is plankton, which is also the primary species affected by thermal effluents (Bamber and Seaby, 2004; Li et al., 2014). The large influx of thermal effluents in NPPs can alter the hydrodynamic conditions and community structure of local seas (Salgueiro et al., 2015; Lee et al., 2018). In this study, station HYH04 was located near the outlet of the thermal effluents and was characterized by high water velocity and relatively drastic changes in temperature. The SST measured at HYH04 was sometimes higher by 2–3°C or more than that at the other stations during 2019 and 2020, and sometimes there were no differences between the SST at HYH04 and that at other stations. The jellyfish exhibited some, but extremely limited, swimming abilities and migrated actively or passively away from the local seas owing to conditions of strong flow expansion and loss of primary food sources. Comparison of the cumulative RB (CRB) and ARB calculated per hour among different stations revealed that the CRB and ARB were lowest at HYH04, for both A. coerulea and N. nomurai (Table 1). The findings could be attributed to the characteristics of the local environmental where the station was located.
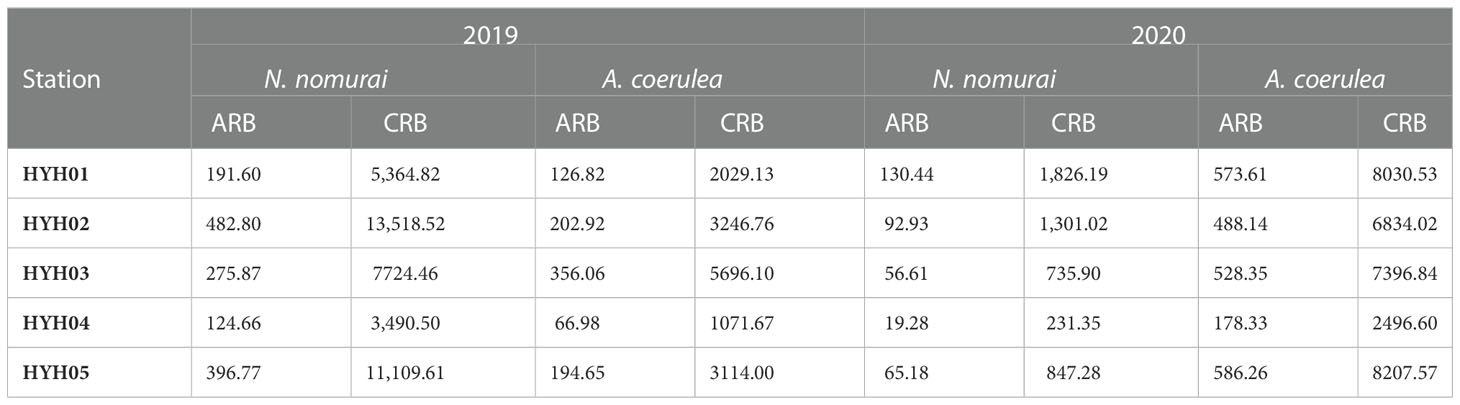
Table 1 The CRB (kg net–1 h–1) and ARB (kg net–1 h–1) of N. nomurai and A. coerulea in 2019 and 2020.
The interannual patterns and bloom dynamics of N. nomurai were linked to processes of the regional climate (Lee et al., 2021). Temperature is an important factor in influencing the growth and development of jellyfish; temperature affects the production of ephyrae during the asexual stage and the growth of medusae during the sexual stage (Baumsteiger et al., 2018; Feng et al., 2020). Optimal temperature is beneficial to the growth of juveniles, and previous studies have demonstrated a significant positive correlation between the RB and SST in many waters (Baumsteiger et al., 2018; Dong et al., 2018). In this study, the results of correlation analyses revealed a significant positive correlation between the RB of N. nomurai and the SST. The RB and BDs of N. nomurai increased rapidly from mid- to late June. The SST decreased slowly after August, while the RB of N. nomurai remained relatively stable and gradually decreased thereafter. The rapid warming in late spring and early summer could lead to the rapid growth and development of jellyfish.
In this study, the results of correlation analyses revealed a significant negative correlation between the DO level and the RB of N. nomurai, which was consistent with the findings of previous studies (Riyas et al., 2021). The DO level decreased sharply from May to early August, which corresponded with the rapid growth and increased RB, and coincided with a rapid increase in the daily interception and capture of jellyfish. This could be attributed to the increased consumption of DO caused by the rapid increase in RB within a short period of time. The phenomenon could also be attributed to artificial jellyfish control measures at the intake area of the NPCS. The presence of large numbers of fragmented and dead jellyfish could affect the local biogeochemical cycle and rapidly reduce the DO levels owing to the high oxygen consumption by microorganisms during decomposition (Pitt et al., 2009; Condon et al., 2011). The practice of fragmenting and discarding jellyfish pieces into the sea water to avoid the risk of blockages inevitably increases the mass of jellyfish fragments and corpses, which in turn accelerates the consumption of DO. As jellyfish have a low metabolism (Rutherford and Thuesen, 2005), artificial jellyfish control measures could play a more significant role in reducing the DO level.
Salinity is another important factor that affects the RB and distribution of jellyfish, and the effect of salinity was more pronounced in the asexual stage. The ephyrae and juveniles of N. nomurai exhibited a preference for low-salinity areas such as estuaries (Yoon et al., 2008; Dong et al., 2018). In this present study, salinity varied in a narrow range, and no significant correlation between SS and RB was detected. Therefore, it is unclear whether distributions of these two species at sexual stages are constrained by salinity. The pH has a limited effect on the RB and distribution of jellyfish, and jellyfish statoliths become significantly smaller at lower pH values (Winans and Purcell, 2010). A reduction in pH affects the pulsing behavior and size of A. coerulea ephyrae (Tills et al., 2016); however, it is generally accepted that acidification is not significantly associated with the abundance of medusae (Richardson and Gibbons, 2008). In this study, the pH values fluctuated between 8.26 and 8.50 during the period of investigation, and there was no significant correlation between the pH and RB of both species of jellyfish.
First, the analysis of environmental factors is an effective strategy for predicting jellyfish blooms (Baumsteiger et al., 2018; Dong et al., 2018; Riyas et al., 2021). Further analyses of metagenic life cycles and real-time online monitoring of environmental indicators would aid in predicting and designing effective measures against the aggregation of different jellyfish species for ensuring the operational safety of NPCSs. Second, ecological theories can provide a theoretical basis for the prevention and control of disasters caused by large jellyfish species. The release of competing species without disturbing the balance of the ecosystem, such as economic fishes, could serve as a suitable strategy for suppressing jellyfish blooms and providing additional economic value. Third, it is necessary to conduct studies on jellyfish blooms in regions beyond the local area of NPCSs. Further efforts are necessary for identifying the sources of jellyfish and understanding their migration patterns for increasing the efficacy of various preventive and control measures. Fourth, the treatment of jellyfish should be enhanced for avoiding negative effects on marine environments (Pitt et al., 2009; Condon et al., 2011), as these would in turn increase the possibility of other related disasters. Fifth, the increased use of unofficial sources or other joint monitoring approaches would increase access to information, provide further evidence for future studies, and aid in ensuring the safety and security of NPCSs (Gutiérrez-Estrada et al., 2021).
The distribution patterns of two jellyfish species were investigated during a survey for two consecutive years at the intake area of an NPCS in Eastern LDB, China. The findings revealed that Jiangjunshi port and the northern region of LDB could be the potential sources of A. coerulea and N. nomurai blooms, respectively. It is necessary to identify the sources of jellyfish species and remove polyps to reinforce the effects. The SST and DO can potentially indicate alterations in N. nomurai populations, and the thermal effluents can reshape the community at the local region. The findings also reveal that the interspecific resource competition may lead to variation of abundance in opposite directions in the two jellyfish species. The introduction of some key economic species could provide a solution to the threat of jellyfish blooms. The increased use of other measures for controlling jellyfish blooms in addition to monitoring and early warning measures can enhance the ease and efficacy of preventing jellyfish disasters.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conceptualization, XW, CG, and HG; data curation, XW, QJ; LY, CJ, and HW; formal analysis, XW and QJ; funding acquisition, XW and CG; investigation, XW, LY, and CJ; methodology, XW, LY, and CJ; validation, XW; writing—original draft, XW; writing—review and editing, XW and CG. All the authors agree to being accountable for the content of this study. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grant number: 42106155), the National Key R&D Program of China (grant number: 2017YFC1404404), and the Doctoral Foundation of National Marine Environmental Monitoring Centre, China (2019-2020 NPCS Safety Guarantee Project).
The authors acknowledge Sen Wang and Chunyang Wu for their help during the collection of samples and environmental data. The authors express their gratitude to Junjian Wang, Yi Sun, and Xiaoyu Cui for their help during data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bamber R. N., Seaby R. M. (2004). The effects of power station entrainment passage on three species of marine planktonic crustacean, Acartia tonsa (Copepoda), Crangon crangon (Decapoda) and Homarus gammarus (Decapoda). Mar. Environ. Res. 57 (4), 281–294. doi: 10.1016/j.marenvres.2003.08.002
Båmstedt U., Wild B., Martinussen M. (2001). Significance of food type for growth of ephyrae Aurelia aurita (Scyphozoa). Mar. Biol. 139 (4), 641–650. doi: 10.1007/s002270100623
Baumsteiger J., O’Rear T. A., Cook J. D., Manfree A. D., Moyle P. B. (2018). Factors affecting distribution and abundance of jellyfish medusae in a temperate estuary: A multi-decadal study. Biol. Invasions 20 (1), 105–119. doi: 10.1007/s10530-017-1518-x
Berwald J. (2017). Spineless: The science of jellyfish and the art of growing a backbone Vol. 132 (New York: Riverhead Books), 304.
Condon R. H., Steinberg D. K., del Giorgio P. A., Bouvier T. C., Bronk D. A., Graham W. M., et al. (2011). Jellyfish blooms result in a major microbial respiratory sink of carbon in marine systems. Proc. Natl. Acad. Sci. 108, 10225–10230. doi: 10.1073/pnas.1015782108
Dawson M. N., Cieciel K., Decker M. B., Hays G. C., Lucas C. H., Pitt K. A. (2015). Population-level perspectives on global change: Genetic and demographic analyses indicate various scales, timing, and causes of scyphozoan jellyfish blooms. Biol. Invasions 17 (3), 851–867. doi: 10.1007/s10530-014-0732-z
Dawson M. N., Hamner W. M. (2009). A character-based analysis of the evolution of jellyfish blooms: Adaptation and exaptation. Hydrobiol. 616, 193–215. doi: 10.1007/978-1-4020-9749-2_13
Dong Z., Liu D., Keesing J. K. (2010). Jellyfish blooms in China: Dominant species, causes and consequences. Mar. pollut. Bull. 60 (7), 954–963. doi: 10.1016/j.marpolbul.2010.04.022
Dong Z., Liu D., Keesing J. K. (2014). “Contrasting trends in populations of rhopilema esculentum and aurelia aurita in Chinese waters,” in In jellyfish blooms (Dordrecht: Springer), 207–218.
Dong J., Sun M., Wang B., Liu H. (2008). Comparison of life cycles and morphology of Cyanea nozakii and other scyphozoans. Plankton Benthos Res. 3 (Supplement), 118–124. doi: 10.3800/pbr.3.118
Dong J., Wang B., Duan Y., Yoon W. D., Wang A., Liu X., et al. (2018). Initial occurrence, ontogenic distribution-shifts and advection of Nemopilema nomurai (Scyphozoa: Rhizostomeae) in liaodong bay, China, from 2005-2015. Mar. Ecol. Prog. Ser. 591, 185–197. doi: 10.3354/meps12272
Feng S., Lin J., Sun S., Zhang F., Li C., Xian W. (2020). Combined effects of seasonal warming and hyposalinity on strobilation of Nemopilema nomurai polyps. J. Exp. Mar. Biol. Ecol. 524, 151316. doi: 10.1016/j.jembe.2020.151316
Feng S., Wang S. W., Sun S., Zhang F., Zhang G. T., Liu M. T., et al. (2018). Strobilation of three scyphozoans (Aurelia coelurea, Nemopilema nomurai, and Rhopilema esculentum) in the field at jiaozhou bay, China. Mar. Ecol. Prog. Ser. 591, 141–153. doi: 10.3354/meps12276
Feng S., Wang S. W., Zhang G. T., Sun S., Zhang F. (2017). Selective suppression of in situ proliferation of scyphozoan polyps by biofouling. Mar. pollut. Bull. 114 (2), 1046–1056. doi: 10.1016/j.marpolbul.2016.10.062
Goldstein J., Steiner U. K. (2020). Ecological drivers of jellyfish blooms–the complex life history of a ‘well-known’medusa (Aurelia aurita). J. Anim. Ecol. 89 (3), 910–920. doi: 10.1111/1365-2656.13147
Greve W. (1994). The 1989 German bight invasion of muggiaea atlantica. ICES J. Mar. Sci. 51, 355–358. doi: 10.1006/jmsc.1994.1037
Gutiérrez-Estrada J. C., Pulido-Calvo I., Peregrín A., García-Gálvez A., Báez J. C., Bellido J. J., et al. (2021). Integrating local environmental data and information from non-driven citizen science to estimate jellyfish abundance in Costa del sol (Southern Spain). Estuar. Coast. Shelf. Sci. 249, 107112. doi: 10.1016/j.ecss.2020.107112
Hamner W., Gilmer R., Hamner P. (1982). The physical, chemical, and biological characteristics of a stratified, saline, sulfide lake in Palau1. Limnol. Oceanogr. 27, 896–909. doi: 10.4319/lo.1982.27.5.0896
Hansson L. J., Moeslund O., Kiørboe T., Riisgård H. U. (2005). Clearance rates of jellyfish and their potential predation impact on zooplankton and fish larvae in a neritic ecosystem (Limfjorden, Denmark). Mar. Ecol. Prog. Ser. 304, 117—131. doi: 10.3354/meps304117
Han C. H., Uye ,. (2010). Combined effects of food supply and temperature on asexual reproduction and somatic growth of polyps of the common jellyfish Aurelia aurita s.l Plankton. Benthos. Res. 5, 98–105. doi: 10.3800/pbr.5.98
Hernroth L., Gröndahl F. (1985). On the biology of Aurelia aurlta (L.): 2. major factors regulating the occurrence of ephyrae and young medusae in the gullmar fjord, western Sweden. B. Mar. Sci. 37, 567–576.
Ishii H., Kojima S., Tanaka Y. (2004). Survivorship and production of Aurelia aurita ephyrae in the innermost part of Tokyo bay, Japan. Plankton Biol. Ecol. 51 (1), 26–35.
Ishii H., Takagi A. (2003). Development time of planula larvae on the oral arms of the scyphomedusa Aurelia aurita. J. Plankton. Res. 25, 1447–1450. doi: 10.1093/plankt/fbg094
Kawahara M., Uye S. I., Ohtsu K., Iizumi H. (2006). Unusual population explosion of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in East Asian waters. Mar. Ecol. Prog. Ser. 307, 161–173. doi: 10.3354/meps307161
Kitajima S., Hasegawa T., Nishiuchi K., Kiyomoto Y., Taneda T., Yamada H. (2020). Temporal fluctuations in abundance and size of the giant jellyfish Nemopilema nomurai medusae in the northern East China Sea 2006–2017. Mar. Biol. 167 (6), 1–10. doi: 10.1007/s00227-020-03682-1
Kroiher M., Siefker B., Berking S. (2000). Induction of segmentation in polyps of Aurelia aurita (Scyphozoa, cnidaria) into medusae and formation of mirror-image medusa anlagen. Int. J. Dev. Biol. 44 (5), 485–490.
Lee S. H., Hwang J. S., Kim K. Y., Molinero J. C. (2021). Contrasting effects of regional and local climate on the interannual variability and phenology of the scyphozoan, aurelia coerulea and nemopilema nomurai in the Korean peninsula. Diversity 13 (5), 214. doi: 10.3390/d13050214
Lee P. W., Tseng L. C., Hwang J. S. (2018). Comparison of mesozooplankton mortality impacted by the cooling systems of two nuclear power plants at the northern Taiwan coast, southern East China Sea. Mar. pollut. Bull. 136, 114–124. doi: 10.1016/j.marpolbul.2018.09.003
Li X. Y., Li B., Sun X. L. (2014). Effects of a coastal power plant thermal discharge on phytoplankton community structure in zhanjiang bay, China. Mar. pollut. Bull. 81, 210–217. doi: 10.1016/j.marpolbul.2013.08.006
Lucas C. H., Dawson M. N. (2014). “What are jellyfishes and thalia-ceans and why do they bloom?,” in Jellyfish blooms. Eds. Pitt I. K. A., Lucas C. H. (Dordrecht, The Netherlands: Springer), 9–44.
Lucas C. H., Pitt K. A., Purcell J. E., Lebrato M., Condon R. H. (2011). What’s in a jellyfish? proximate and elemental composition and biometric relationships for use in biogeochemical studies. Ecol. 92, 1704. doi: 10.1890/11-0302.1
Masilamoni J. G., Jesudoss K. S., Nandakumar K., Satpathy K. K., Nair K. V. K., Azariah J. (2000). Jellyfish ingress: a threat to the smooth operation of coastal power plants. Curr. Sci. 79, 567–569.
Mills C. E. (1995). Medusae, siphonophores, and ctenophores as planktivorous predators in changing global ecosystems. ICES J. Mar. Sci.: J. Conseil 52, 575–581. doi: 10.1016/1054-3139(95)80072-7
Mills C. E. (2001). Jellyfish blooms: Are populations increasing globally in response to changing ocean conditions? Hydrobiologia 451, 55–68. doi: 10.1023/A:1011888006302
Moon J. H., Pang I. C., Yang J. Y., Yoon W. D. (2010). Behavior of the giant jellyfish Nemopilema nomurai in the East China Sea and East/Japan Sea during the summer of 2005: A numerical model approach using a particle-tracking experiment. J. Marine. Syst. 80 (1-2), 101–114. doi: 10.1016/j.jmarsys.2009.10.015
Olesen N. J., Riisgard H. U. (1994). Population dynamic, growth and energetics of jellyfish, Aurelia aurita, in a shallow fjord. Mar. Ecol. Prog. Ser. 105, 9–18. doi: 10.3354/meps105009
Papathanassiou E., Panayotidis P., Anagnostaki K. (1987). Notes on the biology and ecology of the jellyfish Aurelia aurita lam. in elefsis bay (Saronikos gulf, Greece). Mar. Ecol. 8, 49–58. doi: 10.1111/j.1439-0485.1987.tb00174.x
Pitt K. A., Duarte C. M., Lucas C. H., Sutherland K. R., Condon R. H., Mianzan H., et al. (2013). Jellyfish body plans provide allometric advantages beyond low carbon content. PloS One 8 (8), e72683. doi: 10.1371/journal.pone.0072683
Pitt K. A., Welsh D. T., Condon R. H. (2009). Influence of jellyfish blooms on carbon, nitrogen and phosphorus cycling and plankton production. Jellyfish Blooms: Causes Consequences Recent Adv. 616, 133–149. doi: 10.1007/s10750-008-9584-9
Prieto L., Astorga D., Navarro G., Ruiz J. (2010). Environmental control of phase transition and polyp survival of a massive-outbreaker jellyfish. PloS One 5, e13793. doi: 10.1371/journal.pone.0013793
Purcell J. E. (2012). Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Annu. Rev. Mar. Sci. 4, 209–235. doi: 10.1146/annurev-marine-120709-142751
Purcell J. E., Hoover R. A., Schwarck N. T. (2009). Interannual variation of strobilation by the scyphozoan Aurelia labiata in relation to polyp density, temperature, salinity, and light conditions in situ. Mar. Ecol. Prog. Ser. 375, 139–149. doi: 10.3354/MEPS07785
Purcell J. E., Uye S. I., Lo W. T. (2007). Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Mar. Ecol. Prog. Ser. 350, 153–174. doi: 10.3354/meps07093
Quinones J., Chiaverano L. M., Ayon P. (2018). Spatial patterns of large jellyfish chrysaora plocamia blooms in the northern Humboldt upwelling system in relation to biological drivers and climate. ICES J. Mar. Sci. 75, 1405–1415. doi: 10.1093/icesjms/fsy004
Rekstad M. E., Majaneva S., Borgersen Å.L., Aberle N. (2021). Occurrence and habitat characteristics of Aurelia sp. polyps in a high-latitude fjord. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.68463
Richardson A. J., Bakun A., Hays G. C., Gibbons M. J. (2009). The jellyfish joyride: Causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 24 (6), 312–322. doi: 10.1016/j.tree.2009.01.010
Richardson A. J., Gibbons M. J. (2008). Are jellyfish increasing in response to ocean acidification? Limnol. Oceanogr. 53, 2035–2040. doi: 10.4319/lo.2008.53.5.2040
Riyas A., Dahanukar N., Krishnan K. A., Kumar A. B. (2021). Scyphozoan jellyfish blooms and their relationship with environmental factors along the south-eastern Arabian Sea. Mar. Biol. Res. 17 (2), 185–199. doi: 10.1080/17451000.2021.1916034
Russell F. S. (1970). The medusae of the British isles volume II: Pelagic scyphozoa, with a supplement to the first volume of hydromedusae. (London: Cambridge University Press).
Rutherford L. D. Jr., Thuesen E. V. (2005). Metabolic performance and survival of medusae in estuarine hypoxia. Mar. Ecol. Prog. Ser. 294, 189–200. doi: 10.3354/meps294189
Salgueiro D. V., De Pablo H., Nevesa R., Mateus M. (2015). Modelling the thermal effluent of a near coast power plant (Sines, portugal). revista de gestão costeira integrada. J. Integrated Coast. Zone Manage. 15 (4), 533–544. doi: 10.5894/rgci577
Schiariti A., Melica V., Kogovšek T., Malej A. (2015). Density-dependent effects control the reproductive strategy and population growth of Aurelia aurita sl scyphistomae. Mar. Biol. 162 (8), 1665–1672. doi: 10.1007/s00227-015-2704-y
Schneider G. (1992). A comparison of carbon-specific respiration rates in gelatinous and non-gelatinous zooplankton: A search for general rules in zooplankton metabolism. Helgolä nder Meeresuntersuchungen 46, 377–388. doi: 10.1007/BF02367205
Schrope M. (2012). Marine ecology: Attack of the blobs. Nature 482 (7383), 20–21. doi: 10.1038/482020a
Sun S., Li Y., Sun X. (2012). Changes in the small-jellyfish community in recent decades in jiaozhou bay, China. Chin. J. Oceanol. Limnol. 30, 507–518. doi: 10.1007/s00343-012-1179-7
Sun S., Zhang F., Li C., Wang S., Wanh M., Tao Z., et al. (2015). Breeding places, population dynamics, and distribution of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizo - stomeae) in the yellow Sea and the East China Sea. Hydrobiologia 754, 59–74. doi: 10.1007/s10750-015-2266-5
Takizawa M. (2005). Countermeasures for jellyfish attacks at kashiwazaki kariwa [Japan] nuclear power station. Bull. Plankton Soc. Japan 52 (1), 36–38.
Tills O., Sun X., Rundle S. D., Heimbach T., Gibson T., Cartwright A., et al. (2016). Reduced pH affects pulsing behaviour and body size in ephyrae of the moon jellyfish, aurelia aurita. J. Exp. Mar. Biol. Ecol. 480, 54–61. doi: 10.1016/j.jembe.2016.03.014
Wang N., Li C., Liang Y., Shi Y., Lu J. (2014). Prey concentration and temperature effect on budding and strobilation of Aurelia sp. 1 polyps. Hydrobiologia 754, 125–134. doi: 10.1007/s10750-014-1978-2
Wang B., Qi Y. B., Dong J., Li Y. L., Wang W. B., Li Y. P., et al. (2013). Dynamic distribution of nemopilema nomurai in inshore waters of the northern liaodong bay, bohai Sea. Acta Ecol. Sin. 33 (6), 1701–1712. doi: 10.5846/stxb201112081878
Wang Y. T., Sun S. (2015). Population dynamics of Aurelia sp. 1 ephyrae and medusae in jiaozhou bay, China. Hydrobiologia 754 (1), 147–155. doi: 10.1007/s10750-014-2021-3
Wang J., Wang N., Wang Y., Wang X., Li C. (2021). Food composition of common jellyfish species in hongyanhe area revealed by fatty acid biomarkers and stable carbon and nitrogen isotopes. Acta Oceanol. Sin. 52 (1), 132–143. doi: 10.11693/hyhz20200500135
Wang S., Zhang G., Sun S., Wang Y., Zhao Z. (2012). Population dynamics of three scyphozoan jellyfish species during summer of 2011 in jiaozhou bay. Oceanol. ET. Limnol. Sin. 43, 471–479.
Winans A. K., Purcell J. E. (2010). Effects of pH on asexual reproduction and statolith formation of the scyphozoan, Aurelia labiata. Hydrobiologia 645 (1), 39–52. doi: 10.1007/s10750-010-0224-9
Yoon W., Chae J., Koh. B. S., Han. C. (2018). Polyp removal of a bloom forming jellyfish, Aurelia coerulea, in Korean waters and its value evaluation. Ocean. Sci. 53 (3), 499–507. doi: 10.1007/s12601-018-0015-1
Yoon W. D., Lee H. E., Han C., Chang S. J., Lee K. (2014). Abundance and distribution of nemopilema nomurai (Scyphozoa, rhizostomeae), in Korean waters in 2005–2013. Ocean. Sci. J. 49 (3), 183–192. doi: 10.1007/s12601-014-0018-5
Yoon W. D., Yang J. Y., Shim M. B., Kang H. K. (2008). Physical processes influencing the occurrence of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) around jeju island, Korea. J. Plankton. Res. 30, 251–260. doi: 10.1093/plankt/fbm102
Zhang F., Sun S., Jin X., Li C. (2012). “Associations of large jellyfish distributions with temperature and salinity in the Yellow Sea and East China Sea,” In: Purcell J., Mianzan H., Frost J. R. Jellyfish Blooms IV. Developments in Hydrobiology (Dordrecht: Springer), vol. 220. doi: 10.1007/978-94-007-5316-7_7
Keywords: Liaodong Bay, Nemopilema nomurai, jellyfish bloom, nuclear power cooling-water system, Aurelia coerulea, disaster-causing jellyfish
Citation: Wang X, Jin Q, Yang L, Jia C, Guan C, Wang H and Guo H (2023) Aggregation process of two disaster-causing jellyfish species, Nemopilema nomurai and Aurelia coerulea, at the intake area of a nuclear power cooling-water system in Eastern Liaodong Bay, China. Front. Mar. Sci. 9:1098232. doi: 10.3389/fmars.2022.1098232
Received: 14 November 2022; Accepted: 08 December 2022;
Published: 06 January 2023.
Edited by:
Kaizhi Li, South China Sea Institute of Oceanology (CAS), ChinaCopyright © 2023 Wang, Jin, Yang, Jia, Guan, Wang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaocheng Wang, eGN3YW5nQG5tZW1jLm9yZy5jbg==; Chunjiang Guan, Y2pndWFuQG5tZW1jLm9yZy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.