- 1Department of Agricultural Development, Agrofood and Management of Natural Resources, National and Kapodistrian University of Athens, Psachna, Greece
- 2Department of Biology, Marine Ecology Laboratory, University of Crete, Heraklion, Greece
- 3Institute of Oceanography, Hellenic Centre for Marine Research (HCMR), Heraklion, Greece
In addition to food supply, there is a growing recognition of the wider ecosystem benefits of Integrated Multitrophic Aquaculture (IMTA) systems in coastal waters, including regulating services such as carbon sequestration and nutrient remediation. The water trophic status and the co-cultured species combinations affect IMTA productivity. In this study, we examined the ability of different combinations of IMTA organisms to remediate nutrients and the economic/environmental gain for reducing the environmental footprint in potential IMTA systems of the eastern Mediterranean. The results showed that the co-cultivation of organisms can reduce the negative effects on the marine environment of a fish farm both on the water column and the sediment. Meso- and eutrophic water states do not show a high variation in terms of foot print mitigation, with all three of the co-cultivated organisms to perform well. In oligotrophic waters, the obligatory absence of mussels reduces the effectiveness of the IMTA system. As expected, larger-sized IMTA systems have higher production rates and as a result higher percentage of nutrient removal. Finally, bivalve harvesting helps to remove the carbon that is trapped in their shells, contributing to the mitigation of processes related to climate change, such as the acidification of the oceans.
Introduction
Aquaculture is one of the fastest growing food production sectors in recent years and plays a significant role in securing nutritious diets, contributing to 52% of the world supply of aquatic animal-source foods (FAO, 2000) and is expected to cover the food deficit for the human population in this century (Duarte et al., 2009). However, the expansion of aquaculture has caused several environmental and socio-economic issues that influence the sustainability of the sector and could compromise its further development (Massa et al., 2017). The most documented impact of aquaculture on the environment is organic enrichment of the water and sediment. The release of dissolved nutrients (i.e. branchial and urinary losses) and particulate organic matter i.e. uneaten feed and feces to the marine environment can cause adverse biological and geochemical changes and affect the ecosystem services (Kalantzi and Karakassis, 2006; Pitta et al., 2009; Papageorgiou et al., 2010). To this end, integrated management practices (FAO, 2016) are required to mitigate environmental impacts in order to secure sustainable development for the aquaculture industry. Sustainable aquaculture is a dynamic concept integrating three main principles: i.e. it must be economically profitable, environmentally friendly, and socially equitable (FAO, 2017). The concept of IMTA has been proposed to help mitigating the environmental impact of marine fish farming and promote sustainability (Chopin et al., 1999; Neori et al., 2004; Troell et al., 2009).
In IMTA systems extractive (non-fed) aquaculture species (e.g. autotrophs, filter and deposit feeders) are integrated with fed species, so that the wastes of fed species become a nutrient source for extractive ones (Troell et al., 2009; Chopin et al., 2012). In addition to the recycling of waste nutrients there is a harvestable biomass generated that increase the overall productivity of the system and the profit of the farmers (Chopin et al., 2012; Wartenberg et al., 2017). IMTA approach fits well within the global ambition for a reduced “ecological footprint” and circularity in food production and is in agreement with the EU directions for Blue Growth and Blue Economy. IMTA systems can be complex, diverse and affected by multiple drivers. Trophic status of the water column is an important variable for IMTA feasibility (Cranford et al., 2013; Sanz-Lazaro et al., 2018). In addition, optimal species combinations in regard to environmental variables can maximize the production and reduce the environmental impacts of aquaculture.
The concept of IMTA has been used for hundreds of years in Asian countries which now promote IMTA concepts, followed by Canada and North European countries (Chopin et al., 2012). On the contrary, Europe and Mediterranean-bordering countries, have been latecomers to the IMTA concept (Kleitou et al., 2018), despite the fact that the industry is highly developed in those areas (Papageorgiou et al., 2021). This article is part of a project aiming to investigate the potential for using IMTA as a means for reducing environmental impacts of mariculture while increasing the revenues of mariculture farms in the oligotrophic Eastern Mediterranean environment. The objectives of this study were to define: (a) the ability of co-cultivated species to remediate the nutrients released by fish farming, (b) the optimal combination of co-cultivating organisms for efficient utilization of the released nutrients, (c) the ecosystem and economic importance of reducing the environmental footprint of a fish farm through the co-cultivation of other species.
Methods
The analyses presented in this study are divided in two parts. The first part is a desk study where literature data were used to calculate the expected quantities of nitrogen (N) and phosphorus (P) released from a fish farm and then the amount retrieved by harvesting co-cultivated IMTA species. The pilot polyculture systems include aside from fish (sea bream, sea bass), Mediterranean mussels (Mytilus galloprovincialis), pearl oysters (Pinctada imbricata radiata) and holothurians (Holothuria polii).
The calculation of the nutrients (nitrogen, phosphorus) input to the marine ecosystem was calculated for a theoretical fish farm with an annual production of 100 tons. In the calculations it was assumed that the supplied fish feed had a content of 7% nitrogen (N) and 1.4% phosphorus (P), based on the nutrient content of the standard commercial fish feed used in the Greek market. Furthermore, it was assumed that the percentage of N and P in the fish feed that is retained in the harvested fish (seabass and sea bream) is 20% N and 30% P, while the remaining 80% and 70% respectively is released into the environment with their excrement. Soluble ammonia and urea released into the marine environment, represents the 63% of the consumed N and 20% of P, while the rest is in particulate form (fish feces) and will settle in the sediment. All the above ratios were extracted from scientific literature sources for salmonids, trout, seabass and seabream retention experiments and a mean value was calculated with special consideration to experimental studies on seabass and sea bream (Krom et al., 1985; Gowen and Bradbury, 1987; Porter et al., 1987; Folke, 1989; Holby and Hall, 1991; Walain and Hakason, 1991; Hall et al., 1992; Ballestrazzi et al., 1994; Krom et al., 1995; Dosdat et al., 1996; Kaushik, 1998; Lemarié et al., 1998; Lupatsch and Kissil, 1998; Lanari et al., 1999; Karakassis et al., 2005; Tsapakis et al., 2006; García García et al., 2019). It is important to mention that the fine part of the particulate material does not settle but remains suspended in the water column. The percentage of these “never-settling” solid fish wastes is approximately 7% for N and 8% for P (Lupatsch and Kissil, 1998; Tsapakis et al., 2006).
In order to calculate the removed quantities of N and P from the water column through bivalve harvesting, we made the assumption that these organisms use N and P bound in phytoplankton biomass. Sanz-Lazaro and Sanchez-Jerez (2017) found that mussels did not directly assimilate fish farming wastes and thus we considered that the increased dissolved nutrients in the water column near the fish farm induced an increase of the phytoplankton abundance that is then used by bivalves as their primary food source. A proportion, of the nutrients bound in phytoplankton are transferred to the bivalves through active filtration and are utilized for growth.
For the IMTA mussel cultivation exercise, the N, P values content in the harvested mussels were used as reported by Maar et al. (2020). These values are experimental but, as stated by the authors are in agreement with previous studies. In the present study, the lower reported values were used and then adjusted for the theoretical production of 100 t of mussels (1.31% N and 0.12% P). Respectively, averaged values (16.10 kg N/t, 0.61kg P/t) were used also for pearl oysters, as calculated in the literature review of Gifford et al. (2004); Gifford et al. (2005). The bivalve (mussels and oysters) nutrient assimilation capacity was set to 60% (Bouwman et al., 2011; Dabrowski et al., 2013), thus 40% of the nutrients consumed are re-introduced into the marine environment through the pseudo-feces of bivalves. For the sea cucumbers, the volume of N and P concentration in the harvested organisms, as well as the assimilation capacity of them was based on existing literature (Nelson et al., 2012; Hannah et al., 2013; Orr et al., 2014; Shpigel et al., 2018; Israel et al., 2019; Neofitou et al., 2019; Chary et al., 2020). The assimilation capacity for both N & P that can be converted to biomass was set to 0.5 and it was assumed that these organisms fed on the solid material released by a fish farm (100 t fish/y).
The second part estimates the ecosystem value (EV) provided by the IMTA systems by examining the potential of nutrient remediation by co-cultivated IMTA species in commercial Greek fish farms of different size and trophic status. The latter combines information derived from the theoretical exercise with polyculture performance data derived from the study of Chatzivasileiou et al. (2022). In that study pilot IMTA setups for Mediterranean mussels (Mytilus galloprovincialis), pearl oysters (Pinctada imbricata radiata) and holothurians (Holothuria polii) were developed and tested in three fish farms with different trophic status in the eastern Mediterranean. The trophic status was oligotrophic, low mesotrophic, high mesotrophic, based on Simboura et al. (2005). The survival, robustness, growth data and cultivation methodology for each species are presented in detail by Chatzivasileiou et al. (2022). In short, the mussel M. galloprovincialis was successfully cultivated from juvenile to commercial size in the two mesotrophic farms with a condition index of 33% and 42% respectively. The pearl oyster P. imbricata radiata was successfully cultivated from juvenile to commercial size in all locations with a condition index of 19% in oligotrophic, 56% in lower mesotrophic and 53% in higher mesotrophic waters. The H. polii although they had a high survival rate did not show any significant biomass gain. This was probably caused by the fact that for the cultivation were used adult individuals and not offspring. This compromise was made because reared juveniles could not be purchased and it was not possible to find juveniles in such large amounts in natural populations. Thus, the average mean weight of the natural population was used for the calculation 0,11 ± 0,03 kg/individual.To estimate the ecosystem value, we considered the economic value for the nutrient removal (N and P) from the environment through the remediation process of the IMTA organisms. In addition, we considered that the shell of the co-cultivated bivalves constitutes a net sink of carbon through bivalve harvesting, which is very important for mitigating the impacts of climate change and particularly ocean acidification. For removing nitrogen, the alternative cost set in the market was applied (Newell et al., 2005; Beseres Pollack et al., 2013; van der Schatte Olivier et al., 2020). According to it, it costs $ 6.20/kg to reduce nitrogen to 8mg/l, but $ 19.13/kg to reduce nitrogen to 3mg/l. Thus, the average value used in this study was 17290€ for the removal of 1 ton of nitrogen.
For phosphorus, there is no purchase price, and therefore a “shadow” price was used. According to Molinos-Senante et al. (2011) the estimated price of phosphorus remediation for which there is no purchase price is set at 13,118-58,561$/t. The mean value was estimated to 30950 euros for the removal of 1 ton of phosphorus (van der Schatte Olivier et al., 2020). According to Smaal et al. (2019), 95% of the shell of bivalves is composed of calcium carbonate, of which 12% is carbonate. In the calculations, we have considered the shell as a by-product that constitutes a net sink of carbon independent of the CO2 released during the biocalcification and respiration. The equivalent weight of CO2 has been calculated (https://www.epa.gov/energy/greenhouse-gas-equivalencies-calculator) from the shell weight of the total production according to the data from Chatzivasileiou et al. (2022). The economic value of carbon removal depends to a large extent on the price range of removing 1 ton of CO2 (Smaal et al., 2019) with an average price of 24$ (21.5€) per ton of CO2 (average price for Denmark, France, the United Kingdom, British Columbia and Ireland, - (World Bank, 2016; Smaal et al., 2019).
The final calculation of the EV and consequently the reduction of the environmental footprint by IMTA co-cultivated organisms was performed for three commercial Greek fish farms of different size and ambient water trophic status. Three scenarios were examined:(a) small fish farm with leased area of 1ha and fish production of 250t/y; (b) medium fish farm (4 ha leased area) with annual fish production 800 t and (c) large fish farm (leased area 8 ha) and annual production 1600 t fish/year. The first scenario is representative for a standard small business fish farm of Greece, while scenarios (b) and (c) involve larger businesses that lease extended areas with multiple parks.
For each scenario, an oligotrophic, low mesotrophic and a high mesotrophic area are considered as aquaculture sites with different growth rates for the co-cultivated organisms, supported by the data provided by Chatzivasileiou et al. (2022). In addition, IMTA systems were designed within the boundaries of the already leased areas of the farms in order not to be financially burdened with additional costs (e.g. leasing, vessels etc.) other than the expenses of the farming equipment. Of course, this assumption sets a space limit to the production of the co-cultivated organisms according to the aquaculture area leased. Regarding the calculation of the volume of nitrogen (N) and phosphorus (P) released from the fish farming units, the assumption was made that in all scenarios and areas the fish Food Conversion Ratio (FCR) was 1.8.
For the first part of analyses, no statistical package was used since all data applied in the calculations were extracted from published articles with comprehensive statistical analyses performed. For the second part the statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS), (University of Crete, Biology Department, Heraklion, Greece) program. The growth of bivalves (condition index) and survival of Holothurians at the three locations was confirmed by comparing monthly data with one-way ANOVA at p < 0.05 statistical significance after variables were checked for assumptions of normality (Shapiro–Wilk test) and homogeneity of variance (Levene’s test). All mean and standard deviation values were calculated with SPSS from raw data of the study described in Chatzivasileiou et al. (2022). In addition, a residual analysis was performed at the morphometric raw data (weight of bivalves and shells) and a Normal distribution of the residuals was examined by the Normal Probability Plot of Residuals (residuals vs normal expected values). Identified residuals were checked by Mahalanobis distance, and Cook’s distance and occasional outliers were removed.
Results
The values of N and P released for a fish farm with an annual production of 100 t and an FCR of 1.8 reach up to 7358 kg of N (7056 kg dissolved and 302 kg solid) and 1250 kg of P (524 dissolved and 726 solid) annually.
For the IMTA bivalves nutrient remediation, the calculation exercise (Figure 1) showed that near a fish farm with a production of 100 t/y, the co-cultivation with 100 t/y of mussels can remove from the system 19% of nitrogen and 22% of phosphorus derived from the secretions of fish. Respectively, the co-cultivation of fish and 10 t/y of pearl oysters removes 2% N and 1% P. Finally, the annual co-cultivation of 10 t/y sea cucumbers can absorb the 4% particulate N and 2% particulate P derived from the fish excretions.
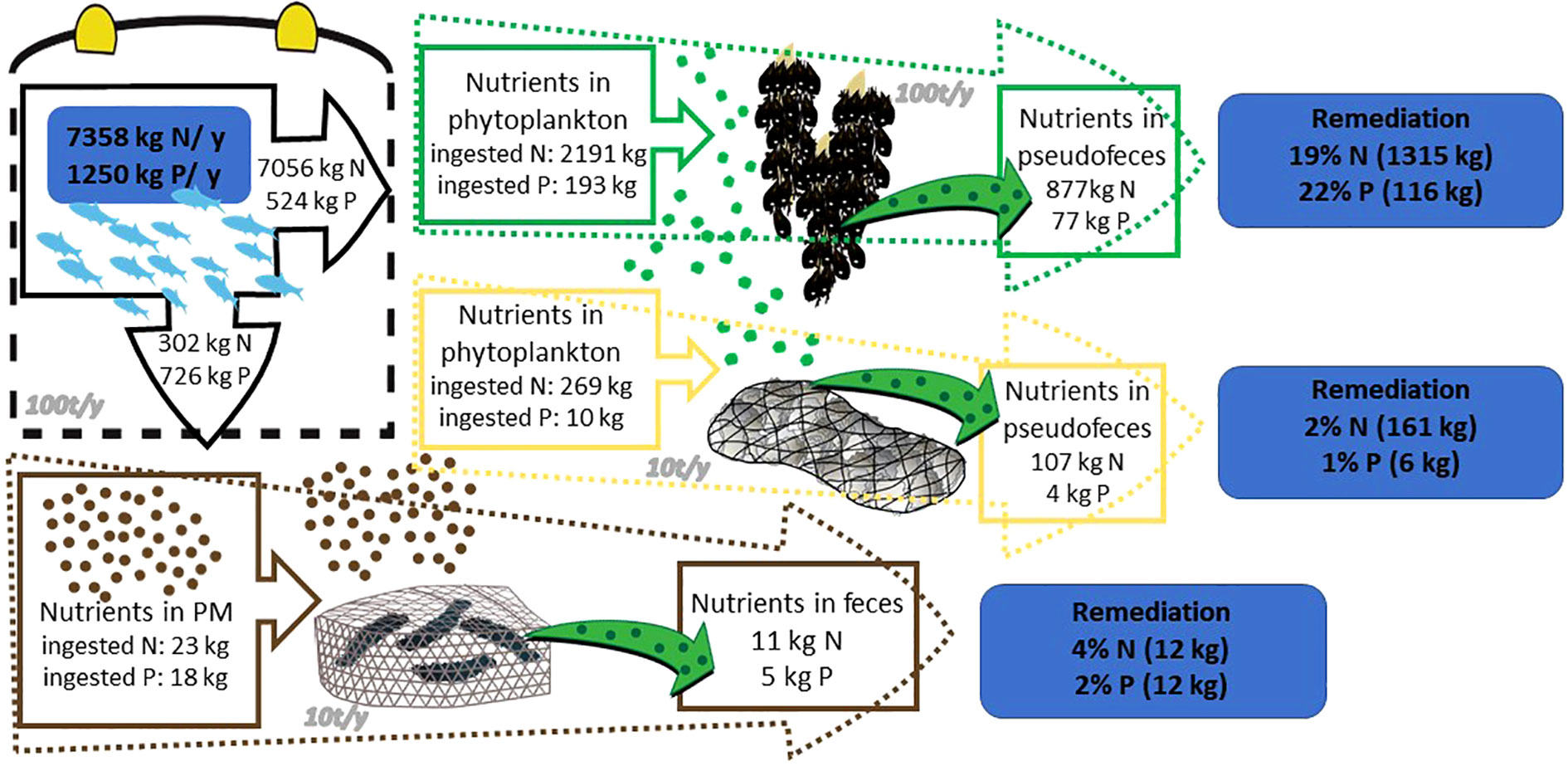
Figure 1 Remediation of dissolved nutrients from a fish farm with the help of mussel co-culture (green text box); pearl oyster co-culture (yellow text box) and sea cucumber co-culture (brown text box). Symbols provided by Integration and Application Network (ian.umces.edu/media-library).
The estimated remediation data of the IMTA systems for different trophic states and fish farm sizes are presented in Table 1. For all scenarios, the high mesotrophic sites show the highest volume of nitrogen and phosphorus remediated followed closely by the low mesotrophic sites. The much lower numbers in the oligotrophic sites are explained by the lower production numbers as long as the absence of the mussels as a third IMTA organism.
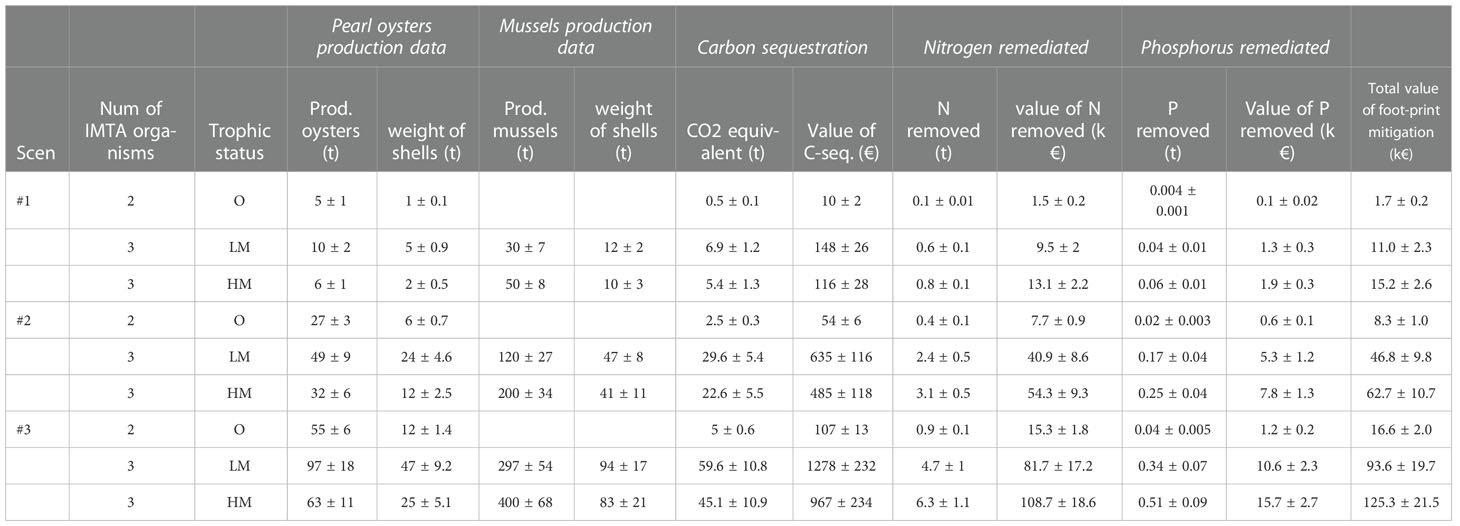
Table 1 Estimation of the footprint mitigation from the application of IMTA systems for three different fish farm sizes (Scenario 1: small fish farm - 250t/y; Scenario 2: medium fish farm - 800t/y; Scenario 3: large fish farm - 1600t/y) and different trophic status (O, oligotrophic; LM, low mesotrophic; HM, high mesotrophic).
Furthermore, the CO2 equivalent value deriving from the volume of sequestrated carbon bound in the cultivated bivalve follows the same patterns.
The ecosystem value of the removal of the nutrients and the carbon sequestration has been converted to economic values and reaches more than50 k€ for the mesotrophic area and around 100k€ for eutrophic areas at scenario 2 and 3, while the lowest values reach just 1.7 ± 0.2 k€ in the oligotrophic site of scenario 1. Comparing the two types of bivalves regarding their efficiency in removing dissolved nutrients from the water column, it can be concluded that 1 g of mussels biomass can remove 1.3 10-2 gr N and 1.2 10-3 gr P. On the other hand, equal biomass of pearl oysters can remove 1.6 10-2 gr N and 0.61 10-3 gr P
Discussion
IMTA can successfully reduce the organic load of fish farms in all three locations and produce profit even in oligotrophic conditions. In conclusion, pearl oysters absorb larger amounts of N, while mussels are more effective at removing P. Holothurians absorb lower quantities of N since for 1 g of biomass they can remove 0.12 10-2 gr N, but they seem to be more efficient in absorbing P (1.2 10-3 gr P for 1 g biomass). In general, their role is important since they exploit the particulate part of the fish farm effluents up to 4% for N and 2% for P. Studies on feeding holothurians with particulate wastes deposited beneath fish cages have shown a reduction in the total organic load released, suggesting that they may play an important role towards a sustainable development of fish farming in the Mediterranean (Neofitou et al., 2019). In addition, the presence of holothurians around fish cages may improve water and sediment quality and consequently affect the benthic community as well as the immune system of the fish (Tolon et al., 2017).
Concerning the IMTA application in different farm areas (scenarios 1, 2, 3), as expected the large IMTA-system (scenario 3) showed the highest percentage of nitrogen and phosphorus removal and the top amount of carbon sequestration. The fact that the area of the farm is much larger than in the other two scenarios gives the advantage of more available space in the leased area to achieve a greater production of co-cultured organisms (sea cucumbers, Mediterranean mussels, pearl oysters). Of course, because fish production is increased in this area, the nutrient removal rates do not differ much from the other areas since the nutrients released from the farm are just as high.
The IMTA systems of examined trophic states do not show important differences between the meso- and more eutrophic areas. On the contrary, the much lower remediation values in the oligotrophic area highlight the absence of mussels of this IMTA system. As an important filter-feeding organism, mussels that can be cultivated in large quantities near the fish cages affect greatly the rate of the nutrient reduction. This is in agreement with studies that showed blue mussel (Mytilus edulis) to be very efficient in compensating fish farming nutrients, acting as bio-filters (Holdt and Edwards, 2014; Maar et al., 2020).
It is also worth noting that in the case of the IMTA systems with only 2 co-cultivated organisms the absence of mussels does not induce a significant reduction in CO2 capture (especially in larger fish farming area), which is justified by the larger and heavier shell of pearl oysters. Therefore, the cultivation of pearl oysters has a significant environmental value associated with the mitigation of acidification of the sea. Accordingly, Higgins et al. (2011) suggest a higher rate of carbon sequestration in oyster beds than in other ecosystem types. However, further work is required to estimate the true potential of shellfish as a reservoir of CO2 (Smaal et al., 2019; van der Schatte Olivier et al., 2020).
The potential economic gain for the mitigation of the fish farming footprint has an important economic value only for larger farms (13000 € at maximum). For smaller farms the costs for establishing and running such IMTA systems is much higher than the potential profit for the mitigation value and the actual net profit of the additional IMTA products. According to (Theodorou et al., 2014), the mussel farm size is critical to the financial viability of the producer, because profitability is very limited for smaller farms (i.e. less than 3 ha).
Conclusions
Regarding the environmental value of IMTA, the results showed that the co-cultivation of organisms could reduce the negative effects of a fish farm on the marine environment. Meso- and eutrophic water states do not show a high variation in terms of footprint mitigation, with all three of the co-cultivated organisms to perform well. Mussels cannot grow in oligotrophic waters and thus their absence reduces the effectiveness of the IMTA system. Larger-sized IMTA systems have higher production rates for all cultured organisms and as a result higher percentages of nutrient removal. But also, a medium sized IMTA farm including the optimum number of three co-cultivated organisms can perform well and yield better profit as biomass and ecosystem services.
The assimilation of nutrients (N and P) released by the fish farm through the nutrition and growth of co-cultured organisms, mitigates the organic enrichment around the fish cages of both the column and the sediment. Part of the particulate matter released by the fish farms it can be consumed by the cultivated sea cucumbers, thus reducing the effects on the benthic system near the fish cages. In the water column, the dissolved nutrients are utilized and increase phytoplankton biomass which is then consumed by the co-cultivated bivalves, reducing the concentration of nutrients, and preventing the overgrowth of phytoplankton. The latter is very important as the existence of harmful algal blooms (HABS) is a growing problem that seems to be linked to climate change. At the same time, the bivalve harvesting helps to remove the carbon that is trapped in their shells, contributing to the mitigation of processes related to climate change, such as the acidification of the oceans.
From the above it can be concluded that the co-cultivation of organisms has a significant environmental value for the marine ecosystem in the naturally oligotrophic waters of the Eastern Mediterranean, but in order to convince fish farmers to invest in IMTA cultures there is a need for an additional economic profit. In Greece there is no refund system for fish farming footprint mitigation thus a reciprocal fee paid by the state or indirectly as an allowed increase in fish production could motivate the farmers to invest in IMTA.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: NP, PD, MT, and IK; methodology: NP, PD, and IK; field data analysis: DC formal analysis: NP; funding acquisition: MT and IK; writing—original draft preparation: NP; writing—review and editing: DC, PD, MT, and IK; All authors have read and agreed to the published version of the manuscript.
Funding
The present work is part of the project “Innovative Development of Multitrophic Aquaculture”, implemented in the context of the “Innovation in Fisheries” EU-Greece Operational Program of Fisheries and Maritime, EPAL 2014–2020 co-funded by Greece and EU (European Maritime, Fisheries and Aquaculture Fund - EMFAF) (grant number 5029294).
Acknowledgments
We thank the CO and personnel of the three fish farms, which officially participated in the IDMA project for providing facilities and equipment for the implementation of the project. We also thank Prof. J. Theodorou and the IDMA-project team members for assisting during the samplings.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ballestrazzi R., Lanari D., D'agaro E., Mion A. (1994). The effect of dietary protein level and source on growth, body composition, total ammonia and reactive phosphate excretion of growing sea bass (Dicentrarchus labrax). Aquaculture 127 (2-3), 197–206. doi: 10.1016/0044-8486(94)90426-X
Beseres Pollack J., Yoskowitz D., Kim H.-C., Montagna P. A. (2013). Role and value of nitrogen regulation provided by oysters (Crassostrea virginica) in the mission-aransas estuary, Texas, USA. PloS One 8 (6), e65314. doi: 10.1371/journal.pone.0065314
Bouwman A. F., Pawłowski M., Liu C., Beusen A. H. W., Shumway S. E., Glibert P. M., et al. (2011). Global hindcasts and future projections of coastal nitrogen and phosphorus loads due to shellfish and seaweed aquaculture. Rev. Fish. Sci. 19 (4), 331–357. doi: 10.1080/10641262.2011.603849
Chary K., Aubin J., Sadoul B., Fiandrino A., Covès D., Callier M. D. (2020). ). integrated multi-trophic aquaculture of red drum (Sciaenops ocellatus) and sea cucumber (Holothuria scabra): Assessing bioremediation and life-cycle impacts. Aquaculture 516, 734621. doi: 10.1016/j.aquaculture.2019.734621
Chatzivasileiou D., Dimitriou P. D., Theodorou J., Kalantzi I., Magiopoulos I., Papageorgiou N., et al. (2022). An IMTA in Greece: Co-culture of fish, bivalves, and holothurians. J. Mar. Sci. Eng. 10 (6), 776. doi: 10.3390/jmse10060776
Chopin T., Cooper J. A., Reid G., Cross S., Moore C. (2012). Open-water integrated multi-trophic aquaculture: environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture. Rev. Aquacult. 4 (4), 209–220. doi: 10.1111/j.1753-5131.2012.01074.x
Chopin T., Yarish C., Wilkes R., Belyea E., Lu S., Mathieson A. (1999). Developing porphyra/salmon integrated aquaculture for bioremediation and diversification of the aquaculture industry. J. Appl. Phycol. 11 (5), 463–472. doi: 10.1023/A:1008114112852
Cranford P. J., Reid G. K., Robinson S. M. (2013). Open water integrated multi-trophic aquaculture: constraints on the effectiveness of mussels as an organic extractive component. Aquacult. Environ. Interact. 4 (2), 163–173. doi: 10.3354/aei00081
Dabrowski T., Lyons K., Curé M., Berry A., Nolan G. (2013). Numerical modelling of spatio-temporal variability of growth of mytilus edulis (L.) and influence of its cultivation on ecosystem functioning. J. Sea Res. 76, 5–21. doi: 10.1016/j.seares.2012.10.012
Dosdat A., Servais F., Metailler R., Huelvan C., Desbruyeres E. (1996). Comparison of nitrogenous losses in five teleost fish species. Aquaculture 141 (1-2), 107–127. doi: 10.1016/0044-8486(95)01209-5
Duarte C. M., Holmer M., Olsen Y., Soto D., Marbà N., Guiu J., et al. (2009). Will the oceans help feed humanity? BioScience 59 (11), 967–976. doi: 10.1525/bio.2009.59.11.8
FAO (2016). The state of world fisheries and aquaculture 2016. Contributing to Food Secur. Nutr. all. Rome.
FAO (2017). Aquaculture development. 7. Aquacult. governance sector Dev. FAO Technical Guidelines for Responsible Fisheries N. 5 Suppl. 7. Rome, Italy, 50pp.
Folke C. (1989). The role of ecosystems for a sustainable development of aquaculture. Ambio 18, 234–243.
García García B., Rosique Jiménez C., Aguado-Giménez F., García García J. (2019). Life cycle assessment of seabass (Dicentrarchus labrax) produced in offshore fish farms: Variability and multiple regression analysis. Sustainability 11 (13), 3523. doi: 10.3390/su11133523
Gifford S., Dunstan R. H., O'Connor W., Roberts T., Toia R. (2004). Pearl aquaculture–profitable environmental remediation? Sci. Total Environ. 319 (1), 27–37. doi: 10.1016/S0048-9697(03)00437-6
Gifford S., Dunstan H., O’Connor W., Macfarlane G. R. (2005). Quantification of in situ nutrient and heavy metal remediation by a small pearl oyster (Pinctada imbricata) farm at port stephens, Australia. Mar. pollut. Bull. 50 (4), 417–422. doi: 10.1016/j.marpolbul.2004.11.024
Gowen R., Bradbury N. (1987). The ecological impact of salmonid farming in coastal waters: a review. Oceanogr. Mar. Biol. 25, 563–575. doi: 10.1016/0198-0254(88)92716-1
Hall P. O. J., Holby O., Kollberg S., Samuelson M. J. (1992). Chemical fluxes and mass balances in a marine fish cage farm. IV. nitrogen. Mar. Ecol. Prog. Ser. 89, 81–91. doi: 10.3354/meps089081
Hannah L., Pearce C. M., Cross S. F. (2013). Growth and survival of California sea cucumbers (Parastichopus californicus) cultivated with sablefish (Anoplopoma fimbria) at an integrated multi-trophic aquaculture site. Aquaculture 406-407, 34–42. doi: 10.1016/j.aquaculture.2013.04.022
Higgins C. B., Stephenson K., Brown B. L. (2011). Nutrient bioassimilation capacity of aquacultured oysters: quantification of an ecosystem service. J. Environ. Qual. 40 (1), 271–277. doi: 10.2134/jeq2010.0203
Holby O., Hall P. O. J. (1991). Chemical fluxes and mass balances in a marine fish cage farm. II. phosphorus. Mar. Ecol. Prog. Ser. 70, 263–272. doi: 10.3354/meps070263
Holdt S. L., Edwards M. D. (2014). Cost-effective IMTA: a comparison of the production efficiencies of mussels and seaweed. J. Appl. Phycol. 26 (2), 933–945. doi: 10.1007/s10811-014-0273-y
Israel D., Lupatsch I., Angel D. L. (2019). Testing the digestibility of seabream wastes in three candidates for integrated multi-trophic aquaculture: Grey mullet, sea urchin and sea cucumber. Aquaculture 510, 364–370. doi: 10.1016/j.aquaculture.2019.06.003
Kalantzi I., Karakassis I. (2006). Benthic impacts of fish farming: meta-analysis of community and geochemical data. Mar. pollut. Bull. 52 (5), 484–493. doi: 10.1016/j.marpolbul.2005.09.034
Karakassis I., Pitta P., Krom M. D. (2005). Contribution of fish farming to the nutrient loading of the Mediterranean. Scientia Marina 69 (2), 313–321. doi: 10.3989/scimar.2005.69n2313
Kaushik S. J. (1998). Nutritional bioenergetics and estimation of waste production in non-salmonids. Aquat. Living Resour. 11 (4), 211–217. doi: 10.1016/S0990-7440(98)89003-7
Kleitou P., Kletou D., David J. (2018). Is Europe ready for integrated multi-trophic aquaculture? a survey on the perspectives of European farmers and scientists with IMTA experience. Aquaculture 490, 136–148. doi: 10.1016/j.aquaculture.2018.02.035
Krom M., Ellner S., Van Rijn J., Neori A. (1995). Nitrogen and phosphorus cycling and transformations in a prototype'non-polluting'integrated mariculture system, eilat, Israel. Mar. Ecol. Prog. Ser., 118 (1/3), 25–36. doi: 10.3354/meps118025
Krom M., Porter C., Gordin H. (1985). Nutrient budget of a marine fish pond in eilat, Israel. Aquaculture 51 (1), 65–80. doi: 10.1016/0044-8486(85)90240-6
Lanari D., Poli B. M., Ballestrazzi R., Lupi P., D'Agaro E., Mecatti M. (1999). The effects of dietary fat and NFE levels on growing European sea bass (Dicentrarchus labrax l.). growth rate, body and fillet composition, carcass traits and nutrient retention efficiency. Aquaculture 179 (1-4), 351–364. doi: 10.1016/S0044-8486(99)00170-2
Lemarié G., Martin J.-L. M., Dutto G., Garidou C. (1998). Nitrogenous and phosphorous waste production in a flow-through land-based farm of European seabass (Dicentrarchus labrax). Aquat. Living Resour. 11 (4), 247–254. doi: 10.1016/S0990-7440(98)89007-4
Lupatsch I., Kissil G. W. (1998). Predicting aquaculture waste from gilthead seabream (Sparus aurata) culture using a nutritional approach. Aquat. Living Resour. 11 (4), 265–268. doi: 10.1016/S0990-7440(98)80010-7
Maar M., Larsen J., von Thenen M., Dahl K. (2020). Site selection of mussel mitigation cultures in relation to efficient nutrient compensation of fish farming. Aquacult. Environ. Interact. 12, 339–358. doi: 10.3354/aei00361
Massa F., Onofri L., Fezzardi D. (2017). “Aquaculture in the Mediterranean and the black Sea: a blue growth perspective,” in Handbook on the economics and management of sustainable oceans (Cheltenham, UK, Northampton, MA, USA: Edward Elgar Publishing), 93–123.
Molinos-Senante M., Hernández-Sancho F., Sala-Garrido R., Garrido-Baserba M. (2011). Economic feasibility study for phosphorus recovery processes. Ambio 40 (4), 408–416. doi: 10.1007/s13280-010-0101-9
Nelson E. J., MacDonald B. A., Robinson S. M. C. (2012). The absorption efficiency of the suspension-feeding sea cucumber, cucumaria frondosa, and its potential as an extractive integrated multi-trophic aquaculture (IMTA) species. Aquaculture 370-371, 19–25. doi: 10.1016/j.aquaculture.2012.09.029
Neofitou N., Lolas A., Ballios I., Skordas K., Tziantziou L., Vafidis D. (2019). Contribution of sea cucumber holothuria tubulosa on organic load reduction from fish farming operation. Aquaculture 501, 97–103. doi: 10.1016/j.aquaculture.2018.10.071
Neori A., Chopin T., Troell M., Buschmann A. H., Kraemer G. P., Halling C., et al. (2004). Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231 (1-4), 361–391. doi: 10.1016/j.aquaculture.2003.11.015
Newell R. I., Fisher T. R., Holyoke R. R., Cornwell J. C. (2005). “Influence of eastern oysters on nitrogen and phosphorus regeneration in Chesapeake bay, USA,” in The comparative roles of suspension-feeders in ecosystems (Netherlands: Springer), 93–120.
Orr L. C., Curtis D. L., Cross S. F., Gurney-Smith H., Shanks A., Pearce C. M. (2014). Ingestion rate, absorption efficiency, oxygen consumption, and fecal production in green sea urchins (Strongylocentrotus droebachiensis) fed waste from sablefish (Anoplopoma fimbria) culture. Aquaculture 422-423, 184–192. doi: 10.1016/j.aquaculture.2013.11.030
Papageorgiou N., Dimitriou P. D., Moraitis M. L., Massa F., Fezzardi D., Karakassis I. (2021). ). changes of the Mediterranean fish farm sector towards a more sustainable approach: A closer look at temporal, spatial and technical shifts. Ocean Coast. Manage. 214, 105903. doi: 10.1016/j.ocecoaman.2021.105903
Papageorgiou N., Kalantzi I., Karakassis I. (2010). Effects of fish farming on the biological and geochemical properties of muddy and sandy sediments in the Mediterranean Sea. Mar. Environ. Res. 69 (5), 326–336. doi: 10.1016/j.marenvres.2009.12.007
Pitta P., Tsapakis M., Apostolaki E. T., Tsagaraki T., Holmer M., Karakassis I. (2009). 'Ghost nutrients' from fish farms are transferred up the food web by phytoplankton grazers. Mar. Ecol. Prog. Ser. 374, 1–6. doi: 10.3354/meps07763
Porter C., Krom M., Robbins M., Brickell L., Davidson A. (1987). Ammonia excretion and total n budget for gilthead seabream (Sparus aurata) and its effect on water quality conditions. Aquaculture 66 (3-4), 287–297. doi: 10.1016/0044-8486(87)90114-1
Sanz-Lazaro C., Fernandez-Gonzalez V., Arechavala-Lopez P., Izquierdo-Gomez D., Martinez-Garcia E., Sanchez-Jerez P. (2018). Depth matters for bivalve culture in integrated multitrophic aquaculture (IMTA) and other polyculture strategies under non-eutrophic conditions. Aquacult. Int., 26 (5), 1161–1170. doi: 10.1007/s10499-018-0276-9
Sanz-Lazaro C., Sanchez-Jerez P. (2017). Mussels do not directly assimilate fish farm wastes: Shifting the rationale of integrated multi-trophic aquaculture to a broader scale. J. Environ. Manage. 201, 82–88. doi: 10.1016/j.jenvman.2017.06.029
Shpigel M., Shauli L., Odintsov V., Ben-Ezra D., Neori A., Guttman L. (2018). The sea urchin, paracentrotus lividus, in an integrated multi-trophic aquaculture (IMTA) system with fish (Sparus aurata) and seaweed (Ulva lactuca): Nitrogen partitioning and proportional configurations. Aquaculture 490, 260–269. doi: 10.1016/j.aquaculture.2018.02.051
Simboura N., Panayotidis P., Papathanassiou E. (2005). A synthesis of the biological quality elements for the implementation of the European water framework directive in the Mediterranean ecoregion: the case of saronikos gulf. Ecol. Indic. 5 (3), 253–266. doi: 10.1016/j.ecolind.2005.03.006
Smaal A. C., Ferreira J. G., Grant J., Petersen J. K., Strand Ø. (2019). Goods and services of marine bivalves (Switzerland: Springer Nature).
Theodorou J. A., Tzovenis I., Adams C. M., Sorgeloos P., Viaene J. (2014). Risk factors affecting the profitability of the Mediterranean mussel (Mytilus galloprovincialis lamarck 1819) farming in Greece. J. Shellfish Res. 33(3) 695-708, 614. doi: 10.2983/035.033.0304
Tolon M. T., Emiroglu D., Gunay D., Ozgul A. (2017). Sea Cucumber (Holothuria tubulosa gmelin 1790) culture under marine fish net cages for potential use in integrated multi-trophic aquaculture (IMTA). Indian J. Geo Mar. Sci. 46 (4), 749–756.
Troell M., Joyce A., Chopin T., Neori A., Buschmann A. H., Fang J.-G. (2009). Ecological engineering in aquaculture — potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 297 (1), 1–9. doi: 10.1016/j.aquaculture.2009.09.010
Tsapakis M., Pitta P., Karakassis I. (2006). Nutrients and fine particulate matter released from sea bass (Dicentrarchus labrax) farming. Aquat. Living Resour. 19 (1), 69–75. doi: 10.1051/alr:2006006
van der Schatte Olivier A., Jones L., Vay L. L., Christie M., Wilson J., Malham S. K. (2020). A global review of the ecosystem services provided by bivalve aquaculture. Rev. Aquacult. 12 (1), 3–25. doi: 10.1111/raq.12301
Walain M., Hakason L. (1991). Nutrient loading models for estimating the environmental effects of marine fish farm. Mar. aquacult. Environ.
Wartenberg R., Feng L., Wu J. J., Mak Y. L., Chan L. L., Telfer T. C., et al. (2017). The impacts of suspended mariculture on coastal zones in China and the scope for integrated multi-trophic aquaculture. Ecosys. Health Sustainability 3 (6), 1340268. doi: 10.1080/20964129.2017.1340268
Keywords: IMTA, finfish-farming, carbon sequestration, nutrient removal, footprint mitigation
Citation: Papageorgiou N, Dimitriou PD, Chatzivasileiou D, Tsapakis M and Karakassis I (2023) Can IMTA provide added ecosystem value services in the fish farms of Greece? Front. Mar. Sci. 9:1083099. doi: 10.3389/fmars.2022.1083099
Received: 28 October 2022; Accepted: 13 December 2022;
Published: 04 January 2023.
Edited by:
Marcel Martinez-Porchas, Consejo Nacional de Ciencia y Tecnología (CONACYT), MexicoReviewed by:
Sílvia C. Gonçalves, Polytechnic Institute of Leiria, PortugalAngel Martin Ortiz-Estrada, State University of Sonora, Mexico
Copyright © 2023 Papageorgiou, Dimitriou, Chatzivasileiou, Tsapakis and Karakassis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nafsika Papageorgiou, bnBhcGFnZW9yZ0B1b2EuZ3I=
 Nafsika Papageorgiou
Nafsika Papageorgiou Panagiotis D. Dimitriou
Panagiotis D. Dimitriou Dimitra Chatzivasileiou
Dimitra Chatzivasileiou Manolis Tsapakis
Manolis Tsapakis Ioannis Karakassis
Ioannis Karakassis