- College of Earth and Planetary Sciences (CEPS), University of Chinese Academy of Sciences (UCAS), Beijing, China
The relationship between temperature (T) and species diversity is one of the most fundamental issues in marine diversity. Although their relationships have been discussed for many years, how species diversity is related to T remains a controversial question. Previous studies have identified three T–diversity relationships: positive, negative, and unimodal. Recently, the unimodal relationship has received great attention. However, these studies may be biased by (1) considering the insufficient T range of database, (2) using a single diversity metric (generally species richness, S), and (3) rarely considering species abundance distribution (SAD) that can better represent diversity. Here, to seek a more comprehensive understanding of T–diversity relationships, their relationships are evaluated according to a global planktonic foraminifera dataset, which is usually considered as a model dataset for exploring diversity pattern. Species diversity are estimated by four most commonly used metrics and a new SAD parameter (p). Results show that S and Shannon’s index support the typical unimodal relationship with T. However, evenness and dominance do not have significant unimodality. Additionally, this study conjectures that the SAD parameter p with increasing T will gradually approach the minimum 1, noting that SAD (Nr/N1, where Nr and N1 are the abundance of the rth and the first species in descending order) tends to be 1:1/2:1/3…. This study suggests that the T–diversity relationship cannot be wholly reflected by S and the other aspects of diversity (especially SAD) should be considered.
Introduction
The relationship between temperature (T) and species diversity is one of the most fundamental issues in ecology (Allen et al., 2002; Tittensor et al., 2010; Peters et al., 2016). Studies on the biotas of forests, grasslands, wetlands, continental shelves, the open ocean, and even the estuarine and coastal areas have all shown the significant correlations between T and diversity (Ray, 1991; Yasuhara and Danovaro, 2016; Henseler et al., 2019). Under the current circumstances of T change in most oceanic regions, their correlations have received more extensive attention from ecologists and climatologists (Currie et al., 2004; Mora et al., 2013; Yasuhara et al., 2020). However, how exactly species diversity is related to T remains a central yet controversial question in ecological research (Danovaro et al., 2004; O’Hara and Tittensor, 2010; Yasuhara and Danovaro, 2016), although many studies and discussions have surrounded the T–diversity relationship for many years (von Humboldt, 1871; Pianka, 1966; Brown, 2014).
Previous studies have identified three apparent T–diversity relationships. (1) Positive: As expected by ecological theories (Allen et al., 2002; Evans et al., 2005), most empirical T–diversity relationships were positive (Rutherford et al., 1999; Tittensor et al., 2010). (2) Negative: Negative relationship had been found on both temporal and spatial scales (Danovaro et al., 2004; O’Hara and Tittensor, 2010), which was supported by climate change science (Cheung et al., 2009; Mora et al., 2013). (3) Unimodal: Yasuhara synthesized two relationships above and stated that T and diversity were more likely to be a unimodal relationship (Yasuhara and Danovaro, 2016; Yasuhara et al., 2020).
The above studies of the T–diversity relationship might be biased, because (1) the temperature range of the datasets was not sufficiently broad (e.g., Danovaro et al., 2004; Hunt et al., 2005); (2) only one diversity metric (generally species richness, S) has been considered (Tittensor et al., 2010; Yasuhara and Danovaro, 2016); and (3) few studies had explored the T–diversity relationship from the perspective of species abundance distribution (SAD), which can better represent species diversity than many other metrics (Mouillot et al., 2000; McGill et al., 2007; Connolly et al., 2009). Thus, it is difficult to draw an overall conclusion on how species diversity relates to T.
The main purpose of this study is to seek a more comprehensive understanding of how species diversity changes with T. A useful model system for this purpose is the microfossils of planktonic foraminifera, because of their complete abundance records, large temperature coverage, and good taxonomic resolution (Rutherford et al., 1999; Siccha and Kucera, 2017; Yasuhara et al., 2017). Thus, the Brown University Foraminiferal Data Base (BFD) (Prell et al., 1999) is used here to explore the correlations between T and species diversity, which records the absolute abundances of 37 extant morpho-species of planktonic foraminifera at 1,265 core-top sites of the global oceans. Species diversity of foraminifera is estimated by four most commonly used diversity metrics and a new SAD parameter (Su, 2016).
Methods
To explore the T–diversity relationship from multiple perspectives, the four most common metrics are used to measure diversity, namely, Species richness (S), Shannon’s index (H’), Simpson’s index (D), and Pielou’s evenness (J) (Pielou, 1975). Additionally, a new SAD parameter (Su, 2016) is also used as a diversity surrogate.
Diversity indices (H’, D, and J)
H’, D, and J are calculated as follows (Pielou, 1975):
where pi is the relative abundance of the ith species (i = 1, 2, 3,…S). As diversity increases, H’ and J increase and D decreases (Pielou, 1975).
The SAD parameter (p)
The parameter p is derived from a new fractal model of SAD (Su, 2016). According to the fractal hypothesis [when K more species appear at each step of the accumulation process, their abundance are k times less abundant and K = k d, where d is a fractal dimension (Su, 2016)], SAD is
where r (= 1, 2, 3,… S) is the rank of species sorted down by abundance; Nr and N1 are the abundance of the rth and the first species; p (= 1/d) is the fractal parameter that determines SAD (e.g., when p = 2 and S = 3, Nr/N1 is 1:1/4:1/9; when p = 1 and S = 3, Nr/N1 is 1:1/2:1/3). The lower the p, the higher the species diversity (Su, 2016).
Let Fr = ln (Nr/N1) and Dr = ln (r). By minimizing the sum of squared error (), p is (Su, 2016)
The sum formula of Eq. 1 is
where NT is the total abundance.
The goodness of fit is measured by the coefficient of determination (R2), which denotes how well the fractal model fits empirical samples. The closer R2 is to 1, the better is the goodness of fit.
Datasets
Species diversity
BFD (Prell et al., 1999) is selected because (1) foraminifera was commonly used as a model taxon of protists to study the effect of T on diversity distribution (Rutherford et al., 1999; Tittensor et al., 2010); (2) they are reliable as they have been reviewed by Brown University for taxonomic consistency (Prell et al., 1999); (3) they contain abundance information of each species, by which SAD in the community can be calculated; (4) the published dataset is easy to recheck; and (5) their samples are extracted sediment core tops raised from global oceans (Prell et al., 1999), so the relationship between T and diversity can be evaluated over a wide T range. Details of BFD can be found in Rutherford et al. (1999).
Temperature
SST denotes the monthly long-term mean (1981–2010) from NOAA optimum interpolation sea surface temperature analysis. It is from https://psl.noaa.gov/data/gridded/data.noaa.oisst.v2.html#source.
The relationship between temperature and species diversity
S, H’, D, and J are calculated by the vegan packages, and S are rarefied to ensure that it is unaffected by the sample size (Oksanen et al., 2020). The fractal p of BFD is estimated by Eq. 5. S, H’, D, J, p, and SST are standardized by taking the average for 5°×5° grids (these terms are below the mean). Since the T–diversity relationship could be unimodal (Yasuhara and Danovaro, 2016), the relationship between T and each diversity metric is divided into two segments according to the piecewise regression model (Muggeo, 2003), by which the unimodal T–diversity relationships can be identified (Table 1). Breakpoint of piecewise regression marks a change in direction from one segment to another. Pearson’s correlation coefficient (r) measures the direction of variation of diversity in two segments (Obilor and Amadi, 2018). P represents the significance of the correlation between T and diversity. When P < 0.05, their correlation is significant. Additionally, the different latitudinal ranges are not represented equally in the dataset, noting that 55.18% of the samples are from −20° to 20°. To circumvent this influence on the results, a bootstrap simulation is used by (1) randomly selecting five original samples from each 5° latitude band, (2) taking the average of T and diversity metrics for 5°×5° grids, and (3) calculating piecewise regression of the T–diversity relationship. The average r of two segments is yielded by repeating these three steps 500 times (with replacement). The results are listed in Supplementary Table 1. All statistical analyses were done in R ver. 4.0.4 (www.r-project.org), and code is publicly archived (Gao and Su, 2021).
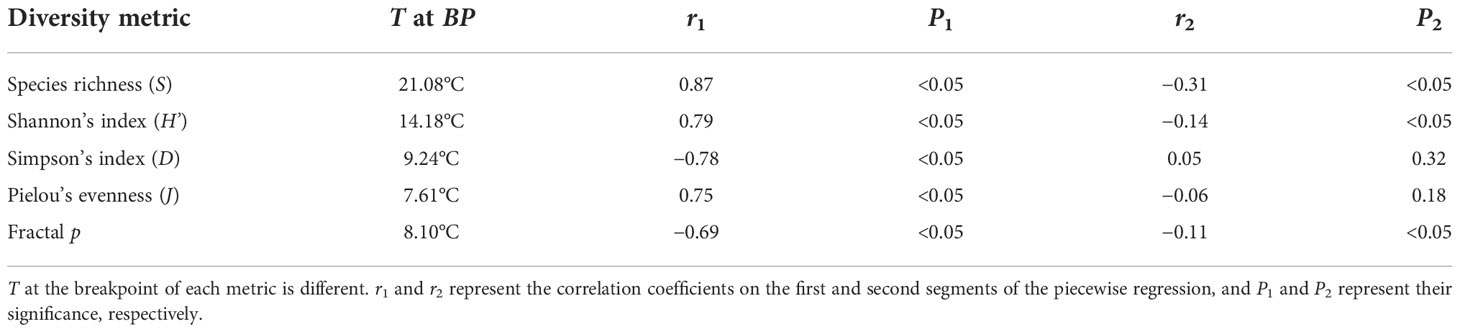
Table 1 The temperature (T) at the breakpoint (BP) of diversity and the correlation between T and diversity.
Results
SST ranges from −0.28 ± 0.01 to 29.60 ± 0.05°C. SST at the breakpoint of S, H’, D, and J are 21.08, 14.18, 9.24 and 7.61°C, respectively (Table 1). In the first segment of the piecewise regression (Figure 1), S, H’, and J are positively correlated with T (rS1 = 0.87, rH’1 = 0.79, rJ1 = 0.75). D is negatively correlated with T (rD1 = −0.78). Four metrics are significantly correlated with SST (P< 0.05). In the second segment of the piecewise regression (Figure 1), S and H’ decrease significantly as SST increases (rS2 = -0.31, rH'2= -0.14, P< 0.05). D and J are not significantly correlated with T (P > 0.05) (Table 1). As T increases, D increases and J decreases (rD2 = 0.05, rJ2 = −0.06).
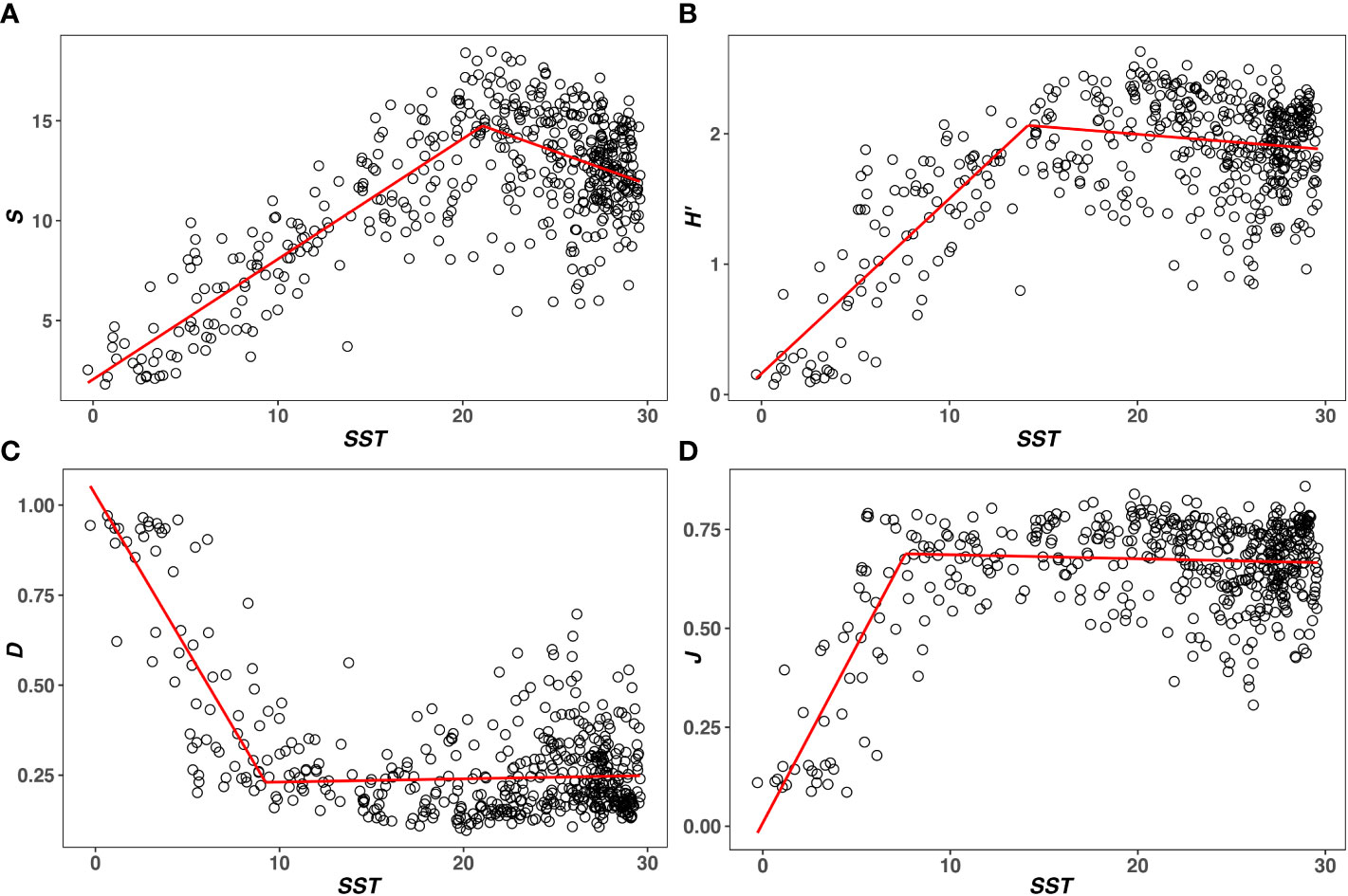
Figure 1 The T–diversity relationships are measured by (A) Species Richness (S), (B) Shannon’s index (H’), (C) Simpson’s index (D), and (D) Pielou’s evenness (J). The red line represents the result of piecewise regression, noting that S, H’, D, and J all show a unimodal relationship with T.
The R2 of the fractal model to the BFD data is from 0.632 to 1.000, and more than 86% is above 0.8 (see Supplementary Table 2). SST at the breakpoint of p is 8.10°C. When SST< 8.10°C, p decreases pronouncedly as SST increases (rp1 = −0.69, P< 0.05). When SST > 8.10°C, p decreases slightly with SST (rp2 = −0.11, P< 0.05) and tends to be flat (Figure 2). The SST–p relationships are significantly negative over the entire SST range (see the non-line regression in Figure 2, P< 0.05).
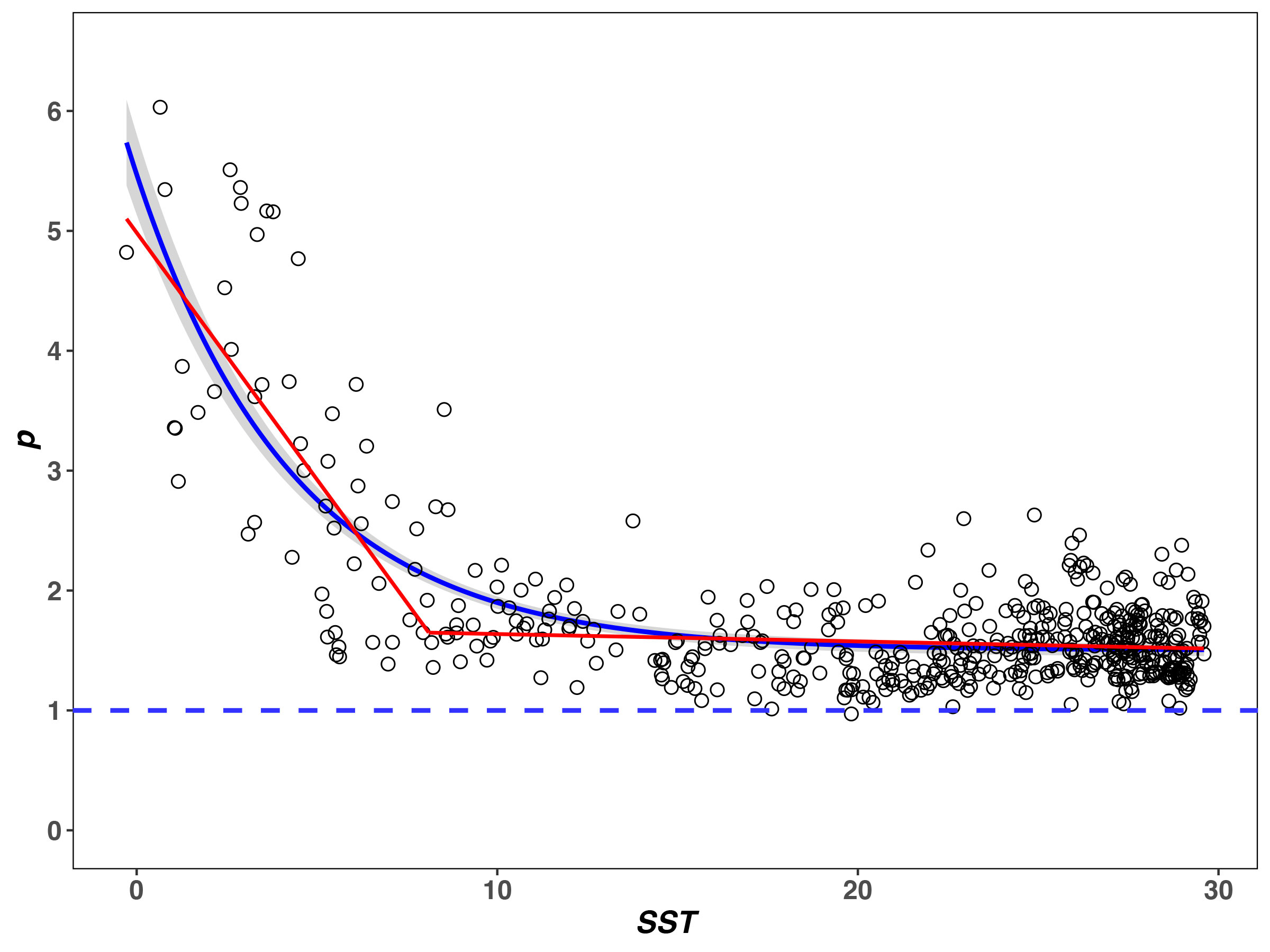
Figure 2 Relationships between SST (T) and fractal p for the BFD data. The red line represents the result of piecewise regression, noting that p changes in the same direction as T increases (rp1 = −0.69, rp2 = −0.11). Non-line regression results are significant (blue line with gray 99% confidence limits, results: y = −3.970e-0.23x + 1.501, R2 = 0.631, P< 0.05).
Although the bootstrap simulation is used, the results are found to be mostly consistent (see Supplementary Table 1). Thus, only the relationships between T and each metric without the bootstrap simulation are presented (Table 1 and Figures 1, 2).
Discussion
The correlation between temperature (T) and species diversity has fascinated and occupied scientists for centuries (von Humboldt, 1871; Pianka, 1966; Brown, 2014). Ecologists generally suggested that increasing T promoted higher rates of speciation or provided more tolerable living conditions leading to greater diversity (Allen et al., 2002; Currie et al., 2004; Tittensor et al., 2010). However, climatologists often implied that climate warming would have negative biological consequences triggering a decline in diversity (Cheung et al., 2009; Mora et al., 2013). Thus, how diversity relates to T has remained controversial (Yasuhara and Danovaro, 2016). Since coastal T may change rapidly in the coming decades (Zhu et al., 2021), a better understanding of the T–diversity relationship is one of the central problems in current research (Cheung et al., 2009; Brown, 2014; Yasuhara et al., 2020).
Yasuhara stated that “when considered over a sufficiently broad T range, the T–diversity relationship appears to be unimodal” (Yasuhara and Danovaro, 2016). This idea stems from a unimodal model, noting that fewer species can physiologically tolerate conditions in extremely cold or warm places than in other sites. Thus, S will show a peak at intermediate T (Currie et al., 2004). The well-known unimodal depth–diversity relationship was considered to support the unimodal model (Yasuhara and Danovaro, 2016; Yasuhara et al., 2020).
In this study, S, H’, and J show similar unimodal relationships with T (Figures 1A, B, D). Their relationships are positive below T at the breakpoint (rS1 = 0.87, rH’1 = 0.79, rJ1 = 0.75) and negative over T at the breakpoint (rS2 = −0.31, rH’2 = −0.14, rJ2 = −0.06). The T–D relationship is opposite to that of the other three metrics in two segments of the piecewise regression (rD1 = −0.78, rD2 = 0.05). However, D represents the species dominance in a community. The lower the D, the higher the diversity (Pielou, 1975). Thus, four metrics are not completely positive or negative with T. Species diversity measured by four metrics increases first and then decreases with increasing T. This is basically consistent with the expectation of the unimodal model (Yasuhara and Danovaro, 2016).
As noted before, the unimodal model is mostly derived based on the relationship between T and S (Yasuhara and Danovaro, 2016). However, S only represents one aspect of species diversity. It has a number of other components, such as dominance (D) and evenness (J) (Hill, 1973; Pielou, 1975; Willig and Presley, 2013). A better understanding of the T–diversity relationship needs to take these components into account (Pielou, 1975; Stevens and Willig, 2002; Willig and Presley, 2013). According to the piecewise regression results (Table 1), T at the breakpoints of D and J are apparently lower than that of S. This means that dominance has started to increase and evenness has started to decrease, when S is still increasing with T. Although the present results based on a single database may not be very universal, they at least imply that S cannot describe some changes that have occurred in species diversity. Therefore, this study suggests that evenness and dominance should be considered when investigating the T–diversity relationship, as they cannot be reflected by S. Additionally, it is worth noting that the change of D and J over T at the breakpoint is less significant than that of S (P > 0.05). This means that the unimodality of dominance and evenness is not as obvious as that of S. Similar results have also been found in the study of New World bat (Stevens and Willig, 2002). Stevens and Willig characterized three aspects of diversity for bat communities and suggested that the evenness and dominance did not change significantly at the warmer lower latitudes (Stevens and Willig, 2002). Thus, whether dominance and evenness can be treated as the typical unimodal relationship also need to be further investigated.
Finally, many ecologists have suggested that the traditional diversity indices (e.g., H’, D, and J) only represent a synthetic characterization of relative abundance in a community (Pielou, 1975; Stevens and Willig, 2002; Willig and Presley, 2013), which may lead to an excessive simplification of diversity (Mouillot et al., 2000; McGill et al., 2007). Since SAD can reflect the relative abundance for each different species encountered within a community (Tokeshi, 1993; Mouillot et al., 2000; McGill et al., 2007; Ulrich et al., 2010), it is a more comprehensive description of diversity (Mouillot et al., 2000; McGill et al., 2007; Connolly et al., 2009). According to Eq. 4, p is the fractal parameter that determines SAD. The T–p relationship shows that the direction of variation of p is the same in two segments of the piecewise regression (rp1 = −0.69, rp2 = −0.11, P< 0.05) (Figure 2) and their relationship is significant (P< 0.05, see the non-line regression in Figure 2). This is totally different from the other metrics (S, H’, D, and J). Accordingly, it is argued here that the fractal p has an asymptotic relationship with T rather than the typical unimodal relationship.
This study assumes that p with increasing T will gradually approach the minimum 1. Firstly, when p = 1, SAD (Nr/N1) is 1:1/2:1/3… (Eq. 4), which is consistent with Zipf’s law (Zipf, 1949). Secondly, in previous studies, Su suggested that Zipf’s law (1:1/2:1/3…) might be a general pattern of SAD (Su, 2018), which is supported by nearly 20,000 community samples. Thirdly, Su (2018) proposed two hypotheses to elucidate this pattern. H1: Species diversity was determined by the entropy that increased with the energy transformation. H2: The total assimilated energy of the community (ET) was finite. These two hypotheses provide a direction for the interpretation of the T–p relationship.
(1) NT/N1 (Eq. 6) is an effective number of species diversity, which is related to Rényi’s entropy (Rényi, 1961; Hill, 1973). According to H1, increasing entropy with T leads to the rise of NT/N1 (Sethna, 2006). (2) According to H2, NT/N1 is finite as NT is usually equivalent to ET (Hutchinson, 1959; Brown, 2014). On the one hand, increasing NT/N1 with T will cause p to decrease (Eq. 6). On the other hand, the finiteness of NT/N1 determines that p ought to be higher than 1 (NT/N1 converges when p > 1, Eq. 6), which means that the theoretical minimum of p is 1. Therefore, if H1 and H2 hold true, their combined effect will make p approach the theoretical minimum 1 with increasing T. Since a lower p means a higher diversity (Su, 2016); this study conjectures that species diversity with increasing T will rise and gradually approach the theoretical maximum, which is Zipf’s law (1:1/2:1/3…).
It should be emphasized that the results of this study depend on the accuracy and representation of the dataset. In fact, although BFD has been used in many studies of diversity (Rutherford et al., 1999; Tittensor et al., 2010), there are some important biases that need to be noted here. Firstly, the “pachyderma-dutertrei intergrade” category is not recognized in BFD (King and Howard, 2003). Secondly, calcium carbonate dissolution in deep-sea sediments of the Pacific and Indian Oceans may decrease the species richness of core-top assemblages (Rutherford et al., 1999). Finally, the representation of planktonic foraminifera to other organisms (especially terrestrial taxa) is still limited (Rutherford et al., 1999; Siccha and Kucera, 2017; Yasuhara et al., 2017). Thus, more details of the T–diversity relationship in planktonic foraminifera, and their pattern in other taxa, still need to be further verified.
Conclusion
When the correlation between T and diversity was mentioned in previous studies, most of the discussions revolved around the potential impact of T on S (Allen et al., 2002; Tittensor et al., 2010; Peters et al., 2016; Yasuhara and Danovaro, 2016). However, this study indicates that (1) the T–diversity relationship is not wholly captured by S and the other aspects of diversity (especially SAD) should be considered; (2) S and H’ support the typical unimodal relationship with T, while dominance and evenness do not have significant unimodality; (3) p with increasing T may decrease and gradually approach the theoretical minimum 1 (Nr/N1 is 1:1/2:1/3…). These points are the biggest difference between previous studies and this paper.
In the future, the relationship between T and the other aspects of diversity (e.g., evenness and dominance) should be discussed more over a broader T range. This can provide feasible guidance for dealing with the potential responses of diversity to global T changes. Additionally, the relationship between T and SAD should receive more attention and investigation. It is particularly noteworthy that the theoretical minimum of fractal p (or the theoretical maximum of diversity) can be supported by more empirical data. Finally, analyzing the T–diversity relationship from multiple aspects and exploring their underlying mechanisms are expected to enrich the theories of community ecology and biogeography.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material. The code supporting the results in the paper have been archived in Figshare (https://doi.org/10.6084/m9.figshare.14933301.v10).
Ethics statement
This study does not involve related issues. Ethics approval was not required for this study.
Author contributions
QS conceived and designed this study. JG developed the mathematical models and performed statistical analyses. JG and QS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 42071137 and 41676113). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1069276/full#supplementary-material
References
Allen A., Brown J., Gillooly J. (2002). Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297, 1545–1548. doi: 10.1126/science.1072380
Brown J. H. (2014). Why are there so many species in the tropics? J. Biogeogr. 41 (1), 8–22. doi: 10.1111/jbi.12228
Cheung W. W., Lam V.W., Sarmiento J.L., Kearney K., Watson R., Pauly D., et al. (2009). Projecting global marine biodiversity impacts under climate change scenarios. Fish Fisheries 10 (3), 235–251. doi: 10.1111/j.1467-2979.2008.00315.x
Connolly S. R., Dornelas M., Bellwood D. R., Hughes T. P. (2009). Testing species abundance models: A new bootstrap approach applied to indo-pacific coral reefs. Ecology 90 (11), 3138–3149. doi: 10.1890/08-1832.1
Currie D., Mittelbach G., Cornell H., Field R., Guégan J.-F., Hawkins B., et al. (2004). Predictions and tests of climate-based hypotheses broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134. doi: 10.1111/j.1461-0248.2004.00671.x
Danovaro R., Dell'Anno A., Pusceddu A. (2004). Biodiversity response to climate change in a warm deep sea. Ecol. Lett. 7 (9), 821–828. doi: 10.1111/j.1461-0248.2004.00634.x
Evans K. L., Warren P.H., Gaston K.J. (2005). Species-energy relationships at the macroecological scale: A review of the mechanisms. Biol. Rev. Cambridge Philos. Soc 80 (1), 1–25. doi: 10.1017/S1464793104006517
Gao J., Su Q. (2021). “The fractal p calculation of program for the community samples,” in Brown university's foraminiferal database.
Henseler C., Nordström M. C., Törnroos A., Snickars M., Pecuchet L., Lindegren M., et al. (2019). Coastal habitats and their importance for the diversity of benthic communities: A species-and trait-based approach. Estuarine Coast. Shelf Sci. 226, 106272. doi: 10.1016/j.ecss.2019.106272
Hill M. O. (1973). Diversity and evenness: A unifying notation and its consequences. Ecology 54 (2), 427–432. doi: 10.2307/1934352
Hunt G., Cronin T. M., Roy K. (2005). Species–energy relationship in the deep sea: A test using the quaternary fossil record. Ecol. Lett. 8 (7), 739–747. doi: 10.1111/j.1461-0248.2005.00778.x
Hutchinson G. (1959). Homage to santa rosalia or why are there so many kinds of animals? Am. Naturalist 93, 145–159. doi: 10.1086/282070
King A. L., Howard W. R. (2003). Planktonic foraminiferal flux seasonality in subantarctic sediment traps: A test for paleoclimate reconstructions. Paleoceanography 18 (1), 1–17. doi: 10.1029/2002PA000839
McGill B. J., Etienne R. S., Gray J. S., Alonso D., Anderson M.J., Benecha H.K., et al. (2007). Species abundance distributions: Moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 10 (10), 995–1015. doi: 10.1111/j.1461-0248.2007.01094.x
Mora C., Wei C.-L., Rollo A., Amaro T., Baco A.R., Billett D., et al. (2013). Biotic and human vulnerability to projected changes in ocean biogeochemistry over the 21st century. PloS Biol. 11 (10), e1001682. doi: 10.1371/journal.pbio.1001682
Mouillot D., Leprêtre A., Andrei-Ruiz M.-C., Viale D. (2000). The fractal model: A new model to describe the species accumulation process and relative abundance distribution (rad). Oikos 90 (2), 333–342. doi: 10.1034/j.1600-0706.2000.900214.x
Muggeo V. M. (2003). Estimating regression models with unknown break-points. Stat Med. 22 (19), 3055–3071. doi: 10.1002/sim.1545
Obilor E. I., Amadi E. C. (2018). Test for significance of pearson’s correlation coefficient. Int. J. Innovative Mathematics Stat Energy Policies 6 (1), 11–23. Available at: https://seahipaj.org/journals/engineering-technology-andenvironment/ijimsep/.
O’Hara T. D., Tittensor D. P. (2010). Environmental drivers of ophiuroid species richness on seamounts. Mar. Ecol. 31, 26–38. doi: 10.1111/j.1439-0485.2010.00373.x
Oksanen J., Blanchet F. G., Friendly M., Kindt R., Legendre P., McGlinn D., et al. (2020). “Vegan: Community ecology package,” in R package version 2. 5-6. 2019. Available at: https://scholar.google.com/scholar?cluster=11712188988930644900&hl=en&oi=scholarr.
Peters M. K., Hemp A., Appelhans T., Behler C., Classen A., Detsch F., et al. (2016). Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat. Commun. 7 (1), 1–11. doi: 10.1038/ncomms13736
Pianka E. R. (1966). Latitudinal gradients in species diversity: A review of concepts. Am. Naturalist 100 (910), 33–46. doi: 10.1086/282398
Prell W., Martin A., Cullen J.L., Trend M. (1999). The brown university foraminiferal data base. IGBP PAGES/World Data Center-A Paleoclimatol. Data Contrib. Series 27, 237–249. doi: 10.1594/PANGAEA.96900
Ray G. C. (1991). Coastal-zone biodiversity patterns. Bioscience 41 (7), 490–498. doi: 10.2307/1311807
Rényi A. (1961). On measures of entropy and information. Proc. Fourth Berkeley Symp. Math. Statist. Prob. Univ. Calif. 1 (5073), 547–561. Available at: https://scholar.google.com/scholar?q=On+measures+of+entropy+and+information&hl=zh-CN&as_sdt=0%2C5&as_vis=1&as_ylo=1961&as_yhi=.
Rutherford S., D'Hondt S., Prell W. (1999). Environmental controls on the geographic distribution of zooplankton diversity. Nature 400 (6746), 749–753. doi: 10.1038/23449
Sethna J. (2006). Statistical mechanics: Entropy, order parameters, and complexity (UK: Oxford University Press).
Siccha M., Kucera M. (2017). Forcens, a curated database of planktonic foraminifera census counts in marine surface sediment samples. Sci. Data 4 (1), 1–12. doi: 10.1038/sdata.2017.109
Stevens R. D., Willig M. R. (2002). Geographical ecology at the community level: Perspectives on the diversity of new world bats. Ecology 83 (2), 545–560. doi: 10.1890/0012-9658(2002)083[0545:GEATCL]2.0.CO;2
Su Q. (2016). Analyzing fractal property of species abundance distribution and diversity indexes. J. Theor. Biol. 392, 107–112. doi: 10.1016/j.jtbi.2015.12.010
Su Q. (2018). A general pattern of the species abundance distribution. PeerJ 6, 1–11. doi: 10.7717/peerj.5928
Tittensor D., Mora C., Jetz W., Lotze H., Ricard D., Vanden Berghe E., et al. (2010). Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098–1101. doi: 10.1038/nature09329
Tokeshi M. (1993). Species abundance patterns and community structure. Adv. Ecol. Res. 24 (08), 111–186. doi: 10.1016/S0065-2504(08)60042-2
Ulrich W., Ollik M., Ugland K. I. (2010). A meta-analysis of species–abundance distributions. Oikos 119 (7), 1149–1155. doi: 10.1111/j.1600-0706.2009.18236.x
von Humboldt A. (1871). Ansichten der natur: Mit wissenschaftlichen erläuterungen (JG Cotta'scher Verlag).
Willig M. R., Presley S. J. (2013). Latitudinal gradients of biodiversity. Levin SA. Encyclopedia Biodiversity 2, 612–626. doi: 10.1016/B978-0-12-384719-5.00086-1
Yasuhara M., Danovaro R. (2016). Temperature impacts on deep-sea biodiversity. Biol. Rev. 91 (2), 275–287. doi: 10.1111/brv.12169
Yasuhara M., Tittensor D. P., Hillebrand H., Worm B.. (2017). Combining marine macroecology and palaeoecology in understanding biodiversity: Microfossils as a model. Biol. Rev. 92 (1), 199–215. doi: 10.1111/brv.12223
Yasuhara M., Wei C.-L., Kucera M., Costello M.J., Tittensor D.P., Kiessling W., et al. (2020). Past and future decline of tropical pelagic biodiversity. Proc. Natl. Acad. Sci. 117 (23), 12891–12896. doi: 10.1073/pnas.1916923117
Zhu Z., Linong L., Xiaona Y., Zhang W., Liu W. (2021). Ar6 climate change 2021: The physical science basis. Available at: https://scholar.google.com/scholar_lookup?title=AR6%20Climate%20Change%202021&publication_year=2021&author=Z.%20Zhongming&author=L.%20Linong&author=Y.%20Xiaona&author=Z.%20Wangqiang&author=L.%20Wei.
Keywords: unimodal relationship, diversity index, species abundance distribution, fractal theory, Zipf’s law
Citation: Gao J and Su Q (2022) A comprehensive analysis of the relationship between temperature and species diversity: The case of planktonic foraminifera. Front. Mar. Sci. 9:1069276. doi: 10.3389/fmars.2022.1069276
Received: 13 October 2022; Accepted: 04 November 2022;
Published: 01 December 2022.
Edited by:
Songlin Liu, South China Sea Institute of Oceanology (CAS), ChinaCopyright © 2022 Gao and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Su, c3FpYW5nQHVjYXMuYWMuY24=
 Junfeng Gao
Junfeng Gao Qiang Su
Qiang Su