
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 20 January 2023
Sec. Marine Ecosystem Ecology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1065514
 Marco Torri1*
Marco Torri1* Stefania Russo1
Stefania Russo1 Federico Falcini2
Federico Falcini2 Biagio De Luca3
Biagio De Luca3 Simone Colella2
Simone Colella2 Gianluca Volpe2
Gianluca Volpe2 Raffaele Corrado2
Raffaele Corrado2 Francesco Placenti3
Francesco Placenti3 Luigi Giaramita3
Luigi Giaramita3 Marianna Musco1,4
Marianna Musco1,4 Tiziana Masullo1
Tiziana Masullo1 Carmelo Bennici1
Carmelo Bennici1 Marilena Vita Di Natale1
Marilena Vita Di Natale1 Bernardo Patti5
Bernardo Patti5 Guglielmo Lacorata2
Guglielmo Lacorata2 Marco Arculeo6†
Marco Arculeo6† Angela Cuttitta1,4†
Angela Cuttitta1,4†The relationship between environmental conditions and early life-history traits of Sardinella aurita are investigated using material collected in two sites of the Central Mediterranean Sea. Individual mean daily growth during the planktonic phase has been determined by using otolith microstructure analysis, while Lagrangian simulation models allowed to estimate the daily position in space and time of each specimen from the hatching to the catch. Generalized Additive Mixed Models (GAMMs) have been implemented to explore the impact of environmental conditions at time t, t-1 day and t-2 days on the mean daily growth rate occurring at time t. Spatial analysis evidenced a wide dispersion of eggs and larvae in the coastal area of both sampling sites in correspondence to relatively warmer and chlorophyll-a enriched waters. Lagrangian simulations detected a complementary larval dispersal pathway able to transport larvae to a known retention area. Temperature at time t was the most important driver affecting the mean daily larval growth, followed by the food availability. On the other hand, models performed on lagged environmental covariates (t-1 and t-2) did not show any significant effect on the growth rate at time t. In addition to the sub-linear positive correlation between temperature and mean daily larval growth, model highlighted a decrease in the otolith core width at higher temperature that can be linked to an earlier stage of ontogeny at hatching. This study provided a useful methodological approach that takes advantage of available remote sensing data to perform ecological studies in support to fisheries management.
Determining the environmental mechanisms sustaining the fish standing stock biomass is a challenging objective of the fisheries sciences, aimed at delivering scientific advice in support to the sustainable exploitation of marine biological resources. Indeed, fish population size can exhibit large interannual fluctuations mainly linked to variations in recruitment success. This is especially the case of the small pelagic fish (Lloret et al., 2001; Patti et al., 2004; Patti et al., 2020), which are typically characterized by large fecundity and population structure mainly composed by few young generations. In these species the population biomass is strongly affected by the impact of recruitment success, which in turn is mainly related to the very high and extremely variable mortality rates characterizing the planktonic stages, i.e., eggs and larvae, representing the most critical phases among the life history stages of fish population (Hjort, 1914). Survival processes and growth are concurrent key factors for the recruitment success (Anderson, 1988; Cowan and Shawn, 2002; Houde, 2009). For instance, fast growth and shortened larval duration phase are considered as factors for supporting survival and recruitment (Miller et al., 1988; Houde, 2008). However, it is well known that growth is a parameter influenced by the environmental conditions experienced by larval stages during their development. In particular, temperature and food availability are considered among the most important drivers able to tune growth and larval development (Blaxter, 1991; Takasuka and Aoki, 2006; Pörtner and Peck, 2010). In the case of temperate marine fish, warm water induces higher metabolic rates (Peck et al., 2013) and it results in general high growth rates (Houde, 1989; Batty and Blaxter, 1992; Benoît et al., 2000) that in turn require a corresponding increase in food supply (Pörtner and Farrell, 2008). However, the determination of the relationship between those parameters is rarely an unambiguous issue as the response of the larval growth to temperature and food availability conditions is strongly related to the species, its life stage and the specific environmental conditions occurring in the field as well as controlled in laboratory experiments (Leggett and Deblois, 1994). Thus, determining the link connecting larval growth rates, temperature and food concentration is a required step to figure out how environmental variability can affect the recruitment and the standing stock biomass fluctuations.
Recent studies have demonstrated the potential of using Lagrangian simulations for the investigation of larval dispersion of small pelagic fish in the Mediterranean, evidencing the key role of the physical forcing in affecting the larval spatial distribution (Palatella et al., 2014; Cuttitta et al., 2018; Torri et al., 2018; Falcini et al., 2020). Moreover, the synergic use of remote sensing allowed to characterize the larval developing environment and provided inferences on the relationships between growth and mortality of early life fish stages and recruitment processes (Falco et al., 2020; Patti et al., 2020; Molina-Valdivia et al., 2021). In this context here we provide a novel approach that takes advantage of both remote sensing data and Lagrangian techniques, in order to study the relationship between the larval growth rate and the environmental conditions experienced by marine fish larvae through an individual-based analysis performed on data collected in situ. In particular, otolith microstructure analysis has been applied to estimate the larval age and the mean daily growth. In addition, Lagrangian simulations were used for estimating the individual back-trajectories, while the remote sensing data allowed to determine the conditions occurring in the larval developing environment, i.e., the marine surface layer, during the advection period. Finally, multivariate statistical models have been implemented to explore the relationship between the larval mean daily growth and environmental conditions occurring during the planktonic phase of fish development.
This approach has been here applied on the case study of the round sardinella (Sardinella aurita, Valenciennes, 1847). This species is an important fishing resource in central Mediterranean Sea and in the Levantine area (FAO-GFCM, 2021) and represents an important component of the summer larval fish assemblage in these areas (Somarakis et al., 2002; Zarrad et al., 2013; Cuttitta et al., 2018; Torri et al., 2018; Patti et al., 2022). Moreover, it is one of the most exploited fish species over the Mediterranean, especially in the Ionian and Levant FAO division fishing areas (FAO-GFCM, 2021). An increasing abundance and gradual northward expansion of this species have been reported along different areas of the Mediterranean in correspondence to the warming of the seawater, with expected effects on the ecosystem and fisheries (Sinovčić et al., 2004; Sabatés et al., 2006; Tsikliras, 2008; Schickele et al., 2021). Our approach was aimed at studying the temperature and food availability effect on the larval growth performances in two separate sites of the Central Mediterranean Sea (CMS), representing thus an interesting scientific question in the context of the changing marine environment on a wide regional scale, with expected effects on the biological resources. The first site is located in the Strait of Sicily, a crucial point for the thermohaline circulation at the basin scale (Gačić et al., 2013; Placenti et al., 2022) and an important hotspot of biodiversity (Cuttitta et al., 2016; Altobelli et al., 2017; di Lorenzo et al., 2018; Patti et al., 2022). The second site is located in the Central-Eastern Tyrrhenian Sea, an area with limited knowledge on the ichthyoplankton assemblages but that hosts a complex ecosystem with a multitude of neritic and coastal fish species (Colloca et al., 2003; Torri et al., 2021).
The objectives of this work are: i) to determine the spatial heterogeneity of the spawning grounds and the larval growth of Sardinella aurita in the CMS; ii) to assess the effect of temperature and food availability on the mean daily growth of Sardinella aurita in different environments and planktonic phases. We aim of pursuing these objectives following an holistic approach, based on Lagrangian simulations, remote sensing, otolith microstructure analysis, and multivariate statistical techniques for ecological studies addressed on the planktonic fish stages.
Two oceanographic cruises have been aimed at collecting ichthyoplankton samples during summer 2013 on board the R/V Urania in a southern site and in a northern site of Central Mediterranean Sea, namely in the Strait of Sicily (11.5-15.5°E; 35-38°N) and in the Central-Eastern Tyrrhenian Sea (13.3-15°E; 40-41.3°N), respectively. Specifically, the two surveys were conducted consecutively, from June 6th to July 16th in the Strait of Sicily and from July 17th to July 25th July in Central-Eastern Tyrrhenian Sea, in correspondence to the spawning period of Sardinella aurita in the Mediterranean Sea (Ben-Tuvia, 1960; Palomera and Sabatés, 1990). A systematic sampling has been carried out following a regular grid of stations (1/10° × 1/10° in both surveys along the continental shelf and 1/5° × 1/5° further offshore only in the Strait of Sicily). During the two cruises, 234 and 76 stations were sampled in the Strait of Sicily and in the Central-Eastern Tyrrhenian Sea, respectively (Figure 1). Planktonic sampling was conducted using a vertical CalVET (one mouth with 25 cm inlet diameter, 150 µm mesh) and an oblique Bongo 40 net (two mouths with 40 cm inlet diameter each, 200 µm mesh, towed at a vessel speed of 2 knots). The nets were hauled from within 5 m above the sea bottom to the surface, or from 100 m to the surface in the deeper stations. Calibrated flow-meters were mounted in each mouth for the estimation of the filtered water volume (m3) and borax-buffered solution of 4% formaldehyde and seawater (for CalVET and mouth 1-Bongo 40 samples) or a solution of 70% ethanol (for mouth 2-Bongo 40 samples) were used to preserve planktonic samples. The identification of Sardinella aurita at egg and larval stage were conducted in a land-based laboratory following the morphological criteria reported in Whitehead (1985) and Ditty et al. (1994). Standard length (SL) of individual larvae was measured to the nearest 0.1 mm using a Zeiss Discovery.V20 stereomicroscope with a PlanApo S 2.3x camera, and Image Pro Plus 6.0 image analysis software (Media Cybernetics).
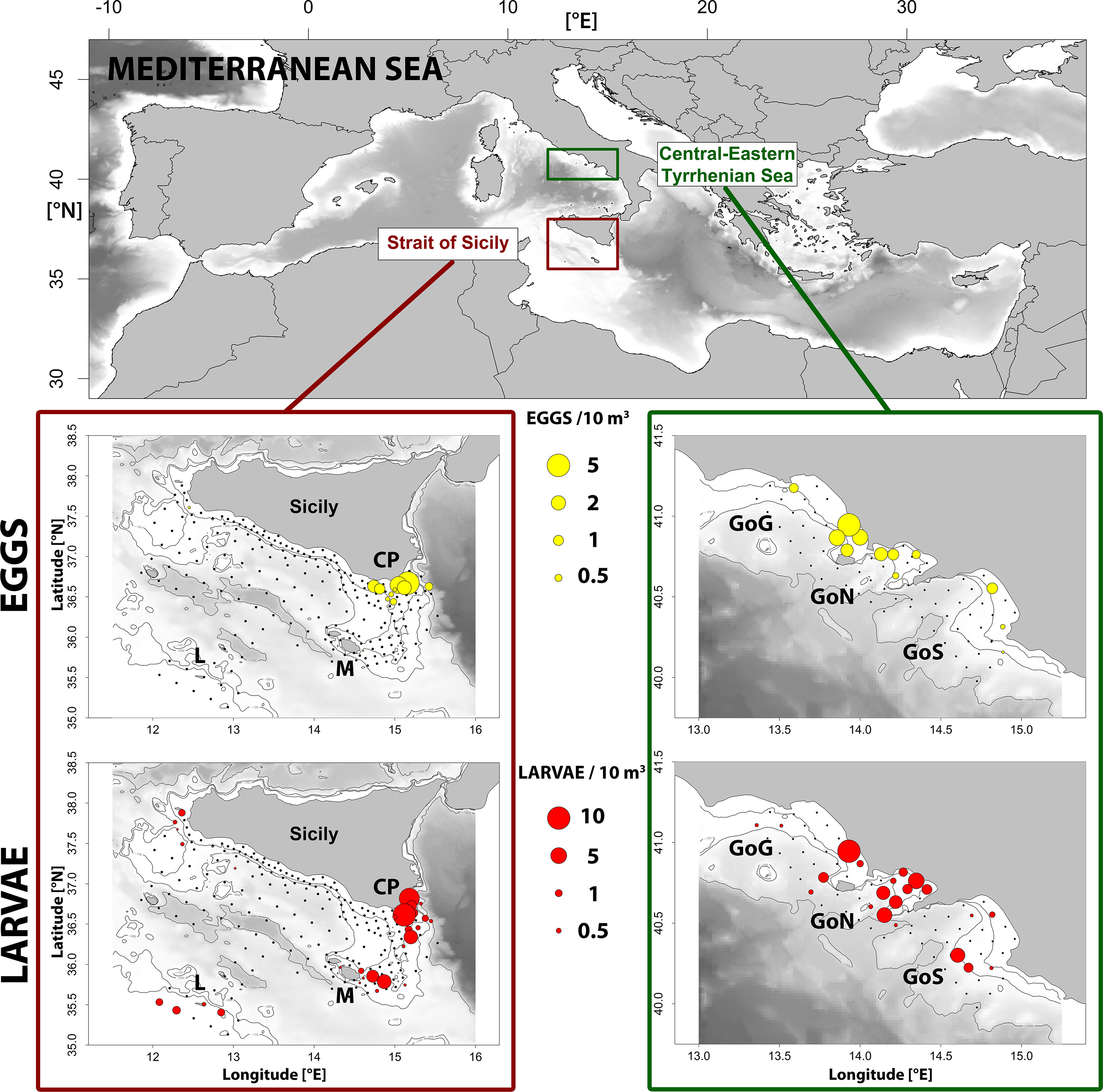
Figure 1 Spatial distribution of eggs and larvae of S. aurita in the Strait of Sicliy (left) and Central-Eastern Tyrrhenian Sea (right). In Strait of Sicily: CP, Capo Passero; M, Malta; L, Lampedusa. In Central-Eastern Tyrrhenian Sea: GoG, Gulf of Gaeta; GoN, Gulf of Naples; GoS, Gulf of Salerno. The isobaths of 100 m, 200 m and 1000 m have been reported in each map.
In order to obtain estimates of ages and larval growth, both sagittae were extracted and fixed on a slide for the otolith readings. Daily deposition of increments in Sardinella aurita was validated according to Balza et al. (2007). Within areas, larvae were chosen randomly from size classes of 1 mm of standard length (SL) with the aim at ensuring an equal representation of all zones and size classes. Otoliths were read at 1000 X magnification (oil immersion) using a ZEISS Axio Lab A1. Daily growth increments were counted in each sagitta, starting from the hatching mark, as described for the larval stage of Sardinella aurita by Balza and Marín (2000). Each otolith was read blindly (i.e., with no information on size or collection location) by a single reader, following the approach described in Sponaugle et al. (2009). This process was repeated twice. If the difference in age between the two readings was <5%, one of the readings was accepted randomly as the final read. Otherwise, the otoliths were read a third time. If a discrepancy ≥5% in the readings remained after a third read, the couple of otoliths was not used for further analysis. Final dataset included information from a total of 118 couples of sagittae (77 selected from the Strait of Sicily, 41 selected from the Central-Eastern Tyrrhenian Sea).
In addition to age determination, by image analysis based on Image-Pro Plus® software (Media Cybernetics, United States) three parameters were obtained from each otolith: the minimum diameter (DMIN); the maximum diameter (DMAX) and the width of the daily increments, the latter one considered as a good proxy for the mean daily larval growth. The width was measured from the core to the edge along the four directions described by DMIN and DMAX. Then, the mean value of the four measures was calculated for each increment. Finally, the mean value of width between the two sagittae has been considered for the subsequent analysis (hereafter called mean “increment Width”, IW). Following the same approach, in addition to the width of the daily increments, which are representative of the larval stage, we also considered the mean “core width” (CW) in order to include the egg stage. It has been defined as the mean distance along the four directions between the primordium and the first hatching mark, following the description provided by Balza and Marín (2000). A reference date has been hence back-calculated from the catch date for each measure of daily width.
The relationships between the diameters and the standard length (SL) or age (number of daily increments), i.e., DMIN-at-SL, DMIN-at-age, DMAX-at-SL and DMAX-at-age, were estimated using linear regression models. Moreover, the growth rate has been calculated from the relationship between the SL and age. In all linear regression models, relationships were compared among two study areas, and the analysis of covariance (ANCOVA) were performed in order to test differences between the two slopes and intercept estimates.
Backward Lagrangian simulations approach was adopted in order to assess the back-trajectory of aged larvae and identify their daily position from the sampling station to the estimated spawning region (i.e., back to age zero). The Mediterranean Forecasting System (MFS; Tonani et al., 2008) has been considered as a model for the Eulerian input. Its domain covers the entire Mediterranean basin. Spatially, MFS is characterized by a horizontal resolution of 1/16 x 1/16 degree (~ 6.5 km) and 72 vertical layers distributed from 1.5 m to 5000 m depth. In order to consider also the atmospheric forcings, 6-hourly wind data from the European Center Medium Weather Forecast (ECMWF) were included in Lagrangian runs. In addition to the large-scale circulation inferred from the MFS model, a 3D convective cell field (i.e., the kinematic model) has been added to the main model in order to compensate for the lack of effective mesoscale turbulent dispersion and vertical migration in the mixed layer [see details on the kinematic model set up, the 3D vertical mixing model, and the 2D mesoscale turbulence model in Palatella et al. (2014) and Lacorata et al. (2014)].
We then evaluated the backward-in-time evolution of Lagrangian particles, starting from the positions where larvae were caught. Field observations are used to generate a large number of virtual larvae (128,000 particles for each simulation), which are individually dispersed as a function of the initial conditions, the large-scale circulation and the subgrid-scale kinematic model (Lacorata et al., 2014; Palatella et al., 2014). The age (in days) has been estimated from the otolith analysis and determined the duration of the backward simulations. Each trajectory’s backward travel ends when its age turns to zero. This approach is fundamental to recognize the daily position as well as the original spawning ground. The boundary conditions of our simulations are open, with rebound conditions of the Lagrangian particles against the coasts (an accurate modelling of the circulation in the proximity of coastal boundaries is outside the capabilities of the Ocean model we used). Larvae were approximated as passive neutrally buoyant particles as during the developmental stages analyzed in this study (from hatch to a maximum age of 11 days) they have not yet acquired the ability to develop long-lasting active swimming capable of significantly influencing their horizontal position with respect to the physical forcing acting on the upper marine layers (Webb and Weihs, 1986; Batty and Blaxter, 1992). As regards Sardinella aurita, a reasonable period of passive advection can be identified during the first 28 days post-hatch (Mbaye et al., 2015).
Finally, in order to reconstruct the spawning areas from larval observations, a probability distribution function (PDF) of arrivals of back-trajectories based on the estimated individual ages (including 24h for the incubation period) has been calculated as the proportion of particles falling inside the cells of a grid whose mesh size is proportional to the extension of the study area (Strait of Sicily: 12-18°W, 35-38°N, 0.05° of cell size; Central-Eastern Tyrrhenian Sea: 13-15°W, 40-41.5°N, 0.025° of cell size).
In all planktonic stations, continuous vertical profiles of temperature, pressure and fluorescence were obtained from the surface to the bottom by means of a CTD SBE 911 plus probe equipped with a Turner fluorimeter (mod. Aquatracka) for chlorophyll-a profiling. The probes were calibrated before and after the cruise at the NURC (NATO Undersea Research Centre) in La Spezia, Italy. The overall accuracies are within 0.001 °C for temperature, 0.001 sm−1 for conductivity, and 0.015% of full scale for pressure. Then, mixed layer temperature, mixed layer salinity and mixed layer chlorophyll-a concentration (the last one considered as a good proxy for food availability) have been calculated as the mean value of the three parameters in the first 20 meters of the water column (the depth of 20 meters being representative of the lower limit for mixed layer, as estimated from the profiles in both areas). This allowed to characterize the environmental conditions of the surface layers where most of the larvae of this species, as well as other fish species, are used to occur and fulfill their feeding needs preying on phytoplankton and zooplankton (Olivar et al., 2014).
In addition, we made use of remote sensing data from Copernicus Marine Environment and Monitoring Service (CMEMS, http://copernicus.marine.eu) to characterize temperature and food availability conditions during the planktonic stage of all sampled larvae in 2013 (i.e., from June 29th to July 22th). Mean daily spatial gap-free data (L4) of Sea Surface Temperature (SST) and Chlorophyll-a concentration (CHL) (0.0625 x 0.0625 degrees of horizontal resolution in both datasets) were used as reference for the evaluation of the environmental conditions in each one of the daily spatio-temporal positions assumed by the simulated Lagrangian particles. Then a unique value per day of simulation has been derived as the mean of the 128,000 values associated to each Lagrangian backward simulation. Finally, environmental and growth data were merged in relation to the following considerations. First, the larval growth occurring at time t could not necessarily be affected by the environmental conditions occurring at time t due to a possible lagged effect of the environmental conditions in previous days (e.g., at times t-1 day and/or t-2 days). Secondly, while the temperature can potentially influence growth during the entire planktonic stage (including the egg stage), growth can be affected by food availability only during the exogenous feeding phase, which begins two days after hatching for Sardinella aurita (Ditty et al., 1994). Thirdly, temperature conditions could affect not only the IWs formed during the larval stage, but also to the CW that is the expression of the egg stage on the otoliths. In agreement with these considerations, four datasets were created in order to test our hypotheses:
● lag0_SST: CW and IWs at time t has been associated with the SST conditions occurring at time t.
● lag0_SST-CHL: IWs related to the exogenous feeding stage, at time t, has been associated with the SST and CHL conditions occurring at time t. Then, CW and the first two increments have been excluded.
● lag1_SST-CHL: IW related to the exogenous feeding stage, at time t, has been associated with the SST and CHL conditions occurring at time t-1 day. CW and the first three increments have been excluded.
● lag2_SST-CHL: IW related to the exogenous feeding, at time t, has been associated with the SST and CHL conditions occurring at time t-2 days. CW and the first four increments have been excluded.
Generalized Additive Mixed Models (GAMM) (Wood, 2011) have been implemented to explore the relationship between growth inferred by otolith analysis and the environmental covariates. Growth increment widths within an otolith are generally an allometric function of the age at which they were formed and can be affected by individual-specific physiological states (Helser et al., 2012). Therefore, all models were tested including the individual factor as random intercepts and the age as random slopes for each specimen, allowing each individual to have its unique growth trajectory for an unbiased estimation of the environmental covariates (Matta et al., 2018). The optimal random effect structure was explored using restricted maximum likelihood (REML) to correct the estimators for the variance terms (Zuur et al., 2009). Then, models were fitted using maximum likelihood (ML) estimation to determine the best intrinsic fixed effect structure. Regarding the fixed effect, in addition to the environmental covariates, the effect of the sampling area (2 levels: “Strait of Sicily”; “C-E Tyrrhenian Sea”) has been tested and subsequently excluded in all models as it was not significant. Regarding the response variables (i.e., the width of the increments), normal, log-normal and inverse gamma distributions were tested in each model. As the gamma distribution produced in all cases the best model in terms of Akaike information criterion (AICc; Akaike, 1998), for the interpretation of the results a Gaussian distribution and an “identity” link between the response variable and the systematic part of the models was considered. The hypothesis of a non-linear relationship between response and continuous covariates was tested by applying natural cubic spline smoothers to SST and CHL parameters included in the datasets. In this regard, we transformed CHL into a natural logarithm to achieve uniform distribution. For model validation, a normal Q-Q plot of the residuals, a plot of residuals versus fitted values and a plot of residuals versus variables considered in the model were used to assess normality distribution of the residuals and the assumptions of homogeneity and independence of the variables (Zuur et al., 2009).
Predictor importance has been used to compare the fixed effects in models implemented on different datasets (i.e., SST and CHL at time t, t-1 day and t-2 days). For this purpose, models from all possible combinations of the covariates have been considered in a model averaging procedure based on the AICc. A model selection has been applied considering ΔAICc<4 as a selection criterion (Burnham et al., 2011). Then, the relative importance of each predictor has been derived as the sum of Akaike weights (∑w; Burnham and Anderson, 2002) over the selected models, including the explanatory variable under estimation. Therefore, ∑w values range between 1 (the predictor is included in all selected models) and 0 (the predictor is excluded in all selected models). This approach has been successfully applied in other studies focused on ichthyoplanktonic species (Torri et al., 2021; Quinci et al., 2022).
All analyses were performed with R software version 4.0.4 (R Core Team, 2022). Statistical models were implemented using the “mgcv” R package (Wood, 2011). The R package “MuMIn” (Barton, 2020) has been used for the estimation of the predictor importance.
Most eggs and larvae of Sardinella aurita were found between the coastline and the isobath of 200 meters both in the Strait of Sicily and the Central-Eastern Tyrrhenian Sea (Figure 1). In the Strait of Sicily eggs were found quite exclusively in correspondence of the waters off Capo Passero (CP), the south-easternmost tip of Sicily Island. The highest abundance of larvae was also detected in the same zone, although further larval specimens were also sampled close to the Maltese archipelago (M), Lampedusa Island (L) and in the north-western part of the Strait of Sicily. The reconstruction of the spawning areas inferred from the Lagrangian simulations evidenced different dispersion pathways in this area (Figure 2A). The PDF of arrivals evidenced an important spawning area occurring along the eastern Sicilian coast. From this zone, runs highlighted a southward advection up to the CP area. Moreover, an additional spawning area has been detected in the north-eastern side of the M area, with a subsequent further eastward transport, and in the L area, both in correspondence of the continental shelf.
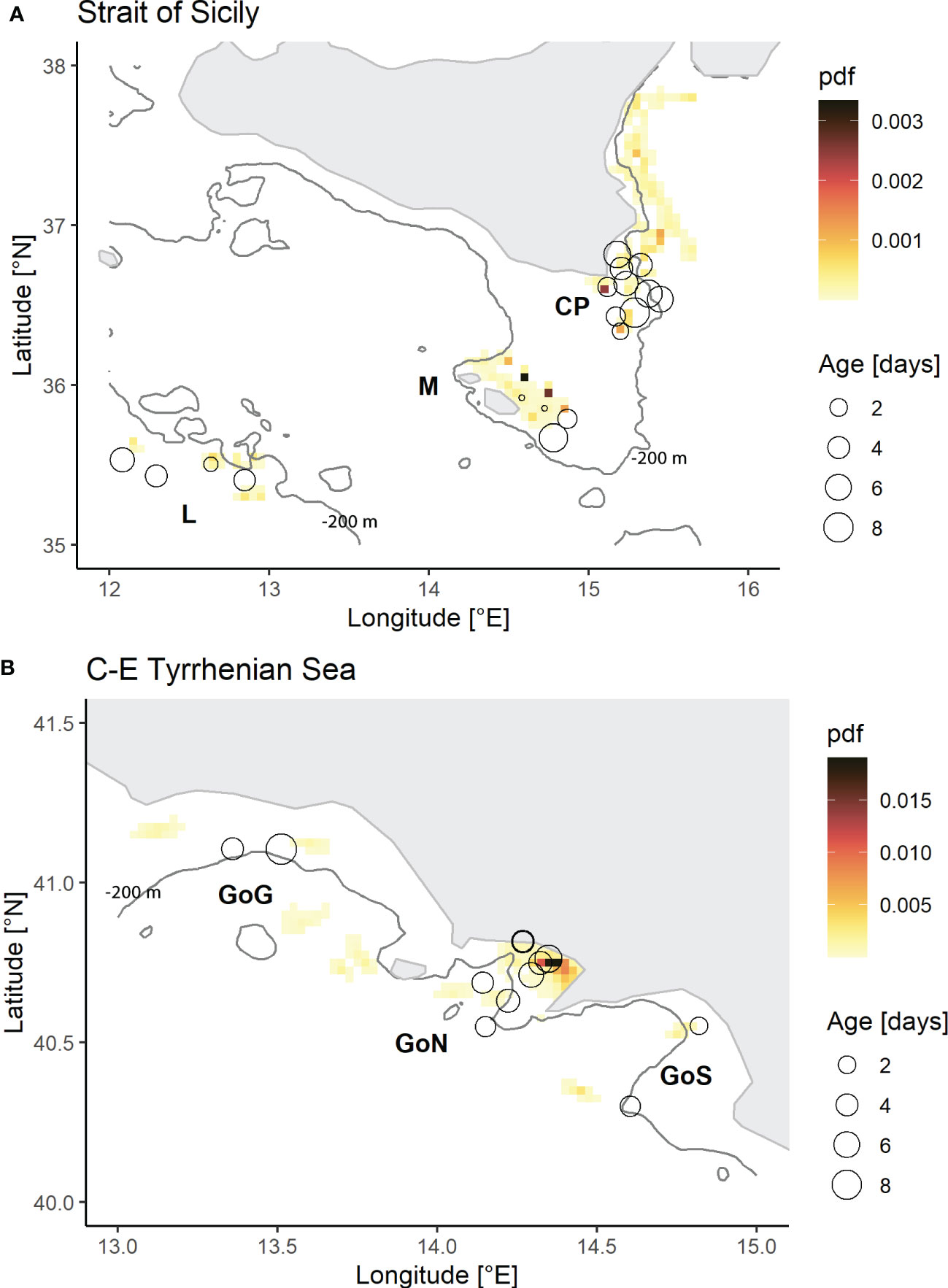
Figure 2 Reconstruction of the spawning areas located in the Strait of Sicily (A) and Central-Eastern Tyrrhenian Sea (B), based on the probability distribution functions (PDFs) derived from Lagrangian simulations. The black circles indicate the release points of the particles in the backward Lagrangian simulations. Their positions correspond to the sampling stations of larvae selected for otolith readings according to the followed methodology, while their sizes are proportional to the mean age of larvae sampled in each release point.
In the Central Tyrrhenian Sea, higher abundance of eggs and larvae were detected within the Gulf of Naples (GoN) (Figure 1). Here the main spawning area has been also identified (Figure 2B). However, planktonic stages and relatively smaller spawning zones were also found in the Gulf of Gaeta (GoG) and the Gulf of Salerno (GoS).
In the Strait of Sicily an increasing south-eastward temperature gradient emerged from CTD data in the mixed layer, ranging from 16°C in the north-western coastal region to 25°C in correspondence to CP and M zones (Figure 3A). A marked salinity front characterized by a rapid increase from 37.75 to 38.50 was evident in the waters off CP (Figure 3C). In addition, relatively saltier waters were detected in the north-western coastal zone. A similar pattern emerged from the distribution of the chlorophyll-a concentration, which showed higher values in the coastal areas of the north-western sector (Figure 3E). Moreover, relatively enhanced trophic conditions emerged in some stations of CP and M area, compared to the offshore zone. Mixed layer waters of the Central-Eastern Tyrrhenian Sea were generally warmer and characterized by higher chlorophyll-a concentrations, especially within the GoG and the GoN (Figure 3B, F). Salinity ranged between 37.5 and 38.2, although differences emerged among the three gulfs, with GoN characterized by relatively fresher waters (Figure 3D).
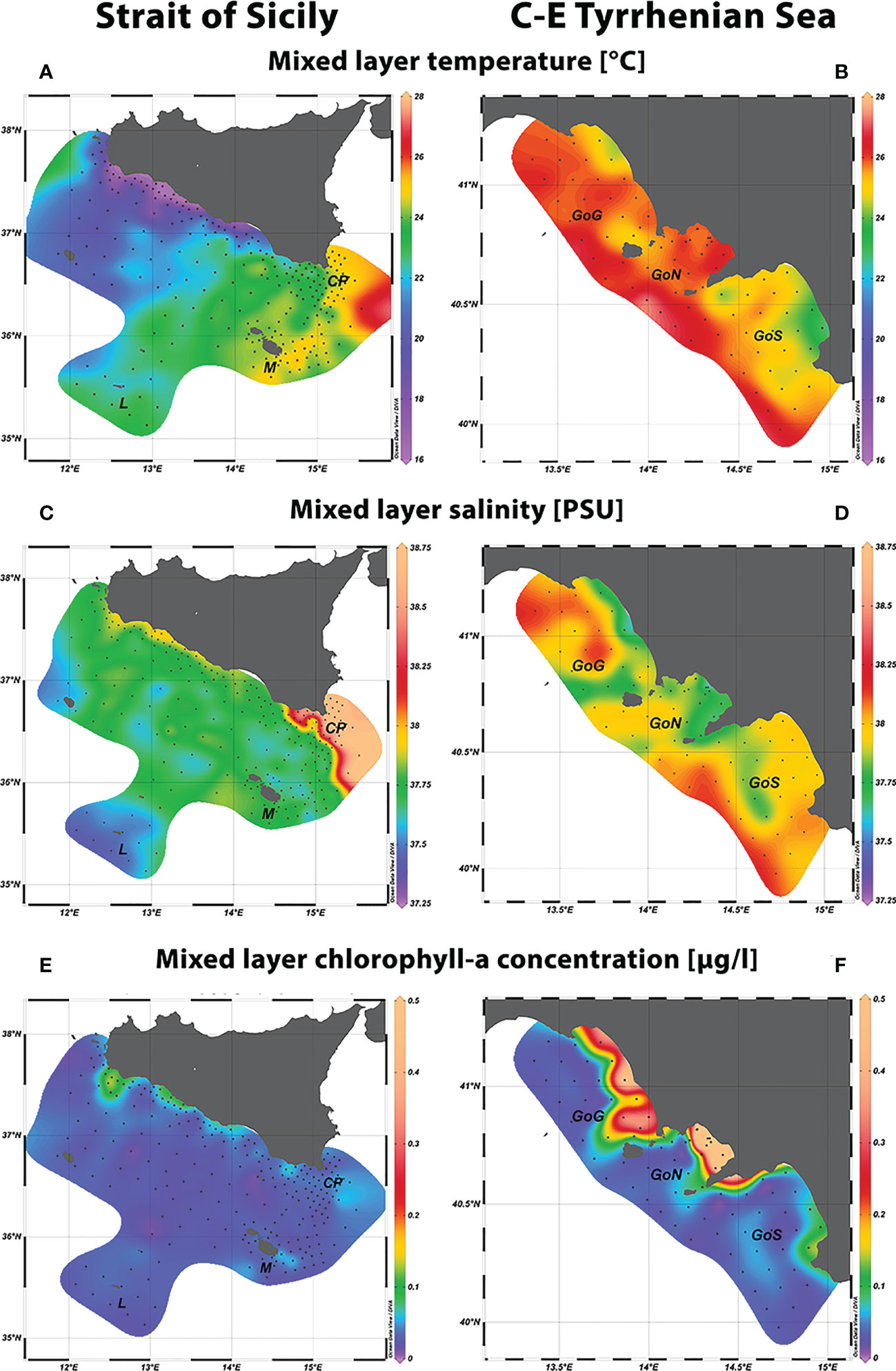
Figure 3 Spatial distribution of mixed layer temperature (A, B), mixed layer salinity (C, D), mixed layer chlorophyll-a concentration (E, F) recorded in situ in the two considered study areas.
Daily time series of sea surface temperature (SST) and chlorophyll-a concentration (CHL) allowed to detect fluctuations among regions. According to the progress of summer season, daily SST showed an increasing trend in both regions, although different patterns emerged among regions. Indeed, SST was higher in the Sicilian sub-area during the first days of July, while an opposite trend followed in the second part of the month (Figure 4A). Differently, CHL showed more variable patterns among regions. It was generally higher and variable in the northern site (especially in GoG and GoN) compared to the Sicilian area, where minimum values were recognized around L island (Figure 4B).
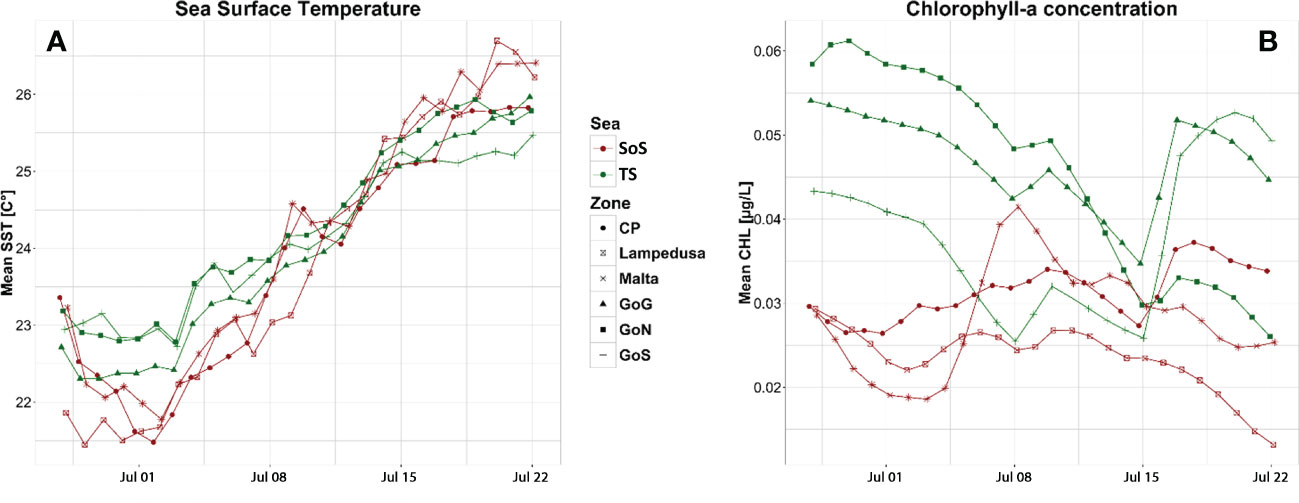
Figure 4 Daily average values of the two satellite parameters, SST (A) and CHL (B), occurring in the identified spawning grounds for S. aurita based on the egg distribution. Time period from June 29th to July 22th was selected based on the duration of planktonic phase of larvae as derived from otolith analysis. TS, Central-Eastern Tyrrhenian Sea; SoS, Strait of Sicily; CP, Capo Passero; GoG, Gulf on Gaeta; GoN, Gulf of Naples; GoS, Gulf of Salerno.
Otolith analysis highlighted a significant linear relationship between age and both maximum and minimum otolith diameters (Dmax and Dmin), while not significant heterogeneity of the slope was found among regions (ANCOVA: p>0.05) (Table 1; Figure 5A, B). Moreover, a significant relationship was found between SL and both diameters (Dmax and Dmin), confirming that otolith increments can be considered as a good proxy for examining the somatic growth in this species at larval stage. However, in this case limited but significant differences were detected among regions (ANCOVA: p<0.01) (Table 1; Figure 5C, D), with steeper trends estimated from the analysis of samples collected in the Strait of Sicily.

Figure 5 Linear regression models of otolith diameters (Dmax and Dmin) -at-age (A, B) and –at-SL (C, D). Separate models by regions are shown in case of significant statistical differences as detected by ANCOVA.
A significant growth rate was obtained from SL-at-age linear regression (Table 1; Figure 6). The obtained instantaneous growth parameter was 1.00 mm/day. Regression parameters do not vary between two regions (ANCOVA: p-values > 0.05).
The mean values of the core width (CW) and increment width (IW) during the first 7 days of larval life in the regions are shown in Figure 7. The difference in CW between the two regions was not statistically significant. In addition, CW showed a lower standard error compared with the other increments. Obtained mean value of CW in this study was 5.27 ± 0.82.
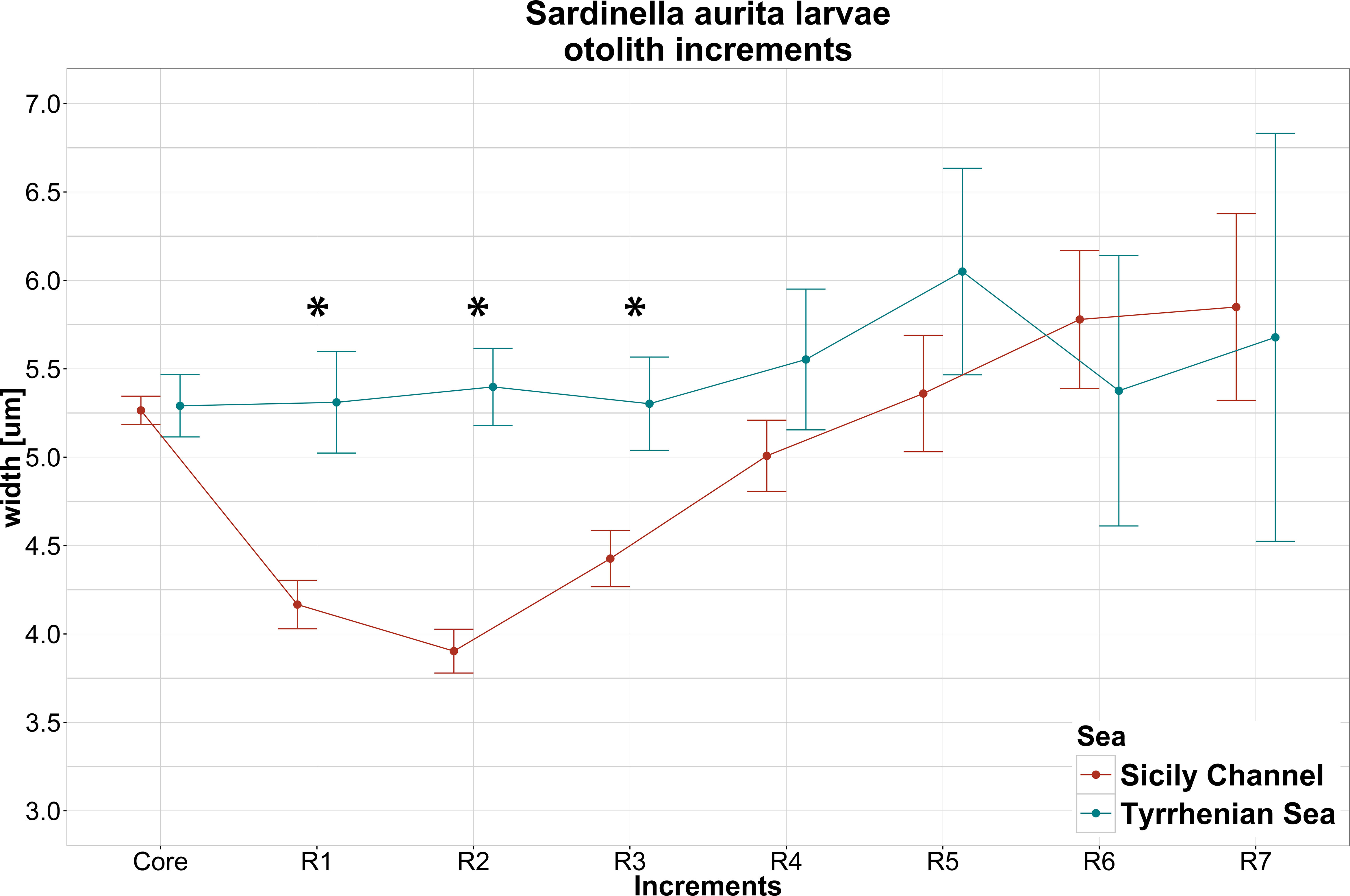
Figure 7 Mean ± SE of core width (Core) and increment width (R1-R7) for larvae collected in Sicily Channel and Tyrrhenian Sea. The significance of Mann–Whitney U test (*= p<0.01) implemented between regions is also shown.
Mann–Whitney U test applied to test difference between regions in terms of mean of otolith increment width (MIW) values showed differences just for the first three daily increments (U: p-value< 0.01), while no significant differences emerged for other increments.
Two GAMMs were implemented to study the temperature effect on the growth at time t (dataset Lag0_SST) (Table 2). In order to assess the effect of the ontogenetic stage, a first model included an interaction term between temperature and the stage (egg or larva) and it has been compared to a second model with SST as the only fixed term. The first GAMM has been identified as the best model in terms of adjusted R-squared, deviance explained and AIC. Significant regression splines evidenced a different relationship between the temperature and the increments formed during the eggs and the larval period. For the egg stage, a negative correlation between temperature and CW emerged for values higher than 23.5°C (Figure 8A). An opposite correlation came out for larval stages. Indeed, a positive non-linear relationship has been evidenced between temperature and the mean daily growth, increasing the slope for values higher than 24°C (Figure 8B). Similarly, a positive sub-linear smoother came out also from the second model that did not consider the interaction term (Figure 8C).
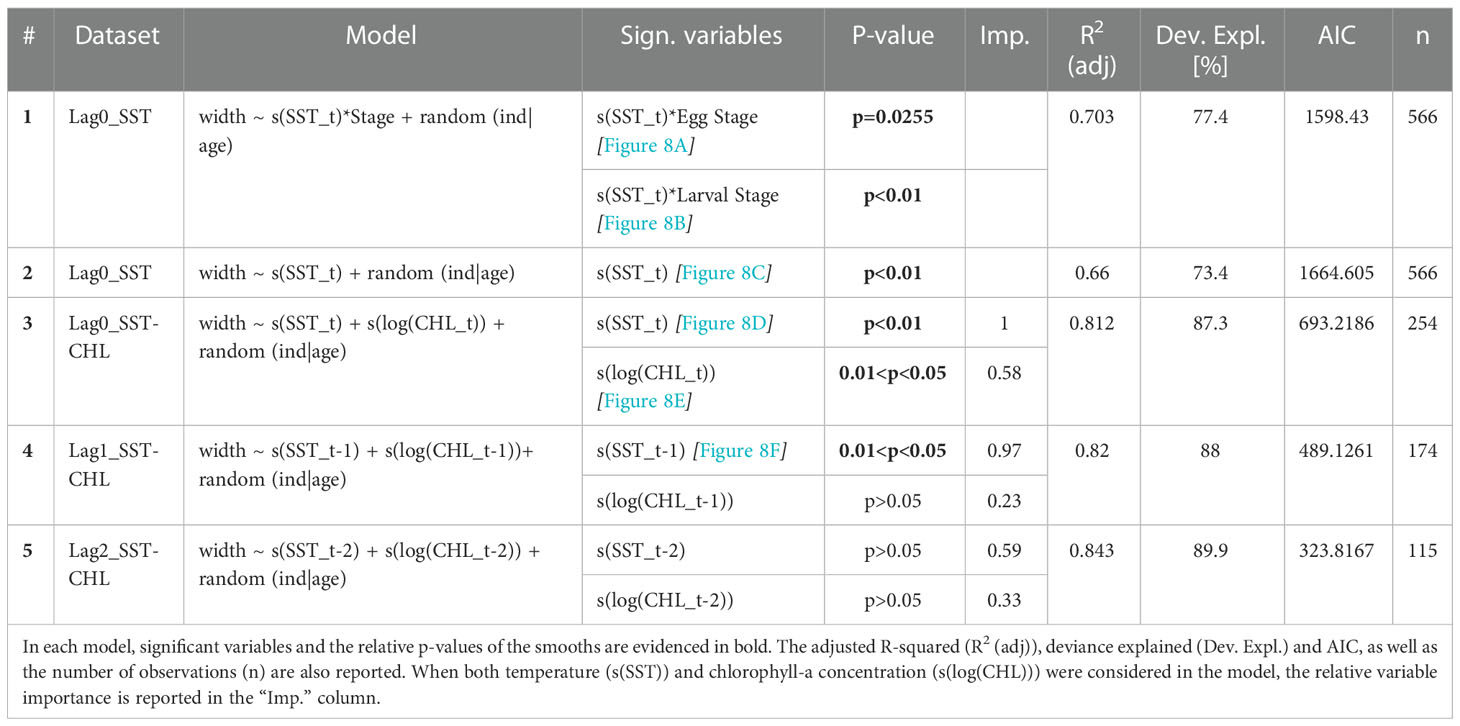
Table 2 Outcomes of the GAMMs including the individual factor as random intercepts and the age as random slopes [in the model: random(ind|age)].
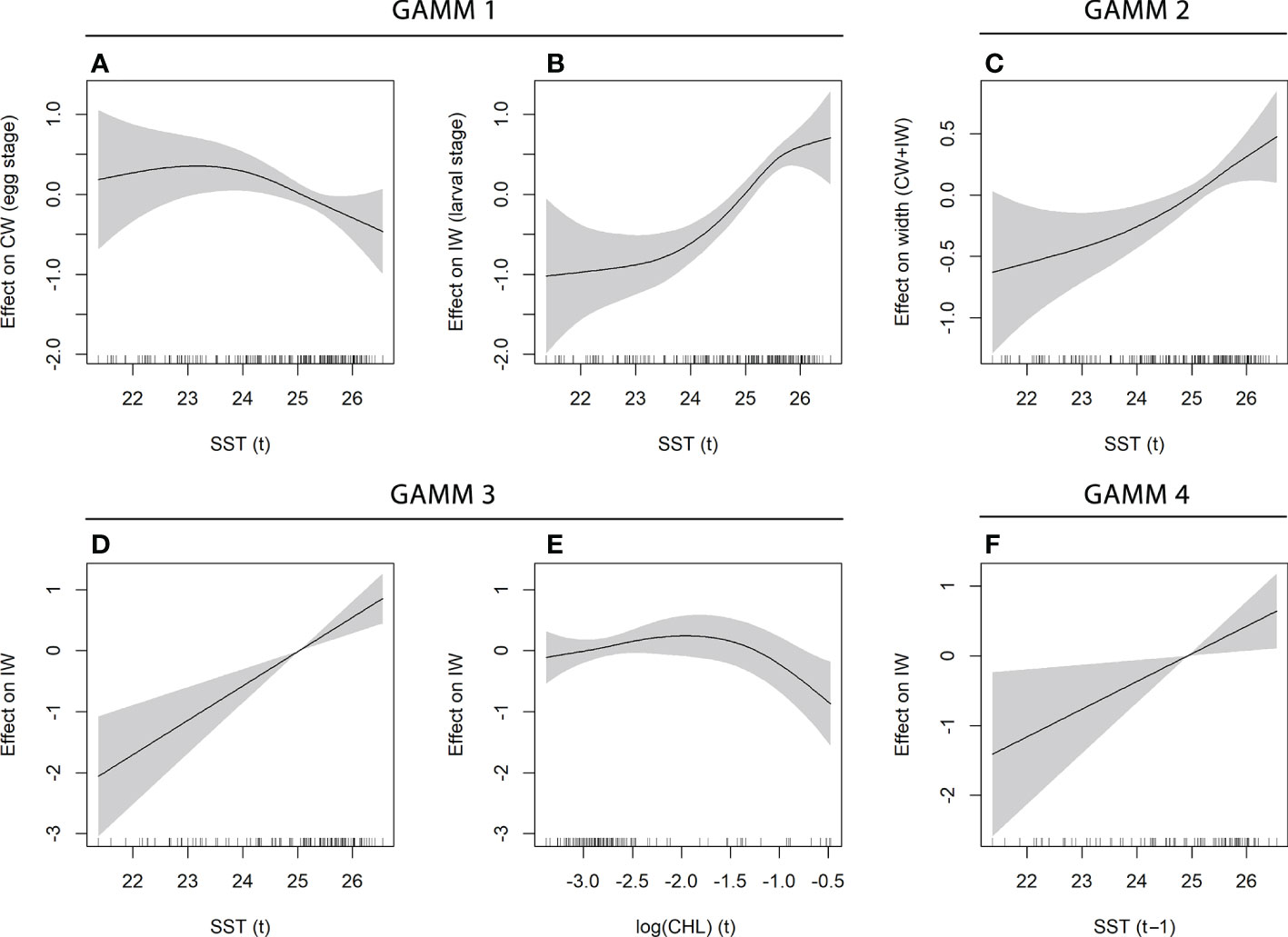
Figure 8 Significant cubic regression smoothers estimated in GAMMs performed on the mean width of otolith increments (IW, response variable) and environmental factors (explanatory variables). Further outcomes are reported in Table 2 (plots for GAMM #5 are not displayed because both covariates were not significant). In each panel, the grey region defines the confidence interval of the estimated smooth term. Vertical ticks over the horizontal axes represent the covariate distribution expressed as a 1-d rug plot.
Moreover, a GAMM including the effect of both SST and food availability (CHL) at time t has been performed on the Lag0_SST-CHL dataset. Results showed a significant effect of both SST and CHL. Among them, temperature emerged as the most important variable compared to food availability (Table 2). The model highlighted a positive linear effect of the temperature on the larval IWs (Figure 8D). Differently, only a weak positive correlation emerged between CHL (proxy for food availability) and IWs for low and medium values of chlorophyll-a concentration, which represented most of the data, while an opposite and more pronounced negative relationships emerged for a few higher values (Figure 8E).
Finally, two additional models have been run to evaluate the potential lagged effect of the temperature and food availability on the mean daily growth. For this purpose, lagged environmental covariates at the time t-1 day and t-2 days have been included in the models, considering the dataset Lag1_SST-CHL and Lag2_SST-CHL, respectively. The outcomes of these GAMMs, although they are not directly comparable with previous models in terms of fitting performance due to the different number of observations, showed a progressively less significant effect of the environmental covariates on the otolith mean increment width at time t in relation to the increase of the delay considered. Indeed, only the temperature was significant in the fourth model (t-1 environmental covariates) (Figure 8F), while no significant effects emerged in the fifth model (t-2 environmental covariates). The importance of both variables progressively decreased in both models (Table 2). However, similarly to the other models, temperature resulted the most important environmental covariate also in the last two models.
The distribution of the eggs of Sardinella aurita in the Strait of Sicily confirmed the presence of an important spawning zone in an already recognized favourable area for larval growth and development of other species, i.e., the coastal zone of CP area (Falco et al., 2020; Cuttitta et al., 2022). However, the PDF of particle arrival obtained from simulation runs allowed to reconstruct a wider spawning zone which includes this area and extends northwards along the eastern Sicilian coast. This finding evidenced a southward dispersion process that appears additional to the one described in most of the literature existing in this study area. Here, many authors identify an advection originating from the western zones of the Strait of Sicily and linked to the south-eastward flow of the fresher surface Atlantic Ionian Stream (AIS), which here dominates the superficial hydrodynamics (Robinson et al., 1999) and plays a key role in transporting planktonic fish stages to waters off CP (García Lafuente et al., 2002; Falcini et al., 2015; Cuttitta et al., 2018; Torri et al., 2018; Patti et al., 2020; Torri et al., 2021; Russo et al., 2022). In this context, CP has been described as an important retention zone due to the presence of a cyclonic structure and a saline front, which create favourable conditions for the recruitment of fish species in the area. The southward larval transport from the eastern coastal zone of Sicily could therefore be interpreted as a complementary transport mechanism towards this important retention area. From the oceanographic point of view, it is interesting that larvae found in this study were located at the eastern side of the saline front highlighted in the Figure 3, in correspondence of saltier waters, making reasonable the hypothesis of a dispersion dynamic unconnected to the AIS. In agreement with this interpretation, the higher ichthyoplankton abundance and diversity that characterizes the Capo Passero area (Cuttitta et al., 2018; Patti et al., 2022) could be the result of different dispersion processes converging in the area. Actually, PDF of arrivals reported in Falcini et al. (2020), in emphasizing the connectivity between the Sicilian area and the Tunisian coasts linked to the AIS also identified the Ionian area as a potential area of origin of European anchovy larvae found in CP area. However, further studies would be needed to quantify the effect of this further process in terms of persistence of the structures underlying the transport dynamics, as well as to study the existence of retention mechanisms in the eastern saltier waters beyond the saline front. Moreover, in agreement with Torri et al. (2018), a spawning area and a subsequent eastward larval advection attributable to AIS has been identified on the continental shelf close to the Maltese archipelago. Finally, larval stages found in Lampedusa have not been associated with significant transport phenomena, evidencing thus the occurrence of a local spawning activity on the African continental shelf.
In addition, our observations provided a spatial representation of the early life stages of this species in a poorly explored area of the Central-Eastern Tyrrhenian Sea, identifying the three gulfs as spawning areas and highlighting the ability of this species to reproduce in different regions of the Mediterranean Sea. Here, respect to the southern site, a lower hydrodynamic linked to the lack of noticeable mesoscale oceanographic structures has been described (Torri et al., 2021) and, in agreement with this scenario, our runs evidenced limited advections generally heading north.
In both regions it is interesting to note an overlap between the occurrence of early life stages and relatively warmer and chl-enriched waters. Specifically, in the Strait of Sicily the presence of an upwelling system in the north-western area, leading colder and nutrient-enriched waters from the bottom to the shallower layers, could limit the reproduction of this species due to its thermophilic features. Previous studies conducted in the north-western Mediterranean indicated that the maturation of the gonads in this species is temperature-dependent and the spawning of this species takes place at temperatures higher than 23°C (Navarro, 1932; Andreu and Rodriguez-Roda, 1951; Palomera and Sabatés, 1990). In the zone corresponding to the upwelling system, coastal temperature values were lower, ranging between 16 and 22°C, partly crossing the sub-lethal temperature range for the larvae of this species that has been estimated to be between 14 and 18°C (Mbaye et al., 2015). Therefore, despite the higher food availability characterizing this area, the lower temperature values could be crucial in this case, forcing adults to shift reproduction in warmer zones, such as the south-eastern part of the study area. Differently, in the Central-Eastern Tyrrhenian Sea we found higher temperature and food availability conditions compared with the southern site. Here, higher densities were spatially correlated with the enhanced food conditions characterizing GoN and the southern part of GoG. In these zones, the temperature was higher than 23°C and food availability could be an important driver affecting the spatial distribution of adults; actually, the latter, being zooplankton feeders, are typically linked to areas characterized by high primary productivity (Ben-Tuvia, 1960; Sabatés et al., 2009). In support of these considerations, the lower density of eggs and larvae collected in the GoS corresponded to lower trophic conditions despite temperature values higher than 23°C.
In spite of the different conditions experienced by larvae in terms of temperature and food availability, no statistically different growth rates emerged by the otolith analysis between regions, although larvae collected in the Central-Eastern Tyrrhenian Sea showed slightly faster growth rates. Actually, the analysis of the mean daily growth evidenced a different mean daily growth rate limited to the first three days of the larval stage, with lower values in larvae collected in the Strait of Sicily. Considering that larvae of Sardinella aurita begin an active feeding behavior by day 2 (Ditty et al., 1994), detected differences could be mainly the result of differences in temperature conditions and only in part linked to food availability. This evidence agrees with the output of GAMM models, which in recognizing a significant and not-lagged effect of both environmental covariates, specifically highlighted the greater importance of the SST compared to CHL. In this framework, the weaker relationship between the chlorophyll-a concentration and the mean daily growth rate for most of our data support the hypothesis of a limited contribution of food availability on the growth rate during the larval stage. A considerable body of evidence on this research topic points out that food is rarely a limiting factor for larval growth and recruitment in nature (see review of Leggett and Deblois, 1994). Indeed, relatively few studies found evidence that growth rates of marine fish larvae are occasionally food limited and, actually, only a small proportions of marine fish larvae starve in the marine environment (Paulsen et al., 2017 and references therein). Jørgensen et al. (2014) evidenced that low prey availability affects the recruitment leading to an increasing of the predation rate due to a behavioural change rather than affecting the larval growth rate. Findings emerging in our study support this thesis and emphasize the effect of temperature as the main environmental factor influencing larval growth in this species.
However, different patterns emerged between the larval and the egg stage, i.e., during the formation of the CW. Compared to the sub-linear positive correlation between temperature and IWs, the regression spline of the first model showed a decrease in the CW at higher temperature that could be the interpretated as a consequence of the reduced time for egg development. Indeed, some studies focused on other small pelagic species evidenced a decrease in the eggs size when the temperature increased (Tsuruta, 1989; Yoneda et al., 2014). In this framework, the high temperatures could lead to early hatching or an early development stage of the larva-at-hatch, resulting in a smaller otolith size. In support to this hypothesis, there are findings from studies focused on mesocosm experiments. For instance, a reduced otolith size at higher incubation temperature has been found in other clupeids, such as the Clupea harengus (Høie et al., 1999), as well as in other fish species (Luczynski et al., 1984; Bengtson et al., 1987). However, as discussed in Høie et al. (1999), our results could not be generalizable to all fish species due to opposite trends in other fish species. Nevertheless, compared to studies carried out in the mesocosm, it is worth noting how our findings emerged from observations collected in situ and suggested a temperature effect on the synchronisation between hatching and embryogenesis, tuning the hatching event to a later or earlier stage of ontogeny.
This study provided an updated overarching methodological approach which combines biological study methods, physical simulations, satellite observations and advanced statistical techniques in order to investigate on the ecology of fish larval stages starting from field observations. Lagrangian simulations allowed to identify new patterns concerning the larval dispersion of larvae in the study areas that could contribute to explain evidences described in the existing literature. Moreover, the modelling approach supported the use of field observations in order to address the possible delayed effect of environmental variables on the mean daily growth. Regarding Sardinella aurita early life stages, the most convincing relationship has been linked to the simultaneous occurrence on a daily scale between growth and marine environmental conditions experienced by larvae, providing useful insights on the physiological response of early life stage to external factors, with potential cascading effects on the recruitment and the biomass of the adult populations. Our holistic approach appears to be facilitated by the availability of a large amount of data deriving from remote sensing, with positive effects on our ability to carry out ecological studies in support of fisheries management. In fact, it is potentially generalizable to other marine areas and target fish species for which it is possible to collect growth data. This study described also reproductive events of Sardinella aurita in different areas of the Central Mediterranean Sea, highlighting their occurrence also in the northernmost region of our study area (40-41.3°N) and suggesting an adaptability of this species to reproduce in different environmental conditions. Temperature and food availability emerged as important factors for the development of Sardinella aurita eggs and larvae. In particular, the temperature acts as an important driver not only at the larval stage, also influencing the size at hatching and hence the probabilities of the newly-hatched larvae to survive in the natural environment. In this context, the expected temperature increase due to climate change could improve the growth performance of this thermophilic species and promote its expansion towards higher latitudes, becoming an increasingly common species in a changing marine environment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval were not required for the animal study because we studied planktonic stages (egg and larvae) of fish in the natural marine environment.
MT, AC and MA, conceptualized the scientific idea and contributed to develop the overall concept of the paper. MT analyzed the data and wrote the manuscript. FF, RC and GL contributed to the implementation and the analyses of the Lagrangian simulations and to the writing of the manuscript. AC and SR contributed to sorting the plankton samples and to writing of the manuscript. FF, SC and GV collected and analyzed remote sensing data. BD, FP, LG, MM, TM, CB, MD and BP participated to the fieldwork and laboratory analyses. All the authors contributed to develop the interpretation of the results and the development of the discussion and the conclusions. All authors contributed to the article and approved the submitted version.
This study has been developed in the framework of the XXVI Ph.D program in Environmental Biology and Biodiversity of the University of Palermo. In addition, this study was supported by the Italian National Research Council (CNR) through USPO office, and by the FAO Regional Project MedSudMed “Assessment and Monitoring of the Fishery Resources and the Ecosystems in the Straits of Sicily”, funded by the Italian Ministry MIPAAF and co-funded by the Directorate-General for Maritime Affairs and Fisheries of the European Commission (DG MARE). The study was also supported by the research projects SSD-PESCA, coordinated by the Ministry of the Education, University and Research (MIUR) and founded by the Ministry of Economic Development (MISE), and the Flagship Project RITMARE - The Italian Research for the Sea, coordinated by the Italian National Research Council and funded by the MIUR within the National Research Program 2011–2013. Activities involved in this study were preparatory to actions planned in the framework of “SevenS” project, financed by the European Maritime Policy, Fisheries and Aquaculture Fund through the PO FEAMP Sicilia 2014-2020 in collaboration with the Dipartimento Pesca Mediterranea - Assessorato Agricoltura, Sviluppo Rurale e Pesca Mediterranea of the Regione Siciliana.
Mr. Emanuele Gentile, Master of the R/V Urania and all his crew are thanked for their work in support to the plankton sampling during the oceanographic cruises. The coordinator of the fieldwork in Central- Eastern Tyrrhenian Sea, Dr. Angelo Bonanno, and all of the participating institutes and scientists who were on-board are gratefully acknowledged for their involvement in the work carried out. We are grateful to Grazia Maria Armeri, Carmelo Buscaino, Gaspare Buffa, Girolama Biondo and Francesca Mangiaracina for their valuable technical support during the sampling and data collection. Finally, special thanks go to Salvatore Mazzola, whose scientific efforts facilitated the start of the sampling surveys in the study area.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akaike H. (1998). Information theory and an extension of the maximum likelihood principle (New York, NY: Springer), 199–213.
Altobelli C., Perzia P., Falautano M., Consoli P., Canese S., Romeo T., et al. (2017). Mediterranean Banks in EBSA area: Hotspots of biodiversity under threat. Mar. Environ. Res. 131, 57–68. doi: 10.1016/j.marenvres.2017.09.005
Anderson J. T. (1988). A review of size dependent survival during pre-recruit stages of fishes in relation to recruitment. J. Northw. All. Fish. Sci. 8, 55–66. doi: 10.2960/J.v8.a6
Andreu B., Rodriguez-Roda J. (1951). Estudio comparativo del ciclo sexual, engrasamiento y repleción estomacal de la sardina, alacha y anchoa del mar catalán, acompañado de relación de pescas de huevos planctónicos de estas especies. Publicaciones Del Instituto Biol. Aplicada 9, 193–232.
Balza M. A., Lemus M., Marín B. (2007). Tasa de crecimiento en larvas de sardinella aurita valencienne 1847 (Pisces: clupeidae) del morro de puerto santo, Venezuela. Interciencia 32, 333–338.
Balza M. A., Marín B. (2000). Verificación de la marca de eclosión en los otolitos sagitales de larvas de Sardinella aurita (Pisces: Clupeidae). Rev. Biol. Trop. 48, 183–186.
Barton K. (2020) MuMIn: Multi-model inference. r package version 1.43.17. Available at: https://CRAN.R-project.org/package=MuMIn.
Batty R. S., Blaxter J. H. S. (1992). The effect of temperature on the burst swimming performance of fish larvae. J. Exp. Biol. 170, 187–201. doi: 10.1242/jeb.170.1.187
Bengtson D. A., Barkman R. C., Berry W. J. (1987). Relationships between maternal size, egg diameter, time of spawning season, temperature, and length at hatch of Atlantic silverside, Menidia menidia. J. Fish. Biol. 31, 697–704. doi: 10.1111/j.1095-8649.1987.tb05272.x
Benoît H. P., Pepin P., Brown J. A. (2000). Patterns of metamorphic age and length in marine fishes, from individuals to taxa. Can. J. Fish. Aquat. Sci. 57, 856–869. doi: 10.1139/f00-019
Ben-Tuvia A. (1960). Synopsis of biological data on Sardinella aurita of the Mediterranean sea and other waters. FAO Fish Biol Synopsis 14, 287–312.
Blaxter J. H. S. (1991). The effect of temperature on larval fishes. Netherlands J. Zool. 42, 336–357. doi: 10.1163/156854291X00379
Burnham K. P., Anderson D. R. (2002). Model selection and multimodel inference: A practical information-theoretic approach (New York: Springer-Verlag).
Burnham K. P., Anderson D. R., Huyvaert K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–35. doi: 10.1007/s00265-010-1029-6
Colloca F., Cardinale M., Belluscio A., Ardizzone G. (2003). Pattern of distribution and diversity of demersal assemblages in the central Mediterranean sea. Estuar. Coast. Shelf Sci. 56, 469–480. doi: 10.1016/S0272-7714(02)00196-8
Cowan J. H. J., Shawn R. F. (2002). “Recruitment,” in Fishery science. the unique contributions of early life stages. Eds. Fuiman L. A., Werner R. G. (Oxford, UK; Blackwell Publishing Ltd), 88–111.
Cuttitta A., Patti B., Musco M., Masullo T., Placenti F., Quinci E. M., et al. (2022). Inferring population structure from early life stage: The case of the European anchovy in the Sicilian and Maltese shelves. Water (Basel) 14, 1427. doi: 10.3390/w14091427
Cuttitta A., Quinci E. M., Patti B., Bonomo S., Bonanno A., Musco M., et al. (2016). Different key roles of mesoscale oceanographic structures and ocean bathymetry in shaping larval fish distribution pattern: A case study in Sicilian waters in summer 2009. J. Sea Res. 115, 6–17. doi: 10.1016/j.seares.2016.04.005
Cuttitta A., Torri M., Zarrad R., Zgozi S., Jarboui O., Quinci E. M., et al. (2018). Linking surface hydrodynamics to planktonic ecosystem: the case study of the ichthyoplanktonic assemblages in the central Mediterranean Sea. Hydrobiologia 821, 191–214. doi: 10.1007/s10750-017-3483-x
di Lorenzo M., Sinerchia M., Colloca F. (2018). The north sector of the strait of Sicily: A priority area for conservation in the Mediterranean Sea. Hydrobiologia 821, 235–253. doi: 10.1007/s10750-017-3389-7
Ditty J. G., Houde E. D., Shaw R. F. (1994). Egg and larval development of Spanish sardine, Sardinella aurita (family clupeidae), with a synopsis of characters to identify clupeid larvae from the northern gulf of Mexico. Bull. Mar. Sci. 54, 367–380.
Falcini F., Corrado R., Torri M., Mangano M. C., Zarrad R., di Cintio A., et al. (2022). Seascape connectivity of European anchovy in the central Mediterranean Sea revealed by weighted Lagrangian backtracking and bio-energetic modelling. Sci. Rep. 10 (1), 1–13. doi: 10.1038/s41598-020-75680-8
Falcini F., Palatella L., Cuttitta A., Nardelli B. B., Lacorata G., Lanotte A. S., et al. (2015). The role of hydrodynamic processes on anchovy eggs and larvae distribution in the Sicily channel (Mediterranean sea): A case study for the 2004 data set. PloS One 10, e0123213. doi: 10.1371/journal.pone.0123213
Falco F., Barra M., Wu G., Dioguardi M., Stincone P., Cuttitta A., et al. (2020). Engraulis encrasicolus larvae from two different environmental spawning areas of the central Mediterranean Sea: first data on amino acid profiles and biochemical evaluations. Eur. Zool. J. 87 (1), 580–590. doi: 10.1080/24750263.2020.1823493
FAO-GFCM (2021). Fisheries and aquaculture software. FishStatJ-software for fishery statistical time series. FAO Fish. Aquacult. Department. (Rome, Italy). Available at: https://www.fao.org/fishery/en/statistics/software/fishstatj.
Gačić M., Schroeder K., Civitarese G., Cosoli S., Vetrano A., Eusebi Borzelli G. L. (2013). Salinity in the Sicily channel corroborates the role of the Adriatic–Ionian bimodal oscillating system (BiOS) in shaping the decadal variability of the Mediterranean overturning circulation. Ocean Sci. 9, 83–90. doi: 10.5194/OS-9-83-2013
García Lafuente J., García A., Mazzola L., Quintanilla J., Delgado J., Cuttitta A., et al. (2002). Hydrographic phenomena influencing early life stages of the Sicilian channel anchovy. Fish. Oceanogr. 11, 31–44. doi: 10.1046/j.1365-2419.2002.00186.x
Høie H., Folkvord A., Johannessen A. (1999). Maternal, paternal and temperature effects on otolith size of young herring (Clupea harengus l.) larvae. J. Exp. Mar. Biol. Ecol. 234, 167–184. doi: 10.1016/S0022-0981(98)00154-3
Helser T. E., Lai H.l., Black B. A. (2012). Bayesian Hierarchical modeling of pacific geoduck growth increment data and climate indices. Ecol. Modell 247, 210–220. doi: 10.1016/J.ECOLMODEL.2012.08.024
Hjort J. (1914). Fluctuations in the great fisheries of northern Europe. Rapports Et Proces-Verbaux La Commission Internationale Pour L’exploration Sci. 20, 1–228.
Houde E. D. (1989). Comparative growth, mortality, and energetics of marine fish larvae: temperature and implied latitudinal effects. Fish. Bull. 87, 471–495.
Houde E. D. (2008). Emerging from hjort’s shadow. J. Northw. Atl. Fish. Sci. 41, 53–70. doi: 10.2960/J.v41.m634
Houde E. D. (2009). “Recruitment variability,” in Fish reproductive biology. Eds. Jakobsen T., Fogarty M. J., Megrey B. A., Moksness E. (Oxford, UK; Blackwell Publishing Ltd), 91–171.
Jørgensen C., Opdal A. F., Fiksen Ø. (2014). Can behavioural ecology unite hypotheses for fish recruitment? ICES J. Mar. Sci. 71, 909–917. doi: 10.1093/icesjms/fst083
Lacorata G., Palatella L., Santoleri R. (2014). Lagrangian Predictability characteristics of an ocean model. J. Geophys. Res. Oceans 119, 8029–8038. doi: 10.1002/2014JC010313
Leggett W. C., Deblois E. (1994). Recruitment in marine fishes: Is it regulated by starvation and predation in the egg and larval stages? Netherlands J. Sea Res. 32, 119–134. doi: 10.1016/0077-7579(94)90036-1
Lloret J., Lleonart J., Solé I., Fromentin J. (2001). Fluctuations of landings and environmental conditions in the north-western Mediterranean Sea. Fish. Oceanogr. 10, 33–50. doi: 10.1046/j.1365-2419.2001.00151.x
Luczynski M., Dlugosz M., Szutkiewicz B., Kirklewska A. (1984). The influence of the incubation temperature on the body length and the yolk sac volume of Coregonus albula (L.) eleutheroembryos. Acta Hydrochim. Hydrobiol. 12, 615–628. doi: 10.1002/aheh.19840120609
Matta M. E., Helser T. E., Black B. A. (2018). Intrinsic and environmental drivers of growth in an alaskan rockfish: an otolith biochronology approach. Environ. Biol. Fish. 101, 1571–1587. doi: 10.1007/s10641-018-0801-8
Mbaye B. C., Brochier T., Echevin V., Lazar A., Lévy M., Mason E., et al. (2015). Do Sardinella aurita spawning seasons match local retention patterns in the Senegalese–Mauritanian upwelling region? Fish. Oceanogr. 24, 69–89. doi: 10.1111/FOG.12094
Miller T. J., Crowder L. B., Rice J. A., Marschall E. A. (1988). Larval size and recruitment mechanisms in fishes: toward a conceptual framework. Can. J. Fish. Aquat. Sci. 45, 1657–1670. doi: 10.1139/f88-197
Molina-Valdivia V., Bustos C. A., Castillo M. I., Search F. V., Plaza G., Landaeta M. F. (2021). Oceanographic influences on the early life stages of a mesopelagic fish across the Chilean Patagonia. Prog. Oceanogr. 195, 102572. doi: 10.1016/j.pocean.2021.102572
Navarro F. (1932). Nuevos estudios sobre la alacha (Sardinella aurita CV) de baleares y de canarias. Notas Resum. Inst. Esp. Oceanogr. 60, 1–35.
Olivar M. P., Sabatés A., Alemany F., Balbín R., Fernández de Puelles M. L., Torres A. P. (2014). Diel-depth distributions of fish larvae off the Balearic islands (western Mediterranean) under two environmental scenarios. J. Mar. Syst. 138, 127–138. doi: 10.1016/j.jmarsys.2013.10.009
Palatella L., Bignami F., Falcini F., Lacorata G., Lanotte A. S., Santoleri R. (2014). Lagrangian Simulations and interannual variability of anchovy egg and larva dispersal in the Sicily channel. J. Geophys. Res. Oceans 119, 1306–1323. doi: 10.1002/2013JC009384
Palomera I., Sabatés A. (1990). Co-Occurrence of Engraulis encrasicolus and Sardinella aurita eggs and larvae in the northwestern Mediterranean. Sci. Mar. 54, 61–67.
Patti B., Bonanno A., Basilone G., Goncharov S., Mazzola S., Buscaino G., et al. (2004). Interannual fluctuations in acoustic biomass estimates and in landings of small pelagic fish populations in relation to hydrology in the strait of Sicily. Chem. Ecol. 20, 365–375. doi: 10.1080/02757540410001727972
Patti B., Torri M., Cuttitta A. (2020). General surface circulation controls the interannual fluctuations of anchovy stock biomass in the central Mediterranean Sea. Sci. Rep. 10, 1554. doi: 10.1038/s41598-020-58028-0
Patti B., Torri M., Cuttitta A. (2022). Interannual summer biodiversity changes in ichthyoplankton assemblages of the strait of Sicily (Central Mediterranean) over the period 2001–2016. Front. Mar. Sci. 9 (1454). doi: 10.3389/fmars.2022.960929
Paulsen M., Clemmesen C., Hammer C., Polte P., Malzahn A. M. (2017). Food-limited growth of larval Atlantic herring Clupea harengus recurrently observed in a coastal nursery area. Helgol. Mar. Res. 70, 1–12. doi: 10.1186/s10152-016-0470-y
Peck M. A., Reglero P., Takahashi M., Catalán I. A. (2013). Life cycle ecophysiology of small pelagic fish and climate-driven changes in populations. Prog. Oceanogr. 116, 220–245. doi: 10.1016/j.pocean.2013.05.012
Placenti F., Torri M., Pessini F., Patti B., Tancredi V., Cuttitta A., et al. (2022). Hydrological and biogeochemical patterns in the Sicily channel: New insights from the last decade, (2010-2020). Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.733540
Pörtner H. O., Farrell A. P. (2008). Physiology and climate change. Sci. (1979) 322, 690–692. doi: 10.1126/science.ll57945
Pörtner H. O., Peck M. A. (2010). Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish. Biol. 77, 1745–1779. doi: 10.1111/j.1095-8649.2010.02783.x
Quinci E. M., Torri M., Cuttitta A., Patti B. (2022). Predicting potential spawning habitat by ensemble species distribution models: The case study of European anchovy (Engraulis encrasicolus) in the strait of Sicily. Water (Basel) 14, 1400. doi: 10.3390/w14091400
R Core Team (2022). R: A language and environment for statistical computing. R. Foundation Stat. Computing. (Vienna, Austria; R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Robinson A. R., Sellschopp J., Warn-Varnas A., Leslie W. G., Lozano C. J., Haley P. J., et al. (1999). The Atlantic Ionian stream. J. Mar. Syst. 20, 129–156. doi: 10.1016/S0924-7963(98)00079-7
Russo S., Torri M., Patti B., Musco M., Masullo T., di Natale M. V., et al. (2022). Environmental conditions along tuna larval dispersion: Insights on the spawning habitat and impact on their development stages. Water (Basel) 14, 1568. doi: 10.3390/w14101568
Sabatés A., Martín P., Lloret J., , Raya. V. (2006). Sea Warming and fish distribution: the case of the small pelagic fish, Sardinella aurita, in the western Mediterranean. Glob. Chang Biol. 12, 2209–2219. doi: 10.1111/j.1365-2486.2006.01246.x
Sabatés A., Salat J., Raya V., Emelianov M., Segura-Noguera M. (2009). Spawning environmental conditions of sardinella aurita at the northern limit of its distribution range, the western Mediterranean. Mar. Ecol. Prog. Ser. 385, 227–236. doi: 10.3354/meps08058
Schickele A., Goberville E., Leroy B., Beaugrand G., Hattab T., Francour P., et al. (2021). European Small pelagic fish distribution under global change scenarios. Fish. Fish. 22, 212–225. doi: 10.1111/faf.12515
Sinovčić G., Franičević M., Čikeš Keč V. (2004). Unusual occurrence and some aspects of biology of juvenile gilt sardine (Sardinella aurita valenciennes 1847) in the zrmanja river estuary (eastern Adriatic). J. Appl. Ichthyol. 20, 53–57. doi: 10.1111/j.1439-0426.2004.00482.x
Somarakis S., Drakopoulos P., Filippou V. (2002). Distribution and abundance of larval fish in the northern Aegean Sea–eastern Mediterranean–in relation to early summer oceanographic conditions. J. Plankton Res. 24, 339–358. doi: 10.1093/plankt/24.4.339
Sponaugle S., Llopiz J. K., Havel L. N., Rankin T. L. (2009). Spatial variation in larval growth and gut fullness in a coral reef fish. Mar. Ecol. Prog. Ser. 383, 239–249. doi: 10.3354/meps07988
Takasuka A., Aoki I. (2006). Environmental determinants of growth rates for larval Japanese anchovy engraulis japonicus in different waters. Fish. Oceanogr. 15, 139–149. doi: 10.1111/j.1365-2419.2005.00385.x
Tonani M., Pinardi N., Dobricic S., Pujol I., Fratianni C. (2008). A high-resolution free-surface model of the Mediterranean Sea. Ocean Sci. 4, 1–14. doi: 10.5194/os-4-1-2008
Torri M., Corrado R., Falcini F., Cuttitta A., Palatella L., Lacorata G., et al. (2018). Planktonic stages of small pelagic fishes (Sardinella aurita and Engraulis encrasicolus) in the central Mediterranean Sea: The key role of physical forcings and implications for fisheries management. Prog. Oceanogr. 162, 25–39. doi: 10.1016/j.pocean.2018.02.009
Torri M., Pappalardo A. M., Ferrito V., Giannì S., Armeri G. M., Patti C., et al. (2021). Signals from the deep-sea: Genetic structure, morphometric analysis, and ecological implications of Cyclothone braueri (Pisces, gonostomatidae) early life stages in the central Mediterranean Sea. Mar. Environ. Res. 169, 105379. doi: 10.1016/J.MARENVRES.2021.105379
Tsikliras A. C. (2008). Climate-related geographic shift and sudden population increase a small pelagic fish (Sardinella aurita) in the eastern Mediterranean Sea. Mar. Biol. Res. 4, 477–481. doi: 10.1080/17451000802291292
Tsuruta Y. (1989). Internal regulation and reproduction in Japanese anchovy (Engraulis japonica) as related to population fluctuation. Canada Spec. Pub. Fish. Aqua. Sci. 108, 111–119.
Webb P. W., Weihs D. (1986). Functional locomotor morphology of early life history stages of fishes. Trans. Am. Fish. Soc. 115, 115–127. doi: 10.1577/1548-8659(1986)115<115:flmoel>2.0.co;2
Whitehead P. J. (1985). FAO species catalogue. clupeoid fishes of the world (suborder clupeioidei). an annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. FAO Fish. Synop. 125 (7), 1–303.
Wood S. N. (2011). Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B. Stat. Methodol. 73, 3–36. doi: 10.1111/j.1467-9868.2010.00749.x
Yoneda M., Kitano H., Tanaka H., Kawamura K., Selvaraj S., Ohshimo S., et al. (2014). Temperature- and income resource availability-mediated variation in reproductive investment in a multiple-batch-spawning Japanese anchovy. Mar. Ecol. Prog. Ser. 516, 251–262. doi: 10.3354/meps10969
Zarrad R., Alemany F., Rodriguez J. M., Jarboui O., Lopez-Jurado J. L., Balbin R. (2013). Influence of summer conditions on the larval fish assemblage in the eastern coast of Tunisia (Ionian Sea, southern Mediterranean). J. Sea Res. 76, 114–125. doi: 10.1016/j.seares.2012.08.001
Keywords: small pelagics, otolith microstructure analysis, mixed effect models, temperature, chlorophyll-a concentration, Tyrrhenian Sea, Strait of Sicily
Citation: Torri M, Russo S, Falcini F, De Luca B, Colella S, Volpe G, Corrado R, Placenti F, Giaramita L, Musco M, Masullo T, Bennici C, Di Natale MV, Patti B, Lacorata G, Arculeo M and Cuttitta A (2023) Coupling Lagrangian simulation models and remote sensing to explore the environmental effect on larval growth rate: The Mediterranean case study of round sardinella (Sardinella aurita) early life stages. Front. Mar. Sci. 9:1065514. doi: 10.3389/fmars.2022.1065514
Received: 09 October 2022; Accepted: 20 December 2022;
Published: 20 January 2023.
Edited by:
Stelios Katsanevakis, University of the Aegean, GreeceReviewed by:
Emilio Hernandez-Garcia, Institute of Interdisciplinary Physics and Complex Systems (CSIC), SpainCopyright © 2023 Torri, Russo, Falcini, De Luca, Colella, Volpe, Corrado, Placenti, Giaramita, Musco, Masullo, Bennici, Di Natale, Patti, Lacorata, Arculeo and Cuttitta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Torri, bWFyY28udG9ycmlAY25yLml0
†These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.