- College of Animal Science and Technology, Northeast Agricultural University, Harbin, China
This study evaluated the potential effects of mannan oligosaccharide (MOS) on the health status of common carp under ammonia stress. The experimental fish were equally divided into four groups. The control group was fed with a basal diet. The ammonia stress group (Am group) was fed with a basal diet and set in the culture environment with 0.15 mg/L NH3. The MOS group was fed 0.2% MOS with a basal diet. The treatment group (MOS/Am group) was fed 0.2% MOS feed under ammonia exposure (NH3, 0.15 mg/L). These groups were fed for 30 d. The results showed that under ammonia stress, the growth performance decreased significantly, and the activities of non-specific immune factors, intestinal digestive enzymes, and antioxidant enzymes decreased significantly. Dietary supplementation of MOS, the growth performance, the activities of non-specific immune factors, intestinal digestive enzymes, and antioxidant enzymes increased. Histopathological studies showed that adding MOS reduced liver, gill, and intestine tissue damage under ammonia exposure. Our study suggests that adding MOS to the diet can improve growth performance, immunity, antioxidant capacity, and intestinal health of the common carp. MOS can effectively alleviate the oxidative damage and inflammatory response caused by ammonia poisoning to common carp.
Introduction
In recent years, due to the adoption of large-scale intensive farming models, the density of aquaculture is too high, the feeding mode is extensive, and the nutrition of compound feed is unbalanced, which causes the concentration of ammonia nitrogen in aquaculture water to exceed the standard (Yuen and Chew, 2010; Badiola et al., 2018). Ammonia is highly toxic to aquatic animals and can cause oxidative stress, immunosuppression, inflammatory response, tissue damage, and even fish death (Paust et al., 2011; Liew et al., 2013; Li et al., 2014). The ability to reduce the harmful effects of ammonia stress on aquatic animals has significant application value. The development and application of aquatic feed additives have received a lot of attention (Tacon, 2020). Feed additives can protect fish health under ammonia stress. Adding myrcene or menthol (Hoseini et al., 2019), garlic (Yousefi et al., 2020), 1,8-cineole (Mirghaed et al., 2019), and olive leaf extract (Rajabiesterabadi et al., 2020) can attenuate oxidative stress and tissue damage during ammonia exposure.
Mannan oligosaccharide (MOS) is a new active antigen substance extracted from yeast cell walls. MOS is relatively stable during feed processing and granulation and can maintain its structural and functional integrity (Sims et al., 2004; Castillo et al., 2008). MOS is considered an ideal substitute to replace antibiotic additives and can improve poultry and piglets’ production performance, intestinal microflora, antioxidant capacity, and immune function (Zhao et al., 2012). As a new type of green feed additive, MOS has gradually being studied in aquaculture. Adding MOS improved the growth performance and immunity of the grass carp (Ctenopharyngodon idella) (Zyl et al., 2020),the common carp (Cyprinus carpio) (Mohammadian et al., 2019), the Nile tilapia (Oreochromis niloticus) (Selim and Reda, 2015), and the Japanese flounder (Paralichthys olivaceusflounder) (Ye et al., 2011).
Organisms have a complete antioxidant defense system. Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH - PX) enzymes are the main antioxidant enzymes that play an important role in scavenging free radicals, relieving oxidative stress, and protecting cell integrity (Li et al., 2014).Adding MOS improved the activity of antioxidant enzymes and reduce the contents of ROS and MDA of the Nile tilapia and the grass carp under ammonia stress. Inflammatory cytokines are endogenous polypeptides produced by immune system cells that can mediate various immune reactions. MOS alleviated the inflammatory reaction of the Japanese flounder, the rainbow trout (Oncorhynchus mykiss) (Estrada et al., 2009), and the Caspian trout (Salmo trutta caspius) (Jami et al., 2019) under ammonia stress.
There is no research on the effect of MOS on the physiological status of aquatic animals exposed to ammonia. The common carp (Cyprinus carpio) is a widely cultivated freshwater bony fish (Xu et al., 2014). Therefore, this study reports whether MOS alleviates the harmful effects of excessive environmental ammonia on the common carp, as well as provides a reference for healthy breeding.
Materials and methods
Experiment management
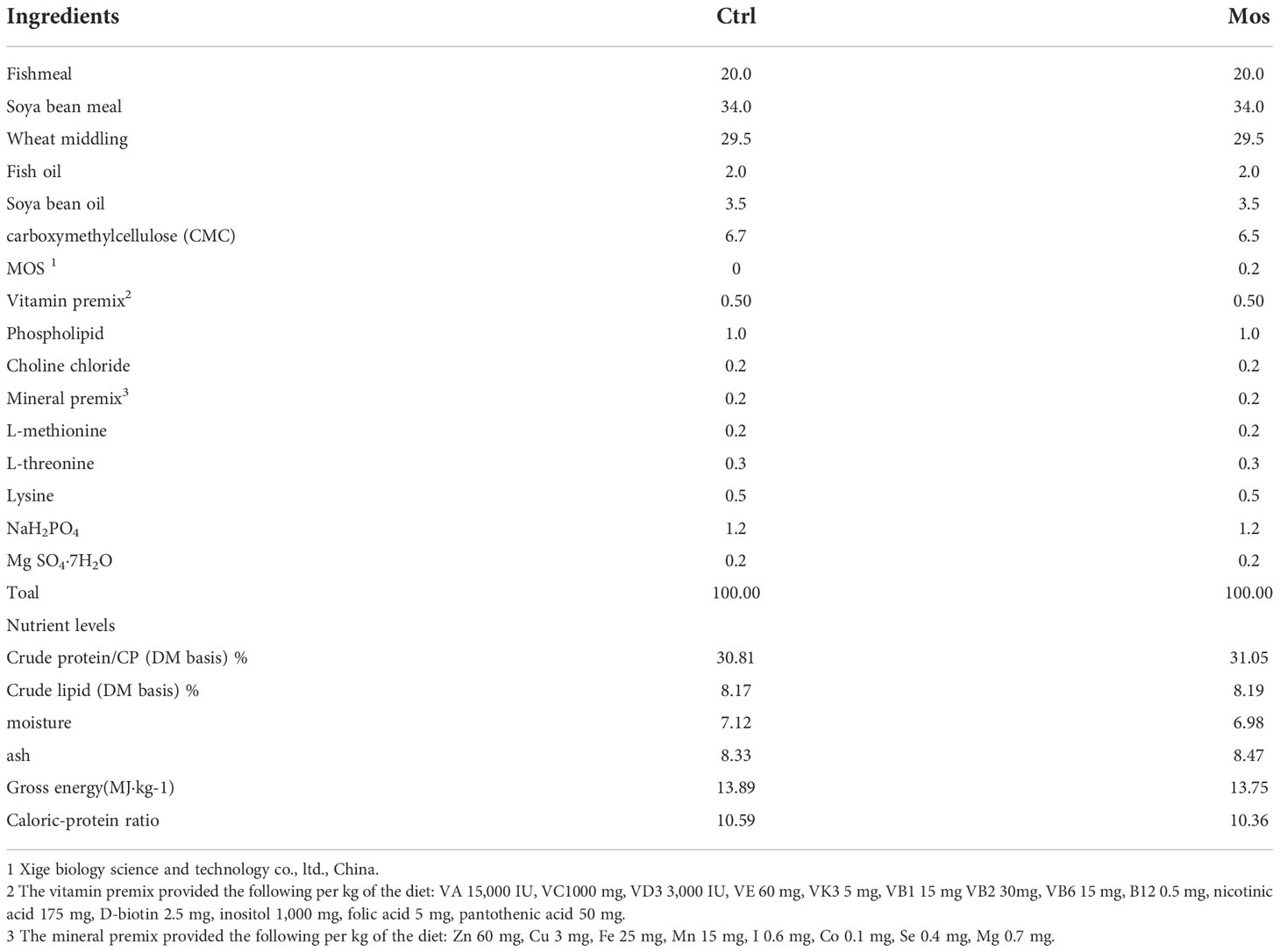
The basic feed formula and production method was based on a prior study conducted in our laboratory (Xue et al., 2022). The experimental feed was prepared by adding 0.2% MOS to the basal feed (Mohammadian et al., 2019). The feed formulas are shown in Table 1. The MOS (purity 99%) was purchased from Shanghai Xige Biology Science and Technology Co., Ltd., China. The experimental common carp were taken from the experimental aquaculture station of Northeast Agricultural University, Harbin, China. A total of 400 common carp fish with an initial weight of 232.51 ± 5.28 g were included in the study. Each experimental group included five replicates, and 20 fish were included in each replicate. The experimental fish were acclimated for 7 d in a circular aquaculture system (300 L) and fed with a basic feed. The experiment was divided into four groups, namely control, Am, MOS, and MOS/Am groups. The control group was fed with a basic feed. The Am group was exposed to ammonia (NH3, 0.15mg/L) and fed a basic feed. The MOS group was fed with experimental feed with 0.2% MOS. The MOS/Am group was exposed to ammonia (NH3, 0.15mg/L) and fed 0.2% MOS. During the experiment, the fish were fed twice daily, and the feeding amount was 3% of the body weight. Aquaculture water met the requirements of APHA (APHA, 2005) (APHA, 2005). The fish were raised for 30 d.
All the procedures were approved by the Laboratory Animal Ethics Committee of Northeast Agricultural University.
Sample collection
Before sampling, the fish were fasted for 24 h. The experimental fish were anesthetized in each group with MS-222 (20mg/L), weighed, and counted. Blood was collected from five fishtail veins. The serum was separated by centrifugation at 5,000 x g for 10 min at 4°C and stored at -80°C for determination of biochemical and immune indices. Viscera were dissected, and the hepatopancreas was separated. Total visceral weight and the hepatopancreas weight were determined. Intestines were stored in liquid nitrogen to determine the activity of intestinal digestive enzymes. Part of the hepatopancreas tissues was frozen with liquid nitrogen for antioxidant enzyme activity determination and related gene mRNA analysis. The gill, hepatopancreas, and intestinal tissues were cut from the same position of experimental fish and then fixed with 4% paraformaldehyde to prepare tissue sections.
Proximate compositions
Proximate compositions of feed were determined according to the AOAC method (AOAC, 1995).
Computational analysis
The growth index calculation formula is shown in Table 2.
Tissue morphology
The tissues of the gill, the hepatopancreas, and the intestinal were cut from the same position as the experimental fish, fixed with 4% paraformaldehyde and embedded with paraffin, sectioned by Wuhan Seville Biotechnology Co., Ltd., China, and stained by hematoxylin and eosin (H&E).
Serum biochemistry and immune parameters
The serum was thawed at 4°C. Serum biochemical parameters were determined using a commercial kit. The activities of growth hormone (GH), insulin-like growth factor (IGF-I), immune enzymes, and cytokines were determined using the commercial kit H091-1-1, H041 (Jiancheng Institute of Biological Engineering, Nanjing, China).
Intestinal digestive enzyme
The whole intestine was weighed, and 0-4°C physiological saline, according to the volume of 1:9, was homogenized and centrifuged to obtain the supernatant. The activities of protease, amylase, and lipase were measured using the commercial kit W060-1-1,C016-1-1,A054-1-1 (Jiancheng Institute of Biological Engineering, Nanjing, China).
Antioxidant activity in hepatopancreas
The hepatopancreas tissue was homogenized with 0-4°C phosphate buffer, and the supernate was collected. Total antioxidant capacity (T-AOC), the activities of SOD, CAT, GSH-Px, and the contents of ROS and MDA were determined using commercial kits A015-2-1, A001-3-2, A007-1-1, A005-1-2, E004-1-1, A003-1-2 (Jiancheng Institute of Biological Engineering,Nanjing, China).
Gene expression analysis
Total RNA of hepatopancreas tissue was extracted using the Trizol method (China Vazyme Biotechnology Co., Ltd.), and RNA was reverse transcribed into complementary DNA (cDNA). The expression level of related genes was analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). The primer information of genes are provided in Table 3. The PCR reaction mix contained 1 μL of upstream primer, 1 μL of downstream primer, 10 μL of SYBR green, 1 μL of complementary DNA (cDNA) template, and 7 μL of ddH2O. The PCR conditions were as follows: 95°C for 30 s, 40 cycles of 95°C for 5 s, and 60°C for 30 s (Xue et al., 2022). Each sample included three biological replicates, and all reactions were performed in triplicate. The internal reference gene β-actin was used. The relative expression was calculated according to the 2-ΔΔCT method.
Data analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 20.0 software, including one-way analysis of variance (ANOVA) and Duncan’s multiple comparisons. All experiments were conducted at least three times, and all data were expressed as mean ± standard error (SE). In the same row, values with no or the same letter superscripts indicated no significant difference (P > 0.05), and values with different letter superscripts indicated a significant difference (P < 0.05).
Results
Growth performance
The specific growth rate (SGR), and weight gain rate (WGR) were decreased in the Am group and increased significantly in the MOS group (P < 0.05). The FCR decreased significantly in the MOS group and increased significantly in the AM group (P < 0.05). However, there was no significant difference in SGR, WGR, and Feed conversion ratio (FCR) between the control and the MOS/Am groups (P > 0.05). Condition factor (CF) and Hepatosomatic index (HSI) increased significantly in the MOS group (P < 0.05). The results are shown in Table 4.

Table 4 Effects of dietary MOS on the growth performance of the common carp under ammonia stress for 30 days.
Serum biochemical and nonspecific immunity
The contents of total protein (TP), albumin (ALB), and globulin (GLB) were decreased significantly in the Am group (P < 0.05) and increased significantly in the MOS group (P < 0.05). The content of urea nitrogen (UN) in the Am group was increased, while it decreased significantly in the MOS group (P < 0.05). The NO content increased significantly in the Am group (P < 0.05). The results are shown in Table 5.
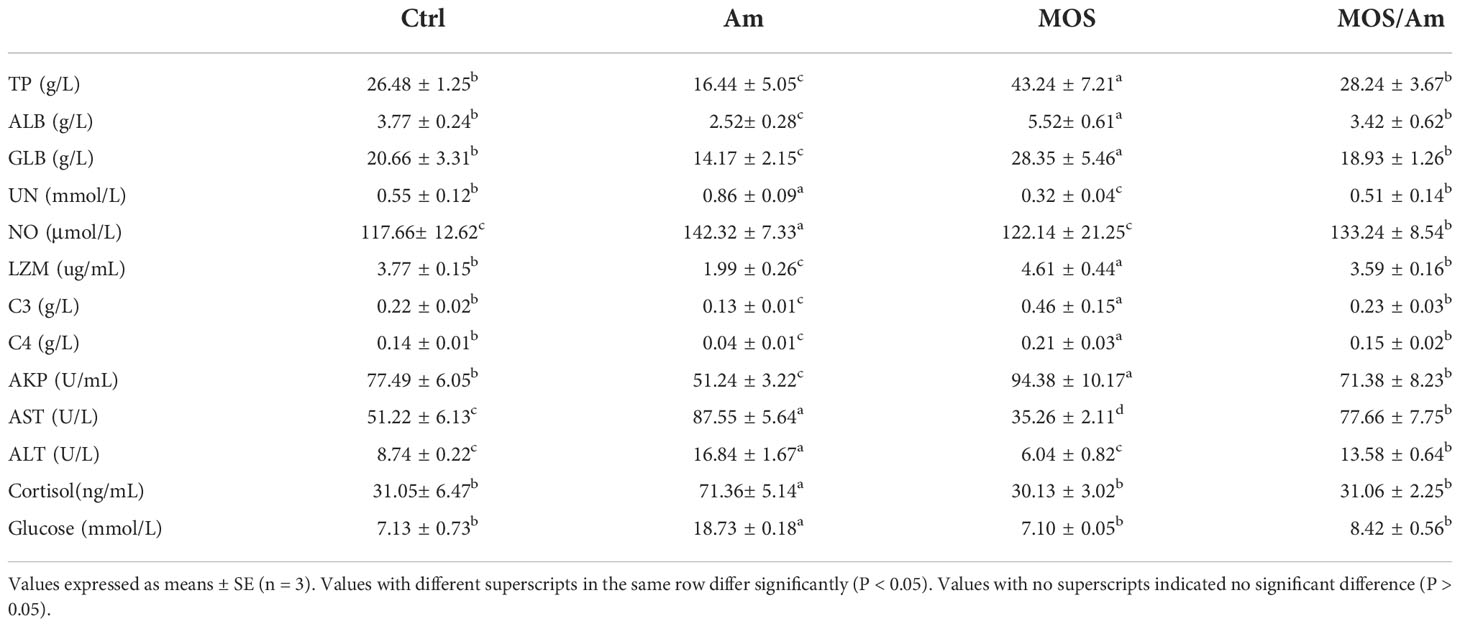
Table 5 Effects of dietary MOS on the serum biochemical and immune parameters of the common carp under ammonia stress for 30 days.
The activities of immune enzymes, such as alysozyme (LZM), complement 3 (C3), complement 4 (C4), and alkaline phosphatase (AKP), decreased significantly in the Am group and increased in the MOS group (P < 0.05). The activities of Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) increased significantly in the AM group (P < 0.05) and decreased in the MOS group (P < 0.05). Cortisol and Glucose contents increased significantly in the AM group (P < 0.05). However, there was no significant difference in the activities of immune enzymes and the content of cortisol and glucose between the control and MOS/Am groups (P >0.05). The results are shown in Table 5.
Digestive enzyme activity
The activities of protease, lipase and amylase increased in the MOS group and decreased in the Am group (P<0.05). There was no difference between the control and MOS/Am groups (P > 0.05). The results are shown in Table 6.

Table 6 Effects of dietary MOS on the intestinal digestive enzyme and intestinal morphology of the common carp under ammonia stress for 30 days.
Growth hormone, antioxidant enzymes, and cytokines
The contents of GH and IGF-I were the highest in the MOS group and the lowest in the Am group (P < 0.05). However, there was no difference in the levels of GH and IGF-1 in serum between the control and MOS/Am groups (P > 0.05) (Figure 1). The activities of antioxidant enzymes (SOD, CAT, GSH-Px, and T-AOC) in the liver increased significantly, and the contents of MDA and ROS decreased significantly in the MOS group (P < 0.05). In the AM group, the activities of antioxidant enzymes decreased significantly, and the contents of MDA and ROS increased significantly (P < 0.05). There was no significant difference in the activity of antioxidant enzymes and ROS content between the control and MOS/Am groups (P > 0.05) (Figure 2). The activities of serum proinflammatory cytokines, such as IL-1β, IL-6, IL-8, IFN-γ, and TNF-α, increased significantly in the AM group, and IL-6, IL-8, TNF- α decreased significantly in the MOS group (P < 0.05). Nevertheless, there were no significant differences in the activities of proinflammatory cytokines between the control and MOS/Am groups (P > 0.05) (Figure 3).
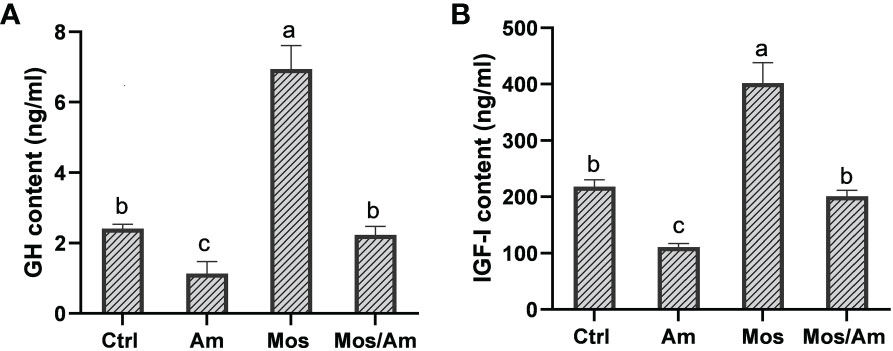
Figure 1 Effects of dietary MOS on the growth hormone content of the common carp under ammonia stress for 30 days. Values are expressed as mean ± standard error (SE) from triplicate groups. Bars with different letters differ significantly from those of other groups (P < 0.05). GH content (A), IGF-I content (B).
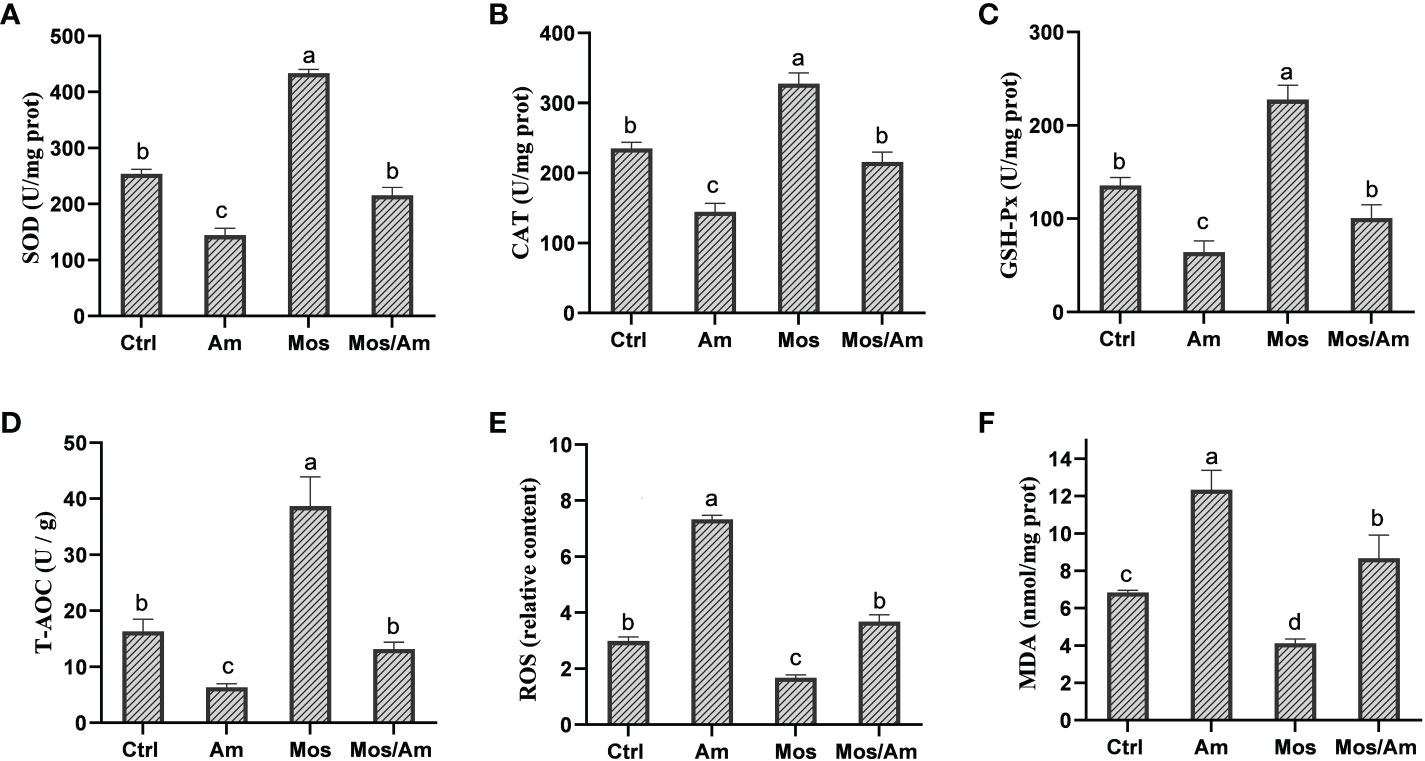
Figure 2 Effects of dietary MOS on the antioxidant capacity of the common carp under ammonia stress for 30 days. Values are expressed as mean ± standard error (SE) from triplicate groups. Bars with different letters differ significantly from those of other groups (P<0.05). SOD activity (A), CAT activity (B), GSH-PX activity (C), T-AOC level (D), ROS content (E), MDA content (F).
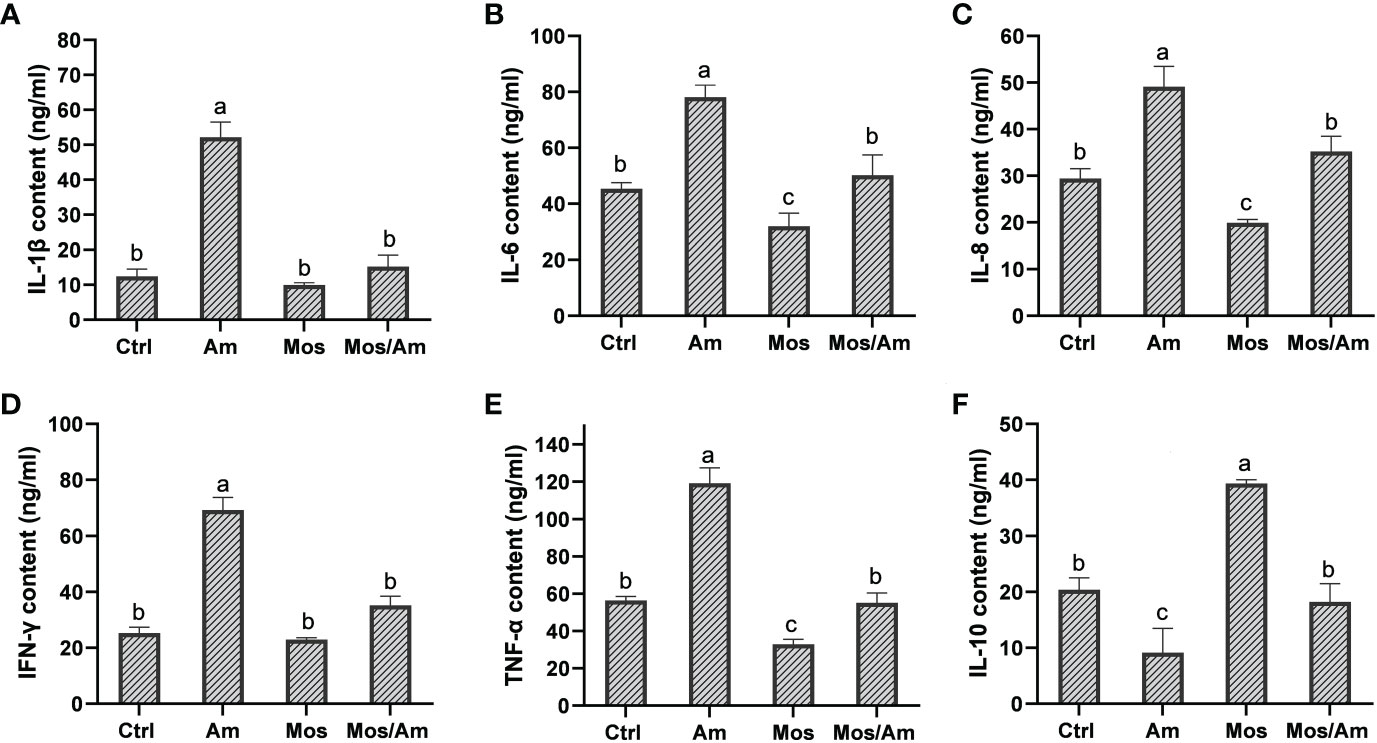
Figure 3 Effects of dietary MOS on the activity levels of inflammatory factors of the common carp under ammonia stress for 30 days. Values are expressed as mean ± standard error (SE) from triplicate groups. Bars with different letters differ significantly from those of other groups (P < 0.05). IL-1β content (A), IL-6 content (B), IL-8 content (C), IFN-γ content (D), TNF-α content (E), IL-10 content (F).
Gene expression
The gene expression levels of GH and IGF-I were the highest in the MOS group. The gene expression levels of Heat shock protein 70 (Hsp70) and Heat shock protein90 (Hsp90) were the highest in the Am group and were not significantly different between the control and MOS groups (P > 0.05) (Figure 4). The gene expression levels of SOD, CAT, and GSH-Px were significantly down-regulated in the AM group and up-regulated in the MOS group (P < 0.05). There was no significant difference between the control and MOS/Am groups (P > 0.05) (Figure 5). The gene expression levels of pro-inflammatory genes were up-regulated in the Am group (P < 0.05), but there was no significant difference between the control, the MOS and the MOS/Am groups (P > 0.05). The expression of the anti-inflammatory gene (interleukin-10, IL-10) was up-regulated in the MOS group and down-regulated in the AM group (P < 0.05) (Figure 6).

Figure 4 Effects of dietary MOS on the mRNA expression of heat shock protein genes of the common carp under ammonia stress for 30 days. Values are expressed as mean ± standard error (SE) from triplicate groups. Bars with different letters differ significantly from those of other groups (P < 0.05). GH relative mRNA expression (A), IGF-I relative mRNA expression (B), Hsp70 relative mRNA expression (C), Hsp90 relative mRNA expression (D).
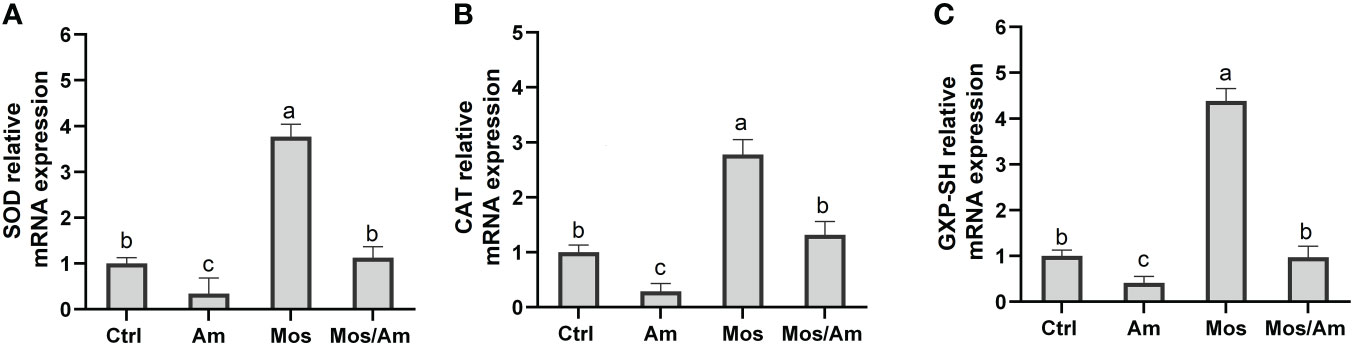
Figure 5 Effects of dietary MOS on the mRNA expression of antioxidative genes of the common carp under ammonia stress for 30 days. Values are expressed as mean ± standard error (SE) from triplicate groups. Bars with different letters differ significantly from those of other groups (P < 0.05). SOD relative mRNA expression (A), CAT relative mRNA expression (B), GSH-PX relative mRNA expression (C).
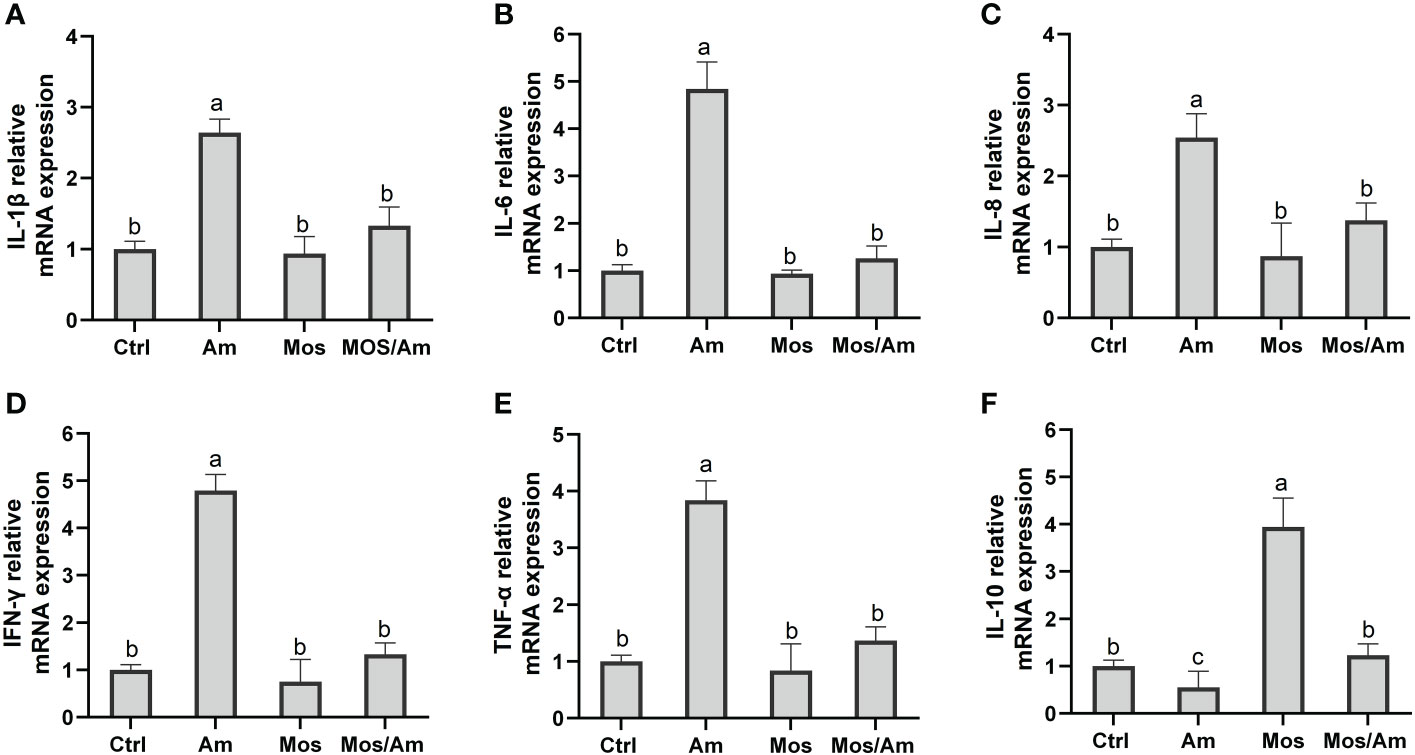
Figure 6 Effects of dietary MOS on the mRNA expression of inflammatory genes of the common carp under ammonia stress for 30 days. Values are expressed as mean ± standard error (SE) from triplicate groups. Bars with different letters differ significantly from those of other groups (P < 0.05). IL-1β relative mRNA expression (A), IL-6 relative mRNA expression (B), IL-8 relative mRNA expression (C), IFN-γ relative mRNA expression (D), TNF-α relative mRNA expression (E), IL-10 relative mRNA expression (F).
Histopathology
The histological observation of the hepatopancreas is shown in Figure 7. The hepatopancreas of the control, the MOS, and the MOS/Am groups were similar in structure. The liver tissue showed a complete arrangement of polyhedral cells. In the Am group, the blood sinus was congested, and there were vacuolized and inflammatory cells. The liver cell in the MOS/Am group showed normal structure with a few vacuolated cells. The observation of gill tissue structure is shown in Figure 8. In the control and the MOS groups, the gills showed normal tissue structure. Gill cells in the AM group had some vacuolated and inflammatory cells. The gills showed a relatively normal structure with slight vascular congestion and few vacuolated cells in the MOS/Am group. Villi height, villi width, and muscular thickness decreased significantly in the Am group (P < 0.05), and villi and muscular thickness were shed. After adding MOS, the intestinal villi height and width of carp significantly increased (P < 0.05). The microvilli structure was complete, and the number of goblet cells increased. In the MOS/Am group, the shedding of intestinal villi was reduced. There was no significant difference in muscular thickness, villi height, and villi width between the control and the MOS/Am groups (P > 0.05) (Figure 9 and Table 6).
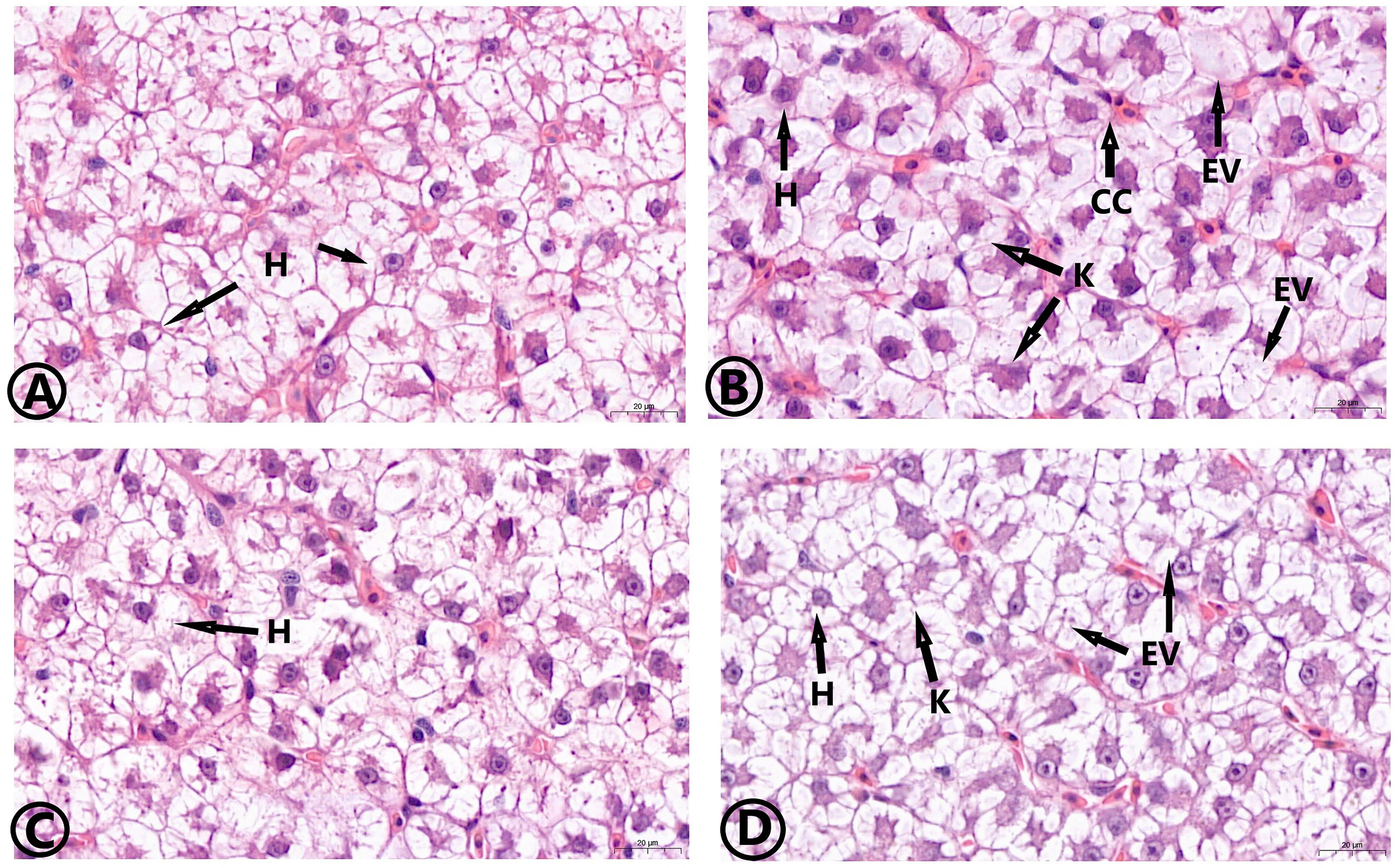
Figure 7 Effects of dietary MOS on the histological structure of hepatopancreas of the common carp under ammonia stress for 30 days. Stain H&E. Bar = 20 μm. 63.00X. Control group (A), ammonia group (B), MOS group (C), and MOS/Am group (D). H, Hepatocyte; K, Cell nuclear deviation; EV, Cell vacuolation; CC, Inflammatory cells.
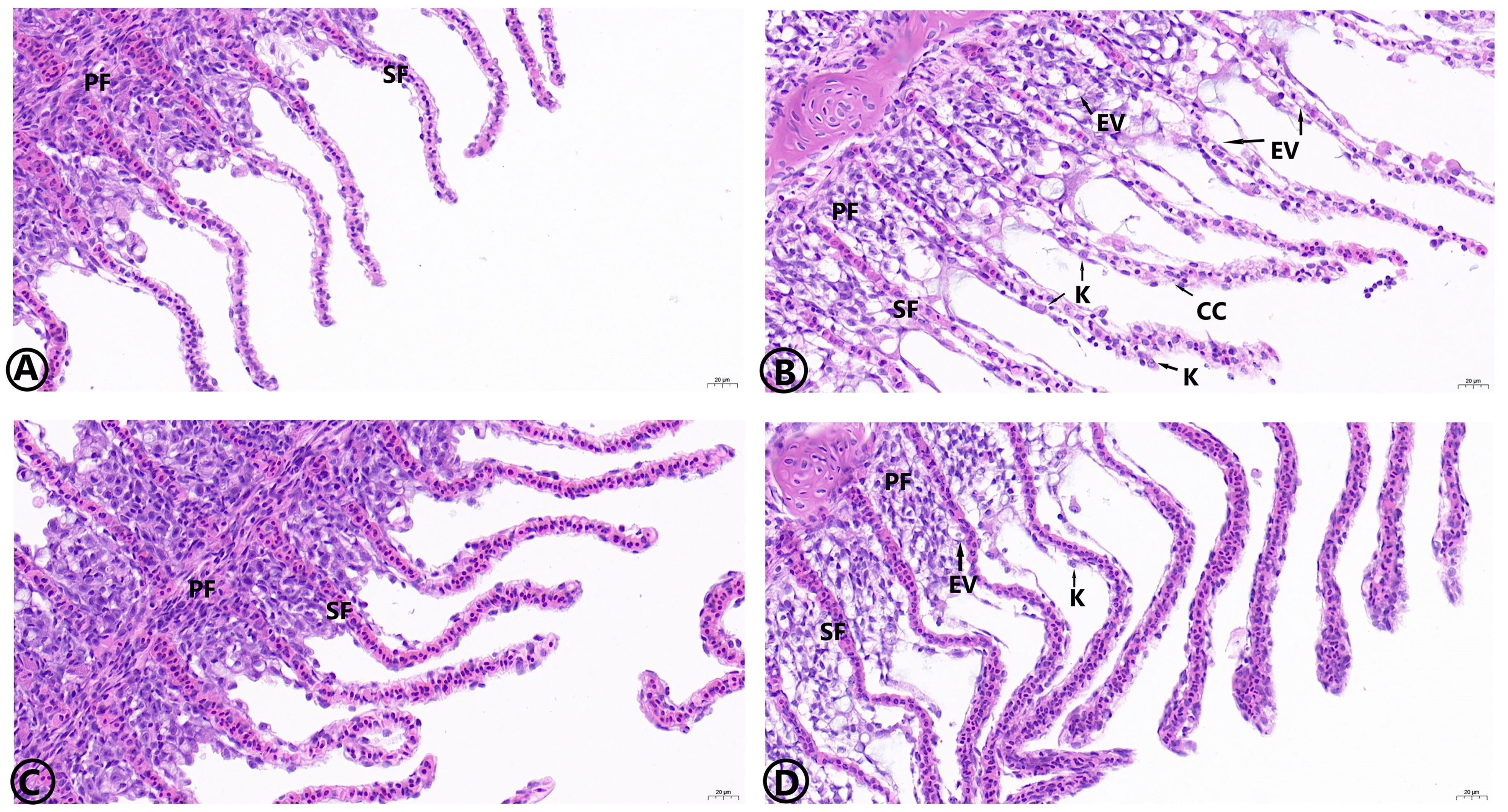
Figure 8 Effects of dietary MOS on the histological structure of gills of the common carp under ammonia stress for 30 days. Stain H&E. Bar = 20 μm, 40.00X. Control group (A), ammonia group (B), MOS group (C), and MOS/Am group (D). PF, Gill filament; SF, Gill patch; EV, Cell vacuolation; K, Cell nuclear deviation; CC, Inflammatory cells.

Figure 9 Effects of dietary MOS on the histological structure of Intestinal of the common carp under ammonia stress for 30 days. Bar = 100 μm, H&E Staining, 10.00X. Control group (A), ammonia group (B), MOS group (C), and MOS/Am group (D). M, Muscular; V, Villi; G, Goblet cell.
Discussion
Excessive ammonia concentration in aquatic animal breeding is a common and severe problem. Excessive ammonia concentration weakens the health of aquatic animals. The feed with the appropriate addition of myrcene or menthol (Hoseini et al., 2019), 1,8-cineole (Rajabiesterabadi et al., 2020), and garlic (Yousefi et al., 2020) improved the ammonia resistance of the common carp. MOS is considered to improve production performance, optimize intestinal microflora, and enhance immunity in livestock, poultry and aquatic animal breeding (Mateo et al., 2000; Zhao et al., 2012; Zyl et al., 2020). To date, there has been no study of the effect of MOS on fish physiology under ambient ammonia.
There were differences in results about the effects of MOS on fish growth performance. In the grass carp, the Nile tilapia, the flounder, the rainbow trout, the Atlantic salmon (Salmo salar) (Grisdale–Helland et al., 2008), the red sea bream (Pagrus major) (Dawood et al., 2020), the hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) (Ren et al., 2020), the Caspian trout (Jami et al., 2019), and the Chinese mitten crab (Eriocheir sinensis) (Lu et al., 2019), MOS can improve SGR, WGR, and reduce feed coefficient. Conversely, in the hybrid tilapia (Oreochromis niloticus×O. aureus), excessive addition of MOS may have a negative impact on the growth of fish (Genc et al., 2007b). In the gilthead seabream (Sparus aurata) (Dimitroglou et al., 2010), and the Mexican sturgeon (Acipenser oxyrinchus desotoi) (Pryor et al., 2003), the addition of MOS in the feed had no significant effect on SGR, feed coefficient and other growth indicators.The differences in the growth effects of MOS on fish may be related to fish species and growth and development stages, breeding environment, and feeding cycle.In this study, ammonia stress reduced the growth performance of common carp, and MOS promoted growth performance and significantly reduce FCR. Adding MOS to feed reduced the negative impact of ammonia stress on the growth performance of common carp. MOS significantly increased CF and HSI of the common carp. HSI and CF are important indicators of the physiological and nutritional status of fish (Xue et al., 2022). Adding MOS can help improve the hepatopancreas function and nutrient accumulation of the common carp. The content and mRNA expression of GH and IGF-I were the highest in the MOS group, thus MOS promoted the mRNA expression of GH and IGF-I, which was consistent with the variation trend of growth traits.
The intestinal digestive enzyme of fish is an important index that reflects the digestion ability and absorption of nutrients (Mohammadian et al., 2019). MOS improved the activities of trypsin, amylase, and lipase in the intestines of the European sea bass (Dicentrarchus labrax) (Torrecillas et al., 2013), the Pacific white shrimp (Litopenaeus vannamei) (Chen et al., 2020), the freshwater crayfish (Paranephrops planifrons) (Sang et al., 2015),the rainbow trout (Dimitroglou et al., 2009), and the Japanese flounder (Ye et al., 2011), which is consistent with the results of this study. Adding MOS to the diet contributes to the growth performance of animals, and its mechanism of action may be attributed to improved intestinal health. From the structural point of view, MOS has a few 1,4 glycosidic bonds, hence it is not easy to be digested and absorbed by fish. MOS enters directly the hindgut of fish as the specific nutrient matrix of beneficial bacteria such as Lactobacillus and Bifidobacterium, which promotes the proliferation of beneficial bacteria and improves the intestinal flora and digestive enzyme activity. The results in a balanced intestinal flora structure, enhanced digestive ability, and improved nutrient absorption efficiency, promoting the growth performance of fish (Mohammadian et al., 2019; Chen et al., 2020).
Serum biochemical factors reflect the metabolism and nutritional status of the protein. TP level was considered to be positively correlated with immune ability (Xue et al., 2022). The content of urea nitrogen (UN) can reflect the metabolism of protein and the balance of amino acids (Mirghaed et al., 2019). AST and ALT are the most important aminotransferase in fish that can be used to evaluate liver health. Elevated levels of AST and ALT reflect abnormal liver function and are used to evaluate the stress response of fish (Xue et al., 2022). Serum cortisol and glucose content are crucial indicators of the state of stress fish. Cortisol can promote gluconeogenesis and glycogen decomposition, as well as accelerate glucose production to meet the increased energy demand of fish under stress (Fevolden, 2010). In this study, MOS promoted the contents of TP, ALB, and GLB, which improved the immunity of the common carp. Adding MOS to the feed could promoted protein synthesis and reduced the serum’s UN content. The activities of AST and ALT increased significantly under ammonia stress but decreased significantly after adding MOS (P < 0.05).There was no significant difference in the activities of AST and ALT between the MOS/Am and the control groups. These results showed that MOS prevented lipid peroxidation of the cell membrane, inhibited the release of AST and ALT enzymes into plasma, and kept the liver cell integrity. Adding MOS to the feed reduced effectively the liver injury caused by ammonia exposure. In this study, MOS inhibited the increase of cortisol and glucose, and reduced the oxidative stress caused by ammonia. These result was similar to those obtained with garlic (Yousefi et al., 2020) and shrimp carotenoids (Rama and Manjabhat, 2014), which inhibited the increase of cortisol content in fish under ammonia pressure.
The immune system of the animal body includes specific immunity and non-specific immunity. When pathogenic microorganisms and foreign bodies invade, non-specific immunity removes pathogenic bacteria and foreign bodies through physical barriers, phagocytosis, bacteriolysis, and agglutination. Lysozyme, complement, and alkaline phosphatase are essential components of the nonspecific immune system (Zhang et al., 2014). Enhancing the activity of immune enzymes can improve the body’s overall immune function (Dalmo et al., 1997) (Xia and Wu, 2018). MOS can enhance the activity of immune enzymes in the serum of the grass carp (Zyl et al., 2020), the Nile tilapia (Selim and Reda, 2015), the rainbow trout (Estrada et al., 2009), and the Chinese mitten crab (Lu et al., 2019). In our study, the activities of immune enzymes were inhibited under ammonia stress and were improved in the MOS group. Adding MOS to the diet can effectively alleviate the immunosuppression of common carp under ammonia stress. MOS can optimize the intestinal microflora and promote the proliferation of beneficial bacteria and the phagocytic ability of macrophages, thereby improving the activity of related immune enzymes and positively impacting the body’s non-specific immunity (Torrecillas et al., 2007). MOS can improve the immune enzyme activity of common carp, thus enhancing its non-specific immune function.
The antioxidant enzyme activities of animals are influenced by both in-vivo and in-vitro environments (Sies, 1997). Ammonia poisoning inhibits the activity of antioxidant enzymes, such as Carassius auratus (Qi et al., 2017) and Litopenaeus vannamei (Lin and Chen, 2001). SOD activity in the liver was not affected by ammonia nitrogen exposure in studies on the rainbow trout, the common carp, the goldfish (C. auratus), and the Dicentrachus labrax (Sinha et al., 2014; Xue et al., 2021). However, SOD activity increased significantly under ammonia exposure in Takifugu obscurus (Wang et al., 2015), Macrobrachium rosenbergii (Zhang et al., 2015), and Pelteobagrus vachelli (Li et al., 2014). The physiological responses of antioxidant enzymes in different fish exposed to an ammonia nitrogen environment are not completely consistent, which may be due to the difference in ammonia nitrogen tolerance among fish species. The level of ROS in fish poisoned by ammonia will increase, leading to the destruction of protein structure and the occurrence of cell membrane peroxidation (Xue et al., 2021). The content of MDA may reflect the degree of oxidative damage. Our study showed that adding MOS improved the activity of antioxidant enzymes and reduced the contents of ROS and MDA under ammonia stress, consequently enhancing the antioxidant capacity and reducing oxidative stress and damage to the common carp.
Ammonia poisoning induces an inflammatory response in the body. Inflammatory cytokines can mediate various immune responses. The activity of inflammatory cytokines is an important index of animal immunity and anti-inflammatory responses (Xue et al., 2022). In our study, ammonia stress caused the inflammatory reaction of the common carp, and MOS could alleviated the inflammatory reaction under ammonia stress, which was consistent with observations stating that MOS improved the expression levels of inflammation-related genes and the anti-infection ability of Allogynogenetic crucian carp (Carassius auratus gibelio) (Liu et al., 2013), and the European sea bass (Dicentrarchus labrax) (Torrecillas et al., 2007). Heat shock protein (Hsp) refers to the protein produced by cells induced by stressors, which has molecular chaperone activity. When external factors stimulate cells, they are induced to generate heat shock proteins, which can improve the stress ability of cells (Wang et al., 2020). The results showed that MOS reduced the negative effects of ammonia stress by regulating the expression of the heat shock protein gene.
Ammonia stress causes pathological damage to the liver, gills, and intestinal tissues of the common carp, while the liver, gills, and intestinal tissues in the Am/Mos group presented normal tissue characteristics.This demonstrates that MOS improved the antioxidant and anti-inflammatory ability of the body and alleviate the tissue damage caused by ammonia stress. The height width of intestinal microvilli increased in the MOS group. MOS promotes the reacylation and synthesis of polar lipids. when the utilization rate of lipids increases, the energy used for growth and cell membrane synthesis increases, which is one of the reasons why MOS improves the integrity of villi (Pryor et al., 2003). At the same time, MOS can protect intestinal villi from stress damage by promoting mucus secretion, thus improving the integrity and height of villi (Dimitroglou et al., 2009). Similar findings have been reported in studies on the hybrid tilapia (Genc et al., 2007b), the rainbow trout (Yilmaz et al., 2007), the gilthead sea bream (Sparus aurata) (Dimitroglou et al., 2010), the white sea bream (Diplodus sargus L.) (Dimitroglou et al., 2010), the larval cobi (Salze et al., 2008), and the Gulf sturgeon in Mexico (Pryor et al., 2003).
Conclusion
Ammonia disrupted the antioxidant enzyme system and immune response of the common carp, causing oxidative damage, immunosuppression, and inflammatory response. MOS can improve growth performance and promote the activity of intestinal digestive enzymes of the common carp. In addition, MOS can regulate the activities of immune enzymes, antioxidant enzymes, and inflammatory cytokines by regulating the genes’ expression of antioxidation, inflammation and stress, improve the capacity of antioxidant and non-specific immune, and effectively relieve tissue damage and inflammatory reaction caused by ammonia poisoning to the common carp.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the Animal Experiment Ethics Committee of Northeast Agricultural University.
Author contributions
SX: Conceptualization, methodology, writing - original draft. BX: Software, formal analysis,writing - review & editing. BZ: Validation, data curation. LL: Validation, investigation. YZ: Formal analysis. ZS: Validation. YX: Validation. YH: Conceptualization, resources. WC: Supervision, project administration. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Ministry of Jixi Fishery Resources and Environment Investigation and Fishery Development Planning Project.
Acknowledgments
We would like to thank MogoEdit (https://www.mogoedit.com) for its English editing during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
APHA (2005). Standard methods for the examination of water and wastewater (Washington, DC, USA: American Public Health Association).
Badiola M., Basurko O. C., Piedrahita R., Hundley P., Mendiola D. (2018). Energy use in recirculating aquaculture systems (RAS): A review. Aquacult. Eng. 81, 57–70. doi: 10.1016/j.aquaeng.2018.03.003
Castillo M., Martin-Orue S. M., Taylor-Pickard J. A., Perez J. F., Gasa J. (2008). Use of mannanoligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: effects on microbiota and gut function. J. Anim. Sci. 86, 94–101. doi: 10.2527/jas.2005-686
Chen M., Chen X. Q., Tian L. X., Liu Y. J., Niu J. (2020). Beneficial impacts on growth, intestinal health, immune responses and ammonia resistance of pacific white shrimp (Litopenaeus vannamei) fed dietary synbiotic (mannan oligosaccharide and bacillus licheniformis). Aquacult. Rep. 17, 100408. doi: 10.1016/j.aqrep.2020.100408
Dalmo R. A., Ingebrigtsen K., Bøgwald J. (1997). Non-specific defense mechanisms in fish, with particular reference to the reticuloendothelial system (RES). J. Fish Dis. 20, 241–273. doi: 10.1046/j.1365-2761.1997.00302.x
Dawood M. A. O., Koshio S., Fadl S. E., Ahmed H. A., Asely A. E., Daim M. M., et al. (2020). The modulatory effect of mannanoligosaccharide on oxidative status selected immune parameters and tolerance against low salinity stress in red sea bream (Pagrus major). Aquacult. Rep. 16, 100278. doi: 10.1016/j.aqrep.2020.100278
Dimitroglou A., Davies S. J., Sweetman J., Divanach P., Chatzifotis (2010). Dietary supple-mentation of mannanoligosaccharide on white sea bream (Diplodus sargus l.) larvae: effects on development, gut morphology and salinity tolerance. Aquacult. Res. 41, 245–251. doi: 10.1111/j.1365-2109.2010.02513.x
Dimitroglou A., Merrifield D. L., Moate R., Davies S. J., Spring P., Sweetman J., et al. (2009). Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Anim. Sci. 87, 3226–3234. doi: 10.2527/jas.2008-1428
Dimitroglou A., Merrifield D. L., Spring P., Sweetman J., Moate R., Davies S. J. (2010). Effects of mannan oligosaccharide (MOS) supplementation on growth perfor-mance, feed utilization, intestinal histology and gut micro-biota of gilthead sea bream (Sparus aurata). Aquaculture 300, 182–188. doi: 10.1016/j.aquaculture.2010.01.015
Estrada U. R., Satoh S., Haga Y., Fushimi H., Sweetman J. (2009). Effects of single and combined supplementation of enterococcus faecalis, mannan oligosaccharide and polyhydroxybutyrate acid on growth performance and immune response of rainbow trout, oncorhynchus mykiss. Aquacult. Sci. 57, 609−617. doi: 10.1007/s10499-007-9096-z
Fevolden S. E. (2010). Cortisol and immune characteristics in rainbow trout (Oncorhynchus mykiss) selected for high or low tolerance to stress. J. Fish Biol. 43, 919–930. doi: 10.1111/j.1095-8649.1993.tb01166.x
Genc M. A., Yilmaz E., Genc E., Aktas M. (2007b). Effects of dietary mannan oligosaccharides (MOS) on growth, body composition, and intestine and liver histology of the hybrid tilapia (Oreochromis niloticus×O. aureus). Isr. J. Aquacult. 59, 10–16. doi: 10.46989/001c.20509
Grisdale–Helland B., Helland S. J., Gatlin D. M. III (2008). The effects of dietary supplementation with mannanoligosaccharide, fruc-tooligosaccharide or galactooligosaccharide on the growth and feed utilization of Atlantic salmon (Salmo salar). Aquaculture 283 (1–4), 163–167. doi: 10.1016/j.aquaculture.2008.07.012
Hoseini S. M., Yousefi M., Hoseinifar S. H., Doan H. V. (2019). Antioxidant, enzymatic and hematological responses of common carp (Cyprinus carpio) fed myrcene- or menthol-supplemented diets and exposed to ambient ammonia. Aquaculture 506, 246–255. doi: 10.1016/j.aquaculture.2019.03.048
Jami M. J., Kenari A. A., Paknejad H., Mohseni M. (2019). Effects of dietary b-glucan, mannan oligosaccharide, lactobacillus plantarum and their combinations on growth performance, immunity and immune related gene expression of Caspian trout, Salmo trutta caspius (Kessler,1877). Fish Shellfish Immunol. 91, 202–208. doi: 10.1016/j.fsi.2019.05.024
Liew H. J., Sinha A. K., Nawata C. M., Blust R., Chris M., Gudrun D. B. (2013). Differential responses in ammonia excretion, sodium fluxes and gill permeability explain different sensitivities to acute high environmental ammonia in three freshwater teleosts. Aquat. Toxicol. 126, 63–76. doi: 10.1016/j.aquatox.2012.10.012
Lin Y. C., Chen J. C. (2001). Acute toxicity of ammonia on litopenaeus vannamei Boone juveniles at different salinity levels. J. Exp. Mar. Biol. Ecol. 259, 109–119. doi: 10.1016/S0022-0981(01)00227-1
Liu B., Xu L., Ge X., Xie J., Xu P., Zhou Q., et al. (2013). Effects of mannan oligosaccharide on the physiological responses, HSP70 gene expression and disease resistance of allogynogenetic crucian carp (Carassius auratus gibelio) under aeromonas hydrophila infection. Fish Shellfish Immunol. 34, 1395–1403. doi: 10.1016/j.fsi.2013.02.028
Li M., Yu N., Qin J. G., Li E., Du Z., Chen L. (2014). Effects of ammonia stress, dietary linseed oil and edwardsiella ictaluri challenge on juvenile darkbarbe catfish Pelteobagrus vachelli. Fish Shellfish Immunol. 38, 158–165. doi: 10.1016/j.fsi.2014.03.015
Lu J. T., Qi C. L., Limbu S. M., Han F. L., Yang L., Wang X. D., et al. (2019). Dietary mannan oligosaccharide (MOS) improves growth performance, antioxidant capacity, non-specific immunity and intestinal histology of juvenile Chinese mitten crabs (Eriocheir sinensis). Aquaculture 510, 337–346. doi: 10.1016/j.aquaculture.2019.05.048
Mateo C. D., Jacques K. A., Harvey J. (2000). Organic chromium, mannan oligosachharides and zinc bacitracin:effects on broiler performance and carcass characteristics. Poultry Sci. 79, 116. doi: 10.21608/ajas.2014.866
Mirghaed A. T., Fayaz S., Hoseini S. M. (2019). Effects of dietary 1,8-cineole supplementation on serum stress and antioxidant markers of common carp (Cyprinus carpio) acutely exposed to ambient ammonia. Aquaculture 509, 8–15. doi: 10.1016/j.aquaculture.2019.04.071
Mohammadian T., Nasirpour M., Tabandeh M. R., Mesbah M. (2019). Synbiotic effects of β- glucan, mannan oligosaccharide and lactobacillus casei on growth performance, intestine enzymes activities, immune-hematological parameters and immune-related gene expression in common carp, Cyprinus carpio: An experimental infection with aeromonas hydrophila. Aquaculture 511, 634197. doi: 10.1016/j.aquaculture.2019.06.011
Paust L. O., Foss A., Imsland A. K. (2011). Effects of chronic and periodic exposure to ammonia on growth, food conver-sion efficiency and blood physiology in juvenile Atlantic halibut (Hippoglossus hippoglossus l.). Aquaculture 315, 400–406. doi: 10.1016/j.aquaculture.2011.03.008
Pryor G. S., Royes J. B., Chapman F. A., Miles R. D. (2003). Mannanoligosaccharides in fish nutrition: effects of dietary supplementation on growth and gastrointestinal villi structure in gulf of Mexico sturgeon. N. Am. J. Aquacult. 65, 106–111. doi: 10.1577/1548-8454(2003)65<106:MIFNEO>2.0.CO;2
Qi X. Z., Xue M. Y., Yang S. B., Zha J. W., Wang G. X., Ling F. (2017). Ammonia exposure alters the expression of immune-related and antioxidant enzymes-related genes and the gut microbial community of crucian carp (Carassius auratus). Fish Shellfish Immunol. 70, 485–490. doi: 10.1016/j.fsi.2017.09.043
Rajabiesterabadi H., Yousefi M., Hoseini S. M. (2020). Enhanced haematological and immune responses in common carp Cyprinus carpio fed olive leaf extract-supplemented diets and subjected to ambient ammonia. Aquacult. Nutr. 26, 763–771. doi: 10.1111/anu.13035
Rama S., Manjabhat S. N. (2014). Protective effect of shrimp carotenoids against ammonia stress in common carp, Cyprinus carpio. Ecotoxicol. Environ. Saf. 107, 207–213. doi: 10.1016/j.ecoenv.2014.06.016
Ren Z. L., Wang S. F., Cai Y., Wu Y., Tian L. J., Wang S. Q., et al. (2020). Effects of dietary mannan oligosaccharide supplementation on growth performance, antioxidant capacity, non-specific immunity and immune-related gene expression of juvenile hybrid grouper (Epinephelus lanceolatus♂ × epinephelus fuscoguttatus♀). Aquaculture 523, 735195. doi: 10.1016/j.aquaculture.2020.735195
Salze G., Mclean E., Schwarz M. H., Craig S. R. (2008). Dietary mannan oligo-saccharide enhances salinity tolerance and gut development of larval cobia. Aquaculture 274, 148–152. doi: 10.1016/j.aquaculture.2007.11.008
Sang H. M., Fotedar R., Filer K. (2015). Effects of dietary mannan oligosaccharide on the survival, growth, immunity and digestive enzyme activity of freshwater crayfish, cherax destructor clark (1936). Aquacult. Nutr. 17, e629–e635. doi: 10.1111/j.1365-2095.2010.00812.x
Selim K. M., Reda R. M. (2015). Beta-glucans and mannan oligosaccharides enhance growth and immunity in Nile tilapia. N. Am. J. Aquac. 77, 22–30. doi: 10.1080/15222055.2014.951812
Sies H. (1997). Oxidative stress: oxidants and antioxidants. Exp. Physiol. 82, 291–295. doi: 10.1113/expphysiol.1997.sp004024
Sims M. D., Dawson K. A., Newman K. E., Spring P., Hoogell D. M. (2004). Effects of dietary mannan oligosaccharide, bacitracin methylene disalicylate, or both on the live performance and intestinal microbiology of turkeys. Poultry Sci. 83, 1148–1154. doi: 10.1093/ps/83.7.1148
Sinha A. K., Elgawad H., Giblen T., Zinta G., Rop M. D., Asard H., et al. (2014). Anti-oxidative defences are modulated differentially in three freshwater teleosts in response to ammonia-induced oxidative stress. PloS One 9, e95319. doi: 10.1371/journal.pone.0095319
Tacon A. G. J. (2020). Trends in global aquaculture and aquafeed production: 2000–2017. Rev. Fish. Sci. Aquacult. 28, 43–56. doi: 10.1080/23308249.2019.1649634
Torrecillas S., Makol A., Betancor M. B., Montero D., Caballero M. J., Sweetman J., et al. (2013). Enhanced intestinal epithelial barrier health status on European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol. 34, 1485–1495. doi: 10.1016/j.fsi.2013.03.351
Torrecillas S., Makol A., Caballero M. J., Montero D., Robaina L., Real F., et al. (2007). Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol. 23, 969–981. doi: 10.1016/j.fsi.2007.03.007
Wang J., Li J., Xu N., Li J., Li Z., Chen Y., et al. (2015). Responses of takifugu obscurus fertilized eggs and larvae to increased ammonia exposure. Environ. Sci. pollut. Res. 44, 15976–15984. doi: 10.1007/s11356-015-4815-x
Wang L., Wu D., Fan Z., Li H., Li J., Zhang Y., et al. (2020). Effect of yucca schidigera extract on the growth performance, intestinal antioxidant status, immune response, and tight junctions of mirror carp (Cyprinus carpio). Fish Shellfish Immunol. 103, 211–219. doi: 10.1016/j.fsi.2020.05.039
Xia Z., Wu S. (2018). Effects of glutathione on the survival, growth performance and non-specific immunity of white shrimps (Litopenaeus vannamei). Fish Shellfish Immunol. 73, 141–144. doi: 10.1016/j.fsi.2017.12.015
Xue S. Q., Chen S. M., Ge Y. X., Ge Y. X., Guan T., Han Y. (2022). Regulation of glutathione on growth performance, biochemical parameters, non-specifific immunity, and related genes of common carp (Cyprinus carpio) exposed to ammonia. Aquaculture 546, 737241. doi: 10.1016/j.aquaculture.2021.737241
Xue S., Lin J., Han Y., Han Y. (2021). Ammonia stress -induced apoptosis by p53–BAX/BCL-2 signal pathway in the hepatopancreas of the common carp (Cyprinus carpio). Aquacult. Int. 29, 1895–1907. doi: 10.1007/s10499-021-00724-3
Xu P., Zhang X., Wang X., Li J., Liu G., Kuang Y., et al. (2014). Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat. Genet. 46, 1212–1219. doi: 10.1038/ng.30980
Ye J. D., Wang K., Li F. D., Sun Y. Z. (2011). Single or combined effects of fructo- and mannan oligosaccharide supplements and bacillus clausii on the growth, feed utilization, body composition, digestive enzyme activity, innate immune response and lipid metabolism of the Japanese flounder paralichthys olivaceus. Aquacult. Nutr. 17, 902–911. doi: 10.1111/j.1365-2095.2011.00863.x
Yilmaz E., Genc M. A., Genc E. (2007). Effects of dietary mannan oligo-saccharides on growth, body composition, and intestine and liver histology of rainbow trout, Oncorhynchus mykiss. Isr. J. Aquacult-bamid 59, 182–189. doi: 10.46989/001c.20509
Yousefi M., Vatnikov Y. A., Kulikov E. V., Plushikov V. G., Drukovsky S. G., Hoseinifar S. H., et al. (2020). The protective effects of dietary garlic on common carp (Cyprinus carpio) exposed to ambient ammonia toxicity. Aquaculture 526, 735400. doi: 10.1016/j.aquaculture.2020.735400
Yuen K., Chew S. F. (2010). Ammonia production, excretion, toxicity, and defense in fish: a review. Front. Physiol. 1. doi: 10.3389/fphys.2010.00134
Zhang Y., Ye C., Wang A., Zhu X., Chen C. H., Xian J. A. (2015). Isolated and combined exposure to ammonia and nitrite in giant freshwater pawn (Macrobrachium rosenbergii): effects on the oxidative stress, antioxidant enzymatic activities and apoptosis in haemocytes. Ecotoxicology 24, 1601–1610. doi: 10.1007/s10646-015-1477-x
Zhang Q., Yu H., Tong T., Tong W. W., Dong L. F., Xu M. Z., et al. (2014). Dietary supplementation of bacillus subtilis and fructooligosaccharide enhance the growth, nonspecifific immunity of juvenile ovate pompano, Trachinotus ovatus and its disease resistance against vibrio vulnifificus. Fish Shellfifish Immun. 38, 7–14. doi: 10.1016/j.fsi.2014.02.008
Zhao P. Y., Jung J. H., Kim I. H. (2012). Effect of mannan oligosaccharides and fructan on growth performance, nutrient digestibility, blood profile, and diarrhea score in weanling pigs. J. Anim. Sci. 90, 833–839. doi: 10.2527/jas.2011-3921
Keywords: mannan oligosaccharide (MOS), common carp, ammonia stress, growth performance, immunity
Citation: Xue S, Xia B, Zhang B, Li L, Zou Y, Shen Z, Xiang Y, Han Y and Chen W (2022) Mannan oligosaccharide (MOS) on growth performance, immunity, inflammatory and antioxidant responses of the common carp (Cyprinus carpio) under ammonia stress. Front. Mar. Sci. 9:1062597. doi: 10.3389/fmars.2022.1062597
Received: 07 October 2022; Accepted: 21 November 2022;
Published: 15 December 2022.
Edited by:
Yafei Duan, South China Sea Fisheries Research Institute, ChinaReviewed by:
Gang Yang, Nanchang University, ChinaZe Fan, Heilongjiang River Fisheries Research Institute, China
Copyright © 2022 Xue, Xia, Zhang, Li, Zou, Shen, Xiang, Han and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weixing Chen, d3hjaGVuQG5lYXUuZWR1LmNu; Ying Han, aGFuZ3lpbmd6b3VAbmVhdS5lZHUuY24=
†These authors have contributed equally to this work
 Shuqun Xue
Shuqun Xue Banghua Xia
Banghua Xia Bitao Zhang
Bitao Zhang