- 1Department of Biological Sciences, University of Manitoba, Winnipeg, MB, Canada
- 2Fish, Food and Allied Workers Union, St. John's, NL, Canada
- 3Northwest Atlantic Fisheries Centre, Fisheries and Oceans Canada, St. John’s, NL, Canada
Introduction: On the Newfoundland-Labrador Shelf, Canada, capelin (Mallotus villosus) is a key forage fish that migrates annually from offshore to spawn within coastal embayments. Although capelin are thought to primarily spawn on beaches in this region, they also spawn subtidally in deeper water (5–40 m), where their eggs remain throughout incubation. The spatial extent of subtidal (i.e. “deep-water”) spawning habitat in coastal Newfoundland is unknown and is a research priority for fishers and management.
Methods: We collaborated with capelin fishers to identify putative deep-water spawning sites as a first step in determining the contribution of deep-water spawning to capelin recruitment. Given limited fine-scale coastal bathymetry and seabed habitat type data, which impeded spatial modeling to determine suitable capelin spawning habitat, this science-industry research collaboration was key to addressing this knowledge gap.
Results: Through two years of multi-bay fisher interviews, 84% of interviewed fishers (56 interviewees) reported having observed deep-water spawning and identified a broad distribution of putative spawning sites throughout coastal Newfoundland. The majority of fishers indicated inter-annual variation in beach and deep-water spawning habitat use, and most interviewees linked this variation to temperature and capelin abundance. Further collaborations with fishers during boat-based surveys, we sampled 136 unique sites within 12 search areas in eastern Placentia Bay and 26 unique sites within six search areas in Bonavista Bay. Underwater video surveys combined with sediment sampling revealed seven previously undocumented deep-water spawning sites.
Conclusion: The deep-water spawning areas derived from these fisher interviews can now be used to build a time series for monitoring capelin spawning habitat use alongside citizen-based beach monitoring data, as a general capelin stock health indicator in a weight of evidence approach for the science advisory process.
Introduction
Many marine systems are described as wasp-waist, whereby energy flow to the higher trophic level is funneled through one or a few forage species at the intermediate trophic level (Bakun, 2006). Thus, alterations in the distributional and density patterns of these forage species can affect marine food webs in unpredictable ways (Coll et al., 2008; Pikitch et al., 2012). Capelin (Mallotus villosus) is a key forage fish species in the Northwest Atlantic that functions as the primary wasp-waist species, or link, between lower and higher trophic levels (Lavigne, 1996; Carscadden and Vilhjalmsson, 2002). In coastal Newfoundland, spawning capelin abundance and distribution directly impact the density and distributional patterns of marine mammals (Whitehead and Carscadden, 1985; Johnson and Davoren, 2021), Atlantic cod (Gadus morhua; Rose and O’Driscoll, 2002), and seabirds (Carscadden et al., 2002; Davoren and Montevecchi, 2003; Davoren, 2013a). However, in the early 1990s, the capelin population on the Newfoundland Shelf (Northwest Atlantic Fisheries Organization [NAFO] Divisions 2J3KL) crashed from a high of 2–6 million tons (Mt) pre-1991 to 0.03–1.0 Mt for the subsequent three decades, reaching an all-time low in 2010 at ~1% of pre-1991 levels (Buren et al., 2014; DFO, 2015). Further, since 1991 there have been minimal signs of recovery (Buren et al., 2019).
Maturing capelin undergo an annual migration from offshore waters to spawn within coastal embayments of Newfoundland and southern Labrador, with beaches considered the primary spawning habitat in coastal regions (Nakashima and Wheeler, 2002). There are, however, historical reports in this region of capelin spawning at subtidal sites in deeper water (< 50 m; hereafter referred to as ‘deep-water sites’ Templeman, 1948). These early records included reports of capelin spawn on traps, trawls and nets from other fisheries and on anchors from 5–45 m depth (Templeman, 1948). Since the early 2000s, deep-water spawning sites have been located and scientifically monitored on an annual basis in Trinity Bay (Nakashima and Wheeler, 2002) and Notre Dame Bay (Davoren et al., 2008) and researchers have studied a variety of topics, including spawning habitat selection (Davoren, 2013b; Crook et al., 2017), genetic divergence between beach and deep-water spawners (Penton et al., 2014; Kenchington et al., 2015), and connectivity between spawning habitats (Davoren and Halden, 2014; Davoren et al., 2015), as well as among embayments (Tripp et al., 2020). In particular, recent studies have shown temperature-dependent shifts between subtidal and intertidal habitats, which results in high inter-annual variation in spawning site use within regions (Davoren, 2013b; Crook et al., 2017). These findings support Templeman's (1948) hypothesis that capelin select between these two spawning habitats based on water temperature, occupying warmer beach habitat in years of cooler water and shifting to deep-water habitat in warmer years. Based on observations of abnormal egg development and low larval emergence at deep-water spawning sites in Trinity Bay, Nakashima and Wheeler (2002) inferred that larval production from this habitat would be limited. More recent studies, however, have shown that both spawning habitats have similar egg densities, temperature-dependent egg development (Penton et al., 2012) and produce larvae in good condition (Penton and Davoren, 2008). Although fishers have reported that deep-water spawning has become more prevalent since 1994 (Nakashima and Clark, 1999) and persistently urge fisheries scientists to quantify the productivity of this habitat (Dawe and Carruthers, 2019), little is currently known about the relative contribution of deep-water and beach habitats to population-level capelin recruitment (Davoren et al., 2008), partly because the province-wide spatial extent of these coastal deep-water spawning sites is unknown.
As capelin typically spawn subtidally in small patches of suitable sediment (0.5–25 mm; Templeman, 1948; Nakashima and Wheeler, 2002; Davoren et al., 2008; Penton et al., 2012; Penton and Davoren, 2013) within a limited temperature range (2–12°C; Carscadden et al., 1989; Davoren, 2013b; Crook et al., 2017), modeling the spatial extent of this habitat in coastal Newfoundland requires both fine-scale (< 100 m) bathymetry and seabed habitat information within the 50 m contour, which is typically within suitable temperature ranges for capelin spawning (Davoren et al., 2006). Unfortunately, both data types have limited spatial coverage within coastal Newfoundland. Therefore, we aimed to collaborate with capelin fishers to identify potential deep-water spawning areas. Fishers’ knowledge (FK) research and, more broadly, Local and Traditional Ecological Knowledge (LEK & TEK), can provide information on species beyond the scope of ongoing scientific observations (e.g., Neis et al., 1999; Silvano et al., 2006; Martinez-Levasseur et al., 2017). In coastal Newfoundland, a long-standing commercial capelin roe fishery occurs during their spawning season with a 100% utilization of both male and female fish since 2006, so fishers collect pre-season samples to determine sex ratios of the schools, female size and roe content (DFO, 2022a). The focus on roe results in an intensive observation window by fishers during spawning which leads to extensive LEK on the location, timing, and duration of beach and deep-water spawning. Additionally, capelin deep-water spawning may overlap with traditional fishing grounds for other species such as cod. Incorporating such fishers’ knowledge has increased our understanding of capelin stock dynamics and ecology in the past (DFO, 1997; Neis and Morris, 2002). In support, FK of other fish stocks has challenged the results/conclusions of stock assessments (e.g., Neis et al., 1999; Hutchings and Ferguson, 2000) and has documented spawning components (e.g., Ames, 2004; DeCelles et al., 2017) as well as fish migration and feeding patterns (e.g., Fraser et al., 2006; Silvano et al., 2006) that either differed from or were not previously documented by fisheries science.
To identify the number and location of deep-water capelin spawning sites throughout the south and east coasts of Newfoundland, we used structured interviews in 2018 and 2019 to document capelin fishers’ knowledge of deep-water spawning areas and then, using information recorded during these interviews, we worked with capelin fishers during boat-based surveys to locate deep-water spawning sites during the 2019 spawning season. We focused our boat-based research surveys on only Bonavista and Placentia bays due to previous knowledge on potential deep-water spawning sites (Placentia Bay, Sjare et al., 2003) and encouragement by fishers based on the economic importance of the local capelin fishery (Bonavista Bay, DFO, 2022a). These bays also represent two different capelin stocks (Placentia Bay: 3Ps; Bonavista Bay: 2J3KL), one of which (3Ps) has a paucity of data. Fisher knowledge interviews were also used to assess inter-annual shifts in the use of beach and deep-water spawning sites, as well as to identify factors likely influencing these shifts in habitat use. Our ultimate goal was to map fishers’ knowledge of the spatial extent of deep-water spawning sites to serve as an important first step in quantifying the contribution of deep-water spawning habitat to capelin recruitment dynamics and to complement ongoing work examining the productivity of capelin spawning habitats (Penton et al., 2012; Davoren et al., 2015) and regions (Tripp et al., 2020) as well as environmental drivers of capelin recruitment (Murphy et al., 2018; Lewis et al., 2019). The results from our study address a research priority for fishers, thereby strengthening fisher-science advisor relationships, and have the potential to be used in the assessment of the stock alongside a citizen-based beach monitoring time series (Murphy, 2022).
Materials and methods
Interview design
We asked capelin fishers a series of questions during in-person interviews. Researcher (ND) from the Fish, Food and Allied Workers Union (FFAW) conducted interviews during January–February 2019 (26 interviewees) in Conception, Trinity, Bonavista, Notre Dame, and White bays, and researcher (LB) from the University of Manitoba conducted interviews during August 2018 in eastern Placentia Bay, St. Mary’s Bay and Southern Avalon (22 interviewees), and July–August 2019 in eastern and western Placentia Bay (19 interviewees). Interview questions were based on an interview template previously used to investigate changes in capelin biology and behavior (Sjare et al., 2003; Table S1). The primary purpose of the interviews was to obtain information on the spatial locations of capelin beach and deep-water spawning activity, while the secondary purpose was to obtain information on the inter-annual changes in use of spawning habitats, as well as factors likely influencing these changes. We identified interviewees by recruitment at the wharf, during which we asked fishers present who in the area had the most experience fishing capelin. Long-time fishers were recommended by their peers as “experts”, that is, particularly knowledgeable representatives of their fleet sector and region (e.g. Davis and Wagner, 2003). We identified additional interviewees using snowball sampling (i.e. asking interviewees to recommend other fishers; e.g. Gunderson and Watson, 2007). Other fishers were recommended for interviews based on their length of time in the fishery (range 8–65 years), fleet sector (fixed or mobile), and region.
We asked interviewees if they knew whether capelin spawned on beaches and/or in deep water currently or historically. Deep-water spawning was defined as subtidal spawning that is not contiguous with a beach or a beach spawning site. We also asked interviewees how they knew deep-water spawning occurred (see Table S1 for specific questions). Physical evidence of deep-water spawning included the presence of capelin eggs adhered to fishing gear (e.g., nets, traps, pots) and capelin spawning visually observed on the seabed in < 5 m depth. Supplemental non-physical evidence that fishers stated to aid in identifying deep-water spawning areas included ‘heard from others’, ‘bird and whale activity’, and ‘aggregations of capelin’ including deep-water spawning behavior observed on echosounders (i.e. stationary aggregations of seabed-associated shoals that were confirmed to be capelin during fishing). Finally, we asked interviewees to draw the location(s) of beach and deep-water spawning on a nautical chart and to only provide information on deep-water spawning locations that were based on physical evidence. These areas identified as putative deep-water spawning areas based on physical evidence (hereafter referred to as ‘putative areas’) were later converted to centroid points for standardization and used to inform boat-based sampling (see below).
As we were also interested in inter-annual changes in the use of beach and deep-water spawning areas, along with fishers’ understanding of factors likely influencing changes in habitat use, we also asked questions related to changes in the timing of spawning, area use, and the duration of spawning at known spawning areas (see Table S1 for specific questions).
Boat-based sampling
As there is high inter-annual variation in the timing of the first day of capelin spawning (3–6 weeks; Crook et al., 2017), the timing of spawning from interviews (in previous years) was not used to determine the initiation of boat-based sampling. Instead, prior to boat-based sampling, we regularly monitored citizen-science social media platforms, including Twitter (#CapelinRoll) and www.ecapelin.ca, where beach spawning observations are posted, to ensure that we began boat-based surveys after capelin had begun beach spawning. This minimized the chances of sampling before deep-water spawning had commenced. Additionally, when spawning at beaches was reported on social media, we sampled them and other nearby beach sites at least once to verify capelin had started spawning. Specifically, we carefully examined beach sediment for adherent eggs and, when eggs were present, we placed the sediment/egg sample into a 20 mL glass scintillation vial with Stockard’s Solution (50 mL formaldehyde, 37% solution; 40 mL glacial acetic acid; 60 mL glycerin; 850 mL sea water). Samples of 50–100 eggs from each site were then examined under a dissecting microscopic (Olympus SZX7) to quantify the number of eggs within six stages of development (as described in Frank and Leggett, 1981; see methods in Penton et al., 2012). The presence of eggs in early developmental stages (Stage I & II) was used to indicate current and recent spawning activity (i.e. 1–5 days, depending on site-specific temperature), whereas later stages (Stages III–VI) indicated spawning activity many days prior to sampling.
To search for deep-water capelin spawning areas, we chartered local fishers for boat-based sampling during July–August 2019. A standard-sized rectangle (4.6 km by 2.3 km) was drawn around the centroid of each putative deep-water spawning area indicated by capelin fishers during interviews. The standardized rectangle size was based on the largest area indicated by an interviewee. If more than one rectangle overlapped, we replaced the overlapping rectangles with a single rectangle which was arranged to cover the area of highest spatial overlap. As the purpose of the interviews was to identify areas in which to search for capelin deep-water spawning, we called these rectangles ‘search areas’. As we did not have fine-scale (< 100 m) bathymetry or seabed habitat type data to refine the search area based on suitable capelin spawning habitat, we used ArcMap 10.3.1 to generate 10 random sites within each search area. The random sites within a search area were at least 500 m apart, based on the size of the sampling vessel (6 m) and expected drift to reduce the risk of resampling another randomly generated site. Other sites (hereafter referred to as ‘adaptive sites’) were added within a search area or outside a search area if we saw evidence suggesting a nearby capelin spawning site while at sea (i.e., dead male capelin on the seabed; stunned, solitary males in the water column; capelin schools on an echosounder or observed from the surface; abundant foraging seabirds and/or whales). All random and adaptive sites were examined carefully regardless of whether they had suitable spawning sediment (0.5–25 mm, Penton and Davoren, 2012), as capelin are also known to spawn on suitable algal species in areas dominated by bedrock in coastal Newfoundland (Bliss and Davoren, 2021).
At each random and adaptive site, we first deployed underwater video cameras to visually determine the presence/absence of capelin eggs. These surveys were conducted during daylight hours, although capelin spawn in both daylight and dark. Underwater video cameras (GoPro Hero 7) were attached to a metal frame (meshless whelk or crab pot), along with a temperature logger (Hoskin Scientific Limited Waterproof TidbiT v2 temperature logger or Star-Oddi DST) that measured temperature every 5–60 s throughout each deployment. The metal frame was lowered to the seabed and left at the bottom for three minutes (‘stationary survey’), during which video footage was continuously recorded. At each site, bottom temperature was characterized by averaging measurements after temperature stabilized at depth. In Placentia Bay only, the metal frame was subsequently lifted ~1 m off the seabed and allowed to drift for up to 250 m (‘drift survey’) to explore more of the adjacent seabed for the presence of capelin eggs. When eggs were determined to be visually present or when egg presence/absence was uncertain, we sampled the sediment using a 15-cm2 Ponar Grab system or a dredge, which consisted of a metal pipe (diameter: 11.4 cm; length [top]: 39.4 cm; length [bottom]: 47 cm) with 150-μm mesh at one end. We identified eggs from sediment samples obtained from deep-water sites as capelin eggs based on egg size and colour (Friðgeirsson, 1976), and compared these eggs to reference capelin egg samples collected from active capelin beach spawning sites. Additionally, we quantified the primary stage of egg development from each sample (described above).
Ethical statement
Animal: The care and use of experimental animals complied with Canadian Council of Animal Care animal welfare laws, guidelines and policies as approved by Canadian Council of Animal Care (Protocol: F16-017).
Human: When we contacted fishers, and before each interview, we described project objectives and how the data would be used, thereby providing the information needed for free and informed consent. Additionally, all interviews were randomly assigned a unique alpha-numeric identifier to ensure anonymity (e.g., A1, B6, F15). We adhered to national ethical research guidelines (e.g., TCPS2 2018), ensuring confidentiality and privacy was protected. Interviews lasted 0.5–3 h, and we recorded responses on prepared interview sheets.
Results
Interviews
Over two years (2018, 2019), we interviewed a total of 67 former or current capelin fishers along the southern and eastern Newfoundland coasts (Figure 1). In 2018, interviewees across all regions (22 interviewees) on average had 34 years (range: 8–65 years) of fishing experience, which was similar to 2019 (45 interviewees; average: 40 years; range: 19–60 years). During exploratory analysis, we found fishing experience did not affect the number of capelin spawning areas reported, likely because we targeted community ‘experts’, and thus, the average interviewee had experience fishing capelin before the stock collapsed (i.e. pre-1991). In 2018, 19/22 interviewees across all regions said that capelin spawn in deep water and 5/19 referred to these areas as “capelin holes” (described as bathymetric depressions); 13/19 indicated one or more deep-water areas on a map. In 2019, the majority of interviewees in all regions (37/45) said that capelin spawn in deep water and 34/37 indicated one or more deep-water areas on a map. Many fishers were not surprised to be asked about the importance of deep-water spawning and clearly had years of experience observing it:
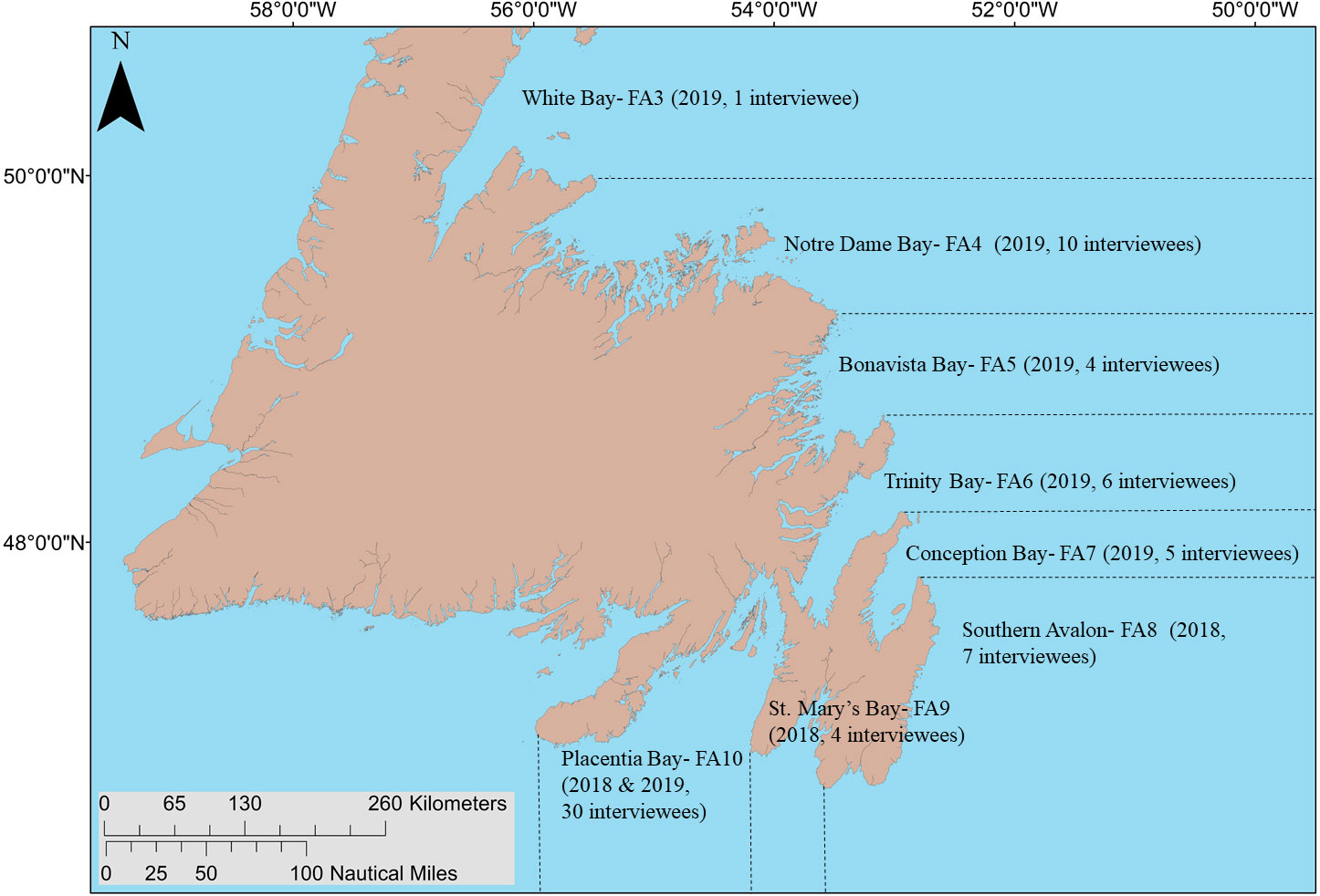
Figure 1 Capelin fishing areas (FA; fishing areas delineated by dashed black lines) covered by interviews of capelin fishers, along with the number of interviewees residing in each fishing area and the year interviews were conducted indicated in parentheses.
“If they don’t hit the beach [to spawn], they’re offshore [spawning]. Now I believe that’s a common occurrence.” (A1)
The interviewed fishers in 2018 and 2019 that reported capelin spawning in deep water (56/67), noted that this knowledge was based on one or more types of information. Fishers that indicated locations of deep-water spawning areas primarily reported observing capelin eggs adhered to their fishing gear, including on trawl foot gear, cod gillnets, lumpfish nets or on crab and lobster pots or visual observations of capelin eggs on the seabed. This physical evidence was often supplemented by non-physical evidence based on observations: the location of capelin spawning behavior as observed on echosounders (8 interviewees; see quotes A2, J7, and F2 below), occurrence of post-spawning capelin and/or capelin eggs in cod stomachs (4), persistent aggregations of whales and gulls during the spawning season (4), and information from other fishers (3). For example:
“When they [capelin] are flat on the bottom, they are spawning and if you put a net there it will be covered by spawn.” (A2)
“See them [capelin] on sounders when they are flat on the bottom. They are spawning. If [you] put a net there [it will be] full of spawn.” (J7)
“[If you] see spawn on the gear [you will] see them on the sounder they stay on the bottom for days.” (F2)
As the capelin spawning migration typically moves northward up the coast of the island, with capelin spawning first in southern bays and subsequently spawn in northern bays as the season progresses (Johnson and Davoren, 2021), many fishers harvest capelin in multiple capelin fishing areas (FA; shown in Figure 1) within a single season. Therefore, our interviewees often identified more than one deep-water spawning location in bays other than their bay of residence. Interviewees identified 101 putative deep-water spawning areas and 193 putative beach spawning areas across eight FAs (see centroids in Figure 2). Due to limited ship time, we focused on 13 putative deep-water spawning areas in eastern Placentia Bay and seven in southern Bonavista Bay. Most search areas in Placentia and Bonavista bays contained a single putative area, with the exception of search areas three (two putative areas) and nine (two putative areas; Figure 3). Therefore, a total of 18 search areas (4.6 km by 2.3 km rectangles) were established for both bays (see rectangle search areas in Figure 3).
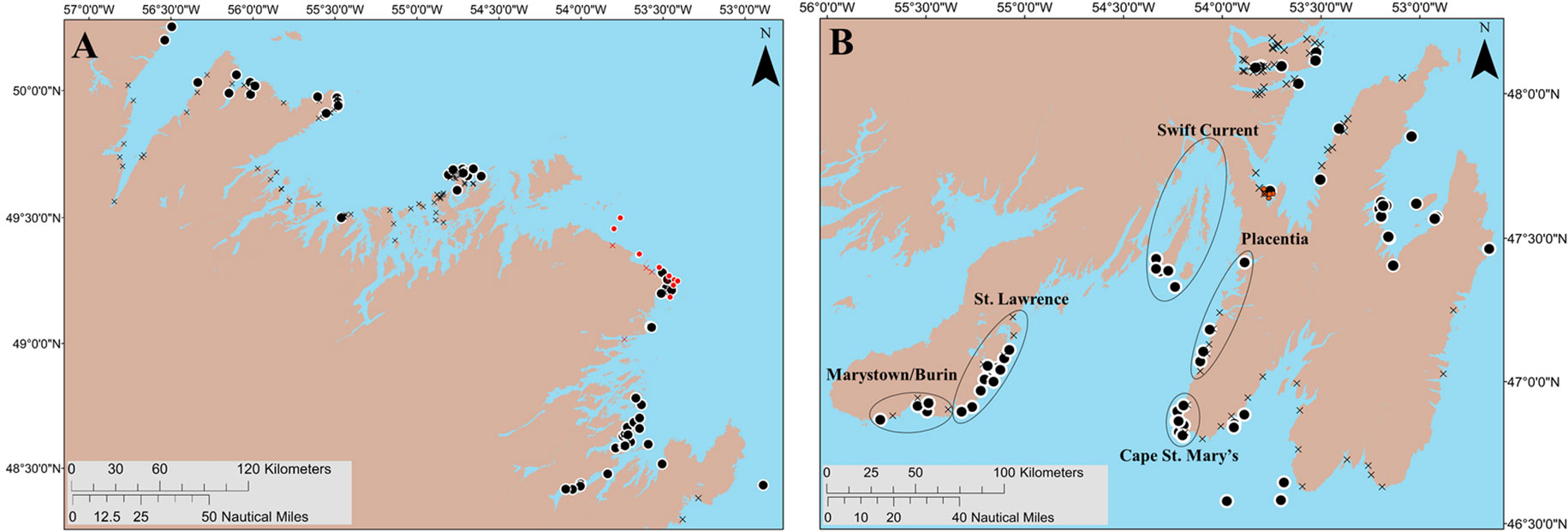
Figure 2 The centroids of putative beach (193; black X symbol) and deep-water (101; black circles) spawning areas identified by interviewees, along with areas previously suggested as important for capelin deep-water spawning by archived fisher interviews in Placentia Bay (black ellipses; based on Sjare et al., 2003), other known and scientifically monitored deep-water spawning sites (16 red circles; based on Nakashima and Wheeler, 2002 and Penton and Davoren, (2012) and beach spawning sites (10; red X symbol). Panel (A) (left): Capelin fishing areas 3–5; Panel (B) (right): Capelin fishing areas 6–10.
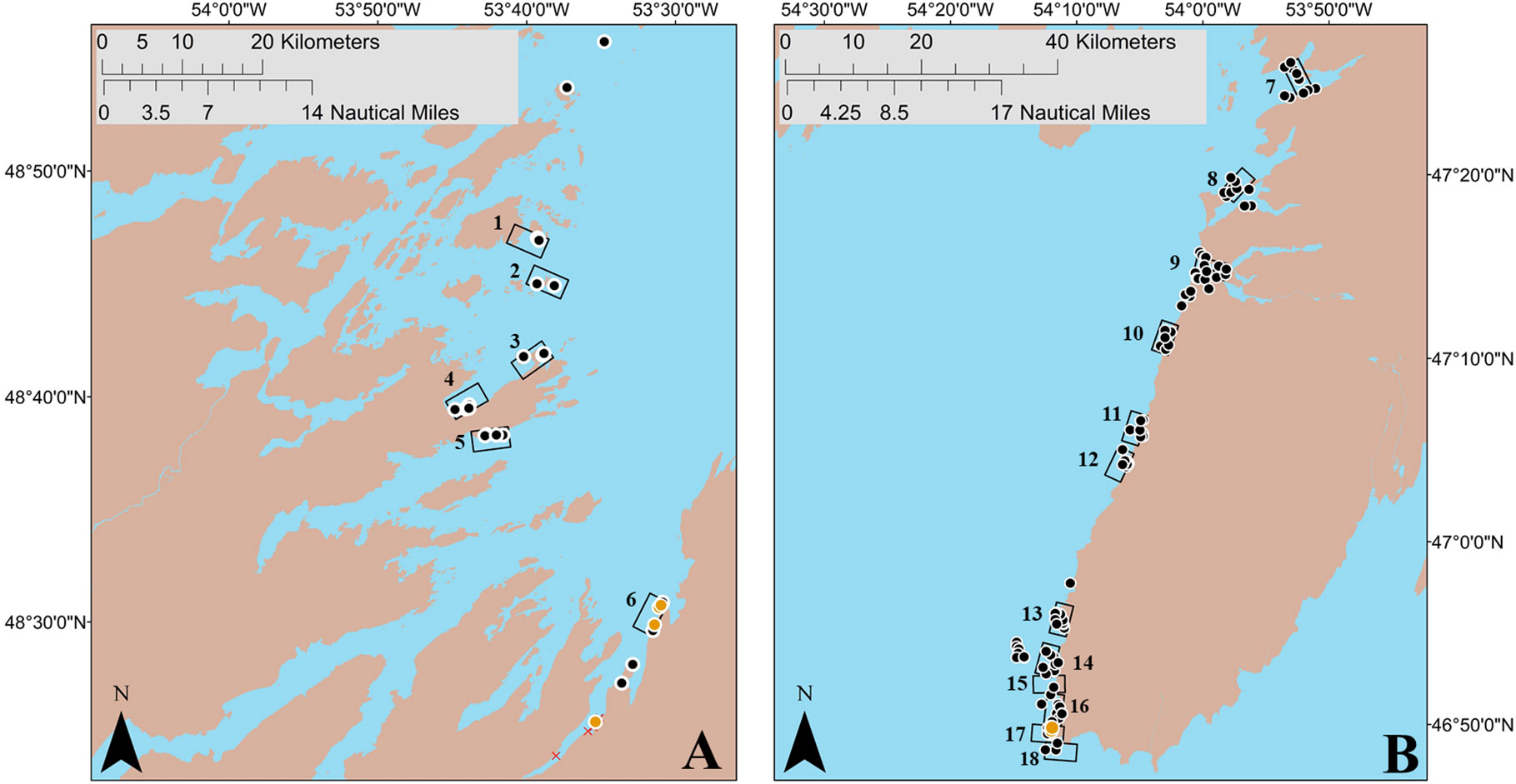
Figure 3 Boat-based search areas (18 areas; rectangles) based on putative deep-water spawning areas identified by interviewees, along with random and adaptive sites (162; black circles), where underwater camera surveys and sediment sampling for capelin spawning (i.e. presence of capelin eggs) were conducted during July–August 2019. Beach sites (21; red X symbol) and deep-water sites where capelin eggs were found (7; orange circles) during July 2019 are also indicated. Panel (A) (left): Bonavista Bay, capelin fishing area 5. Panel (B) (right): Placentia Bay, capelin fishing area 10.
In Placentia Bay, the 12 search areas overlapped with all five of the areas previously indicated as important areas for capelin spawning (i.e., St. Lawrence, Marystown/Burin, Swift Current, Placentia, and Cape St. Mary’s; Sjare et al., 2003) based on fisher interviews (see black ellipses in Figure 2B). Out of the 41 interviewees that indicated deep-water spawning areas in Placentia Bay on a map, 10 interviewees identified putative areas < 5 km from Cape St. Mary’s Ecological Reserve as important for capelin deep-water spawning, seven identified areas near Placentia, six identified areas in Marystown/Burin, and three identified areas in St. Lawrence. Only two of the 41 interviewees had knowledge about deep-water capelin spawning in the Swift Current area (Figure 2B). Fishers also identified putative deep-water spawning areas that have been scientifically documented and monitored along the northeast coast (Penton and Davoren, 2012; Figure 2A, red circles) and six of the putative deep-water spawning areas were within 3.5 km of long-term scientifically monitored deep-water spawning sites in Trinity Bay (red circles; Figure 2B; Nakashima and Wheeler, 2002).
In all bays across both years, 31 of the 56 interviewees who said that capelin spawn in deep water also said that capelin shifted between beach and nearby deep-water habitats within bays annually or on a decadal scale. Eighteen of the 31 interviewees identified factors associated with these shifts: 2/18 suggested the abundance of capelin and 17/18 indicated temperature as the primary factor causing the shift between spawning habitats. For example:
“Temperature has all to do with it. Capelin don’t have to come into the land to spawn. If the temperature is right in 20 fathoms of water, they’ll spawn in 20 fathoms of water.” (B1)
“Temperature has to do with everything. When and where they spawn. [If the] temperature [is] not right by the shore – [capelin will] spawn where the temperature is right.” (B2)
“More capelin spawn in the water now than on beaches. [Capelin] spawned more on beaches in the 1980s. Has to do with the water temperature.” (B3)
Fishers were interviewed along the northeast coast in early 2019, following the 2018 capelin spawning season, which was earlier and more broadly distributed than 2016 and 2017 (DFO, 2021). Among interviewed fishers on the northeast coast who compared 2018 spawning activity to recent years, almost all (17/18) reported an increase in the amount of spawning capelin and/or the number of spawning locations during 2018 relative to recent years.
Boat-based sampling
To confirm capelin were actively spawning prior to or during boat-based sampling during July 2019, we sampled beach sites for capelin spawning in Placentia Bay (three sites), Bonavista Bay (five sites), and nearby beaches along the coast of the Southern Avalon Peninsula (five sites) of Newfoundland (Table 1). In three out of the five initial beach sediment samples from sites in Bonavista Bay, the presence of capelin eggs in later developmental stages (i.e. Stage III–VI; Table 1) indicated that capelin had begun spawning prior to the initial egg sampling date. Similarly, both of the initial beach egg samples in western and eastern Placentia Bay were primarily in later developmental stages, while eggs in early developmental stages (i.e. Stage I–II) in later egg samples from the beaches indicated continued spawning or a second spawning run (Table 1).
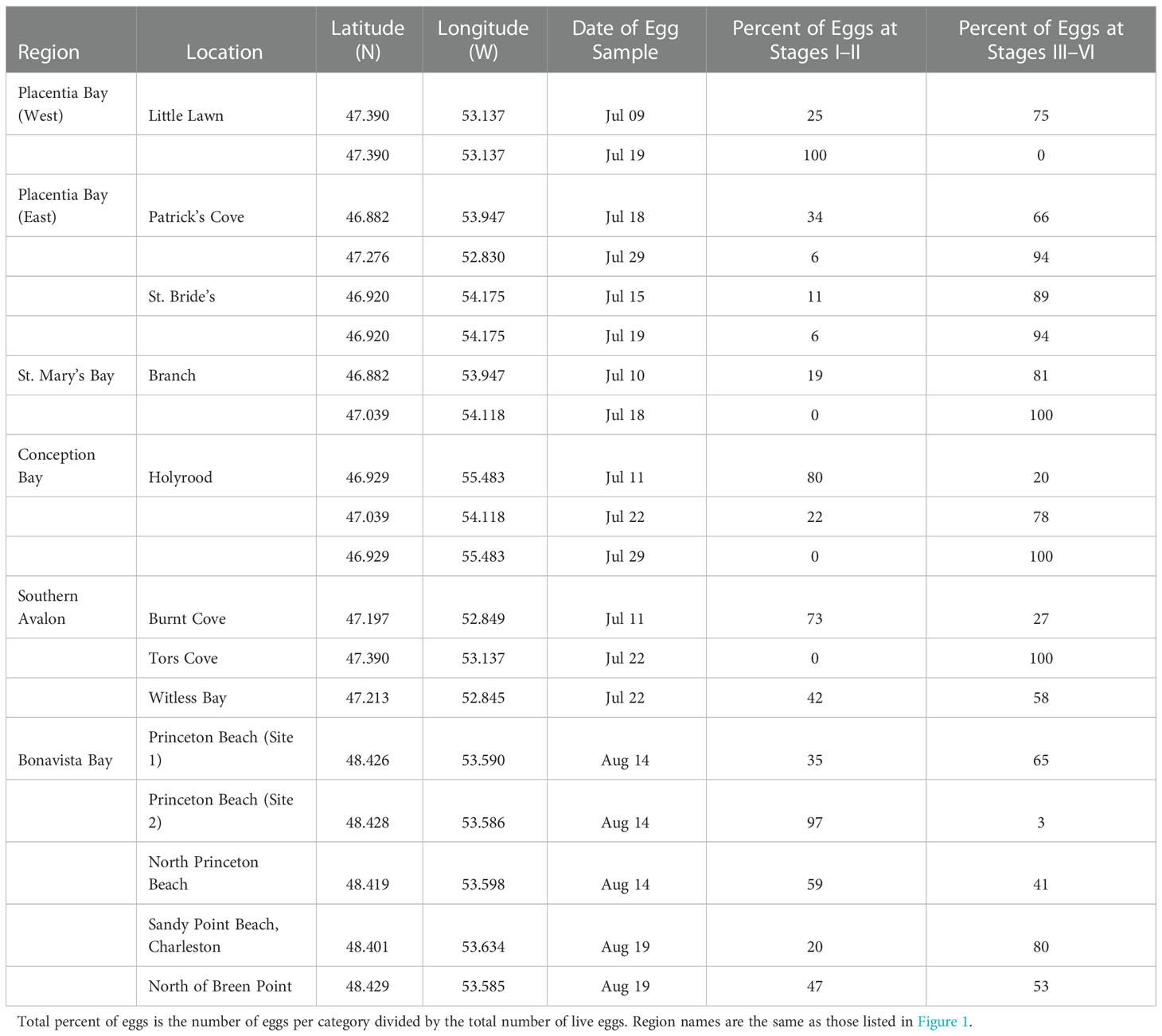
Table 1 Beach capelin egg sampling in Newfoundland during July and August 2019 indicating the sampling location, date and the percentage of capelin eggs in early developmental stages (i.e. Stages I–II) and late developmental stages (i.e. Stages III–VI).
After confirming the presence of spawning capelin in the bays of interest, we sampled for capelin eggs at putative deep-water spawning areas using an underwater camera and/or a bottom grab/dredge at a total of 136 unique sites within 12 search areas in eastern Placentia Bay (Jul 16, 20, 21, 23, 25, 27 and Aug 6, 7, 9, 10, 11, 15) and 26 unique sites within six search areas in Bonavista Bay (Aug 9, 10, 11, 14, 19, 20). These sites included randomly-generated sites (141 sites) and adaptive sites based on evidence of capelin spawning while at sea (21 sites; see methods). We found seven deep-water capelin spawning sites within two search areas (search area 6 in Bonavista Bay; search area 17 in Placentia Bay; Figure 3). These sites were identified from underwater footage based on the presence of dense mats of yellowish fish eggs adhered to gravel/sand, pebbles, or algae (Bliss and Davoren, 2021) which were later confirmed to be capelin eggs. Although dead and live capelin were observed at some of these sites, capelin in the act of spawning were not seen on the video footage. Even though spawning behavior was not directly observed during these surveys, a thorough search of areas adjacent to where capelin eggs were found did not reveal any other egg patches at nearby subtidal or beach sites (Bliss and Davoren, 2021). Overall, we sampled eggs from two of the three deep-water spawning sites located in Placentia Bay and each of the four sites located in Bonavista Bay (Table 2). All seven of these deep-water sites were non-contiguous with the beach, with site-specific depths ranging from 6.1–13.9 m and temperatures ranging from 3.0–15.6°C at the time of egg sampling (Table 2). We measured temperature at the Placentia Bay deep-water spawning sites three times from 20 July to 11 August and the temperature increased at both sites by a maximum of 6.6°C (Table 2).
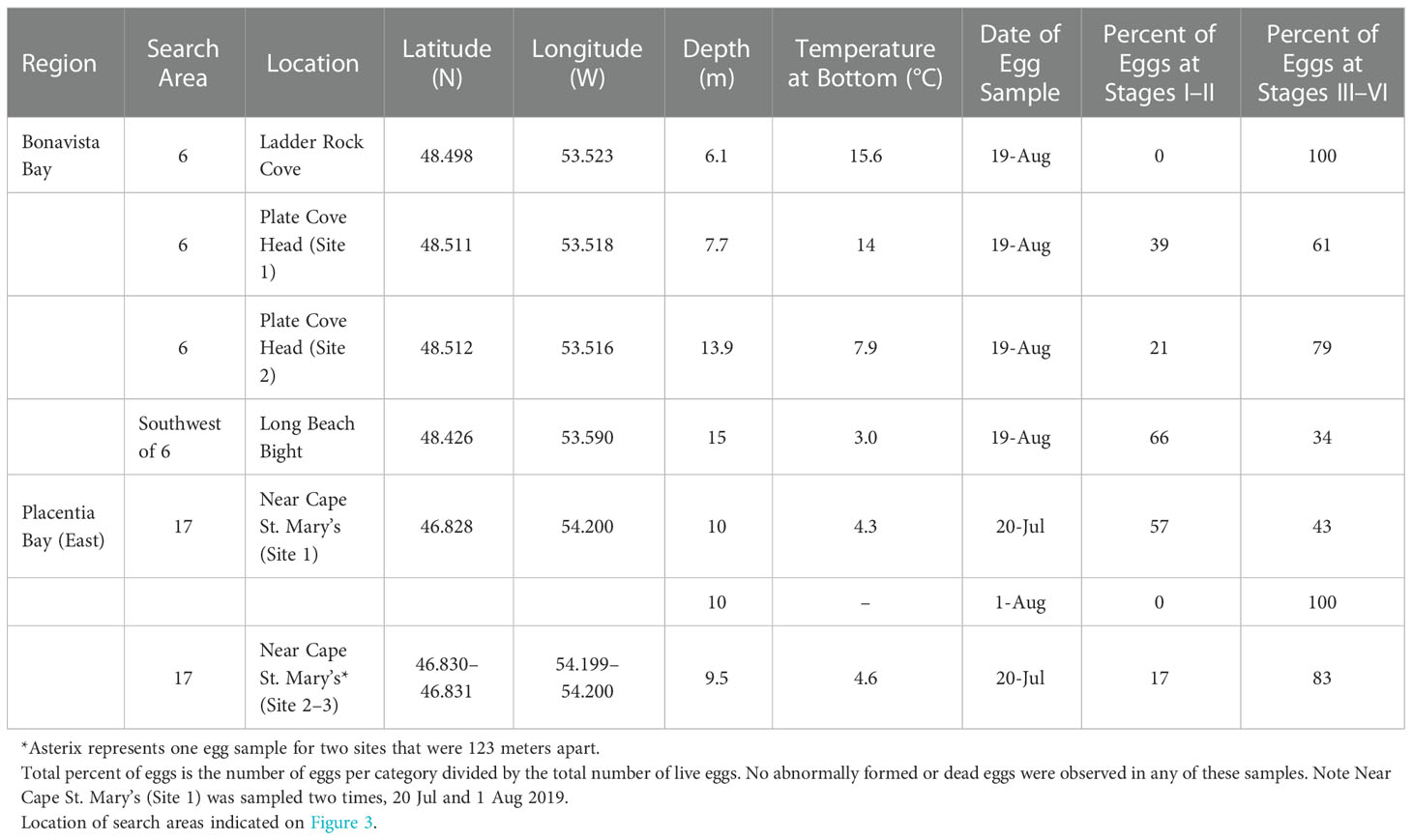
Table 2 Capelin eggs sampled at six of the seven newly found deep-water spawning sites in Placentia Bay and Bonavista Bay during July–August, 2019.
Discussion
During two years of capelin fisher interviews in multiple bays, we found that 84% of interviewed fishers reported knowledge of deep-water spawning, and interviewed fishers identified a broad distribution of putative beach and deep-water spawning sites throughout coastal Newfoundland. Fifty-five percent of fishers indicated inter-annual variation in spawning habitat use; most linked this variation to temperature and some to capelin abundance. By comparing our fisher interview results with similar archived interviews from Placentia Bay in the 1990s (Sjare et al., 2003), fishers identified many of the same deep-water spawning locations in 2018–2019 as in the 1990s. Although the spatial scope of the knowledge of 67 fishers on deep-water and beach spawning spanned across eight bays and two stocks (3Ps, 2J3KL) in coastal Newfoundland, other ecological areas of interest were not covered by our interviews (e.g., Fogo island) and/or boat-based sampling (e.g., Swift Current). We recommend that these areas are considered a priority for further investigation via interviews and boat-based surveys. Based on the high proportion of fishers that identified deep-water spawning areas in this study (56/67) and previous research (Templeman, 1948; Sjare et al., 2003), we conclude that future research may find that deep-water spawning of capelin is widespread throughout coastal Newfoundland.
Although multiple deep-water spawning sites were located that were not previously scientifically documented, we identified active spawning sites during boat-based surveys in only 2 out of the 18 surveyed fisher-interview-based search areas. This result may indicate a disadvantage to using fisher knowledge contributions to inform the location of deep-water spawning sites; however, the low proportion of search areas with active spawning sites may have been due to a number of other reasons. First, as suitable habitat for deep-water spawning can be patchily distributed and suitable patches may be small (Davoren et al., 2008; Penton and Davoren, 2012; Davoren, 2013a; Bliss and Davoren, 2021), active sites may have simply been missed during our boat-based surveys. Second, suitable sediment may be ephemeral at flat sites among years (Penton and Davoren, 2012) and, thus, interviewees may have outlined areas that are no longer suitable for spawning capelin. In contrast, annually persistent deep-water capelin spawning sites are typically found in bathymetric depressions, referred to as “capelin holes” by fishers in this study, where suitable sediment is retained (Penton and Davoren, 2012). Third, the timing of our boat-based surveys began after the start of capelin spawning in both bays. As high abundances of seabirds and whales aggregate at capelin spawning sites during spawning (i.e. multi-species biological hotspots; Davoren, 2013a), they are more easily identified from observations at the ocean surface. If spawning was complete in an area before we began our boat-based surveys, reduced predator activity would make capelin spawning sites more difficult to locate.
Another explanation for the low proportion of active deep-water spawning sites within search areas may be related to inter-annual shifts in beach and deep-water spawning habitat use (Nakashima and Wheeler, 2002; Davoren, 2013b; Penton and Davoren, 2013; Crook et al., 2017). Indeed, habitat suitability and, thus, shifts in the use of habitats and sites (i.e. beach vs. deep-water) and site location within habitats among years appear to be driven by variation in habitat- and site-specific temperature that results in departures from the optimal temperature range for offspring survival (2–12°C; Davoren, 2013b; Penton and Davoren, 2013; Crook et al., 2017). This is supported by the results of our interviews, thereby indicating agreement in fisher-based and science-based knowledge. Indeed, 55% of interviewees that shared knowledge about deep-water spawning also noted shifts between beach and deep-water habitat use among years and 94% of these interviewees stated temperature was the primary factor causing these shifts. However, as water temperatures were similar during 2018 and 2019, and were warmer relative to the local long-term average (Cyr and Galbraith, 2021; DFO, 2021), we would expect extensive use of deep-water spawning sites because beaches may have been too warm (≥ 12°C) later in the summer. Therefore, temperature was likely not a limiting factor for detecting deep-water spawning sites in our study. Inter-annual variation in site-specific temperature, however, may have resulted in some putative spawning areas being unused during our surveys. In support, seabed temperature where D. viridis was found with adhered capelin eggs in Placentia Bay were on average cooler (4.3–4.9°C) than sites without capelin eggs (9.2 ± 3.9°C), indicating that unused sites were at or near the upper temperature threshold for spawning (≥ 12°C). Additionally, it is possible that in warmer years, such as those encountered in 2018 and 2019, capelin may move further offshore into unsurveyed areas where bottom temperature was more suitable for spawning. Monitoring changes in capelin spawning distribution in relation to inter-annual variation in temperature would be an excellent opportunity for further collaboration with fishers.
Inter-annual variation in capelin abundance may also explain the low proportion of detected active deep-water spawning areas. Indeed, density-dependent habitat use (e.g., Basin Model; MacCall, 1990) would result in fewer spawning sites occupied during years of lower capelin abundance, which was found in a recent capelin study (Crook et al., 2017). In support, four interviewees specifically noted the use of fewer sites in both beach and deep-water habitats when capelin abundance is lower. Interestingly, fisher interviews were conducted during two fishing seasons with different perceptions of the state of the capelin stock, with 2018 considered a higher capelin biomass year than both 2017 and 2019 based on spring acoustic surveys of offshore abundance (DFO, 2022b); however, the 2018 capelin biomass was only ~5.5% of pre-1991 levels (DFO, 2021). Ongoing communications with fishers in Bonavista Bay indicated that in 2018 capelin spawned at both beach and deep-water sites where they had not been observed spawning for the past 20 years, whereas in 2019 spawning capelin abundance and distribution was perceived to be more restricted and, thus, more in line with recent, post-collapse years. In support, a citizen science program (www.ecapelin.ca) indicated that the abundance of beach spawning sites was higher with a broader distribution during 2018 than 2019; however, another citizen science program (DFO spawning diaries) found similar use of beach sites in both years. This difference, however, may be due to the methodologies of the two citizen science programs. Both programs aim to capture the spatial and temporal extent of beach spawning, but the former relies on volunteer participation across the island of Newfoundland, whereas the latter is a long-term collaborative monitoring program with a core number of beach sites in NAFO Division 3KLP monitored each year by paid citizen scientists, so does not capture sporadic beach use outside the core area (Murphy, 2022). Overall, lower capelin abundance in 2019 combined with fewer beach spawning sites used suggests that fewer deep-water spawning sites may have been used, thereby resulting in fewer active spawning sites within some search areas.
Given the lack of fine-scale bathymetry and seabed habitat classification in coastal Newfoundland, fishers’ knowledge in this study resulted in the identification of many previously undocumented putative capelin deep-water spawning areas in an otherwise expansive area. Similar to other studies where fishers’ knowledge increased understanding of fish ecology (e.g., Fraser et al., 2006; Silvano et al., 2006; Johannes et al., 2008; Paterson et al., 2018), we found that fishers’ knowledge of capelin spawning behavior in coastal Newfoundland spanned a different temporal, spatial, and behavioral observational lens in comparison to traditional scientific studies. Indeed, interviewed fishers’ 8–65 years of observations in multiple bays was a vast resource of ecological information without which the baseline map of putative deep-water spawning sites herein would not have been possible. Our study illustrates that the long history of fishing capelin for food, bait, roe, and fertilizer in Newfoundland (DFO, 2022a) and the ongoing commercial fisheries for capelin during the spawning period have resulted in extensive LEK of the locations and timing of capelin spawning in coastal Newfoundland along with considerable knowledge of capelin life history (i.e., spawning, migration, survival). Although the Canadian Department of Fisheries and Oceans (DFO) has a longstanding history of collecting this knowledge along with data from citizen scientists on the timing of capelin beach spawning (i.e. spawning diary program; Murphy et al., 2021; DFO, 2022a; Murphy, 2022), we recommend further fisher collaborations to ensure the incorporation of this knowledge in future capelin stock assessments.
In conclusion, by combining fisher interviews and collaborative boat-based sampling, where fishers were directly involved in data collection and choosing non-random adaptive survey sites based on experiential and ecological knowledge of capelin spawning, we generated a baseline map of many putative capelin spawning areas to target for future investigation. Through the integration of fishers’ knowledge and scientific knowledge, we aimed to resolve conflicts between capelin fishers and science advisors and improve stock assessment and management, as shown in previous studies (e.g., Stanley and Rice, 2007; Carruthers and Neis, 2011; Duplisea, 2018). In particular, fishers have reported increasing use of deep-water spawning habitat (Nakashima and Clark, 1999) and have advocated for more scientific studies to incorporate productivity data from this habitat into the Newfoundland capelin stock assessment (Dawe and Carruthers, 2019). The Newfoundland capelin stock assessment already integrates data from a beach spawning diary program, which is used as a general indicator of stock health in a weight of evidence approach to provide scientific advice on stock status. For example, a high number of spawning sites used and earlier spawning are predicted to produce stronger year classes (DFO, 2021; Murphy et al., 2021). The baseline map of deep-water spawning sites generated in this study could similarly be used to build a time series for monitoring deep-water spawning habitat use and shifts between beach and deep-water spawning habitats, providing additional insight into the health of both 3Ps and 2J3KL capelin stocks. As monitoring deep-water spawning sites will be more difficult compared to beach spawning sites, and as monitoring these sites requires vessels, sampling equipment, and expert fisher knowledge, building this time series will require a network of fishers to participate annually. Although this program may be costly, fishers already fishing nearby putative sites during spawning presents an excellent opportunity for further science-industry research collaboration, building on our strong, multi-year collaborative relationships established during this research. To illustrate, through the collaborations and professional relationships established during this study, a fisher volunteered to monitor the deep-water sites near Cape St. Mary’s throughout the COVID-19 pandemic during other fishing activities. Overall, knowledge of capelin spawning habitat, especially persistently used deep-water spawning sites, are key to identifying highly productive coastal areas for capelin. As this is recognized as an important gap in our knowledge on capelin stock dynamics, identifying these key capelin spawning areas will have management implications for the timing and location of the capelin fishery (i.e., which bays are open and when), as well as applications for marine spatial planning and marine conservation efforts.
As climate projections forecast an increase in SST of 0.4°–2.2° C within the next 50 years on the Newfoundland Shelf (Han et al., 2015), if capelin remain in their current range, they are predicted to shift from warm, beach spawning habitat to cooler deep-water habitat to ensure offspring survival (Nakashima and Wheeler, 2002; Davoren, 2013b; Penton and Davoren, 2013; Crook et al., 2017). Although warming conditions might be expected to result in earlier capelin spawning (Buren et al., 2014), the lack of return to pre-collapse timing of spawning (June) relative to post-collapse timing (July) despite the return to pre-collapse oceanographic conditions (Murphy et al., 2021) suggests that capelin phenology might not shift considerably in response to future climate change. In combination with a warming climate, the abrupt and persistent delay in the timing of spawning when the stock collapsed in 1991 may further promote the use of deep-water spawning sites as capelin are now predominately spawning in mid-July when the beaches may be too warm (> 12°C; Crook et al., 2017; Murphy et al., 2021). Therefore, determining the relative contribution of beach and deep-water spawning to capelin recruitment is an important research question. Fishers support these research avenues, whereby 19/56 mentioned the need for increased monitoring and stewardship of beach and deep-water spawning sites and three of these 19 fishers mentioned this need unprompted. Specifically, two fishers requested research on the survivability of larvae at deep-water sites in Bonavista Bay and another requested increased sampling at deep-water sites in Conception Bay. The next step to understanding the contribution of deep-water spawning habitat to capelin recruitment is to continue to examine the spatial extent of deep-water spawning sites, possibly using different technologies (e.g. acoustic-based seabed classification), combined with quantitative estimates of site-specific larval production, as larval production is tightly linked to recruitment (i.e. age-two recruits; Murphy et al., 2018).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The University of Manitoba Research Ethics Board Protocol Number: HS21718 (J2018:021). The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Canadian Council of Animal Care (Protocol: F16-017).
Author contributions
LB was responsible for data generation, survey design, project design, data analysis, fieldwork, and manuscript preparation. Further, LB acquired additional funding from the University of Manitoba. ND aided in survey design and fieldwork such as interviews in northern bays of Newfoundland. HM provided edits and feedback on the project, and contributed to the development of the manuscript and provided expertise on government stock assessments and spawning diaries programs in Newfoundland. EC and GD contributed to project design, acquired the majority of the funding from Fisheries and Oceans Canada through the Coastal Environmental Baseline Program, and provided edits and feedback on the project as it developed and established the original idea for the project. All authors contributed to the article and approved the submitted version.
Funding
Funding was provided by Fisheries and Oceans Canada through their Coastal Restoration Fund Project to World Wildlife Fund Canada (to EHC), and through their Coastal Environmental Baseline Program (to GKD). Additional funding was provided by the University of Manitoba (Graduate Fellowship to LB) and Natural Sciences and Engineering Research Council of Canada (to GKD, 2019-05385). Additional in-kind resources, knowledge and data were provided by Fisheries and Oceans Canada (HMM).
Acknowledgments
We are indebted to the local captains in Newfoundland PL, TL, and AE for their assistance with fieldwork. Thanks to the following people: D. Ivany, J. Marsh, S. Morrison, and K. d’Entremont for assistance with fieldwork. We thank both special issue reviewers for their thoughtful suggestions that greatly improved this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor RS declared a shared affiliation with the author HM at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1061689/full#supplementary-material
Supplementary Table 1 | Interview questions.
References
Ames E. P. (2004). Atlantic Cod stock structure in the gulf of Maine Atlantic in the cod gulf stock structure of Maine from and surveys of retired these derivations are. Fisheries 29, 10–28. doi: 10.1577/1548-8446(2004)29[10:ACSSIT]2.0.CO;2
Bakun A. (2006). Wasp-waist populations and marine ecosystem dynamics: Navigating the ‘predator pit’ topographies. Prog. Oceanography 68, 271–288. doi: 10.1016/j.pocean.2006.02.004
Bliss L. M., Davoren G. K. (2021). Novel observations of capelin (Mallotus villosus) spawning directly on a brown algae species (Desmarestia viridis) in coastal Newfoundland, Canada. J. Northwest Atlantic Fishery Sci. 52, 29–37. doi: 10.2960/J.v52.m734
Buren A. D., Koen-Alonso M., Pepin P., Mowbray F., Nakashima B., Stenson G., et al. (2014). Bottom-up regulation of capelin, a keystone forage species. PLoS One 9, 1–12. doi: 10.1371/journal.pone.0087589
Buren A. D., Murphy H. M., Adamack A. T., Davoren G. K., Koen-Alonso M., Montevecchi W. A., et al. (2019). The collapse and continued low productivity of a keystone forage fish species. Mar. Ecol. Prog. Ser. 616, 155–170. doi: 10.3354/meps12924
Carruthers E. H., Neis B. (2011). Bycatch mitigation in context: Using qualitative interview data to improve assessment and mitigation in a data-rich fishery. Biol. Conserv. 144, 2289–2299. doi: 10.1016/j.biocon.2011.06.007
Carscadden J. E., Frank K. T., Miller D. S. (1989). Capelin (Mallotus villosus) spawning on the southeast shoal: Influence of physical factors past and present. Can. J. Fisheries Aquat. Sci. 46, 1743–1754. doi: 10.1139/f89-221
Carscadden J. E., Montevecchi W. A., Davoren G. K., Nakashima B., Carscadden S. (2002). Trophic relationships among capelin (Mallotus villosus) and seabirds in a changing ecosystem. ICES J. Mar. Sci. 59, 1027–1033. doi: 10.1006/jmsc.2002.1235
Carscadden J., Vilhjalmsson H. (2002). Capelin – what are they good for? introduction. ICES J. Mar. Sci. 59, 863–869. doi: 10.1006/jmsc.2002.1283
Coll M., Libralato S., Tudela S., Palomera I., Pranovi F. (2008). Ecosystem overfishing in the ocean. PLoS One 3, e3881. doi: 10.1371/journal.pone.0003881
Crook K. A., Maxner E., Davoren G. K. (2017). Temperature-based spawning habitat selection by capelin (Mallotus villosus) in Newfoundland. ICES J. Mar. Sci. 74, 1622–1629. doi: 10.1093/icesjms/fsx023
Cyr F., Galbraith P. S. (2021). A climate index for the Newfoundland and Labrador shelf. Earth System Sci. Data 13, 1807–1828. doi: 10.5194/essd-13-1807-2021
Davis A., Wagner J. R. (2003). Who knows? on the importance of identifying ‘experts’ when researching local ecological knowledge. Hum. Ecol. 31, 463–489. doi: 10.1023/A:1025075923297
Davoren G. K. (2013a). Distribution of marine predator hotspots explained by persistent areas of prey. Mar. Biol. 160, 3043–3058. doi: 10.1007/s00227-013-2294-5
Davoren G. K. (2013b). Divergent use of spawning habitat by male capelin (Mallotus villosus) in a warm and cold year. Behav. Ecol. 24, 152–161. doi: 10.1093/beheco/ars147
Davoren G. K., Anderson J. T., Montevecchi W. A. (2006). Shoal behaviour and maturity relations of spawning capelin (Mallotus villosus) off Newfoundland: demersal spawning and diel vertical movement patterns. Can. J. Fisheries Aquat. Sci. 63, 268–284. doi: 10.1139/f05-204
Davoren G. K., Halden N. M. (2014). Connectivity of capelin (Mallotus villosus) between regions and spawning habitats in Newfoundland inferred from otolith chemistry. Fisheries Res. 159, 95–104. doi: 10.1016/j.fishres.2014.05.010
Davoren G. K., May C., Penton P., Reinfort B., Buren A., Burke C., et al. (2008). An ecosystem-based research program for capelin (Mallotus villosus) in the northwest Atlantic: Overview and results. J. Northwest Atlantic Fishery Sci. 39, 35–48. doi: 10.2960/J.v39.m595
Davoren G. K., Montevecchi W. A. (2003). Signals from seabirds indicate changing biology of capelin stocks. Mar. Ecol. Prog. Ser. 258, 253–261. doi: 10.3354/meps258253
Davoren G. K., Woloschiniwsky C. S. A., Halden N. M., Wang F. (2015). Does otolith chemistry indicate the natal habitat of Newfoundland capelin Mallotus villosus? J. Exp. Mar. Biol. Ecol. 464, 88–95. doi: 10.1016/j.jembe.2014.10.025
Dawe N., Carruthers E. H. (2019). Collection of Fisher ecological knowledge on capelin spawning sites interview implementation. Fish Food Allied Workers, 26. (FFAW-Unifor). St. John’s, Newfoundland, Canada.
DeCelles G. R., Martins D., Zemeckis D. R., Cadrin S. X. (2017). Using fishermen’s ecological knowledge to map Atlantic cod spawning grounds on georges bank. ICES J. Mar. Sci. 74, 1587–1601. doi: 10.1093/icesjms/fsx031
DFO (Department of Fisheries and Oceans) (1997). Capelin in SA2 + div. 3KL. Can. Sci. Advisory Secretariat Sci. Advisory Rep. Research Document 97/29. 188.
DFO (Fisheries and Oceans Canada) (2015). Assessment of capelin in subarea 2 and divisions 3KL in 2015. DFO Can. Sci. Advisory Secretariat Sci. Advisory Rep. 2015/036. St. John's, NL, Canada. 21.
DFO (Fisheries and Oceans Canada) (2021). Assessment of 2J3KL capelin in 2020 (DFO Canadian Science Advisory Secretariat Science Advisory Report 2021/045).
DFO (Fisheries and Oceans Canada) (2022a) Integrated fisheries management plan: Capelin (Mallotus villosus) Newfoundland & Labrador region divisions 2+3 (Capelin fishing areas 1-11). Available at: https://www.dfo-mpo.gc.ca/fisheries-peches/ifmp-gmp/capelin-capelan/2019/zone-area_1-11-eng.html
DFO (Department of Fisheries and Oceans) (2022b). Assessment of 2J3KL capelin in 2020 (St. John's, NL, Canada: Canadian Science Advisory Secretariat Science Advisory Report 2022/013).
Duplisea D. E. (2018). Eliminating implausible fisheries assessment models using fishers’ knowledge. Can. J. Fisheries Aquat. Sci. 75, 1280–1290. doi: 10.1139/cjfas-2017-0178
Frank K. T., Leggett W. C. (1981). Prediction of egg development and mortality rates in capelin (Mallotus villosus) from meteorological, hydrographic, and biological factors. Can. J. Fisheries Aquat. Sci. 38, 1327–1338. doi: 10.1139/f81-179
Fraser D. J., Coon T., Prince M. R., Dion R., Bernatchez L. (2006). Integrating traditional and evolutionary knowledge in biodiversity conservation: A population level case study. Ecol. Soc. 11, art4. doi: 10.5751/ES-01754-110204
Friðgeirsson E. (1976). Observations on spawning behaviour and embryonic development of the icelandic capelin. Rit Fiskideildar 5, 1–35.
Gunderson K., Watson A. (2007). Understanding place meanings on the bitterroot national forest, Montana. Soc. Natural Resour. 20, 705–721. doi: 10.1080/08941920701420154
Han G., Colbourne E., Pepin P., Xie Y. (2015). Statistical projections of ocean climate indices off Newfoundland and Labrador. Atmosphere - Ocean 53, 556–570. doi: 10.1080/07055900.2015.1047732
Hutchings J. A., Ferguson M. (2000). Temporal changes in harvesting dynamics of Canadian inshore fisheries for northern Atlantic cod, Gadus morhua. Can. J. Fisheries Aquat. Sci. 57, 805–814. doi: 10.1139/f00-021
Johannes R. E., Freeman M. M. R., Hamilton R. J. (2008). Ignore fishers’ knowledge and miss the boat. Fish Fisheries 1, 257–271. doi: 10.1111/j.1467-2979.2000.00019.x
Johnson K. F., Davoren G. K. (2021). Distributional patterns of humpback whales (Megaptera novaeangliae) along the Newfoundland East coast reflect their main prey, capelin (Mallotus villosus). Mar. Mammal Sci. 37, 80–97. doi: 10.1111/mms.12730
Kenchington E. L., Nakashima B. S., Taggart C. T., Hamilton L. C. (2015). Genetic structure of capelin (Mallotus villosus) in the northwest Atlantic ocean. PLoS One 10, e0122315. doi: 10.1371/journal.pone.0122315
Lavigne D. M. (1996). “Ecological interactions between marine mammals, commercial fisheries and their prey: Unraveling the tangled web,” in Studies of high-latitude seabirds. 4, trophic relationships and energetics of endotherms in cold ocean systems. Ed. Montevecchi. W. A. (Ottawa, Canada: Canadian Wildlife Service Occasional Paper), 59–71.
Lewis K., Buren A., Regular P., Mowbray F., Murphy H. (2019). Forecasting capelin mallotus villosus biomass on the Newfoundland shelf. Mar. Ecol. Prog. Ser. 616, 171–183. doi: 10.3354/meps12930
MacCall A. D. (1990). Dynamic geography of marine fish populations (Seattle, Washington, USA: University of Washington Press).
Martinez-Levasseur L. M., Furgal C. M., Hammill M. O., Burness G. (2017). Challenges and strategies when mapping local ecological knowledge in the Canadian Arctic: the importance of defining the geographic limits of participants’ common areas of observations. Polar Biol. 40, 1501–1513. doi: 10.1007/s00300-016-2071-2
Murphy H. M. (2022). Capelin beach spawning diaries: an analysis of 30 years of citizen science data from the island of Newfoundland, Canada. Cybium Int. J. Ichthyology 46 (4), 357–370. doi: 10.26028/cybium/2022-464-004
Murphy H. M., Adamack A. T., Cyr F. (2021). Identifying possible drivers of the abrupt and persistent delay in capelin spawning timing following the 1991 stock collapse in Newfoundland, Canada. ICES J. Mar. Sci. 78 (8), 2709–2723. doi: 10.1093/icesjms/fsab144
Murphy H. M., Pepin P., Robert D. (2018). Re-visiting the drivers of capelin recruitment in Newfoundland since 1991. Fisheries Res. 200, 1–10. doi: 10.1016/j.fishres.2017.12.005
Nakashima B. S., Clark M. C. (1999). “Results of a telephone opinion survey of fixed gear capelin licence holders for 1998,” In Anon: Capelin in SA2 and Div. 3K. (St. John's, NL, Canada: Canadian Science Advisory Secretariat Res. Doc. 99/206) pp. 1–37.
Nakashima B. S., Wheeler J. P. (2002). Capelin (Mallotus villosus) spawning behaviour in Newfoundland waters - the interaction between beach and demersal spawning. ICES J. Mar. Sci. 59, 909–916. doi: 10.1006/jmsc.2002.1261
Neis B., Morris M. (2002). “Fishers’ ecological knowledge and fisheries science: The capelin Fishery 1975-1996,” in The resilient outport: Ecology, economy and society in rural Newfoundland. Ed. Ommer R. (St. John’s, Newfoundland, Canada: ISER Books), 205–240.
Neis B., Schneider D. C., Felt L., Haedrich R. L., Fischer J., Hutchings J. A. (1999). Fisheries assessment: what can be learned from interviewing resource users? Can. J. Fisheries Aquat. Sci. 56, 1949–1963. doi: 10.1139/f99-115
Paterson B., Neis B., Stephenson R. L. (2018). A social–ecological study of stock structure and fleet dynamics in the Newfoundland herring fishery. ICES J. Mar. Sci. 75, 257–269. doi: 10.1093/icesjms/fsx097
Penton P. M., Davoren G. K. (2008). Patterns of larval emergence of capelin (Mallotus villosus) and environmental cues at demersal spawning sites on the northeastern coast of Newfoundland. Can. J. Fisheries Aquat. Sci. 65, 1135–1143. doi: 10.1139/F08-037
Penton P. M., Davoren G. K. (2012). Physical characteristics of persistent deep-water spawning sites of capelin: Importance for delimiting critical marine habitats. Mar. Biol. Res. 8, 778–783. doi: 10.1080/17451000.2012.678858
Penton P. M., Davoren G. K. (2013). A common garden experiment on capelin (Mallotus villosus) early life history stages to examine use of beach and deep-water spawning habitats. J. Exp. Mar. Biol. Ecol. 439, 54–60. doi: 10.1016/j.jembe.2012.10.009
Penton P. M., Davoren G. K., Montevecchi W. A., Andrews D. W. D. (2012). Beach and demersal spawning in capelin (Mallotus villosus) on the northeast Newfoundland coast: Egg developmental rates and mortality. Can. J. Zoology 90, 248–256. doi: 10.1139/z11-132
Penton P. M., McFarlane C. T., Spice E. K., Docker, M. F., Davoren G. K. (2014). Lack of genetic divergence in capelin (Mallotus villosus) spawning at beach versus subtidal habitats in coastal embayments of newfoundland. Canadian. J. Zoology 92, 377–382. doi: 10.1139/cjz-2013-0261
Pikitch E. K., Rountos K. J., Essington T. E., Santora C., Pauly D., Watson R., et al. (2012). The global contribution of forage fish to marine fisheries and ecosystems. Fish Fisheries 15, 43–64. doi: 10.1111/faf.12004
Rose G. A., O’Driscoll R. L. (2002). Capelin are good for cod: Can the northern stock rebuild without them? ICES J. Mar. Sci. 59, 1018–1026. doi: 10.1006/jmsc.2002.1252
Silvano R. A. M., MacCord P. F. L., Lima R. V., Begossi A. (2006). When does this fish spawn? fishermen’s local knowledge of migration and reproduction of Brazilian coastal fishes. Environ. Biol. Fishes 76, 371–386. doi: 10.1007/s10641-006-9043-2
Sjare B., Nakashima B., Mercer D., Mercer D., Mercer D. (2003). “Integrating scientific and local ecological knowledge to identify potential critical habitats: A case study in placentia bay, Newfoundland,” in Canadian Science advisory secretariat: Department of fisheries and ocean (Canada: St. John’s, Newfoundland).
Stanley R. D., Rice J. (2007). “Fisher Knowledge? why not add their scientific skills while you’re at it? in: Fishers’ knowledge in fisheries science and management,” in Fishers’ knowledge in fisheries science and management. Eds. Haggan N., Neis B., Baird. I. G. (Paris, France: UNESCO Publishing) 335–353.
Templeman W. (1948). The life history of the caplin (Mallotus villosus o. f. müller) in Newfoundland waters Vol. 17 (St. John's, NL, Canada: Newfoundland Government Laboratory), 1–151.
Tripp A., Murphy H. M., Davoren G. K. (2020). Otolith chemistry reveals natal region of larval capelin in coastal Newfoundland, Canada. Front. Mar. Sci. 7, 1–11. doi: 10.3389/fmars.2020.00258
Keywords: fishers’ knowledge research, collaborative research, Newfoundland (Canada), Capelin (Mallotus villosus), knowledge co-creation, fishers’ knowledge
Citation: Bliss LM, Dawe N, Carruthers EH, Murphy HM and Davoren GK (2023) Using fishers’ knowledge to determine the spatial extent of deep-water spawning of capelin (Mallotus villosus) in Newfoundland, Canada. Front. Mar. Sci. 9:1061689. doi: 10.3389/fmars.2022.1061689
Received: 04 October 2022; Accepted: 20 December 2022;
Published: 19 January 2023.
Edited by:
Robert Stephenson, Fisheries and Oceans Canada (DFO), CanadaReviewed by:
Graham Sherwood, Gulf of Maine Research Institute, PortlandBrian R. MacKenzie, Technical University of Denmark, Denmark
Copyright © 2023 Bliss, Dawe, Carruthers, Murphy and Davoren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura M. Bliss, Ymxpc3NsQG15dW1hbml0b2JhLmNh
 Laura M. Bliss
Laura M. Bliss Natalya Dawe2
Natalya Dawe2 Erin H. Carruthers
Erin H. Carruthers Hannah M. Murphy
Hannah M. Murphy Gail K. Davoren
Gail K. Davoren