
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 01 December 2022
Sec. Marine Pollution
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1050957
This article is part of the Research TopicImpact of Marine Debris on Marine Ecosystems and OrganismsView all 6 articles
 Daling Wang1,2,3,4,5†
Daling Wang1,2,3,4,5† Yu Zhen2,4,5†
Yu Zhen2,4,5† Lili Wei1,2,3,4,5
Lili Wei1,2,3,4,5 Yufei Dai1,3
Yufei Dai1,3 Xianyan Wang1,3
Xianyan Wang1,3 Shenhan Tong6
Shenhan Tong6 Liyuan Zhao1,3*
Liyuan Zhao1,3*Microplastics (MPs) pose serious threats to various marine organisms, including many threatened apex predators such as cetaceans. However, information on microplastic contamination in cetaceans from Asian waters is limited. Based on the analysis of Fourier transform infrared spectroscopy (FTIR), we reported MPs from finless porpoise intestinal samples and from their habitats along the Fujian coast of the East China Sea. MPs proved to be ubiquitous in both intestinal and habitat water samples. Most intestinal MPs were fibers (86.90%), transparent (51.19%), small sizes (<1.0 mm, 77.38%), and composed of polyamide (41.67%) or polyethylene terephthalate (45.24%). Seawater MPs were predominantly fibers (90.25%), transparent (82.45%), < 1.0 mm (83.76%) and composed of polypropylene (67.32%). Concentrations of MPs in coastal waters were greater than those in offshore waters. The concentration and composition of fibrous MPs indicate a likely textile industry origin. A recommendation is made to further assess the risks of MPs consumption in threatened species and develop scientific protection and management strategies.
Plastics are cheap and durable, and widely used in industry, agriculture, and daily life (Browne et al., 2011). They are also mass produced, with production nearing ~370 × 106 t in 2019 (Plastics Europe, 2019). Sadly, > 150 × 106 t of plastic waste may already exist in the ocean (Kosior and Mitchell, 2020). Plastic debris fragments into small particles through physical, chemical, and biological processes (Thompson et al., 2004; Cozar et al., 2014). Consequently, concerns regarding microplastics (MPs), plastic particles ≤ 5 mm dimension (Arthur et al., 2009), have attracted increased public attention. MPs are distributed globally, especially in marine environments, where they occur in coastal (Zhu et al., 2020) to pelagic waters, the surface sea (Lorenz et al., 2019) to the deep-sea (Woodall et al., 2014; Monteiro et al., 2018), marine sediments (Zhao et al., 2018; Zhang et al., 2020), and even in the polar regions (Lusher et al., 2015a; Mishra et al., 2021). Coastal areas of China are known to have levels of microplastic pollution (Zhao et al., 2014; Cheung et al., 2016; Fok et al., 2017; Zhang et al., 2020; Wang et al., 2021a).
MPs have been reported from a variety of aquatic animals, such as bivalves (Van Cauwenberghe and Janssen, 2014; Sendra et al., 2021), fish (Vendel et al., 2017; Ma et al., 2020), turtles (Caron et al., 2018; Duncan et al., 2019) and cetaceans (Battaglia et al., 2020; Novillo et al., 2020; Zantis et al., 2021). Their sources have been attributed to direct ingestion or trophic transfer (Nelms et al., 2018; Waring et al., 2018). For low trophic level aquatic animals, ingestion of MPs can reduce energy reserves, feeding capacity, and reproductive outputs, and damage physiological and immune systems (Nabi et al., 2022). For the aquatic mammals in the apex position of the trophic web, although MPs do not cause physical obstacles through entanglement, they can act as carriers of pollutants and toxins, which, combined with biomagnification and bioaccumulation, negatively affect health (Holmes et al., 2012; Avio et al., 2015). Bioaccumulated chemical pollutants transferred by MPs (e.g., heavy metals, pesticides, biocides) can also cause endocrinal gland cancer in aquatic mammals, and MPs-linked pathogens also increase the risk of infectious diseases and mortality (Lauretta et al., 2019; Nabi et al., 2022). Otherwise, micro or nano-plastics are also likely to cross the cell membranes and enter the circulatory system, and then accumulate in organs (such as brain, liver, kidney, placenta) with other pollutants (Prietl et al., 2014; Banerjee and Shelver, 2021; Nabi et al., 2022). Therefore, exposure to MPs and associated toxicants and pathogens in the aquatic environment pose both a serious actual and potential threat to aquatic mammals.
Because cetaceans are apex predators, they are appropriate sentinel species, and study of them can provide insights into or early warning signs of existing or emerging health hazards in the marine environment (Schwacke et al., 2013; Fossi et al., 2018). The European Union Marine Strategy Framework Directive even recommends that cetaceans be used as indicator species for marine plastic debris and other organic pollutants (Galgani et al., 2014; Gui et al., 2016; Jepson et al., 2016). Finless porpoises (genus Neophocaena) include two species according to their morphological characteristics (dorsal ridge, dorsal grooves, and tubercled patch), wide-ridged or Indo-Pacific finless porpoise (Neophocaena phocaenoides) and the narrow-ridged finless porpoise (N. asiaeorientalis), with two subspecies (Yangtze finless porpoise, N. a. asiaeorientalis, and East Asian finless porpoise, N. a. sunameri) (Jefferson and Wang, 2011). They have been listed as vulnerable or endangered species by the International Union for Conservation of Nature (IUCN) (Wang and Reeves, 2017a; Wang and Reeves, 2017b). Although these species and subspecies are protected in China, because they are small and coastal, they are particularly susceptible to anthropogenic disturbance. While MPs have been reported from the intestinal tracts of Yellow and Bohai sea finless porpoises (possibly ingested unintentionally) (Xiong et al., 2018), no comprehensive survey has simultaneously examined microplastic pollution in these porpoises and their habitats.
The hydrography of the Fujian coast in the southern East China Sea and western Taiwan Strait, is influenced by the Kuroshio Current Water, and Zhejiang-Fujian coast current (Wu, 1982; Wu, 1991), and many upwelling regions in the Taiwan Strait (Fu, 1984; Li and Lu, 2008). These hydrological dynamics bring water with high nutrient contents to the surface and create five major fishing grounds. Five major fishing grounds are rich in fishery resources that cover an area of 213,237 km2 occur here (Dai, 2005). And the frequent fishing activities and the increase in the use of plastic fishing gear have introduced MPs into the marine environment (Nelms et al., 2021). The Taiwan Strait is an important habitat for finless porpoises, and the only known zone where both the N. asiaeorientalis and N. phocaenoides co-exist (Jefferson and Wang, 2011). We investigate microplastic pollution in finless porpoises and their habitats along the Fujian coast of the East China Sea. Two objectives are to 1) identify the concentrations and characteristics of MPs (including size, color, shape, and polymer type) in finless porpoises and their habitats; and 2) discuss the potential sources and occurrence of MPs. This investigation provides baseline data on MPs in both surface seawater and cetaceans in China.
Five stranded finless porpoises were collected from April 2019 to January 2020, and the stranded locations were shown in Figure 1. Samples management and dissection were performed in compliance with the introductory guide for the anatomy of marine mammalian necropsy (Pugliares et al., 2007). Among the five stranded finless porpoises, there were one N. phocaenoides and four N. a. sunameri. The states of finless porpoises ranged from fresh carcass (code 2) to moderate decomposition (code 3), as described by Geraci and Lounsbury (2005). After the necropsy, the entire intestines were taken for the MPs´ investigation. To prevent air pollution, the intestines were wrapped with tinfoil, which were stored at -20°C before further analysis. The stranding site, time, and biological information of the finless porpoises were recorded (Table 1). The study was approved by the local fishery administration for autopsy and sampling, and these procedures were systematically conducted following all ethical codes and legal requirements in China.
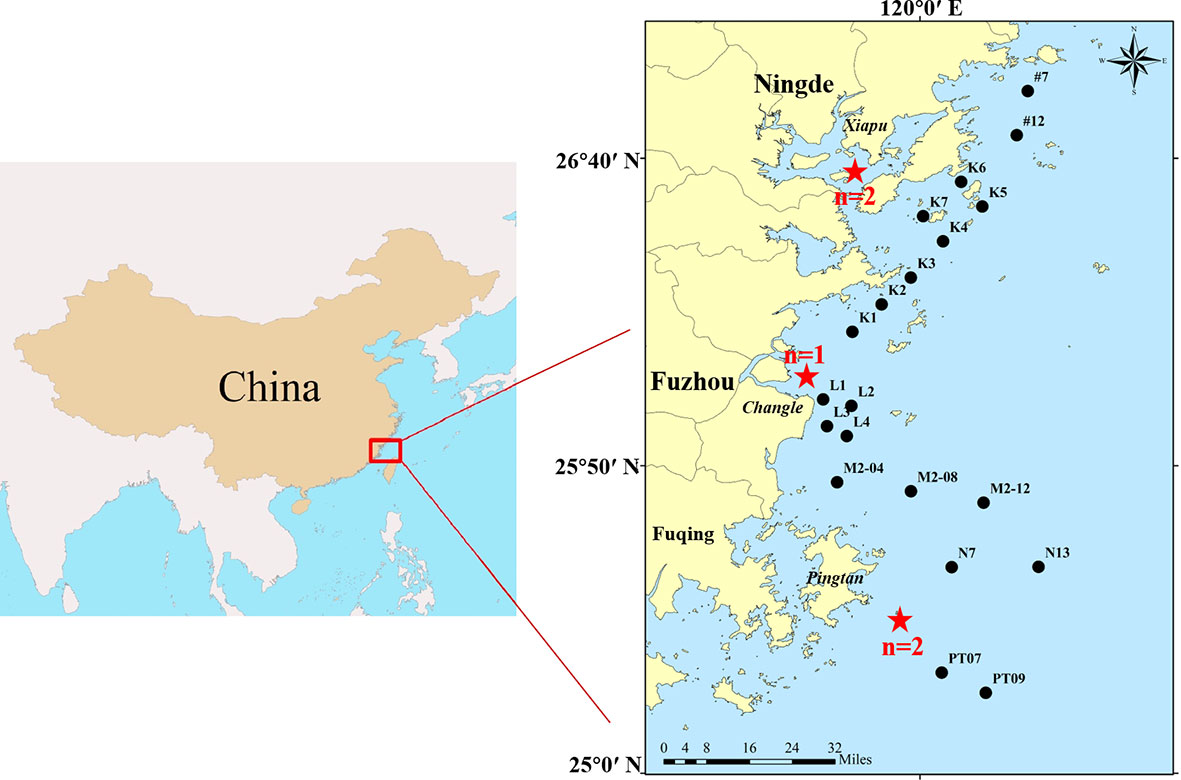
Figure 1 Locations of the stranded finless porpoises and surface seawater sampling sites. The finless porpoises were marked with red stars, the collection number was represented by ′n′, and the seawater sampling sites were labeled with black circles.
The surface seawater was collected for MPs analysis from June 22 to August 29 2020 by trawl in the fishing boat “Minping Yu 6333” (Figure 1). The length and width of the trawl opening were 60 cm and 30 cm, respectively. To avoid those small size MPs lost caused by large pore size nets (Norén, 2007), the horizontal tow behind the boat was performed using the manta net with 70 μm. The mechanical and electronic digital flowmeter (Model 2030R, General Oceanics, Florida, U.S.A) was mounted in the middle of the manta net mouth and kept submerged underwater. The trawling time and speed at each sampling site were about 15 min and 2-2.5 knots, respectively. A station with a distance of fewer than 15 km from the coastline is defined as a nearshore station, otherwise, it is called an offshore station. And the sampling volume of seawater was calculated according to the formula provided by the flowmeter (Pan et al., 2021). Finally, the net was washed repeatedly with the seawater from the outside, and the samples were collected into the glass bottle and stored at 4 °C before laboratory processing. The basic physical parameters (temperature, salinity, and depth) were measured by the multiparameter digital water quality meter (YSI ProDSS, America).
The MPs in the intestines were isolated according to the previous protocol (Lusher et al., 2015b) with some modifications. Briefly, the entire intestine was rinsed with filtered Milli-Q water to prevent contamination from the external environment, and then was placed and stretched neatly on the operating table. The entire intestine was divided into 1-m units to facilitate sample processing and collection. Each segment of the intestine was placed in a set of nested stainless-steel sieves (5 mm, 4 mm, 1 mm, 0.5 mm, 0.1 mm), and cut open by steel scissors. The folded structure between the intestinal walls was carefully rinsed with flowing Milli-Q water to prevent microplastic residue, and the contents of each 3-m intestines were collected and rinsed into the clean glass beakers. Subsequently, the glass beakers were covered with aluminum foil and baked at 90°C for 24h to evaporate the excess water in an oven. The water in the beaker should be careful not to completely dry to avoid MPs breaking. 200-mL filtered 10% KOH solution was added to digest organic matter for 12 h at 60°C and 150 rpm in an oscillation incubator until the biological material was completely digested.
The organic matter in seawater was digested according to the wet peroxide oxidation (WPO) provided by NOAA (Masura et al., 2015) with some modifications. Briefly, the surface seawater was filtered through a set of mesh sieves (5 mm, 0.054 mm), and the retentate on the 5-mm mesh screen were discarded. The retentate on the sieve (0.054 mm) was rinsed into a 1-L clean and dry beaker with filtered Milli-Q water. The beaker covered with aluminum foil to minimize contamination was placed in a drying oven at 90°C for 24 h. Then, 30-50 mL of 30% hydrogen peroxide and an equal volume of 0.05 M FeSO4 solution were added to the beaker, which was heated at 60°C for 12 hours in a water bath. The digestion should be repeated until the organic matter was completely digested. The digestion solution was then passed through a set of sieves (5 mm, 4 mm, 1 mm, 0.5 mm, 0.1 mm, and 0.054 mm), and the retentate on each layer of the sieve was washed into the funnel separately for flotation.
The digestion solution was transferred to the flotation device. The NaI solution (1.8 g/cm3) was added with an equal volume for density separation (Saliu et al., 2018), and the funnel was covered with aluminum foil to prevent MPs pollution from the air. The latex tube was squeezed several times in succession to make the solution evenly mixed and then standing still. After 12 h flotation, the supernatant of the digestion solution was decanted and filtered through a GF/A Whatman filter (diameter =47 mm, pore size =1.6 μm) by using a vacuum filter pump (Jin Teng GM-0.33A), and the filter was collected by using stainless steel tweezers to ensure that all samples were transferred to a glass petri dish, over which lids were placed, for microplastic examination.
Finally, all the suspected MPs on the filters were observed by the Leica stereomicroscope (M205C), and the photographs of MPs with different scales were taken by a Leica DFC425 charge-coupled device (CCD) camera. The abundance, shape, size, and color of suspect MPs were recorded, and the categories of MPs were classified according to a standardized protocol (Frias et al., 2018). The suspected MPs were picked out to make further chemical composition verification with the Fourier transform infrared microscope (Micro-FTIR, Nicolet iN10, Thermo Fisher, USA) (Su et al., 2016). The parameters were set to 16 scans with a resolution of 4 cm-1, the spectral wavenumber range was from 4000-400 cm-1, and the collection time was 3 seconds (Li et al., 2020). By comparing the standard spectra of polymers with the Bruker FTIR library, the composition of the microplastic polymer was accepted if the spectra matching degree was ≥ 70%.
To reduce air pollution during the experiment, the doors and windows were closed and the operating table was covered with aluminum foil. All glassware and scissors were rinsed with the Milli-Q water more than 3 times. The liquid solution must be filtered through GF/A Whatman filters before it was used. Besides, the operator should wear a cotton lab coat and dust-free nitrile gloves during the entire experiment. The procedure blanks were performed simultaneously to monitor airborne contamination. The subsequent digestion, separation steps, and microscopic examination were the same as the sample processing steps. Only 0.33 ± 0.19 items/filter (mean ± SD) were detected in procedure blanks, and final data were corrected by subtracting the blank contamination value.
Statistical analysis was performed by IBM SPSS statistics software (version 24.0). The Mann-Whitney-U test was used to compare the difference in MPs abundance between the nearshore and offshore stations. P < 0.05 was considered to indicate statistical significance. Linear relationships between MPs abundance and the physical parameters of seawater (temperature, salinity) were analyzed by spearman correlation.
Of 398 suspected microplastic particles sorted visually from intestinal samples of 3 male and 2 female porpoises examined by Micro-FTIR individually, 21.11% were confirmed to be MPs. The main basic information of five finless porpoises (body length 1.13-1.54 m, mean=1.34 ± 0.15m) was recorded in Table 1. And the abundance ranged from 10-23 items/individual, with an average abundance of 16.80 ± 2.24 items/individual (1.63 ± 0.14 items/m intestines) (Figure 2).
Various shapes and colors of MPs with different compositions were found in five finless porpoises (Figure 3). Some MPs were transparent, while others were yellow, red, blue, or black (Figure 3A). Transparent fibers accounted for the largest proportion of all finless porpoises’ intestines. Most (86.90%) MPs were fibers, but fragments and films also occurred (Figure 3B).
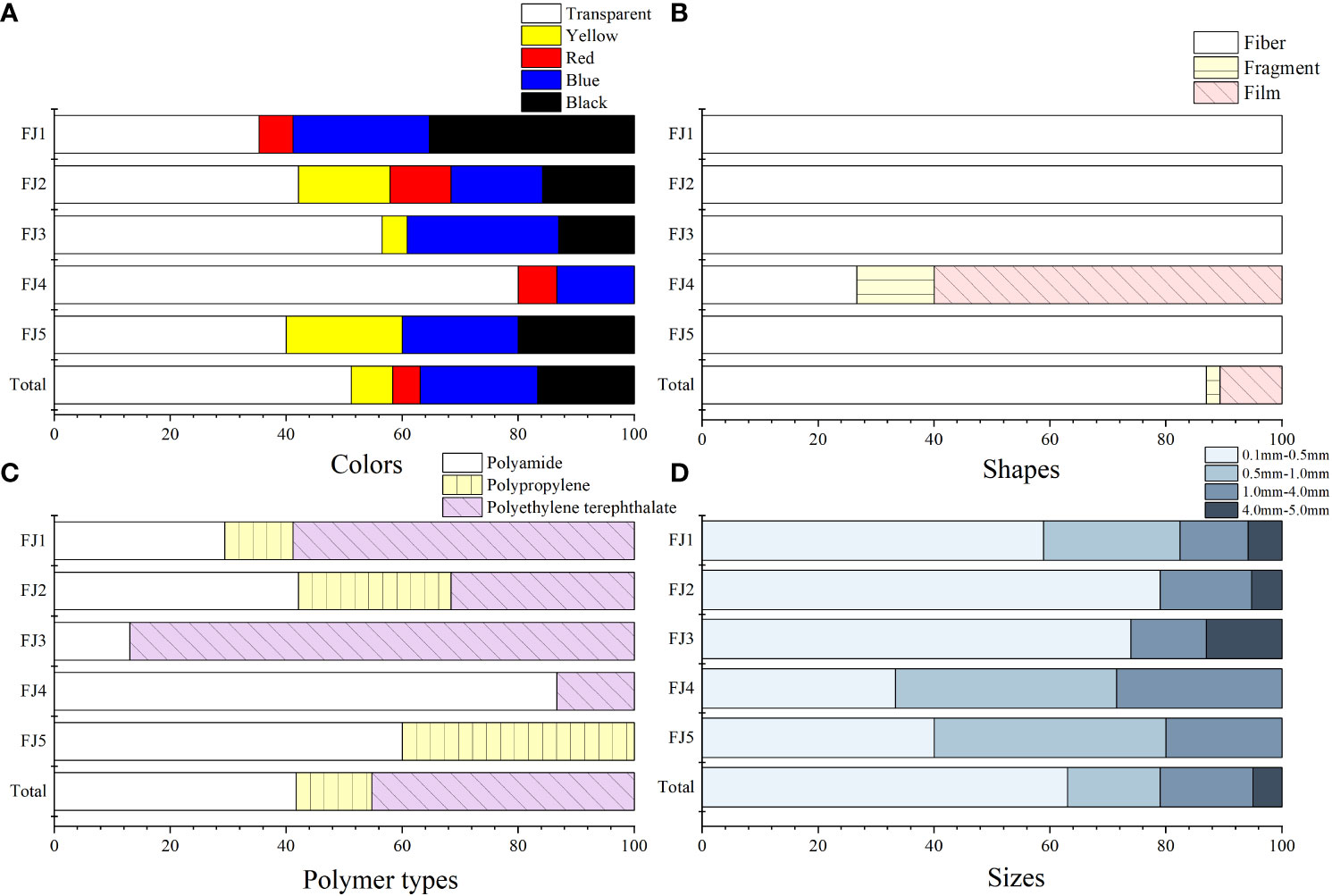
Figure 3 Various MPs were found in finless porpoises. (A) The colors composition; (B) The shapes composition; (C) The polymer types; (D) The sizes composition and distribution.
A Bruker spectral search identified microplastic composition to comprise polyethylene terephthalate (PET), polyamide (PA), and polypropylene (PP), of which PET polymers dominated (45.24%) and PP were the least frequent (13.10%) (Figure 3C). However, PET was not found in specimen FJ5, and PP was not found in the specimens FJ3 and FJ4.
Macro-plastics with a diameter > 5.0 mm were not found in finless porpoises. MPs size ranges were 0.1 mm-0.5 mm, 0.5 mm-1.0 mm, 1.0 mm-4.0 mm and 4.0 mm-5.0 mm. While the range in microplastic size varied among individuals, those of 0.1 mm-0.5 mm were prevalent, comprising 40.00%-78.95% of all samples. Intestines of three (FJ1, FJ2, FJ3) of five contained the large size MPs in the range of 4.0 mm-5.0 mm (Figure 3D).
The sampling information of seawater at each station was recorded (Supplementary Table 1). Surface seawater samples contained 14,856 potential microplastic particles. Of 3255 particles analyzed by Micro-FTIR, 2303 (70.66%) were identified as MPs. It was shown that MPs existed spatial differences and the concentration of MPs in seawater varied by ranging from 1.79-18.48 items/m3, and the average was 10.08 ± 1.19 items/m3 (Figure 4). The mean abundance of MPs in coastal surface seawater (12.30 ± 1.10 items/m3) exceeded that of offshore stations (5.96 ± 2.02 items/m3), Mann-Whitney-U test, P < 0.05 (Figure 5). There were no correlations between water temperature or salinity and the concentration of MPs (P=0.348, P=0.216, respectively).
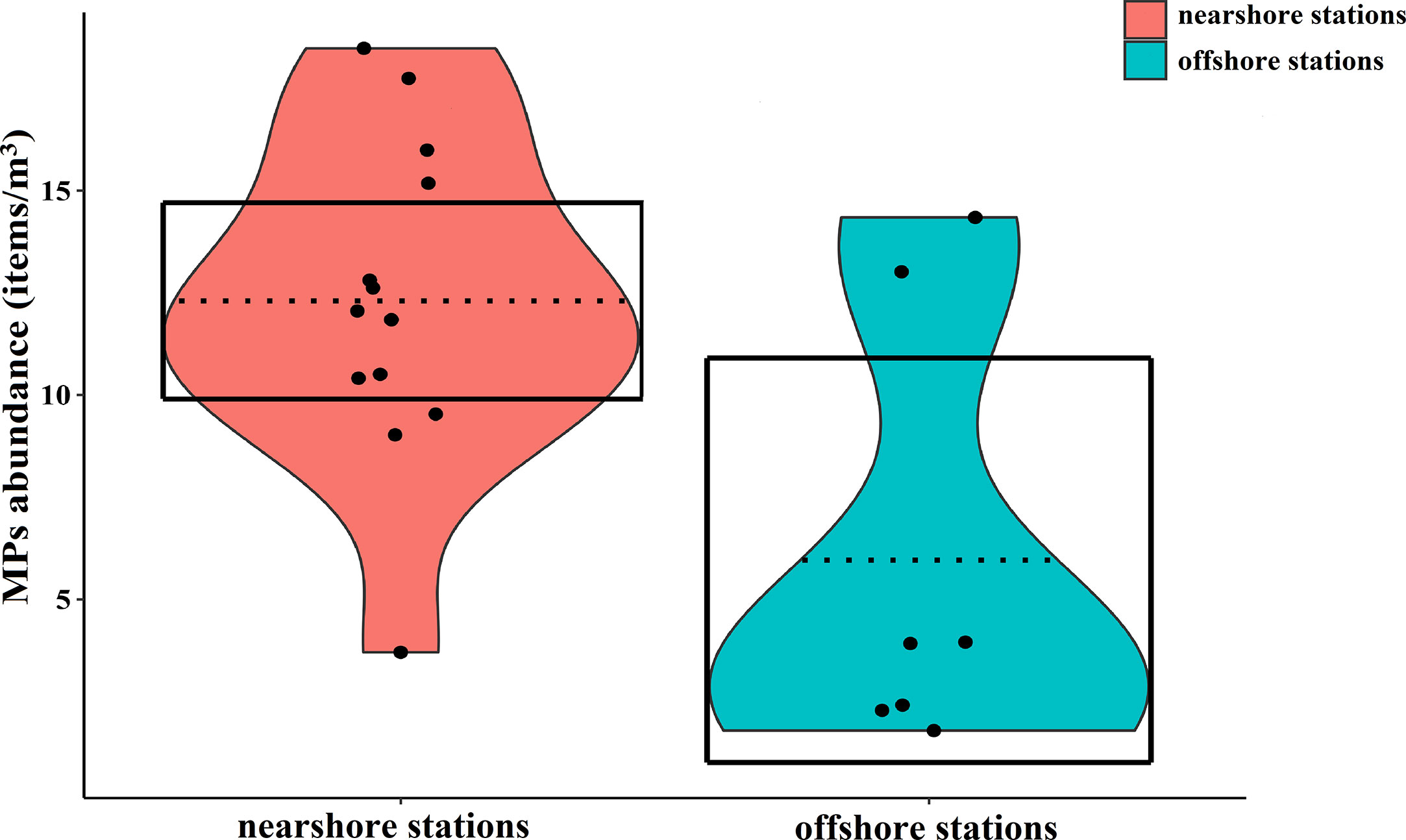
Figure 5 Comparisons of MPs abundance in the surface waters of the nearshore and offshore stations. The points represent the abundance of MPs, and the dashed lines represent the average abundance. The shapes filled with red and blue colors were on behalf of the smoothed densities, and the boxes represent the 95% inference intervals.
MPs collected in seawater were transparent, or colored yellow, red, blue, black, white, or green (Figure 6, Figure 7A). In general, transparent was the most abundant (82.45%), followed by black (7.94%); yellow (0.86%) was the least abundant. Most seawater MPs were fibers (90.25% of samples), but fragments, lines, foam, films, and pellets also occurred (Figure 7B).
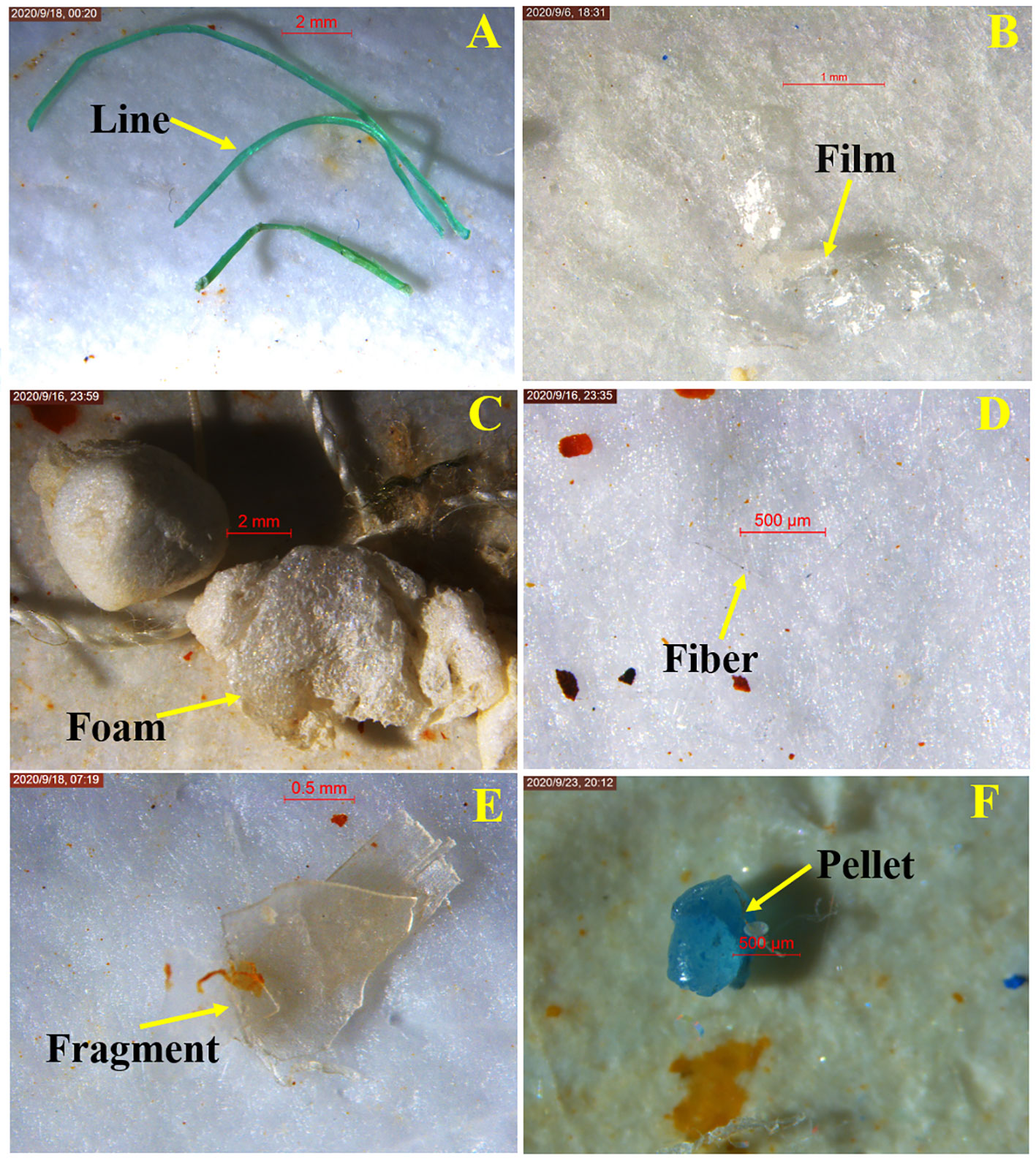
Figure 6 Microscopic images of representative MPs of different shapes collected from the surface seawater. (A) Line; (B) Film; (C) Foam; (D) Fiber; (E) Fragment; (F) Pellet.
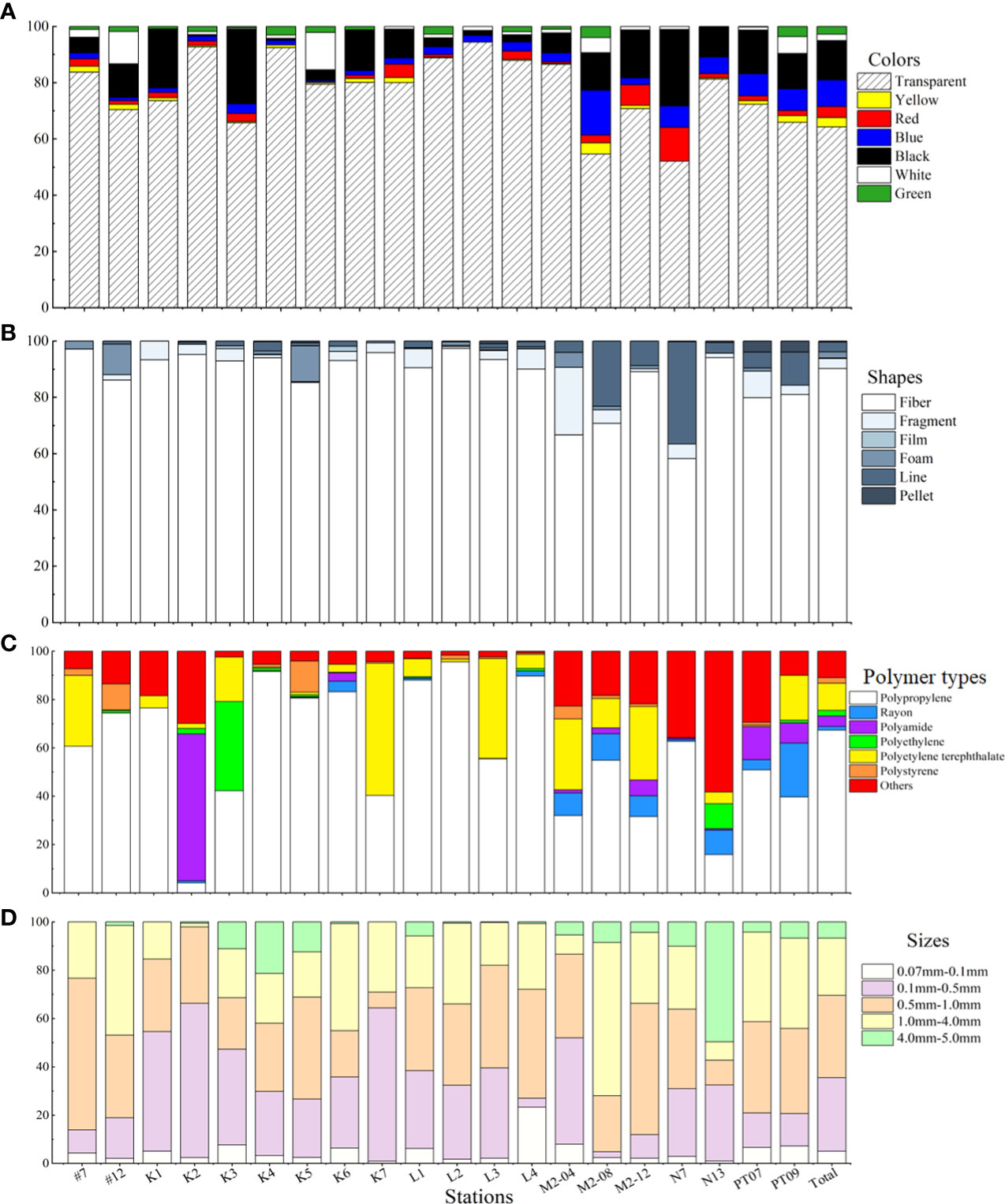
Figure 7 (A) Colors, (B) shapes, (C) polymer types and (D) sizes of MPs in surface seawater of 20 sampling sites in the Fujian coast of the East China Sea.
Micro-Fourier transforms infrared spectroscopy analysis of seawater microplastic samples revealed that rayon, polyethylene (PE), polyethylene terephthalate (PET), polypropylene (PP), polystyrene (PS), and polyamide (PA) were the common polymers (Figure 7C; Supplementary Figure 1). Other different materials of MPs also occurred, including polyester fiber and polyester resin, which were classified as ′others′. Of polymer materials, PP was generally the most abundant (67.32% of samples), followed by PET and others (polyester); rayon was the least abundant.
MPs from seawater were categorized by size ranges: 0.07mm-0.1 mm, 0.1 mm-0.5 mm, 0.5 mm-1.0 mm, 1.0 mm-4.0 mm, and 4.0 mm-5.0 mm. Multiple microplastic size-class occurred at each sampling station (Figure 7D). On the whole, MPs with a diameter < 1.0 mm had the largest proportion, accounting for 83.76%. Compared to other sizes, MPs within the range of 4.0-5.0 mm were less frequent (5.41%) and did not occur at all sampling stations.
Finless porpoises (genus Neophocaena) are vulnerable to both human activities and to aquatic pollution because they occur in shallow coastal waters, estuaries, and within the Yangtze River. Because cetaceans share coastal environments with humans and consume similar foods, they can function as indicators of public health issues, and the health of the marine ecosystem (Bossart, 2011). We reported microplastic contamination in finless porpoises along the Fujian coast of the East China Sea, and provide baseline data on microplastic pollution.
Recently, MPs have been frequently reported from the intestines or stomachs of cetaceans, including toothed whales such as true beaked whales (Mesoplodon mirus), beluga whales (Delphinapterus leucas), pygmy sperm whales (Kogia breviceps), striped dolphins (Stenella coeruleoalba), Indo-Pacific humpback dolphins (Sousa chinensis), and baleen whales such as humpback whales (Megaptera novaeangliae) (Besseling et al., 2015; Lusher et al., 2015b; Lusher et al., 2018; Nelms et al., 2019; Moore et al., 2020; Zhang et al., 2021). However, inter-study variations (types of specimens sampled and habitats) preclude us from comparing previous results with those presented here. We found a slightly lower abundance of MPs in the finless porpoises (16.80 ± 2.24 items/individual) in our study, compared with the finless porpoise from the Yellow and Bohai Sea (19.1 ± 7.2 items/individual) and the Indo-Pacific humpback dolphins from the adjacent Beibu Gulf (25.67 ± 12.60 items/individual), this may be attributed to the differences in habitats and dietary that affect the intake of MPs by cetaceans, and differences in the length and structure of the dolphin intestines (Xiong et al., 2018; Zhu et al., 2019).
The shapes, colors, and polymer types of MPs we retrieved from the intestines of finless porpoises may be used to identify their potential sources (Xiong et al., 2018). Consistent with earlier observations on marine mammals (Hernandez-Gonzalez et al., 2018; Zhang et al., 2019; Battaglia et al., 2020), fibers were more common than fragments or films in Fujian finless porpoises. And the retention of fibers might be also related to the wrinkled structure of the cetacean small intestine. As for most studies on large marine mammals, black and blue MPs were common in the five finless porpoises we examined (Lusher et al., 2018; Duncan et al., 2019; Nelms et al., 2019), although transparent was the most abundant color type in the intestinal MPs in this study. Zhang et al. (2019) also found transparent/white MPs to dominate in the stomach of Sousa chinensis from the Pearl River Estuary. A high proportion of transparent MPs may be a function of weathering from long-distance transportation and immersion, and/or bleaching (whitening and yellowing) caused by physical and chemical processes in the environment (Li et al., 2022). Blue MPs were predominant (> 35%) in finless porpoises from the Yellow and Bohai seas, possibly because of differences in habitat and indirect effects of prey contaminated by MPs (Xiong et al., 2018).
PP, PA, and PET polymers from Fujian finless porpoise intestines have been commonly reported from gastrointestinal tracts of other cetaceans (Lusher et al., 2015b; Lusher et al., 2018; Moore et al., 2020). Whereas PP was a main component of MPs in finless porpoises from the Yellow and Bohai Seas (Xiong et al., 2018), PET and PA were dominant in our study. This may be related to differences in the habitat environment, because many aquaculture zones (Huang and Zuo, 2020) and textile industries occur along the Fujian coast (Huang, 2020). While PA is also often used in automotive, transportation and textile industries and packaging (Queiroz et al., 2022). Its prevalence in finless porpoises may be attributed to its widespread use in aquaculture practices (Do et al., 2022), such as in fishing gear and ropes, as fishing is a main economic activity in this area. And PET is also commonly used in the production of clothing and textiles, or plastic packaging and containers (Lusher et al., 2017; Borges-Ramírez et al., 2020; Wang et al., 2021b).
Most MPs that we retrieved from finless porpoises were small, ranging from 0.1 mm to 1.0 mm. Although small size MPs would not cause physical obstacles to cetaceans through entanglement or swallowing like large plastic fragments, they could embed within tissue and induce local gastrointestinal tract damage (Moore et al., 2020), and even small size MPs may have higher potential for toxic effects (Gomes et al., 2022). Additionally, MPs remaining in the intestines of cetaceans could also act as carriers of toxic substances, such as heavy metals and persistent organic pollutants, which were bioaccumulated in top predators (Bradney et al., 2019; Gao et al., 2019) and could be deleterious to health (Noël et al., 2014; Chen et al., 2019).
We reported MPs from all stations sampled along the Fujian coast of the East China Sea, although their distribution varied (Figure 4). MPs concentrations at the nearshore stations mostly exceeded the average concentration throughout the survey area (10.08 ± 1.19 items/m3). For example, microplastic concentrations at stations L1- L4 were extremely high. The region (L1-L4), located in shallow of Changle, influences by mid-subtropical maritime monsoon climate and has rich fishery grounds. Discarding aquaculture tools and the plastic waste generated during fishery activities contribute to serious microplastic pollution. The Minjiang river enters the sea here, and the Minjiang Estuary is seriously polluted with MPs (Zhao et al., 2015), which could be transported to waters off Changle in currents.
The average concentration of MPs at nearshore stations was significantly higher than those in offshore stations (Mann-Whitney-U test, P < 0.05, Figure 5). A great number of MPs are transferred to the nearshore environment by washing sewage and other human activities (Andrady, 2011; Browne et al., 2011). The many textile factories along the coast of Fujian province (Fang et al., 2019) also release clothing fibers in wastewater that is transported to nearshore in river runoff (Browne et al., 2011). The high microplastic concentrations in nearshore are also closely related to fishing and human activities (Xiong et al., 2022).
The average abundance of MPs in our study (10.08 ± 1.19 items/m3) was higher than that reported from adjacent the East China Sea waters (0.31 piece/m3) (Liu et al., 2018) and the Taiwan Strait (0.026 items/m3) (Wu et al., 2021). This may be because our sampling sites were closer to coastal areas affected by human activities and terrestrial inputs, while sites in previous research were all further offshore (Liu et al., 2018; Wu et al., 2021). Additionally, the mesh that we used (70 μm) to sieve water was finer than that used in previous studies (500 μm, 300 μm), reducing retention of smaller MPs and lowering estimates of microplastic abundance (Norén, 2007). A much higher abundance of MPs (514.3 ± 520.0 particles/m3) in the adjacent sea area in the surface seawater of Xiamen Bay (Tang et al., 2018) may be because of the greater urbanization and population density of Xiamen city than of Fuzhou and Ningde cities (Fujian Statistical Bureau, 2020; Fuzhou Statistical Bureau, 2020; Ningde Statistical Bureau, 2020). Furthermore, Xiamen is also a tourist destination, and the discharge of domestic sewage is likely to be higher and include more MPs.
MPs of different shapes and colors were widely distributed on the surface seawater. Some colors may cause marine organisms to mistake them for prey, with ingestion leading to bioaccumulation or food-chain transfer of these particles (Browne et al., 2008). Consistent with previous findings, most of our MPs were transparent fibers (Zhu et al., 2018; Mu et al., 2019; Jiang et al., 2020b). Fibers, especially synthetic fibers, are the main type of microplastic pollution in waters and sediments (Li et al., 2023). Replacement and discarding of fishing gear during the fishing process and human activities on the land (Deng et al., 2020a; Chen et al., 2020), such as laundry wastewater, would produce a large number of fibrous MPs (Kole et al., 2017; Zhang et al., 2019). More importantly, fibers are also widely used primary raw materials in the textile industry, and microplastic fibers are shed during the process of synthetic textiles, mainly during their washing (Pinlova et al., 2022). Atmospheric deposition is also a non-negligible vector for the transportation of terrestrial fibers to the ocean (Dris et al., 2016). Because of the prevalence of micro-fibers, small size MPs with a diameter < 1.0 mm were predominant (> 83% of all MPs) in surface seawater. It has been reported that the size range of MPs in textile wastewater was 165-1000 μm (Deng et al., 2020a; De Falco et al., 2020), consistent with the major size ranges of MPs fibers in our study.
Like previous studies along Chinese coasts, we reported PET and PP were the main polymer types in surface seawater (Tan et al., 2019; Zheng et al., 2019). PET fibers are important to textile industry, which was estimated that more than 80% PET from 42 × 106 t of synthetic fibers being produced annually (Xu et al., 2022; Pinlova et al., 2022). Most of our PET polymers were fibers, further supporting the possibility of microplastic contamination in the survey area being primarily from textile wastewater. PP, widely used in many fibrous materials such as clothes and rope, also commonly occurs in marine debris and is probably sourced to wastewater from washing clothes and fishing activity (Liao et al., 2021). Polyester fibers, usually used to produce clothing and various textile products (Jiang et al., 2020a), were also common in our seawater samples. While low in abundance, rayon, a regenerated fiber, is routinely used in the clothing industry (Li et al., 2023). Therefore, we further speculate that textile manufacturing activities maybe the important sources of microplastic pollution along the Fujian coast in the East China Sea.
The occurrence, distribution, transport, and fate of MPs in marine environments are affected by human activities, hydrology (e.g., ocean currents, hydrodynamic conditions, tides, salinity), meteorological conditions, and physical, chemical, and biological processes (Fazey and Ryan, 2016; Kanhai et al., 2018; Khatmullina and Chubarenko, 2020; Wang et al., 2021c). The coast we surveyed is located on the west side of the Taiwan Strait, which is a transitional subtropical monsoon climate zone between the East China Sea and the South China Sea. Affected by the monsoon, the Zhejiang-Fujian coast current would descend into the sea area southward along the East China Sea, and during the process, the Oujiang River, Minjiang River, Jinjiang River, and other sea runoffs may provide potential origins of MPs into Fujian coast of the East China Sea (Lin et al., 2012; Zhao et al., 2015; Deng et al., 2020b). Moreover, the Taiwan Strait owns other complex water masses that interact with each other, such as the extension of the Taiwan Warm Current and the invasion of the Kuroshio Current Water, which would cause ocean currents to move northward (Chen and Sheu, 2006; Lee and Takeshi, 2007). Combined with the monsoons (Zhang et al., 2005), these represent a complex potential mechanisms to disperse MPs to the Fujian coast of the East China Sea. However, because of the complex nature of interactions between these hydrographic features, predicting how MPs will be transported to or through this environment is difficult.
The abundance of MPs in the Yellow and the Bohai Sea finless porpoises (19.1 ± 7.2 items/individual and 1.72 ± 0.30 items/m intestines) (Xiong et al., 2018) was greater than our samples collected along the Fujian Coast of the East China Sea (16.80 ± 2.24 items/individual and 1.63 ± 0.14 items/m intestines). The abundance of MPs (545 ± 282 items/m3) in surface seawater close to where finless porpoises previously stranded in the Yellow Sea (Zhu et al., 2018) was also higher than that in our study (10.08 ± 1.19 items/m3). Geographical differences could contribute to differences in microplastic pollution levels between habitats.
We reported transparent to be the most common color, and fibers to be the most common microplastic shape in both surface seawater and from finless porpoise intestines. The polymer types (PA, PET, PP) that we reported from finless porpoise intestines were also common polymers in surface seawater samples. Unintentional uptake of MPs by ingestion from the habitat has been reported for dolphin neonates (Xiong et al., 2018; Zhu et al., 2019). Therefore, direct ingestion and trophic transfer are the two main pathways for MPs to bioaccumulate in higher trophic level organisms (Boerger et al., 2010; Wright et al., 2013; Setälä et al., 2014; Bessa et al., 2018).
We present the first comprehensive published, simultaneous report of microplastic pollution in finless porpoises and their habitat, and we determine MPs to be widespread in both finless porpoise intestinal samples and throughout their habitats along the Fujian coast of the East China Sea. Fiber, transparent, small sizes with a diameter < 1.0 mm were the most common MPs. These data both contribute to our understanding of microplastic pollution in cetaceans and Chinese coastal surface seawaters, and reveal the increased threat of microplastic pollution to top marine predators. Future research should investigate MPs composition in finless porpoise prey to determine mechanisms of transfer and accumulation in apex predators.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the animal study because our manuscript was about the intestinal microplastics research of stranded whales, which did not involve human biomedical research mentioned in the Declaration of Helsinki. Further, the Third Institute of Oceanography, Ministry of Natural Resources is the one of member units of the rescue network for marine rare and endangered wildlife of China. If there are marine animals stranded, the fishery department will inform our institute to rescue them. If the animals die unfortunately, we are permitted to take the samples to do research. We declare that the research has complied with the guidelines or rules for animal care and scientific experiments in China.
DW: methodology, field sampling, data analysis, graphics, and writing the original draft. YZ: conceptualization, formal analysis, writing review and editing, and supervision. LW: methodology, data curation, and software. YD: field sampling, data curation, and investigation. XW: data curation and visualization. ST: data curation and field sampling. LZ: conceptualization, validation, writing review and editing, and supervision. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 42076159), Natural Science Foundation of Fujian Province (2021J06031), the China-ASEAN Maritime Cooperation Fund (No. HX04-210901), the Fundamental Research Funds for Ministry of Natural Resources (No. 2018015), the National Key Research and Development Program of China (2017YFC1404404), and the Scientific and Technological Innovation Project of the Qingdao National Laboratory for Marine Science and Technology (2016ASKJ02).
We are grateful to Renchun Dong from the Xiamen Institute of Aquatic and Land Biology for his assistance in dissecting the samples. We also thank Suying Li and Qingshan Kong from the Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Science for equipment support during sample analysis. We sincerely thank the Administration of Ocean and Fisheries of Fujian for the assistance during sample collection. We thank Steve O’Shea, PhD, from Edanz (https://www.edanz.com/ac), for editing a draft of this manuscript. And we declare that the research has complied with the guidelines or rules for animal care and scientific experiments in China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1050957/full#supplementary-material
Andrady A. L. (2011). Microplastics in the marine environment. Mar. Pollut. Bull. 62 (8), 1596–1605. doi: 10.1016/j.envint.2017.02.013
Arthur C., Baker J., Bamford H. E. (2009). Proceedings of the international research workshop on the occurrence, effects, and fate of microplastic marine debris. NOAA Tech. Memorandum NOS-OR&R-30, 9–11.
Avio C. G., Gorbi S., Milan M., Benedetti M., Fattorini D., D'Errico G., et al. (2015). Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 198, 211–222. doi: 10.1016/j.envpol.2014.12.021
Banerjee A., Shelver W. L. (2021). Micro- and nanoplastic-mediated pathophysiological changes in rodents, rabbits, and chickens: a review. J. Food Prot. 84 (9), 1480–1495. doi: 10.4315/JFP-21-117
Battaglia F. M., Beckingham B. A., Mcfee W. E. (2020). First report from north America of microplastics in the gastrointestinal tract of stranded bottlenose dolphins (Tursiops truncatus). Mar. Pollut. Bull. 160, 111677. doi: 10.1016/j.marpolbul.2020.111677
Bessa F., Barría P., Neto J. M., Frias J. P. G. L., Otero V., Sobral P., et al (2018). Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 128, 575–584. doi: 10.1016/j.marpolbul.2018.01.044
Besseling E., Foekema E. M., Van Franeker J. A., Leopold M. F., Kühn S., Bravo R. E. L., et al. (2015). Microplastic in a macro filter feeder: Humpback whale Megaptera novaeangliae. Mar. Pollut. Bull. 95 (1), 248–252. doi: 10.1016/j.marpolbul.2015.04.007
Boerger C. M., Lattin G. L., Moore S. L., Moore C. J. (2010). Plastic ingestion by planktivorous fishes in the north pacific central gyre. Mar. Pollut. Bull. 60 (12), 2275–2278. doi: 10.1016/j.marpolbul.2010.08.007
Borges-Ramírez M. M., Mendoza-Franco E. F., Escalona-Segura G., Osten J. R. (2020). Plastic density as a key factor in the presence of microplastic in the gastrointestinal tract of commercial fishes from campeche bay, Mexico. Environ. Pollut. 267, 115659. doi: 10.1016/j.envpol.2020.115659
Bossart G. D. (2011). Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 48 (3), 676–690. doi: 10.1177/0300985810388525
Bradney L., Wijesekara H., Palansooriya K. N., Obadamudalige N., Bolan N. S., Ok Y. S., et al. (2019). Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 131, 104937. doi: 10.1016/j.envint.2019.104937
Browne M. A., Crump P., Niven S. J., Teuten E., Tonkin A., Galloway T., et al. (2011). Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ. Sci. Technol. 45 (21), 9175–9179. doi: 10.1021/es201811s
Browne M. A., Dissanayake A., Galloway T. S., Lowe D. M., Thompson R. C. (2008). Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ.Sci. Technol. 42 (13), 5026–5031. doi: 10.1021/es800249a
Caron A. G. M., Thomas C. R., Berry K. L. E., Motti C. A., Ariel E., Brodie J. E. (2018). Ingestion of microplastic debris by green sea turtles (Chelonia mydas) in the great barrier reef: Validation of a sequential extraction protocol. Mar. Pollut. Bull. 127, 743–751. doi: 10.1016/j.marpolbul.2017.12.062
Chen Q., Allgeier A., Yin D., Hollert H. (2019). Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions. Environ. Int. 130, 104938. doi: 10.1016/j.envint.2019.104938
Chen B., Fan Y., Huang W., Rayhan A. B. M. S., Chen K., Cai M. (2020). Observation of microplastics in mariculture water of longjiao bay, southeast China: Influence by human activities. Mar. Pollut. Bull. 160, 111655. doi: 10.1016/j.marpolbul.2020.111655
Chen C. A., Sheu D. D. (2006). Does the Taiwan warm current originate in the Taiwan strait in wintertime? J. Geophysical Res. 111, C04005. doi: 10.1029/2005JC03281
Cheung P. K., Cheung L. T. O., Fok L. (2016). Seasonal variation in the abundance of marine plastic debris in the estuary of a subtropical macro-scale drainage basin in south China. Sci. Total Environ. 562, 658–665. doi: 10.1016/j.scitotenv.2016.04.048
Cozar A., Echevarria F., Gonzalez-Gordillo J. I., Irigoien X., Ubeda B., Hernandez-Leon S., et al. (2014). Plastic debris in the open ocean. Proc. Natl. Acad. Sci. 111 (28), 10239–10244. doi: 10.1073/pnas.1314705111
Dai T. (2005). Sustainable yield of fishery resources in the Taiwan straits and its adjacent waters. Mar. Fisheries Res. (in Chinese). 26 (3), 1–8. doi: 10.1360/biodiv.050121
De Falco F., Cocca M., Avella M., Thompson R. C. (2020). Microfiber release to water, via laundering, and to air, via everyday use: a comparison between polyester clothing with differing textile parameters. Environ. Sci. Technol. 54 (6), 3288–3296. doi: 10.1021/acs.est.9b06892
Deng J., Guo P., Zhang X., Su H., Zhang Y., Wu Y., et al. (2020b). Microplastics and accumulated heavy metals in restored mangrove wetland surface sediments at jinjiang estuary (Fujian, China). Mar. Pollut. Bull. 159, 111482. doi: 10.1016/j.marpolbul.2020.111482
Deng H., Wei R., Luo W., Hu L., Li B., Di Y. N., et al. (2020a). Microplastic pollution in water and sediment in a textile industrial area. Environ. Pollut. 258, 113658. doi: 10.1016/j.envpol.2019.113658
Do V. M., Dang T. T., Le X. T. T., Nguyen D. T., Phung T. V., Vu D. N., et al. (2022). Abundance of microplastics in cultured oysters (Crassostrea gigas) from danang bay of Vietnam. Mar. Pollut. Bull. 180, 113800. doi: 10.1016/j.marpolbul.2022.113800
Dris R., Gasperi J., Saad M., Mirande C., Tassin B. (2016). Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar. Pollut. Bull. 104 (1-2), 290–293. doi: 10.1016/j.marpolbul.2016.01.006
Duncan E. M., Broderick A. C., Fuller W. J., Galloway T. S., Godfrey M. H., Hamann M., et al. (2019). Microplastic ingestion ubiquitous in marine turtles. Global Change Biol. 25 (2), 744–752. doi: 10.1111/gcb.14519
Fang C., Zheng R., Chen H., Hong F., Lin L., Lin H., et al. (2019). Comparison of microplastic contamination in fish and bivalves from two major cities in fujian province, China and the implications for human health. Aquaculture. 512, 734322. doi: 10.1016/j.aquaculture.2019.734322
Fazey F. M. C., Ryan P. G. (2016). Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. Pollut. 210, 354–360. doi: 10.1016/j.envpol.2016.01.026
Fok L., Cheung P. K., Tang G., Li W. C. (2017). Size distribution of stranded small plastic debris on the coast of guangdong, south China. Environ. Pollut. 220 (Part A), 407–412. doi: 10.1016/j.envpol.2016.09.079
Fossi M. C., Panti C., Baini M., Lavers J. L. (2018). A review of plastic-associated pressures: Cetaceans of the Mediterranean Sea and eastern Australian shearwaters as case studies. Front. Mar. Science. 5 (173). doi: 10.3389/fmars.2018.00173
Frias J., Pagter E., Nash R., O’Connor I., Carretero O., Filgueiras A., et al. (2018). Standardised protocol for monitoring microplastics in sediments. JPI-Oceans BASEMAN project. Deliverable 4 (2). doi: 10.13140/RG.2.236256.89601/1
Fujian Statistical Bureau (2020). Fujian statistical yearbook 2020 (BeiJing: China Statistics Press).
Fuzhou Statistical Bureau (2020). Fuzhou statistical yearbook 2020 (BeiJing: China Statistics Press).
Galgani F., Claro F., Depledge M., Fossi C. (2014). Monitoring the impact of litter in large vertebrates in the Mediterranean Sea within the European marine strategy framework directive (MSFD): Constraints, specificities and recommendations. Mar. Environ. Res. 100, 3–9. doi: 10.1016/j.marenvres.2014.02.003
Gao F., Li J., Sun C., Zhang L., Jiang F., Cao W., et al. (2019). Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment. Mar. Pollut. Bull. 144, 61–67. doi: 10.1016/j.marpolbul.2019.04.039
Geraci J. R., Lounsbury V. J. (2005). Marine mammals ashore: a field guide for strandings (Baltimore: National Aquarium).
Gomes T., Bour A., Coutris C., Almeida A. C., Bråte I. L., Wolf R., et al. (2022). “Ecotoxicological impacts of micro- and nanoplastics in terrestrial and aquatic environments,” in Microplastic in the environment: pattern and process. Ed. Bank M. S. (Cham: Springer International Publishing), 199–260. doi: 10.1007/978-3-030-78627-4_7
Gui D., Karczmarski L., Yu R., Plön S., Chen L., Tu Q., et al. (2016). Profiling and spatial variation analysis of persistent organic pollutants in south African delphinids. Environ. Sci. Technol. 50 (7), 4008–4017. doi: 10.1021/acs.est.5b06009
Hernandez-Gonzalez A., Saavedra C., Gago J., Covelo P., Santos M. B., Pierce G. J. (2018). Microplastics in the stomach contents of common dolphin (Delphinus delphis) stranded on the Galician coasts (NW spain 2005–2010). Mar. Pollut. Bull. 137, 526–532. doi: 10.1016/j.marpolbul.2018.10.026
Holmes L. A., Turner A., Thompson R. C. (2012). Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 160, 42–48. doi: 10.1016/j.envpol.2011.08.052
Huang Y. (2020). Analysis of fujian textile and garment export trade. Textile Rep. (in Chinese). 03), 48–51.
Huang J., Zuo C. (2020). Development status and suggestions of typhoon index insurance on aquaculture in fujian province. China Fisheries 12), 59–61.
Jefferson T. A., Wang J. Y. (2011). Revision of the taxonomy of finless porpoises (genus Neophocaena): The existence of two species. J. Mar. Anim. Their Ecology. 4 (1), 3–16.
Jepson P. D., Deaville R., Barber J. L., Aguilar À., Borrell A., Murphy S., et al. (2016). PCB Pollution continues to impact populations of orcas and other dolphins in European waters. Sci. Rep. 6 (1), 18573. doi: 10.1038/srep18573
Jiang Y., Yang F., Zhao Y., Wang J. (2020b). Greenland Sea Gyre increases microplastic pollution in the surface waters of the Nordic seas. Sci. Total Environ. 712, 136484. doi: 10.1016/j.scitotenv.2019.136484
Jiang Y., Zhao Y., Wang X., Yang F., Chen M., Wang J. (2020a). Characterization of microplastics in the surface seawater of the south yellow Sea as affected by season. Sci. Total Environ. 724, 138375. doi: 10.1016/j.scitotenv.2020.138375
Kanhai L. D. K., Gardfeldt K., Lyashevska O., Hassellov M., Thompson R. C., O'Connor I. (2018). Microplastics in sub-surface waters of the Arctic central basin. Mar. Pollut. Bull. 130, 8–18. doi: 10.1016/j.marpolbul.2018.03.011
Khatmullina L., Chubarenko I. (2020). Transport of marine microplastic particles: Why is it so difficult to predict? Mar. Microplastic Pollut. Control (ISMP 2018). 2 (1), 293–305. doi: 10.1139/anc-2018-0024
Kole P. J., Löhr A. J., Van Belleghem F., Ragas A. (2017). Wear And tear of tyres: A stealthy source of microplastics in the environment. Int. J. Env. Res. Pub. He. 14 (10), 1265. doi: 10.3390/ijerph14101265
Kosior E., Mitchell J. (2020). “Chapter 6 - current industry position on plastic production and recycling,” in Plastic waste and recycling. Ed. Letcher T. M. (NEXTEK, Kensingon Gore, London, United Kingdom: Academic Press), 133–162. doi: 10.1016/B978-0-12-817880-5.00006-2
Lauretta R., Sansone A., Sansone M., Romanelli F., Appetecchia M. (2019). Endocrine disrupting chemicals: effects on endocrine glands. Front. Endocrinol. 10. doi: 10.3389/fendo.2019.00178
Lee J., Takeshi M. (2007). Intrusion of kuroshio water onto the continental shelf of the East China Sea. J. Oceanogr. 63, 309–325. doi: 10.1007/s10872-007-0030-9
Liao C., Chiu C., Huang H. (2021). Assessment of microplastics in oysters in coastal areas of Taiwan. Environ. Pollut. 286, 117437. doi: 10.1016/j.envpol.2021.117437
Li J., Chen M., Huang Y., Peng J., Zhao J., Chan F., et al. (2022). Effects of different treatment processes in four municipal wastewater treatment plants on the transport and fate of microplastics. Sci. Total Environ. 831, 154946. doi: 10.3724/SP.J.1035.2008.00038
Li X., Lu Z.. (2008). The productivity of fishery resources and the maximum sustained yield in Fujian coastal water. J. Xiamen Univ. (Nat. Sci.) 47 (04), 596–601. doi: 10.3724/SP.J.1035.2008.00038
Li Y., Lu Q., Xing Y., Liu K., Ling W., Yang J., et al. (2023). Review of research on migration, distribution, biological effects, and analytical methods of microfibers in the environment. Sci. Total Environ. 855, 158922. doi: 10.1016/j.scitotenv.2022.158922
Lin G., Yang Q., Wang Y., Lin W. (2012). Species composition and distribution characteristics of phytoplankton in northern sea of fujian, China during withdraw of zhe-Min coastal current. Chin. J. Appl. Environ. Biol. 18, 411–420. doi: 10.3724/sp.j.1145.2012.00411
Li B., Su L., Zhang H., Deng H., Chen Q., Shi H. (2020). Microplastics in fishes and their living environments surrounding a plastic production area. Sci. Total Environ. 727, 138662. doi: 10.1016/j.scitotenv.2020.138662
Liu T., Sun X., Zhu M., Zhao Y. (2018). Distribution and composition of microplastics in the surface water of the East China Sea. Oceanologia Limnologia Sinica. 49 (01), 62–69. doi: 10.11693/hyhz20170100021
Lorenz C., Roscher L., Meyer M. S., Hildebrandt L., Prume J., Löder M. G. J., et al. (2019). Spatial distribution of microplastics in sediments and surface waters of the southern north Sea. Environ. Pollut. 252 (Part B), 1719–1729. doi: 10.1016/j.envpol.2019.06.093
Lusher A. L., Hernandez-Milian G., Berrow S., Rogan E., O'Connor I. (2018). Incidence of marine debris in cetaceans stranded and bycaught in Ireland: Recent findings and a review of historical knowledge. Environ. Pollut. 232, 467–476. doi: 10.1016/j.envpol.2017.09.070
Lusher A. L., Hernandez-Milian G., O'Brien J., Berrow S., O'Connor I., Officer R. (2015b). Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The true's beaked whale Mesoplodon mirus. Environ. Pollut. 199, 185–191. doi: 10.1016/j.envpol.2015.01.023
Lusher A. L., Hollman P. C., Mendoza-Hill J. (2017). Microplastics in fisheries and aquaculture: Status of knowledge on their occurrence and implications for aquatic organisms and food safety. FAO Fisheries Aquaculture Tech. Paper. 615, 1–126. Available at: https://www.researchgate.net/publication/319644050.
Lusher A. L., Tirelli V., O Connor I., Officer R. (2015a). Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 5 (1), 14947. doi: 10.1038/srep14947
Ma J., Niu X., Zhang D., Lu L., Ye X., Deng W., et al. (2020). High levels of microplastic pollution in aquaculture water of fish ponds in the pearl river estuary of guangzhou, China. Sci. Total Environ. 744, 140679. doi: 10.1016/j.scitotenv.2020.140679
Masura J., Baker J., Foster G., Arthur C. (2015). Laboratory methods for the analysis of microplastics in the marine environment recommendations for quantifying synthetic particles in waters and sediments. Natl. Oceanic Atmospheric Administration (NOAA) Tech. Memorandum NOS-OR&R-48., 1–31. Available at: https://repository.library.noaa.gov/view/noaa/10296.
Mishra A. K., Singh J., Mishra P. P. (2021). Microplastics in polar regions: An early warning to the world's pristine ecosystem. Sci. Total Environ. 784, 147149. doi: 10.1016/j.scitotenv.2021.147149
Monteiro R. C. P., Ivar Do Sul J. A., Costa M. F. (2018). Plastic pollution in islands of the Atlantic ocean. Environ. Pollut. 238, 103–110. doi: 10.1016/j.envpol.2018.01.096
Moore R. C., Loseto L., Noel M., Etemadifar A., Brewster J. D., Macphee S., et al. (2020). Microplastics in beluga whales (Delphinapterus leucas) from the Eastern Beaufort Sea. Mar. Pollut. Bull. 150, 110723. doi: 10.1016/j.marpolbul.2019.110723
Mu J., Zhang S., Qu L., Jin F., Fang C., Ma X., et al. (2019). Microplastics abundance and characteristics in surface waters from the Northwest pacific, the Bering Sea, and the chukchi Sea. Mar. Pollut. Bull. 143, 58–65. doi: 10.1016/j.marpolbul.2019.04.023
Nabi G., Ahmad S., Ullah S., Zada S., Sarfraz M., Guo X., et al. (2022). The adverse health effects of increasing microplastic pollution on aquatic mammals. J. King Saud Univ. - Science. 34 (4), 102006. doi: 10.1016/j.jksus.2022.102006
Nelms S. E., Barnett J., Brownlow A., Davison N. J., Deaville R., Galloway T. S., et al. (2019). Microplastics in marine mammals stranded around the British coast: Ubiquitous but transitory? Sci. Rep. 9 (1), 1075. doi: 10.1038/s41598-018-37428-3
Nelms S. E., Duncan E. M., Patel S., Badola R., Bhola S., Chakma S., et al. (2021). Riverine plastic pollution from fisheries: Insights from the Ganges river system. Sci. Total Environ. 756, 143305. doi: 10.1016/j.scitotenv.2020.143305
Nelms S. E., Galloway T. S., Godley B. J., Jarvis D. S., Lindeque P. K. (2018). Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 238, 999–1007. doi: 10.1016/j.envpol.2018.02.016
Ningde Statistical Bureau (2020). Ningde statistical yearbook 2020 (Beijing: China Statistics Press).
Noël M., Loseto L. L., Helbing C. C., Veldhoen N., Dangerfield N. J., Ross P. S. (2014). PCBs are associated with altered gene transcript profiles in Arctic beluga whales (Delphinapterus leucas). Environ. Sci. Technol. 48 (5), 2942–2951. doi: 10.1021/es403217r
Norén F. (2007). “Small plastic particles in coastal Swedish waters,” in KIMO report(Sweden: N-research), 1–11. Available at: https://www.researchgate.net/publication/284312290.
Novillo O., Raga J. A., Tomás J. (2020). Evaluating the presence of microplastics in striped dolphins (Stenella coeruleoalba) stranded in the Western Mediterranean Sea. Mar. Pollut. Bull. 160, 111557. doi: 10.1016/j.marpolbul.2020.111557
Pan Z., Liu Q., Jiang R., Li W., Sun X., Lin H., et al. (2021). Microplastic pollution and ecological risk assessment in an estuarine environment: The dongshan bay of China. Chemosphere. 262, 127876. doi: 10.1016/j.chemosphere.2020.127876
Pinlova B., Hufenus R., Nowack B. (2022). Systematic study of the presence of microplastic fibers during polyester yarn production. J. Clean Prod. 363, 132247. doi: 10.1016/j.jclepro.2022.132247
Plastics Europe (2019) Plastics-the facts 2019: An analysis of European plastics production, demand and waste data. Available at: http://www.plasticseurope.org/application/files/1115/7236/4388/FINAL_web_version_Plastics_the_facts2019_14102019.pdf.
Prietl B., Meindl C., Roblegg E., Pieber T. R., Lanzer G., Fröhlich E. (2014). Nano-sized and micro-sized polystyrene particles affect phagocyte function. Cell Biol. Toxicol. 30 (1), 1–16. doi: 10.1007/s10565-013-9265-y
Pugliares B. K., Bogomolni A., Touhey K., Herzig S., Harry C., Moore M. (2007). Marine mammal necropsy an introductory guide for stranding responders and field biologists. Woods Hole Oceanographic Institution., 21–27. doi: 10.1575/1912/1823
Queiroz A. F. D. S., Da Conceição A. S., Chelazzi D., Rollnic M., Cincinelli A., Giarrizzo T., et al. (2022). First assessment of microplastic and artificial microfiber contamination in surface waters of the Amazon continental shelf. Sci. Total Environ. 839, 156259. doi: 10.1016/j.scitotenv.2022.156259
Saliu F., Montano S., Garavaglia M. G., Lasagni M., Seveso D., Galli P. (2018). Microplastic and charred microplastic in the faafu atoll, Maldives. Mar. Pollut. Bull. 136, 464–471. doi: 10.1016/j.marpolbul.2018.09.023
Schwacke L. H., Gulland F. M., White S. (2013). “Sentinel species in oceans and human health,” in Environmental toxicology: Selected entries from the encyclopedia of sustainability science and technology. Ed. Meyers R. A. (New York: Springer), 9156–9174. doi: 10.1007/978-1-4419-0851-3_831
Sendra M., Sparaventi E., Novoa B., Figueras A. (2021). An overview of the internalization and effects of microplastics and nanoplastics as pollutants of emerging concern in bivalves. Sci. Total Environ. 753, 142024. doi: 10.1016/j.scitotenv.2020.142024
Setälä O., Fleming-Lehtinen V., Lehtiniemi M. (2014). Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 185, 77–83. doi: 10.1016/j.envpol.2013.10.013
Su L., Xue Y., Li L., Yang D., Kolandhasamy P., Li D., et al. (2016). Microplastics in taihu lake, China. Environ. Pollut. 216, 711–719. doi: 10.1016/j.envpol.2016.06.036
Tang G., Liu M., Zhou Q., He H., Chen K., Zhang H., et al. (2018). Microplastics and polycyclic aromatic hydrocarbons (PAHs) in xiamen coastal areas: Implications for anthropogenic impacts. Sci. Total Environ. 634, 811–820. doi: 10.1016/j.scitotenv.2018.03.336
Tan X., Yu X., Cai L., Wang J., Peng J. (2019). Microplastics and associated PAHs in surface water from the feilaixia reservoir in the beijiang river, China. Chemosphere. 221, 834–840. doi: 10.1016/j.chemosphere.2019.01.022
Thompson R. C., Olsen Y., Mitchell R. P., Davis A., Rowland S. J., John A. W., et al. (2004). Lost at sea: Where is all the plastic? Science 304 (5672), 838. doi: 10.1126/science.1094559
Van Cauwenberghe L., Janssen C. R. (2014). Microplastics in bivalves cultured for human consumption. Environ. Pollut. 193, 65–70. doi: 10.1016/j.envpol.2014.06.010
Vendel A. L., Bessa F., Alves V. E. N., Amorim A. L. A., Patrício J., Palma A. R. T. (2017). Widespread microplastic ingestion by fish assemblages in tropical estuaries subjected to anthropogenic pressures. Mar. Pollut. Bull. 117 (1-2), 448–455. doi: 10.1016/j.marpolbul.2017.01.081
Wang J., Reeves R. (2017a). “Neophocaena asiaeorientalis,” in The IUCN red list of threatened species 2017. doi: 10.2305/IUCN.UK.2017-3.RLTS.T41754A50381766.en
Wang J., Reeves R. (2017b). “Neophocaena asiaeorientalis,” in The IUCN red list of threatened species 2017. doi: 10.2305/IUCN.UK.2017-3.RLTS.T198920A50386795.en
Wang D., Su L., Ruan H. D., Chen J., Lu J., Lee C., et al. (2021a). Quantitative and qualitative determination of microplastics in oyster, seawater and sediment from the coastal areas in zhuhai, China. Mar. Pollut. Bull. 164, 112000. doi: 10.1016/j.marpolbul.2021.112000
Wang C., Zhao J., Xing B. (2021c). Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 407, 124357. doi: 10.1016/j.jhazmat.2020.124357
Wang Q., Zhu X., Hou C., Wu Y., Teng J., Zhang C., et al. (2021b). Microplastic uptake in commercial fishes from the bohai Sea, China. Chemosphere. 263, 127962. doi: 10.1016/j.chemosphere.2020.127962
Waring R. H., Harris R. M., Mitchell S. C. (2018). Plastic contamination of the food chain: A threat to human health? Maturitas. 115, 64–68. doi: 10.1016/j.maturitas.2018.06.010
Woodall L. C., Sanchez-Vidal A., Canals M., Paterson G. L. J., Coppock R., Sleight V., et al. (2014). The deep sea is a major sink for microplastic debris. R. Soc. Open Science. 1 (4), 140317. doi: 10.1098/rsos.140317
Wright S. L., Thompson R. C., Galloway T. S. (2013). The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 178, 483–492. doi: 10.1016/j.envpol.2013.02.031
Wu B. (1982). Some problems on circulation study in the Taiwan strait. J. Appl. Oceanography 1 (1), 1–7.
Wu B. (1991). Kuroshio and circulations in China seas. J. Oceanography Taiwan Strait 10 (1), 25–32. doi: CNKI:SUN:TWHX.0.1991-01-003
Wu Q., Liu S., Chen P., Liu M., Cheng S., Ke H., et al. (2021). Microplastics in seawater and two sides of the Taiwan strait: Reflection of the social-economic development. Mar. Pollut. Bull. 169, 112588. doi: 10.1016/j.marpolbul.2021.112588
Xiong X., Chen X., Zhang K., Mei Z., Hao Y., Zheng J., et al. (2018). Microplastics in the intestinal tracts of East Asian finless porpoises (Neophocaena asiaeorientalis sunameri) from yellow Sea and bohai Sea of China. Mar. Pollut. Bull. 136, 55–60. doi: 10.1016/j.marpolbul.2018.09.006
Xiong W., Mei X., Mi B., Yang H., Han Z., Zhang Y., et al. (2022). Current status and cause analysis of microplastic pollution in sea areas in China. China Geology. 5 (1), 161–172. doi: 10.31035/cg2021072
Xu C., Zhou G., Lu J., Shen C., Dong Z., Yin S., et al. (2022). Spatio-vertical distribution of riverine microplastics: impact of the textile industry. Environ. Res. 211, 112789. doi: 10.1016/j.envres.2022.112789
Zantis L. J., Carroll E. L., Nelms S. E., Bosker T. (2021). Marine mammals and microplastics: A systematic review and call for standardisation. Environ. Pollut. 269, 116142. doi: 10.1016/j.envpol.2020.116142
Zhang X., Luo D., Yu R. Q., Xie Z., He L., Wu Y. (2021). Microplastics in the endangered indo-pacific humpback dolphins (Sousa chinensis) from the pearl river estuary, China. Environ. Pollut. 270, 116057. doi: 10.1016/j.envpol.2020.116057
Zhang C., Shang S., Chen D., Shang S. (2005). Short-term variability of the distribution of zhe-Min coastal water and wind forcing during winter monsoon in the Taiwan strait. J. Remote Sensing. 9, 452–458. doi: 10.11834/jrs.20050465
Zhang W., Zhang S., Zhao Q., Qu L., Ma D., Wang J. (2020). Spatio-temporal distribution of plastic and microplastic debris in the surface water of the bohai Sea, China. Mar. Pollut. Bull. 158, 111343. doi: 10.1016/j.marpolbul.2020.111343
Zhang C., Zhou H., Cui Y., Wang C., Li Y., Zhang D. (2019). Microplastics in offshore sediment in the yellow Sea and East China Sea, China. Environ. Pollut. 244, 827–833. doi: 10.1016/j.envpol.2018.10.102
Zhao J., Ran W., Teng J., Liu Y., Liu H., Yin X., et al. (2018). Microplastic pollution in sediments from the bohai Sea and the yellow Sea, China. Sci. Total Environ. 640-641, 637–645. doi: 10.1016/j.scitotenv.2018.05.346
Zhao S., Zhu L., Li D. (2015). Microplastic in three urban estuaries, China. Environ. Pollut. 206, 597–604. doi: 10.1016/j.envpol.2015.08.027
Zhao S., Zhu L., Wang T., Li D. (2014). Suspended microplastics in the surface water of the Yangtze estuary system, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 86 (1-2), 562–568. doi: 10.1016/j.marpolbul.2014.06.032
Zheng Y., Li J., Cao W., Liu X., Jiang F., Ding J., et al. (2019). Distribution characteristics of microplastics in the seawater and sediment: A case study in jiaozhou bay, China. Sci. Total Environ. 674, 27–35. doi: 10.1016/j.scitotenv.2019.04.008
Zhu L., Bai H., Chen B., Sun X., Qu K., Xia B. (2018). Microplastic pollution in north yellow Sea, China: Observations on occurrence, distribution and identification. Sci. Total Environ. 636, 20–29. doi: 10.1016/j.scitotenv.2018.04.182
Zhu X., Ran W., Teng J., Zhang C., Zhang W., Hou C., et al. (2020). Microplastic pollution in nearshore sediment from the bohai Sea coastline. Bull. Environ. Contamination Toxicology. 107, 665–670. doi: 10.1007/s00128-020-02866-1
Keywords: microplastics, finless porpoises, cetacean, surface water, Fujian coast
Citation: Wang D, Zhen Y, Wei L, Dai Y, Wang X, Tong S and Zhao L (2022) Microplastic pollution in finless porpoises and their habitats along the Fujian coast of the East China Sea. Front. Mar. Sci. 9:1050957. doi: 10.3389/fmars.2022.1050957
Received: 22 September 2022; Accepted: 07 November 2022;
Published: 01 December 2022.
Edited by:
Cristina Panti, University of Siena, ItalyReviewed by:
Giuseppe Suaria, National Research Council (CNR), ItalyCopyright © 2022 Wang, Zhen, Wei, Dai, Wang, Tong and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyuan Zhao, emhhb2xpeXVhbkB0aW8ub3JnLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.