- 1South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou, China
- 2Key Laboratory of South China Sea Fishery Resources Exploitation & Utilization, Ministry of Agriculture and Rural Affairs, Guangzhou, China
- 3Shenzhen Base South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shenzhen, China
Introduction: Antibiotics are widely used in medical and health services, as well as livestock farming. High concentrations of antibiotics are eventually discarded into rivers due to incomplete metabolism and removal. Bays connect rivers to the sea, and have important ecological functions. Although the occurrence, concentrations, and distribution of antibiotics in bays have been widely studied, the temporal variations in the concentration, contamination indicators and sources of antibiotics, as well as related ecological risk factors, remain unclear.
Methods: Within this context, we investigate the sources, concentrations, temporal variations, and ecological risks associated with antibiotics in water and sediment samples from Dapeng Cove, Shenzhen, South China, in three rainy seasons.
Results: Eight and ten different antibiotics were detected in the water and sediment samples, respectively. Of these, the detection rates of erythromycin-H2O (ETM) and clarithromycin were highest in both sample types (100%), while trimethoprim (TMP) and ETM were the most abundant antibiotics in the water and sediment samples, respectively. The total concentration (TC) of antibiotics was highest in water and sediment samples from a local domestic sewage site, at 301.96 ng/L and 18.67 ng/g, respectively. Several environmental factors and the concentrations of the predominant antibiotics TMP and ETM were positively correlated. Multiple linear regression analysis revealed TMP and ETM to be the predominant antibiotics influencing the TC of the target antibiotics, with partial regression coefficients of 1.28 and 1.136, respectively (p < 0.01). Notably, ETM had risk quotients of 0.43–7.91, indicating medium to high risk, while samples from the inner bay and domestic sewage outlet had high ecological risk levels. Redundancy analysis showed that the domestic sewage and inner bay samples were clustered closely but separately from the other samples.
Discussion: The results of our study indicate that domestic sewage is the dominant source of antibiotics in the bay.
Introduction
Large amounts of antibiotics are used in medical and health services, as well as in livestock/poultry farming. In 2013, China used 162,000 tons of antibiotics, 150 and 9 times higher than the amounts used in the UK and USA, respectively (Zhang et al., 2015). It was reported that China produced 231,400 tons of agricultural antibiotics in 2020 (AgroPages, 2021). A 2014 European Centre for Disease Control/European Food Safety Agency/European Medicines Agency joint surveillance report estimated that, across the 28 EU member states, 8927 and 3821 tons of antimicrobial active ingredients were used for agricultural and medical purposes, respectively (EFSA, 2017). Furthermore, by 2030, the global use of antibiotics for livestock farming is estimated to reach 105,596 tons (Van Boeckel et al., 2015). Owing to the incomplete metabolism and inadequate removal of antibiotics at sewage treatment works, large amounts of antibiotics enter rivers (Gao et al., 2012), increasing the risk of antibiotic resistance and threatening ecosystems.
Bays connect rivers to the sea and play an important role in providing vital ecological functions. Several studies have investigated the occurrence and concentrations of antibiotics in bays. For example, in a study on Laizhou Bay, China, it was reported that the antibiotic levels in the river were higher than those in the bay. Additionally, enrofloxacin (EFX) and trimethoprim (TMP) were predominant in the sediment and seawater, respectively, and antibiotics were detected in invertebrates and fish (Zhang et al., 2012; Liu et al., 2017; Han et al., 2021a; Lu et al., 2022). Furthermore, Cheng et al. (2016) reported higher levels of antibiotics in the intertidal zone than in the coastal and offshore areas of Bohai Bay, China, while in Jiaozhou Bay, China, the antibiotic levels in estuarine water were higher than those in seawater (Liu et al., 2020; Lu et al., 2020). In addition, Liu et al. (2018) found the highest concentration of antibiotics in Jiaozhou Bay, China, at the entrance to the Yang River wetland. Notably, Qin et al. (2018) reported that a water diversion project could reduce antibiotic contamination in Gonghu Bay, China. The transport and distribution of antibiotics in bays have also been studied using models (Xing et al., 2020; Liu et al., 2022). Globally, He et al. (2019) detected the presence of fluoroquinolone, macrolide, and sulfonamide antibiotics in water samples in Chesapeake Bay, the largest estuary in the United States, and recorded the highest concentrations for norfloxacin (94.1 ng/L), enrofloxacin (17.8 ng/L), sulfamethoxazole (14.8 ng/L), and clarithromycin (9.7 ng/L). In addition, Kafaei et al. (2018) reported that, in the Persian Gulf on the Bushehr coastline of Iran, the concentrations of antibiotics ranged from 1.21–51.50 ng/L in seawater and 1.40–25.32 ng/g in sediment, and norfloxacin was dominant.
Despite previous studies, the sources of and related temporal variations in antibiotics in bays remain unclear. Within this context, He et al. (2019) and Kafaei et al. (2018) identified hospital and wastewater treatment plant effluent, as well as domestic sewage and agricultural runoff, as potential sources of antibiotics in bays based on adjacent land-use characteristics. Using their findings, we surveyed and investigated the sources of and related variations in antibiotics in the bay of Dapeng Cove, Shenzhen, South China, throughout the wet and dry seasons. Our hypotheses are that 1) the antibiotic concentration varies across different seasons, 2) antibiotics, such as TMP and erythromycin-H2O (ETM), can be selected as indicators of the antibiotic contamination levels, and 3) domestic sewage is a dominant source of antibiotics in the bay. Our study allows for a better understanding of the sources of antibiotics and their temporal variations in bay environments, and can be used to improve the protection of bay ecosystems.
Materials and methods
Study site and sample collection
Dapeng Cove, located in Shenzhen, South China, is a semi-enclosed urbanized bay that covers an area of 17.2 km2. The cove is deep, with an average water depth of 7 m, and serves as a large deep-water wharf. It is rich in aquatic products and serves as a breeding ground for aquatic resources in China. Therefore, Dapeng Cove has high economic and ecological value. We collected water and sediment samples from Dapeng Cove in November 2018 (dry season), April 2019 (dry season), and August 2019 (wet season). Water and sediment samples were collected from the inner bay (IB), open bay (OB), aquaculture zone (AZ), and domestic sewage outlet (DS) at each sampling time (Table S1 and Figure S1). Three samples were collected at each sampling site and mixed thoroughly. We followed the water and sediment sampling methods described by Su et al. (2017). Sediment samples (100 g) were collected from a depth of 0–10 cm using a grab sampler and mixed well in sterile polyethylene bottles.
Pretreatment analysis and instruments analysis for antibiotics
Twenty-two commonly detected antibiotics across seven categories were selected for detection in this study, including tetracyclines (oxytetracycline, chlortetracycline, and tetracycline); sulfonamides (sulfamonomethoxine (SMM), sulfadimethoxine, sulfaquinoxaline, sulfamethazine, sulfamethoxazole, sulfadiazine, and sulfameter); macrolides (clarithromycin (CTM), ETM, and roxithromycin (RTM)); fluoroquinolones (pefloxacin, norfloxacin (NFX), EFX, lomefloxacin, ofloxacin (OFX), and ciprofloxacin (CFX)); and lincomycin, TMP, and salinomycin (SAL).
Sample extraction. For water samples, about 5% (v/v) of methanol was added to each water sample and the sample pH value was adjusted to 3 using 4 M H2SO4. One liter of each water sample was filtered through glass fiber filters (Whatman GF/F, 0.7 μm effective pore size, UK) in order to remove suspended particles, Exactly 100 μL (1.0 mg/L of SMX-D4, CFX-D8, ETM-13C-D3, LIN-D3, TMP-D3, and MC of the internal standard solutions were added into samples. About 0.2 g Na2EDTA was added to chelate with metal ions in water samples. Ten milliliter of HPLC grade methanol and 10 mL HPLC grade water were used for the precondition of solid phase extraction cartridges (Oasis HLB, 6 mL and 500 mg each) prior to use. The filtered water samples passed through the SPE cartridges at a speed of 5 to 10 mL/min. After the water samples were filtered, two aliquots of 50 mL of 5% (v/v) methanol in HPLC grade water were used to rinse the sample bottle twice. Cartridges were pumped under vacuum to remove excess water for 2 h. About 12 mL of methanol was used to elute the target antibiotics from the cartridges. The extracts were dried and re-dissolved in 1 mL of methanol. The final extract was transferred to a 2 mL amber vial with a 0.22 μm membrane, then kept at -20 C until analysis.
For solid samples, 2.0 g of each sediment sample or each feed sample (dry weight) was weighted into a 30 mL glass centrifuge tube. Then 100 μL of the internal standard solutions (1.0 mg/L for each internal standard) was added. The samples were manually mixed well and placed in the fume hood for 4 h to volatilize the solvent, then kept at 4 °C overnight. Each glass tube was added with 10 mL acetonitrile and 10 mL citric acid buffer (pH 3) and vibrated on a vortex mixer for 1 min, then ultrasonicated for 15 min and centrifuged at 3500 rpm for 10 min. The extraction procedure was repeated twice and all the supernatant was transferred into a 250 mL round-bottom flask. The extract in the flask was evaporated at 50°C via rotary evaporation to remove the organic solvent, then diluted to approximately 200 mL with HPLC grade water to ensure that the organic solvent in the solution was less than 5%. SAX cartridges and HLB cartridges were used to clean up and enrich the antibiotics in solid sample extracts. The column set in tandem was preconditioned with 10 mL of methanol and then 10 mL HPLC grade water. After the diluted extract was loaded, the following processes were the same as the water samples described above.
Instrumental analysis. The target antibiotics were analyzed by ultra-high performance liquid chromatography/tandem mass spectrometry system (UPLC-MS/MS) (Acquity UPLC I-Class/Xevo TQ-S Micro, Waters) in multiple reactions monitoring (MRM) mode (Table S2). All target compounds were analyzed in the positive mode. Separation of the compounds was used a Waters ACQUITY UPLC BEH-C18 (50 mm×2.1 mm, 1.7 μm) with a guard column.
The mobile phases were 0.2% (v/v) formic acid aqueous solution with 2 mM ammonium acetate (mobile phase A) and acetonitrile (mobile phase B) for positive mode. The mobile phase gradient was ramped at a flow rate of 0.35 mL/min from 5% - 20% B in 1.8 min, from 20% to 40% B in 1.7 min, ramped from 40% to 90% B in 0.5 min, kept 90%B in 0.5 min, then from 90% to 5%B in 1.5 min, finally kept for 3 min. The column temperature was 40°C. The injection volume was 5 µL. The MS operating conditions in the positive mode were set as follows: capillary, 3.00 kV; cone, 35 V; source desolvation temperature, 550 °C; source gas flow, 900 L/Hr.
The concentrations of the select antibiotics were determined using an internal standard method. The identification of antibiotics was based on the selected parent ion and two corresponding product ions, and the concentrations of target antibiotics were quantified by the calibration standard curves. Calibration curve was constructed for target antibiotics from 1.0 to 200 μg/L (standard concentration levels at 1, 5, 10, 50, 100 and 200 μg/L). Excellent linearity was achieved in these concentration ranges with the correlation coefficients higher than 0.99 for all validation batches.
Field blanks, procedural blanks and an independent check standard were analyzed to control carryover, laboratory contamination, and equipment performance. Both intra- and inter-day precisions of the UPLC-MS/MS instrument were examined. For the intra-day precision, a standard solution (50 μg/L of each compound) was injected successively seven times. For the inter-day experiment, five standard solutions (50 μg/L of each compound) were performed on five different days over one month interval. The relative standard deviation was less than 15%.
The recovery rates of the majority of detected antibiotics were in the range of 70 to 144% for seawater and 70 to 141% for sediment samples (Tables S2-S4). The limit of detection (LOD) and limit of quantitation (LOQ) for each target compound were calculated based on the signal-to-noise ratio (SNR) near the target peak. LOD is defined as three times of SNR, and LOQ is ten times of SNR.
Evaluation of ecological risk for antibiotics
We used the risk quotients (RQs) method based on Van Leeuwen (2003) to evaluate the ecological risks associated with the detected antibiotics. The ecological risks of antibiotics were calculated using the following equation (1):
where MEC is the measured environmental concentration and PNEC is the predicted no-effect concentration of the target antibiotic. Risk levels of 0.01–0.1, 0.1–1.0, and >1.0 indicate low, medium, and high risk, respectively, as described by Hernando et al. (2006).
Data analysis
Significant differences (p < 0.05) between antibiotics and samples were investigated by ANOVA with Tukey’s test using SPSS (V17.0). In addition, we used spearman correlation analysis, multiple linear regression analyses, and redundancy analysis (RDA) to investigate the correlations between environmental factors and antibiotics using SPSS (V17.0) and Canoco 4.5. Pseudo-partitioning coefficients (PPC) were used to explore the partition profiles of antibiotics between the water and sediment samples (Xu et al., 2014).
Results
Environmental factors
The environmental factors did not change greatly in the water samples throughout the sampling period (Table S5). Specifically, the water temperature ranged from 2.5 to 30.9°C, while the concentrations of total organic carbon (TOC), dissolved organic carbon (DOC), total nitrogen (TN), total phosphorus (TP), suspended solids (SS), and chemical oxygen demand (COD) ranged from 16.9 to 29.6 mg/L, 16.7 to 28.9 mg/L, 0.62 to 10.5 mg/L, 0.014 to 0.091 mg/L, 2 to 21 mg/L, and 0.7 to 3.4 mg/L, respectively. Within the collected sediment samples, TN and TP varied greatly, with concentrations ranging from 113 to 1,530 and 49 to 184 mg/kg, respectively (Table S6).
Detection, concentration, and pseudo-partitioning coefficients of identified antibiotics
We detected eight of the target antibiotics in the water samples (Table S7), with the highest variety observed in the DS samples. The detection rates of ETM and CTM were highest among all water samples, at 100%, while TET, OFX, and SMM had the lowest detection rates of 8.33%. Similar to the results from the water samples, the sediment samples from DS also had the highest variety of antibiotics, with 10 of the target antibiotics being detected (Table S8). The detection rates of ETM, CTM, and RTM were highest across all sediment samples at 100%, followed by NFX (66.67%) and OFX (66.67%), while TET and SAL had the lowest detection rates of 8.33%.
Regarding the concentrations of antibiotics in water samples (Figure 1A), TMP exhibited the highest concentration of 184.47 ng/L, followed by ETM (117.79 ng/L) and RTM (47.43 ng/L), with CTM (0.35 ng/L) exhibiting the lowest concentration. Across all samples, those from DS had the highest TC of target antibiotics of 301.96 ng/L, followed by samples from IB and OB, with TCs of 143.29 and 6.81 ng/L, respectively. ETM was the most abundant antibiotic (10.71 ng/g) in the sediment samples, followed by RTM (6.16 ng/g) and CTM (0.07 ng/g) (Figure 1B). Of the collected sediment samples, those from DS had the highest TC of target antibiotics (18.67 ng/g), followed by samples from ES (10.39 ng/g) and AZ (3.66 ng/g).
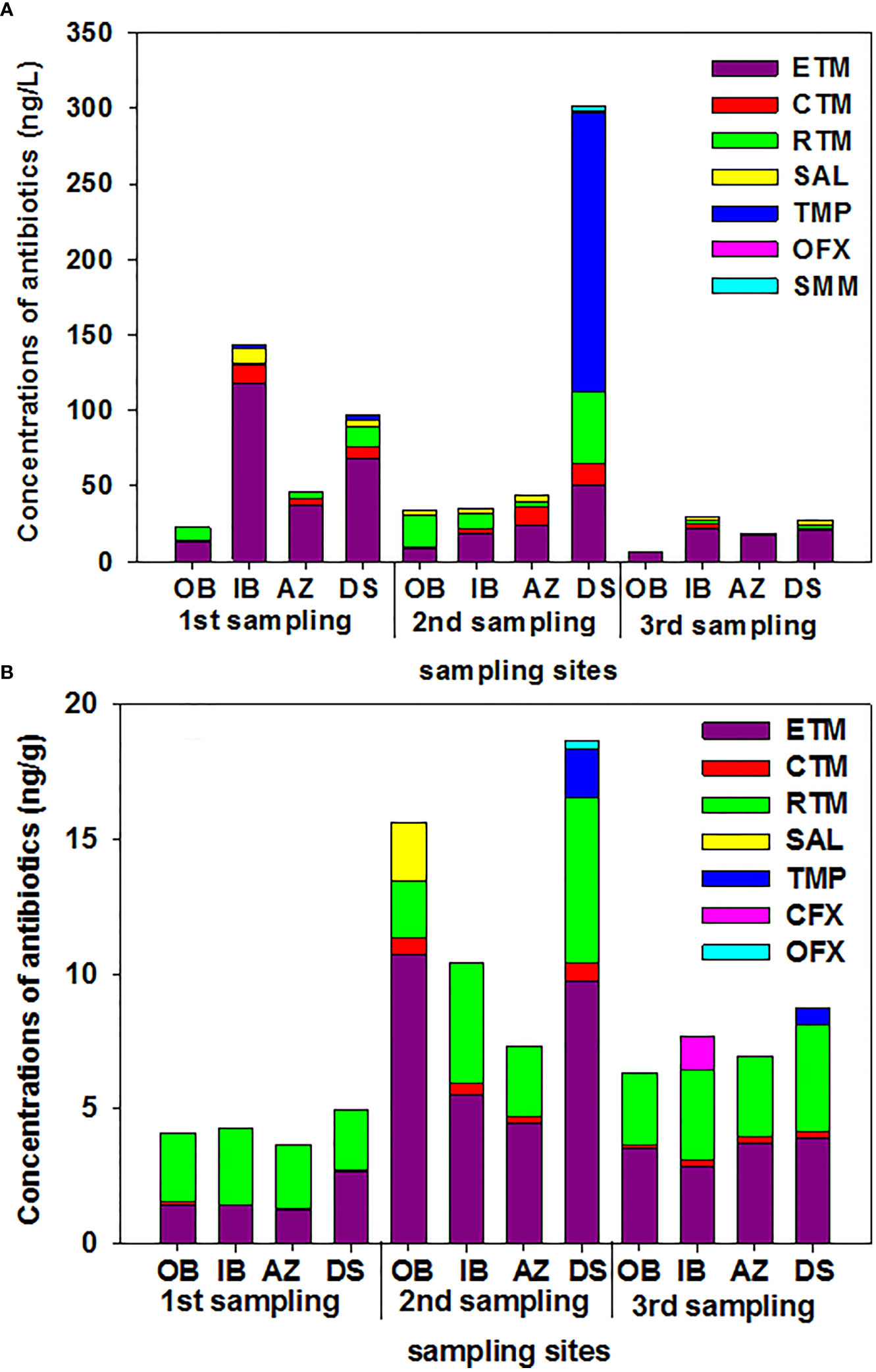
Figure 1 Concentrations of antibiotics in water and sediment samples for three sampling events in Dapeng Cove, Shenzhen, South China. OB, the open bay; IB, the inner bay; AZ, the aquaculture zone; DS, domestic sewage. (A) water samples. (B) sediment samples.
As PPC was not detected in the water or sediment samples, we could not calculate the PPC values for certain target antibiotics. However, RTM was found to have the highest PPC values between the sediment and water samples (Table 1), ranging from 99.58 to 2,890.51 L/kg, followed by ETM (12.23 to 1,295.04 L/kg) and CTM (11.64 to 548.67 L/kg), and TMP had the lowest value (9.65 L/kg).
Ecological risks of antibiotics
We investigated the RQs of the target antibiotics (Table 2); ETM had the highest RQ of 0.43 to 7.91, followed by CTM (0.018 to 0.74) and RTM (0.013 to 0.50), and TMP had the lowest RQ value (0.0013). For the sum RQs of the target antibiotics among all samples, samples from IB had the highest values of 1.48 to 8.55, followed by those from DS (1.49 to 5.10), with those from OB having the lowest sum RQ value (0.45–1.03).
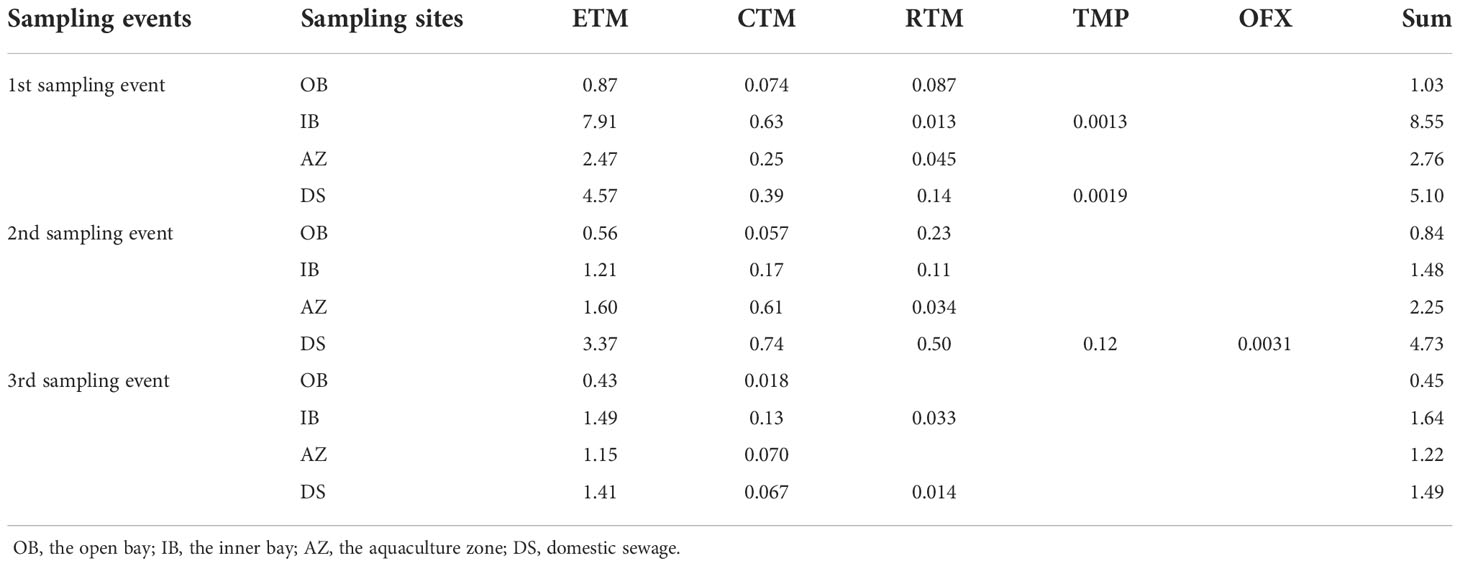
Table 2 The risk quotients of target antibiotics and the total risk quotients of samling sites in this study.
Spearman correlation, RDA, and multiple linear regression analysis
The data did not conform to the normal distribution. Thus, spearman correlation analysis was conducted. Positive correlations between pH and RTM and TP and TMP were observed, with r = 0.580–0.669 (p < 0.05) (Table S9). Positive correlations were also observed between TP and ETM, CTM, and TMP, with r = 0.641–0.718 (p < 0.05). In addition, TN was positively correlated with CTM, ETM, and the TCs of the target antibiotics (r = 0.630–0.653, p < 0.05) in the sediment samples (Table S10).
Using RDA, we investigated the correlations between environmental factors and antibiotics in the water samples (Figure 2A). Of the total variance, 63.4% and 19.0% could be explained by RDA1 and RDA2, respectively. We recorded positive correlations between most of the environmental factors (nitrite, ammonia, TOC, DOC, SS, and TP) and the predominant antibiotics TMP and RTM, as well as between environmental factors (TP and phosphate) and the predominant antibiotic ETM and the TCs of the antibiotics. For sediment samples, RDA1 and RDA2 represented 91.0% and 7.4% of the total variance, respectively (Figure 2B), while TN was positively correlated with the predominant antibiotics, i.e., ETM and RTM, as well as the TCs of target antibiotics.
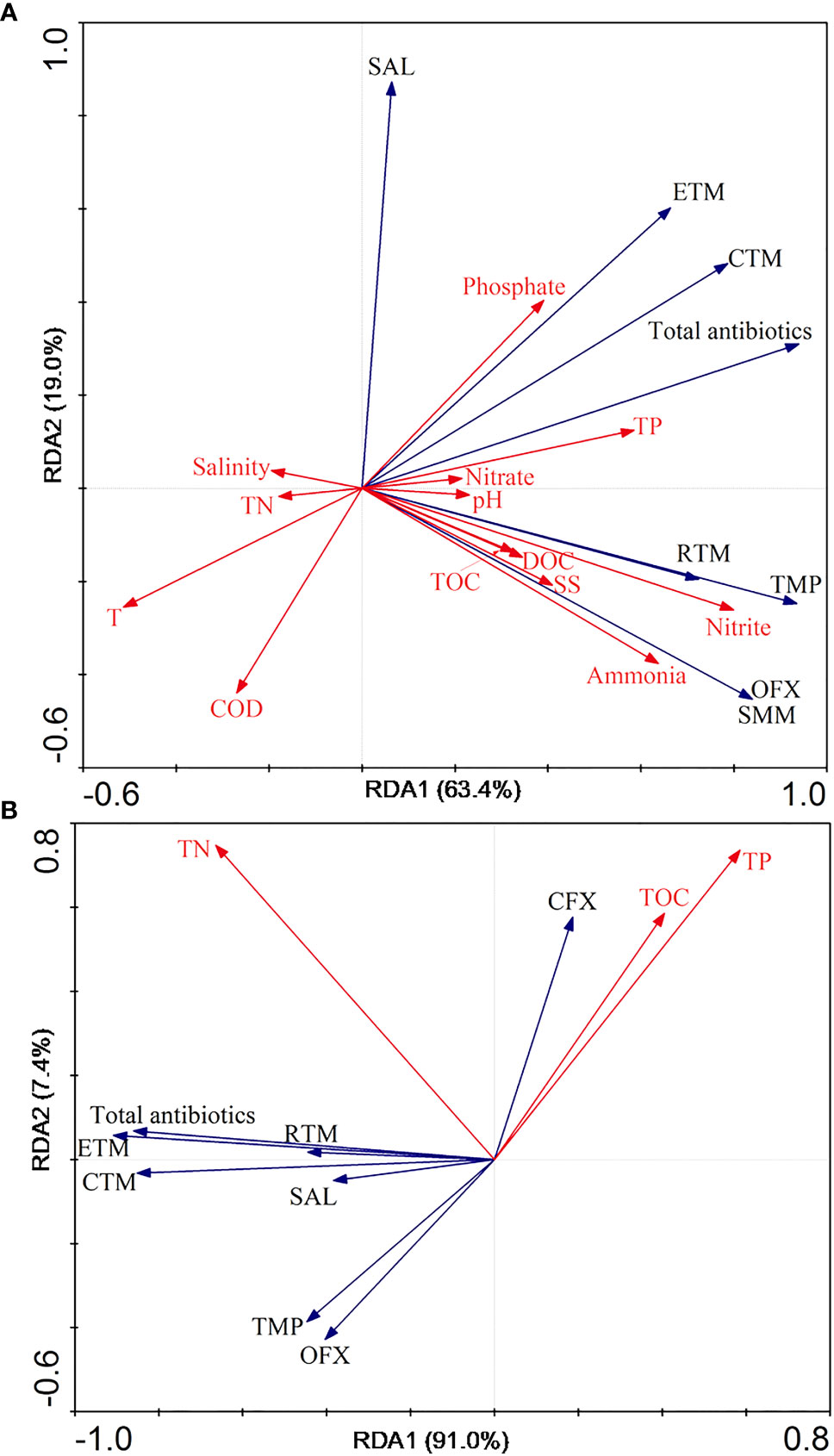
Figure 2 Correlations between environmental factors and antibiotics in water and sediment in Dapeng Cove, Shenzhen, South China with redundancy analysis. (A) water samples. (B) sediment samples.
We investigated the distribution of antibiotics among the samples (Figure 3) and observed three clusters. Specifically, DS-I, DS-II, and IB-I were significantly separated from the other samples, suggesting that the antibiotic profiles of DS and IB were significantly different to those of the other sites. The samples from OB and AZ were clustered together, indicating their antibiotic profiles were similar.
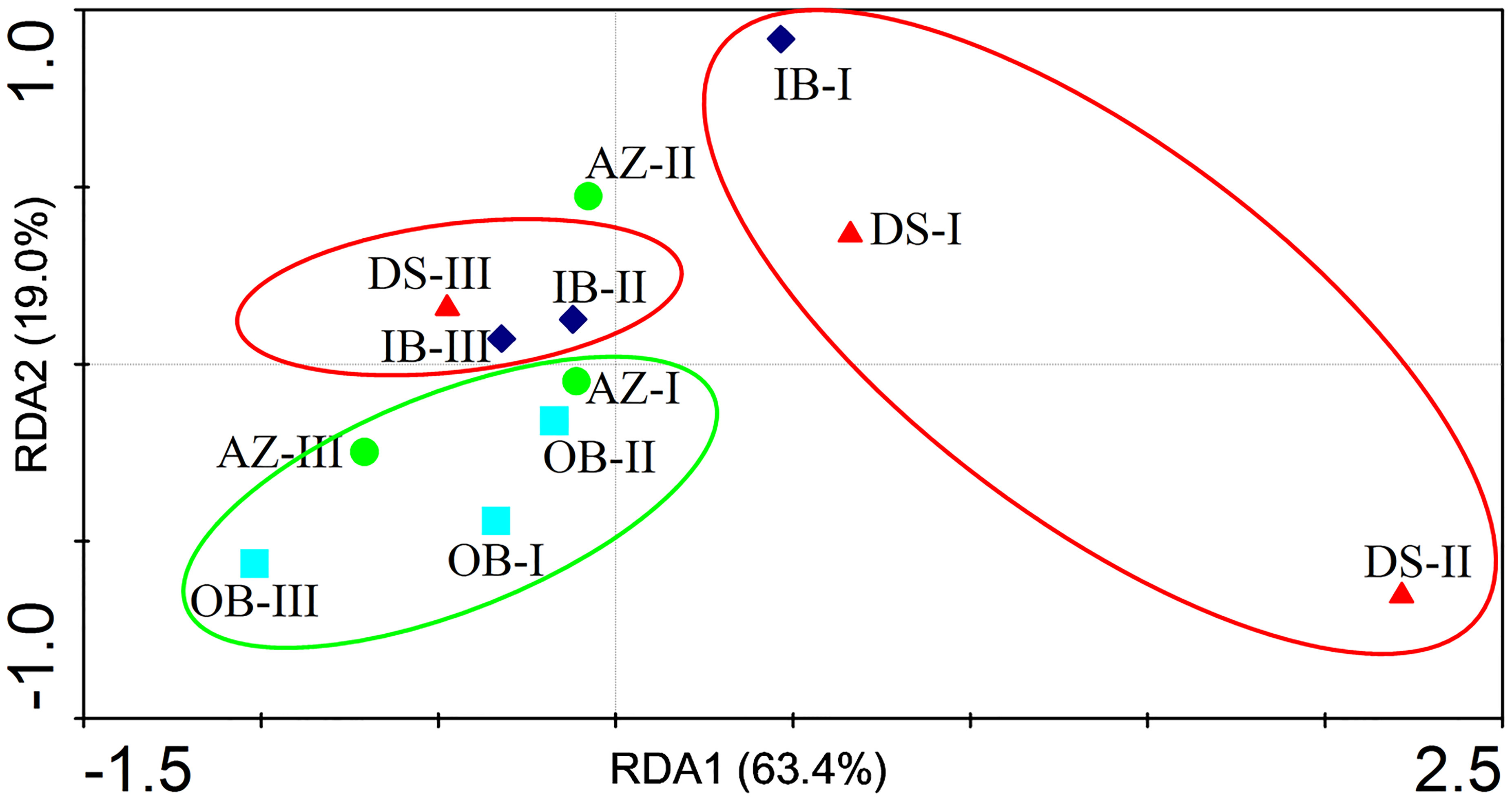
Figure 3 Distribution of antibiotics in different sampling sites for three sampling events in Dapeng Cove, Shenzhen, South China. OB, the open bay; IB, the inner bay; AZ, the aquaculture zone; DS, domestic sewage.
Multiple linear regression analysis revealed that TMP and ETM were the predominant antibiotics influencing the TCs of the target antibiotics in the water samples (Table 3), with partial regression coefficients of 1.28 and 1.136 (p < 0.01). Additionally, CTM, TMP, ETM, and CFX were the four predominant antibiotics influencing the TCs of the target antibiotics in the sediment samples (Table 4), with partial regression coefficients of 6.484, 2.042, 0.857, and 1.107, respectively (p < 0.05).
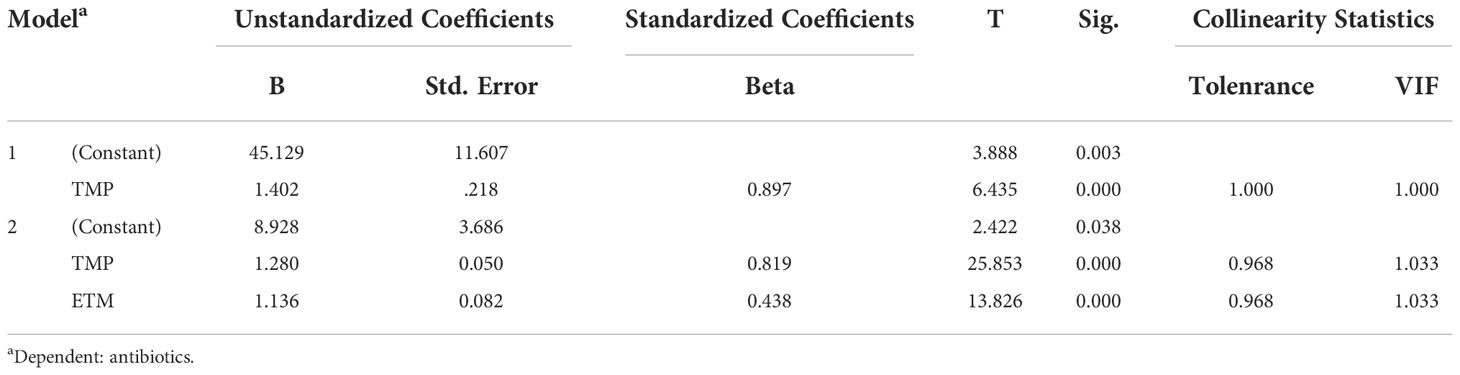
Table 3 The results of multiple linear regression analysis between antibiotics and environmental factors in water samples.
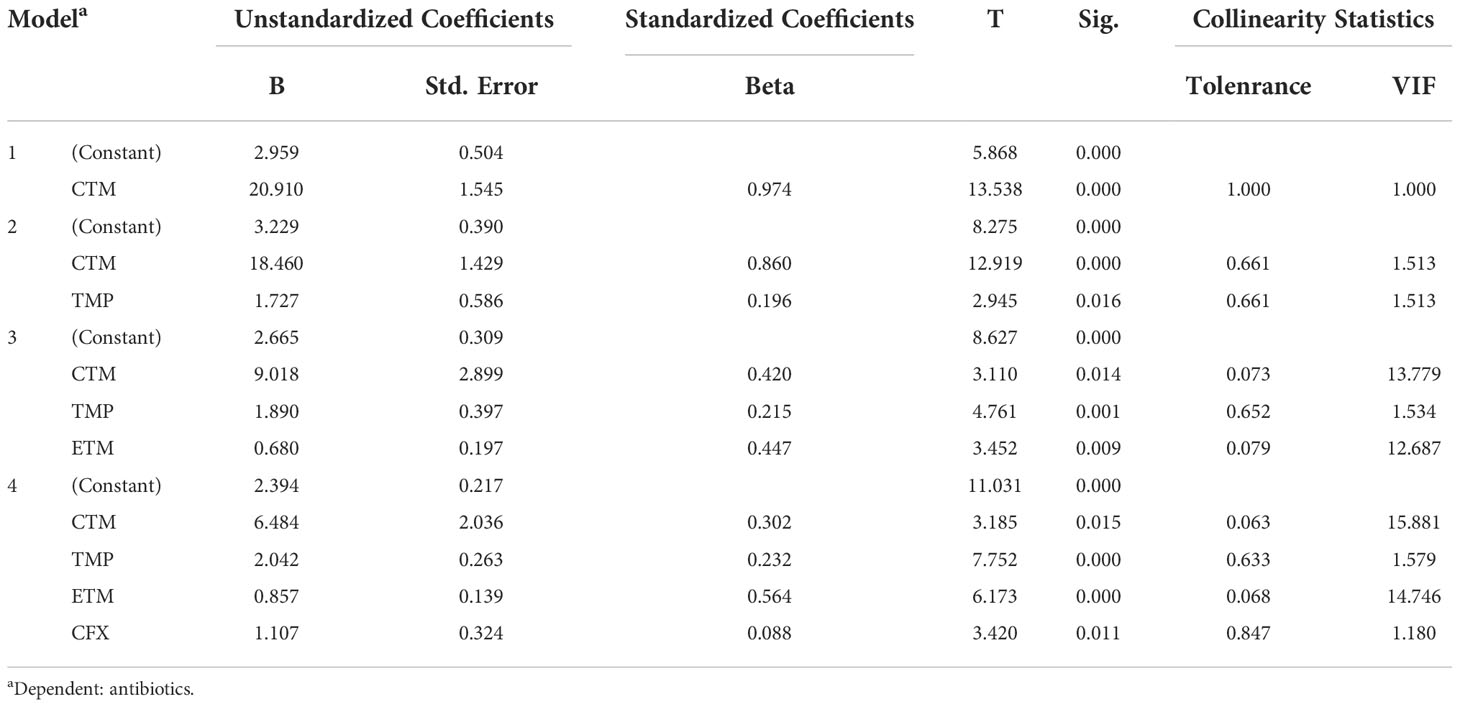
Table 4 The results of multiple linear regression analysis between antibiotics and environmental factors in sediment samples.
Discussion
Temporal variations and high concentrations of antibiotics in the bay posing risks to the marine ecosystem
Our study highlights the seasonal variations in antibiotics in Dapeng Cove for each sampling site. The TCs of antibiotics from samples collected in the dry season (first and second sampling events) were significantly higher than those of samples collected in the wet season (third sampling event) (p < 0.05). Furthermore, samples collected in the dry season exhibited the highest antibiotic concentrations, consistent with the results obtained by Lu et al. (2020) and Kafaei et al. (2018). The lower concentration of antibiotics in samples collected during the wet season could be attributed to dilution by runoff.
Consistent with the findings of Liu et al. (2018), we observed low levels of quinolone antibiotics in the water (0.47 ng/L) and sediment (1.29 ng/g) samples as they are less used. This finding is contrary to the findings of He et al. (2019) and Kafaei et al. (2018), who recorded norfloxacin as the dominant antibiotic in seawater from Chesapeake Bay (94.1 ng/L) and the Persian Gulf (89.43 ng/L); this can be attributed to the high local consumption of norfloxacin in both human and veterinary medicine. In addition, contrary to their findings (Kafaei et al., 2018; He et al., 2019), TMP was the most abundant antibiotic (184.47 ng/L) in our samples, which was lower than that observed by Zhang et al. (2012), who recorded a maximum TMP concentration of 13.6 μg/L in water from Laizhou Bay, China. Notably, in the same bay, Lu et al. (2022) reported a 69–289 ng/L concentration of antibiotics in seawater samples. In water from Hailing Bay, China, (Chen et al., 2015a) observed that the concentration of oxytetracycline was the highest (15,163 ng/L) among all identified antibiotics, while Qin et al. (2018) found TCs of antibiotics in Gonghu Bay, China, of 1,320 to 17,209 ng/L.
In our study, the levels of antibiotics in water from Dapeng Cove were much lower than those reported in the aforementioned studies. The high concentration of TMP recorded in our samples may exert selective pressure on microbes in the seawater of Dapeng Cove, leading to a relatively high risk of antibiotic resistance in the marine ecosystem of this region (Han et al., 2021a; Han et al., 2021b). Liu et al. (2017) reported that TMP can be biomagnified in the food chain; therefore, attention should be paid to the high concentration of TMP in the bay.
Similar to the findings of Liu et al. (2022), we recorded high concentrations of ETM and RTM in both the water and sediment samples, with macrolide antibiotics being predominant. In addition, ETM had the highest RQ values (0.43–7.91), indicating a medium to high risk to the marine ecosystem of Dapeng Cove, followed by CTM and RTM. Although TMP was the most abundant antibiotic in water samples, its risk to the marine ecosystem of Dapeng Cove was low to medium. These results indicate that macrolide antibiotics present a considerable ecological risk to the environment of Dapeng Cove. Furthermore, multiple linear regression analysis revealed that ETM and CTM were the predominant antibiotics influencing the TCs of antibiotics in our study. Thus, our findings, in combination with those of previous studies, indicate that ETM and CTM could be used as risk indicators for antibiotic contamination and ecological health in the Dapeng Cove ecosystem.
Influencing factors, distribution, and sources of antibiotics
Chen et al. (2015b) found that the levels of COD and nitrite were positively correlated with the concentrations of antibiotics in seawater, while Li et al. (2022) reported positive correlations between the concentrations of antibiotics and SS, pH, and ammonia. In our study, we observed positive correlations between some environmental factors (nitrite, ammonia, phosphate, TP, TOC, DOC, and SS) and the concentrations of antibiotics, suggesting that certain environmental factors play an important role in the distribution of antibiotics in seawater.
Regarding the different PPC values for antibiotics, Cheng et al. (2016) reported PPC values ranging from 10 to 591 L/kg in both seawater and sediment samples from the intertidal zone of Bohai Bay, China, while Li et al. (2022) recorded PPC values of 28 to 3,814 and 21 to 2,405 L/kg for sulfadiazine and azithromycin, respectively. Furthermore, Jiang et al. (2014) reported a PPC value of 349 L/kg for ETM in water from the Wangyang River, north China, while in our study, RTM had the largest PPC value (99.58–2,890.51 L/kg) for both the sediment and water samples, followed by ETM (12.23–1,295.04 L/kg) and CTM (11.64–548.67 L/kg). The different PPC values for antibiotics between our study and previous studies may be attributed to the physical and chemical properties of the sampled sediment, as well as the different sorption affinities of the antibiotics (Gao et al., 2012; Jiang et al., 2014).
In our study, the TCs of antibiotics in seawater samples collected from the OB were the lowest among the samples collected across all seasons, and samples from this area were clustered alone in the RDA. This may be due to the higher water exchange and the sampling point being located far away from the antibiotic contamination source. The effluent of wastewater treatment plants can be considered a primary source of antibiotic contamination for water bodies (Kafaei et al., 2018). Domestic sewage is the influent of municipal sewage plants. Notably, in our study, domestic sewage had the highest TC of antibiotics across all samples. Consistent with the findings of Zhang et al. (2012), in our study, the TCs of target antibiotics from DS and IB samples were significantly higher than those of samples from the OB (p < 0.05). Additionally, high levels of ecological risk were identified for DS and IB. However, as IB was close to residential areas, it experienced less water exchange. In the RDA, DS and IB were clustered close together, but separately from the remaining samples. Within this context, several studies have shown domestic sewage to be responsible for high concentrations of antibiotics in bay ecosystems (Chen et al., 2015b; Han et al., 2021a; Liu et al., 2022). This finding is supported by our results, indicating that domestic sewage is the most dominant source of antibiotics in bay environments.
Conclusions
Our study focused on the sources, concentrations, temporal variations, and ecological risks associated with antibiotics in water and sediment samples from Dapeng Cove, Shenzhen, South China, during the wet and dry seasons. In total, eight and ten different types of antibiotics were found in the water and sediment samples, respectively. Of the detected antibiotics, TMP was the most abundant in the water samples, while ETM was the most abundant in the sediment samples. Domestic sewage and sediment from the domestic sewage area had the highest TCs of antibiotics, at 301.96 ng/L and 18.67 ng/g, respectively. In addition, RTM exhibited the highest PPC values of 99.58–2,890.51 L/kg, followed by ETM and CTM. Regarding ecological risk, ETM had a medium to high risk, with RQs of 0.43 to 7.91, followed by CTM and RTM. Positive correlations were observed between some environmental factors and the detected antibiotics. Multiple linear regression analysis revealed that TMP and ETM were the two antibiotics predominantly influencing the TCs of target antibiotics in water. The presence of antibiotics in IB and DS indicated high levels of ecological risk. Furthermore, according to RDA, the DS and IB samples were clustered close together, but separately from the remaining samples. The results of our study indicate that domestic sewage is the most important source of antibiotics in the seawater environment of Dapeng Cove. Our study furthers our understanding of the temporal variations and sources of antibiotics in bays to better protect their ecological health.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
HS and YC designed the research. WL, XH, YX, GW and WX collected samples and conducted experiments. HS and WL analyzed data. HS and YC wrote the paper. All authors reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Key R&D Program of Guangdong Province (2021B0202040001), Central Public-interest Scientific Institution Basal Research Fund, CAFS (NO. 2020TD54), the earmarked fund for CARS-48, Central Public-Interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (2021SD08), Guangdong Provincial Special Fund For Modern Agriculture Industry Technology Innovation Teams (2019KJ149), Rural Science and Technology Commissioner Program of Guangdong Province 2021 (KTP20210297).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1049794/full#supplementary-material
References
AgroPages (2021) Agricultural antibiotics hit 231,400 tons, with 229,200 tons sold in China last year. Available at: https://news.agropages.com/News/NewsDetail—39643.htm.
Cheng D. M., Xie Y. J., Yu Y. J., Liu X. H., Zhao S. N., Cui B. S., et al. (2016). Occurrence and partitioning of antibiotics in the water column and bottom sediments from the intertidal zone in the bohai bay, China. Wetlands 36, S167–S179. doi: 10.1007/s13157-014-0561-y
Chen H., Liu S., Xu X. R., Liu S. S., Zhou G. J., Sun K. Y., et al. (2015a). Antibiotics in typical marine aquaculture farms surrounding hailing island, south China: Occurrence, bioaccumulation and human dietary exposure. Mar. Pollut. Bull. 90, 181–187. doi: 10.1016/j.marpolbul.2014.10.053
Chen H., Liu S., Xu X. R., Zhou G. J., Liu S. S., Yue W. Z., et al. (2015b). Antibiotics in the coastal environment of the hailing bay region, south China Sea: Spatial distribution, source analysis and ecological risks. Mar. Pollut. Bull. 95, 365–373. doi: 10.1016/j.marpolbul.2015.04.025
EFSA (2017). ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. EFSA J. 15, 4872. doi: 10.2903/j.efsa.2017.4872
Gao P., Munir M., Xagoraraki I. (2012). Correlation of tetracycline and sulfonamide antibiotics with corresponding resistance genes and resistant bacteria in a conventional municipal wastewater treatment plant. Sci. Total Environ. 421-422, 173–183. doi: 10.1016/j.scitotenv.2012.01.061
Han Q. F., Song C., Sun X., Zhao S., Wang S. G. (2021a). Spatiotemporal distribution, source apportionment and combined pollution of antibiotics in natural waters adjacent to mariculture areas in the laizhou bay, bohai Sea. Chemosphere 279, 130381. doi: 10.1016/j.chemosphere.2021.130381
Han Q. F., Zhang X. R., Xu X. Y., Wang X. L., Yuan X. Z., Ding Z. J., et al. (2021b). Antibiotics in marine aquaculture farms surrounding laizhou bay, bohai Sea: Distribution characteristics considering various culture modes and organism species. Sci. Total Environ. 760, 143863. doi: 10.1016/j.scitotenv.2020.143863
He K., Hain E., Timm A., Tarnowski M., Blaney L. (2019). Occurrence of antibiotics, estrogenic hormones, and UV-filters in water, sediment, and oyster tissue from the Chesapeake bay. Sci. Total Environ. 650, 3101–3109. doi: 10.1016/j.scitotenv.2018.10.021
Hernando M. D., Mezcua M., Fernandez-Alba A. R., Barcelo D. (2006). Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69, 334–342. doi: 10.1016/j.talanta.2005.09.037
Jiang Y. H., Li M. X., Guo C. S., An D., Xu J., Zhang Y., et al. (2014). Distribution and ecological risk of antibiotics in a typical effluent-receiving river (Wangyang river) in north China. Chemosphere 112, 267–274. doi: 10.1016/j.chemosphere.2014.04.075
Kafaei R., Papari F., Seyedabadi M., Sahebi S., Tahmasebi R., Ahmadi M., et al. (2018). Occurrence, distribution, and potential sources of antibiotics pollution in the water-sediment of the northern coastline of the Persian gulf, Iran. Sci. Total Environ. 627, 703–712. doi: 10.1016/j.scitotenv.2018.01.305
Liu D., Xu Y. Y., Junaid M., Zhu Y. G., Wang J. (2022). Distribution, transfer, ecological and human health risks of antibiotics in bay ecosystems. Environ. Int. 158, 106949. doi: 10.1016/j.envint.2021.106949
Liu K., Yin X. F., Zhang D. L., Yan D. Y., Cui L. J., Zhu Z. G., et al. (2018). Distribution, sources, and ecological risk assessment of quinotone antibiotics in the surface sediments from jiaozhou bay wetland, China. Mar. Pollut. Bull. 129, 859–865. doi: 10.1016/j.marpolbul.2017.10.010
Liu K., Zhang D. L., Xiao X. T., Cui L. J., Zhang H. L. (2020). Occurrence of quinotone antibiotics and their impacts on aquatic environment in typical river-estuary system of jiaozhou bay, China. Ecotoxicol. Environ. Saf. 190, 109993. doi: 10.1016/j.ecoenv.2019.109993
Liu S. S., Zhao H. X., Lehmler H. J., Cai X. Y., Chen J. W. (2017). Antibiotic pollution in marine food webs in laizhou bay, north China: Trophodynamics and human exposure implication. Environ. Sci. Technol. 51, 2392–2400. doi: 10.1021/acs.est.6b04556
Li F. F., Wen D. H., Bao Y. Y., Huang B., Mu Q. L., Chen L. J. (2022). Insights into the distribution, partitioning and influencing factors of antibiotics concentration and ecological risk in typical bays of the East China Sea. Chemosphere 288, 132566. doi: 10.1016/j.chemosphere.2021.132566
Lu S., Lin C. Y., Lei K., Wang B. D., Xin M., Gu X., et al. (2020). Occurrence, spatiotemporal variation, and ecological risk of antibiotics in the water of the semi-enclosed urbanized jiaozhou bay in eastern China. Water Res. 184, 116187. doi: 10.1016/j.watres.2020.116187
Lu S., Lin C. Y., Lei K., Xin M., Gu X., Lian M. S., et al. (2022). Profiling of the spatiotemporal distribution, risks, and prioritization of antibiotics in the waters of laizhou bay, northern China. J. Hazardous Materials 424, 127487. doi: 10.1016/j.jhazmat.2021.127487
Qin Y. W., Wen Q., Ma Y. Q., Yang C. C., Liu Z. C. (2018). Antibiotics pollution in gonghu bay in the period of water diversion from Yangtze river to taihu lake. Environ. Earth Sci. 77, 419. doi: 10.1007/s12665-018-7558-4
Su H., Liu S., Hu X., Xu X., Xu W., Xu Y., et al. (2017). Occurrence and temporal variation of antibiotic resistance genes (ARGs) in shrimp aquaculture: ARGs dissemination from farming source to reared organisms. Sci. Total Environ. 607, 357–366. doi: 10.1016/j.scitotenv.2017.07.040
Van Boeckel T. P., Brower C., Gilbert M., Grenfell B. T., Levin S. A., Robinson T. P., et al. (2015). Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. 112, 5649–5654. doi: 10.1073/pnas.1503141112
Van Leeuwen K. (2003). Technical guidance document on risk assessment in support of commission directive 93/67/EEC on risk assessment for new notified substances and commission regulation (EC) no. 1488/94 on risk assessment for existing substances part II. Euro. Commun. 1–337.
Xing L. M., Liu H. F., Zhou J. G. (2020). Numerical study of the antibiotic transport and distribution in the laizhou bay, China. Environ. Sci. Pollut. Res. 27, 37760–37772. doi: 10.1007/s11356-020-09770-5
Xu J., Zhang Y., Zhou C. B., Guo C. S., Wang D. M., Du P., et al. (2014). Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from taihu lake, China. Sci. Total Environ. 497, 267–273. doi: 10.1016/j.scitotenv.2014.07.114
Zhang Q. Q., Ying G. G., Pan C. G., Liu Y. S., Zhao J. L. (2015). Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 49, 6772–6782. doi: 10.1021/acs.est.5b00729
Keywords: temporal variation, ecological risk, antibiotics, distribution, source
Citation: Su H, Li W, Hu X, Xu W, Xu Y, Wen G and Cao Y (2022) Temporal variations, distribution, ecological risks, and sources of antibiotics in the marine ecosystem of Dapeng Cove, Shenzhen, South China. Front. Mar. Sci. 9:1049794. doi: 10.3389/fmars.2022.1049794
Received: 21 September 2022; Accepted: 16 November 2022;
Published: 29 November 2022.
Edited by:
Hans Uwe Dahms, Kaohsiung Medical University, TaiwanReviewed by:
Jose-Luis Martinez-Guitarte, National University of Distance Education (UNED), SpainChenglian Feng, Chinese Research Academy of Environmental Sciences, China
Copyright © 2022 Su, Li, Hu, Xu, Xu, Wen and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yucheng Cao, Y3ljXzcxNUAxNjMuY29t
 Haochang Su1,2,3
Haochang Su1,2,3 Xiaojuan Hu
Xiaojuan Hu Wujie Xu
Wujie Xu Yucheng Cao
Yucheng Cao