
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 18 November 2022
Sec. Marine Biology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1034152
This article is part of the Research Topic Integration of Development, Physiology and Responses to Environmental Change in Aquatic Invertebrates View all 13 articles
Ammonia nitrogen plays a crucial part in oxidative stress in aquatic animals. To elucidate the effect of ammonia nitrogen stress on the superoxide dismutase (SOD) activity and interferon-induced transmembrane protein 1 (IFITM1) expression in the clam Cyclina sinensis, clams were exposed to ammonia nitrogen (8.07 mg/L) for 768 h (32 days) and then challenged with Vibrio parahaemolyticus. The results showed that the SOD activity in the hepatopancreas of C. sinensis exposed to ammonia nitrogen first increased and then decreased with time, returning to the control group’s normal level at 768 h. Following infection with V. parahaemolyticus, the SOD activity in the hepatopancreas fluctuated over time. The SOD activity in clams infected with V. parahaemolyticus at 144 h did not return to the control group’s normal level. The full-length cDNA of CsIFITM1 was 2,434 bases in length, including a 2,301-bp open reading frame (ORF) encoding 714 amino acids, with a putative molecular weight of 83.86 kDa. CsIFITM1 contains an RNA helicase domain (DEXHc_RLR, DR) and a Helicase_C (HC) domain. The transcriptional levels of CsIFITM1 were upregulated by exposure to ammonia nitrogen and were significantly higher from 6 to 768 h compared to the control (0 h) (p < 0.05). Following infection with V. parahaemolyticus, the transcript levels of CsIFITM1 in the hepatopancreas were upregulated and were significantly higher from 6 to 144 h, in contrast to those of the control (0 h) (p < 0.05). The present data provide the first evidence of the SOD activity and CsIFITM1 transcript levels being able to reflect the effect of ammonia on the clam C. sinensis.
As an unavoidable factor, ammonia nitrogen easily accumulates in the aquaculture system (Barbieri and Bondioli, 2013; Egnew et al., 2019). The accumulation of total ammonia nitrogen (TAN) in the water environment can ultimately lead to severe problems such as oxidative stress (Ge et al., 2022a), gill hyperplasia (Zuffo et al., 2021), inefficient feed utilization (Silva et al., 2018), and poor immune response of aquatic animals (Mangang and Pandey, 2021). As a result, some opportunistic bacteria such as Vibrio parahaemolyticus may cause serious vibriosis (Ni et al., 2020). In some cases, it could even lead to death in aquatic animals (Barbieri et al., 2019). Aquatic animals suffer from oxidative stress, which causes the accumulation of reactive oxygen species (ROS) (Ge et al., 2022a), and the scavenging capacity induces lipid peroxidation (Debbarma et al., 2021). To protect organisms from such oxidative stress, antioxidant enzymes are likely to be activated. Therefore, a change in the antioxidant enzyme activities is one of the reliable and sensitive tools for the evaluation of oxidation resistance in aquatic animals (Ni et al., 2020; Debbarma et al., 2021). Superoxide dismutase (SOD) is one of the key antioxidant enzymes (Ge et al., 2021). It can reduce oxidative damage by eliminating ROS and enhancing the antioxidant capacity (Sinha et al., 2015). Ammonia nitrogen exposure may activate the antioxidant system and alter the activities of antioxidant enzymes (Ghelichpour et al., 2019).
Interferon (IFN) is one of the major multifunctional cytokines that play vital roles in the innate immune response in aquatic animals (Wan and Chen, 2008; Zhang et al., 2020). Among the IFN-responsive genes that collectively regulate the multifunctional effects of IFNs is the interferon-induced transmembrane (IFITM) protein family (Johnson et al., 2006; Wan and Chen, 2008). Members of the IFITM family are likely to be expressed basally in various tissues and cells. They may play a crucial part in the promotion and maintenance of the pluripotent state of an organism’s cells (Johnson et al., 2006). All of the IFITM proteins share a conserved short topology, two transmembrane (TM) domains, and highly variable amino and carboxy termini (Zhang et al., 2020). Thus far, in aquatic animals, IFITM1, IFITM2, IFITM3, and IFITM5 have been annotated in the fish genome (Johnson et al., 2006). The IFITM family comprises the known innate immune effectors involved in the regulation of immunoreaction, such as endocytosis, immune cell signaling, cell physiology, and antioxidative damage (Baird et al., 2001; Zhu et al., 2013). When organisms are under oxidative stress, they may synthesize some proteins, such as IFNs, interleukins, heat shock proteins, and the IFITM proteins (Ghelichpour et al., 2019). In humoral immunity, the transcriptional levels of members of the IFITM family can reflect the current immune status of aquatic animals (Johnson et al., 2006).
As one of the most important economic bivalves, the clam Cyclina sinensis is widely distributed in the coastal areas of East Asia, and the clam industry is growing rapidly (Ge et al., 2022b). The clam grows fast, tastes delicious, and has great market demand (Ge et al., 2021; Liao et al., 2022). The clam is highly adaptable to ammonia nitrogen (Ni et al., 2022). However, when the accumulation of ammonia nitrogen reaches the threshold level, this can have serious effects on the clam, including oxidative stress and poor immune response (Ni et al., 2021; Ge et al., 2022a). Therefore, it is essential to evaluate the effects of ammonia nitrogen on antioxidant enzymes and immunoreaction (Chai et al., 2022). Because a change in the SOD activity and IFITM1 expression level can reflect the current immune status of aquatic animals, we assessed the SOD activity and the IFITM1 transcription response in clams exposed to chronic ammonia nitrogen and following infection with V. parahaemolyticus. The present study provides a theoretical basis for the research on the detoxification mechanisms in marine animals.
C. sinensis (2.99 ± 0.45 g each) from Lianyungang Zhongchuang Aquaculture Company were transported to the experimental base of Jiangsu Ocean University. To acclimate the clams to laboratory conditions, they were stored in concrete tanks (0.8 m × 0.8 m × 0.5 m) with 200 L aerated seawater for 10 days. During the acclimation and the experiment, the seawater temperature was maintained at 24 ± 0.5°C, with pH at 8.0 ± 0.4, dissolved oxygen (DO) ≥ 4.9 mg/L, and TAN < 0.09 mg/L (Chen et al., 2021). Clams were fed twice daily with a mixture of alive microalgae (Isochrysis zhangjiangensis and Nannochloropsis oceanica) at a density of 2 × 104 cells/ml.
Six clams were dissected and various tissues collected, which were then frozen in liquid nitrogen for RNA extraction (Ni et al., 2022).
Seven hundred and twenty selected clams were randomly stored in six concrete tanks (200 L water) at a density of 120 clams per tank, with three replicates for each treatment. According to the 96-h median lethal concentration (LC50-96 h) TAN for C. sinensis (Ni et al., 2022), the TAN level in the experimental group was set at 8.07 mg/L. To achieve the designed level of TAN, a stock solution of NH4Cl (1.0 g/L) was used. The control group was natural seawater. The other management conditions were the same as those used during the temporary rearing period, and the stress experiment was carried out for 768 h. During the test, the seawater was changed twice daily to maintain the concentration of TAN.
Three individuals in each treatment were randomly selected at different time points after the clams were exposed to ammonia nitrogen (0, 3, 6, 12, 24, 48, 96, 192, 384, and 768 h). The hepatopancreas was collected and then frozen in liquid nitrogen for the SOD activity analysis and total RNA isolation. The SOD activity was determined using kits purchased from Nanjing Jiancheng Bioengineering Institute (Ge et al., 2022a).
To determine the effect of ammonia nitrogen stress on the disease resistance of the clam, Vibrio challenge tests were further performed. After the ammonia nitrogen stress experiment, clams in the experimental group were transferred into the normal seawater tank with three replicates and challenged with V. parahaemolyticus. The clams were immersed and infected with V. parahaemolyticus at a level of 1 × 107 CFU/ml for 1 week (Ni et al., 2020). During the infection experiment, to achieve the level of V. parahaemolyticus (1 × 107 CFU/ml), all of the seawater was replaced twice daily. The control group was natural seawater without the addition of V. parahaemolyticus.
Three individuals in each treatment were randomly selected at different time points during the infection experiment (0, 3, 6, 12, 24, 48, 96, 120, and 144 h). The hepatopancreas was collected and then frozen in liquid nitrogen for the SOD activity analysis and total RNA isolation.
The CDS sequence of the IFITM1 gene was derived from the clam whole-genome sequencing complementary DNA (cDNA) library (Wei et al., 2020). To clone the full-length cDNAs, rapid amplification of cDNA ends PCR (RACE-PCR) was conducted (Ni et al., 2022). The primers required for IFITM1 gene cloning are shown in Table 1. The IFITM1 sequence was verified by DNA sequencing and analyzed using the BLAST program (Zhang et al., 2020). The NCBI database was used to predict the ORF of the IFITM1 gene. Sequence homology retrieval and alignment were also performed. The ExPASy ProtParam program was utilized to predict the molecular weight and isoelectric points of the IFITM1 protein. According to a previous report, multiple sequence alignments were generated and a phylogenetic tree was constructed (Ni et al., 2022).
To analyze the transcriptional level of IFITM1 messenger RNA (mRNA), quantitative real-time PCR (qRT-PCR) was carried out with the SYBR method (Ge et al., 2022b). The primers required for qRT-PCR are shown in Table 2. Each sample was in triplicate. The 2−ΔΔCt method was applied to calculate the mRNA transcriptional level with β-actin as the internal control (Ge et al., 2022b).

Table 2 Specific primers for the quantitative real-time PCR (qRT-PCR) of IFITM1 from Cyclina sinensis.
Data were analyzed with one-way ANOVA using SPSS. 18 (Ge et al., 2019). Duncan’s multiple comparison tests were performed when significant differences were detected in the ANOVA. Difierences were considered statistically significant when p < 0.05 (Zhang et al., 2020).
The SOD activity in the hepatopancreas of C. sinensis exposed to ammonia nitrogen first increased and then decreased with time (Figure 1A). The SOD activity in the experimental group increased to the maximum value at 96 h. No significant difference was found at 24 and 48 h (p > 0.05), whereas the SOD activity was significantly higher than that at other time points (p < 0.05). At 768 h, the SOD activity returned to the control group’s normal level. Following infection with V. parahaemolyticus, the SOD activity fluctuated with time (Figure 1B). The SOD activity from 3 to 144 h in the clams infected with V. parahaemolyticus was significantly higher than that in the control group (p < 0.05).

Figure 1 Superoxide dismutase (SOD) activity in the hepatopancreas tissue of Cyclina sinensis under long-term ammonia. (A) SOD activity in C sinensis exposed to long-term ammonia. (B) SOD activity in C sinensis infected with Vibrio parahaemolyticus. The control group in (A) comprised clams raised in natural seawater. The control group in (B) included clams raised in natural seawater without the addition of V. parahaemolyticus. The same lowercase letters indicate non-significant differences between the different stress time points (p > 0.05); otherwise, the difference is significant (p < 0.05).
As shown in Figure 2, the full-length cDNA of CsIFITM1 was 2,434 bases in length, including a 5′ untranslated region (UTR) of 34 bp and a 3′-UTR of 99 bp with a poly(A) sequence. It contained a 2,301-bp ORF encoding 714 amino acids, with a putative molecular weight of 83.86 kDa and a theoretical isoelectric point of 11.18. The CsIFITM1 protein contained putative transmembrane domains, but did not contain a signal peptide (Figure 3). Multiple sequence alignment showed that it contained an RNA helicase domain (DEXHc_RLR, DR) located at amino acid residues 70–265 and a Helicase_C (HC) domain located at amino acid residues 468–564 (Figure 4). Phylogenetic analysis showed that CsIFITM1 formed a cluster with the IFITM1 of Mercenaria mercenaria, Crassostrea gigas, Crassostrea virginica, Dreissena polymorpha, and Mytilus edulis, but not with the IFITM1 of Haliotis rufescens, Haliotis rubra, and Pomacea canaliculata (Figure 5). Expression analysis revealed that the CsIFITM1 mRNA was constitutively expressed in the adductor muscle, mantle, gill, axon foot, hepatopancreas, and gonad, with higher levels of mRNA detected in the hepatopancreas and the adductor muscle (Figure 6).

Figure 2 The cDNA of IFITM1 and the deduced amino acid sequence of Cyclina sinensis. The yellow part indicates the start codon ATG and the stop codon TAA, the underlined area indicates the open reading frame (ORF), and the green part indicates the conserved domain of IFITM1.
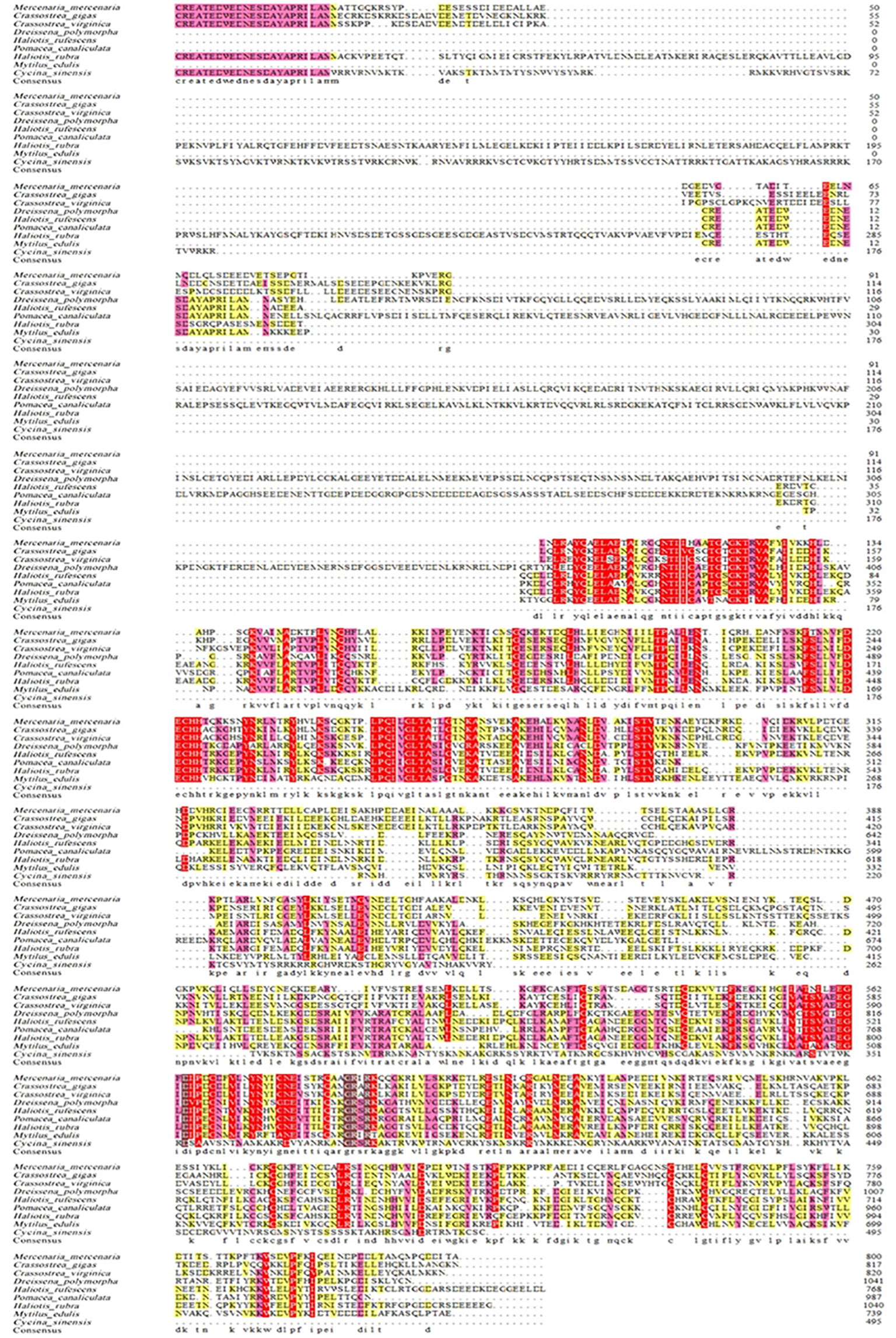
Figure 4 Alignment of the IFITM1 amino acids from Cyclina sinensis and other species. All shaded regions represent residues sharing homology. The light red regions represent homology above 75%, while the dark red regions represent 100% homology. Dots denote amino acid deletion.
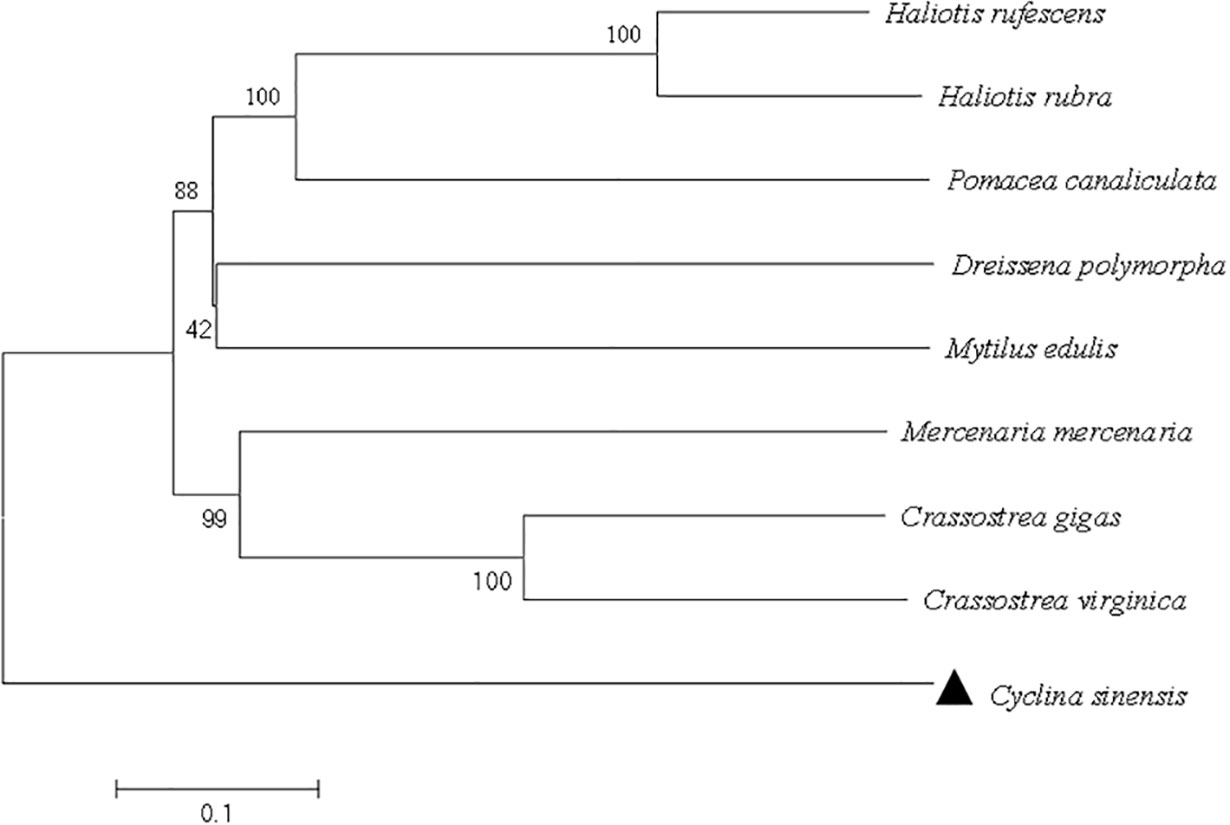
Figure 5 Phylogenetic tree derived from multiple alignment of the IFITM1 amino acids from Cyclina sinensis and other species.
The transcript levels of CsIFITM1 in the hepatopancreas of the clams exposed to ammonia nitrogen were determined at different time points after exposure using qRT-PCR (Figure 7A). Exposure to ammonia nitrogen upregulated the transcriptional levels of CsIFITM1, which reached the peak at 192 h post-exposure. The expression levels of CsIFITM1 were significantly higher from 6 to 768 h in clams exposed to ammonia nitrogen than those of the control (0 h) (p < 0.05).

Figure 7 Expression of the IFITM1 gene in the hepatopancreas tissue of Cyclina sinensis. (A) Gene expression in C sinensis exposed to long-term ammonia. (B) Gene expression in C sinensis infected with Vibrio parahaemolyticus. Different letters in the column indicate significant difference (p < 0.05).
Following infection with V. parahaemolyticus, the transcript levels of CsIFITM1 in the hepatopancreas were upregulated (Figure 7B). The transcriptional levels of CsIFITM1 were also upregulated by V. parahaemolyticus infection, which reached the peak at 48 h post-infection. The transcript levels of CsIFITM1 in clams infected with V. parahaemolyticus from 6 to 144 h were significantly higher, in contrast to those of the control (0 h) (p < 0.05).
The accumulation of ammonia nitrogen in water can ultimately lead to serious oxidative stress (Barbieri and Bondioli, 2013). As one of the key antioxidant enzymes, SOD can reduce oxidative damage (Ge et al., 2021). In the current study, the SOD activity in the hepatopancreas of C. sinensis exposed to ammonia nitrogen first increased and then decreased with time. This may be because aquatic animals have to activate antioxidant enzymes in order to deal with oxidative stress (Ni et al., 2022). This result showed that exposure to ammonia nitrogen could activate the SOD in the clam, and activating SOD can reduce the oxidative damage induced by ammonia nitrogen stress. At 768 h, the SOD activity returned to the control group’s normal level, which is probably due to the toxicity of the low level of ammonia nitrogen (8.07 mg/L) being low. On the other hand, the 768-h recovery period may have been adequate to compensate for the SOD activity in clams exposed to ammonia nitrogen (Ni et al., 2022). This indicated that the SOD activity in clams exposed to ammonia nitrogen (8.07 mg/L) for 768h could return to normal levels. Bacterial infection can cause serious oxidative stress (Kumar, 2021). In the current study, the SOD activity from 3 to 144 h in the clams infected with V. parahaemolyticus was significantly higher than that in the control. This showed that SOD can be activated in clams infected with V. parahaemolyticus. The SOD activity in clams infected with V. parahaemolyticus for 144 h did not return to the control group’s normal level. The reason may be that the bacterial infection may have caused irreparable damage to the antioxidant system, or it could be due to the 144-h recovery time being too short for the recovery of the SOD activity (Ge et al., 2022a).
In the current study, we cloned the IFITM1 gene from the clam C. sinensis. The full-length cDNA of CsIFITM1 was 2,434 bp in length, including a 2,301-bp ORF encoding 714 amino acids, with a putative molecular weight of 83.86 kDa. The CsIFITM1 protein possessed putative transmembrane domains, which showed that the protein contained the typical structural features of IFITMs (Wan and Chen, 2008). This indicated that CsIFITM1 may be a cell surface molecule (Tanaka et al., 2005). As is well known, members of the IFITM family are expressed in various cells in mammals (Bailey et al., 2014). In the present study, the expression analysis showed that, although at a different level, the CsIFITM1 mRNA was constitutively broadly expressed in all the selected tissues, indicating that the IFITM1 gene may be widely distributed among tissues and is expressed in various cells in C. sinensis. This is probably because immune cells (including leukocytes and lymphocytes) are widely distributed in various tissues (Desai et al., 2017).
The IFITM1 gene in mammals is induced by IFNs, and the mRNA transcript level of this gene can increase as much as 100-fold upon induction by IFNs (Wan and Chen, 2008). In the present study, the transcript levels of CsIFITM1 were upregulated by exposure to ammonia nitrogen and were significantly higher from 6 to 768 h in the clams exposed to ammonia nitrogen compared to the control. This result indicated that the gene expression of CsIFITM1 could be induced by ammonia nitrogen stress. This is probably because ammonia nitrogen exposure can stimulate the CsIFITM1 gene (Tanaka et al., 2005) or induce IFNs (Zou et al., 2005). Following infection with V. parahaemolyticus, the transcript levels of CsIFITM1 in hepatopancreas were upregulated, indicating that the gene expression of CsIFITM1 can be induced by infection with V. parahaemolyticus, suggesting that the CsIFITM1 gene may be involved in the immune response of C. sinensis. This observation is consistent with the reported role of IFITM in the large yellow croaker (Larimichthys crocea), which can be stimulated by Vibrio alginolyticus (Zhang et al., 2021).
In summary, exposure to ammonia nitrogen can activate the SOD in the clam, and activating SOD can reduce the oxidative damage induced by ammonia nitrogen stress. Furthermore, infection with V. parahaemolyticus may cause irreparable damage to the antioxidant system. The IFITM1 gene from C. sinensis was cloned, and the CsIFITM1 mRNA was broadly expressed in all the tissues selected. The CsIFITM1 protein possessed the typical structural features of members of the IFITM family. The gene expression of CsIFITM1 can be induced by ammonia nitrogen stress and V. parahaemolyticus infection.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
HG: Conceptualization, methodology, and writing—review and editing. QN: Formal analysis and writing—original draft. JL: Software, validation, and investigation. ZD: Project administration, conceptualization, and funding acquisition. SC: Investigation. All authors contributed to the article and approved the submitted version.
This research was supported by the earmarked fund for CARS (CARS-49); The “JBGS” Project of Seed Industry Revitalization in Jiangsu Province (JBGS[2021]034); China’s National Key Research and Development Projects (2019YFD0900403); Open-End Funds of Jiangsu Key Laboratory of Marine Bioresources and Environment (SH20211209); Jiangsu Agricultural Science and technology Independent Fund (CX(20)3150); and the Priority Academic Program Development of Jiangsu. The funding bodies played a key role in the design of the study, the collection, analysis, and interpretation of data, and in the writing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bailey C., Zhong G., Huang I. C., Farzan M. (2014). IFITM-family proteins: the cell’s first line of antiviral defense. Annu. Rev. Virol. 1, 261–283. doi: 10.1146/annurev-virology-03141-085537
Baird J., Ryan K., Hayes I., Hampson L., Heyworth C., Clark A., et al. (2001). Differentiating embryonal stem cells are a rich source of haemopoietic gene products and suggest erythroid preconditioning of primitive haemopoietic stem cells. J. Biol. Chem. 276, 9189–9198. doi: 10.1074/jbc.M008354200
Barbieri E., Bondioli A. C. V. (2013). Acute toxicity of ammonia in pacu fish (Piaractus mesopotamicus, holmberg 1887) at different temperatures levels. Aquac. Res. 46, 565–571. doi: 10.1111/are.12203
Barbieri E., Lenz R. M., de Nascimento A. A., de Almeida G. L., Roselli L. Y., Henriques M. B. (2019). Lethal and sublethal effects of ammonia in Deuterodon iguape (Eigenmann 1907), potential species for brazilian aquaculture. Bol. Inst. Pesca 45, 1–7. doi: 10.20950/1678-2305.2019.45.1.440
Chai Y., Peng R., Jiang M., Jiang X., Han Q., Han Z. (2022). Effects of ammonia nitrogen stress on the blood cell immunity and liver antioxidant function of Sepia pharaonic. Aquaculture 546, 737417. doi: 10.1016/j.aquaculture.2021.737417
Chen Y., Li H., Ding H., Dong Z., Li J. (2021). Heritability estimation and path analysis for growth traits of the razor clam sinonovacula constricta under high salinity. Aquaculture 545, 737175. doi: 10.1016/j.aquaculture.2021.737175
Debbarma R., Biswas P., Singh S. (2021). An integrated biomarker approach to assess the welfare status of Ompok bimaculatus (Pabda) in biofloc system with altered C/N ratio and subjected to acute ammonia stress. Aquaculture 545, 737184. doi: 10.1016/j.aquaculture.2021.737184
Desai T., Marin M., Mason C., Melikyan G. (2017). pH regulation in early endosomes and interferon-inducible transmembrane proteins control avian retrovirus fusion. J. Biol. Chem. 292 (19), 7817–7827. doi: 10.1074/jbc.M117.783878
Egnew N., Renukdas N., Ramena Y., Yadav A. K., Kelly A. M., Lochmann R. T., et al. (2019). Physiological insights into largemouth bass (Micropterus salmoides) survival during long-term exposure to high environmental ammonia. Aquat. Toxicol. 207, 72–82. doi: 10.1016/j.aquatox.2018.11.027
Ge H., Liang X., Liu J., Cui Z., Guo L., Li L, et al. (2021). Effects of acute ammonia exposure on antioxidant and detoxification metabolism in clam Cyclina sinensis. ecotox. Environ. Safe. 211, 111895. doi: 10.1016/j.ecoenv.2021.111895
Ge H., Liu J., Ni Q., Wang F., Dong Z. (2022a). Effects of acute ammonia exposure and post-exposure recovery on nonspecific immunity in clam Cyclina sinensis. isr. J. Aquacul. Bamid. 74, 1552688. doi: 10.46989/001c.32647
Ge H., Ni Q., Li J., Li J., Chen Z., Zhao F. (2019). Integration of white shrimp (Litopenaeus vannamei) and green seaweed (Ulva prolifera) in minimum-water exchange aquaculture system. J. Appl. Phycol. 31 (1), 1425–1432. doi: 10.1007/s10811-018-1601-4
Ge H., Shi J., Liu J., Guo L., Dong Z. (2022b). Combined analysis of mRNA-miRNA reveals the regulatory roles of miRNAs in the metabolism of clam Cyclina sinensis hepatopancreas during acute ammonia nitrogen stress. Aquacul. Res. 53 (4), 1492–1506. doi: 10.1111/are.15683
Ghelichpour M., Mirghaed A., Hoseinifar S., Khalili M., Yousefid M., Doan H., et al. (2019). Expression of immune, antioxidant and stress related genes in different organs of common carp exposed to indoxacarb. Aquat. Toxicol. 208, 208–216. doi: 10.1016/j.aquatox.2019.01.011
Johnson M., Sangrador-Vegas A., Smith T., Cairns M. (2006). Cloning and characterization of two genes encoding rainbow trout homologues of the IFITM protein family. Vet. Immunol. Immunop. 110, 357–362. doi: 10.1016/j.vetimm.2005.12.007
Kumar N. (2021). Dietary riboflavin enhances immunity and anti-oxidative status against arsenic and high temperature in Pangasianodon hypophthalmus. Aquaculture 533, 736209. doi: 10.1016/j.aquaculture.2020.736209
Liao X., Sun Z., Cui Z., Yan S., Fan S., Xia Q., et al. (2022). Effects of different sources of diet on the growth, survival, biochemical composition and physiological metabolism of clam (Cyclina sinensis). Aquacul. Res. 53 (10), 3797–3806. doi: 10.1111/are.15886
Mangang Y., Pandey P. (2021). Hemato-biochemical responses and histopathological alterations in the gill and kidney tissues of Osteobrama belangeri (Valencienne) exposed to different sub-lethal unionized ammonia. Aquaculture 542, 736887. doi: 10.1016/j.aquaculture.2021.736887
Ni Q., Li W., Jia X., Dong Z., Ge H. (2020). Effect of salinity on growth performance and resistance of the clam Cyclina sinensis against Vibrio parahaemolyticus infection. Isr. J. Aquacul. Bamid. 72, 1124924. doi: 10.46989/IJA.72.2020.1124924
Ni Q., Li W., Liang X., Liu J., Ge H., Dong Z. (2021). Gill transcriptome analysis reveals the molecular response to the acute low-salinity stress in Cyclina sinensis. Aquaculture Rep. 19, 100564. doi: 10.1016/j.aqrep.2020.100564
Ni Q., Wang D., Xie L., Ge H., Dong Z. (2022). Unraveling the characterization of hypoxia-inducible factor-1α (HIF-1α) and antioxidant enzymes in clam Cyclina sinensis in response to hypoxia. Aquaculture Res. 16062. doi: 10.1111/are.16062
Silva M. J., Dos S., Da Costa F. F. B., Leme F. P., Takata R., Costa D. C., et al. (2018). Biological responses of neotropical freshwater fish lophiosilurus alexandri exposed to ammonia and nitrite. Sci. Total Environ., 616-617, 1566–1575. doi: 10.1016/j.scitotenv.2017.10.157
Sinha A. K., Zinta G., AbdElgawad H., Asard H., Blust R., De Boeck G. (2015). High environmental ammonia elicits differential oxidative stress and antioxidant responses in five different organs of a model estuarine teleost (Dicentrarchus labrax). Comp. Biochem. Physiol. C 174, 21–31. doi: 10.1016/j.cbpc.2015.06.002
Tanaka S. S., Yamaguchi L., Tsoi B., Lickert H., Tam P. P. L. (2005). IFITM/Mil/Fragilis family proteins IFITM1 and IFITM3 play distinct roles in mouse primordial germ cell homing and repulsion. Dev. Cell 9, 745–756. doi: 10.1016/j.devcel.2005.10.010
Wan X., Chen X. (2008). Molecular cloning and expression analysis of interferon-inducible transmembrane protein 1 in large yellow croaker Pseudosciaena crocea. vet. Immunol. Immunop. 124, 99–106. doi: 10.1016/j.vetimm.2008.01.004
Wei M., Ge H., Shao C., Yan X., Nie H., Duan H., et al. (2020). Chromosome-level genome assembly of the Venus clam, Cyclina sinensis, helps to elucidate the molecular basis of the adaptation of buried life. iScience 23 (6), 101148. doi: 10.1016/j.isci.2020.101148
Zhang Y., Huang Y., Wang L., Huang L., Zheng J., Huang X., et al. (2020). Grouper interferon-induced transmembrane protein 3 (IFITM3) inhibits the infectivity of iridovirus and nodavirus by restricting viral entry. Fish Shellfish Immun. 104, 172–181. doi: 10.1016/j.fsi.2020.06.001
Zhang W., Zhu C., Chi H., Liu X., Gong H., Xie A., et al. (2021). Early immune response in large yellow croaker (Larimichthys crocea) after immunization with oral vaccine. Mol. Cell. Prob. 56, 101708. doi: 10.1016/j.mcp.2021.101708
Zhu R., Wang J., Lei X., Gui J., Zhang Q. (2013). Evidence for Paralichthys olivaceus IFITM1 antiviral effect by impeding viral entry into target cells. Fish Shellfish Immun. 35, 918–926. doi: 10.1016/j.fsi.2013.07.002
Zou J., Carrington A., Collet B., Dijkstra J. M., Yoshiura Y., Bols N., et al. (2005). Identification and bioactivities of IFN-gamma in rainbow trout (Oncorhynchus mykiss): the first Th1-type cytokine characterized functionally in fish. J. Immunol. 175 (4), 2484–2494. doi: 10.4049/jimmunol.175.4.2484
Keywords: cyclina sinensis, ammonia nitrogen exposure, vibrio challenge, SOD activity, IFITM1 gene
Citation: Ge H, Ni Q, Liu J, Dong Z and Chen S (2022) Effect of chronic ammonia nitrogen stress on the SOD activity and interferon-induced transmembrane protein 1 expression in the clam Cyclina sinensis. Front. Mar. Sci. 9:1034152. doi: 10.3389/fmars.2022.1034152
Received: 01 September 2022; Accepted: 27 September 2022;
Published: 18 November 2022.
Edited by:
Yafei Duan, South China Sea Fisheries Research Institute, ChinaCopyright © 2022 Ge, Ni, Liu, Dong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiguo Dong, ZHpnNzcxMkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.