
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Mar. Sci. , 14 September 2022
Sec. Marine Ecosystem Ecology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1029027
This article is part of the Research Topic The Effects of Environmental Change on Anchialine Ecosystems View all 5 articles
Editorial on the Research Topic
The effects of environmental change on anchialine ecosystems
Anchialine ecosystems comprise interconnected groundwater habitats at the land-sea aquatic continuum within karstic and volcanic geological settings. Here, crevicular and cavernous environments are flooded by the subterranean estuary, the region of coastal aquifers where seawater and terrestrial-borne freshwaters mix (Moore, 1999), creating globally dispersed habitats for characteristic aquatic fauna with subterranean adaptations (Bishop et al., 2015; van Hengstum et al., 2019). These cave-adapted organisms are primarily invertebrates, often endemic, with metabolic, physiologic, and morphologic adaptations that allow them to thrive in dark and energy-limited environments. Historically, these habitats have been considered particularly stable environments (e.g., Sket, 1996). However, there is growing evidence that the functioning of anchialine ecosystems is greatly influenced by external meteorological, hydrological, and oceanic conditions that closely link them with adjacent terrestrial and marine habitats (e.g., Brankovits et al., 2018; Tamalavage et al., 2018). For all these reasons, anchialine ecosystems may be more susceptible to short- and long-term effects of environmental change than previously thought.
Organic matter availability is pivotal to the functioning of freshwater, estuarine, or marine habitats, because it regulates microbial community structure and dissolved oxygen concentrations in the water column and the sediments (e.g., Howarth et al., 2011). Anchialine ecosystems are typically oligotrophic environments with low dissolved oxygen content and, therefore, they can easily transition into anoxic eutrophic habitats when organic matter inputs increase from either terrestrial or marine sources (e.g., at sinkholes, cenotes, or other cave openings) (Pohlman, 2011). The inputs, composition, and bioavailability of organic matter are sensitive to changes in nearby surface habitats, human activities and pressures, tidal fluctuations, seasonal changes in precipitation, and extreme weather events, such as hurricanes and tropical storms (e.g., Brankovits et al., 2021).
This Research Topic was established with the aim to bring together the most recent outcomes and advances from a variety of scientific disciplines that link spatial and temporal changes in the environment to biogeochemical, ecological, and physiological changes at different biological scales in anchialine ecosystems, from microbes and macrofauna to habitat level. We have received contributions from disciplines spanning paleoecology, microbiology, biogeochemistry, and ecophysiology from four geographical regions around the world (Australia, Bermuda, The Bahamas, and the Yucatan Peninsula). Specifically, these studies aimed at characterizing the limits of physiological adaptations of cave-adapted crustaceans, the drivers of organic matter inputs over time, and how organic matter availability regulates microbial community structure and meiofauna assemblages (Figure 1). These contributions expand our understanding of anchialine ecosystem functioning and enable better predictions of future ecological changes within these coastal aquifer habitats.
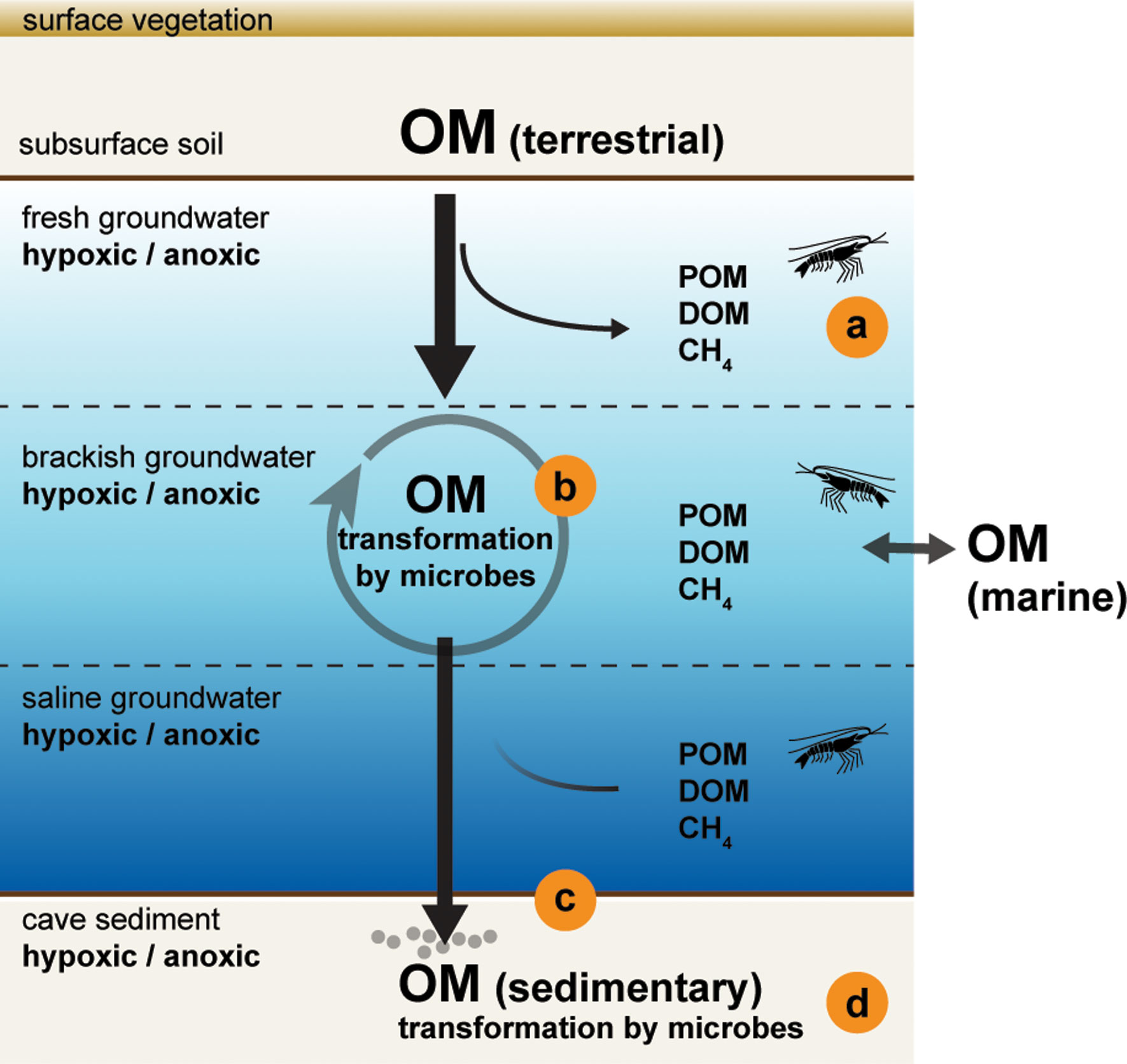
Figure 1 Conceptual model of organic matter (OM) inputs and transformation in a coastal cave environment flooded by fresh and saline groundwaters, a typical anchialine ecosystem. The inputs and consumption of OM affects the concentrations of dissolved oxygen, particulate organic matter (POM), dissolved organic matter (DOM), and methane (CH4) in the water column. The salinity and chemical gradients create a set of habitats in this environment. Studies submitted to this Research Topic include investigations of (A) the physiological adaptations of cave-adapted crustaceans (Chávez-Solís et al., 2022), (B) microbial assemblages in the stratified water column (Elbourne et al., 2022), and the effects of sedimentary OM deposition over time on (C) meiofaunal assemblages (Cresswell and van Hengstum, 2022) and (D) microbial communities (Risley et al., 2022).
Cresswell and van Hengstum (2022) evaluated links between changes in environmental factors and benthic foraminiferal assemblages in Bermuda using sediment cores that are the best-preserved stratigraphic succession currently known from an underwater cave. In addition to marked changes in salinity due to the vertical and horizontal migration of the mixing zone with sea-level fluctuations, the source of organic carbon was an important factor shaping the assemblages of benthic meiofauna over the last 10,000 years. In another study from The Bahamas, Risley et al. (2022) showed that microbial community structure is influenced by the origin of organic carbon at the time of deposition, linking changes in terrestrial vegetation on the surface and microbial sedimentary processes in the subsurface over the past 2,000 years. Beyond the sediments, the stratified water column is also inhabited by a consortium of microbes that regulate organic matter transformation. Using cell population counts and 16S rRNA amplicon analyses, Elbourne et al. (2022) characterized the microbial communities along physicochemical gradients and depth profiles in the stratified water column of the Bundera Sinkhole, Australia. Although the high level of taxonomic novelty made it difficult to attribute metabolic functions to many of the key microbial players, potential chemolithotrophic processes, including sulfur-, ammonia-, and nitrite-oxidation, were supported by the study. This work highlights the effects of increased organic matter loading on microbially-mediated elemental cycling and their influence on shaping habitat variability in anchialine ecosystems. The observed salinity and dissolved oxygen gradients create heterogeneous habitats that affect physiological adaptations within macrofauna species and populations. Through a set of physiological and metabolic parameters, Chávez-Solís et al (2022) showed that closely related cave-shrimp species from the genus Typhlatya in the Yucatan Peninsula have different metabolic capacities that are in correspondence with the salinity they inhabit. This work has implications for understanding the evolutionary history of this cave-restricted genus and for the conservation efforts of anchialine ecosystems.
Collectively, the above contributions highlight how the interplay of environmental factors such as salinity, organic matter loading, and dissolved oxygen content control habitat variability and ecosystem functioning in anchialine ecosystems. Future studies should investigate direct linkages between microbes, biogeochemical processes, and trophic webs, considering the role of macrofauna in these processes. Focus is also needed on a more comprehensive identification of microbial assemblages; investigate regional and sub-regional drivers on the observed biogeochemical processes and differences between ecosystem models from different geographical regions. The use of modern molecular technologies would aid identifying such mechanisms and help shed light on evolutionary and adaptive processes. A conservation approach integrating physiology, niche width, and global environmental change projections for cave-restricted species and their habitats is paramount for the protection of anchialine ecosystems.
LM-O designed the Research Topic. LM-O, EC-S, and DB wrote the manuscript. DB made the figure. All authors contributed to the article and approved the submitted version.
The authors are grateful to all contributors to this Research Topic and to all reviewers whose comments improved the submitted manuscripts.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bishop R. E., Humphreys W. F., Cukrov N., Zic V., Boxshall G. A., Cukrov M., et al. (2015). 'Anchialine' redefined as a subterranean estuary in crevicular or cavernous geological setting. J. Crust. Biol. 35, 511–514. doi: 10.1163/1937240X-00002335
Brankovits D., Little S. N., Winkler T. S., Tamalavage A. E., Mejía-Ortíz L. M., Maupin C. R., et al. (2021). Changes in organic matter sedimentation impact benthic meiofaunal communities in the marine sector of karst subterranean estuaries. Front. Environ. Sci. 9. doi: 10.3389/fenvs.2021.670914
Brankovits D., Pohlman J., Ganju N. K., Iliffe T., Lowell N., Roth E., et al. (2018). Hydrologic controls of methane dynamics in karst subterranean estuaries. Global Biogeochem. Cycles 32, 1759–1775. doi: 10.1029/2018GB006026
Howarth R., Chan F., Conley D. J., Garnier J., Doney S. C., Marino R., et al. (2011). Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 9, 18–26. doi: 10.1890/100008
Moore W. S. (1999). The subterranean estuary: A reaction zone of ground water and sea water. Mar. Chem. 65, 111–125. doi: 10.1016/S0304-4203(99)00014-6
Pohlman J. W. (2011). The biogeochemistry of anchialine caves: Progress and possibilities. Hydrobiologia 677 (1), 33–51. doi: 10.1007/s10750-011-0624-5
Sket B. (1996). The ecology of anchihaline caves. Trends Ecol. Evol. 11, 221–225. doi: 10.1016/0169-5347(96)20031-X
Tamalavage A. E., van Hengstum P. J., Louchouarn P., Molodtsov S., Kaiser K., Donnelly J. P., et al. (2018). Organic matter sources and lateral sedimentation in a Bahamian karst basin (sinkhole) over the late Holocene: Influence of local vegetation and climate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 506, 70–83. doi: 10.1016/j.palaeo.2018.06.014
Keywords: marine connections, anchialine ecosystems, stygofauna, ecology, ecophysiology, organic matter, carbon cycle
Citation: Mejía-Ortíz LM, Chávez-Solís EM and Brankovits D (2022) Editorial: The effects of environmental change on anchialine ecosystems. Front. Mar. Sci. 9:1029027. doi: 10.3389/fmars.2022.1029027
Received: 26 August 2022; Accepted: 02 September 2022;
Published: 14 September 2022.
Edited and Reviewed by:
Angel Borja, Technology Center Expert in Marine and Food Innovation (AZTI), SpainCopyright © 2022 Mejía-Ortíz, Chávez-Solís and Brankovits. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis M. Mejía-Ortíz, bHVpc21lamlhQHVxcm9vLmVkdS5teA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.