- 1Department of Aquaculture, Zhejiang Ocean University, Zhoushan, China
- 2Guangdong Yuehai Feeds Group Company Ltd., Zhanjiang, China
- 3School of Marine Science, Ningbo University, Ningbo, China
This study investigated the effects of different dietary arginine (Arg) levels on the growth, protein synthesis, antioxidant capacity, and immunity of postlarval mud crab Scylla Paramamosain. Six isonitrogenous and isolipidic diets were formulated to contain 1.51%, 1.81%, 2.16%, 2.35%, 2.73%, and 3.07% dietary Arg levels (dry matter). There were four replicates for each diet treatment (26 crabs per replicate, initial body weight: 7.40 ± 0.15 mg). After eight weeks of feeding trial, the survival and molting frequency (MF) of crabs were not affected by the experimental treatment (P>0.05). Crabs fed the 2.50% Arg diet achieved the highest weight gain (WG) and specific growth rate (SGR) (P<0.05). The whole-body protein content of the 2.16% and 2.73% Arg groups were significantly higher than that of the 1.51% Arg group (P<0.05). Crabs in the 2.35% group obtained the highest levels of phenylalanine and leucine (P<0.05). Superoxide dismutase (SOD), catalase (CAT), and total antioxidant capacity (T-AOC) activity in the 2.16%, 2.35% and 2.73% Arg groups were significantly higher than that in other treatments (P<0.05). Malondialdehyde (MDA) concentration and alkaline phosphatase (AKP) activity were not significantly affected by the treatments. The transcript levels of insulin-like growth factor 1 (igf-1), rapamycinin (TOR), S6 kinase-polypeptide 1 (s6k1) in crabs fed with 2.16% and 2.35% dietary Arg were significantly higher than those in crabs fed with 1.51% and 3.07% dietary Arg (P<0.05). The lowest prophenoloxidase (proPO), relish, and lysozyme transcript levels were observed in crabs fed the 1.51% dietary Arg. The current study founded that the Arg requirement for postlaval S.paramamosain was 2.34% (5.20% of the dietary protein), based on the second order polynomial regression analysis of WG.
Introduction
Dietary protein and amino acids (AAs) are essential for the optimum health, growth, development, and survival of animals (Herring et al., 2021; Li et al., 2021b). As a basic AA in physiological body fluids, L-Arginine (Arg) is regarded as an essential amino acid (EAA) and functional AA for aquatic animals (Wu, 2014). It takes part in multiple metabolic pathways in vivo, including the synthesis of protein, nitric oxide, creatine, proline, glutamate, polyamines, and agmatine (Wu et al., 2009; NRC, 2011). Moreover, Arg and its metabolites are important regulators of key metabolic or cell signaling pathways that are essential for vascular regulation, neurotransmission, nutrient sensing, and anti-inflammatory responses of animals (Reyes et al., 1994; Paudel et al., 2021). Arg is also a potent secretagogue of insulin, growth hormone, and igf-1, which can regulate the metabolism of glucose and AAs in various tissues of animals (Newsholme et al., 2005; Wu, 2022). The immune and antioxidant functions of Arg have been extensively reported in different animals (Birmani et al., 2019; Li et al., 2021b).
The functions and requirements of Arg for aquatic animals has been well summarized in recent reviews (Li et al., 2021a; Wu, 2022). Tu et al. (2015) proposed that dietary Arg supplementation could balance AAs and activate the target of TOR signaling pathway sensitive to nutrition, thereby promoting protein synthesis in Carassis auratus gibelio. Gu et al. (2022) and Liang et al. (2016) showed that the expression of TOR and its downstream target s6k1 in muscle and liver of Hemibagrus wyckoiides and Megalobrama amblycephala were improved by dietary Arg supplementation. Like in teleost, Arg is also considered as an essential and functional amino acid in crustaceans (NRC, 2011; Huang et al., 2020). Supplementing an appropriate level of Arg in the diet can enhance growth performance, feed utilization, immune functions, and antioxidant capacity of Litopenaeus vannamei (Zhou et al., 2012). Compared to low Arg diet (1.72%), diets contained 2.73-3.07% Arg improved the growth, survival, antioxidant capacity, immunity, and disease resistance of Eriocheir sinensis (Qi et al., 2019). Similarly, intraperitoneal injection of Arg stimulated the expression of fch-TOR and activated the mTOR signaling pathway in the skeletal muscle of Fenneropenaeus chinensis (Sun et al., 2015). On the other hand, excessive Arg intake has a negative impact on animals, resulting in additional energy consumption for deamination and excretion, and ultimately inhibiting growth (Walton, 1985). Therefore, it is necessary to determine the functions of Arg and its dietary optimal level in different crustaceans.
Due to its high nutritional value, short growth cycle, and strong adaptability, the mud crab Scylla paramamosain has been considered the most crucial marine aquaculture crustaceans in China (Zheng et al., 2020). Recently, related culture methods and technology have been constantly improved (Zheng et al., 2018). The output of mud crab breeds (mostly S. paramamosain) reached 160,616 tons in 2020 (Fishery Bureau of China Agriculture Department, 2020). For mud crabs, dietary requirements of protein, lipid, cholesterol, and phospholipid have also been well studied (Zheng et al., 2018; Xu et al., 2019; Xu et al., 2020; Zheng et al., 2020). To our knowledge, there is limited valuable information on the nutritional and immune aspects of Arg in this species. The lack of relevant information and available data has limited the development of practical formula feeds for S.paramamosain. Therefore, it is vital to determine the proper Arg level in diet formulations for postlaval S. paramamosain.
Materials and methods
Diet formulation
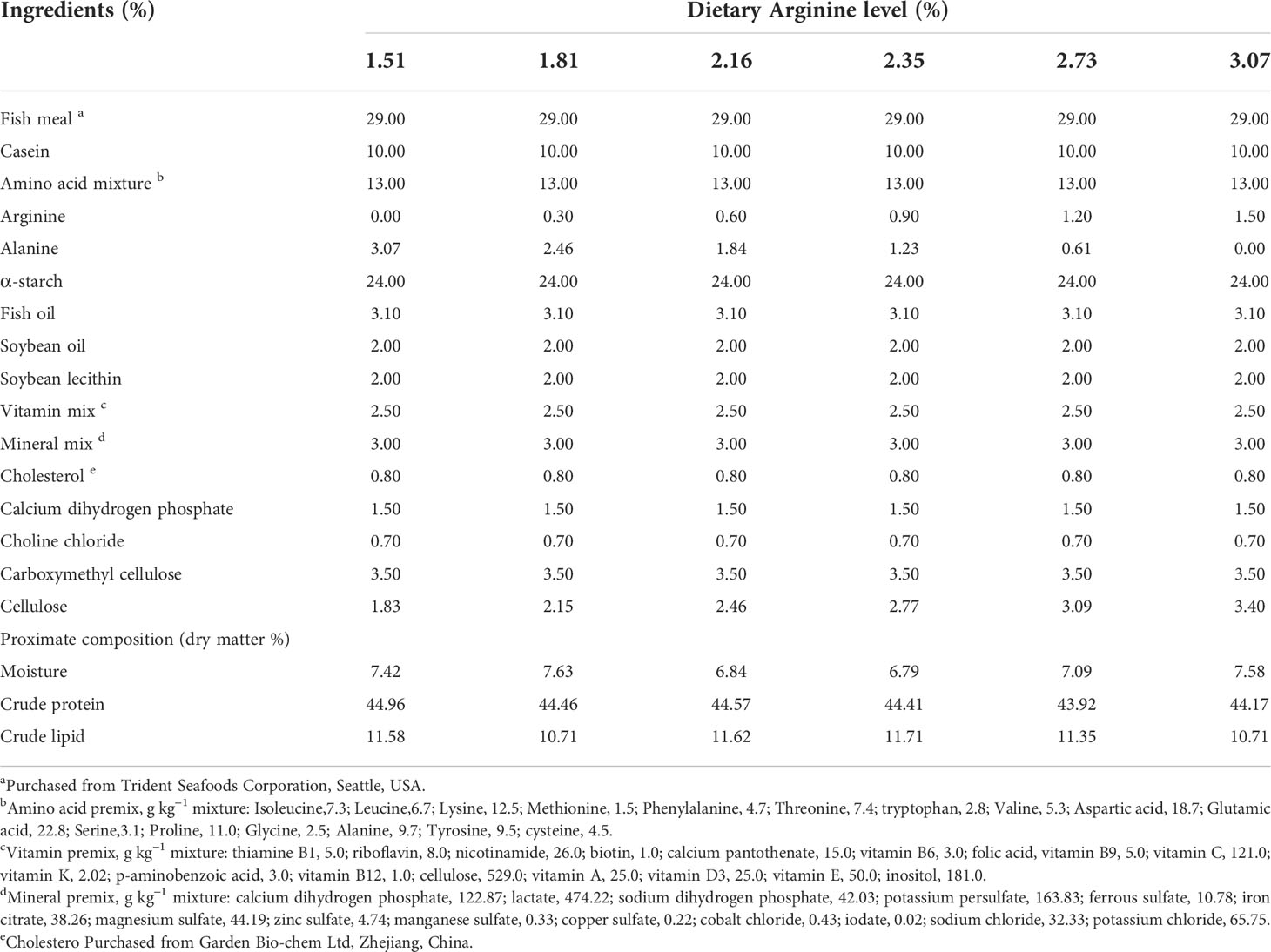
Six isonitrogenous and isolipidic diets were formulated to contain 1.51%, 1.81%, 2.16%, 2.35%, 2.73%, and 3.07% dietary Arg (L-form, purity ≥98.5%; CJ Biological Technology Ltd., Shenyang, China) levels (dry matter). The basal diet contained 1.51% Arg from fish meal and casein, and the other five diets were prepared by adding L-Arg to increase dietary L-Arg levels with alanine being added to keep the diet isonitrogenous (Table 1). All the dry matters were pulverized by a vertical superfine pulverizer (WFS-8, Zhiyang, Jiangsu), passed through a 125 μm mesh and then thoroughly mixed in a mixer. Crystalline amino acids were prepared by the method provided by Alam et al. (2005). The amino acid mixture was coated with carboxymethyl cellulose (CMC) in water at 60°C. Subsequently, the amino acid mixture was mixed with dry matter. After that, soybean lecithin and oil were added to the mixer and fully mixed. Finally, a suitable amount of water was added to the mixture, which was then made into an even dough. The dough was further processed by a laboratory granulator (G-250, the machine factory of South China University of Technology, Guangzhou, China) into 0.8 mm granules. All prepared feed pellets were dried overnight at 45°C to a constant value and then stored in plastics at -20°C until utilization.
Crabs and experimental conditions
The postlaval crabs were purchased from a commercial hatchery in Taizhou, Zhejiang, China. After a week of acclimatization to laboratory conditions, 624 healthy and similarly sized crabs (7.40 ± 0.15 mg) were chosen and individually placed in cylindrical containers (250 ml). There were four replicates for each diet treatment (26 crabs per replicate). The mud crabs were fed the experimental diet daily at 9:00 and 16:00 respectively. After one hour of the feeding, the excess feed was removed and the water was changed 100%. Natural indoor lighting was followed and the lighting of each container consistent was kept the same. Death and molts were recorded daily. Water temperature was controlled at 26°C-29°C, the salinity was at 28-29g L-1, the minimal amount of dissolved oxygen was 6 mg L-1, and the concentration of ammonia nitrogen was not more than 0.05mg L-1.
Sampling and analyzing
After eight weeks of trail, all crabs were fasted for 24 hours, and the survival and wet body weight were measured. Ten crabs from each replicate were selected and frozen at -20°C for body composition analysis, and an additional six crabs were selected for dissection to obtain hepatopancreas and muscle for gene expression analysis. In addition, four more crabs were selected for the analysis of antioxidant parameters. All samples were frozen in liquid nitrogen and stored at -80°C.
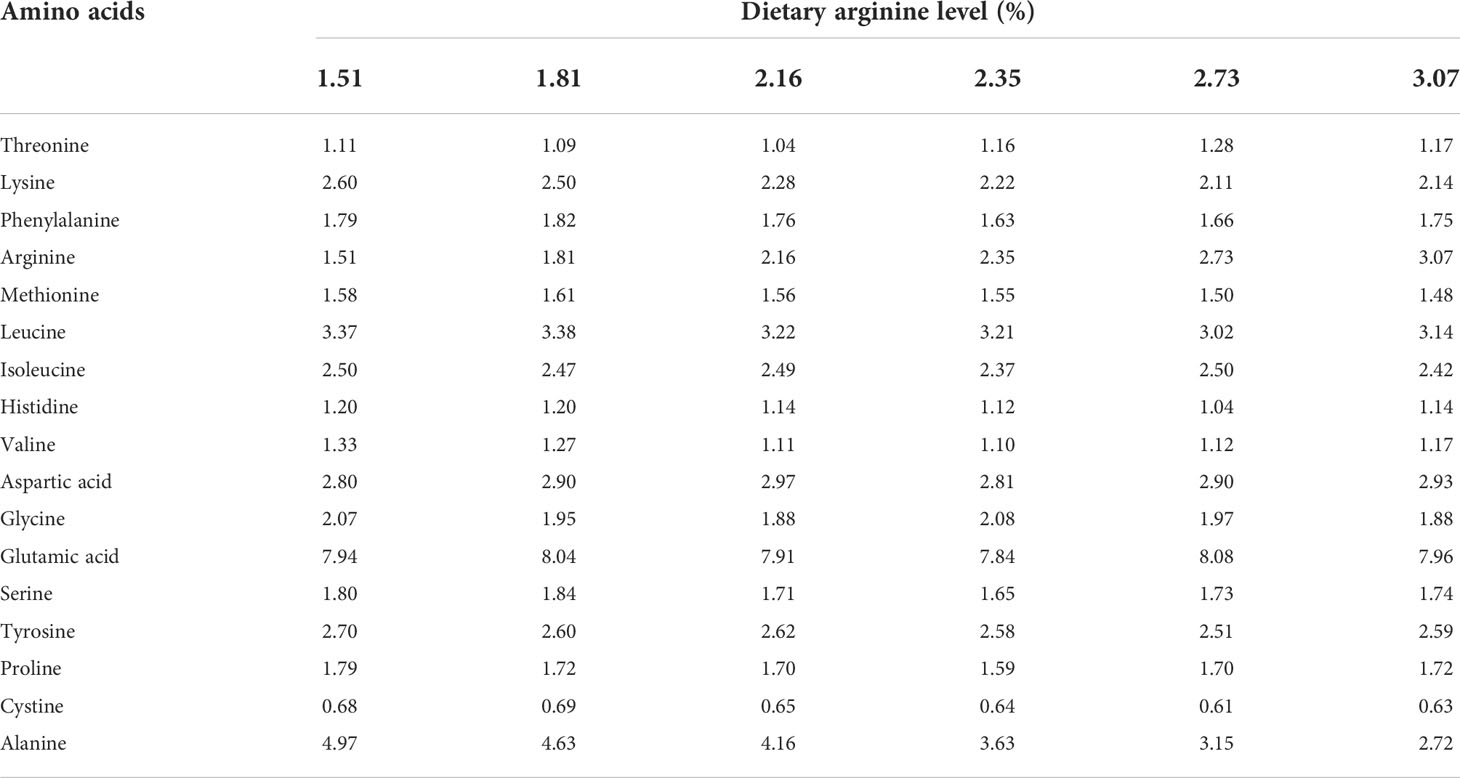
The proximate composition of diets and crabs’ whole-body were analyzed according to standard procedures prescribed in AOAC (1995). Dietary moisture was dried to a constant weight in an oven at 105°C. Whole crab moisture was measured using a freeze dryer (LL1500, Thermo Scientific, Waltham, USA). The Auto Kjeldahl System (K355/K437, Buchi, Flawil, Switzerland) was used to measure the crude protein of feeds and crabs. Total whole-body lipids of crabs were extracted with chloroform/methanol (2/1, v/v) according to the method provided by Folch et al. (1957). To test the amino acid composition of the samples (including experimental diets and whole crab), the amino acid samples were provided to a professional laboratory for measurement using an automatic analyzer (L-8900, HITACHI, Tokyo, Japan; Tables 2, 3).
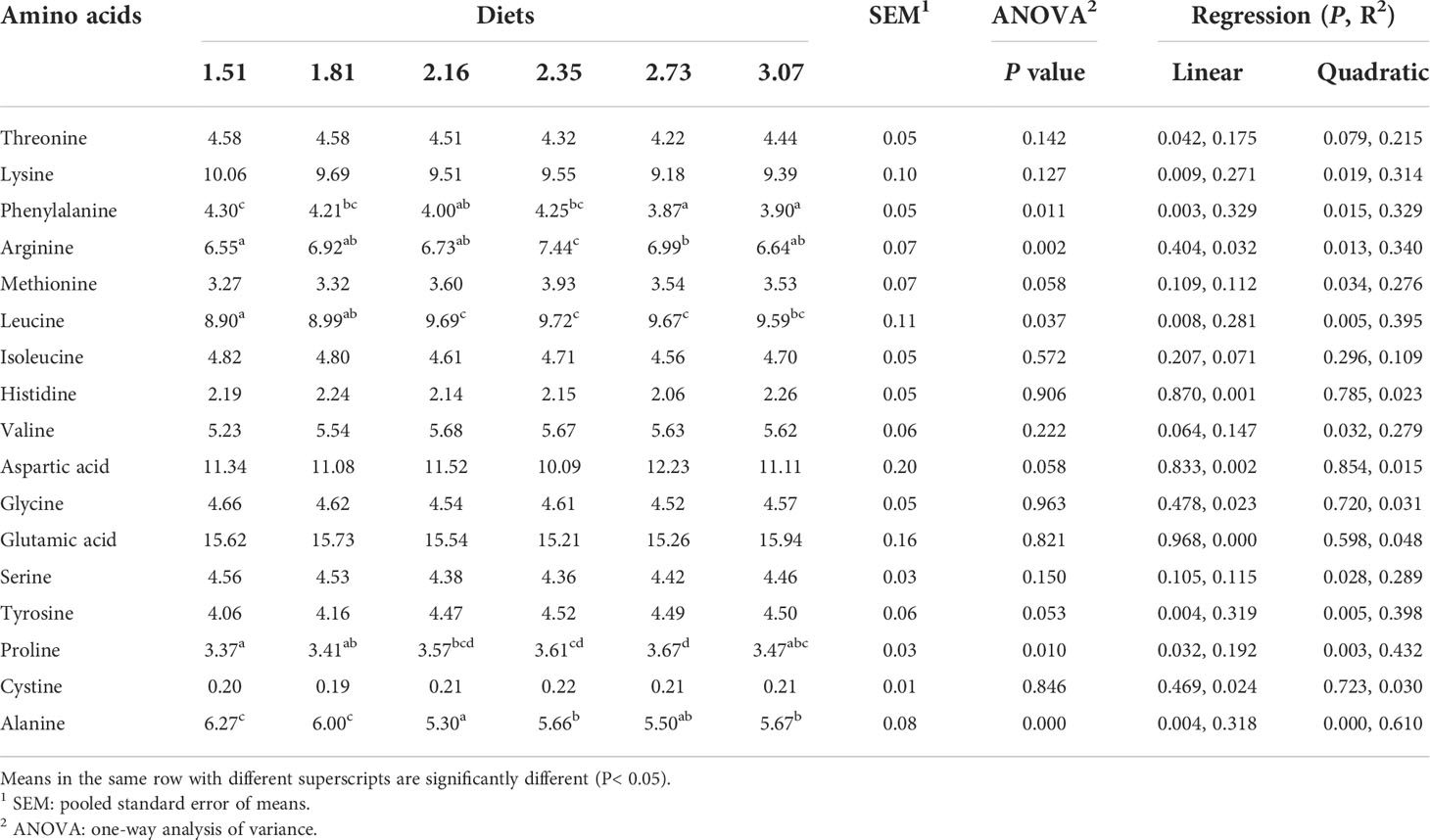
Table 3 Amino acid composition (% total amino acids) in whole-body of postlarval Scylla paramamosain fed with diets containing different arginine levels.
Whole crab supernatants were prepared for enzymatic activity assays according to the method provided by Xu et al. (2018). Alkaline phosphatase (AKP) activity was analyzed using the methods provided by Wei et al. (2014). Total protein concentration was determined following the method of Bradford (1976). Catalase (CAT) activity was measured using the method provided by Góth (1991). Superoxide dismutase (SOD) activity was measured as described by Zhao et al. (2015). Total antioxidant capacity (T-AOC) was determined using the method provided by Benzie and Strain (1996). Malondialdehyde (MDA) concentrations were determined following the method of Zhao et al. (2015). All parameters were determined using a commercial kit (Nanjing Jianchen Bioengineering Institute, Nanjing, China) and a microplate reader (Multiskan Go, Thermo Scientific, Waltham, USA).
Gene expression
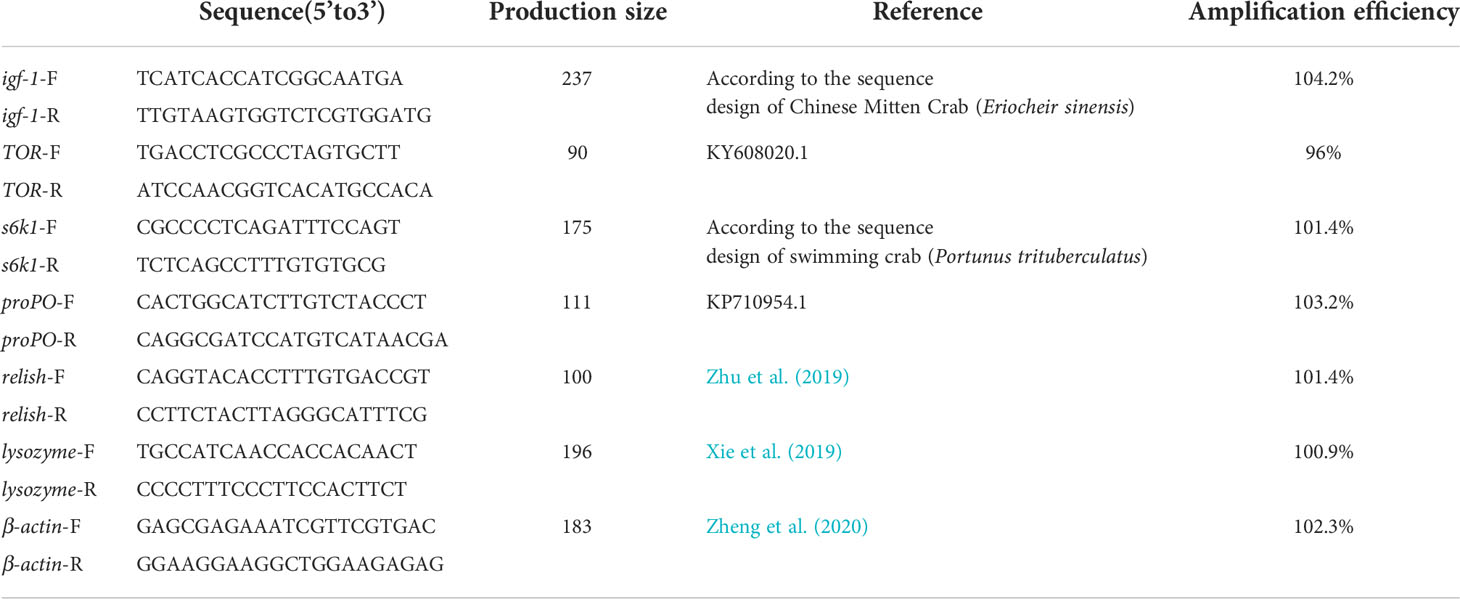
Total RNA was extracted from hepatopancreas and muscle using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and quantified on a 2.0% agarose electrophoresis to assessing RNA concentration. cDNA transcribed from RNA was synthesized using PrimeScript™ RT Kit (Perfect Real Time; Takara, Dalian, China) according to the protocol provided by the manufacturer. The transcription level of Insulin-like growth factor 1 (igf-1), Rapamycinin (TOR), S6 kinase-polypeptide 1 (S6K1), prophenoloxidase (proPO), relish and lysozyme genes were determined by a Real-Time PCR System (QuantStudio™ 6 Flex, Life Technologies, Carlsbad, USA) according to Zheng et al. (2018). Quantitative real-time PCR was performed using the QuantStudio™ 6 Flex Real-Time PCR System (Applied Biosystems). Since β-actin is relatively stable, it was used as a reference gene (Table 4). The transcription level of different genes was calculated using the 2-ΔΔCT equation according to Livak and Schmittgen (2001). Each treatment was performed in quadruplicate and replicated three times on each sample.
Statistical analysis
After all data were tested for homogeneity and normal distribution with Levene’s test and Kolmogorov-Smirnov test, respectively, one-way analysis of variance (ANOVA) was used to determine treatment effects, and treatment deviations were ranked using Duncan’s multiple range test. Linear and quadratic regression models were constructed to test the correlation of results with dietary Arg levels. The statistical significance level was determined at 5% (P<0.05). All parameters were analyzed by SPSS 24.0 (IBM, Chicago, USA) software, and the data were expressed as mean values (n = 4). The second-order polynomial regression model (Robbins et al., 1979) was used to estimate the appropriate supplementation of dietary Arg for S. paramamosain on the basis of WG.
Results
Survival and growth performance
After eight weeks of feeding trial, the survival of postlarval S. paramamosainin ranged from 76.24% to 96.52% (Table 5). Different dietary Arg levels significantly affected the growth performance of S. paramamosain. The highest WG and SGR values were observed in the 2.35% dietary Arg level group (P<0.05). Quadratic regression analysis of WG% against dietary Arg levels indicated that optimal dietary Arg level was 2.34% of dry matter (5.20% of dietary protein) for S. paramamosain (the optimum is the maximum requirement) (Figure 1). Dietary Arg levels had no significant effect on molting frequency (MF; P>0.05).

Table 5 Growth, feed utilization and morphometrical parameters of postlarval Scylla paramamosain fed with diets containing different arginine levels.
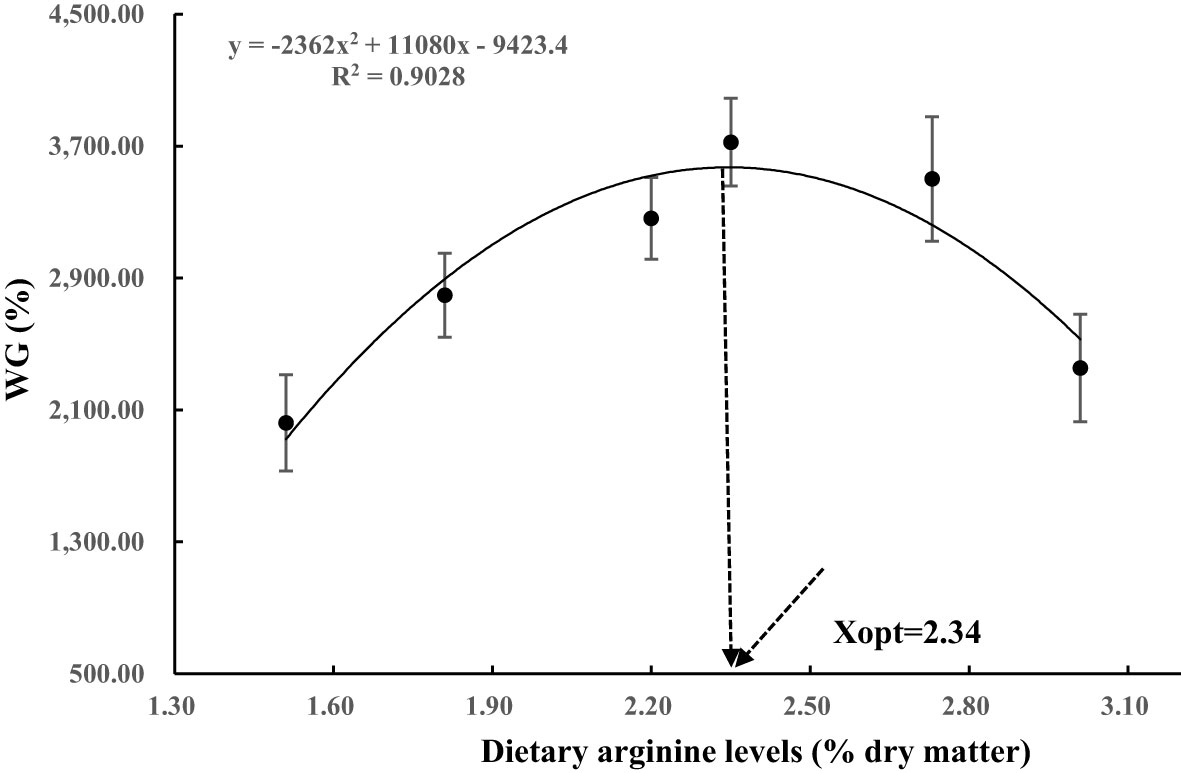
Figure 1 Relationship between the weight gain (WG, %) with the dietary arginine levels, where Xopt represents the optimal dietary Arg level for the maximum WG.
Whole-body proximate composition
The effects of different treatments on the crude lipid content of crabs were not significant (Table 6). The whole-body protein content of the 2.16% and 2.73% Arg groups were significantly higher than that of the 1.51% Arg group (P<0.05). In addition, the effects of different treatments on the content of most AAs in the whole body were not significant, but phenylalanine, Arg, leucine and proline showed linear and quadratic responses to dietary Arg levels (P<0.05).
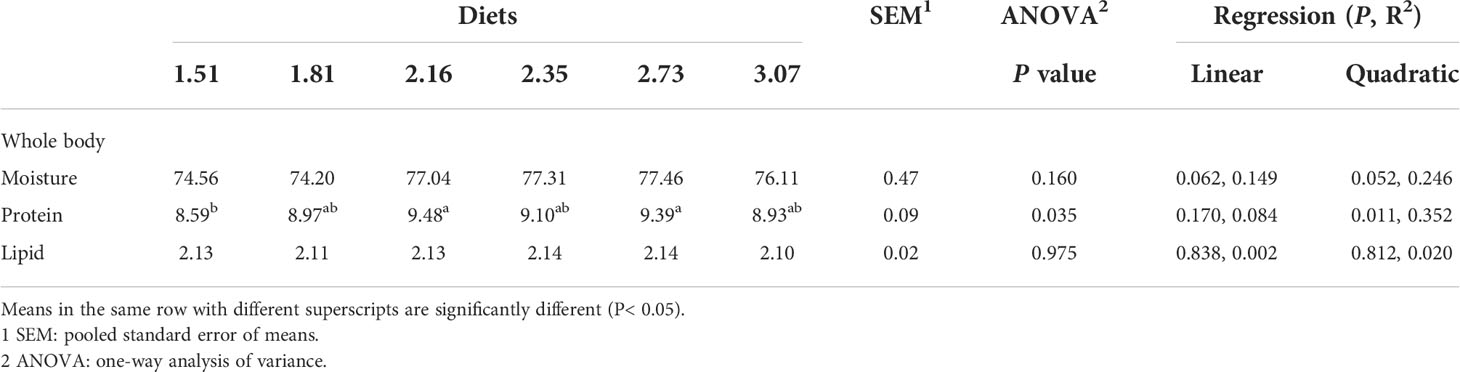
Table 6 Whole-Body composition in postlarval Scylla paramamosain fed with diets containing different arginine levels (in % of wet weight basis).
Antioxidant and immunity parameters
In this experiment, dietary Arg levels significantly affected the antioxidant capacity of crabs (Table 7). The highest SOD activity were observed in the 2.35% Arg group (P<0.05). The lowest CAT and T-AOC activity were observed in the 1.51% Arg group (P<0.05). Although MDA and AKP concentrations were not affected by the experimental treatments, the highest MDA concentration and the lowest AKP concentration were observed in the 1.51% Arg group (P>0.05; Figure 2).
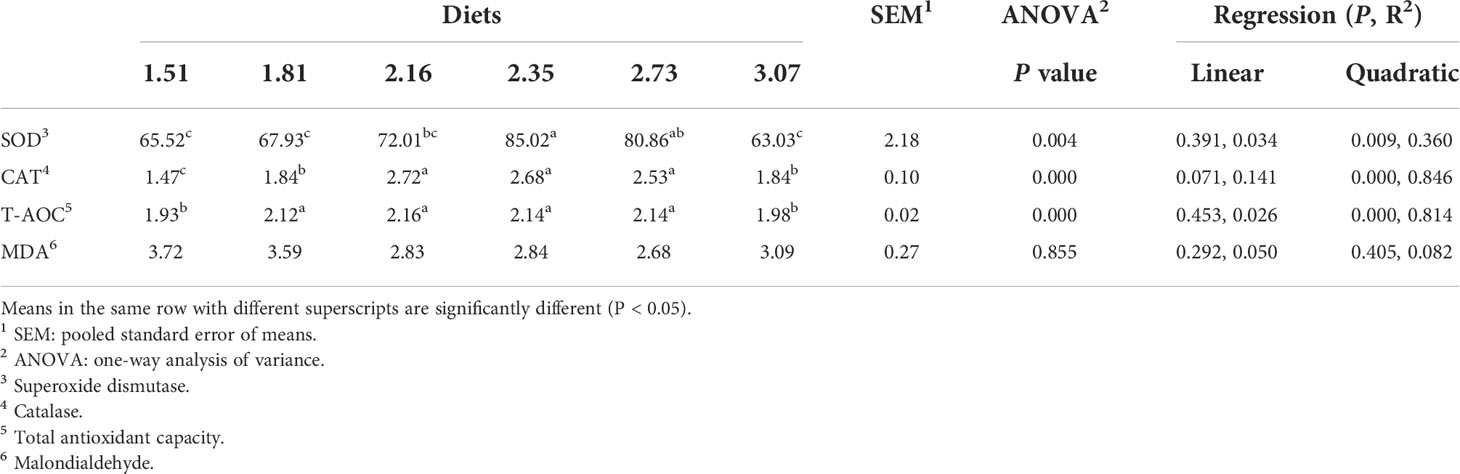
Table 7 Antioxidant parameters in the whole-body of postlarval S. paramamosain fed the experiment diets for 8 weeks.
Figure 2 Activity of AKP in the hepatopancreas of postlarval S. paramamosain fed diets with different arginine levels.
The mRNA expression of igf-1, proPO, relish and lysozyme in hepatopancreas as well as s6k1, TOR in muscle
In this experiment, dietary Arg levels significantly affected transcript levels of igf-1, TOR, s6k1, proPO, relish, and lysozyme (P < 0.05; Figures 3–8). The transcript levels of igf-1, TOR, s6k1 in crabs fed with 2.16% and 2.35% dietary Arg were significantly higher than those in crabs fed with 1.51% and 3.07% dietary Arg (P < 0.05). In addition, the lowest expression of proPO, relish, and lysozyme were observed in the 1.51% dietary Arg group (P < 0.05).
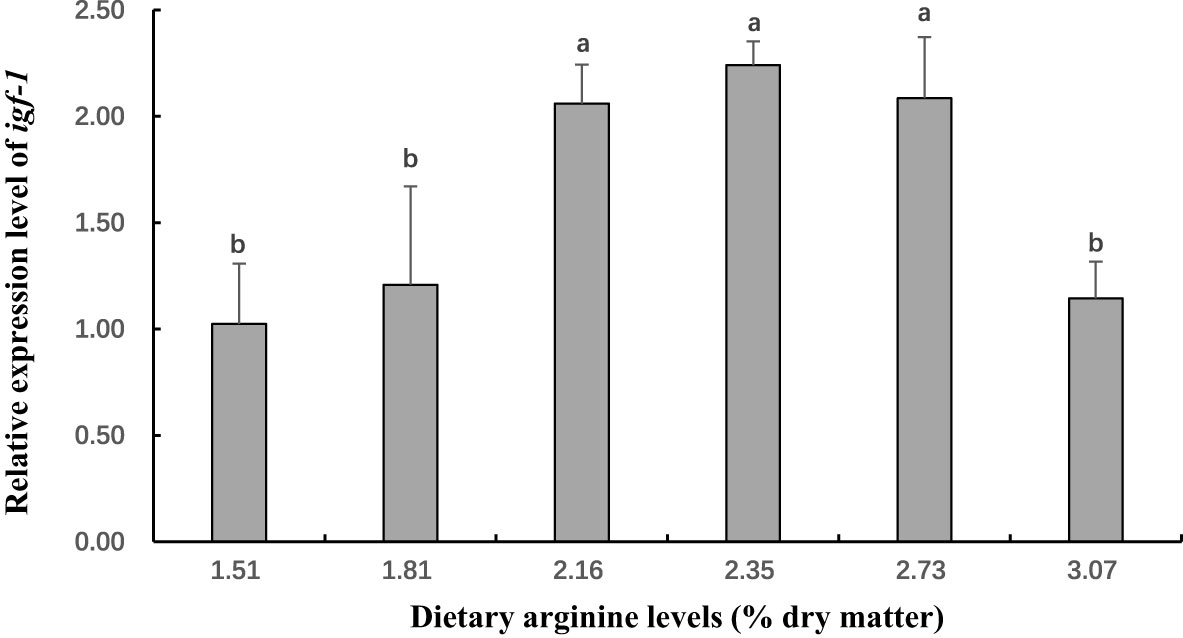
Figure 3 The gene expression (Insulin-like growth factor 1 (igf-1) in hepatopancreas) of postlarval Scylla paramamosain fed diet with different arginine levels. Means in the same row with different superscripts are significantly different (P< 0.05).
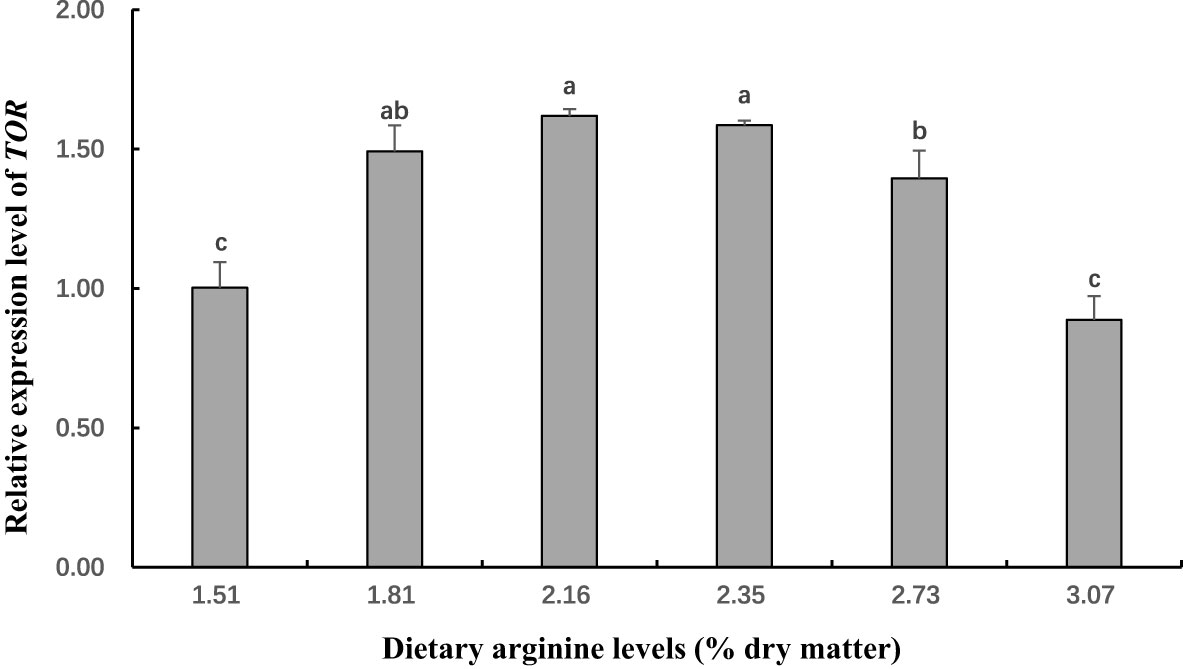
Figure 4 The gene expression (Rapamycinin (TOR) in muscle) of postlarval Scylla paramamosain fed diet with different arginine levels. Means in the same row with different superscripts are significantly different (P< 0.05).
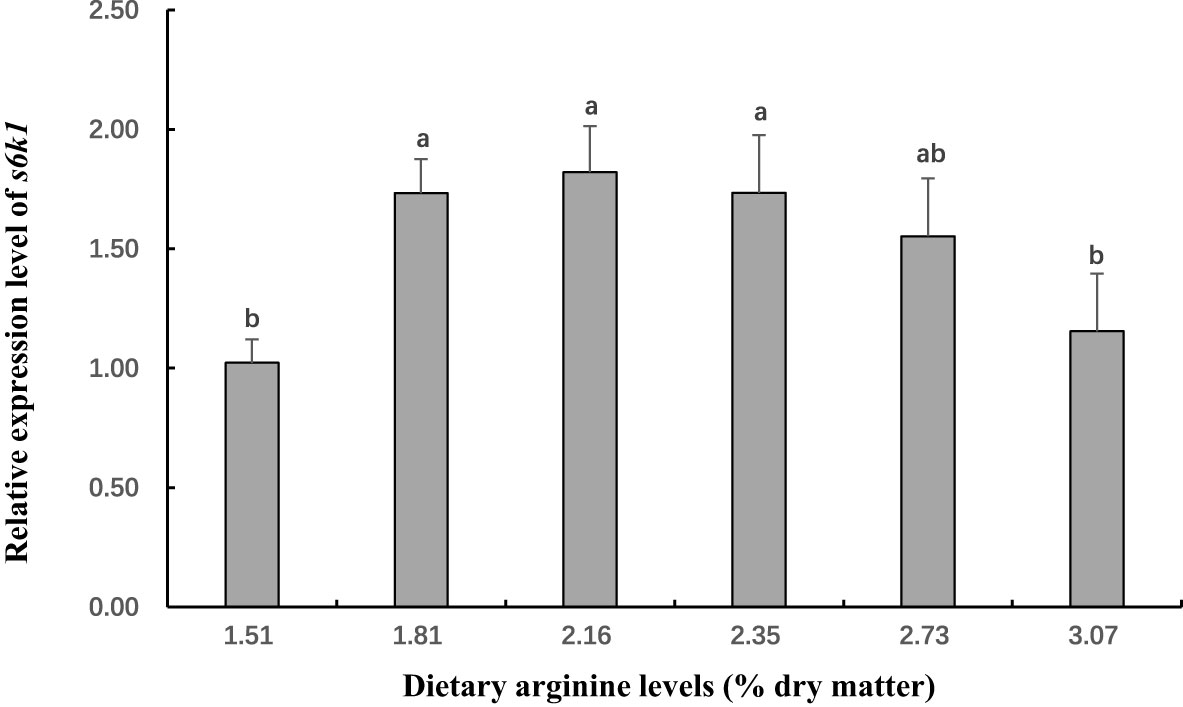
Figure 5 The gene expression (S6 kinase-polypeptide 1 (s6kl) in muscle) of postlarval Scylla paramamosain fed diet with different arginine levels. Means in the same row with different superscripts are significantly different (P< 0.05).
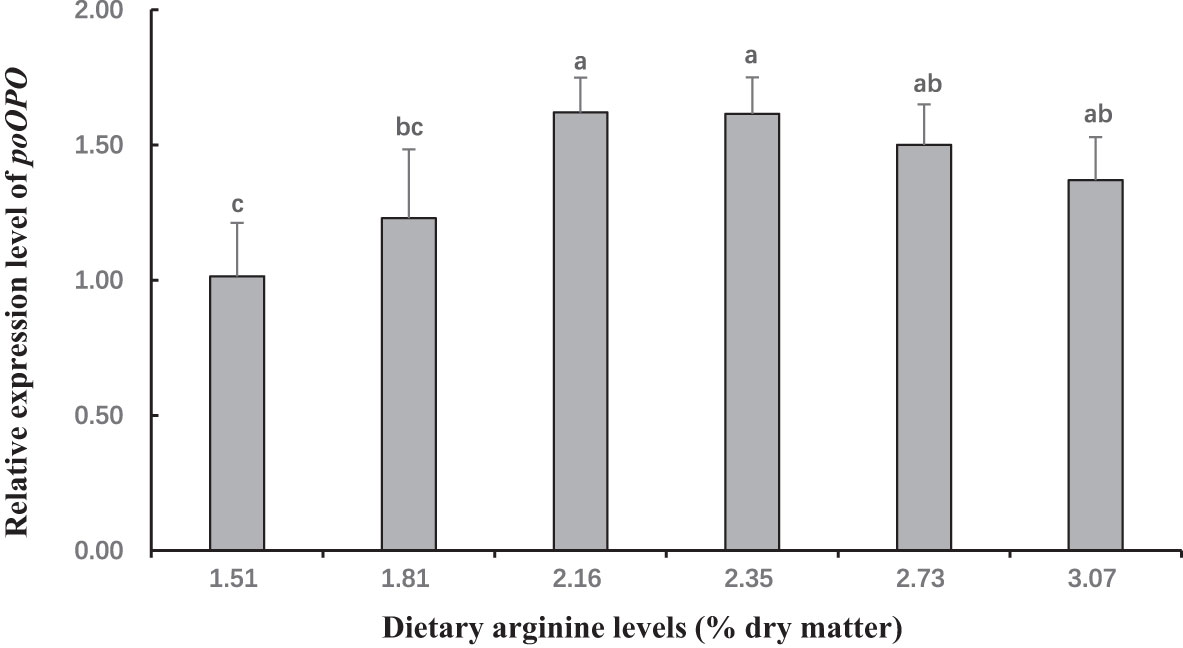
Figure 6 The gene expression (prophenoloxidase (proPO) in hepatopancreas) of postlarval Scylla paramamosain fed diet with different arginine levels. Means in the same row with different superscripts are significantly different (P< 0.05).
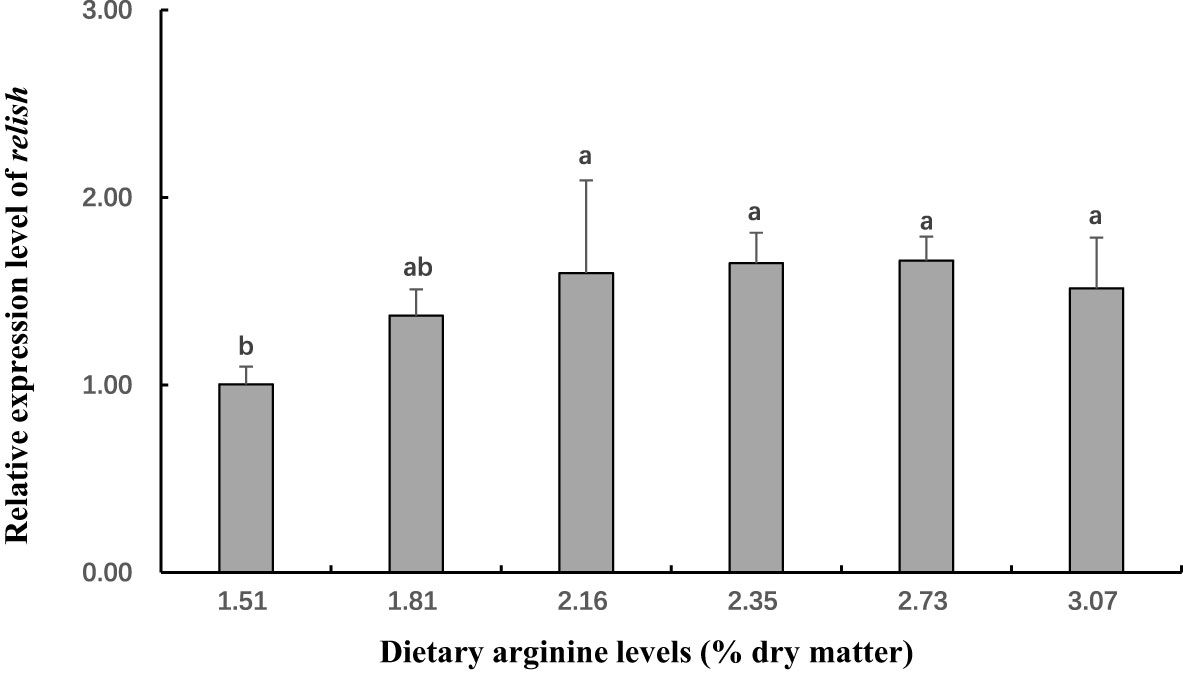
Figure 7 The gene expression (relish on hepatopancreas) of postlarval Scylla paramamosain fed diet with different arginine levels. Means in the same row with different superscripts are significantly different (P< 0.05).
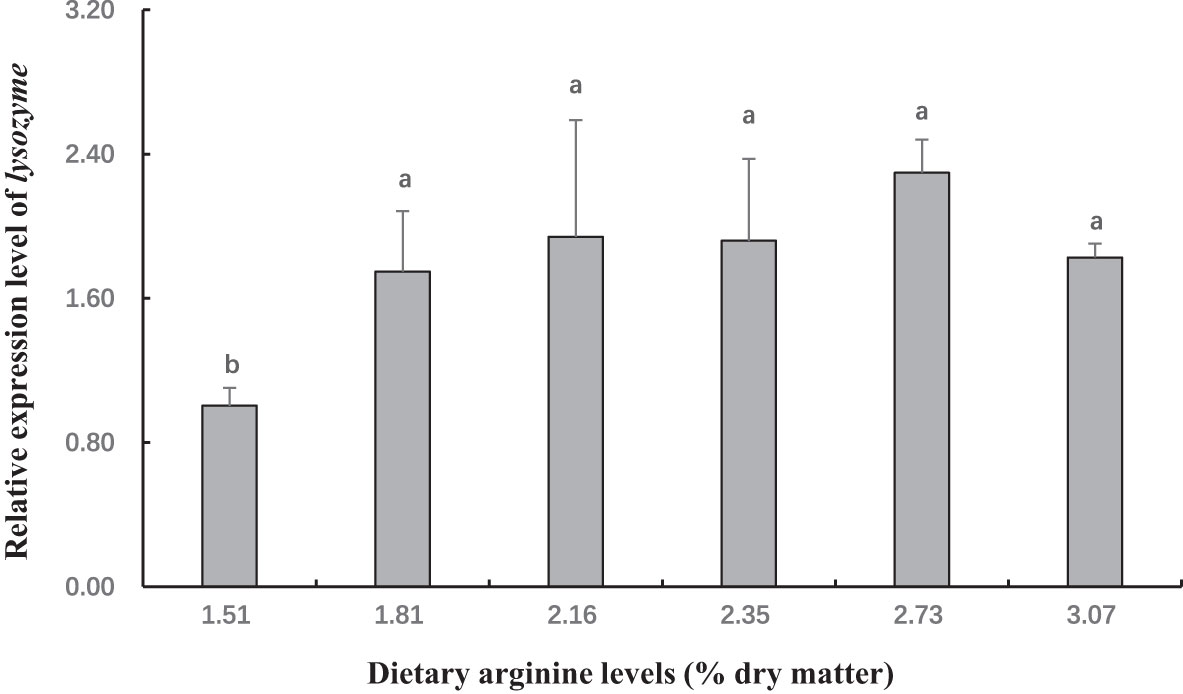
Figure 8 The gene expression (lysozyme in hepatopancreas) of postlarval Scylla paramamosain fed diet with different arginine levels. Means in the same row with different superscripts are significantly different (P< 0.05).
Discussion
A high survival rate is necessary for the success of crustacean farming. Cannibalism is the main reason for the low survival rate of crustacean species in nutritional research experiments (Castine et al., 2008). Generally, it is acceptable that the survival rate exceeds 80% in the feeding trial for crustaceans (Catacutan, 2002). In this study, the survival of postlarval mud crab Scylla paramamosain ranged from 76.24% to 92.52% among different treatments. A similar survival (from 76.19% to 92.86%) was also recorded in a previous study of S. paramamosain (Zheng et al., 2020). This result indicated that the experimental conditions were suitable for the culture of postlarval S. paramamosain in this study.
The growth performance of S. paramamosain was significantly affected by different feed treatments. With the dietary Arg level increased to 2.35% (5.22% of the dietary protein), the WG value of S. paramamosain showed a general increased trend, and then decreased significantly as the dietary Arg level was further increased. A similar tendency was also observed in other species, such as Pacific white shrimp (Litopenaeus vannamei; 4.77% of the dietary protein; Zhou et al., 2012). The deficiency of dietary Arg is characterized by poor growth, low feed utilization, or high mortality (Wilson, 2003). Similarly, the SGR and WG values of the 1.51% and 1.81% Arg groups were significantly lower than those of the 2.35% and 2.73% groups. This is in agreement with the results of juvenile Penaeus monodon (Millamena et al., 1998). On the other hand, crabs fed with the 3.07% Arg diet had significantly lower WG and SGR values than crabs fed with the 2.35% and 2.73% Arg diets. And the survival rate of crabs fed with the 3.07% Arg diet was also the lowest among all groups. In agreement, Fournier et al. (2003) found that excess dietary Arg would adversely impact the growth performance of Oncorhynchus mykiss and Psetta maxima. Based on the second order polynomial regression analysis of WG, the optimum dietary Arg level is 2.34% of the dry diet, which is equivalent to 5.20% of the dietary protein. These values are similar to those reported Arg requirements of other crustaceans, such as Penaeus monodon (5.30% of dietary protein; Millamena et al., 1998) and Marsupenaeus japonicus (5.32% of dietary protein; Alam et al., 2004). However, it is estimated that the Eriocheir sinensis has a higher requirement for Arg (9.18% of dietary protein; Ye et al., 2010). A possible explanation is that water-soluble crystalline AAs were used in the experiment, and the long feeding time of Chinese mitten crabs easily led to the loss of some AAs, so that the real content of AAs in the feed were lower than the values assumed in the experimental design. In contrast, some other crustaceans have lower requirements for Arg (4.2-4.7% of dietary protein; Palaemonetes varians; Palma et al., 2015). This may be attributable to the fact that the experimental diets were pelletized with steam, which resulted in apparently highly water-stable pellets and a low level of dry matter losses. Therefore, the Arg requirements for crustaceans is dependent on different species, size, dietary protein sources and levels, feeding practices, rearing conditions, and experimental design (NRC, 2011).
An imbalanced proportion of EAAs can reduce the absorption and utilization of AAs by animals. In the present study, the content of Arg, leucine, phenylalanine and proline in carb were significantly increased with increasing the dietary Arg level from 1.51% to 2.35%. Similar results were also found in Megalobrama amblycephala and Oncorhynchus mykiss (Yamamoto et al., 2000; Ren et al., 2013). However, no further increases were observed in crab fed with diets containing Arg beyond 2.35%. A possible explanation is that the deamination of Arg was up-regulated in these groups, and excess Arg was excreted through soluble ammonia, as previously reported on Paralichthys olivaceus (Alam et al., 2002). Excess Arg intake also causes additional energy consumption for deamination and excretion (Walton, 1985).
Insulin-like growth factor 1 (igf-1), also called somatomedin C, is a hormone with a molecular structure similar to insulin. igf-1 plays an important role in growth and anabolism (Philipps et al., 1988). igf-1 level is a very useful indicator for measuring the fish growth and response to change in feed nutrient composition (Picha et al., 2008; Izutsu et al., 2022). Dietary Arg promotes hepatic igf-1 transcription, and its positive effect growth has been reported in some fish species, such as Ictalurus punctatus (Pohlenz et al., 2013), Sparus aurata (De Celis et al., 2004) and hybrid Epinephelus fuscoguttatus♀×Epinephelus lanceolatus♂ (Wu et al., 2018). In this study, the mRNA expression of igf-1 in hepatopancreas showed a significantly increase with increasing dietary Arg content to 2.35%. However, excess dietary Arg (3.07%) decreased the mRNA expression of igf-1. It has reported that excessive dietary Arg leads to insulin resistance through a negative feedback mechanism, resulting in phosphorylation of serine/threonine in vivo, thereby affecting the transcription level of igf-1 (Harrington et al., 2004; Um et al., 2006). These results suggest that appropriate dietary Arg levels could activate igf-1 to enhance the growth of S. paramamosain.
Mu et al. (2020) and Gu et al. (2022) reported that Arg could decrease the inhibition of translation and increase TOR activity, resulting in improve the synthesis of proteins in Cromileptes altivelis and Hemibagrus wyckoiides. Furthermore, TOR signaling pathway is also activated by a combination of insulin and AAs (Serrana and Johnston, 2013), and not just by AAs in Oncorhynchus mykiss (Lansard et al., 2010). In addition, Hara et al. (1998) found that in the absence of growth factors such as insulin, the presence of substantial amounts of AA can greatly promote the phosphorylation of s6k1, a key substrate of mTOR. Similarly, Fingar et al. (2002) claimed that TOR increases the translation of 5’ TOP mRNA through phosphorylation of s6k1 to control cell size. Interestingly, the study revealed that overexpression of s6k1 alone enhanced cell size in the absence of rapamycin, whereas they worked together to further increase cell size if co-expressed. In this experiment, dietary Arg levels significantly affected the transcription level of TOR and s6k1 mRNA, which showed a similar trend with whole body protein content in S. paramamosain. Similar results were also reported in the studies on Portunus trituberculatus (Jin et al., 2016). These results indicated that appropriated dietary Arg could activate TOR signaling pathway, which could boost protein synthesis in this species. Similarly, optimal dietary Arg levels could also improve the transcription level of TOR and s6k1 in Cromileptes altivelis (Mu et al., 2020) and Ctenopharyngodon idellus (Chen et al., 2019). However, when the level of dietary Arg exceeded the optimum level (i.e., 3.07%), the transcription level of TOR and s6k1 were be significantly reduced. This phenomenon can be explained by the fact that Arg overload negatively affects insulin signaling through mTOR/s6k1 phosphorylation, leading to insulin resistance (Um et al., 2006; Wullschleger et al., 2006). The specific mechanism of Arg activation of the TOR signaling pathway in crustaceans is complex and needs to be further studied.
Phosphatases and antioxidant enzyme systems are important components of the non-specific immune system and have been widely used to assess the health of crustaceans (Wei et al., 2014; Dong et al., 2018; Fu et al., 2022). Phosphatase participated in many metabolic processes, including chitin secretion, calcium absorption, and calcium phosphate deposition (Robertson, 1937; Travis, 1955; Kobayashi et al., 1983). In this study, AKP activity was highest at 2.73% dietary Arg levels. Furthermore, antioxidant enzymes (e.g., SOD, CAT, and T-AOC) play a direct role in removing excess ROS and protecting cells from oxidative damage (Ke et al., 2011; Ighodaro and Akinloye, 2018). T-AOC can be used as a comprehensive index to assess the antioxidant capacity of the body. In this experiment, crab fed appropriated dietary Arg level (1.81-2.73%) have higher T-AOC activity, indicating the crab had better antioxidant ability. Similarly, the highest SOD activity was observed in crab fed diets with 2.5% Arg. Malondialdehyde (MDA) produced by endogenous oxidative damage in vivo is one of the final metabolites of lipid peroxidation, which can reflect the extent of lipid peroxidation and the extent of cell injure (Grotto et al., 2009). The lowest MDA concentration was observed in the treatment group with appropriate dietary Arg levels. These findings suggested that an adequate Arg content in diet can improve the health of S. paramamosain. Juvenile mud crabs must adapt to various environments from offshores to estuaries and settle on tidal flats or mangrove fringes, experiencing large variations in salinity throughout the entire process. Previous research found that salinity changes stimulate the production of ROS (Wang et al., 2022). Therefore, we suggest that an appropriate dietary Arg level (2.35% group) has benefits for early juvenile S. paramamosain.
Crabs lack adaptive immunity and must rely on innate immunity, to defend themselves against infectious pathogens (Iwanaga and Lee, 2005; Tran et al., 2020). As a crucial component of innate immunity, the prophenoloxidase (proPO) activating system is unique to invertebrates (Cerenius et al., 2008; Chen and Wang, 2019; Tran et al., 2020). In this study, the highest expression of proPO was observed at dietary Arg levels of 2.16% and 2.35%. This indicates that an adequate amount of Arg can boost the immunity of S. paramamosain by stimulating the proPO system. Similarly, it was reported that appropriate Arg levels (2.73% and 3.72%) in the diet can up-regulate proPO mRNA expression to increase the immunity and disease resistance of Eriochier sinensis. On the other hand, the activation and translocation of NF-kB are linked to the expression antibacterial peptide in invertebrates. Peroxinectin is an MPO homologue that can promote the nuclear translocation of the nuclear factor NF-kB (Lau et al., 2005; Lin et al., 2007). Therefore, we examined the gene expression of relish, an NF-B-like transcription factor, as well as antibacterial peptide genes such as lysozyme. The results showed that an appropriate dietary Arg level could increase the expression levels of relish and lysozyme. In summary, supplementing the appropriate Arg content in the diet can boost the immunity of S. paramamosain.
Conclusion
The current study founded that the dietary Arg requirement for postlaval S.paramamosain was 2.34% (5.20% of the dietary protein). Meanwhile, appropriate dietary Arg levels significantly increased the transcription level of igf-1, TOR, and s6k1-related genes, whereas excessive dietary Arg levels suppress the transcription of these genes. Furthermore, this study suggested that appropriated dietary Arg levels improves the antioxidant capacity as well as the immune system of postlarval S.paramamosain. Finally, future research is suggested to explore the specific mechanism behind the regulatory effect of arginine on the immunity in S.paramamosain, which will help with the development of a balanced diet with EAAs and the development of feed formulation for commercial purposes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was reviewed and approved by Institutional Animal Care and Use Committee of Zhejiang Ocean University.
Author contributions
DW designed the experiments with the help of JW. DW performed the experiments and drafted the manuscript. TH provided the experimental laboratory to ensure that the experiments could be carried out properly. WF and TH supervised the experimental procedure. HX and XYL analyzed the data. DW, XL and JW revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The Key Research and Development Program of Zhejiang Key Scientific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties (2021C02069-6) and Zhejiang Province “three agricultural six-party” science and technology collaboration program (2021SNLF030).
Conflict of interest
Author XL is employed by Guangdong Yuehai Feeds Group Company Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alam M. S., Teshima S.-i., Ishikawa M., Hasegawa D., Koshio S. (2004). Dietary arginine requirement of juvenile kuruma shrimp Marsupenaeus japonicus (Bate). Aquac. Res. 35, 842–849. doi: 10.1111/j.1365-2109.2004
Alam M. S., Teshima S.-i., Koshio S., Ishikawa M. (2002). Arginine requirement of juvenile Japanese flounder paralichthys olivaceus estimated by growth and biochemical parameters. Aquaculture 205, 127–140 doi: 10.1016/S0044-8486(01)00670-6
Alam M., Yaniharto D., Sumule O., Ishikawa M., Koshio S. (2005). Assessment of reference dietary amino acid pattern for juvenile red sea bream, Pagrus major. Aquac. Int. 13, 369–379. doi: 10.1007/s10499-005-0614-6
AOAC (1995). “Official methods of analysis of AOAC international,” in Official analytical chemists, 16th ed. Ed. Cunniff P. (Arlington, Virginia: AOAC International), 1141–1154.
Benzie I. F. F., Strain J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of ‘‘Antioxidant power’’: the FRAPA assay. Anal. Biochem. 239, 70–76. doi: 10.1006/abio.1996.0292
Birmani M. W., Raza A., Nawab A., Tang S., Ghani M. W., Li G., et al. (2019). Importance of arginine as immune regulator in animal nutrition. Int. J. Vet. Sci. Res. 5, 1–10. doi: 10.18488/journal.110.2019.51.1.10
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1–2), 248–254. doi: 10.1016/0003-2697(76)90527-3
Castine S., Southgate P. C., Zeng C. (2008). Evaluation of four dietary protein sources for use in microbound diets fed to megalopae of the blue swimmer crab, Portunus pelagicus. Aquaculture 281, 95–99. doi: 10.1016/j.aquaculture.2008.06.008
Catacutan M. R. (2002). Growth and body composition of juvenile mud crab, Scylla serrata, fed different dietary protein and lipid levels and protein to energy ratios. Aquaculture 208, 113–123. doi: 10.1016/S0044-8486(01)00709-8
Cerenius L., Lee B. L., Söderhäll K. (2008). The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 29, 263–271. doi: 10.1016/j.it.2008.02.009
Chen F., Wang K. (2019). Characterization of the innate immunity in the mud crab Scylla paramamosain. Fish Shellfish Immunol. 93, 436–448. doi: 10.1016/j.fsi.2019.07.076
Chen J., Zhang D., Tan Q., Zhou H., Yao J. (2019). Growth performance, intestinal morphology, hepatopancreatic antioxidant capacity and growth-related gene mRNA expressions of juvenile grass carp (Ctenopharyngodon idellus) as affected by graded levels of dietary arginine. Aquac. Nutr. 25, 1124–1134. doi: 10.1111/anu.12928
De Celis S. V.-R., Rojas P., Gómez-Requeni P., Albalat A., Gutiérrez J., Médale F., et al. (2004). Nutritional assessment of somatolactin function in gilthead sea bream (Sparus aurata): concurrent changes in somatotropic axis and pancreatic hormones. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 138, 533–542. doi: 10.1016/j.cbpb.2004.06.007
Dong J., Cheng R., Yang Y., Zhao Y., Wu G., Zhang R., et al. (2018). Effects of dietary taurine on growth, non-specific immunity, anti-oxidative properties and gut immunity in the Chinese mitten crab Eriocheir sinensis. Fish shellfish Immunol. 82, 212–219. doi: 10.1016/j.fsi.2018.08.029
Fingar D. C., Salama S., Tsou C., Harlow E., Blenis J. (2002). Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 16, 1472–1487. doi: 10.1101/gad.995802
Folch J., Lees M., Stanley G. H. S. (1957). A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 226 (1957), 497–509. doi: 10.1016/S0021-9258(18)64849-5
Fournier V., Gouillou-Coustans M., Metailler R., Vachot C., Moriceau J., Le Delliou H., et al. (2003). Excess dietary arginine affects urea excretion but does not improve n utilisation in rainbow trout Oncorhynchus mykiss and turbot Psetta maxima. Aquaculture 217, 559–576. doi: 10.1016/S0044-8486(02)00420-9
Fu C., Cui Z., Shi X., Liu J., Jiang Y., Zhang R. (2022). Effects of dietary glyceryl monolaurate supplementation on growth performance, non-specific immunity, antioxidant status and intestinal microflora of Chinese mitten crabs. Fish Shellfish Immunol. 125, 65–73. doi: 10.1016/j.fsi.2022.05.004
Grotto D., Maria L. S., Valentini J., Paniz C., Schmitt G., Garcia S. C., et al. (2009). Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quimica Nova 32, 169–174. doi: 10.1590/S0100-40422009000100032
Góth L. (1991). A simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta 196 (2-3), 143–152. doi: 10.1016/0009-898(91)90067-M
Gu D., Zhao J., Limbu S. M., Liang Y., Deng J., Bi B., et al. (2022). Arginine supplementation in plant-rich diets affects growth, feed utilization, body composition, blood biochemical indices and gene expressions of the target of rapamycin signaling pathway in juvenile Asian red-tailed catfish (Hemibagrus wyckoiides). J. World Aquac. Soc. 53, 133–150. doi: 10.1111/jwas.12748
Hara K., Yonezawa K., Weng Q.-P., Kozlowski M. T., Belham C., Avruch J. (1998). Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 273, 14484–14494. doi: 10.1074/jbc.273.23.14484
Harrington L. S., Findlay G. M., Gray A., Tolkacheva T., Wigfield S., Rebholz H., et al. (2004). The TSC1-2 tumor suppressor controls insulin–PI3K signaling via regulation of IRS proteins. J. Cell Biol. 166, 213–223. doi: 10.1083/jcb.200403069
Herring C. M., Bazer F. W., Wu G. (2021). Amino acid nutrition for optimum growth, development, reproduction, and health of zoo animals. Adv. Exp. Med. Biol. 1285, 233–253. doi: 10.1007/978-3-030-54462-1_12
Huang Z., Aweya J. J., Zhu C., Tran N. T., Hong Y., Li S., et al. (2020). Modulation of crustacean innate immune response by amino acids and their metabolites: inferences from other species. Front. Immunol. 11. doi: 10.3389/fimmu.2020.574721
Ighodaro O., Akinloye O. (2018). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 54, 287–293. doi: 10.1016/j.ajme.2017.09.001
Iwanaga S., Lee B.-L. (2005). Recent advances in the innate immunity of invertebrate animals. BMB Rep. 38, 128–150. doi: 10.5483/BMBRep.2005.38.2.128
Izutsu A., Tadokoro D., Habara S., Ugachi Y., Shimizu M. (2022). Evaluation of circulating insulin-like growth factor (IGF)-I and IGF-binding proteins as growth indices in rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 320, 114008. doi: 10.1016/j.ygcen.2022.114008
Jin M., Zhou Q., Wang M., Huo Y., Huang W., Mai K. (2016). Dietary arginine requirement of juvenile swimming crab, Portunus trituberculatus. Aquac. Nutr. 22, 1174–1184. doi: 10.1111/anu.12350
Ke C., Sun L., Qiao D., Wang D., Zeng X. (2011). Antioxidant acitivity of low molecular weight hyaluronic acid. Food Chem. Toxicol. 49 (10), 2670–2675. doi: 10.1016/j.fct.2011.07.020
Kobayashi K., Matsui M., Haraguchi H., Fuwa K. (1983). Identification of alkaline phosphatase in sea water. J. inorg. Biochem. 18, 41–47. doi: 10.1016/0162-0134(83)85038-7
Lansard M., Panserat S., Plagnes-Juan E., Seiliez I., Skiba-Cassy S. (2010). Integration of insulin and amino acid signals that regulate hepatic metabolism-related gene expression in rainbow trout: role of TOR. Amino Acids 39, 801–810. doi: 10.1007/s00726-010-0533-3
Lau D., Mollnau H., Eiserich J. P., Freeman B. A., Daiber A., Gehling U. M., et al. (2005). Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc. Natl. Acad. Sci. 102, 431–436. doi: 10.1073/pnas.0405193102
Liang H., Ren M., Habte-Tsion H. M., Ge X., Xie J., Mi H., et al. (2016). Dietary arginine affects growth performance, plasma amino acid contents and gene expressions of the TOR signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 461, 1–8. doi: 10.1016/j.aquaculture.2016.04.009
Li X., Han T., Zheng S., Wu G. (2021a). Nutrition and functions of amino acids in aquatic crustaceans. Amino Acids Nutr. Health 1285, 169–198. doi: 10.1007/978-3-030-54462-1_9
Lin X., Cerenius L., Lee B. L., Söderhäll K. (2007). Purification of properoxinectin, a myeloperoxidase homologue and its activation to a cell adhesion molecule. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1770, 87–93. doi: 10.1016/j.bbagen.2006.06.018
Li X., Zheng S., Wu G. (2021b). Nutrition and functions of amino acids in fish, in: Amino acids in nutrition and health Vol. 1285 (Springer), 133–168. doi: 10.1007/978-3-030-54462-1_8
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25 (4), 402–408. doi: 10.1006/meth.2001.1262
Millamena O. M., Bautista-Teruel M., Reyes O., Kanazawa A. (1998). Requirements of juvenile marine shrimp, Penaeus monodon (Fabricius) for lysine and arginine. Aquaculture 164, 95–104. doi: 10.1016/S0044-8486(98)00179-3
Mu W., Wang X., Wu X., Li X., Dong Y., Geng L, et al. (2020). The optimal arginine requirement in diets for juvenile humpback grouper, Cromileptes altivelis. Aquaculture 514, 734509. doi: 10.1016/j.aquaculture.2019.734509
Newsholme P., Brennan L., Rubi B., Maechler P. (2005). New insights into amino acid metabolism, β-cell function and diabetes. Clin. Sci. 108, 185–194. doi: 10.1042/CS20040290
NRC (2011). Nutrient requirements of fish and shrimp (Washington, DC: National Academy Press). doi: 10.1007/s10499-011-9480-6
Palma J., Andrade J. P., Lemme A., Bureau D. P. (2015). Quantitative dietary requirement of juvenile a tlantic ditch shrimp Palaemonetes varians for lysine, methionine and arginine. Aquac. Res. 46, 1822–1830. doi: 10.1111/are.12335
Paudel S., Wu G., Wang X. (2021). Amino acids in cell signaling: regulation and function. Amino Acids Nutr. Health 1332, 17–33. doi: 10.1007/978-3-030-74180-8_2
Philipps A. F., Persson B., Hall K., Lake M., Skottner A., Sanengen T., et al. (1988). The effects of biosynthetic insulin-like growth factor-1 supplementation on somatic growth, maturation, and erythropoiesis on the neonatal rat. Pediatr. Res. 23, 298–305. doi: 10.1203/00006450-198803000-00014
Picha M. E., Turano M. J., Beckman B. R., Borski R. J. (2008). Endocrine biomarkers of growth and applications to aquaculture: a minireview of growth hormone, insulin-like growth factor (IGF)-I, and IGF-binding proteins as potential growth indicators in fish. North Am. J. aquaculture 70, 196–211. doi: 10.1577/A07-038.1
Pohlenz C., Buentello A., Miller T., Small B. C., MacKenzie D. S., Gatlin D. M. III (2013). Effects of dietary arginine on endocrine growth factors of channel catfish, Ictalurus punctatus. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 166, 215–221. doi: 10.1016/j.cbpa.2013.06.016
Qi C., Wang X., Han F., Jia Y., Lin Z., Wang C., et al. (2019). Arginine supplementation improves growth, antioxidant capacity, immunity and disease resistance of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 93, 463–473. doi: 10.1016/j.fsi.2019.07.082
Ren M., Liao Y., Xie J., Liu B., Zhou Q., Ge X., et al. (2013). Dietary arginine requirement of juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 414, 229–234. doi: 10.1016/j.aquaculture.2013.08.021
Reyes A. A., Karl I. E., Klahr S. (1994). Role of arginine in health and in renal disease. Am. J. Physiol. 267, 331–346. doi: 10.1152/ajprenal.1994.267.3
Robbins K. R., Norton H. W., Baker D. H. (1979). Estimation of nutrient requirements from growth data. J. Nutr. 109 (10), 1710–1714. doi: 10.1093/jn/109.10.1710
Robertson J. D. (1937). Some features of the calcium metabolism of the shore crab (Carcinus maenas pennant). Proc. R. Soc. London Ser. B-Biol. Sci. 124, 162–182. doi: 10.1098/rspb.1937.0080
Serrana D. G. D. L., Johnston I. A. (2013). Expression of heat shock protein (Hsp90) paralogues is regulated by amino acids in skeletal muscle of Atlantic salmon. PloS One 8, e74295. doi: 10.1371/journal.pone.0074295
Sun S., Wang B., Jiang K., Sun J., Liu M., Wang L. (2015). Target of rapamycin (TOR) in Fenneropenaeus chinensis: cDNA cloning, characterization, tissue expression and response to amino acids. Aquac. Nutr. 21, 1–9. doi: 10.1111/anu.12133
Tran N. T., Kong T., Zhang M., Li S. (2020). Pattern recognition receptors and their roles on the innate immune system of mud crab (Scylla paramamosain). Dev. Comp. Immunol. 102, 103469. doi: 10.1016/j.dci.2019.103469
Travis O. F. (1955). The molting cycle of the spiny lobster, panulirus argus latreille. II. pre-ecdysial histological and histochemical changes in the hepatopancreas and integumental tissues. Biol. Bull. 108, 88–112. doi: 10.2307/1538400
Tu Y., Xie S., Han D., Yang Y., Jin J., Zhu X. (2015). Dietary arginine requirement for gibel carp (Carassis auratus gibelio var. CAS III) reduces with fish size from 50 g to 150 g associated with modulation of genes involved in TOR signaling pathway. Aquaculture 449, 37–47. doi: 10.1016/j.aquaculture.2015.02.031
Um S. H., D'Alessio D., Thomas G. (2006). Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 3, 393–402. doi: 10.1016/j.cmet.2006.05.003
Walton M. J. (1985). Aspects of amino acid metabolism in teleost fish. Nutr. feed. fish 1985, 47–67.
Wang X., Yao Q., Zhang D.-m., Lei X.-y., Wang S., Wan J.-w., et al. (2022). Effects of acute salinity stress on osmoregulation, antioxidant capacity and physiological metabolism of female Chinese mitten crabs (Eriocheir sinensis). Aquaculture 552, 737989. doi: 10.1016/j.aquaculture.2022.737989
Wei J., Yu N., Tian W., Zhang F., Wu Q., Li E., et al. (2014). Dietary vitamin B12 requirement and its effect on non-specific immunity and disease resistance in juvenile Chinese mitten crab Eriocheir sinensis. Aquaculture 434, 179–183. doi: 10.1016/j.aquaculture.2014.08.010
Wilson R. P. (2003). Amino acids and proteins, fish nutrition. Amino Acids and Proteins 3, 143–179. doi: 10.1016/B978-012319652-1/50004-5
Wu G. (2014). Amino acid nutrition in animals: Protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2, 387–417. doi: 10.1146/annurev-animal-022513-114113
Wu G. (2022). Nutrition and metabolism: Foundations for animal growth, development, reproduction, and health. Recent Adv. Anim. Nutr. Metab. 1354, 21–24. doi: 10.1007/978-3-030-85686-1_1
Wu G., Bazer F. W., Davis T. A., Kim S. W., Li P., Marc Rhoads J., et al. (2009). Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37, 153–168. doi: 10.1007/s00726-008-0210-y
Wullschleger S., Loewith R., Hall M. N. (2006). TOR signaling in growth and metabolism. Cell 124, 471–484. doi: 10.1016/j.cell.2006.01.016
Wu M., Wu X., Lu S., Gao Y., Yao W., Li X., et al. (2018). Dietary arginine affects growth, gut morphology, oxidation resistance and immunity of hybrid grouper (Epinephelus fuscoguttatus♀× epinephelus lanceolatus♂) juveniles. Br. J. Nutr. 120, 269–282. doi: 10.1017/S0007114518001022
Xie J. W., Cheng C. H., Ma H. L., Feng J., Su Y. L., Deng Y. Q., et al. (2019) Molecular characterization, expression and antimicrobial activities of a c-type lysozyme from the mud crab, Scylla paramamosain. Dev. Comp. Immunol. 98, 54–64. doi: 10.1016/j.dci.2019.04.002
Xu H., Han T., Li X., Wang J., Zheng P., Yin F., et al. (2020). Effects of dietary lipid levels on survival, growth performance, and antioxidant ability of the early juvenile Scylla paramamosain. Aquaculture 528, 735559. doi: 10.1016/j.aquaculture.2020.735559
Xu H. Y., Wang J. T., Han T., Li X. Y., Zheng P. Q., Wang C. L. (2018). Effects of dietary phospholipids levels on growth performance, lipid metabolism, and antioxidant capacity of the early juvenile green mud crab, Scylla paramamosain (Estampador). Aquac. Res. 50 (9), 513–520. doi: 10.1111/are.13922
Xu H., Wang J., Han T., Li X., Zheng P., Yin F., et al. (2019). Effects of dietary phospholipids levels on growth performance, lipid metabolism, and antioxidant capacity of the early juvenile green mud crab, Scylla paramamosain (Estampador). Aquac. Res. 50, 513–520. doi: 10.1111/are.13922
Yamamoto T., Unuma T., Akiyama T. (2000). The influence of dietary protein and fat levels on tissue free amino acid levels of fingerling rainbow trout (Oncorhynchus mykiss). Aquaculture 182, 353–372. doi: 10.1016/S0044-8486(99)00277-X
Ye J., Wang Y., Guo J., Chen J., Pan Q., Shen B. (2010). Lysine, methionine and arginine requirements of juvenile Chinese mitten crab (Eriocheir sinensis). J. Fish. China 34, 1541–1548. doi: 10.3724/SP.J.1231.2010.06944
Zhao J., Wen X., Li S., Zhu D., Li Y. (2015). Effects of dietary lipid levels on growth, feedutilization, body composition and antioxidants of juvenile mud crab Scylla paramamosain (Estampador). Aquaculture 435, 200–206. doi: 10.1016/j.aquaculture.2014.09.018
Zheng P., Han T., Li X., Wang J., Su H., Xu H., et al. (2020). Dietary protein requirement of juvenile mud crab Scylla paramamosain. Aquaculture 518, 734852. doi: 10.1016/j.aquaculture.2019.734852
Zheng P., Wang J., Han T., Yang M., Li X., Wang C. (2018). Effect of dietary cholesterol levels on growth performance, body composition and gene expression of juvenile mud crab Scylla paramamosain. Aquac. Res. 49, 3434–3441. doi: 10.1111/are.13807
Zhou Q. C., Zeng W. P., Wang H. L., Wang T., Wang Y. L., Xie F. J. (2012). Dietary arginine requirement of juvenile pacific white shrimp, Litopenaeus vannamei. Aquaculture 364, 252–258. doi: 10.1016/j.aquaculture.2012.08.020
Keywords: arginine, growth performance, protein synthesis, health status, Scylla paramamosain
Citation: Wu D, Feng W, Li X, Xu H, Luan X, Han T and Wang J (2022) Effect of dietary arginine levels on growth performance, protein synthesis, antioxidant capacity and immunity of postlarval mud crab Scylla paramamosain. Front. Mar. Sci. 9:1025879. doi: 10.3389/fmars.2022.1025879
Received: 23 August 2022; Accepted: 28 September 2022;
Published: 17 October 2022.
Edited by:
Seyyed Morteza Hoseini, Iranian Fisheries Science Research Institute (IFSRI), IranReviewed by:
Morteza Yousefi, Peoples’ Friendship University of Russia, RussiaIgo Guimaraes, Universidade Federal de Jataí, Brazil
Ali Taheri Mirghaed, University of Tehran, Iran
Copyright © 2022 Wu, Feng, Li, Xu, Luan, Han and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiteng Wang, d2FuZ2ppdGVuZzE5NzFAZ21haWwuY29t
 Duoting Wu
Duoting Wu Wenping Feng
Wenping Feng Xinyu Li2
Xinyu Li2 Hanying Xu
Hanying Xu Tao Han
Tao Han Jiteng Wang
Jiteng Wang