
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 14 September 2022
Sec. Marine Biology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1018565
This article is part of the Research Topic Integration of Development, Physiology and Responses to Environmental Change in Aquatic Invertebrates View all 13 articles
Based on the existing research, special plastic baskets were used as anti-injury shelters to explore the hidden behavior and molting growth of Portunus trituberculatus (Swimming crab) different initial body weights (Group A: 5.74 ± 0.11 g, Group B: 12.06 ± 0.15 g, Group C: 24.82 ± 0.41 g, Group D: 49.55 ± 1.12 g and Group E: 94.32 ± 1.19 g). The results showed that the shelter occupancy rate (SOR) during the daytime with all different body weights were significantly higher than that at night (P < 0.05), and the SOR was proportional to the crab’s body weight, among them, SOR in group E was as high as 71.52%. Meanwhile, the territorial consciousness of smaller body weight crabs (Groups A, B and C) was poor, and there was a phenomenon in which multiple individuals occupied the same shelter at the same time, while the individuals with the body weight of approximately 50 g and above (Groups D and E) had strong territorial consciousness, and most of them occupied one shelter alone. In all groups, more individuals chose to molt in the shelter on condition that there existed shelter, and the rate of molting in group B was high up to 81.15% and that in other groups was about 60%. Although the existence of shelter had no significant influence on the molting interphase (MI) of swimming crab in each group, the body weight growth rate (WGR) and carapace width growth rate (WGRC) after molting were increased by shelter compared with those without shelter. In addition, shelter could improve the survival rate (SR) in each group, and the effect of shelter on individuals with large body weight was relatively more obvious, in which the SR in Group D was significantly improved (P < 0.05). In general, the shelter can play a positive role in the whole growth of swimming crab. Therefore, it is necessary to set up the shelter in advance before the seedlings are put into production, which is helpful to increase the yield of swimming crab.
Cannibalism refers to the behavioral characteristics of injury or death caused by mutual attacks, struggles and fighting among individuals of the same species (Polis, 1981; Marshall et al., 2005). It can effectively reduce the waste of space and food by the vulnerable individuals and enable the winners obtain more living materials, survival space, mating rights and other benefits, and have positive effects on selecting the best and most preserving seed (Liu, 2002; Li and Sun, 2013). However, cannibalism has also resulted in massive deaths of aquatic organisms, which is an exceedingly severe problem in the aquaculture industry. Many researchers have found that aquatic cannibalism is caused by a variety of complex factors, including individual size differences, feed density, feed species, stock density, neurochemical factors, temperature, light, water turbidity, and so on (Chen, 2006; Chen et al., 2008; Qin and Li, 2014; Wang et al., 2015).
It has been proved that the use of suitable shelters can reduce the incidence of cannibalism of aquatic organisms, thereby improving the survival rate and yield per unit area. For example, the use of halved PVC agricultural drainage pipes can provide a hiding space for the Salvelinus alpinus, which is conducive to the growth of the S. alpinus and reduces mortality (Benhaïm et al., 2009). The survival rate and growth rate of Callinectes sapidus growing in the vegetated environment were significantly higher than those in the non-vegetated group (Perkins-Visser et al., 1996). In the condition of wavy nets, seaweed, plastic threads and bamboo tubes as shelters, Scylla serrata survived significantly better (Mann et al., 2007; Mirera and Moksnes, 2013). Maja squinado showed a significantly higher growth rate and survival rate in the natural seaweed shelter group than those in the artificial coir fiber shelter group (Gil et al., 2019).
Swimming crab is one of the dominant species of aquaculture in China. Whereas, its aggressive nature, strong territoriality and extremely serious cannibalism lead to low unit yield, which has been hindering the healthy and sustainable development of this species in the aquaculture industry (He et al., 2016). Our previous research found that plastic baskets, as shelters, had an unexpectedly positive effect on reducing cannibalism, improving the survival rate and promoting the growth and development of swimming crab, and the survival rate was up to 68% (He et al., 2017). Moreover, according to this result, a special shelter was developed (Chinese Patent No. ZL2018206226941.X) for swimming crab culture, which is now widely used in China, with an area of 800 ha in Zhejiang Province alone. However, it is uncertain about the confusion at which growth stage of swimming crab the shelter begins to have a better effect, in other words, we are not sure when the best time to place the shelter is. Consequently, this study aims to investigate the hidden behavior and molting growth rule of swimming crab with different body weights, accompanied by the presence of shelters. On the one hand, to understand the hidden habits with different body weights and enrich the theoretical knowledge of the behavioral ecology of swimming crab. On the other hand, to analyze the effect of shelter on the survival and growth at different growth stages. These points will provide a scientific basis for further elucidating the mechanism of shelter in aquaculture production and optimizing the application technology of shelter in aquaculture production of swimming crab.
This experiment was conducted at the test site of Zhejiang Marine Fisheries Research Institute in July 2020. The experimental crabs were captured from the same aquaculture pond by the bait trapping method and placed in the indoor cement pool (6 × 1.8 × 0.7 m) for temporary culture. The experimental water was sand filter seawater, the water depth was approximately 0.4 m, and the oxygen was continuously increased for 24 h. Fresh trash fish were fed at 16: 00 every day, and the feed amount was approximately 5%~10% of the crab’s weight. The following morning at 7:00, the residual feed and excrement were removed, and two-thirds of the water was exchanged. During the temporary culture period, the water temperature was kept at 26.7 ± 1.4°C, the salinity was at 25.9 ± 0.9, and dissolved oxygen (DO) > 5 mg/L.
The experiment was carried out in the indoor cement pool (6 × 1.8 × 0.7 m). Each cement pool was divided into three small pools of equal size, and the small pools adjacent to each other were connected, such that the water could flow freely, but the crabs could not pass freely. The total number of small pools was 6 (3 without and 3 with shelters), and 12 shelters were placed in each of the three pools (The shelter is shown in Figure 1), The arrangement was 3 rows×4 columns, with 35 cm between the front and back and 15 cm between the left and right, and the openings were in the same direction. 5 different body weight groups (A, B, C, D and E) were set up in the experiment. They were 5.74 ± 0.11 g, 12.06 ± 0.15 g, 24.82 ± 0.41 g, 49.55 ± 1.12 g and 94.32 ± 1.19 g, respectively. A comparative study on the hidden behavior and growth rule of five groups (A, B, C, D and E) was carried out successively. In the same weight group, a total of 108 juvenile crabs of similar size and sound appendages were selected from each group. 18 juvenile crabs were randomly placed in each small pool, the weight of the crabs was weighed, and their carapace width was measured before placement, and the crabs were numbered on their carapaces with a marker pen. The mean body weights of the no shelter group (NSG) were 5.67 ± 0.12 g, 12.02 ± 0.07 g, 24.76 ± 0.68 g, 49.59 ± 1.07 g, and 94.09 ± 1.16 g, respectively, while those of the shelter group (SG) were 5.80 ± 0.10 g, 12.10 ± 0.25 g, 24.88 ± 0.08 g, 49.50 ± 1.27 g, and 94.55 ± 1.27 g, respectively.
During the experiment, the water depth in the pool was maintained at 0.4 m, and oxygen was continuously provided. Enough fresh wild fish was fed at 16:00 each afternoon, and then the residual feed was removed and the water was changed by 1/2 at 8:00 the next morning. Moreover, the water temperature was 26.9 ± 1.2°C, salinity was 26.1 ± 0.1, pH was 8.3 ± 0.1, DO > 5 mg/L, and light/dark = 12 h:12 h.
After the experiment began, the activities and hidden behaviors of crabs were observed continuously, and the hidden situation was recorded every 3 hours. In addition, the molt time and molt position of crabs were observed daily. Molting time divided into daytime (6:00-18:00) and night (18:00-6:00), and molting position divided into inside the shelter and outside the shelter. The newly molted crabs were gently dried with a towel after their carapace hardened and weighed and measured for carapace width. After the measurement, renumber the crabs on their carapace and put back into the original pool for further cultivation until all crabs in that group have completed one molt. Afterwards, the shelters occupancy rate (SOR), hidden rate (HR), molting rate during day or night (MRD/MRN), molting rate in the shelter (MRS), molting interphase (MI), survival rate (SR), weight growth rate (WGR), carapace width growth rate (WGRc), weight specific growth rate (SGRw) and carapace width specific growth rate (SGRc) were calculated according to the following formulas.
SOR = occupied shelters/total shelters × 100%
HR = hiding individuals/real-time survivals × 100%
MRD = molting individuals in daytime/total molting individuals × 100%
MRN = molting individuals at night/total molting individuals × 100%
MRS = molting individuals in shelter/total molting individuals × 100%
MI = molting time − initial time
SR = final survivals/initial individuals × 100%
WGR = (final weight − initial weight)/initial weight × 100%
WGRc = (final carapace width − initial carapace width)/initial carapace width ×100%
SGRw = (ln weight after molting − ln initial weight)/molting interphase × 100%
SGRc = (ln carapace width after molting − ln initial carapace width)/molting interphase × 100%
All data are expressed as the mean ± standard deviation. SPSS 22.0 software was used to analyze the experimental data. The homogeneity of variance was detected according to Levene’s method, and the inverse sine or square root processing was performed when the homogeneity variance was not satisfied. Independent samples T test were used to examine the differences in each index between the same group of swimming crab with and without shelters. Origin 2018 software was used for drawing, and P < 0.05 was taken as the significant difference.
After the beginning of the experiment, most crabs swam around the pond and slowly hided into the shelter in the SG, some individuals would hide in the shelter, with their bodies close to the inner wall of the shelter, some individuals were close to the outer wall of the shelter, while others were located near the pool wall. However, all crabs in the NSG were distributed around the wall of the pool, and few crabs moved in the center of the pool.
Figure 2 shows the SOR in the daytime (58.58%~71.52%) was significantly higher than that at night (26.50%~37.92%). Overall, with the increase in the body weight of the crab, the SOR gradually increased during the daytime, especially in Group E, which was as high as 71.52%, a value that was significantly higher than that in Groups A, B and C (P < 0.05). As for the HR, it is also significantly higher in the daytime than at night, but the difference in each group was small, basically between 57.14% and 64.20%, of which Group B had the highest HR in the daytime (64.20%), followed by Group E with 61.40% (Figure 3). In addition, we found that there were different numbers of crabs in a single shelter, including 1 ind/shelter, 2 ind/shelter, 3 ind/shelter, 4 ind/shelter and 5 ind/shelter. In Group B, there were even 6 ind/shelter, while in Groups D and E, there were at most 3 crabs in a shelter, and most individuals occupied one shelter alone. Furthermore, if other crabs try to enter the shelter where crabs were already present, the crab in the shelter will open their chelipeds to deter and drive the invaders away. Therefore, other crabs that fail to occupy the shelter can only be forced to hide around the outer shelter wall or the cement pool wall. By comparison, the proportion of multiple individuals in a shelter in the smaller body weight group (Groups A, B and C) was significantly higher than that in the larger body weight group (Groups D and E), and the probability of 1 ind/shelter in the larger body weight group (Groups D and E) was high up to 77% (Figure 4). As a whole, the proportion of multiple crabs in a shelter decreased as the body weight of crabs increased.
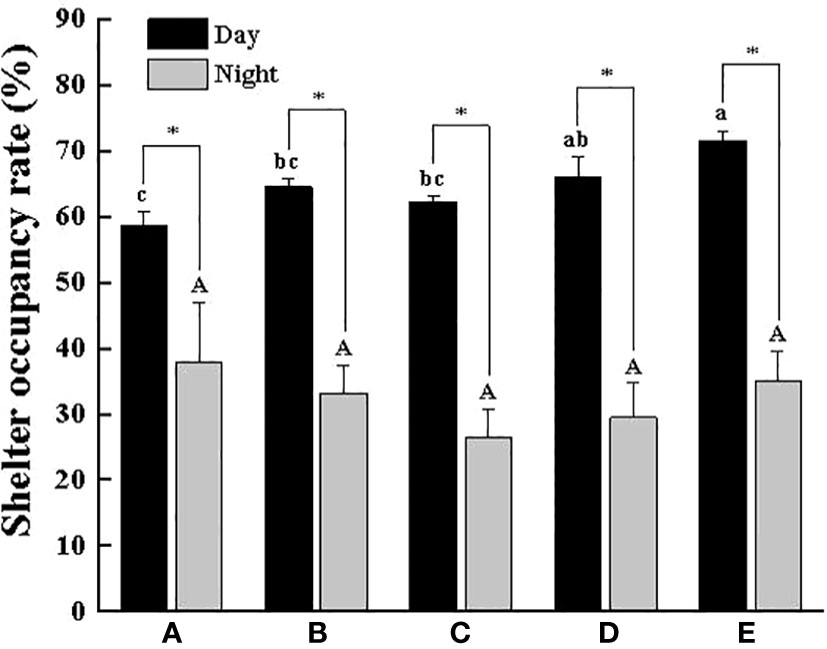
Figure 2 Shelter occupancy rate of swimming crab with different body weight groups in the presence of shelters. Different letters indicate significant differences among different body weight groups (P < 0.05) and “*” indicates significant differences between day and night (P < 0.05).
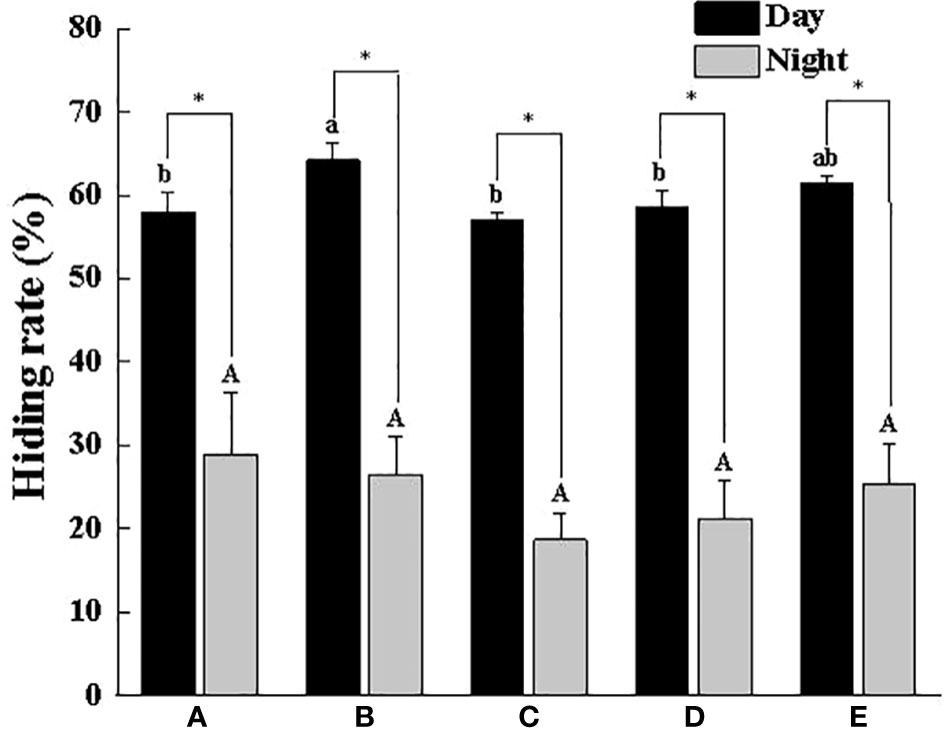
Figure 3 Hidden rate of swimming crab with different body weight groups in the presence of shelters. Different letters indicate significant differences among different body weight groups (P < 0.05) and “*” indicates significant differences between day and night (P < 0.05).
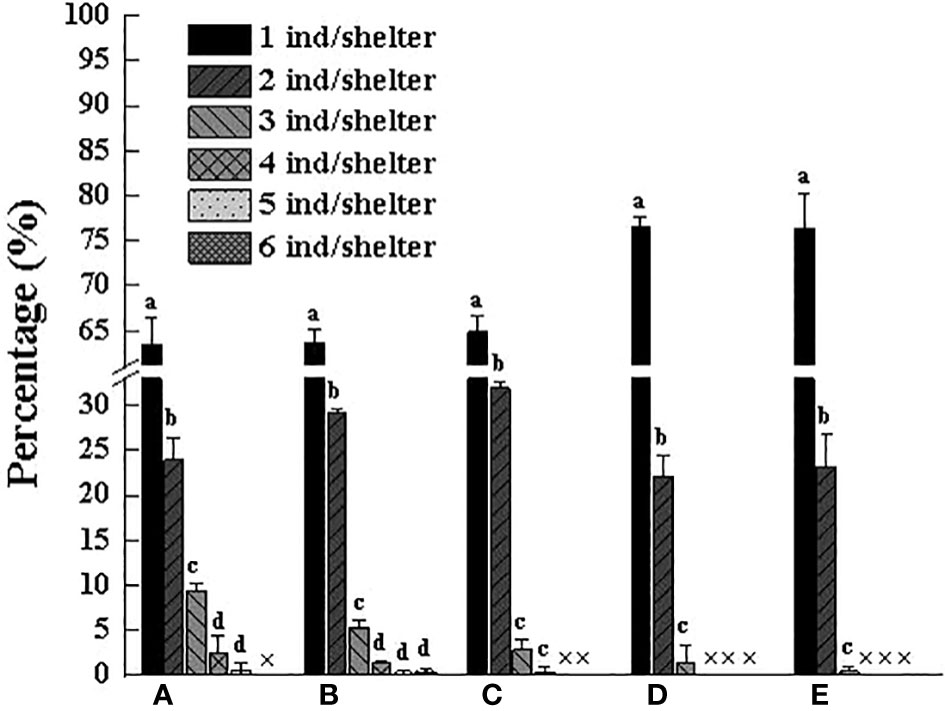
Figure 4 The percentage of the number of swimming crabs in each body weight group in the same shelter at the same time to the total number of the group. Different letters indicate significant differences among different numbers of swimming crab under the same shelter (P < 0.05).
After some intervals, the crabs started to molt. The molting of crabs with different body weights during the day or night was not significantly affected by the shelter on the basis of Figure 5, indicating that the existence of shelter did not change the molting habits of the crab. Additionally, there were some differences in the molting habits of different body weight crabs, especially among Groups A, B, C and D. With the increase of body weight, the MRD was higher.
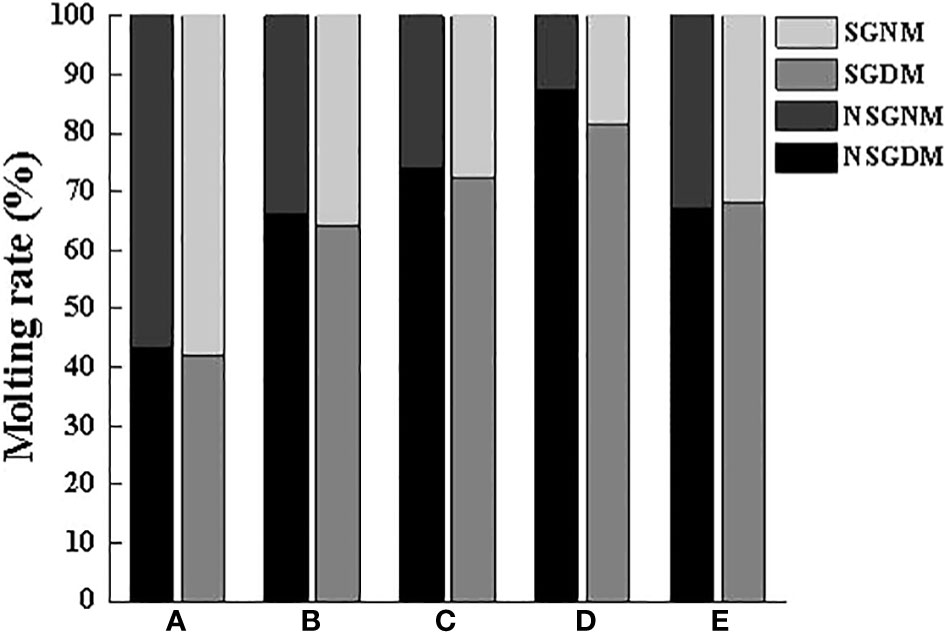
Figure 5 Molting rate of swimming crab in daytime and at night with different body weight groups in the presence and absence of shelters. NSGDM, No shelter group molting during the day; NSGNM, No shelter group molting during the night; SGDM, Shelter group molting during the day; SGNM, Shelter group molting during the night.
Under the condition of the existence of the shelter, most crabs choose to molt inside the shelter. Overall, no matter which body weight group, more than half individuals would choose to molt in the shelter. Among them, Group B had the largest number of individuals molting in the shelter, and the MRS was high up to 81.15%, followed by Group A with 66.01%, and the lowest was 57.70% in Group E (Figure 6). The MI of all groups was less affected by shelter (Figure 7), but the MI in Groups B, D and E was slightly shortened in the presence of shelter. These results demonstrate that the larger the crab’s body weight is, the longer the MI is, regardless of the presence of shelters
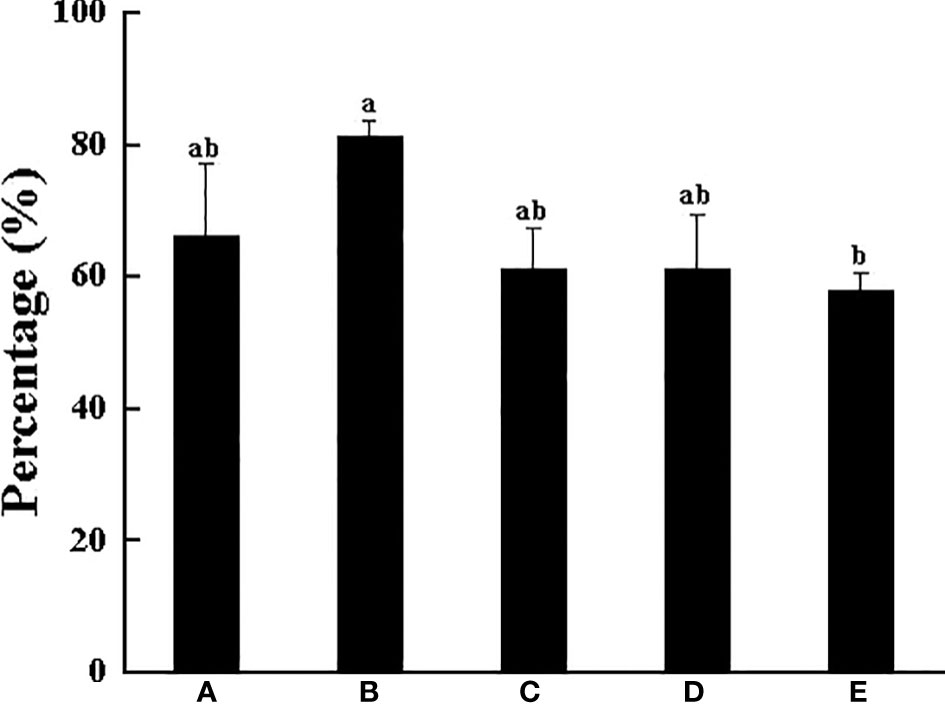
Figure 6 The percentage of swimming crab completing molting under the shelter with different body weight groups. Different letters indicate significant differences among different body weight groups (P < 0.05).
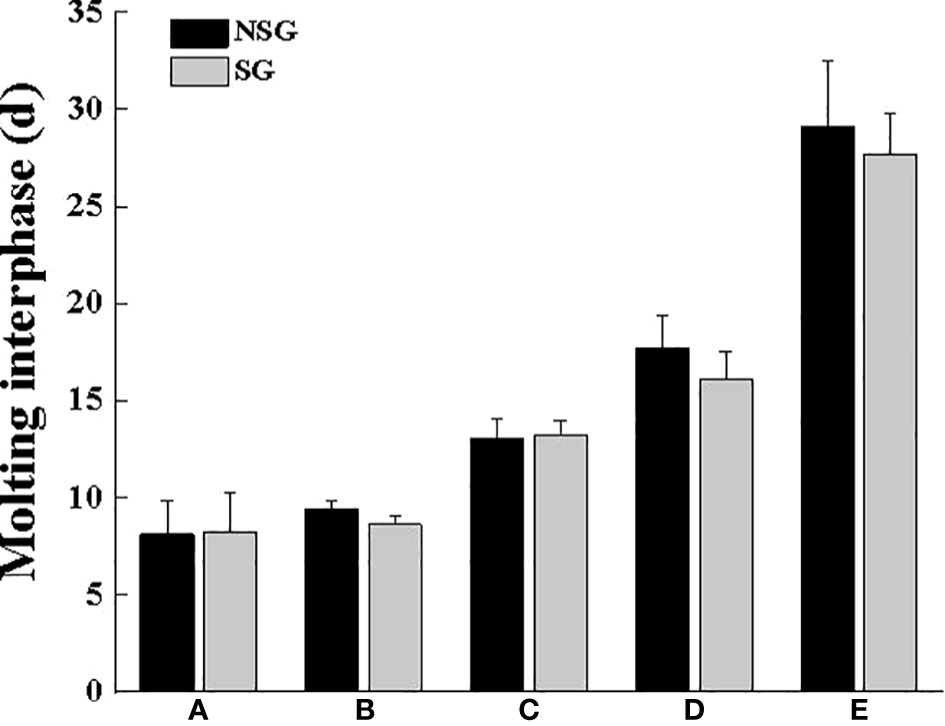
Figure 7 Molting interphase of swimming crab with different body weight groups between NSG and SG. Unmarked letters indicate no significant difference (P > 0.05).
No matter whether there were shelter or not, as long as there was no molting crab, all the crabs get along well. There was basically no aggressive behavior except for normal food grabbing, and several crabs gathered in the corner. During the experiment, no crabs died from the diseases and hunger. However, the intra-specific aggression and predation did cause a high mortality during the molting period, especially in the soft-shell phase. The SR with shelter was higher than that without shelter (Figure 8), In particular, the SR of group D in SG was significantly higher than that in NSG (P <0.05), indicating that the shelter showed a better anti-injury effect. Additionally, whether the shelter existed or not, there was a tendency that the larger the crab weight, the more serious the intraspecific cannibalism phenomenon and the lower the SR
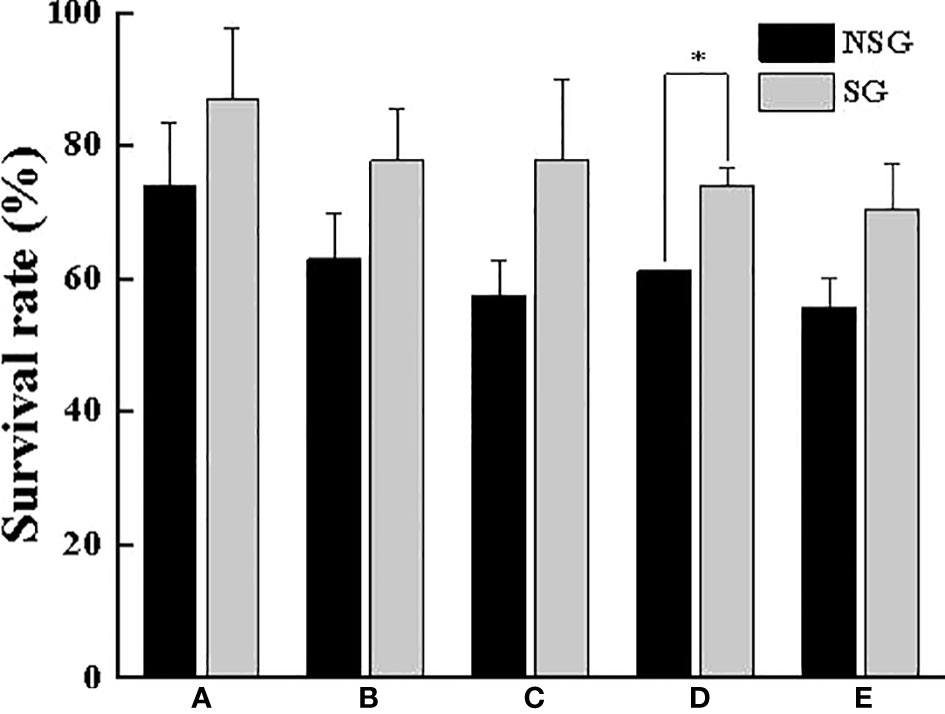
Figure 8 Survival rate of swimming crab with different body weight between NSG and SG. “*” indicates significant difference (P < 0.05).
After one molting, the body weight and carapace width of all surviving crabs changed evidently. The body weight, carapace width, WGR and WGRC of all groups in the SG were higher than those in the NSG (Table 1; Figures 9 and 10). In particular, the WGR and WGRC in Group A in the presence of shelter was significantly higher than that in the absence of shelter (P < 0.05), indicating that shelter has a certain promoting effect on the growth performance of different body weights. Regardless of the presence or absence of shelter, the larger the body weight of crab was, the smaller of the WGR and SGR after molting.
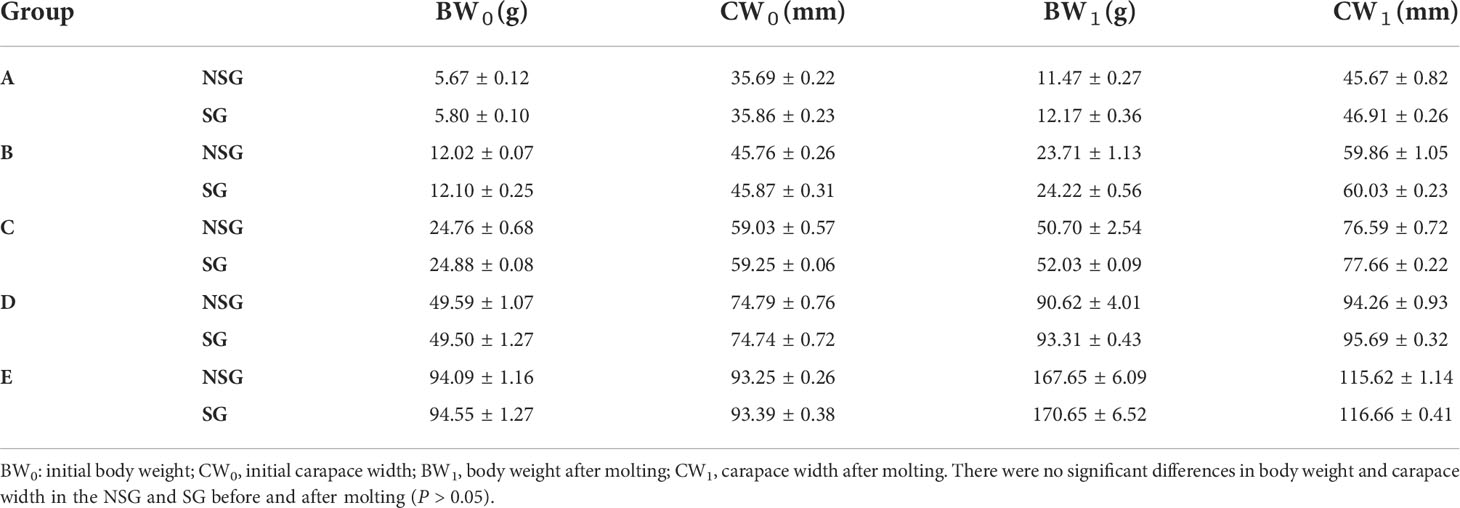
Table 1 Changes in body weight and carapace width of swimming crab with different body weight in the NSG and SG.
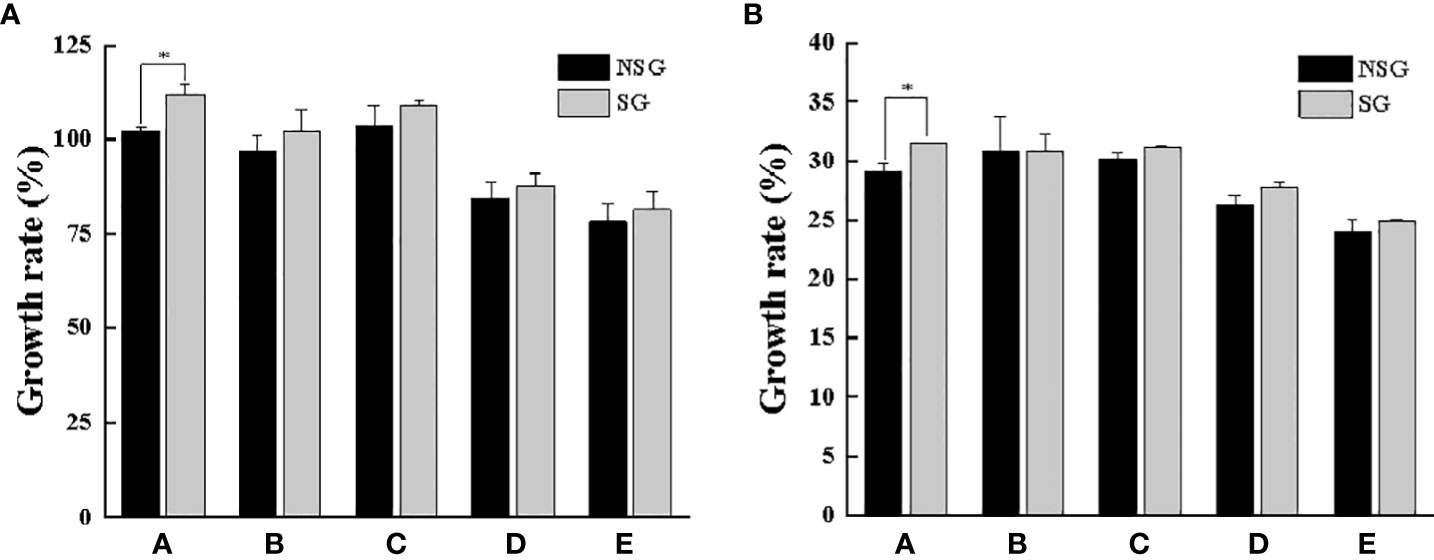
Figure 9 Growth rate of body weight (A) and carapace width (B) of swimming crab with different body weight between NSG and SG. “*” indicates significant differences (P < 0.05).
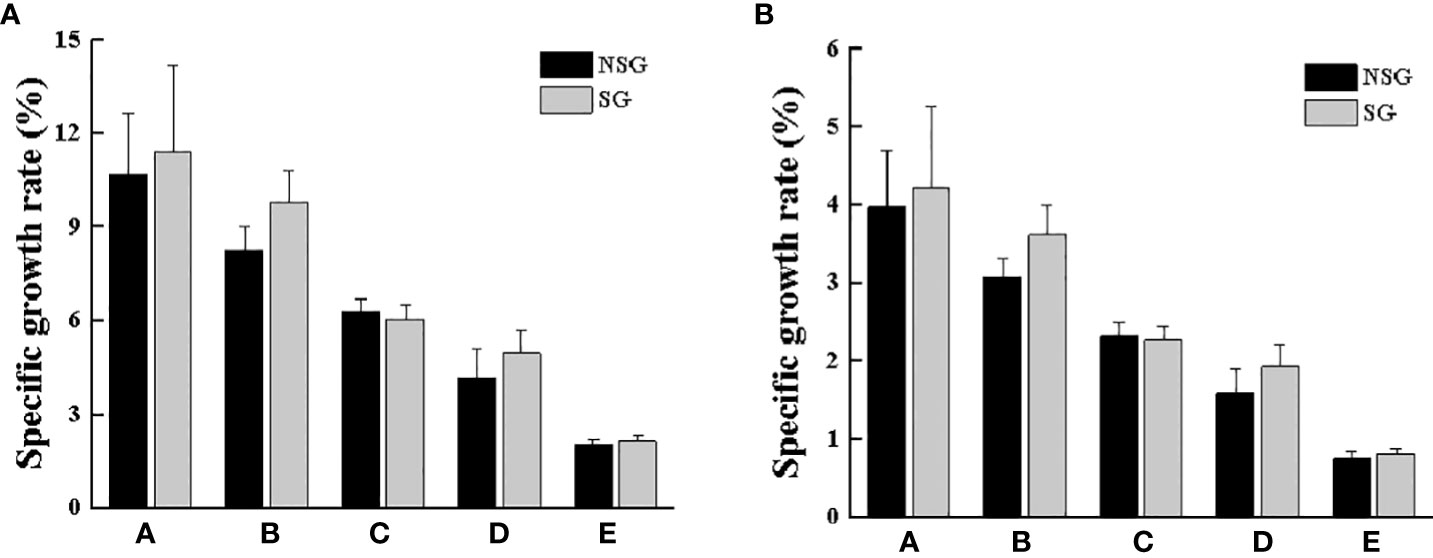
Figure 10 Specific growth rates of body weight (A) and carapace width (B) of swimming crab with different body weight between NSG and SG. Unmarked letters indicate no significant differences (P > 0.05).
In the groups without shelters, crabs with different body weights all preferred to hide in the corners of the cement pool, but rarely in the middle area of the cement pool, suggesting that swimming crab preferred supported and low-light places. Although the preference for shelters varied from group to group in the experiment, most individuals entered the shelters voluntarily and hid under the shelters for an extended, and there was a significant positive correlation between the SOR and body weight, which was consistent with the hidden law of Neogobius melanostomus of different body weights (Stammler and Corkum, 2005). It is noteworthy that the phenomenon of crabs hidden in the same shelter was more frequent in the smaller body weight groups and decreased significantly with increasing body weight, especially in Groups D and E where a shelter was usually occupied by only one crab and when other individuals try to enter the occupied shelter, the occupant would take a defensive posture (using walking legs to lift the body and opening two chelipeds). It showed obvious domain defense behavior, which contained a truth that the territorial consciousness of swimming crab gradually increased with increasing body weight. Similar phenomena have been reported in many crustaceans such as Cancer irroratus, Fiddler crab and Oratosqilla oratoria (Matheson and Gagnon, 2012; Dennenmoser and Christy, 2013; Jian, 2016). This study also manifested that the HR and the SOR with different body weights in the SG remained at approximately 60% during the daytime, while at night crabs would take the initiative to leave the shelters for food and swim freely, thus leading to a reduction in the HR and the SOR, which was consistent with the habit of swimming crab hiding during the day and going out at night (Dai et al., 1977).
Crustaceans such as shrimp and crabs rely on molting to grow. Internal mechanisms and external environment jointly regulate the molting time and molting cycle of crustaceans (Luppi et al., 2001; He et al., 2017). Shelters change the three-dimensional spatial structure in the cement pool, especially increased the complexity of the bottom level, thus playing a positive role in keeping the distance between the swimming crab individuals play a positive role. We still found that only the MRD of the swimming crab with smaller body weight (Group A) was lower than that of MRN, which was quite the opposite to the other groups. Because molting at night can reduce the probability being preyed and killed by similar species, which also indicates that swimming crab can change molting time through their own regulation. With the presence of shelter, more than 50% of individuals would choose to molt in the shelter, especially in Group B, where the molting rate in the shelter was the highest at 81.15%, while the molting rate in the shelter was as low as 57.7% in Group E. This result further illustrates that the setting of shelters has a good attractive effect on all body weights, also confirms that to reduce the visual cues in the population, the numerous crustaceans in the molting process, usually choose relatively hidden spaces to complete molting to avoid the invasion and slaughter of the same species, thereby reducing the risk of cannibalism (Polis, 1981; Moksnes et al., 1997). In addition, the molting interphase in Groups B, D and E was slightly shortened under the condition of the existence of shelter, this means that the setting of shelter accelerated the growth speed of swimming crab.
This study also found that regardless of whether there were shelters or not, when there were no soft-shelled crabs existed, all the crabs were more harmonious with each other, and there would even be multiple crabs gathering in the same corner. Cannibalism mainly occurs in the soft-shell phase. Related studies show that some active chemical factors released by crustaceans during molting are also one of the important factors leading to the intensification of cannibalism, in addition to its soft body, slow swimming speed and poor self-defense ability, leading to the intensification of cannibalism (Bolingbroke and Kass-Simon, 2001). Active chemicals such as amine neurons and 20-hydroxyecdysone have been demonstrated to be essential for the central and peripheral neurotransmission transmission of aggressive behavior in crustaceans (Ruffner et al., 1999; Kravitz, 2000). Soft-shelled crabs that molted in the period before the beginning of this experiment were less likely to be eaten by conspecifics. When individuals with larger molted shell masses were present, those crabs with smaller body weights in the molting process or the soft-shell stage were extremely vulnerable to attack by hard-shelled larger crabs, which means that the asymmetry between individuals was one of the important factors causing the increase of the dynamic stability of cannibalism. There was no individual mortality during the whole experiment due to fighting among the same species caused by food shortage or hunger, disease and other factors, and all mortality was the result of cannibalism. Point view of final SR, the SR was higher in the presence of shelters, especially Group D, which was significantly higher with shelter than that without shelters, revealing those shelters had better anti-injury properties at this body weight stage. Studies on shrimps and crabs have shown that the presence of shelters not only changes the spatial structure of the water column, but also improves the dispersal of individuals (Mirera and Moksnes, 2014). This contributes to reduce the probability of contact and delay the aggressive behavior between them, thus increasing the final SR (Moksens, 2004; He et al., 2017). What is more, regardless of the presence or absence of shelter, there was a trend that the larger the body weight of the crab was, the more severe the intraspecies fratricide and the lower the SR. This is inevitably related to the fact that territorial and hidden consciousness is gradually strengthened as body weight increases. Similar phenomena of intensified fighting behavior and the establishment of intraspecies dominance with increasing body weight have been reported in crustaceans such as Oratosqilla oratoria and Procambarus clarkii (Horner et al., 2008; Jian, 2016). Of particular note was that at smaller body weights, swimming crabs were able to get along amicably even when multiple individuals were hiding inside the same shelter at the same time, and many individuals could safely molt in the shelter. Our study further elucidates that swimming crab with a smaller body weight is weak in territorial consciousness and low in aggression.
Contrary to the previously mentioned fact that the presence of shelter slightly shortened the MI, shelter increase the body weight, carapace width, WGR and WGRC of swimming crab after molting. This illustrates the setting of shelters indirectly changed the growth law based on changing the activity behavior. The authors believe that the main reason for this is that the setting of shelters provides a more suitable living environment for swimming crab, which reduces the energy consumption caused by competing for resources and defending against the same species, such that energy intake is more used for growth. The positive effect of shelter settings on promoting the growth of aquatic animals and improving their growth characteristics has been fully confirmed in varieties such as Salvelinus alpinus and Sebastes schlegelii (Benhaïm et al., 2009; Guo et al., 2015).
Our study proved that swimming crab with different body weights exhibited obvious hiding behavior in the presence of shelter, especially in the daytime, the larger the body weight was, the higher the SOR was. In the case of crabs with a smaller body weight (below 50 g), there were poor territorial consciousness, with multiple individual hiding in the same shelter at the same time. Conversely, in the case of individuals with larger body weight (approximately 50 g or above), there were strong territorial consciousness, and in most cases, a shelter was occupied by a single crab. Furthermore, most individuals of all body weights selected to molt in the shelter. The molting survival rate and growth performance of swimming crab were improved under the protection of the shelter. Accordingly, we recommend that in the process of swimming crab breeding and production, the shelter should be set up in advance before the release of seedlings, which can play a positive role in the whole growth process, and thus improve the survival rate and yield of pond culture.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
JH and HY: Investigation, Formal analysis, Writing - original draft. LW: Conceptualization, Data curation, Formal analysis. DZ: Visualization, Writing - reviewing and editing. WX: Supervision, Funding acquisition, Writing - reviewing and editing. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported by the Basic Public Welfare Program of Zhejiang Province (Grant No. LGN22C190005); the National Science Foundation of China (Grant No. 32172993); the Major Agricultural Technology Cooperation Plan of Zhejiang Province (Grant No. 2020XTTGSC03); the science and technology project of Zhoushan City and Putuo District (Grant No. 2018C31078, 2020JH131); the planned project of Zhejiang Marine Fisheries Research institute (HYS-CZ-202105, HYS-CZ-202209).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Benhaïm D., Leblancb C. A., Lucas G. (2009). Impact of a new artificial shelter on Arctic charr (Salvelinus alpinus, l.) behaviour and culture performance during the endogenous feeding period. Aquaculture 295 (1-2), 38–43. doi: 10.1016/j.aquaculture.2009.06.024
Bolingbroke M., Kass-Simon G. (2001). 20-hydroxyecdysone causes increased aggressiveness in female American lobsters. Homarus americanus. Horm. Behav. 39 (2), 144–156. doi: 10.1006/hbeh.2001.1642
Chen X. (2006). “Behavioural ecology study on megalopae and first juvenile of mud crab, Scylla serrata (Forskål),” (Xiamen University). in Doctoral dissertation, vol. pp 9-pp. 25.
Chen X., Li S., Wang G., Lin Q., Ye H., Ai C. (2008). Study on relationship between cannibalism and resource Availability/Starvation of mud crab, Scylla paramamosain megalopae. J. Xiamen Univer. (Natur. Sci). 47 (1), 99–103. doi: 10.3321/j.issn:0438-0479.2008.01.022
Dai A., Feng Z., Song Y., Huang Z., Wu H. (1977). Fisheries biology of Portunus trituberculatus linneis initialing investigatgtion. Chi. J. Zool. 2, 30–33. doi: 10.13859/j.cjz.1977.02.015
Dennenmoser S., Christy J. H. (2013). The design of a beautiful weapon: compensation for opposing sexual selection on a trait with two functions. Evol 67 (4), 1181–1188. doi: 10.1111/evo.12018
Gil M. D. M., Pastor E., Durán J. (2019). Survival and growth of hatchery-reared mediterranean spider crab juveniles, maja squinado, under different rearing conditions. Aquacult 498, 37–43. doi: 10.1016/j.aquaculture.2018.08.001
Guo H., Zhang X., Gao T. (2015). Effects of artificial shelters and feeding frequency on growth and behavior of juvenile sebastes schlegelii. J. Fish. Sci. Chi. 22 (2), 319–331. doi: 10.3724/SP.J.1118.2015.1422
He J., Gao Y., Wang W., Xie J., Shi H., Wang G., et al. (2016). Limb autotomy patterns in the juvenile swimming crab (Portunus trituberculatus) in earth ponds. Aquaculture 463, 189–192. doi: 10.1016/j.aquaculture.2016.05.043
He J., Gao Y., Xu W., Yu F., Su Z., Xuan F. (2017). Effects of different shelters on the molting, growth and culture performance of Portunus trituberculatus. Aquaculture 481, 133–139. doi: 10.1016/j.aquaculture.2017.08.027
Horner A. J., Schmidt M., Edwards D. H., Derby C. D. (2008). Role of the olfactory pathway in agonistic behavior of crayfish, Procambarus clarkii. Invertebr. Neurosci. 8 (1), 11–18. doi: 10.1007/s10158-007-0063-1
Jian T. (2016). “A preliminary study on the behavior in oratosqilla oratoria,” in Master dissertation (Dalian Ocean University), pp13–pp15.
Kravitz E. A. (2000). Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J. Comp. Physiol. A. 186 (3), 221–238. doi: 10.1007/s003590050423
Li Y., Sun X. (2013). Agonistic behaviors of aquatic animals. Zool. Res. 34 (3), 214–220. doi: 10.11813/j.issn.0254-5853.2013.3.0214
Liu H. (2002). Study on cannibalism of carnivorous fish under culture ecological conditions. Fish. Sci. Tech. Infor. 29 (6), 261–263. doi: 10.3969/j.issn.1001-1994.2002.06.001
Luppi T. A., Spivak E. D., Anger K. (2001). Experimental studies on predation and cannibalism of the settlers of Chasmagnathus granulata and Cyrtograpsus angulatus (Brachyura: Grapsidae). J. Exp. Mar. Biol. Ecol. 265 (1), 29–48. doi: 10.1016/s0022-0981(01)00322-7
Mann D. L., Asakawa T., Kelly B., Lindsay T., Paterson B. (2007). Stocking density and artificial habitat influence stock structure and yield from intensive nursery systems for mud crabs Scylla serrata (Forsskål 1775). Aquacult. Res. 38 (14), 1580–1587. doi: 10.1111/j.1365-2109.2006.01626.x
Marshall S., Warburton K., Paterson B., Mann D. (2005). Cannibalism in juvenile blue-swimmer crabs Portunus pelagicus (Linnaeus 1766): effects of body size, moult stage and refuge availability. Appl. Anim. Behav. Sci. 90 (1), 65–82. doi: 10.1016/j.applanim.2004.07.007
Matheson K., Gagnon P. (2012). Effects of temperature, body size, and chela loss on competition for a limited food resource between indigenous rock crab (Cancer irroratus say) and recently introduced green crab (Carcinus maenas l.). J. Exp. Mar. Biol. Ecol. 428, 49–56. doi: 10.1016/j.jembe.2012.06.003
Mirera D. O., Moksnes P.-O. (2013). Cannibalistic interactions of juvenile mud crabs Scylla serrata: the effect of shelter and crab size. Afr. J. Mar. Sci. 35 (4), 545–553. doi: 10.2989/1814232X.2013.865677
Mirera D. O., Moksnes P.-O. (2014). Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: effect of shelter, crab size and stocking density. Aquacult. Int. 23 1), 155–173. doi: 10.1007/s10499-014-9805-3
Moksnes P.-O. (2004). Self-regulating mechanisms in cannibalistic populations of juvenile shore crabs Carcinus maenas. Ecology 85 (5), 1343–1354. doi: 10.1890/02-0750
Moksnes P.-O., Lipcius R. N., Pihl L., Montfrans J. V. (1997). Cannibal prey dynamics in young juveniles and post larvae of the blue crab. J. Exp. Mar. Biol. Ecol. 215 (2), 157–187. doi: 10.1016/S0022-0981(97)00052-X
Perkins-Visser E., Wolcott T. G., Wolcott D. L. (1996). Nursery role of seagrass beds: enhanced growth of juvenile blue crabs (Callinectes sapidus, rathbun). J. Exp. Mar. Biol. Ecol. 198 (2), 155–173. doi: 10.1016/0022-0981(96)00014-7
Polis G. A. (1981). The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Syst. 12 (1), 225–251. doi: 10.1146/annurev.es.12.110181.001301
Qin H., Li Y. (2014). The effects of stocking density and food on agonistic behavior and growth performance in fenneropenaeus chinensis. Ocean. Limn. Sin. 45 (4), 834–838. doi: 10.11693/hyhz20140400095
Ruffner M. E., Cromarty S. I., Cooper R. I. (1999). Depression of synaptic efficacy in high- and low-output Drosophila neuromuscular junctions by the molting hormone (20-HE). J. Neurophysiol. 81 (2), 788–794.
Stammler K. L., Corkum L. D. (2005). Assessment of fish size on shelter choice and intraspecific interactions by round gobies Neogobius melanostomus. Environ. Biol. Fishes. 73 (2), 117–123. doi: 10.1007/s10641-004-5562-x
Keywords: Portunus trituberculatus, cannibalism, different body weights, shelter, behavior, growth
Citation: He J, Yu H, Wan L, Zhang D and Xu W (2022) At what size do anti-injury shelters start to play a positive role in the culture of Portunus trituberculatus? Front. Mar. Sci. 9:1018565. doi: 10.3389/fmars.2022.1018565
Received: 13 August 2022; Accepted: 29 August 2022;
Published: 14 September 2022.
Edited by:
Zhiguo Dong, Jiangsu Ocean University, ChinaReviewed by:
Xinguo Zhao, Yellow Sea Fisheries Research Institute (CAFS), ChinaCopyright © 2022 He, Yu, Wan, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjun Xu, d2p4dTE5NzFAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.