Abstract
There have been discussions of scaling up offshore seaweed cultivation and sinking it exclusively for carbon sequestration (‘ocean afforestation’) and thereby help mitigate climate change, but is this concept feasible? Here we investigate the feasibility of ocean afforestation across five perspectives: 1) Ecological feasibility; 2) Technical feasibility; 3) Economic feasibility; 4) Co-benefits and risks; and 5) Governance and social considerations. Optimising ecological factors such as species selection and use of currents, alongside the use of low-cost biodegradable rafts in theory could see this concept scaled globally. An area of 400,000km2 or 16.4 billion biodegradable rafts would be needed for 1 gigatonne of CO2 fixation given roughly 16 rafts of 25m2 each would be needed per tonne of CO2 fixation. However, CO2 fixation (calculated from net primary productivity) and carbon sequestration (carbon permanently removed from the atmosphere) are fundamentally different processes, yet this distinction is often overlooked. Quantifying carbon sequestration from ocean afforestation remains elusive given several outstanding oceanic biogeochemical considerations. For example, the displacement of phytoplankton communities and their associated carbon sequestration via nutrient reallocation is a critical knowledge gap in understanding the climate change mitigation potential of ocean afforestation. Ocean afforestation also carries complex risks to marine ecosystems, for example, the impact on benthic communities of seaweed deposition. Additionally, governance and social challenges exist such as the legality of operation in relation to ocean treaties. The concept of ocean afforestation is still in its infancy, and while there are large research gaps, further investment into research should be given before the concept can be adequately compared against the suite of potential ocean-based climate change mitigation strategies.
Introduction
Recently carbon sequestration as a co-benefit from seaweed (macroalgae) aquaculture has gained extensive interest globally in academic, government and industry settings (Duarte et al., 2017; Sondak et al., 2017; Duarte et al., 2021). This has been fueled by a growing interest globally in climate change mitigation. Seaweed aquaculture has been expanding at 6.2% annually from 2000 to 2018, and there are several novel and emerging markets for seaweed products (Duarte et al., 2021). The seaweed aquaculture industry has also been suggested to be a strong contributor to progress on the United Nations Sustainable Development Goals (Duarte et al., 2022). The growth in the seaweed aquaculture industry has included suggestions that offshore (beyond the shelf break) seaweed cultivation could be done on a large scale that would lead to significant carbon sequestration in the ocean termed ‘blue carbon’ (Macreadie et al., 2019). Seaweed carbon capture and sink, a term referred to by Chung et al. (2017), is where seaweed is grown for the sole purpose of carbon offsetting or carbon credits. This has also been referred to as ocean afforestation (Bach et al., 2021; Boyd et al., 2022), which is the term used in this paper.
Several studies have considered seaweed cultivation in the context of geoengineering (Aldridge et al., 2012; Chung et al., 2017; Wood et al., 2017), and a small but increasing number of papers have mentioned the possibility of new seaweed-based carbon capture and storage projects, or ocean afforestation, as they relate to the sale of carbon credits (Chung et al., 2013; N‘Yeurt et al., 2012; Chung et al., 2017; Sondak et al., 2017; Coleman et al., 2022; Troell et al., 2022). Several businesses have recently proposed ocean afforestation as a carbon sequestration strategy (eg. Running tide, The Southern Ocean Carbon Company and Pull to Refresh). In the last two years in a series of papers (Bach et al., 2021; Boyd et al., 2022; Hurd et al., 2022), ocean afforestation was considered in detail. The conclusion from these papers was that the ecological risks of this concept are high and remain to be quantified, likewise there are several significant carbon accounting challenges. More recently in a paper by Ricart et al. (2022) ocean afforestation was deemed to be unethical and lacking scientific backing. However it remains unclear if further investment in research should be made into this concept, i.e, is ocean afforestation a viable and promising future means for climate change mitigation?
Because of seaweeds’ capability for growth on hard substrates, seaweed aquaculture has unique scalability compared to other blue carbon sinks, if offshore aquaculture can be developed (Chung et al., 2017). Given the size of the open ocean, should a method of efficient offshore seaweed cultivation be developed, and scaled, it could in theory have a large contribution to global carbon sequestration. For example, N‘Yeurt et al. (2012) suggested that if 9% of the oceans were arranged for seaweed aquaculture, pre-industrial atmospheric carbon dioxide concentrations could be reached in a few decades. Additionally, Froehlich et al. (2019) calculated an upper estimate of a possible 48 million km2 of the oceans are suitable for seaweed aquaculture, roughly 11% of the total ocean area. Therefore, a theoretical maximum annual sequestration from seaweed aquaculture would be 72 billion tonnes, larger than global annual emissions (based on a theoretical maximum CO2 sequestration of 1,500T CO2 per km2 (Duarte et al., 2017). While this estimate is useful to quantify carbon sequestration on a maximum scale, it assumes all CO2 is sequestered and uses few parameters to constrain seaweed growth. This is unrealistic given over 90% of seaweed products are consumed by humans and a generous estimate for the quantity of seaweed biomass that becomes sequestered during the growth phase by shedding of biomass is 11% (Duarte et al., 2021). Despite these estimates of scale being rudimentary, the scale of opportunity for a potential ocean afforestation industry demands more discussion of the concept.
The lack of scientific development in ocean afforestation research relative to industry interest to date may have been historically attributed to the uncertainties around perceived high costs, engineering constraints and hostile growing conditions of offshore aquaculture, which is where ocean afforestation may be considered for (Fernand et al., 2017). In addition, investors considering seaweed aquaculture may have found a more attractive investment in nearshore aquaculture with an established consumer market, receiving a potentially higher price than if cultivating solely for carbon (van den Burg et al., 2016). Now global interest in climate change solutions is growing and investors are actively looking for scalable climate change mitigation projects. This paper aims to inform businesses and governments considering the potential financial opportunity, climate change mitigation potential and both positive and negative associated impacts of ocean afforestation. Five themes are reviewed and discussed in this paper 1) Ecological feasibility; 2) Technical feasibility; 3) Economic feasibility; 4) Co-benefits and risks; and 5) Governance and social considerations. Importantly in this paper we make a distinction between carbon uptake, carbon fixation, and carbon sequestration. Carbon uptake is CO2 being ‘absorbed’ by the ocean from the atmosphere. Carbon fixation is used to describe the CO2 taken up in primary production (photosynthesis), this carbon fixation in biomass can re-enter the atmosphere. Carbon sequestration is used to describe the permanent (over 100 years) removal of CO2 from the atmosphere in a sink site.
Ecological feasibility
Seaweed beds are amongst the most productive and diverse marine habitats on earth (Mann, 1973; Krause-Jensen et al., 2018). Seaweeds are some of the fastest-growing primary producers on the planet, having equal to or greater rates of productivity than the most productive terrestrial plants (Nellemann et al., 2010). While seaweed aquaculture is well established globally close to the coast in specific regions with a global production of 2.61 × 106 t dry weight in 2014 (Sondak et al., 2017), seaweed aquaculture production is negligible if present at all offshore (García-Poza et al., 2020). Several factors discussed below will need to be investigated to determine the ecological feasability of ocean afforestation.
Species selection
Seaweed species diversity is high and are classified as Rhodophyta (red algae), Chlorophyta (green algae) and Phaeophyceae (brown algae) (Lobban and Harrison, 1994). While growth rates are high for both red and green algae, brown algae (both fucoids and laminaria), can reach a considerably larger size than even the largest red and green algae, and hence may sequester more carbon per individual plant (Lobban and Harrison, 1994). Therefore it is likely brown algae can sequester significantly more carbon per surface area of growth substrate during the cultivation season or per cultivation cycle. Given maximising the ratio of biomass to the surface area of growth substrate will be a key factor for feasibility, brown algae are more likely candidates for ocean afforestation.
Brown algae are large and widely distributed habitat-forming macroalgal species of two orders, Fucales (fucoids) and Laminariales (kelp) (Nelson, 2020). Along temperate rocky shorelines fucoids typically dominate in the intertidal zone, whereas, kelps are more common in the subtidal zone (Chapman, 1987; Lobban and Harrison, 1994). Fucoids generally have slower decomposition rates than kelps because they have more structural tissue and secondary metabolites that may be toxic to grazers and decomposers (Lobban and Harrison, 1994; Lau and Qian, 1997; Nagayama et al., 2002; Birkemeyer et al., 2020). Because of their slower decomposition, the carbon sequestered by fucoids may therefore have a higher likelihood of naturally being deposited in the deep ocean compared to kelps. However, kelp species often have faster growth rates, are large and can be highly abundant, and may therefore also be important blue carbon sources (Duggins et al., 1989; Harrold and Lisin, 1989; Krause-Jensen and Duarte, 2016).
There are many species of brown algae that could be considered for ocean afforestation. Endemic species to that ecoregion should be preferentially considered to minimise the risk of introducing an invasive species. Species will be selected on several metrics including, their ability to grow fast, and take up carbon at a higher ratio to other nutrients than other primary producers like phytoplankton (Bach et al., 2021). Buoyancies of species may also need to be considered to determine the weighting and sinking of seaweed. Because tropical offshore waters are largely more nutrient deficient than temperate waters (Moore et al., 2013) and offshore waters are where ocean afforestation will primarily be considered for, temperate species and locations will be preferential. For discussion on two possible candidate species for ocean afforestation see Box 1.
Box 1 | Candidate species for Ocean Afforestation.
For example, Laminaria saccharina is one of the largest Atlantic seaweed species and for this reason will be preferentially considered for ocean afforestation in the Atlantic (Bekkby and Moy, 2011). Similarly Nereocystis luetkeana is a buoyant, large, canopy forming seaweed species found in the North West pacific (Kruckeberg, 1991). Given its size and growth rates, N. luetkeana may be a possible candidate for ocean afforestation (Springer et al., 2007).
Macrocystis pyrifera - M. pyrifera has a large size, high growth rate and high form-function plasticity (Schiel and Hickford, 2001; Graham et al., 2007; Schiel and Foster, 2015). Furthermore, M. pyrifera can grow more than 30 cm per day under ideal conditions and is the largest macroalgae in the world, reaching well over 15m long (Cribb, 1954; North, 1970; Abbott and Hollenberg, 1976; Graham et al., 2007; Schiel and Foster, 2015). M. pyrifera has been shown to drift and grow for long periods of time at favorable latitudes, suggesting M. pyrifera could grow well on free-floating rafts (Rothäusler et al., 2011). However, M. pyrifera has a relatively low C:N ratio and less structural tissue (Jackson, 1977). This means that given nutrient reallocation considerations (1.2.a), the carbon sequestration potential of M. pyrifera may be reduced. Additionally, M. pyrifera may be more subject to breaking under stressful offshore conditions (Seymour et al., 1989). Given its large size and potential for rapid growth, M. pyrifera could be a candidate for ocean afforestation.
Durvillaea spp. - Durvillaea spp. are sub and intertidal species, native to New Zealand, Tasmania and Chile, with several species endemic to sub-regions (Cheshire et al., 1995; Nelson, 2020). Durvillaea spp. may have a relatively favorable C:N ratio given their high structural integrity, which provides resilience to storms and wave action (Hay, 1977; Dufour et al., 2012). Durvillaea spp. can also be very large, Hay (1977) documented that one species, D. willana can reach up to 40 kg. However, Durvillaea spp. have slow growth rates relative to other large habitat-forming seaweed species (North, 1970; Velásquez et al., 2020). One species, Durvillaea antartica is very buoyant (Fraser et al., 2020), which may merit a different engineering strategy given ocean afforestation for this species. Durvillaea spp. are vulnerable to marine heatwaves and high temperatures which are increasing in occurrence (Thomsen et al., 2019). For this reason, temperature stress should be a primary consideration of any future Durvillaea spp. ocean afforestation.
Limiting factors for growth
Macroalgal growth is primarily driven by light and photosynthesis (Lobban and Harrison, 1994). However, seaweed growth, and therefore carbon sequestration, can also be limited by nutrients (Neori et al., 1991; Kübler et al., 1999), temperature (Kübler and Davison, 1993), grazers (Viejo and Åberg, 2003), diseases (Kumar et al., 2016), irradiance (Mabin et al., 2019), competition (Thirumaran and Anantharaman, 2009), currents (Millar et al., 2020), salinity and, for intertidal species, desiccation (Karsten, 2012; Eckersley and Scrosati, 2020).
-
a. Nutrients - Seaweed growth can be limited by the concentration of various types of, macronutrients, micronutrients and vitamins (Lobban and Harrison, 1994; Harrison and Hurd, 2001). Nutrients in many instances are the key limiting factor for seaweed growth (Roleda and Hurd, 2019; Xiao et al., 2019). In most cases, nutrient availability in the photic zone offshore is lower than in nearshore ecosystems (Fernand et al., 2017). Nutrient concentrations of oceanic (offshore) water in the tropics are lower than temperate latitudes (Moore et al., 2013). However, in some cases, nutrient availability and subsequent growth rates are higher offshore due to stratification, which can also lower nutrient availability nearshore (Chen et al., 2021). While nutrient concentrations are highly variable in offshore and nearshore ecosystems, in the Froehlich et al. (2019) estimates of seaweed aquaculture maximum extent, they found 48 million km2 of the oceans are suitable for seaweed aquaculture both nearshore and offshore. This was constrained by temperature and nutrients. So while the exact amount of offshore ocean area is yet to be accurately assessed, there is likely significant available area offshore for ocean afforestation based solely on nutrient concentration. Nitrogen (N), phosphorous (P) and Iron (Fe) are the most common limiting nutrients for offshore primary production (Moore et al., 2013). P and N are used in a ratio of approximately 30:1 in seaweeds (Atkinson and Smith, 1983). Optimising the P:N ratio is a common limiting factor for seaweed growth (Harrison and Hurd, 2001).
-
Nutrient concentrations can be high in areas of both natural and artificial upwelling, which brings nutrient-rich cold waters that promote seaweed growth (Calvert and Price, 1971; Huyer, 1983; Pan et al., 2016). The use of upwelling greatly supports primary production, fisheries and subsequent carbon fixation (Pan et al., 2016; Zhang et al., 2017). However, there are high costs, ethical concerns, ecological impacts, and potential for the release of deep-sea CO2 from artificial upwelling from deep ocean pumps (Canadell et al., 2021). While nutrient reallocation will be a key concern for ocean afforestation in areas of upwelling, displacement of nutrients and impacts on offshore ecosystems may be relatively proportional to total biomass. Therefore, these potential negative impacts will be present regardless of whether seaweed is limited in growth from nutrients, and hence areas with nutrient concentrations high enough to support fast growth could be preferential. For this reason, nutrient availability should be an important consideration in site selection for potential ocean afforestation. To our knowledge, there has not been any nutrient supplementation proposed for ocean afforestation aside from artificial upwelling.
-
b. Currents - Strong currents support nutrient replacement and provide higher concentrations of CO2 (Hurd, 2000; Stevens et al., 2003). However determining net CO2 flux (1.6) will be more difficult in areas of strong currents. If free-floating structures are proposed for ocean afforestation (3), currents will be one of the essential factors in determining where the structures are released and where they may be retrieved or sink to (Boyd et al., 2022). Currents will result in proposed free-floating structures receiving varying concentrations or levels of nutrients, salinity and temperature during their drift which will be important to understand.
-
c. Disease, grazers and epiphytes - Disease (Largo, 2002), grazers and epiphytes (Vairappan et al., 2008) may impact seaweed growth for ocean afforestation as they have for nearshore aquaculture (Kumar et al., 2016). These and other biosecurity risks will need to be managed in a new way offshore, where principles for managing these issues may be adapted from nearshore seaweed aquaculture systems. Grazing, however, will likely be of minimal concern as grazers rarely consume greater than 10% of living seaweed biomass (Alongi, 2018). Additionally, kelps are primarily grazed by urchins (Miller, 1985; Duffy and Hay, 1990), which will not be present offshore.
-
d. Temperature - All seaweed species have an optimal temperature range where they maximise growth (Lüning, 1990; Eggert, 2012). Given an increase in the frequency of marine heatwaves is likely, there will be a need for ocean afforestation to take place where temperatures are unlikely to exceed temperature constraints, this may be increasingly poleward (Oliver et al., 2018; Smale et al., 2019).
-
e. Irradiance - Irradiance is a limiting factor for seaweed growth (Fortes and Lüning, 1980; Lobban and Wynne, 1981), and therefore maximising light quality and availability will be an important consideration in possible ocean afforestation site selection. While light availability will be mostly dictated by growth substrate depth, localised water clarity and ground level irradiance measures will also be important considerations in optimising irradiance (Gattuso et al., 2006).
-
f. Competition, Salinity and Desiccation - Canopy competition, both within and between species, is a key limiting factor for seaweed growth in wild seaweed forests as it can reduce light and nutrient availability (Carpenter, 1990; Edwards and Connell, 2012). Given the need to optimise growth per unit area, competition between species may limit growth via shading and competition for nutrients, depending on seaweed propagation density. Competition between different species is unlikely to limit growth in a planted aquaculture setting as it does for wild seaweed forests. Salinity and desiccation are unlikely to impact ocean afforestation as they are not likely limiting factors of seaweed growth in temperate offshore environments (Lobban and Wynne, 1981).
Depth
Natural mechanisms facilitate the transport of coastal macroalgae to the deep ocean, these include leaching of dissolved organic matter, pruning and tissue breakage, seasonal partial die-off and enhanced fragmentation – a common strategy for many species (Koop et al., 1982; Duggins et al., 1989; Krause-Jensen and Duarte, 2016). More specifically, Krause-Jensen and Duarte (2016) proposed that seaweed mainly are deposited at deep-sea sites by drifting through submarine canyons or by the sinking of negatively buoyant detritus. Therefore sinking seaweed to this depth could accelerate carbon sequestration. However, the methodology to document macroalgal blue carbon budgets is still to be developed. Large research gaps remain around how much carbon from seaweed makes it into the deep ocean where it becomes a long-term sink, and its potential impacts on deep-sea benthic communities (Filbee-Dexter et al., 2018; Krause-Jensen et al., 2018).
Carbon from seaweed biomass is likely to decompose into surrounding seawater in various forms of dissolved organic carbon and particulate organic carbon (Watanabe et al., 2020). Some organic carbon may also remain in refractory forms that will resist decomposition (Trevathan-Tackett et al., 2015). Additionally, organic carbon from seaweed can become buried in ocean sediments on the seafloor before it can resurface. The primary mechanisms for sedimentation of biomass in the deep sea are direct burial and metabolization by bacteria and other species. Macroalgal biomass has been found digested in the gut of various deep sea creatures below 1,000m depth (Wolff, 1962; Schoener and Rowe, 1970). The seaweed biomass that is ingested could be respired back into the surrounding seawater, or buried in the sediment with the decomposer’s body. Therefore if seaweed tissue deposited at depth is decomposed, released carbon can only re-enter the atmosphere at time scales beyond immediate concern for reducing anthropogenic emissions, i.e. >100 years.
Historical estimates suggested that carbon from marine organic matter, including seaweed, that reaches more than 1,000 m depth (i.e., the bathypelagic zone) becomes a long-term carbon sink (Herzog et al., 2003; Krause-Jensen and Duarte, 2016; DeVries et al., 2017; Ortega et al., 2019). However more recently, Baker et al. (2022) refined these estimates in the North Atlantic and suggested only 66% of carbon remains out of contact with the atmosphere if it is deposited at 1,000m depth and this increases to 94% below 2,000m. Research is still needed to confirm the depths required for permanent carbon sequestration in different locations globally. For this reason, ocean afforestation will likely occur on the surface above the deep ocean if it is to be sunk at the growth site, or grown nearshore and taken offshore to be sunk.
Nutrient competition with phytoplankton
It is important to consider where seaweeds are competing with phytoplankton for nutrients, as this nutrient competition may be reducing carbon sequestration from phytoplankton (Bach et al., 2021). While generally, seaweeds consume carbon more efficiently than phytoplankton relative to their nitrogen and phosphorus consumption (Baird and Middleton, 2004), carbon sequestration via seaweed should subtract the carbon sequestration that would have occurred in phytoplankton communities in the absence of nutrient reallocation (Hurd et al., 2022). Nutrient reallocation may also need to be considered over extended timescales given nutrients may be recycled and transported (Thingstad and Sakshaug, 1990). Questions also remain on the efficiency of alternate nutrient usage via phytoplankton for carbon sequestration (Boyd et al., 2007; Graff et al., 2015). For example, iron fertilisation has been disproved to enhance net carbon sequestration in phytoplankton communities despite enhanced primary productivity (Boyd, 2004; Boyd et al., 2007). Therefore it must be determined if seaweed carbon fixation displaces phytoplankton carbon fixation by monitoring phytoplankton communities at growth sites. Nutrient reallocation considerations are complex and remain relatively unstudied.
Albedo and calcification
Albedo is the measure of the reflectivity of longwave radiation (Twomey, 1974). Seaweed has a higher albedo than oceanic water, therefore lowers warming potential (Bach et al., 2021). Seaweed that is closer to the surface will increase albedo more (Bach et al., 2021). However, this may be variable between species and marine biomes and is relatively unstudied. Seaweeds can also provide habitat for calcifying organisms (Taylor, 1998; Newcombe and Taylor, 2010; Thomsen et al., 2016). While there is carbon fixation from calcification, calcification also reduces seawater alkalinity and therefore lowers the carbon uptake capacity of seawater (Frankignoulle et al., 1994; Macreadie et al., 2017). When seaweed provides habitat for calcifiers and increases their biomass, this may partially offset any CO2 sequestration from seaweed (Bach et al., 2021). Respiration from associated epifauna can also offset CO2 sequestration (Hurd et al., 2022).
Determining net CO2 flux
Importantly, it must be understood if CO2 sequestration through seaweed growth creates a CO2 seawater deficit that proportionally results in additional atmospheric CO2 uptake (Watanabe et al., 2020; Bach et al., 2021). Increased atmospheric CO2 uptake in seawater at ocean afforestation sites is the goal of ocean afforestation and therefore must be demonstrable. This will require tracking changes in oceanic carbon uptake at multiple points in time during seaweed cultivation to validate carbon fixation, and monitoring for this remains complex (Hurd et al., 2022). Timescales at which CO2-deficient seawater will make contact with the surface and increase CO2 uptake remain uncertain (Orr and Sarmiento, 1992; Bach et al., 2021).
Technical feasibility
There have been few proposed methods to grow seaweed specifically as a carbon offset (Froehlich et al., 2019). To our knowledge, all proposed ideas have been early-stage commercial ventures with no supporting research in the peer-reviewed literature. One of the reasons the industry is in its infancy is the significant technical constraints required given seaweed must be sunk in the deep ocean (I.e. 2,000m) to become sequestered (Baker et al., 2022). Various technical concepts exist, and they are broadly defined in the following three categories for technical ocean afforestation.
-
Traditional nearshore seaweed aquaculture, cultivated and dumped offshore (Chung et al., 2017).
-
Offshore tethered growing platform in the deep ocean.
-
Non-tethered growth platforms in the deep ocean, (free floating or automated)
There are several variations of technical design themes that could be used in concert with these three technical categories. Some of these possible technical themes are listed here and possible concepts are illustrated in (Figure 1). It is important to note these are just conceptual designs, and there is a much-needed cost-benefit analysis of each of these potential concepts before application in the field.
-
- Attachment to existing infrastructure, for example, oil and gas or marine wind farms (van den Burg et al., 2020). Hybrid cultivation strategies have been considered for seaweed cultivation as part of integrated multi trophic aquaculture (Flannery, 2017).
-
- Whole structure is sacrificial and biodegradable, to be sunk with seaweed attached.
-
- Platform with biodegradable sacrificial subunits to be released and sunk with seaweed attached. Relying on advection offshore to sink sites.
-
- Self-anchored growth platform with an automated or manual cutting mechanism on a growing platform to cut seaweed that will sink on its own.
-
- Macroalgal biomass could be stored in geosynthetic containers placed in the deep ocean, although the costs for this would be considerable (Boettcher et al., 2019).
Figure 1
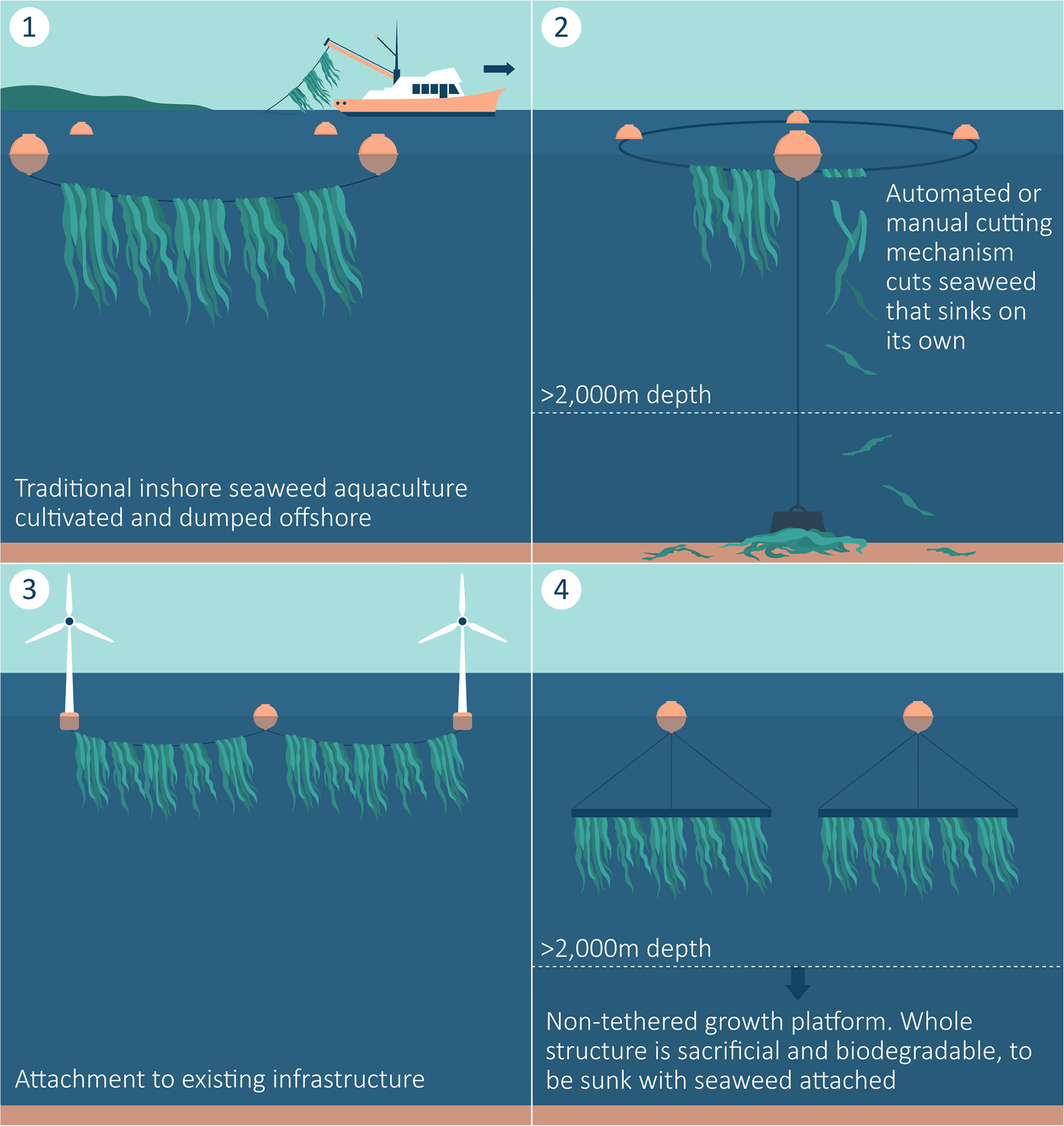
Possible concepts for ocean afforestation. 1. Traditional nearshore aquaculture taken to be dumped offshore. 2. Self-anchored growth platform sunk at the site. 3. Seaweed aquaculture infrastructure attached to existing offshore infrastructure (e.g. oil and gas infrastructure or a wind farm). 4. Free-floating biodegradable platform.
Offshore aquaculture is likely to require significantly more complex infrastructure than coastal or nearshore aquaculture given an increased susceptibility to harsh weather and remoteness (Jansen et al., 2016; Gentry et al., 2017). Therefore the cost of operating offshore is likely much higher than for offshore aquaculture. Due to the difficulty of anchoring a new structure in 2,000m of water, it is unlikely that anchoring a new structure at this depth for ocean afforestation will be viable. In addition, manual harvest and seeding of seaweed will be very difficult in an offshore environment. So in this instance, automated planting and harvesting may be required. One way to get around this however may be ‘sacrificial growth substrates’. This is where the growth rope, or rope and part of the structure are sunk with the seaweed still attached. This means the seaweed can be propagated onto the substrate before being put out to grow, and there is no need to harvest or cut the seaweed again. Here the growth substrate is disconnected from the platform to sink, or let break and sink on its own due to natural degradation. In this way, maintenance and labour costs may be lower for ocean afforestation than for traditional seaweed aquaculture.
While there has been a lack of engineering research for ocean afforestation, there has been considerable research into scalable offshore cultivation for other seaweed markets, like biofuels (Milledge et al., 2014; Cheng et al., 2020). Many of the technical feasibility considerations for growth platforms could be taken from established literature around suggested seaweed biofuel production. As global demand increases for seaweed products and seaweed aquaculture grows (Duarte et al., 2021), this may drive innovation for large-scale seaweed aquaculture and technology which may spill over to ocean afforestation. Additionally, the use of wind farms to co-exist as infrastructure for seaweed aquaculture has been considered (van den Burg et al., 2020), however, few, if any wind farms exist in the deep ocean so a harvest and deposition model may be needed for ocean afforestation if co-existence on existing infrastructure is to be used.
Additionally, the installation of offshore infrastructure is costly, so if permanent structures are to be established, the full lifecycle of infrastructure should be considered. This includes installation, removal and recycling. Importantly, the carbon footprint of infrastructure, including fuel used in boats, for ocean afforestation must be offset by the venture itself. For this reason, an infrastructure system with a low-emission build would be preferable. This is an advantage for sacrificial biodegradable growth substrates, where they may be carbon sinks on their own, depending on the material used (i.e. hemp, pine and bamboo). Regardless of the design used, one of the main constraints will be minimising the energy required to transport and maintain a potential structure offshore.
Free-floating structures will take away the need for an anchor and may result in less maintenance and transport need. Free-floating structures could also be automated so they can dive during storms and when ships are near, and so they can stay within designated areas. However, if free-floating structures are automated and require propulsion, the cost may be significant for each growth structure and may not justify returns. In the interest of simplicity and cost saving, free-floating structures may be biodegradable and subject to ocean currents. They could be pieces of biodegradable rope floating on the surface, or wooden rafts that degrade and sink, along with the seaweed grown, after an estimated amount of time-based on degradation rates. Free-floating rafts could also be remotely triggered for release from bioplastic floats. If individual rafts are used it may be harder to transfer disease or epiphytes at a large scale given the separation between rafts. While free-floating sacrificial structures have a basic level of technical feasibility and therefore are potentially a lower-cost option, monitoring of growth, and susceptibility to currents, wind and waves may be two significant liabilities to this concept. While large-scale oceanic drifting experiments have taken place, to our knowledge none have been for an aquaculture purpose (Whitaker and Carter, 1954; Christensen et al., 2018). Free-floating rafts have been visualised in Figure 2.
Figure 2
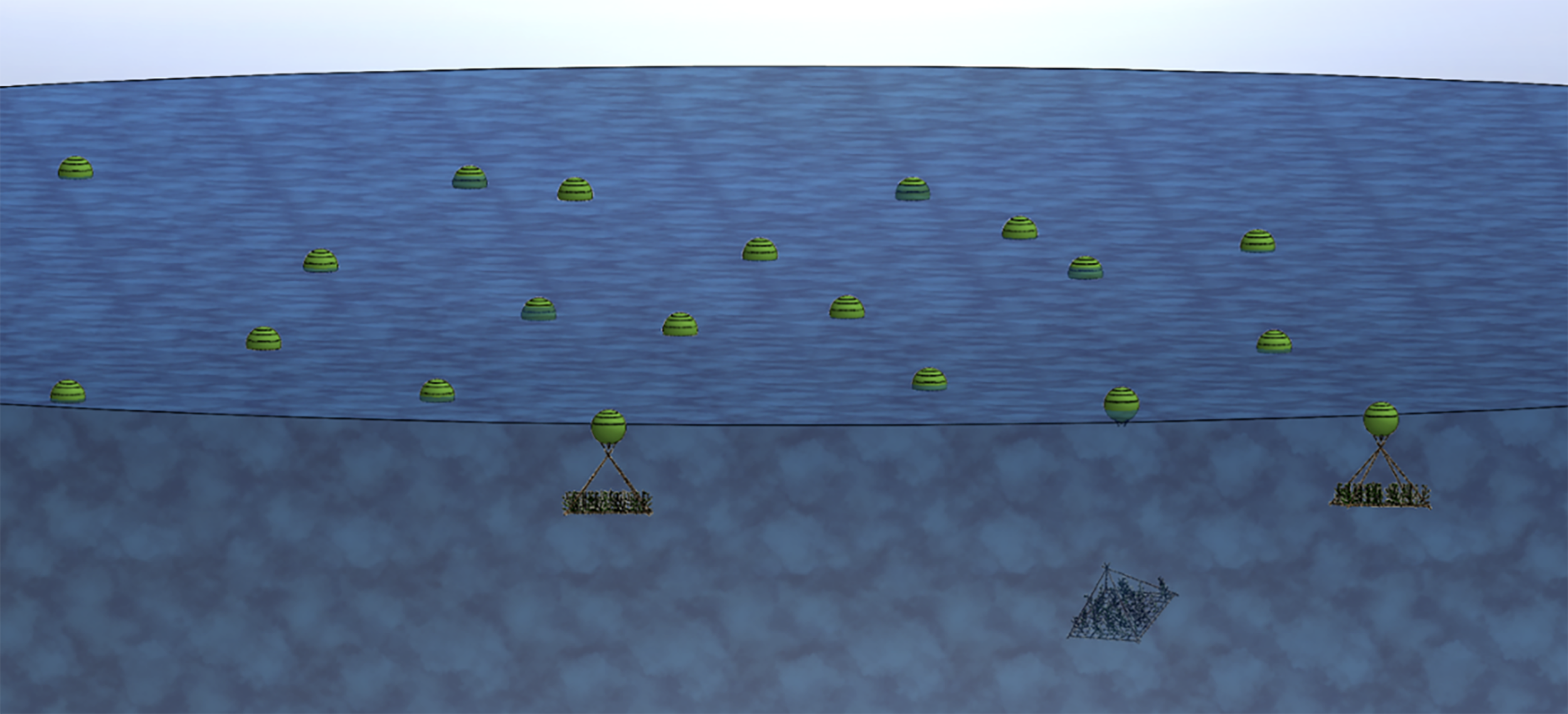
Free floating raft concept visualization.
A key operational consideration will be the sourcing of young kelp/sporelings. The planting of these at sea will be very difficult given weather limitations. So seeding ropes in onshore or nearshore facilities should be considered before ropes are put offshore. At what life stage these ropes are seeded, or how old the sporelings are, can be adapted from existing seaweed aquaculture depending on the species. One of the key challenges will be having a supply of sporelings available. A large supply of sporelings will require a significant amount of nursery infrastructure, which can be costly (Yarish et al., 2017). So the use of community nurseries or cooperatives setting up nurseries supplying individual aquaculture businesses could be considered to gain economies of scale.
Economic feasibility
The feasibility for ocean afforestation is still largely unknown (Boettcher et al., 2019). For ocean afforestation to be deemed viable and have an impact at scale it must be proved to be cost-efficient and an attractive business model. Given ocean afforestation is not an established practice, and technology development is limited, costs have been largely unknown (Froehlich et al., 2019; Troell et al., 2022). Ultimately if ocean afforestation is to operate unsubsidized it may have to compete with terrestrial carbon sequestration schemes to provide a marketable carbon offset at a low price. There are, however, novel carbon sequestration ventures operating at well above the market price, for example, direct air capture with a 2021 price of $149.0 - 248.6 USD/t CO2 (Breyer et al., 2019). Global carbon prices are much lower, for example in 2017 the global average carbon price was USD $5.1 for the forest and land use sector (Hamrick and Gallant, 2017). Novel carbon sequestration technologies may have the intention of improving efficiency, gaining economies of scale, and then lowering the price. Certainly, a considerable hurdle to scaling ocean afforestation will be seaweed carbon credits being competitive on the global market in the long term. Seaweed offsetting potentially has a much faster sequestration timeline than terrestrial offset schemes, with planted biomass potentially reaching a maximum in less than a year, therefore receiving an earlier return on investment than terrestrial carbon sinks like forests. There is also generally less competition for space in coastal environments than for terrestrial carbon sequestration sites.
Recently Coleman et al. (2022) provided the first detailed published estimates on the costs of a potential ocean afforestation via a bio-techno-economic model. They found that there would be a cost of $17,048 tCO2eq-1, but by optimising parameters on ocean afforestation this could be reduced to $1,257 tCO2eq-1. This price is considerably higher than the global averages for carbon prices mentioned previously. However, this study investigated the cultivation of nearshore seaweed and subsequent dumping and sinking offshore, option 1. in Figure 1. The other methods described in 2. (technical feasibility) may cost more or less than this approach to implement per tonne of carbon sequestration. This approach to ocean afforestation due to the boat hours required to cultivate, harvest and move the seaweed will likely carry significant costs and emissions (see 2. for pros and cons of each approach). Importantly, the carbon footprint (i.e. boat fuel emissions and construction of aquaculture infrastructure) of the operation of any ocean afforestation project should be subtracted from any potential carbon sequestration via ocean afforestation (Coleman et al., 2022). Coleman et al. (2022) calculated that potential CO2 sequestration was reduced from 638 tons to 244 tons of net emissions once operational emissions were subtracted and that did not include boat emissions to transport to the sink site [Figure 5 in Coleman et al. (2022)]. This suggests operational emissions may be considerable for some types of ocean afforestation operations. Therefore in developing a potential business case for ocean afforestation it will be critical to ensure sufficiently low operational emissions. At present emissions from different types of potential ocean afforestation operations are yet to be constrained based on live projects, and it remains to be seen if potential carbon sequestration from ocean afforestation will be sufficiently greater than operational emissions.
Financial viability will most importantly be determined by high ocean afforestation biomass yield and subsequent carbon sequestration in comparison with implementation cost. Froehlich et al. (2019) calculated the medium current cost of carbon sequestration to be USD $543 per tonne and a minimum of USD 71 per tonne of CO2. N‘Yeurt et al. (2012) calculated an average yield of 23.5 t/ha of ash-free weight from available data in the literature with a range of 6-90 t/ha, and used a conservative value of 18 t/ha. N‘Yeurt et al. (2012) use an ash-free rate of 68% of dry weight, so we can assume an average yield of 26.5 dry weight ha/yr-1. Using an average carbon content of 24.8% of seaweed dry weight (Duarte et al., 2017), we can use 24.2 t/CO2/yr-1 fixation as an average value for seaweed aquaculture. Using rafts of 5x5m, 0.061 t/CO2/yr-1 could be used as an average value of CO2/yr-1 absorbed per raft. Therefore, in theory, 16.4 billion of these rafts would need to be deployed for one gigatonne of CO2 fixation. However, while highly productive seaweed species may be selected it is worth noting that seaweed growth is not consistent throughout the year (Lobban and Harrison, 1994), and can be limited in some offshore environments due to low nutrient concentrations offshore. Additionally, if these rafts are free floating they may become fouled or reach the end of their drift before one year (Boyd et al., 2022), so this value is still highly speculative. Additionally, as has been discussed, the CO2 fixation described here can not currently be equated to carbon sequestration.
Co-benefits and risks
Seaweed ocean afforestation may have several co-benefits and risks that will need to be considered. While these are discussed in concept here, there may be other impacts that are yet to be considered. There has historically been relatively little investigation into the ecological or biosecurity risks of ocean afforestation (Russell et al., 2012), until recently by Boyd et al. (2022). These impacts will also largely be scale-dependent and may be challenging to quantify until ocean afforestation deployment at scale (Boyd et al., 2022). Additionally, the evidence for co-benefits from seaweed aquaculture to date has been associated with nearshore seaweed aquaculture, it remains to be seen if these co-benefits will extend offshore.
Possible co-benefits of ocean afforestation may include;
-
Habitat creation for macrofauna or possible fisheries benefits (Walls et al., 2016; Theuerkauf et al., 2021).
-
Nutrient alleviation can occur because of seaweed aquaculture, particularly in regions with excess anthropogenic nutrient loading or eutrophication, via absorbing nutrients or competition with photosynthetic microalgae that cause hypoxic conditions (Fei, 2004; He et al., 2008; Xiao et al., 2017; Visch et al., 2020).
-
Seaweed can also alleviate ocean acidification at local levels, which can protect nearby calcifying organisms (Chung et al., 2017; Clements and Chopin, 2017; Xiao et al., 2021). Additionally, acidification can lead to reduced carbon sequestration from phytoplankton, so alleviating acidification locally could potentially support localised phytoplankton carbon sequestration (Petrou et al., 2019).
Several possible negative impacts of ocean afforestation have recently been well described by Boyd et al. (2022) and include but are not limited to;
-
Allelopathy or chemical competition between phyto-plankton and seaweed should be considered as it impacts offshore ecological function (Boyd et al., 2022).
-
An increased abundance of organic matter will consume oxygen while decomposing, this could result in lower oxygen concentrations or hypoxia (Wyrtki, 1962). Therefore dumping of seaweed could lead to hypoxia in some locations (Lapointe et al., 2018). It remains to be seen if methane emissions from seaweed decomposing in hypoxic areas will be lower or higher (Naqvi et al., 2010).
-
Seaweed releases dissolved organic carbon (DOC), so their cultivation could lead to an increase in DOC in offshore ecosystems. Changes to offshore quantity and quality of DOC may impact offshore communities, for example by changing the dynamics of oceanic heterotrophic bacterial communities; however, those impacts are poorly documented (Boyd et al., 2022).
-
Entanglement of marine life, seabirds and mammals should be considered as a possible risk of offshore afforestation given entanglement has occurred with other aquaculture operations (Lobban and Harrison, 1994; Würsig and Gailey, 2002; Young, 2015). There is also potential for visual pollution from structures washing ashore or as floating debris.
-
Some deep sea microorganisms convert carbon to methane in the deep sea, so there may be a risk of methane being released due to carbon deposition from ocean afforestation (Whiticar, 1999; Kotelnikova, 2002). However, methanogenesis can be reduced at higher sulphate concentrations in the deep ocean (Sivan et al., 2007). Currently little is known about these complex deep-sea chemical pathways for potential seaweed decomposition.
-
Impact of seaweed and associated debris on deep-sea benthic communities which will be difficult to monitor (Danovaro et al., 2020), and may be significant (Wolff, 1962; Filbee-Dexter and Scheibling, 2014; Baker et al., 2018). However, the impact could be reduced if deposition can be directed to hypoxic areas with low biodiversity (Levin, 2002; Helly and Levin, 2004).
-
Seaweed will likely be befouled on the way offshore and can transport their microbiome community, which could interact with offshore phytoplankton communities (Fraser et al., 2011; Boyd et al., 2022).
Much more testing is needed to gauge potential negative impacts and offshore biosecurity risk of offshore afforestation as they are currently poorly constrained by location and various conditions (Boyd et al., 2022). Additionally, if large-scale ocean afforestation is to take place, the possible impacts on kelp forests of an abundance of spores reaching the coast should be considered, given various seaweed species could be considered a threat as an invader (Wikström and Kautsky, 2004; Williams and Smith, 2007). This will depend on the location of ocean afforestation compared to coastal seaweed ecosystems and the life histories of cultivated seaweed species. Therefore, cultivating native or endemic macroalga species could reduce the chance of invasion from non-native species and their associated epifauna (Boettcher et al., 2019; Boyd et al., 2022). However, some wild kelp forest ecosystems are experiencing a significant decline (Smale and Wernberg, 2013; Thomsen et al., 2019; Layton et al., 2020). The release of spores from nearby ocean afforestation operations could in theory in some cases support kelp forest recovery. If spores will reach the coast at a greater abundance than was naturally occurring, unknown ecological implications should be considered. This discussion should include genetically modified or engineered seaweed species which may face major regulatory challenges in the aquaculture industry, given the potential ecological risk of invasion in coastal ecosystems (Robinson et al., 2013; Kim et al., 2017; Cheney et al., 2019).
Governance and social considerations
Job creation and social or economic developed from a new ocean afforestation industry could benefit local communities (Valderrama, 2012; Boettcher et al., 2019; Larson et al., 2021). There may be an opportunity for high skilled engineering, business and science jobs. And potentially low-skilled jobs in developing countries, depending on how labour-intensive the chosen cultivation method is. For cultivation methods using biodegradable substructures and growth substrates, e.g. hemp, bamboo or pine, further economic development opportunities may come from supporting these primary industries. Importantly the environmental and social impact from any industries where ocean afforestation sources materials from should also be considered.
Coastal seaweed aquaculture is an important food industry, a source of increasingly diverse low-carbon products and will help us achieve the United Nations Sustainable Development Goals (Duarte et al., 2021). Ricart et al. (2022) asserted that ocean afforestation may jeopardise the positive effects of coastal seaweed aquaculture on society if biomass is deposited offshore instead of being used for other markets. This is an important consideration, however, this may only be the case if ocean afforestation is conducted to the detriment of nearshore seaweed aquaculture and if these two industries compete for physical resources and human expertise. If ocean afforestation is conducted fully offshore and did not compete for resources with nearshore seaweed aquaculture there would be a lower risk of ocean afforestation having a negative impact on the important contribution of coastal seaweed aquaculture to a sustainable low-carbon future.
In developing coastal communities, alternative livelihoods through ocean afforestation industry support via nurseries for example, to exploitive, or non-sustainable sources of income like unsustainable commercial fishing, may be preferable (Valderrama, 2012). However, countries and regions with coastline within the majority of the kelp biome (New Zealand, Australia, North America, Europe, Chile, Japan, South Korea) are for the majority more developed economies (South Africa and Namibia being the exemption) (Fund, 2017). If ocean afforestation is considered primarily for kelp species, the social development impact may be less than for an industry operating in developing countries. However there still may be development opportunities with new forms of aquaculture like ocean afforestation, for example, they could be used for re-skilling fishermen or land-based farmers whose livelihoods are increasingly at-risk of the impacts of climate change.
A key consideration for ocean afforestation feasibility will be legality, consent and permits to operate (Barbier et al., 2019; Kim et al., 2019; van den Burg et al., 2020). To our knowledge, no country yet has a protocol for ocean afforestation within their economic exclusive zone. For international waters, there are a range of ocean-related treaties including; The London Protocol, The International Seabed Authority, the International Maritime Organization, The Law of the Sea and the Convention on Biological Diversity. Under the Law of the Sea, there are details regarding marine research in international waters, however little to no laws to specifically govern ocean afforestation that we could determine. The London Convention is an international treaty designed to protect the marine environment from pollution caused by dumping into the ocean., Article III b ii) of the London Convention states: “Dumping” does not include: Placement of matter for a purpose other than the mere disposal thereof, provided that such placement is not contrary to the aims of this Convention.” It remains to be seen if ocean afforestation falls in this category. The socio-political risks of ocean afforestation were considered to be medium by Boettcher et al. (2019). Additionally the impacts on biodiversity in international waters should also be considered as they relate to the UN convention on biodiversity (Boettcher et al., 2019). Particularly given sink sites may be in different locations from sites of seaweed growth.
Linking ocean afforestation to carbon credit opportunities around the world
Demand for carbon credits is growing internationally (O'connor et al., 2019; Kreibich and Hermwille, 2021). As countries are looking to lower GHG budgets nationally, various countries may look to invest in blue carbon projects as they relate to their national greenhouse gas contributions (Herr and Landis, 2016). There have been internationally recognised blue carbon accounting frameworks compiled that include blue carbon habitats, however, they have so far excluded seaweed (Hiraishi et al., 2014; Emmer et al., 2015; Sapkota and White, 2020). While there has been thought given to a methodology for near-shore seaweed aquaculture carbon budgets (Duarte et al., 2017; Sondak et al., 2017), there has not been any carbon sequestration methodological research to our knowledge for offshore seaweed cultivation. However, because via ocean afforestation seaweed may be sunk directly, down to the storage site, a methodology to document this transport is potentially simpler than mapping detritus export and sediment transport pathways from nearshore environments to offshore sink sites. This timescale may be quicker than the difficult-to-track current pathways for seaweed carbon sequestration, given the carbon could be more easily monitored and quantified (Chung et al., 2017). But the challenges of monitoring carbon storage sites and quantifying carbon sequestration in the deep ocean will remain.
While biomass accumulation with seaweed will be comparatively simple, mapping the pathway for viable sequestration remains a large challenge with ocean afforestation (Boettcher et al., 2019). With any carbon offset project or proposal, it is very important to provide an accurate methodology to determine the quantity of carbon sequestered (Chung et al., 2017). Any verification methodology will need to meet international standards for carbon sequestration such as in the UN Framework Convention on Climate Change, to enable ocean afforestation to be eligible for carbon credits (Michaelowa et al., 2019). This includes criteria around proof of additionality, permanence and accuracy. There will be costs associated with methodological development for the verification of ocean afforestation projects. A method for documenting detailed sequestration pathways, permanence and monitoring of sink sites would be needed for the implementation of ocean afforestation. To develop a methodology for measuring carbon budgets from ocean afforestation Hurd et al. (2022), proposed a framework for this called ‘Forensic carbon accounting’, which will be useful to build a methodology upon if ocean afforestation projects are developed further.
Conclusion
While it is clear seaweed cultivation can yield significant biomass and ‘carbon fixation’, the pathways for this to result in long-term effective carbon sequestration remain unclear. It seems highly likely that seaweed can be grown in abundance and deposited offshore, with some carbon being permanently sequestered at depth, however many fundamental questions must be addressed first to determine the viability of ocean afforestation. Pressing research topics include, understanding potential nutrient reallocation and oceanic biogeochemical feedback mechanisms, meeting carbon accounting criteria, cost-efficient infrastructure for ocean afforestation, meeting oceanic regulatory requirements and minimising environmental impacts. Importantly, once these questions have been addressed, ocean afforestation must be price competitive in sequestration of carbon in comparison to other offset schemes, if it is to be viable at scale. While there are many research gaps to overcome before the possibility of an ocean afforestation industry, the potential scale of ocean afforestation is extensive. A combination of modelling, lab and ocean-based experiments are needed to overcome the key research gaps remaining if we are to confirm or deny the viability of ocean afforestation for climate change mitigation.
Funding
This project was supported by Deakin University and Sea Green Pte Ltd. PIM thanks the support of the Australian Research Council Discovery Project (DP200100575).
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
All authors contributed to the editing and writing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
Author PT is employed by Sea Green Pte Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Sea Green Pte Ltd. The funder had the following involvement in the study: financial sponsorship of FR's PhD of which this paper was part of, and advisory role in design and preparation of the paper.
References
1
Abbott I. Hollenberg G. (1976). Marine algae of California (Stanford, California: Stanford University Press).
2
Aldridge J. van der Molen J. Forster R. (2012). Wider ecological implications of macroalgae cultivation Vol. 95 (Marine Estate Research Report: The Crown Estate).
3
Alongi D. M. (2018). “Kelp forests,” in Blue carbon (Springer), 53–57.
4
Atkinson M. Smith S. (1983). C: N: P ratios of benthic marine plants 1. Limnol Oceanogr28, 568–574. doi: 10.4319/lo.1983.28.3.0568
5
Bach L. T. Tamsitt V. Gower J. Hurd C. L. Raven J. A. Boyd P. W. (2021). Testing the climate intervention potential of ocean afforestation using the great Atlantic Sargassum belt. Nat. Commun.12, 1–10. doi: 10.1038/s41467-021-22837-2
6
Baird M. E. Middleton J. H. (2004). On relating physical limits to the carbon: nitrogen ratio of unicellular algae and benthic plants. J. Mar. Syst.49, 169–175. doi: 10.1016/j.jmarsys.2003.10.007
7
Baker C. A. Martin A. P. Yool A. Popova E. (2022). “Biological carbon pump sequestration efficiency in the north Atlantic: A leaky or a long-term sink?,” in Global biogeochemical cycles, vol. 36.
8
Baker P. Minzlaff U. Schoenle A. Schwabe E. Hohlfeld M. Jeuck A. et al . (2018). Potential contribution of surface-dwelling Sargassum algae to deep-sea ecosystems in the southern north Atlantic. Deep Sea Res. Part II: Topical Stud. Oceanogr148, 21–34. doi: 10.1016/j.dsr2.2017.10.002
9
Barbier M. Charrier B. Araujo R. Holdt S. Jacquemin B. Rebours C. et al . (2019). PEGASUS-PHYCOMORPH European guidelines for a sustainable aquaculture of seaweeds (Roscoff, France: COST action FA1406).
10
Bekkby T. Moy F. E. (2011). Developing spatial models of sugar kelp (Saccharina latissima) potential distribution under natural conditions and areas of its disappearance in skagerrak. Estuarine Coast. Shelf Sci.95, 477–483. doi: 10.1016/j.ecss.2011.10.029
11
Birkemeyer C. Lemesheva V. Billig S. Tarakhovskaya E. (2020). Composition of intracellular and cell wall-bound phlorotannin fractions in fucoid algae indicates specific functions of these metabolites dependent on the chemical structure. Metabolites10, 369. doi: 10.3390/metabo10090369
12
Boettcher M. Chai F. Cullen J. Goeschl T. Lampitt R. Lenton A. et al . (2019). High level review of a wide range of proposed marine geoengineering techniquesLondon: International Maritime Organization.
13
Boyd P. (2004). Ironing out algal issues in the southern ocean. Science304, 396–397. doi: 10.1126/science.1092677
14
Boyd P. W. Bach L. T. Hurd C. L. Paine E. Raven J. A. Tamsitt V. (2022). Potential negative effects of ocean afforestation on offshore ecosystems. Nat. Ecol. Evol., 1–9. doi: 10.1038/s41559-022-01722-1
15
Boyd P. W. Jickells T. Law C. Blain S. Boyle E. Buesseler K. et al . (2007). Mesoscale iron enrichment experiments 1993-2005: synthesis and future directions. Science315, 612–617. doi: 10.1126/science.1131669
16
Breyer C. Fasihi M. Bajamundi C. Creutzig F. (2019). Direct air capture of CO2: a key technology for ambitious climate change mitigation. Joule3, 2053–2057. doi: 10.1016/j.joule.2019.08.010
17
Calvert S. Price N. (1971). “upwelling and nutrient regeneration in the benguela current, octobe,” in Deep Sea research and oceanographic abstracts (Elsevier) 18 (5), 505–523.
18
Canadell J. G. Monteiro P. M. Costa M. H. Da Cunha L. C. Cox P. M. Alexey V. et al . (2021). Global carbon and other biogeochemical cycles and feedbacksIPCC.
19
Carpenter R. C. (1990). Competition among marine macroalgae: a physiological perspective. J. Phycol26, 6–12. doi: 10.1111/j.0022-3646.1990.00006.x
20
Chapman A. R. O. (1987). “Population and community ecology of seaweeds,” in Advances in marine biology (Elsevier), 1–161.
21
Cheney D. Mathieson A. Schubert D. (2019). “The application of genetic improvement techniques to seaweed cultivation: I. strain selection in the carrageenophyte chondrus crispus,” in International seaweed symposium (Xth) (Göteborg, Sweden: De Gruyter), 559–568.
22
Cheng L. Abraham J. Zhu J. Trenberth K. E. Fasullo J. Boyer T. et al . (2020). Record-setting ocean warmth continued in 2019. Advances In Atmospheric Sciences (Springer) 37, 137–142.
23
Chen Z. Sun J. Gu T. Zhang G. Wei Y. (2021). Nutrient ratios driven by vertical stratification regulate phytoplankton community structure in the oligotrophic western pacific ocean. Ocean Sci.17, 1775–1789. doi: 10.5194/os-17-1775-2021
24
Cheshire A. C. Conran J. G. Hallam N. D. (1995). A cladistic analysis of the evolution and biogeography of durvillaea (Phaeophyta). J. Phycol31, 644–655. doi: 10.1111/j.1529-8817.1995.tb02561.x
25
Christensen K. H. Breivik Ø. Dagestad K.-F. Röhrs J. Ward B. (2018). Short-term predictions of oceanic drift. Oceanography31, 59–67. doi: 10.5670/oceanog.2018.310
26
Chung I. K. Oak J. H. Lee J. A. Shin J. A. Kim J. G. Park K.-S. (2013). Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean project overview. ICES J. Mar. Sci.70, 1038–1044. doi: 10.1093/icesjms/fss206
27
Chung I. K. Sondak C. F. Beardall J. (2017). The future of seaweed aquaculture in a rapidly changing world. Eur. J. Phycol52, 495–505. doi: 10.1080/09670262.2017.1359678
28
Clements J. C. Chopin T. (2017). Ocean acidification and marine aquaculture in north America: potential impacts and mitigation strategies. Rev. Aquacult9, 326–341. doi: 10.1111/raq.12140
29
Coleman S. Dewhurst T. Fredriksson D. W. St Gelais A. Cole K. MacNicoll M. et al . (2022). Quantifying baseline costs and cataloging potential optimization strategies for kelp aquaculture carbon dioxide removal. Front. Mar. Sci., 1460. doi: 10.3389/fmars.2022.966304
30
Cribb A. (1954). Macrocystis pyrifera (L.) ag. in Tasmanian waters. Mar. Freshw. Res.5, 1–34. doi: 10.1071/MF9540001
31
Danovaro R. Fanelli E. Aguzzi J. Billett D. Carugati L. Corinaldesi C. et al . (2020). Ecological variables for developing a global deep-ocean monitoring and conservation strategy. Nat. Ecol. Evol.4, 181–192. doi: 10.1038/s41559-019-1091-z
32
DeVries T. Holzer M. Primeau F. (2017). Recent increase in oceanic carbon uptake driven by weaker upper-ocean overturning. Nature542, 215–218. doi: 10.1038/nature21068
33
Duarte C. M. Bruhn A. Krause-Jensen D. (2021). A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustainabil (Springer Science and Business Media LLC), 1–9. doi: 10.1038/s41893-021-00773-9
34
Duarte C. M. Gattuso J. P. Hancke K. Gundersen H. Filbee-Dexter K. Pedersen M. F. et al . (2022). Global estimates of the extent and production of macroalgal forests. Global Ecol. Biogeogr. doi: 10.1111/geb.13515
35
Duarte C. M. Wu J. Xiao X. Bruhn A. Krause-Jensen D. (2017). Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci.4, 100.
36
Duffy J. E. Hay M. E. (1990). Seaweed adaptations to herbivory (BioScience) 40 (5).
37
Dufour C. Probert P. Savage C. (2012). Macrofaunal colonisation of stranded durvillaea antarctica on a southern new Zealand exposed sandy beach. New Z. J. Mar. Freshw. Res.46, 369–383. doi: 10.1080/00288330.2012.676557
38
Duggins D. Simenstad C. Estes J. (1989). Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science245, 170–173. doi: 10.1126/science.245.4914.170
39
Eckersley L. K. Scrosati R. A. (2020). Temperature, desiccation, and species performance trends along an intertidal elevation gradient5 (2), 59–73. doi: 10.31230/osf.io/y9ph3
40
Edwards M. S. Connell S. D. (2012). “Competition, a major factor structuring seaweed communities,” in Seaweed biology, vol. 135-156. (Verlag Berlin Heidelberg: Springer).
41
Eggert A. (2012). “Seaweed responses to temperature,” in Seaweed biology (ECOLSTUD: Springer), 47–66.
42
Emmer I. Needelman B. Emmett-Mattox S. Crooks S. Megonigal P. Myers D. et al . (2015). Methodology for tidal wetland and seagrass restoration (Verified Carbon Standard), VM0033. Available at: https://verra.org/methodology/vm0033-methodology-for-tidal-wetland-and-seagrass-restoration-v2-0/.
43
Fei X. (2004). “Solving the coastal eutrophication problem by large scale seaweed cultivation,” in Asian Pacific phycology in the 21st century: Prospects and challenges (NetherlandsSpringer), 145–151.
44
Fernand F. Israel A. Skjermo J. Wichard T. Timmermans K. R. Golberg A. (2017). Offshore macroalgae biomass for bioenergy production: Environmental aspects, technological achievements and challenges. Renewable Sustain. Energy Rev.75, 35–45. doi: 10.1016/j.rser.2016.10.046
45
Filbee-Dexter K. Scheibling R. (2014). Detrital kelp subsidy supports high reproductive condition of deep-living sea urchins in a sedimentary basin. Aquat. Biol.23, 71–86. doi: 10.3354/ab00607
46
Filbee-Dexter K. Wernberg T. Norderhaug K. M. Ramirez-Llodra E. Pedersen M. F. (2018). Movement of pulsed resource subsidies from kelp forests to deep fjords. Oecologia187, 291–304. doi: 10.1007/s00442-018-4121-7
47
Flannery T. (2017). Sunlight and seaweed: an argument for how to feed, power and clean up the world (Melbourne: Text Publishing).
48
Fortes M. Lüning K. (1980). Growth rates of north Sea macroalgae in relation to temperature, irradiance and photoperiod. Helgoländer Meeresuntersuchungen34, 15–29. doi: 10.1007/BF01983538
49
Frankignoulle M. Canon C. Gattuso J. P. (1994). Marine calcification as a source of carbon dioxide: Positive feedback of increasing atmospheric CO2. Limnol Oceanogr39, 458–462. doi: 10.4319/lo.1994.39.2.0458
50
Fraser C. I. Nikula R. Waters J. M. (2011). Oceanic rafting by a coastal community. Proc. R. Soc. B: Biol. Sci.278, 649–655. doi: 10.1098/rspb.2010.1117
51
Fraser C. I. Velásquez M. Nelson W. A. Macaya E. C. Hay C. H. (2020). The biogeographic importance of buoyancy in macroalgae: A case study of the southern bull-kelp genus Durvillaea (Phaeophyceae), including descriptions of two new Species1. J. Phycol56, 23–36. doi: 10.1111/jpy.12939
52
Froehlich H. E. Afflerbach J. C. Frazier M. Halpern B. S. (2019). Blue growth potential to mitigate climate change through seaweed offsetting. Curr. Biol.29, 3087–3093.e3083. doi: 10.1016/j.cub.2019.07.041
53
Fund I. M. (2017). World economic outlook database (World Econ Finance Survey). Available at: https://www.imf.org/en/Publications/WEO/weo-database/2017/April.
54
García-Poza S. Leandro A. Cotas C. Cotas J. Marques J. C. Pereira L. et al . (2020). The evolution road of seaweed aquaculture: Cultivation technologies and the industry 4.0. Int. J. Environ. Res. Public Health17, 6528. doi: 10.3390/ijerph17186528. Available at: https://www.forest-trends.org/who-we-are/initiatives/who-we-areinitiativesecosystem-marketplace/.
55
Gattuso J.-P. Gentili B. Duarte C. M. Kleypas J. Middelburg J. J. Antoine D. (2006). Light availability in the coastal ocean: impact on the distribution of benthic photosynthetic organisms and their contribution to primary production. Biogeosciences3, 489–513. doi: 10.5194/bg-3-489-2006
56
Gentry R. R. Lester S. E. Kappel C. V. White C. Bell T. W. Stevens J. et al . (2017). Offshore aquaculture: spatial planning principles for sustainable development. Ecol. Evol.7, 733–743. doi: 10.1002/ece3.2637
57
Graff J. R. Westberry T. K. Milligan A. J. Brown M. B. Dall’Olmo G. van Dongen-Vogels V. et al . (2015). “Analytical phytoplankton carbon measurements spanning diverse ecosystems,” in Deep Sea research part I: Oceanographic research papers (Elsevier Ltd), vol. 102. , 16–25. doi: 10.1016/j.dsr.2015.04.
58
Graham M. H. Vasquez J. A. Buschmann A. H. (2007). Global ecology of the giant kelp Macrocystis: from ecotypes to ecosystems. Oceanogr Mar. Biol.45, 39.
59
Hamrick K. Gallant M. (2017). Unlocking potential: state of the voluntary carbon markets 2017 (Ecosystem marketplace). Available at: https://www.forest-trends.org/who-we-are/initiatives/who-we-areinitiativesecosystem-marketplace/.
60
Harrison P. J. Hurd C. L. (2001). Nutrient physiology of seaweeds: application of concepts to aquaculture. Cahiers biologie Mar.42, 71–82.
61
Harrold C. Lisin S. (1989). Radio-tracking rafts of giant kelp: local production and regional transport. J. Exp. Mar. Biol. Ecol.130, 237–251. doi: 10.1016/0022-0981(89)90166-4
62
Hay C. H. (1977). A biological study of durvillaea antarctica (Chamisso) Hariot and D Willana Lindauer in New Zealand (Thesis and Dissertation University of Canterbury) .
63
Helly J. J. Levin L. A. (2004). “Global distribution of naturally occurring marine hypoxia on continental margins,” in Deep Sea research part I: Oceanographic research papers (Elsevier Ltd), vol. 51. , 1159–1168. 10.1016/j.dsr.2004.03.009
64
Herr D. Landis E. (2016). Coastal blue carbon ecosystems. opportunities for nationally determined contributions. policy brief (Gland, Switzerland: IUCN). Washington, DC: TNC.
65
Herzog H. Caldeira K. Reilly J. (2003). An issue of permanence: Assessing the effectiveness of temporary carbon storage. Climatic Change59, 293–310. doi: 10.1023/A:1024801618900
66
He P. Xu S. Zhang H. Wen S. Dai Y. Lin S. et al . (2008). Bioremediation efficiency in the removal of dissolved inorganic nutrients by the red seaweed, porphyra yezoensis, cultivated in the open sea. Water Res.42, 1281–1289. doi: 10.1016/j.watres.2007.09.023
67
Hiraishi T. Krug T. Tanabe K. Srivastava N. Baasansuren J. Fukuda M. et al . (2014). 2013 supplement to the 2006 IPCC guidelines for national greenhouse gas inventories (Wetlands: IPCC, Switzerland).
68
Hurd C. L. (2000). Water motion, marine macroalgal physiology, and production. J. Phycol36, 453–472. doi: 10.1046/j.1529-8817.2000.99139.x
69
Hurd C. L. Law C. S. Bach L. T. Britton D. Hovenden M. Paine E. et al . (2022). Forensic carbon accounting: Assessing the role of seaweeds for carbon sequestration. J. Phycol. doi: 10.1111/jpy.13249
70
Huyer A. (1983). Coastal upwelling in the California current system. Prog. Oceanogr12, 259–284. doi: 10.1016/0079-6611(83)90010-1
71
Jackson G. A. (1977). Nutrients and production of giant kelp, macrocystis pyrifera, off southern California 1. Limnol Oceanogr22, 979–995. doi: 10.4319/lo.1977.22.6.0979
72
Jansen H. M. Van Den Burg S. Bolman B. Jak R. G. Kamermans P. Poelman M. et al . (2016). The feasibility of offshore aquaculture and its potential for multi-use in the north Sea. Aquacult Int.24, 735–756. doi: 10.1007/s10499-016-9987-y
73
Karsten U. (2012). “Seaweed acclimation to salinity and desiccation stress,” in Seaweed biology (Berlin, Heidelberg: Springer), 87–107.
74
Kim J. Stekoll M. Yarish C. (2019). Opportunities, challenges and future directions of open-water seaweed aquaculture in the united states. Phycologia58, 446–461. doi: 10.1080/00318884.2019.1625611
75
Kim J. K. Yarish C. Hwang E. K. Park M. Kim Y. (2017). Seaweed aquaculture: cultivation technologies, challenges and its ecosystem services. Algae32, 1–13. doi: 10.4490/algae.2017.32.3.
76
Koop K. Newell R. C. Lucas M. (1982). Biodegradation and carbon flow based on kelp (Ecklonia maxima) debris in a sandy beach microcosm. Mar. Ecol. Prog. Ser.7, 315–326.
77
Kotelnikova S. (2002). Microbial production and oxidation of methane in deep subsurface. Earth-Sci Rev.58, 367–395. doi: 10.1016/S0012-8252(01)00082-4
78
Krause-Jensen D. Duarte C. M. (2016). Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci.9, 737–742. doi: 10.3389/fmars.2019.00375
79
Krause-Jensen D. Lavery P. Serrano O. Marba N. Masque P. Duarte C. M. (2018). Sequestration of macroalgal carbon: The elephant in the blue carbon room. Biol. Lett.14. doi: 10.1098/rsbl.2018.0236
80
Kreibich N. Hermwille L. (2021). Caught in between: credibility and feasibility of the voluntary carbon market post-2020 (Climate Policy) 7, 939–957 doi: 10.1080/14693062.2021.1948384.
81
Kruckeberg A. R. (1991). The natural history of puget sound country (University of Washington Press).
82
Kübler J. E. Davison I. R. (1993). High-temperature tolerance of photosynthesis in the red alga Chondrus crispus. Mar. Biol.117, 327–335. doi: 10.1007/BF00345678
83
Kübler J. E. Johnston A. M. Raven J. A. (1999). “The effects of reduced and elevated CO2 and O2 on the seaweed lomentaria articulata,” in Plant, cell & environment (Hoboken, New Jersey, United States: Blackwell Science Ltf. Headquarters), vol. 22. , 1303–1310.
84
Kumar V. Zozaya-Valdes E. Kjelleberg S. Thomas T. Egan S. (2016). Multiple opportunistic pathogens can cause a bleaching disease in the red seaweed delisea pulchra. Environ. Microbiol.18, 3962–3975. doi: 10.1111/1462-2920.13403
85
Lapointe B. E. Burkholder J. M. Van Alstyne K. L. (2018). “Harmful macroalgal blooms in a changing world: causes, impacts, and management,” in Harmful algal blooms: a compendium desk reference, 515–560. doi: 10.1002/9781118994672.ch15
86
Largo D. B. (2002). Recent developments in seaweed diseases. Proceedings of the National Seaweed Planning Workshop
87
Larson S. Stoeckl N. Fachry M. E. Mustafa M. D. Lapong I. Purnomo A. H. et al . (2021). Women's well-being and household benefits from seaweed farming in Indonesia. Aquaculture530, 735711. doi: 10.1016/j.aquaculture.2020.735711
88
Lau S. C. Qian P.-Y. (1997). Phlorotannins and related compounds as larval settlement inhibitors of the tube-building polychaete Hydroides elegans. Mar. Ecol. Prog. Ser.159, 219–227. doi: 10.3354/meps159219
89
Layton C. Coleman M. A. Marzinelli E. M. Steinberg P. D. Swearer S. E. Vergés A. et al . (2020). Kelp forest restoration in Australia. Front. Mar. Sci.7, 74. doi: 10.3389/fmars.2020.00074
90
Levin L. A. (2002). “Deep-ocean life where oxygen is scarce: oxygen-deprived zones are common and might become more so with climate change. here life hangs on, with some unusual adaptations,” in American ScientistThe Scientific Research, vol. 90. , 436–444.
91
Lobban C. S. Harrison P. J. (1994). Seaweed ecology and physiology (Cambridge UK: Cambridge University Press).
92
Lobban C. S. Wynne M. J. (1981). The biology of seaweeds (Berkely and Los Angeles: Univ of California Press).
93
Lüning K. (1990). Seaweeds: their environment, biogeography, and ecophysiology (Canada: John Wiley & Sons).
94
Mabin C. J. Johnson C. R. Wright J. T. (2019). Physiological response to temperature, light, and nitrates in the giant kelp Macrocystis pyrifera from Tasmania, Australia. Mar. Ecol. Prog. Ser.614, 1–19. doi: 10.3354/meps12900
95
Macreadie P. I. Anton A. Raven J. A. Beaumont N. Connolly R. M. Friess D. A. et al . (2019). The future of blue carbon science. Nat. Commun.10, 1–13. doi: 10.1038/s41467-019-11693-w
96
Macreadie P. I. Serrano O. Maher D. T. Duarte C. M. Beardall J. (2017). Addressing calcium carbonate cycling in blue carbon accounting. Limnol Oceanogr Lett.2, 195–201. doi: 10.1002/lol2.10052
97
Mann K. (1973). Seaweeds: their productivity and strategy for growth. Science182, 975–981. doi: 10.1126/science.182.4116.975
98
Michaelowa A. Hermwille L. Obergassel W. Butzengeiger S. (2019). Additionality revisited: guarding the integrity of market mechanisms under the Paris agreement. Climate Policy19, 1211–1224. doi: 10.1080/14693062.2019.1628695
99
Millar R. V. Houghton J. D. Elsäβer B. Mensink P. J. Kregting L. (2020). Influence of waves and currents on the growth rate of the kelp laminaria digitata (Phaeophyceae). J. Phycol56, 198–207. doi: 10.1111/jpy.12943
100
Milledge J. J. Smith B. Dyer P. W. Harvey P. (2014). Macroalgae-derived biofuel: a review of methods of energy extraction from seaweed biomass. Energies7, 7194–7222. doi: 10.3390/en7117194
101
Miller R. (1985). Succession in sea urchin and seaweed abundance in Nova Scotia, Canada. Mar. Biol.84, 275–286. doi: 10.1007/BF00392497
102
Moore C. Mills M. Arrigo K. Berman-Frank I. Bopp L. Boyd P. et al . (2013). Processes and patterns of oceanic nutrient limitation. Nat. Geosci.6, 701–710. doi: 10.1038/ngeo1765
103
Nagayama K. Iwamura Y. Shibata T. Hirayama I. Nakamura T. (2002). Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrobial Chemother50, 889–893. doi: 10.1093/jac/dkf222
104
Naqvi S. Bange H. W. Farías L. Monteiro P. Scranton M. Zhang J. (2010). Marine hypoxia/anoxia as a source of CH 4 and n 2 O. Biogeosciences7, 2159–2190. doi: 10.5194/bg-7-2159-2010
105
Nellemann C. Corcoran E. Duarte C. M. De Young C. Fonseca L. E. Grimsdith G. (2010). Blue carbon: the role of healthy oceans in binding carbon. Center for Coastal and Ocean Mapping132.
106
Nelson W. A. (2020). New Zealand seaweeds: an illustrated guide (Wellington: Te Papa Press).
107
Neori A. Cohen I. Gordin H. (1991). Ulva lactuca biofilters for marine fishpond effluents. II. growth rate, yield and c: N ratio. Botanica Marina34, 483–490. doi: 10.1515/botm.1991.34.6.483
108
Newcombe E. M. Taylor R. B. (2010). Trophic cascade in a seaweed-epifauna-fish food chain. Mar. Ecol. Prog. Ser.408, 161–167. doi: 10.3354/meps08589
109
North W. J. (1970). The biology of giant kelp beds (Macrocystis) in California (Nova Hedwigia, Beihefte, Beih) 32.
110
N‘Yeurt A. R. Chynoweth D. P. Capron M. E. Stewart J. R. Hasan M. A. (2012). Negative carbon via ocean afforestation. Process Saf. Environ. Prot.90, 467–474. doi: 10.1016/j.psep.2012.10.008
111
O'connor P. Summers D. Connor J. Stirling E. Cavagnaro T. (2019). Assessing south Australian carbon offset supply and policy for co-beneficial offsets: Policy context (Adelaide: The Goyder Institute for Water Research).
112
Oliver E. C. Donat M. G. Burrows M. T. Moore P. J. Smale D. A. Alexander L. V. et al . (2018). Longer and more frequent marine heatwaves over the past century. Nat. Commun.9, 1–12. doi: 10.1038/s41467-018-03732-9
113
Orr J. C. Sarmiento J. L. (1992). Potential of marine macroalgae as a sink for CO 2: constraints from a 3-d general circulation model of the global ocean. Water Air Soil pollut.64, 405–421. doi: 10.1007/BF00477113
114
Ortega A. Geraldi N. R. Alam I. Kamau A. A. Acinas S. G. Logares R. et al . (2019). Important contribution of macroalgae to oceanic carbon sequestration. Nat. Geosci.12, 748–754. doi: 10.1038/s41561-019-0421-8
115
Pan Y. Fan W. Zhang D. Chen J. Huang H. Liu S. et al . (2016). Research progress in artificial upwelling and its potential environmental effects. Sci. China Earth Sci.59, 236–248. doi: 10.1007/s11430-015-5195-2
116
Petrou K. Baker K. G. Nielsen D. A. Hancock A. M. Schulz K. G. Davidson A. T. (2019). Acidification diminishes diatom silica production in the southern ocean. Nat. Climate Change9, 781–786. doi: 10.1038/s41558-019-0557-y
117
Ricart A. M. Krause-Jensen D. Hancke K. Price N. N. Masque P. Duarte C. M. (2022). Sinking seaweed in the deep ocean for carbon neutrality is ahead of science and beyond the ethics. Environ. Res. Lett. doi: 10.1088/1748-9326/ac82ff
118
Robinson N. Winberg P. Kirkendale L. (2013). Genetic improvement of macroalgae: status to date and needs for the future. J. Appl. Phycol25, 703–716. doi: 10.1007/s10811-012-9950-x
119
Roleda M. Y. Hurd C. L. (2019). Seaweed nutrient physiology: application of concepts to aquaculture and bioremediation. Phycologia58, 552–562. doi: 10.1080/00318884.2019.1622920
120
Rothäusler E. Gómez I. Karsten U. Tala F. Thiel M. (2011). Physiological acclimation of floating macrocystis pyrifera to temperature and irradiance ensures long-term persistence at the sea surface at mid-latitudes. J. Exp. Mar. Biol. Ecol.405, 33–41. doi: 10.1016/j.jembe.2011.05.018
121
Russell L. M. Rasch P. J. Mace G. M. Jackson R. B. Shepherd J. Liss P. et al . (2012). Ecosystem impacts of geoengineering: a review for developing a science plan. Ambio41, 350–369. doi: 10.1007/s13280-012-0258-5
122
Sapkota Y. White J. R. (2020). Carbon offset market methodologies applicable for coastal wetland restoration and conservation in the united states: A review. Sci. Total Environ.701, 134497. doi: 10.1016/j.scitotenv.2019.134497
123
Schiel D. R. Foster M. S. (2015). The biology and ecology of giant kelp forests (Oakland California: Univ of California Press).
124
Schiel D. R. Hickford M. J. H. (2001). Biological structure of nearshore rocky subtidal habitats in southern new Zealand (Wellington, New Zealand: Department of Conservation).
125
Schoener A. Rowe G. (1970). “Pelagic sargassum and its presence among the deep-sea benthos,” in Deep Sea research and oceanographic abstracts17 (5), 923. doi: 10.1016/0011-7471(70)90010-0
126
Seymour R. Tegner M. Dayton P. Parnell P. (1989). Storm wave induced mortality of giant kelp, Macrocystis pyrifera, in southern California. Estuarine Coast. Shelf Sci.28, 277–292. doi: 10.1016/0272-7714(89)90018-8
127
Sivan O. Schrag D. Murray R. (2007). Rates of methanogenesis and methanotrophy in deep-sea sediments. Geobiology5, 141–151. doi: 10.1111/j.1472-4669.2007.00098.x
128
Smale D. A. Wernberg T. (2013). Extreme climatic event drives range contraction of a habitat-forming species. Proc. R. Soc. B: Biol. Sci.280, 20122829. doi: 10.1098/rspb.2012.2829
129
Smale D. A. Wernberg T. Oliver E. C. Thomsen M. Harvey B. P. Straub S. C. et al . (2019). Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Climate Change9, 306–312. doi: 10.1038/s41558-019-0412-1
130
Sondak C. F. Ang P. O. Beardall J. Bellgrove A. Boo S. M. Gerung G. S. et al . (2017). Carbon dioxide mitigation potential of seaweed aquaculture beds (SABs). J. Appl. Phycol29, 2363–2373. doi: 10.1007/s10811-016-1022-1
131
Springer Y. Hays C. Carr M. Mackey M. Bloeser J. (2007). Ecology and management of the bull kelp, nereocystis luetkeana (Washington, DC: Lenfest Ocean Program).
132
Stevens C. L. Hurd C. L. Isachsen P. E. (2003). Modelling of diffusion boundary-layers in subtidal macroalgal canopies: The response to waves and currents. Aquat. Sci.65, 81–91. doi: 10.1007/s000270300007
133
Taylor R. B. (1998). Short-term dynamics of a seaweed epifaunal assemblage. J. Exp. Mar. Biol. Ecol.227, 67–82. doi: 10.1016/S0022-0981(97)00262-1
134
Theuerkauf S. J. Barrett L. T. Alleway H. K. Costa-Pierce B. A. St. Gelais A. Jones R. C. (2021). Habitat value of bivalve shellfish and seaweed aquaculture for fish and invertebrates: Pathways, synthesis and next steps. Rev. Aquacult. doi: 10.1111/raq.12584
135
Thingstad T. F. Sakshaug E. (1990). Control of phytoplankton growth in nutrient recycling ecosystems. theory and terminology. Mar. Ecol. Prog. Ser.63, 261–272. doi: 10.3354/meps063261
136
Thirumaran G. Anantharaman P. (2009). Daily growth rate of field farming seaweed Kappaphycus alvarezii (Doty) doty ex. p. Silva in vellar estuary. World J. Fish Mar. Sci.1, 144–153.
137
Thomsen M. S. Hildebrand T. South P. M. Foster T. Siciliano A. Oldach E. et al . (2016). A sixth-level habitat cascade increases biodiversity in an intertidal estuary. Ecol. Evol.6, 8291–8303. doi: 10.1002/ece3.2499
138
Thomsen M. S. Mondardini L. Alestra T. Gerrity S. Tait L. South P. M. et al . (2019). Local extinction of bull kelp (Durvillaea spp.) due to a marine heatwave. Front. Mar. Sci.6, 84. doi: 10.3389/fmars.2019.00084
139
Trevathan-Tackett S. M. Kelleway J. Macreadie P. I. Beardall J. Ralph P. Bellgrove A. (2015). Comparison of marine macrophytes for their contributions to blue carbon sequestration. Ecology96, 3043–3057. doi: 10.1890/15-0149.1
140
Troell M. Henriksson P. J. Buschmann A. Chopin T. Quahe S. (2022). Farming the ocean–seaweeds as a quick fix for the climate? (Taylor & Francis), 1–11.
141
Twomey S. (1974). Pollution and the planetary albedo. Atmospheric Environ. 19678, 1251–1256. doi: 10.1016/0004-6981(74)90004-3
142
Vairappan C. S. Chung C. S. Hurtado A. Soya F. E. Lhonneur G. B. Critchley A. (2008). Distribution and symptoms of epiphyte infection in major carrageenophyte-producing farms. J. Appl. Phycol20, 477–483. doi: 10.1007/s10811-007-9299-8
143
Valderrama D. (2012). Social and economic dimensions of seaweed farming: a global review.
144
van den Burg S. W. Röckmann C. Banach J. L. van Hoof L. (2020). Governing risks of multi-use: seaweed aquaculture at offshore wind farms. Front. Mar. Sci. (International Institute of Fisheries Economics and Trade) 7, 60. doi: 10.3389/fmars.2020.00060
145
van den Burg S. W. van Duijn A. P. Bartelings H. van Krimpen M. M. Poelman M. (2016). The economic feasibility of seaweed production in the north Sea. Aquacult Economics Manage.20, 235–252. doi: 10.1080/13657305.2016.1177859
146
Velásquez M. Fraser C. I. Nelson W. A. Tala F. Macaya E. C. (2020). Concise review of the genus durvillaea bory de saint-vincen. J. Appl. Phycol32, 3–21. doi: 10.1007/s10811-019-01875-w
147
Viejo R. M. Åberg P. (2003). Temporal and spatial variation in the density of mobile epifauna and grazing damage on the seaweed ascophyllum nodosum. Mar. Biol.142, 1229–1241. doi: 10.1007/s00227-002-0994-3
148
Visch W. Kononets M. Hall P. O. Nylund G. M. Pavia H. (2020). Environmental impact of kelp (Saccharina latissima) aquaculture. Mar. pollut. Bull.155, 110962. doi: 10.1016/j.marpolbul.2020.110962
149
Walls A. Kennedy R. Fitzgerald R. Blight A. J. Johnson M. Edwards M. (2016). Potential novel habitat created by holdfasts from cultivated laminaria digitata: assessing the macroinvertebrate assemblages. Aquacult Environ. Interact.8, 157–169. doi: 10.3354/aei00170
150
Watanabe K. Yoshida G. Hori M. Umezawa Y. Moki H. Kuwae T. (2020). Macroalgal metabolism and lateral carbon flows can create significant carbon sinks. Biogeosciences17, 2425–2440. doi: 10.5194/bg-17-2425-2020
151
Whitaker T. W. Carter G. F. (1954). Oceanic drift of gourds-experimental observations. Am. J. Bot.41, 697–700. doi: 10.1002/j.1537-2197.1954.tb14397.x
152
Whiticar M. J. (1999). Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geology161, 291–314. doi: 10.1016/S0009-2541(99)00092-3
153
Wikström S. A. Kautsky L. (2004). Invasion of a habitat-forming seaweed: effects on associated biota. Biol. Invasions6, 141–150. doi: 10.1023/B:BINV.0000022132.00398.14
154
Williams S. L. Smith J. E. (2007). A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu. Rev. Ecol. Evol. Syst.38, 327–359. doi: 10.1146/annurev.ecolsys.38.091206.095543
155
Wolff T. (1962). The systematics and biology of bathyal and abyssal isopoda asellota. Galathea Rep.6, 1–320.
156
Wood D. Capuzzo E. Kirby D. Mooney-McAuley K. Kerrison P. (2017). UK Macroalgae aquaculture: What are the key environmental and licensing considerations? Mar. Policy83, 29–39. doi: 10.1016/j.marpol.2017.05.021
157
Würsig B. Gailey G. A. (2002). Aquaculture: Conflicts and potential resolutions. Responsible Mar. aquacult61. doi: 10.1079/9780851996042.0045.
158
Wyrtki K. (1962). “The oxygen minima in relation to ocean circulation,” in Deep Sea research and oceanographic abstracts (Elsevier), 11–23. doi: 10.1016/0011-7471(62)90243-7
159
Xiao X. Agusti S. Lin F. Li K. Pan Y. Yu Y. et al . (2017). Nutrient removal from Chinese coastal waters by large-scale seaweed aquaculture. Sci. Rep.7, 1–6. doi: 10.1038/srep46613
160
Xiao X. Agusti S. Lin F. Yu Y. Pan Y. Li K. et al . (2019). Resource (light and nitrogen) and density-dependence of seaweed growth. Front. Mar. Sci.6, 618. doi: 10.3389/fmars.2019.00618
161
Xiao X. Agustí S. Yu Y. Huang Y. Chen W. Hu J. et al . (2021). Seaweed farms provide refugia from ocean acidification. Sci. Total Environ.776, 145192. doi: 10.1016/j.scitotenv.2021.145192
162
Yarish C. Kim J. K. Lindell S. Kite-Powell H. (2017). Developing an environmentally and economically sustainable sugar kelp aquaculture industry in southern new England: From seed to market. EEB38. Available at: https://opencommons.uconn.edu/eeb_articles/38.
163
Young M. O. (2015). Marine animal entanglements in mussel aquaculture gear (Ísafjörður, Iceland: University of Akureyri).
164
Zhang Y. Zhao M. Cui Q. Fan W. Qi J. Chen Y. et al . (2017). Processes of coastal ecosystem carbon sequestration and approaches for increasing carbon sink. Sci. China Earth Sci.60, 809–820. doi: 10.1007/s11430-016-9010-9
Summary
Keywords
climate change, blue carbon, seaweed, aquaculture, ocean afforestation, macroalgae, climate mitigation, climate solution
Citation
Ross F, Tarbuck P and Macreadie PI (2022) Seaweed afforestation at large-scales exclusively for carbon sequestration: Critical assessment of risks, viability and the state of knowledge. Front. Mar. Sci. 9:1015612. doi: 10.3389/fmars.2022.1015612
Received
09 August 2022
Accepted
20 October 2022
Published
18 November 2022
Volume
9 - 2022
Edited by
Pamela A. Fernández, Universidad de Los Lagos, Chile
Reviewed by
Meiron Zollmann, Tel Aviv University, Israel; John F. Bruno, University of North Carolina at Chapel Hill, United States
Updates
Copyright
© 2022 Ross, Tarbuck and Macreadie.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Finnley Ross, fwross@deakin.edu.au
This article was submitted to Ocean Solutions, a section of the journal Frontiers in Marine Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.