- 1Laboratory of Marine Organism Taxonomy and Phylogeny, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 2Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, China
- 3Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao, China
- 4Independent Researcher, Aarschot, Belgium
During two scientific expeditions to the seamounts near the Mariana Trench in the tropical western Pacific, two undescribed gastropod species belonging to the genus Amiantofusus Fraussen et al., 2007 were collected from the upper bathyal zone. In the present study, we describe and illustrate them as new species. Amiantofusus granulus sp. nov. was collected from Magellan and Caroline Seamounts at depths of 1357–1473 m, and Amiantofusus tchangsii sp. nov. was discovered from Caroline Seamounts at depths of 1893–2291 m. The new species are distinguished from each other and congeners by shell morphology. Phylogenetic analyses based on the cytochrome oxidase c subunit I (COI) gene using Bayesian inference indicate that Amiantofusus granulus sp. nov. is a sister group to other congeners, and Amiantofusus tchangsii sp. nov. shows a close relationship with Amiantofusus sp. JQ950210 from the Philippines. The results provide additional support for the assignment of the new species to the genus Amiantofusus and their separation from congeners. In addition, our molecular analysis reveals that Amiantofusus candoris Fraussen et al., 2007 and Amiantofusus sebalis Fraussen et al., 2007 have almost identical COI sequences. Their taxonomic relationship is briefly discussed, and it is concluded that A. candoris should be regarded as a junior synonym of A. sebalis.
ZooBank registration LSIDs
Article: urn:lsid:zoobank.org:pub:BA88C7BB-B164-4960-BEEE-5CC03DD4D3AB
Amiantofusus granulus sp. nov.: urn:lsid:zoobank.org:act:C756BA82-9D30-4610-8FD6-0A80FFCE0442
Amiantofusus tchangsii sp. nov.: urn:lsid:zoobank.org:act:B43E0C27-6F7B-429F-BC33-165A9FF3BA01
Introduction
Members of the genus Amiantofusus possess shells that are quite similar to those of the family Buccinidae but differ in having a particular protoconch morphology with striking semilunar axial riblets, fasciolariid-like radula, and a distinctive soft-part anatomy (Fraussen et al., 2007; Couto et al., 2016; Couto and Simone, 2019). The genus was originally proposed by Fraussen et al. (2007) to accommodate a small group of deep-sea fasciolariid species. In that publication, Fraussen et al. (2007) designated Fusus amiantus Dall, 1889 from the Atlantic Ocean as the type species and described seven other species from the Indo-west Pacific region, including Amiantofusus borbonicus Fraussen et al., 2007 and Amiantofusus cartilago Fraussen et al., 2007, from the western Indian Ocean and the other five species from the western Pacific. Since then, no additional species have been formally introduced to the genus, although several undescribed species have been reported (Fraussen et al., 2007; Kantor et al., 2012). To date, all known species inhabit the water ranging from relatively shallow depths (down to 300 m) to the upper bathyal zone [1665 m, represented by Amiantofusus amiantus (Dall, 1889)].
During two scientific expeditions conducted by the Institute of Oceanology, Chinese Academy of Sciences (IOCAS), two and five specimens belonging to the family Fasciolariidae were collected from two seamounts near the Mariana Trench with the aid of the submersible ROV FAXIAN. Examinations of the shells and radulae revealed that these specimens represent two new species that belong to the genus Amiantofusus. During the molecular analysis, we also found that Amiantofusus sebalis Fraussen et al., 2007 is genetically very close to Amiantofusus candoris Fraussen et al., 2007. In the present study, we formally describe the species and regard A. candoris as a junior synonym of A. sebalis.
Materials and methods
Sample collection and preservation
Specimens were collected from the upper bathyal zone during the two dives of the Remotely Operated Vehicle (ROV) Faxian (IOCAS; mother ship R/V KEXUE) at the two seamounts near the Mariana Trench (Figure 1). The specimens were photographed in situ before being sampled by the ROV. Soon after collection, the specimens were fixed in 99.5% ethanol. All examined specimens have been deposited in the Marine Biological Museum of Chinese Academy of Sciences (MBMCAS) at Qingdao, China.
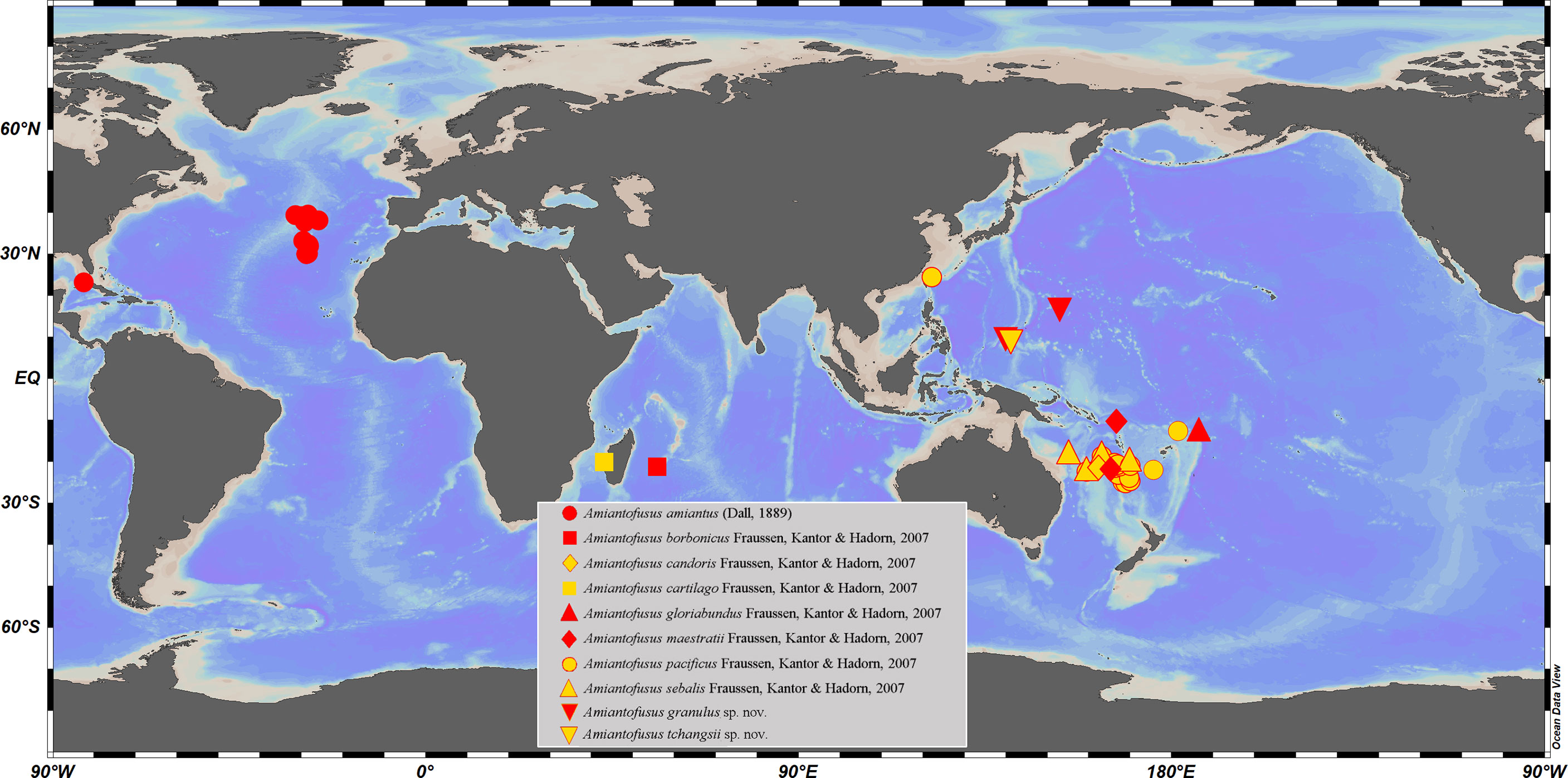
Figure 1 Geographic distribution of known Amiantofusus species. Data from Dall (1889); Gofas (2000); Fraussen et al. (2007), and the present study.
Light and scanning electron microscopies
Shells and soft parts were examined via a stereo dissecting microscope (Zeiss SteREO Discovery. V12). For SEM studies, radulae were extracted from the buccal mass by gross dissection, cleaned using 10% NaOH for 1–2 h to remove the surrounding tissue, rinsed in distilled water, air-dried, coated with gold, and examined with a Hitachi S-3400N SEM with an accelerating voltage of 5 kV. For the identity and terminology of the radula, we followed Fraussen et al. (2007) and Couto and Simone (2019).
DNA extraction and sequencing
One specimen of Amiantofusus granulus sp. nov. and three specimens of Amiantofusus tchangsii sp. nov. were subjected to molecular analysis. Genomic DNA was extracted with a Column Genomic DNA Isolation Kit (Beijing TIANGEN, China) according to the manufacturer’s instructions. DNA was eluted in an elution buffer and stored at –20°C until use. The COI region was amplified by polymerase chain reaction (PCR) using the primers LCO1490 (forward: 5’-GGTCAACAAATCATAAAGATATTGG-3’) and HCO2198 (reverse: 5’-TTAACTTCAGGGTGACCAAAAAATCA-3’) (Folmer et al., 1994). PCRs were carried out in a total volume of 25 μl, including 2 μl DNA template, 0.5 μl of each 10 μM primers, 0.5 μl of 10 mM dNTPs, 2.5 μl of 10× buffer, and 0.5 U Taq DNA polymerase. Thermal cycling was performed under the following conditions: 95°C for 3 min (initial denaturation), followed by 35 cycles of 95°C for 30 s (denaturation), 42°C for 45 s (annealing), 72°C for 60 s (extension), and a final extension at 72°C for 10 min. The PCR products were verified on a GelRed-stained 1.5% agarose gel and purified with a Column PCR Product Purification Kit (Shanghai Sangon, China), and sequencing was performed with TsingKe Biological Technology (TsingKe Biotech, Beijing, China).
Phylogenetic analyses and genetic distance
For phylogenetic analyses, COI sequences from the present study and representative COI sequences of the family Fasciolariidae downloaded from GenBank were used (Table 1). The species used for phylogenetic analyses represent all the genera currently recognized. Sequence alignments were generated with MAFFT 7 (Katoh and Standley, 2013) using the “G-INS-i (accurate)” strategy. No stop codons, insertions, or deletions were observed in the COI sequences. Ragged parts at both ends were removed with trimAl (Capella-Gutierrez et al., 2009) using the “-automated1” command. ModelFinder (Kalyaanamoorthy et al., 2017) was used to select the best-fit model using the AICc criterion. Bayesian inference phylogenies were inferred using MrBayes 3.2.7 (Ronquist et al., 2012) under the GTR+I+G model. Metropolis-coupled Monte Carlo Markov chains were run using the following parameters: ngen=5,000,000, nchains=4, samplefreq=1000, diagnfreq=1000, and temp=0.05, with split frequencies of less than 0.01 before the analyses were terminated. A 25% burn-in was applied before constructing the majority-rule consensus tree. The tree log was checked to ensure that all chain swap information scores fell into the range 0.1–0.7. Tracer 1.7 (Rambaut et al., 2018) was used to assess the convergence. We followed Couto et al. (2016) to use the species of Buccinum (Buccinidae) to root the tree. The results were visualized using FigTree v. 1.4.4. The software MEGA X (Kumar et al., 2018) was employed to calculate the pairwise distances of COI sequences using a Kimura 2-parameter model.
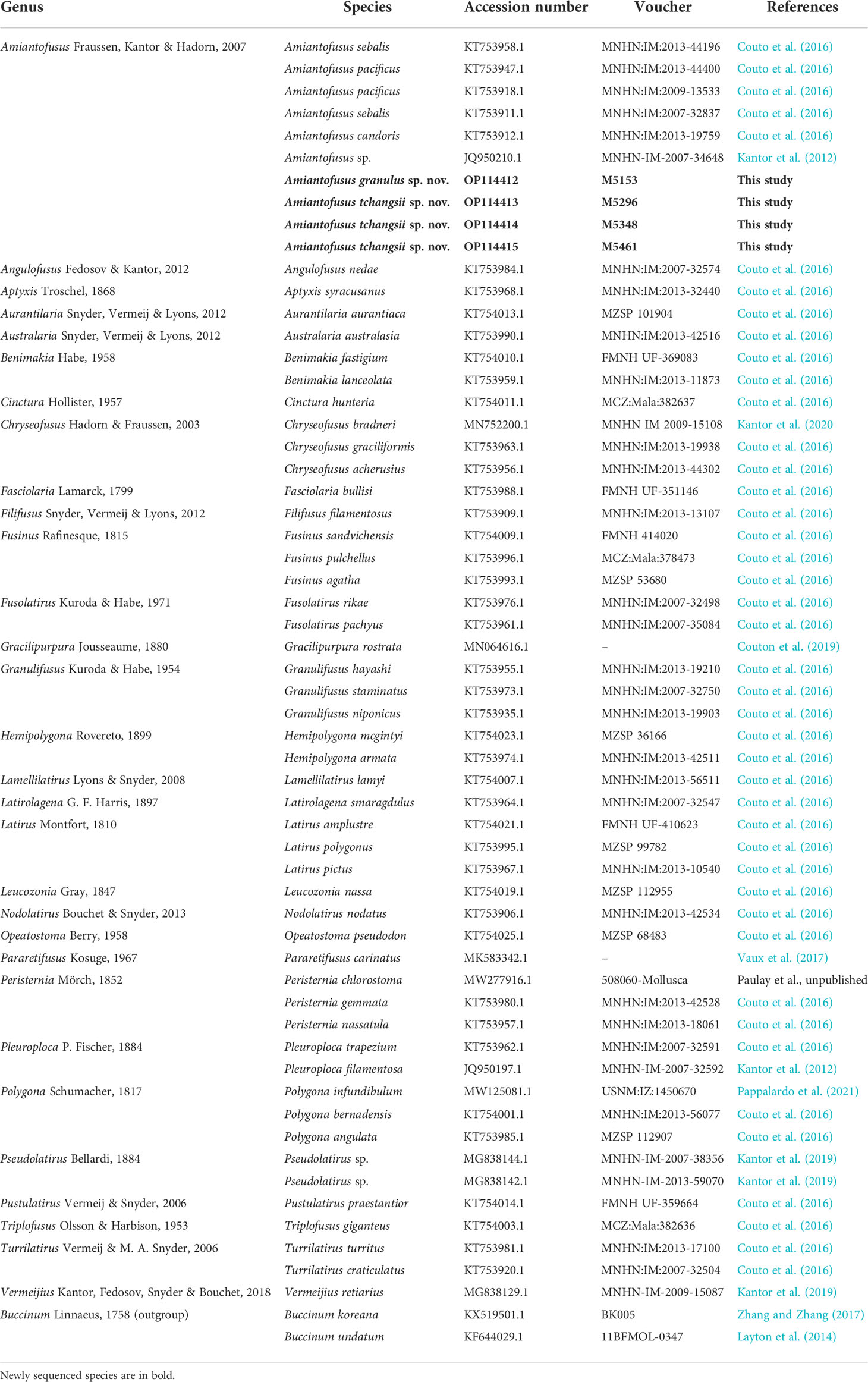
Table 1 List of representatives of the family Fasciolariidae and outgroup species used for phylogenetic analysis using the COI gene, with GenBank accession number, voucher number (where available), and original references.
Results
Systematics
Order Neogastropoda Wenz, 1938
Superfamily Buccinoidea Rafinesque, 1815
Family Fasciolariidae Gray, 1853
Subfamily Fusininae Wrigley, 1927
Genus Amiantofusus Fraussen et al., 2007
Type species. Fusus amiantus Dall, 1889, Atlantic, type by original designation
Amiantofusus granulus sp. nov.
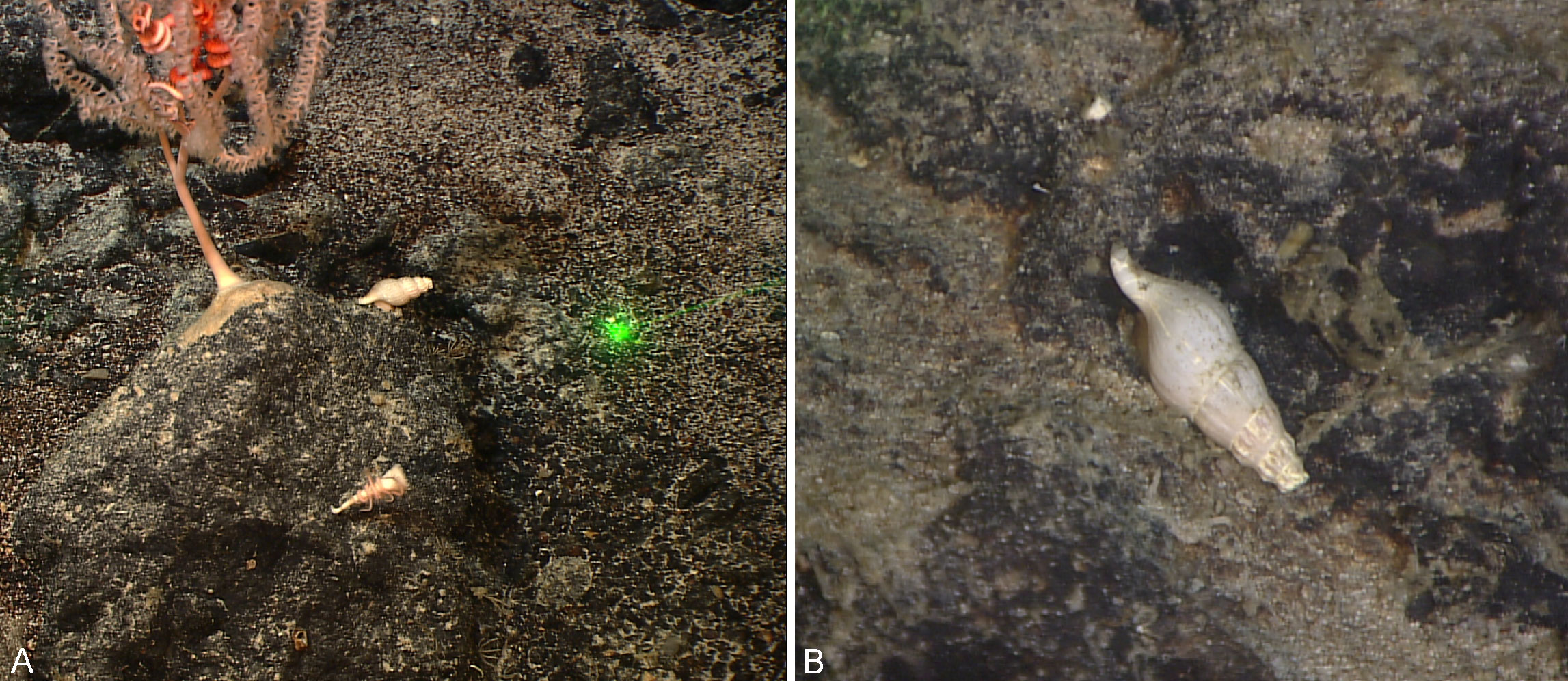
Figure 2 In situ image of Amiantofusus species on natural substrate. (A) Amiantofusus granulus sp. nov.; (B) Amiantofusus tchangsii sp. nov.

Figure 3 Amiantofusus species. (A–E) Amiantofusus granulus sp. nov. (A–C) Holotype, MBM286510, 32.8 mm; (D, E) paratype, MBM286711, 21.8 mm; (F–K) Amiantofusus tchangsii sp. nov. (F–H) Holotype, MBM287288, 30.6 mm; (I) paratype 1, MBM287289, 35.9 mm; (J) paratype 2, MBM287280, 36.7 mm; (K) paratype 3, MBM287291, 36.3 mm. Scale bars = 10 mm.
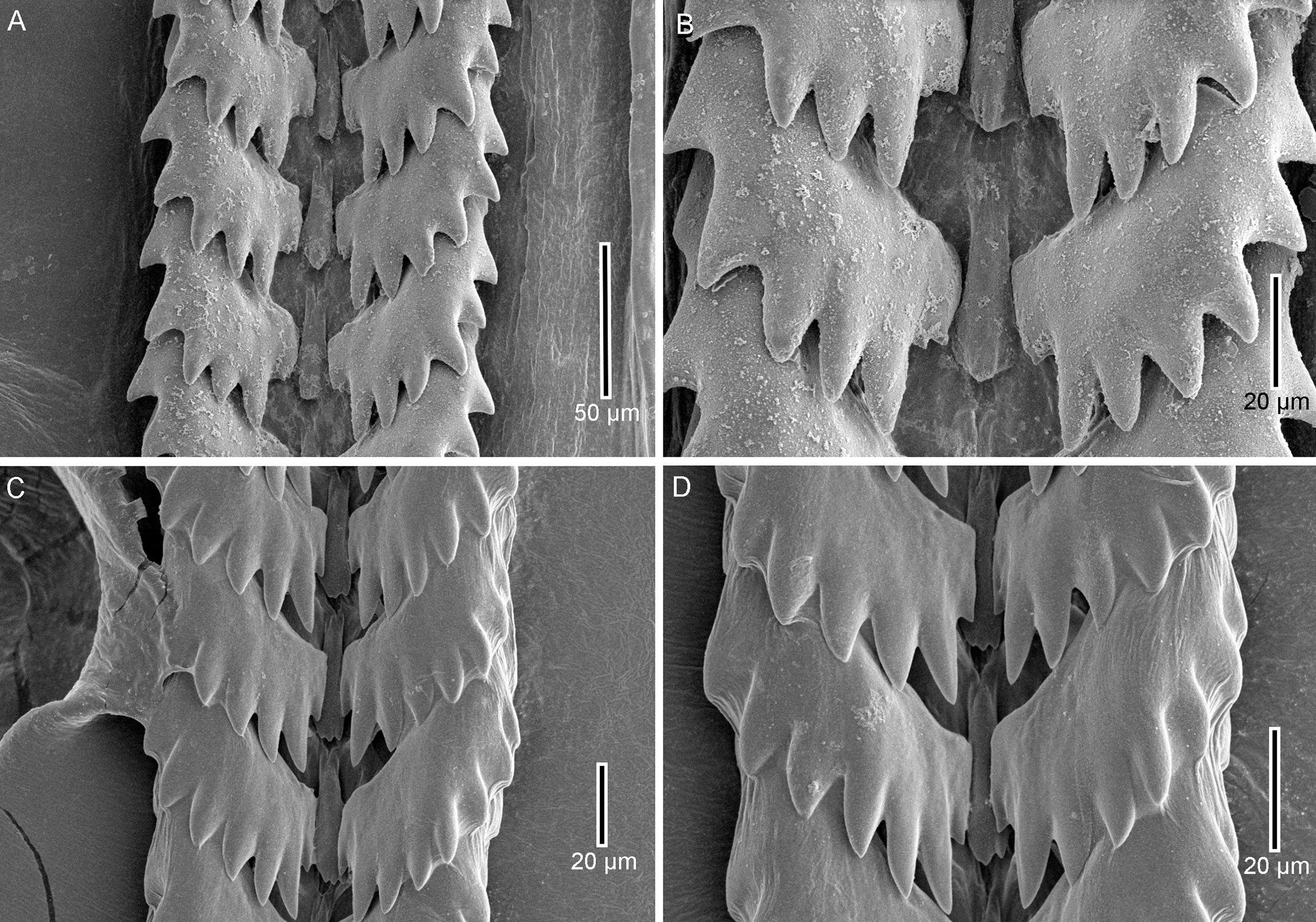
Figure 4 Radulae. (A, B) Radula of Amiantofusus granulus sp. nov., MBM286510; (C, D) radula of Amiantofusus tchangsii sp. nov., MBM287291.
Material examined
Holotype: MBM286510; collection number: M5153; 33.7 mm, 10°01′N 140°06′E, 1473 m, 29 May 2019, from type locality.
Paratype: MBM286711; collection number: M1032; one specimen, 21.9 mm, 17°05′N 153°08′E, ~1357 m, 8 April, 2018.
Description
Shell (Figures 3A–E) fusiform, relatively thick, white or yellowish in color. Protoconch and upper teleoconch whorls lost, with only last four whorls remaining. Suture thin, shallow. Teleoconch whorls convex, subsutural area slightly constricted. Axial sculpture of thin, regularly spaced ribs, numbering 19 and 21 on penultimate and the last whorl, respectively. Spiral sculpture with numerous prominent cords of different strength, primary ones forming small, rounded granules on the axial ribs. Both axial and spiral sculptures becoming weak on the shell base. Aperture large, ovate, inner surface white, outer lip sharped; columellar lip weakly callused, anterior part slightly curved. Siphonal canal relatively long, semitubular.
Radula (Figures 4A, B) rachiglossan, with a formula of 1 + 1+1, asymmetric. Rachidian teeth small, narrow, with an elongated base; distal end simple, with a large central cusp. Right lateral teeth with six major cusps along the posterior margin and a small knob at the inner end, left lateral teeth with five major cusps and a small knob.
Operculum (Figure 3A) semitransparent, pale brown, very thin, elongate-oval, with terminal nucleus.
Type locality: Caroline seamounts, near the Mariana Trench, 1473 m deep, 10°01′N 140°06′E.
Etymology: The name of the new species refers to the nature of the axial ribs with granules.
Distribution and habitat: To date, this species is known from the two seamounts near the Mariana Trench—Kocebu Guyot and Caroline seamounts, where it lives on basalt rock at depths of 1375–1473 m.
Remarks: Amiantofusus granulus sp. nov. is characterized by its large shell sculptured with broad but weak spiral cords separated by fine interspaces and thin, regularly spaced axial ribs and numerous, small, rounded granules on the axial ribs. Within the genus, only one species, Amiantofusus gloriabundus Fraussen et al., 2007, has similar granules on the axial ribs. However, the latter can be clearly separated from the new species in having a slightly broader shell (a width/height ratio of the last whorl of 0.72 instead of 0.64) with a more convex outline of the last whorl, the much more developed spiral cords separated by well-defined interspaces and the weaker axial ribs that are slightly lower in number (17 on penultimate and 20 on the last whorl vs. 19 on penultimate and 21 on the last whorl).
Amiantofusus tchangsii sp. nov.
Material examined
Holotype: MBM287288; collection number: M5296; 30.6 mm, 10°05′N 140°10′E, 1237 m, 1 June 2019, from type locality;
Paratype 1: MBM287289; collection number: M5461; 35.9 mm, 10°03′N 140°09′E, 1816–2291 m, 9 June 2019;
Paratype 2: MBM287280; collection number: M5348; 36.7 mm, 10°04′N 140°11′E, 893 m, 2 June 2019;
Paratype 3: MBM287291; collection number: M7041; 36.3 mm, 10°05′N 140°15′E, 1087 m, 8 June 2019;
Paratype 4: MBM287292; collection number: M7042; 40.6 mm, 10°05′N 140°15′E, 1084 m, 8 June 2019.
Description
Shell (Figures 3F–K) fusiform, relatively thick, white or yellowish in color. Protoconch and upper teleoconch whorls lost, with only last four or five whorls remaining. Suture thin, shallow. Teleoconch whorls convex, subsutural area slightly constricted. Axial ribs thin and sharp, widely spaced, numbering 16–17 on penultimate whorl, becoming reduced and almost absent on the ventral and dorsal sides of the last whorl. Spire whorls with two weak primary spiral cords, forming spiny nodules on the axial ribs. Secondary spiral cords numerous, densely spaced. Aperture large, ovate, inner surface whitish, outer lip sharped; columellar lip weakly callused, anterior part slightly curved. Siphonal canal relatively long, semitubular.
Radula (Figures 4C, D) rachiglossan, with a formula of 1 + 1+1, asymmetric. Rachidian teeth small, narrow, with an elongated base; distal end tricuspid. Right lateral teeth with seven major cusps along the posterior margin and a small knob at the inner end, left lateral teeth with six major cusps and a small knob.
Operculum (Figure 3K) semitransparent, light yellow, very thin, ovate, with terminal nucleus.
Type locality: Caroline seamounts, near the Mariana Trench, 1237 m deep, 10°05′N 140°10′E.
Etymology: The new species is named after the late professor Si Tchang (Xi Zhang) for his pioneering work on malacology in China.
Distribution and habitat: To date, this species is only known from the Caroline seamounts, where it lives on basalt rock at depths of 893–2291 m.
Remarks: In general, the shell shape of Amiantofusus tchangsii sp. nov. is similar to that of Amiantofusus pacificus Fraussen et al., 2007. The latter species has a remarkable degree of variability, and six forms were mentioned by Fraussen et al. (2007). Nonetheless, all the forms have strong axial ribs and spiral cords, which are clearly different from those of Amiantofusus tchangsii sp. nov. In addition, Amiantofusus pacificus differs from the new species in having less major cusps (four instead of six to seven) on the lateral teeth (see Couto and Simone, 2019). In addition, our phylogenetic tree shows that Amiantofusus tchangsii sp. nov. together with an unnamed species formed a sister group with Amiantofusus pacificus (see below). The COI pairwise distance between Amiantofusus tchangsii sp. nov. and Amiantofusus pacificus ranges from 3.6% to 4.1%, a divergence much higher than the known intraspecific variation of Amiantofusus spp. (0.2–1.0%).
Amiantofusus tchangsii sp. nov. has a similar appearance in sculpture to Amiantofusus species 2 Fraussen et al., 2007, which is known from a single shell from Vanuatu. That shell has three to four primary spiral cords along the spire whorls instead of two and has a slender shape.
Phylogenetic analysis and genetic distance
One and three partial sequences for the COI region were successfully amplified and sequenced from Amiantofusus granulus sp. nov. and Amiantofusus tchangsii sp. nov., respectively, and have been deposited in GenBank (accession numbers: OP114412–OP114415). The Bayesian phylogenetic tree (Figure 5) was reconstructed using available COI sequences from the present study and GenBank. In our phylogenetic analyses, none of the three currently recognized subfamilies (Fasciolariinae, Fusininae, and Peristerniinae) was recovered as monophyly. Fasciolariinae and Peristerniinae were clustered together: some species of Peristerniinae (e.g., Hemipolygona armata, Nodolatirus nodatus, and Benimakia fastigium) nested within Fasciolariinae, and Fusininae formed a paraphyletic clade. In the clade Fusininae, the two new species fell into the genus Amiantofusus, which forms a monophyletic sister group (PP = 1) with Granulifusus + Pseudolatirus + Angulofusus. This topology is consistent with the ML analysis of Couto et al. (2016) based on multiple genes. Within the genus, Amiantofusus granulus sp. nov. independently formed a sister group with all other congeners (PP = 0.99), while Amiantofusus tchangsii sp. nov. together with an unnamed species, Amiantofusus sp. from the Philippines, formed a sister group with Amiantofusus pacificus (PP = 0.56).
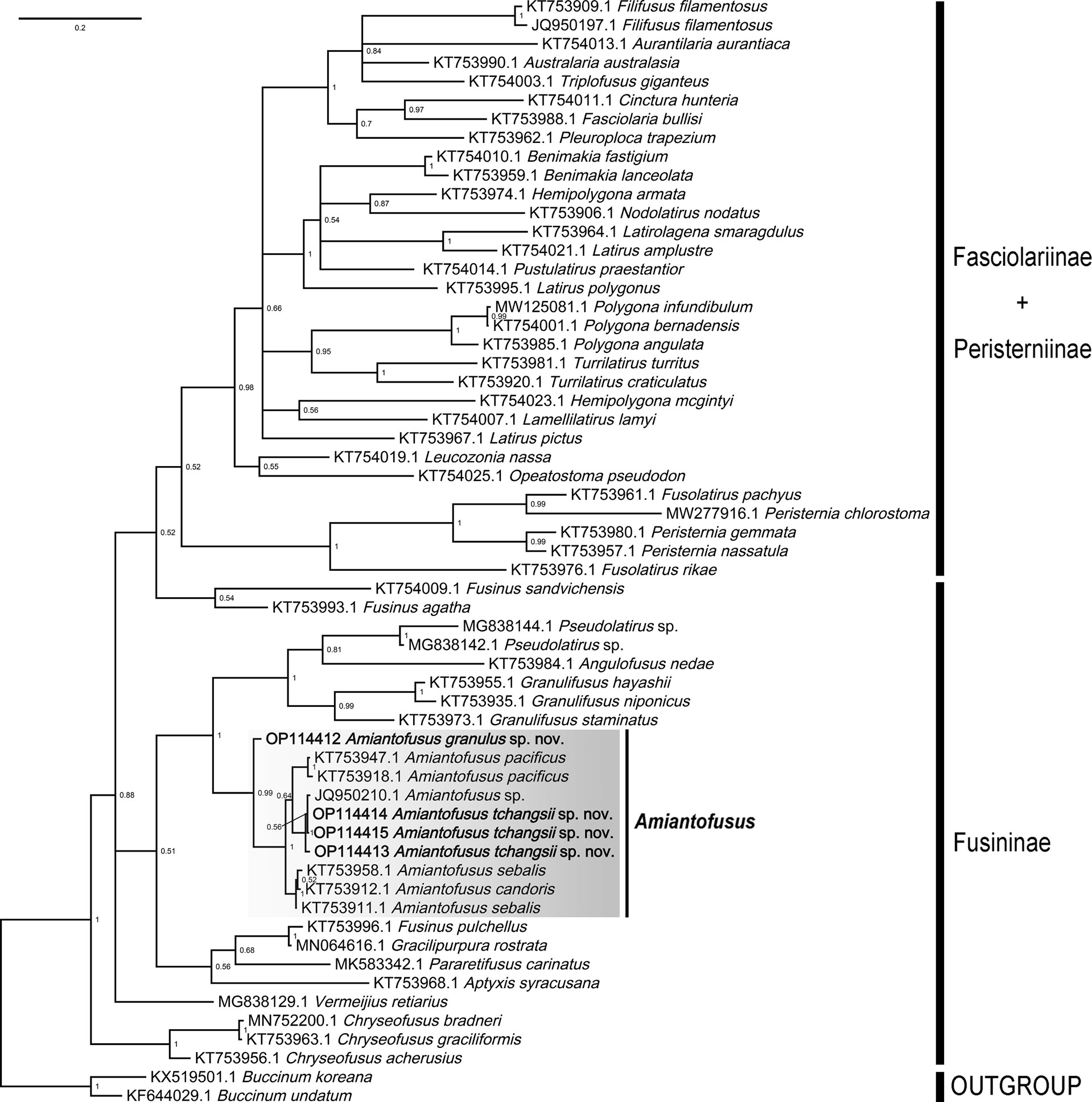
Figure 5 Phylogenetic tree inferred by Bayesian analysis (BI) showing the systematic position of Amiantofusus granulus sp. nov. and Amiantofusus tchangsii sp. nov. within the family Fasciolariidae. Numbers adjacent to nodes refer to BI posterior probability.
The inter- and intraspecific genetic divergences of the COI were calculated to investigate the genetic distances in Amiantofusus. For the COI alignment, the interspecific distances range from 3.1% to 5.8%, and the intraspecific distances are in the range of 0.2–1% (Table 2).
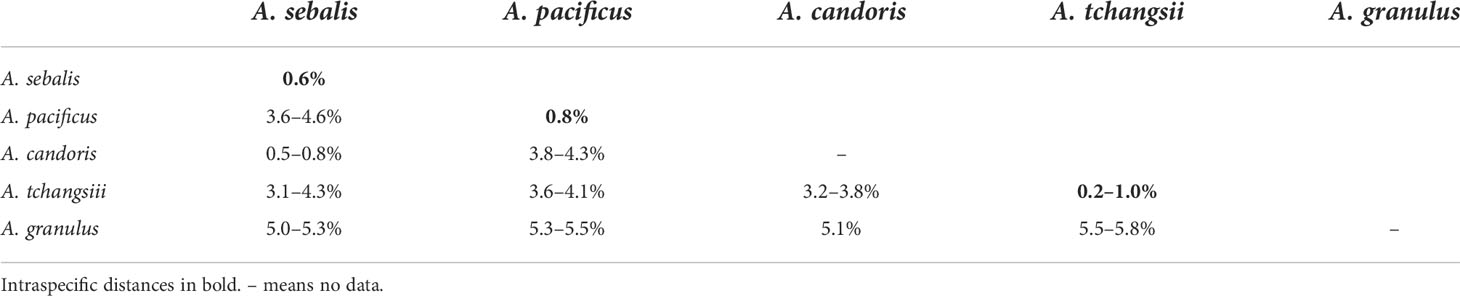
Table 2 Estimates of p-distances of the mitochondrial COI gene among Amiantofusus species and studied sequences.
Discussion
Generic assignment and species delineation
Both the morphology and molecular phylogenetic analyses support the assignment of the two new species to the genus Amiantofusus Fraussen et al., 2007. Amiantofusus species are conchologically quite similar to Buccinidae but have a fasciolariid radula (Fraussen et al., 2007; Couto and Simone, 2019). The radula has a small, narrow, tricuspid central tooth with an elongated base and broad, slightly curved lateral teeth with several (usually four to seven) major cusps accompanied by a small knob or cusp at both ends. The two new species described in the present study conform to these characteristics. In our molecular phylogenetic tree, the genus Amiantofusus, including the two new species, was recovered as a monophyletic group with full support (PP=1). This result provides additional support for the systematic placement of the new species in the genus Amiantofusus.
Amiantofusus granulus sp. nov. and Amiantofusus tchangsii sp. nov. can be clearly separated from other congeners by the shell shape, the sculpture, and the number of cusps on the lateral teeth. The separations were confirmed by the p-distance analyses, which showed that the uncorrected p-distance for the COI among Amiantofusus granulus sp. nov. and other congeners is 5.0–5.8%; among Amiantofusus tchangsii sp. nov. and other congeners it is 3.1-5.8%. These divergences are both much higher than the known intraspecific variation of Amiantofusus spp. (0.2–1%) (see Table 2) and thus warrant separations of Amiantofusus granulus sp. nov. and Amiantofusus tchangsii sp. nov. from other congeners.
Our phylogenetic tree showed that Amiantofusus tchangsii sp. nov. together with an unnamed species, Amiantofusus sp. from the Philippines, formed a fully supported branch (PP = 1). This unnamed species, deposited in the Muséum National d’Histoire Naturelle, Paris (voucher number: MNHN-IM-2007-34648), may be conspecific with Amiantofusus tchangsii sp. nov., as the COI p-distance between the two is 0.2–1%. The divergences fall within the range of intraspecific variation of the genus (0.2–1%; see Table 2).
Geographic distribution of Amiantofusus species
Among the 10 currently known species of Amiantofusus, A. amiantus (Dall, 1889), the type species of the genus, is the only species recorded from the Atlantic Ocean (Fraussen et al., 2007). All other species are known from the Indo-west Pacific region: A. borbonicus Fraussen et al., 2007 and A. cartilago Fraussen et al., 2007 from the western Indian Ocean and the other seven species including the present two new species from the Western Pacific (Figure 1). Such a wide and disjunct distribution indicates that there are likely many more species awaiting discovery. Amiantofusus species have a multispiral protoconch with a large diameter, indicating planktotrophic larval development (Gofas, 2000; Fraussen et al., 2007). Larvae with this developmental mode typically have a high dispersion potential and, consequently, broad geographic distribution (Bouchet and Warén, 1979; Lima and Lutz, 1990; Barroso et al., 2022). This is clearly reflected in several Amiantofusus species. The most evident case is A. pacificus Fraussen et al., 2007, which has a fairly wide distribution range from Taiwan, China via the North Fiji Basin to the northern and southern New Caledonia, the southern Coral Sea, Vanuatu, and Tonga (Fraussen et al., 2007). Another example is A. amiantus (Dall, 1889), which exhibits an amphi-Atlantic distribution. If the unnamed species Amiantofusus sp. from the Philippines, as mentioned above, is indeed conspecific with Amiantofusus tchangsii sp. nov., it would largely extend the distribution of A. tchangsii sp. nov. from the Caroline seamounts to the Philippines.
On a vertical scale, all species inhabit the water from relatively shallow depths (down to 300 m) to the upper bathyal zone. Amiantofusus tchangsii sp. nov. represents the deepest record of the genus (down to 1816–2291 m depth), with A. pacificus Fraussen et al., 2007 being the shallowest (minimum depth of 364 m).
Synonym
During the genetic analyses, it came to light that Amiantofusus sebalis Fraussen et al., 2007 is genetically very close to Amiantofusus candoris Fraussen et al., 2007. The COI pairwise distance between the two is only 0.5–0.8%, which falls within the range of intraspecific variation of the genus (0.2–1.0%; see Table 2). This result indicates that the two species are likely conspecific. In the original description, Fraussen et al. (2007) compared the two species morphologically and concluded that Amiantofusus sebalis differs from Amiantofusus candoris in terms of the strength of the sculpture, the size of the protoconch, and the shell shape. Some of these differences, however, are also observed between different individuals of Amiantofusus tchangsii sp. nov., e.g., the shell shape varies from broad (Figures 3F–I) to slender (Figures 3J–K), and axial ribs being prominent on the penultimate and body whorl in some individuals (Figures 3F–I) but reduced (Figure 3J) or almost obsolete in others (Figure 3K). Thus, it is reasonable to conclude that the differences mentioned by Fraussen et al. (2007) are, in fact, intraspecific variations. We consider Amiantofusus candoris to be a morph of Amiantofusus sebalis, probably connected to shallower waters. As Amiantofusus sebalis was described based on many more specimens, while the few specimens of Amiantofusus candoris cover a limited range, we herein regard A. candoris Fraussen et al., 2007 as a junior synonym of A. sebalis Fraussen et al., 2007.
Conclusion
We describe two new species, Amiantofusus granulus sp. nov. and Amiantofusus tchangsii sp. nov., from the seamounts in the tropical western Pacific. Both their morphology and molecular phylogenetic analyses support the assignment of the two species to the genus Amiantofusus, and with separation from other congeners. Based on the very close COI p-distance between Amiantofusus sebalis and A. candoris, the two species are suggested to be synonymized. Amiantofusus has a wide but sparse distribution: one species from Atlantic, two from the western Indian Ocean, and the other seven species including the present two new species from the western Pacific. Such a broad and disjunct distribution indicates that there may well be many more species awaiting discovery. More explorations are needed to evaluate the species diversity and the geographic distribution of these deep-sea gastropods.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article.
Author contributions
SQZ conceived of and designed the study. SQZ and KF analyzed the morphological and molecular data and drafted the original manuscript, which was revised and improved by SPZ. All authors gave final approval for submission and publication.
Funding
This study is supported by the Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (No. 2022QNLM050102), the Major Program of National Natural Science Foundation of China (No. 42090044), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB42000000), and the Senior User Project of RV Kexue (No. Kexue2020G02)
Acknowledgments
We would like to express our sincere thanks to the crews of R/V Kexue for their assistance during the survey. We thank the three reviewers for their comments and suggestions that helped improve an earlier version of our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barroso C. X., Lotufo T. M. D., Matos A. S., de Macedo Carneiro P. B., Matthews-Cascon H. (2022). The distribution of marine gastropods is more influenced by larval development than by adult characteristics. Mar. Biol. 169, 83. doi: 10.1007/s00227-022-04069-0
Bouchet P., Warén A. (1979). Planktotrophic larval development in deep-water gastropods. Sarsia 64, 37–40. doi: 10.1080/00364827.1979.10411360
Capella-Gutierrez S., Silla-Martinez J. M., Gabaldon T. (2009). trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973. doi: 10.1093/bioinformatics/btp348
Couto D. R., Bouchet P., Kantor Y., Simone L. R. L., Giribet G. (2016). A multilocus molecular phylogeny of fasciolariidae (Neogastropoda: Buccinoidea). Mol. Phylogenet. Evol. 99, 309–322. doi: 10.1016/j.ympev.2016.03.025
Couton M., Comtet T., Le Cam S., Corre E., Viard F. (2019). Metabarcoding on planktonic larval stages: an efficient approach for detecting and investigating life cycle dynamics of benthic aliens. Manage. Biol. Invasion 10, 657–689. doi: 10.3391/mbi.2019.10.4.06
Couto D. R., Simone L. R. L. (2019). A morphology-based phylogenetic analysis of fasciolariidae (Gastropoda: Buccinoidea). Zootaxa 4684, 001–065. doi: 10.11646/zootaxa.4684.1.1
Dall W. H. (1889). Reports on the results of dredgings under the supervision of Alexander agassiz in the gulf of Mexico, (1877-78) and in the Caribbean sea, (1879-80) by the U.S. coast survey steamer Blake: No. 29, report on the Mollusca, part 2, Gastropoda and scaphopoda. Bull. Mus. Comp. Zool. 18, 1–492.
Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. (1994). DNA Primers for amplification of mitochondrial cytpchrome c oxidase submit from diverse metazoan invertebrate. Mol. Mar. Biol. Biotechnol. 3, 294–299.
Fraussen K., Kantor Y., Hadorn R. (2007). Amiantofusus gen. nov. for fusinus amiantus dall 1889 (Mollusca: Gastropoda: Fasciolariidae) with description of a new and extensive indo-West pacific radiation. Novapex 8, 79–101.
Gofas S. (2000). Four species of the family fasciolariidae (Gastropoda) from the north Atlantic seamounts. J. Conchol. 37, 7–16.
Kalyaanamoorthy S., Minh B. Q., Wong T. K. F., von Haeseler A., Jermiin L. S. (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589. doi: 10.1038/nmeth.4285
Kantor Y., Fedosov A. E., Snyder M. A., Bouchet P. (2019). Pseudolatirus bellardi 1884 revisited, with the description of two new genera and five new species (Neogastropoda: Fasciolariidae). Eur. J. Taxon. 33, 1–57. doi: 10.5852/ejt.2018.433
Kantor Y., Kosyan A., Sorokin P., Herbert D. G., Fedosov A. (2020). Review of the abysso-hadal genus Bayerius (Gastropoda: Neogastropoda: Buccinidae) from the north-West pacific, with description of two new species. Deep-Sea Res. PT. I 160, 103256. doi: 10.1016/j.dsr.2020.103256
Kantor Y., Puillandre N., Rivasseau A., Bouchet P. (2012). Neither a buccinid nor a turrid: A new family of deep-sea snails for Belomitra p. fischer 1883 (Mollusca, neogastropoda), with a review of recent indo-pacific species. Zootaxa 3496, 1–64. doi: 10.11646/zootaxa.3496.1.1
Katoh K., Standley D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kumar S., Stecher G., Li M., Knyaz C., Tamura K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Layton K. K., Martel A. L., Hebert P. D. (2014). Patterns of DNA barcode variation in Canadian marine molluscs. PloS One 9, E95003. doi: 10.1371/journal.pone.0095003
Lima G., Lutz R. (1990). The relationship of larval shell morphology to mode of development in marine prosobranch gastropods. J. Mar. Biolog. Assoc. U.K. 70, 611–637. doi: 10.1017/S0025315400036626
Pappalardo P., Collins A. G., Pagenkopp Lohan K. M., Hanson K. M., Truskey S. B., Jaeckle W., et al. (2021). The role of taxonomic expertise in interpretation of metabarcoding studies. ICES J. Mar. Sci. 78, 3397–3410. doi: 10.1093/icesjms/fsab082
Rambaut A., Drummond A. J., Xie D., Baele G., Suchard M. A. (2018). Posterior summarization in Bayesian phylogenetics using tracer 1.7. Syst. Biol. 67, 901–904. doi: 10.1093/sysbio/syy032
Ronquist F., Teslenko M., Mark P. V. D., Ayres D. L., Darling A., Hohna S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Vaux F., Hills S. F. K., Marshall B. A., Trewick S. A., Morgan-Richards M. (2017). A phylogeny of southern hemisphere whelks (Gastropoda: Buccinulidae) and concordance with the fossil record. Mol. Phylogenet. Evol. 114, 367–381. doi: 10.1016/j.ympev.2017.06.018
Keywords: Buccinoidea, Mariana Trench, deep sea, phylogeny, diversity
Citation: Zhang S, Fraussen K and Zhang S (2022) Two new species of the genus Amiantofusus (Gastropoda: Fasciolariidae) from seamounts in the tropical western Pacific, with remarks on the taxonomy of A. candoris and A. sebalis. Front. Mar. Sci. 9:1009707. doi: 10.3389/fmars.2022.1009707
Received: 02 August 2022; Accepted: 01 September 2022;
Published: 26 September 2022.
Edited by:
Jin Sun, Ocean University of China, ChinaReviewed by:
Daniel Cavallari, University of São Paulo, BrazilGuoyi Zhang, University of Nottingham, United Kingdom
Michael Gemmell, Ministry for Primary Industries, New Zealand
Copyright © 2022 Zhang, Fraussen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuqian Zhang, enNxdGF4b25AcWRpby5hYy5jbg==
 Shuqian Zhang
Shuqian Zhang Koen Fraussen4
Koen Fraussen4