- 1Marine Biology Branch, Zoology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt
- 2Zoology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt
- 3Marine Chemistry Department, National Institute of Oceanography and Fisheries, NIOF, Alexandria, Egypt
- 4Department of Biology, College of Science, Taif University, Taif, Saudi Arabia
- 5Department of clinical pharmacology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
- 6Faculty of Medicine, Zagazig University, Zagazig, Egypt
Seaweed draws a lot of attention for its vital role in aquaculture as it contains beneficial biological compounds that undoubtedly might help in the development of this field. The current study sheds light on the potential efficiency of dietary supplements of Grateloupia acuminata and G. doryphore (Halymeniaceae) nanoparticles (GNS) at different levels with bionanocomposite cellulose acetate membranes (CA/bio-AgNps) on improved growth performance, digestive enzyme activity, immunity, antioxidative, resistance against infectious pathogens, and characterization of water quality treated with CA/bio-AgNps that is used in rearing Nile tilapia (Oreochromis niloticus). Four concentrations (0.1, 0.25, 0.5, and 1.0 ml/L) of GNS extract were tested as potential anti-bacterial and for the efficacy of being parasitic. Fish with an average weight (24.46 ± 0. 50 g) were apportioned into six experimental groups (T0, T1, T2, T3, T4, and T5) represented as 0.0%, 0.0%, 0.1%, 0.25%, 0.5%, and 1.0% GNS in diets with CA/bio-AgNps, respectively. Injection of fish with Aeromonas hydrophila was performed at the end of the trial. Chemical and bacteriological water indices significantly showed improvement after being treated with CA/bio-AgNps than the control group. Growth, carcass composition, digestive enzyme, and hematological and biochemical indices were significantly noticed positive (p< 0.05), especially T4 and T5, than the control group. In parallel, a significant improvement was noticed in serum lysozyme, total immunoglobulin, complement C3, antioxidative enzyme, and the relative expression of hepatic and inflammatory genes with an increased level of GNS (p< 0.05) are upregulated than the control group. Remarkably, GNS-supplemented diets and extracts provided positive efficacy against A. hydrophila with a decreased percentage of fish mortality, besides efficacy on antibacterial strains and Cichlidogyrus tilapiae, respectively. To sum up, the seaweed extract with CA/bio-AgNps resulted in better growth performance of fish, antipathogenic effect, and health status. Furthermore, CA/bio-AgNps were vital in improving water characteristics. They should be studied and applied more in the future.
Introduction
In response to the increasing world population, aquaculture activities have increased rapidly and grown into a significant supply of low-cost protein across the world (Dawood et al., 2021). Effective aquaculture methods are preferable in order to reduce any potential production loss in volatile ecological settings (Yukgehnaish et al., 2020). Aquaculture in Egypt suffers from several issues, including the integrity of the freshwater setting, prevalent fish diseases, feed, seed resources, and genetic supplies, which are negatively affecting financial profits (Nasr-Allah et al., 2020 and Radwan et al., 2022b). Researchers endeavored to advance several strategies quickly in order to control fish diseases, including using antimicrobial agents and immunizations during production stages (Ashour et al., 2021). Still, synthetic antimicrobial substances are continually used. As a result of the evolution and accumulation of multiresistance bacteria in edible tissues, recent unfavorable impacts on people and the environment have emerged (Dawood and Koshio, 2016). An active strategy to foster sustainable aquaculture is using phytobiotic substances/extracts in the aquaculture field in order to promote an immune response to environmental materials (Abdelhamid et al., 2021).
As a highly significant component of aquatic environments, the environmental importance of seaweed for its bioactive substances is growing, and the commercial uses of seaweed substances are expanding worldwide (Yang et al., 2015). Many studies have been conducted on the impact of algal cells and/or extracts on marine animals as they involve improving growth performance, feed efficacy, modulating gut microbiota, increasing resistance to diseases, and stimulating immunity (Cantelli et al., 2019; Zaki et al., 2021). On the other hand, seaweeds are considered immunostimulators that play a major part in the improved growth performance of marine organisms by enhancing feed efficacy, digestion, and use (Ringø et al., 2012). Additionally, marine algae were used to synthesize nanoparticles, which are safer than chemical methods (Ingale and Chaudhari, 2013). Nowadays, marine algae-based biogenic synthesis of nanoparticles has great potential for environmental solutions. It is also a cost-effective and eco-friendly method for producing stable metallic nanoparticles (Kanchana and Zantye, 2018).
Likewise, seaweeds are widespread, readily available, much safer to handle, and act as a source of several metabolites. Nanoparticle synthesis using marine algae extracts is a widely accepted method to produce green, cheap, eco-friendly nanoparticles (Mondal et al., 2011). Extracts of marine algae are rich in secondary metabolites such as alkaloids, flavonoids, proteins, phenolic acids, and terpenoids, which are capable of reducing ionic metals and help in the formation of metallic nanoparticles (Aromal and Philip, 2012).
Nanoparticles display a wide variety of applications in water desalination, aquaculture, and the environment, which can be synthesized by chemical, physical, and biological methods but were fabricated using biological methods because of their high efficiency in controlling diseases with fewer side effects (Kuppusamy et al., 2016). Applying nanotechnology in the aquaculture field entails the preparation of several nanosized beneficial substances to be used as feed additives, medicinal agents, and vaccine preparations (Dar et al., 2020). Because of their small-sized particles and massive surface area, the nanoform of trace minerals has an effective impact, which increases its permeability and functionality on aquatic animal performances (Dawit Moges et al., 2020). The extensive studies on these materials demonstrated the important impacts of the nanosized feeding supplies on promoting the flesh quality, immunity, health, and growth rates of Nile tilapia (Korni and Khalil, 2017; Abdel-Razek et al., 2019). Moreover, they improve aquaculture production, disease control, feeding formulation, fish nutrient absorption, and biofouling control (Fajardo et al., 2022).
Metal nanoparticles have gained attention as powerful antibacterial agents due to their durability, resistance, selectivity, and specificity (Swain et al., 2014). Biogenic silver nanoparticles (bio-AgNps) have provided disease control in aquaculture due to their antipathogen properties (Camacho-Jiménez et al., 2020). Sadrzadeh and Mohammadi (2019) declared that bionanocomposite membranes were synthesized by the incorporation of bionanomaterials into the polymeric membrane matrix and offer superior performance in terms of both water flux and salt rejection percentage and increase the permeability, selectivity, and stability of the membrane, which are the key factors for water treatment. Furthermore, these particles have antibacterial properties on the surface due to their catalytic behavior, as well as electrical and magnetic properties and antibiofouling behavior, and are considered an addition of metallic nanoparticles to polymers that improves mechanical strength, thermal stability, hydrophilicity, and membrane performance. The newly developed bionanocomposite membranes are being studied deeply for major separation processes, such as microfiltration (MF), ultrafiltration (UF), and reverse osmosis (RO), which have the capability of removing the dissolved solids, bacteria, viruses, and other germs contained in the raw water (Tewari, 2015).
In the family of halymeniaceae, Grateloupia is considered to be a considerable source of food and lambda carrageenan, having many commercial uses (Kim et al., 2013). Grateloupia sp. showed antibacterial (García-Bueno et al., 2015), antifungal (Plouguerné et al., 2008), antiviral (Hudson et al., 1999), anticoagulant (Shanmugam and Mody, 2000), and antioxidant (Liu and Pang, 2010) activities. Capacity makes this alga an excellent candidate for integration into the animal culture to hinder the proliferation of possible pathogenic bacteria (Pang et al., 2006). Also, researchers reported that seaweeds could cause specific health benefits other than basic nutrition; seaweeds have prospective as well as functional feed (Holdt and Kraan, 2011; Mendis and Kim, 2011).
A few studies investigated the feeding strategies of dietary supplements of Grateloupia acuminata and G. doryphore (Halymeniaceae) nanoparticles (GNS) to Nile tilapia. Therefore, this paper evaluates the potential feeding strategies of GNS nanoparticles with biogenic nanocomposite cellulose acetate membranes (CA/bio-AgNps) on the antioxidative status, hematobiochemical parameters, growth rate, and immune-related genes, as well as resistance against bacteria and parasitic pathogens of O. niloticus.
Materials and methods
Collection and preparation of algal powder
Seaweeds were picked manually during the spring season from the eastern harbor along the Mediterranean coastline between 31° 20′ N latitude and 29° 88′ E longitude, Alexandria, Egypt. They were washed several times with distilled water and air dried. The seaweed mixture of species was equal (1:10), and the algae were extracted by adding 20 ml of double-distilled water to 2 g of powdered algae and mixing for 1 h on a rotary shaker, then boiling for 15 min. The obtained extract was filtered using Whatman filter paper and used as a reducing agent (Negm et al., 2018; Ashour et al., 2020).
GC-MS of aqueous algal extract
The bioactive constituents present in the aqueous extract of marine algae were analyzed by gas chromatography-mass spectrometry (Agilent 7890A Series GC system interfaced to 5975C inert MSD with Triple-Axis detector with 7697A an autosampler (Agilent Technologies, Inc., Stevens Creek Blvd, Santa Clara, CA, United States)) and an HP-5MS 5% phenyl methyl Silox-bonded phase column (30 m long × 250 μm diameter × 0.25 μm film thickness) (Agilent Technologies, USA). The total GC run time was 62 min, with helium as the carrier gas at 1.22 ml/min flow rate and 22.231 psi constant pressure. After setting the initial oven temperature to 90°C for 1 min, it was increased to 205°C for 1 min at a rate of 8 ml/min. It was then increased to 240°C for 1 min at a rate of 5 ml/min. Finally, it was set to 300°C for 30 min at a rate of 8 ml/min. The injection volume was set at 1 μl. The authors conducted compound identification by comparing them to chromatographic retention features, a mass spectral library of the GC-MS data system (Sigma-Aldrich), and quantifying them using the total ion peak area and external criteria calibration curves.
Biosynthesis of silver nanoparticles using aqueous algal extract
The authors synthesized colloidal AgNps through these procedures. They added 10 ml of algae crude extract dropwise into a 90-ml Ag NO3 aqueous solution of 1 mM that was constantly stirred. After 48 h of vigorous stirring, the color of biosilver nanoparticles changed from yellow to dark brown (Negm et al., 2018).
Characterization of silver nanoparticles
The typical characterization techniques of nanoparticles include UV–visible spectrophotometry and Fourier transform infrared spectroscopy (FTIR). The authors obtained the optical absorption spectra of the biosilver nanoparticle suspension using a Jenway 6800 UV/VIS scanning spectrometer in the wavelength range of 300–700 nm. Utilizing quartz cuvettes with an optical path length of 10 m, the presence of different components was confirmed using the FTIR spectrophotometer Vertex 70 by Bruker (Germany).
Synthesis of pure and modified cellulose acetate membranes
The pure cellulose acetate (CA) membrane was prepared according to Ebrahim et al. (2016). First, the authors cast the solution with a 6-s evaporation time. They then immersed the CA membrane cast onto the glass plate for 15 min in a bath of deionized water ice. After that, they placed the formed CA membrane in a water bath at about 4°C for 2 h to eliminate the effect of capillary pressure and washed it using distilled water to obliterate the residual solvents. The formed CA-RO membranes were annealed for 10 min at 80°C. They soaked these membranes in deionized water for 24 h and dried them in the air for 24 h before characterization (Morsy et al., 2016). The authors dispersed 2.5 mg of biosilver nanoparticles in solvents and sonicated the solutions of bio-AgNps for 5 min. After that, CA (8.45 g) was added gradually to the silver nanoparticle solution and stirred for 24 h at room temperature until the complete solution of the CA and the CA/bio-Ag Np nanocomposite polymer dope was formed (Morsy et al., 2016).
Water analysis
The water parameter was measured before and after entering the concert ponds through the membrane (CA/bio-AgNps) throughout the experimental period. The oxygen thermometer apparatus, YSI model 58 (Yellow Spring Instrument Co. Yellow Springs, OH, USA), was used to measure the dissolved oxygen (DO). The pH value was estimated using a digital pH meter. Colorimetric methods were adopted to estimate the total levels of ammonia, nitrite (NO2), nitrate (NO3), and phosphate (PO4). The total alkalinity and hardness were determined using titration methods. Water samples were chemically analyzed and microbiologically examined in accordance with APHA (1998).
Diet preparation and fish husbandry
A control diet was prepared with 30% crude protein. Table 1 shows the feeding elements and proximate chemical structure. Using GNS, the enrichment of the control diet was done at 0.0% (control), 0.1%, 0.25%, 0.5%, and 1.0%/kg diet levels. Nevertheless, we suspended GNS in 100 ml of distilled water and mixed it with the compounds based on uniform spraying. We then mixed them well for 30 min and pelleted them (1 ml diameter). After that, we stored the formed experimental diets in plastic bags at 4°C until use. They analyzed the formed diets chemically following the methods of AOAC (2012).
O. niloticus fry were obtained from a private fish farm in Abbassa, Sharkia Governorate, Egypt. The authors transported the specimens in polyethylene bags filled with dechlorinated water and provided with aeration to an experimental unit of a private fish farm in the same area. When the specimens arrived at the experimental unit, they were placed in 1 × 1 × 1 m3 fiberglass tanks for 14 days of acclimatization and fed a basal control diet twice daily. They replenished water in ponds at a 10% weekly rate and provided new freshwater. All specimens were examined visually and found to be healthy, with no lesions or injuries (Schmitt et al., 2004).
After acclimatization, fish with an average weight of 24.46 ± 0.50 g were equally and arbitrarily allocated into six experimental groups (three replicates per group). Each group comprised 60 specimens and was evenly quadrupled (20 specimens/replicate). After that, specimens were put in 1.50 × 1.50 × 1.10 m3 experimental concrete ponds. They were fed the prepared diet two times a day, at 9:00 and 14:00, for 60 days in May and June 2022.
Growth indices and whole body analysis
After 60 days of the feed experiment, the weight of the fish of each concrete pond was estimated, calculated, and bulk-weighed. Growth performance was estimated, and feeding utilization was determined according to Doan et al. (2020). At the end of the experiment, the final whole-body proximate composition of fish underwent analysis in triplicate following AOAC (2003).
Sample collection
Blood sampling was performed after fasting the fish for 24 h at the end of the trial. Blood sampling was collected from the caudal vein in a 3-ml syringe from three fish per pond. Half of the collected blood containing EDTA was used for counting RBCs, WBCs, hematocrit (Hct) value, and hemoglobin (Hb) level, and the other half was without anticoagulant for separating serum. The collected serum was kept at −20°C for biochemical and antioxidant analysis, including ALAT, ASAT, total protein, albumin, globulin, catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and malondialdehyde (MDA) measurement.
Hematobiochemical analysis
Brown’s methods were used for estimating the red blood cells (RBCs) and white blood cells (WBCs) by the Neubauer slide using a light microscope (Brown and Keith, 1993). The hemoglobin (Hb; g/dl) concentrations were estimated by colorimetric methods by Van Kampen and Zijlstra (1983). The hematocrit (Hct; %) value was measured based on Brown and Keith (1993) methods. Blood smears were formed using fresh blood, dried in the air, fixed with methanol for 3 min, and stained with Giemsa. The slides were explored using a light microscope, and at least 200 leucocytes were estimated and distinguished as monocytes, lymphocytes, and neutrophils. After that, the percentages of the cell types were estimated.
Specific commercial kits were used to analyze total protein (TP) and albumin (ALB) (Biodiagnostic Co., Giza, Egypt), following those introduced by Gornall et al. (1949) and Doumas et al., 1971). The globulin (GLO) value was calculated mathematically by the subtraction of ALB levels from TP values. The techniques of Reitman and Frankel (1975) were determined to describe the serum alanine (ALAT) and aspartate aminotransferase (ASAT) activities.
Digestive enzyme activity
As illustrated by Abdel-Tawwab et al. (2018), enzyme actions were analyzed by the diagnostic reagent kits following the manufacturer’s instructions (Cusabio Biotech Co. Ltd., Wuhan, Hubei, China).
Serum antioxidant and innate immunity assay
Enzymatic antioxidants including GPx, CAT, and SOD were analyzed in fish serum by the diagnostic kits (Biodiagnostic Co., Egypt) according to Paglia and Valentine (1967); Aebi (1984), and Kakkar et al. (1984). The concentration of serum MDA as a lipid peroxidation biomarker was determined, according to Yoshioka et al. (1979).
Innate immunity indices in serum samples were calculated. Micrococcus luteus was used as a substrate to estimate lysozyme (LYZ) actions turbidimetrically (Ellis, 1990). A unit of LYZ actions as the number of enzymes that cause the decline of 0.001OD/min in absorbing 1 ml of serum is determined. The total immunoglobulin (total Ig) concentrations were estimated following Siwicki and Anderson (1993). Moreover, they used commercial immune-turbidimetry to estimate complement C3 activity (Tang et al., 2008).
Western blot analysis
Forty micrograms of protein extracted from O. niloticus, liver or spleen, was dissolved over 8%–12% polyacrylamide gels and transferred to a nitrocellulose membrane. The authors blocked each blot in a blocking buffer (7% nonfat dry milk/1% Tween 20; in 20 mmol/L TBS (pH 7.6)) for 1 h at room temperature before incubating it with primary antibodies Hsp70 (Abcam, USA), CAT (Biorbyt, USA), SOD (Sigma-Aldrich, USA), TNF-α (Thermo Fisher Scientific, USA), IL-10 (Santa Cruz Biotechnology, USA), IL-9 (Abcam, USA), GAPDH, β-actin, and vinculin (Santa Cruz Biotechnology, USA) in blocking buffer for 2 h at room temperature or overnight at 4°C, followed by incubation with anti-rabbit IRDye 800CW-labeled secondary antibody (Abcam, USA). Blots were subjected to improved chemiluminescence (Thermo Scientific Pierce, USA) and autoradiography using the BioRad imaging system (Hercules, CA). Quantity One (BioRad) was used by the authors to perform a densitometric measurement of the bands in the Western blot analysis. The treatment protocol was carried out at least three times, and the analysis of individual protein expressions was carried three times and with similar results (Chen et al., 2017).
Bacterium challenge test
Aeromonas hydrophila was isolated and prepared according to Abdel-Razek et al. (2019). At the end of the feed trial, fish representing each subgroup had 10 fish in 100-L tanks in replicas. The first subgroup was challenged with pathogenic A. hydrophila by a sublethal dose illustrated by Schäperclaus (1992), in which they intraperitoneally (IP) injected a dose of 0.1 ml of 24 h broth from virulent A. hydrophila (5 × 105 CFU/ml). They IP injected the second subgroup with 0.1 ml of saline solution as the control group. They fed fish on matching diets throughout the challenge test in each treatment. Mortality data were utilized to estimate the relative live percentage (RLP) following Amend (1981) equation:
In vitro antibacterial activity of GNS
Four GNS levels (0.1%, 0.2%, 0.5%, and 1.0% ml/L) were investigated to identify the antibacterial ability of three chosen bacteria. The three bacterial strains tested were Aeromonas sobria, Pseudomonas fluorescens, and Streptococcus agalactiae from the Department of Microbiology, Faculty of Science, Al-Azhar University, Egypt.
In vitro antiparasitic activity of GNS
Cichlidogyrus tilapiae was isolated, identified, and prepared as shown in Radwan (2022a); Radwan et al., (2022c). Four GNS extract concentrations (0.1, 0.25, 0.50, and 1 ml/L for 60 min, four duplicates each) were utilized against C. tilapiae in the case of setting the timing to zero. There were control wells with distilled water in each treatment without adding GNS. The parasites were observed every 10 min by a dissecting microscope with recording rates. Considering the parasites dead depends on their lack of response to touch or showing a reaction when moving them to clean wells with distilled water. Zhang et al. (2014) concluded that treatment would be effective when achieving 100% parasite mortality in 24 h. In the end, the antiparasitic efficacy was calculated based on the formula of Wang et al. (2009):
where AP denotes the antiparasitic efficacy, T1 represents the mean survival in the control group, and T2 denotes the treatment group’s mean survival.
Statistical analysis
The means with their standard error (SE) represented the data that were analyzed using one-way ANOVA via SPSS 22.0 (SPSS V.22, SPSS Inc., IL, USA). After that, we used Duncan’s multiple range test in order to identify differences in the treatments with a significance value of p< 0.05.
Results
Identified components by GC-MS of aqueous algal extract
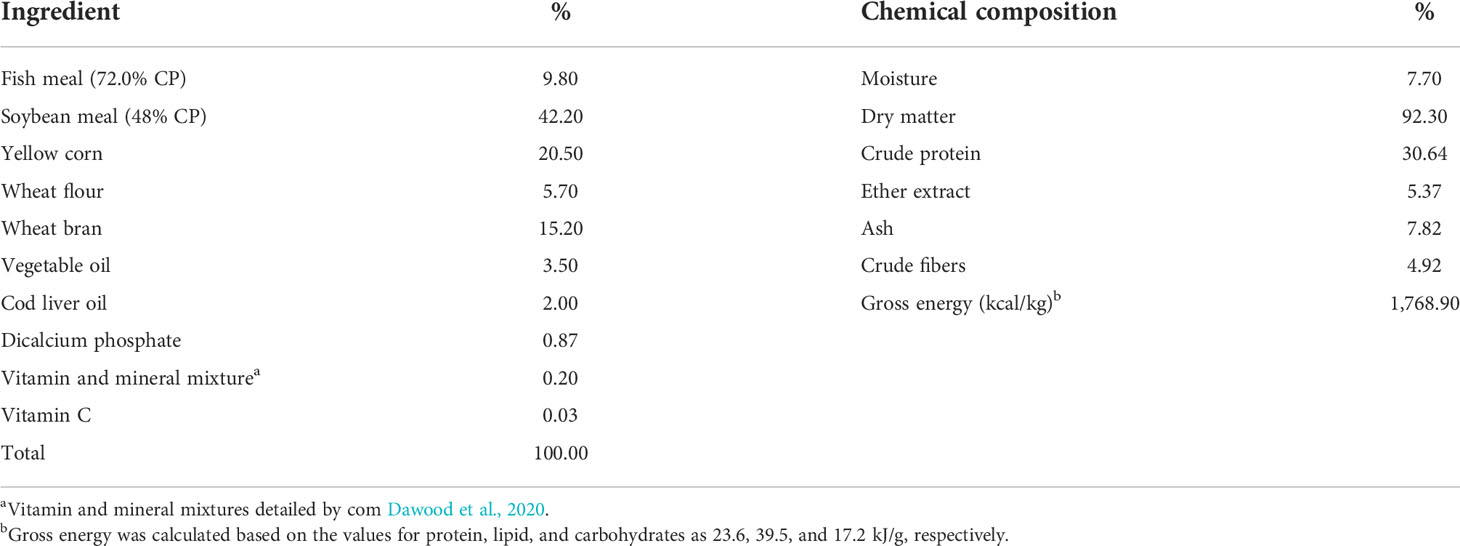
Analysis of aqueous algal extract by GC-MS illustrates 20 phytochemical extracts from 10 biochemical groups. Four of the extracts were of fatty acid nature (pentatonic acid, 4-oxo-, ethyl ester; oleic acid; pentadecanoic acid, ethyl ester; ethyl 9-hexadecenoate), found compounds individually whose nature was polysaccharide (α-D-glucopyranoside, O-α-D-glucopyranosyl (1.fwdarw.3)-β-D-fructofuranosyl), ester (androstan-17-one,3-ethyl-3-hydroxy-, (5à)-), alkaloid (1H-pyrrole, 1-pentyl-), phenols (caffeic acid), amino acid (undecanoic acid, 11-amino-), and vitamin (retinol). Also, there were two of each of these compounds, and their nature was alcohols (ethyl iso-allocholate; phytol), steroids (cholest-5-En-3-Ol (3á)-, acetate; cholest-5-en-3-ol (3β)-, tetradecanoate), and carotenoides (9-octadecenoic acid,1,2,3-propanetriyl ester, (E,E,E)-; rhodopin). In the context, three compounds were aldehydes (5-acetoxymethyl-2-furaldehyde; 5-octadecenal; 1-butanamine, 2-methyl-N-(2-methylbutylidene)-, and milbemycin-oxime as antibiotics (Table 2; Figure 1A).
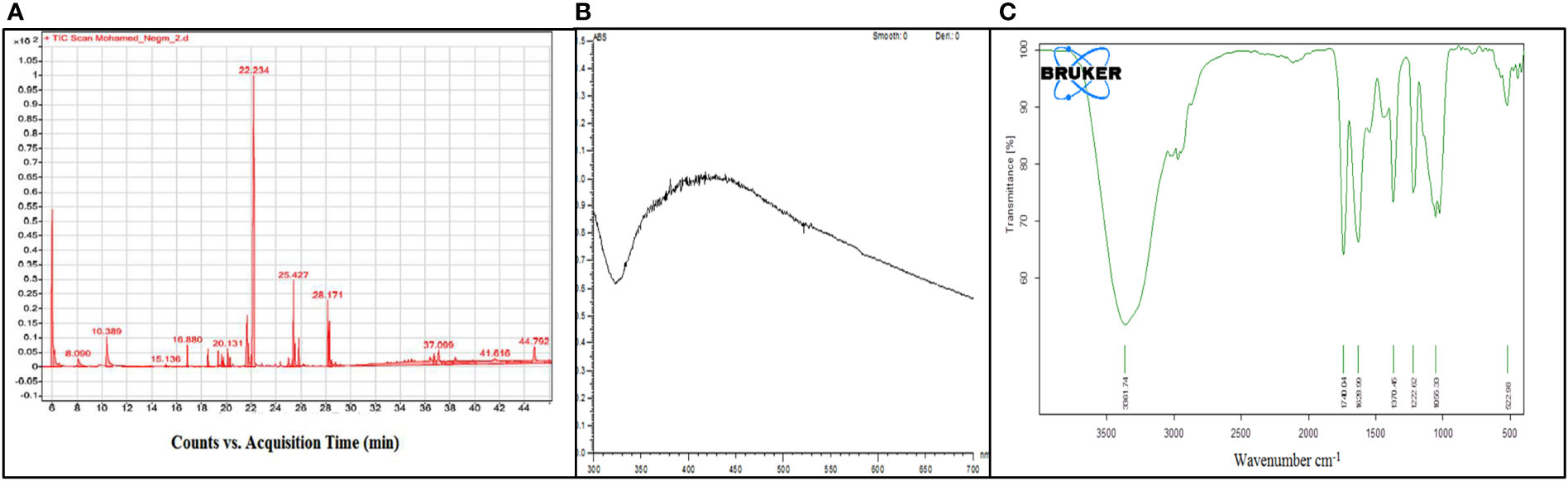
Figure 1 (A) GC-MS analysis of the observed components. (B, C) UV–visible and FTIR spectra of AgNps synthesized from the Grateloupia sp. alga-distilled water extract.
UV and FTIR characterization
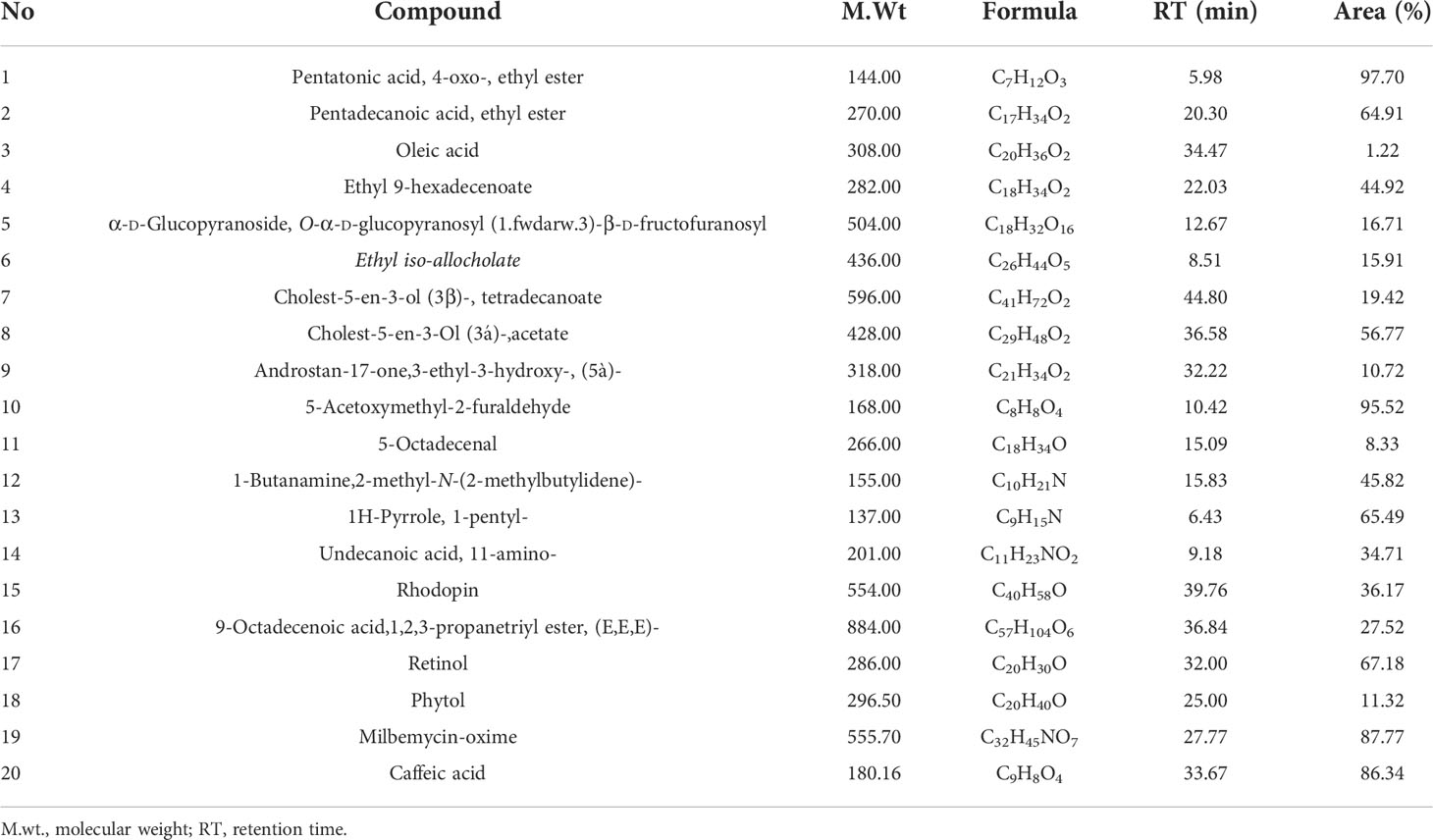
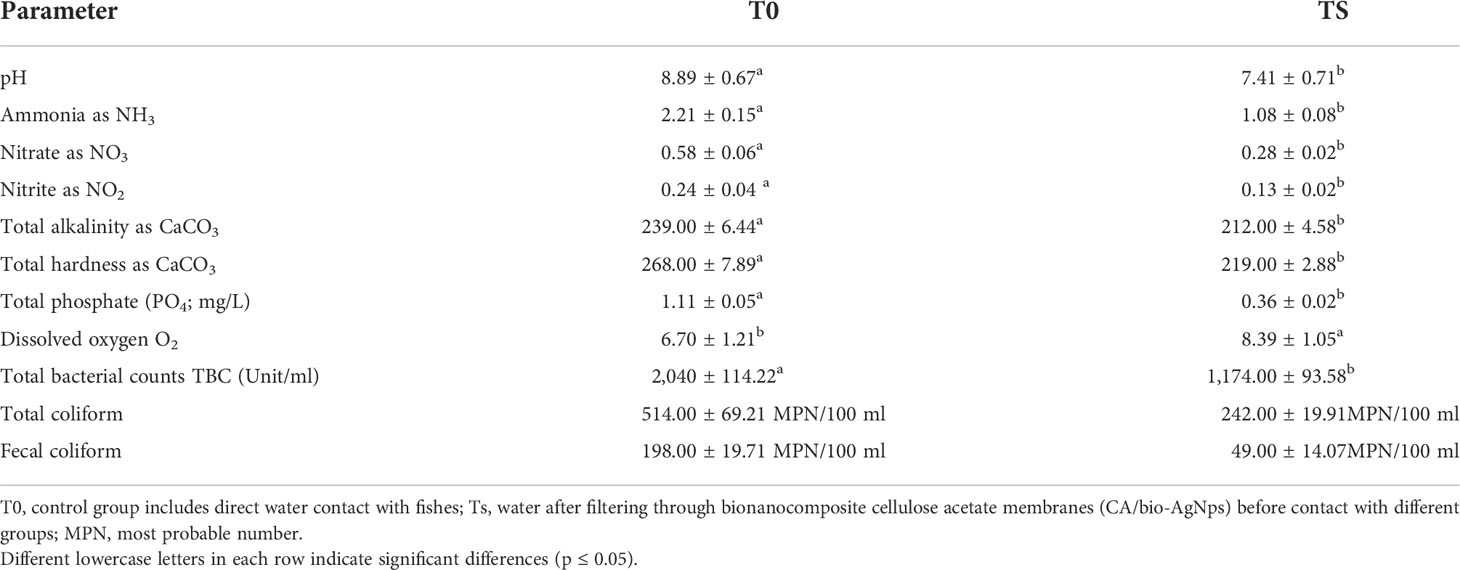
Data showed UV–vis absorption spectra monitored forming silver nanoparticles at 300 to 700 nm, where an intense band was clearly detected at 430 nm, confirming the formation of silver nanoparticles (Figure 1B). On the same line, FTIR is used in order to determine the potential biomolecules accountable for the stabilization, reducing Ag+ ions and limiting the synthesized bioreduced AgNps. Chromatograms are presented in Figure 1C. The IR chromatogram of the SGDW showed an absorption band at 3,361 cm−1 matching N–H stretching vibrations of (NH2) peptide linkages and hydroxyl (OH) stretch vibrations of carboxylic acid groups, demonstrating polyphenols. In contrast, the absorption bands were observed at 1,740 and 1,628 cm−1.
Synthesis and performance of pure and modified cellulose acetate membranes
As shown in Table 3, the pure cellulose acetate membrane had the highest contact angle of 66° and the addition of CA/bio-AgNps increased surface hydrophilicity of the cellulose acetate membrane at a contact angle of 52° and reduced the surface roughness, leading to significantly improved antifouling performance. In parallel, the addition of a small number of biogenic silver nanoparticles (2.5 mg) decreased pore size, increased salt rejection, and increased water flux of the CA membranes from 3.1 to 7.78 L/m2 h at 10 bar and effectively improved the water flux.
Water investigation
Physicochemical and microbial analyses displayed a statistically significant improvement (p< 0.05) among all parameters in the water after being treated with CA/bio-AgNps. Data from water analysis and microbial analyses are summarized in Table 4.
Growth performance and carcass structure activity
According to Table 5, the final body weight (FBW), weight gain (WG), and specific growth rate (SGR) of Nile tilapia fed with GNS for 60 days were significantly increased compared to those of the fish fed base diet (p< 0.05), especially at 0.5% and 1.0% feed rates. In contrast, there was a significant reduction in the feed conversion ratio (FCR) in the T5 group, unlike in the control fish. The survival rate registered a significant peak, especially in T4 and T5 (p< 0.05) compared to the control group. Furthermore, all fish dietary GNS supplements with CA/bio-AgNps had more CP (%) and EE (%) and less ash (%). The noticeable improvement was recorded in the T5 and the lowest in T0.
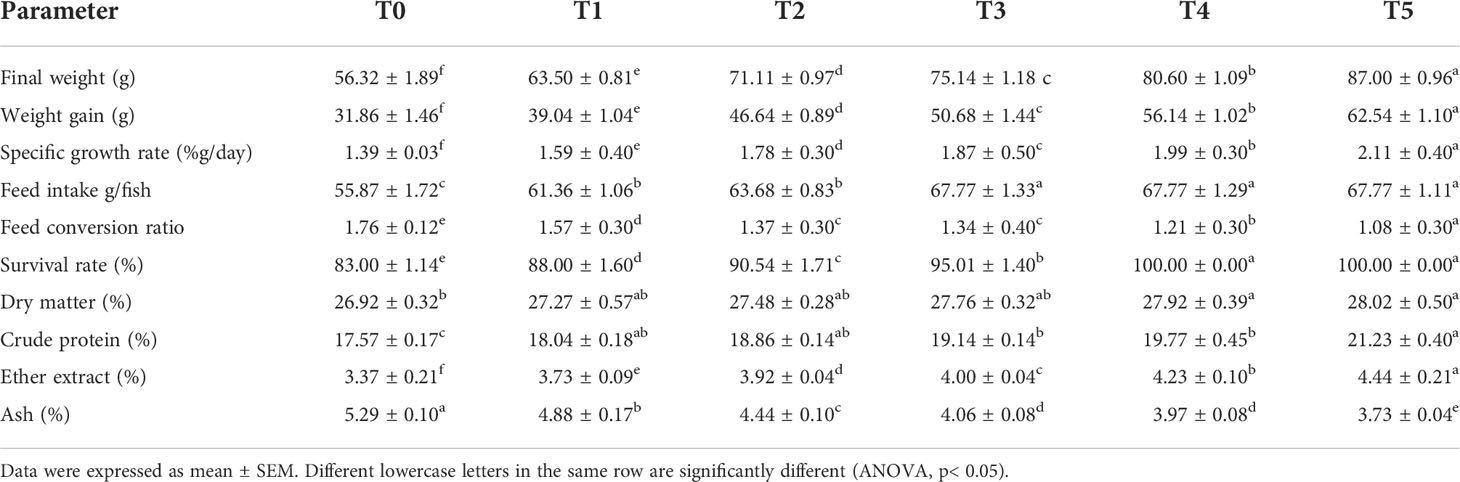
Table 5 Survival, feed utilization, growth performance, and carcass structure (on a wet weight basis) of Nile tilapia fed diets with various levels of GNS with CA/bio-AgNps for 60 days.
Digestive enzyme activities and serum immunity
The activity of protease, amylase, and lipase in Figure 2 shows a significant improvement of Nile tilapia feed GNS after a 60-day experiment period; maximum values were recorded in T5. On the contrary, the fish fed basal diet (T0) had the lowest performance compared to its analogs (p< 0.05). Remarkably, applying the diet gave substantially greater serum LYZ, Ig, and complement C3 activities than the other groups (p ≤ 0.05), especially T4 and T5 (Figure 3).
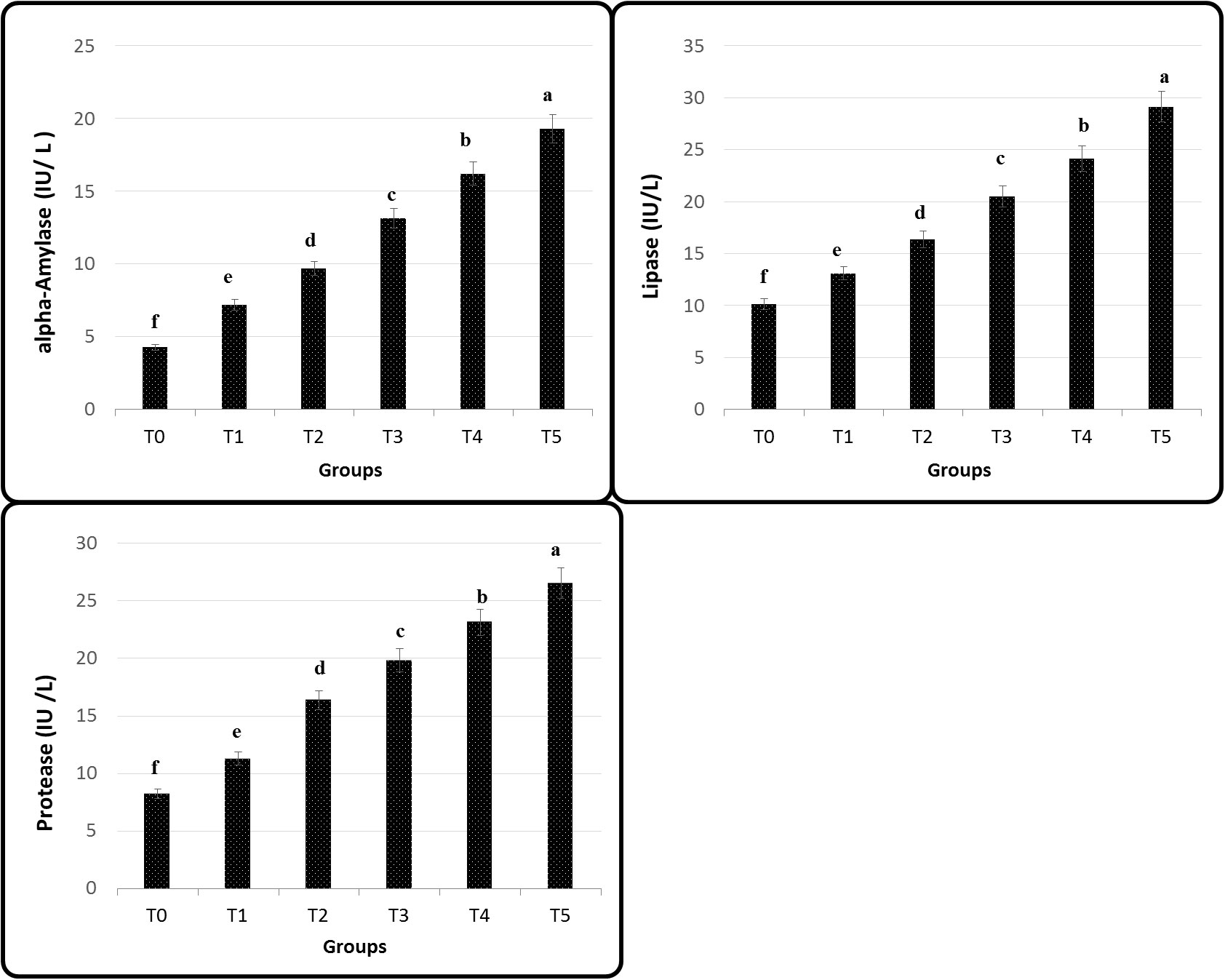
Figure 2 Intestinal digestive enzymes of Nile tilapia fed on diets containing different levels of GNS with CA/bio-AgNps for 60 days. Data were expressed as mean ± SE. Different superscripts refer to differences between all groups for each parameter (p< 0.05).
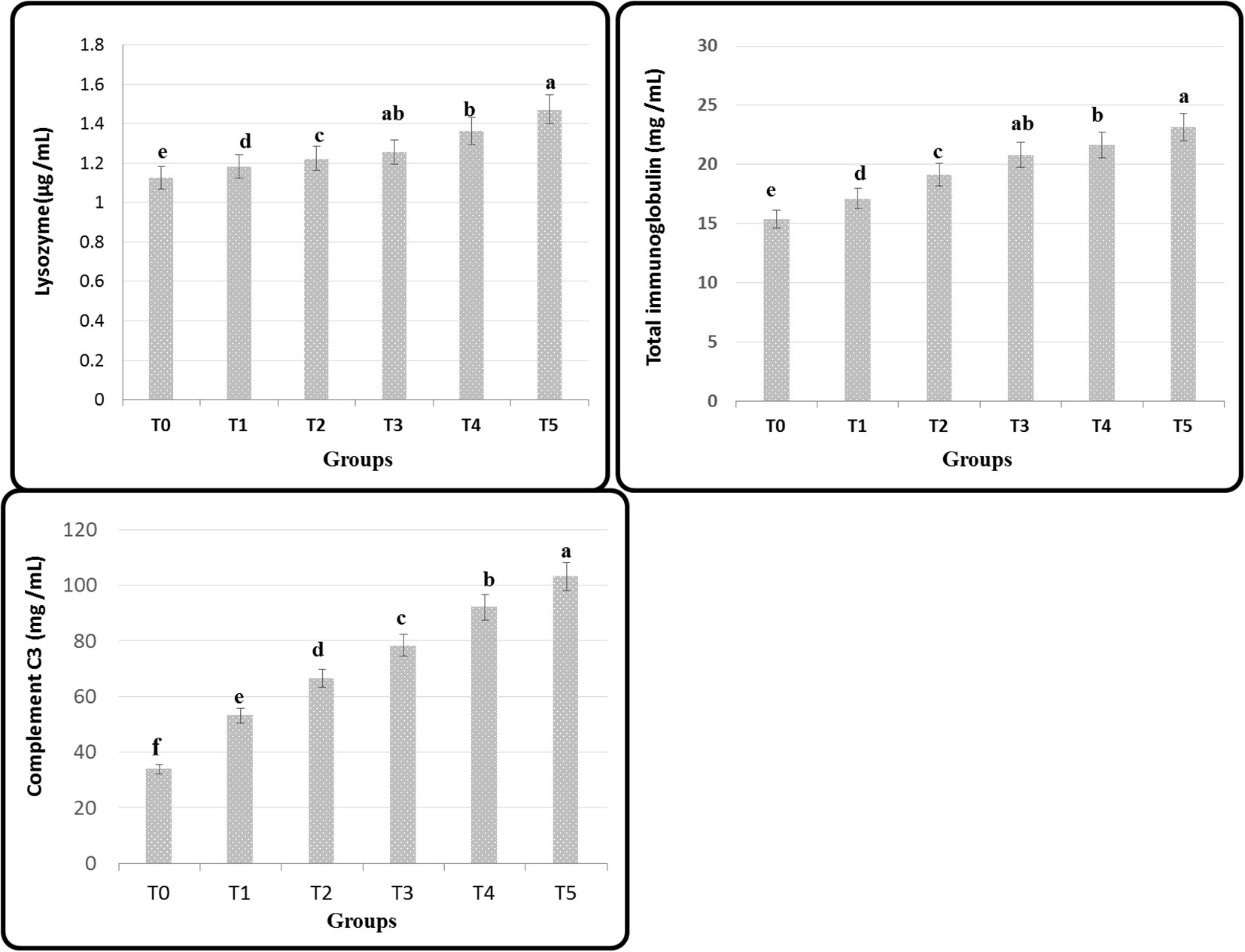
Figure 3 Serum immune indices of Nile tilapia fed on diets containing many GNS levels with CA/bio-AgNps for 60days. Data were expressed as mean ± SE. Different superscripts refer to differences between all groups for each parameter (p< 0.05).
Hematobiochemical parameters
The hematological profile of O. niloticus fed with various GNS-enhanced diets throughout 60 days with CA/bio-AgNps showed tangible improvement in RBCs, Hb, and Hct than the control group, particularly at T4 and T5, which showed the greatest values. The WBC number experienced a significant (p< 0.05) increase with graded GNS levels. Concerning differential leukocyte numbers, neutrophils demonstrated higher percentages, but lymphocytes had lower percentages at T4 and T5. In the meantime, monocyte cells showed more significant changes (p< 0.05), especially in T4- and T5-treated groups, than in the control group. Serum TP, ALB, and GLO values had a significant (p< 0.05) increase in GNS-fed fish with CA/bio-AgNps, unlike the control group. In contrast, the dietary supplement of GNS to O. niloticus decreased considerably (p< 0.05) in the activity of ASAT and ALAT, especially at T4 and T5 (Table 6).
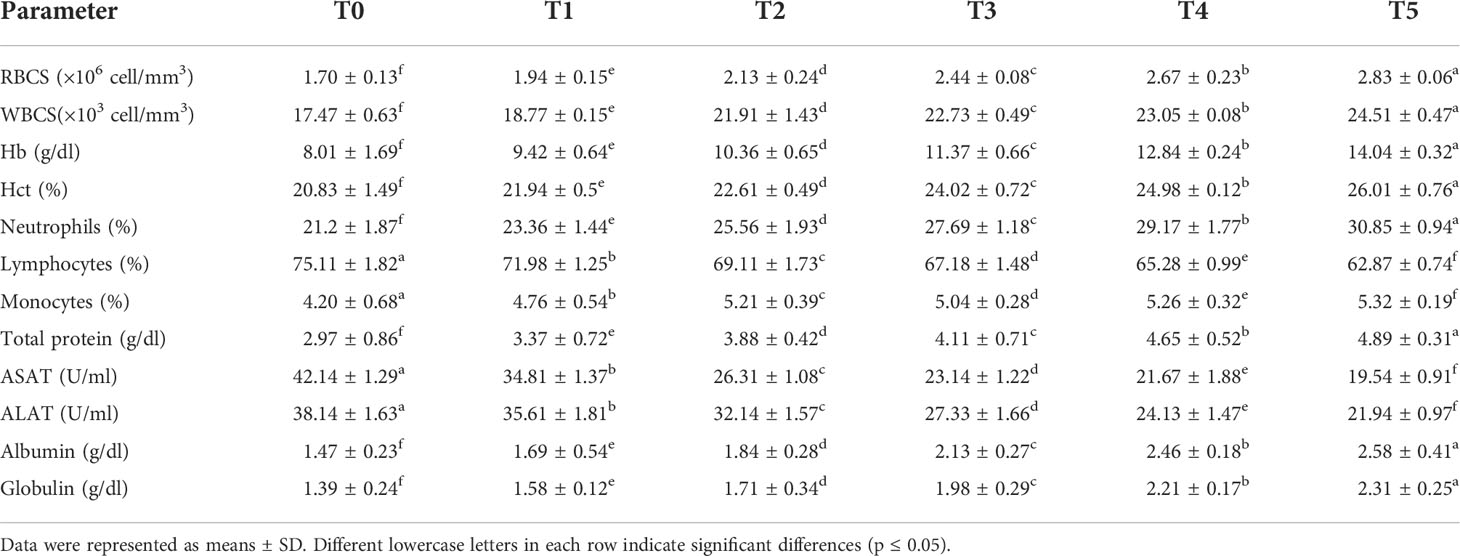
Table 6 Hematobiochemical determinants of Nile tilapia fed diets having many GNS levels with CA/bio-AgNps for 60 days.
Stress biomarkers, lipid peroxidation, and stress-related genes
Figure 4 illustrates that in all groups, SOD, CAT, and GPx had a significant increase when adding GNS, but the levels of MDA decreased (p< 0.05) particularly in T4 and T5. Interestingly, the levels of hepatic and splenic HSP70, SOD, and CAT antioxidative and stress-related genes were upregulated in fish diet GNS with CA/bio-AgNps gradually compared to the control group, especially T4 and T5 (Figure 5). Similarly, the levels of hepatic and splenic IL-8, IL-10, and TNF-α inflammatory-related genes were increased in fish diet GNS with CA/bio-AgNps gradually compared to the control group (Figure 6).
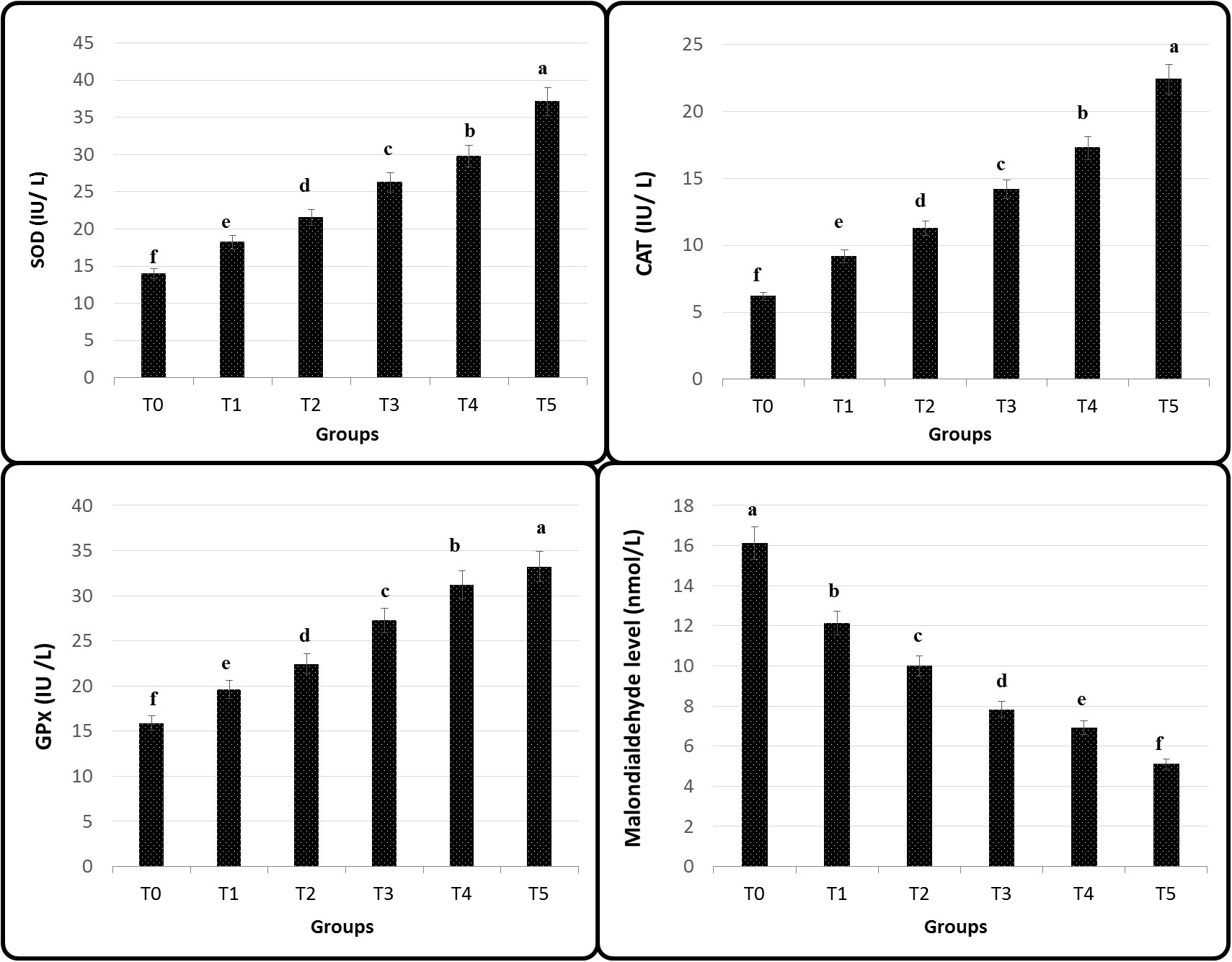
Figure 4 Antioxidants of Nile tilapia fed on diets having different GNS levels with CA/bio-AgNps for 60 days. Data were expressed as mean ± SE. Different superscripts refer to differences between all groups for each parameter (p< 0.05).
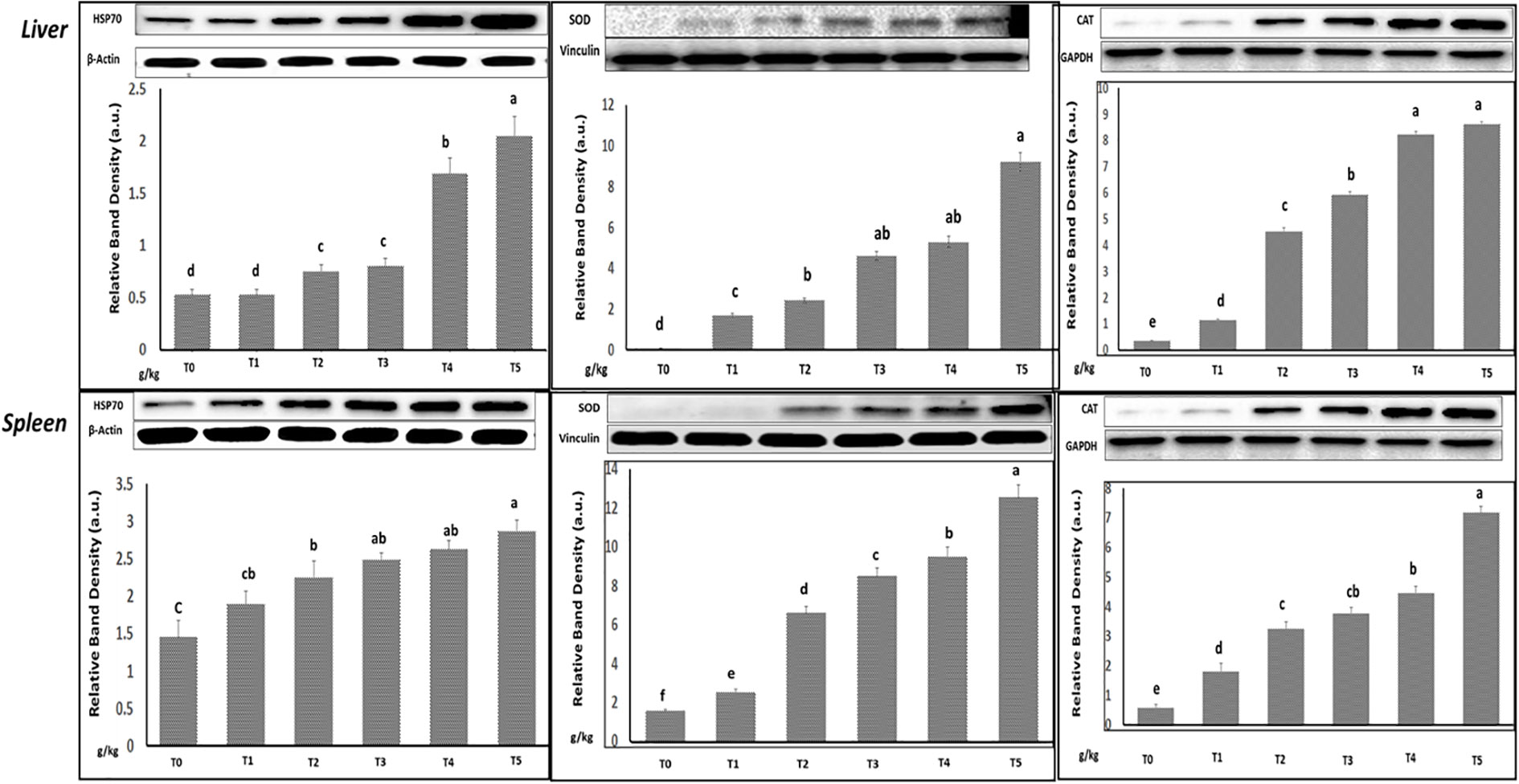
Figure 5 Relative transcription of heat shock protein 70, antioxidative (SOD and CAT), and β-actin: housekeeping genes in the liver and spleen of Nile tilapia fed on diets having many GNS levels with CA/bio-AgNps for 60 days. The observed immunoblots represent three independent trials with similar findings. Bars show the means ± SD. Different letters indicate significant differences (p< 0.05).

Figure 6 The relative transcription of (A) interleukin 8 (IL-8), (B) interleukin 10 (IL-10), tumor necrosis factor-alpha (TNF-α), and β-actin: housekeeping gene in liver and spleen of Nile tilapia fed on diets having different GNS levels with CA/bio-AgNps for 60 days. The observed immunoblots represent three independent trials with similar findings. Bars illustrate the means ± SD. Different letters indicate significant differences (p< 0.05).
A. hydrophila challenge
The findings showed that after 10 days of the bacterial injection, the best RPS value was noticed at T5 (91%), followed by T4 (80%), T3 (76%), T2 (71%), T2 (61%), and T1 (61%). The lowest RPS was in the control group (11% (see, Figure 7).
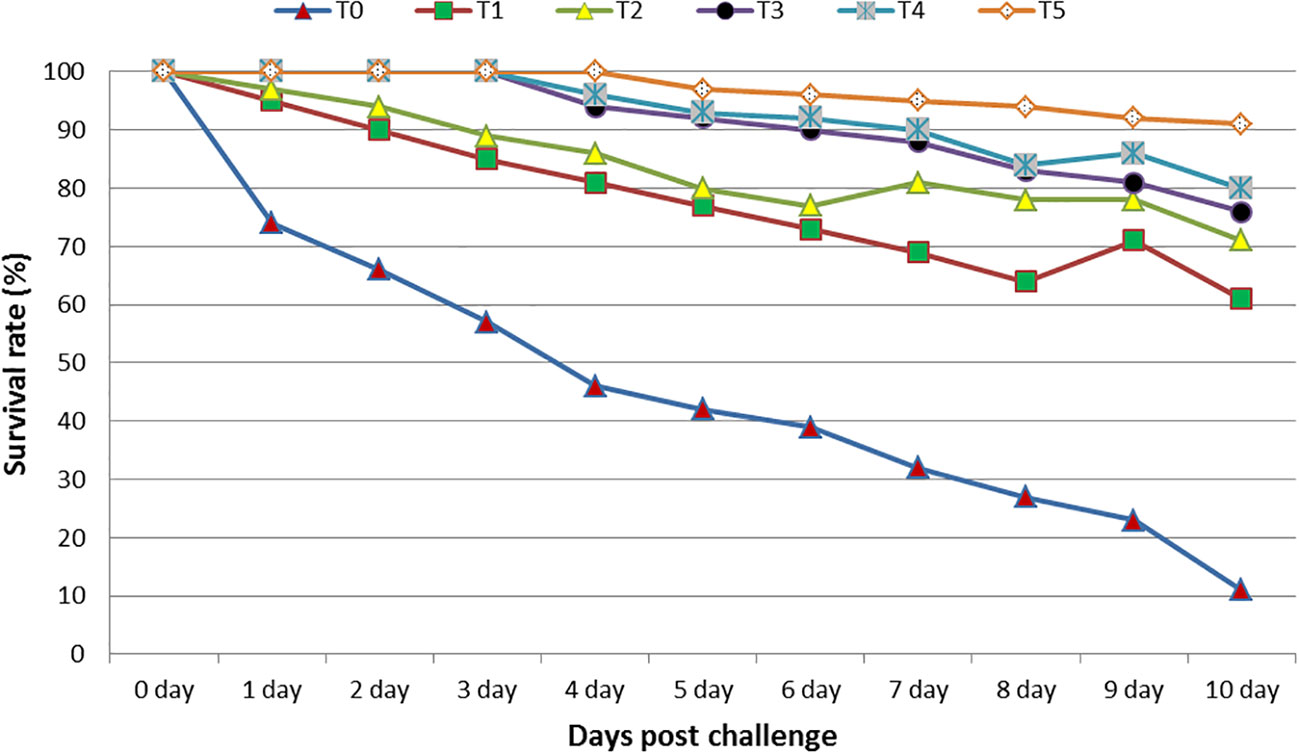
Figure 7 The relative percentage of the survival of tilapia, O. niloticus, fed diets containing various levels of GNS with CA/bio-AgNps for 60 days, during the 10 days of postchallenge with A. hydrophila. Data were expressed as mean ± SE.
In vitro antibacterial and antiparasitic activity of GNS
Figure 8 shows the results of the antibacterial examination, demonstrating the higher efficiency of the GNS against bacterial strains. With more GNS concentration, a higher antibacterial action was demonstrated in the gram-positive and gram-negative bacteria. In a parallel trend, GNS extract has a positive impact on a reduced number of C. tilapiae parasites. The effect on parasites differed from the GNS and control groups because the dead parasite count increased with higher GNS concentrations. In the control group, the dead parasite count shifted from 12% after 30 min to 42% after 60 min. At 1.0 ml/L GNS concentration, 43% of dead parasites were reported after 10 min. After 40 min, complete parasite death took place (Table 7).
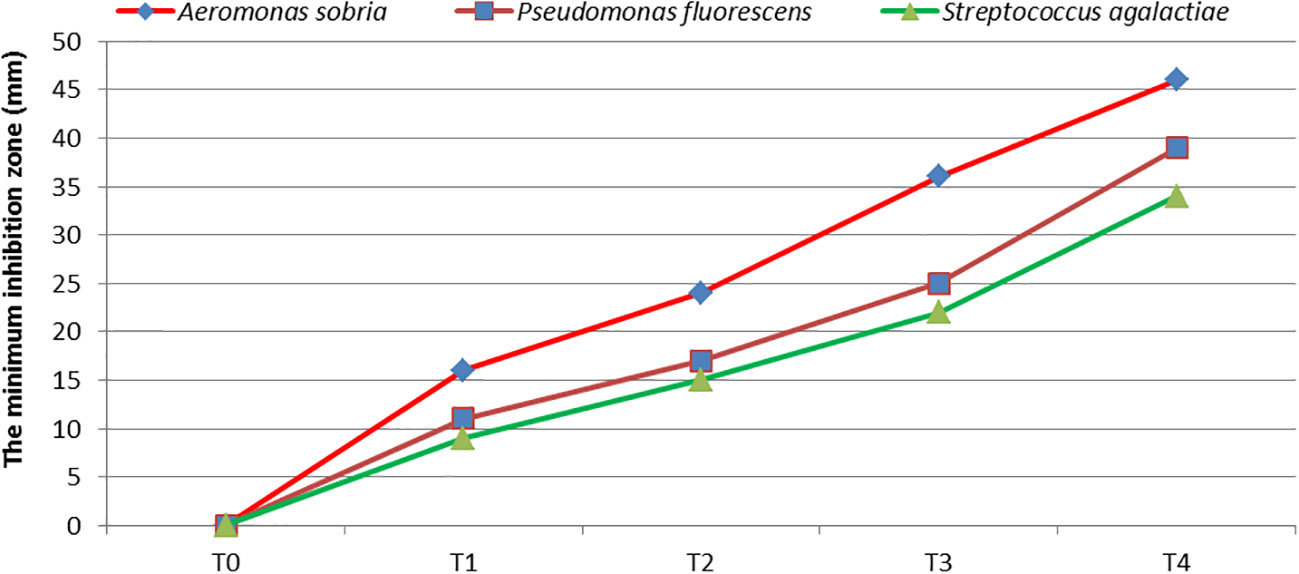
Figure 8 The antibacterial action (safe zone, mm) of the GNS compound against three chosen bacterial strains. Mean ± SD showed data representation.
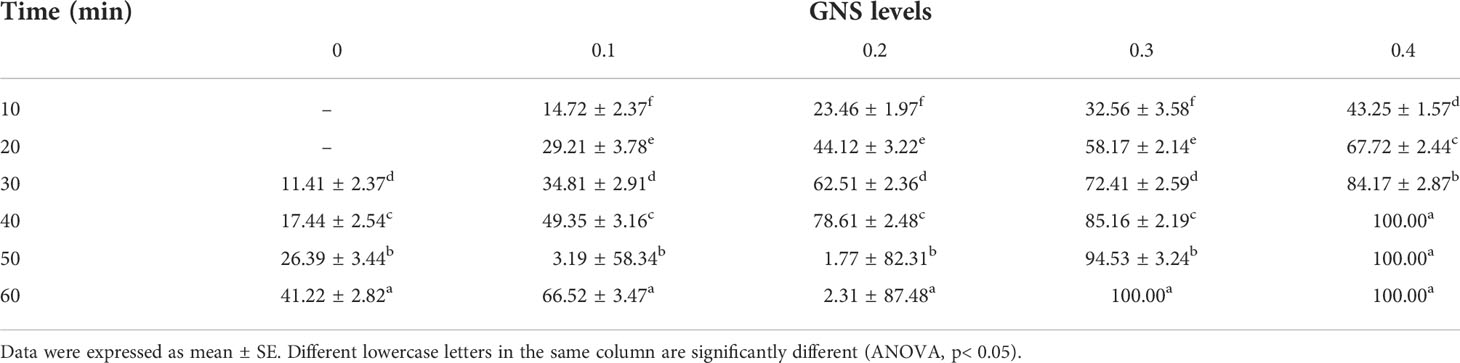
Table 7 Antiparasitic efficiency of various GNS concentrations on time after being treated (min) to the death of C. tilapiae (%).
Discussion
The characterization of UV–vis illustrated the association of this range’s band with bio-AgNps, suggesting that the spherical or roughly spherical CA/bio-AgNps were unchanged during the reaction period. In other words, particles diffused in the water solution with a lack of aggregation evidence (Saifuddin et al., 2009). Moreover, FTIR characterization of biosilver nanoparticles declared that a peak at 1,055 cm−1 showed an alcoholic group, suggesting the phenolic elements in seaweed compounds reduced the metallic salt silver nanoparticles (Sunitha et al., 2015). In parallel, chemical and microbial water analysis were conducted, which was treated through (CA/bio-AgNps) effective improvement of water quality. It was explained that the presence of the multiple high-polar groups (–COO-, –NH2, and –OH) on the modified membrane surface would make it easy to transport aqueous molecules through the membrane, decrease pore size, and effectively enhance the water flux (Morsy et al., 2016). Moreover, microorganisms are relatively hydrophobic and usually negatively charged. They are easily attached to a hydrophobic surface but prefer rough surfaces (Ebrahim et al., 2016). In this study, CA/bio-AgNps have moderate hydrophilicity, which increased slightly with low content addition of biogenic silver nanoparticles (2.5 mg), and more negative surface charge, which increases with biogenic silver nanoparticle concentration. Therefore, the antibiofouling behavior could be explained by the presence of biogenic silver nanoparticles (Gzara et al., 2016). Recently, Fayed et al. (2019) explored the impact of plant compounds as aqueous additives on enhancing water quality indices, growth performance, and health status in Nile tilapia.
Lately, the utilization of algal compounds as an aquaculture feeding additive improved the growth performance, feeding utilization, and immune response of marine animals (Sharawy et al., 2020). According to the obtained data, using GNS, especially 0.5% and 1.0% concentrations, caused positive impacts on the growth performances, carcass composition, and digestive enzyme actions of O. niloticus fry. The obtained results could be explained by the fact that GNS had several bioactive elements, including carotenoids, fatty acids, polysaccharides, and amino acids, which improved the feeding palatability to consume more diets (Sattanathan et al., 2020). Those bioactive elements can enhance the secretion of digestive enzymes that improve feeding digestibility and nutrient assimilation (Abdelhamid et al., 2021). Furthermore, steroidal and saponins help in increasing nutrient absorption by enhancing intestinal barriers’ permeability (Dawood et al., 2021). Increases in the enzymatic amylase, lipase, and protease can be achieved because the algal cells improved the secretion of digestive enzymes after rupture and/or increase feeding consumption (Abdel-Tawwab et al., 2022a). Moreover, Silva et al. (2015) and Ashour et al. (2021) reported that using diets in the feed of tilapia significantly enhanced growth performance.
The present paper showed that hematobiochemical, especially with 0.5% and 1.0% GNS diets, significantly increased Hct, Hb, and RBC values. This finding was concluded because of better erythropoietin production and erythrocytic stability. Moreover, increasing RBC counts suggested the blood’s high oxygen-carrying capacity. This finding agreed with results obtained for gilthead sea bream (Vizcaíno et al., 2016; Guerreiro et al., 2019; Ashour et al., 2021). WBCs showed greater numbers with graded GNS levels. Moreover, while neutrophil counts increased at different levels of GNS diets, lymphocyte counts decreased. The concluded findings could be attributed to enhanced T-cell maturation, stimulated lymphoid tissues, and regenerated lymphoid follicles in the spleen and thymus (Shoemaker et al., 2015; Khalafalla and El-Hais, 2015).
GLO, ALB, and TP values are considerably improved in GNS-fed fish and decreased in ALAT and ASAT activities compared with the control group. The findings highlight the positive impact of GNS on having improved the immune response of O. niloticus. GNS can exert good influences on the mobilization of energy in protein synthesis, illustrating in part the higher serum protein, ALB, and GLO and decreased ALAT and ASAT activities. It acted as a hepatoprotective agent (Heneash et al., 2015; Akbary and Aminikhoei, 2018; Ashour et al., 2020). In previous studies with other microalgae, Mahmoud et al. (2020) and Abdel-Tawwab et al. (2022b) argued that the serum ALAT and ASAT levels declined significantly in the fish because of dietary Chlorella.
In this paper, GNS dietary supplements efficiently decreased the hepatic MDA level, the final output of lipid peroxidation. It significantly increased hepatic GPx, CAT, and SOD actions compared with the control group. GNS is positively affected because of its bioactive phytochemical components, including polysaccharides and fatty acids, especially the polyphenol compound, with antioxidant features (Abdelhamid et al., 2021). Chen and Zhang (2019) stated that the antioxidant enzyme action was enhanced by the polysaccharide taken from Porphyra yezoensis in diets for grass carp in comparison with the control fish. According to Hu et al. (2008), the algal carotenoid compound demonstrated a considerable antioxidant action. Moreover, diets with the red algae (Laurencia caspica) hydroalcoholic compound enhanced the rainbow trout’s antioxidant performance (Kiadaliri et al., 2020).
Data illustrated the immune-stimulating and antioxidant impacts of dietary GNS because it contains high levels of flavonoids and phenolic compounds, such as caffeic acid. This finding is in line with Ahmadifar et al. (2021) that these components have immune-stimulating and antioxidant impacts in several marine animals. Also, Arguelles (2018) stated that these components in algae had effective antioxidant features. According to del Rocío Quezada-Rodríguez and Fajer-Ávila (2017), polysaccharides in algae may improve immune responses. Moreover, Vazirzadeh et al. (2020) reported enhanced immune action in juvenile rainbow trout because fish diets include seaweed. However, Yilmaz (2019) concluded that the dietary supplement of caffeic acid appreciably improved the immune response, enhanced the expression of immune as well as antioxidant-associated genes and caused higher resistance of Nile tilapia against A. veronii infection. In sum, the GNS diet-enhanced antioxidative condition is attributable to more phenols and polyphenols with natural antioxidative actions by causing over-ROS degeneration (Ahmadifar et al., 2021). In parallel, polyphenols inhibited ROS formulation, ROS scavenging, and induction of Nrf2 activation (Kumar and Pandey, 2013). Moreover, polyphenol bioactive components demonstrating better antioxidant characteristics influence fish immunity by enhancing the protection of immune cells (Mohammadi et al., 2020).
The positive impact of GNS on fish health is interpreted by the anti-inflammatory and antioxidative properties. It reduced the inflammatory effects by mediating the hepatic enzymes and antioxidative reaction and regulating anti-inflammatory, proinflammatory, and stress-related genes. This finding matches the finding of Saleh et al. (2020); Abdel-Tawwab et al. (2021), and Dawood et al. (2021). However, Thépot et al. (2021) declared that red seaweed is known as a natural antioxidative agent and immunostimulant. In previous studies, the dietary supplementation with other algal species, such as C. vulgaris and spirulina, caused a significant modulation of the antioxidant capacity and improved innate immunity in Nile tilapia (Abdelghany et al., 2020; Abdel-Tawwab et al., 2021).
O. niloticus confronted with A. hydrophila showed lower mortality rates when a diet was fed, especially T4 and T5. In parallel, in vitro GNS extract has a positive effect against the tested strains, especially with increased concentration. These results may be due to having more natural bioactive substances with evident antimicrobial actions and useful features against fish pathogens (Vatsos and Rebours, 2015; Abdelhamid et al., 2021). Otherwise, Ashour et al. (2020) showed that Nile tilapia confronted with A. hydrophila illustrated a lower mortality rate in the case of using a diet supplemented with seaweed extract. According to Abdel-Tawwab and Ahmad (2009), dietary supplements with live spirulina caused a lower mortality rate in challenged Nile tilapia with A. hydrophila. Moreover, several studies showed that the carotenoid compound, phytol, demonstrated antimicrobial properties (Santos et al., 2013; Pinto et al., 2017).
Concerning the findings of in vitro antiparasitic trials, GNS extract illustrated an antiparasitic efficiency against C. tilapiae. Higher GNS concentrations allowed more antiparasitic efficacy, indicating an optimum concentration of 0.5% and 1.0% for the ultimate antiparasitic action. This study’s general antiparasitic GNS actions were ascribed to the immunostimulation features of peptidoglycans or lipopolysaccharides in seaweed substances that enhance fish resistance against some parasitic diseases (Thanigaivel et al., 2016). Indeed, the general extracts of many seaweeds have pronounced antiparasitic properties; however, in the current study, they may be attributed to polysaccharides present in red algae (Besednova et al., 2021). Moreover, the inhibition is attributable to the milbemycin-oxime in GNS with high insecticidal, anthelminthic, and antiparasitic actions (Kumar et al., 2015). The study results match those of Prichard et al. (2012) regarding the extensive use of milbemycins for resisting parasite infection in aquatic animals.
Conclusion
According to the study results, supplementing GNS diets, especially 0.5% and 1.0%/kg diets with bionanocomposite cellulose acetate membranes (CA/bio-AgNps), improved the growth performance, digestive enzyme actions, and overall health status remarkably. It also enhanced Nile tilapia fingerlings’ resistance against common bacterial fish pathogens. CA/bio-AgNps played a role in improving water quality. Additionally, GNS feeding resulted in regulating proinflammatory and stress-related genes. In vitro, the extract showed a significant positive effect as an antiparasitic. Nevertheless, further research can amplify the benefits of using phytochemical compounds of seaweed extracts as natural phytobiotics in aquaculture for other fish types in the farm with a wider trail to improved water entry into ponds.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
All applicable guidelines (international, national, and/or institutional) for the care of fish were closely followed by the authors during the experimental works.
Author contributions
MR: Writing – original draft, Formal analysis, Methodology, Investigation, Conceptualization, review and editing. ME: Formal analysis, Statistical analysis, Methodology, Editing. MN: Formal analysis, Statistical analysis, Methodology, Editing. AmM: Formal analysis, Methodology, Conceptualization, Editing. JM: Formal analysis, Methodology, Visualization, Editing. AA: Formal analysis, Methodology, Conceptualization, Editing. AhM: Writing – review- Formal analysis, Statistical analysis and editing. SY: Writing – review- Formal analysis, Statistical analysis and editing. MB: Writing – original draft, Methodology, Conceptualization.
Funding
This research was funded by the Taif University researchers, supporting project number TURSP-2020/299, Taif University, Taif, Saudi Arabia.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research, Taif University for the financial support. The authors would also like to thank the research staff of the Department of Zoology, Al-Azhar University for their scientific guidance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1008397/full#supplementary-material
References
Abdelghany M. F., El-Sawy H. B., Abd El-Hameed S. A. A., Khames M. K., Abdel-Latif H. M. R., Naiel M. A. (2020). Effects of dietary nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and resistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus). Fish. Shellfish Immunol. 107, 277–288. doi: 10.1016/j.fsi.2020.10.015
Abdelhamid A. F., Ayoub H. F., Abd El-Gawad E. A., Abdelghany M. F., Abdel-Tawwab M. (2021). Potential effects of dietary seaweeds mixture on the growth performance, antioxidant status, immunity response, and resistance of striped catfish (Pangasianodon hypophthalmus) against aeromonas hydrophila infection. Fish. Shellfish Immunol. 119, 76–83. doi: 10.1016/j.fsi.2021.09.043
Abdel-Razek N., Awad S. M., Abdel-Tawwab M. (2019). Effect of dietary purslane (Portulaca oleracea l.) leaves powder on growth, immunostimulation, and protection of Nile tilapia, Oreochromis niloticus against Aeromonas hydrophila infection. Fish. Physiol. Biochem. 45 (6), 1907–1917. doi: 10.1007/s10695-019-00685-8
Abdel-Tawwab M., Ahmad M. H. (2009). Live spirulina (Arthrospira platensis) as a growth and immunity promoter for Nile tilapia, Oreochromis niloticus (L.), challenged with pathogenic Aeromonas hydrophila. Aquac. Res. 40 (9), 1037–1046. doi: 10.1111/j.1365-2109.2009.02195.x
Abdel-Tawwab M., Eissa E. S. H., Tawfik W. A., Abd Elnabi H. E., Saadony S., Bazina W. K., et al. (2022a). Dietary curcumin nanoparticles promoted the performance, antioxidant activity, and humoral immunity, and modulated the hepatic and intestinal histology of Nile tilapia fingerlings. Fish. Physiol. Biochem. 48 (3), 585–601. doi: 10.1007/s10695-022-01066-4
Abdel-Tawwab M., El-Saadawy H. A., El-Belbasi H. I., Abd El-Hameed S. A. A., Attia ,. A. A. (2021). Dietary spirulina (Arthrospira platenesis) mitigated the adverse effects of imidacloprid insecticide on the growth performance, haemato-biochemical, antioxidant, and immune responses of Nile tilapia. Comp. Biochem. Physiol. Part C 247, 109067. doi: 10.1016/j.cbpc.2021.109067
Abdel-Tawwab M., Mousa M. A., Mamoon A., Abdelghany M. F., Abdel-Hamid E. A., Abdel-Razek N., et al. (2022b). Dietary chlorella vulgaris modulates the performance, antioxidant capacity, innate immunity, and disease resistance capability of Nile tilapia fingerlings fed on plant-based diets. Anim. Feed Sci. Technol. 283, 115181. doi: 10.1016/j.anifeedsci.2021.115181
Abdel-Tawwab M., Samir F., Abd El-Naby A. S., Monier ,. M. N. (2018). Antioxidative and immunostimulatory effect of dietary cinnamon nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to hypoxia stress and aeromonas hydrophila infection. Fish. Shellfish Immunol. 78, 346–354. doi: 10.1016/j.fsi.2017.12.033
Aebi H. (1984). Catalase in vitro. In: Methods in Enzymology Elsevier 105, 121–126. doi: 10.1016/S0076-6879(84)05016-3
Ahmadifar E., Yousefi M., Karimi M., Fadaei Raieni R., Dadar M., Yilmaz S., et al. (2021). Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: an overview. Rev. Fish. Sci. Aquacult. 29 (4), 478–511. doi: 10.1080/23308249.2020.1818689
Akbary P., Aminikhoei Z. (2018). Effect of water-soluble polysaccharide extract from the green alga Ulva rigida on growth performance, antioxidant enzyme activity, and immune stimulation of grey mullet Mugil cephalus. J. Appl. Phycol. 30, 1345–1353. doi: 10.1007/s10811-017-1299-8
Amend D. F. (1981). Potency testing of fish vaccines. Fish. Biol: Serodiagnostics and Vaccines 49, 447–454.
AOAC (2003). Official methods of analysis of the association of official analytical chemists, 17th edn (Arlington, Virginia: Association of Official Analytical Chemists).
AOAC (2012). Official methods of analysis. (15th ed.) (Washington, DC USA: Association of official analytical chemists Inc), 478.
APHA (1998). Standard methods for the examination of water and waste water. 20th edition (Washington, DC: American Public Health Association, American Water Work Association, Water Environment Federation).
Arguelles E. D. L. R. (2018). Proximate analysis, antibacterial activity, total phenolic content and antioxidant capacity of a green microalga scenedesmus quadricauda (Turpin) br´ebisson. As. J. Microbiol. Biotech. Environ. Sci. 20 (1), 150–158.
Aromal S. A., Philip D. (2012). Green synthesis of gold nanoparticles using trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochimica Acta Part A: Mol. biomol. Spectrosc. 97, 1–5. doi: 10.1016/j.saa.2012.05.083
Ashour M., Mabrouk M. M., Ayoub H. F., El-Feky M. M. M., Sharawy Z. Z., Van Doan H., et al. (2020). Effect of dietary seaweed extract supplementation on growth, feed utilization, hematological indices and non-specific immunity of Nile tilapia, Oreochromis niloticus challenged with Aeromonas hydrophila. J. Appl. Phycol. 32, 3467–3479. doi: 10.1007/s10811-020-02178-1
Ashour M., Mabrouk M. M., Ayoub H. F., El-Feky M. M. M., Zaki S. Z., Hoseinifar ,. S. H., et al. (2021). Effect of dietary seaweed extract supplementation on growth, feed utilization, hematological indices, and non-specific immunity of Nile tilapia, Oreochromis niloticus challenged with Aeromonas hydrophila. J. Appl. Phycol. 32, 3467–3479. doi: 10.1016/j.aquaculture.2021.736915
Besednova N. N., Zaporozhets T. S., Andryukov B. G., Kryzhanovsky S. P., Ermakova S. P., Kuznetsova T. A., et al. (2021). Antiparasitic effects of sulfated polysaccharides from marine hydrobionts. Mar. Drugs 19, 637. doi: 10.3390/md19110637
Brown S. P., Keith ,. W. B. (1993). The effects of acute exercise on levels of erythrocyte 2,3-bisphosphoglycerate: a brief review. J. Sports Sci. 11, 479–484. doi: 10.1080/02640419308730016
Camacho-Jiménez L., Álvarez-Sánchez A. R., Mejía-Ruíz ,. C. H. (2020). Silver nanoparticles (AgNps) as antimicrobials in marine shrimp farming: A review. Aquacult. Rep. 18, 100512. doi: 10.1016/j.aqrep.2020.100512
Cantelli L., Goncalves P., Guertler C., Kayser M., Pilotto M. R., Barracco M. A., et al. (2019). Dietary supplementation with sulfated polysaccharides from Gracilaria birdiae promotes a delayed immunostimulation in marine shrimp challenged by the white spot syndrome virus. Aquac. Int. 27 (2), 349–367. doi: 10.1007/s10499-018-0328-1
Chen N., Wu M., Tang G. P., Wang H. J., Huang C. X., Wu X. J., et al. (2017). Effects of acute hypoxia and reoxygenation on physiological and immune responses and redox balance of wuchang bream (Megalobrama amblycephala yi). Front. Physiol. 8. doi: 10.3389/fphys.2017.00375
Chen L., Zhang Y. (2019). The growth performance and nonspecific immunity of juvenile grass carp (Ctenopharyngodon idella) affected by dietary porphyra yezoensis polysaccharide supplementation. Fish. shellfish Immunol. 87, 615–619. doi: 10.1016/j.fsi.2019.02.013
Dar A. H., Rashid N., Majid I., Hussain S., Dar M. A. (2020). Nanotechnology interventions in aquaculture and seafood preservation. Crit. Rev. Food Sci. Nutr. 60, 1912–1921. doi: 10.1080/10408398.2019.1617232
Dawit Moges F., Patel P., Parashar S. K. S., Das B. (2020). Mechanistic insights into diverse nano-based strategies for aquaculture enhancement: A holistic review. Aquaculture 519, 734770. doi: 10.1016/j.aquaculture.2019.734770
Dawood M. A. (2021). Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquacult. 13 (1), 642–663. doi: 10.1111/raq.12492
Dawood ,. M. A., Gewaily ,. M. S., Monier ,. M. N., Younis ,. E. M., Doan ,. H., Sewilam ,. H. (2021). The regulatory roles of yucca extract on the growth rate, hepato-renal function, histopathological alterations, and immune-related genes in common carp exposed with acute ammonia stress. Aquaculture 534, 736287. doi: 10.1016/j.aquaculture.2020.736287
Dawood M. A. O., Koshio S. (2016). Recent advances in the role of probiotics and prebiotics in carp aquaculture: a review. Aquaculture 454, 243–251. doi: 10.1016/j.aquaculture.2015.12.033
Dawood M. A., Metwally A. E. S., El-Sharawy M. E., Ghozlan A. M., Abdel-Latif H. M., Van Doan H., et al. (2020). The influences of ferulic acid on the growth performance, haemato-immunological responses, and immune-related genes of Nile tilapia (Oreochromis niloticus) exposed to heat stress. Aquaculture 525, 735320. doi: 10.1016/j.aquaculture.2020.735320
del Rocío Quezada-Rodríguez P., Fajer-Ávila E. J. (2017). The dietary effect of ulvan from ulva clathrata on hematological-immunological parameters and growth of tilapia (Oreochromis niloticus). J. Appl. Phycol. 29 (1), pp.423–pp.431. doi: 10.1007/s10811-016-0903-7
Doan H. V., Hoseinifar S. H., Jaturasitha S., Dawood M. A. O., Harikrishnan R. (2020). The effects of berberine powder supplementation on growth performance, skin mucus immune response, serum immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 520, 734927. doi: 10.1016/j.aquaculture.2020.734927
Doumas B. T., Watson W. A., Biggs H. G. (1971). : Albumin standard and the measurement of serum albumin with bromocresol green. Clin. Chim. Acta 31, 87–96. doi: 10.1016/0009-8981(71)90365-2
Ebrahim S., Morsy A., Kenawy E., Abdel-Fattah T., Kandil S. (2016). Reverse osmosis membranes for water desalination based on cellulose acetate extracted from Egyptian rice straw. Desalination Water Treat 57 (44), 20738–20748. doi: 10.1080/19443994.2015.1110052
Ellis A. E. (1990). “Lysozyme assays,” in Techniques in fish immunology, vol. pp . Eds. Stolen J. S., Fletcher T. C., Anderson D. P., Roberson B. S., Van Muiswinkel ,. W. B. (Fair Haven, NJ: SOS Publications), 101–103.
Fajardo C., Martinez-Rodriguez G., Blasco J., Mancera J. M., Thomas B., De Donato M. (2022) Nanotechnology in aquaculture: Applications, perspectives and regulatory challenges Aquacult. Fish. 7 (2) 185–200. doi: 10.1016/j.aaf.2021.12.006
Fayed W. M., Khalil R. H., Sallam G. R., Mansour A. T., Elkhayat B. K., Omar E. A. (2019). Estimating the effective level of yucca schidigera extract for improvement of the survival, haematological parameters, immunological responses and water quality of European seabass juveniles (Dicentrarchus labrax). Aquac. Rep. 15, 100208. doi: 10.1016/j.aqrep.2019.100208
García-Bueno N., Decottignies P., Turpin V., Dumay J., Paillard C., Stiger-Pouvreau V., et al. (2015). Seasonal variation in the antivibrio activity of two organic extracts from two red seaweed: Palmaria palmata and the introduced Grateloupia turuturu against the abalone pathogen vibrio harveyi. Aquat. Living Resour. 27, 83–89. doi: 10.1051/alr/2016003
Gornall A. G., Bardawill C. J., David ,. M. M. (1949). : Determination of serum protein by means of the biuret reagent. J. Biol. Chem. 177, 751.
Guerreiro I., Magalhães R., Coutinho F., Couto A., Sousa S., Delerue-Matos C., et al. (2019). Evaluation of the seaweeds Chondrus crispus and ulva lactuca as functional ingredients in gilthead seabream (Sparus aurata). J. Appl. Phycol. 31 (3), 2115–2124. doi: 10.1007/s10811-018-1708-7
Gzara L., Rehan Z. A., Khan S. B., Alamry K. A., Albeirutty M. H., El-Shahawi M. S., et al. (2016). Preparation and characterization of PES-cobalt nanocomposite membranes with enhanced anti-fouling properties and performances. J. Taiwan Inst. Chem. Eng. 65, 405–419. doi: 10.1016/j.jtice.2016.04.012
Heneash A., Ashour M., Matar M. (2015). Effect of un-live microalgal diet, nannochloropsis oculata and arthrospira (Spirulina) platensis, comparing to yeast on population of rotifer, brachionus plicatilis. Mediterr. Aquac. J. 7 (1), 48–54. doi: 10.21608/MAJ.2015.4632
Holdt S. L., Kraan S. (2011). Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol. 23, 543–597. doi: 10.1007/s10811-010-9632-5
Hudson J. B., Kim J. H., Lee M. K., DeWreede R. E., Hong Y. K. (1999). Antiviral compounds in extracts of Korean seaweeds: evidence for multiple activities. J. Appl. Phycol. 10, 427–434. doi: 10.1023/A:1008004117305
Hu C. C., Lin J. T., Lu F. J., Chou F. P., Yang ,. D. J. (2008). Determination of carotenoids in dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem. 15 (109), 439–446. doi: 10.1016/j
Ingale A. G., Chaudhari ,. A. N. (2013). Biogenic synthesis of nanoparticles and potential applications: an eco-friendly approach. J. Nanomed. Nanotechol. 4 (165), 1–7. doi: 10.4172/2157-7439.1000165
Kakkar P., Das B., Viswanathan ,. P. N. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 21 (2), 130–132.
Kanchana R., Zantye P. (2018). Synthesis, characterization and applications of biogenic iron oxide nanoparticles. Int. J. Res. Appl. Sci. Eng. Technol. 3, 709–716. doi: 10.22214/ijraset.2018.3115
Khalafalla M. M., El-Hais A. E. M. (2015). Evaluation of seaweeds ulva rigida and pterocladia capillaceaas dietary supplements in Nile tilapia fingerlings. J. Aquacult. Res. Dev. 6 (3), 1–5. doi: 10.4172/2155-9546.1000312
Kiadaliri M., Firouzbakhsh F., Deldar H. (2020). Effects of feeding with red algae (Laurencia caspica) hydroalcoholic extract on antioxidant defense, immune responses, and immune gene expression of kidney in rainbow trout (Oncorhynchus mykiss) infected with Aeromonas hydrophila. Aquaculture 526, 735361. doi: 10.1016/j.aquaculture.2020.735361
Kim ,. S. Y., Han E. G., Kim S. M., Park J. K., Boo S. M. (2013). Grateloupia jejuensis (Halymeniales, rhodophyta): a new species previously confused with g. elata and g. cornea in Korea. Algae 28, 233–240. doi: 10.4490/algae.2013.28.3.233
Korni F. M. M., Khalil F. (2017). Effect of ginger and its nanoparticles on growth performance, cognition capability, immunity and prevention of motile Aeromonas septicaemia in Cyprinus carpio fingerlings. Aquacult. Nutr. 23, 1492–1499. doi: 10.1111/anu.12526
Kumar S., Pandey A. K. (2013). Chemistry and biological activities of flavonoids: an overview. Sci. World J 2013, 16. doi: 10.1155/2013/162750
Kumar S., Sahoo D., Levine I. (2015). Assessment of nutritional value in a brown seaweed sargassum wightii and their seasonal variations. Algal. Res. 9, 117–125. doi: 10.1016/j.algal.2015.02.024
Kuppusamy P., Yusoff M. M., Maniam G. P., Govindan N. (2016). Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications–an updated report. Saudi Pharm. J. 24 (4), 473–484. doi: 10.1016/j.jsps.2014.11.013
Liu F., Pang S. J. (2010). Stress tolerance and antioxidant enzymatic activities in the metabolisms of the reactive oxygen species in two intertidal red algae Grateloupia turuturu and palmaria palmata. J. Exp. Mar. Biol. Ecol. 382, 82–87. doi: 10.1016/j.jembe.2009.11.005
Mahmoud E. A., El-Sayed B. M., Mahsoub Y. H., El-Murr A. I., Neamat-Allah A. N. F. (2020). Effect of Chlorella vulgaris enriched diet on growth performance, hematoimmunological responses, antioxidant and transcriptomics profile disorders caused by deltamethrin toxicity in Nile tilapia (Oreochromis niloticus). Fish. Shellfish Immunol. 102, 422–429. doi: 10.1016/j.fsi.2020.04.061
Mendis E., Kim S.-K. (2011). Present and future prospects of seaweeds in developing functional foods. Adv. Food Nutr. Res. 64, 1–15. doi: 10.1016/B978-0-12-387669-0.00001-6
Mohammadi G., Rafiee G., El Basuini M. F., Abdel-Latif H. M., Dawood M. A. (2020). The growth performance, antioxidant capacity, immunological responses, and the resistance against Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus) fed Pistacia vera hulls derived polysaccharide. Fish. Shellfish Immunol. 106, 36–43. doi: 10.1016/j.fsi.2020.07.064
Mondal S., Roy N., Laskar R. A., Sk I., Basu S., Mandal D., et al. (2011). Biogenic synthesis of Ag, au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ.) leaves. . Colloids surfaces B: biointerfaces 82 (2), 497–504. doi: 10.1016/j.colsurfb.2010.10.007
Morsy A., Ebrahim S., Kenawy E. R., Abdel-Fattah T., Kandil S. (2016). Grafted cellulose acetate reverse osmosis membrane using 2-acrylamido-2-methylpropanesulfonic acid for water desalination. Water Sci. Technol.: Water Supply 16 (4), 1046–1056. doi: 10.2166/ws.2016.025
Nasr-Allah A., Gasparatos A., Karanja A., Dompreh E. B., Murphy S., Rossignoli C. M., et al. (2020). Employment generation in the Egyptian aquaculture value chain: implications for meeting the sustainable development goals (SDGs). Aquaculture 520, 734940. doi: 10.1016/j.aquaculture.2020.734940
Negm M. A., Ibrahim H. A. H., Shaltout N. A., Shawky H. A., Abdel-mottaleb M. S., Hamdona ,. S. K. (2018). Green synthesis of silver nanoparticles using marine algae extract and their antibacterial activity. Middle East J. Appl. Sci. 8 (3), 957–970.
Paglia D. E., Valentine ,. W. N. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70, 158–168.
Pang S. J., Xiao T., Bao Y. (2006). Dynamic changes of total bacteria and vibrio in an integrated seaweed–abalone culture system. Aquaculture 252 (2–4), 289–97. doi: 10.1016/j.aquaculture.2005.06.050
Pinto M. E., Araujo S. G., Morais M. I., Sá N. P., Lima C. M., Rosa C. A., et al. (2017). Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. Anais. Acad. Bras. Ciˆenc 89, 1671–1681. doi: 10.1590/0001-3765201720160908.
Plouguerné E., Hellio C., Deslandes E., Veron B., Stiger-Pouvreau V. (2008). Anti-microfouling activities in extracts of two invasive algae: Grateloupia turuturu and Sargassum muticum. Bot. Mar. 51, 202–208. doi: 10.1515/BOT.2008.026
Prichard R., Ménez C., Lespine A. (2012). Moxidectin and the avermectins: consanguinity but not identity. Int. J. Parasitol.: Drugs Drug Resist. 2, 134–153. doi: 10.1016/j.ijpddr.2012.04.001
Radwan M. (2022a). Vital economic threat of predatory birds and parasites to cultivated fishes in Egypt. Aquaculture 548, 737666. doi: 10.1016/j.aquaculture.2021.737666
Radwan M., Abbas M. M. M., Afifi M. A. M., Mohammadein A., Al Malki J. S. (2022c). Fish parasites and heavy metals relationship in wild and cultivated fish as potential health risk assessment in Egypt. Front. Environ. Sci. 10. doi: 10.3389/fenvs.2022.890039
Radwan M., Abbas M. M. M., Mohammadein A., Al Malki J. S., Elraey S. M. A., Magdy. M. (2022b). Growth performance, immune response, antioxidative status, and antiparasitic and antibacterial capacity of the Nile tilapia (Oreochromis niloticus) after dietary supplementation with bottle gourd (Lagenaria siceraria, Molina) seed powder. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.901439
Reitman S., Frankel ,. S. A. (1975). A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Path. 28, 56–65. doi: 10.1093/ajcp/28.1.56
Ringø E., Olsen R. E., Vecino J. L. G., Wadsworth S., Song S. K. (2012). Use of immunostimulants and nucleotides in aquaculture: a review. J. Mar. Sci. Res. Dev. 2, 104. doi: 10.4172/2155-9910.1000104
Sadrzadeh M., Mohammadi T. (Eds.) (2019). Nanocomposite membranes for water and gas separation (Elsevier: Matthew Deans), 524 9780128168813.
Saifuddin N., Wong C. W., Yasumira A. A. (2009). Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E-journal Chem. 6 (1), 61–70. doi: 10.1155/2009/734264
Saleh N. E., Ismail R. F., Sayed A. E. H., Zaghloul E. H. (2020). Comprehensive assessment of benthic diatom (Amphora coffeaeformis) as a feed additive in Nile tilapia (Oreochromis niloticus) diet. Aquac. Res. 51, 3506–3519. doi: 10.1111/are.14686
Santos C. C., Salvadori M. S., Mota V. G., Costa L. M., de Almeida A. A. C., de Oliveira G. A. L., et al. (2013). Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013, 949452–949459. doi: 10.1155/2013/949452
Sattanathan G., Palanisamy T., Padmapriya S., Arumugam V. A., Park S., Kim I. H., et al. (2020). Influences of dietary inclusion of algae chaetomorpha aerea enhanced growth performance, immunity, haematological response and disease resistance of labeo rohita challenged with Aeromonas hydrophila. Aquac. Rep. 17, 100353. doi: 10.1016/j.aqrep.2020.100353
Schmitt C. J., Dethloff G. M., Hinck J. E., Bartish T. M., Blazer V. S., Coyle J. J., et al. (2004). “Biomonitoring of environmental status and trends (BEST) program: Environmental contaminants and their effects on fish in the Rio grande basin,” in U.S. geological survey, Columbia environmental research center (Columbia, Missouri: Scientific investigations report). (Reston, VA: U.S. Geological Survey). doi: 10.3133/sir20045108
Shanmugam M., Mody K. H. (2000). “Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents,” in Current science, vol. 79. (Bangalor: Current Science Association), 1672–1683. Available at: http://www.jstor.org/stable/24104127.
Sharawy Z. Z., Ashour M., Abbas E., Ashry O., Helal M., Nazmi H., et al. (2020). Effects of dietary marine microalgae, tetraselmis suecica on production, gene expression, protein markers and bacterial count of pacific white shrimp litopenaeus vannamei. Aquac. Res. 51 (6), 2216–2228. doi: 10.1111/are.14566
Shoemaker C., Xu D. H., LaFrentz B., LaPatra S. (2015). Overview of fish immune system and infectious diseases. Diet. nutr. additives fish Health Wiley Canada, 1–24. doi: 10.1002/9781119005568.ch1
Silva T. F. A., Petrillo T. R., Yunis-Aguinaga J., Marcusso P. F., da Silva Claudiano G., de Moraes F. R., et al. (2015). Effects of the probiotic bacillus amyloliquefaciens on growth performance, hematology and intestinal morphometry in cage-reared Nile tilapia. Lat. Am. J. Aquat. Res. 43 (5), 963–971. doi: 10.3856/vol43-issue5-fulltext-16
Siwicki A. K., Anderson D. P. (1993). “Nonspecific defense mechanisms assay in fish. II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin (Ig) level in serum,” in Fish diseases diagnosis and preventions methods (Olsztyn, Poland).
Sunitha S., Rao A. N., Abraham L. S., Dhayalan E., Thirugnanasambandam R., Kumar V. G. (2015). Enhanced bactericidal effect of silver nanoparticles synthesized using marine brown macro algae. J. Chem. Pharma. Res. 7 (3), 191–195.
Swain P., Nayak S. K., Sasmal A., Behera T., Barik S. K., Swain S. K., Jayasankar P., et al (2014). Antimicrobial activity of metal based nanoparticles against microbes associated with diseases in aquaculture. World Journal of Microbiology and Biotechnology 30 (9), 2491–2502. doi: 10.1007/s11274-014-1674-4
Tang H. G., Wu T. X., Zhao Z. Y., Pan X. D. (2008). Effects of fish protein hydrolysate on growth performance and humoral immune response in large yellow croaker (Pseudosciaena crocea r.). J. Zhejiang Univ. Sci. B 9, 684–690. doi: 10.1631/jzus.B0820088
Tewari P. K. (2015). Nanocomposite membrane technology: Fundamentals and applications (Boca Raton: CRC Press), 325. doi: 10.1201/b19213
Thanigaivel S., Chandrasekaran N., Mukherjee A., Thomas J. (2016). Seaweeds as an alternative therapeutic source for aquatic disease management. Aquaculture 464, 529–536. doi: 10.1016/j.aquaculture.2016.08.001
Thépot V., Campbell A. H., Rimmer M. A., Paul N. A. (2021). Effects of a seaweed feed inclusion on different life stages of the mottled rabbitfish Siganus fuscescens. Aquacult. Res. 52 (12), 6626–6640. doi: 10.1111/are.15533
Van Kampen E. J., Zijlstra W. G. (1983). Spectrophotometry of hemoglobin and hemoglobin derivatives. Adv. Clin. Chem. 23, 199–257. doi: 10.1016/S0065-2423(08)60401-1
Vatsos I. N., Rebours C. (2015). Seaweed extracts as antimicrobial agents in aquaculture. J. Appl. Phycol. 27 (5), 2017–2035. 2017–2035. doi: 10.1007/s10811-014-0506-0
Vazirzadeh A., Marhamati A., Rabiee R., Faggio C. (2020). Immunomodulation, antioxidant enhancement and immune genes up-regulation in rainbow trout (Oncorhynchus mykiss) fed on seaweeds included diets. Fish. Shellfish Immunol. 106, 852–858. doi: 10.1016/j.fsi.2020.08.048
Vizcaíno A. J., Mendes S. I., Varela J. L., Ruiz-Jarabo I., Rico R., Figueroa F. L., et al. (2016). Growth, tissue metabolites and digestive functionality in sparus aurata juveniles fed different levels of macroalgae, gracilaria cornea and ulva rigida. Aquacult. Res. 47 (10), 3224–3238. doi: 10.1111/are.12774
Wang G. X., Han J., Cheng C., Feng T. T., Fu-yuan L., Zhu B. (2009). Bioassayguided isolation and identification of active compounds from fructus cnidii against dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). ParasitolRes 106, 247–255. doi: 10.1007/s00436-009-1659-7
Yang Y., Chai Z., Wang Q., Chen W., He Z., Jiang S. (2015). Cultivation of seaweed gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal. Res. 9, 236–244. doi: 10.1016/j.alga.2015.03.017
Yilmaz S. (2019). Effects of dietary caffeic acid supplement on antioxidant, immunological and liver gene expression responses, and resistance of Nile tilapia, Oreochromis niloticus to Aeromonas veronii. Fish. Shellfish Immunol. 86, 384–392. doi: 10.1016/j.fsi.2018.11.068
Yoshioka T., Kawada K., Shimada T., Mori M. (1979). Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. J. obstet. Gynecol. 135, 372–376. doi: 10.1016/0002-9378(79)90708-7
Yukgehnaish K., Kumar P., Sivachandran P., Marimuthu K., Arshad A., Paray B. A., et al. (2020). Gut microbiota metagenomics in aquaculture: factors influencing gut microbiome and its physiological role in fish. Rev. Aquacult. 121903–1927. doi: 10.1111/raq.12416
Zaki M. A., Ashour M., Heneash A. M. M., Mabrouk M. M., Alprol A. E., Khairy H. M., et al. (2021). Potential applications of native cyanobacterium isolate (Arthrospira platensis NIOF17/003) for biodiesel production and utilization of its byproduct in marine rotifer (Brachionus plicatilis) production. Sustainability 13, 1769. doi: 10.3390/su13041769
Keywords: seaweed, Nile tilapia feed, innate immunity, gene expression, antibacterial and parasitic
Citation: Radwan M, El-Sharkawy MA, Negm MA, Mohammadein A, Malki JSA, Al-Thomali AW, Mohamed AM, Yassir S and Bashar MAE (2022) Dual effect of dietary seaweed of extract nanoparticles (GNS) with bionanocomposite cellulose acetate membranes (CA/bio-AgNps) on growth performance and health status of the Nile tilapia (Oreochromis niloticus): Specification on feed utilization, immune system, and antiparasitic action. Front. Mar. Sci. 9:1008397. doi: 10.3389/fmars.2022.1008397
Received: 31 July 2022; Accepted: 04 October 2022;
Published: 27 October 2022.
Edited by:
Sladana Rakita, University of Novi Sad, SerbiaReviewed by:
Zsuzsanna J. Sandor, Hungarian University of Agricultural and Life Sciences, HungaryMarko Stanković, University of Belgrade, Serbia
Copyright © 2022 Radwan, El-Sharkawy, Negm, Mohammadein, Malki, Al-Thomali, Mohamed, Yassir and Bashar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahmoud Radwan, TWFobW91ZEtocml5LjIwMUBhemhhci5lZHUuZWc=
 Mahmoud Radwan
Mahmoud Radwan Mahmoud A. El-Sharkawy2
Mahmoud A. El-Sharkawy2 Mohammed A. Negm
Mohammed A. Negm Mansour A.E. Bashar
Mansour A.E. Bashar