
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 17 October 2022
Sec. Marine Megafauna
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1005649
This article is part of the Research Topic Risks, Threats, and Conservation Status of Cetaceans in the Mediterranean and Black Seas View all 15 articles
 Roberto Carlucci1,2
Roberto Carlucci1,2 Giulia Cipriano1,2*
Giulia Cipriano1,2* Daniela Cascione2
Daniela Cascione2 Maurizio Ingrosso1
Maurizio Ingrosso1 Tommaso Russo2,3
Tommaso Russo2,3 Alice Sbrana3
Alice Sbrana3 Carmelo Fanizza4
Carmelo Fanizza4 Pasquale Ricci1,2
Pasquale Ricci1,2The assessment of the spatial overlap between eligible cetacean conservation areas (CCAs) and fishing grounds could be a strategic element in the implementation of effective conservation measures in the pelagic offshore areas. A multi-species bio-economic modelling approach has been applied to estimate the fishing traits in eligible CCAs in the Northern Ionian Sea (NIS, Central Mediterranean Sea) between 10-800 m of depth, adopting the Spatial MAnagement of demersal Resources for Trawl fisheries model (SMART). Four possible CCAs were defined according to the distribution of cetacean species, their bio-ecological needs, as well as socio-economic needs of human activities, identifying a Blue, Red, Orange and Green CCAs in the NIS. SMART spatial domain was a grid with 500 square cells (15×15 NM). The analysis was conducted for the period 2016-2019, considering the Otter Trawl Bottom (OTB) fleet activities in the study areas through the Vessel Monitoring System. The spatial extension of fishing activities, hourly fishing effort (h), landings (tons) and economic value (euros) for each CCA and the NIS were estimated as yearly median values. Fishing activities were absent in the Blue CCA, where the presence of the submarine canyon head does not offer accessible fishing grounds. The hourly fishing effort in the Green area accounted for about 22% (3443 h) of the total hourly effort of the NIS, while the Orange and Red areas were about 8% (1226 h) and 2% (295 h), respectively. The Green CCA corresponded to about 14% (36 tons) of the total landings in the NIS, whereas the Orange and Red areas represented about 9% (22 tons) and 6% (16 tons), respectively. The Green CCA accounted for about 13% (156 thousand euros) of the total economic value of the NIS, while the Orange and Red areas represented about 6% (69 thousand euros) and 4% (44thousand euros), respectively. Results showed no or negligible negative effects on trawl activities by potential spatial restrictions due to the establishment of CCAs highlighting the importance to consider spatially integrated information during the establishment process of conservation areas for cetacean biodiversity according to the principles of Ecosystem Based Management.
Spatial analysis of the distribution of key species and their interaction with human activities at sea is a key aspect of any ecosystem-based marine spatial planning (MSP, Foley et al., 2010). Moreover, the MSP approach emphasizes the importance of including both direct and indirect relationships with the legal, socio-economic and ecological complexity of governance when assigning a marine area to a specific use, as there is often a space of overlap between conflicting components that can and must be buffered in advance through measures of appropriate sizing, mitigation and compensation, ensuring greater acceptance and above all a real effectiveness in the conservation of marine biodiversity (Ehler and Douvere, 2009). Being able to harmonize these aspects is crucial if Blue Growth (https://s3platform.jrc.ec.europa.eu/blue-growth) is to be effectively supported. For their bio-ecological traits, the effectiveness of protection measures for cetaceans can be represented by the establishment of conservation areas that encompass large portions of the pelagic domain. Although Marine Protected Areas (MPAs) have been widely adopted in several marine ecosystems (Claudet, 2011), the institution of spatial conservation measures dedicated to the protection of the pelagic domain on a large scale is poorly applied (Wood et al., 2008; Game et al., 2010; Kaplan et al., 2010). On the contrary, the protection of coastal and pelagic offshore areas seems to be a fundamental corner for effective biodiversity conservation, because pelagic MPAs can ensure ecological connectivity between different coastal protected areas, such as those distributed in gulfs and bays (Guidetti et al., 2013). In addition, the ecological benefits derived from coastal MPAs are also often accompanied by positive effects on the rebuilding of fishing stocks, which have economic fallouts on the fishery and other associated activities, such as tourism (Stelzenmüller et al., 2007; Russo et al., 2019). Even more relevant could be the contribution of deep-sea pelagic conservation areas, as the restriction of fishing over large areas could result in minimal economic losses for the sector, but with the advantage of ensuring a more effective remedy against the processes of extinction and loss of diversity and key ecosystem services (Sumaila et al., 2007). In this regard, cetaceans have proven to be of maximum importance in the stability and resiliency of the marine ecosystems (Tromeur and Loeuille, 2017) and in the support of several ecosystem services (Pace et al., 2015), with positive reflection even on climate change (Sergio et al., 2008; Hooker et al., 2011; Roman et al., 2014; Mazzoldi et al., 2019). Therefore, due to threats to and risk of degradation in their status in the Mediterranean Sea (ACCOBAMS, 2020) there is a very urgent need to provide action favouring the maintenance of their critical habitat. However, the planning of cetacean conservation areas represents a real challenge mostly because the spatial overlap between the distribution of cetacean critical habitat and fishing activities is wide. In fact, the feeding preferences of cetaceans and their behavioural strategies could cause conflicts with the fishing activities that are classified as a competition for food resources (Bearzi, 2002; Jusufovski et al., 2019).
Under the umbrella of the European Common fisheries policy (CFP), the management of fishery resources in the Mediterranean Sea is largely based on the regulation of the spatial fishing effort distribution, that is the identification of areas in which to prohibit some or all types of fishing to protect the environment and resources (https://oceans-and-fisheries.ec.europa.eu/policy/common-fisheries-policy-cfp_en). However, the establishment of Fishery-Restricted Area leads to reallocation of fishing effort (displacement from closed areas to adjacent or new ones) that can significantly influence the final effects of this kind of management measures, both in biological and economic aspects (Bastardie et al., 2018; Russo et al., 2019; D’Andrea et al., 2020). Therefore, understanding the allocation of fishing effort displacement is an information to be taken into account when management regulations, which are characterized by temporal and/or spatial banning of fishery, need to be implemented. Several studies have adopted spatial modelling approaches to investigate the fishing exploitation pattern in terms of effort distribution, catches and economic production (Russo et al., 2014; Quijano Quiñones et al., 2021), to simulate possible spatial management scenarios of trawl fishery (Russo et al., 2019) or to investigate the risks of interaction between cetaceans and the fishery (Breen et al., 2017).
In the Northern Ionian Sea (NIS, Central Mediterranean Sea), cetaceans represent key elements in the ecosystem functioning supporting trophic regulations of the entire food web (Ricci et al., 2020a; Carlucci et al., 2021a). Several bio-ecological traits of cetacean species distributed in the NIS (Carlucci et al., 2018a; Carlucci et al., 2018b; Carlucci et al., 2020a; Carlucci et al., 2020b; Cipriano et al., 2022) have been investigated, as well as the potential competition with local fishing activities (Ricci et al., 2020b; Ricci et al., 2021a). This ecological knowledge acquired in the last decade is a focal point in the assessment of cetacean distribution and the interaction with anthropogenic impacts in the Gulf of Taranto, the northernmost part of the NIS (Carlucci et al., 2021b). This information supports the possibility to propose area-based management tools (ABMTs) for cetacean conservation (Notarbartolo di Sciara et al., 2016), such as Cetacean Conservation Areas (CCAs, Carlucci et al., 2021c), aimed at protecting these species and their critical habitats, mitigating anthropogenic impacts and promoting the sustainable development of human maritime activities. In particular, the NIS can be considered an eligible area for the implementation of ABMTs and CCAs, where underwater noise, marine litter, ship collision, and competition for prey by fishery are the main disturbances involved in interacting with cetaceans (Carlucci et al., 2021b). Although direct fishing impacts on the cetaceans (e.g. by-catches) are not recorded in the area (Ricci et al., 2021a), potential competition for food resources could arise with local fishing activities (Carlucci et al., 2021a). However, an investigation into the potential spatial interactions between fishing activities and eligible CCAs has never been explored, although long time series of data are available on species distribution and their life history traits (Maiorano et al., 2010; Capezzuto et al., 2010; Carlucci et al., 2018c; Ricci et al., 2021b) and the characterization of fishing grounds (Russo et al., 2017). Therefore, the main objective of this study is to set up an assessment of the spatial overlap between eligible CCAs and fishing grounds in the NIS using a multi-species bio-economic modelling approach. In particular, the assessment was conducted in the period 2016-2019 using the Spatial MAnagement of demersal Resources for Trawl fisheries model (SMART, Russo et al., 2014; D’Andrea et al., 2020). SMART was selected since it allows reconstruct, using a combination of different data sources, the spatial and temporal origin of catches or landings and their final faith in terms of landing harbour. In addition, given that is a spatial bio-economic model, SMART allows to estimate the economic indicators associated to different patterns of fishing effort, at the scales of both single vessels and fleets. In this way, SMART can be used to assess the economic and the biological value of a given fishing area, supporting quantitative analyses and evaluation in the framework of marine spatial planning. In this paper, SMART has been applied on the harbour-specific fleets of bottom trawlers operating in the study area in order to obtain an assessment of the potential bio-economic impacts of different spatial management actions involving the CCAs. According to Carlucci et al. (2021a), bottom otter trawling (OTB) represents by far the main fishery in the Northern Ionian Sea, both in terms of landings and profits and impacts.
CCAs were described through fishing traits inherent to the otter bottom trawl fleet, by using several indicators, such as fishing effort, landing, economic incomes, and the landing flows from the fishing grounds included within the CCAs towards the main harbours of the study area.
The study area extends from Punta Alice to Santa Maria di Leuca covering a surface of about 14000 km² and reaching 1500 m in depth in the Northern Ionian Sea (NIS) (Central Mediterranean Sea). The hydrographic features of the area are characterized by up-welling systems (Bakun and Agostini, 2001) and decadal processes of deep-water circulation inversion with effects on the energy exchanges between benthic and pelagic domain (Ricci et al., 2022). The NIS includes several important habitats from a conservation point of view in shallow (including seagrass meadows and coralligenous outcrops), pelagic (upwelling sites) and deep-sea areas (including submarine canyon and cold-water coral banks) (Capezzuto et al., 2010; Bo et al., 2011; D’Onghia et al., 2016; Carlucci et al., 2018c; Castellan et al., 2019; Chimienti et al., 2019) ensuring favourable conditions for the support of a high biological diversity and providing diverse ecological services (Carlucci et al., 2021a). Moreover, the study area has been widely recognized as a critical area for the day-to-day life of striped dolphin Stenella coeruleoalba and common bottlenose Tursiops truncatus (Carlucci et al., 2016a; Carlucci et al., 2017; Carlucci et al., 2018b; Carlucci et al., 2018d; Ciccarese et al., 2019; Santacesaria et al., 2019; Azzolin et al., 2020). In particular, the spatial distribution and areas where these dolphins realize feeding, resting, socializing, and traveling activities have been identified (Carlucci et al., 2018b; Papale et al., 2020). In addition, other cetacean species occur in the NIS, such as the Risso’s dolphin (Grampus griseus, Maglietta et al., 2020; Maglietta et al., 2022; Maglietta et al., 2018; Renò et al., 2019; Carlucci et al., 2020a), the sperm whale (Physeter macrocephalus, Bellomo et al., 2019), the Cuvier’s beaked whale (Ziphius cavirostris, Podestà et al., 2016; Carlucci et al., 2020b) and the fin whale (Balaenoptera physalus, Dimatteo et al., 2011; Fanizza et al., 2014).
The habitat complexity of this NIS is accompanied by several anthropogenic pressures, which are represented by fishing activity and marine traffic, as well as the occurrence of navy exercises areas, and industrial activities (Carlucci et al., 2021b; Carlucci et al., 2016a). In particular, the geo-morphological and biological heterogeneity described so far, strongly influences the distribution of the fishing effort, distribution and the typologies of fishing gears adopted in the area. Fishing boats are frequently registered as polyvalent fishing vessels, often changing type of fishing according to the season and sea/weather conditions as well as to the variability in the availability of resources and market demand (Carlucci et al., 2016b). In particular, fishing occurs from coastal waters to about 800 m in depth and it is mainly characterized by the bottom otter trawls, that mostly exploit the shelf break and slope, and the small-scale fishery operating on coastal grounds (Russo et al., 2017). The most important fishing resources are the red mullet (Mullus barbatus) on the continental shelf, the European hake (Merluccius merluccius), the deep-water rose shrimp (Parapenaeus longirostris) and the Norway lobster (Nephrops norvegicus) on a wide bathymetric range; as regards the bathyal grounds, the shrimps (Aristeus antennatus and Aristaeomorpha foliacea) are the most important resources (Carlucci et al., 2018c; Maiorano et al., 2010; Carlucci et al., 2016b; Russo et al., 2017).
Information and data on the cetofauna occurring in the NIS have been available since 2009. This knowledge has led to the hypothesis of a delimitation of four possible CCAs based on different assumptions related to the distribution of cetacean species according to their bio-ecological needs as well as socio-economic constraints (Figure 1). The former area, hereafter called the “Red area”, has an extension of approximately 715 km2 and includes the persistent critical habitats of the striped dolphin (Carlucci et al., 2018d). The second, hereafter called the “Orange area”, encompasses approximately 1530 km2 being enlarged to include all the areas where behavioural activities of the striped dolphin population were observed from 2009 to 2017 (Carlucci et al., 2018d). The third, hereafter called the “Green area”, covers approximately 3170 km2 and includes areas where the highest abundances of both striped and common bottlenose dolphins were estimated through habitat modelling techniques (Carlucci et al., 2018a), together with all the sightings recorded up to 2020 for both the Risso’s dolphin and the sperm whale. The latter CCA, hereafter called the “Blue area”, covers 615 km2 and has been delimited according to the specific spatial needs indicated by the main stakeholders (e.g. maritime authority, navy, municipality, NGOs, research institutions) operating in the study area.
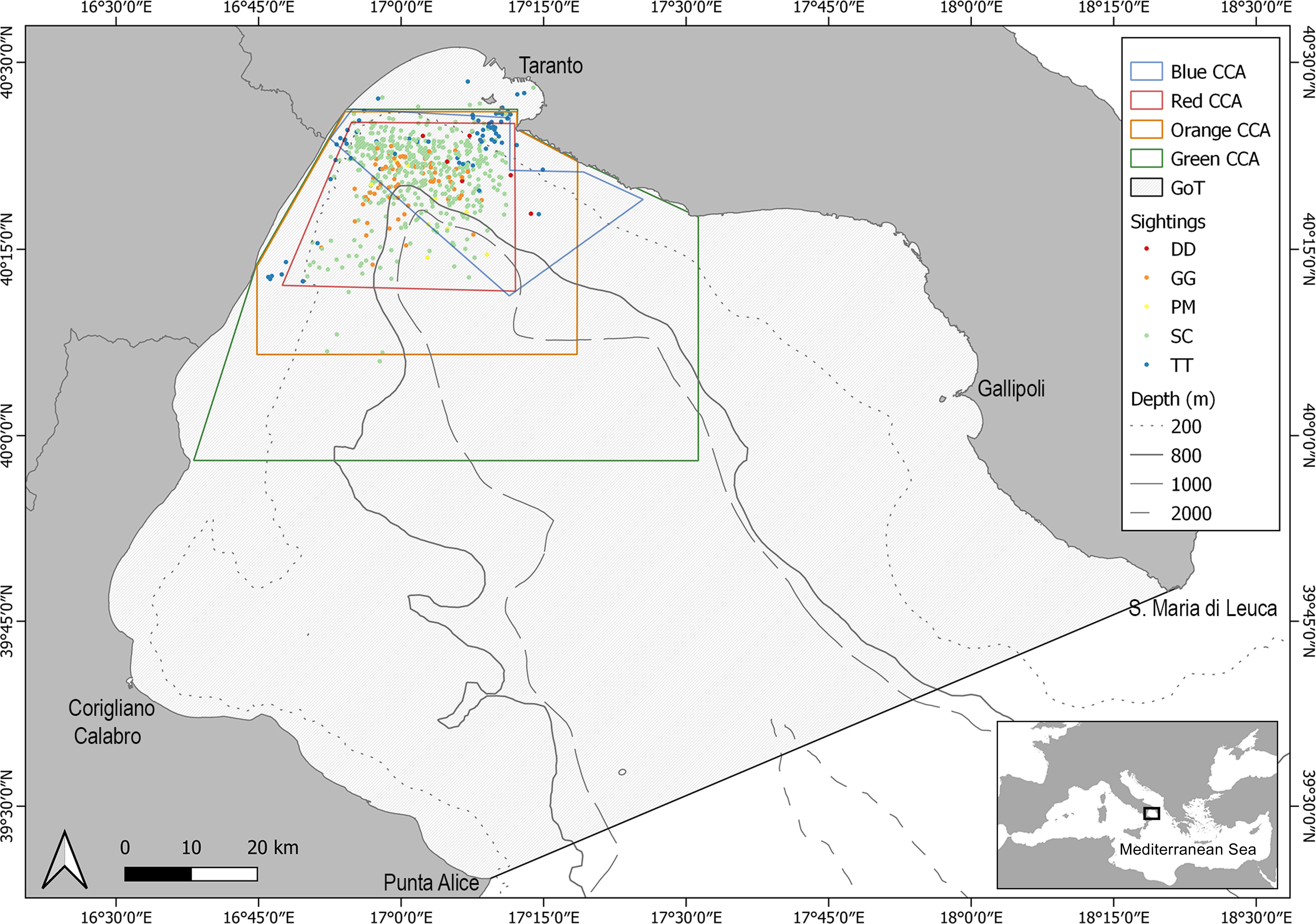
Figure 1 Map of the Northern Ionian Sea (Central Mediterranean Sea) with sightings distribution of S. coeruleoalba (SC), D. delphis (DD), G. griseus (GG), T. truncatus (TT), P. macrocephalus (PM) and the spatial limits of CCAs (the black line indicates the border of the gulf).
The assessment of fishing traits of each CCA and the Northern Ionian Sea was carried out through the SMART modelling approach, a method able to reconstruct the spatial and temporal fluxes of landings coming from well-defined areas (fishing grounds) and times to harbors to which they are delivered for sale (Russo et al., 2018; Russo et al., 2014; D’Andrea et al., 2020). The modelling of spatial fishing effort is based on the use of information obtained by the Vessel Monitoring System (VMS), which is applied to the remote control of fishing vessels with length overall (LOA) ≥ 15 m in European waters (EC, 2011). The VMS data are combined with information on landings acquired from fishing logbooks, where information on landing by species and harbors are reported by the fishers (Gerritsen and Lordan, 2011). All these data are collected within the Data Collection Framework since 2006 and they are provided by the Italian “Ministry of the Agricultural, Alimentary and Forestry Politics” (Russo et al., 2014). Starting from this information on the spatial effort and landings, it is possible to estimate the Landing Per Unit of Effort (LPUE, kg h-1 km-2) for each vessel length (D’Andrea et al., 2020). In addition, a reconstruction of the effort and production data for vessels with a LOA < 15 m was carried out using the European Common Fleet Register (EC, 2010) according to the method reported in Russo et al. (2018).
The spatial domain of the SMART model for the investigated area was defined as a grid with 500 square cells (15 × 15 nautical miles). The rationale of the model, as well as the workflow of the smartR R package, can be summarized in the following logical steps:
1. Analysing VMS data to assess the fishing effort by vessel/cell/time;
2. Processing landings data, combined with VMS data, to estimate the spatial/temporal productivity of each cell (or spatial unit), in terms of mean monthly LPUE by species, according to the method described and applied in Russo et al. (2018);
3. Estimating the cost per vessel/time associated with a given effort pattern and the related revenues, as a function of the landings by vessel/species/length class/time;
4. Combining costs and revenues by vessel, on the yearly scale, to obtain the profit, which is the proxy of the vessel performance.
Each of these steps corresponds to a different module of the smartR package (D’Andrea et al., 2020). A detailed description of the SMART workflow is reported in Russo et al. (2019) and D’Andrea et al. (2020), while a diagram of the approach used in this paper is represented in Figure 2.
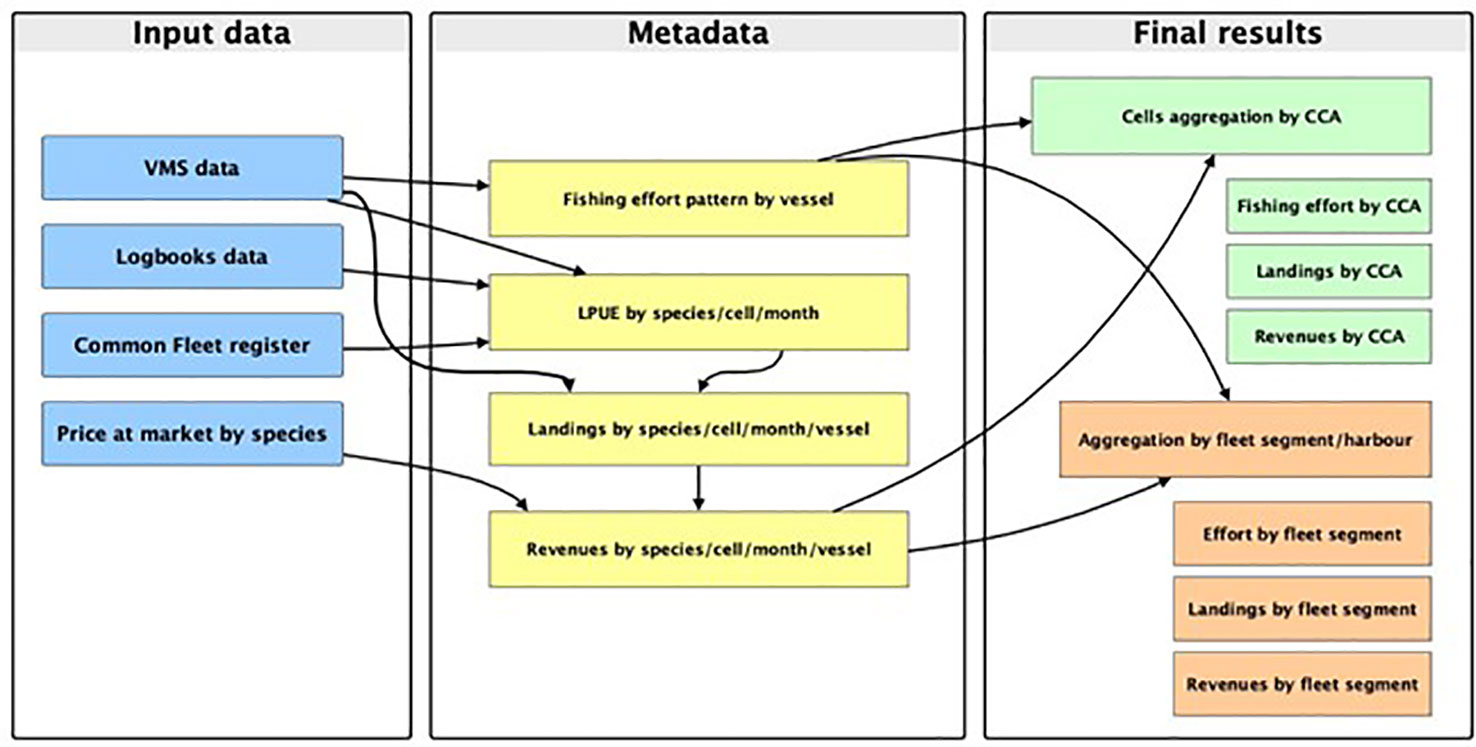
Figure 2 Diagram of the workflow, from input data to final output, applied in this study to assess the value of CCAs for the trawl fisheries operating in the area of study.
The analysis was conducted for the period 2016-2019 (48 months), considering the fleets of vessel performing bottom otter trawling (OTB) in the Northern Ionian Sea and belonging to three fleet segments defined by vessel length-over-all (namely LOA <12, LOA between 12-18 and LOA between18-24 m), being the main segments operating in the study areas (Maiorano et al., 2019; Maiorano et al., 2010; Russo et al., 2017). To avoid anomalies in the fishing production induced by fishing effort variations at the national scale which occurred during the SARS-CoV-2 coronavirus pandemic period (Russo et al., 2022) the years 2020-2021 were excluded from the analysis.
The combination of the different data sources (i.e. VMS data, Logbooks data, Common Fleet register and Price at market by species) allowed to estimate the Fishing effort and the related landings and revenues for the different CCAs. In addition, being known the harbour of departure/landing of each trawler, it was possible to estimate fishing effort, landings and revenues by harbour-specific fleets. In this way, the results of this modelling approaches represent estimates of the real values but simulations. Moreover, considering that the displacement of effort potentially determined by the CCAs was not predicted, the results of this study represent an assessment of the status quo (i.e. the present values of CCAs for trawl fishing).
The assessment of the OTB fleet fishing exploitation in the CCAs and the NIS was carried out considering the spatial distribution of fishing activities, the hourly fishing effort, and the production in terms of landing and economic revenue during the period 2016-2019. The spatial extension of fishing activities (expressed as km2) was calculated as the median of annual values estimated through VMS data for all OTB LOA segments. OTB swept areas by LOA segment were estimated in each year in the range of depth between 10-800 m. In addition, annual trends of the spatial coverage (%) of each VL segment were analysed.
The hourly fishing effort and the yield (landings) were estimated using data provided by the SMART model considering available OTB LOA segments during the investigated period. Therefore, monthly landings were combined with VMS data (using the fishing vessel and temporal range of the fishing activity as references) to estimate the monthly LPUE for each species and cell in the grid (see Russo et al., 2018, for an extensive description of this procedure). The LPUEs obtained were aggregated by the target species of the trawling (Table 1). In addition, data provided by the SMART model were used to calculate several indicators, such as the hourly effort (hours) by OTB LOA segment, the landing, the spatial LPUE and the economic value for each CCA and the NIS. Economic incomes of fishing landing were calculated by multiplying the landing value (kg) of main target species of trawl segment by their price (expressed as mean value in euros kg-1 per species) (Table 1).
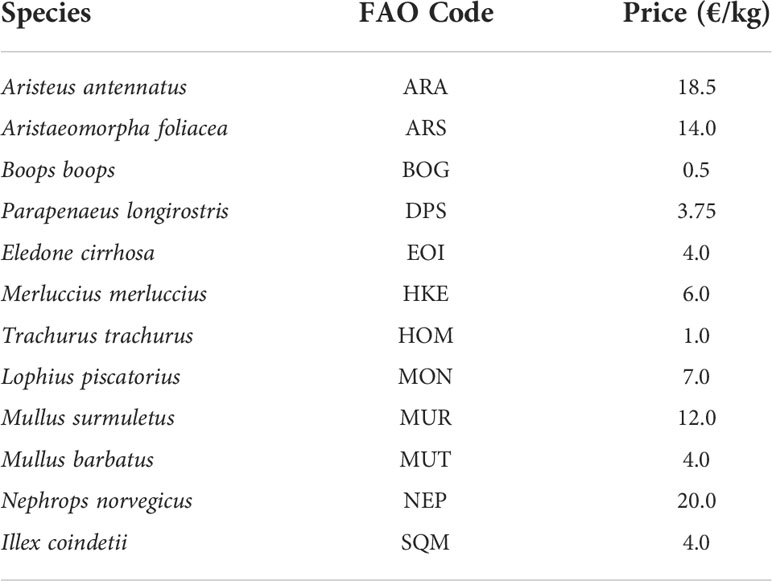
Table 1 Mean sales prices for target species indicated by FAO 3alpha code considered in the analysis.
Further analysis involved the estimation of production flows from the study areas to the main fishing harbours (Crotone, Cariati, Corigliano, Taranto, Gallipoli, Otranto) of the NIS, according to the method reported in Russo et al. (2018). Fishing harbours were aggregated at regional level (Calabrian and Apulian), which are precisely divided by the Taranto valley in the Gulf of Taranto, occupying the southwestern and north-eastern zones, respectively (Rossi and Gabbianelli, 1978). This choice is due to the difference in bottom trawling fleets in the two areas in terms of capacity and effort (Maiorano et al., 2022; Maiorano et al., 2010; Russo et al., 2017), as well as to the structure of the demersal assemblage (Carlucci et al., 2018c) and the food webs (Ricci et al., 2019).
Therefore, median, minimum, and maximum, interquartile range (IR) and the percentage values (% calculated on the median value) of each production indicator for CCAs and the NIS were analysed. A statistical comparison of the fishing and production indicators (hourly effort, landing and economic values) among all CCAs and the NIS was carried out using the multiple non-parametric Mann–Whitney (U) post hoc test, based on the Bonferroni correction (McDonald, 2014). The selection of the KW test was due to the non-normal distribution of the data tested by the Shapiro–Wilk test (Shapiro and Wilk, 1965) (Table S1). The statistical analysis was carried out using PAST 4.03 (Hammer et al., 2001).
The aggregated fishing footprint distributed in potential fishable areas between 10-800 m of depth is reported, together with the different CCAs of interest, in Figures 3–5 and Supp. Materials (Table S2; Figure S1).
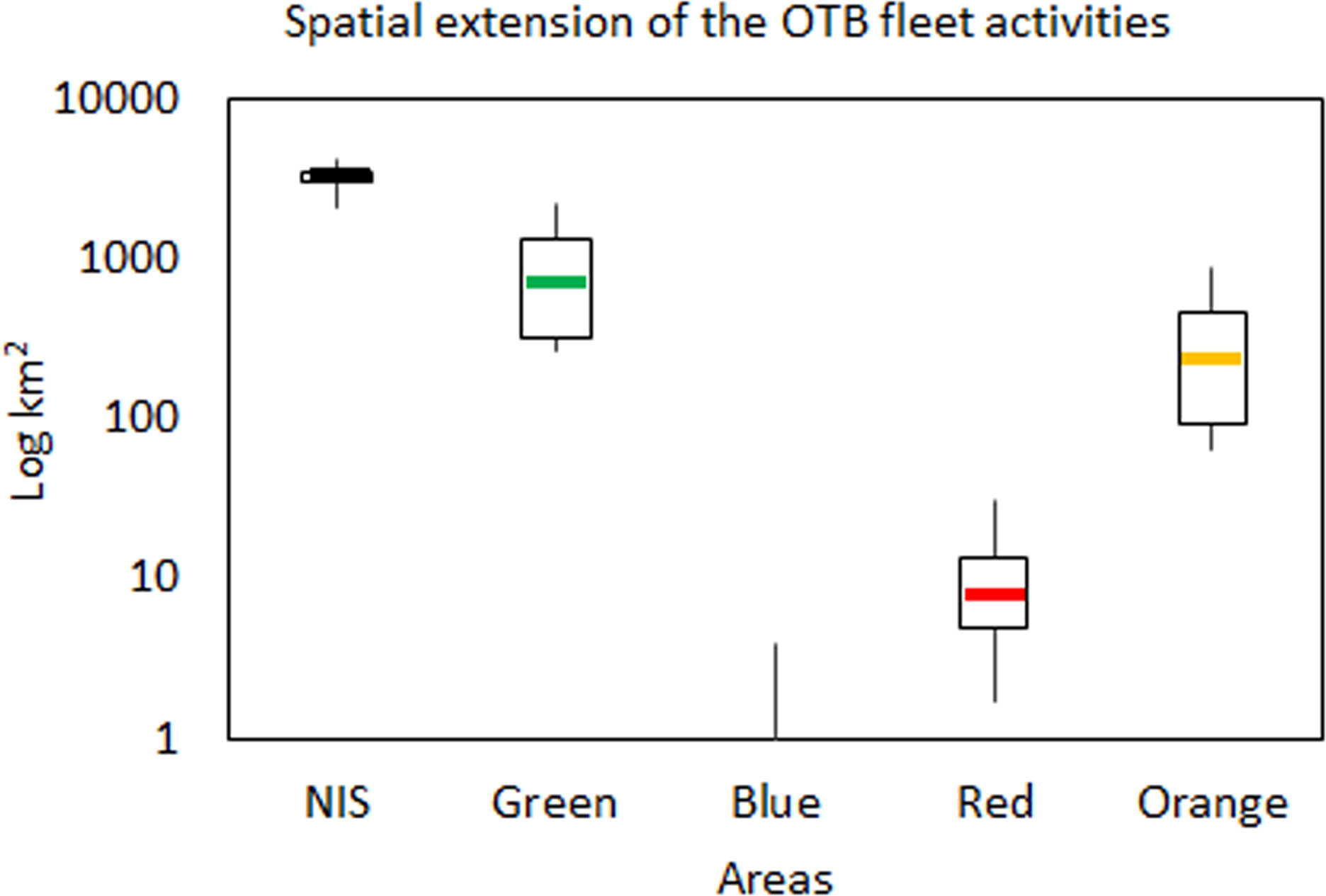
Figure 3 The total yearly spatial extension (km2 in Log scale) of the OTB fleet within CCAs and the NIS during the investigated period (2016-2019). Boxplots report the median (midline), quartiles (box limits); minimum and maximum values (whiskers out of boxes).
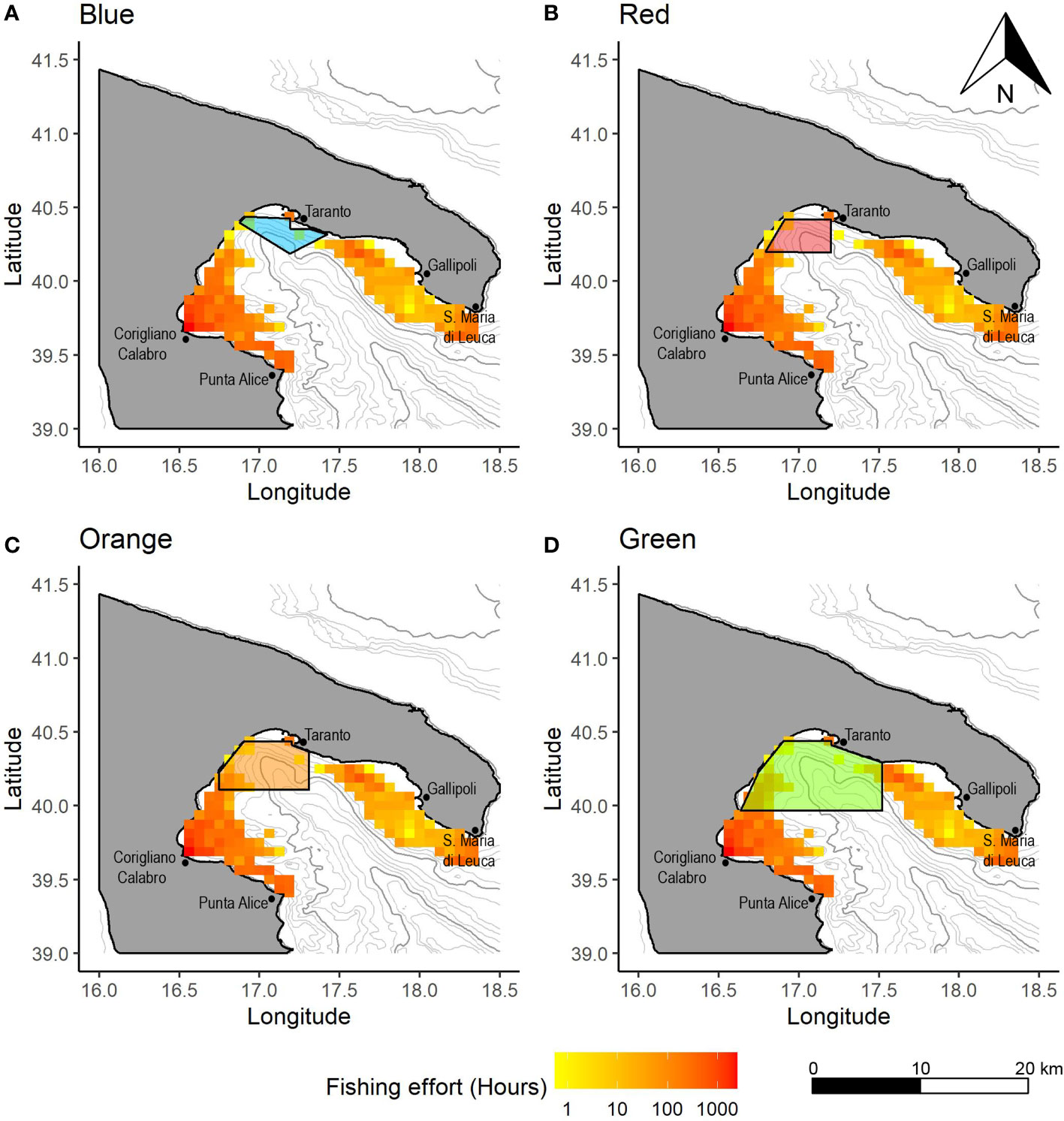
Figure 4 Spatial distribution of the OTB effort (in hours) by VL 12-18 showing the overlap with the (A) Blue, (B) Red, (C) Orange and (D) Green CCAs. Values in hours are calculated as yearly averages for the period 2016-2019.
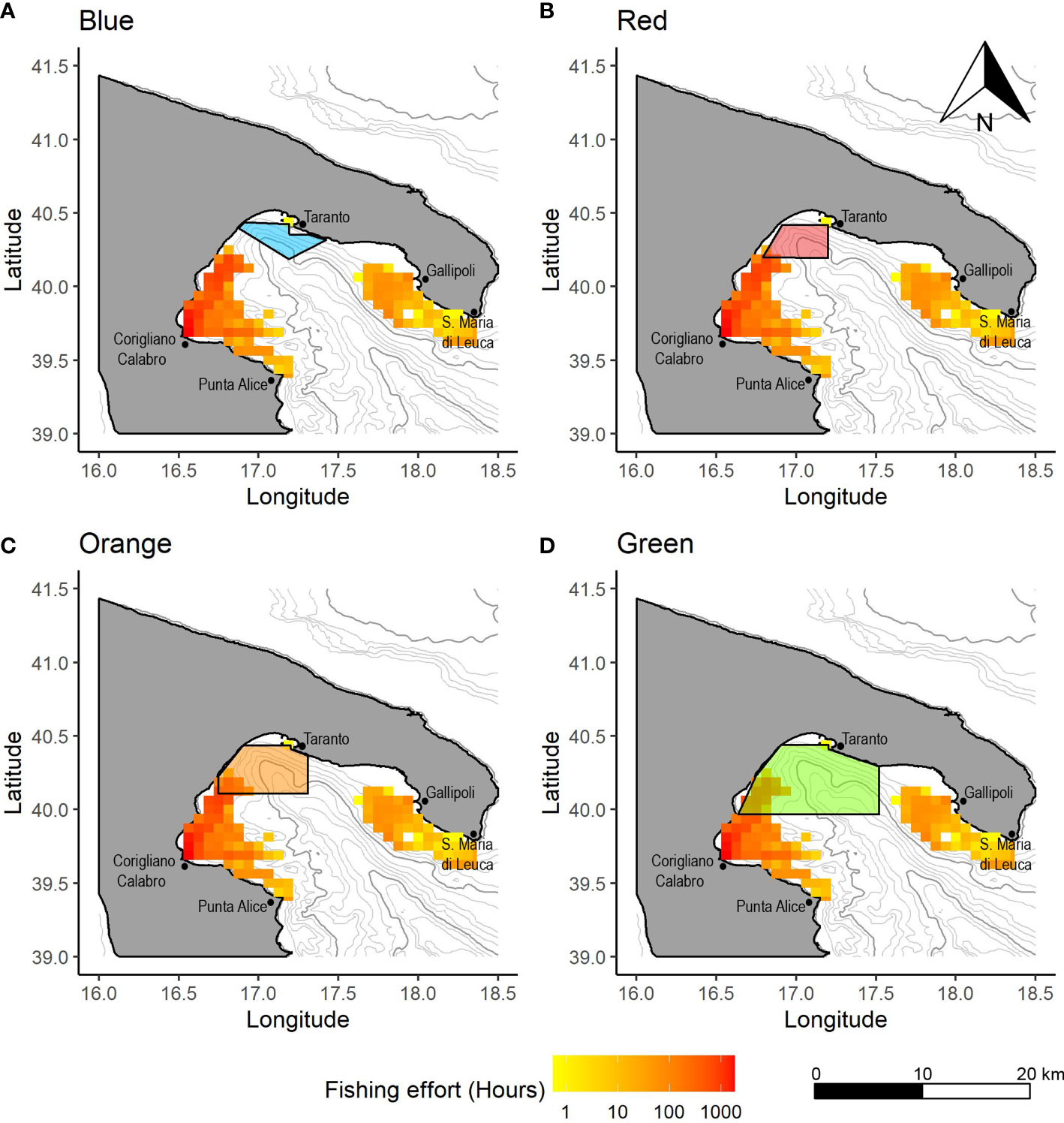
Figure 5 Spatial distribution of the OTB effort (in hours) by VL 18-24 showing the overlap with the (A) Blue, (B) Red, (C) Orange and (D) Green CCAs. Values in hours are calculated as yearly averages for the period 2016-2019.
In the Blue CCA, fishing activity was detected for the OTB VL 15-18 only in 2018, with an absolute extent of 4 km2 (Figure 3). This indicated that the fishing activities are substantially absent in this area. In the Red CCA, the median spatial extent of the entire OTB fleet showed a value of 8 km2 (IR=8), representing a percentage of spatial extension of 0.9% with respect to the whole potential fishable area (Figure 3). In addition, the maximum percentage value of spatial extension was estimated for the OTB VL_18-24 in 2016 (32 km2, 3.7%), while other values in the remaining years were lower than 1.5% (Figure S1). In the Orange CCA, the spatial extension of the OTB fleet showed a median value of 237 km2 (IR=380; 17.0% of the Orange area total) (Figure 3). The highest spatial extension was estimated for the OTB VL 18-24 with a percentage value of 64.1% in 2016, while the lowest was estimated in 2018 (26.8%) (Figure S1). In the Green area, the median spatial extension was of 704 km2 (IR=992; 26.5% of the Green CCA total) and the highest value was observed in 2016 (82.9%) for the OTB VL 18-24 (Figure 3). Also in this area, percentage values decreased over time with the lowest value in 2018 (36.5%) (Figure S1). In the NIS, the spatial extension of the OTB fleet showed a median value of 3469 km2 (IR=522; 36.0% of the total NIS area) (Figure 3). The OTB VL 18-24 segment showed the highest percentage values in 2016, with values of 44.1%, while the lowest was detected in 2019 (28.5%) (Figure S1). In addition, OTB VL 12-18 vessel showed a spatial extension lower than approximately 36% in all years.
In the NIS, the mean total yearly effort of the whole trawl fleet showed a median value of 15925 hours (IR= 5174) (Figure 6A, Table S3). Considering the CC areas, the highest was estimated for the Green area (median value of 3443 hours, IR=3070), followed by the Orange area (median value of 1226 hours, IR=1385) and the Red area (median value of 295 hours; IR=199), which were significantly different between them (p<0.001; Table S4). In addition, the fishing effort in the Green area accounted for 21.6% of the total hourly effort of the NIS, and the Orange and Red areas were 7.7% and 1.9%, respectively.
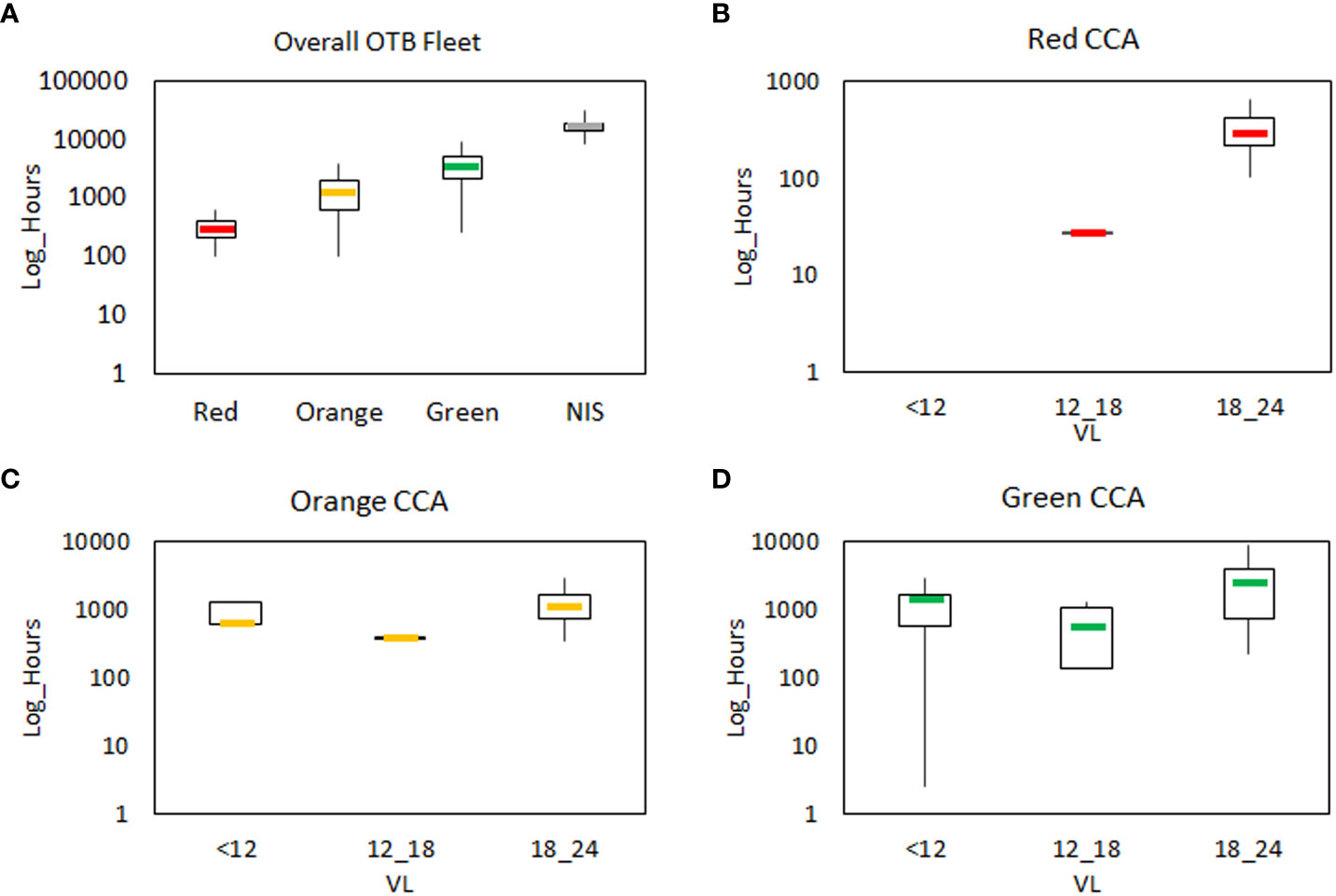
Figure 6 The total yearly fishing effort (hours in Log scale) of the OTB fleet estimated by SMART model. In (A) the hourly effort is reported for the overall OTB fleet in the CCAs and Northern Ionian Sea. The hourly effort by LOA segments is reported in (B) for the Red CCA, in (C) for the Orange CCA and in (D) for the Green CCA. LOA segments are split into vessels lower than 12 m (<12), vessels between 12-18 m (12_18) and those between 18-24 m (18_24). Boxplots report the median (midline), quartiles (box limits); minimum and maximum values (whiskers out of boxes).
Considering the hourly effort by VL segments, fishing activities in the Red CCA were almost exclusively performed by OTB 18-24 vessels (median value of 295 hours, IR=197; 91.4% of the total hourly effort) (Figure 6B), Differently, other VL segments were absent, as VL <12, or characterized by very negligible activities (less than 30 fishing hours estimated for VL 12-18). Similarly, the hourly effort in the Orange CCA showed the highest median value of 1102 hours (IR=865) for the OTB 18-24 segment, representing 52.7% of the total hourly effort in the CCA (Figure 6C). Lower median values were detected for other segments, where OTB<12 and OTB 12-18 vessels accounted for 18.2% (median value of 380 hours; IR=36) and 29.1% (median value of 609 hours; IR=725), respectively. In the Green CCA, the division of fishing effort by VL segments showed the highest median value for OTB VL 18-24 (2499 hours; IR=3205) accounting for 56.5% of the total fishing hourly effort of the CCA (Figure 6D). In the NIS, the OTB VL<12 vessels showed the highest median value of hourly effort (7535 hours, IR=3269) representing 49.0% of the total effort, followed by OTB 12-18 vessels (median value 4900 hours, IR=3761, 31.9%) and the OTB18-24 fleet (median value 2938, IR=5642, 19.1%).
In the NIS, the median estimated production as landings and landing values corresponded to 254.8 tons (IR=124.6) and 1.253 mln euro (IR=580.1), respectively (Table S3). In the CCAs, the highest median value of landings was estimated for the Green area (36.1 tons, IR=26), followed by the Orange area (22.4 tons, IR=13.7) and the Red area (16.1 tons, IR=11.1). Thus, the Green area corresponded to 14.2% of the total landings in the NIS, and the Orange and Red areas represented 8.8% and 6.3%, respectively. Considering the economic revenue, median values estimated for the CCA were 156.3 thousand euros (IR=107.9) for the Green area, 69.2 thousand euros (IR=45.2) for the Orange area and 43.9 thousand euros (IR=25.4) for the Red one. Thus, the Green CCA accounts for 12.5% of the total economic value of the NIS, and the Orange and Red areas represented 5.5% and 3.5%, respectively. All median values calculated for the production indicators were significantly different between the CCAs and NIS (p<0.01) (Table S4).
Considering the composition of landings in the NIS, the most landed species were B. boops with a median value of 41.2 tons (IR=19.2; 16.5% of the total landing in the NIS), followed by T. mediterraneus (33.6 tons, IR=22.53, 13.4%), M. merluccius (32.4 tons, IR=14.37, 13%), M. barbatus (29.8 tons, IR=16.87, 11.9%), I. coindettii (26.2 tons, IR=20.18, 10.5%) and P. longirostris (25.9 tons, IR=12.34, 10.3%) (Figure 7 left plots; Table S5). In term of economic yield, A. antennatus was the most important species, with the median revenue value of 254.7 thousand euros (IR=132.47, 20.7%), followed by M. merluccius (151.0 tons, IR=67.06, 12.3%) (Figure 7 right plots). In addition, N. norvegicus, M. barbatus and I. coindetii each account for between 8 and 10% of the total economic value of the NIS.

Figure 7 Landings (tons) and economic value (mln euro) by commercial species estimated for each CCA and the Northern Ionian Sea (NIS). Code species are reported in the Table 1. Boxplots report the median (midline), quartiles (box limits); minimum and maximum values (whiskers out of boxes).
The production pattern showed some differences in the CCAs compared to the NIS. In particular, the Red CCA showed the lowest number of species in the landing. P. longirostris was the most important landed and economic species, with median values of 7.2 tons (IR=4; 47.7% of the total landing in the Red CCA) and 26.8 thousand euros (IR=15.04; 65.5% of the total economic yield of Red CCA). Other relevant species in the landing were T. mediterraneus (4.0 tons; 26.5%) and B. boops (2.1 tons, 14.1%), but they were characterized by a very low economic value.
In the Orange and Green CCAs, the most important species in the landings were always P. longirostris, T. mediterraneus and B. boops, but an increase in the number of landed species was observed. Deep resources (A. foliacea, A. antennatus and N. norvegicus) were found, as well as commercial cephalopods (I. coindettii and E. cirrhosa). In the Orange CCA, P. longirostris showed the highest median values of landing (7.93 tons, IR=5.28; 36.1% of the total landing in the CCA) and economic yield (29.7 thousand euros, IR=19.8, 37.4% of the total economic value in the Orange CCA). In addition, A. foliacea and A. antennatus accounted for 12.4% and 11.2% of the total economic value in the Orange CCA (median values of 9.87 thousand euros, IR=5.2 and 8.92 thousand euros, IR=10.4, respectively). In the Green CCA, P. longirostris showed the highest median production values account for 25.8% of the landing in the investigated CCA (median value 9.19; IR=5.93) and for 22% of the economic value in the CCA (median value of 34.47 thousand euros, IR=22.25). In addition, A. antennatus and A. foliacea showed high median revenue values equal to 25.3 thousand euros (IR=24.30, 16.3%) and 23.0 thousand euros (IR=19.26; 14.8%), respectively.
The analysis of landing flows by species towards the main fishing harbours showed differences in the pattern of production among the CCAs and the NIS (Figures 8, 9; Table S6). Considering landing flows in the NIS, most of the production was landed in the Apulian region, with the highest median total landing of 108.93 tons in Gallipoli (43.4% of the total production of the NIS), followed by that of Taranto (median total value equal to 43.91 tons; 17.5%), and a very small fraction in Otranto (median total value equal to 6.29 tons; 2.5%) (Figure 9D). In the Calabria region, the landings flows were directed towards Corigliano (median total value equal to 65.82 tons; 26.2%) and Crotone (median total value equal to 26.18 tons; 10.4%). The main landed species in Gallipoli were B. boops (median value of 20.87 tons, IR=11.86; 8.3%), M. merluccius (median value of 16.22 tons, IR=9.17; 6.5%), M. barbatus (median value of 14.33 tons, IR=6.84; 5.7%) and I. coindetii and T. mediterraneus, each accounting for about 4-5% (Figure 8). A similar pattern was observed for the landing species composition in the remaining harbours with lower percentage values. Economic yields showed similar percentage values to the landing flows, with the highest total median value for the landing in Gallipoli (516.35 thousand euros, 41.6% of the total economic production from the NIS). Considering the species, A. antennatus showed the highest economic values in all harbours, excepted for Taranto, where A. foliacea showed the highest median value equal to 49.75 thousand euros (18.95). Other relevant species were M. merluccius, N. norvegicus, M. barbatus and I. coidettii in all harbours.

Figure 8 Landing flows by species (expressed in %) from the CCAs and NIS towards the main fishing harbours. Species codes are reported in Table 1.
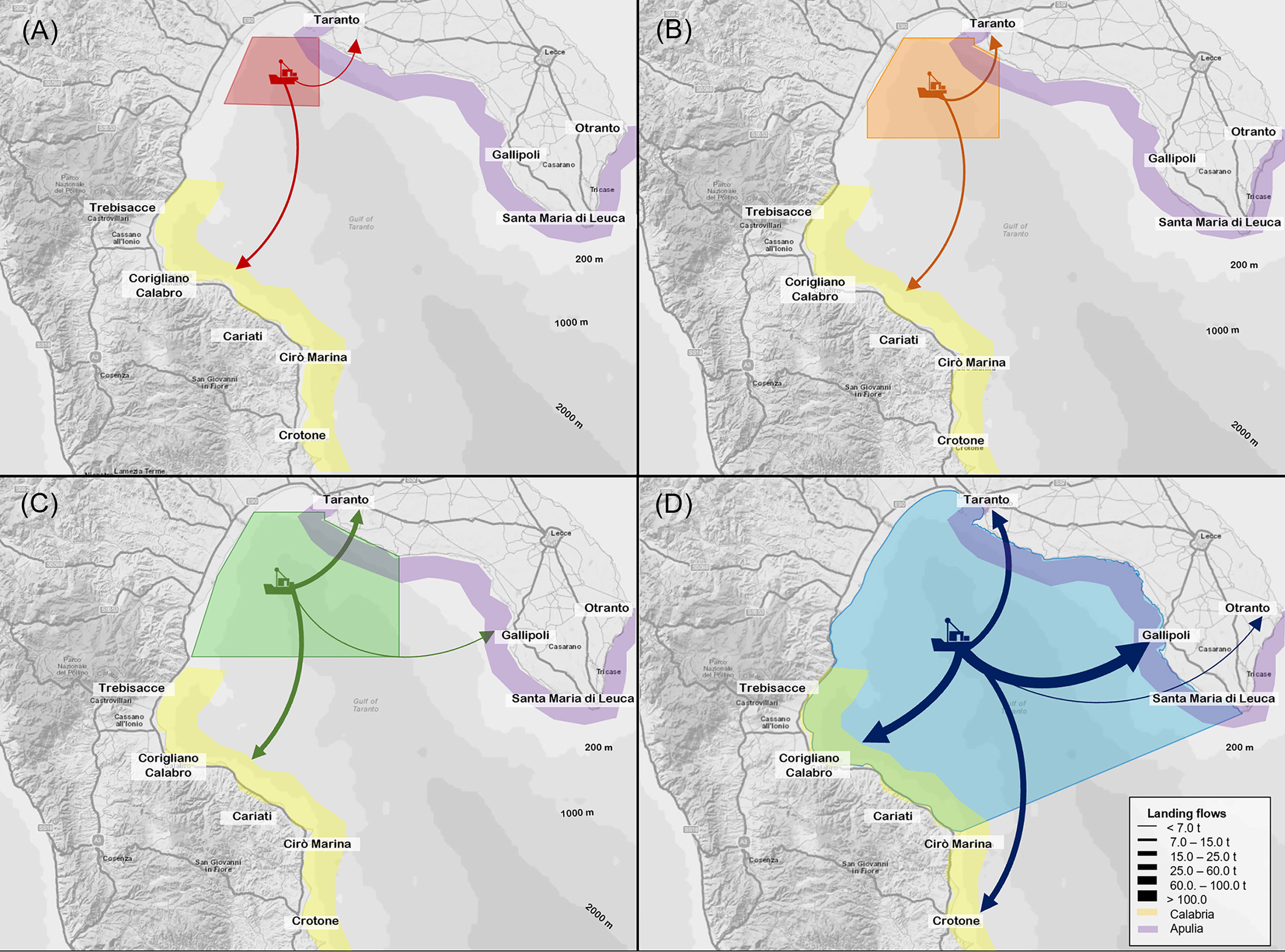
Figure 9 Landings flows (tons) from (A) the Red CCA, (B) Orange CCA, (C) Green CCA and (D) the Northern Ionian Sea towards the main fishing harbours aggregated in the Calabrian (yellow) and Apulian region (violet). Thickness of arrows is proportional to the magnitude of flows.
In the Red CCA, P longirostris showed the highest fraction in the Taranto landings (median value 4.28 tons; IR=3.39) accounted for 27.5% of the total landing flow from this CCA (Figure 8). This species together with B. boops represented all species landed in Taranto from the Red CCA. In Corigliano harbour, T. mediterraneus showed the highest fraction in the landings (median value 3.99 tons, IR= 6.04; 25.6%), followed by P longirostris (median value of 3.03 tons, IR=2.33; 19.5%) and B. boops (median value 1.63 tons, IR=1.35; 10.5%). Other species landed in Corigliano were M. merluccius, Lophius spp. and M. barbatus.
Concerning the economic yield, P. longirostris was also the most important species in both harbours, showing median values of 16.06 thousand euros (IR=12.73; 38.5% of the total economic value) in Taranto and 11.36 thousand euros (IR=8.72; 27%) in Corigliano, respectively.
Overall, the median total landing from the Red CCA was higher within Corigliano harbour (66.9%) than Taranto harbour (33.1%) (Figure 9A). Similarly, the economic production accounted for 60.5% (25.23 thousand euros) in the Corigliano harbour and 39.5% (16.50 thousand euros) in Taranto, respectively.
In the Orange area, the most important species in the landing flows towards Corigliano harbour were T. mediterraneus (median value of 4.65 tons; IR=6.22; 19.8% of the total production of the area), P. longirostris (median value of 3.13 tons; IR=2.53; 13.3%) and B. boops (median value of 1.84 tons; IR=1.72; 7.8%). In addition, small amounts of A. foliacea (median value of 0.71 tons; IR=0.37; 3.0%) and A. antennatus (median value of 0.26 tons; IR=0.35; 1.1%) were detected in the landings (Figure 8). In Taranto harbour, the main landed species were always P. longirostris (median value of 4.70 tons, IR=3.44; 20.1%) and B. boops (median value of 2.45 tons, IR=2.52; 10.5%). Other relevant species were M. barbatus, M. merluccius and I. coindetii, and A. antennatus was detected in the landing. Concerning the economic revenue, P. longirostris, A. foliacea and A. antennatus were the main important species in the production flows of both harbours. The former species accounted for 20.5% (17.63 thousand euros) in Taranto and for 13.7% (11.74 thousand euros) in Corigliano harbour, respectively, as well as A. antennatus being equal to 8.4% (7.20 thousand euros) for the Taranto production and 5.6% (4.84 thousand euros) in that of the Corigliano. Finally, A foliacea represented 11.5% (9.87 thousand euros) in Corigliano. Overall, the median total landing from the Orange CCA was split into 13.66 tons in Corigliano (58.2% of the total landing from the CCA) and 9.80 tons in Taranto (41.8%), respectively (Figure 9B). Median total revenues were slightly higher in the Corigliano (56.7%) than in Taranto (43.3%).
The landings flow from the Green area was mainly directed to the Corigliano harbour (49.5% of the Green CCA total landing) and to Taranto (43.7%), while the lowest fraction was landed in Gallipoli (6.8%) (Figure 9C). In all harbours, P. longirostris and B. boops were the main landed species (Figure 8). In addition, T. mediterraneus showed a high median value exclusively in Corigliano (4.65 tons, IR=5.78; 11.3% of the total landing production). Other relevant species were M. merluccius, M. barbatus and A. foliacea with values which ranged between 3-4% in both Corigliano and Taranto harbours. Considering the economic yield, as observed in the Orange CCA, P. longirostris, A. foliacea and A. antennatus were the most important species in all harbours, representing overall about 54% of the total economic revenue (24.2% in Corigliano harbour, 25.8% in Taranto and 3.9% in Gallipoli). Other relevant species were M. merluccius and Lophius spp. in the production Corigliano harbour, with values of 4.0% and 4.5%, respectively. Overall, the median total economic yield from the Green CCA was slightly higher in Corigliano harbour (92.65 thousand euros, 47.7%), than in Taranto (86.60 thousand euros 44.6%), while Gallipoli accounted for 7.8% (15.10 thousand euros).
The analysis developed in this study through a multi-species bio-economic modelling approach is the first attempt to quantify fishing exploitation patterns within eligible CCAs identified with the explicit purpose of protecting cetacean species in the Northern Ionian Sea. The SMART approach was used to obtain a quantitative reconstruction of fishing activities in the study area and to provide a baseline for the planning of spatial conservation measures, as well as for sustainable management of fishery. Indeed, the output obtained could be considered in the application of measures required for a sustainable management of the trawl fishery, as required by the Multi-annual Plan for the Fisheries exploiting demersal stocks (Sánchez Lizaso et al., 2020). In fact, effective management aimed at reducing discards and mortality of both target and non-target species require integrated strategies based on several kinds of regulation (Colloca et al., 2013). Although the main Annual Multiplan Fishery regulations are based on effort reduction in terms of fishing days, other restrictions could be planned to adopt spatial conservation tools able to synthetize multiple targets in the conservation of marine biodiversity and ecosystems (Pérez-Ruzafa et al., 2017; Russo et al., 2019).
The first main relevant output is the absence of fishing activities in the smallest CCA (blue) where the head of the submarine canyon does not offer accessible grounds. Thus, the area can be an interesting space for the planning of spatial conservation actions, without conflicts with the local fishery. In addition, the proximity of the Blue CCA to the coast should be evaluated from the socio-economic perspective, stimulating the involvement of different stakeholders for the planning of regulatory measures (Heck et al., 2011). Indeed, the growing interest in citizen science activities can find opportunities to develop ocean literacy activities in suitable locations such as marine protected areas and to promote Sustainable Development Goals in coastal communities (Ferreira et al., 2021). However, a critical issue could be represented by the small size of this CCA, which partially covers the head of canyons and could not provide and exhaustive protection of some cetacean species, such as Z. cavirostris, which is distributed in the offshore area of the NIS (Carlucci et al., 2020b). However, the level of conservation represented by the Blue CCA could be very beneficial for the protection of cetaceans and easily implemented in the area given the involvement of the main stakeholders. From an operational perspective, an increase in knowledge regarding other human pressures impacting in the CCA, such as naval traffic, should be acquired. Indeed, this pressure is an important source of impact for cetaceans, such as the accidental strikes (Pennino et al., 2017), which could be regulated through the adoption of specific spatial measures on the routes and speeds of the naval traffic (Guzman et al., 2020).
Considering the eligible CCAs, the results highlighted a growing fishing exploitation pattern moving from the Red to the Green CCA. This increase in fishing effort and production is expected, because the spatial dimensions of CCAs grow from red to green area, with the consequence of including additional fishing grounds. Furthermore, small changes in the species composition of the landing in each CCA were observed, showing the absence of the exploitation of deep commercial resources in the red CCA. Indeed, its landings composition is exclusively characterized by the shallowest species, such as P. longirostris, T. mediterraneus and B. boops. Though not negligible, the last two species are characterized by a low economic yield, and a high discard rate from trawl catches (Maiorano et al., 2019), and at the same time, they are prey of T. truncatus (Bearzi et al., 2010; Ricci et al., 2020a). Moreover, the fishing pressure in this CCA showed the lowest level of spatial coverage and hourly fishing effort, in line with the knowledge on the fishing effort displacement in the Northern Ionian Sea. Here, some fishing grounds are in the south-western zone between Taranto and the Calabrian area and in the south-eastern zone off Gallipoli (Russo et al., 2017). The western border of the CCA is located at the end of the former fishing ground, where the trawl vessels stop their hauls at the head of the canyon slope. This condition forces the trawl vessels towards the shelf platform up to 200 m in depth. Thus, potential fishing interactions in the Red CCA could interest mainly the common bottlenose dolphin, since the species is distributed in shallower areas (Carlucci et al., 2018a; Carlucci et al., 2016a) showing a trophic overlap with commercial species caught by several fishing gears (Ricci et al., 2020a; Carlucci et al., 2021a). A further noteworthy point is that the Red CCA, which defines a conservation level aimed at protecting the persistent critical habitats of the striped dolphin (Carlucci et al., 2018d), is partially overlapping with the blue area, covering the totally canyon head. Thus, the establishment of this CCA does not seem to particularly interfere with local fishing activities and the potential economic losses due to the banning of the area are very scarce. At same time, this CCA represents an efficient conservation level for the life cycle of the striped dolphin, as well as for deep-sea habitats requiring protection actions (Manea et al., 2020). Considering the importance of deep-sea habitats, future studies should investigate the occurrence of other impacts on the area, providing specific regulations of the human activities.
The Green and Orange CCAs differ from the Red one by a higher intensity of fishing activities and in the landing species composition, especially the occurrence of deep commercial species in the catches, which are target species of the Northern Ionian Sea (Maiorano et al., 2022). However, P. longirostris is always the main species in terms of landing amount and sale in both CCAs, and only in the Green area do deep-water shrimps seem to achieve an economic yield similar to that of the deep-water rose shrimp. This condition could be affected by the geographic traits and the position of this CCA, which is the only area that partially overlaps with south-eastern grounds, where the main exploited species are A. foliacea and A. antennatus (Russo et al., 2017; Maiorano et al., 2022). Observations of landings flows to harbours also support this explanation, which in the Green area also show the presence of the Gallipoli fleet, which is known for its exploitation of deep water shrimp on the south-eastern slope the Gulf (D’Onghia et al., 2005; Maiorano et al., 2022). Concerning fishery performances in the Green and Orange CCAs, hourly fishing effort in the former CCA was more than twice that of the latter (3342 against 1226 fishing hours, respectively). However, the landing and economic yield in proportion to the employed effort was lower in the Green CCA than the Orange one. This observation, like that observed at the global scale by Sumaila et al. (2007), should be considered within an overall costs and benefits assessment addressed to planning effective spatial measures to conserve the cetaceans and biodiversity. Indeed, in a scenario of low losses for the fishing industry, other incomes could be acquired by other ecosystem services, such as those performed by small cetaceans (Kiszka et al., 2022), compensating and improving the ecological conditions and the sustainability of the socio-economic systems linked to the marine resources (Hammershalg et al., 2019).
The need to establish conservation areas for cetaceans is a fundamental goal of several international protocols, such as Important Marine Mammal Areas (IMMAs, Notarbartolo di Sciara et al., 2016; Hoyt and Notarbartolo di Sciara, 2021) or the Cetaceans Critical Habitats (CCHs, Notarbartolo di Sciara, 2002). These protocols required several selection criteria of the eligible areas. For instance, IMMA selection criteria are focused on the distribution and abundance of cetacean populations, the key life cycle activities occurring in the considered areas, as well as the assessment of vulnerability of the resident cetacean populations (Tetley et al., 2022). Similarly, CCHs require information on the fishery interactions with the cetaceans to identify suitable habitats for these organisms (ACCOBAMS-ECS-WK Threats, 2017; IUCN Marine Mammal Protected Areas Task Force, 2018). In addition, other international initiatives in the Ionian basin, such as the EU Strategy for the Adriatic-Ionian Region (EUSAIR, 2014), includes among its objectives the implementation of MPAs, with particular attention to the identification of areas to create new MPAs or areas requiring special measures for the conservation of biodiversity, as well as the proposal of complementary measures for sustainable fishing in the conservation areas of the Adriatic-Ionian ecoregion (EUSAIR, 2021). These proposals as a whole should be driven by the application of quantitative methodologies useful to provide both information on the ecological consequences of the establishment of CCAs and the socio-economic effects linked to conservation areas. The quantification of these aspects is important because conservation plans often conflict with fishing activities and other uses of the sea (Grip and Blomqvist, 2020). This is particularly true in such complex exploited systems as the Gulf of Taranto, with multiple human use of the maritime space, relevant sensitive habitats, and high biodiversity at all ecosystem levels (Carlucci et al., 2021b). No less relevant, urgent planning of spatial conservation measures is required in the Adriatic-Ionian region because it is one of the least-protected areas in the Mediterranean Sea (EUSAIR, 2021). Despite all these factors, the analysis shows no or very negligible negative effects on trawling due to potential spatial restrictions on the establishment of CCAs, especially within the Blue and Red area delimitations. At the same time, the ecological benefits for cetofauna diversity provided by a more extensive protection level, such as that of the Orange and Green CCAs, could be accompanied by effects on demersal stock repopulation, reduction of fishing discards, as well as increased ecotourism activities with positive spill-over effects on other economic activities in a more sustainable use of maritime space. Therefore, the planning of spatial conservation measures for cetaceans could find points of agreement with a redefinition of the fishing areas in the Northern Ionian Sea without generating socio-economic conflicts.
The quantification of both fishing pressures and production in terms of economic value from CCAs is an important strength. Indeed, the identification of the level of fishing pressure in space and time, the amounts of landings and their species composition, could provide insight into the intensity of fishing disturbances to the cetaceans, due to competition for food resources (Kaschner and Pauly, 2005). Furthermore, such knowledge may provide data required in the processes of assessing the conservation status of cetaceans and their habitats (ACCOBAMS ECS‐WK Threats, 2017; Breen et al., 2017), as well as in the use of indicators that classify the environmental state of the marine ecosystem through cetacean biodiversity (Azzellino et al., 2014). On the other hand, the quantification of economic value represents a way of assessing the ecosystem service represented by the fishing resources production (Holmlund and Hammer, 1999; Pope et al., 2016). Such information could make it possible to manage and mitigate possible conflicts between the need for biodiversity conservation and fishing exploitation, especially in the case of fishing restrictions. In this regard, the results obtained from the SMART model allow for a better understanding of the dynamics of trawling activity in areas important for cetaceans living in the NIS. Not less important, future investigations should be addressed to quantify collateral impacts on cetaceans and the ecosystem derived from trawling activities, such as the underwater noise pollution produced by trawling vessels (Daly and White, 2021) and the spatial redistribution of the trawling fishing effort in response to the establishment of spatial closures (Powers and Abeare, 2009). The former impact represents a critical disturbance for cetaceans, which should be assessed in the framework of the of Marine Strategy Framework Directive (Descriptor 11) (EU, 2017) while the latter could lead to a potential increase in the fishing pressure around the banned areas and on other grounds (Elhani et al., 2018). However, it should be noted that the Blue and Red CCAs investigated in this study should not influence the redistribution of fishing effort, since trawling activity is almost entirely absent. Moreover, potential effects of spatial conservation measures adopted for cetaceans on the population dynamics of demersal resources (e.g. spill-over effects from CCAs) represent an important aspects to investigate through fisheries management scenarios.
The assessment of the value of conservation areas could be considered as part of the broader framework of assessing the ecosystem services provided by specific maritime areas with high biodiversity. To provide spatially integrated information on the fishing effort and the economic value could be a key point in planning based on the principles of EBM (Essington et al., 2018).
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Italian Ministry of Ecological Transition.
RC, PR, TR: Conceptualization. RC, PR, TR: methodology. TR, AS, DC, PR: formal analysis. CF, RC, PR, GC: data investigation and sampling design. PR, DC, MI, GC: writing—original draft preparation. RC, PR, TR DC, MI, AS, CF, GC: writing—review and editing. RC, PR: supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1005649/full#supplementary-material
ACCOBAMS (2020). “Assessment of the conservation status of cetaceans in the ACCOBAMS area,” in Thirteenth meeting of the ACCOBAMS scientific committee, Monaco, ACCOBAMS-SC13/2020/Doc 10Rev1, 9. (Monaco:ACCOBAMS).
ACCOBAMS-ECS-WK Threats (2017)Inputs to the ACCOBAMS ongoing effort to map human threats on cetaceans in the Mediterranean and black seas ACCOBAMS-ECS-WK Threats/2017/Report. In: . Available at: https://www.accobams.org/wp-content/uploads/2016/06/Report_workshop_Threats_31_ECS.pdf (Accessed June 3, 2020).
Azzellino A., Fossi M. C., Gaspari S., Lanfredi C., Lauriano G., Marsili L., et al. (2014). An index based on the biodiversity of cetacean species to assess the environmental status of marine ecosystems. Mar. Environ. Res. 100, 94–111. doi: 10.1016/j.marenvres.2014.06.003
Azzolin M., Arcangeli A., Cipriano G., Crosti R., Maglietta R., Pietroluongo G., et al. (2020). Spatial distribution modelling of striped dolphin (Stenella coeruleoalba) at different geographical scales within the EU Adriatic and Ionian Sea region, central-eastern Mediterranean Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 30, 1194–1207. doi: 10.1002/aqc.3314
Bakun A., Agostini V. N. (2001). Seasonal patterns of wind-induced upwelling/downwelling in the Mediterranean Sea. Sci. Mar. 65, 243–257. doi: 10.3989/scimar.2001.65n3243
Bastardie F., Rufener M., Nielsen J., Maina I., Kavadas S., Vassilopoulou C., et al. (2018). “Modelling spatial interactions among fish communities, fishers and other marine activities: comparing five European case-studies,” in Conference of the international institute of fisheries economics and trade (IIFET)(Seattle, US:International Institute of Fisheries Economics & Trade)2018. Session on Marine Spatial Planning and Multiple Use Management.
Bearzi G. (2002). “Interactions between cetaceans and fisheries in the Mediterranean Sea,” in “Cetaceans of Mediterranean and black seas: State of knowledge and conservation Strategies2 in a report to the ACCOBAMS secretariat, vol. 20 . Ed. Notarbartolo di Sciara G. (Monaco: ACCOBAMS).
Bearzi G., Agazzi S., Gonzalvo J., Bonizzoni S., Costa M., Petroselli A. (2010). Biomass removal by dolphins and fisheries in a Mediterranean Sea coastal area: do dolphins have an ecological impact on fisheries? Aquat. Conserv. Mar. Freshw. Ecosyst. 20, 549–559. doi: 10.1002/aqc.1123
Bellomo S., Santacesaria F. C., Fanizza C., Cipriano G., Renò V., Carlucci R., et al. (2019). “Photo-identification of physeter macrocephalus in the gulf of taranto (Northern Ionian Sea, central-eastern Mediterranean Sea),” in Proceedings IEEE metrology for the Sea 2019 IMEKO TC-19 international workshop on metrology for the Sea(Genoa, Italy:IMEKO), 2019 33–37.
Bo M., Bavestrello G., Canese S. (2011). Coral assemblage off the calabrian coast (South Italy) with new observations on living colonies of Antipathes dichotoma. Ital J. Zool. 78, 231–242. doi: 10.1080/11250001003652619
Breen P., Brown S., Reid D., Rogan E. (2017). Where is the risk? integrating a spatial distribution model and a risk assessment to identify areas of cetacean interaction with fisheries in the northeast Atlantic. Ocean Coast. Manage. 136, 148–155. doi: 10.1016/j.ocecoaman.2016.12.001
Capezzuto F., Carlucci R., Maiorano P., Sion L., Battista D., Giove A., et al. (2010). The bathyal benthopelagic fauna in the north-western Ionian Sea: structure, patterns and interactions”. Chem. Ecol. 26, 199–217. doi: 10.1080/0275754100-3639188
Carlucci R., Akkaya Baş A., Liebig P., Renò V., Santacesaria F. C., Bellomo S., et al. (2020a). Residency patterns and site fidelity of Grampus griseus (Cuvier 1812) in the gulf of taranto (Northern Ionian Sea, central eastern Mediterranean Sea). Mamm. Res. 65 (3), 445–455. doi: 10.1007/s13364-02000485-z
Carlucci R., Akkaya Baş A., Maglietta R., Renò V., Fanizza C., Rizzo A., et al. (2018b). “Site fidelity, residency and habitat use of the risso’s dolphin grampus griseus in the gulf of taranto (Northern Ionian Sea, central-eastern Mediterranean Sea) by photo-identification,” in Proceedings IEEE metrology for the Sea, vol. 2018d. (Bari, Italy:IEEE), 173–177.
Carlucci R., Bandelj V., Ricci P., Capezzuto F., Sion L., Maiorano P., et al. (2018c). Exploring spatio-temporal changes of the demersal and benthopelagic assemblages of the north-western Ionian Sea (Central Mediterranean Sea). Mar. Ecol. Progr. Ser. 598, 1–19. doi: 10.3354/meps12613
Carlucci R., Capezzuto F., Cipriano G., D’Onghia G., Fanizza C., Libralato S., et al. (2021a). Assessment of cetacean–fishery interactions in the marine food web of the gulf of taranto (Northern Ionian Sea, central Mediterranean Sea). Rev. Fish Biol. Fish. 31 (1), 135–156. doi: 10.1007/s11160-020-09623-x
Carlucci R., Cipriano G., Fattoruso G., Oliveri M. G., Ricci P., Squillante M. (2021c). “Parsimonious AHP applied to the conservation of cetaceans: a case study in the gulf of taranto,” in Proceedings IEEE international workshop on metrology for the Sea: Learning to measure Sea health parameters, (MetroSea: IEEE) 2021., 1–5.
Carlucci R., Cipriano G., Paoli C., Ricci P., Fanizza C., Capezzuto F., et al. (2018a). Random forest population modelling of striped and common-bottlenose dolphins in the gulf of taranto (Northern Ionian Sea, central-eastern Mediterranean Sea). Estuar. Coast. Shelf Sci. 204, 177–192. doi: 10.1016/j.ecss.2018.02.034
Carlucci R., Cipriano G., Santacesaria F. C., Ricci P., Maglietta R., Petrella A., et al. (2020b). Exploring data from an individual stranding of a cuvier's beaked whale in the gulf of taranto (Editoriale Scientifica: Northern Ionian Sea, central-eastern Mediterranean Sea). J. Exp. Mar. Biol. Ecol. 553, 151473. doi: 10.1016/j.jembe.2020.151473
Carlucci R., Fanizza C., Cipriano G., Paoli C., Russo T., Vassallo P. (2016a). Modeling the spatial distribution of the striped dolphin (Stenella coeruleoalba) and common bottlenose dolphin (Tursiops truncatus) in the gulf of taranto (Editoriale Scientifica: Northern Ionian Sea, central-eastern Mediterranean sea)”. Ecol. Indic. 69, 707–721. doi: 10.1016/j.ecolind.2016.05.035
Carlucci R., Maglietta L., Buscaino G., Cipriano G., Milella A., Pollazzon V., et al. (2017). “Review on research studies and monitoring system applied to cetaceans in the gulf of taranto (northern Ionian Sea, central-eastern Mediterranean Sea),” in Proceedings 14th IEEE international conference on advanced video and signal based surveillance (AVSS)(Lecce:IEEE). doi: 10.1109/AVSS.2017.8078473
Carlucci R., Maiorano P., Sion L., D’Onghia G., Tursi A. (2016b). ““The sustainability of fishing in the southern Adriatic and northern Ionian seas”,” in Governance of the Adriatic and Ionian marine space. Ed. Caligiuri A. (Editoriale Scientifica), 149–159.
Carlucci R., Manea E., Ricci P., Cipriano G., Fanizza C., Maglietta R., et al. (2021b). Managing multiple pressures for cetaceans’ conservation with an ecosystem-based marine spatial planning approach. J. Environ. Manage. 287, 112240. doi: 10.1016/j.jenvman.2021.112240
Carlucci R., Ricci P., Cipriano G., Fanizza C. (2018d). Abundance, activity and critical habitat of the striped dolphin Stenella coeruleoalba in the gulf of taranto (Northern Ionian Sea, central Mediterranean Sea). Aquat. Conserv. Mar. Freshw. Ecosyst. 28, 324–336. doi: 10.1002/aqc.2867
Castellan G., Angeletti L., Taviani M., Montagna P. (2019). The yellow coral Dendrophyllia cornigera in a warming ocean. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00692
Chimienti G., Bo M., Taviani M., Mastrototaro F. (2019). “Occurrence and biogeography of Mediterranean cold-water corals,” in Mediterranean Cold-water corals: Past, present and future (Cham, CH: Springer International Publishing), 213–243.
Ciccarese S., Carlucci R., Ciani E., Corcella E., Cosentino A., Fanizza C., et al. (2019). Cytochrome b marker reveals an independent lineage of Stenella coeruleoalba in the gulf of taranto. PloS One 14 (3), e0213826. doi: 10.1371/journal.pone.0213826
Cipriano G., Carlucci R., Bellomo S., Santacesaria F. C., Fanizza C., Ricci P., et al. (2022). Behavioral pattern of risso’s dolphin (Grampus griseus) in the gulf of taranto (Northern Ionian Sea, central-Eastern Mediterranean Sea). J. Mar. Sci. Eng. 10 (2), 175. doi: 10.3390/jmse10020175
Claudet J. (2011). Marine protected areas - a multidisciplinary approach (Cambridge: Cambridge University Press).
Colloca F., Cardinale M., Maynou F., Giannoulaki M., Scarcella G., Jenko K., et al. (2013). Rebuilding Mediterranean fisheries: a new paradigm for ecological sustainability. Fish Fish. 14, 89–109. doi: 10.1111/j.1467-2979.2011.00453.x
Daly E., White M. (2021). Bottom trawling noise: Are fishing vessels polluting to deeper acoustic habitats? Mar. pollut. Bull. 162, 111877. doi: 10.1016/j.marpolbul.2020.111877
D’Andrea L., Parisi A., Fiorentino F., Garofalo G., Gristina M., Russo T., et al. (2020). smartR: a r package for spatial modelling of fisheries and simulation of effort management. Environ. Monit. Assess. 192 (12), 1–17. doi: 10.1111/2041-210X.13394
Dimatteo S., Siniscalchi M., Esposito L., Prunella V., Bondanese P., Bearzi G., et al. (2011). Encounters with pelagic and continental slope cetacean species near the northern shore of the gulf of taranto, Italy. Ital. J. Zool. 78 (1), 130–132. doi: 10.1080/11250003.2010.532161
D’Onghia G., Calculli E., Capezzuto F., Carlucci R., Carluccio A., Maiorano P. (2016). New records of cold-water coral sites and fish fauna characterization of a potential network existing in the Mediterranean Sea. Mar. Ecol. 37, 1398–1422. doi: 10.1111/maec.12356
D’Onghia G., Capezzuto F., Mytilineou C., Maiorano P., Kapiris K., Carlucci R., et al. (2005). Comparison of the population structure and dynamics of Aristeus antennatus (Riss between exploited and unexploited areas in the Mediterranean Sea. Fish. Res. 76 (1), 22–38. doi: 10.1016/j.fishres.2005.05.007
EC (2010). Fleet register on the net. Available at: http://ec.europa.eu/fisheries/fleet/index.cfm/.
EC. (2011). Commission Implementing Regulation (EU) No. 404/2011 of 8 April 2011 laying down detailed rules for the implementation of Council Regulation (EC) No 1224/2009 establishing a Community control system for ensuring compliance with the rules of the Common Fisheries Policy. Off. J. Eur. Union L112, 1–30. Available at: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R0404&from=EN
Ehler C., Douvere F. (2009). Marine spatial planning: a step-by-step approach toward ecosystem-based management (Paris, UNESCO: Intergovernmental Oceanographic Commission and Man and the Biosphere Programme).
Elahi R., Ferretti F., Bastari A., Cerrano C., Colloca F., Kowalik J., et al (2018). Leveraging vessel traffic data and a temporary fishing closure to informmarine management Front. Ecol. Environ. 16(8):1–7 doi: 10.1002/fee.19
Essington T. E., Sanchirico J. N., Baskett M. L. (2018). Economic value of ecological information in ecosystem-based natural resource management depends on exploitation history. Proc. Natl. Acad. Sci. U.S.A. 115, 1658–1663. doi: 10.1073/pnas.1716858115
EU (2011). Impact assessment of discard reducing policies. Available at: https://ec.europa.eu/fisheries/documentation/studies/discards_en/.
EU (2017). Commission decision (EU) 2017/848 of 17 may 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardised methods for monitoring and assessment, and repealing decision 2010/477/EU. Official Journal of the European Union L. 125 (43), 32
EUSAIR (2014). Communication from the commission to the European parliament, the council, the European economic and social committee and the committee of the regions (Brussels:European Commission). 17.6.2014 SWD, 190 final.
EUSAIR (2021). Analysis of marine (water) protected areas in EUSAIR and proposals for corrective measures. (Ljubljana, Slovenia:Government Office of the Republic of Slovenia for Development and European Cohesion Policy). 77.
Fanizza C., Dimatteo S., Pollazzon V., Prunella V., Carlucci R. (2014). An update of cetaceans occurrence in the gulf of taranto (Western-central Mediterranean Sea). Biol. Mar. Mediterr. 21 (1), 373–374.
Ferreira J. C., Vasconcelos L., Monteiro R., Silva F. Z., Duarte C. M., Ferreira F. (2021). Ocean literacy to promote sustainable development goals and agenda 2030 in coastal communities. Educ. Sci. 11, 62. doi: 10.3390/educsci11020062
Foley M. M., Halpern B. S., Micheli F., Armsby M. H., Caldwell M. R., Crain C. M., et al. (2010). Guiding ecological principles for marine spatial planning. Mar. Pol. 34 (5), 955–966. doi: 10.1016/j.marpol.2010.02.001
Game E. T., Grantham H. S., Hobday A. J., Pressey R. L., Lombard A. T., Beckley L. E., et al. (2010). Pelagic MPAs: the devil you know. Trends Ecol. Evol. 25, 63–64. doi: 10.1016/j.tree.2009.09.002
Gerritsen H., Lordan C. (2011). Integrating vessel monitoring systems (VMS) data with daily catch data from logbooks to explore the spatial distribution of catch and effort at high resolution. ICES J. Mar. Sci. 68, 245–252. doi: 10.1093/icesjms/fsq137
Grip K., Blomqvist S. (2020). Marine nature conservation and conflicts with fisheries. Ambio 49, 1328–1340. doi: 10.1007/s13280-019-01279-7
Guidetti P., Notarbartolo-Di-Sciara G., Agardy T. (2013). Integrating pelagic and coastal MPAs into large-scale ecosystem-wide management. Aquat. Conserv: Mar. Freshw. Ecosyst. 23, 179–182. doi: 10.1002/aqc.2314
Guzman H. M., Hinojosa N., Kaiser S. (2020). Ship’s compliance with a traffic separation scheme and speed limit in the gulf of Panama and implications for the risk to humpback whales. Mar. Policy 120, 104113. doi: 10.1016/j.marpol.2020.104113
Hammer Ø., Harper D. A. T., Ryan P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 4–9.
Hammerschlag N., Schmitz O. J., Flecker A. S., Lafferty K. D., Sih A., Atwood T. B., et al. (2019). Ecosystem function and services of aquatic predators in the anthropocene. Trends Ecol. Evol. 34 (4), 369–383. doi: 10.1016/j.tree.2019.01.005
Heck N., Dearden P., McDonald A. (2011). Stakeholders’ expectations towards a proposed marine protected area: a multi-criteria analysis of MPA performance criteria. Ocean Coast. Manage. 54 (9), 687–695. doi: 10.1016/j.ocecoaman.2011.07.003
Holmlund C. M., Hammer M. (1999). Ecosystem services generated by fish populations. Ecol. Econ. 29, 253–268. doi: 10.1016/S0921-8009(99)00015-4
Hooker S. K., Cañadas A., Hyrenbach K. D., Corrigan C., Polovina J. J., Reeves R. R. (2011). Making protected area networks effective for marine top predators. Endang. Species Res. 13, 203–218. doi: 10.3354/esr00322
Hoyt E., Notarbartolo di Sciara G. (2021). Important marine mammal areas: a spatial tool for marine mammal conservation. Oryx 55, 330. doi: 10.1017/S0030605321000272
IUCN Marine Mammal Protected Areas Task Force (2018). Guidance on the use of selection criteria for the identification of important marine mammal areas (IMMAs). version. 82.
Jusufovski D., Saavedra C., Kuparinen A. (2019). Competition between marine mammals and fisheries in contemporary harvested marine ecosystems. Mar. Ecol. Prog. Ser. 627, 207–232. doi: 10.3354/meps13068
Kaplan D. M., Chassot E., Gruss A., Fonteneau A. (2010). Pelagic MPAs: the devil is in the details. Trends Ecol. Evol. 25, 62–63. doi: 10.1016/j.tree.2009.09.003
Kaschner K., Pauly D. (2005). ““Competition between marine mammals and fisheries: Food for thought”,” in The state of the animals III, eds. D.J. Salem and A.N. Rowan (Washington, DC: Humane Society Press), 95–117.
Kiszka J. J., Woodstock M. S., Heithaus M. R. (2022). Functional roles and ecological importance of small cetaceans in aquatic ecosystems. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.803173
Maglietta R., Carlucci R., Fanizza C., Dimauro G. (2022). Machine learning and image processing methods for cetacean photo identification: a systematic review. IEEE Access 10, 80195–80207. doi: 10.1109/ACCESS.2022.3195218
Maglietta R., Renò V., Caccioppoli R., Seller E., Bellomo S., Santacesaria F. C., et al. (2020). Convolutional neural networks for risso’s dolphins identification. IEEE Access 8, 80195–80206. doi: 10.1109/ACCESS.2020.2990427
Maglietta R., Renò V., Cipriano G., Fanizza C., Milella A., Stella E., et al. (2018). DolFin: an innovative digital platform for studying risso’s dolphins from the northern Ionian Sea (Central-Eastern Mediterranean). Sci. Rep. 8, 17185. doi: 10.1038/s41598-018-35492-3
Maiorano P., Capezzuto F., Carluccio A., Calculli C., Cipriano G., Carlucci R., et al. (2022). Food from the depths of the Mediterranean: The role of habitats, changes in the Sea-bottom temperature and fishing pressure. Foods 11, 1420. doi: 10.3390/foods11101420
Maiorano P., Sabatella R. F., Marzocchi B. M. (2019). Annuario sullo stato delle risorse e sulle strutture produttive dei mari italiani. Biol. Mar. Mediterranea 22 (1 Suppl.), 358.
Maiorano P., Sion L., Carlucci R., Capezzuto F., Giove A., Costantino G., et al. (2010). The demersal faunal assemblage of the north-Western Ionian Sea (Central mediterranean): present knowledge and perspectives. Chem. Ecol. 26 (1), 219–240. doi: 10.1080/02757541003693987
Manea E., Bianchelli S., Fanelli E., Danovaro R., Gissi E. (2020). Towards an ecosystem-based marine spatial planning in the deep Mediterranean Sea. Sci. Total Environ. 715, 1–15. doi: 10.1016/j.scitotenv.2020.136884
Mazzoldi C., Bearzi G., Brito C., Carvalho I., Desiderà E., Endrizzi L., et al. (2019). From sea monsters to charismatic megafauna: changes in perception and use of large marine animals. PloS One 14 (12), e0226810. doi: 10.1371/journal.pone.0226810
McDonald J. H. (2014). Handbook of biological statistics. 3rd edition (Baltimore, Maryland, U.S.A: Sparky House Publishing).
Notarbartolo di Sciara G. (2002). Cetaceans of the Mediterranean and black seas: State of knowledge and conservation strategies. a report to the ACCOBAMS secretariat (Monaco: ACCOBAMS) Vol. Section 1, 5.
Notarbartolo di Sciara G., Hoyt E., Reeves R., Ardron J., Marsh H., Vongraven D., et al. (2016). Place-based approaches to marine mammal conservation. Aquat. Conserv. 26, 85–100. doi: 10.1002/aqc.2642
Pace D. S., Tizzi R., Mussi B. (2015). Cetaceans value and conservation in the Mediterranean Sea. J. Biodivers. Endanger. Species S1, S1004. doi: 10.4172/2332-2543.S1.004
Papale E., Fanizza C., Buscaino G., Maria C., Cipriano G., Crugliano R., et al. (2020). The social role of vocal complexity in striped dolphins. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.584301
Pennino M. G., Arcangeli A., Prado Fonseca V., Campana I., Pierce G. J., Rotta A., et al. (2017). A spatially explicit risk assessment approach: Cetaceans and marine traffic in the pelagos sanctuary (Mediterranean Sea). PloS One 12 (6), e0179686. doi: 10.1371/journal.pone.0179686
Pérez-Ruzafa A., García-Charton J. A., Marcos C. (2017). North East Atlantic vs. Mediterranean marine protected areas as fisheries management tool. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00245
Podestà M., Azzellino A., Cañadas A., Frantzis A., Moulins A., Rosso M. P., et al. (2016). ““Cuvier's beaked whale, ziphius cavirostris, distribution and occurrence in the Mediterranean Sea: high-use areas and conservation threats”,” in Mediterranean Marine mammal ecology and conservation, vol. 75 . Eds. Notarbartolo di Sciara G., Podestà M., Curry B. E. (Amsterdam: Elsevier), 103–140.
Pope K. L., Pegg M. A., Cole N. W., Siddons S. F., Fedele A. D., Harmon B. S., et al. (2016). Fishing for ecosystem services. J. Environ. Manage. 183, 408–417. doi: 10.1016/j.jenvman.2016.04.024
Powers J. E., Abeare S. M. (2009). Fishing effort redistribution in response to area closures. Fish. Res. 99, 216–225. doi: 10.1016/j.fishres.2009.06.011
Quijano Quiñones D. R., López-Rocha J. A., Hernández-Herrera I., Torres-Irineo E. (2021). Spatial dynamics modeling of small-scale fishing fleets with a random walk approach. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.669112
Renó V., Dimauro G., Labate G., Stella E., Fanizza C., Cipriano G., et al. (2019). A SIFT-based software system for the photo-identification of the risso’s dolphin. Ecol. Inform. 50, 95–101. doi: 10.1016/j.ecoinf.2019.01.006
Ricci P., Carlucci R., Capezzuto F., Carluccio A., Cipriano G., D’Onghia G., et al. (2022). Contribution of intermediate and high trophic level species to benthic-pelagic coupling: insights from modelling analysis. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.887464
Ricci P., Ingrosso M., Carlucci R., Fanizza C., Maglietta R., Santacesaria F. C., et al. (2020a). “Quantifying the dolphins-fishery competition in the gulf of taranto (Northern Ionian Sea, central Mediterranean Sea),” in Proceedings IMEKO Tc-19 metrology for the Sea(Naples, Italy:IMEKO).
Ricci P., Ingrosso M., Cipriano G., Fanizza C., Maglietta R., Renò V., et al. (2020b). “Top down cascading effects driven by the odontocetes in the gulf of taranto (Northern Ionian Sea, central Mediterranean Sea),” in Proceedings IMEKO Tc-19 metrology for the Sea(Naples, Italy:IMEKO).
Ricci P., Libralato S., Capezzuto F., D’Onghia G., Maiorano P., Sion L., et al. (2019). Ecosystem functioning of two marine food webs in the north-Western Ionian Sea (Central Mediterranean Sea). Ecol. Evol. 9 (18), 10198–10212. doi: 10.1002/ece3.5527
Ricci P., Manea E., Cipriano G., Cascione D., D’Onghia G., Ingrosso M., et al. (2021a). Addressing cetacean–fishery interactions to inform a deep-sea ecosystem-based management in the gulf of taranto (Northern Ionian Sea, central Mediterranean Sea). J. Mar. Sci. Eng. 9, 872. doi: 10.3390/jmse9080872
Ricci P., Sion L., Capezzuto F., Cipriano G., D'Onghia G., Libralato S. (2021b). Modelling the trophic roles of the demersal Chondrichthyes in the northern Ionian Sea (Central Mediterranean Sea). Ecol. Modell. 444, 109468. doi: 10.1016/j.ecolmodel.2021.109468
Roman J., Estes J. A., Morissette L., Smith G., Costa D., McCarthy J., et al. (2014). Whales as marine ecosystem engineers. Front. Ecol. Environ. 12, 377–385. doi: 10.1890/130220
Rossi S., Gabbianelli G. (1978). Geomorfologia del golfo di taranto. Boll. Soc Geol. Ital. 97 (4), 423–437.
Russo T., Bitetto E., Carbonara P., Carlucci R., D'Andrea L., Facchini M. T., et al. (2017). A holistic approach to fishery management: evidence and insights from a central Mediterranean case study (Western Ionian Sea). Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00193
Russo T., Catucci E., Franceschini S., Labanchi L., Libralato S., Sabatella E. C., et al. (2022). Defend as you can, react quickly: the effects of the COVID-19 shock on a large fishery of the Mediterranean Sea. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.824857
Russo T., D’Andrea L., Franceschini S., Accadia P., Cucco A., Garofalo G., et al. (2019). Simulating the effects of alternative management measures of trawl fisheries in the central Mediterranean Sea: Application of a multi-species bio-economic modeling approach. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00542
Russo T., Morello E. B., Parisi A., Scarcella G., Angelini S., Labanchi L., et al. (2018). A model combining landings and VMS data to estimate landings by fishing ground and harbor. Fish. Res. 199, 218–230. doi: 10.1016/j.fishres.2017.11.002
Russo T., Parisi A., Garofalo G., Gristina M., Cataudella S., Fiorentino F. (2014). SMART: A spatially explicit bio-economic model for assessing and managing demersal fisheries, with an application to Italian trawlers in the strait of Sicily. PloS One 9 (1), e86222. doi: 10.1371/journal.pone.0086222
Sánchez Lizaso J. L., Sola I., Guijarro-García E., Bellido J. M., Franquesa R. (2020). A new management framework for western Mediterranean demersal fisheries. Mar. Policy 112, 103772. doi: 10.1016/J.MARPOL.2019.103772
Santacesaria F. C., Bellomo S., Fanizza C., Maglietta R., Renò V., Cipriano G., et al. (2019). Long-term residency of Tursiops truncatus in the Gulf of Taranto (Northern Ionian Sea, Central-eastern Mediterranean Sea). Genoa Italy 2019, 28–32.
Sergio F., Caro T., Brown D., Clucas B., Hunter J., Ketchum J., et al. (2008). Top predators as conservation tools: ecological rationale, assumptions, and efficacy. Annu. Rev. Ecol. Evol. 39, 1–19. doi: 10.1146/annurev.ecolsys.39.110707.173545
Shapiro S. S., Wilk M. B. (1965). An analysis of variance test for normality (complete samples). Biometrika 52, 591–611. doi: 10.1093/biomet/52.3-4.591
Stelzenmüller V., Maynou F., Martin P. (2007). Spatial assessment of benefits of a coastal Mediterranean marine protected area. Biol. Conserv. 136, 571–583. doi: 10.1016/j.biocon.2007.01.002
Sumaila U. R., Zeller D., Watson R., Alder J., Pauly D. (2007). Potential costs and benefits of marine reserves in the high seas. Mar. Ecol. Prog. Ser. 345, 305–310. doi: 10.3354/meps07065
Tetley M. J., Braulik G. T., Lanfredi C., Minton G., Panigada S., Politi E., et al. (2022). The important marine mammal area network: A tool for systematic spatial planning in response to the marine mammal habitat conservation crisis. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.841789
Tromeur E., Loeuille N. (2017). Balancing yield with resilience and conservation objectives in harvested predator–prey communities. Oikos 126 (12), 1780–1789. doi: 10.1111/oik.03985
Keywords: SMART model, fishing effort, fishing production, conservation, MPA, dolphins and whales
Citation: Carlucci R, Cipriano G, Cascione D, Ingrosso M, Russo T, Sbrana A, Fanizza C and Ricci P (2022) Application of a multi-species bio-economic modelling approach to explore fishing traits within eligible cetacean conservation areas in the Northern Ionian Sea (Central Mediterranean Sea). Front. Mar. Sci. 9:1005649. doi: 10.3389/fmars.2022.1005649
Received: 28 July 2022; Accepted: 27 September 2022;
Published: 17 October 2022.
Edited by:
Ibon Galparsoro, Technological Center Expert in Marine and Food Innovation (AZTI), SpainReviewed by:
Stefanos G. Kavadas, Hellenic Centre for Marine Research (HCMR), GreeceCopyright © 2022 Carlucci, Cipriano, Cascione, Ingrosso, Russo, Sbrana, Fanizza and Ricci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulia Cipriano, Z2l1bGlhLmNpcHJpYW5vQHVuaWJhLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.