- 1School of Ecology and Nature Conservation, Beijing Forestry University, Beijing, China
- 2Center for East Asian-Australasian Flyway Studies, Beijing Forestry University, Beijing, China
- 3Rudi Drent Chair in Global Flyway Ecology, Conservation Ecology Group, Groningen Institute for Evolutionary Life Sciences (GELIFES), University of Groningen, Groningen, Netherlands
- 4Department of Coastal Systems, Netherlands Institute for Sea Research (NIOZ) Royal Netherlands Institute for Sea Research, Texel, Netherlands
- 5Global Flyway Network, Broome, WA, Australia
- 6Shenzhen Mangrove Wetlands Conservation Foundation, Shenzhen, China
- 7Zhanjiang Bird Watching Society, Zhanjiang, China
- 8Guangximen Beili, Beijing, China
Leizhou Bay in Guangdong Province is the most important wintering site in China for the critically endangered Spoon-billed Sandpipers (Calidris pygmaea). As food is usually a strong predictor of presence, in the winters of 2019-2022 we studied arthropod food resources and diet on the intertidal mudflats at the Tujiao and Hebei mudflats in Leizhou Bay. In December 2020, using a sampling device that encloses mobile epibenthic prey before the human sampler would disturb them in shallow pools, we visited 34 stations in their core foraging area at Tujiao. A total of 15 mobile benthic species were identified, including 13 arthropod and 2 fish species, with a total density of 106 animals/m2 (range= 0.2-48 animals/m2), with the lengths of the animals ranging from 1-19 mm. Two amphipod and one cumacean species contributed 85%. On the basis of photographs of foraging during low tide in 2019-2022, the visibly ingested prey items appeared to mainly consist of small shrimp, but also included crabs and fish. At 27 mm (compared with the 22 mm long bill of Spoon-billed Sandpipers) the average visibly ingested prey showed a strong size bias. Among the measured environmental covarying factors (sediment pH, salinity, TOC content, median particle size and distance from the seawall etc.) potentiually affecting the mobile epibenthic prey in shallow pools, only distance from the seawall was significantly and negatively correlated. Densities were higher within 1 km of the seawall (126 animals/m2) than further offshore (69 animals/m2). This may relate to the mangrove forests growing in abundance near the seawall providing released minerals, nutrients, bacterial production and diatoms for the benthic community in the adjacent mudflats. However, the potential negative impact of artificial mangrove expansion in Leizhou mudflats need to be carefully monitored and assessed to balance both mangrove and Spoon-billed Sandpipers conservation.
Introduction
Animals that do not eat will die, so for good reasons, studies of food and foraging define to a considerable extent migration ecology, of which diet and food resources are an important part (Ma et al., 2005; Piersma, 2007). Among all the parameters determine habitat selection of a particular species, energy management on the basis of diet and food availability is the most crucial to understand (Piersma, 2012). Shorebirds are migratory birds that feed mainly on bottom-dwelling invertebrates (benthos) in wetlands including coastal intertidal mudflats (van de Kam et al., 2004). The distribution of shorebirds during the non-breeding season is largely a function of available food resources (Goss-Custard, 1977; Bryant, 1979; Zwarts and Wanink, 1993; Ribeiro et al., 2004); densities of shorebirds are often correlated with the quantity and quality of available macrobenthos (Boettcher et al., 1995; Butler et al., 2001; Placyk and Harrington, 2004; van Gils et al., 2005; Nuka et al., 2005; Folmer et al., 2010; Folmer and Piersma, 2012; Folmer et al., 2012; Zharikov and Skilleter, 2016). Therefore, the status of benthos resources is an important aspect to consider in the conservation of a particular species (Piersma et al., 2001; Kraan et al., 2009).
The East Asia-Australasian Flyway is home to the largest number of shorebird species in the world, with stretches of coastal mudflats supporting huge numbers of shorebirds through the non-breeding season (Stroud et al., 2006; Bamford et al., 2008). A wide range of food resources is available in these intertidal mudflats, including a variety of bivalves, gastropods, polychaetes, arthropods, and small fish (Zhao, 2001; van de Kam et al., 2004). Studies on the food choices and intake rates of shorebirds such as Red Knots (Calidris canutus), Great Knots (Calidris tenuirostris), and Bar-tailed Godwits (Limosa lapponica) have revealed that these birds, which migrate over long distances with only a few staging sites, display certain food preferences during their time in the Yellow Sea Ecoregion, and intertidal mudflats with high-quality or high-density benthos are their preferred staging sites (Yang, 2012; Choi, 2015; Zhang et al., 2019). The history of long-term monitoring of food resources in important non-breeding areas in the East Asia-Australasian Flyway is not long, but it provided essential information for the conservation of shorebirds and their habitats (Choi et al., 2014; Yang et al., 2016; Zhang et al., 2018).
The Spoon-billed Sandpiper (Calidris pygmaea), which only occurs in the East Asia-Australasian Flyway, is one of the world’s most critically endangered species with very low and declining numbers (Zöckler et al., 2010; Amano et al., 2012; Aung et al., 2018). The latest estimate of the Spoon-billed Sandpiper population was 490 mature individuals or 773 individuals of all ages (Green et al., 2021). Therefore, conservation of this endangered species and its habitats is currently one of the most important tasks for biodiversity conservation in the East Asia-Australasian Flyway.
Spoon-billed Sandpipers breed mainly on the coast of the Chukotsk Peninsula in the Russian Arctic (Flint et al., 1977; Tomkovich et al., 2002). The wintering areas of this bird range from southern China, with stable populations in the coastal areas of Fujian, Guangdong, Guangxi, and Hainan provinces, to southeastern and southern Asia, including Vietnam, Thailand, Myanmar, and Bangladesh. Among them, the Gulf of Mottama in Myanmar is home to 60% of the global population of Spoon-billed Sandpipers (Bird et al., 2010; Zöckler et al., 2010; Chowdhury, 2011; BirdLife International, 2016; Zöckler et al., 2016; Chowdhury et al., 2017; SBS Conservation Alliance, 2022). Based on wintering population surveys conducted over the past three years, the four most important wintering grounds of Spoon-billed Sandpipers include the Gulf of Mottama, Leizhou Bay in China, Nanthar Island in Myanmar, and Sonadia Island in Bangladesh (Tables 1, 2) (EAAFP SBS Task Force, prep.; SBS Conservation Alliance, 2022).

Table 1 The wintering population and the proportion to the global population of Spoon-billed Sandpipers in the top four important wintering areas in the world*.
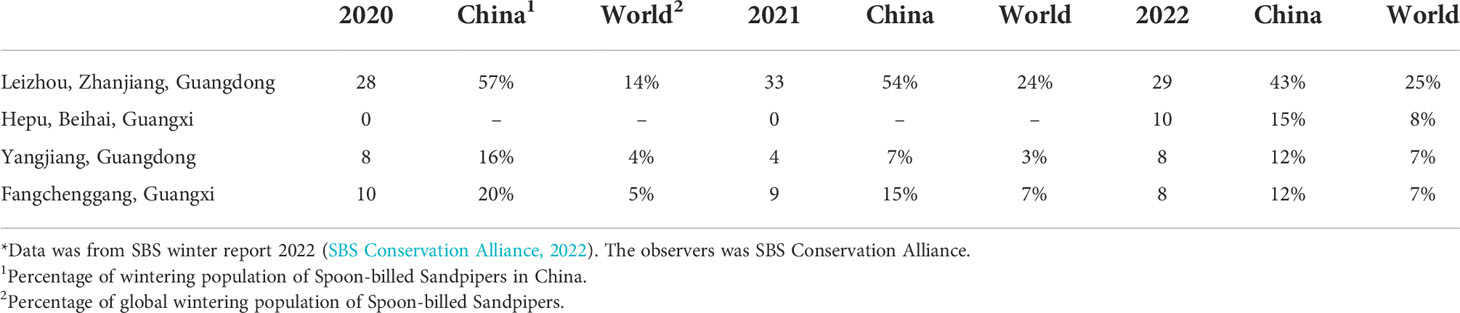
Table 2 Wintering population of Spoon-billed Sandpipers in the top four important wintering sites in southern China*.
Due to the rarity of Spoon-billed Sandpipers and difficulty in monitoring individual prey catches, few studies have reported their diet and the status of food resources in their critical habitats (Dixon, 1918; Portenko, 1957; Portenko, 1968; Voronov, 1980; Portenko, 1981; Cha and Young, 1990; Kelly et al., 2017; Aung et al., 2022; Yang, 2022). Diet descriptions mention crustaceans, small fish, polychaetes, mollusks, and insects (Dixon, 1918; Portenko, 1957; Portenko, 1968; Voronov, 1980; Portenko, 1981; Cha and Young, 1990; Kelly et al., 2017). Although Spoon-billed Sandpipers forage in a variety of substrate types, they prefer mixed sand and mud substrates and often forage in small shallow pools remaining after the ebbing tide, which contain crabs, shrimp, amphipods, and polychaetes (Burton, 1971; Piersma, 1986; Bird et al., 2010; Chowdhury et al., 2017; Kelly et al., 2017; Aung et al., 2022). Previous studies investigating feeding behavior reported that foraging Spoon-billed Sandpipers do not deeply probed the sediment (Piersma, 1986; Swennen and Marteijn, 1988; Kelly et al., 2017; Aung et al., 2022; L Cheng 2022, personal observation; DM Li 2022, personal communication), implying that their main food is not mollusks or sandworms buried within the sediment. In southern Jiangsu, China, which is the globally most important staging site for Spoon-billed Sandpipers, reported that foraging birds show a significant preference for areas with high amphipod densities in areas with abundant tide pools in which amphipods congregate at low tide (Shepherd, 1995; Drolet and Barbeau, 2009; Yang, 2022). Therefore, shallow tidal pools are key foraging habitat for Spoon-billed Sandpipers and the mobile epibenthos (benthos live on the surface of the mudflats) living in it are the known food resource.
Leizhou Bay in Guangdong Province is the most important wintering area for Spoon-billed Sandpipers in China (SBS Conservation Alliance, 2022). At low tide, the intertidal mudflats are widely covered with shallow pools (Figure 1), and Spoon-billed Sandpipers have been observed to feed here on crabs (Kelly et al., 2017). Here we set out assessing food abundance of Spoon-billed Sandpipers in this area as well as providing a very preliminary description of diet. The food resources were explored by sampling macrobenthos in shallow pools at 34 sampling stations using sampling devices and methods specifically designed to target mobile epibenthos, e.g., arthropods and small fish. Meanwhile, the sediment characteristics were measured to explore the correlations with potential prey densities. To describe the diet we photographed food items held in the bill of Spoon-billed Sandpipers.
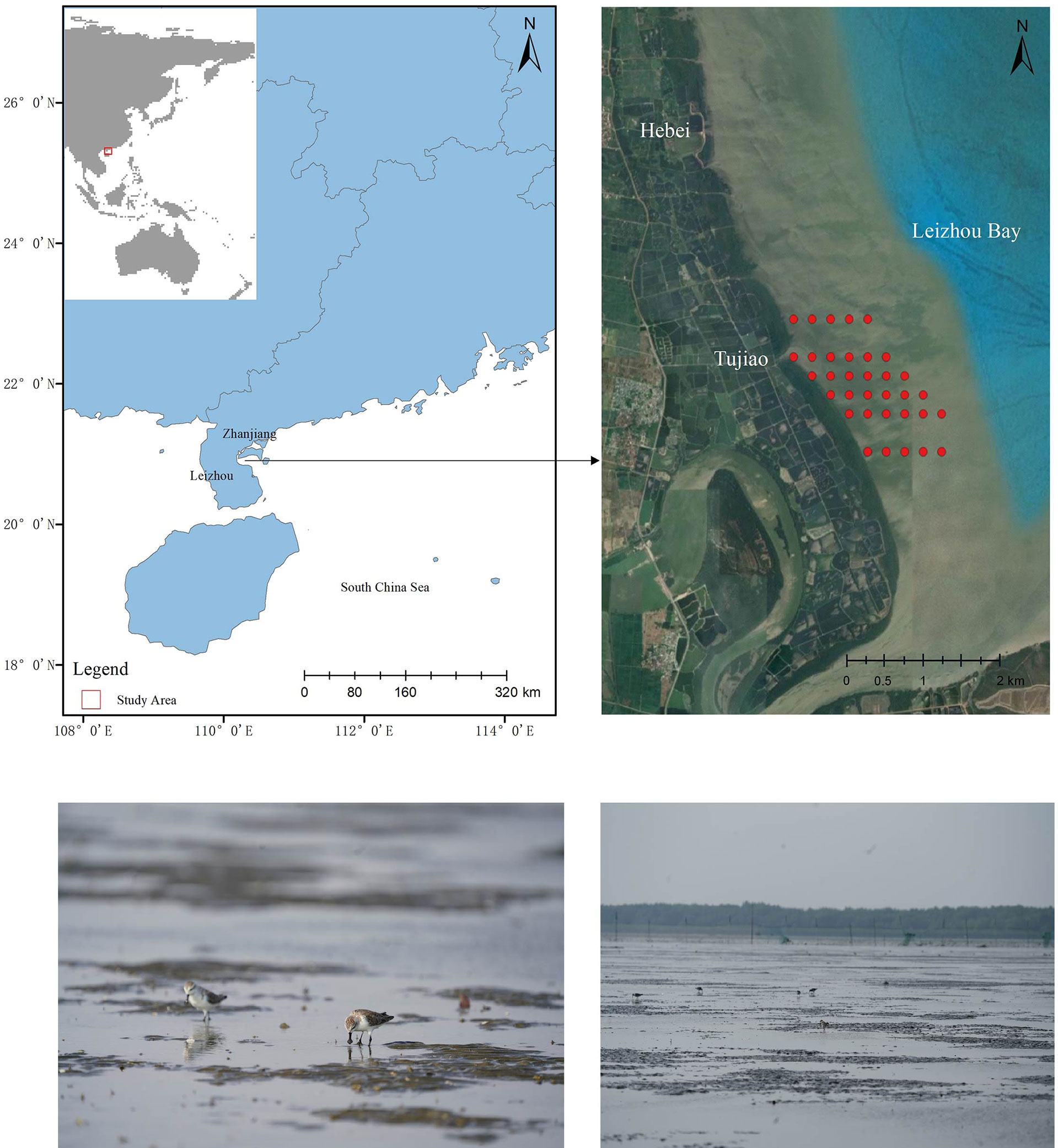
Figure 1 Map (above) and landscape (below) of the study sites situated in Leizhou Bay in the South China Sea: Tujiao and Hebei. Benthos and sediment sampling stations along transects in the intertidal mudflats are shown.
Materials and methods
Study area
The study area was located in the Guangdong Zhanjiang Mangrove National Nature Reserve (20°51’-55’N, 110°10’-12’E) (Figure 1), which is at the southernmost tip of mainland China and divided into over 40 patches, which are scattered in bands along the coastal intertidal zone on both sides of Leizhou Bay on the Leizhou Peninsula, Guangdong Province (Chen and Chen, 2012). The Leizhou Peninsula is the third largest peninsula in China and has a northern tropical marine monsoon climate with approximately 2,000 h of sunshine annually and no frost or snow cover in ‘winter’. The average annual temperature is 22°C, with an average temperature of 15°C in January and 29°C in August. The dry and wet seasons are distinct, with an average annual rainfall of 1100-1800 mm concentrated in the rainy season from May to September, with the most rainfall occurring in the coastal summer when typhoons are frequent (Agriculture Office of Guangdong Provincial People’s Government, 1996). Most of the tidal flats on the Leizhou Peninsula are sandy, and only the estuaries and inner bays have muddy and sandy substrates. The east coast of the peninsula experiences an irregular semidiurnal tide, while the west coast experiences a regular diurnal tide. The annual average tidal range is 1.77 m, with an annual average maximum tidal range of 3.52 m (Huang and Ye, 1995).
Leizhou Bay, located on the east side of the Leizhou Peninsula, is 50 km wide and 75 km length, containing extensive intertidal mudflats along the coast. It is the largest natural bay on the Leizhou Peninsula, with 22 internal rivers in the bay and 17 rivers flowing into the sea. The Nandu River is the largest river on the Leizhou Peninsula with a total length of 65 km and has the largest downstream coastal alluvial plain. The alluvial coast at the estuary is formed by silt accumulation and contains a concentrated mangrove area (Guangdong Zhanjiang Mangrove National Nature Reserve Administration (GZMNNRA), prep. 1 and prep. 2; Jin et al., 1992).
The study area included the mudflats in Tujiao and Hebei villages, located in the Fucheng (town) section of the reserve on the west side of Leizhou Bay. The sediment was muddy to sandy and influenced by the Nandu River, with sandy mudflats on the offshore side and a small slope. A dense mangrove belt is distributed near the seawall. In recent years, patches of mangrove have become established in the mudflats. Additionally, an invasive species of cordgrass, Spartina alterniflora, has expanded its range in the mudflats outside the mangroves, although most patches have been cleaned up and replaced with mangrove seedlings as a measure of invasive plant control and mangrove protection as well. Aquaculture ponds and returning-aquaculture-pond-to-sea areas are located inside the seawall (Guangdong Zhanjiang Mangrove National Nature Reserve Administration (GZMNNRA), prep. 1 and prep. 2). The mudflats in Tujiao and Hebei are popular feeding grounds in the reserve for the wintering population of Spoon-billed Sandpipers. In mid-December 2020 and 2021, 22 and 25 individuals were recorded in the mudflats of Tujiao, respectively.
Benthos sampling
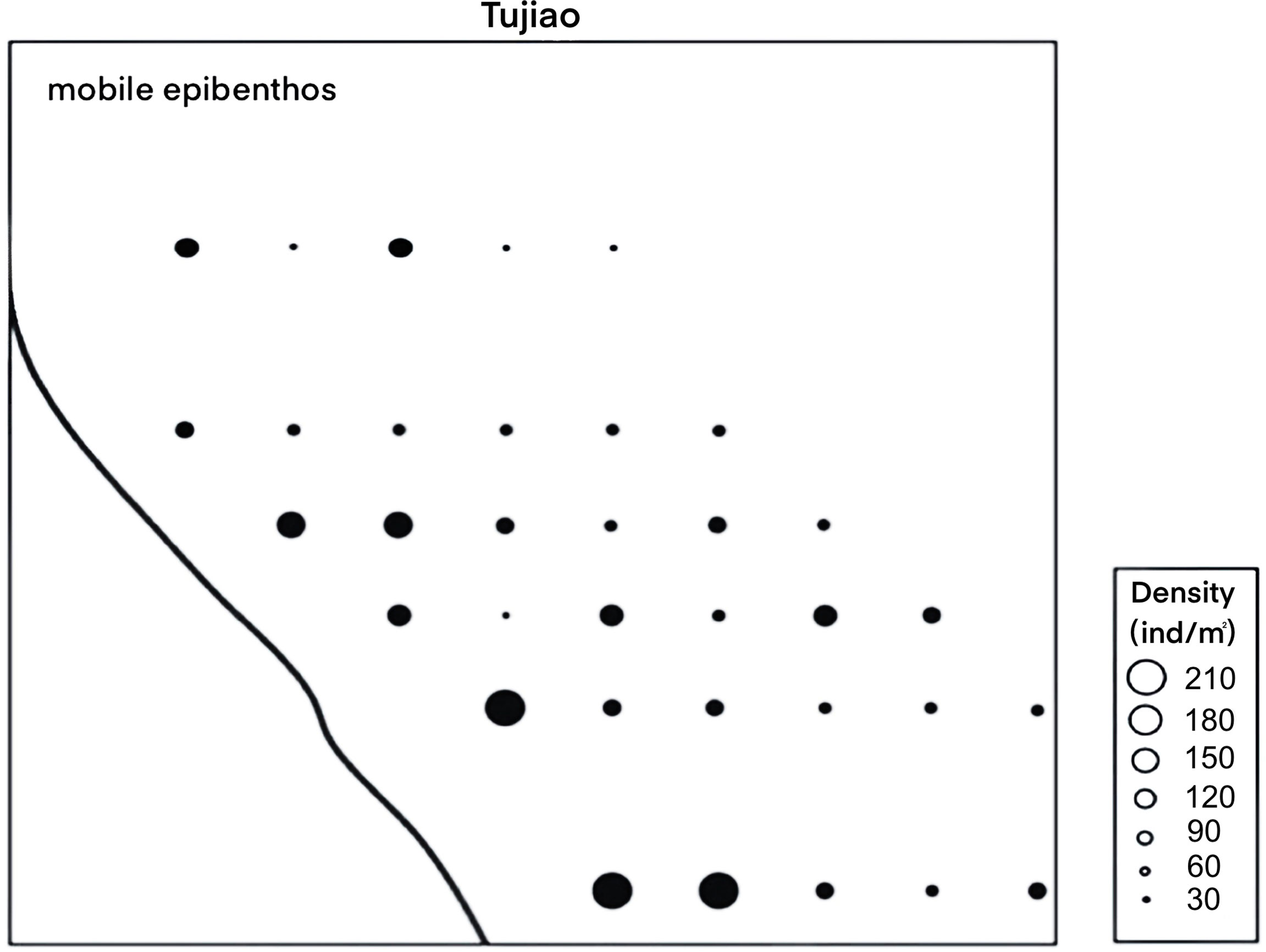
Macrobenthos were sampled in the Tujiao mudflats oncefrom December 15 to 17, 2020. Sampling stations were deployed using the grid sampling method, according to the intertidal width (Figure 1) (Bijleveld et al., 2012). Six transects were set up parallel to the latitudinal lines, with the middle four transects spaced 250 m apart, and two transects at both ends spaced 500 m apart. Grid-like sampling stations spaced 250 m apart were set up along each transect, parallel to the longitudinal lines. A total of 34 stations were sampled from the six transects. The distance between each sampling station and the seawall was determined using Google Earth software based on GPS data.
As Spoon-billed Sandpipers prefer to feed in shallow pools, a shallow pool has been chosen at each station to sample the benthos. To avoid disturbing the mobile epibenthos (arthropods and fish) in shallow pools, a 40 × 40 cm2 metal sampling frame was lifted into the air at a height of 2 m above the ground with a 4-m-long pole, reaching 10 m from the sampling station, and then slowly brought toward the shallow pool. The frame was then pushed into the mudflat surface, and the lower part of the frame was immediately pressed down into the sediment to prevent benthos inside the frame from escaping (Penning et al. MS 1). A 30 cm D-shaped hand net was used to scrape the top 4 cm of sediment from the mudflat inside the sampling frame, while filtering the water inside the frame to capture the benthos within. The contents of the hand net were poured into a 1 mm aperture metal sieve and washed. The benthos remaining in the sieve were placed in labeled self-sealing bags, transported to the laboratory, and stored at -20°C for further analysis.
The collected macrobenthos were identified at the species level, measured for size, and counted using a stereomicroscope (Phoenix Optical Technologies, Shangrao, China). Because polychaetes were not easily counted as they were small and often broken into several segments during collection, they were not included in the analysis.
Estimation of shallow pool area proportion
A photograph of the mudflat surface was taken upon arrival at the sampling station, and the proportion of the shallow pool area to the total area at each station was estimated. This proportion was used to calculate the density of benthos in shallow pools in the sample frame for shrimp, gammarids, hooded shrimp, and fish using the following formula: Di=Ni/(0.16×R), where Di is the density of species i in a sample frame (ind/m2), Ni is the number of species i in the sample frame, R is the proportion of the shallow pool area in the sample station to the total area of the station (%), and 0.16 is the sample frame area (m2).
Sediment analysis
Sediment samples were obtained at 34 sampling stations, although the sample from the most seaward station of the second transect on the north side was lost. Therefore, 33 sediment samples were analyzed. Sediment cores were collected from the surface at a depth of 4 cm using a 2.3 cm diameter sampling tube in the undisturbed area of each station, transported to the laboratory, and stored at -20°C for further analysis. The pH of the sediment was measured using a pH meter (Sartorius AG, Gottingen, Germany) (according to the manufacturer’s instructions). Sediment salinity was determined by measuring the weight of the soluble salts (McAllister, 1958). The average particle size of the sediment and distribution of sediment particles of different diameters were measured using a Mastersizer 2000 laser particle size analyzer (Malvern Panalytical, Worcestershire, UK) (according to the manufacturer’s instructions). The total organic carbon (TOC) concentration in the sediment was measured using a FlashEA 1112 elemental analyzer (Thermo Fisher Scientific, Waltham, MA, USA) (according to the manufacturer’s instructions).
Prey of Spoon-billed Sandpipers
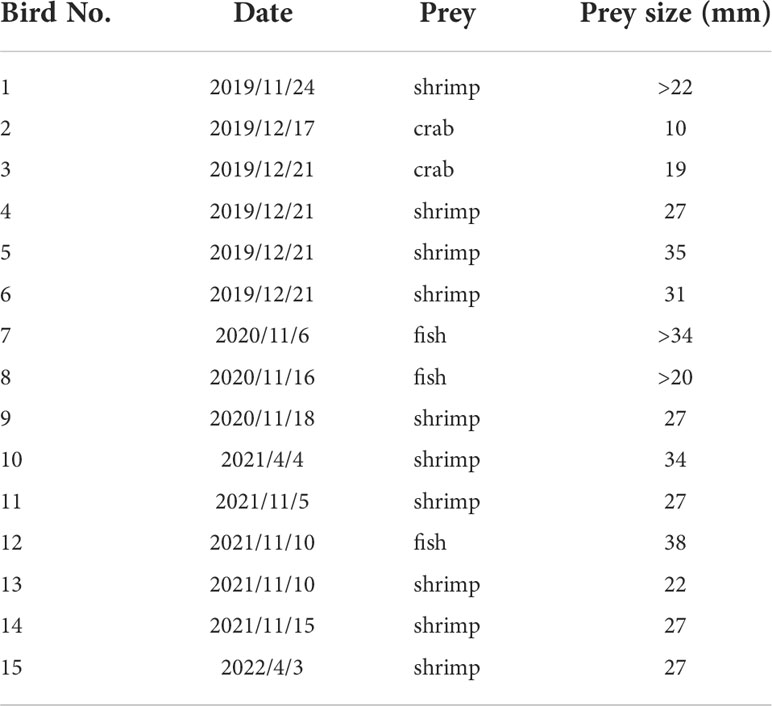
The mudflats in the study area were visited regularly during the overwintering period of Spoon-billed Sandpipers in 2019–2022. The photographer visited the mudflats 30 times in each overwintering period and spent an average of 6 hours to follow the foraging Spoon-billed Sandpipers at each time. Photographs of foraging birds were captured at a suitable distance to avoid disturbance using a Canon 1DX Mark II camera with a Canon EF 600 mm f/4L IS II USM lens (Canon Inc., Tokyo, Japan). Eventually it took a total of 540 hours to catch 15 different Spoon-billed Sandpiper individuals with prey in their bills during the three overwintering periods. Prey were identified from images, and their sizes were estimated by the ratio of the prey to the length of the bird’s bill (22 mm according to Wang et al., 2006). Note that the prey recorded by the photographer were only the big visible ones, so the methods are quiet bias towards big prey with long handling times.
Data analysis
In this study, the sample size was determined to be sufficient to adequately characterize arthropod taxa by plotting a species accumulation curve. The composition of the mobile epibenthos community in the study area was analyzed by combining both density (number of individuals per unit area) and frequency of occurrence (the proportion of samples in which a species occurred among all samples). The dominance of a species was calculated using the following formula: Yi = (Ni/N)×fi, where Yi is the dominance of species i, Ni is the number of species i, N is the total number of mobile epibenthos, and fi is the frequency of occurrence for species i. A species was identified as dominant when Y > 0.02.
Redundancy analysis (RDA) was used to analyze the relationship between arthropod density and environmental factors (sediment median particle size, TOC content, pH, salinity, and distance from the seawall). Stepwise regression was used to analyze the main environmental factors affecting arthropod density. Pearson correlation analysis was used to analyze the relationships between arthropod density and environmental factors, and between the density of dominant species and environmental factors.
Statistical analysis was preformed using SPSS Statistics software and the results were plotted using the R Studio tool in R software (R Core Team, Vienna, Austria).
Results
Sediment characteristics
The mean value of the median particle size of the sediment samples was 64.0 ± 17.6 μm (N = 33) with a median particle size ranging between 19.3-88.3 μm. The composition of the sediment of all samples, from large to small particle size, was 10.7% fine sand (particle size >125 μm and <250 μm), 61.7% very fine sand (particle size >63 μm and <125 μm), 16.3% silt (particle size >4 μm and <63 μm), and 1.3% clay (particle size <4 μm). Therefore, the sediments in the mudflats of Tujiao contained mixed sand and mud substrates. The median particle size did not differ significantly among sediment samples from sampling stations located at different distances from the seawall (Figure 2). However, 11 sampling stations in four transects on the south side of the study area had relatively larger proportions of sediment with a particle size < 2 μm (ranging between 1.1–4.5%) and all sediment samples from these stations had a small median particle size (ranging between 19.3–67.3 μm). The average pH of all sediment samples was 8.2 ± 0.33, the average salinity was 1.21 ± 3.36%, and the average TOC content was 0.21 ± 0.07%. Sediment pH, salinity, and TOC content did not differ significantly at sampling stations located at different distances from the seawall (Figure 2).
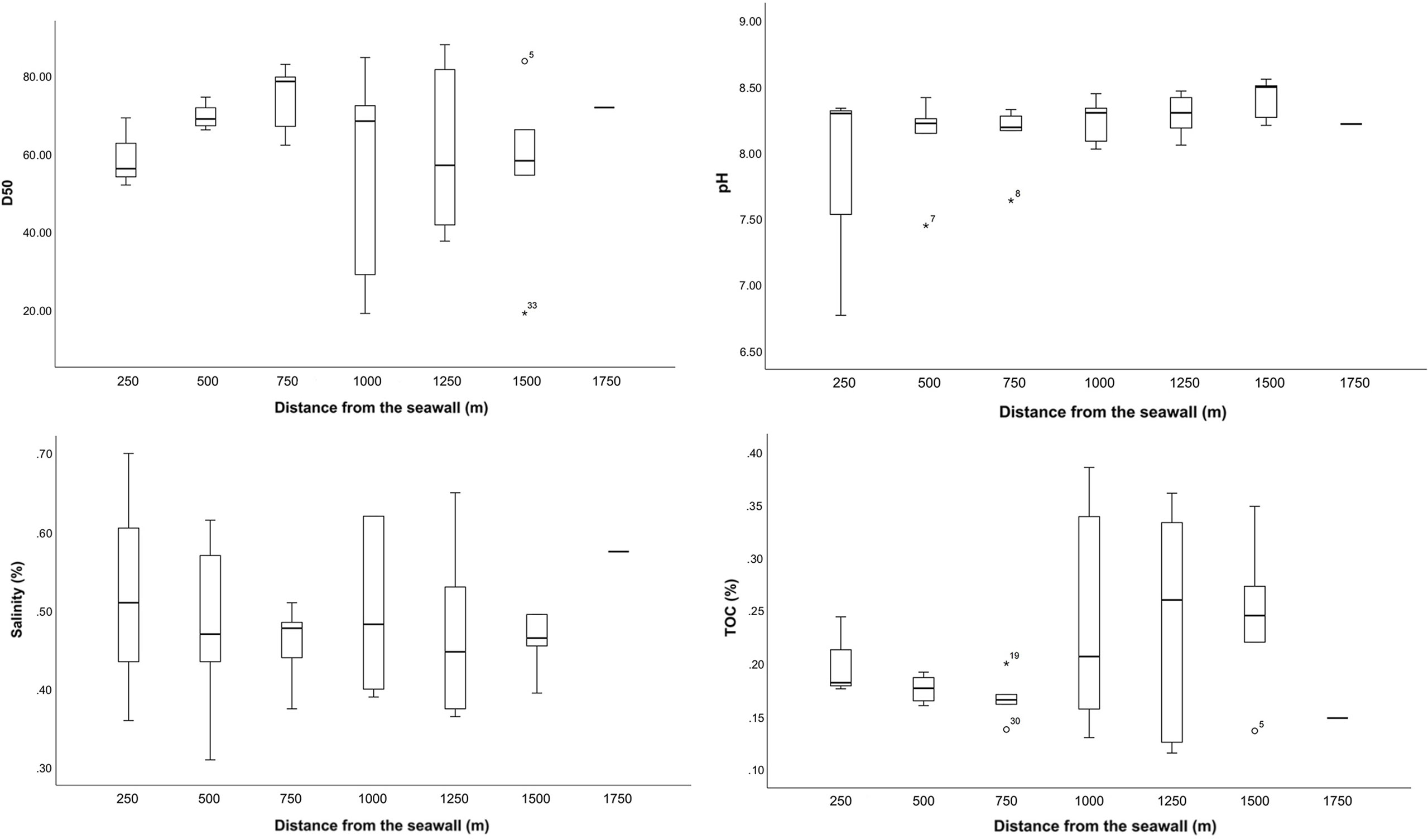
Figure 2 Median particle size (D50), pH, salinity, and total organic carbon (TOC) content of sediment samples. * symbol represents the extreme value in the data.
Food resources
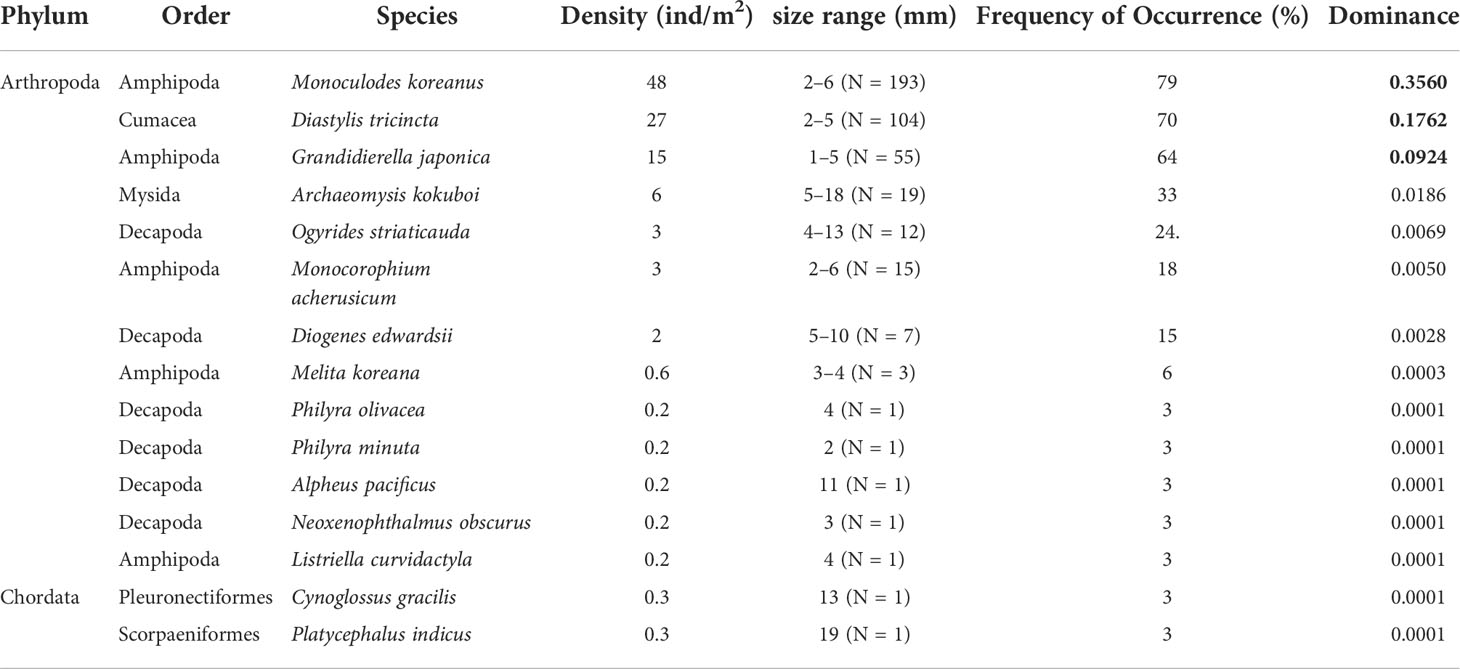
A total of 58 macrobenthic species with an average density of 257 ind/m2 were found in six transects in the Tujiao mudflats of the Leizhou Bay (Table 3). A total of 15 species of mobile epibenthos were recorded, including 13 arthropod species (one species of hooded shrimp, five species of gammarid, one species of opossum shrimp, two species of shrimp, and four species of crab) and two species of fish (Table 4). The species accumulation curves of the dominant arthropod taxa indicated that the sample size used in this study was adequate (Figure 3). The density of mobile epibenthos in shallow pools was 106 ind/m2, accounting for 41% of the total benthos. The average length of each species was 1–7 mm, with a length range of 1–19 mm, which were ingestible for Spoon-billed Sandpipers (Table 4), except for Diogenes edwardsii, a hermit crab that is unlikely to be food for Spoon-billed Sandpipers as it hides in a heavy shell.
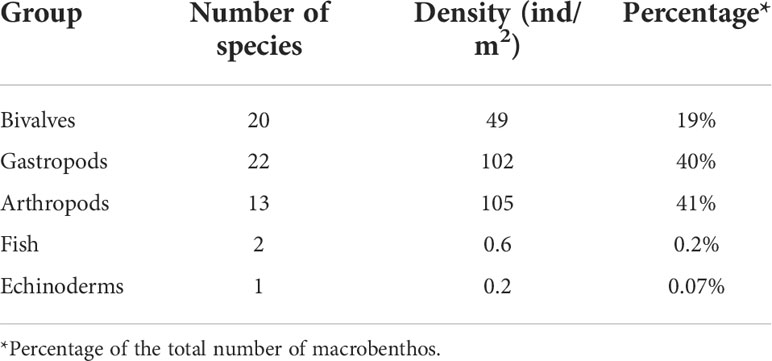
Table 3 Number of species, average density and the percentage of different marobenthic groups in mudflats of Tujiao in December 2020.
Three amphipod and cumacean species (Monoculodes koreanus, Diastylis tricincta, and Grandidierella japonica) were dominant among the mobile epibenthos collected, accounting for 85% of mobile epibenthic animals recorded. Among them, the average density of M. koreanus reached 48 ind/m2 and the species was distributed in 79% of the sampling stations, while the average density of D. tricincta reached 27 ind/m2 and the species was distributed in 70% of the sampling stations, and the average density of G. japonica reached 15 ind/m2 and the species was distributed in 64% of the sampling stations. Other species (shrimp, crabs, and other amphipods) were less abundant and had a relatively small distribution range. Among them, Philyra olivacea, P. minuta, Alpheus pacificus, Neoxenophthalmus obscurus, and Listriella curvidactyla were recorded in only 3% of the sampling stations, while Archaeomysis kokuboi was distributed in 33% of the sampling stations. The density of the two fish species was 0.6 ind/m2 and their frequency of occurrence in the sampling stations was 6%.
The distribution of these mobile epibenthos was heterogeneous in the mudflats (Figure 4) and their density was significantly greater on mudflats within 1 km of the seawall (126 animals/m2) than those beyond 1 km (69 animals/m2), with > 80% of mobile epibenthos distributed on mudflats within 1 km of the seawall.
Prey of Spoon-billed Sandpipers
Photographs of 15 different individual Spoon-billed Sandpipers with clear prey items were obtained (Table 5). Among them, six birds had been previously banded in breeding, migratory, and wintering grounds. The food sources included shrimp (10/15, 66.7%), fish (3/15, 20.0%), and crabs (2/15, 13.3%) (Figure 5, also see Appendix). The average sizes of the shrimp, fish, and crab were 28 mm (N=10), 30 mm (N=3), and 15 mm (N=2), respectively, while the total average prey size was 27 mm (N=15).
Discussion
Food resources
The mixed sand and mud substrates of the Leizhou mudflats are consistent with those in the preferred foraging sites of Spoon-billed Sandpipers in other overwintering areas (Burton, 1971; Bird et al., 2010; Chowdhury et al., 2017; Kelly et al., 2017; Aung et al., 2022). More than half of the mudflat surface in the study area was covered by shallow pools at low tide and the rest of the area was also very wet. Small arthropods were widely and abundantly distributed in the Tujiao mudflats in winter. Interestingly, one small crab species (2-7 mm in length) is abundantly and evenly distributed in the mixed sand and mud substrates of the Gulf of Mottama, the world’s most important wintering site for Spoon-billed Sandpipers (Aung et al., 2022). As the second most important wintering site for Spoon-billed Sandpipers in the world at present, the mudflat shallow pools of Leizhou Bay in winter contained 13 arthropod species (1–19 mm in length) with an average density of 105 ind/m2, which was less dense than the small crabs in the Mottama mudflats in winter (approximately 181 ind/m2), but relatively more dense than the winter benthos of coastal mudflats in four other overwintering areas of southern China (Huang et al., 2002; Lin, 2003; Huang, 2010; Xia, 2015; State Oceanic Administration, 2016; Tang et al., 2019).
Environmental factors affecting arthropod distribution
The RDA results showed that among abiotic factors, median particle size, TOC content, and distance from the seawall had a greater effect on arthropod density, whereas sediment pH and salinity had a smaller effect on arthropod density (Figure 6). Among biotic factors, the presence of bivalves and gastropods had a smaller effect on arthropod density, with bivalves having a slightly greater effect on arthropod density than gastropods. Among environmental variables, median particle size was negatively correlated with sediment pH, salinity, and TOC content as well as distance from the seawall, while the rest of the variables were positively correlated. Spearman’s correlation analysis showed that arthropod density was significantly and negatively correlated with distance from the seawall (p<0.05). Among environmental variables, sediment pH and salinity were significantly and negatively correlated (p<0.05), while TOC content and median particle size were significantly and negatively correlated (p<0.05). Furthermore, sediment pH was significantly and positively correlated with distance from the seawall (p<0.05). Sediment salinity was significantly and positively correlated with TOC content (p<0.05), but significantly and negatively correlated with median particle size (p<0.05). Sediment TOC content was significantly and negatively correlated with median particle size (p<0.05).
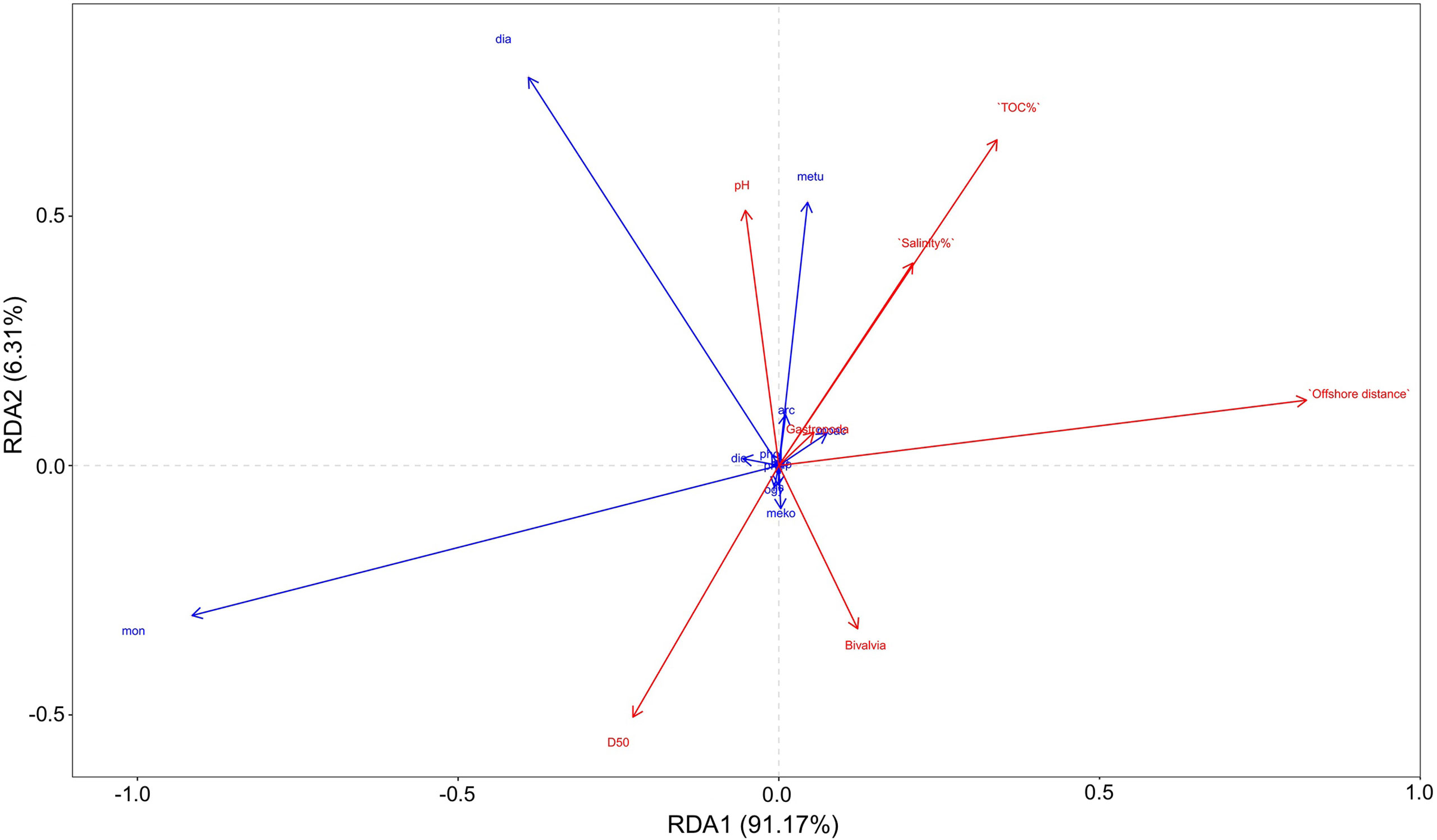
Figure 6 Redundancy analysis results of the relationship between arthropod density and environmental factors.
Among all environmental factors, the results indicated that only distance from the seawall had a significant impact on the distribution of arthropods; that is, the closer to the seawall, the greater the density of arthropods. It is possibly because of the longer exposure of shallow pools near the seawall, resulting in a more adequate food supply (Ysebaert et al., 2005; Choi et al., 2010). Moreover, mangrove forests grow in abundance near the seawall may provide released minerals, nutrients, and bacterial production to support the benthos community in the adjacent mudflats (Wolff et al., 2000; Koch and Wolff, 2002; Henriques et al., 2021; Meijer et al., 2021). Additionally, mangrove forests are rich in diatoms (Reyes-Vasquez, 1975; Du and Jin, 1983; Chen, 2004), which may provide a rich food source for mudflat surface-dwelling arthropods (Leh and Sasekumar, 1985; Marples et al., 1988; Wah and Wee, 1988; Vicente, 1990).
Prey of Spoon-billed Sandpipers
Spoon-billed Sandpipers in the study area fed on shrimp, crabs, and fish, which are all highly mobile benthos and thus the focus of this study. Among them, shrimp was the main food resource. The sizes of all prey in the images were larger than the bill length of Spoon-billed Sandpipers, but less than twice their bill length. Previous researchers have observed Spoon-billed Sandpipers foraging mainly in mud pools or shallow water pools in mudflats, but catching prey was not observed most of the time (Jahn, 1942; Piersma, 1986; Kelly et al., 2017; Aung et al., 2022; Z Liu and DM Li 2022, personal communication). In the current study, Spoon-billed Sandpipers were extensively followed over three winters in the study area, and only occasionally photographed feeding on this larger prey. Based on the total of 540 hours tracking foraging birds and only 15 individual prey caught, the average hours to catch one big prey was 36 hours. Researchers in the Tiaozini mudflats (Yancheng, Jiangsu Province) during migration season and in the Xichang mudflats (Hepu, Beihai, Guangxi Province) during the non-breeding period reported similar findings (Kelly et al., 2017; Z Liu and DM Li 2022, personal communication). Most prey that the above researchers witnessed were also larger shrimp (normally larger than bill length). Therefore, we hypothesized that Spoon-billed Sandpipers likely feed primarily on smaller benthos in shallow pools.
In addition, previous studies have suggested that Spoon-billed Sandpipers and spoonbills (Platalea) share many similarities, such as mouth shape, dense Herbst corpuscles in their bills, and certain specific foraging methods (e.g., open mouth scanning in liquids such as water or pulpy mud, and preferring to scan for food in small groups), and spoonbills are known to feed on animals that escape easily in the water (Burton, 1971; Piersma, 1986; van de Kam et al., 2004; Bird et al., 2010; Kelly et al., 2017). Spoon-billed Sandpipers forage in liquids on the surface of mudflats by sweep-stitching (Kelly et al., 2017; Aung et al., 2022), suggesting that their targets are likely escaping prey living in water or sloppy mud.
In summary, we hypothesize that arthropods living in tidal pools, sloppy mud, and shallow water in mudflats are likely the main prey of Spoon-billed Sandpipers in non-breeding areas in China. The preference of Spoon-billed Sandpipers for feeding grounds with mixed sand and mud substrates is likely because these sediment characteristics help the birds to successfully capture slippery mobile epibenthos, which are best caught by their unique bills (Kelly et al., 2017).
Conservation implications
Degradation, pollution, and human activities at important staging and wintering sites along the migration route are considered to be important factors that negatively affect the population of Spoon-billed Sandpipers (Peng et al., 2017). Since 2018, with the cessation of large-scale coastal reclamation, the invasion of S. alterniflora has gradually become one of the main threats to coastal mudflats in China. The rapid spread of this stubborn plant species has accelerated the degradation and disappearance of intertidal mudflats (Wang et al., 2006; Lin et al., 2015; Okoye et al., 2020; Zeng et al., 2020). Although the expansion of this invasive plant is not as severe in the wintering grounds of Leizhou as in southern Jiangsu, the most important staging area for Spoon-billed Sandpipers in the world, managing S. alterniflora growth in Leizhou and Hepu is imperative (Choi et al., 2022; RJ Sun 2022, personal communication).
In addition, the impact of artificial mangrove expansion on intertidal mudflats in the coastal areas of southern China is another concern for Spoon-billed Sandpiper conservation. The mangroves in the East Asian-Australasian Flyway account for 45.6% of the total global mangrove area (Giri et al., 2011). The dense vegetation of mangroves often acts as a barrier to shorebird foraging, resulting in the loss of suitable habitats (Zwarts, 1988; Choi et al., 2017). The restoration of mangrove forests in many parts of mainland China since the last century has led to a continuous increase in mangrove area, and some important waterbird habitats have been affected, including on the Leizhou Peninsula. Thus, mangroves are likely to have an impact on the largest wintering population of Spoon-billed Sandpipers in China (Choi et al., 2022). The potential negative impact of mangrove restoration on shorebirds needs to be carefully monitored and assessed to balance both mangrove and waterbird conservation, thus controlling the harmful expansion of mangroves when necessary to protect the biodiversity of coastal areas under the premise of diversification.
To date, a paucity of winter benthos data has been reported in important and potential habitats of Spoon-billed Sandpipers in China, and no targeted and systematic investigations have been conducted. We hope that the current study will inspire researchers to further explore the diet and status of food resources of Spoon-billed Sandpipers in critical habitats. The following aspects warrant further research: (1) comprehensive and in-depth analysis using environmental DNA and isotopes to determine the diet of Spoon-billed Sandpipers in various habitats in order to more accurately assess and monitor the quality of their foraging habitats (Penning et al.MS 2); (2) applying the sampling method used in this study to other foraging sites of Spoon-billed Sandpipers to obtain more accurate data on benthos; (3) comprehensive measuring of environmental factors associated with potential food resources of Spoon-billed Sandpipers, especially variables related to mobile epibenthos in shallow pools, in order to identify key factors influencing the potential food resources of Spoon-billed Sandpipers. Acquiring this knowledge will fill a crucial knowledge gap, which is urgently needed to protect important habitats and facilitate the conservation of Spoon-billed Sandpipers (Aung et al., 2022).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
XL: data analysis and article writing. HY: experimental design, benthic and sediment sample collection, benthic sample laboratory processing, data analysis and article writing. TP: article review and edit. LS: article review and edit. QC: article review and edit. YJ: article writing-review and edit, project administration. GL: supervision, article edit. LC: taking photos of Spoon-billed Sandpiper foraging. XR: benthic and sediment sample collection. All authors contributed to the article and approved the submitted version.
Funding
Supported by the “Saving Spoon-billed Sandpiper” of Shenzhen Mangrove Wetlands Conservation Foundation (MCF), and the National Natural Science Foundation of China (No. 31971400).
Acknowledgments
We acknowledge the support for our field research from Guangdong Zhanjiang Mangrove National Nature Reserve Administration, Guangdong Province, China. We thank Tao He and Chi-Yeung Jimmy Choi for their enthusiastic guiding to the study site in Leizhou Bay and sharing the local knowledge. We thank Mengling Liu and Lijun Wang for helping with the identification of benthos specimens. We thank Hebo Peng and Ting Fu for helping draw the map of study sites. We thank Xianghan Liu for helping editing the graphs. The manuscript was improved with help from anonymous reviewers, Jia Guo, Sicheng Ren, Waner Liang. We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agriculture Office of Guangdong Provincial People’s Government (1996). Climate and agriculture in guangdong (Guangzhou: Guangdong Provincial People’s Government).
Amano T., Székely T., Koyama K., Amano H., Sutherland W. J. (2012). A framework for monitoring the status of populations: An example from wader populations in the East Asian-Australasian flyway. Biol. Conserv. 145 (9), 278–295. doi: 10.1016/j.biocon.2011.06.006
Aung P. P., Buchanan G. M., Round P. D., Zockler C., Kelly C., Tantipisanuh N., et al. (2022). Foraging microhabitat selection of spoon-billed sandpiper in the upper gulf of mottama, Myanmar - ScienceDirect. Global Ecol. Conserv. 35, e02077. doi: 10.1016/j.gecco.2022.e02077
Aung P. P., Moses S., Clark N. A., Anderson G. Q. A., Green R. E. (2018). Recent changes in the number of spoon-billed sandpipers Calidris pygmaea wintering on the upper gulf of mottama in Myanmar. Oryx 54 (1), 1–7. doi: 10.1017/S0030605318000698
Bamford M., Watkins D., Bancroft W., Tischler G., Wahl J. (2008). Migartory shorebirds of the East Asian-Australasian flyway: Population estimates and internationally important sites (Canberra, Australia: Wetlands International - Oceania).
Bijleveld A. I., van Gils J. A., van der Meer J., Dekinga A., Kraan C., van der Veer H. W., et al. (2012). Designing a benthic monitoring programme with multiple conflicting objectives. Methods Ecol. Evol. 3, 526–536. doi: 10.1111/j.2041-210X.2012.00192.x
Bird J. P., Lees A. C., Chowdhury S. U., Martin R., Haque E. U. (2010). A survey of the critically endangered spoon-billed sandpiper Eurynorhynchus pygmeus in Bangladesh and key future research and conservation recommendations. Forktail 26 (26), 1–8.
BirdLife International (2016) Important bird and biodiversity area factsheet: Ganges-Brahmaputra-Meghna delta. Available at: http://www.birdlife.org (Accessed April 2, 2022).
Boettcher R., Haig S. M., Bridges W. C. Jr. (1995). Habitat-related factors affecting the distribution of nonbreeding American avocets in coastal south Carolina. Condor 97 (1), 68–81. doi: 10.2307/1368984
Bryant D. M. (1979). Effects of prey density and site character on estuary usage by overwintering waders (Charadrii). Estuar. Coast. Mar. Sci. 9 (4), 369–384. doi: 10.1016/0302-3524(79)90012-4
Burton P. (1971). Comparative anatomy of head and neck in the spoon-billed sandpiper, Eurynorhynchus pygmeus and its allies. Proc. Zoological Soc. London 163 (2), 145–163. doi: 10.1111/j.1469-7998.1971.tb04529.x
Butler R. W., Davidson N. C., Morrison R. G. (2001). Global-scale shorebird distribution in relation to productivity of nearshore ocean waters. Waterbirds 24, 224–232. doi: 10.2307/1522034
Cha M. W., Young L. (1990). “Food of the spoon-billed sandpiper in Hong Kong,” in Hong Kong:Hong Kong Bird report, vol. 1990, 192–193.
Chen C. (2004). Ecological distribution of diatoms in some mangrove areas along the coast of fujian and guangdong province and influence of six heavy matals on extracellular polymeric substances (Xiamen:Xiamen University).
Chen Y., Chen J. (2012). Resource management strategy of mangrove national nature reserve in zhanjiang, guangdong Vol. 1) (Beijing:Wetland science and management), 3.
Choi C. Y. (2015). The northward migration stopover ecology of bar-tailed godwits and great knots in the yalu jiang estuary, national nature reserve, China (New Zealand: Massey University).
Choi C. Y., Battley P. F., Potter M. A., Ma Z., Liu W. (2014). Factors affecting the distribution patterns of benthic invertebrates at a major shorebird staging site in the yellow Sea, China. Wetlands 34 (6), 1085–1096. doi: 10.1007/s13157-014-0568-4
Choi C. Y., Coleman J., Klaassen M., Moffitt D., Rogers D., Skilleter G., et al. (2017). Final report: migratory shorebird monitoring–understanding ecological impact (CA12000284). Gladstone: Report produced for the ecosystem research and monitoring program advisory panel as part of Gladstone ports corporation’s ecosystem research and monitoring program (Brisbane Australia: Uniquest).
Choi K. H., Lee S. M., Lim S. M., Park W. (2010). Benthic habitat quality change as measured by macroinfauna community in a tidal flat on the west coast of Korea. J. Oceanography 66, 307–317. doi: 10.1007/s10872-010-0027-7
Choi C. Y., Xiao H., Jia M., Jackson M. V., Lai Y. C., Murray N. J., et al. (2022). An emerging coastal wetland management dilemma between mangrove expansion and shorebird conservation. Conserv. Biol, e13905. doi: 10.1111/cobi.13905
Chowdhury S. U. (2011). A pictorial field guide to the shorebirds of Bangladesh (England: The Eric Hosking Trust).
Chowdhury S. U., Foysal M., Diyan M. A. A., Ahmed S. (2017). Discovery of an important wintering site of the critically endangered spoon-billed sandpiper Calidris pygmaea in the meghna estuary, Bangladesh. Bird Conserv. Int. 28, 251–262. doi: 10.1017/S0959270917000247
Dixon J. (1918). The nesting grounds and nesting habits of the spoon-billed sandpiper. Auk 25, 387–404. doi: 10.2307/4073213
Drolet D., Barbeau M. A. (2009). Differential emigration causes aggregation of the amphipod Corophium volutator (Pallas) in tide pools on mudflats of the upper bay of fundy, Canada. J. Exp. Mar. Biol. Ecol. 370 (1-2), 41–47. doi: 10.1016/j.jembe.2008.11.012
Du Q., Jin D. (1983). Attached diatoms on marine plants in the intertidal zone of jiulong estuary, fujian province. Taiwan Strait 2 (01), 78–100.
Flint V. E., Kondratiev A. Y., Voinstvenski M. A. (1977). An experience of evaluating of the total number of a rare stenotypic species (Spoon-billed sandpiper Eurynorhynchus pygmeus as an example). 7th All-Union Ornithol. Conf. Abstracts talks 2, 250.
Folmer E. O., Olff H., Piersma T. (2010). How well do food distributions predict spatial distributions of shorebirds with different degrees of self-organization? J. Anim. Ecol. 79, 747–756. doi: 10.1111/j.1365-2656.2010.01680.x
Folmer E. O., Olff H., Piersma T. (2012). The spatial distribution of flocking foragers: disentangling the effects of food availability, interference and conspecific attraction by means of spatial autoregressive modelling. Oikos 121, 551–561. doi: 10.1111/j.1600-0706.2011.19739.x
Folmer E. O., Piersma T. (2012). The contributions of resource availability and social forces to foraging distributions: A spatial lag modelling approach. Anim. Behav. 84, 1371–1380. doi: 10.1016/j.anbehav.2012.08.031
Giri C., Ochieng E., Tieszen L. L., Zhu Z., Singh A., Loveland T., et al. (2011). Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecol. Biogeography 20 (1), 154–159. doi: 10.1111/j.1466-8238.2010.00584.x
Goss-Custard J. D. (1977). The ecology of the wash. [England] 3. density-related behaviour and the possible effects of a loss of feeding grounds on wading birds (Charadrii). J. Appl. Ecol. 14 (3), 681–700. doi: 10.2307/2402803
Green R. E., Syroechkovskiy E. E., Anderson G., Chang Q., Clark N. A. (2021). New estimates of the size and trend of the world population of the spoon-billed sandpiper using three independent statistical models. International Wader Study Group 128, 22–35. doi: 10.18194/ws.00218
Henriques M., Granadeiro J. P., Piersma T., Leão S., Pontes S., Catry T. (2021). Assessing the contribution of mangrove carbon and of other basal sources to intertidal flats adjacent to one of the largest West African mangrove forests. Mar. Environ. Res. 169, 105331. doi: 10.1016/j.marenvres.2021.105331
Huang Y. Q. (2010). Study on the biodiversity of macrobenthic fauna in the wetland of the luoyangjiang mangrove nature reserve (Xiamen: Third Institute of Oceanography, Ministry of Natural Resources).
Huang F., Ye C. C. (1995). Marine hydrology of guangdong island (Guangzhou: Guangdong Science & Technology Press).
Huang B., Zhang B., Lu J., Ou Z., Xing Z. (2002). Studies on macrobenthic ecology and beach aquculture holding capacity in dongzhai bay mangrove areas i. number and density of macrobenthos in surface lay of mangrove region. Mar. science-Qingdao-Chinese edition 26 (3), 65–67. doi: 10.3969/j.issn.1000-3096.2002.03.019
Jahn H. (1942). Zur oekologie und biologie der vögel japans. J. Orn. 90, 277–278. doi: 10.1007/BF01916289
Jin Z., Liang L. Q., Yao G. C. (1992). Experimental report on the proliferation of Macrobrachium rosenbergii in nandu river. Inland Fisheries 18 (6), 3.
Kelly C., Zöckler C., Scampion B., Syroechkovskiy E. E. (2017). Hammer, filter or microphone: How does the spoon-billed sandpiper Calidris pygmaea use its bill to feed? Wader Study 124 (2), 99–111. doi: 10.18194/ws.00076
Koch V., Wolff M. (2002). Energy budget and ecological role of mangrove epibenthos in the caet´e estuary, north Brazil. Mar. Ecol. Prog. Ser. 228, 119–130. doi: 10.3354/meps228119
Kraan C., van Gils J. A. V., Spaans B., Dekinga A., Bijleveld A. I., Roomen M. V. (2009). Landscape-scale experiment demonstrates that wadden Sea intertidal flats are used to capacity by molluscivore migrant shorebirds. J. Anim. Ecol. 78 (6), 1259–1268. doi: 10.1111/j.1365-2656.2009.01564.x
Leh C., Sasekumar A. (1985). The food of sesarmid crabs in Malaysian mangrove forests. Malayan Nat. J. 39, 135–145.
Lin Q. (2003). Study on birds and their relationship with macrobenthos in mangrove areas along the coast of southern fujian (Xiamen: Xiamen University).
Lin H. J., Hsu C. B., Liao S. H., Chen C. P., Hsieh H. L. (2015). Effects of Spartina alterniflora invasion on the abundance and community of meiofauna in a subtropical wetland. Wetlands 35 (3), 547–556. doi: 10.1007/s13157-015-0643-5
Ma Z. J., Li B., Chen J. K. (2005). Study on the utilitizaion of stopover sites and migration strategies of migratory birds. J. Ecol. 25 (6), 9. doi: 10.3321/j.issn:1000-0933.2005.06.027
Marples T. G., Nicholas W. L., Stewart A. C. (1988). Field and laboratory studies of Desmodora cazca gerlach 1956 (Desmodoridae: Nematoda) rrom mangrove mud-flats. Nematologica 34 (3), 331–349. doi: 10.1163/002825988X00189
McAllister R. F. (1958). Rapid removal of marine salts from sediment samples. J. Sedimentary Res. 28 (2), 231. doi: 10.1306/74D707C2-2B21-11D7-8648000102C1865D
Meijer K. J., El-Hacen E. H. M., Govers L. L., Lavaleye M., Piersma T., Olff H. (2021). Mangrove-mudflat connectivity shapes benthic communities in a tropical intertidal system. Ecol. Indic. 130, 108030. doi: 10.1016/j.ecolind.2021.108030
Nuka T., Norman C. P., Kuwabara K., Miyazaki T. (2005). Feeding behavior and effect of prey availability on sanderling Calidris alba distribution on kujukuri beach. Ornithologicalence 4 (2), 139–146. doi: 10.2326/osj.4.139
Okoye O. K., Li H., Gong Z. (2020). Retraction of invasive Spartina alterniflora and its effect on the habitat loss of endangered migratory bird species and their decline in YNNR using remote sensing technology. Ecol. Evol. 10 (24), 1–15. doi: 10.1002/ece3.6971
Peng H. B., Choi C. Y., Zhang L., Gan X. J., Liu W. L., LI J., et al. (2017). Distribution and conservation status of the spoon-billed sandpiper in China. J. Zoology 52 (1), 9. doi: 10.13859/j.cjz.201701021
Penning E., Ersoy S., Govers L., Dekinga A., Holthuijsen S., Piersma T., et al. Measuring low tide densities of mobile epibenthos in the wadden Sea.
Penning E., Verkuil Y., Klunder L., Reneerkens J.Sanderlings use a diverse spectrum of prey worldwide but primarily rely on brown shrimp in the wadden Sea.
Piersma T. (1986). Feeding method of spoon-billed sandpipers on a mudflat in south Korea. J. Bombay Natural History Soc. 83 Suppl, 206–208.
Piersma T. (2007). Using the power of comparison to explain habitat use and migration strategies of shorebirds worldwide. J. Ornithology 148 (s1), 45–59. doi: 10.1007/s10336-007-0240-3
Piersma T. (2012). “What is habitat quality? dissecting a research portfolio on shorebirds,” in Birds and habitat: Relationships in changing landscapes. ecological reviews. Ed. Fuller R. J. (Cambridge: Cambridge University Press), 383–407. doi: 10.1017/CBO9781139021654.019
Piersma T., Koolhaas. A., Dekinga. A., Beukema J. J., Dekker R., Essink K. (2001). Long-term indirect effects of mechanical cockle-dredging on intertidal bivalve stocks in the wadden Sea. J. Appl. Ecol. 38 (5), 976–990. doi: 10.1046/j.1365-2664.2001.00652.x
Placyk J. S., Harrington B. A. (2004). Prey abundance and habitat use by migratory shorebirds at coastal stopover site in Connecticut. J. Field Ornithology 75 (3), 223–231. doi: 10.1648/0273-8570-75.3.223
Portenko L. A. (1957). Studien an einigen seltenen limikolen aus dem nördlichen und ostlichen siberiën. i. die löffelschnepfe-Eurynorhynchus pygmaeus. J. Orn. 93, 454–466.
Portenko L. A. (1968). Studien an einigen seltenen limicolen aus dem nördlichen und östlichen sibirien III. J. Ornithologie 109, 141–172. doi: 10.1007/BF01678109
Portenko L. A. (1981). Birds of the chukchi penisula and wrangal island. vol. 1. (Translated from Russian 1972) (New Delhi: Amerind).
Reyes-Vasquez G. (1975). Littoral diatoms of the family naviculaceae from la restinga lagoon Vol. 14 (Margarita Island, Venezuela: Boletin del Instituto Oceano grafico de la Universidad de Oriente), 199–225.
Ribeiro P. D., Iribarne O. O., Navarro D., Jaureguy L. (2004). Environmental heterogeneity, spatial segregation of prey, and the utilization of southwest Atlantic mudflats by migratory shorebirds. Ibis 146 (4), 672–682. doi: 10.1111/j.1474-919X.2004.00301.x
Shepherd P. (1995). Changes in sediment types and invertebrate fauna in the intertidal mudflats of the bay of fundy between 1977 and 1994 (Atlantic Region: Canadian Wildlife Service, Environmental Conservation Branch).
State Oceanic Administration (2016). Atlas of china’s offshore oceans: Marine biology and ecology (Beijing: China Ocean Press).
Stroud D. A., Baker A., Blanco D. E., Davidson N. C., Zöckler C. (2006). “The conservation and population status of the world’s waders at the turn of the millennium,” in Waterbirds around the world, Edinburgh Eds. Boere G. C., Galbraith C. A., Stroud D. A. (Edinburgh, UK: The Stationery Office)
Swennen C., Marteijn E. (1988). Foraging behaviour of spoon-billed sandpipers Eurynorhynchus pygmaeus on a mudflat in penisular Thailand. Nat. Hist. Bull. Siam Soc 36, 85–88.
Tang Y., Cui Z., Huang P., Zhong X., Pang M., Li Q., et al. (2019). Community structure of macrobenthos in mangrove in haizhu wetland, guangzhou. J. Guangdong Univ. Educ. 39 (5), 35–42.
Tomkovich P. S., Syroechkovski E. E., Lappo E. G., Zöckler C. (2002). First indications of a sharp population decline in the globally threatened spoon-billed sandpiper Eurynorhynchus pygmeus. Bird Conserv. Int. 12 (01), 1–18. doi: 10.1017/S0959270902002010
van de Kam J., Ens B., Piersma T., Zwarts L. (2004). Shorebirds. an illustrated behavioural ecology (Utrecht, The Netherlands: KNNV Publishers).
van Gils J. A., de Rooij S. R., van Belle J., van der Meer J., Dekinga A., Piersma T. (2005). ). digestive bottleneck affects foraging decisions in red knots Calidris canutus. i. prey choice. J. Anim. Ecol. 74, 105–119. doi: 10.1111/j.1365-2656.2004.00903.x
Vicente H. (1990). Monthly population density fluctuation and vertical distribution of meiofauna community in tropical muddy substrate (Tokyo: The Asian Fisheries Society).
Voronov V. G. (1980). “Observations on the feeding of the spoonbill stint (Eurynorhynchus pygmaeus),” in New studies on the biology and distribution of waders. Ed. Flint V. (Russian: Nauka, Moscow), 138–139.
Wah T. T., Wee Y. C. (1988). Diatoms from mangrove environments of Singapore and southern peninsular Malaysia. Botanica Marina 31 (4), 317–328. doi: 10.1515/botm.1988.31.4.317
Wang Q., An S., Ma Z., Zhao B., Chen J., Li B. (2006). Invasive plant Spartina alterniflora–—Biology, ecology and management. J. systematics\&\evolution 44 (5), 559–588. doi: 10.1360/aps06044
Wang Q., Ma M., Gao Y. R. (2006). Fauna sinica Aves vol. 5 gruiformes chardriforme lariformes (Beijing: Science Press).
Wolff M., Koch V., Isaac V. (2000). A trophic flow model of the caet´e mangrove estuary (North Brazil) with considerations for the sustainable use of its resources. Estuar. Coast. Shelf Sci. 50 (6), 789–803. doi: 10.1006/ecss.2000.0611
Yang H. Y. (2012). Usage of tidal mudflats by red knots (Calidris canutus) in northern bohai bay as a stopover site during spring migration (Beijing: Normal University).
Yang H. Y., Chen B., Piersma T., Zhang Z., Ding C. (2016). Molluscs of an intertidal soft-sediment area in China: Does overfishing explain a high density but low diversity community that benefits staging shorebirds? J. Sea Res. 109 (mar.), 20–28. doi: 10.1016/j.seares.2016.01.006
Ysebaert T., Fettweis M., Meire P., Sas M. (2005). Benthic variability in intertidal soft-sediments in the mesohaline part of the schelde estuary. Hydrobiologia 540 (1-3), 197–216. doi: 10.1007/s10750-004-7144-5
Zeng A., Hu W., Zeng C., Sun Z., Gao D. (2020). Litter decomposition and nutrient dynamics of native species (Cyperus malaccensis) and alien invasive species (Spartina alterniflora) in a typical subtropical estuary (Min river) in China. Estuaries Coasts 43 (7), 1873–1883. doi: 10.1007/s12237-020-00744-x
Zhang S. D., Ma Z., Choi C. Y., Peng H. B., Bai Q. Q., Liu W. L., et al. (2018). Persistent use of a shorebird staging site in the yellow Sea despite severe declines in food resources implies a lack of alternatives. Bird Conserv. Int. 28, 1–15. doi: 10.1017/S0959270917000430
Zhang S., Ma Z., Feng C., Melville D. S., Piersma T. (2019). Individual diet differences in a molluscivore shorebird are associated with the size of body instruments for internal processing rather than for feeding. J. Avian Biol 50, e02255. doi: 10.1111/jav.02255
Zharikov Y., Skilleter G. A. (2016). Nonbreeding eastern curlews numenius madagascariensis do not increase the rate of intake or digestive efficiency before long-distance migration because of an apparent digestive constraint. Physiol. Biochem. Zoology 76 (5), 704–715. doi: 10.1086/376427
Zöckler C., Beresford A. E., Bunting G., Chowdhury S. U., Buchanan G. M. (2016). The winter distribution of the spoon-billed sandpiper Calidris pygmaeus. Bird Conserv. Int. 26, 476–489. doi: 10.1017/S0959270915000295
Zöckler C., Hla T. H., Clark N., Syroechkovskiy E., Robinson R. (2010). Hunting in Myanmar is probably the main cause of the decline of the spoon-billed sandpiper Calidris pygmeus. Wader Study 117 (1), 1–8.
Keywords: spoon-billed sandpiper, food resources, mobile epibenthos, arthropods, diet, leizhou
Citation: Lu X, Yang H, Piersma T, Sun L, Chen Q, Jia Y, Lei G, Cheng L and Rao X (2022) Food resources for Spoon-billed Sandpipers (Calidris pygmaea) in the mudflats of Leizhou Bay, southern China. Front. Mar. Sci. 9:1005327. doi: 10.3389/fmars.2022.1005327
Received: 28 July 2022; Accepted: 22 August 2022;
Published: 15 September 2022.
Edited by:
Tian Xie, Beijing Normal University, ChinaCopyright © 2022 Lu, Yang, Piersma, Sun, Chen, Jia, Lei, Cheng and Rao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yifei Jia, amlheWlmZWlAYmpmdS5lZHUuY24=; Guangchun Lei, Z3VhbmdjaHVuLmxlaUBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
 Xiuyuan Lu
Xiuyuan Lu Hongyan Yang1,2†
Hongyan Yang1,2† Theunis Piersma
Theunis Piersma Yifei Jia
Yifei Jia Guangchun Lei
Guangchun Lei