
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 27 January 2022
Sec. Marine Biology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.782524
 Paulo F. Lagos1*
Paulo F. Lagos1* Alva Curtsdotter2
Alva Curtsdotter2 Antonio Agüera3
Antonio Agüera3 Amandine J. M. Sabadel4
Amandine J. M. Sabadel4 David J. Burrit5
David J. Burrit5 Miles D. Lamare1
Miles D. Lamare1
A Dynamic Energy Budget (DEB) model is applied to predict rapid metabolic shifts in an ecologically important krill, Nyctiphanes australis, in response to temperature and ultraviolet radiation (UVR). Specifically, we predict changes in fatty acids, amino acids and respiration rate in response to several light and temperature treatments. Environmental variability can alter the metabolic equilibrium and the mechanisms marine ectotherms used to obtain energy, which is a topical point given the current level of environmental change. Environmental variability also includes multiple stressors, which can have additive, antagonistic or synergistic effects on metabolism. In consequence, disentangling and quantifying the effects of multiple stressors on metabolism and the energy balance of ecthothermal species, such as krill, can be challenging. Here we apply a DEB model to direct measurements of fatty acids, amino acids and respiration rate of krill experimentally exposed simultaneously to several doses of UVR and temperatures. We found that on average light escalates metabolic rates by a factor of two, and temperature has an effect 1.35 times greater than the effect of light over respiration rates at temperatures from 9 to 19°C. The DEB model predicted shifts in metabolic function and indicated that the combined effect of light and elevated temperatures decrease the total of fatty acid concentrations at a higher rate than amino acids when krill are exposed to environmentally relevant temperatures and light treatments. Our results demonstrate that, when krill experience warmer conditions and higher levels of solar radiation, the mobilization of energy-relevant metabolites from the reserves increases by up to 36% and increase the total energetic cost by up to 45%. These findings suggest that ectothermal species with a fast metabolism, such as krill, quickly deplete energy reserves to compensate for changes in the environment. This renders krill susceptible to the effects of climate variability if the current climatic trend for the region continues to show temperature increases, even if solar radiation levels remain unchanged.
Variability in the marine environment, such as in temperature and ultraviolet radiation (UVR), present challenges for marine ectotherms, which frequently need to survive adverse abiotic conditions caused by multiple stressors. This is especially true when shifts in distribution or behavior are insufficient to mitigate the increased levels of stress created by global climate change (Sokolova, 2021). Physiological adaptability can play an essential part in improving survival chances under stressful conditions (McNamara et al., 2005; Lutz et al., 2008; Norin and Metcalfe, 2019). However, the physiological mechanisms involved in improved adaptation to environmental stress can be complex, especially when animals are subjected to multiple stressors such as a combination of increased temperatures, acidification, oxygen limitation, or UVR (Pecorino et al., 2014; Kazerouni et al., 2016; Lee et al., 2018; Rubalcaba et al., 2020), making it challenging to determine mechanisms based on empirical data alone. Process-based modeling approaches, informed by empirical data, can be powerful tools to explore biological mechanisms. Here, we take such an approach to gain insight into the metabolic response of krill exposed to multiple environmental stressors.
Multiple stressors can have additive, antagonistic or synergistic effects on metabolism (Przeslawski et al., 2005; Sokolova, 2021), and are a key factor in driving global change impacts on species ecosystems due to the inevitable constraints they put on the rates at which energy is acquired, assimilated, and converted into usable metabolites (Sokolova, 2013). These constraints can result in an overall decrease in fitness or loss of metabolic homeostasis (Klein et al., 2018; Toft et al., 2018; Sokolova, 2021). For instance, depletion of energy-relevant compounds, including fatty acids (FAs) and amino acids (AAs) in response to environmental stress is usually found in marine ectotherms, and the reduction of such compounds may act as a signal of transition into a bioenergetically unstable condition that might alter life-history traits and reduce fitness (Issartel et al., 2005; Sokolova, 2013; Kazerouni et al., 2016). How the interaction between ocean warming and other stressors such as ocean acidification and UVR deplete energy-relevant compounds and shape organism responses to climate variability is an active area of research (Valles-Regino et al., 2015; Wolinski et al., 2016; Lee et al., 2018; Wang et al., 2020; Heinze et al., 2021). This is due to the fundamental role energy transformation and energy fluxes within individuals play in the maintenance of life-history processes and the link they provide between an organism’s physiology and ecological processes (Chen and Nielsen, 2019).
Two of the most important drivers of change in marine organisms are temperature and UVR. Temperature plays a direct role in controlling physiological rates at the individual level, with warmer temperatures increasing metabolic cost (Hirche, 1984; Kooijman, 2010; Romero et al., 2010). Similarly, UVR can escalate physiological rates and can alter metabolism due to the formation of damaging free radicals or reactive oxygen species (ROS). This can ultimately decrease fitness by imposing energetic costs associated with repair mechanisms (Lesser et al., 2004; Moresino and Helbling, 2010; Tartarotti et al., 2014; Alves and Agustí, 2020; Sokolova, 2021) or by breaking down energy-relevant compounds, such as FAs and AAs. This process, known as photo-oxidation, can be relatively rapid and occur in a matter of hours for FAs and AAs (Dhams et al., 2011; Souza et al., 2012; Won et al., 2014; Kazerouni et al., 2016; Dur et al., 2021). The increase in energetic costs associated with environmental stress and the rapid decrease of energy-relevant metabolites, due to photo-oxidation or defense mechanisms, can disrupt metabolic homeostasis by introducing a trade-off between the available amount of energy necessary to maintain essential metabolic function and the energy necessary to produce metabolic defenses against environmental stress.
Many studies show temperature or UVR acting as a stressor for marine ectotherms (Tedetti and Sempéré, 2006; Lagos et al., 2015; Burritt and Lamare, 2016), however, there are few studies into their combined effect (Burritt and Lamare, 2016). These studies clearly show that the individual effect of temperature stress or UVR usually have strong effects on the physiology and ecology of marine life. Examples include, shortening the growing seasons of phytoplankton (Williamson et al., 2010), increasing mortality (Przeslawski et al., 2005), increasing crustaceans respiration rates (Fischer et al., 2006), or lowering fatty and amino acid concentrations (Arts et al., 2012). These studies are insightful, but their generality is less certain because the response to variation in temperature and UVR is species-specific (Saborowski et al., 2000, 2002; Atkinson et al., 2006; Jager et al., 2016; Klein et al., 2018), and the studies available are not enough to provide a robust understanding. For temperature and UVR, the challenge involves quantifying the relative effect of temperature and UVR on metabolic rates, as well as disentangling the general impact of UVR on metabolic rates from the compound-specific effects related to photo-oxidation. Furthermore, while we know that these stressors can escalate metabolic rates and reduce the amount of energy-relevant compounds, our understanding of the specific compound dynamics and their bioenergetic consequences is still limited.
Few studies have assessed the interactive effects of UVR with other environmental stressors in marine taxa (Wolinski et al., 2016; Liao et al., 2019; Williamson et al., 2019). However, these studies have found that both light and temperature tend to increase overall metabolism, as evidenced by increased respiration rates, and to decrease the concentrations of compounds.
Evaluating metabolic shifts and describing the energetic consequences of altered metabolism in marine ectotherms in response to temperature and light can be made through Dynamic Energy Budget (DEB) theory, developed to explain the link between physiological adaptability and the underlying processes fuelling metabolism. DEB models are proven frameworks successfully applied to interpret and predict the effect of environmental variability in the bioenergetics and life-history of marine ectotherms (Jager and Ravagnan, 2015; Gourault et al., 2019), and can be used to explain at ecosystemic, community and individual level, how these systems will reconfigure with changing environmental conditions (Beaugrand and Kirby, 2018). This includes the application of DEB models to infer patterns of lipid storage in copepods (Jager et al., 2017). A DEB is used here, for the first time, to connect the theory behind the usage of compounds to observations on how ectotherms use several types of energy-relevant compounds under multiple environmental stressors.
Here, we use a multiple stressor approach based on laboratory observations to find relevant patterns in the usage of compounds and a dynamic process-based model to assess how environmental variability affects krill metabolism associated with energy-relevant compounds and the overall energy balance. To date, few studies have connected energetic aspects of crustacean metabolism to essential metabolites such FAs and AAs (Issartel et al., 2005; Werbrouck et al., 2016; Lee et al., 2018). By applying a DEB model for the krill Nyctiphanes australis (Euphausiacea: Euphausiidae), the current research aims to (1) find stress threshold points, at which the combined effects of temperature and UVR affect the intermediate metabolism of krill, and (2) predict changes in the energy balance of krill, related to the rate of decrease of energy-relevant compounds such as fatty acids and amino acids under different temperature and light scenarios.
For this, we subjected krill to ecologically relevant levels of temperature and UV radiation, and measured the response in krill respiration rate and in the concentration of FAs and AAs. We model the effect of temperature and light (white and UV light) on respiration and on the dynamics of several compounds, using a DEB model. The modeling approach allows us to separate those compound decreases that stem from an overall increase in metabolism from those that may occur at a compound-specific rate due to photo-oxidation or from preferential use of certain compounds under light stress. Finally, we assess the multiple stressor effect of temperature and light on krill bioenergetics, by converting the mass flows in the DEB model to flows of energy. The DEB model is used to test the hypothesis that as temperature and doses of UVR increase, a rapid increase in metabolic rate is followed by a quick increase in the rate at which FAs and AAs are metabolized, ultimately decreasing the levels of energy stored as reserves.
Krill were exposed to a fully factorial design that combined the effect of temperature and light. Non-reproductive male and female adult krill (11–21 mm body length) were collected during summer and winter at night from two areas around the Otago Harbour, New Zealand (45°49′40″S; 170°38′23″E) (Figure 1) and kept starved in dark (no light) conditions for 24 h at ambient sea temperatures (Supplementary Figure A.1) inside a 250 L fiberglass holding tank. Krill individuals in the best visible physiological conditions (e.g., normal swimming activity) were manually selected and placed in the experimental tanks. The tanks were then heated to the selected experimental temperature at an approximate rate of 0.025–0.05°C/min depending on the ocean temperatures at the time of sampling. The krill were then acclimated for 1 h to the final experimental conditions which match temperatures typically found across a year (8, 12, 13, and 16°C) and a higher temperature reflecting occasional peaks (19°C) projected to increase in frequency along the Otago coast (Supplementary Figure A.1). In addition to temperature, krill were exposed to a combination of light treatments, no light (DARK), white light (WH), a low dose of UVR (19.2 J cm–2 UVA and 2.4 J cm–2 UVB), or a high dose of UVR (31 J cm–2 UVA; 3.2 J cm–2 UVB) (Supplementary Material A). Exposure to UVR was 2 h for the respiration experiment and 8 h in the compound concentration experiment. The maximum intensities of UV light (0.05–0.3 mW cm–2 UVB; 0.4–3.4 mW cm–2), was adjusted so that krill were exposed to the same total doses of UVR at 2 h in the respiration experiment as they were at 8 h in the compound concentration experiment. All experimental doses of UVR were adjusted to closely match typically found UVR doses across a year in the Otago Harbour (Supplementary Figure A.2).
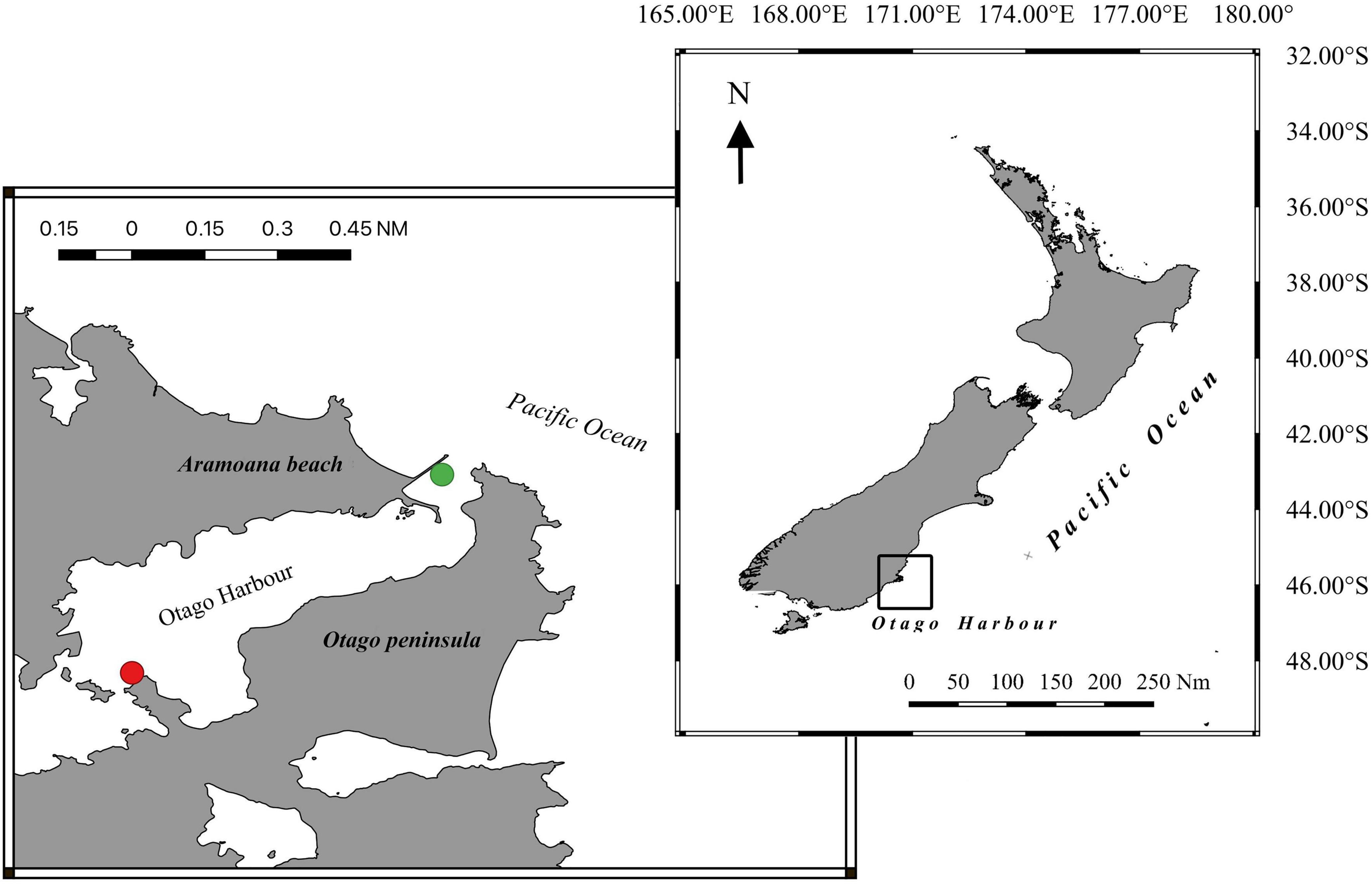
Figure 1. Locations used to collect krill inside the Otago Harbour, New Zealand. (Green dot) Aramoana point. (Red dot) Portobello Marine Laboratory.
The respiration rate data used here is the mass-specific oxygen consumption rate (μg O2 h–1 g–1 AFDW) measured using a previously calibrated fiber optic oxygen meter (PreSens®, Fibox 3 oxygen meter) on a closed respirometry system. We placed three active krill adults inside each of nine independent respirometry chambers (10 cm length × 3.5 cm diameter; replication: UV × 3, WH × 3, Dark × 3). Respirometry was repeated a total of eight times with oxygen consumption rates averaged across the three replicates for each treatment.
The compounds were extracted from pooled dry krill samples (∼200 ind. per sample) and concentrations (μg compound g–1 AFDW) were measured prior to starvation as well as at 4 and 8 h of light exposure. Measurements by gas chromatography with a flame ionisation detector (GC-FID) (Agilent Technologies 6890) followed GC methods described in Sabadel et al. (2019) and Kolodzey et al. (2021) for FAs and AAs, respectively. Due to the large number of individual FAs (n = 24) and AAs (n = 11) isolated from krill, all compounds were grouped by type. The fatty acids were grouped into four major types: monounsaturated (MUFA), polyunsaturated (PUFA), saturated (SAFA), and highly unsaturated (HUFA), and amino acids were grouped as: essential (EAA) and non-essential (NEAA). For consistency with units in the DEB model, the concentration of each individual FA and AA was transformed, using its molecular weight, to its equivalent amount in moles of carbon per gram of krill dry weight (C-mol g–1 DW). For full experimental method description see Supplementary Material A.
Dynamic Energy Budget theory is a robust and accepted framework that mechanistically describes the bioenergetics of an organism (Kooijman, 2010). DEB models are mathematical implementations of this theory, and describe how food is converted into reserves, and how reserves are mobilized and allocated to growth, reproduction, and maintenance (Figure 2A). We calibrated a standard DEB model for N. australis using growth data from Hosie (1982), Haywood and Burns (2003), and Lagos et al. (under review)1 and reproduction data obtained from Hosie and Ritz (1983) in conjunction with data of respiration rates and energy-relevant compounds from krill not exposed to light treatments (details in: Supplementary Material B). This provided us with core model parameters needed to subsequently model the compound dynamics and ensured that the compound dynamics were coherent with the overall DEB model.
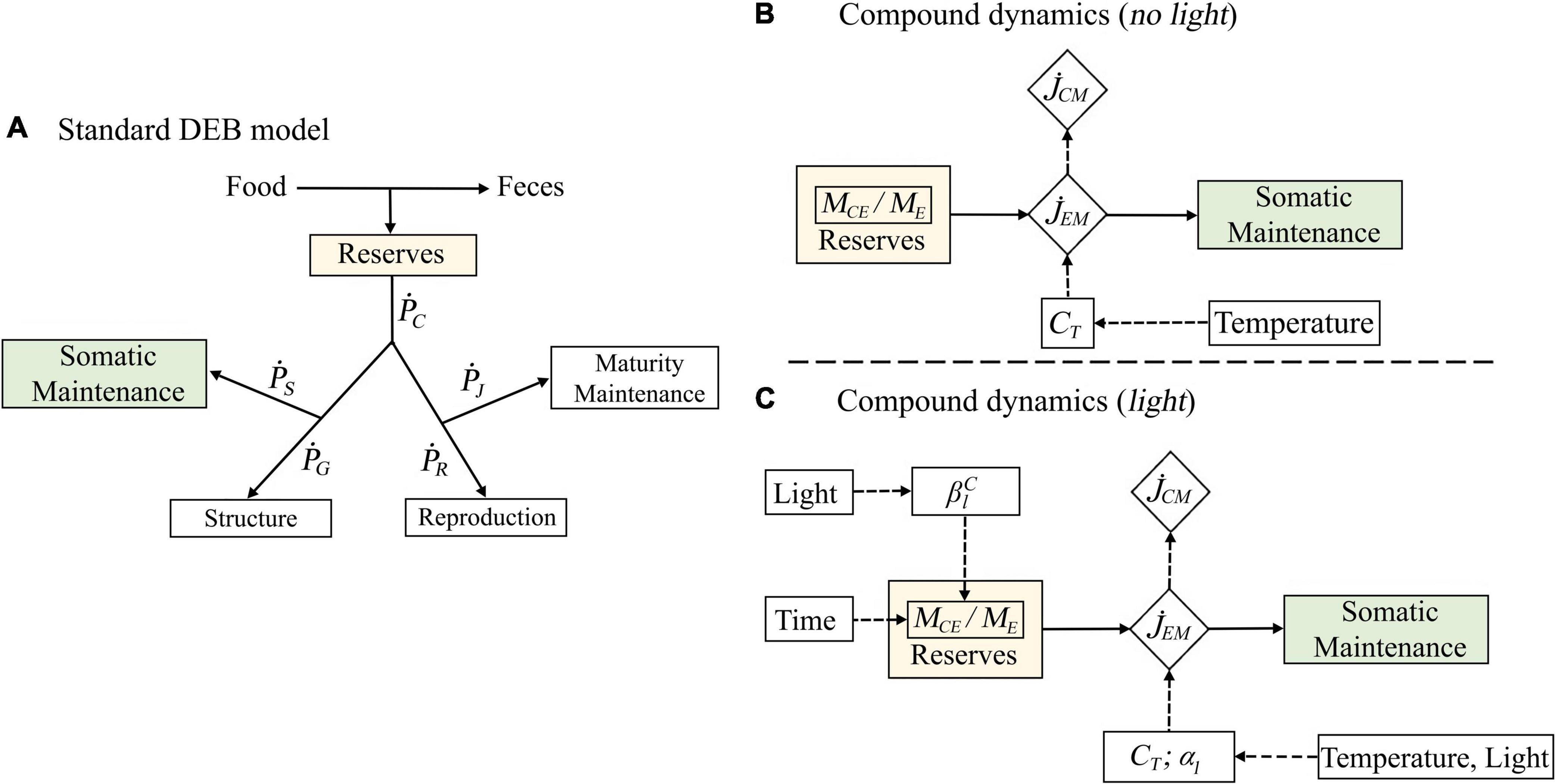
Figure 2. Schematic representation of the three steps implemented for the DEB model parametrization. The components of the standard DEB model that are used to predict the reduction of compounds in time are indicated as green and yellow rectangles (A). The control model describes how the temperature correction factor (CT) affect the flux of compounds () and the total outflux of energy from the reserves for maintenance purposes () (B). The second DEB model describes how the temperature correction factor (CT) and two light parameters () affect the rate of decrease of compounds. However, here both and are time-dependent, therefore, affecting the ratio of compounds in the reserves (MCE/ME) and the total outflux of energy for maintenance () (C).
In DEB theory, temperature affects all rates, including the mobilization rate of compounds and the respiration rates, in the same way. The relationship between rates and temperature is based on the Arrhenius concept of enzyme activation (Kooijman, 2010; Jager and Ravagnan, 2015; Jager et al., 2017). We quantified the effect of temperature on respiration by fitting the Arrhenius equation to the respiration rate data (Eq. 1). The effect of light on respiration was captured by estimating light factors applied to the Arrhenius equation. Thus, in biological terms, light was assumed to act on metabolism and respiration by amplifying its temperature response as shown in Kazerouni et al. (2016) and Williamson et al. (2019). We use a subset of the standard DEB model to describe the krill compound dynamics under starvation conditions, assuming that under such conditions, energy is mobilized from the reserve only to cover somatic maintenance costs (Figures 2B,C). Although the state variables of the model are usually termed in units of volume or energy, they can easily be converted to units of mass, as we do here for the compound dynamics. The model formulation for the compound dynamics is slightly different for the 24 h period of dark conditions (Figure 2B) and the subsequent 8 h period of light exposure (Figure 2C). In the model formulation for dark conditions (Figure 2B), mass is mobilized from the reserve to cover somatic maintenance costs. The rate of this mobilization is affected by temperature, as described by the Arrhenius equation. The mobilization rate of specific compounds is simply a product of the total reserve mobilization rate and the relative proportion of the compound in the reserve. This proportion is assumed to be constant over time, in accordance with the assumption of strong homeostasis. This assumption is central in DEB theory and states that the chemical composition of the organism is constant.
Dynamic Energy Budget theory does not consider the effect of ultraviolet and visible light on organism bioenergetics. Therefore, to model the decrease of energy-relevant compounds in the presence of light, we modify the DEB model subset as illustrated in Figure 2C. Firstly, the reserve mobilization rate is amplified by the light factors that we estimated for the respiration rate. Thus, biologically speaking, light and temperature are assumed to increase the total reserve mobilization rate the same way that they affect respiration. Secondly, we assume that the effect of light may disrupt the strong homeostasis; in other words, the composition of the reserve and, hence, the relative proportion of compounds in the reserve, is allowed to change in time. The disruption of the strong homeostasis proposed here is based on our hypothesis that in crustaceans the internal homeostasis of compounds can change due to the photo-oxidation of energy-relevant compounds or due to the differential usage of compounds to fuel defense mechanisms against environmental stress. We consider this to be a compound-specific process that can occur very quickly in time and precede physiological responses such as the increase in respiration rates as theoretically illustrated in Supplementary Figure C.1 and empirically supported for ectotherms (Fields et al., 1998; Won et al., 2014; Duan et al., 2016; Abol-Munafi et al., 2020). Finally, we convert the mass flows of the compound dynamics into energy, to evaluate the effect of temperature and light on the overall krill energetics.
The krill DEB model used here is the standard DEB model as first applied to N. australis (see text footnote 1). Briefly, the dynamics model (Figure 2A) can be explained as follows: food is assimilated into the energy reserve, from which energy is mobilized, and the reserve mobilization rate is divided in two parts; a constant fraction, k, is allocated to growth, and somatic maintenance, , and the remainder, 1 − k, is allocated to reproduction and maturity maintenance (Figure 2A). The equations describing the dynamics of the state variables and the energy fluxes of the model are given in Supplementary Table B.1. The full list of parameters of the DEB model are given in Supplementary Table B.2 and the full list of equations for model prediction are given in Supplementary Table B.3. The temperature dependence of DEB parameters that represent rates is achieved by multiplying the default parameter value, i.e., the rate at Tref, by a temperature correction factor, CT, derived from the Arrhenius concept:
where TA is the Arrhenius temperature, TL and TH are the upper and lower tolerance boundary, and TAH and TAL are the Arrhenius temperatures for the rate of decrease at both boundaries (Kooijman, 2010) (detailed methods in: Supplementary Material E). The effect of light on metabolic rates and on the reserve mobilization rate was captured by a light factor, αl, the value of which is specific for each light treatment (αWH for WH light, αUVL for the low UVR doses, and αUVH for the high UVR dose). αl escalates the rate for any given temperature, such that the combined effect of temperature and light on a rate was described as:
In the absence of light, the fatty acids and amino acids decrease linearly in time as described for starvation conditions in Kooijman (2010):
with:
MC(t), the amount of compound in C-mol at time t, is thus a direct function of MC0, the initial amount of compound in the organism, and , the compound-specific rate of decrease. This rate of decrease is in turn a product of the temperature-dependent , the total mass flux mobilized from the reserve to cover maintenance, and MCE/ME, the relative proportion of compound in the reserve. The mass flux corresponds to the total somatic maintenance cost, , in the standard DEB model for N. australis (Figure 2A), simply converted from units of energy to units of mass. In the presence of light (WH or UVR), the fatty acids and amino acids decrease non-linearly in time:
αl, is the effect of light on metabolic rates and, , is the effect of light on compounds due to photo-oxidation or differential usage of compounds to fuel defense mechanisms against environmental stress and, tl, is the time when krill is exposed to light. The effect of temperature and light on krill bioenergetics was assessed by converting the compound concentrations and fluxes of the model from units of mass (C-mol) to units of energy (kJ), using the conversion parameters in Table 3.
The parameterization of the model was a three-step procedure. In the first step, we parameterized the Arrhenius equation of the model using the software R v3.6 (R Development Core Team, 2019) and the function “nlsLM” of the package “minpak.lm” to fit a non-linear least squares regression to the scaled respiration rate of krill at several temperatures from the dark treatment (methods in: Supplementary Material D). In the second step, we calibrated the DEB model, which included parameterizing the DEB model core parameters, estimating the αl parameters, as well as the parameters pertaining to the compound dynamics under dark conditions. The αl parameters were estimated by fitting Eq. 2 to the respiration data from the light treatments. For the compound dynamics, the initial amount of compound, MC0, was known from FA and AA measurements, and only the compound specific values for needed to be parameterized, by fitting Eq. 3 to the compound data from the dark treatments. In the third step, we estimated the parameters, by fitting Eq. 5 to the compound data from the light treatments. The other parameters in Eq. 5 were already known from the previous parameterization steps; in addition to and αl already mentioned, can be calculated from the DEB core parameters, and MCE/ME can then be obtained from Eq. 4. Since MCE/ME is constant under dark conditions, the MCE/ME in Eq. 4 equals in Eq. 5. Thus, in the third parameterization step, all parameters were fixed except for the compound- and light-specific parameter. It was necessary to divide the parameterization of the compound dynamics into these two steps; otherwise, it would not have been possible to separate the effect of light on overall reserve mobilization from the effect of light on the relative proportion of compounds due to photo-oxidation or preferential use. The MATLAB® code used for model predictions is freely available2.
A previously developed DEB model was used to predict general life-history traits of N. australis, including growth and reproduction (see text footnote 1). When new data on oxygen consumption rates and fatty and amino acid concentrations were added to the data previously used to parameterize the model, the model performed well at predicting respiration rates, fatty acids and amino acids concentrations. The addition of respiration rates and energy-relevant compounds to the model increased the accuracy in prediction of life-span. The model predicted the respiration rates under dark conditions well (MRE = 0.20) (Supplementary Figure A.1), and the model performance for fatty acid and amino acid dynamics in the absence of light was in most cases good (MRE = 0.10–0.21). Higher errors were only observed at the warmest temperature for amino acids (MRE = 0.48 at 19°C) and at the coolest temperature for fatty acids (MRE = 0.12 at 8°C) (Figures 2, 3). Age and length at birth were largely underestimated by the model, with an overall error of more than 22% (Supplementary Table A.4). With the additional data, the model maintained a low relative error (MRE = 0.135) and a low symmetric mean squared error (SMSE = 0.194), values that represent a reduction of 20% in the MRE and an increase of 10% of the SMSE respectively, compared to the previously described parametrization for the species (Table 1).
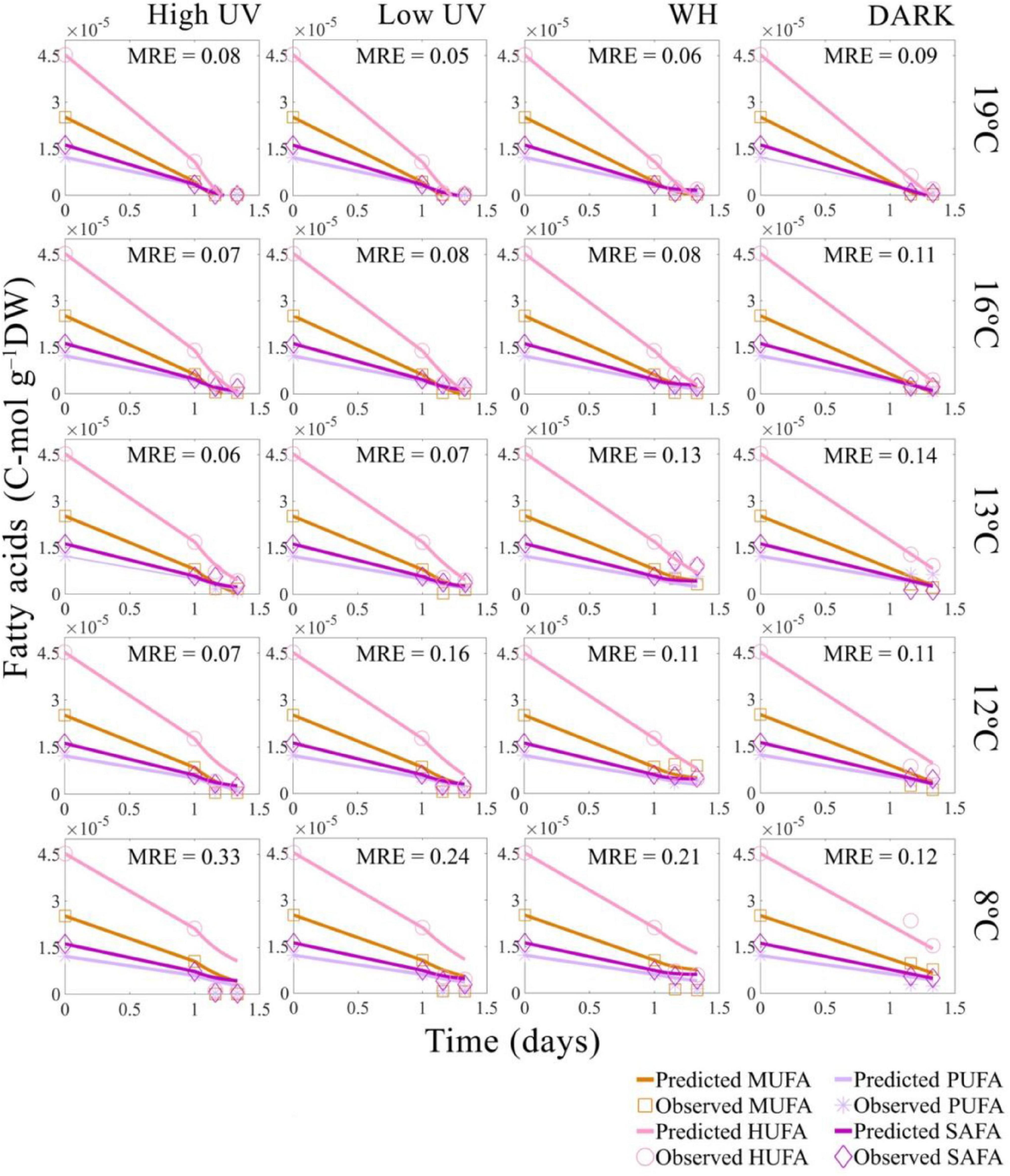
Figure 3. Predicted and observed decrease in Nyctiphanes australis fatty acid concentration in time for all temperatures and light treatments: high UV, low UV, PAR, and DARK with the mean across fatty acids types relative error of the DEB model predictions (MRE). Values used for the DEB model are the total sum of fatty acids converted into C-moles. For model predictions of UV and PAR treatments the value of fatty acids at 1 day (24 h) of starvation was interpolated from the negative control data, which is krill not experimentally exposed to light.
Adding the proposed escalation parameter αl and , the model successfully predicted changes in oxygen consumption at different temperatures and light conditions, with low mean relative errors for the low UVR dose and high UVR dose treatments. The model slightly underestimated the oxygen consumption by krill at 19° and 16°C for high UVR exposed krill (Figure 4A), but maintained good predictions at low UVR doses and good predictions when krill is under WH light and DARK conditions across all temperatures.
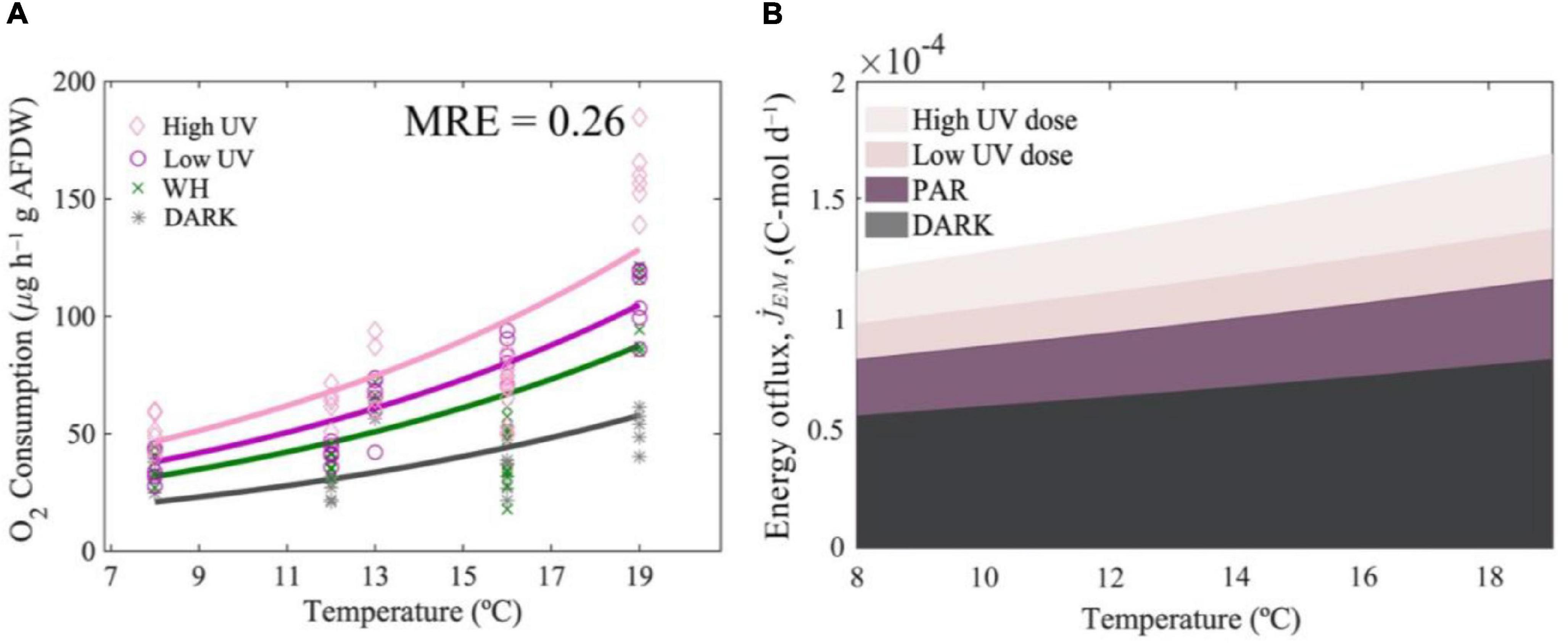
Figure 4. Metabolic responses to light and temperature. Predicted changes in respiration rates of Nyctiphanes australis with light and temperature (A). Effect of temperature on the total outflux of energy from the reserves to cover somatic maintenance at different levels of UVR and at different light condition (B).
Overall, the light amplification factors indicate that UVR has a moderate effect on the rate at which oxygen consumption increases with temperature. For instance, light amplification parameters predicted by the model (Table 2) indicate that, compared to the DARK exposed krill, light in general escalates metabolic rates by a factor of 2. The strongest escalation was by the high UV light treatment, compared to the escalation of WH light and low intensity UV light, which was 36% and 18% lower, respectively (Figure 4). In contrast, model predictions under DARK and WH light conditions indicate that temperature increases respiration rates by a factor of 2.7 between 8 and 19°C and under low doses of UVR increases respiration by a factor of 2.6 between 8 and 19°C. Therefore, by comparing predictions across temperature ranges, temperature has an effect 1.35 times higher than the effect in respiration rates produced by light.
The DEB model successfully predicted the rate of decrease of FAs in time, with error estimates for the predicted decrease of FAs in time ranging between MRE = 0.05–0.33 (Figure 3) and error estimates for the decrease of AAs in time ranging between MRE = 0.02–0.48 (Figure 5). Overall, the relative errors of the model for FAs and AAs in the absence of light (DARK) were relatively low, with a total MRE = 0.149 and an SMSE = 0.185 (Table 1).
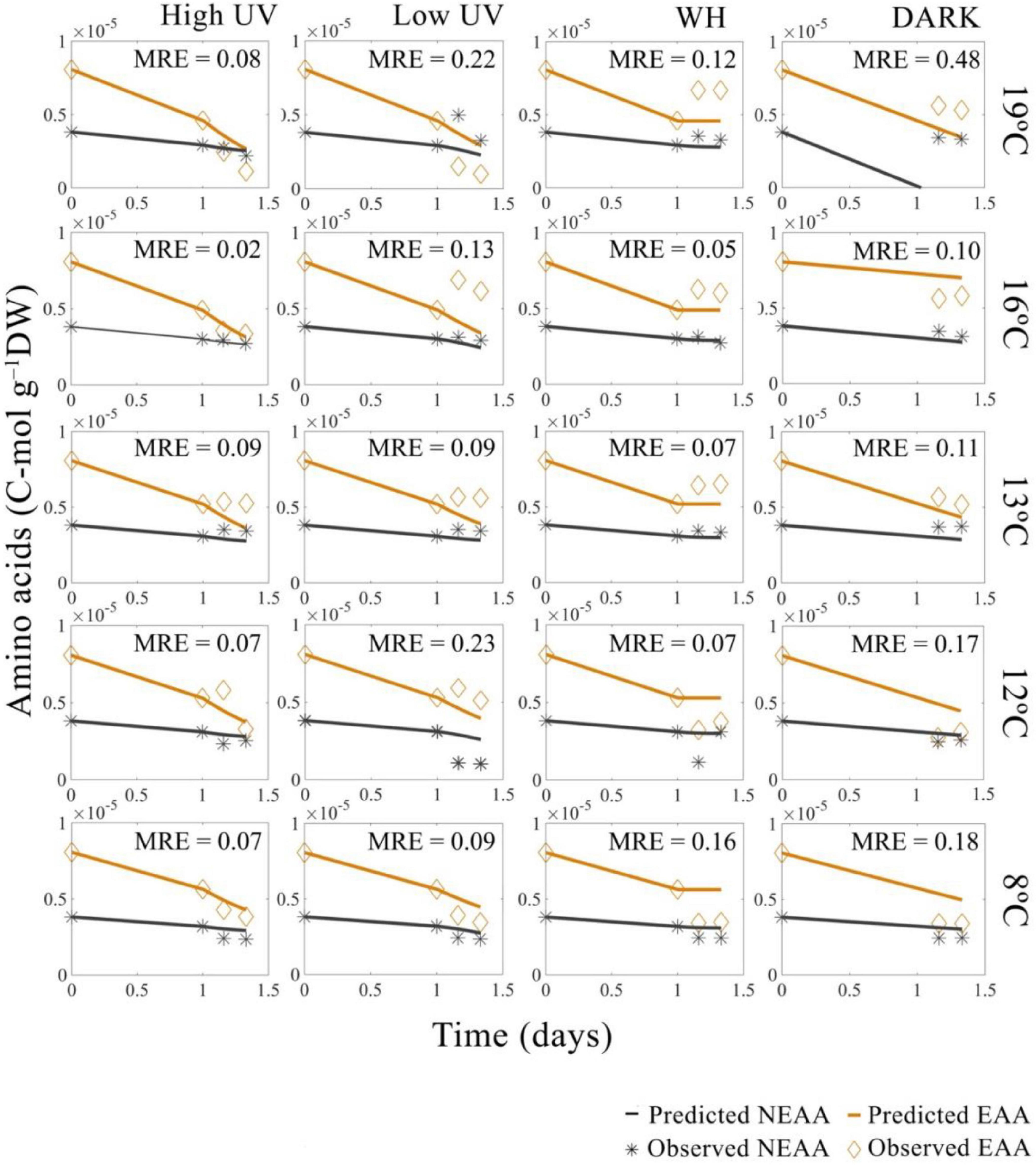
Figure 5. Predicted and observed decrease in Nyctiphanes australis amino acid concentrations in time for all temperatures and light treatments: high UV, low UV, PAR, and DARK. MRE represent the mean relative error across amino acids type (EAA and NEAA) for each DEB model prediction. All observed values used for DEB prediction are the total sum of amino acids converted into C-moles. For model predictions of UV and PAR treatments the value of amino acids at 1 day (24 h) of starvation was interpolated from the negative control data, which represent krill not exposed to light.
The model predictions indicated a faster rate of decrease of compounds when UVR and WH light are present. This rate of decrease was greater when doses of UVR are above 31 J cm–2 UVA and 3.2 J cm–2 UVB and when the temperature is above 16°C, slowing down at temperatures below 16°C. Additionally, model results indicate that compounds consistently decrease at different rates when temperature and light interact. For instance, the predicted rate of decrease of HUFA was 3.5e–05 C-mol g–1, which is an order of magnitude higher than the rate of decrease of the remaining PUFA. In contrast, the predicted rate of decrease of MUFA and SAFA was on average 1.7e–06 C-mol g–1 (Table 2). Similarly, the predicted rate of decrease of AA was higher for EAA, with a rate of decrease of 3.58e–06 C-mol g–1 and smaller for NEAA, with a rate of decrease of 9.13e–07 C-mol g–1.
Based on error estimates, the model had difficulties predicting the rate of decrease of FAs at 8°C, with error estimates ranging between MRE = 0.12–0.33, and not accurately predicting the decrease of MUFA’s and PUFA’s at the lower temperature. However, the model performed better predicting the rate of decrease of EAAs and NEAAs, with lower error estimates of MRE = 0.07–0.18 at 8°C. Overall, the model predicted a mean specific rate of decrease of fatty acids of 0.03 C-μmol day–1, which was higher than the mean specific rate of decrease of amino acids 0.002 C-μmol day–1. The model also predicted dose-dependent differences in the way light affects compounds. For instance, the difference between primary drivers of reductions of energy-relevant compounds (i.e., effect of light on metabolic activity) and secondary drivers of reductions in energy-relevant compounds (i.e., Photooxidation of compounds) was 0.3 times smaller when high doses of UVR (31 J cm–2 UVA; 3.2 J cm–2 UVB) are present and 0.8 times higher when low doses of UVR (19.2 J cm–2 UVA and 2.4 J cm–2 UVB) are present, with a total effect of UVR dose greater on SAFAs, HUFAs, and NEAAs, and less on MUFAs, PUFAs, and EAAs (Table 2). The modeling also predicted that visible light is 0.78 times less effective in producing FA and AA decreases. However, the model overestimated the compound-specific impact of WH light. For instance, WH light predicted effect were stronger for NEAAs, EAAs, and SAFAs with minor impact in MUFAs and PUFA’s (Table 2).
Using mass energy conversions (Table 3), predictions of the DEB model show that the total energy content stored in FAs and AAs in N. australis is around 0.061 kJ. Most of the available energy for N. australis is stored in the form of HUFAs and MUFAs, which contain a combined energy content of 0.043 kJ, approximately ∼71% of the total energy stored in all compounds measured. At the same time, SAFA and PUFA constituted ∼29% of the total energy content and essential and non-essential amino acids only constitute 0.63% of the total, with a combined energy content of 0.0004 kJ.
The total energy expenditure of N. australis that comes from the mobilization of all compounds stored as reserves to cover somatic maintenance costs, assuming we have most of the compounds krill uses as a source of energy, is 0.049 kJ day–1 at the reference temperature of 20°C, and in the absence of WH or UV light. This energetic cost to cover somatic maintenance changes with temperature and light as a result of the decrease of compounds in the reserve and as a result of changes in the mobilization flux of compounds from the reserves (Figure 4B). For instance, model results indicate that at 19°C and high doses of UV, the total maintenance costs are considerably higher compared to maintenance cost at temperatures below 19°C or when krill do not experience high doses of UVR (Figure 4B). The rate of change in the mean mobilization flux of compounds from the reserves also increases with temperature, and is higher when krill are exposed to higher doses of UVR, which indicates that the mobilization of compounds occurs at a higher pace at elevated temperatures and when doses of UVR are higher, therefore compounds decrease rapidly over time under these circumstances (Figure 6).
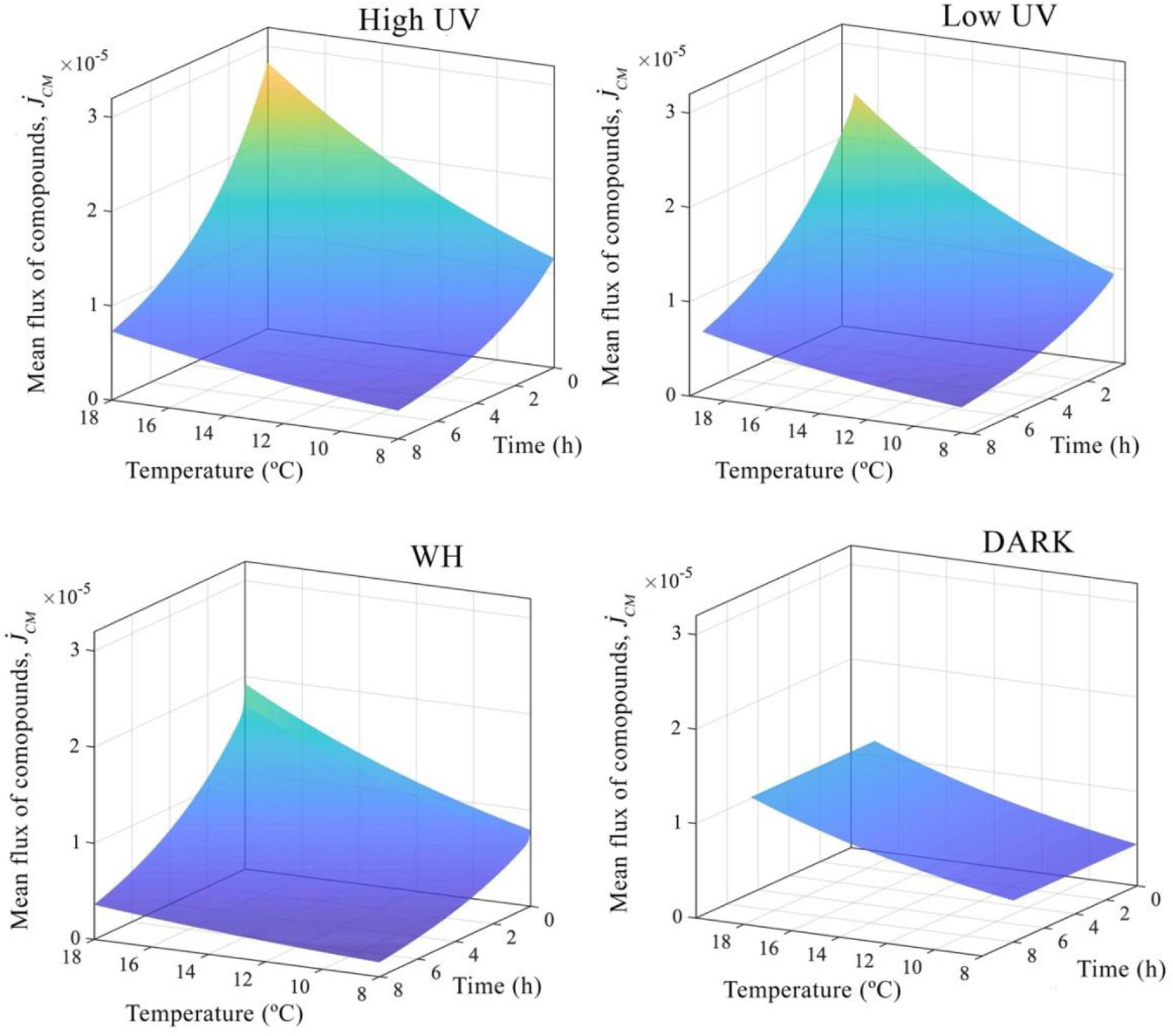
Figure 6. Mean changes in energy fluxes. Nyctiphanes australis energy fluxes from the reserve to cover somatic maintenance.
In terms of the total energy balance, by using mass energy conversion values for fatty acids and amino acids (Table 3), the higher rate at which the flux of compounds changes at temperatures above 16°C and high doses of UVR increases the total energy maintenance cost from 0.018 to 0.059 kJ day–1. Similarly, at intermediate temperatures from 12 to 16°C, the mean total energetic costs associated with the flux of compounds from reserves is 0.039 kJ day–1 at high UVR doses and 0.032 kJ day–1 at low UVR doses, which represents an increase of 44% compared to krill not exposed to any light treatment and an increase of 13% compared to krill exposed to WH light. In contrast, at the lower temperature of 8°C the energetic cost of maintenance for krill at low doses of UVR are 0.018 kJ day–1, and 0.022 kJ day–1 at high doses of UVR, which is a total reduction of 43% in the mean energetic cost for somatic maintenance compared to krill at 19°C and higher doses of UVR. Finally, visible light (WH) had a smaller effect on the energetic balance of N. australis, imposing a total energetic cost to cover somatic maintenance of 0.027 kJ day–1 at temperatures between 8 and 16°C and 0.43 kJ day–1 at 19°C with total changes in the flux or mobilization of compounds from the reserves smaller than changes produced by UV light (Figure 4). Looking across all light and temperature conditions that krill were exposed to, the rate of decrease of compounds over time in reserve indicates that close to 40% of the total energy available is mobilized within the first hours and the remaining energy is mobilized at a slower rate until reserves are depleted.
The DEB model predicted rapid changes in N. australis utilization of compounds, such as fatty acids and amino acids, to cover the energetic cost of maintaining metabolic activity under the combined exposure to light and temperature. The model results show that when krill are exposed to high temperatures and UV doses (31–39 J cm–2 UVA; 3.2–4.2 J cm–2 UVB) and irradiances (3.9 mW cm–2 UVA; 0.35 mW cm–2 UVB) of UVR, comparable to the maximum doses (25–35 J cm–2 UVA; 3.3–6.9 J cm–2 UVB), and irradiances (1.4–3.9 mW cm–2 UVA; 0.18–0.36 mW cm–2 UVB) naturally found during summer, the concentration of energy-relevant compounds in krill decrease rapidly, while the metabolic rates increase. However, model predictions only partially support our hypothesis that as temperature and doses of UVR increase, a rapid increase in metabolic rates is followed by a quick increase in the rate at which all compounds are metabolized. For instance, the model was not fully able to capture the differential rate of decrease between FAs and AAs at lower temperatures or the lower decrease rate of compounds in the dark treatments. Nevertheless, we accurately predict that the effect of temperature on the respiration rate and reserve mobilization is of comparable magnitude to the effect of UVR on these rates, while the compound-specific effect of UVR can be either larger or smaller than the effect on the overall metabolism (Figures 3, 5). From a bioenergetic perspective this means that under higher temperature and UVR doses, krill would increase the mobilization flux of compounds from the reserves (Figure 6). The accuracy of model predictions at high levels of UVR above 16°C suggest that this temperature is likely to be the optimum upper metabolic threshold at which the mobilization of energy from the reserves occur at a pace fast enough to cope with environmental exposure to higher doses of UVR, but slow enough to maintain an energetic balance that can sustain the extra expenditure of energy for longer periods of time. However, the model was not entirely able to reproduce the full dynamics of fatty acids and amino acids in time in the lower temperatures, especially in the absence of light.
The model indicates that at temperatures below 19°C, krill have the metabolic adaptability to cope with decreases in temperatures even if UVR levels are high. This is evidenced by the lower rate at which compounds are mobilized below 16°C and by the lower metabolic effect of light predicted by the model compared to the effect produced by temperature. Model predictions of energy fluxes at the lower temperatures intervals support this and indicate that energy demands below 16°C are lower, which is evidenced by the lower fluxes of compounds from the reserves and the higher content of energy-relevant compounds. This indicates that krill should be more tolerant to additional environmental stress below 16°C, due to the lower energetic demands and the higher amount of FAs and AAs that can be used in stress defense. These results agree with studies showing similar tolerance responses to environmental variability in ectotherms, in which lower metabolic demands lead to higher stress tolerance (Vajedsamiei et al., 2021). However, the mean error of the model predictions at 8°C and high UV levels were greater than mean errors at intermediate temperatures. Therefore, it is also possible that N. australis could be closer to its lower temperatures tolerance limit than predicted by the model, which suggests that animals that don’t possess large reserves, could potentially be at risk of not surviving extended periods of time at temperatures below 8°C as well as above 19°C, when levels of solar radiation are high.
Generally speaking, in marine ectotherms the capacity to survive adverse conditions is directly proportional to the internal reserve formation and mobilization, which is generally less temperature-dependent than the metabolic demands (Kooijman, 2010). From an energy balance perspective, the DEB model indicates that under environmentally stressful conditions, krill would quickly utilize all energy-relevant compounds available to maintain metabolic functions, which in turn decreases the overall energetic content at a fast rate, leaving the animals with energy reserves that last for a period no longer than 2 days (Supplementary Figure F1). The predictions of the model indicate that the rapid decrease of compounds occurs together with rapid compositional changes in the reserves due to UVR. For instance, while degradation and utilization of HUFAs and PUFAs occur more slowly, MUFAs and SAFAs are severely damaged by UVR, as predicted by the high values of the specific light correction factors for these compounds, which are higher than the values of the parameter that represents the normal metabolic use of the compounds (Table 2). These findings are consistent with research in fish showing empirically that MUFAs are greatly reduced when the fish are exposed to UVR (Arts et al., 2012) and imply that photo-oxidative stress processes have the potential to reduce MUFA and SAFA at a faster rate than the organism is able to metabolize the compounds to maintain normal metabolic functions. Although there is not enough evidence to support that this is a general response in marine organisms to temperature and light, reductions of FAs can extend from the individual to the ecosystem level, by reducing the amount of available FAs that are transferred to higher trophic levels (Brett et al., 2009; Jiang et al., 2020).
Previous studies evaluating the antioxidant capacity and fatty acid photo-oxidation due to UVR in the copepods Paracyclopina nana (Won et al., 2014), Schmackeria inopinus (Yu et al., 2009), and Eudiaptomus gracilis (Souza et al., 2012) have shown that doses of UVR between 0.3 and 0.4 kJ cm–2 significantly increase the activity of antioxidant enzymes but still strongly decrease the amount of MUFAs and PUFAs (Won et al., 2014), reducing adaptability to environmental variability. The model results presented here are in agreement with the previous studies performed on copepods and also indicate that photo-oxidation due to UVR is one of the causes behind the rapid reduction of FAs, not only for MUFAs and SAFAs but also for PUFAs and HUFAs. In addition, model results suggest that reductions in FA driven by temperature could enhance these reductions. This is most likely due to the higher metabolic turnover of compounds produced by higher temperatures, which can be explained by the predicted increase in respiration rates found when krill are exposed to temperatures above 16°C and high doses of UVR. These changes in composition and rapid reductions of FAs by light ultimately limit the thermal tolerance at the higher temperatures by imposing energetic cost that cannot be sustained by the reserve capacity.
Further studies examining the role of oxidative stress associated with daily migration patterns of the sister species Nyctiphanes simplex have shown that N. simplex cannot migrate through high stress areas of the water column (elevated water temperature >23°C; low dissolved oxygen concentrations <2.5 mL O2 L–1) and higher temperature significantly increased oxidative stress and affected krill metabolic homeostasis (Tremblay et al., 2010). Our results are in agreement with observations made for other Nyctiphanes species and demonstrate from a different perspective how members of the genus do not possess the physiological mechanism to cope with high oxidative stress environmental scenarios. Alternatively, it appears that Nyctiphanes may try to offset damage produced by oxidative stress by rapidly mobilizing energy reserves, a metabolic adaptation that could have serious repercussions for the only known genus of krill that form large daytime surface swarms, if surface ocean temperature keep increasing. However, it is uncertain whether Nyctiphanes would stay in the surface, to feed and escape predators as hypothesized by Gendron (1992), when high temperatures and levels of UVR are present or if they would actively avoid the surface of the water column. It is clear that Nyctiphanes species face the dilemma of food limitations in deeper waters or high stress at the surface and shallow areas where N. australis are normally found in the Otago Harbour
As for fatty acids, model predictions suggest that amino acid composition can change considerably under environmental stress, but with overall amino acid levels decreasing at a lower rate than fatty acids at any given temperature or light treatment. Light had different effects on the overall amino acid content. For instance the model predicted that when krill are exposed to high UV doses, AA levels decrease at a faster rate compared to krill exposed to low UVR doses or WH light, with EAAs decreasing faster than NEAAs, which is in agreement with the empirical observations. For temperature, the model predicted reductions in AAs, as expected based on the implementation of the Arrhenius concept. This effect of temperature fit the data well for the two UVR treatments but had variable success in the WH and DARK treatments. In particular, the observed phenomena in these two treatments that the model could not capture was the high amounts of both EAAs and NEAAs at higher temperatures and the low amount of EAAs at low temperatures. The reason for this is that the data describe a temperature trend in amino acid concentration opposite than is expected based on the general effect of temperature on metabolic rates. However, the trend observed in the data is in line with what has been found for freshwater crustacean, that showed an increase in temperature of 5°C tends to increase the AA content, but produce an overall decrease in proteins levels (Cogo et al., 2018). These responses may be caused by a higher degree of proteolysis at elevated temperatures. However, we caution that the AA patterns predicted by the model should not be discarded too quickly, as they well could be an species-specific response to temperature not previously observed in marine ectotherms.
Although, the levels of AAs at both temperature extremes predicted by the model partially contrast with the general responses to temperature of AAs levels in crustaceans, which show a general increase of AAs at lower temperatures (Issartel et al., 2005), the predicted reduction of FAs are in agreement with studies showing similar reduction of FAs with temperature extremes (Wen et al., 2017; Abol-Munafi et al., 2020) and with the levels measured here. DEB model predictions and data also indicate that the generality of the response of AA at lower temperature could shift when krill is exposed to multiple environmental stressors, ultimately reducing AAs as shown by the good fit of the model when AAs are affected by UVR. However, we acknowledge that there is high specificity in the role AAs play in response to cold and warm temperatures, and how multiple stressors affect AAs levels is an area of research that still remains largely unknown for crustaceans. The DEB approach taken here provides a mechanistic foundation to explain patterns of reduction of AAs in ectotherms exposed simultaneously to elevated temperatures and doses of UVR.
Model predictions indicate that krill could undergo metabolic shifts due to temperature and UVR that could trigger a bioenergetic imbalance between the necessary energy to sustain metabolism and the mobilization rate of available energy from the reserves at higher temperature (Figure 6). This is due to the elevated rate at which krill use fatty acids and amino acids, which imply that krill can potentially use gluconeogenetic pathways to gain rapid access to stored energy, which has already been reported for other species of crustaceans (Ferreira et al., 2005; Foucreau et al., 2014). However, this hypothesis will require further testing and evaluation of levels of proteins and glycogen. These compounds are typically used by crustaceans during changes in environmental conditions or periods of increased activity, evidenced in eye ablated crustaceans, who show a decrease in glycogen levels followed by increases in protein levels (Bhaskar, 2016).
Based on model predictions on AA reduction, we propose some interesting hypotheses and conclusions. For instance, it is likely that the reduction of AAs in time predicted by the models are not caused by thermal decomposition, as amino acids degradation is a process that has been demonstrated to only occur at temperatures above 100°C (Weiss et al., 2018). It is reasonable to expect that the reduction in AAs after exposure to thermal and UVR stress could be due to krill simply using all available sources of energy. This could explain the sharp decrease in EAAs at 19°C, but does not necessarily explain the changes in NEAAs in time, which at lower temperatures remain almost unaltered. The rate of decrease of NEAA in time at 19°C remained almost unaltered regardless of the light treatments. But using the model formulation presented in this study the concentrations of NEAA decrease quickly above 16°C. This could be a clear indication that either krill would metabolize EAA and NEAA differently with temperature and light and indicate from a modeling perspective, that there are still biological mechanisms that we can’t currently describe. However, there are no empirical studies that have shown this response in crustaceans.
Regardless of the biological mechanisms behind the different rates of use of AAs with temperature and light, we believe that future modelling approaches attempting to predict amino acid dynamics under multiple stressors would be improved by adding feedback loops to the amino acids pool due to protein degradation and synthesis (Mente et al., 2010). Moreover, because EAAs cannot be synthesized by krill and are incorporated directly from the diet, a rapid reduction of such compounds is expected (Carter and Mente, 2014). On the other hand, NEAAs can be formed from different sources, therefore we might expect a slower reduction over time (Wu, 2013; Wu et al., 2013). This is likely due to the fact that under more favorable conditions, when metabolic stress still remains low, some level of recycling of compounds can occur as krill do not need to meet high energy demanding processes. Therefore, future model approximations that establish positive feedback loops in the flux of energy relevant compounds such as amino acids coming out from the reserve to cover somatic maintenance could benefit from such approaches and establish even more accurate models. Future modeling approaches that consider feedback loops in the dynamics of amino acids reserve, should include the use of an Arrhenius function that extent beyond the thermal limits of normal functioning of the species, as this could help solve problems in predictions at the thermal extremes.
Regardless of the model approach, the results presented in this study accurately predict reduction on most of the compounds used by krill. The implication of the present findings is that higher doses of UVR and elevated temperatures accelerate the degradation of MUFAs and SAFAs at a rate fast enough to disrupt normal metabolic activity. This could increase susceptibility of krill to climatic variations by accelerating the fluxes of compounds from the reserves that otherwise would be destined to maintenance of metabolic functions. At the ecosystem level the decreased availability of fatty acids under current warming conditions (Jin et al., 2020) is already affecting the fitness of top predators by decreasing spawning and egg quality, fertilization and hatching rates, and consequently ecosystem stability (Parrish, 2013; Colombo et al., 2020). Therefore, specifically targeted investigations of alterations in the composition and content of energy-relevant compounds can provide insights into changes in cellular function, biochemical mechanisms of stress and impaired health processes under climate warming.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://github.com/paulagos/DEB_Nyctiphanes_australis.git.
PL, ML, and AA have developed the idea. PL led the writing of the manuscript and figures while AC and PL developed the code and some of the mathematics behind. AS performed the amino acids measurements. All authors contributed to the editing of the manuscript and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Amanda N. Laubmeier from the Mathematics and Statistics department of the Texas Tech University for her contribution to the present work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.782524/full#supplementary-material
Abol-Munafi, A. B., Ikhwanuddin, M., and Azra, M. N. (2020). Effects of temperature on the whole body fatty acid composition and histological changes of the gills in blue swimmer crabs, Portunus Pelagicus. Aquac. Rep. 16:100270. doi: 10.1016/j.aqrep.2019.100270
Alves, R. N., and Agustí, S. (2020). Effect of ultraviolet radiation (UVR) on the life stages of fish. Rev. Fish Biol. Fish. 30, 335–372. doi: 10.1007/s11160-020-09603-1
Arts, M. T., Palmer, M. E., Skiftesvik, A. B., Jokinen, I. E., and Browman, H. I. (2012). UVB radiation variably affects N-3 fatty acids but elevated temperature reduces n-3 fatty acids in juvenile atlantic Salmon (Salmo Salar). Lipids 47, 1181–1192. doi: 10.1007/s11745-012-3719-5
Atkinson, A., Shreeve, R. S., Hirst, A. G., Rothery, P., Tarling, G., Pond, D. W., et al. (2006). Natural growth rates in antarctic krill (Euphausia Superba): II. predictive models based on food, temperature, body length, sex, and maturity stage. Limnol. Oceanogr. 51, 973–987. doi: 10.4319/lo.2006.51.2.0973
Beaugrand, G., and Kirby, R. R. (2018). How do marine pelagic species respond to climate change? theories and observations. Annu. Rev. Mar. Sci. 10, 169–197. doi: 10.1146/annurev-marine-121916-063304
Bhaskar, L. V. K. S. (2016). Bioenergetics and Energy Metabolism of Crustaceans. Morrisville, NC: Lulu Press.
Brett, M. T., Muller-Navarra, D., and Persson, J. (2009). “Crustacean zooplankton fatty acid composition,” in Lipids in Aquatic Ecosystems, eds M. Arts, M. Brett, and M. Kainz (Berlin: Springer Science & Business Media), 115–146. doi: 10.1007/978-0-387-89366-2_6
Burritt, D. J., and Lamare, M. D. (2016). “The cellular responses of marine algae and invertebrates to ultraviolet radiation, alone and in combination with other common abiotic stressors,” in Stressors in the Marine Environment, eds M. Solan and N. Whiteley (Oxford: Oxford University Press), 117–134.
Carter, C. G., and Mente, E. (2014). Protein synthesis in crustaceans: a review focused on feeding and nutrition. Cent. Eur. J. Biol. 9, 1–10. doi: 10.2478/s11535-013-0134-0
Chen, Y., and Nielsen, J. (2019). Energy metabolism controls phenotypes by protein efficiency and allocation. Proc. Natl. Acad. Sci. U.S.A. 116, 17592–17597. doi: 10.1073/pnas.1906569116
Cogo, G. B., Biasi, C., Severo, E. S., Loro, V., and Santos, S. (2018). Environmental warming induces behavioral and metabolic changes in a freshwater crustacean - Aeglids as a model organism. Ann. Limnol. 54, 1–7. doi: 10.1051/limn/2017032
Colombo, S. M., Rodgers, T. F. M., Diamond, M. L., Bazinet, R. P., and Arts, M. T. (2020). Projected declines in global DHA availability for human consumption as a result of global warming. Ambio 49, 865–880. doi: 10.1007/s13280-019-01234-6
Dhams, H., Dobretsov, S., and Lee, J.-S. (2011). Effects of UV radiation on marine ectotherms in polar regions. Comp. Biochem. Physiol. Physiol. C Toxicol. Pharmacol. 153, 363–371. doi: 10.1016/j.cbpc.2011.01.004
Duan, Y., Li, F., Li, Y., Tang, Y., Kong, X., Zemeng, F., et al. (2016). The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 48, 41–51. doi: 10.1007/s00726-015-2067-1
Dur, G., Won, E. J., Han, J., Lee, J. S., and Souissi, S. (2021). An individual-based model for evaluating post-exposure effects of UV-B radiation on zooplankton reproduction. Ecol. Model. 441:109379. doi: 10.1016/j.ecolmodel.2020.109379
Ferreira, B. D. P., Hack, C., de Oliveira, G. T., and Bond-Buckup, G. (2005). Perfil metabólico de Aegla platensis schmitt, (Crustacea, Aeglidae, Anomura) submetida a dietas ricas em carboidratos ou proteínas. Rev. Bras. Zool. 22, 161–168. doi: 10.1590/s0101-81752005000100018
Fields, P. G., Fleurat-Lessard, F., Lavenseau, L., Febvay, G., Peypelut, L., and Bonnot, G. (1998). The effect of cold acclimation and deacclimation on cold tolerance, trehalose and free amino acid levels in Sitophilus granarius and Cryptolestes ferrugineus (Coleoptera). J. Insect Physiol. 44, 955–965. doi: 10.1016/S0022-1910(98)00055-9
Fischer, J. M., Fields, P. A., Pryzbylkowski, P. G., Nicolai, J. L., and Neale, P. J. (2006). Sublethal exposure to UV radiation affects respiration rates of the freshwater cladoceran Daphnia catawba. Photochem. Photobiol. 82, 547–550. doi: 10.1562/2005-08-30-RA-664
Foucreau, N., Cottin, D., Piscart, C., and Hervant, F. (2014). Physiological and metabolic responses to rising temperature in Gammarus pulex (Crustacea) populations living under continental or mediterranean climates. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 168, 69–75. doi: 10.1016/j.cbpa.2013.11.006
Gendron, D. (1992). Population structure of daytime surface swarms of Nyctiphanes simplex (Crustacea: Euphausiacea) in the Gulf of California, Mexico. Mar. Ecol. Prog. Ser. 87, 1–6.
Gourault, M., Petton, S., Thomas, Y., Pecquerie, L., Marques, G. M., Cassou, C., et al. (2019). Modeling reproductive traits of an invasive bivalve species under contrasting climate scenarios from 1960 to 2100. J. Sea Res. 143, 128–139. doi: 10.1016/j.seares.2018.05.005
Haywood, G. J., and Burns, C. W. (2003). Growth of Nyctiphanes (Euphausiacea) on different diets. J. Exp. Mar. Biol. Ecol. 289, 139–151.
Heinze, C., Blenckner, T., Martins, H., Rusiecka, D., Döscher, R., Gehlen, M., et al. (2021). The quiet crossing of ocean tipping points. Proc. Natl. Acad. Sci. U.S.A. 118, e2008478118. doi: 10.1073/pnas.2008478118
Hirche, H. (1984). Temperature and metabolism of Plankton - I. respiration of antarctic zooplankton at different temperatures with comparison of antarctic and nordic krill. Comp. Biochem. Physio. 77A, 361–368. doi: 10.1016/0300-9629(84)90074-4
Hosie, G. W. (1982). Biology and Production of Nyctiphanes Australis G.O. Sars, in the Coastal Waters of S.E. Tasmania. Ph.D. thesis. Hobart TAS: University of Tasmania.
Hosie, G. W., and Ritz, D. A. (1983). Contribution of moulting and eggs to secondary production in Nyctiphanes australis (Crustacea: Euphausiacea). Mar. Biol. 77, 215–220. doi: 10.1007/BF00395809
Issartel, J., Renault, D., Voituron, Y., Bouchereau, A., Vernon, P., and Hervant, F. (2005). Metabolic responses to cold in subterranean crustaceans. J. Exp. Biol. 208, 2923–2929. doi: 10.1242/jeb.01737
Jager, T., and Ravagnan, E. (2015). Parameterising a generic model for the dynamic energy budget of Antarctic krill Euphausia superba. Mar. Ecol. Prog. Ser. 519, 115–128.
Jager, T., Ravagnan, E., and Dupont, S. (2016). Near-future ocean acidification impacts maintenance costs in sea-urchin larvae: identification of stress factors and tipping points using a DEB modelling approach. J. Exp. Mar. Biol. Ecol. 474, 11–17. doi: 10.1016/j.jembe.2015.09.016
Jager, T., Salaberria, I., Altin, D., and Nordtug, T. (2017). Modelling the dynamics of growth, development and lipid storage in the marine copepod Calanus Finmarchicus. Mar. Biol. 164, 1–15. doi: 10.1007/s00227-016-3030-8
Jiang, Y., Jiao, H., Sun, P., Yin, F., and Tang, B. (2020). Metabolic response of Scapharca subcrenata to heat stress using GC/MS-based metabolomics. PeerJ 8, 1–20. doi: 10.7717/peerj.8445
Jin, P., Gonzàlez, G., and Agustí, S. (2020). Long-term exposure to increasing temperature can offset predicted losses in marine food quality (Fatty Acids) caused by ocean warming. Evol. Appl. 13, 2497–2506. doi: 10.1111/eva.13059
Kazerouni, G. E., Franklin, C. E., and Seebacher, F. (2016). UV-B radiation Interacts with temperature to determine animal performance. Funct. Ecol. 30, 584–595. doi: 10.1098/rsbl.2016.0258
Klein, E. S., Hill, S. L., Hinke, J. T., Phillips, T., and Watters, G. M. (2018). Impacts of rising sea temperature on krill increase risks for predators in the Scotia Sea. PLoS One 13:e0191011. doi: 10.1371/journal.pone.0191011
Kolodzey, S., Durante, L. M., Sabadel, A. J. M., and Wing, S. R. (2021). Larval quality and fecundity trade-off are linked to the maternal environment in sea perch (Helicolenus percoides, Sebastidae). J. Exp. Mar. Biol. Ecol. 537:151525. doi: 10.1016/j.jembe.2021.151525
Kooijman, S. A. L. M. (2010). Dynamic Energy Budget Theory for Metabolic Organisation, 3rd Edn. New York, NY: Cambridge University Press.
Lagos, P. F., Valdés, M. J., and Manríquez, K. (2015). Effects of UV radiation on the RNA/DNA ratio of copepods from antarctica and chile. Adv. Polar Sci. 26, 147–157.
Lee, M. C., Park, J. C., and Lee, J. S. (2018). Effects of environmental stressors on lipid metabolism in aquatic invertebrates. Aquat. Toxicol. 200, 83–92. doi: 10.1016/j.aquatox.2018.04.016
Lesser, M. P., Lamare, M. D., and Barker, M. F. (2004). Transmission of ultraviolet radiation through the Antarctic annual sea ice and its biological effects on sea urchin embryos. Limnol. Oceanogr. 49, 1957–1963. doi: 10.4319/lo.2004.49.6.1957
Liao, J., Xu, J., Yuan, X., Liang, Y., Guo, Y., Zhou, W., et al. (2019). Interactive effects of ultraviolet radiation and dissolved organic carbon on phytoplankton growth and photosynthesis in sanya bay, northern south china sea. Ocean Sci. J. 54, 581–593. doi: 10.1007/s12601-019-0033-7
Lutz, A., Freier, U., Lopata, A., and Meyer, B. (2008). Physiological and morphological colour change in antarctic krill, Euphausia Superba: a field study in the lazarev sea. J. Exp. Biol. 211, 3850–3858. doi: 10.1242/jeb.024232
McNamara, J. M., Buchanan, K. L., and Place, P. (2005). Stress, resource allocation, and mortality. Behav. Ecol. 16, 1008–1017. doi: 10.1093/beheco/ari087
Mente, E., Davidson, I., Karapanagiotidis, I. T., Fountoulaki, E., and Nengas, I. (2010). Amino acid analysis in the shore crab Carcinus maenas (Decapoda: Brachyura). J. Crust. Biol. 30, 643–650.
Moresino, R. D., and Helbling, E. W. (2010). Combined effects of UVR and temperature on the survival of crab larvae (Zoea I) from Patagonia: the role of UV-absorbing compounds. Mar. Drugs 8, 1681–1698. doi: 10.3390/md8051681
Norin, T., and Metcalfe, N. B. (2019). Ecological and evolutionary consequences of metabolic rate plasticity in response to environmental change. Philos. Trans. R. Soc. B Biol. Sci. 374:20180180. doi: 10.1098/rstb.2018.0180
Parrish, C. C. (2013). Lipids in marine ecosystems. ISRN Oceanogr. 2013, 1–16. doi: 10.5402/2013/604045
Pecorino, D., Barker, M. F., Dworjanyn, S. A., Byrne, M., and Lamare, M. D. (2014). Impacts of near future sea surface pH and temperature conditions on fertilisation and embryonic development in Centrostephanus rodgersii from northern New Zealand and northern New South Wales, Australia. Mar. Biol. 161, 101–110. doi: 10.1007/s00227-013-2318-1
Przeslawski, R., Davis, A. R., and Benkendorff, K. (2005). Synergistic effects associated with climate change and the development of rocky shore molluscs. Glob. Change Biol. 11, 515–522. doi: 10.1111/j.1365-2486.2005.00918.x
R Development Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Romero, M. C., Tapella, F., Stevens, B., and Buck, C. L. (2010). Effects of reproductive stage and temperature on rates of oxygen consumption in Paralithodes Platypus (Decapoda: Anomura). J. Crust. Biol. 30, 393–400.
Rubalcaba, J. G., Verberk, W. C. E. P., Jan Hendriks, A., Saris, B., and Woods, A. H. (2020). Oxygen limitation may affect the temperature and size dependence of metabolism in aquatic ectotherms. Proc. Natl. Acad. Sci. U.S.A. 117, 31963–31968. doi: 10.1073/pnas.2003292117
Sabadel, A. J. M., Van Oostende, N., Ward, B. B., Woodward, E. M. S., Van Hale, R., and Frew, R. D. (2019). Characterization of particulate organic matter cycling during a summer north atlantic phytoplankton bloom using amino acid C and N Stable Isotopes. Mar. Chem. 214, 1–12.
Saborowski, R., Bröhl, S., Tarling, G. A., and Buchholz, F. (2002). Metabolic properties of Northern krill, Meganyctiphanes norvegica, from different climatic zones. I. Respiration and excretion. Mari. Biol. 140, 547–556.
Saborowski, R., Salomon, M., and Buchholz, F. (2000). The Physiological response of northern krill (Meganyctiphanes Norvegica) to temperature gradients in the kattegat. Hydrobiologia 426, 157–160. doi: 10.1007/978-94-011-4148-2_14
Sokolova, I. (2013). Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integrat. Comp. Biol. 53, 597–608. doi: 10.1093/icb/ict028
Sokolova, I. (2021). Bioenergetics in environmental adaptation and stress tolerance of aquatic ectotherms: linking physiology and ecology in a multi-stressor landscape. J. Exp. Biol. 224(Pt Suppl. 1):jeb236802. doi: 10.1242/jeb.236802
Souza, M. S., Hansson, L., Hylander, S., Modenutti, B. E., and Balseiro, E. G. (2012). Rapid enzymatic response to compensate UV radiation in copepods. PLoS One 7:e32046. doi: 10.1371/journal.pone.0032046
Tartarotti, B., Saul, N., Chakrabarti, S., Trattner, F., Steinberg, C. E. W., and Sommaruga, R. (2014). UV-induced DNA damage in cyclops Abyssorum Tatricus populations from clear and turbid alpine lakes. J. Plankton Res. 36, 557–566. doi: 10.1093/plankt/fbt109
Tedetti, M., and Sempéré, R. (2006). Penetration of ultraviolet radiation in the marine environment. A review. Photochem. Photobiol. 82, 389–397. doi: 10.1562/2005-11-09-IR-733
Toft, J. D., Munsch, S. H., Cordell, J. R., Siitari, K., Brett, V. H., Holycross, L. M., et al. (2018). Impact of multiple stressors on juvenile fish in estuaries of the northeast pacific. Glob. Change Biol. 24, 2008–2020. doi: 10.1111/gcb.14055
Tremblay, N., Gómez-Gutiérrez, J., Zenteno-Savín, T., Robinson, C. J., and Sánchez-Velascoa, L. (2010). Role of oxidative stress in seasonal and daily vertical migration of three krill species in the Gulf of California. Limnol. Oceanogr. 55, 2570–2584. doi: 10.4319/lo.2010.55.6.2570
Vajedsamiei, J., Wahl, M., Schmidt, A. L., Yazdanpanahan, M., and Pansch, C. (2021). The higher the needs, the lower the tolerance: extreme events may select ectotherm recruits with lower metabolic demand and heat sensitivity. Front. Mar. Sci. 8:660427. doi: 10.3389/fmars.2021.660427
Valles-Regino, R., Tate, R., Kelaher, B., Savins, D., Dowell, A., and Benkendorff, K. (2015). Ocean warming and CO2-induced acidification impact the lipid content of a marine predatory gastropod. Mar. Drugs 13, 6019–6037. doi: 10.3390/md13106019
Wang, X., Huang, Z., Wang, C., Qi, C., Gu, Z., Li, E., et al. (2020). A comparative study on growth and metabolism of Eriocheir sinensis juveniles under chronically low and high pH stress. Front. Physiol. 11:885. doi: 10.3389/fphys.2020.00885
Weiss, I. M., Muth, C., Drumm, R., and Kirchner, H. O. K. (2018). Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 11:2. doi: 10.1186/s13628-018-0042-4
Wen, B., Jin, S. R., Chen, Z. Z., Gao, J. Z., Wang, L., Liu, Y., et al. (2017). Plasticity of energy reserves and metabolic performance of discus fish (Symphysodon aequifasciatus) exposed to low-temperature stress. Aquaculture 481, 169–176.
Werbrouck, E., Gansbeke, D., Vanreusel, A., and De Troch, M. (2016). Temperature affects the use of storage fatty acids as energy source in a benthic copepod (Platychelipus Littoralis, Harpacticoida). PLoS One 11:e0151779. doi: 10.1371/journal.pone.0151779
Williamson, C. E., Neale, P. J., Hylander, S., Rose, K. C., Figueroa, F. L., Robinson, S. A., et al. (2019). The interactive effects of stratospheric ozone depletion, UV radiation, and climate change on aquatic ecosystems. Photochem. Photobiol. Sci. 18, 717–746. doi: 10.1039/c8pp90062k
Williamson, C. E., Salm, C., Cooke, S. L., and Saros, J. E. (2010). How do UV radiation, temperature, and zooplankton influence the dynamics of alpine phytoplankton communities? Hydrobiologia 648, 73–81.
Wolinski, L., Modenutti, B., Souza, M. S., and Balseiro, E. (2016). Interactive effects of temperature, ultraviolet radiation and food quality on zooplankton alkaline phosphatase activity. Environ. Pollut. 213, 135–142. doi: 10.1016/j.envpol.2016.02.016
Won, E. J., Lee, Y., Han, J., Hwang, U., Shin, K. H., Park, H. G., et al. (2014). Effects of UV radiation on hatching, lipid peroxidation, and fatty acid composition in the copepod Paracyclopina Nana. Comp. Biochem. Physiol. Part C 165, 60–66. doi: 10.1016/j.cbpc.2014.06.001
Wu, G., Wu, Z., Dai, Z., Yang, Y., Wang, W., Liu, C., et al. (2013). Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 44, 1107–1113. doi: 10.1007/s00726-012-1444-2
Yu, J., Yang, G., and Tian, J. (2009). Effects of UV-B radiation on ingestion, fecundity, population dynamics and antioxidant enzyme activities of Schmackeria inopinus (Copepoda Calanoida). J. Exp. Mar. Biol. Ecol. 381, 74–81. doi: 10.1016/j.jembe.2009.09.020
Zhu, C., Gao, Y., Li, H., Meng, S., Li, L., Francisco, J. S., et al. (2016). Characterizing hydrophobicity of amino acid side chains in a protein environment via measuring contact angle of a water nanodroplet on a planar peptide network. Proc. Natl. Acad. Sci. U.S.A. 113, 12946–12951. doi: 10.1073/pnas.1616138113
Keywords: DEB model, environmental variability, fatty acids, amino acid, ultraviolet radiation (UVR), bioenergetics, temperature, light
Citation: Lagos PF, Curtsdotter A, Agüera A, Sabadel AJM, Burrit DJ and Lamare MD (2022) Fast Changes in the Bioenergetic Balance of Krill in Response to Environmental Stress. Front. Mar. Sci. 8:782524. doi: 10.3389/fmars.2021.782524
Received: 24 September 2021; Accepted: 14 December 2021;
Published: 27 January 2022.
Edited by:
Menghong Hu, Shanghai Ocean University, ChinaReviewed by:
Nelly Tremblay, Maurice Lamontagne Institute, Fisheries and Oceans Canada, CanadaCopyright © 2022 Lagos, Curtsdotter, Agüera, Sabadel, Burrit and Lamare. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paulo F. Lagos, cGF1LmxhZ29zakBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.