- 1Guangzhou Key Laboratory of Aquatic Animal Diseases and Waterfowl Breeding, Guangdong Provincial Water Environment and Aquatic Products Security Engineering Technology Research Center, College of Animal Sciences and Technology, Zhongkai University of Agriculture and Engineering, Guangzhou, China
- 2Guangdong Provincial Key Laboratory for Healthy and Safe Aquaculture, College of Life Science, South China Normal University, Guangzhou, China
Gonad-inhibiting hormone (GIH) belongs to a family of neuropeptides that are released from the eyestalks of male crustaceans and plays key roles in gonadal maturity, reproduction, and molting. However, the detailed mechanisms underlying the effects of GIH on sexual regulation have yet to be elucidated. In the present study, we aimed to demonstrate how GIH mediate the activity of the androgenic gland (AG) to affect sexual regulation. To do this, we cloned and characterized a GIH sequence from Macrobrachium rosenbergii (MrGIH). The open reading frame (ORF) of MrGIH was 360 bp and codes for a polypeptide of 119 amino acids and a putative protein of 13.56 KDa. Tissue analysis showed that MrGIH is widely expressed in a range of tissues but particularly, the eyestalk, intestine, and nerve cord. Following the dsRNA silencing of MrGIH for 24 h, the expression levels of MrGIH were down-regulated in both the eyestalk and AG when compared with the negative control, but significantly increased the expression of Macrobrachium rosenbergii insulin-like androgenic gland hormone-binding protein (MrIAGBP) in AG, thus suggesting that MrGIH is an inhibitory factor for MrIAGBP. In addition, we found that eyestalk removal on certain days led to increased expression levels of MrIAGBP expression. The expression levels of MrIAGBP peaked at 2 d in the AG after unilateral and bilateral eyestalk ablation, exhibiting a 7.27- and 6.03-fold increase, respectively. Afterward, the expression of GIH protein levels were down-regulated and IAGBP protein levels were up-regulated after GIH silencing using immunohistochemistry method, combined with the increase of IAGBP protein levels after eyestalk ablation, confirming that MrGIH is an inhibitory factor that can moderately regulate AG development and IAGBP expression. Collectively, our findings enriched the mechanisms that control the sexual regulation pathway of male M. rosenbergii, and provided significant information for further explorations of the mechanism underlying sex regulation in other decapod crustaceans.
Introduction
The mechanisms of sex determination and differentiation in Macrobrachium rosenbergii, a commercially important species of giant freshwater prawn, have been the subject of significant research interest in terms of evolutionary biology and endocrinology. Previous work, based on the pattern of sexual dimorphism in many species of crustaceans, led to the use of monosex culture (all-male or all-female) in the commercial sector as this was the best strategy to achieve the highest yield (Ventura and Sagi, 2012; Levy et al., 2016). In addition, following sexual maturation, growth rate decline and more energy is partitioned toward reproductive events (Wang and Xu, 2019). Recent research has shown that environmental factors have led to the onset of sexual maturation in some crustaceans at a much earlier time than normal, thus resulting in slow growth and reduced production (Wilder et al., 2010). Therefore, it is of great significance to study the mechanisms associated with sex differentiation that involves gonad-related gene expression as this may be advantageous with regards to improving yield. However, the dynamic processes underlying the regulation of gonad-inhibiting factors in the giant freshwater prawn have yet to be elucidated.
In crustaceans, sexual maturation is an elaborate molecular process that is regulated by an endocrine network (Huberman, 2000). Gonad-related hormones are closely involved with sexual regulation and have the activity to inhibit spermatogenesis (Fingerman, 1997a) in the androgenic gland (AG) of male crustaceans. In mammals, spermatogenesis relies upon a number of hormones that act within the hypothalamic-pituitary-gonadal axis (Godwin, 2010; Kanda, 2019). As with the mammalian hypothalamic nervous system, decapodal crustaceans also use an endocrine axis to regulate male sex differentiation; this is referred to as the eyestalk-androgenic gland (AG)-testis (Khalaila et al., 2002). The AG is an endocrine organ that is unique to male crustaceans and exerts functionality by secreting a sex-controlling switch element known as insulin-like androgenic gland (IAG) hormone (Levy and Sagi, 2020). A series of experiments have proven that AG is able to control the sexual differentiation of the male reproductive system. For example, the transplantation of the AG into females can lead to the masculinization of females while the removal of the AG from males can result in the feminization of males (Suzuki and Yamasaki, 1991; Sagi et al., 1999; Hoang et al., 2006). Furthermore, the eyestalk is also an endocrine organ and contains an endocrine regulatory center-X-organ-Sinus gland (XO-SG) complex (Hanström, 1931). The XO-SG mainly secretes neurohormones from the crustacean hyperglycemic hormone (CHH)-superfamily; some of these are involved in a range of complex biological activities (Webster et al., 2012).
Gonad-inhibiting hormone (GIH) is a key member of the CHH-superfamily and is produced by the XO-SG complex in the eyestalk (Hopkins, 2012). GIH is also called vitellogenesis-inhibiting hormone (VIH) in females for its main function to inhibit the vitellogensis of female shrimps and crabs (Medina et al., 1996; Yano et al., 1996). GIH is indispensable because it can inhibit gonad-relate hormones in male crustaceans. Previously, GIH has been studied with regards to reproductive function (Warrier et al., 2001; Treerattrakool et al., 2008); such studies have revealed that GIH plays roles in ovarian maturation and testicular development. In vivo research involving Scylla serrata (Haihui et al., 2006), Procambarus clarkii (Kulkarni et al., 1991), and Parapenaeus longirostris (Tom et al., 1987) demonstrated that the GIH gene can exert direct influence on the ovary by inhibiting the expression of vitellogenin. A previous study also demonstrated that GIH exhibited the same activity of molt-inhibiting hormone (MIH) which also belongs to the CHH superfamily (Chang et al., 1987; de Kleijn et al., 1994; Gu et al., 2002). It has been well established that sex differentiation in crustaceans is jointly regulated by neuropeptides and hormones, and that the endocrine organs that are involved in the pathway of sexual regulation mainly include the eyestalk and glands. Surprisingly, GIH acts directly on the AG rather than the testis in male crustaceans. The GIH gene interacts with other AG genes containing insulin-like androgenic gland hormone (IAG) (Ventura et al., 2011), insulin-like androgenic gland hormone receptor (IAGR) (Sharabi et al., 2016), and insulin-like androgenic gland hormone-binding protein (IAGBP) (Yang et al., 2020) to regulate sexual reproduction and the maturation of gonads (Fingerman, 1997a,b). The GIH gene, which encodes a specific eyestalk-derived hormone, exerts action on the eyestalk-androgenic gland (AG)-testis axis to mediate the activity of the AG (Khalaila et al., 2002). However, the regulatory mechanisms underlying the role of GIH in the sexual differentiation of M. rosenbergii have yet to be systematically reported.
In this study, we cloned a cDNA encoding GIH from Macrobrachium rosenbergii (MrGIH) and investigated its potential role on the eyestalk-androgenic gland (AG)-testis axis. We also used double-stranded (ds)RNA to create a functional knockdown of MrGIH. We also applied eyestalk ablation to demonstrate the effects of GIH on IAG, IAGR, and IAGBP expression in the eyestalk and AG of male M. rosenbergii. Our research sheds light on the mechanisms underlying the regulation of sexual reproduction and provides evidence that GIH plays a critical role in a number of key processes.
Materials and Methods
Animals, Reagents, and Experimental Design
Male giant freshwater prawns (M. rosenbergii), weighing 15–40 g in weight and 10–20 cm in length, were obtained from Jin Yang Aquaculture Co., Ltd. (Guangzhou, China) and then cultured in a recirculating tank system at 28 ± 2°C with uninterrupted aeration in our laboratory. Young males, weighing 35 ± 5 g, were separated and had their eyestalks surgically removed. A group of smaller individuals, weighing 20 ± 5 g, were separated and used for dsGIH gene knockdown. These animals were grown in a feed-free culture system after manipulation. Total RNAs (1 μg) were extracted using RNAiso plus (TaKaRa) and first−strand cDNA was synthesized using HIScript® Q Select RT SuperMix for qPCR (Vazyme, Dalian, China) in accordance with the manufacturer’s instructions. The specific primers used in this study are shown in Table 1.
RNAiso Plus was purchased from TaKaRa Bio (TaKaRa, Japan). HIScript® Q Select RT SuperMix and ChamQ SYBR qPCR Master Mix were both obtained from Vazyme (Nanjing, China). Rabbit polyclonal antibodies against MrGIH and MrIAGBP (Yang et al., 2020) were produced and purified by Frdbio Bioscience and Technology Company (Wuhan, China) and stored at −80°C prior to use.
Nucleotide Sequences and Bioinformatic Analysis of MrGIH
Once identified, we used the full-length of MrGIH nucleotide sequence as a basis to predict the open reading frame (OFR) of MrGIH and its amino acid (AA) sequence by Emboss.1 The signal peptide prediction of MrGIH was used the SignalP-5.02 (Chen et al., 2014). SMART servicer3 was used to identify putative protein domains from the MrGIH sequence and SWISS-MODEL4 was used to model protein structure homology (Lu et al., 2020). Clustal X 2.0 program and DNAMAN software package were then used to perform multiple sequence alignments. We also generated a phylogenetic tree, based on the ORF AA sequences of MrGIH proteins; this was carried out by the neighbor-joining (NJ) method using the molecular evolution genetics analysis tool (MEGA 6.0).
Tissue Distribution Profile of MrGIH in M. rosenbergii
Next, we investigated the tissue distribution of MrGIH in M. rosenbergii by collecting a range of tissues from healthy prawns, including brain (B), heart (Ht), stomach (S), gill (G), testis (Te), androgenic gland (AG), eyestalk (Es), intestine (In), and ventral nerve cord (VNC). Once harvested, these tissues were stored at −80°C for RNA extraction with the RNAiso Plus system (TaKaRa, Dalian, China). First-strand cDNA was synthesized by reverse transcription, in accordance with the manufacturer’s instructions, using the HiScript® Q Select RT SuperMix for qPCR (+gDNA wiper) kit. In brief, the expression profile of MrGIH was tested in each of the tissue types by quantitative polymerase chain reaction (qPCR). The β−actin gene (Yang et al., 2020) was used as an internal reference and the 2–ΔΔCT method was used to calculate the relative expression levels of MrGIH.
The Preparation of dsRNA and MrGIH Silencing
To silence the function of MrGIH in vivo, we used specific primer sequences (Table 1) that were linked to the T7 promoter by using a commercial transcription T7 kit (Fermentas, United States) and followed previously described methods (Qin et al., 2019a). In order to determine the gene silencing efficiency, a group of small individuals (20 ± 5 g) were divided into three groups (n = 4); animals from two groups were injected with dsGIH-1 or dsGIH-2 (5 μg/g body weight), respectively (Sharabi et al., 2016), while individuals from the other group were injected with dsGFP to serve as a control group. We evaluated the silencing efficacy of MrGIH and its effect on three gonad-related genes (MrIAG, MrIAGR, MrIAGBP) by performing a single injection into the abdominal muscles between the prawn’s 4th and 5th pleopods (Levy et al., 2016) and then collecting a range of target tissues 24 h post-injection for gene expression analysis by quantitative real-time (qRT)–polymerase chain reaction (PCR) as described in our previous report (Lu et al., 2021).
Eyestalk Ablation Assays
Young males were equally divided into three groups (n = 44 per group): (1) a unilateral eyestalk ablation group; (2) a bilateral eyestalk ablation group; and (3) a control group (intact individuals). We performed treatments on different days (2, 4, 6, and 8 days). Following eyestalk removal, we acquired AG tissues from both the experimental group and the control group, respectively. In total, we cultured M. rosenbergii which had undergone eyestalk ablation for 8 days; four individuals during were dissected and sampled every 2 days. Then, relative gene expression levels were determined by qRT-PCR.
Immunofluorescence Assays
To monitor the release of MrGIH and MrIAGBP protein from target sections following dsGIH knockdown and eyestalk ablation, we conducted immunofluorescence signal assessment, as described previously (Qin et al., 2019b) but with minor modifications. Following dsRNA silencing and surgical removal experiments, we harvested eyestalk and AG tissues and fixed these at 4°C for 24 h in 4% paraformaldehyde solution. These tissues were then rehydrated in ethyl alcohol, dewaxed in xylene, and then cut into small sections. Later, the samples were incubated with anti-MrGIH, anti-MrIAGBP and dye-linked goat Cy3 conjugated anti-rabbit IgG (Guge Biotech, China), successively. DAPI was used to stain nuclei. Immunofluorescence signals were monitored by fluorescence microscopy (Leica DMI8, Germany).
Statistical Analyses
All data are represented as mean ± standard deviation (SD; n = 3). Statistical analyses were performed using SSPS version 23.0. Statistical significance was determined by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test. Figures were created by GraphPad Prism 7 and statistical significance was set at p < 0.05.
Results
Sequence Analysis and Molecular Characteristics of the MrGIH Gene
Sequence analysis showed that the ORF of MrGIH was 360 bp in length and encoded a MrGIH protein that was 13.56 kDa in size and 119 amino acids (AAs) in length, while has a 40-aa signal peptide (Figure 1A). Moreover, SMART showed that MrGIH was a crustacean neurohormone. Protein modeling revealed that the three-dimensional (3D) structure of the MrGIH gene featured alpha helices in the N-terminal region and shared 51.28% sequence identity with MIH from the Kuruma prawn (Figure 1B).
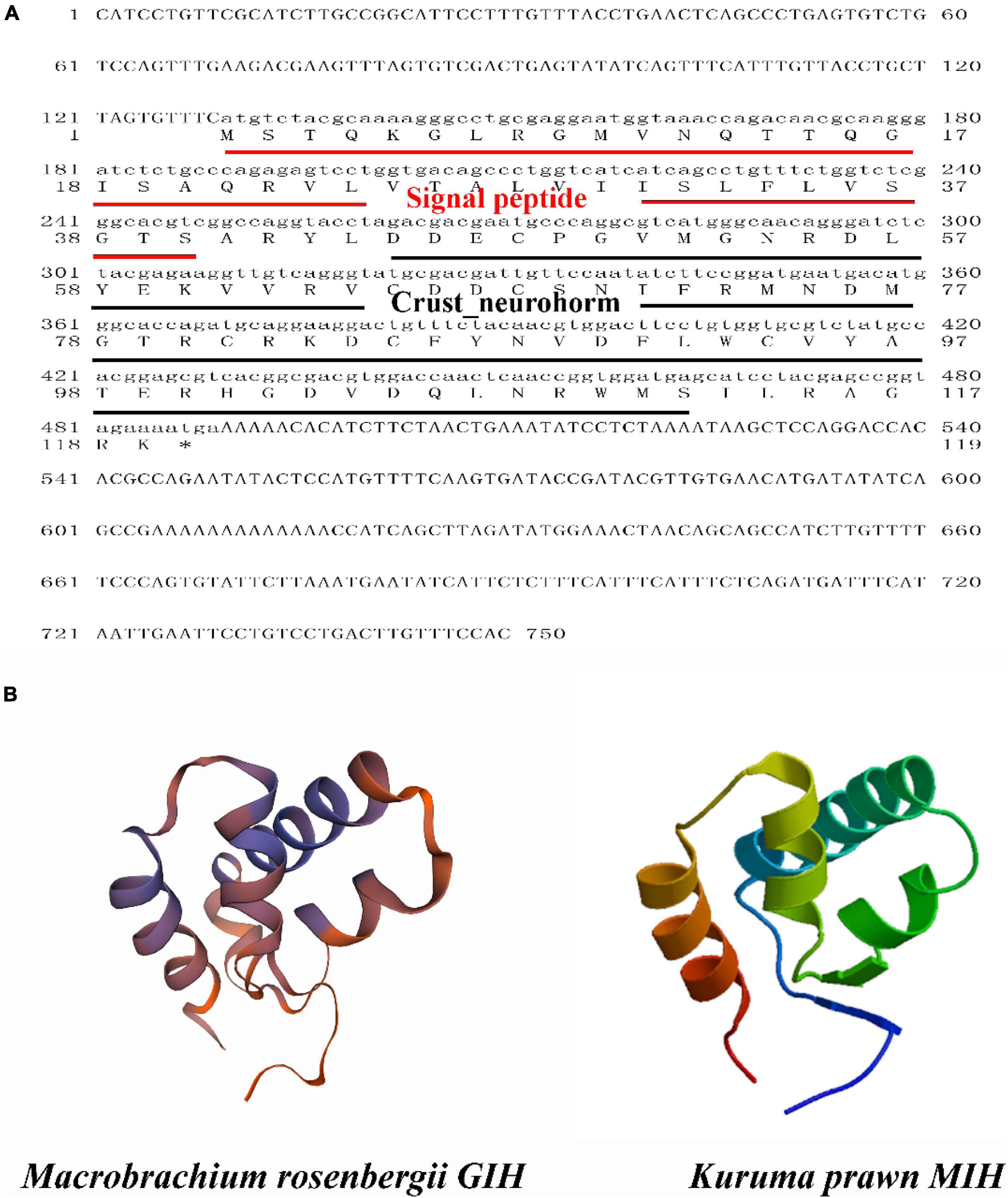
Figure 1. The sequence analysis of MrGIH. (A) The full-length ORF nucleotide sequence and deduced amino acid sequence of MrGIH. Signal peptide was underlined in red lines. Crustacean neurohormone was underlined in black lines. (B) Protein structural homology of MrGIH was modeled by SWISS-MODEL service, which built a high quality of 3D model with kuruma prawn MIH protein.
Multiple Alignment and Phylogenetic Analysis of MrGIH
Multiple alignment showed that the amino acids of MrGIH shared 98.18% identity with Macrobrachium nipponense (AEJ54622.1), 89.09% identity with Palaemon carinicauda (AIJ49750.1), 83.64% identity with Chorismus antarcticus (ANQ38670.1), 58.93% identity with Homarus gammarus (ABA42181.1), 48.21% identity with Charybdis japonica (ACD11361.1), 47.22% identity with Eriocheir sinensis (AAQ81640.1), 48.05% identity with Cancer pagurus (CAB61424.1), 44.93% identity with Trachypenaeus curvirostris (AAL55259.1), 34.62% identity with Cherax quadricarinatus (AWK57516.1), and 31.67% identity with Litopenaeus vannamei (KF879913.1) (Figure 2). Phylogenetic analysis further showed that in the GIH of M. rosenbergii was most similar to MIH from M. nipponense (with a sequence similarity of 98.18%), followed by GIH from P. carinicauda (with a sequence similarity of 89.09%) (Figure 3).
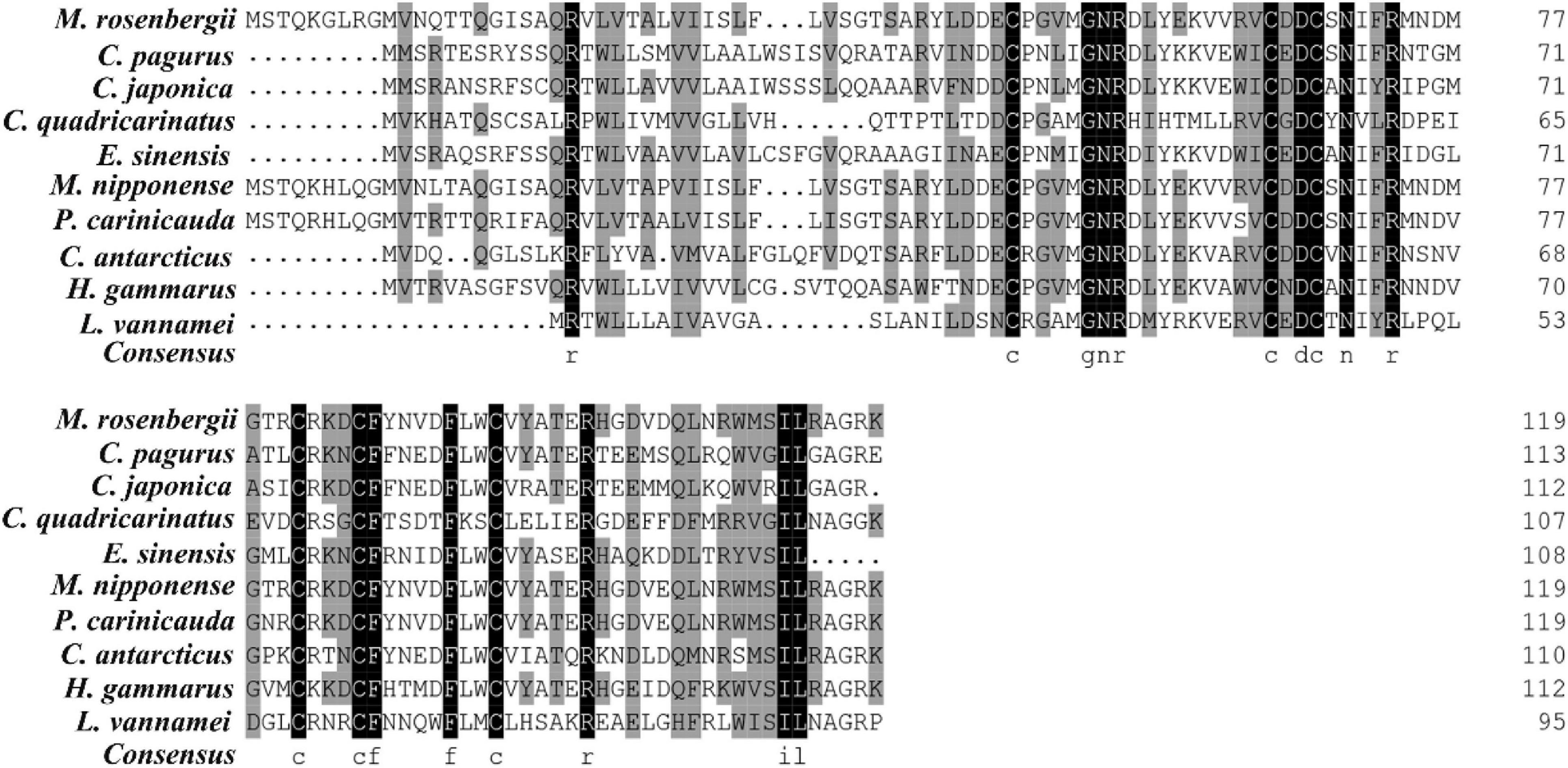
Figure 2. Multiple-sequence alignments of MrGIH with other crustaceans. Species of Cancer pagurus (CAB61424.1), Charybdis japonica (ACD11361.1), Cherax quadricarinatus (AWK57516.1), Eriocheir sinensis (AAQ81640.1), Macrobrachium nipponense (AEJ54622.1), Palaemon carinicauda (AIJ49750.1), Chorismus antarcticus (ANQ38670.1), Homarus gammarus (ABA42181.1), Litopenaeus vannamei (KF879913.1) were aligned. Highly conserved amino acids were represented by black background.
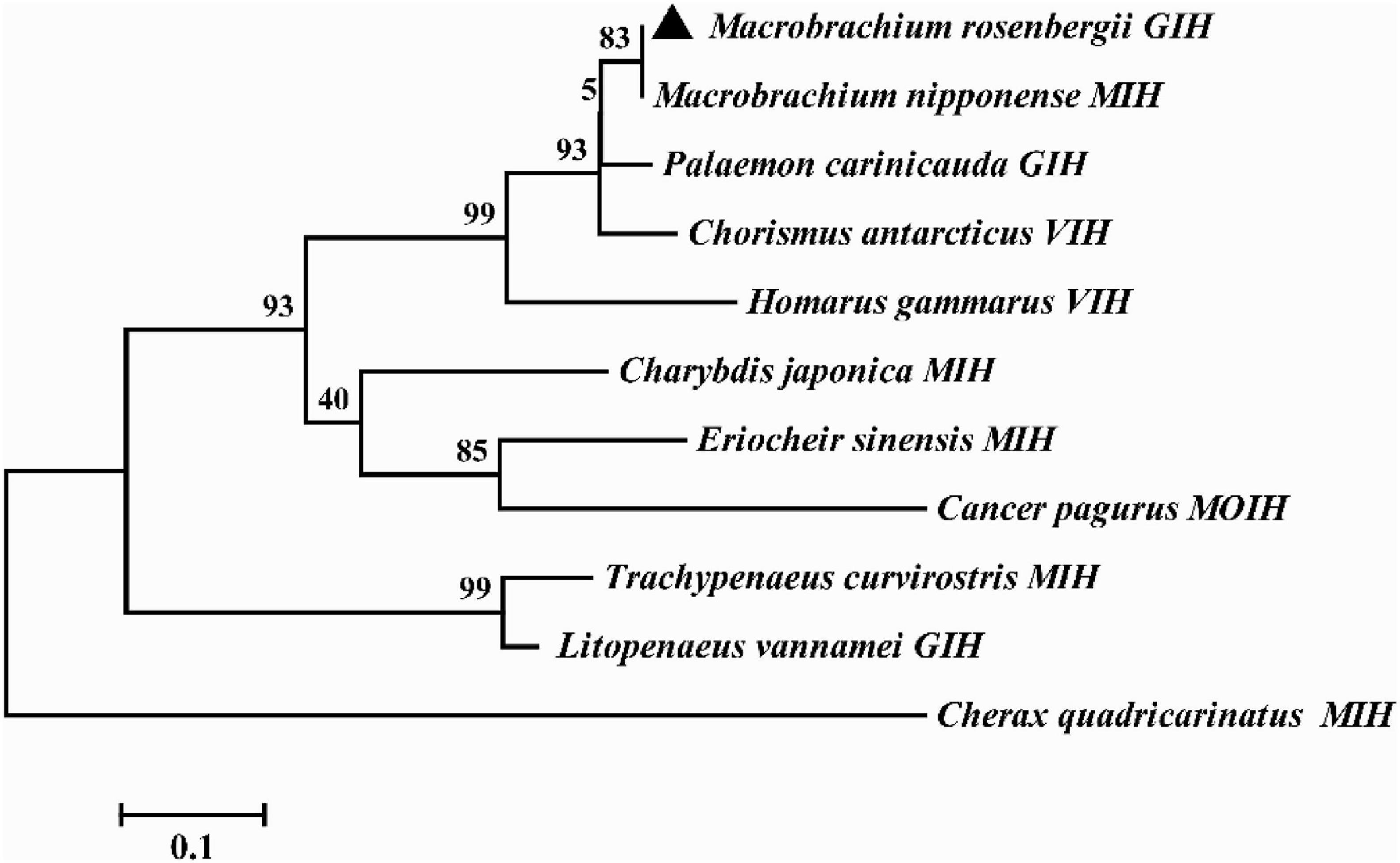
Figure 3. Phylogenetic tree of MrGIH with other homologous species. The amino acid sequences of MrGIH from M. rosenbergii (marked with a triangle) and other species are listed below: Macrobrachium nipponense (AEJ54622.1), Palaemon carinicauda (AIJ49750.1), Chorismus antarcticus (ANQ38670.1), Homarus gammarus (ABA42181.1), Charybdis japonica (ACD11361.1), Eriocheir sinensis (AAQ81640.1), Cancer pagurus (CAB61424.1), Trachypenaeus curvirostris (AAL55259.1), Cherax quadricarinatus (AWK57516.1), Litopenaeus vannamei (KF879913.1).
Tissue Expression Profile of MrGIH
Target tissues were harvested and qRT-PCR was used to determine the spatial distribution profile of MrGIH. We found that MrGIH mRNA was expressed at very high levels in the eyestalk both of females and males; Interestingly in the brain, female GIH is highly expressed, while male GIH is low expressed; MrGIH was also expressed in a range of other tissues, but with lower levels; for example, in the androgenic gland, ovary, testis, stomach, gill, and heart (Figure 4).
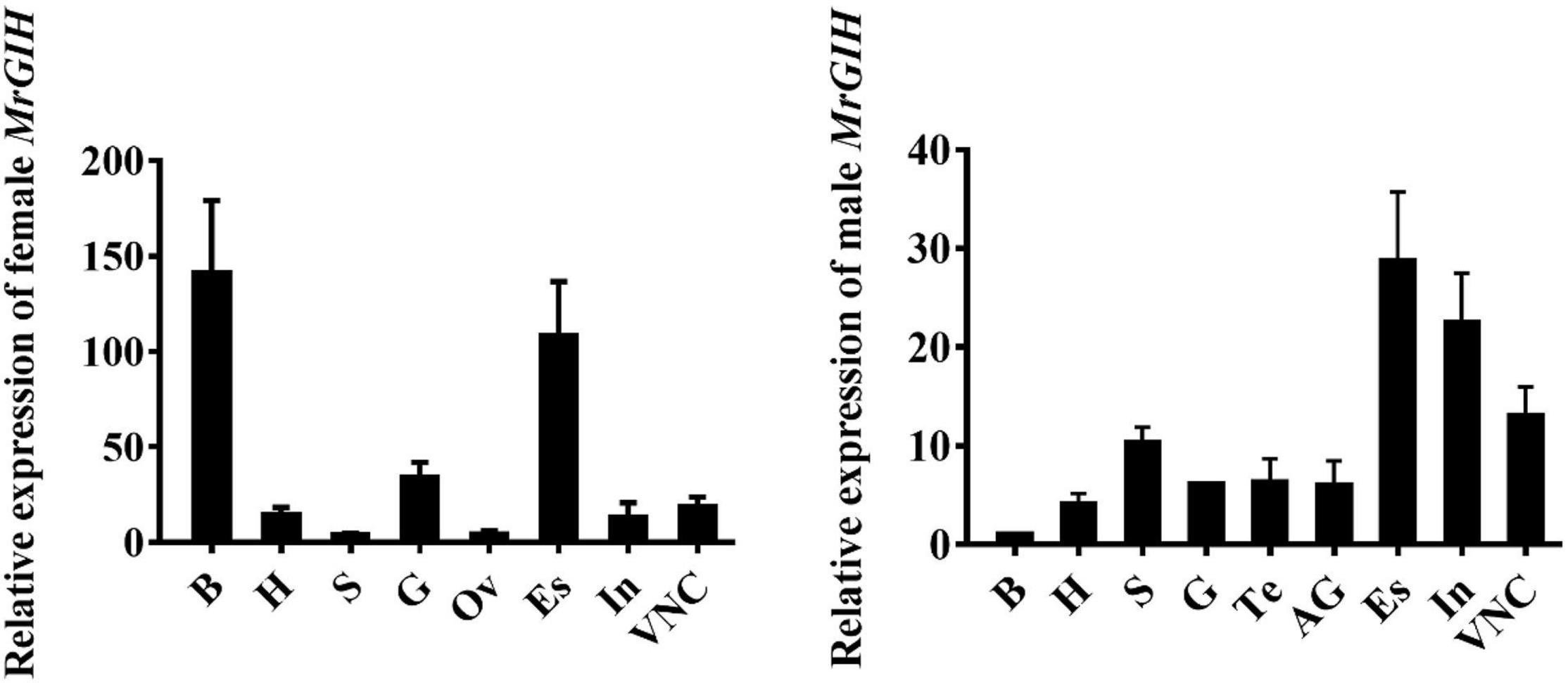
Figure 4. Tissue distribution analysis of MrGIH. Different tissues from healthy female and male M. rosenbergii were as followed: Brain (B), Heart (Ht), Stomach (S), Gills (G), Testis (Te), Ovary (Ov), Androgenic gland (AG), Eyestalk (Es), Intestine (In), and Ventral Nerve Cord (VNC). β-actin gene is as an internal reference to normalize MrGIH mRNA.
The Effects of dsRNA-Induced MrGIH Silencing on Sexual Regulation
Next, we performed experiments to investigate the effects of MrGIH silencing on sexual regulation in the eyestalk and AG. First, we investigated the efficiency of the two dsRNAs (dsGIH-1, dsGIH-2) to influence the expression of MrGIH in the eyestalk and AG. Analysis showed that dsGIH-1 resulted in a knockdown efficiency of 39% in the eyestalk and 90% in the AG; the dsGIH-2 approach resulted in a knockdown efficiency of MrGIH expression by 37% in the eyestalk and 98% in the AG (Figure 5), thus suggesting that the dsRNAs created by our special primers led to a significant reduction in the expression of MrGIH. It is suggested that dsGIH-2 should be used to detect MrGIH expression in the subsequent immunofluorescence assays. As shown in Figure 6, the expression of MrGIH was down-regulated by dsGIH-2 in the eyestalk and AG when compared to the dsGFP group.
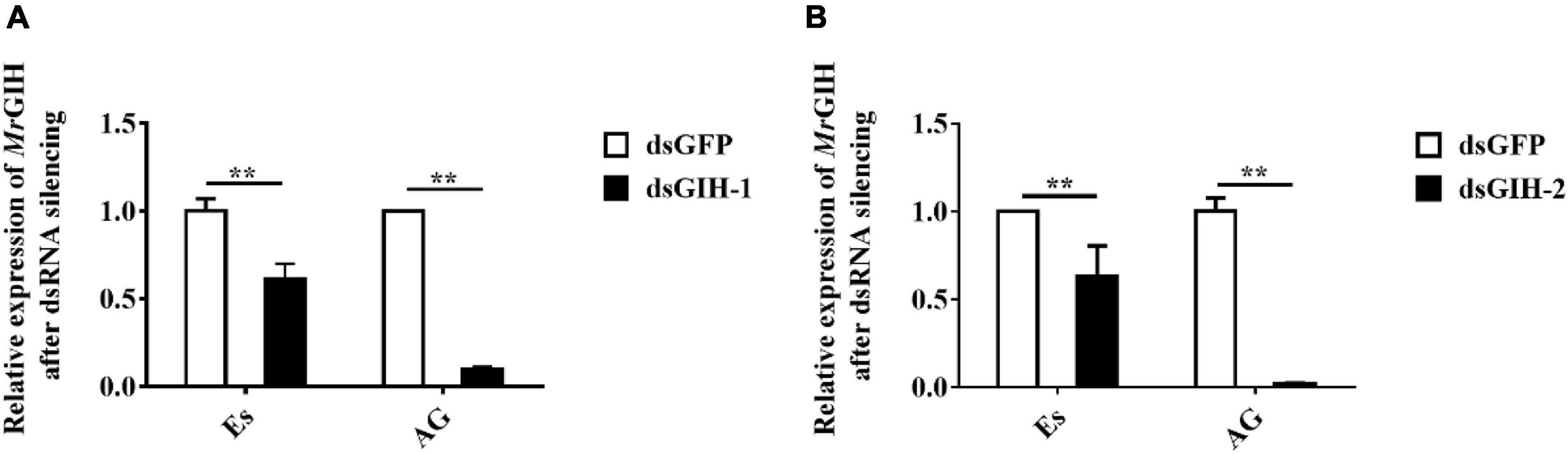
Figure 5. The mRNA expression levels of MrGIH after gene silencing. (A) The silencing efficiency of MrGIH injected with dsMrGIH-1. (B) The silencing efficiency of MrGIH injected with dsMrGIH-2, **P < 0.001.
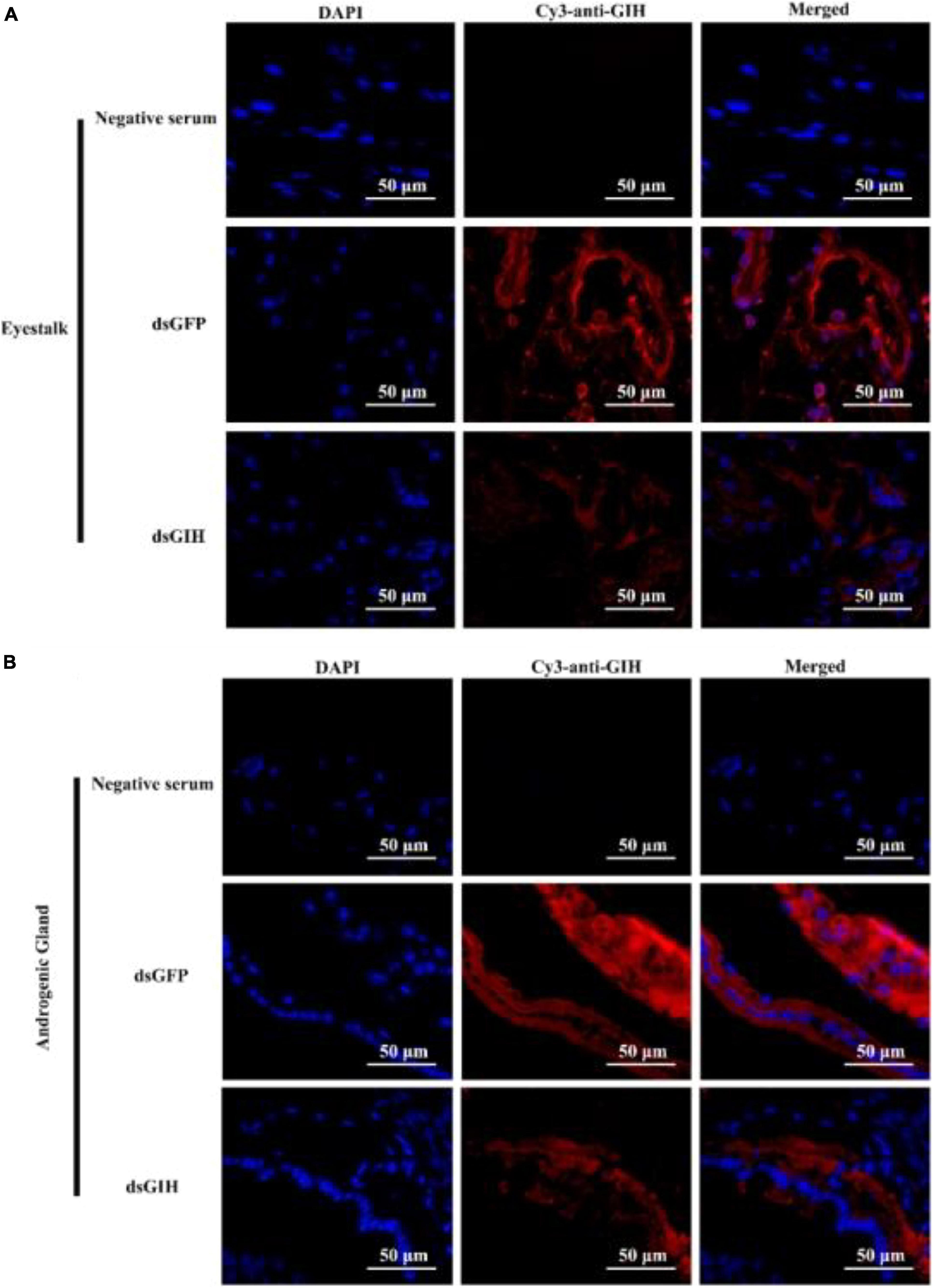
Figure 6. The protein expression levels of MrGIH after gene silencing using immunohistochemistry. dsGFP, injected with dsGFP RNA; dsGIH, injected with dsGIH RNA. MrGIH was in red fluorescence signals. The protein expression levels of MrGIH in the eyestalk (A) and androgenic gland (B) after injected with dsMrGIH at 24 h. Negative serum served was as the control. Scale bar was indicated 50 μm.
Twenty-four hours after the knockdown of MrGIH, qRT-PCR showed that the mRNA expression of MrIAG and MrIAGR had increased significantly in the AG; there was no significant difference with regards to the expression levels of these two mRNAs in the eyestalk (Figures 7A,B). Surprisingly, the expression levels of MrIAGBP mRNA were significantly upregulated in both the AG and the eyestalk (Figure 7C). Therefore, we carried out further immunofluorescence assays to investigate the specific relationship between the MrGIH and MrIAGBP proteins. Analysis showed that MrIAGBP protein expression increased in the eyestalk and AG following the knockdown of MrGIH (Figures 7D,E). Collectively, these results indicate that MrGIH is an inhibitory factor that inhibits sex-related genes in the “eyestalk-AG-testis” pathway.
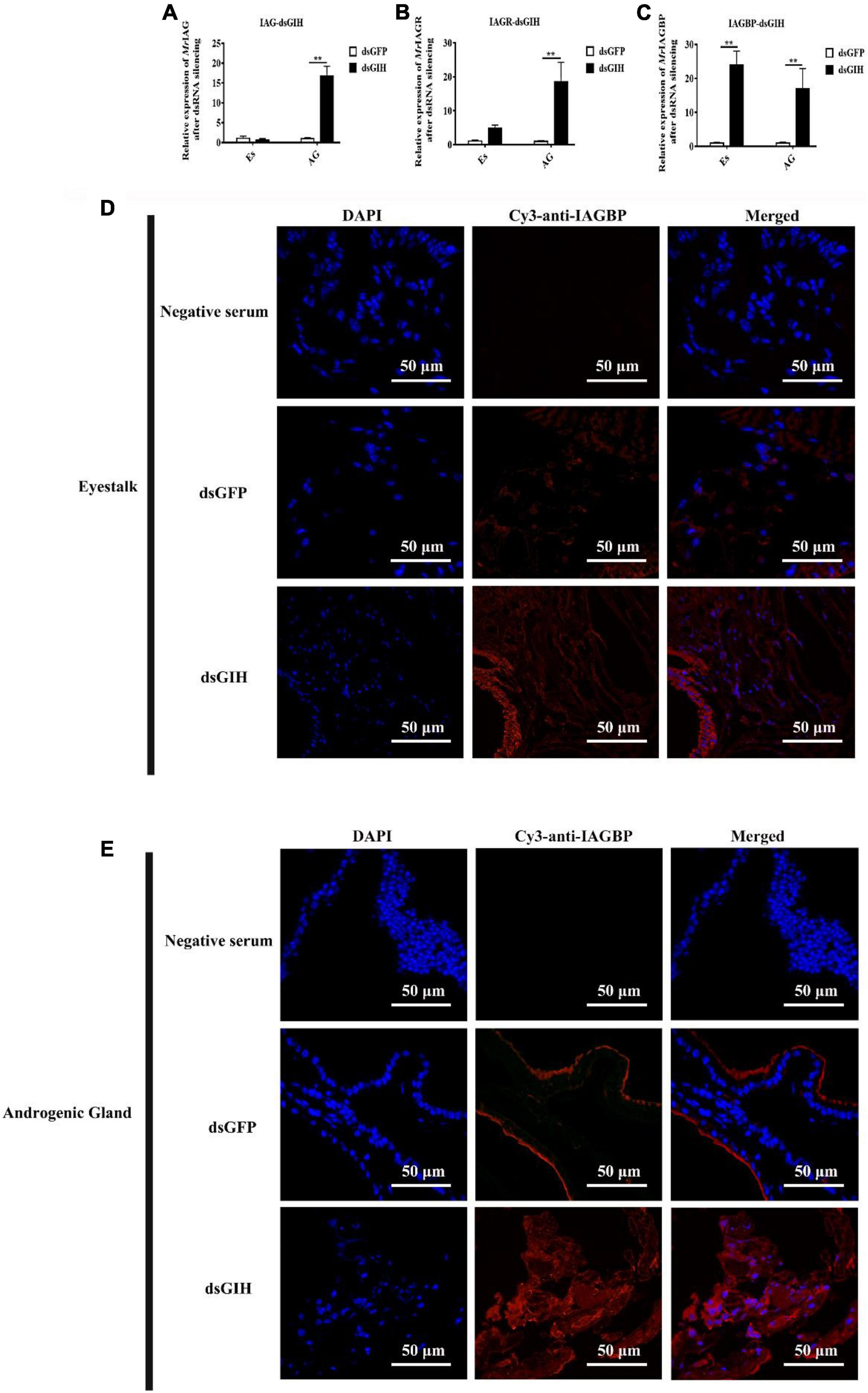
Figure 7. Effects of dsRNA-MrGIH injection. The mRNA expression levels of MrIAG (A), MrIAGR (B), and MrIAGBP (C) in eyestalk (Es) and androgenic gland (AG) after MrGIH knockdown at 24 h. dsGFP, injected with dsGFP RNA; dsGIH, injected with dsGIH RNA. The protein expression levels of MrIAGBP in the eyestalk (D) and androgenic gland (E) after injected with dsMrGIH at 24 h. MrIAGBP was in red fluorescence signals. Negative serum served was as the control. Scale bar was indicated 50 μm. **P < 0.001.
Surgical Removal of the Eyestalk in M. rosenbergii
As shown in Figure 4, the highest expression levels of MrGIH were identified in the eyestalks of healthy male prawns. To further investigate the regulatory activity of MrGIH hormone in more detail, we designed an experiment involving unilateral or bilateral eyestalk removal in male M. rosenbergii. Following unilateral eyestalk ablation, the expression levels of MrIAG in the AG were significantly up-regulated at 4 and 8 d, exhibiting a 14.77- and 5.96-fold increase, respectively (Figure 8A). The expression levels of MrIAGR in the AG peaked (a 15.51-fold increase) at 2 d and remained at high levels (14. 57-, 11. 86-, and 9.84-fold increases at 4, 6, and 8 days, respectively) (Figure 8B). In the AG, the expression levels of MrIAGBP increased by 7.27, 5. 80-, 4. 04-, and 5.18-fold at 2, 4, 6, and 8 days, respectively (Figure 8C).
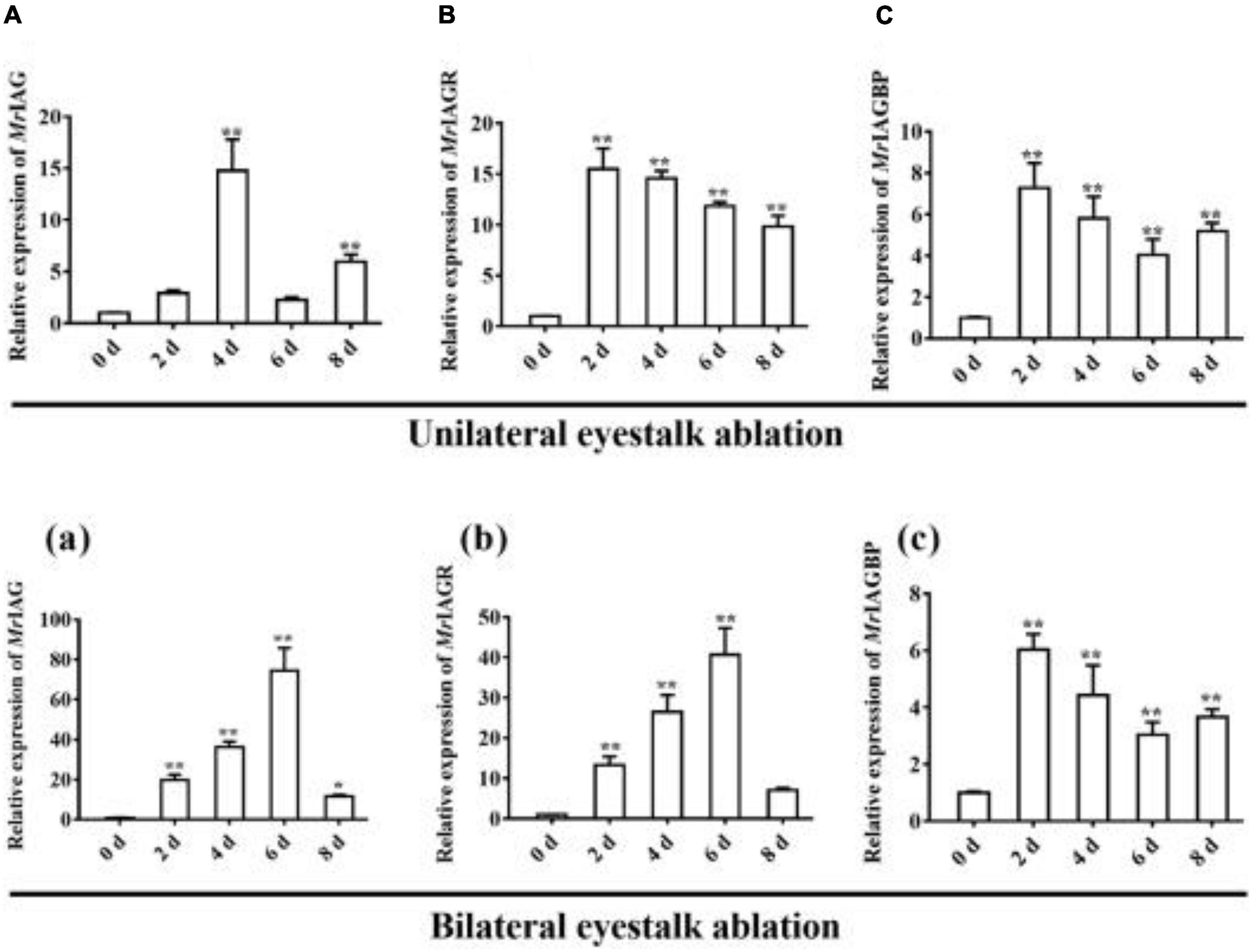
Figure 8. Expression profile of IAG, IAGR, and IAGBP gene on different days in androgenic gland after eyestalk ablation. (A–C) unilateral eyestalk ablation, (A) MrIAG, (B) MrIAGR, (C) MrIAGBP. (a–c): bilateral eyestalk ablation, (a) MrIAG, (b) MrIAGR, (c) MrIAGBP. MrIAGBP was in red fluorescence signals. mRNA levels were analyzed by qRT-PCR and normalized to β-actin. qRT-PCR data are shown as means ± SD (standard deviation). *P < 0.05, **P < 0.001.
Following bilateral eyestalk ablation, the expression levels of MrIAG in the AG increased significantly at 2 and 4 days, exhibiting a 19.78- and 36.27-fold increase, respectively. Levels were highest (a 74.36-fold increase) at the 6 d timepoint; by the 8 days timepoint, there was an 11.68-fold increase (Figure 8a). The expression levels of MrIAGR exhibited a 13. 31-, 26. 50-, 40. 65-, and 7.06-fold increase at 2, 4, 6, and 8 days, respectively (Figure 8b). The expression levels of MrIAGBP in the AG peaked at 2 d (a 6.03-fold increase) and remained high from 4 to 8 days, exhibiting a 4. 42-, 3. 03-, and 3.65-fold increase at 4, 6, and 8 days, respectively (Figure 8c). To further investigate the role of MrGIH protein under unilateral or bilateral eyestalk ablation in male M. rosenbergii, we evaluated the expression levels of MrIAGBP protein in the AG at 2, 4, 6, and 8 days by immunohistochemistry analyses; we used animals with intact eyestalks as controls. Our results showed strong immunostaining for MrIAGBP (red staining) in animals undergoing both unilateral (Figure 9) and bilateral eyestalk ablation (Figure 10) in AG tissues at 2, 4, 6, and 8 days. These results were confirmed by qRT-PCR, which also provided evidence for high expression levels of MrIAGBP in the AG following eyestalk ablation.
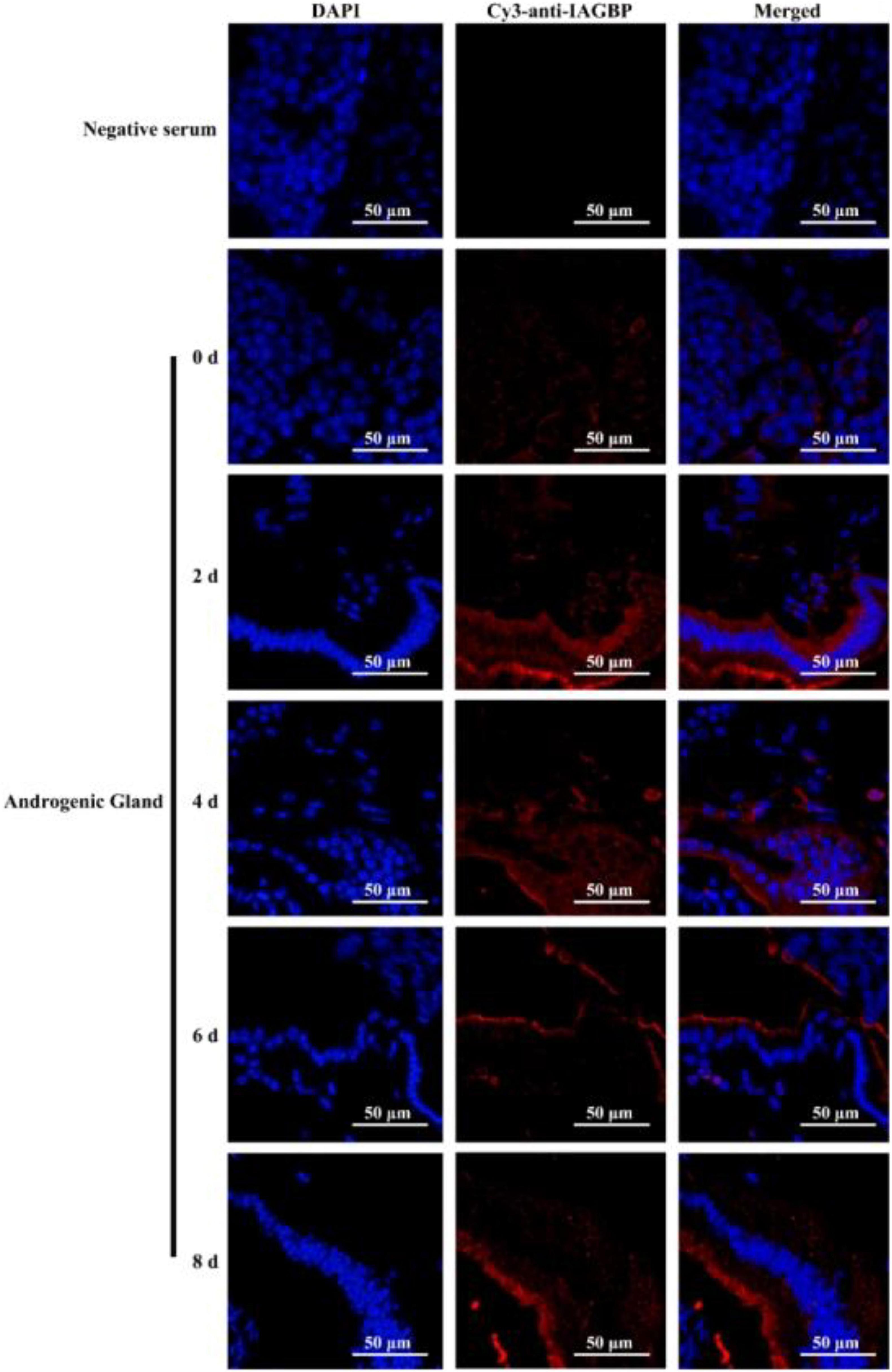
Figure 9. The protein expression levels of MrIAGBP on different days in androgenic gland after unilateral eyestalk ablation. MrIAGBP was in red fluorescence signals. Negative serum served was as the control. Scale bar was indicated 50 μm.
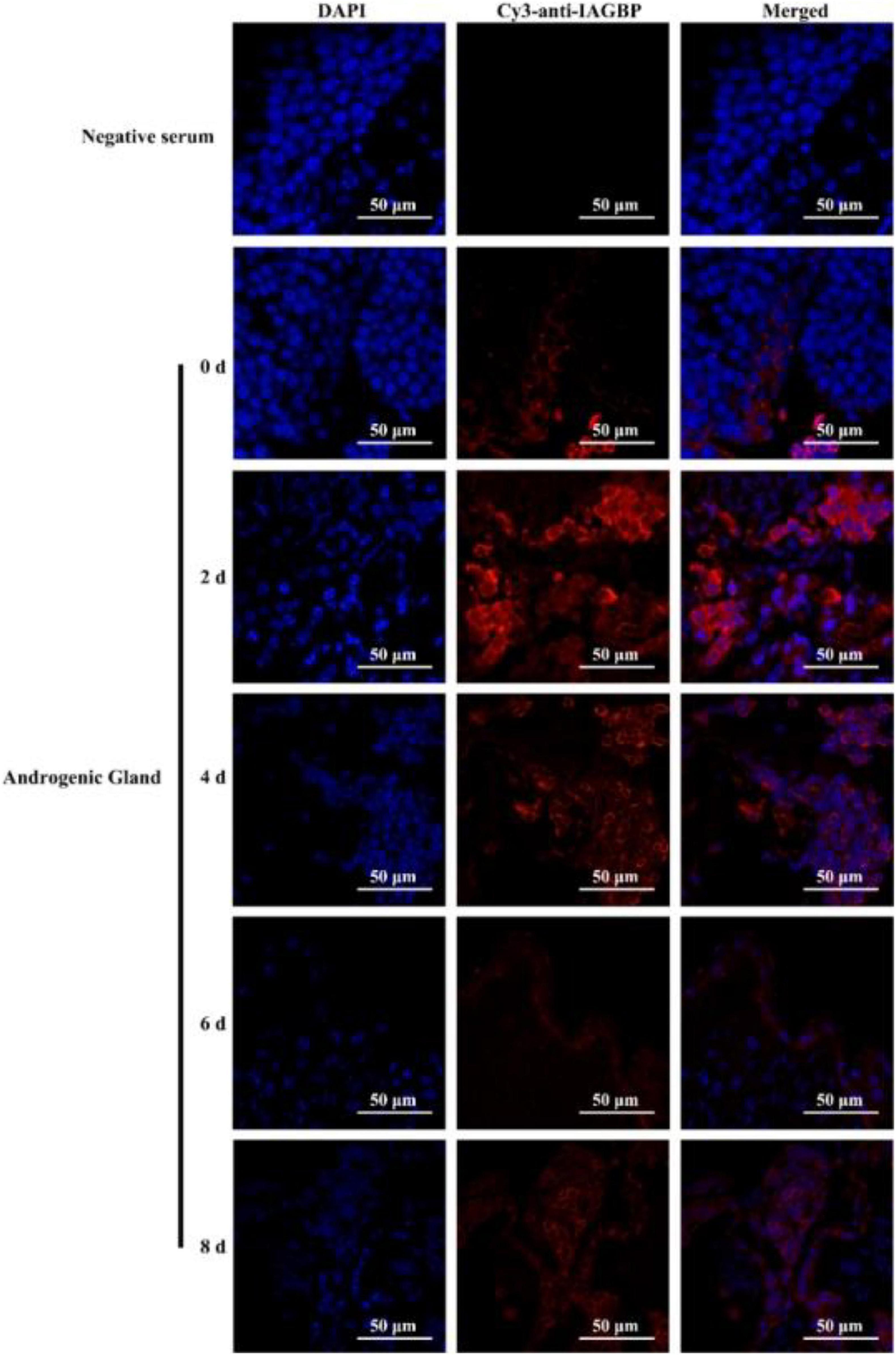
Figure 10. The protein expression levels of MrIAGBP on different days in androgenic gland after bilateral eyestalk ablation. MrIAGBP was in red fluorescence signals. Negative serum served was as the control. Scale bar was indicated 50 μm.
Discussion
Secretory hormones play an important role in sex determination and sex differentiation in crustaceans, particularly AG genes and genes from the CHH superfamily. The eyestalk-AG-testis axis is also known to be a significant signaling pathway in male crustaceans and plays a key role in regulating sexual determination (Khalaila et al., 2002). The IAG signaling pathway has been investigated in different species of crustaceans (Rosen et al., 2010; Chung et al., 2011; Herran et al., 2020; Tan et al., 2020; Liu et al., 2021). However, previous research did not identify the precise relationship between GIH-mediated activity in the AG and sex differentiation in Macrobrachium rosenbergii. Therefore, in the present study, we isolated and characterized the GIH gene from M. rosenbergii (referred to herein as MrGIH) and investigated its potential role in sexual regulation. As a member of the CHH superfamily II, MrGIH possess several conserved CHH domains, including the crust_neurohorm domain (Davey et al., 2000) but without a precursor-related peptide (Chung et al., 2010).
The ORF for the MrGIH cDNA was 360 bp in length and encoded a protein containing 119 amino acids, as also reported in previous studies (Shi et al., 2018; Chen et al., 2020). In addition, multiple sequence alignment and the generation of a phylogenetic tree indicated that MrGIH shared a high degree of similarity to MIH from Macrobrachium nipponense (Qiao et al., 2018). This suggests that in addition to its characteristic ability to inhibit gonads, the GIH gene can also regulate molting activity, as previously reported in Homarus americanus (de Kleijn et al., 1994). In the present study, we found that the MrGIH gene was mainly expressed in the eyestalks of Macrobrachium rosenbergii, although the transcription of GIH was also detected in the gills, mid-gut gland, hemolymph, nerve cord, thoracic ganglion, ovary, testis, muscle, and spermatophore, as previously reported for Nephrops norvegicus (Edomi et al., 2002), Penaeus monodon (Treerattrakool et al., 2008), and Fenneropenaeus (Shi et al., 2018).
The IAG signaling pathway is directly activated by the binding of insulin-like peptides to their receptors, including IAGR and IAGBP. For example, the knockdown of IAGBP in M. rosenbergii (Yang et al., 2020) and the knockdown of IAGR in M. nipponense (Li et al., 2015a) lead to the downregulation of IAG expression. The XO-SG, located at the base of the eyestalk, is the central-hub of the neuroendocrine system in crustaceans (Dircksen et al., 2001). Neurohormones of the CHH family are structurally related peptides that are encoded by a multigene family that is specific to arthropods (Hopkins, 2012). In decapods, these neurohormones are mainly produced by the XO-SG system, a major neuroendocrine organ situated in the eyestalk (De Kleijn and Van Herp, 1995). These hormones play important roles in metabolism, reproduction, and development in animals (Escamilla-Chimal et al., 2001). We aimed to investigate the role of GIH mediation in AG activity and establish whether eyestalk-derived genes regulate the sex-related genes in the eyestalk-AG-testis endocrine axis. In our study, we found that MrGIH was a member of the CHH subfamily II, as reported previously (Soyez, 1997; Chen et al., 2020). In this research, we propose that MrGIH, which serves as an upstream gene for the eyestalk-AG-testis endocrine axis and is responsible for inhibiting sexual maturation and sex differentiation in male M. rosenbergii. Thus, we performed dsRNA-MrGIH knockdown. We found that the knockdown of MrGIH facilitated the expression of MrIAG, MrIAGR, and MrIAGBP hormones and promoted the expression of MrIAGBP protein. Collectively, these data indicated that MrGIH was associated with sexual regulation systems in the male eyestalk, and acts as an inhibitory factor that regulates sex-related genes.
Eyestalk-derived factors, acting under hormonal stimulation, can also regulate other physiological processes (Chen et al., 2020). As part of the eyestalk-AG-testis pathway, the XO-SG complex can activate the expression of multiple target genes. In the crayfish Cherax quadricarinatus, the addition of sinus gland extract led to the significant inhibition of amino acid incorporation into the AG (Khalaila et al., 2002). Furthermore, eyestalk ablation led to hypertrophic glands and the overexpression of IAG hormones (Chung et al., 2011; Li et al., 2015b; Guo et al., 2019). In our study, we found that the unilateral and bilateral removal of eyestalks in M. rosenbergii led to an increase in the expression levels of MrIAG, MrIAGR, and MrIAGBP. This data proved that the presence of neuropeptides in the eyestalk could regulate the expression of the MrIAG, MrIAGR, and MrIAGBP genes. Our data suggest that MrGIH plays an essential role in regulating upstream components in the eyestalk-AG-testis pathway of prawns.
Collectively, we isolated and characterized GIH in M. rosenbergii and demonstrated its role as a key regulator of sexual differentiation and an inhibitor for the expression of sex-related genes. Furthermore, our findings have significant aquacultural implications because they highlight a pathway enabling that allows us to identify and analyze the GIH-induced mediation of AG activity and interactions with sex-regulatory mechanisms. Although previous research has described the effects of the eyestalk-AG-testis pathway on sexual activity in decapod crustaceans, the specific mechanisms underlying the effects of AG inhibitory factors produced by eyestalk and the axis itself still remain to be characterized and elucidated.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
LL and ZQ conceived and designed the experiments. MT and ZL performed the experiments and wrote the manuscript. GY, VB, and ZX analyzed the data. All authors read the manuscript and approved it in its final version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (nos. 31902409, 31872606, 31572657, and U1701233), the Foundation of Guangdong Provincial Marine and Fisheries Bureau (nos. GDME-2018C006 and D21822202), the Foundation of China-ASEAN Maritime Cooperation (no. CAMC-2018F), and the Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (no. 2019KJ141). JL was supported by a Pearl River Scholarship from Guangdong Province.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ https://www.bioinformatics.nl/emboss-explorer/
- ^ http://www.cbs.dtu.dk/services/SignalP/
- ^ http://smart.embl.de/
- ^ https://swissmodel.expasy.org/
References
Chang, E. S., Bruce, M. J., and Newcomb, R. W. (1987). Purification and amino acid composition of a peptide with molt-inhibiting activity from the lobster, Homarus americanus. General Comparat. Endocrinol. 65, 56–64. doi: 10.1016/0016-6480(87)90222-X
Chen, H. Y., Toullec, J. Y., and Lee, C. Y. (2020). The Crustacean Hyperglycemic Hormone Superfamily: Progress Made in the Past Decade. Front. Endocrinol. 11:578958. doi: 10.3389/fendo.2020.578958
Chen, T., Zhang, L. P., Wong, N. K., Zhong, M., Ren, C. H., and Hu, C. Q. (2014). Pacific white shrimp (Litopenaeus vannamei) vitellogenesis-inhibiting hormone (VIH) is predominantly expressed in the brain and negatively regulates hepatopancreatic vitellogenin (VTG) gene expression. Biol. Reproduct. 90:47. doi: 10.1095/biolreprod.113.115030
Chung, J. S., Manor, R., and Sagi, A. (2011). Cloning of an insulin-like androgenic gland factor (IAG) from the blue crab, Callinectes sapidus: implications for eyestalk regulation of IAG expression. General Comparat. Endocrinol. 173, 4–10. doi: 10.1016/j.ygcen.2011.04.017
Chung, J. S., Zmora, N., Katayama, H., and Tsutsui, N. (2010). Crustacean hyperglycemic hormone (CHH) neuropeptidesfamily: Functions, titer, and binding to target tissues. General Comparat. Endocrinol. 166, 447–454. doi: 10.1016/j.ygcen.2009.12.011
Davey, M. L., Hall, M. R., Willis, R. H., Oliver, R. W., Thurn, M. J., and Wilson, K. J. (2000). Five Crustacean Hyperglycemic Family Hormones of Penaeus monodon: Complementary DNA Sequence and Identification in Single Sinus Glands by Electrospray Ionization-Fourier Transform Mass Spectrometry. Mar. Biotechnol. 2, 80–91. doi: 10.1007/s101269900011
De Kleijn, D. P., and Van Herp, F. (1995). Molecular biology of neurohormone precursors in the eyestalk of Crustacea. Comparat. Biochem. Physiol. Part B Biochem. Mol. Biol. 112, 573–579. doi: 10.1016/0305-0491(95)00126-3
de Kleijn, D. P., Sleutels, F. J., Martens, G. J., and Van Herp, F. (1994). Cloning and expression of mRNA encoding prepro-gonad-inhibiting hormone (GIH) in the lobster Homarus americanus. FEBS Lett. 353, 255–258. doi: 10.1016/0014-5793(94)01055-2
Dircksen, H., Böcking, D., Heyn, U., Mandel, C., Chung, J. S., Baggerman, G., et al. (2001). Crustacean hyperglycaemic hormone (CHH)-like peptides and CHH-precursor-related peptides from pericardial organ neurosecretory cells in the shore crab, Carcinus maenas, are putatively spliced and modified products of multiple genes. Biochem. J. 356, 159–170. doi: 10.1042/bj3560159
Edomi, P., Azzoni, E., Mettulio, R., Pandolfelli, N., Ferrero, E. A., and Giulianini, P. G. (2002). Gonad-inhibiting hormone of the Norway lobster (Nephrops norvegicus): cDNA cloning, expression, recombinant protein production, and immunolocalization. Gene 284, 93–102. doi: 10.1016/S0378-1119(02)00389-X
Escamilla-Chimal, E. G., Van Herp, F., and Fanjul-Moles, M. L. (2001). Daily variations in crustacean hyperglycaemic hormone and serotonin immunoreactivity during the development of crayfish. J. Exp. Biol. 204, 1073–1081. doi: 10.1242/jeb.204.6.1073
Fingerman, M. (1997a). Crustacean endocrinology: a retrospective, prospective, and introspective analysis. Physiol. Zool. 70, 257–269. doi: 10.1086/639593
Fingerman, M. (1997b). Roles of neurotransmitters in regulating reproductive hormone release and gonadal maturation in decapod crustaceans. Int. J. Invertebrate Reproduct. 31, 47–54. doi: 10.1080/07924259.1997.9672562
Godwin, J. (2010). Neuroendocrinology of sexual plasticity in teleost fishes. Front. Neuroendocrinol. 31, 203–216. doi: 10.1016/j.yfrne.2010.02.002
Gu, P. L., Tobe, S. S., Chow, B. K., Chu, K. H., He, J. G., and Chan, S. M. (2002). Characterization of an additional molt inhibiting hormone-like neuropeptide from the shrimp Metapenaeus ensis. Peptides 23, 1875–1883. doi: 10.1016/S0196-9781(02)00178-X
Guo, Q., Li, S., Lv, X., Xiang, J., Manor, R., Sagi, A., et al. (2019). Sex-Biased CHHs and Their Putative Receptor Regulate the Expression of IAG Gene in the Shrimp Litopenaeus vannamei. Front. Physiol. 10:1525. doi: 10.3389/fphys.2019.01525
Haihui, Y., Huiyang, H., Shaojing, L., Guizhong, W., and Qifu, L. (2006). In vitro study of neuroendocrine regulation over the testicular development in mud crabs Scylla serrata. Chin. J. Oceanol. Limnol. 24, 142–146. doi: 10.1007/BF02842813
Hanström, B. (1931). Neue untersuchungen über sinnesorgane und nervensystem der crustaceen. I. Zeitschrift Morphol. Ökol. Tiere 23, 80–236. doi: 10.1007/BF00446350
Herran, B., Geniez, S., Delaunay, C., Raimond, M., Lesobre, J., Bertaux, J., et al. (2020). The shutting down of the insulin pathway: a developmental window for Wolbachia load and feminization. Sci. Rep. 10:10551. doi: 10.1038/s41598-020-67428-1
Hoang, E. D. A. T., Nguyen, V. H., Lam, Q., Nguyen, D. M., Trinh, Q. S., Raviv, S., et al. (2006). A novel two-step procedure for mass production of all-male populations of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 256, 468–478. doi: 10.1016/j.aquaculture.2006.01.035
Hopkins, P. M. (2012). The eyes have it: A brief history of crustacean neuroendocrinology. General Comparat. Endocrinol. 175, 357–366. doi: 10.1016/j.ygcen.2011.12.002
Huberman, A. (2000). Shrimp endocrinology. A review. Aquaculture 191, 191–208. doi: 10.1016/S0044-8486(00)00428-2
Kanda, S. (2019). Evolution of the regulatory mechanisms for the hypothalamic-pituitary-gonadal axis in vertebrates-hypothesis from a comparative view. General Comparat. Endocrinol. 284:113075. doi: 10.1016/j.ygcen.2018.11.014
Khalaila, I., Manor, R., Weil, S., Granot, Y., Keller, R., and Sagi, A. (2002). The eyestalk-androgenic gland-testis endocrine axis in the crayfish Cherax quadricarinatus. General Comparat. Endocrinol. 127, 147–156. doi: 10.1016/S0016-6480(02)00031-X
Kulkarni, G. K., Glade, L., and Fingerman, M. (1991). Oogensis and effects of neuroendocrine tissue on in vitro synthesis of protein by the ovary of the red swamp crayfish, Procambarus clarkii (Girard). J. Crust. Biol. 11, 513–522. doi: 10.2307/1548520
Levy, T., and Sagi, A. (2020). The “IAG-Switch”-A Key Controlling Element in Decapod Crustacean Sex Differentiation. Front. Endocrinol. 11:651. doi: 10.3389/fendo.2020.00651
Levy, T., Rosen, O., Eilam, B., Azulay, D., Aflalo, E. D., Manor, R., et al. (2016). A Single Injection of Hypertrophied Androgenic Gland Cells Produces All-Female Aquaculture. Mar. Biotechnol. 18, 554–563. doi: 10.1007/s10126-016-9717-5
Li, F., Bai, H., Xiong, Y., Fu, H., Jiang, S., Jiang, F., et al. (2015a). Molecular characterization of insulin-like androgenic gland hormone-binding protein gene from the oriental river prawn Macrobrachium nipponense and investigation of its transcriptional relationship with the insulin-like androgenic gland hormone gene. General Comparat. Endocrinol. 216, 152–160. doi: 10.1016/j.ygcen.2014.12.007
Li, F., Bai, H., Zhang, W., Fu, H., Jiang, F., Liang, G., et al. (2015b). Cloning of genomic sequences of three crustacean hyperglycemic hormone superfamily genes and elucidation of their roles of regulating insulin-like androgenic gland hormone gene. Gene 561, 68–75. doi: 10.1016/j.gene.2015.02.012
Liu, F., Shi, W., Ye, H., Zeng, C., and Zhu, Z. (2021). Insulin-like androgenic gland hormone 1 (IAG1) regulates sexual differentiation in a hermaphrodite shrimp through feedback to neuroendocrine factors. General Comparat. Endocrinol. 303:113706. doi: 10.1016/j.ygcen.2020.113706
Lu, Z., Tang, M., Li, Y., Shi, F., Zhan, F., Zhang, M., et al. (2021). Molecular cloning and characterization of FADD from the grass carp (Ctenopharyngodon idellus) in response to bacterial infection. Aquaculture 542:736829. doi: 10.1016/j.aquaculture.2021.736829
Medina, A., Vila, Y., Mourente, G., and Rodríguez, A. (1996). A comparative study of the ovarian development in wild and pond-reared shrimp, Penaeus kerathurus (Forskal, 1775). Aquaculture 148, 63–75. doi: 10.1016/S0044-8486(96)01408-1
Qiao, H., Jiang, F., Xiong, Y., Jiang, S., Fu, H., Li, F., et al. (2018). Characterization, expression patterns of molt-inhibiting hormone gene of Macrobrachium nipponense and its roles in molting and growth. PLoS One 13:e0198861. doi: 10.1371/journal.pone.0198861
Qin, Z., Sarath Babu, V., Lin, H., Dai, Y., Kou, H., Chen, L., et al. (2019a). The immune function of prophenoloxidase from red swamp crayfish (Procambarus clarkii) in response to bacterial infection. Fish Shellf. Immunol. 92, 83–90. doi: 10.1016/j.fsi.2019.05.005
Qin, Z., Vijayaraman, S. B., Lin, H., Dai, Y., Zhao, L., Xie, J., et al. (2019b). Antibacterial activity of erythrocyte from grass carp (Ctenopharyngodon idella) is associated with phagocytosis and reactive oxygen species generation. Fish Shellf. Immunol. 92, 331–340. doi: 10.1016/j.fsi.2019.06.008
Rosen, O., Manor, R., Weil, S., Gafni, O., Linial, A., Aflalo, E. D., et al. (2010). A sexual shift induced by silencing of a single insulin-like gene in crayfish: ovarian upregulation and testicular degeneration. PLoS One 5:e15281. doi: 10.1371/journal.pone.0015281
Sagi, A., Khalaila, I., Abdu, U., Shoukrun, R., and Weil, S. (1999). A newly established ELISA showing the effect of the androgenic gland on secondary-vitellogenic-specific protein in the hemolymph of the crayfish Cherax quadricarinatus. General Comparat. Endocrinol. 115, 37–45. doi: 10.1006/gcen.1999.7277
Sharabi, O., Manor, R., Weil, S., Aflalo, E. D., Lezer, Y., Levy, T., et al. (2016). Identification and Characterization of an Insulin-Like Receptor Involved in Crustacean Reproduction. Endocrinology 157, 928–941. doi: 10.1210/en.2015-1391
Shi, L., Li, B., Zhou, T. T., Wang, W., and Chan, S. F. (2018). Functional and evolutionary implications from the molecular characterization of five spermatophore CHH/MIH/GIH genes in the shrimp Fenneropenaeus merguiensis. PLoS One 13:e0193375. doi: 10.1371/journal.pone.0193375
Soyez, D. (1997). Occurrence and diversity of neuropeptides from the crustacean hyperglycemic hormone family in arthropods. A short review. Ann. N Y. Acad. Sci. 814, 319–323. doi: 10.1111/j.1749-6632.1997.tb46174.x
Suzuki, S., and Yamasaki, K. (1991). Sex-reversal of male Armadillidium vulgare (Isopoda, Malacostraca, Crustacea) following andrectomy and partial gonadectomy. General Comparat. Endocrinol. 83, 375–378. doi: 10.1016/0016-6480(91)90142-S
Tan, K., Zhou, M., Jiang, H., Jiang, D., Li, Y., and Wang, W. (2020). siRNA-Mediated MrIAG Silencing Induces Sex Reversal in Macrobrachium rosenbergii. Mar. Biotechnol. 22, 456–466. doi: 10.1007/s10126-020-09965-4
Tom, M., Goren, M., and Ovadia, M. (1987). Purification and partial characterization of vitellin from the ovaries of Parapenaeus longirostris (Crustacea, Decapoda, Penaeidae). Comparat. Biochem. Physiol. Part B Comparat. Biochem. 87, 17–23. doi: 10.1016/0305-0491(87)90464-0
Treerattrakool, S., Panyim, S., Chan, S. M., Withyachumnarnkul, B., and Udomkit, A. (2008). Molecular characterization of gonad-inhibiting hormone of Penaeus monodon and elucidation of its inhibitory role in vitellogenin expression by RNA interference. FEBS J. 275, 970–980. doi: 10.1111/j.1742-4658.2008.06266.x
Ventura, T., and Sagi, A. (2012). The insulin-like androgenic gland hormone in crustaceans: From a single gene silencing to a wide array of sexual manipulation-based biotechnologies. Biotechnol. Adv. 30, 1543–1550. doi: 10.1016/j.biotechadv.2012.04.008
Ventura, T., Manor, R., Aflalo, E. D., Weil, S., Khalaila, I., Rosen, O., et al. (2011). Expression of an Androgenic Gland-Specific Insulin-Like Peptide during the Course of Prawn Sexual and Morphotypic Differentiation. ISRN Endocrinol. 2011:476283. doi: 10.5402/2011/476283
Wang, C., and Xu, Y. (2019). Mechanisms for Sex Differences in Energy Homeostasis. J. Mol. Endocrinol. 62, R129–R143. doi: 10.1530/JME-18-0165
Warrier, S. R., Tirumalai, R., and Subramoniam, T. (2001). Occurrence of vertebrate steroids, estradiol 17beta and progesterone in the reproducing females of the mud crab Scylla serrata. Comparative biochemistry and physiology. Part A Mol. Integrat. Physiol. 130, 283–294. doi: 10.1016/S1095-6433(01)00385-3
Webster, S. G., Keller, R., and Dircksen, H. (2012). The CHH-superfamily of multifunctional peptide hormones controlling crustacean metabolism, osmoregulation, moulting, and reproduction. General Comparat. Endocrinol. 175, 217–233. doi: 10.1016/j.ygcen.2011.11.035
Wilder, M., Okumura, T., and Tsutsui, N. (2010). Reproductive Mechanisms in Crustacea Focusing on Selected Prawn Species: Vitellogenin Structure, Processing and Synthetic Control. Aqua BioSci. Monogr. 3, 73–110. doi: 10.5047/absm.2010.00303.0073
Yang, G., Lu, Z., Qin, Z., Zhao, L., Pan, G., Shen, H., et al. (2020). Insight into the Regulatory Relationships between the Insulin-Like Androgenic Gland Hormone Gene and the Insulin-Like Androgenic Gland Hormone-binding Protein Gene in Giant Freshwater Prawns (Macrobrachium rosenbergii). Int. J. Mol. Sci. 21:4207. doi: 10.3390/ijms21124207
Yano, I., Krol, R. M., Overstreet, R. M., and Hawkins, W. E. (1996). Route of egg yolk protein uptake in the oocytes of kuruma prawn, Penaeus japonicus. Mar. Biol. 125, 773–781. doi: 10.1007/BF00349260
Lu, Z., Guang, Y., Zhendong, Q., Haiyang, S., Menglan, Z., Fei, S., et al. (2020). Glutamate related osmoregulation of guanine nucleotide-binding protein G (I) α2 from giant freshwater prawn (Macrobrachium rosenbergii) during molting and salinity stress. Aquaculture 521:735000. doi: 10.1016/j.aquaculture.2020.735000
Keywords: Macrobrachium rosenbergii, GIH, IAGBP, eyestalk-AG-testis, sex-biased regulation
Citation: Tang M, Lu Z, Babu S V, Yang G, Li Y, Xu Z, Pan G, Qin Z and Lin L (2021) The Regulatory Relationships Between the Gonad-Inhibiting Hormone and Insulin-Like Androgenic Gland Hormone-Binding Protein Genes in the Eyestalk-Androgenic Gland-Testis Axis of Macrobrachium rosenbergii. Front. Mar. Sci. 8:775191. doi: 10.3389/fmars.2021.775191
Received: 13 September 2021; Accepted: 11 October 2021;
Published: 29 October 2021.
Edited by:
Shengming Sun, Shanghai Ocean University, ChinaReviewed by:
Takayoshi Ubuka, Cancer Medical Service, JapanTing Chen, South China Sea Institute of Oceanology, Chinese Academy of Sciences (CAS), China
Copyright © 2021 Tang, Lu, Babu, Yang, Li, Xu, Pan, Qin and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhendong Qin, qinzhendongsc@163.com; Li Lin, linli@zhku.edu.cn
†These authors have contributed equally to this work
 Meizhen Tang
Meizhen Tang