
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci., 19 November 2021
Sec. Marine Biology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.770963
 Shunyang Chen1,2,3,4†
Shunyang Chen1,2,3,4† Bingpeng Xing1†
Bingpeng Xing1† Weiwei Yu1,2,3
Weiwei Yu1,2,3 Bin Chen1,2,3,4
Bin Chen1,2,3,4 Jianji Liao1,2
Jianji Liao1,2 Wenshuo An1,2,3
Wenshuo An1,2,3 Guangcheng Chen1,2,3,4*
Guangcheng Chen1,2,3,4*The species within the genus Mainwaringia in Littorininae have been recognized as being strongly associated with mangroves; however, their abundance and distribution patterns in mangroves have rarely been reported. In this study, we reported Mainwaringia leithii specimens collected from young, rehabilitated mangroves in Xiamen city as a newly recorded Mainwaringia species in China, based on their DNA barcoding and morphological taxonomy characteristics. The recruitment pattern of this species and its relationships with mangrove species in the early stage of mangrove rehabilitation were also investigated. The snails were mainly collected from the backsides of leaves and from some leaf nodes. Continuous sampling showed the rapid recruitment of M. leithii following mangrove rehabilitation, with a density up to 278 ind m–2 in a 2.5-year-old mangrove site; however, declining densities were observed and snails were only occasionally collected at mangrove sites 4 years after rehabilitation. Aegiceras corniculatum mangroves in shrub form could support a snail abundance higher than that supported by Kandelia obovata mangroves of the same age. The present study suggests that M. leithii could be common in mangrove forests and that intensive changes in its assemblage occurs in the early stage of mangrove rehabilitation. Moreover, the recruitment of M. leithii following mangrove rehabilitation is related to the planting of different mangrove species. The spatio-temporal patterns of M. leithii distribution are likely owing to the variability in habitat characteristics related to mangrove species and stand age. Future studies should give more attention to the ecology of this species in mangrove forests.
Mangroves sustain a rich biodiversity of benthic fauna because they provide critical energy and nutrients for these fauna as well as the physical structures inhabited by benthic fauna, protecting the fauna from environmental stressors and predators (Nagelkerken et al., 2008; Hajializadeh et al., 2020; Tolhurst et al., 2020). Therefore, changes in the compositions and abundances of benthic fauna result from the development of vegetation after mangroves are rehabilitated, and certain faunal communities are thus related to specific mangrove species (Chen et al., 2017; Salmo et al., 2017). Obtaining detailed information on benthic faunal communities following mangrove rehabilitation is essential because this information indicates the state of rehabilitated mangroves (Bosire et al., 2008; Ellison et al., 2020), and the recruitment of a faunal community is one of the main criteria used to judge the success of rehabilitation programs (Field, 1999; Teal and Weishar, 2005). Currently, numerous studies have been conducted to investigate how the recruitment of benthic faunal communities in rehabilitated mangroves varies with forest age (Chen et al., 2007; Salmo et al., 2017; Al-Khayat et al., 2019) or among mangrove species (Macintosh et al., 2002; Chen et al., 2013; Li et al., 2015). However, few studies have reported the recruitment patterns of benthic fauna in the early stage (i.e., a few years after plantation) of mangrove rehabilitation.
Mainwaringia and Littoraria are the only two littorinid clades of Littorininae species to have been recognized as being strongly associated with mangroves (Reid et al., 2010, 2012). Currently, only three species, Mainwaringia leithii, M. rhizophila and M. dantaae, have been recorded (Reid, 1986; Fang et al., 2012; Reid et al., 2012). M. leithii was recorded from India to Vietnam (Annanale and Prashad, 1919; Reid, 1986), and M. rhizophila was recorded from Malaysia to Hong Kong (Morton and Morton, 1983; Reid, 1986). A Mainwaringia species, M. dantaae Fang, Peng, Zhang and He, n. sp., was collected in a mangrove forest in South China and identified as a new species based on its morphological characteristics (Fang et al., 2012; Liu, 2013). However, the Mainwaringia genus has received less attention than the Littoraria genus (Reid et al., 2010), and ecological information characterizing Mainwaringia species in mangroves, including the abundance and distribution patterns of these species, is rare.
During our field investigations on macro-benthic faunal communities in rehabilitated mangroves conducted in recent years, we found a large number of individuals of a snail species with morphological characteristics similar to those of Mainwaringia species. In this study, we identified this species based on its morphological characteristics and DNA sequencing information. The abundance of this species and its temporal variation with stand age were also investigated in two young, rehabilitated mangrove sites where plantations were performed using two different mangrove species, Kandelia obovata and Aegiceras corniculatum. We aimed to test whether the abundance of the snail species may be related to the associated mangrove species and stand age. We hypothesized that plantation of A. corniculatum as a shrub species would favor more Mainwaringia snails than what K. obovata performs, and the snail abundance was higher at younger stands while decreasing with stand age.
Investigations were carried out in the Xiatanwei mangrove wetland (24°38′46.06″N, 118°12′7.61″E) in Xiamen, China. The region has a subtropical monsoon climate, and monthly mean temperature ranges from 13°C to 28°C from 2009 to 2007, with the highest temperature recorded in July and August (Weather China, 2021). While the monthly mean precipitation ranges from 51 to 189 mm, and the annual precipitation was 1291 mm. The tides in this area are semi-diurnal, with an annual tidal range of 3.98 m. The Xiatanwei mangrove wetland is a rehabilitated area, and most of the rehabilitation activities were conducted in 2012 or 2013, mainly using K. obovata (KO), a dominant mangrove species in Xiamen and the surrounding Jiulong River Estuary; A. corniculatum (AC) was planted with mono species in a certain area (∼600 m2) in June of 2012. In this study, the AC site and an adjacent KO site (∼250 m apart from each other) planted in 2012 with a same planting density of 0.5 m × 0.8 m were selected for investigation (Supplementary Figure 1). These two mangrove sites had a similar mean vertical soil surface elevation (1.6 m, Yellow Sea elevation) before plantation, and their substrates had a comparable physicochemical condition according to the investigation conducted in July 2014 (Supplementary Table 1).
Continuous samplings were conducted during ebb tide periods from July 2014, that is, approximately 2 year after rehabilitation. Biannual samplings in Summer (July) and Winter (December) were performed each year until December 2016 when the mangrove sites reached 4.5-year-old. At each site, three 4-m × 4-m sampling quadrats were setup, at least 10 m apart from each other, for vegetation investigation. Plant height and crown diameter of 12 plants were measured within each quadrat during each sampling campaign, and canopy coverage was estimated based on the density and the mean crown diameter of each quadrat. All snail individuals within three 1-m × 1-m sampling quadrats were carefully collected from the stems, leaves and prop roots of mangrove saplings and then transported alive to laboratory for analysis. In the laboratory, the sampled individuals were cleaned of mud, and the total number and fresh weight of snails for each quadrat were measured. The shell heights and fresh weight were recorded for all snail samples collected from November 2015 to November 2016. While during the samplings between July 2014 and July 2015, only those collected from one random plant within each quadrat were used for the measurements, regarding the large number of snails in the whole quadrat.
A fragment of the mitochondrial gene cytochrome oxidase subunit I was amplified from each using the barcoding primers (LCO1490 and HCO2198) and thermal program described by Williams et al. (2003). The PCR samples were screened for the existence of PCR products on a 1.0% agarose gel. Sequencing in both directions was performed by Sangon Biotech (Shanghai). The SeqMan application of DNAStar software was used to splice the forward and reverse sequencing results obtained for each sample, and the findings were then submitted to GenBank. The sequences were then arranged by ClustalW in MEGA6.0 software. All sequences were compared with the reference sequences in both the National Center for Biotechnology Information (NCBI) and Barcode of Life Data (BOLD) systems. Top matches with sequence similarities of at least 98% were used to designate potential species identifications (Barbuto et al., 2010). The sampled individuals were identified using known taxonomy keys described by Reid (1986) and the DNA barcoding result above.
The normality and homogeneity of variables was checked using the Shapiro-Wilk test and Levene’s test, respectively. For the canopy coverage with a normal distribution and homogeneity, and snail density and biomass which followed after transformed with the Blom method, a parametric two-way analysis of variance (ANOVA) was conducted to test for any effects of the mangrove species and stand age, and pairwise comparisons of interactive effects were performed using post hoc Tukey’s honestly significant difference (HSD) test. When a significant interaction was observed, a one-way ANOVA (post hoc Tukey’s test) with Bonferonni-adjusted alpha was used to investigate the effect of the stand age separately for each mangrove site, and the differences between the two sites were examined by t-test. For the variables without a normal distribution and homogeneity even after transformation, Scheirer-Ray-Hare two-way non-parametric ANOVA of ranks was used to test the effects of stand age and mangrove species (Li et al., 2019). The regression between shell height and weight of the snail samples were also examined for each mangrove species. All statistical analyses were performed using IBM SPSS Statistics (version 22.0, Chicago, IL, United States).
The two mangrove sites had similar crown diameters (H = 0.2, p > 0.05) and canopy coverages (F = 1.27, p > 0.05) throughout the investigation (Figure 1). The canopy coverage was <65% in the 2-year-old sites (July 2014), increased to >82% in the 2.5-year-old sites, and was sustained at a similar level in subsequent measurements (F = 13.12, p < 0.001). The crown diameter showed a similar temporal trend to that of the canopy coverage and increased from ∼50 cm in the 2-year-old sites to ∼70 cm in the 2.5-year-old sites (H = 33.3, p < 0.001). Increased plant heights with stand age were observed at the mangrove sites (H = 15.8, p < 0.01), but the KO site had a significantly higher plant height than that simultaneously measured at the AC site during the samplings (H = 6.9, p < 0.01).
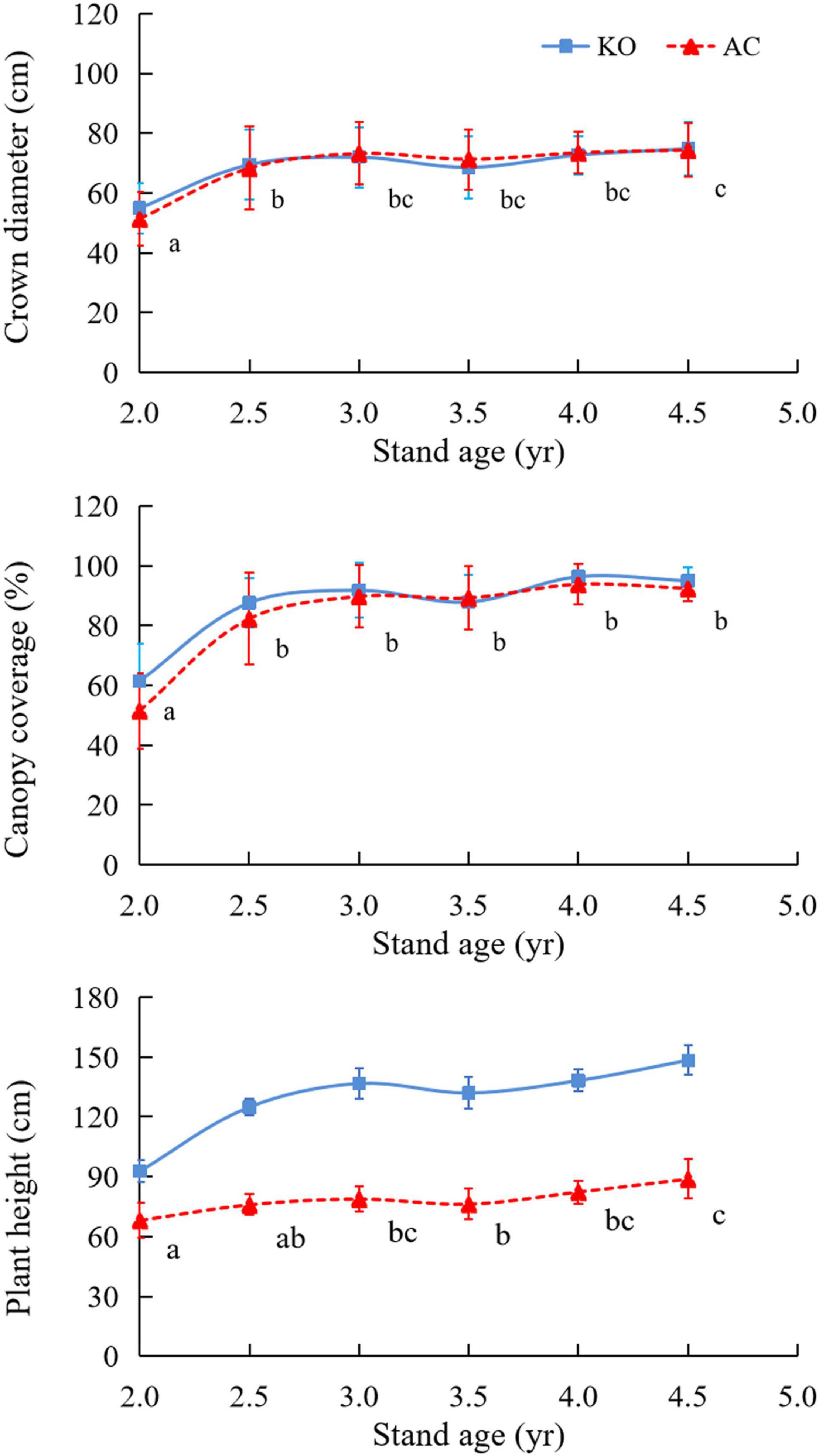
Figure 1. Crown diameters, canopy coverages, and plant heights of the two mangrove sites. Different letters indicate significant differences among the stand ages at p < 0.05.
In this study, 5 DNA barcodes were successfully sequenced from the collected snail samples (GenBank accession numbers MZ801729–MZ801733). After editing, the consensus length of all barcode sequences was 658 bp, and no stop codons, insertions or deletions were observed in any of the sequences. Through a comparison with the NCBI database, all the analyzed sequences matched M. leithii (serial number: AJ488610) with a similarity of 98.38–100% (Williams et al., 2003).
Based on the DNA barcoding results and taxonomic keys of the samples (Figure 2), the studied mangrove snail species was identified as follows:
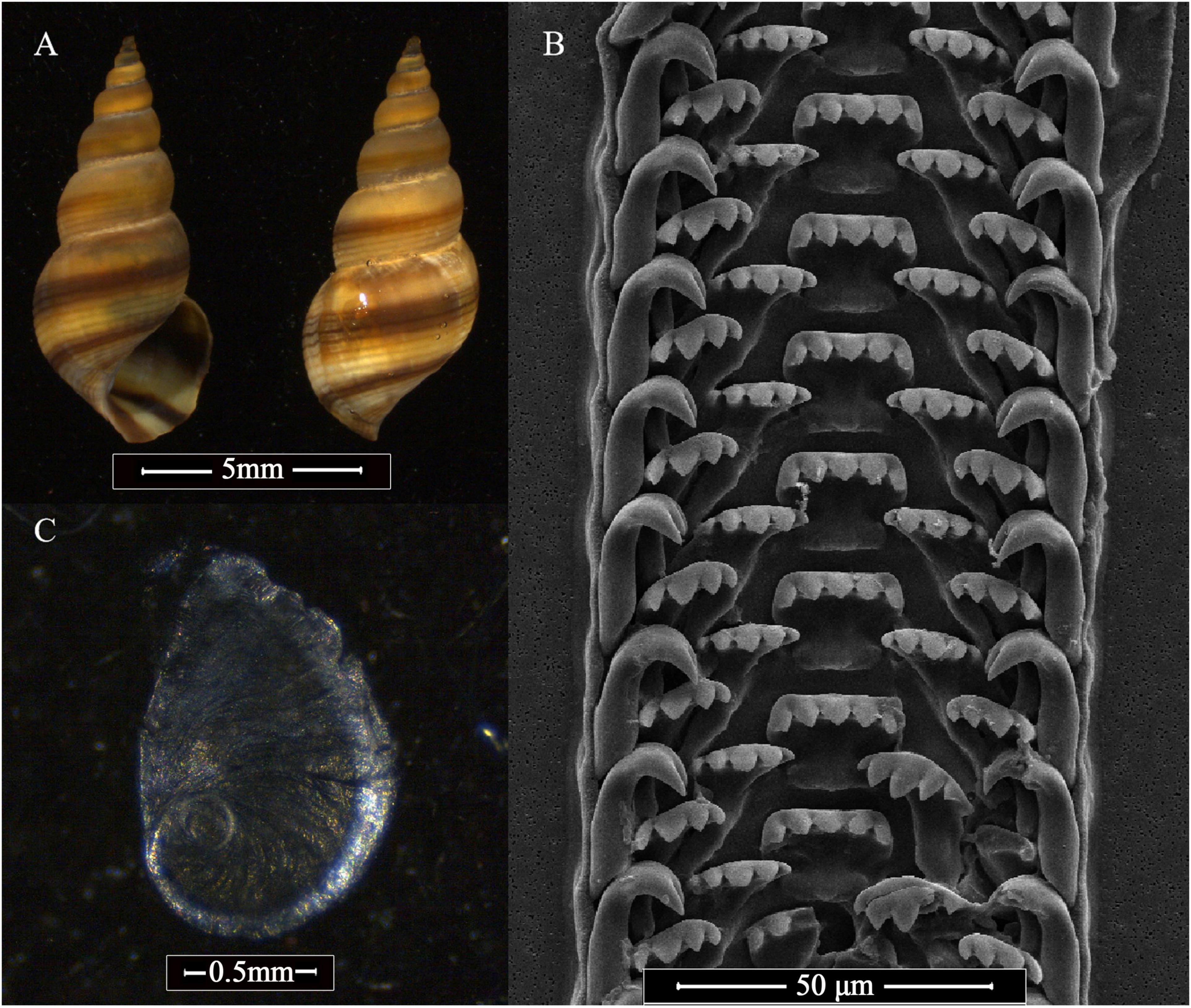
Figure 2. Shell shape (A), rachidian tooth (B), and operculum (C) of Mainwaringia leithii collected from rehabilitated mangroves in the present study.
Family Littorinidae Children, 1834
Genus Mainwaringia G. Nevill, 1885
Mainwaringia leithii
http://www.marinespecies.org/aphia.php?p = taxdetails& id = 446280
New records. Mangrove in Xiatanwei, Xiamen, Fujian Province, China. 24°38′46.06″N, 118°12′7.61″E.
Morphological characters. Teleoconch 6 whorls. Shell thin, spire tall, outline slightly convex; whorls well rounded; sutures impressed. Last whorl rounded, similar in shape to the previous whorl, only the color is darker than the other whorl. Outline of aperture oval; labral underdeveloped, not flared, without denticles. Columella narrow, not folds. Spiral ribs on the first teleoconch are not very obvious, but are more distinctly observable within the body whorl. The number of the grooves increase until 22–30 on the body whorl. Grooves are narrow, approximately parallel, more obvious at the middle and the lower part of the whorls. The color of the shell is yellow to light brown, with the four brown spiral bands on body whorl. The aperture color pattern is same as the exterior part. The operculum is thin, transparent, and consists of about three volutions, and its nucleus is situated at about one fourth of the entire length from the inferior margin. Rachidian tooth with 5 subequal cusps, outer 2 slightly smaller; posterior and lateral edges raised, posterior edge almost straight. Lateral tooth with 5 cusps, central 3 largest; base with deep notch to receive inner marginal. Inner marginal with 4 subequal cusps. Outer marginal rather broad and hook-shaped in side view, bearing 3 subequal cusps. The morphological characters of the snails are the same as those described by Reid (1986).
M. leithii samples were mainly collected from the backsides of mangrove leaves, and some snails were found on leaf nodes or infrequently collected from the front sides of leaves (Supplementary Figure 2). Interactions between mangrove species and stand age were found for the snail density (F = 3.55, p < 0.05). The M. leithii density was 85.05 ± 18.35 ind m–2 in the 2-year-old AC site, and the value increased to 284.31 ± 75.76 ind m–2 in the next sampling campaign and then decreased gradually to 9.72 ± 4.21 ind m–2 in the 3.5-year-old site (Figure 3). The KO site had a comparable density to that of the AC site at the beginning and then experienced a decreasing density with stand age. Almost no snail individuals (<3 ind m–2) were collected at either site 4 years after plantation. The biomasses of the snails at both sites showed decreasing trends with stand age (F = 20.06, p < 0.001), and the biomass was not affected by the mangrove species (F = 2.50, p > 0.05). The shell heights of the samples ranged from 2.60 to 9.53 mm during the study (Figure 3), and the mean value decreased from >6 to ∼4 mm when the stand age increased from 2 years to 3 years in both sites and increased to up to 8.5 mm in the AC site at the subsequent sampling dates (H = 90.94, p < 0.001). There was no significant difference in the shell height between the two sites (H = 0.2, p > 0.05). However, the two sites had a different snail size composition. Snail samples were dominated by those with shell height falling within 3–4 mm (55.8%) in AC site, while 58.0% of snails in the KO site had individual shell height ranging from 6 to 8 mm (Figure 4). The individual weight presents a quadratic increase with shell height of the snails at both sites (Figure 4).
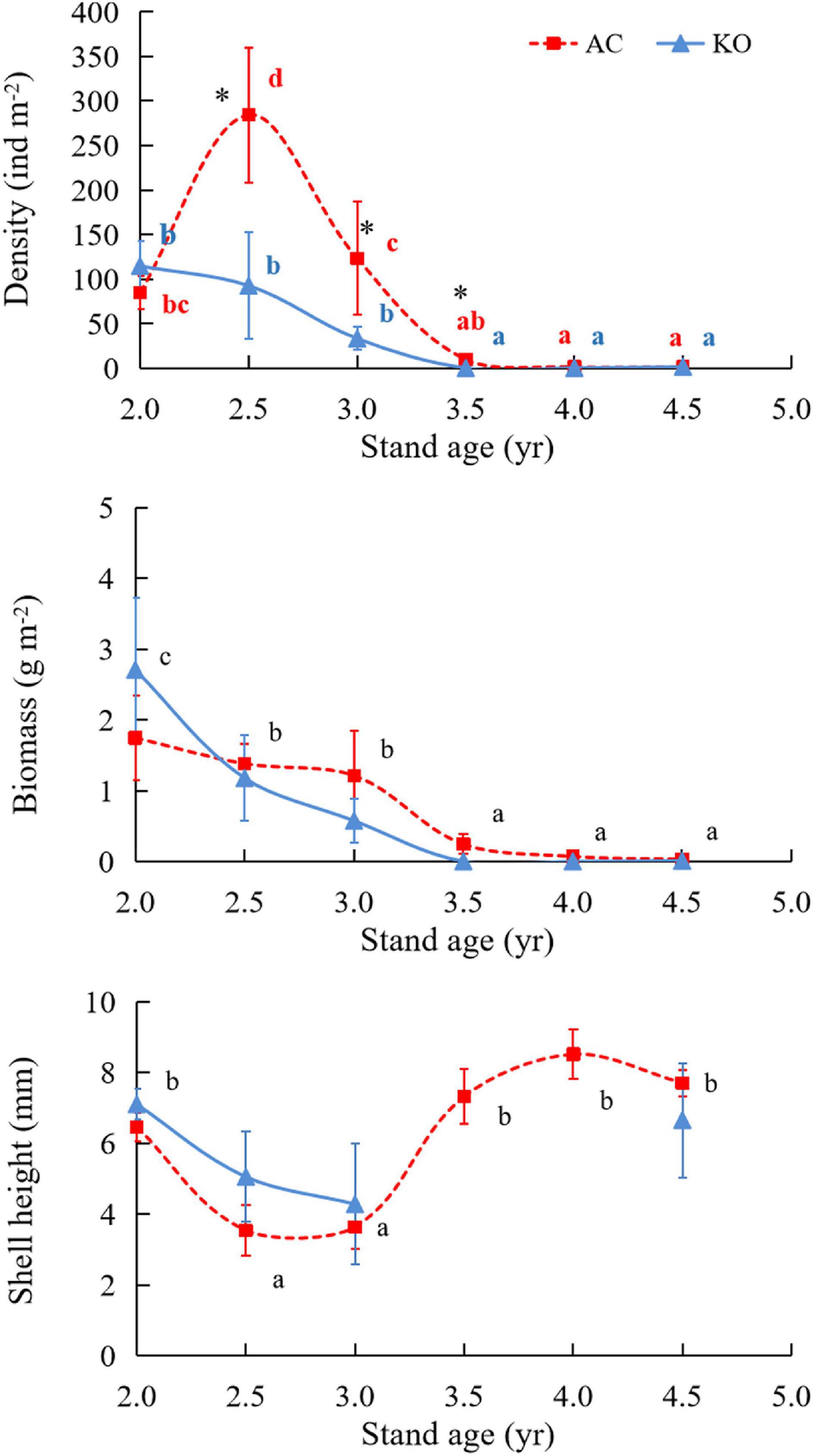
Figure 3. Densities, biomasses, and shell heights of Mainwaringia leithii in the two mangrove sites. For biomasses and shell heights, different letters indicate significant differences among the stand ages at p < 0.05. For density, the asterisk (∗) symbol indicates significant differences between the two mangrove sites according to t-tests at p < 0.05, and different letters in red and blue indicate significant differences among the stand ages at p < 0.05 in AC and KO sites, respectively.
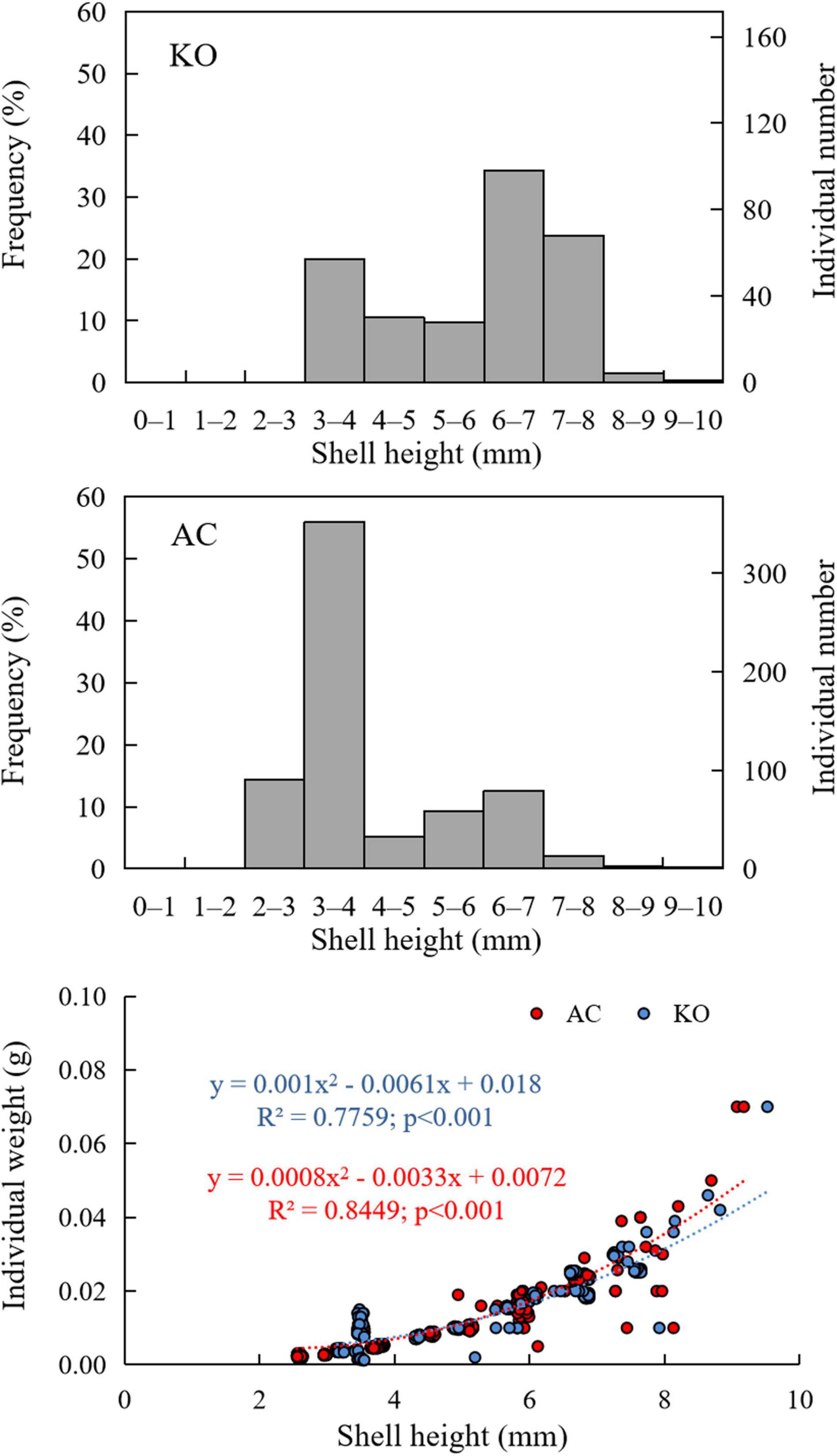
Figure 4. Size distributions of the Mainwaringia leithii specimens collected in the two mangrove sites and the relationship between the shell height and fresh weight of the snails.
In the present study, we identified the snail species collected in the subtropical Xiatanwei mangroves as M. leithii based on their morphological characteristics and DNA barcoding. The present study was the first record of M. leithii in China. The results also supported our hypothesis that the snail abundance in the young mangrove sites varied with the mangrove species and stand age, with overall more snails in the AC site than in KO site, and the abundance was higher at younger stands. We also found a different size distribution of M. leithii between the two mangrove sites.
Previous studies have suggested that M. leithii is mainly distributed in India and Southeast Asia, and this species has been recorded in India, the Malay Peninsula and Vietnam (Reid, 1986; Williams et al., 2003). However, this species and its congener M. rhizophila have been suggested to be relatively uncommon in their habitats on mangrove trees, in contrast to many other littorinid snails that occur in large numbers (Williams et al., 2003). Our continuous surveys in Xiatanwei found that the M. leithii density could reach up to 278 ind m–2 at young, rehabilitated mangrove sites, much higher than the Littoraria melanostoma density (32.7 ind m–2) simultaneously measured at the two mangrove sites (Chen et al., 2017). In the present study and during our field observations in other mangroves, small M. leithii snails adhered to the backsides of mangrove leaves and were likely ignored during field surveys. This can probably explain the scarce collections of this species reported in previous studies conducted in mangrove forests. A recent study also reported occurrences of M. dantaae in two mangroves in Hainan Province in South China (Ma et al., 2018) following Liu (2013), who reported the collection of this new species in a Gaoqiao mangrove site. These results suggest that Mainwaringia snails could be common in mangrove forests and could also be closely associated with mangroves; thus, these snails are worthy of attention in future studies.
Changes in the macrofaunal community structure with forest age have been recorded in mangrove forests (Morrisey et al., 2003; Chen et al., 2007; Salmo et al., 2017). Snails of the families Neritidae and Ellobiidae are abundant in mature forests, whereas Littoriinidae, Assimineidae and Potamidae species are more representative of younger sites (Macintosh et al., 2002). We observed a rapid recruitment of M. leithii snails following the rehabilitation of mangroves, and the M. leithii density reached ∼100 ind m–2 at the 2-year-old mangrove sites. However, 3.5 years after rehabilitation, snails were only occasionally collected at both sites, suggesting that this species is more associated with mangrove seedlings than with older mangroves. This observation is similar to previous findings in which M. rhizophila was reported in the leaves and stems of K. obovata seedlings (Reid, 1986). Younger K. obovata mangrove sites (4 years in age) were also found to support high L. melanostoma abundances, while these abundances decreased as mangrove vegetation developed (Chen et al., 2007). The scarcity of M. leithii snails in the aged and mature mangrove forests may also explain the limited records of this species in previous studies as concluded by Williams et al. (2003). M. leithii seems to be sensitive to tide exposure because this species has been found to adhere tightly to the trunks and aerating roots of mangrove trees at low tide and become active when submerged by tidal water (Annanale and Prashad, 1919). We suspect that the increased plant height (and consequently increased canopy height) that occurs as mangrove stands age reduces the accessibility of small Mainwaringia snails on the mangrove leaves to tidal floodwaters, thus increasing their environmental stress. Future studies with the vertical distribution of the snails on the plants in mangrove sites taken into account are deserved to test whether the snail distribution might be related to tidal flooding. Adhering to the backsides of mangrove leaves could likely protect snails from environmental stressors, including high heat and desiccation.
In addition, the decrease in M. leithii population may also be attributed to the occurrence of predators in the older sites. Previous studies have suggested that abundance of predators, including the predatory arboreal crabs, could influence the spatial distribution of mangrove littorinids (Reid, 1985; Duncan and Szelistowski, 1998; Lee and Williams, 2002). Increase in abundance of omnivorous sesarmid crabs have been reported in association with the aging of rehabilitated mangroves (Macintosh et al., 2002; Chen et al., 2007; Lin et al., 2016). Although sesarmid crabs mainly feed on mangrove materials with high C:N ratio, they also graze other invertebrates to supplement nitrogen sources (Wu et al., 2020). In South Fujian, Parasesarma plicatum is the most common sesarmid crab in mangroves (Chen et al., 2007), and was observed common within our sampling sites. We suspect that the P. plicatum crabs might predate the M. leithii snails and the increase in P. plicatum abundance with stand age would also result in the decline in M. leithii abundance. We therefore suggest future studies to investigate the variations of the crab predators with the stand age and mangrove species, and their role in regulating the M. leithii abundance in rehabilitated mangroves.
Previous studies have demonstrated that the succession trajectory of macro-benthic fauna is dependent on the mangrove species because the performances of mangroves in modifying their environmental setting and regulating the distribution of benthic fauna vary among mangrove species (Lee and Williams, 2002; Chen et al., 2007; Li et al., 2015). The results reported in the present study further confirmed the impact of mangrove species on the recruitment of M. leithii to rehabilitated mangroves. The M. leithii density in the K. obovata site decreased with the stand age. M. leithii was more abundant in the A. corniculatum mangrove than in the K. obovata mangrove and had a peak density in the 2.5-year-old site. Because these two mangrove sites had similar site conditions before seedlings were planted, the observed fluctuations in snail abundance could be attributed to the planting of mangrove species. The shrub form of A. corniculatum is dwarf and dense, with more twigs than the K. obovata site, providing more inhabitable space and better accessibility to tidal flooding for snails. Our previous studies also observed similar temporal trends of L. melanostoma abundances in K. obovata and A. corniculatum mangrove sites (Chen et al., 2007; Chen and Ye, 2011). We therefore suggest that the high abundance of Littorinidae snails observed within A. corniculatum forests might be an inherent attribute of the ecosystem. The overall decrease of M. leithii abundance through time might relate to the fact that the mangrove plants becoming higher makes it difficult for the snails to access the tidal flooding. The more relatively small snails in the AC sites than the KO sites, and smaller snails in younger sites than the older sites suggest that the small individuals are less migratory and tolerant to the harsh condition resulting from the less tidal flooding.
In the present study, we collected a Mainwaringia species identified as M. leithii from rehabilitated mangroves; this identification represents a new record from China. This species prefers to adhere to the backsides of mangrove leaves and was found to be abundant in 2-year-old mangrove sites but was only found more occasionally in older sites, suggesting rapid recruitment and a decline in its population within a few years following mangrove rehabilitation. Mangrove species regulate the M. leithii assemblages in rehabilitated mangrove sites, and the shrub form of A. corniculatum supports a higher abundance of snails than K. obovata mangroves of the same age. The distribution of M. leithii might be related to tidal flooding, which could explain the change in its abundance observed with stand age and relevant differences observed between the two mangrove sites. The present study also suggests that intensive changes in M. leithii assemblage occur in the early stage of mangrove rehabilitation, and future studies should give more attention to faunal assemblages in young mangroves. The species-related performance and mechanism of mangrove vegetation in sustaining faunal biodiversity and abundance following plantation also deserves attention in rehabilitating mangrove sites to promote biodiversity.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI (accession: MZ801729–MZ801733).
GC contributed to the study’s conception. SC, JL, and WA performed the sample collection and data acquisition. WY and BC performed the data analysis. BX performed the molecular analysis and species identification. All authors contributed to the article and approved the submitted version.
The work described in this article was funded by the National Natural Science Foundation of China (42176171 and 42076163). The China-ASEAN Maritime Collaboration Fund and Provincial Natural Science Foundation of Fujian (2016J01159) also contributed to this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to Ganlin Chen, Zinong Zheng, Yan Huang, and Meiling Xie for their assistance with field sampling and laboratory analysis. The authors also thank Qiong Wu, Li Gu, and Jianjia Wang for their contributions to photographing the snails.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.770963/full#supplementary-material
Al-Khayat, J. A., Abdulla, M. A., and Alatalo, J. M. (2019). Diversity of benthic macrofauna and physical parameters of sediments in natural mangroves and in afforested mangroves three decades after compensatory planting. Aquat. Sci. 81:4. doi: 10.1007/s00027-018-0599-7
Annanale, N., and Prashad, B. (1919). Some gastropod molluscs from the Gangetic delta. Rec. Indian Mus. 16, 241–257.
Barbuto, M., Galimberti, A., Ferri, E., Labra, M., Malandra, R., Galli, P., et al. (2010). DNA barcoding reveals fraudulent substitutions in shark seafood products: the Italian case of “palombo” (Mustelus spp.). Food Res. Int. 43, 376–381. doi: 10.1016/j.foodres.2009.10.009
Bosire, J. O., Dahdouh-Guebas, F., Waton, M., Crona, B. I., Lewis III, R. R., et al. (2008). Functionality of restored mangroves: a review. Aquat. Bot. 89, 251–259. doi: 10.1016/j.aquabot.2008.03.010
Chen, G., and Ye, Y. (2011). Restoration of Aegiceras corniculatum mangroves in Jiulongjiang estuary changed macro benthic faunal community. Ecol. Eng. 37, 224–228. doi: 10.1016/j.ecoleng.2010.10.003
Chen, G., Ye, Y., and Lu, C. (2007). Changes of macro-benthic faunal community with stand age of rehabilitated Kandelia candel mangrove in Jiulongjiang estuary, China. Ecol. Eng. 31, 215–224. doi: 10.1016/j.ecoleng.2007.07.002
Chen, G., Yu, D., Ye, Y., and Chen, B. (2013). Impacts of mangrove vegetation on macro-benthic faunal communities. Acta Ecol. Sin. 33, 327–336.
Chen, S., Chen, B., Liao, J., Chen, G., Huang, Y., and Chen, G. (2017). Composition and distribution pattern of Littorinid snails in young rehabilitated mangroves. Chin. J. Ecol. 36, 460–467. doi: 10.13292/j.1000-4890.201702.022
Duncan, R. S., and Szelistowski, W. A. (1998). Influence of puffer predation on vertical distribution of mangrove littorinids in the Gulf of Nicoya, Costa Rica. Oecologia 117, 433–442. doi: 10.1007/s004420050678
Ellison, A. M., Felson, A. J., and Friess, D. A. (2020). Mangrove rehabilitation and restoration as experimental adaptive management. Front. Mar. Sci. 7:327. doi: 10.3389/fmars.2020.00327
Fang, Y., Peng, Y., Zhang, G., and He, J. (2012). Description of a new species (Gastropoda: Littorinidae) from South China Sea. Shell Discov. 1:34.
Field, C. D. (1999). Rehabilitation of mangrove ecosystems: an overview. Mar. Pollut. Bull. 37, 383–392. doi: 10.1016/S0025-326X(99)00106-X
Hajializadeh, P., Safaie, M., Naderloo, R., Shojaei, M. G., Gammal, J., Villnäs, A., et al. (2020). Species composition and functional traits of macrofauna in different mangrove habitats in the Persian Gulf. Front. Mar. Sci. 7:575480. doi: 10.3389/fmars.2020.575480
Lee, O. H. K., and Williams, G. A. (2002). Locomotor activity patterns of the mangrove littorinids, Littoraria ardouiniana and L. melanostoma, in Hong Kong. J. Molluscan Stud. 68, 235–241. doi: 10.1093/mollus/68.3.235
Li, J., Shan, Y., Yin, S., Wang, M., Sun, L., and Wang, D. (2019). Nonparametric multivariate analysis of variance for affecting factors on the extent of forest fire damage in Jilin Province, China. J. For. Res. 30, 209–221. doi: 10.1007/s11676-019-00958-1
Li, W., Cui, L., Zhang, M., Wang, Y., Zhang, Y., Lei, Y., et al. (2015). Effect of mangrove restoration on crab burrow density in Luoyangjiang estuary, China. For. Ecosyst. 2:21. doi: 10.1186/s40663-015-0046-3
Lin, J., He, X., Wang, J., Lin, H., Huang, Y., Liu, K., et al. (2016). Macrobenthic diversity and seasonal changes in the mangrove swamp of Luoyangjiang estuary, Fujian Province. Biodiv. Sci. 24, 791–801. doi: 10.17520/biods.2015328
Liu, Y. (2013). Ecological Studies on Mangrove Molluscs Based on Typical Mangrove Communities. Ph.D. thesis. Xiamen: Xiamen University.
Ma, W., Wang, M., Wang, W., Liu, Y., Luo, L., and Tang, C. (2018). Biodiversity of mangrove mollusks in the west coast of Hainan Island, China. Biodiv. Sci. 26, 707–716. doi: 10.17520/biods.2018104
Macintosh, D. J., Ashton, E. C., and Havanon, S. (2002). Mangrove rehabilitation and intertidal biodiversity: a study in the Ranong mangrove ecosystem, Thailand. Estuar. Coast. Shelf Sci. 55, 331–345. doi: 10.1006/ecss.2001.0896
Morrisey, D. J., Skilleter, G. A., Ellis, J. I., Burns, B. R., Kemp, C. E., and Burt, K. (2003). Differences in benthic fauna and sediment among mangrove (Avicennia marina var. australasica) stands of different ages in New Zealand. Estuar. Coast. Shelf Sci. 56, 581–592. doi: 10.1016/S0272-7714(02)00208-1
Morton, B., and Morton, J. (1983). The Sea Shore Ecology of Hong Kong. Hong Kong: Hong Kong University Press.
Nagelkerken, I., Blaber, S. J. M., Bouillon, S., Green, P., Haywood, M., Kirton, L. G., et al. (2008). The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat. Bot. 89, 155–185. doi: 10.1016/j.aquabot.2007.12.007
Reid, D. G. (1985). Habitat and zonation patterns of Littoraria species (Gastropoda: Littorinidae) in Indo-Pacific mangrove forests. Biol. J. Linn. Soc. 26, 39–68.
Reid, D. G. (1986). Mainwarigia Nevill, 1885, a Littorinid genus from Asiatic mangrove forestsm, and a case of protandrous hermaphroditism. J. Molluscan Stud. 52, 225–242.
Reid, D. G., Dyal, P., and Williams, S. T. (2010). Global diversification of mangrove fauna a molecular phylogeny of Littoraria (Gastropoda: Littorinidae). Mol. Phylogenet. Evol. 55, 185–201. doi: 10.1016/j.ympev.2009.09.036
Reid, D. G., Dyal, P., and Williams, S. T. (2012). A global molecular phylogeny of 147 periwinkle species (Gastropoda, Littorininae). Norwegian Acad. Sci. Lett. 41, 125–136. doi: 10.1111/j.1463-6409.2011.00505.x
Salmo III, S. G., Tibbetts, I., and Duke, N. C. (2017). Colonization and shift of mollusc assemblages as a restoration indicator in planted mangroves in the Philippines. Biodiv. Conserv. 26, 865–881. doi: 10.1007/s10531-016-1276-6
Teal, J. M., and Weishar, L. (2005). Ecological engineering, adaptive management, and restoration management in delaware bay salt marsh restoration. Ecol. Eng. 25, 304–314. doi: 10.1016/j.ecoleng.2005.04.009
Tolhurst, T. J., Chapman, M. G., and Murphy, R. J. (2020). The effect of shading and nutrient addition on the microphytobenthos, macrofauna, and biogeochemical properties of intertidal flat sediments. Front. Mar. Sci. 7:419. doi: 10.3389/fmars.2020.00419
Weather China (2021). History.shtml. Available online at: http://www.weather.com.cn/forecast/history.shtml (accessed October 15, 2021)
Williams, S. T., Reid, D. G., and Littlewood, D. T. J. (2003). A molecular phylogeny of the Littorininae (Gastropoda: Littorinidae): unequal evolutionary rates, morphological parallelism and biogeography of the Southern Ocean. Mol. Phylogenet. Evol. 28, 60–86. doi: 10.1016/S1055-7903(03)00038-1
Keywords: mangrove rehabilitation, stand age, mangrove species, vegetation characteristics, Mainwaringia
Citation: Chen S, Xing B, Yu W, Chen B, Liao J, An W and Chen G (2021) Distribution of a Newly Recorded Gastropod Species, Mainwaringia leithii (Gastropoda, Littorinidae), in Young, Rehabilitated Mangroves in China. Front. Mar. Sci. 8:770963. doi: 10.3389/fmars.2021.770963
Received: 05 September 2021; Accepted: 28 October 2021;
Published: 19 November 2021.
Edited by:
Menghong Hu, Shanghai Ocean University, ChinaReviewed by:
Wenzhe Xu, Tianjin University of Science and Technology, ChinaCopyright © 2021 Chen, Xing, Yu, Chen, Liao, An and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangcheng Chen, Z2MuY2hlbkB0aW8ub3JnLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.