
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 29 November 2021
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.770180
Animal personality refers to individual behavioral and physiological differences that are consistent over time and across context. Recently, the fish personality has gained increasing attention, especially from the perspective of aquaculture production. Here, we used an important aquaculture species, black rockfish Sebastes schlegelii, as the target animal, and conducted a series of experiments to explore the relationships among fish boldness, aggressiveness, locomotor activity, opercular beat rate, standard metabolic rate, and cortisol level. Generally, the results showed that the boldness of black rockfish was significantly, positively correlated with fish aggressiveness, stressed locomotor activity, and standard metabolic rate, while was negatively correlated with stressed opercular beat rate. Bold fish had significantly higher aggressiveness, standard metabolic rate, and stressed locomotor activity but lower stressed opercular beat rate. However, there were no significant correlations between boldness and basal locomotor activity or between boldness and basal cortisol level. These results preliminarily constructed the behavioral and physiological spectrum of black rockfish in the context of fish personality and clearly indicated that the boldness could be used as a discrimination tool to predict fish aggressiveness and metabolic rate, which may have valuable applications for decreasing fish harmful aggression and increasing fish welfare in the aquaculture industry.
Differences among organisms widely exist in diverse levels, such as ecosystem, community, species, and population (Sih et al., 2004a). In recent years, the differences in the individual level have gained increasing attention (Sih et al., 2004b; Castanheira et al., 2017; Wilson et al., 2019). Commonly, individual behavioral and physiological differences that are consistent over time and across context are referred to as animal personality (Dingemanse et al., 2010; Réale et al., 2010a,b; Toms et al., 2010; Wilson et al., 2019). Alternatively, it is also called temperament (Réale et al., 2007), behavioral syndromes (Sih et al., 2004b, a), and coping styles (Koolhaas et al., 1999, 2010; Øverli et al., 2007; Castanheira et al., 2017). In spite of the differences in terminology, the connotation of them is relatively consistent, that is, the individual variation is consistent and biologically meaningful, and individual differences in certain behavioral or physiological traits are predictive of other behaviors or physiological responses (Castanheira et al., 2017).
Animal personality has been widely studied in terrestrial mammals, and based on an important behavioral trait, boldness, it has been well established that bold animals have lower attack latency, higher routine formation, lower behavioral flexibility, lower hypothalamic-pituitary-adrenal axis activity, and higher sympathetic reactivity compared with shy animals (Koolhaas et al., 1999, 2010; Stamps, 2016). Then similar phenomenon was also found in marine animals (Mittelbach et al., 2014; Castanheira et al., 2017). For example, proactive Nile tilapia (Oreochromis niloticus) individuals seem to exhibit a faster recovery of feed intake after being transferred into a novel environment and use feed resources more efficiently (Martins et al., 2011). But to date, the relevant studies that focused on fish were relatively scarce compared with terrestrial studies, and sometimes, the results for the same relevance among fish species were contradictory. For example, Backström’s research showed that in rainbow trout Oncorhynchus mykiss, the basal cortisol concentration in shy fish was significantly higher than that in bold fish (Backström et al., 2011). But others found no significant difference in basal cortisol concentration between bold and shy fish, both in rainbow trout and in Atlantic salmon Salmo salar (Øverli et al., 2002a, b; Vindas et al., 2017). This representative discrepancy indicates that fish personality may be highly species- and context-dependent, and the extrapolation seems to be improper given the wide range of fish species and rearing conditions. Furthermore, in recent years, the viewpoint that fish personality studies should put more focus on the practical application has been repeatedly proposed (Castanheira et al., 2017; Wilson et al., 2019), but research works the starting point of which was linking fish personality and aquaculture application are still few.
Based on these backgrounds, in this study, we conducted a series of experiments to elucidate the relationships among diverse personality traits which were crucial in the aquaculture industry, in an important aquaculture species, black rockfish (Sebastes schlegelii). In the wild, black rockfish is widely distributed in the coastal regions of China, the Korean peninsula, and Japan (Xi et al., 2017a; Zhang et al., 2019). In recent years, aquaculture of the black rockfish in China, especially in northern China, has rapidly developed (Zhang et al., 2020a,b, 2021). However, the severe social interactions (e.g., aggressive behavior) among individuals during the hatchery and aquaculture often cause serious body damage, suppressed growth performance, stimulated physiological stress, and even higher cannibalism and mortality (Xi et al., 2017a, b; Zhang et al., 2020a,b). Here, we used the framework of fish personality to ascertain whether black rockfish exhibits individual variation in boldness and whether boldness is correlated with aggressiveness, locomotor activity, opercular beat rate, cortisol, and standard metabolic rate. The results may lay the foundation for applying fish personality for improving fish welfare and increasing the production in aquaculture industry.
In order to elucidate the relationships among behaviors (boldness, aggressiveness, activity, and respiration) and physiology (metabolism and hormone) in the context of fish personality, three experiments were conducted using a typical rockfish species S. schlegelii during a period of 3 years. Generally, the first experiment aimed to explore the relationships among boldness, aggressiveness, basal locomotor activity, and basal cortisol level. In this experiment, fish boldness was determined by a “box-emerging test.” The second experiment aimed to test the relationships among boldness, stressed locomotor activity, stressed opercular beat rate, and standard metabolic rate. In this experiment, fish boldness was measured based on an “open-field test.” The third experiment was conducted to examine the validation of the two behavioral apparatuses used for determining fish boldness in this study. Through these three experiments, we could broadly construct a behavioral-physiological spectrum for black rockfish.
The first experiment was conducted from October 2018 to December 2018. Black rockfish juveniles were obtained from a hatchery in Qingdao, Shandong, China. Fifty-three fish (mean body length 7.53 ± 0.11 cm, mean body weight 9.75 ± 0.27 g) were individually acclimated to the experimental condition (i.e., glass tank, 40 cm × 30 cm × 30 cm) for 2 weeks before the formal experiment. The formal experiment included four parts according to priority (Figure 1A): (I) observing fish basal locomotor activity under a normal condition, (II) evaluating fish aggressiveness using the “mirror-image test,” (III) evaluating fish boldness using the “box-emerging test,” and (IV) sampling fish to measure basal cortisol concentration.
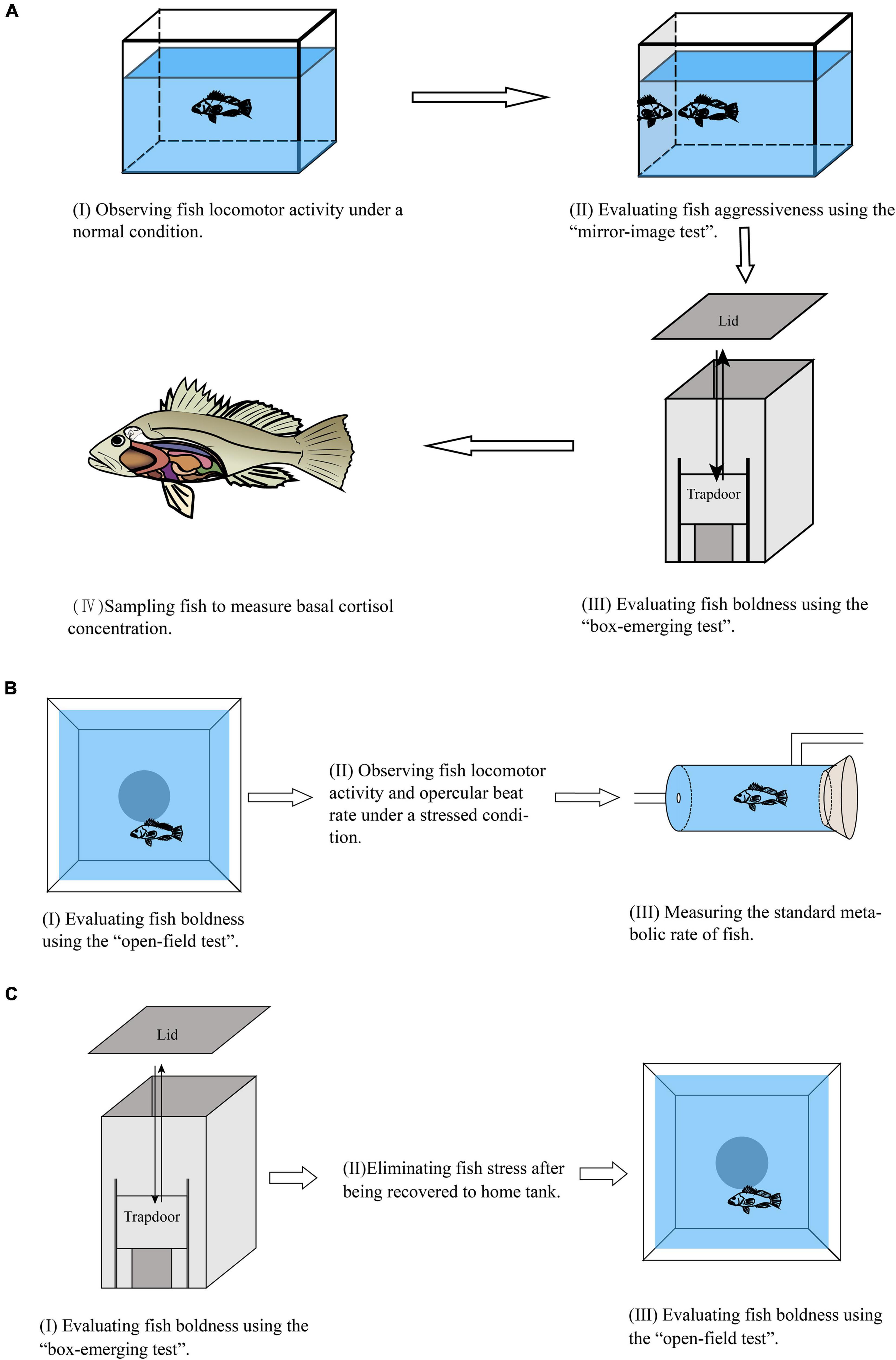
Figure 1. The flow chart of the experimental design. (A) Experiment I. (B) Experiment II. (C) Experiment III.
Based on our prior experience and careful observations, the 2-week acclimation period was sufficient for fish to resume normal behaviors and physiology (Zhang et al., 2021). Therefore, the fish locomotor activity in glass tanks during the formal experiment could be assumed to be under a normal condition, and we called this parameter basal locomotor activity. In detail, a camera (HDR-AS100V, Sony Corporation, Tokyo, Japan) was placed in front of each glass tank, and the videos were recorded from 8:00 a.m. to 10:00 a.m., during which no human interference occurred (Figure 1A). Each tank was filmed for 10 min, and then only the middle 5 min of each video was used to determine the basal locomotor activity to completely eliminate the possible disturbance from experimenters (Zhang et al., 2020a, b, 2021). The basal locomotor activity in this study was defined as time spent moving in five minutes, which has been proven to accurately and effectively reflect the basal locomotor activity of fish (Øverli et al., 2002a, b, 2006).
After observing fish basal locomotor activity, a mirror-image test, which has been verified to be valid in several fish species (Woodward et al., 2019; Da Silva-Pinto et al., 2020; Thore et al., 2020; Xu et al., 2021), was adopted to determine fish aggressiveness. In brief, a mirror (27 cm × 30 cm) was gently placed perpendicular to the bottom of the tank on a narrow side of each glass tank (Figure 1A). Videos were recorded for 12 min from the moment the mirror was introduced, and then the middle 10 min of each video was selected to measure the aggressive behavior of the fish. Fish aggressiveness was estimated by counting the number of instances of chasing, nipping, and biting of the target fish for the mirror (Woodward et al., 2019; Zhang et al., 2020b; Xu et al., 2021).
We used the “box-emerging test” to evaluate fish boldness, and the behavioral apparatus and experimental procedure followed the descriptions of previous studies (Brown and Braithwaite, 2004; Brown et al., 2005, 2007; Roberts et al., 2011; Zhang et al., 2019). In short, the apparatus (Figure 1A) mainly consisted of an opaque start box (10 cm × 10 cm × 20 cm) that was placed in one side of a plastic tank (50 cm × 30 cm × 30 cm). The start box was equipped with a vertically sliding trapdoor that could be opened and closed via a fine monofilament, and the top lid of the start box could be removed if required. When starting the trial, a fish was gently transferred from a glass tank to the start box using a hand-netting. After a 2 min acclimatization period, the trapdoor was slowly lifted, and the fish was free to emerge. The time taken by the fish to completely emerge from the start box was recorded. If the fish did not leave the shelter after 10 min, we removed the lid of the box. If the fish still did not leave the shelter after 15 min, we terminated the trial, and they were allocated the maximum time score of 900 s. In this test, the more time duration the fish needed to emerge from the start box, the lower boldness the fish had. One fish was tested at a time, and the seawater in the apparatus was refreshed after each trial.
To measure the basal cortisol levels of fish, all of the fish that had completed the box-emerging test were put back in the original glass tanks for 1 week. This further acclimation period allowed fish to recover to their normal behavioral and physiological status from possible handling stress. Then these fish were euthanized with an overdose of anesthetic (Tricaine methanesulfonate, MS-222, 100 mg/L), and the body weight (precision 0.01 mg) and body length (precision 0.01 mm) were recorded. Subsequently, fish were rapidly dissected, and the whole visceral masses (this was performed because the juveniles were too small to draw blood; Figure 1A) (Guo et al., 2017; Zhang et al., 2020a) were removed and frozen in liquid nitrogen and then stored at −80∘C until analyzing the cortisol concentrations. All sampling procedures were completed within 1 min.
The visceral mass samples were homogenized with normal saline, diluted 10 times, and centrifuged at 4∘C in a refrigerated centrifuge for 20 min at 3000 rpm. The supernatants were collected and used for analysis. The cortisol concentrations were measured using commercial ELISA Assay Kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer’s guidelines. The ELISA assay kit has been validated previously (Zhang et al., 2020a, 2021).
The second experiment was conducted from September 2019 to October 2019. Black rockfish juveniles were obtained from a hatchery in Wendeng, Shandong, China. Twenty-nine fish (mean body length 8.05 ± 0.19 cm, mean body weight 10.41 ± 0.34 g) were individually acclimated to the experimental condition (same to experiment I) for 2 weeks before the formal experiment. The formal experiment consisted of three parts (Figure 1B): (I) evaluating fish boldness using the “open-field test,” (II) observing fish stressed locomotor activity and opercular beat rate under a stressed condition (see below for further explanation), and (III) measuring the standard metabolic rate of fish.
Based on the observations of experiment I, we found that the fish were more willing to hide in the starting box rather than emerge from the shelter during the given testing period, and this phenomenon may lower the discrimination degree for boldness among the experimental fish. Therefore, in experiment II, another classical method, open-field test (Archard et al., 2012; Bergendahl et al., 2016; Salvanes, 2017; Thore et al., 2020), was adopted to evaluate fish boldness. The behavioral apparatus mainly included a quadrate tank (30 cm × 30 cm × 30 cm), which had a circle (diameter 10 cm) in the center of the tank bottom (Figure 1B). Each fish was taken out of the glass tank with a hand-netting and placed into the center circle of the test tank. A camera was fixed above the tank. Then a 10-min video was recorded from the time point when the fish were put in. The fish boldness was estimated by the time duration that fish head was in the center circle during the 10-min video. The more time duration the fish head was in the center circle, the higher boldness the fish had.
Based on the videos filmed in the open-field test, we also determined fish stressed locomotor activity and opercular beat rate. Notably, when conducting the open-field test, the fish were in a state of stress, given that each fish was transferred from a home tank to a relatively unfamiliar experimental tank using a hand-netting. Therefore, we defined the relevant behaviors as stressed behaviors (i.e., stressed locomotor activity and stressed opercular beat rate). Similarly, only the middle 5 min of each 10-min video was analyzed to determine the interested behaviors. The stressed locomotor activity was defined as time spent moving in 5 min, which was the same as experiment I. The opercular beat rate was measured as the number of opercular beats per minute (Braithwaite and Salvanes, 2005; Zhang et al., 2020a).
After completing the open-field test, all fish were recovered to their home tank and were fasted for 1 day. Then, each fish was introduced into a continuous-flow respirometer (Figure 1B) to measure the standard metabolic rate. The experimental apparatus and procedure followed the classical method, which was repeatedly evidenced to be valid in several studies (Xie and Sun, 1990; Fu et al., 2005). In brief, the apparatus consisted of 11 parallel respiratory chambers, one of which did not contain fish and was used as a control. The whole apparatus was covered by opaque sheets to completely eliminate the disturbances from the surrounding environment. An oxygen meter (S9-Field kit, METTLER TOLEDO, China) was used to measure the oxygen consumption rate of the experimental fish. The fish were introduced into the respiratory chambers at 20:00 on the last day and were given one night to recover from the possible handling stress. Then, the dissolved oxygen contents of water outlets were measured at 10:00, 12:00, 14:00, 16:00, and 20:00 on the next day, and the average value of the five measurements was used to calculate the standard metabolic rate of the experimental fish. The calculation formula of the standard metabolic rate (SMR) was SMR = △O2⋅V/m. In the formula, SMR [mg/(kg⋅h)] was the standard metabolic rate, △O2 (mg/L) was the difference between the dissolved oxygen content in the respiration chamber and the control respiration chamber, V (L/h) was the flow velocity in the respiration chamber, which was obtained by measuring the flow volume at the outlet of the respiration chamber for 1 min, and m (kg) was the weight of the experimental fish.
The third experiment was conducted in September 2020. Black rockfish juveniles were obtained from a hatchery in Wendeng, Shandong, China. Fifty-four fish (mean body length 9.10 ± 0.61 cm, mean body weight 12.46 ± 1.86 g) were individually acclimated to the experimental condition (same as experiment I) for 2 weeks before the formal experiment. In the formal experiment, the boldness of each fish was first evaluated by the box-emerging test (Figure 1C) and then recovered to their home tank for 1 day to eliminate the possible handling stress. After this acclimation period, the boldness of the same fish was subsequently evaluated by the open-field test (Figure 1C). The detailed procedures were the same as experiments I and II.
First, we classified each fish into the “bold fish” category or “shy fish” category using the hierarchical clustering analysis (Castanheira et al., 2013), based on the boldness value that was determined by box-emerging test in experiment I or open-field test in experiment II. The principle of the algorithm is: at the beginning, all the data points themselves are used as clusters, and then a specific algorithm is used to find the two closest clusters and merge them into one, and the above steps are repeated until the preset number of clusters is reached (Murtagh and Contreras, 2011a, b). Then, an independent-samples t-test was used to examine the difference of above-mentioned behavioral and physiological parameters (i.e., aggressiveness, locomotor activity, opercular beat rate, standard metabolic rate, and cortisol level) between the generated clusters (i.e., “bold fish” vs. “shy fish”). To further clarify the relationships among the boldness, aggressiveness, activity, respiration, metabolism, and cortisol, linear fitting analysis and Pearson’s correlation test were performed to pooled data. The same analytical method was also applied to clarify the relationships between the boldness values determined by the two boldness test apparatuses (experiment III). Normality of the data was tested with Kolmogorov–Smirnov’s test, and the homogeneity of variance in the data was tested using Levene’s test prior to independent-samples t-test. When data failed to meet independent-samples t-test assumptions (i.e., normality and homogeneity), transformations were applied [i.e., logarithmic transformation log10(1+x) for number of aggressive behavior, time spent moving (experiment I:box-emerging test)]. Statistical analyses were performed using SPSS 22.0 for windows. Differences were considered significant at a probability level of 0.05 (P < 0.05). The results were expressed as mean ± SE.
We generated two groups (bold fish, n = 9; shy fish, n = 44) using the hierarchical cluster analysis based on the latency to emerge from the start box in experiment I. Bold fish showed significantly more aggressive behavior compared to shy fish (t51 = 3.358, P = 0.001; Figure 2A). However, there were no significant differences in basal locomotor activity (t51 = 1.344, P = 0.185; Figure 3A) and basal cortisol levels (t51 = 0.546, P = 0.587; Figure 4A) between the two clusters. Fitting analysis showed that the emerging latency was significantly, negatively correlated with the number of aggressive behavior (Figure 2B). However, Pearson’s correlations between emerging latency and time spent moving under the normal condition (Figure 3B) or between emerging latency and basal cortisol level (Figure 4B) were not statistically significant (P > 0.05).
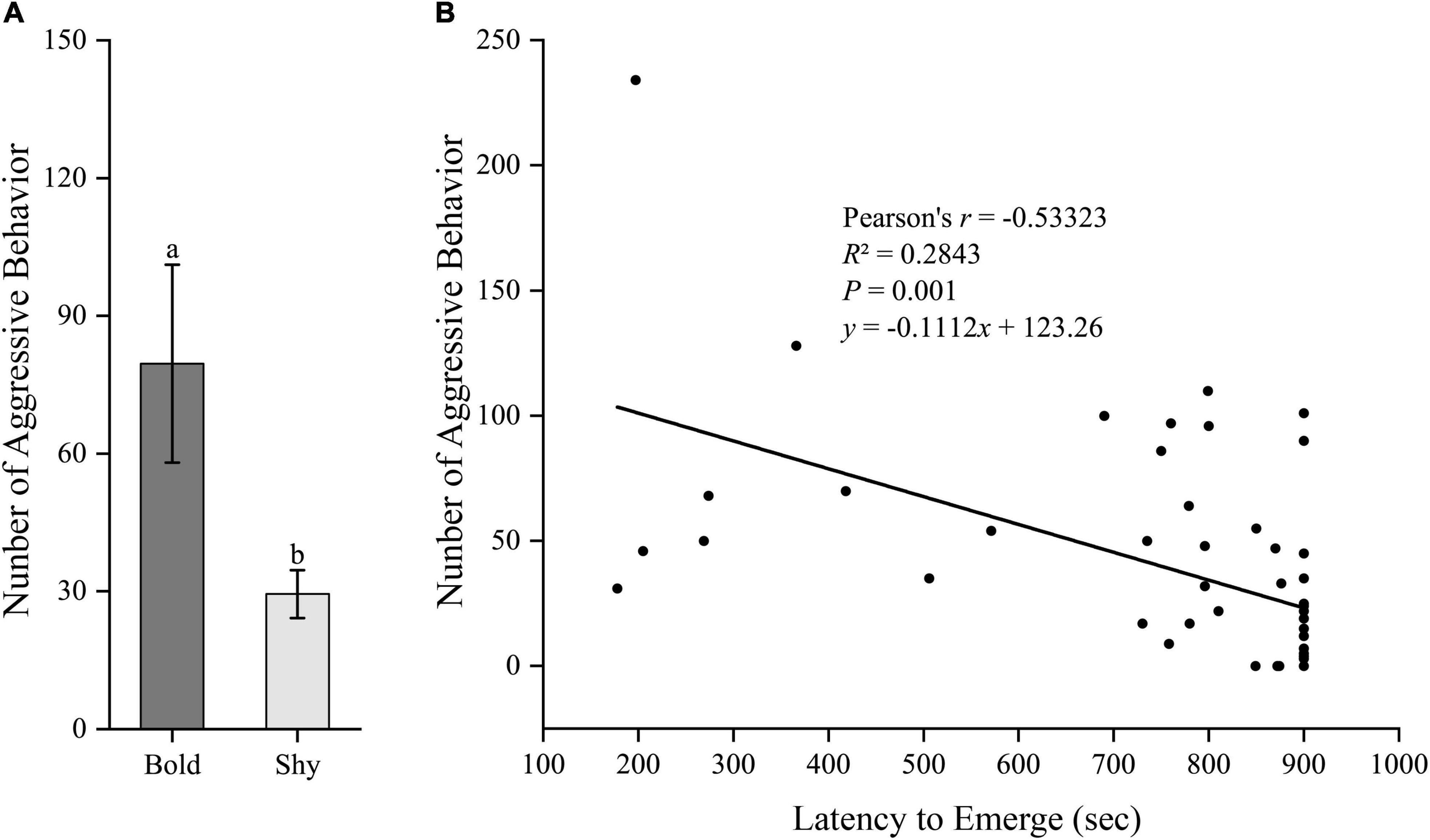
Figure 2. The boldness and aggressiveness of black rockfish. (A) The number of aggressive behavior in bold (n = 9) and shy (n = 44) groups. Different letters indicate significant differences (P < 0.05; independent samples t-test). (B) Linear fit of the latency to emerge from the start box (box-emerging boldness test) and the number of aggressive behavior (n = 53).
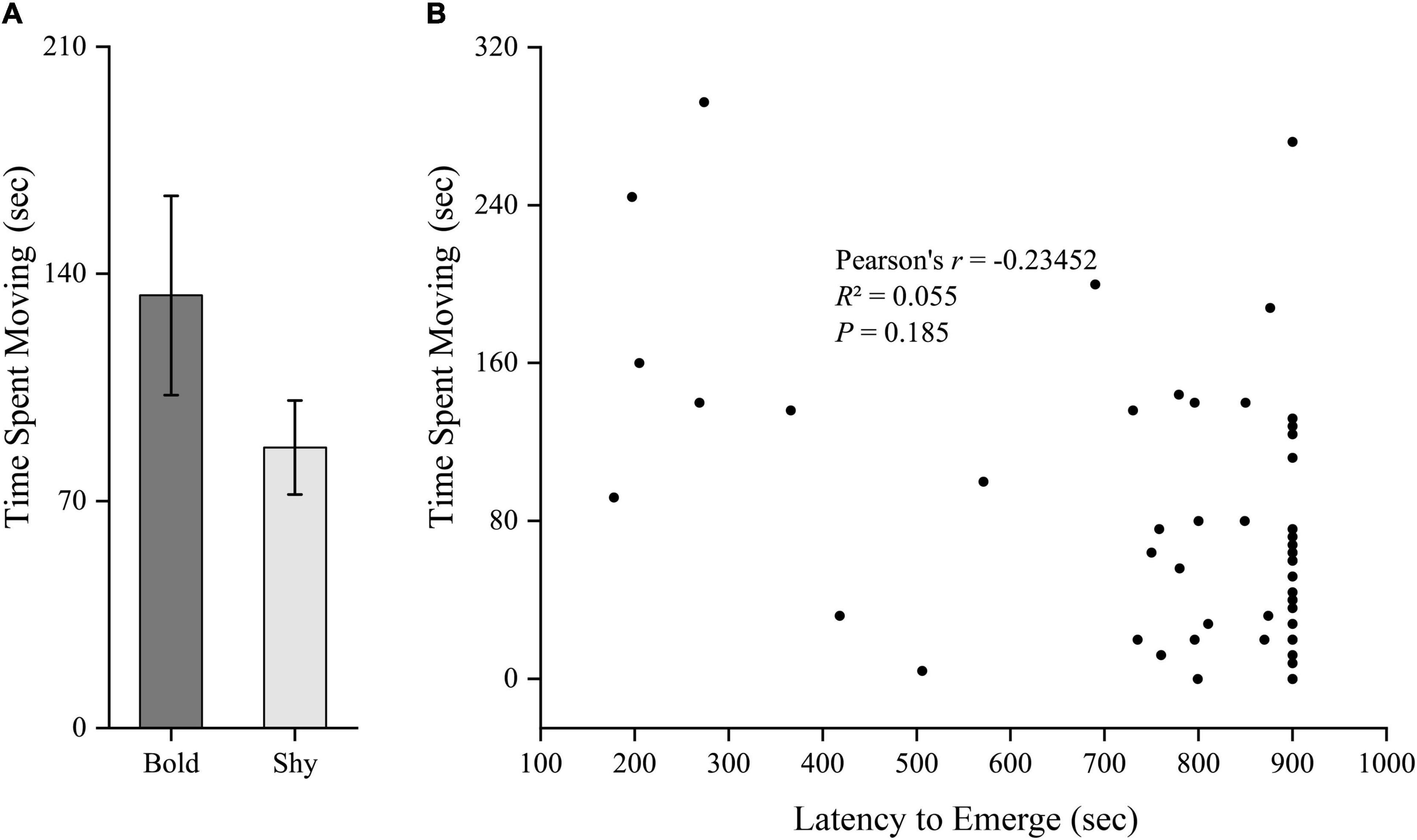
Figure 3. The boldness and basal locomotor activity of black rockfish. (A) The time spent moving (under normal condition) in bold (n = 9) and shy (n = 44) groups. (P > 0.05; independent samples t-test). (B) Pearson’s correlation between the latency to emerge from the start box (box-emerging boldness test) and the time spent moving (under normal condition) (n = 53).
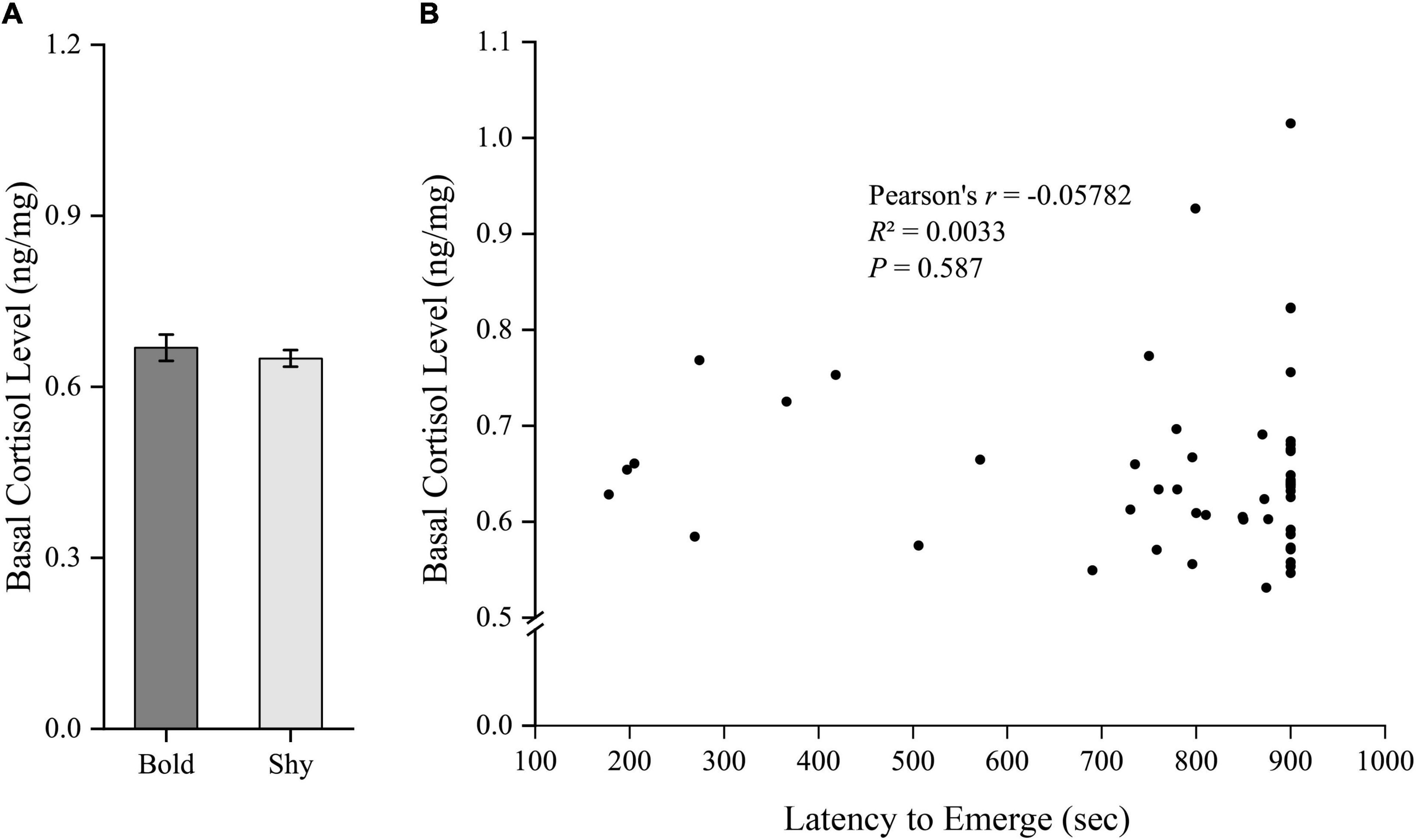
Figure 4. The boldness and basal cortisol level of black rockfish. (A) The basal cortisol level in bold (n = 9) and shy (n = 44) groups. (P > 0.05; independent samples t-test). (B) Pearson’s correlation between the latency to emerge from the start box (box-emerging boldness test) and the basal cortisol level (n = 53).
Similarly, we first generated two groups (bold fish, n = 9; shy fish, n = 20) using the hierarchical cluster analysis based on the time duration in the center circle in experiment II. Bold fish had significantly higher standard metabolic rate (t27 = 2.96, P = 0.006; Figure 5A) and stressed locomotor activity (t27 = 2.222, P = 0.035; Figure 6A), while had significantly lower stressed opercular beat rate (t27 = −2.078, P = 0.047; Figure 7A) compared to shy fish. Fitting analysis showed that the duration in center circle was significantly, positively correlated with standard metabolic rate (Figure 5B), and positively correlated with time spent moving under the stressed condition (Figure 6B), but negatively correlated with stressed opercular beat rate (Figure 7B).
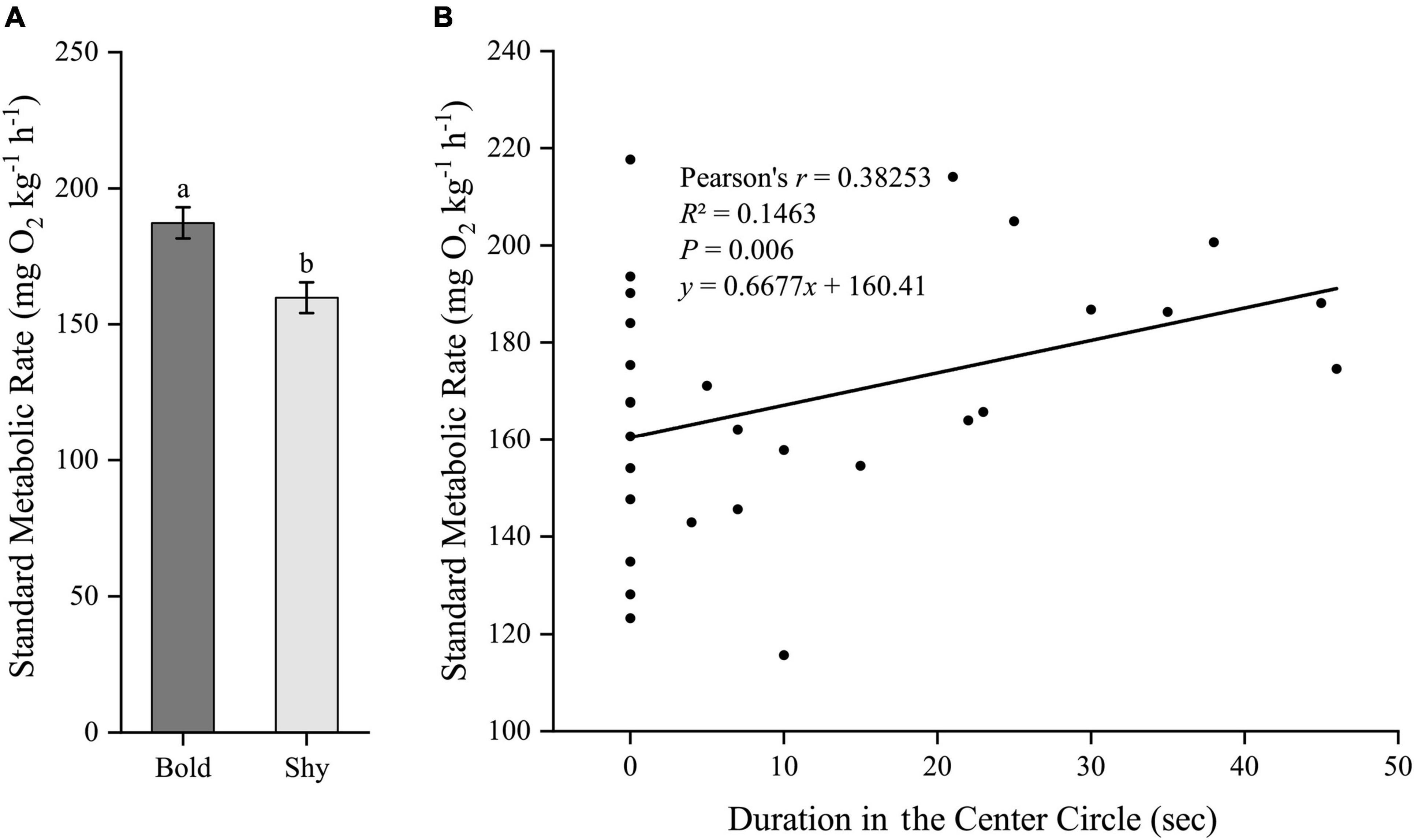
Figure 5. The boldness and standard metabolic rate of black rockfish. (A) The standard metabolic rate in bold (n = 9) and shy (n = 20) groups. Different letters indicate significant differences (P < 0.05; independent samples t-test). (B) Linear fit of the time duration in center circle (open-field boldness test) and the standard metabolic rate (n = 29).
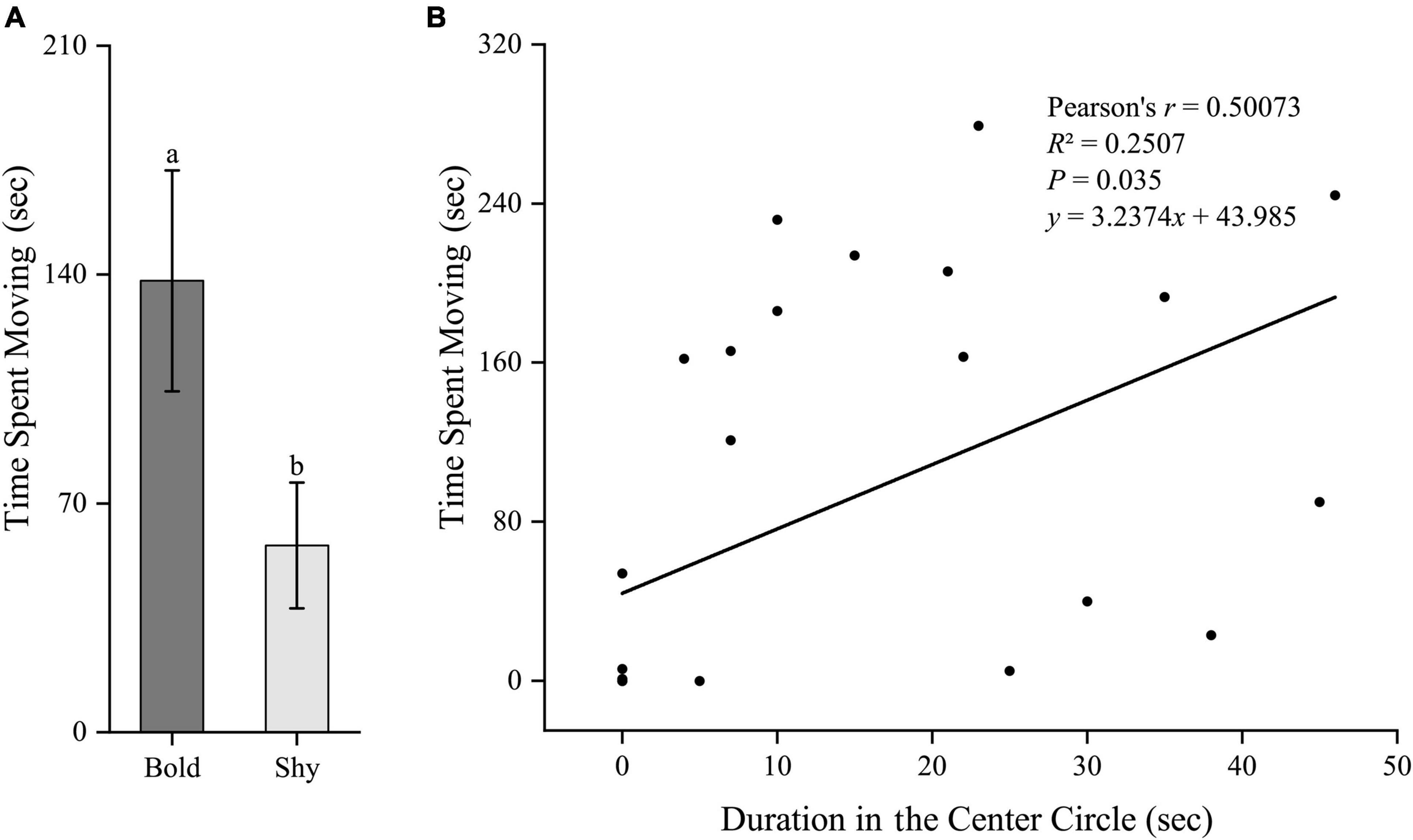
Figure 6. The boldness and stressed locomotor activity of black rockfish. (A) The time spent moving (under stressed condition) in bold (n = 9) and shy (n = 20) groups. Different letters indicate significant differences (P < 0.05; independent samples t-test). (B) Linear fit of the time duration in center circle (open-field boldness test) and the time spent moving (under stressed condition) (n = 29).
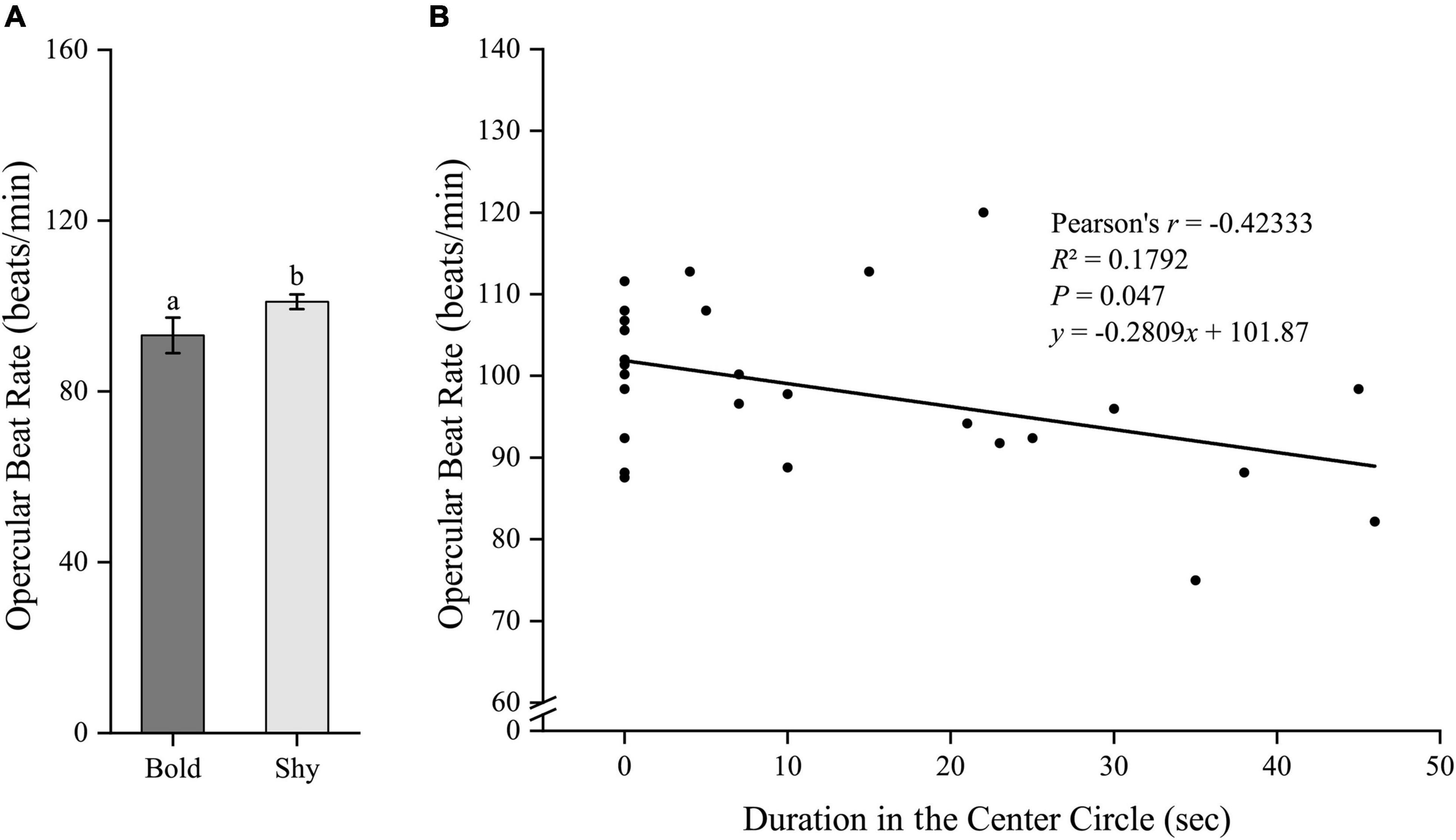
Figure 7. The boldness and stressed opercular beat rate of black rockfish. (A) The stressed opercular beat rate in bold (n = 9) and shy (n = 20) groups. Different letters indicate significant differences (P < 0.05; independent samples t-test). (B) Linear fit of the time duration in center circle (open-field boldness test) and the opercular beat rate (under stressed condition) (n = 29).
A significantly negative correlation between the duration in center circle in the open-field test and the emerging latency in the box-emerging test was detected (r = −0.275, P = 0.044, y = −4.650 x + 804.3; Figure 8A), which indicated that both boldness determination apparatuses were sensitive and valid to estimate the boldness of black rockfish.
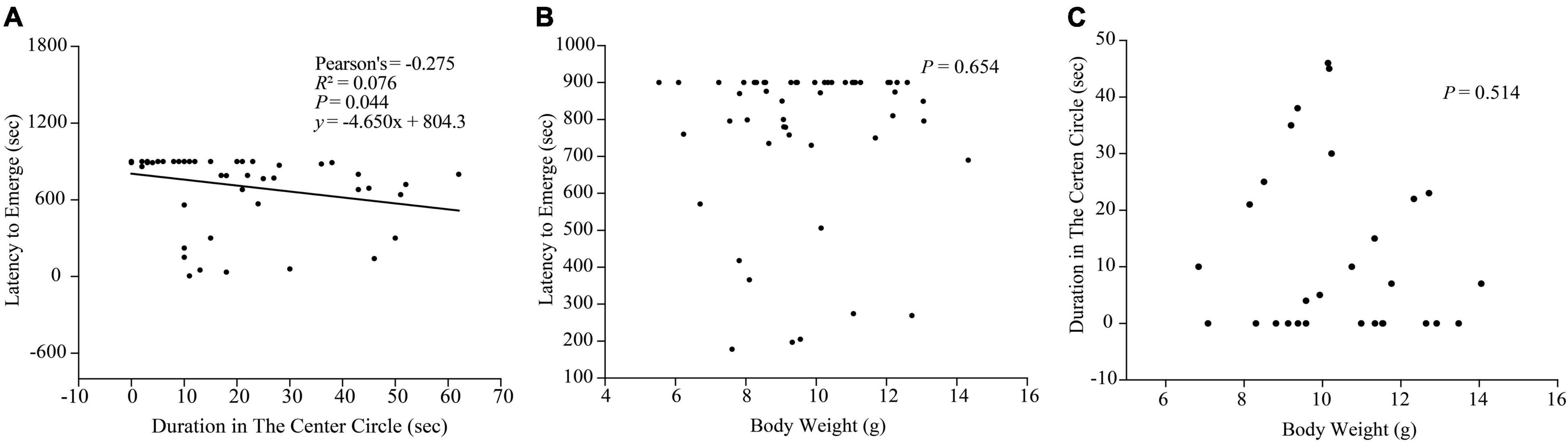
Figure 8. (A) Correlation between the duration in center circle in the open-field test and the emerging latency in the box-emerging test. (B) Correlation between body weight and latency to emerge in the box-emerging test (n = 53). (C) Correlation between body weight and duration in the center circle in the open-field test (n = 29).
Since we controlled the size of the experimental fish within a small gap, we did not find a significant relationship between the size and boldness of the experimental fish in the two boldness experiments (P > 0.05; Figures 8B,C).
The main results of this study showed that the boldness of black rockfish was significantly, positively correlated with fish aggressiveness, stressed locomotor activity, and standard metabolic rate, while was negatively correlated with stressed opercular beat rate. There were no significant correlations between boldness and basal locomotor activity or between boldness and basal cortisol level. These results preliminarily constructed the behavioral and physiological spectrum of black rockfish in the context of fish personality.
Aggressiveness has been linked with a variety of aquaculture problems including decreased feed intake, higher body/fin damage, stimulated chronic stress, and lower disease resistance, which may impair fish welfare, growth performance, and fish quality (Bonga, 1997; Mommsen et al., 1999; Conte, 2004; Ashley, 2007; Näslund and Johnsson, 2014). Several previous studies attempted to decrease fish aggression by manipulating environmental (rearing) factors, such as environmental enrichment (Näslund and Johnsson, 2014; Zhang et al., 2020a, b) and light intensity (Dou et al., 2000; Xi et al., 2017a). However, relatively little research has been conducted on the relationship between fish aggression and other personality traits. Here, we attempted to link fish aggressiveness with fish boldness. We found that the boldness was positively correlated with fish aggressiveness, and bold fish expressed significantly higher aggressive behavior for the mirror image than shy fish, which were consistent with previous findings (Colléter and Brown, 2011; Dahlbom et al., 2011; Castanheira et al., 2017). These results may have potential application value, which was, if the farmers attempted to decrease intraspecific aggression, they could screen relatively shy individuals through laboratory experiments or other methods for the subsequent rearing. Obviously, this is just a preliminary assumption, and a series of further research is needed, for example, how to rapidly and efficiently identify fish boldness, and how to control fish boldness through environmental manipulation or other methods? On the other hand, bold fish may have stronger adaptability and survivability in the wild. Therefore, for the stock conservation and supplementation projects that fish are destined to be released into wild, which personality is beneficial for fish fitness is also a valuable question to be solved in future research.
The relationships between boldness and activity and between boldness and stress were inconsistent between experiment I and experiment II. In experiment I, we did not find correlations between boldness and basal locomotor activity or between boldness and cortisol level (an indicator of physiological stress). In experiment II, we detected significant correlations between boldness and stressed locomotor activity and between boldness and opercular beat rate (an indicator of behavioral stress). These discrepancies may result from the differences in experimental environments. As mentioned above, the basal locomotor activity and cortisol level in experiment I were determined after fish have adapted to the environment for 2 weeks, so these two parameters could be seen as the basal status. But in experiment II, the stressed locomotor activity and opercular beat rate were determined immediately after fish were introduced into the experimental tank, so these two parameters could be seen as the stressed status. Therefore, the above results could be summarized as follows: fish boldness was significantly correlated with stressed activity and stressed respiration, while there were no significant correlations between boldness and basal activity or between boldness and basal stress. These were similar to the results of several previous studies (Øverli et al., 2002b; Barreto and Volpato, 2011; Vindas et al., 2017), but did not agree with others (Silva et al., 2010; Backström et al., 2011). These discrepancies may mainly come from differences in fish species and experimental situations. For example, bold individuals had lower basal cortisol levels than shy individuals in rainbow trout and Senegalese sole Solea senegalensis (Silva et al., 2010; Backström et al., 2011), but no significant correlations were detected in Atlantic salmon (Vindas et al., 2017). In gilthead seabream Sparus aurata, boldness (indicated by latency to take risks) was negatively correlated to swimming activity after confinement (an acute stress) (Herrera et al., 2014). In Nile tilapia O. niloticus, the ventilation rates of fish correlated with the rate of feeding resumption following transfer to a novel social-isolation environment, i.e., bold fish had lower baseline ventilation rates (Barreto and Volpato, 2011). These results strongly suggest that fish personality is highly species- and context-dependent, and the research for specific species and context is necessary, to a certain degree.
Our result showed that boldness was significantly correlated with SMR, and bold fish had higher SMR than shy fish. This result was in accordance with common carp Cyprinus carpio (Huntingford et al., 2010). One alternative explanation for this phenomenon was that bold fish adopted an energetically expensive strategy, while shy fish was energetically conservative. In normal conditions, bold fish expressed higher aggressiveness, sometimes higher exploration, and all these biological processes mean increased energy requirement and subsequently higher metabolic rate. From the contrary aspect, to maintain their higher metabolism, bold fish had to explore in a higher frequency and express higher aggressiveness to get sufficient food (Biro and Stamps, 2010; Burton et al., 2011).
Although both boldness determination apparatuses in experiment I and experiment II could successfully distinguish the boldness of black rockfish and a significant correlation between boldness values was detected in experiment III, only nine of the 53 fish were identified to be bold using the box-emerging test, whereas nine of the 29 fish were identified to be bold using the open-field test. The reason for this difference may be that black rockfish is a kind of relatively timid and sedentary fish. Based on our careful observations, they like a habitat that has a dark shelter. In the box-emerging test, the start box was opaque, which created a dark space to hide for black rockfish. On the contrary, the experimental apparatus in the open-field test was a transparent glass tank, almost no shadows, which inspired the exploration of black rockfish. This hints us that when we design the experimental apparatus to estimate fish boldness, in order to get a higher discrimination degree, it is essential to fully consider the ecological habits of the target fish species.
Overall, this study clearly showed that a behavioral syndrome exists in black rockfish. Bold fish had significantly higher aggressiveness, standard metabolic rate, and stressed locomotor activity but lower stressed opercular beat rate. These results indicated that the boldness of black rockfish could be used as a discrimination tool to predict fish aggressiveness and metabolic rate, which may have valuable applications for decreasing fish harmful aggression and increasing fish welfare in the aquaculture industry. Boldness was linked to aggression as well as other behavioral and physiological traits in this study. However, growth traits that are one of the most important characteristics in aquaculture are not involved in the article. Therefore, future research should combine the short-term behavioral experiments and long-term rearing experiments to comprehensively explore the interaction effects or trade-offs of boldness on fish aggressiveness and growth performance.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of the Ocean University of China.
YF wrote the first draft of the manuscript and performed the experiments and statistical analysis. YF and ZoZ contributed to the conception and design of the study. All authors contributed to manuscript revision, read, and approved the submitted version.
This research was funded by the National Natural Science Foundation of China (32072966) and the Province Key Research and Development Program of Zhejiang (2021C02047).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Archard, G. A., Earley, R. L., Hanninen, A. F., and Braithwaite, V. A. (2012). Correlated behaviour and stress physiology in fish exposed to different levels of predation pressure. Funct. Ecol. 26, 637–645. doi: 10.1111/j.1365-2435.2012.01968.x
Ashley, P. J. (2007). Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 104, 199–235. doi: 10.1016/j.applanim.2006.09.001
Backström, T., Schjolden, J., Øverli, Ø, Thörnqvist, P., and Winberg, S. (2011). Stress effects on AVT and CRF systems in two strains of rainbow trout (Oncorhynchus mykiss) divergent in stress responsiveness. Horm. Behav 59, 180–186. doi: 10.1016/j.yhbeh.2010.11.008
Barreto, R. E., and Volpato, G. L. (2011). Ventilation rates indicate stress-coping styles in Nile tilapia. J. Biosciences. 36, 851–855. doi: 10.1007/s12038-011-9111-4
Bergendahl, I. A., Salvanes, A. G. V., and Braithwaite, V. A. (2016). Determining the effects of duration and recency of exposure to environmental enrichment. Appl. Anim. Behav. Sci. 176, 163–169. doi: 10.1016/j.applanim.2015.11.002
Biro, P. A., and Stamps, J. A. (2010). Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol. Evol. 25, 653–659. doi: 10.1016/j.tree.2010.08.003
Bonga, S. E. W. (1997). The stress response in fish. Physiol. Rev. 77, 591–625. doi: 10.1152/physrev.1997.77.3.591
Braithwaite, V. A., and Salvanes, A. G. (2005). Environmental variability in the early rearing environment generates behaviourally flexible cod: Implications for rehabilitating wild populations. Philos. Trans. R. Soc. B: Biol. Sci. 272, 1107–1113. doi: 10.1098/rspb.2005.3062
Brown, C., and Braithwaite, V. A. (2004). Size matters: A test of boldness in eight populations of the poeciliid Brachyraphis episcopi. Anim. Behav. 68, 1325–1329. doi: 10.1016/j.anbehav.2004.04.004
Brown, C., Burgess, F., and Braithwaite, V. A. (2007). Heritable and experiential effects on boldness in a tropical poeciliid. Behav. Ecol. Sociobiol. 62, 237–243. doi: 10.1007/s00265-007-0458-3
Brown, C., Jones, F. C., and Braithwaite, V. A. (2005). In situ examination of boldness–shyness traits in the tropical poeciliid, Brachyraphis episcopi. Anim. Behav. 70, 1003–1009. doi: 10.1016/j.anbehav.2004.12.022
Burton, T., Killen, S. S., Armstrong, J. D., and Metcalfe, N. B. (2011). What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Philos. Trans. R. Soc. B: Biol. Sci. 1724, 3465–3473. doi: 10.2307/41315096
Castanheira, M. F., Conceição, L. E. C., Millot, S., Rey, S., Bégout, M. L., DamsgAard, B., et al. (2017). Coping styles in farmed fish: Consequences for aquaculture. Rev. Aquacult. 9, 23–41. doi: 10.1111/raq.12100
Castanheira, M. F., Herrera, M., Costas, B., Conceição, L. E. C., and Martins, C. I. M. (2013). Can we predict personality in fish? Searching for consistency over time and across contexts. PLoS One. 8:e62037. doi: 10.1371/journal.pone.0062037
Colléter, M., and Brown, C. (2011). Personality traits predict hierarchy rank in male rainbowfish social groups. Anim. Behav. 81, 1231–1237. doi: 10.1016/j.anbehav.2011.03.011
Conte, F. S. (2004). Stress and the welfare of cultured fish. Appl. Anim. Behav. Sci. 86, 205–223. doi: 10.1016/j.applanim.2004.02.003
Da Silva-Pinto, T., Silveira, M. M., de Souza, J. F., Moreira, A. L. P., Vieira, E. A., Longo, G. O., et al. (2020). Damselfish face climate change: Impact of temperature and habitat structure on agonistic behavior. PLoS One. 15:e0235389. doi: 10.1371/journal.pone.0235389
Dahlbom, S. J., Lagman, D., Lundstedt-Enkel, K., Sundström, L. F., and Winberg, S. (2011). Boldness predicts social status in zebrafish (Danio rerio). PLoS One. 6:e23565. doi: 10.1371/journal.pone.0023565
Dingemanse, N. J., Kazem, A. J. N., Réale, D., and Wright, J. (2010). Behavioural reaction norms: Animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. doi: 10.1016/j.tree.2009.07.013
Dou, S., Seikai, T., and Tsukamoto, K. (2000). Cannibalism in Japanese flounder juveniles, Paralichthys olivaceus, reared under controlled conditions. Aquaculture. 182, 149–159. doi: 10.1016/s0044-8486(99)00256-2
Fu, S., Xie, X., and Cao, Z. (2005). Effect of fasting and repeat feeding on metabolic rate in southern catfish, Silurus meridionalis Chen. Mar. Freshw. Behav. Phy. 38, 191–198. doi: 10.1080/10236240500231532
Guo, H., Zhang, X., and Johnsson, J. I. (2017). Effects of size distribution on social interactions and growth of juvenile black rockfish (Sebastes schlegelii). Appl. Anim. Behav. Sci. 194, 135–142. doi: 10.1016/j.applanim.2017.05.004
Herrera, M., Castanheira, M. F., Conceição, L. E., and Martins, C. I. (2014). Linking risk taking and the behavioral and metabolic responses to confinement stress in gilthead seabream Sparus aurata. Appl. Anim. Behav. Sci. 155, 101–108. doi: 10.1016/j.applanim.2014.03.001
Huntingford, F. A., Andrew, G., Mackenzie, S., Morera, D., Coyle, S. M., Pilarczyk, M., et al. (2010). Coping strategies in a strongly schooling fish, the common carp Cyprinus carpio. J. Fish Biol. 76, 1576–1591. doi: 10.1111/j.1095-8649.2010.02582.x
Koolhaas, J. M., De Boer, S. F., Coppens, C. M., and Buwalda, B. (2010). Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Front. Neuroendocrin. 31:307–321. doi: 10.1016/j.yfrne.2010.04.001
Koolhaas, J. M., Korte, S. M., De Boer, S. F., Van Der Vegt, B. J., Van Reenen, C. G., Hopster, H., et al. (1999). Coping styles in animals: Current status in behavior and stress-physiology. Neurosci. Biobehav. R. 23, 925–935. doi: 10.1016/s0149-7634(99)00026-3
Martins, C. I. M., Silva, P. I. M., Conceição, L. E. C., Costas, B., Höglund, E., and Øverli, Ø, et al. (2011). Linking fearfulness and coping styles in fish. PLoS One 6:e28084. doi: 10.1371/journal.pone.0028084
Mittelbach, G. G., Ballew, N. G., and Kjelvik, M. K. (2014). Fish behavioral types and their ecological consequences. Can. J. Fish. Aquat. Sci. 71, 927–944. doi: 10.1139/cjfas-2013-0558
Mommsen, T. P., Vijayan, M. M., and Moon, T. W. (1999). Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fisher. 9, 211–268. doi: 10.1023/A:1008924418720
Murtagh, F., and Contreras, P. (2011a). Algorithms for hierarchical clustering: An overview. Wires. Data Min. Knowl. 2, 86–97. doi: 10.1002/widm.53
Murtagh, F., and Contreras, P. (2011b). Methods of hierarchical clustering. arXiv [Preprint] arXiv: 1105.0121,
Näslund, J., and Johnsson, J. I. (2014). Environmental enrichment for fish in captive environments: Effects of physical structures and substrates. Fish Fish. 17, 1–30. doi: 10.1111/faf.12088
Øverli, Ø, Kotzian, S., and Winberg, S. (2002a). Effects of cortisol on aggression and locomotor activity in rainbow trout. Horm. Behav 42, 53–61. doi: 10.1006/hbeh.2002.1796
Øverli, Ø, Pottinger, T. G., Carrick, T. R., Øverli, E., and Winberg, S. (2002b). Differences in behaviour between rainbow trout selected for high- and low-stress responsiveness. J. Exp. Biol. 205, 391–395. doi: 10.1242/jeb.205.3.391
Øverli, Ø, Sørensen, C., and Nilsson, G. (2006). Behavioral indicators of stress-coping style in rainbow trout: Do males and females react differently to novelty? Physiol. Behav 87, 506–512. doi: 10.1016/j.physbeh.2005.11.012
Øverli, Ø, Sørensen, C., Pulman, K. G., Pottinger, T. G., Korzan, W., Summers, C. H., et al. (2007). Evolutionary background for stress-coping styles: Relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci. Biobehav. Rev 31, 396–412. doi: 10.1016/j.neubiorev.2006.10.006
Réale, D., Dingemanse, N. J., Kazem, A. J., and Wright, J. (2010a). Evolutionary and ecological approaches to the study of personality. Philos. Trans. R. Soc. B: Biol. Sci. 365, 3937–3946. doi: 10.1098/rstb.2010.0222
Réale, D., Garant, D., Humphries, M. M., Bergeron, P., Careau, V., and Montiglio, P. (2010b). Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos. Trans. R. Soc. B: Biol. Sci. 365, 4051–4063. doi: 10.1098/rstb.2010.0208
Réale, D., Reader, S. M., Sol, D., McDougall, P. T., and Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. doi: 10.1111/j.1469-185x.2007.00010.x
Roberts, L. J., Taylor, J., and de Leaniz, C. G. (2011). Environmental enrichment reduces maladaptive risk-taking behavior in salmon reared for conservation. Biol. Conserv. 144, 1972–1979. doi: 10.1016/j.biocon.2011.04.017
Salvanes, A. (2017). Are antipredator behaviours of hatchery Salmo salar juveniles similar to wild juveniles? J. Fish Biol. 90, 1785–1796. doi: 10.1111/jfb.13268
Sih, A., Bell, A. M., and Johnson, J. C. (2004a). Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. doi: 10.1016/j.tree.2004.04.009
Sih, A., Bell, A. M., Johnson, J. C., and Ziemba, R. E. (2004b). Behavioral syndromes: An integrative overview. Q. Rew. Biol. 79, 241–277. doi: 10.1086/422893
Silva, P. I. M., Martins, C. I, Engrola, S., Marino, G., Øverli, Ø, and Conceição, L. E. (2010). Individual differences in cortisol levels and behaviour of Senegalese sole (Solea senegalensis) juveniles: Evidence for coping styles. Appl. Anim. Behav. Sci 124, 75–81. doi: 10.1016/j.applanim.2010.01.008
Stamps, J. A. (2016). Individual differences in behavioural plasticities. Biol. Rev. 91, 534–567. doi: 10.1111/brv.12186
Thore, E. S. J., Brendonck, L., and Pinceel, T. (2020). Conspecific density and environmental complexity impact behaviour of turquoise killifish (Nothobranchius furzeri). J. Fish Biol. 97, 1448–1461. doi: 10.1111/jfb.14512
Toms, C. N., Echevarria, D. J., and Jouandot, D. J. (2010). A methodological review of personality-related studies in fish: Focus on the shy-bold axis of behavior. Int. J. Comp. Psychol. 23, 1–25.
Vindas, M. A., Gorissen, M., Höglund, E., Flik, G., Tronci, V., Damsgård, B., et al. (2017). How do individuals cope with stress? Behavioural, physiological and neuronal differences between proactive and reactive coping styles in fish. J. Exp. Biol. 220, 1524–1532. doi: 10.1242/jeb.153213
Wilson, V., Guenther, A., Øverli, Ø, Seltmann, M. W., and Altschul, D. (2019). Future directions for personality research: Contributing new insights to the understanding of animal behavior. Animals 9, 240. doi: 10.3390/ani9050240
Woodward, M. A., Winder, L. A., and Watt, P. J. (2019). Enrichment increases aggression in zebrafish. Fishes 4, ∗∗∗q, doi: 10.3390/fishes4010022
Xi, D., Zhang, X., Lü, H., and Zhang, Z. (2017a). Cannibalism in juvenile black rockfish, Sebastes schlegelii (Hilgendorf, 1880), reared under controlled conditions. Aquaculture. 479, 682–689. doi: 10.1016/j.aquaculture.2017.07.007
Xi, D., Zhang, X., Lü, H., and Zhang, Z. (2017b). Prediction of cannibalism in juvenile black rockfish, Sebastes schlegelii (Hilgendorf, 1880), based on morphometric characteristics and paired trials. Aquac. Res. 48, 3198–3206. doi: 10.1111/are.13150
Xie, X., and Sun, R. (1990). The bioenergetics of the southern catfish (Silurus meridionalis Chen). I. Resting metabolic rate as a function of body weight and temperature. Physiol. Zool. 63, 1181–1195. doi: 10.1086/physzool.63.6.30152639
Xu, X., Guo, H., Zhang, Z., Wang, Y., Qin, J., and Zhang, X. (2021). Impact of pre-aggressive experience on behavior and physiology of black rockfish (Sebastes schlegelii). Aquaculture. 536, 736416. doi: 10.1016/j.aquaculture.2021.736416
Zhang, Z., Bai, Q., Xu, X., Guo, H., and Zhang, X. (2020a). Effects of environmental enrichment on the welfare of juvenile black rockfish Sebastes schlegelii: Growth, behavior and physiology. Aquaculture. 518, 734782. doi: 10.1016/j.aquaculture.2019.734782
Zhang, Z., Xu, X., Wang, Y., and Zhang, X. (2020b). Effects of environmental enrichment on growth performance, aggressive behavior and stress-induced changes in cortisol release and neurogenesis of black rockfish Sebastes schlegelii. Aquaculture. 528, 735483. doi: 10.1016/j.aquaculture.2020.73548
Zhang, Z., Fu, Y., Guo, H., and Zhang, X. (2021). Effect of environmental enrichment on the stress response of juvenile black rockfish Sebastes schlegelii. Aquaculture. 533, 736088. doi: 10.1016/j.aquaculture.2020.736088
Keywords: personality, boldness, aggressiveness, standard metabolic rate, Sebastes schlegelii
Citation: Fu Y, Zhang Z, Zhang Z, Shen F, Xu X, Li Z, Zhang Y and Zhang X (2021) Boldness Predicts Aggressiveness, Metabolism, and Activity in Black Rockfish Sebastes schlegelii. Front. Mar. Sci. 8:770180. doi: 10.3389/fmars.2021.770180
Received: 03 September 2021; Accepted: 29 October 2021;
Published: 29 November 2021.
Edited by:
Huang Wei, Second Institute of Oceanography, Ministry of Natural Resources, ChinaReviewed by:
Fawen Hu, Marine Biology Institute of Shandong Province, ChinaCopyright © 2021 Fu, Zhang, Zhang, Shen, Xu, Li, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiumei Zhang, eGl1bWVpMTIyN0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.